Abstract
Background
Staphylococcus aureus is a leading cause of human bacterial infections worldwide. It is the most common causative agent of skin and soft tissue infections, and can also cause various other infections, including pneumonia, osteomyelitis, as well as life-threatening infections, such as sepsis and infective endocarditis. The pathogen can also asymptomatically colonize human skin, nasal cavity, and the intestine. S. aureus colonizes approximately 20–30% of human nostrils, being an opportunistic pathogen for subsequent infection. Its strong ability to silently spread via human contact makes it difficult to eradicate S. aureus. A major concern with S. aureus is its capacity to develop antibiotic resistance and adapt to diverse environmental conditions. The variability in the accessory gene regulator (Agr) region of the genome contributes to a spectrum of phenotypes within the bacterial population, enhancing the likelihood of survival in different environments. Agr functions as a central quorum sensing (QS) system in S. aureus, allowing bacteria to adjust gene expression in response to population density. Depending on Agr expression, S. aureus secretes various toxins, contributing to virulence in infectious diseases. Paradoxically, expressing Agr may be disadvantageous in certain situations, such as in hospitals, causing S. aureus to generate Agr mutants responsible for infections in healthcare settings.
Main body
This review aims to demonstrate the molecular mechanisms governing the diverse phenotypes of S. aureus, ranging from a harmless colonizer to an organism capable of infecting various human organs. Emphasis will be placed on QS and its role in orchestrating S. aureus behavior across different contexts.
Short conclusion
The pathophysiology of S. aureus infection is substantially influenced by phenotypic changes resulting from factors beyond Agr. Future studies are expected to give the comprehensive understanding of S. aureus overall profile in various settings.
Keywords: Staphylococcus aureus, Accessory gene regulator, Quorum sensing, Infectious diseases, Skin infection, Atopic dermatitis, Systemic infection
Background
S. aureus resistance and adaptation to the ecological niche
Throughout human history, we have consistently battled bacteria. However, bacteria have persistently sought out vulnerabilities in our attempt and adapted to their ever-changing environment. The mortality rate from systemic S. aureus infections was approximately 80% before the discovery of antibiotics [1]. The discovery of penicillin in 1928 [2] and its clinical use temporarily decreased the death toll due to bacterial pneumonia and meningitis during World War II. However, only 2 years after the clinical introduction of penicillin, the penicillin-resistant S. aureus strains developed [1] and became predominant worldwide: 80% of clinical isolates were resistant to penicillin by 1945 and penicillin-resistant S. aureus became a pandemic throughout the late 1950s and early 1960s [1]. These strains encode β-lactamase, which is capable of hydrolyzing the β-lactam ring of penicillin [1]. To overcome this, methicillin, the semisynthetic β-lactamase-resistant antibiotic, was developed in 1959, although it led to the emergence of methicillin-resistant S. aureus (MRSA) soon after in 1961 [3]. MRSA arises due to the presence of the mecA gene. This gene encodes a modified penicillin-binding protein with reduced affinity for methicillin and other β-lactam antibiotics [3]. Importantly, mecA is situated within the Staphylococcal cassette chromosome mec (SCCmec), a mobile genetic element (MGE) that has the capability to transfer between bacterial strains [3]. This transferability of SCCmec contributes to the spread of methicillin resistance among S. aureus strains. MRSA spread worldwide over the next several decades and started to cause an endemic in hospitals and healthcare facilities, affecting immune-compromised hosts and causing life-threatening infections. Starting from the 1980s, MRSA spread globally to such an extent that many countries now report MRSA rates of 50% or higher among infective S. aureus isolates in hospitals [4]. For a while, MRSA was confined to only hospitals and healthcare settings. However, since early 1990s, MRSA started to cause outbreaks among otherwise healthy individual outside of the hospital settings, such as in sports teams, army recruits, or prisoners [5]. These novel MRSA strains, capable of infecting healthy individuals within the community, have been designated as community-associated (CA)-MRSA strains. In contrast, the traditional strains prevalent in hospital settings are referred to as hospital-acquired (HA)-MRSA strains. CA-MRSA infections are now prevalent and widespread worldwide [4]. Notably, CA-MRSA strains are more virulent and transmissible than are traditional HA-MRSA strains [6]. CA-MRSA gains methicillin resistance via small size SCCmec, type IV and V, which is attributed to less metabolic burdens of protein synthesis during replication, whereas HA-MRSA carries other large SCCmec types [7, 8]. It became clear that HA-MRSA and CA-MRSA differ in host selectivity and virulence. As the history proves, a formidable ability to adapt to a specific ecological niche, with the host immune system and environment, seems to be the core characteristics of S. aureus survival strategy. Currently, S. aureus is gaining new resistance to different antibiotics [9]. Multi-resistant S. aureus in hospitals not only leads to death and disability of immunocompromised hosts, but also prolongs illness of those who survive and requires more expensive medication, posing a financial challenge [10]. Understanding the fundamental bacterial property that enables MRSA to adapt to various environments and eventually gain resistance is an urgent need to fight against this bacterium. Recent advances in genome sequencing enabled us to understand bacterial genomic transition in detail [11]. In particular, the variation in the accessory gene regulator (Agr) region on the genome seems to generate a kaleidoscopic of phenotypes within the bacterial population and increases the likelihood of bacterial survival in versatile environments [12–14]. In this review, we will discuss how Agr regulates the bacterial phenotype in various infectious diseases.
Agr quorum sensing in S. aureus
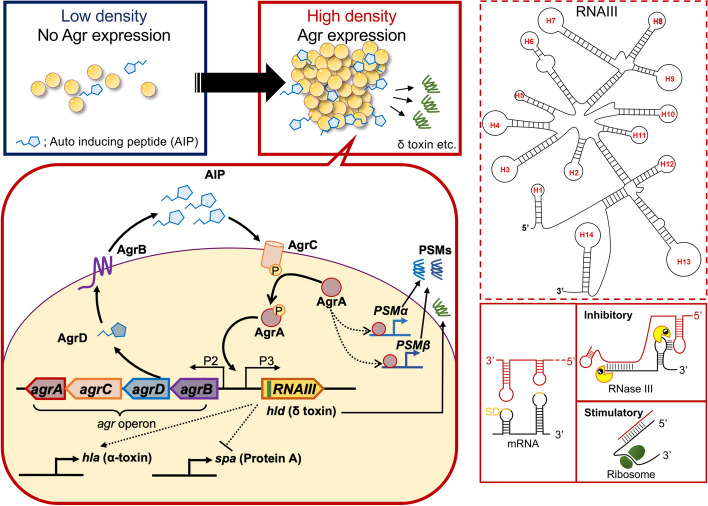
The quorum sensing (QS) system is the ability of bacteria to adjust gene expressions in response to their population density [15]. Many bacteria secrete chemical signaling molecules, called autoinducers, which vary between species [15]. When a bacterial population increases and the corresponding autoinducers reaches a threshold concentration, the signal activates a regulator that can induce or repress target genes [15]. Among the many traits controlled by QS is the expression of virulence factors, conjugation, biofilm formation [15]. S. aureus possesses an auto-regulatory operon, Agr system, as a QS function (Fig. 1) [16]. S. aureus consistently releases an exocrine auto-inducing peptide (AIP). AgrD is the 45–47 residue peptide precursor of AIP [16], which is proteolytically processed by AgrB, a trans-membrane peptidase. AgrB-mediated cleavage of AgrD results in the formation of AIP [17–19], which is transported to the extracellular space [20]. This peptide is typically seven to nine amino acids in length and features a distinctive five-residue thiolactone ring formed between the C-terminal end and a conserved cysteine residue [16]. When AIP reaches the threshold, the transmembrane receptor on the cell surface, AgrC, is activated via autophosphorylation of its histidine protein kinase (HPK) domain [16]. The phosphate of HPK is transferred to AgrA, which in turn binds and activates two bidirectional promoters, P2 and P3 in agr operon [16]. The P2 promoter drives the autoregulation circuit of Agr, by inducing the expression of the agrBDCA operon, that encodes the machinery of the QS system. In contrast, the P3 promoter regulates various toxins via RNAIII, a large regulatory RNA which has a complex secondary structure with several C-rich hairpin loops to interact with its target mRNAs [16, 21]. RNAIII also encodes the delta-hemolysin gene (hld), known as δ-toxin [16]. In the following section, we will discuss various toxins regulated by the Agr system. Their pathological role in infections will be discussed in subsequent sections.
Fig. 1.
Agr quorum sensing in Staphylococcus aureus. S. aureus consistently releases AIP. When the population density and corresponding AIP reaches the threshold, the receptor on the cell surface, AgrC, is activated via autophosphorylation of the histidine kinase. The phosphate is transferred to AgrA, which in turn binds and activates two bidirectional promoters, P2 and P3 in agr operon. The P2 promoter drives the autoregulation circuit of Agr, by inducing the expression of agrBDCA operon, that encodes the machinery of the QS system. AgrD is cleaved by AgrB, a trans-membrane peptidase, to form of AIP. The P3 promoter regulates various toxins via RNAIII, which is a large regulatory RNA. RNAIII exhibits a complex secondary structure with several C-rich hairpin loops, many of which align with the Shine-Dalgarno sequence of targeted genes. These interactions can manifest as either inhibitory or stimulatory. RNAIII also encodes hld, a gene responsible for δ-toxin. Agr accessory gene receptor, AIP auto-inducing peptide, QS quorum-sensing, SD Shine-Dalgarno sequence
SarA, the upstream regulator of Agr
In addition to AgrA, other regulators are known to control agr expression [22]. Among these are SarA and SarR, both winged-helix DNA-binding proteins. SarA binds to the conserved regions, Sar boxes, within the promoter region of targeted genes [23, 24]. In association with Agr, SarA binds to the P2-P3 intergenic region in agr operon, induces DNA bending, and thus allow interaction of two AgrA dimers to result in the efficient recruitment of RNA polymerase and augmentation of RNAII transcription [25]. Without effective SarA enhancement, Agr operon can be only weakly activated [25]. Additionally, SarA binds to promoter regions of α-toxin and fibronectin-binding protein A to enhance their expressions [23, 24]. Therefore, SarA controls regulation of certain Agr-regulated virulence factors both directly and indirectly [26]. Contrary to SarA, SarR functions as a brake to attenuate Agr expression during the stationary phase, when cell growth reaches a confluence. During the post-exponential phase, SarR accumulates and binds to the agr promoter at a site that overlaps with SarA. This binding results in the displacement of SarA and the reversal of DNA bending [25]. Although not directly affected, agr P3 promoter is indirectly affected by the SarA/R system via its regulation on the P2 promoter and resulting agr operon machinery [25].
The SarA protein family is a collection of DNA-binding proteins homologous to SarA (SarR, SarS, SarT, SarU, SarV, SarX, SarZ, MgrA, and Rot) [22]. While each protein acts on various gene expressions independently, the SarA family also interact each other in a complexed manner and create a hierarchical regulatory cascade, affecting Agr expression in a complicated way. The interplay of the SarA family and its effect on Agr is only partially understood [20, 22].
Phenol-soluble modulin
Phenol-soluble modulins (PSMs) are a family of small (2–5 kDa), amphipathic, α-helical peptides, including PSMα, PSMβ, and PSMγ (also called δ-toxin and delta-hemolysin) [27, 28] (Table 1). In addition to activating P2 and P3 promoter in the agr region, AgrA is capable of directly binding to the promoters of the PSMα (encoding PSMα1-α4) and PSMβ (encoding PSMβ1 and β2) [29]. The gene locus of δ-toxin (hld) is located in RNAIII region of agr operon thus transcribed by P3 promoter [30]. The essential virulence of PSM peptides rely on its cytolytic property, although not all PSMs from S. aureus are cytolytic. PSMα, especially PSMα3, has a pronounced ability to lyse human leukocytes, erythrocytes, and epithelial cells; δ-toxin has moderate cytolytic activity; and PSMβ peptides are non-cytolytic [31]. Although the exact mechanism of PSM toxicity against host cells is still unclear, the characteristic amyloid protofilaments assembled by stacking of amphipathic helices, which gives them surfactant-like characteristics, are proposed to result in the pathogenic activity in PSMα and PSMβ [32, 33].
Table 1.
