Abstract
Interleukin 10 (IL-10) is a pleiotropic cytokine with well known antiinflammatory, immunosuppressive, and immunostimulatory properties. Chronic allograft rejection, characterized by vascular neointimal proliferation, is a major cause of organ transplant loss, particularly in heart and kidney transplant recipients. In a Dark Agouti to Lewis rat model of aortic transplantation, we evaluated the effects of a single intramuscular injection of a recombinant adeno-associated viral vector (serotype 1) encoding IL-10 (rAAV1-IL-10) on neointimal proliferation and inflammation. rAAV1-IL-10 treatment resulted in a significant reduction of neointimal proliferation and graft infiltration with macrophages and T and B lymphocytes. The mechanism underlying the protective effects of IL-10 in aortic allografts involved heme oxygenase 1 (HO-1) because inhibition of HO activity reversed not only neointimal proliferation but also inflammatory cell infiltration. Our results indicate that IL-10 attenuates neointimal proliferation and inflammatory infiltration and strongly imply that HO-1 is an important intermediary through which IL-10 regulates the inflammatory responses associated with chronic vascular rejection.
Keywords: aortic transplantation, chronic transplant rejection, recombinant adeno-associated virus, transplant arteriosclerosis, vascular injury
Chronic vascular rejection is a major cause of the loss of functioning solid organ transplants. Typical lesions, such as intimal thickening, proliferation of vascular smooth muscle cells (SMC), and adventitial inflammation (1), are observed in chronic vasculopathy and are also characteristic of arteriosclerosis. These lesions occur because of immune- and nonimmune-mediated injury to the vascular endothelium which, in turn, leads to a narrowing of the vascular lumen, ischemia, and the development of fibrosis. A variety of lymphocyte- and monocyte-derived cytokines have shown an influence in the development of such vascular changes. In particular, interleukin 10 (IL-10) has gained significant attention because of its suppressive influence on inflammatory vascular changes such as arteriosclerosis, as well as immune processes leading to allograft rejection.
IL-10 is a pleiotropic cytokine with well known antiinflammatory, immunosuppressive, and immunostimulatory properties (2). One of its best known roles involves the ability to inhibit cytokine production by T cells (e.g., IL-2), natural killer cells (e.g., IFN-γ), and monocyte/macrophages (e.g., IL-1α and IL-1β, IL-6, IL-8, IL-12, TNF-α, and granulocyte-macrophage colony-stimulating factor) and induce anergy in T cells (2, 3). Quelling the cytokine milieu and effector functions of alloreactive T cells may represent an important method for preventing allograft rejection; however, IL-10 may also induce peripheral tolerance through its effect on antigen-presenting cells, particularly those involving dendritic cells (4). Furthermore, a subset of CD4+ cells that possess T regulatory cell properties (CD4+CD25+ phenotype) rely heavily on IL-10 for their development (5). IL-10 can also impair processes of allograft rejection by down-regulation of class II major histocompatibility complex (MHC) expression on monocytes and B7 costimulatory ligands on macrophages (6, 7). Importantly, it has been shown that IL-10 prevents the development of intimal hyperplasia and atherosclerosis (8-11) through its powerful inhibitory effects on monocytes (12-14), and directly, on activation and growth of vascular SMC (15, 16). It has been demonstrated recently that the antiinflammatory effects of IL-10 in a sepsis model are mediated by the expression of heme oxygenase 1 (HO-1) (17).
HO catalyzes the rate-limiting step in degradation of cellular heme producing equimolar quantities of biliverdin, iron, and carbon monoxide (CO) (18). Biliverdin is then converted to bilirubin. Two major isoforms of HO have been described: an inducible isoform, HO-1, and a constitutive isoform, HO-2. HO-1 is activated by a variety of oxidant stimuli, including cytokines and growth factors, and HO-1's induction serves as an adaptive and protective response to injury (reviewed in ref. 19). Evidence suggests that the induction of HO-1 is protective against organ transplant rejection and vascular neointimal proliferation after balloon injury (reviewed in refs. 20-22). However, a direct link between IL-10 and HO-1 in the transplant setting has not been examined.
In this study, we examined the effects of systemic IL-10 by using a recombinant adeno-associated viral vector (serotype 1) (rAAV1-IL-10) delivered by a single intramuscular injection in a rat model of chronic vascular rejection. We chose rAAV as a delivery vehicle because of its capacity for persistent transgene expression, the absence of viral coding sequences, and the relatively low potential for adverse immune responses and toxicity (reviewed in ref. 23). The mechanism underlying the therapeutic effects of IL-10, particularly the potential involvement of the HO-1 pathway, was also explored in these studies.
Methods
Vector Construction and Production. rAAV vectors expressing rat IL-10 (provided by Linda Watkins, University of Colorado, Boulder) or green fluorescence protein (GFP) were generated and purified according to methods described in ref. 24. The vector cassette consisted of a cytomegalovirus chicken β-actin hybrid promoter, rat IL-10, or GFP cDNAs, and a simian virus 40 polyadenylation sequence, flanked by AAV2 inverted terminal repeats. Vectors were transcapsidated, by using the helper plasmid pXYZ1, to provide viral capsids for the AAV1 serotype and purified by iodixanol gradient centrifugation and anion exchange (Q-Sepharose, Amersham Biosciences) chromatography (24). The physical titers of vector preparations were assessed by quantitative competitive PCR and dot-blot analysis.
