Abstract
Phlomoides is one of the largest genera of Lamiaceae with approximately 150–170 species distributed mainly in Eurasia. In this study, we describe and illustrate a new species, P.henryi, which was previously misidentified as P.bracteosa, from Yunnan Province, southwest China. Molecular phylogenetic analyses revealed that P.henryi is found within a clade in which most species lack basal leaves. In this clade, the new species is morphologically distinct from P.rotata in having an obvious stem and, from the rest, by having transparent to white trichomes inside the upper corolla lip. In addition, micro-features of trichomes on the calyx and leaf epidermis can differentiate the new species from other species grouped in the same clade and a key, based on trichome morphology for these species, is provided. The findings demonstrate that the use of scanning electron microscopy can reveal inconspicuous morphological affinities amongst morphologically similar species and play an important role in the taxonomic study of the genus Phlomoides.
Key words: Micromorphology, Phlomoides , Phlomis , Phlomideae, taxonomy, trichomes
Introduction
The tribe Phlomideae (Lamiaceae, Lamioideae) was originally established by Scheen et al. (2010) to include seven genera, Eremostachys Bunge, Lamiophlomis Kudô, Notochaete Benth., Phlomis L., Phlomoides Moench, Paraeremostachys Adylov et al. and Pseuderemostachys Popov. Subsequent phylogenetic and taxonomic studies (Bendiksby et al. 2011; Mathiesen et al. 2011; Salmaki et al. 2012b) have revised generic boundaries and Phlomoides was expanded to include Eremostachys, Lamiophlomis, Notochaete, Paraeremostachys and Pseuderemostachys. Recently, two monotypic genera, Metastachydium Airy Shaw ex C.Y. Wu & H.W. Li and Pseudomarrubium Popov were also transferred to Phlomoides (Zhao et al. 2023a, b), leaving only two genera retained in Phlomideae, Phlomoides and Phlomis. The re-defined Phlomoides is easily distinguished from its sister genus Phlomis by: (1) being generally perennial herbs (vs. small shrubs, occasionally perennial herbs); (2) having leaves cordate to triangular-ovate, simple or laciniate to bipinnatisect (vs. lanceolate to oblong-lanceolate, not deeply lobed); (3) the upper lip of corolla dome-shaped with apex hairy or fringed-incised (vs. laterally compressed, flattened, sickle-shaped, apex not fringed-incised) and (4) a basic chromosome number of x = 11 (vs. x = 10) (Azizian and Cutler 1982; Astanova 1984; Ghaffari 2006; Fang et al. 2007; Mathiesen et al. 2011; Salmaki et al. 2012a; Zhao et al. 2021b).
As currently defined, Phlomoides consists of approximately 150–170 species and ranks the second largest genus within subfamily Lamioideae (Salmaki et al. 2012a, b; F Zhao et al. 2021). Species of Phlomoides are mainly distributed from central Europe to the Russian Far East, but highly diversified in three regions: Central Asia (59 spp.; Czerepanov (1995)), the Iranian highlands (ca. 41 spp.; Salmaki et al. (2012a)) and China (58 spp.; Xiang et al. (2014); Zhao et al. (2021a, 2024)). In China, most species are found in the southwest region and 29 species and 11 varieties are endemic and geographically restricted (Li and Hedge 1994). The existing infrageneric classification of Chinese Phlomoides (= PhlomissectionPhlomoides Briq.) was established by Hsuan (1977), who divided Chinese species into two subsections and 17 series, based on external morphology (e.g. the absence/presence of the basal leaves, shape of stem leaves, length and density of trichomes on stems and leaves etc.). However, most infrageneric categories were not recovered as monophyletic (Zhao et al. 2024) and those external and quantitative characters used for traditional taxonomy are highly variable amongst different species or at different populations for the same species. In contrast, some micro-features probably have taxonomic significance within Phlomoides. For example, Seyedi and Salmaki (2015) and Khosroshahi and Salmaki (2019) found trichome morphology to be important for the delimitation of sections and species of Phlomoides. In addition, trichome characters have significant taxonomic values in other genera of Lamiaceae (Gairola et al. 2009; Xiang et al. 2010; Hu et al. 2012; Yao et al. 2013). However, micro-features of trichomes and other characters of Chinese Phlomoides species are poorly known.
During the past ten years, phylogenetic and taxonomic studies have focused on Phlomoides from China (Xiang et al. 2014; Zhao et al. 2021a, b, 2023a, b, 2024) resolving some taxonomic puzzles. In the process of the continuing taxonomic study of the genus, two collections attracted our attention when investigating historical specimens. One collection with three sheets were collected by Augustine Henry in 1898 (A. Henry 10216) from Mengtze (now Mengzi County), Yunnan Province and were identified as P.bracteosa (Royle ex Benth.) Kamelin & Makhm. (= Phlomisbracteosa Royle ex Benth.). Two sheets were deposited at K (A. Henry 10216A, K000928267; A. Henry 10216 K, without barcode) and another at LE (without barcode). Another collection was collected by F. Ducloux in 11 August 1907 (F. Ducloux 369) from Yunnan “Lao Kouy [Chan]” deposited at E (without barcode) without any identification, but external morphology indicates that this specimen represents the same species as the Henry’s collections at K and LE. However, characters (e.g. floral leaves with petioles 5–15 mm long, bracts subulate, simple long trichomes on calyces, bracts and both sides of leaves) shown in these specimens are obviously different from those of P.bracteosa (upper floral leaves sessile, lower floral leaves with petioles up to 20 mm long, bracts lanceolate-linear, branched trichomes on calyces, bracts and both sides of leaves). Fortunately, we re-discovered the plant in the wild from the possible locality where specimens were collected by Henry, after more than 125 years since the first collection in 1887. Molecular phylogenetic analyses and macro- and micro- morphological studies demonstrate that the species is a new species, P.henryi and we describe and illustrate it in this study.
Materials and methods
Taxon sampling
In total, we sampled 49 out of 58 (84.48%) Chinese species of Phlomoides for molecular phylogenetic analyses. Sampling is primarily based on previous molecular phylogenetic studies of Phlomoides (Zhao et al. 2024) and only samples of the potential new species and P.bracteosa were newly sequenced. Fresh leaves of the putative new species (P.henryi) were collected and dried with silica-gel in the field (Jianshui County, Yunnan Province) and herbarium materials of P.bracteosa were collected from the herbarium BM.
In addition, six species from the subclade comprising the potential new species, as well as P.bracteosa, were sampled to investigate macro-micro-features of trichomes on flora bracts and leaves. The list of sampled species and their origins are given in Table 1 and voucher specimens were deposited in the Herbarium of the Kunming Institute of Botany (KUN) and Institute of Botany (PE), Chinese Academy of Sciences.
Table 1.
List of sampled Phlomoides species to investigate macro/micro features of trichomes and their voucher information.
| Taxon | Geographical origin | Voucher information | Collection date |
|---|---|---|---|
| P.henryi | China, Yunnan Province, Honghe Hani and Yi Autonomous Prefecture, Jianshui County, on the forest edge, 23°57′52.54″N, 102°59′49.47″E, alt. 1279 m. | F. Zhao et al. XCL2222 (KUN) | 3 Sep. 2022 |
| P.bracteosa | India, Uttarakhand, Bhyundar Valley, alt. 3430 m. | Anonymous 6583 (PE) | 16 Aug. 1975 |
| P.breviflora | China, Tibet, Nielamu County, Qu Town, 28°4′44″N, 86°0′2.109″E, alt. 3246 m | Y.P. Chen et al. EM1139 (KUN) | 12 Sep. 2019 |
| P.macrophylla | China, Xizang Province, Yadong County, on the way from Yadong to Nathu La Pass. | Y.P. Chen et al. EM1094 (KUN) | 9 Sep. 2019 |
| P.nyalamensis | China, Xizang Province, Nyalam County, Zhangmu Town, on the way from Lixin to Xuebugang, 27°56′37.0356″N, 85°58′28.1712″E, alt. 2896 m. | Y.P. Chen et al. EM1145 (KUN) | 13 Sep. 2019 |
| P.tibetica | China, Xizang Province, Linzhi City, Shergyla Mountain. | C.L. Xiang et al. XCL1458 (KUN) | 15 Sep. 2016 |
| P.milingensis | China, Xizang Province, Linzhi City, Miling County, Lilong Town, Lilonggou, 29°1′45.6″N, 93°53′34.7″E, alt. 3188 m. | C.L. Xiang et al. XCL1469 (KUN) | 16 Sep. 2016 |
| P.rotata | China, Xizang Province, Changdu City, Zuogong County, Dondara Mountain, 29°42′59.9″N, 98°1′7.3″E, alt. 5034 m. | C.L. Xiang et al. XCL1419 (KUN) | 12 Sep. 2016 |
DNA extraction, selection of markers and molecular phylogenetic analyses
Total genomic DNA was extracted using the CTAB method (Doyle and Doyle 1987). Previous studies revealed that plastid DNA phylogeny can better resolve relationships of Phlomoides than the tree inferred from the nuclear ribosomal internal and external transcribed spacer regions (nrITS and nrETS) (Zhao et al. 2023a, b; 2024). In order to test systematic placement of the new species, nine plastid DNA regions (atpB-rbcL, psbA-trnH, rpl16, rpl32-trnL, rps16, trnK, trnL-trnF, trnS-trnG, trnT-L) were selected for phylogenetic reconstruction. Primers, polymerase chain reaction (PCR), sequencing and alignment followed those described in Zhao et al. (2024). The sequences newly generated in this study together with their GenBank accession numbers are listed in Appendix 1.
