Abstract
The skin often reflects the presence of internal diseases. Acrokeratosis neoplastica (Bazex syndrome) is a unique skin manifestation characterized by its erythematous hyperkeratosis with yellowish, adherent scales on the palm, sole, or other acral locations. There is a potentially high association between Bazex syndrome and malignant pathology, especially squamous cell carcinomas (SCC). To date, various skin conditions have been recognized as diagnostic indicators of insidious malignancies. The recognition of paraneoplastic dermatoses has a strong potential for prompt cancer detection and early therapeutic intervention. Here we describe clinical and forensic cases of Bazex syndrome that are associated with SCC of the glottis and lung. Bazex syndrome has been reported to be associated with a variety of cancers in addition to SCC. We review the clinical manifestations of Bazex syndrome and include updated knowledge on disease pathogenesis.
Keywords: Acrokeratosis neoplastica, Bazex syndrome, Paraneoplastic dermatosis, Squamous cell carcinoma, Case report
Highlights
-
•
Acrokeratosis neoplastica (Bazex syndrome) is a skin manifestation indicative of malignancies.
-
•
Squamous cell carcinoma is highly associated with Bazex syndrome.
-
•
Lung and glottis squamous cell carcinomas were found together with Bazex syndrome.
-
•
Hidden neoplasms should be primarily investigated in cases with Bazex syndrome.
1. Introduction
The skin often reflects the presence of underlying systemic disease. Some malignancies can trigger paraneoplastic dermatoses. It is well known that Leser-Trélat syndrome or acanthosis nigricans maligna is associated with gastrointestinal adenocarcinoma [1,2]. Currently, more than 50 skin manifestations have been proposed as potential markers of internal malignancy [2]. These dermatologic conditions have been widely used to identify hidden neoplasms. Acrokeratosis neoplastica (Bazex syndrome) is a well-described but rare paraneoplastic dermatosis characterized by symmetrical scaly psoriasiform-like eruptions on acral locations, commonly the ear helix, nose, hands, and feet [3,4]. The soles often show diffuse thickening and scaling that resemble keratoderma. Bazex et al. first suggested that these characteristic skin manifestations are associated with malignancies of the laryngopharyngeal region [3]. To date, approximately 50% of the underlying malignancies with Bazex syndrome are squamous cell carcinoma (SCC) of the aerodigestive tract [4]. Bazex syndrome is the third most common (5%) paraneoplastic condition that is associated with SCCs, after hypercalcemia (78%) and anemia (10%) [5]. The recognition of Bazex syndrome provides an opportunity to detect insidious SCC and early therapeutic intervention. Here, we describe clinical and forensic cases of Bazex syndrome and review the clinical manifestations of Bazex syndrome, and include updated knowledge on disease pathogenesis.
2. Case presentation
2.1. Case 1
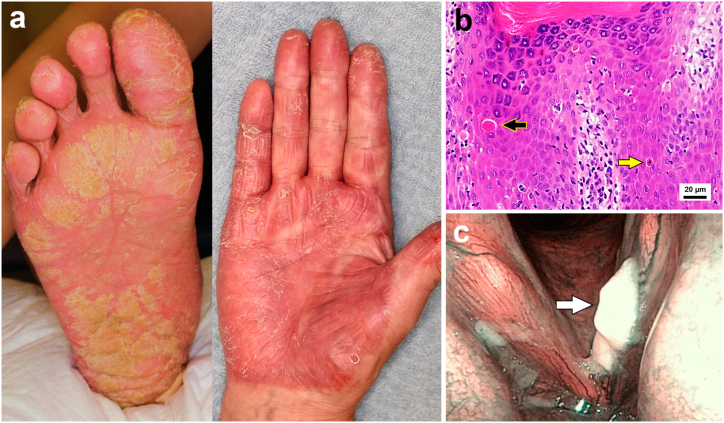
A 78-year-old man presented with an 11-month history of hyperkeratotic erythematous plaques on his bilateral palms and soles that were unresponsive to topical steroids, antifungal medications, and urea cream (Fig. 1a). Initially, keratinization and erythema of the palms appeared, followed by keratinization of the soles four months later. Although a mild cough and hoarseness were noted, he was unaware of its new onset or exacerbation because of heavy smoking. His medical history included hypertension, hyperuricemia, and a prostate cancer resection four years earlier. Serology for hepatitis B and C, and syphilis were negative. A skin biopsy of the soles found hyperkeratosis, acanthosis, and perivascular lymphocyte infiltration in the upper dermis. Necrotic keratinocytes were observed within the epidermis, with additional rounded homogenous eosinophilic masses in the lower epidermis and at the dermo-epidermal junction that resembled Civatte bodies (Fig. 1b). Because the clinical course and histology differed from typical psoriasis or eczema, visceral malignancies were primarily investigated to rule out Bazex syndrome. Systemic evaluation that included Thoracic X-ray and computed tomography scans for any malignancies showed no remarkable results. Because of the high incidence of oropharyngeal cancers reported with Bazex syndrome, a laryngoscopy was subsequently performed, which revealed glottal SCC (Fig. 1c). After radiotherapy of 70 Gy in 35 fractions, the glottal SCC went into remission, and the skin symptoms spontaneously subsided. There has been no recurrence of either cancer or skin rash in the past 6 years.
