Abstract
The literature on large‐scale resting‐state functional brain networks across the adult lifespan was systematically reviewed. Studies published between 1986 and July 2021 were retrieved from PubMed. After reviewing 2938 records, 144 studies were included. Results on 11 network measures were summarized and assessed for certainty of the evidence using a modified GRADE method. The evidence provides high certainty that older adults display reduced within‐network and increased between‐network functional connectivity. Older adults also show lower segregation, modularity, efficiency and hub function, and decreased lateralization and a posterior to anterior shift at rest. Higher‐order functional networks reliably showed age differences, whereas primary sensory and motor networks showed more variable results. The inflection point for network changes is often the third or fourth decade of life. Age effects were found with moderate certainty for within‐ and between‐network altered patterns and speed of dynamic connectivity. Research on within‐subject bold variability and connectivity using glucose uptake provides low certainty of age differences but warrants further study. Taken together, these age‐related changes may contribute to the cognitive decline often seen in older adults.
Keywords: aging, fMRI, functional connectivity, large‐scale networks, lifespan, PET, PRISMA, resting‐state networks, systematic review
Short abstract
Although the literature on large‐scale, resting state functional networks in aging has been reviewed previously, we offer the first systematic qualitative and quantitative synthesis of the evidence. The novel synthesis stems from the adoption of PRISMA method and the breadth of network measures reviewed. The review offers a contemporary summary of the strength of the evidence, theoretical implications, and recommendations for further research.
1. INTRODUCTION
The number and proportion of older adults in the community is projected to increase significantly over the coming decades. It is estimated that the global population of adults aged over 65 years will increase from 703 million in 2019 to 1.5 billion by 2050 (United Nations, 2019). The rise in the number and proportion of older people stems from declining birth and fertility rates and an increase in the average lifespan across the world (United Nations, 2019).
An aging population is expected to drive an increased societal burden from cognitive decline. Aging is a major risk factor for cognitive decline, as well as many chronic and neurodegenerative diseases. Hence, an aging population is likely to be associated with a growth in age‐related diseases that will exact a significant health, social and financial toll on individuals and society (United Nations, 2019). These costs raise important questions about what can be done to ensure that the expected increase in the aging population is accompanied by optimal cognitive aging, and how age‐related illnesses and their associated healthcare and social impacts can be minimized.
It is well established that extensive brain changes occur with age, from the molecular to the functional level. Age‐related brain changes include alterations in the structure, function and metabolic processes of the brain, often with an associated decline in cognitive function (Salthouse, 2019; Smith et al., 2020; Wu et al., 2011). Older adults typically show a decline in a number of cognitive domains compared with younger adults (for reviews, see Brown & Park, 2003; Glisky, 2007). These cognitive domains have been shown to peak in the third decade of life and then gradually decline (Harada et al., 2013). They include executive function and attention, such as the ability to think abstractly, reason and problem‐solve. In contrast, tasks relying on predominantly automatic or well‐practiced processes are less impacted by age or may even increase slightly across the lifespan, such as vocabulary and general knowledge (Harada et al., 2013). Speech and language processing tend to be relatively stable with age (Salthouse, 2019), although processing time may be slower in older adults due to more generalized reductions in processing speed. Together, cognitive changes seen in the aging process can have a widespread impact on an older individual's daily function and quality of life.
1.1. The brain as a network
Understanding the complex, multi‐layered structure and function of the brain has been an area of scientific enquiry for many decades and has led to a variety of schema for describing the organization of the cortex. Early approaches provided insight into the functional specialization of brain areas and how age‐related and degenerative changes occur with local neuronal alterations. However, it soon became apparent that the function of brain regions also relates to their connectivity with other regions (Genon et al., 2018).
In the last two decades or so, there has been a rapid increase in the number of studies in which the brain is modeled as a complex network that consist of units (e.g., brain systems, regions, sub‐regions, neurons) linked by structural connectivity, functional connectivity, or both (Damoiseaux, 2017; Liao et al., 2017; Wig, 2017). In this research, “functional connectivity” is typically defined as the temporal dependency between spatially remote neurophysiological events (Shen, 2015). A large body of research has led to the understanding that communication in the brain is organized according to a topology that combines local information processing with global information integration across networks of functionally interacting regions. This combination of functional properties enables intricate, synchronized dynamics across multiple spatiotemporal scales (Fornito & Bullmore, 2015; Sala & Perani, 2019).
In the neuroimaging field, the term “network” is often used to refer to a group of voxels or brain regions that have a consistent pattern of correlated activity in a resting‐state or during a task. The construction of networks and their analyses draws on the concept of “small world” networks and graph theory (Rubinov & Sporns, 2010; Sporns, 2018; Sun et al., 2012; Wig et al., 2011). The key concepts and measures used in functional network and graph theory analyses and which form the basis for this review are defined in Table 1 and depicted in Figure 1. In graph theory analyses, the brain can be modeled as a group of networks, consisting of a set of nodes that represent the units of the system, and edges that denote the interactions between nodes (Liao et al., 2017; Wig et al., 2011). The nodes can be neurons, neuronal populations or brain regions, depending on the spatial scales of interest, and the edges represent the structural or functional connectivity that links the nodes (Liao et al., 2017). In this review, we focus on large‐scale networks of brain regions, involving tens to hundreds of nodes (across centimeters), as well as whole‐brain, voxel‐wise network studies of thousands of nodes (i.e., voxels of 1–2 millimeters).
TABLE 1.
Network and graph theory properties, definitions, and measures
| Property | Definition | Example graph measure |
|---|---|---|
| Functional connectivity | Temporal correlation between time‐series of neurophysiological events | The average of the weight of node edges, e.g., BOLD correlation or PET tracer uptake |
| Path length | Distance between nodes in terms of edges traversed | Average minimum path length is the shortest mean distance from a particular node to all other nodes in the network (in terms of edges traversed), and is a measure of integration |
| Local efficiency | Efficiency of information transfer between nodes within a network | Inverse of the average shortest path length of each node to its neighbors |
| Global efficiency | Efficiency of information transfer across the entire network system | Inverse of the average shortest path length between networks in the system |
| Modularity | Degree to which the network is divided into distinct communities. Dense within relative to sparse between network connections. A modular network exhibits a ‘clustering’ of its nodes into multiple distinct subnetworks | Fraction of connections (edges) within the communities of a network compared to if the connections were distributed at random across the network, e.g., ICA or thresholded correlations aim to reflect modularity |
| Segregation | The amount of partitioning between distinct regions of a network. Reflects the ability for specialized processing to occur within densely interconnected groups of brain regions. Similar to modularity, except that it directly quantifies within versus between network connections | Dense or strong connectivity among nodes in the same network (high within‐network connectivity) coupled with sparse or weak connections between nodes belonging to different networks (low between‐network connectivity), e.g., clustering coefficient |
| Clustering | Nodes in the same network are more similar to each other than to those in other networks | Clustering coefficient is a measure of the proportion of a node's neighbors that are themselves neighbors and is a measure of segregation |
| Integration | Reflects the degree of integration among multiple brain regions. The ability to rapidly combine specialized information from distributed brain regions | The average number of edges of the shortest paths that connect nodes over the whole network. A smaller path length represents greater integration. Measures include shortest path length and node degree. Also related to global efficiency, i.e., as the inverse of the average shortest path length |
| Hubs | Nodes that have highly centralized connections and strong relationships with each other via long‐distance connection. A hub can be defined as a node that has many important connections running through it (is a member of the shortest path between many nodes), or a node that associates with (correlates to) many other networks | Measures of centrality, including how many (degree) and weight (strength) of the connections, together with the shortest paths within the network pass through a given node (betweenness). For example, participation coefficient measure the connections that are not within a node's own network or that are across networks. When the between node connections are denser than expected by chance, they are form “rich clubs”, serving as “way stations” for high volume transfer |
| “Small World” network | High level of local and global efficiency in information processing, with economical “sparse” wiring costs. High segregation and integration | In graph theoretical terms, a graph is “small world” if the path length is similar to that of a random graph, and the clustering coefficient is much greater than a random graph |
FIGURE 1.
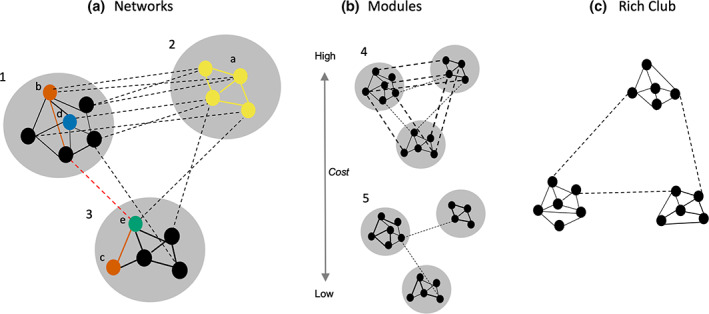
Illustration of functional networks and small‐world metrics. (a) Larger gray circles are networks of the graph (networks 1, 2 and 3). The dots within each of the networks are nodes and the lines are the edges (significant correlation between nodes in resting‐state networks); solid lines are within‐network edges and dashed lines between‐network edges. Yellow nodes and edges depict a network displaying within‐network connectivity. Connectivity strength of node a is the average weight of the edges linking with it; and local efficiency is the inverse of the average shortest path length of each node to its neighbors. Red edges depict the shortest path between the red node b and red node c. the shorter the average path length across all pairs of nodes, the higher the global efficiency. The blue node d depicts a provincial hub (strongly connects nodes within the same network); the green node e depicts a connector hub (connects nodes between different networks). (b) Modules reflect the division of larger networks into smaller “building blocks” or communities, typically reflecting anatomically connected or functionally related regions. The human brain displays “small world” properties that balances an economical trade‐off between efficiency, physical connection cost and maximizing topological value. In module 4, the networks are wired for global efficiency and integrative processing, with each node likely to connect to multiple other nodes within‐ and between‐networks, minimizing path length and optimizing global efficiency. However, this topology comes at a high wiring cost owing to the large number of connections across the system, particularly long‐range connections. In comparison, in module 5, the networks are wired to minimize cost and support functional specialization, with each node having within‐network near neighbors topologically and spatially. However, insufficient topologically direct connections or edges between networks limits global integration and efficiency. Efficiency is reduced through information needing to transverse a high number of long‐range paths or edges between nodes in different networks. Relative to module 4, module 5 is also higher in segregation (stronger or denser links within‐networks but weaker or sparser links between‐networks) and modularity (higher proportion of within‐network connections, or higher average path length, across the system). (c) Rich club network is indicated by functional connections between dense networks (Adapted from Cohen & D'Esposito, 2016; Wig, 2017).
1.2. Functional networks in aging as an early marker of cognitive decline
Although cognitive decline is generally considered a normal consequence of aging, it is not in fact an inevitable consequence (Salat, 2011). While some older adults show clear signs of cognitive decline by age 60, others retain excellent cognitive function well into their 1980s and beyond, performing as well or better than younger adults (Glisky, 2007). Improved understanding of the heterogeneity of the cognitive aging process is an important empirical and clinical matter, as neural changes can begin to occur several decades before the onset of overt cognitive decline and/or disease symptoms (Chen, 2019; Coupé et al., 2019). Better characterization of the timeframe and profile of normal aging and cognitive decline may provide an opportunity to delay, slow or even reverse or prevent cognitive decline and disease trajectories.
Conceptualizing the brain as a multifaceted network has provided a useful framework to examine how neural information processing relates to cognition and behavior, and how it may be altered in aging and diseases (van den Heuvel & Hulshoff Pol, 2010). Graph theory posits that the interplay between integration, segregation and “small world” properties of networks will have implications for cognitive aging. In this framework, the heterogeneity in cognitive aging is an emergent property of network interactions involving multiple brain regions and information processing capacities. Connections within‐ and between‐networks may change over time in number, strength, configuration and efficiency and as a function of learning, age or disease states (Genon et al., 2018). Dysfunction can arise from alterations to connected regions, leading to cascades seen in degenerative disease, or more gradual changes seen in “normal” aging (Fornito & Bullmore, 2015). A complex interaction of both maladaptive and compensatory mechanisms can follow, resulting in a heterogeneous expression of cognition and behavior depending on the time, location, and scale of the underlying changes (Fornito et al., 2015; Naik et al., 2017).
The typology of the connections of a functional network will also dictate how age‐related changes or damage impact the networks and their associated functions. Alterations to a “rich club” network hub, such as the executive control or the default mode networks, will have diffuse impacts across the system. In contrast, alterations to a more local or provincial hub will result in more specific deficits (Fornito et al., 2015). For example, in a meta‐analysis of a large magnetic resonance imaging (MRI) database of 20,000 subjects with 26 brain disorders, Crossley et al. (2014) found that that perturbances to white matter “rich club” network hubs, or their longer distance edges, are especially likely to degrade system‐wide global efficiency and are critical to communication across the network.
Individual differences in brain network alterations in aging may also be mediated by shared pathways in disease processes. For example, alterations to brain network function and an increased risk of cognitive decline have been associated with metabolic diseases, such as diabetes and insulin resistance (Akintola & van Heemst, 2015; Arvanitakis et al., 2016; Bello‐Chavolla et al., 2019; Ekblad et al., 2017). Blood flow throughout the brain is a central player in neuronal function and cognition and changes with age and certain diseases. With age, arterial stiffness, neurovascular uncoupling and blood–brain barrier damage can impact the dynamics of brain blood flow and local perfusion (Kalaria et al., 2019). The potential role of shared pathways of aging and disease in functional brain network changes and their implications for research in cognitive aging are discussed in more detail in Section 4.5.
1.3. Resting‐state functional networks are valuable for understanding cognitive aging
It has been suggested that humans spend as much as half of their waking lives in cognitive states that are not directed towards a specific task (Lurie et al., 2020). In 1995, Biswal et al. 1995 published a landmark neuroimaging study of the brain at rest. It was predicted that the recorded neural activity would be largely random, as it was not directed towards a specific task. However, Biswal et al. found synchrony between brain regions that were known to share functional properties (also see Biswal et al., 2010). Over the next two decades, investigation of the connectivity of brain activity in a resting, task‐free state has been one of the primary methods for understanding the brain's network structure and function (Mill et al., 2020). A large body of research has shown that resting‐state fluctuations across brain regions and at multiple scales originate, at least in part, from spontaneous neural activity. It is also now well established that brain activity at rest is organized into functional resting‐state networks, defined by their spatiotemporal configuration and functional roles (Beckmann et al., 2005; Calhoun et al., 2008).
An important advantage of resting‐state analyses over task analyses is that it avoids the need to understand the response and source of differences between groups in behavior, cognitive strategy or performance, especially where performance can be supported by more than one cognitive mechanism (Rugg, 2016). Nonetheless, resting‐state functional connectivity has been questioned for its ecological and cognitive relevance. For this reason, the field has also extensively examined age differences in functional connectivity when undertaking cognitive tasks (Campbell & Schacter, 2017). A detailed review of task‐related functional connectivity is beyond the scope of this review (see Lurie et al., 2020). It is worth mentioning that research has shown that resting‐state functional connectivity patterns are often similar to cognitive task activation patterns, with as much as 80% shared variance (Cole et al., 2014, 2016; also see Chan et al., 2017) and that patterns of functional connectivity are relatively stable across tasks, with similarity estimates ranging between r = 0.5 and r = 0.9 (Lurie et al., 2020; Medaglia et al., 2015). This suggests functional network architecture in the resting state likely reflects at least some of the underlying “map” or “circuitry” by which activity flows during cognitive task performance. This shared circuitry supports the importance of studying resting‐state functional connectivity as a means to better understand cognitive aging (Cieri & Esposito, 2018; Ferreira & Busatto, 2013).
1.4. Resting‐state functional networks and theories of cognitive aging
In systematically reviewing the literature, we also relate the findings to theories that have been proposed to explain functional network differences in aging. These include the dedifferentiation hypothesis and the compensation hypothesis (Grady, 2012). These hypotheses were originally developed to explain differences in task performance and task‐related brain activity between younger and older adults. However, the compensation and dedifferentiation hypotheses are likely applicable to resting state functional connectivity because, as noted above, the same functional architecture implicated in task‐related processing also applies in the resting state.
Dedifferentiation describes the loss of functional specialization in networks that are engaged during the performance of a task (Cabeza & Dennis, 2012; Park et al., 2004; Rajah & D'Esposito, 2005). Dedifferentiation is underpinned by more diffuse, nonspecific recruitment of brain regions (Fornito et al., 2015). It can reflect the capacity of structurally distinct components of the network system to provide the same contributions to a given output, providing functional “plasticity” as well as potentially compensation to perturbance or damage (Fornito et al., 2015).
The compensation hypothesis in aging postulates that older adults are able to recruit higher levels of activity in comparison to young subjects in some brain areas to compensate for functional deficits located in other regions. The compensation‐related utilization of neural circuits hypothesis (CRUNCH; Reuter‐Lorenz & Cappell, 2008; Schneider‐Garces et al., 2010; but see Jamadar et al., 2010) proposes that, older adults recruit higher levels of neural resources than young adults even at the same level of cognitive demand. As will be outlined below, this increased neural activity among older adults is often seen in the frontal areas of the brain both at rest and during task performance.
The theory of coordination dynamics (Tognoli & Kelso, 2014) proposes that brain networks transiently connect when people attend to a stimulus or undertake cognitive and behavioral tasks. “Metastability” is central concept in the coordination dynamics theory. Metastability refers to the human brain's ability to integrate several functional parts and to produce neural fluctuations in a coordinated manner, providing the basis for cognitive function and behavior. Coordination dynamics theory proposes that the tendency for brain regions to express their individual specialized functions (segregation, modularity) coexist with tendencies to couple and coordinate globally for multiple functions (integration). Hence, metastability reflects a balance between integration and segregation, with signal variability within the network enabling the dynamic shift between integration and segregation (Naik et al., 2017; Nomi et al., 2017).
Another theoretical focus in cognitive aging has centered on the overall efficiency in the recruitment and deployment of neuronal resources, sometimes referred to as “neural reuse” theories (see Anderson & Finlay). One such theory, the scaffolding theory of aging and cognition (STAC; Reuter‐Lorenz & Park, 2014), suggests that the recruitment of additional neural resources via network reorganization provides the foundation to preserve cognitive function in the face of structural and functional decrements with age. According to scaffolding theory, dedifferentiation and compensation are two sides of the same coin (Naik et al., 2017), and metastable network dynamics are the outcome of functional brain interactions constrained by modular structural connectivity. In other words, network dedifferentiation in older age can be considered compensation for a change in the underlying structural and functional “scaffolding” of the brain (Naik et al., 2017).
The cognitive reserve theory (Stern, 2002) posits that functional brain architecture may support cognitive performance in the face of other age‐related brain changes, including structural brain changes. Factors such as life experiences, education and physical activity have been identified to increase reserve, although the biological processes underpinning these effects remain largely unknown and debated (Perneczky, 2019; Varangis et al., 2019). Evidence suggests that older people with substantial cognitive reserve can compensate for brain changes to maintain their overall cognitive performance, even in the presence of significant alterations.
It is well established that brain regions show functional lateralization, with the left hemisphere predominantly involved with language, analytical and logical functions, and the right hemisphere with non‐verbal visuospatial, intuitive and sensory tasks (Agcaoglu et al., 2015). Moreover, the sensorimotor cortex exhibits stronger between hemisphere resting‐state functional connectivity than the prefrontal and temporoparietal regions (Zuo et al., 2010), which is believed to be an evolutionarily conserved mechanism that supports fast and efficient information processing (Chen, Xia et al., 2019). The degree of lateralization has been found to differ with age (e.g., Agcaoglu et al., 2015). Greater recruitment of prefrontal cortical regions involved in executive functions is also frequently reported in older adulthood compared to younger adults. These lateralization and frontal recruitment patterns have been described as hemispheric asymmetry reductions in old age (HAROLD) and a posterior‐to‐anterior shift in aging (PASA) (see Cabeza, 2002; Davis et al., 2008, 2012; Spreng & Turner, 2019, for review of these theories). Although HAROLD and PASA were developed to explain task‐related changes in aging, research has also shown their relevance in resting‐state studies of aging. The research assessing the evidence for HAROLD and PASA across the adult lifespan is reviewed in Section 3.8.
1.5. Scope of the current review
The objective of this paper is to systematically review the imaging literature on large‐scale resting‐state network function across the adult lifespan. The novel synthesis stems from the systematic review method of PRISMA and the breadth of network measures, which has not been undertaken previously for resting state functional connectivity in aging. We aim to address whether age differences exist at rest in the static and dynamic functional connectivity within‐ and between‐networks; the local and global efficiency of functional networks; the network segregation, integration, modularity and hub structure; the topological connectivity pattern of lateralization across the hemispheres and between the anterior and posterior regions of the brain; and “metabolic connectivity” based on positron emission tomography (PET) imaging.
Oxygen and glucose in the brain are central players in brain physiology and function. They also underly two widely‐used neuroimaging methods, MRI and FDG‐PET imaging, respectively. For detailed reviews of the physiological bases of these methods, refer to, for example, (Chen, 2019; Cipolla, 2009; Grayson et al., 2013; Mergenthaler et al., 2013). Suffice to say that many of the advances in our understanding of brain connectivity in health and disease have come from research using MRI and PET imaging. MRI has been widely used to study resting‐state functional connectivity, as it provides exquisite spatial resolution with moderate temporal resolution (sub‐second resolution is now obtainable with fast imaging techniques like multiband acquisition; Feinberg et al., 2010, Feinberg & Setsompop, 2013; see Risk et al., 2021 for resting‐state multiband fMRI). FDG‐PET has also been used to identifying specific patterns of glucose metabolism and the “metabolic connectivity” of regions of the brain. Both of these imaging methods also have limitations that can present challenges for research in aging populations, which are discussed further below.
2. METHOD
2.1. Type of studies and participants
Studies of large‐scale brain network function across the adult lifespan were retrieved from PubMed published between 1986 and 2021, following the PRISMA 2020 statement (Page et al., 2021). The identification, screening and selection process is summarized in Figure 2. We included studies in which participants were adults aged between 18 and at least 70 years of age. In some studies, the age of participants extended into the 1980s. Studies of older adults only were also included if they covered at least three decades of life from the 1940s or 1950s to at least the 1970s or 1980s.
FIGURE 2.
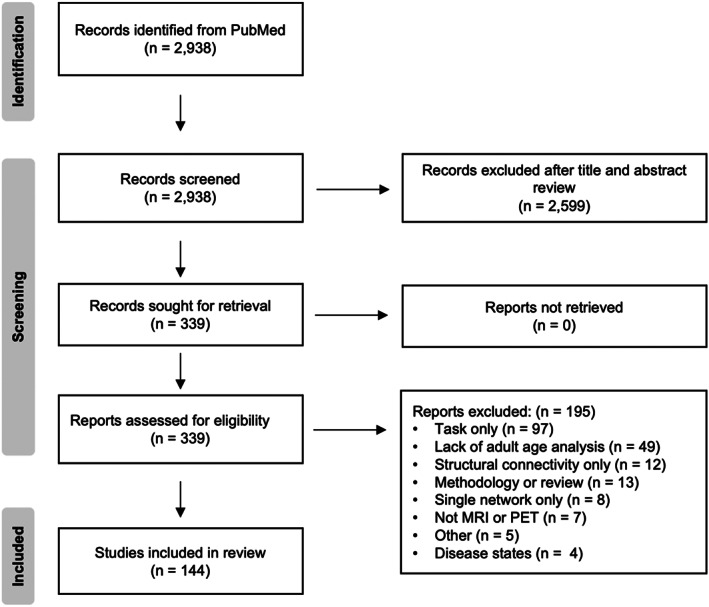
The identification, screening and selection process of studies included in the systematic review.
2.2. Search strategy
Search criteria were based on combinations of the following keywords and terms: functional connectivity, resting‐state, resting‐state connectivity, large‐scale networks, brain networks, intrinsic networks, functional network, functional brain, brain systems, functional architecture, functional organization, fMRI, MRI, resting‐state fMRI, metabolic connectivity, simultaneous PET, multimodal PET, functional PET, fPET; together with age, aging, aging, lifespan, age‐related, and older adults. Additional papers were identified from the citations from the retrieved references. Studies were included if they were published in a peer‐reviewed journal in English and used human subjects.
2.3. Exclusion criteria
Papers were excluded if the sole focus was on a single brain region or associated cognitive domain (e.g., executive, attention, visual, frontal, motor regions). Studies examining connectivity while performing a task in scanner or subjects with dementia were excluded, unless they also included a resting‐state condition and a healthy cohort for which age differences were reported separately. Gray and white matter structural connectivity papers were excluded, unless they also reported resting‐state functional connectivity separately. Disease states (mental disorders including developmental disorders, dementias, epilepsy), training and intervention studies were excluded in the search criteria.
