Abstract
Alzheimer's disease is a neurological disorder that causes increased memory loss, mood swings, behavioral disorders, and disruptions in daily activities. Polymer scaffolds for the brain have been grown under laboratory, physiological, and pathological circumstances because of the limitations of conventional treatments for patients with central nervous system diseases. The blood-brain barrier prevents medications from entering the brain, challenging AD treatment. Numerous biomaterials such as biomolecules, polymers, inorganic metals, and metal oxide nanoparticles have been used to transport therapeutic medicines into the nervous system. Incorporating biocompatible materials that support neurogenesis through a combination of topographical, pharmacological, and mechanical stimuli has also shown promise for the transfer of cells to replenish dopaminergic neurons. Components made of naturally occurring biodegradable polymers are appropriate for the regeneration of nerve tissue. The ability of natural-based materials (biomaterials) has been shown to promote endogenous cell development after implantation. Also, strategic functionalization of polymeric nanocarriers could be employed for treating AD. In particular, nanoparticles could resolve Aβ aggregation and thus help cure Alzheimer's disease. Drug moieties can be effectively directed to the brain by utilizing nano-based systems and diverse colloidal carriers, including hydrogels and biodegradable scaffolds. Notably, early investigations employing neural stem cells have yielded promising results, further emphasizing the potential advancements in this field. Few studies have fully leveraged the combination of cells with cutting-edge biomaterials. This study provides a comprehensive overview of prior research, highlighting the pivotal role of biomaterials as sophisticated drug carriers. It delves into various intelligent drug delivery systems, encompassing pH and thermo-triggered mechanisms, polymeric and lipid carriers, inorganic nanoparticles, and other vectors. The discussion synthesizes existing knowledge and underscores the transformative impact of these biomaterials in devising innovative strategies, augmenting current therapeutic methodologies, and shaping new paradigms in the realm of Alzheimer's disease treatment.
Keywords: Natural biomaterials, Alzheimer's disease, Scaffold, Liposome, Hydrogel
1. Introduction
The most complex systems of the human body are the central nervous system (CNS) and the peripheral nervous system [1]. The CNS is affected by neurological diseases, traumatic injuries, and strokes [1]. Approximately 6.8 million people are affected by neurological diseases every year, which is expected to increase to 130 million by 2050 [2]. Despite the progress in studying environmental conditions, brain disorders are not well treatable, and their treatment is challenging [1,3]. Unfortunately, the existing diagnostic and treatment methods for neurodegenerative diseases (NDs), including Alzheimer's disease (AD), amyotrophic lateral sclerosis, Huntington's disease, and Parkinson's disease, are incomplete, and no treatment methods have been found for nerve regeneration [4,5]. Nerve allografts and autografts, which can be a golden solution for treatment, have limitations associated with immune and infectious diseases, lack of donor nerves, and donor site morbidity, among others [6].
Medications used for treatment of NDs, including AD and Parkinson's disease, slow disease progression. Cell therapy may be a valuable strategy for the treatment of NDs; neural tissue can be repaired or healthy glial cells can be protected from further damage by replacing differentiated cells with stem cells [7]. However, this method is constrained by its time- and labor-intensive nature. Therefore, there is a compelling need for the development of highly efficient and cost-effective methods to facilitate nerve repair and regeneration [5,8]. A comparison of clinical and autopsy findings shows that the clinical diagnosis of neurodegenerative diseases may not be performed correctly; therefore, prognostic biomarkers are needed to detect and evaluate damage in the early stages of the patient's blood [9,10]. In recent years, investigations on blood biomarkers that assess the value of serum or plasma neurofilament light (NfL), which is detected in the cerebrospinal fluid and blood, as a primary biomarker of neurodegeneration for Alzheimer's illness have increased significantly. Nevertheless, there hasn't been a lot of efforts to generate existing findings to evaluate the utility blood NfL's potential as biomarker of neurodegeneration for an Alzheimer's illness and there are still limitations to NfL research [10,11]. Because of the limitations of traditional treatments for CNS disorders in clinical practice, researchers have used polymer scaffolds for neural growth under laboratory, physiological, and pathological conditions [12]. Numerous polymers have been investigated as support matrices in nerve tissue engineering using regenerative medical polymers [13,14].
The disorder of Alzheimer's is hallmarked by advancing athophysiological neurodegeneration. The amyloid-beta (Aβ) peptides are amino acid peptides that are present in the amyloid plaques that are discovered in the brains of individuals who have Alzheimer's disease (AD). There is no cure for AD with current medications, and the effects associated with AD are decreased. The combination of polymer-based biomaterials, molecular detection, and efficient medication targeting provides a high sensitivity for resolving Aβ aggregation, which in turn aids in the treatment of Alzheimer's disease [15].
Generally, natural biomaterials are derived from biological sources and are similar to tissues. They exhibit enhanced biocompatibility and potential to elicit adverse reactions within the human body [1]. Furthermore, natural biomaterials that emulate the extracellular matrix milieu can facilitate the proliferation and viability of nascent brain cells, thus retarding the progression of AD [3]. Moreover, natural biomaterials possess inherent protective properties and represent a noninvasive modality that can attenuate the progression of AD. Notably, investigational studies have provided evidence that cationic substances incorporating chitosan can ameliorate oxidative stress associated with AD [4]. Additionally, it has been documented that cationic biopolymer nanoparticles (NPs), which are functionalized with lutein, can mitigate oxidative stress in a noninvasive manner [5]. The utilization of both synthetic and natural biomaterials has been observed in various tissue engineering studies in areas such as neurite growth, bridging of neural gaps, and the differentiation of human neural stem cells. More specifically, the incorporation of natural biomaterials, including polymers, polysaccharides, and proteins, has been found to affect nerve growth and regeneration. For instance, the combination of hyaluronic acid with other natural polymers has proven advantageous for promoting and regenerating tissues. Additionally, neural tissue regeneration and neural cell regrowth have been achieved using neural stem cells, growth factors, neural progenitor cells, and various scaffolds. However, it is important to note that the selection of a biomaterial for specific purposes is contingent on the properties of the scaffold, and no single biomaterial can adequately facilitate nerve regeneration [[6], [7], [8], [9]]. In this review, we examine recent advances in the use of natural biomaterials for Alzheimer's treatment.
2. Alzheimer's disease
AD, a neurodegenerative disease, is the most common form of dementia. It is characterized by gradual progressive brain degeneration caused by the development of plaques outside the cell in the hippocampus, the presence of amyloid-beta peptide (Aβ), and a significant loss of neurons in the inner temporal lobe and neocortical regions [16,17]. Brain disorders such as AD and factors including infections, poisoning, abnormalities in the pulmonary system, blood circulation, disruption of the brain's oxygen flow, and deficiency of vitamin B12 and nutrients ultimately lead to the loss of cognitive function [18,19].
According to previous studies, vitamin B12 deficiency is associated with neurodegenerative disorders and increased risk of AD [20,21]. Increased homocysteine levels are a specific indicator of vitamin B12 deficiency, and can cause brain damage through oxidative stress, increased calcium influx, and apoptosis [22,23]. Serum vitamin B12 levels, as well as complete blood count and serum homocysteine level tests, can be used to diagnose vitamin B12 deficiency [24,25]. AD is likely when neuropsychological tests show dementia, memory loss, difficulty performing daily tasks, and other symptoms such as aphasia, apraxia, and agnosia (loss of perception) [26]. In the absence of any systemic or brain disease, these symptoms can appear at any time between the ages of 40 and 90 years [27].
AD is a complex neurodegenerative disease with a multifactorial etiology and thus, the exact cause of the disorder remains elusive. Several mechanisms are implicated in the pathogenesis of AD and there is a crosstalk between these mechanisms that contribute to the progressive cognitive decline in AD patients. The main mechanisms include Aβ accumulation, hyperphosphorylation of tau protein resulting in its aggregation and the formation of intracellular neurofibrillary tangles, oxidative stress due to increased ROS and decreased antioxidant capacity of neuronal cells, mitochondrial dysfunction inflammation, vascular and genetic factors, and synaptic loss [28,29]. Mitochondrial dysfunction and oxidative stress due to mitochondrial ROS accumulation are involved in the pathogenesis of AD [30]. Extracellular and intracellular Aβ has been shown to induce mitochondrial dysfunction in neuronal cells [31]. Furthermore, AD enhances Aβ accumulation in peripherical organs and tissues inducing their dysfunction. We have shown that Aβ affect metabolomic profiles [32] and impairs mitochondrial function associated with significant increases in mitochondrial and decreased membrane potential and calcium retention capacity [33] in cardiomyocytes and coronary endothelial cells. Diversities in etiology, and mechanisms involved in the pathogenesis of AD challenge the effectiveness of different therapeutic approaches for treatment of AD patients [34,35] (see Fig. 1).
Fig. 1.
Common risk factors of AD.
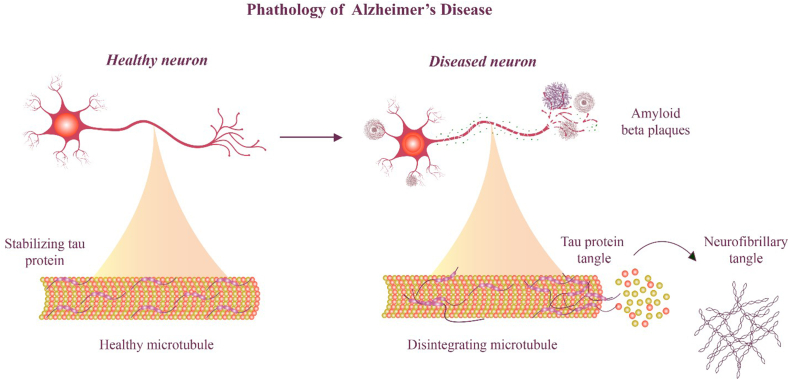
The National Institute of Aging and Alzheimer's Association proposed two categories of biomarkers as criteria for AD in 2011, in addition to clinical biomarkers: a) amyloid markers of the brain, such as the cerebrospinal fluid and positron emission tomography (PET), and b) neurological markers, such as magnetic resonance imaging, to measure atrophy and fluorodeoxyglucose (FDG) and cerebrospinal fluid tau for metabolic activity [[36], [37], [38]]. The pathology of AD presents two types of neuropathological changes in the progression of AD and its symptoms; the first change is related to positive lesions (due to accumulation) with amyloid plaques, accumulation of neurofibrillary tangles, dystrophies, neuritis, and other deposits. It was found in the brains of patients with AD (Fig. 2).
Fig. 2.
Comparison of healthy neuron and neuron of Alzheimer's patient.
The second change is linked to two negative lesions that are characterized by significant atrophy caused by neuronal, neuropil, and synaptic losses. Oxidative stress, neuroinflammation, and damage to cholinergic neurons are other variables that may result in neurodegeneration [[39], [40], [41]].
3. Treatment for AD
Currently, there are no established approaches for treating neurodegenerative illnesses. The lack of the effectiveness of existing therapeutic approaches is attributed not only to a lack of medication efficacy but also to the existence of different barriers in the drug delivery. In the last ten years, few clinical trials have been conducted on AD patients. Physical activity has been demonstrated to improve cognitive function in older persons by preventing AD, promoting brain health, and stimulating neurogenesis, while reducing inflammation by inhibiting Aβ synthesis [16]. Another study demonstrated that a multidomain approach involving lifestyle (cognitive training, exercise, and diet), controlling cardiovascular risk factors, and the reduction of AD symptoms can improve or preserve cognitive performance and prevent the development of new cases of AD in elderly people [42].
To date, only two classes of inhibitors, including N-methyl-d-aspartate antagonists (NMDA) and acetylcholinesterase inhibitors (AChEIs) (naturally occurring, synthetic, and hybrid versions) have been permitted as a AD remedy. N-methyl-d-aspartate receptor (NMDAR) antagonists reduce NMDAR glutamate receptor overactivation, thereby reducing Ca2+ influx and restoring normal activity. Excessive NMDAR activation causes an increase in the Ca2+ influx, which promotes cell death and synaptic dysfunction. Regardless of their beneficial effects, these drugs only cure the AD symptoms [16,43]. Inhibitors of AChE, including donepezil (Aricept), tacrine (Cognex), galantamine (Razadyne and Reminyl) and rivastigmine (Exelon) have been investigated and accepted by Food and Drug Administration (FDA) [44]. AChEIs stop butyrylcholinesterase and cholinesterase enzymes from degrading ACH, resulting in an increase in ACH levels in the synaptic cleft [16,45,46]. The principal location of the effect of these medications once passing physical and metabolic barriers is the brain; hence, present oral formulations of these medications are prescribed in greater dosages to ensure that a considerable quantity reaches the brain [47]. Nevertheless, acetylcholinesterase inhibitors are generally ineffective as a treatment method owing to their poor dissolution, low bioavailability, and inability to cross the blood-brain barrier (BBB).
The BBB plays a critical role in the treatment of certain conditions and in the pathogenesis of AD and is one of the most important barriers between the blood and the brain [48,49]. Only a small percentage of medications can penetrate the BBB while maintaining their actions in the CNS [50]. Therefore, medication accessibility in the CNS is restricted by the BBB, which limits treatment chemical availability to the CNS [51]. Thus, efficient BBB strategies for the transfer of curative agents to the brain, develop effective delivery methods for medications that traverse the BBB, and comprehension of BBB transportation, are required [52].
A biomaterial, according to Williams 2009, is “a material aimed for communicating with systems of biology to investigate, cure, augment, or substitute any tissue, organ, or functioning of the body [53]. Applications using biomaterials as a mediated medicine delivery system for AD are one of the newest methods for improving CNS penetration for both diagnostic and therapeutic purposes. Their being able to open the BBB's tight junctions, penetrate the BBB system, and also absorb via endothelial cell tight junctions makes effective absorption by BBB cell types and targeted drug accumulation possible. Moreover, biomaterials are attractive options because they can shield pharmaceuticals from enzymatic hydrolysis by efficiently hiding the membrane barrier, prolonging drug release, and limiting the characterization of drug molecules [54].
