Abstract
Background:
Nintedanib and pirfenidone are preferred pharmacological therapies for patients with idiopathic pulmonary fibrosis (IPF). However, evidence favoring antifibrotic therapy in patients with non-IPF fibrosing interstitial lung diseases (ILD) is limited.
Objective:
To investigate the effects of antifibrotic therapy on disease progression, all-cause mortality, and acute exacerbation (AE) risk in patients with non-IPF fibrosing ILDs.
Design:
Meta-analysis.
Data sources and methods:
Electronic databases were searched for articles published before 28 February 2023. Studies that evaluated the efficacy of antifibrotic agents in patients with fibrosing ILDs were selected. The primary outcome was the disease progression risk, and the secondary outcomes included all-cause mortality and AE risk. The GRADE criteria were used for the certainty of evidence assessment.
Results:
Nine studies with 1990 participants were included. Antifibrotic therapy reduced the rate of patients with disease progression (five trials with 1741 subjects; relative risk (RR), 0.56; 95% CI, 0.42–0.75; p < 0.0001; I2 = 0; high-certainty evidence). Antifibrotic therapy did not significantly decrease all-cause mortality (nine trials with 1990 subjects; RR, 0.76; 95% CI, 0.55–1.03; p = 0.08; I2 = 0; low-certainty evidence). However, in patients with progressive fibrosing ILDs (PF-ILD), antifibrotic therapy decreased all-cause mortality (four trials with 1100 subjects; RR, 0.69; 95% CI, 0.48–0.98; p = 0.04; I2 = 0; low-certainty evidence).
Conclusion:
Our study supports the use of antifibrotic agents in patients with PF-ILDs, which could slow disease progression and decrease all-cause mortality.
Trial registration:
This study protocol was registered with PROSPERO (registration number: CRD42023411272).
Keywords: antifibrotic therapy, disease progression, fibrosing interstitial lung disease, meta-analysis, mortality
Background
Interstitial lung diseases (ILDs) are a series of disorders, most, but not all, of which are characterized by interstitial inflammation or fibrosis. 1 It is estimated that over 200 separate conditions can lead to ILDs; however, disease progression, respiratory failure, and eventual death are inevitable in many patients with ILDs, especially in those manifesting as fibrosing ILDs.
Idiopathic pulmonary fibrosis (IPF) is the most common and severe form of fibrosing ILD, with a median untreated survival of only 3–5 years after diagnosis. 2 In recent years, nintedanib and pirfenidone have been shown to delay lung function decline and reduce mortality and acute exacerbations (AE) risk in patients with IPF.3–5 As for other fibrosing ILDs, while appropriate management is efficacious in improving or stabilizing clinical symptoms, some patients still suffer from progressive fibrosis. 6 Considering the pathophysiological similarities between these diseases, researchers have speculated that non-IPF-fibrosing ILDs would have similar treatment responses to antifibrotic agents. However, the results of these clinical trials were not satisfactory. The SENSCIS and INBUILD trials showed that nintedanib could delay the decline in forced vital capacity (FVC) with no other clinical benefits observed in patients with systemic sclerosis-associated ILD (SSc-ILD) or progressive fibrosing ILDs (PF-ILD).7,8 Meanwhile, two pirfenidone trials were prematurely terminated because of poor recruitment, and the conclusions may thereby be underpowered.9,10 Although a meta-analysis suggested that the efficacy of antifibrotic therapy on changes in FVC between IPF and non-IPF PF-ILDs was similar, 11 two other recently published pooled analyses underlined that the current evidence favoring antifibrotic therapy in non-IPF PF-ILDs is weak.12,13
Therefore, we conducted this meta-analysis to further evaluate the efficacy of antifibrotic drugs in non-IPF-fibrosing ILDs. In contrast to previous studies, we mainly focused on the following outcomes: (1) progression of ILDs, (2) AE risk, and (3) all-cause mortality.
Methods
We performed and reported the meta-analysis and systematic review in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. 14 The PRISMA checklist is provided in Supplemental Appendix E1. The PROSPERO registration number for this meta-analysis is CRD42023411272.
Two researchers were independently responsible for literature retrieval, data extraction, risk of bias evaluation, and certainty of evidence assessment of outcomes. A third researcher was consulted if a disputation could not be resolved through discussion.
Literature search and study selection
We performed a literature search without language restrictions using the following electronic databases: PubMed, Embase, Cochrane Library, and Web of Science. Articles published before 28 February 2023 were retrieved. The whole search strategy is presented in Supplemental Appendix E2. We also reviewed the references of previous publications related to our topic to avoid missing eligible studies.
Randomized controlled trials (RCT) and prospective controlled studies evaluating the efficacy of antifibrotic agents (nintedanib or pirfenidone) in patients with fibrosing ILDs were selected. Fibrosing ILDs included autoimmune-related, exposure-related, unclassifiable ILD.1,15 Other ILDs characterized by chronic progressive fibrosis were also considered. The following studies were excluded: (1) those recruited patients aged <18 years; (2) those without complete data related to outcomes; and (3) published in the form of letters, comments, or conference abstracts.
