Abstract
Chlorophyll b is synthesized from chlorophyllide a, catalyzed by chlorophyllide a oxygenase (CAO). To examine whether reduced chlorophyll b content regulates chlorophyll (Chl) synthesis and photosynthesis, we raised CAO transgenic tobacco plants with antisense CAO expression, which had lower chlorophyll b content and, thus, higher Chl a/b ratio. Further, these plants had (i) lower chlorophyll b and total Chl content, whether they were grown under low or high light; (ii) decreased steady-state levels of chlorophyll biosynthetic intermediates, due, perhaps, to a feedback-controlled reduction in enzyme expressions/activities; (iii) reduced electron transport rates in their intact leaves, and reduced Photosystem (PS) I, PS II and whole chain electron transport activities in their isolated thylakoids; (iv) decreased carbon assimilation in plants grown under low or high light. We suggest that reduced synthesis of chlorophyll b by antisense expression of CAO, acting at the end of Chl biosynthesis pathway, downregulates the chlorophyll b biosynthesis, resulting in decreased Chl b, total chlorophylls and increased Chl a/b. We have previously shown that the controlled up-regulation of chlorophyll b biosynthesis and decreased Chl a/b ratio by over expression of CAO enhance the rates of electron transport and CO2 assimilation in tobacco. Conversely, our data, presented here, demonstrate that-antisense expression of CAO in tobacco, which decreases Chl b biosynthesis and increases Chl a/b ratio, leads to reduced photosynthetic electron transport and carbon assimilation rates, both under low and high light. We conclude that Chl b modulates photosynthesis; its controlled down regulation/ up regulation decreases/ increases light-harvesting, rates of electron transport, and carbon assimilation.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12298-023-01395-5.
Keywords: Chlorophyll b, Chlorophyllide a oxygenase, Electron transport, Light intensity, Photosynthesis
Introduction
Chlorophylls (Chls) are essential for light harnessing in photosynthesis. Chl a is present in both photosynthetic reaction centers and light-harvesting complex proteins (LHCP) (Björn et al. 2009). Chl b is associated with LHCP that harvests and transfers light energy efficiently (~ 100%) to Chl a present in both Photosystem I (PS I) and PS II (Grossman et al. 1995; Shevela et al. 2019). Chl biosynthesis is an important metabolic process for the greening of plants as well as for photosynthesis; it is synthesized from a simple amino acid glutamate by several Chl biosynthetic enzymes (for reviews, see Tanaka and Tanaka 2006, 2007; Tripathy and Pattanayak 2012; Brzezowski et al. 2015). Chl biosynthesis pathway intermediates are known to act as photosensitizers (see e.g., Tripathy and Patttanayak 2010; Tripathy and Oelmüller 2012), and therefore, their synthesis in higher plants is tightly regulated (Mochizuki et al. 2010; Larkin 2016). The enzymes of the Chl synthesis pathway are regulated at transcriptional (Kobayashi and Masuda 2016), and posttranslational levels (Wang et al. 2020), as well as by the redox state of the cell (Richter et al. 2018). Chlorophyllide b is synthesized by the oxidation of a methyl group of Chlorophyllide a to formyl group by the enzyme chlorophyllide a oxygenase (CAO) (Von Wettstein et al. 1995; Tanaka et al. 1998; Oster et al. 2000). The chloropyllide a and chloropyllide b are esterified with the phytol by chlorophyll synthase to form Chl a and Chl b respectively.
Light intensity strongly modulates the gene expression of CAO (Masuda et al. 2003; Harper et al. 2004; Pattanayak et al. 2005; Tanaka and Tanaka 2005). Interestingly, the CAO enzyme is also regulated by its product i.e., Chl b (Yamasato et al. 2005; Nakagawara et al. 2007; Sakuraba et al. 2009). Excess Chl b production is bad for the plants; it makes the plant susceptible to photodamage immediately after etiolation (Yamasato et al. 2008), or when grown under high light (HL) (Hirashima et al. 2006).
Chlorophyll b is essential for the formation and stabilization of light harvesting Chl protein (LHCP) complex in the thylakoid membranes (Thornber and Highkin 1974; Bellemare et al. 1982). In tobacco, Biswal et al. (2012) have shown that the controlled up-regulation of Chl b biosynthesis and the decreased Chl a/b ratio, by overexpression of intact AtCAO, enhance not only the expression of light-harvesting complex, but the electron transport chain components of PSII (OEC33, D1 and D2), PSI components, (psaG, psaF, and psaE), and the intersystem electron transport components of cytochrome b/f complex, as well as the rate of CO2 assimilation.
The amount of LHC proteins decreases sharply in Chl b lacking mutants (Murray and Kohorn 1991; Król et al. 1995), partly due to proteases degrading the unbound LHC apoproteins (Hoober and Eggink 2001). Similarly, Chl b-lacking (ch11) rice plants are known to downregulate two (Osa_107276047 and Osa_4342395) PsbR (chloroplast photosystem II subunit R) protein genes, resulting in reduced photosynthetic capacity (Nguyen et al. 2020). The Chl b mutants, lacking Chl b, or being deficient (i.e., containing reduced levels) in Chl b, have highly reduced photosynthesis, and biomass that often affect plant survival (Thornber and Highkin 1974; Murray and Kohorn 1991; Harrison et al. 1993; Kim et al. 2009; Dall’Osto et al. 2010; Ramel et al. 2013). The leaves of Chl b-less Arabidopsis mutant (chlorina1 [ch1]) are devoid of PSII Chl-protein antenna complexes and have a very low capacity of nonphotochemical quenching (NPQ) of Chl fluorescence (Havaux et al. 2007; Ramel et al. 2013). Furthermore, the divinyl protochlorophyllide 8-vinyl reductase (pcb2; pale-green and chlorophyll b reduced 2) mutant lines of Arabidopsis have an increased divinyl Chl a, reduced chlorophyll b and a decreased number of grana stacks (Nakanishi et al. 2005). Similarly, in Chl b less (chlorina1 [ch1]) mutant, there is oxidative stress induced by singlet oxygen production, leading to poor growth (Ramel et al. 2013; Voitsekhovskaja and Tyutereva 2015). Conversely, over expression of CAO with A domain removed or Prochlorothrix hollandica CAO having no A domain leads to excessive synthesis of Chl b increased oxidative stress, decreased photosynthesis and reduced biomass (Nagata et al. 2004). The synthesis of excess Chl b results in Chl a/b ratio less than or equal to 1. Consequently, the Chl b is incorporated into the PSI/PSII core complex, leading to the inactivation of photosynthetic electron transport and the generation of reactive oxygen species (ROS) (Sakuraba et al. 2010).
From these studies, it is evident that either too much or too little of Chl b is deleterious to plants. However, very little information is available about the impact of reduced expression of CAO (so that transgenic plants have less Chl b) on the metabolic control of the entire Chl biosynthesis pathway and photosynthesis.
Thus, in the present study, we have reduced Chl b synthesis, in tobacco plants, by antisense CAO expression, and then measured its impact on the metabolic flux of the Chl biosynthetic pathway, as well on photosynthesis in these CAO antisense (CAOas) tobacco plants grown under low and high light intensities. We demonstrate that downregulation of CAO expression (in CAOas plants) results in lower Chl b content, higher Chl a/b ratio, decreased synthesis of Chl intermediate metabolites and total Chl content, which is, probably, due to a feedback-controlled reduction in the expression/ activities of several enzymes involved in chlorophyll biosynthesis. Further, the photosynthetic potential of CAOas plants decreased due to their reduced Chl b content. Data presented in this study, along with our previous results (Biswal et al. 2012), show that amount of Chl b indeed modulates photosynthesis; its optimal upregulation increases, and its downregulation decreases Chl biosynthesis intermediates and Chl content, light-harvesting capacity, rates of electron transport and, above all, carbon assimilation.
Materials and methods
Plant growth conditions
Tobacco (Nicotiana tabacum cv. Petit Havana) plants were grown in a greenhouse under natural photoperiod, as described by Biswal et al. (2012). Plants were grown under light intensity of 300 μmol photons m−2 s−1 at 22 °C ± 2 °C for 30 days. These plants were then transferred either to low light (LL) (70–80 μmol photons m−2 s−1) or to high light (HL) (700–800 μmol photons m−2 s−1) for 4 more weeks.
Construction of plasmid and plant transformation
AtCAO cDNA (1611 bp) fragment was amplified from A. thaliana ecotype Columbia cDNA, using a pair of primers: 5’-gc gaa ttc atg aac gcc gcc gtg ttt ag-3’ and 5’-gc gaa ttc tta gcc gga gaa agg tag-3’. EcoRI restriction sites were introduced into both the primers (underlined). The amplified cDNA fragment was ligated into pGEMT-Easy. Subsequently, the EcoRI digested CAO cDNA fragment was excised from cloned pGEMT-Easy and inserted into the modified pCAMBIA 2301 (Pattanayak et al. 2005) in an antisense orientation under the control of the constitutive cauliflower mosaic virus (CaMV) 35S promoter. The modified pCAMBIA 2301contained npt II as a selection marker (Fig. 1A). The antisense orientation of AtCAO (CAOas) in pCAMBIA 2301 plasmid was verified through PCR, restriction digestion, and DNA sequencing methods.
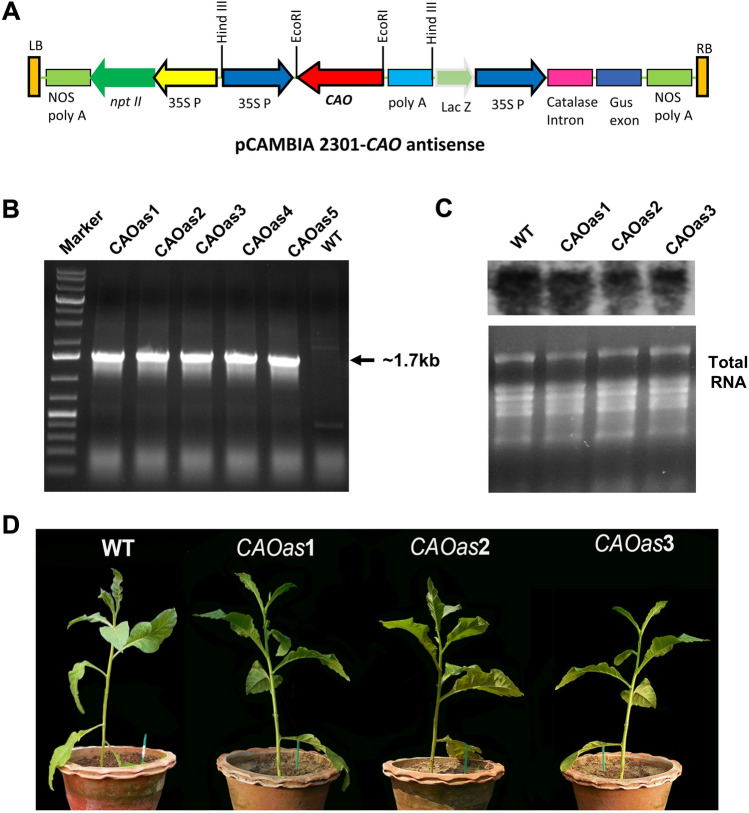
Fig. 1.
