Abstract
A screen for host mutations which increase the rate of transposition of Ty1 and Ty2 into a chromosomal target was used to identify factors influencing retroelement transposition. The fortuitous presence of a mutation in the CAC3 gene in the strain in which this screen was undertaken enabled us to discover that double mutaions of cac3 and hir3, but neither of the two single mutations, caused a dramatic increase in the rate of retrotransposition. We further showed that this effect was not due to an increase in the overall level of Ty1 mRNA. Two subtle cac3 phenotypes, slight methyl methanesulfonate (MMS) sensitivity and reduction of telomeric silencing, were significantly enhanced in the cac3 hir3 double mutant. In addition, the growth rate of the double mutant was reduced. HIR3 belongs to a class of HIR genes that regulate the transcription of histones, while Cac3p, together with Cac1p and Cac2p, forms chromatin assembly factor I. Other combinations of mutations in cac and hir genes (cac3 hir1, cac3 hir2, and cac2 hir3) also increase Ty transposition and MMS sensitivity and reduce the growth rate. A model explaining the synergistic interaction between cac and hir mutations in terms of alterations in chromatin structure is proposed.
Retroviruses are currently under intensive study because they can elicit malignant tumors and cause AIDS. Furthermore, at least 0.1 to 0.6% of the human genome is composed of endogenous retroviruses and long-terminal-repeat (LTR)-containing retrotransposons, which resemble retroviruses in their structural organization and mode of transposition (38). The life cycle of these elements begins with the transcription of an integrated DNA copy of the element and the incorporation of the transcribed RNA into a viruslike particle composed of element-encoded proteins, including the capsid protein, protease, reverse transcriptase, and integrase. The RNA is then reverse transcribed into cDNA, which integrates into a new chromosomal location in the host cell (for a review, see reference 5). Such integration is required for retroviruses to induce disease, for example, by activating a nearby cellular proto-oncogene (70). Likewise, insertions of the yeast Ty1 element (5) can alter the regulation of nearby cellular genes.
Common laboratory yeast (Saccharomyces cerevisiae) strains contain five types of Ty elements composed of central regions of DNA flanked by LTRs. Structural proteins and enzymatic activity are encoded within the central region (for reviews, see references 5 and 22). Ty1, Ty2, Ty4, and Ty5 elements are members of the copia class of retrotransposons, while Ty3 elements belong to the gypsy class. Ty1 and Ty2 elements are respectively present at about 30 to 35 and 5 to 15 copies per genome, contain the same LTR sequences (called delta elements), and have very similar internal regions. The more distantly related retrotransposons Ty3, Ty4, and Ty5 have different sets of LTRs, are present in lower copy numbers, and have not been observed to be insertional mutagens.
Yeast retrotransposons provide an attractive model system in which to define host functions required for retroviral transposition. Mutations that reduce Ty1 and Ty2 transcription levels also reduce transposition (6, 18, 19, 73, 74), since Ty elements transpose through RNA intermediates (3). Transcription of Ty1 and Ty2 elements is also inhibited in mating-incompetent cells (52) and by growth on glycerol rather than glucose (69). Also, both the Ty1 and Ty2 RNA levels and transposition rates are increased by DNA damage (7). In addition, a number of conditions and mutations alter Ty1 transposition via posttranscriptional mechanisms, including translation of Ty-encoded open reading frames (ORFs) (22), reverse transcription of the mRNA into a cDNA copy (10, 11), and growth at 20°C (51, 52).
The ubiquitin-conjugating enzyme Rad6p (Ubc2p) also affects Ty1 transposition. Mutations in RAD6 increase the overall rate of Ty1 transposition into CAN1 and SUP4 without causing an increase in the level of total Ty1 RNA (8, 31, 54). However, when genetically marked Ty1 elements were used, it was determined that the deletion of RAD6 affected the transposition of some, but not other, elements, and these effects were at the transcriptional level (8). The recent findings that mutations in RAD6 release transcriptional silencing at telomeres and HM loci (28) and that rad6 mutations enhance the transcription of marked Ty1 elements located in silent rDNA chromatin regions (8) are consistent with the hypothesis that mutations in RAD6 cause alterations in chromatin structure in certain chromosomal regions, making it easier for Ty1 elements to integrate. Ubiquitination has also been shown to affect Ty3 transposition. Cellular stress, induced by growth at high temperature or in ethanol, inhibits transposition of Ty3 without affecting Ty3 transcription. This inhibition can be reversed by overexpression of a protease that cleaves ubiquitin off proteins (43).
In this paper, we describe a genetic screen designed to identify mutations that increase the rate of transposition of Ty1 and/or the closely related Ty2 elements (referred to henceforth together as Ty1 for simplicity). Using this screen, we have uncovered a synthetic interaction between mutations in CAC3 and HIR3 that leads to a dramatic increase in Ty1 transposition rates without affecting the overall levels of Ty1 transcription. Simultaneous loss of both genes also increases methyl methanesulfonate (MMS) and UV sensitivities and reduces telomeric silencing and the growth rate. Since CAC3 encodes a component of chromatin assembly factor I (CAF-I) (34) and HIR3 controls the levels and balance of histone mRNAs (45, 50, 57), our results suggest that alterations in chromatin structure can increase the efficiency of integration of Ty1 cDNA into the host genome.
MATERIALS AND METHODS
Strains, cultivation conditions, and scoring for markers.
Standard yeast cultivation conditions were used (66). Cells were grown on organic complete medium (yeast extract-peptone-dextrose [YPD]) or synthetic complete medium (SC) lacking nutrients (e.g., uracil [−Ura]). Ura− colonies were selected on medium containing 1 g of 5-fluoro-orotic acid and 12 mg of uracil per liter (+FOA) (4).
Yeast strains used in this study are listed in Table 1. L1356 was made by replacing the LYS2 locus in SL984-6B (his3-Δ200) with a lys2::his3-Δ4 allele. SL984-6B colonies transformed with pCB1 (carrying lys2::his3-Δ4 and URA3), which had been linearized with BstEII to target integration into the chromosomal LYS2 locus, were selected on −Ura. Selection for plasmid excision on +FOA medium and subsequent screening for Lys− colonies resulted in the desired transplacement (Fig. 1), which was verified by DNA blot analysis (for details, see reference 9). SL1006 meiotic segregants are from a cross of L1356 with SL1005-54. SL1005-54 is a random spore obtained from a cross of L1356 with DC042.
TABLE 1.
Yeast strains used in this study
| Straina | Genotype |
|---|---|
| SL984-6B | MATa ura3-52 his3-Δ200 leu2-1 met8-1 ilv1-1 trp1-901 cac3-1 |
| L1356 | MATa ura3-52 his3-Δ200 leu2-1 met8-1 ilv1-1 trp1-901 cac3-1 lys2::his3Δ4 |
| L1561 | L1356 hir3-2 (also called htr1) |
| L1562 | L1356 rad1::LEU2 |
| L1563 | L1356 rad52::URA3 |
| L1635 | L1356 rad6::LEU2 |
| L1675 | L1356 hir3::LEU2 |
| L1680 | L1356 hir1::URA3 |
| L1681 | L1356 hir2::URA3 |
| L1685 | L1356 cac2::TRP1 hir3::LEU2 [pCAC3] |
| SL1005-54 | MATα his3-Δ200 leu2-1 lys2::his3-Δ4 ade1 cac3-1 |
| SL1006-1B | MATα his3-Δ200 leu2-1 lys2::his3-Δ4 ura3-52 trp1-901 met8-1 cac3-1 hir3-2 |
| SL1006-1D | MATα his3-Δ200 leu2-1 lys2::his3-Δ4 trp1-901 met8-1 cac3-1 |
| SL1006-7B | MATα his3-Δ200 leu2-1 lys2::his3-Δ4 trp1-901 ade1 cac3-1 hir3-2 |
| DC042 | MATα ade1 leu2 |
| W303 | MATa ura3-1 leu2-3,112 ade2-1 trp1-1 his3-11,15 can1-100 |
| W303Δ3 | MATa hir3::HIS3 ura3-1 leu2-3,112 ade2-1 trp1-1 his3-11,15 can1-100 |
| UCC4543 | MATa ura3-52 lys2-801 ade2-101 trp1-Δ63 his3-Δ200 leu2-Δ1 adh4::SUP4-o-TELb |
| L1698 | UCC4543 hir1::URA3 |
| L1699 | UCC4543 hir2::URA3 |
| L1687 | UCC4543 hir3::LEU2 |
| L1678 | UCC4543 cac3::hisG-URA3-kan-hisG |
| L1688 | L1678 hir3::LEU2 |
| L1700 | UCC4543 cac3::hisG |
| L1701 | L1700 hir1::URA3 |
| L1702 | L1700 hir2::URA3 |
| JC364 | MATa ura3-167 his3-Δ200 leu2Δ-hisG trp1Δ-hisG Ty588::neo Ty146(tyb::lacZ) Ty1-270his3-AI |
| L1689 | JC364 cac3::hisG-URA3-kan-hisG |
| L1690 | L1689 hir3::LEU2 |
| YJB2306 | MATa/MATα ade2-1/ade2-1 can1-100/can1-100 his3-11,15/his3-11,15 leu2-3,112/leu2-3,112 trp1-1/trp1-1 ura3-1/ura3-1 |
DC042, W303, W303Δ3, UCC4543, and JC364 were kindly provided by M. Olson, R. Rothstein, M. A. Osley, D. Gottschling, and D. Garfinkel, respectively.