Major virulent mechanisms of Agr-related toxins
| Toxin (gene) | Contribution of Agr expression on genes | Molecular character | Major virulent mechanism | References |
|---|---|---|---|---|
| PSMα (PSMα) | Activation (AgrA enhances PSMα promoter) | Amyloid protofilaments assembled by stacking of amphipathic helices | -Low concentration: stimulate leukocytes FPR2, leading to inflammatory response | [34] |
| -High concentration:Cytolytic against leuckocytes, keratinocytes, and erythrocytes | [31, 35] | |||
| PSMβ (PSMβ) | Activation (AgrA enhances PSMβ promoter) | Unknown | ||
| δ-toxin (hld) | Activation (hld encoded in RNAIII) | Mast cell degranulation | [36] | |
| α-toxin (hla) | Activation (RNAIII initiate hla translation) | Pore-forming | -Pore forming cytotoxicity via ADAM10 binding in epithelial, endothelial, and immune cells | [37, 38] |
| -Platelets aggregation | [39] | |||
| -Inflammasome response in macrophages | [40] | |||
| protein A (spa) | Downregulation (RNAIII inhibit spa mRNA, RNAIII-spa mRNA degraded by RNAse III) | Five homologous Ig-binding domains | -Resist opsonization by Fc binding | [41, 42] |
| -Activate TNFR1 | [43] |
PSMα plays a major role in the bacterial interaction with neutrophils. Neutrophils can directly respond to specific bacterial molecules, ‘‘pathogen-associated molecular patterns,’’ via Toll-like receptors (TLRs) [44] or certain G protein-coupled receptors (GPCRs), such as the formyl peptide receptor (FPR) family [45]. FPR recognize the formylated bacterial peptides and can activate host cells as well as elicit chemotactic migration. A formylated methionine is a hallmark of bacteria since only bacterial cells start protein biosynthesis with formylated methionine, whereas the human cells use an unmodified methionine for the initiation of translation [45]. At sub-cytolytic concentration, PSMα stimulate leukocytes via FPR2 and initiate pro-inflammatory responses, including neutrophil chemoattraction activation, and the release of interleukin (IL)-8 [34]. Once S. aureus is recognized by TLRs or GPCRs of immune cells, neutrophils migrate from vessels into tissues, phagocyte bacteria, and kill the bacteria. PSMα at high concentrations is able to lyse neutrophils after phagocytosis and induce a marked proinflammatory response while promoting bacterial survival [46, 47].
In contrast to the pro-inflammatory response in neutrophils, PSMα3 modulates monocyte-derived dendritic cells (moDC) to a tolerogenic phenotype in vitro. PSMα3 incubation with moDC led to impaired TLR2/4-induced maturation, decreased pro- and anti-inflammatory cytokine secretion, as well as reduced antigen uptake, and thus possibly increased the immune-tolerance toward the bacteria [48, 49].
Although very little is known about the role of PSMβ, it seems like PSMβ partly reverses the effect of PSMα on neutrophils and alleviate inflammation. This anti-inflammatory effect was seen in some reports, as measured by serum IL-6, neutrophil apoptosis in vitro, resulting in decreased host mortality in a mouse sepsis model [50] and a smaller dermonecrotic area in a subcutaneous injection model [31]. Meanwhile, δ-toxin is known to directly activate mast cells to degranulate [36].
In addition to the psm locus found in the core genome, some strains, particularly HA-MRSA, possess psm-mec. This is a mobile genetic element that contains both the psm and mecA genes. PSM-mec is responsible for antibiotic resistance and cytolytic capacity at the protein level. Interestingly, the psm-mec locus also encodes a regulatory RNA that inhibits the translation of the agrA gene [51]. The impact of this gene cassette on enhancing or inhibiting PSM expression is highly dependent on the specific strain, possibly due to the counteracting effects of the PSM-mec peptide and the RNA-controlled inhibitory effects of psm-mec [52, 53].
Alpha-toxin
α-Toxin or α-hemolysin (hla) is a pore-forming toxin secreted as a soluble monomer [38]. α-Toxin assembles upon contact with the host disintegrin and metalloprotease 10 (ADAM10) to heptameric, β-barrel structure creating a cytolytic pore [37, 38]. Caveolin-1, the main component of the cell membranes, interacts with α-toxin and stabilizes the pore [54]. The hla mRNA normally forms a hairpin loop that prevents the ribosome from accessing its ribosome-binding site. RNAIII in the agr operon can bind to the hla mRNA, relieving the hairpin loop structure and allowing the ribosome to recognize the binding site for hla translation initiation [55]. Additional to Agr, hla expression levels can also be enhanced by the SarA regulatory systems as described above [24, 25, 56]. Although Agr appears to be the main regulator of hla expression, how other regulatory circuits contribute to hla expression in vivo remains unclear [57].
α-Toxin was initially named as α-hemolysin based on its property to lyse rabbit red blood cell, although later works revealed that human erythrocytes are devoid of ADAM10 and thus are insensitive to α-toxin [38, 58]. Rather, α-toxin intoxicates a wide range of human cell types via ADAM10 binding, including epithelial [38], endothelial [59], and immune cells, including T cells, monocytes, macrophages, and neutrophils [38, 60]. Additionally, recent works emphasize on α-toxin ability to cause the human platelets aggregation through ADAM10 interaction [39]. In vivo, α-toxin is an important virulence determinant contributing to skin necrosis in a subdermal injection model [61, 62], lethality in pneumonia [63, 64], and high bacterial burden in a brain abscess model [65]. α-Toxin contributes to the host lethal outcome in bloodstream infections [59] through disseminated thrombosis caused by platelets and neutrophil intoxication [39, 66]. The strong virulence of α-toxin depends on direct cell lysis as well as its ability to elicit host inflammatory responses. Intoxication with α-toxin induces inflammasome activation and result in IL-1β secretion and cell death in macrophages and monocytes [67]. The following inflammatory response leads to recruitment of various immune cells and reaction, leading to necrotic tissue injury [68].
Consistent with murine experiments, α-toxin-ADAM10 interaction poses a deteriorating effect on human. In patients with OTULIN (a linear deubiquitinase) haploinsufficiency, increased levels of linear ubiquitin caused the accumulation of caveolin-1 complexes in dermal fibroblasts, not in leukocytes [69]. Caveolin-1 accumulation enhanced the cytotoxicity of α-toxin and resulted in a life-threatening Staphylococcal disease of the skin and lungs [69]. The good news is, α-toxin-neutralizing antibodies could rescue the impaired cell-intrinsic immunity to α-toxin in these patients [69]. Meanwhile, human possesses an innate immune mechanism utilizing autophagy machinery to counteract α-toxin-induced toxicity. Upon recognition of bacterial and CpG DNA, host cells transfer ADAM10-bearing exosomes to the cell surface and expose decoy ADAM10 to trap α-toxin [70]. These studies suggest that genetic differences in α-toxin-ADAM10 signaling may produce phenotypic variation in human S. aureus infections.
Notably, although α-toxin contribution to host organ damage and lethality is evident, some studies report α-toxin as not being responsible for high bacterial load in the infection model. In a peritoneal infection model, α-toxin contributed to high mice lethality, but did not affect the remaining bacterial load in peritoneal cavity [71]. Additionally, in a corneal infection model, α-toxin contributed to high corneal damage, but did not affect bacterial load on the cornea [72]. α-Toxin deletion led to a small abscess formation in a subcutaneous injection model, but did not change the bacterial load [73]. Moreover, conditional knockout of ADAM10 in lung alveolar epithelium led to increased survival, but did not alter bacterial load in S. aureus pneumonia [64]. Thus, α-toxin-ADAM10 interaction is essential for progressive lethal disease, although it may not affect toxin-mediated control of the tissue bacterial load, depending on the conditions and model for in vivo assay (Table 1).
Other Agr-regulated toxins
Transcriptome analysis revealed other toxins and enzymes positively regulated by the Agr system, such as serine proteases (SplA-F, SspA), cysteine proteases (ScpA, SspB), gamma-hemolysin (Hlg), and lipase (Geh) [26]. Among them, some proteases are known to contribute to virulence through proteolytic activity against specific targets. For instance, SspA targets the Fc region of immunoglobulins, degrading it and disrupting the effector function of antibodies [3]. This action leads to a partial loss of antigenic determinants of the antibody. Moreover, SspA damages tight junctions on keratinocytes, contributing to the development of atopic dermatitis. Another set of proteases, the six serine protease-like proteins (SplA-SplF), encoded in a single operon, trigger Th2 cytokines and induce the production of IgE antibodies in response to allergens [3]. This immune response is implicated in the development of various chronic airway diseases, including asthma and pneumonia.
Toxins downregulated by Agr system
In contrast, RNAIII downregulates some surface proteins, including protein A (Spa) [26]. Protein A interferes with immune cells by (1) non-specifically binding to the Fc portion of IgG and escape phagocytes opsonization and (2) binding to the Fab region of IgM to serve as a B cell superantigen and cause B cell apoptosis [41, 42]. Protein A also activates tumor necrosis factor receptor (TNFR)1, a receptor for TNF-α on airway epithelium even without IgG, and elicits inflammatory response [43] (Table 1).
Spa gene expression is negatively controlled by Agr expression through two distinct mechanisms. First, RNAIII directly inhibits spa mRNA by RNA-RNA interactions, inhibiting access to the ribosome binding site [74]. Second, the complex formed between RNAIII and spa mRNA is also a substrate for RNAse III, thus RNAIII can also inhibit Protein A production by enhancing the degradation of spa mRNA [74]. Additionally, spa is repressed by SarA by binding and altering the mRNA turnover [75] (Table 1). Thus, Agr expression may sacrifice some virulence factors associated with surface proteins.
Other than Protein A, Agr has been described to generally downregulate adhesion factors, collectively referred to as microbial surface components recognizing adhesive matrix molecules (MSCRAMMs). However, more recent studies revealed that Agr does not regulate most MSCRAMMs in clinical strains [76].
Interspecies quorum sensing between bacteria
In addition to S. aureus, other staphylococcal species also employ AIPs for Agr Quorum Sensing. Despite these species sharing a common AIP structure, there are variations in the amino acid sequences of several AIPs. Consequently, staphylococci with different AIP types engage in competitive interactions. They upregulate the expression of Agr in bacteria with the same AIP type, while concurrently downregulating the expression of Agr in other staphylococci with different AIP types [77].
S. aureus necessitates functional Agr to colonize skin and cause Th2-driven skin inflammation in atopic dermatitis
In addition to its role as an opportunistic pathogen, Staphylococcus aureus can establish colonization on various human sites such as the skin, nares, and intestine. Notably, S. aureus skin colonization is strongly associated with atopic dermatitis (AD), a condition influenced by environmental factors, Th2 cell-skewed immunity, and deficiencies in the skin barrier [78]. The pathogenesis of AD is further complicated by alterations in the skin microbiome, known as dysbiosis. In fact, a significant percentage (30–100%) of AD patients are found to be colonized with S. aureus, in contrast to an approximate 20% prevalence in healthy control subjects [79, 80]. Moreover, the bacterial loads of S. aureus on the skin have been observed to correlate with the severity of AD [81, 82]. Despite these associations, the specific contribution of S. aureus to the pathogenesis of AD remained unclear until recent developments in research (Fig. 2).
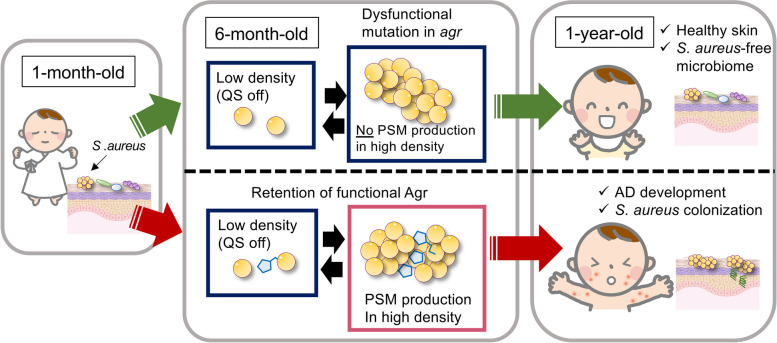
Fig. 2.
