Abstract
Objective
Depression is a common complication in Takayasu arteritis (TA). Disorders of the immune system play an important role in both diseases. This study aimed to clarify the feature of cytokines in TA patients with depression.
Methods
In this cross-sectional study, serum cytokines were tested in 40 TA patients and 11 healthy controls using the Bio-Plex Magpix System (Bio-Rad®). The state of depression was measured by the Zung Self-Rating Depression Scale (SDS) in TA patients. Logistic regression analysis was performed to find the risk factors of depression in patients with TA.
Results
TA patients with depression had higher ESR, hsCRP, NIH, and ITAS.A than patients without depression (16.00 [10.00, 58.50]mm/H vs. 7.50 [4.50, 17.75]mm/H, p = 0.013; 7.60 [2.32, 46.52]mg/L vs. 0.71 [0.32, 4.37]mg/L, p = 0.001; 2.00 [2.00, 3.00] vs. 1.00 [0.00, 2.00], p = 0.007; 7.00 [4.00, 9.50] vs. 1.50 [0.00, 5.75], p = 0.012, respectively). Additionally, the lower age of onset and levels of IL-4, IL-13, eotaxin, and IP-10 were observed in the depressed group compared with the non-depressed (23.50 [19.25, 32.50]pg./ml vs. 37.00 [23.25, 42.50]pg./ml, p = 0.017; 2.80 [2.17, 3.18]pg./ml vs. 3.51 [3.22, 4.66]pg./ml, p < 0.001; 0.66 [0.60, 1.12]pg./ml vs. 1.04 [0.82, 1.25]pg./ml, p = 0.008; 46.48 [37.06, 61.75]pg./ml vs. 69.14 [59.30, 92.80]pg./ml, p = 0.001; 184.50 [138.23, 257.25]pg./ml vs. 322.32 [241.98, 412.60]pg./ml, p = 0.005, respectively). The lower level of IL-4 and age of onset were the independent risk factors for depression in TA patients (OR [95% CI] 0.124 [0.018, 0.827], p = 0.031; 0.870 [0.765, 0.990], p = 0.035, respectively).
Conclusion
Our data suggested that lower cytokine levels, especially IL-4, might be involved in the development of TA patients with depression. Clinicians can probably use serum IL-4 level testing as a potential indicator of depression in TA.
Keywords: Takayasu arteritis, mental psychology, depression, cytokines, disease activity
Introduction
Takayasu arteritis (TA) is a chronic inflammatory vasculitis that affects the aorta and its major branches, predominantly occurring in young women in 80–90% of cases (1). Inflammation within the vasculature can result in segmental stenosis, occlusion, dilatation, and/or aneurysm formation (2). Existing evidence strongly indicates that cell-mediated immunity is a crucial factor in the pathogenic cascade leading to these lesions (3, 4). However, the quantitative and qualitative changes in cytokine production observed in TA remain incompletely elucidated in current studies.
Pathophysiological investigations into depression have elucidated a strong association between neuroimmune inflammatory pathways and the cytokine-mediated activation of cellular immunity (5). Individuals with major depression often manifest elevated levels of inflammatory mediators, including cytokines, chemokines, and acute-phase proteins in blood or cerebrospinal fluid (6). It is proposed that the onset and persistence of depression are substantially influenced by the secretion of peripheral pro-inflammatory cytokines and other factors. This activation initiates localized, low-grade, yet chronic inflammation within the central nervous system (6, 7). It is now recognized that the cytokines themselves are capable of triggering or inhibiting the release of other cytokines (8). Against the background of inflammation, cytokines act beyond the normal regulatory range. The abnormal phenomenon, in turn, leads to an increase in oxidative stress, thus maintaining, even enhancing chronic inflammation till depression happens (9).
TA is a disease caused by a dysregulation of the systemic immune system (10). In previous studies, a certain proportion of TA patients would have developed depressive co-morbidities (11). Though the mechanism of the disease is not fully defined, it is generally believed that the pathogenic process includes disruption of the cytokine network, which may underlie the pathophysiology of combined depression in TA (12). Previous studies have identified dysregulation of pro- and anti-inflammatory cytokines during inflammatory disease progression as an underlying factor in depression (13). However, the cytokine levels in depression TA patients were unclear. In our study, we detected the serum cytokines (27 types) in depression TA patients. The authors aimed to clarify the characteristics and risk factors of depression in TA patients and provided new evidence for the clinical manifestations and mechanisms of such co-morbidities in the context of systemic inflammatory states.
Materials and methods
Patients
A cross-sectional study was carried out to determine whether there is a relationship between cytokines and depression in TA patients. The authors consecutively enrolled 40 hospitalized patients with TA who attended Beijing Anzhen Hospital from March 2021 to September 2022, along with 11 healthy controls recruited from the general population. Cytokine profiles were compared with non-depressed healthy controls to examine the differences between these populations. Clinical and biological data were collected concurrently with the recruitment process of the patient registry.
Inclusion and exclusion criteria
TA patients who had been treated for more than 6 months were recruited, fulfilling the classification criteria of TA developed by the American College of Rheumatology (ACR)/EULAR in 2022 (14). The following were the exclusion criteria: (1) chronic or current infections, (2) tumors, (3) other autoimmune diseases, and (4) other psychiatric diseases or a history of psychiatric diseases. The authors also included healthy controls with matched age–sex ratios and no previous history of illness, which were evaluated by the SDS to confirm the absence of depressive symptoms.
Ethics statement
This study was conducted in accordance with the ethical principles Helsinki Declaration and was approved by the Ethics Committee of Beijing Anzhen Hospital (Approval Number: 2023183X).
Collection of clinical data and laboratory parameters
The authors collected the age of onset, gender, education level, and body mass index (BMI) as demographic variables. Clinical parameters consisted of NIH (15), ITAS.A, and ITAS2010 (16), which were defined to evaluate disease activity and Numano type (17). The authors also recorded in detail the typical clinical symptoms presented during the course of the disease, such as fever, neck pain, chest tightness/chest pain, bilateral pulse inequality, intermittent claudication, dizziness/headache, and abdominal pain (18). All patients were treated with medications, including glucocorticoids, conventional synthetic disease-modifying antirheumatic drugs (cDMARDs), and biologic disease-modifying antirheumatic drugs (btDMARDs). The authors documented the glucocorticoid dosage at the time of enrollment and converted it to prednisone equivalents. The dosages and methods of administration of DMARDs were methotrexate 15 mg Qw, martimecrolide 0.75 g bid, cyclophosphamide 100 mg Qod, hydroxychloroquine sulfate 0.2 g Qd, azathioprine 100 mg Qd, tocilizumab 8 mg/kg Qm, adalimumab 40 mg Q2w, and baricitinib 2 mg Qd. Laboratory parameters included white blood cell count (WBC), neutrophils (NEUT), hemoglobin (Hb), platelets (PLT), erythrocyte sedimentation rate (ESR), and high-sensitivity c-reactive protein (hsCRP), which were assessed at the same time as the estimation of psychological status by the laboratory medicine of Anzhen Hospital.
Evaluation of depression
Depressive symptoms were evaluated by using the Zung Self-Rating Depression Scale (SDS), a well-established and valid instrument for identifying depression in primary care patients (19). Participants with SDS scores equal to or exceeding 52 were categorized as experiencing depression, while those with SDS scores below 52 were classified as not having depression (20). The healthy controls were also screened for depressive tendencies through the SDS. None reported the presence of depressive symptoms.
