Abstract
All cells possess an innate ability to respond to a range of mechanical stimuli through their complex internal machinery. This comprises various mechanosensory elements that detect these mechanical cues and diverse cytoskeletal structures that transmit the force to different parts of the cell, where they are transcribed into complex transcriptomic and signaling events that determine their response and fate. In contrast to static (or steady) mechanostimuli primarily involving constant-force loading such as compression, tension, and shear (or forces applied at very low oscillatory frequencies ( Hz) that essentially render their effects quasi-static), dynamic mechanostimuli comprising more complex vibrational forms (e.g., time-dependent, i.e., periodic, forcing) at higher frequencies are less well understood in comparison. We review the mechanotransductive processes associated with such acoustic forcing, typically at ultrasonic frequencies ( kHz), and discuss the various applications that arise from the cellular responses that are generated, particularly for regenerative therapeutics, such as exosome biogenesis, stem cell differentiation, and endothelial barrier modulation. Finally, we offer perspectives on the possible existence of a universal mechanism that is common across all forms of acoustically driven mechanostimuli that underscores the central role of the cell membrane as the key effector, and calcium as the dominant second messenger, in the mechanotransduction process.
I. INTRODUCTION
Tissue, bone, and organs continuously grow and remodel as part of the natural process by which living organisms develop and adapt to their environment and to therapeutic intervention.1 Central to these processes are cellular level responses to external physical, chemical, and biological cues. Although the role of mechanical forces constituting diverse physical cues that cells respond to in organogenesis has long been known,1–3 it has only been of late that the mechanostimulatory effects on physiological and pathological conditions have been increasingly studied, given their therapeutic implications, such as for nanomedicine and regenerative medicine.2,3
The mechanisms by which cells sense these mechanical forces and translate such cues into a cascade of cellular and molecular signaling events that ultimately determine their biological response and fate is, however, extremely complex.2–4 The majority of investigations in this area have primarily employed the application of static forms of mechanical forcing, be it compressional, tensile, or shear forcing,4,5 in which the applied loads were predominantly static, with the exception of a few “dynamic” studies,5 although these cases only involved mechanical forcing at very low repetition frequencies ( Hz) such that the loads are essentially at constant force and the cells' mechanoresponse do not differ significantly from that observed for the steady, static cases.
Cells have nevertheless also been known to respond to vibration in which the applied force is time-dependent and essentially periodic, although this again has mostly been conducted at oscillation frequencies of several Hz—understandably given that this is the characteristic frequency associated with typical human or organism locomotion. More recently, though, it has been shown that more complex dynamic mechanical forcing in the form of acoustically driven vibrational stimuli, both at audible (20 Hz–20 kHz) and, curiously, even ultrasonic (20 kHz–1 GHz) and hypersonic ( GHz), frequencies can also invoke a variety of cellular responses.
Existing reviews on mechanotransduction—the cellular process by which mechanical stimuli to the cell are transducted into biochemical signals6–8—have not just largely focused on static mechanical forcing but have also been primarily confined to their effects, in particular, on sensory cells. While this is because such cells function specifically to detect mechanical cues and are hence ideal models to study these effects,8 they do not capture the myriad of effects observed when other types of cells are mechanostimulated. There also generally appears to be a patchwork of investigations on different types of mechanostimuli with little attempt to understand the similarities or differences between the cellular responses and the underlying mechanotransduction mechanisms between them.
We, therefore, attempt to address this by providing a systematic perspective on the various cellular mechanostimulation strategies employing sound wave vibration that have been examined to date, together with their associated mechanisms, phenomena, and applications (touched on briefly by Rufo et al.9) particularly since this is considerably more complex and relatively less understood compared to their conventional mechanostimulation counterparts involving steady, constant-force compressional, tensile, or shear forcing. We begin in Sec. II by briefly revisiting the principles that underpin mechanotransduction. We then proceed to summarize the different forms of mechanostimuli that have been employed, beginning first with a brief overview of static constant-force mechanical loading in Sec. III, followed by a more extensive treatise on dynamic (periodic) mechanostimulation driven by sound wave vibration over a wide range of frequencies and the signaling pathways they induce in Sec. IV. We subsequently discuss their various applications, particularly for, albeit not limited to, nanomedicine and regenerative medicine (namely, cell-free and cell-based therapies, and cell/tissue reorganization) in Sec. V and show in Sec. VI that it is possible to adopt a unified mechanistic framework centered around membrane-tension-driven second messenger calcium-induced signaling that holistically captures the way different cells perceive various forms of acoustically driven mechanostimuli irrespective of the applied frequency and intensity.
II. MECHANOTRANSDUCTION
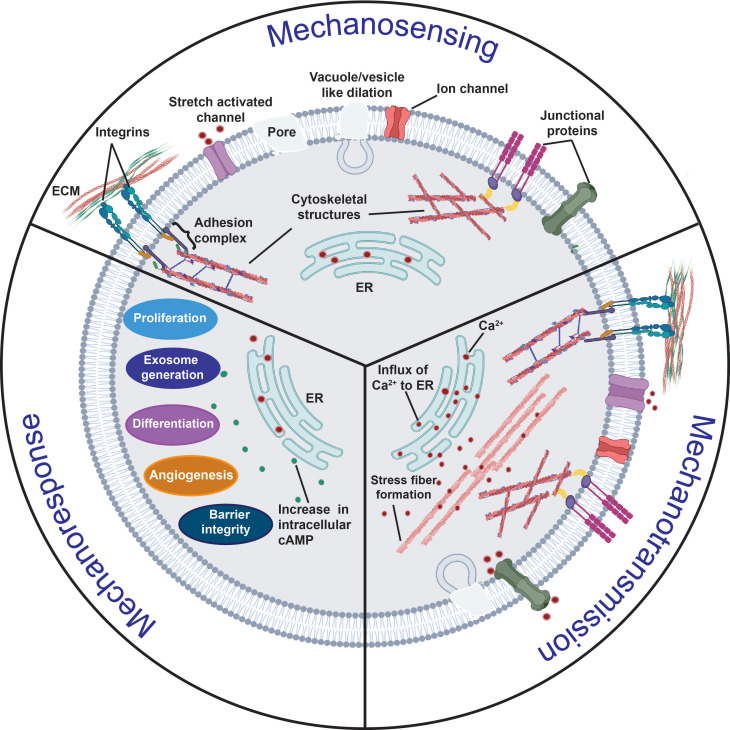
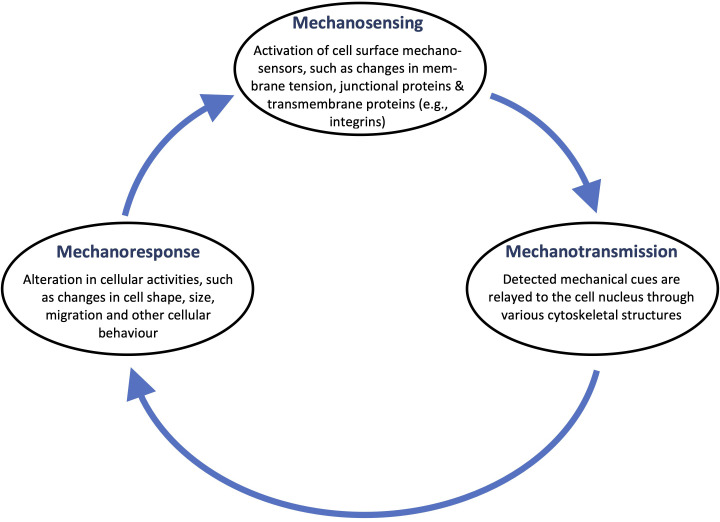
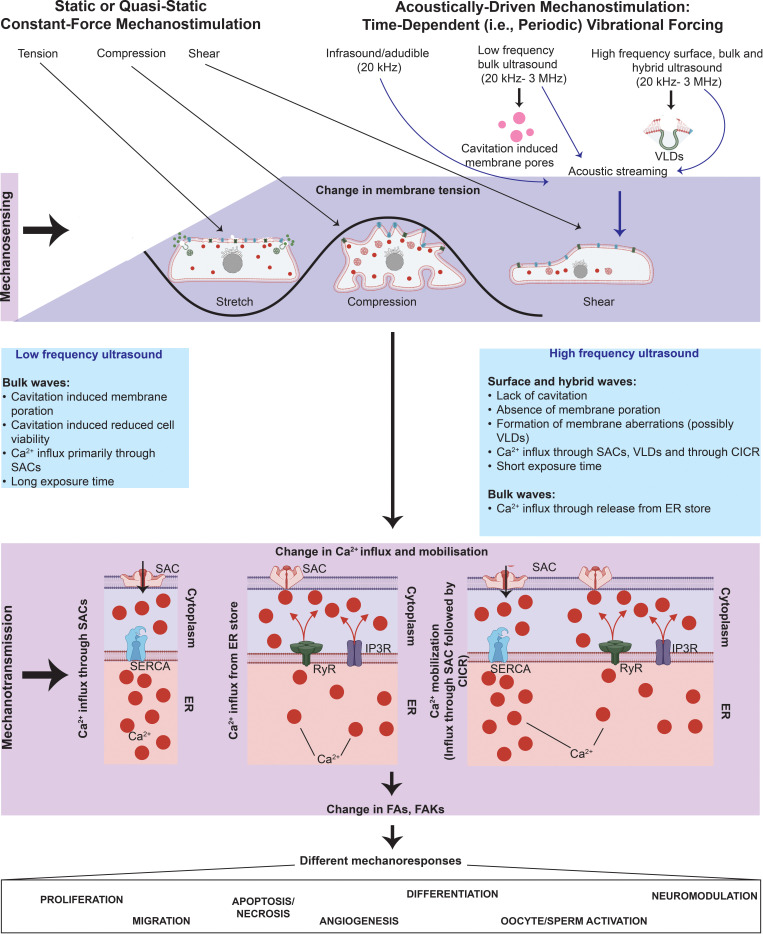
Mechanotransduction—in which cells respond to mechanical stimuli over millisecond timescales10 associated with the time it takes for the cues to propagate to the nucleus11,12 where they are converted into autocrine, paracrine, or endocrine signals, which, in turn, trigger downstream local or global events that maintain homeostasis or direct cellular fate—is a particularly complex phenomenon. At a fundamental level, the mechanotransduction process can be viewed as comprising three distinct stages involving mechanosensing, mechanotransmission, and mechanoresponse steps (Fig. 1). In simpler models, these are considered to be in series, although more complex descriptions allow for dynamic coupling between the stages to account for regulation of the signaling pathways by the applied mechanical load.13
FIG. 1.
Overview of the mechanotransduction process in which a cell recognizes and responds to mechanical cues through three distinct stages. In the mechanosensing stage, the cell detects mechanical cues from the extracellular milieu primarily through local changes or perturbations along its plasma membrane, such as vacuole/vesicle-like dilations (VLDs) or pore formation, that arise as a consequence of the external force on the cell; alternatively, or additionally, other key mechanosensors such as transmembrane proteins (e.g., integrins) or junctional proteins [e.g., adherens junctions (AJs) such as cadherins, or, gap junctions (GJs) such as connexins] can also be involved in the process. In the mechanotransduction stage, the aforementioned conformational changes in the cytoskeletal structures associated with the mechanosensors as a result of the mechanical cues are then relayed to intracellular sensing structures; phosphorylation, ion transport (primarily Ca2+), and the activation of other second messengers, such as cyclic adenosine monophosphate (cAMP), also play a crucial role in the process. These transmitted cues subsequently trigger multiple downstream signaling cascades in the mechanoresponse stage, resulting in the activation of different cellular activities, such as cell migration, proliferation and differentiation, angiogenesis, exosome biogenesis, and barrier integrity maintenance.
A. Mechanosensing
There are two categories of mechanosensors that facilitate cellular perception of a force applied to it through a change in either their molecular assembly or a conformational change in its protein structure: passive (outside–in), wherein the mechanosensing element detects an external force imparted on it and transmits the signal into the cell, or, active (inside–out), in which forces are generated by the mechanosensing element to detect changes in the extracellular matrix (ECM).6,14 Various mechanosensory elements exist in the form of proteins embedded at the cell–ECM interface that are sensitive to membrane stiffness changes [e.g., focal adhesions (FAs), membrane receptor proteins such as G-protein coupled receptors, adherens junctions (AJs), and stress activated channels (SACs)], all of which are involved in either or both passive or active sensing.
FA protein complexes under the cell membrane, for example, play a role in both forms of mechanosensing by constituting the bridge between the ECM and the intracellular domain. This connectivity occurs through the recruitment of transmembrane reporters known as integrins, whose intracellular tails are connected to the actin cytoskeleton by resident adaptor proteins, such as vinculin and talin, and whose extracellular head domains bind to proteins in the ECM6—the latter being regulated by the mechanical stimuli, which induces a conformational change, i.e., unbending of integrins that result in the exposure of the binding sites.15
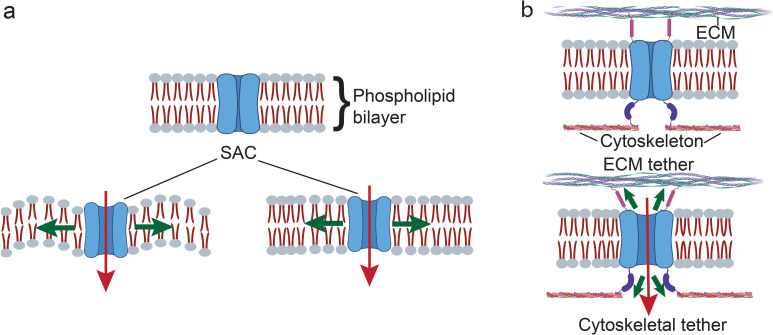
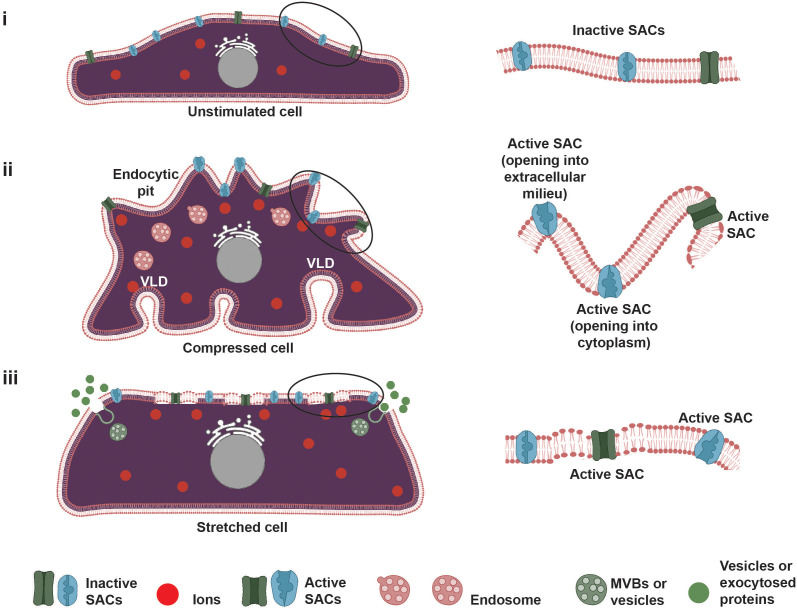
SACs or mechanosensitive ion channels, which facilitate gating of the ion transport in and out of the cell, constitute another dominant type of mechanosensor,16 especially given the role of calcium (Ca2+) as a key second messenger and signaling molecule that is critical to the dynamic triggering of downstream transcriptomic changes;17 intracellular Ca2+ concentrations in particular also play a central role in regulating a wide range of cellular processes.17 Two different models exist to describe the gating mechanism by which SACs operate (Fig. 2). In the stretch model [also known as the force-from-lipid (FFL) model18], tension imposed by any mechanical perturbation to the cell membrane leads to the reorganization of the lipids in the bilayer around the transmembrane SACs as well as a conformal change in its proteins that results in the opening of the channel.19 The tether, or force-from-filament (FFF), model, on the other hand, suggests that the SACs are physically connected directly to the ECM or indirectly through intracellular accessories such as the cytosekeleton.20 These tethers then interact directly with channel or auxiliary proteins, indirectly imposing tension on the channel and allowing for long-range propagation of membrane tension, therefore facilitating long-distance sensing via the cytoskeletal structure.20–22
FIG. 2.
Two accepted models for SAC gating. (a) In the force-from-lipid (FFL) model, the reorganization of the lipid bilayer structure of the cell membrane in response to the applied mechanical loading exerts a force on the SAC to regulate its gating. (b) In the force-from-filament (FFF) model, the SACs are tethered to the extracellular matrix (ECM) or the cytoskeleton, through which the force is exerted.
In eukaryotes, four main types of SACs have been identified: two-pore-domain (K2P) potassium channels, degenerin–epithelial sodium–acid-sensing ion channels (DEG–ENaC–ASIC), transient receptor potential (TRP) channels, and piezo channels.23 The K2P family of ion channels consists of 15 members (KNCK genes), which can be distributed within six subfamilies: TWIK (tandem of pore domains in a weak inward rectifying K+ channel), TREK (TWIK related K+ channels), TASK (TWIK related acid-sensitive K+ channels), TALK (TWIK-related alkaline pH-activated K+ channels), THIK (tandem pore domain halothane inhibited K+ channels), and TRESK (TWIK related spinal cord K+ channel).24 DEG–ENaC–ASIC channels in vertebrates comprise two subgroups: ENaC and ASIC; while ENaC is known to be sensitive to osmotic swelling, shrinking, and shear stress, the mechanosensitivity of ASIC has not as yet been fully explored.25 The TRP channel family, on the other hand, consists of 28 channels that are grouped into 6 subfamilies, namely, canonical TRP (TRPC), vanilloid TRP (TRPV), melastatin-related TRP (TRPM), ankyrin TRP (TRPA), mucolipin TRP (TRPML), and polycystic TRP (TRPP).26,27
The gating mechanisms involved in all of these ion channels, and, in particular, the DEG–ENaC–ASIC channels, are not completely understood.28 However, it is likely that membrane tension plays a key role in controlling the opening and closing of these channels, although we note that the factors initiating the gating through membrane tension varies between each subfamily. For instance, TREK and TRAAK channels were found to be sensitive to shear stress, negative membrane pressure, and temperature,29 whereas TRP channels are known to be insensitive to membrane tension induced by cell membrane stretching.30 The TRPV, TRPM, and TRPA subfamilies, in contrast, are thermally activated, while exocytosis was found to activate the TRPC, TRPV, and TRPM channels.27
Piezo channels—vertebrate mechanically activated nonselective cation channels—are a more recently discovered member of the SACs family,31 whose role has been widely implicated in mechanosensing. Whereas Piezo1 initiates Ca2+ signals predominantly in various non-excitable cells,31 Piezo2 mainly functions in sensory neurons and specialized cell types, such as tactile epithelial cells (e.g., Merkel cells31). Piezo channels can be activated by membrane tension (force-from-lipid), indicating that any physiological force that can cause indentation of the cell membrane on the apical surface-lumen facing side and hence alter the membrane tension, such as that produced with a blunt glass probe or suction to the membrane, can robustly activate the piezo channel.32 It has also been noted that mechanical perturbations imposed onto the basolateral surface of the membrane, for instance, those at the cell–cell and cell–matrix interfaces produced by stretching cells seeded on tensile substrates or on elastomeric pillar arrays, or by compressing the membrane using an atomic force microscopy (AFM) probe, can also activate these channels, thereby alluding to the key role that piezo channels play in sensing of the microenvironment.33
Both localized and whole-cell responses can be mediated through Piezo1 regardless of the endogenous or exogenous origin of the mechanical stimulus.34 As long-range propagation of membrane tension in intact cell membranes is limited, long-distance sensing is enabled through biochemical and functional tethering of the piezo channel to the actin cytoskeleton via the mechanotransduction complex comprising the AJ complex (cadherin and -catenin) and cytoskeletal-associated proteins (vinculin), which allow complementary force-from-filament gating via the cytoskeletal network.34
Upon their activation, piezo channels generate cationic nonselective currents; the dominant cation that arises is however dependent on the cell type.35 For example, piezo channel activation in cells such as sensory neurons leads to membrane depolarization owing to Na+ influx, with Ca2+ playing a minor role,36 whereas Ca2+-induced signaling cascades are elicited in endothelial cells (ECs)37 and mesenchymal stem cells (MSCs).38 As such, piezo channels have been recognized to possess different roles along diverse mechanotransduction pathways depending on the physiological environment that they are exposed to.
B. Mechanotransmission
A hierarchy of load-bearing cytoskeletal structures, such as F-actin, intermediate filaments, and microtubules, are critical for continuous propagation of the force across the entire length of the cell, either from within it, i.e., the nucleus, through the perinuclear space to the ECM, or vice versa. Such transmission occurs over time scales on the order of a tenth of a second10 and is regulated by a “molecular clutch” mechanism in which the size of the FAs along which the signals are transmitted are dependent on the applied force.39 Key interactions implicated in the transmission of force across the cell include myosin-induced actin contraction and molecular bond (e.g., actin–adaptor proteins, adaptor protein–integrin, or integrin–ECM) formation and dissociation;13 the rate of the latter determining the efficiency of transmission.13
C. Mechanoresponse
The final mechanotransduction stage, which involves processes such as calcium signaling, phosphorylation cascades, and DNA transcription factor binding encompasses the ability of the cell to transcribe the detected and transmitted mechanical cues into complex transcriptomic and signaling events. It is quite typical that the entire mechanotransduction process comprises a transcription feedback loop (Fig. 3) in which the cells' response is often transmitted back to the mechanosensitive structures that first produced the response.13,40
FIG. 3.
The closed-feedback-loop nature of the mechanotransduction process. Changes in mechanosensors are relayed to the nucleus, which alter various cellular activities and behavior. These include changes to cell shape and size, as well as the propensity of the cell to migrate, which, in turn, can act as a trigger for further sensing to maintain cellular homeostasis.
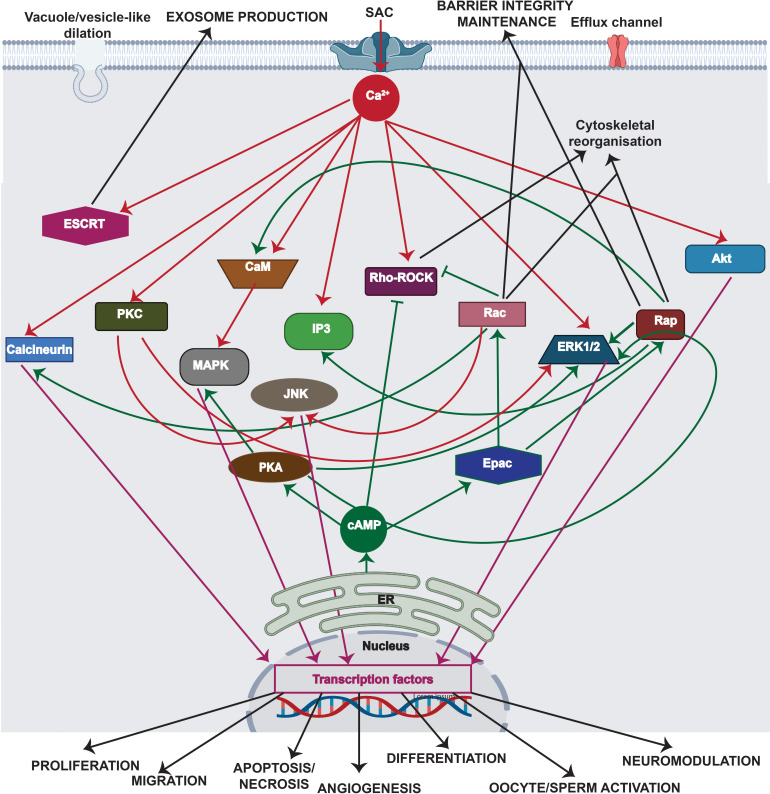
Unlike mechanosensing and mechanotransmission, which occur rapidly on the order of milliseconds, mechanoresponses take place over significantly longer time scales: the activation of signaling pathways, for example, occurs immediately over minutes, whereas the induction of gene expression pathways is typically delayed and occurs over hours or days.41 Depending on the magnitude and duration of the imposed mechanical cue, these delayed responses can lead to transient changes in vesicle trafficking or cellular morphology, permanent compositional or structural alterations in adhesion complexes and cytoskeletal structures, and/or the triggering of secondary signaling cascades that facilitate cell migration or proliferation, cancer progression, angiogenesis, or differentiation.3,42 Key mechanoresponses are depicted in Fig. 4.
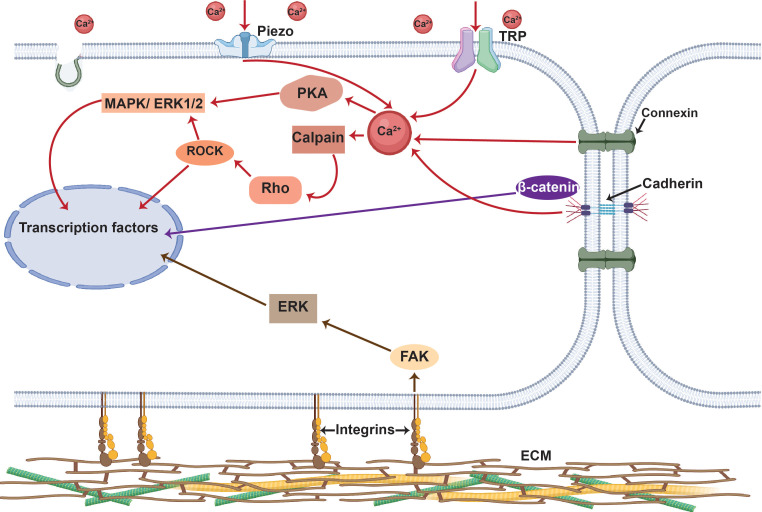
FIG. 4.
Major signaling cascades associated with the mechanotransduction process (for simplicity, only the key pathways involved in mechanotransduction are shown). These can be broadly categorized into Ca2+-facilitated pathways (red lines) and cAMP-assisted pathways (green lines) that either result in transient (e.g., cytoskeletal reorganization, barrier integrity maintenance, or exosome production) or permanent (e.g., cell migration, proliferation, apoptosis, angiogenesis and differentiation, or cellular activation or neuromodulation) changes in cell behavior. Transient changes can be initiated by phosphorylation of substrates associated with the Rho–ROCK proteins and through ESCRT pathways. Permanent changes, on the other hand, can be initiated by signaling cascades, such as the MAPK, JNK, ERK1/2, or Akt pathways, which activate transcription factors, such as c-Fos, c-Jun or CREB, that are triggered by both Ca2+ and cAMP. The normal arrows depict the activation of signaling cascades, whereas the inhibitory arrows ⊣ denote inhibitory effects. (Rho: Ras homologous protein; ROCK: Rho-associated protein kinase; MAPK: mitogen-activated protein kinase; ESCRT: endosomal sorting complexes required for transport; Rap: Ras-related protein; PKC: protein kinase C; CaM: calmodulin; IP3: inositol 1,4,5-trisphosphate; Rac: Ras-related C3 botulinum toxin substrate; ERK: extracellular signal-regulated kinase; Akt: protein kinase B; cAMP: cyclic adenosine monophosphate; Epac: exchange factor directly activated by cAMP; PKA: cAMP-dependent protein kinase; c-Fos: AP-1 transcription factor subunit; c-Jun: transcription factor AP-1; CREB: cAMP responsive element binding protein; JNK: c-Jun N-terminal protein kinases).
III. STATIC OR QUASI-STATIC CONSTANT-FORCE MECHANOSTIMULATION
As alluded to in Sec. II, cells have the ability to sense and respond to both exogeneous as well as endogenous forces. The literature to date has therefore found it convenient to broadly delineate these into extrinsic and intrinsic stimuli—the former constituting external forces physically exerted on the cell, and the latter being the cues “felt” by the cell from its surrounding environment, namely, the ECM. Both are known to regulate cell function. In reality, however, both extrinsic and intrinsic cues are intricately interrelated since mechanotransduction is essentially bidirectional, wherein nuclear mechanoresponses are transmitted back to the mechanosensors and cytoskeletal structures at the cell–ECM interface to give rise to a feedback loop, as discussed above. As such, the cell response to intrinsic stimuli cannot, therefore, be easily or simply decoupled from its extrinsic counterpart. Nevertheless, such a categorization provides a simple and convenient framework to understand the effects of different stimuli on cell fate.
Given our focus in this review on external mechanostimulatory cues, and, in particular, those arising through acoustic forcing, we refer the reader to the excellent articles that provide extensive commentary on intrinsic stimulations.40,43–46 Briefly, intrinsic stimulation in the cell can arise due to changes to its shape and density, ECM, or substrate topology.40 Altering the cell shape and density, both of which are interrelated, has, for example, long been known to affect cell spreading and proliferation: densely seeded cells tend to assume a rounder shape, whereas low seeding densities allow cells to spread and grow.47,48 In stem cells, this was reported to promote osteogenesis over adipogenesis.49 Restricting the cell shape through geometrical confinement or through the introduction of topology, for instance, through micropatterning of the substrate on which the cells are grown, on the other hand, can lead to apoptosis.50,51 Cues arising from changes to the mechanical property of the ECM (e.g., stiffness and viscoelasticity), on the other hand, are detected through the FA adhesion complex that anchors the cell to ECM proteins;52 the accompanying conformation change in the integrin-binding domains (Sec. II A) is relayed to the cytoskeletal complex, triggering a signaling cascade involving the Rho family of small GTPases (RhoA, Rac and Cdc42) that are responsible for cytoskeletal organization. These, along with their downstream effectors, Rho-associated protein kinase (ROCK), myosin light chain (MLC) kinase and yes-associated protein (YAP), or transcriptional coactivator with PDZ binding motifs (TAZ), are known to induce cell migration and cell cycle progression apoptosis, or to direct stem cell differentiation.40
For extrinsic mechanostimulation, the bulk of work carried out to date has primarily involved the application of static or steady forms of mechanical loading under compression, tension, or shear (Fig. 5). Periodic forcing, typically in the form of oscillatory vibrations or sound waves, has only been more recently explored and constitutes the subject of the present review. We nevertheless first provide here an overview of the existing work involving constant-force mechanical loading (Table I) to provide the reader with a contextual basis toward a better understanding of the more complex dynamic nature of their time-dependent (periodic forcing) counterpart to be discussed subsequently.
FIG. 5.
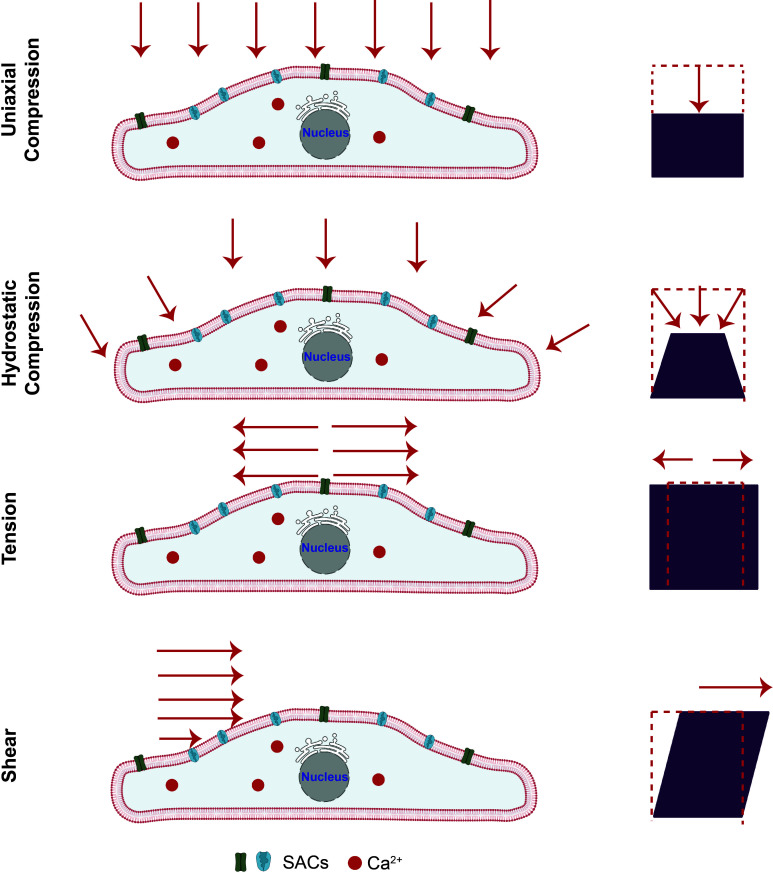
Static forms of mechanostimulation. Schematic depiction of different extrinsic mechanical load, which can induce compression (uniaxial compression or hydrostatic compression), tension or shear stress, on the cells. Adapted from Ref. 40.