Retention of Agr in infant skin is related to atopic dermatitis. In a Japanese cohort study, S. aureus colonization at 1 month old did not affect the development of AD at 1 year old. However, possessing S. aureus on skin at 6 months old substantially increases the risk of developing AD at 1 year old. The whole-genome sequencing revealed that S. aureus on skin of the infants who did not develop AD by the age of one year acquired loss-of-function mutations in the agr locus between one and six months of age, whereas retention of a functional Agr is crucial for S. aureus to colonize the infants’ skin and cause AD. AD atopic dermatitis
The human skin microbiome is composed of bacteria, archaea, viruses, and fungi, and differ in communities at different body sites [83]. The infant skin microbiome is affected by various factors including delivery mode and neonatal skin barrier [84, 85], although the long-term consequences of these initial perturbations are not known. The early life microbiome undergoes frequent strain replacements over time [86]. During puberty, the sebaceous glands increase sebum production, and postpubescent skin favors lipophilic organisms [80, 87, 88]. The skin microbial communities in healthy adults remains stable, regardless of environmental perturbations [89]. However, dysbiosis associated with AD is characterized by decreased microbial diversity and an increase in Staphylococcus in general, especially S. aureus [79, 90–94]. Why and when S. aureus particularly colonizes AD-infected skin, especially on the lesional skin, remains unclear. One cohort study analyzed infants skin microbiome sequentially during 1 to 6 months after birth and found that approximately 45% of the infants were colonized with S. aureus in the cheek at 1 month, whether or not infants developed AD later in their life [13]. However, possessing S. aureus on skin at 6 months old substantially increases the risk of developing AD later in life [13]. Whole-genome sequencing of the bacterial genome showed that having a properly functioning Agr is crucial for S. aureus to colonize the infants’ skin and cause AD [13]. Additionally, a murine model of epicutaneous S. aureus colonization demonstrated that the Agr system plays a critical role in the epidermal colonization [13] (Fig. 3). Moreover, only Agr-positive strains could induce AD-like eczematous skin, as measured by skin disease score and histological analysis. Another study utilizing Staphylococcus caprae to inhibit the S. aureus Agr QS via AIP competition also exhibited that Agr-expressing S. aureus colonized on the skin of mice more efficiently than the Agr-suppressed strain [95]. Thus, functional agr seems to be necessary for S. aureus to colonize skin. This may explain why S. aureus on AD-infected skin can be a silent colonizer in a steady state, but also transform into a pathogenic phase with increase in number during an AD flare. Certain toxins reportedly playing key roles in the AD development are actually regulated by Agr, thus are only expressed when the bacteria reach high population densities. Specifically, δ-toxin and PSMα, which are regulated under the Agr system, are proven to elicit skin inflammation in AD. δ-toxin is a potent inducer of mast cell degranulation, contributing to the T helper 2 (Th2)-driven skin inflammation represented by IgE and IL-4 production in mice epicutaneous S. aureus infection models (Fig. 3, Table 1) [36]. Concomitant with mice data, S. aureus isolates recovered from patients with AD produced high levels of δ-toxin [36]. On the other hand, another study utilized AIPs derived from coagulase-negative staphylococci (CoNS), specifically S. epidermidis, to inhibit the Agr system of S. aureus and alleviated skin symptoms (M. R. [96]. This competitive interference highlights the intricate dynamics among different staphylococcal species and their impact on skin health.
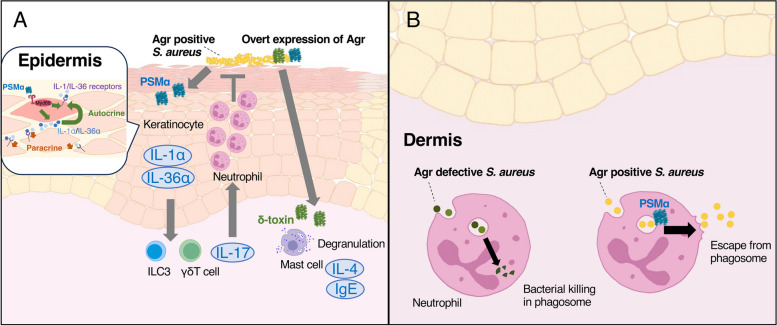
Fig. 3.
Agr-regulated toxins in atopic dermatitis. A Upon contact with S. aureus, keratinocytes detect PSMα, triggering the release of alarming signals, such as IL-1α and IL-36α. The receptors IL-1R and IL-36R become amplified during the inflammatory response in immunocompetent cells, leading to the induction of IL-17-producing γδ T cells and ILC3. IL-17 plays a crucial role in protective immunity against bacteria by promoting neutrophil recruitment. δ-toxin is a potent inducer of mast cell degranulation, contributing to the Th2-driven skin inflammation represented by IgE and IL-4 production. B Once bacteria reach the dermis, S. aureus utilizes PSMα to escape from phagosomes into the cytosol and limit both oxidative and non-oxidative pathogen killing after neutrophil engulfment. This leads to bacterial growth and consecutive inflammation in dermis. PSM: phenol-soluble modulin
Keratinocytes serve as the frontline defense against bacteria, actively sensing microbial presence beyond their role as a physical barrier. Upon contact with S. aureus, keratinocytes detect PSMα, triggering the release of alarming signals, such as IL-1α and IL-36α, with skin barrier disruption [35]. The receptors, IL-1R and IL-36R, become amplified during the inflammatory response, leading to the induction of IL-17-producing γδ T cells and Type 3 innate lymphoid cells [35] (Fig. 3, Table 1). IL-17 plays a crucial role in protective immunity against bacteria by promoting neutrophil recruitment, antimicrobial peptide production, and enhancing barrier function [97], among which neutrophils are essential for preventing S. aureus from invading the dermis [98]. Thus, PSMα-induced keratinocytes inflammatory response contributes to the protective immunity against S. aureus. Meanwhile, epicutaneous S. aureus colonization/infection enhances Th2-driven skin inflammation and skin barrier disruption, two important hallmarks of AD, via Agr-regulated toxins (Fig. 3).
Surprisingly, in neutrophil-deficient mice, S. aureus penetrates the epidermis with only mild Th2-driven skin inflammation and then grows in the dermis [98]. This epidermal penetration was dependent Agr and PSMα [98]. Once S. aureus reaches into subdermis, pathogen grow and expand more severely than neutrophil-sufficient mice, depending on saeR/S but no on Agr [98]. This discrepancy may at least partially explain why S. aureus requires Agr to cause SSTI in immunocompetent patients, but can infect immunocompromised hosts without the need for Agr.
Agr system in skin and soft tissue infections
Clinical features of CA-MRSA
The most frequent disease manifestation associated with CA-MRSA is the skin and soft tissue infections (SSTI), accounting for at least 90% of CA-MRSA infections [99]. CA-MRSA SSTI are usually severe and often very painful. Up to 4% cases of CA-MRSA infection manifest as very potentially life-threatening skin infections, such as necrotizing fasciitis, whereas HA-MRSA rarely leads to such an invasive SSTI [100]. CA-MRSA strains also cause various infections such as osteomyelitis, pneumonia, sepsis, and urinary tract infections. The observation that CA-MRSA strains have the capacity to infect otherwise healthy people had indicated enhanced virulence. Many genetic and phenotypical analyses were attempted to establish the key differences of CA-MRSA and HA-MRSA, revealing Agr system as a significant key player in virulence.
Molecular background of strong virulence in CA-MRSA
The predominant factors of enhanced virulence in CA-MRSA was initially believed to rely on the bacterial ability to evade phagocytes killing by Panton–Valentine leukocidin (PVL), a pore-forming toxin to kill immune cells [6, 101]. However, more recent research have questioned the importance of PVL as a major contributor to CA-MRSA virulence [63, 102–105] since an increasing number of CA-MRSA clones do not contain lukSF genes responsible for PVL [106] and lukSF-deficient clones are not less virulent than lukSF-containing CA-MRSA clones in animal experiments [107]. Rather, recent papers emphasize that the Agr system has a crucial role in CA-MRSA infection [62, 108, 109]. Many epidemiological studies report that dysfunctional Agr is higher among HA-MRSA (25–30%) versus CA-MRSA (up to 5%) [110–112]. Therefore, Agr plays a key role in CA-MRSA SSTI in vivo.
Agr system in mice subcutaneous injection models
S. aureus can invade the host skin from minor scratches or wounds and may cause skin infections to become invasive [113]. However, most mouse experiments for S. aureus SSTI rely on subcutaneous bacterial injection to resemble cellulitis in human clinical settings [114]. SSTI in humans also occur without apparent skin barrier impairment, for example at hair follicles (folliculitis), deep (furuncles), or confluent abscesses (carbuncles) [115]. With subcutaneous bacterial injection model using Agr whole-knock out S. aureus, numerous studies established that Agr positive strains cause dramatically strong skin inflammatory responses, leading to abscess formation, skin necrosis, and ulcers with high bacterial load in the skin [108, 116, 117]. Hence, functional Agr seems to have an essential contribution on CA-MRSA virulence, in contrast to HA-MRSA. Among many toxins regulated by Agr, PSMα is proven to have a strong impact on bacterial burden and abscess formation in mice intradermal injection experiments [31, 62]. S. aureus relies on PSMα to escape from phagosomes into the cytosol and limit both oxidative and non-oxidative pathogen killing after neutrophil engulfment, to promote bacterial growth within the dermal layer (Fig. 3) [98]. Another study showed that agr whole-knock out strains show considerably less abscess formation and bacterial survival than PSM knockout strains, suggesting other Agr-regulated toxins than PSMα are responsible for S. aureus virulence in mice subdermal injection models [118].
In addition to invading host skin from minor scratches [113], S. aureus may be capable of actively disrupting the epithelial barrier function. α-Toxin activates ADAM10 on epithelial cells, thereby cleaving E-cadherin, which is one of the most important molecules in cell–cell adhesion [119]. Many studies report that α-toxin is an important virulence determinant in mice subcutaneous injection models, especially in eliciting skin necrosis [61, 119]. However, whether α-toxin is critical in disrupting intact skin barrier to invade and cause subcutaneous infection remains to be elucidated. A recent study has emphasized PSMα function in S. aureus penetrating epidermis to the dermis in neutrophil-deficient mice [98].
S. aureus Agr system in bacteremia
S. aureus is one of the most common causes of bloodstream infections worldwide [120]. The all-cause mortality rate from S. aureus sepsis in high-income countries has been reported to be up to 20–50% [120–123] and the recurrence rate reported to be 5–10% [124]. Entry of S. aureus into the bloodstream occurs mostly via colonization of intravenous catheters or dissemination from skin and soft tissue infections [125, 126]. S. aureus bacteremia can lead to secondary infectious foci in almost any tissue, resulting in a diverse range of infections, including infective endocarditis, tissue abscesses, meningitis, osteomyelitis, and septic arthritis. The bacterial capacity to infiltrate and disseminate to a broad range of second host tissue infections is the distinct characteristics of S. aureus infection. The extensive array of toxins supporting bacterial virulence are collectively termed by their function as, adhesins (attachment to host cells), invasins (penetration into host cells), and evasin (evasion of the host’s immune response), of which some of these effector molecules are at least partially regulated by the Agr system [127, 128]. Additionally, S. aureus bacteremia can lead to endothelial damage, platelet aggregation, and overt inflammatory responses, resulting in life-threatening disseminated intravascular coagulation (DIC). The DIC microthrombi further damage the endothelium and block blood flow, resulting in oxygen depletion in organs, as well as depleting available clotting factors and paradoxically causing hemorrhages [129]. In this pathophysiology, endothelial damage and platelet activation are related to α-toxin under Agr regulation.
It is therefore easy to understand that the Agr system is required for systemic infection, as numerous studies have shown in different animal models of infection. However, in the real-world settings, 3–82% of cases of S. aureus bacteremia are caused by strains lacking detectable Agr activity [130–132]. Moreover, dysfunctional Agr is reported to be an independent risk factor for MRSA bacteremia-attributed mortality [8]. Notably, S. aureus sepsis and systemic infections mostly happen in patients in the hospital settings, not in healthy individuals. Some epidemiological studies revealed that the healthcare environment selects for loss of Agr function and carriage of agr-defective strains is strongly associated with a hospital stay or prior use of antibiotics [111, 133–135]. Some studies using clinical isolates revealed that agr mutants do not transfer between patients [111] and the dysfunctional mutation of Agr occurs newly in every individual infection rather than in a population-wide process [135]. However, we still do not know why the lack of Agr can be beneficial for S. aureus to cause bacteremia and other diseases in immunocompromised patients. There seems to be limitations with animal models analyzing each virulence factor independently to approach the dramatic pathogenesis of infections in the host. Nevertheless, taking various data into consideration, it appears that S. aureus benefits from both expressing and not expressing Agr, depending on the specific phase of the infection and the location of the pathogen within the host [136]. In the bloodstream, apolipoprotein B in serum sequesters AIP, blocking Agr activity, and any produced toxins will be quickly diluted. Therefore, Agr-regulated toxins do not contribute to sepsis severity [137]. Meanwhile in organs, maintaining a functional Agr system is useful for surviving inside phagocytes and establishing a niche in the host [136]. When S. aureus clumps or reside inside host cells, high bacterial density allow them to activate Agr and produce toxins. At the later stage of local infection, bacteria benefit from not expressing Agr to have strong adhesion to organs and avoid eliciting immune responses [136]. We will separately review the benefit of expressing and not expressing Agr in systemic infections in the following section.
