Abstract
To monitor relative vaccine effectiveness (rVE) against COVID-19-related hospitalisation of the first, second and third COVID-19 booster (vs complete primary vaccination), we performed monthly Cox regression models using retrospective cohorts constructed from electronic health registries in eight European countries, October 2021–July 2023. Within 12 weeks of administration, each booster showed high rVE (≥ 70% for second and third boosters). However, as of July 2023, most of the relative benefit has waned, particularly in persons ≥ 80-years-old, while some protection remained in 65–79-year-olds.
Keywords: COVID-19, SARS-CoV-2, vaccine effectiveness, hospitalisation, cohort design, electronic health records, multi-country study
Since 2021, the Vaccine Effectiveness, Burden and Impact Studies of coronavirus disease 2019 (COVID-19) and influenza (VEBIS) project monitors vaccine effectiveness (VE) in real-world conditions to inform vaccination programmes in the European Union/European Economic Area (EU/EEA) countries [1]. One project aims to monitor real-time COVID-19 VE using electronic health registries (EHR) in multiple countries, with initial findings previously published [2-4]. We report pooled VE results against hospitalisation due to COVID-19 by number of doses received and time since vaccination in a community-dwelling resident population aged ≥ 65 years between October 2021 and July 2023.
Design of a monitoring framework to evaluate vaccine effectiveness of COVID-19
The study period covers 22 months, including the sequential circulation of different Omicron BA subvariants and the administration of adapted vaccines against Omicron BA subvariants (Phylogenetic Assignment of Named Global Outbreak (Pango) lineage designation B.1.1.529), in the autumn 2022. See Supplementary Figure S1 for the variation in the proportion of selected severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants over the study period in the participating sites.
Eight sites participated in the study: Denmark, Navarra (Spain), Norway and Portugal from October 2021, Belgium from July 2022, Luxembourg between July 2022 and February 2023, the Netherlands from November 2022 and Italy from December 2022. Retrospective population-cohorts were constructed at each site following a common protocol [5,6]. Individual deterministic linkage was used to cross-match administrative databases with registries for COVID-19 vaccination, SARS-CoV-2 testing, hospitalisations and, in some instances, surveillance data. Estimates of VE were produced monthly to have a near real-time monitoring system of COVID-19 VE and to be able to detect changes in VE for informing public health decisions. To accumulate sufficient events to support VE estimation, each monthly estimate included an observation period of 8 weeks, with a lag of 1 month for data consolidation (i.e. estimates produced in January 2023 covered October–November 2022). This resulted in an overlap in the observation periods, which were rolled 1 month forward for each successive monthly estimate.
The detailed methodology is published elsewhere [5,6]. Briefly, hospitalisation due to COVID-19 was defined as a hospital admission due to a severe acute respiratory infection with a SARS-CoV-2 positive test from 14 days before to 1 day after admission [7] or as COVID-19 as the main diagnosis in admission or discharge records. Vaccination status was time-varying and categorised as: (i) complete primary vaccination administered ≥ 24 weeks earlier (reference category in all models), (ii) first booster administered ≥ 90 days after primary vaccination, (iii) second booster administered ≥ 90 days after the first booster and (iv) third booster administered ≥ 90 days after the second booster. Time after each booster was categorised as: (i) 0 to < 12 weeks, (ii) 12 to < 24 weeks and (iii) ≥ 24 weeks. Time 0 was defined as the end of the induction period, i.e. 14 days after administration of the first dose.
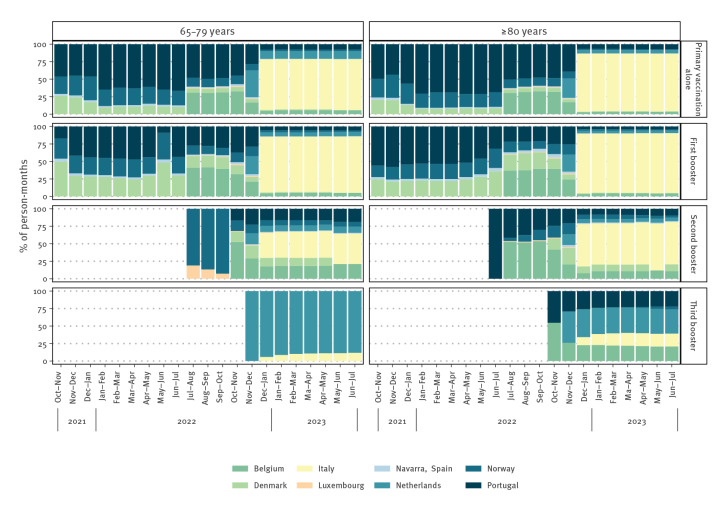
For each 8-week observation period, follow-up started on the first day and ended at the earliest date of either hospitalisation due to COVID-19, death, emigration outside the country or end of the observation period. Figure 1 shows the cumulative number of person-months by age, vaccination status and study site, reflecting the participation of each site and the timing of booster administration.
