Abstract
The annual cost of hospital care services in the US has risen to over $1 trillion despite relatively worse health outcomes compared to similar nations. These trends accentuate a growing need for innovative care delivery models that reduce costs and improve outcomes. HaH—a program that provides patients acute-level hospital care at home—has made significant progress over the past two decades. Technological advancements in remote patient monitoring, wearable sensors, health information technology infrastructure, and multimodal health data processing have contributed to its rise across hospitals. More recently, the COVID-19 pandemic brought HaH into the mainstream, especially in the US, with reimbursement waivers that made the model financially acceptable for hospitals and payors. However, HaH continues to face serious challenges to gain widespread adoption. In this review, we evaluate the peer-reviewed evidence and discuss the promises, challenges, and what it would take to tap into the future potential of HaH.
Subject terms: Health services, Diagnostic markers, Health care economics, Health policy
Introduction
Prior to the 19th century, hospitals in the United States (US) primarily served poor and marginalized communities while the upper class received medical and surgical care at home1. The mid to late 19th century brought about the technological sophistication of medical practice and the professionalization of healthcare, notably nursing, which reshaped “the day-to-day texture of hospital life” and improved clinical outcomes, thereby driving the transition from acute home care to hospital care2. The formation of academic medical centers and centralization of emerging medical technologies cemented the hospital’s role as the primary site of care for sick patients by the 20th century3,4. However, record-high hospital expenditures and increasing patient volumes has not necessarily translated into better health outcomes, spotlighting problems associated with inpatient hospital stays, such as nosocomial infection, iatrogenic complications, and medical errors5–10. With over $1 trillion spent on hospital services in 2021 and projected increases in the coming years, there is an urgent need to explore more efficient care delivery models11. Growing evidence and advancements in digital health technologies have positioned the Hospital at Home (HaH) model as a promising strategy to reduce health care spending and improve patient outcomes. A recent comprehensive meta-analysis of randomized trials of HaH versus hospital care demonstrated lower risk of readmission or long-term care admission and higher patient satisfaction with at-home acute care12. This begs the question: does the hospital need to be the primary venue of care for those who aren’t critically ill?
Out of necessity rather than intent, the COVID-19 pandemic tested this hypothesis. The pandemic presented hospitals with the challenge of caring for (and getting reimbursed for) sick patients while limiting admissions to only those who required critical care. While prior attempts to utilize home-based care to shorten hospital stays and reduce hospital admissions had already demonstrated clinical utility, in the US there was a scarcity of adoption due to lack of perceived need, compelling evidence of safety, challenges in altering health care delivery, or supportive reimbursement models13–15. The absence of sustained strategies to reduce hospital admissions after the peak of the COVID-19 pandemic reveals some of the obstacles that must be addressed for HaH models to gain wider acceptance in the US. Globally, HaH studies have reported noninferior and even superior patient outcomes, higher quality of life, and cost effectiveness compared to inpatient care for select clinical conditions16–23. Notably, in countries like Australia and Norway, in-person at-home acute care has gained significant traction and has been offered in most hospitals for over 15 years24,25.
Regardless of adoption, the primary application for at-home care programs continues to be management of chronic disease and the primary intervention remains in-person clinical staff with a gradual implementation of virtual care, and despite the availability, there has not been much evaluation of digital remote monitoring tools even though there was rampant use for acute care delivery during the COVID-19 pandemic. Further, a new crop of health technology and care delivery companies has risen to help health systems set up HaH programs, utilizing care delivery approaches ranging from in-person to completely virtual. In this manuscript we provide an assessment of the major US published and ongoing studies in the field of HaH, highlighting the promises and challenges that HaH programs face to scale and become a mainstream alternative form of acute care delivery. For the purposes of this manuscript, we defined HaH as fully substituting acute inpatient hospital admission with at-home care, whether it be delivered by in-person, virtual or hybrid (i.e., in-person with virtual elements) care models.
Historical context
The HaH model is not a new one. The first published trials come from the United Kingdom in the late 1970s. The first US-based prospective study of providing hospital-level care in the home, in which physicians made in-person visits, was published in the late 1990s26 (Supplementary Table 1). This single-site study demonstrated the feasibility, safety, and cost-effectiveness of an HaH program in participants >65 in four different acute medical conditions requiring hospital care (community acquired pneumonia (CAP), exacerbations of chronic heart failure (CHF) and chronic obstructive pulmonary disease (COPD) and cellulitis).
In-person
This early foray in US HaH was characterized by a reliance on a clinician’s physical presence in the home. In the initial HaH study26, after a patient was deemed appropriate for admission by an emergency room physician, the patient was consented, sent home in an ambulance and unless the patient refused, a nurse was present to provide 1:1 care for the first 24 h, then every 8 h, followed by as needed. A HaH physician did at least daily in-person visits and determined the frequency of monitoring as well as appropriateness for discharge. These visits were found to be 40% cheaper than an inpatient hospitalization and with higher patient satisfaction26. To ensure the results were not provider/site specific, a follow-up national study included 4 facilities (3 Medicare managed care entities and 1 by the Veterans Health Administration)27. In this study, patients were given a choice between an inpatient hospital admission or acute care at home in a HaH model after an emergency physician had determined they needed to be admitted; most participants selected HaH. The HaH group had a shorter duration of acute care, fewer procedures, less in-home medical devices, fewer complications, and higher levels of satisfaction at a lower cost with better clinical outcomes27. Subsequently, between 2005 and 2009, several separate publications reported on patient satisfaction, functional outcomes, caregiver stress, and HaH program costs28–31. All reports showed favorable results for the HaH cohorts compared to hospitalized controls, acknowledging limitations due to selection bias, absence of randomization, and data missingness.
