Abstract
While loss-of-function (LoF) variants in KCNQ2 are associated with a spectrum of neonatal-onset epilepsies, gain-of-function (GoF) variants cause a more complex phenotype that precludes neonatal-onset epilepsy. In the present work, the clinical features of three patients carrying a de novo KCNQ2 Y141N (n = 1) or G239S variant (n = 2) respectively, are described. All three patients had a mild global developmental delay, with prominent language deficits, and strong activation of interictal epileptic activity during sleep. Epileptic seizures were not reported. The absence of neonatal seizures suggested a GoF effect and prompted functional testing of the variants. In vitro whole-cell patch-clamp electrophysiological experiments in Chinese Hamster Ovary cells transiently-transfected with the cDNAs encoding Kv7.2 subunits carrying the Y141N or G239S variants in homomeric or heteromeric configurations with Kv7.2 subunits, revealed that currents from channels incorporating mutant subunits displayed increased current densities and hyperpolarizing shifts of about 10 mV in activation gating; both these functional features are consistent with an in vitro GoF phenotype. The antidepressant drug amitriptyline induced a reversible and concentration-dependent inhibition of current carried by Kv7.2 Y141N and G239S mutant channels. Based on in vitro results, amitriptyline was prescribed in one patient (G239S), prompting a significant improvement in motor, verbal, social, sensory and adaptive behavior skillsduring the two-year-treatment period. Thus, our results suggest that KCNQ2 GoF variants Y141N and G239S cause a mild DD with prominent language deficits in the absence of neonatal seizures and that treatment with the Kv7 channel blocker amitriptyline might represent a potential targeted treatment for patients with KCNQ2 GoF variants.
Keywords: Voltage-gated potassium channel, Developmental encephalopathy, Genotype-phenotype, Amitriptyline, Gain of function
Introduction
KCNQ2 and KCNQ3 encode Kv7.2 and Kv7.3 voltage-gated potassium channel subunits, respectively, that assemble into homo- or hetero-tetrameric channels mediating the M-current [1,2], a critical determinant of neuronal excitability and response to synaptic input [1,3]. Similar to other Kv subunits, Kv7.2 and Kv7.3 subunits display a characteristic structure with six transmembrane segments (S1–S6) and intracellular N- and C-termini; the S5–S6 region encompasses the pore domain (PD) where critical structural determinants for ion conductance and selectivity are located, while the S1–S4 region forms the voltage-sensing domain (VSD), where positively-charged amino acids in the S4 transmembrane segment play a critical role in voltage sensing [4].
Pathogenic KCNQ2 variants are among the most common causes of neonatal onset genetic epilepsies, and the incidence of KCNQ2-related epilepsy is reported to be 1/17.000 live births [5]. A broad spectrum of neonatal-onset phenotypes, ranging from self-limited familial neonatal epilepsy, to devastating developmental and epileptic encephalopathy (DEE) with treatment-resistant neonatal onset seizures and intellectual disability (ID) are associated to pathogenic KCNQ2 variants prompting loss-of-function (LoF) effects in vitro [6]. In comparison, rare gain-of-function (GoF) variants have been reported to cause a wider range of epileptic and neurodevelopmental phenotypes. So far, GoF KCNQ2 variants affect residues located within the VSD of Kv7.2 and include R144Q/W/G, [7,8] V175L, [9,10] R198Q [11] and R201C/H [7,12]. While the R198Q variant leads to infantile onset (4–6 months) of epileptic spasms and developmental delay (DD) in previously healthy children [11,13], variants at the V175 and R201 residues cause neonatal encephalopathy with DD, non-epileptic myoclonus, infantile onset epilepsy, and a high risk of early mortality [6,12,13]. The R144Q/W/G variants causes a neurodevelopmental disorder with cognitive and developmental impairment, autism spectrum disorder (ASD), infantile to childhood onset epilepsy in some patients, and sleep-activated epileptic activity during sleep [8].
So far only few patients with a pathogenic KCNQ2 GoF variant [8,9,11,12,[14], [15], [16], [17], [18], [19], [20]] have been described and knowledge about the natural history associated with such variants remains limited. While epilepsy due to LoF variants in KCNQ2 generally responds to sodium channel blockers and retigabine is currently being trialed as a potential precision treatment [13], no specific treatments have been described for disease due to KCNQ2 GoF variants. This highlights an urgent need for highly-targeted and genotype-specific therapy.
In the present study, the clinical features of three patients affected with mild global DD, prominent language deficits, and strong activation of interictal epileptic activity during sleep but no epileptic seizures, carrying a two de novo variants in KCNQ2 (Y141N, n = 1; G239S variant, n = 2) are described. In vitro functional testing with whole-cell patch-clamp electrophysiology revealed both variants prompted an in vitro GoF phenotype. The antidepressant drug amitriptyline induced a reversible and concentration-dependent inhibition of current carried by Kv7.2 Y141N and G239S mutant channels. Treatment with amitriptyline in one patient (G239S) led to a significant improvement in motor, verbal, social, sensory and adaptive behaviour skills. Thus, our results suggest that treatment with the Kv7 channel blocker amitriptyline might represent a potential targeted treatment for patients with KCNQ2 GoF variants.
Methods
Patient collection
Patients were recruited through specialized epilepsy centers and a KCNQ channel patient registry (www.rikee.org). Clinical data including birth parameters, epilepsy, developmental histories and physical examinations were collected from the local healthcare providers. The study was conducted in agreement with the Declaration of Helsinki. Authors consented each patient using research protocols approved by their local human research ethics committees. Probands were minors and had cognitive impairment, thus informed consent was given by their parents.
Evaluation of motor, verbal and neuropsychological features in one patient treated with amitriptyline
Cognitive and adaptive level of functioning
The Wechslers Intelligence Scale test (WPPSI-IV) [21] was administered at baseline, at 12 months and at 24 months after initiation of amitriptyline. Subtests targeting children younger than the study-participant were administered if the patient was unable to complete age-appropriate subtests. Testing was undertaken by a trained neuropsychologist. The questionnaires Vineland Adaptive Behavior Scales, Second Edition (Vineland-II) [22], and the Childrens Communication Checklist (CCC-2) [23] were administered by parents, assessing adaptive level of function and pragmatic language, respectively. The scales were administered at baseline, at 6, 12 and 24-months of follow-up. The Beery-Buktenica Developmental Test of Visual-Motor Integration test (BeeryWMI) [24] assessing visual-motor integration, and the Connors Kiddie Continous performance Test (K-CPT 2) [25] assessing attention were administered to the patient at 6, 12 and 24-months of follow-up.
Behavior
The Social Responsiveness Scale (SRS-2) [26], the Social Communication Questionnaire (SCQ) [27] and the Quality of Life in Childhood Epilepsy-55 (QoLCE-55) [28] were administered by parents at baseline, 6, 12, and 24-months of follow-up.
Motor function
The Canadian Occupational Performance Measure (COPM) [29], the Gross Motor Function Measure (GMFM-88) [30], Movement ABC-2 (MABC-2) and the Child Sensory Profile-2 [31] were used to assess motor ability at baseline and 12 and 24 months of follow-up.
