Abstract
Introduction
The decision to shorten the duration of DAPT following PCI in patients with ACS remains controversial because of the concern for increased ischemic events.
Methods
We performed a comprehensive literature search in seven databases to explore the efficacy of 1–3 months of DAPT in patients who underwent PCI for ACS. Randomized controlled trials that compared 1–3 months with 6–12 months of DAPT after PCI for ACS were identified. Integrated hazard ratio (HR) and 95% confidence interval (CI) were calculated by random effects model for each prespecified outcome of interest. Meta-regression analyses were performed to examine the association of outcomes with select patient characteristics.
Results
A total of 9 randomized controlled trials consisting of 25,907 patients were included. There was no difference in the hazard of NACE (HR 0.92, 95% CI 0.79–1.07) and MACE (HR 0.96, 95% CI 0.78–1.17) between 1–3 months of DAPT and 6–12 months of DAPT. However, implementing 1–3 months of DAPT was associated with lower hazard of both any bleeding (HR 0.55, 95% CI 0.46–0.66) and major bleeding (HR 0.47, 95% CI 0.36–0.62). Meta-regression revealed a non-significant but increasing trend of both NACE and MACE with greater proportion of left main and left anterior descending coronary artery lesions and greater proportion of STEMI included the trials.
Conclusion
Our findings suggest that 1–3 months of DAPT has similar efficacy for preventing ischemic events with reduced bleeding risk compared with 6–12 months of DAPT.
Keywords: DAPT, ACS, PCI, stent, short, abbreviated
Introduction
Dual antiplatelet therapy (DAPT) remains the mainstay of treatment after percutaneous coronary intervention (PCI) of either stable ischemic heart disease or acute coronary syndrome (ACS).1 In the last decade, many trials have explored deescalating DAPT to a single antiplatelet agent at an earlier time but concerns about higher ischemic events have persisted, especially after PCI for ACS.2 Two previous trials exclusively randomized patients with ACS to shorter duration of DAPT, although no significant difference in composite outcomes was identified3, 4. The REDUCE trial noted numerically higher rates of mortality and stent thrombosis in those receiving just 3 months of DAPT, while the TICO trial was underpowered for ischemic events and limited to patients from South Korea. More recently, the STOPDAPT-2 ACS trial reported that 1 to 2 months of DAPT failed to establish noninferiority to 12 months of DAPT for the hazard of cardiovascular (CV) death, myocardial infarction (MI), definite stent thrombosis, stroke, or bleeding because of a greater increase in CV events compared to reduction in bleeding events.5 Given these findings, uncertainty remains over whether shortening the duration of DAPT is an efficacious strategy in ACS patients following drug-eluting stent (DES) placement. Therefore, we performed an updated systematic review and meta-analysis of randomized clinical trials (RCTs) on the efficacy and safety of 1 to 3 months of DAPT compared to 6 to 12 months in patients who have undergone PCI for ACS.
Methods
The authors declare that all supporting data are available within the article. This systematic review was conducted according to a published protocol pending registration on PROSPERO and available on Open Science Framework (10.17605/OSF.IO/MW3VY). Our study followed the Preferred Reporting Items for Systematic Reviews guideline for reporting (Table S1)6.
Search Strategy and Inclusion Criteria
A systematic search was performed in the following databases: Cochrane Library, Google Scholar, Ovid Embase, Ovid MEDLINE, PubMed, Scopus, and Web of Science Core Collection from the inception of database to March 25, 2022. The search was formulated using controlled vocabulary and keywords with synonyms for percutaneous coronary intervention, dual anti-platelet therapy, duration of treatment, and randomized controlled trials. Full search strategies for all databases can be found in Table S2. Relevant websites (www.escardio.org, www.tctmd.com, www.europcr.com, www.scai.org, and www.acc.org) and the reference lists of each included study using CitationChaser were searched for additional relevant literature.
Studies were included only if they met the following criteria: (1) RCT; (2) comparison of 1 to 3 months of DAPT (case group) with 6 to 12 months of DAPT (control group); (3) inclusion only of patients who underwent PCI for ACS; (4) follow-up duration of at least 12 months after index PCI; (5) written in English language. Subgroup analysis or post-hoc analysis of RCTs and official abstracts published in well-known international conferences (ACC, AHA, SCAI, TCT, TVT, ESC, EuroPCR) were allowed. Duplicative studies were excluded.
Citations from the initial search were imported into the Endnote 20 database. After removing duplicates using the Yale Reference Deduplicator Tool, the remaining articles were uploaded into Covidence.7 Two authors (D.P. and P.W.) independently screened papers in title and abstract, and selected relevant papers by their full manuscripts and supplementary appendices after assessing for eligibility. Selected studies were re-examined for appropriateness and disagreements were settled by a third author (M.N.). We utilized the Cochrane collaboration’s tool to assess the risk of bias for each trial and the GRADE system to evaluate the quality of each pooled outcome.8, 9 Our study was exempt from institutional review board’s approval as only publicly published data were used.
Data Acquisition and Outcomes of Interest
Baseline characteristics of studies, patients, and procedures were extracted by 2 authors (D.P. and S.A.) and validated by a third author (M.N.). Primary outcomes of interest were net adverse clinical events (NACE) and major adverse cardiovascular events (MACE). Secondary outcomes included any bleeding, major bleeding, all-cause mortality, CV mortality, myocardial infarction (MI), stroke, definite or probable stent thrombosis, and repeat revascularization. Of note, the definitions of MACE and NACE differed across the included trials. The definition of outcomes in each trial are summarized in Table S3.
Statistical analysis
Integrated hazard ratio (HR) and 95% confidence intervals (CI) were generated by applying random effects model based on the DerSimonian-Laird method. Higgins and Thompson’s I2 statistics and τ2 were calculated to assess the heterogeneity among the studies. Funnel plots showing the scatter plot of the hazard ratios against the standard error in a logarithmic scale were visualized to evaluate for publication bias. Begg-Mazumdar and Egger tests were then applied. For meta-regression analysis, mixed-effects logistic regression was used to examine the association of outcomes with the proportion of left main (LM) and left anterior descending (LAD) coronary artery lesions and the proportion of ST-elevation myocardial infarction (STEMI). Beta coefficient with its corresponding P value, τ2, I2, H2, and R2 indexes were generated from all meta-regression models. All statistical analyses were performed using the meta package in R version 4.0.5 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Our initial literature search yielded 3,012 potential studies after removing duplicate publications (Figure 1). Of these, 62 papers were evaluated in full-text and 53 papers were excluded for duplicate study data, wrong setting, or wrong patient population (Table S4). Nine studies met the inclusion criteria. Publication years spanned from 2012 to 2022 (Table 1). Four RCTs were intention-to-treat analysis3, 5, 10, 11, while 3 were post-hoc analyses12–14, and 2 were per-protocol analyses.4, 15 Data from one trial was from a conference publication as a full paper had not yet been published.12 Three trials exclusively enrolled patients who presented with ACS3–5, while others reported outcomes from the subgroup of patients who had ACS by either subgroup analysis or post-hoc analysis. Two trials compared 1 month with 12 months of DAPT.4, 14 Five trials compared 3 months with 12 months of DAPT.3, 10, 11, 13, 15 One trial compared 1 to 2 months of DAPT with 12 months of DAPT5, and another trial compared 1 month with 6 months of DAPT.16 Seven of the 9 trials administered P2Y12 inhibitor monotherapy after given period of dual-antiplatelet therapy. Two trials did not report NACE and another two trials did not report MACE. Among the trials that reported these two outcomes, the component events were not identical, with differences in the inclusion of stent thrombosis and target vessel revascularization for MACE and differences in the inclusion of cardiovascular death, stroke, and target vessel revascularization for NACE (Table S3). Risk of biases in the trials were largely low to moderate, and quality of evidence ranged from high to moderate (Table S5–S6). Publication bias was not observed (Table S7).
Figure 1. PRISMA flow diagram of this meta-analysis.