2.4. Selection process and included studies
The literature search retrieved 2938 unique records. Titles and abstracts were independently screened and reviewed by two raters (HD & RDP) for inclusion, reducing the articles for review against the inclusion criteria to 339. Where differences between raters were found, the articles were discussed and reconciled. As a result, 144 references were included in the systematic review.
2.5. Network measures
The studies included in the review reported one or a combination of the functional network measures in Table 2. Studies were entered into tables and the direction and nature of age differences classified across all reported measures, both positive and negative. For each measure, counts were made of the total number of studies assessing age differences and the number of studies showing a consistent direction and pattern of statistically significant age effects. Counts were undertaken for the overall pattern of age differences across all resting‐state networks and for specific networks where patterns of results varied by network and aided explanation of the heterogeneity of findings. Counts were also independently undertaken by two raters (HD & RDP) and differences reconciled.
TABLE 2.
Summary table of systematic review findings for functional connectivity and graph theory measures. Age effects are counts (%) of studies
| Measure | Number of Studies | Total N‐Size | Negative effect of age for all or some networks, together with absence of any positive age effects | No age differences in any networks | Positive effect of age for all or some networks, together with absence of any negative age effects | Varied direction of age effect by network | Level of certainty |
|---|---|---|---|---|---|---|---|
| Within‐network connectivity (static) | 50 | 21,110 | 36 (72%) | 0 (0%) | 1 (2%) | 13 (26%) | High a |
| Between‐network connectivity (static) | 37 | 16,518 | 5 (14%) | 1 (3%) | 20 (54%) | 11 (30%) | High a |
| Segregation, modularity, integration | 32 | 9330 | 30 (94%) | 0 (0%) | 1 (3%) | 1 (3%) | High |
| Local efficiency | 9 | 1145 | 7 (78%) b | 0 (0%) | 0 (0%) | 2 (22%) | High |
| Global efficiency | 13 | 1768 | 7 (54%) c | 3 (23%) | 3 (23%) | 0 (0%) | High |
| Within‐network connectivity (dynamic) | 7 | 993 | 7 (100%) d | 0 (0%) | 0 (0%) | 0 (0%) | Moderate |
| Between‐network connectivity (dynamic) | 8 | 1709 | 0 (0%) | 0 (0%) | 8 (100%) d | 0 (0%) | Moderate |
| Lateralization of functional connectivity at rest | 9 | 2889 | 8 (89%) | 0 (0%) | 1 (11%) | 0 (0%) | High |
| Posterior–anterior shift in functional connectivity at rest | 8 | 2124 | 0 (0%) | 0 (0%) | 7 (88%) | 1 (12%) e | High |
| Within‐subject BOLD variability | 6 | 1321 | 3 (50%) | 0 (0%) | 1 (17%) | 2 (33%) | Low |
| Metabolic connectivity | 9 | 827 | 1 (11%) | 0 | 4 (44%) | 4 (44%) | Low |
Consistency of findings was considered high, with distinct and consistent age effects seen in higher‐order and primary processing networks, see Sections 3.2.1 and 3.2.2.
100% of studies reported reduced local efficiency in higher‐order networks, see Section 3.7.2.
When nodal scale is held constant at 90–114, consistency of findings increased to 83% of studies showing negative effect of age, see section 3.4.2 and Table S8.
For dynamic within‐ and between‐network connectivity, the measures vary across studies although the direction of age effect was consistent, see section 3.6.
For posterior–anterior shift, “varied” direction of age effects indicates partial support for the HAROLD and PASA models, respectively, see section 3.5.
2.6. Certainty of the evidence
We classified the overall level of certainty or strength of evidence for each network measure as high, moderate or low using a modified version of the GRADE method (Guyatt et al., 2008; Murad et al., 2017). GRADE was originally developed to provide a framework for presenting summaries of clinical trial evidence and an assessment of certainty when making clinical recommendations. We considered the following four criteria when assessing the strength of the evidence across the available literature: (1) The number and cumulative sample sizes of the studies and the amount of functional data; (2) the risk of bias from the study samples and recruitment procedures; (3) consistency of findings across the studies; and (4) directness of age comparisons across the adult lifespan.
For each network measure, the four GRADE criteria were scored as either 5 (high), 3 (moderate), or 1 (low), except risk of bias, for which scoring was reversed so higher score corresponds to low risk of bias. Scoring was undertaken by one author (HD), independently reviewed by the remaining authors and any differences discussed and resolved based on cut‐points that defined the scores.
Scan length and the amount of functional data collected are important consideration in the analysis of brain network metrics. Research has indicated that scans of at least five minutes are needed to generate moderate reliability (Van Dijk et al., 2012) and that increasing scan duration to between 13 (Anderson et al., 2011) and 15 (Birn et al., 2013) minutes greatly improves reliability. We report the scan length and volumes of data collected for each study (see Tables S1–S7) as well as their average, minimum and maximum for each network measure (Table S8).
We considered a combination of the number, cumulative sample size and average volumes and scan length in the GRADE assessment. To score “High”, more than 10 studies were required with a cumulative sample size above 1000 and at least 200 average volumes collected across an average seven minute or longer resting‐state scan; to score “Moderate”, 6–10 studies were required with a cumulative sample size of 500 or more and at least 180 average volumes across a 5 min or longer scan; and to score “Low”, five or fewer studies with a cumulative sample size less than 500 and fewer than 180 average volumes in a less than 5 min scan.
For consistency of findings, we considered the percentage of studies reporting an age‐related network change in a consistent direction. To score “High”, at least 70% of studies were required to have reported a consistent age‐related result; to score “Moderate”, 51–69% of studies; and to score “Low”, 50% or fewer studies.
The risk of bias assessment was based on the geographical and public database diversity. The absolute number of countries and databases and their diversity were considered. More than 15 unique countries and databases were required to score “Low” for risk of bias; 7–14 for “Moderate”; and 6 or fewer for “High”. It is also worth noting that the participant populations in the studies is not always mutually exclusive, as several authors obtained samples from the same open source databases (see Supplementary Tables). The risk of circularity or biased results from the inclusion of the same datasets was considered in the “risk of bias” assessment. Specifically, no single database accounted for more than 13% of at least 32 studies for any network measure rated as having low “risk of bias” (e.g., within‐ and between‐network static connectivity; segregation, modularity, integration; lateralization and posterior–anterior shift); whereas on other measures (e.g., dynamic connectivity and within‐subject BOLD variability), up to three of six studies (50%) used the same dataset and were rated as having a high “risk of bias”.
For directness of age comparisons, we counted the number of studies that included participants across the adult lifespan. Ten or more studies using a full adult lifespan sample was required to score “High”; 4–9 for “Moderate”; and 3 or fewer for “Low”.
The total GRADE score was calculated by weighting the four criteria scores as follows: Number and size of studies (0.25), consistency of findings (0.35), risk of bias (0.2) and full adult lifespan represented (0.2). The final overall GRADE score for each measure could potentially range from 1 to 5. The final GRADE score for certainty of the evidence for each measure was based on the thresholds of: 1.0–2.5 (low certainty), 2.6–3.4 (moderate certainty), and 3.5–5.0 (high certainty).
Head motion is a well document confounder in resting‐state fMRI based assessments of the network properties including those based on graph theoretical analysis (Power et al., 2012, 2013; Satterthwaite et al., 2013; Yan et al., 2013). We report the method(s) used by the authors of each study to correct for head motion (Tables S1–S6 and S8). All authors included head motion correction in their pre‐processing pipeline, and across the network measures 96–100% of authors took additional steps to control for head motion. The additional steps included one or a combination of scrubbing of volumes with excessive motion; regressing out head motion from the functional time‐series, partialling out head motion in independent component analyses, and using head‐motion parameters in group‐level analyses. Because of the range of approaches taken and the very high percentage of studies correcting for head motion, we did not directly include head motion in the GRADE assessment.
3. RESULTS
3.1. Summary tables and figures
Table 2 provides a summary and Figure 3 a visual representation of the systematic review findings. The data in Table 2 presents a global summary of the detailed study data in Supplementary Tables S1–S7 for each network measures and Table S8 for the GRADE assessment. As some studies reported multiple network measures, the studies listed in Tables S1–S8 are not mutually exclusive.
FIGURE 3.
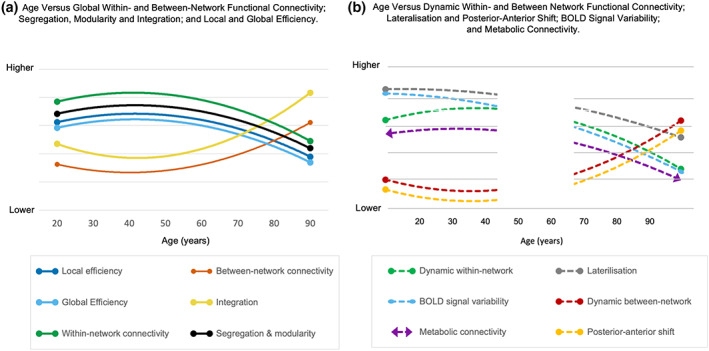
Visual representation of systematic review findings on network measures reported in Table 2. The figures represent conceptual rather than quantified summaries of each measure to illustrate the relative pattern across the lifespan (refer to Table 1 for definitions of metrics and their units of measurement). For panels a and b, a solid line with circular ends indicates high certainty of evidence; the dashed line with circular ends indicates moderate certainty; and dashed lines with arrow ends indicate low certainty. For panel b, the blank area from ages approximately 45–65 years indicates a smaller number of studies explicitly including these ages (see Table S8) and an absence of testing for non‐linear relationships across the adult lifespan.
Across the network measures, approximately 50% of studies do not include middle‐aged individuals (see Table S8), which limits the age differences that can be characterized across the adult lifespan. As will be shown below, quadratic trajectories of age differences likely have an inflection point somewhere in the third to fifth decade of life. However, the lack of middle aged adult studies precludes the rigorous identification and quantification of aging trajectories for adults from younger to older ages. This issue is most evident for the network measures where we assessed the strength of the evidence to be moderate to low (e.g., within‐subject BOLD signal variability and dynamic connectivity), as a smaller number of studies using these measures across the adult lifespan were available for review. The relative scarcity of studies examining middle age especially limits the ability to identify any quadratic relationships that exist between the network measures and age (see Figure 3b). In contrast, the measures for which we rated the certainty of evidence to be high (e.g., within‐ and between‐network connectivity, segregation and integration), were used in a sufficiently large number of studies that covered the full adult lifespan to draw more certain conclusions.
3.2. Older adults show reduced within‐network functional connectivity compared to younger adults
The economic “small world” organization of brain regions, characterized by local clustering with efficient local and global information transfer, emerges in the first decades of life. A detailed review of early age research can be found in (e.g., Edde et al., 2021; Keunen et al., 2017; Liao et al., 2017). Briefly, the “small world” organization is present as early as 30 weeks of gestation, and is strengthened across the first two decades of life. Networks also progressively shift from isolated local regions to a more distributed organization in infancy, before displaying additional and more subtle integration changes during early adulthood. The networks reach their most stable period in the third or fourth decade of life (Edde et al., 2021) and become a more interconnected system that extends over longer distances. The networks tend to follow a development sequence that is believed to reflect an adaptation to environmental demands (Fornito et al., 2015) from an initial consolidation of primary sensory and motor networks by late childhood and higher‐order networks in late adolescence.
Compared to younger adults, older adults show differences in the functional connectivity of large‐scale resting‐state networks. We assessed the strength of the evidence as high for decreased within‐network connectivity among older adults. Thirty six of the 50 studies (72%) that assessed adult age differences in within‐network connectivity reported lower connectivity in all or some of the networks analyzed among older subjects, with an absence of any increased connectivity in older age (see Table S1 for details). Thirteen studies (26%) reported age‐related patterns of varying increases and decreases for specific networks. Consistency of the findings is also increased when age effects are assessed separately for higher‐order and primary sensory and motor networks.
3.2.1. Higher‐order and lower‐order networks show varied within‐network functional connectivity trajectories
The varied within‐network connectivity results across some studies may reflect differences in the trajectories of specific functional network differences with age. These different trajectories are illustrated in Figure 4. The primary sensory (e.g., visual, auditory) and motor networks, as well as the sub‐cortical and attention networks, appear to be more variably impacted by age than higher‐order functional networks (e.g., default mode, front‐parietal, executive control, and cingulo‐opercular networks). Across the 50 studies assessing within‐network connectivity, none reported increases within the default mode network with age. Moreover, among the 23 studies reporting results for the default mode network separately, 22 (96%) reported lower within‐connectivity among older adults; the remaining study reported no age difference (Jockwitz et al., 2017). Twenty one of the 23 (91%) studies reporting other associative networks separately, such as the cingulo‐opercular and fronto‐parietal networks, reported lower within‐network connectivity among older adults.
FIGURE 4.
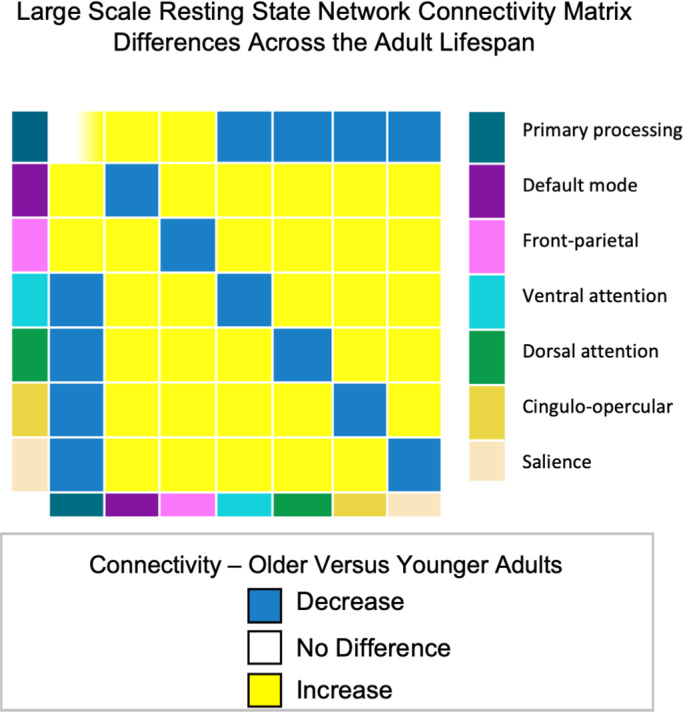
Network connectivity matrix differences across the adult lifespan. The figure represents conceptual rather than a quantified connectivity measure (correlation) to illustrate the relative pattern across the lifespan. Diagonal cells show within‐network connectivity differences with age; off‐diagonal shows between‐network connectivity (primary processing includes sensorimotor, visual and auditory networks). Cells on the diagonal of the matrix with graded shading reflects where study findings are variable in the effect of age, either showing no age difference (white) or increased connectivity among older compared to younger adults (yellow). Off‐diagonal cells for the primary processing networks reflects mixed patterns of increased connectivity to higher order networks (yellow) and decreased (blue) connectivity to attention and control networks for older compared to younger adults.
There is more variability in results for the primary sensory and motor networks where they were reported separately. Nine of 24 (42%) studies reported no age differences or higher within‐network connectivity among older adults in the sensorimotor network (Cao et al., 2014; Chan et al., 2014; Onoda & Yamaguchi, 2013; Siman‐Tov et al., 2017; Song et al., 2014; Tomasi & Volklow, 2012; Viviano et al., 2017; Wen, Dong, et al., 2020; Zhai & Li, 2019), three of 14 (21%) in the visual system (Farras‐Permanyer et al., 2019; Petrican et al., 2017; Zhai & Li, 2019; Zonneveld et al., 2019) and two of two (100%) in the emotional network (Nashiro et al., 2017; Wang et al., 2012). Age‐related reductions in integration within the sensorimotor network have also been reported (He et al., 2020; Roski et al., 2013).
3.3. Older adults show increased between‐network functional connectivity compared to younger adults
Of the 37 studies that investigated between‐network connectivity changes with age, 20 (54%) reported increases in connectivity with an absence of any decreased connectivity among older adults compared to young adults. Five studies (14%) reported a negative age effect, and 11 studies (30%) reported age‐related patterns of varying increases and decreases for specific networks.
We assessed the strength of the evidence as high for increased between‐network connectivity among older adults. This assessment reflects the relatively large number of studies, cumulative sample sizes, direct testing of age differences across the adult lifespan, and relative consistency of the findings. Consistency of the findings is also increased when age effects are assessed separately for higher‐order and primary processing networks.
3.3.1. Higher‐order and lower‐order networks show different age trajectories of between‐network connectivity
Decreases in between‐network connectivity of associative networks was relatively uncommon, reported in two of the 37 (5%) studies reviewed (Luo et al., 2020; Wen, Dong, et al., 2020). Seventeen studies reported on the default mode network separately, with 15 (88%) reporting increased connectivity to other large‐scale networks. In contrast, 12 studies reported networks with decreased connectivity to primary sensory, motor and attention networks, such as between the motor regions and other resting‐state networks (Allen et al., 2011; Geerligs et al., 2015; Hou et al., 2019; Luo et al., 2020; Wang et al., 2012; Zhai & Li, 2019), between the salience network and the visual, auditory and sensorimotor networks (Hou et al., 2019; Monteiro et al., 2019; Onoda et al., 2012; Vij et al., 2018), and between sub‐regions of the default mode, visual, auditory and dorsal attention networks (Huang et al., 2015; Spreng et al., 2016; Viviano et al., 2017; Zhai & Li, 2019; Zonneveld et al., 2019). Lower connectivity between the supplementary motor area and left anterior insular cortex has also been reported with increasing age (Li et al., 2015).
3.4. The trajectory of age differences in functional connectivity are non‐linear
There is evidence that the trajectory of age‐related differences in resting‐state functional network connectivity are non‐linear, typically showing a quadratic pattern with age. Investigation of these non‐linear relationships was reported in eight studies (see Table S1) at varying spatial scales, showing an inflection point in the third decade for within‐network connectivity, and in the fourth decade for between‐network connectivity (Betzel et al., 2014; Cao et al., 2014; Chen et al., 2018; Luo et al., 2020; Vij et al., 2018; Wang et al., 2012; Wei et al., 2018; Zhai & Li, 2019). For example, Wei et al. (2018) found within‐network connectivity followed a negative quadratic (inverted U‐shape) relationship from 20–80 years of age across the whole brain, starting to decline around 30 years, and accelerating from around 40 years of age.
In large sample of 5967 subjects aged 13 to 72 years, Luo et al. (2020) found varied patterns of network correlations with age. Within‐network connectivity decreased linearly, particularly in the visual and default mode networks. Connectivity between the default mode network and fronto‐parietal network exhibited a positive quadratic (U‐shape) relationship from the second to eight decades of life, with the lowest level of connectivity occurring in the fourth decade. Luo et al. noted that this pattern is consistent with a “last‐in‐first‐out” configuration during maturation, in which the later‐maturing higher‐order brain regions are more sensitive to earlier age‐related decline. Of note, Luo et al. (2020) also found coherence between the timing and pattern of corresponding structural network changes with age, suggesting that structural changes may mediate or moderate at least some of the functional changes in aging, consistent with the STAC theory of cognitive aging.
3.5. Networks are less segregated, less modular and more integrated
Brain network modules divide larger brain systems into basic “building blocks” of internally densely connected clusters that are relatively weakly interconnected among each other (Sporns, 2017). Modularity is also closely related to two fundamental principles of functional organization: segregation and integration (Sun et al., 2012). Segregation describes the fact the cerebral cortex is heterogeneous and can be divided into regionally distinct cortical areas, based on functional and structural properties (Genon et al., 2018). Integration relates to the notion that cognitive abilities rely on a dynamic interplay and exchange of information between different regions (Genon et al., 2018). The dense within‐module connections increase the local clustering and thus facilitate functional specialization within the module, whereas the sparse (but not necessarily absent) between‐module connections optimize the path length of the network and provide the basis for global information integration (Chan et al., 2014).
Excess integration or segregation can be deleterious (see Figure 1b). Too much integration can lead to inefficiencies in information transfer and even rapid spreading of disease, whereas too much segregation can result in diminished network interaction (Wig, 2017). From a graph network perspective, although segregation is similar to modularity, it is different in that it directly quantifies the proportions of both within‐ and between‐network connections in the network (Wig, 2017).
It was noted earlier that the “small world” properties of brain networks emerge and strengthen over the first two decades of life. The coherence between structural and functional connectivity also strengthens during this period (Hagmann et al., 2010). In other words, there is a strengthening of the correlation between structural and functional connectivity with age suggesting that white matter connectivity plays an increasingly important role in creating brain‐wide organization. The developmental period of “plasticity” provides the foundation for functional specialization and connectivity that supports the development of higher‐order executive functions and other cognitive abilities. It is built on a foundation or “anatomical backbone” of myelinated white‐matter pathways (Baum et al., 2020). Functional networks undergo a parallel change in the first two decades of life to those seen in the structural networks. The refinement of structural and functional connectivity during development results in a more modular system that balances low‐cost neuronal resources with highly efficient information transmission (Figures 1b and 5). A more modular system supports the dynamic and efficient control of attention and behavior, aligned with functional specialization (Baum et al., 2020; Edde et al., 2020; Keulers et al., 2019).
FIGURE 5.
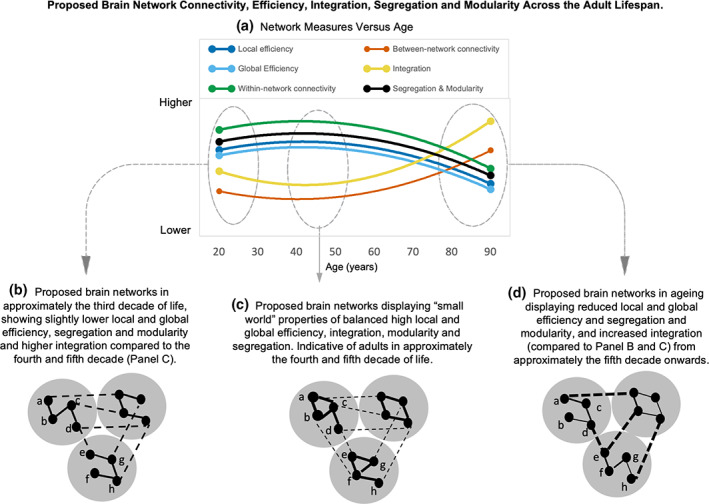
Proposed brain network differences across the adult lifespan. Panel a shows network measures from Figure 3a across the adult lifespan. All measures had “high” certainty of age effects from the GRADE assessment, except for global efficiency, which was rated as “moderate”. Panel c illustrates how the human brain sits between the extremes of module 4 and module 5 in Figure 1b, and displays “small world” properties, peaking in the fourth or fifth decade with local efficiency complemented by global efficiency. Local, short‐distance connections reflect the capacity for information transfer between nodes across a short path length and are complemented by sparse long‐distance connections that incur a higher wiring cost. Topologically direct connections between spatially remote brain regions are expected to yield benefits in terms of flexible function and behavior. The system balances modularity, integration, and segregation by balancing strength or density of within‐ and between‐network connections and relatively short average path lengths. As shown in panel b, brain networks in approximately the third decade of life show somewhat lower local and global efficiency and integration compared to the fourth and fifth decade (panel c). Panel d, compared with younger adults (panel b and c), older adults display reduced local efficiency (increased path length to neighboring nodes) and global efficiency (loss of long‐range paths or longer average path lengths) between nodes across networks. For example, in panel c, local efficiency is seen in the direct path from node a to node b traversing one edge only; whereas in panel d the path from node a to node b traverses three edges (node a to c to d to b). In terms of global efficiency, in panel c, the path length from node a to node h is via three edges: Node a to b to f to h; whereas in panel d, it is via six edges: Node a to c to d to e to f to g to h. within‐network connectivity strength is also reduced in older adults in panel d compared to younger adults in panel b and c (indicated by thinner black lines in panel d within networks) and between‐network connectivity increased (indicated by thicker black lines across networks in panel d compared to panel b and c), leading to a less segregated and more modular, integrated system. (Adapted from Bullmore & Sporns, 2012).
3.5.1. Networks are less segregated and modular among older adults
Modularity and segregation are related concepts, both measuring the degree of separation between the nodes within a network (Wig, 2017). Segregation is calculated as the difference in mean within‐ versus between‐network connections (strength or density), relative to the mean within‐network connections (Wig, 2017). Modularity is the fraction of connections (edges) within the nodes of a network compared to if the connections were distributed at random across the network (Wig, 2017).