Genetic identification and analysis
Patients 1 and 2 were investigated by whole exome sequencing ordered by the treating physician. Segregation analysis of selected variants was done by deep Amplicon or Sanger sequencing. Patient 3 was diagnosed by trio exome sequencing. KCNQ2 variants were annotated using the transcript NM_172107.4 (GRCh37/hg19) and classified based on the American College of Medical Genetics and Genomics and the Association for Molecular Pathology joint guidelines [32].
Mutagenesis and heterologous expression of Kv7.2 and Kv7.3 cDNAs
Variants were engineered in human Kv7.2 cDNAs cloned into pcDNA3.1 by QuickChange site-directed mutagenesis (Agilent Technologies), as described [7]. Channel subunits were expressed in Chinese Hamster Ovary (CHO) cells by transient transfection. CHO cells were grown in 100 mm plastic Petri dishes in Dulbecco's Modified Eagle's Medium (DMEM) containing 10 % Fetal Bovine Serum (FBS), penicillin (50 U/ml), and streptomycin (50 g/ml) in a humidified atmosphere at 37 °C with 5 % CO2. For electrophysiological experiments, cells were seeded on glass coverslips (Carolina Biological Supply) and transfected on the next day with the appropriate cDNAs using Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocol. A plasmid encoding for enhanced green fluorescent protein (Clontech) was used as transfection marker; total cDNA in the transfection mixture was kept constant at 4 g.
Whole-cell electrophysiology
Currents from CHO cells were recorded at room temperature (20 °C–22 °C) 1–2 d after transfection, using a commercially available amplifier (Axopatch 200B, Molecular Devices) and the whole-cell configuration of the patch-clamp technique, with glass micropipettes of 3–5 MΩ resistance. The extracellular solution contained (in mM) the following: 138 NaCl, 5.4 KCl, 2 CaCl2, 1 MgCl2, 10 glucose, and 10 HEPES, pH 7.4 with NaOH; the pipette (intracellular) solution contained (in mM) the following: 140 KCl, 2 MgCl2, 10 EGTA, 10 HEPES, 5 Mg-ATP, pH 7.3–7.4 with KOH. The pCLAMP software (version 10.0.2) was used for data acquisition and analysis. Current densities (expressed in picoamperes per picofarad) were calculated as peak K+ currents at 0 mV divided by cell capacitance (C). Data were acquired at 0.5–2 kHz and filtered at 1–5 kHz with the 4-pole low-pass Bessel filter of the amplifier. No corrections were made for liquid junction potentials. To generate conductance-voltage curves, the cells were held at −80 mV, then depolarized for 1.5 s from −80 mV to +20 in 10 mV increments, followed by an isopotential pulse at 0 mV of 300 ms duration; the current values recorded at the beginning of the 0 mV pulse were measured, normalized, and expressed as a function of the preceding voltages. The data were then fit to a Boltzmann distribution of the following form: y = max/[1 exp(V1/2 -V)/k], where V is the test potential, V1/2 the half-activation potential, and k the slope factor.
A stock solution (10 mM) of amitriptyline (Sigma-Aldrich, Germany) was prepared in dimethyl sulfoxide (DMSO); extracellular bathing solution was used for subsequent drug dilutions. Maximal DMSO concentration in the final solution was 0.1 %; this concentration, which did not affect current size and kinetics, was also added to the control (no drug) solution. The effect of amitriptyline (1 and 10 μM) was investigated using a ramp protocol in which currents were activated by 3s voltage ramps from −80 to +20 mV applied every 15 s. Currents at +20 mV were measured before and after drug application, and the effect of amitriptyline was expressed as % of blockade.
Statistics
Data are expressed as the mean ± SEM. Statistically significant differences between the data were evaluated with the Student's t-test (p < 0.05).
Results
In this study, three unrelated patients carrying de novo and previously unreported missense variants in KCNQ2 were included: one with the Y141N and two with the G239S variant.
Clinical features and genetic results
Patient 1 is a 7-year-old girl, born from non-consanguineous parents. She has one healthy younger sister. During pregnancy, poor fetal movements were reported. She was born at term, with a birth weight of 3160 g (0 SD), length of 52 cm (0 SD), and head circumference of 35 cm (0 SD). Apgar score was 10. From birth, delayed motor development was noted. She was hypotonic, with an absent sucking reflex at birth, and very weak and disorganized sucking from day 3. Head stabilization occurred at 5 months, sitting at 9 months, crawling at 13 months, pincer grasp at 18 months, and independent walking at 22 months. Speech development was also delayed and she said her first word at 18 months of life. By the time she was 3 years old she was speaking about 100 two-syllable words. At the age of 14 months, psychomotor delay with muscle hypotonia was diagnosed and intensive rehabilitation was initiated, including physiotherapy, speech therapy, and sensory therapy. At 4 years of age, oral electrostimulation was started. Neuropsychological evaluation at the age of 3 years showed a Leiter scale Intelligence Quotient of 82, and a disharmonious development with significant delay in the development of active speech. Speech comprehension and social skills were within the norm, and she was diagnosed with developmental dysphasia. At present time she walks independently, is able to walk on tiptoes and heels, and squats and stands up without support. She is still showing constant motor improvement, but fine motor skills remain poor. She is able to cut circles with scissors, draws circles and lines. She makes 20-piece puzzles, plays with dolls, and draws simple pictures. She currently speaks 3-5-word sentences (level of a 3 years-old), with errors at pronunciation. She counts to 10, can write her name, and adds and subtracts using her fingers. Social development is normal, without obvious behavioral problems. She has a normal head circumference of 50 cm. She is still hypotonic with ligament laxity and brisk reflexes; further neurological exam is normal. She is not taking any medication. Brain MRI performed at the age of 2.5 years was normal. Several EEGs were performed between the age of 2.5 and 6.5 years due to DD and showed interictal sharp wave and spike and slow wave activity in the posterior regions with maximum at the right side and strong accentuation during sleep. She never had clinical seizures. Whole exome sequencing revealed a novel missense variant (c.421T>A, p.Tyr141Asn) in KCNQ2 arising de novo in the patient.
Patient 2 is a 10-year-old male with no significant family history. He was born at term (38 + 6) following a normal pregnancy and was delivered via planned caesarian section due to breech presentation. Birthweight was 3276 gr (0 SD), length 50 cm (0 SD) and head circumference 35 cm (0 SD). Head stabilization occurred at 3 months, sitting at 8 months, and independent walking at the age of one year, albeit with a clumsy and unsteady gait. He developed a pincer grasp at 14 months and learned to use utensils at 7 years of life. His speech development was delayed as at the age of 2 years he was still babbling without using any actual words. At the age of three years, he was diagnosed with verbal dyspraxia. He was diagnosed with cognitive impairment and an autism spectrum disorder at the age of 4 years and 8 months. He was easily fixated on favorite objects or routines and disliked changes. He had a low anger threshold with tantrums and occasional aggressive outbursts. He had a short attention span and a poor concentration. Understanding social situations and making friends were difficult. He displayed symptoms suggestive of a sensory integrative dysfunction which interfered with learning, playing, social interaction, and completing daily activities of life. A neuropsychological evaluation with the Wechsler Preschool and Primary Scale of Intelligence fourth edition (WPPSI-IV) was performed at age 5 years and 8 months in order to determine the level of support needed at school; the results were suggestive of a mild intellectual disability (supplementary file). At present he attends a special needs school. The EEG performed around the second year of life due to staring spells, showed interictal paroxysmal epileptiform activity that accentuated during sleep but no ictal discharges. Intermixed beta activity was observed in the fronto-temporal derivations. Staring spells were considered to be not epileptic. Brain MRI performed at the age of five years was normal. Exome sequencing revealed a de novo KCNQ2 missense variant (c.715G>A; p.G239S).