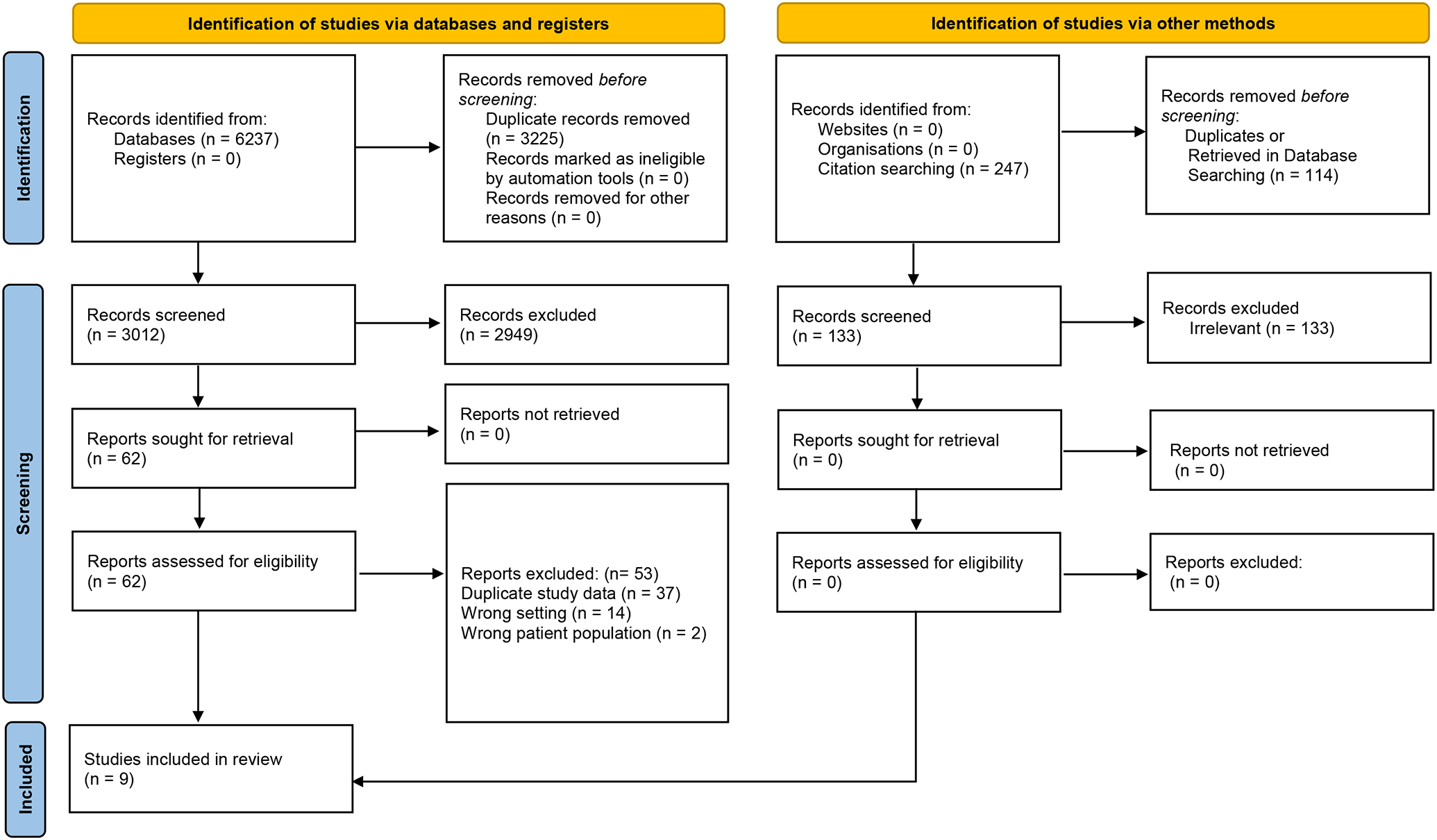
The flow diagram shows the process of how the trials included in this meta-analysis were selected. All steps adhered to PRISMA guidelines.
Table 1.
Characteristics of the selected trials
| Trial | Author | Yeara | Analysis | Case (Short-term DAPT) | Control (Standard-term DAPT) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Size (N) |
DAPTb (Mo.) |
Drugsc | Stent | Size (N) |
DAPTb (Mo.) |
Drugsd | Stent | ||||
| STOPDAPT-2 ACS | Watanabe et al. | 2022 | ITT | 2058 | 1–2 | Clopidogrel | Cobalt-chromium EES | 2078 | 12 | Clopidogrel | Cobalt-chromium EES |
| MASTER DAPT | Valgimigli et al. | 2021 | PHA | 914 | 1 | Clopidogrel, aspirin | SES | 866 | 6e | Clopidogrel | SES |
| TICO | Kim et al. | 2020 | PP | 1527 | 1 | Ticagrelor | SES | 1529 | 12 | Ticagrelor | SES |
| TWILIGHT-ACS | Baber et al. | 2020 | PHA | 2273 | 3 | Ticagrelor | 2G DESf | 2341 | 12 | Ticagrelor | 2G DESf |
| GLOBAL LEADERS | Tomaniak et al. | 2019 | PHA | 3750 | 1 | Ticagrelor | BES | 3737 | 12 | Ticagrelor | BES |
| SMART-CHOICE | Hahn et al. | 2019 | ITT, SA | 870 | 3 | Clopidogrel | EES, SES | 871 | 12 | Clopidogrel | EES, SES |
| REDUCE | De Luca et al. | 2019 | ITT | 751 | 3 | Aspirin | Combo CD34+ antibody-coated SES | 745 | 12 | Prasugrel, ticagrelor, clopidogrel | Combo CD34+ antibody-coated SES |
| OPTIMIZE | Feres et al. | 2013 | ITT, SA | 494 | 3 | Aspirin | ZES | 502 | 12 | Clopidogrel | ZES |
| RESET | Kim et al. | 2012 | PP, SA | 301 | 3 | Clopidogrel | ZES | 300 | 12 | Clopidogrel | SES, EES, ZES |
Year study was published
Duration of dual-antiplatelet therapy
Single antiplatelet agent used after given period of dual-antiplatelet therapy
Antiplatelet agent used in combination with aspirin
Interquartile range of 102 to 366 days
Second-generation drug-eluting stent: durable polymer cobalt-chromium EES, durable polymer platinum-chromium EES, durable polymer ZES, durable polymer cobalt-chromium SES, biodegradable polymer DES, polymer-free DES, bioresorbable vascular scaffold, sirolimus-eluting self-apposing stent, tacrolimus-eluting carbostent
Abbreviations: BES = biolimus-eluting stent; DAPT = dual anti-platelet therapy; EES = everolimus-eluting stent; ITT = intention to treat; Mo = month(s); PHA = post-hoc analysis; PP = per protocol; SA = subgroup analysis; SES = sirolimus-eluting stent; ZES = zotarolimus-eluting stent
A total of 12,938 patients underwent 1 to 3 months of DAPT while 12,969 patients underwent 6 to 12 months of DAPT. The sample size included in our study represented the number of patients with ACS and not the total enrollment in 6 out of 9 trials as only the STOPDAPT-2 ACS, TICO, and REDUCE trials exclusively enrolled patients with ACS. However, the STOPDAPT-2 ACS trial did include the ACS subgroup of the initial STOPDAPT-2 trial who comprised 28% of the total sample size. The proportion of patients with stable ischemic heart disease in original trials that were not limited to ACS are shown in Figure S1. Types of dual antiplatelet agent and stents were variable among trials, but only the RESET trial deployed first-generation drug-eluting stents in a fraction of the patients.15 Demographics and comorbidities also widely varied among the trials (Table 2). Reasons for undergoing PCI consisted of 35.7% unstable angina, 34.7% non-ST-elevation MI (NSTEMI), and 30.5% STEMI. However, RESET trial did not distinguish between NSTEMI and STEMI, so it was excluded when calculating the percentages.15 TWILIGHT-ACS trial and OPTIMIZE trial did not include patients with STEMI.10, 13 Proportion of STEMI was heterogenous in the remaining 7 trials, comprising >70% in the STOPDAPT-2 ACS trial and <20% in the SMART-CHOICE trial.5, 11 Procedural characteristics are summarized in Table 3.
Table 2.