SUP4-o-TEL, SUP4-o located near telomere VII-L.
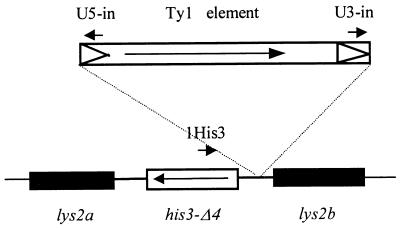
FIG. 1.
Ty1 transposition assay. The most common way for the His− strains containing the promoterless his3-Δ4 allele diagrammed above to revert to His+ is by the insertion of an upstream Ty1 element. Such insertions were detected by the appearance of 400- to 500-bp PCR amplicons when the HIS3 (1His3) and delta element (U5-in) primers were used. No insertions were detected when the HIS3 (1His3) and delta element (U3-in) primers were used, indicating that the Ty1 elements had to be in the indicated orientation to cause a His+ reversion. Arrows in boxes indicate the direction of transcription.
The htr1 transposition phenotype was scored by estimating the frequency of His+ revertants in independent colonies of approximately equal sizes. Strains were scored for the hir3 phenotype as described previously (67).
Deletions-disruptions of RAD1, RAD6, RAD52, CAC2, CAC3, HIR1, and HIR2 were made in L1356 or UCC4543 (Table 1) by using the one-step transplacement method (62) and the appropriate plasmid (described below). Null alleles of HIR3 were made by a previously described sticky-end PCR method (41), using pZJ31 as a template and primer pairs 170 (CCCCGGCGCGCCCCCCCAAATAGACGGTAGCAAGGC)-171 (CTCTTGAAGATGCGAGCCAG) and 172 (CAAGACAAGAGCTACGTGGA)-173 (GGGGGGCGCGCCGGGGCACACTTCTTCCAAAATATG). Sticky extensions (indicated by underlines) carried an AscI site. The annealed fragments were then amplified with primers 171 and 172, and the product was ligated into SmaI-digested YIplac128 (LEU2) (24) (kindly supplied by R. Gietz) to generate pZJ49. AscI-linearized pZJ49 was then transformed into L1356 to make hir3 deletions. Yeast transformations were performed by the lithium acetate procedure (29). The rad1, rad6, and rad52 deletions were confirmed by complementation tests. All other deletions were confirmed by PCR.
Plasmids.
The URA3-integrative plasmid pCB1 (9) contains a 1.37-kb SmaI-BamHI fragment from pAB100 (63) carrying his3-Δ4, which replaces the internal HpaI-BamHI fragment of a LYS2 gene on the plasmid. The YCp50 (61)-based CEN-URA3 plasmids pHIR3 (which contains HIR3) and pCAC3 (which carries CAC3) were partially digested with EcoRI or HindIII and self-ligated to generate the deletion plasmids diagrammed in Fig. 2B. To generate CAC3 deletion plasmid pZJ13, a BglII-BamHI fragment containing hisG-URA3-hisG (1) (kindly supplied by E. Alani), cloned into the BglII site of the XbaI-SalI subclone of pCAC3 in Bluescript II KS, was partially digested with ClaI and self-ligated.
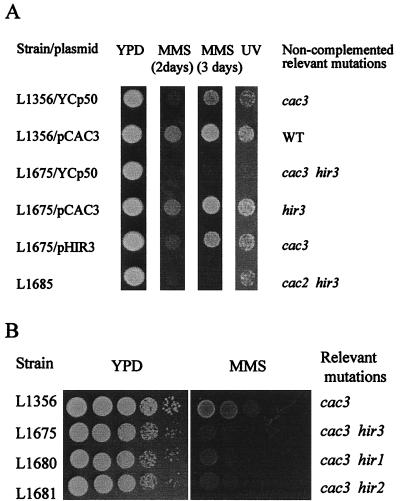
FIG. 2.
Characterization of the htr1 mutant. (A) Complementation of htr1 MMS sensitivity. Transformants of L1356 (wild type [WT]) and L1561 (htr1) with the indicated plasmids (YCp50, pCAC3, and pHIR3) were spotted from −Ura onto YPD and YPD plus 0.02% MMS. (B) Localization of htr1-complementing activity. Restriction maps of the cloned fragments in plasmids pCAC3 (top) and pHIR3 (bottom) and subcloned DNA fragments described in the text are shown. +, MMS sensitivity was complemented by the fragment; −, no complementation occurred. Arrows indicate the direction of transcription. Restriction sites are abbreviated as follows: B, BamHI; Bg, BglII; Bs, BssHII; C, ClaI; E, EcoRI; H, HindIII; K, KpnI; S, SalI; Sn, SnaBI; Sp, SphI; P, PstI; X, XhoI; and Xb, XbaI. (C) Deletion of HIR3 does not affect levels of Ty1 mRNA. RNA blot analysis of Ty1 (and Ty2) versus TEF1 (internal control) mRNAs from L1356 and the isogenic hir3::LEU2 deletion strain, L1675.
The following plasmids were used to create deletions-disruptions: pRR46 (58) (kindly supplied by L. Prakash), containing rad1::LEU2; pSM22 (kindly supplied by D. Schild), containing rad52::URA3; pJJ105, containing rad6::LEU2 (46) (kindly supplied by L. Prakash); pZJ13, containing cac3::URA3 (Fig. 2B); pPK104, containing cac3::hisG-URA3-kan-hisG (34) (kindly supplied by P. Kaufman); and pHIR1::URA3 and pHIR2::URA3 (68) (kindly supplied by M. A. Osley).
pHIR3-HA, containing a gene with the normal HIR3 promoter encoding Hir3p tagged with three HA moieties right before the stop codon, was constructed by previously described PCR methods (41, 64). Primer pair 156 (TATTAGTGGATAAGATCACGAGGGAACAAAG [underlined sequence is complementary to that of primer 160)-157 (GTACTTATCTAGAGCGGCCGCACTG) used the pMPY-3xHA template. The end of the HIR3 ORF, including the BssHII site (Fig. 2B), was amplified with primers 159 (CAACGACGAAGGTCTTGCAT) and 160 (CGTGATCTTATCCACTAATAG). The annealed fragments were then amplified with primers 159 and 157, and the product was ligated into SmaI-digested YEplac195 (24) to generate pZJ53. The KpnI-BssHI fragment in PZJ52 (containing a 10.5-kb KpnI-PstI pHIR3 fragment in YEplac195) was replaced by the KpnI-BssHI piece from pZJ53 to generate pHIR3-HA.
Screen for mutants with high rates of Ty1 transposition.
L1356 mutagenized with ethyl methanesulfonate to 20 to 40% survival was incubated on YPD at 20°C for 2 weeks. Colonies consisting of approximately 5 × 107 to 1 × 108 cells were spread on −His (or −His containing 0.0125% Casamino Acids so that His− cells could divide for a few generations). After 3 days at 20°C, which increased the transposition rate to a level more easily measured, the plates were incubated at 30°C for another week and then the number of His+ revertants per plate was counted.
Transposition rate measurements.
Most transposition rates were measured by the his3-Δ4 assay. In this case, rates of mutation to His+ were calculated by fluctuation tests, using the equation P0 = e−Nμ (40), or by the method of the median, using the equation μ = f/ln(Nμ) (17). Cells spread on −Ura (to retain plasmids) or YPD were incubated at 20°C until the colonies consisted of about 108 cells (on YPD) or 5 × 107 cells (on −Ura). For each rate measurement, 10 (or in some cases 20) colonies of equal size were scooped up and each entire colony was spread on a −His plate. Two or three additional colonies of equal size were used to determine cell viability. We used PCR to estimate how many of the His+ colonies resulted from a Ty1 transposition (Fig. 1). Representative His+ colonies were analyzed with the HIS3 primer 1His3 (TGTAATACGCTTTACTAGG) and Ty primers U5-in (ATTGTTGGGATTCCATT) and U3-in (ATATTATCATATACGGTGTT). Since Ty1 insertions were found in 90% or more of the His+ revertants, the His+ revertant rate was used as an estimate of the Ty1 transposition rate.
Transposition was also assayed as described by Curcio and Garfinkel (14, 15), using a genomic Ty1 element containing the his3-AI retrotransposition indicator gene (77). The inversely oriented his3-AI gene marking the Ty1 element is inactive because it is disrupted by an intron in the antisense orientation relative to the his3 gene, but the intron is spliced out during Ty1 transposition, leading to the appearance of a functional HIS3 gene. Strain JC364 (77) (kindly supplied by D. Garfinkel), which contains the Ty1-270his3-AI transposition reporter, was disrupted first at the CAC3 gene and then also at the HIR3 gene, with pPK104 and pZJ49, respectively, to make L1689 and L1690. Strains JC364, L1689, and L1690 were grown to saturation in liquid SC at 20°C. The His+ frequencies (the number of His+ prototrophs divided by the total number of colonies) of three independent cultures were averaged to assay the transposition efficiency.
The final Ty1 transposition assay has been described by us previously (39, 54) and involves determining the rate at which normal endogenous Ty1 elements inactivate the CAN1 gene at 20°C. Rates of mutation to canavanine resistance were calculated by the method of the median, using equation μ = f/ln(Nμ) (17), and the fraction of can1 mutants that contained Ty1 elements was determined by DNA blot analysis.
Analyses of DNA, RNA, and protein.