TABLE I.
The different forms of mechanostimuli and their key mechanosensing and mechanoresponsive pathways.
| Mechanostimulation | Relevant mechanosensors | Key mechanoresponsive signaling pathways |
|---|---|---|
| Compression | Integrins,53 piezo channels,54,55 TRP channels,54,55 FAs53 | Wnt/ -catenin,56 YAP/TAZ57 |
| Tension | Integrins,58,59 piezo channels,60 TRP channels,61,62 FAs,63–65 and cytoskeletal elements66 | Rho–ROCK,66 MAPK,63 and ERK63 |
| Shear | Integrins,62,67 TRP channels,62,68 FAs,69 G-protein and G-protein receptors,70 tyrosine kinase receptors,71,72 lipid rafts,73,74 primary cilia,75 and GJs76,77 | Rho–ROCK,78,79 MAPK,80 ERK,81 and Wnt/ -catenin82,83 |
| Acoustic stimulation | Piezo channels,84 TRP channels,85 FAs,86 AJs,87 and GJs88 | Rho–ROCK,84 MAPK,89 ERK,90 Wnt,91 ESCRT,92 Epac–Rap,87 YAP,93 and PI3K/Akt94 |
A. Compression
In studies to date, cells have generally been subjected to two different forms of compressive loading: hydrostatic compression/pressure and direct uniaxial compression.95,96 In the former, compression is applied to the media, and hence, the applied pressure on the cells is isotropic, whereas directly compressing the cell-laden scaffold uniaxially in the latter maintains the applied force in a specific direction. Unlike direct compression, hydrostatic compression/pressure can initiate volumetric membrane deformation without shear induced deformation.97 Yet, despite this difference, both forms of compression appear to induce similar mechanosensing and mechanotransmission mechanisms involving integrins, FAs, SACs, and Ca2+-signaling. In particular, compression induces distortion of cell surface receptors and integrins, through which the signal is transferred along the FA complex via the involvement of both vinculin and mature tight junctions.53,98 Additionally, mechanosensing through SACs such as piezo and TRP channels has also been implicated in both forms of compression to drive an influx of Ca2+ in the cell, and, in some cases, activation of actin-related protein (ARP2/3) complexes, which, in turn, mediates actin remodeling within the cell.54,55
Compression-induced downstream signaling is, however, not well understood to date,57 although YAP and TAZ signaling are among suggested downstream cascades induced by compressive mechanostimulation,57 which, in turn, affect the cells' ability to proliferate and undergo apoptosis, although these effects are largely dependent on the cell type.97,99–103 Additionally, compressive loads along with biochemical factors (interleukin-6) have also been shown to promote ECM remodeling while mediating epithelial-to-mesenchymal transition.56 In adipocytes, compression was observed to trigger Wnt/ -catenin signaling that, in turn, induces dedifferentiation of the cells, resulting in a distinct transcriptome profile, long-term self-renewal, and serial clonogenicity (including osteogenesis, adipogenesis, myogenesis, chondrogenesis, and myofibrogenesis).104
B. Tension
Numerous techniques have been developed to mechanically stretch a cell. These differ primarily based on the type of actuation scheme used to generate the tensile load.105 Tensile stretching has been reported to influence cell polarizaion/migration, morphology, proliferation, lineage commitment, and differentiation.106,107 The exact response that is observed, however, depends on both cell type108 as well as the mode by which the stretch is generated, in addition to the properties of the ECM and the presence of soluble factors.109–112 Major stretch-induced mechanotransduction signaling elements include integrins, force-sensitive kinases and proteins, cytoskeletal elements,66 and SACs,60–62 although the exact mechanism by which stretch-induced mechanotransduction occurs has not yet been clearly elucidated due to the complexity of the various processes and their dependence on a number of different parameters.58,59,63–65 In all cases though, the stretched-induced stimuli are relayed into the cells through a change in intracellular Ca2+ signaling. In particular, it was reported that mechanically stretched cells exhibited intracellular Ca2+ oscillations through SAC activation, which is manifested in F-actin assembly, actomyosin contractility, and associated Rho–ROCK signaling;66 the downstream effector of these being the mitogen-activated protein kinase (MAPK) and extracellular signal-regulated kinase (ERK)-induced signaling cascades. Similarly, various stretch-induced signaling cascades have been found to initiate osteoblast differentiation and regulation of morphogenesis, angiogenesis, heart remodeling, and neuritogenesis.113–115
C. Shear
There have been a number of studies on the mechanotransductive effect of shear given its prevalence in physiological conditions where cells in the endothelium are constantly subjected to such stresses arising as a consequence of vasculatory flow. Studies employing flow channels to investigate the response of ECs under flow over a range of exposure durations from seconds to hours have now also been extended toward smaller scales to mimic conditions in the microvasculature with more recent advances in microfluidics.116
At the fundamental conceptual level, shear stress involves the activation of various intracellular and extracellular mechanosensors, such as transmembrane integrins,62,67 ion channels and SACs (in particular, TRP channels),62,68 G-proteins and their receptors,70 tyrosine kinase receptors,71,72 lipid rafts,73,74 and primary cilia,75 among others. More specifically, shear stresses and their gradients have been reported to upregulate v with integrin subunits117–119 and their association with FAs, force–modulated through lipid raft diffusion,69 and with the adaptor protein Shc.118 Shear flow is also known to drive upregulation in GJs, as well as the activation of TRP channels, to allow modulation of cations, particularly Ca2+,76,77 which, in turn, results in the triggering of important downstream signaling pathways. These, depending on cell type, include, although are not limited to, the Rho–ROCK pathway in osteoblasts and osteocytes,78,79 the ERK1/2 MAPK pathway in stem cells80,81 and ECs,120,121 or the Wnt/ -catenin pathway in ECs and fibroblasts,82,83 all of which play a role in modulating multiple cellular activities.122–128
IV. ACOUSTICALLY DRIVEN MECHANOSTIMULATION: TIME-DEPENDENT (PERIODIC) VIBRATIONAL FORCING
Unlike the static forms of steady mechanical loading discussed in Sec. III, acoustic forcing provides an alternative and versatile means for cell stimulation. In addition to the ability of harnessing different forms of sound waves, the periodic nature of the vibrational forcing introduces further possibilities for dynamic stimulation that can be tuned over a broad range of applied frequencies, as will be discussed below.
Sound, in essence, is a mechanical wave generated by a vibrating surface or object. It mainly propagates through an elastic media as longitudinal (pressure; vibration displacement parallel to the direction of wave propagation) waves that involve the compression and rarefaction of the molecules in that medium. As it is possible to support vibrations in other directions in solids, transverse (shear) waves can also exist where the vibration displacement is transverse to the direction of wave propagation.
The sound wavelength is related to the frequency f by the speed at which the sound wave propagates through the medium c, which is dependent on its density and elasticity. Given this relationship and the broad spectrum of sound frequencies—ranging from infrasound ( Hz), audible (20 Hz–20 kHz), ultrasound (20 kHz–1 GHz) to hypersound ( GHz)—together with the different configurations of sound wave generation devices, different wave modes—i.e., the different ways sound waves can propagate through the media—can arise.129,130
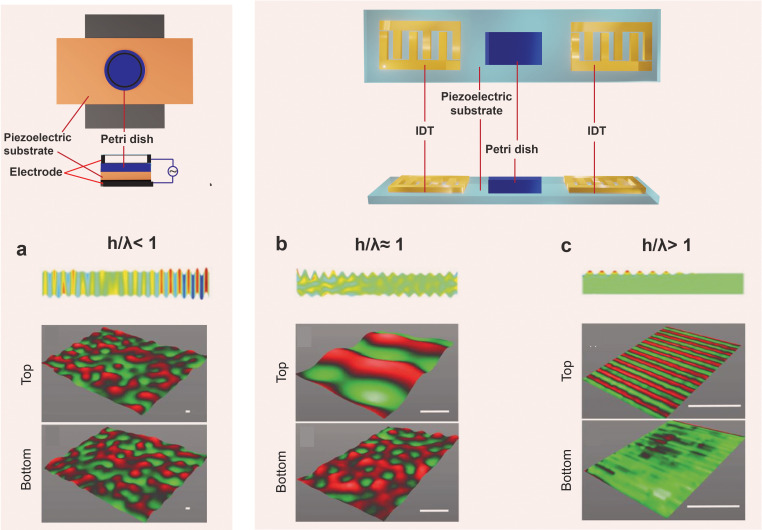
Bulk acoustic waves [BAWs; Fig. 6(a)] do not only take the form of longitudinal (pressure) or transverse (waves). Thin solid sheets with thicknesses (or ), for example, can support plate waves that propagate parallel to its surface and through the thickness of the material. If the plate is infinitely wide, only a thickness mode exists, whereas a plate with finite width gives rise to symmetric (extensional) or asymmetric (flexural) Lamb waves that comprise both thickness and width modes. Surface acoustic waves [SAWs; Fig. 6(c)], which are also known as Rayleigh waves, in contrast, occur in piezoelectric substrates whose thicknesses are much greater than the acoustic wavelength, i.e., or > 1, and supports a combination of both longitudinal and transverse waves. A hybrid surface and bulk wave known as surface reflected bulk waves [SRBWs or pseudo-SAWs; Fig. 6(b)], on the other hand, can also exist in the intermediate transition regime where or 1.131
FIG. 6.
The different sound wave modes and the way in which they are generated on piezoelectric substrates: (a) bulk acoustic waves (BAWs), (b) hybrid surface and bulk acoustic waves [known as surface reflected bulk waves (SRBWs)131], and (c) surface acoustic waves (SAWs). The top row comprises top and side view sketches illustrating how these different wave forms are generated on piezoelectric (often, lithium niobate is used) substrates: (a) BAWs are generated by applying an AC electric field through a conducting layer or strip on the top and bottom surfaces of the piezoelectric substrate, whereas (b) SRBWs and (c) SAWs are typically generated using the same setup in which interdigitated transducer (IDT) electrodes are patterned on the piezoelectric substrate to which the AC electric field is applied; the thickness of the substrate h relative to the wavelength and hence resonant frequency is what determines which wave form is generated: (a) BAWs usually arise when , (b) SRBWs when , and (c) SAWs when .131 The bottom row shows actual laser Doppler vibrometry scans measuring the displacement velocity on the top and bottom surfaces of the piezoelectric substrate for each case. Reproduced in part with permission from Rezk et al., Adv. Mater. 28, 1970 (2016). Copyright 2016 Wiley-VCH GmbH & Co. KGaA.
In practice, the coupling of sound waves to laboratory cell cultureware can be achieved with the use of a transducer. At low frequencies in the infrasound and audible range, longitudinal bulk acoustic waves can be induced in a culture chamber by coupling the piston-like vibration generated with a conventional sound transducer akin to that found in loudspeakers. To generate BAWs at ultrasound frequencies up to several MHz, piezoceramic transducers are typically used, on which electrode pads are patterned [alternatively, higher frequency (MHz) BAWs can also be generated on piezoelectric substrates, as illustrated in Fig. 6(a)132]. The thickness of the transducer or substrate then determines its resonant frequency, and hence, the frequency of the bulk waves that are produced. While it is possible to generate bulk vibration at higher frequencies beyond several MHz, this requires considerably thinner resonant structures or typically thin piezoelectric film resonators with thicknesses d that are equal to half the resonant wavelength (i.e., the resonant frequency , wherein c is the sound speed in the piezoelectric film) mounted atop either an air gap (thin film bulk acoustic resonators, or FBAR) or multiple reflection layers (solid mounted resonators, or SMR).133
For SAWs and SRBWs, a chipscale piezoelectric substrate is employed, on which interdigitated transducers (IDTs)—electrodes patterned in an interleaved pattern—are photolithographically patterned, whose gap and spacing determines the resonant frequency of the device and hence the wavelength and frequency of the SAW or SRBW that propagates along the substrate [Figs. 6(b) and 6(c)]. Whether an SRBW [Fig. 6(b)] or SAW [Fig. 6(c)] is generated depends on this resonant frequency and hence wavelength relative to the substrate thickness.131
A. Bulk waves at infrasound and audible frequencies ( kHz)
There has only been limited work to date on mechanostimulation using bulk waves at infrasound ( Hz) and audible (20 Hz–20 kHz) frequencies. This is likely because of the relative ease by which it is possible to generate other mechanical stimuli at Hz-order frequencies (which are the frequencies typically experienced by cells in their local physiological environment during human motion, e.g., walking and running), for example, using oscillatory versions of mechanical forcing such as the laminar shear or stretching discussed in Sec. III. Similarly, it is relatively simple to excite cells at ultrasonic frequencies at tens to hundreds of kHz given decades of progress in ultrasound technology, borrowing from the extensive advances from imaging and diagnostic and imaging modalities, which will be discussed in Sec. IV B.
Most of the work on acoustic cell stimuli in the infrasound and audible ranges has primarily been limited to cell proliferation,134–138 cell migration,138,139 and even apoptosis.85 Neuronal degeneration has also been reported in astrocytes, neurons, and cardiomyocytes exposed to 16 Hz infrasound,85,140 whereas upregulation in neuronal differentiation was observed with 1 kHz mechanostimulated bone marrow-derived stem cells (BMD-SCs),141 although we note that the latter appeared to be dependent on the inclusion of specific biochemical additives, such as dexamethasone, ascorbate, -glycerophosphate, bone morphogenetic protein (BMP), 3-isobutyl-1-methylxanthine, insulin, transferrin, valproic acid, forskolin, neural supplement and human transforming growth factor 1, among others.142
It should be noted though that even for acoustic stimulation in the same frequency range, the mechanoresponse varies considerably between cell types. NIH/3T3 mouse immortalized fibroblasts and NB2a mouse neuroblastoma cells, for example, were found to be insensitive to audible sound wave excitation over the range 55 Hz–4 kHz, whereas genes such as prostaglandin-endoperoxide synthase 2 (Ptgs2)—an osteoblastic differentiation gene, connective tissue growth factor (CTGF), and ECM protein tenascin-C (TNC) were observed in ST2 (mouse stromal) and C2C12 (mouse immortalized myoblast) cells, indicating that while normal cells may be responsive to acoustic mechanostimulation, cancer cells, and immortalized cells (depending on the tissue source) are resistant to bulk vibrational stimuli, at least in the audible range.143
In any case, the majority of these studies do not explicate the mechanotransductive mechanisms responsible for these responses, although some have noted the involvement of intracellular Ca2+.85,141 More specifically, upregulation in TRPV4 (transient receptor potential cation channel subfamily V member 4) expression has been observed, which led to an increase in intracellular Ca2+ concentration in cells exposed to infrasound at 16 Hz for more than 7 days.85 Increases in intracellular Ca2+ concentration in astrocytes at this frequency have also been attributed to the opening of GJ proteins (connexin-43).136 Such Ca2+-induced signal transduction has been noted to activate the calmodulin and protein kinase C pathways, resulting in the release of glutamate136 or pro-inflammatory cytokines, both of which result in neuronal death.140,144 Similarly, ryanodine receptor (RyR)-induced Ca2+ signaling has also been reported in non-auditory cells such as MSCs when stimulated by audible sound waves (1 kHz),141 leading to the alteration of the cytoskeletal structure that, in turn, initiated a downstream chain of mechanotransductive events,139,143 primarily those related to intracellular Ca2+-induced signaling cascades, such as the ERK pathway.141
B. Low frequency ultrasonic bulk waves (20 kHz–3 MHz)
The main bulk of the studies on vibrationally induced mechanotransduction has been carried out using bulk sound wave excitation at tens to hundreds of kHz. For convenience, we have opted to use the terminology “low frequency ultrasound” to refer to sound wave excitation at frequencies in this range, specifically from 20 kHz to approximately 3 MHz, which is typically the upper limit at which BAWs at ultrasonic frequencies can be generated.145 We have done so to distinguish this from the “high frequency ultrasound” mechanostimulation work carried out above this frequency range from several MHz up to 1 GHz that will be discussed in Sec. IV C given the considerable mechanistic differences that arise between them. Not least, high frequency ultrasonic excitation, in contrast, typically comprises surface or hybrid modes (SAWs or SRBWs), as introduced in the preamble of this section.
In the medical ultrasound imaging literature, low frequency ultrasonic bulk wave excitation has commonly been viewed to interact with biological matter through thermal and non-thermal effects.146 At the cellular level, thermal effects, which arise as a consequence of the dissipation of sound energy as heat upon its absorption in tissue, can be extremely detrimental to cells, which typically denature above 40 °C cells due to hyperthermia.147–150 Stem cells are even more sensitive to thermal effects and suffer from heat shock with temperature rises of merely 4 °C (even within the physiological range).151 Non-lethal thermal effects, on the other hand, include the release of heat shock proteins,152 and altered protein conformation, leading to misfolding or nonspecific aggregation of proteins, which result in cell cycle arrest or impaired growth rates.147 Further, temperature variations can give rise to changes in the cell membrane tension, that, in turn, triggers the activation of ion channels such as TRP (particularly TRPV)27 and K2P channels (TREK and TRAAK),29 which alters the intracellular Ca2+ concentration and potentially instigates various downstream signaling cascades.152 As such, it is extremely important to control the intensity as well as the duration of the mechanostimulation to minimize these adverse effects on cells, which can also include poor reseeding viabilities and colony-forming ability,153 although we acknowledge that a moderate increase in temperature has, in certain circumstances, been known to induce thermal mechanotransductive responses.152
Non-thermal effects, on the other hand, arise as a consequence of (1) the acoustic radiation pressure that is imposed on a cell when the sound waves encounter it along their propagation pathway; (2) the normal and shear stresses experienced by a cell due to the fluid flow that is generated by the sound wave propagation (known as “acoustic streaming”) through the cell media; and (3) the extreme pressures and temperatures that the cell is subject to during cavitation that typically accompanies low frequency ultrasonic excitation.146
The acoustic radiation pressure is a steady, time-averaged normal force that is imparted on a cell as a consequence of its presence in an acoustic field. More specifically, it consists of the transfer of momentum from the sound wave to the cell when the propagating wave encounters it. The magnitude of the acoustic radiation pressure depends on the sound wave intensity, the cell dimension, the wave characteristics (wavelength, sound speed through the medium), and an acoustic contrast factor, which accounts for the relative densities and compressibilities between the medium and the cell. For the most part, cells, being of a dimension much smaller than typical sound wavelengths, at least in the low frequency ultrasound range below 1 MHz, tend to either be forced to translate under the influence of the acoustic radiation pressure in what is known as “acoustophoresis.”154 In a standing wave field, which typically arises due to reflections and hence superpositioning of waves along the confines of the cell chamber, the cells then end up being trapped at the nodes or antinodes of the standing wave.155 At high intensities, though, it is nevertheless possible for cells to fragment under the extreme mechanical stresses exerted on them by the acoustic radiation pressure.156
Acoustic streaming, on the other hand, comprises the flow, or momentum flux, that arises from pressure and velocity fluctuations that are generated in the liquid media due to viscous dissipation of the energy of the sound wave upon its attenuation as it propagates and is absorbed within the liquid. Again, at low ultrasonic frequencies, acoustic streaming mainly results in the translation of the cells along flow streamlines as a consequence of the viscous drag imposed on the cells. Other effects of either or both acoustic radiation pressure and acoustic streaming on cells can typically arise at much higher frequencies when the sound wavelength becomes comparable to the cell dimension, which we discuss in Sec. IV C.
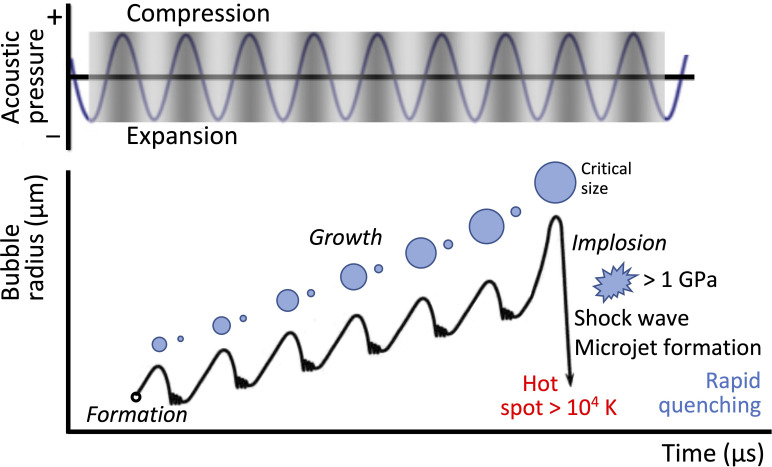
Among these effects, however, cavitation—the rapid growth, oscillation, and implosion of a bubble or an ensemble of bubbles beyond a critical sound wave intensity (Fig. 7)—by far, constitutes the central feature of low frequency ultrasonic mechanostimulation. Not only does the violent event associated with the rapid collapse of the bubble(s) lead to the formation of shock waves157 and high velocity microjets that induce intense local shear within the vicinity,158 extreme pressures ( GPa), and temperatures ( K) are also generated which can be extremely detrimental to cells.147,159,160 Additionally, cells are known to suffer from post-cavitation DNA damage when exposed to the free radicals that are typically produced during cavitation that lead to the production of reactive oxygen species (ROS).161
FIG. 7.
Acoustic cavitation. Schematic illustration depicting the formation, growth, and implosion of cavitation bubbles under low frequency ultrasonic excitation. Rezk et al., Adv. Sci. 8, 2001983 (2021); licensed under a Creative Commons Attribution (CC BY) license.
If moderate cell viabilities can be maintained at lower sound intensities, particularly at the higher end of the low frequency ultrasound range, whatever sufficient deformation experienced by the cell due to any of the aforementioned effects can potentially give rise to various mechanically activated cell signaling pathways.149,150 While the exact mechanism by which such mechanotransduction arises is as yet unclear, the deformation of the cell membrane likely plays a key role in the mechanosensory process. Pore formation in the cell membrane (sonoporation), which is commonly observed in low frequency ultrasonic excitation particularly as a consequence of cavitation,162,163 and which constitutes the dominant mechanism that underpins ultrasound-induced intracellular delivery,164 is expected to alter the membrane curvature, which, in turn, can activate SACs, such as the TRP and piezo channels.165–167 Additionally, transmembrane receptors such as integrins ( and ) in many cells (including fibroblasts, osteoblasts, and chondrocytes)168–170 and FAs169,171 have been reported to alter with low frequency acoustic stimulation.89,172
These effects, and, in some cases, even cavitation-induced ROS production,162,173,174 have been claimed to alter intracellular Ca2+ concentration, leading to cytoskeletal reorganization both with or without FAK activation,175,176 while integrin activation is suggested to trigger the PI3K/Akt (phosphatidylinositol-3-kinase/serine–threonine kinase) pathway.169 In addition, low frequency ultrasonic mechanostimulation has also been reported to regulate the ERK/MAPK,168,170,177 nuclear factor kappa B (NF- B),178,179 Rho–ROCK/ERK,177 and Wnt/ -catenin pathways,91,180 in addition to calcineurin–NFAT (nuclear factor for activated T cells) activation179 and YAP nuclear translocation in MSCs.181 In ECs, regulation of expression of vascular endothelial growth factor (VEGF) and interleukin-8 (IL-8) has been reported.182,183
The translation of these signaling pathways can be observed in various downstream effects and cell fate in response to the low frequency ultrasonic mechanostimulation, such as MSC proliferation and upregulation of many cyclins,180,184 along with improvement in cell migration and an increase in adhesion molecules such as CXCR4, integrin‐1 , and CCR2.172,185 Additionally, upregulation in Nanog expression has also been observed with such stimuli to thereby maintain the stemness of mouse primary osteoblasts and mesenchymal stromal stem cells.186 Depending on the culture conditions (e.g., the presence of biochemicals), low frequency ultrasound stimulation of multipotent stem cells can promote osteogenesis,187 chondrogenesis,188 neurogenesis,189,190 and adipogenesis.191 The commitment to osteogenic lineage in MSCs in the absence of chemical differentiation factors under low frequency ultrasound (stimulated for 28 days) has also been reported.167,192–195 In ECs, low frequency ultrasonic stimulation has been shown to improve proliferation and adhesion,183,196 in addition to stimulating angiogenesis182,183 to promote tube formation in human umbilical vein endothelial cells (HUVECs).197
A drawback, nevertheless, of low-frequency ultrasonic mechanostimulation is the long times (typically days) that are commonly required for the cells to be exposed to the irradiation to evoke more complex cellular responses.90,192 Such prolonged exposure often evokes deleterious effects on the cells, which include a considerable reduction in cell viability as well as a greater chance of contamination.
C. High frequency ultrasonic bulk, surface, and hybrid waves (1 MHz–1 GHz)
High frequency ultrasonic waves have yielded completely distinct and often nonlinear phenomena when coupled into fluids and materials,145 stemming from the smaller sound wavelengths that approach the characteristic system length scale—in this case, that of the cell dimension or the confinement they are present within. Moreover, cavitation, which tends to be the dominant mechanism responsible for a host of phenomena driven by low frequency ultrasonic excitation, such as membrane poration, which we have seen in Sec. IV B to trigger a variety of mechanotransduction cascades, becomes increasingly non-existent at higher frequencies since the threshold sound intensity required to generate cavitation scales as the inverse of frequency.145,198 In addition to therefore involving an alternative mechanosensory mechanism for the excitation of mechanotransductive responses in the cell, the absence of cavitation allows for considerably greater retention of cell viabilities (typically 90% for high frequency ultrasound compared to around 60%–80% for low frequency ultrasound has generally been reported).199
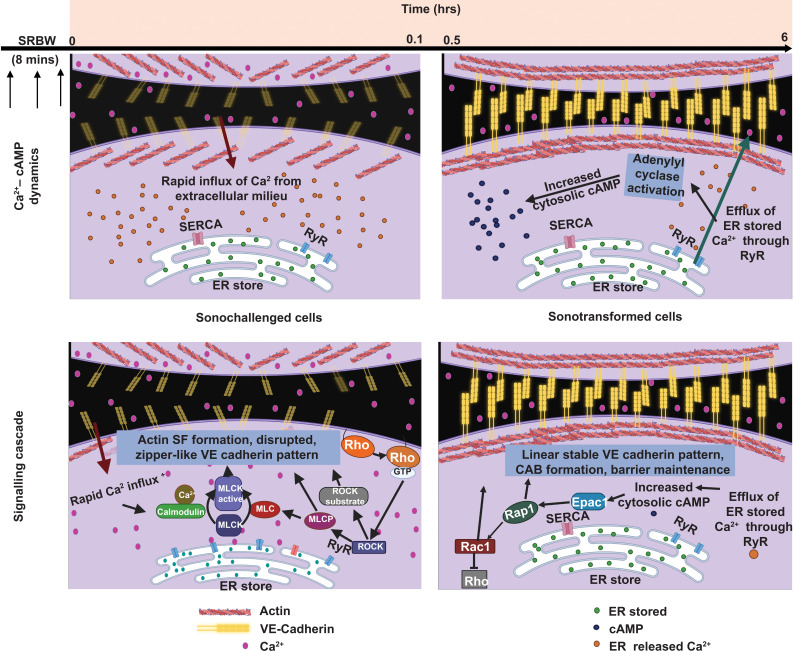
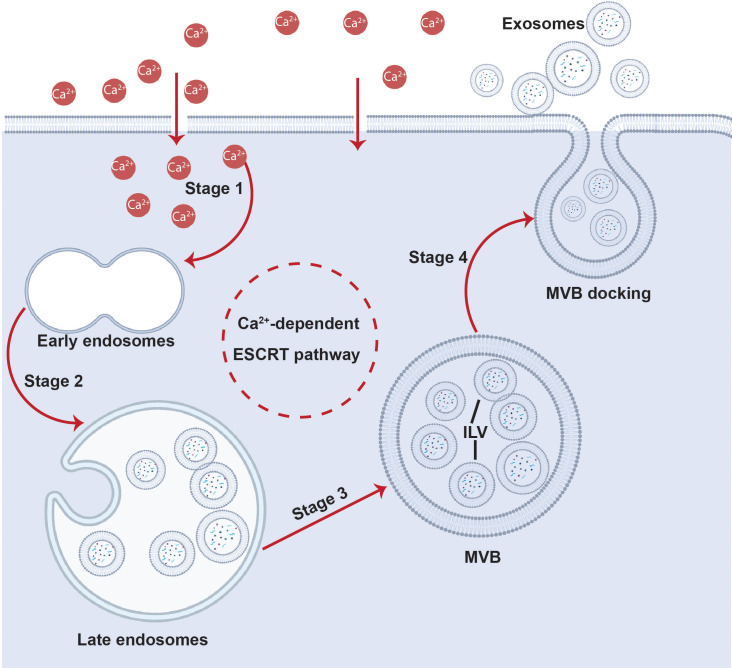
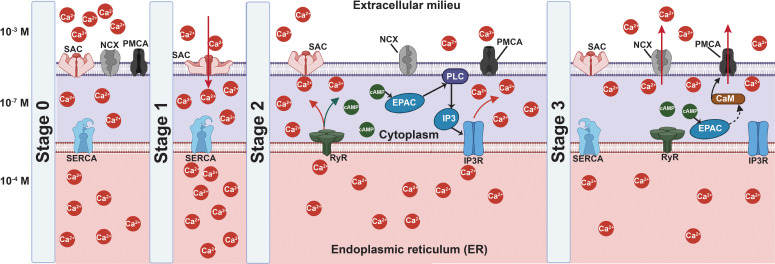
Moreover, only short-duration exposure (typically up to 10 min per day for several days) of 10 MHz order surface (SAW) and hybrid (SRBW) wave excitation, for example, was generally required to evoke similar cell mechanoresponses84,92 that would have otherwise necessitated considerably longer exposure times with low frequency ultrasound (typically continuous excitation over several days). In these instances, perturbation of the cell membrane as a result of the SAW or SRBW excitation was reported to lead to activation of the SACs, and, in particular, the piezo channels,84,200 to drive an influx of extracellular Ca2+ into the cytoplasm. This, in turn, resulted in a transient decrease in the Ca2+ concentration in the immediate vicinity between the intercellular junctions to faciliate rearrangement of the AJ (e.g., cadherin) structure.87 Such cytoskeletal rearrangement is then responsible for triggering a series of downstream signaling cascades. In MSCs, MAPK signaling, for example, was seen to be activated when chemical factors were absent, whereas the Wnt/ -catenin pathway appeared to dominate in the presence of chemical cues.84 Concurrently, the transient increase in the Ca2+ concentration in the cytoplasm promotes storage of intracellular Ca2+ in the endoplasmic reticulum (ER) by activating the sarcoplasmic reticulum Ca2+-ATPase (SERCA) pump,92 which subsequently led to activation of IP3R (inositol 1,4,5-trisphosphate receptor) and RyR to trigger signaling by the secondary second messenger cyclic adenosine monophosphate (cAMP). Downstream effects that transpire then include Rho–ROCK, ERK1/2 MAPK, and BMP2 signaling, as well as activation of the ESCRT (endosomal sorting complexes required for transport) and Epac1 (cAMP-activated guanine nucleotide exchange protein 1)–Rap1 (Ras-related protein 1) pathways to drive a variety of cellular activities depending on the type of cell that was stimulated84,92 (to be discussed in Sec. V).