Baseline demographics of the selected trials
| Case/Control, % | STOPDAPT-2 ACS | MASTER DAPTa | TICO | TWILIGHT-ACS | GLOBAL LEADERSa | SMART- CHOICEa | REDUCE | OPTIMIZEa | RESETa |
|---|---|---|---|---|---|---|---|---|---|
| Age, year | 67.0/66.6 | 76.1/76.0 | 61.0/61.0 | 64.2/64.2 | 64.9/64.8 | 64.6/64.4 | 61.0/60.0 | 61.3/61.9 | 62.4/62.4 |
| Female | 20.8/20.6 | 30.7/30.8 | 21.0/20.0 | 25.5/24.8 | 23.2/22.9 | 27.3/25.8 | 17.4/22.7 | 36.5/36.9 | 35.6/37.1 |
| BMI, mean | 24.1/24.2 | 27.3/27.4 | 24.9/24.9 | 28.4/28.4 | – | 24..5/24.7 | 26.6/26.6 | – | 25.0/24.9 |
| Diabetes mellitus | 29.5/29.9 | 32.9/34.3 | 27.0/27.0 | 35.6/34.3 | 21.6/21.2 | 38.2/36.8 | 21.6/19.5 | 35.4/35.3 | 29.8/28.8 |
| Hypertension | 67.8/68.1 | 76.9/78.2 | 50.0/51.0 | 67.5/67.4 | 68.6/67.9 | 61.6/61.3 | 50.7/50.7 | 86.4/88.2 | 62.3/61.4 |
| Dyslipidemia | 66.7/66.9 | 67.2/68.1 | 61.0/60.0 | – | 60.8/62.0 | 45.1/45.5 | 46.3/44.9 | 63.2/63.7 | 57.7/59.9 |
| Current smoking | 34.9/33.8 | 10.0/8.1 | – | 23.3/26.6 | 34.3/33.6 | 28.4/24.5 | 42.1/42.7 | 18.6/17.3 | 25.2/22.8 |
| Impaired renal fx | 3.3/3.4 | 18.2/20.1 | 19.0/22.0 | 14.6/15.1 | 13.4/12.5 | 2.9/3.5 | – | 7.4/5.8 | – |
| PAD | 1.9/2.0 | – | – | 5.7/5.6 | 5.1/5.3 | – | – | 2.8/3.0 | – |
| Previous MI | 6.6/5.3 | 18.9/18.8 | 4.0/3.0 | 25.4/25.2 | 18.3/18.6 | 4.1/4.3 | – | 34.6/34.8 | 1.8/1.6 |
| Previous ACS | – | – | – | – | – | – | 12.5/11.8 | 31.6/32.3 | – |
| Previous PCI | 10.9/9.7 | 25.9/26.0 | – | 34.2/34.4 | 22.8/23.4 | 11.5/11.8 | 11.7/9.8 | 20.9/19.1 | 3.5/3.0 |
| Previous CABG | 0.4/0.9 | 7.4/7.5 | 1.0/1.0 | 8.8/8.5 | 3.5/3.9 | – | 2.8/2.8 | 7.1/8.2 | 0.2/0.6 |
| Previous CVA | 4.8/4.6 | – | 4.0/4.0 | – | 2.2/2.5 | 6.6/6.8 | 1.5/2.0 | 2.5/2.5 | – |
| Previous bleeding | 0.9/0.7 | 7.2/6.8 | – | 0.9/0.7 | 0.6/0.6 | – | – | 0.6/0.6 | – |
| LVEF, mean | 56.7/56.9 | 53.5/53.0 | – | – | – | 60.0/59.9 | – | – | 64.2/63.9 |
| MVD | – | – | 55.0/56.0 | 61.9/59.5 | – | 50.1/49.0 | 36.1/33.8 | – | 43.1/42.9 |
| Unstable angina b | 23.3/24.4 | 23.0/24.1 | 29.0/32.0 | 54.9/53.1 | 26.8/27.2 | 53.6/56.4 | 15.2/13.8 | 82.9/83.3 | 73.5/74.3 |
| NSTEMI b | 2.0/2.8 | 52.7/51.5 | 35.0/32.0 | 45.1/46.9 | 44.9/45.2 | 27.5/26.5 | 35.6/41.0 | 17.1/16.7 | 26.5/25.7 |
| STEMI b | 74.7/72.8 | 24.2/24.5 | 36.0/36.0 | – | 28.3/27.6 | 18.9/17.2 | 49.3/45.2 | – |
Demographics and comorbidities of the entire sample, including all silent ischemia, stable angina, and acute coronary syndrome
Clinical presentation
Includes both unstable angina and STEMI
Abbreviations: ACS = acute coronary syndrome; BMI = body mass index; CABG = coronary artery bypass graft; CAD = coronary artery disease; CVA = cerebrovascular disease; fx = function; LVEF = left ventricular ejection fraction; MI = myocardial infarction; MVD = multivessel disease; NSTEMI = non-ST segment elevation myocardial infarction; PAD = peripheral vascular disease; PCI = percutaneous coronary intervention; STEMI = ST segment elevation myocardial infarction
Table 3.
Procedural characteristics in the selected trials
| Case/Control, % | STOPDAPT-2 ACS | MASTER DAPTa | TICO | GLOBAL LEADERSa | SMART- CHOICEa | TWILIGHT-ACS | REDUCE | OPTIMIZEa | RESETa |
|---|---|---|---|---|---|---|---|---|---|
| Radial approach | 89.0/89.7 | 84.1/86.9 | 55/56 | 73.0/73.8 | 73.0/72.8 | 76.7/76.3 | 76.1/76.9 | 40/40 | - |
| Staged approach | 13.6/15.3 | - | - | - | - | - | - | 6.7/7.3 | - |
| Culprit vessels | |||||||||
| LAD | 54.0/53.2 | 42.3/42.9 | 48/48 | 40.8/40.0 | 48.8/50.4 | 57.7/58.4 | 48.0/44.2 | 47.9/46.6 | 52.7/53.6 |
| LCx | 13.8/13.1 | 22.1/23.0 | 19/19 | 24.9/25.3 | 21.6/19.9 | 32.6/32.9 | 19.5/22.0 | 23.4/24.3 | 21.0/19.2 |
| Left main | 1.3/0.9 | 3.9/4.0 | 3/2 | 2.0/2.2 | 1.2/1.9 | 5.2/5.1 | 1.3/0.8 | 1.2/1.5 | 0/0 |
| RCA | 30.8/32.7 | 30.5/28.8 | 30/31 | 31.3/31.5 | 28.2/27.8 | 34.9/33.9 | 31.2/33.0 | 27.6/27.7 | 26.3/27.1 |
| Bypass graft | 0.1/0.1 | 1.3/1.3 | - | 0.9/1.0 | - | - | - | - | - |
| Bifurcation lesion | 26.8/26.4 | 3.6/4.4 | 14/15 | 11.5/11.7 | 13.3/12.1 | 12.5/12.6 | - | 14.7/14.9 | - |
| Total occlusion | 3.2/3.0 | - | - | - | - | 5.6/6.1 | 26.9/24.3 | 4.2/3.6 | - |
| Target lesions, mean | 1.27/1.28 | 1.29/1.32 | 1.23/1.24 | 1.29/1.30 | 1.24/1.26 | 1.5/1.5 | - | 1.32/1.33 | 1.27/1.27 |
| Multivessel treatment | 16.7/18.8 | 25.2/27.8 | 17/18 | 14.2/14.7 | 22.5/24.6 | - | - | 25.3/26.5 | 22.0/23.4 |
| IVUS | 87.3/86.2 | - | - | - | 25.0/27.2 | - | - | - | - |
| OCT | 13.6/14.9 | - | - | - | - | - | - | - | - |
| Stents placed, mean | 1.40/1.41 | 1.74/1.76 | 1.37/1.37 | 1.52/1.54 | - | - | 1.20/1.21 | 1.6/1.6 | - |
| Stent diameter, mm | 3.01/3.02 | 3.00/2.99 | 3.13/3.14 | 3.0/3.0 | - | 2.9/2.9 | - | 2.7/2.7 | 3.18/3.17 |
| Stent length, mm | 34.3/34.6 | 39.3/39.7 | 35/35 | 24.3/24.3 | 38.0/37.8 | 40.5/39.8 | 23.0/23.0 | - | 22.7/22.9 |
Demographics and comorbidities of the entire sample, including all silent ischemia, stable angina, and acute coronary syndrome
Abbreviations: IVUS = intravascular ultrasound; LAD = left anterior descending coronary artery; LCx = left circumflex coronary artery; OCT = optical coherence tomography; RCA = right coronary artery
There was no difference in the hazard of NACE (HR 0.92, 95% CI 0.79–1.07, p=0.26) and MACE (HR 0.96, 95% CI 0.78–1.17, p=0.69) between 1 to 3 months of DAPT and 6 to 12 months of DAPT (Figure 2). However, implementing 1 to 3 months of DAPT was associated with lower hazard of both any bleeding (HR 0.55, 95% CI 0.46–0.66, p<0.01) and major bleeding (HR 0.47, 95% CI 0.36–0.62, p<0.01) (Figure 3). No differences were found in the outcomes of all-cause mortality (HR 0.95, 95% CI 0.63–1.43, p=0.81), CV mortality (HR 1.07, 95% CI 0.54–2.13, p=0.84), myocardial infarction (HR 1.08, 95% CI 0.84–1.39, p=0.55), and stroke (HR 1.19, 95% CI 0.79–1.78, p=0.40) (Figure 4 and Figure S1). The hazard of definite or probable stent thrombosis (HR 1.41, 95% CI 0.70–2.87, p=0.34) and repeat revascularization (HR 1.22, 95% CI 0.89–1.67, p=0.22) also did not differ between 1 to 3 months of DAPT and 6 to 12 months of DAPT (Figure S3). Sensitivity analysis of RCTs that exclusively enrolled patients with ACS produced similar findings (Figure S4). Sensitivity analysis excluding the MASTER DAPT trial, whose ACS subgroup analysis has not yet been published as a full manuscript, revealed similar results (Table S8). Additional sensitivity analysis excluding the RESET trial, which employed first-generation drug-eluting stents in a portion of its patients, yielded similar results (Table S9). Finally, sensitivity analysis to newer (ticagrelor, prasugrel) or older (clopidogrel, aspirin) antiplatelet agents also produced similar results in primary outcomes (Figure S5). However, the numerical HR was lower in the former for both NACE and MACE.
