Abstract
There is an increased risk of adverse perinatal outcomes in the ∼17% of women with asthma during pregnancy. The mechanisms linking maternal asthma and adverse outcomes are largely unknown, but reflect joint effects of genetics and prenatal exposure to maternal asthma. Animal models are essential to understand the underlying mechanisms independent of genetics and comorbidities, and enable safe testing of interventions. This scoping review aimed to explore the methodology, phenotype, characteristics, outcomes and quality of published studies using preclinical maternal asthma models. MEDLINE (PubMed), Embase (Elsevier) and Web of Science were systematically searched using previously validated search strings for maternal asthma and for animal models. Two reviewers independently screened titles and abstracts, full texts, and then extracted and assessed the quality of each study using the Animal Research: Reporting of In Vivo Experiments (ARRIVE) 2.0 guidelines. Out of 3618 studies identified, 39 were eligible for extraction. Most studies were in rodents (86%) and all were models of allergic asthma. Maternal and progeny outcomes included airway hyperresponsiveness, airway resistance, inflammation, lung immune cells, lung structure and serum immunoglobulins and cytokines. Experimental design (100%), procedural details (97%) and rationale (100%) were most often reported. Conversely, data exclusion (21%), blinding (18%) and adverse events (8%) were reported in a minority of studies. Species differences in physiology and timing of development, the use of allergens not relevant to humans and a lack of comparable outcome measures may impede clinical translation. Future studies exploring models of maternal asthma should adhere to the minimum core outcomes set presented in this review.
Tweetable abstract
Asthma during pregnancy impacts the unborn baby, but the mechanisms are largely unknown. This scoping review evaluates current preclinical research and explains a set of minimum reporting requirements for future animal studies of asthma in pregnancy. https://bit.ly/3RFpSlY
Introduction
Asthma affects up to 17% of pregnancies worldwide [1, 2] and is characterised by airway hyperresponsiveness, eosinophil influx, mucus accumulation and immunoglobulin (Ig)E production [3, 4]. Asthma is classified in phenotypes according to the specific underlying immune mediators [3, 5], the broadest classifications being allergic and nonallergic asthma [6]. In women of reproductive age, atopic sensitisation associated with allergic asthma is higher at age 20–27 years (OR 1.52, 95% CI 1.27–1.82) and 28–35 years (OR 1.27, 95% CI 1.07–1.51) than 36–44 years [7].
Maternal asthma is associated with increased risks of adverse maternal and progeny outcomes [8–10]. Pregnant women with asthma are at increased risk of gestational diabetes (risk ratio 1.39, 95% CI 1.17–1.66), pre-eclampsia (risk ratio 1.54, 95% CI 1.32–1.81), Caesarean section (risk ratio 1.31, 95% CI 1.22–1.39) and preterm birth (risk ratio 1.41, 95% CI 1.22–1.61) [9, 11, 12]. Similarly, babies born to asthmatic mothers are at increased risk of being born small for gestational age (risk ratio 1.22, 95% CI 1.14–1.31), or having transient tachypnoea of the newborn (adjusted (a)OR 1.10, 95% CI 1.02–1.19), respiratory distress syndrome (aOR 1.09, 95% CI 1.01–1.19), requiring neonatal hospitalisation (aOR 1.50, 95% CI 1.03–2.20) and neonatal death (aOR 1.49, 95% CI 1.11–2.00) [8–12]. Furthermore, the impact of maternal asthma persists after birth. Children of mothers with asthma are more likely to have wheeze (hazard ratio 1.67, 95% CI 1.20–2.31), asthma (aOR 2.5, 95% CI 1.9–3.1), pneumonia (OR 1.3, 95% CI 1.06–1.66) and general respiratory disease (aOR 1.6, 95% CI 1.4–1.9) [13–15]. Adults (aged ≥18 years) are more likely to have asthma if their mother ever had asthma (OR 5.33, 95% CI 2.51–11.3), compared to offspring of nonasthmatic mothers [16].
Despite clear evidence for associations between maternal asthma and poor offspring outcomes, the mechanisms are largely unknown. In mothers with well-controlled maternal asthma, the risk of several adverse perinatal outcomes is lower than in women whose asthma is poorly controlled compared to mothers with well controlled asthma [17, 18]. Additionally, maternal asthma (OR 3.04, 95% CI 2.59–3.56) conveys a greater risk of childhood asthma than paternal asthma (OR 2.44, 95% CI 2.14–2.79; p=0.037) [16], demonstrating that although genetics play a role, asthma risk in progeny is influenced by in utero exposure to maternal asthma. Nevertheless, environmental exposures are difficult to investigate in humans due to multiple confounding environmental factors. For example, asthmatic women are more likely than nonasthmatic women to have a high body mass index, hypertension, cardiac disease and to smoke [12, 17, 19, 20], and these concurrent risk factors probably contribute to poor outcomes. For instance, the progeny of asthmatic smokers have higher risks of growth restriction, admission to neonatal intensive or special care and neonatal oxygen therapy, but not the progeny of asthmatic nonsmokers, relative to progeny of nonasthmatic nonsmokers [20].
Noncompliance with asthma medication is another key confounder in pregnancy, as 39% of women reduce or stop taking their asthma medication due to concerns about harm to the fetus (82%) [21, 22]. The same hesitancy to prescribe asthma medication during pregnancy may be present in treating physicians. In the setting of asthma exacerbations in the emergency department, pregnant asthmatics were ∼20% less likely to be treated with systemic corticosteroids and ∼30% less likely to receive steroid prescriptions for their asthma [23]. These data suggest a lack of patient and clinician awareness about the importance and safety of asthma medications during pregnancy [24]. Assessing the impact of better asthma control is further complicated by the interaction between asthma control and severity [17]. Women with severe asthma before pregnancy are more likely to experience uncontrolled asthma during pregnancy (risk ratio 2.40, 95% CI 1.53–5.58) [17]. Animal models remove these confounders and interactions while enabling mechanistic studies of complex maternal–placental–fetal physiology [25, 26], for which in vitro, ex vivo and in silico models are insufficient.
Preclinical models of asthma have been developed in multiple species including mice (Mus musculus L.), rats (Rattus norvegicus Berkenhout), guinea pigs (Cavia porcellus L.), rabbits (Oryctolagus cuniculus L.), cats (Felis catus L.), dogs (Canis lupus familiaris), sheep (Ovis aries L.), monkeys (Macaca mulatta Zimmermann) and horses (Equus ferus caballus L.) [27–29]. Preclinical allergic asthma can be induced by allergen administration, usually with adjuvants to strengthen the immune response, followed by confirmation of allergic status [30]. Subsequent aerosol challenges stimulate a lung-specific allergic immune response to induce the asthma phenotype [30]. Allergic asthma in rodent models is characterised by elevated lung eosinophils, airway remodelling and high circulating IgE concentrations, similar to human asthma [3, 31, 32]. In large animal models, horses and monkeys can naturally develop respiratory sensitivities, with acute airway obstruction similar to human asthma [27]. Induction of allergic asthma in sheep also results in comparable outcomes to human asthma, including elevated eosinophils, reduced lung function and airway remodelling [3, 30, 33].
Another consideration for appropriate model selection is the timing of fetal organ development in different species [25, 26]. For instance, lung alveoli develop by ∼90% of term gestation in humans, with similar prenatal timing in sheep (∼86% of term) and in guinea pigs (∼81%) [34–36]. In contrast, alveoli appear just prior to birth in rabbits, and 4 days postnatally in rats [34]. If fetal organs are exposed to maternal asthma at a different development stage to a human pregnancy [25], the clinical translation of the findings is limited.
To facilitate future studies and clinical translation, the primary aim of this scoping review was to describe preclinical models of maternal asthma and their outcomes [37]. Maternal, placental, pregnancy and fetal, and postnatal progeny outcomes were explored. A core outcomes set was developed for the findings, an approach used in human clinical trials to improve relevance, consistency and reporting, enabling greater comparison between studies [38]. The secondary aim was to assess the methodological rigour of existing studies [37]. Each study was evaluated according to the Animal Research: Reporting of In Vivo Experiments (ARRIVE) 2.0 guidelines [39]. The ARRIVE guidelines bring together many previously published guidelines for animal research and to improve the transparency, reliability and repeatability of animal research [39–41]. These guidelines prioritise basic minimum reporting requirements, the “essential 10”, including elements of study design, sample size, experimental animals, procedures and statistical methods [39]. An additional “recommended set” of reporting requirements add further context and content for studies, including details on the background, ethical statement, animal husbandry and generalisability [39].