A few studies have also found similar increases in intracellular Ca2+ in various cells, including HUVECs,201 Chinese hamster ovary (CHO) cells, and both invasive and noninvasive cancer cells,202 under high-frequency bulk waves, albeit with longer exposure durations over several hours. This was attributed to SACs gating, such as that of piezo channels, when both CHO and epithelial human breast cancer (MDA-MB-231) cells were stimulated at approximately 40–50 MHz at intensities of approximately 90 W/cm2.202 The involvement of TRP channels in cancer cells203 or connexin-43 in MSCs88 has also been reported. In the latter, the opening of connexin-43 is accompanied by the release of adenosine triphosphate (ATP) into the extracellular space, which, in turn, results in the activation of phospholipase C followed by Ca2+ release from the ER.88 As with other mechanostimulation studies using different frequencies and types of waves, it is expected that a myriad of downstream signaling cascades can be activated, although most investigations involving high-frequency bulk wave excitation (with the exception of Ref. 88) have focused primarily on demonstrating their effect on cell proliferation (see, for example, Refs. 204 and 205) and therefore its potential utility, for example, for the development of cancer therapeutics.206
V. APPLICATIONS OF ACOUSTICALLY DRIVEN MECHANOSTIMULATION
Given the diverse mechanoresponses of cells to mechanostimulation driven by acoustic excitation in Sec. IV, sound waves, particularly at ultrasonic frequencies, have been employed to modulate a range of cellular activities that include cell growth, adhesion, proliferation, migration, death, and lysis.149,207–209 Additionally, there is a large body of literature on acoustically mediated intracellular delivery, enabled primarily by poration of the membrane by the acoustic forcing (i.e., sonoporation) that arises predominantly through cavitation (see, for example, the reviews by Coussios and Roy,210 Sitta and Howard,164,211 and Rich et al.212) although poration-free acoustic membrane permeabilization methods for intracellular delivery using high frequency (>10 MHz) excitation that are equally as efficient but also facilitate significantly higher retention in cellular viability have also been recently reported.199,213–216
In this section, we discuss a number of exemplary applications where acoustically driven mechanostimulation of different cell types can be deployed. These have primarily been focused on applications in regenerative therapeutics given the considerable work that has been carried out to date on the application of mechanical forces to control in vitro cell behavior for tissue remodeling and regeneration,217–219 although we also dedicate a brief discussion at the end of this section to other applications. As the scope of the present work is limited to acoustic modulation of internal cellular processes, we have excluded applications of ultrasonic technology for the physical manipulation of cells, such as cell trapping and sorting, or rotation (e.g., for spheroid generation220–222), for which we refer the reader to the various reviews available on this subject (see, for example, Refs. 9, 220, 223, and 206).
A. Cell proliferation
Given their multipotency, trophicity, immunosuppressive properties, and capacity for self-renewal, stem cells, and, in particular, MSCs, are attractive candidates for regenerative therapy. Depending on the targeted disease/application, the required stem cell dose can range from to cells, and often involves multiple treatment courses.224 As such, the isolation and processing of these required numbers of cells constitutes the current bottleneck in practical stem cell therapies. Effective techniques for high-throughput in vitro stem cell expansion that can also address challenges associated with donor-related heterogeneity are therefore required.225 As constituents within the expansion media itself can potentially alter the characteristics of the expanded cells,226 efforts have predominantly been focused on developing mechanical intervention methods as an alternative strategy to chemically augmented cell proliferation.
Acoustic stimulation ranging from tens of Hz to approximately 150 MHz, as short as 5 min/day for one day through to continuous treatment over 12 days, has been noted to enhance the proliferation rate in various types of cells, irrespective of species or tissue source (Table II). While the propensity for cells to proliferate typically increases with vibration-induced stimuli, it is difficult to generally conclude how cell proliferation is affected by specific system parameters (frequency, intensity or exposure duration) given that these studies were conducted on widely varying cell types and conditions, often with markedly distinct trends observed. For example, while the proliferation rate of primary ECs was seen to be generally enhanced irrespective of applied frequency or intensity, that of nonmalignant cells tended to increase under low intensities on the order of 10 W/cm2, while malignant cells appeared to be more responsive to high intensity stimulation on the order 100–1000 W/cm2 (Table II). As such, cell type is likely to be a key determinant in the cell proliferation response to acoustic stimulation.
TABLE II.
Summary of studies demonstrating modulation of cell proliferation through acoustic mechanostimuli.
| Cell | Frequency | Intensity | Exposure time | Summary |
|---|---|---|---|---|
| Fibroblasts232 | 45 kHz | 15 and 50 mW/cm2 | 5 min | 30% and 43% increase in DNA content within 18 h of post-exposure incubation, as an indicator of the increase in cell proliferation |
| Osteoblasts232 | 45 kHz | 5 and 30 mW/cm2 | 5 min | 32% and 35% increase in DNA content within 18 h of post-exposure incubation, as an indicator of the increase in cell proliferation |
| Human amnion-derived mesenchymal stem cells (hAD-MSC)94 | 250 kHz | 30 mW/cm2 | 30 min/day for a day | Increase in DNA content, as an indicator of increased cell proliferation |
| Mouse myoblasts (C2C12)228 | 0.5, 1, 3, 5 MHz | 250–1000 mW/cm2 | 24 h | Improved proliferation |
| Bovine aortic endothelial cells229 | 0.5, 1, 3.5, and 5 MHz | 1.2 W/cm2 | 5, 15, and 30 min/day for 3 days | Improved proliferation was detected within 24 h of post-exposure incubation although the proliferation rate was dependent on intensity |
| Human cardiac microvascular endothelial cells (hcMEC)227 | 0.51, 0.994, and 4.36 MHz | 3, 25, 50, and 600 W/cm2 | 10 min (low intensity), 20 min (high intensity) | Enhanced proliferation at all frequencies irrespective of intensity |
| Fibroblasts232 | 1 MHz | 0.7 and 1.0 W/cm2 | 5 min | 47% and 37% increase in DNA content within 18 h of post-exposure incubation, as an indicator of the increase in cell proliferation |
| Osteoblasts232 | 1 MHz | 0.7 and 1.0 W/cm2 | 5 min | 34% and 52% increase in DNA content within 18 h of post-exposure incubation, as an indicator of the increase in cell proliferation |
| Rat Schwann cells234 | 1 MHz | 27.25 W/cm2 | 10 min/day for 5 days | Improved proliferation |
| Rat pheochromocytoma cells (PC-12)235 | 1.48 MHz | 0.1 MPa | 5, 10, 20, and 30 min/day for 3 days | 138%–166% increase in proliferation |
| Human nucleus pulposus cells (HNPSV-1)236 | 1.5 MHz | 7.5, 15, 30, 60, and 120 mW/cm2 | 20 min/day for 5 or 12 days | Improved cell proliferation |
| Human bone marrow derived mesenchymal stem cells (hBMD-MSC)233 | 1.5 MHz | 30, 40, 50, 60, and 80 mW/cm2 | 5–20 min/day for 4 days | Increase in proliferation with 50 and 60 mW/cm2 with at least 5 min incubation per day |
| Mouse myoblasts (C2C12)237 | 1.5 MHz | 30 mW/cm2 | 20 min/day for 8 days | Increased proliferation rate and cell number |
| Primary skin fibroblasts168 | 1.5 MHz | 30 mW/cm2 | 6–11 min/day for 7 days | Improved proliferation with 11 min stimulation |
| Rabbit knee synovial membrane cells (HIG-82)238 | 3 MHz | 30 mW/cm2 | 15 min | No effect on cell proliferation |
| Mouse neuroblastoma cells (Neuro2A), Human colon adenocarcinoma cells (HT29)227 | 0.51, 0.994, and 4.36 MHz | 3, 25, 50, and 600 W/cm2 | 10 min (low intensity), 20 min (high intensity) | Positive response to 4.36 MHz, 600 W/cm2 but decreased proliferation at 50 W/cm2; in both cell lines, the proliferation rate decreased after 120 h |
| Madin–Darby canine kidney epithelial cells (MDCK)227 | 0.51, 0.994, and 4.36 MHz | 3, 25, and 50 W/cm2 | 10 min (low intensity), 20 min (high intensity) | Unresponsive to 4.36 MHz, 50 W/cm2 but improved proliferation rate at 4.36 MHz, 25 W/cm2; highest proliferation rate at 3 W/cm2 |
| Human mesenchymal stem cells (hMSC)184 | 5 MHz | 20 mW/cm2 | 5 min/day for 14 days | Increase in proliferation |
| Human monocytes cell line (U-937)239 | 48.8 MHz | 467 mW | 48 h | 36% increase in cell proliferation |
| Madin–Darby canine kidney cells (MDCK-II), Human osteosarcoma sarcoma osteogenic cells (SaOs-2), human embryonic kidney cells (T-REx-293), Human monocytic tumor cells (U-937)201 | 100 MHz | 80 mW/cm2 | 5 min–27 h | Increase in cell proliferation |
| Human osteosarcoma cells (SaOs-2)240 | 159 MHz | 300 mW/cm2 | 5 h | Increase in proliferation |
Moreover, the exact mechanisms by which cell proliferation is altered by acoustic stimulation are still not well understood. As heat is generated due to viscous absorption of the sound wave as it is transmitted through the media, most studies involving acoustic mechanostimulation incorporate some attempt to control fluctuations in temperature, as these can trigger thermally dependent mechanotransductive processes.227,228 Even then, the various mechanisms associated with non-thermal mechanical effects remain complex. Given the ubiquitous involvement of Ca2+ upregulation and Rho–ROCK activation across all forms of acoustic stimulation, as discussed in Sec. IV, and the corresponding changes to the cytoskeletal structure and FAs,168,229–231 it is probable that Ca2+-induced signaling triggers upregulation of YAP that, in turn, enhances actin nucleation and stability, cytokinesis, and cell cycle progression181 along with improved expression of growth factor receptors and cyclins, such as cyclin D1, E1, A2, and B1.94,184,232 Other possible signals triggered by acoustic stimulation that have also been implicated in mediating cell proliferation include the ERK1/2, Akt serine/threonine kinase,94,233 and glycogen synthase kinase-3 (GSK- )/ -catenin234 pathways. However, we caution that careful consideration of the extent of stimulation applied is necessary given that prolonged exposure is known to inhibit cell growth or to induce apoptosis.233
B. Cell migration
Cell migration is a complex process involving changes to tension in the cell membrane in addition to rearrangement of the cytoskeletal structure241 that is critical to various cellular activities, including homeostasis, morphogenesis, immune cell trafficking, cancer metastasis, and wound healing.242 In stem cells, mobilization (migration and homing of cells) to defective regions is critical for successful regeneration.243 In the bid to improve regenerative therapy, various mechanical loads, including acoustic forcing at frequencies ranging from a few Hz to MHz with intensities between 20 and 250 mW/cm2 (Table III) together with the addition of biochemical stimuli, have been explored to regulate cell migration.242–245 Based on the available data, higher frequencies, particularly that at MHz order, appeared to facilitate more cell migration over lower frequencies. A lone study nevertheless found that 20 kHz acoustic stimulation, in contrast, reduced migration of smooth muscle cells, with the baseline migration only being restored after 24 h of post-exposure incubation.246
TABLE III.
Summary of studies demonstrating modulation of cell migration through acoustic mechanostimuli.
| Cell | Frequency | Intensity | Exposure time | Summary |
|---|---|---|---|---|
| Immortalized mouse olfactory ensheathing cells (OECs)253 | 20, 60, and 80 Hz | 2.173g | “Long durations” (not specified; 6, 12, and 24 h in prior study) | 60 Hz stimulation produced larger spheroids (70-fold), indicating better migration |
| Human lung fibroblasts (LL24), Mouse fibroblasts (L929)139 | 100 Hz–1.6 kHz | 0.2 W | 5 min | Observation over 4 h showed frequency-dependent improvement in cell migration and migration distance; both cells showed increased migration (approximately 10%) at 100 Hz, although this decreased unsteadily with increasing frequency |
| Mouse fibroblasts (L929)247 | 11.2 kHz | 2, 4 V | 24 h | Vibration in the orthogonal direction to the gap of the scratch in the wound healing assay: twofold increase in migration with 2 V vibration but decrease in vibration at 4 V; vibration parallel to the gap: both 2 and 4 V vibration led to reduced migration rate |
| Bovine aortic smooth muscle cell (SMC)246 | 20 kHz | 1.5 W | 15 s | Observations at 0.2, 2, and 24 h post-exposure incubation showed 2.4-fold decrease in migration after sonication immediately following stimulation; this recovered slowly with post-exposure incubation but still remained at levels below that of untreated cells |
| Odontoblast-like cells (MDPC-23)248 | 45 kHz | 25 mW/cm2 | 30 min | Cell proliferation and rate of wound healing significantly reduced in the presence of cell proliferation inhibitor mitomycin C (MMC), although not fully quelled; no effect on cell migration |
| Mouse calvarial derived osteoblasts (MC3T3-E1)249 | 45 kHz (continuous), 1 MHz (pulsed) | 25 mW/cm2 (45 kHz), 250 mW/cm2 (1 MHz) | 30 min | Both frequencies promoted migration in the presence of MMC (which has no significant effect of migration), although MHz stimulation displayed greater increase |
| Human epidermal keratinocyte cells (HaCaT)252 | 0.5 MHz | 0.3 MPa | 1 min | Migration improved by approximately 50% |
| Immortalized human chondrocytes (C-28/I2)254 | 1 MHz | 30 mW/cm2 | 24, 48, and 72 h | Increased migration rate with or without the presence of cytokines (IL-1b) |
| Mouse calvarial derived osteoblasts (MC3T3-E1), Mouse osteosarcoma cells (LM8), Human osteosarcoma cells (SaOs-2), Human renal cancer cells (786-O), Human prostate cancer cells (PC-3), Human lung cancer cells (A549)255 | 1.5 MHz | 30 mW/cm2 | 20 min/day for 3 days | Migration of MC3T3-E1 increased by 8.7% and 9.4% at 6 and 12 h, respectively, whereas the other cells were unaffected |
| Human periodontal ligament stem cells (PDLSC)256 | 1.5 MHz | 30, 60, and 90 mW/cm2 | 30 min | Migration rate improved after 24 h post-exposure incubation |
| Normal human urothelial cells (NHU)257 | 1.5 MHz | 30 mW/cm2 | 20 min/day for 2 days | No effect on migration |
| Rat bone marrow derived stem cells258 | 3 MHz | 20, 30, 40, and 50 mW/cm2 | 20 min/day for 10 days | Increased migration rate, particularly at 50 mW/cm2 |
| Mouse embryonic fibroblasts (NIH-3T3)230 | 14 MHz | 59.3 mW cm2 | 4–8 h | Migration speed increased by 42% after 4 h post-exposure incubation although significant retraction of cells observed at higher intensities |
| Madin–Darby canine kidney cells (MDCK-II)201 | 100 MHz | 80 mW cm2 | 27 h | Increased migration rate by 135% in addition to a significant increase in the rate of cell growth |
| Human osteosarcoma cells (SaOs-2)240 | 159 MHz | 2–4 mW | 48 h | Migration rate increased as a function of intensity with no preference in direction |
In addition to promoting migration, continuous acoustic stimuli have been reported to orientate cells along the direction of the acoustic wave propagation, at least in fibroblasts,201,230,247 although this observation was not always universal. For example, while osteosarcoma exposed to 159 MHz at an intensity of 300 mW/cm2 displayed improved migration, there was no preference for its orientation.240
The fundamental mechanisms that govern such random or directed collective migration under acoustic stimulation are unclear. A complication is that migration effects are typically accompanied by cell proliferation and is often unclear if the improvement in migration is due to the increased proliferation rate or the actual movement of the cell. At least in one study, it was seen that acoustic stimulation at 45 kHz and 1.5 MHz frequencies under intensities between 25 and 250 mW/cm2 led to reductions in the migration rate of odontoblast and osteoblast cells in the presence of mitomycin C (MMC)—a cell proliferation inhibitor, suggesting that the proliferative capacity of the cells under acoustic stimuli dominates their propensity to migrate.248,249
Similarly, little is understood about the mechanotransductive processes that govern the migration of cells under acoustic stimuli. What is known to date is the involvement of the ECM, FA and cytoskeletal structures.250 In particular, acoustic stimuli have been reported to activate FA proteins, vinculin and tubulin, that, in turn, give rise to rearrangement of the cytoskeletal network.246,251 Additionally, both FAK and Ras-related protein 5 (Rab5) have been found to regulate cell migration driven by the acoustic stimulation,86 whereas mechanosensitive molecules such as Ca2+ and ROS (reactive oxygen species) are known to regulate the activation of PI3K/Akt and JNK (c-Jun N-terminal kinase) pathways, in addition to Rac1 (Rac Family Small GTPase 1) signaling,201,251,252 which increase the formation of lamellipodia and membrane ruffling to result in increased cell spreading and the formation of actin filopodia to drive cellular migration.139
C. Angiogenesis
Acoustically induced mechanical loading has been shown to influence angiogenesis183,196,229,259—the in situ formation of new vasculature through a complex process that involves the proliferation, migration, and differentiation of vascular ECs—to thereby facilitate tissue regeneration and wound healing. Exposure of these cells to 0.5–1 MHz acoustic stimulation at intensities between 0.3 and 2.2 W/cm2 for 5–30 min, for example, displayed enhanced propensity for proliferation and migration, that, in turn, promoted sprouting of new capillaries.183,196,229 It has been claimed that the microstreaming from cavitation-induced microbubbles generated as a consequence of acoustic stimulation at low ultrasonic frequencies (1 MHz) also leads to improved cell proliferation and migration, both of which are crucial for angiogenesis, although it is unclear if this was a direct consequence of either the vibrational stimuli on the cells and the membrane deformation and corresponding SAC activation that is induced, or an indirect consequence of the shear stress imposed by the microstreaming.150
In any case, the exact fundamental mechanism by which angiogenesis is triggered by mechanostimuli, in general, is not well understood, although the role of mechanosensitive molecules, such as Ca2+, nitric oxide (NO) integrins, FAKs, and caveolin, have been implicated in the process.260 In particular, acoustic stimulation has been shown to increase intracellular Ca2+ and NO to effect cytoskeletal rearrangement in ECs.261,262 It is then possible that these induce nuclear translocation of YAP and activation of the Akt signaling cascade to result in improved vascular endothelial growth factor (VEGF) expression that accompanies angiogenesis.93,259 However, due care has to be taken when exposing the ECs to these low frequency ultrasonic waves to avoid inducing apoptosis in the cells.
D. Differentiation
Mechanical stimuli involving laminar shear or cyclic stretching/pressure have long been adopted to guide the differentiation fate of stem and progenitor cells, particularly toward osteogenic lineages,126,263,264 although these have typically been administered in the presence of at least one biochemical stimulant. Bulk acoustic stimuli at frequencies ranging from Hz to MHz have also been used to instigate various differentiation fates, particularly osteogenesis, in stem cell or progenitor cells, albeit typically in the presence of biochemical factors as well (Table IV). The focus of the majority of these studies has, nevertheless, been primarily rooted in the demonstration of the mechanostimuli for stem cell differentiation, rather than explicating the fundamental mechanisms by which the acoustic stimulation drives the process. That the mechanostimuli have been applied in the presence of the biochemical differentiation factors, however, has led to the prevalent thought that the observed changes in the protein profile can primarily be attributed to the chemical rather than the acoustic stimuli.265
TABLE IV.
Summary of studies demonstrating acoustically induced osteogenesis in various stem and progenitor cells.
| Cell | Frequency | Intensity/acceleration/amplitude | Culture condition | Exposure time | Summary |
|---|---|---|---|---|---|
| Human bone marrow derived mesenchymal stem cells (hBMD-MSC)292 | 30, 400, and 800 Hz | 0.3g | Osteogenic media | 30 min/day for 14 days | Improved osteogenesis with 800 Hz stimulation |
| Human mesenchymal stem cells (hMSC)193 | 50 Hz–1 kHz | 10 and 20 V | Basal media | 7 days continuous stimulation | Moderate improvement of RUNX2 expression (0.4-fold) with 1 kHz stimulation |
| Rat bone marrow derived mesenchymal stem cells (rBMD-MSC)293 | 60 Hz | 0.3g | Osteogenic media | 1 h/day on days 0, 1, 2, 4, 5, and 6 | Observations at day 14 showed improved osteogenesis |
| Human mesenchymal stem cells (hMSC)195 | 500 Hz–10 kHz | 3.6 and 20g | Basal media | 21 days of continuous stimulation with nanotopography | Synergistic action of nanovibrations and square-shaped nanotopography with viteronectin improved osteogenesis based on the frequency of nanovibration |
| Co-culture (human bone marrow hematopoietic cells, osteoclast precursors, mesenchymal stromal cells, osteoprogenitors, osteoblasts, and osteocytes)266 | 1 kHz | 44.4 nm | Basal media | 7 days continuous stimulation | Both 2D and 3D culture (collagen matrix with collagen 2–5 mg/ml) showed improved osteogenesis and inhibited osteoclastiogenesis in hBMD-MSC/hBMHC |
| Skeltol stem cells selected from bone marrow stroma192 | 1 kHz | 30 and 90 nm | Basal media with collagen hydrogel (2–5 mg/ml) of stiffness 26 Pa | 9 days continuous stimulation | Improved osteogenesis |
| Human bone marrow derived mesenchymal stem cells (hBMD-MSC)167 | 1 kHz | 20 nm, 80 V | Basal media with collagen-based hydrogel scaffolds (2.05 mg/ml) | 21 days continuous stimulation | Improved osteogenesis |
| Rat bone marrow derived mesenchymal stem cells (rBMD-MSC)294 | 0.6 MHz | 30 mW/cm2 | Osteogenic media | 20 min/day for 13 days | Improved osteogenesis |
| Rat bone marrow derived mesenchymal stem cells (rBMD-MSC)295 | 1 MHz | 400 mW/cm2 | Osteogenic media with -tricalcium phosphate and poly(L-lactic acid) scaffold | 20 min/day for 16 days | Improved osteogenesis |
| Human mesenchymal stem cells (hMSC)296 | 1 MHz | 200 mW/cm2 | Osteogenic media with arginin-glycine-aspartate (RGD)-grafted oxidized sodium alginate/N-succinyl chitosan | 10 min/day for 28 days | Along with RGD, mechanostimulation improved osteogenesis |
| Human adipose derived mesenchymal stem cells (hAD-MSC)297 | 1 MHz | 30 mW/cm2 | Osteogenic media under microgravity | 20 min/day for 12 days | Under microgravity, osteogenesis reduced significantly but was restored under mechanostimulation |
| Human alveolar bone-derived mesenchymal stem cells (hABD-MSC)298 | 1 MHz | 50 mW/cm2 | Osteogenic media | 10 min/day for 3 weeks | Improved osteogenesis |
| Human mesenchymal stem cells (hMSC)177 | 1.5 MHz | 30 mW/cm2 | Osteogenic media with magnesium-doped hydroxyapatite/collagen hybrid scaffold | 20 min/day for 7 and 14 days | Improved osteogenesis |
| Human bone marrow derived mesenchymal stem cells (hBMD-MSC)299 | 1.5 MHz | 150 mW/cm2 | Osteogenic media with arginin-glycine-aspartate-serene and nanochrystaline hydroxyapatite | 5 min/day for 3 weeks | Improved osteogenesis with synergistic effect with 3D printed bioactive scaffolds |
| Human mesenchymal stem cells (hMSCs)265 | 1.5 MHz | 30 mW/cm2 | Basal and osteogenic media | 20 min/day for five consecutive days per week, up to 3 weeks | Improved osteogenesis in osteogenic media |
| Mouse preadipocyte cells (3T3-L1), Mouse mesenchymal stem cells [ST2 and 10 T(1/2)], Subclone 4 preosteoblasts (MC3T3-E1), Osteoblasts300 | 1.5 MHz | 30 mW/cm2 | Adipogenic and osteogenic media | 20 min/day for 15 days | Adipogenesis was suppressed in preadipocytes and MSCs upon stimulation; osteogenesis was improved in MSCs |
| Rat bone marrow derived stromal cells301 | 1.5 MHz | 30 mW/cm2 | Basal media with ascorbic acid as osteogenic stimulant | 20 min single treatment | Observation at 0.5, 1, 3, 6, and 12 h post-exposure showed improved osteogenesis |
| Human mandibular fracture haematoma-derived cells (hMHCs) | 1.5 MHz | 30 mW/cm2 | Osteogenic media | 20 min/day for 20 days | Improved osteogenesis |
| Human adipose derived stem cells (hAD-SC)302 | 2 MHz | 20 and 30 mW/cm2 | Osteogenic media | 30 min/day for 21 days | Improved osteogenesis with increase in heat shock protein-70 and heat shock protein-90 expression and BMP signaling pathway activation |
| Neonatal mice osteoblasts303 | 3 MHz | 30 mW/cm2 | Basal media | 15 min/day for 10 days | Improved proliferation and mineralization; enhanced heat shock protein-90 expression |
| Human bone marrow derived mesenchymal stem cells (hBMD-MSC), Human adipose derived stem cells (hAD-SC), Human umbilical cord derived stem cells (hUCD-SC)84 | 10 MHz | 2.5 W | Basal and osteogenic media | 10 min/day for 5 days | Improved osteogenesis in both basal and osteogenic media; early osteogenic commitment with basal media while stimulated cells at ostegenic media showed similar patterns to that for control osteogenic media |
An exception to the aforementioned studies is the work by Dalby and co-workers, who showed the possibility of directing MSCs toward osteogenic lineage using nanoscale-amplitude acoustic stimuli at kHz-order (50 Hz–10 kHz) frequencies without requiring any biochemical factors. In their studies, a modest improvement in the expression of the osteogenic marker RUNX2 (Runt-related transcription factor 2) was reported, particularly with 1 kHz mechanostimulation continuously for 7 days.193 This was ameliorated by co-culturing stem cells with osteoprogenitors, osteoblasts, and osteocytes, and also in collagen-based soft gel (0.26 Pa) scaffolds and on nanotopographically manipulated surfaces.167,192,195,266 The acoustic stimulation, delivered continuously for 27 days, resulted in upregulation of various osteogenic markers, such as RUNX2, bone morphogenic protein (BMP-2), osteocalcin (OCN), osteopontin (OPN), osteonectin (ONN), and osterix.
Of particular note is the finding in the aforementioned studies of little upregulation in osteogenic markers beyond 1 kHz, and hence the assertion that there is little benefit in operating at higher frequencies.195 Contrary to those findings, however, it has recently been shown that 10 MHz mechanostimulation involving SRBWs not just results in significant osteogenic marker upregulation in human MSCs from different tissue sources and donors without requiring chemical/biochemical stimuli, but that it also does so considerably earlier at day 3 following commencement of the stimulation, and with just 10 min of stimulation per day for 5 days.84 Despite such short stimulation periods, the cells are nevertheless observed to retain their long-term osteogenic potential, even after trypsinization and reseeding after 5 days. As such, there are appreciable advantages in using these higher frequencies since the early osteogenic commitment lowers the dedifferentiation risk in the stem cells.267 Moreover, the considerably shorter, non-continuous stimulation not only increases cell viability and proliferability but also offers a simpler and more cost-effective solution for clinical translation. In addition, the early, yet persistent, differentiation of SRBW-stimulated cells facilitates easier and more effective incorporation of committed cells into scaffolds for implantation84,268 or delivery direct to the injury site.269 This is because such early commitment allows the embedded or delivered cells to grow and adapt within the naturally evolving niche in real time, therefore circumventing the cumbersome need for designing and developing multicomponent scaffolds that mimic the natural niche during ex vivo or in vitro differentiation,270–273 in addition to facilitating the adaptation of the cells to different physiological needs for the development of personalized therapeutics.
The exact mechanostimulation-induced signaling cascade involved in osteogenesis is complex due to the involvement of multiple mechanosensors and pathways, and their interconnectivity. With mechanical loading, integrins,59,172,274,275 SACs such as TRP124,167,276 and piezo channels,84,167,277 cadherins, and connexins have been implicated in the process. The integrins trigger mechanotransductive processes that lead to activation of kinase pathways169,278 involving FAs such as paxillin and vinculin, whereas activation of SACs and the opening of connexin hemichannels result in increases in intracellular Ca2+ that initiates the MAPK pathway.279,280 On the other hand, mechanostimulation is also known to dissociate cadherins from auxiliary proteins such as -catenin, whose concentration increase in the intracellular domains initiates the Wnt/ -catenin pathway.281,282
It was suggested that the low frequency (50 Hz–10 kHz) acoustic stimulation, in particular, directed activation of TRPV channels (which, being insensitive to membrane stretch,30 are gated through changes in temperature that affects the membrane phospholipids to drive changes in the membrane tension26,27,283) that, in turn, triggered the Wnt/ -catenin pathway. In addition to such temperature-induced ion channel gating, it is also probable that the osteogenic differentiation in these studies could also be induced through the influence of the surface nanotopography of the substrates employed, or due to the presence of collagen, even though the scaffold used was considerably softer than those known to promote osteogenesis, since both the ECM and patterned substrates are known to participate in osteogenic differentiation through Wnt/ -catenin and MAPK signaling.284–287 A different signaling transduction cascade, however, has been explicated for the SRBW mechanostimulation in the absence of any biochemicals, wherein the transient membrane aberrations that are induced by the excitation are suggested to activate piezo channels that, in turn, lead to Rho–ROCK or calpain activation to trigger bone morphogenetic protein-2 (BMP-2) production.84 Regardless, all of these pathways typically upregulate RUNX2, which commits the cells toward osteogenesis (Fig. 8).288,289
FIG. 8.
Schematic representation of the typical osteogenic pathways triggered by acoustically driven mechanostimulation of stem cells. The stimulation activates SACs, such as piezo and TRP channels, in addition to cell junctional proteins, such as connexins and cadherins, that facilitate influx of Ca2+. The change in the intracellular Ca2+ profile is responsible for initiating various Ca2+-signaling cascades (red arrows), such as Rho–ROCK (possibly through calpain) and PKA (cAMP-dependent protein kinase) signaling. While the former is responsible for regulating nuclear translocation of osteogenic factors such as TAZ and RUNX2, it can also initiate ERK/MAPK signaling such as PKA to instigate transcription factors, such as c-Fos, to induce osteogenesis. Concurrently, mechanostimulated integrins initiate the kinase pathway (brown arrows) through FAKs. The dissociation of -catenin from cadherin, on the other hand, activates the Wnt canonical signaling pathway (purple arrows). ERK: extracellular signal-related kinase; FAK: focal adhesion kinase; MAPK: mitogen-activated protein kinase, Rho: Ras homologous protein; ROCK: Rho-associated protein kinase; TRP: transient receptor potential channel.
In addition to osteogenesis, acoustic stimuli have also been shown to direct stem cells toward other cellular fates, such as neurogenesis, adipogenesis, and chondrogenesis (Table V), although this always necessitated the inclusion of the specific biochemical differentiation factor. Interestingly, the incubation period between consecutive acoustic exposure at 90 Hz on embryonic MSCs was shown to have an inhibitory effect on adipogenesis,290 although another work at the same frequency on BMD-SCs, in the absence of such incubation periods between stimuli but again with the addition of adipogenic differentiation media, resulted in commitment to an adipogenic lineage.191 Most of these studies, however, do not examine the mechanotransduction pathways that are involved in the process, although it has been suggested that the ERK/MAPK pathway is activated in acoustically triggered neurogenesis in BMD-SCs.189 More broadly, it is likely from various studies that MAPK signaling can induce either osteogenesis, neurogenesis, chondrogenesis, or adipogenesis in BMD-SCs or chondrocytes depending on the biochemical factor that is added during the mechanostimulation.141,191,290,291
TABLE V.
