Abstract
Replication factor C (RF-C), an auxiliary factor for DNA polymerases δ and ɛ, is a multiprotein complex consisting of five different polypeptides. It recognizes a primer on a template DNA, binds to a primer terminus, and helps load proliferating cell nuclear antigen onto the DNA template. The RFC2 gene encodes the third-largest subunit of the RF-C complex. To elucidate the role of this subunit in DNA metabolism, we isolated a thermosensitive mutation (rfc2-1) in the RFC2 gene. It was shown that mutant cells having the rfc2-1 mutation exhibit (i) temperature-sensitive cell growth; (ii) defects in the integrity of chromosomal DNA at restrictive temperatures; (iii) progression through cell cycle without definitive terminal morphology and rapid loss of cell viability at restrictive temperatures; (iv) sensitivity to hydroxyurea, methyl methanesulfonate, and UV light; and (v) increased rate of spontaneous mitotic recombination and chromosome loss. These phenotypes of the mutant suggest that the RFC2 gene product is required not only for chromosomal DNA replication but also for a cell cycle checkpoint. It was also shown that the rfc2-1 mutation is synthetically lethal with either the cdc44-1 or rfc5-1 mutation and that the restrictive temperature of rfc2-1 mutant cells can be lowered by combining either with the cdc2-2 or pol2-11 mutation. Finally, it was shown that the temperature-sensitive cell growth phenotype and checkpoint defect of the rfc2-1 mutation can be suppressed by a multicopy plasmid containing the RFC5 gene. These results suggest that the RFC2 gene product interacts with the CDC44/RFC1 and RFC5 gene products in the RF-C complex and with both DNA polymerases δ and ɛ during chromosomal DNA replication.
In the yeast Saccharomyces cerevisiae, three distinct DNA polymerases, I (α), II (ɛ), and III (δ), are required for chromosomal DNA replication (7, 35). It has been believed that polymerase I (α)-DNA primase complex is required to initiate the synthesis of the leading strand and to prime each Okazaki fragment during the synthesis of the lagging strand, while polymerase II (ɛ) and polymerase III (δ) participate in the subsequent elongation step of DNA replication (28, 35). To switch DNA synthesis from polymerase α to polymerase δ or polymerase ɛ, two accessory proteins, proliferating cell nuclear antigen (PCNA) and replication factor C (RF-C), are believed to be required. PCNA is a processivity factor for polymerases δ and ɛ, and RF-C is an auxiliary protein for DNA polymerases δ and ɛ that recognizes the primer-template junction, binds to the primer terminus, and helps load PCNA onto the DNA template (7, 12, 36). Subsequently, either polymerase δ or polymerase ɛ binds to primed DNA template through PCNA and processively elongates a DNA strand.
Yeast RF-C complex consists of five different polypeptides (12–14). Recently, the genes for all RF-C subunits (CDC44/RFC1, RFC2, RFC3, RFC4, and RFC5) were cloned and sequenced (9, 13, 20, 24, 25, 30). The deduced amino acid sequences show significant homology with each other. Despite this sequence similarity, each subunit is essential for cell proliferation, suggesting that each subunit plays an important role in RF-C function during DNA replication (9, 13, 20, 24, 25, 30). Besides its role in DNA replication, the RF-C complex might be required for other cellular processes, such as DNA repair and recombination. It is also possible that some of the RF-C subunits are a target for regulatory mechanisms acting at the S- to M-phase transition during the cell cycle.
Isolation of conditionally lethal mutations in the RF-C genes might be helpful to understand the function of each subunit in vivo. Cells having a cold-sensitive mutation in the CDC44 gene, which encodes the largest subunit of RF-C, showed the terminal morphology that is typical of DNA replication mutants, arresting as a large-budded cell with a single undivided nucleus at restrictive temperatures (20). The mutant cells also exhibited spontaneous mutator- and hyperrecombinogenic phenotypes and were more sensitive than a wild-type strain to DNA-damaging agents. Thus, it was concluded that the largest subunit of RF-C is required not only for DNA replication but also for DNA repair (20, 26, 27). Growth defects of cold-sensitive cdc44 mutant cells could be suppressed by point mutations in the POL30/PCNA gene, suggesting that the largest subunit of the RF-C complex interacts with PCNA (26).
A temperature-sensitive mutant (rfc5-1) in the RFC5 gene encoding the second-largest subunit of RF-C has been isolated during screening for conditionally lethal mutations that can be suppressed by a multicopy plasmid of the SPK1 (RAD53/SAD1/MEC2) protein kinase gene (34). In rfc5-1 mutant cells, DNA replication was also stalled at restrictive temperatures, but in contrast to cdc44 mutants as well as other DNA replication mutants, rfc5-1 mutant cells proceeded through the cell cycle without arresting at the G2 phase and subsequently died. Furthermore, mutant cells started mitosis in the presence of a DNA synthesis inhibitor, hydroxyurea (HU). Thus, it was concluded that the rfc5-1 mutant has defects in both DNA replication and an S-phase checkpoint. Growth defects, but not cell-cycle checkpoint defects of the mutant could be suppressed by a multicopy plasmid of POL30/PCNA, similar to defects in the cdc44 mutants (33, 34).
In this study we describe the isolation and characterization of a thermosensitive mutation (rfc2-1) of the RFC2 gene, encoding the third-largest subunit of the RF-C complex. Our results suggest that the rfc2-1 mutant has defects in both DNA replication and a cell cycle checkpoint. The phenotypes of rfc2-1 mutant cells are very similar to those of rfc5-1 mutant cells. Moreover, both growth and checkpoint defects of thermosensitive rfc2-1 mutant cells can be suppressed by a multicopy plasmid of the RFC5 gene, suggesting that Rfc2 interacts with Rfc5 in the RF-C complex and is in the same pathway as the cell cycle checkpoint. However, unlike the rfc5-1 mutation, the mutation was not suppressed by overproduction of SPK1 (RAD53/MEC2/SAD1). Synthetic lethality analysis with the rfc2-1 mutation strongly supports the notion that Rfc2 interacts with Rfc1/Cdc44, Rfc5, and DNA polymerases δ and ɛ during chromosomal DNA replication.
MATERIALS AND METHODS
Yeast strains and media.
The S. cerevisiae strains used in this study are listed in Table 1. Strain YVN1 was constructed from strain YHA1 by transformation with plasmid YEpRFC2, followed by one-step replacement of one copy of the RFC2 gene with the LEU2 gene. The correct gene replacement was verified by Southern blot hybridization. Strain YVN2 is a meiotic segregant of diploid YVN1. Strains YVN3 and YVN4 were derived from strain YVN2 by plasmid shuffling. Strain YVN11 is a meiotic segregant of diploid created by crossing YVN2 with 15Dαu. Strains YVN12 and YVN13 were derived from strain YVN11 by plasmid shuffling. Strain YVN21 was obtained from strain SLD101 by transformation with plasmid YEprfc2-1, followed by disruption of one copy of the chromosomal RFC2 gene with the LEU2 gene. Strain YVN22 is a mitotic segregant of YVN21 strain. Strain YVN23 was derived from strain YVN22 by plasmid shuffling. The remaining strains are meiotic segregants isolated from the following crosses: CH609 × 15Dau (9B-YVN15), 9B-YVN15 × YVN11 (7C-YVN16), SS111-pol2-11 × L1-CG379 (4B-YVN25), 4B-YVN25 × YVN11 (3C-YVN27), 2749-2-2 × L1-CG379 (3D-YVN26), 3D-YVN26 × YVN11 (1B-YVN28), SS111-pol1-17 × L1-CG379 (1A-YVN29), 1A-YVN29 × YVN11 (12C-YVN30), and KSC766 × YVN11 (6B-YVN31).
TABLE 1.