Telemedicine
With increasing telemedicine acceptance there was a move from in-person to telemedicine via phone or video visit. In 2004, during the nascent stages of globalizing high-speed internet, a small prospective study on HaH solely using telemedicine showed that out of 25 study participants who required hospital admission (but not intensive care unit admission) for community acquired pneumonia, cellulitis, or urinary tract infection, 8 avoided index hospital admission and received 100% of their acute care at home. The other 17 were admitted to the hospital with early transfer to HaH to complete their hospitalization at home32, demonstrating the feasibility of HaH by telemedicine. The duration of acute care, readmissions, and costs were all lower in the HaH group compared to matched controls of hospitalized patients32.
In a typical study32, the patient’s set up required a nearby telephone outlet, and a second member of the telemedicine team communicated with the patient from a central station at the hospital. The patient was taught to obtain their vital signs using a blood pressure cuff, a stethoscope on the chest, and a pulse oximeter on the finger. Note that the technology required to do this had already been around for decades, the only novelty was virtual communication of biometrics performed either by a care provider or the patient themselves. Interestingly, despite the introduction of the smartphone, medical apps, electronic health records (EHR), and wearable sensors for biometric monitoring, it was not until 2015 that their potential was evaluated for HaH care33.
From analog to digital
The remote patient monitoring movement, which began in the 1970s (STARPAHC program), had already seen an evolution of novel sensors that could collect biometrics longitudinally with high fidelity34. Initially it was activity trackers with step count and heart metrics in the early 2000s, transmitting data via Cellular to the cloud or Bluetooth to their respective apps35–37. Since then, the adoption of wearables has continued to grow exponentially38 covering everything from internal physiologic biometrics to external exposome measures. Given the need for higher frequency monitoring in acute care with optimal patient adherence to ensure high-level data quality, HaH programs provided a natural entry point for device and software manufacturers. The first HaH study to utilize wireless biometrics came in 2015 and performed a virtual physician visit in 50 HaH patients compared it to 52 hospitalized controls. To emphasize the significance of scalability, the study suggested that a scalable substitutive model of HaH using biometrically enhanced 2-way tele-video, virtual physician visits were safe, feasible, and highly satisfactory33. The primary change was 2-way communication, the use of wireless devices to collect vital signs, and a circumscribed period of 34 days (including the hospital care admission at home and then followed by 30 days of post-acute care at home). The vital signs were collected from the devices and then uploaded to a third-party website, circumventing the need for a care provider to collect and manually upload the numbers into the care portal to be reviewed. This approach shifted the burden of data entry away from the provider and the patient to the device.
Remote Patient Monitoring (RPM) sensors
With the plethora of FDA cleared or CE marked sensors collecting different biomarkers, remote patient monitoring companies had the option to include multiple devices or find devices with multiple capabilities or work with companies that aggregated different biometric signals. Largely, wearables can be divided into biophysical or biochemical sensors39. Biophysical sensors use photoplethysmography, acoustic, mechanical, electrical, bioimpedance, thermal, and other signals to derive biometrics like heart rate, blood pressure, respiratory rate, temperature, pulse oximetry, activity, gait, sleep, heart rhythm, hydration, brain activity, muscle activity, and other metrics. Biochemical sensors on the other hand utilize a combination of noninvasive or minimally invasively derived biofluids to measure biofluid chemistry such as glucose, electrolytes, vitamins, lactic acid, creatinine, alcohol, urea, levodopa, cortisol, and other biomarkers. With time, sensor forms have evolved from the commonly accepted wrist bands, weighing scales, and finger clips to chest or arm patches, contact lenses, mouth guards, necklaces, and even tattoos40–42. Prior to the COVID-19 pandemic, the medical wearable sensor ecosystem had grown substantially, however, other than some ambulatory electrocardiography and glucose measurement devices, most of these novel sensors were left out of diagnostic tools and acute care management and were mostly utilized by Quantified Self enthusiasts with some momentum towards chronic care management43.
Remote patient monitoring platforms
With the increasing number of sensors generating data, there was a need for remote patient monitoring companies to aggregate and harmonize this data for return of information to the user and other stakeholders. Device manufactures with single or multiparameter capabilities built out their own care platforms. Additionally, large consumer wrist wearable companies like Apple and Google released their own research kits44,45. Patients were able to connect different personal health monitoring apps that separately collected different data streams that fed into the research kits, enabling applications in wellness and athletic performance monitoring as well as offering a glimpse into chronic disease management46,47. However, despite the potential, there was not much implementation in the HaH space, largely due to missing design features specific to HaH use cases, inadequate backend algorithms to address signal noise, and limited EHR integration.
Virtual care
Payors have been slowly adapting to the idea of virtual care delivery, but only for primary care and chronic conditions. From the provider side, EHR systems were allowing third party developers and EHR integration companies to facilitate health system integration, which in most cases used scanned lists of biometrics48. Companies like Amwell and Teladoc laid inroads facilitating hybrid (in-person and virtual) care models, but the applications continued to remain limited, such as specialist consultations, particularly in specialties which required visual pattern recognition (such as dermatology and radiology) and mostly in rural communities.