Data extraction and risk of bias assessment
The extracted data included author name, publication year, region, study design, population characteristics of included study, sample size, intervention for treatment and control groups, and duration of follow-up.
We assessed the risk of bias for the RCTs using a tool recommended by the Cochrane Collaboration. 16 Each trial was considered to have a low, high, or unclear risk of bias according to the following criteria: random sequence generation, allocation concealment, blinding of participants and personnel to the study protocol, blinding of outcome assessment, incomplete outcome data, selective reporting, and other biases. A trial was regarded as high-risk if it had high risk of bias in any of the domains mentioned above. Trials with a low risk in all domains were regarded as having a low risk of bias. The other trials were categorized as having an unclear risk. The Newcastle-Ottawa Scale (NOS) was used for quality evaluation of prospective controlled studies.17,18 Details are presented in Supplemental Table E1. The scale consists of three parts, including the assessment of selection bias, information bias, and confounding bias. Additionally, publication bias was evaluated using Egger’s test.
Outcomes and certainty of evidence evaluation
The primary outcome of this meta-analysis was the rate of disease progression. Secondary outcomes included all-cause mortality and risk of AE. The certainty of evidence was assessed using the GRADE criteria and classified as high, moderate, low, and very low according to risk of bias, inconsistency of results, indirectness of evidence, imprecision, and reporting bias. 19
Data synthesis and statistical analysis
The Mantel-Haenszel method was used to pool the individual data. The random-effects model was selected considering the clinical heterogeneity across studies. The relative risk (RR) with a 95% confidence interval (95% CI) was selected as the effect measure. Forest plots were used to show individual and pooled results. I-squared (I2) statistics were used to assess the heterogeneity. A leave-one-out sensitivity analysis was performed to check the robustness of the results. Subgroup analyses were conducted according to the drug (nintedanib vs. pirfenidone) and the type of fibrosing ILDs (non-progressive fibrosing ILDs versus PF-ILDs). PF-ILDs were defined based on worsening respiratory symptoms and physiological or radiological evidence of disease progression. We conducted all statistical analyses using Review Manager 5.3 (Nordic Cochrane Centre) and Stata 15.0 (StataCorp, College Station, Texas).
Results
Study selection and characteristics of eligible studies
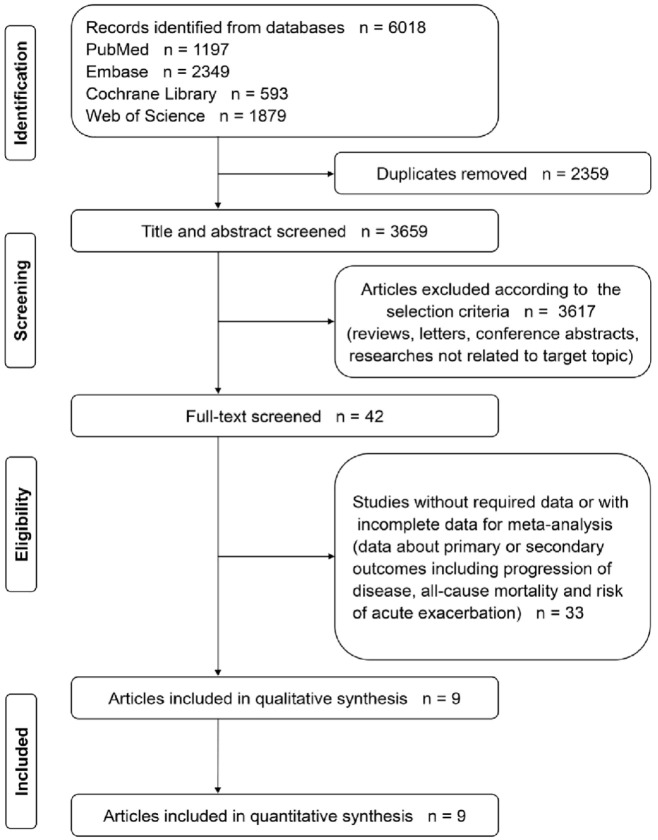
6018 records in all were obtained. We screened the titles and abstracts of the articles and obtained 42 potentially eligible studies. We then reviewed the full text and included nine studies for quantitative synthesis. The study selection process is illustrated in Figure 1.
Figure 1.
The flowchart of study selection.
Seven RCTs and two prospective controlled studies were included, and the details are presented in Table 1. Two studies were from the United States,20,21 two from China,22,23 one from Germany, 9 and the other four were global multicenter studies.7,8,10,24 These studies were published between 2002 and 2023. The participants were patients with fibrosing ILDs, and the sample sizes ranged from 21 to 663. The follow-up duration ranged from 24 to 52 weeks. The population characteristics of each study, including age, gender, lung function at baseline, and background therapy, are summarized in Table 2.
Table 1.