Detection of cytokines
Venous blood (5 mL) was drawn into a vacuum blood collection tube on the day of assessing depressive symptoms. The clotted blood stood for 30 min before centrifugation at 2,000 × g for 10 min at room temperature. The serum was aliquoted and stored in an −80°C freezer for subsequent analysis.
Cytokine levels were determined by the Bio-Plex Pro Human Inflammation and Treg Cytokine Assays (United States) on the Bio-Plex Magpix System (Bio-Rad®). The panel analyzed 27cytokines, including IL-1β, IL-1rα, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12(p70), IL-13, IL-15, IL-17, eotaxin, FGF basic, G-CSF, GM-CSF, IFN-γ, IP-10, MCP-1, MIP-1α, MIP-1β, PDGF-BB, RANTES, TNF-α, and VEGF. The samples were processed according to the Bio-Plex Pro Human Inflammation Panel I Assay Quick Guide (Bio-Rad®, 10,044,282). The concentrations of the analytes in the test samples were determined by the standard curves of the different concentrations of cytokines.
Statistical analysis
The demographic and clinical data were expressed as median and IQR as the data results did not conform to normal distribution. The Wilcoxon test was used to determine the significance. The ANOVA test was used to analyze the relationship between the levels of each cytokine profile and the different groups. Spearman’s test was used to determine the correlation between SDS scores, ESR, hsCRP, and cytokines. Multivariate logistic regression analysis was performed to find out the risk factors of TA patients with depression. The analysis and visualization of all statistical tests were exhibited by R version 3.1.1 and SPSS.26.
Results
Sociodemographic characteristics and clinical differences in the depressed TA group versus the non-depressed group
In our research, 40 TA patients who had received pharmacological treatment for more than 6 months were recruited. The sociodemographic and clinical data are shown in Table 1; 12 patients with SDS scores ≥52 were categorized as the depressed group and 28 patients with SDS scores lower than 52 were recognized as the non-depressed. The authors found that the age of onset in the depressed group was significantly lower than the non-depressed group in TA patients (23.50 [19.25, 32.50] vs. 37.00 [23.25, 42.50], p = 0.017). There were no significant differences between the depressed and non-depressed groups in terms of disease duration, percentage of females, duration of education, BMI, and medications. On the other hand, no differences were observed in Numano types, coronary, pulmonary, intracranial artery involvement, nor in the typical clinical symptoms in TA (Table 1).
Table 1.
Clinical summary for TA patients with or without depression.
| Variable | TA with depression (n = 12) | TA without depression (n = 28) | p-value |
|---|---|---|---|
| Age of onset, years | 23.50 (19.25, 32.50) | 37.00 (23.25, 42.50) | 0.017* |
| Female, n (%) | 12 (100.0) | 25 (89.3) | 0.332 |
| Duration, months | 45.00 (15.75, 156.00) | 72.00 (24.00, 120.00) | 0.738 |
| Education, months | 14.00 (12.00, 16.00) | 13.00 (9.75, 14.00) | 0.236 |
| BMI (kg/m2) | 23.03 (20.63, 26.59) | 23.40 (21.51, 27.05) | 0.738 |
| Medication paradigm, n (%) | |||
| Glucocorticoid | 9 (75.0) | 15 (53.6) | 0.181 |
| Prednisone equivalent, mg | 10.00 (6.25, 15.00) | 2.50 (0.00, 15.00) | 0.260 |
| cDMARDs | 8 (66.7) | 18 (64.3) | 0.591 |
| MTX | 5 (41.7) | 13 (46.4) | 0.529 |
| CTX | 0 (0.0) | 1 (3.6) | 0.700 |
| MMF | 3 (25.0) | 8 (28.6) | 0.570 |
| AZA | 2 (16.7) | 1 (3.6) | 0.209 |
| HCQ | 0 (0.0) | 2 (7.1) | 0.485 |
| btDMARDs | 5 (41.7) | 9 (32.1) | 0.409 |
| TCZ | 4 (33.3) | 7 (25.0) | 0.430 |
| ADA | 1 (8.3) | 1 (3.6) | 0.515 |
| Baricitinib | 0 (0.0) | 2 (7.1) | 0.485 |
| Numano type, n (%) | |||
| I | 2 (16.7) | 2 (7.1) | 0.346 |
| IIa | 0 (0.0) | 1 (3.6) | 0.700 |
| IIb | 2 (16.7) | 10 (35.7) | 0.207 |
| III | 0 (0.0) | 0 (0.0) | |
| IV | 0 (0.0) | 1 (3.6) | 0.700 |
| V | 8 (66.7) | 14 (50.0) | 0.268 |
| Coronary artery | 4 (33.3) | 14 (50.0) | 0.268 |
| Pulmonary artery | 4 (33.3) | 6 (21.4) | 0.337 |
| Intracranial artery | 5 (41.7) | 12 (42.9) | 0.612 |
BMI, body mass index; cDMARD, conventional synthetic disease-modifying antirheumatic drugs; btDMARDs, biologic disease-modifying antirheumatic drugs; MTX, methotrexate; CTX, cyclophosphamide; MMF, Mycophenolate mofetil; AZA, azathioprine; HCQ, hydroxychloroquine; TCZ, tocilizumab; ADA, adalimumab; * represents p-value < 0.05.
Laboratory indicators for TA patients with or without depression
In the comparison of laboratory indicators, the authors found that the depressed group in TA had higher levels of WBC, NEUT, PLT, ESR, and hsCRP than the non-depressed group (9.58 [6.92, 10.52] ×109/L vs. 6.53 [4.99, 9.10]×109/L, p = 0.036; 69.05 [63.83, 76.38]% vs. 60.80 [51.33, 68.68]%, p = 0.033; 291.00 [232.50, 443.25]×109/L vs. 231.00 [208.00, 278.75]×109/L, p = 0.024; 16.00 [10.00, 58.50]mm/H vs. 7.50 [4.50, 17.75]mm/H, p = 0.013; 7.60 [2.32, 46.52]mg/L vs. 0.71 [0.32, 4.37]mg/L, p = 0.001), while the level of Hb was lower in the depressed group (104.50 [97.25, 126.00]g/L vs. 124.00 [115.50, 133.00]g/L, p = 0.009). The authors also compared the indexes of activity between the two groups. The levels of NIH and ITAS.A suggested significant differences (2.00 [2.00, 3.00] vs. 1.00 [0.00, 2.00], p = 0.007; 7.00 [4.00, 9.50] vs. 1.50 [0.00, 5.75], p = 0.012), while the level of ITAS2010 also emerged on a trend toward higher in the depressed group despite p > 0.05 (4.00 [3.25, 6.50] vs. 1.00 [0.00, 5.00], p = 0.056) (Table 2).
Table 2.