Methods
A protocol was developed, published [37] and registered online at Open Science Framework (https://doi.org/10.17605/OSF.IO/89BQC) before commencing this review. This review complies with JBI methodology [42] and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for scoping reviews (PRISMA-ScR) guidelines [43].
Search strategy
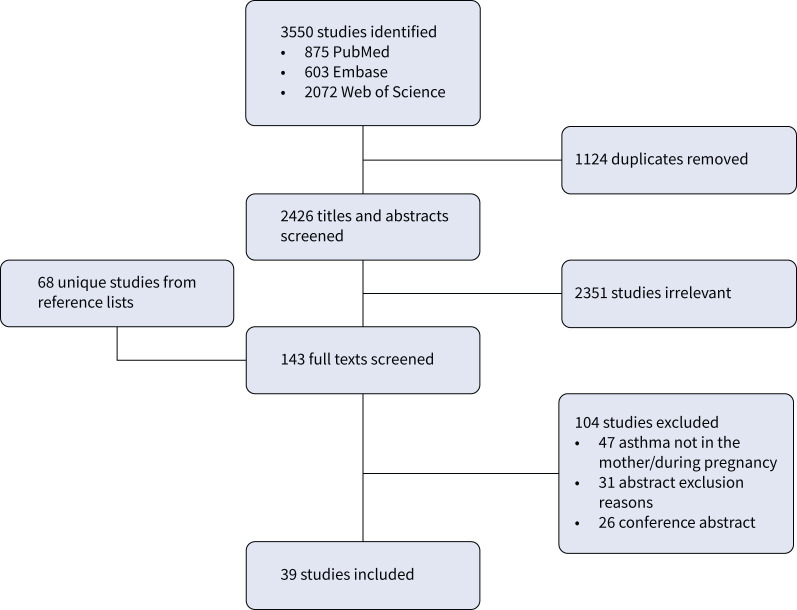
The literature search was performed using the search string from the published protocol [37], which utilised validated search strings for animal models combined with previously published search terms for maternal asthma (supplementary table S1) [44–47]. We searched PubMed (MEDLINE), Embase (Elsevier) and Web of Science, the three most relevant databases for accessing publications describing preclinical animal studies [44]. Initial searches were conducted on 20 September 2022 and updated on 10 January 2023. Papers published in hard copy or electronically up to the end of 2022 were included. In addition, the reference lists of included studies and relevant reviews were screened for relevant papers, and these were added for assessment at full-text stage (figure 1).
FIGURE 1.
Preferred Reporting Items for Systematic Reviews – Scoping Review (PRISMA-ScR) flow diagram for database search of studies utilising a nonhuman model of maternal asthma.
Study inclusion and exclusion
All screening was conducted independently by two reviewers, and any conflicts were decided by consensus with the input of a third reviewer. Publications screened at title and abstract stage were included for full-text screening if the paper mentioned pregnancy, explored asthma, allergy in the lung or hyperresponsiveness, described an in vivo study in a nonhuman mammalian animal, was a primary research paper and was published in English [37]. We excluded reviews, editorials, case studies and conference abstracts. Full texts of relevant studies were included for extraction if the study was specifically of asthma in the mother during pregnancy. Comparators were a healthy nonasthmatic mother and her progeny from the same population [37].
Data extraction
Extraction was conducted independently by two reviewers, and any conflicts were decided by consensus with the input of a third reviewer. Data extraction was completed using a template designed in Covidence review software (Veritas Health Innovation, Melbourne, Australia) and published in an appendix to the protocol [37]. This template included background details of the study, animal species and strain, treatment groups, method of inducing maternal asthma, timing of induction, measured characteristics of maternal asthma, and outcomes measured in mothers, placentae, fetuses and postnatal progeny.
Quality assessment
Quality of each study was assessed by comparing the published information against the ARRIVE 2.0 guidelines (https://arriveguidelines.org/) [39]. Study quality was assessed by two independent reviewers, and any conflicts were decided by consensus with the input of a third reviewer. Each study was assessed for compliance with each subpoint of the essential 10 and the recommended set of best reporting practice from the ARRIVE 2.0 guidelines, giving a total of 38 quality assessment criteria (supplementary table S2) [39].
Synthesis of results
Data extracted included species, method of maternal asthma induction and allergens, and timing of measurements, and were summarised into four outcome types: maternal, pregnancy and fetal, placental and postnatal progeny outcomes. Outcomes were classified into immune, respiratory, other (nonlung) organs, endocrine, size, genetic/epigenetic, behavioural, survival and placental anatomical outcomes. A mixed-methods approach using the ARRIVE guidelines, commonly reported or omitted outcomes and opinion of the experts named on this study was used to develop a core outcome set for studies of maternal asthma models. Descriptive statistics and one-way ANOVA for quality assessment over time were generated using SPSS version 28 (IBM Corporation, Armonk, NY, USA). A p-value <0.05 was considered significant.
Results
Out of the 3550 studies found, 1124 were excluded as duplicates, and 2426 articles were screened at title and abstract stage (figure 1). After excluding 2351 abstracts as irrelevant, 75 papers were identified for full-text review, in addition to 68 papers identified from screening reference lists. These 143 full texts were screened, resulting in exclusion of 104, primarily due to asthma not being in the mother or during pregnancy. Extraction and quality assessment were performed on 39 eligible studies (figure 1).
Study characteristics
The earliest study was published in 2001 with a publication average of approximately two per year up to 2022. Experimental studies were largely nonrandomised (n=25, 64%) and only one was a pilot study (supplementary table S3) [48]. A variety of species were used: 29 (74%) studies in mice, four (10%) in sheep, four (10%) in rats, one in dogs and one in cats (supplementary table S3). Predominant mouse strains were BALB/c and C57BL/6, and rat strains were Sprague Dawley, Brown Norway and Lewis (supplementary table S2). Genetically modified knockout mice were used in three studies (supplementary table S3). Progeny were delivered at term in 20 (51%) studies, but gestational age at delivery was not explicitly stated in 15 (39%) studies. Animals were delivered late preterm in four articles on sheep (140±1 days, term 147 days) and one mouse paper (18 days, term 21 days) (supplementary table S3) [33, 49]. Maternal outcomes were reported in 25 (64%) studies, placental outcomes in five (12%) studies, pregnancy and fetal outcomes in eight (21%) studies and postnatal progeny outcomes in 32 (82%) studies (supplementary table S3). Two (5%) studies only reported maternal outcomes and 12 (31%) studies only reported postnatal progeny outcomes; 63% of studies reported multiple outcome types. Of the included studies, 32 (82%) reported immune, 17 (44%) respiratory, eight (21%) other (nonlung) organs, eight (19%) endocrine, seven (18%) size, six (15%) genetic/epigenetic, four (10%) behavioural, three (8%) survival, and two (5%) reported placental anatomical outcomes. There were no studies that included clinical data or samples in conjunction with preclinical models.
Maternal asthma induction
All the studies induced an allergic asthma phenotype through allergic sensitisation and 30 (77%) studies reported using adjuvants including aluminium hydroxide, potassium aluminium sulphate and heat-killed Bordetella pertussis (supplementary table S3). One study compared two different adjuvants, complete Freund's adjuvant and aluminium hydroxide to stimulate different maternal immune responses [49]. Another study explored spontaneous asthma after allergen exposure in a feline model, not requiring immune sensitisation [29]. Lung challenges with aerosolised allergens involved nebulised allergen in a sealed chamber for a specified duration or allergen instillation into the trachea or lungs directly. One study investigated whether early or late maternal allergen aerosol challenges impacted outcomes [50]. The predominant allergens were ovalbumin (OVA; an egg-white protein, n=29, 74%) and house dust mite extract (HDM; Dermatophagoides pteronyssinus Trouessart, n=6, 15%), with casein (a protein in cow's milk) used in comparison to OVA in one study [51]. Ragweed (Ambrosia artemisiifolia L.) extract; mycelium, spores and enzyme extract of green muscardine fungus (Metarhizium anisopliae UKSI) and Bermuda grass (Cynodon dactylon L.) allergen were used in the other four (10%) studies. In mice and rats, OVA was the predominant allergen used to induce maternal allergy and asthma, and sheep studies used HDM exclusively [33]. Maternal asthma was induced before pregnancy in the majority of studies (n=35, 90%), but in some studies maternal asthma was induced during early (n=3, 8%) or late (n=1, 3%) pregnancy. Only a minority (n=11, 28%) of studies validated the maternal allergic or asthmatic phenotype of the cohort either in the article itself or in one of the cited references (where the study described additional outcomes in a cohort of animals described previously). Measures of maternal asthmatic phenotype included airway hyperresponsiveness, airway resistance, inflammation, lung-specific IgE and other immunoglobulins (e.g. IgG), bronchoalveolar lavage immune cells and lung cytokine profile.