Characteristics of included studies.
| Study | Publication year | Region | Study design | Population | Sample size | Intervention, TG/CG | Treatment duration |
|---|---|---|---|---|---|---|---|
| INBUILD 7 | 2019 | Multicenter, 15 countries | Randomized, double-blind, placebo-controlled | Patients with non-IPF PF-ILD | 663 | Nintedanib (300 mg, daily)/placebo | 52-week |
| SENSCIS 8 | 2019 | Multicenter, 32 countries | Randomized, double-blind, placebo-controlled | Patients with SSc-ILD | 576 | Nintedanib (300 mg, daily)/placebo | 52-week |
| RELIEF 9 | 2021 | Multicenter, Germany | Randomized, double-blind, placebo-controlled | Patients with non-IPF PF-ILD | 127 | Pirfenidone (2403 mg daily)/placebo | 48-week |
| TRAIL110 | 2023 | Multicenter, 4 countries | Randomized, double-blind, placebo-controlled | Patients with RA-ILD | 123 | Pirfenidone (2403 mg daily)/placebo | 52-week |
| Maher et al. 24 | 2020 | Multicenter, 14 countries | Randomized, double-blind, placebo-controlled | Patients with unclassifiable PF-ILD | 253 | Pirfenidone (2403 mg daily)/placebo | 24-week |
| Wang et al. 22 | 2022 | Single-center, China | Prospective, open-label, controlled | Patients with CTD-ILD | 111 | Pirfenidone (1800 mg daily)/ no intervention | 24-week |
| Li et al. 23 | 2016 | Single-center, China | Prospective, open-label, controlled | Patients with rapidly progressive ADM-ILD | 57 | Pirfenidone (1800 mg daily)/placebo | 12-month |
| O’Brien et al.20 | 2011 | Single-center, United States | Randomized, double-blind, placebo-controlled | Patients with HPS-1 pulmonary fibrosis | 35 | Pirfenidone (2403 mg daily)/placebo | 12-month |
| Gahl et al. 21 | 2002 | Single-center, United States | Randomized, double-blind, placebo-controlled | Patients with HPS-1 pulmonary fibrosis | 21 | Pirfenidone (2400 mg daily)/placebo | TG: 18.8 ± 14.8 months; CG: 23.2 ± 14.0 months |
ADM-ILD, amyopathic dermatomyositis-associated interstitial lung disease; CG, control group; CTD-ILD, connective tissue disease-associated interstitial lung disease; HPS, Hermansky-Pudlak syndrome; IPF, idiopathic pulmonary fibrosis; PF-ILD, progressive fibrosing interstitial lung disease; RA-ILD, rheumatoid arthritis-associated interstitial lung disease; SSc-ILD, systemic sclerosis-associated interstitial lung disease; TG, treatment group.
Table 2.
Baseline information of subjects in each study.
| Study | Age | Gender, male (%) | Lung function | Background therapy |
|---|---|---|---|---|
| INBUILD 7 | ⩾18 years | TG: 53.9% CG: 53.5% |
FVC ⩾ 45%, and DLCO 30–80% | Use of azathioprine, cyclosporine, mycophenolate mofetil, tacrolimus, rituximab, cyclophosphamide, or glucocorticoids was allowed after 6 months of trial treatment |
| SENSCIS 8 | ⩾18 years | TG: 23.3% CG: 26.3% |
FVC ⩾ 40%, and DLCO 30–89% |
Prednisone (10 mg/day) or mycophenolate or methotrexate for more than6 months |
| RELIEF 9 | 18–80 years | TG: 67% CG: 51% |
FVC 40–90%, and DLCO 10–90% | NA |
| TRAIL110 | 18–85 years | TG: 60.3% CG: 65.0% |
FVC ⩾ 40, and DLCO ⩾ 30% | Any management of RA-related pulmonary manifestations (e.g. cytotoxic, immunosuppressive) was not allowed |
| Maher et al. 24 | 18–85 years | TG: 55% CG: 55% |
FVC ⩾ 45, and DLCO ⩾ 30% | NA |
| Wang et al. 22 | ⩾18 years | 16.2% | FVC ⩽ 80%, or DLCO ⩽ 80% | Glucocorticoid and/or immunosuppressant at baseline |
| Li et al. 23 | TG: 46.3 ± 11.3 years CG: 51.8 ± 7.8 years |
TG: 33.3% CG: 44.4% |
NA | Glucocorticoid and/or immunosuppressant at baseline |
| O’Brien et al. 20 | TG: 41.5 ± 12.1 years CG: 34.0 ± 9.2 years |
TG: 34.8% CG: 50.0% |
FVC 51–85% | NA |
| Gahl et al. 21 | 19–55 years | TG: 45.5% CG: 40.0% |
FVC 40–75% | Use of high-dose steroids at baseline was not allowed |
CG, control group; DLCO, diffusion lung capacity for carbon monoxide; FVC, forced vital capacity; ILD, interstitial lung disease; NA, not available; SD, standard deviation; TG, treatment group.
As there are currently no recognized criteria, the definitions of the progression of fibrosing ILDs differ. In four trials, disease progression was identified by the investigators based on the worsening of symptoms and lung function, as well as the extent of fibrosis on HRCT.7,9,10,24 In the SENSCIS trial, 8 disease progression was defined as an absolute decline in FVC ⩾ 10%. An absolute decline in FVC ⩾ 10% has been consistently reported to be a strong death predictor in patients with fibrosing ILDs,25–27 and it has been considered as evidence of progression in several clinical trials. 7
Risk of bias assessment
Supplemental Figure E1 shows the results of the quality evaluation. Six trials7–10,21,24 were considered to have a low risk of bias. One trial 20 was terminated prematurely because of futility and was therefore considered to have a high risk of bias. According to the NOS, two prospective controlled studies22,23 were considered to be of high quality. Please see the details in Supplemental Table E2.
