Abstract
Peroxisome proliferator‐activated receptors (PPARs) are transcription factors belonging to the nuclear receptor family. There are three subtypes of PPARs, including PPAR‐α, PPAR‐β/δ and PPAR‐γ. They are expressed in different tissues and act by regulating the expression of target genes in the form of binding to ligands. Various subtypes of PPAR have been shown to have significant roles in a wide range of biological processes including lipid metabolism, body energy homeostasis, cell proliferation and differentiation, bone formation, tissue repair and remodelling. Recent studies have found that PPARs are closely related to tumours. They are involved in cancer cell growth, angiogenesis and tumour immune response, and are essential components in tumour progression and metastasis. As such, they have become a target for cancer therapy research. In this review, we discussed the current state of knowledge on the involvement of PPARs in cancer, including their role in tumourigenesis, the impact of PPARs in tumour microenvironment and the potential of using PPARs combinational therapy to treat cancer by targeting essential signal pathways, or as adjuvants to boost the effects of current chemo and immunotherapies. Our review highlights the complexity of PPARs in cancer and the need for a better understanding of the mechanism in order to design effective cancer therapies.
Keywords: cancer, combinational therapy, PPARs, therapeutic targets, tumor microenvironment
1. INTRODUCTION: PPAR ISOFORMS IN HEALTH AND DISEASES
Peroxisome proliferator‐activated receptors (PPARs) are transcription factors that belong to the nuclear receptor family, they can be activated by endogenous unsaturated and saturated fatty acids or synthetic ligands. 1 , 2 , 3 , 4 PPAR‐α, the first isoform of PPAR, was successfully cloned from the mouse liver in 1990 and is a new nuclear receptor that plays a key role in triglyceride and cholesterol homeostasis. 2 , 5 Two years later, all three PPAR isoforms, namely PPAR‐α, PPAR‐β/δ and PPAR‐γ, were isolated from the ovary and liver of Xenopus laevis. 5 , 6 Since then, 30 years of in‐depth research on PPAR has gradually unveiled its mystery. The expression of each PPAR isoform was found to be tissue specific. PPAR‐α is mainly expressed in liver, kidney and tissues involved in lipid oxidation. PPAR‐γ are found in macrophages, adipose tissue, vascular smooth muscle and tumours of various organ origins. Different from PPAR‐α and PPAR‐γ, the expression of PPAR‐β/δ has been reported in skeletal muscle, adipose tissue, heart, etc. 4 , 7 After ligand binding, PPARs form a heterodimer with nuclear receptor and regulate target gene expression by binding to specific consensus DNA sequences in the promoter, namely peroxisome proliferator response elements (PPREs) (Figure 1). 4 , 8 The function of PPARs is primarily accomplished through ligand binding. In the repressed state, the heterodimer binds to corepressor proteins, forming multi‐protein complexes containing histone deacetylase activity, which ultimately represses target gene transcription. Upon ligand binding, the heterodimer undergoes a conformational change, releasing the corepressor and enhancing the coactivator binding. 9 Additionally, various post‐translational modifications (PTMs) also regulate the functions of PPARs. These modifications include phosphorylation, SUMOylation, ubiquitination, acetylation and O‐GlcNAcylation, which are found at multiple modification sites. The addition of these PTMs can have a wide spectrum of consequences on protein stability, transactivation function and co‐factor interaction. Furthermore, specific PTMs in PPAR proteins have complex roles in cancer and metabolism, as described in detail in this review. 10
FIGURE 1.
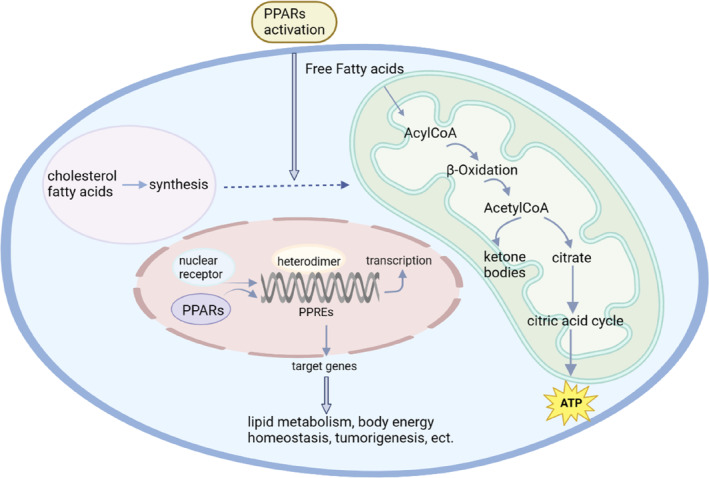
Activation of peroxisome proliferator‐activated receptors (PPARs). Upon activation, PPARs form heterodimers with nuclear receptors by binding to their ligands. The heterodimers then bind to peroxisome proliferator response elements (PPREs) in the promoter region, which regulates the expression of downstream target genes. These genes play crucial roles in various biological processes, such as lipid metabolism, body energy homeostasis and tumourigenesis. Figure was generated with BioRender.
Currently, it has been established that PPAR isoforms are important in metabolism and body energy homeostasis. 11 PPAR‐α regulates genes involved in fatty acid uptake, β‐oxidation and ω‐oxidation. It not only downregulates apolipoprotein C‐III, which regulates lipoprotein lipase hydrolysis, but also regulates genes involved in reverse cholesterol transport, such as apolipoprotein A‐I and apolipoprotein A‐II. 12 PPAR‐δ activation can regulate HDL cholesterol levels and affect glycaemic control. 13 Activation of PPAR‐δ significantly improves glucose tolerance and insulin resistance. 14 PPAR‐γ is a major regulator of adipocyte differentiation, but recent studies have shown that its activation is also associated with the expression of many important genes that affect energy metabolism, including TNF‐α, leptin and adiponectin. 15 PPAR‐γ can also induce cell cycle arrest by inhibiting cyclin‐dependent kinase activity in several tumour cell lines. 16
PPAR agonists have been used in clinical practice for various purposes. For example, PPAR‐α agonists, such as fibrates, are used clinically to lower lipids and prevent atherosclerosis and cardiovascular disease, 11 , 17 whereas PPAR‐γ agonists, such as thiazolidinediones, reduce blood glucose levels mainly in skeletal muscle and adipose tissue by increasing insulin sensitivity. 18 In addition, recent studies have found that PPAR isoforms are also crucial in a broad spectrum of biological processes, including cell proliferation and differentiation, signalling pathways involving fatty acid and eicosanoid, bone formation, tissue repair and remodelling. 19 Therefore, the PPAR agonists have gained considerable interest as potential therapeutic candidates for neurodegenerative diseases, 20 psychiatric disorders such as addiction and depression, 21 , 22 , 23 liver 24 , 25 and kidney diseases 26 , 27 and autoimmune and inflammatory diseases. 28 , 29 , 30 Furthermore, PPARs are closely related to cancer. Increasing evidence indicates that PPARs are involved in cancer cell growth, angiogenesis and tumour immune response and are essential in tumour progression and metastasis. In the scope of this review, we explored the effects and possible mechanisms of PPAR agonists in tumourigenesis and the tumour microenvironment (TME), we also analysed the latest evidence on the co‐administration of PPAR agonists with chemo, immune or other therapies and conducted a critical assessment of the existing knowledge gaps and progress in this area.
2. ROLES OF PPARs IN TUMOURIGENESIS
Tumourigenesis is the process by which normal cells undergo a transformation and gain the malignant properties of proliferation, differentiation and metastasis. 31 PPARs have been recognized as potential cancer therapies due to their key roles in metabolism and proliferation. Notably, different isoforms of PPARs play distinct roles in tumour progression across various cancer types. For example, the role of PPAR‐β/δ is controversial in multiple studies under different condition‐based disease types and research models. PPARs are widely involved in abnormal metabolism progress and could potentially act as a therapy option in various cancer types through versatile strategies. Here, we mainly focus on the roles of PPARs in cancer, specifically on cancer proliferation, metabolism and metastasis (Figure 2).
FIGURE 2.
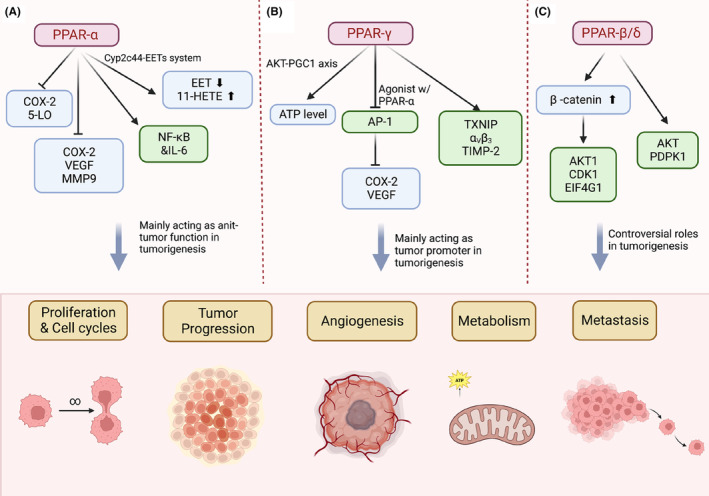
Roles of peroxisome proliferator‐activated receptors (PPARs) in tumourigenesis. Act as nuclear receptor, PPARs regulate lipid metabolism and are involved in tumour progression. (A) The isoform PPAR‐α regulates nuclear receptor NF‐κB and COX‐2‐related metabolism progression. PPAR‐α mainly plays an antitumour role during tumour progression. (B) The isoform PPAR‐γ increases ATP level through AKT involved signalling and reduces the expression of COX‐2/vascular endothelial growth factor (VEGF) by inhibiting AP‐1. PPAR‐γ mainly acts as a tumour promoter during tumour progression. (C) The isoform PPAR‐β/δ affects AKT mediated signalling and plays controversial roles in tumourigenesis. Figure was generated with BioRender.
2.1. PPARs involve tumourigenesis through metabolism reprogramming
The dysregulated metabolism has been recognized as the hallmark of cancer 32 and it has been shown that the fatty acid biosynthetic pathway is involved in an early stage of tumour progression. 33 As nuclear receptors, PPARs involve in metabolic progress, which regulate the energy level and cell fate. Based on the different isoform and cancer type, PPARs exert a dual function in tumour progression, and the function of PPARs are determined by the various cancer types and research models. For example, a study using the orthograft prostate cancer model demonstrated that PPAR‐γ activation could stimulate the AKT‐PGC1 axis, resulting in increased ATP levels and enhanced mitochondrial biogenesis activities. The elevated ATP levels create an energetically favourable environment for tumour growth and metastasis. 34 Moreover, in combination therapy, the co‐administration of PPAR‐α and PPAR‐γ agonists was found to inhibit activator protein‐1 (AP‐1), leading to reduced expression of cyclooxygenase‐2 (COX‐2) and vascular endothelial growth factor (VEGF). This combination of agonists was shown to reduce angiogenesis and induce apoptosis in a mouse model of OVCAR‐3 ovarian tumours. The activation of PPAR‐α and PPAR‐γ could also suppress the progression of solid ovarian tumour. 35 In addition, PARA‐γ could support the cancer growth through the metabolism reprogramming of cancer‐associated fibroblasts (CAFs) and adipocytes. 5
PPARs regulate multiple metabolic pathways which may further impact the cellular proliferation and promote metastasis in cancer progress. For example, activated PPAR‐α is deeply involved in the regulation of lipid metabolism in many healthy organs. 36 Under the context of cancer, PPAR‐α regulates proliferation and cell cycles of tumour cells through inhibition of the prostaglandin biosynthesis and arachidonic acid metabolic pathway‐related enzyme COX‐2 and 5‐lipoxygenase (5‐LO). 37 Similarly, in non‐small cell lung cancer, the activation of PPARα ligand reduced the production of proangiogenic epoxyeicosatrienoic acids (EET) and increased the hydroxyl 11‐hydroxyeicosatetraenoic acids (11‐HETE) through Cyp2c44‐EETs system, thus inhibiting tumour progression and metastasis (Figure 2A). 38 More details on how PPARs involve tumourigenesis on these two aspects are as below.