Laboratory indicators for TA patients with or without depression.
| Variable | TA with depression (n = 12) | TA without depression (n = 28) | p-value |
|---|---|---|---|
| WBC (×109/L) | 9.58 (6.92, 10.52) | 6.53 (4.99, 9.10) | 0.036* |
| NEUT (%) | 69.05 (63.83, 76.38) | 60.80 (51.33, 68.68) | 0.033* |
| LYM (%) | 24.60 (20.50, 32.88) | 30.20 (24.15, 36.20) | 0.172 |
| Hb (g/L) | 104.50 (97.25, 126.00) | 124.00 (115.50, 133.00) | 0.009** |
| PLT (×109/L) | 291.00 (232.50, 443.25) | 231.00 (208.00, 278.75) | 0.024* |
| Cr (μmol/L) | 54.25 (45.45, 65.00) | 56.30 (52.10, 64.58) | 0.328 |
| ALT (U/L) | 21.00 (9.00, 28.75) | 14.00 (10.00, 24.00) | 0.570 |
| ESR (mm/H) | 16.00 (10.00, 58.50) | 7.50 (4.50, 17.75) | 0.013* |
| hsCRP (mg/L) | 7.60 (2.32, 46.52) | 0.71 (0.32, 4.37) | 0.001** |
| IgA (g/L) | 2.78 (1.82, 3.69) | 2.23 (1.47, 2.92) | 0.202 |
| IgG (g/L) | 9.69 (8.57, 13.11) | 12.01 (10.33, 14.91) | 0.074 |
| IgM (g/L) | 1.42 (0.96, 1.66) | 1.06 (0.85, 1.51) | 0.328 |
| C3 (g/L) | 1.31 (0.95, 1.53) | 1.18 (1.00, 1.38) | 0.342 |
| C4 (g/L) | 0.24 (0.18, 0.32) | 0.24 (0.14, 0.31) | 0.694 |
| NIH | 2.00 (2.00, 3.00) | 1.00 (0.00, 2.00) | 0.007** |
| ITAS2010 | 4.00 (3.25, 6.50) | 1.00 (0.00, 5.00) | 0.056 |
| ITAS.A | 7.00 (4.00, 9.50) | 1.50 (0.00, 5.75) | 0.012* |
Numano type is divided according to Hata and Numano’s criteria; WBC, white blood cell count; NEUT, neutrophil count; Hb, hemoglobin; PLT, platelet count; ALT, alanine aminotransferase; Cr, creatinine; ESR, erythrocyte sedimentation rate; hsCRP, high-sensitivity C-reactive protein; C3, complement component 3; C4, complement component 4; Ig, immunoglobulin; * represents p-value < 0.05,** represents p-value < 0.010.
The differences in cytokine profiles between TA patients and healthy controls
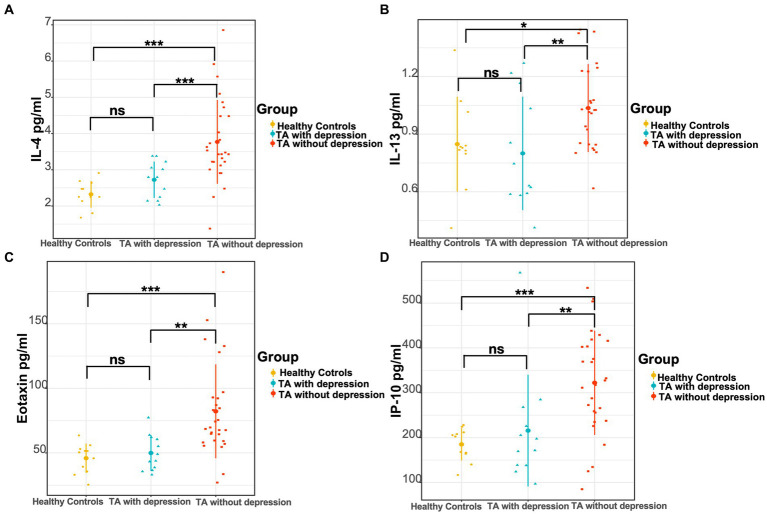
As in previously known studies, many cytokines were elevated in TA patients compared to healthy controls, which was consistent with the inflammatory essence of the disease. The levels of IL-1rα, IL-2, IL-4, IL-6, IL-8, IL-12 (p70), eotaxin, FGF basic, G-CSF, IP-10, MCP-1, MIP-1α, and TNF-α were significantly higher in TA patients than the levels of 13 cytokines in the healthy controls (Table 3). No heterogeneity was found in the other 14 cytokines between TA patients and controls in our study. The authors further compared the cytokine profiles between depressed and non-depressed groups in TA patients. The levels of IL-4, IL-13, eotaxin, and IP-10 were significantly lower in the depressed group than in the non-depressed group (2.80 [2.17, 3.18]pg./ml vs. 3.51 [3.22, 4.66]pg./ml, p < 0.001; 0.66 [0.60, 1.12]pg./ml vs. 1.04 [0.82, 1.25]pg./ml, p = 0.008; 46.48 [37.06, 61.75]pg./ml vs. 69.14 [59.30, 92.80]pg./ml, p = 0.001; 184.50 [138.23, 257.25]pg./ml vs. 322.32 [241.98, 412.60]pg./ml, p = 0.005). However, there were no significant differences between the depressed TA group and healthy controls in the level of IL-4, IL-13, eotaxin, IP-10 (2.80 [2.17, 3.18]pg./ml vs. 2.25 [2.14, 2.64]pg./ml, p = 0.091; 0.66 [0.60, 1.12]pg./ml vs. 0.82 [0.82, 1.04]pg./ml, p = 0.487; 46.48 [37.06, 61.75]pg./ml vs. 50.45 [35.63, 53.05]pg./ml, p = 0.608; 184.50 [138.23, 257.25]pg./ml vs. 202.44 [163.94, 211.78]pg./ml, p = 0.976). In contrast, the levels of IL-4, IL-13, eotaxin, and IP-10 were significantly higher in the non-depressed TA group than in the healthy control group (3.51 [3.22, 4.66]pg./ml vs. 2.25 [2.14, 2.64]pg./ml, p < 0.001; 1.04 [0.82, 1.25]pg./ml vs. 0.82 [0.82, 1.04]pg./ml, p = 0.015; 69.14 [59.30, 92.80]pg./ml vs. 50.45 [35.63, 53.05]pg./ml, p < 0.001; 322.32 [241.98, 412.60]pg./ml vs. 202.44 [163.94, 211.78]pg./ml, p < 0.001) (Figure 1 and Table 3).
Table 3.
Baseline levels of cytokines and chemokines in TA patients with depression, without depression, and healthy controls.