The combined dataset of nine plastid DNA regions was analysed using Bayesian Inference (BI) and Maximum Likelihood (ML). Three species of Phlomis were selected as outgroup, based on previous studies (Zhao et al. 2023a). The best-fit substitution model was selected by the jModelTest v.2.1.7 (Darriba et al. 2012) under the Akaike Information Criterion (AIC) score. BI and ML analyses were conducted on the Cyberinfrastructure for Phylogenetic Research Science (CIPRES) Gateway v.3.3 (Miller et al. 2010), using MrBayes (Ronquist et al. 2012) and RAxMLv.8.2.9 (Stamatakis 2014), respectively. Details for parameter settings follow the previous study of Zhao et al. (2021). All the phylogenetic trees with posterior probabilities (PP) and bootstrap values (BS) were exhibited and annotated in FigTree v.1.4.2 (Rambaut 2014).
Morphological investigations
Species concept, definitions of characters and depiction generally follow Li and Hedge (1994). Type specimens and protologues for all species of Phlomoides in China were collated. Morphological features were based on herbarium as well as field investigations. Specimens at B, BM, C, CDBI, E, FI, GH, HIB, IBSC, K, KUN, LE, M, MA, MAO, MICH, MO, MW, NAS, P, PE, S, SG, TI, W, WUK and XJBI (herbarium acronyms followed Thiers 2022) and our collections from the field were examined for characterisation and morphological comparison. Additional morphological information (including habit, habitat, root, leaf, calyx, flower etc.) was taken from field observations, as well as literature (Hsuan 1977; Wu et al. 1977; Li 1985; Li and Hedge 1994).
Micro-features of leaf epidermis and floral bracts were investigated using Light Microscopy (LM) and Scanning Electron Microscopy (SEM). Photographs and morphological observations were taken using a Leica DM2500 light microscope (Leica Microsystems GmbH, Wetzlar, Germany). Mature leaves and floral bracts were taken from our collection (Table 1) for SEM investigation. Materials were mounted on to stubs and coated with gold, using a ZEISS EVO LS10 scanning electron microscope (Carl ZEISS NTS, Germany) with 10 kV voltage (Kunming Institute of Botany, Yunnan, China). Terminology of morphological characteristics of trichomes followed Khosroshahi and Salmaki (2019).
Results
Molecular phylogeny and systematic placement of Phlomoideshenryi
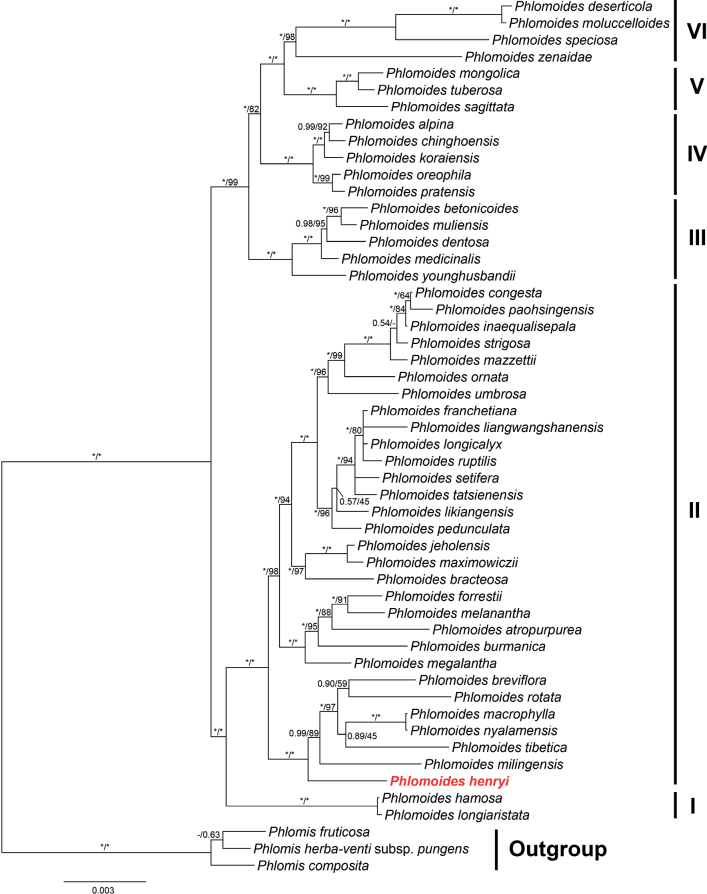
A total of 18 sequences were newly sequenced in the present study and they were submitted to GenBank under accession nos. OR674852–OR674869. The aligned length of the combined plastid dataset was 9259 bp (2380 bp for atpB-rbcL, 421 bp for psbA-trnH, 1361 bp for rpl16, 681 bp for rpl32-trnL, 967 bp for rps16, 958 bp for trnK, 868 bp for trnL-trnF, 831 bp for trnS-trnG and 792 bp for trnT-L), respectively. The topologies of the BI and ML trees were consistent with each other, only the Bayesian 50% majority-rule consensus tree being presented, with the posterior probabilities (PP) and Bootstrap support (BS) and values being superimposed near the nodes (Fig. 1).
Figure 1.
Phylogeny of Phlomoides inferred by Bayesian Inference (BI), based on the combined plastid dataset cpDNA. Support values displayed on the branches follow the order BI-PP/ML-BS (“ * ” indicates PP = 1.00 or BS = 100%, "-" indicates incongruent relationship between BI and ML tree.
Monophyly of the genus Phlomoides was recovered (Fig. 1: PP =1.00/BS = 100%). The backbone topologies of Phlomoides recovered in present study are largely consistent with those of previous studies (Zhao et al. 2024), clade I is sister to Clade II with strong support values (Fig. 1: 1.00/100%), then sister to a large clade consisting of Clades III, IV, V and VI. Chinese Phlomoides species can subdivided into six clades (Fig. 1).
As shown in Fig. 1, the new species, Phlomoideshenryi is distantly related to P.bracteosa. Instead, P.henryi is sister to a subclade (Fig. 1: 1.00/100%) comprising P.milingensis (C.Y. Wu & H.W. Li) Kamelin & Makhm., P.tibetica (C. Marquand & Airy Shaw) Kamelin & Makhm., P.macrophylla (Wall.) Kamelin & Makhm., P.nyalamensis (H.W. Li) Y. Zhao & C.L. Xiang, P.breviflora (Benth.) Kamelin & Makhm., and P.rotata (Benth. ex Hook.f.) Mathiesen, while P.bracteosa is sister to P.maximowiczii (Regel) Kamelin & Makhm. and P.jeholensis (Nakai & Kitag.) Kamelin & Makhm.