We found no significant publication bias based on the results of the funnel plots and Egger’s test (p = 0.598). Please see the details of Supplemental Figures E2 and E3.
Meta-analysis
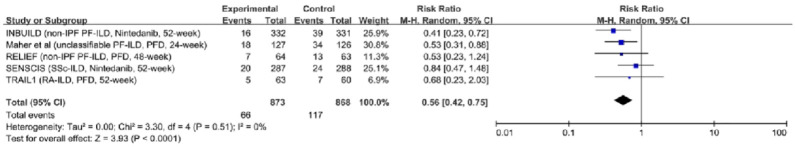
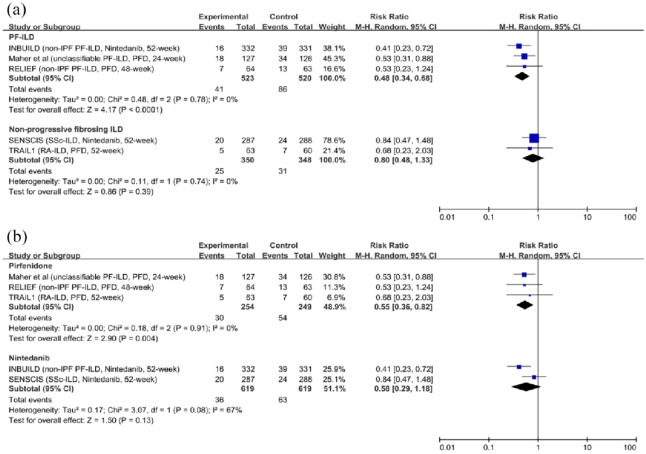
Disease progression: Five trials with 1741 participants reported results, including measurements of progression of ILDs.7–10,24 Compared with placebo, antifibrotic agents reduced the rate of patients with disease progression (RR, 0.56; 95% CI, 0.42–0.75; p < 0.0001; I2 = 0; see Figure 2). Additionally, the sensitivity analysis suggested the results were robust and unlikely to be influenced by any single trial (see Supplemental Figure E4). The results were consistent in patients with PF-ILDs [RR, 0.48; 95% CI, 0.34–0.68; p < 0.0001; I2 = 0; see Figure 3(a)]. However, antifibrotic agents failed to significantly delay the disease progression in patients with non-progressive fibrosing ILDs [RR, 0.80; 95% CI, 0.48–1.33; p = 0.39; I2 = 0; see Figure 3(a)]. Pirfenidone reduced the rate of patients with disease progression [RR, 0.55; 95% CI, 0.36–0.82; p = 0.004; I2 = 0; see Figure 3(b)] while Nintedanib failed to significantly delay the disease progression [RR, 0.58; 95% CI, 0.29–1.18; p = 0.13; I2 = 67; see Figure 3(b)].
Figure 2.
The relative risk of antifibrotic therapy on disease progression in patients with fibrosing interstitial lung diseases.
Figure 3.
Subgroup analyses for the relative risk of disease progression. (a) The relative risk of antifibrotic therapy on disease progression in patients with progressive fibrosing interstitial lung diseases and non-progressive fibrosing interstitial lung diseases. (b) The relative risk of pirfenidone and nintedanib on disease progression in patients with fibrosing interstitial lung diseases.
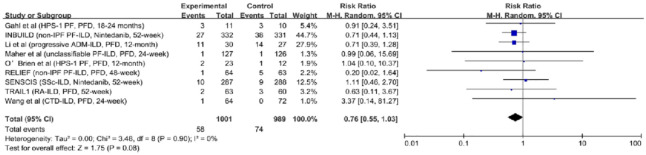
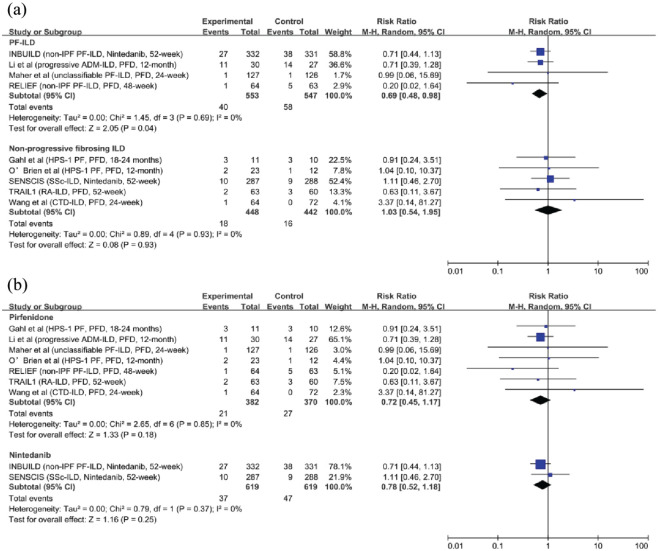
All-cause mortality: Data on all-cause mortality were provided in nine studies (1900 participants).7–10,20–24 In the overall population, antifibrotic therapy did not show benefits on survival (RR, 0.76; 95% CI, 0.55–1.03; p = 0.08; I2 = 0; see Figure 4). We performed subgroup analyses according to the drug, and the results were consistent [RR, 0.72; 95% CI, 0.45–1.17; p = 0.18; I2 = 0; and RR, 0.78; 95% CI, 0.52–1.18; p = 0.25; I2 = 0; see Figure 5(b)]. Antifibrotic therapy had no impact in patients with non-progressive fibrosing ILDs [RR, 1.03; 95% CI, 0.54–1.95; p = 0.93; I2 = 0; see Figure 5(a)]. However, in patients with PF-ILDs, antifibrotic therapy decreased all-cause mortality [RR, 0.69; 95% CI, 0.48–0.98; p = 0.04; I2 = 0; see Figure 5(a)].