IL-10 Gene Delivery and Aortic Transplantation. For full MHC incompatibility in the aortic transplant model, we chose Lewis rats as recipients and Dark Agouti (DA) rats as donors. Animals were housed at the animal care facility (University of Florida and University of Alabama at Birmingham), and the studies were approved by the Institutional Animal Care and Use Committee. rAAV1-IL10 or rAAV1-GFP (100 μl of PBS containing 1 × 1011 viral particles per 200 g of body weight) was administered by intramuscular injection (50 μl in each thigh) into recipient rats 8 weeks before aortic transplantation. An additional group received an equal volume of PBS. To determine the role of HO-1 in mediating the effects of IL-10, we blocked HO enzyme activity in a separate group of rats receiving rAAV1-IL10 by weekly s.c. injections of tin protoporphyrin (SnPP, 45 μmol/kg of body weight; Frontier Scientific, Logan, UT) beginning 1 day before transplantation through the end of the experiment. Each group consisted of five to eight animals.
Eight weeks after rAAV vector delivery, a segment of thoracic aorta was removed from DA rats under sterile conditions and preserved at 4°C in normal saline. The aortic segment was transplanted into Lewis rats below the renal arteries and above the iliac bifurcation as described in ref. 25. No immunosuppression was used. To control for the effects of vector administration and the surgical procedure, we also performed isografts (Lewis to Lewis) that were treated with an equivalent dose of rAAV1-GFP. The animals were followed for 8 weeks after transplantation and then killed, and the transplanted aortic grafts were harvested for analysis.
Measurement of IL-10. Blood samples were collected at 0, 4, 8, and 16 weeks after rAAV1-IL10, rAAV1-GFP, or PBS injection, and sera were analyzed for the presence of rat IL-10 by using a commercially available ELISA kit (OPTEIA kit, BD Biosciences Pharmingen) according to the supplier's instructions. The IL-10 concentrations were interpolated by using the softmax pro software (Molecular Devices) against the linear range on the standard curve.
Measurement of Neointimal Area of Aortic Allografts. For determination of vascular wall changes, aortic cross sections were stained with hematoxylin/eosin (H&E). Histomorphometric analyses were performed on images taken with a DMR Leica microscope (Leica, Bannockburn, IL) and image pro software (Media Cybernetics, Silver Spring, MD). Neointimal and medial areas were calculated in three to five sections per aortic graft. The medial area was unchanged, as expected, and was used to control for interanimal variability.
Phenotypic Analyses of the Infiltrate. An immunohistochemical analysis of transplanted aortic segments was performed to determine the influence of IL-10 on the intensity of the infiltrate as well as on particular subtypes of graft-infiltrating cells. Five rings from each aortic allograft were fixed overnight in formalin and embedded in paraffin. Next, 4-μm sections were deparaf-finized, blocked for endogenous peroxidase, and incubated at 95°C in Trilogy solution (Cell Marque, Hot Springs, AR) for antigen retrieval. Slides were incubated with primary antibodies against T cells (CD3), T helper cells (CD4), cytotoxic T cells (CD8), B cells (CD45RA) (all from BD Biosciences Pharmingen) and monocytes (CD68, DAKO). Appropriate secondary antibodies were used based on the origin of the primary antibodies. Detection was achieved by using an avidin-biotin conjugate kit (Vector Laboratories). The total area of positively stained cells/total adventitial area for each of the antigens was determined by using image pro software and a DMR Leica microscope. Such counts were performed on three to five consecutive aortic sections for each aortic transplant. Counts were performed by two separate observers on blinded sections to ensure validity of the method.
Western Blot Analysis. A segment of the aortic graft was washed twice with ice-cold PBS and lysed in a buffer containing a broad-spectrum mixture of protease inhibitors and Triton X-100. Immunoblot analysis was performed as described in ref. 26 by using an anti-HO-1 antibody (1:500 dilution, Stressgen Biotechnologies, Victoria, BC, Canada) followed by incubation with peroxidase-conjugated goat anti-rabbit IgG antibody (1:10,000 dilution) for 1 h. The membranes were reprobed with an anti-actin antibody (1:1,000; Sigma) to confirm equal loading.
HO Enzyme Activity Assay. Splenic HO activity was measured by bilirubin generation as described in ref. 27. Briefly, a microsomal fraction prepared from splenic tissue by differential centrifugation was added to a reaction mixture (400 μl) containing 3 mg of rat liver cytosol (a source for biliverdin reductase), 20 μM hemin, 2 mM glucose 6-phosphate, 0.2 unit of glucose-6-phosphate dehydrogenase, and 0.8 mM β-NADPH and incubated at 37°C for 1 h in the dark. One milliliter of chloroform was added to extract the bilirubin, and the change in absorbance at 464-530 nm was measured. The concentration of bilirubin was calculated by using the extinction coefficient 40 mM-1·cm-1, and enzyme activity was expressed as pmol of bilirubin formed per 60 min per mg of protein.