Trichome morphology of bracts and leaf epidermis
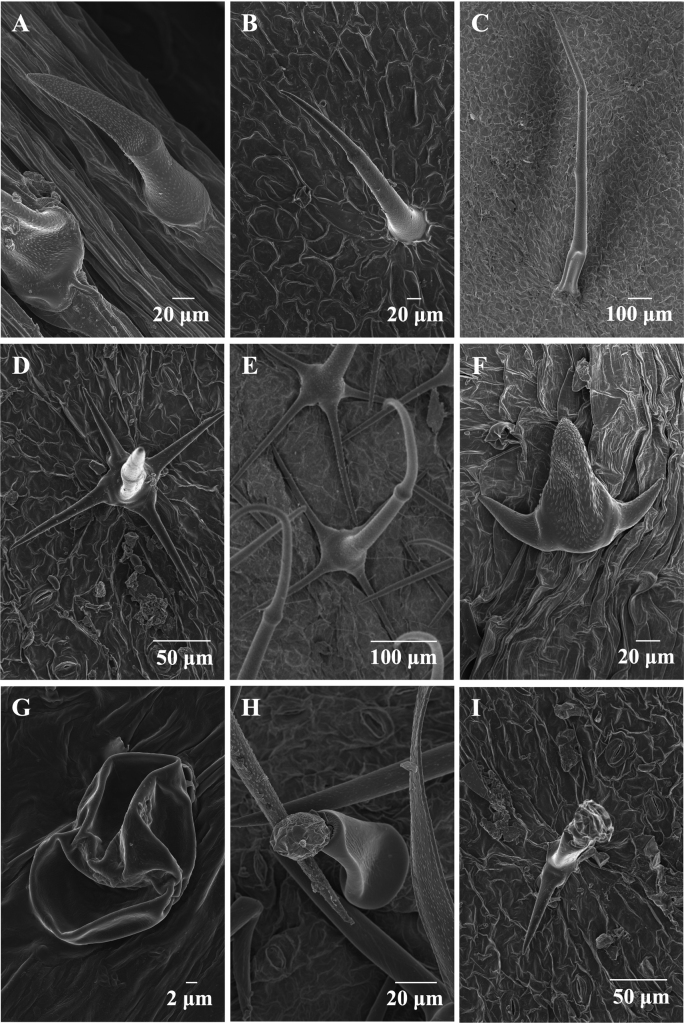
Two basic types of trichomes can be observed in Phlomoides: eglandular hairs and glandular hairs, as reported by Khosroshahi and Salmaki (2019). Both kinds of hairs can be divided into simple (unbranched) and branched trichomes. Simple eglandular trichomes were subdivided by length: short (< 500 μm) (Fig. 2A, B), long (500–2000 μm) (Fig. 2C) and extremely long (> 2000 μm); branched eglandular trichomes were subdivided into symmetrically stellate (Fig. 2D), stellate with central long branch (Fig. 2E) and bi- or trifurcate (Fig. 2F). Simple glandular trichomes were subdivided by stalk length: sub-sessile/sessile glandular trichomes (Fig. 2G), short-stalked glandular trichomes (< 500 μm) (Fig. 2H) and long-stalked glandular trichomes (> 500 μm). Branched glandular trichomes contain only one type (Fig. 2I). For the eight species examined in this study, extremely long simple non-glandular trichomes and long-stalked glandular trichomes were not observed.
Figure 2.
Different types of trichomes of PhlomoidesA short simple non-glandular trichomes (P.macrophylla) B short simple non-glandular trichomes (P.breviflora) C long simple non-glandular trichomes (P.henryi) D symmetrically non-glandular stellate (P.breviflora) E non-glandular stellate with central long branch (P.bracteosa) F bi- or trifurcate non-glandular stellate (P.nyalamensis) G sub-sessile/ sessile glandular trichomes (P.macrophylla) H simple glandular trichomes of (P.bracteosa) I branched glandular trichomes (P.breviflora).
Figs 3, 4 and Table 2 show the morphology and distribution of trichomes on leaves and bracts of the investigated taxa. Sub-sessile/sessile glandular trichomes occur widely in every part of each species of Phlomoides (Table 2). Short stalked glandular trichomes were observed on the abaxial leaf surface in five species and on the bracts of only one species, i.e. P.breviflora. Branched glandular trichomes were only recorded on the abaxial leaf surface of P.breviflora.
Figure 3.
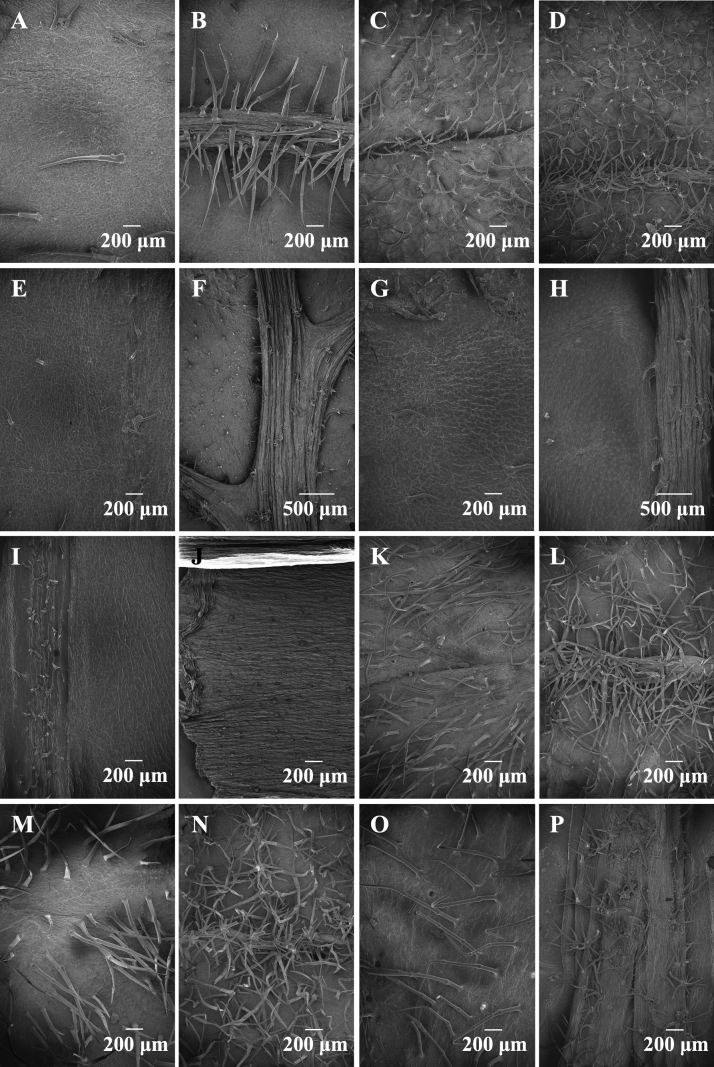
Photos of bracts, SEM of bracts of Phlomoideshenryi and related species A, BP.henryiC, DP.bracteosaE, FP.brevifloraG, HP.macrophyllaI, JP.nyalamensisK, LP.tibeticaM, NP.milingensisO, PP.rotata. A, C, E, G, I, K, M, O photos of bracts B, D, F, H, J, L, N, PSEM of bracts.
Figure 4.
SEM of both sides of leaves of Phlomoideshenryi and related species A, BP.henryiC, DP.bracteosaE, FP.brevifloraG, HP.macrophyllaI, JP.nyalamensisK, LP.tibeticaM, NP.milingensisO, PP.rotataA, C, E, G, I, K, M, OSEM of adaxial leaves B, D, F, H, J, L, N, PSEM of abaxial leaves.
Table 2.
Distribution of different types of trichome in the examined Phlomoides spp.
| Tissue | Species | Eglandular hairs | Glandular hairs | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Simple | Branched | Simple | Branched | ||||||
| Short | Long | Symmetrically stellate | Stellate with central long branch | Bi- or trifurcate | Sub-sessile/ sessile | Short stalked | |||
| Adaxial leaf | P.henryi | + | + | – | – | – | + | – | – |
| P.bracteosa | + | – | + | + | + | + | – | – | |
| P.breviflora | + | – | – | – | – | + | – | – | |
| P.macrophylla | + | – | – | – | – | + | – | – | |
| P.nyalamensis | + | – | – | – | – | + | – | – | |
| P.tibetica | + | + | – | – | – | + | – | – | |
| P.milingensis | + | + | – | – | – | + | – | – | |
| P.rotata | + | + | – | – | – | + | – | – | |
| Abaxial leaf | P.henryi | + | + | – | – | – | + | – | – |
| P.bracteosa | + | – | + | + | + | + | + | – | |
| P.breviflora | + | – | + | + | + | + | + | + | |
| P.macrophylla | + | – | + | + | + | + | + | – | |
| P.nyalamensis | – | – | – | – | + | + | – | – | |
| P.tibetica | + | + | – | + | – | + | + | – | |
| P.milingensis | + | – | – | + | + | + | + | – | |
| P.rotata | + | – | + | + | + | + | – | – | |
| Bract | P.henryi | + | + | – | – | – | + | – | – |
| P.bracteosa | + | + | + | + | + | + | – | – | |
| P.breviflora | + | – | + | + | – | + | + | – | |
| P.macrophylla | + | + | – | – | – | + | – | – | |
| P.nyalamensis | + | + | – | – | – | + | – | – | |
| P.tibetica | + | + | – | – | – | + | – | – | |
| P.milingensis | + | + | – | + | + | + | – | – | |
| P.rotata | + | + | – | – | – | + | – | – | |
Simple short eglandular trichomes were observed in every species on leaf and bract surface, but were missing in the abaxial leaf of Phlomoidesnyalamensis, since it was nearly glabrous (Fig. 4J). Adaxial leaf surfaces were often covered by simple eglandular trichomes, except for P.bracteosa (Fig. 4C), which has dense branched eglandular trichomes on the adaxial leaf surface. Simple long eglandular trichomes were most common on bracts (Fig. 3B, H, J, L, O). Abaxial leaf surfaces often had branched eglandular trichomes, but these are not present in the new species (Fig. 4B).
