Abstract
Osteoclasts are the principal cells that efficiently resorb bone. Numerous studies have attempted to reveal the molecular pathways leading to the differentiation and activation of osteoclasts to improve the treatment and prevention of osteoporosis and other bone-destructive diseases. While the cumulative knowledge of osteoclast regulatory molecules, such as receptor activator of nuclear factor-kB ligand (RANKL) and nuclear factor of activated T cells 1 (NFATc1), contributes to the understanding of the developmental progression of osteoclasts, little is known about how the discrete steps of osteoclastogenesis modify osteoclast status but not the absolute number of osteoclasts. The regulatory mechanisms involved in osteoclast maturation but not those involved in differentiation deserve special attention due to their potential use in establishing a more effective treatment strategy: targeting late-phase differentiation while preserving coupled bone formation. Recent studies have shed light on the molecules that govern late-phase osteoclast differentiation and maturation, as well as the metabolic changes needed to adapt to shifting metabolic demands. This review outlines the current understanding of the regulation of osteoclast differentiation, as well as osteoclast metabolic adaptation as a differentiation control mechanism. Additionally, this review introduces molecules that regulate the late-phase osteoclast differentiation and thus minimally impact coupled bone formation.
Subject terms: Cell biology, Cell signalling
Metabolic adaptation in osteoclasts: a novel approach to bone disease therapy
This study explores the process of bone remodeling, particularly the role of osteoclasts (cells that break down bone) and osteoblasts (cells that build new bone). There is a lack of understanding about the biology of the osteoclast differentiation process (the process by which a cell changes from one type to another), which is vital for maintaining bone health. The scientists reviewed how osteoclast differentiation is regulated, focusing on cell signaling (communication between cells) and metabolic adaptation (how cells change their metabolism to survive). They discovered certain molecules could change osteoclast-mediated bone breakdown without affecting bone formation, potentially serving as treatment targets for diseases that destroy bone. The study concludes that understanding the molecular complexities of late-phase osteoclast differentiation will help improve the treatment and prevention of osteoporosis and other bone-destructive diseases.
This summary was initially drafted using artificial intelligence, then revised and fact-checked by the author.
Introduction
Skeletal bone is maintained by bone remodeling, in which old and damaged bone is replaced with new bone through continuous cellular processes that are mainly controlled by bone-resorbing osteoclasts and bone-forming osteoblasts1,2. During bone remodeling, osteoclast formation is initiated, and a tiny amount of bone is removed, which is then replaced by new bone formed by osteoblasts; after that, the new bone is mineralized. Bone remodeling takes place at sites known as basic multicellular units, which contain all bone cells, including osteoclasts and osteoblasts, and are asynchronously distributed throughout the skeleton1,2. Osteoblast-osteoclast interactions are necessary and must be coordinated in time and space to maintain the balance of focal bone remodeling. The communication mechanism by which bone formation follows bone resorption is known as coupling. Researchers have discovered several coupling mechanisms, such as (1) the release of osteoblast precursor recruitment factors and growth-promoting factors, which are stored in large amounts in the bone matrix through osteoclastic bone resorption3–13; (2) the secretion of coupling factors by osteoclasts14–20; (3) the expression of coupling factors on the osteoclast cell membrane21–23; and (4) the production of extracellular vesicles, which contain coupling factors synthesized by osteoclasts24,25. Through these mechanisms, osteoclasts signal osteoblast precursors to prepare for bone formation. A functional imbalance in osteoclasts and osteoblasts unbalances coupling, causing abnormal bone homeostasis.
Various diseases and metabolic abnormalities adversely affect bone health. Activation of osteoclastic bone resorption is a common factor in the pathogenesis of bone loss and fractures. Antiresorptive agents such as bisphosphonate and anti-receptor activator of nuclear factor-kB ligand (RANKL) antibodies (denosumab) are used as treatments. Bisphosphonate specifically inhibits farnesyl diphosphate synthase, an enzyme in the cholesterol biosynthesis pathway, in osteoclasts. This inhibition inactivates osteoclasts, decreases their formation, and increases their apoptosis26,27. Denosumab is a humanized monoclonal antibody that binds to and inhibits RANKL, blocking osteoclast formation28–30. These osteoclast-targeting treatments are efficiently antiresorptive. However, their long-term use compromises bone strength because inhibiting osteoclast formation and activity also inhibits osteoclast-mediated release of coupling factors, thereby inhibiting osteoblast differentiation and not promoting bone formation31,32. Since bone health in the adult skeleton depends on the balance of bone remodeling, understanding the biology of osteoclast differentiation is crucial. Targeting only late-phase differentiation while preserving early osteoclast differentiation, which is necessary for coupled bone formation, would aid in establishing a more effective treatment strategy. Multiple studies have demonstrated that osteoclasts that fail to achieve multinucleation, which is a hallmark of osteoclast maturation, retain their osteoclastic phenotype; these osteoclasts continue expressing osteoclast-related markers such as tartrate-resistant acid phosphatase (TRAP) and cathepsin K and exhibit low levels of bone resorption33–39. The evidence indicates that targeting osteoclast maturation could selectively control bone destruction, circumventing the negative effects of general osteoblast suppression.
In this review, we will examine the updated regulatory mechanisms of osteoclast differentiation and focus on cell signaling and metabolic adaptation as key differentiation control mechanisms. Additionally, we will describe the molecules needed for osteoclast maturation, which are essential for the process but dispensable for coupled bone formation.
Osteoclast differentiation and signaling pathways
Osteoclasts are the primary bone-resorbing cells. These cells are highly specific, multinucleated, phagocytic cells of hematopoietic origin and are characterized by distinct features such as a ruffled border that facilitates demineralization and degradation of the bone matrix. Osteoclast differentiation requires two cytokines. The first is macrophage colony-stimulating factor (M-CSF), which is produced by a variety of cells, including lymphocytes and monocytes, and is necessary for the differentiation of hematopoietic stem cells into monocyte/macrophage lineages and the induction of receptor activator of nuclear factor-kB (RANK)40–44. The other is RANKL, which is produced mainly by osteoblasts and osteocytes45,46 and is essential for promoting and sustaining osteoclast differentiation47. During osteoclast differentiation, an osteoclast undergoes various changes, including changes in gene expression patterns, morphology, and metabolic processes. The developmental progression of osteoclasts can be divided into roughly three phases: commitment, maturation, and resorption.
Commitment
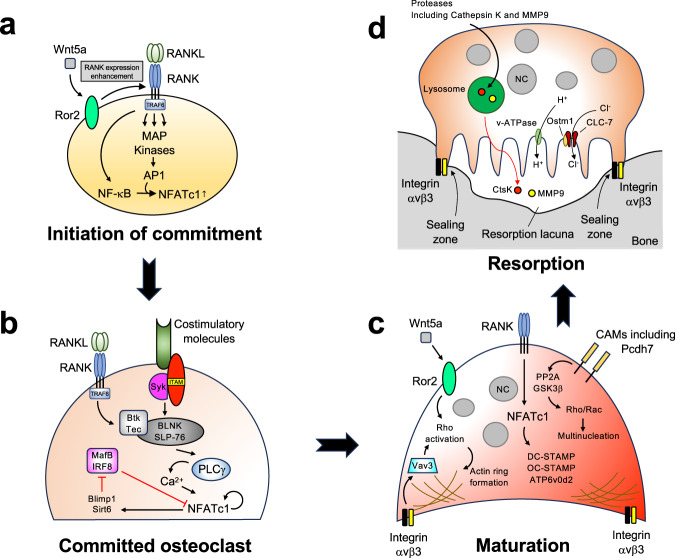
The initiation of commitment to the osteoclast lineage from monocyte/macrophage lineage precursors is driven mainly by RANK trimerization and activation48 and the recruitment of adaptor proteins, including TNF receptor associated factor 6 (TRAF6), through its intracellular binding sites for the adaptor protein TRAF49,50, thereby initiating the downstream signaling cascade. The recruitment of adaptors converges on kinase activation and promotes nuclear translocation and the activation of the critical transcription factors nuclear factor kappa B (NF-κB)51,52 and AP1 (consisting of a variety of dimers composed of Fos, Jun, and ATF)53–55, which contribute to the initial induction of nuclear factor of activated T cells 1 (NFATc1)56–60. NFATc1, NF-κB, and AP1 are the master regulators of osteoclast-specific transcriptional programs. Additional factors, including the noncanonical Wnt ligand Wnt5a61, can enhance RANK expression in osteoclast precursors, thereby promoting RANKL-induced osteoclast formation. RANK signaling functions with costimulatory signals via immunoglobulin-like receptor/immunoreceptor tyrosine-based activation motif (ITAM)-motif-containing proteins, such as DNAX-activation protein 12 (DAP12) and Fc receptor gamma-chain (FcRγ)62, which associate with the cell surface receptors osteoclast-associated receptor (OSCAR)63, paired immunoglobulin-like receptor A (PIR-A)62, or triggering receptor expressed on myeloid cells 2 (TREM-2)64,65. Activation of costimulatory molecules and tyrosine phosphorylation of ITAM form and activate a complex including spleen tyrosine kinase (Syk), Bruton’s tyrosine kinases (Btk)/Tek, adaptor molecules B-cell linker protein (BLNK), and Src homology 2 domain-containing leukocyte protein of 76 kDa (SLP-76)66. This complex integrates both RANK and ITAM downstream signaling, thereby promoting phospholipase Cγ (PLCγ)-mediated Ca2+ signaling, which is essential for robust amplification of NFATc1 and its translocation to the nucleus66. NFATc1, as well as the transcription factors c-Fos and NF-κB, activates the expression of genes critical to osteoclast activation, including TRAP, cathepsin K, calcitonin receptor, and c-myc67. RANKL signaling also downregulates negative regulators of NFATc1, such as v-maf musculoaponeurotic fibrosarcoma oncogene family protein B (MafB)68, interferon regulatory factor-8 (IRF-8)69, and B-cell lymphoma 6 (Bcl6)70. Downregulation of these negative regulators is mediated by the transcriptional repressor B-lymphocyte-induced maturation protein 1 (Blimp1) and sirtuin 6 (Sirt6)70,71, both of which are induced by RANKL signaling. The RANKL-RANK signaling pathway orchestrates the expression of these genes, leading committed osteoclasts to progress toward a maturation phase characterized by cell adhesion, fusion, motility, and actin ring formation (Fig. 1a, b).