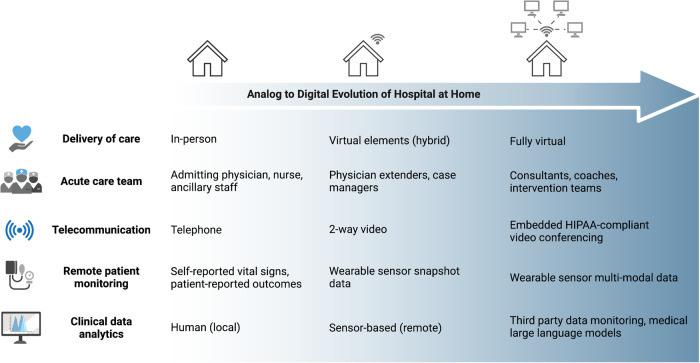
The idea of providing care through a 2-way virtual platform was already catching on prior to COVID-19, especially for primary care with the growth of companies like Teladoc (founded in 2002), Carbon Health (founded in 2015), Firefly health (founded in 2016), and others. Additionally, condition-specific virtual platforms like Livongo (founded in 2008) and Omada (founded in 2011) were on the rise, and the idea of having a virtual provider on-demand for a wellness or low acuity visit was catching on. However, prior to COVID-19, only a few companies were attempting to support virtual at-home acute care like Current Health (founded in 2014), Medically Home (founded in 2016), DispatchHealth (founded in 2013) with an at-home urgent care as well as Biofourmis (founded in 2015) and Heartbeat Health (founded in 2016) which were aimed at providing virtual specialty care. The modern HaH model has several core elements, all of which have significantly evolved over the last 20 years (Fig. 1).
Fig. 1. Analog to digital evolution of hospital at home.
From left to right, the expansion of assets and advancements in technologies continue to evolve for acute care hospital at home. Physician extenders include providers such as nurse practitioners and physician assistants. Intervention team includes several types of medical professionals such as paramedics, physical therapists, and phlebotomists.
COVID-19 and acute care at home
With the global pandemic requiring mandatory shutdowns and the overwhelming shortage of hospital beds, virtual medical care (at the time primarily pigeon-holed into rural or remote care) rapidly became mainstream. This situation was made significantly more palatable for hospitals once most payors agreed to reimburse virtual visits13. Reimbursement was further expanded when an Emergency Use Authorization (EUA) waiver of certain regulations began on November 25, 2020, supporting the AHCaH initiative. Prior to this waiver, the Centers for Medicare, and Medicaid Services required around-the-clock presence of a registered nurse for parity in reimbursement for a patient admitted to an HaH program. This requirement was waived by the EUA waiver, and as of July 2023, 125 health systems and 290 hospitals in 37 states have been approved for the waiver.
Decentralized clinical studies had already demonstrated the use of wearables to identify COVID-19 infection49,50. Therefore, device and vital sign monitoring companies pivoted to the diagnosis and monitoring of COVID-19 patients51. Hospitals relied on video calling platforms like Zoom, Microsoft Teams, Skype, Twilio and Whatsapp, eventually leading to partnerships like the ones between Microsoft and Amwell or Doximity and Twilio52. As volumes increased, communications companies turned into large telehealth companies. Ultimately, the remote/virtual first option gained popularity and companies started looking to incorporate novel sensors like home physical exam devices in different forms in lieu of the in-person physical exam, leading to large-scale mergers like Livongo and Teladoc, Best Buy and Current Health, and Amazon and One Medical53.
The clinical need to manage COVID positive patients at home was evident. Studies demonstrated the potential of HaH programs to increase hospital surge capacity, reduce nosocomial spread of the virus, and ultimately provide noninferior acute care at home in acutely ill COVID patients54–56. The need to provide continuous monitoring with a user-friendly platform created a doorway for technology companies to enter the HaH space with remote wearable technologies. The Medically Home and Kaiser Permanente partnership began before COVID -19 to treat a range of conditions, and thus was well-positioned to treat over 2000 COVID positive patients at home. The program subsequently expanded to cover other conditions like acute exacerbations of COPD exacerbation, CHF and infections like pneumonia, urinary tract infection and cellulitis57. HaH companies have raised millions of dollars demonstrating their feasibility and validating their care delivery approach, yet the field is nascent, and we hope that large-scale prospective evaluation of clinical outcomes beyond COVID-19 are soon to come.
Challenges
The Centers for Medicare and Medicaid Services AHCaH waiver that catalyzed the creation of many HaH programs is set to expire in late 202458, and while we have highlighted the many promises of HaH programs, here we present the consistently cited challenges that need to be addressed before HaH programs can cement their role in care delivery.
Evidence
To date we have identified 7 HaH studies utilizing digital sensors, platforms, and mostly virtual care evaluating the clinical outcomes of acute care HaH programs56,59–64. Three of these studies demonstrated the ability to provide COVID-19 acute hospital care in the home and increase surge capacity without adverse outcomes. A retrospective study on 1031 participants admitted for acute respiratory disease compared HaH care versus hospital care and found that HaH was safe and effective for patients in the 30day period post discharge61. A randomized controlled trial of 172 HaH patients showed virtual physician visits were non-inferior to in-home physician visits62. One study that looked at hybrid care (in person plus virtual care) of 679 acutely ill patients in Florida and Wisconsin with the use of a single command center demonstrated that the Acute Care at Home Hybrid model was able to provide high acuity care and post-acute care without major difference in length of stay, mortality, discharge, and readmission metrics64.