4. Nanoparticle-based approaches for brain drug delivery
4.1. Special challenges and conditions for brain-targeting medications transfer
4.1.1. Blood circulation
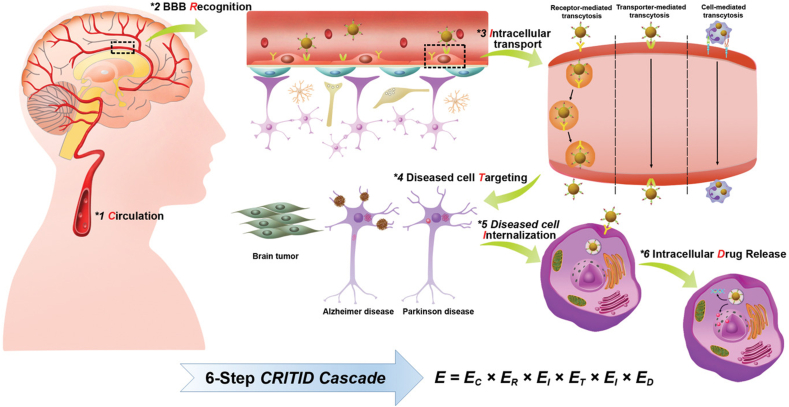
Owing to inadequate accumulation of medications in brain illness cells and off-target side consequences, appreciation of the relationships among NPs and biological mechanisms is required for managing their biodistribution. NPs for targeting of brain have the potential to enhance the therapeutic effectiveness of medicines used for treating brain disorders. In this section, we offer an extensive outline of the drug delivery mechanism of NPs and demonstrate the six-step critical events in the CRITID (systemic circulation, BBB recognition by receptors, transporters, adsorptive mechanisms, etc, BBB intracellular transport, diseased cell targeting after entering the brain parenchyma, internalization by diseased cells, intracellular drug release) delivery cascade, which contain: circulation in systemic blood, recognizing receptors on the BBB, intracellular transport, and diseased cell targeting after parenchyma entry (Fig. 3). The CRITID delivery cascade can create more effective and targeted brain-targeting medication-delivery systems [55].
Fig. 3.
Diagrammatic representation of the suggested mechanism for delivering brain-targeting NPs via the CRITID delivery cascade [55].
Subcutaneously administered brain-targeting NPs pass through the blood circulation before their destination in the brain. Hence, it is crucial to plan a delivery system for brain-targeted NPs that can deliver medications to sick cells more precisely and successfully. The capacity of nano-drugs to gather at the target place is closely connected to their time of circulation in blood [56]. After NPs enter the systemic circulation, compounds, and proteins quickly compete for sorption on the outer layer of the NP, resulting in the creation of a protein corona [57]. After intravenous administration, a considerable fraction of the NPs is quickly caught through the blood circulation by the mononuclear phagocytic system (MPS) via opsonization-mediated phagocytosis [58]. An extensively approved strategy for enhancing the duration of circulation of NPs is to modify their physical and chemical features [e.g., size, shape, and surface charge], which are crucial for influencing opsonization-directed MPS phagocytosis and elimination [59]. For example, modifying the surface, such as cell-membrane coating [60], biomimetic self-marker CD47-functionalization [61] and PEGylation [62] has come to be an appropriate approach for decreasing uptake and as a result extending the time that NPs spend in blood circulation. The utilization of endogenous transporters or receptors throughout the BBB endothelium for designing NPs can be used to detect certain transporters and/or receptors present on BECs, providing possibilities to act as possible targets [63]. Thus, it is important to develop a brain-targeting NPs delivery system that can more precisely and efficiently deliver medications to diseased cells to maximize the beneficial effect of NPs and minimize the toxicity [64]. For instance, a delivery method based on NPs has been developed and tested. Chunhong Gao et al. have produced curcumin-loaded red blood cell (RBC) membrane-coated PLGA NPs functionalized with DSPEPEG3400-T807 [65]. The lipid insertion method was used to conjugate T807-and RBCm-coated NPs. Findings revealed that, by reducing neuronal cell death, loaded NPs may be able to suppress the pathogenic process of AD [66]. Consequently, creating delivery methods for brain-targeting should not merely consider the conditions of brain-targeted transport and BBB transcytosis, but the delivery in the brain parenchyma, brain endothelial cells, circulation, and disease cells [64].
4.2. Recognizing receptors on BBB
One of the most serious issues in the treatment of neurological conditions is the release of medication. The tight junction proteins and elevated selectivity of the BBB serve as physical barriers that restrict the movement of substances among the blood and neural tissues. Therefore, understanding BBB transport processes is crucial for creating new drug carriers that can cross the BBB. In comparison to other areas of circulation, firm BBB connections among endothelial cells protecting cerebral capillaries are more widespread, and endothelial cell-restricted pinocytosis [51]. These processes lead to undermining of the BBB, which then leads to neuronal and synaptic dysfunctions. In AD, cerebrovascular malfunction culminates in mental deterioration which may result in cerebral amyloid angiopathy. Additionally, it leads to the buildup of brain Aβ peptides. Thus, the BBB plays a fundamental role in the etiology of AD [48].
The BBB is crucial for the control of Aβ transit to the brain with two major receptors, the minimal concentration of lipoprotein receptor-related protein 1 (LRP1) and lipoprotein receptor-related protein 2 (LRP 2) receptor for developed ending results of glycation (RAGE) [67]. The inappropriate clearing of Ab through irregular RAGE/LRP1 receptors, vascular malfunction, and defective angiogenesis may lead to Ab buildup, brain hypoperfusion, neurovascular uncoupling, cerebrovascular regression, and neurovascular infection. Ultimately, these processes undermine the BBB, which leads to neuronal and synaptic dysfunction [68]. Thus, owing to the BBB, efficient strategies for the transfer of curative agents to the brain are required. For example, a combination of nanocarriers (polymeric, lipid, micelles liposomes) that are covered with thiamine and have high affinity for the BBB, directing the components to the BBB thiamine transporter [69]. Therefore, carriers can induce active medication and transport of drugs via the BBB.
5. Biomaterials
There have been substantial advancements in the design and functionalization of implantable biomaterials, and every year, more of these materials are released into the market [70,71]. To create cutting-edge therapeutic medical goods, biomaterials are frequently combined with cells, synthetic materials, and therapeutic chemicals in tissue engineering solutions [72,73]. Cells and physiologically active components are frequently delivered using a three-dimensional polymeric scaffold as the support framework. Owing to their beneficial qualities, these materials are increasingly used as starting materials in tissue engineering. They are well tolerated and promote cellular adhesion and subsequent tissue creation to improve body integration, whereas their biodegradability enables tissue remodeling. To improve cell infiltration, nutrient transport, and metabolite removal, the design of an exogenous construct must match the interconnected network of the original tissue. These natural polymers can be modified to withstand compressive stresses, match tissue properties, and mechanically excite cells [74].
Innate biological activity, cell recognition induced by natural polymers, and subsequent signaling cascades are not typically considered throughout the scaffold design process, and the properties and functions of the finished scaffold are poorly described [75]. Complex macromolecules, including proteoglycans, polysaccharides, proteins, lipids, and nucleic acids are examples of natural polymers. Biological activities can be produced using mammalian/non-mammalian based natural polymers including cellulose [76].
The most common naturally occurring material not derived from mammals, cellulose is an essential part of plant cell walls and is produced in biofilm form by some bacterial species [77]. It is made up of linear β-d-glucose linked by β (1 → 4) bond; the cellobiose repeating unit of biocellulose (BC) is made up of two glucose molecules. Modified biocellulose tubes for peripheral nerve grafts were shown to be biocompatible with a minimum immunogenic reaction and promising axon guidance and neutrophic factor delivery in rats. Biocellulose has been described several times for peripheral nerve regeneration [78].
In the biomedical products, the most commonly utilized cellulose ethers are carboxymethyl cellulose, methyl cellulose, and hydroxyethyl cellulose [79]. being the first FDA-approved cellulose-based substance and having a number of appropriate characteristics mark carboxymethyl cellulose an excellent cellulose-based compound. Its commercial application is facilitated by its easy preparation using inexpensive materials and straightforward synthesis methods [80]. Carboxymethyl cellulose is biodegradabile, chemically stabile, nontoxic, and water soluble [81].
Collagen, the most common mammalian polymer, accounts for approximately 30% of the body's total protein content. Moreover, they constitute the majority of the extracellular matrix in both soft and hard mammalian tissues [82,83]. As key structural protein in soft and hard tissues, collagen has a wide range of sources (skin, nerve, bone, blood vessel, cartilage), and it is also important for keeping the structural and biological integrity of extracellular matrix [84]. Collagen also has low immunogenicity, a biodegradable, biocompatible, permeable and porous structure and it regulates the differentiation, migration, adhesion, and morphology of cells [85,86].
Protein biomaterials includes silk fibroin, collagen, gelatin, keratin, fibronectin, etc.). Polysaccharide biomaterials are often the two groups under which natural biomaterials fall (e.g., cellulose, hyaluronic acid, alginate, chondroitin, glucose, chitin, and its derivative chitosan). While dextran and its derivatives, as well as bioactive molecules that mimic the extracellular environment, are primarily derived from microbial sources (such as algae, Agar, and alginate), protein-based biomaterials are typically obtained from animal and human sources [[87], [88], [89]].
Different types of biomaterials can be used as smart carriers for drugs consisting of magnetic, electro-, photo-, thermo-, pH triggered systems; lipid carriers (liposomes, nanoemulsions, SLN and NLC), inorganic NPs (gold NPs, quantum dots, etc.), and additional medicine vectors (hydrogels, biodegradable scaffolds, and carbon nanotubes) for the AD treatment. Of these NPs, gold, magnetic iron oxide, and quantum dots are widely utilized for imaging or diagnostic applications, dendrimers, nanoclusters, and polymerosomes are also employed in medicines delivery systems [90]. Additionally, new kinds of nanomaterials called cationic and anionic nanobubbles are now being exploited for both applications [91].
Physiological temperature, pH, ROS, enzymes, ionic concentration, and redox reactions are examples of endogenous stimulus-responsive materials being studied in AD therapy mainly for therapeutic purposes, that is, as a novel carrier system to carry pharmaceutical cargo to the affected area of the brain. Conversely, endogenous biomaterials have been studied for their use as taggers or marker particles in diagnostic applications [92]. Therefore, by enhancing the functional characteristics of the BBB, the application of nanomaterials offers new prospects for AD treatment. Nanomaterials appear to be useful building blocks for scaffolds that offer mechanical, biological, and structural support for permeation through the BBB. Overall, it has been determined that key elements in biomaterial engineering are the characteristics of scaffolds, such as their porosity, 3D design, degradability, and biocompatibility [93].
6. Using nanoparticles for drug delivery across the BBB in AD treatment
The intricate structure of the BBB makes it possible to thoroughly filter substances to protect the brain and the central nervous system. Although filtration is required, it is difficult to distribute medications to cure various CNS illnesses. Absence of fenestration in their endothelial cells is one unique characteristic that sets BBB capillaries apart from capillaries found in other regions of the body [94]. The endothelial cells are joined by adherens junctions (AJs) and tight junctions (TJs) and display minimum transcellular activity, due to absence of fenestrations and the existence of efficient efflux pumps in higher densities. Moreover, paracellular transport is further constrained by the significantly greater electrical resistance of the brain endothelium than that of other endothelial cells [95]. Only lipophilic, and extremely small molecules (less than 400 Da) may permeate the cell membrane's lipid bilayer [95].
NPs have the ability to encapsulate AD remedies including memantine, galantamine, rivastigmine donepezil and conjugate antibodies on their surface, thereby achieving significant surface functionalization to increase bioavailability, targetability, drug-loading capacity and penetration to BBB [96]. Quantitative real-time checking of various NPs will also be useful in selecting the right NPs to cross the BBB and in optimizing the beneficial regimen for patients with AD.
So far, various methods that can be employed to mediate the transcytosis of biomaterials throughout the BBB have been developed to aid in the passage of compounds or proteins into the brain. Receptor-mediated transcytosis (RMT), adsorptive-mediated transcytosis (AMT), carrier-mediated transcytosis and passive diffusion were observed (Fig. 2) [97].
6.1. Passive diffusion
Passive diffusion is a transportation method that can only be utilized for tiny lipid-soluble compounds. These compounds are freely dispersed throughout the BBB via lipid-mediated diffusion [94]. For instance, curcumin, resveratrol, and other lipophilic anti-Alzheimer's compounds pass the blood-brain barrier by transcellular diffusion, whilst hydrophilic compounds such epigallocatechin-3-gallate (EGCG) penetrate with tight junctions utilizing paracellular routes [98].
6.2. Carrier-mediated transport
In carrier-mediated transfer, materials penetrate the endothelial cells through the appropriate transmembrane proteins on the cell membrane. It is the most common transport method which contains multiple transporter systems that actively and discriminatively permit the flow of needed compounds, such as endogenous compounds, and nourishment like peptides, amino acids, and glucose, which are essential for brain activity and metabolic rate [99]. In addition to being able to filter out the majority of medication compounds, this barrier can exclude most medication molecules [100]. One example is the active transfer of glucose across the BBB via glucose transporter type 1 (GLUT1). A basic glucose substitute, p-aminophenyl-d-mannopyranoside, was attached to the surface of liposomes to investigate the possibility of crossing the BBB [101]. Cell absorption was shown to be enhanced in C6 glioma cells and cells overexpressing GLUT1 and GLUT3 [98]. Another example is BBB-mediated transport of phenylalanine by the large neutral amino acid transporter type 1 (LAT1). LAT1 has also been used as a medicinal delivery method. l-DOPA can successfully penetrate the BBB through LAT1 [102].
