Abstract
The transcription factor CCAAT/enhancer binding protein α (C/EBPα) regulates a number of myeloid cell-specific genes. To delineate the role of C/EBPα in human granulopoiesis, we studied its expression and function in human primary cells and bipotential (granulocytic/monocytic) myeloid cell lines. We show that the expression of C/EBPα initiates with the commitment of multipotential precursors to the myeloid lineage, is specifically upregulated during granulocytic differentiation, and is rapidly downregulated during the alternative monocytic pathway. Conditional expression of C/EBPα alone in stably transfected bipotential cells triggers neutrophilic differentiation, concomitant with upregulation of the granulocyte-specific granulocyte colony-stimulating factor receptor and secondary granule protein genes. Moreover, induced expression of C/EBPα in bipotential precursors blocks their monocytic differentiation program. These results indicate that C/EBPα serves as a myeloid differentiation switch acting on bipotential precursors and directing them to mature to granulocytes.
According to the current view of hematopoiesis, all blood cell types derive from a common pluripotent stem cell (65). In the adult, the stem cells are found in bone marrow, where they divide to produce more stem cells (self-renewal) and various precursor cells committed to a single lineage which terminally differentiate to morphologically and functionally distinct erythroid, myeloid, or lymphoid cells. Within the myeloid compartment, the same committed precursor can give rise to monocytic or granulocytic cells. This raises a question: what are the molecular mechanisms that dictate the fate of the common precursor to one or the other of these two diverse myeloid lineages?
Genetic manipulations such as gene knockout and gene transfer experiments provide increasing evidence that transcription factors are involved in execution of the differentiation program of a cell. Determination of the developmental role of a number of transcription factors in blood development has been achieved by gene disruption experiments. These studies indicated that the GATA-1 and GATA-2, SCL, PU.1, Ikaros, c-myb, and AML1 genes, among others, are key regulators of hematopoiesis (reviewed in references 63 and 68). Inactivation of these genes had multilineage effects. Complementary to these knockout experiments are expression studies which identified certain transcription factors as master regulators of development, defined as genes which once activated would establish a specific cell type. For example, ectopic expression of MyoD in diverse cell types converts them to muscle cells (17). Similarly, expression of the B-cell transcription factor Oct-2 or the helix-loop-helix protein E47 in non-B cells induces B cell-like phenotypes (55, 59). In addition, thanks to advances in cell and molecular biological techniques, isolation and analysis of single primary cells, including functional stem cells, is feasible, and their developmental patterns can be studied as well (12).
CCAAT/enhancer binding protein α (C/EBPα) was initially identified in liver and adipose tissue, where it was found to be important for terminal differentiation (8, 16, 22, 39, 44, 75). More recently it was shown to be also expressed in early myeloid cells (12, 61, 68). In addition, a number of granulocyte-specific genes, including granulocyte colony-stimulating factor (G-CSF) receptor (64), neutrophil elastase (52), and myeloperoxidase (23, 52) genes, have been shown to be regulated by C/EBPα. Gene targeting experiments revealed a specific defect in the hematopoietic system of C/EBPα knockout mice. The C/EBPα null phenotype was characterized by lack of mature granulocytes, with all the other blood cell types present, including monocytes and peritoneal macrophages (81). These results strongly point to a critical role of C/EBPα in granulocytic differentiation (reviewed in reference 68).
In the present study, we have investigated the expression pattern of C/EBPα in the hematopoietic system during monocytic and granulocytic differentiation. We also examined the effect of induced overexpression of C/EBPα on the differentiation program of early bipotential myeloid cells. Our results demonstrate that the C/EBPα gene is activated at the stage of myeloid commitment and is specifically expressed in granulocytic cells. Increased levels of C/EBPα expressed from an inducible promoter construct directed differentiation along the granulocytic pathway, as determined by morphological criteria. Furthermore, ectopic expression of C/EBPα resulted in upregulation of mRNA of the granulocyte-restricted genes encoding the G-CSF receptor and C/EBPɛ, as well as secondary granule protein genes lactoferrin and human neutrophil collagenase. Our findings identify C/EBPα as the molecular switch during early hematopoietic developmental events that directs cells to the granulocytic pathway.
MATERIALS AND METHODS
Cell lines.
Human myeloid U937 (American Type Culture Collection [ATCC] no. CRL 1593; ATCC, Rockville, Md.) and Mono Mac 6 (82), promyelocytic HL-60 (ATCC no. CCL 240) and NB4 (40), erythroleukemic K562 (ATCC no. CCL 246), immature myeloid KG1a (ATCC no. CCL 246.1), T-cell Jurkat (ATCC no. TIB 152), and B-cell Raji (ATCC no. CCL 86) and BJA-B (34) cell lines were grown in RPMI 1604 medium (Gibco, Grand Island, N.Y.) containing 10% fetal bovine serum (FBS; HyClone, Logan, Utah) and 2 mM l-glutamine. Human cervical carcinoma HeLa cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM; Gibco) supplemented with 10% FBS and 2 mM l-glutamine. The murine myeloid progenitor line 416B (19) was cultured in DMEM with 15% FBS. Interleukin-3 (IL-3)-dependent murine BaF3 cells (54) were grown in DMEM (Gibco)–10% FBS (HyClone) with 10% WEHI-3 conditioned medium as a source of murine IL-3.
Primary cell purification.
Human single bone marrow cells, G0 phase arrested, CD34+ CD33−, CD34+ CD33+, CD11b+, CD3+, and CD19+, were purified as described previously (6). Peripheral blood monocytes and neutrophils were isolated from healthy donors by passage through a Ficoll-Hypaque gradient followed by erythrocyte lysis according to a procedure described earlier (11).
In vitro differentiation.
To induce erythrocytic differentiation, K562 cells were cultured in medium supplemented with 1.5% dimethyl sulfoxide (DMSO) for up to 5 days. U937 and HL-60 cells were treated for 2 days with 1.3 × 10−7 M tetradecanoyl phorbol acetate (TPA) (for monocytic differentiation) or with 10−6 M all-trans-retinoic acid (Sigma) for 4 days (granulocytic differentiation). Stock solutions of TPA and retinoic acid were prepared in DMSO; to yield working concentrations, they were diluted 1:7,700 (TPA) and 1:1,000 (retinoic acid) in media. Similar dilutions of vehicle (DMSO) alone did not induce morphologic differentiation. At the end of each differentiation experiment, approximately 104 cells were centrifuged at 500 rpm for 5 min onto a glass slide and stained with Wright-Giemsa stain, and differential cell counts were performed.
Reverse transcription-PCR and Southern blot analysis.
cDNA synthesis from isolated single bone marrow cells, amplification, and Southern blotting procedures were described previously (6, 7, 12). The human C/EBPα 3′ untranslated region was used as a probe (see below). Hybridization conditions were as for Northern blots (see below) except that the last wash was with 0.2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)–0.5% sodium dodecyl sulfate (SDS).
Plasmids and transfections.
The expression vector pPC22 containing the rat C/EBPα cDNA under the control of the human metallothionein promoter, as well as the empty vector pPC18, was described previously (76). Both plasmids contained a neomycin phosphotransferase gene as a selectable marker. DNA for stable transfections was purified on cesium chloride gradients. A total of 2 × 107 cells were electroporated in a Gene Pulser apparatus (Bio-Rad, Melville, N.Y.) with 10 μg of XmnI-linearized plasmid at 960 μF in 0.4-cm cuvettes (BTX). Voltages were 250 V for U937 and HL-60 cells and 270 V for 416B cells. Cells were plated on 96-well plates at 105 cells/well. Selection with G418 (850 μg/ml [active concentration]) began 48 h posttransfection. The expression of rat C/EBPα gene was induced by adding 100 μM ZnSO4 to the culture medium.
Apoptosis assay.
Programmed cell death (apoptosis) was detected by the terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) assay (27) (In Situ Cell Death Detection kit; Boehringer Mannheim) according to the manufacturer’s protocol.
NBT assay.
Reduction of the nitroblue tetrazolium (NBT) by respiratory burst products was performed by a slide test as described previously (51).
RNA isolation and Northern blot analysis.
Total RNA was isolated by the guanidinium thiocyanate method (13). In each lane, 20 μg of RNA was denatured in formamide and fractionated on 1% agarose–2.2 M formaldehyde gels (58). RNA was transferred to Biotrans (ICN, East Hills, N.Y.) or MagnaGraph (Micron Separations, Inc., Westborough, Mass.) membranes in 20× SSC, and the blots were hybridized at 40°C in 50% formamide–7.5× Denhardt’s solution (50× Denhardt’s solution is 5 g of Ficoll, 5 g of polyvinylpyrrolidone, and 5 g of bovine serum albumin in 500 ml)–5× SSC–50 mM NaPO4 (pH 6.8)–100 μg of salmon sperm DNA per ml–0.5% SDS. DNA fragments were labeled by the random primer method (21). The blots were washed once in 1× SSC–0.5% SDS for 15 min at room temperature, once in 1× SSC–0.5% SDS for 15 min at 42°C, and once in 0.1× SSC–1% SDS for 30 min at 65°C. The 700-bp EcoRI-HindIII fragment of pG28B5.0 (4) and a fragment encompassing bp 289 to 1024 of the human G-CSF receptor cDNA (25, 64) served as probes for human C/EBPα and G-CSF receptor mRNAs, respectively. The IL-8 receptor B probe was a 1.8-kb XhoI-EcoRI fragment of pIL8RB (1, 10), and the CD18 probe was a 2.7-kb BamHI-ClaI insert of clone J9 (41, 57). Rat C/EBPα mRNA was detected with a HincII-BamHI 300-bp fragment from pPC22 (76). The human lactoferrin probe was a 2.3-kb EcoRI cDNA insert (plasmid 39) (32), the human neutrophil collagenase probe was a 2.4-kb BamHI-EcoRI cDNA insert (plasmid 59) (18), and the human C/EBPɛ probe was a 0.5-kb PstI fragment of the pJurkat1 clone (3). To ensure uniform levels and integrity of RNA samples loaded in each lane, the blot was stripped and rehybridized to probes specific for 18S and 28S RNAs. The oligonucleotide for the 18S RNA corresponded to bp 938 to 921 of the human 18S rRNA gene (5′-TCGGGCCTGCTTTGAACA-3′) (71). 28S RNA was detected with an oligonucleotide containing bp 4036 to 4020 (5′-AGGTAGCCAAATGCCTC-3′) of the human 28S rRNA gene (5). Autoradiography was performed by using Kodak XAR-5 film at −80°C with Dupont Cronex Lightning Plus screens except as otherwise noted. Quantitation of relative mRNA levels was performed with a Molecular Dynamics PhosphorImager and software.
Protein extract preparation and Western blotting.