2.2. Effects of PPARs in tumourigenesis involve tumour cell proliferation and survival
The abnormal proliferation is another hallmark of cancer. Cellular proliferation is a fundamental function of cells, enabling them to perform essential roles and support organ survival. 39 During tumourigenesis, normal cells acquire malignant properties, including fast proliferation. 40 PPARs, as transcription factors, play a role in cell proliferation and the dysregulation of apoptosis, consequently leading to tumourigenesis 41 ; however, the isoform of PPARs contribute to tumour progression in different aspects.
PPAR‐α has been reported as a tumour promoter for the regulation of proliferation and cell death through lipid metabolic modulation in a breast cancer cell. 42 Papi et al. reported the activation of PPAR‐α promoted multi‐signalling pathways, including nuclear receptor κB (NF‐κB)/interleukin‐6 (IL‐6) axis, and resulted in clonal expansion of breast cancer mammospheres. 43 The dual role of PPAR‐γ in tumour survival is more complex than PPAR‐α. The anti‐proliferative and pro‐apoptotic properties of PPAR‐ γ have been widely reported in colon, oesophageal, breast, lung and prostate cancer. 44 , 45 , 46 As a differentiation‐promoting factor, PPAR‐γ has been found as an antitumour target in breast cancer, although it is also associated with poor prognosis patients with Cox‐1 negative primary breast cancers. 47 , 48 Many studies have shown that the existing antidiabetic drug thiazolidinediones (TZDs), also a synthetic agonists of PPAR‐γ, exert a beneficial effect on breast cancer treatment in both a pre‐clinical mouse models and clinical trials. 49 For instance, Khandekar et al. found PPAR‐γ ligands can induce cell death through accumulated DNA damage, which sensitize cancer cells to cytotoxic chemotherapy. 50 However, in a Phase II clinical trial involving thyroid cancer, rosiglitazone, another member of the TZD family, did not show any relationship with the expression of PPAR‐γ. 51 Similarly, PPAR‐γ play a dual role in prostate cancer and it may relate with complex factors including the research models and different stage of cancer progression. For example, PPAR‐γ was considered as a tumour suppressor due to its ability 52 to inhibit tumour cell proliferation. However, it has been shown that a high level of PPAR‐γ expression is associated with late‐staged or high‐graded prostate cancer, suggesting its role in promoting prostate cancer. 53 Furthermore, PPAR‐γ antagonists have also shown the anticancer effects in various epithelial cancer. The selective PPAR‐γ antagonists, T007, has been demonstrated the anticancer effect in breast cell lines MD‐231 and MCF‐7. It has also been shown that T007 is involved the apoptotic pathways in hepatocellular carcinoma cells in a certain concentration. 54
3. PPARs INVOLVE TUMOUR DEVELOPMENT THROUGH METASTASIS
Metastasis is the process that cancer cells move from the primary site to form a new tumour in other parts of the body, which is usually associated with late‐stage cancer. 55 In colon cancer, PPAR‐α promoted metastasis by inhibiting the expression of Cox‐2 and VEGF and TGF‐induced matrix metalloproteinase (MMP)‐9, both factors are highly implicated in metastasis promotion. 56 , 57 A study in melanoma revealed that the activation of PPAR‐γ is involved in the melanoma cell invasiveness through regulating thioredoxin‐interacting protein (TXNIP), integrin alpha‐v/beta‐3 and the tissue inhibitors of metalloproteinases (TIMP)‐2, all of which promoted the progression of metastasis in melanoma cancer (Figure 2B). 58 It has been shown that both PPAR‐α and PPAR‐γ are widely involved in the late‐stage of cancer and promote metastasis. Especially, PPAR‐γ has been recognized as a therapeutic target for tumour angiogenesis and metastasis in multiple steps. For example, PPAR‐γ agonists could inhibit angiogenetic factors including FGF2 and VEGF, thus inducing endothelial cell apoptosis in several cancer cell lines. 52
Many studies have shown that PPAR‐β/δ is associated with the progression of tumour metastasis across different cancer types. The activation of PPAR‐β/δ receptor has been widely shown to have a pro‐tumourigenic role in colon cancer progression, especially the APC mutation‐driven colorectal cancer progression 59 (Figure 2C). A study showed that overexpression of PPAR‐β/δ is associated with poor prognosis colorectal cancer with potent of distant liver metastases. 60 One possible explanation is that the PPAR‐β/δ receptor may be involved in cell proliferation. For example, the knock down of PPAR‐β/δ promotes the growth of colon cancer by reducing the differentiation and accelerating the proliferation in cell lines and xenograft mouse model. 61 , 62 Besides that, overexpression of PPAR‐β/δ can also increase migration and metastasis in breast cancer through elevated expression of antioxidant proteins and AKT‐mediated signalling, which are involved in the survival of breast cancer cells. 63 In addition, PPAR‐β/δ receptor can stimulate the activation of β‐catenin and enhance invasiveness through PDGFR β, AKT1, EIF4G1 and CDK1 pathways. 64 , 65 Another explanation could be that the activation of PPAR‐β/δ receptor involves in the proinflammatory modulating and proangiogenic molecules across different cancer types. 59
4. THE ROLE OF PPARs TME
The TME comprises nonmalignant cells including fibroblasts, endothelial cells, immune cells, as well as the acellular components like extracellular matrix, secreted chemokines, cytokines and cell metabolites. Over the past decades, the role of TME in tumour progression and the therapeutic resistance have become evident. Increasing appreciation gradually raised in the regulations of PPARs in TME cells which directly and indirectly exerted certain impacts on cancer progression.
4.1. PPARs in cancer stromal microenvironment
Under the paracrine influences of cancer cells, stromal cells such as CAFs and tumour‐associated endothelial cells can become the nutrients donor of tumour cells, providing fuels such as glutamine, L‐lactate, fatty acids and ketone bodies. PPARs govern many processes involved in the metabolic remodelling of stromal cells and further influent tumour cells.
4.1.1. Fibroblast
As energy regulators, PPARs interact closely with metabolic regulator elements to reprogram the cell metabolism, and cell fate of fibroblasts. The PPARs‐regulated metabolic reprogramming in CAFs is correlated to tumour initiation, proliferation and progression through the epithelial–mesenchymal communication (Figure 4A). Clinically, the expression of PPAR‐γ is significantly upregulated in CAFs of cutaneous skin squamous cell carcinoma and colon adenocarcinoma. 66 Avena et al. reported the overexpression of PPAR‐γ reprogrammed CAFs to the autophagic and glycolytic metabolism, which accelerates tumour growth in breast cancer xenograft mouse model when co‐implanted with transgenic PPAR‐γ‐high fibroblasts. 67 PPAR‐β/δ in CAFs controls the redox homeostasis and affects tumourigenesis through stromal‐epithelial crosstalk. In skin tumour mouse model, Tan et al. showed, PPARβ/δ‐knockout fibroblasts remarkably increased the H2O2 production in the adjacent epidermis, subsequently triggering an RAF/MEK‐mediated NRF2 activation that elicits a strong antioxidant and cytoprotective response, which resulted in fewer and smaller skin tumours when comparing to wild‐type mice exposed to topical carcinogens. 68 The expression of LRG1was upregulated by PPAR‐β/δ in fibroblasts. LRG1 interferes with TGF‐β1‐dependent redox homeostasis, resulting in oncogenic transformation in the surrounding epithelium. 69 , 70 , 71
FIGURE 4.
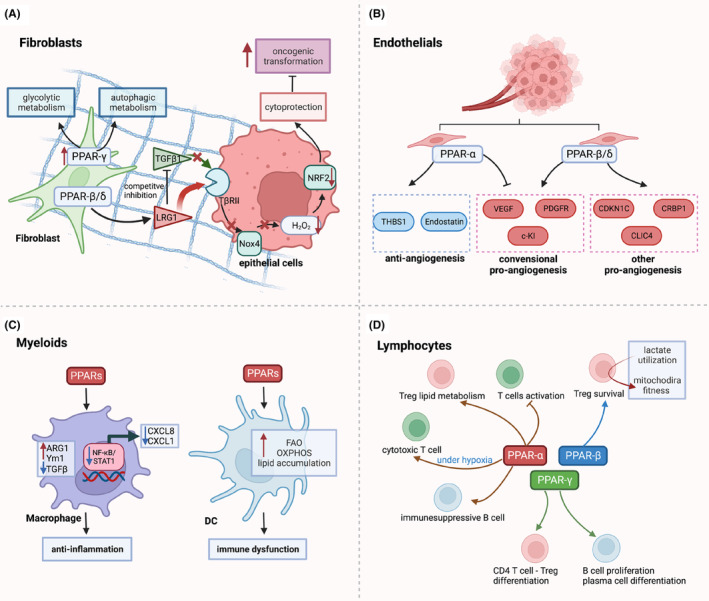
Role of peroxisome proliferator‐activated receptors (PPARs) in tumour microenvironment. (A) PPARs reprogram the metabolism of fibroblasts. overexpression of PPAR‐γ reprogrammed cancer‐associated fibroblasts to the autophagic and glycolytic metabolism, which accelerates tumour growth. PPAR‐β/δ activate LRG1 which interferes with TGF‐β1‐dependent redox homeostasis, resulting in oncogenic transformation in the surrounding epithelium. (B) In the endothelial cells, PPAR‐α exhibits an anti‐angiogenic effect by promoting anti‐angiogensis genes and inhibiting pro‐angiogenesis factors, while PPAR‐β/δ is more pro‐angiogenic, promoting endothelial proliferation and vascular maturation. (C) PPARs prime anti‐inflammatory M2‐like macrophage polarisation which causes anti‐inflammatory response; PPARs regulate DCs lipid metabolism, resulting in immune dysfunction. (D) PPARs involve in activation, differentiation, metabolic reprogramming of T cell and B cell. Figure was generated with BioRender.
4.1.2. Endothelium
Rapid tumour growth often induces hypoxic regions with poor oxygen perfusion and insufficient nutrients from the existing vasculature. This can be mitigated by secreted pro‐angiogenic factors which is promoted by PPAR‐β/δ, but inhibited PPAR‐α (Figure 4B). The pro‐angiogenic effects of PPAR‐β/δ activation have been revealed in previous studies. In PPAR‐β/δ knockout mouse model, the endothelial cells formed the immature microvessels in the tumours, leading to abnormal microvasculature and restricted blood flow into the tumours. 72 The activation of PPAR‐β/δ in endothelial resulted in upregulated biosynthesis of VEGF, PDGFR and c‐KI, which accelerated endothelial cell proliferation and vascular formation. 73 Beside conventional growth factors, other potential PPAR‐β/δ‐dependent angiogenic mediators include CDKN1C, 73 IL‐8, 74 CLIC4 and CRBP1. 75 In contrast to PPAR‐β/δ, PPAR‐α is an anti‐angiogenic nuclear receptor. Activated PPAR‐α in stromal cells attenuated tumour angiogenesis and tumour xenograft growth by upregulating the expression of anti‐angiogenic factors, including thrombospondin‐1 and endostatin, which suppress endothelial cell proliferation and neovascularisation. 76 , 77 , 78 PPAR‐γ was reported to maintain endothelium homeostasis through the interaction with key regulators of DNA repair signalling. Activated PPAR‐γ binds to DNA damage sensor MRE11‐RAD50‐NBS1 (MRN) and the E3 ubiquitin ligase UBR5, which promote ATM activation and DNA repair. 79
4.2. PPARs in inflammation and cancer immune microenvironment
PPARs and their endogenous ligand lipids are closely related to the anti‐inflammation and immuno‐suppressive phenotype transformation of tumour‐infiltrated immune cells, which can be modulated either directly through regulating immunomodulatory gene expression or indirectly by altering lipid metabolism.