Figure 1.
Estimated person-months in VEBIS studies against COVID-19 hospitalisation in persons aged 65–79 and ≥ 80 years, by observation period, vaccination status, age-group and study site, eight European Union/European Economic Area countriesa, October 2021–July 2023
COVID-19: coronavirus disease 2019; VEBIS: Vaccine Effectiveness (VE), Burden and Impact Studies of COVID-19 and influenza.
a Participation in the study: Belgium (July 2022–July 2023), Denmark (October 2021–July 2023), Italy (December 2022–July 2023), Luxembourg (July 2022–March 2023), the Netherlands (November 2022–July 2023), Norway (October 2021–July 2023), Portugal (October 2021–July 2023) and Spain (Navarra) (October 2021–July 2023).
We used Cox proportional hazards models to estimate hazard ratios (HR) and 95% confidence intervals (CI) adjusted by sex, age group (in 5-year age bands), previous SARS-CoV-2 infection, comorbidities and other variables as relevant at each site [3,4] We computed relative VE (rVE) as (1-HR) × 100%. Estimates were pooled using Paule-Mandel random-effect meta-analysis [8].
Relative vaccine effectiveness by time since the last dose
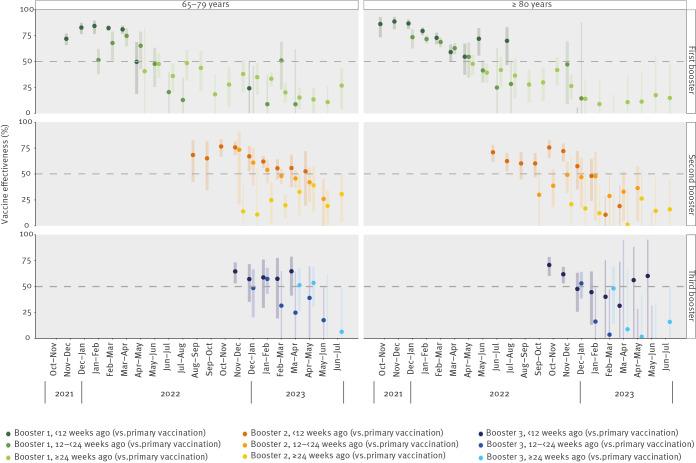
In persons aged ≥ 80 years, rVE of the first booster administered < 12 weeks earlier (Figure 2, Table 1), declined from 86% (95% CI: 73–93) in October–November 2021 to 55% (95% CI: 37–67) in April–May 2022. Estimates were inconsistent thereafter, possibly reflecting some individuals receiving the first booster during spring vaccination campaigns in 2022 (Belgium and Portugal). The relative VE of the first booster administered 12 to < 24 weeks earlier decreased from 73% (95% CI: 63–81) in December 2021–January 2022 to ≤ 50% in May–June 2022, with high uncertainty afterwards. When the first booster was administered ≥ 24 weeks earlier, the rVE was ≤ 50% throughout the study period and dropped to 15% (95% CI: −38 to 48) in June–July 2023. Similar estimates and overall trends were observed in persons in the age group of 65–79-years-old (Table 2).
Figure 2.
Estimated relative vaccine effectiveness against COVID-19 hospitalisation in persons aged 65–79 and ≥ 80 years in overlapping observation intervals of 8 weeks by number of vaccine doses and time since the latest dose, eight European Union/European Economic Area countriesa, October 2021–July 2023
a Participation in the study: Belgium (July 2022–July 2023), Denmark (October 2021–July 2023), Italy (December 2022–July 2023), Luxembourg (July 2022–March 2023), the Netherlands (November 2022–July 2023), Norway (October 2021–July 2023), Portugal (October 2021–July 2023) and Spain (Navarra) (October 2021–July 2023).