Summary of studies demonstrating acoustically induced stem cell differentiation toward non-osteogenic lineages.
| Cell | Frequency | Intensity/acceleration | Culture condition | Exposure time | Summary |
|---|---|---|---|---|---|
| Rat bone marrow derived mesenchymal stem cells (rBMD-MSC)191 | 40 Hz | 0.3g | Adipogenic media | 15 min/day for 14 days | Improved adipogenesis |
| C3H10T1/2 embryonic MSCs290 | 90 Hz | 0.7g | Adipogenic/multipotential media | Varying exposure ranging between 20 min/day to 6 h/day for 7–8 days | Two 20 min exposure with 1 h refractory period between each exposure suppressed adipogenesis; osteogenesis at day 8 improved by increasing refractory period to 3 h |
| Human bone marrow derived mesenchymal stem cells (hBMD-MSC)189 | 0.04 MHz | 50 mW/cm2 | Neuronal differentiation media | 60 min/day for 3 and 7 days | Improved neurogenesis |
| Neural crest stem cells (NCSC) derived from human induced pluripotent stem cells (iPSC)304 | 1 MHz | 500 mW/cm2 | Neuronal differentiation media | 10 min/day up to 4 days | Improved neuronal differentiation |
| Rat neural stem cells (rNSC)305 | 1 MHz | 69.3 mW/cm2 | Neuronal differentiation media | 5 min/day for three consecutive days, 72 h from seeding | Observations at day 7 showed improved differentiation by modulating the Notch signaling pathway |
| Human mesenchymal stem cells (hMSC)187 | 1 MHz | 200 mW/cm2 | Chondrogenic/osteogenic media | 20 min/day for 4 weeks | Improved differentiation to respective lineage based on media used |
| Rat bone marrow derived mesenchymal stem cells (rBMD-MSC)190 | 1 MHz | 10, 30, 50, and 70 mW/cm2 | Neuronal differentiation media | 3 min/day for 3 day | Inhibited gliosis differentiation; did not promote neuronal differentiation |
| Murine embryonic stem cells306 | 1 MHz | 21 and 147 mW/cm2 | Embryoid body media containing cardiogenol | 10 min/day from days 11 to 21 | Increased late cardiac gene expression, indicating cardiomyocyte differentiation |
| C28/I2 human chondrocyte cells291 | 1.0 MHz | 30 mW/cm2 | Basal media | 10 and 20 min | Upregulation in chondrogenic markers up to 1 day after stimulation |
| Human mesenchymal stem cells (hMSC)307 | 1.5 MHz | 100 mW/cm2 | Chondrogenic media with poly-(ethylene glycol) diacrylate scaffold | 3 min for 3 weeks | Improved proliferation and chondrogenesis |
| Human mesenchymal stem cells (hMSC)308 | 1.5 MHz | 30 mW/cm2 | Chondrogenic media with poly-(ethylene glycol) diacrylate scaffold and microbubbles | 3 min/day for 3 weeks | Improved chondrogenesis |
| Human gingival progenitor cells (HGPC)309 | 1.5 MHz | 30 mW/cm2 | Neuronal differentiation media | 10 min/day for 3 days | Improved neurodifferentiation at day 4 |
| Human embryonic stem cells (hESC)310 | 19.69 MHz | 24, 31, and 39 V | Neuronal differentiation media | 10 min/day for 12 days | Improved neurodifferenitation |
| Rat bone marrow derived cells (rBMDC)311 | 3 MHz | 20, 30, 40, and 50 mW/cm2 | Chondrogenic media | 20 min/day for 10 days | Improved chondrogenesis |
| Rat bone marrow derived cells (rBMDC)312 | 3 MHz | 20, 30, 40, and 50 mW/cm2 | Chondrogenic media | 20 min/day for 10 days | Improved chondrogenesis |
| Human chondrocytic cells (HCS)-2/8, Rat primary epiphyseal and articular cartilage cells, Ccn2-deficient chondrocytes313 | 3 MHz | 60 mW/cm2 | Basal media | 20 min | Observations between 30 min and 5 h showed upregulation in chondrocyte differentiation markers |
E. Endothelial barrier modulation
The endothelial barrier, which partitions the intravascular and interstitial tissue compartments, plays a vital role in controlling the passage of solute and nutrient molecules in and out of organs. To enable such transport, its permeability is regulated by interactions between neighboring ECs that facilitate dynamic remodeling of the cytoskeletal structure through the modification of the associated junctional proteins. Given we have seen that cytoskeletal perturbation constitutes a dominant cellular response to various mechanostimuli, it then stands to reason that acoustic stimuli can potentially constitute a key driver of cytoskeletal remodeling and is hence a potent modulator of endothelial barrier function, which is crucial for tissure remodeling.
Indeed, 1 MHz-order bulk acoustic waves, either through direct mechanostimulation of ECs, or through the generation of cavitational microbubbles, have been exploited for enhancing the permeability of the endothelial barrier in both in vitro and in vivo conditions to facilitate transport of therapeutic molecules across the barrier.162,314–316 In either case, the acoustic stimuli, through the modification of the associated junctional proteins, were observed to drive influx of Ca2+ into the cell to induce the formation of actin stress fibers across the cell that, in turn, alters its contractility, leading to a change in the intracellular gap and hence the permeability of the barrier.314–317 We note the effect to be considerably larger in the case of bubble generation given their propensity to porate the cell membrane. In both cases, though, only slight increases in intracellular Ca2+ within the cell were observed, given that higher Ca2+ concentrations ( M) were associated with the inability for cells to recover from the imposed disturbance.318
More recently, 10 MHz SRBW mechanostimulation of endothelial monolayers has also been observed to facilitate modulation of endothelial barrier integrity: up to around fourfold increase in transendothelial electrical resistance (TEER), corresponding to a decrease in paracellular permeability by 5% that was sustained for over 4 h, was observed with just 8 min of SRBW excitation of ECs from different tissue sources (human umbilical vein, human saphenous vein, human coronary artery, and human aorta cells). Moreover, the effect was able to be sustained over longer durations through repeated treatments every 6 h.87
The same increase in intracellular Ca2+ influx into the cell as a result of transient membrane permeabilization and piezo channel activation under SRBW mechanostimulation in Sec. V D for stem cells, was observed to induce a local depreciation of Ca2+ near the junctions between the ECs, causing a reversible invagination of the immature zipper-like vascular endothelial (VE)-cadherin structure that forms the backbone of the AJs anchoring together the actin cytoskeletons of the ECs to form its barrier (termed the “sonochallenge” phase). This leads to the formation of mature linear VE-cadherin structures as a consequence of activation of the Ca2+-induced cAMP-activated guanine nucleotide exchange protein 1 (Epac1)–Ras-related protein 1 (Rap1) pathway (termed the “sonotransformation” phase). This, together with Ca2+ remodeling of the actin cytoskeletal structure, in which actin stress fibers across the cytosol were progressively replaced by circumferential actin bundles lining the cell periphery near its junctions, then resulted in a reduction in barrier permeability.87
The fourfold improvement in TEER with SRBW mechanostimulation is considerably higher than the 1.1–1.2-fold enhancement reported for chemical stimulation, therefore alluding to the SRBW as a potent means for improving endothelial barrier capacity in the development of in vitro models that more faithfully recapitulate in vivo barrier function—a current limitation of endothelial tissue models such as the blood–brain barrier, blood–retinal barrier or pulmonary air–liquid interface, and hence a bottleneck in the development and testing of therapeutic interventions and delivery strategies.87
The mechanotransduction process involved in the SRBW endothelial barrier modulation, illustrated in Fig. 9, clearly shows how acoustic stimulation is able to invoke the activation of multiple signaling cascades. Upon SRBW stimulation, permeabilization of the membrane and SAC activation leads to a rapid influx of Ca2+ into the cell. Such an increase in intracellular Ca2+ results in immediate Rho–ROCK activation given that Rho signaling simply involves phosphorylation/dephosphorylation, which triggers the formation of actin stress fibers in the initial sonochallenge phase. The disruption of the AJ structure into zipper-like patterns that ensues then leads to contraction of the cell and a corresponding increase in the intercellular gap that results in permeabilization of the endothelial barrier. This response is however transient, since the increase in intracellular Ca2+ also activates the SERCA pump to facilitate storage of surplus Ca2+ within the ER to maintain cytoplasmic Ca2+ homeostasis, as described in Sec. IV C. Upon relaxation of the SRBW excitation, the stored Ca2+ is subsequently released from the ER back into the extracellular milieu over time primarily through RyR activation. This is accompanied by activation of the Epac1–Rap1 pathway to result in formation of circumferential actin bundles and stable linear AJs in the sonotransformation phase, consequently resulting in an increase in the integrity of the endothelial barrier.87
FIG. 9.
Calcium–cAMP signaling cascades induced by SRBW mechanostimulation. The immediate response of the SRBW-challenged cells is the sudden influx in intracellular Ca2+ as a consequence of membrane aberrations and piezo channel activation, which reduces Ca2+ levels in the immediate vicinity of the cells and drives transient invagination of VE-cadherin, while inducing Rho–ROCK signaling that causes actin stress fibers to be produced. Consequently, immature zipper-like VE-cadherin conformation ensues in the initial sonochallenge phase immediately following the 8 min SRBW excitation. As the cell subsequently relaxes, the increased intracellular Ca2+ level is ameliorated by their storage in the ER through SERCA, after which Ca2+ is slowly released back into the extracellular milieu through RyR activation. cAMP, formed by RyR activation, then initiates the Epac1–Rap1 pathway which further triggers the formation of circumferential actin bundles and mature VE-cadherin conformation in this subsequent, though simultaneous, sonotransformation phase in which the endothelial barrier integrity is enhanced. Reproduced with permission from Ambattu et al., Biomaterials 292, 121866 (2023). Copyright 2023 Elsevier Ltd.
F. Exosome stimulation
In a manner akin to chemical intervention (e.g., subjecting cells to chemical additives such as calcium ionophores or calcium phosphate) or physical insults (e.g., inducing hypoxia or exposing cells to radiation), all of which serve to increase Ca2+ levels in the cell, the increase in influx of Ca2+ into cancer cells as a consequence of SRBW mechanostimulation due to aberrations generated on the cell membrane and piezo channel activation has been observed to activate various signaling pathways associated with cellular homeostasis. More specifically, the elevation of Ca2+ in the cytoplasmic matrix under the SRBW was observed to trigger sequestration of the accessory protein ALIX [ALG-2 (apoptosis-linked gene 2)-interacting protein X]319 at the site of the membrane aberrations to initiate exosome biogenesis,92 in which the membrane is remodeled to facilitate protein cargo sorting, plasma and nuclear envelope membrane repair, and the formation of multivesicular bodies (MVBs). In particular, membrane invagination resulted in the generation of intraluminal vesicles within these MVBs, which upon fusion with the plasma membrane, are released into the ECM as exosomes.320,321
As such, the SRBW-facilitated stimulation of exosome production can be viewed from the perspective of the cells' attempt to maintain homeostasis by repairing the transient membrane aberrations induced by the mechanical stimulation. In this process, the cell membrane acts as the mechanosensor with the intracellular Ca2+ as the mechanotransmission element and the ESCRT pathway as the mechanoresponse (Fig. 10). As with the endothelial and stem cell treatments in Secs. V D–V F, we note again that only short exposure to the SRBW (8 min) is sufficient to drive longer term effects of exosome release within a half hour post-exposure period. Given similarly high post-exposure cell viabilities (90+%), it is then possible to envisage cycling the SRBW irradiation to achieve tenfold enrichment in the exosome concentration to a homogeneous yield of around 1.7–2.1 fold/h, thereby constituting a facile but effective method for circumventing the perennial limitation of insufficient homogeneous exosome yield that constitutes the bottleneck in exosome diagnostics and therapeutics.92
FIG. 10.
Proposed mechanism for SRBW-facilitated exosome production. The Ca2+ influx through membrane aberrations or piezo channel activation (stage 1) promotes the recruitment of ALIX—an accessory protein that makes up part of the ESCRT machinery—to the site along the membrane where the perturbation is introduced. The resultant ESCRT activation leads to the formation of early endosomes (stage 2) and their maturation into late endosomes (stage 3). These then transform into MVBs that subsequently dock onto the cell membrane (stage 4) to release the intraluminal vesicles (ILVs) within them as exosomes, all the while during which the aberrations in the cell membrane are being healed.92
G. Applications beyond regenerative therapeutics
While the majority of applications involving acoustic mechanostimulation have predominantly been confined to those within the regenerative therapeutics space, there are also a number of other applications in which the utility of such stimulation has proven beneficial for the manipulation of a diverse range of cells, including immune cells [e.g., natural killer (NK) cells], neurons, oocytes, embryos, and sperm.322–327 Acoustically stimulating oocytes, for example, at 20 Hz and between 1 and 3 MHz with intensities of approximately 2 W/cm2, have been observed to maintain higher numbers of cells in the blastocyst (suggesting that the stimulation prevented cellular damage during oocyte activation), in addition to facilitating nuclear activation, which is dependent on the extracellular Ca2+ concentration,328,329 noting that 0.1–0.5 mM is optimal for oocyte activation.323 In fact, oocyte activation deficiency is the primary reason for total fertilization failure (TFF), with abnormal Ca2+ signaling being a key characteristic of the condition.330 Interestingly, similar increases in the rate at which embryos develop as a consequence of their activation have been observed when fused embryos are exposed to 1 MHz mechanostimulation at an intensity of 2 W/cm2 for just 30 s per day.322
Short duration (seconds) exposure of sperms to 19.28, 48.5, and 100 MHz SAWs with intensities ranging between 250 mW and 2 W, on the other hand, has been shown to influence their motility without compromising their structure or function.325 The influence of the acoustic stimulation on sperm motility was suggested to arise through the ability of the acoustic stimulation to mediate transport of Na+, H+, Ca2+, and/or HCO3− into the cells by activating various ion channels, although the authors were compelled to attribute a lesser role to Ca2+ transport given their observations that sperm motility remained elevated even under Ca2+-free conditions. We note from their results though that the motility did appear to decrease from 34% to 19% in the absence of Ca2+, therefore implying that Ca2+ transport still has an effect on sperm motility, which would be consistent with prior studies reporting Ca2+ regulation of intracellular ATP and cAMP, both of which play a central role in sperm motility.331,332 While a large influx of Ca2+ is known to produce hypermotile sperm,325 we note Ca2+ influx to be a key driver in the activation of the cAMP/PKA pathway that regulates sperm motility.325,331 Given that it is the ratio of intracellular to extracellular Ca2+ that regulates the energy (ATP) required for sperms to swim,333 it is therefore likely that the magnitude of Ca2+ influx constitutes a key parameter in regulating sperm motility.
Acoustic stimulation has also been shown to offer a nondestructive, reversible, and adjustable means of manipulating neurons,334 giving rise to an entire field of sonogenetic neuromodulation for the regulation of the central, peripheral, or autonomic nervous system.207,324,326,335–338 Sciatic nerves and cortical neurons, excited by 100–800 MPa acoustic pressure at 0.8 and 27 MHz, for example, can be activated by acoustic stimulation,339,340 leading to an increase in their conduction velocity and action potential, thereby altering their excitability. Similar effects were noted in acoustically stimulated sciatic nerves at 2–7 MHz and 100–800 W/cm2.341 Meanwhile, a reduction in the action potential of sciatic nerves due to thermal effects was reported with acoustic stimulation at 0.661 and 1.986 MHz with an intensity of 44 W/cm2.342
Increased neural activity of cells in the superficial layer of salamander retina with 43 MHz bulk ultrasound has, in addition, been reported, although the effect failed to be reproduced in retinal ganglion cells.343 Although the precise mechanism by which neuron excitability is modulated through acoustic stimulation was not explicated in this study, it is possible that their observations can be attributed to piezo channel activation, which was reported in HEK293 cells exposed to bulk ultrasound excitation at similar frequencies,202 and since SACs such as piezo channels are endogenously expressed in neurons.31 Moreover, the excitation of mouse neurons under 500 kHz bulk mechanostimulation at intensities of 0.1–0.5 MPa for 20 min has been reported, wherein Ca2+ influx upon activation of the piezo channel as a result of the stimuli was seen to promote upregulation in calcium/calmodulin-dependent protein kinase II (CaMKII), cAMP Response Element-Binding protein (CREB) and c-Fos,166 the latter having been implicated in the induction of neuron excitability.341,344
Recently, it was as well noted that exposing natural killer (NK-92) cells to 96.7 MHz SAWs at an applied voltage of 3.6 Vpp for 30 min with a 5% duty cycle led to an increase in intracellular Ca2+. While the increase, in itself, was insufficient to activate the NK cells alone, which is known to occur through calcium signaling, it was shown that the SAW mechanostimulation led to an approximate 1.5-fold enhancement in the expression of lysosomal-associated membrane protein 1 (LAMP-1)—a marker of NK cell degranulation and hence activation—when applied in conjunction with the addition of phorbol 12-myristate 13-acetate (PMA) that is commonly employed to trigger protein kinase C (PKC) signaling for initiating T-cell activation.327
VI. UNIVERSAL MECHANOTRANSDUCTION MECHANISM
Despite (1) the myriad of mechanosensors that can be activated, (2) the multiple signaling pathways that arise, and (3) the variety of downstream mechanoresponses observed—all as a consequence of the diverse forms of the application of acoustically driven mechanostimulation modes on different cells across a range of frequencies that have been discussed in Sec. IV F, we make the case here for the possibility of a mechanotransduction mechanism that is universal across all such acoustically driven mechanostimulation, at least for both low and high ultrasonic frequencies.
A. The cell membrane as a universal transducer/effector
The cell membrane, which comprises an asymmetric bilayer containing glycerophospholipids, sphingolipids, cholesterol, and carbohydrates, in addition to high amounts of transmembrane or peripheral proteins,345,346 compartmentalizes the cell from its surrounding matrix. As the interface between the microenvironment and the cellular metabolism, it therefore plays an important role in the transduction of mechanical cues into the cell. Specifically, the cell membrane couples directly with effector molecules. These include transmembrane proteins, such as SACs, AJ proteins, GJ proteins,347–349 and, mechanosensitive proteins, such as FA proteins.350 Any perturbation imposed on the fluidic cell membrane is therefore expected to alter these transmembrane proteins and associated cytoskeletal structures.
These mechanosensory structures on the cell membrane constantly interact with its surrounding microenvironment, allowing the cell to adapt to mechanical stimulation. This is facilitated by tension within the membrane that is generated as a response to the application of an external force on the membrane surface. Membrane tension develops within lipid bilayers and constitutes the basis that defines the structure of all biological membranes. Unlike in pure lipid vesicles, the membrane tension of a cell can vary locally owing to peripheral protein binding, the presence of transmembrane proteins, and interactions with the underlying actomyosin cortex.351,352 These interactions provide additional resistance to changes in the membrane area and can constrain such changes to a localized area of the cell. Moreover, they also facilitate mechanotransduction and therefore have the ability to modulate various cellular processes such as endocytosis, exocytosis, cell migration, cytokinesis, mitosis, and intracellular trafficking. For example, changes in membrane tension have been suggested to induce ligand-independent integrin signaling that arises during cell migration.353
When a cell is mechanically stimulated in a way that changes its membrane tension and shape, the primary response of the cell is to counteract the effect and attempt to restore the membrane tension in order for it to maintain homeostasis, particularly to avoid apoptosis. The actual response depends on the nature and level of the mechanical stimulation since different stimuli alter the membrane tension and shape differently. Mechanical stimuli that constricts the cell leads to membrane compression [Fig. 11(ii)], which reduces the cell size by forming membrane folds of different shapes and sizes with lipid packaging defects in highly curved areas and phosphoinositide clusters.354–356 Such changes in membrane curvature are often accompanied by an increase in endocytosis owing to a decrease in membrane tension. To counteract the reduction in membrane tension, vacuole/vesicle-like dilations (VLDs) can also be formed. The formation of these endocytic pits and/or VLDs, in turn, can activate SACs.54,357
FIG. 11.
Response of the cell membrane, originally at rest under homeostatic conditions in which the SACs are (i) inactive, to subsequent mechanical stimuli involving (ii) compression or (iii) stretch; the illustrations in the right column are a magnification of the lipid bilayer structure along a representative portion of the cell membrane as indicated by the circle in the illustrations in the left column. Under (ii) compression, the cell membrane folds to form VLDs and endocytic pits to counteract the decrease in membrane tension, leading to the activation of SACs through the FFL gating mechanism illustrated in Fig. 2. Depending on the location of the SAC with respect to the deformation region, it may either open into the ECM to allow ions to bind to the SAC, or into the cytoplasm to release ions into the cell. In contrast, the membrane aberrations and pores that form in cells under (iiii) stretch or tension activate the SACs directly due to the increase in membrane tension, again through the FFL mechanism, leading to an increased propensity for the fusion of multivesicular bodies (MVB) to ease the membrane tension, and resulting in exocytosis and exosome release.
In contrast, any form of mechanical stimuli which increases the cell area (e.g., stretching the cell) causes both direct activation of SACs through the force-from-lipid model, as well as the flattening of membrane folds, to accommodate the change in tension [Fig. 11(iii)]. The corresponding increase in membrane area involves the use of lipid reserves that, in turn, increases the membrane tension and order, in addition to increasing the propensity for vesicles to fuse to the membrane to increase its area. Both effects result in transient membrane aberrations or perturbations, which also lead to the activation of SACs through the force-from-filament model along with actomyosin contraction.34,357–359
In short, all forms of mechanical stimulation—be it static stretch (as a result of tension or shear), compression, or a periodic combination of both effects to constitute more complex dynamical forms of mechanical stimuli such as those due to cavitational-driven poration at low ultrasonic frequencies, or membrane oscillation under SAWs or SRBWs that possess both in-plane and out-of-plane vibrational displacement—lead to some form of disruption to the cell membrane, whether it be to reduce or increase its tension. 84,87,92,199,360,361 [While some studies have proposed that mechanical stimuli may induce intra-membrane cavitation, which, in turn, can modulate transmembrane proteins (SACs) and the cytoskeletal structure, they do not explicitly rule out the involvement of the membrane itself since the periodic expansion and contraction of the intra-membrane spaces as a result of such cavitation can itself also lead to stresses on the membrane (Refs. 362 and 363).] Whichever the case, the resultant change in membrane tension drives activation of the SACs, leading to an increase in intracellular Ca2+ and triggers downstream effects associated with various Ca2+-induced signaling cascades that constitute the cell's mechanoresponse. We note that such mechanosensitivity also applies conversely when the membrane correspondingly relaxes upon cessation of the mechanostimulation.
B. Ca2+ as the key mechanotransduction element
1. Ca2+ chemistry
For it to invoke a wide range of biological effects, a metal ion must interact with biological ligands such as proteins, lipids, and carbohydrates by binding to commonly available reaction centers in these biomolecules. Unlike Mg2+ and Zn2+, which bind with greater affinity to nitrogen ligands, Ca2+ has the most affinity for carboxylate oxygen, and, as such, is able bind to these groups in aspartic and glutamic acid, and to the oxygen atoms in Asn (asparagine), Gln (glutamine), Ser (serine), Thr (threonine), and Tyr (tyrosine).364
Another relevant parameter that is critical for an efficient second messenger is its binding kinetics. Ca2+ binds to and dissociates from a protein a hundred or so times faster than Mg2+.365 Moreover, its high coordination number (6–8) and often irregular (i.e., protein-induced) coordination geometry allow it to be accommodated into a larger number of proteins.364 Ca2+-mediated cross-linking is moreover reversible and thus responsive to a change of conditions,366 thus allowing cells to easily regulate their concentrations of free and sequestered Ca2+ both in time and space by utilizing their calcium stores and calcium-specific ion channels.367 Furthermore, Ca2+ can either operate within small cellular compartments or act on the entire cytoplasm, with signaling that can last from microseconds to hours, occurring either transiently or in a pulsatile manner.
2. Ca2+ mobilization
The underlying principle of Ca2+ signaling is the cellular response to a change in its internal Ca2+ concentration. In general, the stimulation of a cell leads to an acute increase in its internal Ca2+ concentration above the basal level. Upon removal of the stimulus, this concentration returns back to the resting state. The intensity and duration of the stimulus determines the extent of the calcium dynamics. Increases in internal Ca2+ concentration can either originate from Ca2+ release from intracellular stores and/or Ca2+ influx from the extracellular space, assisted by ion channels or transporters on the cell membrane or the ER within the cell.
As large and sustained elevations in internal Ca2+ concentration can lead to a variety of deleterious cellular effects, the cell naturally attempts to remove extra Ca2+ from the cytoplasm in an effort to return the Ca2+ concentration back to its basal level to maintain homeostasis.368 This is regulated by the simultaneous interplay of multiple counteracting processes known as Ca2+ “on” and “off” mechanisms. During the “on” mechanism, ion channels located on the cell membrane regulate the supply of Ca2+ from the ECM, and ion channels on the ER from the intracellular storage space. In the “off” mechanism, cells remove Ca2+ from the cytoplasm either by storing extra Ca2+ in its intracellular stores such as the ER or by effluxing Ca2+ back into the extracellular milieu through Ca2+ATPases on the cell membrane and the ER through activation of its ion channel receptors, such as inositol 1,4,5-trisphosphate (IP3R) and ryanodine (RyR), along with ion exchangers that utilize gradients of other ions to provide the energy to transport Ca2+ out of the cell.17
At highly elevated Ca2+ levels, quick restoration of the cytosolic Ca2+ concentration to its basal level occurs through the movement of intracellular Ca2+ via the SERCA pump that mediates its redistribution within the ER (Fig. 12).369 As such, the influx of Ca2+ from the extracellular milieu can initiate Ca2+-induced Ca2+ release (CICR) and RyR activation when the stores' Ca2+ content exceeds normal physiological values (i.e., “store overload”),370 releasing Ca2+ primarily into the cytosol where it may be released back into the extracellular milieu.369 The RyR-mediated Ca2+ release, in turn, activates IP3R-mediated Ca2+ release from the ER.371 Such Ca2+ release from the ER could then enhance the Ca2+ efflux activity of sodium/calcium exchangers (NCX) and plasma membrane calcium ATPase (PMCA) pumps.372 This process by which a cell maintains its internal Ca2+ concentration spatiotemporally through various receptors and ion channels is known as Ca2+ mobilization.
FIG. 12.
Calcium mobilization stages and the various signaling cascades they induce. Stage 0: The cytoplasmic Ca2+ level is maintained at relatively low levels ( M) in resting cells by influx and efflux through plasma membrane Ca2+ ATPase (PMCA) pumps and Na+/Ca2+ exchangers (NCX) along the cell membrane and via smooth endoplasmic reticular Ca2+ ATPase (SERCA) transporters along the ER membrane. Stage 1: With a change in membrane tension, SACs are activated, leading to an influx of Ca2+ into the cytoplasm that increases the cytoplasmic Ca2+ levels. This is rapidly restored by activation of the SERCA pumps that transport the Ca2+ ions into internal stores, mainly the ER. Stage 2: The increase in intracellular Ca2+ is followed by slow release of Ca2+ back into cytoplasm through calcium-induced calcium release (CICR) primarily from the ER via Ca2+-sensitive ryanodine receptors (RyR), accompanied by an increase in intracellular cAMP that activates the Epac signaling cascade. This, in turn, triggers the formation of the phospholipase C (PLC) pathway to activate the IP3 receptor (IP3R) that stimulates further release of Ca2+ from the ER store. Stage 3: The Ca2+ that is released from the ER is slowly effluxed back into the ECM through NCX. Meanwhile, Epac activates Ca2+/calmodulin (CaM) to trigger Ca2+ efflux via PMCA back into the ECM.
Such a unique ability of the cellular machinery to spatiotemporally modulate intracellular Ca2+ concentration renders Ca2+ an ideal messenger for mechanotransduction. The versatility of Ca2+ signaling, which is evidenced from the fact that Ca2+ controls almost every aspect of cellular activity from its infancy to death, and its diversity in directing different activities depending on the cell type stemming from non-transcriptional events (phosphorylation/dephosphorylation events to activate proteins) or transcriptional effects,373 arises from its potential to interact with a wide range of molecules, including cAMP, nitric oxide, phosphatidylinositol-3-OH kinase, and MAPK. As seen in Fig. 4, these interactions trigger a multitude of signaling cascades, such as the phosphatidyl inositol (PI), phospholipase C (PLC), inositol triphosphate (IP3), protein kinase C (PKC), and calmodulin (CaM) or calmodulin kinase II pathways, and can crosstalk with G protein-coupled receptor (GPCR) signaling [e.g., protein kinase A (PKA) and receptor tyrosine kinase (RTK)] pathways to control almost every cellular activity, from short-term effects (e.g., gene transcription, and, cell contraction and secretion) to longer-term effects regulating cell development and regeneration (e.g., cell proliferation, migration, differentiation, apoptosis, and necrosis).
Interestingly, downstream effectors of Ca2+ signaling can also, in turn, regulate Ca2+ mobilization. For instance, RyR-mediated Ca2+ release from the ER is accompanied by activation of adenylyl cyclase (AC) and an increase in cAMP, which then initiates the Epac-mediated signaling cascade. RyR activation can also trigger Epac directly that initiates PLC signaling to result in the formation of IP3 that initiates IP3R-triggered Ca2+ release from the calcium store.374 Essentially, any signaling cascade triggering the formation of IP3 is able to activate IP3R-triggered Ca2+ release from the ER, whereas increases in intracellular Ca2+ alone triggers RyR activation.371 Whichever the case, Ca2+ release from the ER enhances the Ca2+ efflux activity of NCX372 while Ca2+ efflux through plasma membrane calcium ATPase (PMCA) is regulated by calmodulin, PKC, and PKA (products of the cAMP–Epac pathway).375 In short, intracellular Ca2+ not only regulates cellular activities but also its own mobilization, and, as such, initiates multiple signaling cascades at any stage of the mobilization.
A possible explanation for this exquisite behavior could be the plethora of Ca2+-binding proteins (which regulate Ca2+ homeostasis to activate different Ca2+ signaling cascades that determine downstream cell response and fate) that exist, and the wide range of affinities they possess (which vary by 106+-fold), depending on their location and function.376 Based on a study conducted on effectors of calcium-binding proteins such as Ca2+/calmodulin-dependent protein kinase II (CAMKII) and PKC, different cellular activities were observed to be determined by the magnitude and frequency of the Ca2+ oscillation:377–380 brief Ca2+ oscillations/spikes evoke simpler cellular activities such as cytoskeletal rearrangement, vesicle trafficking (including neurotransmitter release), and contraction,17 whereas complete Ca2+ mobilization has the propensity to trigger more complex processes such as differentiation381–383 and angiogenesis,384–386 or even oocyte activation.387,388
C. Acoustic stimulation
All forms of acoustic stimulation, irrespective of frequency, are able to modulate intracellular Ca2+ to drive its mobilization within the cell in one way or another by altering the tension of the cell membrane, as described in Sec. VI A and illustrated in Fig. 13. At lower (Hz- to kHz-order) frequencies where cavitation is prevalent, this could occur either directly through the formation of membrane pores or via activation of SACs such as the TRPV channels. At higher (MHz-order) frequencies where cavitation is suppressed, the influx of Ca2+ is primarily driven through transient formation of VLDs or the activation of piezo channels (the involvement of voltage-gated ion channels has also been implicated at GHz frequencies389) with Ca2+ efflux from the ER back into the extracellular milieu being dependent on, or, in some instances,390 independent of, the extracellular Ca2+ concentration.
FIG. 13.
Proposed universal mechanism that underpins a cell's response to different forms of mechanostimuli, wherein the cell membrane plays a central role as a universal transducer/effector, and the second messenger Ca2+ as a key mechanotransduction mechanism. Static or quasi-static constant force mechanostimuli in the form of compression, stretch, or shear directly alter membrane fluidity to drive influx of Ca2+ into the cell that, in turn, trigger different downstream signaling pathways to elicit a variety of mechanoresponses. More complex dynamical forms of mechanostimuli, such as those driven acoustically, invoke changes to the membrane tension indirectly by either inducing cavitation, perturbations or aberrations to the membrane, or the generation of acoustic streaming in the cell media. They nevertheless lead to similar mechanoresponses given that these effectively result in compression, stretch, or shear to the cell, or more complex combinations thereof. Where mechanoresponses differ, however, is a consequence of how Ca2+ is regulated and hence mobilized within the cell in response to these mechanotransductive processes.