Payors and regulatory authorities continue to ask for more compelling evidence, with more studies looking at comparisons between different forms of care delivery (in-person to completely virtual) looking at endpoints of morbidity, mortality as well as cost effectiveness and quality of care measures. Additionally, there is a need to define the ideal HaH model including equipment requirements. We need to extend beyond the 30-day period, which is one of Centers for Medicare and Medicaid Services criteria for readmission penalties, and look at the 90 day period65. As the field matures, the capability of technology will eventually advance predictive analytics to intervention and therapeutics, and these will need to be evaluated as well. As large hospital systems continue to build HaH programs, two things are clear: the HaH movement has demonstrated feasibility, efficacy, and effectiveness; and, if given the choice, many patients would select it. However, it remains to be seen how best to customize HaH treatment, and whether remote patient monitoring needs a more individualized approach based on each patient’s clinical presentation, social support, and other demographic factors.
Analytical
In the era of digital sensors there is an ocean of possible multimodal snapshot, episodic, and continuous data available to inform clinical insight66. Ingesting this data, harmonizing it, building pipelines to share and analyze it, and turning it into clinically actionable insight presents a major data quality challenge. A master clinician typically does this in the hospital, using vital signs, imaging, prior history, and biomarkers to determine a management plan. Today, we have computing and processing power to automate this as evidenced by large language models passing medical licensing exams and the concept of general medical artificial intelligence (GMAI)67. The HaH program provides a potential test model given its relatively short time frame, continuity with a hospitalization, and multiple touchpoints. Artificial intelligence is currently focused on making repetitive tasks more efficient, but with efforts to collect, adjudicate and harmonize multimodal data and inclusive pre-training, it is reasonable to expect the future development of an AI-based tool to support remote medical decision making, adverse event prediction and detection, and patient education to help develop trust in novel models of care delivery.
Structural
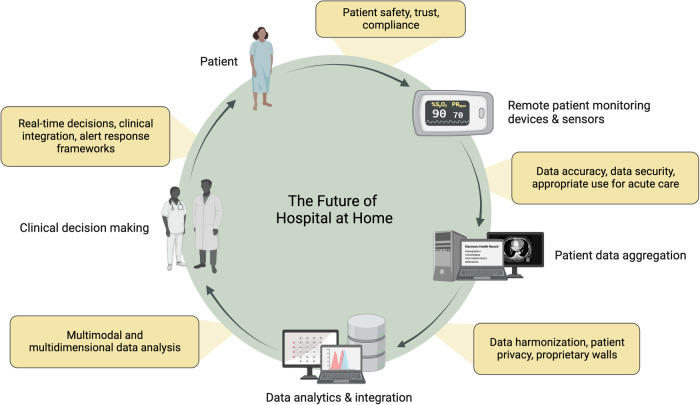
Most hospitals in the US digitized their health records after it was mandated by the HITECH Act in 200968. Electronic health records have slowly increased their capabilities, however, while the internal-facing hospital/clinic interface is evolving, the patient and device manufacturer-facing interface leave more to be desired69. There are services that help collect home-based digital sensor information and input it into the EHR through an application programming interface (API), usually as a portable document format (PDF) file or a spreadsheet that is typically overlooked during a busy clinic visit70. In many countries, tapping the full potential of the EHR is still a large barrier, which is slowly being overcome71. There is a glaring need for platforms that ingest this data from an HaH perspective and turn into a personalized clinical decision support tool compliant with local privacy rules to help inform the clinical management plan. Additionally, many current hospital programs have post-discharge follow-up phone call programs. HaH programs should strive to align with current care models as they assess their technical and workforce needs and iterate based on their concerns to effectively extend acute care to the home (Fig. 2).
Fig. 2. The Future of Hospital at Home.
The data life cycle for a future Hospital at Home model with practical and technological barriers for broad implementation. Multimodal patient data is aggregated, analyzed, integrated, interpreted, and applied to provide acute-level care in the home.
Incentive conflict
The US healthcare system is great at managing sick care, but this has had the unfortunate side effect of incentivizing more healthcare visits and procedures. Hospitals are a major lobbying force, and they bring in over $ 1 trillion in revenue in the US per year. The value-based care system has slowly moved the needle towards preventing dollars spent by focusing on dollars saved, but it is not ubiquitous72. HaH companies would effectively help reduce hospital utilization and allow hospitals an alternative form of care delivery to address the health problems of our continually aging population and overall population morbidity. While this is ideal, we need more studies to rigorously evaluate and publicize the hard clinical outcomes and cost effectiveness of HaH programs. At a societal level, HaH could help avoid billions in capital costs to build more brick-and-mortar hospitals, without alienating the existing hospital infrastructure. Further, nursing organizations have expressed concern over quality-of-care delivery with HaH programs. Engaging nursing and hospital leaders as stakeholders, understanding their concerns and expectations, and defining their role within the HaH model will be needed to overcome potentially negative views on HaH programs. Overall, there is already an alignment on the desire to keep patients healthy, but there now needs to be more alignment of incentives around keeping the patients that qualify for HaH out of the hospital.
Privacy and scalability
These are consistently stated problems for any novel health technology and care delivery solution involving transfer of data especially in the context of protected health information (PHI). Fortunately, there have already been significant developments like privacy preserving frameworks, deidentification, developing and updating security and data use infrastructure, and building out governance tokens for large datasets. Scalability was an issue for in-person HaH programs given the reliance on clinical staffing, however, with digital sensors, virtual care delivery platforms and GMAI models to help with data analytics and processing, the concept of acute care with a virtual medical assistant linked to a care delivery team is possible.
