Abstract
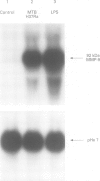
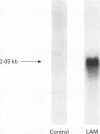
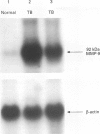
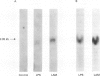
BACKGROUND: Pulmonary tuberculosis is associated with caseating necrosis, parenchymal lung destruction, and cavity formation. It was hypothesised that tuberculous lung destruction is mediated, at least in part, by the participation of matrix metalloproteinases released by mononuclear phagocytes. METHODS: Cells of the myelomonocytic leukaemia cell line THP-1 were incubated with lipoarabinomannan (LAM), the major antigenic cell wall component, and with Mycobacterium tuberculosis and analysed by Northern blot analysis. Two patients with active cavitary tuberculosis also underwent bronchoalveolar lavage and the cells were analysed by Northern blotting. RESULTS: Incubation of THP-1 cells with LAM resulted in the stimulated release of matrix metalloproteinase-9 (MMP-9), a 92 kDa gelatinase, by 24 hours in a dose-dependent fashion. In addition, Northern analysis revealed that LAM upregulated the gene for MMP-9 by 24 hours, but not the gene for the 72 kDa gelatinase MMP-2. Heat killed M tuberculosis H37Ra also upregulated the MMP-9 gene. Bronchoalveolar lavage of the two patients with active cavitary tuberculosis showed striking upregulation of the MMP-9 gene compared with a normal control using Northern analysis. LAM also upregulated the type I interstitial collagenase (MMP-1) gene by 24 hours in both THP-1 cells and peripheral blood monocytes. CONCLUSIONS: These data suggest that M tuberculosis and its major cell antigenic component, LAM, stimulate the release of MMP-9 and upregulate the expression of genes for MMP-1 and MMP-9. It is possible that M tuberculosis and its components contribute directly to cavity formation by their ability to stimulate macrophages to release matrix metallo-proteinases that digest collagens I-IV, and indirectly by stimulating the release of the cytokines interleukin 1 beta and tumour necrosis factor alpha that induce fibroblasts to amplify the release of matrix metalloproteinases.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bar-Shavit Z., Teitelbaum S. L., Stricklin G. P., Eisen A. Z., Kahn A. J., Welgus H. G. Differentiation of a human leukemia cell line and expression of collagenase inhibitor. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5380–5384. doi: 10.1073/pnas.82.16.5380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes P. F., Chatterjee D., Abrams J. S., Lu S., Wang E., Yamamura M., Brennan P. J., Modlin R. L. Cytokine production induced by Mycobacterium tuberculosis lipoarabinomannan. Relationship to chemical structure. J Immunol. 1992 Jul 15;149(2):541–547. [PubMed] [Google Scholar]
- Birkedal-Hansen H., Moore W. G., Bodden M. K., Windsor L. J., Birkedal-Hansen B., DeCarlo A., Engler J. A. Matrix metalloproteinases: a review. Crit Rev Oral Biol Med. 1993;4(2):197–250. doi: 10.1177/10454411930040020401. [DOI] [PubMed] [Google Scholar]
- Brenner D. A., O'Hara M., Angel P., Chojkier M., Karin M. Prolonged activation of jun and collagenase genes by tumour necrosis factor-alpha. Nature. 1989 Feb 16;337(6208):661–663. doi: 10.1038/337661a0. [DOI] [PubMed] [Google Scholar]
- Campbell E. J., Cury J. D., Lazarus C. J., Welgus H. G. Monocyte procollagenase and tissue inhibitor of metalloproteinases. Identification, characterization, and regulation of secretion. J Biol Chem. 1987 Nov 25;262(33):15862–15868. [PubMed] [Google Scholar]
- Chang J. C., Jagirdar J., Lesser M. Long-term evolution of BCG- and CFA-induced granulomas in rat lungs. Correlation of histologic features with cells in bronchoalveolar lavage samples. Am J Pathol. 1986 Oct;125(1):16–27. [PMC free article] [PubMed] [Google Scholar]
- Chatterjee D., Roberts A. D., Lowell K., Brennan P. J., Orme I. M. Structural basis of capacity of lipoarabinomannan to induce secretion of tumor necrosis factor. Infect Immun. 1992 Mar;60(3):1249–1253. doi: 10.1128/iai.60.3.1249-1253.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chensue S. W., Davey M. P., Remick D. G., Kunkel S. L. Release of interleukin-1 by peripheral blood mononuclear cells in patients with tuberculosis and active inflammation. Infect Immun. 1986 Apr;52(1):341–343. doi: 10.1128/iai.52.1.341-343.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark S. D., Wilhelm S. M., Stricklin G. P., Welgus H. G. Coregulation of collagenase and collagenase inhibitor production by phorbol myristate acetate in human skin fibroblasts. Arch Biochem Biophys. 1985 Aug 15;241(1):36–44. doi: 10.1016/0003-9861(85)90358-3. [DOI] [PubMed] [Google Scholar]
- Dannenberg A. M., Jr Cellular hypersensitivity and cellular immunity in the pathogensis of tuberculosis: specificity, systemic and local nature, and associated macrophage enzymes. Bacteriol Rev. 1968 Jun;32(2):85–102. doi: 10.1128/br.32.2.85-102.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannenberg A. M., Jr Pathogenesis of pulmonary tuberculosis. Am Rev Respir Dis. 1982 Mar;125(3 Pt 2):25–29. doi: 10.1164/arrd.1982.125.3P2.25. [DOI] [PubMed] [Google Scholar]