Restriction fragments to be sequenced were subcloned into Bluescript II KS (Stratagene) and sequenced by the dideoxy chain termination method, using Sequenase version 2.0 (U.S. Biochemical Corp.). The cac3 alleles from L1356 and L1561 were cloned for sequencing by gap repair (48) of BssHII- and SnaBI-digested pCAC3.
RNA and DNA blot analyses were performed as described previously (9, 54). The probes used to detect Ty1 and TEF1 mRNAs were, respectively, a 5.6-kb XhoI fragment isolated from pNN166 and an 840-bp EcoRI-HindIII fragment from pSP36. Western blot analysis was performed on cells transformed with pHIR3-HA or pHIR3, using a 1:1,000 dilution of primary antihemagglutinin (anti-HA) mouse monoclonal antibody (BAbCo, Inc., Berkeley, Calif.).
Indirect immunofluorescence and immunogold labeling.
Indirect immunofluorescence was performed as previously described (21), using a 1:5,000 dilution of anti-HA mouse monoclonal antibody, rabbit polyclonal anti-Rap1, and anti-nuclear pore (BAbCo, Inc.) as well as a fluorescein isothiocyanate-conjugated secondary antibody. Processing and immunogold labeling were performed as described elsewhere (2) with the changes noted below. Cells fixed overnight at 4°C in buffer (4% sucrose in 100 mM sodium phosphate [pH 7.5]) containing 1% acrolein and 4% paraformaldehyde were washed twice with this same buffer for 10 min and then dehydrated at room temperature in an ethylene glycol concentration gradient (25, 50, 75, 90, and 90% for 15, 15, 15, 10, and 10 min, respectively). Infiltration was carried out over a period of 3 days. The primary antibody, HA mouse monoclonal antibody, was used at a 1:1,000 dilution. The secondary antibody was 10-mm gold particles attached to goat anti-mouse antibody (Nanoprobe, Inc., no. CG1001). Grids stained with 1% (wt/vol) uranyl acetate (aqueous) for 10 min and then washed three times for 5 min each in distilled water were stained for 4 min with lead citrate.
RESULTS
An assay for Ty1 transposition.
The assay used to determine Ty1 transposition rates was based on the observation that His+ revertants of a plasmidborne his3-Δ4 allele that lacks a promoter result either from insertions of Ty1 into the region upstream of his3-Δ4 or from plasmid rearrangements (3, 63). To eliminate the occurrence of His+ revertants due to plasmid rearrangements, we made a haploid strain, L1356, that bears a large deletion at the normal HIS3 locus and an insertion of the his3-Δ4 allele in its genomic LYS2 locus (Fig. 1). By using DNA blot and PCR analyses, we found that 38 of 41 independent His+ revertants derived from L1356 colonies grown at 20°C contained Ty1 insertions in the 5′ region of his3-Δ4. As expected, all Ty1 elements detected among His+ revertants were in the orientation which enabled the enhancer sequences in this element to activate the promoterless his3-Δ4 gene (Fig. 1). Furthermore, as reported for other assay systems (51, 52), the transposition rate in our assay was increased at low temperature (data not shown).
Isolation of mutants with a high Ty1 transposition rate phenotype and characterization of one mutant strain, L1561.
To isolate mutations that increase the rate of Ty1 transposition, we screened mutagenized L1356 (his3-Δ4) cells for increased levels of His+ revertants (see Materials and Methods). Colonies which gave rise to more than five His+ revertants (on average, there was about 0.75 His+ revertant/colony) were retested. Of 1,000 colonies screened, 7 candidates repeatedly gave rise to an increased number of His+ revertants. Of these seven, two were MMS sensitive and two were temperature sensitive. Each of these four was crossed with SL1005-54, which also contains the his3-Δ4 transposition assay system. The MMS or temperature sensitivity failed to segregate with the high transposition rate phenotype in three of these crosses.
In the cross involving the fourth mutant strain, designated L1561, the high transposition rate and MMS sensitivity (Mmss) phenotypes cosegregated. Similar results were obtained when a high transposition rate, Mmss segregant (SL1006-7B) from this cross was backcrossed with the parental strain, L1356. Although the Mmss phenotype could not be scored unambiguously in every tetrad, possibly due to the segregation of heterogeneous background genes, MMS sensitivity clearly segregated at a 2:2 ratio in 16 of 18 tetrads examined in the two crosses. Ten of these tetrads were also scored for the high transposition rate phenotype, which clearly segregated 2:2 in nine tetrads and always cosegregated with Mmss. Thus, it appeared that the segregation of a single Mendelian mutation, which we originally named htr1 (and renamed hir3-2 [see below]), was responsible for the Mmss and high transposition rate phenotypes in the strains produced by these crosses.
The diploid resulting from crossing the L1356 parental strain with a high transposition rate, MMS-sensitive segregant, SL1006-1B, was not sensitive to MMS, indicating that htr1(hir3-2) is recessive for MMS sensitivity. We could not determine if the mutation was recessive for the high transposition rate phenotype because Ty1 transcription is down-regulated in MATa/MATα diploid cells (52, 75).
In addition to being MMS sensitive (Fig. 2A), the L1561 mutant was slightly UV sensitive relative to the L1356 parent, but both strains were equally viable following exposure to gamma-ray doses that cause 70 to 85% lethality (data not shown). The rate of Ty1 transposition in L1561 (4.1 × 10−7) was increased about 60-fold relative to that in the L1356 parental strain (6.4 × 10−9).
CAC3 and ORF YJR140c were cloned by functional complementation of MMS sensitivity.
The L1561 mutant was transformed with a genomic S. cerevisiae library made in the CEN-URA3 vector YCp50 (61). Transformants obtained on −Ura or −Ura supplemented with 0.01% MMS were tested for resistance to 0.025% MMS in YPD, and 21 resistant transformants that regained MMS sensitivity when the plasmid was forced out by growth on +FOA medium were recovered. Plasmid pCAC3, isolated from one of these transformants, was retransformed into L1561, in which it complemented both MMS sensitivity (Fig. 2A) and the high Ty1 transposition rate (see below). Restriction mapping and partial sequencing of the insert in pCAC3 identified a fragment from chromosome II containing six ORFs, including CAC3. By deletion analysis, CAC3 was identified as the complementing gene (Fig. 2B). PCR analysis showed that 16 of the complementing plasmids contained CAC3.
The other five complementing plasmids were identical and have been designated pHIR3. Plasmid pHIR3 complements L1561 for MMS sensitivity (Fig. 2A) and for the high transposition rate phenotype (see below). Restriction mapping and partial sequencing of the insert in pHIR3 identified a 14-kb insertion from chromosome X with four ORFs. Deletion analysis showed that YJR140c is responsible for the complementing activity (Fig. 2B). YJR140c encodes a potential polypeptide of 1,648 amino acids with a predicted molecular mass of 191.7 kDa. A search of databases originally revealed no significant similarity to any known protein. When this project was nearing completion, YJR140c was identified as the HIR3 gene in the Saccharomyces genome database (49).
Deletion of HIR3, but not CAC3, in parental strain L1356 causes MMS sensitivity and a high rate of transposition.
L1675, a hir3::LEU2 deletion mutant created in our original L1356 strain, was MMS sensitive (see Fig. 4) and had an average Ty1 transposition rate of 1.3 × 10−7, approximately 20-fold higher than the rate in L1356. In contrast, when CAC3 was disrupted in L1356, the null mutant did not display any increase in MMS sensitivity or Ty1 transposition rate (data not shown).
FIG. 4.
Double mutations of hir and cac cause MMS sensitivity. (A) Deletion of HIR3 causes MMS sensitivity in the presence of cac3 or cac2. L1356 was found to contain a relevant mutation, cac3-1. L1356 (cac3 HIR3) was transformed with YCp50 or pCAC3, and L1675 (cac3 hir3) was transformed with YCp50, pCAC3, or pHIR3. Cells were grown on −Ura medium and were then spotted onto YPD and YPD plus 0.025% MMS. UV irradiation was at 90 J/m2. (B) Deletion of HIR3, HIR1, or HIR2 in L1356 causes MMS sensitivity. MMS sensitivity was scored by spotting 10-fold serial dilutions of cells on YPD with 0.02% MMS (incubation at 30°C for 2 days).
Since the Ty1 transposition rate is reduced in mutants with lower levels of Ty1 transcription (6, 74), we tested whether the L1675 hir3 deletion mutant had an altered level of Ty1 mRNA relative to that of the L1356 parental strain. No differences were found when the strains were grown at 30°C, the normal incubation temperature (Fig. 2C), or at 20°C, the incubation temperature used for transposition rate determinations (data not shown).
The htr1 mutation is an allele of HIR3.
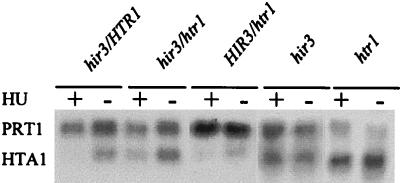
Since a deletion of HIR3 reproduced the phenotype of the htr1 mutation, we suspected that htr1 was allelic to HIR3. This hypothesis was confirmed by genetic complementation. SL1006-1B (htr1) was crossed with both W303 (HIR3) and W303Δ3 (hir3::HIS3) (kindly supplied by M. A. Osley), and SL106-1D was crossed with W303Δ3. The diploids were then examined for the hir3 phenotype. Mutations in HIR3 cause cells to lose their ability to repress the transcription of histone HTA1-HTB1 mRNA in response to the DNA replication-inhibiting drug hydroxyurea (HU) (50). As shown in Fig. 3, htr1 failed to complement hir3, since the hir3/htr1 diploid (as well as the htr1 haploid) constitutively expressed HTA1 in the presence of HU while transcription of HTA1 was repressed by HU in the hir3/HTR1 and HIR3/htr1 diploids. This identified htr1 as an allele of hir3, now called hir3-2.