While many think that the HaH model is a major paradigm shift in acute care delivery that requires rewiring of the care delivery system, the reality is that the current infrastructure is completely able to deliver this type of care. Hospitals have already started developing digital command centers for both inpatient and outpatient monitoring73,74, health systems are already using third party vendors for remote monitoring of outpatient high-risk patients, and the remote monitoring ecosystem has matured to be able to provide real-time clinically actionable insights. None of these challenges are insurmountable and HaH programs already have the tools and evidence to answer these questions that can address quality of care concerns, improve trust, save time and dollars, and ultimately provide more patient-centric care. We believe the ideal program would be one that feels as seamless as an admission to the hospital, with a workforce that is specifically trained to provide culturally compatible care, technology that is vetted to provide clinically actionable insight and programmatic buy-in from the system, community, and individual level.
Conclusion
HaH models have significant promise with novel innovations in digital sensors, large language models for data processing, and data pipelines built for remote care delivery during COVID-19; it is now time to rigorously evaluate their ability to become a permanent alternative form of acute care management.
Search strategy and selection criteria
We performed a web-based literature search to identify peer-reviewed publications that substituted inpatient hospital admission with acute-level at-home care. Using the National Center for Biotechnology Information, PubMed, and Medical Subject Heading (Mesh) databases were queried on March 3, 2023. The PubMed search included the following keywords: hospital at home; hospital-at-home; home hospitalization; virtual hospital; acute care at home; and hospital-level care at home. The PubMed queries generated a total of 3 MeSH search terms (hospitalization; hospitals; home environment), and a MeSH database search generated a MeSH Unique ID (D018575) with the Mesh Heading, “home care services, hospital based.” Using this Heading, a PubMed MeSH search was performed, and articles were subsequently filtered by article type. If a PubMed key word search did not generate MeSH terms (acute care at home; hospital-at-home), then a filtered PubMed search was performed. Articles that reported on in-person, virtual and hybrid care models were included. Search filters included the following: US-based, English language; human species; substitutive care for inpatient admission; article types (clinical study, clinical trial, comparative study, controlled clinical trial, evaluation study, multicenter study, observational study, pragmatic clinical trial, randomized controlled trial). Predictive modeling studies, palliative care and mental health applications, meta-analyses, case reports, and systematic reviews were excluded. Furthermore, in preparation for this manuscript, the current authors shared various peer-reviewed HaH-related articles outside of the PubMed and MeSH database queries, and the shared articles that met the above criteria were included.
Supplementary information
Supplementary Table 1. Summary of peer-reviewed publications on hospital-at-home research studies.
Acknowledgements
Funding for J.A.P. and E.J.T. was provided in part by the National Institutes of Health Clinical and Translational Science Award (NIH/CTSA) grant UL1TR002550.
Author contributions
J.A.P. contributed to the design of the work, provided oversight of the work, and was a major contributor in writing the manuscript. J.B.P. was a major contributor in the writing and editing of the manuscript including figure and table design. B.L. contributed to the design, writing, and editing of the manuscript. E.J.T. contributed to the design, writing and editing of the manuscript. All authors read and approved the final manuscript.
Data availability
No datasets were generated or analyzed for the creation of this work.
Competing interests
J.A.P. has a paid consulting relationship with QuidelOrtho, receives payment or honoraria from Niterra, serves on an advisory board for Cardiosense, Preciseli, Bodyport, Angiotensin Tx, and Lytic, and has stock options through advisory positions with Cardiosense, Preciseli, Bodyport, and Angiotensin Tx. B.L. has paid consulting relationships with Medically Home, Chartis, and Kenes and has stock options with Medically Home and Dispatch Health. J.B.P and E.J.T. declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41746-024-01040-9.
References
- 1.Starr P. (eds) The Social Transformation of American Medicine (Basic Books, 2017).
- 2.Rosenberg C. E. (eds) The Care of Strangers : The Rise of America’s Hospital System (Johns Hopkins University Press, 1995).
- 3.Sorenson C, Drummond M, Bhuiyan Khan B. Medical technology as a key driver of rising health expenditure: disentangling the relationship. Clinicoecon. Outcomes Res. 2013;5:223–234. doi: 10.2147/CEOR.S39634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kisacky J. An architectural history of US community hospitals. AMA. J. Ethics. 2019;21:E288–E296. doi: 10.1001/amajethics.2019.288. [DOI] [PubMed] [Google Scholar]
- 5.Brennan TA, et al. Incidence of adverse events and negligence in hospitalized patients - results of the harvard medical practice study I. N. Engl. J. Med. 1991;324:370–376. doi: 10.1056/NEJM199102073240604. [DOI] [PubMed] [Google Scholar]
- 6.Lefevre F, et al. Iatrogenic complications in high-risk, elderly patients. Arch. Intern. Med. 1992;152:2074–2080. [PubMed] [Google Scholar]
- 7.Hirsch CH, Sommers L, Olsen A, Mullen L, Winograd CH. The natural history of functional morbidity in hospitalized older patients. J. Am. Geriatr. Soc. 1990;38:1296–1303. doi: 10.1111/j.1532-5415.1990.tb03451.x. [DOI] [PubMed] [Google Scholar]
- 8.Creditor MC. Hazards of hospitalization of the elderly. Ann. Intern. Med. 1993;118:219–223. doi: 10.7326/0003-4819-118-3-199302010-00011. [DOI] [PubMed] [Google Scholar]
- 9.Loyd C, et al. Prevalence of hospital-asociated disability in older adults: a meta-analysis. J. Am. Med Dir. Assoc. 2020;21:455–61 e5. doi: 10.1016/j.jamda.2019.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bates DW, et al. The safety of inpatient health care. N. Engl. J. Med. 2023;388:142–153. doi: 10.1056/NEJMsa2206117. [DOI] [PubMed] [Google Scholar]
- 11.Services CfMM. National Health Expenditure Fact Sheet.https://www.cms.gov/data-research/statistics-trends-and-reports/national-health-expenditure-data/nhe-fact-sheet (2023).