- Dayer J. M., Beutler B., Cerami A. Cachectin/tumor necrosis factor stimulates collagenase and prostaglandin E2 production by human synovial cells and dermal fibroblasts. J Exp Med. 1985 Dec 1;162(6):2163–2168. doi: 10.1084/jem.162.6.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayer J. M., de Rochemonteix B., Burrus B., Demczuk S., Dinarello C. A. Human recombinant interleukin 1 stimulates collagenase and prostaglandin E2 production by human synovial cells. J Clin Invest. 1986 Feb;77(2):645–648. doi: 10.1172/JCI112350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desrochers P. E., Jeffrey J. J., Weiss S. J. Interstitial collagenase (matrix metalloproteinase-1) expresses serpinase activity. J Clin Invest. 1991 Jun;87(6):2258–2265. doi: 10.1172/JCI115262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter S. W., Brennan P. J. Evidence for the presence of a phosphatidylinositol anchor on the lipoarabinomannan and lipomannan of Mycobacterium tuberculosis. J Biol Chem. 1990 Jun 5;265(16):9272–9279. [PubMed] [Google Scholar]
- MacNaul K. L., Chartrain N., Lark M., Tocci M. J., Hutchinson N. I. Discoordinate expression of stromelysin, collagenase, and tissue inhibitor of metalloproteinases-1 in rheumatoid human synovial fibroblasts. Synergistic effects of interleukin-1 and tumor necrosis factor-alpha on stromelysin expression. J Biol Chem. 1990 Oct 5;265(28):17238–17245. [PubMed] [Google Scholar]
- Matrisian L. M. Metalloproteinases and their inhibitors in matrix remodeling. Trends Genet. 1990 Apr;6(4):121–125. doi: 10.1016/0168-9525(90)90126-q. [DOI] [PubMed] [Google Scholar]
- Moreno C., Taverne J., Mehlert A., Bate C. A., Brealey R. J., Meager A., Rook G. A., Playfair J. H. Lipoarabinomannan from Mycobacterium tuberculosis induces the production of tumour necrosis factor from human and murine macrophages. Clin Exp Immunol. 1989 May;76(2):240–245. [PMC free article] [PubMed] [Google Scholar]
- Reynolds J. J. The molecular and cellular interactions involved in connective tissue destruction. Br J Dermatol. 1985 Jun;112(6):715–723. doi: 10.1111/j.1365-2133.1985.tb02343.x. [DOI] [PubMed] [Google Scholar]
- Senior R. M., Campbell E. J., Landis J. A., Cox F. R., Kuhn C., Koren H. S. Elastase of U-937 monocytelike cells. Comparisons with elastases derived from human monocytes and neutrophils and murine macrophagelike cells. J Clin Invest. 1982 Feb;69(2):384–393. doi: 10.1172/JCI110462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior R. M., Griffin G. L., Fliszar C. J., Shapiro S. D., Goldberg G. I., Welgus H. G. Human 92- and 72-kilodalton type IV collagenases are elastases. J Biol Chem. 1991 Apr 25;266(12):7870–7875. [PubMed] [Google Scholar]
- Takashima T., Ueta C., Tsuyuguchi I., Kishimoto S. Production of tumor necrosis factor alpha by monocytes from patients with pulmonary tuberculosis. Infect Immun. 1990 Oct;58(10):3286–3292. doi: 10.1128/iai.58.10.3286-3292.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis R. S., Amir-Tahmasseb M., Ellner J. J. Induction of interleukin 1 and tumor necrosis factor by mycobacterial proteins: the monocyte western blot. Proc Natl Acad Sci U S A. 1990 May;87(9):3348–3352. doi: 10.1073/pnas.87.9.3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welgus H. G., Campbell E. J., Cury J. D., Eisen A. Z., Senior R. M., Wilhelm S. M., Goldberg G. I. Neutral metalloproteinases produced by human mononuclear phagocytes. Enzyme profile, regulation, and expression during cellular development. J Clin Invest. 1990 Nov;86(5):1496–1502. doi: 10.1172/JCI114867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welgus H. G., Connolly N. L., Senior R. M. 12-o-Tetradecanoyl-phorbol-13-acetate-differentiated U937 cells express a macrophage-like profile of neutral proteinases. High levels of secreted collagenase and collagenase inhibitor accompany low levels of intracellular elastase and cathepsin G. J Clin Invest. 1986 May;77(5):1675–1681. doi: 10.1172/JCI112485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welgus H. G., Fliszar C. J., Seltzer J. L., Schmid T. M., Jeffrey J. J. Differential susceptibility of type X collagen to cleavage by two mammalian interstitial collagenases and 72-kDa type IV collagenase. J Biol Chem. 1990 Aug 15;265(23):13521–13527. [PubMed] [Google Scholar]
- Werb Z., Banda M. J., Jones P. A. Degradation of connective tissue matrices by macrophages. I. Proteolysis of elastin, glycoproteins, and collagen by proteinases isolated from macrophages. J Exp Med. 1980 Nov 1;152(5):1340–1357. doi: 10.1084/jem.152.5.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Doerfler M., Lee T. C., Guillemin B., Rom W. N. Mechanisms of stimulation of interleukin-1 beta and tumor necrosis factor-alpha by Mycobacterium tuberculosis components. J Clin Invest. 1993 May;91(5):2076–2083. doi: 10.1172/JCI116430. [DOI] [PMC free article] [PubMed] [Google Scholar]