Abstract
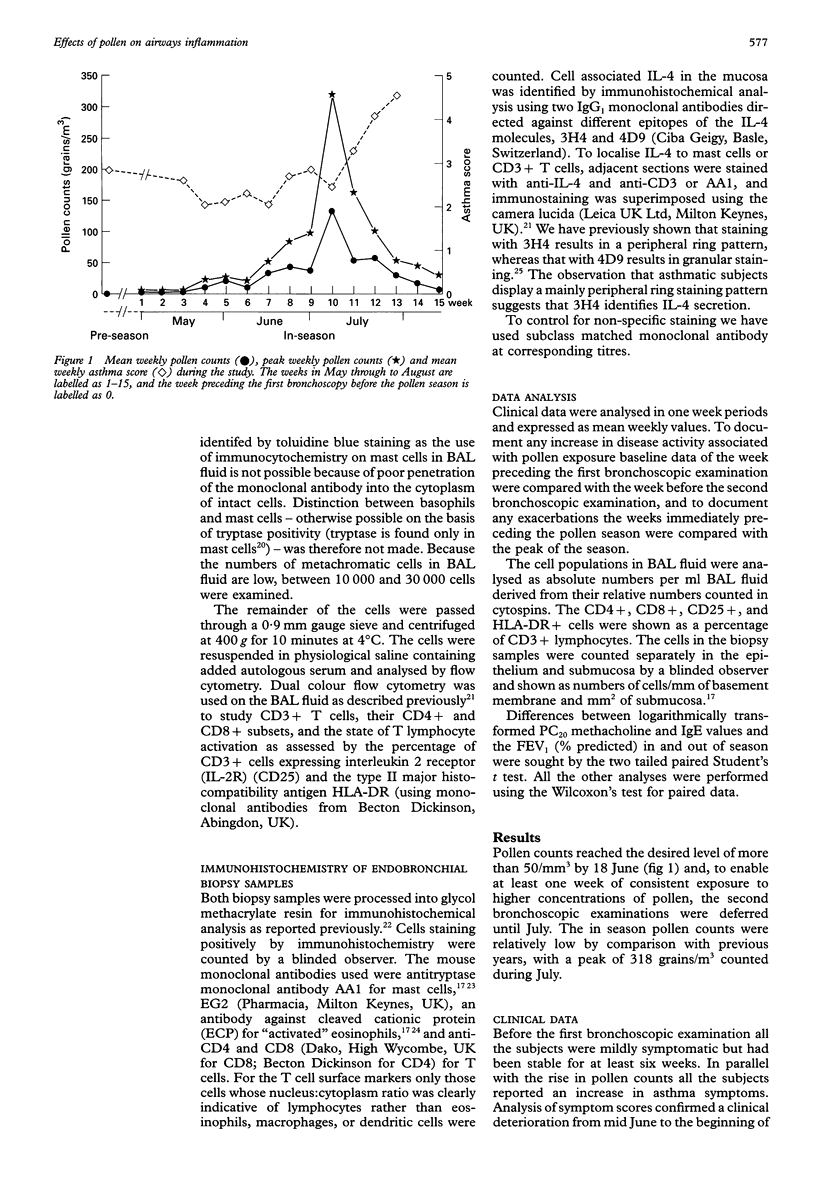
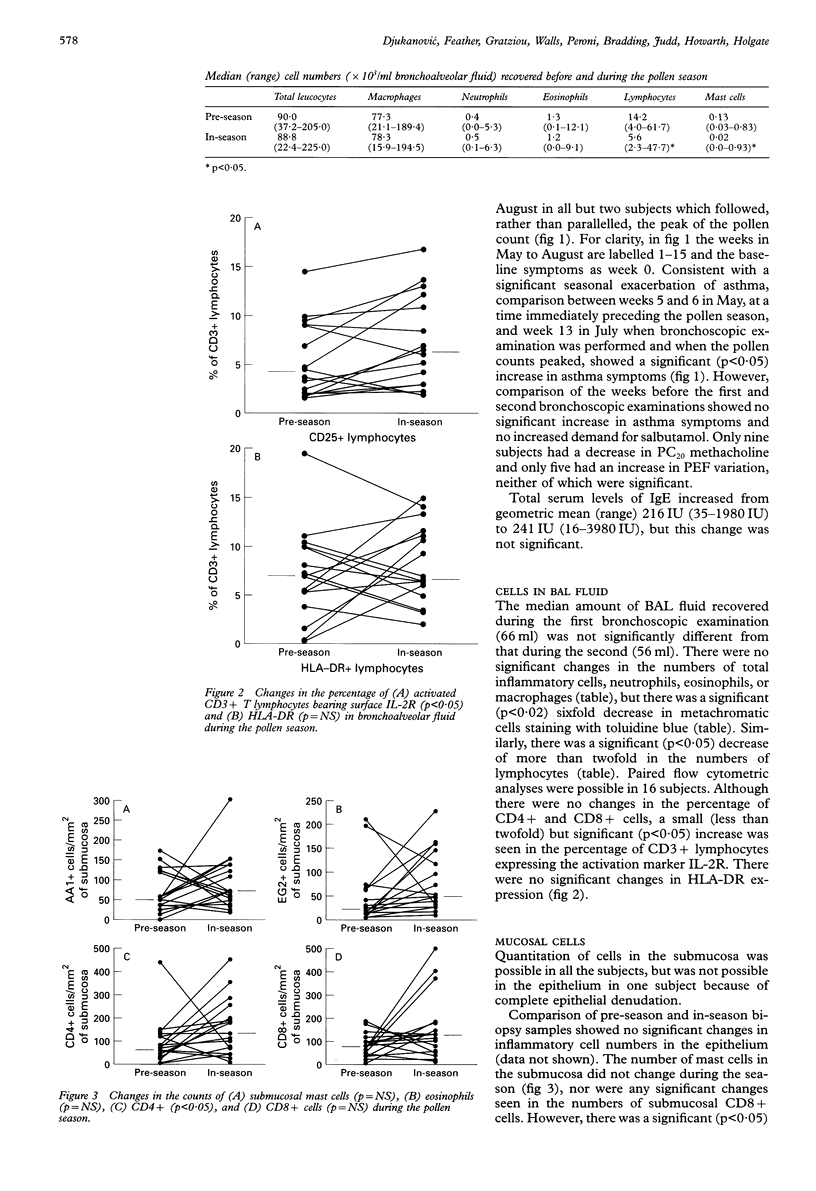
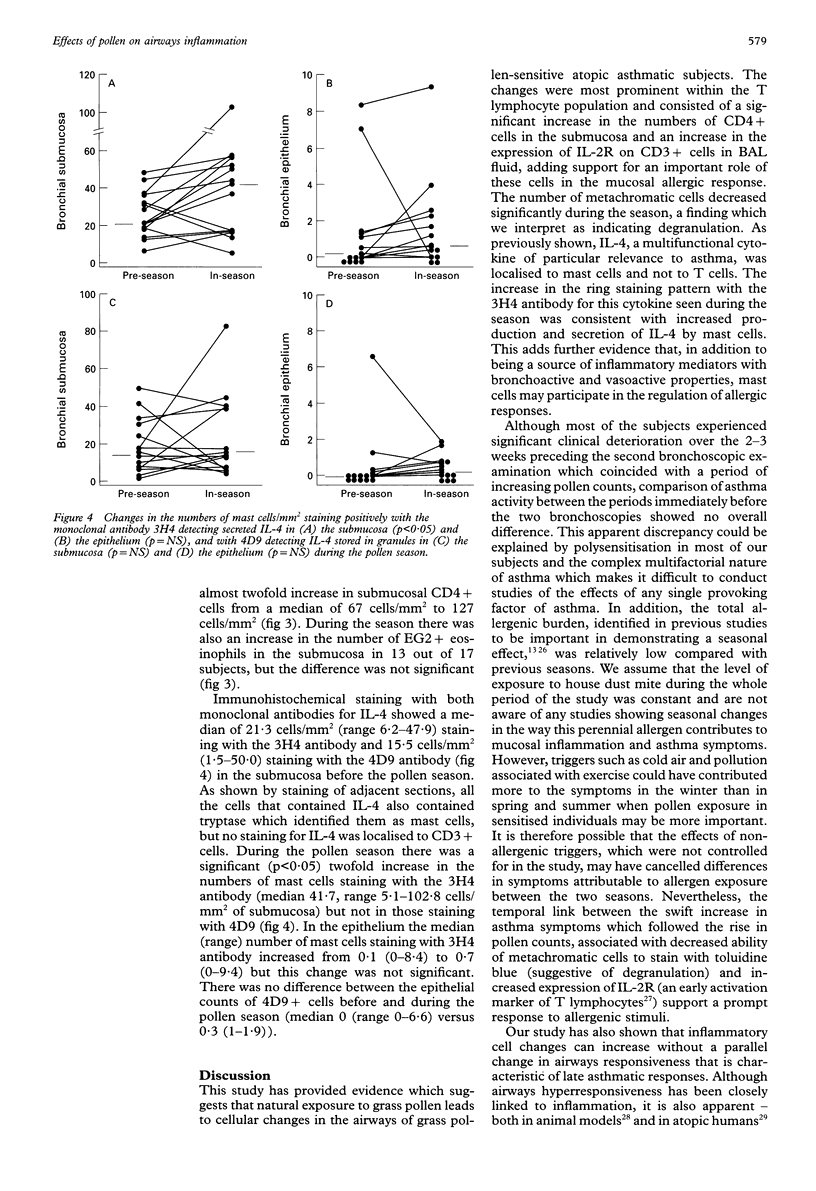
BACKGROUND: Bronchial challenge with allergen causes a specific form of airways inflammation consisting of an influx of neutrophils, eosinophils, and T cells. Because the relevance of the challenge model to clinical asthma is uncertain, the cellular changes that occur in the lungs of asthmatic subjects during natural seasonal allergen exposure were investigated. METHODS: Seventeen grass pollen sensitive asthmatic subjects with previously reported seasonal exacerbations of asthma kept records of symptoms and underwent fibreoptic bronchoscopy with bronchoalveolar lavage (BAL) and endobronchial biopsy before and during the peak of the grass pollen season. The BAL cells were analysed for differential cell counts and by flow cytometry for T cell subsets and surface activation markers. The biopsy samples were processed into glycol methacrylate resin and immunohistochemical analysis was performed for mast cells, activated eosinophils, T cells and interleukin 4 (IL-4), a cytokine with a pivotal role in allergen-induced inflammation. RESULTS: In the pollen season there was an increase in T lymphocyte activation in the BAL fluid as identified by increased expression of interleukin 2 receptor (IL-2R). In the submucosa these changes were paralleled by an increase in CD4+ T cells. By contrast, the numbers of metachromatic cells in BAL fluid staining with toluidine blue were reduced, possibly because of degranulation following allergen stimulation. In keeping with mast cell activation, the number of mucosal mast cells staining for secreted IL-4 increased during the season. In comparison with the period shortly before the onset of the season, all but two subjects experienced an asthma exacerbation which followed the rise in pollen counts but, compared with the period preceding the first bronchoscopic examination, asthma symptoms were not increased during the pollen season. CONCLUSIONS: The data suggest that natural allergen exposure, leading to a clinical exacerbation of asthma, may induce an inflammatory response involving T cells, mast cells and eosinophils. The relationship between allergen exposure, cellular infiltration and activation, and clinical symptoms appears to be complex, with factors other than allergen also contributing to asthmatic activity.