Combinations of maternal asthma with other exposures or interventions
The combination of maternal asthma with other maternal exposures or interventions was explored in 13 (32%) studies. Maternal asthma was studied in combination with dietary interventions (α-tocopherol or γ-tocopherol, which respectively have anti-inflammatory and pro-inflammatory effects on lung) [52, 53], environmental exposures (cigarette smoke) [48], treatments (anti-asthma simplified herbal medicine) [54], dexamethasone [54], nerve growth factor treatment [31], antibodies (anti-interleukin (IL)-4 [55]) and immune stimuli (immunisation with Mycobacterium vaccae [56], non-CpG oligodeoxynucleotides or CpG oligodeoxynucleotides [57]). In another study, the maternal asthma phenotype was modified by adoptive transfer of allergen-specific T-cells from donor spleens to elucidate the role of T-cells in the transmission of asthma risk [58]. Genetically modified mouse models were used to understand how asthmatic mothers confer risk to offspring through breastmilk IgG uptake in intestinal epithelial cells [59, 60].
The combination of maternal asthma with additional offspring exposures was reported in 23 (59%) studies. The most common offspring exposure was an allergic stimulus and challenge similar to the maternal asthma induction (n=20, 51%). Other studies explored whether progeny responses differed when progeny were challenged with the same or a different allergen as had been used to induce asthma in the mother [55], and progeny responses to different allergen doses or number of challenges [55, 61]. Further offspring manipulations assessed in combination with maternal asthma were donor dendritic cell injection [62] or antibody injection (anti-CTLA-4 and anti-GITR [63]).
Maternal outcomes
Of the 41 extracted maternal outcomes, 24 were immune, eight respiratory, five in other (nonlung) organs, and four were endocrine outcomes (supplementary table S4). Immune outcomes included serum, amniotic and breastmilk immunoglobulins and cytokines [48, 53, 64, 65], and lung cellular immune response and inflammation [33, 55]. Respiratory outcomes included airway responsiveness [31, 33, 54, 55, 58] and lung histology [31, 33, 66]. Other (nonlung) organ outcomes included concentration of α- and γ-tocopherol in the liver [52, 53], and histological and molecular responses of the adrenal medulla [31]. Endocrine outcomes included serum concentrations of corticosterone and adrenaline (supplementary table S3) [31, 50].
Placental outcomes
Of the nine extracted placental outcomes, there were four anatomical, three endocrine and two immune outcomes (supplementary table S5). All of the anatomical and endocrine outcomes were from a sheep model of HDM-induced maternal asthma, including placental weight [33, 67], placentome maturity [33, 68] and the isoforms and subcellular localisation of glucocorticoid [67] and androgen receptor isoforms [67, 69]. The immune outcomes were from two mouse studies that measured cytokine production by placental lymphocytes [56, 70].
Pregnancy and fetal outcomes
Of the 17 extracted pregnancy and fetal outcomes, there were six size, four immune, four survival, one endocrine, one genetic/epigenetic and one respiratory outcome (supplementary table S6). Size outcomes included fetal weights [33, 48, 52, 53, 67] and fetal to maternal weight ratio [67]. Immune outcomes include cytokines in whole brain [70] and plasma [65], and immune cells in lung, thymus and spleen [65]. Survival outcomes included pup viability [53, 71] and average litter size [48, 71]. Other outcomes included fetal plasma cortisol concentration [65], lung gene expression of surfactant proteins [33] and organ weights [65]
Postnatal progeny outcomes
Of the 121 postnatal progeny outcomes, there were 62 immune, 21 respiratory, 13 behavioural, 12 nonlung organs, seven genetic/epigenetic, five endocrine and one size (supplementary table S7). Immune outcomes include lung cellular immune response [55, 72], serum and bronchoalveolar lavage immunoglobulins and cytokines [29, 54], lung histological inflammation [61] and phenotyping of thymic and splenic immune cells [65]. Respiratory outcomes included airway responsiveness and lung histology [55, 64]. Behavioural outcomes included locomotion [50, 73, 74], social activity [50, 73, 74], anxiety-like behaviours [50, 73, 74] and repetitive behaviours [73, 74]. Nonlung organ outcomes include neuronal morphology [32, 72] and adrenal medulla histology and protein expression [31, 75]. Genetic and epigenetic outcomes included whole-genome DNA methylation changes [62], genotype profiling of splenic dendritic cells [62], gene expression in airways [76] and the microglial transcriptome [77]. Other outcomes were growth trajectories of offspring [73], serum adrenaline and corticosterone concentration [31, 75].
Sex-specific outcomes
Of the 12 studies that reported sex-specific analyses and outcomes, eight reported outcomes in both sexes, two in males only and two in females only (supplementary tables S5–S7). No maternal outcomes were reported by fetal sex. One placental outcome was analysed by sex in a study which reported placental cytokines in male and female placentae (supplementary table S4). The three pregnancy and fetal outcomes (supplementary table S5) analysed by sex were differences in mass of pups per litter, average litter size [53] and whole-brain fetal cytokines [70]. Sex-specific data were reported for 22 postnatal progeny outcomes (supplementary table S6). Outcomes reported in both sexes include fetal brain cytokines [70], exploratory and sexual behaviours [32, 50], mast cell number [32], immunoglobulins and cytokine concentration [50, 71] and airway responsiveness [71]. Outcomes reported in males only included serum adrenaline and corticosterone [31] and mRNA of hyperresponsive airways [76]. Outcomes reported in females only included microglial transcriptome [77], serum immunoglobulins [51] and numbers of T-regulatory cells [51].
Quality of studies
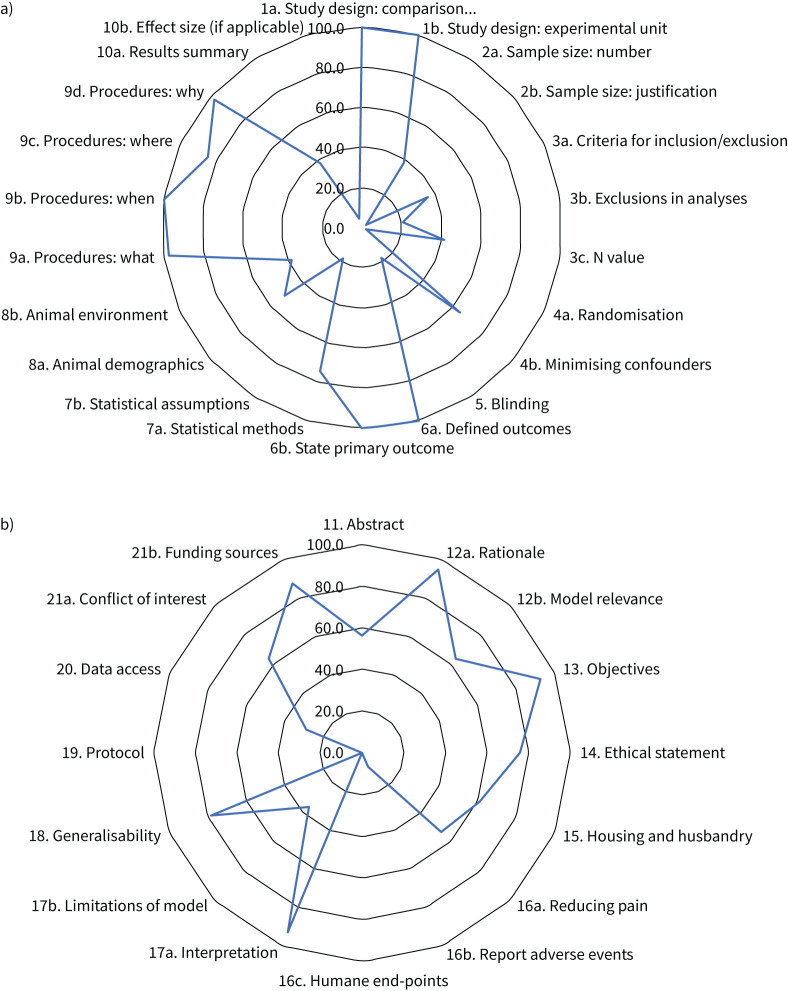
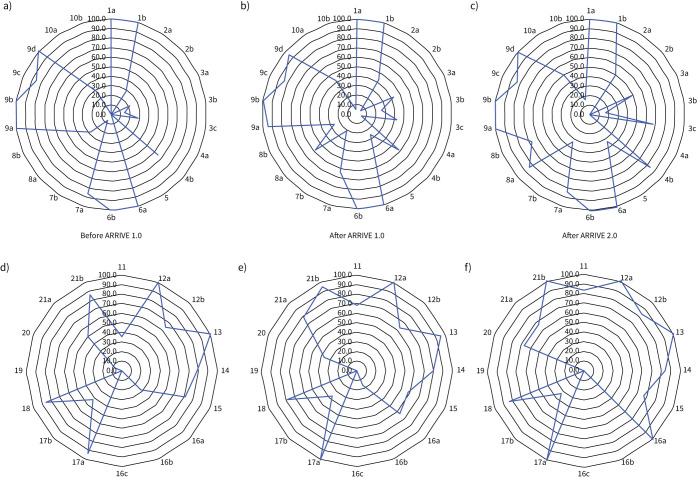
Of the ARRIVE 2.0 essential 10, well-reported criteria (reported in >80% of studies) (figure 2a, supplementary table S8) were details of experimental design (n=39, 100%), outcome measures (n=41, 100%), the primary hypothesis-driven measure (n=39, 100%) and procedural details for each experimental group (n=38, 97%), including when (n=39, 100%), where (n=33, 85%) and why (n=38, 97%) procedures were performed. Conversely, several of the ARRIVE 2.0 essential 10 criteria were poorly reported (<20% of studies) (figure 2b): sample size justification (n=1, 2%), randomisation statement including method (n=1, 2%), data points excluded from the analysis (n=8, 21%), blinding and operator awareness of group allocation (n=7, 18%) and description of methods to test whether the data met the assumptions of the statistical approach (n=7, 18%). In studies published before 2010 when ARRIVE 1.0 was implemented, reporting score for the essential 10 was 52%; 56% in studies published from 2010–2019; and 64% in studies published from 2020 onwards after the release of the ARRIVE 2.0 guidelines (figure 3a–c), but the change over time was not significant (p=0.116).