6.3. Adsorptive-mediated transcytosis
Adsorptive-mediated transcytosis (AMT) is transmitted via clathrin-dependent endocytosis, and it only goes unidimensional from the blood to the brain [97]. In adsorptive-mediated transcytosis, biomaterials cling to the cell membrane and the first affinity between biomaterials and the cell membrane affect the cellular uptake levels as well as the ability to facilitate biomaterial transport throughout the BBB while exhibiting poor selectivity. It depends on the electrostatic association between the positively charged biomaterials and negatively charged BBB endothelium. Several medication delivery systems have been developed to deliver medications across the BBB by conjugating and coating them with biomaterials. For instance, poly (ethylenimine) (PEI) and poly (propylene imine) (PPI) are cationic polymers that can compact nucleic acids by ionic contact and effectively carry genes by utilizing proton buffering capacity [103]. Chitosan can also be utilized as a potential drug-loading matrix to enhance electrostatic contact with the cell surface [104]. After combining with chitosan, nanocarriers exhibit increased penetration and sustained aggregation of neuronal cells [105].
6.4. Receptor-mediated transcytosis
The other most prevalent transport mechanism is the receptor-mediated transport (RMT). In comparison with AMT, the interaction attachment in RMT has a particular target and a higher affinity for attachment between the ligands and receptors. In RMT, peptide receptors on the cell membrane, in preference for transmembrane transporters, mediate transcytosis of ligands. The external layer of the transport system in the RMT does not need to be positively charged and may be negatively or neutrally charged. In RMT, after the attachment of a ligand to its receptor, receptor-mediated endocytosis causes transport of ligand-conjugated drug vehicles outside or inside the cells. Without transferring into the brain parenchyma, this mechanism facilitates blood-to-brain transport and conversely, blood-to-brain capillary endothelium transit [94]. Several receptors are implicated in RMT, depending on the ligand type and particular application region, including albumin-binding, insulin receptor, lactoferrin (Lf) receptor and transferrin (Tf) receptor protein. For example, transferrin-related ligands can be joined to polylactic acid (PLA)-D-PEG or PLGA. All these medication carriers have improved BBB penetrability and a considerably faster rate of movement [106].
In another study, NPs of magnetic material coupled with polyethylene glycol (PEG) were initially modified via the lactoferrin (Lf) receptor and then infused into the circulatory system of rats. The potential of Lf-Fe3O4 NPs to pass through the BBB via the Lf-receptor-mediated route was demonstrated by magnetic resonance imaging of blood vessels in the brain [107].
Therefore, carrier-mediated transfer and RMT are the most prevalent methods that play a role in active targeting for delivering brain-targeting biomaterials, illustrating the best choice. Thus, to select brain-targeting delivery and an efficient BBB, it is essential to provide brain-targeting biomaterials with the capacity to detect transporters or receptors on the BBB. Therefore, enhancing the affinity of brain-targeting biomaterials for BBB receptors to reduce the likelihood that they may become trapped within BECs is the simplest strategy [108].
7. AD treatment by different polymer-based biomaterials
Nano-based systems are a promising scheme for brain delivery of bioactives through the BBB. These systems provide many advantages, including the eluding of drugs from the gastrointestinal and passing the metabolism of the drugs to achieve a therapeutic impact. So, biomaterials as drug carriers can offer an accurate and progressive method to be applied for brain targeting with different carriers and dementia management by diagnostic to delivering patient focused treatment, which appears to be the fundamental gap in the current system. Given, molecules attached to the outermost layer of biomaterials must be carefully constructed such that they can be transported by certain carriers [92]. Several biomaterials with specialized physicochemical property-based brain delivery systems with specific ligands target have been constructed to successfully cross the BBB and deliver medicines directly to brain-sick lesions.
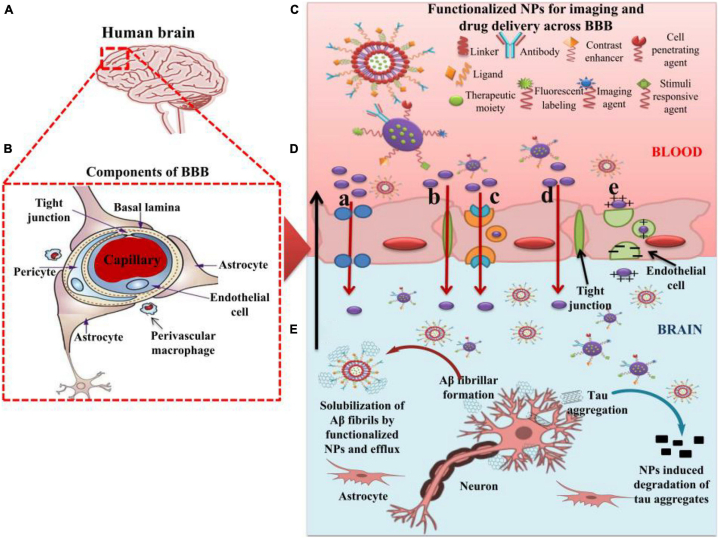
Modified nanomaterial-based medicine delivery systems and related compounds permeate the BBB and deliver medications to the brain through carrier-mediated transcytosis [109]. The modification of the surface through specific ligands such as transferrin, lactoferrin, glutathione, and others allows medicines to enter the brain by active transport mechanisms such as receptor or carrier-mediated transport [110]. For example, drug nanocarriers surface-modified by cationic compounds and ligands demonstrate higher BBB permeability and brain accumulation through AMT and RMT [111]. In addition, the cellular incorporation of biomaterials may include both passive transport (direct penetration) and active transport (either endocytosis or phagocytosis) depending on the physiological chemical characteristics of the biomaterials and the structure of the target cells (Fig. 4) [112]. Phagocytosis generally proceeds when specialized phagocytes interact with NPs, particularly those larger than 500 nm [113]. On the contrary, endocytosis happens in all cells and comprises caveolae-mediated endocytosis, micropinocytosis, clathrin-mediated endocytosis, receptor-mediated endocytosis (RME) and other endocytosis pathways unrelated to clathrin and caveolae [112]. The main mechanism responsible for the internalization of ligand-modified NPs is RME [114]. The ligand-modified NPs that are internalized by RME typically travel through the trafficking of vesicles to endosomes or lysosomes [112]. Thus, recognizing the variables that influence the performance of RME is crucial in delivery of NPs to cells.
Fig. 4.
Shows how functioning NPs have been used to go over the BBB, making use of several transport processes to provide anti-AD effects in the transported payloads [111].
Moreover, in Alzheimer's disease, when nanoparticles are used in patients, numerous studies have shown that NPs may stimulate, reduce, or even postpone the kinetic development of amyloid plaques. So that, the nanoparticles can prevent the accumulation of amyloid plaques during the efficient delivery of the drug to the cells to treat Alzheimer's disease. Among numerous preclinical applications, for instance, Cheng et al. evaluated a stable curcumin NP formulation consisting of polyvinylpyrrolidone (PVP) and polyethylene glycol-polylactic acid (PEG-PLA) co-block polymer in an in vitro BBB model and in the AD mice model Tg2576 oral administration. In opposite to controls, the combination of nanocurcumin was able to reduce the amount of amyloid plaque in the brains of AD mice, which improved cue and working memory more than regular curcumin did in several behavioral tests [115]. In another preclinical investigation, curcumin-conjugated nanoliposomes were injected into the neocortex and hippocampal regions of a double transgenic APP/PS1 mice model of AD. Researchers came to the conclusion that these particular nanoliposomes may both downregulate the release of the amyloid peptide in vitro and precisely identify Ab deposits in vivo in AD mice brains [116]. In a different example, mice were given injections of polyethylene glycol-gold (PEG-AuNPs) as a test model. They discover that Aβ peptide in vitro agglomeration is reduced by nanoparticles' ability to enter cells. Consequently, in the mouse AD model, the toxicity of the accumulated Ab peptide was similarly reduced [117].
Additionally, there are several limitations to polymeric nanoparticles, such as the buildup of biological materials in the brain, non-biodegradability, and systemic toxicity under certain circumstances. Thus, multiple attempts have been made to create viable polymeric NPs with desirable characteristics [118]. One of these strategies focused on the utilization of intranasal carotenoid (lutein) transport using cationic chitosan-modified PLGA core/shell NPs for the treatment of AD. Intranasal administration of chitosan NPs to the brain is encouraging and offers an efficient, non-invasive approach for managing AD. For example, lutein has been recognized as an anti-AD medication owing to its antioxidant and anti-inflammatory properties. However, inadequate solubility and bioavailability limit its curative potency. To enhance bioavailability and solubility and accomplish brain targeting, it was inserted into PLGA NPs. The electrostatic interactions between cationic chitosan-coated NPs and endothelial cells were caused by internalization of the nanocarriers. Chitosan-coated lutein-PLGA NPs successfully delivered the medicinal carrier to the brain and were highly capable of crossing the nasal mucosal barrier, where lutein affects nerve cells therapeutically [119]. Another investigation revealed the transfer of alendronate from the nose to the brain using chitosan NPs. Alendronate inhibits cholesterol and AChE synthesis The reaction of a group of amino acids with the negatively charged cell surface occurs to the carrier, providing persistent delivery of a medication where the primary release was less than 39% in 30 min [120]. Numerous studies have shown that NPs may stimulate, reduce, or even postpone the kinetic development of amyloid plaques in patients with AD. Additionally, there are several limitations, such as the buildup of biological materials in the brain, non-biodegradability, and systemic toxicity under certain circumstances. Thus, multiple attempts have been made to create viable polymeric NPs with desirable characteristics [118].
One of these strategies focused on the utilization of intranasal carotenoid (lutein) transport using cationic chitosan-modified PLGA core/shell NPs for the treatment of AD. Intranasal administration of chitosan NPs to the brain is encouraging and offers an efficient, non-invasive approach for managing AD. For example, lutein has been recognized as an anti-AD medication owing to its antioxidant and anti-inflammatory properties. However, inadequate solubility and bioavailability limit its curative potency. To enhance bioavailability and solubility and accomplish brain targeting, it was inserted into PLGA NPs. The electrostatic interactions between cationic chitosan-coated NPs and endothelial cells were caused by internalization of the nanocarriers. Chitosan-coated lutein-PLGA NPs successfully delivered the medicinal carrier to the brain and were highly capable of crossing the nasomucosal barrier, where lutein affects nerve cells therapeutically [119].
Another investigation revealed the transfer of alendronate from the nose to the brain using chitosan NPs. Alendronate inhibits cholesterol and AChE synthesis, and the reaction of a group of amino acids with the negatively charged cell surface occurs in the carrier, providing persistent delivery of a medication where the primary release was less than 39% in 30 min [120].
Moreover, owing to their pharmacological characteristics, including neuroprotective, anti-amyloid antioxidant anti-inflammatory activities of natural agents such as curcumin (CCM) have been examined as capable agents in AD treatment [121]. The antioxidant capabilities of CCM work against the free radicals involved in neurodegenerative processes, preventing lipid peroxidation in the brain and tau protein hyperphosphorylation [122,123]. Moreover, CCM inhibits the production of proinflammatory cytokines, which affects neuroinflammation [124] and lowers the development of amyloid-beta plaques in vivo [123]. Its water insolubility, substantial metabolism, limited instability, and bioavailability in the biological milieu make it difficult to use in clinical settings [125].
Given these restrictions, delivery systems based on nanotechnology may enable the use of natural substances to treat AD. Owing to their exceptional qualities, such as physicochemical stability, variable volume and pore diameter, wide surface area, and ease of functionalization, mesoporous silica NPs (MSNs) have demonstrated tremendous potential as drug delivery systems [126]. MSNs have the potential to encapsulate a high concentration of CCM, improve its bioavailability and solubility in the biological milieu, shield it from deterioration or early leakage, and support controlled drug release [127]. MSNs also have excellent biological properties such as biodegradability, biocompatibility, and low toxicity [128].
Intranasal drug delivery, a non-invasive method to administer drugs, has been used for a long time to treat mostly local nasal diseases. Because it distributes medication directly to the cerebrospinal fluid, bypassing the BBB, and keeping the medication intact, this conventional therapy has recently gained popularity [129]. Further advantages of intranasal delivery include an improved drug bioavailability, more vascularized epithelium, and larger surface area for drug absorption [130]. Treatments for neurodegenerative diseases are also receiving more attention because they can be administered through the nose [131]. Moreover, the intranasal route provides a novel method for medication administration from the nose to the brain through the trigeminal and olfactory pathways.
Despite these benefits, this method has certain drawbacks, such as the brief residence period of the formulation in the nasal cavity due to the mucociliary clearance mechanism [132]. The development of thermo-sensitive hydrogels based on poloxamer 407 and chitosan appears to be a promising remedy for overcoming these limitations because of their increased mucoadhesiveness and environmental responsiveness (temperature and nasal cavity pH) [133]. In one study, mesoporous silica NPs were developed as curcumin nanocontainers.
7.1. Hydrogels-based nanocomposites
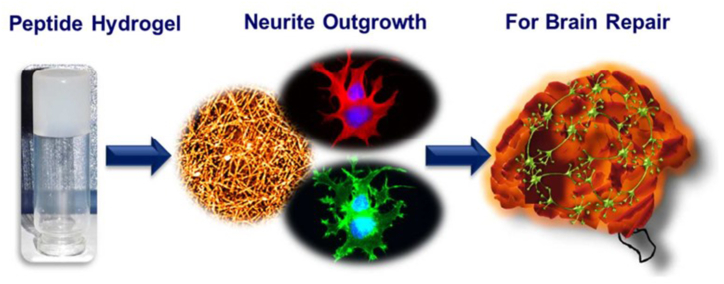
Crosslinked hydrophilic polymer networks with a high water content are called hydrogels. Previous works showed that the temperature-responsive hydrogels with good mechanical, viscoelastic, mucoadhesive properties that bypass the mucociliary clearance mechanism, and were reflected sufficient for intranasal administration (Fig. 5). The final formulation (HG@MSN-CCM) and nanosystem (MSNs and MSN-CCM) were deemed biocompatible based on in vitro results. The ex vivo permeation experiments proved that HG@MSN-CCM increased CCM permeation through the porcine nasal mucosa compared to MSN-CCM. An in vivo assay demonstrated that both MSN-CCM and HG@MSN-CCM were capable of reversing cognitive loss in mice caused by the AD model produced by streptozotocin. The results support the formulation of HG@MSN-CCM and MSN-CCM as promising cutting-edge methods for treating AD [134].