| Healthy controls (n = 11) | TA patients (n = 40) | TA with depression (n = 12) | TA without depression (n = 28) | p1 | p2 | p3 | p4 | |
|---|---|---|---|---|---|---|---|---|
| IL-1β (pg/mL) | 1.27 (1.18, 1.37) | 1.37 (1.18, 1.77) | 1.37 (1.18, 1.74) | 1.42 (1.18, 1.91) | 0.221 | 0.449 | 0.221 | 0.631 |
| IL-1rα (pg/mL) | 274.33 (203.45, 335.63) | 390.90 (349.45, 489.60) | 390.90 (349.45, 460.05) | 416.39 (349.45, 534.71) | <0.001*** | <0.001*** | <0.001*** | 0.457 |
| IL-2 (pg/mL) | 0.73 (0.00, 1.10) | 1.46 (0.73, 2.51) | 1.64 (1.10, 1.82) | 1.10 (0.35, 2.51) | 0.034* | 0.032* | 0.078 | 0.694 |
| IL-4 (pg/mL) | 2.25 (2.14, 2.64) | 3.35 (2.91, 3.98) | 2.80 (2.17, 3.18) | 3.51 (3.22, 4.66) | <0.001*** | 0.091 | <0.001*** | <0.001*** |
| IL-5 (pg/mL) | 26.02 (17.77, 73.84) | 58.11 (26.02, 88.45) | 60.02 (36.16, 92.32) | 58.11 (13.59, 82.61) | 0.353 | 0.069 | 0.747 | 0.224 |
| IL-6 (pg/mL) | 1.34 (0.39, 1.94) | 7.54 (3.30, 14.60) | 7.92 (4.55, 22.61) | 6.52 (3.10, 14.06) | <0.001*** | <0.001*** | <0.001*** | 0.439 |
| IL-7 (pg/mL) | 1.93 (0.00, 7.17) | 2.68 (1.93, 7.17) | 1.93 (1.93, 4.75) | 4.75 (1.93, 7.17) | 0.214 | 0.740 | 0.149 | 0.154 |
| IL-8 (pg/mL) | 7.91 (7.91, 11.73) | 13.60 (8.56, 20.29) | 9.20 (7.59, 15.30) | 13.76 (9.84, 21.93) | 0.014* | 0.316 | 0.004** | 0.084 |
| IL-9 (pg/mL) | 426.64 (401.70, 440.39) | 431.99 (413.65, 468.08) | 426.89 (422.31,4 57.73) | 434.79 (401.95, 468.84) | 0.243 | 0.211 | 0.346 | 0.942 |
| IL-10 (pg/mL) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.08) | 0.00 (0.00, 0.00) | 0.088 | 0.190 | 0.396 | 0.570 |
| IL-12p70 (pg/mL) | 2.54 (2.54, 3.53) | 3.53 (2.54, 6.22) | 3.53 (2.54, 4.51) | 4.02 (2.54, 6.46) | 0.029* | 0.091 | 0.040* | 0.550 |
| IL-13 (pg/mL) | 0.82 (0.82, 1.04) | 1.04 (0.82, 1.25) | 0.66 (0.60, 1.12) | 1.04 (0.82, 1.25) | 0.133 | 0.487 | 0.015* | 0.008** |
| IL-15 (pg/mL) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | 0.280 | 0.525 | 0.747 | 0.694 |
| IL-17 (pg/mL) | 3.30 (0.18, 6.31) | 4.53 (3.30, 6.87) | 4.06 (2.73, 4.81) | 4.81 (3.49, 9.20) | 0.051 | 0.379 | 0.026* | 0.065 |
| Eotaxin (pg/mL) | 50.45 (35.63, 53.05) | 64.61 (54.78, 84.24) | 46.48 (37.06, 61.75) | 69.14 (59.30, 92.80) | 0.002** | 0.608 | <0.001*** | 0.001** |
| FGF basic (pg/mL) | 18.79 (18.79, 21.42) | 23.89 (20.13, 26.22) | 21.42 (20.13, 23.89) | 23.89 (19.45, 26.22) | 0.042* | 0.134 | 0.050 | 0.172 |
| G-CSF (pg/mL) | 90.53 (82.32, 101.37) | 138.57 (112.10, 183.98) | 120.09 (108.09, 165.29) | 150.35 (114.76, 236.15) | <0.001*** | 0.001** | <0.001*** | 0.260 |
| GM-CSF (pg/mL) | 0.68 (0.11, 1.40) | 1.17 (0.45, 2.48) | 1.29 (0.68, 2.48) | 1.05 (0.42, 2.48) | 0.161 | 0.169 | 0.246 | 0.716 |
| IFN-γ (pg/mL) | 4.82 (3.95, 7.73) | 6.50 (4.82, 9.67) | 5.66 (4.82, 7.32) | 7.12 (4.93, 11.02) | 0.121 | 0.525 | 0.078 | 0.130 |
| IP-10 (pg/mL) | 202.44 (163.94, 211.78) | 270.27 (187.35, 395.70) | 184.50 (138.23, 257.25) | 322.32 (241.98, 412.60) | 0.007** | 0.976 | <0.001* | 0.005** |
| MCP-1 (pg/mL) | 23.28 (16.55, 29.61) | 31.06 (25.30, 44.00) | 31.27 (26.27, 36.72) | 30.96 (24.96, 48.67) | 0.003** | 0.011* | 0.005** | 0.805 |
| MIP-1α (pg/mL) | 2.12 (1.61, 2.82) | 4.23 (3.22, 5.92) | 4.09 (2.67, 5.04) | 4.35 (3.25, 10.15) | <0.001*** | 0.002** | <0.001*** | 0.247 |
| PDGF-BB (pg/mL) | 363.45 (237.83, 433.46) | 415.79 (312.23, 504.89) | 469.12 (320.27, 582.11) | 411.82 (304.91, 486.81) | 0.131 | 0.104 | 0.223 | 0.422 |
| MIP-1β (pg/mL) | 221.12 (215.93, 235.72) | 236.19 (223.85, 249.95) | 237.57 (226.79, 256.82) | 236.19 (220.12, 245.60) | 0.140 | 0.104 | 0.246 | 0.550 |
| RANTES (pg/mL) | 7581.99 (6942.25, 8470.32) | 8178.82 (7689.31, 9070.81) | 7987.64 (7702.62, 8887.28) | 8265.12 (7583.14, 9209.47) | 0.074 | 0.169 | 0.089 | 0.631 |
| TNF-α (pg/mL) | 46.57 (42.61, 49.20) | 51.39 (46.68, 58.57) | 50.51 (46.68, 54.65) | 52.70 (46.79, 61.05) | 0.015* | 0.051 | 0.020* | 0.439 |
| VEGF (pg/mL) | 0.00 (0.00, 0.00) | 0.00 (0.00, 52.66) | 0.00 (0.00, 0.00) | 0.00 (0.00, 65.88) | 0.054 | 0.525 | 0.124 | 0.475 |
p1: TA patients vs. healthy controls.
p2: TA with depression vs. healthy controls.
p3: TA without depression vs. healthy controls.
p4: TA with depression vs. TA without depression.
IL, interleukin; IFN-γ, interferon gamma; IP-10, interferon gamma-induced protein-10; FGF basic, fibroblast growth factor basic; G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte macrophage colony-stimulating factor; MCP-1, monocyte chemotactic protein-1; MIP-1α, macrophage inflammatory protein-1alpha; MIP-1β, macrophage inflammatory protein-1 beta; PDGF-BB, platelet-derived growth factor-BB; RANTES, regulated upon activation, normal T cells expressed, and secreted; TNF-α, tumor necrosis factor-alpha; VEGF, vascular endothelial growth factor. *represents p-value < 0.05,** represents p-value < 0.010, *** represents p-value < 0.001.
Figure 1.
Comparison of three groups of cytokine levels. (A) The levels of IL-4, (B) IL-13, (C) eotaxin, and (D) IP-10 in the TA with depression, TA without depression, and healthy controls, respectively. *p < 0.05; **p < 0.01; ***p < 0.001.