4.2.1. Inflammation
As transcription factors, activated PPARs bind to their recognition sequences and regulate the expression of genes involved in inflammation (Figure 3A). PPAR‐γ could stimulate the trans‐repression on proinflammatory genes like NF‐κB through a type of PTM called SUMOylation or through the conjugation with small ubiquitin‐like modifier (SUMO). 80 Similarly, PPAR‐α could also downregulate inflammation by gene transrepression. It was found that PPARα directly bound key transcription factors of IL‐6, the NF‐κB subunit p65, c‐Jun and c‐AMP response element‐binding protein‐binding protein (CBP). 81 To note, unlike the other two subtypes, PPAR‐β/δ ligand repress the inflammatory genes indirectly. Bartish et al showed activation of PPAR‐β/δ released BCL‐6 and trans‐repress the expression of inflammatory genes like CCL12, IL‐1β, TNFα, IL‐6. 82
FIGURE 3.
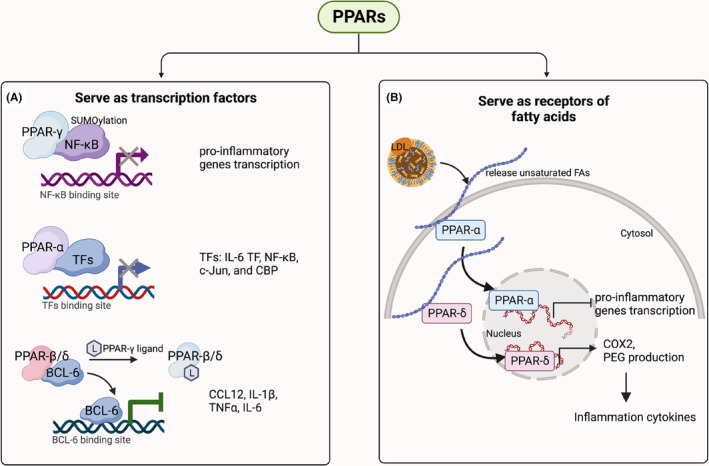
Role of peroxisome proliferator‐activated receptors (PPARs) in tumour inflammation. (A) PPARs serve as transcriptional regulators directly or indirectly repressing the expression of proinflammatory genes. Top, PPAR‐γ binds NF‐κB thus affect the transcription of the genes regulated by NF‐κB; middle, PPAR‐α binds transcription factors thus interfere the expression of IL‐6, the NF‐κB subunit p65, c‐Jun and c‐AMP response element‐binding protein‐binding protein (CBP); bottom, ligands activated PPAR‐β/δ releases BCL‐6 thus transrepress the expression of inflammatory genes. (B) PPARs are activated by lipid ligands further regulating the production of inflammatory factors. PPAR‐α is activated by FAs then translocate to nuclear repress the transcription of proinflammatory genes. In contrast, FAs activated PPAR‐δ induced the production of COX2 and PEG2 thus promoted the inflammation. Figure was generated with BioRender.
As receptors of fatty acid‐derived signals, PPARs are capable of transducing lipid‐mediated inflammatory signalling events 83 , 84 (Figure 3B). PPARs can directly modify the intracellular and extracellular lipid pool and alter the lipid microenvironment to initiate further inflammatory regulatory processes. It is noted that three subtypes of PPARs regulate lipid homeostasis in different ways. PPAR‐α provides energy from lipid catabolism during starvation, PPAR‐γ is activated in well‐fed state and initiate the synthesis of fatty acids, while PPAR‐β/δ regulates fatty acids level to ensure enough energy for muscles. 85 Therefore, their regulations on inflammation are distinct. Many studies reported that activation of PPAR‐α and PPAR‐β/δ regulated the inflammatory process. 86 For instance, low‐density lipoprotein (LDL) can release hydroxyoctadecadienoic acids (HODEs), known as PPAR‐α agonists, further reversing the proinflammatory responses of LDL. 87 The proinflammation role of PPAR‐δ in tumours has been studied by various groups, especially in relation to lipid signalling like prostaglandin E2 (PGE2) induced inflammation process. 88 Activation of PPAR‐δ in colon cancer cell lines upregulated COX‐2 expression and PGE2 production, subsequently increasing macrophage production of proinflammatory cytokines including CXCL1, CXCL2, CXCL4 and IL‐1β. 89
4.2.2. Cancer immune microenvironment
Myeloid
Activation of PPARs in macrophages favours an anti‐inflammatory tumour‐associated macrophage (TAM) phenotype (Figure 4C). 90 PPARs regulated the phenotypic changes of TAMs by gene transcriptomic modulation and enhanced fatty acid oxidation. In macrophages, PPAR‐α agonist and PPAR‐γ agonist‐induced M2 macrophage transformation by enhancing the expression of ARG1, Ym1 mannose receptor, TGF‐β and increasing phagocytic capacity. 91 In primary human monocyte‐derived macrophages, PPAR‐δ ligands were reported to repress inflammation‐associated NF‐κB and signal transducer and activator of transcription 1 (STAT1)‐targeted genes, including CXCL8 and CXCL1, yielding the M2‐like macrophage phenotype. 92 Recent studies have shown PPAR‐γ and PPAR‐δ primes M2 macrophage transformation by improving FAs metabolism and insulin sensitivity. 93 , 94 High concentrations of linoleic acid, arachidonic acid and lipid droplets in the TME can activate PPAR‐δ and polarize the pro‐tumoural TAMs in breast cancer and ovarian cancer. 95 , 96 Tumour‐infiltrating DCs (TIDCs) are critical in orchestrating antitumour immunity. DCs are more prone to adapt OXPHOS and FAO regulated by PPARs. Abnormal lipid accumulation is one of the emerging features of immune dysfunction of TIDCs. Yin et al. report that multitypes of tumour cells can activate PPAR‐α in TIDCs by secreting FA‐containing exosomes, resulting in lipid accumulation and FAO metabolic shift in TIDCs, ultimately leading to DC immune dysfunction. 97
T and B lymphocyte
PPARs have been shown to regulate the activation, proliferation and differentiations in T cell and B cell. 98 Unlike the dominant expression of PPAR‐γ in myeloids, PPARα is mainly found to express in T and B lymphocytes. It was reported that ligand activation of lymphocyte PPAR‐α antagonized NF‐κB and cytokine production then inhibited T‐cell activation. 99 In addition, Wang et al. revealed that CD36–PPAR‐β signal orchestrates metabolic adaptation to lactate utilisation, which sustains survival and functional fitness of intra‐tumoural Treg cells, promoting survival of intra‐tumoural. 100 Furthermore, activation of PPAR‐α and PPAR‐γ induced CD4+CD25− T cells differentiated to functional Tregs by TGF‐β. 101 PPARα signalling was reported to preserve cytotoxic function of tumour infiltrating CD8+ T cells through FAs catabolism under hypoglycaemic and hypoxic TME. 102 Compared to T cells, the role of PPARs in B cells is not well explored. PPAR‐γ activation enhanced B‐cell proliferation and significantly stimulated plasma cell differentiation as well as Ab production. 103 In contrast, Wejksza et al. reported that in breast cancer, PPAR‐α could be activated by tumour lipid metabolites, inducing immunosuppressive regulatory B cells and finally leading to distant metastasis (Figure 4D). 104
5. PPARs COMBINATIONAL THERAPY TO TREAT CANCER
Despite the promising preclinical evidence, over the decade, monotherapy of PPARs agonists has not yielded exciting results for the treatment of advanced cancer, as many clinical trials showed mixed results. For example, troglitazone monotherapy showed little to no improvement on metastatic colon cancer and breast cancer, 105 , 106 and rosiglitazone had no or minimum effects on clinical Phase II studies for prostate 107 and thyroid cancers. 108 However, the use of PPAR agonists in combination with chemotherapy or other compounds has shown more promise. In this paper, we reviewed the potential of PPAR agonists to target signalling pathways and receptors for the treatment of cancer (Figure 5), as well as their potential for use in combination with immunotherapy and other cancer treatments to improve therapeutic efficacy. We also discussed how PPAR modulator could be combined with immunotherapy and other cancer treatments to boost the therapeutic efficacy (Table 1).
FIGURE 5.
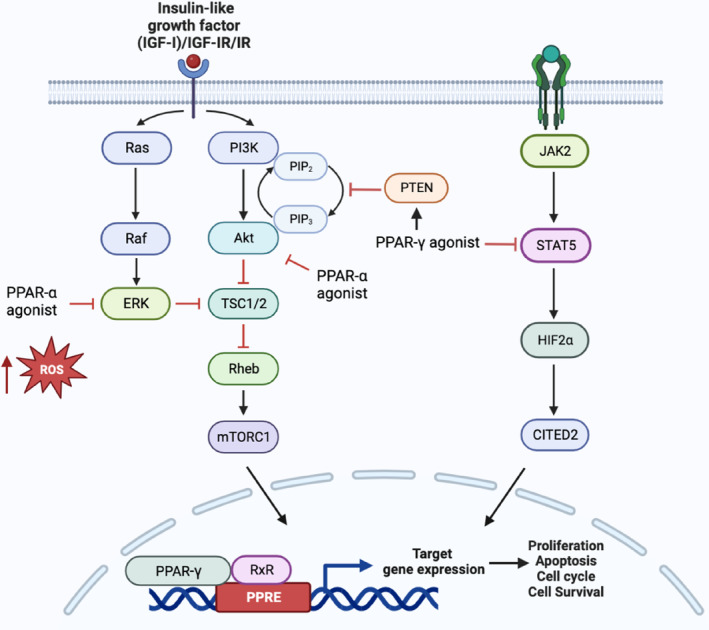
Schematic overview of the interaction of peroxisome proliferator‐activated receptor (PPAR) agonists with key signalling pathways. PPAR‐γ agonist enhance the expression of PTEN, which subsequently inhibits PI3K/AKT/mTORC1 pathway, resulting reduced tumour expansion and progression. Additionally, PPAR‐γ agonist can also downregulate the expression of STAT5 expression and its downstream targets, HIF2a and CITED2, promoting tumour quiescence. When combined with RXR agonist, PPAR‐γ agonist can potentiate their effects, leading to decreased cell proliferation and increased apoptosis by regulating the transcriptional activity of genes controlling these processes. On the other hand, PPAR‐α agonist, contribute to anticancer activity by elevating reactive oxygen species levels and inhibiting IGF‐I receptor signalling, thereby hindering tumour growth. Figure was generated with BioRender.
TABLE 1.
Summary of peroxisome proliferator‐activated receptors (PPAR) combinational therapies.