Figure 2. Comparison of primary outcomes in 1–3 months versus 6–12 months of DAPT.
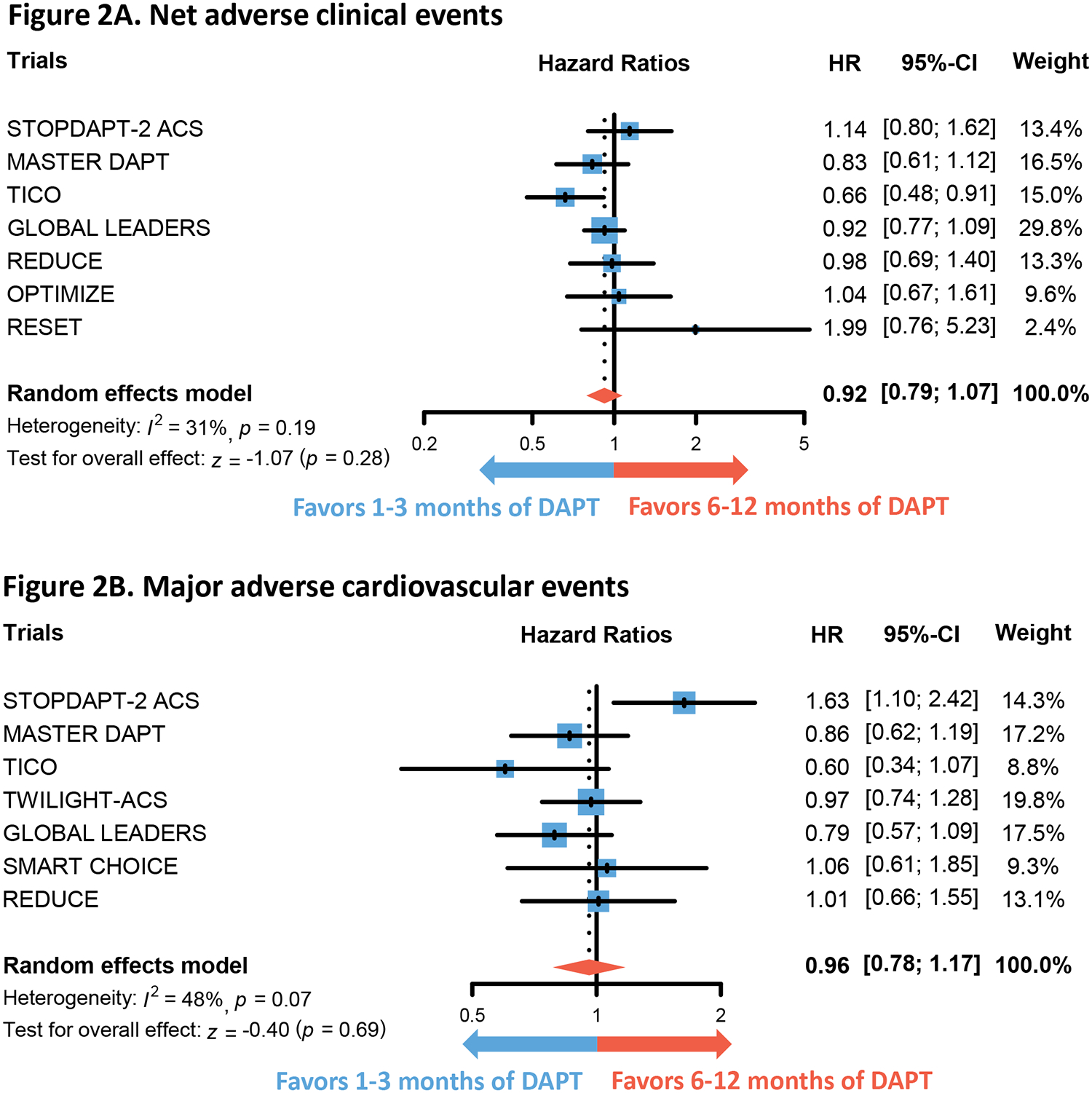
Forest plots show the integrated hazard ratio for the outcomes of net adverse clinical events (Figure 2A) and major adverse cardiovascular events (Figure 2B). Hazard ratio below 1 favors 1 to 3 months of DAPT whereas that above 1 favors 6–12 months of DAPT. Please note that the trials included in Figure 2A and Figure 2B are not entirely identical.
Abbreviations: DAPT = dual anti-platelet therapy
Figure 3. Comparison of bleeding outcomes in 1–3 months versus 6–12 months of DAPT.
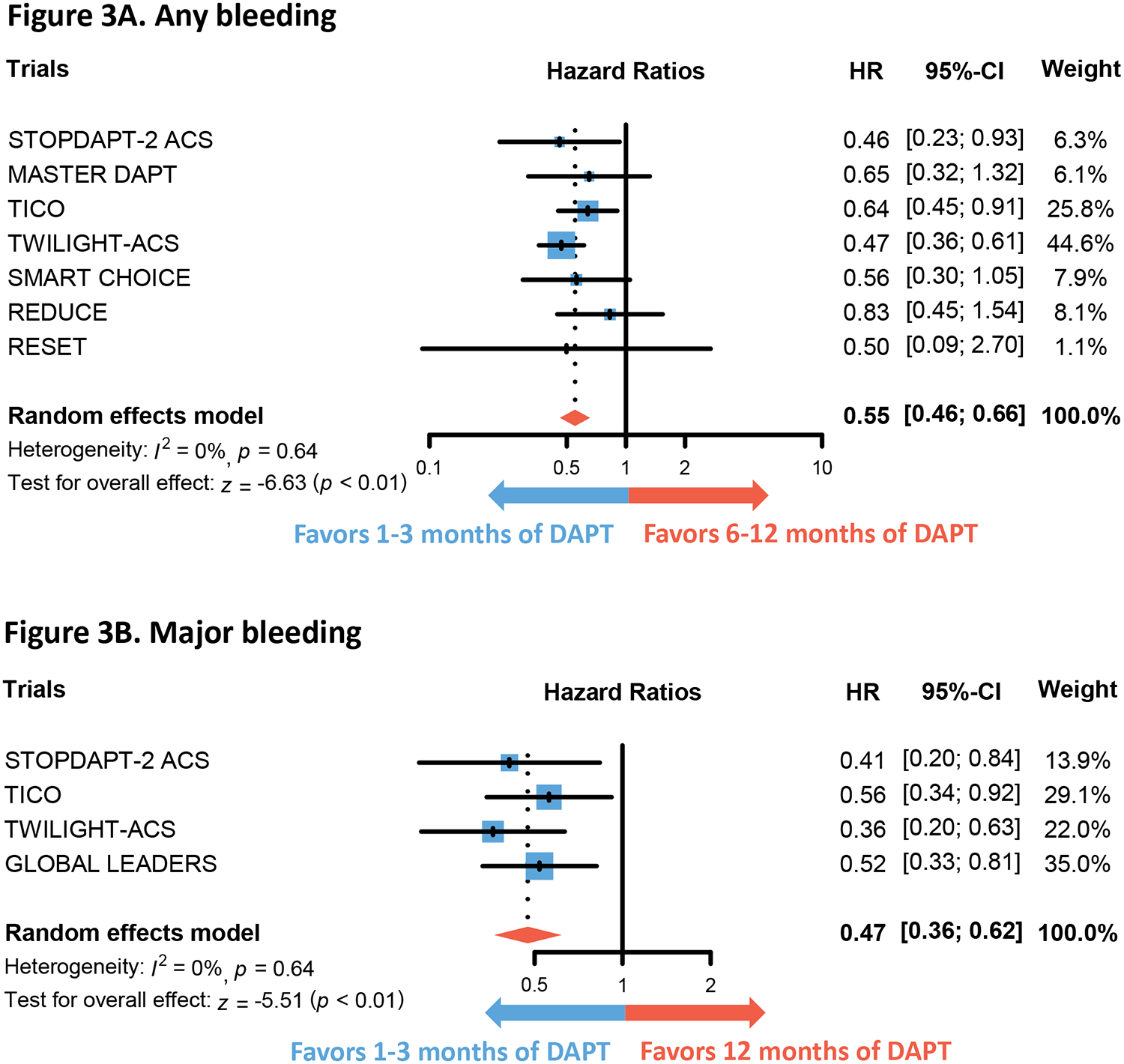
Forest plots show the integrated hazard ratio for the outcomes of any bleeding (Figure 3A) and major bleeding (Figure 3B). Hazard ratio below 1 favors 1 to 3 months of DAPT whereas that above 1 favors 6–12 months of DAPT.