Correlation between self-rating depression scale and cytokines, ESR, hsCRP, and disease activity
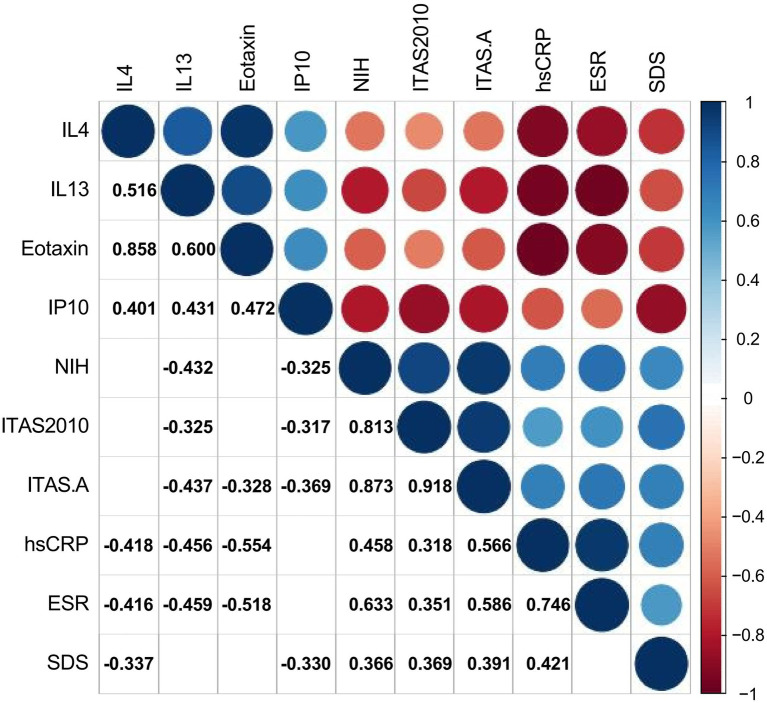
The authors used Spearman’s test as appropriate to assess the correlations between SDS and other significant variables in TA patients. The authors presented statistically correlated parameters in Figure 2 (p-value < 0.05) and the correlation coefficient r was shown in the form, while the blank indicated no statistical association. SDS was negatively correlated with the levels of IL-4 and IP-10 (r = −0.337, p = 0.033; −0.330, p = 0.038), while positively correlated with hsCRP, NIH, ITAS.A and ITAS2010 (r = 0.421, p = 0.007; r = 0.366, p = 0.020; r = 0.391, p = 0.013; r = 0.369, p = 0.019, respectively). In the meantime, the levels of IL-4, IL-13 and eotaxin were negatively associated with ESR (r = −0.416, p = 0.008; r = −0.459, p = 0.003; r = −0.518, p = 0.001) and hsCRP (r = −0.418, p = 0.007; r = −0.456, p = 0.003; r = −0.554, p < 0.001).
Figure 2.
Correlations between cytokines, ESR, hsCRP, disease activity, and Self-Rating Depression Scale. The number and color in each box represented the correlation coefficient for two covariates corresponding to the box, of which blank indicated a non-statistically significant association.
Risk factors of depression in TA patients
To identify independent predictors of depression in TA, the authors use multivariate logistic regression analysis to confirm the value of cytokines, which were significantly different in univariate analysis (p < 0.05) (Table 4). The univariate logistic regression analysis showed that age of onset and the levels of IL-4, IL-13, eotaxin, and IP-10 were protective in the depressed TA group (OR [95% CI] 0.926 [0.864, 0.992], p = 0.028; 0.176 [0.045, 0.689], p = 0.013; 0.021 [0.001, 0.555], p = 0.021; OR [95% CI] 0.932 [0.883, 0.983], p = 0.010; 0.992 [0.985, 0.999], p = 0.028, respectively), while ESR and NIH, ITAS.A as potential risk factors (OR [95% CI] 1.053 [1.004, 1.104], p = 0.032; 2.654 [1.251, 5.631], p = 0.011; OR [95% CI] 1.246 [1.035, 1.500], p = 0.020, respectively). In order to exclude confounding factors and build a multivariate regression model to better identify risk factors in the depressed group, the authors included traditional demographic risk factors, adjusted for gender, education, and disease duration. The results suggested that both the lower level of IL-4 and age of onset were risk factors for depression in TA patients (OR [95% CI] 0.124 [0.018, 0.827], p = 0.031; 0.870 [0.765, 0.990], p = 0.035, respectively).
Table 4.
Multiple logistic regression model with cytokines and confounding factors.
| Univariable analysis | Multivariable analysis | |||
|---|---|---|---|---|
| Odds ratio (95% CI) | p-value | Odds ratio (95% CI) | p-value | |
| Age of onset | 0.926 (0.864, 0.992) | 0.028* | 0.870 (0.765, 0.990) | 0.035* |
| Female | 0.999 | |||
| Disease duration | 0.999 (0.999, 1.005) | 0.716 | ||
| Education time | 1.170 (0.941, 1.456) | 0.158 | ||
| ESR | 1.053 (1.004, 1.104) | 0.032* | ||
| hsCRP | 1.095 (0.984, 1.219) | 0.096 | ||
| NIH | 2.654 (1.251, 5.631) | 0.011* | ||
| ITAS.A | 1.246 (1.035, 1.500) | 0.020* | ||
| ITAS2010 | 1.187 (0.967, 1.457) | 0.102 | ||
| IL-4 | 0.176 (0.045, 0.689) | 0.013* | 0.124 (0.018, 0.827) | 0.031* |
| IL-13 | 0.021 (0.001, 0.555) | 0.021* | ||
| Eotaxin | 0.932 (0.883, 0.983) | 0.010* | ||
| IP-10 | 0.992 (0.985, 0.999) | 0.028* | ||
*represents p-value < 0.05.
Discussion
In this research, the authors recorded the levels of 27 cytokines, laboratory, and clinical data in patients with Takayasu arteritis (TA). Our study has reported the age of onset and IL-4 were risk factors for depressed TA patients and showed the depression group was more active in the disease for the first time. The authors found that depression was correlated with lower levels of IL-4, IL-13, eotaxin, and IP-10, suggesting the abnormal immune responses contributing to the evolution of depression in TA.
The authors found the depressed group was more active in TA patients. There were higher levels of ESR, hsCRP, NIH, ITAS.A, and ITAS2010 in TA with depression compared with non-depression. Previous studies have found depression and inflammation are intertwined, which depression facilitates inflammatory responses and inflammation promotes depression (21). Due to the inflammatory properties of autoimmune disease, many research studies devoted to studying the bidirectional inflammatory pathway between autoimmune diseases and depression (22). Imran et al. (23) identified a positive correlation between the severity of depression and the disease activity in rheumatoid arthritis (RA). Similar to other autoimmune disorders such as Behçet disease and rheumatoid arthritis (23, 24), the disease activity also showed a positive correlation with depression scores in TA (11, 25), which was consistent with our results.
Our study then compared the clinical characterizations between the depressed and non-depressed groups in TA patients. There was no heterogeneity in disease duration, BMI, educational time, and the female ratio. The younger age of onset was identified as an independent risk factor for depression in TA patients. It was shown that the younger age had well-known associations with depression (26). This can also be observed in autoimmune diseases. Previous research has shown that younger age in people under 40 years old had a significant interaction with RA in terms of depression risk (27, 28). In the existing life quality reports on TA, it was argued that age was negatively associated with emotional scores (29). However, studies on the relationship between age in TA patients with depression are lacking. Consistent with RA, the authors found that the younger age of onset showed a significantly positive correlation with depression in TA. This may be related to the fact that young patients are often actively involved in social work. The physical discomfort of TA combined with work stress can have a negative impact on the individual and trigger negative emotions.