Differences in Ca2+ mobilization between the frequency regimes can, nevertheless, provide an explanation for why prolonged exposure times are required with low frequency acoustic stimulation whereas significantly shorter durations are sufficient to invoke similar cellular responses at high frequencies, particularly that with SRBW mechanostimulation. Moreover, given the different stages of Ca2+ mobilization that exist, a plethora of signaling cascades can be evoked, which can explain the distinct mechanoresponses observed for the various forms of acoustic stimuli on different cell types, or even that observed with the same acoustic stimuli but under different excitation parameters. As such, a deeper understanding of the Ca2+ mobilization induced for each specific case is required to design and develop more effective and targeted therapeutic strategies.
VII. SUMMARY
Sound wave excitation offers a considerably broader means of mechanically stimulating cells. Unlike steady forms of mechanical loading (e.g., compression, tension, or shear) applied on the cells under constant loads, either statically or quasi-statically at frequencies of several Hz, dynamic vibrational stimuli can be excited in distinct forms of bulk, surface, or hybrid acoustic waves across a broad amplitude and frequency spectrum to not only evoke far richer, albeit more complex, cellular responses, but to also allow tunability for a diverse range of applications.
Low frequency (Hz to kHz) excitation, for example, has longer characteristic length scales over which the acoustic energy decays, thus allowing greater penetration, particularly through skin and tissue, and thereby enabling their use for in vivo applications. A corollary of the use of low frequency mechanostimulation, however, is the existence of cavitational effects, which, besides exerting considerable stress on cells, can also induce poration along the cell membrane. The larger intensities and prolonged exposure durations that are typically required with low frequency acoustic excitation can also introduce considerable thermal effects that could potentially induce the cells to produce heat shock proteins.
These effects can be circumvented by operating at higher frequencies (MHz order and beyond), whose wavelengths are more closely matched to the characteristic cell dimension and hence allows for localization of the mechanostimulation signal, thereby reducing the intensity required to elicit the same effect. This reduction in power and hence heating as a consequence of absorption of the acoustic energy, together with the diminished propensity for cavitation, allows significantly greater retention in cell viability. The considerably shorter penetration depths associated with high frequency excitation however precludes their use for in vivo applications unless implanted at the local site to be targeted. Nevertheless, the much shorter exposure durations that have been demonstrated with the use of surface and hybrid waves—which have been shown to facilitate both efficient poration-free cellular uptake as well as exosome release in addition to stem cell differentiation and endothelial barrier modeling—together with the possibility for parallelization of the chipscale technology can be appealing for large-scale cellular engineering.
In addition to providing an overview of the advances of acoustically driven cell mechanostimulation across these frequencies to date, and their utility for various applications, particularly in, but not limited to, regenerative therapeutics and nanomedicine, we postulate that the mechanoresponses stemming from such stimuli, particularly those driven at ultrasonic frequencies across 10 kHz–1 GHz, arise from a universal mechanotransductive mechanism regardless of the considerable differences between the underlying mechanostimulation modalities (e.g., constant force, static or quasi-static stimuli such as compression, tension or shear, or, more complex dynamic stimuli involving time-dependent vibrational forcing in the form of bulk, surface or hybrid acoustic waves) across a wide range of frequencies.
Broadly, the cellular response to acoustic forcing, and, mechanostimulation, in general, arises as an outcome of the cells' attempt to maintain both membrane and Ca2+ homeostasis. We posit that the cell membrane plays a central mechanosensory role and that the mechanoresponses that are observed can be attributed to the effect of the mechanical cue, whatever its form, imparted on the cell membrane. Be it static compression or tension (stretching) of the membrane, or a dynamic combination of these constituents as a consequence of the time-dependent, i.e., periodic, vibrational forcing associated with the acoustic excitation, the resultant membrane aberrations (e.g., VLDs) or pores that arise lead to a change in the membrane tension that then drives the activation of SACs. It is primarily these SACs, together with auxiliary gating mechanisms such as other transmembrane proteins (e.g., integrins) and cell junctional proteins (e.g., connexins and cadherins), that facilitate the transmission of the imparted mechanical force through the rest of the cell via its cytoskeletal network to trigger a cascade of downstream molecular signaling events.
More specifically, we observe the second messenger Ca2+ to play a central role in maintaining cellular homeostasis in response to the mechanical insult. In the first Ca2+ mobilization stage, Ca2+ influx into the cell facilitated by the SAC and cell junctional proteins trigger the activation of a series of signaling cascades that include Rho–ROCK signaling and ESCRT pathways. In the second stage of Ca2+ mobilization, the aforementioned influx of Ca2+ into the cell as a consequence of the mechanical load is regulated by channeling it into internal stores primarily through SERCA pumps or returning it to the extracellular milieu through calcium transporters on the membrane. Upon Ca2+ overload in the internal store, Ca2+ is further released through RyR activation, which results in the production of other second messengers such as cAMP and IP3. These second messengers further initiate other signaling cascades (e.g., the Epac1-Rap1 pathway) to facilitate activation of transcription factors, including c-Jun, c-Fos, or ERK1/2, which regulate a diverse set of cellular activities such as cell proliferation, migration, and exosome generation, in addition to other cell-specific processes such as stem cell differentiation, endothelial barrier modulation, neuromodulation, and oocyte activation.
The translation of mechanostimulation to practical living systems for clinical therapeutic applications, however, remains a challenge. Unlike their in vitro counterparts, in vivo systems are considerably more complex due to the lack of appropriate models and means to study the collective behavior arising from the number of different cell types that are simultaneously stimulated, and the multitude of mechanoresponses over widely varying response timescales that can arise.391 As such, there is, at present, insufficient conclusive evidence for coordinated mechanotransduction in vivo, and a thorough understanding of the underlying mechanisms that are involved is still elusive.392,393 In addition, the vast number of systems and protocols associated with multicellular bodies make it significantly more difficult to compare and interpret the data required to upscale these platforms for practical applications. Nevertheless, a better understanding of the in vitro mechanotransduction mechanisms stemming from that discussed in this review would hopefully constitute a start in advancing the technology toward clinical translation.
ACKNOWLEDGMENTS
L.Y.Y. is grateful for funding through Australian Research Council Discovery Project DP210101720. Some images were created using BioRender.
AUTHOR DECLARATIONS
Conflict of Interest
The authors have no conflicts to disclose.
Author Contributions
Lizebona August Ambattu: Conceptualization (equal); Data curation (lead); Writing – original draft (lead). Leslie Yeo: Conceptualization (equal); Funding acquisition (lead); Methodology (equal); Project administration (lead); Resources (lead); Supervision (lead); Validation (lead); Writing – review & editing (lead).
DATA AVAILABILITY
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
References
- 1. Ambrosi D., Ben Amar M., Cyron C. J., DeSimone A., Goriely A., Humphrey J. D., and Kuhl E., “ Growth and remodelling of living tissues: Perspectives, challenges and opportunities,” J. R. Soc. Interface 16, 20190233 (2019). 10.1098/rsif.2019.0233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ingber D. E., “ Mechanobiology and diseases of mechanotransduction,” Ann. Med 35, 564–577 (2003). 10.1080/07853890310016333 [DOI] [PubMed] [Google Scholar]
- 3. Shah N., Morsi Y., and Manasseh R., “ From mechanical stimulation to biological pathways in the regulation of stem cell fate,” Cell Biochem. Funct 32, 309–325 (2014). 10.1002/cbf.3027 [DOI] [PubMed] [Google Scholar]
- 4. Bukoreshtliev N. V., Haase K., and Pelling A. E., “ Mechanical cues in cellular signalling and communication,” Cell Tissue Res 352, 77–94 (2013). 10.1007/s00441-012-1531-4 [DOI] [PubMed] [Google Scholar]
- 5. Wang J., Lu D., Mao D., and Long M., “ Mechanomics: An emerging field between biology and biomechanics,” Protein Cell 5, 518–531 (2014). 10.1007/s13238-014-0057-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Martino F., Perestrelo A. R., Vinarsky V., Pagliari S., and Forte G., “ Cellular mechanotransduction: From tension to function,” Front. Physiol. 9, 824 (2018). 10.3389/fphys.2018.00824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Burridge K., Monaghan-Benson E., and Graham D. M., “ Mechanotransduction: From the cell surface to the nucleus via RhoA,” Philos. Trans. R. Soc., B 374, 20180229 (2019). 10.1098/rstb.2018.0229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gillespie P. G. and Walker R. G., “ Molecular basis of mechanosensory transduction,” Nature 413, 194–202 (2001). 10.1038/35093011 [DOI] [PubMed] [Google Scholar]
- 9. Rufo J., Cai F., Friend J., Wiklund M., and Huang T. J., “ Acoustofluidics for biomedical applications,” Nat. Rev. Methods Primers 2, 30 (2022). 10.1038/s43586-022-00109-7 [DOI] [Google Scholar]
- 10. Na S., Collin O., Chowdhury F., Tay B., Ouyang M., Wang Y., and Wang N., “ Rapid signal transduction in living cells is a unique feature of mechanotransduction,” Proc. Natl. Acad. Sci. U. S. A. 105, 6626–6631 (2008). 10.1073/pnas.0711704105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Maurer M. and Lammerding J., “ The driving force: Nuclear mechanotransduction in cellular function, fate, and disease,” Annu. Rev. Biomed. Eng. 21, 443–468 (2019). 10.1146/annurev-bioeng-060418-052139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Janota C. S., Calero-Cuenca F. J., and Gomes E. R., “ The role of the cell nucleus in mechanotransduction,” Curr. Opin. Cell Biol. 63, 204–211 (2020). 10.1016/j.ceb.2020.03.001 [DOI] [PubMed] [Google Scholar]
- 13. Hoffman B. D., Grashoff C., and Schwartz M. A., “ Dynamic molecular processes mediate cellular mechanotransduction,” Nature 475, 316–323 (2011). 10.1038/nature10316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Holle A. W. and Engler A. J., “ More than a feeling: Discovering, understanding, and influencing mechanosensing pathways,” Curr. Opin. Biotechnol. 22, 648–654 (2011). 10.1016/j.copbio.2011.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen W., Lou J., Evans E. A., and Zhu C., “ Observing force-regulated conformational changes and ligand dissociation from a single integrin on cells,” J. Cell Biol. 199, 497–512 (2012). 10.1083/jcb.201201091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sachs F., “ Stretch-activated ion channels: What are they?,” Physiology 25, 50–56 (2010). 10.1152/physiol.00042.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Berridge M. J., Lipp P., and Bootman M. D., “ The versatility and universality of calcium signalling,” Nat. Rev. Mol. Cell Biol. 1, 11–21 (2000). 10.1038/35036035 [DOI] [PubMed] [Google Scholar]
- 18. Martinac B., Adler J., and Kung C., “ Mechanosensitive ion channels of E. coli activated by amphipaths,” Nature 348, 261–263 (1990). 10.1038/348261a0 [DOI] [PubMed] [Google Scholar]
- 19. Anishkin A., Loukin S. H., Teng J., and Kung C., “ Feeling the hidden mechanical forces in lipid bilayer is an original sense,” Proc. Natl. Acad. Sci. U. S. A. 111, 7898–7905 (2014). 10.1073/pnas.1313364111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cox C. D., Bavi N., and Martinac B., “ Biophysical principles of ion-channel-mediated mechanosensory transduction,” Cell Rep. 29, 1–12 (2019). 10.1016/j.celrep.2019.08.075 [DOI] [PubMed] [Google Scholar]
- 21. Wang N. and Suo Z., “ Long-distance propagation of forces in a cell,” Biochem. Biophys. Res. Commun. 328, 1133–1138 (2005). 10.1016/j.bbrc.2005.01.070 [DOI] [PubMed] [Google Scholar]
- 22. Matthews B. D., Thodeti C. K., and Ingber D. E., “ Activation of mechanosensitive ion channels by forces transmitted through integrins and the cytoskeleton,” Curr. Top. Membr. 58, 59–85 (2007). 10.1016/S1063-5823(06)58003-2 [DOI] [Google Scholar]
- 23. Murthy S. E., Dubin A. E., and Patapoutian A., “ Piezos thrive under pressure: Mechanically activated ion channels in health and disease,” Nat. Rev. Mol. Cell Biol. 18, 771–783 (2017). 10.1038/nrm.2017.92 [DOI] [PubMed] [Google Scholar]
- 24. Enyedi P. and Czirják G., “ Molecular background of leak K+ currents: Two-pore domain potassium channels,” Physiol. Rev. 90, 559–605 (2010). 10.1152/physrev.00029.2009 [DOI] [PubMed] [Google Scholar]
- 25. Drummond H. A., Jernigan N. L., and Grifoni S. C., “ Sensing tension,” Hypertension 51, 1265–1271 (2008). 10.1161/HYPERTENSIONAHA.107.093401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Samanta A., Hughes T. E. T., and Moiseenkova-Bell V. Y., in Membrane Protein Complexes: Structure and Function, edited by Harris J. R. and Boekema E. J. ( Springer, Singapore, 2018), pp. 141–165. [Google Scholar]
- 27. Venkatachalam K. and Montell C., “ TRP channels,” Annu. Rev. Biochem. 76, 387–417 (2007). 10.1146/annurev.biochem.75.103004.142819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chuang Y. C. and Chen C. C., “ Force from filaments: The role of the cytoskeleton and extracellular matrix in the gating of mechanosensitive channels,” Front Cell Dev. Biol. 10, 886048 (2022). 10.3389/fcell.2022.886048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Noël J., Sandoz G., and Lesage F., “ Molecular regulations governing TREK and TRAAK channel functions,” Channels 5, 402–409 (2011). 10.4161/chan.5.5.16469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nikolaev Y. A., Cox C. D., Ridone P., Rohde P. R., Cordero-Morales J. F., Vasquez V., Laver D. R., and Martinac B., “ Mammalian TRP ion channels are insensitive to membrane stretch,” J. Cell Sci. 132, jcs238360 (2019). 10.1242/jcs.238360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Xiong H., Yang J., Guo J., Ma A., Wang B., and Kang Y., “ Mechanosensitive Piezo channels mediate the physiological and pathophysiological changes in the respiratory system,” Respir. Res. 23, 196 (2022). 10.1186/s12931-022-02122-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Young M., Lewis A. H., and Grandl J., “ Physics of mechanotransduction by Piezo ion channels,” J. Gen. Physiol. 154, e202113044 (2022). 10.1085/jgp.202113044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wu J., Lewis A. H., and Grandl J., “ Touch, tension, and transduction—The function and regulation of piezo ion channels,” Trends Biochem. Sci. 42, 57–71 (2017). 10.1016/j.tibs.2016.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang J., Jiang J., Yang X., Zhou G., Wang L., and Xiao B., “ Tethering Piezo channels to the actin cytoskeleton for mechanogating via the cadherin–beta-catenin mechanotransduction complex,” Cell Rep. 38, 110342 (2022). 10.1016/j.celrep.2022.110342 [DOI] [PubMed] [Google Scholar]
- 35. Parpaite T. and Coste B., “ Piezo channels,” Curr. Biol. 27, R250–R252 (2017). 10.1016/j.cub.2017.01.048 [DOI] [PubMed] [Google Scholar]
- 36. Woo S. H., Lukacs V., de Nooij J. C., Zaytseva D., Criddle C. R., Francisco A., Jessell T. M., Wilkinson K. A., and Patapoutian A., “ Piezo2 is the principal mechanotransduction channel for proprioception,” Nat. Neurosci. 18, 1756–1762 (2015). 10.1038/nn.4162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lai A., Chen Y. C., Cox C. D., Jaworowski A., Peter K., and Baratchi S., “ Analyzing the shear-induced sensitization of mechanosensitive ion channel piezo-1 in human aortic endothelial cells,” J. Cell Physiol. 236, 2976–2987 (2021). 10.1002/jcp.30056 [DOI] [PubMed] [Google Scholar]
- 38. Chubinskiy-Nadezhdin V. I., Vasileva V. Y., Pugovkina N. A., Vassilieva I. O., Morachevskaya E. A., Nikolsky N. N., and Negulyaev Y. A., “ Local calcium signalling is mediated by mechanosensitive ion channels in mesenchymal stem cells,” Biochem. Biophys. Res. Commun. 482, 563–568 (2017). 10.1016/j.bbrc.2016.11.074 [DOI] [PubMed] [Google Scholar]
- 39. Stricker J., Aratyn-Schaus Y., Oakes P., and Gardel M., “ Spatiotemporal constraints on the force–dependent growth of focal adhesions,” Biophys. J. 100, 2883–2893 (2011). 10.1016/j.bpj.2011.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Petzold J. and Gentleman E., “ Intrinsic mechanical cues and their impact on stem cells and embryogenesis,” Front. Cell Dev. Biol. 9, 761871 (2021). 10.3389/fcell.2021.761871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Olson E. N. and Nordheim A., “ Linking actin dynamics and gene transcription to drive cellular motile functions,” Nat. Rev. Mol. Cell Biol. 11, 353–365 (2010). 10.1038/nrm2890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Huang C., Akaishi S., and Ogawa R., “ Mechanosignaling pathways in cutaneous scarring,” Arch. Dermatol. Res. 304, 589–597 (2012). 10.1007/s00403-012-1278-5 [DOI] [PubMed] [Google Scholar]
- 43. Nemec S. and Kilian K. A., “ Materials control of the epigenetics underlying cell plasticity,” Nat. Rev. Mater. 6, 69–83 (2021). 10.1038/s41578-020-00238-z [DOI] [Google Scholar]
- 44. Hoang P. and Ma Z., “ Biomaterial-guided stem cell organoid engineering for modeling development and diseases,” Acta Biomater. 132, 23–36 (2021). 10.1016/j.actbio.2021.01.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Carthew J., Taylor J. B. J., Garcia-Cruz M. R., Kiaie N., Voelcker N. H., Cadarso V. J., and Frith J. E., “ The bumpy road to stem cell therapies: Rational design of surface topographies to dictate stem cell mechanotransduction and fate,” ACS Appl. Mater. Interfaces 14, 23066–23101 (2022). 10.1021/acsami.1c22109 [DOI] [PubMed] [Google Scholar]
- 46. Han P., Gomez G. A., Duda G. N., Ivanovski S., and Poh P. S., “ Scaffold geometry modulation of mechanotransduction and its influence on epigenetics,” Acta Biomater. (published online). 10.1016/j.actbio.2022.01.020 [DOI] [PubMed] [Google Scholar]
- 47. Folkman J. and Moscona A., “ Role of cell shape in growth control,” Nature 273, 345–349 (1978). 10.1038/273345a0 [DOI] [PubMed] [Google Scholar]
- 48. Singhvi R., Kumar A., Lopez G. P., Stephanopoulos G. N., Wang D. I., Whitesides G. M., and Ingber D. E., “ Engineering cell shape and function,” Science 264, 696–698 (1994). 10.1126/science.8171320 [DOI] [PubMed] [Google Scholar]
- 49. McBeath R., Pirone D. M., Nelson C. M., Bhadriraju K., and Chen C. S., “ Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment,” Dev. Cell 6, 483–495 (2004). 10.1016/S1534-5807(04)00075-9 [DOI] [PubMed] [Google Scholar]
- 50. Chen C. S., Mrksich M., Huang S., Whitesides G. M., and Ingber D. E., “ Geometric control of cell life and death,” Science 276, 1425–1428 (1997). 10.1126/science.276.5317.1425 [DOI] [PubMed] [Google Scholar]
- 51. Engler A. J., Griffin M. A., Sen S., Bonnemann C. G., Sweeney H. L., and Discher D. E., “ Myotubes differentiate optimally on substrates with tissue-like stiffness: Pathological implications for soft or stiff microenvironments,” J. Cell Biol. 166, 877–887 (2004). 10.1083/jcb.200405004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Urbanczyk M., Layland S. L., and Schenke-Layland K., “ The role of extracellular matrix in biomechanics and its impact on bioengineering of cells and 3D tissues,” Matrix Biol. 85–86, 1–14 (2020). 10.1016/j.matbio.2019.11.005 [DOI] [PubMed] [Google Scholar]
- 53. Millward-Sadler S. J. and Salter D. M., “ Integrin-dependent signal cascades in chondrocyte mechanotransduction,” Ann. Biomed. Eng. 32, 435–446 (2004). 10.1023/B:ABME.0000017538.72511.48 [DOI] [PubMed] [Google Scholar]
- 54. Roberts S. R., Knight M. M., Lee D. A., and Bader D. L., “ Mechanical compression influences intracellular Ca2+ signaling in chondrocytes seeded in agarose constructs,” J. Appl. Physiol. 90, 1385–1391 (2001). 10.1152/jappl.2001.90.4.1385 [DOI] [PubMed] [Google Scholar]
- 55. Quiroga X., Walani N., Chavero A., Mittens A., Disanza A., Tebar F., Trepat X., Parton R. G., Scita G., Geli M. I. et al. , “ A mechanosensing mechanism mediated by IRSp53 controls plasma membrane shape homeostasis at the nanoscale,” bioRxiv (2021). [DOI] [PMC free article] [PubMed]
- 56. Chen Q., Yang D., Zong H., Zhu L., Wang L., Wang X., Zhu X., Song X., and Wang J., “ Growth-induced stress enhances epithelial–mesenchymal transition induced by IL-6 in clear cell renal cell carcinoma via the Akt/GSK-3beta/beta-catenin signaling pathway,” Oncogenesis 6, e375 (2017). 10.1038/oncsis.2017.74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Cai X., Wang K.-C., and Meng Z., “ Mechanoregulation of YAP and TAZ in cellular homeostasis and disease progression,” Front. Cell Dev. Biol. 9, 673599 (2021). 10.3389/fcell.2021.673599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Li B., Moshfegh C., Lin Z., Albuschies J., and Vogel V., “ Mesenchymal stem cells exploit extracellular matrix as mechanotransducer,” Sci. Rep. 3, 2425 (2013). 10.1038/srep02425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Peng Y., Qu R., Feng Y., Huang X., Yang Y., Fan T., Sun B., Khan A. U., Wu S., Dai J. et al. , “ Regulation of the integrin actin filaments axis in early osteogenesis of human fibroblasts under cyclic tensile stress,” Stem Cell Res. Ther. 12, 523 (2021). 10.1186/s13287-021-02597-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Miyamoto T., Mochizuki T., Nakagomi H., Kira S., Watanabe M., Takayama Y., Suzuki Y., Koizumi S., Takeda M., and Tominaga M., “ Functional role for Piezo1 in stretch–evoked Ca2+ influx and ATP release in urothelial cell cultures,” J. Biol. Chem. 289, 16565–16575 (2014). 10.1074/jbc.M113.528638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Thodeti C. K., Matthews B., Ravi A., Mammoto A., Ghosh K., Bracha A. L., and Ingber D. E., “ TRPV4 channels mediate cyclic strain-induced endothelial cell reorientation through integrin-to-integrin signaling,” Circ. Res. 104, 1123–1130 (2009). 10.1161/CIRCRESAHA.108.192930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kuipers A. J., Middelbeek J., and van Leeuwen F. N., “ Mechanoregulation of cytoskeletal dynamics by TRP channels,” Eur. J. Cell Biol. 91, 834–846 (2012). 10.1016/j.ejcb.2012.05.006 [DOI] [PubMed] [Google Scholar]
- 63. Wang J. G., Miyazu M., Matsushita E., Sokabe M., and Naruse K., “ Uniaxial cyclic stretch induces focal adhesion kinase (FAK) tyrosine phosphorylation followed by mitogen-activated protein kinase (MAPK) activation,” Biochem. Biophys. Res. Commun. 288, 356–361 (2001). 10.1006/bbrc.2001.5775 [DOI] [PubMed] [Google Scholar]
- 64. De R., “ A general model of focal adhesion orientation dynamics in response to static and cyclic stretch,” Commun. Biol. 1, 81 (2018). 10.1038/s42003-018-0084-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Na S., Trache A., Trzeciakowski J., Sun Z., Meininger G. A., and Humphrey J. D., “ Time-dependent changes in smooth muscle cell stiffness and focal adhesion area in response to cyclic equibiaxial stretch,” Ann. Biomed. Eng. 36, 369–380 (2008). 10.1007/s10439-008-9438-7 [DOI] [PubMed] [Google Scholar]
- 66. Xu B., Song G., Ju Y., Li X., Song Y., and Watanabe S., “ RhoA/ROCK, cytoskeletal dynamics, and focal adhesion kinase are required for mechanical stretch-induced tenogenic differentiation of human mesenchymal stem cells,” J. Cell Physiol. 227, 2722–2729 (2012). 10.1002/jcp.23016 [DOI] [PubMed] [Google Scholar]
- 67. Ross T. D., Coon B. G., Yun S., Baeyens N., Tanaka K., Ouyang M., and Schwartz M. A., “ Integrins in mechanotransduction,” Curr. Opin. Cell Biol. 25, 613–618 (2013). 10.1016/j.ceb.2013.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Baratchi S., Almazi J. G., Darby W., Tovar-Lopez F. J., Mitchell A., and McIntyre P., “ Shear stress mediates exocytosis of functional TRPV4 channels in endothelial cells,” Cell Mol. Life Sci. 73, 649–666 (2016). 10.1007/s00018-015-2018-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Fuentes D. E. and Butler P. J., “ Coordinated mechanosensitivity of membrane rafts and focal adhesions,” Cell Mol. Bioeng. 5, 143–154 (2012). 10.1007/s12195-012-0225-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Chachisvilis M., Zhang Y. L., and Frangos J. A., “ G protein-coupled receptors sense fluid shear stress in endothelial cells,” Proc. Natl. Acad. Sci. U. S. A. 103, 15463–15468 (2006). 10.1073/pnas.0607224103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Li H., Zhou W. Y., Xia Y. Y., and Zhang J. X., “ Endothelial mechanosensors for atheroprone and atheroprotective shear stress signals,” J. Inflamm. Res. 15, 1771–1783 (2022). 10.2147/JIR.S355158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Lee H. J. and Koh G. Y., “ Shear stress activates Tie2 receptor tyrosine kinase in human endothelial cells,” Biochem. Biophys. Res. Commun. 304, 399–404 (2003). 10.1016/s0006-291x(03)00592-8 [DOI] [PubMed] [Google Scholar]
- 73. Das J., Maji S., Agarwal T., Chakraborty S., and Maiti T. K., “ Hemodynamic shear stress induces protective autophagy in HeLa cells through lipid raft-mediated mechanotransduction,” Clin. Exp. Metastasis 35, 135–148 (2018). 10.1007/s10585-018-9887-9 [DOI] [PubMed] [Google Scholar]
- 74. Das J. and Maiti T. K., “ Fluid shear stress influences invasiveness of HeLa cells through the induction of autophagy,” Clin. Exp. Metastasis 39, 495–504 (2022). 10.1007/s10585-022-10156-9 [DOI] [PubMed] [Google Scholar]
- 75. Nauli S. M., Alenghat F. J., Luo Y., Williams E., Vassilev P., Li X., Elia A. E., Lu W., Brown E. M., Quinn S. J. et al. , “ Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells,” Nat. Genet. 33, 129–137 (2003). 10.1038/ng1076 [DOI] [PubMed] [Google Scholar]
- 76. Le Q. A., Kim J. C., Kim K. H., Van Vu A. T., and Woo S. H., “ Distinct shear-induced Ca2+ signaling in the left and right atrial myocytes: Role of P2 receptor context,” J. Mol. Cell. Cardiol. 143, 38–50 (2020). 10.1016/j.yjmcc.2020.04.018 [DOI] [PubMed] [Google Scholar]
- 77. Park M. G., Jang H., Lee S. H., and Lee C. J., “ Flow shear stress enhances the proliferative potential of cultured radial glial cells possibly via an activation of mechanosensitive calcium channel,” Exp. Neurobiol. 26, 71–81 (2017). 10.5607/en.2017.26.2.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Hamamura K., Swarnkar G., Tanjung N., Cho E., Li J., Na S., and Yokota H., “ RhoA-mediated signaling in mechanotransduction of osteoblasts,” Connect. Tissue Res. 53, 398–406 (2012). 10.3109/03008207.2012.671398 [DOI] [PubMed] [Google Scholar]
- 79. Simfia I., Schiavi J., and McNamara L. M., “ Alterations in osteocyte mediated osteoclastogenesis during estrogen deficiency and under ROCK-II inhibition: An in vitro study using a novel postmenopausal multicellular niche model,” Exp. Cell Res. 392, 112005 (2020). 10.1016/j.yexcr.2020.112005 [DOI] [PubMed] [Google Scholar]
- 80. Yuan L., Sakamoto N., Song G., and Sato M., “ Low–level shear stress induces human mesenchymal stem cell migration through the SDF-1/CXCR4 axis via MAPK signaling pathways,” Stem Cells Dev. 22, 2384–2393 (2013). 10.1089/scd.2012.0717 [DOI] [PubMed] [Google Scholar]
- 81. Choi H. Y., Yang G.-M., Dayem A. A., Saha S. K., Kim K., Yoo Y., Hong K., Kim J.-H., Yee C., Lee K.-M. et al. , “ Hydrodynamic shear stress promotes epithelial–mesenchymal transition by downregulating ERK and GSK3β activities,” Breast Cancer Res. 21, 6 (2019). 10.1186/s13058-018-1071-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Abuammah A., Maimari N., Towhidi L., Frueh J., Chooi K. Y., Warboys C., and Krams R., “ New developments in mechanotransduction: Cross talk of the Wnt, TGF-β and Notch signalling pathways in reaction to shear stress,” Curr. Opin. Biomed. Eng. 5, 96–104 (2018). 10.1016/j.cobme.2018.03.003 [DOI] [Google Scholar]