Patient 3 is a 10-year-old female. Family history included two older male siblings, one was late to talk (age 3) and subsequently diagnosed with attention deficit disorder; a paternal uncle was also late in talking (4 years) and had mild learning problems as a child. Patient 3 was born at term [39] following a normal pregnancy and was delivered via planned caesarian section due to prior C-section. Birthweight was 3062 gr (0 SD) while length and head circumference were unknown. Early motor milestones were achieved normally (walked at 11 months, ran at 15 months) but both expressive and receptive language were noted to be delayed prompting evaluation. At age 17.5 months, a mixed receptive-expressive language disorder was diagnosed. She was referred to speech therapy but only attended for 3–4 months due to lack of progress. Re-assessed at 3.5 years, she spoke only five words, was generally happy but distractible and inattentive, with some repetitive head banging, hitting, biting, and hair-pulling when frustrated. She could follow only one step commands, and was unable to nod yes or no. Formal testing included the Broch-League receptive expressive emergent language teste (REEL-3; receptive: 1 percentile, equivalent to 7 months; expressive: 3rd percentile, equivalent to 8 months). One provider noted good eye contact, but other signs concerning for autism risk. Another performed the Autism Diagnostic Observation Schedule (ADOS)-II, Module 1, the Child Behavioral Checklist, and a comprehensive neurological and physical exam, leading to an ASD and DD diagnosis. At age 9 years, the parent and ABA therapist were concerned that staring spells might be epileptic seizures. A 72 h ambulatory EEG was performed. Recurring left parietal sharp waves were noted during drowsiness, and, with more frequency, during sleep, but two typical spells during wakefulness were noted and were not accompanied by ictal EEG changes. Brain MRI performed at age 9.5 years was normal. Trio exome sequencing revealed a de novo missense variant KCNQ2 c.715G>A; p.G239S.
Functional analysis of Kv7.2 Y141N and G239S mutant channels
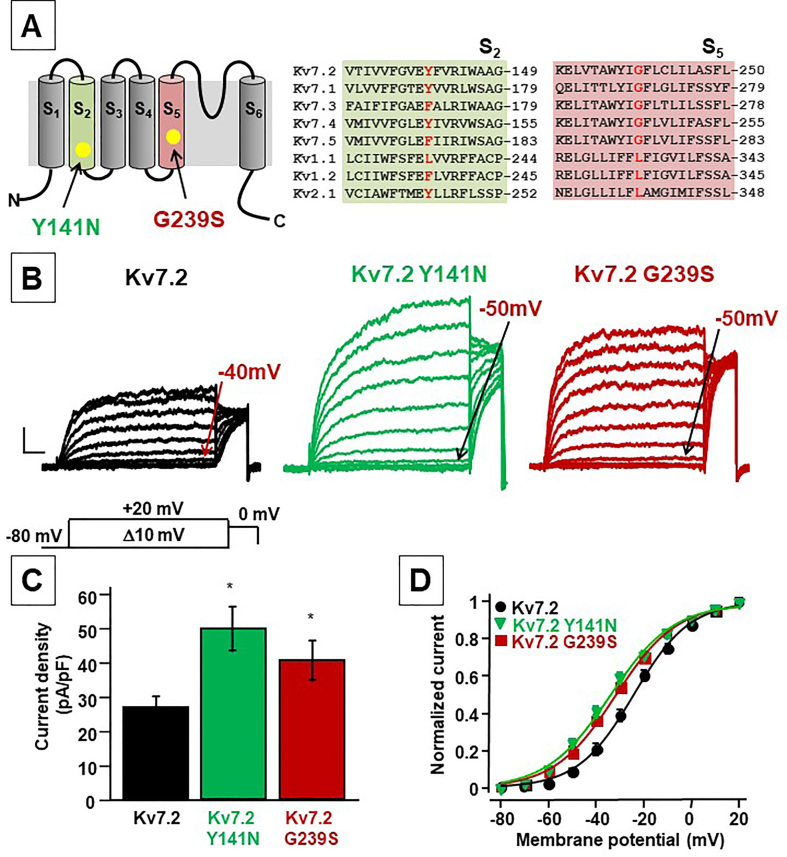
The Y141N is located in S2 of the VSD while the G239 residue is located in the middle of the S5 segment of the PD (Fig. 1A). The G239 residue is conserved among all Kv7 channels, but not in other Kv channels; whereas the Y141 residue is present in Kv7.1, Kv7.2 and Kv7.4 subunits (Fig. 1B).
Fig. 1.
Schematic representation of a single Kv7.2 subunit, alignment and functional analysis of mutations at position Y141 and G239. A. Cartoon depicting the six transmembrane arrangement of a single Kv7.2 subunit (left panel) and sequence alignments of the S2 and S5 (right panel) regions among different Kv subunits. B. Macroscopic currents from Kv7.2, Kv7.2 Y141N and Kv7.2 G239S channels, in response to the indicated voltage protocol. Current scale, 200 pA; time scale, 200 ms. C. Current density from currents from Kv7.2, Kv7.2 Y141N and Kv7.2 G239S channels calculated at 0 mV. D. Conductance/voltage curves for the indicated channels. Continuous lines are Boltzmann fits to the experimental data.
When expressed in CHO cells, homomeric Kv7.2 channels generated slowly activating and deactivating K+-selective currents in response to membrane depolarization from −80 to +20 mV, characterized by a voltage threshold for current activation around −40/−50 mV (Fig. 1B). When compared to those carried by homomeric Kv7.2 channels, currents recorded in cells expressing Kv7.2 subunits carrying the Y141N or the G239S mutations showed higher densities and a small but statistically-significant hyperpolarizing shift (∼10 mV) in activation gating (Fig. 1C–D). Both the increased current densities and the negative shifts (∼10 mV) in activation voltage-sensitivity strongly suggest that both Y141N and G239S missense substitutions trigger mild GoF effects on channel behaviour. The gating changes of homomeric Kv7.2 mutant channels were rather small quantitatively; as a matter of fact, we were unable to detect significant changes in activation and deactivation kinetics among experimental groups analysed (data not shown).