The coexistence of immune-mediated inflammatory diseases and depression has long been recognized (7). Therefore, the authors focused on the analysis of the link between cytokines and depression in TA. In our study, the authors found the levels of IL-4, IL-13, eotaxin, and IP-10 were significantly lower in the depression of TA patients. Furthermore, the lower level of IL-4 proved to be an independent risk factor for depressed TA patients. IL-4 is an important anti-inflammatory factor produced by activated Th2 cells, thereby reducing the inflammatory response (30). Previous studies have proved that deficiencies in IL-4 can lead to an inability to recover in depressed mice caused by stress (31). It was shown that IL-4 reprogrammed the microglia, which activated the brain-derived neurotrophic factor signaling pathways to protect hippocampal nerves in depressed mice (32). These results indicated that IL-4 exerted neuroprotective function to fight depression. Current research found an elevated level of IL-4 in depressed populations and rats after exposure to chronic mild stress (33, 34) to suppress the inflammatory response. Recent findings suggested that IL-4 and IL-13 might play a significant role in the downregulation of inflammatory processes underlying RA pathology and beneficially modulate the course of the disease (35). In autoimmune disease, scholars have found that an increase in the ratio of Th1/Th2 cells could promote the production of depressive symptoms in collagen-induced arthritis mice (36). Hyperactive or hypoactive stress systems through modulation of the Th1/Th2 cytokine balance might be associated with abnormalities of the “systemic anti-inflammatory feedback,” contributing to the pathogenesis of depression and autoimmune diseases (37). This can explain that a decrease in IL-4 may indicate a lower proportion of Th2 cells, leading to an increase in the Th1/Th2 ratio, which can lead to TA patients with depression. It was shown that TA patients had higher serum levels of IL-4 and IL-13 than healthy controls. However, Gao et al. (38), in their investigation of Th2 cell characteristics in TA, identified there was not a significant difference in IL-4 between active and inactive groups. This aligns with our discovery that IL-4 levels did not show a correlation with NIH/ITAS.A/ITAS2010, which implies that the reduction of IL-4 in TA patients with depression might be specific to this condition.
The cytokine IL-13 is grouped together with IL-4 as anti-inflammatory cytokines, both of which are closely linked in the genome (39, 40). However, IL-13 was proven only one-tenth of the inhibiting effect of IL-4 in RA (41), without a direct correlation with depression. A cross-sectional study on depression in patients with breast cancer identified that the level of IL-13 was lower in the depressed group than in the non-depressed (13), a lack of further research to clarify the pathological mechanisms. Previous studies have found that IL-13 is significantly elevated in TA (38). Our result that the lower level of IL-13 was associated with depression might be related to the synergy of IL-4 reduction, with the need for further research to prove it. Eotaxin is a potent eosinophil chemoattractant cytokine (42). Its main receptor, the CC chemokine receptor 3 (43–45), is expressed on Th2 lymphocytes, implicated in the production of the Th2 cytokines (IL-4, IL-13) (46). Consequently, eotaxin has been proven to tend to a Th2 response (47). However, the current research studies remains contradictory about the role of eotaxin in depression (46). In previous studies, eotaxin was found to be higher in depressed patients than in healthy controls, but still remained elevated from baseline after 8 weeks of effective treatment (48). The determination of chemokines found no significant difference in eotaxin between TA and healthy controls (49). The authors confirmed that the lower level of eotaxin had a suggestive effect on TA patients with depression to a certain extent, which might be consistent with the declined IL-4. IP-10 is a Th1-related chemokine, secreted by T lymphocytes, NK cells, and monocytes (50) and demonstrated to be associated with multiple rheumatic immune disease activity (51). It was known that the HIV+ subjects with depression had increased levels of IP-10 compared to their non-depressed counterparts (52). Similar findings have not been identified in autoimmune diseases. The authors first found the declined level of IP-10 attributed to the depression in TA, the mechanism remaining a mystery.
Given the rarity of TA, our study is limited by a small sample size. Additionally, being a retrospective and cross-sectional study, it lacks a prospective plan and a follow-up cohort. In future, the authors will expand the number of patients and collect samples from patients with other systemic inflammatory diseases for more rigorous research.
Conclusion
This study presented that the serum levels of IL-4 were significantly decreased in TA patients with depression. It suggests that IL-4 may play an important part in the development of depression in TA patients. Clinicians need to be vigilant about the decline of IL-4 in order to predict the potential risk of depression in TA patients and respond positively.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Beijing Anzhen Hospital (Approval Number: 2023183X). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
YZ: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft. SY: Data curation, Writing – original draft. AF: Investigation, Methodology, Writing – original draft. JD: Data curation, Writing – original draft. NG: Funding acquisition, Validation, Writing – review & editing. LP: Conceptualization, Funding acquisition, Writing – review & editing. TL: Conceptualization, Data curation, Funding acquisition, Validation, Writing – review & editing.
Glossary
| TA | Takayasu arteritis |
| SDS | The Zung Self-Rating Depression Scale |
| OR | Odds ratio |
| CI | Confidence interval |
| BMI | Body mass index |
| NIH | National Institutes of Health criteria |
| ITAS | Indian Takayasu’s Arteritis Activity Score |
| ITAS.A | ITAS with acute-phase reactants |
| ALT | Alanine aminotransferase |
| CR | Creatinine |
| ESR | Erythrocyte sedimentation rate |
| hsCRP | High-sensitivity C-reactive protein |
| IL | Interleukin |
| IFN-γ | Interferon gamma |
| IP-10 | Interferon gamma-induced protein-10 |
| FGF basic | Fibroblast growth factor basic |
| G-CSF | Granulocyte colony-stimulating factor |
| GM-CSF | Granulocyte macrophage colony-stimulating factor |
| MCP-1 | Monocyte chemotactic protein-1 |
| MIP-1α | Macrophage inflammatory protein-1alpha |
| MIP-1β | Macrophage inflammatory protein-1 beta |
| PDGF-BB | Platelet-derived growth factor-BB |
| RANTES | Regulated upon activation, normal T cells expressed, and secreted |
| TNF-α | Tumor necrosis factor-alpha |
| VEGF | Vascular endothelial growth factor |
| C3 | Complement component 3 |
| C4 | Complement component 4 |
| Ig | Immunoglobulin |
| WBC | White blood cell |
| RBC | Red blood cell |
| NEUT | Neutrophil count |
| PLT | Platelet count |