FIGURE 2.
Assessment of quality for all included studies (n=41) based on the Animal Research: Reporting of In Vivo Experiments (ARRIVE) 2.0 guidelines for a) the essential 10 and b) the recommended set. Data are percentages of studies that met each specific ARRIVE 2.0 recommendation.
FIGURE 3.
Assessment of quality for included studies based on the Animal Research: Reporting of In Vivo Experiments (ARRIVE) 2.0 guidelines for a–c) the essential 10 and d–f) the recommended set separated into studies published before ARRIVE 1.0 (n=14, before 2010; a, d), after ARRIVE 1.0 (n=21, 2010–2019; b, e) and after ARRIVE 2.0 (n=6, 2020–2022; c, f). Data are percentages of studies that met each specific ARRIVE 2.0 recommendation.
Of the ARRIVE 2.0 recommended set, well-reported criteria (>80% of studies) (figure 2b, supplementary table S9) were scientific background on the study rationale (n=31, 100%), describing the results question and objectives (n=38, 97%), interpreting the results within the scope of the current literature (n=38, 97%), commenting on generalisability of findings (n=32, 82%) and stating the funding bodies (n=36, 92%). Conversely, multiple criteria within the ARRIVE 2.0 recommended set were poorly reported (<20% of studies) (figure 2b): reporting of adverse events (n=3, 8%), descriptions of humane end-points and monitoring (n=0, 0%) and reference to a pre-published protocol (n=0, 0%). In studies published before 2010 when ARRIVE 1.0 was implemented, reporting score for the recommended set was 53%; 61% in studies published from 2010 to 2019 and 67% in studies published from 2020 onwards after the release of the ARRIVE 2.0 guidelines (figure 3d–f), increasing over time (p=0.049).
Discussion
This scoping review highlights that few studies have explored preclinical models of maternal asthma (n=39). Furthermore, the majority of published preclinical data describing maternal asthma are from studies of OVA-induced asthma in rodent models (73%), with a limited range of allergens used to induce asthma, and studies conducted in only a few species. The reported studies focussed on immunological outcomes, reported in 82% of studies, elucidating immune mechanisms underlying maternal asthma and perinatal outcomes. These mechanisms included T-helper cell and dendritic cell mediation increasing the susceptibility of offspring to allergic airway disease and asthma [58, 62]. While findings could inform likely interventions, a greater diversity of maternal asthma models is needed to ensure these are common mechanistic pathways across species [25], before clinical translation is possible. Largely unexplored areas include large animal models of maternal asthma with more similar fetal developmental timing relative to human pregnancy and a broader range of allergens to induce asthma.
Airway hyperresponsiveness and airway remodelling are key characteristics of asthma [4]. In the included studies, maternal measures of asthma phenotype described airway responsiveness and airway remodelling: increased airway subepithelial smooth muscle [33, 66], basement membrane thickness [66] and epithelial thickness in asthmatic mothers and progeny of asthmatic mothers compared to controls (supplementary table S3) [66]. Only 11 (28%) studies validated maternal asthma consistent with our recommended minimum core reporting set (table 1). Other studies measured serum immunoglobulins (such as IgE) or cytokines [32, 74]. However, measuring serum immunoglobulins or cytokines alone is insufficient to validate asthma, being indicative of allergy, but not necessarily asthma [30]. Allergic asthma involves both a systemic immune response and localised respiratory immune mediators [3], and lung-specific measures of IgE and related cytokines are required to indicate asthma [3]. Furthermore, validation is important since the animal strain can impact the response to the asthma induction [78]. One study comparing rat strains demonstrated that OVA airway challenge elicited a response to that included elevated lung resistance, and higher bronchoalveolar lavage eosinophil numbers, IL-1β and tumour necrosis factor-α concentration in Brown Norway rats, but no responses in Lewis rats that underwent the same asthma induction protocol [78]. Similarly, in mice, offspring of C57 strain asthmatic mothers exhibited behavioural changes including reduced social time and body sniffing, but offspring of FVB/Ant asthmatic mothers exhibited behavioural changes in the opposite direction [74]. Each cohort must be independently validated to confirm the successful induction of maternal asthma to ensure the outcomes are reliable and recapitulate clinical findings in asthma (table 1).
TABLE 1.
Core minimum reporting items and outcomes for animal models of maternal asthma derived from this scoping review and the Animal Research: Reporting of In Vivo Experiments (ARRIVE) 2.0 guidelines
| General | |
| Species | Choice of animal species (and strain, if applicable), justification/generalisability of outcomes, developmental timing of offspring and organs of interest |
| Animal source and management | Source of animals, housing including group size, feed type and availability, frequency of handling |
| Animal numbers | Total number of animals, total number of pregnant mothers, total number of offspring, number of litters and average number per litter (if applicable), number of offspring analysed from each pregnancy |
| Animal phenotype | Age, weight, parity |
| Treatment groups | Study design should include a maternal asthma-only group, and analysis should compare the maternal asthma group with a concurrent control group |
| Experimental protocol | Protocol information should include dose(s) of allergens used, route of administration and timing of outcome assessments (time of day and relative timing) |
| Animal welfare | Describe environmental enrichment, humane end-points and any adverse outcomes |
| Maternal | |
| Pregnancy information | Normal pregnancy duration for the species (strain) and stage of pregnancy for outcome measurements |
| Maternal asthma phenotype | Maternal asthma phenotype should be assessed in each study, given impacts of variable environment Outcomes should include airway function, airway remodelling, lung-specific immune response and other immune responses (immune cells and/or cytokines) |
| Pregnancy, placental and fetal | |
| Pregnancy health | Total and viable litter size, placental weights, implantation number |
| Sex | Numbers within each sex should be provided and sex-specific analysis of fetal and placental outcomes performed where possible, including sex×intervention interactions |
| Fetal growth | Fetal weight |
| Placenta | Placental weight |
| Postnatal progeny | |
| Sex | Progeny numbers within each sex should be provided and sex-specific analysis performed where possible, including sex×intervention interactions |
| Developmental stage at assessment | Gestational age at birth and postnatal ages relative to age at adulthood |
| Progeny health | Survival, any adverse health outcomes, any animal losses explained |
| Growth | Weight at regular intervals |
| For studies of immune outcomes | Bronchoalveolar lavage eosinophils and total leukocytes, serum immunoglobulin E, cytokines |
| For studies of lung development | Expression of surfactant protein B, lung structure (histology) |
| For studies of lung function | Lung compliance and/or airway responsiveness to allergen or bronchoconstrictor |
All of the reported studies described models of allergic asthma, which is only one of the aetiologies of asthma in humans [5], but arguably the most relevant to women of reproductive age [7]. Interestingly, OVA was the allergen used in 74% of studies, but is a common food allergen and not a trigger for asthma in humans [79, 80]. Fewer studies (15%) sensitised and challenged mothers with HDM, which is the most prevalent allergen in human asthma [80]. Allergic asthma can be further divided into a mild–moderate phenotype driven by T-helper type 2 cells (Th2), or a moderate–severe phenotype driven by T-helper type 1 or 17 cells (Th1/Th17) [72, 81]. Importantly, the specific cells involved in the allergic immune response can alter progeny outcomes. One included study used different adjuvants to develop Th1 and Th2 maternal asthma phenotypes and found a reduced immune response in the offspring of Th1 asthmatic mothers, but not in the offspring of Th2 asthmatic mothers, relative to controls [49]. Future studies of maternal asthma should be careful to assess the maternal and progeny immune responses and consider whether the progeny outcomes vary with maternal asthma phenotype (table 1).