Nuclear extracts were prepared as described (20) with minor modifications. Briefly, 5 × 107 cells were washed twice with 1× phosphate-buffered saline, resuspended in 1 volume of packed cells of ice-cold buffer A (10 mM HEPES [pH 7.9], 1.5 mM MgCl2, 10 mM KCl, 0.5 mM dithiothreitol, 0.5 mM phenylmethylsulfonyl fluoride [PMSF]), and allowed to swell on ice for 15 min. Cells were lysed by vortexing for 10 s and centrifuged in a microcentrifuge at 14,000 rpm for 10 s at 4°C. After removal of the supernatant, nuclei were lysed by addition of 80 μl of buffer C (20 mM HEPES [pH 7.9], 25% glycerol, 0.42 M NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 0.5 mM dithiothreitol, 0.5 mM PMSF) for 30 min at 4°C. After centrifugation in a microcentrifuge at 14,000 for 5 min, supernatants were aliquoted and frozen at −80°C. For whole-cell extracts, 5 × 107 cells were harvested by centrifugation, washed twice in phosphate-buffered saline, and lysed by addition of 300 μl of radioimmunoprecipitation assay buffer (150 mM NaCl, 1% Nonidet P-40, 0.5% deoxycholate, 0.1% SDS, 50 mM Tris [pH 7.5], 0.5 mM PMSF, 1% aprotinin), passaged twice through an 18-gauge needle, and placed on ice. DNA was digested by adding 0.1 volume of DNase I digestion buffer and 30 U of DNase I (both reagents from Boehringer Mannheim). The digestion was allowed to proceed for 30 min on ice, and the resulting lysate was vortexed for 10 s, aliquoted, and stored frozen at −70°C. Alternatively, washed cells (3 × 106) were lysed directly in 10 μl of 1× Laemmli sample buffer.
For Western blot analysis, protein extracts (20 to 40 μg) were diluted 1:1 with the Laemmli sample buffer, boiled for 10 min, and electrophoresed on SDS–10 to 12% discontinuous polyacrylamide gels (38). Proteins were transferred by electroblotting to Immobilon P (Millipore Corp., Bedford, Mass.) or nitrocellulose membranes. Human and rat C/EBPα proteins were detected after 1 h of incubation with a 1:1,000 dilution of a rabbit polyclonal anti-rat C/EBPα antibody (Santa Cruz Biotechnology, Santa Cruz, Calif.) or a 1:2,000 dilution of C103 antibody (kindly provided by Pernille Rorth) and visualized by enhanced chemiluminescence (ECL kit; Amersham) according to the manufacturer’s protocol, using a horseradish peroxidase-conjugated secondary antibody (diluted 1:1,000). Quantitation of relative protein levels was performed with a Molecular Dynamics PhosphorImager. Equivalent loading of lanes was determined by Ponceau S staining and staining of the membranes with anti-β-tubulin antibody (1 μg/μl; Boehringer Mannheim) followed by sheep anti-mouse immunoglobulin G-horseradish peroxidase (1:1,000 dilution; Amersham).
RESULTS
C/EBPα mRNA is present in myeloid but not in lymphoid cells.
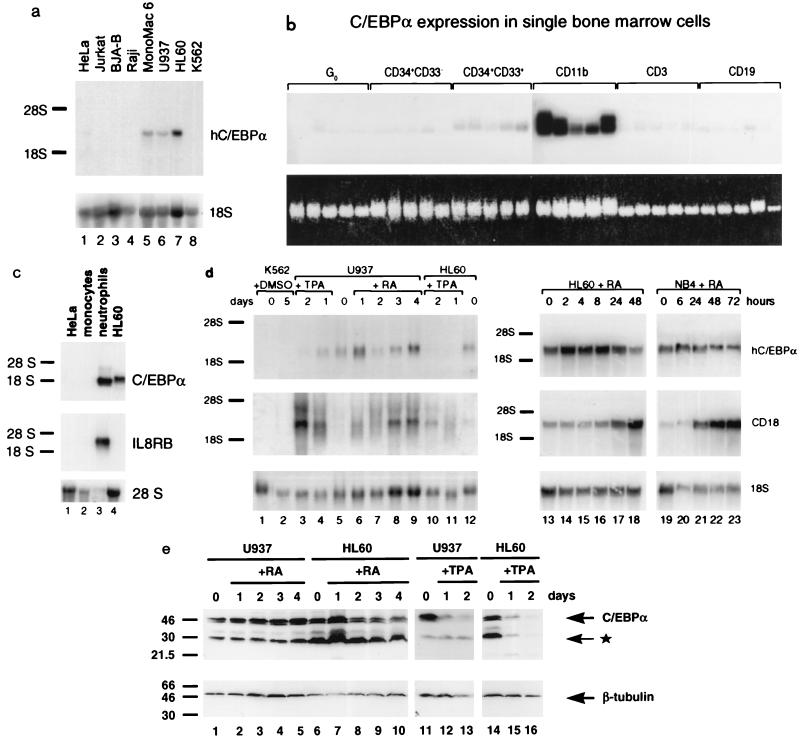
C/EBPα mRNA is expressed in a number of different tissues (4), and within the hematopoietic system it had been shown by Western blot analysis that C/EBPα is regulated during differentiation of myelomonocytic cells (61). We extended these analyses and examined the mRNA expression of C/EBPα by using a panel of myeloid and lymphoid cell lines. As shown in Fig. 1a, Northern blot hybridization to a specific human C/EBPα 3′ untranslated region probe (4) revealed human C/EBPα transcripts of 2.9 kb in myeloid cell lines Mono Mac 6, U937, and HL-60. Very low levels of C/EBPα were detected in KG1a cells, which represent a very immature myeloid line (26, 35) (data not shown), as well as in the nonhematopoietic cervical carcinoma line, HeLa (Fig. 1a and c). In contrast, no C/EBPα mRNA was seen in Jurkat T cells, BJA-B and Raji B cells, or erythroleukemic K562 cells. These results complement previous studies using Western blot analysis (61) and demonstrate that C/EBPα mRNA is specifically expressed in myeloid and not lymphoid lines.
FIG. 1.
Human C/EBPα mRNA and protein increase with granulocytic and shut off with monocytic differentiation. (a) Human C/EBPα mRNA is expressed specifically in cells of the myeloid lineage, as determined by Northern blotting of total RNA isolated from nonhematopoietic HeLa cells (lane 1), Jurkat T cells (lane 2), BJA-B (lane 3) and Raji (lane 4) B cells, promyelocytic Mono Mac 6 (lane 5), U937 (lane 6), and HL-60 (lane 7) cells, and K562 erythroleukemia cells (lane 8). The same blot was hybridized sequentially with a probe for human C/EBPα (hC/EBPα) and for 18S rRNA, as indicated to the right. Positions of migration of 28S and 18S rRNAs are marked on the left. Exposure was for 5 days with an intensifying screen. (b) The C/EBPα gene is activated at the stage of hematopoietic stem cell commitment to the myeloid lineage. Single human bone marrow cells were isolated as described in Materials and Methods and analyzed by Southern blotting of PCR-amplified cDNA. Shown are hybridization of the blot to a human C/EBPα-specific probe (top) and ethidium bromide staining of the gel before blotting (bottom). Each panel (bracketed on top) contains cDNA from five individual cells representing various stages of differentiation (marked above each bracket). Exposure was for 42 h with an intensifying screen. (c) C/EBPα is preferentially expressed in primary granulocytic cells, as determined by Northern blotting of total RNA purified from normal human peripheral blood monocytes and neutrophils. HeLa and HL-60 RNAs were included as negative and positive controls, respectively. The top panel shows results from hybridization to the human C/EBPα probe. Exposure was for 3 days with an intensifying screen. The same blot was stripped and rehybridized to an IL-8 receptor B (IL8RB; neutrophil-specific) probe (middle panel; 2-day exposure) and then to a 28S oligonucleotide as an internal control (bottom panel). (d) Expression of C/EBPα mRNA increases during granulocytic induction of myeloid cell lines and shuts off with monocytic differentiation. K562, U937, HL-60, and NB4 cells were stimulated to erythroid differentiation with DMSO, to monocytic differentiation with TPA, or to granulocytic differentiation with retinoic acid (RA); cell aliquots were withdrawn at the time points indicated above the blot, and RNA was analyzed by Northern blotting. Top, expression of human C/EBPα; middle, expression of the myeloid maturation marker CD18; bottom, 18S RNA as a control for RNA loading and quantitation of C/EBPα expression. Exposure was for 3 days (left) and 4 days (right) for C/EBPα and 4 days (left) and 18 h (right) for CD18, using an intensifying screen. (e) C/EBPα protein increases with retinoic acid-induced granulocytic induction of myeloid cell lines and decreases with TPA-induced monocytic differentiation. Shown is a Western blot of whole-cell extracts from uninduced U937 (lanes 1 and 11) and HL-60 (lanes 6 and 14) cells and retinoic acid (lanes 2 to 5 and 7 to 10)- or TPA (lanes 12, 13, 15, and 16)-induced cells. The cell lines and the inducers, as well as the times of induction in days, are indicated above the lanes. In the top panel, the blot was stained with anti-C/EBPα antibody; in the bottom panel, the same blot was stripped and subsequently stained with anti-β-tubulin antibody. Molecular size standards are shown in kilodaltons to the left. Arrows on the right indicate full-length C/EBPα and β-tubulin, and the asterisk indicates truncated C/EBPα protein (45, 53).
C/EBPα gene expression is initiated at the time of commitment of stem cells to differentiate to the myeloid lineage.
We examined how early during normal hematopoiesis is C/EBPα expressed. To do this, we examined cDNA synthesized from RNA of single isolated human bone marrow cells. Individual quiescent (G0-arrested) multipotent hematopoietic stem cells were selected by treatment of bone marrow cells with stem cell factor, IL-3, and the antimetabolite 5-fluorouracil (6, 12). Cells representing various stages of hematopoietic differentiation were isolated by fluorescence-activated cell sorting (FACS) using antibodies against specific antigens. CD34 is a marker of stem cells and precursors (2, 36). Concurrent expression of CD34 and early myeloid marker, CD33, distinguishes progenitors committed to the myeloid lineage (28). CD11b, CD3, and CD19 are the markers of mature myeloid, T, and committed B lymphoid cells, respectively (28, 66, 74). Each panel in Fig. 1b contains cDNA synthesized from five individual FACS-sorted cells representing each group. Hybridization to a human C/EBPα-specific probe demonstrates that C/EBPα is not expressed in G0-arrested stem cells or uncommitted progenitors (CD34+ CD33−). However, all five committed myeloid (CD34+ CD33+) precursor cells had detectable C/EBPα mRNA. CD11b+ mature myeloid cells (which in bone marrow are almost entirely neutrophils with rare monocytes) showed the highest expression of C/EBPα. Background levels of C/EBPα were observed in CD3+ T cells and CD19+ B cells. These results indicate that C/EBPα is first expressed at the time of commitment of myeloid precursors and persists in mature myeloid but not lymphoid cells.