Fig. 1.
(a) Hyperkeratotic erythematous plaque on the right sole and right palm. (b) Skin biopsy (hematoxylin and eosin staining) of hyperkeratotic erythema on the right sole revealing hyperkeratosis, acanthosis, and perivascular lymphocyte infiltration in the upper dermis. Individual cell keratinization (yellow arrow) and necrotic eosinophilic masses resemble Civatte bodies (black arrow) within the epidermis. (c) Laryngoscopy revealing glottal squamous cell carcinoma (white arrow).
2.2. Case 2
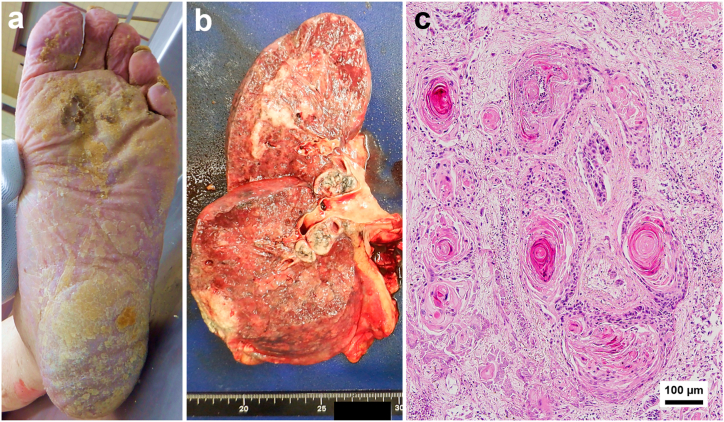
An 84-year-old man died in his home. He had no specific pre-existing condition until a month before his death, when he was suddenly discovered to have suspected terminal lung cancer. However, he had breathing difficulties; he did not undergo aggressive treatment and remained at home alone. He was found lying dead on his back. A judicial autopsy was performed approximately 26 hours after his death. The deceased was 162.5 cm tall, weighed 60.2 kg, and had no signs of cachexia. Bluish-purple livor mortis had mildly developed on his back. The soles showed diffuse thickening and scaling with violaceous erythema, and yellowish crusts with a honeycomb appearance (Fig. 2a). Internally, the cut surface of the right lung showed multiple white-toned nodules with bulky perihilar lymph nodes (Fig. 2b). A direct metastatic lesion was observed in the right diaphragm. Similar findings, though to a lesser degree, were observed in the left lung indicating intrathoracic metastasis. Pathologically, squamous cell metaplasia of alveolar epithelium exhibited a growth pattern in cell clusters or irregular nests with keratin pearls (Fig. 2c). Furthermore, microscopic arterial invasion was observed. Toxicological screening and ethanol levels measurements in the blood showed no significant findings. The cause of death was concluded to be a severe respiratory failure from advanced lung squamous cell carcinoma.
Fig. 2.
(a) Diffuse thickening and scaling with violaceous erythema, and yellowish crusts with a honeycomb appearance on the left sole. (b) The cut surface of the right lung shows multiple white-toned nodules with bulky perihilar lymph nodes. (c) Squamous cell carcinoma component showing keratin pearls (hematoxylin and eosin staining).