Fig. 5.
Schematic of neuro-compatible Peptide Hydrogel for Alzheimer's treatment.
7.2. Liposomal biomaterial
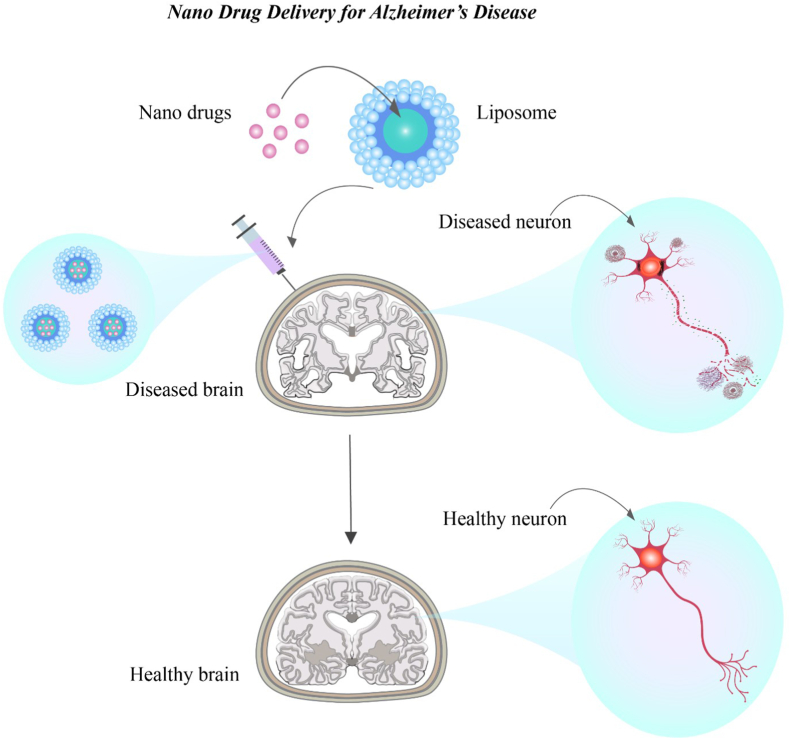
Liposomes are spherical vesicles with an internal water component surrounded by at least one impermeable lipid bilayer. They often include cholesterol and phospholipids such as phosphatidylcholine. Liposomes are of great interest because of their ability to carry both hydrophilic and hydrophobic compounds, biocompatibility, and nontoxicity. The water and lipid components of liposomes contain hydrophilic and hydrophobic substances, respectively. The introduction of liposomes into biological systems, even though they are foreign materials, does not result in adverse biological reactions. In addition, liposomes are often non-immunogenic, non-thrombogenic, non-carcinogenic, and biodegradable [135]. Liposomes have been used as nanocarriers for drug delivery to the brain. Thus, diverse components that can be encapsulated in liposomes are adequately shielded from breakdown by plasma enzymes and the removal of the reticuloendothelial system (Fig. 6). The ability of liposomes to bind to biological membranes, cross cell membranes via endocytosis, and cross the BBB are their most significant properties [135,136].
Fig. 6.
Liposomal nano-drug carrier in the rapid delivery of drugs targeting the brain of AD patients.
NPs, which are created materials with unique physiochemical properties and nanoscale diameters, have been extensively explored as cutting-edge therapeutic modalities to slow the progression of AD over the past ten years. Although patient compliance and formulation factors are considered, the oral route has some disadvantages for poorly soluble drug molecules and medicines that are unstable in GI fluids. Hence, a new nanoplatform is required to avoid gastrointestinal degradation of the therapeutic moiety and improve its solubility and bioavailability to address the difficulties outlined above. In the field of health, the science of NPs and their distribution is a relatively new concept that is rapidly developing. To boost solubility, target delivery with fewer adverse effects, and improve bioavailability, various nanoformulations are routinely used in drug delivery studies [137].
The most well-known and extensively studied novel vesicular mechanism for enhanced drug delivery involves the use of liposomes. Both the hydrophilic and lipophilic medications were successfully administered. Liposomes are spherical vesicles that contain an inner-core hydrophilic partition enclosed by several compact lipid layers. Traditional lipid carriers have several advantages. Additionally, they experience serious concerns regarding stability, reproducibility, sterilization techniques, inadequate drug entrapment, and particle size management. During formulation and storage, instability can occur in the form of drug leakage or phospholipid layer separation. Because of enzyme activity, pH, and bile salts in the gastrointestinal tract (GIT), conventional liposomes are not recommended for oral administration [138].
Stability is a crucial factor in determining the quality, effectiveness, and safety of dosage forms. Several techniques, including stabilization, lipid composition modification, surface coating, and interior liposomal layer thickening can be used to further increase the stability and integrity of conventional liposomes. One such modification involves liposomes coated with chitosan, which are widely used for non-parenteral medication administration [139]. Chitosan is a cheap and abundant biopolymer with various drug delivery capabilities. Glucosamine and acetyl-glucosamine residues were joined by–1-4 linkage to produce chitosan. The positively charged amino group and negatively charged phospholipid group of chitosan combine electrostatically to form a solid biopolymer coat that surrounds the liposome, increasing its stability and decreasing drug leakage. The mucoadhesive property of chitosan is due to its surface's positive charge, which also prolongs drug interactions at the intestinal absorption site and aids in the sustained release of the medication [140].
Maintaining a stable colloidal liposomal suspension requires optimal repulsive interactions to prevent drug aggregation. The liposomal layer of PEG coating achieves steric stabilization, which reduces aggregation and improves the stability. Owing to the reduced reticuloendothelial system uptake, satirical liposomes exhibit excellent drug distribution and bioavailability. Centella Asiatics, popularly known as Brahmi, is a well-known herb for memory improvement and as a neuroprotective agent. Asiatic acid (AA) is a pentacyclic triterpene derived from this plant. Beta-site amyloid precursor protein-cleaving enzyme (BACE) 1, which is responsible for producing the A protein, is an enzymatic pathway that is successfully modulated by the bioactive chemical AA. Owing to its poor solubility and absorption in aqueous environments and low bioavailability, AA's therapeutic application of AA is limited. Its use in terms of bioavailability can be enhanced by fitting it into a nanovesicular system [141].
For this purpose, AA was prepared and evaluated in three different liposomal forms: regular liposomes, chitosan-coated liposomes, and stealth liposomes. Compared to AA liposomes (AAL) and AA, chitosan-coated AA liposomes (CAAL) and stealth AA liposomes (SAAL) have a higher drug release rate and longer duration of action. Compared with SAAL and AAL, which showed poor stability and increased turbidity in simulated gastric fluid (SGF), CAAL showed improved stability. This suggests that phospholipid layers degrade when an acidic pH is present. CAAL, AAL, and SAAL formulations are inappropriate for oral medication administration. Data from stability studies of AAL, CAAL, and SAAL over three months revealed that, in contrast to AAL, the size, drug content, and percentage entrapment efficiency of the vesicles in CAAL and SAAL remained constant. The goal of this study was to improve surface-modified lipid carriers and to assess their in vitro characteristics. However, in-depth research is necessary to verify the potential outcomes of product formulations [142].
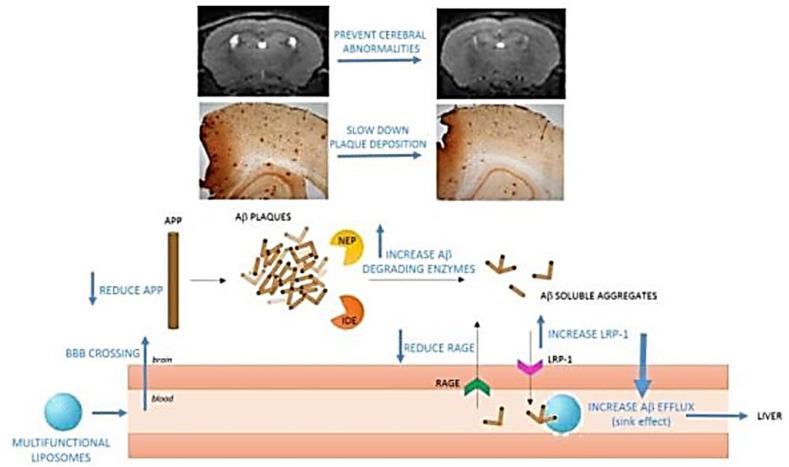
In another study, young mice were utilized as a model of the presymptomatic stage of the illness to examine the efficacy of treatment utilizing Aβ-targeting liposomes to postpone or stop the emergence of the symptoms of AD, such as brain Aβ accumulation, alterations in cerebral morphology, and memory impairment. To target and cross the BBB, liposomes created to treat AD (mApoE-PA-LIP) were dual-functionalized with a synthetic peptide (mApoE) containing the receptor-binding domain of apolipoprotein-E and phosphatidic acid (PA) for Aβ binding [143,144]. Previously, aged symptomatic Tg mice, which were employed as a severe AD model [103], were abruptly treated with these liposomes. The frequency and duration of therapy were adapted to this specific objective in the current investigation to assess the efficacy of these liposomes for treating AD at the presymptomatic stage using an animal model. These results indicate that mApoE-PA-LIP is a novel, all-encompassing, multifunctional approach that may be useful for treating AD at the presymptomatic stage, delaying the development of cognitive decline and brain structural abnormalities, and slowing the progression of Aβ accumulation. In addition, they argued that medications that target Aβ peptide accumulation, which have not been clinically beneficial to date, ought to be reevaluated at this stage of the disease (Fig. 7) [145].
Fig. 7.
When administered to pre-symptomatic Alzheimer's mice, multifunctional liposomes penetrated the BBB, decreased APP, boosted A-degrading enzymes (IDE, NEP), and promoted brain A-clearance via the sink effect, delaying plaque buildup, and preventing anomalies in the cerebellum.
One potential approach for augmenting therapeutic efficacy involves augmenting the bioactivity of naturally occurring biomaterials. Recent advances in biomaterials have demonstrated their capacity to enhance the precise delivery of therapeutic compounds and cells to the brain. Furthermore, augmentation of bioactivity in natural biomaterials has the potential to improve therapeutic effectiveness in the context of AD. As such, researchers have investigated various biomaterials with the aim of developing potential vectors or other delivery systems that can specifically target bioactive components within the brain while simultaneously preserving their inherent properties.
8. The new uses and directions of natural polymers in AD
8.1. pH-responsive biomaterials
A vast array of naturally occurring polymers, including cellulose, albumin, gelatin, and chitosan, exhibit pH-sensitive properties. pH-responsive bioactive biomaterials can transport medication packages to pathological conditions or target locations, including tumor tissues, inflammatory sites, and amyloid aggregates. The acidic environment of the amyloid plaque, harmed nerve cells, or inflamed neurons controls the physical or chemical release of medications from this carrier or treatment at the targeted location. The pH-responsive delivery system lowers systemic exposure to anti-AD medications and increases drug concentrations in brain areas harmed by medications whose pH is lower than that of the general circulation [146].
8.2. Thermoresponsive biomaterials
In a recent study, ruthenium NPs altered with a phase-changing material were investigated to increase permeability across the BBB and produce a thermoresponsive (responds to variations in the outside temperature) release of nerve growth factor (NGF). NGF is a potent neurotrophin that enhances memory and learning while reducing nerve injury by preventing tau hyperphosphorylation [147]. The created nanocarrier's thermoresponsive release characteristic prevented the medication from being released too soon and extended the duration of blood circulation. It successfully reduced several elements that contribute to AD development, such as tau hyperphosphorylation, oxidative stress, and neuronal injury [147].
Georgieva et al. in order to cure AD, showed how Poly (N-isopropylacrylamide) (PNIPAM)'s thermos-responsive activity may be used to distribute galantamine (a well-known anti-AD medication) transdermally. Several polymeric network films containing galantamine were created, assessed, and loaded. Excellent thermo-responsive behavior was provided by the PNIPAM-based formulation, which allowed the transdermal delivery of the drug to prevent systemic exposure and ensuing side effects [148]. In another investigation, a thermoresponsive new conjugated polymer-based micelle's capacity to target amyloid was described for the treatment of AD. The two PEG analogs that make up the smart polymer, PMO37 and POEG38, show exceptional thermoresponsiveness, biocompatibility, mechanical strength in comparison to PNIPAM, including its limited. Temperature shock converts the polymeric conjugate from a hydrophilic to a hydrophobic state, further trapping the Aβ fibril and preventing the formation of dangerous Aβ plaques.
8.3. Photothermal biomaterials
Photothermal biomaterials exhibit the capacity to release heat energy when activated by a light stimulation. This thermal energy causes the biomaterial to modify, eliminate damaged cells or protein clumps, and stimulate surrounding tissues [92]. Li et al. investigated the light-regulated release characteristics of a microscopic hydrogel containing polypyrrole (PPy) NPs loaded with a neurotransmitter that was activated by near-infrared radiation. When an NIR irradiation stimulus induced remote-controlled localized glutamate injection in an AD rat model, there was an increase in auditory cortex activity [149].
8.4. Dendrimers
Dendrimers are another type of polymeric carrier that have become increasingly prominent in the last decade as a unique medication delivery system for the brain. With the use of combination biomaterials such as triazine, PAMAM, PPI50, carbosilane, and PLL, a variety of dendrimers have been made, and Igartua et al. used PAMAM dendrimers to carry carbamazepine to the brain in an effort to decrease dosage, frequency of administration, side effects, and therapeutic costs. The solubility of the medication was greatly increased, approximately three times, by complexation with the PAMAM dendrimer [150]. Additionally, they created a methanol solution of dendrimers [DG 4.0 and 4.5] and tacrine, an approved anti-AD medication, and investigated the interaction. Additionally, they documented the co-administration of tacrine and PAMAM dendrimers to reduce the toxicity of medication and enhance its therapeutic effectiveness.