Generation of CAO antisense (CAOas) plants in tobacco (Nicotiana tabacum). A Schematic representation of the construct used for the antisense expression of AtCAO in the tobacco plant. B PCR analysis revealed the integration of CaMV35S-AtCAO into tobacco plant’s genome. A likely, 1.7 kb DNA fragment, was observed in all the 5 transgenics used (listed above the data), when the PCR was done using 35S-promoter internal forward primer and AtCAO forward primer. Further, as expected, 1.7 kb band was absent in the wildtype (WT) genomic DNA. C Northern blot analysis of CAO of WT and CAOas tobacco plants (CAOas1, CAOas2, CAOas3) grown under 300 µmol photons m−2 s−1 light (natural sun light + metal halide lamps) for 30 days. D Phenotype of WT and CAOas plants grown for 30 days under 300 μmol photons m−2 s−1light
The LBA4404 strain of Agrobacterium tumefaciens, carrying the gene-construct p35S: AtCAOas: nos poly (A) was used to transform tobacco; three independent transformed lines, i.e., the CAOas1, CAOas2 and CAOas3, were selected for further studies as described earlier (Biswal et al. 2012).
A number of primary transformants were selected on kanamycin plates (30 mg/L), and allowed to grow to the flowering stage and then to the seed stage. In the T1 generation, genomic DNA was extracted, and the antisense orientation of AtCAO was confirmed by PCR using genomic DNA from the T1 plants, and the internal forward primers for the 35S CaMV promoter (5’-ccc act atc ctt cgc aag ac-3’), as well as the AtCAO cDNA (5’-gc gaa ttc atg aac gcc gcc gtg ttt ag-3’). The stably transformed T3 generation plants, derived from the CAOas primary transformants, were used in the subsequent studies.
Northern blot analysis
Northern blot analysis was done as described by Pattanayak et al. (2005).
Chlorophyll, chlorophyll biosynthesis intermediates and protein content
The chlorophyll content was measured in 90% acetone, as described by Porra et al. (1989). Leaves were homogenised in 90% ammonical acetone (90 ml acetone: 10 ml 0.1 N NH4OH), and the homogenate was centrifuged at 4ºC for 10 min. The pellet was white; the supernatant was used for the estimation of Chl and other Chl biosynthesis intermediates. The fluorescence emission measurements (excited by 400 nm (E400), 420 nm (E420) and 440 nm (E440)) were used for the estimation of Chl biosynthesis intermediates, i.e., protoporphyrin (Proto) IX, Mg-protoporphyrin IX monoester (MPE) and protochlorophyllide (Pchlide), as described earlier (Hukmani and Tripathy 1992; Pattanayak and Tripathy 2002). Further, The Bradford assay was used for the estimation of protein content of the leaves and of the thylakoid membranes (Bradford 1976). The estimation of Chl was performed with seven biological replicates, and the measurement of Chl biosynthesis intermediates were performed with four biological replicates.
Estimation of 5-aminolevulinic acid (ALA) and glutamate-1-semialdehyde (GSA)
ALA was estimated from leaf samples as described by Tewari and Tripathy (1998). Leaves (200 mg) were incubated in 50 mM levulinic acid, dissolved in 50 mM MES, pH 7.0, either in dark or light (30 μmol photons m−2 s−1) for 6 h, then hand -homogenized under green safe light in 5 ml of ice cold 4% trichloroacetic acid, and the homogenate was centrifuged at 10,000 rpm for 10 min at 4 °C. The supernatant was used to estimate ALA, as described earlier (Tewari and Tripathy 1998). Results were expressed as net ALA synthesis in response to low light or high light (ALA synthesis in dark subtracted from ALA synthesis in light). Experiments were performed with four replicates.
For the estimation of GSA, leaves (50 mg) were incubated in the presence of GSA-AT inhibitor, 500 µM gabaculine dissolved in MES 0.1 M (pH 7.0), for 6 h under light. The leaf tissue was hand homogenized in 5.0 mL of cold HCl (0.1 N), and centrifuged at 10,000 rpm for 10 min at 4 °C. The supernatant was used to estimate GSA (extinction coefficient 16.9 mM−1; Kannagara and Schouboe 1985; Sood et al. 2005). These experiments were performed using four biological replicates.
Enzyme assays
5-Aminolevulinic acid dehydratase (ALAD)
Leaves (250 mg) were collected from WT and CAOas plants and hand homogenized in 5 ml of 0.1 M Tris–HCL (pH 7.6) and 0.01 M β-mercaptoethanol solution at 4 °C. The homogenate was centrifuged at 10,000 rpm for 10 min at 4 °C. The supernatant was taken and the activity of the ALAD enzyme was determined by measuring the amount of porphobilinogen (PBG) formed in 1.0 ml of reaction mixture, as described earlier (Shemin 1962; Tewari and Tripathy 1998). The incubation mixture consisted of 60 mM Tris, 0.2 mM ALA, 1 mM EDTA, 15 mM MgCl2, 0.5% BSA (w/v), and 0.33 M Sucrose, pH 7.5, and the extracts from the leaves. After 10 min of pre-incubation, the reaction was started by adding the substrate ALA, and the incubation was carried out for 1 h at 28 °C. The amount of PBG formed was measured at 553 nm, and calculated by using 6.2 × 104 M−1, as absorption coefficient (Bogorad 1962; Hukmani and Tripathy 1994). These experiments were performed using four biological replicates.
Porphobilinogen deaminase (PBGD)
The enzymatic activity of porphobilinogen deaminase (PBGD) was assayed as described by e.g., Bogorad (1962). Leaves (250 mg) of LL- and HL- grown WT and CAOas plants were collected and hand homogenized in 5 ml of 0.1 M Tris–HCL (pH 7.6) at 4 °C. The supernatant was taken for the assay and then the homogenate was centrifuged at 10,000 rpm for 10 min at 4 °C. The enzyme activity was assayed as the amount of porphyrin synthesized in 1.0 ml of reaction mixture, which contained 140 µg PBG, 3 mM EDTA, 100 mM Tris–HCL (pH 7.6) and 600 µl of the enzyme extract (Bogorad 1962; Hukmani and Tripathy 1994; Sood et al. 2005;). For calculation of the enzyme activity, an extinction coefficient of 5.48 × 105 M−1 cm−1 (at 405.5 nm) was used. These experiments were performed using four biological replicates.
Protoporphyrinogen oxidase, Mg-Chelatase and MPE Cyclase
For the estimation of these enzymes, we first isolated chloroplasts, as described below. Five grams of leaves from WT and CAOas plants were hand homogenized using 5 volumes of cold grinding buffer (pH 7.6) consisting of 50 mM HEPES, 0.33 M sorbitol, 1 mM MgCl2, 1 mM MnCl2, 2 mM Na2EDTA, 0.1% BSA, and 0.025% isoascorbate (Gupta and Tripathy 2010). The homogenate was filtered through two layers of cheesecloth, and the resulting filtrate underwent centrifugation at 4000 rpm for 7 min at 4 °C. After discarding the supernatant, the pellet was suspended in 2 mL of grinding buffer using a paintbrush. Subsequently, the suspended pellets were added to 50% Percoll solution, and the contents were mixed gently by inverting the tubes, which was sealed with parafilm. The material was centrifuged at 15,000 rpm for 15 min. After centrifugation, the lower green band, comprising intact chloroplasts, was carefully collected, washed with wash buffer (grinding buffer without bovine serum albumin) and re-suspended in the same buffer. These experiments were performed using four biological replicates.
Protoporphyrinogen oxidase (Proto IX) assay was then performed as described by Jacobs et al. (1989) and Tewari and Tripathy (1998). The intact chloroplasts isolated from leaves of LL- and HL-grown WT and CAOas plants were lysed in a buffer containing 10 mM Tris–HCl (pH 7.7), 20 mM MgCl2 and 2.5 mM Na2EDTA. These lysed plastids were centrifuged at 5,000 rpm for 3 min at 4 °C and the supernatant was used for the enzyme assay. To do this, the following components were added to the supernatant (containing 100 μg chloroplastic protein): 6 mM ATP, 5 mM DTT, and 55 µl of protogen. Proto IX was estimated by spectrofluorometry as described earlier (Hukmani and Tripathy 1992; Tewari and Tripathy 1998). The enzyme, inactivated by heat, was obtained by keeping the supernatant in boiling water bath for 10 min, which was used as a control (blank). These experiments were performed using four biological replicates.
For Mg-Chelatase assay, intact chloroplasts were suspended, at room temperature, in a buffer containing 0.5 M sucrose, 0.2 M Tris–HCL (pH 7.7), 20 mM MgCl2, 2.5 mM Na2EDTA, 20 mM ATP, 20 mM NAD and 8 mM methanol. The reaction was started by the addition of 1.5 μM ProtoIX to the reaction mixture, which was then left for 1 h at 28°C in the dark, while the sample was continuously shaken, and finally the reaction was stopped by adding 1.7 ml of ice-cold acetone. The samples were extracted with hexane and the hexane extracted acetone solvent mixture (HEAR), at the bottom, was used to estimate MPE by a spectrofluorometer (Hukmani and Tripathy 1992). These experiments were performed using four biological replicates.
For MPE Cyclase, intact chloroplasts were suspended in a suspension buffer containing 0.5 M sucrose, 0.2 M Tris–HCl (pH 7.7), 20 mM MgCl2, 2.5 mM Na2EDTA and 20 mM ATP. The reaction mixture consisted of 100 uL of this chloroplast suspension, 100 uL of additional suspension buffer without chloroplasts, and 1.5 μM MPE. The incubation took place at room temperature in the dark for 1 h. Subsequently, 1.7 ml of ice-cold 90% ammonical acetone was introduced to halt the reaction. The hexane-extracted acetone residue (HEAR) was prepared from the acetone extract, and the quantity of Pchlide formed was assessed using spectrofluorimetry (Hukmani and Tripathy 1992). These experiments were performed using four biological replicates.
Protochlorophyllide oxidoreductase (POR) assay
Plants were initially kept in dark for 6 h and then exposed to cool white fluorescent light (80 μmol photons m−2 s−1) for 15 min to measure the photo-transformation of Pchlide (POR activity). From the 6-h dark-incubated and 6-h dark + 15 min light-illuminated WT and CAOas plants, 50 mg of leaf tissues were selected, and their Pchlide contents were monitored using the methods previously described by Pattanayak and Tripathy (2002, 2011). The percent of phototransformation of Pchlide to Chlide in LL-and HL-WT and CAOas samples was calculated as described by Pattanayak and Tripathy (2011):[(Pchlide content before phototransformation)—(Pchlide content after phototransformation)] /(Pchlide content before phototransformation) × 100]. These experiments were performed using four biological replicates.