3. Discussion
Bazex syndrome is a well-described but rare paraneoplastic dermatosis. Large analytical reviews were performed by Bolognia et al. and Räßler et al. [4,6]. Following those reports, cases in which age, gender, and underlying malignancy were clearly described were selected. Additionally, the current literature about Bazex syndrome was tabulated by searching for indexed articles between January 2016 and January 2024 in MEDLINE via PubMed using the following keywords: “Bazex syndrome” OR “Acrokeratosis neoplastica” NOT "Bazex-Dupre-Christol syndrome". A total of 20 cases, including the present two cases, were newly identified. Table 1 provides an overview of the Bazex syndrome patient profile [4,[6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22]]. The mean age was 63 years with a higher frequency of males. The majority of associated malignancies were SCCs, accounting for 62.8% of cases, and were especially common in the oropharynx/larynx, esophagus, and lungs. SCCs of cervical lymph node metastasis of unknown primary origins were also observed in 6.6% of cases, suggesting the presence of occult SCCs of head and neck lesions. Particular attention should be paid to the possibility of SCCs in the head, and neck lesions. Associations with additional cancers are frequently observed in the aerodigestive tract. About 9.8% of cases were associated with adenocarcinoma or small-cell carcinoma in the lungs. Moreover, adenocarcinoma of the esophagus accounts for 4.4% of cases. Furthermore, hematological neoplasms, as well as solid cancers, should be considered, suggesting the need for a careful and thorough systemic evaluation. The cancers behind the Bazex syndrome may reflect regional characteristics. According to the analytical review of Japanese domestic journals, lung cancer is the most common cause of Bazex syndrome, followed by cancers of the esophagus and stomach, with adenocarcinoma (34%) exceeding SCC (27%) [23]. These differences may partially explain the higher incidence of gastric cancer because of H. pylori in Japan [24]. Typically, the skin manifestations are known to develop in advance of the diagnosis of internal malignancies. In 47%–67% of cases, skin manifestations predict cancer diagnosis [4,6]. The cutaneous lesions precede the initial diagnosis of the underlying malignancy by an average of 11 months [6]. Bazex syndrome can represent the earliest manifestation of insidious malignancies. The skin manifestations most commonly involved the hands (38%), nose (33%), feet (30%), fingers (29%), and ears (28%) [4]. Differential diagnoses primarily include psoriasis, eczemas, and tinea. Although the lesions often mimic psoriasiform, they less frequently occur on the face (49%), palms, and soles (12%–16%) with psoriasis [25]. Furthermore, Bazex syndrome is generally resistant to a variety of topical treatments, which may provide the opportunity to facilitate further investigation. The lack of histopathological features distinguishes Bazex syndrome from psoriasis or other inflammatory dermatoses. Although the histological features of Bazex syndrome are non-specific, including hyperkeratosis, parakeratosis, and acanthosis, several reports propose the existence of dyskeratotic keratinocytes [4,6]. Those necrotic keratinocytes often accompany vacuolization and subsequently form subepidermal clefts that clinically present as bullae [4]. When accompanied by severe interface dermatitis, the condition may resemble lichenoid diseases such as lichen planus, lupus erythematous, or lichenoid drug eruption [4]. Consistent with those reports, necrotic keratinocytes were conspicuous in our case (Fig. 1b). Although the pathogenesis of Bazex syndrome has not been fully elucidated, the involvement of cytokines and hormones released by the tumor and immunological cross-reactivity of tumor antigens to the epidermis have been proposed to be involved [2,5]. Overexpression of epidermal growth factor receptor (EGFR), also known as tumor growth factor-α (TGF-α), is known to induce hyperplasia of keratinocytes [26]. Consistent with that theory, expression levels of TGF-α were decreased in lesional keratinocytes after cancer removal [8]. Parathyroid hormone-related protein secreted by SCC can also induce epithelial differentiation and proliferation [5]. Anti-tumor antibodies may cross-react with the epidermis via an autoreactive T-cell-mediated immune response. In paraneoplastic pemphigus, a gene-centric approach identified the epidermal protein transglutaminase 1 as a common autoantigen [27]. Deposits of immunoglobulins and C3 have been reported in Bazex syndrome, suggesting an autoimmune response [28]. Alternative theories include dyskeratosis from a vitamin A or zinc deficiency associated with cancer [4]. In our cohort, significant sex differences in the incidence of Bazex syndrome are highlighted, with males predominating in 85.8% of cases. There are several possible reasons for this. The higher incidence of SCCs of the head and neck and aerodigestive tract in males has been attributed to the high consumption of tobacco and alcohol by men. The onset of Bazex syndrome correlates with puberty and adulthood, suggesting a possible role for sex hormones in promoting or exacerbating this disease. Testosterone has been suggested to be an inducer of tumor cell proliferation and migration in several non-reproductive cancers [29]. X chromosome inactivation and epigenetic mechanisms of disease susceptibility must be considered for their potential contribution to this apparent sex difference.
Table 1.