In an associated study, Gothwal et al. assessed the effectiveness of Lf53 conjugated PAMAM dendrimer in brain targeting in in vivo AD model. The size of the drug-loaded PAMAM dendrimers grew significantly from 11.5 to 131.7 nm when conjugated with Lf. Lactoferrin's cationic character raised its zeta potential, and the Lf surface coating slowed the drug's release while achieving the intended delayed release. The Lf-conjugated dendrimer demonstrated improvements in behavioral and memory function in an AD mouse model and effectively transported the medication with increased bioavailability to the brain [151]. Another remarkable study by Al-Azzawi et al. revealed how to utilize the PEL54 dendrimer to transport flurbiprofen, an anti-inflammatory medication authorized by the United States Food and USFDA for the treatment of AD. The delivery of medicines to the target location via hydrolysis was facilitated by medication integration with the PEL dendrimer, which also improved BBB permeability and was successfully directed by the formulation to the required location on the γ-secretase enzyme [152].
8.5. Solid Lipid Nanoparticles
Solid Lipid Nanoparticles (SLN), the newest delivery method, provide viable carriers for brain targeting. Delivering proteins, peptides, and other hydrophobic medicines with a significant molecular weight is the main use of SLN; however, they are also appropriate for all other kinds of bioactive compounds. Sathya et al. described how to reduce Aβ and lessen neurotoxicity by targeting the brain with α-bisabolol. SLN were prepared by homogenizing cholesterol, Tween 80 and soy lecithin in a heating process. According to Sathya et al. the system significantly lowers AChE activity, oxidative stress, and neurotoxicity as well as Aβ aggregation [153].
9. Drug intracellular release
To generate an elicit therapeutic reaction, free form of drugs must be released into certain subcellular sections, usually the nucleus or cytoplasm of the diseased cell. After integration, NP-based compositions are inclined via a diffusion-controlled release route to passively release drug payloads, which results in inadequate concentrations of drugs throughout the targeted time frame. Additionally, the ligand-modified NPs that are internalized by RME typically travel through the trafficking of vesicles to endosomes or lysosomes [112], whereas the cytoplasm, mitochondria, or nucleus are the target of the treatment of the discharged medications, and the acidic pH and numerous proteolytic enzymes destroy released medications [154]. Consequently, upon internalization into diseased cells, a quick and substantial release of medication with the capacity to escape from the lysosome is critical.
10. Clinical trials
Natural and synthetic lipids are biomaterials that are used in the preparation of nanocarriers. These nanocarriers have gained popularity owing to their notable characteristics, including biodegradability, compatibility, lower toxicity compared to mineral and polymer materials, enhanced penetration into biological membranes, and drug loading capabilities in comparison to alternative nanocarrier systems. Remarkably, lipid carriers were used in the development of over 70% of nanomedicines in clinical trials up until 2019. Moreover, biopharmaceutical compounds play a crucial role in facilitating the transfer of therapeutic contents across the BBB and enabling their targeting to pathological sites within the brain. Additionally, they contribute to the preservation of drug properties, minimization of side effects, and the establishment of cost-effective treatment options. However, despite the exceptional properties exhibited by biomaterials, a suitable treatment for AD has yet to be identified, and most research has remained confined to successful preclinical stages without progressing to various clinical trials. Although certain strategies are currently being investigated in clinical studies, they often fail to demonstrate favorable effects.
The majority of therapeutic approaches concentrate on the mild reduction of AD symptoms, with only a few groups exploring targeted gene therapy and neuroregenerative treatments that have the potential to eradicate the disease. Nevertheless, these studies are still in their preliminary stages and their advancement is exceedingly complex. Moreover, the advancement of efficacious therapies for AD, including those that leverage natural biomaterials, has the potential to yield economic advantages through the alleviation of the financial strain associated with the ailment [1,3,12,13]. However, the utilization of natural biomaterials in the management of AD gives rise to numerous ethical concerns pertaining to the acquisition of informed consent, prompt diagnosis, fair and equal availability, personal privacy and confidentiality, the principle of doing good and avoiding harm, and the establishment of regulatory measures and supervision. This necessitates meticulous deliberation and resolution to guarantee the safety, efficacy, and universal accessibility of treatment for all individuals requiring it. Therefore, a comprehensive strategy that encompasses cooperation among healthcare professionals, researchers, patient advocacy organizations, regulatory bodies, and the general public has the potential to enhance knowledge regarding the possible advantages and drawbacks of utilizing natural biomaterials for the management of AD. Consequently, maybe can be said the utilization of diverse biomaterials, in conjunction with meticulous strategies, extensive research, and advanced technologies in the near future, holds promise as a viable solution for AD treatment [1,14,16,18,19].
11. Challenges and complexities
Natural biomaterials have been investigated as prospective remedies for AD; however, numerous obstacles must be overcome to ensure their effective utilization in patients. Ongoing research and development efforts are being undertaken to address these challenges. One of the primary impediments is delivery of biomaterials to the brain. The BBB, which serves as a selective barrier, restricts the entry of numerous substances, including certain biomaterials. Researchers have explored diverse approaches to overcome this hurdle, such as employing NPs or modifying biomaterials to enhance their permeability across the BBB. Another challenge is to ensure the biocompatibility of biomaterials, thereby averting any immune response or other detrimental effects. Some natural biomaterials possess immunogenic properties that elicit immune responses within the body. Researchers have endeavored to modify biomaterials to diminish their immunogenicity and enhance their biocompatibility. Additionally, natural biomaterials may undergo degradation over time, thereby restricting their efficacy as therapeutic interventions. To address this concern, researchers are exploring methodologies to modify biomaterials, thereby augmenting their stability and extending their lifespans. Moreover, AD manifests as a complex condition that affects distinct regions of the brain. Researchers are actively engaged in developing biomaterials capable of targeting specific brain regions affected by disease, a prospect that could enhance the effectiveness of treatment. Lastly, any novel remedy for AD must undergo arduous testing and secure regulatory approval prior to its application in patients. This process is arduous and costly, with no guarantee of successful approval of a new treatment [24,25,27,[36], [37], [38]].
Additionally, despite extensive scientific endeavors, numerous unfulfilled criteria and loopholes still exist in the currently available treatment and diagnostic strategies, thereby constraining their effectiveness. One potential approach to augment therapeutic efficacy involves augmenting the bioactivity of naturally occurring biomaterials. Recent advances in biomaterials have demonstrated their capacity to enhance the precise delivery of therapeutic compounds and cells to the brain. Furthermore, augmentation of bioactivity in natural biomaterials has the potential to improve therapeutic effectiveness in the context of AD. As such, researchers have investigated various biomaterials with the aim of developing potential vectors or other delivery systems that can specifically target bioactive components within the brain while simultaneously preserving their inherent properties. This approach seeks to address existing limitations such as poor solubility, permeability, and bioavailability, as well as minimizing any associated side effects [1,10,[38], [39], [40], [41]].
Moreover, ensuring the safety of natural biomaterials is of utmost importance for biomedical applications. Guaranteeing the safety of natural biomaterials involves consideration of various factors. The initial step involves characterizing the material to determine its inherent bioactivity. This process involves analyzing the chemical composition, structure, and mechanical properties of the material. The manner in which natural biomaterials are processed can significantly affect bioactivity. Techniques, such as cross-linking, decellularization, and purification, can be employed to optimize and preserve the bioactive components of the material. To ensure the safety of biomaterials, the American Society for Testing and Materials (ASTM) has established standards that specify the chemical composition, phase determination, grain size, and impurities of ceramic materials. Finally, a pathway for biomaterial evaluation was devised to guarantee safety and consistency within the biomaterial research community. This pathway encompasses preclinical testing, clinical trials, and postmarket surveillance. Therefore, the safety of natural biomaterials can be improved by considering these factor [25,34,38,43,45,46].
More importantly, the activation of the immune system, known as immune rejection, can be triggered by the utilization of natural biomaterials, resulting in their rejection of said biomaterial. This is a cause of concern when employing natural biomaterials in transplantation or implantation procedures. However, the utilization of natural biomaterials introduces additional difficulties, owing to their variability and complexity. Further investigation is imperative to gain a comprehensive understanding of the risks and advantages associated with the use of natural biomaterials in the treatment of neurodegenerative disorders [3,10,35,38,42].
In addition, certain challenges arise when attempting to commercialize natural biomaterials for the treatment of AD. For example, the limited availability of these materials stems from their derivation from plants or animals, thereby constraining their accessibility and scalability in terms of commercial production. Furthermore, the composition and characteristics of natural biomaterials are subject to variation and influenced by factors such as their source, season, and processing methods. Consequently, ensuring consistent quality and efficacy is challenging. Moreover, the commercialization of biomaterials for medical purposes necessitates regulatory approval, an arduous and expensive process. Additional regulatory hurdles may be encountered with natural biomaterials because of concerns regarding their safety and efficacy. Finally, the production of natural biomaterials tends to be costlier than that of synthetic materials, thereby diminishing their market competitiveness [1,3,4,12,25,36].
One of the biggest challenges that lie ahead is defining information needs related to a medicinal product's nano-specific properties that may affect its quality, safety, and efficacy. As of right now, there are very few approved methods from standardization bodies specifically addressing the application of nanotechnology in the health sector, which may contribute to national and regional variations in information needs. Satalkar et al.'s (2016) survey [155] supports this theory by finding that respondents confirmed regional and national differences of individual requirements, which pose serious difficulties for researchers conducting multicentrical international clinical trials. Good manufacturing practice (GMP) rules should be strictly adhered to and the production process should be scalable and robust. The criticality of particle size, surface morphology, drug loading, release, and probably a few others necessitates a variety of quality control assays in addition to the usual array of quality checks, even if quality control must be strict for any medicinal product, nanomedicine or not. Particle size, size distribution, charge and morphology, drug encapsulation and release, and other crucial quality aspects need to be considered from the very beginning of formulation design. In fact, certain safety concerns were pertinently brought up in conjunction with these newly developted tactics. The safety evaluation of nanomaterials is emphasized despite the lack of specific regulatory assessments. This is because it is outlined in two places: (i) the ISO 10993–1:2009 and ISO/TR 13014:2012 guidelines, which describe the general framework for biological evaluation of medical devices, and (ii) the ISO 10993-22 standard, which goes into greater detail.
All things considered, the risk assessment of these nanomaterials has to involve the following: (i) a thorough description of their physicochemical characteristics, as stated in ISO 10993-22 guideline; (ii) a meticulous biological evaluation, which includes well-reasoned potential deviations from the standard recommendations.
12. Perspective and future outlook
The utilization of natural biomaterials for the management of AD is an ongoing area of research. In this field, there are several prospective avenues for future investigation. One of these involves the utilization of nanoparticle-based drug delivery systems, wherein it is recommended that future studies be conducted to enhance the effectiveness of natural biomaterials in the treatment of AD. Additionally, it is crucial to develop new strategies for the treatment of inflammation in AD, which can impede disease progression. Furthermore, it is imperative to explore a wide range of natural and synthetic biomaterials in order to design promising systems for the transportation of vectors or drugs to the brain, with the ultimate goal of discovering a cure for AD [10,12,42,49,50,52]. Considering the significant involvement of mitochondrial dysfunction in the pathogenesis of AD, a promising avenue lies in the development of highly effective nanoparticle-based mitochondria-targeting compounds. These compounds, specifically tailored to engage mitochondria, can potentially impede mitochondrial ROS production, permeability transition pore opening, and DNA mutation. Furthermore, they may enhance the biogenesis and dynamics of mitochondria, and improve mitochondrial bioenergetics both in the brain and peripheral tissues of individuals impacted by AD [156].
In addition, there are various approaches through which the research community can achieve improved coordination to expedite the progress of natural biomaterials for the management of AD. First, collaboration among researchers from diverse fields, including bioengineering, clinical research, healthcare providers, funding and community partners, policymakers, and educators, has tremendous potential. By engaging in collaborative discussions, these individuals can jointly address the present-day impact of bioengineering on resolving global health challenges and establish connections with communities to facilitate the implementation of solutions. Second, it is imperative to organize conferences and events that foster an environment conducive to open exchange. Such gatherings would facilitate meaningful discussions among researchers, clinicians, and other relevant stakeholders, thereby promoting the dissemination of the latest advancements in the realm of biomaterials for AD treatment. Third, funding agencies should prioritize the allocation of resources towards research endeavors focused on the development of biomaterials for AD treatment. In doing so, these agencies can motivate a greater number of researchers to contribute to this particular area of study. This would ensure that their work was accessible to a wider audience, consequently enhancing the dissemination of knowledge in the field. Finally, educators should design educational programs aimed at training the next generation of researchers in the field of biomaterials for AD treatment. By equipping individuals with the necessary skills and knowledge, they can contribute to the accelerated development of natural biomaterials to combat this disease. By implementing these strategies, the research community can achieve improved coordination and expedite the development of natural biomaterials for AD treatment [1,40].
However, there are still several significant gaps in our knowledge that must be addressed to enhance the utilization of natural biomaterials for the management of AD. These gaps encompass the understanding of the immune response and the application of immunotherapy involving nanomaterials. Although natural biomaterials have been utilized for an extended period of time, there is still a lack of understanding regarding immune response and immunotherapy involving nanomaterials. Furthermore, the development of efficacious biomaterials for neuroregeneration is a priority of researchers. They combine natural biomaterials with synthetic or electro-conductive polymers to create effective biomaterials for neuroregeneration. Nevertheless, it is imperative to identify the most optimal natural biomaterials and develop strategies to optimize their utilization. In addition, the identification of the most effective natural biomaterials for neural tissue engineering is crucial. Natural biomaterials can be derived from extracellular matrix proteins or polysaccharides that are synthesized by other organisms. Although their biologically active nature and ability to promote cell adhesion and growth render them popular, there is still a need to identify the most effective natural biomaterials for neural tissue engineering. Finally, obstacles associated with sourcing natural biomaterials must be overcome. Certain natural biomaterials such as collagen have limitations in terms of sourcing. Researchers are actively exploring approaches to overcome these limitations, such as the development of synthetic alternatives or discovery of novel sources of natural biomaterials [1,36,40,47,51].