A reduction of within‐network and an increase in between‐network connectivity across the adult lifespan means that networks evolve to become less segregated, less modular and more integrated in older age. We assessed the certainty of the evidence to be high for age‐related changes in network segregation, integration and modularity (see Table 2). Of the 32 studies assessing segregation, integration or modularity, 30 (94%) showed increased integration, or a loss of segregation, modularity, or both (see Table S2 for details). One study reported varied results by network (Petrican et al., 2017), with decreased segregation of three higher‐order networks (i.e., fronto‐parietal, salience, cingulo‐opercular) and increased segregation of networks for external processing (e.g., language, subcortical). The other study reported an increase in segregation only, possibly because of the more limited age range of adults studied (mean, 65; standard deviation, 12 years) and a high dimension brain parcellation of 90 regions (Sala‐Llonch et al., 2014).
Chan et al. (2014) found the sensorimotor and association networks exhibit distinct patterns of age differences in segregation. The sensorimotor networks comprised hand, visual, mouth and auditory networks; whereas the association network comprised the default mode, fronto‐parietal, ventral and dorsal attention, and salience and cingulo‐opercular networks. A linear association was found between decreasing sensorimotor system segregation and increasing age. The sensorimotor systems also exhibited linear age‐related reductions in segregation with other systems, such as the visual system. In contrast, a negative quadratic relationship was found with the association system and age, with the inflection point of accelerated reductions in segregation at approximately 50 years. Han et al. (2018) and Pedersen et al. (2021) found largely similar non‐linear patterns between age and network segregation.
Longitudinal studies have shown that adults aged 65 years and over without a college degree had lower resting‐state system segregation compared to college‐educated peers, and that system segregation predicted changes in dementia severity up to 10 years later (Chan et al., 2021). Socioeconomic status has also been found to relate to the brain's functional network organization. Across adults aged 35–64 years, lower education and occupation stats was associated with reduced resting‐state system segregation (Chan et al., 2018). Taken together these results suggest that cognitive reserve, as measured by education status, likely impacts the trajectory of an adult's functional network organization.
The decreased segregation in older adults compared to younger adults during rest has also been found to carry over into task states (Chou et al., 2013; Crowell et al., 2020; Geerligs et al., 2012, 2014; Tsvetanov et al., 2018). For example, the composition of modules identified during rest changed more during an n‐back task in older adults than young adults, particularly under higher task demands (Gallen et al., 2016). Older adults recruited additional between‐module connections at all levels of task demand, whereas young adults only did so when task demands were highest. This is compatible with the CRUNCH model of cognitive compensation, whereby older adults recruit additional regions compared to younger adults at all levels of cognitive demands (Reuter‐Lorenz & Cappell, 2008; Schneider‐Garces et al., 2010).
Thirteen studies reported measures of modularity of the resting‐state networks. All (100%) studies reported a decrease among older adults; namely, a reduction in the degree of centralized connections or the strength of their long‐range connections (see Table S2 for details). For example, Meunier et al. (2009) derived 90 cortical and subcortical regions from fMRI data in a young (mean 24 years) and older (mean 67 years) group of participants. They found that some modules that were single, coherent regions in the young group were fractured into two modules in the older group. Based on its high inter‐module connections, a frontal‐striatal module appeared to be critical for coordinating information transfer in the younger group. However, the same pattern was not found in the older group, with the same functional role appearing to be taken‐up by the posterior module.
3.5.2. Network hubs are less modular among older adults
Hubs are a particular type of node that have many important connections running through them and strong relationships with each other. They are also a key player in communication between different brain regions due to the number and positioning of their connections in a network. Hubs are often connected via long‐distance neural pathways so that they can maintain information integration and coordination across spatially distributed regions, optimizing the balance between the cost of neuronal resources and the efficiency of information transmission (Liao et al., 2017; Sporns, 2018). The spatial distance of the edges connecting hubs to the rest of the network is also considered to reflect their “wiring cost”. Hubs have high rates of cerebral blood flow, aerobic glycolysis and oxidative glucose metabolism, suggesting that they are “biologically costly”, although with associated high value for the integration of information processing (Crossley et al., 2014).
An established functional hub architecture is evident in late childhood, and strengthens by early adulthood, as connections between hubs and other regions increase (Hwang et al., 2013). In parallel with the maturation of the functional hub architecture is the alteration in the influence of white matter hubs and structural brain networks, providing the “scaffolding” for information transfer (Puxeddu et al., 2020). In normal aging this is revealed by topological restructuring between‐ and within‐hubs, with cognitively normal older adults displaying reduced connections running through the frontal and parietal hubs compared with younger adults (Ash & Rapp, 2014; Wu et al., 2012; Zhu et al., 2012; Zimmermann et al., 2016). Reduced “hubness” also predicts whether communication efficiency is compromised between networks in aging (Betzel et al., 2014).
Some hub regions play a key role in the overall network organization and participate in multiple communities across the network. These hubs and their connections have been termed “rich club” because they are more densely connected than are nodes of a lower degree of connections (see Figure 1c). They tend to interconnect strongly with each other, forming the “rich clubs”. Because “rich club” connections link spatially distributed and remote hub nodes, their overall network cost is high, but this high cost is offset by their disproportionately large contribution to efficient communication (van den Heuvel et al., 2012). Compared to younger adults, older adults show a reduced hub structure, including “rich club” connectivity (Cao et al., 2014). These networks show a negative quadratic “rich club” connectivity pattern and a linear decrease in modular organization with age, mainly localized to hubs in the frontal, parietal and occipital lobes. Because these networks are central in flexibly allocating resources to produce goal‐directed behavior, they are likely to have a relatively global impact on the cognitive aging process. Indeed, these hubs support higher order executive functions that require high levels of information integration across the brain and have been shown to discriminate individuals' executive function, IQ and behavior (Finn et al., 2015; Miranda‐Dominguez et al., 2018).
3.6. Age differences in functional connectivity, modularity, segregation, integration and hubs function: Summary and implications
Taken together, studies of (a) within‐ and between‐network connectivity; (b) segregation, modularity and integration; and (c) hub differences across the lifespan support the notion that functionally related regions emerge during development, optimize during early adulthood and deteriorate in older age. This pattern of functional differences is illustrated in Figure 5 (comparing Panel C with Panel B and D). Differences between younger and older adults in terms of within‐ and between‐network functional connectivity at rest may drive differences in functional network communication and possibly contribute to the decreased cognitive performance often seen in older adults, particularly in higher‐order processes, such as executive function. Owing to its widespread role in human cognition and behavior, executive function is central in theories of brain aging, such as the frontal lobe hypothesis of aging (Morrison & Baxter, 2012). Older adults tend to show less flexible thinking, such as forming new concepts and abstract thinking, response inhibition, as well as verbal and numeric reasoning (Darowski et al., 2008; Harada et al., 2013; Salthouse, 2019; Wecker et al., 2000). These executive function changes can be seen first in adults in their fifth decade of life (Singh‐Manoux et al., 2012), consistent with the findings of the systematic review that functional network connectivity changes reach their inflection point in the fourth and fifth decade.
The finding that older adults show increased between‐network connectivity (Section 3.2) and integration (Section 3.3) compared to younger adults is compatible with the “dedifferentiating” hypothesis (Cabeza & Dennis, 2012), in which more neural systems are activated in parallel in older adults during rest reflecting a more diffuse, nonspecific recruitment of brain regions. The literature reviewed in Section 3.3 also support the STAC theory by indicating that a loss of segregation with age impacts how brain regions integrate during rest (and task). These findings are also consistent with a decline in executive functions but maintenance of primary information processing in “normal” aging, which implies an underlying compensation mechanism in aging to support higher‐level cognitive functioning (Singh‐Manoux et al., 2012). Age‐related dedifferentiation is also seen in perceptual processing regions and may drive the need to recruit more neural resources further down the processing stream (Goh, 2011). In contrast, primary sensory and motor functions, such as visual information processing, benefit from clustered connections between adjacent areas. As these nodes are well connected, information exchange is more segregated, and the networks are more resilient to disruptions in connectivity with age (Geerligs et al., 2015; Song et al., 2014).
The results in Section 3,3 may also help explain the heterogeneity between individuals in cognitive aging trajectories. Cognition and behavior depend on the information flow sent and received between brain areas (Genon et al., 2018) and the interplay between network integration and segregation mediates this flow. As noted earlier, connections within‐ and between‐modules may change in strength, configuration, and number, and may change over time as a function of learning, age or disease states. Some modules may remain relatively stable (e.g., primary sensory and motor) and others may vary substantially (e.g., associative regions), connecting and disconnecting (Medaglia et al., 2015). At the same time, hubs are biologically expensive and deteriorate with reduced metabolic activity in aging (Arneman et al., 2018; Dai et al., 2015; Liang et al., 2013; Tomasi et al., 2013). Alteration to “rich club” network hubs in particular, such as the default mode network or executive control network, including their long range connections, reduce the communication between brain regions, a prerequisite for the fast and effective higher order cognitive functions. This complex interplay will dictate, at least in part, the differential cognitive expression of underlying functional changes with age. This is consistent with results that show that tasks relying on predominantly automatic or well‐practiced processes are less impacted by age than higher‐order functions, or may even improve slightly across the life span (Grady, 2017; Harada et al., 2013).
3.7. Local and global efficiency are lower in older adults
3.7.1. The brain is “wired” for “small world” local and global efficiency
Brain networks with “small world” properties balance a high level of local and global efficiency in information processing, with economical “sparse” wiring costs (Bullmore & Sporns, 2012; see Figure 1b). Minimization of cost is achieved by dividing the brain into localized modules that are close in space, with the nodes within each module having a short average path length of connection, increasing signal transmission speed and promoting local efficiency (Barbey, 2018). Local efficiency is complemented by global efficiency, which reflects the capacity for information transfer between any two nodes across the shortest possible path length. The complementary need for both local and global efficiency creates a need for long distance connections that incur a higher “wiring cost”. Thus, an efficient system is achieved by balancing competing constraints on brain organization, demanding a decrease in the wiring cost for local specialization and an opposing need to increase the connection distance to facilitate global, system‐wide function (Barbey, 2018).
Higher order executive functions that require high levels of information integration across the brain benefit from global efficiency of long‐range connections. Graph theory analyses indicate that older age is associated with reduced functional connectivity of long‐range connections and higher connectivity of short‐range connections (Sala‐Llonch et al., 2014). Global efficiency of long‐range connections is particularly important in the frontoparietal network and has been shown to discriminate individuals' executive function, working memory, task switching and general intelligence (Finn et al., 2015; Hakun et al., 2015; Miranda‐Dominguez et al., 2018; Rypma et al., 2005; Santarnecchi et al., 2014; Stanley et al., 2015). Processing speed is also usually reduced in older adults, at least in part due to the need to traverse more nodes, leading to greater neural activity (between network connectivity) but less efficient, slower processing.
3.7.2. Older adults show reduced local and global efficiency of large‐scale networks
In line with the reduced within‐network connectivity among older adults noted in Section 3.2, a corresponding loss of local efficiency (Table 2) is also reported consistently when compared to younger adults. Seven of nine studies (78%) that included a local efficiency metric reported lower efficiency among older adults compared to young adults in all or the majority of networks studied (see Table S3 for details). Two studies (22%) reported results that varied by network, both reporting lower local efficiency among older adults compared to younger adults in higher‐order networks (e.g., fronto‐parietal, default mode, cingulo‐opercular), but higher local efficiency in the sensorimotor and visual networks (Geerligs et al., 2015; Song et al., 2014). There is also some evidence that there is an inverted‐U shaped relation between age and local efficiency (Cao et al., 2014; Xie et al., 2020), with efficiency peaking around the third or fourth decade of life (Figure 3a).
The overall GRADE score for local and global efficiency was at or close to the border of a “moderate” and “high” rating (i.e., a score of 3.7 for local and 3.5 for global efficiency; see Table S8). Given that 100% of studies reported loss of local efficiency in the associative networks with age, we assessed the consistency of the findings to be high for those networks. In comparison to local efficiency, there was less consistency in the direction of age group effects for global efficiency. Seven of 13 studies (54%) reported lower global efficiency among older adults than younger adults; three studies reporting no age differences (23%); and three (23%) reported higher efficiency among older adults.
There are several possible reasons for the less consistent findings in global efficiency compared to local efficiency. Aging‐related effects on global efficiency have been found to be of smaller effect size than local efficiency (e.g., Chong et al., 2019; Varangis et al., 2019), and so are likely to be more difficult to detect. This conclusion is aligned with our finding that the effects of aging on local efficiency are more robust than on global efficiency.
Variation in image pre‐processing, nodal scale (i.e., number of nodes within networks) and parcellation approaches may also have impacted the consistency of the global efficiency results. Research has shown that global efficiency is one of the most sensitive graph theoretic metrics to nodal scale, as it can alter the number and length of edges between nodes (Stanley et al., 2013; Zalesky et al., 2010). The studies reviewed here used nodal scales ranging from 90 (e.g., Onoda & Yamaguchi, 2013) to 1204 (Cao et al., 2014) nodes. The variance in nodal scale will impact the path length of edges between nodes. Indeed, when nodal scale is largely constant, the results are consistent. Specifically, at a scale of 90 to 114 nodes, five out of six studies (83%) found decreased global efficiency among older compared to younger adults (Achard & Bullmore, 2007; Chong et al., 2019; Hou et al., 2019; Li et al., 2016; Onoda & Yamaguchi, 2013; Sala‐Llonch et al., 2014). Although this subset of studies increased the consistency of findings, only two studies covered the full adult lifespan. Taken together with the fact that the overall GRADE score for global efficiency was at the boundary between a “moderate” and “high” rating, we suggest that additional research is required to explore the stability of global efficiency and age differences across nodal scales.
3.7.3. Network efficiency among older adults: Summary and implications for cognitive performance and theories of cognitive aging
Taken together, the results of the systematic review suggest that the balance of local and global efficiency indicative of “small world” brain networks is present across the lifespan, although it likely peaks in the fourth or fifth decade of life and then declines. As depicted in Figure 5, older adults display reduced local efficiency (increased path length to neighboring nodes) and global efficiency (loss of long‐range paths or longer average path lengths) between nodes across networks. Age differences in efficiency are likely contributing to the decline often seen in executive function, working memory and processing speed in aging.
At least some of the differences in functional network efficiency with age may be driven by changes in anatomical connectivity and energy demands (Salat, 2011). Changes in structural efficiency based on graph theoretical measures of gray matter volume have been reported among older adults both longitudinally and cross sectionally (e.g., Wu et al., 2012, 2013) and reported to explain 83% of the age‐related reductions in executive function (Fjell et al., 2017). These changes in structural efficiency are also in line with the “neuronal resource” and “scaffolding” theories of aging outlined earlier. According to these theories, the efficiency of the transmission of neuronal communication is reliant on the integrity of white matter. Age‐related functional changes are paralleled white matter changes that create a reduced capacity for global network integration and decreasing global efficiency. Reduced efficiency is mediated by alterations to the path length over which information needs to flow between nodes in different networks, as well as reductions in white matter global and local efficiency that provides a “scaffolding” for efficiency in the human brain (Burzynska et al., 2013; Niu et al., 2019; Zhu et al., 2015). Metabolic efficiency and cellular change that occur with age may also impact on network efficiency (Ramchandran et al., 2019).
3.8. Older adults show posterior–anterior and inter‐hemisphere functional connectivity alterations at rest
According to the HAROLD model, younger adults show mostly unilateral prefrontal connectivity patterns, whereas older adults show bilateral connectivity of the prefrontal cortex. Therefore, in grading the certainty of the evidence for lateralization and the HAROLD model, we focused on frontal lobe connectivity. It is also worth noting that eight of the nine studies testing HAROLD used quantitative metrics to assess the degree of laterality among younger and older adults, such as measures that directly compare or subtract the connectivity correlations in the hemispheres, or graph theory metrics in contralateral voxels and networks (see Table S4 for details).
The available literature for lateralization of connectivity in aging is summarized in Table 2, and indicates a high degree of certainty for age effects in line with HAROLD. Nine studies addressed age differences in the degree of lateralization across the hemispheres (see Table S4 for details). Eight studies (89%) provide support for decreased lateralization with age in the frontal regions (Agcaoglu et al., 2015; Chen et al., 2016, 2017; Jiang et al., 2020; Li et al., 2009; Sala‐Llonch et al., 2014; Yao et al., 2013; Zuo et al., 2010). For example, Yao et al. (2013) used a novel measure of brain entropy (i.e., the degree of underlying randomness) of the BOLD signal during rest. Variables with small entropy have a high level of predictability and low level of randomness. They pooled 26 fMRI resting‐state datasets, with a total of 1248 participants aged 6 to 76 years. Functional entropy of the BOLD activity increased with age: correlations in BOLD activity became more widely distributed among older adults. The increase in entropy was found in the connections between the hemispheres, suggesting that the hemispheres become more symmetric in functional connectivity with age, which is compatible with the HAROLD pattern. Zuo et al. (2010) found a positive quadratic pattern of age and the homotopic connectivity in frontal, temporal, parietal, and occipital lobes and subcortical region. The lowest point of synchrony in connectivity in contralateral regions was around the fifth decade of life, increase from approximately 50 years of age into the 1970s and 1980s as the activity in the lobes was less lateralized.
The available literature for a posterior–anterior shift in aging is summarized in Table 2, and indicates a high degree of certainty for age effects in line with PASA. Eight studies (see Table S4) reported results related to PASA, with seven (88%) studies showing support (Chen et al., 2016, 2017, 2018; McCarthy et al., 2014; Sala‐Llonch et al., 2014; Yao et al., 2013; Zhang et al., 2017); and one (12%) failing to show support (Lee et al., 2015). For example, lower clustering and local efficiency have been found in posterior regions, together with an increase in anterior regions, in both task and resting‐state networks for older compared to younger adults (McCarthy et al., 2014; O'Connell & Basak, 2018; Zhang et al., 2017). This includes functional hub dynamics and dynamic connectivity changes with an age‐related posterior‐to‐anterior shift (Zhang et al., 2017).
In summary, although the HAROLD and PASA theories were originally developed to explain task‐based performance in older adults, the results of the studies summarized here indicate with high certainty that older age is also associated with a less lateralized hemisphere function and a posterior–anterior shift in functional connectivity during rest. The presence of these patterns at rest is compatible with the argument that the resting‐state forms a baseline “framework” of the brain, upon which task‐ and goal‐directed activity builds (e.g., Jamadar et al., 2016; Raichle, 2011). The HAROLD‐ and PASA‐like effects in resting‐state connectivity suggest that older adults are able to recruit higher levels of activity contralaterally and in the anterior regions of the brain, possibly to compensate for functional deficits located in other regions or because of a loss of efficiency across the brain.
3.9. Dynamic connectivity
3.9.1. Dynamic resting‐state functional connectivity is different in older adults
Traditional analytic approaches to functional connectivity analysis implicitly assume that the functional connectivity over the course of the scan period is static.1 In other words, the correlation of time courses between regions is temporally invariant (or “stationary”) across the scan period. However, the brain itself is highly dynamic, and dynamically shifts between states across time (Lehmann et al., 1987; see reviews by e.g., Tang et al., 2012; Michel & Koenig, 2018). “Chronnectomic” approaches to connectivity analysis examine the temporal dynamics of region‐to‐region coupling across the scan period (Calhoun et al., 2014; Liégeois et al., 2017). The most common approach to assessing dynamic functional connectivity is the “sliding‐window” approach, which involves segmenting the time‐course from nodes into a set of temporal windows, inside which their connectivity is analyzed (Bijsterbosch et al., 2020). Dynamic functional connectivity analyses have revealed that resting‐state networks undergo fluctuations between states of higher integration and segregation, or modularity, both at rest and during tasks.
There are methodological considerations in the study of dynamic functional connectivity, not least of which is that temporal dynamics are better measured using fast neuroimaging methods like electroencephalography (EEG). While EEG provides millisecond temporal resolution, fMRI provides moderate temporal resolution between several hundred milliseconds (with multiband fMRI; Feinberg et al., 2010, Feinberg & Setsompop, 2013) and seconds. PET is even slower, with temporal resolution of a few seconds in fPET (Jamadar et al., 2021; Rischka et al., 2018), and equal to the scan duration in typical PET studies (Jamadar et al., 2021). Systematic review of EEG studies of dynamic connectivity in aging is beyond the scope of this review, but see Courtney and Hinault (2021) for a recent review.
For fMRI‐based studies of dynamic connectivity in aging, relevant methodological considerations include the choice of the analytical approach, sliding‐window length, and the measures used to assess connectivity within the windows. Metrics can include connectivity strength, spatial network organization, topological variations, and graph metrics (see Preti & Van De Ville, 2017 for a review). Indeed, across the 16 studies assessing age‐related differences in dynamic functional connectivity, various analytic methods, window lengths and network measures have been used, which potentially impact the conclusions that can be drawn across the studies (see Table S7). The number of studies is also relatively small; hence, we assess the weight of the evidence for age differences in dynamic functional connectivity as moderate (Table 2).
All seven of the studies (100%) that assessed dynamic within‐network connectivity found altered dynamics in older adults compared to younger adults in all or some of the networks investigated (see Table S5 for details). Older adults showed reduced connectivity strength and reduced signal variability compared to younger adults (Chen et al., 2018; Madhyastha & Grabowski, 2014; Park et al., 2017; Qin et al., 2015; Tian et al., 2018; Wen, Dong, et al., 2020). All eight of the studies (100%) that assessed dynamic between‐network connectivity also found different dynamics with age (Chen et al., 2018; Davison et al., 2016; Qin et al., 2015; Tian et al., 2018; Viviano et al., 2017; Wen, Dong, et al., 2020). Three studies examined other measures, such as segregation and integration (He et al., 2020), network stability (Mujica‐Parodi et al., 2020), and network hub properties (Schaefer et al., 2014) and all found altered states in older adults compared to younger adults.
Research has indicated that age differences in dynamic resting state functional connectivity are non‐linear. For example, Chen et al. (2018) reported a negative quadratic pattern in dynamic between network functional connectivity, particularly between the fronto‐parietal network and the default mode and occipital networks. Chen et al. (2019) used cluster analysis to define connectivity patterns of micro‐states that exhibited distinct intrinsic patterns of functional connectivity. They reported a negative quadratic pattern across the adult lifespan for changes in most between‐network connections in the micro‐states.
Eight studies assessed the nature and speed of transition between connectivity states, and all (100%) revealed age differences. The differences included a lower number of transitions between states (defined by specific connectivity patterns of networks), and slower transitions between states for older compared to younger adults (Chen et al., 2018, 2019; Viviano et al., 2017; Xia et al., 2019). Dynamic functional connectivity becomes less efficient and more random with increasing age (Battaglia et al., 2020; Ezaki et al., 2018), with older adults also spending more time in functional connectivity states with weaker connectivity throughout the networks (Tian et al., 2018).
Five studies also reported age‐related dynamic connectivity differences using graph theory metrics, including reduced efficiency (Ezaki et al., 2018), alterations to the dynamic participation of nodes within hubs (Schaefer et al., 2014) and increased segregation and reduced integration during dynamic states (Davison et al., 2016; He et al., 2020). For example, Mujica‐Parodi et al. (2020) assessed the portion of nodes in a network module that switched modules between window periods of 24 s. In two large‐scale fMRI lifespan datasets, age‐associated degradation in network stability was sigmoidal, peaking around age 60 years but starting 13 years earlier. Schaefer et al. (2014) used connectivity clustering to show that the degree of hub integration into different networks was lower in older adults. Older adults also showed a reduced ability to access changeable substates of dynamic resting‐state networks that reflect a “rich club” organization (Escrichs et al., 2021), suggesting an impairment in the capacity to flexibly connect and serve as “relay stations” and in achieving changeable functional states that are key for efficient global communication.
Age‐related changes in dynamic functional connectivity are likely representative of a reduction in the dynamic network changes required to adapt to an array of cognitive states or environmental demands. Effective task performance is likely best accomplished by a dynamic reduction in segregation, or greater integration, of the task‐relevant systems that support the processing goals of the task. This suggests a “push–pull dichotomy” between segregation and integration in functional brain networks during rest versus task (Wig, 2017).