Yeast strains used in the present study
| Strain | Genotype | Reference or source |
|---|---|---|
| YHA1 | MATa/MATα leu2-3,112/leu2-3,112 ura3-52/ura3-52 trp1-289/trp1-289 ade5-1/ade5-1 HIS7/his7-2 CAN1/can1 | 1 |
| YVN1 | Same as YHA1 but RFC2/rfc2Δ::LEU2 [YEpRFC2] | This study |
| YVN2 | MATa his7-2 trp1-289 leu2-3,112 ura3-52 ade5-1 rfc2Δ::LEU2 [YEpRFC2] | This study |
| YVN3 | MATa his7-2 trp1-289 leu2-3,112 ura3-52 ade5-1 rfc2Δ::LEU2 [YCpRFC2] | This study |
| YVN4 | MATa his7-2 trp1-289 leu2-3,112 ura3-52 ade5-1 rfc2Δ::LEU2 [YCprfc2-1] | This study |
| YVN11 | MATα leu2 trp1 ura3 ade5-1 his2 his7 rfc2Δ::LEU2 [YEpRFC2] | This study |
| YVN12 | MATα leu2 trp1 ura3 ade5-1 his2 his7 rfc2Δ::LEU2 [YCpRFC2] | This study |
| YVN13 | MATα leu2 trp1 ura3 ade5-1 his2 his7 rfc2Δ::LEU2 [YCprfc2-1] | This study |
| 15Dau | MATa ade1 his2 leu2 trp1 ura3 | 8 |
| 15Dαu | MATα ade1 his2 leu2 trp1 ura3 | 8 |
| SLD101 | MATa/MATα lys2/lys2 ura3/ura3 leu2::hisG/leu2::his trp1::hisG/trp1::hisG his4X/his4B | 22 |
| YVN21 | Same as SLD101 but RFC2/rfc2Δ::LEU2 [YEprfc2-1] | This study |
| YVN22 | Same as SLD101 but rfc2Δ::LEU2/rfc2Δ::LEU2 [YCprfc2-1] | This study |
| YVN23 | Same as SLD101 but rfc2Δ::LEU2/rfc2Δ::LEU2 [YCpRFC2] | This study |
| CH609 | MATα his4 cdc44-1 | 20 |
| KSC766 | MATa ade2 his2 ura3 trp1 lys2 leu2 rfc5-1 | 34 |
| SS111-pol1-17 | MATa ura3-1,2 trp1-289 ade2-101 his3 gal2 can1 pol1-17 | 4 |
| SS111-pol2-11 | MATa ura3-1,2 trp1-289 ade2-101 his3 gal2 can1 pol2-1 | 5 |
| 2749-2-2 | MATa his7 leu2 cdc2-2 | 18 |
| L1-CG379 | MATα ade5-1 his7 leu2-3,112 trp1-289 ura3-52 lys2::Tn5-13 | 16 |
| 9B-YVN15 | MATa trp1 leu2 ura3 cdc44-1 | This study |
| 7C-YVN16 | MATa trp1 ura3 leu2 ade5-1 cdc44-1 rfc2Δ::LEU2 [YEpRFC2] | This study |
| 4B-YVN25 | MATa leu2-3,112 ura3 trp1-289 ade5-1 lys2::Tn5-13 pol2-11 | This study |
| 3D-YVN26 | MATa his7 leu2-3,112 ura3 trp1-289 lys2::Tn5-13 cdc2-2 | This study |
| 3C-YVN27 | MATa trp1 ura3 leu2 ade5-1 lys2::Tn5-13 pol2-11 rfc2Δ::LEU2 [YEpRFC2] | This study |
| 1B-YVN28 | MATα trp1 ura3 leu2 ade5-1 his7 his2 cdc2 rfc2Δ::LEU2 [YEpRFC2] | This study |
| 1A-YVN29 | MATa leu2-3,112 ura3 trp1-289 lys2::Tn5-13 pol1-17 | This study |
| 12C-YVN30 | MATa trp1 ura3 leu2 lys2::Tn5-13 pol1-17 rfc2Δ::LEU2 [YEpRFC2] | This study |
| 6B-YVN31 | MATa trp1 ura3 leu2 his2 ade2 lys2 rfc5-1rfc2Δ::LEU2 [YEpRFC2] | This study |
Standard rich (yeast extract-peptone-dextrose [YPD]) and synthetic dextrose minimal (SD) media were used (21). SD medium supplemented with 40 μg of l-canavanine sulfate (Sigma) per ml was used for selection of can1 mutants. Medium containing 5-fluoroorotic acid (5-FOA) (3) was used for plasmid shuffling.
Plasmids and yeast genomic DNA library.
Vectors pBluescript II SK(+), YEplac195, and YCplac22 (15) were used for plasmid construction. The plasmid for disruption of the RFC2 gene, pBS rfc2Δ::LEU2, was constructed as follows. To remove the SspI sites from the vector, pBluescript IISK(+) DNA was digested with SspI and SmaI and then self ligated. To the resulting plasmid DNA, the 2.5-kb HindIII-XhoI fragment from originally isolated RFC2 clone (30) was inserted. Finally, the SspI fragment in the RFC2 gene was substituted with the 2.0-kb HpaI fragment of the LEU2 gene. Plasmids YEpRFC2 and YCpRFC2 were constructed by inserting the 1.24-kb HindIII-BamHI fragment containing the RFC2 gene into a respective vector. Both HindIII and BamHI restriction sites were introduced into both ends of the RFC2 gene when it was amplified by PCR. Plasmids YEprfc2-1 and YCprfc2-1 were constructed the same way as plasmids YEpRFC2 and YCpRFC2 except that the mutant rfc2-1 gene was used. Plasmids YEpRFC1, YEpRFC3, and YEpRFC4 were constructed by subcloning the RFC1, RFC2, and RFC3 genes (9), obtained from G. Cullman and B. Stillman (Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.), into vector YEplac195. YEpRFC1 contains the RFC1 gene on the 3.5-kb SacI-KpnI fragment from plasmid pKSRFC1; YEpRFC3 contains the RFC3 gene on the 2.3-kb SphI-PstI fragment from plasmid pSKRFC3; and YEpRFC4 contains the RFC4 gene on the 1.3-kb HindIII fragment from plasmid pSKRFC4H-1. Plasmids YEpSPK1 (34), pRS426-TEL1, and pRS426-MEC1 were gifts from K. Sugimoto (Nagoya University). Yeast genomic DNA library for plasmid YEp24 was provided by H. Ogawa (Osaka University).
Random mutagenesis of the RFC2 gene.
The RFC2 gene was mutagenized by modified PCR (23). Oligonucleotides N-1, 5′-ATCAGGAAGCTTCTCAAGCGAACAAGTCAA-3′ (corresponding to nucleotides 776 to 805) (GenBank accession no. D28499) and N-2, 5′-AGGTACCGGATCCGATAAGAGGAATTATGGATAGA-3′ (corresponding to nucleotides 2020 to 1998 of the sequence [GenBank accession no. 28499]) were used to amplify the RFC2 gene (35 cycles of 94°C for 1 min, 50°C for 1 min, and 72°C for 3 min) with the cloned RFC2 gene (30) as a template. The HindIII and BamHI restriction sites introduced into the oligonucleotides are underlined. The resulting 1,258-bp PCR product was digested with HindIII and BamHI, ligated with the HindIII-BamHI-digested vector YCplac22, and used for Escherichia coli transformation. Approximately 5,000 ampicillin-resistant transformants were obtained. Plasmid DNA was extracted from them and used to transform yeast strain YVN2 to Trp+. The transformants were tested for their temperature-sensitive cell growth by replica plating onto three sets of SD-Trp-Ura plates, which were then incubated either at 16, 25, or 37°C. No temperature-sensitive colonies were observed. Then the transformants grown at 25°C were replica plated onto medium containing 5-FOA to select colonies containing only the mutagenized RFC2 gene. Of the 2,000 5-FOA-resistant colonies assayed, one transformant which was able to grow at 25 and 16°C, but not at 37°C, was obtained. From this transformant, plasmid DNA was recovered and named YCprfc2-1.