Table 1. Estimated relative vaccine effectiveness against COVID-19 hospitalisation in persons aged ≥ 80 years in overlapping observation intervals of 8 weeks by number of vaccine doses and time since the latest dose, eight European Union/European Economic Area countriesa, October 2021–July 2023.
| Period | Time since the last dose | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| < 12 weeks | 12 to < 24 weeks | ≥ 24 weeks | |||||||
| rVE (%) | 95% CI | Countries | rVE (%) | 95% CI | Countries | rVE (%) | 95% CI | Countries | |
| First boosterb | |||||||||
| 1 Oct–25 Nov 2021 | 86 | 73 to 93 | DK, ES, NO, PT | NA | NA | ||||
| 1 Nov–26 Dec 2021 | 89 | 81 to 93 | NA | NA | |||||
| 1 Dec 2021–25 Jan 2022 | 87 | 81 to 90 | 73 | 63 to 81 | DK, ES, PT | NA | |||
| 1 Jan–25 Feb 2022 | 79 | 75 to 83 | 72 | 68 to 75 | DK, ES, NO, PT | NA | |||
| 1 Feb–28 Mar 2022 | 73 | 67 to 78 | 69 | 64 to 73 | NA | ||||
| 1 Mar–25 Apr 2022 | 59 | 50 to 66 | DK, NO, PT | 63 | 57 to 68 | 50 | 2 to 75 | DK, ES, NO, PT | |
| 1 Apr–26 May 2022 | 55 | 37 to 67 | 55 | 34 to 68 | 48 | 37 to 57 | |||
| 1 May–25 Jun 2022 | 72 | 56 to 82 | PT | 41 | 30 to 51 | 39 | 29 to 48 | ||
| 1 Jun–26 Jul 2022 | NA | 25 | −53 to 63 | DK, NO, PT | 42 | 25 to 55 | |||
| 1 Jul–25 Aug 2022 | 70 | 46 to 83 | PT | 28 | −3 to 50 | BE, DK, NO, PT | 36 | 15 to 53 | |
| 1 Aug–25 Sep 2022 | NA | NA | 28 | 10 to 42 | BE, DK, ES, NO, PT | ||||
| 1 Sep–26 Oct 2022 | NA | NA | 30 | 12 to 44 | BE, ES, LU, NO, PT | ||||
| 1 Oct–25 Nov 2022 | NA | NA | 42 | 27 to 54 | BE, DK, ES, LU, NO, PT | ||||
| 1 Nov–26 Dec 2022 | NA | 47 | 9 to 69 | NO, PT | 26 | 13 to 37 | BE, DK, ES, LU, NL, NO, PT | ||
| 1 Dec 2022–25 Jan 2023 | −39 | −104 to 6 | IT | 14 | −502 to 88 | IT, NO | 14 | −10 to 33 | BE, DK, ES, IT, LU, NL, NO, PT |
| 1 Jan–25 Feb 2023 | NA | −79 | −179 to −15 | IT | 9 | −23 to 33 | |||
| 1 Feb–28 Mar 2023 | NA | NA | -8 | −42 to 18 | BE, DK, IT, LU, NL, NO, PT | ||||
| 1 Mar–25 Apr 2023 | NA | −82 | −206 to −8 | IT | 11 | −27 to 37 | BE, DK, IT, NL, NO, PT | ||
| 1 Apr–26 May 2023 | NA | −166 | −342 to −60 | IT | 11 | −31 to 40 | |||
| 1 May–25 Jun 2023 | NA | NA | 18 | −53 to 56 | BE, IT, NL, NO, PT | ||||
| 1 Jun–26 Jul 2023 | NA | NA | 15 | −38 to 48 | BE, ES, IT, NL, NO, PT | ||||
| Second boosterc | |||||||||
| 1 Jun–26 Jul 2022 | 71 | 61 to 78 | DK, PT | NA | NA | ||||
| 1 Jul–25 Aug 2022 | 62 | 53 to 70 | DK, NO, PT | NA | NA | ||||
| 1 Aug–25 Sep 2022 | 60 | 45 to 71 | BE, DK, NO, PT | NA | NA | ||||
| 1 Sep–26 Oct 2022 | 60 | 47 to 70 | BE, NO, PT | 30 | −7 to 54 | BE, LU, PT | NA | ||
| 1 Oct–25 Nov 2022 | 76 | 65 to 83 | 39 | 18 to 54 | BE, LU, NO, PT | NA | |||
| 1 Nov–26 Dec 2022 | 72 | 62 to 79 | BE, ES, NL, NO, PT | 49 | 32 to 62 | BE, LU, NL, NO, PT | 21 | −100 to 69 | LU, NL |
| 1 Dec 2022–25 Jan 2023 | 58 | 35 to 72 | BE, ES, IT, NL, NO, PT | 47 | 17 to 66 | BE, IT, LU, NL, NO, PT | 17 | −49 to 53 | BE, IT, LU, NL, PT |