Fig. 1. Osteoclast differentiation and signaling pathways.
a Commitment to osteoclast differentiation is initiated by the engagement of RANKL with RANK. RANK activation recruits adaptor proteins, including TRAF6, thereby initiating the downstream signaling cascade and inducing the expression of osteoclast master regulators. The expression of RANK can be enhanced by additional factors to promote RANKL-induced osteoclast formation. b In committed osteoclasts, RANKL-RANK signaling cooperates with costimulatory molecules and promotes PLCγ-mediated Ca2+ signaling, which robustly amplifies NFATc1 levels. RANKL-RANK signaling also downregulates the negative regulators of NFATc1. c During maturation, committed osteoclasts undergo pronounced morphological changes. Cell adhesion molecules, including integrins and cadherins, regulate the activation of Rho family small GTPases and cytoskeletal organization, contributing to the formation of an actin ring/sealing zone. Additionally, RANKL-RANK signaling induces the expression of osteoclast fusion molecules through the activation of NFATc1, thereby contributing to multinucleation. d During resorption, osteoclasts digest inorganic and organic bone matrix by transporting protons and hydrolases through the ruffled border, as well as via endosomal/lysosomal vesicle trafficking. Transcytosis removes the resorbed bone material from the resorption pit. NC nucleus.
Maturation
During maturation, osteoclasts undergo a pronounced morphological change known as multinucleation, which is a hallmark of their maturation. Osteoclasts that cannot achieve multinucleation exhibit diminished bone-resorbing activity, demonstrating the important role of multinucleation in osteoclast function. Multinucleation is a multistep process that is mainly mediated by cell‒cell fusion and repeated incomplete cytokinesis. Committed osteoclasts undergo proliferation72,73, migration, cellular adhesion, cytoskeletal rearrangement74, and eventually cell‒cell fusion75. The transient increase in proliferation induced by RANKL leads to incomplete cytokinesis, contributing to the formation of multinucleated cells72,73, which is regulated by Akt activation73. The chemoattractant (C-C) ligand-2 (CCL2) and its receptor CCR2 are needed to form multinucleated osteoclasts76,77. Cell adhesion molecules (CAMs), such as integrins and cadherins, are needed for committed osteoclasts to adhere to each other and establish close membrane contact and cytoskeletal organization76,78,79. CAMs also coordinate RANKL-induced osteoclast differentiation as signal transduction molecules80,81. Extensive cytoskeletal rearrangements occur during this phase. Eventually, committed osteoclasts undergo cell‒cell fusion. Several molecules have been shown to be involved in osteoclast fusion, including dendritic cell-specific transmembrane protein (DC-STAMP)38,82, osteoclast stimulatory transmembrane protein (OC-STAMP)36, and v-ATPase subunit d2 (Atp6v0d2)83. NFATc1 and c-Fos, which is downstream of RANKL-RANK signaling, regulates the expression of these fusion-related molecules56 (Fig. 1c).
During maturation, osteoclasts adhere to the bone surface, and the resorptive machinery of osteoclasts polarizes toward the bone-cell interface84. Signaling mediators, including Rho family small GTPases (RhoA, Rac1, and Cdc42), regulate the extensive cytoskeletal rearrangement that occurs during this phase85–87. Integrin αvβ3 mediates the bone-cell interaction and recognizes the arginyl-glycyl-aspartic acid (RGD) sequence present in various bone matrix proteins, such as osteopontin, vitronectin, and bone sialoprotein88. The binding of integrin αvβ3 to its ligands phosphorylates and activates c-Src, which phosphorylates Syk in an ITAM-containing protein (DAP12 and FcRγ)-dependent manner89. Activated Syk regulates guanine nucleotide exchange factors (GEFs), including Vav390, which eventually activates Rho family small GTPases and regulates cytoskeletal organization. A mature osteoclast eventually forms F-actin-rich adhesive structures, which form a sealing zone (actin ring) on the ventral membranes in contact with the bone surface. This sealing is designed to release protons and proteases for demineralization and to break down the bone matrix91. Syk and c-Src are critical for sealing zone formation and bone resorption89,92,93 (Fig. 1c).
Resorption
The functional resorption phase involves the formation of a ruffled border surrounded by the sealing zone84. The ruffled border is a highly folded and invaginated membrane structure that increases the surface area of osteoclasts in contact with bone47. Through this border, protons and hydrolases that solubilize and digest inorganic and organic bone matrix are transported, enabling mature osteoclasts to efficiently resorb bone. Initially, osteoclasts produce hydrochloric acid, which dissolves the bone mineral. The α3 subunit of v-ATPases, the Cl-/H+ antiporter chloride voltage-gated channel 7 (CLC-7), and its β-subunit osteopetrosis associated transmembrane protein 1 (OSTM1) localize to the lysosome and the ruffled border, where they acidify secretory lysosomes and the space between the ruffled border and the bone surface94–97. This local acidosis dissolves inorganic minerals such as calcium, exposing organic matrix components such as collagen in the connective bone tissue. The decalcified organic matrix is subsequently degraded by lysosomal proteases such as collagenases, cathepsin K, and matrix metalloproteinases (MMPs)98,99. The secretory lysosome pathway, which delivers proteases, is regulated by Rab7, a small GTPase that regulates endosomal/lysosomal vesicle trafficking100. Synaptotagmin VII, which is a member of the synaptotagmin family that mediates the Ca2+-triggered fusion of cytoplasmic/synaptic vesicles to the plasma membrane, mediates the fusion of secretory lysosomes with the ruffled border101. The autophagy-related protein Atg5 also participates in the secretion of lysosomal contents by directing lysosomes to fuse with the plasma membrane102. Mutations in genes involved in matrix demineralization and dissolution account for most human cases of osteopetrosis103. The resorbed material is removed from the resorption pit by transcytosis through osteoclasts104,105 (Fig. 1d).
Osteoclast differentiation and metabolic adaptation
The differentiation of osteoclasts and their bone-resorbing function are energy-intensive processes. Along with signaling pathways and osteoclast-specific gene expression programs, osteoclast differentiation activates metabolic programs. While our understanding of these pathways and gene expression programs during osteoclast differentiation has significantly advanced, the investigation of energy metabolism and its regulation in osteoclasts is in its infancy. However, recent evidence shows that osteoclasts display remarkable metabolic adaptation during differentiation, highlighting the importance of cell metabolism as a differentiation control mechanism (Table 1).
Table 1.
Key molecules involved in osteoclast differentiation and metabolic adaptation.