13. Conclusion
With much research being done on natural biomaterials to treat Alzheimer's, it is possible to investigate newly discovered and created natural biomaterials to improve treatment effectiveness. Smart biomaterials, because of their small size and excellent penetration through the BBB, offer an accurate and progressive method to deliver improved patient-focused treatment with regard to target-specific and targeted treatment, which is the fundamental gap in the current system and a promising technique for delivering bioactive compounds to the brain. Moreover, the rapid creation and introduction of natural biomaterials into clinical studies will encourage the creation of novel biomaterials, which could aid in the discovery of effective AD treatments. Therefore, biomaterials can be utilized for AD treatment by specialized diagnosis or treatment techniques in purpose-designed device scaffolds. Nano-based systems have many advantages, such as avoiding gastrointestinal and first-pass metabolism of the drug, and using lower medication dosages to achieve an equivalent or superior therapeutic impact compared to conventional methods of oral delivery. Specific ligands, such as transferrin, lactoferrin, and glutathione, can modify the surface, allowing drugs to enter the brain through active delivery mechanisms, including receptor- or carrier-mediated transportation. Further research into the synergistic impact of carrier-mediated transcytosis, AMT, and RMT on BBB drug delivery as well as the identification of suitable nanomaterial-medicine pairings could enable a broader variety of functions in the imaging diagnosis of CNS illnesses and efficient therapy via medication systems for delivery. In the past few years, a major development in the management and treatment of numerous neurological illnesses, including neurodegeneration, inflammatory, autoimmune, and cognitive disorders, is anticipated due to the continued rapid advancement of pharmaceutical nanotechnology and the advancement of medication delivery through brain barriers directly to the brain. In summary, nanomaterials have confirmed their capacity to cross the BBB and their potential in BBB-involved systems for medication delivery. In conclusion, natural biomaterials have excellent potential for growing their market in the field of treating AD, restoring damaged brain tissue, and enhancing post-injury neural regeneration. Therefore, we reviewed the current developments in natural biomaterials for the treatment of AD individuals.
Funding
This work was supported by Bashkir State Medical University Strategic Academic Leadership Program (PRIORITY-2030). This work also was supported by the Research Center for Pharmaceutical Nanotechnology, Biomedicine Institute of Tabriz University of Medical Sciences, Tabriz, Iran (Grant no: 73829).
Data availability
This is a review; therefore, all data are included in the manuscript.
CRediT authorship contribution statement
Tayebeh Zivari-Ghader: Writing – original draft, Writing – review & editing, Investigation, Conceptualization. Ferzane Valioglu: Funding acquisition, Formal analysis, Data curation, Conceptualization. Aziz Eftekhari: Resources, Funding acquisition, Formal analysis, Data curation, Conceptualization. Immi Aliyeva: Writing – original draft, Writing – review & editing, Visualization, Formal analysis, Data curation, Conceptualization. Ozal Beylerli: Writing – review & editing, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation. Soodabeh Davran: Writing – original draft, Writing – review & editing, Visualization, Validation. William C. Cho: Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. Aferin Beilerli: Writing – review & editing, Visualization, Formal analysis, Data curation. Rovshan Khalilov: Writing – original draft, Writing – review & editing, Visualization, Validation. Sabzali Javadov: Supervision, Software, Resources, Investigation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors are thankful to Research Center for Pharmaceutical Nanotechnology, Biomedicine Institute of Tabriz University of Medical Sciences, Tabriz, Iran (Grant no: 73829).
Contributor Information
Aziz Eftekhari, Email: eftekharia@tbzmed.ac.ir.
Soodabeh Davran, Email: davaran@tbzmed.ac.ir.
Abbreviations
- AA
asiatic acid
- AAL
AA liposomes
- Aβ
amyloid beta
- AChEIs
acetylcholinesterase inhibitors
- AD
Alzheimer's disease
- AJs
adherens junctions
- AMT
adsorptive-mediated transcytosis
- BACE1
beta-site amyloid precursor protein cleaving enzyme 1
- BBB
blood-brain barrier
- BECS
brain endothelial cells
- CAAL
chitosan-coated AA liposomes
- CCM
curcumin
- CNS
central nervous system
- CRITID
systemic circulation, BBB recognition by receptors, transporters, adsorptive mechanisms, etc, BBB intracellular transport, diseased cell targeting after entering the brain parenchyma, internalization by diseased cells, intracellular drug release
- FDA
Food and Drug Administration
- FDG
fluorodeoxyglucose
- FL
neurofilament light
- GLUT1
glucose transporter type 1
- LAT1
large neutral amino-acid transporter type 1
- Lf
lactoferrin
- LRP1
lipoprotein receptor related protein 1
- LRP 2
lipoprotein receptor-related protein 2
- MPS
mononuclear phagocytic system
- MSNs
mesoporous silica nanoparticles
- NDs
neurodegenerative diseases
- NFs
nanofibers
- NfL
neurofilament light
- NMDA
N-methyl-d-aspartate
- NMDAR
N-Methyl-d-aspartate receptor
- NPs
nanoparticles
- PA
phosphatidic acid
- PEG
polyethylene glycol
- PEI
poly(ethylenimine)
- PET
positron emission tomography
- PLA
polylactic acid
- PPI
poly (propylene imine)
- PVP
polyvinylpyrrolidone
- RAGE
receptor for advanced glycation end products
- RME
receptor-mediated endocytosis
- RMT
receptor-mediated transcytosis
- SAAL
Stealth AA liposomes
- SGF
simulated gastric fluid
- SLN
solid lipid nanoparticles
- TJs
tight junctions
References
- 1.Papadimitriou L., et al. Biofabrication for neural tissue engineering applications. Materials Today Bio. 2020;6 doi: 10.1016/j.mtbio.2020.100043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malik S., et al. Synthesis, characterization, in-silico, and pharmacological evaluation of new 2-amino-6-trifluoromethoxy benzothiazole derivatives. Bioorg. Chem. 2023;130 doi: 10.1016/j.bioorg.2022.106175. [DOI] [PubMed] [Google Scholar]
- 3.Montazersaheb Soheila, et al. Emerging Nanotherapeutic Alzheimer’s Disease. Front. Clin. Drug Res. 2021;2 [Google Scholar]
- 4.Zulfugarova Parvin, et al. A mechanistic review of pharmacological activities of homeopathic medicine licorice against neural diseases. Front. Neurosci. 2023;17 doi: 10.3389/fnins.2023.1148258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dwivedi N., et al. Dendrimer-mediated approaches for the treatment of brain tumor. J. Biomater. Sci. Polym. Ed. 2016;27(7):557–580. doi: 10.1080/09205063.2015.1133155. [DOI] [PubMed] [Google Scholar]
- 6.Ahmed S., Ali A., Sheikh J. A review on chitosan centred scaffolds and their applications in tissue engineering. Int. J. Biol. Macromol. 2018;116:849–862. doi: 10.1016/j.ijbiomac.2018.04.176. [DOI] [PubMed] [Google Scholar]
- 7.Ahmadian Elham, et al. The role and therapeutic potential of connexins, pannexins and their channels in Parkinson’s disease. Cell. Signal. 2019;58:111–118. doi: 10.1016/j.cellsig.2019.03.010. [DOI] [PubMed] [Google Scholar]
- 8.Poovaiah N., et al. Treatment of neurodegenerative disorders through the blood–brain barrier using nanocarriers. Nanoscale. 2018;10(36):16962–16983. doi: 10.1039/c8nr04073g. [DOI] [PubMed] [Google Scholar]
- 9.Rizzo G., et al. Accuracy of clinical diagnosis of dementia with Lewy bodies: a systematic review and meta-analysis. J. Neurol. Neurosurg. Psychiatr. 2018;89(4):358–366. doi: 10.1136/jnnp-2017-316844. [DOI] [PubMed] [Google Scholar]
- 10.Simrén J., et al. An update on fluid biomarkers for neurodegenerative diseases: recent success and challenges ahead. Curr. Opin. Neurobiol. 2020;61:29–39. doi: 10.1016/j.conb.2019.11.019. [DOI] [PubMed] [Google Scholar]
- 11.Hansson O. Biomarkers for neurodegenerative diseases. Nat. Med. 2021;27(6):954–963. doi: 10.1038/s41591-021-01382-x. [DOI] [PubMed] [Google Scholar]
- 12.Madhusudanan P., Raju G., Shankarappa S. Hydrogel systems and their role in neural tissue engineering. J. R. Soc. Interface. 2020;17(162) doi: 10.1098/rsif.2019.0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Biswal T. Biopolymers for tissue engineering applications: a review. Mater. Today: Proc. 2021;41:397–402. [Google Scholar]
- 14.Boni R., et al. Current and novel polymeric biomaterials for neural tissue engineering. J. Biomed. Sci. 2018;25:1–21. doi: 10.1186/s12929-018-0491-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luo S., et al. Application of iron oxide nanoparticles in the diagnosis and treatment of neurodegenerative diseases with emphasis on Alzheimer's disease. Front. Cell. Neurosci. 2020;14:21. doi: 10.3389/fncel.2020.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Breijyeh Z., Karaman R. Comprehensive review on Alzheimer's disease: causes and treatment. Molecules. 2020;25(24):5789. doi: 10.3390/molecules25245789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sweeney M.D., Sagare A.P., Zlokovic B.V. Cerebrospinal fluid biomarkers of neurovascular dysfunction in mild dementia and Alzheimer's disease. J. Cerebr. Blood Flow Metabol. 2015;35(7):1055–1068. doi: 10.1038/jcbfm.2015.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Terry R.D., Davies P. Dementia of the alzheimer type. Annu. Rev. Neurosci. 1980;3(1):77–95. doi: 10.1146/annurev.ne.03.030180.000453. [DOI] [PubMed] [Google Scholar]
- 19.Rathmann K.L., Conner C.S. Alzheimer's disease: clinical features, pathogenesis, and treatment. Ann. Pharmacother. 2007;41(9):1499–1504. doi: 10.1345/aph.140065. [DOI] [PubMed] [Google Scholar]
- 20.Luthra N.S., et al. Vitamin B12 measurements across neurodegenerative disorders. Journal of clinical movement disorders. 2020;7(1):1–6. doi: 10.1186/s40734-020-00085-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stanger O., et al. Homocysteine, folate and vitamin B12 in neuropsychiatric diseases: review and treatment recommendations. Expert Rev. Neurother. 2009;9(9):1393–1412. doi: 10.1586/ern.09.75. [DOI] [PubMed] [Google Scholar]
- 22.Wan C., Zong R.-Y., Chen X.-S. The new mechanism of cognitive decline induced by hypertension: high homocysteine-mediated aberrant DNA methylation. Frontiers in Cardiovascular Medicine. 2022;9 doi: 10.3389/fcvm.2022.928701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ojha R.P., et al. Homocysteine Metabolism in Health and Disease. Springer; 2022. Homocysteine determinants as risk markers for neurological diseases; pp. 205–228. [Google Scholar]
- 24.Jatoi S., et al. Low vitamin B12 levels: an underestimated cause of minimal cognitive impairment and dementia. Cureus. 2020;12(2) doi: 10.7759/cureus.6976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cho H.S., et al. Suboptimal baseline serum vitamin B12 is associated with cognitive decline in people with Alzheimer's disease undergoing cholinesterase inhibitor treatment. Front. Neurol. 2018;9:325. doi: 10.3389/fneur.2018.00325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perani D. The Dementias. CRC Press; 2016. Neuropsychological screening and advanced neuropsychological tests; pp. 51–90. [Google Scholar]
- 27.Cipriani G., et al. Alzheimer and his disease: a brief history. Neurol. Sci. 2011;32:275–279. doi: 10.1007/s10072-010-0454-7. [DOI] [PubMed] [Google Scholar]
- 28.Selkoe D.J., Hardy J. The amyloid hypothesis of Alzheimer's disease at 25 years. EMBO Mol. Med. 2016;8(6):595–608. doi: 10.15252/emmm.201606210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Long J.M., Holtzman D.M. Alzheimer disease: an update on pathobiology and treatment strategies. Cell. 2019;179(2):312–339. doi: 10.1016/j.cell.2019.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tobore T.O. On the central role of mitochondria dysfunction and oxidative stress in Alzheimer's disease. Neurol. Sci. 2019;40:1527–1540. doi: 10.1007/s10072-019-03863-x. [DOI] [PubMed] [Google Scholar]
- 31.Guo L., et al. Cyclophilin D deficiency rescues axonal mitochondrial transport in Alzheimer's neurons. PLoS One. 2013;8(1) doi: 10.1371/journal.pone.0054914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jang S., et al. The effects of amyloid-β on metabolomic profiles of cardiomyocytes and coronary endothelial cells. J. Alzheim. Dis. 2023:1–13. doi: 10.3233/JAD-221199. (Preprint) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jang S., et al. Beta-amyloid instigates dysfunction of mitochondria in cardiac cells. Cells. 2022;11(3):373. doi: 10.3390/cells11030373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Briggs R., Kennelly S.P., O'Neill D. Drug treatments in Alzheimer's disease. Clin. Med. 2016;16(3):247. doi: 10.7861/clinmedicine.16-3-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumar A., Sidhu J., Goyal A.S. StatPearls Publishing; Treasure Island, FL, USA: 2020. Alzheimer Disease.https://www.ncbi.nlm.nih.gov/books/NBK499922/ [Google Scholar]