Abbreviations: DAPT = dual anti-platelet therapy
Figure 4. Comparison of mortalities in 1–3 months versus 12 months of DAPT.
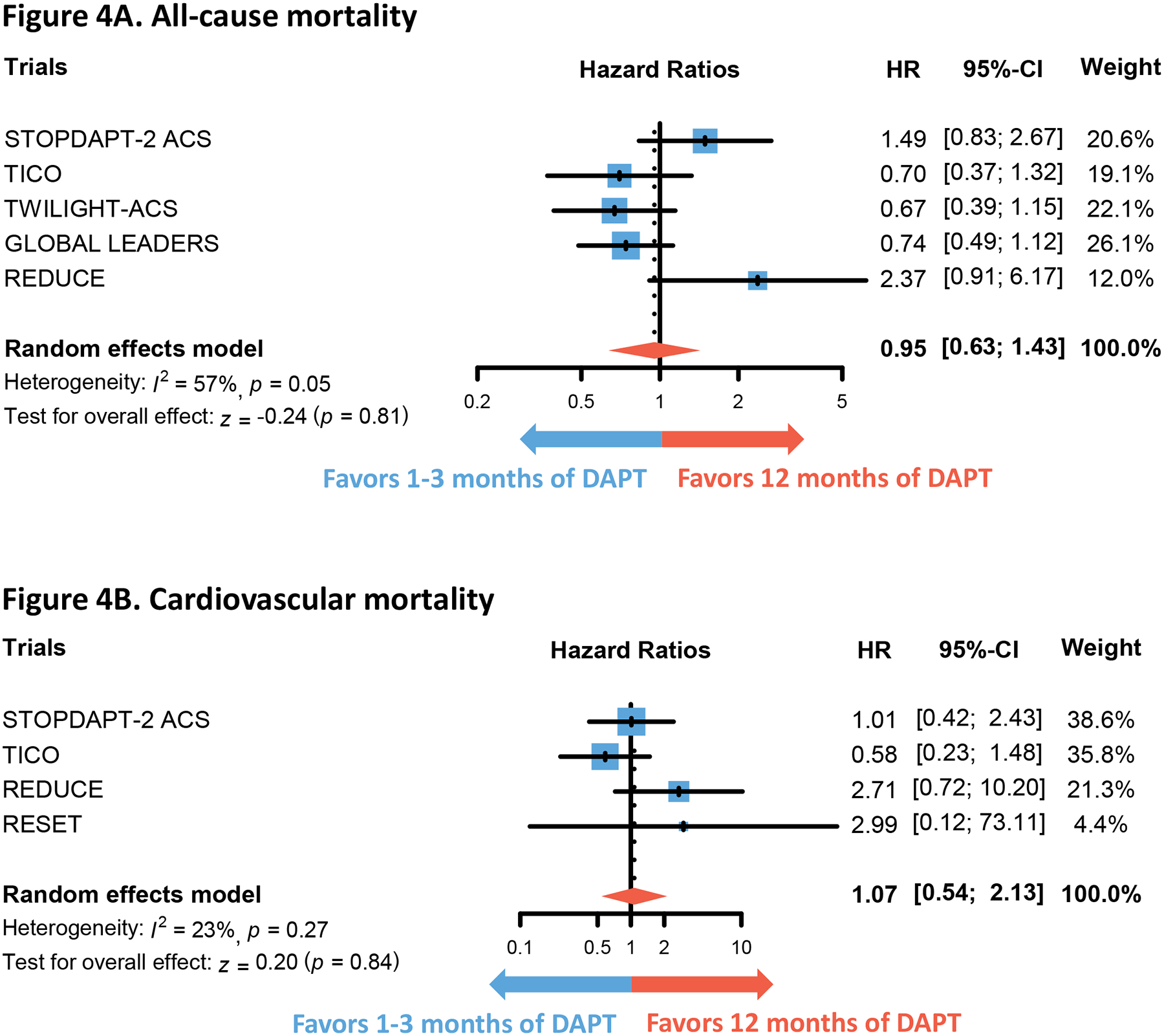
Forest plots show the integrated hazard ratio for the outcomes of all-cause mortality (Figure 4A) and cardiovascular mortality (Figure 4B). Hazard ratio below 1 favors 1 to 3 months of DAPT whereas that above 1 favors 12 months of DAPT. Note that 6 months of DAPT is not included in the control group as MASTER DAPT trial did not provide data on specified outcome.
Abbreviations: DAPT = dual anti-platelet therapy
Meta-regression revealed a non-significant but increasing trend of both NACE (β=0.003, R2=0%, p=0.76) and MACE (β=0.013, R2=26.4%, p=0.19) with greater proportion of LM and LAD coronary artery lesions in the trials (Figure 5). Increasing trends of NACE (β=0.006, R2=21.4%, p=0.15) and MACE (β=0.025, R2=0%, p=0.51), albeit non-significant, were also observed with increasing proportion of STEMI included in the trials (Figure 6). On the other hand, major bleeding showed an inconsistent and non-significant trend with increasing proportion of LM and LAD coronary artery lesions (β= −0.003, R2=0.00%, p=0.83) and STEMI (β=0.002, R2=0.00%, p=0.70) (Figure S6–S7). Details of the meta-regression analysis are shown in Table S10.
Figure 5. Meta-regression of primary outcomes to proportion of left main and left anterior descending coronary artery lesions.