| Pathway | Subtypes | PPARs agonists | Combination | Cancer cells/function | Citation |
|---|---|---|---|---|---|
| PI3K/AKT/mTOR pathway | PPAR‐α | Clofibrate | Breast cancer cell | [37] | |
| Clofibric acid | Pioglitazone | Ovarian cancer | [35] | ||
| PPAR‐γ | Balaglitazone | Celecoxib | Hepatocellular carcinoma expansion | [109, 110] | |
| Rosiglitazone | mTOR inhibitor rapamycin | Non‐small cell lung carcinoma cell | [111] | ||
| N‐(9‐fluorenyl‐methyloxycarbonyl)‐l‐leucine (F‐L‐Leu) | Celecoxib | N1‐S1 cells, breast cancer, Huh7 cells | [110, 112] | ||
| Pioglitazone | Arsenic trioxide | Leukaemia | [113] | ||
| Pioglitazone | Trofosfamide, rofecoxib (a COX‐2 inhibitor) | Chemo‐refractory melanoma, soft tissue sarcoma, advanced vascular malignancies | [114, 115] | ||
| STAT5 | PPAR‐γ | Glitazones | Imatinib mesylate | Chronic myeloid Leukaemia | [116, 117, 118, 119, 120] |
| Retinoid X receptor/retinoic acid receptor | PPAR‐γ | Ciglitazone, pioglitazone | Rexinoid 6‐OH‐11‐O‐hydroxyphenantrene (IIF) | Colon cancer cell lines | [121] |
| Pioglitazone | IIF | Glioma cell lines, a murine glioma in vivo model | [122] | ||
| Rosiglitazone, 15‐deoxy‐Delta 12,14‐prostaglandin J, triterpenoid 2‐cyano‐3,12‐dioxooleana‐1,9‐dien‐28‐oic acid | RXR agonist: LG100268 | U937 and HL‐60; and lymphoid cells, including Su‐DHL, Sup‐M2, Ramos, Raji, Hodgkin's cell lines | [123] | ||
| 2‐cyano‐3,12‐dioxooleana‐1,9‐dien‐28‐oic acid | Bcl‐2 inhibitor HA14‐1 | Bcl‐2‐overexpressing chronic lymphocytic leukaemia cells | [123] | ||
| Insulin‐like growth factor | PPAR‐α | Fenofibrate | Medulloblastoma cell line | [124] | |
| Fenofibrate | Glioma cells | [125] | |||
| Immunotherapy | PPAR‐α | Fenofibrate | PD‐1 blocker | Reprogramming the metabolism of effector T cells | [102] |
| PPAR‐γ | Bezafibrate | Increase fatty acid oxidation and mitochondrial respiratory capacity in CD8+ T lymphocytes | [126] | ||
| Ciglitazone | Lovastatin, phenylbutyrate | Trigger TNF‐α‐related apoptosis | [127] | ||
| Chemotherapy | PPAR‐γ | Pioglitazone | Cisplatinum | Orthotopic xenograft (PDOX) models of osteosarcoma | [128] |
| Efatutazone | Paclitaxel | Advanced anaplastic thyroid carcinoma | [100, 129] | ||
| Troglitazone | Tamoxifen | MCF‐7 cells | [130] | ||
| Troglitazone | Lovastatin | DBTRG 05 MG (glioblastoma) and CL1‐0 (lung) | [131] | ||
| Troglitazone | Aspirin | Lung cancer CL1‐0 and A549 cells | [132] | ||
| Troglitazone | Radiation | Cervix cancer cells | [133] | ||
| Troglitazone | Lovastatin | Human anaplastic thyroid cancer cell Line. Mouse xenograft model | [134] | ||
| Troglitazone | TNF‐related apoptosis inducing ligand (TRAIL) | Breast cancer cell | [135] | ||
| Troglitazone | Heregulin | Breast cancer cell | [136] | ||
| Ciglitazone | TRAIL | Ca Ski cells containing HPV type 16 | [137] | ||
| Pioglitazone | Prevent radiation‐induced cognitive decline (RICD) | [138] |
5.1. PPARs combinational therapy with targeted cancer essential signal pathways
5.1.1. PI3K/AKT/mTOR pathway
The phosphatidylinositol 3‐kinase (PI3K)/AKT/mammalian target of rapamycin (mTOR) signalling pathway is essential in regulating cell proliferation, growth, metabolism, motility and cell size. 139 The phosphatase and tensin homologue (PTEN) promoter is a natural inhibitor of PI3K/AKT pathway, and studies have shown that targeting PPARs can induce tumour regression by inducing PTEN expression. Two putative PPAR binding sites have been identified within PTEN promotor, suggesting that PPAR agonists may be able to modulate the PI3K/AKT/mTOR pathway and potentially have antitumour effects. 140 A recent study suggests that Balaglitazone, a PPAR‐γ agonist, can reverse P‐glycoprotein‐mediated multidrug resistance by upregulating PTEN in a in leukaemia cell. 109 The use of celecoxib, a COX‐2 inhibitor, has been shown to upregulate PTEN gene by activation of PPAR‐γ, leading to the inhibition of AKT and disruption of hepatocellular carcinoma expansion. 110 Similarly, in a mouse model of breast cancer, the combination of celecoxib and the PPAR‐γ agonist N‐(9‐fluorenyl‐methyloxycarbonyl)‐l‐leucine (F‐L‐Leu) significantly delayed the tumour progression. 112 Some clinical trials have been conducted to test the efficacy of PPAR‐γ as an adjuvant agent in treating patients with refractory or advanced cancer. For instance, a combinational of trofosfamide, rofecoxib (a COX‐2 inhibitor) and pioglitazone was found to have exerted encouraging results in patients with chemo‐refractory melanoma, soft tissue sarcoma, as well as advanced vascular malignancies, leading to stabilisation and remission. 114 , 115 Besides inducing PTEN expression, pioglitazone also intensified the tumour‐killing effect of arsenic trioxide (ATO) in leukaemia via the suppression of PI3K/AKT pathway, 113 and mTOR inhibitor rapamycin has been shown to enhance the effects of rosiglitazone in inhibiting non‐small cell lung carcinoma (NSCLC) cell proliferation in vivo. 111
In addition to PPAR‐γ, activation of PPAR‐α by its agonist clofibrate has also been shown to downregulate the inflammatory activity of COX‐2 and 5‐LO, and inhibits cell cycle‐related kinases and breast cancer cell survival. 37 Another study in ovarian cancer showed that the combination of clofibric acid and pioglitazone significantly decreased the expression of COX‐2 and VEGF, leading to reduced tumour angiogenesis, tumour growth and induction of apoptosis through the inhibition of AP‐1. 35
5.1.2. STAT5
STAT5 proteins are recognized as major drivers in the development and/or maintenance of chronic myeloid leukaemia (CML). 116 The development of tyrosine kinase inhibitors such as imatinib mesylate has revolutionized the treatment of CML; however, some patients do not respond well to this treatment due to high levels of STAT5 expression. Activation of PPAR‐γ by glitazones can re‐sensitize imatinib‐resistant CML to treatment by downregulating STAT5 expression and its downstream targets HIF2a and CITED2, two key guardians involved with quiescence and stemness of CML leukaemia stem cells. In a small trial, when pioglitazone was given temporarily to CML patients in chronic residual disease despite continuous treatment with imatinib, all of them achieved sustained complete molecular response even after withdrawal of the drug. 117 , 118 , 119 The initial clinical Phase 1/2 trial investigating the combination of pioglitazone and imatinib in the treatment of CML patients was proven to be feasible and safe, although the efficacy of this therapy is being evaluated 120 (Clinicaltrials.gov: NCT02852486).
5.1.3. Retinoid X receptor/retinoic acid receptor
Many studies have shown that combination therapy using retinoid X receptor (RXR) agonist and PPAR‐γ agonist holds promise as novel therapy against cancers. In a study on colon cancer cell lines, the RXR agonist rexinoid 6‐OH‐11‐O‐hydroxyphenantrene (IIF) potentiated the antitumoural properties of PPAR‐γ agonist ciglitazone and pioglitazone in inhibiting cell growth and inducing apoptosis. 121 The combination of IIF and pioglitazone also markedly reduced proliferation and induced apoptosis in three glioma cell lines, and reduced tumour volume and proliferation in a murine glioma in vivo model. 122 PPAR‐γ ligation alone and in combination with either RXR agonist like LG100268 or a retinoic acid receptor agonist like all‐trans‐retinoic acid, has been shown to inhibit growth and enhance differentiating in myeloid cells (U937 and HL‐60) lymphoid cells (Su‐DHL, Sup‐M2, Ramos, Raji, Hodgkin's cell lines) and primary chronic lymphocytic leukaemia cells, by activating the transcriptional activity of target genes controlling apoptosis and differentiation in leukaemias. 123
5.1.4. Insulin‐like growth factor
It is now widely accepted that dysregulation of insulin‐like growth factor (IGF) signalling is involved in cancer development, progression and resistance. This signalling pathway involves the autocrine/paracrine production of IGFs (IGF‐I and IGF‐II) and overexpression of their cognate receptors (IGF‐I receptor, IGF‐insulin receptor (IR) and IR). 141 , 142 , 143 , 144 Fenofibrate, a PPAR‐α agonist, exerts an anticancer effect by accumulating reactive oxygen species (ROS) and inhibiting IGF‐I receptor signalling in glioma cells in vitro. 125 It also attenuates IGF‐I‐induced phosphorylation of IRS‐1, AKT, ERKs and GSK3beta, and inhibits tumour growth in medulloblastoma cell lines. 124 These findings suggest that the combination therapies of PPAR agonists may be effective in targeting IGF signalling in the treatment of cancer.
5.2. Combination of PPARs modulator with immunotherapy
In the recent decades, the cancer treatment era has been revolutionized by immunotherapy through modulating immune responses against tumour cells to overcome insufficient therapy such as radiotherapy and chemotherapy. 145 However, there are still limitations to the use of immunotherapy. For example, while PD‐1 blockade can restore the function of effector T cells, these cells can still die from terminal differentiation and energy restriction in the tumour microenvironment. To address this, researchers have suggested combining drugs that modulate T cell metabolism with anti‐PD‐1 immunotherapy to enhance the antitumour activity of immunotherapy. 146 , 147 , 148 , 149 As a key regulator in tumour metabolism, PPAR agonists are good candidates for adjuvant to be used with immunotherapy to enhance their antitumour activity of active T cells, even in an immunosuppressive TME. Studies have shown that the PPAR‐α agonist fenofibrate can work synergistically with PD‐1 blockers in the immunotherapy of cancer by reprogramming the metabolism of effector T cells. 102 Bezafibrate, an agonist of the PPAR‐γ coactivator 1α (PGC‐1α)/PPAR complex, has been shown to increase fatty acid oxidation and mitochondrial respiratory capacity in CD8+ T lymphocytes. This leads to an increase in mitochondrial oxidative phosphorylation and glycolysis, which can enhance antitumour immunity during PD‐1 blockade. 126 Moreover, the co‐administration of ciglitazone, the first thiazolidinediones with drugs such as lovastatin and phenylbutyrate, which are not traditionally used as cancer medications, can trigger TNF‐α‐related apoptosis inducing‐ligand, enhance the effects of gamma‐radiation and lead to a decreased cancer cell viability, 127 suggesting that these drug combinations could potentially be used as a treatment approach for cancer.
5.3. Other PPAR combinational therapies
PPAR agonists have been shown to have synergic effects when used in combination with chemotherapy, radiotherapy or even prevent side effects of these treatment regimens. Chemotherapy agents that induce the production of ROS have been shown to have higher efficacy when combined with a PPAR agonist. For example, in a patient‐derived orthotopic xenograft model of osteosarcoma, a combination of cisplatinum (CDDP)–pioglitazone (PIO) resulted in the greatest reduction in tumour volume and the most necrosis observed in histological sections. 128 A recent Phase I clinical trial using efatutazone, a highly selective PPAR‐γ agonist, 129 in combination with the microtubule inhibitor paclitaxel, demonstrated positive results in terms of safety, disease control and disease stability in patients with advanced anaplastic thyroid carcinoma (Clinicaltrials.gov: NCT00603941). 150 Troglitazone has also been shown to have synergistic effects when used in combination with the lovastatin, nonsteroidal anti‐inflammatory drugs such as aspirin, the oestrogen modulator tamoxifen and x‐ray therapy in the treatment of thyroid, glioblastoma, lung, breast and cervix cancers. 130 , 131 , 132 , 133 , 134 The combined administration of cell signalling molecules, such as TNF‐related apoptosis‐inducing ligand and troglitazone achieved promising apoptotic results synergistically in ovarian and breast cancer cell lines that are resistant to conventional therapies. 135 , 136 , 137 Furthermore, the PPAR agonist pioglitazone has also been studied for its ability to prevent radiation‐induced cognitive decline in non‐diabetic patients undergoing radiotherapy in a Phase I clinical trial (Clinicaltrials.gov: NCT01151670), 138 suggesting huge potentials of PPAR agonist as an adjuvant when combined with other therapies for improving the effectiveness and reducing the side effects of cancer treatments.
6. CONCLUSION AND FUTURE PERSPECTIVE
In this paper, we review the basic understanding of PPARs and recent research on their roles in tumourigenesis and microenvironment. Recent development of cancer therapies such as chemo, radio and immunotherapy have significantly improved cure rates for many patients. Nevertheless, for some patients who are less responsive to these established therapies, the clinical response rate remains unsatisfactory and this is where the potential of PPARs modulators becomes particularly relevant.