Statistical Analysis. Statistical analysis was performed by using ANOVA and the Student-Neuman-Keuls posttest analysis. All data are presented as the mean ± SEM. Statistical significance was defined as P < 0.05.
Results
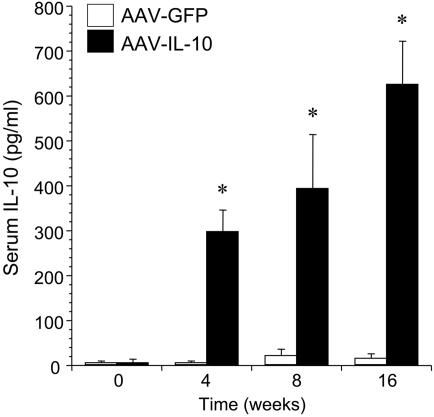
Effect of rAAV-Mediated IL-10 Treatment on Neointimal Proliferation in Rat Aortic Transplantation. To achieve elevated levels of systemic IL-10, recipient Lewis rats received a single intramuscular administration of a rAAV1-IL10 vector 8 weeks before aortic transplantation. As shown in Fig. 1, a significant increase in serum levels of IL-10 was observed at 4, 8, and 16 weeks after the intramuscular injection of rAAV1-IL10, compared with the control group of animals treated with rAAV1-GFP. The vector dose was determined based on pilot studies to provide serum IL-10 levels of 400-600 pg/ml at the time of transplantation.
Fig. 1.
Serum levels of IL-10 in recipient Lewis rats at 0, 4, 8, and 16 weeks after a single intramuscular administration of rAAV1-IL-10 or rAAV1-GFP (100 μl of PBS containing 1 × 1011 viral particles per 200 g of body weight). *, P < 0.01 for rAAV1-IL-10-injected animals versus rAAV1-GFP control at respective time points; n = 5-8 per group.
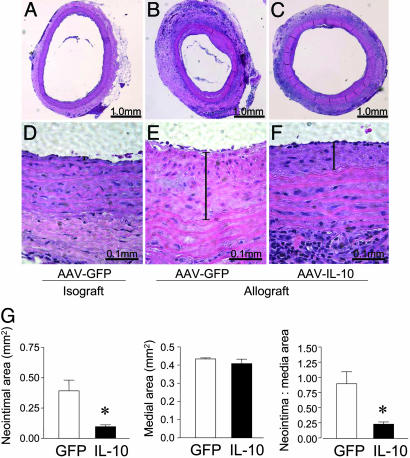
The effects of IL-10 on the vascular changes in the rat aortic grafts were analyzed by histology and morphometry at 8 weeks after transplantation. As demonstrated in Fig. 2 C and F, IL-10 markedly reduced the neointimal proliferation that was observed clearly in the control GFP group (Fig. 2 B and E). Neointimal proliferation in the PBS-treated animals receiving aortic allografts was similar to the rAAV1-GFP group (data not shown). No neointimal proliferation was seen in aortic isografts from animals treated with rAAV1-GFP (Fig. 2 A and D). A quantitative analysis of the aortic allografts showed a significant (P < 0.01) reduction in the neointimal area as well as the neointimal/medial ratio in the IL-10 group as compared with the GFP controls (Fig. 2G).
Fig. 2.
The effect of IL-10 treatment on neointimal proliferation in aortic allografts. (A-C) H&E staining of explanted aortic grafts (Lewis-to-Lewis isografts and DA-to-Lewis allografts) 8 weeks after transplantation. (D-F) Higher-magnification images showing the neointimal layer (vertical bar) in the respective groups. (G) Morphometric analysis of neointimal and medial area performed on explanted aortic grafts. Three to five sections per aortic segment were examined. Data are presented as mean ± SEM from five or six animals per group, *, P < 0.01 for rAAV1-IL-10 versus rAAV1-GFP.
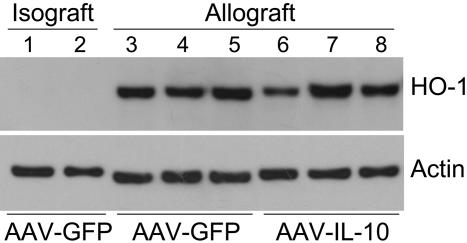
Role of HO-1 Expression and Activity in IL-10-Mediated Inhibition of Neointimal Proliferation. Both IL-10 (8-11) and the expression of HO-1 (28, 29) have been demonstrated to exert antiinflammatory properties resulting in vascular protection in allograft rejection as well as in balloon-induced vascular injury. However, a clear link between the effects of IL-10 and HO activity in chronic rejection has not yet been established. In this study, we first examined HO-1 protein expression in the explanted grafts. As demonstrated in Fig. 3, aortic allografts from both IL-10 and GFP groups demonstrated markedly elevated levels of HO-1. No difference in HO-1 expression was observed between the IL-10 and GFP groups. Consistent with observations from ref. 30, the expression of HO-1 was localized predominantly to infiltrating cells (data not shown). In the isografts, however, HO-1 levels were undetectable, suggesting that the immune injury associated with allograft rejection in both groups led to maximal local expression of HO-1, which was not affected by IL-10.