Electron transport assay
Thylakoid membranes were extracted in a buffer containing 0.01 M Tris and 1 mM EDTA (pH 7.5). The homogenate was centrifuged at 5000 g for 5 min, and the resulting pellet, which contained thylakoid membranes, was resuspended in a buffer composed of 0.4 M sorbitol, 0.05 M Tris (pH 7.5), 1 mM MgCl 2, and 1 mM EDTA (Mohapatra and Tripathy 2003; Gupta and Tripathy 2010). Assays to measure electron transport activity of whole chain, PSII and PSI were conducted using thylakoid membranes isolated from plants. These assays were carried out in a glass cuvette fitted within a Clark-type oxygen electrode from Hansatech, UK, as described earlier (Jilani et al. 1996; Chakraborty and Tripathy 1992a, 1992b; Kandoi et al. 2022). The reactions were maintained at 25ºC using a temperature controlled water bath, and the samples were illuminated for 20 s with tungsten light (1500 µmol photons m−2 s−1).
The whole chain electron transport, from water to methylviologen (MV) (1 mM), was monitored as O2 uptake. The assay mixture (3 ml) consisted of 50 mM HEPES (pH 7.5), 10 mM NaCl, 1 mM NH4Cl, 3 mM MgCl2, 1.0 mM sodium azide (NaN3), and 0.5 mM methyl viologen and 50 µg chlorophyll. PSII activity was observed as O2 evolution using the H2O to p-phenylenediamine (PD) system. The reaction mixture (3 mL) for PD supported O2 evolution assay consisted of 50 mm HEPES (pH 7.3) buffer, 3 mM MgCl2, 10 mM NaCl, and freshly prepared PD (0.5 mM). The partial electron transport chain through PSI was assessed by monitoring oxygen consumption using ascorbate (1 mM)/ DCIP (0.1 mM) as an electron donor, along with MV (1 mM), 1.0 mM sodium azide (NaN3). Electron flow from PSII was inhibited by adding 20 µM of 3-(3, 4-dichlorophenyl) 1, 1-dimethyl urea (DCMU). These experiments were conducted with five biological replicates.
Chlorophyll a fluorescence
Chl a fluorescence in the dark-adapted leaves was measured by a PAM-2100 (Walz, Germany) fluorometer, at room temperature, following the protocol as described by Genty et al. (1989). For a comprehensive understanding of Chl a fluorescence and its relation to photosynthesis (including definition and use of ∆F/Fm’, mentioned below), see Govindjee (1995, 2005), Schreiber et al. (1995), and Baker (2008). Before fluorescence measurement, the leaves were dark-adapted for 20 min. The effective PSII quantum yield (фPSII) was calculated according to Genty et al. (1989) by the formula фPSII = (Fm′ − Ft) / Fm′, where Fm′ is defined as the maximal fluorescence yield in a pulse of saturating light, when the sample is pre-illuminated and Ft represents the measured fluorescence yield at any given time (t). The relative electron transport rate (ETR), expressed as μmol electrons m−2 s−1, was estimated as [(∆F/Fm’) × 0.5 × 0.84 x PAR], where ∆F/Fm’ = (Fm’-F) / Fm’, and photosynthetically active radiation (PAR) was measured in μmol photons m−2 s−1. The factor 0.5 represents our estimate that the light incident on the leaf surface is absorbed equally by PSII and PSI, whereas, the factor 0.84 is the fractional absorption of solar energy by leaves (Dutta et al. 2009; Kandoi et al. 2018). Non-photochemical quenching (NPQ) of the excited state of Chl a was calculated from the formula: NPQ = (Fm − Fm′) / Fm′ (Schreiber 2004). These experiments were performed using five biological replicates.
Carbon assimilation
Photosynthetic gas exchange was measured using an infrared gas analyzer (IRGA), LI-COR 6400-XT portable photosynthesis system, as described by Kandoi et al. (2016). In the leaf chamber, CO2 concentration was maintained at 380 µL L−1 with a flow rate of 400 µmol s−1 and air temperature at 25 °C. Before monitoring CO2 assimilation, both WT and CAOas leaves were exposed to 700 µmol photons m−2 s−1 (HL-grown plants) or to 200 µmol photons m−2 s−1 (LL-grown plants), for 20 min. The rates of CO2 uptake of the attached leaves of both the WT and CAOas plants were monitored using IRGA, at limiting (80 µmoles photons m−2 s−1), intermediate (400 µmoles photons m−2 s−1) and near-saturating light intensity (800 µmoles photons m−2 s−1). These experiments were performed using five biological replicates.
Statistical analysis
For statistical analysis, using appropriate data, one-way/two- way ANOVA along with Dunnett’s post hoc test were used to determine statistical significance (P < 0.05; P < 0.01) (Kandoi et al. 2022). Statistically significant differences have been calculated between WT grown in LL and CAOas grown in LL, as well as between WT grown in HL and CAOas grown in HL.
Results
Generation of transgenic tobacco lines
To study the effects of down-regulation of CAO gene expression on Chl biosynthesis pathway, and on photosynthesis, AtCAO cDNA was introduced, in tobacco, in antisense orientation (Fig. 1A). In the T1generation, genomic DNA was extracted and the antisense orientation of AtCAO was confirmed by PCR that yielded a fragment of ~ 1.7 kb, suggesting that the transgene had been stably integrated into the host genome (Fig. 1B). We then checked the CAO expression in the transformed T3 generation homozygous antisense transgenic lines (CAOas1, CAOas2, CAOas3) by Northern blot analysis. All the three antisense lines showed reduced CAO expression (Fig. 1C). The CAOas3 was phenotypically slightly smaller in height than the WT under the growth conditions used (Fig. 1D).
Antisense expression of CAO altered the chlorophyll a/b ratio and the metabolic flux of chlorophyll biosynthesis pathway
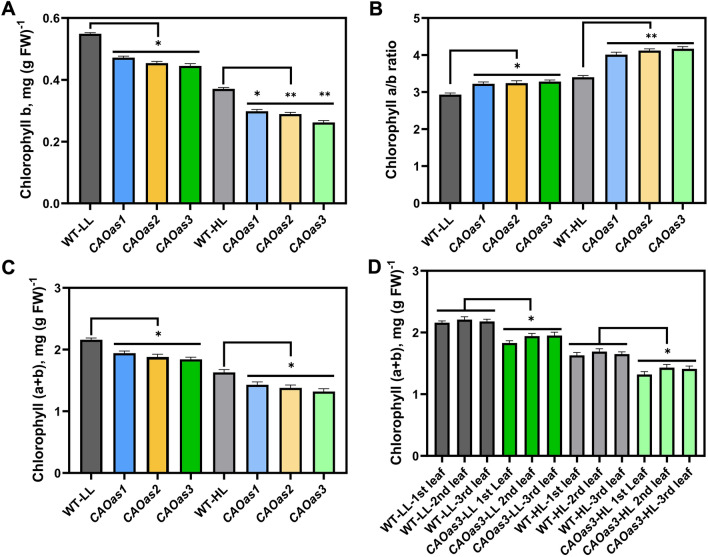
The downregulation of CAO, in CAOas plants, led to reduced accumulation of Chl b in all the three CAOas lines (CAOas1, CAOas2, CAOas3) grown in LL (14%-19%) or HL (20%-29%) regime (Fig. 2A). The Chl a content was higher in LL than in HL grown WT and transgenic plants. Further, the Chl a content did not decline significantly in CAOas plants in HL and LL (Fig. S1). Consequently, the Chl a/b ratio increased by 10% to 12% (in CAOas-LL) and 18 to 23% (in CAOas-HL) compared to WT-LL and WT-HL plants, respectively (Fig. 2B). The total Chl content in LL- and HL-grown CAOas plants (CAOas1, CAOas2, CAOas3) was lower by 10%-15% or 12%-19% than in the WT plants, grown in LL or HL (Fig. 2C). To maintain consistency in our experiments, we always selected the 1st leaf from the top of the plant. We also measured the total Chl content from the 1st, 2nd, and the 3rd leaf of the WT, and of all the 3 antisense lines. We observed a decreased Chl content of all the leaves in all the 3 transgenic lines (Fig. 2D).
Fig. 2.
Chlorophyll content of wild-type (WT) and CAO antisense (CAOas) tobacco plants grown under low-light (LL) and high-light (HL) in the greenhouse A chlorophyll b, B chlorophyll a/b ratio, C Total chlorophyll content, and, D Total chlorophyll content of the 2nd, 3rd and 4th leaf from the top, from WT and CAOas plants. Plants were grown for up to 30 days under light intensity of 300 μmol photons m−2 s−1; then, they were transferred to LL (70–80 μmol photons m−2 s−1) and HL (700–800 μmol photons m−2 s−1) for additional 4 weeks. Each data point is the average of seven replicates and error bars represent the ± SE; asterisks indicate significant differences determined by ANOVA-test along with Dunnett’s post hoc test compared to WT (*P < 0.05, **P < 0.01). FW: Fresh weight. Statistical tests were conducted between WT and mutant within the same treatment. Lines have been drawn between WT and transgenic lines to show statistical differences between them
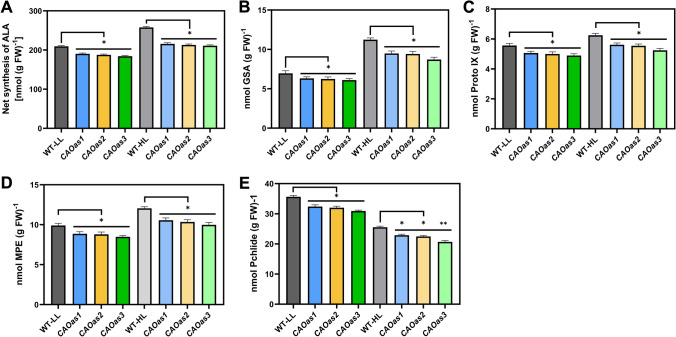
We then examined how the downregulation of CAO affected the metabolic intermediates of Chl biosynthesis pathway in the 3rd leaf of the WT and the transgenics. For this, we measured the steady state accumulation of the early precursors of Chl biosynthesis, i.e., glutamate semialdehyde (GSA) and 5-aminolevulinic acid (ALA) in LL as well as HL- grown WT and CAOas plants. The synthesis of ALA was reduced in different CAOas lines by 9%-12% and 16%-18% grown in LL and HL, respectively (Fig. 3A). As compared to WT, GSA was lower by 9–12% in CAOas-LL and by 16–22% in CAOas-HL (Fig. 3B).
Fig. 3.