It is worth noting that monotherapies of PPARs agonist have yield a mixed results and are not used in the clinic. For instance, studies on patients with tenosynovial giant cell tumour treated with PPAR‐γ agonist zaltoprofen (UMIN‐CTR: UMIN000025901) 151 or patients with metastatic colon cancer and breast cancer treated with TGZ 105 , 106 , 152 have shown little to no improvement. The reasons for the failed response to PPAR modulators are complex, and one possible explanation is that many of the patients enrolled in these trials are heavily treated with many other chemo or biological therapies, making it difficult to evaluate the clinical effects of PPARs therapies. 105 In addition, PPAR modulators may have more of a preventive than a therapeutic role in cancer. For example, a study suggests that PPAR‐ γ may play a role in the prevention of APC‐related colorectal carcinogenesis. 153
Although PPAR modulators alone have shown limited efficacy, the co‐administration of PPAR agonists with chemotherapy or immunotherapy could open up new possibilities for increasing the effectiveness and accountability of cancer treatment. Here, we also illuminated how PPARs, when used as an adjuvant or in combination with other conventional cancer therapies, can exert antitumour effects.
Many PPAR agonists have strong safety profiles and have been widely used for a long time, making them convenient candidates for repurposing as cancer treatments. However, there are some limitations to their use as adjuvant in cancer treatment. Current studies have revealed conflicting results on the role of the different isoforms of PPARs in different types of tumours. For example, pioglitazone has been shown to potentially increase the risk of bladder cancer in patients with type II diabetes with a strong dosage effect. 154 , 155 , 156 , 157 , 158 Therefore, more studies and clinical trials are needed to assess their efficacy and safety in different kinds of cancers. Additionally, PPARs agonists are often combined with many other therapeutics in cancer treatment, 159 making it difficult to delineate the specific contribution of each component to the overall effect. To address this, it will be necessary to use biomarkers or more robust analysis methods to target the effects of PPAR agonists and determine whether they have additive or synergistic effects in combination therapy, which may also provide insights for future trial designs. 160 Moreover, safety profiles of PPARs combinational therapy should be carefully examined before and during any trials, given the potential for increased adverse effects with combinational therapy. 161
AUTHOR CONTRIBUTIONS
Yuqing Wang: Conceptualization (lead); investigation (equal); writing – original draft (equal). Feifei Lei: Conceptualization (equal); investigation (supporting); writing – original draft (supporting). Yiyun Lin: Conceptualization (equal); investigation (equal); writing – original draft (equal); writing – review and editing (equal). Yuru Han: Conceptualization (equal); investigation (equal); writing – original draft (equal); writing – review and editing (equal). Lei Yang: Conceptualization (equal); investigation (equal); project administration (lead); writing – original draft (equal); writing – review and editing (equal). Huabing Tan: Project administration (lead); writing – review and editing (equal).
CONFLICT OF INTEREST STATEMENT
The authors declare that there is no conflict of interest regarding the publication of this paper.
Wang Y, Lei F, Lin Y, Han Y, Yang L, Tan H. Peroxisome proliferator‐activated receptors as therapeutic target for cancer. J Cell Mol Med. 2024;28:e17931. doi: 10.1111/jcmm.17931
Yuqing Wang, Feifei Lei and Yiyun Lin contributed equally to this work.
Contributor Information
Lei Yang, Email: lyang15@mdanderson.org.
Huabing Tan, Email: tanhb_2013@163.com.
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are available in material of this article.
REFERENCES
- 1. Sher T, Yi HF, McBride OW, Gonzalez FJ. cDNA cloning, chromosomal mapping, and functional characterization of the human peroxisome proliferator activated receptor. Biochemistry. 1993;32:5598‐5604. doi: 10.1021/bi00072a015 [DOI] [PubMed] [Google Scholar]
- 2. Issemann I, Green S. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature. 1990;347:645‐650. doi: 10.1038/347645a0 [DOI] [PubMed] [Google Scholar]
- 3. Yasmin S, Jayaprakash V. Thiazolidinediones and PPAR orchestra as antidiabetic agents: from past to present. Eur J Med Chem. 2017;126:879‐893. doi: 10.1016/j.ejmech.2016.12.020 [DOI] [PubMed] [Google Scholar]
- 4. Miyachi H. Structural biology‐based exploration of subtype‐selective agonists for peroxisome proliferator‐activated receptors. Int J Mol Sci. 2021;22(17):9223. doi: 10.3390/ijms22179223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cheng HS, Yip YS, Lim EKY, Wahli W, Tan NS. PPARs and tumor microenvironment: the emerging roles of the metabolic master regulators in tumor stromal‐epithelial crosstalk and carcinogenesis. Cancers (Basel). 2021;13(9):2153. doi: 10.3390/cancers13092153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dreyer C, Krey G, Keller H, Givel F, Helftenbein G, Wahli W. Control of the peroxisomal beta‐oxidation pathway by a novel family of nuclear hormone receptors. Cell. 1992;68:879‐887. doi: 10.1016/0092-8674(92)90031-7 [DOI] [PubMed] [Google Scholar]
- 7. Wagner KD, Wagner N. Peroxisome proliferator‐activated receptor beta/delta (PPARbeta/delta) acts as regulator of metabolism linked to multiple cellular functions. Pharmacol Ther. 2010;125:423‐435. doi: 10.1016/j.pharmthera.2009.12.001 [DOI] [PubMed] [Google Scholar]
- 8. Keller H, Dreyer C, Medin J, Mahfoudi A, Ozato K, Wahli W. Fatty acids and retinoids control lipid metabolism through activation of peroxisome proliferator‐activated receptor‐retinoid X receptor heterodimers. Proc Natl Acad Sci U S A. 1993;90:2160‐2164. doi: 10.1073/pnas.90.6.2160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Amber‐Vitos O, Chaturvedi N, Nachliel E, Gutman M, Tsfadia Y. The effect of regulating molecules on the structure of the PPAR‐RXR complex. Biochim Biophys Acta. 2016;1861:1852‐1863. doi: 10.1016/j.bbalip.2016.09.003 [DOI] [PubMed] [Google Scholar]
- 10. Brunmeir R, Xu F. Functional regulation of PPARs through post‐translational modifications. Int J Mol Sci. 2018;19(6):1738. doi: 10.3390/ijms19061738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lamichane S, Dahal Lamichane B, Kwon SM. Pivotal roles of peroxisome proliferator‐activated receptors (PPARs) and their signal cascade for cellular and whole‐body energy homeostasis. Int J Mol Sci. 2018;19(4):949. doi: 10.3390/ijms19040949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Staels B, Auwerx J. Role of PPAR in the pharmacological regulation of lipoprotein metabolism by fibrates and thiazolidinediones. Curr Pharm des. 1997;3:1‐14. [Google Scholar]
- 13. Oliver WR Jr, Shenk JL, Snaith MR, et al. A selective peroxisome proliferator‐activated receptor delta agonist promotes reverse cholesterol transport. Proc Natl Acad Sci U S A. 2001;98:5306‐5311. doi: 10.1073/pnas.091021198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tanaka T, Yamamoto J, Iwasaki S, et al. Activation of peroxisome proliferator‐activated receptor delta induces fatty acid beta‐oxidation in skeletal muscle and attenuates metabolic syndrome. Proc Natl Acad Sci U S A. 2003;100:15924‐15929. doi: 10.1073/pnas.0306981100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Okuno A, Tamemoto H, Tobe K, et al. Troglitazone increases the number of small adipocytes without the change of white adipose tissue mass in obese Zucker rats. J Clin Invest. 1998;101:1354‐1361. doi: 10.1172/jci1235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huang JW, Shiau CW, Yang J, et al. Development of small‐molecule cyclin D1‐ablative agents. J Med Chem. 2006;49:4684‐4689. doi: 10.1021/jm060057h [DOI] [PubMed] [Google Scholar]
- 17. Wagner KD, Wagner N. PPARs and myocardial infarction. Int J Mol Sci. 2020;21(24):9436. doi: 10.3390/ijms21249436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Marx N, Davies MJ, Grant PJ, et al. Guideline recommendations and the positioning of newer drugs in type 2 diabetes care. Lancet Diabetes Endocrinol. 2021;9:46‐52. doi: 10.1016/s2213-8587(20)30343-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Duszka K, Gregor A, Guillou H, König J, Wahli W. Peroxisome proliferator‐activated receptors and caloric restriction‐common pathways affecting metabolism, health, and longevity. Cell. 2020;9(7):1708. doi: 10.3390/cells9071708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sáez‐Orellana F, Octave JN, Pierrot N. Alzheimer's disease, a lipid story: involvement of peroxisome proliferator‐activated receptor α. Cell. 2020;9(5):1215. doi: 10.3390/cells9051215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Matheson J, Le Foll B. Therapeutic potential of peroxisome proliferator‐activated receptor (PPAR) agonists in substance use disorders: a synthesis of preclinical and human evidence. Cell. 2020;9(5):1196. doi: 10.3390/cells9051196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Elias E, Zhang AY, Manners MT. Novel pharmacological approaches to the treatment of depression. Life (Basel). 2022;12(2):196. doi: 10.3390/life12020196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Quiroga C, Barberena JJ, Alcaraz‐Silva J, et al. The role of peroxisome proliferator‐activated receptor in addiction: a novel drug target. Curr Top Med Chem. 2021;21:964‐975. doi: 10.2174/1568026621666210521165532 [DOI] [PubMed] [Google Scholar]
- 24. Fougerat A, Montagner A, Loiseau N, Guillou H, Wahli W. Peroxisome proliferator‐activated receptors and their novel ligands as candidates for the treatment of non‐alcoholic fatty liver disease. Cell. 2020;9(7):1638. doi: 10.3390/cells9071638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mantovani A, Byrne CD, Targher G. Efficacy of peroxisome proliferator‐activated receptor agonists, glucagon‐like peptide‐1 receptor agonists, or sodium‐glucose cotransporter‐2 inhibitors for treatment of non‐alcoholic fatty liver disease: a systematic review. Lancet Gastroenterol Hepatol. 2022;7:367‐378. doi: 10.1016/s2468-1253(21)00261-2 [DOI] [PubMed] [Google Scholar]
- 26. Kökény G, Calvier L, Hansmann G. PPARγ and TGFβ‐major regulators of metabolism, inflammation, and fibrosis in the lungs and kidneys. Int J Mol Sci. 2021;22(19):10431. doi: 10.3390/ijms221910431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Luan ZL, Zhang C, Ming WH, Huang YZ, Guan YF, Zhang XY. Nuclear receptors in renal health and disease. EBioMedicine. 2022;76:103855. doi: 10.1016/j.ebiom.2022.103855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Toobian D, Ghosh P, Katkar GD. Parsing the role of PPARs in macrophage processes. Front Immunol. 2021;12:783780. doi: 10.3389/fimmu.2021.783780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liu Y, Wang J, Luo S, Zhan Y, Lu Q. The roles of PPARγ and its agonists in autoimmune diseases: a comprehensive review. J Autoimmun. 2020;113:102510. doi: 10.1016/j.jaut.2020.102510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rzemieniec J, Castiglioni L, Gelosa P, Muluhie M, Mercuriali B, Sironi L. Nuclear receptors in myocardial and cerebral ischemia‐mechanisms of action and therapeutic strategies. Int J Mol Sci. 2021;22(22):12326. doi: 10.3390/ijms222212326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cao Y. Tumorigenesis as a process of gradual loss of original cell identity and gain of properties of neural precursor/progenitor cells. Cell Biosci. 2017;7:61. doi: 10.1186/s13578-017-0188-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhang F, Du G. Dysregulated lipid metabolism in cancer. World J Biol Chem. 2012;3:167‐174. doi: 10.4331/wjbc.v3.i8.167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Menendez JA, Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer. 2007;7:763‐777. doi: 10.1038/nrc2222 [DOI] [PubMed] [Google Scholar]