Measurement of DNA synthesis.
YVN4 (rfc2-1) cells grown in YPD medium at 25°C to 3 × 106 cells/ml were divided into two portions. Each portion was incubated at either 25 or 37°C, and a 10-ml aliquot was withdrawn at 1-hour intervals. Cells were then harvested by centrifugation, and total DNA was measured by the diphenylamine procedure (32, 32a).
Measurement of spontaneous mutation and recombination rates.
The frequency of spontaneous mutation at CAN was measured as previously described (31). Spontaneous frequency of gene conversion events in the HIS4 locus was determined as follows. Nine independent colonies of each strain were inoculated into 5 ml of YPD medium and incubated at 25°C for 2 days. Cells were collected by centrifugation, resuspended in distilled water, and sonicated. After appropriate dilutions, cells were plated onto SD-His and YPD medium and incubated at 25°C for 5 days. Frequencies of His+ appearance in nine independent cultures from one strain were compared with those of a wild-type strain by the Wilcoxon-Mann-Whitney nonparametric criterion (10).
Measurement of EMS-induced mutation frequencies.
Strains to be tested were grown in YPD medium overnight at 25°C. Cells were harvested by centrifugation, resuspended in fresh YPD medium to 2 × 107 cells/ml, and divided into portions of 5 ml each. Ethyl methanesulfonate (EMS) was added into cell suspensions. Cells were incubated at 25°C for 4 h with extensive shaking, collected by centrifugation, and washed twice with distilled water. Finally, the cells were resuspended in 0.5 ml of water, sonicated, and plated onto canavanine-containing SD and YPD plates, which were incubated at 25°C for 5 days.
Other procedures.
Cells were prepared for fluorescence-activated cell sorter analysis and stained with propidium iodide as previously described (2, 34). All media and growth conditions were as described previously (2, 34) unless otherwise noted.
RESULTS
Isolation of thermosensitive rfc2 mutants.
To generate temperature-sensitive mutations in the RFC2 gene encoding the third-largest subunit of the RF-C complex, we used a random PCR mutagenesis procedure (see Materials and Methods). A mutagenized RFC2 gene library was used to transform S. cerevisiae strain YVN2, in which the RFC2 gene is deleted from the chromosome but is on a multicopy plasmid. After plasmid shuffling, the transformants carrying the mutagenized RFC2 gene on the centromere plasmid were selected and examined for temperature sensitivity for cell growth. Among 2,000 transformants tested, only one colony which was able to grow at 16°C and 25°C, but not at 37°C, was obtained. From this transformant, plasmid DNA was isolated and named YCprfc2-1. To confirm that the mutation conferring thermosensitive growth is in the RFC2 gene but not in the vector DNA, the 1.24-kb HindIII-BamHI fragment from plasmid YCprfc2-1 was recloned into vector YCplac22. The resulting plasmid was used to transform strain YVN2. Transformants were replica plated onto the 5-FOA medium to select for the transformants containing only YCprfc2-1. All 5-FOA-resistant colonies were able to grow at 25°C but not at 37°C. Thus, the rfc2-1 mutation conferring thermosensitive cell growth was in the HindIII-BamHI fragment.
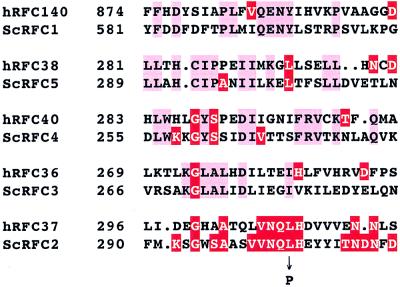
DNA sequencing of the rfc2-1 allele revealed a single-base-pair substitution, AT→GC, resulting in an amino acid change of Leu304 to Pro304 (Fig. 1) in the coding region of RFC2 (30). This region of the RFC2 gene shows no significant homology with other subunits of the yeast RF-C (9). However, it has extensive similarity with the 37-kDa subunit of human RF-C (9, 30), suggesting that this region is important for its specific function. In this context, it is interesting to note that the Leu304 residue in this region is conserved in RFC5 (Fig. 1).
FIG. 1.
Amino acid change caused by the rfc2-1 mutation. The mutation converts leucine at position 304 into proline. The region of S. cerevisiae (Sc) Rfc2p in the vicinity of the mutation site has a high level of similarity with an appropriate region of the 37-kDa RF-C subunit of human (h). The corresponding regions of amino acid sequence of other subunits of both human and S. cerevisiae RF-C complex are shown. Identical amino acids are shown by red boxes. Amino acid sequence is shown by the single-letter code.
Phenotype of the rfc2-1 mutant.
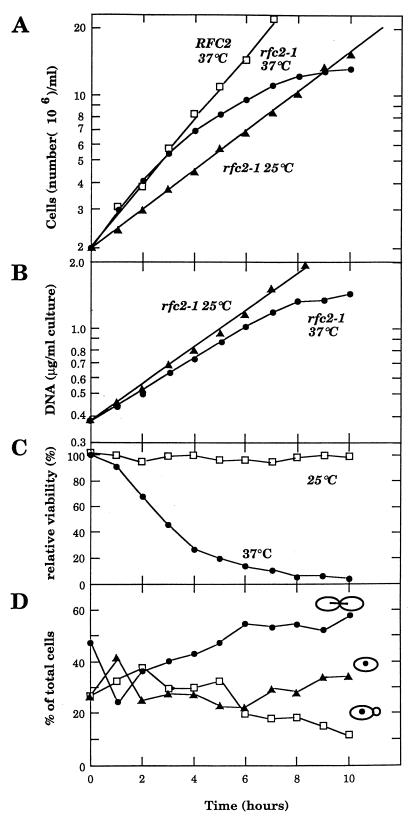
It has been shown that DNA polymerases δ and ɛ require RF-C activity together with PCNA and replication protein A (RP-A) when they elongate a DNA strand on a singly primed single-stranded DNA in vitro (6). Therefore, together with the fact that the gene encoding each subunit of the RF-C complex is essential for cell growth, it has been believed that they are required for chromosomal DNA replication. To examine whether DNA replication is defective in the rfc2-1 mutant, rfc2-1 cells grown at 25°C were divided into two portions, and each was incubated at either 25 or 37°C. During incubation, cell number, amount of DNA, and cell viability were measured. As shown in Fig. 2A, the growth rate of the mutant was faster at 37°C than at 25°C, and the number of cells in the mutant increased sevenfold at 37°C before leveling off, indicating that mutant cells undergo cell division two or three times after temperature shifts up. On the other hand, the total amount of DNA in the mutant cells was consistently lower at 37°C than that at 25°C after the temperature shifted up (Fig. 2B), but the increase in the total DNA in the mutant cells ceased in about 8 h at 37°C. Cell viability measurement indicated that mutant cells quickly lost their viability during incubation at the restrictive temperature (only 16% of the cells were viable after 6 h) (Fig. 2C). This quick loss of viability was prevented by treating mutant cells with α-factor (late G1 arrest) or benomyl (G2 arrest) before shifting to the restrictive temperature. After 6 h of incubation at 37°C, viabilities of the G1-arrested cells and the benomyl-treated cells were 55 and 85%, respectively, suggesting that loss of viability is associated with cell cycle progression, either from M to G1 phase and/or from S to M phase. The viability of cells arrested at G1 was lower than that of cells arrested at G2 because of the poor synchronization of the mutant with the α-factor (data not shown).