It has been suggested that dynamic functional connectivity may simply reflect a variability expected in random data or noise in fMRI scanning (Hutchison et al., 2013). However, multimodal techniques have indicated that dynamic functional connectivity is related to both behavior and neural activity, showing a strong correlation with electrophysiology data in EEG (Tagliazucchi et al., 2012) and MEG (Brookes et al., 2011). Although the coherence between multimodal data supports the reliability of dynamic functional connectivity, it is also possible to apply additional techniques to test that the dynamic functional network properties can be attributed to system dynamics, rather than overall statistical properties, noise or random patterns of activity. The techniques include testing for significant variation from a null or random model (see Table S5 for the approaches taken by the authors of each study in the review). Across the dynamic functional network measures, a relatively high percentage of studies reported significant variation from a null model or the reliability of the network measures employed: 5 of 7 studies (71%) for dynamic within‐network connectivity; 5 of 8 studies (63%) for dynamic between‐network connectivity; 6 of 8 studies (75%) for the transition between states; and 3 of 5 studies (67%) for graph theory metrics.
This systematic review indicates with moderate certainty that older adults show a reduced ability to dynamically shift between states both during and in the transitions between states, and spend more time in weaker states of connectivity. When they do transition between states, they do so with reduced speed. Dynamic connectivity states likely become less complex and more random with increasing age. These changes are likely dysfunctional in terms of older adults being able to rapidly respond to a changing environment and oscillate between integration and segregation to manage complex environments and task demands.
3.9.2. Within‐subject BOLD variability is lower in older adults
It was noted earlier that the theory of coordination dynamics (Tognoli & Kelso, 2014) proposes that “metastable” brain networks can shift flexibly between integrated and segregated configurations. In contrast, networks with high integration or segregation without variability cannot flexibly shift between states. In this theory, neurons activating the same way to stimuli over time would not adapt to changing demands or environmental conditions. Brain function variability enables stable output, greater information coding capacity and protection to disruption (Grady & Garrett, 2018). When variability is too low, there is reduced capacity for the system to shift between states. An optimal level of variability facilitates neural function, with too little or too much leading to a less efficient network system (Garrett et al., 2010).
According to the coordination dynamics framework, age‐related loss of brain network efficiency and deterioration in processing speed and cognitive performance are potentially mediated by a loss of brain variability. Bold signal variability has been studied during task performance (see Huettel & McCarthy, 2000, Huettel et al., 2001; West et al., 2019). There is a relatively small literature of six studies (see Table S6) that investigated the age differences in the variability of the BOLD signal within‐subjects during rest. The authors of these studies used different spatial scales and measures of the BOLD signal variability and did not consistently include participants across the full adult lifespan. Hence, the level of certainty of age differences in this literature is low (Table 2).
Of the six studies assessing within‐subject BOLD variability, three studies (50%) reported lower BOLD variability or amplitude modulation in all or most networks of older compared to younger adults (Chen et al., 2017; Grady & Garrett, 2018; Yang et al., 2018). Grady and Garrett (2018) used the standard deviation of the BOLD signal to find reduced within‐subject variability at rest among old adults. Age‐related reductions in frequency and amplitude modulation of resting‐state fMRI signals have also been found in the default mode network and salience network across the lifespan (Yang et al., 2018). When the brain was analyzed at a voxel level (Garrett et al., 2010) and parcellated into 90 regions, the pattern of variability in BOLD signal differed by spatial location, and showed both positive and negative correlations between the variability and global functional connectivity of the BOLD signal (Xie et al., 2020). These studies also suggest that a cortical–subcortical distinction may exist, with subcortical areas increasing in variability across age compared with cortical areas (also see Garrett et al., 2013).
Several studies have also compared BOLD variability to mean BOLD measures as a statistical predictor of cognition in aging. These studies suggest that signal variability within‐subjects can be a powerful predictor of individual differences in cognition across the adult lifespan (Garrett et al., 2011). In fact, some have found that BOLD variability within‐subjects accounts for almost all the variance in age‐related fMRI differences and are largely uncorrelated with age‐related mean‐based BOLD patterns (Garrett et al., 2010). This suggests that brain signal variability potentially provides a largely independent measure to mean brain activity in cognitive aging and that BOLD variability should be considered for wider use in fMRI studies.
The relatively small number of studies reviewed in this section suggest that older adults may display less variability than younger adults in BOLD signal in most, if not all, large‐scale resting‐state networks at rest. Reduced BOLD variability in older adults likely reflects a state of reduced metastability to integrate functional regions and to produce neural fluctuations needed for optimal cognitive function and behavior in response to complex environmental demands. Further research is need to establish whether less variability in BOLD signal impacts functional performance in aging and whether changes in variability patterns are a necessary condition for age‐related connectivity changes to become evident (Garrett et al., 2010).
3.10. Metabolic connectivity differences are evident in older adults
The concept of “metabolic connectivity” actually predates the concept of functional connectivity, with the first analyses of region‐to‐region covariance in neuroimaging signal conducted in FDG‐PET data (Horwitz et al., 1984, 1986; Metter et al., 1984). Glucose metabolism provides the primary source of energy for brain function and maintenance, as well as the production of neurotransmitters (Mergenthaler et al., 2013). The adult brain accounts for approximately 2% of total body weight but requires approximately 20% of total glucose supply (Clarke & Sokoloff, 1999). Moreover, the brain cannot store glucose and so requires a readily available source via the arterial blood supply. Hence, there are complex and dynamic changes in the energy consumption of brain cells from moment‐to‐moment as the brain performs its vast array of functions (Yellen, 2018). Brain glucose metabolism is tightly coupled to cerebral blood flow and oxygen supply to the degree that local blood flow is highest in regions with the highest glucose metabolism. An intricate interplay exists between the brain, endocrine system, central and peripheral energy supply, and energy utilization. This requires a highly responsive system that is well controlled by both feedback and feedforward processes that produce choreographed changes in the flow through biochemical pathways (Yellen, 2018).
Brain glucose metabolism has been well studied in aging and shown to differ with age. For example, Kuhl et al. (1982) used FGD‐PET to study brain glucose utilization in subjects from 18 to 78 years of age, and found that whole‐brain cerebral rate of glucose metabolism was, on average, 26% less at 78 years than at age 18 years of age. The difference was greater than that reported for mean cerebral oxygen utilization. The lower cerebral glucose metabolism with age was seen most in frontal, temporal, and subcortical regions, as well as in the cingulate and insula (Nugent et al., 2014; Trotta et al., 2016), which are heavily involved in higher‐order cognitive functions.
The brain's dependence on glucose, and changes in brain glucose metabolism with age, suggest that FDG‐PET imaging is a potential biomarker for the early identification of cognitive aging and neurodegenerative disease. Indeed, there is a broader literature on FDG‐PET in older adults beyond connectivity and in age‐related diseases, such as Alzheimer's disease. FDG‐PET is typically used to measure neuronal activity, specifically the local concentrations of radioactive tracer that is metabolically trapped in neurons or glial cells after being metabolized in glucose‐6‐phosphate, with the slope of the time‐activity curve used to measure glucose metabolism (Herzog et al., 2019). Voxel‐based analyses and semi‐quantitative measures of FDG uptake can be used for the identification of specific patterns of glucose uptake and the “metabolic connectivity” of brain regions.
Before discussing the studies summarized in Table 2, several considerations should be noted about their design. First, the use of FDG‐PET has historically been limited by poor temporal resolution, with studies acquiring single images that index the rate of glucose uptake across the entire scan period. As such, most studies purporting to study “metabolic connectivity” have actually measured metabolic covariance across subjects. Metabolic covariance is a poor predictor of metabolic connectivity (Jamadar et al., 2021). To be comparable to “functional connectivity” measures, it is necessary to measure the time‐course of FDG‐PET activity across the scan for each individual subject, and then correlate those time‐courses across regions to form a connectome. Recent advances in FDG‐PET data acquisition have made it possible to measure the time course of FDG uptake over the course of a scan (Hahn et al., 2020; Jamadar et al., 2019; Villien et al., 2014); and this “fPET” method has been applied to characterize metabolic connectivity in younger adults (Jamadar et al., 2020, 2021). We showed that metabolic connectivity yields correlated, but unique information, to fMRI‐based measures of functional connectivity. To date, the high temporal resolution fPET approach has not been applied to study resting‐state metabolic connectivity in aging, so existing studies use a combination of metabolic covariance measures, or correlation of network properties estimated using fMRI with FDG‐PET based measures of glucose uptake or the cerebral metabolic rate of glucose (CRMGLC).
The second consideration is that although there is a clear correlation between FDG‐PET signal and glucose consumption in the brain, the measured concentration of FDG uptake is not a direct measurement of the metabolic function (Berti et al., 2013). Absolute quantification of brain glucose utilization is a more complex procedure that is able to provide fully quantitative estimates of CRMGLC. In order to extract the biological measurement of interest, PET data need to be evaluated using a mathematical model that takes into account both the delivery of the tracer to the tissue and its subsequent fate in brain tissue (Berti et al., 2013). However, this quantitative approach was not undertaken and reported in all studies reported here.
A small literature was identified as part of the systematic review that specifically investigated resting‐state network using FDG‐PET in healthy aging (see Table S7 for details). Due to differences in the metrics reported, drawing clear conclusions about metabolic connectivity in aging from these studies is difficult and certainty in the results is low (Table 2). However, the nine studies, together with other select research investigating brain glucose, point to glucose as a key player in “normal” age‐related brain functional network changes and as a promising avenue for further research.
The metabolic basis for changes in functional network covariance and segregation was examined in eight studies, suggesting age differences across the adult lifespan (Arnemann et al., 2018; Azari et al., 1992; Horwitz et al., 1986; Liu et al., 2014; Manza et al., 2020; Moeller et al., 1996; Trotta et al., 2016; Zuendorf et al., 2003). For example, older subjects display reduced metabolic covariance within most networks, together with a more heterogeneous pattern of high and low correlations between‐networks (Liu et al., 2014; also see Azari et al., 1992). A strong correspondence has also been found between large‐scale resting‐state network connectivity and glucose network covariance in younger and older adults (Bernier et al., 2017; Di et al., 2012; Li et al., 2020; Tomasi et al., 2013, 2017) and in animals (Amend et al., 2019). Varied networks measures, analytic and parcellation approaches used across the studies limits assessment of consistency of age effects (see Table S7).
Research has demonstrated the existence of “small world” properties in metabolic functional brain networks (Hu et al., 2015), including in older adults (Di et al., 2017). The correlation between glucose metabolism and local and global connectivity is especially high for hubs with high between network connectivity, indicating a topology optimized for maximal communication speed with minimal energy consumption (Tomasi et al., 2013). Network hubs have greater metabolic rates compared to non‐hubs (Dai et al., 2015; Liang et al., 2013). Moreover, older adults show increased clustering and decreased efficiency of metabolic networks; which implies a degeneration process in which the brain shifts from small‐world segregated networks to a more local and less distributed organization with age (Arnemann et al., 2018). Longitudinal changes in metabolic covariance have also been found among older adults (Di et al., 2019). The orbital frontal cortex and anterior temporal lobe showed a significant reduced metabolic activity in aging, and causally influenced many other regions, which were widespread and included regions that did not show age‐related reductions in metabolic activity.
Preserved brain glucose metabolism may be a mechanism underlying “reserve” theories of aging. In other words, efficient metabolism is neuroprotective as it supports the brain with the necessary energy it needs to function, whereas impaired metabolism inhibits this normal brain function (Bastin et al., 2012; Stranahan & Mattson, 2012; Yoshizawa et al., 2014). Kim et al. (2015) examined the mediating role of education on functional covariance using regional cerebral glucose metabolism rates (rCRMGLC). The results showed that participants with higher education also had higher rCRMGLC in the regions responsible for memory and language. A lower education level was associated with higher rCRMGLC in motor and somatosensory areas. Graph theory analyses indicated that individuals with higher education were more efficient and displayed greater small‐world characteristics, that is, relatively local clustering with sparse between network connections. These results suggest that glucose metabolism is a likely mechanism mediating changes in functional connectivity across the lifespan and may also contribute to the “reserve” found in people with higher education.
In summary, although the literature on metabolic connectivity is relatively small, it points to glucose as a potentially central player in age‐related changes in brain network function that may contribute to age‐related changes in cognitive performance. Specifically, metabolic brain networks appear to show strong coherence with fMRI networks, including “small world” properties, although additional research using graph metrics is needed. Further research is also needed to confirm that aging is associated with metabolic “dedifferentiation” of large‐scale networks, and that glucose metabolism is tied to network connectivity. Like fMRI studies, metabolic connectivity studies suggest that hub networks associated with higher order cognitive processes are most vulnerable to age‐related alterations, although additional work is needed to fully elucidate these changes across large scale resting‐state networks.
4. DISCUSSION, METHODOLOGICAL CONSIDERATIONS, AND FUTURE RESEARCH DIRECTIONS
4.1. The systematic review findings largely support theories of cognitive aging
The literature reviewed here is largely consistent with theories of cognitive aging. A less segregated, less modular and less efficient network organization among older adults my reflect dedifferentiation and compensation, underpinned by more diffuse, nonspecific recruitment of brain regions. Large‐scale network segregation in aging may also be moderated by cognitive reserve, as measured by factors such as education and occupational levels. Older age is also associated with a less lateralized and posterior–anterior shift in functional connectivity during rest in line with the HAROLD and PASA theories of cognitive aging. These differences in older adults' functional networks likely reflect impaired coordination dynamics, namely, the ability to access metastable states between integration and segregation, with reduced signal variability compromising this dynamic shift.
At least some of the differences in functional network connectivity and efficiency with age are likely driven by changes in anatomical connectivity and energy demands. Age‐related functional changes are paralleled by gray and white matter changes that alter the scaffolding of the brain and reduce the capacity for global network integration and decrease global efficiency. Although the literature reviewed here does not relate functional connectivity to measures of cognitive reserve directly, it does suggest that some of the individual variability in cognitive functioning in older adults can be accounted for by a differential deployment of specific brain networks or that preserved glucose metabolism contributes to the “reserve” found in certain older adult cohorts (e.g., higher education, professional careers). Future research is needed to elucidate whether the relationships between functional connectivity and cognitive performance may be influenced by cognitive reserve.
4.2. Limitations of cross‐sectional studies
One of the limitations of the studies reviewed so far is their cross‐sectional nature, with approximately half of the studies comparing younger and older adults cohorts only, and half analyzing age as a continuous variable across the adult lifespan. Cross‐sectional studies are highly informative about age‐related differences in resting state networks and their underlying neural correlates. However, because they are correlational in nature, they are less informative about the causal relationship between age‐related brain changes in the aging process and associated alterations to function. In other words, cross‐sectional studies allow the examination of age‐related differences in connectivity, not age‐related changes. Drawing conclusions about causal pathways in aging requires longitudinal and causal analyses, which were rarely undertaken in the research reviewed here.
There are also other considerations in the research designs of the studies reviewed, such as comparability of measures of brain activity obtained from different age groups, and the choice of the study populations (see Rugg, 2016 for review). Cross‐sectional comparisons raise the possibility that differences found between study groups reflect underlying cohort differences rather than age‐related changes. For example, perhaps differences between younger and older adults are due to generational differences in psychosocial experiences (younger adults experience a developmental environment with substantial technological integration not experienced by the older generation in their youth), rather than differences related to aging per se. Or perhaps age‐related differences reflect different cognitive strategies employed by younger and older adults during tasks or at rest. Cross‐sectional studies cannot rule out these alternative explanations of age‐related differences in behavior and brain connectivity.
The influence of variables that also correlate with age and age‐related diseases may confound fMRI‐based measures of connectivity. Older adults show greater individual variability in cortical thickness, vascular integrity, and undergo metabolic and hemodynamic changes, which affect neurovascular coupling and the MR signal independently from its effects on cognition, brain activity, and connectivity (D'Esposito, 1999; Gazzaley & D'Esposito, 2003; Ward et al., 2020). Although some evidence suggests that age influences connectivity both within and between networks over and above the effects on neurovascular coupling (Tsvetanov et al., 2016), between‐group differences in neurovascular coupling and fMRI signal intensity is an important confound in cross‐sectional studies of connectivity in aging, and great caution should be taken when comparing fMRI activity or connectivity between groups (Samanez‐Larkin & D'Esposito, 2008; Ward et al., 2020).
4.2.1. Longitudinal studies also show reduced connectivity in older adults
Longitudinal studies address at least some of the potential cohort differences by tracking changes within‐subjects over time. Longitudinal studies suggest that the age‐related changes in large‐scale networks, such as the fronto‐parietal, sensorimotor network and default mode network, occur in as little as two to four years in older adults (Chong et al., 2019; Li et al., 2020; Ng et al., 2016; Oschmann & Gawryluk, 2020; Staffaroni et al., 2018; Zonneveld et al., 2019), with the fronto‐parietal changes associated with reduce processing speed (Malagurski et al., 2020). Global network segregation, integration, and module distinctiveness also decrease over a two year period in older adults (but not younger adults) particularly in the higher‐order cognitive hubs of the cingulo‐opercular, default mode, salience and ventral attention networks (Chong et al., 2019). The strength of long‐range connections in the default mode and dorsal attention networks decrease 6% and 3% per decade of life, respectively (Tomasi & Volkow, 2012). The reduction was even more pronounced at 12% in the posterior cingulate and ventral precuneus. Taken together, these studies suggest that changes in the higher‐order networks occur as part of the aging process and are consistent with the reduced resting‐state activity in these networks when comparing older and young adults reported earlier.
4.2.2. The need for additional studies across the entire adult lifespan
It was noted earlier that some studies do not include middle‐aged individuals, which means that full adult lifespan differences in functional connectivity cannot be characterized from their findings. For example, inverted‐U or negative quadratic trajectories of age‐related changes likely have an inflection point in the fourth and fifth decade of life; however, studies that compare young and old groups only cannot investigate these trajectories. This issue is most evident for the network measures where we assessed the strength of the evidence to be moderate to low (e.g. within‐subject BOLD signal variability), as a smaller number of studies was available for review. Additional research across the adult lifespan is needed to investigate whether quadratic relationships exist between these network measures and age.
4.3. Resting‐state studies are consistent with task studies, although both remain valuable
Although there is considerable overlap between resting‐state networks and task‐based networks, patterns of connectivity can have meaningful differences across a range of domains and task (Davis et al., 2017). Brain regions can also be involved in multiple domains, with different patterns of connectivity depending on the task, and can vary based on task difficulty or load (Antonenko & Flöel, 2014). This suggests that comparing and contrasting rest and task‐based networks in aging remains important for understanding the complex interplay of functional networks. In fact, to account for the complex network reorganizations and mechanisms in aging and their behavioral relevance, it is still valuable to consider the structural, functional and metabolic properties at both rest and during task performance.
4.4. Potential physiological confounds
The physiological properties that underlie the BOLD hemodynamic response are known to change with age. Thus, measures of BOLD‐fMRI activity and connectivity are potentially confounded by physiological changes in age that affect the signal but may not affect neuronal activity. Factors like changes in neurovascular coupling, vascular integrity, breathing, hemoglobin, and head movement impact BOLD signal changes with age (Geerligs et al., 2017; Samanez‐Larkin & D'Esposito, 2008; West et al., 2019). The magnetic resonance relaxation rate that underlies the BOLD response is also influenced by the levels of hemoglobin in the blood, which also vary across the lifespan, affect measures of connectivity and its relationship with cognitive measures (Ward et al., 2020). Age‐related decrements in global and regional cerebral blood flow impact the measurement of brain activity throughout the brain (Samanez‐Larkin & D'Esposito, 2008), and estimates of connectivity (Galiano et al., 2020). These underlying changes in neurovascular coupling with aging mean that group differences in BOLD measures alone cannot always be interpreted as changes in neural activity.
Simultaneous MRI‐PET scanning has showed utility in demonstrating the presence of bioenergetic coupling between glucose utilization and rapid transmission of neural information, with some initial research showing utility in aging, and a broader literature demonstrating its reduction in mild cognitive impairment and AD (Marchitelli et al., 2018). This approach offers great promise for understanding metabolic and functional connectivity or dysconnectivity between brain regions across the aging and disease spectrum. It also means that it is now possible to calculate region‐to‐region metabolic connectivity within individuals, which opens the possibility of using this metric more widely as a biomarker in aging and neurodegenerative disease. To date, this emerging field has not examined brain metabolic connectivity across the lifespan.
Some studies have indicated that structural changes that occur in aging, rather than functional connectivity organization, may mediate at least some of the brain metabolic changes in aging (Bullmore & Sporns, 2012; Chételat et al., 2013; Curiati et al., 2011; Hedden et al., 2016). As further research is undertaken on metabolic connectivity in aging, it will be important to control for the changes in brain volume that also occur with age.
Head motion is known to impact the reliability of resting‐state fMRI data (Power et al., 2012, 2013, 2014; Satterthwaite et al., 2013; Yan et al., 2013). All authors included head motion correction in their pre‐processing pipeline, and across network measures 96–100% took additional steps to control for head motion (see Table S1–S6 for individual studies). These steps support the reliability of the results reported here, although the possibility remains that head motion may have impacted the strength and consistency of the findings.
4.5. Research is needed to further elucidate the shared pathways in aging and disease
There is a large body of research looking at brain changes along the spectrum from cognitive aging to dementia (Titov et al., 2017), as well as in vascular and metabolic disease states (Love & Miners, 2016). This research suggests that aging and disease processes likely overlap, differing in degree rather than in the underlying mechanisms (Cole et al., 2019). For example, there is an increased risk of cognitive decline and dementia related to hypoglycemia, glycemic control, metabolic syndrome, and insulin resistance (Akintola & van Heemst, 2015; Arvanitakis et al., 2016; Bello‐Chavolla et al., 2019; Ekblad et al., 2017).
Vascular dysfunction is also an important and early event in age‐related cognitive decline and neurodegenerative disease (see Zimmerman et al., 2021, for review), and is believed to be responsible for more than 50% of cases of dementia worldwide (Raz & Rodrigue, 2006; Sweeney et al., 2019). Blood pressure tends to increase with age due to alterations in the vasculature, increasing the possibility of stroke, ischemia and white matter lesions, particularly in the myelinated regions of the frontal lobes (Riddle et al., 2003). Changes in cerebral vasculature with age can impact cognitive function, as the ability of the microvasculature to respond to the metabolic demands falls (Riddle et al., 2003). With age, arterial stiffness, neurovascular uncoupling and blood–brain barrier (BBB) damage can impact the dynamics of brain blood flow and local perfusion (Kalaria et al., 2019). Cerebral hypoperfusion in older age has been implicated as a key element in white matter changes and cognitive decline (Hase et al., 2019).
Although there is a large body of epidemiological evidence linking metabolic and vascular health to aging and disease, the relationship to functional connectivity is still in its infancy. Some imaging studies have shown that connectivity is altered in states of metabolic and vascular dysfunction (e.g. Biessels & Reijmer, 2014; Biessels et al., 2014; Carnevale et al., 2020; Cui et al., 2014, 2016; Debette & Markus, 2010; Giorgio et al., 2020; Iozzo & Guzzardi, 2019; Liu et al., 2020; Manschot et al., 2006; Moran et al., 2017; Musen et al., 2012; Quevenco et al., 2020; Reijmer et al., 2015; Sang et al., 2018; Spielberg et al., 2017; Viviano et al., 2017). Given the rising rates of chronic diseases that impact vascular and metabolism in the periphery and brain, such as obesity (Afshin et al., 2017) and diabetes (Tao et al., 2015), it is likely that future research in cognitive aging may find an important role of peripheral factors, particularly as age itself is a key risk factor for these diseases. Moreover, many of these diseases sit along a spectrum from health to clinical dysfunction and hence understanding their influence on cognitive aging may also allow them to be used as early markers for age‐related structural and functional changes. Of course, the concepts in psychophysiology that underscore the relationship between central and peripheral systems are not new (see Fabiani, 2015 for a review). However, with the increasing availability of tools to measure network function, assessing the relationships between mechanisms known to affect the brain and to change with age and disease states, such as cerebral blood flow, hormonal status and metabolism, presents important avenues for further research.
Age‐related changes that impair cerebrovascular integrity and function also potentially introducing confounds when interpreting hemodynamic responses in fMRI (Yabluchanskiy et al., 2021). Recent research has validated a number of approaches for use in aging research to evaluate and separate neuronal and vascular signals in brain network changes (e.g., arterial stiffness, cerebral blood flow, brain barrier permeability, blood pressure). The approaches include arterial spin labelling to assess cerebral blood flow (Tsvetanov et al., 2020), arterial pulse based on diffuse optical tomography to assess cerebral arterial elasticity (Kong et al., 2020) and functional near‐infrared spectroscopy to assess neurovascular coupling (see Yabluchanskiy et al., 2021, for review).