C/EBPα mRNA is highly expressed in granulocytes but not peripheral blood monocytes.
Our studies (see above) showed that C/EBPα was highly expressed in mature myeloid cells (CD11b+) but did not indicate whether there was preferential expression in monocytes or granulocytes. To address the question of how C/EBPα mRNA is expressed in terminally differentiated primary cells, we purified peripheral blood human monocytes and neutrophils and analyzed total RNA by Northern blotting as shown in Fig. 1c. Very high levels of C/EBPα were detected in neutrophils. Control hybridization to the neutrophil-specific IL-8 receptor B probe confirmed the identity of neutrophil RNA (1, 10). No C/EBPα transcripts were noted in purified human peripheral blood monocytes, which express mRNA of the late-stage monocytic marker, CD14 (data not shown). We conclude that C/EBPα is expressed at high levels in mature neutrophils and is undetectable in mature peripheral blood monocytes.
Granulocyte-specific upregulation of C/EBPα expression is recapitulated in myeloid cell lines.
We were interested in whether the granulocyte-restricted pattern of C/EBPα expression observed in primary cells would also be found in myeloid cell lines, which are good models for studying differentiation (35). K562 erythroleukemia cells were induced to erythroid differentiation with DMSO. U937 and HL-60 promyelocytic cells were treated with TPA to induce monocytic differentiation or with retinoic acid to stimulate granulocytic differentiation. At different time points, cell aliquots were withdrawn for preparation of cytocentrifuged slides for morphologic analysis and preparation of total RNA. The effectiveness of each differentiation induction was verified by microscopic inspection of Wright-Giemsa-stained slides, which showed that at the final time points over 90% of U937 and HL-60 cells were differentiated to monocytic and granulocytic lineages, respectively. To determine whether the morphological changes observed were associated with alterations in C/EBPα gene expression, we measured C/EBPα mRNA (Fig. 1d). A clear decrease in C/EBPα mRNA was apparent in U937 and HL-60 cells undergoing in vitro monocytic differentiation (compare lane 5 to lanes 3 and 4 and lane 12 to lanes 10 and 11). Normalization of the C/EBPα hybridization signals to the 18S RNA internal control revealed that TPA treatment of HL-60 cells led to a decrease of C/EBPα mRNA levels by approximately 90%. The same treatment of U937 cells resulted in a reduction of expression by 70%. Control hybridization showed a significant increase in accumulation of mRNA for CD18, a marker of myeloid maturation, demonstrating induction of differentiation of the cultures.
A different picture emerged when the cells were treated with retinoic acid, which induces granulocytic differentiation of U937 and HL-60 cells (35, 50). Both cell lines demonstrated twofold upregulation of C/EBPα mRNA following 1 day of culture in the presence of retinoic acid (Fig. 1d). Maximum levels of C/EBPα mRNA were noted during the first 2 to 8 h of treatment of HL-60 cells (Fig. 1d, lanes 14 to 16), and they decreased to original levels at 48 h of treatment (Fig. 1d; compare lanes 13 and 18). A similar pattern of C/EBPα mRNA expression was observed during granulocytic differentiation of another promyelocytic cell line, NB4 (Fig. 1d, lanes 19 to 23). In the case of U937 cells, C/EBPα mRNA was upregulated on day 1 of granulocytic differentiation, followed by a slight downregulation on day 2 and then by a gradual increase after an additional 2 days of culture with retinoic acid. This biphasic pattern of expression was observed repeatedly. As a control for induction of myeloid differentiation, the amount of CD18 mRNA was noted to increase (Fig. 1d). As shown earlier (Fig. 1a, lane 8), no C/EBPα transcripts were seen in unstimulated K562 cells, and no upregulation was observed during DMSO-induced erythrocytic differentiation (Fig. 1d). In addition to investigating C/EBPα mRNA, we also determined the expression of C/EBPα protein during monocytic and granulocytic differentiation by Western blot analysis. C/EBPα protein was downregulated (fivefold at day 1) with TPA-induced monocytic differentiation. In contrast, C/EBPα protein levels were increased twofold after 1 day of retinoic acid treatment in both HL-60 and U937 cells. At later time points, protein levels were maintained at initial levels (Fig. 1e). In addition, C/EBP DNA binding activity (as assessed by gel shift assays) was upregulated 3-fold with retinoic acid and downregulated 2.5-fold with TPA treatment (55a). In summary, the granulocyte-specific pattern of C/EBPα expression seen in primary human cells was observed in bipotential cell lines induced to differentiate along the two major myeloid pathways. Specifically, C/EBPα was highly expressed during retinoic acid-induced granulocytic differentiation and rapidly downregulated with respect to mRNA and protein levels and DNA binding activity with TPA-induced monocytic differentiation.
Conditional expression of C/EBPα is sufficient to induce granulocytic differentiation of bipotential cells.
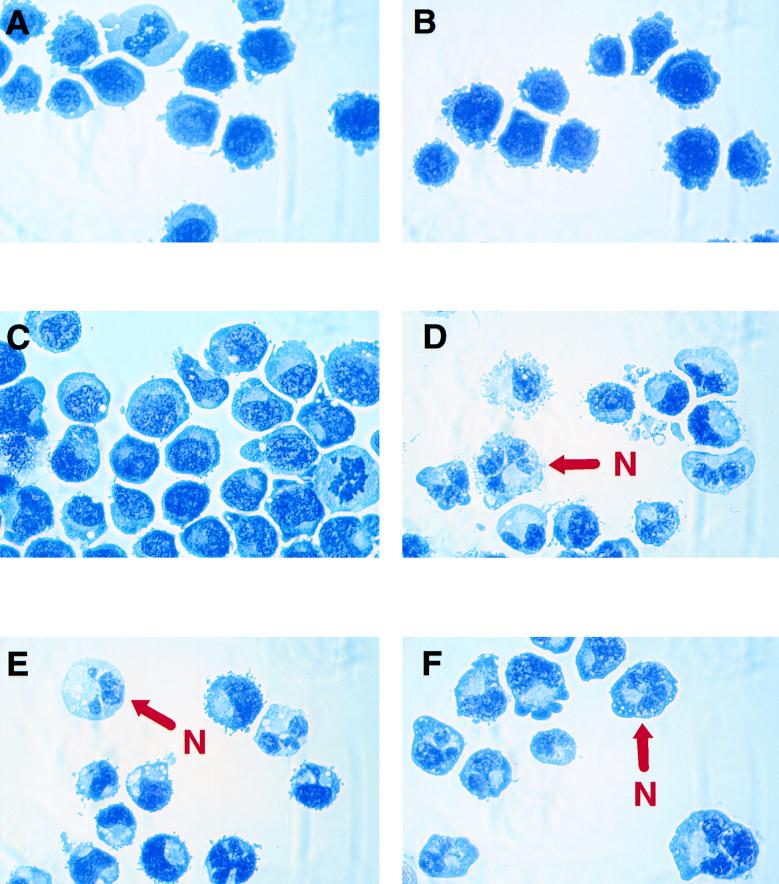
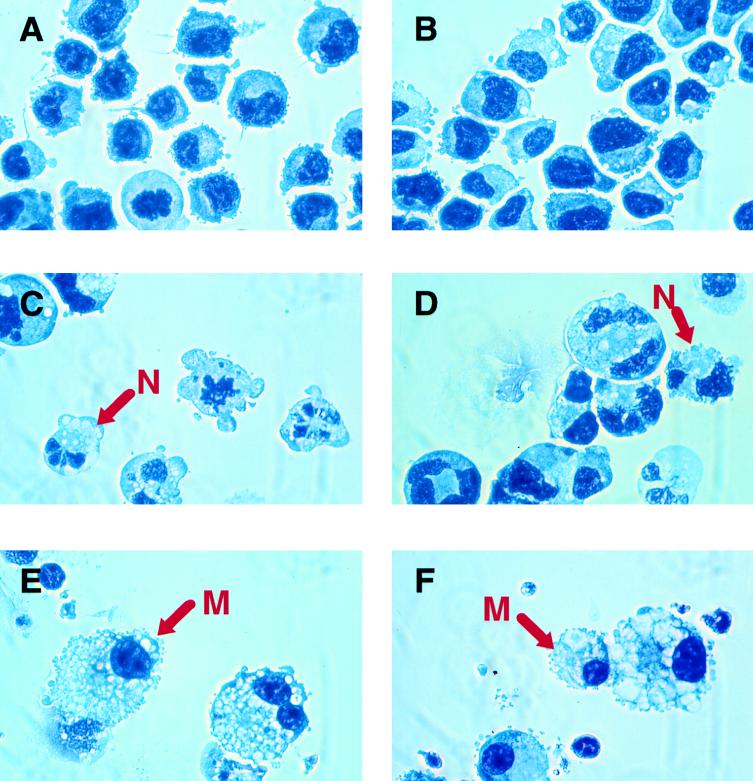
Recently we reported that mice homozygous for a C/EBPα null mutation have an absolute lack of mature neutrophils but not monocytes (81). This effect together with granulocyte-limited expression of C/EBPα suggested that this transcription factor is essential for granulopoiesis. To investigate the possibility that C/EBPα acts as a molecular switch, directing bipotential cells to granulocytic differentiation, we generated stable lines of U937 and HL-60 cells containing a rat C/EBPα cDNA expressed by the zinc-inducible human metallothionein promoter. For controls, the same cell lines were also stably transfected with a C/EBPα-deficient plasmid containing the promoter only. Stable clones were isolated by resistance to G418. Several individual clones were cultured in regular growth medium or medium supplemented with 100 μM ZnSO4 for up to 20 days. Cell morphology was monitored at various times by observation of Wright-Giemsa-stained cytocentrifuged cells. Figure 2 shows representative cytospin preparations. Cells from two independent clones of U937 with an empty vector [U937(vect)#1 and U937(vect)#3] grown in zinc-containing medium for 17 days showed no gross morphological changes compared to the untransfected parental line (Fig. 2A and B and data not shown). These cells had characteristics of myeloblasts: relatively large, rather oblong nuclei with finely granular chromatin and surrounded by narrow rims of deeply staining cytoplasm (Fig. 2A and B). Actively dividing cells representing various stages of mitosis were encountered frequently. U937 cells transfected with the inducible C/EBPα expression vector (U937α#2) and maintained for several weeks in medium without zinc resembled promyelocytes (Fig. 2C). They were somewhat larger than U937(vect)#1 and U937(vect)#3 cells and had slightly more abundant cytoplasm. Overall, however, they still had an appearance of immature myeloid cells (promyelocytes or myelocytes). Addition of zinc into the medium and culture for 17 days caused a remarkable change in cell morphology of this clone (Fig. 2D). The great majority of cells represented terminal stages of granulocytic differentiation. Typical mature neutrophilic cells with segmented nuclei and faintly stained cytoplasm were predominant. In addition, cells were positive for neutrophil-specific respiratory burst enzyme activity as assessed by the NBT assay (data not shown). Mitotic cells were decreased over 80% compared to cells cultured in the absence of zinc. Two additional independent lines of U937 with the C/EBPα expression vector (U937α#3 and U937α#5 [Fig. 2E and F, respectively]) also exhibited the morphological characteristics of polymorphonuclear neutrophils upon induction with zinc. The same phenomenon was seen with HL-60 stable clones, although the morphological changes were slightly less dramatic (data not shown). The murine multipotential progenitor cell line 416B, stably transfected with the metallothionein-C/EBPα construct and treated with 100 μM ZnSO4, also underwent granulocytic differentiation as judged by lobular nuclei and lightly stained cytoplasm (data not shown). Since C/EBPα has been shown to possess growth-inhibitory activity (29, 70, 76), we also compared the proliferation rates of C/EBPα transfectants cultured in zinc-containing and zinc-deficient media. The U937(vect)#1 and U937(vect)#3 clones grew indistinguishably regardless of the presence or absence of zinc throughout the entire 20-day culture period. In contrast, the C/EBPα transfectants U937α#2 and U937α#5 showed a moderate reduction in proliferation beginning at day 17 of the zinc treatment, consistent with their morphological differentiation at that time (data not shown). It could be argued that the lower rate of proliferation together with the increased convolution of the nucleus might be a result of apoptosis rather than genuine granulocytic maturation. Accordingly, we performed a TUNEL assay (27) using one C/EBPα-transfected U937 line (U937α#2) and one clone with the empty expression vector [U937(vect)#1]. As shown in Fig. 3, no apoptotic cells were noted in the culture of U937 cells containing the empty expression vector for 17 days in the zinc-supplemented medium or in the culture of U937α#2 cells (containing the rat C/EBPα expression cDNA) in the absence of zinc. When U937α#2 cells were grown in the presence of zinc, only a very few cells became apoptotic (indicated by a red arrow in Fig. 3F). The darker shadows of the nuclei of the nonapoptotic cells clearly show their granulocytic morphology. Therefore, we conclude that the morphologic changes we observe are not secondary to increased apoptosis. In summary, following zinc treatment, bipotential myeloid cells stably transfected with a zinc-inducible C/EBPα expression vector underwent morphological changes indicative of terminal stages of granulocytic differentiation.