Figure 4.
The relative risk of all-cause mortality in patients with fibrosing interstitial lung diseases who received antifibrotic therapy.
Figure 5.
Subgroup analyses for the relative risk of all-cause mortality. (a) The relative risk of antifibrotic therapy on all-cause mortality in patients with progressive fibrosing interstitial lung diseases and non-progressive fibrosing interstitial lung diseases. (b) The relative risk of pirfenidone and nintedanib on all-cause mortality in patients with fibrosing interstitial lung diseases.
Risk of AE: A double-blind, placebo-controlled trial conducted in 15 countries recruited 663 patients with non-IPF PF-ILDs. 7 The incidence of acute exacerbation or death at 52 weeks was 7.8% (26/332) in the treatment group (TG; nintedanib) and 9.7% (32/331) in the control group. The differences between the groups were not statistically significant (HR, 0.80; 95% CI, 0.48–1.34). Another multicenter, double-blind trial recruited 123 patients with RA-ILD from four countries. 10 The incidence of respiratory exacerbations at 52 weeks was 1.6% (1/63) in the TG (pirfenidone) and 3.3% (2/60) in the control group. The difference between the groups was not statistically significant (p = 0.62).
Certainty of evidence classification for outcomes
The GRADE evidence levels are presented in Table 3. For the RR of disease progression, the certainty of the evidence was regarded as high. For subgroup analyses of the RR of disease progression, the certainty of the evidence ranged from low to moderate. For the RR of all-cause mortality, the certainty of the evidence was low, owing to the risk of bias. For subgroup analyses of the RR of all-cause mortality, the certainty of the evidence ranged from low to moderate.
Table 3.
Certainty of evidence for each outcome.
| Outcome | Number of patients | RR | 95% CI | Risk of bias | Inconsistency | Indirectness | Imprecision | Publication bias | Certainty of evidence |
|---|---|---|---|---|---|---|---|---|---|
| Relative risk of disease progression | 1741 | 0.56 | 0.42–0.75 | No | No | No | No | No | High |
| Relative risk of disease progression in patients with PF-ILDs | 1043 | 0.48 | 0.34–0.68 | No | No | No | Yes | No | Moderate |
| Relative risk of disease progression in patients with non-progressive ILDs | 698 | 0.80 | 0.48–1.33 | No | No | No | Yes | No | Moderate |
| Relative risk of pirfenidone on disease progression in patients with fibrosing ILDs. | 503 | 0.55 | 0.36–0.82 | No | No | No | Yes | No | Moderate |
| Relative risk of nintedanib on disease progression in patients with fibrosing ILDs | 1238 | 0.58 | 0.29–1.18 | No | Yes | No | Yes | No | Low |
| Relative risk of all-cause mortality | 1990 | 0.76 | 0.55–1.03 | Yes | No | No | No | No | Low |
| Relative risk of all-cause mortality in patients with PF-ILDs | 1100 | 0.69 | 0.48–0.98 | Yes | No | No | No | No | Low |
| Relative risk of all-cause mortality in patients with non-progressive fibrosing ILDs | 890 | 1.03 | 0.54–1.95 | Yes | No | No | No | No | Low |
| Relative risk of pirfenidone on all-cause mortality in patients with fibrosing ILDs. | 752 | 0.72 | 0.45–1.17 | Yes | No | No | No | No | Low |
| Relative risk of nintedanib on all-cause mortality in patients with fibrosing ILDs | 1238 | 0.78 | 0.52–1.18 | No | No | No | Yes | No | Moderate |
AE, acute exacerbation; CI, confidence interval; ILD, interstitial lung diseases; PF-ILD, progressive fibrosing interstitial lung disease; RR, relative risk.
Discussion
Main findings
After a systematic review of the current literature and meta-analyses of available data, we found that (1) antifibrotic therapy could slow the progression of fibrosing ILDs, (2) antifibrotic treatment might decrease the all-cause mortality in patients with PF-ILDs, and (3) no evidence currently supports that antifibrotic agents could decrease AE risk in patients with fibrosing ILDs.