Fig. 3.
HO-1 protein expression by Western blot analysis in aortic grafts 8 weeks after transplantation. Lanes 1 and 2, aortic isografts (Lewis-to-Lewis transplants) from animals treated with AAV-GFP; lanes 3-5, aortic allografts (DA-to-Lewis transplants) from animals treated with AAV-GFP; lanes 6-8, aortic allografts from animals treated with AAV-IL-10. Each lane represents an individual transplant and was loaded with 20 μg of protein.
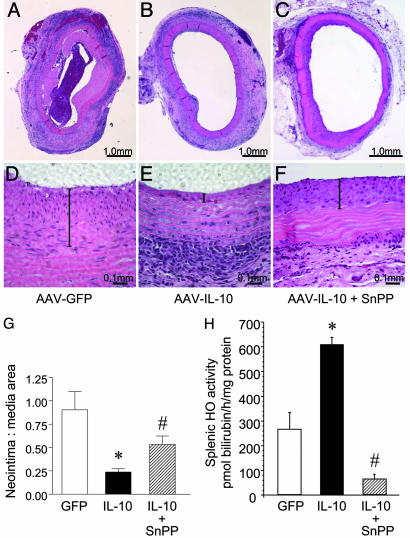
To explore the effects of systemic HO inhibition on the protective effects of IL-10 in preventing neointimal proliferation, aortic allograft recipients were treated with SnPP. IL-10 levels in the IL-10 plus SnPP-treated animals were not significantly different from the IL-10-alone group (245 ± 31 pg/ml at 4 weeks; 365 ± 108 pg/ml at 8 weeks; and 438 ± 77 pg/ml at 16 weeks). As shown in Fig. 4 C and F, blockade of HO reversed the decrease in neointimal proliferation observed in the IL-10-treated animals (Fig. 4 B and E), a quantitative analysis of which is shown in Fig. 4G. Inhibition of HO enzyme activity by SnPP (Fig. 4H) was confirmed in splenic microsomes, a site for constitutive expression of HO-1 (31). Interestingly, IL-10 significantly increased splenic HO activity, an effect abolished by SnPP (Fig. 4H).
Fig. 4.
The effects of HO blockade on neointimal proliferation in aortic allografts. To block HO activity, Lewis recipients of DA aortic segments received weekly s.c. injections of SnPP. (A-C) H&E staining of explanted aortic allografts 8 weeks after transplantation. (D-F) Higher-magnification images of the neointimal layer (vertical bar) in the respective groups. (G) Morphometric analysis of neointimal-medial area performed on explanted aortic grafts. Three to five sections per aortic segment were examined. Data are presented as mean ± SEM from five or six animals per group, *, P < 0.001, IL-10 versus GFP control; #, P < 0.05, IL-10 + SnPP versus IL-10 group. (H) Effect of SnPP on HO activity in aortic allograft recipients treated with rAAV1-GFP, rAAV1-IL-10, or rAAV1-IL-10 + SnPP. HO activity was determined in splenic microsomes as described in Methods. Data are presented as mean ± SEM; n = 5 or 6 animals per group; *, P < 0.01, IL-10 versus GFP control, #, P < 0.01, IL-10 + SnPP versus IL-10 and GFP groups.
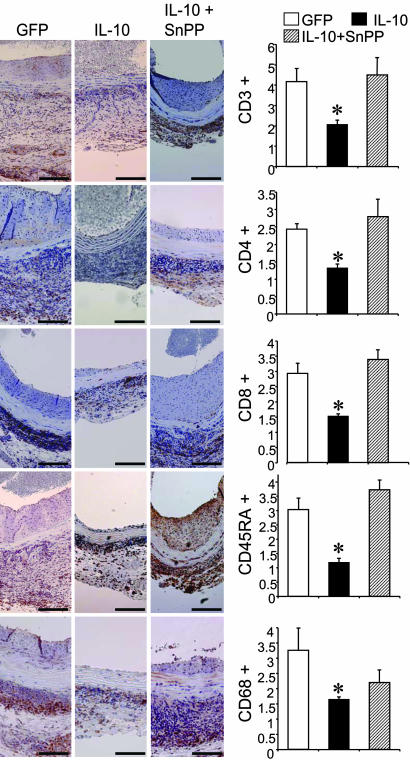
Influence of HO-1 on IL-10-Mediated Suppression of Adventitial Infiltrate in Aortic Allografts. Adventitial infiltration with lymphocytes and monocytes/macrophages is a consistent feature of immune-mediated injury in rat aortic allografts. As shown in Fig. 5, the explanted grafts from control recipients treated with GFP displayed a marked adventitial infiltration with T cells (CD3+) with its helper (CD4+) and effector (CD8+) subsets, B cells (CD45RA+), and monocytes/macrophages (CD68+). As expected, the IL-10-treated animals showed significant reduction in the adventitial infiltrate for all of the above cell populations by 50% (for CD3+), 47% (for CD4+), 48% (for CD8+), 62% (for CD45RA+), and 50% (for CD68+). Interestingly, systemic HO blockade in recipient rats with SnPP resulted in a complete reversal of this protective effect for CD3+, CD4+, CD8+, and CD45RA+ infiltrating cells. Although there was an increase in the density of CD68+ cells in the IL-10 + SnPP group, it did not reach statistical significance. Taken together, these results demonstrate that HO activity, at least in part, mediates the modulatory effects of IL-10 on chronic rejection of rat aortic allografts in terms of both neointimal proliferation and inflammation.