At early developmental stages expression pattern of Kv7.2 and Kv7.3 subunits seems not to be identical, with Kv7.2 being expressed at earlier time points [33]; thus, in addition to Kv7.2/3 heteromers, Kv7.2 homomeric channels likely exist in vivo [34]. Moreover, the clinical features of Kv7.2-DEE appear to more closely correlate with Kv7.2 homomer dysfunction, rather than Kv7.2/3 heteromers [35]. Given these premises, we performed electrophysiological experiments co-expressing mutant subunits (Kv7.2 G239S and Kv7.2 Y141N) with WT Kv7.2 (mutant/WT Kv7.2; 1:1 transfection ratio). The results obtained revealed a slightly but statistically-significant leftward shift of the V½ of about 5–6 mV in the Kv7.2+Kv7.2 Y141N and Kv7.2+Kv7.2 G239S, when compared to homomeric Kv7.2 channels (Table 1).
Table 1.
Biophysical and pharmacological properties of mutant Kv7.2 channels.
| Blockade by amitriptyline (%) |
||||||
|---|---|---|---|---|---|---|
| Construct(s) | n | Half-activation potential, V½ (mV) | Slope factor, k (mV/e-fold) | Current density (pA/pF) | 1 μM | 10 μM |
| Kv7.2 | 28 | −22.3 ± 0.9 | 12.6 ± 0.6 | 26.9 ± 3.2 | – | – |
| Kv7.2 Y141N | 22 | −35.4 ± 0.6∗ | 14.9 ± 0.6∗ | 50.1 ± 6.4∗ | ||
| Kv7.2 G239S | 16 | −30.5 ± 1.7∗ | 14.3 ± 0.7∗ | 38.2 ± 5.5∗ | – | – |
| Kv7.2 + Kv7.3 | 10 | −27.1 ± 1.0 | 10.6 ± 0.5 | 112.4 ± 15.1 | 8.1 ± 1.2 | 40.0 ± 3.8 |
| Kv7.2 + Kv7.2 Y141N + Kv7.3 |
13 | −28.7 ± 1.1 | 11.3 ± 0.6 | 99.9 ± 17.0 | 9.7 ± 2.5 | 39.8 ± 3.0 |
| Kv7.2 + Kv7.2 G239S + Kv7.3 |
11 | −28.7 ± 1.6 | 13.9 ± 1.0 | 135.5 ± 30.4 | 11.8 ± 3.6 | 64.6 ± 5.2∗∗ |
∗p < 0.05 versus Kv7.2; ∗∗p < 0.05 versus Kv7.2+Kv7.3.
Finally, since IKM in mature neurons is mainly formed by Kv7.2/Kv7.3 heteromeric channels [1], and to replicate in vitro the genetic combination occurring in the affected family members who are heterozygous for the pathogenic allele, functional studies were also carried out upon transfection of cDNAs of Kv7.2+Kv7.2Y141N+Kv7.3 and Kv7.2+Kv7.2G239S+Kv7.3 at a cDNA ratio of 0.5:0.5:1. However, in these experimental settings no significant differences in either current size or gating were detected when Kv7.2 Y141N or G239S subunits were co-expressed in heterotetrameric channels with Kv7.2 and Kv7.3 subunits, a result possibly due to the relatively small functional changes prompted by the two variants, (Table 1).
Blockade of Kv7.2 Y141N and Kv7.2 G239S mutant channels by amitriptyline
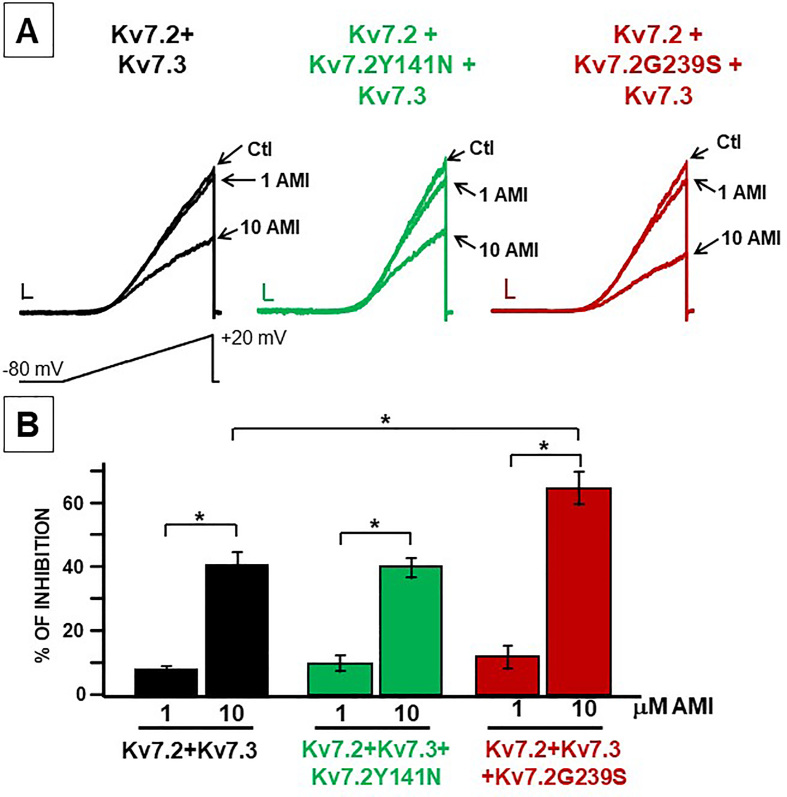
In order to pharmacologically reverse the GoF consequences triggered by the Y141N and G239S variants in Kv7.2, the ability of amitriptyline [36] to block heteromeric channels containing wild-type and mutant Kv7.2 subunits was investigated. Perfusion with 1 and 10 μM amitriptyline dose-dependently inhibited currents recorded from Kv7.2+Kv7.3, Kv7.2+Kv7.2Y141N+Kv7.3-, and Kv7.2+Kv7.2G239S+Kv7.3-expressing cells. Interestingly, 10 μM amitriptyline showed higher efficacy in inhibiting Kv7.2+Kv7.2G239S+Kv7.3 when compared to Kv7.2+Kv7.3 currents (Fig. 2; Table 1).
Fig. 2.
Effect of amitriptyline on heteromeric channels incorporating Kv7.2 mutant subunits. A. Representative current traces in response to the voltage ramp protocol before and during amitriptyline (AMI) exposure (1 and 10 μM) from cells expressing the indicated subunit combinations. Current scale, 200 pA; time scale, 200 ms. B. % of current inhibition upon amitriptyline application.∗ indicate values significantly different from each respective control (p < 0.05).
Response to amitriptyline treatment in patient 2
Based on the described in vitro results, and after parental consent, patient 2 was titrated to a daily dosage of 1 mg/kg/day amitriptyline at the age 8 years and 8 months. He has adhered to the treatment for the past 24 months without experiencing any adverse effects.