FIG. 2.
Growth characteristics of the rfc2 mutant at restrictive temperatures. YVN3 (RFC2) and YVN4 (rfc2-1) cells grown at 25°C were divided and incubated either at 25 or 37°C. An aliquot of cell suspension was withdrawn every hour, the number of cells was counted, and total amount of DNA, cell viability, and cell morphology were assessed. (A) Growth rate of yeast cells at 25 and 37°C was measured with an automated Coulter counter. (B) DNA synthesis in rfc2 cells at 25 and 37°C was measured as described in Materials and Methods. (C) Viability of the rfc2 mutant at 25 and 37°C was determined by plating diluted cell suspension onto YPD medium at 25°C. (D) Cell morphology (unbudded cell, small-budded cell, and large-budded [dumbbell-shaped]) was monitored with a microscope.
Cell morphology of the mutant was also observed by microscopy. Although the population of dumbbell-shaped cells increased at the restrictive temperature, mutant cells did not exhibit any distinct terminal cell morphology, unlike mutants in the typical cell cycle. After 10 h at 37°C, about 60% of rfc2-1 cells arrested as large-budded cells, and cell viability rapidly dropped to 1% (Fig. 2D).
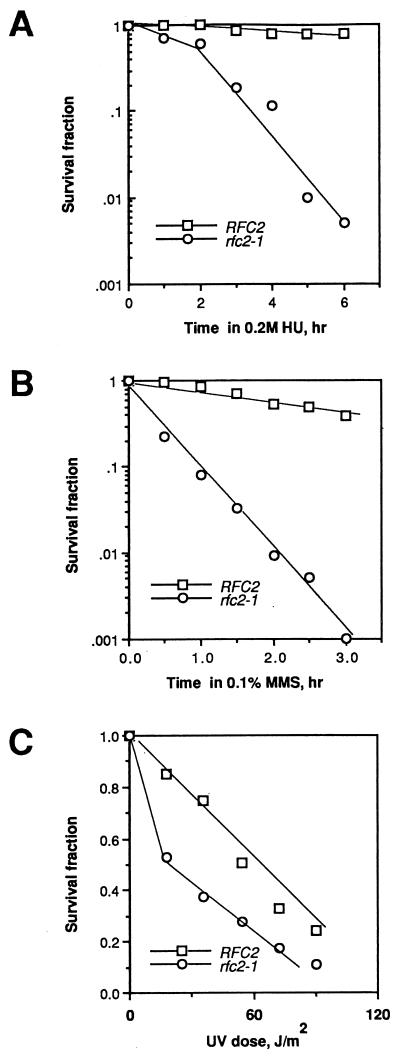
We also examined cell sensitivity to a DNA synthesis inhibitor, HU, and to DNA-damaging agents methyl methanesulfonate (MMS) and UV light. As shown in Fig. 3, rfc2-1 mutant cells were extremely sensitive to HU and MMS and moderately sensitive to UV light.
FIG. 3.
The rfc2 mutant is sensitive to HU and DNA-damaging agents. (A) Sensitivity of YVN3 (RFC2) and YVN4 (rfc2-1) strains to HU. Both strains were grown at 25°C to a density of 2 × 106 cells/ml, and 0.2 M HU was added. During incubation in the presence of HU, 1-ml aliquots were withdrawn every hour, and the total number of cells was determined with an automated Coulter counter. Viable cell number was estimated by plating diluted cell suspension onto YPD medium and incubating at 25°C for 3 days. (B) Sensitivity of strains YVN3 and YVN4 to MMS. Cells grown at 25°C were harvested and resuspended in 50 mM sodium phosphate buffer, pH 7.0, and MMS was added to the suspension at a final concentration of 0.1%. One-milliliter aliquots were withdrawn every 30 min at 25°C, washed with distilled water, made into various dilutions, and plated onto YPD plates. The plates were incubated at 25°C for 3 days, and the colonies were counted. (C) Sensitivities of strains YVN3 and YVN4 to UV light. Cell suspensions grown at 25°C were diluted and spread onto YPD plates. The plates were exposed to UV irradiation as indicated and incubated at 25°C for 3 days.
rfc2-1 mutant has a defect in DNA integrity.
As shown in Fig. 2B, we could not detect any obvious defect in DNA synthesis in rfc2-1 mutant cells at the restrictive temperature. Nevertheless, chromosomal DNA from rfc2-1 mutant cells was also analyzed by pulsed-field agarose gel electrophoresis. In this assay, only fully replicated chromosomes enter the gel and migrate properly (19). Chromosomes prepared from the rfc2-1 strain incubated at the restrictive temperature entered into the gel with reduced efficiency compared with that of the chromosomes prepared from wild-type cells grown at 25 or 37°C. In a control, chromosomes of wild-type cells in the presence of HU did not enter into the gel (data not shown). These results suggest that mutant rfc2-1 shows no obvious change in DNA synthesis but has a defect in DNA integrity at the restrictive temperature.
rfc2-1 mutant has a defect in S-phase checkpoint.
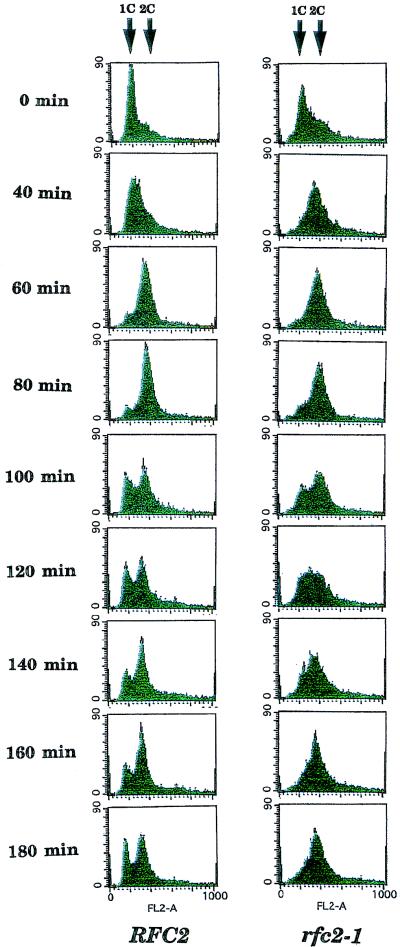
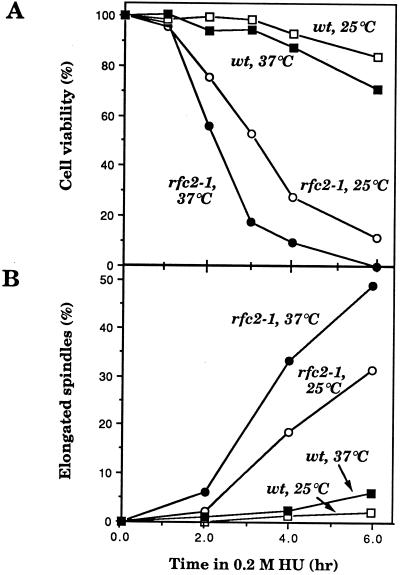
Wild-type and rfc2-1 mutant cells grown at 25°C were arrested with α-factor released at 37°C. Aliquots of cells were withdrawn, and DNA content was analyzed by flow cytometry. As shown in Fig. 4, mutant cells proceeded through the cell cycle as well as wild-type cells, and no significant defect in the mutant’s DNA synthesis could be detected during the first round of the cell cycle at 37°C. Taken together with the results of the pulsed-field agarose gel electrophoresis, these results suggest that the S-phase checkpoint is defective in the mutant at the restrictive temperature. This S-phase checkpoint defect was tested more directly with the DNA synthesis inhibitor HU. Wild-type and rfc2-1 mutant cells grown at 25°C were arrested with α-factor released into HU at 37°C. HU-treated cells defective at the S-phase checkpoint should enter into mitosis, as evidenced by partial spindle elongation before completion of DNA replication. DNA replication was efficiently blocked in wild-type and rfc2-1 mutant cells 2 h after release into HU (data not shown). Under these conditions, most wild-type cells (90%) arrested as large-budded cells with short spindles, and more than 80% of the cells remained viable for 6 h after release (Fig. 5). In contrast, 50% of rfc2-1 cells exhibited partially elongated spindles, and less than 1% of mutant cells were variable during the incubation (Fig. 5). These results support the notion that the rfc2-1 mutation is defective in the S-phase checkpoint in response to HU.