α-Toxin and PSMα enable S. aureus to survive intracellularly in phagocytes
Recent research revealed the role of liver Kupffer cells and peritoneal macrophages as infectious reservoirs in S. aureus bacteremia [138, 139]. In mice S. aureus sepsis experiments, bacteria are trapped in Kupffer cells, but survive and multiply within cells, escape to the peritoneum and become trapped in peritoneal macrophage, and eventually disseminate to other organs (Fig. 4) [138, 139]. This intracellular survival is a critical mechanism that determines the development of subsequent S. aureus bacteremia and the establishment of infection in other organs [140]. In the initial phase of Kupffer cells engulfing S. aureus, platelets rapidly bind to the Kupffer cells, preventing escape of the pathogen [141]. At the later phase of Kupffer cell-S. aureus interaction, platelet aggregation caused by α-toxin induces microthrombi and subsequent liver damage [66]. Also in human macrophages, α-toxin is a key effector molecule essential for S. aureus intracellular survival [40]. Besides, S. aureus PSMα can lyse neutrophils within 2 to 4 h after phagocytosis, resulting in re-entry of the pathogen into the bloodstream (Fig. 4) [46, 47]. Bacteria may repeat this cycle by being taken up by nearby healthy neutrophils or, alternatively, disseminate to other sites causing secondary infection foci [142]. Thus, Agr plays a critical role in S. aureus sepsis by facilitating bacterial survival inside phagocytes and even using phagocytes as carriers for dissemination. Additionally, α-toxin also activates platelets and endothelial cells in sepsis, ultimately leading to DIC (Fig. 4).
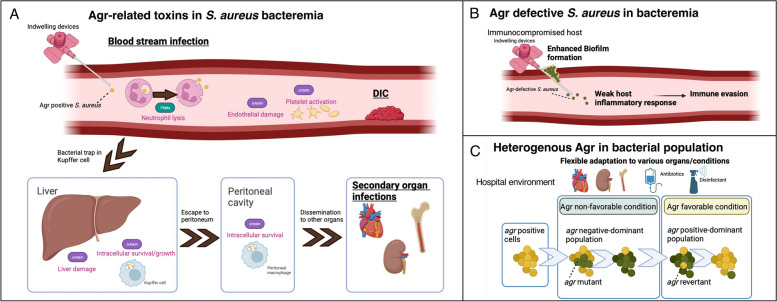
Fig. 4.
Agr system in systemic infection. A In the bloodstream infection, S. aureus PSMα can lyse neutrophils after phagocytosis, resulting in re-entry of the pathogen into the bloodstream. Additionally, S. aureus α-toxin can lead to endothelial damage, platelet aggregation, and overt inflammatory responses, resulting in life-threatening disseminated intravascular coagulation (DIC). In mice S. aureus sepsis experiments, bacteria are trapped in Kupffer cells, but survive and multiply within cells, escape to the peritoneum and become trapped in peritoneal macrophage, and eventually disseminate to other organs. α-toxin is involved in the liver damage and intracellular survival in this pathogenesis. B Agr defective S. aureus reportedly produces dense biofilm and escapes from immune attack by not eliciting strong inflammatory response. C S. aureus population is not always homogeneous in Agr activity but can produce an agr mutant or revertant within them to coordinately survive in various conditions in hospitals. PSM phenol-soluble modulin
Benefit of not expressing Agr in bacteremia
Agr defective clinical isolates seem to arise in a low-cell-density state particularly in cases with endocarditis, osteomyelitis, and bacteremia [143–145]. Despite the loss of toxin production, there is evidence that agr-defective strains are considerably more likely to cause persistent infection than agr-competent strains, resulting in an increased rate of secondary infections and mortality [130, 131, 146–149]. The deregulation of Agr seems to somehow confer an advantage in certain host niches and many studies attempt to reveal how these mutations enable S. aureus to infect the host. Some plausible explanations for the prevalence of agr-defective S. aureus strains causing bacteremia are (1) Agr-regulated toxins may not be necessary for infecting immune-compromised hosts, (2) agr-defective S. aureus have enhanced ability to form a biofilm, and (3) S. aureus benefit from other toxins than Agr-regulated toxins.
Agr-defective S. aureus escape host neutrophil attack
Notably, Agr expression enables bacteria to successfully escape from phagocytes killing; however, it supposedly leads to a strong immune response, which eventually reduces bacterial survival (Fig. 4). In vitro studies reported that Agr-positive strains trigger a strong pro-inflammatory response in neutrophils, including IL-8 and TNF-α expression, compared with that of Agr negative strains [46]. However, in mice sepsis models, S. aureus lineage with an attenuated form of PSMα elicited increased bacterial burden on bloodstream, with diminished cytolytic and chemotactic activity toward human neutrophils [27, 28]. Thus, Agr defective strains may evade recognition and subsequent elimination by host neutrophils, thereby successfully disseminating during blood infection [27, 28].
Biofilm formation in agr mutants
The contamination of indwelling medical devices is another route of infection that occurs frequently in the hospital setting [150]. S. aureus can form biofilms, which is a multicellular bacteria embedded in an extracellular matrix to protect them from phagocyte attacks and killing. S. aureus can form biofilms on various types of abiotic surfaces, such as indwelling medical devices, as well as tissue surfaces, such as heart valves in the case of endocarditis [150]. Biofilm formation starts from bacterial attachment to a surface, production of the extracellular matrix, and the disassembly of the biofilm to disseminate to other sites [150]. Many studies attempted to reveal molecular mechanisms of biofilm formation, although this dynamic process seems to be orchestrated by a complex network and the whole process remains unclear [150]. Among many regulatory systems involved in biofilm formation, Agr reportedly affects biofilm formation either negatively or positively depending on the formation step [150].
Many studies showed that the Agr system is necessary for efficient S. aureus dissemination from a biofilm infection and subsequent spreading into neighboring tissues, mainly depending on PSMs [151–153]. Another study reported S. aureus Agr activation, particularly the PSM production, is also a key component in biofilm structuring [152]. An in vivo study using a murine orthopedic implant biofilm infection model showed that macrophage phagocytosis and cytotoxicity decrease with the biofilm, which is partially dependent on Agr [154]. However, Agr-dysfunctional strains formed dense and enlarged biofilms [155]. Moreover, clinically isolated S. aureus gained agr mutation during infection to cause device-associated infection and increased biofilm formation [135]. Some possible explanations of contraindications in reports are (1) Agr may function differently on biofilm production and dispersal [156]; (2) the S. aureus population in a biofilm is not always homogeneous in Agr activity, but can produce an agr mutant or revertant within them to coordinately form biofilm [118, 135]; and (3) many environmental conditions such as pH, glucose level, and attached surface affect biofilm formation, thus making culture-based experiments difficult to reproduce the same environment as in vivo [150].
To overcome this, recent work used the subcutaneous catheter infection model in which catheter pieces were coated with bacteria and inserted under the dorsal skin of mice for 6 days before bacterial loads in the biofilm were analyzed (Fig. 4) [118]. In this model, Agr-dysfunctional cells formed larger biofilms than that of Agr-positive cells and had increased resistance toward neutrophil attacks in immunocompetent mice [118]. Additionally, in a subcutaneous catheter-associated and prosthetic joint-associated infection model, sub-inhibitory concentrations of antibiotics increased the incidence of agr mutation, leading to a considerable increase in bacteria and the bacterial load [157]. In these animal models relevant to clinical situations in hospitals, Agr-defective strains seem to succeed in creating dense biofilm and eventually cause bacteria compared to Agr-positive strains. However, this thesis remains to be further assessed with other infection models, since human serum affects the S. aureus transcriptome and behavior [158], thus biofilm production may differ in the subcutaneous space from blood vessels. A suitable model of catheter-associated biofilm infection, such as a study inserting an indwelling device in blood vessels [159], can be used for further study.
Uninhibited protein A production in Agr-defective S. aureus
As mentioned in the previous section, Agr activation suppresses some toxin expression. Among them, protein A functions as an essential virulence factor in S. aureus platelet aggregation, forming an abscess, and eliciting inflammation. Protein A activates platelet aggregation via its binding to von Willebrand factor [160] and is a virulence determinant in endovascular infection in a rabbit model of endocarditis [161]. Additionally, protein A mutants are unable to form abscesses, although the mechanism remain unknown [162]. As abscess formation shield bacteria from host immune cells by a surrounding pseudo-capsule and enable bacteria to replicate inside, lack of abscess formation leads to quick bacterial elimination [163]. Moreover, in airway epithelium, protein A stimulates TNFR1, and contributes to pathogenesis of Staphylococcus-related pneumonia [43]. Thus, expressing protein A instead of Agr-regulated toxins may contribute to virulence in various settings. The prophylactic or therapeutic use of anti-protein A succeeded in improving the survival rate and diminishing bacterial load in mice sepsis and peritoneal infection model, suggesting that protein A is critical in these infection models [164].
In another study analyzing a clinically isolated agr defective strain, there were multiple genetic changes in virulence factors (such as the S. aureus ESAT6-like secretion system) other than the agr system, which resulted in increased virulence in a murine model of bloodstream infection. Thus, there was a partial compensation for the absence of conventional agr-mediated virulence with another virulence factor [133]. Although the functional Agr is likely crucial in infection, its importance may be substantially diminished in some situations, and Agr-defective strains may cause mortality through other virulence factors [123, 165–167].
Conclusions
Despite extensive research on S. aureus, there is still a notable absence of effective treatments or preventive measures for bacterial infections caused by this organism, apart from antibiotic therapy. The challenge stems from the complex expression or suppression of numerous virulence factors by this bacterium, influenced by diverse environmental conditions such as host immunity, organ specificity, and antibiotic utilization, resulting in the wide array of intricate phenotypes. Another challenge in understanding the pathogenicity of S. aureus lies in the reliance on mouse infection models for many research efforts, despite mice not being the natural host for S. aureus. Additionally, certain secreted factors exhibit toxicity in a strain-specific manner [e.g., CHIPS (a chemotaxis inhibitory protein of staphylococci) and PVL], further complicating the understanding of the pathogenic mechanisms [168, 169].
This review aimed to enhance our understanding of S. aureus pathogenesis by focusing on Agr, as a critical gene regulation system impacting the bacterial phenotype. Altogether, Agr-regulated toxins are essential in causing SSTI in immune-competent patients. However, agr-defective strains seem to cause sepsis and secondary infections in immunocompromised hosts, of which bacteria utilize multiple toxins to move between organs and live in a specific niche. Notably, while some infection types may select for entirely agr-functional or agr-defective populations, other infections yield mixed populations (Fig. 4) [170]. Moreover, agr mutations occur while bacteria are infecting patients, to cope with selective pressures [135]. In vitro, the agr revertant rise within a population possibly because bacteria cannot acquire essential nutrients in a population completely devoid of Agr-controlled secreted degradative enzymes [118]. Thus, instead of conducting experiments that directly compare Agr-expressing and non-expressing strains, a more realistic approach may involve a model where a fixed percentage of both Agr-expressing and non-expressing strains are mixed in a population. This dynamic system, where the percentage changes over time, could provide insights into more realistic phenomena and the dynamic interactions between these strains and hosts. The pathophysiology of S. aureus infection is substantially influenced by phenotypic changes resulting from factors beyond Agr. Future studies are expected to give the comprehensive understanding of S. aureus overall profile in various settings.
Acknowledgements
Not applicable.
Abbreviations
- AD
Atopic dermatitis
- Agr
Accessory gene regulator
- AIP
Auto-inducing peptide
- CA-MRSA
Community-associated MRSA
- DIC
Disseminated intravascular coagulation
- FPR
Formyl peptide receptor
- GPCR
G protein coupled receptor
- HA-MRSA
Hospital-acquired MRSA
- hla
α-Hemolysin
- hld
Delta-hemolysin
- HPK
Histidine protein kinase
- IL
Interleukin
- moDC
Monocyte-derived dendritic cell
- MRSA
Methicillin-resistant Staphylococcus aureus
- PSM
Phenol-soluble modulin
- QS
Quorum sensing
- SSTI
Skin and soft tissue infection
- TLR
Toll-like receptor
Authors’ contributions
Writing—original draft: YY, TI, YN. Writing—review and editing: YY, MT, SN, YN.