Trichomes were transparent to white or brown to black in Phlomoides. Trichomes inside the upper corolla lip of the new species (P.henryi), P.bracteosa and P.rotata were transparent to white, while the other five species were brown to black. Bract trichomes of P.tibetica and P.milingensis were brown to black (Fig. 3K, M), the other six species were transparent to white (Fig. 3A, C, E, G, I, O).
Discussion
Herbaria house millions of specimens that embody the plant diversity on the Earth. Many new species have been lurking in herbaria for many years before being published. Bebber et al. (2010) estimated that 84% of new species’ descriptions were from old specimens collected more than five years prior to publication and 25% from specimens more than 50 years old. During the taxonomic review of some groups of Lamiaceae, we have also found some new species from old herbarium specimens (Chen et al. 2014; Dong et al. 2015), indicating taxonomic work, based on herbaria, is still a very important resource for the discovery of new taxa.
The resulting phylogenetic tree of Phlomoides in this study was similar to that in previous study (Zhao et al. 2024). The new species, P.henryi, was nested within Clade II and formed a separate branch (Fig. 1: 1.00/100%) that is sister to a subclade containing P.rotata and five species with brown to black trichomes on the upper corolla. Geographically, Phlomoideshenryi is distributed in southern Yunnan, while the other six species in this subclade were mainly distributed in the Qinghai-Tibetan Plateau and Himalaya. The new species is morphologically distinct from the other six species in this subclade. For example, trichomes on the upper corolla lip of P.henryi and P.rotata are colourless and perceptually transparent to white, but brown to black in the other five species. Morphologically, P.rotata is distinct from all other species of Phlomoides by the very short stem producing a rosette of leaves with the plant often less than 10 cm high, while P.henryi is generally taller than 1 m. As we observed, all the species with trichomes brown to black were embedded within this subclade. The sister clade to that containing P.henryi contains 23 species that are mainly distributed in Hengduan Mountains. Phlomoideshenryi is similar to other species in Clade I and Clade II in lacking basal leaves. Only four species have basal leaves in Clade II, i.e. P.rotata, P.tibetica, P.milingensis and P.atropurpurea, while all the species in Clades III–VI have basal leaves.
As above mentioned, we believe that the differences merit recognition of the new species and we describe it below.
Phlomoides is a morphologically diverse and taxonomically difficult group with many characters used for traditional taxonomy being highly variable. In this study, we investigated trichome micro-morphology on bracts and leaves of Phlomoideshenryi and related species. We found that trichomes are a useful character to distinguish some morphologically similar species. Based on the colour of trichomes, we can separate two groups of those species. Phlomoidesnyalamensis, P.macrophylla, P.tibetica, P.milingensis and P.breviflora have brown to black trichomes on the upper corolla lip, while the other species (P.rotata, P.bracteosa and the new species described here, P.henryi) have transparent to white trichomes on the upper corolla lip. Trichome density and bract trichome colour can separate P.tibetica from the similar P.milingensis. Both species are distributed in Xizang at an altitude from 3500–4500 m and Hsuan (1977) placed them within Series Tibeticae. Phlomoidestibetica has floral bracts with black simple trichomes and no branched trichomes, while P.milingensis has floral bracts with brown simple and branched trichomes. Similarly, the new species described here, P.henryi, can be distinguished from the six related species in the subclade by the absence of branched trichomes on the abaxial leaf surface (Fig. 5B). Phlomoidesbracteosa can easily be separated from these six species by having branched trichomes on the adaxial leaves (Fig. 5C). Azizian and Cutler (1982) have found that adaxial and abaxial leaf surfaces have different trichome types, but in that work, Phlomoides was treated as a section of Phlomis and they only discussed the differences amongst Phlomissect.Phlomis, Phlomissect.Phlomoides and Eremostachys and not at the species level. Subsequent studies did not observe trichomes on different structures (Seyedi and Salmaki 2015; Khosroshahi and Salmaki 2019). However, here we found different structures were covered with significantly different trichomes and these differences can be used as evidence to separate morphologically similar species. Future studies should focus on micro-morphological investigation of trichomes and other characters (i.e. appendages, calyces, roots, mericarps) and those micro-features are probably helpful for taxonomy and species identification of Phlomoides species.
Figure 5.
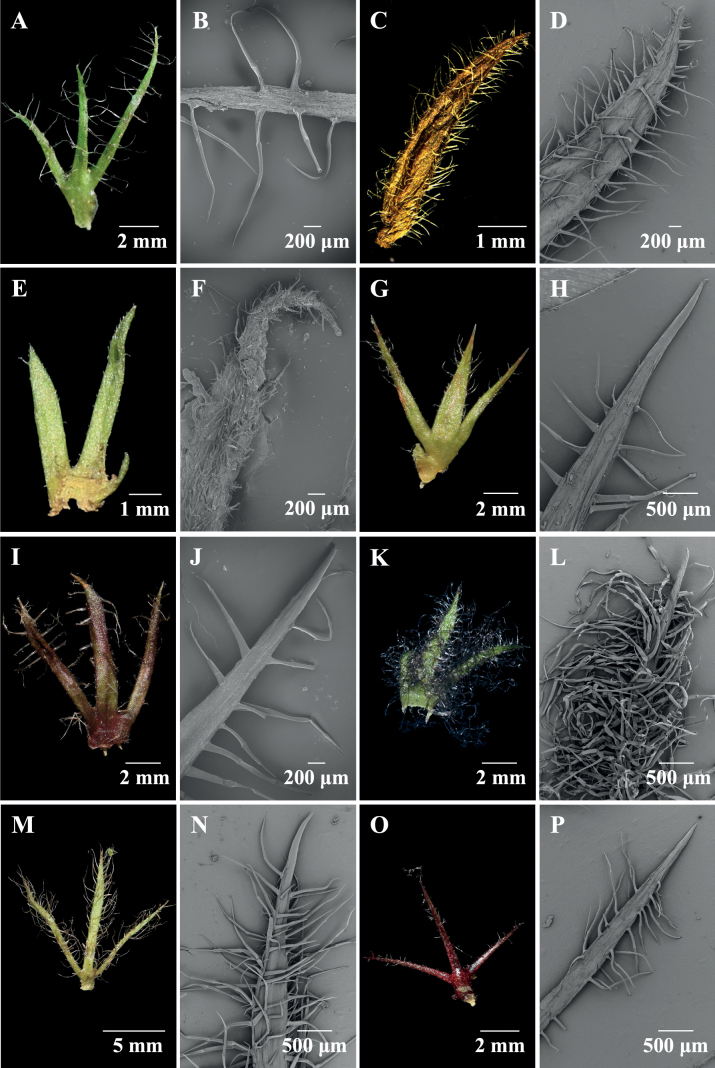
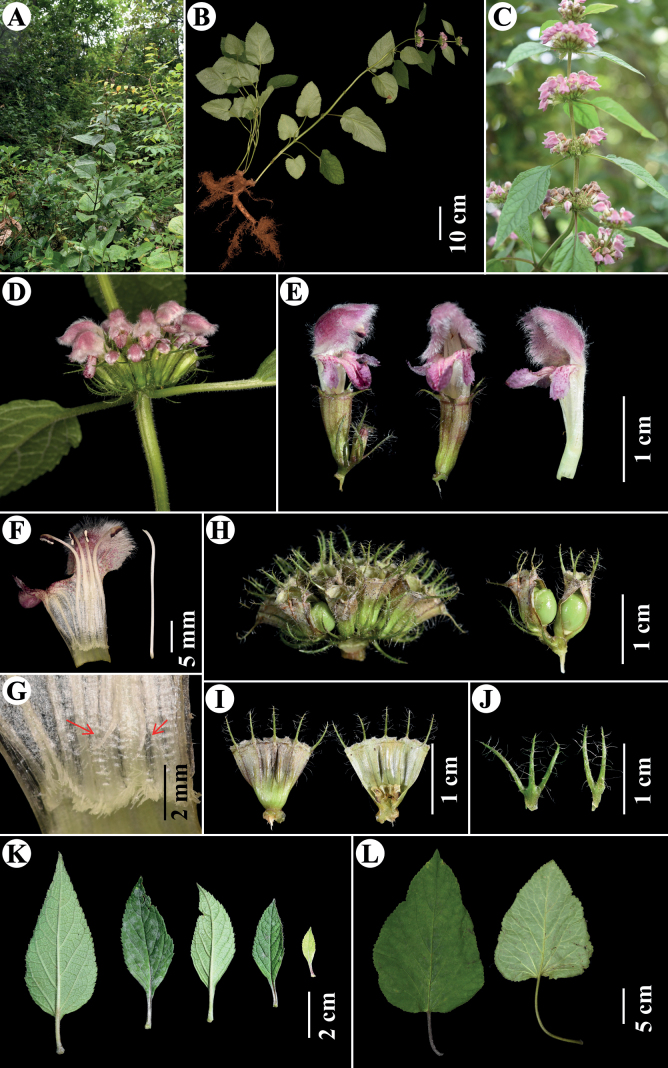
Phlomoideshenryi Y.Zhao & C.L.Xiang A habitat B plant with linear-tuberous roots C inflorescence D verticillaster E flowers F dissected flower G appendages at base of posterior filaments H fruiting calyces I dissected calyces J bracts K floral leaves L stem leaves. Photographs by Yue Zhao, except C by Li Chen.