- 12.Arsenault-Lapierre G, Henein M, Gaid D, Le Berre M, Gore G, Vedel I. Hospital-at-home interventions vs in-hospital stay for patients with chronic disease who present to the emergency department: a systematic review and meta-analysis. JAMA. Netw. Open. 2021;4:e2111568. doi: 10.1001/jamanetworkopen.2021.11568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Topol EJ, et al. A randomized controlled trial of hospital discharge three days after myocardial infarction in the era of reperfusion. N. Engl. J. Med. 1988;318:1083–1088. doi: 10.1056/NEJM198804283181702. [DOI] [PubMed] [Google Scholar]
- 14.Damiani G, Pinnarelli L, Sommella L, Vena V, Magrini P, Ricciardi W. The short stay unit as a new option for hospitals: a review of the scientific literature. Med. Sci. Monit. 2011;17:SR15–SR19. doi: 10.12659/MSM.881791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siddique SM, et al. Reducing hospital admissions for paracentesis: a quality improvement intervention. Clin. Gastroenterol. Hepatol. 2019;17:2630–3.e2. doi: 10.1016/j.cgh.2019.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lim AKH, De Silva ML, Wang RSH, Nicholson AJ, Rogers BA. Observational study of the incidence and factors associated with patient readmission from home-based care under the hospital in the home programme. Intern. Med. J. 2021;51:1497–1504. doi: 10.1111/imj.15213. [DOI] [PubMed] [Google Scholar]
- 17.Shepperd S, et al. Is comprehensive geriatric assessment admission avoidance hospital at home an alternative to hospital admission for older persons : a randomized trial. Ann. Intern. Med. 2021;174:889–898. doi: 10.7326/M20-5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miro O, et al. Frequency, profile and outcomes of patients with acute heart failure transferred directly to home hospitalization from emergency departments. Rev. Clin. Esp. (Barc.) 2021;221:1–8. doi: 10.1016/j.rceng.2020.11.002. [DOI] [PubMed] [Google Scholar]
- 19.Jakobsen AS, et al. Home-based telehealth hospitalization for exacerbation of chronic obstructive pulmonary disease: findings from “the virtual hospital” trial. Telemed. J. E. Health. 2015;21:364–373. doi: 10.1089/tmj.2014.0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aimonino Ricauda N, et al. Substitutive “hospital at home” versus inpatient care for elderly patients with exacerbations of chronic obstructive pulmonary disease: a prospective randomized, controlled trial. J. Am. Geriatr. Soc. 2008;56:493–500. doi: 10.1111/j.1532-5415.2007.01562.x. [DOI] [PubMed] [Google Scholar]
- 21.Vianello A, et al. “Hospital at home” for neuromuscular disease patients with respiratory tract infection: a pilot study. Respir. Care. 2013;58:2061–2068. doi: 10.4187/respcare.02501. [DOI] [PubMed] [Google Scholar]
- 22.Tibaldi V, et al. Hospital at home for elderly patients with acute decompensation of chronic heart failure: a prospective randomized controlled trial. Arch. Intern. Med. 2009;169:1569–1575. doi: 10.1001/archinternmed.2009.267. [DOI] [PubMed] [Google Scholar]
- 23.Davies L, Wilkinson M, Bonner S, Calverley PM, Angus RM. Hospital at home” versus hospital care in patients with exacerbations of chronic obstructive pulmonary disease: prospective randomised controlled trial. BMJ. 2000;321:1265–1268. doi: 10.1136/bmj.321.7271.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Montalto M. The 500-bed hospital that isn’t there: the victorian department of health review of the hospital in the home program. Med. J. Aust. 2010;193:598–601. doi: 10.5694/j.1326-5377.2010.tb04070.x. [DOI] [PubMed] [Google Scholar]
- 25.Montalto M, McElduff P, Hardy K. Home ward bound: features of hospital in the home use by major Australian hospitals, 2011-2017. Med. J. Aust. 2020;213:22–27. doi: 10.5694/mja2.50599. [DOI] [PubMed] [Google Scholar]
- 26.Leff B, Burton L, Guido S, Greenough WB, Steinwachs D, Burton JR. Home hospital program: a pilot study. J. Am. Geriatr. Soc. 1999;47:697–702. doi: 10.1111/j.1532-5415.1999.tb01592.x. [DOI] [PubMed] [Google Scholar]
- 27.Leff B, et al. Hospital at home: feasibility and outcomes of a program to provide hospital-level care at home for acutely ill older patients. Ann. Intern. Med. 2005;143:798–808. doi: 10.7326/0003-4819-143-11-200512060-00008. [DOI] [PubMed] [Google Scholar]
- 28.Leff B, et al. Satisfaction with hospital at home care. J. Am. Geriatr. Soc. 2006;54:1355–1363. doi: 10.1111/j.1532-5415.2006.00855.x. [DOI] [PubMed] [Google Scholar]
- 29.Leff B, et al. Comparison of stress experienced by family members of patients treated in hospital at home with that of those receiving traditional acute hospital care. J. Am. Geriatr. Soc. 2008;56:117–123. doi: 10.1111/j.1532-5415.2007.01459.x. [DOI] [PubMed] [Google Scholar]
- 30.Leff B, et al. Comparison of functional outcomes associated with hospital at home care and traditional acute hospital care. J. Am. Geriatr. Soc. 2009;57:273–278. doi: 10.1111/j.1532-5415.2008.02103.x. [DOI] [PubMed] [Google Scholar]