- 36.McKhann G.M., et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's Dementia. 2011;7(3):263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mayeux R., Stern Y. Epidemiology of alzheimer disease. Cold Spring Harbor perspectives in medicine. 2012;2(8):a006239. doi: 10.1101/cshperspect.a006239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yaari R., Fleisher A.S., Tariot P.N. Updates to diagnostic guidelines for Alzheimer's disease. The Primary Care Companion for CNS Disorders. 2011;13(5) doi: 10.4088/PCC.11f01262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Serrano-Pozo A., et al. Neuropathological alterations in Alzheimer disease. Cold Spring Harbor perspectives in medicine. 2011;1(1):a006189. doi: 10.1101/cshperspect.a006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spires-Jones T.L., Hyman B.T. The intersection of amyloid beta and tau at synapses in Alzheimer's disease. Neuron. 2014;82(4):756–771. doi: 10.1016/j.neuron.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Singh S.K., et al. Overview of Alzheimer's disease and some therapeutic approaches targeting Aβ by using several synthetic and herbal compounds. Oxid. Med. Cell. Longev. 2016;2016 doi: 10.1155/2016/7361613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Crous-Bou M., et al. Alzheimer's disease prevention: from risk factors to early intervention. Alzheimer's Res. Ther. 2017;9:1–9. doi: 10.1186/s13195-017-0297-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang R., Reddy P.H. Role of glutamate and NMDA receptors in Alzheimer's disease. J. Alzheim. Dis. 2017;57(4):1041–1048. doi: 10.3233/JAD-160763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Musardo S., et al. Trafficking in neurons: searching for new targets for Alzheimer's disease future therapies. Eur. J. Pharmacol. 2013;719(1–3):84–106. doi: 10.1016/j.ejphar.2013.07.019. [DOI] [PubMed] [Google Scholar]
- 45.Eldufani J., Blaise G. The role of acetylcholinesterase inhibitors such as neostigmine and rivastigmine on chronic pain and cognitive function in aging: a review of recent clinical applications. Alzheimer's & Dementia. Translational Research & Clinical Interventions. 2019;5:175–183. doi: 10.1016/j.trci.2019.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sharma K. Cholinesterase inhibitors as Alzheimer's therapeutics. Mol. Med. Rep. 2019;20(2):1479–1487. doi: 10.3892/mmr.2019.10374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao Y., et al. The role of genetics in Parkinson's disease: a large cohort study in Chinese mainland population. Brain. 2020;143(7):2220–2234. doi: 10.1093/brain/awaa167. [DOI] [PubMed] [Google Scholar]
- 48.Fonseca-Santos B., Gremião M.P.D., Chorilli M. Nanotechnology-based drug delivery systems for the treatment of Alzheimer's disease. Int. J. Nanomed. 2015:4981–5003. doi: 10.2147/IJN.S87148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nguyen P.H., et al. Amyloid oligomers: a joint experimental/computational perspective on Alzheimer's disease, Parkinson's disease, type II diabetes, and amyotrophic lateral sclerosis. Chem. Rev. 2021;121(4):2545–2647. doi: 10.1021/acs.chemrev.0c01122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xie J., et al. Nanomaterial-based blood-brain-barrier (BBB) crossing strategies. Biomaterials. 2019;224 doi: 10.1016/j.biomaterials.2019.119491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ballabh P., Braun A., Nedergaard M. The blood–brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol. Dis. 2004;16(1):1–13. doi: 10.1016/j.nbd.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 52.Reddy P.H., et al. A critical evaluation of neuroprotective and neurodegenerative MicroRNAs in Alzheimer's disease. Biochem. Biophys. Res. Commun. 2017;483(4):1156–1165. doi: 10.1016/j.bbrc.2016.08.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Williams D.F. On the nature of biomaterials. Biomaterials. 2009;30(30):5897–5909. doi: 10.1016/j.biomaterials.2009.07.027. [DOI] [PubMed] [Google Scholar]
- 54.Mazayen Z.M., et al. Pharmaceutical nanotechnology: from the bench to the market. Future journal of pharmaceutical sciences. 2022;8(1):12. doi: 10.1186/s43094-022-00400-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ruan S., et al. Rethinking CRITID procedure of brain targeting drug delivery: circulation, blood brain barrier recognition, intracellular transport, diseased cell targeting, internalization, and drug release. Adv. Sci. 2021;8(9) doi: 10.1002/advs.202004025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yoo J.-W., Chambers E., Mitragotri S. Factors that control the circulation time of nanoparticles in blood: challenges, solutions and future prospects. Curr. Pharmaceut. Des. 2010;16(21):2298–2307. doi: 10.2174/138161210791920496. [DOI] [PubMed] [Google Scholar]
- 57.Röcker C., et al. A quantitative fluorescence study of protein monolayer formation on colloidal nanoparticles. Nat. Nanotechnol. 2009;4(9):577–580. doi: 10.1038/nnano.2009.195. [DOI] [PubMed] [Google Scholar]
- 58.Longmire M., Choyke P.L., Kobayashi H. 2008. Clearance Properties of Nano-Sized Particles and Molecules as Imaging Agents: Considerations and Caveats. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kwon I.K., et al. Analysis on the current status of targeted drug delivery to tumors. J. Contr. Release. 2012;164(2):108–114. doi: 10.1016/j.jconrel.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Parodi A., et al. King's research portal. Nat. Nanotechnol. 2013;8(1):61–68. doi: 10.1038/nnano.2012.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rodriguez P.L., et al. Minimal" Self" peptides that inhibit phagocytic clearance and enhance delivery of nanoparticles. Science. 2013;339(6122):971–975. doi: 10.1126/science.1229568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dai Y., et al. Nanoparticle design strategies for enhanced anticancer therapy by exploiting the tumour microenvironment. Chem. Soc. Rev. 2017;46(12):3830–3852. doi: 10.1039/c6cs00592f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Michikawa M. The role of cholesterol in pathogenesis of Alzheimer's disease: dual metabolic interaction between amyloid β-protein and cholesterol. Mol. Neurobiol. 2003;27:1–12. doi: 10.1385/MN:27:1:1. [DOI] [PubMed] [Google Scholar]
- 64.Thomsen M.S., et al. Blood–Brain barrier transport of transferrin Receptor-Targeted nanoparticles. Pharmaceutics. 2022;14(10):2237. doi: 10.3390/pharmaceutics14102237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Panghal Archna. Nanotechnology in the diagnostic and therapy for Alzheimer’s disease. Biochim. Biophys. Acta - Gen. Subj. 2024:130559. doi: 10.1016/j.bbagen.2024.130559. [DOI] [PubMed] [Google Scholar]
- 66.Paramanick D., Singh V.D., Singh V.K. Neuroprotective effect of phytoconstituents via nanotechnology for treatment of Alzheimer diseases. J. Contr. Release. 2022;351:638–655. doi: 10.1016/j.jconrel.2022.09.058. [DOI] [PubMed] [Google Scholar]
- 67.Van Tellingen O., et al. Overcoming the blood–brain tumor barrier for effective glioblastoma treatment. Drug Resist. Updates. 2015;19:1–12. doi: 10.1016/j.drup.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 68.Deane R., Zlokovic B.V. Role of the blood-brain barrier in the pathogenesis of Alzheimer's disease. Curr. Alzheimer Res. 2007;4(2):191–197. doi: 10.2174/156720507780362245. [DOI] [PubMed] [Google Scholar]
- 69.Lockman P.R., et al. Brain uptake of thiamine-coated nanoparticles. J. Contr. Release. 2003;93(3):271–282. doi: 10.1016/j.jconrel.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 70.Chen R., et al. Biomaterial-assisted scalable cell production for cell therapy. Biomaterials. 2020;230 doi: 10.1016/j.biomaterials.2019.119627. [DOI] [PubMed] [Google Scholar]
- 71.Zhang X., Williams D. Elsevier; 2019. Definitions of Biomaterials for the Twenty-First Century. [Google Scholar]
- 72.Zeugolis D.I., Pandit A. ACS Publications; 2015. Biofunctional Biomaterials–The Next Frontier; p. 1157. 1157. [DOI] [PubMed] [Google Scholar]
- 73.Zeugolis D.I., Pandit A. Scaffolds, cells, biologics: at the crossroads of musculoskeletal repair. Adv. Drug Deliv. Rev. 2015;84:v–vi. doi: 10.1016/j.addr.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 74.Bhattarai D.P., et al. A review on properties of natural and synthetic based electrospun fibrous materials for bone tissue engineering. Membranes. 2018;8(3):62. doi: 10.3390/membranes8030062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sanjay M., et al. Characterization and properties of natural fiber polymer composites: a comprehensive review. J. Clean. Prod. 2018;172:566–581. [Google Scholar]
- 76.Thomas S., et al. CRC press; 2012. Natural Polymers, Biopolymers, Biomaterials, and Their Composites, Blends, and IPNs. [Google Scholar]
- 77.Stumpf T.R., et al. In situ and ex situ modifications of bacterial cellulose for applications in tissue engineering. Mater. Sci. Eng. C. 2018;82:372–383. doi: 10.1016/j.msec.2016.11.121. [DOI] [PubMed] [Google Scholar]
- 78.Mohammad S.M., et al. An overview of biocellulose production using Acetobacter xylinum culture. Adv. Biol. Res. 2014;8(6):307–313. [Google Scholar]
- 79.Seddiqi H., et al. Cellulose and its derivatives: towards biomedical applications. Cellulose. 2021;28(4):1893–1931. [Google Scholar]
- 80.Mu R., et al. Recent trends and applications of cellulose nanocrystals in food industry. Trends Food Sci. Technol. 2019;93:136–144. [Google Scholar]
- 81.Rahman M.S., et al. Recent developments of carboxymethyl cellulose. Polymers. 2021;13(8):1345. doi: 10.3390/polym13081345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li P., Wu G. Roles of dietary glycine, proline, and hydroxyproline in collagen synthesis and animal growth. Amino Acids. 2018;50:29–38. doi: 10.1007/s00726-017-2490-6. [DOI] [PubMed] [Google Scholar]
- 83.Joyce K., et al. Bioactive potential of natural biomaterials: identification, retention and assessment of biological properties. Signal Transduct. Targeted Ther. 2021;6(1):122. doi: 10.1038/s41392-021-00512-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Burgeson R.E., Nimni M.E. Collagen types. Molecular structure and tissue distribution. Clin. Orthop. Relat. Res. 1992;(282):250–272. [PubMed] [Google Scholar]
- 85.Chevallay B., Herbage D. Collagen-based biomaterials as 3D scaffold for cell cultures: applications for tissue engineering and gene therapy. Med. Biol. Eng. Comput. 2000;38:211–218. doi: 10.1007/BF02344779. [DOI] [PubMed] [Google Scholar]
- 86.Wolf K., et al. Seminars in Cell & Developmental Biology. Elsevier; 2009. Collagen-based cell migration models in vitro and in vivo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wu X., et al. Tissue engineering in female pelvic floor reconstruction. Eng. Life Sci. 2020;20(7):275–286. doi: 10.1002/elsc.202000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chow D., et al. Peptide-based biopolymers in biomedicine and biotechnology. Mater. Sci. Eng. R Rep. 2008;62(4):125–155. doi: 10.1016/j.mser.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cui F.-Z., Li Y., Ge J. Self-assembly of mineralized collagen composites. Mater. Sci. Eng. R Rep. 2007;57(1–6):1–27. [Google Scholar]
- 90.Barreto J.A., et al. Nanomaterials: applications in cancer imaging and therapy. Adv. Mater. 2011;23(12):H18–H40. doi: 10.1002/adma.201100140. [DOI] [PubMed] [Google Scholar]
- 91.Pan T.-L., et al. Toxicological effects of cationic nanobubbles on the liver and kidneys: biomarkers for predicting the risk. Food Chem. Toxicol. 2012;50(11):3892–3901. doi: 10.1016/j.fct.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 92.Shan D., Ma C., Yang J. Enabling biodegradable functional biomaterials for the management of neurological disorders. Adv. Drug Deliv. Rev. 2019;148:219–238. doi: 10.1016/j.addr.2019.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Abdollahiyan P., Oroojalian F., Mokhtarzadeh A. The triad of nanotechnology, cell signalling, and scaffold implantation for the successful repair of damaged organs: an overview on soft-tissue engineering. J. Contr. Release. 2021;332:460–492. doi: 10.1016/j.jconrel.2021.02.036. [DOI] [PubMed] [Google Scholar]
- 94.Grabrucker A.M., et al. Nanoparticle transport across the blood brain barrier. Tissue Barriers. 2016;4(1) doi: 10.1080/21688370.2016.1153568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Abbott N.J., et al. Structure and function of the blood–brain barrier. Neurobiol. Dis. 2010;37(1):13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 96.Topal G.R., et al. ApoE-targeting increases the transfer of solid lipid nanoparticles with donepezil cargo across a culture model of the blood–brain barrier. Pharmaceutics. 2020;13(1):38. doi: 10.3390/pharmaceutics13010038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen Y., Liu L. Modern methods for delivery of drugs across the blood–brain barrier. Adv. Drug Deliv. Rev. 2012;64(7):640–665. doi: 10.1016/j.addr.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 98.Cano A., et al. Current advances in the development of novel polymeric nanoparticles for the treatment of neurodegenerative diseases. Nanomedicine. 2020;15(12):1239–1261. doi: 10.2217/nnm-2019-0443. [DOI] [PubMed] [Google Scholar]
- 99.Pardridge W.M. Blood-brain barrier carrier-mediated transport and brain metabolism of amino acids. Neurochem. Res. 1998;23:635–644. doi: 10.1023/a:1022482604276. [DOI] [PubMed] [Google Scholar]