Another consideration when selecting animal models of maternal asthma is the appropriate species selection to maximise relevance and clinical translation of findings [25], since maternal asthma may impact a different fetal developmental stage than would occur during human pregnancy [25, 26]. In our review, the majority of included studies (86%) were rodent models of maternal asthma. Rodents are cost-efficient, readily available, and may be the most appropriate for questions of genetics and genetic modification, and multigenerational studies due to the shorter intergeneration timeframe [25]. However, disparities exist between clinical and preclinical studies that may arise from the developmental timing differences between rodents and humans. For example, in rodents the majority of brain myelination and lung alveoli develop postnatally, but these events occur prenatally in humans [25, 26, 34]. Similar to a systematic review in humans [82], some preclinical studies of behavioural outcomes found a minimal impact of maternal asthma on child cognitive and behavioural development, but other reduced sociable and increased anxiety behaviours were reported in other studies (supplementary table S7) [50, 73, 74]. Respiratory outcomes reported in rodent studies include increased airway responsiveness and remodelling in progeny of asthmatic mothers, similar to human studies demonstrating increased wheeze and asthma in children of asthmatic mothers [16]. However, the lower surfactant expression observed in fetal sheep from asthmatic mothers [65] would not be observable in rodents, since surfactant synthesis begins postnatally in rodents but prenatally in humans [34]. Surfactant deficiency at birth causes respiratory distress syndrome and is therefore a clinically important observation [83]. Hence, for particular research questions, larger-animal models may be more appropriate to investigate the impact of maternal asthma on the later stages of fetal organ development. Previous findings, timing of exposures, interventions and outcome measures relative to species-specific organ maturation should be considered to ensure the most appropriate preclinical model selection, reflecting clinically relevant outcomes (table 1).
Likewise, investigators must consider that maternal asthma impacts offspring in a sex-specific manner. Across the sources eligible for inclusion in this scoping review, only eight studies reported analyses in both sexes separately. Of the four studies that explored only male or only female outcomes, the reason for selecting one sex was given in one study that investigated the impact of maternal asthma on microglia and referenced higher microglial reactivity in females [77]. In contrast, other studies did not provide a rationale for selecting only one sex in which to investigate progeny outcomes [31, 51, 76]. This is an important omission, because effects of maternal asthma are sex-specific effects in humans. Females born to asthmatic mothers are more likely to be small at birth [84], partially mediated by changes in placental vascular function [85]. Male infants born to asthmatic mothers have reduced lung function at 5–6 weeks of age [86], predictive of respiratory morbidities in later life [87]. Thus, studies of maternal asthma may be missing important sex-specific outcomes, especially since male offspring dominate animal biomedical research (males:females, 5.5:1), leading to outcomes impacted by sex bias [88]. This evidence justifies sex-specific analyses in all future studies of preclinical maternal asthma models, where possible (table 1).
This scoping review uses the ARRIVE 2.0 guidelines for quality assessment, the most recent reporting guidelines for in vivo animal experiments [39, 41]. Updates from the ARRIVE 1.0 guidelines include improved clarity, prioritisation of criteria into the essential 10 and recommended set, and consultation with an international working group to ensure global relevance [39, 89]. Further details were added to the ARRIVE 2.0 guidelines such as inclusion and exclusion criteria, protocol registration and data access. In this scoping review, some included studies (n=14, 34%) were published before the ARRIVE 1.0 guidelines (published in 2010) [40] and most (n=33, 85%) before the ARRIVE 2.0 guidelines (published in 2020) [39]. Publication before the guidelines may partially explain the lack of compliance with reporting guidelines, including no mentions of pre-published protocols, and few mentions of access to experimental data. However, as 66% of papers were published after at least the first iteration of ARRIVE guidelines, poor reporting of certain criteria highlights a lack of awareness and adherence to the guidelines overall [90]. In particular, the quality assessment in this scoping review highlights poor reporting of total animal numbers, numbers in each analysis, randomisation details, blinding during analysis and statistical assumptions. Previous assessments of ARRIVE 2.0 utilisation concur with this scoping review, particularly the lack of clarity on numbers and poor reporting of animal welfare [90, 91]. Further studies using animal models of maternal asthma should pay particular attention to reporting of animal numbers and animal welfare (table 1).
The core outcomes determined in this scoping review represent minimal details for reporting in future studies of maternal asthma models (table 1). Comparable outcomes allow for greater transparency and similarity between studies, therefore allowing evidence synthesis and improving clinical translation [92]. The core outcomes we consider most critical include species justification, assessment of the maternal asthma phenotype, timing of measurements and sex-specific reporting. We also recommend reporting of aspects such as specific numbers and animal welfare, identified during quality assessment as missing in many published studies. A review of animal models in Developmental Origins of Health and Disease (DOHaD) research provides further recommendations included in our core outcome set: species-appropriate environmental enrichment, testing for an effect of sex and interaction, consistent age and time of day of samples or data collection, and concurrent controls in each cohort [25]. The careful selection of appropriate species is facilitated by reviews comparing the developmental physiology of DOHaD models, contrasting the strengths and limitations of common species used as animal models [25, 26, 36].
Strengths of this scoping review include a systematic literature search using validated search strings and databases most relevant for animal studies [44, 93]. The search strings were developed by a well published research group in this area, having done extensive work on systematic searching and assessment of animal models [44–46, 94, 95]. Our methodologies complied with current best practices for scoping reviews in line with JBI methodology [42] and the PRISMA-ScR guidelines [43]. We used the updated ARRIVE 2.0 guidelines to assess study quality, aligning with the latest guidelines for transparent and accurate reporting [89]. Limitations of the scoping review include the heterogeneity of terms used to describe models of maternal asthma, making identification of all relevant studies more difficult. The number of studies may have been impacted by only including studies in English and only using three databases, although our study design matched published methodologies for comprehensive searching of animal models [44, 93]. In addition, the quality of studies in this scoping review was variable, although all outcomes were reported without regard to the overall quality score.
These findings will inform the selection of appropriate animal models for the study of asthma in pregnancy, including species, method of asthma induction, as well as core outcomes for assessment. Current unexplored questions in maternal asthma models include the impact of maternal asthma on the progeny cardiovascular system and the exploration of how asthma treatment improves maternal and offspring outcomes. Future studies of preclinical models of maternal asthma should consider: species selection, the timing of fetal development for studies of progeny outcome, validation and classification of the maternal asthma phenotype, and adherence to the ARRIVE guidelines. By improving preclinical studies of maternal asthma, clinical translation will be easier, and the adverse perinatal outcomes associated with maternal asthma can be reduced through better-informed interventions.
Points for future research
The appropriate selection of animal models is paramount in understanding the mechanisms underlying maternal asthma and associated adverse perinatal outcomes, and to ensure greater clinical translation utility.
Model selection should consider species physiology, timing of organ development and comparable asthma induction to humans.
Outcomes assessed should include the minimum reporting requirements stated in this scoping review.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERR-0174-2023.SUPPLEMENT (1.2MB, pdf)
Acknowledgements
We thank Vikki Langton (University of Adelaide, Adelaide, Australia) for guidance in developing the search strategy for this review.