Sites of underlying malignancies in 183 patients with Bazex syndrome.
| Until 2015a | 2016–2024a, b | Total (%) | |
|---|---|---|---|
| Age (mean) | 62 | 63 | 63 |
| Male/Female | 144/19 | 13/7 | 157/26 |
| Underlying Malignancies | |||
| SCCs | 103 | 12 | 115 (62.8) |
| SCC of head & neck | 67 | 8 | 75 (41.0) |
| SCC of esophagus | 12 | 12 (6.6) | |
| SCC of cervical LN MUO | 11 | 1 | 12 (6.6) |
| SCC of lung | 8 | 2 | 10 (5.5) |
| SCC of skin | 3 | 1 | 4 (2.2) |
| SCC of Thymus | 2 | 2 (1.1) | |
| Other lung carcinoma | 17 | 1 | 18 (9.8) |
| Other LN MTS MUO | 10 | 10 (5.5) | |
| Other esophagus carcinoma | 8 | 8 (4.4) | |
| Hematological neoplasms | 4 | 3 | 7 (3.8) |
| Stomach | 4 | 4 (2.2) | |
| Colon | 3 | 3 (1.6) | |
| Liver | 2 | 1 | 3 (1.6) |
| Prostate | 3 | 3 (1.6) | |
| Breast | 3 | 3 (1.6) | |
| Pancreas | 2 | 2 (1.1) | |
| Bile duct | 1 | 1 (0.5) | |
| Bladder | 1 | 1 (0.5) | |
| Uterus | 1 | 1 (0.5) | |
| Ovary | 1 | 1 (0.5) | |
| Thyroid | 1 | 1 (0.5) | |
| Neuroendcrine tumor | 1 | 1 (0.5) | |
| Liposarcoma | 1 | 1 (0.5) | |
In forensics, skin examinations are of great importance for evaluating postmortem phenomena, trauma assessment, and personal identification. There has been strong advocacy for potentially integrating advanced dermatology into standard forensic practice [[30], [31], [32], [33]]. Dermatologists are specially trained in visual assessment, which provides not only an accurate diagnosis but also helps with evaluating the patient's background, including their physical condition, comorbidities, habits, and lifestyle. This dermatologic theory has an affinity with forensic cases that have no clinical history or life background.
4. Conclusion
Here, we demonstrated cases of Bazex syndrome that provide insight into the detection of SCC of the glottis and lung. Cancers of the head, neck, esophagus, and lung are most common, and these sites should be prioritized in the diagnostic workup. Bazex syndrome often presents as a chronic keratoderma, raising a challenge for the dermatologist to determine whether a further examination for an occult neoplasm is warranted or whether conservative management is appropriate. Because Bazex syndrome can often predict cancer up to one year in advance, multidisciplinary collaboration between dermatology, otolaryngology, pulmonology, and oncology for optimal screening is necessary, even in the absence of overt symptoms. Screening should not be delayed or refused because of patient reluctance. The skin can reflect the presence of systemic diseases, leading to an unexpected diagnosis of certain conditions. All clinicians should be familiarized with paraneoplastic dermatoses to perform an early diagnosis of the underlying neoplasm.
Funding
None.
Ethics approval
This study was conducted in accordance with the principles of the Declaration of Helsinki. Study approval was granted by the ethics committee of our university (registration number: 1159-01).