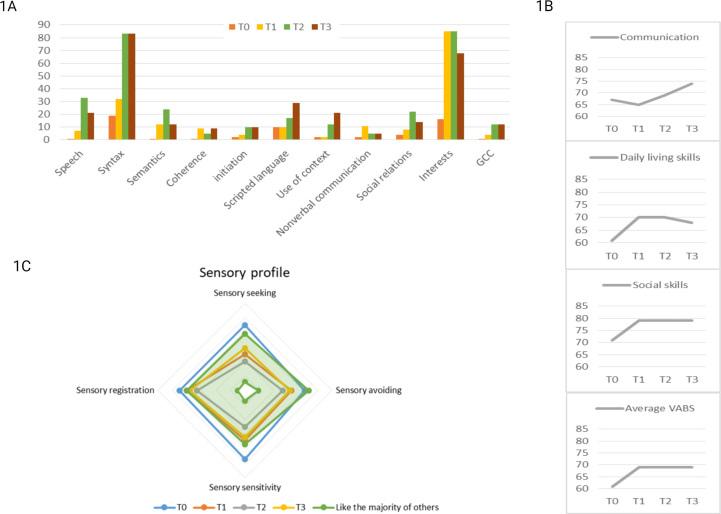
A baseline cognitive testing was done before initiating amitriptyline at the age of 8 years. At this time, he was unable to join two puzzle pieces, do jumping jacks or understand both simple and more complex tasks. He failed to attend an age-appropriate test due to attention deficits and cognitive impairment and was instead subjected to the WPPSI-IV, the Beery WMi and the computer-based test K-CPT 2. He was difficult to regulate and despite support, the K-CPT 2 test ended prematurely. He scored in the category “more than others” in three out of four areas at baseline using the Sensory Profile 2 questionnaire; sensory seeking, sensory sensitivity and sensory registration (Fig. 3). His score on the GMFM-88 was 97 % at baseline, and he performed at percentile 0.5 in MABC-2 (supplementary file). The cognitive test results and the parent-proxy evaluation of adaptive functions (Vineland II and CCC-2) suggested that he had a mild intellectual disability, poor cognitive efficiency and attention, and was more sensitive, more sensory seeking and registered more sensory input compared to peers (supplementary file).
Fig. 3.
Results from neuropsychological profiling in patient 2 treated with amitriptyline. Results are based on Vineland Adaptive Behavior Scales, Second Edition (1A), the Childrens Communication Checklist (1B) and Child Sensory Profile-2. (GCC = Children's Communication Checklist; T0 = baseline; T1 = after 6 months of amitriptylin treatment; T2 = after 12 months of amitriptylin treatment; T3 = after 24 months of amitriptylin treatment. VABS = Vineland Adaptive Behavior Scales).
Within three months after initiation of amitriptyline he had learned to do 9-piece puzzles, do jumping jacks and undertake tasks that he had previously been unable to do. Supplementary video 1 shows gross motor skills at baseline and after 3 months of amitriptyline treatment.
At six months, parents reported that he no longer needed diapers during sleep, his social interaction with other children had improved, and that his verbal skills had improved enabling him to engage in conversations during dinner time. According to the parents, his focus, attention, and sensory profile had improved while treated with amitriptyline (Fig. 3 and supplementary file). All scores within the sensory profile were in the area of “same like others” (Fig. 3) and his score on the GMFM-88 had improved to 98 %.
One year after initiation of amitriptyline, he was submitted to a second WPPSI-IV, K-CPT 2 and Beery WMI test. Although frequent pauses and support was needed, he now managed to finish the testing; raw scores were improved in most subtests (supplementary file) and parent-proxy evaluations of adaptive level of functioning, communication and social skills were markedly improved (Fig. 3 and supplementary file). The sensory profiling and GMFM-88 was unchanged compared to the evaluation after 6 months (Fig. 3).
His cognitive efficiency and attention had significantly improved after 2 years of treatment. For the first time, he could engage in the test sessions with a reasonably concentration and persistence for his age and made only a few mistakes due to inattentiveness (supplementary file). The sensory profiling was unchanged and his score on the GMFM-88 had improved to 100 %. His hand-motoric scores improved to percentile 5 at 6 and 12 month after initiation, and improved further to percentile 25 at 24 month.
At baseline, the EEG showed interictal paroxysmal epileptiform activity that accentuated to 49 % of non-rapid-eye-movement sleep, while after 12 and 24 months of treatment the paroxysmal activity dropped to 27 and 31 %, respectively.
Discussion
KCNQ2 related disorders encompass a wide spectrum of epileptic and/or neurodevelopmental disorders. While most pathogenic variants have a LoF effect and lead to neonatal onset epilepsy with or without neurodevelopmental problems, rare GoF variants have been described that lead to neurodevelopmental disorders without neonatal onset seizures. All currently known disease-causing GoF KCNQ2 variants affect residues located within the VSD of Kv7.2 and include R144Q/W/S [7,8], V175L [9,10], R198Q [11] and R201C/H [7,12]. In this study, we describe three patients carrying two novel de novo missense variants, Y141N (1 patient) and G239S (2 patients). All patients had neurodevelopmental impairment without seizures. Additional comorbidities included verbal dysphasia or dyspraxia, and sleep-activated epileptic activity, resembling phenotypes caused by the R144W variant in KCNQ2, and GoF variants in the homologue gene KCNQ3 [2]. We confirmed that Y141N and G239S have mild GoF effects in vitro, through an increase of the maximal current density and a negative shift in the activation gating. Noteworthy, quantitatively similar shifts in activation voltage were described in homomeric channels carrying the R144W variant in Kv7.2 [8]. The G239S variant represents the first reported mild GoF variant located outside the VSD of Kv7.2. Interestingly, in a complete functional dataset of all possible single-nucleotide variants (SNVs) encoding missense mutations for Kv7.4 channels identified in patients with autosomal dominant non-syndromic hearing loss, several variants at positions paralogous to Y141 and G239 in Kv7.2 (Y147 and G245, respectively) have been described. Functional analysis, which was carried out both in homomeric mutant channels and in heteromeric configuration with wtKv7.4 channels, revealed that some of these variants also showed similar in vitro mild GoF effects [37], suggesting that substitution at these two positions might play an important role in the gating properties of Kv7 channels.
While LoF variants in both KCNQ2 and KCNQ3 can potentially be treated with sodium channel blockers or retigabine [13] this may not be the case for KCNQ2 and KCNQ3 GoF variants. Currently, despite a large number of antiseizure medications available, no drugs are licensed for the treatment of patients suffering from DEEs (such as infantile epileptic spasms syndrome) caused by GoF variants in KCNQ2. Investigating the use of approved drugs for a different indication, a process called drug repurposing, offers the opportunity to speed up the traditional process of drug discovery and to more quickly tackle the unmet clinical needs of patients suffering from rare diseases like those associated with the KCNQ2 GoF spectrum. In this context, amitriptyline, a tricyclic antidepressant that mainly inhibits the reuptake of serotonin and norepinephrine, has been described to be also a potent blocker of heteromeric Kv7.2/Kv7.3 channels [36].
Amitriptyline is a commonly used drug to treat several conditions including depression, obsessive–compulsive disorder and neuropathic pain and it has an acceptable safety profile [38]. Moreover, it is licensed for use in children of 6 years and older, mostly to treat nocturnal enuresis [39]. In this study, we showed that amitriptyline also blocks heteromeric channels containing mutant Kv7.2 subunits at concentrations of 1–10 μM. The therapeutic plasma concentrations of amitriptyline, when used for its known indications, are in the range of 0.4–0.9 μM [40]. However, previous studies in rats suggest that amitriptyline accumulates in the brain, thus reaching concentrations which are up to ten times higher than those achieved in the plasma during standard dosing [41]. Such distribution is dependent upon the protein binding and lipid solubility characteristics of the drug; these do not vary appreciably between different mammalian species [42], suggesting that the plasma/brain ratio distribution of amitriptyline observed in rodents is valid also for humans. Accordingly, the concentrations of amitriptyline (1–10 μM) shown to block both wild-type and mutant heteromeric channels in this study are close to those reached in the brain during conventional therapy with the drug.