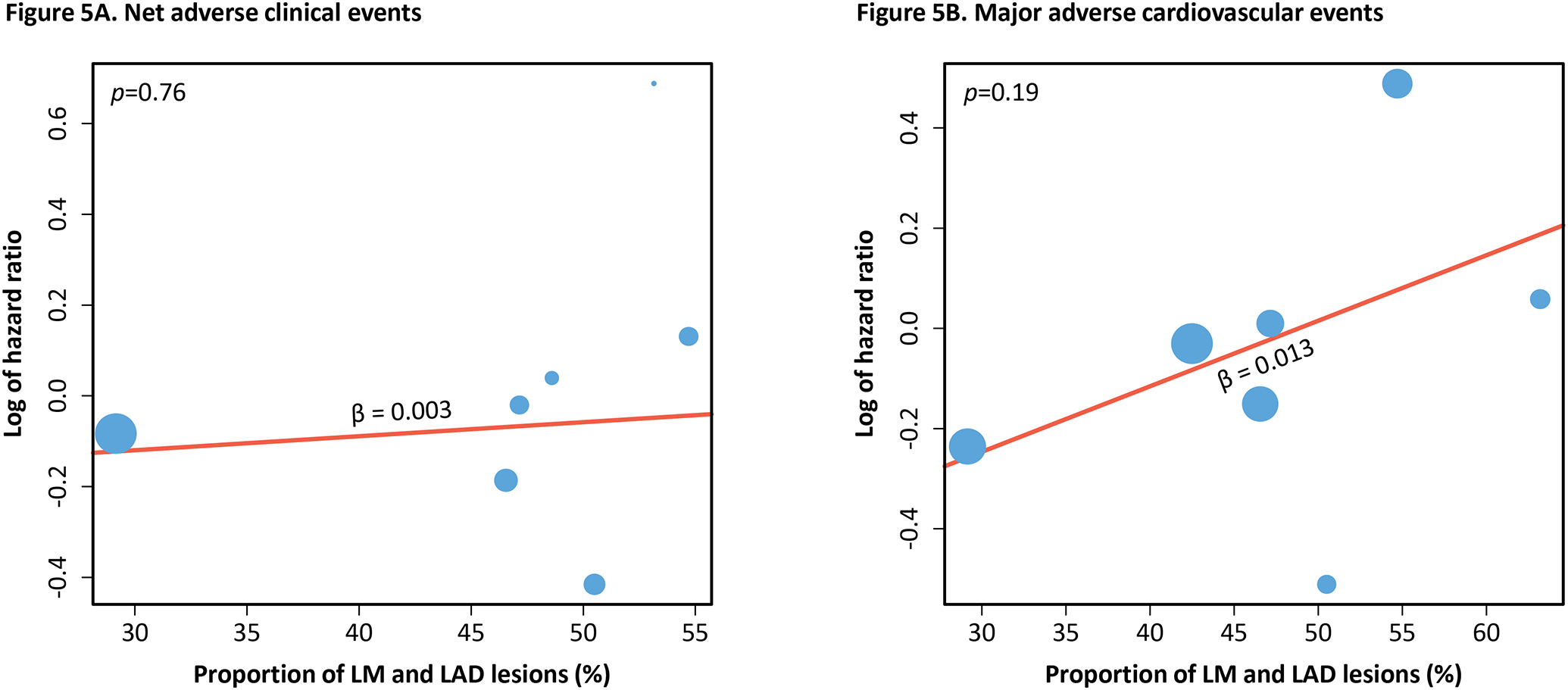
The two graphs above show the association of the hazard ratio of net adverse clinical events (Figure 5A) and major adverse cardiovascular events (Figure 5B) with the proportion of LM and LAD coronary artery lesions included in the trials. Positive beta coefficient signifies positive correlation and vice versa. P-value of the trend is shown in the top left corner of each graph.
Abbreviations: LM = left main coronary artery; LAD = left anterior descending coronary artery
Figure 6. Meta-regression of primary outcomes to proportion of ST-elevation myocardial infarction.
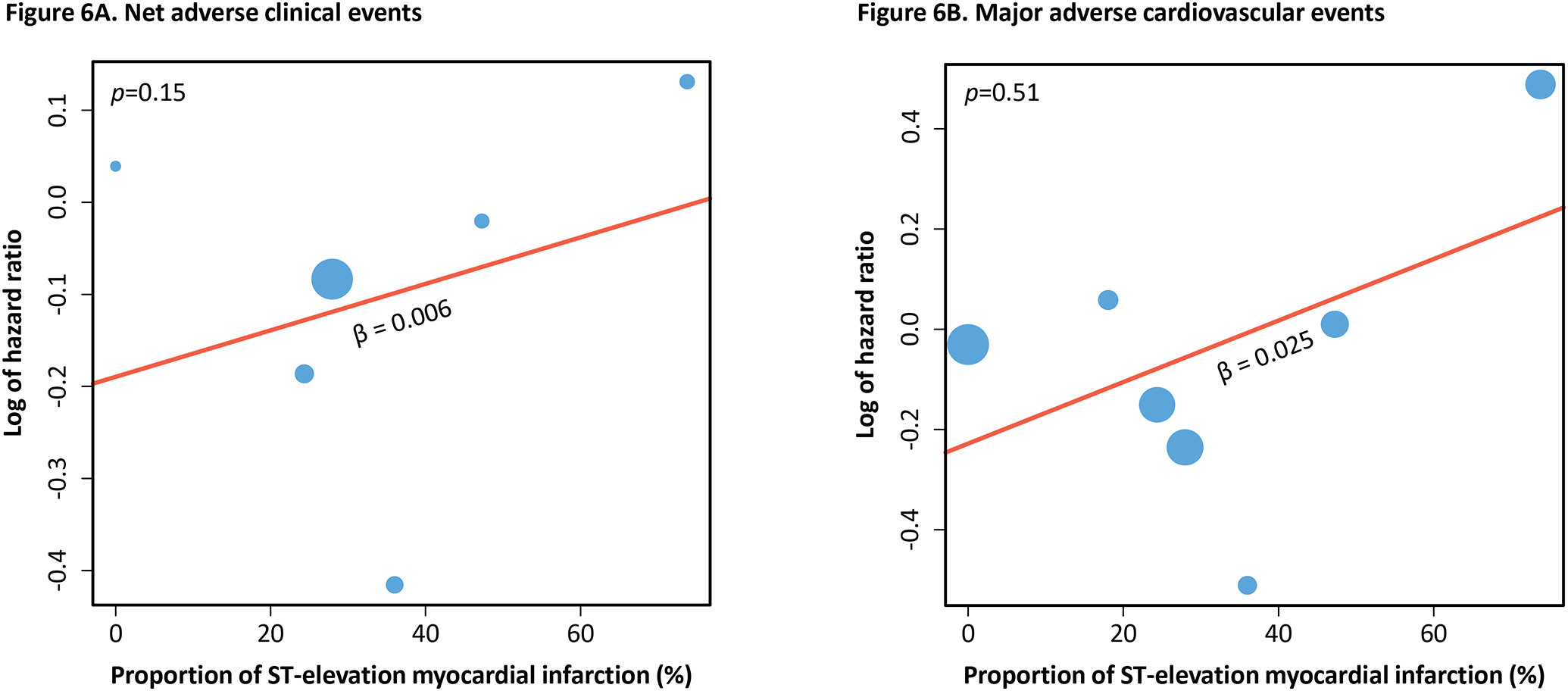
The two graphs above show the association of the hazard ratio of net adverse clinical events (Figure 6A) and major adverse cardiovascular events (Figure 6B) with the proportion of ST-elevation myocardial infarction included in the trials. Positive beta coefficient signifies positive correlation and vice versa. P-value of the trend is shown in the top left corner of each graph.
Discussion
We present the first meta-analysis of shorter-duration DAPT post-DES in ACS versus standard longer-duration DAPT since the publication of the STOPDAPT-2 ACS trial and presentation of the MASTER-DAPT results. We detected no statistical difference in the primary outcomes of NACE and MACE when comparing the two approaches. As expected, there was a significant decrease of any bleeding and major bleeding in patients receiving a shorter duration of DAPT versus a longer duration of DAPT. We also detected a non-significant trend toward increasing risk of both NACE and MACE in trials with increased proportion of high-risk patients, including those with STEMI, LM and LAD lesions. Although these findings do not definitively establish the existence of a subgroup in which ischemic risk exceeds bleeding risk, they collectively raise the question of ischemic risks in those patients with high-risk features, such as left main or left anterior descending lesions and STEMI. Thus, the results of our analysis substantiate and add precision to the estimate of bleeding reduction with a shortened DAPT strategy but cannot conclusively settle the uncertainty around ischemic risk in patients with higher risk features. Dedicated future investigations into the safety of a shortened DAPT strategy in patients with the highest ischemic risk will be necessary to address this evidence gap.