Comparison with previous meta-analyses
We noticed two recently published meta-analyses (one for nintedanib and one for pirfenidone) that were related to this topic. 12 These two studies mainly focused on lung-function-related outcomes, similar to the initial trials. However, the certainty of evidence for most outcomes ranged from very low to low owing to the limited number of included studies (two in each). Considering the similar antifibrotic effects of nintedanib and pirfenidone, we pooled data from studies related to both drugs to investigate the impact of antifibrotic therapy on fibrosing ILDs. The present meta-analysis included five trials with a low risk of bias and indicated that antifibrotic agents delayed the disease progression (high-certainty evidence). We found no significant heterogeneity across the trials, and the results of the sensitivity analysis were consistent with those of the primary quantitative synthesis. Further sub-analyses indicated that the effect of antifibrotic therapy on disease progression differed between the groups. However, the certainty of the evidence for these sub-analyses was downgraded due to imprecision, and the conclusion may be statistically insignificant.
Although mortality is a vital outcome for the efficacy evaluation of antifibrotic agents in patients with fibrosing ILDs, there are only a few records of death in initial trials owing to insufficient sample size and follow-up. A meta-analysis by Petnak et al. showed that antifibrotic therapy decreases AE risk and mortality in patients with IPF. 5 The present study indicated that patients with fibrosing ILDs did not benefit from antifibrotic treatment on survival, which is coincidence with those of a previous pooled analysis. 11 However, our meta-analysis included more studies, and the subgroup analysis suggested that mortality decreased significantly in patients with PF-ILDs treated with antifibrotic agents. Although one prospective controlled study was also pooled for quantitative analysis, in which the risk of bias was high due to the lack of blinding, the outcome (i.e. mortality) was less likely to be influenced. In addition, only two studies were included in the analysis of AE risk, and the conclusions may lack statistical power.
Implications for clinical practice
According to the recent ATS/ERS/JRS/LATS clinical practice guidelines, nintedanib and pirfenidone are the preferred pharmacological therapies for patients with IPF. 27 With regard to PF-ILDs, the guideline committee merely made a ‘conditional recommendation’ for the use of nintedanib and suggested the need for more research. Our meta-analysis suggests that patients with PF-ILDs could benefit from antifibrotic treatment in terms of total survival and maintenance of their condition. Therefore, the timely identification of patients whose fibrosing ILDs are progressing is of great importance, and antifibrotic agents could be a potential therapeutic strategy for these patients. A previous study found that patients with IPF treated with nintedanib had a higher risk of respiratory-related hospitalization and all-cause mortality. 29 However, another observational study identified no differences in patient-related outcomes between the two drugs. 30 Currently, it is difficult to determine which agent is superior, and further studies are required.
Additionally, current evidence favoring antifibrotic treatment in patients with non-progressive fibrosing ILDs is limited. Although the SENSCIS trial and another multicenter real-world study showed that nintedanib could slow the decline in lung function in patients with SSc-ILD,8,31 significant adverse gastrointestinal events should also be considered. Thus, clinicians should fully weigh the advantages and disadvantages while managing these patients, which is critical to prognosis.
Strength and limitations
This is the latest and most comprehensive meta-analysis designed to evaluate the effects of antifibrotic agents on disease progression, mortality, and the risk of AE. We performed the analyses in strict compliance with the PRISMA guidelines and identified potential participants who would show a better treatment response to antifibrotic agents. Moreover, the GRADE score was used to assess the certainty of evidence for the outcomes to make clinical decisions.
An obvious limitation of our meta-analysis is that the findings were based on a pooled analysis of aggregate data reported in previous studies rather than individual data. The definitions of disease progression differed among the studies, which could have led to bias. Selection bias may also exist, considering that the diagnosis of PF-ILD varied among the trials. Although different criteria were employed, the included studies recruited eligible participants based on worsening respiratory symptoms and physiological or radiological evidence of disease progression. This is roughly consistent with the consensus recommendations, 28 and could help identify patients whose disease has progressed similarly. Therefore, the results of our study may be applicable to patients with ILDs who manifest with progressive fibrosis.
Furthermore, because the included studies were limited, stratified analyses according to different baseline characteristics were not conducted. Several subgroup analyses of the INBUILD and SENSCIS trials have indicated that the protective effects of nintedanib on lung function are not subject to race, background treatment, and cause of ILDs.32–34 Nevertheless, whether the association between antifibrotic therapy and reduced mortality is modified by specific factors needs to be clarified in future studies.
Conclusion
Antifibrotic treatments can reduce the rate of disease progression. However, it should be noted that the definition of disease progression was established mainly based on the worsening of symptoms, lung function, and extent of fibrosis on HRCT. We also found that antifibrotic treatment might decrease all-cause mortality in patients with PF-ILDs, although the certainty of the evidence is low. Our study supports the routine use of antifibrotic agents in these patients, as no preferred therapeutic strategies are currently available.
Supplemental Material
Supplemental material, sj-docx-1-tar-10.1177_17534666241232561 for Impact of antifibrotic therapy on disease progression, all-cause mortality, and risk of acute exacerbation in non-IPF fibrosing interstitial lung diseases: evidence from a meta-analysis of randomized controlled trials and prospective controlled studies by De-yu Li, Xin Liu, Jing-yi Huang, Wen-lu Hang, Gu-ran Yu and Yong Xu in Therapeutic Advances in Respiratory Disease
Acknowledgments
None.