| Molecule | Function in osteoclasts | References |
|---|---|---|
| PGC1β | Regulating mitochondrial biogenesis | 107 |
| Tfam | Regulating mitochondrial DNA metabolism and mitochondrial biogenesis | 113 |
| Ndufs4 | An accessory subunit of mitochondrial respiratory chain complex I | 117 |
| Myc | Regulating mitochondrial respiratory capacity | 116 |
| PKM2 | Regulating glycolysis. Downregulation is required for late phase osteoclast differentiation | 123 |
| HIF1α | Regulating gene expression (especially glycolysis-involved genes) in response to hypoxic stimuli. Important under pathological conditions | 106,133 |
| COMMD1 | Negatively regulating osteoclast differentiation, which is inhibited by hypoxia. Important under pathological conditions | 124 |
Oxidative phosphorylation and osteoclast numbers
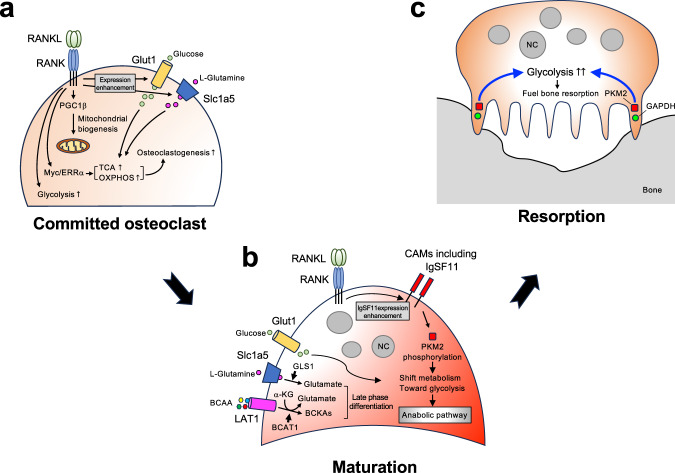
Osteoclast differentiation is associated with a substantial increase in biomass106. High metabolic activity and adenosine triphosphate (ATP) production are required for these processes. The energy produced by a cell mainly depends on glucose. Glucose is used to produce ATP through the glycolysis pathway, which occurs in the cytoplasm, and the tricarboxylic acid cycle (TCA cycle) and oxidative phosphorylation, both of which occur in mitochondria. Several studies have suggested that both oxidative phosphorylation and glycolysis are increased during osteoclast differentiation. When osteoclast precursors receive RANKL signals, mitochondrial biogenesis and, consequently, mitochondrial size and numbers increase107,108. The increase in oxidative phosphorylation is mediated at least in part by an increase in mitochondrial biogenesis orchestrated by peroxisome proliferator-activated receptor-g coactivator 1β (PGC1β)107. Mitochondrial DNA is critical for mitochondrial biogenesis, and disruptions in mitochondrial transcription factor A (Tfam) cause severe respiratory chain deficiency and reduced ATP production109–112. Tfam deficiency in osteoclasts significantly reduces osteoclast numbers113. However, the prerequisite of mitochondrial biogenesis for osteoclast differentiation and activity remains in question, as osteoclast lineage-specific PGC1β-deficient mice have a normal bone phenotype, and Tfam-deficient osteoclasts have increased resorption activity and accelerated apoptosis despite the significant decrease in mitochondrial biogenesis levels113,114. Along with increased mitochondrial biogenesis, RANKL stimulation upregulates the expression of the genes involved in the TCA cycle and oxidative phosphorylation108,115,116. This accelerates mitochondrial respiration and produces high levels of ATP. Oxidative phosphorylation has been identified as the primary bioenergetic source for osteoclast formation. NADH:ubiquinone oxidoreductase iron-sulfur protein 4 (Ndufs4) is a subunit of mitochondrial membrane respiratory chain complex I. Ndufs4 deficiency suppresses mitochondrial complex I activity, inhibiting osteoclast differentiation and thereby increasing bone mass in vivo117. The RANKL-induced increase in the expression of oxidative phosphorylation-involved genes and enhanced mitochondrial respiration depend on the Myc-ERRα signaling axis116. In addition to oxidative phosphorylation, S-adenosylmethionine (SAM)-mediated DNA methylation by Dnmt3a regulates osteoclast differentiation via epigenetic repression of anti-osteoclastogenic genes, including IRF-8115. Myc deficiency in osteoclasts severely impairs mitochondrial respiratory capacity and completely stops osteoclastogenesis, thereby increasing bone mass in vivo116. These observations strongly indicate that oxidative phosphorylation is required for osteoclast differentiation. Moreover, disturbing oxidative phosphorylation in osteoclasts changes the bone phenotype, specifically by decreasing osteoclast numbers (Fig. 2a).
Fig. 2. Osteoclast differentiation and metabolic adaptation.
a In committed osteoclasts, RANKL-RANK signaling enhances mitochondrial biogenesis, which is regulated in part by PGC1β. In addition to increasing mitochondrial biogenesis, RANKL stimulation upregulates TCA cycle- and oxidative phosphorylation-related genes, enhances mitochondrial respiration, and results in the production of high levels of ATP. The Myc-ERRα signaling axis mediates this process. Oxidative phosphorylation is needed for osteoclast differentiation, thereby contributing to osteoclast numbers. Moreover, RANKL-RANK signaling enhances glycolysis. b Mature osteoclasts have a high glycolytic rate. RANKL-RANK signaling enhances the expression of IgSF11, which regulates the activity of the glycolysis rate-limiting enzyme PKM2. Consequently, glucose metabolism shifts toward an anabolic pathway rather than energy production. Moreover, alternative energy substrates, such as glutamine and BCAAs, fuel osteoclast differentiation. c Glycolysis fuels bone resorption. The glycolysis-associated enzymes PKM2 and GAPDH were detected in sealing zones. NC nucleus.
Glycolysis, osteoclast maturation and resorption
Glycolysis is the metabolic pathway that oxidizes glucose for energy production and anabolic processes such as protein, lipid, and nucleic acid synthesis and supports proliferation and growth118–120. RANKL stimulation induces the expression of glycolysis-involved genes, including hexokinase (HK), phosphofructokinase (PFK), and pyruvate kinase (PKM), which are rate-limiting enzymes that control the pace of glycolysis and the outflow from the glycolytic pathway to the TCA cycle106. RANKL also induces lactate dehydrogenase (LDH), which catalyzes the transformation of pyruvate into lactate121, as well as the glucose transporter Glut1106. During RANKL-induced osteoclast differentiation, glucose consumption and lactate production are elevated, indicating an increase in glycolysis122,123. The importance of glycolysis for osteoclast differentiation has been demonstrated using specific inhibitors and/or activators. The molecule 2-deoxy-D-glucose (2-DG) is a modified glucose molecule with the 2-hydroxyl group replaced by hydrogen. This factor accumulates in the cell and inhibits glucose phosphorylation by HK, preventing further glycolysis. Treatment with 2-DG severely abrogates osteoclast differentiation116,124. PKM isoform 2 (PKM2) is a rate-limiting glycolysis enzyme whose activity can be negatively regulated118,125. PKM2 catalyzes the conversion of phosphoenolpyruvate to pyruvate. Active PKM2 contributes to glucose metabolism by directing it toward mitochondrial oxidative phosphorylation, thereby increasing ATP production through the mitochondrial respiratory chain. Conversely, when PKM2 is negatively regulated, glucose metabolism shifts toward aerobic glycolysis. Thus, glucose metabolites are increased and directed toward anabolic pathways120,126. Activation of PKM2 using the specific activator TEPP46 reduces glycolysis (i.e., shifts glucose metabolism toward oxidative phosphorylation) and inhibits the formation of large multinucleated osteoclasts, whereas PKM2 inhibition by the specific inhibitor shikonin enhances osteoclast differentiation123. Phosphorylation of PKM2 at tyrosine 105 (Y105) can negatively regulate its enzymatic activity125. Increased phosphorylation of PKM2 Y105 is observed during RANKL-induced osteoclast differentiation, especially in the late phase (maturation)123. These results suggest that glycolysis plays a key role in osteoclast differentiation, especially during maturation. Glycolysis is also an influential factor in the bone resorption phase. Osteoclastic resorption is significantly decreased when osteoclasts are fueled by galactose instead of glucose, which forces cells to depend on oxidative phosphorylation to generate sufficient ATP by reducing the glycolysis rate108. The impaired resorption activity in the presence of galactose can be restored with a nontoxic dose of rotenone, an inhibitor of mitochondrial complex I108. These observations indicate that glycolysis is the preferred energetic pathway driving the resorption activity of mature osteoclasts. Notably, localization of the glycolysis-associated enzymes PKM2 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) has been detected in proximity to the sealing zones of mature osteoclasts108. This observation supports the idea that glycolysis fuels the bone resorption process (Fig. 2a–c).
Oxygen tension is very low in bone, especially in resorption lacunae, where osteoclasts reside. Therefore, oxygen availability may dictate the metabolic profile and modulate osteoclast formation and activation. Hypoxia is a critical stimulator of osteoclast differentiation124, although conflicting data exist127. The hypoxia-inducible transcription factor HIF is the main regulator of the hypoxic response and plays a crucial role in osteoclast regulation. HIF is a heterodimer composed of an inducible alpha subunit (HIF-1α and HIF-2α) and a constitutively expressed beta subunit (HIF-1β). In hypoxic microenvironments, HIF proteins are stabilized, establishing an active transcriptional complex. Hypoxia stabilizes HIF-1α in osteoclasts128–132, and RANKL-induced osteoclast differentiation increases HIF-1α expression128,133. HIF-1α activation induces the transcription of Glut1 and rate-limiting glycolytic enzymes such as lactate dehydrogenase A (LDHA) and phosphofructokinase liver type (PFKL), thereby promoting glucose uptake and glycolysis in osteoclasts106. Inhibiting HIF-1α significantly inhibits osteoclastic resorption despite enhancing the formation of large multinucleated osteoclasts106. Hypoxia also augments osteoclast differentiation by increasing the glycolysis metabolic pathway by suppressing the negative regulator copper metabolism MURR1 domain-containing 1 (COMMD1)124. Importantly, osteoclast-specific HIF-1α deficiency or COMMD1 deficiency in mice or treatment with the HIF-1α inhibitor 2-methoxyestradiol results in little change in bone volume or osteoclast numbers on the bone surface under normal conditions124,133. However, under conditions of osteoclast activation, such as estrogen deficiency and inflammation, bone loss is prevented by suppressing osteoclast activation124,133. These observations indicate the important role of HIF-1α and COMMD1 (and possibly the molecule-dependent increase in glycolysis) under pathological rather than physiological conditions.