Arterial stiffness has been shown to be highly correlated with the extent of reduced segregation in healthy older adults (Kong et al., 2020). Some evidence also suggests that age‐related differences in resting state BOLD signal variability are partly explained by cardiovascular health, as measured by pulse oximetry and electrocardiograph (Tsvetanov et al., 2015), and cerebrovascular factors, as measured by arterial spin labelling and CO2 inhalation‐induced hypercapnia (Garrett et al., 2017). The effects of age on resting‐state BOLD signal variability have also been found to be explained by the combination of cardiovascular and cerebrovascular factors (Tsvetanov et al., 2020). Additional research is needed to apply these techniques together with fMRI to further assess the relative hemodynamic and neuronal changes contributing to functional network changes in aging.
4.6. Impact of scan length, volume of data and network definition
The average scan time across studies and network metrics was seven to 10 min (see Table S8). This is consistent with research suggesting that scans of at least five minutes are needed to generate moderate reliability (Van Dijk et al., 2012; but also see Anderson et al., 2011; Birn et al., 2013). Some recent evidence suggests that shorter scans can lead to unreliable network measures (Gordon et al., 2017). It is also worth noting that longer scan times are likely needed to acquire data on single subject level (e.g., in clinical settings), whereas the potential confounding of shorter scan times is at least somewhat mitigated in research group comparisons where true groups effects are large enough to be identified and the groups matched for quantity of motion‐corrected data. Moreover, although longer scan times may provide increased reliability, a limiting factor is the subject's ability to tolerate prolonged scanning, particularly older adults.
An important consideration when reviewing the brain network literature is how regions and networks are defined and parceled (Stanley et al., 2013). For example, graph theory metrics have been shown to vary as a function of parcellation decisions (Wig et al., 2011; Zalesky et al., 2010). Across the studies, there is variation in image pre‐processing, nodal scale (i.e., number of nodes within networks) and parcellation approaches (based on a structural or functional criteria; templates or data‐driven approaches, e.g., ICA) (see Tables S1–S8). These factors will influence the derived functional network measures and study results and may explain some of the variability in findings reported here.
It is worth noting that the studies that tested for PASA in aging used a variety of approaches to assess the posterior–anterior shift in activity among older adults. For example, some used connectivity matrices to test for patterns of age differences at the lobular level (e.g., Chen et al., 2016) or large‐scale network level (Chen et al., 2018; Lee et al., 2015), and other used graph theoretical analysis (McCarthy et al., 2014). Although the varied approaches and network scales yielded largely consistent results in terms of increased anterior and decreased posterior activity, additional research is needed to validate these findings. We recommend that in line with the PASA theory, connections between the pre‐frontal cortex and other large‐scale resting state networks are consistently assessed to strengthen the comparability of findings.
5. CONCLUSIONS
The brain undergoes significant structural, functional, and metabolic changes with age, with associated alterations in cognition and behavior. During the early years of life, there is a rapid organization of functional brain networks. A further refinement of the functional networks then takes place until around the third and fourth decade of life. A multifaceted interplay of potentially harmful and compensatory changes can follow in aging. Moreover, these changes can lead to a variety of expressions of cognition and behavior, depending on the time, location and extent of the changes. The strength of connections, the topology by which regions are connected, and the efficiency of the connections are important determinants in the function of the brain networks and their potential impact of cognition and behavior across the adult lifespan. When investigated across the whole brain at rest, the literature suggests with high certainty that older adults display lower within‐network connectivity and higher between‐network connectivity than younger adults. These changes begin to occur as early as the third or fourth decade of life, with the trajectory of alterations varying by network. Some networks may remain relatively stable (e.g., primary sensory and motor) and others may vary substantially (e.g., associative regions).
The typology of the connections of a functional network also dictates how age‐related changes or damage impact the networks and their associated functions. The literature suggests with high certainty that older adults show a less segregated, less modular and more integrated system of networks than younger adults, presenting as diffuse and less specialized configurations of functional connections. A lower level of local and global network efficiency is also reported consistently in older adults compared to younger adults, particularly in the higher‐order networks. Alterations to a “rich club” network hub, such as the executive control or the default mode networks, have a diffuse impact across the system by reducing the communication between brain regions, a prerequisite for efficient and effective higher order cognitive functions. Their importance may lie primarily in the between‐network connectivity that support long‐range connections. They are known for their key role in efficient brain‐wide information processing, functional integration of diverse cognitive functions, and are vulnerable to aging. These functional network changes are likely driving at least part of the changes often seen in aging in higher‐order cognitive processes.
On the other network measures reviewed, relatively consistent results were reported although certainty in the results is moderate to low due to smaller bodies of literature, various methods and fewer studies that include participants across the full adult lifespan. Studies assessing dynamic network connectivity found with moderate certainty age differences in all or some of the networks, both during and in the transitions between states, and occurring at a slower rate in older adults.
Functional networks based on glucose metabolism appear to show covariance differences across the adult lifespan, although the certainty of the evidence is low based on the available literature at this time. Nonetheless, metabolic brain networks have economical “small world” properties, and brain glucose metabolism is highly correlated with local and global connectivity, especially for hubs with high between‐network connectivity. This and a broader literature on brain glucose suggests that it is a key contributor to age‐related changes in connectivity and cognitive performance and warrants further study.
Simultaneous MR‐PET measurements have also been used recently and shown underlying synchrony between hemodynamic processes and glucose uptake. Their high sensitivity and regional specificity offer promise for functional and multimodal brain imaging. To date, they have not been widely applied across aging and cognitive decline and present a promising avenue for future research. There also appears to be a complex interaction between brain glucose, age and peripheral physiology, including cardiovascular and metabolic factors that are well documented in cognitive aging, and for which age is a major risk factor. With a rise in these conditions in the global population, future research will also need to further characterize the role of these peripheral factors in cognitive aging.
AUTHOR CONTRIBUTIONS
Hamish Deery: Conceptualization, Data curation, Formal analysis, Methodology, Validation, Writing – original draft, Writing – review & editing. Robert Di Paolo: Data curation; formal analysis; validation; writing – review and editing. Chris Moran: Conceptualization; methodology; supervision; validation; writing – review and editing. Gary Egan: Conceptualization; methodology; supervision; validation; writing – review and editing. Sharna Jamadar: Conceptualization, Methodology, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
CONFLICT OF INTEREST
The authors declare no conflict of interest. The funding source had no involvement in the review design and production.
Supporting information
Appendix S1 Supporting information
ACKNOWLEDGMENTS
Jamadar is supported by an Australian National Health and Medical Research Council (NHMRC) Fellowship (APP1174164). Egan and Jamadar are supported by the Australian Research Council Centre of Excellence for Integrative Brain Function (CE114100007).
Deery, H. A. , Di Paolo, R. , Moran, C. , Egan, G. F. , & Jamadar, S. D. (2023). The older adult brain is less modular, more integrated, and less efficient at rest: A systematic review of large‐scale resting‐state functional brain networks in aging. Psychophysiology, 60, e14159. 10.1111/psyp.14159
Footnotes
There are many conflicting definitions of the terms ‘static’ and ‘dynamic’ in the neuroimaging literature. We have clearly defined our use of the terms here, but see Jamadar et al. (2021) and Liegeois et al. (2017) for discussion of alternative definitions of the terms.
DATA AVAILABILITY STATEMENT
The data used in this paper are publicly available from published journals articles.
REFERENCES
- Achard, S. , & Bullmore, E. (2007). Efficiency and cost of economical brain functional networks. PLoS Computational Biology, 3(2), e17. 10.1371/journal.pcbi.0030017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afshin, A. , Forouzanfar, M. H. , Reitsma, M. B. , Sur, P. , Estep, K. , Lee, A. , Marczak, L. , Mokdad, A. H. , Moradi‐Lakeh, M. , Naghavi, M. , Salama, J. S. , Vos, T. , Abate, K. H. , Abbafati, C. , Ahmed, M. B. , Al‐Aly, Z. , Alkerwi, A.'a. , Al‐Raddadi, R. , Amare, A. T. , … Murray, C. (2017). Health effects of overweight and obesity in 195 countries over 25 years. The New England Journal of Medicine, 377(1), 13–27. 10.1056/NEJMoa1614362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agcaoglu, O. , Miller, R. , Mayer, A. R. , Hugdahl, K. , & Calhoun, V. D. (2015). Lateralization of resting state networks and relationship to age and gender. NeuroImage, 104, 310–325. 10.1016/j.neuroimage.2014.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akintola, A. A. , & van Heemst, D. (2015). Insulin, aging, and the brain: Mechanisms and implications. Frontiers in Endocrinology, 6, 13. 10.3389/fendo.2015.00013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen, E. A. , Erhardt, E. B. , Damaraju, E. , Gruner, W. , Segall, J. M. , Silva, R. F. , Havlicek, M. , Rachakonda, S. , Fries, J. , Kalyanam, R. , Michael, A. M. , Caprihan, A. , Turner, J. A. , Eichele, T. , Adelsheim, S. , Bryan, A. D. , Bustillo, J. , Clark, V. P. , Feldstein Ewing, S. W. , … Calhoun, V. D. (2011). A baseline for the multivariate comparison of resting‐state networks. Frontiers in Systems Neuroscience, 5, 2. 10.3389/fnsys.2011.00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amend, M. , Ionescu, T. M. , Di, X. , Pichler, B. J. , Biswal, B. B. , & Wehrl, H. F. (2019). Functional resting‐state brain connectivity is accompanied by dynamic correlations of application‐dependent [18F]FDG PET‐tracer fluctuations. NeuroImage, 196, 161–172. 10.1016/j.neuroimage.2019.04.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson, J. S. , Ferguson, M. A. , Lopez‐Larson, M. , & Yurgelun‐Todd, D. (2011). Reproducibility of single‐subject functional connectivity measurements. American Journal of Neuroradiology, 32(3), 548–555. 10.3174/ajnr.A2330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson, M. L. , & Finlay, B. L. (2014). Allocating structure to function: The strong links between neuroplasticity and natural selection. Frontiers in Human Neuroscience, 7, 918. 10.3389/fnhum.2013.00918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonenko, D. , & Flöel, A. (2014). Healthy aging by staying selectively connected: A mini‐review. Gerontology, 60(1), 3–9. 10.1159/000354376 [DOI] [PubMed] [Google Scholar]
- Tsvetanov, K. A. , Henson, R. , Jones, P. S. , Mutsaerts, H. , Fuhrmann, D. , Tyler, L. K. , Cam‐CAN, & Rowe, J. B. (2020). The effects of age on resting‐state BOLD signal variability is explained by cardiovascular and cerebrovascular factors. Psychophysiology, 58(7), e13714. 10.1111/psyp.13714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsvetanov, K. A. , Henson, R. N. A. , Tyler, L. K. , Davis, S. W. , Shafto, M. A. , Taylor, J. R. , Williams, N. , Cam‐Can, & Rowe, J. B. (2015). The effect of ageing on fMRI: Correction for the confounding effects of vascular reactivity evaluated by joint fMRI and MEG in 335 adults. Human Brain Mapping, 36(6), 2248–2269. 10.1002/hbm.22768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quevenco, F. C. , van Bergen, J. M. , Treyer, V. , Studer, S. T. , Kagerer, S. M. , Meyer, R. , Gietl, Kaufmann, P. A. , Nitsch, R. M. , Hock, C. , & Unschuld, P. G. (2020). Functional brain network connectivity patterns associated with normal cognition at old‐age, local β‐amyloid, tau, and APOE4. Frontiers in Aging Neuroscience, 12, 46. 10.3389/fnagi.2020.00046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer, J. A. , Lee, A. , Qiu, A. , & Chen, S. H. (2016). A comprehensive analysis of connectivity and aging over the adult life span. Brain Connectivity, 6(2), 169–185. 10.1089/brain.2015.0345 [DOI] [PubMed] [Google Scholar]
- Arnemann, K. L. , Stöber, F. , Narayan, S. , Rabinovici, G. D. , & Jagust, W. J. (2018). Metabolic brain networks in aging and preclinical Alzheimer's disease. NeuroImage: Clinical, 17, 987–999. 10.1016/j.nicl.2017.12.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvanitakis, Z. , Capuano, A. W. , Leurgans, S. E. , Bennett, D. A. , & Schneider, J. A. (2016). Relation of cerebral vessel disease to Alzheimer's disease dementia and cognitive function in elderly people: A cross‐sectional study. The Lancet: Neurology, 15(9), 934–943. 10.1016/S1474-4422(16)30029-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ash, J. A. , & Rapp, P. R. (2014). A quantitative neural network approach to understanding aging phenotypes. Ageing Research Reviews, 15, 44–50. 10.1016/j.arr.2014.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azari, N. P. , Rapoport, S. I. , Salerno, J. A. , Grady, C. L. , Gonzalez‐Aviles, A. , Schapiro, M. B. , & Horwitz, B. (1992). Interregional correlations of resting cerebral glucose metabolism in old and young women. Brain Research, 589(2), 279–290. 10.1016/0006-8993(92)91288-p [DOI] [PubMed] [Google Scholar]
- Bagarinao, E. , Watanabe, H. , Maesawa, S. , Mori, D. , Hara, K. , Kawabata, K. , Yoneyama, N. , Ohdake, R. , Imai, K. , Masuda, M. , Yokoi, T. , Ogura, A. , Taoka, T. , Koyama, S. , Tanabe, H. C. , Katsuno, M. , Wakabayashi, T. , Kuzuya, M. , Ozaki, N. , … Sobue, G. (2019). Reorganization of brain networks and its association with general cognitive performance over the adult lifespan. Scientific Reports, 9(1), 11352. 10.1038/s41598-019-47922-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsters, J. H. , O'Connell, R. G. , Galli, A. , Nolan, H. , Greco, E. , Kilcullen, S. M. , Bokde, A. L. W. , Lai, R. , Upton, N. , & Robertson, I. H. (2013). Changes in resting connectivity with age: A simultaneous electroencephalogram and functional magnetic resonance imaging investigation. Neurobiology of Aging, 34(9), 2194–2207. 10.1016/j.neurobiolaging.2013.03.004 [DOI] [PubMed] [Google Scholar]
- Barbey, A. K. (2018). Network neuroscience theory of human intelligence. Trends in Cognitive Sciences, 22(1), 8–20. 10.1016/j.tics.2017.10.001 [DOI] [PubMed] [Google Scholar]
- Bastin, C. , Yakushev, I. , Bahri, M. A. , Fellgiebel, A. , Eustache, F. , Landeau, B. , Scheurich, A. , Feyers, D. , Collette, F. , Chételat, G. , & Salmon, E. (2012). Cognitive reserve impacts on inter‐individual variability in resting‐state cerebral metabolism in normal aging. NeuroImage, 63(2), 713–722. 10.1016/j.neuroimage.2012.06.074 [DOI] [PubMed] [Google Scholar]
- Battaglia, D. , Boudou, T. , Hansen, E. , Lombardo, D. , Chettouf, S. , Daffertshofer, A. , McIntosh, A. R. , Zimmermann, J. , Ritter, P. , & Jirsa, V. (2020). Dynamic functional connectivity between order and randomness and its evolution across the human adult lifespan. NeuroImage, 222, 117156. 10.1016/j.neuroimage.2020.117156 [DOI] [PubMed] [Google Scholar]
- Baum, G. L. , Cui, Z. , Roalf, D. R. , Ciric, R. , Betzel, R. F. , Larsen, B. , Cieslak, M. , Cook, P. A. , Xia, C. H. , Moore, T. M. , Ruparel, K. , Oathes, D. J. , Alexander‐Bloch, A. F. , Shinohara, R. T. , Raznahan, A. , Gur, R. E. , Gur, R. C. , Bassett, D. S. , & Satterthwaite, T. D. (2020). Development of structure‐function coupling in human brain networks during youth. Proceedings of the National Academy of Sciences of the United States of America, 117(1), 771–778. 10.1073/pnas.1912034117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann, C. F. , DeLuca, M. , Devlin, J. T. , & Smith, S. M. (2005). Investigations into resting‐state connectivity using independent component analysis. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences, 360(1457), 1001–1013. 10.1098/rstb.2005.1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello‐Chavolla, O. Y. , Antonio‐Villa, N. E. , Vargas‐Vázquez, A. , Ávila‐Funes, J. A. , & Aguilar‐Salinas, C. A. (2019). Pathophysiological mechanisms linking type 2 diabetes and dementia: Review of evidence from clinical, translational and epidemiological research. Current Diabetes Reviews, 15(6), 456–470. 10.2174/1573399815666190129155654 [DOI] [PubMed] [Google Scholar]
- Bernier, M. , Croteau, E. , Castellano, C. A. , Cunnane, S. C. , & Whittingstall, K. (2017). Spatial distribution of resting‐state BOLD regional homogeneity as a predictor of brain glucose uptake: A study in healthy aging. NeuroImage, 150, 14–22. 10.1016/j.neuroimage.2017.01.055 [DOI] [PubMed] [Google Scholar]
- Berti, V. , Vanzi, E. , Polito, C. , & Pupi, A. (2013). Back to the future: The absolute quantification of cerebral metabolic rate of glucose. Clinical and Translational Imaging, 1, 289–296. 10.1007/s40336-013-0030-2 [DOI] [Google Scholar]
- Bethlehem, R. , Paquola, C. , Seidlitz, J. , Ronan, L. , Bernhardt, B. , Consortium, C. C. , & Tsvetanov, K. A. (2020). Dispersion of functional gradients across the adult lifespan. NeuroImage, 222, 117299. 10.1016/j.neuroimage.2020.117299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betzel, R. F. , Byrge, L. , He, Y. , Goñi, J. , Zuo, X. N. , & Sporns, O. (2014). Changes in structural and functional connectivity among resting‐state networks across the human lifespan. NeuroImage, 102(2), 345–357. 10.1016/j.neuroimage.2014.07.067 [DOI] [PubMed] [Google Scholar]
- Biessels, G. J. , & Reijmer, Y. D. (2014). Brain changes underlying cognitive dysfunction in diabetes: What can we learn from MRI? Diabetes, 63(7), 2244–2252. 10.2337/db14-0348 [DOI] [PubMed] [Google Scholar]
- Biessels, G. J. , Strachan, M. W. , Visseren, F. L. , Kappelle, L. J. , & Whitmer, R. A. (2014). Dementia and cognitive decline in type 2 diabetes and prediabetic stages: Towards targeted interventions. The Lancet: Diabetes & Endocrinology, 2(3), 246–255. 10.1016/S2213-8587(13)70088-3 [DOI] [PubMed] [Google Scholar]
- Bijsterbosch, J. , Harrison, S. J. , Jbabdi, S. , Woolrich, M. , Beckmann, C. , Smith, S. , & Duff, E. P. (2020). Challenges and future directions for representations of functional brain organization. Nature Neuroscience, 23(12), 1484–1495. 10.1038/s41593-020-00726-z [DOI] [PubMed] [Google Scholar]
- Birn, R. M. , Molloy, E. K. , Patriat, R. , Parker, T. , Meier, T. B. , Kirk, G. R. , Nair, V. A. , Meyerand, M. E. , & Prabhakaran, V. (2013). The effect of scan length on the reliability of resting‐state fMRI connectivity estimates. NeuroImage, 83, 550–558. 10.1016/j.neuroimage.2013.05.099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal, B. B. , Mennes, M. , Zuo, X.‐N. , Gohel, S. , Kelly, C. , Smith, S. M. , Beckmann, C. F. , Adelstein, J. S. , Buckner, R. L. , Colcombe, S. , Dogonowski, A. M. , Ernst, M. , Fair, D. , Hampson, M. , Hoptman, M. J. , Hyde, J. S. , Kiviniemi, V. J. , Kötter, R. , Li, S. J. , … Milham, M. P. (2010). Toward discovery science of human brain function. Proceedings of the National Academy of Sciences of the United States of America, 107(10), 4734–4739. 10.1073/pnas.0911855107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal, B. , Yetkin, F. Z. , Haughton, V. M. , & Hyde, J. S. (1995). Functional connectivity in the motor cortex of resting human brain using echo‐planar MRI. Magnetic Resonance in Medicine, 34(4), 537–541. 10.1002/mrm.1910340409 [DOI] [PubMed] [Google Scholar]
- Brookes, M. J. , Woolrich, M. , Luckhoo, H. , Price, D. , Hale, J. R. , Stephenson, M. C. , Barnes, G. R. , Smith, S. M. , & Morris, P. G. (2011). Investigating the electrophysiological basis of resting state networks using magnetoencephalography. Proceedings of the National Academy of Sciences of the United States of America, 108(40), 16783–16788. 10.1073/pnas.1112685108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, S. C. , & Park, D. C. (2003). Theoretical models of cognitive aging and implications for translational research in medicine. The Gerontologist, 43 spec no 1, 57–67. 10.1093/geront/43.suppl_1.57 [DOI] [PubMed] [Google Scholar]
- Bullmore, E. , & Sporns, O. (2012). The economy of brain network organization. Nature reviews. Neuroscience, 13(5), 336–349. 10.1038/nrn3214 [DOI] [PubMed] [Google Scholar]
- Burzynska, A. Z. , Garrett, D. D. , Preuschhof, C. , Nagel, I. E. , Li, S. C. , Bäckman, L. , Heekeren, H. R. , & Lindenberger, U. (2013). A scaffold for efficiency in the human brain. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 33(43), 17150–17159. 10.1523/JNEUROSCI.1426-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza, R. (2002). Hemispheric asymmetry reduction in older adults: The HAROLD model. Psychology and Aging, 17(1), 85–100. 10.1037//0882-7974.17.1.85 [DOI] [PubMed] [Google Scholar]
- Cabeza, R. , & Dennis, N. A. (2012). Frontal lobes and aging. In Stuss D. & Knight T. (Eds.), Principles of frontal lobes function (pp. 628–652). Oxford University Press. 10.1093/med/9780199837755.001.0001 [DOI] [Google Scholar]
- Calhoun, V. D. , Kiehl, K. A. , & Pearlson, G. D. (2008). Modulation of temporally coherent brain networks estimated using ICA at rest and during cognitive tasks. Human Brain Mapping, 29(7), 828–838. 10.1002/hbm.20581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun, V. D. , Miller, R. , Pearlson, G. , & Adalı, T. (2014). The chronnectome: Time‐varying connectivity networks as the next frontier in fMRI data discovery. Neuron, 84(2), 262–274. 10.1016/j.neuron.2014.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell, K. L. , & Schacter, D. L. (2017). Aging and the resting state: Is cognition obsolete? Language. Cognition and Neuroscience, 32(6), 661–668. 10.1080/23273798.2016.1227858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, M. , Wang, J. H. , Dai, Z. J. , Cao, X. Y. , Jiang, L. L. , Fan, F. M. , Song, X. W. , Xia, M. R. , Shu, N. , Dong, Q. , Milham, M. P. , Castellanos, F. X. , Zuo, X. N. , & He, Y. (2014). Topological organization of the human brain functional connectome across the lifespan. Developmental Cognitive Neuroscience, 7, 76–93. 10.1016/j.dcn.2013.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnevale, L. , Maffei, A. , Landolfi, A. , Grillea, G. , Carnevale, D. , & Lembo, G. (2020). Brain functional magnetic resonance imaging highlights altered connections and functional networks in patients with hypertension. Hypertension, 76(5), 1480–1490. 10.1161/HYPERTENSIONAHA.120.15296 [DOI] [PubMed] [Google Scholar]