FIG. 3.
The htr1 mutation depressed transcription of HTA1-HTB1 and failed to complement hir3. RNA blot analysis was performed after 30 min of growth in the absence (−) or presence (+) of HU. The diploids were formed by crossing SL1006-1B (htr1) and SL1006-1D (HTR1) with W303 (HIR3) and W303Δ3 (hir3). The constitutively transcribed gene PRT1 was used as an internal loading control since its abundance is not affected by HU.
The MMS sensitivity and high Ty1 transposition rate caused by hir3 are dependent on an additional mutation.
Transformation with pCAC3 complemented the MMS sensitivity (Fig. 2A and 4A) and high Ty1 transposition rate (Table 2) phenotypes associated with both the L1561 hir3-2 mutant strain and strain L1675, which contains a hir3::LEU2 deletion made in the parental strain L1356. We originally thought that pCAC3 was an extra-copy suppressor of hir3-2. However, to our surprise, we found that the L1356 parental strain contained a mutation in CAC3. The CAC3 alleles in L1356 and L1561 were cloned by gap repair and sequenced. The CAC3 gene cloned from these strains contained, relative to the database sequence, a G-to-C change at position 877 and an A inserted between 877 and 878, resulting in a frameshift causing the loss of about 30% of the Cac3p. Additional differences were silent or neutral. The sequence of the PCR-amplified CAC3 gene from SL1005-54 (used in the original cross with L1561 to score for segregation of htr1) indicated the presence of the same frameshift allele, which we have called cac3-1.
TABLE 2.
Mutations in hir and cac genes cause a synthetic increase in the rate of Ty1 transposition
| Strain/plasmida | Relevant noncomplemented mutation(s) | Transposition rateb | Avg. increase (fold) in transposition rate |
|---|---|---|---|
| L1356/pCAC3 | Wild type | 1.9 (5.0) × 10−8 | 1.0 |
| L1356/YCp50 | cac3-1 | 4.5 × 10−8 ± 2.4 × 10−8 | 1.3 |
| L1561/pHIR3 | cac3-1 | 4.0 × 10−8 ± 1.6 × 10−8 | 1.1 |
| L1675/pHIR3 | cac3-1 | 3.5 (4.3) × 10−8 | 1.1 |
| L1561/pCAC3 | hir3-2 | 4.8 × 10−8 ± 2.0 × 10−8 | 1.4 |
| L1675/pCAC3 | hir3::LEU2 | 3.1 (4.9) × 10−8 | 1.2 |
| L1561/YCp50 | cac3-1 hir3-2 | 6.2 × 10−7 ± 3.0 × 10−7 | 18.0 |
| L1675/YCp50 | cac3-1 hir3::LEU2 | 3.7 (3.3) × 10−7 | 10.1 |
| L1685/pCAC3 | cac2::TRP1 hir3::LEU2 | 5.8 (4.5) × 10−7 | 14.9 |
| L1635/pCAC3 | rad6::LEU2 | 6.0 × 10−8 ± 4.0 × 10−8 | 1.7 |
All strains are derivatives of L1356, which was found to contain a relevant mutation, cac3-1. Strains were transformed with the indicated plasmids.
Ty1 transposition rates were determined during growth on −URA in order to maintain selection for the plasmids (see Materials and Methods). When three or more determinations were made, the average and standard deviation are indicated; when two determinations were made, the duplicate value is shown in parentheses.
To distinguish the roles of the hir3 and cac3 mutations in causing MMS sensitivity and high Ty1 transposition rates, the isogenic strains L1356 (cac3-1), L1561 (cac3-1 hir3-2), and L1675 (cac3-1 hir3::LEU2) were transformed with pCAC3, pHIR3, or YCp50 (Table 2). The resulting isogenic transformants, mutant for neither CAC3 nor HIR3, either CAC3 or HIR3, or both CAC3 and HIR3, were compared for MMS sensitivity and Ty1 transposition rates (Fig. 4A; Table 2). The results indicate that the cac3, but not the hir3, single mutant is slightly sensitive to MMS and that the single cac3 or hir3 mutation did not increase the Ty1 transposition rate. In contrast, the double cac3 hir3 mutant strains were much more sensitive to MMS and had increased Ty1 transposition rates relative to those of wild-type or single-mutant strains.
Mutations in other HIR and CAC genes also cause MMS sensitivity and high rates of Ty1 transposition when combined with cac3 and hir3 mutations, respectively.
Since mutations in HIR1, HIR2, and HIR3 all have the same effects on the regulation of histone expression (50, 68), we tested whether deletions of HIR1 and HIR2 in our parental cac3-1 strain, L1356, would increase the MMS sensitivity and the Ty1 transposition rate as did mutations in HIR3. Indeed, in the cac3-1 strain, deletion of either HIR1 or HIR2 led to an increase in the sensitivity to MMS (Fig. 4B) and caused a dramatic increase in the Ty1 transposition rate (Table 3). Likewise, cac3 hir1 and cac3 hir2 double deletions in UCC4543 caused increased sensitivity to MMS, while single hir deletions did not (data not shown).
TABLE 3.
Ty1 transposition rates are dramatically increased by hir1, hir2, or hir3 mutations in a cac3-1 strain
| Straina | Relevant genotype | Transposition rateb | Avg. increase (fold) in trans- position rate |
|---|---|---|---|
| L1356 | cac3-1 | 6.4 × 10−9 ± 2.4 × 10−9 | 1 |
| L1561 | cac3-1 hir3-2 | 4.1 × 10−7 ± 0.6 × 10−7 | 60 |
| L1675 | cac3-1 hir3::LEU2 | 1.3 × 10−7 ± .06 × 10−7 | 20 |
| L1681 | cac3-1 hir2::URA3 | 1.6 (1.6) × 10−7 | 25 |
| L1680 | cac3-1 hir1::URA3 | 2.0 (2.0) × 10−7 | 30 |
| L1635 | cac3-1 rad6::LEU2 | 2.0 (1.2) × 10−8 | 2.5 |
| L1562 | cac3-1 rad1::LEU2 | 2.1 × 10−8 ± 1.1 × 10−8 | 3.3 |
| L1563 | cac3-1 rad52::URA3 | 2.8 × 10−8 ± 1.5 × 10−8 | 4.3 |
All strains are derivatives of L1356.
Transposition rates were determined during growth in YPD (see Materials and Methods). When three or more determinations were made, the average and standard deviation are indicated; when two determinations were made, the duplicate value is indicated in parentheses.
Since Cac3p complexes with Cac1p and Cac2p to form CAF-I (34, 71), we asked whether a cac2::TRP1 deletion could substitute for the cac3-1 mutation in causing MMS sensitivity and a high transposition rate in conjunction with a hir3::LEU2 deletion. Indeed, L1685 (cac2::TRP1 hir3::LEU2) is MMS sensitive and has an increased transposition rate (Table 2 and Fig. 4A).
Since the cac3 hir double mutants are sensitive to MMS and therefore deficient in DNA repair, we tested the possibility that the increase in Ty1 transposition is a secondary effect of the DNA repair deficiency by deleting a gene of the excision repair pathway (RAD1), the error-prone repair pathway (RAD6), or the recombination repair pathway (RAD52) in L1356 (cac3-1). The Ty1 transposition rates in these rad cac3-1 double mutant strains were determined, and all showed a marginal increase (two- to fourfold) over that of L1356 (Tables 2 and 3).
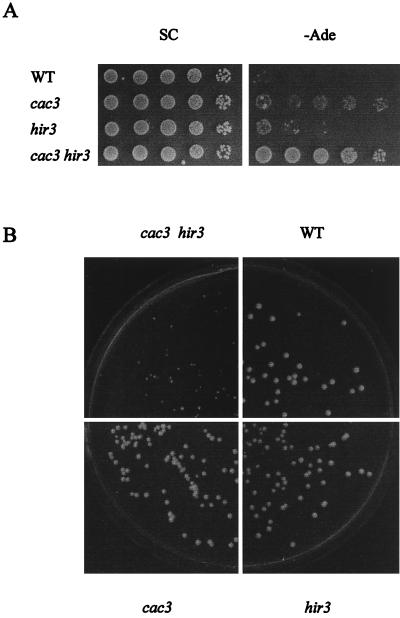
Simultaneous deletion of HIR3 and CAC3 has a dramatic effect on telomeric silencing and growth rate.
To examine the effects of mutations in HIR3 and CAC3 on telomeric silencing, we used strain UCC4543, which contains the RNA polymerase III (RNAP III)-transcribed gene SUP4-o placed close to telomere VII-L (28). Telomeric silencing inhibits the expression of SUP4-o, a tyrosyl-tRNA ochre suppressor, thereby causing the loss of suppression of the ade2-101 ochre marker, leading to red colony color and the absence of growth on −Ade. As shown in Fig. 5A, silencing of SUP4-o was reduced in cac3 hir3 double mutants, while deletions of CAC3 alone reduced silencing to a much lesser extent and deletions of HIR3 alone had no effect on telomeric silencing. Six, three, and two independent cac3 hir3, cac3, and hir3 deletion strains were examined, respectively. Similar data was obtained with single hir1 or hir2 and double hir1 cac3 or hir2 cac3 deletions (data not shown).