- 100.Geldenhuys W., et al. Brain-targeted delivery of paclitaxel using glutathione-coated nanoparticles for brain cancers. J. Drug Target. 2011;19(9):837–845. doi: 10.3109/1061186X.2011.589435. [DOI] [PubMed] [Google Scholar]
- 101.Du D., et al. The role of glucose transporters in the distribution of p-aminophenyl-α-d-mannopyranoside modified liposomes within mice brain. J. Contr. Release. 2014;182:99–110. doi: 10.1016/j.jconrel.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 102.Puris E., et al. L-Type amino acid transporter 1 as a target for drug delivery. Pharmaceut. Res. 2020;37:1–17. doi: 10.1007/s11095-020-02826-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Park T.-E., et al. Enhanced BBB permeability of osmotically active poly (mannitol-co-PEI) modified with rabies virus glycoprotein via selective stimulation of caveolar endocytosis for RNAi therapeutics in Alzheimer's disease. Biomaterials. 2015;38:61–71. doi: 10.1016/j.biomaterials.2014.10.068. [DOI] [PubMed] [Google Scholar]
- 104.Monsalve Y., et al. PEG-g-chitosan nanoparticles functionalized with the monoclonal antibody OX26 for brain drug targeting. Nanomedicine. 2015;10(11):1735–1750. doi: 10.2217/nnm.15.29. [DOI] [PubMed] [Google Scholar]
- 105.Muniswamy V.J., et al. ‘Dendrimer-Cationized-Albumin’encrusted polymeric nanoparticle improves BBB penetration and anticancer activity of doxorubicin. Int. J. Pharm. 2019;555:77–99. doi: 10.1016/j.ijpharm.2018.11.035. [DOI] [PubMed] [Google Scholar]
- 106.Fornaguera C., et al. PLGA nanoparticles prepared by nano-emulsion templating using low-energy methods as efficient nanocarriers for drug delivery across the blood–brain barrier. J. Contr. Release. 2015;211:134–143. doi: 10.1016/j.jconrel.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 107.Zhang Y., Lu C., Zhang J. Lactoferrin and its detection methods: a review. Nutrients. 2021;13(8):2492. doi: 10.3390/nu13082492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cui J., et al. Gather wisdom to overcome barriers: well-designed nano-drug delivery systems for treating gliomas. Acta Pharm. Sin. B. 2022;12(3):1100–1125. doi: 10.1016/j.apsb.2021.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Spindler K.R., Hsu T.-H. Viral disruption of the blood–brain barrier. Trends Microbiol. 2012;20(6):282–290. doi: 10.1016/j.tim.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Orive G., et al. Biomaterials for promoting brain protection, repair and regeneration. Nat. Rev. Neurosci. 2009;10(9):682–692. doi: 10.1038/nrn2685. [DOI] [PubMed] [Google Scholar]
- 111.Khan N.H., et al. Nanomedicine: a Promising way to manage Alzheimer's disease. Front. Bioeng. Biotechnol. 2021;9 doi: 10.3389/fbioe.2021.630055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hillaireau H., Couvreur P. Nanocarriers' entry into the cell: relevance to drug delivery. Cell. Mol. Life Sci. 2009;66:2873–2896. doi: 10.1007/s00018-009-0053-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shang L., Nienhaus K., Nienhaus G.U. Engineered nanoparticles interacting with cells: size matters. J. Nanobiotechnol. 2014;12(1):1–11. doi: 10.1186/1477-3155-12-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Vácha R., Martinez-Veracoechea F.J., Frenkel D. Receptor-mediated endocytosis of nanoparticles of various shapes. Nano Lett. 2011;11(12):5391–5395. doi: 10.1021/nl2030213. [DOI] [PubMed] [Google Scholar]
- 115.Cheng K.K., et al. Highly stabilized curcumin nanoparticles tested in an in vitro blood–brain barrier model and in Alzheimer's disease Tg2576 mice. AAPS J. 2013;15:324–336. doi: 10.1208/s12248-012-9444-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lazar A.N., et al. Curcumin-conjugated nanoliposomes with high affinity for Aβ deposits: possible applications to Alzheimer disease. Nanomed. Nanotechnol. Biol. Med. 2013;9(5):712–721. doi: 10.1016/j.nano.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 117.Ali T., et al. Anthocyanin-loaded PEG-gold nanoparticles enhanced the neuroprotection of anthocyanins in an Aβ 1–42 mouse model of Alzheimer's disease. Mol. Neurobiol. 2017;54:6490–6506. doi: 10.1007/s12035-016-0136-4. [DOI] [PubMed] [Google Scholar]
- 118.Chakraborty A., et al. Impact of nanoparticles on amyloid β-induced Alzheimer's disease, tuberculosis, leprosy and cancer: a systematic review. Biosci. Rep. 2023;43(2) doi: 10.1042/BSR20220324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dhas N., Mehta T. Cationic biopolymer functionalized nanoparticles encapsulating lutein to attenuate oxidative stress in effective treatment of Alzheimer's disease: a non-invasive approach. Int. J. Pharm. 2020;586 doi: 10.1016/j.ijpharm.2020.119553. [DOI] [PubMed] [Google Scholar]
- 120.Zameer S., et al. Development, optimisation and evaluation of chitosan nanoparticles of alendronate against Alzheimer's disease in intracerebroventricular streptozotocin model for brain delivery. J. Drug Target. 2021;29(2):199–216. doi: 10.1080/1061186X.2020.1817041. [DOI] [PubMed] [Google Scholar]
- 121.Vaz G.R., et al. Development of nasal lipid nanocarriers containing curcumin for brain targeting. J. Alzheim. Dis. 2017;59(3):961–974. doi: 10.3233/JAD-160355. [DOI] [PubMed] [Google Scholar]
- 122.Park S.-Y., et al. Curcumin protected PC12 cells against beta-amyloid-induced toxicity through the inhibition of oxidative damage and tau hyperphosphorylation. Food Chem. Toxicol. 2008;46(8):2881–2887. doi: 10.1016/j.fct.2008.05.030. [DOI] [PubMed] [Google Scholar]
- 123.Yang F., et al. Curcumin inhibits formation of amyloid β oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J. Biol. Chem. 2005;280(7):5892–5901. doi: 10.1074/jbc.M404751200. [DOI] [PubMed] [Google Scholar]
- 124.Begum A.N., et al. Curcumin structure-function, bioavailability, and efficacy in models of neuroinflammation and Alzheimer's disease. J. Pharmacol. Exp. Therapeut. 2008;326(1):196–208. doi: 10.1124/jpet.108.137455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Liu W., et al. Oral bioavailability of curcumin: problems and advancements. J. Drug Target. 2016;24(8):694–702. doi: 10.3109/1061186X.2016.1157883. [DOI] [PubMed] [Google Scholar]
- 126.Taleghani A.S., et al. Mesoporous silica nanoparticles as a versatile nanocarrier for cancer treatment: a review. J. Mol. Liq. 2021;328 [Google Scholar]
- 127.Bollu V.S., et al. Curcumin-loaded silica-based mesoporous materials: synthesis, characterization and cytotoxic properties against cancer cells. Mater. Sci. Eng. C. 2016;63:393–410. doi: 10.1016/j.msec.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 128.Sabio R.M., et al. New insights towards mesoporous silica nanoparticles as a technological platform for chemotherapeutic drugs delivery. Int. J. Pharm. 2019;564:379–409. doi: 10.1016/j.ijpharm.2019.04.067. [DOI] [PubMed] [Google Scholar]
- 129.Long Y., et al. Nose to brain drug delivery-a promising strategy for active components from herbal medicine for treating cerebral ischemia reperfusion. Pharmacol. Res. 2020;159 doi: 10.1016/j.phrs.2020.104795. [DOI] [PubMed] [Google Scholar]
- 130.Kumar R., et al. Advances in nanotechnology and nanomaterials based strategies for neural tissue engineering. J. Drug Deliv. Sci. Technol. 2020;57 [Google Scholar]
- 131.Agrawal M., et al. Nose-to-brain drug delivery: an update on clinical challenges and progress towards approval of anti-Alzheimer drugs. J. Contr. Release. 2018;281:139–177. doi: 10.1016/j.jconrel.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 132.Aderibigbe B.A., Naki T. Chitosan-based nanocarriers for nose to brain delivery. Appl. Sci. 2019;9(11):2219. [Google Scholar]
- 133.Agrawal M., et al. Stimuli-responsive in situ gelling system for nose-to-brain drug delivery. J. Contr. Release. 2020;327:235–265. doi: 10.1016/j.jconrel.2020.07.044. [DOI] [PubMed] [Google Scholar]
- 134.Ribeiro T.d.C., et al. Curcumin-loaded mesoporous silica nanoparticles dispersed in thermo-responsive hydrogel as potential alzheimer disease therapy. Pharmaceutics. 2022;14(9):1976. doi: 10.3390/pharmaceutics14091976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Agrawal M., et al. Biomaterials in treatment of Alzheimer's disease. Neurochem. Int. 2021;145 doi: 10.1016/j.neuint.2021.105008. [DOI] [PubMed] [Google Scholar]
- 136.Sanati M., et al. Impact of gold nanoparticles on amyloid β-induced Alzheimer's disease in a rat animal model: involvement of STIM proteins. ACS Chem. Neurosci. 2019;10(5):2299–2309. doi: 10.1021/acschemneuro.8b00622. [DOI] [PubMed] [Google Scholar]
- 137.Patra J.K., et al. Nano based drug delivery systems: recent developments and future prospects. J. Nanobiotechnol. 2018;16(1):1–33. doi: 10.1186/s12951-018-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Laouini A., et al. Preparation, characterization and applications of liposomes: state of the art. Journal of colloid Science and Biotechnology. 2012;1(2):147–168. [Google Scholar]
- 139.Malam Y., Loizidou M., Seifalian A.M. Liposomes and nanoparticles: nanosized vehicles for drug delivery in cancer. Trends Pharmacol. Sci. 2009;30(11):592–599. doi: 10.1016/j.tips.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 140.Le-Vinh B., et al. Chitosan based micelle with zeta potential changing property for effective mucosal drug delivery. Int. J. Biol. Macromol. 2019;133:647–655. doi: 10.1016/j.ijbiomac.2019.04.081. [DOI] [PubMed] [Google Scholar]
- 141.Borhan M., et al. Green extraction: enhanced extraction yield of asiatic acid from Centella asiatica (L.) nanopowders. J. Appl. Chem. 2013;2013 [Google Scholar]
- 142.Dhas N., et al. Factorial design-based fabrication of biopolymer-functionalized Asiatic acid-embedded liposomes: in-vitro characterization and evaluation. J. Appl. Pharmaceut. Sci. 2022;12(11):71–81. [Google Scholar]
- 143.Re F., Gregori M., Masserini M. Nanotechnology for neurodegenerative disorders. Maturitas. 2012;73(1):45–51. doi: 10.1016/j.maturitas.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 144.Bana L., et al. Liposomes bi-functionalized with phosphatidic acid and an ApoE-derived peptide affect Aβ aggregation features and cross the blood–brain-barrier: implications for therapy of Alzheimer disease. Nanomed. Nanotechnol. Biol. Med. 2014;10(7):1583–1590. doi: 10.1016/j.nano.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 145.Mancini S., et al. Multifunctional liposomes delay phenotype progression and prevent memory impairment in a presymptomatic stage mouse model of Alzheimer disease. J. Contr. Release. 2017;258:121–129. doi: 10.1016/j.jconrel.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 146.Wells C.M., et al. Stimuli-responsive drug release from smart polymers. J. Funct. Biomater. 2019;10(3):34. doi: 10.3390/jfb10030034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zhuo S., et al. pH-sensitive biomaterials for drug delivery. Molecules. 2020;25(23):5649. doi: 10.3390/molecules25235649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Georgieva D., et al. Thermoresponsive poly (N-isopropylacrylamide) copolymer networks for galantamine hydrobromide delivery. Colloid Polym. Sci. 2020;298:377–384. [Google Scholar]
- 149.Li W., et al. Remote modulation of neural activities via near-infrared triggered release of biomolecules. Biomaterials. 2015;65:76–85. doi: 10.1016/j.biomaterials.2015.06.041. [DOI] [PubMed] [Google Scholar]
- 150.Igartúa D.E., et al. PAMAM dendrimers as a carbamazepine delivery system for neurodegenerative diseases: a biophysical and nanotoxicological characterization. Int. J. Pharm. 2018;544(1):191–202. doi: 10.1016/j.ijpharm.2018.04.032. [DOI] [PubMed] [Google Scholar]
- 151.Gothwal A., et al. Lactoferrin coupled lower generation PAMAM dendrimers for brain targeted delivery of memantine in aluminum-chloride-induced Alzheimer's disease in mice. Bioconjugate Chem. 2019;30(10):2573–2583. doi: 10.1021/acs.bioconjchem.9b00505. [DOI] [PubMed] [Google Scholar]
- 152.Al-Azzawi S., et al. Dendrimeric poly (Epsilon-Lysine) delivery systems for the enhanced permeability of flurbiprofen across the blood-brain barrier in Alzheimer's disease. Int. J. Mol. Sci. 2018;19(10):3224. doi: 10.3390/ijms19103224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sathya S., Shanmuganathan B., Devi K.P. Deciphering the anti-apoptotic potential of α-bisabolol loaded solid lipid nanoparticles against Aβ induced neurotoxicity in Neuro-2a cells. Colloids Surf. B Biointerfaces. 2020;190 doi: 10.1016/j.colsurfb.2020.110948. [DOI] [PubMed] [Google Scholar]
- 154.Ciechanover A. Proteolysis: from the lysosome to ubiquitin and the proteasome. Nat. Rev. Mol. Cell Biol. 2005;6(1):79–87. doi: 10.1038/nrm1552. [DOI] [PubMed] [Google Scholar]
- 155.Dorbeck‐Jung B.R., Chowdhury N. Is the European medical products authorisation regulation equipped to cope with the challenges of nanomedicines? Law Pol. 2011;33(2):276–303. [Google Scholar]
- 156.Blagov A.V., et al. Role of impaired mitochondrial dynamics processes in the pathogenesis of Alzheimer's disease. Int. J. Mol. Sci. 2022;23(13):6954. doi: 10.3390/ijms23136954. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This is a review; therefore, all data are included in the manuscript.