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbato A., Pisetta F., Ragusa A., Marcer G., Zacchello F. Modification of bronchial hyperreactivity during pollen season in children allergic to grass. Ann Allergy. 1987 Feb;58(2):121–124. [PubMed] [Google Scholar]
- Boulet L. P., Cartier A., Thomson N. C., Roberts R. S., Dolovich J., Hargreave F. E. Asthma and increases in nonallergic bronchial responsiveness from seasonal pollen exposure. J Allergy Clin Immunol. 1983 Apr;71(4):399–406. doi: 10.1016/0091-6749(83)90069-6. [DOI] [PubMed] [Google Scholar]
- Bradding P., Feather I. H., Howarth P. H., Mueller R., Roberts J. A., Britten K., Bews J. P., Hunt T. C., Okayama Y., Heusser C. H. Interleukin 4 is localized to and released by human mast cells. J Exp Med. 1992 Nov 1;176(5):1381–1386. doi: 10.1084/jem.176.5.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradding P., Feather I. H., Wilson S., Bardin P. G., Heusser C. H., Holgate S. T., Howarth P. H. Immunolocalization of cytokines in the nasal mucosa of normal and perennial rhinitic subjects. The mast cell as a source of IL-4, IL-5, and IL-6 in human allergic mucosal inflammation. J Immunol. 1993 Oct 1;151(7):3853–3865. [PubMed] [Google Scholar]
- Bradding P., Roberts J. A., Britten K. M., Montefort S., Djukanovic R., Mueller R., Heusser C. H., Howarth P. H., Holgate S. T. Interleukin-4, -5, and -6 and tumor necrosis factor-alpha in normal and asthmatic airways: evidence for the human mast cell as a source of these cytokines. Am J Respir Cell Mol Biol. 1994 May;10(5):471–480. doi: 10.1165/ajrcmb.10.5.8179909. [DOI] [PubMed] [Google Scholar]
- Bradley B. L., Azzawi M., Jacobson M., Assoufi B., Collins J. V., Irani A. M., Schwartz L. B., Durham S. R., Jeffery P. K., Kay A. B. Eosinophils, T-lymphocytes, mast cells, neutrophils, and macrophages in bronchial biopsy specimens from atopic subjects with asthma: comparison with biopsy specimens from atopic subjects without asthma and normal control subjects and relationship to bronchial hyperresponsiveness. J Allergy Clin Immunol. 1991 Oct;88(4):661–674. doi: 10.1016/0091-6749(91)90160-p. [DOI] [PubMed] [Google Scholar]