Alternative energy substrates that fuel osteoclast differentiation
Although glucose is a major nutrient for osteoclasts, limiting glycolysis by omitting glucose from the culture media or knocking down HIF-1α does not impair osteoclast formation106, indicating alternative energy substrates that support the differentiation process. During osteoclast differentiation, osteoclasts take up a considerable amount of amino acids, including glutamine, arginine, serine, and branched-chain amino acids (BCAAs)134. Glutamine is the most bountiful and flexible amino acid in the body. L-glutamine significantly impacts osteoclast differentiation, and its depletion abrogates the induction of osteoclast formation106. Supplementation with L-glutamine during the later phase of differentiation has more impact than during the early phase106, indicating its significant role in the later phase of differentiation. The high-affinity Na+-dependent L-glutamine transporter solute carrier family 1 member 5 (Slc1a5) mediates L-glutamine uptake, which is catalyzed to form glutamate by glutaminase (Gls1). RANKL stimulation induces both Slc1a5 and Gls1 in osteoclasts. Although the metabolism of glutamate in osteoclasts remains unclear, it is thought to be converted to α-ketoglutarate (α-KG) and subsequently fuels other metabolic pathways, such as the TCA cycle, as a substrate of anaplerosis during osteoclast differentiation135. Arginine, a conditionally essential amino acid, has been shown to control RANKL-induced osteoclast formation. Extracellular arginine supports TCA cycle activity and oxidative phosphorylation136. Depletion of arginine by recombinant arginase 1 during differentiation decreases RANKL-induced expression of NFATc1 and Fos at an early timepoint and completely blocks osteoclastogenesis136. BCAAs and branched-chain aminotransferase 1 (BCAT1) play important roles in osteoclast differentiation. BCAT1 is the cytoplasmic BCAT isoform that converts BCAAs into branched-chain ketoacids, generating glutamate from α-KG137. BCAT1 deficiency in mice increases bone mass by decreasing osteoclast differentiation and resorption activity138. Pharmacological inhibition of BCAT1 prevents osteoclast differentiation and inflammation-induced bone loss139. The depletion of BCAAs during the later phase of differentiation significantly decreases osteoclast maturation, whereas depletion in the early phase has no effects on osteoclast differentiation or maturation139, indicating the role of BCAAs in the relatively late phase of osteoclast differentiation. Moreover, conflicting data on the role of BCAAs in osteoclast differentiation arise from osteoclast-specific deletion of LAT1, an L-type amino acid transporter 1 that mediates the uptake of BCAAs (also known as Slc7a5), resulting in enhanced osteoclast differentiation and function, which are attributed to impaired mTOR signaling140. Further studies are needed to clarify the impact of BCAA metabolism and BCAA uptake on osteoclast differentiation (Fig. 2a, b).
Molecules regulating late-phase osteoclast differentiation with minimal impacts on coupled bone formation
Studies have identified molecules whose functional disturbance causes defective osteoclastic bone resorption without affecting osteoblastic bone formation. These molecules can be promising targets to aid in establishing a more effective treatment strategy for late-phase osteoclast differentiation while preserving coupled bone formation (Table 2).
Table 2.
Key molecules involved in osteoclast late-phase differentiation with a minimal impact on coupled bone formation.
| Molecule | Function in osteoclasts | References |
|---|---|---|
| BTK/Tec | Integrating RANK and ITAM signaling and promoting PLCγ-mediated Ca2+ signaling. Essential for osteoclast differentiation | 66 |
| Ror2 | Promoting osteoclast differentiation by enhancing the expression of RANK in osteoclast precursors. Required for osteoclast differentiation | 61,142 |
| Pkn3 | Enhancing c-Src activity. Required for osteoclastic bone resorbing activity | 142,143 |
| Pcdh7 | Regulating small GTPases. Required for osteoclast multinucleation | 81,144,145 |
| IgSF11 | Regulating PKM2 activity. Required for osteoclast differentiation | 80,123 |
| PKM2 | Regulating glycolysis. Downregulation is required for late phase osteoclast differentiation | 123 |
Btk and Tec are critical signaling molecules for osteoclast differentiation
The tyrosine kinases BTK and Tec are critical for integrating RANK and ITAM downstream signaling and promoting PLCγ-mediated Ca2+ signaling66. Osteoclasts derived from Xid mice, which possess a natural mutation in the BTK gene, exhibit impaired multinucleated osteoclast formation in vitro due to impaired fusion of preosteoclasts141, although no significant defects were detected in BTK-deficient mice in terms of osteoclast numbers, resorption activity, and osteoblastic bone formation. However, Tec and BTK double-deficient mice exhibit striking defects in osteoclast numbers and bone resorption66. Notably, osteoblastic bone formation remains intact in double-deficient mice66. Local administration of the Tec kinase inhibitor LFM-A13 attenuates inflammation-induced bone loss or RANKL-induced bone loss by decreasing osteoclast numbers on the bone surface but does not affect inflammatory responses66. These observations suggest that targeting Tec family kinases can suppress osteoclastogenesis while preserving coupled bone formation in vivo.
PKN3 is a signaling mediator of the Wnt5a-Ror2 signaling pathway
Cytoskeletal reorganization in osteoclasts to form sealing zones is imperative for the attachment of these cells to bone and the resorption of bone matrices. Wnt5a and receptor tyrosine kinase-like orphan receptor 2 (Ror2) signaling promote osteoclast differentiation by enhancing the expression of RANK in osteoclast precursors by activating c-Jun N-terminal kinase (Jnk)61. Additionally, Wnt5a-Ror2 signaling enhances osteoclastic bone-resorbing activity in mature osteoclasts through protein kinase N3 (Pkn3)142. Mechanistically, the engagement of Wnt5a with Ror2 stimulates the small GTPase Rho and its effector kinase Pkn3 and eventually enhances c-Src activity. Osteoclasts that lack Ror2 and/or Pkn3 expression have impaired actin ring formation142. Heterozygous mutations in Ror2 increase bone volume due to decreased numbers of osteoclasts on the bone surface, and osteoblastic bone formation is normal61. Pkn3 deficiency in mice increases bone mass due to impaired osteoclastic bone resorption activity, which is characterized by decreases in the eroded surface and erosion depth on the bone surface, but not by osteoclast differentiation, as the number of osteoclasts on the bone surface is comparable to that in control mice142. Osteoblastic bone formation is intact in the absence of Pkn3142. These results suggest that Ror2 is required for osteoclast differentiation, whereas Pkn3 is dispensable for osteoclast differentiation but essential for the maturation and resorption activity of osteoclasts. Of note, both Ror2 and Pkn3 are dispensable for coupled osteoblastic bone formation61,142. Recently, the Pkn3 inhibitor SB202190 has been shown to inhibit osteoclast activity but not osteoclast differentiation and coupled bone formation in vivo, thereby preventing ovariectomy-induced bone loss143. These findings indicate that targeting Pkn3 with SB202190 can be a promising treatment. These findings also suggest that the signaling pathway that regulates the formation of a sealing zone can be a target of a novel anti-bone resorption therapy.
Pcdh7 is a signal transduction molecule for the activation of small GTPases
Osteoclast differentiation requires costimulatory molecules in addition to the signals of RANKL. Protocadherin-7 (Pcdh7) is a member of the protocadherin family, which is a subgroup of the cadherin superfamily. This factor acts as a signaling receptor for osteoclast differentiation81,144,145. Mechanistically, Pcdh7 ligation, which is thought to occur through homophilic interactions, activates protein phosphatase 2 A (PP2A) during RANKL-induced osteoclast differentiation. PP2A dephosphorylates and activates GSK3β, which, in turn, activates small GTPases, including RhoA, inducing osteoclast differentiation and multinucleation. Pcdh7 deficiency in mice reduces osteoclast numbers, thereby increasing bone mass144. Pcdh7 deficiency does not affect coupled bone formation144. Of note, Pcdh7 deficiency impairs the formation of large multinucleated osteoclasts, although it does not affect the expression of differentiation markers such as Acp5 and Cathepsin K or osteoclastic resorption functions144. Pcdh7 may be required during the osteoclast maturation phase to form large multinucleated osteoclasts. These findings suggest that multinucleation is a potential target for regulating osteoclastic bone resorption without affecting coupled bone formation.
IgSF11 and PKM2 regulate glycolysis
Glucose metabolism, including glycolysis and oxidative phosphorylation, is accelerated during osteoclast differentiation, and it exhibits potential as a target for the regulation of osteoclastic bone resorption. The glycolysis rate-limiting enzyme PKM2 regulates osteoclast differentiation as a signal mediator that is downstream of immunoglobulin superfamily 11 (IgSF11)80,123. Stimulation of IgSF11, which is mediated by homophilic interactions, phosphorylates PKM2 at Y105 through multiple src family kinases, including c-Src, thereby inhibiting PKM2 activity. RANKL stimulation induces expression of IgSF11, which peaks during the late phase of differentiation, and tyrosine phosphorylation of PKM2 also peaks during the late phase of differentiation. PKM2 phosphorylation and lactate production during osteoclast differentiation are decreased in the absence of IgSF11, suggesting IgSF11-mediated regulation of PKM2 activity and, thereby, glycolysis123. IgSF11 deficiency impairs osteoclast differentiation and resorption activity without affecting early-phase signaling pathways. Consistently, IgSF11 deficiency increases bone mass due to decreased numbers of osteoclasts on the bone surface80. Additionally, systemic administration of the PKM2 activator TEPP46 increases bone mass due to decreased numbers and sizes of osteoclasts on the bone surface123. IgSF11 deficiency and/or the administration of TEPP46 do not affect coupled bone formation80,123. Notably, the inhibitory effect of TEPP46 on osteoclast differentiation is more prominent under inflammatory conditions and do not affect inflammation or coupled bone formation123. These findings indicate that IgSF11-PKM2 signaling axis-mediated regulation of glucose metabolism is a promising target for selectively inhibiting bone loss. More importantly, metabolic adaptation is a mechanism controlling osteoclast differentiation and has potential as a target for the treatment and prevention of bone-destructive diseases.