- 83. Gelfand B. D., Meller J., Pryor A. W., Kahn M., Bortz P. D. S., Wamhoff B. R., and Blackman B. R., “ Hemodynamic activation of β-catenin and T-cell-specific transcription factor signaling in vascular endothelium regulates fibronectin expression,” Arterioscler. Thromb. Vasc. Biol. 31, 1625–1633 (2011). 10.1161/ATVBAHA.111.227827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Ambattu L. A., Gelmi A., and Yeo L. Y., “ Short-duration high frequency megaHertz–order nanomechanostimulation drives early and persistent osteogenic differentiation in mesenchymal stem cells,” Small 18, e2106823 (2022). 10.1002/smll.202106823 [DOI] [PubMed] [Google Scholar]
- 85. Shi M., Du F., Liu Y., Li L., Cai J., Zhang G.-F., Xu X.-F., Lin T., Cheng H.-R., Liu X.-D. et al. , “ Glial cell-expressed mechanosensitive channel TRPV4 mediates infrasound-induced neuronal impairment,” Acta Neuropathol. 126, 725–739 (2013). 10.1007/s00401-013-1166-x [DOI] [PubMed] [Google Scholar]
- 86. Jang K. W., Ding L., Seol D., Lim T. H., Buckwalter J. A., and Martin J. A., “ Low-intensity pulsed ultrasound promotes chondrogenic progenitor cell migration via focal adhesion kinase pathway,” Ultrasound Med. Biol. 40, 1177–1186 (2014). 10.1016/j.ultrasmedbio.2013.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Ambattu L. A., Gelmi A., Keng-hui L., and Yeo L. Y., “ Calcium-dependent cAMP mediates mechanoresponsive behaviour of endothelial cells to high-frequency nanomechanostimulation,” Biomaterials 292, 121866 (2023). 10.1016/j.biomaterials.2022.121866 [DOI] [PubMed] [Google Scholar]
- 88. Yoon C. W., Jung H., Goo K., Moon S., Koo K. M., Lee N. S., Weitz A. C., and Shung K. K., “ Low-intensity ultrasound modulates Ca2+ dynamics in human mesenchymal stem cells via connexin 43 hemichannel,” Ann. Biomed. Eng. 46, 48–59 (2018). 10.1007/s10439-017-1949-7 [DOI] [PubMed] [Google Scholar]
- 89. Xia P., Ren S., Lin Q., Cheng K., Shen S., Gao M., and Li X., “ Low-intensity pulsed ultrasound affects chondrocyte extracellular matrix production via an integrin-mediated p38 MAPK signaling pathway,” Ultrasound Med. Biol. 41, 1690–1700 (2015). 10.1016/j.ultrasmedbio.2015.01.014 [DOI] [PubMed] [Google Scholar]
- 90. Chen J., Jiang J., Wang W., Qin J., Chen J., Chen W., and Wang Y., “ Low intensity pulsed ultrasound promotes the migration of bone marrow-derived mesenchymal stem cells via activating FAK-ERK1/2 signalling pathway,” Artif. Cells Nanomed. Biotechnol. 47, 3603–3613 (2019). 10.1080/21691401.2019.1657878 [DOI] [PubMed] [Google Scholar]
- 91. Liao B., Guan M., Tan Q., Wang G., Zhang R., Huang J., Liu M., Chen H., Li K., Bai D. et al. , “ Low-intensity pulsed ultrasound inhibits fibroblast-like synoviocyte proliferation and reduces synovial fibrosis by regulating Wnt/β-catenin signaling,” J. Orthop. Transl. 30, 41–50 (2021). 10.1016/j.jot.2021.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Ambattu L. A., Ramesan S., Dekiwadia C., Hanssen E., Li H., and Yeo L. Y., “ High frequency acoustic cell stimulation promotes exosome generation regulated by a calcium-dependent mechanism,” Commun. Biol. 3, 553 (2020). 10.1038/s42003-020-01277-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Xu X. M., Xu T. M., Wei Y. B., Gao X. X., Sun J. C., Wang Y., Kong Q. J., and Shi J. G., “ Low-intensity pulsed ultrasound treatment accelerates angiogenesis by activating YAP/TAZ in human umbilical vein endothelial cells,” Ultrasound Med. Biol. 44, 2655–2661 (2018). 10.1016/j.ultrasmedbio.2018.07.007 [DOI] [PubMed] [Google Scholar]
- 94. Ling L., Wei T., He L., Wang Y., Wang Y., Feng X., Zhang W., and Xiong Z., “ Low-intensity pulsed ultrasound activates ERK1/2 and PI3K-Akt signalling pathways and promotes the proliferation of human amnion-derived mesenchymal stem cells,” Cell Prolif. 50, e12383 (2017). 10.1111/cpr.12383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Brown T. D., “ Techniques for mechanical stimulation of cells in vitro: A review,” J. Biomech. 33, 3–14 (2000). 10.1016/S0021-9290(99)00177-3 [DOI] [PubMed] [Google Scholar]
- 96. Natenstedt J., Kok A. C., Dankelman J., and Tuijthof G. J., “ What quantitative mechanical loading stimulates in vitro cultivation best?,” J. Exp. Orthop. 2, 15 (2015). 10.1186/s40634-015-0029-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Liu S., Tao R., Wang M., Tian J., Genin G. M., Lu T. J., and Xu F., “ Regulation of cell behavior by hydrostatic pressure,” Appl. Mech. Rev. 71, 040803 (2019). 10.1115/1.4043947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Crenshaw H. C., Allen J. A., Skeen V., Harris A., and Salmon E. D., “ Hydrostatic pressure has different effects on the assembly of tubulin, actin, myosin II, vinculin, talin, vimentin, and cytokeratin in mammalian tissue cells,” Exp. Cell Res. 227, 285–297 (1996). 10.1006/excr.1996.0278 [DOI] [PubMed] [Google Scholar]
- 99. Nathan S. S., DiResta G. R., Casas-Ganem J. E., Hoang B. H., Sowers R., Yang R., Huvos A. G., Gorlick R., and Healey J. H., “ Elevated physiologic tumor pressure promotes proliferation and chemosensitivity in human osteosarcoma,” Clin. Cancer Res. 11, 2389–2397 (2005). 10.1158/1078-0432.CCR-04-2048 [DOI] [PubMed] [Google Scholar]
- 100. Wei T. Q., Luo D. Y., Chen L., Wu T., and Wang K. J., “ Cyclic hydrodynamic pressure induced proliferation of bladder smooth muscle cells via integrin alpha5 and FAK,” Physiol. Res. 63, 127–134 (2014). 10.33549/physiolres.932506 [DOI] [PubMed] [Google Scholar]
- 101. Frey B., Janko C., Ebel N., Meister S., Schlucker E., Meyer-Pittroff R., Fietkau R., Herrmann M., and Gaipl U. S., “ Cells under pressure—Treatment of eukaryotic cells with high hydrostatic pressure, from physiologic aspects to pressure induced cell death,” Curr. Med. Chem. 15, 2329–2336 (2008). 10.2174/092986708785909166 [DOI] [PubMed] [Google Scholar]
- 102. Schwartz E. A., Bizios R., Medow M. S., and Gerritsen M. E., “ Exposure of human vascular endothelial cells to sustained hydrostatic pressure stimulates proliferation: Involvement of the v integrins,” Circ. Res. 84, 315–322 (1999). 10.1161/01.RES.84.3.315 [DOI] [PubMed] [Google Scholar]
- 103. Ju W. K., Kim K. Y., Lindsey J. D., Angert M., Patel A., Scott R. T., Liu Q., Crowston J. G., Ellisman M. H., Perkins G. A. et al. , “ Elevated hydrostatic pressure triggers release of OPA1 and cytochrome C, and induces apoptotic cell death in differentiated RGC–5 cells,” Mol. Vis. 15, 120–134 (2009). [PMC free article] [PubMed] [Google Scholar]
- 104. Li Y., Mao A. S., Seo B. R., Zhao X., Gupta S. K., Chen M., Han Y. L., Shih T. Y., Mooney D. J., and Guo M., “ Compression-induced dedifferentiation of adipocytes promotes tumor progression,” Sci. Adv. 6, eaax5611 (2020). 10.1126/sciadv.aax5611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Kamble H., Barton M. J., Jun M., Park S., and Nguyen N.-T., “ Cell stretching devices as research tools: Engineering and biological considerations,” Lab Chip 16, 3193–3203 (2016). 10.1039/C6LC00607H [DOI] [PubMed] [Google Scholar]
- 106. Lee W. C., Maul T. M., Vorp D. A., Rubin J. P., and Marra K. G., “ Effects of uniaxial cyclic strain on adipose-derived stem cell morphology, proliferation, and differentiation,” Biomech. Model. Mechanobiol. 6, 265–273 (2007). 10.1007/s10237-006-0053-y [DOI] [PubMed] [Google Scholar]
- 107. Riehl B. D., Park J. H., Kwon I. K., and Lim J. Y., “ Mechanical stretching for tissue engineering: Two-dimensional and three-dimensional constructs,” Tissue Eng., Part B 18, 288–300 (2012). 10.1089/ten.teb.2011.0465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Mikuni-Takagaki Y., Suzuki Y., Kawase T., and Saito S., “ Distinct responses of different populations of bone cells to mechanical stress,” Endocrinology 137, 2028–2035 (1996). 10.1210/endo.137.5.8612544 [DOI] [PubMed] [Google Scholar]
- 109. Katsumi A., Milanini J., Kiosses W. B., del Pozo M. A., Kaunas R., Chien S., Hahn K. M., and Schwartz M. A., “ Effects of cell tension on the small GTPase Rac,” J. Cell Biol. 158, 153–164 (2002). 10.1083/jcb.200201105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Liegibel U. M., Sommer U., Tomakidi P., Hilscher U., Van Den Heuvel L., Pirzer R., Hillmeier J., Nawroth P., and Kasperk C., “ Concerted action of androgens and mechanical strain shifts bone metabolism from high turnover into an osteoanabolic mode,” J. Exp. Med. 196, 1387–1392 (2002). 10.1084/jem.20021017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Bhatt K. A., Chang E. I., Warren S. M., Lin S. E., Bastidas N., Ghali S., Thibboneir A., Capla J. M., McCarthy J. G., and Gurtner G. C., “ Uniaxial mechanical strain: An in vitro correlate to distraction osteogenesis,” J. Surg. Res. 143, 329–336 (2007). 10.1016/j.jss.2007.01.023 [DOI] [PubMed] [Google Scholar]
- 112. Huang C. H., Chen M. H., Young T. H., Jeng J. H., and Chen Y. J., “ Interactive effects of mechanical stretching and extracellular matrix proteins on initiating osteogenic differentiation of human mesenchymal stem cells,” J. Cell Biochem. 108, 1263–1273 (2009). 10.1002/jcb.22356 [DOI] [PubMed] [Google Scholar]
- 113. van der Flier A., Badu-Nkansah K., Whittaker C. A., Crowley D., Bronson R. T., Lacy-Hulbert A., and Hynes R. O., “ Endothelial alpha5 and alphav integrins cooperate in remodeling of the vasculature during development,” Development 137, 2439–2449 (2010). 10.1242/dev.049551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Gupton S. L. and Gertler F. B., “ Integrin signaling switches the cytoskeletal and exocytic machinery that drives neuritogenesis,” Dev. Cell 18, 725–736 (2010). 10.1016/j.devcel.2010.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Jung Y., Kissil J. L., and McCarty J. H., “ β8 integrin and band 4.1B cooperatively regulate morphogenesis of the embryonic heart,” Dev. Dyn. 240, 271–277 (2011). 10.1002/dvdy.22513 [DOI] [PubMed] [Google Scholar]
- 116. Griffith C. M., Huang S. A., Cho C., Khare T. M., Rich M., Lee G.-H., Ligler F. S., Diekman B. O., and Polacheck W. J., “ Microfluidics for the study of mechanotransduction,” J. Phys. D 53, 224004 (2020). 10.1088/1361-6463/ab78d4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Urbich C., Walter D. H., Zeiher A. M., and Dimmeler S., “ Laminar shear stress upregulates integrin expression: Role in endothelial cell adhesion and apoptosis,” Circ. Res. 87, 683–689 (2000). 10.1161/01.RES.87.8.683 [DOI] [PubMed] [Google Scholar]
- 118. Chen K. D., Li Y. S., Kim M., Li S., Yuan S., Chien S., and Shyy J. Y., “ Mechanotransduction in response to shear stress. Roles of receptor tyrosine kinases, integrins, and shc,” J. Biol. Chem. 274, 18393–18400 (1999). 10.1074/jbc.274.26.18393 [DOI] [PubMed] [Google Scholar]
- 119. Cui X., Zhang X., Guan X., Li H., Li X., Lu H., and Cheng M., “ Shear stress augments the endothelial cell differentiation marker expression in late EPCs by upregulating integrins,” Biochem. Biophys. Res. Commun. 425, 419–425 (2012). 10.1016/j.bbrc.2012.07.115 [DOI] [PubMed] [Google Scholar]
- 120. Li Y.-S. J., Haga J. H., and Chien S., “ Molecular basis of the effects of shear stress on vascular endothelial cells,” J. Biomech. 38, 1949–1971 (2005). 10.1016/j.jbiomech.2004.09.030 [DOI] [PubMed] [Google Scholar]
- 121. Ruze A., Zhao Y., Li H., Gulireba X., Li J., Lei D., Dai H., Wu J., Zhao X., and Nie Y., “ Low shear stress upregulates the expression of fractalkine through the activation of mitogen-activated protein kinases in endothelial cells,” Blood Coagul. Fibrinol. 29, 361–368 (2018). 10.1097/MBC.0000000000000701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Chistiakov D. A., Orekhov A. N., and Bobryshev Y. V., “ Effects of shear stress on endothelial cells: Go with the flow,” Acta Physiol. 219, 382–408 (2017). 10.1111/apha.12725 [DOI] [PubMed] [Google Scholar]
- 123. Chien S., “ Mechanotransduction and endothelial cell homeostasis: The wisdom of the cell,” Am. J. Physiol. Heart Circ. Physiol. 292, H1209–H1224 (2007). 10.1152/ajpheart.01047.2006 [DOI] [PubMed] [Google Scholar]
- 124. Hu K., Sun H., Gui B., and Sui C., “ TRPV4 functions in flow shear stress induced early osteogenic differentiation of human bone marrow mesenchymal stem cells,” Biomed. Pharmacother. 91, 841–848 (2017). 10.1016/j.biopha.2017.04.094 [DOI] [PubMed] [Google Scholar]
- 125. Hosseini M. S., Tafazzoli-Shadpour M., Haghighipour N., Aghdami N., and Goodarzi A., “ The synergistic effects of shear stress and cyclic hydrostatic pressure modulate chondrogenic induction of human mesenchymal stem cells,” Int. J. Artif. Organs 38, 557–564 (2015). 10.5301/ijao.5000433 [DOI] [PubMed] [Google Scholar]
- 126. Arora S., Srinivasan A., Leung C. M., and Toh Y. C., “ Bio–mimicking shear stress environments for enhancing mesenchymal stem cell differentiation,” Curr. Stem Cell Res. Ther. 15, 414–427 (2020). 10.2174/1574888X15666200408113630 [DOI] [PubMed] [Google Scholar]
- 127. Kim D. H., Heo S.-J., Kang Y. G., Shin J. W., Park S. H., and Shin J.-W., “ Shear stress and circumferential stretch by pulsatile flow direct vascular endothelial lineage commitment of mesenchymal stem cells in engineered blood vessels,” J. Mater. Sci.: Mater. Med 27, 60 (2016). 10.1007/s10856-016-5670-0 [DOI] [PubMed] [Google Scholar]
- 128. Wolfe R. P. and Ahsan T., “ Shear stress during early embryonic stem cell differentiation promotes hematopoietic and endothelial phenotypes,” Biotechnol. Bioeng. 110, 1231–1242 (2013). 10.1002/bit.24782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Auld B. A., Acoustic Fields and Waves in Solids, John Wiley & Sons: New York, 1973. [Google Scholar]
- 130. Dieulesaint E. and Royer D., Elastic Waves in Solids I: Free and Guided Propagation ( Springer, New York, 2000). [Google Scholar]
- 131. Rezk A. R., Tan J. K., and Yeo L. Y., “ HYbriD resonant acoustics (HYDRA),” Adv. Mater. 28, 1970–1975 (2016). 10.1002/adma.201504861 [DOI] [PubMed] [Google Scholar]
- 132. Rezk A. R., Friend J. R., and Yeo L. Y., “ Simple, low cost MHz-order acoustomicrofluidics using aluminium foil electrodes,” Lab Chip 14, 1802–1805 (2014). 10.1039/C4LC00182F [DOI] [PubMed] [Google Scholar]
- 133. Liu Y., Cai Y., Zhang Y., Tovstopyat A., Liu S., and Sun C., “ Materials, design, and characteristics of bulk acoustic wave resonator: A review,” Micromachines 11, 630 (2020). 10.3390/mi11070630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Li K., Fan J., and Bao Y., “ The effect of infrasound on the proliferation of HL-60 cells,” Chin. J. Rehabil. Med. 22, 212–214 (2007). [Google Scholar]
- 135. Pei Z.-H., Chen B.-Y., Tie R., Zhang H.-F., Zhao G., Qu P., Zhu X.-X., Zhu M.-Z., and Yu J., “ Infrasound exposure induces apoptosis of rat cardiac myocytes by regulating the expression of apoptosis–related proteins,” Cardiovasc. Toxicol 11, 341–346 (2011). 10.1007/s12012-011-9126-y [DOI] [PubMed] [Google Scholar]
- 136. Jiang S., Wang Y. Q., Xu C. F., Li Y. N., Guo R., and Li L., “ Involvement of connexin43 in the infrasonic noise-induced glutamate release by cultured astrocytes,” Neurochem. Res 39, 833–842 (2014). 10.1007/s11064-014-1277-3 [DOI] [PubMed] [Google Scholar]
- 137. Nahum R., Jacob H. P., Orit R., and Haim A., “ Determination of biophysical parameters for enhancement of human osteoblast proliferation by mechanical vibration in the infrasonic frequency range,” bioRxiv 448225 (2018).
- 138. Mehrafsar A. and Mokhtari M. J., “ Effect of exposure to quran recitation on cell viability, cell migration, and BCL2L12 gene expression of human prostate adenocarcinoma cell line in culture,” Health Spiritual. Med. Ethics 5, 46–52 (2018). 10.29252/jhsme.5.4.46 [DOI] [Google Scholar]
- 139. Mohammed T., Murphy M. F., Lilley F., Burton D. R., and Bezombes F., “ The effects of acoustic vibration on fibroblast cell migration,” Mater. Sci. Eng., C 69, 1256–1262 (2016). 10.1016/j.msec.2016.07.037 [DOI] [PubMed] [Google Scholar]
- 140. Cheng H., Wang B., Tang C., Feng G., Zhang C., Li L., Lin T., Du F., Duan H., Shi M. et al. , “ Infrasonic noise induces axonal degeneration of cultured neurons via a Ca2+ influx pathway,” Toxicol. Lett 212, 190–197 (2012). 10.1016/j.toxlet.2012.05.015 [DOI] [PubMed] [Google Scholar]
- 141. Choi Y., Park J. E., Jeong J. S., Park J. K., Kim J., and Jeon S., “ Sound waves induce neural differentiation of human bone marrow-derived mesenchymal stem cells via ryanodine receptor-induced calcium release and Pyk2 activation,” Appl. Biochem. Biotechnol 180, 682–694 (2016). 10.1007/s12010-016-2124-6 [DOI] [PubMed] [Google Scholar]
- 142. Ciuffreda M. C., Malpasso G., Musarò P., Turco V., and Gnecchi M., in Mesenchymal Stem Cells: Methods and Protocols, edited by Gnecchi M. ( Springer, New York, 2016), pp 149–158. [DOI] [PubMed] [Google Scholar]
- 143. Kumeta M., Takahashi D., Takeyasu K., and Yoshimura S. H., “ Cell type-specific suppression of mechanosensitive genes by audible sound stimulation,” PLoS One 13, e0188764 (2018). 10.1371/journal.pone.0188764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Liu Z., Gong L., Li X., Ye L., Wang B., Liu J., Qiu J., Jiao H., Zhang W., Chen J., and Wang J., “ Infrasound increases intracellular calcium concentration and induces apoptosis in hippocampi of adult rats,” Mol. Med. Rep 5, 73–77 (2012). 10.3892/mmr.2011.597 [DOI] [PubMed] [Google Scholar]
- 145. Rezk A. R., Ahmed H., Ramesan S., and Yeo L. Y., “ High frequency sonoprocessing: A new field of cavitation–free acoustic materials synthesis, processing, and manipulation,” Adv. Sci. 8, 2001983 (2020). 10.1002/advs.202001983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Dalecki D., “ Mechanical bioeffects of ultrasound,” Annu. Rev. Biomed. Eng. 6, 229–248 (2004). 10.1146/annurev.bioeng.6.040803.140126 [DOI] [PubMed] [Google Scholar]
- 147. Wiklund M., “ Acoustofluidics 12: Biocompatibility and cell viability in microfluidic acoustic resonators,” Lab Chip 12, 2018–2028 (2012). 10.1039/c2lc40201g [DOI] [PubMed] [Google Scholar]
- 148. Levario-Diaz V., Bhaskar P., Galan M. C., and Barnes A. C., “ Effect of acoustic standing waves on cellular viability and metabolic activity,” Sci. Rep. 10, 8493 (2020). 10.1038/s41598-020-65241-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Wu J. and Nyborg W. L., “ Ultrasound, cavitation bubbles and their interaction with cells,” Adv. Drug Delivery Rev. 60, 1103–1116 (2008). 10.1016/j.addr.2008.03.009 [DOI] [PubMed] [Google Scholar]
- 150. Karthikesh M. S. and Yang X., “ The effect of ultrasound cavitation on endothelial cells,” Exp. Biol. Med. 246, 758–770 (2021). 10.1177/1535370220982301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Shimoni C., Goldstein M., Ribarski-Chorev I., Schauten I., Nir D., Strauss C., and Schlesinger S., “ Heat shock alters mesenchymal stem cell identity and induces premature senescence,” Front. Cell Dev. Biol. 8, 565970 (2020). 10.3389/fcell.2020.565970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Park H., Han S., Oh S., and Kang H., “ Cellular responses to mild heat stress,” Cell. Mol. Life Sci. 62, 10–23 (2005). 10.1007/s00018-004-4208-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Kaufman G. E., Miller M. W., Griffiths T. D., Ciaravino V., and Carstensen E. L., “ Lysis and viability of cultured mammalian cells exposed to 1 MHz ultrasound,” Ultrasound Med. Biol. 3, 21–25 (1977). 10.1016/0301-5629(77)90117-X [DOI] [PubMed] [Google Scholar]
- 154. Lenshof A. and Laurell T., in Encyclopedia of Nanotechnology, edited by Bhushan B. ( Springer, Dordrecht, 2012), pp 45–50. [Google Scholar]
- 155. Evander M. and Laurell T., in Encyclopedia of Nanotechnology, edited by Bhushan B. ( Springer, Dordrecht, 2012), pp 41–45. [Google Scholar]
- 156. Rubin D. M., Anderton N., Smalberger C., Polliack J., Nathan M., and Postema M., “ On the behaviour of living cells under the influence of ultrasound,” Fluids 3, 82 (2018). 10.3390/fluids3040082 [DOI] [Google Scholar]
- 157. Pecha R. and Gompf B., “ Microimplosions: Cavitation collapse and shock wave emission on a nanosecond time scale,” Phys. Rev. Lett. 84, 1328–1330 (2000). 10.1103/PhysRevLett.84.1328 [DOI] [PubMed] [Google Scholar]
- 158. Vogel A., Lauterborn W., and Timm R., “ Optical and acoustic investigations of the dynamics of laser-produced cavitation bubbles near a solid boundary,” J. Fluid Mech. 206, 299–338 (1989). 10.1017/S0022112089002314 [DOI] [Google Scholar]
- 159. Prentice P., Cuschieri A., Dholakia K., Prausnitz M., and Campbell P., “ Membrane disruption by optically controlled microbubble cavitation,” Nat. Phys. 1, 107–110 (2005). 10.1038/nphys148 [DOI] [Google Scholar]
- 160. Rossmanna C. and Haemmerich D., “ Review of temperature dependence of thermal properties, dielectric properties, and perfusion of biological tissues at hyperthermic and ablation temperatures,” Crit. Rev. Biomed. Eng. 42, 467–492 (2014). 10.1615/CritRevBiomedEng.2015012486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Milowska K. and Gabryelak T., “ Reactive oxygen species and DNA damage after ultrasound exposure,” Biomol. Eng 24, 263–267 (2007). 10.1016/j.bioeng.2007.02.001 [DOI] [PubMed] [Google Scholar]
- 162. Lentacker I., De Cock I., Deckers R., De Smedt S. C., and Moonen C. T., “ Understanding ultrasound induced sonoporation: Definitions and underlying mechanisms,” Adv. Drug Delivery Rev. 72, 49–64 (2014). 10.1016/j.addr.2013.11.008 [DOI] [PubMed] [Google Scholar]
- 163. Helfield B., Chen X., Watkins S. C., and Villanueva F. S., “ Biophysical insight into mechanisms of sonoporation,” Proc. Natl. Acad. Sci. U. S. A. 113, 9983–9988 (2016). 10.1073/pnas.1606915113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Tu J. and Yu A. C. H., “ Ultrasound-mediated drug delivery: Sonoporation mechanisms, biophysics, and critical factors,” BME Front. 2022, 9807347. 10.34133/2022/9807347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Chu Y.-C., Lim J., Chien A., Chen C.-C., and Wang J.-L., “ Activation of mechanosensitive ion channels by ultrasound,” Ultrasound Med. Biol. 48, 1981–1994 (2022). 10.1016/j.ultrasmedbio.2022.06.008 [DOI] [PubMed] [Google Scholar]
- 166. Qiu Z., Guo J., Kala S., Zhu J., Xian Q., Qiu W., Li G., Zhu T., Meng L., Zhang R. et al. , “ The mechanosensitive ion channel piezo1 significantly mediates in vitro ultrasonic stimulation of neurons,” iScience 21, 448–457 (2019). 10.1016/j.isci.2019.10.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Tsimbouri P. M., Childs P. G., Pemberton G. D., Yang J., Jayawarna V., Orapiriyakul W., Burgess K., González-García C., Blackburn G., Thomas D. et al. , “ Stimulation of 3D osteogenesis by mesenchymal stem cells using a nanovibrational bioreactor,” Nat. Biomed. Eng. 1, 758–770 (2017). 10.1038/s41551-017-0127-4 [DOI] [PubMed] [Google Scholar]
- 168. Zhou S., Schmelz A., Seufferlein T., Li Y., Zhao J., and Bachem M. G., “ Molecular mechanisms of low intensity pulsed ultrasound in human skin fibroblasts,” J. Biol. Chem. 279, 54463–54469 (2004). 10.1074/jbc.M404786200 [DOI] [PubMed] [Google Scholar]
- 169. Tang C. H., Yang R. S., Huang T. H., Lu D. Y., Chuang W. J., Huang T. F., and Fu W. M., “ Ultrasound stimulates cyclooxygenase–2 expression and increases bone formation through integrin, focal adhesion kinase, phosphatidylinositol 3–kinase, and Akt pathway in osteoblasts,” Mol. Pharmacol. 69, 2047–2057 (2006). 10.1124/mol.105.022160 [DOI] [PubMed] [Google Scholar]
- 170. Whitney N. P., Lamb A. C., Louw T. M., and Subramanian A., “ Integrin-mediated mechanotransduction pathway of low-intensity continuous ultrasound in human chondrocytes,” Ultrasound Med. Biol. 38, 1734–1743 (2012). 10.1016/j.ultrasmedbio.2012.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Wu C., “ Focal adhesion,” Cell Adh. Migr 1, 13–18 (2007). 10.4161/cam.4081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Xiao W., Xu Q., Zhu Z., Li L., and Chen W., “ Different performances of CXCR4, integrin-1β and CCR-2 in bone marrow stromal cells (BMSCs) migration by low-intensity pulsed ultrasound stimulation,” Biomed. Tech. 62, 89–95 (2017). 10.1515/bmt-2015-0166 [DOI] [PubMed] [Google Scholar]
- 173. VanBavel E., “ Effects of shear stress on endothelial cells: Possible relevance for ultrasound applications,” Prog. Biophys. Mol. Biol. 93, 374–383 (2007). 10.1016/j.pbiomolbio.2006.07.017 [DOI] [PubMed] [Google Scholar]
- 174. Basta G., Venneri L., Lazzerini G., Pasanisi E., Pianelli M., Vesentini N., Del Turco S., Kusmic C., and Picano E., “ In vitro modulation of intracellular oxidative stress of endothelial cells by diagnostic cardiac ultrasound,” Cardiovasc. Res. 58, 156–161 (2003). 10.1016/S0008-6363(02)00665-X [DOI] [PubMed] [Google Scholar]
- 175. Samandari M., Abrinia K., Mokhtari-Dizaji M., and Tamayol A., “ Ultrasound induced strain cytoskeleton rearrangement: An experimental and simulation study,” J. Biomech. 60, 39–47 (2017). 10.1016/j.jbiomech.2017.06.003 [DOI] [PubMed] [Google Scholar]
- 176. Rwei P., Gong C.-S. A., Luo L.-J., Lin M.-B., Lai J.-Y., and Liu H.-L., “ In vitro investigation of ultrasound-induced oxidative stress on human lens epithelial cells,” Biochem. Biophys. Res. Commun. 482, 954–960 (2017). 10.1016/j.bbrc.2016.11.139 [DOI] [PubMed] [Google Scholar]
- 177. Carina V., Costa V., Raimondi L., Pagani S., Sartori M., Figallo E., Setti S., Alessandro R., Fini M., and Giavaresi G., “ Effect of low-intensity pulsed ultrasound on osteogenic human mesenchymal stem cells commitment in a new bone scaffold,” J. Appl. Biomater. Funct. Mater. 15, e215–e222 (2017). 10.5301/jabfm.5000342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Yi W., Chen Q., Liu C., Li K., Tao B., Tian G., Zhou L., Li X., Shen J., Liu B. et al. , “ LIPUS inhibits inflammation and catabolism through the pathway in human degenerative nucleus pulposus cells,” J. Orthop. Surg. Res. 16, 619 (2021). 10.1186/s13018-021-02739-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Truong T.-T., Chiu W.-T., Lai Y.-S., Huang H., Jiang X., and Huang C.-C., “ Ca2+ signaling-mediated low-intensity pulsed ultrasound–induced proliferation and activation of motor neuron cells,” Ultrasonics 124, 106739 (2022). 10.1016/j.ultras.2022.106739 [DOI] [PubMed] [Google Scholar]
- 180. Li F., Liu Y., Cai Y., Li X., Bai M., Sun T., and Du L., “ Ultrasound irradiation combined with hepatocyte growth factor accelerate the hepatic differentiation of human bone marrow mesenchymal stem cells,” Ultrasound Med. Biol. 44, 1044–1052 (2018). 10.1016/j.ultrasmedbio.2018.01.005 [DOI] [PubMed] [Google Scholar]
- 181. Puts R., Rikeit P., Ruschke K., Knaus P., Schreivogel S., and Raum K., “ Functional regulation of YAP mechanosensitive transcriptional coactivator by focused low-intensity pulsed ultrasound (FLIPUS) enhances proliferation of murine mesenchymal precursors,” PLoS One 13, e0206041 (2018). 10.1371/journal.pone.0206041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Reher P., Doan N., Bradnock B., Meghji S., and Harris M., “ Effect of ultrasound on the production of IL-8, basic FGF and VEGF,” Cytokine 11, 416–423 (1999). 10.1006/cyto.1998.0444 [DOI] [PubMed] [Google Scholar]
- 183. Mizrahi N., Seliktar D., and Kimmel E., “ Ultrasound-induced angiogenic response in endothelial cells,” Ultrasound Med. Biol. 33, 1818–1829 (2007). 10.1016/j.ultrasmedbio.2007.05.007 [DOI] [PubMed] [Google Scholar]
- 184. Budhiraja G., Sahu N., and Subramanian A., “ Low-intensity ultrasound upregulates the expression of cyclin-D1 and promotes cellular proliferation in human mesenchymal stem cells,” Biotechnol. J. 13, e1700382 (2018). 10.1002/biot.201700382 [DOI] [PubMed] [Google Scholar]
- 185. Wei F.-Y., Leung K.-S., Li G., Qin J., Chow S. K.-H., Huang S., Sun M.-H., Qin L., and Cheung W.-H., “ Low intensity pulsed ultrasound enhanced mesenchymal stem cell recruitment through stromal derived factor–1 signaling in fracture healing,” PLoS One 9, e106722 (2014). 10.1371/journal.pone.0106722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Kusuyama J., Seong C., Makarewicz N. S., Ohnishi T., Shima K., Semba I., Bandow K., and Matsuguchi T., “ Low intensity pulsed ultrasound (LIPUS) maintains osteogenic potency by the increased expression and stability of Nanog through spleen tyrosine kinase (Syk) activation,” Cell Signal 62, 109345 (2019). 10.1016/j.cellsig.2019.109345 [DOI] [PubMed] [Google Scholar]
- 187. Lai C. H., Chen S. C., Chiu L. H., Yang C. B., Tsai Y. H., Zuo C. S., Chang W. H., and Lai W. F., “ Effects of low-intensity pulsed ultrasound, dexamethasone/TGF-β1 and/or BMP-2 on the transcriptional expression of genes in human mesenchymal stem cells: Chondrogenic vs. osteogenic differentiation,” Ultrasound Med. Biol. 36, 1022–1033 (2010). 10.1016/j.ultrasmedbio.2010.03.014 [DOI] [PubMed] [Google Scholar]