Metabolites of chlorophyll biosynthesis pathway of WT and CAOas plants grown under LL and HL. The content of tetrapyrrole intermediates, as measured from WT and CAOas plants grown under LL and HL, was measured as described under Materials and Methods; also see the legend of Fig. 2. A ALA, B Glutamate semialdehyde (GSA), C protoporphyrin IX (Proto IX), D Mg-protoporphyrin IX monoester {MP(E)}, and E protochlorophyllide (Pchlide). Each data point is the average of four replicates, error bars represent the mean ± SE; asterisks indicate significant differences determined by ANOVA-test along with Dunnett’s post hoc test compared to WT (*P < 0.05, **P < 0.01). Statistical tests were conducted between WT and mutant within the same treatment
In addition to GSA, we measured the tetrapyrrole intermediates, i.e., protoporphyrin IX (Proto IX), Mg-protoporphyrin IX monomethyl ester MP(E) and protochlorophyllide (Pchlide) from both the WT and the CAOas plants. As compared to WT, Proto IX was reduced in CAOas3 plants by 9%–12% in LL, and 10%–16% in HL plants (Fig. 3C). Both MP(E) and Pchlide were also reduced by ~ 10–20% in the CAOas3 plants, grown under LL and HL-regimes (see Fig. 3D and E).
Modulation of enzymes involved in Chl biosynthesis in WT and CAOas plants, grown under LL or HL regime
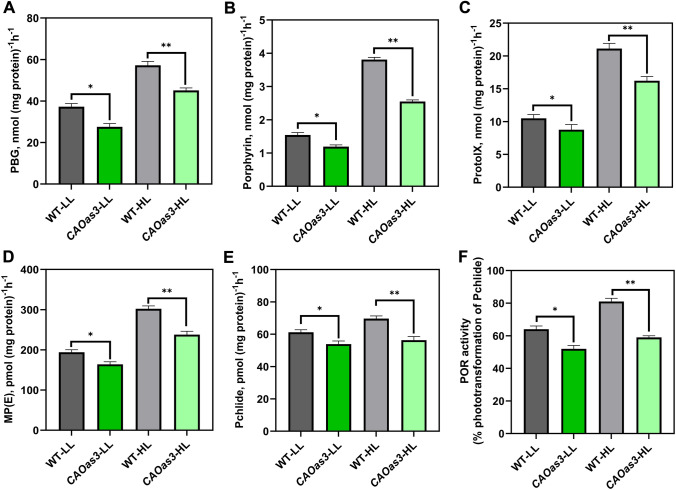
To examine the synthesis of tetrapyrroles in CAOas in plants, grown in LL and HL regimes, we measured (see Fig. 4A-F) the activities of Chl biosynthesis pathway enzymes (e.g., ALA dehydratase (ALAD), Porphobilinogen deaminase (PBGD), Protoporphyrinogen oxidase (PROTOX), Mg-Chelatase, Mg-protoporphyrin IX monoester (MPE) cyclase and Protochlorophyllide oxidoreductase, POR). Among the three, CAOas3 plants showed the maximum decrease in Chl b, total Chl content and amounts of Chl biosynthetic intermediates; therefore, we monitored Chl biosynthetic enzyme in these plants.
Fig. 4.
Enzyme activities of chlorophyll biosynthesis pathway of WT and CAOas plants grown under different light regimes. A ALA dehydratase, B PBG deaminase, C Protoporphyrinogen oxidase, D Mg-chelatase, E MP(E) cyclase, measured from both LL- and HL- grown WT and CAOas plants (see Material and Methods). Plastids from different plants were isolated, enzymatic assays were performed, and ProtoX, Mg-chelatase and MPE cyclase were expressed per mg protein per hour, F Protochlorophyllide oxidoreductase activity (POR) determined in dark. Both LL- and HL-grown WT and CAOas plants were incubated in dark for 8 h. Plants were exposed to light (100 μmol photons m−2 s−1) for 15 min after dark incubation, and their Pchlide concentration was measured, as well as phototransformation (%) of Pchlide to Chlide. Each data point is the average of four replicate, error bars represent the ± SE; asterisks indicate significant differences determined by ANOVA-test along with Dunnett’s post hoc test compared to WT (*P < 0.05, **P < 0.01). Statistical tests were conducted between WT and mutant within the same treatment
ALA dehydratase activity was 27% and 21% lower in CAOas-LL and CAOas-HL than in WT-LL and WT-HL- plants (Fig. 4A). Further, the next enzyme of the Chl biosynthesis pathway, porphobilinogen deaminase (PBDG), was also reduced by 33% and 22% in CAOas plants grown in HL and LL regimes. The activity of protoporphyrinogen oxidase (protox), that converts protoporphyrinogen IX to protoporphyrin IX, also decreased by 16% to 23% in the CAOas plants, as compared to the WT plants (Fig. 4B-C). Further, when we checked the enzymatic activities of the Mg-branch of tetrapyrrole biosynthesis, which leads to Chl synthesis, we observed that the activity of Mg-chelatase, which inserts Mg on protoporphyrin IX to produce Mg-protoporphyrin IX, decreased by 15% and 21% in LL- and HL-grown transgenic plants (Fig. 4D). In addition, the Mg-protoporphyrin IX monoester (MPE) cyclase, which produces Pchlide, was reduced by 12% and 19% in LL and HL plants (Fig. 4E).
When we estimated the enzymatic function of protochlorophyllide oxidoreductase (POR), we found that its activity increased in response to growth in HL, both in WT and CAOas plants compared to plants grown in LL. However, the POR activity decreased by 19% and 27%, respectively, under LL and HL conditions in CAOas plants compared to WT (Fig. 4F).
Photosynthetic responses of CAOas plants grown under LL or HL regime
To check if decreased Chl b content and a higher Chl a/b ratio modulates the function of photosynthetic apparatus, both the primary processes of photosynthesis and carbon assimilation were monitored in WT and CAOas plants.
Chl a fluorescence was monitored as a non-invasive signature of photosynthesis (Govindjee 1995; Nellaepalli et al. 2012). The light response curve of PSII-supported electron transport rate (ETR; µmol electrons m−2 s−1) of intact leaves, measured in LL-grown plants, saturated at ~ 550 µmol photons m−2 s−1, whereas plants grown under HL regime attained a maximum rate at ~ 800 µmol photons m−2 s−1 (Fig. 5). The relative ETR in limiting as well as saturating light intensities was lower, as compared to the WT, in all the 3 transgenic lines of CAOas plants grown both in LL- and HL-regimes. At saturating light intensity, the relative ETR of LL- and HL-grown CAOas3 plants was reduced by 20%–25% (Fig. 5). Although, we used a factor 0.5 for the calculation of ETR, assuming equal distribution of absorbed light between PSI and PSII, this factor could have been changed in our actual study where the Chl b content decreased in CAOas plants, resulting in a changed absorption ratio of PSII and PSI. Therefore, we have used relative ETR for measurements in intact leaves.
Fig. 5.
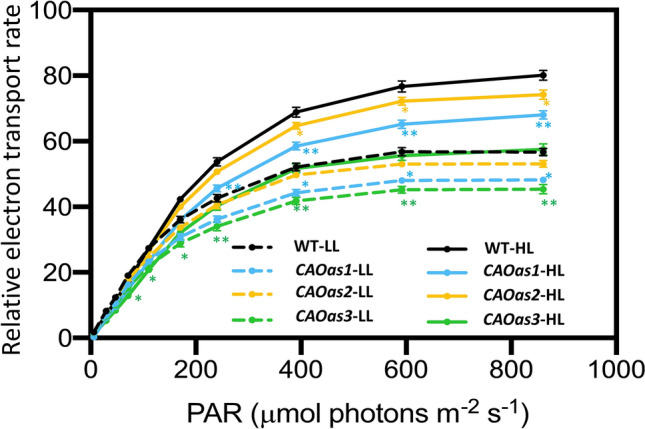
Electron transport rate of WT and CAOas plants. LL- and HL-grown WT and CAOas plants were dark adapted for 20 min before measurements were made, using PAM 2100 fluorometer. ETR was estimated from data obtained by this fluorometer at different light intensities (up to 900 µmol photons m−2 s−1) as described in the methods. Each data point is the average of five replicates; error bars represent the ± SE; asterisks indicate significant differences determined by ANOVA-test along with Dunnett’s post hoc test compared to WT (*P < 0.05, **P < 0.01). Statistical tests were conducted between WT and mutant within the same treatment
The quantum yield of PSII (ΦPSII) decreased in response to increasing light intensity in both HL- and LL- grown WT and CAOas plants. In LL-grown WT plants, the effective фPSII at limiting light intensity was slightly higher than in HL-grown WT plants (Fig. S2). The effective фPSII of LL- and HL-grown CAOas plants was lower than in the WT under both limiting and saturating light intensities. Furthermore, the LL- grown CAOas plants had lower effective фPSII than HL-grown CAOas plants under limiting and saturating light intensities (Fig. S2).
The non-photochemical quenching (NPQ) of Chl a fluorescence increased in response to increasing light intensity both in HL and LL grown WT and CAOas. However, the NPQ of Chl a fluorescence was higher in CAOas plants than WT grown in HL and LL regimes (Fig. S3). [ Note that both the ΦPSII yield and NPQ were calculated from the fluorescence data as described in the Material and Methods.]
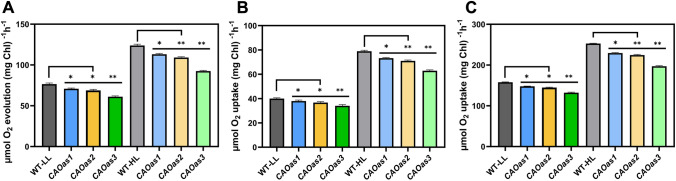
Whole chain, PSII and PSI reactions of LL- and HL-grown WT and CAOas plants
To further check the effect of reduced Chl b content, coupled with higher Chl a/b ratio, on photosynthetic electron transport chain, we measured the light-saturated whole chain electron transport (H2O MV, methyl viologen), the partial reaction of PSII (H2O PD, phenylenediamine) and that of PSI (ascorbate/DCIP, dichlorophenolindophenol MV) in the thylakoid membranes, isolated from chloroplasts of LL- and HL-grown WT and CAOas plants. The light-saturated whole chain electron transport and partial reactions of PSI, and PSII, measured from LL-grown WT plants, were lower than that in HL-grown WT plants (Fig. 5). All the 3 transgenic antisense lines had lower PSII, PSI and whole chain electron transport rates than the WT, expressed on an equal Chl basis; the % decrease of activities at saturating light intensity was maximum for CAOas3 lines. At saturating light, and for plants grown under LL, CAOas-3 plants, as compared to WT, had lower (16–18%) PSII, PSI and whole chain electron activities, whereas for plants grown under HL, these activities were much lower (20–25%; see Fig. 6A, B and C).
Fig. 6.