FIG. 2.
Conditional expression of C/EBPα is sufficient for induction of granulocytic differentiation of bipotential U937 cells. Two individual stable clones of U937 cells with an empty expression vector, U937(vect)#1 (A) and U937(vect)#3 (B), were grown in zinc-containing medium for 17 days. Three individual clones of U937 cells were transfected with an inducible C/EBPα expression vector and grown in the absence of zinc (C [U937α#2]) or the presence of zinc for 17 days (D [U937α#2], E [U937α#3], and F [U937α#5]). Cell aliquots were centrifuged onto glass slides and stained with Wright-Giemsa stain. N, representative cell with the appearance of a polymorphonuclear granulocyte.
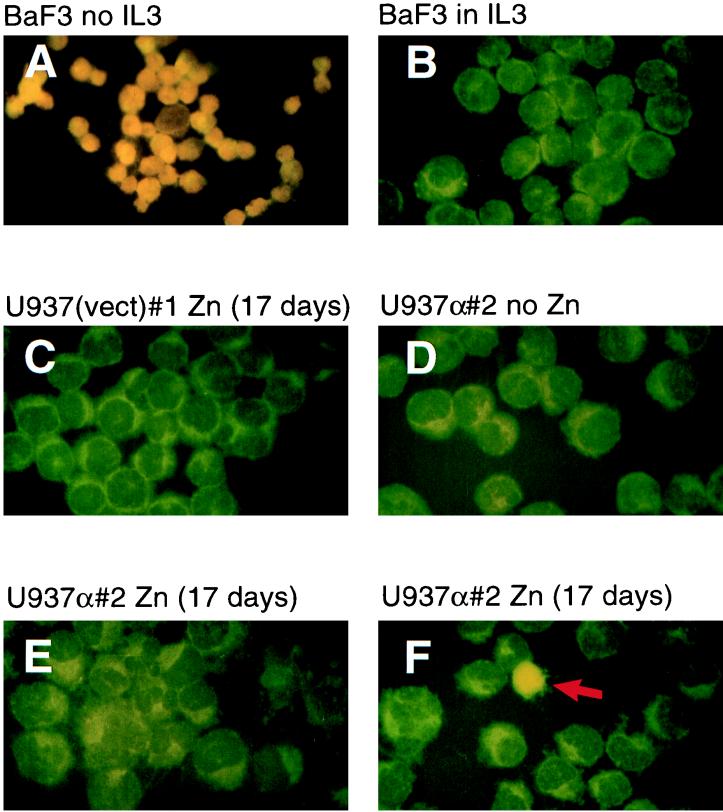
FIG. 3.
Induced expression of C/EBPα does not lead to apoptosis. IL-3-dependent BaF3 cells cultured in medium supplemented with IL-3 (B; negative control), or in conditions such that IL-3 was withdrawn for 24 h (A; positive control, showing apoptosis of nearly all cells as demonstrated by typical orange-yellow staining of the cell nuclei), as well as U937 cells stably transfected with the empty expression vector [U937(vect)#1] and cultured for 17 days in the presence of zinc (C), or transfected with the rat C/EBPα construct (U937α#2) and grown in the absence (D) or presence of zinc (E and F), were tested by TUNEL assay for apoptosis. Panels E and F show different fields from the same slide. The red arrow in panel F indicates an apoptotic cell.
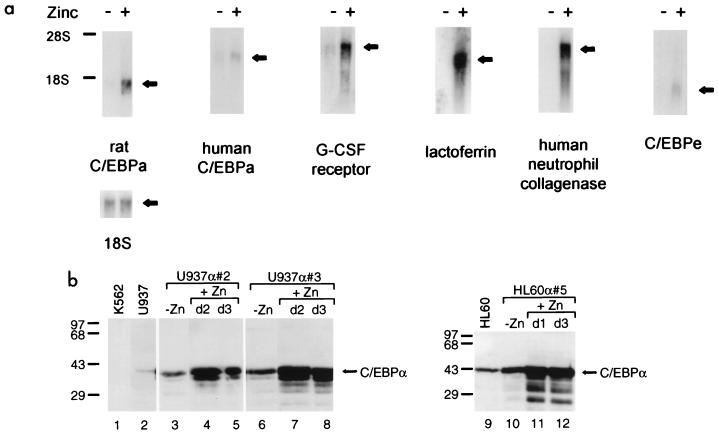
To confirm that this effect was attributable to zinc induction of ectopic C/EBPα expression, cells were harvested for RNA after 13 days of culture in zinc-free and zinc-supplemented media. After 13 days of zinc treatment, most of the cells resembled metamyelocytes (bands) or polymorphonuclear neutrophils, whereas none of the cells in the absence of zinc had this morphology. Low levels of rat C/EBPα expression were detectable even in some clones grown in zinc-deficient medium. However, addition of zinc augmented expression of the transfected C/EBPα considerably. There were in U937α#2 over 100-fold, in U937α#3 almost 3-fold, and in an HL-60 transfectant (HL60α#5) nearly 9-fold more rat C/EBPα transcripts after zinc induction (Fig. 4a and data not shown). Overall, the highest induced expression was seen in clone U937α#2, which reached approximately fourfold-higher expression of rat C/EBPα mRNA than did clones U937α#3 and HL60α#5. The latter two lines produced roughly equivalent amounts of rat C/EBPα mRNA.
FIG. 4.
Induced expression of C/EBPα causes upregulation of granulocytic maturation marker genes. (a) Northern blot with total RNA isolated from the U937 cell line stably transfected with the inducible rat C/EBPα expression gene (clone α#2). Prior to RNA extraction cells were cultured for 13 days in zinc-deficient (−) or zinc-supplemented (+) medium, as indicated at the top. The blot was sequentially hybridized to probes indicated below each panel. Hybridization to 18S rRNA served as an internal control for loading and RNA transfer. The migration of 28S and 18S rRNAs is indicated to the left. Similar results were obtained with other stable clones of U937 and HL-60 cells. (b) Western blot of protein extracts from parental untransfected lines (U937 [lane 2] and HL-60 [lane 9]) and their C/EBPα transfectants (U937α#2, U937α#3, and HL60α#5). C/EBPα-negative K562 extract served as a control of antibody specificity (lane 1). Cells were grown in the absence of zinc (−Zn) or in the presence of zinc (+Zn) for 1 day (d1), 2 days (d2), or 3 days (d3), as indicated. Positions of molecular size markers are shown in kilodaltons on the left, and the arrow on the right marks the migration of the 42-kDa C/EBPα protein detected by the anti-rat C/EBPα antibody, which also detects the endogenous human C/EBPα protein. All lanes except lane 9 contained 20 μg of protein extract; in lane 9, 40 μg of total protein was loaded.
Consistent with the Northern blot data, Western blot analyses of C/EBPα protein showed that untransfected U937 and HL-60 cells expressed low but detectable levels of endogenous C/EBPα protein (Fig. 4b, lanes 2 and 9). In zinc-deficient medium, all three stable clones tested (U937α#2, U937α#3, and HL60α#5) produced threefold more total (endogenous human and ectopic rat) C/EBPα protein than the corresponding untransfected parental lines (Fig. 4b; compare lanes 3 and 6 with lane 2 and lane 10 with lane 9). This result was expected since leaky rat C/EBPα mRNA expression was noted by Northern blot analysis (Fig. 4a and data not shown). However, after 2 days of culture in the presence of zinc, U937α#2 and U937α#3 cells produced 10-fold more total C/EBPα protein than the parental untransfected U937 line (Fig. 4b; compare lanes 4 and 7 to lane 2). Approximately eightfold more C/EBPα protein was detected in HL60α#5 cells treated with zinc for 24 h (compare lane 11 to lane 9; 40 μg of protein extract was loaded in lane 9, while all other lanes contain 20 μg).