Selection of DAPT therapy duration following DES placement in patients with ACS is of critical importance as it impacts both efficacy and safety outcomes. Since both ischemic risks, measured by MACE, and bleeding risks are associated with poorer outcomes following DES in ACS, it is important to maintain the proper balance to maximize safety and efficacy. The efficacy of long-term DAPT following PCI, irrespective of clinical presentation, has been well-established. Universal guidelines recommend 12 months of DAPT with aspirin and a P2Y12 inhibitor.1, 17, 18 Recent guideline recommendations acknowledge that a shorter duration of DAPT is reasonable in patients with stable ischemic heart disease following PCI. On the other hand, shorter duration of DAPT has not been recognized as an equivalent strategy for patients with ACS, unless clinically indicated in patients with high bleeding risk1. Advancement of pharmacological therapy, improvement of stent technology, and innovative ancillary devices such as intravascular ultrasound and optical coherence tomography have raised the question: can DAPT also be safely shortened in patients with ACS following PCI while maintaining efficacy? Our results suggest the answer may be yes for many patients, as we observed similar NACE and MACE between shorter- and longer-duration DAPT groups. Considering that many patients may have high bleeding risk, this abbreviated DAPT strategy may be valuable and especially relevant given that thrombotic risk of patients with ACS leading to ischemic events is known to decrease over time.19
Some prior studies have suggested the benefit of short-duration DAPT in reducing bleeding events without increasing CV events.20–23 However, one of the strengths of this meta-analysis is the inclusion of the most recent RCT results from STOPDAPT-2 ACS and the recently presented MASTER DAPT. Importantly, STOPDAPT-2 ACS demonstrated that a 1-month DAPT strategy did not meet non-inferiority criteria for the composite of CV or bleeding events compared with 12 months DAPT. Specifically, shorter duration of DAPT was associated with a reduction in major bleeding events, but with an increase in CV events. The presented results of MASTER DAPT, while not yet published, suggested a non-significant trend toward improvement in NACE, MACE, and any bleeding with a shortened duration of DAPT treatment. Ultimately, even with the inclusion of data from STOPDAPT-2 ACS, there was no statistically significant difference in MACE, CV mortality, MI. However, these results must be interpreted in the context of insufficient power to detect potentially meaningful differences. Thus, high ischemic risk subgroups may require more individualized care, particularly when considering the upper limits of the 95% confidence intervals (MACE 1.17, MI 1.39, CV mortality 2.13) (Figure 3).
The non-significant trends toward increasing risk of both NACE and MACE in trials with increased proportion of high-risk patients highlight an area of residual uncertainty. Hesitancy over a shortened DAPT approach in ACS patient populations is based on the heterogeneity of the trials in this space as well as the clinical, anatomic, and biochemical differences from those with stable ischemic heart disease, all which can increase the risk of major ischemic events. Most trials did not exclusively enroll patients with ACS and lacked data on lesion-specific characteristics and the complexity of the PCI performed, all of which may influence outcome observations. Only three of the included trials exclusively enrolled ACS patients,3–5 with the rest synthesized via subgroup analysis or post-hoc analyses. Moreover, the proportion of STEMI was also heterogeneous across trials. However, on a sensitivity analysis of the RCTs that exclusively enrolled patients with ACS, we found no significant statistical difference in the ischemic outcomes of interest. A meta-regression analysis of primary outcomes to proportion of STEMI also did not yield any statistically significant difference but did detect a positive trend in both NACE and MACE. Similarly, a meta-regression assessing studies by proportion of LM and LAD lesions detected a non-significant trend toward increasing NACE and MACE as the proportion of LM and LAD lesions increased. These findings suggest that patients presenting with high-risk ACS (STEMI) and/or high-risk lesions (LM and LAD disease) may not glean the same benefit from short-term DAPT as the general ACS population. They should be viewed as hypothesis-generating and merit further dedicated investigation. This may include future patient-level meta-analyses to further delineate specific patient, presentation and/or procedural characteristics associated with increased risk to inform clinical decision-making. Ultimately, our results may justify future large clinical trials, focusing on the highest-risk cohorts of patients, including those with STEMI and those with high-risk anatomy. In the interim, the established 12 months DAPT duration should remain the standard for most patients considered high-risk for ischemic events, including those with STEMI or LM and LAD disease.
Unsurprisingly, we observed a significant improvement in safety among patients receiving shorter duration of DAPT compared with a longer duration of DAPT, with a 41% decrease in any bleeding and a 53% decrease in major bleeding. Notably TICO, TWILIGHT ACS, and STOPDAPT-2 ACS all had shown significant reduction in any bleeding as well as major bleeding, while GLOBAL LEADERS demonstrated a significant reduction in major bleeding. Interestingly, with such a significant decrease in bleeding events, our results did not demonstrate a significant reduction in all-cause mortality. Explanation remains unclear, although it could be influenced by the competing risk of other secondary outcomes.
Choice of P2Y12 inhibitor varied across the trials included in this study. Although the use of clopidogrel in ACS has been evaluated many times with proof of its efficacy and was used in many of the studies included in this analysis, the newer P2Y12 inhibitors have become the standard-of-care treatment for ACS patients. Ticagrelor significantly reduced the rate of CV death, MI, or stroke without increasing overall major bleeding events when compared with clopidogrel in patients with ACS.24 Prasugrel significantly reduced CV morbidity and mortality at the expense of increased bleeding when compared with clopidogrel.25 Our sensitivity analysis stratified to older and newer antiplatelets also revealed a numerically higher HR of NACE and MACE in the aspirin/clopidogrel group compared with the ticagrelor/prasugrel group. Although these findings must be interpreted in the context of the limited number of studies including newer agents, which corresponds to a higher risk of type 2 error, they may be considered hypothesis-generating. Similarly, the failure to prove noninferiority of efficacy in the STOPDAPT-2 ACS trial could be partially due to the use of clopidogrel, an older generation P2Y12. This contrasts with other studies that used ticagrelor, such as TICO, TWILIGHT ACS, and GLOBAL LEADERS, whose sensitivity analysis demonstrated numerically lower hazard of primary outcomes compared to RCTs that used clopidogrel or aspirin (Figure S4). In addition, clopidogrel has been known to have high resistance amongst Asians, with certain communities reaching rates up to 70%.26 STOPDAPT-2 ACS was conducted in a Japanese patient population, and unfortunately did not include clopidogrel resistance testing as part of the trial. This casts further uncertainty around the efficacy outcomes of STOPDAPT-2 ACS.
Results of this meta-analysis contrast with the current guidelines which advocate for longer duration of DAPT following DES in ACS according to the ACC/AHA as well as ESC guidelines. Although there appears to be a potential trend toward decreased efficacy in the highest risk (STEMI, LAD and LM) subgroups regardless of baseline patient profile, ischemic risks, types and locations of lesions, clinical presentation, and number of stents placed, there was no statistically significant difference in efficacy endpoints. This study reinforces the bleeding risk reduction benefits associated with shorter duration DAPT, but cannot entirely dispel the ischemic risk uncertainty, especially when higher risk ischemic features are identified.
Limitations
The results of this meta-analysis should be considered with the following limitations.
First, we did not have access to patient-level data which would have allowed more granular analyses of specific subgroups and assessment of independent patient and procedural characteristics that may have influenced our observations. Many of the trials were open label which can lead to increased performance bias. Varying types of DES were also used across studies, with first-generation DES used in RESET, while others used second-generation DES and bioresorbable polymer DES. The newer generation stents have significantly lower rates of restenosis and stent thrombosis when compared to first generation stents.27 A large portion of the study population were of East Asian descent, which may compromise the generalizability of the findings to people of other ethnicities. In particular, there exists an ethnic-based difference in patient response to P2Y12 inhibitors. In East Asians, the association between platelet reactivity and both ischemic and bleeding outcomes may differ from that of Westerners.28 Bleeding definitions differed across RCTs, with BARC versus TIMI criteria used, and some not reporting major bleeding at all. Primary outcome reporting also differed as MACE was not reported in OPTIMIZE and RESET, while NACE was not reported in TWILIGHT ACS and SMART-CHOICE. These differences in outcome reporting can add imprecision to the meta-analysis results. The RCTs also differed in their analytic strategy, with four studies utilizing intention to treat and two studies using per protocol analysis, the latter of which may introduce bias. Results of the meta-regression were largely driven by the STOPDAPT-2 ACS trial, which had higher proportion of STEMI as well as LM or LAD coronary artery disease. Therefore, what appears to be two distinct factors may be one colinear marker of elevated ischemic risk. This trial also had its own limitations, including heterogeneity in design, restriction to the East Asian population, and potential reduced efficacy of clopidogrel, which could have biased the results seen in meta-regression analyses. Lastly, moderate heterogeneity was detected in all-cause mortality and repeat revascularization outcomes. Differences in baseline patient characteristics and individual risks, procedural characteristics including stent type used, trial design, and different types of P2Y12 inhibitor use could potentially explain the heterogeneity. Even with random effects method applied, heterogeneity was present in the outcomes, suggesting that more data may be needed.
Conclusion
Amongst patients receiving DES in ACS, this meta-analysis demonstrates that an abbreviated duration of DAPT is associated with reduced bleeding and a pooled estimate suggesting no significant difference in ischemic events when compared with standard duration of DAPT. However, uncertainty remains surrounding the risk of ischemic events with a shortened DAPT strategy for populations with higher risk ischemic features. Further studies will need to be conducted to definitively define the most appropriate management in the population at highest risk for ischemic events.