| 1 Jan–25 Feb 2023 | 48 | 24 to 65 | 48 | 5 to 71 | BE, IT, NL, NO, PT | 12 | −29 to 40 | BE, IT, LU, NL, NO, PT | |
| 1 Feb–28 Mar 2023 | 11 | −12 to 29 | IT, LU | 29 | −2 to 50 | BE, ES, IT, LU, NL, NO, PT | −2 | −55 to 32 | |
| 1 Mar–25 Apr 2023 | 19 | −8 to 39 | DK, IT | 33 | 5 to 53 | BE, DK, ES, IT, NL, NO, PT | 1 | −53 to 36 | BE, IT, NL, NO, PT |
| 1 Apr–26 May 2023 | NA | 38 | 1 to 60 | 26 | −25 to 56 | BE, DK, ES, IT, NL, NO, PT | |||
| 1 May–25 Jun 2023 | NA | −16 | −57 to 14 | IT | 14 | −39 to 47 | BE, ES, IT, NL, NO, PT | ||
| 1 Jun–26 Jul 2023 | NA | NA | 16 | −26 to 44 | BE, DK, ES, IT, NL, NO, PT | ||||
| Third boosterd | |||||||||
| 1 Oct–25 Nov 2022 | 71 | 60 to 79 | BE | NA | NA | ||||
| 1 Nov–26 Dec 2022 | 62 | 53 to 69 | BE, NL, PT | NA | NA | ||||
| 1 Dec 2022–25 Jan 2023 | 48 | 26 to 63 | BE, IT, NL, PT | 53 | 38 to 64 | BE, NL, PT | NA | ||
| 1 Jan–25 Feb 2023 | 44 | 13 to 65 | 16 | −39 to 49 | BE, IT, NL, PT | NA | |||
| 1 Feb–28 Mar 2023 | 40 | −45 to 75 | 3 | −77 to 47 | 48 | 14 to 69 | BE, NL | ||
| 1 Mar–25 Apr 2023 | 31 | −81 to 74 | BE, IT, NL | −1,043 | −253,127 to 95 | 9 | −153 to 67 | BE, NL, NO, PT | |
| 1 Apr–26 May 2023 | 56 | −64 to 88 | BE, IT, NO | −1 | −42 to 28 | 1 | −67 to 42 | BE, IT, NL, PT | |
| 1 May–25 Jun 2023 | 60 | −237 to 95 | −69 | −319 to 32 | BE, IT, PT | 8 | −77 to 34 | ||
| 1 Jun–26 Jul 2023 | NA | NA | 16 | −42 to 50 | |||||
BE: Belgium; CI: confidence interval; DK: Denmark; ES: Spain (Navarra); IT: Italy; LU: Luxembourg; NA: not available; NL: the Netherlands; NO: Norway; PT: Portugal; rVE: relative vaccine effectiveness.
a Participation in the study: Belgium (July 2022–July 2023), Denmark (October 2021–July 2023), Italy (December 2022–July 2023), Luxembourg (July 2022–March 2023), the Netherlands (November 2022–July 2023), Norway (October 2021–July 2023), Portugal (October 2021–July 2023) and Spain (Navarra) (October 2021–July 2023).
b First booster rollout date: Belgium (September 2021), Denmark (October 2021), Italy (September 2021), Luxembourg (July 2021), the Netherlands (November 2021), Norway (October 2021), Portugal (October 2021) and Spain (Navarra) (October 2021).
c Second booster rollout date: Belgium (July 2022), Denmark (September 2022), Italy (April 2022), Luxembourg (April 2022), the Netherlands (February 2022), Norway (June 2022), Portugal (May 2022) and Spain (Navarra) (October 2022).
d Third booster rollout date: Belgium (September 2022), Italy (October 2022), the Netherlands (September 2022), Norway (March 2023) and Portugal (September 2022).
Countries with five events were included in the analysis.
Table 2. Estimated relative vaccine effectiveness against COVID-19 hospitalisation in persons aged 65–79 years, in overlapping observation intervals of 8 weeks by number of vaccine doses and time since the last dose, eight European Union/European Economic Area countriesa, November 2021–July 2023.