- Cantrell D. A., Smith K. A. Transient expression of interleukin 2 receptors. Consequences for T cell growth. J Exp Med. 1983 Dec 1;158(6):1895–1911. doi: 10.1084/jem.158.6.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartier A., Thomson N. C., Frith P. A., Roberts R., Hargreave F. E. Allergen-induced increase in bronchial responsiveness to histamine: relationship to the late asthmatic response and change in airway caliber. J Allergy Clin Immunol. 1982 Sep;70(3):170–177. doi: 10.1016/0091-6749(82)90038-0. [DOI] [PubMed] [Google Scholar]
- Chai H., Farr R. S., Froehlich L. A., Mathison D. A., McLean J. A., Rosenthal R. R., Sheffer A. L., Spector S. L., Townley R. G. Standardization of bronchial inhalation challenge procedures. J Allergy Clin Immunol. 1975 Oct;56(4):323–327. doi: 10.1016/0091-6749(75)90107-4. [DOI] [PubMed] [Google Scholar]
- Chapman I. D., Foster A., Morley J. The relationship between inflammation and hyperreactivity of the airways in asthma. Clin Exp Allergy. 1993 Mar;23(3):168–171. doi: 10.1111/j.1365-2222.1993.tb00877.x. [DOI] [PubMed] [Google Scholar]
- Claman H. N., Choi K. L., Sujansky W., Vatter A. E. Mast cell "disappearance" in chronic murine graft-vs-host disease (GVHD)-ultrastructural demonstration of "phantom mast cells". J Immunol. 1986 Sep 15;137(6):2009–2013. [PubMed] [Google Scholar]
- Cotner T., Williams J. M., Christenson L., Shapiro H. M., Strom T. B., Strominger J. Simultaneous flow cytometric analysis of human T cell activation antigen expression and DNA content. J Exp Med. 1983 Feb 1;157(2):461–472. doi: 10.1084/jem.157.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Monchy J. G., Kauffman H. F., Venge P., Koëter G. H., Jansen H. M., Sluiter H. J., De Vries K. Bronchoalveolar eosinophilia during allergen-induced late asthmatic reactions. Am Rev Respir Dis. 1985 Mar;131(3):373–376. doi: 10.1164/arrd.1985.131.3.373. [DOI] [PubMed] [Google Scholar]