Supplementary Material
Highlights.
Shortened DAPT is associated with reduced bleeding risk compared with longer DAPT
While ischemic events are not increased with shortened DAPT compared with longer DAPT in the overall ACS population, uncertainty remains around patients with higher ischemic risk.
Funding:
no grants, contracts, or other forms of financial support was received
Dr. Rao reports institutional research funding from Bayer AG for role on steering committee. Dr. Nanna reports funding from the American College of Cardiology Foundation supported by the George F. and Ann Harris Bellows Foundation and from the National Institute on Aging/National Institutes of Health from R03AG074067 (GEMSSTAR award).
Footnotes
Disclosures:
Park DY: None
Wang P: None
An S: None
Frampton J: None
Conflict of Interest
Ohman M: Research Chiesi USA, Abiomed; Consulting: Cara Therapeutics, Cytokinetics, Milestone, Neurocrine, Otsuka, Pfizer, XyloCor Therapeutics.
Data Used
Data included in this study can be found as published articles in online databases.
Ethical approval
This study was exempt from ethics approval as only data from previously published studies were retrieved and synthesized.
References
- 1.Writing Committee M, Lawton JS, Tamis-Holland JE, Bangalore S, Bates ER, Beckie TM, et al. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol 2022;79(2):e21–e129. [DOI] [PubMed] [Google Scholar]
- 2.Hahn JY, Song YB, Oh JH, Cho DK, Lee JB, Doh JH, et al. 6-month versus 12-month or longer dual antiplatelet therapy after percutaneous coronary intervention in patients with acute coronary syndrome (SMART-DATE): a randomised, open-label, non-inferiority trial. Lancet 2018;391(10127):1274–1284. [DOI] [PubMed] [Google Scholar]
- 3.De Luca G, Damen SA, Camaro C, Benit E, Verdoia M, Rasoul S, et al. Final results of the randomised evaluation of short-term dual antiplatelet therapy in patients with acute coronary syndrome treated with a new-generation stent (REDUCE trial). EuroIntervention 2019;15(11):e990–e998. [DOI] [PubMed] [Google Scholar]
- 4.Kim BK, Hong SJ, Cho YH, Yun KH, Kim YH, Suh Y, et al. Effect of Ticagrelor Monotherapy vs Ticagrelor With Aspirin on Major Bleeding and Cardiovascular Events in Patients With Acute Coronary Syndrome: The TICO Randomized Clinical Trial. JAMA 2020;323(23):2407–2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Watanabe H, Morimoto T, Natsuaki M, Yamamoto K, Obayashi Y, Ogita M, et al. Comparison of Clopidogrel Monotherapy After 1 to 2 Months of Dual Antiplatelet Therapy With 12 Months of Dual Antiplatelet Therapy in Patients With Acute Coronary Syndrome: The STOPDAPT-2 ACS Randomized Clinical Trial. JAMA Cardiol 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yale University. Reference Deduplicator. In; 2022.
- 8.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. Bmj 2008;336(7650):924–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feres F, Costa RA, Abizaid A, Leon MB, Marin-Neto JA, Botelho RV, et al. Three vs twelve months of dual antiplatelet therapy after zotarolimus-eluting stents: the OPTIMIZE randomized trial. JAMA 2013;310(23):2510–22. [DOI] [PubMed] [Google Scholar]
- 11.Hahn JY, Song YB, Oh JH, Chun WJ, Park YH, Jang WJ, et al. Effect of P2Y12 Inhibitor Monotherapy vs Dual Antiplatelet Therapy on Cardiovascular Events in Patients Undergoing Percutaneous Coronary Intervention: The SMART-CHOICE Randomized Clinical Trial. JAMA 2019;321(24):2428–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.MASTER-DAPT—Coverage of TCT 2021. In. TCT 2021: Society of Cardiovascular Angiography & Interventions. [Google Scholar]
- 13.Baber U, Dangas G, Angiolillo DJ, Cohen DJ, Sharma SK, Nicolas J, et al. Ticagrelor alone vs. ticagrelor plus aspirin following percutaneous coronary intervention in patients with non-ST-segment elevation acute coronary syndromes: TWILIGHT-ACS. Eur Heart J 2020;41(37):3533–3545. [DOI] [PubMed] [Google Scholar]
- 14.Tomaniak M, Chichareon P, Onuma Y, Deliargyris EN, Takahashi K, Kogame N, et al. Benefit and Risks of Aspirin in Addition to Ticagrelor in Acute Coronary Syndromes: A Post Hoc Analysis of the Randomized GLOBAL LEADERS Trial. JAMA Cardiol 2019;4(11):1092–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim BK, Hong MK, Shin DH, Nam CM, Kim JS, Ko YG, et al. A new strategy for discontinuation of dual antiplatelet therapy: the RESET Trial (REal Safety and Efficacy of 3-month dual antiplatelet Therapy following Endeavor zotarolimus-eluting stent implantation). J Am Coll Cardiol 2012;60(15):1340–8. [DOI] [PubMed] [Google Scholar]
- 16.Valgimigli M, Frigoli E, Heg D, Tijssen J, Juni P, Vranckx P, et al. Dual Antiplatelet Therapy after PCI in Patients at High Bleeding Risk. N Engl J Med 2021;385(18):1643–1655. [DOI] [PubMed] [Google Scholar]
- 17.Valgimigli M, Bueno H, Byrne RA, Collet JP, Costa F, Jeppsson A, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2018;39(3):213–260. [DOI] [PubMed] [Google Scholar]
- 18.Levine GN, Bates ER, Bittl JA, Brindis RG, Fihn SD, Fleisher LA, et al. 2016 ACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients With Coronary Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2016;68(10):1082–115. [DOI] [PubMed] [Google Scholar]
- 19.Rodriguez F, Harrington RA. Management of Antithrombotic Therapy after Acute Coronary Syndromes. N Engl J Med 2021;384(5):452–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhong PY, Shang YS, Bai N, Ma Y, Niu Y, Wang ZL. Efficacy and Safety of Very Short-Term Dual Antiplatelet Therapy After Drug-Eluting Stents Implantation for Acute Coronary Syndrome: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Front Cardiovasc Med 2021;8:660360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benenati S, Galli M, De Marzo V, Pescetelli F, Toma M, Andreotti F, et al. Very short vs. long dual antiplatelet therapy after second generation drug-eluting stents in 35 785 patients undergoing percutaneous coronary interventions: a meta-analysis of randomized controlled trials. Eur Heart J Cardiovasc Pharmacother 2021;7(2):86–93. [DOI] [PubMed] [Google Scholar]
- 22.Misumida N, Abo-Aly M, Kim SM, Ogunbayo GO, Abdel-Latif A, Ziada KM. Efficacy and safety of short-term dual antiplatelet therapy (≤6 months) after percutaneous coronary intervention for acute coronary syndrome: A systematic review and meta-analysis of randomized controlled trials. Clin Cardiol 2018;41(11):1455–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khan SU, Singh M, Valavoor S, Khan MU, Lone AN, Khan MZ, et al. Dual Antiplatelet Therapy After Percutaneous Coronary Intervention and Drug-Eluting Stents: A Systematic Review and Network Meta-Analysis. Circulation 2020;142(15):1425–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2009;361(11):1045–57. [DOI] [PubMed] [Google Scholar]
- 25.Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2007;357(20):2001–15. [DOI] [PubMed] [Google Scholar]
- 26.Hasan MS, Basri HB, Hin LP, Stanslas J. Genetic polymorphisms and drug interactions leading to clopidogrel resistance: why the Asian population requires special attention. Int J Neurosci 2013;123(3):143–54. [DOI] [PubMed] [Google Scholar]
- 27.Tada T, Byrne RA, Simunovic I, King LA, Cassese S, Joner M, et al. Risk of stent thrombosis among bare-metal stents, first-generation drug-eluting stents, and second-generation drug-eluting stents: results from a registry of 18,334 patients. JACC Cardiovasc Interv 2013;6(12):1267–74. [DOI] [PubMed] [Google Scholar]
- 28.Jeong YH. “East asian paradox”: challenge for the current antiplatelet strategy of “one-guideline-fits-all races” in acute coronary syndrome. Curr Cardiol Rep 2014;16(5):485. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