| Period | Time since the last dose | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| < 12 weeks | 12 to < 24 weeks | ≥ 24 weeks | |||||||
| rVE (%) | 95% CI | Countries | rVE (%) | 95% CI | Countries | rVE (%) | 95% CI | Countries | |
| First boosterb | |||||||||
| 1 Nov–26 Dec 2021 | 72 | 66 to 77 | DK, ES, NO, PT | NA | NA | ||||
| 1 Dec 2021–25 Jan 2022 | 83 | 77 to 87 | NA | NA | |||||
| 1 Jan–25 Feb 2022 | 84 | 77 to 89 | 51 | 38 to 62 | DK, ES, NO, PT | NA | |||
| 1 Feb–28 Mar 2022 | 82 | 79 to 85 | 68 | 51 to 79 | NA | ||||
| 1 Mar–25 Apr 2022 | 81 | 77 to 85 | DK, NO, PT | 75 | 65 to 82 | NA | |||
| 1 Apr–26 May 2022 | 50 | 19 to 69 | 65 | 43 to 79 | 41 | −98 to 82 | NO, PT | ||
| 1 May–25 Jun 2022 | NA | 48 | 25 to 63 | 48 | 34 to 58 | DK, ES, NO, PT | |||
| 1 Jun–26 Jul 2022 | NA | 20 | −4 to 40 | DK, NO, PT | 36 | 21 to 48 | |||
| 1 Jul–25 Aug 2022 | NA | 13 | −16 to 35 | BE, DK, LU, NO, PT | 48 | 32 to 61 | BE, DK, ES, LU, NO, PT | ||
| 1 Aug–25 Sep 2022 | NA | NA | 44 | 21 to 60 | |||||
| 1 Sep–26 Oct 2022 | NA | NA | 18 | −71 to 38 | |||||
| 1 Oct–25 Nov 2022 | NA | NA | 28 | 6 to 44 | |||||
| 1 Nov–26 Dec 2022 | NA | NA | 38 | 20 to 52 | BE, DK, ES, LU, NL, NO, PT | ||||
| 1 Dec 2022–25 Jan 2023 | 24 | −15 to 50 | DK, IT | -3 | −97 to 46 | IT, NO | 35 | 18 to 48 | BE, DK, ES, IT, LU, NL, NO, PT |
| 1 Jan–25 Feb 2023 | NA | 9 | −32 to 37 | IT, NL, NO | 33 | 26 to 40 | BE, DK, ES, IT, NL, NO, PT | ||
| 1 Feb–28 Mar 2023 | NA | 51 | 23 to 69 | DK, IT, LU, NL, PT | 20 | 10 to 29 | BE, DK, ES, IT, LU, NL, NO, PT | ||
| 1 Mar–25 Apr 2023 | NA | 9 | −119 to 62 | DK, IT, PT | 15 | 4 to 25 | BE, DK, ES, IT, NL, NO, PT | ||
| 1 Apr–26 May 2023 | NA | NA | 15 | −1 to 28 | BE, DK, IT, NL, NO, PT | ||||
| 1 May–25 Jun 2023 | NA | NA | 11 | −8 to 27 | BE, ES, IT, NL, NO, PT | ||||
| 1 Jun–26 Jul 2023 | NA | NA | 27 | 4 to 44 | |||||
| Second boosterc | |||||||||
| 1 Aug–25 Sep 2022 | 68 | 42 to 83 | NO | NA | NA | ||||
| 1 Sep–26 Oct 2022 | 65 | 34 to 81 | LU, NO | NA | NA | ||||
| 1 Oct–25 Nov 2022 | 77 | 66 to 83 | LU, NO, PT | NA | NA | ||||
| 1 Nov–26 Dec 2022 | 76 | 68 to 81 | BE, ES, NL, NO, PT | 73 | 21 to 91 | LU, NL, NO | 14 | −22 to 39 | NL |
| 1 Dec 2022–25 Jan 2023 | 67 | 53 to 77 | BE, DK, IT, LU, NL, NO, PT | 61 | 39 to 75 | IT, LU, NL, NO, PT | 11 | −76 to 55 | IT, NL |
| 1 Jan–25 Feb 2023 | 62 | 56 to 67 | BE, DK, IT, NL, NO, PT | 54 | 41 to 64 | BE, DK, IT, NL, NO, PT | 25 | 2 to 42 | DK, IT, NL, NO |
| 1 Feb–28 Mar 2023 | 56 | 45 to 64 | BE, IT, LU, NL, NO, PT | 48 | 40 to 55 | BE, ES, IT, LU, NL, NO, PT | 20 | 8 to 30 | IT, LU, NL, NO |
| 1 Mar–25 Apr 2023 | 56 | 37 to 69 | IT | 46 | 36 to 54 | BE, DK, ES, IT, NL, NO, PT | 33 | 10 to 50 | BE, IT, NL, NO, PT |
| 1 Apr–26 May 2023 | 53 | 19 to 72 | ES, IT | 42 | 19 to 59 | BE, DK, IT, NL, NO, PT | 38 | 8 to 58 | BE, DK, IT, NL, NO, PT |
| 1 May–25 Jun 2023 | NA | 26 | 0 to 45 | BE, IT, NO, PT | 19 | 1 to 34 | BE, DK, ES, IT, NL, NO, PT | ||
| 1 Jun–26 Jul 2023 | NA | NA | 31 | 3 to 50 | BE, ES, IT, NL, NO, PT | ||||
| Third boosterd | |||||||||
| 1 Nov–26 Dec 2022 | 65 | 53 to 73 | NL | NA | NA | ||||
| 1 Dec 2022–25 Jan 2023 | 57 | 35 to 72 | IT, NL | 48 | 20 to 67 | NL | NA | ||
| 1 Jan–25 Feb 2023 | 59 | 29 to 76 | 57 | 43 to 68 | NA | ||||
| 1 Feb–28 Mar 2023 | 57 | 19 to 78 | 31 | −34 to 65 | IT, NL | NA | |||
| 1 Mar–25 Apr 2023 | 65 | 41 to 79 | 25 | −60 to 65 | 51 | 26 to 68 | NL | ||
| 1 Apr–26 May 2023 | NA | 39 | −22 to 69 | 53 | 30 to 69 | ||||
| 1 May–25 Jun 2023 | NA | 17 | −35 to 49 | IT | 0 | −168 to 62 | IT, NL | ||
| 1 Jun–26 Jul 2023 | NA | NA | 6 | −71 to 49 | |||||
BE: Belgium; CI: confidence interval; DK: Denmark; ES: Spain (Navarra); IT: Italy; LU: Luxembourg; NA: not available; NL: the Netherlands; NO: Norway; PT: Portugal; rVE: relative vaccine effectiveness.