Electron transport reactions of thylakoid membranes isolated from wild-type and CAOas plants. A Electron transport through PSII (oxygen evolution; water to PD); B whole chain (water to MV; oxygen uptake); and C PSI (ascorbate to MV; oxygen uptake). The oxygen evolution/uptake was measured polarographically, using a Hansatech oxygen electrode. Each data point is the average of five replicates, error bars represent the mean ± SE; asterisks indicate significant differences determined by ANOVA-test along with Dunnett’s post hoc test compared to WT (*P < 0.05, **P < 0.01). Statistical tests were conducted between WT and mutant within the same treatment
Carbon assimilation
To assess whether the decreased electron transport rates, which results in lower reducing power in the form of NADPH, as well as ATP, for CO2 reduction, indeed lead to reduced net carbon assimilation in the antisense plants, we examined the rates of CO2 uptake in intact leaves. The measurement was conducted at ambient level CO2, using IRGA under three different light intensities: limiting (80 µmoles photons m−2 s−1), partially saturating (400 µmoles photons m−2 s−1) and almost saturating (800 µmol photons m−2 s−1). Among all the 3 antisense lines in CAOas3 plants, photosynthetic CO2 assimilation rates at all the three light intensities were reduced by 15% and 20% in LL- and HL-growth regimes (Fig. 7).
Fig. 7.
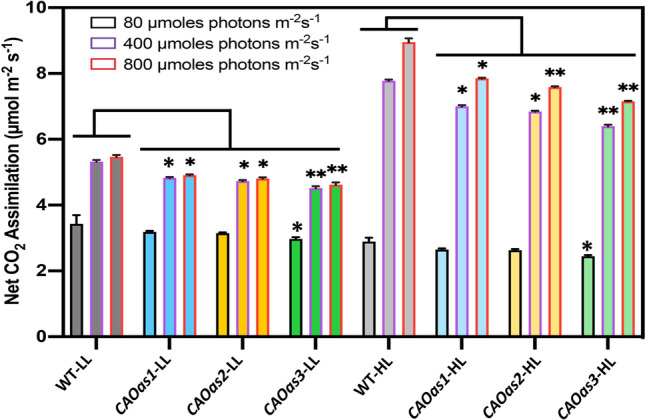
Net CO2 assimilation rates of the attached leaves of WT and CAOas plants grown in LL and HL conditions. Net CO2 assimilation rates were monitored with an infrared gas analyzer (Licor 6400-XT portable photosynthetic system) at ambient CO2 at different light intensities (80, 400, 800 µmol photons m−2 s−1). These experiments were done three times, error bars represent the ± SE; asterisks indicate significant differences determined by ANOVA-test along with Dunnett’s post hoc test compared to WT (*P < 0.05, **P < 0.01). Statistical tests were conducted between WT and mutant within the same treatment.
Discussion
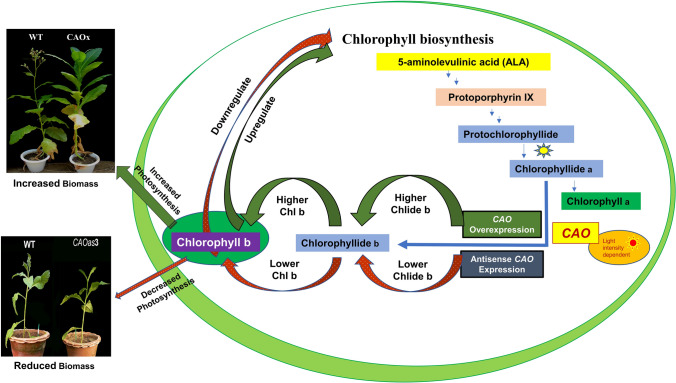
In the present study, we demonstrated that antisense expression of CAO in tobacco results in decreased Chl b, and consequently higher Chl a/b ratio in all the 3 antisense lines (Fig. 2). We then attempted to ascertain if the down regulation of CAO expression, that resulted in reduced Chl b content, affected the steady state levels of Chl metabolic intermediates (see Fig. 8). Our results indeed demonstrate that reduced CAO expression decreases activities of both the early enzymes, i.e., ALA dehydratase (ALAD), Porphobilinogen deaminase (PBGD), Protoporphyrinogen oxidase (PROTOX), and the late enzymes, i.e., Mg-Chelatase, Mg-protoporphyrin IX monoester (MPE) cyclase and protochlorophyllide oxidoreductase (POR) of Chl biosynthesis pathway (Fig. 4). This leads to reduced synthesis of Chl biosynthesis intermediates. Similarly, the rice Chl b-deficient mutant (chlorina 11, ch11), having very low amounts of Chl b, is shown to down-regulate the POR gene expression in the Chl biosynthesis pathway, although it does not affect an early enzyme of Chl biosynthesis (Nguyen et al. 2021). Conversely, The Chl b mutant (Chlorina 1, ch1), which completely lacks Chl b, has reduced expression of the early enzyme of Chl biosynthesis, i.e., glutamyl-tRNA reductase, GluTR, without affecting the gene expression of the late enzyme POR (Nguyen et al. 2020). The gene expression of certain other enzymes in the Chl biosynthesis pathway is upregulated or not significantly affected. This results in reduced synthesis of Chl a and/or Chl b (Nguyen et al. 2021). In our study, where CAO expression is partially reduced, it affects most of the enzymatic activity of the Chl biosynthetic pathway, resulting in reduced synthesis of Chl. The inconsistency between gene expression reported in Chl b mutants (Nguyen et al. 2021) and our present study, where most of the enzymatic reactions of Chl biosynthesis pathway are down-regulated, may be due to their post-transcriptional regulation. This demonstrates that the reduced expression of CAO, that acts at the penultimate step of Chl biosynthesis to divert Chlide a for Chlide b synthesis, had indeed affected the metabolic flux of pyrroles and its derivatives in the Chl biosynthesis pathway by downregulating the Chl biosynthetic enzymes. This demonstrates the presence of regulatory network of Chl biosynthetic genes (Pattanayak and Tripathy 2011; Biswal et al. 2012; Wang and Grimm 2021).
Fig. 8.
Schematic diagram demonstrating the central role of Chlorophyll b in the upregulation and the downregulation of photosynthesis and on the biomass of the plants
In an earlier study, we found that overexpression of CAO results in increased content of Chl b, Chl a and total Chl (Biswal et al. 2012). This increase was due to upregulation of gene expression, higher protein abundance, and increased activities of most of the enzymes involved in Chl biosynthesis, in both LL- and HL-grown plants. Furthermore, due to higher Chl b synthesis, the CAO overexpressors had lower Chl a/b ratio than the WT. Our present results demonstrate that increase or decrease of Chl b synthesis modulates Chl biosynthetic enzymes leading to increases or decreases in the Chl content of plants. Chl biosynthetic pathway intermediates are photodynamic in nature (Rebeiz et al. 1990). If the pathway of Chl synthesis is impaired or blocked due to genetic mutation or to environmental stresses, it is expected to result in the accumulation of porphyrin intermediates that are toxic to plants (Rebeiz et al. 1990; Tripathy and Chakraborty 1991; Chakraborty and Tripathy 1992a; Mochizuki et al. 2010; Ambastha et al. 2020). Therefore, we suggest that the reduced expression of CAO may attenuate ALA synthesis to prevent the over-accumulation of photodynamic tetrapyrroles of the pathway that have the potential to generate singlet oxygen (1O2) (Chakraborty and Tripathy 1992a; Tripathy et al. 2007; Tripathy and Pattanayak 2010; Pattanayak and Tripathy 2011; op den Camp et al. 2013). Chl b -less mutants, upon transfer to HL, have been shown to be photo-damaged and to generate high amounts of singlet oxygen as well as MDA due to the absence of antenna located protection mechanisms (Ramel et al. 2013; Voitsekhovskaja and Tyutereva 2015). Similarly, excess Chl b synthesis is known to lead to uncontrolled light absorption, damaging cells when kept under high light (Yamasato et al. 2008). Unlike other Chl b-less mutant studies, where there was no Chl b, the CAOas plants that were used in this study had significant amount(s) of Chl b; and there was only a small reduction in Chl b biosynthesis and therefore, there was no visible photo-damage to plants under high light.
Sun and shade plants respond differently to varying light intensities (LL and HL). Shade plants exhibit a reduced Chl a/b ratio, higher levels of LHCP II, resulting in a larger antenna size of PSII (Anderson et al. 1988; Melis 1991; Zivcak et al. 2014). Conversely, HL-grown plants display an increased Chl a/b ratio, elevated PSII, ATP synthase, and Rubisco levels, but reduced chlorophyll and LHC contents (Leong and Anderson 1984; Bailey et al. 2001; Tanaka and Tanaka 2005). Our previous study (Biswal et al. 2012) has shown that CAO overexpression results in greener leaves due to the accumulation of higher amounts of Chl; however, the increase in Chl content in CAO overexpressers was higher than that in the WT in both LL and HL regimes. Conversely, our present study demonstrates that downregulation of CAO through antisense expression results in reduced Chl accumulation both in LL and HL grown plants. However, the total Chl content in CAOas plant was also lower in HL as compared to that in LL. These demonstrate the presence of a regulatory network of Chl biosynthetic genes which is not suppressed either by CAO overexpression or antisense expression. Further, at higher light intensities, all the CAOas lines had reduced ETR compared to the WT plants. This reduction in ETR in CAOas lines may be attributed to decreases in abundance and the presence of non-functional PSII and higher NPQ.
Ort et al. (2015) purposed that photosynthetic potential of plants can be increased by reducing Chl content. In our study, the observed changes in PSII-dependent ETR, measured in intact leaves, demonstrate that the reduced CAO expression, with lower Chl b and higher Chl a/b ratio, led to diminished efficiency of energy capture and its utilization in all the 3 transgenic lines (Fig. 5). This was accompanied by a decrease in the light-saturated PSI, PSII and whole chain electron transport activities of thylakoid membranes isolated from LL- and HL-grown plants (Fig. 6). Conversely, plants overexpressing CAO had higher light saturated PSI and PSII reaction rates than the WT due to increased abundance of electron transport complexes, D1, D2, the oxygen-evolving complex protein OEC33, cytochrome b/f complex, cytochrome f and Rieske proteins (Biswal et al. 2012) and delayed senescence (Sakuraba et al. 2012).
The reduced amount of the NADPH and ATP due to lower ETR could have resulted in reduced carbon assimilation in all the 3 CAOas lines (Fig. 7). An increased expression of photosynthetic genes and proteins were observed in CAO over expressers (Biswal et al. 2012). The reduced photosynthetic carbon assimilation rates in all our 3 CAOas lines, under both limiting and saturating light intensities, agree with the results of Friedland et al. (2019) on 2 of 3 CAO RNAi lines of Camelina sativa in their research. We speculate that the difference in the results for the RNAi line 1 where they had obtained higher photosynthetic rate could be due to the use of different species, the growth light intensity or the degree of gene silencing. Conversely, the CAO overexpression, in tobacco, has been shown to have decreased Chl a/b ratio, increased Chl content and higher carbon assimilation rate per unit leaf area (Biswal et al. 2012). However, algae grow as a population and could attenuate light intensity due to larger light-harvesting antenna in deeper layers and consequently could have higher biomass when the light-harvesting antenna are reduced (Perrine et al. 2012; for other factors, see e.g., Maltsev et al. 2021). In our study of tobacco, the leaf thickness was not high enough to substantially attenuate light intensity. Thus, we suggest that this was the reason why we did not see the beneficial impact of reduced Chl b on photosynthesis of intact leaves of a stand-alone transgenic plant.