FIG. 4.
YVN3 (RFC2) and YVN4 (rfc2-1) cells were grown at 25°C and treated with 5 μg of α-factor per ml for 2.5 h. Then 0.1 mg of actinase per ml was added to the cell culture, and the culture was incubated at 37°C. At the indicated times, aliquots of cells were withdrawn, cells were collected by centrifugation and fixed with ethanol, and DNA was stained as described previously (34). The DNA content of cells was analyzed with a Becton Dickinson fluorescence-activated cell sorter. Arrows indicate the positions of 1C and 2C cells.
FIG. 5.
Spindle abnormalities observed in the rfc2-1 mutant during incubation in the presence of HU. YVN3 (RFC2) and YVN4 (rfc2-1) cells were synchronized by α-factor (5 μg/ml) for 2.5 h and released from the factor by treatment with actinase (0.1 mg/ml), and 0.2 M HU was added to the culture. Cells were divided into two portions and cultivated at 25 or 37°C. At the indicated time after release from α-factor treatment, aliquots were withdrawn to examine cell viability (A) and nuclear and spindle morphologies (B and C). Nuclear and microtubulin structures were visualized with 4′,6-diamidino-2-phenylindole (DAPI) and anti-tubulin antibodies, respectively, as before (32, 34). (A and B) Open and closed circles, mutant cells grown at 25 and 37°C, respectively; open and closed squares, wild-type (wt) cells grown at 25 and 37°C, respectively.
Spontaneous and EMS-induced mutagenesis.
Since the rfc2-1 mutant was more sensitive to DNA-damaging agents than a wild-type strain, it is possible that Rfc2p is also involved in DNA repair. We therefore examined the effect of the rfc2-1 mutation on spontaneous and EMS-induced mutagenesis. To determine whether the rfc2-1 mutation affects spontaneous mutagenesis, we measured mutation frequency at the CAN1 locus in rfc2-1 (YVN13) mutant and isogenic wild-type (YVN12) cells. The results of the fluctuation test are summarized in Table 2. Statistical analysis indicated that the difference in spontaneous mutation frequencies between the two strains is not significant (P > 0.05).
TABLE 2.
Effect of the rfc2-1 mutation on spontaneous mutation and mitotic recombination rates
| Strain | Relevant genotypea | Scored event | Frequencyb | rfc2-1/ RFC2 |
|---|---|---|---|---|
| YVN12 | rfc2Δ [YCpRFC2] | Forward mutations in CAN1 locus | 3.45 × 10−6 | 1.6 |
| YVN13 | rfc2Δ [YCprfc2-1] | 5.48 × 10−6 | ||
| YVN23 | rfc2Δ/rfc2Δ [YCpRFC2] | Gene conversion events in the HIS4 locus | 0.60 × 10−4 | 4.1 |
| YVN22 | rfc2Δ/rfc2Δ [YCprfc2-1] | 2.46 × 10−4 |
The rest of the genotypes are listed in Table 1.
Frequencies were measured at 25°C. Nine independent clones were analyzed for each strain. Only median values of the frequencies are presented.
As shown in Table 3, although EMS was more toxic to the rfc2 mutant than to the wild-type strain, the frequency of can1 mutation in the mutant was not significantly different from that in a wild-type strain. These results suggest that the rfc2-1 mutant is not defective in the repair of EMS-induced DNA damage. Rather, the increased sensitivity of the rfc2-1 mutant to EMS may be attributed to its deficiency at the DNA damage checkpoint.
TABLE 3.
EMS-induced mutation frequencies in the wild-type and rfc2-1 mutant strains
| Strain | EMS dose (μg/ml) | Survival fraction (%) | Frequency of Canr mutants (105)a |
|---|---|---|---|
| YVN12 (wild type) | 0 | 100.0 | 0.48 ± 0.10 |
| 100 | 97.2 | 2.70 ± 0.27 | |
| 200 | 99.8 | 3.69 ± 0.32 | |
| 400 | 84.4 | 6.56 ± 0.51 | |
| 800 | 79.6 | 8.26 ± 0.62 | |
| 1,600 | 72.3 | 16.34 ± 1.09 | |
| YVN13 (rfc2-1) | 0 | 100.0 | 1.28 ± 0.16 |
| 100 | 77.6 | 3.75 ± 0.35 | |
| 200 | 64.1 | 7.24 ± 0.60 | |
| 400 | 63.5 | 9.29 ± 0.73 | |
| 800 | 46.4 | 14.01 ± 1.16 | |
| 1,600 | 24.8 | 20.83 ± 2.21 |
Mutation frequencies, expressed as means ± standard deviations, were measured at 25°C.
Mitotic recombination and chromosome stability.
To compare the level of spontaneous mitotic recombination in the HIS4 locus in the rfc2-1 mutant (YVN22) and an isogenic wild-type strain (YVN23), we performed the fluctuation test. As shown in Table 2, the median value of recombination frequencies was 4.1-fold higher in rfc2-1 mutant cells than in wild-type cells. Although this increase was relatively small compared with that observed for mutants with other replication defects (20), the difference was statistically significant (P < 0.05).
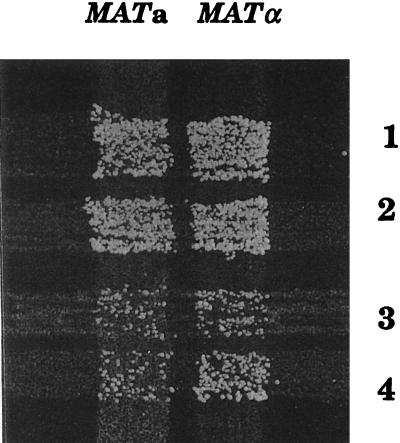
To estimate the frequency of chromosome loss in rfc2-1 mutant cells, we crossed independent clones of YVN22 diploid strain homozygous for the rfc2-1 mutation and transformants of YVN22 strain harboring plasmid YCpRFC2 with MATa and MATα testers. As shown in Fig. 6, the rfc2-1 diploid strain could efficiently mate with both tester strains, while the addition of a wild-type copy of the RFC2 gene greatly reduced its efficiency. Since the mating ability of the mutant is likely due to chromosome III loss (17), these results imply that chromosomes in rfc2-1 mutant cells are unstable even at the permissive temperature of 25°C.
FIG. 6.
rfc2-1 mutation causes a chromosome loss. Two clones of the YVN22 (rfc2-1) diploid strain (streaks 1 and 2) and two transformants of YVN22 harboring YCpRFC2 plasmid (streaks 3 and 4) were crossed with MATa lys2 and MATα lys2 tester strains. Crosses were grown at 25°C overnight on complete YPD medium, replica plated on minimal SD medium, and incubated at 25°C for 2 days. Growth of colonies suggests loss of chromosome III.
Multicopy suppressor of the rfc2-1 mutation.