a Participation in the study: Belgium (July 2022–July 2023), Denmark (October 2021–July 2023), Italy (December 2022–July 2023), Luxembourg (July 2022–March 2023), the Netherlands (November 2022–July 2023), Norway (October 2021–July 2023), Portugal (October 2021–July 2023) and Spain (Navarra) (October 2021–July 2023).
b First booster rollout date: Belgium (September 2021), Denmark (October 2021), Italy (September 2021), Luxembourg (July 2021), the Netherlands (November 2021), Norway (October 2021), Portugal (October 2021) and Spain (Navarra) (October 2021).
c Second booster rollout date: Belgium (July 2022), Denmark (September 2022), Italy (April 2022), Luxembourg (April 2022), the Netherlands (February 2022), Norway (June 2022), Portugal (May 2022) and Spain (Navarra) (October 2022).
d Third booster rollout date: Belgium (September 2022), Italy (October 2022), the Netherlands (September 2022), Norway (March 2023) and Portugal (September 2022).
Countries with five events were included in the analysis.
In persons ≥ 80-years-old, rVE of the second booster administered < 12 weeks earlier (Figure 2, Table 1) fluctuated between 71 and 60% from June to October 2022, following spring campaigns (Belgium and Portugal) and between 76 and 72% from October to December 2022, following autumn campaigns and decreasing thereafter. For a second booster 12 to < 24 weeks earlier, rVE varied without a specific time-trend between a maximum of 49% (95% CI: 32–62) and no relative protection (−16%; 95% CI: −57 to 14). No relative protective effect was estimated for a second booster ≥ 24 weeks earlier. In 65–79-year-olds, a similar overall trend was observed, although rVE < 12 weeks earlier remained ≥ 50% until April–May 2023. Estimated rVE of the second booster was 26% (95% CI: 0–45) when administered 12 to < 24 weeks earlier (estimate for May–June 2023) and 31% (95% CI: 3–50) when administered ≥ 24 weeks earlier (estimate for June–July 2023).
The third booster has been recommended to persons aged ≥ 80 years in the Netherlands since September 2022, in Belgium, Italy and Portugal since October 2022, and in Norway since April 2023. When administered < 12 weeks earlier, rVE of the third booster ranged between 71% (95% CI: 60–79) in October–November 2022 and 44% (95% CI: 13–65) in January–February 2023. However, it waned rapidly, with mostly null rVE beyond 12 weeks, albeit with high uncertainty. In persons 65–79-years-old, the third booster had been rolled out only in Italy and the Netherlands since October 2022. Between November 2022 and April 2023, rVE of the third booster administered < 12 weeks earlier ranged between 57 and 65%, although no relative protective effect of the third booster was observed between 12 and < 24 weeks (since February–March 2023) nor beyond 24 weeks (since May–June 2023).
Discussion
Our results have implications for COVID-19 vaccination roll-out and the monitoring framework. They suggest that successive booster vaccine doses were important for restoring individual protection against COVID-19 hospitalisation in the context of waning effectiveness. However, as of July 2023, most of the relative benefit has waned, particularly in individuals ≥ 80 years, while low relative protection was still observed in persons aged 65–79 years with a first or second booster.