In order to distinguish those species grouped with the new species in the phylogenetic tree (Fig. 2), as well as P.bracteosa, we provide a key, mainly based on macro- and micro-morphological trichomes.
Key to P.henryi and morphologically similar species
| 1 | Upper corolla lip with trichome transparent to white | 2 |
| – | Upper corolla lip with trichomes brown to black | 4 |
| 2 | No branched trichomes on leaf and bract | P.henryi |
| – | With branched trichomes on leaf and bract | 3 |
| 3 | Floral bracts with branched trichomes, bracts linear to lanceolate | P.bracteosa |
| – | Floral bracts with simple trichomes only, bracts needle-like to subulate | P.rotata |
| 4 | Floral bracts with trichomes brown to black | 5 |
| – | Floral bracts with trichomes transparent to white | 6 |
| 5 | Floral bracts with black simple trichomes, no branched trichomes | P.tibetica |
| – | Floral bracts with brown simple and branched trichomes | P.milingensis |
| 6 | Floral bracts less than 1 cm long, with branched trichomes | P.breviflora |
| – | Floral bracts more than 1 cm long, with only simple trichomes | 7 |
| 7 | Flower purple | P.nyalamensis |
| – | Flower white | P.macrophylla |
Taxonomic treatment
. Phlomoides henryi
Y.Zhao & C.L.Xiang sp. nov.
952A4EFD-8DF8-5080-AC6E-666D6472F87A
urn:lsid:ipni.org:names:77330835-1
Type.
China, Yunnan Province, Honghe Hani and Yi Autonomous Prefecture, Jianshui County, on the forest edge, 23°57′52.54″N, 102°59′49.47″E, alt. 1279 m, 3 Sep 2022, F. Zhao, Y. Zhao & C.L. Xiang XCL2222 (holotype: KUN!; isotypes: KUN!, CSH!).
Diagnosis.
Within the subclade, Phlomoideshenryi is morphologically similar to P.rotata for having transparent to white trichomes inside the upper corolla lip rather than brown to black and is distinct from all other species by lacking branched hairs. P.bracteosa has similar transparent to white trichomes inside the upper corolla lip, but with branched trichomes on both sides of leaves and floral bracts. The differences between P.henryi, P.rotata and P.bracteosa are listed in Table 3.
Table 3.
Morphological comparisons amongst Phlomoideshenryi, P.rotata and P.bracteosa.
| Characters | P.henryi | P.rotata | P.bracteosa |
|---|---|---|---|
| Height | 100–150 cm | 2.5–10 cm | 20–50 (–100) cm |
| Basal leaves | absent | rosette basal leaves | absent |
| Floral leaf shape | narrowly lanceolate | lanceolate, oblanceolate, or linear | Ovate to lanceolate |
| Floral leaf petiole length | 5–35 mm | Lack obvious petiole | Upper floral leaves sessile, lower floral leaves with petiole 5–20 mm long |
| Floral bracts shape | Subulate | Needle-like to subulate | Linear to lanceolate, often with enlarged bracts |
| Branched trichomes | No branched trichomes | With branched trichomes on abaxial leaves | With branched trichomes on both sides of leaves and bracts |
Perennial herbs.
Roots robust, linear-tuberous. Stems 1–1.5 m tall, subquadrangular, robust, simple pilose. Basal leaves absent; stem leaves with petioles 4–15 cm long, with simple trichomes, broadly ovate to ovate-oblong, papery, 10–18 × 15–24 cm, adaxially green with sparse simple trichomes, abaxially light green, with sparse simple trichomes, denser and longer on the main vein, base cordate, margin serrate or crenate, apex acute to acuminate. Verticillasters axillary, 8–20-flowered; floral leaves with petioles 5–35 mm long, lanceolate, base rounded to cuneate, 1–6 × 0.5–4 cm, gradually reduced upwards; bracts subulate, 6–10 mm long, with sparse long simple trichomes, ca. 2 mm long. Calyx tubular, 10–11 × 4–5 mm, pubescent outside with sparse long simple trichomes on veins, conspicuously 10-veined; teeth 5, truncate, ca. 1.5 mm long, apical spines 3–4 mm long, with sparse long simple trichomes. Corolla light purple to pink, ca. 2.1 cm long, 2-lipped; posterior lip ca. 7 mm long, galeate, densely stellate tomentose outside, margin denticulate, bearded inside; anterior lip 3-lobed, ca. 7 × 8 mm, middle lobe largest, oblong, ca. 5 × 3 mm, lateral lobes ovate; tube glabrous outside, ca. 1.5 cm, annulate pilose inside. Stamens 4, included, with cobwebby indumentum, posterior filaments with reflexed appendages at base. Style unequally 2-lobed. Nutlets oblong-globose, glabrous.
Etymology.
The new species is named after the collector Augustine Henry (1857–1930), who collected more than 15,000 dry specimens and seeds from China.
Phenology.
Flowering from July to September and fruiting from October to December.
Distribution, habitat and ecology.
Based on present collections, P.henryi is only known from its type locality, i.e. Muyang Mountain in Jianshui County, Yunnan Province, China. It is restricted to the edge of the forest at an elevation near 1280 m.
Chinese name.
jiàn shuǐ cǎo cāo sū (建水草糙苏).
Additional specimen examined.
Phlomoideshenryi: Paratypes. China, Yunnan Province, Honghe Hani and Yi Autonomous Prefecture, Jianshui County, 6 September 2019, Jianshui Exped. 2164 (KUN); Yunnan Province, Mengtze, 1898, A. Henry 10216 (K000928267, K without barcode, LE without barcode); Yunnan Province, “Lao Kouy [Chan], 11 August 1907, F. Ducloux 369 (E).
Phlomoidesbracteosa: India. Choor & Kidarlonta, 1832, J.F. Royle 633 (Type: K, K000894384); State of Punjab: Kangra, Lahul, Bhaga Valley, alt. 3000 m, 26 July 1933, T.R. Chand 74A (MICH, 1519061); State of Uttarakhand: Bhyundar Valley – Valley of flowers (N.E. road Josimath to Badrinath), alt. 3430 m, 16 August 1975, Anonymous 6583 (PE, 1290791).
Phlomoidesbreviflora: China. Xizang Province: Yadong County, on the way from Yadong to Dingga, alt. 2850 m, 11 June 1975, Qinghai-Tibet Exped. 750283 (Holotype: KUN, 1218974!; Isotype: PE, 00835569!, 00835570!); Nielamu County, Qu Town, alt. 3240 m, 12 September 2019, Y.P. Chen, Y. Zhao & B.Y. Zhang EM1139 (KUN). India. Sikkim: Nayathang-Phalut, alt. 3500 m, 2 June 1960, H. Hara et al. 5551 (TI); West Bengal: Darjeeling, Phalut, alt. 3500 m, 5 June 1960, H. Hara et al. 5555 (TI); Darjeeling, Garibans-Tanglu, alt. 2700 m, 7 June 1969, H. Hara et al. 5557 (TI). Nepal. Bagmati Province: Rasuwa District, Gossain than (Gosainkund), Benth in Wall. Cat. Herb. Ind. n. 2066 (Type: K, K001115039!).
Phlomoidesmacrophylla: Nepal. Gandaki Province: Mustang, Annapurna Conservation area, Trekking route Jomosom-Nayapul, Near Ghorepani village (way to Tikhedhunga). China. Xizang Province: Yadong County, Shang Yadong Village, alt. 3448 m, 30 August 2023, Y. Zhao et al. XCL2703 (KUN); Yadong County, on the way from Yadong County to the Pass of Naiduila Mountain, 9 September 2019, Y.P. Chen, Y. Zhao & B.Y. Zhang EM1145 (KUN).