| cDMARD | Conventional synthetic disease-modifying antirheumatic drugs |
| btDMARDs | Biologic disease-modifying antirheumatic drugs |
| MTX | Methotrexate |
| CTX | Cyclophosphamide |
| MMF | Mycophenolate mofetil |
| AZA | Azathioprine |
| HCQ | Hydroxychloroquine |
| TCZ | Tocilizumab |
| ADA | Adalimumab |
Funding Statement
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was sponsored by the National Natural Science Foundation of China (nos. 82270427, 81900448); Beijing Natural Science Foundation (nos. 7222043, 7232038).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1.Saadoun D, Garrido M, Comarmond C, Desbois AC, Domont F, Savey L, et al. Th1 and Th17 cytokines drive inflammation in Takayasu arteritis. Arthritis Rheumatol. (2015) 67:1353–60. doi: 10.1002/art.39037, PMID: [DOI] [PubMed] [Google Scholar]
- 2.Numano F, Okawara M, Inomata H, Kobayashi Y. Takayasu's arteritis. Lancet. (2000) 356:1023–5. doi: 10.1016/s0140-6736(00)02701-x [DOI] [PubMed] [Google Scholar]
- 3.Dai X, Sun Y, Ma L, Hou J, Wang L, Gong Y, et al. A novel molecular mechanism of vascular fibrosis in Takayasu arteritis: macrophage-derived GPNMB promoting adventitial fibroblast extracellular matrix production in the aorta. Transl Res. (2023) 255:128–39. doi: 10.1016/j.trsl.2022.12.004, PMID: [DOI] [PubMed] [Google Scholar]
- 4.Savioli B, Abdulahad WH, Brouwer E, Kallenberg CGM, de Souza AWS. Are cytokines and chemokines suitable biomarkers for Takayasu arteritis? Autoimmun Rev. (2017) 16:1071–8. doi: 10.1016/j.autrev.2017.07.023, PMID: [DOI] [PubMed] [Google Scholar]
- 5.Beurel E, Toups M, Nemeroff CB. The bidirectional relationship of depression and inflammation: double trouble. Neuron. (2020) 107:234–56. doi: 10.1016/j.neuron.2020.06.002, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mindt S, Neumaier M, Hoyer C, Sartorius A, Kranaster L. Cytokine-mediated cellular immune activation in electroconvulsive therapy: a CSF study in patients with treatment-resistant depression. World J Biol Psychiatry. (2020) 21:139–47. doi: 10.1080/15622975.2019.1618494, PMID: [DOI] [PubMed] [Google Scholar]
- 7.Nerurkar L, Siebert S, McInnes IB, Cavanagh J. Rheumatoid arthritis and depression: an inflammatory perspective. Lancet Psychiatry. (2019) 6:164–73. doi: 10.1016/s2215-0366(18)30255-4, PMID: [DOI] [PubMed] [Google Scholar]
- 8.Opal SM, DePalo VA. Anti-inflammatory cytokines. Chest. (2000) 117:1162–72. doi: 10.1378/chest.117.4.1162 [DOI] [PubMed] [Google Scholar]
- 9.Howard Tripp N, Tarn J, Natasari A, Gillespie C, Mitchell S, Hackett KL, et al. Fatigue in primary Sjögren's syndrome is associated with lower levels of proinflammatory cytokines. RMD Open. (2016) 2:e000282. doi: 10.1136/rmdopen-2016-000282, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wen D, Feng L, Du X, Dong JZ, Ma CS. Biomarkers in Takayasu arteritis. Int J Cardiol. (2023) 371:413–7. doi: 10.1016/j.ijcard.2022.08.058 [DOI] [PubMed] [Google Scholar]
- 11.Yilmaz N, Can M, Oner FA, Kalfa M, Emmungil H, Karadag O, et al. Impaired quality of life, disability and mental health in Takayasu's arteritis. Rheumatology (Oxford). (2013) 52:1898–04. doi: 10.1093/rheumatology/ket238, PMID: [DOI] [PubMed] [Google Scholar]
- 12.Leighton SP, Nerurkar L, Krishnadas R, Johnman C, Graham GJ, Cavanagh J. Chemokines in depression in health and in inflammatory illness: a systematic review and meta-analysis. Mol Psychiatry. (2018) 23:48–58. doi: 10.1038/mp.2017.205, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ho HY, Chin-Hung Chen V, Tzang BS, Hsieh CC, Wang WK, Weng YP, et al. Circulating cytokines as predictors of depression in patients with breast cancer. J Psychiatr Res. (2021) 136:306–11. doi: 10.1016/j.jpsychires.2021.02.037, PMID: [DOI] [PubMed] [Google Scholar]
- 14.Grayson PC, Ponte C, Suppiah R, Robson JC, Gribbons KB, Judge A, et al. 2022 American College of Rheumatology/EULAR classification criteria for Takayasu arteritis. Ann Rheum Dis. (2022) 81:1654–60. doi: 10.1136/ard-2022-223482, PMID: [DOI] [PubMed] [Google Scholar]
- 15.Kerr GS, Hallahan CW, Giordano J, Leavitt RY, Fauci AS, Rottem M, et al. Takayasu arteritis. Ann Intern Med. (1994) 120:919–29. doi: 10.7326/0003-4819-120-11-199406010-00004 [DOI] [PubMed] [Google Scholar]
- 16.Misra R, Danda D, Rajappa SM, Ghosh A, Gupta R, Mahendranath KM, et al. Development and initial validation of the Indian Takayasu clinical activity score (ITAS2010). Rheumatology (Oxford). (2013) 52:1795–01. doi: 10.1093/rheumatology/ket128, PMID: [DOI] [PubMed] [Google Scholar]
- 17.Hata A, Noda M, Moriwaki R, Numano F. Angiographic findings of Takayasu arteritis: new classification. Int J Cardiol. (1996) 54:S155–63. doi: 10.1016/s0167-5273(96)02813-6, PMID: [DOI] [PubMed] [Google Scholar]
- 18.Hellmich B, Agueda A, Monti S, Buttgereit F, de Boysson H, Brouwer E, et al. 2018 update of the EULAR recommendations for the management of large vessel vasculitis. Ann Rheum Dis. (2020) 79:19–30. doi: 10.1136/annrheumdis-2019-215672, PMID: [DOI] [PubMed] [Google Scholar]
- 19.Dunstan DA, Scott N, Todd AK. Screening for anxiety and depression: reassessing the utility of the Zung scales. BMC Psychiatry. (2017) 17:329. doi: 10.1186/s12888-017-1489-6, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zung WW. A self-rating depression scale. Arch Gen Psychiatry. (1965) 12:63–70. doi: 10.1001/archpsyc.1965.01720310065008 [DOI] [PubMed] [Google Scholar]
- 21.Kiecolt-Glaser JK, Derry HM, Fagundes CP. Inflammation: depression fans the flames and feasts on the heat. Am J Psychiatry. (2015) 172:1075–91. doi: 10.1176/appi.ajp.2015.15020152, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matcham F, Rayner L, Steer S, Hotopf M. The prevalence of depression in rheumatoid arthritis: a systematic review and meta-analysis. Rheumatology (Oxford). (2013) 52:2136–48. doi: 10.1093/rheumatology/ket169, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Imran MY, Saira Khan EA, Ahmad NM, Farman Raja S, Saeed MA, Ijaz HI. Depression in rheumatoid arthritis and its relation to disease activity. Pak J Med Sci. (2015) 31:393–7. doi: 10.12669/pjms.312.6589, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee J, Kim SS, Jeong HJ, Son CN, Kim JM, Cho YW, et al. Association of sleep quality in Behcet disease with disease activity, depression, and quality of life in Korean population. Korean J Intern Med. (2017) 32:352–9. doi: 10.3904/kjim.2016.367, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Erdal S, Nalbantoğlu B, Gür MB, Yıldırım M, Kılıçarslan A, Kaymaz-Tahra S, et al. HADS-depression score is a mediator for illness perception and daily life impairment in Takayasu's arteritis. Clin Rheumatol. (2021) 40:4109–16. doi: 10.1007/s10067-021-05719-2, PMID: [DOI] [PubMed] [Google Scholar]
- 26.Margaretten M, Julian L, Katz P, Yelin E. Depression in patients with rheumatoid arthritis: description, causes and mechanisms. Int J Clin Rheumtol. (2011) 6:617–23. doi: 10.2217/ijr.11.6, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu MC, Guo HR, Lin MC, Livneh H, Lai NS, Tsai TY. Bidirectional associations between rheumatoid arthritis and depression: a nationwide longitudinal study. Sci Rep. (2016) 6:20647. doi: 10.1038/srep20647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fifield J, Reisine S, Sheehan TJ, McQuillan J. Gender, paid work, and symptoms of emotional distress in rheumatoid arthritis patients. Arthritis Rheum. (1996) 39:427–35. doi: 10.1002/art.1780390310, PMID: [DOI] [PubMed] [Google Scholar]