- 188. Lee H. J., Choi B. H., Min B. H., Son Y. S., and Park S. R., “ Low-intensity ultrasound stimulation enhances chondrogenic differentiation in alginate culture of mesenchymal stem cells,” Artif. Organs 30, 707–715 (2006). 10.1111/j.1525-1594.2006.00288.x [DOI] [PubMed] [Google Scholar]
- 189. Cho S. E., Kim Y. M., Jeong J. S., and Seo Y. K., “ The effect of ultrasound for increasing neural differentiation in hBM-MSCs and inducing neurogenesis in ischemic stroke model,” Life Sci. 165, 35–42 (2016). 10.1016/j.lfs.2016.08.029 [DOI] [PubMed] [Google Scholar]
- 190. Ning G.-Z., Song W.-Y., Xu H., Zhu R.-S., Wu Q.-L., Wu Y., Zhu S.-B., Li J.-Q., Wang M., Qu Z.-G. et al. , “ Bone marrow mesenchymal stem cells stimulated with low-intensity pulsed ultrasound: Better choice of transplantation treatment for spinal cord injury,” CNS Neurosci. Ther. 25, 496–508 (2019). 10.1111/cns.13071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Zhao Q., Lu Y., Yu H., and Gan X., “ Low magnitude high frequency vibration promotes adipogenic differentiation of bone marrow stem cells via P38 MAPK signal,” PLoS One 12, e0172954 (2017). 10.1371/journal.pone.0172954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Orapiriyakul W., Tsimbouri M. P., Childs P., Campsie P., Wells J., Fernandez-Yague M. A., Burgess K., Tanner K. E., Tassieri M., Meek D. et al. , “ Nanovibrational stimulation of mesenchymal stem cells induces therapeutic reactive oxygen species and inflammation for three-dimensional bone tissue engineering,” ACS Nano 14, 10027–10044 (2020). 10.1021/acsnano.0c03130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Nikukar H., Reid S., Tsimbouri P. M., Riehle M. O., Curtis A. S., and Dalby M. J., “ Osteogenesis of mesenchymal stem cells by nanoscale mechanotransduction,” ACS Nano 7, 2758–2767 (2013). 10.1021/nn400202j [DOI] [PubMed] [Google Scholar]
- 194. Hodgkinson T., Tsimbouri P. M., Llopis-Hernandez V., Campsie P., Scurr D., Childs P. G., Phillips D., Donnelly S., Wells J. A., O'Brien F. J. et al. , “ The use of nanovibration to discover specific and potent bioactive metabolites that stimulate osteogenic differentiation in mesenchymal stem cells,” Sci. Adv. 7, eabb7921 (2021). 10.1126/sciadv.abb7921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Pemberton G. D., Childs P., Reid S., Nikukar H., Tsimbouri P. M., Gadegaard N., Curtis A. S., and Dalby M. J., “ Nanoscale stimulation of osteoblastogenesis from mesenchymal stem cells: Nanotopography and nanokicking,” Nanomedicine 10, 547–560 (2015). 10.2217/nnm.14.134 [DOI] [PubMed] [Google Scholar]
- 196. Liu X., Wang B., Ding H., Shi H., Liu J., and Sun H., “ Low-intensity pulsed ultrasound in combination with SonoVue induces cytotoxicity of human renal glomerular endothelial cells via repression of the ERK1/2 signaling pathway,” Renal Failure 40, 458–465 (2018). 10.1080/0886022X.2018.1487868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Zhou X.-Y., Wu S.-Y., Zhang Z.-C., Wang F., Yang Y.-L., Li M., and Wei X.-Z., “ Low-intensity pulsed ultrasound promotes endothelial cell-mediated osteogenesis in a conditioned medium coculture system with osteoblasts,” Medicine 96, e8397 (2017). 10.1097/MD.0000000000008397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Rezk A. R., Ahmed H., Brain T. L., Castro J. O., Tan M. K., Langley J., Cox N., Mondal J., Li W., Ashokkumar M. et al. , “ Free radical generation from high-frequency electromechanical dissociation of pure water,” J. Phys. Chem. Lett. 11, 4655–4661 (2020). 10.1021/acs.jpclett.0c01227 [DOI] [PubMed] [Google Scholar]
- 199. Ramesan S., Rezk A. R., Dekiwadia C., Cortez-Jugo C., and Yeo L. Y., “ Acoustically-mediated intracellular delivery,” Nanoscale 10, 13165–13178 (2018). 10.1039/C8NR02898B [DOI] [PubMed] [Google Scholar]
- 200. Liao D., Li F., Lu D., and Zhong P., “ Activation of Piezo1 mechanosensitive ion channel in HEK293T cells by 30 MHz vertically deployed surface acoustic waves,” Biochem. Biophys. Res. Commun. 518, 541–547 (2019). 10.1016/j.bbrc.2019.08.078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Brugger M. S., Baumgartner K., Mauritz S. C. F., Gerlach S. C., Roder F., Schlosser C., Fluhrer R., Wixforth A., and Westerhausen C., “ Vibration enhanced cell growth induced by surface acoustic waves as in vitro wound–healing model,” Proc. Natl. Acad. Sci. U. S. A. 117, 31603–31613 (2020). 10.1073/pnas.2005203117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Prieto M. L., Firouzi K., Khuri-Yakub B. T., and Maduke M., “ Activation of Piezo1 but not NaV1.2 Channels by ultrasound at 43 MHz,” Ultrasound Med. Biol 44, 1217–1232 (2018). 10.1016/j.ultrasmedbio.2017.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Weitz A. C., Lee N. S., Yoon C. W., Bonyad A., Goo K. S., Kim S., Moon S., Jung H., Zhou Q., Chow R. H. et al. , “ Functional assay of cancer cell invasion potential based on mechanotransduction of focused ultrasound,” Front. Oncol. 7, 161 (2017). 10.3389/fonc.2017.00161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Choe S. W. and Choi H., “ Suppression technique of HeLa cell proliferation using ultrasonic power amplifiers integrated with a series-diode linearizer,” Sensors 18, 4248 (2018). 10.3390/s18124248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Choi H., Ryu J.-M., and woon Choe S., “ A novel therapeutic instrument using an ultrasound-light-emitting diode with an adjustable telephoto lens for suppression of tumor cell proliferation,” Measurement 147, 106865 (2019). 10.1016/j.measurement.2019.106865 [DOI] [Google Scholar]
- 206. Figarol A., Olive L., Joubert O., Ferrari L., Rihn B. H., Sarry F., and Beyssen D., “ Biological effects and applications of bulk and surface acoustic waves on in vitro cultured mammal cells: New insights,” Biomedicines 10, 1166 (2022). 10.3390/biomedicines10051166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Pan Y., Yoon S., Zhu L., and Wang Y., “ Acoustic mechanogenetics,” Curr. Opin. Biomed. Eng. 7, 64–70 (2018). 10.1016/j.cobme.2018.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Du M., Li Y., Zhang Q., Zhang J., Ouyang S., and Chen Z., “ The impact of low intensity ultrasound on cells: Underlying mechanisms and current status,” Prog. Biophys. Mol. 174, 41–49 (2022). 10.1016/j.pbiomolbio.2022.06.004 [DOI] [PubMed] [Google Scholar]
- 209. Devendran C., Carthew J., Frith J. E., and Neild A., “ Cell adhesion, morphology, and metabolism variation via acoustic exposure within microfluidic cell handling systems,” Adv. Sci. 6, 1902326 (2019). 10.1002/advs.201902326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Coussios C. C. and Roy R. A., “ Applications of acoustics and cavitation to noninvasive therapy and drug delivery,” Annu. Rev. Fluid Mech. 40, 395–420 (2008). 10.1146/annurev.fluid.40.111406.102116 [DOI] [Google Scholar]
- 211. Sitta J. and Howard C. M., “ Applications of ultrasound-mediated drug delivery and gene therapy,” Int. J. Mol. Sci. 22, 11491 (2021). 10.3390/ijms222111491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Rich J., Tian Z., and Huang T. J., “ Sonoporation: Past, present, and future,” Adv. Mater. Technol. 7, 2100885 (2022). 10.1002/admt.202100885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Yoon S., Kim M. G., Chiu C. T., Hwang J. Y., Kim H. H., Wang Y., and Shung K. K., “ Direct and sustained intracellular delivery of exogenous molecules using acoustic-transfection with high frequency ultrasound,” Sci. Rep. 6, 20477 (2016). 10.1038/srep20477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Yoon S., Wang P., Peng Q., Wang Y., and Shung K. K., “ Acoustic-transfection for genomic manipulation of single-cells using high frequency ultrasound,” Sci. Rep. 7, 5275 (2017). 10.1038/s41598-017-05722-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Ramesan S., Rezk A. R., Cevaal P. M., Cortez-Jugo C., Symons J., and Yeo L. Y., “ Acoustofection: High-frequency vibrational membrane permeabilization for intracellular siRNA delivery into nonadherent cells,” ACS Appl. Bio Mater. 4, 2781–2789 (2021). 10.1021/acsabm.1c00003 [DOI] [PubMed] [Google Scholar]
- 216. Kamenac A., Schilberth F. L., Wagner E., Wixforth A., Lächelt U., and Westerhausen C., “ Transient permeabilization of living cells: Combining shear flow and acoustofluidic trapping for the facilitated uptake of molecules,” Processes 9, 913 (2021). 10.3390/pr9060913 [DOI] [Google Scholar]
- 217. Sheng R., Jiang Y., Backman L. J., Zhang W., and Chen J., “ The application of mechanical stimulations in tendon tissue engineering,” Stem Cells Int. 2020, 8824783. 10.1155/2020/8824783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Belly H. D., Paluch E. K., and Chalut K. J., “ Interplay between mechanics and signalling in regulating cell fate,” Nat. Rev. Mol. Cell Biol. 23, 465–480 (2022). 10.1038/s41580-022-00472-z [DOI] [PubMed] [Google Scholar]
- 219. Liang W., Wu X., Dong Y., Chen X., Zhou P., and Xu F., “ Mechanical stimuli-mediated modulation of bone cell function—Implications for bone remodeling and angiogenesis,” Cell Tissue Res 386, 445–454 (2021). 10.1007/s00441-021-03532-6 [DOI] [PubMed] [Google Scholar]
- 220. Guex A. G., Di Marzio N., Eglin D., Alini M., and Serra T., “ The waves that make the pattern: A review on acoustic manipulation in biomedical research,” Mater. Today Bio 10, 100110 (2021). 10.1016/j.mtbio.2021.100110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Cai H., Ao Z., Hu L., Moon Y., Wu Z., Lu H.-C., Kim J., and Guo F., “ Acoustofluidic assembly of 3D neurospheroids to model Alzheimer's disease,” Analyst 145, 6243–6253 (2020). 10.1039/D0AN01373K [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Jiang D., Liu J., Pan Y., Zhuang L., and Wang P., “ Surface acoustic wave (SAW) techniques in tissue engineering,” Cell Tissue Res. 386, 215–226 (2021). 10.1007/s00441-020-03397-1 [DOI] [PubMed] [Google Scholar]
- 223. Destgeer G. and Sung H. J., “ Recent advances in microfluidic actuation and micro-object manipulation via surface acoustic waves,” Lab Chip 15, 2722–2738 (2015). 10.1039/C5LC00265F [DOI] [PubMed] [Google Scholar]
- 224. Borlongan C. V., “ Concise review: Stem cell therapy for stroke patients: Are we there yet? Stem Cells,” Transl. Med 8, 983–988 (2019). 10.1002/sctm.19-0076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Jin Y. Z. and Lee J. H., “ Mesenchymal stem cell therapy for bone regeneration,” Clin. Orthop. Surg. 10, 271–278 (2018). 10.4055/cios.2018.10.3.271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Shahdadfar A., Fronsdal K., Haug T., Reinholt F. P., and Brinchmann J. E., “ In vitro expansion of human mesenchymal stem cells: Choice of serum is a determinant of cell proliferation, differentiation, gene expression, and transcriptome stability,” Stem Cells 23, 1357–1366 (2005). 10.1634/stemcells.2005-0094 [DOI] [PubMed] [Google Scholar]
- 227. Schuster A., Schwab T., Bischof M., Klotz M., Lemor R., Degel C., and Schafer K. H., “ Cell specific ultrasound effects are dose and frequency dependent,” Ann. Anat 195, 57–67 (2013). 10.1016/j.aanat.2012.03.008 [DOI] [PubMed] [Google Scholar]
- 228. Salgarella A. R., Cafarelli A., Ricotti L., Capineri L., Dario P., and Menciassi A., “ Optimal ultrasound exposure conditions for maximizing C2C12 muscle cell proliferation and differentiation,” Ultrasound Med. Biol. 43, 1452–1465 (2017). 10.1016/j.ultrasmedbio.2017.03.003 [DOI] [PubMed] [Google Scholar]
- 229. Raz D., Zaretsky U., Einav S., and Elad D., “ Cellular alterations in cultured endothelial cells exposed to therapeutic ultrasound irradiation,” Endothelium 12, 201–213 (2005). 10.1080/10623320500227317 [DOI] [PubMed] [Google Scholar]
- 230. Imashiro C., Kang B., Lee Y., Hwang Y. H., Im S., Kim D. E., Takemura K., and Lee H., “ Propagating acoustic waves on a culture substrate regulate the directional collective cell migration,” Microsyst. Nanoeng. 7, 90 (2021). 10.1038/s41378-021-00304-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Sharma R. V., Chapleau M. W., Hajduczok G., Wachtel R. E., Waite L. J., Bhalla R. C., and Abboud F. M., “ Mechanical stimulation increases intracellular calcium concentration in nodose sensory neurons,” Neuroscience 66, 433–441 (1995). 10.1016/0306-4522(94)00560-R [DOI] [PubMed] [Google Scholar]
- 232. Doan N., Reher P., Meghji S., and Harris M., “ In vitro effects of therapeutic ultrasound on cell proliferation, protein synthesis, and cytokine production by human fibroblasts, osteoblasts, and monocytes,” J. Oral Maxillofac. Surg. 57, 409–420 (1999). 10.1016/S0278-2391(99)90281-1 [DOI] [PubMed] [Google Scholar]
- 233. Xie S., Jiang X., Wang R., Xie S., Hua Y., Zhou S., Yang Y., and Zhang J., “ Low-intensity pulsed ultrasound promotes the proliferation of human bone mesenchymal stem cells by activating PI3K/AKt signaling pathways,” J. Cell Biochem. 120, 15823–15833 (2019). 10.1002/jcb.28853 [DOI] [PubMed] [Google Scholar]
- 234. Ren C., Chen X., Du N., Geng S., Hu Y., Liu X., Wu X., Lin Y., Bai X., Yin W. et al. , “ Low-intensity pulsed ultrasound promotes Schwann cell viability and proliferation via the GSK-3β/β-catenin signaling pathway,” Int. J. Biol. Sci. 14, 497–507 (2018). 10.7150/ijbs.22409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Lee W., Yoo S., Jung J., Kang W., Wang W., Moon C., and Choi H., “ All-in-one low-intensity pulsed ultrasound stimulation system using piezoelectric micromachined ultrasonic transducer (pMUT) arrays for targeted cell stimulation,” Biomed. Microdev. 19, 86 (2017). 10.1007/s10544-017-0228-6 [DOI] [PubMed] [Google Scholar]
- 236. Kobayashi Y., Sakai D., Iwashina T., Iwabuchi S., and Mochida J., “ Low-intensity pulsed ultrasound stimulates cell proliferation, proteoglycan synthesis and expression of growth factor-related genes in human nucleus pulposus cell,” Eur. Cell Mater. 17, 15–22 (2009). 10.22203/eCM.v017a02 [DOI] [PubMed] [Google Scholar]
- 237. Chan Y. S., Hsu K. Y., Kuo C. H., Lee S. D., Chen S. C., Chen W. J., and Ueng S. W., “ Using low-intensity pulsed ultrasound to improve muscle healing after laceration injury: An in vitro and in vivo study,” Ultrasound Med. Biol. 36, 743–751 (2010). 10.1016/j.ultrasmedbio.2010.02.010 [DOI] [PubMed] [Google Scholar]
- 238. Nakamura T., Fujihara S., Yamamoto-Nagata K., Katsura T., Inubushi T., and Tanaka E., “ Low-intensity pulsed ultrasound reduces the inflammatory activity of synovitis,” Ann. Biomed. Eng. 39, 2964–2971 (2011). 10.1007/s10439-011-0408-0 [DOI] [PubMed] [Google Scholar]
- 239. Greco G., Agostini M., Tonazzini I., Sallemi D., Barone S., and Cecchini M., “ Surface-acoustic-wave (SAW)-driven device for dynamic cell cultures,” Anal. Chem. 90, 7450–7457 (2018). 10.1021/acs.analchem.8b00972 [DOI] [PubMed] [Google Scholar]
- 240. Stamp M. E. M., Brugger M. S., Wixforth A., and Westerhausen C., “ Acoustotaxis—In vitro stimulation in a wound healing assay employing surface acoustic waves,” Biomater. Sci. 4, 1092–1099 (2016). 10.1039/C6BM00125D [DOI] [PubMed] [Google Scholar]
- 241. Seetharaman S. and Etienne-Manneville S., “ Cytoskeletal crosstalk in cell migration,” Trends Cell Biol. 30, 720–735 (2020). 10.1016/j.tcb.2020.06.004 [DOI] [PubMed] [Google Scholar]
- 242. Merino-Casallo F., Gomez-Benito M. J., Hervas-Raluy S., and Garcia-Aznar J. M., “ Unravelling cell migration: Defining movement from the cell surface,” Cell Adh. Migr. 16, 25–64 (2022). 10.1080/19336918.2022.2055520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. de Lucas B., Perez L. M., and Galvez B. G., “ Importance and regulation of adult stem cell migration,” J. Cell Mol. Med. 22, 746–754 (2018). 10.1111/jcmm.13422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Smart N. and Riley P. R., “ The stem cell movement,” Circ. Res. 102, 1155–1168 (2008). 10.1161/CIRCRESAHA.108.175158 [DOI] [PubMed] [Google Scholar]
- 245. Zhang Y., Yan J., Xu H., Yang Y., Li W., Wu H., and Liu C., “ Extremely low frequency electromagnetic fields promote mesenchymal stem cell migration by increasing intracellular Ca2+ and activating the FAK/Rho GTPases signaling pathways in vitro,” Stem Cell Res. Ther. 9, 143 (2018). 10.1186/s13287-018-0883-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Alter A., Rozenszajn L. A., Miller H. I., and Rosenschein U., “ Ultrasound inhibits the adhesion and migration of smooth muscle cells in vitro,” Ultrasound Med. Biol. 24, 711–721 (1998). 10.1016/S0301-5629(98)00030-1 [DOI] [PubMed] [Google Scholar]
- 247. Enomoto U., Imashiro C., and Takemura K., “ Collective cell migration of fibroblasts is affected by horizontal vibration of the cell culture dish,” Eng. Life Sci. 20, 402–411 (2020). 10.1002/elsc.202000013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Man J., Shelton R. M., Cooper P. R., and Scheven B. A., “ Low-intensity low-frequency ultrasound promotes proliferation and differentiation of odontoblast-like cells,” J. Endod. 38, 608–613 (2012). 10.1016/j.joen.2012.01.015 [DOI] [PubMed] [Google Scholar]
- 249. Man J., Shelton R. M., Cooper P. R., Landini G., and Scheven B. A., “ Low intensity ultrasound stimulates osteoblast migration at different frequencies,” J. Bone Miner. Metab. 30, 602–607 (2012). 10.1007/s00774-012-0368-y [DOI] [PubMed] [Google Scholar]
- 250. Kuo J. C., “ Mechanotransduction at focal adhesions: Integrating cytoskeletal mechanics in migrating cells,” J. Cell Mol. Med. 17, 704–712 (2013). 10.1111/jcmm.12054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Atherton P., Lausecker F., Harrison A., and Ballestrem C., “ Low-intensity pulsed ultrasound promotes cell motility through vinculin-controlled Rac1 GTPase activity,” J. Cell Sci. 130, 2277–2291 (2017). 10.1242/jcs.192781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Leng X., Shang J., Gao D., and Wu J., “ Low-intensity pulsed ultrasound promotes proliferation and migration of HaCaT keratinocytes through the PI3K/AKT and JNK pathways,” Braz. J. Med. Biol. Res. 51, e7862 (2018). 10.1590/1414-431x20187862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Beckingham L. J., Todorovic M., Velasquez J. T., Vial M.-L., Chen M., Ekberg J. A. K., and John J. A. S., “ Three-dimensional cell culture can be regulated by vibration: Low-frequency vibration increases the size of olfactory ensheathing cell spheroids,” J. Biol. Eng. 13, 41 (2019). 10.1186/s13036-019-0176-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Uddin S. M., Richbourgh B., Ding Y., Hettinghouse A., Komatsu D. E., Qin Y. X., and Liu C. J., “ Chondro–protective effects of low intensity pulsed ultrasound,” Osteoarthritis Cartilage 24, 1989–1998 (2016). 10.1016/j.joca.2016.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Sawai Y., Murata H., Koto K., Matsui T., Horie N., Ashihara E., Maekawa T., Fushiki S., and Kubo T., “ Effects of low-intensity pulsed ultrasound on osteosarcoma and cancer cells,” Oncol. Rep. 28, 481–486 (2012). 10.3892/or.2012.1816 [DOI] [PubMed] [Google Scholar]
- 256. Li Y. Wang, J., Qiu Y., Hu B., Chen J., Fu T., Zhou P., and Song J., “ Low intensity pulsed ultrasound promotes periodontal ligament stem cell migration through TWIST1 mediated SDF 1 expression,” Int. J. Mol. Med. 42, 322–330 (2018). 10.3892/ijmm.2018.3592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Hill G. E., Fenwick S., Matthews B. J., Chivers R. A., and Southgate J., “ The effect of low-intensity pulsed ultrasound on repair of epithelial cell monolayers in vitro,” Ultrasound Med. Biol. 31, 1701–1706 (2005). 10.1016/j.ultrasmedbio.2005.08.001 [DOI] [PubMed] [Google Scholar]
- 258. Xia P., Wang X., Wang Q., Wang X., Lin Q., Cheng K., and Li X., “ Low-intensity pulsed ultrasound promotes autophagy-mediated migration of mesenchymal stem cells and cartilage repair,” Cell Transplant. 30, 0963689720986142 (2021). 10.1177/0963689720986142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Li J., Guo W., Yu F., Liu L., Wang X., Li L., Fang B., and Xia L., “ Low-intensity pulsed ultrasound promotes angiogenesis via the AKT pathway and DNA methylation in human umbilical vein endothelial cells,” Ultrasonics 118, 106561 (2022). 10.1016/j.ultras.2021.106561 [DOI] [PubMed] [Google Scholar]
- 260. Lamalice L., Boeuf F. L., and Huot J., “ Endothelial cell migration during angiogenesis,” Circ. Res. 100, 782–794 (2007). 10.1161/01.RES.0000259593.07661.1e [DOI] [PubMed] [Google Scholar]
- 261. Sharifpanah F., Behr S., Wartenberg M., and Sauer H., “ Mechanical strain stimulates vasculogenesis and expression of angiogenesis guidance molecules of embryonic stem cells through elevation of intracellular calcium, reactive oxygen species and nitric oxide generation,” Biochim. Biophys. Acta 1863, 3096–3105 (2016). 10.1016/j.bbamcr.2016.10.001 [DOI] [PubMed] [Google Scholar]
- 262. Hsu S. H. and Huang T. B., “ Bioeffect of ultrasound on endothelial cells in vitro,” Biomol. Eng. 21, 99–104 (2004). 10.1016/j.bioeng.2004.06.001 [DOI] [PubMed] [Google Scholar]
- 263. Manokawinchoke J., Pavasant P., Limjeerajarus C. N., Limjeerajarus N., Osathanon T., and Egusa H., “ Mechanical loading and the control of stem cell behavior,” Arch. Oral Biol. 125, 105092 (2021). 10.1016/j.archoralbio.2021.105092 [DOI] [PubMed] [Google Scholar]
- 264. Rayat Pisheh H., Ansari M., and Eslami H., “ How is mechanobiology involved in bone regenerative medicine?,” Tissue Cell 76, 101821 (2022). 10.1016/j.tice.2022.101821 [DOI] [PubMed] [Google Scholar]
- 265. Costa V., Carina V., Fontana S., De Luca A., Monteleone F., Pagani S., Sartori M., Setti S., Faldini C., Alessandro R. et al. , “ Osteogenic commitment and differentiation of human mesenchymal stem cells by low-intensity pulsed ultrasound stimulation,” J. Cell Physiol. 233, 1558–1573 (2018). 10.1002/jcp.26058 [DOI] [PubMed] [Google Scholar]
- 266. Kennedy J. W., Tsimbouri P. M., Campsie P., Sood S., Childs P. G., Reid S., Young P. S., Meek D. R. M., Goodyear C. S., and Dalby M. J., “ Nanovibrational stimulation inhibits osteoclastogenesis and enhances osteogenesis in co-cultures,” Sci. Rep. 11, 22741 (2021). 10.1038/s41598-021-02139-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Lee J., Abdeen A. A., and Kilian K. A., “ Rewiring mesenchymal stem cell lineage specification by switching the biophysical microenvironment,” Sci. Rep. 4, 5188 (2014). 10.1038/srep05188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Place E. S., Evans N. D., and Stevens M. M., “ Complexity in biomaterials for tissue engineering,” Nat. Mater. 8, 457–470 (2009). 10.1038/nmat2441 [DOI] [PubMed] [Google Scholar]
- 269. Griffith L. G. and Naughton G., “ Tissue engineering–current challenges and expanding opportunities,” Science 295, 1009–1014 (2002). 10.1126/science.1069210 [DOI] [PubMed] [Google Scholar]
- 270. Discher D. E., Mooney D. J., and Zandstra P. W., “ Growth factors, matrices, and forces combine and control stem cells,” Science 324, 1673–1677 (2009). 10.1126/science.1171643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Ooi H. W., Hafeez S., van Blitterswijk C. A., Moroni L., and Baker M. B., “ Hydrogels that listen to cells: A review of cell-responsive strategies in biomaterial design for tissue regeneration,” Mater. Horiz. 4, 1020–1040 (2017). 10.1039/C7MH00373K [DOI] [Google Scholar]
- 272. Xia H., Li X., Gao W., Fu X., Fang R. H., Zhang L., and Zhang K., “ Tissue repair and regeneration with endogenous stem cells,” Nat. Rev. Mater. 3, 174–193 (2018). 10.1038/s41578-018-0027-6 [DOI] [Google Scholar]
- 273. Donnelly H., Salmeron-Sanchez M., and Dalby M. J., “ Designing stem cell niches for differentiation and self-renewal,” J. R. Soc. Interface 15, 20180388 (2018). 10.1098/rsif.2018.0388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274. Wang L., Zheng F., Song R., Zhuang L., Yang M., Suo J., and Li L., “ Integrins in the regulation of mesenchymal stem cell differentiation by mechanical signals,” Stem Cell Rev. Rep. 18, 126–141 (2022). 10.1007/s12015-021-10260-5 [DOI] [PubMed] [Google Scholar]
- 275. Hu B., Zhang Y., Zhou J., Li J., Deng F., Wang Z., and Song J., “ Low-intensity pulsed ultrasound stimulation facilitates osteogenic differentiation of human periodontal ligament cells,” PLoS One 9, e95168 (2014). 10.1371/journal.pone.0095168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Xiao E., Yang H. Q., Gan Y. H., Duan D. H., He L. H., Guo Y., Wang S. Q., and Zhang Y., “ Brief reports: TRPM7 senses mechanical stimulation inducing osteogenesis in human bone marrow mesenchymal stem cells,” Stem Cells 33, 615–621 (2015). 10.1002/stem.1858 [DOI] [PubMed] [Google Scholar]
- 277. Sugimoto A., Miyazaki A., Kawarabayashi K., Shono M., Akazawa Y., Hasegawa T., Ueda-Yamaguchi K., Kitamura T., Yoshizaki K., Fukumoto S. et al. , “ Piezo type mechanosensitive ion channel component 1 functions as a regulator of the cell fate determination of mesenchymal stem cells,” Sci. Rep. 7, 17696 (2017). 10.1038/s41598-017-18089-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278. Hamidouche Z., Fromigue O., Ringe J., Haupl T., Vaudin P., Pages J. C., Srouji S., Livne E., and Marie P. J., “ Priming integrin alpha5 promotes human mesenchymal stromal cell osteoblast differentiation and osteogenesis,” Proc. Natl. Acad. Sci. U. S. A. 106, 18587–18591 (2009). 10.1073/pnas.0812334106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Decrock E., Vinken M., Bol M., D'Herde K., Rogiers V., Vandenabeele P., Krysko D. V., Bultynck G., and Leybaert L., “ Calcium and connexin-based intercellular communication, a deadly catch?,” Cell Calcium 50, 310–321 (2011). 10.1016/j.ceca.2011.05.007 [DOI] [PubMed] [Google Scholar]
- 280. Khatiwala C. B., Kim P. D., Peyton S. R., and Putnam A. J., “ ECM compliance regulates osteogenesis by influencing MAPK signaling downstream of RhoA and ROCK,” J. Bone Miner. Res. 24, 886–898 (2009). 10.1359/jbmr.081240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Hamidouche Z., Hay E., Vaudin P., Charbord P., Schule R., Marie P. J., and Fromigue O., “ FHL2 mediates dexamethasone-induced mesenchymal cell differentiation into osteoblasts by activating Wnt/beta-catenin signaling-dependent Runx2 expression,” FASEB J. 22, 3813–3822 (2008). 10.1096/fj.08-106302 [DOI] [PubMed] [Google Scholar]
- 282. Gaur T., Lengner C. J., Hovhannisyan H., Bhat R. A., Bodine P. V., Komm B. S., Javed A., van Wijnen A. J., Stein J. L., Stein G. S. et al. , “ Canonical WNT signaling promotes osteogenesis by directly stimulating Runx2 gene expression,” J. Biol. Chem. 280, 33132–33140 (2005). 10.1074/jbc.M500608200 [DOI] [PubMed] [Google Scholar]
- 283. Dhaka A., Viswanath V., and Patapoutian A., “ TRP ion channels and temperature sensation,” Annu. Rev. Neurosci. 29, 135–161 (2006). 10.1146/annurev.neuro.29.051605.112958 [DOI] [PubMed] [Google Scholar]
- 284. Elango J., Robinson J., Zhang J., Bao B., Ma N., de Val J., and Wu W., “ Collagen peptide upregulates osteoblastogenesis from bone marrow mesenchymal stem cells through MAPK– Runx2,” Cells 8, 446 (2019). 10.3390/cells8050446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285. Akhir H. M. and Teoh P. L., “ Collagen type I promotes osteogenic differentiation of amniotic membrane-derived mesenchymal stromal cells in basal and induction media,” Biosci. Rep. 40, BSR20201325 (2020). 10.1042/BSR20201325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286. Zhou Q., Lyu S., Bertrand A. A., Hu A. C., Chan C. H., Ren X., Dewey M. J., Tiffany A. S., Harley B. A. C., and Lee J. C., “ Stiffness of nanoparticulate mineralized collagen scaffolds triggers osteogenesis via mechanotransduction and canonical Wnt signaling,” Macromol. Biosci. 21, e2000370 (2021). 10.1002/mabi.202000370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287. Barlian A. and Vanya K., “ Nanotopography in directing osteogenic differentiation of mesenchymal stem cells: Potency and future perspective,” Future Sci. OA 8, FSO765 (2022). 10.2144/fsoa-2021-0097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288. Schroeder T. M., Jensen E. D., and Westendorf J. J., “ Runx2: A master organizer of gene transcription in developing and maturing osteoblasts,” Birth Defects Res., Part C 75, 213–225 (2005). 10.1002/bdrc.20043 [DOI] [PubMed] [Google Scholar]
- 289. Hassan M. Q., Tare R. S., Lee S. H., Mandeville M., Morasso M. I., Javed A., van Wijnen A. J., Stein J. L., Stein G. S., and Lian J. B., “ BMP2 commitment to the osteogenic lineage involves activation of Runx2 by DLX3 and a homeodomain transcriptional network,” J. Biol. Chem. 281, 40515–40526 (2006). 10.1074/jbc.M604508200 [DOI] [PubMed] [Google Scholar]