- Chan, M. Y. , Alhazmi, F. H. , Park, D. C. , Savalia, N. K. , & Wig, G. S. (2017). Resting‐state network topology differentiates task signals across the adult life span. The Journal of Neuroscience, 37(10), 2734–2745. 10.1523/JNEUROSCI.2406-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan, M. Y. , Han, L. , Carreno, C. A. , Zhang, Z. , Rodriguez, R. M. , LaRose, M. , Hassenstab, J. , & Wig, G. S. (2021). Long‐term prognosis and educational determinants of brain network decline in older adult individuals. Nature Aging, 1(11), 1053–1067. 10.1038/s43587-021-00125-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan, M. Y. , Na, J. , Agres, P. F. , Savalia, N. K. , Park, D. C. , & Wig, G. S. (2018). Socioeconomic status moderates age‐related differences in the brain's functional network organization and anatomy across the adult lifespan. Proceedings of the National Academy of Sciences of the United States of America, 115(22), E5144–E5153. 10.1073/pnas.1714021115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan, M. Y. , Park, D. C. , Savalia, N. K. , Petersen, S. E. , & Wig, G. S. (2014). Decreased segregation of brain systems across the healthy adult lifespan. Proceedings of the National Academy of Sciences of the United States of America, 111(46), E4997–E5006. 10.1073/pnas.1415122111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, J. J. (2019). Functional MRI of brain physiology in aging and neurodegenerative diseases. NeuroImage, 187, 209–225. 10.1016/j.neuroimage.2018.05.050 [DOI] [PubMed] [Google Scholar]
- Chen, P. Y. , Chiou, J. M. , Yang, Y. F. , Chen, Y. T. , Hsieh, H. L. , Chang, Y. L. , & Tseng, W. I. (2016). Heterogeneous aging effects on functional connectivity in different cortical regions: A resting‐state functional MRI study using functional data analysis. PLoS One, 11(9), e0162028. 10.1371/journal.pone.0162028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Q. , Xia, Y. , Zhuang, K. , Wu, X. , Liu, G. , & Qiu, J. (2019). Decreased inter‐hemispheric interactions but increased intra‐hemispheric integration during typical aging. Aging, 11(22), 10100–10115. 10.18632/aging.10242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y. , Liu, Y. N. , Zhou, P. , Zhang, X. , Wu, Q. , Zhao, X. , & Ming, D. (2019). The transitions between dynamic micro‐states reveal age‐related functional network reorganization. Frontiers in Physiology, 9, 1852. 10.3389/fphys.2018.01852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y. , Wang, W. , Zhao, X. , Sha, M. , Liu, Y. , Zhang, X. , Ma, J. , Ni, H. , & Ming, D. (2017). Age‐related decline in the variation of dynamic functional connectivity: A resting state analysis. Frontiers in Aging Neuroscience, 9, 203. 10.3389/fnagi.2017.00203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y. , Zhao, X. , Zhang, X. , Liu, Y. , Zhou, P. , Ni, H. , Ma, J. , & Ming, D. (2018). Age‐related early/late variations of functional connectivity across the human lifespan. Neuroradiology, 60(4), 403–412. 10.1007/s00234-017-1973-1 [DOI] [PubMed] [Google Scholar]
- Chong, J. , Ng, K. K. , Tandi, J. , Wang, C. , Poh, J. H. , Lo, J. C. , Chee, M. , & Zhou, J. H. (2019). Longitudinal changes in the cerebral cortex functional organization of healthy elderly. The Journal of Neuroscience, 39(28), 5534–5550. 10.1523/JNEUROSCI.1451-18.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou, Y. H. , Chen, N. K. , & Madden, D. J. (2013). Functional brain connectivity and cognition: Effects of adult age and task demands. Neurobiology of Aging, 34(8), 1925–1934. 10.1016/j.neurobiolaging.2013.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chételat, G. , Landeau, B. , Salmon, E. , Yakushev, I. , Bahri, M. A. , Mézenge, F. , Perrotin, A. , Bastin, C. , Manrique, A. , Scheurich, A. , Scheckenberger, M. , Desgranges, B. , Eustache, F. , & Fellgiebel, A. (2013). Relationships between brain metabolism decrease in normal aging and changes in structural and functional connectivity. NeuroImage, 76, 167–177. 10.1016/j.neuroimage.2013.03.009 [DOI] [PubMed] [Google Scholar]
- Cieri, F. , & Esposito, R. (2018). Neuroaging through the lens of the resting state networks. BioMed Research International, 2018, 5080981. 10.1155/2018/5080981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipolla, M. J. (2009). The cerebral circulation. Morgan & Claypool Life Sciences; chapter 5, Control of Cerebral Blood Flow. https://www.ncbi.nlm.nih.gov/books/NBK53081/ [PubMed] [Google Scholar]
- Clarke, D. D. , & Sokoloff, L. (1999). Regulation of cerebral metabolic rate. In Siegel G. J., Agranoff B. W., & Albers R. W. (Eds.), Basic neurochemistry: Molecular, cellular and medical aspects. 6th edition. Lippincott‐Raven; ISBN‐10: 0–397‐51820‐X. [Google Scholar]
- Cohen, J. R. , & D'Esposito, M. (2016). The segregation and integration of distinct brain networks and their relationship to cognition. The Journal of Neuroscience, 36(48), 12083–12094. 10.1523/JNEUROSCI.2965-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole, J. H. , Marioni, R. E. , Harris, S. E. , & Deary, I. J. (2019). Brain age and other bodily ‘ages’: Implications for neuropsychiatry. Molecular Psychiatry, 24(2), 266–281. 10.1038/s41380-018-0098-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole, M. W. , Bassett, D. S. , Power, J. D. , Braver, T. S. , & Petersen, S. E. (2014). Intrinsic and task‐evoked network architectures of the human brain. Neuron, 83(1), 238–251. 10.1016/j.neuron.2014.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole, M. W. , Ito, T. , Bassett, D. S. , & Schultz, D. H. (2016). Activity flow over resting‐state networks shapes cognitive task activations. Nature Neuroscience, 19(12), 1718–1726. 10.1038/nn.4406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coupé, P. , Manjón, J. V. , Lanuza, E. , & Catheline, G. (2019). Lifespan changes of the human brain in Alzheimer's disease. Scientific Reports, 9(1), 3998. 10.1038/s41598-019-39809-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney, S. M. , & Hinault, T. (2021). When the time is right: Temporal dynamics of brain activity in healthy aging and dementia. Progress in Neurobiology, 203, 102076. 10.1016/j.pneurobio.2021.102076 [DOI] [PubMed] [Google Scholar]
- Crossley, N. A. , Mechelli, A. , Scott, J. , Carletti, F. , Fox, P. T. , McGuire, P. , & Bullmore, E. T. (2014). The hubs of the human connectome are generally implicated in the anatomy of brain disorders. Brain: A Journal of Neurology, 137(Pt 8), 2382–2395. 10.1093/brain/awu132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowell, C. A. , Davis, S. W. , Beynel, L. , Deng, L. , Lakhlani, D. , Hilbig, S. A. , Palmer, H. , Brito, A. , Peterchev, A. V. , Luber, B. , Lisanby, S. H. , Appelbaum, L. G. , & Cabeza, R. (2020). Older adults benefit from more widespread brain network integration during working memory. NeuroImage, 218, 116959. 10.1016/j.neuroimage.2020.116959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui, Y. , Jiao, Y. , Chen, Y. C. , Wang, K. , Gao, B. , Wen, S. , Ju, S. , & Teng, G. J. (2014). Altered spontaneous brain activity in type 2 diabetes: A resting‐state functional MRI study. Diabetes, 63(2), 749–760. 10.2337/db13-0519 [DOI] [PubMed] [Google Scholar]
- Cui, Y. , Li, S. F. , Gu, H. , Hu, Y. Z. , Liang, X. , Lu, C. Q. , Cai, Y. , Wang, C. X. , Yang, Y. , & Teng, G. J. (2016). Disrupted brain connectivity patterns in patients with type 2 diabetes. American Journal of Neuroradiology, 37(11), 2115–2122. 10.3174/ajnr.A4858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curiati, P. K. , Tamashiro‐Duran, J. H. , Duran, F. L. , Buchpiguel, C. A. , Squarzoni, P. , Romano, D. C. , Vallada, H. , Menezes, P. R. , Scazufca, M. , Busatto, G. F. , & Alves, T. C. (2011). Age‐related metabolic profiles in cognitively healthy elders: Results from a voxel‐based [18F]fluorodeoxyglucose‐positron‐emission tomography study with partial volume effects correction. American Journal of Neuroradiology, 32(3), 560–565. 10.3174/ajnr.A2321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Esposito, M. (1999). Cognitive aging: New answers to old questions. Current Biology, 9(24), R939–R941. 10.1016/s0960-9822(00)80110-4 [DOI] [PubMed] [Google Scholar]
- Dai, Z. J. , Bi, Y. C. , & He, Y. (2015). With great brain hub connectivity comes great vulnerability. CNS Neuroscience & Therapeutics, 21(7), 541–542. 10.1111/cns.12407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux, J. S. (2017). Effects of aging on functional and structural brain connectivity. NeuroImage, 160, 32–40. 10.1016/j.neuroimage.2017.01.077 [DOI] [PubMed] [Google Scholar]
- Darowski, E. S. , Helder, E. , Zacks, R. T. , Hasher, L. , & Hambrick, D. Z. (2008). Age‐related differences in cognition: The role of distraction control. Neuropsychology, 22(5), 638–644. 10.1037/0894-4105.22.5.638 [DOI] [PubMed] [Google Scholar]
- Das, M. , Singh, V. , Uddin, L. Q. , Banerjee, A. , & Roy, D. (2021). Reconfiguration of directed functional connectivity among neurocognitive networks with aging: Considering the role of thalamo‐cortical interactions. Cerebral Cortex, 31(4), 1970–1986. 10.1093/cercor/bhaa334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, S. W. , Dennis, N. A. , Daselaar, S. M. , Fleck, M. S. , & Cabeza, R. (2008). Que PASA? The posterior‐anterior shift in aging. Cerebral Cortex, 18(5), 1201–1209. 10.1093/cercor/bhm155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, S. W. , Kragel, J. E. , Madden, D. J. , & Cabeza, R. (2012). The architecture of cross‐hemispheric communication in the aging brain: Linking behavior to functional and structural connectivity. Cerebral Cortex, 22(1), 232–242. 10.1093/cercor/bhr123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, S. W. , Stanley, M. L. , Moscovitch, M. , & Cabeza, R. (2017). Resting‐state networks do not determine cognitive function networks: A commentary on Campbell and Schacter (2016). Language. Cognition and Neuroscience, 32(6), 669–673. 10.1080/23273798.2016.1252847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison, E. N. , Turner, B. O. , Schlesinger, K. J. , Miller, M. B. , Grafton, S. T. , Bassett, D. S. , & Carlson, J. M. (2016). Individual differences in dynamic functional brain connectivity across the human lifespan. PLoS Computational Biology, 12(11), e1005178. 10.1371/journal.pcbi.1005178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debette, S. , & Markus, H. S. (2010). The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: Systematic review and meta‐analysis. British Medical Journa (Clinical Research ed.), 341, c3666. 10.1136/bmj.c3666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di, X. , Biswal, B. B. , & Alzheimer's Disease Neuroimaging Initiative . (2012). Metabolic brain covariant networks as revealed by FDG‐PET with reference to resting‐state fMRI networks. Brain Connectivity, 2(5), 275–283. 10.1089/brain.2012.0086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di, X. , Gohel, S. , Thielcke, A. , Wehrl, H. F. , Biswal, B. B. , & Alzheimer's Disease Neuroimaging Initiative . (2017). Do all roads lead to Rome? A comparison of brain networks derived from inter‐subject volumetric and metabolic covariance and moment‐to‐moment hemodynamic correlations in old individuals. Brain Structure & Function, 222(8), 3833–3845. 10.1007/s00429-017-1438-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di, X. , Wölfer, M. , Amend, M. , Wehrl, H. , Ionescu, T. M. , Pichler, B. J. , Biswal, B. B. , & Alzheimer's Disease Neuroimaging Initiative . (2019). Interregional causal influences of brain metabolic activity reveal the spread of aging effects during normal aging. Human Brain Mapping, 40(16), 4657–4668. 10.1002/hbm.24728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edde, M. , Leroux, G. , Altena, E. , & Chanraud, S. (2020). Functional brain connectivity changes across the human life span: From fetal development to old age. Journal of Neuroscience Research, 99(1), 236–262. 10.1002/jnr.24669 [DOI] [PubMed] [Google Scholar]
- Edde, M. , Leroux, G. , Altena, E. , & Chanraud, S. (2021). Functional brain connectivity changes across the human life span: From fetal development to old age. Journal of Neuroscience Research, 99(1), 236–262. 10.1002/jnr.24669 [DOI] [PubMed] [Google Scholar]
- Ekblad, L. L. , Rinne, J. O. , Puukka, P. , Laine, H. , Ahtiluoto, S. , Sulkava, R. , Viitanen, M. , & Jula, A. (2017). Insulin resistance predicts cognitive decline: An 11‐year follow‐up of a nationally representative adult population sample. Diabetes Care, 40(6), 751–758. 10.2337/dc16-2001 [DOI] [PubMed] [Google Scholar]
- Escrichs, A. , Biarnes, C. , Garre‐Olmo, J. , Fernández‐Real, J. M. , Ramos, R. , Pamplona, R. , Brugada, R. , Serena, J. , Ramió‐Torrentà, L. , Coll‐de‐Tuero, G. , Gallart, L. , Barretina, J. , Vilanova, J. C. , Mayneris‐Perxachs, J. , Essig, M. , Figley, C. R. , Pedraza, S. , Puig, J. , & Deco, G. (2021). Whole‐brain dynamics in aging: Disruptions in functional connectivity and the role of the rich club. Cerebral Cortex, 31(5), 2466–2481. 10.1093/cercor/bhaa367 [DOI] [PubMed] [Google Scholar]
- Ezaki, T. , Sakaki, M. , Watanabe, T. , & Masuda, N. (2018). Age‐related changes in the ease of dynamical transitions in human brain activity. Human Brain Mapping, 39(6), 2673–2688. 10.1002/hbm.24033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farras‐Permanyer, L. , Mancho‐Fora, N. , Montalà‐Flaquer, M. , Bartrés‐Faz, D. , Vaqué‐Alcázar, L. , Peró‐Cebollero, M. , & Guàrdia‐Olmos, J. (2019). Age‐related changes in resting‐state functional connectivity in older adults. Neural Regeneration Research, 14(9), 1544–1555. 10.4103/1673-5374.255976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg, D. A. , & Setsompop, K. (2013). Ultra‐fast MRI of the human brain with simultaneous multi‐slice imaging. Journal of Magnetic Resonance, 229, 90–100. 10.1016/j.jmr.2013.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg, D. A. , Moeller, S. , Smith, S. M. , Auerbach, E. , Ramanna, S. , Gunther, M. , Glasser, M. F. , Miller, K. L. , Ugurbil, K. , & Yacoub, E. (2010). Multiplexed echo planar imaging for sub‐second whole brain FMRI and fast diffusion imaging. PLoS One, 5(12), e15710. 10.1371/journal.pone.0015710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira, L. K. , & Busatto, G. F. (2013). Resting‐state functional connectivity in normal brain aging. Neuroscience and Biobehavioral Reviews, 37(3), 384–400. 10.1016/j.neubiorev.2013.01.017 [DOI] [PubMed] [Google Scholar]
- Ferreira, L. K. , Regina, A. C. , Kovacevic, N. , Martin Mda, G. , Santos, P. P. , Carneiro Cde, G. , Kerr, D. S. , Amaro, E. , McIntosh, A. , & Busatto, G. F. (2016). Aging effects on whole‐brain functional connectivity in adults free of cognitive and psychiatric disorders. Cerebral Cortex, 26(9), 3851–3865. 10.1093/cercor/bhv190 [DOI] [PubMed] [Google Scholar]
- Finn, E. S. , Shen, X. , Scheinost, D. , Rosenberg, M. D. , Huang, J. , Chun, M. M. , Papademetris, X. , & Constable, R. T. (2015). Functional connectome fingerprinting: Identifying individuals using patterns of brain connectivity. Nature Neuroscience, 18(11), 1664–1671. 10.1038/nn.4135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell, A. M. , Sneve, M. H. , Grydeland, H. , Storsve, A. B. , & Walhovd, K. B. (2017). The disconnected brain and executive function decline in aging. Cerebral Cortex, 27(3), 2303–2317. 10.1093/cercor/bhw082 [DOI] [PubMed] [Google Scholar]
- Fornito, A. , & Bullmore, E. T. (2015). Connectomics: A new paradigm for understanding brain disease. European Neuropsychopharmacology: The Journal of the European College of Neuropsychopharmacology, 25(5), 733–748. 10.1016/j.euroneuro.2014.02.011 [DOI] [PubMed] [Google Scholar]
- Fornito, A. , Zalesky, A. , & Breakspear, M. (2015). The connectomics of brain disorders. Nature reviews. Neuroscience, 16(3), 159–172. 10.1038/nrn390 [DOI] [PubMed] [Google Scholar]
- Galiano, A. , Mengual, E. , García de Eulate, R. , Galdeano, I. , Vidorreta, M. , Recio, M. , Riverol, M. , Zubieta, J. L. , & Fernández‐Seara, M. A. (2020). Coupling of cerebral blood flow and functional connectivity is decreased in healthy aging. Brain Imaging and Behavior, 14(2), 436–450. 10.1007/s11682-019-00157-w [DOI] [PubMed] [Google Scholar]
- Gallen, C. L. , Turner, G. R. , Adnan, A. , & D'Esposito, M. (2016). Reconfiguration of brain network architecture to support executive control in aging. Neurobiology of Aging, 44, 42–52. 10.1016/j.neurobiolaging.2016.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett, D. D. , Kovacevic, N. , McIntosh, A. R. , & Grady, C. L. (2010). Blood oxygen level‐dependent signal variability is more than just noise. Journal of Neuroscience, 30(14), 4914–4921. 10.1523/jneurosci.5166-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett, D. D. , Kovacevic, N. , McIntosh, A. R. , & Grady, C. L. (2010). Blood oxygen level‐dependent signal variability is more than just noise. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 30(14), 4914–4921. 10.1523/JNEUROSCI.5166-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett, D. D. , Kovacevic, N. , McIntosh, A. R. , & Grady, C. L. (2011). The importance of being variable. The Journal of Neuroscience, 31(12), 4496–4503. 10.1523/JNEUROSCI.5641-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett, D. D. , Lindenberger, U. , Hoge, R. D. , & Gauthier, C. J. (2017). Age differences in brain signal variability are robust to multiple vascular controls. Scientific Reports, 7(1), 10149. 10.1038/s41598-017-09752-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzaley, A. , & D'Esposito, M. (2003). The contribution of functional brain imaging to our understanding of cognitive aging. Science of Aging Knowledge Environment: SAGE KE, 2003(4), PE2. 10.1126/sageke.2003.4.pe2 [DOI] [PubMed] [Google Scholar]
- Geerligs, L. , Maurits, N. M. , Renken, R. J. , & Lorist, M. M. (2014). Reduced specificity of functional connectivity in the aging brain during task performance. Human Brain Mapping, 35(1), 319–330. 10.1002/hbm.22175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerligs, L. , Renken, R. J. , Saliasi, E. , Maurits, N. M. , & Lorist, M. M. (2015). A brain‐wide study of age‐related changes in functional connectivity. Cerebral Cortex, 25(7), 1987–1999. 10.1093/cercor/bhu012 [DOI] [PubMed] [Google Scholar]
- Geerligs, L. , Saliasi, E. , Maurits, N. M. , & Lorist, M. M. (2012). Compensation through increased functional connectivity: Neural correlates of inhibition in old and young. Journal of Cognitive Neuroscience, 24(10), 2057–2069. 10.1162/jocn_a_00270 [DOI] [PubMed] [Google Scholar]
- Geerligs, L. , Tsvetanov, K. A. , & Cam‐Can, & Henson, R. N. (2017). Challenges in measuring individual differences in functional connectivity using fMRI: The case of healthy aging. Human Brain Mapping, 38(8), 4125–4156. 10.1002/hbm.23653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genon, S. , Reid, A. , Langner, R. , Amunts, K. , & Eickhoff, S. B. (2018). How to characterize the function of a brain region. Trends in Cognitive Sciences, 22(4), 350–364. 10.1016/j.tics.2018.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgio, A. , Zhang, J. , Costantino, F. , De Stefano, N. , & Frezzotti, P. (2020). Altered large‐scale brain functional connectivity in ocular hypertension. Frontiers in Neuroscience, 14, 146. 10.3389/fnins.2020.00146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glisky, E. L. (2007). Changes in cognitive function in human aging. In Riddle D. R. (Ed.), Brain aging: Models, methods, and mechanisms. CRC Press/Taylor & Francis; 2007. Chapter 1. ISBN‐10: 0–8493–3818‐2. [Google Scholar]
- Goh, J. O. (2011). Functional dedifferentiation and altered connectivity in older adults: Neural accounts of cognitive aging. Aging and Disease, 2(1), 30–48 ISSN: 2152‐5250. [PMC free article] [PubMed] [Google Scholar]
- Goldstone, A. , Mayhew, S. D. , Przezdzik, I. , Wilson, R. S. , Hale, J. R. , & Bagshaw, A. P. (2016). Gender specific re‐organization of resting‐state networks in older age. Frontiers in Aging Neuroscience, 8, 285. 10.3389/fnagi.2016.00285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon, E. M. , Laumann, T. O. , Gilmore, A. W. , Newbold, D. J. , Greene, D. J. , Berg, J. J. , Ortega, M. , Hoyt‐Drazen, C. , Gratton, C. , Sun, H. , Hampton, J. M. , Coalson, R. S. , Nguyen, A. L. , McDermott, K. B. , Shimony, J. S. , Snyder, A. Z. , Schlagga, B. L. , Petersen, S. E. , Nelson, S. M. , & Dosenbach, N. (2017). Precision functional mapping of individual human brains. Neuron, 95(4), 791–807.e7. 10.1016/j.neuron.2017.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady, C. (2012). The cognitive neuroscience of ageing. Nature Reviews Neuroscience, 13(7), 491–505. 10.1038/nrn3256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady, C. L. (2017). Age differences in functional connectivity at rest and during cognitive tasks. In Cabeza R., Nyberg L., & Park D. (Eds.), Cognitive neuroscience of aging: Linking cognitive and cerebral aging. Published to Oxford Scholarship Online, 2009. 10.1093/acprof:oso/9780195156744.001.0001 [DOI] [Google Scholar]
- Grady, C. L. , & Garrett, D. D. (2018). Brain signal variability is modulated as a function of internal and external demand in younger and older adults. NeuroImage, 169, 510–523. 10.1016/j.neuroimage.2017.12.031 [DOI] [PubMed] [Google Scholar]
- Grady, C. , Sarraf, S. , Saverino, C. , & Campbell, K. (2016). Age differences in the functional interactions among the default, frontoparietal control, and dorsal attention networks. Neurobiology of Aging, 41, 159–172. 10.1016/j.neurobiolaging.2016.02.020 [DOI] [PubMed] [Google Scholar]