These considerations prompted us to initiate intervention with amitriptyline in one patient described in this study. The promising results of the amitriptyline treatment of patient 2, carrying the G239S KCNQ2 GoF variant, appear consistent with the above-mentioned considerations concerning its pharmacokinetic profile. Twelve months of treatment with amitriptyline indeed led to a notable improvement in sensory profile and level of adaptive behavioral functioning, communication, and social skills, as supported by an improvement on most subtests of a standardized neuropsychological testing battery. We observed a striking developmental improvement within 6 months of intervention, which is beyond what would be expected from natural development. He then followed the developmental trajectories starting from this new level of functioning before taking another leap during the second year of intervention. The second improvement was within areas such as attention, motor control and cognitive efficiency. His developmental trajectories will be studied in the years to come and we speculate that the second improvement provides a foundation for learning and development onwards and enhances his possibility to improve his abilities within his cognitive capacity.
Our study has several strengths but also important limitations; systematic neuropsychological evaluation was done before and during amitriptyline treatment but only a single family was able to participate in a trial that was neither placebo-controlled nor blinded. Since the study lacks a control group, it remains difficult to determine to which extend the clinical improvement was an age-related maturation or a result of the study intervention. However, before amitriptyline treatment patient 2 struggled to understand and perform both simple and more complex tasks. Within three months after initiation of amitriptyline he could undertake tasks that he had previously been unable to do. We believe that such a drastic change is more likely to be attributed to the intervention than to spontaneous age-related improvement. Another limitation was that the baseline and 12- and 24 months intelligence scale test were done by two different and unblinded neuropsychologists. Both were skilled and trained test supervisors but we can not rule out that there might have been a subtle difference in support during the tests. Since large randomized controlled trials are not feasible in rare disorders, N-of-1 trials have been advocated to be a valid methodological alternative for this type of study population [43]. Such type of studies will be important to further validate the role of amitriptyline in the treatment of patients with GoF KCNQ2 variants, and to explore if there is an age-dependent window of opportunity for the treatment of the behavioral and cognitive difficulties in this rare disorder [44]. N-of-1 trials are based on multiple cross-over trial designs, wherein a single participant alternates between being on and off a given intervention at least twice [43]. Finally, it should be highlighted that amitriptyline cannot be considered a Kv7.2-specific blocker, since it also blocks Kv1.1 potassium channels with equal potency [36] as well as KCNQ1/KCNE1 cardiac potassium channel responsible for controlling the length of ventricular plateau potentials and mutated in long-QT syndrome [45]. In addition, tricyclic antidepressants with similar molecular structures, such as imipramine, are well known to potently block EAG-class potassium channels [46,47]. At the dosage administered in this study, if it acts by blocking Kv7.2-derived channels, it most likely also blocks many other molecular subclasses of potassium channels in the brain, making a direct causal relationship between drug-dependent blockade of Kv7.2 currents and amelioration of GOF Kv7.2 phenotypes, difficult to interpret. Finally, although amitriptyline is FDA and EMA approved, caution should be noted, since it can potentially cause adverse side-effects due to the block of the cardiac-specific potassium channels, KCNQ1/KCNE1 and HERG, critical for ventricular repolarization.
In conclusion, this study shows that the KCNQ2 variants Y141N and G239S lead to a phenotype associated with a mild cognitive and developmental impairment, language deficits, and sleep-activated epileptic activity in the absence of seizures. We show that both variants lead to a mild GoF in vitro and thus confirm that identification of a de novo KCNQ2 variant in patients with a neurodevelopmental disorder without neonatal seizures should raise suspicion of a GoF variant. In vitro, amitriptyline induces a reversible and concentration-dependent inhibition of Kv7.2 heteromeric channels carrying variants Y141N or G239S subunits, and we document the neurodevelopmental improvements following amitriptyline treatment of a single patient harbouring the G239S variant. Systematic multicenter studies examining the short- and long-term effects of amitriptyline in additional patients with KCNQ2 GoF variants are warranted to study the role of this potentially tailored treatment approach.
Author contributions
A. Bayat and R.S.M. Steensbjerre conceptualized the study. A. Bayat and E.C. Cooper were responsible for collecting medical records. M. Taglialatela, S. Lavarone and F. Miceli performed the in vitro experiments. A. Jakobsen was responsible for follow-up neuropsychological testing and the overall interpretation of test results of subject 2. A. Bayat drafted the manuscript. All authors were involved in the ongoing revision of the manuscript. All authors contributed to the revision of the final manuscript.
Data availability
Anonymized data including data not published in this article will be made available upon request by any qualified investigator.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Allan Bayat reports financial support was provided by Novo Nordisk Foundation. Francesco Miceli reports financial support was provided by Ministry of Education and Merit. Edward C. Cooper reports financial support was provided by Jack Pribaz Foundation and Miles Family Fund. Sarah Weckhuysen reports financial support was provided by FWO, GSKE, KCNQ2-Cure, Jack Pribaz Foundation, KCNQ2e.v., European Joint Programme on Rare Disease JTC 2020 (TreatKCNQ). Maurizio Taglialatela reports financial support was provided by Ministry of Education and Merit. Maurizio Taglialatela reports financial support was provided by Italian Ministry of Health. Maurizio Taglialatela reports was provided by European Joint Programme on Rare Disease JTC 2020 (TreatKCNQ). Sarah Weckhuysen reports a relationship with UCB, Xenon Pharmaceuticals, Lundbeck, Knopp Biosciences, Angelini Pharma, Roche, Biohaven and Encoded Therapeutics that includes: consulting or advisory. Edward C. Cooper reports a relationship with Xenon Pharmaceuticals and Knopp Biosciences that includes: consulting or advisory and funding grants. Rikke Steensbjerre Moller reports a relationship with EISAI, UCB, Orion and Angelini Pharma that includes: consulting or advisory and speaking and lecture fees.
Acknowledgements
The authors would like to thank the patients and their families for their participation in our research.
AB is funded by a BRIDGE - Translational Excellence Programme grant funded by the Novo Nordisk Foundation, grant agreement number: NNF20SA0064340.
FM received funding from the Italian Ministry for University and Research (MIUR) (PRIN2017YH3SXK).
ECC received funding from the Jack Pribaz Foundation and Miles Family Fund.
SW received funding from FWO (1861419 N, G041821 N and G056122 N), GSKE, KCNQ2-Cure, Jack Pribaz Foundation, KCNQ2e.v., European Joint Programme on Rare Disease JTC 2020 (TreatKCNQ).