To identify proteins interacting with the RFC2 gene product, we looked for genes on a multicopy plasmid which suppress the thermosensitive growth phenotype of the rfc2-1 mutant. Strain YVN4 having the rfc2-1 mutation was transformed with a multicopy-based yeast genomic DNA library, plated onto SD-Ura medium, and incubated at 25°C until small colonies appeared. Then the plates were further incubated at 34.5°C for another 4 days. Of approximately 5,000 Ura+ transformants, two colonies were able to grow at 34.5°C. Plasmids recovered from these two transformants were subjected to restriction endonuclease analysis. It was revealed that both plasmids contain overlapping regions of a yeast DNA insert. The overlapping region of the insert DNA contained two genes, POL30 (PCNA) and RFC5 (Fig. 7A). Deletion analysis of the region revealed that the RFC5 gene, but not POL30 (PCNA), was responsible for the suppression (Fig. 7A). The RFC5 gene on a low-copy-number plasmid could also rescue the thermosensitivity of the rfc2-1 mutation, but the suppression was much less than that of the RFC5 gene on a multicopy plasmid (Fig. 7B). Thus, suppression of the rfc2-1 mutation was dependent on the copy number of the RFC5 gene. The RFC5 gene on a multicopy plasmid also suppressed the sensitivity of the rfc2-1 mutant to HU and MMS (data not shown). These results are consistent with the notion that the RFC2 gene product (Rfc2p) is in the RF-C complex and interacts with the RFC5 gene product.
FIG. 7.
The RFC5 gene can suppress thermosensitive growth caused by the rfc2-1 mutation. (A) The insert DNA fragment carrying a suppressor which was originally isolated and its deletion derivatives are represented by open rectangles. The representative restriction enzyme sites and the open reading frames predicted from the nucleotide sequence of the region are also indicated. The growth phenotype of strain YVN4 (rfc2-1) transformed with different plasmids is shown at the right. (B) Dependence of efficiency of suppression on a copy number of the RFC5 gene. Strains YVN12 (wild type) and YVN13 (rfc2-1) and two transformants of YVN13 harboring either plasmid YCpRFC5 or plasmid YEpRFC5 were streaked onto YPD plates and incubated at different temperatures for 4 days.
As yeast RF-C complex consists of five different polypeptides (Rfc1/Cdc44, Rfc2, Rfc3, Rfc4, and Rfc5), we also examined whether overproduction of other RF-C subunits could suppress the temperature sensitivity of the rfc2-1 mutation. The YVN4 strain was transformed with either plasmid YEpRFC1, YEpRFC3, or YEpRFC4, and transformants were tested for growth on YPD plates at various temperatures, as were those with the RFC5 gene. None of these genes on a multicopy plasmid was able to suppress the temperature-sensitive growth phenotype of the rfc2-1 mutation. Thus, the suppression is specific for the RFC5 gene.
A thermosensitive mutation in the RFC5 gene (rfc5-1) can be suppressed by a multicopy plasmid of the SPK1 gene. Furthermore, the thermosensitive-growth phenotype, but not the S-phase checkpoint defect, was suppressed by PCNA on a multicopy plasmid (34). Therefore, we also tested whether overproduction of Spk1 or PCNA suppresses the rfc2-1 mutation. It was found that neither SPK1 nor PCNA suppresses the thermosensitive-growth phenotype, S-phase checkpoint defects, and sensitivities of the rfc2-1 mutant to HU, MMS, and UV light (data not shown). We also tested whether TEL1 and MEC1 on a multicopy plasmid suppress the rfc2-1 mutation. These two genes failed to suppress either temperature-sensitive cell growth or HU, MMS, or UV light sensitivities of the mutation (data not shown).
The rfc2-1 mutation is synthetically lethal with either the cdc44-1, rfc5-1, cdc2-2, or pol2-11 mutations.
It was shown that the cell cycle arrest of cdc44-1 mutant cells at restrictive temperatures requires the RAD9, MEC1, and MEC2 checkpoint genes (20). To determine whether the cell cycle arrest of the cdc44-1 mutant under restrictive conditions is RFC2 gene dependent, we attempted to construct a double mutant carrying the cdc44-1 and rfc2-1 mutations. Strain 7C-YVN16, in which disruption of the chromosomal copy of the RFC2 gene is complemented by YEpRFC2, was transformed with either plasmid YCpRFC2 or plasmid YCprfc2-1. Transformants were transferred onto 5-FOA plates and incubated at either 20, 25, or 30°C for 5 days. Although all transformants carrying plasmid YCpRFC2 yielded 5-FOA-resistant colonies at 25 and 30°C, the transformants carrying plasmid YCprfc2-1 did not grow on 5-FOA plates at all temperatures tested. Therefore, it was concluded that cdc44-1 is synthetically lethal with the rfc2-1 mutation at all temperatures.
We further tested the synthetic lethality of the rfc2-1 mutation in combination with the rfc5-1, pol2-11, cdc2-2, and pol1-17 mutations. As shown in Table 4, double mutant rfc2-1 rfc5-1 was inviable at all temperatures tested. Furthermore, the restrictive temperature of the rfc2-1 mutant was lowered by introduction of either the pol2-11 or the cdc2-2 mutation but not by the pol1-17 mutation. At 25°C, the double mutants pol2-11 rfc2-1 and cdc2-2 rfc2-1 did not grow, while a single mutant was able to grow at the same temperature. These double mutants, however, could grow at 20°C, unlike double mutants rfc2-1 rfc5-1 and rfc2-1 cdc44-1, which did not grow at all temperatures. These results are consistent with biochemical data indicating that the RF-C complex interacts with each subunit of the complex and with both DNA polymerases ɛ and δ during yeast chromosomal DNA replication.
TABLE 4.
Growth of double mutants on 5-FOA medium at different temperatures
| Straina | Growth at indicated temperature (°C)
|
||
|---|---|---|---|
| 20 | 25 | 30 | |
| rfc2-1 | + | + | − |
| cdc44-1 | − | + | + |
| cdc44-1 rfc2-1 | − | − | − |
| rfc5-1 | + | + | + |
| rfc5-1 rfc2-1 | − | − | − |
| pol1-17 | + | + | + |
| pol1-17 rfc2-1 | + | + | − |
| pol2-11 | + | + | − |
| pol2-11 rfc2-1 | + | − | − |
| cdc2-2 | + | + | + |
| cdc2-2 rfc2-1 | + | − | − |
The strains that were used in this experiment are YVN11 (rfc2Δ::LEU2 [YEpRFC2]), 7C-YVN16 (cdc44-1 rfc2Δ::LEU2 [YEpRFC2]), 12C-YVN30 (pol1-17 rfc2Δ::LEU2 [YEpRFC2]), 6B-YVN31 (rfc5-1rfc2Δ::LEU2 [YEpRFC2]), 3C-YVN27 (pol2-11 rfc2Δ::LEU2 [YEpRFC2]), and 1B-YVN28 (cdc2-2 rfc2Δ::LEU2 [YEpRFC2]).