The upregulation of exogenous C/EBPα was paralleled by an increase of endogenous G-CSF receptor mRNA levels. The magnitude of G-CSF receptor mRNA correlated with the extent of rat C/EBPα mRNA induction, suggesting a dosage-dependent effect (Fig. 4a and data not shown). In contrast, upregulation of ectopic C/EBPα had no significant effect on the expression of endogenous C/EBPα mRNA in our stable lines (the C/EBPα mRNA levels correlated exactly with the amount of total RNA loaded in each lane as assessed by hybridization to the 18S rRNA probe). In addition, induction of ectopic C/EBPα expression also paralleled with the upregulation of another C/EBP family member, C/EBPɛ. C/EBPɛ was shown recently to exhibit a granulocyte-restricted pattern of expression (14, 79) and to be essential for late-stage (metamyelocyte-to-segmented granulocyte) differentiation of neutrophils (78), and therefore acts downstream of C/EBPα function. Most interestingly, however, increased expression of C/EBPα resulted in activation of the neutrophil-specific genes lactoferrin and human neutrophil collagenase (Fig. 4a), which were shown to remain silent in human leukemic cell lines even after induction of their granulocytic differentiation by retinoic acid or DMSO (32, 33). In summary, overexpression of C/EBPα alone had an impact on the genetic program of the U937 and HL-60 cells by acting as a molecular switch, which triggered their granulocytic differentiation program through upregulation of myeloid genes such as those encoding G-CSF receptor, C/EBPɛ, and secondary granule proteins, and was associated with marked neutrophilic morphologic changes.
Provisionally expressed C/EBPα prevents TPA-induced macrophage differentiation.
The data described above suggest a fundamental role of C/EBPα as a granulocytic differentiation determinant. The following experiment was performed to establish whether the expression of C/EBPα can prevent macrophage differentiation. The stable clone U937α#2 was chosen because the transfected C/EBPα gene was the least active in zinc-deficient medium and the addition of zinc to this cell line induced the highest levels of rat C/EBPα among all stable clones tested (Fig. 4 and data not shown). U937α#2 cells were grown in zinc-supplemented medium, and at 2- to 3-day intervals TPA was added to individual cell cultures. The cultures were allowed to grow in the continued presence of zinc for 2 more days. Changes in cell morphology were monitored by observation of Wright-Giemsa-stained cytospin preparations. As shown in Fig. 5B, 9 days of culture in the presence of zinc did not bring any discernible changes in cell morphology compared to untreated immature cells (Fig. 5A). When U937α#2 cells were pretreated with zinc for 7 days and then induced with TPA (in the continuous presence of zinc) for 2 days, no mature macrophages were observed and all cells resembled neutrophils (Fig. 5C and D). Interestingly, although TPA has been known exclusively as a macrophage differentiation inducer, in the latter experiment it enhanced neutrophil maturation (compare Fig. 5C and D to Fig. 5B). In contrast, TPA treatment alone led to terminal macrophage differentiation of U937α#2 cells grown in zinc-deficient medium (Fig. 5E and F); the morphological changes observed here were indistinguishable from those observed in TPA-stimulated parental U937 cells (data not shown). Simultaneous addition of zinc and TPA or 3-day zinc pretreatment prior to TPA stimulation did not prevent macrophage differentiation (data not shown). After zinc treatment for 5 days and thereafter with TPA, a mixture of phenotypically different cells emerged; among the predominantly macrophage-like cells, occasional polymorphonuclear neutrophils were also apparent (data not shown). These results indicate that forced C/EBPα expression can block the monocytic differentiation pathway and that 5 to 7 days of zinc pretreatment was required to obtain this effect.
FIG. 5.
Induced expression of C/EBPα prevents monocytic differentiation. Shown are Wright-Giemsa-stained cytospin preparations of U937α#2 cells. (A) Cells grown in zinc-deficient medium for 9 days. (B) Cells grown in zinc-containing medium for 9 days. (C and D) Cells cultured in the presence of zinc for 7 days, with TPA then added for 2 days, in the continued presence of zinc (total of 9 days of zinc treatment). N, representative cell with the appearance of a polymorphonuclear granulocyte. (E and F) Cells treated with TPA for 2 days in the absence of zinc. M, representative cell with the morphology of a macrophage.
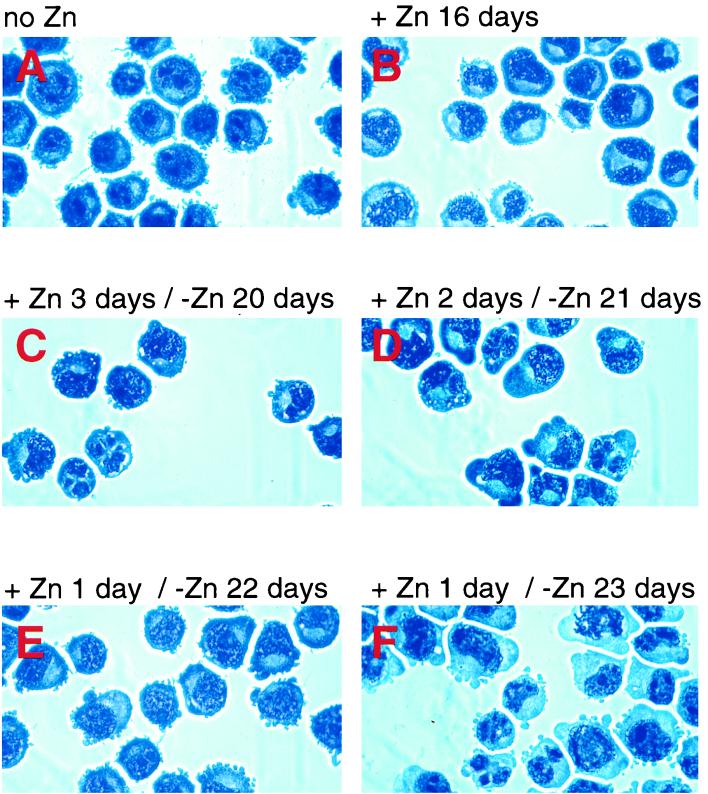
Transient overexpression of C/EBPα is sufficient for induction and progress of granulocytic differentiation.
Northern and Western blot data shown in Fig. 1d and e indicate that retinoic acid-prompted granulocytic differentiation of myeloid cell lines (NB4, HL-60, and U937) resulted initially in a twofold increase in endogenous C/EBPα levels during the first 24 h, which subsequently decreased to the original levels. We wanted to determine whether such transient upregulation of C/EBPα expression was sufficient to trigger granulocytic differentiation. To test this, we cultured the exogenous C/EBPα-expressing U937α#2 cells in the presence of zinc for 1, 2, or 3 days, subsequently washed them twice in zinc-deficient medium, and then continued culture in the absence of zinc. Granulocytic differentiation was assessed by monitoring morphological changes at various time points. Figure 6 shows Wright-Giemsa-stained cytospin preparations. Morphological changes in U937α#2 cells were first noted on day 16 of continued culture in the presence of zinc. When U937α#2 cells were treated with zinc for 1 to 3 days, granulocytic morphologic changes (appearance of polymorphonuclear cells) took place at progressively shorter intervals. As little as 1 day of zinc treatment induced granulocytic differentiation, although it occurred 1 week later (23 to 24 days of culture in total) than in the parallel culture in the continued presence of zinc. Differentiated cells induced by transient expression of C/EBPα showed the same decreases in mitotic activity (fivefold) as was observed for continually induced cells. No changes in the endogenous C/EBPα mRNA expression levels were noted. Again, no differentiation was observed in zinc-treated cells transfected with the empty vector. Therefore, continued induced expression of C/EBPα is not necessary to induce granulocytic differentiation; only a brief exposure can set the differentiation program in motion.
FIG. 6.
Short-term induction of C/EBPα expression in U937 cells is sufficient for morphological granulocytic maturation. C/EBPα-transfected U937 cells (clone U937α#2) were cultured in the absence (A) or continued presence (B) of zinc for 16 days. The same cell line was also initially seeded in medium containing zinc, subsequently washed after 1 day (E and F), 2 days (D), or 3 days (C), and maintained in zinc-deficient medium for an additional 20 days (C), 21 days (D), 22 days (E), or 23 days (F).
DISCUSSION
In this report we have investigated the role of C/EBPα as a regulatory switch in hematopoietic cells. Earlier studies in a murine granulocytic cell line, 32Dcl3, had suggested early upregulation and subsequent downregulation of C/EBPα with granulocytic differentiation (61). We have extended these studies to primary hematopoietic cells and shown that the C/EBPα gene is activated during commitment of multipotential cells to the myeloid lineage. In addition, we demonstrated that as bipotential myeloid progenitors are stimulated toward monocyte/macrophage differentiation, C/EBPα is consistently and rapidly downregulated to undetectable levels (Fig. 1). This finding is in agreement with the increase in C/EBPα seen in developing granulocytic but not monocytic colonies in culture (12). In contrast, induction to the granulocytic pathway results in initial upregulation and then subsequent slight downregulation of C/EBPα expression, consistently seen in multiple human and murine cell lines (Fig. 1) (61). Consistent with this pattern of C/EBPα expression is the finding that a short pulse of C/EBPα expression can induce morphologic granulocyte differentiation weeks later (Fig. 6). Relatively high levels of C/EBPα mRNA were noted in primary mature neutrophils (Fig. 1d), suggesting that C/EBPα is further upregulated with terminal differentiation. This observation is consistent with the secondary upregulation of C/EBPα mRNA observed during retinoic acid-mediated granulocytic differentiation of U937 cells (Fig. 1d). This biphasic pattern of C/EBPα mRNA expression and the effects of induced expression in myeloid lines suggest that C/EBPα might serve different functions in early multipotential cells, in which it induces a switch in differentiation to granulocytes versus monocytes, than in terminally differentiated granulocytes, in which it might serve as an antiproliferation factor (29, 70, 76).
These expression data, combined with studies of C/EBPα knockout mice (81), in which granulocytic but not monocytic differentiation is blocked, lead to the question of whether C/EBPα gene activation is a prerequisite for or a consequence of granulocytic differentiation. The C/EBPα knockout model does not completely answer this question, and therefore we turned our attention to studies in which C/EBPα was overexpressed in early bipotential myeloid precursors. We estimated that a threefold increase in total C/EBPα (endogenous human plus exogenous rat) protein was sufficient to induce terminal polymorphonuclear differentiation (Fig. 4b). The time required for granulocytic maturation (17 days) is similar to the time estimated for myeloid maturation in normal bone marrow (9, 49). Moreover, the extent of differentiation among individual clones correlated with the degree of induction of C/EBPα mRNA. Since the forced expression of C/EBPα instructed bipotential myeloid cells to granulocytes rather than monocytes, these results showed that C/EBPα expression is a necessary prerequisite for this process.