Funding
JST FOREST JPMJFR200Y (YN). AMED-CREST 23gm1610004h0003 (YN). AMED 23ek0410105s0101 (YN).
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lowy FD. Antimicrobial resistance: the example of Staphylococcus aureus. J Clin Invest. 2003;111(9):1265–1273. doi: 10.1172/JCI18535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gaynes R. The Discovery of Penicillin—new insights after more than 75 years of clinical use. Emerg Infect Dis. 2017;23(5):4. doi: 10.3201/eid2305.161556. [DOI] [Google Scholar]
- 3.Singh V, Phukan UJ. Interaction of host and Staphylococcus aureus protease-system regulates virulence and pathogenicity. Med Microbiol Immunol. 2019;208(5):585–607. doi: 10.1007/s00430-018-0573-y. [DOI] [PubMed] [Google Scholar]
- 4.Lee AS, de Lencastre H, Garau J, Kluytmans J, Malhotra-Kumar S, Peschel A, Harbarth S. Methicillin-resistant Staphylococcus aureus. Nat Rev Dis Primers. 2018;4:18033. doi: 10.1038/nrdp.2018.33. [DOI] [PubMed] [Google Scholar]
- 5.DeLeo FR, Otto M, Kreiswirth BN, Chambers HF. Community-associated meticillin-resistant Staphylococcus aureus. Lancet. 2010;375(9725):1557–1568. doi: 10.1016/S0140-6736(09)61999-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Otto M. Community-associated MRSA: what makes them special? Int J Med Microbiol. 2013;303(6–7):324–330. doi: 10.1016/j.ijmm.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feng Y, Chen CJ, Su LH, Hu S, Yu J, Chiu CH. Evolution and pathogenesis of Staphylococcus aureus: lessons learned from genotyping and comparative genomics. FEMS Microbiol Rev. 2008;32(1):23–37. doi: 10.1111/j.1574-6976.2007.00086.x. [DOI] [PubMed] [Google Scholar]
- 8.Kang CK, Cho JE, Choi YJ, Jung Y, Kim NH, Kim CJ, Kim HB. agr dysfunction affects staphylococcal cassette chromosome mec type-dependent clinical outcomes in methicillin-resistant Staphylococcus aureus bacteremia. Antimicrob Agents Chemother. 2015;59(6):3125–3132. doi: 10.1128/AAC.04962-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gardete S, Tomasz A. Mechanisms of vancomycin resistance in Staphylococcus aureus. J Clin Invest. 2014;124(7):2836–2840. doi: 10.1172/JCI68834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee BY, Singh A, David MZ, Bartsch SM, Slayton RB, Huang SS, Daum RS. The economic burden of community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA) Clin Microbiol Infect. 2013;19(6):528–36. doi: 10.1111/j.1469-0691.2012.03914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harris SR, Cartwright EJ, Torok ME, Holden MT, Brown NM, Ogilvy-Stuart AL, Peacock SJ. Whole-genome sequencing for analysis of an outbreak of meticillin-resistant Staphylococcus aureus: a descriptive study. Lancet Infect Dis. 2013;13(2):130–136. doi: 10.1016/S1473-3099(12)70268-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huber C, Stamm I, Ziebuhr W, Marincola G, Bischoff M, Strommenger B, Walther B. Silence as a way of niche adaptation: mecC-MRSA with variations in the accessory gene regulator (agr) functionality express kaleidoscopic phenotypes. Sci Rep. 2020;10(1):14787. doi: 10.1038/s41598-020-71640-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raghuram V, Alexander AM, Loo HQ, Petit RA, 3rd, Goldberg JB, Read TD. Species-wide phylogenomics of the Staphylococcus aureus Agr operon revealed convergent evolution of frameshift mutations. Microbiol Spectr. 2022;10(1):e0133421. doi: 10.1128/spectrum.01334-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakamura Y, Takahashi H, Takaya A, Inoue Y, Katayama Y, Kusuya Y, Shimojo N. Staphylococcus Agr virulence is critical for epidermal colonization and associates with atopic dermatitis development. Sci Transl Med. 2020;12(551). 10.1126/scitranslmed.aay4068. [DOI] [PMC free article] [PubMed]
- 15.Rutherford ST, Bassler BL. Bacterial quorum sensing: its role in virulence and possibilities for its control. Cold Spring Harb Perspect Med. 2012;2(11):a012427. doi: 10.1101/cshperspect.a012427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Novick RP, Geisinger E. Quorum sensing in staphylococci. Annu Rev Genet. 2008;42:541–564. doi: 10.1146/annurev.genet.42.110807.091640. [DOI] [PubMed] [Google Scholar]
- 17.Thoendel M, Horswill AR. Random mutagenesis and topology analysis of the autoinducing peptide biosynthesis proteins in Staphylococcus aureus. Mol Microbiol. 2013;87(2):318–337. doi: 10.1111/mmi.12100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thoendel M, Horswill AR. Identification of Staphylococcus aureus AgrD residues required for autoinducing peptide biosynthesis. J Biol Chem. 2009;284(33):21828–21838. doi: 10.1074/jbc.M109.031757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thoendel M, Horswill AR. Biosynthesis of peptide signals in gram-positive bacteria. Adv Appl Microbiol. 2010;71:91–112. doi: 10.1016/S0065-2164(10)71004-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jenul C, Horswill AR. Regulation of Staphylococcus aureus Virulence. Microbiol Spectr. 2019;7(2). 10.1128/microbiolspec.GPP3-0031-2018. [DOI] [PMC free article] [PubMed]
- 21.Geisinger E, Adhikari RP, Jin R, Ross HF, Novick RP. Inhibition of rot translation by RNAIII, a key feature of agr function. Mol Microbiol. 2006;61(4):1038–1048. doi: 10.1111/j.1365-2958.2006.05292.x. [DOI] [PubMed] [Google Scholar]
- 22.Cheung AL, Nishina KA, Trotonda MP, Tamber S. The SarA protein family of Staphylococcus aureus. Int J Biochem Cell Biol. 2008;40(3):355–361. doi: 10.1016/j.biocel.2007.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wolz C, Pohlmann-Dietze P, Steinhuber A, Chien YT, Manna A, van Wamel W, Cheung A. Agr-independent regulation of fibronectin-binding protein(s) by the regulatory locus sar in Staphylococcus aureus. Mol Microbiol. 2000;36(1):230–243. doi: 10.1046/j.1365-2958.2000.01853.x. [DOI] [PubMed] [Google Scholar]
- 24.Chien Y, Manna AC, Projan SJ, Cheung AL. SarA, a global regulator of virulence determinants in Staphylococcus aureus, binds to a conserved motif essential for sar-dependent gene regulation. J Biol Chem. 1999;274(52):37169–37176. doi: 10.1074/jbc.274.52.37169. [DOI] [PubMed] [Google Scholar]
- 25.Reyes D, Andrey DO, Monod A, Kelley WL, Zhang G, Cheung AL. Coordinated regulation by AgrA, SarA, and SarR to control agr expression in Staphylococcus aureus. J Bacteriol. 2011;193(21):6020–6031. doi: 10.1128/JB.05436-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dunman PM, Murphy E, Haney S, Palacios D, Tucker-Kellogg G, Wu S, Projan SJ. Transcription profiling-based identification of Staphylococcus aureus genes regulated by the agr and/or sarA loci. J Bacteriol. 2001;183(24):7341–7353. doi: 10.1128/JB.183.24.7341-7353.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheung GY, Joo HS, Chatterjee SS, Otto M. Phenol-soluble modulins—critical determinants of staphylococcal virulence. FEMS Microbiol Rev. 2014;38(4):698–719. doi: 10.1111/1574-6976.12057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Queck SY, Jameson-Lee M, Villaruz AE, Bach TH, Khan BA, Sturdevant DE, Otto M. RNAIII-independent target gene control by the agr quorum-sensing system: insight into the evolution of virulence regulation in Staphylococcus aureus. Mol Cell. 2008;32(1):150–8. doi: 10.1016/j.molcel.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Novick RP, Ross HF, Projan SJ, Kornblum J, Kreiswirth B, Moghazeh S. Synthesis of staphylococcal virulence factors is controlled by a regulatory RNA molecule. EMBO J. 1993;12(10):3967–3975. doi: 10.1002/j.1460-2075.1993.tb06074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang R, Braughton KR, Kretschmer D, Bach TH, Queck SY, Li M, Otto M. Identification of novel cytolytic peptides as key virulence determinants for community-associated MRSA. Nat Med. 2007;13(12):1510–4. doi: 10.1038/nm1656. [DOI] [PubMed] [Google Scholar]
- 31.Tayeb-Fligelman E, Tabachnikov O, Moshe A, Goldshmidt-Tran O, Sawaya MR, Coquelle N, Landau M. The cytotoxic Staphylococcus aureus PSMalpha3 reveals a cross-alpha amyloid-like fibril. Science. 2017;355(6327):831–833. doi: 10.1126/science.aaf4901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kreutzberger MAB, Wang S, Beltran LC, Tuachi A, Zuo X, Egelman EH, Conticello VP. Phenol-soluble modulins PSMalpha3 and PSMbeta2 form nanotubes that are cross-alpha amyloids. Proc Natl Acad Sci U S A. 2022;119(20):e2121586119. doi: 10.1073/pnas.2121586119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Uematsu S, Akira S. Toll-like receptors and innate immunity. J Mol Med (Berl) 2006;84(9):712–725. doi: 10.1007/s00109-006-0084-y. [DOI] [PubMed] [Google Scholar]
- 34.Le Y, Murphy PM, Wang JM. Formyl-peptide receptors revisited. Trends Immunol. 2002;23(11):541–548. doi: 10.1016/s1471-4906(02)02316-5. [DOI] [PubMed] [Google Scholar]
- 35.Kretschmer D, Gleske AK, Rautenberg M, Wang R, Köberle M, Bohn E, Peschel A. Human formyl peptide receptor 2 senses highly pathogenic Staphylococcus aureus. Cell Host Microbe. 2010;7(6):463–73. doi: 10.1016/j.chom.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Surewaard BG, de Haas CJ, Vervoort F, Rigby KM, DeLeo FR, Otto M, Nijland R. Staphylococcal alpha-phenol soluble modulins contribute to neutrophil lysis after phagocytosis. Cell Microbiol. 2013;15(8):1427–1437. doi: 10.1111/cmi.12130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kobayashi SD, Braughton KR, Palazzolo-Ballance AM, Kennedy AD, Sampaio E, Kristosturyan E, DeLeo FR. Rapid neutrophil destruction following phagocytosis of Staphylococcus aureus. J Innate Immun. 2010;2(6):560–75. doi: 10.1159/000317134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schreiner J, Kretschmer D, Klenk J, Otto M, Bühring HJ, Stevanovic S, Autenrieth SE. Staphylococcus aureus phenol-soluble modulin peptides modulate dendritic cell functions and increase in vitro priming of regulatory T cells. J Immunol. 2013;190(7):3417–26. doi: 10.4049/jimmunol.1202563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Richardson JR, Armbruster NS, Gunter M, Henes J, Autenrieth SE. Staphylococcus aureus PSM peptides modulate human monocyte-derived dendritic cells to prime regulatory T cells. Front Immunol. 2018;9:2603. doi: 10.3389/fimmu.2018.02603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hu Z, Kopparapu PK, Ebner P, Mohammad M, Lind S, Jarneborn A, Jin T. Phenol-soluble modulin alpha and beta display divergent roles in mice with staphylococcal septic arthritis. Commun Biol. 2022;5(1):910. doi: 10.1038/s42003-022-03839-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakamura Y, Oscherwitz J, Cease KB, Chan SM, Munoz-Planillo R, Hasegawa M, Nunez G. Staphylococcus delta-toxin induces allergic skin disease by activating mast cells. Nature. 2013;503(7476):397–401. doi: 10.1038/nature12655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaito C, Saito Y, Ikuo M, Omae Y, Mao H, Nagano G, Sekimizu K. Mobile genetic element SCCmec-encoded psm-mec RNA suppresses translation of agrA and attenuates MRSA virulence. PLoS Pathog. 2013;9(4):e1003269. doi: 10.1371/journal.ppat.1003269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chatterjee SS, Chen L, Joo HS, Cheung GY, Kreiswirth BN, Otto M. Distribution and regulation of the mobile genetic element-encoded phenol-soluble modulin PSM-mec in methicillin-resistant Staphylococcus aureus. PLoS ONE. 2011;6(12):e28781. doi: 10.1371/journal.pone.0028781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qin L, McCausland JW, Cheung GY, Otto M. PSM-Mec-a virulence determinant that connects transcriptional regulation, virulence, and antibiotic resistance in Staphylococci. Front Microbiol. 