- 34. Galbraith LCA, Mui E, Nixon C, et al. PPAR‐gamma induced AKT3 expression increases levels of mitochondrial biogenesis driving prostate cancer. Oncogene. 2021;40:2355‐2366. doi: 10.1038/s41388-021-01707-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shigeto T, Yokoyama Y, Xin B, Mizunuma H. Peroxisome proliferator‐activated receptor alpha and gamma ligands inhibit the growth of human ovarian cancer. Oncol Rep. 2007;18:833‐840. [PubMed] [Google Scholar]
- 36. Luci S, Giemsa B, Kluge H, Eder K. Clofibrate causes an upregulation of PPAR‐{alpha} target genes but does not alter expression of SREBP target genes in liver and adipose tissue of pigs. Am J Physiol Regul Integr Comp Physiol. 2007;293:R70‐R77. doi: 10.1152/ajpregu.00603.2006 [DOI] [PubMed] [Google Scholar]
- 37. Chandran K, Goswami S, Sharma‐Walia N. Implications of a peroxisome proliferator‐activated receptor alpha (PPARα) ligand clofibrate in breast cancer. Oncotarget. 2016;7:15577‐15599. doi: 10.18632/oncotarget.6402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wu L, Wang W, Dai M, Li H, Chen C, Wang D. PPARα ligand, AVE8134, and cyclooxygenase inhibitor therapy synergistically suppress lung cancer growth and metastasis. BMC Cancer. 2019;19:1166. doi: 10.1186/s12885-019-6379-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fares J, Fares MY, Khachfe HH, Salhab HA, Fares Y. Molecular principles of metastasis: a hallmark of cancer revisited. Signal Transduct Target Ther. 2020;5:28. doi: 10.1038/s41392-020-0134-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646‐674. doi: 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 41. Martinasso G, Oraldi M, Trombetta A, et al. Involvement of PPARs in cell proliferation and apoptosis in human colon cancer specimens and in normal and cancer cell lines. PPAR Res. 2007;2007:93416. doi: 10.1155/2007/93416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Castelli V, Catanesi M, Alfonsetti M, et al. PPARα‐selective antagonist GW6471 inhibits cell growth in breast cancer stem cells inducing energy imbalance and metabolic stress. Biomedicine. 2021;9(2):127. doi: 10.3390/biomedicines9020127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Papi A, Guarnieri T, Storci G, et al. Nuclear receptors agonists exert opposing effects on the inflammation dependent survival of breast cancer stem cells. Cell Death Differ. 2012;19:1208‐1219. doi: 10.1038/cdd.2011.207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ferrari SM, Materazzi G, Baldini E, et al. Antineoplastic effects of PPARγ agonists, with a special focus on thyroid cancer. Curr Med Chem. 2016;23:636‐649. doi: 10.2174/0929867323666160203114607 [DOI] [PubMed] [Google Scholar]
- 45. Ravi Kiran Ammu VVV, Garikapati KK, Krishnamurthy PT, Chintamaneni PK, Pindiprolu S. Possible role of PPAR‐γ and COX‐2 receptor modulators in the treatment of non‐small cell lung carcinoma. Med Hypotheses. 2019;124:98‐100. doi: 10.1016/j.mehy.2019.02.024 [DOI] [PubMed] [Google Scholar]
- 46. Catalano S, Mauro L, Bonofiglio D, et al. In vivo and in vitro evidence that PPARγ ligands are antagonists of leptin signaling in breast cancer. Am J Pathol. 2011;179:1030‐1040. doi: 10.1016/j.ajpath.2011.04.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kotta‐Loizou I, Giaginis C, Theocharis S. The role of peroxisome proliferator‐activated receptor‐γ in breast cancer. Anticancer Agents Med Chem. 2012;12:1025‐1044. doi: 10.2174/187152012803529664 [DOI] [PubMed] [Google Scholar]
- 48. Shao W, Kuhn C, Mayr D, et al. Cytoplasmic PPARγ is a marker of poor prognosis in patients with cox‐1 negative primary breast cancers. J Transl Med. 2020;18:94. doi: 10.1186/s12967-020-02271-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jarrar MH, Baranova A. PPARgamma activation by thiazolidinediones (TZDs) may modulate breast carcinoma outcome: the importance of interplay with TGFbeta signalling. J Cell Mol Med. 2007;11:71‐87. doi: 10.1111/j.1582-4934.2007.00003.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Khandekar MJ, Banks AS, Laznik‐Bogoslavski D, et al. Noncanonical agonist PPARγ ligands modulate the response to DNA damage and sensitize cancer cells to cytotoxic chemotherapy. Proc Natl Acad Sci U S A. 2018;115:561‐566. doi: 10.1073/pnas.1717776115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tepmongkol S, Keelawat S, Honsawek S, Ruangvejvorachai P. Rosiglitazone effect on radioiodine uptake in thyroid carcinoma patients with high thyroglobulin but negative total body scan: a correlation with the expression of peroxisome proliferator‐activated receptor‐gamma. Thyroid. 2008;18:697‐704. doi: 10.1089/thy.2008.0056 [DOI] [PubMed] [Google Scholar]
- 52. Panigrahy D, Singer S, Shen LQ, et al. PPARgamma ligands inhibit primary tumor growth and metastasis by inhibiting angiogenesis. J Clin Invest. 2002;110:923‐932. doi: 10.1172/jci15634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Elix C, Pal SK, Jones JO. The role of peroxisome proliferator‐activated receptor gamma in prostate cancer. Asian J Androl. 2018;20:238‐243. doi: 10.4103/aja.aja_15_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zaytseva YY, Wallis NK, Southard RC, Kilgore MW. The PPARgamma antagonist T0070907 suppresses breast cancer cell proliferation and motility via both PPARgamma‐dependent and ‐independent mechanisms. Anticancer Res. 2011;31:813‐823. [PubMed] [Google Scholar]
- 55. Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331:1559‐1564. doi: 10.1126/science.1203543 [DOI] [PubMed] [Google Scholar]
- 56. Grau R, Punzón C, Fresno M, Iñiguez MA. Peroxisome‐proliferator‐activated receptor alpha agonists inhibit cyclo‐oxygenase 2 and vascular endothelial growth factor transcriptional activation in human colorectal carcinoma cells via inhibition of activator protein‐1. Biochem J. 2006;395:81‐88. doi: 10.1042/bj20050964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Meissner M, Berlinski B, Gille J, Doll M, Kaufmann R. Peroxisome proliferator activated receptor‐α agonists suppress transforming growth factor‐α‐induced matrix metalloproteinase‐9 expression in human keratinocytes. Clin Exp Dermatol. 2011;36:911‐914. doi: 10.1111/j.1365-2230.2011.04125.x [DOI] [PubMed] [Google Scholar]
- 58. Meylan P, Pich C, Winkler C, et al. Low expression of the PPARγ‐regulated gene thioredoxin‐interacting protein accompanies human melanoma progression and promotes experimental lung metastases. Sci Rep. 2021;11:7847. doi: 10.1038/s41598-021-86329-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wagner N, Wagner KD. PPAR beta/delta and the hallmarks of cancer. Cell. 2020;9(5):1133. doi: 10.3390/cells9051133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yoshinaga M, Taki K, Somada S, et al. The expression of both peroxisome proliferator‐activated receptor delta and cyclooxygenase‐2 in tissues is associated with poor prognosis in colorectal cancer patients. Dig Dis Sci. 2011;56:1194‐1200. doi: 10.1007/s10620-010-1389-9 [DOI] [PubMed] [Google Scholar]
- 61. Yang L, Zhou J, Ma Q, et al. Knockdown of PPAR δ gene promotes the growth of colon cancer and reduces the sensitivity to bevacizumab in nude mice model. PLoS One. 2013;8:e60715. doi: 10.1371/journal.pone.0060715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Yang L, Olsson B, Pfeifer D, et al. Knockdown of peroxisome proliferator‐activated receptor‐beta induces less differentiation and enhances cell‐fibronectin adhesion of colon cancer cells. Oncogene. 2010;29:516‐526. doi: 10.1038/onc.2009.370 [DOI] [PubMed] [Google Scholar]
- 63. Wang X, Wang G, Shi Y, et al. PPAR‐delta promotes survival of breast cancer cells in harsh metabolic conditions. Oncogenesis. 2016;5:e232. doi: 10.1038/oncsis.2016.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. He TC, Chan TA, Vogelstein B, Kinzler KW. PPARdelta is an APC‐regulated target of nonsteroidal anti‐inflammatory drugs. Cell. 1999;99:335‐345. doi: 10.1016/s0092-8674(00)81664-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Liu Y, Deguchi Y, Tian R, et al. Pleiotropic effects of PPARD accelerate colorectal tumorigenesis, progression, and invasion. Cancer Res. 2019;79:954‐969. doi: 10.1158/0008-5472.Can-18-1790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Chan JSK, Sng MK, Teo ZQ, Chong HC, Twang JS, Tan NS. Targeting nuclear receptors in cancer‐associated fibroblasts as concurrent therapy to inhibit development of chemoresistant tumors. Oncogene. 2018;37:160‐173. doi: 10.1038/onc.2017.319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Avena P, Anselmo W, Whitaker‐Menezes D, et al. Compartment‐specific activation of PPARgamma governs breast cancer tumor growth, via metabolic reprogramming and symbiosis. Cell Cycle. 2013;12:1360‐1370. doi: 10.4161/cc.24289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Tan MWY, Sng MK, Cheng HS, et al. Deficiency in fibroblast PPARbeta/delta reduces nonmelanoma skin cancers in mice. Cell Death Differ. 2020;27:2668‐2680. doi: 10.1038/s41418-020-0535-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sng MK, Chan JSK, Teo Z, et al. Selective deletion of PPARbeta/delta in fibroblasts causes dermal fibrosis by attenuated LRG1 expression. Cell Discov. 2018;4:15. doi: 10.1038/s41421-018-0014-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Xie ZB, Zhang YF, Jin C, Mao YS, Fu DL. LRG‐1 promotes pancreatic cancer growth and metastasis via modulation of the EGFR/p38 signaling. J Exp Clin Cancer Res. 2019;38:75. doi: 10.1186/s13046-019-1088-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zhou Y, Zhang X, Zhang J, Fang J, Ge Z, Li X. LRG1 promotes proliferation and inhibits apoptosis in colorectal cancer cells via RUNX1 activation. PLoS One. 2017;12:e0175122. doi: 10.1371/journal.pone.0175122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Muller‐Brusselbach S, Kömhoff M, Rieck M, et al. Deregulation of tumor angiogenesis and blockade of tumor growth in PPARbeta‐deficient mice. EMBO J. 2007;26:3686‐3698. doi: 10.1038/sj.emboj.7601803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Piqueras L, Reynolds AR, Hodivala‐Dilke KM, et al. Activation of PPARbeta/delta induces endothelial cell proliferation and angiogenesis. Arterioscler Thromb Vasc Biol. 2007;27:63‐69. doi: 10.1161/01.ATV.0000250972.83623.61 [DOI] [PubMed] [Google Scholar]
- 74. Meissner M, Hrgovic I, Doll M, et al. Peroxisome proliferator‐activated receptor delta activators induce IL‐8 expression in nonstimulated endothelial cells in a transcriptional and posttranscriptional manner. J Biol Chem. 2010;285:33797‐33804. doi: 10.1074/jbc.M110.137943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Adamkiewicz J, Kaddatz K, Rieck M, Wilke B, Müller‐Brüsselbach S, Müller R. Proteomic profile of mouse fibroblasts with a targeted disruption of the peroxisome proliferator activated receptor‐beta/delta gene. Proteomics. 2007;7:1208‐1216. doi: 10.1002/pmic.200601003 [DOI] [PubMed] [Google Scholar]