Conditional expression of ectopic C/EBPα can induce differentiation of bipotential myeloid cells and adipogenesis (24, 44, 73, 80). However, one possible difference between the adipocyte and granulocyte differentiation systems is that it is thought that in the case of adipocyte differentiation, a critical early event is induction of C/EBPβ expression prior to subsequent activation of C/EBPα (60, 77, 80), indicating the importance of C/EBPβ function in adipocyte development. In contrast, gene targeting of C/EBPβ (62, 67) and conditional expression of avian C/EBPβ (NF-M) in a multipotential hematopoietic cell line (47) indicate that it is neither necessary nor sufficient for development of monocytes and neutrophils. Another difference between these systems is that the retinoic acid receptor pathway blocks adipogenesis by blocking C/EBP-mediated transcription (60) but is a positive regulator of granulocyte development (15, 72), and it will be of interest to investigate the effects of liganded retinoic acid receptor on C/EBPα transcriptional activation of myeloid target genes, such as those encoding the G-CSF receptor (64) and C/EBPα itself (69). In bipotential myeloid cell lines, retinoic acid signaling itself leads to upregulation of C/EBPα expression (Fig. 1), and this could be the primary mechanism of retinoic acid-induced granulocyte differentiation.
While we clearly observe upregulation of C/EBPα mRNA and protein with retinoic acid-induced differentiation of myeloid cell lines, other possible mechanisms of granulocytic induction of myeloid cell lines exist. For example, neutrophilic differentiation of HL-60 cells with DMSO treatment was reported to lead to a decrease in C/EBPα protein (61), and we observed the same results in Western blots of the HL-60 cells used in our studies (data not shown). Clearly, DMSO and retinoic acid evoke granulocytic differentiation by different molecular pathways and C/EBPα upregulation is not essential for DMSO-induced differentiation. For example, the lack of upregulation of C/EBPα in DMSO-treated HL-60 cells might be compensated by some other transcription factor(s), such as the granulocyte-specific C/EBPɛ (79) or by C/EBPβ, which is also highly upregulated during granulocytic differentiation (55a). Whether the mechanisms observed in DMSO-induced HL-60 granulocytic differentiation extend to primary cells is not known, since C/EBPα is clearly critical for granulocyte maturation in vivo (81). We also observed a decrease in C/EBPα mRNA and protein levels with TPA-induced monocytic differentiation of cell lines. We and others (9a) also observed no mRNA detectable by Northern blot analysis of peripheral blood monocytes. However, it is clear that C/EBPα can be expressed in murine bone marrow-derived macrophages (31), murine peritoneal macrophages (81a), and human alveolar macrophages (9b), raising the possibility that C/EBPα is downregulated as multipotential myeloid precursors differentiate into monocytes but upregulated as monocytes further differentiate to macrophages.
Induced expression of exogenous C/EBPα also enhanced expression of the granulocyte-specific G-CSF receptor (Fig. 4a). This finding is consistent with the findings that the G-CSF receptor promoter is regulated by C/EBPα (64), and G-CSF receptor mRNA is selectively absent in C/EBPα knockout mice (81). Induction of G-CSF receptor expression in immature granulocytic cells is critical for early proliferation and viability, as removal of growth factors can lead to apoptosis (56), which was not observed in our cells (Fig. 3). Another set of important granulocytic mRNAs which are induced in these cells (and absent from C/EBPα−/− murine fetal liver cells) are those encoding neutrophil-specific secondary granule proteins (such as lactoferrin and collagenase [Fig. 4a]). These genes could not previously be induced during granulocytic differentiation of myeloid cells lines by using retinoic acid alone (32, 33), suggesting that induced expression of C/EBPα leads to a stage of maturation more similar to actual neutrophils.
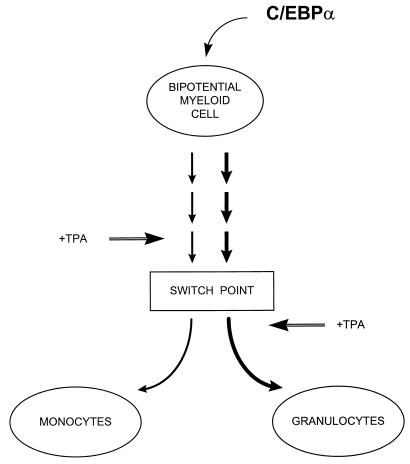
In the absence of C/EBPα, granulocytes did not develop in C/EBPα knockout mice (81), and provision of high levels of C/EBPα to bipotential myeloid precursors directed them to differentiate along the granulocytic pathway (this report). Taken together, these studies show that expression of C/EBPα is sufficient and necessary for granulocytic differentiation and suggest a model of how bipotential myeloid precursors are induced to become either monocytes or granulocytes (Fig. 7). Myeloid precursors can develop into either granulocytes or monocytes. If they encounter a stimulus which induces upregulation of C/EBPα expression, then the outcome will result in granulocyte development. One possible stimulus is signaling through the G-CSF receptor itself, which can induce upregulation of C/EBPα mRNA in multipotential cell lines (64a). Conversely, a different stimulus might induce expression of a monocytic factor resulting in monocytic development (Fig. 7). One candidate for such a monocytic factor is egr-1 (37, 48), although the finding that egr-1 knockout mice can make macrophages demonstrates that in contrast to C/EBPα-induced granulocytic development, egr-1 is not absolutely necessary for monocytic differentiation (42). Another candidate monocyte regulator is PU.1, since PU.1 knockout mice have an absolute block in monocyte development but can make neutrophilic cells, albeit their development is delayed (46).
FIG. 7.
Model of bipotential myeloid lineage differentiation. The expression of increased levels of C/EBPα in a bipotential myeloid precursor induces genetic changes over the course of a week that lead to irreversible granulocytic differentiation. Prior to this switch point, monocytic differentiation agents such as TPA can induce macrophage development, but subsequent to this point the same agents can accelerate granulocytic differentiation. After the switch point, C/EBPα silencing is required for the monocytic pathway to proceed, as forced expression of C/EBPα prevents monocytic differentiation. At present, the factors which direct monocytic maturation of the bipotential myeloid precursor cell are not clearly defined, but candidate genes include the egr-1 family (48) and/or PU.1 (30).
During TPA stimulation of monocytic development, endogenous C/EBPα was downregulated, and it is likely that this downregulation is required in order for the cells to differentiate along the monocytic pathway. Subsequent to the ectopic C/EBPα induction for 7 days, treatment with TPA was unable to downregulate the endogenous C/EBPα expression. As a result, forced expression of C/EBPα prevented monocytic differentiation normally achieved by TPA treatment alone (Fig. 5). This block is likely due to the cells having been committed to the granulocytic pathway, rather than elevated levels of C/EBPα protein per se. It might be possible that C/EBPα acts as a repressor of genes which play a role in monocytic differentiation, such as egr-1. Moreover, if ectopic C/EBPα was induced and allowed to be expressed for a sufficient time (7 days), TPA treatment accelerated granulocytic maturation. This observation suggests that C/EBPα is a particularly powerful differentiation factor, as it has been shown that HL-60 cells which had been treated for 5 days with DMSO to induce granulocytic cells could be still differentiated to macrophages by subsequent treatment with TPA (43). One possible explanation could be that C/EBPα expression affects the expression of other factors fundamental for granulocytic maturation which act either independently or in cooperation with C/EBPα. A likely candidate for such cooperative interactions might be the recently cloned and predominantly granulocytic-specific factor C/EBPɛ (3, 14, 79). C/EBPɛ appears to act downstream of C/EBPα, in that in C/EBPɛ−/− mice, granulocytic differentiation proceeds beyond the immature blast stage observed in C/EBPα−/− mice but is blocked at the very terminal stages of metamyelocyte-to-segmented granulocyte differentiation (78).
Our results suggest that genetic changes toward irreversible granulocytic differentiation occur during the first week of C/EBPα expression, and it will be of great importance to identify which presently unknown C/EBPα target genes are upregulated in these C/EBPα cells during the neutrophilic commitment period, prior to the switch point (Fig. 7). In addition, complete myeloid maturation takes at least another 10 days beyond this first 7-day commitment phase; the total time required (17 days) is similar to the time estimated for myeloid maturation in normal bone marrow (9, 49). TPA itself, normally a monocytic inducer, can accelerate granulocytic maturation of cells in which C/EBPα has been expressed at increased levels for 7 days to induce granulocytic commitment (Fig. 5). Therefore, it will also be of great interest to identify which genes are differentially induced during TPA-mediated monocytic differentiation of these lines versus TPA-induced granulocytic maturation of cells in which C/EBPα has already been expressed for 7 days to trigger granulocytic commitment but not maturation. The cell lines used in these studies should be of great value in dissecting the genetic and signaling pathways leading from bipotential myeloid precursors to granulocytes, as well as the mechanisms leading to the differentiation block observed in leukemic cells.
ACKNOWLEDGMENTS
We acknowledge the assistance of Jeff Marx of Baxter Healthcare Corporation for his generous gifts of purified cells and cell culture reagents; J. Patrick Condreay for his kind gift of plasmids pPC18 and pPC22 and valuable discussions; Mathieu Cellier for allowing us to cite his unpublished data; Kleanthis Xanthopoulos for the human C/EBPα cDNA probe; Jane Visvader for 416B cells; Pernille Rorth for C103 anti-C/EBPα antibody; David Gonzalez, Laura T. Smith, Kristina Rhoades, and Chaker N. Adra for assistance with the expression studies; Stuart Orkin, Kleanthis Xanthopoulos, Gretchen Darlington, Len Zon, Claus Nerlov, Laura Smith, Atsushi Iwama, and Milton Datta for critical reading of the manuscript; and all members of the Tenen laboratory who contributed to this work by numerous discussions and helpful suggestions.
This work was supported by fellowship award DK09721 from the National Institutes of Health to H.S.R. and grants CA41456 and HL56745 (to D.G.T.) and HL44851 and DK50234 (to D.T.S.). C.S.H. is a recipient of a fellowship from the Deutsche Forschungsgemeinschaft.
REFERENCES
- 1.Ahuja S K, Shetty A, Tiffany H L, Murphy P M. Comparison of the genomic organization and promoter function for human interleukin-8 receptors A and B. J Biol Chem. 1994;269:26381–26389. [PubMed] [Google Scholar]
- 2.Andrews R G, Bryant E M, Bartelmez S H, Muirhead D Y, Knitter G H, Bensinger W, Strong D M, Bernstein I D. CD34+ marrow cells, devoid of T and B lymphocytes, reconstitute stable lymphopoiesis and myelopoiesis in lethally irradiated allogeneic baboons. Blood. 1992;80:1693–1701. [PubMed] [Google Scholar]
- 3.Antonson P, Stellan B, Yamanaka R, Xanthopoulos K G. A novel human CCAAT/enhancer binding protein gene, C/EBPɛ, is expressed in cells of lymphoid and myeloid lineages and is localized on chromosome 14q11.2 close to the T-cell receptor α/δ locus. Genomics. 1996;35:30–38. doi: 10.1006/geno.1996.0319. [DOI] [PubMed] [Google Scholar]
- 4.Antonson P, Xanthopoulos K G. Molecular cloning, sequence, and expression patterns of the human gene encoding CCAAT/enhancer binding protein α (C/EBPα) Biochem Biophys Res Commun. 1995;215:106–113. doi: 10.1006/bbrc.1995.2439. [DOI] [PubMed] [Google Scholar]
- 5.Barbu V, Dautry F. Northern blot normalization with a 28S rRNA oligonucleotide probe. Nucleic Acids Res. 1989;17:7115. doi: 10.1093/nar/17.17.7115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berardi A C, Wang A L, Levine J D, Lopez P, Scadden D T. Functional isolation and characterization of human hematopoietic stem cells. Science. 1995;267:104–108. doi: 10.1126/science.7528940. [DOI] [PubMed] [Google Scholar]
- 7.Brady G, Barbara M, Iscove N N. Representative in vitro cDNA amplification from individual hemopoietic cells and colonies. Methods Mol Cell Biol. 1990;2:17–25. [Google Scholar]
- 8.Cao Z, Umek R M, McKnight S L. Regulated expression of three C/EBP isoforms during adipose conversion of 3T3-L1 cells. Genes Dev. 1991;5:1538–1552. doi: 10.1101/gad.5.9.1538. [DOI] [PubMed] [Google Scholar]
- 9.Cartwright G E, Athens J W, Wintrobe M M. The kinetics of granulopoiesis in normal man. Blood. 1964;24:780–803. [PubMed] [Google Scholar]
- 9a.Cellier, M. Personal communication.