MT received funding from the Italian Ministry for University and Research (MIUR) (PRIN 2017ALCR7C; National Recovery and Resilience Plan (NRRP), Mission 4 Component 2 Investment 1.3 – PE12 Neuroscience “A multiscale integrated approach to the study of the nervous system in healt and disease” (MNESYS)”); the Italian Ministry of Health (Project RF-2019-12370491); the European Commission H2020 (UNICOM – 875299); European Joint Programme on Rare Disease JTC 2020 (TreatKCNQ).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.neurot.2023.10.006.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Wang H.S., Pan Z., Shi W., Brown B.S., Wymore R.S., Cohen I.S., et al. KCNQ2 and KCNQ3 potassium channel subunits: molecular correlates of the M-channel. Science. 1998;282(5395):1890–1893. doi: 10.1126/science.282.5395.1890. [DOI] [PubMed] [Google Scholar]
- 2.Sands T.T., Miceli F., Lesca G., Beck A.E., Sadleir L.G., Arrington D.K., et al. Autism and developmental disability caused by KCNQ3 gain-of-function variants. Ann Neurol. 2019;86(2):181–192. doi: 10.1002/ana.25522. [DOI] [PubMed] [Google Scholar]
- 3.Yue C., Yaari Y. KCNQ/M channels control spike afterdepolarization and burst generation in hippocampal neurons. J Neurosci. 2004;24(19):4614–4624. doi: 10.1523/JNEUROSCI.0765-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soldovieri M.V., Miceli F., Taglialatela M. Driving with no brakes: molecular pathophysiology of Kv7 potassium channels. Physiology. 2011;26(5):365–4624. doi: 10.1152/physiol.00009.2011. [DOI] [PubMed] [Google Scholar]
- 5.Symonds J.D., Zuberi S.M., Stewart K., McLellan A., O'Regan M., MacLeod S., et al. Incidence and phenotypes of childhood-onset genetic epilepsies: a prospective population-based national cohort. Brain. 2019;142(8):2303–2318. doi: 10.1093/brain/awz195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miceli F., Soldovieri M.V., Weckhuysen S., Cooper E., Taglialatela M. 2010. KCNQ2-Related Disorders. (GeneReviews®) [PubMed] [Google Scholar]
- 7.Miceli F., Soldovieri M.V., Ambrosino P., De Maria M., Migliore M., Migliore R., et al. Early-onset epileptic encephalopathy caused by gain-of-function mutations in the voltage sensor of Kv7.2 and Kv7.3 potassium channel subunits. J Neurosci. 2015;35(9):3782–3793. doi: 10.1523/JNEUROSCI.4423-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miceli F., Millevert C., Soldovieri M.V., Mosca I., Ambrosino P., Carotenuto L., et al. KCNQ2 R144 variants cause neurodevelopmental disability with language impairment and autistic features without neonatal seizures through a gain-of-function mechanism. EBioMedicine. 2022;81 doi: 10.1016/j.ebiom.2022.104130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Devaux J., Abidi A., Roubertie A., Molinari F., Becq H., Lacoste C., et al. A Kv7.2 mutation associated with early onset epileptic encephalopathy with suppression-burst enhances Kv7/M channel activity. Epilepsia. 2016;57(5):e87–e93. doi: 10.1111/epi.13366. [DOI] [PubMed] [Google Scholar]
- 10.Samanta D., Ramakrishnaiah R., Willis E., Frye R.E. Myoclonic epilepsy evolved into West syndrome: a patient with a novel de novo KCNQ2 mutation. Acta Neurol Belg. 2015;115(3):475–478. doi: 10.1007/s13760-014-0344-5. [DOI] [PubMed] [Google Scholar]
- 11.Millichap J.J., Miceli F., De Maria M., Keator C., Joshi N., Tran B., et al. Infantile spasms and encephalopathy without preceding neonatal seizures caused by KCNQ2 R198Q, a gain-of-function variant. Epilepsia. 2017;58(1):e10–e15. doi: 10.1111/epi.13601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mulkey S.B., Ben-Zeev B., Nicolai J., Carroll J.L., Grønborg S., Jiang Y.H., et al. Neonatal nonepileptic myoclonus is a prominent clinical feature of KCNQ2 gain-of-function variants R201C and R201H. Epilepsia. 2017;58(3):436–445. doi: 10.1111/epi.13676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weckhuysen S., Ivanovic V., Hendrickx R., Van Coster R., Hjalgrim H., Moller R.S., et al. Extending the KCNQ2 encephalopathy spectrum: clinical and neuroimaging findings in 17 patients. Neurology. 2013;81(19):1697–1703. doi: 10.1212/01.wnl.0000435296.72400.a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weckhuysen S., Mandelstam S., Suls A., Audenaert D., Deconinck T., Claes L.R., et al. KCNQ2 encephalopathy: emerging phenotype of a neonatal epileptic encephalopathy. Ann Neurol. 2012;71(1):15–25. doi: 10.1002/ana.22644. [DOI] [PubMed] [Google Scholar]
- 15.Trump N., McTague A., Brittain H., Papandreou A., Meyer E., Ngoh A., et al. Improving diagnosis and broadening the phenotypes in early-onset seizure and severe developmental delay disorders through gene panel analysis. J Med Genet. 2016;53(5):310–317. doi: 10.1136/jmedgenet-2015-103263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kojima K., Shirai K., Kobayashi M., Miyauchi A., Saitsu H., Matsumoto N., et al. A patient with early myoclonic encephalopathy (EME) with a de novo KCNQ2 mutation. Brain Dev. 2018;40(1):69–73. doi: 10.1016/j.braindev.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 17.Hortiguela M., Fernandez-Marmiesse A., Cantarin V., Gouveia S., Garcia-Penas J.J., Fons C., et al. Clinical and genetic features of 13 Spanish patients with KCNQ2 mutations. J Hum Genet. 2017;62(2):185–189. doi: 10.1038/jhg.2016.104. [DOI] [PubMed] [Google Scholar]
- 18.Kim H.J., Yang D., Kim S.H., Won D., Kim H.D., Lee J.S., et al. Clinical characteristics of KCNQ2 encephalopathy. Brain Dev. 2021;43(2):244–250. doi: 10.1016/j.braindev.2020.08.015. [DOI] [PubMed] [Google Scholar]
- 19.Olson H.E., Kelly M., LaCoursiere C.M., Pinsky R., Tambunan D., Shain C., et al. Genetics and genotype-phenotype correlations in early onset epileptic encephalopathy with burst suppression. Ann Neurol. 2017;81(3):419–429. doi: 10.1002/ana.24883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mary L., Nourisson E., Feger C., Laugel V., Chaigne D., Keren B., et al. Pathogenic variants in KCNQ2 cause intellectual deficiency without epilepsy: broadening the phenotypic spectrum of a potassium channelopathy. Am J Med Genet. 2021;185(6):1803–1815. doi: 10.1002/ajmg.a.62181. [DOI] [PubMed] [Google Scholar]
- 21.Wechsler D. The Psychological Corporation; San Antonio, TX: 2012. Wechsler Preschool and Primary Scale of Intelligence—Fourth Edition. [Google Scholar]