FIG. 5.
Silencing and growth defects in hir3 cac3 double-deletion mutants. Strain UCC4543 (wild type [WT]) and its deletion derivatives L1678 (cac3), L1687 (hir3), and L1688 (cac3 hir3) were used. (A) Silencing of telomere VII-L-located SUP4-o (SUP4-o-TEL) is released in hir3 cac3 double-deletion mutants. Expression of SUP4-o-TEL was assayed by spotting 10-fold serial dilutions on SC (control) and −Ade and incubating the plates at 30°C for 4 days. The slight increase in growth of the hir3 strain shown on −Ade was not reproducible. (B) Deletion of both HIR3 and CAC3 retards cell growth. The indicated strains were diluted, plated on YPD, and incubated at 20°C.
Deletions of HIR1, HIR2, or HIR3 in L1356 (cac3-1) resulted in a reduced colony size (data not shown). Likewise, cac3 hir3 double deletions in strain UCC4543 reduced the colony size while single cac3 or hir3 deletions in UCC4543 had no detectable effect (Fig. 5B). This colony size difference was easily observed when plates were incubated at 20°C and could also be detected at 30°C during the first 2 days of growth. When grown in liquid YPD, strain UCC4543 had doubling times of 3.5 and 1.8 h at 20 and 30°C, respectively, while the cac3 hir3 deletion derivative, L1688, had doubling times of 5.4 and 2.5 h at these respective temperatures.
Effects of cac3 hir3 double mutations on Ty1 transposition at other loci.
Above we showed that cac3 hir3 double mutations increase the rate of Ty1 transposition into his3-Δ4, located at the LYS2 locus. To determine the effect of cac3 hir3 double mutations on Ty1 transposition into other loci, we used two previously published assays. In the his3AI assay, the generation of His+ prototrophs is indicative of the transposition of a single marked Ty1 element to any position in the genome (14, 15). Since the majority of transpositions occur in a few genomic hot spots (16, 30), this assay essentially measures the frequency of transposition into these hot spots. In strain JC364 (CAC3 HIR3), the frequency (mean ± standard deviation) of His+ prototrophs was 5.07 × 10−7 ± 0.15 × 10−7. Deletion of CAC3 alone did not increase the frequency of His+ prototrophs. However, the simultaneous deletion of CAC3 and HIR3 (L1690 [cac3::hisG-URA3-hisG hir3::LEU2]) resulted in a His+ frequency of 2.27 × 10−6 ± 0.43 × 10−6, representing a three- to fivefold increase in transposition rate relative to that of the CAC3 HIR3 and cac3 HIR3 parents. Transposition into CAN1 increased about 10-fold in L1675 (cac3 hir3) relative to that in L1356 (cac3 HIR3), from 1.0 × 10−7 (duplicate, 1.05 × 10−7) to 1.15 × 10−6 (duplicate, 9.0 × 10−7).
Localization of Hir3p.
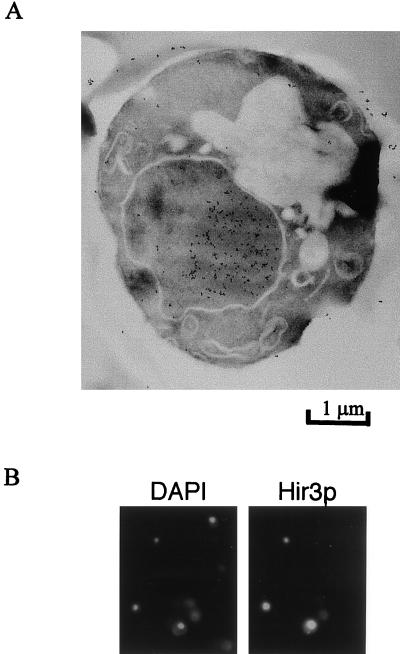
To determine the cellular location of Hir3p, we fused three repeats of the HA epitope tag to the 3′ end of the HIR3 ORF and placed the tagged gene on a multicopy 2μm plasmid, pHIR3-HA. pHIR3-HA transformants of the MMS-sensitive strain L1675 (cac3-1 hir3::LEU2) became resistant to 0.02% MMS (data not shown), demonstrating that the tagged Hir3p (Hir3p-HA) was at least partially functional. The size of Hir3p-HA, as determined by Western blot analysis, was consistent with the predicted size of 191.7 kDa (data not shown). YJB2306 and L1675 transformed with pHIR3-HA (or pHIR3 as a control) were respectively examined by indirect immunofluorescence microscopy and immunoelectron microscopy, using antibodies specific for the HA epitope. Both techniques localized Hir3p-HA to the nucleus (Fig. 6). About 50 and 10%, respectively, of the YJB2306 and L1675 pHIR3-HA transformants were labeled with the HA antibody, and Hir3p-HA was clearly present in the nucleus, in all of the labeled cells. In contrast, only background immunofluorescence and immunogold labeling were observed in cells transformed with the pHIR3 plasmid and stained with the HA epitope antibody. In double-labeling immunofluorescence experiments with HA antibody and either Rap1p antibody (21) or nuclear pore antibody (MAB414, BAbCo, Inc.), the nuclear Hir3p-HA signal did not colocalize with either Rap1p or the nuclear pores (data not shown).
FIG. 6.
Nuclear localization of HA-tagged Hir3p. (A) Immunogold labeling of HA-Hir3p in L1675. (B) Indirect immunofluorescence and 4′,6-diamidino-2-phenylindole (DAPI) staining of HA-Hir3p in YJB2306.
DISCUSSION
During the analysis of a genetic screen for mutations that increase the rate of transposition of Ty1 into a promoterless his3-Δ4 target, we discovered that the parental strain in which the search was undertaken contained a frameshift mutation in a gene (CAC3) encoding a component of yeast CAF-I (yCAF-I). Since the cac3 mutant phenotype is very subtle, it is unknown how widespread this mutation is in laboratory stocks. Our search revealed that a newly induced hir3 mutation in this strain caused a dramatic increase in the Ty1 transposition rate. Mutations in HIR3 are known to affect the regulation of histone synthesis (45, 50, 68). The observed effect on Ty1 transposition was dependent on the simultaneous presence of both the cac3 and hir3 mutations. The two mutations also caused a synthetic increase in growth inhibition as well as in MMS, UV, and cold sensitivity but did not cause gamma-ray sensitivity. The combined presence of mutations in CAC3 and HIR3 had no effect on the overall level of Ty1 mRNA, so the increase in the Ty1 transposition rate must result from another mechanism. We propose that this mechanism is an alteration in chromatin structure which makes the DNA more available for Ty1 integration. In support of this hypothesis, silencing of a telomere-proximal RNAP III-transcribed gene was reduced by a combination of cac3 and hir3 mutations much more than by the single cac3 mutation alone. Likewise, in the accompanying paper (32), mutations in CAC and HIR genes are shown to have a synergistic effect on the release of telomeric silencing of an RNAP II-transcribed gene.
The role of CAC3 in Ty1 transposition is as a component of CAF-I.
yCAF-I (composed of Cac1p, Cac2p, and Cac3p) and human CAF-I (hCAF-I) show a high degree of conservation. Both yCAF-I and hCAF-I preferentially assemble nucleosomes on replicating DNA (33, 34). hCAF-I is thought to be involved in the first step of the nucleosome assembly process, bringing newly synthesized, acetylated isoforms of H3 and H4 tetramers to replicating DNA. Nucleosome assembly is then completed by the addition of H2A-H2B dimers. Mutations in any of the yeast CAC genes cause UV but not X-ray sensitivity and a reduction in telomeric silencing (21, 34, 44). Furthermore, the localization of Rap1p, a DNA binding protein which is a significant component of telomeric chromatin, is altered in cac1 mutants (21). Our finding that a cac2 deletion (in a hir3 background) causes increases in Ty1 transposition and MMS sensitivity similar to those observed in the cac3 hir3 strains suggests that these phenotypes are caused by inactivation of the CAF-I activity. In addition, our finding that mutations in CAC3 or CAC2 (synergistically with hir3) cause an increase in transposition of Ty1 into a nontelomeric region (lys2::his3-Δ4 is, respectively, 141 and 58 centimorgans from its telomere and centromere) supports the hypothesis that CAF-I has a general role in chromatin assembly that is not restricted to the formation of heterochromatin.
The findings that deletions of the CAC genes are nonlethal and that such deletions do not cause a significant growth defect (34) imply that either replacements for the CAF-I subunits exist or newly replicated DNA can be assembled into chromatin without CAF-I by a parallel pathway. Hat2p has previously been identified to be highly similar to Cac3p (53). A search of the yeast database revealed that Cac3p was 31.3 and 25.1% similar and 23.4 and 18.7% identical to the histone acetylase Hat2p and an uncharacterized ORF, YMR131C, respectively, with BLAST (1a) probabilities of 3.0 × 10−30 and 9.0 × 10−18, respectively. It is possible that Hat2p or Ymr131cp could substitute for Cac3p in the CAF-I complex. The yeast protein most similar to Cac2p is Hir1p (32.3% similarity and 18.2% identity; BLAST probability, 8.0 × 10−17), and the yeast protein most similar to Hir1p is Hir2p. Cac2p is only about half the size of Hir1p or Hir2p, and the homology between them exists within the N-terminal half of Hir1p. Thus, it is possible that Cac2p and Hir1p have partially overlapping functions.