Conclusions
In this review, we provided an overview of cell signaling and metabolic adaptations as regulatory mechanisms of osteoclast differentiation and specifically focused on understanding these mechanisms in the context of the osteoclast differentiation phases. Through the use of gene-edited mice, we have gained valuable insights into the phases of osteoclast differentiation and identified regulatory molecules that can modulate osteoclast-mediated bone resorption without affecting coupled bone formation. These include (1) the multinucleation process, as characterized by gene deletion of Pcdh7; (2) sealing zone (actin ring) formation, as characterized by Pkn3 deficiency; and (3) RANKL-induced glycolysis, as characterized by the modulation of PKM2 activity. Note that caution should be exercised due to the potential involvement of these molecules in other tissues. Indeed, in mice, Pkn3 deficiency could impact angiogenesis146, and bioinformatic analysis identified Pcdh7 as a key gene related to the development of sarcopenia and osteoporosis147. Understanding the molecular intricacies underlying late-phase osteoclast differentiation, in addition to its physiological roles, will assist in improving the treatment and prevention of osteoporosis and other bone-destructive diseases.
It is important to acknowledge that while many studies have explored the impact of gene functions on osteoclast differentiation and activity, they mainly focused on the role of genes in intrinsic cell functions. As the coupling between osteoclasts and osteoblasts can only be observed in vivo, some molecules that can potentially impact late-phase osteoclast differentiation (i.e., maturation) without affecting osteoblastic bone formation might have been overlooked. To gain a comprehensive understanding, future studies should reinvestigate the function of these molecules from the perspective of their involvement in coupling mechanisms. This will pave the way for further advancements in the field and potentially unveil novel therapeutic targets for bone-destructive diseases.
Acknowledgements
This work was supported in part by NIH grants (AR077526 and AR080021).
Author contributions
N.T., H.K., and Y.C.: conceptualization; N.T. and H.K.: mansucript writing; Y.C.: supervision.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Noriko Takegahara, Hyunsoo Kim.
References
- 1.Sims NA, Martin TJ. Osteoclasts provide coupling signals to osteoblast lineage cells through multiple mechanisms. Annu. Rev. Physiol. 2020;82:507–529. doi: 10.1146/annurev-physiol-021119-034425. [DOI] [PubMed] [Google Scholar]
- 2.Bolamperti S, Villa I, Rubinacci A. Bone remodeling: an operational process ensuring survival and bone mechanical competence. Bone Res. 2022;10:48. doi: 10.1038/s41413-022-00219-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tang Y, et al. TGF-beta1-induced migration of bone mesenchymal stem cells couples bone resorption with formation. Nat. Med. 2009;15:757–765. doi: 10.1038/nm.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xian L, et al. Matrix IGF-1 maintains bone mass by activation of mTOR in mesenchymal stem cells. Nat. Med. 2012;18:1095–1101. doi: 10.1038/nm.2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centrella M, Canalis E. Local regulators of skeletal growth: a perspective. Endocr. Rev. 1985;6:544–551. doi: 10.1210/edrv-6-4-544. [DOI] [PubMed] [Google Scholar]
- 6.Hock JM, Canalis E. Platelet-derived growth factor enhances bone cell replication, but not differentiated function of osteoblasts. Endocrinology. 1994;134:1423–1428. doi: 10.1210/endo.134.3.8119182. [DOI] [PubMed] [Google Scholar]
- 7.Sanchez-Fernandez MA, Gallois A, Riedl T, Jurdic P, Hoflack B. Osteoclasts control osteoblast chemotaxis via PDGF-BB/PDGF receptor beta signaling. PLoS One. 2008;3:e3537. doi: 10.1371/journal.pone.0003537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitlak BH, et al. The effect of systemically administered PDGF-BB on the rodent skeleton. J. Bone Miner. Res. 1996;11:238–247. doi: 10.1002/jbmr.5650110213. [DOI] [PubMed] [Google Scholar]
- 9.Oreffo RO, Mundy GR, Seyedin SM, Bonewald LF. Activation of the bone-derived latent TGF beta complex by isolated osteoclasts. Biochem. Biophys. Res. Commun. 1989;158:817–823. doi: 10.1016/0006-291X(89)92795-2. [DOI] [PubMed] [Google Scholar]
- 10.Fiedler J, Roderer G, Gunther KP, Brenner RE. BMP-2, BMP-4, and PDGF-bb stimulate chemotactic migration of primary human mesenchymal progenitor cells. J. Cell. Biochem. 2002;87:305–312. doi: 10.1002/jcb.10309. [DOI] [PubMed] [Google Scholar]
- 11.Rickard DJ, Sullivan TA, Shenker BJ, Leboy PS, Kazhdan I. Induction of rapid osteoblast differentiation in rat bone marrow stromal cell cultures by dexamethasone and BMP-2. Dev. Biol. 1994;161:218–228. doi: 10.1006/dbio.1994.1022. [DOI] [PubMed] [Google Scholar]
- 12.Robey PG, et al. Osteoblasts synthesize and respond to transforming growth factor-type beta (TGF-beta) in vitro. J. Cell. Biol. 1987;105:457–463. doi: 10.1083/jcb.105.1.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hock JM, Canalis E, Centrella M. Transforming growth factor-beta stimulates bone matrix apposition and bone cell replication in cultured fetal rat calvariae. Endocrinology. 1990;126:421–426. doi: 10.1210/endo-126-1-421. [DOI] [PubMed] [Google Scholar]
- 14.Pederson L, Ruan M, Westendorf JJ, Khosla S, Oursler MJ. Regulation of bone formation by osteoclasts involves Wnt/BMP signaling and the chemokine sphingosine-1-phosphate. Proc. Natl. Acad. Sci. USA. 2008;105:20764–20769. doi: 10.1073/pnas.0805133106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walker EC, et al. Cardiotrophin-1 is an osteoclast-derived stimulus of bone formation required for normal bone remodeling. J. Bone Miner. Res. 2008;23:2025–2032. doi: 10.1359/jbmr.080706. [DOI] [PubMed] [Google Scholar]
- 16.Takeshita S, et al. Osteoclast-secreted CTHRC1 in the coupling of bone resorption to formation. J. Clin. Investig. 2013;123:3914–3924. doi: 10.1172/JCI69493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garimella R, et al. Expression and synthesis of bone morphogenetic proteins by osteoclasts: a possible path to anabolic bone remodeling. J. Histochem. Cytochem. 2008;56:569–577. doi: 10.1369/jhc.2008.950394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsuoka K, Park KA, Ito M, Ikeda K, Takeshita S. Osteoclast-derived complement component 3a stimulates osteoblast differentiation. J. Bone Miner. Res. 2014;29:1522–1530. doi: 10.1002/jbmr.2187. [DOI] [PubMed] [Google Scholar]
- 19.Kim BJ, et al. Osteoclast-secreted SLIT3 coordinates bone resorption and formation. J. Clin. Investig. 2018;128:1429–1441. doi: 10.1172/JCI91086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ota K, et al. Transforming growth factor beta 1 induces CXCL16 and leukemia inhibitory factor expression in osteoclasts to modulate migration of osteoblast progenitors. Bone. 2013;57:68–75. doi: 10.1016/j.bone.2013.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao C, et al. Bidirectional ephrinB2-EphB4 signaling controls bone homeostasis. Cell Metab. 2006;4:111–121. doi: 10.1016/j.cmet.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 22.Negishi-Koga T, et al. Suppression of bone formation by osteoclastic expression of semaphorin 4D. Nat. Med. 2011;17:1473–1480. doi: 10.1038/nm.2489. [DOI] [PubMed] [Google Scholar]
- 23.Tonna S, et al. EphrinB2 signaling in osteoblasts promotes bone mineralization by preventing apoptosis. FASEB J. 2014;28:4482–4496. doi: 10.1096/fj.14-254300. [DOI] [PubMed] [Google Scholar]
- 24.Ikebuchi Y, et al. Coupling of bone resorption and formation by RANKL reverse signalling. Nature. 2018;561:195–200. doi: 10.1038/s41586-018-0482-7. [DOI] [PubMed] [Google Scholar]
- 25.Li D, et al. Osteoclast-derived exosomal miR-214-3p inhibits osteoblastic bone formation. Nat. Commun. 2016;7:10872. doi: 10.1038/ncomms10872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reszka AA, Rodan GA. Bisphosphonate mechanism of action. Curr. Rheumatol. Rep. 2003;5:65–74. doi: 10.1007/s11926-003-0085-6. [DOI] [PubMed] [Google Scholar]
- 27.Drake MT, Clarke BL, Khosla S. Bisphosphonates: mechanism of action and role in clinical practice. Mayo Clin. Proc. 2008;83:1032–1045. doi: 10.4065/83.9.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takegahara N, Kim H, Choi Y. RANKL biology. Bone. 2022;159:116353. doi: 10.1016/j.bone.2022.116353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miyazaki T, Tokimura F, Tanaka S. A review of denosumab for the treatment of osteoporosis. Patient Prefer. Adher. 2014;8:463–471. doi: 10.2147/PPA.S46192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lacey DL, et al. Bench to bedside: elucidation of the OPG-RANK-RANKL pathway and the development of denosumab. Nat. Rev. Drug Discov. 2012;11:401–419. doi: 10.1038/nrd3705. [DOI] [PubMed] [Google Scholar]