Footnotes
ORCID iD: Yong Xu  https://orcid.org/0000-0003-3990-6013
https://orcid.org/0000-0003-3990-6013
Supplemental material: Supplemental material for this article is available online.
Contributor Information
De-yu Li, Affiliated Hospital of Nanjing University of Chinese Medicine, Nanjing, China.
Xin Liu, Affiliated Hospital of Nanjing University of Chinese Medicine, Nanjing, China.
Jing-yi Huang, Baoshan Branch, Shuguang Hospital Affiliated to Shanghai University of Traditional Chinese Medicine, Shanghai, China.
Wen-lu Hang, Department of Respiratory Medicine, Second Affiliated Hospital of Xuzhou Medical University, Xuzhou, China.
Gu-ran Yu, Affiliated Hospital of Nanjing University of Chinese Medicine, 155 Hanzhong Road, Nanjing 210029, China.
Yong Xu, School of Chinese Medicine, Nanjing University of Chinese Medicine, 138 Xianlin Road, Nanjing 210046, China.
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Author contributions: De-yu Li: Investigation; Methodology; Writing – original draft.
Xin Liu: Data curation; Formal analysis; Investigation; Methodology.
Jing-yi Huang: Investigation; Methodology; Resources.
Wen-lu Hang: Writing – review & editing.
Gu-ran Yu: Conceptualization.
Yong Xu: Conceptualization.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: NATCM’s Project of High-level Construction of Key TCM Disciplines.
The authors declare that there is no conflict of interest.
Availability of data and materials: All data relevant to the study are included in the article or uploaded as supplementary imformation.
References
- 1. Wijsenbeek M, Suzuki A, Maher TM. Interstitial lung diseases. Lancet 2022; 400: 769–786. [DOI] [PubMed] [Google Scholar]
- 2. Behr J, Prasse A, Wirtz H, et al. Survival and course of lung function in the presence or absence of antifibrotic treatment in patients with idiopathic pulmonary fibrosis: long-term results of the INSIGHTS-IPF registry. Eur Respir J 2020; 56: 1902279. [DOI] [PubMed] [Google Scholar]
- 3. Richeldi L, du Bois RM, Raghu G, et al.; INPULSIS Trial Investigators. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med 2014; 370: 2071–2082. [DOI] [PubMed] [Google Scholar]
- 4. King TE, Jr, Bradford WZ, Castro-Bernardini S, et al.; ASCEND Study Group. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med 2014; 370: 2083–2092. [DOI] [PubMed] [Google Scholar]
- 5. Petnak T, Lertjitbanjong P, Thongprayoon C, et al. Impact of antifibrotic therapy on mortality and acute exacerbation in idiopathic pulmonary fibrosis: a systematic review and meta-analysis. Chest 2021; 160: 1751–1763. [DOI] [PubMed] [Google Scholar]
- 6. George PM, Spagnolo P, Kreuter M, et al.; Erice ILD working group. Progressive fibrosing interstitial lung disease: clinical uncertainties, consensus recommendations, and research priorities. Lancet Respir Med 2020; 8: 925–934. [DOI] [PubMed] [Google Scholar]
- 7. Flaherty KR, Wells AU, Cottin V, et al.; INBUILD Trial Investigators. Nintedanib in progressive fibrosing interstitial lung diseases. N Engl J Med 2019; 381: 1718–1727. [DOI] [PubMed] [Google Scholar]
- 8. Distler O, Highland KB, Gahlemann M, et al.; SENSCIS Trial Investigators. Nintedanib for systemic sclerosis-associated interstitial lung disease. N Engl J Med 2019; 380: 2518–2528. [DOI] [PubMed] [Google Scholar]
- 9. Behr J, Prasse A, Kreuter M, et al.; RELIEF investigators. Pirfenidone in patients with progressive fibrotic interstitial lung diseases other than idiopathic pulmonary fibrosis (RELIEF): a double-blind, randomised, placebo-controlled, phase 2b trial. Lancet Respir Med 2021; 9: 476–486. [DOI] [PubMed] [Google Scholar]
- 10. Solomon JJ, Danoff SK, Woodhead FA, et al.; TRAIL1 Network Investigators. Safety, tolerability, and efficacy of pirfenidone in patients with rheumatoid arthritis-associated interstitial lung disease: a randomised, double-blind, placebo-controlled, phase 2 study. Lancet Respir Med 2023; 11: 87–96. [DOI] [PubMed] [Google Scholar]
- 11. Finnerty JP, Ponnuswamy A, Dutta P, et al. Efficacy of antifibrotic drugs, nintedanib and pirfenidone, in treatment of progressive pulmonary fibrosis in both idiopathic pulmonary fibrosis (IPF) and non-IPF: a systematic review and meta-analysis. BMC Pulm Med 2021; 21: 411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ghazipura M, Mammen MJ, Bissell BD, et al. Pirfenidone in progressive pulmonary fibrosis: a systematic review and meta-analysis. Ann Am Thorac Soc 2022; 19: 1030–1039. [DOI] [PubMed] [Google Scholar]
- 13. Ghazipura M, Mammen MJ, Herman DD, et al. Nintedanib in progressive pulmonary fibrosis: a systematic review and meta-analysis. Ann Am Thorac Soc 2022; 19: 1040–1049. [DOI] [PubMed] [Google Scholar]
- 14. Moher D, Liberati A, Tetzlaff J, et al.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ Clin Res Ed 2009; 339: b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wong AW, Ryerson CJ, Guler SA. Progression of fibrosing interstitial lung disease. Respir Res 2020; 21: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Higgins JP, Altman DG, Gøtzsche PC, et al.; Cochrane Bias Methods Group and Cochrane Statistical Methods Group. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ Clin Res Ed 2011; 343: d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wells GA, O’Connell D, Peterson J, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses, http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed April, 27, 2023).