2016;7:1293. doi: 10.3389/fmicb.2016.01293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wilke GA, BubeckWardenburg J. Role of a disintegrin and metalloprotease 10 in Staphylococcus aureus alpha-hemolysin-mediated cellular injury. Proc Natl Acad Sci U S A. 2010;107(30):13473–13478. doi: 10.1073/pnas.1001815107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fussle R, Bhakdi S, Sziegoleit A, Tranum-Jensen J, Kranz T, Wellensiek HJ. On the mechanism of membrane damage by Staphylococcus aureus alpha-toxin. J Cell Biol. 1981;91(1):83–94. doi: 10.1083/jcb.91.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Seilie ES, BubeckWardenburg J. Staphylococcus aureus pore-forming toxins: The interface of pathogen and host complexity. Semin Cell Dev Biol. 2017;72:101–116. doi: 10.1016/j.semcdb.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morfeldt E, Taylor D, von Gabain A, Arvidson S. Activation of alpha-toxin translation in Staphylococcus aureus by the trans-encoded antisense RNA. RNAIII EMBO J. 1995;14(18):4569–4577. doi: 10.1002/j.1460-2075.1995.tb00136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cheung AL, Eberhardt KJ, Chung E, Yeaman MR, Sullam PM, Ramos M, Bayer AS. Diminished virulence of a sar-/agr- mutant of Staphylococcus aureus in the rabbit model of endocarditis. J Clin Invest. 1994;94(5):1815–1822. doi: 10.1172/JCI117530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xiong YQ, Willard J, Yeaman MR, Cheung AL, Bayer AS. Regulation of Staphylococcus aureus alpha-toxin gene (hla) expression by agr, sarA, and sae in vitro and in experimental infective endocarditis. J Infect Dis. 2006;194(9):1267–1275. doi: 10.1086/508210. [DOI] [PubMed] [Google Scholar]
- 51.Berube BJ, BubeckWardenburg J. Staphylococcus aureus alpha-toxin: nearly a century of intrigue. Toxins (Basel) 2013;5(6):1140–1166. doi: 10.3390/toxins5061140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Powers ME, Kim HK, Wang Y, BubeckWardenburg J. ADAM10 mediates vascular injury induced by Staphylococcus aureus alpha-hemolysin. J Infect Dis. 2012;206(3):352–356. doi: 10.1093/infdis/jis192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nygaard TK, Pallister KB, DuMont AL, DeWald M, Watkins RL, Pallister EQ, Voyich JM. Alpha-toxin induces programmed cell death of human T cells, B cells, and monocytes during USA300 infection. PLoS One. 2012;7(5):e36532. 10.1371/journal.pone.0036532. [DOI] [PMC free article] [PubMed]
- 54.Powers ME, Becker RE, Sailer A, Turner JR, BubeckWardenburg J. Synergistic action of Staphylococcus aureus alpha-toxin on platelets and myeloid lineage cells contributes to lethal sepsis. Cell Host Microbe. 2015;17(6):775–787. doi: 10.1016/j.chom.2015.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kennedy AD, Bubeck Wardenburg J, Gardner DJ, Long D Whitney AR, Braughton KR, DeLeo FR. Targeting of alpha-hemolysin by active or passive immunization decreases severity of USA300 skin infection in a mouse model. J Infect Dis. 2010;202(7):1050–8. 10.1086/656043. [DOI] [PMC free article] [PubMed]
- 56.Kobayashi SD, Malachowa N, Whitney AR, Braughton KR, Gardner DJ, Long D, DeLeo FR. Comparative analysis of USA300 virulence determinants in a rabbit model of skin and soft tissue infection. J Infect Dis. 2011;204(6):937–41. doi: 10.1093/infdis/jir441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.BubeckWardenburg J, Patel RJ, Schneewind O. Surface proteins and exotoxins are required for the pathogenesis of Staphylococcus aureus pneumonia. Infect Immun. 2007;75(2):1040–1044. doi: 10.1128/IAI.01313-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Inoshima I, Inoshima N, Wilke GA, Powers ME, Frank KM, Wang Y, BubeckWardenburg J. A Staphylococcus aureus pore-forming toxin subverts the activity of ADAM10 to cause lethal infection in mice. Nat Med. 2011;17(10):1310–1314. doi: 10.1038/nm.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kielian T, Cheung A, Hickey WF. Diminished virulence of an alpha-toxin mutant of Staphylococcus aureus in experimental brain abscesses. Infect Immun. 2001;69(11):6902–6911. doi: 10.1128/IAI.69.11.6902-6911.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Surewaard BGJ, Thanabalasuriar A, Zeng Z, Tkaczyk C, Cohen TS, Bardoel BW, Kubes P. Alpha-toxin induces platelet aggregation and liver injury during Staphylococcus aureus sepsis. Cell Host Microbe. 2018;24(2):271–284e273. doi: 10.1016/j.chom.2018.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Craven RR, Gao X, Allen IC, Gris D, BubeckWardenburg J, McElvania-Tekippe E, Duncan JA. Staphylococcus aureus alpha-hemolysin activates the NLRP3-inflammasome in human and mouse monocytic cells. PLoS One. 2009;4(10):e7446. doi: 10.1371/journal.pone.0007446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kebaier C, hamberland RR, Allen IC, Gao X, Broglie PM, Hall JD, Duncan JA. Staphylococcus aureus alpha-hemolysin mediates virulence in a murine model of severe pneumonia through activation of the NLRP3 inflammasome. J Infect Dis. 2012;205(5):807–817. doi: 10.1093/infdis/jir846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Spaan AN, Neehus AL, Laplantine E, Staels F, Ogishi M, Seeleuthner Y, Casanova J. Human OTULIN haploinsufficiency impairs cell-intrinsic immunity to staphylococcal alpha-toxin. Science. 2022;376:eabm6380. doi: 10.1126/science.abm6380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Keller MD, Ching KL, Liang FX, Dhabaria A, Tam K, Ueberheide, BM, CadwellK. Decoy exosomes provide protection against bacterial toxins. Nature. 2020;579(7798):260–4. 10.1038/s41586-020-2066-6. [DOI] [PMC free article] [PubMed]
- 65.Rauch S, DeDent AC, Kim HK, BubeckWardenburg J, Missiakas DM, Schneewind O. Abscess formation and alpha-hemolysin induced toxicity in a mouse model of Staphylococcus aureus peritoneal infection. Infect Immun. 2012;80(10):3721–3732. doi: 10.1128/IAI.00442-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.O'Callaghan RJ, Callegan MC, Moreau JM, Green LC, Foster TJ, Hartford OM, Hill JM. Specific roles of alpha-toxin and beta-toxin during Staphylococcus aureus corneal infection. Infect Immun. 1997;65(5):1571–1578. doi: 10.1128/iai.65.5.1571-1578.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Berube BJ, Sampedro GR, Otto M, BubeckWardenburg J. The psmalpha locus regulates production of Staphylococcus aureus alpha-toxin during infection. Infect Immun. 2014;82(8):3350–3358. doi: 10.1128/IAI.00089-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Elbashir MI, Nilson BH, Akesson P, Bjorck L, Akerstrom B. Antibody response in immunized rabbits measured with bacterial immunoglobulin-binding proteins. J Immunol Methods. 1990;135(1–2):171–179. doi: 10.1016/0022-1759(90)90270-6. [DOI] [PubMed] [Google Scholar]
- 69.Graille M, Stura EA, Corper AL, Sutton BJ, Taussig MJ, Charbonnier JB, Silverman GJ. Crystal structure of a Staphylococcus aureus protein A domain complexed with the Fab fragment of a human IgM antibody: structural basis for recognition of B-cell receptors and superantigen activity. Proc Natl Acad Sci U S A. 2000;97(10):5399–5404. doi: 10.1073/pnas.97.10.5399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gomez MI, Lee A, Reddy B, Muir A, Soong G, Pitt A, Prince A. Staphylococcus aureus protein A induces airway epithelial inflammatory responses by activating TNFR1. Nat Med. 2004;10(8):842–848. doi: 10.1038/nm1079. [DOI] [PubMed] [Google Scholar]
- 71.Huntzinger E, Boisset S, Saveanu C, Benito Y, Geissmann T, Namane A, Romby P. Staphylococcus aureus RNAIII and the endoribonuclease III coordinately regulate spa gene expression. EMBO J. 2005;24(4):824–835. doi: 10.1038/sj.emboj.7600572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Morrison JM, Anderson KL, Beenken KE, Smeltzer MS, Dunman PM. The staphylococcal accessory regulator, SarA, is an RNA-binding protein that modulates the mRNA turnover properties of late-exponential and stationary phase Staphylococcus aureus cells. Front Cell Infect Microbiol. 2012;2:26. doi: 10.3389/fcimb.2012.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cheung GYC, Bae JS, Otto M. Pathogenicity and virulence of Staphylococcus aureus. Virulence. 2021;12(1):547–569. doi: 10.1080/21505594.2021.1878688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Le KY, Otto M. Quorum-sensing regulation in staphylococci-an overview. Front Microbiol. 2015;6:1174. doi: 10.3389/fmicb.2015.01174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Williams H, Flohr C. How epidemiology has challenged 3 prevailing concepts about atopic dermatitis. J Allergy Clin Immunol. 2006;118(1):209–213. doi: 10.1016/j.jaci.2006.04.043. [DOI] [PubMed] [Google Scholar]
- 76.Kong HH, Oh J, Deming C, Conlan S, Grice EA, Beatson MA, Segre JA. Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Res. 2012;22(5):850–9. doi: 10.1101/gr.131029.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Oh J, Conlan S, Polley EC, Segre JA, Kong HH. Shifts in human skin and nares microbiota of healthy children and adults. Genome Med. 2012;4(10):77. doi: 10.1186/gm378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tauber M, Balica S, Hsu CY, Jean-Decoster C, Lauze C, Redoules D, Paul CF. Staphylococcus aureus density on lesional and nonlesional skin is strongly associated with disease severity in atopic dermatitis. J Allergy Clin Immunol. 2016;137(4):1272–1274 e1273. doi: 10.1016/j.jaci.2015.07.052. [DOI] [PubMed] [Google Scholar]
- 79.Totte JE, van der Feltz WT, Hennekam M, van Belkum A, van Zuuren EJ, Pasmans SG. Prevalence and odds of Staphylococcus aureus carriage in atopic dermatitis: a systematic review and meta-analysis. Br J Dermatol. 2016;175(4):687–695. doi: 10.1111/bjd.14566. [DOI] [PubMed] [Google Scholar]
- 80.Townsend EC, Kalan LR. The dynamic balance of the skin microbiome across the lifespan. Biochem Soc Trans. 2023;51(1):71–86. doi: 10.1042/BST20220216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dwyer LR, Scharschmidt TC. Early life host-microbe interactions in skin. Cell Host Microbe. 2022;30(5):684–695. doi: 10.1016/j.chom.2022.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hourigan SK, Dominguez-Bello MG, Mueller NT. Can maternal-child microbial seeding interventions improve the health of infants delivered by Cesarean section? Cell Host Microbe. 2022;30(5):607–611. doi: 10.1016/j.chom.2022.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Casterline BW, Paller AS. Early development of the skin microbiome: therapeutic opportunities. Pediatr Res. 2021;90(4):731–737. doi: 10.1038/s41390-020-01146-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jo JH, Deming C, Kennedy EA, Conlan S, Polley EC, Ng WI, Kong HH. Diverse human skin fungal communities in children converge in adulthood. J Invest Dermatol. 2016;136(12):2356–63. doi: 10.1016/j.jid.2016.05.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jo JH, Kennedy EA, Kong HH. Topographical and physiological differences of the skin mycobiome in health and disease. Virulence. 2017;8(3):324–333. doi: 10.1080/21505594.2016.1249093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Byrd AL, Belkaid Y, Segre JA. The human skin microbiome. Nat Rev Microbiol. 2018;16(3):143–155. doi: 10.1038/nrmicro.2017.157. [DOI] [PubMed] [Google Scholar]