- 29.Alibaz-Oner F, Sreih AG, Merkel PA, Direskeneli H. Patient-reported outcomes in Takayasu's arteritis. Presse Med. (2017) 46:e225–7. doi: 10.1016/j.lpm.2017.03.023 [DOI] [PubMed] [Google Scholar]
- 30.Woodward EA, Prêle CM, Nicholson SE, Kolesnik TB, Hart PH. The anti-inflammatory effects of interleukin-4 are not mediated by suppressor of cytokine signalling-1 (SOCS1). Immunology. (2010) 131:118–27. doi: 10.1111/j.1365-2567.2010.03281.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wachholz S, Knorr A, Mengert L, Plümper J, Sommer R, Juckel G, et al. Interleukin-4 is a participant in the regulation of depressive-like behavior. Behav Brain Res. (2017) 326:165–72. doi: 10.1016/j.bbr.2017.03.020, PMID: [DOI] [PubMed] [Google Scholar]
- 32.Zhang J, Rong P, Zhang L, He H, Zhou T, Fan Y, et al. IL4-driven microglia modulate stress resilience through BDNF-dependent neurogenesis. Sci Adv. (2021) 7. doi: 10.1126/sciadv.abb9888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang YL, Han QQ, Gong WQ, Pan DH, Wang LZ, Hu W, et al. Microglial activation mediates chronic mild stress-induced depressive-and anxiety-like behavior in adult rats. J Neuroinflamm. (2018) 15. doi: 10.1186/s12974-018-1054-3, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xie XH, Lai WT, Xu SX, di Forti M, Zhang JY, Chen MM, et al. Hyper-inflammation of astrocytes in patients of major depressive disorder: evidence from serum astrocyte-derived extracellular vesicles. Brain Behav Immun. (2023) 109:51–62. doi: 10.1016/j.bbi.2022.12.014, PMID: [DOI] [PubMed] [Google Scholar]
- 35.Iwaszko M, Biały S, Bogunia-Kubik K. Significance of interleukin (IL)-4 and IL-13 in inflammatory arthritis. Cell. (2021) 10. doi: 10.3390/cells10113000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miossec P, Briolay J, Dechanet J, Wijdenes J, Martinez-Valdez H, Banchereau J. Inhibition of the production of proinflammatory cytokines and immunoglobulins by interleukin-4 in an ex vivo model of rheumatoid synovitis. Arthritis Rheum. (1992) 35:874–83. doi: 10.1002/art.1780350805, PMID: [DOI] [PubMed] [Google Scholar]
- 37.Calcagni E, Elenkov I. Stress system activity, innate and T helper cytokines, and susceptibility to immune-related diseases. Ann N Y Acad Sci. (2006) 1069:62–76. doi: 10.1196/annals.1351.006 [DOI] [PubMed] [Google Scholar]
- 38.Gao N, Cui W, Zhao LM, Li TT, Zhang JH, Pan LL. Contribution of Th2-like Treg cells to the pathogenesis of Takayasu's arteritis. Clin Exp Rheumatol. (2020) 38:48–54. [PubMed] [Google Scholar]
- 39.de Waal Malefyt R, Figdor CG, Huijbens R, et al. Effects of IL-13 on phenotype, cytokine production, and cytotoxic function of human monocytes. Comparison with IL-4 and modulation by IFN-gamma or IL-10. J Immunol. (1993) 151:6370–81. [PubMed] [Google Scholar]
- 40.Zurawski G, de Vries JE. Interleukin 13, an interleukin 4-like cytokine that acts on monocytes and B cells, but not on T cells. Immunol Today. (1994) 15:19–26. doi: 10.1016/0167-5699(94)90021-3, PMID: [DOI] [PubMed] [Google Scholar]
- 41.Morita Y, Yamamura M, Kawashima M, Aita T, Harada S, Okamoto H, et al. Differential in vitro effects of IL-4, IL-10, and IL-13 on proinflammatory cytokine production and fibroblast proliferation in rheumatoid synovium. Rheumatol Int. (2001) 20:49–54. doi: 10.1007/s002960000074, PMID: [DOI] [PubMed] [Google Scholar]
- 42.Sirivichayakul S, Kanchanatawan B, Thika S, Carvalho AF, Maes M. Eotaxin, an endogenous cognitive deteriorating chemokine (ECDC), is a major contributor to cognitive decline in Normal people and to executive, memory, and sustained attention deficits, formal thought disorders, and psychopathology in schizophrenia patients. Neurotox Res. (2019) 35:122–38. doi: 10.1007/s12640-018-9937-8, PMID: [DOI] [PubMed] [Google Scholar]
- 43.Kitaura M, Nakajima T, Imai T, Harada S, Combadiere C, Tiffany TL. Molecular cloning of human eotaxin, an eosinophil-selective CC chemokine, and identification of a specific eosinophil eotaxin receptor, CC chemokine receptor 3. J Biol Chem. (1996) 271:7725–30. doi: 10.1074/jbc.271.13.7725 [DOI] [PubMed] [Google Scholar]
- 44.Ponath PD, Qin S, Ringler DJ, Clark-Lewis I, Wang J, Kassam NJ, et al. Cloning of the human eosinophil chemoattractant, eotaxin. Expression, receptor binding, and functional properties suggest a mechanism for the selective recruitment of eosinophils. J Clin Invest. (1996) 97:604–12. doi: 10.1172/jci118456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Teixeira MM, Wells TN, Lukacs NW, Proudfoot AE, Kunkel SL, Williams TJ, et al. Chemokine-induced eosinophil recruitment. Evidence of a role for endogenous eotaxin in an in vivo allergy model in mouse skin. J Clin Invest. (1997) 100:1657–66. doi: 10.1172/jci119690, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Teixeira AL, Gama CS, Rocha NP, Teixeira MM. Revisiting the role of Eotaxin-1/CCL11 in psychiatric disorders. Front Psych. (2018) 9:241. doi: 10.3389/fpsyt.2018.00241, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gutierrez-Ramos JC, Lloyd C, Gonzalo JA. Eotaxin: from an eosinophilic chemokine to a major regulator of allergic reactions. Immunol Today. (1999) 20:500–4. doi: 10.1016/s0167-5699(99)01522-4, PMID: [DOI] [PubMed] [Google Scholar]
- 48.de la Peña FR, Cruz-Fuentes C, Palacios L, Girón-Pérez MI, Medina-Rivero E, Ponce-Regalado MD, et al. Serum levels of chemokines in adolescents with major depression treated with fluoxetine. World J Psych. (2020) 10:175–86. doi: 10.5498/wjp.v10.i8.175, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dong H, Zhang Y, Zou Y, Chen Y, Yue J, Liu H, et al. Elevated chemokines concentration is associated with disease activity in Takayasu arteritis. Cytokine. (2021) 143:155515. doi: 10.1016/j.cyto.2021.155515, PMID: [DOI] [PubMed] [Google Scholar]
- 50.Dufour JH, Dziejman M, Liu MT, Leung JH, Lane TE, Luster AD. IFN-gamma-inducible protein 10 (IP-10; CXCL10)-deficient mice reveal a role for IP-10 in effector T cell generation and trafficking. J Immunol. (2002) 168:3195–04. doi: 10.4049/jimmunol.168.7.3195 [DOI] [PubMed] [Google Scholar]
- 51.van Hooij A, Boeters DM, Tjon Kon Fat EM, van den Eeden SJF, Corstjens PL, et al. Longitudinal IP-10 serum levels are associated with the course of disease activity and remission in patients with rheumatoid arthritis. Clin Vaccine Immunol. (2017) 24. doi: 10.1128/CVI.00060-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rivera-Rivera Y, García Y, Toro V, Cappas N, López P, Yamamura Y, et al. Depression correlates with increased plasma levels of inflammatory cytokines and a dysregulated oxidant/antioxidant balance in HIV-1-infected subjects undergoing antiretroviral therapy. J Clin Cell Immunol. (2014) 5. doi: 10.4172/2155-9899.1000276 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.