Consent to publish
Written informed consent was obtained from the patient in case 1 to use the individual photographs for academic publication. Written informed consent was obtained from the bereaved family in case 2 to use the individual data for academic publication.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
CRediT authorship contribution statement
Atsushi Yamada: Conceptualization, Data curation, Formal analysis, Investigation, Validation, Writing – original draft, Writing – review & editing. Naoka Umemoto: Data curation, Formal analysis, Investigation, Validation, Writing – review & editing. Toshio Demitsu: Data curation, Formal analysis, Investigation, Supervision, Validation, Writing – review & editing. Osamu Kitamura: Supervision, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank J. Iacona, Ph.D., from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
References
- 1.Poole S., Fenske N.A. Cutaneous markers of internal malignancy. II. Paraneoplastic dermatoses and environmental carcinogens. J. Am. Acad. Dermatol. 1993;28(2 Pt 1):147–164. doi: 10.1016/0190-9622(93)70022-l. [DOI] [PubMed] [Google Scholar]
- 2.Silva J.A., Mesquita Kde C., Igreja A.C., Lucas I.C., Freitas A.F., Oliveira S.M., et al. Paraneoplastic cutaneous manifestations: concepts and updates. An. Bras. Dermatol. 2013;88(1):9–22. doi: 10.1590/s0365-05962013000100001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bazex A., Griffiths A. Acrokeratosis paraneoplastica a new cutaneous marker of malignancy. Br. J. Dermatol. 1980;103(3):301–306. doi: 10.1111/j.1365-2133.1980.tb07248.x. [DOI] [PubMed] [Google Scholar]
- 4.Räßler F., Goetze S., Elsner P. Acrokeratosis paraneoplastica (Bazex syndrome) - a systematic review on risk factors, diagnosis, prognosis and management. J. Eur. Acad. Dermatol. Venereol. 2017;31(7):1119–1136. doi: 10.1111/jdv.14199. [DOI] [PubMed] [Google Scholar]
- 5.Vlachos C., Tziortzioti C., Bassukas I.D. Paraneoplastic syndromes in patients with keratinocyte skin cancer. Cancers. 2022;14(1):249. doi: 10.3390/cancers14010249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bolognia J.L., Brewer Y.P., Cooper D.L. Bazex syndrome (acrokeratosis paraneoplastica). An analytic review. Medicine (Baltim.) 1991;70(4):269–280. doi: 10.1097/00005792-199107000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Matsui H., Iwae S., Hirayama Y., Yonezawa K., Shigeji J. Bazex syndrome with hypoalbuminemia and severe ascites. Case Rep. Oncol. 2016;9(2):405–408. doi: 10.1159/000448168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amano M., Hanafusa T., Chikazawa S., Ueno M., Namiki T., Igawa K., et al. Bazex syndrome in lung squamous cell carcinoma: high expression of epidermal growth factor receptor in lesional keratinocytes with Th2 immune shift. Case Rep. Dermatol. 2016;8(3):358–362. doi: 10.1159/000452827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Squires B., Daveluy S.D., Joiner M.C., Hurst N., Bishop M., Miller S.R. Acrokeratosis paraneoplastica associated with cervical squamous cell carcinoma. Case Rep Dermatol Med. 2016;2016 doi: 10.1155/2016/7137691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dabas G., De D., Handa S., Chatterjee D., Radotra B.D. Acrokeratosis paraneoplastica (Bazex syndrome) QJM. 2018;111(1):63–64. doi: 10.1093/qjmed/hcx187. [DOI] [PubMed] [Google Scholar]
- 11.Iwanami K., Nakai M., Kitamura K. Bazex syndrome. Intern Med. 2018;57(10):1501–1502. doi: 10.2169/internalmedicine.9771-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McClatchey T.M., Haynes D., Korcheva V.B., Keller J. Acrokeratosis paraneoplastica (Bazex syndrome) associated with peripheral T-cell lymphoma. JAAD Case Rep. 2018;5(1):86–88. doi: 10.1016/j.jdcr.2018.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nguyen S., Grosber M., Gutermuth J. Acrokeratosis can Be a warning sign of an underlying malignancy. Eur J Case Rep Intern Med. 2019;6(5) doi: 10.12890/2019_001108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mititelu R., Powell M. A case report of resolution of acrokeratosis paraneoplastica (Bazex syndrome) post resection of non-small-cell lung carcinoma. SAGE Open Med Case Rep. 2019;7 doi: 10.1177/2050313X19881595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Narasimha S., Shah J., Drame A. From the outside looking in: psoriasiform dermatitis presenting as a paraneoplastic syndrome for pancreatic adenocarcinoma. Cureus. 2020;12(7) doi: 10.7759/cureus.9067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eckstein J., Healy E., Jain A., Hawkins D., Ho Q.A., Agrawal A., et al. A series of typical and atypical cases of Bazex syndrome: identifying the red herring to avoid delaying cancer treatment. Clin Case Rep. 2020;8(11):2259–2264. doi: 10.1002/ccr3.3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Macca L., Manuella L., Taibi R., Guarneri C. Acrokeratosis of Bazex as a sign of thyroid cancer: first description and review of thyroid-associated paraneoplastic dermatoses. Eur. Rev. Med. Pharmacol. Sci. 2022;26(12):4367–4370. doi: 10.26355/eurrev_202206_29075. [DOI] [PubMed] [Google Scholar]