- 87.Seite S, Flores GE, Henley JB, Martin R, Zelenkova H, Aguilar L, Fierer N. Microbiome of affected and unaffected skin of patients with atopic dermatitis before and after emollient treatment. J Drugs Dermatol. 2014;13(11):1365–1372. [PubMed] [Google Scholar]
- 88.Geoghegan JA, Irvine AD, Foster TJ. Staphylococcus aureus and atopic dermatitis: a complex and evolving relationship. Trends Microbiol. 2018;26(6):484–497. doi: 10.1016/j.tim.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 89.Gonzalez ME, Schaffer JV, Orlow SJ, Gao Z, Li H, Alekseyenko AV, Blaser MJ. Cutaneous microbiome effects of fluticasone propionate cream and adjunctive bleach baths in childhood atopic dermatitis. J Am Acad Dermatol. 2016;75(3):481–493e488. doi: 10.1016/j.jaad.2016.04.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Byrd AL, Deming C, Cassidy SKB, Harrison OJ, Ng WI, Conlan S, Kong HH. Staphylococcus aureus and Staphylococcus epidermidis strain diversity underlying pediatric atopic dermatitis. Sci Transl Med. 2017;9(397). 10.1126/scitranslmed.aal4651. [DOI] [PMC free article] [PubMed]
- 91.Shi B, Bangayan NJ, Curd E, Taylor PA, Gallo RL, Leung DYM, Li H. The skin microbiome is different in pediatric versus adult atopic dermatitis. J Allergy Clin Immunol. 2016;138(4):1233–1236. doi: 10.1016/j.jaci.2016.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Paharik AE, Parlet CP, Chung N, Todd DA, Rodriguez EI, Van Dyke MJ, Horswill AR. Coagulase-negative Staphylococcal Strain prevents Staphylococcus aureus colonization and skin infection by blocking quorum sensing. Cell Host Microbe. 2017;22(6):746–756e745. doi: 10.1016/j.chom.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Williams MR, Costa SK, Zaramela LS, Khalil S, Todd DA, Winter HL, Gallo RL. Quorum sensing between bacterial species on the skin protects against epidermal injury in atopic dermatitis. Sci Transl Med. 2019;11(490):eaat8329. doi: 10.1126/scitranslmed.aat8329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nakaawa S, Matsumoto M, Katayama Y, Oguma R, Wakabayashi S, Nygaard T, Nakamura Y. Staphylococcus aureus virulent PSMalpha peptides induce keratinocyte alarmin release to orchestrate IL-17-dependent skin inflammation. Cell Host Microbe. 2017;22(5):667–677e665. doi: 10.1016/j.chom.2017.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mills KHG. IL-17 and IL-17-producing cells in protection versus pathology. Nat Rev Immunol. 2023;23(1):38–54. doi: 10.1038/s41577-022-00746-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Matsumoto M, Nakagawa S, Zhang L, Nakamura Y, Villaruz AE, Otto M, Nunez G. Interaction between Staphylococcus Agr virulence and neutrophils regulates pathogen expansion in the skin. Cell Host Microbe. 2021;29(6):930–40. doi: 10.1016/j.chom.2021.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fridkin SK, Hageman JC, Morrison M, Sanza LT, Como-Sabetti K, Jernigan J.A, Active bacterial core surveillance program of the emerging infections program N Methicillin-resistant Staphylococcus aureus disease in three communities. N Engl J Med. 2005;352(14):1436–1444. doi: 10.1056/NEJMoa043252. [DOI] [PubMed] [Google Scholar]
- 98.Miller LG, Perdreau-Remington F, Rieg G, Mehdi S, Perlroth J, Bayer AS, Spellberg B. Necrotizing fasciitis caused by community-associated methicillin-resistant Staphylococcus aureus in Los Angeles. N Engl J Med. 2005;352(14):1445–53. doi: 10.1056/NEJMoa042683. [DOI] [PubMed] [Google Scholar]
- 99.Spaan AN, van Strijp JAG, Torres VJ. Leukocidins: staphylococcal bi-component pore-forming toxins find their receptors. Nat Rev Microbiol. 2017;15(7):435–447. doi: 10.1038/nrmicro.2017.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Villaruz AE, Wardenburg JB, Khan BA, Whitney AR, Sturdevant DE, Gardner DJ, DeLeo FR, Otto M. A point mutation in the agr locus rather than expression of the Panton-Valentine leukocidin caused previously reported phenotypes in Staphylococcus aureus pneumonia and gene regulation. J Infect Dis. 2009;200(5):724–34. doi: 10.1086/604728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Voyich JM, Otto M, Mathema B, Braughton KR, Whitney AR, Welty D, DeLeo FR. Is Panton-Valentine leukocidin the major virulence determinant in community-associated methicillin-resistant Staphylococcus aureus disease? J Infect Dis. 2006;194(12):1761–1770. doi: 10.1086/509506. [DOI] [PubMed] [Google Scholar]
- 102.BubeckWardenburg J, Bae T, Otto M, Deleo FR, Schneewind O. Poring over pores: alpha-hemolysin and Panton-Valentine leukocidin in Staphylococcus aureus pneumonia. Nat Med. 2007;13(12):1405–1406. doi: 10.1038/nm1207-1405. [DOI] [PubMed] [Google Scholar]
- 103.BubeckWardenburg J, Palazzolo-Ballance AM, Otto M, Schneewind O, DeLeo FR. Panton-Valentine leukocidin is not a virulence determinant in murine models of community-associated methicillin-resistant Staphylococcus aureus disease. J Infect Dis. 2008;198(8):1166–1170. doi: 10.1086/592053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Otto M. Basis of virulence in community-associated methicillin-resistant Staphylococcus aureus. Annu Rev Microbiol. 2010;64:143–162. doi: 10.1146/annurev.micro.112408.134309. [DOI] [PubMed] [Google Scholar]
- 105.Li M, Cheung GY, Hu J, Wang D, Joo HS, Deleo FR, Otto M. Comparative analysis of virulence and toxin expression of global community-associated methicillin-resistant Staphylococcus aureus strains. J Infect Dis. 2010;202(12):1866–1876. doi: 10.1086/657419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Li M, Diep BA, Villaruz AE, Braughton KR, Jiang X, DeLeo FR, Chambers HF, Lu Y, Otto M. Evolution of virulence in epidemic community-associated methicillin-resistant Staphylococcus aureus. Proc Natl Acad Sci U S A. 2009;106(14):5883–8. doi: 10.1073/pnas.0900743106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tsuji BT, MacLean RD, Dresser LD, McGavin MJ, Simor AE. Impact of accessory gene regulator (agr) dysfunction on vancomycin pharmacodynamics among Canadian community and health-care associated methicillin-resistant Staphylococcus aureus. Ann Clin Microbiol Antimicrob. 2011;10:20. doi: 10.1186/1476-0711-10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shopsin B, Drlica-Wagner A, Mathema B, Adhikari RP, Kreiswirth BN, Novick RP. Prevalence of agr dysfunction among colonizing Staphylococcus aureus strains. J Infect Dis. 2008;198(8):1171–1174. doi: 10.1086/592051. [DOI] [PubMed] [Google Scholar]
- 109.Pynnonen M, Stephenson RE, Schwartz K, Hernandez M, Boles BR. Hemoglobin promotes Staphylococcus aureus nasal colonization. PLoS Pathog. 2011;7(7):e1002104. doi: 10.1371/journal.ppat.1002104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Stryjewski ME, Chambers HF. Skin and soft-tissue infections caused by community-acquired methicillin-resistant Staphylococcus aureus. Clin Infect Dis. 2008;46(Suppl 5):S368–377. doi: 10.1086/533593. [DOI] [PubMed] [Google Scholar]
- 111.Kim HK, Missiakas D, Schneewind O. Mouse models for infectious diseases caused by Staphylococcus aureus. J Immunol Methods. 2014;410:88–99. doi: 10.1016/j.jim.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lowy FD. Staphylococcus aureus infections. N Engl J Med. 1998;339(8):520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 113.Mayville P, Ji G, Beavis R, Yang H, Goger M, Novick RP, Muir TW. Structure-activity analysis of synthetic autoinducing thiolactone peptides from Staphylococcus aureus responsible for virulence. Proc Natl Acad Sci U S A. 1999;96(4):1218–1223. doi: 10.1073/pnas.96.4.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Montgomery CP, Boyle-Vavra S, Daum RS. Importance of the global regulators Agr and SaeRS in the pathogenesis of CA-MRSA USA300 infection. PLoS ONE. 2010;5(12):e15177. doi: 10.1371/journal.pone.0015177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cheung GY, Wang R, Khan BA, Sturdevant DE, Otto M. Role of the accessory gene regulator agr in community-associated methicillin-resistant Staphylococcus aureus pathogenesis. Infect Immun. 2011;79(5):1927–1935. doi: 10.1128/IAI.00046-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.He L, Le KY, Khan BA, Nguyen TH, Hunt RL, Bae JS, Otto M. Resistance to leukocytes ties benefits of quorum sensing dysfunctionality to biofilm infection. Nat Microbiol. 2019;4(7):1114–1119. doi: 10.1038/s41564-019-0413-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Inoshima N, Wang Y, BubeckWardenburg J. Genetic requirement for ADAM10 in severe Staphylococcus aureus skin infection. J Invest Dermatol. 2012;132(5):1513–1516. doi: 10.1038/jid.2011.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kern WV, Rieg S. Burden of bacterial bloodstream infection-a brief update on epidemiology and significance of multidrug-resistant pathogens. Clin Microbiol Infect. 2020;26(2):151–157. doi: 10.1016/j.cmi.2019.10.031. [DOI] [PubMed] [Google Scholar]
- 119.Jacobsson G, Gustafsson E, Andersson R. Outcome for invasive Staphylococcus aureus infections. Eur J Clin Microbiol Infect Dis. 2008;27(9):839–848. doi: 10.1007/s10096-008-0515-5. [DOI] [PubMed] [Google Scholar]
- 120.Anantha RV, Jegatheswaran J, Pepe DL, Priestap F, Delport J, Haeryfar SM, McCormick JK, Mele T. Risk factors for mortality among patients with Staphylococcus aureus bacteremia: a single-centre retrospective cohort study. CMAJ Open. 2014;2(4):E352–359. doi: 10.9778/cmajo.20140018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kaasch AJ, Barlow G, Edgeworth JD, Fowler VG, Jr, Hellmich M, Hopkins S, Colleagues Staphylococcus aureus bloodstream infection: a pooled analysis of five prospective, observational studies. J Infect. 2014;68(3):242–251. doi: 10.1016/j.jinf.2013.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gradel KO, Jensen US, Schonheyder HC, Ostergaard C, Knudsen JD, Wehberg S, Danish Collaborative Bacteraemia N Impact of appropriate empirical antibiotic treatment on recurrence and mortality in patients with bacteraemia: a population-based cohort study. BMC Infect Dis. 2017;17(1):122. doi: 10.1186/s12879-017-2233-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Fowler VG, Olsen MK, Corey GR, Woods CW, Cabell CH, Reller LB, Oddone EZ. Clinical identifiers of complicated Staphylococcus aureus bacteremia. Arch Intern Med. 2003;163(17):2066–72. doi: 10.1001/archinte.163.17.2066. [DOI] [PubMed] [Google Scholar]
- 124.Wilson J, Guy R, Elgohari S, Sheridan E, Davies J, Lamagni T, Pearson A. Trends in sources of meticillin-resistant Staphylococcus aureus (MRSA) bacteraemia: data from the national mandatory surveillance of MRSA bacteraemia in England, 2006–2009. J Hosp Infect. 2011;79(3):211–217. doi: 10.1016/j.jhin.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 125.Foster TJ, Geoghegan JA, Ganesh VK, Hook M. Adhesion, invasion and evasion: the many functions of the surface proteins of Staphylococcus aureus. Nat Rev Microbiol. 2014;12(1):49–62. doi: 10.1038/nrmicro3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Otto M. Staphylococcus aureus toxins. Curr Opin Microbiol. 2014;17:32–37. doi: 10.1016/j.mib.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med. 2013;369(9):840–851. doi: 10.1056/NEJMra1208623. [DOI] [PubMed] [Google Scholar]