Our earlier data of CAO overexpression in tobacco having increased Chl b and total Chl has demonstrated an enhancement in photosynthesis and biomass production (Biswal et al. 2012). Similarly, an increase in Chl b and total Chl due to CAO overexpression in rice has been shown to not only increase the rate of photosynthesis but also the seed yield (Ping et al. 2023). Conversely, the present data demonstrate that a small decrease of Chl b amount, increased Chl a/b ratio accompanied by lower chlorophyll content, could diminish the rate of photosynthesis both in low light and high light. Therefore, we suggest that an increase in Chl b content, and larger antenna, could enhance photosynthetic efficiency of a stand-alone plant. However, this concept needs to be tested under field conditions to check if the reduced light intensity reaching the bottom of the crop canopy due to attenuation by increased light absorption by light-harvesting complex of the upper layers of the canopy would negatively impact crop productivity. It is well known that the increase in the leaf area index beyond a certain point could reduce crop productivity due to mutual shading (Tanaka and Kawano 1966; Li et al. 2014). Conversely, under field conditions, higher penetration of light through a crop canopy, having reduced Chl b and smaller light-harvesting antenna, could beneficially impact photosynthesis of the bottom leaves and increase plant productivity. Such an experiment could not, yet, be done as we did not have the official permission to grow transgenics in the field conditions. However, there is a need to test these concepts under field conditions to study the impact of increased or decreased Chl b on photosynthesis of crop canopy and biomass production.
Supplementary Information
Below is the link to the electronic supplementary material.
Fig. S1. Chlorophyll a content of wild-type (WT) and CAO antisense (CAOas) tobacco plants grown under low-light (LL) and high-light (HL) in the greenhouse. Plants were grown for up to 30 days under light intensity of 300 µmol photons m-2s-1; then, they were transferred to LL (70–80 µmol photons m-2s-1) and HL (700–800 µmol photons m-2s-1) for additional 4 weeks. Each data point is the average of seven replicates and error bars represent the mean ±SE; asterisks indicate significant differences determined by ANOVA-test along with Dunnett’s post hoc test compared to WT (*P < 0.05, **P < 0.01). Statistical tests were conducted between WT and mutant within the same treatment. Fig. S2. Effective quantum yield of PSII (φPSII) of WT and CAOas plants. LL- and HL-grown WT and CAOas plants were dark adapted for 20 min before measurements were made, using PAM 2100 fluorometer. фPSII was estimated from data obtained by this fluorometer at different light intensities (up to 900 µmol photons m-2s-1) as described in the methods. Each data point is the average of five replicates; error bars represent the ±SE; asterisks indicate significant differences determined by ANOVA-test along with Dunnett’s post hoc test compared to WT (*P < 0.05, **P < 0.01). Statistical tests were conducted between WT and mutant within the same treatment. Fig. S3. Non-photochemical quenching (NPQ) of WT and CAOas plants. LL- and HL-grown WT and CAOas plants were dark adapted for 20 min before measurements were made, using PAM 2100 fluorometer. NPQ of excited states of chlorophyll was estimated from data obtained by this fluorometer at different light intensities (up to 900 µmol photons m-2s-1) as described in the methods. Each data point is the average of five replicates; error bars represent the ±SE; asterisks indicate significant differences determined by ANOVA-test along with Dunnett’s post hoc test compared to WT (*P < 0.05, **P < 0.01). The experiment was done three times. Statistical tests were conducted between WT and mutant within the same treatment. (PPTX 68 kb)
Acknowledgements
This work was supported by J.C. Bose Fellowship grant from the Science and Engineering Research Board (SERB), Government of India to BCT.
Abbreviations
- ALA
5-Aminolevulinic acid
- ALAD
5-Aminolevulinic acid dehydratase
- cDNA
Complementary DNA
- CAO
Chlorophyllide a oxygenase
- CAOas
Chlorophyllide a oxygenase antisense
- CaMV
Cauliflower mosaic virus
- Chl
Chlorophyll
- Chlide
Chlorophyllide
- ETR
Electron Transport Rate
- GSA
Glutamate-1-semialdehyde
- IRGA
Infra-Red Gas Analyzer
- LHCP
Light harvesting Chl protein
- PAM
Pulse amplitude modulation
- PBGD
Porphobilinogen deaminase
- PCR
Polymerase chain reaction
- POR
Protochlorophyllide oxidoreductase
- Proto IX
Protoporphyrin IX
- PROTOX
Protoporphyrinogen oxidase
- ROS
Reactive oxygen species
- LL
Low light
- HL
High light
- MPE
Mg-protoporphyrin IX monoester
- WT
Wild type
Author contributions
AKB, GKP, KR, SSM and SL carried out experiments. AKB, GKP and BCT designed the experiments and analyzed the data. AKB, GKP, DK, GG, and BCT wrote the manuscript.
Declarations
Conflict of interest
The authors declare no competing financial and non-financial interests.
Footnotes
Submitted to the Special issue in honour of P.V. Sane.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ajaya K. Biswal and Gopal K. Pattanayak have contributed equally to this article.
References
- Ambastha V, Chauhan G, Tiwari BS, Tripathy BC. Execution of programmed cell death by singlet oxygen generated inside the chloroplasts of Arabidopsis thaliana. Protoplasma. 2020;257:841–851. doi: 10.1007/s00709-019-01467-y. [DOI] [PubMed] [Google Scholar]
- Anderson J, Chow W, Goodchild D. Thylakoid membrane organization in sun/shade acclimation. Aust J Plant Physiol. 1988;15:11–26. [Google Scholar]
- Bailey S, Walters RG, Jansson S, Horton P. Acclimation of Arabidopsis thaliana to the light environment: the existence of separate low light and high light responses. Planta. 2001;213:794–801. doi: 10.1007/s004250100556. [DOI] [PubMed] [Google Scholar]
- Baker NR. Chlorophyll fluorescence: a probe of photosynthesis in vivo. Annu Rev Plant Biol. 2008;59:89–113. doi: 10.1146/annurev.arplant.59.032607.092759. [DOI] [PubMed] [Google Scholar]
- Bellemare G, Bartlett SG, Chua NH. Biosynthesis of chlorophyll a/b-binding polypeptides in wild type and the chlorina f2 mutant of barley. J Biol Chem. 1982;257:7762–7767. doi: 10.1016/S0021-9258(18)34446-6. [DOI] [PubMed] [Google Scholar]
- Biswal AK, Pattanayak GK, Pandey SS, Leelavathi S, Reddy VS, Govindjee G, Tripathy BC. Light intensity-dependent modulation of chlorophyll b biosynthesis and photosynthesis by overexpression of chlorophyllide a oxygenase in tobacco. Plant Physiol. 2012;159:433–449. doi: 10.1104/pp.112.195859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björn LO, Papageorgiou GC, Blankenship R, Govindjee G. A viewpoint: why chlorophyll a? Photosynth Res. 2009;99:85–98. doi: 10.1007/s11120-008-9395-x. [DOI] [PubMed] [Google Scholar]
- Bogorad L. Porphyrin synthesis. Methods Enzymol. 1962;5:885–895. doi: 10.1016/S0076-6879(62)05334-3. [DOI] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brzezowski P, Richter AS, Grimm B. Regulation and function of tetrapyrrole biosynthesis in plants and algae. Biochim Biophys Acta. 2015;1847:968–985. doi: 10.1016/j.bbabio.2015.05.007. [DOI] [PubMed] [Google Scholar]
- Chakraborty N, Tripathy BC. Involvement of singlet oxygen in 5-aminolevulinic acid-induced photodynamic damage of cucumber (Cucumis sativus L.) chloroplasts. Plant Physiol. 1992;98:7–11. doi: 10.1104/pp.98.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty N, Tripathy BC. 5-aminolevulinic acid-induced photodynamic reactions in thylakoid membranes of cucumber (Cucumis sativus L.) cotyledons. J Plant Biochem Biotech. 1992;1:65–68. doi: 10.1007/BF03262898. [DOI] [Google Scholar]
- Dall’Osto L, Cazzaniga S, Havaux M, Bassi R. Enhanced photoprotection by protein-bound vs free xanthophyll pools: a comparative analysis of chlorophyll b and xanthophyll biosynthesis mutants. Mol Plant. 2010;3:576–593. doi: 10.1093/mp/ssp117. [DOI] [PubMed] [Google Scholar]
- Dutta S, Mohanty S, Tripathy BC. Role of temperature stress on chloroplast biogenesis and protein import in Pea. Plant Physiol. 2009;150:1050–1061. doi: 10.1104/pp.109.137265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedland N, Negi S, Vinogradova-Shah T, Wu G, Ma L, Flynn S, Kumssa T, Lee CH, Sayre RT. Fine-tuning the photosynthetic light harvesting apparatus for improved photosynthetic efficiency and biomass yield. Sci Reports. 2019;9(1):13028. doi: 10.1038/s41598-019-49545-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genty B, Briantais JM, Baker NR. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochem Biophys Acta. 1989;990:87–92. doi: 10.1016/S0304-4165(89)80016-9. [DOI] [Google Scholar]
- Govindjee G. Sixty-three years since Kautsky: chlorophyll a fluorescence. Aust J Plant Physiol. 1995;22:131–160. [Google Scholar]
- Govindjee G. Chlorophyll a fluorescence: a bit of basics and history. In: Papageorgiou GC, Govindjee, editors. Chlorophyll a Fluoroscence: A Signature of Photosynthesis, Advances in Photosynthesis and Respiration. Cham: Springer; 2005. pp. 1–42. [Google Scholar]
- Grossman AR, Bhaya D, Apt KE, Kehoe DM. Light-harvesting complexes in oxygenic photosynthesis: diversity, control, and evolution. Annu Rev Genet. 1995;29:231–288. doi: 10.1146/annurev.ge.29.120195.001311. [DOI] [PubMed] [Google Scholar]
- Gupta V, Tripathy BC. Effect of light quality on chlorophyll accumulation and protein expression in wheat (Triticum aestivum L.) seedlings. Int J Biotechnol Biochem. 2010;6:521–536. [Google Scholar]
- Harper AL, von Gesjen SE, Linford AS, Peterson MP, Faircloth RS, Thissen MM, Brusslan JA. Chlorophyllide a oxygenase mRNA and protein levels correlate with the chlorophyll a/b ratio in Arabidopsis thaliana. Photosynth Res. 2004;79:149–159. doi: 10.1023/B:PRES.0000015375.40167.76. [DOI] [PubMed] [Google Scholar]