Fig. 5.
Effects of HO blockade on IL-10-mediated attenuation of inflammatory infiltrate in aortic allografts. Immunohistochemical analysis was performed on aortic allografts 8 weeks after transplantation. (Left) Immunostaining for T cells (CD3, CD4, and CD8), B cells (CD45RA) and macrophages (CD68) in the indicated groups. (Magnification bars: 0.1 mm.) (Right) Morphometric analysis of the area of positive adventitial staining for the respective marker in relation to the total adventitial area. Three to five sections per aortic segment were examined. Data are presented as mean ± SEM from n = 3-7 animals per group; *, P < 0.05, IL-10 versus GFP and IL-10 + SnPP groups for all markers except CD68 where *, P < 0.05 only for IL-10 versus GFP group.
Discussion
Neointimal proliferation and inflammation are characteristic features of chronic vascular rejection, a process that leads to significant loss of functioning organ transplants. The results of this study show that IL-10, delivered by a single intramuscular administration of a rAAV serotype 1 vector, can effectively prevent vascular changes associated with chronic rejection in a rat model of aortic transplantation. The findings show that HO-1 mediates the beneficial effects of IL-10 in aortic allografts because inhibition of HO activity reverses not only neointimal proliferation, but also inflammatory cell infiltration. This study demonstrates a mechanistic link between the protective effects of IL-10 and HO-1 in transplantation.
IL-10 has been an attractive candidate for immunomodulatory therapies aimed at improving graft survival because of its ability to promote Th2-type immune responses with concomitant suppression of Th1 responses. The other effects of IL-10 that promote allograft survival include suppression of leukocyte adhesion and migration, promotion of regulatory T cell development, inhibition of antigen-specific responses, and regulation of IL-10 receptor levels (2, 32). IL-10-deficient mice receiving cardiac allografts exhibit significantly greater leukocyte infiltration and vascular neointimal proliferation compared with wild-type recipients (2, 32). On the other hand, increased IL-10 levels achieved by the administration of a recombinant protein or by using a gene delivery system inhibits vascular neointimal proliferation after balloon injury and transplant-related arteriosclerosis (10, 11, 32). Our approach involving the use of a rAAV vector for IL-10 delivery eliminates the need for repeated administration as well as the adverse immune effects related to other viral vectors. It also allows for constitutive and sustained levels of expression not observed in previous studies of IL-10 administration.
The IL-10 receptor is expressed on vascular SMC, and IL-10 has direct inhibitory effects on SMC proliferation in vitro and in vivo in balloon injury-induced intimal hyperplasia (16). However, the intracellular mechanisms of these effects are incompletely understood. Interestingly, the vascular protective effects of HO-1 follow a pattern very similar to that of IL-10 (22, 33, 34), which prompted us to investigate the link between IL-10 and HO-1 in our model. The protective effects of HO-1 have been demonstrated in several models of transplantation (reviewed in refs. 21, 35, and 36). Chemical or genetic manipulation to increase HO-1 expression showed significant reduction in neointimal proliferation in a model of aortic transplantation (33) and in mechanically induced vascular injury (22, 34, 37). In addition, HO-1 has been shown to mediate the antiinflammatory properties of IL-10 in endotoxin-induced septic shock (17). Our studies extend these important observations to chronic allograft rejection.
Transplant rejection is associated with increased expression of HO-1, which is localized predominantly to infiltrating cells (30). This induction is attributed to several stimuli, including heme, cytokines, growth factors, and nitric oxide (30), which are all relevant to immune-mediated tissue injury. Although we observed increased local expression of HO-1 in allografts, the difference between the IL-10 and GFP-treated grafts was not significant. However, IL-10 treatment resulted in increased splenic HO activity and was associated with graft protection. These results are consistent with Araujo et al. (38) that systemic, rather than local, expression of HO-1 is more important for allograft survival. Using HO-1 transgenic mice, Araujo et al. reported that hearts transplanted from MHC-mismatched donors into transgenic recipients displayed significantly longer survival rates than when transgenic hearts were transplanted into MHC-mismatched recipient animals. Several recent studies have suggested that the neointimal lesion in transplant vasculopathy consists predominantly of myointimal cells derived from circulating progenitor cells (39, 40). We speculate that IL-10 or the IL-10-HO-1 pathway modulates recruitment of progenitor cells to the site of vascular injury and modifies progression of the neointimal lesion.
The protective properties of HO-1 have been ascribed to the antiinflammatory, antiapoptotic, and antiproliferative effects of one or more of its products (41, 42). Increased HO activity leads to the generation of CO and biliverdin, both of which, when administered systemically, are associated with prolonged graft survival (28, 43). CO, among other functions, has antiapoptotic effects (44) and also suppresses SMC proliferation (37). Recent studies have also linked the protective effects of HO-1 to concomitant up-regulation of the cell cycle regulatory protein p21 in renal epithelial cells (45) and SMC (34), an effect shared by IL-10, as shown in macrophages (46). Otterbein et al. (47) have demonstrated that CO exerts antiinflammatory effects, in part, by increasing macrophage IL-10 production. Inoue et al. (48) have corroborated these findings by providing evidence that the overexpression of HO-1 in macrophages leads to a significant increase in macrophage-derived IL-10 levels.