- 31.Seeman E, Martin TJ. Antiresorptive and anabolic agents in the prevention and reversal of bone fragility. Nat. Rev. Rheumatol. 2019;15:225–236. doi: 10.1038/s41584-019-0172-3. [DOI] [PubMed] [Google Scholar]
- 32.Sims NA, Ng KW. Implications of osteoblast-osteoclast interactions in the management of osteoporosis by antiresorptive agents denosumab and odanacatib. Curr. Osteoporos. Rep. 2014;12:98–106. doi: 10.1007/s11914-014-0196-1. [DOI] [PubMed] [Google Scholar]
- 33.Dou C, et al. Graphene-based MicroRNA transfection blocks preosteoclast fusion to increase bone formation and vascularization. Adv. Sci. 2018;5:1700578. doi: 10.1002/advs.201700578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chiu YH, et al. Dendritic cell-specific transmembrane protein (DC-STAMP) regulates osteoclast differentiation via the Ca(2+) /NFATc1 Axis. J. Cell. Physiol. 2017;232:2538–2549. doi: 10.1002/jcp.25638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Witwicka H, et al. Studies of OC-STAMP in osteoclast fusion: a new knockout mouse model, rescue of cell fusion, and transmembrane topology. PLoS One. 2015;10:e0128275. doi: 10.1371/journal.pone.0128275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miyamoto H, et al. Osteoclast stimulatory transmembrane protein and dendritic cell-specific transmembrane protein cooperatively modulate cell-cell fusion to form osteoclasts and foreign body giant cells. J. Bone Miner. Res. 2012;27:1289–1297. doi: 10.1002/jbmr.1575. [DOI] [PubMed] [Google Scholar]
- 37.Yang M, et al. Osteoclast stimulatory transmembrane protein (OC-STAMP), a novel protein induced by RANKL that promotes osteoclast differentiation. J. Cell. Physiol. 2008;215:497–505. doi: 10.1002/jcp.21331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yagi M, et al. DC-STAMP is essential for cell-cell fusion in osteoclasts and foreign body giant cells. J. Exp. Med. 2005;202:345–351. doi: 10.1084/jem.20050645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kodama J, Kaito T. Osteoclast multinucleation: review of current literature. Int. J. Mol. Sci. 2020;21:5685. doi: 10.3390/ijms21165685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ross FP. M-CSF, c-Fms, and signaling in osteoclasts and their precursors. Ann. N. Y Acad. Sci. 2006;1068:110–116. doi: 10.1196/annals.1346.014. [DOI] [PubMed] [Google Scholar]
- 41.Hamilton JA. Colony-stimulating factors in inflammation and autoimmunity. Nat. Rev. Immunol. 2008;8:533–544. doi: 10.1038/nri2356. [DOI] [PubMed] [Google Scholar]
- 42.Ross FP, Teitelbaum S. L. alphavbeta3 and macrophage colony-stimulating factor: partners in osteoclast biology. Immunol. Rev. 2005;208:88–105. doi: 10.1111/j.0105-2896.2005.00331.x. [DOI] [PubMed] [Google Scholar]
- 43.Pixley FJ, Stanley ER. CSF-1 regulation of the wandering macrophage: complexity in action. Trends Cell Biol. 2004;14:628–638. doi: 10.1016/j.tcb.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 44.Arai F, et al. Commitment and differentiation of osteoclast precursor cells by the sequential expression of c-Fms and receptor activator of nuclear factor kappaB (RANK) receptors. J. Exp. Med. 1999;190:1741–1754. doi: 10.1084/jem.190.12.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nakashima T, et al. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat. Med. 2011;17:1231–1234. doi: 10.1038/nm.2452. [DOI] [PubMed] [Google Scholar]
- 46.Xiong J, et al. Matrix-embedded cells control osteoclast formation. Nat. Med. 2011;17:1235–1241. doi: 10.1038/nm.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boyce BF. Advances in the regulation of osteoclasts and osteoclast functions. J. Dent. Res. 2013;92:860–867. doi: 10.1177/0022034513500306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kanazawa K, Kudo A. Self-assembled RANK induces osteoclastogenesis ligand-independently. J. Bone Miner. Res. 2005;20:2053–2060. doi: 10.1359/JBMR.050706. [DOI] [PubMed] [Google Scholar]
- 49.Wong BR, et al. The TRAF family of signal transducers mediates NF-kappaB activation by the TRANCE receptor. J. Biol. Chem. 1998;273:28355–28359. doi: 10.1074/jbc.273.43.28355. [DOI] [PubMed] [Google Scholar]
- 50.Kobayashi N, et al. Segregation of TRAF6-mediated signaling pathways clarifies its role in osteoclastogenesis. EMBO J. 2001;20:1271–1280. doi: 10.1093/emboj/20.6.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Abu-Amer Y. NF-kappaB signaling and bone resorption. Osteoporos. Int. 2013;24:2377–2386. doi: 10.1007/s00198-013-2313-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Franzoso G, et al. Requirement for NF-kappaB in osteoclast and B-cell development. Genes Dev. 1997;11:3482–3496. doi: 10.1101/gad.11.24.3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Matsuo K, et al. Fosl1 is a transcriptional target of c-Fos during osteoclast differentiation. Nat. Genet. 2000;24:184–187. doi: 10.1038/72855. [DOI] [PubMed] [Google Scholar]
- 54.Wagner EF. Functions of AP1 (Fos/Jun) in bone development. Ann. Rheum. Dis. 2002;61:ii40–ii42. doi: 10.1136/ard.61.suppl_2.ii40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grigoriadis AE, et al. c-Fos: a key regulator of osteoclast-macrophage lineage determination and bone remodeling. Science. 1994;266:443–448. doi: 10.1126/science.7939685. [DOI] [PubMed] [Google Scholar]
- 56.Kim K, Lee SH, Ha Kim J, Choi Y, Kim N. NFATc1 induces osteoclast fusion via up-regulation of Atp6v0d2 and the dendritic cell-specific transmembrane protein (DC-STAMP) Mol. Endocrinol. 2008;22:176–185. doi: 10.1210/me.2007-0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ishida N, et al. Large scale gene expression analysis of osteoclastogenesis in vitro and elucidation of NFAT2 as a key regulator. J. Biol. Chem. 2002;277:41147–41156. doi: 10.1074/jbc.M205063200. [DOI] [PubMed] [Google Scholar]
- 58.Takayanagi H, et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev. Cell. 2002;3:889–901. doi: 10.1016/S1534-5807(02)00369-6. [DOI] [PubMed] [Google Scholar]
- 59.Asagiri M, et al. Autoamplification of NFATc1 expression determines its essential role in bone homeostasis. J. Exp. Med. 2005;202:1261–1269. doi: 10.1084/jem.20051150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yamashita T, et al. NF-kappaB p50 and p52 regulate receptor activator of NF-kappaB ligand (RANKL) and tumor necrosis factor-induced osteoclast precursor differentiation by activating c-Fos and NFATc1. J. Biol. Chem. 2007;282:18245–18253. doi: 10.1074/jbc.M610701200. [DOI] [PubMed] [Google Scholar]
- 61.Maeda K, et al. Wnt5a-Ror2 signaling between osteoblast-lineage cells and osteoclast precursors enhances osteoclastogenesis. Nat. Med. 2012;18:405–412. doi: 10.1038/nm.2653. [DOI] [PubMed] [Google Scholar]
- 62.Koga T, et al. Costimulatory signals mediated by the ITAM motif cooperate with RANKL for bone homeostasis. Nature. 2004;428:758–763. doi: 10.1038/nature02444. [DOI] [PubMed] [Google Scholar]
- 63.Kim N, Takami M, Rho J, Josien R, Choi Y. A novel member of the leukocyte receptor complex regulates osteoclast differentiation. J. Exp. Med. 2002;195:201–209. doi: 10.1084/jem.20011681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Humphrey MB, et al. TREM2, a DAP12-associated receptor, regulates osteoclast differentiation and function. J. Bone Miner. Res. 2006;21:237–245. doi: 10.1359/JBMR.051016. [DOI] [PubMed] [Google Scholar]
- 65.Cella M, et al. Impaired differentiation of osteoclasts in TREM-2-deficient individuals. J. Exp. Med. 2003;198:645–651. doi: 10.1084/jem.20022220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shinohara M, et al. Tyrosine kinases Btk and Tec regulate osteoclast differentiation by linking RANK and ITAM signals. Cell. 2008;132:794–806. doi: 10.1016/j.cell.2007.12.037. [DOI] [PubMed] [Google Scholar]
- 67.Walsh MC, Choi Y. Biology of the RANKL-RANK-OPG system in immunity, bone, and beyond. Front. Immunol. 2014;5:511. doi: 10.3389/fimmu.2014.00511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim K, et al. MafB negatively regulates RANKL-mediated osteoclast differentiation. Blood. 2007;109:3253–3259. doi: 10.1182/blood-2006-09-048249. [DOI] [PubMed] [Google Scholar]
- 69.Zhao B, et al. Interferon regulatory factor-8 regulates bone metabolism by suppressing osteoclastogenesis. Nat. Med. 2009;15:1066–1071. doi: 10.1038/nm.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Miyauchi Y, et al. The Blimp1-Bcl6 axis is critical to regulate osteoclast differentiation and bone homeostasis. J. Exp. Med. 2010;207:751–762. doi: 10.1084/jem.20091957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Park SJ, et al. Sirt6 cooperates with Blimp1 to positively regulate osteoclast differentiation. Sci. Rep. 2016;6:26186. doi: 10.1038/srep26186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Takegahara, N. Osteoclast Cytokinesis. In: Zaidi, M (ed.) Encyclopedia of Bone Biology. Oxford: Academic Press; 2020. Vol. 1 p. 221–235.