- 9b.Cellier, M. Unpublished data.
- 10.Cellier M, Shustik C, Dalton W, Rich E, Hu J X, Malo D, Schurr E, Gros P. Expression of the human NRAMP1 gene in professional primary phagocytes: studies in blood cells and in HL-60 promyelocytic leukemia. J Leukocyte Biol. 1997;61:96–105. doi: 10.1002/jlb.61.1.96. [DOI] [PubMed] [Google Scholar]
- 11.Chen H M, Zhang P, Voso M T, Hohaus S, Gonzalez D A, Glass C K, Zhang D-E, Tenen D G. Neutrophils and monocytes express high levels of PU.1 (Spi-1) but not Spi-B. Blood. 1995;85:2918–2928. [PubMed] [Google Scholar]
- 12.Cheng T, Shen H, Giokas D, Gere J, Tenen D G, Scadden D T. Temporal mapping of gene expression levels during the differentiation of individual primary hematopoietic cells. Proc Natl Acad Sci USA. 1996;93:13158–13163. doi: 10.1073/pnas.93.23.13158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 14.Chumakov A M, Grillier I, Chumakova E, Chih D, Slater J, Koeffler H P. Cloning of the novel human myeloid-cell-specific C/EBP-epsilon transcription factor. Mol Cell Biol. 1997;17:1375–1386. doi: 10.1128/mcb.17.3.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Collins S J, Robertson K A, Mueller L. Retinoic acid-induced granulocytic differentiation of HL-60 myeloid leukemia cells is mediated directly through the retinoic acid receptor (RARα) Mol Cell Biol. 1990;10:2154–2163. doi: 10.1128/mcb.10.5.2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Costa R H, Grayson D R, Xanthopoulos K G, Darnell J E., Jr A liver-specific DNA-binding protein recognizes multiple nucleotide sites in regulatory regions of transthyretin, α 1-antitrypsin, albumin, and simian virus 40 genes. Proc Natl Acad Sci USA. 1988;85:3840–3844. doi: 10.1073/pnas.85.11.3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davis R L, Weintraub H, Lassar A B. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 1987;51:987–1000. doi: 10.1016/0092-8674(87)90585-x. [DOI] [PubMed] [Google Scholar]
- 18.Devarajan P, Mookhtiar K, Van Wart H, Berliner N. Structure and expression of the cDNA encoding human neutrophil collagenase. Blood. 1991;77:2731–2738. [PubMed] [Google Scholar]
- 19.Dexter T M, Allen T D, Scott D, Teich N M. Isolation and characterisation of a bipotential haematopoietic cell line. Nature. 1979;277:471–474. doi: 10.1038/277471a0. [DOI] [PubMed] [Google Scholar]
- 20.Dignam J D, Lebovitz R M, Roeder R G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983;11:1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feinberg A P, Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983;132:6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- 22.Flodby P, Barlow C, Kylefjord H, Ahrlund-Richter L, Xanthopoulos K G. Increased hepatic cell proliferation and lung abnormalities in mice deficient in CCAAT/enhancer binding protein α. J Biol Chem. 1996;271:24753–24760. doi: 10.1074/jbc.271.40.24753. [DOI] [PubMed] [Google Scholar]
- 23.Ford A M, Bennett C A, Healy L E, Towatari M, Greaves M F, Enver T. Regulation of the myeloperoxidase enhancer binding proteins PU.1, CEBPα, β, and δ during granulocytic-lineage specification. Proc Natl Acad Sci USA. 1996;93:10838–10843. doi: 10.1073/pnas.93.20.10838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Freytag S O, Geddes T J. Reciprocal regulation of adipogenesis by Myc and C/EBP alpha. Science. 1992;256:379–382. doi: 10.1126/science.256.5055.379. [DOI] [PubMed] [Google Scholar]
- 25.Fukunaga R, Seto Y, Mizushima S, Nagata S. Three different mRNAs encoding human granulocyte colony-stimulating factor receptor. Proc Natl Acad Sci USA. 1990;87:8702–8706. doi: 10.1073/pnas.87.22.8702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Furley A J, Reeves B R, Mizutani S, Altass L J, Watt S M, Jacob M C, van den Elsen P, Terhorst C, Greaves M F. Divergent molecular phenotypes of KG1 and KG1a myeloid cell lines. Blood. 1986;68:1101–1107. [PubMed] [Google Scholar]
- 27.Gavrieli Y, Sherman Y, Ben-Sasson S A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Griffin J D, Ritz J, Nadler L M, Schlossman S F. Expression of myeloid differentiation antigens on normal and malignant myeloid cells. J Clin Invest. 1981;68:932–941. doi: 10.1172/JCI110348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hendricks-Taylor L R, Darlington G J. Inhibition of cell proliferation by C/EBPα occurs in many cell types, does not require the presence of p53 or Rb, and is not affected by large T-antigen. Nucleic Acids Res. 1995;23:4726–4733. doi: 10.1093/nar/23.22.4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Henkel G W, McKercher S R, Yamamoto H, Anderson K L, Oshima R G, Maki R A. PU.1 but not Ets-2 is essential for macrophage development from ES cells. Blood. 1996;88:2917–2926. [PubMed] [Google Scholar]
- 31.Hu H-M, Baer M, Williams S C, Johnson P F, Schwartz R C. Redundancy of C/EBPα, -β, and -δ in supporting the lipopolysaccharide-induced transcription of IL-6 and monocyte chemoattractant protein-1. J Immunol. 1998;160:2334–2342. [PubMed] [Google Scholar]
- 32.Johnston J J, Rintels P, Chung J, Sather J, Benz E J, Jr, Berliner N. Lactoferrin gene promoter: structural integrity and nonexpression in HL60 cells. Blood. 1992;79:2998–3006. [PubMed] [Google Scholar]
- 33.Khanna-Gupta A, Kolibaba K, Zibello T A, Berliner N. NB4 cells show bilineage potential and an aberrant pattern of neutrophil secondary granule protein gene expression. Blood. 1994;84:294–302. [PubMed] [Google Scholar]
- 34.Klein G, Lindahl T, Jondal M, Leibold W, Menezes J, Nilsson K, Sundstrom C. Continuous lymphoid cell lines with characteristics of B cells (bone-marrow-derived), lacking the Epstein-Barr virus genome and derived from three human lymphomas. Proc Natl Acad Sci USA. 1974;71:3283–3286. doi: 10.1073/pnas.71.8.3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koeffler H P, Golde D W. Human myeloid leukemia cell lines: a review. Blood. 1980;56:344–350. [PubMed] [Google Scholar]
- 36.Krause D S, Fackler M J, Civin C I, May W S. CD34: structure, biology, and clinical utility. Blood. 1996;87:1–13. [PubMed] [Google Scholar]
- 37.Krishnaraju K, Nguyen H Q, Liebermann D A, Hoffman B. The zinc finger transcription factor Egr-1 potentiates macrophage differentiation of hematopoietic cells. Mol Cell Biol. 1995;15:5499–5507. doi: 10.1128/mcb.15.10.5499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 39.Landschulz W H, Johnson P F, Adashi E Y, Graves B J, McKnight S L. Isolation of a recombinant copy of the gene encoding C/EBP. Genes Dev. 1988;2:786–800. doi: 10.1101/gad.2.7.786. [DOI] [PubMed] [Google Scholar]
- 40.Lanotte M, Martin-Thouvenin V, Najman S, Balerini P, Valensi F, Berger R. NB4, a maturation inducible cell line with t(15;17) marker isolated from a human acute promyelocytic leukemia (M3) Blood. 1991;77:1080–1086. [PubMed] [Google Scholar]
- 41.Law S K, Gagnon J, Hildreth J E, Wells C E, Willis A C, Wong A J. The primary structure of the beta-subunit of the cell surface adhesion glycoproteins LFA-1, CR3 and p150,95 and its relationship to the fibronectin receptor. EMBO J. 1987;6:915–919. doi: 10.1002/j.1460-2075.1987.tb04838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee S L, Wang Y, Milbrandt J. Unimpaired macrophage differentiation and activation in mice lacking the zinc finger transactivation factor NGFI-A (EGR1) Mol Cell Biol. 1996;16:4566–4572. doi: 10.1128/mcb.16.8.4566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liebermann D, Hoffman-Liebermann B, Sachs L. Regulation of gene expression by tumor promoters. II. Control of cell shape and developmental programs for macrophages and granulocytes in human myeloid leukemic cells. Int J Cancer. 1981;28:285–291. doi: 10.1002/ijc.2910280306. [DOI] [PubMed] [Google Scholar]
- 44.Lin F T, Lane M D. CCAAT/enhancer binding protein α is sufficient to initiate the 3T3-L1 adipocyte differentiation program. Proc Natl Acad Sci USA. 1994;91:8757–8761. doi: 10.1073/pnas.91.19.8757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lin F T, MacDougald O A, Diehl A M, Lane M D. A 30-kDa alternative translation product of the CCAAT/enhancer binding protein α message: transcriptional activator lacking antimitotic activity. Proc Natl Acad Sci USA. 1993;90:9606–9610. doi: 10.1073/pnas.90.20.9606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McKercher S R, Torbett B E, Anderson K L, Henkel G W, Vestal D J, Baribault H, Klemsz M, Feeney A J, Wu G E, Paige C J, Maki R A. Targeted disruption of the PU.1 gene results in multiple hematopoietic abnormalities. EMBO J. 1996;15:5647–5658. [PMC free article] [PubMed] [Google Scholar]
- 47.Muller C, Kowenz-Leutz E, Grieser-Ade S, Graf T, Leutz A. NF-M (chicken C/EBPβ) induces eosinophilic differentiation and apoptosis in a hematopoietic progenitor cell line. EMBO J. 1995;14:6127–6135. doi: 10.1002/j.1460-2075.1995.tb00303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nguyen H Q, Hoffman-Liebermann B, Liebermann D A. The zinc finger transcription factor Egr-1 is essential for and restricts differentiation along the macrophage lineage. Cell. 1993;72:197–209. doi: 10.1016/0092-8674(93)90660-i. [DOI] [PubMed] [Google Scholar]
- 49.Nimer S D, Golde D W. Molecular, cellular, and clinical biology of phagocytic cells. In: Handin R I, Lux S E, Stossel T P, editors. Blood: principles and practice of hematology. J. B. Philadelphia, Pa: Lippincott Company; 1995. pp. 513–541. [Google Scholar]
- 50.Oberg F, Botling J, Nilsson K. Functional antagonism between vitamin-D3 and retinoic acid in the regulation of CD14 and CD23 expression during monocytic differentiation of U937 cells. J Immunol. 1993;150:3487–3495. [PubMed] [Google Scholar]
- 51.Ochs H D, Igo R P. The NBT slide test: a simple screening method for detecting chronic granulomatous disease and female carriers. J Pediatr. 1973;83:77–82. doi: 10.1016/s0022-3476(73)80316-6. [DOI] [PubMed] [Google Scholar]
- 52.Oelgeschlager M, Nuchprayoon I, Luscher B, Friedman A D. C/EBP, c-Myb, and PU.1 cooperate to regulate the neutrophil elastase promoter. Mol Cell Biol. 1996;16:4717–4725. doi: 10.1128/mcb.16.9.4717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ossipow V, Descombes P, Schibler U. CCAAT/enhancer-binding protein mRNA is translated into multiple proteins with different transcription activation potentials. Proc Natl Acad Sci USA. 1993;90:8219–8223. doi: 10.1073/pnas.90.17.8219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Palacios R, Steinmetz M. Il-3-dependent mouse clones that express B-220 surface antigen, contain Ig genes in germ-line configuration, and generate B lymphocytes in vivo. Cell. 1985;41:727–734. doi: 10.1016/s0092-8674(85)80053-2. [DOI] [PubMed] [Google Scholar]
- 55.Radomska H S, Shen C P, Kadesch T, Eckhardt L A. Constitutively expressed Oct-2 prevents immunoglobulin gene silencing in myeloma × T cell hybrids. Immunity. 1994;1:623–634. doi: 10.1016/1074-7613(94)90034-5. [DOI] [PubMed] [Google Scholar]
- 55a.Radomska, H. S., and D. G. Tenen. Unpublished data.