- 31.Frick KD, et al. Substitutive hospital at home for older persons: effects on costs. Am. J. Manag. Care. 2009;15:49–56. [PubMed] [Google Scholar]
- 32.Eron L, King P, Marineau M, Yonehara C. Treating acute infections by telemedicine in the home. Clin. Infect. Dis. 2004;39:1175–1181. doi: 10.1086/424671. [DOI] [PubMed] [Google Scholar]
- 33.Summerfelt WT, Sulo S, Robinson A, Chess D, Catanzano K. Scalable hospital at home with virtual physician visits: pilot study. Am. J. Manag. Care. 2015;21:675–684. [PubMed] [Google Scholar]
- 34.Freiburger G, Holcomb M, Piper D. The STARPAHC collection: part of an archive of the history of telemedicine. J. Telemed. Telecare. 2007;13:221–223. doi: 10.1258/135763307781458949. [DOI] [PubMed] [Google Scholar]
- 35.Al-Rousan M, Al-Ali AR, Eberlein A. Remote patient monitoring and information system. Int. J. Electron Health. 2006;2:231–249. doi: 10.1504/IJEH.2006.009271. [DOI] [PubMed] [Google Scholar]
- 36.Lu L, et al. Wearable health devices in health care: narrative systematic review. JMIR. Mhealth Uhealth. 2020;8:e18907. doi: 10.2196/18907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perez AJ, Zeadally S. Recent advances in wearable sensing technologies. Sens. (Basel) 2021;21:6828. doi: 10.3390/s21206828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vogels E. About one-in-five Americans use a Smart Watch or Fitness Tracker. https://www.pewresearch.org/fact-tank/2020/01/09/about-one-in-five-americans-use-a-smart-watch-or-fitness-tracker/ (2023).
- 39.Xu S, Kim J, Walter JR, Ghaffari R, Rogers JA. Translational gaps and opportunities for medical wearables in digital health. Sci. Transl. Med. 2022;14:eabn6036. doi: 10.1126/scitranslmed.abn6036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu S, Jayaraman A, Rogers JA. Skin sensors are the future of health care. Nature. 2019;571:319–321. doi: 10.1038/d41586-019-02143-0. [DOI] [PubMed] [Google Scholar]
- 41.Yazdi D, Patel S, Ozonat K, Fudim M, Smith S, Centen C. Feasibility of a cardiac scale in measuring blood pressure. J. Cardiovasc Transl. Res. 2022;15:1212–1214. doi: 10.1007/s12265-022-10243-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Topol EJ, Steinhubl SR, Torkamani A. Digital medical tools and sensors. JAMAA. 2015;313:353–354. doi: 10.1001/jama.2014.17125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Adapa A, Nah FF-H, Hall RH, Siau KL, Smith SN. Factors influencing the adoption of smart wearable devices. Int. J. Hum.–Comput. Interact. 2018;34:399–409. [Google Scholar]
- 44.Jardine J, Fisher J, Carrick B. Apple’s researchKit: smart data collection for the smartphone era. J. R. Soc. Med. 2015;108:294–296. doi: 10.1177/0141076815600673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ali Y, Khan HU. A survey on harnessing the applications of mobile computing in healthcare during the COVID-19 pndemic: challenges and solutions. Comput. Netw. 2023;224:109605. doi: 10.1016/j.comnet.2023.109605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Falter M, Budts W, Goetschalckx K, Cornelissen V, Buys R. Accuracy of apple watch measurements for heart rate and energy expenditure in patients with cardiovascular disease: cross-sectional study. JMIR. mHealth uHealth. 2019;7:e11889. doi: 10.2196/11889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jim HSL, et al. Innovations in research and clinical care using patient-generated health data. CA. Cancer J. Clin. 2020;70:182–199. doi: 10.3322/caac.21608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Griffin AC, et al. Clinical, technical and implementation characteristics of real-world health applications using FHIR. JAMIA. Open. 2022;5:ooac077. doi: 10.1093/jamiaopen/ooac077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Radin JM, et al. Assessment of prolonged physiological and behavioral changes associated with COVID-19 infection. JAMA. Netw. Open. 2021;4:e2115959. doi: 10.1001/jamanetworkopen.2021.15959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gadaleta M, et al. Passive detection of COVID-19 with wearable sensors and explainable machine learning algorithms. NPJ. Digit Med. 2021;4:166. doi: 10.1038/s41746-021-00533-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pandit JA, Radin JM, Quer G, Topol EJ. Smartphone apps in the COVID-19 pandemic. Nat. Biotechnol. 2022;40:1013–1022. doi: 10.1038/s41587-022-01350-x. [DOI] [PubMed] [Google Scholar]
- 52.Jimenez-Rodriguez D, Santillan Garcia A, Montoro Robles J, Rodriguez Salvador MDM, Munoz Ronda FJ, Arrogante O. Increase in video consultations during the COVID-19 pandemic: healthcare professionals’ perceptions about their implementation and adequate management. Int. J. Environ. Res. Public Health. 2020;17:5112. doi: 10.3390/ijerph17145112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ansary AM, Martinez JN, Scott JD. The virtual physical exam in the 21st century. J. Telemed. Telecare. 2021;27:382–392. doi: 10.1177/1357633X19878330. [DOI] [PubMed] [Google Scholar]