Provenance: Submitted article, peer reviewed.
Conflict of interest: The authors declare no conflicts of interest.
Support statement: J.L. Robinson is supported by an Australian Government Research Training Program Scholarship and a Healthy Development Adelaide/Channel 7 Children's Research Foundation PhD Excellence Supplementary Scholarship. A.J. Roff is supported by an Australian Government Research Training Program Scholarship. V.L. Clifton is supported by a National Health and Medical Research Council Senior Research Fellowship (APP1136100).
References
- 1.Das J, Andrews C, Flenady V, et al. Maternal asthma during pregnancy and extremes of body mass index increase the risk of perinatal mortality: a retrospective cohort study. J Asthma 2021; 59: 2108–2116. doi: 10.1080/02770903.2021.1993249 [DOI] [PubMed] [Google Scholar]
- 2.Robijn AL, Murphy VE, Gibson PG. Recent developments in asthma in pregnancy. Curr Opin Pulm Med 2019; 25: 11–17. doi: 10.1097/MCP.0000000000000538 [DOI] [PubMed] [Google Scholar]
- 3.Hammad H, Lambrecht BN. The basic immunology of asthma. Cell 2021; 184: 1469–1485. doi: 10.1016/j.cell.2021.02.016 [DOI] [PubMed] [Google Scholar]
- 4.Lambrecht BN, Hammad H. The airway epithelium in asthma. Nat Med 2012; 18: 684–692. doi: 10.1038/nm.2737 [DOI] [PubMed] [Google Scholar]
- 5.Agache I, Akdis C, Jutel M, et al. Untangling asthma phenotypes and endotypes. Allergy 2012; 67: 835–846. doi: 10.1111/j.1398-9995.2012.02832.x [DOI] [PubMed] [Google Scholar]
- 6.Rackemann FM. A working classification of asthma. Am J Med 1947; 3: 601–606. doi: 10.1016/0002-9343(47)90204-0 [DOI] [PubMed] [Google Scholar]
- 7.Leynaert B, Sunyer J, Garcia-Esteban R, et al. Gender differences in prevalence, diagnosis and incidence of allergic and non-allergic asthma: a population-based cohort. Thorax 2012; 67: 625–631. doi: 10.1136/thoraxjnl-2011-201249 [DOI] [PubMed] [Google Scholar]
- 8.Murphy VE, Wang G, Namazy JA, et al. The risk of congenital malformations, perinatal mortality and neonatal hospitalisation among pregnant women with asthma: a systematic review and meta-analysis. BJOG 2013; 120: 812–822. doi: 10.1111/1471-0528.12224 [DOI] [PubMed] [Google Scholar]
- 9.Murphy VE, Namazy JA, Powell H, et al. A meta-analysis of adverse perinatal outcomes in women with asthma. BJOG 2011; 118: 1314–1323. doi: 10.1111/j.1471-0528.2011.03055.x [DOI] [PubMed] [Google Scholar]
- 10.Mendola P, Männistö TI, Leishear K, et al. Neonatal health of infants born to mothers with asthma. J Allergy Clin Immunol 2014; 133: 85–90. doi: 10.1016/j.jaci.2013.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang G, Murphy VE, Namazy J, et al. The risk of maternal and placental complications in pregnant women with asthma: a systematic review and meta-analysis. J Matern Fetal Neonatal Med 2014; 27: 934–942. doi: 10.3109/14767058.2013.847080 [DOI] [PubMed] [Google Scholar]
- 12.Robinson JL, Gatford KL, Hurst CP, et al. Do improvements in clinical practice guidelines alter pregnancy outcomes in asthmatic women? A single-center retrospective cohort study. J Asthma 2023; 60: 1907–1917. doi: 10.1080/02770903.2023.2200824 [DOI] [PubMed] [Google Scholar]
- 13.Spiegel E, Shoham-Vardi I, Goldbart A, et al. Maternal asthma is an independent risk factor for long-term respiratory morbidity of the offspring. Am J Perinatol 2018; 35: 1065–1070. doi: 10.1055/s-0038-1639507 [DOI] [PubMed] [Google Scholar]
- 14.Liu X, Agerbo E, Schlünssen V, et al. Maternal asthma severity and control during pregnancy and risk of offspring asthma. J Allergy Clin Immunol 2018; 141: 886–892. doi: 10.1016/j.jaci.2017.05.016 [DOI] [PubMed] [Google Scholar]
- 15.Venter C, Palumbo MP, Sauder KA, et al. Incidence and timing of offspring asthma, wheeze, allergic rhinitis, atopic dermatitis, and food allergy and association with maternal history of asthma and allergic rhinitis. World Allergy Organ J 2021; 14: 100526. doi: 10.1016/j.waojou.2021.100526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lim RH, Kobzik L, Dahl M. Risk for asthma in offspring of asthmatic mothers versus fathers: a meta-analysis. PLoS One 2010; 5: e10134. doi: 10.1371/journal.pone.0010134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grzeskowiak LE, Smith B, Roy A, et al. Patterns, predictors and outcomes of asthma control and exacerbations during pregnancy: a prospective cohort study. ERJ Open Res 2016; 2: 00054-02015. doi: 10.1183/23120541.00054-2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abdullah K, Zhu J, Gershon A, et al. Effect of asthma exacerbation during pregnancy in women with asthma: a population-based cohort study. Eur Respir J 2020; 55: 1901335. doi: 10.1183/13993003.01335-2019 [DOI] [PubMed] [Google Scholar]
- 19.Clifton VL, Engel P, Smith R, et al. Maternal and neonatal outcomes of pregnancies complicated by asthma in an Australian population. Aust NZ J Obstet Gynaecol 2009; 49: 619–626. doi: 10.1111/j.1479-828X.2009.01077.x [DOI] [PubMed] [Google Scholar]
- 20.Hodyl NA, Stark MJ, Scheil W, et al. Perinatal outcomes following maternal asthma and cigarette smoking during pregnancy. Eur Respir J 2014; 43: 704–716. doi: 10.1183/09031936.00054913 [DOI] [PubMed] [Google Scholar]
- 21.Giles W, Murphy V. Asthma in pregnancy: a review. Obstet Med 2013; 6: 58–63. doi: 10.1258/om.2012.120008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chambers K. Asthma education and outcomes for women of childbearing age. Case Manager 2003; 14: 58–61. doi: 10.1016/j.casemgr.2003.09.003 [DOI] [PubMed] [Google Scholar]
- 23.McCallister JW, Benninger CG, Frey HA, et al. Pregnancy related treatment disparities of acute asthma exacerbations in the emergency department. Respir Med 2011; 105: 1434–1440. doi: 10.1016/j.rmed.2011.05.015 [DOI] [PubMed] [Google Scholar]
- 24.Chen C, Taing M-W, Burr L, et al. Improving antenatal asthma management: a complex journey. EMJ Respir 2020; 8: 97–107. doi: 10.33590/emjrespir/20-00143 [DOI] [Google Scholar]
- 25.Dickinson H, Moss TJ, Gatford KL, et al. A review of fundamental principles for animal models of DOHaD research: an Australian perspective. J Dev Orig Health Dis 2016; 7: 449–472. doi: 10.1017/S2040174416000477 [DOI] [PubMed] [Google Scholar]
- 26.Morrison JL, Berry MJ, Botting KJ, et al. Improving pregnancy outcomes in humans through studies in sheep. Am J Physiol Regul Integr Comp Physiol 2018; 315: R1123–R1153. doi: 10.1152/ajpregu.00391.2017 [DOI] [PubMed] [Google Scholar]
- 27.Shin YS, Takeda K, Gelfand EW. Understanding asthma using animal models. Allergy Asthma Immunol Res 2009; 1: 10–18. doi: 10.4168/aair.2009.1.1.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barrett EG, Rudolph K, Bowen LE, et al. Parental allergic status influences the risk of developing allergic sensitization and an asthmatic-like phenotype in canine offspring. Immunology 2003; 110: 493–500. doi: 10.1111/j.1365-2567.2003.01757.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heller MC, Lee-Fowler TM, Liu H, et al. Neonatal aerosol exposure to Bermuda grass allergen prevents subsequent induction of experimental allergic feline asthma: evidence for establishing early immunologic tolerance. Vet Immunol Immunopathol 2014; 160: 20–25. doi: 10.1016/j.vetimm.2014.03.006 [DOI] [PubMed] [Google Scholar]
- 30.Bischof RJ, Snibson K, Shaw R, et al. Induction of allergic inflammation in the lungs of sensitized sheep after local challenge with house dust mite. Clin Exp Allergy 2003; 33: 367–375. doi: 10.1046/j.1365-2222.2003.01534.x [DOI] [PubMed] [Google Scholar]