Up-regulation of HO activity has been shown to interfere with leukocyte adhesion to vascular endothelium by changing the expression of various adhesion molecules (49-51), a phenomenon that has been attributed to biliverdin and/or bilirubin, rather than CO (49, 50). Recent studies have shown that treatment of graft recipients with biliverdin decreases intragraft inflammation and inhibits T cell proliferation (43), an effect also observed with CO (52, 53). Together, these findings suggest a significant role of HO-1 in regulating the processes involved in allograft rejection.
In summary, our studies demonstrate that the protective effects of IL-10, administered by using a rAAV vector, are mediated through a HO-1-dependent mechanism in a model of aortic transplantation. Our studies further substantiate the role of HO-1 as a “therapeutic funnel” (54) because it appears to mediate the action of several other molecules that have been used to improve graft survival, including rapamycin (55) and statins (56).
Acknowledgments
We acknowledge Dr. Linda Watkins (University of Colorado, Boulder, CO) for providing rat IL-10 cDNA. We thank Richard Snyder and Vince Chiodo for help with vector packaging. The work was supported by Grant 4-2000-947 from the Juvenile Diabetes Research Foundation.
Author contributions: K.I.B., C.C.T., and A.A. designed research; S.C., M.H.K., and O.Y.G. performed research; C.W., M.C.-T., J.S.D., R.J., P.E.C., W.W.H., K.M.M., B.P.C., K.I.B., M.A.A., and T.R.F. contributed new reagents/analytic tools; S.C., M.H.K., C.W., and A.A. analyzed data; and S.C., M.H.K., and A.A. wrote the paper.
Abbreviations: H&E, hematoxylin/eosin; HO-1, heme oxygenase 1; rAAV, recombinant adeno-associated virus; SMC, smooth muscle cells; SnPP, tin protoporphyrin; DA, Dark Agouti.
References
- 1.Demetris, A. J., Zerbe, T. & Banner, B. (1989) Transplant. Proc. 21, 3667-3669. [PubMed] [Google Scholar]
- 2.Moore, K. W., de Waal Malefyt, R., Coffman, R. L. & O'Garra, A. (2001) Annu. Rev. Immunol. 19, 683-765. [DOI] [PubMed] [Google Scholar]
- 3.Ding, Y., Chen, D., Tarcsafalvi, A., Su, R., Qin, L. & Bromberg, J. S. (2003) J. Immunol. 170, 1383-1391. [DOI] [PubMed] [Google Scholar]
- 4.Muller, G., Muller, A., Tuting, T., Steinbrink, K., Saloga, J., Szalma, C., Knop, J. & Enk, A. H. (2002) J. Invest. Dermatol. 119, 836-841. [DOI] [PubMed] [Google Scholar]
- 5.Maloy, K. J., Salaun, L., Cahill, R., Dougan, G., Saunders, N. J. & Powrie, F. (2003) J. Exp. Med. 197, 111-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Waal Malefyt, R., Haanen, J., Spits, H., Roncarolo, M. G., te Velde, A., Figdor, C., Johnson, K., Kastelein, R., Yssel, H. & de Vries, J. E. (1991) J. Exp. Med. 174, 915-924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ding, L., Linsley, P. S., Huang, L. Y., Germain, R. N. & Shevach, E. M. (1993) J. Immunol. 151, 1224-1234. [PubMed] [Google Scholar]
- 8.Caligiuri, G., Rudling, M., Ollivier, V., Jacob, M. P., Michel, J. B., Hansson, G. K. & Nicoletti, A. (2003) Mol. Med. 9, 10-17. [PMC free article] [PubMed] [Google Scholar]
- 9.Mallat, Z., Besnard, S., Duriez, M., Deleuze, V., Emmanuel, F., Bureau, M. F., Soubrier, F., Esposito, B., Duez, H., Fievet, C., et al. (1999) Circ. Res. 85, e17-24. [DOI] [PubMed] [Google Scholar]
- 10.Pinderski Oslund, L. J., Hedrick, C. C., Olvera, T., Hagenbaugh, A., Territo, M., Berliner, J. A. & Fyfe, A. I. (1999) Arterioscler. Thromb. Vasc. Biol. 19, 2847-2853. [DOI] [PubMed] [Google Scholar]
- 11.Feldman, L. J., Aguirre, L., Ziol, M., Bridou, J. P., Nevo, N., Michel, J. B. & Steg, P. G. (2000) Circulation 101, 908-916. [DOI] [PubMed] [Google Scholar]