- 76. Panigrahy D, Kaipainen A, Huang S, et al. PPARalpha agonist fenofibrate suppresses tumor growth through direct and indirect angiogenesis inhibition. Proc Natl Acad Sci U S A. 2008;105:985‐990. doi: 10.1073/pnas.0711281105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Pozzi A, Ibanez MR, Gatica AE, et al. Peroxisomal proliferator‐activated receptor‐alpha‐dependent inhibition of endothelial cell proliferation and tumorigenesis. J Biol Chem. 2007;282:17685‐17695. doi: 10.1074/jbc.M701429200 [DOI] [PubMed] [Google Scholar]
- 78. Arima T, Uchiyama M, Nakano Y, et al. Peroxisome proliferator‐activated receptor alpha agonist suppresses neovascularization by reducing both vascular endothelial growth factor and angiopoietin‐2 in corneal alkali burn. Sci Rep. 2017;7:17763. doi: 10.1038/s41598-017-18113-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Li CG, Mahon C, Sweeney NM, et al. PPARγ interaction with UBR5/ATMIN promotes DNA repair to maintain endothelial homeostasis. Cell Rep. 2019;26:1333‐1343.e7. doi: 10.1016/j.celrep.2019.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Cai W, Yang T, Liu H, et al. Peroxisome proliferator‐activated receptor gamma (PPARgamma): a master gatekeeper in CNS injury and repair. Prog Neurobiol. 2018;163‐164:27‐58. doi: 10.1016/j.pneurobio.2017.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Delerive P, de Bosscher K, Besnard S, et al. Peroxisome proliferator‐activated receptor alpha negatively regulates the vascular inflammatory gene response by negative cross‐talk with transcription factors NF‐kappaB and AP‐1. J Biol Chem. 1999;274:32048‐32054. doi: 10.1074/jbc.274.45.32048 [DOI] [PubMed] [Google Scholar]
- 82. Barish GD, Atkins AR, Downes M, et al. PPARdelta regulates multiple proinflammatory pathways to suppress atherosclerosis. Proc Natl Acad Sci U S A. 2008;105:4271‐4276. doi: 10.1073/pnas.0711875105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Devchand PR, Keller H, Peters JM, Vazquez M, Gonzalez FJ, Wahli W. The PPARalpha‐leukotriene B4 pathway to inflammation control. Nature. 1996;384:39‐43. doi: 10.1038/384039a0 [DOI] [PubMed] [Google Scholar]
- 84. Kobayashi Y, Ueki S, Mahemuti G, et al. Physiological levels of 15‐deoxy‐Delta12,14‐prostaglandin J2 prime eotaxin‐induced chemotaxis on human eosinophils through peroxisome proliferator‐activated receptor‐gamma ligation. J Immunol. 2005;175:5744‐5750. doi: 10.4049/jimmunol.175.9.5744 [DOI] [PubMed] [Google Scholar]
- 85. Pawlak M, Lefebvre P, Staels B. Molecular mechanism of PPARalpha action and its impact on lipid metabolism, inflammation and fibrosis in non‐alcoholic fatty liver disease. J Hepatol. 2015;62:720‐733. doi: 10.1016/j.jhep.2014.10.039 [DOI] [PubMed] [Google Scholar]
- 86. Vargas‐Sanchez K, Vargas L, Urrutia Y, et al. PPARalpha and PPARbeta/delta are negatively correlated with proinflammatory markers in leukocytes of an obese pediatric population. J Inflamm (Lond). 2020;17:35. doi: 10.1186/s12950-020-00264-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Ahmed W, Orasanu G, Nehra V, et al. High‐density lipoprotein hydrolysis by endothelial lipase activates PPARalpha: a candidate mechanism for high‐density lipoprotein‐mediated repression of leukocyte adhesion. Circ Res. 2006;98:490‐498. doi: 10.1161/01.RES.0000205846.46812.be [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Wang D, DuBois RN. PPARdelta and PGE(2) signaling pathways communicate and connect inflammation to colorectal cancer. Inflamm Cell Signal. 2014;1(6). doi: 10.14800/ics.338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Wang D, Fu L, Ning W, et al. Peroxisome proliferator‐activated receptor delta promotes colonic inflammation and tumor growth. Proc Natl Acad Sci U S A. 2014;111:7084‐7089. doi: 10.1073/pnas.1324233111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Christofides A, Konstantinidou E, Jani C, Boussiotis VA. The role of peroxisome proliferator‐activated receptors (PPAR) in immune responses. Metabolism. 2021;114:154338. doi: 10.1016/j.metabol.2020.154338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Penas F, Mirkin GA, Vera M, et al. Treatment in vitro with PPARalpha and PPARgamma ligands drives M1‐to‐M2 polarization of macrophages from T. cruzi‐infected mice. Biochim Biophys Acta. 2015;1852:893‐904. doi: 10.1016/j.bbadis.2014.12.019 [DOI] [PubMed] [Google Scholar]
- 92. Adhikary T, Wortmann A, Schumann T, et al. The transcriptional PPARbeta/delta network in human macrophages defines a unique agonist‐induced activation state. Nucleic Acids Res. 2015;43:5033‐5051. doi: 10.1093/nar/gkv331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Schug TT, Li X. PPARdelta‐mediated macrophage activation: a matter of fat. Dis Model Mech. 2009;2:421‐422. doi: 10.1242/dmm.003913 [DOI] [PubMed] [Google Scholar]
- 94. Nelson VL, Nguyen HCB, Garcìa‐Cañaveras JC, et al. PPARgamma is a nexus controlling alternative activation of macrophages via glutamine metabolism. Genes Dev. 2018;32:1035‐1044. doi: 10.1101/gad.312355.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Niu Z, Shi Q, Zhang W, et al. Caspase‐1 cleaves PPARgamma for potentiating the pro‐tumor action of TAMs. Nat Commun. 2017;8:766. doi: 10.1038/s41467-017-00523-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Schumann T, Adhikary T, Wortmann A, et al. Deregulation of PPARbeta/delta target genes in tumor‐associated macrophages by fatty acid ligands in the ovarian cancer microenvironment. Oncotarget. 2015;6:13416‐13433. doi: 10.18632/oncotarget.3826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Yin X, Zeng W, Wu B, et al. PPARalpha inhibition overcomes tumor‐derived exosomal lipid‐induced dendritic cell dysfunction. Cell Rep. 2020;33:108278. doi: 10.1016/j.celrep.2020.108278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Choi JM, Bothwell AL. The nuclear receptor PPARs as important regulators of T‐cell functions and autoimmune diseases. Mol Cells. 2012;33:217‐222. doi: 10.1007/s10059-012-2297-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Jones DC, Ding X, Daynes RA. Nuclear receptor peroxisome proliferator‐activated receptor alpha (PPARalpha) is expressed in resting murine lymphocytes. The PPARalpha in T and B lymphocytes is both transactivation and transrepression competent. J Biol Chem. 2002;277:6838‐6845. doi: 10.1074/jbc.M106908200 [DOI] [PubMed] [Google Scholar]
- 100. Wang H, Franco F, Tsui YC, et al. CD36‐mediated metabolic adaptation supports regulatory T cell survival and function in tumors. Nat Immunol. 2020;21:298‐308. doi: 10.1038/s41590-019-0589-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Lei J, Hasegawa H, Matsumoto T, Yasukawa M. Peroxisome proliferator‐activated receptor alpha and gamma agonists together with TGF‐beta convert human CD4+CD25− T cells into functional Foxp3+ regulatory T cells. J Immunol. 2010;185:7186‐7198. doi: 10.4049/jimmunol.1001437 [DOI] [PubMed] [Google Scholar]
- 102. Zhang Y, Kurupati R, Liu L, et al. Enhancing CD8(+) T cell fatty acid catabolism within a metabolically challenging tumor microenvironment increases the efficacy of melanoma immunotherapy. Cancer Cell. 2017;32:377‐391.e9. doi: 10.1016/j.ccell.2017.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Garcia‐Bates TM, Baglole CJ, Bernard MP, Murant TI, Simpson‐Haidaris PJ, Phipps RP. Peroxisome proliferator‐activated receptor gamma ligands enhance human B cell antibody production and differentiation. J Immunol. 2009;183:6903‐6912. doi: 10.4049/jimmunol.0900324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Wejksza K, Lee‐Chang C, Bodogai M, et al. Cancer‐produced metabolites of 5‐lipoxygenase induce tumor‐evoked regulatory B cells via peroxisome proliferator‐activated receptor alpha. J Immunol. 2013;190:2575‐2584. doi: 10.4049/jimmunol.1201920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Burstein HJ, Demetri GD, Mueller E, Sarraf P, Spiegelman BM, Winer EP. Use of the peroxisome proliferator‐activated receptor (PPAR) gamma ligand troglitazone as treatment for refractory breast cancer: a phase II study. Breast Cancer Res Treat. 2003;79:391‐397. doi: 10.1023/a:1024038127156 [DOI] [PubMed] [Google Scholar]
- 106. Kulke MH, Demetri GD, Sharpless NE, et al. A phase II study of troglitazone, an activator of the PPARgamma receptor, in patients with chemotherapy‐resistant metastatic colorectal cancer. Cancer J. 2002;8:395‐399. doi: 10.1097/00130404-200209000-00010 [DOI] [PubMed] [Google Scholar]
- 107. Smith MR, Manola J, Kaufman DS, et al. Rosiglitazone versus placebo for men with prostate carcinoma and a rising serum prostate‐specific antigen level after radical prostatectomy and/or radiation therapy. Cancer. 2004;101:1569‐1574. doi: 10.1002/cncr.20493 [DOI] [PubMed] [Google Scholar]
- 108. Kebebew E, Peng M, Reiff E, et al. A phase II trial of rosiglitazone in patients with thyroglobulin‐positive and radioiodine‐negative differentiated thyroid cancer. Surgery. 2006;140:960‐966; discussion 966‐967. doi: 10.1016/j.surg.2006.07.038 [DOI] [PubMed] [Google Scholar]
- 109. Yousefi B, Azimi A, Majidinia M, et al. Balaglitazone reverses P‐glycoprotein‐mediated multidrug resistance via upregulation of PTEN in a PPARγ‐dependent manner in leukemia cells. Tumour Biol. 2017;39:1010428317716501. doi: 10.1177/1010428317716501 [DOI] [PubMed] [Google Scholar]
- 110. Chu TH, Chan HH, Kuo HM, et al. Celecoxib suppresses hepatoma stemness and progression by up‐regulating PTEN. Oncotarget. 2014;5:1475‐1490. doi: 10.18632/oncotarget.1745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Han S, Roman J. Rosiglitazone suppresses human lung carcinoma cell growth through PPARgamma‐dependent and PPARgamma‐independent signal pathways. Mol Cancer Ther. 2006;5:430‐437. doi: 10.1158/1535-7163.Mct-05-0347 [DOI] [PubMed] [Google Scholar]
- 112. Mustafa A, Kruger WD. Suppression of tumor formation by a cyclooxygenase‐2 inhibitor and a peroxisome proliferator‐activated receptor gamma agonist in an in vivo mouse model of spontaneous breast cancer. Clin Cancer Res. 2008;14:4935‐4942. doi: 10.1158/1078-0432.Ccr-08-0958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Esmaeili S, Safaroghli‐azar A, Pourbagheri‐Sigaroodi A, et al. Activation of PPARγ intensified the effects of arsenic trioxide in acute promyelocytic leukemia through the suppression of PI3K/Akt pathway: proposing a novel anticancer effect for pioglitazone. Int J Biochem Cell Biol. 2020;122:105739. doi: 10.1016/j.biocel.2020.105739 [DOI] [PubMed] [Google Scholar]
- 114. Vogt T, Hafner C, Bross K, et al. Antiangiogenetic therapy with pioglitazone, rofecoxib, and metronomic trofosfamide in patients with advanced malignant vascular tumors. Cancer. 2003;98:2251‐2256. doi: 10.1002/cncr.11775 [DOI] [PubMed] [Google Scholar]
- 115. Reichle A, Bross K, Vogt T, et al. Pioglitazone and rofecoxib combined with angiostatically scheduled trofosfamide in the treatment of far‐advanced melanoma and soft tissue sarcoma. Cancer. 2004;101:2247‐2256. doi: 10.1002/cncr.20574 [DOI] [PubMed] [Google Scholar]
- 116. Rani A, Murphy JJ. STAT5 in cancer and immunity. J Interferon Cytokine Res. 2016;36:226‐237. doi: 10.1089/jir.2015.0054 [DOI] [PubMed] [Google Scholar]