- 18. Farrah K, Young K, Tunis MC, et al. Risk of bias tools in systematic reviews of health interventions: an analysis of PROSPERO-registered protocols. Syst Rev 2019; 8: 280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Guyatt GH, Oxman AD, Vist GE, et al.; GRADE Working Group. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ Clin Res Ed 2008; 336: 924–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. O’Brien K, Troendle J, Gochuico BR, et al. Pirfenidone for the treatment of Hermansky-Pudlak syndrome pulmonary fibrosis. Mol Genet Metab 2011; 103: 128–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gahl WA, Brantly M, Troendle J, et al. Effect of pirfenidone on the pulmonary fibrosis of Hermansky-Pudlak syndrome. Mol Genet Metab 2002; 76: 234–242. [DOI] [PubMed] [Google Scholar]
- 22. Wang J, Wang X, Qi X, et al. The efficacy and safety of pirfenidone combined with immunosuppressant therapy in connective tissue disease-associated interstitial lung disease: a 24-week prospective controlled Cohort Study. Front Med 2022; 9: 871861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li T, Guo L, Chen Z, et al. Pirfenidone in patients with rapidly progressive interstitial lung disease associated with clinically amyopathic dermatomyositis. Sci Rep 2016; 6: 33226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Maher TM, Corte TJ, Fischer A, et al. Pirfenidone in patients with unclassifiable progressive fibrosing interstitial lung disease: a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet Respir Med 2020; 8: 147–157. [DOI] [PubMed] [Google Scholar]
- 25. Goh NS, Hoyles RK, Denton CP, et al. Short-Term pulmonary function trends are predictive of mortality in interstitial lung disease associated with systemic sclerosis. Arthritis Rheumatol 2017; 69: 1670–1678. [DOI] [PubMed] [Google Scholar]
- 26. Solomon JJ, Chung JH, Cosgrove GP, et al. Predictors of mortality in rheumatoid arthritis-associated interstitial lung disease. Eur Respir J 2016; 47: 588–596. [DOI] [PubMed] [Google Scholar]
- 27. Gimenez A, Storrer K, Kuranishi L, et al. Change in FVC and survival in chronic fibrotic hypersensitivity pneumonitis. Thorax 2018; 73: 391–392. [DOI] [PubMed] [Google Scholar]
- 28. Raghu G, Remy-Jardin M, Richeldi L, et al. Idiopathic pulmonary fibrosis (an update) and progressive pulmonary fibrosis in adults: an official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med 2022; 205: e18–e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Belhassen M, Dalon F, Nolin M, et al. Comparative outcomes in patients receiving pirfenidone or nintedanib for idiopathic pulmonary fibrosis. Respir Res 2021; 22: 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Marijic P, Schwarzkopf L, Schwettmann L, et al. Pirfenidone vs. Nintedanib in patients with idiopathic pulmonary fibrosis: a retrospective cohort study. Respir Res 2021; 22: 268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Campochiaro C, De Luca G, Lazzaroni MG, et al. Real-life efficacy and safety of nintedanib in systemic sclerosis-interstitial lung disease: data from an Italian multicentre study. RMD 2023; 9: e002850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Matteson EL, Kelly C, Distler JH, et al.; the INBUILD Trial Investigators. Nintedanib in patients with autoimmune disease–related progressive fibrosing interstitial lung diseases: subgroup analysis of the inbuild trial. Arthrit Rheumatol (Hoboken, N.J) 2022; 74: 1039–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Highland KB, Distler O, Kuwana M, et al.; SENSCIS trial investigators. Efficacy and safety of nintedanib in patients with systemic sclerosis-associated interstitial lung disease treated with mycophenolate: a subgroup analysis of the SENSCIS trial. Lancet Respir Med 2021; 9: 96–106. [DOI] [PubMed] [Google Scholar]
- 34. Azuma A, Chung L, Behera D, et al.; SENSCIS trial investigators. Efficacy and safety of nintedanib in Asian patients with systemic sclerosis-associated interstitial lung disease: subgroup analysis of the SENSCIS trial. Respir Investig 2021; 59: 252–259. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tar-10.1177_17534666241232561 for Impact of antifibrotic therapy on disease progression, all-cause mortality, and risk of acute exacerbation in non-IPF fibrosing interstitial lung diseases: evidence from a meta-analysis of randomized controlled trials and prospective controlled studies by De-yu Li, Xin Liu, Jing-yi Huang, Wen-lu Hang, Gu-ran Yu and Yong Xu in Therapeutic Advances in Respiratory Disease