- Grayson, B. E. , Seeley, R. J. , & Sandoval, D. A. (2013). Wired on sugar: The role of the CNS in the regulation of glucose homeostasis. Nature reviews. Neuroscience, 14(1), 24–37. 10.1038/nrn3409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyatt, G. H. , Oxman, A. D. , Vist, G. E. , Kunz, R. , Falck‐Ytter, Y. , Alonso‐Coello, P. , Schünemann, H. J. , & GRADE Working Group . (2008). GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. British Medical Journal (Clinical Research Ed.), 336(7650), 924–926. https://doi.org/10.1136/bmj.39489.470347.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagmann, P. , Sporns, O. , Madan, N. , Cammoun, L. , Pienaar, R. , Wedeen, V. J. , Meuli, R. , Thiran, J. P. , & Grant, P. E. (2010). White matter maturation reshapes structural connectivity in the late developing human brain. Proceedings of the National Academy of Sciences of the United States of America, 107(44), 19067–19072. 10.1073/pnas.1009073107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn, A. , Breakspear, M. , Rischka, L. , Wadsak, W. , Godbersen, G. M. , Pichler, V. , Michenthaler, P. , Vanicek, T. , Hacker, M. , Kasper, S. , Lanzenberger, R. , & Cocchi, L. (2020). Reconfiguration of functional brain networks and metabolic cost converge during task performance. eLife, 9, e52443. 10.7554/eLife.52443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakun, J. G. , Zhu, Z. , Johnson, N. F. , & Gold, B. T. (2015). Evidence for reduced efficiency and successful compensation in older adults during task switching. Cortex, 64, 352–362. 10.1016/j.cortex.2014.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, L. , Savalia, N. K. , Chan, M. Y. , Agres, P. F. , Nair, A. S. , & Wig, G. S. (2018). Functional parcellation of the cerebral cortex across the human adult lifespan. Cerebral Cortex, 28(12), 4403–4423. 10.1093/cercor/bhy218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada, C. N. , Natelson Love, M. C. , & Triebel, K. L. (2013). Normal cognitive aging. Clinics in Geriatric Medicine, 29(4), 737–752. 10.1016/j.cger.2013.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hase, Y. , Ding, R. , Harrison, G. , Hawthorne, E. , King, A. , Gettings, S. , Platten, C. , Stevenson, W. , Craggs, L. J. L. , & Kalaria, R. N. (2019). White matter capillaries in vascular and neurodegenerative dementias. Acta Neuropathologica Communications, 7(1), 1–12. 10.1186/s40478-019-0666-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, L. , Wang, X. , Zhuang, K. , & Qiu, J. (2020). Decreased dynamic segregation but increased dynamic integration of the resting‐state functional networks during normal aging. Neuroscience, 437, 54–63. 10.1016/j.neuroscience.2020.04.030 [DOI] [PubMed] [Google Scholar]
- Hedden, T. , Schultz, A. P. , Rieckmann, A. , Mormino, E. C. , Johnson, K. A. , Sperling, R. A. , & Buckner, R. L. (2016). Multiple brain markers are linked to age‐related variation in cognition. Cerebral Cortex, 26(4), 1388–1400. 10.1093/cercor/bhu238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog, H. , Iida, H. , & Caldeira, L. (2019). Kinetic modelling and extraction of metabolic. In Shah N. J. (Ed.), Hybrid MR‐PET imaging: Systems, methods and applications. Royal Society of Chemistry. 10.1039/9781788013062 [DOI] [Google Scholar]
- Horwitz, B. , Duara, R. , & Rapoport, S. I. (1984). Intercorrelations of glucose metabolic rates between brain regions: Application to healthy males in a state of reduced sensory input. Journal of Cerebral Blood Flow and Metabolism, 4(4), 484–499. 10.1038/jcbfm.1984.73 [DOI] [PubMed] [Google Scholar]
- Horwitz, B. , Duara, R. , & Rapoport, S. I. (1986). Age differences in intercorrelations between regional cerebral metabolic rates for glucose. Annals of Neurology, 19(1), 60–67. 10.1002/ana.410190111 [DOI] [PubMed] [Google Scholar]
- Hou, X. , Liu, P. , Gu, H. , Chan, M. , Li, Y. , Peng, S. L. , Wig, G. , Yang, Y. , Park, D. , & Lu, H. (2019). Estimation of brain functional connectivity from hypercapnia BOLD MRI data: Validation in a lifespan cohort of 170 subjects. NeuroImage, 186, 455–463. 10.1016/j.neuroimage.2018.11.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrybouski, S. , Cribben, I. , McGonigle, J. , Olsen, F. , Carter, R. , Seres, P. , Madan, C. R. , & Malykhin, N. V. (2021). Investigating the effects of healthy cognitive aging on brain functional connectivity using 4.7 T resting‐state functional magnetic resonance imaging. Brain Structure & Function, 226(4), 1067–1098. 10.1007/s00429-021-02226-7 [DOI] [PubMed] [Google Scholar]
- Hu, Y. , Xu, Q. , Shen, J. , Li, K. , Zhu, H. , Zhang, Z. , & Lu, G. (2015). Small‐worldness and gender differences of large scale brain metabolic covariance networks in young adults: A FDG PET study of 400 subjects. Acta Radiologica, 56(2), 204–213. 10.1177/0284185114529106 [DOI] [PubMed] [Google Scholar]
- Huang, C. C. , Hsieh, W. J. , Lee, P. L. , Peng, L. N. , Liu, L. K. , Lee, W. J. , Huang, J. K. , Chen, L. K. , & Lin, C. P. (2015). Age‐related changes in resting‐state networks of a large sample size of healthy elderly. CNS Neuroscience & Therapeutics, 21(10), 817–825. 10.1111/cns.12396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huettel, S. A. , & McCarthy, G. (2000). Evidence for a refractory period in the hemodynamic response to visual stimuli as measured by MRI. NeuroImage, 11(5 Pt 1), 547–553. 10.1006/nimg.2000.0553 [DOI] [PubMed] [Google Scholar]
- Huettel, S. A. , Singerman, J. D. , & McCarthy, G. (2001). The effects of aging upon the hemodynamic response measured by functional MRI. NeuroImage, 13(1), 161–175. 10.1006/nimg.2000.0675 [DOI] [PubMed] [Google Scholar]
- Hughes, C. , Faskowitz, J. , Cassidy, B. S. , Sporns, O. , & Krendl, A. C. (2020). Aging relates to a disproportionately weaker functional architecture of brain networks during rest and task states. NeuroImage, 209, 116521. 10.1016/j.neuroimage.2020.116521 [DOI] [PubMed] [Google Scholar]
- Hutchison, R. M. , Womelsdorf, T. , Allen, E. A. , Bandettini, P. A. , Calhoun, V. D. , Corbetta, M. , Della Penna, S. , Duyn, J. H. , Glover, G. H. , Gonzalez‐Castillo, J. , Handwerker, D. A. , Keilholz, S. , Kiviniemi, V. , Leopold, D. A. , de Pasquale, F. , Sporns, O. , Walter, M. , & Chang, C. (2013). Dynamic functional connectivity: Promise, issues, and interpretations. NeuroImage, 80, 360–378. 10.1016/j.neuroimage.2013.05.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang, K. , Hallquist, M. N. , & Luna, B. (2013). The development of hub architecture in the human functional brain network. Cerebral Cortex, 23(10), 2380–2393. 10.1093/cercor/bhs227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iordan, A. D. , Cooke, K. A. , Moored, K. D. , Katz, B. , Buschkuehl, M. , Jaeggi, S. M. , Jonides, J. , Peltier, S. J. , Polk, T. A. , & Reuter‐Lorenz, P. A. (2018). Aging and network properties: Stability over time and links with learning during working memory training. Frontiers in Aging Meuroscience, 9, 419. 10.3389/fnagi.2017.00419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iozzo, P. , & Guzzardi, M. A. (2019). Imaging of brain glucose uptake by PET in obesity and cognitive dysfunction: Life‐course perspective. Endocrine Connections, 8(11), R169–R183. 10.1530/EC-19-0348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamadar, S. D. , Egan, G. F. , Calhoun, V. D. , Johnson, B. , & Fielding, J. (2016). Intrinsic connectivity provides the baseline framework for variability in motor performance: A multivariate fusion analysis of low‐ and high‐frequency resting‐state oscillations and antisaccade performance. Brain Connectivity, 6(6), 505–517. 10.1089/brain.2015.0411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamadar, S. D. , Ward, P. G. D. , Liang, E. X. , Orchard, E. R. , Chen, Z. , & Egan, G. F. (2021). Metabolic and hemodynamic resting‐state connectivity of the human rain: A high‐temporal resolution simultaneous BOLD‐fMRI and FDG‐fPET multimodality study. Cerebral Cortex, 31(6), 2855–2867. 10.1093/cercor/bhaa393 [DOI] [PubMed] [Google Scholar]
- Jamadar, S. D. , Ward, P. G. , Li, S. , Sforazzini, F. , Baran, J. , Chen, Z. , & Egan, G. F. (2019). Simultaneous task‐based BOLD‐fMRI and [18‐F] FDG functional PET for measurement of neuronal metabolism in the human visual cortex. NeuroImage, 189, 258–266. 10.1016/j.neuroimage.2019.01.003 [DOI] [PubMed] [Google Scholar]
- Jamadar, S. D. , Ward, P. , Close, T. G. , Fornito, A. , Premaratne, M. , O'Brien, K. , Stäb, D. , Chen, Z. , Shah, N. J. , & Egan, G. F. (2020). Simultaneous BOLD‐fMRI and constant infusion FDG‐PET data of the resting human brain. Scientific Data, 7(1), 363. 10.1038/s41597-020-00699-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamadar, S. , Michie, P. , & Karayanidis, F. (2010). Compensatory mechanisms underlie intact task‐switching performance in schizophrenia. Neuropsychologia, 48(5), 1305–1323. 10.1016/j.neuropsychologia.2009.12.034 [DOI] [PubMed] [Google Scholar]
- Jiang, J. , Liu, T. , Crawford, J. D. , Kochan, N. A. , Brodaty, H. , Sachdev, P. S. , & Wen, W. (2020). Stronger bilateral functional connectivity of the frontoparietal control network in near‐centenarians and centenarians without dementia. NeuroImage, 215, 116855. 10.1016/j.neuroimage.2020.116855 [DOI] [PubMed] [Google Scholar]
- Jockwitz, C. , Caspers, S. , Lux, S. , Eickhoff, S. B. , Jütten, K. , Lenzen, S. , Moebus, S. , Pundt, N. , Reid, A. , Hoffstaedter, F. , Jöckel, K. H. , Erbel, R. , Cichon, S. , Nöthen, M. M. , Shah, N. J. , Zilles, K. , & Amunts, K. (2017). Influence of age and cognitive performance on resting‐state brain networks of older adults in a population‐based cohort. Cortex, 89, 28–44. 10.1016/j.cortex.2017.01.008 [DOI] [PubMed] [Google Scholar]
- Kalaria, R. N. , Hase, Y. , & Ihara, M. (2019). The rise and rise of cerebral small vessel disease: Implications for vascular cognitive impairment and dementia. Future Neurology, 14(2), FNL11. 10.2217/fnl-2019-0004 [DOI] [Google Scholar]
- Keulers, E. , Birkisdóttir, M. B. , Falbo, L. , de Bruin, A. , & Stiers, P. (2019). Age‐related differences in task‐induced brain activation is not task specific: Multivariate pattern generalization between metacognition, cognition and perception. NeuroImage, 188, 309–321. 10.1016/j.neuroimage.2018.12.014 [DOI] [PubMed] [Google Scholar]
- Keunen, K. , Counsell, S. J. , & Benders, M. (2017). The emergence of functional architecture during early brain development. NeuroImage, 160, 2–14. 10.1016/j.neuroimage.2017.01.047 [DOI] [PubMed] [Google Scholar]
- Kim, J. , Chey, J. , Kim, S. E. , & Kim, H. (2015). The effect of education on regional brain metabolism and its functional connectivity in an aged population utilizing positron emission tomography. Neuroscience Research, 94, 50–61. 10.1016/j.neures.2014.12.009 [DOI] [PubMed] [Google Scholar]
- King, B. R. , van Ruitenbeek, P. , Leunissen, I. , Cuypers, K. , Heise, K. F. , Santos Monteiro, T. , Hermans, L. , Levin, O. , Albouy, G. , Mantini, D. , & Swinnen, S. P. (2018). Age‐related declines in motor performance are associated with decreased segregation of large‐scale resting state brain networks. Cerebral Cortex, 28(12), 4390–4402. 10.1093/cercor/bhx297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong, T. S. , Gratton, C. , Low, K. A. , Tan, C. H. , Chiarelli, A. M. , Fletcher, M. A. , Zimmerman, B. , Maclin, E. L. , Sutton, B. P. , Gratton, G. , & Fabiani, M. (2020). Age‐related differences in functional brain network segregation are consistent with a cascade of cerebrovascular, structural, and cognitive effects. Network Neuroscience, 4(1), 89–114. 10.1162/netn_a_00110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl, D. E. , Metter, E. J. , Riege, W. H. , & Phelps, M. E. (1982). Effects of human aging on patterns of local cerebral glucose utilization determined by the [18F]fluorodeoxyglucose method. Journal of Cerebral Blood Flow and Metabolism, 2(2), 163–171. 10.1038/jcbfm.1982.15 [DOI] [PubMed] [Google Scholar]
- Lee, A. , Ratnarajah, N. , Tuan, T. A. , Chen, S. H. , & Qiu, A. (2015). Adaptation of brain functional and structural networks in aging. PLoS One, 10(4), e0123462. 10.1371/journal.pone.0123462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann, D. , Ozaki, H. , & Pal, I. (1987). EEG alpha map series: Brain micro‐states by space‐oriented adaptive segmentation. Electroencephalography and Clinical Neurophysiology, 67(3), 271–288. 10.1016/0013-4694(87)90025-3 [DOI] [PubMed] [Google Scholar]
- Li, Q. , Dong, C. , Liu, T. , Chen, X. , Perry, A. , Jiang, J. , Cheng, J. , Niu, H. , Kochan, N. A. , Brodaty, H. , Sachdev, P. S. , & Wen, W. (2020). Longitudinal changes in whole‐brain functional connectivity strength patterns and the relationship with the global cognitive decline in older adults. Frontiers in Aging Neuroscience, 12, 71. 10.3389/fnagi.2020.00071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, S. , Jamadar, S. D. , Ward, P. , Premaratne, M. , Egan, G. F. , & Chen, Z. (2020). Analysis of continuous infusion functional PET (fPET) in the human brain. NeuroImage, 213, 116720. 10.1016/j.neuroimage.2020.116720 [DOI] [PubMed] [Google Scholar]
- Li, W. , Wang, M. , Li, Y. , Huang, Y. , & Chen, X. (2016). A novel brain network construction method for exploring age‐related functional reorganization. Computational Intelligence and Neuroscience, 2016, 2429691. 10.1155/2016/2429691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y. , Li, C. , Wu, Q. , Xu, Z. , Kurata, T. , Ohno, S. , Kanazawa, S. , Abe, K. , & Wu, J. (2015). Decreased resting‐state connections within the visuospatial attention‐related network in advanced aging. Neuroscience Letters, 597, 13–18. 10.1016/j.neulet.2015.03.047 [DOI] [PubMed] [Google Scholar]
- Li, Z. , Moore, A. B. , Tyner, C. , & Hu, X. (2009). Asymmetric connectivity reduction and its relationship to “HAROLD” in aging brain. Brain Research, 1295, 149–158. 10.1016/j.brainres.2009.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang, X. , Zou, Q. , He, Y. , & Yang, Y. (2013). Coupling of functional connectivity and regional cerebral blood flow reveals a physiological basis for network hubs of the human brain. Proceedings of the National Academy of Sciences of the United States of America, 110(5), 1929–1934. 10.1073/pnas.1214900110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao, X. , Vasilakos, A. V. , & He, Y. (2017). Small‐world human brain networks: Perspectives and challenges. Neuroscience and Biobehavioral Reviews, 77, 286–300. 10.1016/j.neubiorev.2017.03.018 [DOI] [PubMed] [Google Scholar]
- Lindbergh, C. A. , Zhao, Y. , Lv, J. , Mewborn, C. M. , Puente, A. N. , Terry, D. P. , Renzi‐Hammond, L. M. , Hammond, B. R. , Liu, T. , & Miller, L. S. (2019). Intelligence moderates the relationship between age and inter‐connectivity of resting state networks in older adults. Neurobiology of Aging, 78, 121–129. 10.1016/j.neurobiolaging.2019.02.014 [DOI] [PubMed] [Google Scholar]
- Liu, D. , Duan, S. , Wei, P. , Chen, L. , Wang, J. , & Zhang, J. (2020). Aberrant brain spontaneous activity and synchronization in type 2 diabetes mellitus patients: A resting‐state functional MRI study. Frontiers in Aging Neuroscience, 12, 181. 10.3389/fnagi.2020.00181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Z. , Ke, L. , Liu, H. , Huang, W. , & Hu, Z. (2014). Changes in topological organization of functional PET brain network with normal aging. PLoS One, 9(2), e88690. 10.1371/journal.pone.0088690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liégeois, R. , Laumann, T. O. , Snyder, A. Z. , Zhou, J. , & Yeo, B. (2017). Interpreting temporal fluctuations in resting‐state functional connectivity MRI. NeuroImage, 163, 437–455. 10.1016/j.neuroimage.2017.09.012 [DOI] [PubMed] [Google Scholar]
- Love, S. , & Miners, J. S. (2016). Cerebrovascular disease in ageing and Alzheimer's disease. Acta Neuropathologica, 131(5), 645–658. 10.1007/s00401-015-1522-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo, N. , Sui, J. , Abrol, A. , Lin, D. , Chen, J. , Vergara, V. M. , Fu, Z. , Du, Y. , Damaraju, E. , Xu, Y. , Turner, J. A. , & Calhoun, V. D. (2020). Age‐related structural and functional variations in 5,967 individuals across the adult lifespan. Human Brain Mapping, 41(7), 1725–1737. 10.1002/hbm.24905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lurie, D. J. , Kessler, D. , Bassett, D. S. , Betzel, R. F. , Breakspear, M. , Kheilholz, S. , Kucyi, A. , Liégeois, R. , Lindquist, M. A. , McIntosh, A. , Poldrack, R. A. , Shine, J. M. , Thompson, W. H. , Bielczyk, N. Z. , Douw, L. , Kraft, D. , Miller, R. L. , Muthuraman, M. , Pasquini, L. , … Calhoun, V. D. (2020). Questions and controversies in the study of time‐varying functional connectivity in resting fMRI. Network Neuroscience, 4(1), 30–69. 10.1162/netn_a_00116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden, D. J. , Parks, E. L. , Tallman, C. W. , Boylan, M. A. , Hoagey, D. A. , Cocjin, S. B. , Packard, L. E. , Johnson, M. A. , Chou, Y. H. , Potter, G. G. , Chen, N. K. , Siciliano, R. E. , Monge, Z. A. , Honig, J. A. , & Diaz, M. T. (2017). Sources of disconnection in neurocognitive aging: Cerebral white‐matter integrity, resting‐state functional connectivity, and white‐matter hyperintensity volume. Neurobiology of Aging, 54, 199–213. 10.1016/j.neurobiolaging.2017.01.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhyastha, T. M. , & Grabowski, T. J. (2014). Age‐related differences in the dynamic architecture of intrinsic networks. Brain Connectivity, 4(4), 231–241. 10.1089/brain.2013.0205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malagurski, B. , Liem, F. , Oschwald, J. , Mérillat, S. , & Jäncke, L. (2020). Functional dedifferentiation of associative resting state networks in older adults ‐ A longitudinal study. NeuroImage, 214, 116680. 10.1016/j.neuroimage.2020.116680 [DOI] [PubMed] [Google Scholar]
- Manschot, S. M. , Brands, A. M. , van der Grond, J. , Kessels, R. P. , Algra, A. , Kappelle, L. J. , Biessels, G. J. , & Utrecht Diabetic Encephalopathy Study Group . (2006). Brain magnetic resonance imaging correlates of impaired cognition in patients with type 2 diabetes. Diabetes, 55(4), 1106–1113. 10.2337/diabetes.55.04.06.db05-1323 [DOI] [PubMed] [Google Scholar]
- Manza, P. , Wiers, C. E. , Shokri‐Kojori, E. , Kroll, D. , Feldman, D. , Schwandt, M. , Wang, G. J. , Tomasi, D. , & Volkow, N. D. (2020). Brain network segregation and glucose energy utilization: Relevance for age‐eelated differences in cognitive function. Cerebral Cortex, 30(11), 5930–5942. 10.1093/cercor/bhaa167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marstaller, L. , Williams, M. , Rich, A. , Savage, G. , & Burianová, H. (2015). Aging and large‐scale functional networks: White matter integrity, gray matter volume, and functional connectivity in the resting state. Neuroscience, 290, 369–378. 10.1016/j.neuroscience.2015.01.049 [DOI] [PubMed] [Google Scholar]
- McCarthy, P. , Benuskova, L. , & Franz, E. A. (2014). The age‐related posterior‐anterior shift as revealed by voxelwise analysis of functional brain networks. Frontiers in Aging Neuroscience, 6, 301. 10.3389/fnagi.2014.00301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medaglia, J. D. , Lynall, M.‐E. , & Bassett, D. S. (2015). Cognitive network neuroscience. Journal of Cognitive Neuroscience, 27(8), 1471–1491. 10.1162/jocn_a_00810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mergenthaler, P. , Lindauer, U. , Dienel, G. A. , & Meisel, A. (2013). Sugar for the brain: The role of glucose in physiological and pathological brain function. Trends in Neurosciences, 36(10), 587–597. 10.1016/j.tins.2013.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metter, E. J. , Riege, W. H. , Kuhl, D. E. , & Phelps, M. E. (1984). Cerebral metabolic relationships for selected brain regions in healthy adults. Journal of Cerebral Blood Flow and Metabolism, 4(1), 1–7. 10.1038/jcbfm.1984.1 [DOI] [PubMed] [Google Scholar]
- Meunier, D. , Achard, S. , Morcom, A. , & Bullmore, E. (2009). Age‐related changes in modular organization of human brain functional networks. NeuroImage, 44(3), 715–723. 10.1016/j.neuroimage.2008.09.062 [DOI] [PubMed] [Google Scholar]
- Michel, C. M. , & Koenig, T. (2018). EEG microstates as a tool for studying the temporal dynamics of whole‐brain neuronal networks: A review. NeuroImage, 180(Pt B), 577–593. 10.1016/j.neuroimage.2017.11.062 [DOI] [PubMed] [Google Scholar]
- Mill, R. D. , Gordon, B. A. , Balota, D. A. , & Cole, M. W. (2020). Predicting dysfunctional age‐related task activations from resting‐state network alterations. NeuroImage, 221, 117167. 10.1016/j.neuroimage.2020.117167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda‐Dominguez, O. , Feczko, E. , Grayson, D. S. , Walum, H. , Nigg, J. T. , & Fair, D. A. (2018). Heritability of the human connectome: A connectotyping study. Network Neuroscience, 2(2), 175–199. 10.1162/netn_a_00029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller, J. R. , Ishikawa, T. , Dhawan, V. , Spetsieris, P. , Mandel, F. , Alexander, G. E. , Grady, C. , Pietrini, P. , & Eidelberg, D. (1996). The metabolic topography of normal aging. Journal of Cerebral Blood Flow and Metabolism, 16(3), 385–398. 10.1097/00004647-199605000-00005 [DOI] [PubMed] [Google Scholar]
- Monteiro, T. S. , King, B. R. , Zivari Adab, H. , Mantini, D. , & Swinnen, S. P. (2019). Age‐related differences in network flexibility and segregation at rest and during motor performance. NeuroImage, 194, 93–104. 10.1016/j.neuroimage.2019.03.015 [DOI] [PubMed] [Google Scholar]
- Monti, R. P. , Gibberd, A. , Roy, S. , Nunes, M. , Lorenz, R. , Leech, R. , Ogawa, T. , Kawanabe, M. , & Hyvärinen, A. (2020). Interpretable brain age prediction using linear latent variable models of functional connectivity. PLoS One, 15(6), e0232296. 10.1371/journal.pone.0232296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran, C. , Beare, R. , Phan, T. , Starkstein, S. , Bruce, D. , Romina, M. , & Srikanth, V. (2017). Neuroimaging and its relevance to understanding pathways linking diabetes and cognitive dysfunction. Journal of Alzheimer's Disease, 59(2), 405–419. 10.3233/JAD-161166 [DOI] [PubMed] [Google Scholar]
- Morrison, J. H. , & Baxter, M. G. (2012). The ageing cortical synapse: Hallmarks and implications for cognitive decline. Nature reviews. Neuroscience, 13(4), 240–250. 10.1038/nrn3200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowinckel, A. M. , Espeseth, T. , & Westlye, L. T. (2012). Network‐specific effects of age and in‐scanner subject motion: A resting‐state fMRI study of 238 healthy adults. NeuroImage, 63(3), 1364–1373. 10.1016/j.neuroimage.2012.08.004 [DOI] [PubMed] [Google Scholar]
- Mujica‐Parodi, L. R. , Amgalan, A. , Sultan, S. F. , Antal, B. , Sun, X. , Skiena, S. , Lithen, A. , Adra, N. , Ratai, E. M. , Weistuch, C. , Govindarajan, S. T. , Strey, H. H. , Dill, K. A. , Stufflebeam, S. M. , Veech, R. L. , & Clarke, K. (2020). Diet modulates brain network stability, a biomarker for brain aging, in young adults. Proceedings of the National Academy of Sciences of the United States of America, 117(11), 6170–6177. 10.1073/pnas.1913042117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murad, M. H. , Mustafa, R. A. , Schünemann, H. J. , Sultan, S. , & Santesso, N. (2017). Rating the certainty in evidence in the absence of a single estimate of effect. Evidence‐Based Medicine, 22(3), 85–87. 10.1136/ebmed-2017-110668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musen, G. , Jacobson, A. M. , Bolo, N. R. , Simonson, D. C. , Shenton, M. E. , McCartney, R. L. , Flores, V. L. , & Hoogenboom, W. S. (2012). Resting‐state brain functional connectivity is altered in type 2 diabetes. Diabetes, 61(9), 2375–2379. 10.2337/db11-1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik, S. , Banerjee, A. , Bapi, R. S. , Deco, G. , & Roy, D. (2017). Metastability in senescence. Trends in Cognitive Sciences, 21(7), 509–521. 10.1016/j.tics.2017.04.007 [DOI] [PubMed] [Google Scholar]
- Nashiro, K. , Sakaki, M. , Braskie, M. N. , & Mather, M. (2017). Resting‐state networks associated with cognitive processing show more age‐related decline than those associated with emotional processing. Neurobiology of Aging, 54, 152–162. 10.1016/j.neurobiolaging.2017.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng, K. K. , Lo, J. C. , Lim, J. , Chee, M. , & Zhou, J. (2016). Reduced functional segregation between the default mode network and the executive control network in healthy older adults: A longitudinal study. NeuroImage, 133, 321–330. 10.1016/j.neuroimage.2016.03.029 [DOI] [PubMed] [Google Scholar]