- 18.Gaurav V., Grover C. Bazex syndrome associated with squamous cell carcinoma of the lip: a rare paraneoplastic acrokeratosis with nail dystrophy. Skin Appendage Disord. 2022;8(4):317–321. doi: 10.1159/000521269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holzgruber J., Oberneder-Popper J., Guenova E., Hötzenecker W. Acrokeratosis paraneoplastica (Bazex syndrome): a case report. Case Rep. Dermatol. 2022;14(3):307–312. doi: 10.1159/000525381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oka S., Ono K. Bazex Syndrome in acute myeloid leukemia. Intern Med. 2023;62(21):3259–3260. doi: 10.2169/internalmedicine.1654-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alband N., Danila I., Oliver C., Bardsley V., Banfield C., Van-De-Velde V. Acrokeratosis paraneoplastica in tonsillar carcinoma. Eur. J. Dermatol. 2023;33(1):62–64. doi: 10.1684/ejd.2023.4437. [DOI] [PubMed] [Google Scholar]
- 22.Couselo-Rodríguez C., Galego-Fernández R., Núñez-Fernández M.J. Complete resolution of Bazex syndrome after surgical treatment of squamous cell carcinoma of the larynx. Actas Dermosifiliogr. 2024;115(1):86. doi: 10.1016/j.ad.2022.08.035. [DOI] [PubMed] [Google Scholar]
- 23.Sugiura M., Iwata Y., Tanaka Y., Sugiura K. A case of Bazex syndrome accompanied by intrahepatic cholangiocarcinoma. Rinsho Hifuka. 2023;77(10):795–801. doi: 10.11477/mf.1412207098. [DOI] [Google Scholar]
- 24.World Health Organization cancer country profiles. 2020. https://www.who.int/teams/noncommunicable-diseases/surveillance/data/cancer-profiles
- 25.Dopytalska K., Sobolewski P., Błaszczak A., Szymańska E., Walecka I. Psoriasis in special localizations. Reumatologia. 2018;56(6):392–398. doi: 10.5114/reum.2018.80718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnston A., Gudjonsson J.E., Aphale A., Guzman A.M., Stoll S.W., Elder J.T. EGFR and IL-1 signaling synergistically promote keratinocyte antimicrobial defenses in a differentiation-dependent manner. J. Invest. Dermatol. 2011;131(2):329–337. doi: 10.1038/jid.2010.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Landegren N., Ishii N., Aranda-Guillén M., Gunnarsson H.I., Sardh F., Hallgren Å., Ståhle M., Hagforsen E., Bradley M., Edqvist P.D., Pontén F., Mäkitie O., Eidsmo L., Norlén L., Achour A., Dahlbom I., Korponay-Szabó I., Agardh D., Alimohammadi M., Eriksson D., Hashimoto T., Kämpe O. A gene-centric approach to biomarker discovery identifies transglutaminase 1 as an epidermal autoantigen. Proc Natl Acad Sci U S A. 2021;118(51) doi: 10.1073/pnas.2100687118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mutasim D.F., Meiri G. Bazex syndrome mimicking a primary autoimmune bullous disorder. J. Am. Acad. Dermatol. 1999 doi: 10.1053/jd.1999.v40.a95652. 822–5. [DOI] [PubMed] [Google Scholar]
- 29.Costa A.R., Lança de Oliveira M., Cruz I., Gonçalves I., Cascalheira J.F., Santos C.R.A. The sex bias of cancer. Trends Endocrinol Metab. 2020;31(10):785–799. doi: 10.1016/j.tem.2020.07.002. [DOI] [PubMed] [Google Scholar]
- 30.Cocks M., Sander I., Crain B. Frequency of dermatologic findings at autopsy. J. Forensic Sci. 2018;63(6):1867–1869. doi: 10.1111/1556-4029.13772. [DOI] [PubMed] [Google Scholar]
- 31.Hammer U., Boy D., Rothaupt D., Büttner A. Distinction between forensic evidence and dermatological findings. J Forensic Leg Med. 2015;33:1–4. doi: 10.1016/j.jflm.2015.02.018. [DOI] [PubMed] [Google Scholar]
- 32.Yamada A., Demitsu T., Umemoto N., Kitamura O. Video image of genital melanosis provides strong evidence to support identification of a sexual offender. Forensic Sci. Med. Pathol. 2021;17(3):510–512. doi: 10.1007/s12024-021-00364-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deps P.D., Aborghetti H.P., Zambon T.L., Costa V.C., Dos Santos J.D., Collin S.M., Charlier P. Assessing signs of torture: a review of clinical forensic dermatology. J. Am. Acad. Dermatol. 2022;87(2):375–380. doi: 10.1016/j.jaad.2020.09.031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.