- 56.Rodel J E, Link D C. Suppression of apoptosis during cytokine deprivation of 32D cells is not sufficient to induce complete granulocytic differentiation. Blood. 1996;87:858–864. [PubMed] [Google Scholar]
- 57.Rosmarin A G, Weil S C, Rosner G L, Griffin J D, Arnaout M A, Tenen D G. Differential expression of CD11b/CD18 (Mo1) and myeloperoxidase genes during myeloid differentiation. Blood. 1989;73:131–136. [PubMed] [Google Scholar]
- 58.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 59.Schlissel M, Voronova A, Baltimore D. Helix-loop-helix transcription factor E47 activates germ-line immunoglobulin heavy-chain gene transcription and rearrangement in a pre-T-cell line. Genes Dev. 1991;5:1367–1376. doi: 10.1101/gad.5.8.1367. [DOI] [PubMed] [Google Scholar]
- 60.Schwarz E J, Reginato M J, Shao D, Krakow S L, Lazar M A. Retinoic acid blocks adipogenesis by inhibiting C/EBPβ-mediated transcription. Mol Cell Biol. 1997;17:1552–1561. doi: 10.1128/mcb.17.3.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Scott L M, Civin C I, Rorth P, Friedman A D. A novel temporal expression pattern of three C/EBP family members in differentiating myelomonocytic cells. Blood. 1992;80:1725–1735. [PubMed] [Google Scholar]
- 62.Screpanti I, Romani L, Musiani P, Modesti A, Fattori E, Lazzaro D, Sellitto C, Scarpa S, Bellavia D, Lattanzio G, Bistoni F, Frati L, Cortese R, Gulino A, Ciliberto G, Costantini F, Poli V. Lymphoproliferative disorder and imbalanced T-helper response in C/EBPβ-deficient mice. EMBO J. 1995;14:1932–1941. doi: 10.1002/j.1460-2075.1995.tb07185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shivdasani R A, Orkin S H. The transcriptional control of hematopoiesis. Blood. 1996;87:4025–4039. [PubMed] [Google Scholar]
- 64.Smith L T, Hohaus S, Gonzalez D A, Dziennis S E, Tenen D G. PU.1 (Spi-1) and C/EBPα regulate the granulocyte colony-stimulating factor receptor promoter in myeloid cells. Blood. 1996;88:1234–1247. [PubMed] [Google Scholar]
- 64a.Smith, L. T., and D. G. Tenen. Unpublished data.
- 65.Spangrude G J, Smith L, Uchida N, Ikuta K, Heimfeld S, Friedman J, Weissman I L. Mouse hematopoietic stem cells. Blood. 1991;78:1395–1402. [PubMed] [Google Scholar]
- 66.Stamenkovic I, Seed B. CD19, the earliest differentiation antigen of the B cell lineage, bears three extracellular immunoglobulin-like domains and an Epstein-Barr virus-related cytoplasmic tail. J Exp Med. 1988;168:1205–1210. doi: 10.1084/jem.168.3.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tanaka T, Akira S, Yoshida K, Umemoto M, Yoneda Y, Shirafuji N, Fujiwara H, Suematsu S, Yoshida N, Kishimoto T. Targeted disruption of the NF-IL6 gene discloses its essential role in bacteria killing and tumor cytotoxicity by macrophages. Cell. 1995;80:353–361. doi: 10.1016/0092-8674(95)90418-2. [DOI] [PubMed] [Google Scholar]
- 68.Tenen D G, Hromas R, Licht J D, Zhang D-E. Transcription factors, normal myeloid development, and leukemia. Blood. 1997;90:489–519. [PubMed] [Google Scholar]
- 69.Timchenko N, Wilson D R, Taylor L R, Abdelsayed S, Wilde M, Sawadogo M, Darlington G J. Autoregulation of the human C/EBPα gene by stimulation of upstream stimulatory factor binding. Mol Cell Biol. 1995;15:1192–1202. doi: 10.1128/mcb.15.3.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Timchenko N A, Wilde M, Nakanishi M, Smith J R, Darlington G J. CCAAT/enhancer-binding protein α (C/EBPα) inhibits cell proliferation through the p21 (WAF-1/CIP-1/SDI-1) protein. Genes Dev. 1996;10:804–815. doi: 10.1101/gad.10.7.804. [DOI] [PubMed] [Google Scholar]
- 71.Torczynski R M, Fuke M, Bollon A P. Cloning and sequencing of a human 18S ribosomal RNA gene. DNA. 1985;4:283–291. doi: 10.1089/dna.1985.4.283. [DOI] [PubMed] [Google Scholar]
- 72.Tsai S, Collins S J. A dominant negative retinoic acid receptor blocks neutrophil differentiation at the promyelocyte stage. Proc Natl Acad Sci USA. 1993;90:7153–7157. doi: 10.1073/pnas.90.15.7153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Umek R M, Friedman A D, McKnight S L. CCAAT-enhancer binding protein: a component of a differentiation switch. Science. 1991;251:288–292. doi: 10.1126/science.1987644. [DOI] [PubMed] [Google Scholar]
- 74.van den Elsen P, Shepley B A, Borst J, Coligan J E, Markham A F, Orkin S, Terhorst C. Isolation of cDNA clones encoding the 20K T3 glycoprotein of human T-cell receptor complex. Nature. 1984;312:413–418. doi: 10.1038/312413a0. [DOI] [PubMed] [Google Scholar]
- 75.Wang N D, Finegold M J, Bradley A, Ou C N, Abdelsayed S V, Wilde M D, Taylor L R, Wilson D R, Darlington G J. Impaired energy homeostasis in C/EBPα knockout mice. Science. 1995;269:1108–1112. doi: 10.1126/science.7652557. [DOI] [PubMed] [Google Scholar]
- 76.Watkins P J, Condreay J P, Huber B E, Jacobs S J, Adams D J. Impaired proliferation and tumorigenicity induced by CCAAT/enhancer-binding protein. Cancer Res. 1996;56:1063–1067. [PubMed] [Google Scholar]
- 77.Wu Z, Xie Y, Bucher N L R, Farmer S R. Conditional ectopic expression of C/EBPβ in NIH-3T3 cells induces PPARΓ and stimulates adipogenesis. Genes Dev. 1995;9:2350–2363. doi: 10.1101/gad.9.19.2350. [DOI] [PubMed] [Google Scholar]
- 78.Yamanaka R, Barlow C, Lekstrom-Himes J, Castilia L H, Liu P P, Eckhaus M, Decker T, Wynshaw-Boris A, Xanthopoulos K G. Impaired granulopoiesis, myelodysplasia and early lethality in CCAAT/enhancer binding protein ɛ-deficient mice. Proc Natl Acad Sci USA. 1997;94:13187–13192. doi: 10.1073/pnas.94.24.13187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yamanaka R, Kim G-D, Radomska H S, Lekstrom-Himes J, Smith L T, Antonson P, Tenen D G, Xanthopoulos K G. CCAAT/enhancer binding protein ɛ is preferentially upregulated during granulocytic differentiation and its functional versatility is determined by alternative use of promoters and differential splicing. Proc Natl Acad Sci USA. 1997;94:6462–6467. doi: 10.1073/pnas.94.12.6462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yeh W C, Cao Z, Classon M, McKnight S L. Cascade regulation of terminal adipocyte differentiation by three members of the C/EBP family of leucine zipper proteins. Genes Dev. 1995;9:168–181. doi: 10.1101/gad.9.2.168. [DOI] [PubMed] [Google Scholar]
- 81.Zhang D-E, Zhang P, Wang N D, Hetherington C J, Darlington G J, Tenen D G. Absence of granulocyte colony-stimulating factor signaling and neutrophil development in CCAAT enhancer binding protein α-deficient mice. Proc Natl Acad Sci USA. 1997;94:569–574. doi: 10.1073/pnas.94.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81a.Zhang, P., and D. G. Tenen. Unpublished data.
- 82.Ziegler-Heitbrock H W, Thiel E, Futterer A, Herzog V, Wirtz A, Riethmuller G. Establishment of a human cell line (Mono Mac 6) with characteristics of mature monocytes. Int J Cancer. 1988;41:456–461. doi: 10.1002/ijc.2910410324. [DOI] [PubMed] [Google Scholar]