- 31.Wu XM, Hu CP, Li XZ, et al. Asthma pregnancy alters postnatal development of chromaffin cells in the rat adrenal medulla. PLoS One 2011; 6: e20337. doi: 10.1371/journal.pone.0020337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lenz KM, Pickett LA, Wright CL, et al. Prenatal allergen exposure perturbs sexual differentiation and programs lifelong changes in adult social and sexual behavior. Sci Rep 2019; 9: 4837. doi: 10.1038/s41598-019-41258-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clifton VL, Moss TJ, Wooldridge AL, et al. Development of an experimental model of maternal allergic asthma during pregnancy. J Physiol 2016; 594: 1311–1325. doi: 10.1113/JP270752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pringle KC. Human fetal lung development and related animal models. Clin Obstet Gynecol 1986; 29: 502–513. doi: 10.1097/00003081-198609000-00006 [DOI] [PubMed] [Google Scholar]
- 35.Lock M, McGillick EV, Orgeig S, et al. Regulation of fetal lung development in response to maternal overnutrition. Clin Exp Pharmacol Physiol 2013; 40: 803–816. doi: 10.1111/1440-1681.12166 [DOI] [PubMed] [Google Scholar]
- 36.Morrison JL, Botting KJ, Darby JRT, et al. Guinea pig models for translation of the developmental origins of health and disease hypothesis into the clinic. J Physiol 2018; 596: 5535–5569. doi: 10.1113/JP274948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robinson JL, Gatford KL, Clifton VL, et al. Preclinical models of maternal asthma and progeny outcomes: a scoping review protocol. JBI Evid Synth 2023; 21: 2115–2126. doi: 10.11124/JBIES-11123-00006 [DOI] [PubMed] [Google Scholar]
- 38.Chiarotto A, Ostelo RW, Turk DC, et al. Core outcome sets for research and clinical practice. Braz J Phys Ther 2017; 21: 77–84. doi: 10.1016/j.bjpt.2017.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Percie du Sert N, Hurst V, Ahluwalia A, et al. The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. Br J Pharmacol 2020; 177: 3617–3624. doi: 10.1111/bph.15193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kilkenny C, Browne W, Cuthill IC, et al. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol 2010; 160: 1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Percie du Sert N, Hurst V, Ahluwalia A, et al. Revision of the ARRIVE guidelines: rationale and scope. BMJ Open Sci 2018; 2: e000002. doi: 10.1136/bmjos-2018-000002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aromataris E, Munn Z. JBI Manual for Evidence Synthesis. 2020. https://synthesismanual.jbi.global. Doi: 10.46658/JBIMES-20-01 [DOI]
- 43.Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med 2018; 169: 467–473. doi: 10.7326/M18-0850 [DOI] [PubMed] [Google Scholar]
- 44.de Vries RB, Hooijmans CR, Tillema A, et al. A search filter for increasing the retrieval of animal studies in Embase. Lab Anim 2011; 45: 268–270. doi: 10.1258/la.2011.011056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hooijmans CR, Tillema A, Leenaars M, et al. Enhancing search efficiency by means of a search filter for finding all studies on animal experimentation in PubMed. Lab Anim 2010; 44: 170–175. doi: 10.1258/la.2010.009117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de Vries RBM, Hooijmans CR, Tillema A, et al. Updated version of the Embase search filter for animal studies. Lab Anim 2013; 48: 88. doi: 10.1177/0023677213494374 [DOI] [PubMed] [Google Scholar]
- 47.Roff AJ, Morrison JL, Tai A, et al. Maternal asthma during pregnancy and risks of allergy and asthma in progeny: a systematic review protocol. JBI Evid Synth 2021; 19: 2007–2013. doi: 10.11124/JBIES-20-00328 [DOI] [PubMed] [Google Scholar]
- 48.Allina J, Grabowski J, Doherty-Lyons S, et al. Maternal allergy acts synergistically with cigarette smoke exposure during pregnancy to induce hepatic fibrosis in adult male offspring. J Immunotoxicol 2011; 8: 258–264. doi: 10.3109/1547691X.2011.589412 [DOI] [PubMed] [Google Scholar]
- 49.Matson AP, Zhu L, Lingenheld EG, et al. Maternal transmission of resistance to development of allergic airway disease. J Immunol 2007; 179: 1282–1291. doi: 10.4049/jimmunol.179.2.1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Church JS, Tamayo JM, Ashwood P, et al. Repeated allergic asthma in early versus late pregnancy differentially impacts offspring brain and behavior development. Brain Behav Immun 2021; 93: 66–79. doi: 10.1016/j.bbi.2020.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sodemann EB, Dähling S, Klopfleisch R, et al. Maternal asthma is associated with persistent changes in allergic offspring antibody glycosylation. Clin Exp Allergy 2020; 50: 520–531. doi: 10.1111/cea.13559 [DOI] [PubMed] [Google Scholar]
- 52.Abdala-Valencia H, Berdnikovs S, Soveg FW, et al. α-Tocopherol supplementation of allergic female mice inhibits development of CD11c+CD11b+ dendritic cells in utero and allergic inflammation in neonates. Am J Physiol Lung Cell Mol Physiol 2014; 307: L482–L496. doi: 10.1152/ajplung.00132.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Abdala-Valencia H, Soveg F, Cook-Mills JM. γ-Tocopherol supplementation of allergic female mice augments development of CD11c+CD11b+ dendritic cells in utero and allergic inflammation in neonates. Am J Physiol Lung Cell Mol Physiol 2016; 310: L759–L771. doi: 10.1152/ajplung.00301.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.López-Expósito I, Srivastava KD, Birmingham N, et al. Maternal Antiasthma Simplified Herbal Medicine Intervention therapy prevents airway inflammation and modulates pulmonary innate immune responses in young offspring mice. Ann Allergy Asthma Immunol 2015; 114: 43–51. doi: 10.1016/j.anai.2014.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hamada K, Suzaki Y, Goldman A, et al. Allergen-independent maternal transmission of asthma susceptibility. J Immunol 2003; 170: 1683–1689. doi: 10.4049/jimmunol.170.4.1683 [DOI] [PubMed] [Google Scholar]
- 56.Akkoc T, Eifan AO, Ozdemir C, et al. Mycobacterium vaccae immunization to OVA sensitized pregnant BALB/c mice suppressed placental and postnatal IL-5 and inducing IFN-γ secretion. Immunopharmacol Immunotoxicol 2008; 30: 1–11. doi: 10.1080/08923970701812159 [DOI] [PubMed] [Google Scholar]
- 57.Fedulov A, Silverman E, Xiang Y, et al. Immunostimulatory CpG oligonucleotides abrogate allergic susceptibility in a murine model of maternal asthma transmission. J Immunol 2005; 175: 4292–4300. doi: 10.4049/jimmunol.175.7.4292 [DOI] [PubMed] [Google Scholar]
- 58.Hubeau C, Apostolou I, Kobzik L. Adoptively transferred allergen-specific T cells cause maternal transmission of asthma risk. Am J Pathol 2006; 168: 1931–1939. doi: 10.2353/ajpath.2006.051231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Matson AP, Thrall RS, Rafti E, et al. IgG transmitted from allergic mothers decreases allergic sensitization in breastfed offspring. Clin Mol Allergy 2010; 8: 9. doi: 10.1186/1476-7961-8-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nakata K, Kobayashi K, Ishikawa Y, et al. The transfer of maternal antigen-specific IgG regulates the development of allergic airway inflammation early in life in an FcRn-dependent manner. Biochem Biophys Res Commun 2010; 395: 238–243. doi: 10.1016/j.bbrc.2010.03.170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fedulov AV, Leme AS, Kobzik L. Duration of allergic susceptibility in maternal transmission of asthma risk. Am J Reprod Immunol 2007; 58: 120–128. doi: 10.1111/j.1600-0897.2007.00496.x [DOI] [PubMed] [Google Scholar]
- 62.Fedulov AV, Kobzik L. Allergy risk is mediated by dendritic cells with congenital epigenetic changes. Am J Respir Cell Mol Biol 2011; 44: 285–292. doi: 10.1165/rcmb.2009-0400OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hubeau C, Apostolou I, Kobzik L. Targeting of CD25 and glucocorticoid-induced TNF receptor family-related gene-expressing T cells differentially modulates asthma risk in offspring of asthmatic and normal mother mice. J Immunol 2007; 178: 1477–1487. doi: 10.4049/jimmunol.178.3.1477 [DOI] [PubMed] [Google Scholar]