- 12.Bogdan, C., Vodovotz, Y. & Nathan, C. (1991) J. Exp. Med. 174, 1549-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Waal Malefyt, R., Abrams, J., Bennett, B., Figdor, C. G. & de Vries, J. E. (1991) J. Exp. Med. 174, 1209-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fiorentino, D. F., Zlotnik, A., Mosmann, T. R., Howard, M. & O'Garra, A. (1991) J. Immunol. 147, 3815-3822. [PubMed] [Google Scholar]
- 15.Selzman, C. H., McIntyre, R. C., Jr., Shames, B. D., Whitehill, T. A., Banerjee, A. & Harken, A. H. (1998) J. Mol. Cell. Cardiol. 30, 889-896. [DOI] [PubMed] [Google Scholar]
- 16.Mazighi, M., Pelle, A., Gonzalez, W., Mtairag, E. M., Philippe, M., Henin, D., Michel, J. B. & Feldman, L. J. (2004) Am. J. Physiol. 287, H866-H871. [DOI] [PubMed] [Google Scholar]
- 17.Lee, T. S. & Chau, L. Y. (2002) Nat. Med. 8, 240-246. [DOI] [PubMed] [Google Scholar]
- 18.Maines, M. D. (1997) Annu. Rev. Pharmacol. Toxicol. 37, 517-554. [DOI] [PubMed] [Google Scholar]
- 19.Platt, J. L. & Nath, K. A. (1998) Nat. Med. 4, 1364-1365. [DOI] [PubMed] [Google Scholar]
- 20.Durante, W. (2003) J. Cell. Physiol. 195, 373-382. [DOI] [PubMed] [Google Scholar]
- 21.Katori, M., Busuttil, R. W. & Kupiec-Weglinski, J. W. (2002) Transplantation 74, 905-912. [DOI] [PubMed] [Google Scholar]
- 22.Tulis, D. A., Durante, W., Peyton, K. J., Evans, A. J. & Schafer, A. I. (2001) Atherosclerosis (Shannon, Ireland) 155, 113-122. [DOI] [PubMed] [Google Scholar]
- 23.Kapturczak, M. H., Flotte, T. & Atkinson, M. A. (2001) Curr. Mol. Med. 1, 245-258. [DOI] [PubMed] [Google Scholar]
- 24.Zolotukhin, S., Potter, M., Zolotukhin, I., Sakai, Y., Loiler, S., Fraites, T. J., Jr., Chiodo, V. A., Phillipsberg, T., Muzyczka, N., Hauswirth, W. W., et al. (2002) Methods 28, 158-167. [DOI] [PubMed] [Google Scholar]
- 25.Mennander, A., Tiisala, S., Halttunen, J., Yilmaz, S., Paavonen, T. & Hayry, P. (1991) Arteriosclerosis (Dallas) 11, 671-680. [DOI] [PubMed] [Google Scholar]
- 26.Kapturczak, M., Wasserfall, C., Brusko, T., Campbell-Thompson, M., Ellis, T., Atkinson, M. & Agarwal, A. (2004) Am. J. Pathol. 165, 1045-1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Agarwal, A., Balla, J., Alam, J., Croatt, A. J. & Nath, K. A. (1995) Kidney Int. 48, 1298-1307. [DOI] [PubMed] [Google Scholar]
- 28.Otterbein, L. E., Zuckerbraun, B. S., Haga, M., Liu, F., Song, R., Usheva, A., Stachulak, C., Bodyak, N., Smith, R. N., Csizmadia, E., et al. (2003) Nat. Med. 9, 183-190. [DOI] [PubMed] [Google Scholar]
- 29.Chauveau, C., Bouchet, D., Roussel, J. C., Mathieu, P., Braudeau, C., Renaudin, K., Tesson, L., Soulillou, J. P., Iyer, S., Buelow, R. & Anegon, I. (2002) Am. J. Transplant. 2, 581-592. [DOI] [PubMed] [Google Scholar]
- 30.Agarwal, A., Kim, Y., Matas, A. J., Alam, J. & Nath, K. A. (1996) Transplantation 61, 93-98. [DOI] [PubMed] [Google Scholar]
- 31.Braggins, P. E., Trakshel, G. M., Kutty, R. K. & Maines, M. D. (1986) Biochem. Biophys. Res. Commun. 141, 528-533. [DOI] [PubMed] [Google Scholar]
- 32.Raisanen-Sokolowski, A., Glysing-Jensen, T. & Russell, M. E. (1998) Am. J. Pathol. 153, 1491-1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bouche, D., Chauveau, C., Roussel, J. C., Mathieu, P., Braudeau, C., Tesson, L., Soulillou, J. P., Iyer, S., Buelow, R. & Anegon, I. (2002) Transplant Immunol. 9, 235-238. [DOI] [PubMed] [Google Scholar]
- 34.Duckers, H. J., Boehm, M., True, A. L., Yet, S. F., San, H., Park, J. L., Clinton Webb, R., Lee, M. E., Nabel, G. J. & Nabel, E. G. (2001) Nat. Med. 7, 693-698. [DOI] [PubMed] [Google Scholar]
- 35.Agarwal, A. & Nick, H. S. (2000) J. Am. Soc. Nephrol. 11, 965-973. [DOI] [PubMed] [Google Scholar]