- Harrison MA, Nemson JA, Melis A. Assembly and composition of the chlorophyll a-b light harvesting complex of barley (Hordeum vulgare L.): immunochemical analysis of chlorophyll b-less and chlorophyll b-deficient mutants. Photosynth Res. 1993;38:141–151. doi: 10.1007/BF00146413. [DOI] [PubMed] [Google Scholar]
- Havaux M, Dall'osto L, Bassi R. Zeaxanthin has enhanced antioxidant capacity with respect to all other xanthophylls in Arabidopsis leaves and functions independent of binding to PSII antennae. Plant Physiol. 2007;145:1506–1520. doi: 10.1104/pp.107.108480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirashima M, Satoh S, Tanaka R, Tanaka A. Pigment shuffling in antenna systems achieved by expressing prokaryotic chlorophyllide a oxygenase in Arabidopsis. J Biol Chem. 2006;281:15385–15393. doi: 10.1074/jbc.M602903200. [DOI] [PubMed] [Google Scholar]
- Hoober JK, Eggink LL. A potential role of chlorophylls b and c in assembly of light-harvesting complexes. FEBS Lett. 2001;489:1–3. doi: 10.1016/S0014-5793(00)02410-8. [DOI] [PubMed] [Google Scholar]
- Hukmani P, Tripathy BC. Spectrofluorometric estimation of intermediates of chlorophyll biosynthesis: protoporphyrin IX, Mg-protoporphyrin, and protochlorophyllide. Anal Biochem. 1992;206:125–130. doi: 10.1016/S0003-2697(05)80021-1. [DOI] [PubMed] [Google Scholar]
- Hukmani P, Tripathy BC. Chlorophyll biosynthetic reactions during senescence of excised barley (Hordeum vulgare L. cv. IB 65) leaves. Plant Physiol. 1994;105:1295–1300. doi: 10.1104/pp.105.4.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs NJ, Borotz SE, Guerinot ML. Protoporphyrinogen Oxidation, a Step in Heme Synthesis in Soybean Root Nodules and Free-Living Rhizobia. J Bacteriol. 1989;171:573–576. doi: 10.1128/jb.171.1.573-576.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jilani A, Kar S, Bose S, Tripathy BC. Regulation of the carotenoid content and chloroplast development by levulinic acid. Physiol Plant. 1996;96:139–145. doi: 10.1111/j.1399-3054.1996.tb00194.x. [DOI] [Google Scholar]
- Kandoi D, Mohanty S, Govindjee G, Tripathy BC. Towards efficient photosynthesis: overexpression of Zea mays phosphoenolpyruvate carboxylase in Arabidopsis thaliana. Photosynth Res. 2016;130:47–72. doi: 10.1007/s11120-016-0224-3. [DOI] [PubMed] [Google Scholar]
- Kandoi D, Mohanty S, Tripathy BC. Overexpression of plastidic maize NADP-malate dehydrogenase (ZmNADP-MDH) in Arabidopsis thaliana confers tolerance to salt stress. Protoplasma. 2018;255:547–563. doi: 10.1007/s00709-017-1168-y. [DOI] [PubMed] [Google Scholar]
- Kandoi D, Ruhil K, Govindjee G, Tripathy BC. Overexpression of cytoplasmic C4 Flaveria bidentis carbonic anhydrase in C3 Arabidopsis thaliana increases amino acids photosynthetic potential and biomass. Plant Biotechnol J. 2022;20(8):1518–1532. doi: 10.1111/pbi.13830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannagara CG, Schouboe A. Biosynthesis of ∆-ALA in greening barley laves.VII.Glutamate-1-semialdehyde accumulation in gabaculine treated leaves. Carlsberg Res Com. 1985;50:179–191. doi: 10.1007/BF02907144. [DOI] [Google Scholar]
- Kim EH, Li XP, Razeghifard R, Anderson JM, Niyogi K, Pogson BJ, Chow WS. The multiple roles of light-harvesting chlorophyll a/b-protein complexes define structure and optimize function of Arabidopsis chloroplasts: a study using two chlorophyll b-less mutants. Biochim Biophys Acta. 2009;1787:973–984. doi: 10.1016/j.bbabio.2009.04.009. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Masuda T. Transcriptional regulation of tetrapyrrole biosynthesis in Arabidopsis thaliana. Front Plant Sci. 2016;7:1811. doi: 10.3389/fpls.2016.01811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Król M, Spangfort MD, Huner NP, Oquist G, Gustafsson P, Jansson S. Chlorophyll a/b-binding proteins, pigment conversions, and early light-induced proteins in a chlorophyll b-less barley mutant. Plant Physiol. 1995;107:873–883. doi: 10.1104/pp.107.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin RM. Tetrapyrrole signaling in plants. Front Plant Sci. 2016;7:1586. doi: 10.3389/fpls.2016.01586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong T-Y, Anderson JM. Adaptation of the thylakoid membranes of pea chloroplasts to light intensities. I. Study on the distribution of chlorophyll–protein complexes. Photosynth Res. 1984;5:105–115. doi: 10.1007/BF00028524. [DOI] [PubMed] [Google Scholar]
- Li T, Liu LN, Jiang CD, Liu YJ, Shi L. Effects of mutual shading on the regulation of photosynthesisin field-grown sorghum. J Photochem Photobiol B Biol. 2014;137:31–38. doi: 10.1016/j.jphotobiol.2014.04.022. [DOI] [PubMed] [Google Scholar]
- Maltsev Y, Maltseva K, Kulikovskiy M, Svetlana Maltseva S. Influence of light conditions on microalgae growth and content of lipids, carotenoids, and fatty acid composition. Biology (basel). 2021;10(10):1060. doi: 10.3390/biology10101060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda T, Tanaka A, Melis A. Chlorophyll antenna size adjustments by irradiance in Dunaliella salina involve coordinate regulation of chlorophyll a oxygenase (CAO) and Lhcb gene expression. Plant Mol Biol. 2003;51:757–771. doi: 10.1023/A:1022545118212. [DOI] [PubMed] [Google Scholar]
- Melis A. Dynamics of photosynthetic membrane composition and function. Biochim Biophys Acta. 1991;1058:87–106. doi: 10.1016/S0005-2728(05)80225-7. [DOI] [Google Scholar]
- Mochizuki N, Tanaka R, Grimm B, Masuda T, Moulin M, Smith AG, Tanaka A, Terry MJ. The cell biology of tetrapyrroles: a life and death struggle. Trends Plant Sci. 2010;15:488–498. doi: 10.1016/j.tplants.2010.05.012. [DOI] [PubMed] [Google Scholar]
- Mohapatra A, Tripathy BC. Developmental changes in sub-plastidic distribution of chlorophyll biosynthetic intermediates in Cucumber (Cucumis sativus L.) J Plant Physiol. 2003;160:9–15. doi: 10.1078/0176-1617-00848. [DOI] [PubMed] [Google Scholar]
- Murray DL, Kohorn BD. Chloroplasts of Arabidopsis thaliana homozygous for the ch-1 locus lack chlorophyll b, lack stable LHCPII and have stacked thylakoids. Plant Mol Biol. 1991;16:71–79. doi: 10.1007/BF00017918. [DOI] [PubMed] [Google Scholar]
- Nagata N, Satoh S, Tanaka R, Tanaka A. Domain structures of chlorophyllide a oxygenase of green plants and Prochlorothrix hollandica in relation to catalytic functions. Planta. 2004;218:1019–1025. doi: 10.1007/s00425-003-1181-6. [DOI] [PubMed] [Google Scholar]
- Nakagawara E, Sakuraba Y, Yamasato A, Tanaka R, Tanaka A. Clp protease controls chlorophyll b synthesis by regulating the level of chlorophyllide a oxygenase. Plant J. 2007;49:800–809. doi: 10.1111/j.1365-313X.2006.02996.x. [DOI] [PubMed] [Google Scholar]
- Nakanishi H, Nozue H, Suzuki K, Kaneko Y, Taguchi G, Hayashida N. Characterization of the Arabidopsis thaliana mutant pcb2 which accumulates divinyl chlorophylls. Plant and Cell Physiol. 2005;46:467–473. doi: 10.1093/pcp/pci053. [DOI] [PubMed] [Google Scholar]
- Nellaepalli S, Kodru S, Tirupathi M, Subramanyam R. Anaerobiosis induced state transition: a non-photochemical reduction of PQ pool mediated by NDH in Arabidopsis thaliana. PLoS ONE. 2012;7:e49839. doi: 10.1371/journal.pone.0049839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen MK, Shih TH, Lin SH, Huang WD, Yang CM. Transcription analysis of chlorophyll biosynthesis in wildtype and chlorophyll b-lacking rice (Oryza sativa L.) Photosynthetica. 2020;58:702–711. doi: 10.32615/ps.2020.022. [DOI] [Google Scholar]
- Nguyen MK, Shih TH, Lin SH, Lin JW, Nguyen HC, Yang ZW, Yang CM. Transcription profile analysis of chlorophyll biosynthesis in leaves of wild-type and chlorophyll b-deficient rice (Oryza sativa L.) Agriculture. 2021;11:401. doi: 10.3390/agriculture11050401. [DOI] [Google Scholar]
- op den Camp RG, Przybyla D, Ochsenbein C, Laloi C, Kim C, Danon A, Wagner D, Hideg E, Göbel C, Feussner I, Nater M, Apel K. Rapid induction of distinct stress responses after the release of singlet oxygen in Arabidopsis. Plant Cell. 2013;15:2320–2332. doi: 10.1105/tpc.014662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ort DR, Merchant SS, Alric J, Barkan A, Blankenship RE, Bock R, Croce R, Hanson MR, Hibberd JM, Long SP, Moore TA, Moroney J, Niyogi KK, Parry MA, Peralta-Yahya PP, Prince RC, Redding KE, Spalding MH, van Wijk KJ, Vermaas WF, von Caemmerer S, Weber AP, Yeates TO, Yuan JS, Zhu XG. Redesigning photosynthesis to sustainably meet global food and bioenergy demand. Proc Natl Acad Sci U S A. 2015;112:8529–8536. doi: 10.1073/pnas.1424031112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oster U, Tanaka R, Tanaka A, Rudiger W. Cloning and functional expression of the gene encoding the key enzyme for chlorophyll b biosynthesis (CAO) from Arabidopsis thaliana. Plant J. 2000;21:305–310. doi: 10.1046/j.1365-313x.2000.00672.x. [DOI] [PubMed] [Google Scholar]
- Pattanayak GK, Tripathy BC. Catalytic function of a novel protein protochlorophyllide oxidoreductase C of Arabidopsis thaliana. Biochem Biophys Res Commun. 2002;291:921–924. doi: 10.1006/bbrc.2002.6543. [DOI] [PubMed] [Google Scholar]
- Pattanayak GK, Tripathy BC. Overexpression of protochlorophyllide oxidoreductase C regulates oxidative stress in Arabidopsis. PLoS ONE. 2011;6:e26532. doi: 10.1371/journal.pone.0026532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattanayak GK, Biswal AK, Reddy VS, Tripathy BC. Light-dependent regulation of chlorophyll b biosynthesis in chlorophyllide a oxygenase overexpressing tobacco plants. Biochem Biophys Res Com. 2005;326:466–471. doi: 10.1016/j.bbrc.2004.11.049. [DOI] [PubMed] [Google Scholar]
- Perrine Z, Negi S, Sayre RT. Optimization of photosynthetic light energy utilization by microalgae. Algal Res. 2012;1:134–142. doi: 10.1016/j.algal.2012.07.002. [DOI] [Google Scholar]