- 64.Leme AS, Hubeau C, Xiang YH, et al. Role of breast milk in a mouse model of maternal transmission of asthma susceptibility. J Immunol 2006; 176: 762–769. doi: 10.4049/jimmunol.176.2.762 [DOI] [PubMed] [Google Scholar]
- 65.Wooldridge AL, Clifton VL, Moss TJM, et al. Maternal allergic asthma during pregnancy alters fetal lung and immune development in sheep: potential mechanisms for programming asthma and allergy. J Physiol 2019; 597: 4251–4262. doi: 10.1113/JP277952 [DOI] [PubMed] [Google Scholar]
- 66.Babayiğit A, Ölmez D, Erbil G, et al. Histopathologic changes of the lung in newborn mice born from asthmatic mothers. Türkiye Klinikleri J Med Sci 2008; 28: 606–611. [Google Scholar]
- 67.Clifton VL, McDonald M, Morrison JL, et al. Placental glucocorticoid receptor isoforms in a sheep model of maternal allergic asthma. Placenta 2019; 83: 33–36. doi: 10.1016/j.placenta.2019.06.380 [DOI] [PubMed] [Google Scholar]
- 68.Clifton VL. Managing asthma in pregnancy: effects on future child health. Lancet Respir Med 2019; 7: 485–486. doi: 10.1016/S2213-2600(19)30149-3 [DOI] [PubMed] [Google Scholar]
- 69.Meakin AS, Morrison JL, Bradshaw EL, et al. Identification of placental androgen receptor isoforms in a sheep model of maternal allergic asthma. Placenta 2021; 104: 232–235. doi: 10.1016/j.placenta.2021.01.003 [DOI] [PubMed] [Google Scholar]
- 70.Tamayo JM, Rose D, Church JS, et al. Maternal allergic asthma induces prenatal neuroinflammation. Brain Sci 2022; 12: 1041. doi: 10.3390/brainsci12081041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pucheu-Haston CM, Copeland LB, Haykal-Coates N, et al. Maternal respiratory sensitization and gestational allergen exposure does not affect subsequent pup responses to homologous or heterologous allergen. J Immunotoxicol 2010; 7: 57–67. doi: 10.3109/15476910903373440 [DOI] [PubMed] [Google Scholar]
- 72.Lebold KM, Drake MG, Pincus AB, et al. Unique allergic asthma phenotypes in offspring of house dust mite-exposed mice. Am J Respir Cell Mol Biol 2022; 67: 89–98. doi: 10.1165/rcmb.2021-0535OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schwartzer JJ, Careaga M, Chang C, et al. Allergic fetal priming leads to developmental, behavioral and neurobiological changes in mice. Transl Psychiatry 2015; 5: e543. doi: 10.1038/tp.2015.40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schwartzer JJ, Careaga M, Coburn MA, et al. Behavioral impact of maternal allergic-asthma in two genetically distinct mouse strains. Brain Behav Immun 2017; 63: 99–107. doi: 10.1016/j.bbi.2016.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Feng JT, Wu XM, Li XZ, et al. Transformation of adrenal medullary chromaffin cells increases asthmatic susceptibility in pups from allergen-sensitized rats. Respir Res 2012; 13: 99. doi: 10.1186/1465-9921-13-99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pulczinski JC, Shang Y, Dao T, et al. Multigenerational epigenetic regulation of allergic diseases: utilizing an experimental dust mite-induced asthma model. Front Genet 2021; 12: 624561. doi: 10.3389/fgene.2021.624561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vogel Ciernia A, Careaga M, LaSalle JM, et al. Microglia from offspring of dams with allergic asthma exhibit epigenomic alterations in genes dysregulated in autism. Glia 2018; 66: 505–521. doi: 10.1002/glia.23261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Carpe N, Mandeville I, Kho AT, et al. Maternal allergen exposure reprograms the developmental lung transcriptome in atopic and normoresponsive rat pups. Am J Physiol Lung Cell Mol Physiol 2012; 303: L899–L911. doi: 10.1152/ajplung.00179.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bates JH, Rincon M, Irvin CG. Animal models of asthma. Am J Physiol Lung Cell Mol Physiol 2009; 297: L401–L410. doi: 10.1152/ajplung.00027.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Calderón MA, Linneberg A, Kleine-Tebbe J, et al. Respiratory allergy caused by house dust mites: what do we really know? J Allergy Clin Immunol 2015; 136: 38–48. doi: 10.1016/j.jaci.2014.10.012 [DOI] [PubMed] [Google Scholar]
- 81.Luo W, Hu J, Xu W, et al. Distinct spatial and temporal roles for Th1, Th2, and Th17 cells in asthma. Front Immunol 2022; 13: 974066. doi: 10.3389/fimmu.2022.974066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Whalen OM, Karayanidis F, Murphy VE, et al. The effects of maternal asthma during pregnancy on child cognitive and behavioral development: a systematic review. J Asthma 2019; 56: 130–141. doi: 10.1080/02770903.2018.1437174 [DOI] [PubMed] [Google Scholar]
- 83.Reuter S, Moser C, Baack M. Respiratory distress in the newborn. Pediatr Rev 2014; 35: 417–429. doi: 10.1542/pir.35.10.417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Murphy VE, Gibson PG, Giles WB, et al. Maternal asthma is associated with reduced female fetal growth. Am J Respir Crit Care Med 2003; 168: 1317–1323. doi: 10.1164/rccm.200303-374OC [DOI] [PubMed] [Google Scholar]
- 85.Meakin AS, Saif Z, Seedat N, et al. The impact of maternal asthma during pregnancy on fetal growth and development: a review. Expert Rev Respir Med 2020; 14: 1207–1216. doi: 10.1080/17476348.2020.1814148 [DOI] [PubMed] [Google Scholar]
- 86.de Gouveia Belinelo P, Collison AM, Murphy VE, et al. Maternal asthma is associated with reduced lung function in male infants in a combined analysis of the BLT and BILD cohorts. Thorax 2021; 76: 996–1001. doi: 10.1136/thoraxjnl-2020-215526 [DOI] [PubMed] [Google Scholar]
- 87.Berry CE, Billheimer D, Jenkins IC, et al. A distinct low lung function trajectory from childhood to the fourth decade of life. Am J Respir Crit Care Med 2016; 194: 607–612. doi: 10.1164/rccm.201604-0753OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Beery AK, Zucker I. Sex bias in neuroscience and biomedical research. Neurosci Biobehav Rev 2011; 35: 565–572. doi: 10.1016/j.neubiorev.2010.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Percie du Sert N, Ahluwalia A, Alam S, et al. Reporting animal research: explanation and elaboration for the ARRIVE guidelines 2.0. PLoS Biol 2020; 18: e3000411. doi: 10.1371/journal.pbio.3000411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.McGrath JC, Lilley E. Implementing guidelines on reporting research using animals (ARRIVE etc.): new requirements for publication in BJP. Br J Pharmacol 2015; 172: 3189–3193. doi: 10.1111/bph.12955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Avey MT, Moher D, Sullivan KJ, et al. The devil is in the details: incomplete reporting in preclinical animal research. PLoS One 2016; 11: e0166733. doi: 10.1371/journal.pone.0166733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ritskes-Hoitinga M, Leenaars M, Avey M, et al. Systematic reviews of preclinical animal studies can make significant contributions to health care and more transparent translational medicine. Cochrane Database Syst Rev 2014; 3: ED000078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Leenaars M, Hooijmans CR, van Veggel N, et al. A step-by-step guide to systematically identify all relevant animal studies. Lab Anim 2012; 46: 24–31. doi: 10.1258/la.2011.011087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hooijmans CR, Rovers MM, de Vries RB, et al. SYRCLE's risk of bias tool for animal studies. BMC Med Res Methodol 2014; 14: 43. doi: 10.1186/1471-2288-14-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hooijmans CR, de Vries RBM, Ritskes-Hoitinga M, et al. Facilitating healthcare decisions by assessing the certainty in the evidence from preclinical animal studies. PLoS One 2018; 13: e0187271. doi: 10.1371/journal.pone.0187271 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERR-0174-2023.SUPPLEMENT (1.2MB, pdf)