- 290. Sen B., Xie Z., Case N., Styner M., Rubin C. T., and Rubin J., “ Mechanical signal influence on mesenchymal stem cell fate is enhanced by incorporation of refractory periods into the loading regimen,” J. Biomech. 44, 593–599 (2011). 10.1016/j.jbiomech.2010.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291. Kaur H., Siraki A. G., Sharma M., Uludag H., Dederich D. N., Flood P., and El-Bialy T., “ Reactive oxygen species mediate therapeutic ultrasound-induced, mitogen-activated protein kinase activation in C28/I2 chondrocytes,” Ultrasound Med. Biol. 44, 2105–2114 (2018). 10.1016/j.ultrasmedbio.2018.05.025 [DOI] [PubMed] [Google Scholar]
- 292. Chen X., He F., Zhong D.-Y., and Luo Z.-P., “ Acoustic-frequency vibratory stimulation regulates the balance between osteogenesis and adipogenesis of human bone marrow-derived mesenchymal stem cells,” Biomed. Res. Int. 2015, 540731. 10.1155/2015/540731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Lau E., Lee W. D., Li J., Xiao A., Davies J. E., Wu Q., Wang L., and You L., “ Effect of low-magnitude, high-frequency vibration on osteogenic differentiation of rat mesenchymal stromal cells,” J. Orthop. Res. 29, 1075–1080 (2011). 10.1002/jor.21334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294. He R., Chen J., Jiang J., Liu B., Liang D., Zhou W., Chen W., and Wang Y., “ Synergies of accelerating differentiation of bone marrow mesenchymal stem cells induced by low intensity pulsed ultrasound, osteogenic and endothelial inductive agent,” Artif. Cells Nanomed. Biotechnol. 47, 673–683 (2019). 10.1080/21691401.2019.1576704 [DOI] [PubMed] [Google Scholar]
- 295. Chiu C.-Y., Tsai T.-L., R. Vanderby, Jr. , Bradica G., Lou S.-L., and Li W.-J., “ Osteoblastogenesis of mesenchymal stem cells in 3-D culture enhanced by low-intensity pulsed ultrasound through soluble receptor activator of nuclear factor kappa B ligand,” Ultrasound Med. Biol. 41, 1842–1852 (2015). 10.1016/j.ultrasmedbio.2015.03.017 [DOI] [PubMed] [Google Scholar]
- 296. Wang Y., Peng W., Liu X., Zhu M., Sun T., Peng Q., Zeng Y., Feng B., Zhi W., Weng J. et al. , “ Study of bilineage differentiation of human-bone-marrow-derived mesenchymal stem cells in oxidized sodium alginate/N-succinyl chitosan hydrogels and synergistic effects of RGD modification and low-intensity pulsed ultrasound,” Acta Biomater. 10, 2518–2528 (2014). 10.1016/j.actbio.2013.12.052 [DOI] [PubMed] [Google Scholar]
- 297. Uddin S. M. and Qin Y. X., “ Enhancement of osteogenic differentiation and proliferation in human mesenchymal stem cells by a modified low intensity ultrasound stimulation under simulated microgravity,” PLoS One 8, e73914 (2013). 10.1371/journal.pone.0073914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298. Lim K., Kim J., Seonwoo H., Park S. H., Choung P.-H., and Chung J. H., “ In vitro effects of low-intensity pulsed ultrasound stimulation on the osteogenic differentiation of human alveolar bone-derived mesenchymal stem cells for tooth tissue engineering,” Biomed Res. Int. 2013, 269724. 10.1155/2013/269724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299. Zhou X., Castro N. J., Zhu W., Cui H., Aliabouzar M., Sarkar K., and Zhang L. G., “ Improved human bone marrow mesenchymal stem cell osteogenesis in 3D bioprinted tissue scaffolds with low intensity pulsed ultrasound stimulation,” Sci. Rep. 6, 32876 (2016). 10.1038/srep32876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300. Kusuyama J., Bandow K., Shamoto M., Kakimoto K., Ohnishi T., and Matsuguchi T., “ Low intensity pulsed ultrasound (LIPUS) influences the multilineage differentiation of mesenchymal stem and progenitor cell lines through ROCK-Cot/Tpl2-MEK-ERK signaling pathway,” J. Biol. Chem. 289, 10330–10344 (2014). 10.1074/jbc.M113.546382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301. Sena K., Leven R. M., Mazhar K., Sumner D. R., and Virdi A. S., “ Early gene response to low-intensity pulsed ultrasound in rat osteoblastic cells,” Ultrasound Med. Biol. 31, 703–708 (2005). 10.1016/j.ultrasmedbio.2005.01.013 [DOI] [PubMed] [Google Scholar]
- 302. Zhang Z., Ma Y., Guo S., He Y., Bai G., and Zhang W., “ Low-intensity pulsed ultrasound stimulation facilitates in vitro osteogenic differentiation of human adipose-derived stem cells via up-regulation of heat shock protein (HSP)70, HSP90, and bone morphogenetic protein (BMP) signaling pathway,” Biosci. Rep. 38, BSR20180087 (2018). 10.1042/BSR20180087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303. Miyasaka M., Nakata H., Hao J., Kim Y. K., Kasugai S., and Kuroda S., “ Low-intensity pulsed ultrasound stimulation enhances heat-shock protein 90 and mineralized nodule formation in mouse calvaria-derived osteoblasts,” Tissue Eng., Part A 21, 2829–2839 (2015). 10.1089/ten.tea.2015.0234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304. Lv Y., Zhao P., Chen G., Sha Y., and Yang L., “ Effects of low-intensity pulsed ultrasound on cell viability, proliferation and neural differentiation of induced pluripotent stem cells-derived neural crest stem cells,” Biotechnol. Lett. 35, 2201–2212 (2013). 10.1007/s10529-013-1313-4 [DOI] [PubMed] [Google Scholar]
- 305. Wu Y., Gao Q., Zhu S., Wu Q., Zhu R., Zhong H., Xing C., Qu H., Wang D., Li B. et al. , “ Low-intensity pulsed ultrasound regulates proliferation and differentiation of neural stem cells through notch signaling pathway,” Biochem. Biophys. Res. Commun. 526, 793–798 (2020). 10.1016/j.bbrc.2020.03.142 [DOI] [PubMed] [Google Scholar]
- 306. Teo A., Morshedi A., Wang J. C., Zhou Y., and Lim M., “ Enhancement of cardiomyogenesis in murine stem cells by low-intensity ultrasound,” J. Ultrasound Med. 36, 1693–1706 (2017). 10.7863/ultra.16.12042 [DOI] [PubMed] [Google Scholar]
- 307. Aliabouzar M., Lee S. J., Zhou X., Zhang G. L., and Sarkar K., “ Effects of scaffold microstructure and low intensity pulsed ultrasound on chondrogenic differentiation of human mesenchymal stem cells,” Biotechnol. Bioeng. 115, 495–506 (2018). 10.1002/bit.26480 [DOI] [PubMed] [Google Scholar]
- 308. Aliabouzar M., Zhang L. G., and Sarkar K., “ Lipid coated microbubbles and low intensity pulsed ultrasound enhance chondrogenesis of human mesenchymal stem cells in 3D printed scaffolds,” Sci. Rep. 6, 37728 (2016). 10.1038/srep37728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309. El-Bialy T., Alhadlaq A., Wong B., and Kucharski C., “ Ultrasound effect on neural differentiation of gingival stem/progenitor cells,” Ann. Biomed. Eng. 42, 1406–1412 (2014). 10.1007/s10439-014-1013-9 [DOI] [PubMed] [Google Scholar]
- 310. Sun C., Dong Y., Wei J., Cai M., Liang D., Fu Y., Zhou Y., Sui Y., Wu F., Mikhaylov R. et al. , “ Acoustically accelerated neural differentiation of human embryonic stem cells,” Acta Biomater. 151, 333–345 (2022). 10.1016/j.actbio.2022.07.041 [DOI] [PubMed] [Google Scholar]
- 311. Wang X., Lin Q., Zhang T., Wang X., Cheng K., Gao M., Xia P., and Li X., “ Low-intensity pulsed ultrasound promotes chondrogenesis of mesenchymal stem cells via regulation of autophagy,” Stem Cell Res. Ther. 10, 41 (2019). 10.1186/s13287-019-1142-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312. Xia P., Wang X., Qu Y., Lin Q., Cheng K., Gao M., Ren S., Zhang T., and Li X., “ TGF- -induced chondrogenesis of bone marrow mesenchymal stem cells is promoted by low-intensity pulsed ultrasound through the integrin-mTOR signaling pathway,” Stem Cell Res. Ther. 8, 281 (2017). 10.1186/s13287-017-0733-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313. Nishida T., Kubota S., Aoyama E., Yamanaka N., Lyons K. M., and Takigawa M., “ Low-intensity pulsed ultrasound (LIPUS) treatment of cultured chondrocytes stimulates production of CCN family protein 2 (CCN2), a protein involved in the regeneration of articular cartilage: Mechanism underlying this stimulation,” Osteoarthr. Cartil. 25, 759–769 (2017). 10.1016/j.joca.2016.10.003 [DOI] [PubMed] [Google Scholar]
- 314. Juffermans L. J., Meijering B. D., Henning R. H., and Deelman L. E., “ Ultrasound and microbubble-targeted delivery of small interfering RNA into primary endothelial cells is more effective than delivery of plasmid DNA,” Ultrasound Med. Biol. 40, 532–540 (2014). 10.1016/j.ultrasmedbio.2013.09.031 [DOI] [PubMed] [Google Scholar]
- 315. Xia C. Y., Liu Y. H., Wang P., and Xue Y. X., “ Low-frequency ultrasound irradiation increases blood-tumor barrier permeability by transcellular pathway in a rat glioma model,” J. Mol. Neurosci. 48, 281–290 (2012). 10.1007/s12031-012-9770-0 [DOI] [PubMed] [Google Scholar]
- 316. Sheikov N., McDannold N., Sharma S., and Hynynen K., “ Effect of focused ultrasound applied with an ultrasound contrast agent on the tight junctional integrity of the brain microvascular endothelium,” Ultrasound Med. Biol. 34, 1093–1104 (2008). 10.1016/j.ultrasmedbio.2007.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317. Xie F., Boska M. D., Lof J., Uberti M. G., Tsutsui J. M., and Porter T. R., “ Effects of transcranial ultrasound and intravenous microbubbles on blood brain barrier permeability in a large animal model,” Ultrasound Med. Biol. 34, 2028–2034 (2008). 10.1016/j.ultrasmedbio.2008.05.004 [DOI] [PubMed] [Google Scholar]
- 318. Babiychuk E. B., Monastyrskaya K., Potez S., and Draeger A., “ Intracellular Ca2+ operates a switch between repair and lysis of streptolysin O-perforated cells,” Cell Death Differ. 16, 1126–1134 (2009). 10.1038/cdd.2009.30 [DOI] [PubMed] [Google Scholar]
- 319. Jimenez A. J., Maiuri P., Lafaurie-Janvore J., Divoux S., Piel M., and Perez F., “ ESCRT machinery is required for plasma membrane repair,” Science 343, 1247136 (2014). 10.1126/science.1247136 [DOI] [PubMed] [Google Scholar]
- 320. Thery C., Zitvogel L., and Amigorena S., “ Exosomes: Composition, biogenesis and function,” Nat. Rev. Immunol. 2, 569–579 (2002). 10.1038/nri855 [DOI] [PubMed] [Google Scholar]
- 321. Huotari J. and Helenius A., “ Endosome maturation,” EMBO J. 30, 3481–3500 (2011). 10.1038/emboj.2011.286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322. Miyoshi K., Sato K., and Yoshida M., “ In vitro development of cloned embryos derived from miniature pig somatic cells after activation by ultrasound stimulation,” Cloning Stem Cells 8, 159–165 (2006). 10.1089/clo.2006.8.159 [DOI] [PubMed] [Google Scholar]
- 323. Miyoshi K., Fujimoto Y., Mori H., and Yoshida M., “ Activation and parthenogenetic development of pig oocytes exposed to ultrasound in media containing different concentrations of Ca,” J. Reprod. Dev. 54, 42–45 (2008). 10.1262/jrd.19034 [DOI] [PubMed] [Google Scholar]
- 324. Zhou W., Wang J., Wang K., Huang B., Niu L., Li F., Cai F., Chen Y., Liu X., Zhang X. et al. , “ Ultrasound neuro-modulation chip: Activation of sensory neurons in Caenorhabditis elegans by surface acoustic waves,” Lab Chip 17, 1725–1731 (2017). 10.1039/C7LC00163K [DOI] [PubMed] [Google Scholar]
- 325. Gai J., Dervisevic E., Devendran C., Cadarso V. J., O'Bryan M. K., Nosrati R., and Neild A., “ High-frequency ultrasound boosts bull and human sperm motility,” Adv. Sci. 9, e2104362 (2022). 10.1002/advs.202104362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326. Duque M. et al. , “ Sonogenetic control of mammalian cells using exogenous Transient Receptor Potential A1 channels,” Nat. Commun. 13, 600 (2022). 10.1038/s41467-022-28205-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327. Kim S., Nam H., Cha B., Park J., Sung H. J., and Jeon J. S., “ Acoustofluidic stimulation of functional immune cells in a microreactor,” Adv. Sci. 9, 2105809 (2022). 10.1002/advs.202105809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328. Isachenko E., Maettner R., Isachenko V., Roth S., Kreienberg R., and Sterzik K., “ Mechanical agitation during the in vitro culture of human pre-implantation embryos drastically increases the pregnancy rate,” Clin. Lab 56, 569–576 (2010). [PubMed] [Google Scholar]
- 329. Sato K., Yoshida M., and Miyoshi K., “ Utility of ultrasound stimulation for activation of pig oocytes matured in vitro,” Mol. Reprod. Dev. 72, 396–403 (2005). 10.1002/mrd.20352 [DOI] [PubMed] [Google Scholar]
- 330. Sun B. and Yeh J., “ Calcium oscillatory patterns and oocyte activation during fertilization: A possible mechanism for total fertilization failure (TFF) in human in vitro fertilization?,” Reprod. Sci. 28, 639–648 (2021). 10.1007/s43032-020-00293-5 [DOI] [PubMed] [Google Scholar]
- 331. Lindemann C. B. and Kanous K. S., “ Regulation of mammalian sperm motility,” Arch. Androl. 23, 1–22 (1989). 10.3109/01485018908986783 [DOI] [PubMed] [Google Scholar]
- 332. Torres-Flores V., Picazo-Juarez G., Hernandez-Rueda Y., Darszon A., and Gonzalez-Martinez M. T., “ Sodium influx induced by external calcium chelation decreases human sperm motility,” Hum. Reprod. 26, 2626–2635 (2011). 10.1093/humrep/der237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333. Li X., Wang L., Li Y., Zhao N., Zhen L., Fu J., and Yang Q., “ Calcium regulates motility and protein phosphorylation by changing cAMP and ATP concentrations in boar sperm in vitro,” Anim. Reprod. Sci. 172, 39–51 (2016). 10.1016/j.anireprosci.2016.07.001 [DOI] [PubMed] [Google Scholar]
- 334. Krames E., Peckham P., Rezai A., and Aboelsaad F., Neuromodulation ( Academic Press: London, 2009), Vol. 1, pp. 3–8. [Google Scholar]
- 335. Ibsen S., Tong A., Schutt C., Esener S., and Chalasani S. H., “ Sonogenetics is a non–invasive approach to activating neurons in Caenorhabditis elegans,” Nat. Commun. 6, 8264 (2015). 10.1038/ncomms9264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336. Ye J., Tang S., Meng L., Li X., Wen X., Chen S., Niu L., Li X., Qiu W., Hu H. et al. , “ Ultrasonic control of neural activity through activation of the mechanosensitive channel MscL,” Nano Lett. 18, 4148–4155 (2018). 10.1021/acs.nanolett.8b00935 [DOI] [PubMed] [Google Scholar]
- 337. Maresca D., Lakshmanan A., Abedi M., Bar-Zion A., Farhadi A., Lu G. J., Szablowski J. O., Wu D., Yoo S., and Shapiro M. G., “ Biomolecular ultrasound and sonogenetics,” Annu. Rev. Chem. Biomol. Eng. 9, 229–252 (2018). 10.1146/annurev-chembioeng-060817-084034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338. Wang S., Meng W., Ren Z., Li B., Zhu T., Chen H., Wang Z., He B., Zhao D., and Jiang H., “ Ultrasonic neuromodulation and sonogenetics: A new era for neural modulation,” Front. Physiol. 11, 00787 (2020). 10.3389/fphys.2020.00787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339. Tsui P. H., Wang S. H., and Huang C. C., “ In vitro effects of ultrasound with different energies on the conduction properties of neural tissue,” Ultrasonics 43, 560–565 (2005). 10.1016/j.ultras.2004.12.003 [DOI] [PubMed] [Google Scholar]
- 340. Qi X., Lyu K., Meng L., Li C., Zhang H., Niu L., Lin Z., Zheng H., and Tang J., “ Low-intensity ultrasound causes direct excitation of auditory cortical neurons,” Neural Plast. 2021, 8855055. 10.1155/2021/8855055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341. Mihran R. T., Barnes F. S., and Wachtel H., “ Temporally-specific modification of myelinated axon excitability in vitro following a single ultrasound pulse,” Ultrasound Med. Biol. 16, 297–309 (1990). 10.1016/0301-5629(90)90008-Z [DOI] [PubMed] [Google Scholar]
- 342. Colucci V., Strichartz G., Jolesz F., Vykhodtseva N., and Hynynen K., “ Focused ultrasound effects on nerve action potential in vitro,” Ultrasound Med. Biol. 35, 1737–1747 (2009). 10.1016/j.ultrasmedbio.2009.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343. Menz M. D., Oralkan Ö., Khuri-Yakub P. T., and Baccus S. A., “ Precise neural stimulation in the retina using focused ultrasound,” J. Neurosci. 33, 4550–4560 (2013). 10.1523/JNEUROSCI.3521-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344. Hou X., Qiu Z., Xian Q., Kala S., Jing J., Wong K. F., Zhu J., Guo J., Zhu T., Yang M. et al. , “ Precise ultrasound neuromodulation in a deep brain region using nano gas vesicles as actuators,” Adv. Sci. 8, e2101934 (2021). 10.1002/advs.202101934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345. Tan S., Tan H. T., and Chung M. C., “ Membrane proteins and membrane proteomics,” Proteomics 8, 3924–3932 (2008). 10.1002/pmic.200800597 [DOI] [PubMed] [Google Scholar]
- 346. Nicolson G. L. and Ferreira de Mattos G., “ A brief introduction to some aspects of the fluid–mosaic model of cell membrane structure and its importance in membrane lipid replacement,” Membranes 11, 947 (2021). 10.3390/membranes11120947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347. Perozo E., Kloda A., Cortes D. M., and Martinac B., “ Physical principles underlying the transduction of bilayer deformation forces during mechanosensitive channel gating,” Nat. Struct. Biol. 9, 696–703 (2002). 10.1038/nsb827 [DOI] [PubMed] [Google Scholar]
- 348. Ranade S. S., Syeda R., and Patapoutian A., “ Mechanically activated ion channels,” Neuron 87, 1162–1179 (2015). 10.1016/j.neuron.2015.08.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349. Inman A. and Smutny M., “ Feeling the force: Multiscale force sensing and transduction at the cell–cell interface,” Semin. Cell Dev. Biol. 120, 53–65 (2021). 10.1016/j.semcdb.2021.06.006 [DOI] [PubMed] [Google Scholar]
- 350. Grashoff C., Hoffman B. D., Brenner M. D., Zhou R., Parsons M., Yang M. T., McLean M. A., Sligar S. G., Chen C. S., Ha T. et al. , “ Measuring mechanical tension across vinculin reveals regulation of focal adhesion dynamics,” Nature 466, 263–266 (2010). 10.1038/nature09198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351. Sitarska E. and Diz-Munoz A., “ Pay attention to membrane tension: Mechanobiology of the cell surface,” Curr. Opin. Cell Biol. 66, 11–18 (2020). 10.1016/j.ceb.2020.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352. Murase K., Fujiwara T., Umemura Y., Suzuki K., Iino R., Yamashita H., Saito M., Murakoshi H., Ritchie K., and Kusumi A., “ Ultrafine membrane compartments for molecular diffusion as revealed by single molecule techniques,” Biophys. J. 86, 4075–4093 (2004). 10.1529/biophysj.103.035717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353. Bottcher R. T. and Fassler R., “ Membrane tension drives ligand-independent integrin signaling,” EMBO J. 33, 2439–2441 (2014). 10.15252/embj.201489886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354. Apodaca G., “ Modulation of membrane traffic by mechanical stimuli,” Am. J. Physiol. Renal Physiol. 282, F179–F190 (2002). 10.1152/ajprenal.2002.282.2.F179 [DOI] [PubMed] [Google Scholar]
- 355. Pinot M., Vanni S., Ambroggio E., Guet D., Goud B., and Manneville J.-B., “ Feedback between membrane tension, lipid shape and curvature in the formation of packing defects,” bioRxiv 389627 (2018).
- 356. Le Roux A. L., Quiroga X., Walani N., Arroyo M., and Roca-Cusachs P., “ The plasma membrane as a mechanochemical transducer,” Philos. Trans. R. Soc., B 374, 20180221 (2019). 10.1098/rstb.2018.0221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357. Lewis A. H. and Grandl J., “ Mechanical sensitivity of Piezo1 ion channels can be tuned by cellular membrane tension,” eLife 4, e12088 (2015). 10.7554/eLife.12088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358. Jiang Y., Yang X., Jiang J., and Xiao B., “ Structural designs and mechanogating mechanisms of the mechanosensitive piezo channels,” Trends Biochem. Sci. 46, 472–488 (2021). 10.1016/j.tibs.2021.01.008 [DOI] [PubMed] [Google Scholar]
- 359. Bavi O., Cox C. D., Vossoughi M., Naghdabadi R., Jamali Y., and Martinac B., “ Influence of global and local membrane curvature on mechanosensitive ion channels: A finite element approach,” Membranes 6, 14 (2016). 10.3390/membranes6010014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360. Wang M., Zhang Y., Cai C., Tu J., Guo X., and Zhang D., “ Sonoporation-induced cell membrane permeabilization and cytoskeleton disassembly at varied acoustic and microbubble-cell parameters,” Sci. Rep. 8, 3885 (2018). 10.1038/s41598-018-22056-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361. Reusch T., Schülein F. J. R., Nicolas J. D., Osterhoff M., Beerlink A., Krenner H. J., Müller M., Wixforth A., and Salditt T., “ Collective lipid bilayer dynamics excited by surface acoustic waves,” Phys. Rev. Lett. 113, 118102 (2014). 10.1103/PhysRevLett.113.118102 [DOI] [PubMed] [Google Scholar]
- 362. Krasovitski B., Frenkel V., Shoham S., and Kimmel E., “ Intramembrane cavitation as a unifying mechanism for ultrasound-induced bioeffects,” Proc. Natl. Acad. Sci. U. S. A. 108, 3258–3263 (2011). 10.1073/pnas.1015771108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363. Mizrahi N., Zhou E. H., Lenormand G., Krishnan R., Weihs D., Butler J. P., Weitz D. A., Fredberg J. J., and Kimmel E., “ Low intensity ultrasound perturbs cytoskeleton dynamics,” Soft Matter 8, 2438–2443 (2012). 10.1039/c2sm07246g [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Encyclopedia of Metalloproteins, edited by Kretsinger R. H., Uversky V. N., and Permyakov E. A ( Springer, New York, 2013), p. 521. [Google Scholar]
- 365. Ochiai E.-I., “ Why calcium? Principles and applications in bioorganic chemistry–IV,” J. Chem. Educ. 68, 10 (1991). 10.1021/ed068p10 [DOI] [Google Scholar]
- 366. Kilhoffer M. C., Roberts D. M., Adibi A. O., Watterson D. M., and Haiech J., “ Investigation of the mechanism of calcium binding to calmodulin. Use of an isofunctional mutant with a tryptophan introduced by site–directed mutagenesis,” J. Biol. Chem 263, 17023–17029 (1988). 10.1016/S0021-9258(18)37492-1 [DOI] [PubMed] [Google Scholar]
- 367. Carafoli E. and Krebs J., “ Why calcium? How calcium became the best communicator,” J. Biol. Chem 291, 20849–20857 (2016). 10.1074/jbc.R116.735894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368. Bootman M. D. and Bultynck G., “ Fundamentals of cellular calcium signaling: A primer,” Cold Spring Harb. Perspect. Biol. 12, a038802 (2020). 10.1101/cshperspect.a038802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369. Fierro L., DiPolo R., and Llano I., “ Intracellular calcium clearance in Purkinje cell somata from rat cerebellar slices,” J. Physiol. 510, 499–512 (1998). 10.1111/j.1469-7793.1998.499bk.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370. ZhuGe R., Tuft R. A., Fogarty K. E., Bellve K., Fay F. S., and Walsh J. J. V., “ The influence of sarcoplasmic reticulum Ca2+ concentration on Ca2+ sparks and spontaneous transient outward currents in single smooth muscle cells,” J. Gen. Physiol. 113, 215–228 (1999). 10.1085/jgp.113.2.215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371. Woll K. A. and Van Petegem F., “ Calcium-release channels: Structure and function of IP3 receptors and ryanodine receptors,” Physiol. Rev. 102, 209–268 (2022). 10.1152/physrev.00033.2020 [DOI] [PubMed] [Google Scholar]
- 372. Brini M. and Carafoli E., “ The plasma membrane Ca2+ ATPase and the plasma membrane sodium calcium exchanger cooperate in the regulation of cell calcium,” Cold Spring Harb. Perspect. Biol. 3, a004168 (2011). 10.1101/cshperspect.a004168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373. Berridge M. J., “ Calcium signal transduction and cellular control mechanisms,” Biochim. Biophys. Acta 1742, 3–7 (2004). 10.1016/j.bbamcr.2004.08.012 [DOI] [PubMed] [Google Scholar]
- 374. Ruiz-Hurtado G., Morel E., Dominguez-Rodriguez A., Llach A., Lezoualc'h F., Benitah J. P., and Gomez A. M., “ Epac in cardiac calcium signaling,” J. Mol. Cell. Cardiol. 58, 162–171 (2013). 10.1016/j.yjmcc.2012.11.021 [DOI] [PubMed] [Google Scholar]
- 375. Brini M., “ Plasma membrane Ca2+-ATPase: From a housekeeping function to a versatile signaling role,” Pflug. Arch. 457, 657–664 (2009). 10.1007/s00424-008-0505-6 [DOI] [PubMed] [Google Scholar]
- 376. Zhou Y., Xue S., and Yang J. J., “ Calciomics: Integrative studies of Ca2+-binding proteins and their interactomes in biological systems,” Metallomics 5, 29–42 (2013). 10.1039/C2MT20009K [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377. De Koninck P. and Schulman H., “ Sensitivity of CaM kinase II to the frequency of Ca2+ oscillations,” Science 279, 227–230 (1998). 10.1126/science.279.5348.227 [DOI] [PubMed] [Google Scholar]
- 378. Li W., Llopis J., Whitney M., Zlokarnik G., and Tsien R. Y., “ Cell-permeant caged InsP3 ester shows that Ca2+ spike frequency can optimize gene expression,” Nature 392, 936–941 (1998). 10.1038/31965 [DOI] [PubMed] [Google Scholar]
- 379. Dolmetsch R. E., Xu K., and Lewis R. S., “ Calcium oscillations increase the efficiency and specificity of gene expression,” Nature 392, 933–936 (1998). 10.1038/31960 [DOI] [PubMed] [Google Scholar]
- 380. Oancea E. and Meyer T., “ Protein kinase C as a molecular machine for decoding calcium and diacylglycerol signals,” Cell 95, 307–318 (1998). 10.1016/S0092-8674(00)81763-8 [DOI] [PubMed] [Google Scholar]
- 381. Pinto M. C., Tonelli F. M., Vieira A. L., Kihara A. H., Ulrich H., and Resende R. R., “ Studying complex system: Calcium oscillations as attractor of cell differentiation,” Integr. Biol. 8, 130–148 (2016). 10.1039/c5ib00285k [DOI] [PubMed] [Google Scholar]
- 382. Suzuki T., Notomi T., Miyajima D., Mizoguchi F., Hayata T., Nakamoto T., Hanyu R., Kamolratanakul P., Mizuno A., Suzuki M. et al. , “ Osteoblastic differentiation enhances expression of TRPV4 that is required for calcium oscillation induced by mechanical force,” Bone 54, 172–178 (2013). 10.1016/j.bone.2013.01.001 [DOI] [PubMed] [Google Scholar]
- 383. Li P., Bian X., Liu C., Wang S., Guo M., Tao Y., and Huo B., “ STIM1 and TRPV4 regulate fluid flow-induced calcium oscillation at early and late stages of osteoclast differentiation,” Cell Calcium 71, 45–52 (2018). 10.1016/j.ceca.2017.12.001 [DOI] [PubMed] [Google Scholar]
- 384. Yokota Y., Nakajima H., Wakayama Y., Muto A., Kawakami K., Fukuhara S., and Mochizuki N., “ Endothelial Ca2+ oscillations reflect VEGFR signaling–regulated angiogenic capacity in vivo,” eLife 4, e08817 (2015). 10.7554/eLife.08817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385. Page D., Thuret R., Venkatraman L., Takahashi T., Bentley K., and Herbert S., “ Positive feedback defines the timing, magnitude, and robustness of angiogenesis,” Cell Rep. 27, 3139–3151 (2019). 10.1016/j.celrep.2019.05.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386. Savage A. M., Kurusamy S., Chen Y., Jiang Z., Chhabria K., MacDonald R. B., Kim H. R., Wilson H. L., van Eeden F. J. M., Armesilla A. L. et al. , “ tmem33 is essential for VEGF-mediated endothelial calcium oscillations and angiogenesis,” Nat. Commun. 10, 732 (2019). 10.1038/s41467-019-08590-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387. Keating T. J., Cork R. J., and Robinson K. R., “ Intracellular free calcium oscillations in normal and cleavage-blocked embryos and artificially activated eggs of Xenopus laevis,” J. Cell Sci. 107, 2229–2237 (1994). 10.1242/jcs.107.8.2229 [DOI] [PubMed] [Google Scholar]
- 388. Kubota H. Y., Yoshimoto Y., and Hiramoto Y., “ Oscillation of intracellular free calcium in cleaving and cleavage-arrested embryos of Xenopus laevis,” Dev. Biol. 160, 512–518 (1993). 10.1006/dbio.1993.1325 [DOI] [PubMed] [Google Scholar]
- 389. Balasubramanian P. S., Singh A., Xu C., and Lal A., “ GHz ultrasonic chip-scale device induces ion channel stimulation in human neural cells,” Sci. Rep. 10, 3075 (2020). 10.1038/s41598-020-58133-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390. Lee N. S., Yoon C. W., Wang Q., Moon S., Koo K. M., Jung H., Chen R., Jiang L., Lu G., Fernandez A. et al. , “ Focused ultrasound stimulates ER localized mechanosensitive PANNEXIN–1 to mediate intracellular calcium release in invasive cancer cells,” Front. Cell Dev. Biol. 8, 00504 (2020). 10.3389/fcell.2020.00504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391. Hinton P. V., Rackard S. M., and Kennedy O. D., “ In vivo osteocyte mechanotransduction: Recent developments and future directions,” Curr. Osteoporos. Rep. 16, 746–753 (2018). 10.1007/s11914-018-0485-1 [DOI] [PubMed] [Google Scholar]
- 392. Schwartz M. A. and DeSimone D. W., “ Cell adhesion receptors in mechanotransduction,” Curr. Opin. Cell Biol. 20, 551–556 (2008). 10.1016/j.ceb.2008.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393. Willett N. J., Long R. C., Maiellaro-Rafferty K., Sutliff R. L., Shafer R., Oshinski J. N., Giddens D. P., Guldberg R. E., and Taylor W. R., “ An in vivo murine model of low-magnitude oscillatory wall shear stress to address the molecular mechanisms of mechanotransduction—Brief report,” Arterioscler. Thromb. Vasc. Biol. 30, 2099–2102 (2010). 10.1161/ATVBAHA.110.211532 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.