- Del Prete G., Maggi E., Parronchi P., Chrétien I., Tiri A., Macchia D., Ricci M., Banchereau J., De Vries J., Romagnani S. IL-4 is an essential factor for the IgE synthesis induced in vitro by human T cell clones and their supernatants. J Immunol. 1988 Jun 15;140(12):4193–4198. [PubMed] [Google Scholar]
- Diaz P., Gonzalez M. C., Galleguillos F. R., Ancic P., Cromwell O., Shepherd D., Durham S. R., Gleich G. J., Kay A. B. Leukocytes and mediators in bronchoalveolar lavage during allergen-induced late-phase asthmatic reactions. Am Rev Respir Dis. 1989 Jun;139(6):1383–1389. doi: 10.1164/ajrccm/139.6.1383. [DOI] [PubMed] [Google Scholar]
- Djukanović R., Lai C. K., Wilson J. W., Britten K. M., Wilson S. J., Roche W. R., Howarth P. H., Holgate S. T. Bronchial mucosal manifestations of atopy: a comparison of markers of inflammation between atopic asthmatics, atopic nonasthmatics and healthy controls. Eur Respir J. 1992 May;5(5):538–544. [PubMed] [Google Scholar]
- Djukanović R., Wilson J. W., Britten K. M., Wilson S. J., Walls A. F., Roche W. R., Howarth P. H., Holgate S. T. Quantitation of mast cells and eosinophils in the bronchial mucosa of symptomatic atopic asthmatics and healthy control subjects using immunohistochemistry. Am Rev Respir Dis. 1990 Oct;142(4):863–871. doi: 10.1164/ajrccm/142.4.863. [DOI] [PubMed] [Google Scholar]
- Djukanović R., Wilson J. W., Lai C. K., Holgate S. T., Howarth P. H. The safety aspects of fiberoptic bronchoscopy, bronchoalveolar lavage, and endobronchial biopsy in asthma. Am Rev Respir Dis. 1991 Apr;143(4 Pt 1):772–777. doi: 10.1164/ajrccm/143.4_Pt_1.772. [DOI] [PubMed] [Google Scholar]
- Enerbäck L., Pipkorn U., Olofsson A. Intraepithelial migration of mucosal mast cells in hay fever: ultrastructural observations. Int Arch Allergy Appl Immunol. 1986;81(4):289–297. doi: 10.1159/000234152. [DOI] [PubMed] [Google Scholar]
- Frew A. J., Kay A. B. The relationship between infiltrating CD4+ lymphocytes, activated eosinophils, and the magnitude of the allergen-induced late phase cutaneous reaction in man. J Immunol. 1988 Dec 15;141(12):4158–4164. [PubMed] [Google Scholar]