- Ping H, Jie M, Shujing K, Sanfeng L, Xianmei W, Longjun Z, CAOlin L, Rui H, Huiying H, Shan L, Yuchun R, Xudong Z, Guosheng X, Qian Q, Guo L, Yuexing W. Chlorophyllide-a Oxygenase 1 (OsCAO1) over-expression affects rice photosynthetic rate and grain yield. Rice Sci. 2023;30:87–91. doi: 10.1016/j.rsci.2022.05.006. [DOI] [Google Scholar]
- Porra RJ, Thompson WA, Kriedmann PA. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim Biophys Acta. 1989;975:384–394. doi: 10.1016/S0005-2728(89)80347-0. [DOI] [Google Scholar]
- Ramel F, Ksas B, Akkari E, Mialoundama AS, Monnet F, Krieger-Liszkay A, Ravanat JL, Mueller MJ, Bouvier F, Havaux M. Light-induced acclimation of the Arabidopsis chlorina1 mutant to singlet oxygen. Plant Cell. 2013;25:1445–1462. doi: 10.1105/tpc.113.109827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebeiz CA, Reddy KN, Nandihalli UB, Velu J. Tetrapyrrole-dependent photodynamic herbicides. Photochem Photobiol. 1990;52:1099–1117. doi: 10.1111/j.1751-1097.1990.tb08451.x. [DOI] [Google Scholar]
- Richter AS, Pérez-Ruiz JM, Cejudo FJ, Grimm B. Redox-control of chlorophyll biosynthesis mainly depends on thioredoxins. FEBS Lett. 2018;592(18):3111–3115. doi: 10.1002/1873-3468.13216. [DOI] [PubMed] [Google Scholar]
- Sakuraba Y, Tanaka R, Yamasato A, Tanaka A. Determination of a chloroplast degron in the regulatory domain of chlorophyllide a oxygenase. J Biol Chem. 2009;284:36689–36699. doi: 10.1074/jbc.M109.008144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuraba Y, Yokono M, Akimoto S, Tanaka R, Tanaka A. Deregulated chlorophyll b synthesis reduces the energy transfer rate between photosynthetic pigments and induces photodamage in Arabidopsis thaliana. Plant Cell Physiol. 2010;51:1055–1065. doi: 10.1093/pcp/pcq050. [DOI] [PubMed] [Google Scholar]
- Sakuraba Y, Balazadeh S, Tanaka R, Mueller-Roeber B, Tanaka A. Overproduction of chl B retards senescence through transcriptional reprogramming in Arabidopsis. Plant Cell Physiol. 2012;53:505–517. doi: 10.1093/pcp/pcs006. [DOI] [PubMed] [Google Scholar]
- Schreiber U. Pulse-amplitude-modulation (PAM) fluorometry and saturation pulse method an overview. In: PapageorgiouGovindjee GC, editor. Chlorophyll a fluorescence: a signature of photosynthesis Advances in photosynthesis and respiration. Dordrecht: Springer; 2004. pp. 279–319. [Google Scholar]
- Schreiber U, Bilger W, Neubaue C. Chlorophyll fluorescence as a nonintrusive indicator for rapid assessment of in vivo photosynthesis. In: Schulze ED, Caldwell MM, editors. Ecophysiology of Photosynthesis. Springer, Berlin, Heidelberg: Springer Study Edition; 1995. pp. 49–70. [Google Scholar]
- Shemin D. δ-Aminolevulinic acid dehydrase from Rhodopseudomonas spheroids. In: Press A, editor. Methods in Enzymology Colowick SP and Kaplan NO. New York: Inc; 1962. pp. 883–884. [Google Scholar]
- Shevela D, Bjorn L, Govindjee G. Photosynthesis: Solar Energy for Life. Singapore: World Scientific; 2019. [Google Scholar]
- Sood S, Gupta V, Tripathy BC. Photoregulation of the greening process of wheat seedlings grown in red light. Plant Mol Biol. 2005;59:269–287. doi: 10.1007/s11103-005-8880-2. [DOI] [PubMed] [Google Scholar]
- Tanaka A, Kawano K. Effect of mutual shading on dry-matter production in the tropical rice plant. Plant Soil. 1966;24:128–144. doi: 10.1007/BF01373079. [DOI] [Google Scholar]
- Tanaka R, Tanaka A. Effects of chlorophyllide a oxygenase overexpression on light acclimation in Arabidopsis thaliana. Photosynth Res. 2005;85:327–340. doi: 10.1007/s11120-005-6807-z. [DOI] [PubMed] [Google Scholar]
- Tanaka A, Tanaka R. Chlorophyll metabolism. Curr Opin Plant Biol. 2006;9:248–255. doi: 10.1016/j.pbi.2006.03.011. [DOI] [PubMed] [Google Scholar]
- Tanaka R, Tanaka A. Tetrapyrrole biosynthesis in higher plants. Annu Rev Plant Biol. 2007;58:321–346. doi: 10.1146/annurev.arplant.57.032905.105448. [DOI] [PubMed] [Google Scholar]
- Tanaka A, Ito H, Tanaka R, Tanaka NK, Yoshida K, Okada K. Chlorophyll a oxygenase (CAO) is involved in chlorophyll b formation from chlorophyll a. Proc Natl Acad Sci USA. 1998;95:12719–12723. doi: 10.1073/pnas.95.21.12719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewari AK, Tripathy BC. Temperature-stress-induced impairment of chlorophyll biosynthetic reactions in cucumber and wheat. Plant Physiol. 1998;117:851–858. doi: 10.1104/pp.117.3.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornber JP, Highkin HR. Composition of the photosynthetic apparatus of normal barley leaves and a mutant lacking chlorophyll b. Eur J Biochem. 1974;41:109–116. doi: 10.1111/j.1432-1033.1974.tb03250.x. [DOI] [PubMed] [Google Scholar]
- Tripathy BC, Chakraborty N. 5-Aminolevulinic acid induced photodynamic damage of the photosynthetic electron transport chain of cucumber (Cucumis sativus L.) cotyledons. Plant Physiol. 1991;96:761–767. doi: 10.1104/pp.96.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathy BC, Oelmüller R. Reactive oxygen species generation and signaling in plants. Plant Signaling Behavior. 2012;7:1621–1633. doi: 10.4161/psb.22455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathy BC, Pattanayak GK. Singlet oxygen-induced stress in plants. In: Rebeiz CA, Benning C, Bohnert HJ, Daniell H, Hoober JK, Lichtenthaler HK, Portis AR, Tripathy BC, editors. The Chloroplast: Basics and Applications. Cham: Springer; 2010. pp. 397–412. [Google Scholar]
- Tripathy BC, Pattanayak GK. Chlorophyll biosynthesis in higher plants. In: Eaton-Rye JJ, Tripathy BC, Sharkey TD, editors. Photosynthesis: Plastid Biology, Energy Conversion and Carbon Assimilation. Cham: Springer; 2012. pp. 63–94. [Google Scholar]
- Tripathy BC, Mohapatra A, Gupta I. Impairment of the photosynthetic apparatus by oxidative stress induced by photosensitization reaction of protoporphyrin IX. Biochim Biophys Acta. 2007;1767:860–868. doi: 10.1016/j.bbabio.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Voitsekhovskaja OV, Tyutereva EV. Chlorophyll b in angiosperms: functions in photosynthesis, signaling and ontogenetic regulation. J PlAnt Physiol. 2015;189:51–64. doi: 10.1016/j.jplph.2015.09.013. [DOI] [PubMed] [Google Scholar]
- Von Wettstein D, Giugh S, Kannangara CG. Chlorophyll biosynthesis. Plant Cell. 1995;7:1039–1057. doi: 10.1105/tpc.7.7.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Grimm B. Connecting chlorophyll metabolism with accumulation of the photosynthetic apparatus. Trends Plant Sci. 2021;26:484–495. doi: 10.1016/j.tplants.2020.12.005. [DOI] [PubMed] [Google Scholar]
- Wang P, Richter AS, Kleeberg JR, Geimer S, Grimm B. Post-translational coordination of chlorophyll biosynthesis and breakdown by BCMs maintains chlorophyll homeostasis during leaf development. Nat Commun. 2020;11(1):1254. doi: 10.1038/s41467-020-14992-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasato A, Nagata N, Tanaka R, Tanaka A. The N-terminal domain of chlorophyllide a oxygenase confers protein instability in response to chlorophyll b accumulation in Arabidopsis. Plant Cell. 2005;17:1585–1597. doi: 10.1105/tpc.105.031518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasato A, Tanaka R, Tanaka A. Loss of the N-terminal domain of chlorophyllide a oxygenase induces photodamage during greening of Arabidopsis seedlings. BMC Plant Biol. 2008;8:64. doi: 10.1186/1471-2229-8-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zivcak M, Brestic M, Kalaji HM, Govindjee. Photosynthetic responses of sun-and shade-grown barley leaves to high light: is the lower PSII connectivity in shade leaves associated with protection against excess of light? Photosynth Res. 2014;119:339–354. doi: 10.1007/s11120-014-9969-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Chlorophyll a content of wild-type (WT) and CAO antisense (CAOas) tobacco plants grown under low-light (LL) and high-light (HL) in the greenhouse. Plants were grown for up to 30 days under light intensity of 300 µmol photons m-2s-1; then, they were transferred to LL (70–80 µmol photons m-2s-1) and HL (700–800 µmol photons m-2s-1) for additional 4 weeks. Each data point is the average of seven replicates and error bars represent the mean ±SE; asterisks indicate significant differences determined by ANOVA-test along with Dunnett’s post hoc test compared to WT (*P < 0.05, **P < 0.01). Statistical tests were conducted between WT and mutant within the same treatment. Fig. S2. Effective quantum yield of PSII (φPSII) of WT and CAOas plants. LL- and HL-grown WT and CAOas plants were dark adapted for 20 min before measurements were made, using PAM 2100 fluorometer. фPSII was estimated from data obtained by this fluorometer at different light intensities (up to 900 µmol photons m-2s-1) as described in the methods. Each data point is the average of five replicates; error bars represent the ±SE; asterisks indicate significant differences determined by ANOVA-test along with Dunnett’s post hoc test compared to WT (*P < 0.05, **P < 0.01). Statistical tests were conducted between WT and mutant within the same treatment. Fig. S3. Non-photochemical quenching (NPQ) of WT and CAOas plants. LL- and HL-grown WT and CAOas plants were dark adapted for 20 min before measurements were made, using PAM 2100 fluorometer. NPQ of excited states of chlorophyll was estimated from data obtained by this fluorometer at different light intensities (up to 900 µmol photons m-2s-1) as described in the methods. Each data point is the average of five replicates; error bars represent the ±SE; asterisks indicate significant differences determined by ANOVA-test along with Dunnett’s post hoc test compared to WT (*P < 0.05, **P < 0.01). The experiment was done three times. Statistical tests were conducted between WT and mutant within the same treatment. (PPTX 68 kb)