- 73.Takegahara N, et al. Involvement of receptor activator of nuclear Factor-kappaB Ligand (RANKL)-induced incomplete cytokinesis in the polyploidization of osteoclasts. J. Biol. Chem. 2016;291:3439–3454. doi: 10.1074/jbc.M115.677427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kikuta J, Ishii M. Osteoclast migration, differentiation and function: novel therapeutic targets for rheumatic diseases. Rheumatology. 2013;52:226–234. doi: 10.1093/rheumatology/kes259. [DOI] [PubMed] [Google Scholar]
- 75.Pereira M, et al. Common signalling pathways in macrophage and osteoclast multinucleation. J. Cell. Sci. 2018;131:jcs216267. doi: 10.1242/jcs.216267. [DOI] [PubMed] [Google Scholar]
- 76.Khan UA, Hashimi SM, Bakr MM, Forwood MR, Morrison NA. CCL2 and CCR2 are essential for the formation of osteoclasts and foreign body giant cells. J. Cell. Biochem. 2016;117:382–389. doi: 10.1002/jcb.25282. [DOI] [PubMed] [Google Scholar]
- 77.Sul OJ, et al. Absence of MCP-1 leads to elevated bone mass via impaired actin ring formation. J. Cell. Physiol. 2012;227:1619–1627. doi: 10.1002/jcp.22879. [DOI] [PubMed] [Google Scholar]
- 78.McHugh KP, et al. Mice lacking beta3 integrins are osteosclerotic because of dysfunctional osteoclasts. J. Clin. Investig. 2000;105:433–440. doi: 10.1172/JCI8905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fiorino C, Harrison RE. E-cadherin is important for cell differentiation during osteoclastogenesis. Bone. 2016;86:106–118. doi: 10.1016/j.bone.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 80.Kim H, et al. IgSF11 regulates osteoclast differentiation through association with the scaffold protein PSD-95. Bone Res. 2020;8:5. doi: 10.1038/s41413-019-0080-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kim H, Takegahara N, Choi Y. Protocadherin-7 regulates osteoclast differentiation through intracellular SET-binding domain-mediated RhoA and Rac1 activation. Int. J. Mol. Sci. 2021;22:13117. doi: 10.3390/ijms222313117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kukita T, et al. RANKL-induced DC-STAMP is essential for osteoclastogenesis. J. Exp. Med. 2004;200:941–946. doi: 10.1084/jem.20040518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee SH, et al. v-ATPase V0 subunit d2-deficient mice exhibit impaired osteoclast fusion and increased bone formation. Nat. Med. 2006;12:1403–1409. doi: 10.1038/nm1514. [DOI] [PubMed] [Google Scholar]
- 84.Feng X, Teitelbaum SL. Osteoclasts: new Insights. Bone Res. 2013;1:11–26. doi: 10.4248/BR201301003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Weivoda MM, Oursler MJ. The roles of small GTPases in osteoclast biology. Orthop. Muscular Syst. 2014;3:1000161. doi: 10.4172/2161-0533.1000161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Croke M, et al. Rac deletion in osteoclasts causes severe osteopetrosis. J. Cell. Sci. 2011;124:3811–3821. doi: 10.1242/jcs.086280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Razzouk S, Lieberherr M, Cournot G. Rac-GTPase, osteoclast cytoskeleton and bone resorption. Eur. J. Cell Biol. 1999;78:249–255. doi: 10.1016/S0171-9335(99)80058-2. [DOI] [PubMed] [Google Scholar]
- 88.Duong LT, Lakkakorpi P, Nakamura I, Rodan GA. Integrins and signaling in osteoclast function. Matrix Biol. 2000;19:97–105. doi: 10.1016/S0945-053X(00)00051-2. [DOI] [PubMed] [Google Scholar]
- 89.Zou W, et al. Syk, c-Src, the alphavbeta3 integrin, and ITAM immunoreceptors, in concert, regulate osteoclastic bone resorption. J. Cell. Biol. 2007;176:877–888. doi: 10.1083/jcb.200611083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Faccio R, et al. Vav3 regulates osteoclast function and bone mass. Nat. Med. 2005;11:284–290. doi: 10.1038/nm1194. [DOI] [PubMed] [Google Scholar]
- 91.Novack DV, Teitelbaum SL. The osteoclast: friend or foe? Annu Rev. Pathol. 2008;3:457–484. doi: 10.1146/annurev.pathmechdis.3.121806.151431. [DOI] [PubMed] [Google Scholar]
- 92.Boyce BF, Yoneda T, Lowe C, Soriano P, Mundy GR. Requirement of pp60c-src expression for osteoclasts to form ruffled borders and resorb bone in mice. J. Clin. Investig. 1992;90:1622–1627. doi: 10.1172/JCI116032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Miyazaki T, et al. Src kinase activity is essential for osteoclast function. J. Biol. Chem. 2004;279:17660–17666. doi: 10.1074/jbc.M311032200. [DOI] [PubMed] [Google Scholar]
- 94.Vacher J, Bruccoleri M, Pata M. Ostm1 from mouse to human: insights into osteoclast maturation. Int. J. Mol. Sci. 2020;21:5600. doi: 10.3390/ijms21165600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Neutzsky-Wulff AV, et al. Severe developmental bone phenotype in ClC-7 deficient mice. Dev. Biol. 2010;344:1001–1010. doi: 10.1016/j.ydbio.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 96.Kornak U, et al. Mutations in the a3 subunit of the vacuolar H(+)-ATPase cause infantile malignant osteopetrosis. Hum. Mol. Genet. 2000;9:2059–2063. doi: 10.1093/hmg/9.13.2059. [DOI] [PubMed] [Google Scholar]
- 97.Kornak U, et al. Loss of the ClC-7 chloride channel leads to osteopetrosis in mice and man. Cell. 2001;104:205–215. doi: 10.1016/S0092-8674(01)00206-9. [DOI] [PubMed] [Google Scholar]
- 98.Teitelbaum SL, Ross FP. Genetic regulation of osteoclast development and function. Nat. Rev. Genet. 2003;4:638–649. doi: 10.1038/nrg1122. [DOI] [PubMed] [Google Scholar]
- 99.Takayanagi H. Osteoclast differentiation and activation. Clin. Calcium. 2007;17:484–492. [PubMed] [Google Scholar]
- 100.Roy M, Roux S. Rab GTPases in osteoclastic bone resorption and autophagy. Int. J. Mol. Sci. 2020;21:7655. doi: 10.3390/ijms21207655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhao H, et al. Synaptotagmin VII regulates bone remodeling by modulating osteoclast and osteoblast secretion. Dev. Cell. 2008;14:914–925. doi: 10.1016/j.devcel.2008.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.DeSelm CJ, et al. Autophagy proteins regulate the secretory component of osteoclastic bone resorption. Dev. Cell. 2011;21:966–974. doi: 10.1016/j.devcel.2011.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sobacchi C, Schulz A, Coxon FP, Villa A, Helfrich MH. Osteopetrosis: genetics, treatment and new insights into osteoclast function. Nat. Rev. Endocrinol. 2013;9:522–536. doi: 10.1038/nrendo.2013.137. [DOI] [PubMed] [Google Scholar]
- 104.Nesbitt SA, Horton MA. Trafficking of matrix collagens through bone-resorbing osteoclasts. Science. 1997;276:266–269. doi: 10.1126/science.276.5310.266. [DOI] [PubMed] [Google Scholar]
- 105.Salo J, Lehenkari P, Mulari M, Metsikko K, Vaananen HK. Removal of osteoclast bone resorption products by transcytosis. Science. 1997;276:270–273. doi: 10.1126/science.276.5310.270. [DOI] [PubMed] [Google Scholar]
- 106.Indo Y, et al. Metabolic regulation of osteoclast differentiation and function. J. Bone Miner. Res. 2013;28:2392–2399. doi: 10.1002/jbmr.1976. [DOI] [PubMed] [Google Scholar]
- 107.Ishii KA, et al. Coordination of PGC-1beta and iron uptake in mitochondrial biogenesis and osteoclast activation. Nat. Med. 2009;15:259–266. doi: 10.1038/nm.1910. [DOI] [PubMed] [Google Scholar]
- 108.Lemma S, et al. Energy metabolism in osteoclast formation and activity. Int. J. Biochem. Cell Biol. 2016;79:168–180. doi: 10.1016/j.biocel.2016.08.034. [DOI] [PubMed] [Google Scholar]
- 109.Jeng JY, et al. Maintenance of mitochondrial DNA copy number and expression are essential for preservation of mitochondrial function and cell growth. J. Cell. Biochem. 2008;103:347–357. doi: 10.1002/jcb.21625. [DOI] [PubMed] [Google Scholar]
- 110.Larsson NG, et al. Mitochondrial transcription factor A is necessary for mtDNA maintenance and embryogenesis in mice. Nat. Genet. 1998;18:231–236. doi: 10.1038/ng0398-231. [DOI] [PubMed] [Google Scholar]
- 111.Wang J, et al. Dilated cardiomyopathy and atrioventricular conduction blocks induced by heart-specific inactivation of mitochondrial DNA gene expression. Nat. Genet. 1999;21:133–137. doi: 10.1038/5089. [DOI] [PubMed] [Google Scholar]