- 36.Hill-Kapturczak, N., Chang, S. H. & Agarwal, A. (2002) DNA Cell Biol. 21, 307-321. [DOI] [PubMed] [Google Scholar]
- 37.Togane, Y., Morita, T., Suematsu, M., Ishimura, Y., Yamazaki, J. I. & Katayama, S. (2000) Am. J. Physiol. 278, H623-H632. [DOI] [PubMed] [Google Scholar]
- 38.Araujo, J. A., Meng, L., Tward, A. D., Hancock, W. W., Zhai, Y., Lee, A., Ishikawa, K., Iyer, S., Buelow, R., Busuttil, R. W., et al. (2003) J. Immunol. 171, 1572-1580. [DOI] [PubMed] [Google Scholar]
- 39.Kong, D., Melo, L. G., Mangi, A. A., Zhang, L., Lopez-Ilasaca, M., Perrella, M. A., Liew, C. C., Pratt, R. E. & Dzau, V. J. (2004) Circulation 109, 1769-1775. [DOI] [PubMed] [Google Scholar]
- 40.Hillebrands, J. L., Klatter, F. A., van den Hurk, B. M., Popa, E. R., Nieuwenhuis, P. & Rozing, J. (2001) J. Clin. Invest. 107, 1411-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Balla, J., Jacob, H. S., Balla, G., Nath, K., Eaton, J. W. & Vercellotti, G. M. (1993) Proc. Natl. Acad. Sci. USA 90, 9285-9289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nath, K. A., Grande, J. P., Croatt, A. J., Likely, S., Hebbel, R. P. & Enright, H. (1998) Kidney Int. 53, 100-111. [DOI] [PubMed] [Google Scholar]
- 43.Yamashita, K., McDaid, J., Ollinger, R., Tsui, T. Y., Berberat, P. O., Usheva, A., Csizmadia, E., Smith, R. N., Soares, M. P. & Bach, F. H. (2004) FASEB J. 18, 765-767. [DOI] [PubMed] [Google Scholar]
- 44.Thom, S. R., Fisher, D., Xu, Y. A., Notarfrancesco, K. & Ischiropoulos, H. (2000) Proc. Natl. Acad. Sci. USA 97, 1305-1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Inguaggiato, P., Gonzalez-Michaca, L., Croatt, A. J., Haggard, J. J., Alam, J. & Nath, K. A. (2001) Kidney Int. 60, 2181-2191. [DOI] [PubMed] [Google Scholar]
- 46.O'Farrell, A. M., Parry, D. A., Zindy, F., Roussel, M. F., Lees, E., Moore, K. W. & Mui, A. L. (2000) J. Immunol. 164, 4607-4615. [DOI] [PubMed] [Google Scholar]
- 47.Otterbein, L. E., Bach, F. H., Alam, J., Soares, M., Tao Lu, H., Wysk, M., Davis, R. J., Flavell, R. A. & Choi, A. M. (2000) Nat. Med. 6, 422-428. [DOI] [PubMed] [Google Scholar]
- 48.Inoue, S., Suzuki, M., Nagashima, Y., Suzuki, S., Hashiba, T., Tsuburai, T., Ikehara, K., Matsuse, T. & Ishigatsubo, Y. (2001) Hum. Gene Ther. 12, 967-979. [DOI] [PubMed] [Google Scholar]
- 49.Hayashi, S., Takamiya, R., Yamaguchi, T., Matsumoto, K., Tojo, S. J., Tamatani, T., Kitajima, M., Makino, N., Ishimura, Y. & Suematsu, M. (1999) Circ. Res. 85, 663-671. [DOI] [PubMed] [Google Scholar]
- 50.Vachharajani, T. J., Work, J., Issekutz, A. C. & Granger, D. N. (2000) Am. J. Physiol. 278, H1613-H1617. [DOI] [PubMed] [Google Scholar]
- 51.Wagener, F. A., Eggert, A., Boerman, O. C., Oyen, W. J., Verhofstad, A., Abraham, N. G., Adema, G., van Kooyk, Y., de Witte, T. & Figdor, C. G. (2001) Blood 98, 1802-1811. [DOI] [PubMed] [Google Scholar]
- 52.Song, R., Mahidhara, R. S., Zhou, Z., Hoffman, R. A., Seol, D. W., Flavell, R. A., Billiar, T. R., Otterbein, L. E. & Choi, A. M. (2004) J. Immunol. 172, 1220-1226. [DOI] [PubMed] [Google Scholar]
- 53.Pae, H. O., Oh, G. S., Choi, B. M., Chae, S. C., Kim, Y. M., Chung, K. R. & Chung, H. T. (2004) J. Immunol. 172, 4744-4751. [DOI] [PubMed] [Google Scholar]
- 54.Otterbein, L. E., Soares, M. P., Yamashita, K. & Bach, F. H. (2003) Trends Immunol. 24, 449-455. [DOI] [PubMed] [Google Scholar]
- 55.Visner, G. A., Lu, F., Zhou, H., Liu, J., Kazemfar, K. & Agarwal, A. (2003) Circulation 107, 911-916. [DOI] [PubMed] [Google Scholar]
- 56.Lee, T. S., Chang, C. C., Zhu, Y. & Shyy, J. Y. (2004) Circulation 110, 1296-1302. [DOI] [PubMed] [Google Scholar]