Adapted vaccines against Omicron BA subvariants administered in the autumn 2022 vaccination campaign showed similar rVE when given as the second (76%) or third booster (71%), suggesting that the time since the latest dose is more important for protection than the total number of doses administered. After 12 weeks, rVE declined, especially for the third booster in persons ≥ 80-years-old. These estimates and waning patterns have been observed in other studies [9-13]. Since March 2023, the circulation of the Omicron XBB.1.5 sublineage, with a higher immunity evasion capacity [14], has been increasing, reaching around or above 50% of the isolates in the EU/EEA in July 2023 [15]. This could also partially explain the decrease in the rVE, as other studies suggested [9,16,17].
A multi-country approach can provide representative estimates for the EU/EEA and assess different vaccination roll-out strategies (more details on the roll-out of booster doses by country are provided in Supplementary Table S1). However, estimates may vary due to countries contributing differently and over time, challenging the interpretation of the time-trends. Despite using national databases, a low number of events during certain observation periods, as seen in Supplementary Tables S2–3, resulted in high uncertainty. Additionally, misclassification of hospitalisations not due to COVID-19 cannot be ruled out, despite the specific case-definition. Finally, with increasing number of doses and decreasing coverage of the last dose, comparability across different vaccination statuses regarding vulnerability and past infection can be compromised, despite adjusting for comorbidities, socioeconomic variables and registered SARS-CoV-2 infections (where available). Differential depletion of susceptible persons can also lead to spurious or overestimated waning results [18].
Although the effect of vaccination is likely to differ with respect to prior SARS-CoV-2 infection, we did not stratify by this variable due to suspected high misclassification. This would result from decreasing SARS-CoV-2 testing and increasing use of self-tests along the study period, particularly after the arrival of Omicron in 2021. Therefore, our estimates could be interpreted as an average population effect of the vaccine in country populations with given pre-existing immunity levels. Moreover, because vaccine recommendations are not expected to be made depending on previous infection status we believe our results are relevant from a public health perspective.
To conclude, our results suggest that time since the last booster dose is more important for the risk of hospitalisation than the number of booster doses. This supports seasonal COVID-19 vaccination in population ≥ 65 years at the start of periods with increased expected disease burden. Monitoring focused on the effectiveness of the seasonal vaccine dose is probably more appropriate for the current context.
Acknowledgements
The authors would like to thank all persons from the study sites involved in the data collection and the estimate production, as without their work these results wouldn’t be available for the scientific community and the public.
Supplementary Data
Conflict of interest: None declared.
Authors’ contributions: SM, AN, MFV, EK, NN and BN conceived the study and the methods. All authors from Public Health institutions at each study site were responsible for the data management and analysis at the site level. MFV and SM were in charge of pooling site estimates. MFV and SM drafted the first version of the manuscript, with the help of EK and AN. All the authors contributed to the interpretation of the results and critically reviewed the manuscript. All the authors approved the final version of this manuscript. All the authors within the VEBIS-Lot4 working group made a substantial contribution to the conception or design of the work, critically revised the manuscript, provided their final approval of the version to be published, and agreed to be accountable for all aspects of the work.
References
- 1.Epiconcept. Vaccine Effectiveness, Burden and Impact Studies (VEBIS) of COVID-19 and Influenza. Paris: Epiconcept. [Accessed 6 Oct 2023]. Available from: https://www.epiconcept.fr/en/epidemio-project/vaccine-effectiveness-burden-and-impact-studies-vebis-of-covid-19-and-influenza
- 2.Sentís A, Kislaya I, Nicolay N, Meijerink H, Starrfelt J, Martínez-Baz I, et al. Estimation of COVID-19 vaccine effectiveness against hospitalisation in individuals aged ≥ 65 years using electronic health registries; a pilot study in four EU/EEA countries, October 2021 to March 2022. Euro Surveill. 2022;27(30):2200551. 10.2807/1560-7917.ES.2022.27.30.2200551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kislaya I, Sentís A, Starrfelt J, Nunes B, Martínez-Baz I, Nielsen KF, et al. Monitoring COVID-19 vaccine effectiveness against COVID-19 hospitalisation and death using electronic health registries in ≥65 years old population in six European countries, October 2021 to November 2022. Influenza Other Respir Viruses. 2023;17(11):e13195. 10.1111/irv.13195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.European Centre for Disease Prevention and Control (ECDC). Interim analysis of COVID-19 vaccine effectiveness against hospitalisation and death using electronic health records in six European countries. Stockholm: ECDC; 30 Nov 2023. Available from: https://www.ecdc.europa.eu/en/publications-data/interim-analysis-covid-19-vaccine-effectiveness-against-hospitalisation-and-death