- 117. Prost S, Relouzat F, Spentchian M, et al. Erosion of the chronic myeloid leukaemia stem cell pool by PPARγ agonists. Nature. 2015;525:380‐383. doi: 10.1038/nature15248 [DOI] [PubMed] [Google Scholar]
- 118. Liu H, Zang C, Fenner MH, et al. Growth inhibition and apoptosis in human Philadelphia chromosome‐positive lymphoblastic leukemia cell lines by treatment with the dual PPARα/γ ligand TZD18. Blood. 2006;107:3683‐3692. doi: 10.1182/blood-2005-05-2103 [DOI] [PubMed] [Google Scholar]
- 119. Bertz J, Zang C, Liu H, et al. Compound 48, a novel dual PPAR alpha/gamma ligand, inhibits the growth of human CML cell lines and enhances the anticancer‐effects of imatinib. Leuk Res. 2009;33:686‐692. doi: 10.1016/j.leukres.2008.11.023 [DOI] [PubMed] [Google Scholar]
- 120. Pagnano KBB, Lopes ABP, Miranda EC, et al. Efficacy and safety of pioglitazone in a phase 1/2 imatinib discontinuation trial (EDI‐PIO) in chronic myeloid leukemia with deep molecular response. Am J Hematol. 2020;95:E321‐E323. doi: 10.1002/ajh.25986 [DOI] [PubMed] [Google Scholar]
- 121. Papi A, Rocchi P, Ferreri AM, Orlandi M. RXRgamma and PPARgamma ligands in combination to inhibit proliferation and invasiveness in colon cancer cells. Cancer Lett. 2010;297:65‐74. doi: 10.1016/j.canlet.2010.04.026 [DOI] [PubMed] [Google Scholar]
- 122. Papi A, Tatenhorst L, Terwel D, et al. PPARgamma and RXRgamma ligands act synergistically as potent antineoplastic agents in vitro and in vivo glioma models. J Neurochem. 2009;109:1779‐1790. doi: 10.1111/j.1471-4159.2009.06111.x [DOI] [PubMed] [Google Scholar]
- 123. Konopleva M, Elstner E, McQueen TJ, et al. Peroxisome proliferator‐activated receptor gamma and retinoid X receptor ligands are potent inducers of differentiation and apoptosis in leukemias. Mol Cancer Ther. 2004;3:1249‐1262. [PubMed] [Google Scholar]
- 124. Urbanska K, Pannizzo P, Grabacka M, et al. Activation of PPARalpha inhibits IGF‐I‐mediated growth and survival responses in medulloblastoma cell lines. Int J Cancer. 2008;123:1015‐1024. doi: 10.1002/ijc.23588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Drukala J, Urbanska K, Wilk A, et al. ROS accumulation and IGF‐IR inhibition contribute to fenofibrate/PPARalpha ‐mediated inhibition of glioma cell motility in vitro. Mol Cancer. 2010;9:159. doi: 10.1186/1476-4598-9-159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Chowdhury PS, Chamoto K, Kumar A, Honjo T. PPAR‐induced fatty acid oxidation in T cells increases the number of tumor‐reactive CD8(+) T cells and facilitates anti‐PD‐1 therapy. Cancer Immunol Res. 2018;6:1375‐1387. doi: 10.1158/2326-6066.Cir-18-0095 [DOI] [PubMed] [Google Scholar]
- 127. Fröhlich E, Wahl R. Chemotherapy and chemoprevention by thiazolidinediones. Biomed Res Int. 2015;2015:845340. doi: 10.1155/2015/845340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Higuchi T, Yamamoto J, Sugisawa N, et al. PPARγ agonist pioglitazone in combination with cisplatinum arrests a chemotherapy‐resistant osteosarcoma PDOX model. Cancer Genomics Proteomics. 2020;17:35‐40. doi: 10.21873/cgp.20165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Chen L, Bush CR, Necela BM, et al. RS5444, a novel PPARgamma agonist, regulates aspects of the differentiated phenotype in nontransformed intestinal epithelial cells. Mol Cell Endocrinol. 2006;251:17‐32. doi: 10.1016/j.mce.2006.02.006 [DOI] [PubMed] [Google Scholar]
- 130. Yu HN, Noh EM, Lee YR, et al. Troglitazone enhances tamoxifen‐induced growth inhibitory activity of MCF‐7 cells. Biochem Biophys Res Commun. 2008;377:242‐247. doi: 10.1016/j.bbrc.2008.09.111 [DOI] [PubMed] [Google Scholar]
- 131. Yao CJ, Lai GM, Chan CF, Cheng AL, Yang YY, Chuang SE. Dramatic synergistic anticancer effect of clinically achievable doses of lovastatin and troglitazone. Int J Cancer. 2006;118:773‐779. doi: 10.1002/ijc.21361 [DOI] [PubMed] [Google Scholar]
- 132. Yan KH, Yao CJ, Chang HY, Lai GM, Cheng AL, Chuang SE. The synergistic anticancer effect of troglitazone combined with aspirin causes cell cycle arrest and apoptosis in human lung cancer cells. Mol Carcinog. 2010;49:235‐246. doi: 10.1002/mc.20593 [DOI] [PubMed] [Google Scholar]
- 133. An Z, Liu X, Song H, et al. Effect of troglitazone on radiation sensitivity in cervix cancer cells. Radiat Oncol J. 2012;30:78‐87. doi: 10.3857/roj.2012.30.2.78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Zhong WB, Tsai YC, Chin LH, et al. A synergistic anti‐cancer effect of troglitazone and lovastatin in a human anaplastic thyroid cancer cell line and in a mouse xenograft model. Int J Mol Sci. 2018;19(7):1834. doi: 10.3390/ijms19071834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Bräutigam K, Biernath‐Wüpping J, Bauerschlag DO, et al. Combined treatment with TRAIL and PPARγ ligands overcomes chemoresistance of ovarian cancer cell lines. J Cancer Res Clin Oncol. 2011;137:875‐886. doi: 10.1007/s00432-010-0952-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Park BH, Lee SB, Stolz DB, Lee YJ, Lee BC. Synergistic interactions between heregulin and peroxisome proliferator‐activated receptor‐gamma (PPARgamma) agonist in breast cancer cells. J Biol Chem. 2011;286:20087‐20099. doi: 10.1074/jbc.M110.191718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Plissonnier ML, Fauconnet S, Bittard H, Mougin C, Rommelaere J, Lascombe I. Cell death and restoration of TRAIL‐sensitivity by ciglitazone in resistant cervical cancer cells. Oncotarget. 2017;8:107744‐107762. doi: 10.18632/oncotarget.22632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Cramer CK, Alphonse‐Sullivan N, Isom S, et al. Safety of pioglitazone during and after radiation therapy in patients with brain tumors: a phase I clinical trial. J Cancer Res Clin Oncol. 2019;145:337‐344. doi: 10.1007/s00432-018-2791-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Alzahrani AS. PI3K/Akt/mTOR inhibitors in cancer: at the bench and bedside. Semin Cancer Biol. 2019;59:125‐132. doi: 10.1016/j.semcancer.2019.07.009 [DOI] [PubMed] [Google Scholar]
- 140. Teresi RE, Waite KA. PPARgamma, PTEN, and the fight against cancer. PPAR Res. 2008;2008:932632. doi: 10.1155/2008/932632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Belfiore A, Frasca F, Pandini G, Sciacca L, Vigneri R. Insulin receptor isoforms and insulin receptor/insulin‐like growth factor receptor hybrids in physiology and disease. Endocr Rev. 2009;30:586‐623. doi: 10.1210/er.2008-0047 [DOI] [PubMed] [Google Scholar]
- 142. Pollak M. Insulin and insulin‐like growth factor signalling in neoplasia. Nat Rev Cancer. 2008;8:915‐928. doi: 10.1038/nrc2536 [DOI] [PubMed] [Google Scholar]
- 143. Pollak MN, Schernhammer ES, Hankinson SE. Insulin‐like growth factors and neoplasia. Nat Rev Cancer. 2004;4:505‐518. doi: 10.1038/nrc1387 [DOI] [PubMed] [Google Scholar]
- 144. Belfiore A, Malaguarnera R. Insulin receptor and cancer. Endocr Relat Cancer. 2011;18:R125‐R147. doi: 10.1530/erc-11-0074 [DOI] [PubMed] [Google Scholar]
- 145. Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27:450‐461. doi: 10.1016/j.ccell.2015.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Bahrambeigi S, Molaparast M, Sohrabi F, et al. Targeting PPAR ligands as possible approaches for metabolic reprogramming of T cells in cancer immunotherapy. Immunol Lett. 2020;220:32‐37. doi: 10.1016/j.imlet.2020.01.006 [DOI] [PubMed] [Google Scholar]
- 147. Odorizzi PM, Pauken KE, Paley MA, Sharpe A, Wherry EJ. Genetic absence of PD‐1 promotes accumulation of terminally differentiated exhausted CD8+ T cells. J Exp Med. 2015;212:1125‐1137. doi: 10.1084/jem.20142237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Tumeh PC, Harview CL, Yearley JH, et al. PD‐1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568‐571. doi: 10.1038/nature13954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Chowdhury PS, Chamoto K, Honjo T. Combination therapy strategies for improving PD‐1 blockade efficacy: a new era in cancer immunotherapy. J Intern Med. 2018;283:110‐120. doi: 10.1111/joim.12708 [DOI] [PubMed] [Google Scholar]
- 150. Smallridge RC, Copland JA, Brose MS, et al. Efatutazone, an oral PPAR‐γ agonist, in combination with paclitaxel in anaplastic thyroid cancer: results of a multicenter phase 1 trial. J Clin Endocrinol Metab. 2013;98:2392‐2400. doi: 10.1210/jc.2013-1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Takeuchi A, Endo M, Kawai A, et al. Randomized placebo‐controlled double‐blind phase II study of zaltoprofen for patients with diffuse‐type and unresectable localized tenosynovial giant cell tumors: the REALIZE study. Front Oncol. 2022;12:900010. doi: 10.3389/fonc.2022.900010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Mueller E, Smith M, Sarraf P, et al. Effects of ligand activation of peroxisome proliferator‐activated receptor gamma in human prostate cancer. Proc Natl Acad Sci U S A. 2000;97:10990‐10995. doi: 10.1073/pnas.180329197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Girnun GD, Smith WM, Drori S, et al. APC‐dependent suppression of colon carcinogenesis by PPARgamma. Proc Natl Acad Sci U S A. 2002;99:13771‐13776. doi: 10.1073/pnas.162480299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Yang DR, Lin SJ, Ding XF, et al. Higher expression of peroxisome proliferator‐activated receptor γ or its activation by agonist thiazolidinedione‐rosiglitazone promotes bladder cancer cell migration and invasion. Urology. 2013;81(5):1109.e1‐6. doi: 10.1016/j.urology.2012.12.027 [DOI] [PubMed] [Google Scholar]
- 155. Kostapanos MS, Elisaf MS, Mikhailidis DP. Pioglitazone and cancer: angel or demon? Curr Pharm des. 2013;19:4913‐4929. doi: 10.2174/13816128113199990294 [DOI] [PubMed] [Google Scholar]
- 156. Jin SM, Song SO, Jung CH, et al. Risk of bladder cancer among patients with diabetes treated with a 15 mg pioglitazone dose in Korea: a multi‐center retrospective cohort study. J Korean Med Sci. 2014;29:238‐242. doi: 10.3346/jkms.2014.29.2.238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Azoulay L, Yin H, Filion KB, et al. The use of pioglitazone and the risk of bladder cancer in people with type 2 diabetes: nested case‐control study. BMJ. 2012;344:e3645. doi: 10.1136/bmj.e3645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Zhu Z, Shen Z, Lu Y, Zhong S, Xu C. Increased risk of bladder cancer with pioglitazone therapy in patients with diabetes: a meta‐analysis. Diabetes Res Clin Pract. 2012;98:159‐163. doi: 10.1016/j.diabres.2012.05.006 [DOI] [PubMed] [Google Scholar]
- 159. Heudobler D, Rechenmacher M, Lüke F, et al. Peroxisome proliferator‐activated receptors (PPAR)γ agonists as master modulators of tumor tissue. Int J Mol Sci. 2018;19(11):3540. doi: 10.3390/ijms19113540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Oprea TI, Bauman JE, Bologa CG, et al. Drug repurposing from an academic perspective. Drug Discov Today Ther Strateg. 2011;8:61‐69. doi: 10.1016/j.ddstr.2011.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Oettle H, Richards D, Ramanathan RK, et al. A phase III trial of pemetrexed plus gemcitabine versus gemcitabine in patients with unresectable or metastatic pancreatic cancer. Ann Oncol. 2005;16:1639‐1645. doi: 10.1093/annonc/mdi309 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that supports the findings of this study are available in material of this article.