- Gerblich A. A., Salik H., Schuyler M. R. Dynamic T-cell changes in peripheral blood and bronchoalveolar lavage after antigen bronchoprovocation in asthmatics. Am Rev Respir Dis. 1991 Mar;143(3):533–537. doi: 10.1164/ajrccm/143.3.533. [DOI] [PubMed] [Google Scholar]
- Gleich G. J. The late phase of the immunoglobulin E-mediated reaction: a link between anaphylaxis and common allergic disease? J Allergy Clin Immunol. 1982 Sep;70(3):160–169. doi: 10.1016/0091-6749(82)90037-9. [DOI] [PubMed] [Google Scholar]
- Gonzalez M. C., Diaz P., Galleguillos F. R., Ancic P., Cromwell O., Kay A. B. Allergen-induced recruitment of bronchoalveolar helper (OKT4) and suppressor (OKT8) T-cells in asthma. Relative increases in OKT8 cells in single early responders compared with those in late-phase responders. Am Rev Respir Dis. 1987 Sep;136(3):600–604. doi: 10.1164/ajrccm/136.3.600. [DOI] [PubMed] [Google Scholar]
- Metzger W. J., Richerson H. B., Worden K., Monick M., Hunninghake G. W. Bronchoalveolar lavage of allergic asthmatic patients following allergen bronchoprovocation. Chest. 1986 Apr;89(4):477–483. doi: 10.1378/chest.89.4.477. [DOI] [PubMed] [Google Scholar]
- Metzger W. J., Zavala D., Richerson H. B., Moseley P., Iwamota P., Monick M., Sjoerdsma K., Hunninghake G. W. Local allergen challenge and bronchoalveolar lavage of allergic asthmatic lungs. Description of the model and local airway inflammation. Am Rev Respir Dis. 1987 Feb;135(2):433–440. doi: 10.1164/arrd.1987.135.2.433. [DOI] [PubMed] [Google Scholar]
- Pipkorn U., Karlsson G., Enerback L. The cellular response of the human allergic mucosa to natural allergen exposure. J Allergy Clin Immunol. 1988 Dec;82(6):1046–1054. doi: 10.1016/0091-6749(88)90143-1. [DOI] [PubMed] [Google Scholar]
- Rak S., Björnson A., Håkanson L., Sörenson S., Venge P. The effect of immunotherapy on eosinophil accumulation and production of eosinophil chemotactic activity in the lung of subjects with asthma during natural pollen exposure. J Allergy Clin Immunol. 1991 Dec;88(6):878–888. doi: 10.1016/0091-6749(91)90244-i. [DOI] [PubMed] [Google Scholar]