- 5.European Centre for Disease Prevention and Control (ECDC). Pilot protocol for a COVID-19 vaccine effectiveness study using health data registries: version 1.0. Stockholm: ECDC; 19 Sep 2022. Available from: https://www.ecdc.europa.eu/en/publications-data/covid-19-pilot-protocol-vaccine-effectiveness-study-using-health-data-registries
- 6.European Centre for Disease Prevention and Control (ECDC). Protocol for a COVID-19 vaccine effectiveness study using health data registries. Stockholm: ECDC; 30 Jan 2023. Available from: https://www.ecdc.europa.eu/en/publications-data/protocol-covid-19-vaccine-effectiveness-study-using-health-data-registries
- 7.European Centre for Disease Prevention and Control (ECDC). Case definition for COVID-19. Stockholm: ECDC; 31 May 2023. Available from: https://www.ecdc.europa.eu/en/covid-19/surveillance/case-definition
- 8.Deeks J, Higgins J, Altman D. Analysing data and undertaking meta-analyses. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page M, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.4. Cochrane: 2023. Available from www.training.cochrane.org/handbook
- 9.Lin DY, Xu Y, Gu Y, Zeng D, Sunny SK, Moore Z. Durability of bivalent boosters against Omicron subvariants. N Engl J Med. 2023;388(19):1818-20. 10.1056/NEJMc2302462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lau JJ, Cheng SMS, Leung K, Lee CK, Hachim A, Tsang LCH, et al. Real-world COVID-19 vaccine effectiveness against the Omicron BA.2 variant in a SARS-CoV-2 infection-naive population. Nat Med. 2023;29(2):348-57. 10.1038/s41591-023-02219-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kirsebom FCM, Andrews N, Stowe J, Ramsay M, Lopez Bernal J. Duration of protection of ancestral-strain monovalent vaccines and effectiveness of bivalent BA.1 boosters against COVID-19 hospitalisation in England: a test-negative case-control study. Lancet Infect Dis. 2023;23(11):1235-43. 10.1016/S1473-3099(23)00365-1 [DOI] [PubMed] [Google Scholar]
- 12.Andersson NW, Thiesson EM, Baum U, Pihlström N, Starrfelt J, Faksová K, et al. Comparative effectiveness of bivalent BA.4-5 and BA.1 mRNA booster vaccines among adults aged ≥50 years in Nordic countries: nationwide cohort study. BMJ. 2023;382:e075286. 10.1136/bmj-2022-075286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grewal R, Buchan SA, Nguyen L, Nasreen S, Austin PC, Brown KA, et al. Effectiveness of mRNA COVID-19 monovalent and bivalent vaccine booster doses against Omicron severe outcomes among adults aged ≥50 years in Ontario, Canada: a Canadian Immunization Research Network (CIRN) Study. J Infect Dis. 2023:jiad419. 10.1093/infdis/jiad419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tamura T, Ito J, Uriu K, Zahradnik J, Kida I, Anraku Y, et al. Virological characteristics of the SARS-CoV-2 XBB variant derived from recombination of two Omicron subvariants. Nat Commun. 2023;14(1):2800. 10.1038/s41467-023-38435-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.European Centre for Disease Prevention and Control (ECDC). Epidemiological update: COVID-19 transmission in the EU/EEA, SARS-CoV-2 variants, and public health considerations for Autumn 2023. Stockholm: ECDC; 7 Sep 2023. Available from: https://www.ecdc.europa.eu/en/news-events/epidemiological-update-covid-19-transmission-eueea-sars-cov-2-variants-and-public
- 16.Machado A, Kislaya I, Soares P, Magalhães S, Rodrigues AP, Franco R, et al. Bivalent mRNA vaccine effectiveness against COVID-19 infections, hospitalisations and deaths in Portugal: a cohort study based on electronic health records, September 2022 to May 2023. medRxiv. 2023:2023.09.05.23295025. Preprint. http://medrxiv.org/lookup/doi/10.1101/2023.09.05.23295025 http://dx.doi.org/ 10.1101/2023.09.05.23295025 [DOI]
- 17.Fabiani M, Mateo-Urdiales A, Sacco C, Rota MC, Fotakis EA, Petrone D, et al. Relative effectiveness of bivalent Original/Omicron BA.4-5 mRNA vaccine in preventing severe COVID-19 in persons 60 years and above during SARS-CoV-2 Omicron XBB.1.5 and other XBB sublineages circulation, Italy, April to June 2023. Euro Surveill. 2023;28(32):2300397. 10.2807/1560-7917.ES.2023.28.32.2300397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lipsitch M. Challenges of vaccine effectiveness and waning studies. Clin Infect Dis. 2019;68(10):1631-3. 10.1093/cid/ciy773 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.