- 112.Li H, et al. Genetic modification of survival in tissue-specific knockout mice with mitochondrial cardiomyopathy. Proc. Natl. Acad. Sci. USA. 2000;97:3467–3472. doi: 10.1073/pnas.97.7.3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Miyazaki T, et al. Intracellular and extracellular ATP coordinately regulate the inverse correlation between osteoclast survival and bone resorption. J. Biol. Chem. 2012;287:37808–37823. doi: 10.1074/jbc.M112.385369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wei W, et al. PGC1beta mediates PPARgamma activation of osteoclastogenesis and rosiglitazone-induced bone loss. Cell Metab. 2010;11:503–516. doi: 10.1016/j.cmet.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nishikawa K, et al. DNA methyltransferase 3a regulates osteoclast differentiation by coupling to an S-adenosylmethionine-producing metabolic pathway. Nat. Med. 2015;21:281–287. doi: 10.1038/nm.3774. [DOI] [PubMed] [Google Scholar]
- 116.Bae S, et al. MYC-dependent oxidative metabolism regulates osteoclastogenesis via nuclear receptor ERRalpha. J. Clin. Investig. 2017;127:2555–2568. doi: 10.1172/JCI89935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jin Z, Wei W, Yang M, Du Y, Wan Y. Mitochondrial complex I activity suppresses inflammation and enhances bone resorption by shifting macrophage-osteoclast polarization. Cell Metab. 2014;20:483–498. doi: 10.1016/j.cmet.2014.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Christofk HR, Vander Heiden MG, Wu N, Asara JM, Cantley LC. Pyruvate kinase M2 is a phosphotyrosine-binding protein. Nature. 2008;452:181–186. doi: 10.1038/nature06667. [DOI] [PubMed] [Google Scholar]
- 119.Liberti MV, Locasale JW. The warburg effect: how does it benefit cancer cells? Trends Biochem. Sci. 2016;41:211–218. doi: 10.1016/j.tibs.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg Effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ahn H, et al. Accelerated lactate dehydrogenase activity potentiates osteoclastogenesis via NFATc1 signaling. PLoS One. 2016;11:e0153886. doi: 10.1371/journal.pone.0153886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kim JM, et al. Osteoclast precursors display dynamic metabolic shifts toward accelerated glucose metabolism at an early stage of RANKL-stimulated osteoclast differentiation. Cell Physiol. Biochem. 2007;20:935–946. doi: 10.1159/000110454. [DOI] [PubMed] [Google Scholar]
- 123.Kim H, Takegahara N, Choi Y. IgSF11-mediated phosphorylation of pyruvate kinase M2 regulates osteoclast differentiation and prevents pathological bone loss. Bone Res. 2023;11:17. doi: 10.1038/s41413-023-00251-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Murata K, et al. Hypoxia-Sensitive COMMD1 integrates signaling and cellular metabolism in human macrophages and suppresses osteoclastogenesis. Immunity. 2017;47:66–79.e65. doi: 10.1016/j.immuni.2017.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hitosugi T, et al. Tyrosine phosphorylation inhibits PKM2 to promote the Warburg effect and tumor growth. Sci. Signal. 2009;2:ra73. doi: 10.1126/scisignal.2000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ward PS, Thompson CB. Metabolic reprogramming: a cancer hallmark even warburg did not anticipate. Cancer Cell. 2012;21:297–308. doi: 10.1016/j.ccr.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nishikawa K, et al. Osteoclasts adapt to physioxia perturbation through DNA demethylation. EMBO Rep. 2021;22:e53035. doi: 10.15252/embr.202153035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Utting JC, Flanagan AM, Brandao-Burch A, Orriss IR, Arnett TR. Hypoxia stimulates osteoclast formation from human peripheral blood. Cell Biochem. Funct. 2010;28:374–380. doi: 10.1002/cbf.1660. [DOI] [PubMed] [Google Scholar]
- 129.Knowles HJ, Athanasou NA. Acute hypoxia and osteoclast activity: a balance between enhanced resorption and increased apoptosis. J. Pathol. 2009;218:256–264. doi: 10.1002/path.2534. [DOI] [PubMed] [Google Scholar]
- 130.Knowles HJ, Cleton-Jansen AM, Korsching E, Athanasou NA. Hypoxia-inducible factor regulates osteoclast-mediated bone resorption: role of angiopoietin-like 4. FASEB J. 2010;24:4648–4659. doi: 10.1096/fj.10-162230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Morten KJ, Badder L, Knowles HJ. Differential regulation of HIF-mediated pathways increases mitochondrial metabolism and ATP production in hypoxic osteoclasts. J. Pathol. 2013;229:755–764. doi: 10.1002/path.4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Knowles HJ, Athanasou NA. Hypoxia-inducible factor is expressed in giant cell tumour of bone and mediates paracrine effects of hypoxia on monocyte-osteoclast differentiation via induction of VEGF. J. Pathol. 2008;215:56–66. doi: 10.1002/path.2319. [DOI] [PubMed] [Google Scholar]
- 133.Miyauchi Y, et al. HIF1alpha is required for osteoclast activation by estrogen deficiency in postmenopausal osteoporosis. Proc. Natl. Acad. Sci. USA. 2013;110:16568–16573. doi: 10.1073/pnas.1308755110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Devignes CS, Carmeliet G, Stegen S. Amino acid metabolism in skeletal cells. Bone Rep. 2022;17:101620. doi: 10.1016/j.bonr.2022.101620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008;7:11–20. doi: 10.1016/j.cmet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 136.Brunner JS, et al. Environmental arginine controls multinuclear giant cell metabolism and formation. Nat. Commun. 2020;11:431. doi: 10.1038/s41467-020-14285-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Brosnan JT, Brosnan ME. Branched-chain amino acids: enzyme and substrate regulation. J. Nutr. 2006;136:207S–211S. doi: 10.1093/jn/136.1.207S. [DOI] [PubMed] [Google Scholar]
- 138.Pereira M, et al. A trans-eQTL network regulates osteoclast multinucleation and bone mass. Elife. 2020;9:e55549. doi: 10.7554/eLife.55549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Go M, et al. BCAT1 promotes osteoclast maturation by regulating branched-chain amino acid metabolism. Exp. Mol. Med. 2022;54:825–833. doi: 10.1038/s12276-022-00775-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ozaki K, et al. The L-type amino acid transporter LAT1 inhibits osteoclastogenesis and maintains bone homeostasis through the mTORC1 pathway. Sci. Signal. 2019;12:eaaw3921. doi: 10.1126/scisignal.aaw3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lee SH, Kim T, Jeong D, Kim N, Choi Y. The tec family tyrosine kinase Btk Regulates RANKL-induced osteoclast maturation. J. Biol. Chem. 2008;283:11526–11534. doi: 10.1074/jbc.M708935200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Uehara S, et al. Protein kinase N3 promotes bone resorption by osteoclasts in response to Wnt5a-Ror2 signaling. Sci. Signal. 2017;10:eaan0023. doi: 10.1126/scisignal.aan0023. [DOI] [PubMed] [Google Scholar]
- 143.Uehara S, et al. Inhibitor of protein kinase N3 suppresses excessive bone resorption in ovariectomized mice. J. Bone Min. Metab. 2022;40:251–261. doi: 10.1007/s00774-021-01296-1. [DOI] [PubMed] [Google Scholar]
- 144.Kim H, et al. Protocadherin-7 contributes to maintenance of bone homeostasis through regulation of osteoclast multinucleation. Bmb Rep. 2020;53:472–477. doi: 10.5483/BMBRep.2020.53.9.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kim H, Takegahara N, Choi Y. PP2A-Mediated GSK3β Dephosphorylation Is Required for Protocadherin-7-dependent regulation of small GTPase RhoA in Osteoclasts. Cells. 2023;12:1967. doi: 10.3390/cells12151967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mukai H, et al. PKN3 is the major regulator of angiogenesis and tumor metastasis in mice. Sci. Rep. 2016;6:18979. doi: 10.1038/srep18979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Liu M, et al. PCDH7 as the key gene related to the co-occurrence of sarcopenia and osteoporosis. Front. Genet. 2023;14:1163162. doi: 10.3389/fgene.2023.1163162. [DOI] [PMC free article] [PubMed] [Google Scholar]