Phlomoidesnyalamensis: China. Xizang Province: Nyalam County, Zhangmu Town, on the way from Lixin to Xuebugang, open spaces in forests, alt. 2700–2800 m, 29 June 1975, Qinghai-Xizang Comp. Exped. 6622 (Holotype, KUN 1218985! Isotype, KUN 1218984!); Nyalam County, Zhangmu Town, Lixin Village, damp hillside, alt. 2800 m, 24 August 1972, Tibetan herbal medicine Exped. 1461 (PE, 00835826); Nyalam County, Zhangmu Town, on the way from Lixin to Xuebugang, edge of forests, alt. 2896 m, 13 September 2019, Y.P. Chen, Y. Zhao & B.Y. Zhang EM1145 (KUN).
Phlomoidestibetica: China. Xizang Province: in open alpine pastures of turf in well drained situations, alt. 4200–4500 m, 6 July 1924, F. K. Ward 5901 (Holotype: K, K000894378!; isotype: BM, BM000950520, E, E00301982!); Linzhi City, Bomi County, Northern Galongla Mountain, 3800–3900 m, 16 August 1982, S.Z. Cheng & B.S. Li 00160 (PE, 00923472); Linzhi City, on the way from Lulang Town to the Pass of Sejila Mountain, 4108 m, 15 September 2016, C.L. Xiang et al. 1456 (KUN). Bhutan. Trashigang District, Shingbe Town, 3800 m, 27 May 1949, F. Ludlow, G. Sherriff & J.H. Hicks 20673 (BM).
Phlomoidesmilingensis: China. Xizang Province: Mainling County, Zedanggang, alt. 4400 m, 26 July 1972, Tibetan herbal medicine Exped. 3883 (holotype: PE, 00835583; isotype: PE, 00923457); Mainling County, Nanyi Mountain, alt. 3400–3500 m, 28 July 1972, Tibetan herbal medicine Exped. 4228 (PE, 00835584; PE, 00923459); Gongbo’gyamda County, Xueka, 10 August 1974, Anonymous 2129 (PE, 00832244).
Phlomoidesrotata: China: Xizang, Shannan City, Cona County, near radar station, alt. 4280 m, 17 July 1975, C.Y. Wu et al. 75-893 (KUN, 0216402); Naqu City, Lhari County, meteorological station, alt. 4500 m, 8 June 1976, Qinghai-Xizang Comp. Exped. 10491 (KUN, 0216409); Nyingchi City, Gongbo’gyamda County, Mira pass, alt. 4920 m, 30 August 1974, Qinghai-Xizang Comp. Exped. 74-2027 (KUN, 0216384); Sichuan, Liangshan Yi Autonomous Prefecture, Muli County, on the way from Mogalaji to Nonsa pasture, alt. 4312 m, 30 August 2015, C.L. Xiang 1219 (KUN, 1264607); Yunnan, Diqing Tibetan Autonomous Prefecture, Deqin County, Baimaxueshan, alt. 4300 m, 4 June 2000, Z.K. Zhou et al. 159 (KUN, 0699320); Qinghai, Yushu Tibetan Autonomous Prefecture, Yushu County, Hongtu Mountain, alt. 4900 m, 3 September 2013, J.W. Zhang et al. Zh678 (KUN, 1260768).
Supplementary Material
Acknowledgements
The authors are very grateful to the curators of the following herbaria for their kind assistance in research facilities: K, CDBI, E, GXMI, HIB, IBK, IBSC, K, KUN, KYO, MW, NAS, PE, SZ, TI. We also thank Mr. Zhi-Jia Gu for his technical assistance with SEM.
Appendix 1
Specimen information (taxon, voucher, herbarium, country) for samples newly-sequenced in the present study with GenBank accession numbers for atpB-rbcL, psbA-trnH, rpl16, rpl32-trnL, rps16, trnK, trnL-trnF, trnS-trnG and trnT-L, respectively. Herbarium abbreviations are listed after the vouchers. The accession numbers marked with an asterisk represent sequences newly generated. Only GenBank accession numbers are listed for sequences downloaded from NCBI.
Phlomiscomposita Pau, ON820555, ON820616, ON835580, ON835627, ON835674, ON835721, ON843184, OR632134, ON843231; Phlomisfruticosa L., ON820556, ON820615, ON835581, ON835628, ON835675, ON835722, ON843185, OR632135, ON843232; Phlomisherba-venti subsp. pungens (Willd.) Maire ex DeFilipps, ON820557, ON820614, ON835582, ON835629, ON835676, ON835723, ON843186, ON815620, ON843233; Phlomoidesalpina (Pall.) Adylov, Kamelin & Makhm., OR631925, OR631967, OR642305, OR632009, OR632051, OR642347, OR642389, OR632137, OR632093; Phlomoidesatropurpurea (Dunn) Kamelin & Makhm., OQ672946, OQ672946, OQ672946, OQ672946, OQ672946, OQ672946, OQ672946, OQ672946, OQ672946; Phlomoidesbetonicoides (Diels) Kamelin & Makhm., MN617020, MN617020, MN617020, MN617020, MN617020, MN617020, MN617020, MN617020, MN617020; Phlomoidesbracteosa (Royle ex Benth.) Kamelin & Makhm., C.R. Lancaster 72 (BM), India, Srinagar, Pahalgam, Gully, alt. 2286 m, OR674852*, OR674855*, OR674856*, OR674858*, OR674860*, OR674863*, OR674864*, OR674867*, OR674869*; Phlomoidesbreviflora (Benth.) Kamelin & Makhm., OQ672923, OQ672923, OQ672923, OQ672923, OQ672923, OQ672923, OQ672923, OQ672923, OQ672923; Phlomoidesburmanica (Mukerjee) Kamelin & Makhm., ON820563, ON820630, ON835588, ON835635, ON835682, ON835729, ON843192, OR632142, ON843239; Phlomoideschinghoensis (C.Y. Wu) Kamelin & Makhm., ON820580, ON820611, ON835605, ON835652, ON835699, ON835746, ON843209, ON815622, ON843256; Phlomoidescongesta (C.Y. Wu) Kamelin & Makhm., ON820567, ON820608, ON835592, ON835639, ON835686, ON835733, ON843196, OR632145, ON843243; Phlomoidesdentosa (Franch.) Kamelin & Makhm., OR631929, OR631973, OR642309, OR632015, OR632057, OR642351, OR642395, OR632149, OR632099; Phlomoidesdeserticola Sennikov, OQ672935, OQ672935, OQ672935, OQ672935, OQ672935, OQ672935, OQ672935, OQ672935, OQ672935; Phlomoidesforrestii (Diels) Kamelin & Makhm., OR631934, OR631976, OR642313, OR632018, OR632060, OR642355, OR642398, OR632153, OR632102; Phlomoidesfranchetiana (Diels) Kamelin & Makhm., ON820561, ON820621, ON835586, ON835633, ON835680, ON835727, ON843190, OR632155, ON843237; Phlomoideshamosa (Benth.) Mathiesen, ON820558, ON820604, ON835583, ON835630, ON835677, ON835724, ON843187, OQ672937, ON843234; Phlomoideshenryi Y.Zhao & C.L.Xiang, F. Zhao, Y. Zhao & C.L. Xiang XCL2222 (KUN), China, Yunnan Province, Jianshui County, Limin Town, Muyang Mountain, alt. 2179 m, OR674853*, OR674854*, OR674857*, OR674859*, OR674861*, OR674862*, OR674865*, OR674866*, OR674868*; Phlomoidesinaequalisepala (C.Y. Wu) Kamelin & Makhm., OR631937, OR631979, OR642317, OR632021, OR632063, OR642359, OR642401, OR632160, OR632105; Phlomoidesjeholensis (Nakai & Kitag.) Kamelin & Makhm., OR631938, OR631980, OR642318, OR632022, OR632064, OR642360, OR642402, OR632162, OR632106; Phlomoideskoraiensis (Nakai) Kamelin & Makhm., OR631939, OR631981, OR642319, OR632023, OR632065, OR642361, OR642403, OR632163, OR632107; Phlomoidesliangwangshanensis Y. Zhao, H.L. Zheng & C.L. Xiang, OR631940, OR631982, OR642320, OR632024, OR632066, OR642362, OR642404, OR632165, OR632108; Phlomoideslikiangensis (C.Y. Wu) Kamelin & Makhm., OR631942, OR631984, OR642322, OR632026, OR632068, OR642364, OR642406, OR632167, OR632110; Phlomoideslongiaristata (C.Y. Wu & H.W. Li) Salmaki, ON820559, ON820603, ON835584, ON835631, ON835678, ON835725, ON843188, ON815625, ON843235; Phlomoideslongicalyx (C.Y. Wu) Kamelin & Makhm., OR631943, OR631985, OR642323, OR632027, OR632069, OR642365, OR642407, OR632168, OR632111; Phlomoidesmacrophylla (Benth.) Kamelin & Makhm., OR631944, OR631986, OR642324, OR632028, OR632070, OR642366, OR642408, OR632169, OR632112; Phlomoidesmaximowiczii (Regel) Kamelin & Makhm., ON820565, ON820622, ON835590, ON835637, ON835684, ON835731, ON843194, OR632170, ON843241; Phlomoidesmazzettii Lazkov, OR631945, OR631987, OR642325, OR632029, OR632071, OR642367, OR642409, OR632171, OR632113; Phlomoidesmedicinalis (Diels) Kamelin & Makhm., OR631946, OR631988, OR642326, OR632030, OR632072, OR642368, OR642410, OR632172, OR632114; Phlomoidesmegalantha (Diels) Kamelin & Makhm., OR631947, OR631989, OR642327, OR632031, OR632073, OR642369, OR642411, OR632173, OR632115; Phlomoidesmelanantha (Diels) Kamelin & Makhm., OR631948, OR631990, OR642328, OR632032, OR632074, OR642370, OR642412, OR632174, OR632116; Phlomoidesmilingensis (C.Y. Wu & H.W. Li) Kamelin & Makhm., OR631949, OR631991, OR642329, OR632033, OR632075, OR642371, OR642413, OR632175, OR632117; Phlomoidesmoluccelloides (Bunge) Salmaki, OQ672938, OQ672938, OQ672938, OQ672938, OQ672938, OQ672938, OQ672938, OQ672938, OQ672938; Phlomoidesmongolica (Turcz.) Kamelin & A.L. Budantzev, ON820576, ON820617, ON835601, ON835648, ON835695, ON835742, ON843205, OR632176, ON843252; Phlomoidesmuliensis (C.Y. Wu) Kamelin & Makhm., OR631950, OR631992, OR642330, OR632034, OR632076, OR642372, OR642414, OR632177, OR632118; Phlomoidesnyalamensis (H.W. Li) Y. Zhao & C.L. Xiang, OR631952, OR631994, OR642332, OR632036, OR632078, OR642374, OR642416, OR632179, OR632120; Phlomoidesoreophila (Kar. & Kir.) Adylov, Kamelin & Makhm., OR631953, OR631995, OR642333, OR632037, OR632079, OR642375, OR642417, OR632180, OR632121; Phlomoidesornata (C.Y. Wu) Kamelin & Makhm., ON820570, ON820618, ON835595, ON835642, ON835689, ON835736, ON843199, OR632181, ON843246; Phlomoidespaohsingensis (C.Y. Wu) Kamelin & Makhm., OR631954, OR631996, OR642334, OR632038, OR632080, OR642376, OR642418, OR632182, OR632122; Phlomoidespedunculata (Y.Z.Sun) Kamelin & Makhm., OQ672936, OQ672936, OQ672936, OQ672936, OQ672936, OQ672936, OQ672936, OQ672936, OQ672936; Phlomoidespratensis (Kar. & Kir.) Adylov, Kamelin & Makhm., ON820579, ON820612, ON835604, ON835651, ON835698, ON835745, ON843208, ON815626, ON843255; Phlomoidesrotata (Benth. ex Hook.f.) Mathiesen, ON820564, ON820602, ON835589, ON835636, ON835683, ON835730, ON843193, ON815627, ON843240; Phlomoidesruptilis (C.Y. Wu) Kamelin & Makhm., OR631955, OR631997, OR642335, OR632039, OR632081, OR642377, OR642419, OR632183, OR632123; Phlomoidessagittata (Regel) C.L.Xiang & Y.Zhao, ON820578, ON820620, ON835603, ON835650, ON835697, ON835744, ON843207, ON815617, ON843254; Phlomoidessetifera (Bureau & Franch.) Kamelin & Makhm., OR631956, OR631998, OR642336, OR632040, OR632082, OR642378, OR642420, OR632186, OR632124; Phlomoidesspeciosa (Rupr.) Adylov, Kamelin & Makhm., ON820592, ON820631, ON835617, ON835664, ON835711, ON835758, ON843221, ON815629, ON843268; Phlomoidesstrigosa (C.Y. Wu) Kamelin & Makhm., OR631957, OR631999, OR642337, OR632041, OR632083, OR642379, OR642421, OR632187, OR632125; Phlomoidestatsienensis (Bureau & Franch.) Kamelin & Makhm., OR631960, OR632002, OR642340, OR632044, OR632086, OR642382, OR642424, OR632190, OR632128; Phlomoidestibetica (C. Marquand & Airy Shaw) Kamelin & Makhm., OR631961, OR632003, OR642341, OR632045, OR632087, OR642383, OR642425, OR632193, OR632129; Phlomoidestuberosa (L.) Moench, ON820575, ON820624, ON835600, ON835647, ON835694, ON835741, ON843204, ON815631, ON843251; Phlomoidesumbrosa (Turcz.) Kamelin & Makhm., ON820571, ON820605, ON835596, ON835643, ON835690, ON835737, ON843200, OR632198, ON843247; Phlomoidesyounghusbandii (Mukerjee) Kamelin & Makhm., MW405448, MW405448, MW405448, MW405448, MW405448, MW405448, MW405448, MW405448, MW405448; Phlomoideszenaidae (Popov) Adylov, Kamelin & Makhm., OQ672945, OQ672945, OQ672945, OQ672945, OQ672945, OQ672945, OQ672945, OQ672945, OQ672945.
Citation
Zhao Y, Zhao F, Paton AJ, Xiao J-F, Chen Y-P, Xiang C-L (2024) Using scanning electron microscopy and molecular data to discover a new species from old herbarium collections: The case of Phlomoides henryi (Lamiaceae, Lamioideae). PhytoKeys 238: 127–146. https://doi.org/10.3897/phytokeys.238.117180
Additional information
Conflict of interest
The authors have declared that no competing interests exist.
Ethical statement
No ethical statement was reported.
Funding
This study was supported by the National Natural Science Foundation of China (Grant Nos. 32161143015, 31872648), International Partnership Program of Chinese Academy of Sciences (Grant No. 070GJHZ202211FN), the Yunnan Fundamental Research Projects (Grant No. 202001AS070016) awarded to CLX, the Postdoctoral Research Funding Projects of Yunnan Province and the Postdoctoral Directional Training Foundation of Yunnan Province awarded to FZ.
Author contributions
YZ and CLX conceived this research. YZ, FZ, JFX, YPC and CLX collected materials. YZ and FZ performed the experiments. YZ, AP, YPC and CLX analysed the data. All co-authors contributed to the manuscript and revised it critically.
Author ORCIDs
Yue Zhao https://orcid.org/0000-0001-6850-5070
Fei Zhao https://orcid.org/0000-0001-7328-2262
Alan J. Paton http://orcid.org/0000-0002-6052-6675
Jin-Fei Xiao https://orcid.org/0000-0001-7729-2548
Ya-Ping Chen http://orcid.org/0000-0002-7502-1848
Chun-Lei Xiang https://orcid.org/0000-0001-8775-6967
Data availability
DNA sequences associated with this work are available from GenBank (https://www.ncbi.nlm.nih.gov/genbank/) or from the corresponding author upon reasonable request.
References
- Astanova SB. (1984) Chromosome numbers in the species of the families AlliaceaeAsteraceaeCaryophyllaceaeEbenaceaeLinaceaeOleaceaeLamiaceae from Tadjikistan. Botanicheskii Zhurnal 69(11): 1563–1564. [Google Scholar]
- Azizian D, Cutler DF. (1982) Anatomical, cytological and phytochemical studies on Phlomis L. and Eremostachys Bunge (Labiatae). Botanical Journal of the Linnean Society 85(4): 249–281. 10.1111/j.1095-8339.1982.tb00373.x [DOI] [Google Scholar]
- Bebber DP, Carine MA, Wood JRI, Wortley AH, Harris DJ, Prance GT, Davidse G, Paige J, Pennington TD, Robson NKB, Scotland RW. (2010) Herbaria are a major frontier for species discovery. Proceedings of the National Academy of Sciences of the United States of America 107(51): 22169–22171. 10.1073/pnas.1011841108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendiksby M, Thorbek L, Scheen A-C, Lindqvist C, Ryding O. (2011) An updated phylogeny and classification of Lamiaceae subfamily Lamioideae. Taxon 60(2): 471–484. 10.1002/tax.602015 [DOI] [Google Scholar]
- Chen YP, Hu GX, Xiang CL. (2014) Isodondelavayi (Ocimeae, Nepetoideae, Lamiaceae): A new species from Yunnan Province, Southwest China. Phytotaxa 156(5): 291–297. 10.11646/phytotaxa.156.5.5 [DOI] [Google Scholar]
- Czerepanov SK. (1995) Labiatae. In: Czerepanov SK. (Ed.) Vascular Plants of Russia and Adjacent States.Cambridge University Press, Cambridge, 295–305.