The effect of mutations in HIR3 on Ty1 transposition is mimicked by mutations in HIR1 and HIR2.
The HIR1, HIR2, and HIR3 genes are required for the proper balance of the core histones H2A, H2B, H3, and H4 (45). These histones are encoded by four pairs of divergently transcribed genes, HTA1-HTB1 and HTA2-HTB2 (each encoding H2A and H2B) and HHT1-HHF1 and HHT2-HHF2 (each encoding H3 and H4). Often the consequence of an imbalance of histones is altered chromatin structure (47), which appears to lead to altered transcription of numerous genes (12, 25, 26) and altered chromosome segregation (42). Hir1p, Hir2p, and Hir3p are required for a feedback control system which autogenously regulates transcription of the HTA1-HTB1 locus in response to intracellular H2A and H2B levels. Consistent with its role as a transcriptional repressor, Hir2p was previously shown to be located in the nucleus (68). Here we showed that overexpressed Hir3p-HA also localizes to the nucleus. Despite the presence of seven hypothetical transmembrane domains in Hir3p (60), there was no indication from either the immunofluorescence or immunogold labeling studies that Hir3p-HA is associated with a membrane.
The synthesis of histones is coordinated with DNA replication by transcriptional repression of three of the four histone loci, HTA1-HTB1, HHT1-HHF1, and HHT2-HHF2. Derepression of these loci occurs during late G1 or early S phase. Mutations in HIR1, HIR2, and HIR3 selectively derepress the synthesis of the three histone loci, and such mutations do not appear to be general transcriptional repressors (50, 76).
Mutations in the HIR genes suppress the his4-912δ and lys2-128δ alleles, which are mutant due to the presence of a Ty1 delta sequence in their promoter regions. The hir mutations shift the transcription initiation site away from the delta insertion and back to the normal HIS4 or LYS2 start site. Yet mutations in the HIR genes do not alter the overall levels of Ty1 mRNA (67). Deletion of HTA1-HTB1 or overexpression of any one of the four histone loci likewise suppresses delta insertion mutations (12). This is consistent with the idea that the hir mutations cause an imbalance in the levels of histones and that in a cac3 background this imbalance enhances the rate of Ty1 transposition.
The dramatic increase in Ty1 transposition associated with cac3 hir double mutations is not a property of other DNA repair mutations.
Each of the cac3 hir double mutants is sensitive to MMS and therefore deficient in DNA repair. It is thus possible that the increase in Ty1 transposition associated with cac3 hir mutations is a secondary effect of the DNA repair deficiency and results from unrepaired lesions in the DNA. However, using the promoterless his3 assay, we found that combinations of cac3 and deletions of genes from the excision repair pathway (RAD1), the error-prone repair pathway (RAD6), or the recombination repair pathway (RAD52) cause only marginal increases in Ty1 transposition. Since rad6 and rad52 mutants are very MMS sensitive, this suggests that the increase in Ty1 transposition caused by the hir cac3 double mutations is not simply due to unrepaired DNA lesions.
We previously reported that a deletion of RAD6 dramatically increases the rate of transposition of Ty1 into the CAN1 gene (54). This is in contrast to our finding, presented here, that deletions of RAD6 cause only a marginal increase in the transposition of Ty1 into the promoterless his3 target. To reconcile this discrepancy, we suggest that mutations in RAD6 affect the chromatin structure of different regions of DNA differently. Indeed, mutations in other genes have been shown to have differential effects on chromatin structure in different loci (47). The cac3 hir3 double deletions consistently increased the rate of Ty1 transposition when three different assays employing different targets were used.
Other investigators have also examined the effects of various rad mutations on the rate of transposition of Ty1 into different targets and obtained different results. Transposition of Ty1 into a plasmidborne copy of the tyrosyl-tRNA SUP4-o gene was increased 5- and 20-fold by deletions of RAD1 and RAD6, respectively (31, 36), and appeared to be eliminated by a deletion of RAD52 (37). In contrast, other investigators found that deletion of RAD52 did not decrease Ty1 transposition (63, 65).
In addition to its role in duplicating chromosomes, CAF-I has been proposed to be involved in replication-dependent DNA repair mechanisms (34). Indeed, hCAF-I has been shown to participate in excision repair in vitro (23). Our finding that cac3 hir1, cac3 hir2, cac3 hir3, and cac2 hir3 double mutants are MMS sensitive suggests that CAF-I may also be involved in the repair of MMS damage. It is also possible that an imbalance of histones together with an inactive CAF-I causes an alteration in chromatin structure which makes DNA bases more accessible to UV and MMS damage.
Possible mechanisms for the synergistic effects of cac and hir mutations on silencing and the rate of Ty1 transposition.
Wild-type cells use CAF-I to assemble nascent histones into new nucleosomes in replicated DNA. In cac mutant strains, the assembly of new nucleosomes must be accomplished by another pathway. One way to explain the finding that cac mutants reduce silencing (21, 34, 44) is to propose that the assembly of new nucleosomes is slower in the absence of CAF-I, allowing time for silencing factors to diffuse away, for acetylases to act on recycled nucleosomes, and for activators to bind to newly replicated DNA (20). Likewise, the synergistic interaction between cac and hir mutations could be explained if the nucleosome assembly activity substituting for CAF-I in cac mutants were further slowed by the hir mutations (possibly due to an imbalance in histones) while the bona fide CAF-I activity in CAC strains was relatively resistant to the effects of the hir mutations. A longer delay in chromatin reassembly in cac hir strains would increase the chance that dissociated proteins diffuse away and that recycled histones are modified. After several generations, the result would be a steady-state structure in cac hir strains that differed from that in CAC HIR strains in both histone modification levels and DNA binding proteins. In silent regions, this could cause a reduction of silencing; in other regions of the genome, it could result in increased availability for Ty transposition.
The increased transposition rate in cac hir strains could result from histone modifications and DNA binding proteins that make the DNA structure more available for transposition. Indeed, in vitro experiments suggest that human immunodeficiency virus integration is enhanced by nucleosome-promoted distortions in the DNA double helix which make the major groove more accessible than it is in naked DNA (55, 56). Previous results are consistent with the idea that the conformation of chromatin can affect the integration of Ty1 elements. Mutations in RAD6 which affect silencing (8, 28) also dramatically reduce the bias for Ty1 integrations to occur preferentially in the promoter rather than in the coding regions of a variety of genes (27, 31, 39, 72). Deletion of HTA1-HTB1 relaxes (59) the normally strong orientation bias of Ty1 elements that transpose into the promoter region of CAN1 (72).
Hot spots for integration of various Ty elements depend on host protein complexes assembled on the DNA. Ty5 elements are preferentially targeted for integration by the protein complex assembled at silenced regions (78). Ty3 integration requires binding of the transcription factors TFIIIB and TFIIIC but is inhibited by RNAP III, suggesting that the Ty3 integration machinery competes with RNAP III for interaction with TFIIIB and TFIIIC (13, 35). Likewise, Ty1 has also been shown to have a strong preference for integration into regions upstream of genes transcribed by RNAP III (16). We suggest that the combination of cac and hir mutations may enhance the rate of integration of Ty1 into the promoterless his3 gene used in this study by creating conditions under which proteins that encourage Ty1 integration bind to the his3 upstream region.
ACKNOWLEDGMENTS
We are grateful to P. D. Kaufman and M. A. Osley for strains and plasmids and for sharing unpublished data. We also thank L. Prakash, D. Schild, K. Struhl, R. Gietz, M. Rose, and E. Alani for plasmids, strains, and libraries and I. Derkatch and S. Prakash for helpful comments on the manuscript. We are indebted to Jack Gibbons for help with the immunogold electron microscopy, to M. Gerami-Nejad for assistance with immunofluorescence microscopy, to A. Kagan for help with the mutant screen, and to the employees of the University of Florida sequencing facility.
This work was supported by National Institutes of Health (NIH) grants GM50365 to S.W.L. and GM38626 to J.B.