- Rossi G. A., Crimi E., Lantero S., Gianiorio P., Oddera S., Crimi P., Brusasco V. Late-phase asthmatic reaction to inhaled allergen is associated with early recruitment of eosinophils in the airways. Am Rev Respir Dis. 1991 Aug;144(2):379–383. doi: 10.1164/ajrccm/144.2.379. [DOI] [PubMed] [Google Scholar]
- Schleimer R. P., Sterbinsky S. A., Kaiser J., Bickel C. A., Klunk D. A., Tomioka K., Newman W., Luscinskas F. W., Gimbrone M. A., Jr, McIntyre B. W. IL-4 induces adherence of human eosinophils and basophils but not neutrophils to endothelium. Association with expression of VCAM-1. J Immunol. 1992 Feb 15;148(4):1086–1092. [PubMed] [Google Scholar]
- Tai P. C., Spry C. J., Peterson C., Venge P., Olsson I. Monoclonal antibodies distinguish between storage and secreted forms of eosinophil cationic protein. Nature. 1984 May 10;309(5964):182–184. doi: 10.1038/309182a0. [DOI] [PubMed] [Google Scholar]
- Thornhill M. H., Wellicome S. M., Mahiouz D. L., Lanchbury J. S., Kyan-Aung U., Haskard D. O. Tumor necrosis factor combines with IL-4 or IFN-gamma to selectively enhance endothelial cell adhesiveness for T cells. The contribution of vascular cell adhesion molecule-1-dependent and -independent binding mechanisms. J Immunol. 1991 Jan 15;146(2):592–598. [PubMed] [Google Scholar]
- Varney V. A., Jacobson M. R., Sudderick R. M., Robinson D. S., Irani A. M., Schwartz L. B., Mackay I. S., Kay A. B., Durham S. R. Immunohistology of the nasal mucosa following allergen-induced rhinitis. Identification of activated T lymphocytes, eosinophils, and neutrophils. Am Rev Respir Dis. 1992 Jul;146(1):170–176. doi: 10.1164/ajrccm/146.1.170. [DOI] [PubMed] [Google Scholar]
- Walls A. F., Jones D. B., Williams J. H., Church M. K., Holgate S. T. Immunohistochemical identification of mast cells in formaldehyde-fixed tissue using monoclonal antibodies specific for tryptase. J Pathol. 1990 Oct;162(2):119–126. doi: 10.1002/path.1711620204. [DOI] [PubMed] [Google Scholar]
- Wilson J. W., Djukanović R., Howarth P. H., Holgate S. T. Lymphocyte activation in bronchoalveolar lavage and peripheral blood in atopic asthma. Am Rev Respir Dis. 1992 Apr;145(4 Pt 1):958–960. doi: 10.1164/ajrccm/145.4_Pt_1.958. [DOI] [PubMed] [Google Scholar]