- Darriba D, Taboada GL, Doallo R, Posada D. (2012) jModel-Test 2: More models, new heuristics and parallel computing. Nature Methods 9(8): e772. 10.1038/nmeth.2109 [DOI] [PMC free article] [PubMed]
- Dong HJ, Jamzad Z, Xiang CL. (2015) Nepetawuana (Nepetinae, Nepetoideae, Lamiaceae), a new species from Shanxi, China. Iranian Journal of Botany 21(1): 13–18. [Google Scholar]
- Doyle JJ, Doyle JD. (1987) A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochemical Bulletin 19(1): 11–15. [Google Scholar]
- Fang LQ, Pan YZ, Gong X. (2007) A karyomorphological study in the monotypic genus Lamiophlomis and five species in Phlomis (Lamiaceae). Zhiwu Fenlei Xuebao 45(5): 627–632. 10.1360/aps050192 [DOI] [Google Scholar]
- Gairola S, Naidoo Y, Bhatt A, Nicholas A. (2009) An investigation of the foliar trichomes of Tetradeniariparia (Hochst.) Codd (Lamiaceae): An important medicinal plant of Southern Africa. Flora 204(4): 325–330. 10.1016/j.flora.2008.04.002 [DOI] [Google Scholar]
- Ghaffari SM. (2006) New or rare chromosome counts of some angiosperm species from Iran. Iranian Journal of Botany 11(2): 185–192. [Google Scholar]
- Hsuan SJ. (1977) Phlomis. In: Wu CY, Li HW. (Eds) Flora Reipublicae Popularis Sinicae (Vol.65). Science Press, Beijing, 428–478.
- Hu GX, Balangcod TD, Xiang CL. (2012) Trichome micromorphology of the Chinese-Himalayan genus Colquhounia (Lamiaceae), with emphasis on taxonomic implications. Biologia 67(5): 867–874. 10.2478/s11756-012-0076-z [DOI] [Google Scholar]
- Khosroshahi EE, Salmaki Y. (2019) Evolution of trichome types and its systematic significance in the genus Phlomoides (Lamioideae-Lamiaceae). Nordic Journal of Botany 37(5): 40–48. 10.1111/njb.02132 [DOI] [Google Scholar]
- Li HW. (1985) Labiatae. In: Wu CY. (Ed.) Flora Xizangica (Vol.4). Science Press, Beijing, 98–299.
- Li HW, Hedge IC. (1994) Lamiaceae. In: Wu CY, Raven PH. (Eds) Flora of China (Vol.17). Science Press, Beijing & Missouri Botanical Garden Press, St. Louis, 50–299.
- Mathiesen C, Scheen A-C, Lindqvist C. (2011) Phylogeny and biogeography of the lamioid genus Phlomis (Lamiaceae). Kew Bulletin 66(1): 83–99. 10.1007/s12225-011-9257-0 [DOI] [Google Scholar]
- Miller MA, Pfeiffer W, Schwartz T. (2010) . Creating the CIPRES Science Gateway for inference of large phylogenetic trees. Proceedings of the Gateway Computing Environments Workshop (GCE), New Orleans, 8 pp. 10.1109/GCE.2010.5676129 [DOI] [Google Scholar]
- Rambaut A. (2014) FigTree, v.1.4.2. http://tree.bio.ed.ac.uk/software/figtree/
- Ronquist F, Teslenko M, Van der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP. (2012) MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61(3): 539–542. 10.1093/sysbio/sys029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmaki Y, Zarre S, Heubl G. (2012a) The genus Phlomoides Moench (Lamiaceae; Lamioideae; Phlomideae) in Iran: An updated synopsis. Iranian Journal of Botany 18(2): 207–219. [Google Scholar]
- Salmaki Y, Zarre S, Ryding O, Lindqvist C, Scheunert A, Bräuchler C, Günther H. (2012b) Phylogeny of the tribe Phlomideae (Lamioideae: Lamiaceae) with special focus on Eremostachys and Phlomoides: New insights from nuclear and chloroplast sequences. Taxon 61(1): 161–179. 10.1002/tax.611012 [DOI] [Google Scholar]
- Scheen A-C, Bendiksby M, Ryding O, Mathiesen C, Albert VA, Lindqvist C. (2010) Molecular phylogenetics, character evolution and suprageneric classification of Lamioideae (Lamiaceae). Annals of the Missouri Botanical Garden 97(2): 191–219. 10.3417/2007174 [DOI] [Google Scholar]
- Seyedi Z, Salmaki Y. (2015) Trichome morphology and its significance in the systematics of Phlomoides (Lamiaceae; Lamioideae; Phlomideae). Flora 213: 40–48. 10.1016/j.flora.2015.04.003 [DOI] [Google Scholar]
- Stamatakis A. (2014) RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30(9): 1312–1313. 10.1093/bioinformatics/btu033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiers B. (2022) Index Herbariorum: a global directory of public herbaria and associated staff. New York Botanical Garden’s Virtual Herbarium.
- Wu CY, Li HW, Chen J, Huang SQ, Li H, Fang RZ, Huang SH, Zhuang X, Li YR, Bai PY. (1977) Lamiaceae. In: Wu CY. (Ed.) Flora Yunnanica (Vol.1). Science Press, Beijing, 497–817.
- Xiang CL, Dong ZH, Peng H, Liu ZW. (2010) Trichome micromorphology of the East Asiatic genus Chelonopsis (Lamiaceae) and its systematic implications. Flora 205(7): 434–441. 10.1016/j.flora.2009.12.007 [DOI] [Google Scholar]
- Xiang CL, Dong HJ, Hu GX, Zhou J, Peng H. (2014) Taxonomic notes on the genus Phlomoides (Lamiaceae: Lamioideae) from China. Plant Diversity Resources 36: 551–560. [Google Scholar]
- Yao G, Deng YF, Ge XJ. (2013) Validation of the name Pogostemonpetelotii (Lamiaceae). Phytotaxa 81(1): 38–44. 10.11646/phytotaxa.81.1.11 [DOI] [Google Scholar]
- Zhao F, Chen YP, Salmaki Y, Drew BT, Wilson TC, Scheen A-C, Celep F, Bräuchler C, Bendiksby M, Wang Q, Min DZ, Peng H, Olmstead RG, Li B, Xiang CL. (2021) An updated tribal classification of Lamiaceae based on plastome phylogenomics. BMC Biology 19(1): 1–2. 10.1186/s12915-020-00931-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Chen YP, Liu DT, Turginov OT, Liang CZ, Xiang CL. (2021a) The identity of Phlomoidespararotata (Lamiaceae, Lamioideae). Phytotaxa 524(2): 107–113. 10.11646/phytotaxa.524.2.5 [DOI] [Google Scholar]
- Zhao Y, Chen YP, Zhen HL, Peng H, Xiang CL. (2021b) Phlomoidesliangwangshanensis (Lamiaceae), a new species from Yunnan, China. Phytotaxa 491(1): 72–78. 10.11646/phytotaxa.491.1.8 [DOI] [Google Scholar]
- Zhao Y, Chen YP, Yuan JC, Paton AJ, Nuraliev MS, Zhao F, Drew BT, Salmaki Y, Turginov OT, Sun M, Sennikov AN, Yu XQ, Li B, Xiang CL. (2023a) Museomics in Lamiaceae: Resolving the taxonomic mystery of Pseudomarrubium. Current Plant Biology 35(35–36): e100300. 10.1016/j.cpb.2023.100300 [DOI]
- Zhao Y, Zhao F, Salmaki Y, Paton A, Cai J, Drew BT, Chonour M, Efimov P, Turginov OT, Liang CZ, Chen YP, Xiang CL. (2023b) Home at last: Molecular phylogenetic analyses support subsuming Metastachydium within Phlomoides (Lamiaceae). Taxon 72(3): 590–606. 10.1002/tax.12935 [DOI] [Google Scholar]
- Zhao Y, Chen YP, Drew BT, Zhao F, Almasi M, Turginov OT, Xiao JF, Salmaki Y, Yu XQ, Xiang CL. (2024) (in press) Molecular phylogeny and taxonomy of Phlomoides (Lamiaceae subfamily Lamioideae) in China: Insights from molecular and morphological data. Plant Diversity. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
DNA sequences associated with this work are available from GenBank (https://www.ncbi.nlm.nih.gov/genbank/) or from the corresponding author upon reasonable request.