REFERENCES
- 1.Alani E, Cao L, Kleckner N. A method for gene disruption that allows repeated use of URA3 selection in the construction of multiply disrupted yeast strains. Genetics. 1987;116:541–545. doi: 10.1534/genetics.112.541.test. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1a.Altschul S F, Madden T L, Schäffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson L E, Wang X, Gibbons J T. Three enzymes of carbon metabolism or their antigenic analogs in pea leaf nuclei. Plant Physiol. 1995;108:659–667. doi: 10.1104/pp.108.2.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boeke J D, Garfinkel D J, Styles C A, Fink G R. Ty elements transpose through an RNA intermediate. Cell. 1985;40:491–500. doi: 10.1016/0092-8674(85)90197-7. [DOI] [PubMed] [Google Scholar]
- 4.Boeke J D, LaCroute F, Fink G R. A positive selection for mutants lacking orotidine-5′-phosphate decarboxylase activity in yeast: 5-fluoro-orotic acid resistance. Mol Gen Genet. 1984;197:345–346. doi: 10.1007/BF00330984. [DOI] [PubMed] [Google Scholar]
- 5.Boeke J D, Sandmeyer S B. Yeast transposable elements. In: Jones E W, Pringle J R, Broach J R, editors. The molecular biology of the yeast Saccharomyces. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1991. pp. 193–261. [Google Scholar]
- 6.Boeke J D, Styles C A, Fink G R. Saccharomyces cerevisiae SPT3 gene is required for transposition and transpositional recombination of chromosomal Ty elements. Mol Cell Biol. 1986;6:3575–3581. doi: 10.1128/mcb.6.11.3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bradshaw V A, McEntee K. DNA damage activates transcription and transposition of yeast Ty retrotransposons. Mol Gen Genet. 1989;218:465–474. doi: 10.1007/BF00332411. [DOI] [PubMed] [Google Scholar]
- 8.Bryk M, Banerjee M, Murphy M, Knudsen K E, Garfinkel D J, Curcio M J. Transcriptional silencing of Ty1 elements in the RDN1 locus of yeast. Genes Dev. 1997;11:255–269. doi: 10.1101/gad.11.2.255. [DOI] [PubMed] [Google Scholar]
- 9.Burck C L. Ph.D. thesis. Chicago: University of Illinois; 1996. [Google Scholar]
- 10.Chapman K B, Boeke J D. Isolation and characterization of the gene encoding yeast debranching enzyme. Cell. 1991;65:483–492. doi: 10.1016/0092-8674(91)90466-c. [DOI] [PubMed] [Google Scholar]
- 11.Chapman K B, Bystrom A S, Boeke J D. Initiator methionine tRNA is essential for Ty1 transposition in yeast. Proc Natl Acad Sci USA. 1992;89:3236–3240. doi: 10.1073/pnas.89.8.3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clark-Adams C D, Norris D, Osley M A, Fassler J S, Winston F. Changes in histone gene dosage alter transcription in yeast. Genes Dev. 1988;2:150–159. doi: 10.1101/gad.2.2.150. [DOI] [PubMed] [Google Scholar]
- 13.Connolly C M, Sandmeyer S B. RNA polymerase III interferes with Ty3 integration. FEBS Lett. 1997;405:305–311. doi: 10.1016/s0014-5793(97)00200-7. [DOI] [PubMed] [Google Scholar]
- 14.Curcio M J, Garfinkel D J. Posttranslational control of Ty1 retrotransposition occurs at the level of protein processing. Mol Cell Biol. 1992;12:2813–2825. doi: 10.1128/mcb.12.6.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Curcio M J, Garfinkel D J. Single-step selection for Ty1 element retrotransposition. Proc Natl Acad Sci USA. 1991;88:936–940. doi: 10.1073/pnas.88.3.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Devine S E, Boeke J D. Integration of the yeast retrotransposon Ty1 is targeted to regions upstream of genes transcribed by RNA polymerase III. Genes Dev. 1996;10:620–633. doi: 10.1101/gad.10.5.620. [DOI] [PubMed] [Google Scholar]
- 17.Drake J W. A constant rate of spontaneous mutation in DNA-based microbes. Proc Natl Acad Sci USA. 1991;88:7160–7164. doi: 10.1073/pnas.88.16.7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eisenmann D M, Arndt K M, Ricupero S L, Rooney J W, Winston F. SPT3 interacts with TFIID to allow normal transcription in Saccharomyces cerevisiae. Genes Dev. 1992;6:1319–1331. doi: 10.1101/gad.6.7.1319. [DOI] [PubMed] [Google Scholar]
- 19.Eisenmann D M, Dollard C, Winston F. SPT15, the gene encoding the yeast TATA binding factor TFIID, is required for normal transcription initiation in vivo. Cell. 1989;58:1183–1191. doi: 10.1016/0092-8674(89)90516-3. [DOI] [PubMed] [Google Scholar]
- 20.Enomoto S, Berman J. Chromatin assembly factor I contributes to the maintenance, but not the reestablishment, of silencing at the yeast silent mating loci. Genes Dev. 1998;12:219–232. doi: 10.1101/gad.12.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Enomoto S, McCune-Zierath P D, Gerami-Nejad M, Sanders M A, Berman J. RLF2, a subunit of yeast chromatin assembly factor-I, is required for telomeric chromatin function in vivo. Genes Dev. 1997;11:358–370. doi: 10.1101/gad.11.3.358. [DOI] [PubMed] [Google Scholar]
- 22.Farabaugh P J. Post-transcriptional regulation of transposition by Ty retrotransposons of Saccharomyces cerevisiae. J Biol Chem. 1995;270:10361–10364. doi: 10.1074/jbc.270.18.10361. [DOI] [PubMed] [Google Scholar]
- 23.Gaillard P H, Martini E M, Kaufman P D, Stillman B, Moustacchi E, Almouzni G. Chromatin assembly coupled to DNA repair: a new role for chromatin assembly factor I. Cell. 1996;86:887–896. doi: 10.1016/s0092-8674(00)80164-6. [DOI] [PubMed] [Google Scholar]
- 24.Gietz R D, Sugino A. New yeast-Escherichia coli shuttle vectors constructed with in vitro mutagenized yeast genes lacking six-base pair restriction sites. Gene. 1988;74:527–534. doi: 10.1016/0378-1119(88)90185-0. [DOI] [PubMed] [Google Scholar]
- 25.Han M, Grunstein M. Nucleosome loss activates yeast downstream promoters in vivo. Cell. 1988;55:1137–1145. doi: 10.1016/0092-8674(88)90258-9. [DOI] [PubMed] [Google Scholar]
- 26.Han M, Kim U J, Kayne P, Grunstein M. Depletion of histone H4 and nucleosomes activates the PHO5 gene in Saccharomyces cerevisiae. EMBO J. 1988;7:2221–2228. doi: 10.1002/j.1460-2075.1988.tb03061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang, H., J. Y. Yong, C. Burck, and S. W. Liebman. Host genes that affect the target-site distribution of the yeast retrotransposon, Ty1. Submitted for publication. [DOI] [PMC free article] [PubMed]
- 28.Huang H, Kahana A, Gottschling D E, Prakash L, Liebman S W. The ubiquitin-conjugating enzyme Rad6 (Ubc2) is required for silencing in Saccharomyces cerevisiae. Mol Cell Biol. 1997;17:6693–6699. doi: 10.1128/mcb.17.11.6693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ito H, Fukuda Y, Murata K, Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983;153:163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ji H, Moore D P, Blomberg M A, Braiterman L T, Voytas D F, Natsoulis G, Boeke J D. Hotspots for unselected Ty1 transposition events on yeast chromosome III are near tRNA genes and LTR sequences. Cell. 1993;73:1007–1018. doi: 10.1016/0092-8674(93)90278-x. [DOI] [PubMed] [Google Scholar]
- 31.Kang X L, Yadao F, Gietz R D, Kunz B A. Elimination of the yeast RAD6 ubiquitin conjugase enhances base-pair transitions and G.C—T.A transversions as well as transposition of the Ty element: implications for the control of spontaneous mutation. Genetics. 1992;130:285–294. doi: 10.1093/genetics/130.2.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaufman P D, Cohen J L, Osley M A. Hir proteins are required for position-dependent gene silencing in Saccharomyces cerevisiae in the absence of chromatin assembly factor I. Mol Cell Biol. 1998;18:4793–4806. doi: 10.1128/mcb.18.8.4793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaufman P D, Kobayashi R, Kessler N, Stillman B. The p150 and p60 subunits of chromatin assembly factor I: a molecular link between newly synthesized histones and DNA replication. Cell. 1995;81:1105–1114. doi: 10.1016/s0092-8674(05)80015-7. [DOI] [PubMed] [Google Scholar]
- 34.Kaufman P D, Kobayashi R, Stillman B. Ultraviolet radiation sensitivity and reduction of telomeric silencing in Saccharomyces cerevisiae cells lacking chromatin assembly factor-I. Genes Dev. 1997;11:345–357. doi: 10.1101/gad.11.3.345. [DOI] [PubMed] [Google Scholar]
- 35.Kirchner J, Connolly C M, Sandmeyer S B. Requirement of RNA polymerase III transcription factors for in vitro position-specific integration of a retroviruslike element. Science. 1995;267:1488–1491. doi: 10.1126/science.7878467. [DOI] [PubMed] [Google Scholar]
- 36.Kunz B A, Kohalmi L, Kang X, Magnusson K A. Specificity of the mutator effect caused by disruption of the RAD1 excision repair gene of Saccharomyces cerevisiae. J Bacteriol. 1990;172:3009–3014. doi: 10.1128/jb.172.6.3009-3014.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kunz B A, Peters M G, Kohalmi S E, Armstrong J D, Glattke M, Badiani K. Disruption of the RAD52 gene alters the spectrum of spontaneous SUP4-o mutations in Saccharomyces cerevisiae. Genetics. 1989;122:535–542. doi: 10.1093/genetics/122.3.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leib-Mosch C, Seifarth W. Evolution and biological significance of human retroelements. Virus Genes. 1995;11:133–145. doi: 10.1007/BF01728654. [DOI] [PubMed] [Google Scholar]
- 39.Liebman S W, Newnam G. A ubiquitin-conjugating enzyme, RAD6, affects the distribution of Ty1 retrotransposon integration positions. Genetics. 1993;133:499–508. doi: 10.1093/genetics/133.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luria S E, Delbruck M. Mutations of bacteria from virus sensitivity to virus resistance. Genetics. 1943;28:491–511. doi: 10.1093/genetics/28.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maftahi M, Gaillardin C, Nicaud J M. Sticky-end polymerase chain reaction method for systematic gene disruption in Saccharomyces cerevisiae. Yeast. 1996;12:859–868. doi: 10.1002/(SICI)1097-0061(199607)12:9%3C859::AID-YEA978%3E3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 42.Meeks-Wagner D, Hartwell L H. Normal stoichiometry of histone dimer sets is necessary for high fidelity of mitotic chromosome transmission. Cell. 1986;44:43–52. doi: 10.1016/0092-8674(86)90483-6. [DOI] [PubMed] [Google Scholar]
- 43.Menees T M, Sandmeyer S B. Cellular stress inhibits transposition of the yeast retrovirus-like element Ty3 by a ubiquitin-dependent block of virus-like particle formation. Proc Natl Acad Sci USA. 1996;93:5629–5634. doi: 10.1073/pnas.93.11.5629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Monson E K, de Bruin D, Zakian V A. The yeast Cac1 protein is required for the stable inheritance of transcriptionally repressed chromatin at telomeres. Proc Natl Acad Sci USA. 1997;94:13081–13086. doi: 10.1073/pnas.94.24.13081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moran L, Norris D, Osley M A. A yeast H2A-H2B promoter can be regulated by changes in histone gene copy number. Genes Dev. 1990;4:752–763. doi: 10.1101/gad.4.5.752. [DOI] [PubMed] [Google Scholar]
- 46.Morrison A, Miller E J, Prakash L. Domain structure and functional analysis of the carboxyl-terminal polyacidic sequence of the RAD6 protein of Saccharomyces cerevisiae. Mol Cell Biol. 1988;8:1179–1185. doi: 10.1128/mcb.8.3.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Norris D, Dunn B, Osley M A. The effect of histone gene deletions on chromatin structure in Saccharomyces cerevisiae. Science. 1988;242:759–761. doi: 10.1126/science.2847314. [DOI] [PubMed] [Google Scholar]
- 48.Orr-Weaver T L, Szostak J W. Yeast recombination: the association between double-strand gap repair and crossing-over. Proc Natl Acad Sci USA. 1983;80:4417–4421. doi: 10.1073/pnas.80.14.4417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Osley, M. A. 1997. Saccharomyces genome database. http://genome-www.stanford.edu/cgi-bin/dbrun/SacchDB?find+Locus+%22hir3%22.