- 54.Miyamoto Y, et al. Hospital at home for elderly COVID-19 patients: a preliminary report with 100 patients. J. Clin. Med. 2022;11:1850. doi: 10.3390/jcm11071850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Heller DJ, et al. Adapting a hospital-at-home care model to respond to New York city’s COVID-19 crisis. J. Am. Geriatr. Soc. 2020;68:1915–1916. doi: 10.1111/jgs.16725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sitammagari K, et al. Insights from rapid deployment of a “virtual hospital” as standard care during the COVID-19 pandemic. Ann. Intern. Med. 2021;174:192–199. doi: 10.7326/M20-4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Batt R, Appelbaum E. The new hospital-at-home movement: opportunity or threat for patient care. Public Policy Aging Rep. 2023;33:63–69. [Google Scholar]
- 58.Adams D, et al. Initial findings from an acute hospital care at home waiver initiative. JAMA. Health Forum. 2023;4:e233667. doi: 10.1001/jamahealthforum.2023.3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chou SH, et al. Factors associated with risk for care escalation among patients with COVID-19 receiving home-based hospital care. Ann. Intern. Med. 2021;174:1188–1191. doi: 10.7326/M21-0409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Siu AL, et al. Health equity in hospital at home: outcomes for economically disadvantaged and non-disadvantaged patients. J. Am. Geriatr. Soc. 2022;70:2153–2156. doi: 10.1111/jgs.17759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hernandez C, et al. Hospital-level care at home for patients with acute respiratory disease: a aescriptive analysis. Chest. 2023;163:891–901. doi: 10.1016/j.chest.2022.11.006. [DOI] [PubMed] [Google Scholar]
- 62.Levine DM, et al. Remote vs in-home physician visits for hospital-level care at home: a randomized clinical trial. JAMA. Netw. Open. 2022;5:e2229067. doi: 10.1001/jamanetworkopen.2022.29067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu TL, et al. Evaluating racial/ethnic differences in care escalation among COVID-19 patients in a home-based hospital. J. Racial Ethn. Health Disparities. 2023;10:817–825. doi: 10.1007/s40615-022-01270-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Paulson MR, et al. Implementation of a virtual and in-person hybrid hospital-at-home model in two geographically separate regions utilizing a single command center: a descriptive cohort study. BMC. Health Serv. Res. 2023;23:139. doi: 10.1186/s12913-023-09144-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Services CfMM. Hospital Readmissions Reduction Program (HRRP)https://www.cms.gov/medicare/payment/prospective-payment-systems/acute-inpatient-pps/hospital-readmissions-reduction-program-hrrp (2023).
- 66.Subbiah V. The next generation of evidence-based medicine. Nat. Med. 2023;29:49–58. doi: 10.1038/s41591-022-02160-z. [DOI] [PubMed] [Google Scholar]
- 67.Moor M, et al. Foundation models for generalist medical artificial intelligence. Nature. 2023;616:259–265. doi: 10.1038/s41586-023-05881-4. [DOI] [PubMed] [Google Scholar]
- 68.Burde H. Health law the hitech act-an overview. Virtual Mentor. 2011;13:172–175. doi: 10.1001/virtualmentor.2011.13.3.hlaw1-1103. [DOI] [PubMed] [Google Scholar]
- 69.Pawelek J, Baca-Motes K, Pandit JA, Berk BB, Ramos E. The power of patient engagement with electronic health records as research participants. JMIR Med. Inf. 2022;10:e39145. doi: 10.2196/39145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dinh-Le C, Chuang R, Chokshi S, Mann D. Wearable health technology and electronic health record integration: scoping review and future directions. JMIR. Mhealth Uhealth. 2019;7:e12861. doi: 10.2196/12861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Thompson H. Remote monitoring of patients in France moves into the mainstream. The Connexion (4 July 2023).
- 72.Miller HD. From volume to value: better ways to pay for health care. Health Aff. (Millwood) 2009;28:1418–1428. doi: 10.1377/hlthaff.28.5.1418. [DOI] [PubMed] [Google Scholar]
- 73.Ohannessian R, Duong TA, Odone A. Global telemedicine implementation and integration within health systems to fight the COVID-19 pandemic: a call to action. JMIR. Public Health Surveill. 2020;6:e18810. doi: 10.2196/18810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vilendrer S, et al. Rapid deployment of inpatient telemedicine in response to COVID-19 across three health systems. J. Am. Med. Inf. Assoc. 2020;27:1102–1109. doi: 10.1093/jamia/ocaa077. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Summary of peer-reviewed publications on hospital-at-home research studies.
Data Availability Statement
No datasets were generated or analyzed for the creation of this work.