- 22.Sparrow S.S., Cicchetti D., Balla D.A. second ed. 2005. Vineland Adaptive Behavior Scales. [Google Scholar]
- 23.Bishop D.V. Psychological Corporation London; 2003. The Children's Communication Checklist. [Google Scholar]
- 24.Beery K.E., Beery N.A. 2010. The Beery-Buktenica Developmental Test of Visual-Motor Integration (Beery VMI): With Supplemental Developmental Tests of Visual Perception and Motor Coordination and Stepping Stones Age Norms from Birth to Age Six: Administration, Scoring, and Teaching Manual: Pearson. [Google Scholar]
- 25.Conners C.K., Staff M., Connelly V., Campbell S., MacLean M., Barnes J. vol. 29. Multi-Health Syst Inc; 2000. pp. 175–196. (Conners' Continuous Performance Test II (CPT II V. 5)). [Google Scholar]
- 26.Constantino J.N., Gruber C.P. Western Psychological Services Torrance; CA: 2012. Social Responsiveness Scale: SRS-2. [Google Scholar]
- 27.Lord C., Rutter M. WPS; Torrance, CA: 2003. Social Communication Questionnaire (SCQ) [Google Scholar]
- 28.Goodwin S.W., Lambrinos A.I., Ferro M.A., Sabaz M., Speechley K.N. Development and assessment of a shortened quality of life in childhood epilepsy questionnaire (QOLCE-55) Epilepsia. 2015;56(6):864–872. doi: 10.1111/epi.13000. [DOI] [PubMed] [Google Scholar]
- 29.Mcaoot L. Canadian Association of Occupational Therapists (CAOT); Ottawa: 2014. Canadian Occupational Performance Measure (COPM) [Google Scholar]
- 30.Michaelis U. Gross Motor Function Measure (GMFM-66 & GMFM 88) User's Manual 2nd Edition Clinics in Developmental Medicine Edited by Dianne J Russell, Peter L Rosenbaum, Marilyn Wright, Lisa M Avery London, UK: Mac Keith Press, 2013 £70.00 (Spiral Binding), pp 290 ISBN: 978-1-908316-88-2. Dev Med Child Neurol. 2015;57(12):1188. [Google Scholar]
- 31.Dunn W. Psych Corp.; Bloomington, MN: 2014. Sensory Profile 2 : User's Manual. [Google Scholar]
- 32.Richards S., Aziz N., Bale S., Bick D., Das S., Gastier-Foster J., et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of medical genetics and Genomics and the association for molecular Pathology. Genet Med. 2015;17(5):405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dirkx N., Miceli F., Taglialatela M., Weckhuysen S. The role of Kv7.2 in neurodevelopment: insights and gaps in our understanding. Front Physiol. 2020;11 doi: 10.3389/fphys.2020.570588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Devaux J.J., Kleopa K.A., Cooper E.C., Scherer S.S. KCNQ2 is a nodal K+ channel. J Neurosci. 2004;24(5):1236–1244. doi: 10.1523/JNEUROSCI.4512-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gomis-Pérez C., Urrutia J., Marcé-Grau A., Malo C., López-Laso E., Felipe-Rucián A., et al. Homomeric Kv7.2 current suppression is a common feature in KCNQ2 epileptic encephalopathy. Epilepsia. 2019;60(1):139–148. doi: 10.1111/epi.14609. [DOI] [PubMed] [Google Scholar]
- 36.Punke M.A., Friederich P. Amitriptyline is a potent blocker of human Kv1.1 and Kv7.2/7.3 channels. Anesth Analg. 2007;104(5):1256–1264. doi: 10.1213/01.ane.0000260310.63117.a2. (tables of contents) [DOI] [PubMed] [Google Scholar]
- 37.Zheng H., Yan X., Li G., Lin H., Deng S., Zhuang W., et al. Proactive functional classification of all possible missense single-nucleotide variants in KCNQ4. Genome Res. 2022;32(8):1573–1584. doi: 10.1101/gr.276562.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hicks J.K., Swen J.J., Thorn C.F., Sangkuhl K., Kharasch E.D., Ellingrod V.L., et al. Clinical Pharmacogenetics Implementation Consortium guideline for CYP2D6 and CYP2C19 genotypes and dosing of tricyclic antidepressants. Clin Pharmacol Ther. 2013;93(5):402–408. doi: 10.1038/clpt.2013.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Caldwell P.H., Sureshkumar P., Wong W.C. Tricyclic and related drugs for nocturnal enuresis in children. Cochrane Database Syst Rev. 2016;1:CD002117. doi: 10.1002/14651858.CD002117.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang G.K., Russell C., Wang S.Y. State-dependent block of voltage-gated Na+ channels by amitriptyline via the local anesthetic receptor and its implication for neuropathic pain. Pain. 2004;110(1-2):166–174. doi: 10.1016/j.pain.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 41.Glotzbach R.K., Preskorn S.H. Brain concentrations of tricyclic antidepressants: single-dose kinetics and relationship to plasma concentrations in chronically dosed rats. Psychopharmacology (Berl) 1982;78(1):25–27. doi: 10.1007/BF00470582. [DOI] [PubMed] [Google Scholar]
- 42.Borga O., Azarnoff D.L., Sjoqvist F. Species differences in the plasma protein binding of desipramine. J Pharm Pharmacol. 1968;20(7):571. doi: 10.1111/j.2042-7158.1968.tb09809.x. [DOI] [PubMed] [Google Scholar]
- 43.Muller A.R., Brands M., van de Ven P.M., Roes K.C.B., Cornel M.C., van Karnebeek C.D.M., et al. Systematic review of N-of-1 studies in rare genetic neurodevelopmental disorders: the power of 1. Neurology. 2021;96(11):529–540. doi: 10.1212/WNL.0000000000011597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Porcino A.J., Punja S., Chan A.W., Kravitz R., Orkin A., Ravaud P., et al. Protocol for a systematic review of N-of-1 trial protocol guidelines and protocol reporting guidelines. Syst Rev. 2017;6(1):132. doi: 10.1186/s13643-017-0525-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Villatoro-Gomez K., Pacheco-Rojas D.O., Moreno-Galindo E.G., Navarro-Polanco R.A., Tristani-Firouzi M., Gazgalis D., et al. Molecular determinants of Kv7.1/KCNE1 channel inhibition by amitriptyline. Biochem Pharmacol. 2018;152:264–271. doi: 10.1016/j.bcp.2018.03.016. [DOI] [PubMed] [Google Scholar]
- 46.Weinshenker D., Wei A., Salkoff L., Thomas J.H. Block of an ether-a-go-go-like K(+) channel by imipramine rescues egl-2 excitation defects in Caenorhabditis elegans. J Neurosci. 1999;19(22):9831–9840. doi: 10.1523/JNEUROSCI.19-22-09831.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Garcia-Ferreiro R.E., Kerschensteiner D., Major F., Monje F., Stuhmer W., Pardo L.A. Mechanism of block of hEag1 K+ channels by imipramine and astemizole. J Gen Physiol. 2004;124(4):301–317. doi: 10.1085/jgp.200409041. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymized data including data not published in this article will be made available upon request by any qualified investigator.