- 50.Osley M A, Lycan D. trans-acting regulatory mutations that alter transcription of Saccharomyces cerevisiae histone genes. Mol Cell Biol. 1987;7:4204–4210. doi: 10.1128/mcb.7.12.4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paquin C E, Williamson V M. Temperature effects on the rate of Ty transposition. Science. 1984;226:53–55. doi: 10.1126/science.226.4670.53. [DOI] [PubMed] [Google Scholar]
- 52.Paquin C E, Williamson V M. Ty insertions at two loci account for most of the spontaneous antimycin A resistance mutations during growth at 15°C of Saccharomyces cerevisiae strains lacking ADH1. Mol Cell Biol. 1986;6:70–79. doi: 10.1128/mcb.6.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Parthun M R, Widom J, Gottschling D E. The major cytoplasmic histone acetyltransferase in yeast: links to chromatin replication and histone metabolism. Cell. 1996;87:85–94. doi: 10.1016/s0092-8674(00)81325-2. [DOI] [PubMed] [Google Scholar]
- 54.Picologlou S, Brown N, Liebman S W. Mutations in RAD6, a yeast gene encoding a ubiquitin-conjugating enzyme, stimulate retrotransposition. Mol Cell Biol. 1990;10:1017–1022. doi: 10.1128/mcb.10.3.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pruss D, Bushman F D, Wolffe A P. Human immunodeficiency virus integrase directs integration to sites of severe DNA distortion within the nucleosome core. Proc Natl Acad Sci USA. 1994;91:5913–5917. doi: 10.1073/pnas.91.13.5913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pryciak P M, Varmus H E. Nucleosomes, DNA-binding proteins, and DNA sequence modulate retroviral integration target site selection. Cell. 1992;69:769–780. doi: 10.1016/0092-8674(92)90289-o. [DOI] [PubMed] [Google Scholar]
- 57.Recht J, Dunn B, Raff A, Osley M A. Functional analysis of histones H2A and H2B in transcriptional repression in Saccharomyces cerevisiae. Mol Cell Biol. 1996;16:2545–2553. doi: 10.1128/mcb.16.6.2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reynolds P, Prakash L, Prakash S. Nucleotide sequence and functional analysis of the RAD1 gene of Saccharomyces cerevisiae. Mol Cell Biol. 1987;7:1012–1020. doi: 10.1128/mcb.7.3.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rinckel L A, Garfinkel D J. Influences of histone stoichiometry on the target site preference of retrotransposons Ty1 and Ty2 in Saccharomyces cerevisiae. Genetics. 1996;142:761–776. doi: 10.1093/genetics/142.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rose, M., P. Koetter, and K. D. Entian. 1995. Hypothetical 191.7 KD protein in HOM6-PMT4 intergenic region. http://www.expasy.ch/cgi-bin/sprot-search-ac?P47171.
- 61.Rose M D, Novick P, Thomas J H, Botstein D, Fink G R. A Saccharomyces cerevisiae genomic plasmid bank based on a centromere-containing shuttle vector. Gene. 1987;60:237–243. doi: 10.1016/0378-1119(87)90232-0. [DOI] [PubMed] [Google Scholar]
- 62.Rothstein R J. One-step gene disruption in yeast. Methods Enzymol. 1983;101:202–211. doi: 10.1016/0076-6879(83)01015-0. [DOI] [PubMed] [Google Scholar]
- 63.Scherer S, Mann C, Davis R W. Reversion of a promoter deletion in yeast. Nature. 1982;298:815–819. doi: 10.1038/298815a0. [DOI] [PubMed] [Google Scholar]
- 64.Schneider B L, Seufert W, Steiner B, Yang Q H, Futcher A B. Use of polymerase chain reaction epitope tagging for protein tagging in Saccharomyces cerevisiae. Yeast. 1995;11:1265–1274. doi: 10.1002/yea.320111306. [DOI] [PubMed] [Google Scholar]
- 65.Sharon G, Burkett T J, Garfinkel D J. Efficient homologous recombination of Ty1 element cDNA when integration is blocked. Mol Cell Biol. 1994;14:6540–6551. doi: 10.1128/mcb.14.10.6540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sherman F, Fink G R, Hicks J B. Saccharomyces. In: King R C, editor. Handbook of genetics. New York, N.Y: Plenum Publishing Corp.; 1986. pp. 359–393. [Google Scholar]
- 67.Sherwood P W, Osley M A. Histone regulatory (hir) mutations suppress delta insertion alleles in Saccharomyces cerevisiae. Genetics. 1991;128:729–738. doi: 10.1093/genetics/128.4.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sherwood P W, Tsang S V-M, Osley M A. Characterization of HIR1 and HIR2, two genes required for regulation of histone gene transcription in Saccharomyces cerevisiae. Mol Cell Biol. 1993;13:28–38. doi: 10.1128/mcb.13.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Taguchi A K W, Ciriacy M, Young E T. Carbon source dependence of transposable element-associated gene activation in Saccharomyces cerevisiae. Mol Cell Biol. 1984;4:61–68. doi: 10.1128/mcb.4.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Varmus H. Lessons from the life cycle of retrovirus. Harvey Lect. 1989;83:35–56. [PubMed] [Google Scholar]
- 71.Verreault A, Kaufman P D, Kobayashi R, Stillman B. Nucleosome assembly by a complex of CAF-1 and acetylated histones H3/H4. Cell. 1996;87:95–104. doi: 10.1016/s0092-8674(00)81326-4. [DOI] [PubMed] [Google Scholar]
- 72.Wilke C M, Heidler S H, Brown N, Liebman S W. Analysis of yeast retrotransposon Ty insertions at the CAN1 locus. Genetics. 1989;123:655–665. doi: 10.1093/genetics/123.4.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Winston F, Chaleff D T, Valent B, Fink G R. Mutations affecting Ty-mediated expression of the HIS4 gene of Saccharomyces cerevisiae. Genetics. 1984;107:179–197. doi: 10.1093/genetics/107.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Winston F, Dollard C, Malone E A, Clare J, Kapakos J G, Farabaugh P, Minehart P L. Three genes are required for trans-activation of Ty transcription in yeast. Genetics. 1987;115:649–656. doi: 10.1093/genetics/115.4.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xu H, Boeke J D. Inhibition of Ty1 transposition by mating pheromones in Saccharomyces cerevisiae. Mol Cell Biol. 1991;11:2736–2743. doi: 10.1128/mcb.11.5.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xu H, Kim U-J, Schuster T, Grunstein M. Identification of a new set of cell cycle-regulatory genes that regulate S-phase transcription of histone genes in Saccharomyces cerevisiae. Mol Cell Biol. 1992;12:5249–5259. doi: 10.1128/mcb.12.11.5249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang S, Burkett T J, Yamashita I, Garfinkel D J. Genetic redundancy between SPT23 and MGA2: regulators of Ty-induced mutations and Ty1 transcription in Saccharomyces cerevisiae. Mol Cell Biol. 1997;17:4718–4729. doi: 10.1128/mcb.17.8.4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zou S, Voytas D F. Silent chromatin determines target preference of the Saccharomyces retrotransposon Ty5. Proc Natl Acad Sci USA. 1997;94:7412–7416. doi: 10.1073/pnas.94.14.7412. [DOI] [PMC free article] [PubMed] [Google Scholar]