DISCUSSION
In this study, we have generated and characterized a temperature-sensitive mutation (rfc2-1) in the RFC2 gene, encoding the third-largest subunit of the RF-C complex of S. cerevisiae. Based on the biochemical properties of the RF-C complex (6, 12), it was expected that a temperature-sensitive mutation in the RF-C complex would cause a severe defect in the elongation step of chromosomal DNA replication and that DNA synthesis would cease quickly after the restrictive temperatures were reached. However, the temperature-sensitive rfc2-1 mutation we obtained showed only a modest defect in chromosomal DNA replication at restrictive temperatures (Fig. 2B), as did the rfc5-1 mutation, which has a mutation in the RFC5 gene, encoding the second-largest subunit of the RF-C complex (34). This result might be explained by the leakiness of the mutation. Alternatively, it is possible that once the complex is formed, it becomes more temperature resistant, although the mutation is tight. This result may also be explained by a partial takeover of its function by other subunits of the complex, as they have extensive similarity to the amino acid sequence in the protein. However, the specific function of each subunit cannot be taken over genetically by other subunits, since each protein is essential for cell growth (9, 13, 20, 24, 25, 30). Thus, this last possibility is less likely. The chromosomes from mutant cells at restrictive temperatures did not enter into the gel during electrophoresis (data not shown). This result suggests that chromosomal DNA synthesized in rfc2-1 mutant cells accumulates structures which prevent entry of the chromosomal DNA into a gel, presumably nicks, gaps, and/or stalled replication forks. These structures should be recognized by the S-phase checkpoint machinery in which polymerase ɛ (29), Dpb11 (2), and Rfc5 (32, 34) are involved. Alternatively, these structures might be analogous to those generated by treatment with DNA-damaging agents and may be recognized by the DNA damage checkpoint machinery. Thus, it would be expected that the terminal phenotype of mutant cells is a dumbbell shape, which is typical for DNA replication mutants. Although an increase in dumbbell-shaped cells was observed at restrictive temperatures, rfc2-1 mutant cells proceeded through the cell cycle without completion of chromosomal DNA replication, resulting in a rapid loss of their viability (Fig. 2 and 4). Furthermore, HU-treated mutant cells entered into mitosis, as evidenced by partial spindle elongation before completion of DNA replication (Fig. 5). These results suggest that Rfc2 has a direct role in sensing incomplete DNA replication and transmitting the signal to the checkpoint machinery. In a previous study, we showed that the rfc2 deletion mutant spores germinated, divided two to three times, and generated microcolonies (30). This phenotype of the deletion mutant can be explained by loss of a cell cycle checkpoint. Consistent with this notion, mutant cells were very sensitive to HU and MMS and moderately sensitive to UV light (Fig. 3), and a loss of cell viability was prevented by either G1 arrest with α-factor or G2 arrest with benomyl treatment before temperature shift. Furthermore, this sensitivity to DNA-damaging agents of the mutant might not be due to a defect in DNA repair or recombination, as mutagenesis induced by EMS damage was as normal in mutant cells as in wild-type cells.
To date, many genes which are involved in the control of the S-phase checkpoint have been identified and characterized in the yeast S. cerevisiae (2, 11, 29, 34). These are POL2, which encodes the catalytic subunit of DNA polymerase II (ɛ), DPB11, which interacts with DNA polymerase II (ɛ), and RFC5, which encodes the second-largest subunit of the RF-C complex. The RFC2 gene seems also to be involved in the S phase checkpoint as demonstrated in this study. The temperature-sensitive growth phenotype of the rfc5-1 mutant was suppressed by a multicopy plasmid of yeast PCNA and SPK1, while the HU and DNA-damaging sensitivities were suppressed by overproduction of either the SPK1 (RAD53) or the TEL1 gene but not by that of PCNA (32, 34). It remains unclear how overexpression of SPK1 (RAD53) and TEL1 suppresses the growth defect of rfc5-1. Since TEL1 overexpression restored Rad53 modification and RNR3 induction in response to MMS in the rfc5-1 mutant, the suppression could be regulated by the checkpoint control (34). These results suggest that the checkpoint pathway in which Rfc5 is involved has regulatory components overlapping with those of the DNA damage checkpoint (34). On the other hand, the temperature-sensitive growth phenotype and HU- and DNA-sensitive phenotypes of the rfc2-1 mutant were suppressed by a multicopy plasmid containing the RFC5 gene, but neither phenotype was suppressed by a multicopy plasmid containing either PCNA, SPK1, TEL1, or MEC1. Thus, the RFC2 gene may be located upstream of Rfc5 in the checkpoint pathway. Nevertheless, it becomes clear that there are many replication proteins which recognize a stalled replication fork and generate an S-phase checkpoint signal in S. cerevisiae. However, the nature of the stalled replication structure which is recognized by each protein is not yet known. Furthermore, it is not known whether each protein recognizes the same structure, although the structure generated by HU treatment is recognized by each protein.
So far, a limited number of conditionally lethal mutations in the RFC1/CDC44, RFC2, and RFC5 genes in the RF-C complex have been identified. However, our synthetic lethality experiments described in this study strongly suggest that Rfc2p interacts with both Rfc1p and Rfc5p in the RF-C complex. Further, the introduction of either the cdc2-2 or the pol2-11 mutation, but not the pol1-17 mutation, into the rfc2-1 strain lowered its restrictive temperature. This partial synthetic lethality of double mutants rfc2-1 cdc2-2 and rfc2-1 pol2-11 is the first genetic evidence that the RF-C complex is required for the chromosomal DNA replication catalyzed by both DNA polymerases ɛ and δ. This evidence is consistent with the in vitro data that the RF-C complex is required for a processive DNA synthesis catalyzed by both DNA polymerases on a singly primed single-stranded circular template DNA (6).
The RF-C complex consists of five different polypeptides (Rfc1 to Rfc5) and has primer-template DNA-dependent ATPase activity which is stimulated by PCNA (12, 36). Interestingly, the deduced amino acid sequences, among them the nucleotide-binding consensus sequence motif, show significant homology to each other. Among those polypeptide subunits, Rfc3p has been shown to have ATPase activity (24), while Rfc2 has the activity which preferentially binds to primed single-stranded DNA and weak ATP-binding activity (30). The thermosensitive rfc5-1 mutation causes a Gly-to-Glu substitution just within the ATP-binding consensus, Gly X X Gly X Gly Lys, suggesting that the mutation likely affects the ATPase or ATP-binding activity of Rfc5 (34). A similar mutation was introduced into the RFC2 gene, resulting in cell lethality (data not shown). This result strongly suggests that the ATP-binding consensus sequence of Rfc2 plays a crucial role in chromosomal DNA replication.
ACKNOWLEDGMENTS
We thank K. Sugimoto and K. Matsumoto for unpublished data, yeast strains, and plasmid DNA. We also thank G. Cullman, B. Stillman, C. Holm, and H. Ogawa for yeast strains and plasmid DNA.
This work was supported by grants-in-aid for scientific research on priority areas from the Ministry of Education, Science, Sports and Culture of Japan. V. Noskov was supported by a fellowship from the Ciba-Geigy Research Foundation.