- 128.Priest NK, Rudkin JK, Feil EJ, Van Den Elsen JM, Cheung A, Peacock SJ, Laabei M, Massey RC. From genotype to phenotype: can systems biology be used to predict Staphylococcus aureus virulence? Nat Rev Microbiol. 2012;10(11):791–7. doi: 10.1038/nrmicro2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chong YP, Kim ES, Park SJ, Park KH, Kim T, Kim MN, Kim YS. Accessory gene regulator (agr) dysfunction in Staphylococcus aureus bloodstream isolates from South Korean patients. Antimicrob Agents Chemother. 2013;57(3):1509–12. doi: 10.1128/AAC.01260-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Butterfield JM, Tsuji BT, Brown J, Ashley ED, Hardy D, Brown K, Forrest A, Lodise TP. Predictors of agr dysfunction in methicillin-resistant Staphylococcus aureus (MRSA) isolates among patients with MRSA bloodstream infections. Antimicrob Agents Chemother. 2011;55(12):5433–7. doi: 10.1128/AAC.00407-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Altman DR, Sullivan MJ, Chacko KI, Balasubramanian D, Pak TR, Sause WE, van Bakel H. Genome plasticity of agr-defective Staphylococcus aureus during clinical infection. Infect Immun. 2018;86(10). 10.1128/IAI.00331-18. [DOI] [PMC free article] [PubMed]
- 132.He L, Zhang F, Jian Y, Lv H, Hamushan M, Liu J, Otto M. Key role of quorum-sensing mutations in the development of Staphylococcus aureus clinical device-associated infection. Clin Transl Med. 2022;12(4):e801. doi: 10.1002/ctm2.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kwiecinski JM, Horswill AR. Staphylococcus aureus bloodstream infections: pathogenesis and regulatory mechanisms. Curr Opin Microbiol. 2020;53:51–60. doi: 10.1016/j.mib.2020.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Painter KL, Krishna A, Wigneshweraraj S, Edwards AM. What role does the quorum-sensing accessory gene regulator system play during Staphylococcus aureus bacteremia? Trends Microbiol. 2014;22(12):676–685. doi: 10.1016/j.tim.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 135.Surewaard BG, Deniset JF, Zemp FJ, Amrein M, Otto M, Conly J, Omri A, Yates RM, Kubes P. Identification and treatment of the Staphylococcus aureus reservoir in vivo. J Exp Med. 2016;213(7):1141–51. doi: 10.1084/jem.20160334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Jorch SK, Surewaard BG, Hossain M, Peiseler M, Deppermann C, Deng J, Kubes P. Peritoneal GATA6+ macrophages function as a portal for Staphylococcus aureus dissemination. J Clin Invest. 2019;129(11):4643–56. doi: 10.1172/JCI127286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Pollitt EJG, Szkuta PT, Burns N, Foster SJ. Staphylococcus aureus infection dynamics. PLoS Pathog. 2018;14(6):e1007112. doi: 10.1371/journal.ppat.1007112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wong CH, Jenne CN, Petri B, Chrobok NL, Kubes P. Nucleation of platelets with blood-borne pathogens on Kupffer cells precedes other innate immunity and contributes to bacterial clearance. Nat Immunol. 2013;14(8):785–792. doi: 10.1038/ni.2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kubica M., Guzik K, Koziel J, Zarebski M, Richter W, Gajkowska B, Potempa J. A potential new pathway for Staphylococcus aureus dissemination: the silent survival of S. aureus phagocytosed by human monocyte-derived macrophages. PLoS One. 2008;3(1):e1409. doi: 10.1371/journal.pone.0001409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Edwards AM, Massey RC. How does Staphylococcus aureus escape the bloodstream? Trends Microbiol. 2011;19(4):184–190. doi: 10.1016/j.tim.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 141.Mwangi MM, Wu SW, Zhou Y, Sieradzki K, de Lencastre H, Richardson P, Tomasz A. Tracking the in vivo evolution of multidrug resistance in Staphylococcus aureus by whole-genome sequencing. Proc Natl Acad Sci U S A. 2007;2007(22):9451–9456. doi: 10.1073/pnas.0609839104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Smyth DS, Kafer JM, Wasserman GA, Velickovic L, Mathema B, Holzman RS, Shopsin B. Nasal carriage as a source of agr-defective Staphylococcus aureus bacteremia. J Infect Dis. 2012;206(8):1168–77. doi: 10.1093/infdis/jis483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Suligoy CM, Lattar SM, Noto Llana M, Gonzalez CD, Alvarez LP, Robinson DA, Sordelli DO. Mutation of Agr is associated with the adaptation of Staphylococcus aureus to the host during chronic osteomyelitis. Front Cell Infect Microbiol. 2018;8:18. doi: 10.3389/fcimb.2018.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chong YP, Park SJ, Kim HS, Kim ES, Kim MN, Park KH, Kim YS. Persistent Staphylococcus aureus bacteremia: a prospective analysis of risk factors, outcomes, and microbiologic and genotypic characteristics of isolates. Medicine. 2013;92(2):98. doi: 10.1097/MD.0b013e318289ff1e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Park SY, Chong YP, Park HJ, Park KH, Moon SM, Jeong JY, Kim YS. agr Dysfunction and persistent methicillin-resistant Staphylococcus aureus bacteremia in patients with removed eradicable foci. Infection. 2013;41(1):111–119. doi: 10.1007/s15010-012-0348-0. [DOI] [PubMed] [Google Scholar]
- 146.Fowler VG, Jr, Sakoulas G, McIntyre LM, Meka VG, Arbeit RD, Cabell CH, Bayer AS. Persistent bacteremia due to methicillin-resistant Staphylococcus aureus infection is associated with agr dysfunction and low-level in vitro resistance to thrombin-induced platelet microbicidal protein. J Infect Dis. 2004;190(6):1140–1149. doi: 10.1086/423145. [DOI] [PubMed] [Google Scholar]
- 147.Schweizer ML, Furuno JP, Sakoulas G, Johnson JK, Harris AD, Shardell MD, Perencevich EN. Increased mortality with accessory gene regulator (agr) dysfunction in Staphylococcus aureus among bacteremic patients. Antimicrob Agents Chemother. 2011;55(3):1082–7. doi: 10.1128/AAC.00918-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Moise PA, Sakoulas G, Forrest A, Schentag JJ. Vancomycin in vitro bactericidal activity and its relationship to efficacy in clearance of methicillin-resistant Staphylococcus aureus bacteremia. Antimicrob Agents Chemother. 2007;51(7):2582–2586. doi: 10.1128/AAC.00939-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Cheung GY, Kretschmer D, Duong AC, Yeh AJ, Ho TV, Chen Y, Joo HS, Kreiswirth BN, Peschel A, Otto M. Production of an attenuated phenol-soluble modulin variant unique to the MRSA clonal complex 30 increases severity of bloodstream infection. PLoS Pathog. 2014;10(8):e1004298. doi: 10.1371/journal.ppat.1004298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Schilcher K, Horswill AR. Staphylococcal Biofilm Development: Structure, Regulation, and Treatment Strategies. Microbiol Mol Biol Rev. 2020;84(3):10–128. doi: 10.1128/MMBR.00026-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Boles BR, Horswill AR. Agr-mediated dispersal of Staphylococcus aureus biofilms. PLoS Pathog. 2008;4(4):e1000052. doi: 10.1371/journal.ppat.1000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Periasamy S, Joo HS, Duong AC, Bach TH, Tan VY, Chatterjee SS, Otto M. How Staphylococcus aureus biofilms develop their characteristic structure. Proc Natl Acad Sci U S A. 2012;109(4):1281–1286. doi: 10.1073/pnas.1115006109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Le KY, Dastgheyb S, Ho TV, Otto M. Molecular determinants of staphylococcal biofilm dispersal and structuring. Front Cell Infect Microbiol. 2014;4:167. doi: 10.3389/fcimb.2014.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Scherr TD, Hanke ML, Huang O, James DB, Horswill AR, Bayles KW, Fey PD, Torres VJ, Kielian T. Staphylococcus aureus biofilms induce macrophage dysfunction through leukocidin AB and alpha-toxin. MBio. 2015;6(4):10–128. doi: 10.1128/mBio.01021-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Vuong C, Saenz HL, Gotz F, Otto M. Impact of the agr quorum-sensing system on adherence to polystyrene in Staphylococcus aureus. J Infect Dis. 2000;182(6):1688–1693. doi: 10.1086/317606. [DOI] [PubMed] [Google Scholar]
- 156.Moormeier DE, Bayles KW. Staphylococcus aureus biofilm: a complex developmental organism. Mol Microbiol. 2017;104(3):365–376. doi: 10.1111/mmi.13634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.He L, Lv H, Wang Y, Jiang F, Liu Q, Zhang F, Li M. Antibiotic treatment can exacerbate biofilm-associated infection by promoting quorum cheater development. NPJ Biofilms Microbiomes. 2023;9(1):26. doi: 10.1038/s41522-023-00394-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Malachowa N, Whitney AR, Kobayashi SD, Sturdevant DE, Kennedy AD, Braughton KR, DeLeo FR. Global changes in Staphylococcus aureus gene expression in human blood. PloS one. 2011;6(4):e18617. doi: 10.1371/journal.pone.0018617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Heim CE, Hanke ML, Kielian T. A mouse model of Staphylococcus catheter-associated biofilm infection. Methods Mol Biol. 2014;1106:183–191. doi: 10.1007/978-1-62703-736-5_17. [DOI] [PubMed] [Google Scholar]
- 160.Hartleib J, Kohler N, Dickinson RB, Chhatwal GS, Sixma JJ, Hartford OM, Herrmann M. Protein A is the von Willebrand factor binding protein on Staphylococcus aureus. Blood. 2000;96(6):2149–2156. [PubMed] [Google Scholar]
- 161.Siboo IR, Chambers HF, Sullam PM. Role of SraP, a Serine-rich surface protein of Staphylococcus aureus, in binding to human platelets. Infect Immun. 2005;73(4):2273–2280. doi: 10.1128/IAI.73.4.2273-2280.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Cheng AG, Kim HK, Burts ML, Krausz T, Schneewind O, Missiakas DM. Genetic requirements for Staphylococcus aureus abscess formation and persistence in host tissues. FASEB J. 2009;23(10):3393–3404. doi: 10.1096/fj.09-135467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Cheng AG, DeDent AC, Schneewind O, Missiakas D. A play in four acts: Staphylococcus aureus abscess formation. Trends Microbiol. 2011;19(5):225–232. doi: 10.1016/j.tim.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Yang Y, Qian M, Yi S, Liu S, Li B, Yu R, Chen W. Monoclonal antibody targeting Staphylococcus aureus surface protein A (SasA) protect against Staphylococcus aureus sepsis and peritonitis in mice. PLoS One. 2016;11(2):e0149460. doi: 10.1371/journal.pone.0149460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Khatib R, Johnson LB, Sharma M, Fakih MG, Ganga R, Riederer K. Persistent Staphylococcus aureus bacteremia: incidence and outcome trends over time. Scand J Infect Dis. 2009;41(1):4–9. doi: 10.1080/00365540802441711. [DOI] [PubMed] [Google Scholar]
- 166.Park S, Rich J, Hanses F, Lee JC. Defects in innate immunity predispose C57BL/6J-Leprdb/Leprdb mice to infection by Staphylococcus aureus. Infect Immun. 2009;77(3):1008–1014. doi: 10.1128/IAI.00976-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Tseng CW, Kyme PA, Arruda A, Ramanujan VK, Tawackoli W, Liu GY. Innate immune dysfunctions in aged mice facilitate the systemic dissemination of methicillin-resistant S. aureus. PloS One. 2012;7(7):e41454.. doi: 10.1371/journal.pone.0041454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Rooijakkers SH, Ruyken M, Roos A, Daha MR, Presanis JS, Sim RB, Van Strijp JA. Immune evasion by a staphylococcal complement inhibitor that acts on C3 convertases. Nat Immunol. 2005;6(9):920–7. doi: 10.1038/ni1235. [DOI] [PubMed] [Google Scholar]
- 169.Spaan AN, Surewaard BG, Nijland R, van Strijp JA. Neutrophils versus Staphylococcus aureus: a biological tug of war. Annu Rev Microbiol. 2013;67:629–650. doi: 10.1146/annurev-micro-092412-155746. [DOI] [PubMed] [Google Scholar]
- 170.Traber KE, Lee E, Benson S, Corrigan R, Cantera M, Shopsin B, Novick RP. agr function in clinical Staphylococcus aureus isolates. Microbiology (Reading) 2008;154(Pt 8):2265–2274. doi: 10.1099/mic.0.2007/011874-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.