REFERENCES
- 1.Araki H, Ropp P A, Sugino A. DPB2, the gene encoding DNA polymerase II subunit B, is required for chromosome replication in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1991;88:4601–4605. doi: 10.1073/pnas.88.11.4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Araki H, Leem S-H, Phongdara A, Sugino A. Dpb11, which interacts with DNA polymerase II (ɛ) in Saccharomyces cerevisiae, has a dual role in S-phase progression and at a cell cycle checkpoint. Proc Natl Acad Sci USA. 1995;92:11791–11795. doi: 10.1073/pnas.92.25.11791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boeke J D, Lacroute F, Fink G R. A positive selection for mutants lacking orotidine-5′-phosphate decarboxylase activity in yeast: 5-fluoroorotic acid resistance. Mol Gen Genet. 1984;197:345–346. doi: 10.1007/BF00330984. [DOI] [PubMed] [Google Scholar]
- 4.Budd M E, Campbell J L. Temperature-sensitive mutations in the yeast DNA polymerase I gene. Proc Natl Acad Sci USA. 1987;84:2838–2842. doi: 10.1073/pnas.84.9.2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Budd M E, Campbell J L. DNA polymerases δ and ɛ are required for chromosomal replication in Saccharomyces cerevisiae. Mol Cell Biol. 1993;13:496–505. doi: 10.1128/mcb.13.1.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burgers P M J. Saccharomyces cerevisiae replication factor C. II. Formation and activity of complexes with proliferating cell nuclear antigen and with DNA polymerases δ and ɛ. J Biol Chem. 1991;266:22698–22706. [PubMed] [Google Scholar]
- 7.Campbell J L. Yeast DNA replication. J Biol Chem. 1993;268:25261–25264. [PubMed] [Google Scholar]
- 8.Cole G M, Stone D E, Reed S I. Stoichiometry of G protein subunits affects the Saccharomyces cerevisiae mating pheromone signal transduction pathway. Mol Cell Biol. 1990;10:510–517. doi: 10.1128/mcb.10.2.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cullmann G, Fien K, Kobayashi R, Stillman B. Characterization of the five replication factor C genes of Saccharomyces cerevisiae. Mol Cell Biol. 1995;15:4661–4671. doi: 10.1128/mcb.15.9.4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dixon W J, Massey F J., Jr . Introduction to statistical analysis. New York, N.Y: McGraw-Hill, Inc.; 1969. [Google Scholar]
- 11.Elledge S J. Cell cycle checkpoints: preventing an identity crisis. Science. 1997;274:1664–1671. doi: 10.1126/science.274.5293.1664. [DOI] [PubMed] [Google Scholar]
- 12.Fien K, Stillman B. Identification of replication factor C from Saccharomyces cerevisiae: a component of the leading-strand DNA replication complex. Mol Cell Biol. 1992;12:155–163. doi: 10.1128/mcb.12.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gary S L, Burgers P M J. Identification of the fifth subunit of Saccharomyces cerevisiae replication factor C. Nucleic Acids Res. 1995;23:4986–4991. doi: 10.1093/nar/23.24.4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gerik K J, Garry S L, Burgers P M J. Overproduction and affinity purification of Saccharomyces cerevisiae replication factor C. J Biol Chem. 1997;272:1256–1262. doi: 10.1074/jbc.272.2.1256. [DOI] [PubMed] [Google Scholar]
- 15.Gietz R D, Sugino A. New yeast-Escherichia coli shuttle vectors constructed with in vitro mutagenized yeast genes lacking six-base pair restriction sites. Gene. 1988;74:527–534. doi: 10.1016/0378-1119(88)90185-0. [DOI] [PubMed] [Google Scholar]
- 16.Gordenin D A, Malkova A L, Petersen A, Kulikov V N, Pavlov Y I, Perkins A, Resnick M A. Transposon Tn5 excision in yeast: influence of DNA polymerase α, δ and ɛ and DNA repair genes. Proc Natl Acad Sci USA. 1992;89:3785–3789. doi: 10.1073/pnas.89.9.3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haber J E. Bisexual mating behavior in diploid of S. cerevisiae: evidence for genetically controlled non-random chromosome loss during vegetative growth. Genetics. 1974;78:843–858. doi: 10.1093/genetics/78.3.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hartwell L H, Smith D. Altered fidelity of mitotic chromosome transmission in cell cycle mutants of S. cerevisiae. Genetics. 1985;110:381–395. doi: 10.1093/genetics/110.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hennessy K M, Lee A, Chen E, Botstein D. A group of interacting yeast DNA replication genes. Genes Dev. 1991;5:958–969. doi: 10.1101/gad.5.6.958. [DOI] [PubMed] [Google Scholar]
- 20.Howell E A, McAlear M A, Rose D, Holm C. CDC44: a putative nucleotide-binding protein required for cell cycle progression that has homology to subunits of replication factor C. Mol Cell Biol. 1994;14:255–267. doi: 10.1128/mcb.14.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaiser C, Michaelis S, Mitchell A. Methods in yeast genetics. A laboratory course manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1994. [Google Scholar]
- 22.Leem S-H, Ropp P A, Sugino A. The yeast Saccharomyces cerevisiae DNA polymerase IV: possible involvement in double strand break DNA repair. Nucleic Acids Res. 1994;22:3011–3017. doi: 10.1093/nar/22.15.3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leung D W, Chen E, Goeddel D V. Method for random mutagenesis of defined DNA segment using a modified polymerase chain reaction. Technique. 1989;1:11–15. [Google Scholar]
- 24.Li X, Burgers P M J. Molecular cloning and expression of the Saccharomyces cerevisiae RFC3 gene, an essential component of replication factor C. Proc Natl Acad Sci USA. 1994;91:868–872. doi: 10.1073/pnas.91.3.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li X, Burgers P M J. Cloning and characterization of the essential Saccharomyces cerevisiae RFC4 gene encoding the 37-kDa subunit of replication factor C. J Biol Chem. 1994;269:21880–21884. [PubMed] [Google Scholar]
- 26.McAlear M A, Howell E A, Espenshade K K, Holm C. Proliferating cell nuclear antigen (pol30) mutations suppress cdc 44 mutations and identify potential regions of interaction between the two encoded proteins. Mol Cell Biol. 1994;14:4390–4397. doi: 10.1128/mcb.14.7.4390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McAlear M A, Tuffo K, Holm C. The large subunit of replication factor C (Rfc1p/Cdc44p) is required for DNA replication and DNA repair in Saccharomyces cerevisiae. Genetics. 1996;142:65–78. doi: 10.1093/genetics/142.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morrison A, Sugino A. The 3′→5′ exonucleases of both DNA polymerases δ and ɛ participate in correcting errors of DNA replication in Saccharomyces cerevisiae. Mol Gen Genet. 1994;242:289–296. doi: 10.1007/BF00280418. [DOI] [PubMed] [Google Scholar]
- 29.Navas T A, Zhou Z, Elledge S J. DNA polymerase ɛ links the DNA replication machinery to the S phase checkpoint. Cell. 1995;80:29–39. doi: 10.1016/0092-8674(95)90448-4. [DOI] [PubMed] [Google Scholar]
- 30.Noskov V, Maki S, Kawasaki Y, Leem S-H, Ono B-I, Araki H, Pavlov Y, Sugino A. The RFC2 gene encoding a subunit of replication factor C of Saccharomyces cerevisiae. Nucleic Acids Res. 1994;22:1527–1535. doi: 10.1093/nar/22.9.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Noskov V N, Staak K, Shcherbakova P V, Kosmin S G, Negishi K, Ono B-I, Hayatsu H, Pavlov Y I. HAM1, the gene controlling 6-N-hydroxylaminopurine sensitivity and mutagenesis in the yeast Saccharomyces cerevisiae. Yeast. 1996;12:17–29. doi: 10.1002/(SICI)1097-0061(199601)12:1%3C17::AID-YEA875%3E3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 32.Stewart P R. Analytical methods for yeast. In: Prescott D M, editor. Methods in cell biology. XII. New York, N.Y: Academic Press; 1975. pp. 122–123. [Google Scholar]
- 32a.Subden R E, Krizus A. Correction factors for the diphenylamine test for deoxyribonucleic acid in yeast. Microbios. 1985;43:233–243. [PubMed] [Google Scholar]
- 33.Sugimoto K, Ando S, Shimomura T, Matsumoto K. Rfc5, a replication factor C component, is required for regulation of Rad53 protein kinase in the yeast checkpoint pathway. Mol Cell Biol. 1997;17:5905–5914. doi: 10.1128/mcb.17.10.5905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sugimoto K, Shimomura T, Hashimoto K, Araki H, Sugino A, Matsumoto K. Rfc5, a small subunit of replication factor C complex, couples DNA replication and mitosis in budding yeast. Proc Natl Acad Sci USA. 1996;93:7048–7052. doi: 10.1073/pnas.93.14.7048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sugino A. Yeast DNA polymerases and their role at the replication fork. Trends Biochem Sci. 1995;20:319–323. doi: 10.1016/s0968-0004(00)89059-3. [DOI] [PubMed] [Google Scholar]
- 36.Yoder B L, Burgers P M J. Saccharomyces cerevisiae replication factor C. I. Purification and characterization of its ATPase activity. J Biol Chem. 1991;266:22689–22697. [PubMed] [Google Scholar]