- Niu, H. , Zhu, J. , Wang, C. , Zhu, L. , & Wu, J. (2019). Changes in white‐matter functional network efficiency across the adult lifespan. Neuroreport, 30(8), 600–604. 10.1097/WNR.0000000000001255 [DOI] [PubMed] [Google Scholar]
- Nomi, J. S. , Bolt, T. S. , Ezie, C. , Uddin, L. Q. , & Heller, A. S. (2017). Moment‐to‐moment BOLD signal variability reflects regional changes in neural flexibility across the lifespan. The Journal of Neuroscience, 37(22), 5539–5548. 10.1523/JNEUROSCI.3408-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent, S. , Tremblay, S. , Chen, K. W. , Ayutyanont, N. , Roontiva, A. , Castellano, C. A. , Fortier, M. , Roy, M. , Courchesne‐Loyer, A. , Bocti, C. , Lepage, M. , Turcotte, E. , Fulop, T. , Reiman, E. M. , & Cunnane, S. C. (2014). Brain glucose and acetoacetate metabolism: A comparison of young and older adults. Neurobiology of Aging, 35(6), 1386–1395. 10.1016/j.neurobiolaging.2013.11.027 [DOI] [PubMed] [Google Scholar]
- O'Connell, M. A. , & Basak, C. (2018). Effects of task complexity and age‐differences on task‐related functional connectivity of attentional networks. Neuropsychologia, 114, 50–64. 10.1016/j.neuropsychologia.2018.04.013 [DOI] [PubMed] [Google Scholar]
- Onoda, K. , & Yamaguchi, S. (2013). Small‐worldness and modularity of the resting‐state functional brain network decrease with aging. Neuroscience Letters, 556, 104–108. 10.1016/j.neulet.2013.10.023 [DOI] [PubMed] [Google Scholar]
- Onoda, K. , Ishihara, M. , & Yamaguchi, S. (2012). Decreased functional connectivity by aging is associated with cognitive decline. Journal of Cognitive Neuroscience, 24(11), 2186–2198. 10.1162/jocn_a_00269 [DOI] [PubMed] [Google Scholar]
- Oschmann, M. , & Gawryluk, J. R. (2020). A longitudinal study of changes in resting‐state functional magnetic resonance imaging functional connectivity networks during healthy aging. Brain Connectivity, 10(7), 377–384. 10.1089/brain.2019.0724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page, M. J. , McKenzie, J. E. , Bossuyt, P. M. , Boutron, I. , Hoffmann, T. C. , Mulrow, C. D. , Shamseer, L. , Tetzlaff, J. M. , Akl, E. A. , Brennan, S. E. , Chou, R. , Glanville, J. , Grimshaw, J. M. , Hróbjartsson, A. , Lalu, M. M. , Li, T. , Loder, E. W. , Mayo‐Wilson, E. , McDonald, S. , … Moher, D. (2021). The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. British Medical Journal (Clinical Research Ed.), 372, n71. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, D. C. , Polk, T. A. , Park, R. , Minear, M. , Savage, A. , & Smith, M. R. (2004). Aging reduces neural specialization in ventral visual cortex. Proceedings of the National Academy of Sciences of the United States of America, 101(35), 13091–13095. 10.1073/pnas.0405148101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, J. E. , Jung, S. C. , Ryu, K. H. , Oh, J. Y. , Kim, H. S. , Choi, C. G. , Kim, S. J. , & Shim, W. H. (2017). Differences in dynamic and static functional connectivity between young and elderly healthy adults. Neuroradiology, 59(8), 781–789. 10.1007/s00234-017-1875-2 [DOI] [PubMed] [Google Scholar]
- Pedersen, R. , Geerligs, L. , Andersson, M. , Gorbach, T. , Avelar‐Pereira, B. , Wåhlin, A. , Rieckmann, A. , Nyberg, L. , & Salami, A. (2021). When functional blurring becomes deleterious: Reduced system segregation is associated with less white matter integrity and cognitive decline in aging. NeuroImage, 242, 118449. 10.1016/j.neuroimage.2021.118449 [DOI] [PubMed] [Google Scholar]
- Perneczky, R. (2019). Dementia prevention and reserve against neurodegenerative disease. Dialogues in Clinical Neuroscience, 21(1), 53–60. 10.31887/DCNS.2019.21.1/rperneczky2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrican, R. , Taylor, M. J. , & Grady, C. L. (2017). Trajectories of brain system maturation from childhood to older adulthood: Implications for lifespan cognitive functioning. NeuroImage, 163, 125–149. 10.1016/j.neuroimage.2017.09.025 [DOI] [PubMed] [Google Scholar]
- Power, J. D. , Barnes, K. A. , Snyder, A. Z. , Schlaggar, B. L. , & Petersen, S. E. (2012). Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage, 59(3), 2142–2154. 10.1016/j.neuroimage.2011.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power, J. D. , Barnes, K. A. , Snyder, A. Z. , Schlaggar, B. L. , & Petersen, S. E. (2013). Steps toward optimizing motion artifact removal in functional connectivity MRI; A reply to Carp. Neuroimage, 76, 439–441. 10.1016/j.neuroimage.2012.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power, J. D. , Mitra, A. , Laumann, T. O. , Snyder, A. Z. , Schlaggar, B. L. , & Petersen, S. E. (2014). Methods to detect, characterize, and remove motion artifact in resting state fMRI. NeuroImage, 84, 320–341. 10.1016/j.neuroimage.2013.08.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preti, M. G. , & Van De Ville, D. (2017). Dynamics of functional connectivity at high spatial resolution reveal long‐range interactions and fine‐scale organization. Scientific Reports, 7(1), 12773. 10.1038/s41598-017-12993-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puxeddu, M. G. , Faskowitz, J. , Betzel, R. F. , Petti, M. , Astolfi, L. , & Sporns, O. (2020). The modular organization of brain cortical connectivity across the human lifespan. NeuroImage, 218, 116974. 10.1016/j.neuroimage.2020.116974 [DOI] [PubMed] [Google Scholar]
- Qin, J. , Chen, S. G. , Hu, D. , Zeng, L. L. , Fan, Y. M. , Chen, X. P. , & Shen, H. (2015). Predicting individual brain maturity using dynamic functional connectivity. Frontiers in Human Neuroscience, 9, 418. 10.3389/fnhum.2015.00418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle, M. E. (2011). The restless brain. Brain Connectivity, 1(1), 3–12. 10.1089/brain.2011.0019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajah, M. N. , & D'Esposito, M. (2005). Region‐specific changes in prefrontal function with age: A review of PET and fMRI studies on working and episodic memory. Brain, 128(Pt 9), 1964–1983. 10.1093/brain/awh608 [DOI] [PubMed] [Google Scholar]
- Ramchandran, K. , Zeien, E. , & Andreasen, N. C. (2019). Distributed neural efficiency: Intelligence and age modulate adaptive allocation of resources in the brain. Trends in Neuroscience and Education, 15, 48–61. 10.1016/j.tine.2019.02.006 [DOI] [PubMed] [Google Scholar]
- Raz, N. , & Rodrigue, K. M. (2006). Differential aging of the brain: Patterns, cognitive correlates and modifiers. Neuroscience and Biobehavioral Reviews, 30(6), 730–748. 10.1016/j.neubiorev.2006.07.00 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reijmer, Y. D. , Schultz, A. P. , Leemans, A. , O'Sullivan, M. J. , Gurol, M. E. , Sperling, R. , Greenberg, S. M. , Viswanathan, A. , & Hedden, T. (2015). Decoupling of structural and functional brain connectivity in older adults with white matter hyperintensities. NeuroImage, 117, 222–229. 10.1016/j.neuroimage.2015.05.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter‐Lorenz P.A. and, Cappell, K.A. (2008) Neurocognitive aging and the compensation hypothesis. Current Directions in Psychological Science, 17, 177–182. 10.1111/j.1467-8721.2008.00570.x [DOI] [Google Scholar]
- Reuter‐Lorenz, P. A. , & Park, D. C. (2014). How does it STAC up? Revisiting the scaffolding theory of aging and cognition. Neuropsychology Review, 24(3), 355–370. 10.1007/s11065-014-9270-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle, D. R. , Sonntag, W. E. , & Lichtenwalner, R. J. (2003). Microvascular plasticity in aging. Ageing Research Reviews, 2(2), 149–168. 10.1016/s1568-1637(02)00064-8 [DOI] [PubMed] [Google Scholar]
- Risk, B. B. , Murden, R. J. , Wu, J. , Nebel, M. B. , Venkataraman, A. , Zhang, Z. , & Qiu, D. (2021). Which multiband factor should you choose for your resting‐state fMRI study? NeuroImage, 234, 117965. 10.1016/j.neuroimage.2021.117965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roski, C. , Caspers, S. , Langner, R. , Laird, A. R. , Fox, P. T. , Zilles, K. , Amunts, K. , & Eickhoff, S. B. (2013). Adult age‐dependent differences in resting‐state connectivity within and between visual‐attention and sensorimotor networks. Frontiers in Aging Neuroscience, 5, 67. 10.3389/fnagi.2013.00067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinov, M. , & Sporns, O. (2010). Complex network measures of brain connectivity: Uses and interpretations. NeuroImage, 52(3), 1059–1069. 10.1016/j.neuroimage.2009.10.003 [DOI] [PubMed] [Google Scholar]
- Rugg, M. D. (2016). Interpreting age‐related differences in memory‐related neural activity. Cognitive Neuroscience of Aging, 183–204. 10.1093/acprof:oso/9780199372935.003.0008 [DOI] [Google Scholar]
- Rypma, B. , Berger, J. S. , Genova, H. M. , Rebbechi, D. , & D'Esposito, M. (2005). Dissociating age‐related changes in cognitive strategy and neural efficiency using event‐related fMRI. Cortex, 41(4), 582–594. 10.1016/s0010-9452(08)70198-9 [DOI] [PubMed] [Google Scholar]
- Sala, A. , & Perani, D. (2019). Brain molecular connectivity in neurodegenerative diseases: Recent advances and new perspectives using positron emission tomography. Frontiers in Neuroscience, 13, 617. 10.3389/fnins.2019.00617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salat, D. H. (2011). The declining infrastructure of the aging brain. Brain Connectivity, 1(4), 279–293. 10.1089/brain.2011.0056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala‐Llonch, R. , Junqué, C. , Arenaza‐Urquijo, E. M. , Vidal‐Piñeiro, D. , Valls‐Pedret, C. , Palacios, E. , Domènech, S. , Salvà, A. , Bargalló, N. , & Bartrés‐Faz, D. (2014). Changes in whole‐brain functional networks and memory performance in aging. Neurobiology of Aging, 35(10), 2193–2202. 10.1016/j.neurobiolaging.2014.04.007 [DOI] [PubMed] [Google Scholar]
- Salthouse, T. A. (2019). Trajectories of normal cognitive aging. Psychology and Aging, 34(1), 17–24. 10.1037/pag0000288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanez‐Larkin, G. R. , & D'Esposito, M. (2008). Group comparisons: Imaging the aging brain. Social Cognitive and Affective Neuroscience, 3(3), 290–297. 10.1093/scan/nsn029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sang, L. , Chen, L. , Wang, L. , Zhang, J. , Zhang, Y. , Li, P. , Li, C. , & Qiu, M. (2018). Progressively disrupted brain functional connectivity network in subcortical ischemic vascular cognitive impairment patients. Frontiers in Neurology, 9, 94. 10.3389/fneur.2018.00094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarnecchi, E. , Galli, G. , Polizzotto, N. R. , Rossi, A. , & Rossi, S. (2014). Efficiency of weak brain connections support general cognitive functioning. Human Brain Mapping, 35(9), 4566–4582. 10.1002/hbm.22495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite, T. D. , Elliott, M. A. , Gerraty, R. T. , Ruparel, K. , Loughead, J. , Calkins, M. E. , Eickhoff, S. B. , Hakonarson, H. , Gur, R. C. , Gur, R. E. , & Wolf, D. H. (2013). An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting‐state functional connectivity data. NeuroImage, 64, 240–256. 10.1016/j.neuroimage.2012.08.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer, A. , Margulies, D. S. , Lohmann, G. , Gorgolewski, K. J. , Smallwood, J. , Kiebel, S. J. , & Villringer, A. (2014). Dynamic network participation of functional connectivity hubs assessed by resting‐state fMRI. Frontiers in Human Neuroscience, 8, 195. 10.3389/fnhum.2014.00195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheinost, D. , Finn, E. S. , Tokoglu, F. , Shen, X. , Papademetris, X. , Hampson, M. , & Constable, R. T. (2015). Sex differences in normal age trajectories of functional brain networks. Human Brain Mapping, 36(4), 1524–1535. 10.1002/hbm.22720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider‐Garces, N. J. , Gordon, B. A. , Brumback‐Peltz, C. R. , Shin, E. , Lee, Y. , Sutton, B. P. , Maclin, E. L. , Gratton, G. , & Fabiani, M. (2010). Span, CRUNCH, and beyond: Working memory capacity and the aging brain. Journal of Cognitive Neuroscience, 22(4), 655–669. 10.1162/jocn.2009.21230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen, H. H. (2015). Core concept: Resting‐state connectivity. Proceedings of the National Academy of Sciences of the United States of America, 112(46), 14115–14116. 10.1073/pnas.1518785112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siman‐Tov, T. , Bosak, N. , Sprecher, E. , Paz, R. , Eran, A. , Aharon‐Peretz, J. , & Kahn, I. (2017). Early age‐related functional connectivity decline in high‐order cognitive networks. Frontiers in Aging Neuroscience, 8, 330. 10.3389/fnagi.2016.00330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh‐Manoux, A. , Kivimaki, M. , Glymour, M. M. , Elbaz, A. , Berr, C. , Ebmeier, K. P. , Ferrie, J. E. , & Dugravot, A. (2012). Timing of onset of cognitive decline: Results from Whitehall II prospective cohort study. British Medical Journal (Clinical Research Ed.), 344, d7622. 10.1136/bmj.d7622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, S. M. , Elliott, L. T. , Alfaro‐Almagro, F. , McCarthy, P. , Nichols, T. E. , Douaud, G. , & Miller, K. L. (2020). Brain aging comprises many modes of structural and functional change with distinct genetic and biophysical associations. eLife, 9, e52677. 10.7554/eLife.52677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, J. , Birn, R. M. , Boly, M. , Meier, T. B. , Nair, V. A. , Meyerand, M. E. , & Prabhakaran, V. (2014). Age‐related reorganizational changes in modularity and functional connectivity of human brain networks. Brain Connectivity, 4(9), 662–676. 10.1089/brain.2014.0286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberg, J. M. , Sadeh, N. , Leritz, E. C. , McGlinchey, R. E. , Milberg, W. P. , Hayes, J. P. , & Salat, D. H. (2017). Higher serum cholesterol is associated with intensified age‐related neural network decoupling and cognitive decline in early‐ to mid‐life. Human Brain Mapping, 38(6), 3249–3261. 10.1002/hbm.23587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporns, O. (2017). The future of network neuroscience. Network Neuroscience, 1(1), 1–2. 10.1162/netn_e_00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporns, O. (2018). Graph theory methods: Applications in brain networks. Dialogues in Clinical Neuroscience, 20(2), 111–121. 10.31887/DCNS.2018.20.2/osporns [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng, R. N. , & Turner, G. R. (2019). The shifting architecture of cognition and brain function in older adulthood. Perspectives on Psychological Science, 14(4), 523–542. 10.1177/1745691619827511 [DOI] [PubMed] [Google Scholar]
- Spreng, R. N. , Stevens, W. D. , Viviano, J. D. , & Schacter, D. L. (2016). Attenuated anticorrelation between the default and dorsal attention networks with aging: Evidence from task and rest. Neurobiology of Aging, 45, 149–160. 10.1016/j.neurobiolaging.2016.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staffaroni, A. M. , Brown, J. A. , Casaletto, K. B. , Elahi, F. M. , Deng, J. , Neuhaus, J. , Cobigo, Y. , Mumford, P. S. , Walters, S. , Saloner, R. , Karydas, A. , Coppola, G. , Rosen, H. J. , Miller, B. L. , Seeley, W. W. , & Kramer, J. H. (2018). The longitudinal trajectory of default mode network connectivity in healthy older adults varies as a function of age and is associated with changes in episodic memory and processing speed. The Journal of Neuroscience, 38(11), 2809–2817. 10.1523/JNEUROSCI.3067-17.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley, M. L. , Moussa, M. N. , Paolini, B. M. , Lyday, R. G. , Burdette, J. H. , & Laurienti, P. J. (2013). Defining nodes in complex brain networks. Frontiers in Computational Neuroscience, 7, 169. 10.3389/fncom.2013.00169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley, M. L. , Simpson, S. L. , Dagenbach, D. , Lyday, R. G. , Burdette, J. H. , & Laurienti, P. J. (2015). Changes in brain network efficiency and working memory performance in aging. PLoS One, 10(4), e0123950. 10.1371/journal.pone.0123950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern, Y. (2002). What is cognitive reserve? Theory and research application of the reserve concept. Journal of the International Neuropsychological Society, 8(3), 448–460 PMID: 11939702. [PubMed] [Google Scholar]
- Stranahan, A. M. , & Mattson, M. P. (2012). Metabolic reserve as a determinant of cognitive aging. Journal of Alzheimer's Disease, 30(Suppl 2), S5–S13. 10.3233/JAD-2011-110899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumme, J. , Jockwitz, C. , Hoffstaedter, F. , Amunts, K. , & Caspers, S. (2020). Functional network reorganization in older adults: Graph‐theoretical analyses of age, cognition and sex. NeuroImage, 214, 116756. 10.1016/j.neuroimage.2020.116756 [DOI] [PubMed] [Google Scholar]
- Sun, J. , Tong, S. , & Yang, G. Y. (2012). Reorganization of brain networks in aging and age‐related diseases. Aging and Disease, 3(2), 181–193 PMID: 22724079. [PMC free article] [PubMed] [Google Scholar]
- Sweeney, M. D. , Montagne, A. , Sagare, A. P. , Nation, D. A. , Schneider, L. S. , Chui, H. C. , Harrington, M. , Pa, J. , Law, M. , Wang, D. J. J. , Jacobs, R. E. , Doubal, F. N. , Ramirez, J. , Black, S. E. , Nedergaard, M. , Benveniste, H. , Dichgans, M. , Iadecola, C. , Love, S. , … Zlokovic, B. V. (2019). Vascular dysfunction ‐ the disregarded partner of Alzheimer's disease. Alzheimer's & Dementia, 15(1), 158–167. 10.1016/j.jalz.2018.07.222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagliazucchi, E. , von Wegner, F. , Morzelewski, A. , Brodbeck, V. , & Laufs, H. (2012). Dynamic BOLD functional connectivity in humans and its electrophysiological correlates. Frontiers in Human Neuroscience, 6, 339. 10.3389/fnhum.2012.00339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, Y. Y. , Rothbart, M. K. , & Posner, M. I. (2012). Neural correlates of establishing, maintaining, and switching brain states. Trends in Cognitive Sciences, 16(6), 330–337. 10.1016/j.tics.2012.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao, Z. , Shi, A. , & Zhao, J. (2015). Epidemiological perspectives of diabetes. Cell Biochemistry and Biophysics, 73(1), 181–185. 10.1007/s12013-015-0598-4 [DOI] [PubMed] [Google Scholar]
- Tian, L. , Li, Q. , Wang, C. , & Yu, J. (2018). Changes in dynamic functional connections with aging. NeuroImage, 172, 31–39. 10.1016/j.neuroimage.2018.01.040 [DOI] [PubMed] [Google Scholar]
- Titov, D. , Diehl‐Schmid, J. , Shi, K. , Perneczky, R. , Zou, N. , Grimmer, T. , Li, J. , Drzezga, A. , & Yakushev, I. (2017). Metabolic connectivity for differential diagnosis of dementing disorders. Journal of Cerebral Blood Flow and Metabolism, 37(1), 252–262. 10.1177/0271678X15622465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tognoli, E. , & Kelso, J. A. (2014). The metastable brain. Neuron, 81(1), 35–48. 10.1016/j.neuron.2013.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi, D. G. , Shokri‐Kojori, E. , Wiers, C. E. , Kim, S. W. , Demiral, Ş. B. , Cabrera, E. A. , Lindgren, E. , Miller, G. , Wang, G. J. , & Volkow, N. D. (2017). Dynamic brain glucose metabolism identifies anti‐correlated cortical‐cerebellar networks at rest. Journal of Cerebral Blood Flow and Metabolism, 37(12), 3659–3670. 10.1177/0271678X17708692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi, D. , & Volkow, N. D. (2011). Aging and functional brain networks. Molecular Psychiatry, 17(5), 549–558. 10.1038/mp.2011.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi, D. , Wang, G. J. , & Volkow, N. D. (2013). Energetic cost of brain functional connectivity. Proceedings of the National Academy of Sciences of the United States of America, 110(33), 13642–13647. 10.1073/pnas.1303346110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotta, N. , Archambaud, F. , Goldman, S. , Baete, K. , van Laere, K. , Wens, V. , van Bogaert, P. , Chiron, C. , & de Tiège, X. (2016). Functional integration changes in regional brain glucose metabolism from childhood to adulthood. Human Brain Mapping, 37(8), 3017–3030. 10.1002/hbm.23223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsvetanov, K. A. , Henson, R. N. , Tyler, L. K. , Razi, A. , Geerligs, L. , Ham, T. E. , Rowe, J. B. , & Cambridge Centre for Ageing and Neuroscience . (2016). Extrinsic and intrinsic brain network connectivity maintains cognition across the lifespan despite accelerated decay of regional brain activation. The Journal of Neuroscience, 36(11), 3115–3126. 10.1523/JNEUROSCI.2733-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsvetanov, K. A. , Ye, Z. , Hughes, L. , Samu, D. , Treder, M. S. , Wolpe, N. , Tyler, L. K. , Rowe, J. B. , & Cambridge Centre for Ageing and Neuroscience . (2018). Activity and connectivity differences underlying inhibitory control across the adult life span. The Journal of Neuroscience, 38(36), 7887–7900. 10.1523/JNEUROSCI.2919-17.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Nations, Department of Economic and Social Affairs, Population Division (2019). World population ageing. United Nations. ISBN: 978‐92‐1‐148325‐3. https://www.unpopulation.org [Google Scholar]
- van den Heuvel, M. P. , & Hulshoff Pol, H. E. (2010). Exploring the brain network: A review on resting‐state fMRI functional connectivity. European Neuropsychopharmacology, 20(8), 519–534. 10.1016/j.euroneuro.2010.03.008 [DOI] [PubMed] [Google Scholar]
- van den Heuvel, M. P. , Kahn, R. S. , Goñi, J. , & Sporns, O. (2012). High‐cost, high‐capacity backbone for global brain communication. Proceedings of the National Academy of Sciences of the United States of America, 109(28), 11372–11377. 10.1073/pnas.1203593109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk, K. R. , Sabuncu, M. R. , & Buckner, R. L. (2012). The influence of head motion on intrinsic functional connectivity MRI. NeuroImage, 59(1), 431–438. 10.1016/j.neuroimage.2011.07.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varangis, E. , Habeck, C. G. , Razlighi, Q. R. , & Stern, Y. (2019). The effect of aging on resting state connectivity of predefined networks in the brain. Frontiers in Aging Neuroscience, 11, 234. 10.3389/fnagi.2019.00234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vij, S. G. , Nomi, J. S. , Dajani, D. R. , & Uddin, L. Q. (2018). Evolution of spatial and temporal features of functional brain networks across the lifespan. NeuroImage, 173, 498–508. 10.1016/j.neuroimage.2018.02.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villien, M. , Wey, H. Y. , Mandeville, J. B. , Catana, C. , Polimeni, J. R. , Sander, C. Y. , Zürcher, N. R. , Chonde, D. B. , Fowler, J. S. , Rosen, B. R. , & Hooker, J. M. (2014). Dynamic functional imaging of brain glucose utilization using fPET‐FDG. NeuroImage, 100, 192–199. 10.1016/j.neuroimage.2014.06.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viviano, R. P. , Raz, N. , Yuan, P. , & Damoiseaux, J. S. (2017). Associations between dynamic functional connectivity and age, metabolic risk, and cognitive performance. Neurobiology of Aging, 59, 135–143. 10.1016/j.neurobiolaging.2017.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, L. , Su, L. , Shen, H. , & Hu, D. (2012). Decoding lifespan changes of the human brain using resting‐state functional connectivity MRI. PLoS One, 7(8), e44530. 10.1371/journal.pone.0044530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward, P. , Orchard, E. R. , Oldham, S. , Arnatkevičiūtė, A. , Sforazzini, F. , Fornito, A. , Storey, E. , Egan, G. F. , & Jamadar, S. D. (2020). Individual differences in haemoglobin concentration influence bold fMRI functional connectivity and its correlation with cognition. NeuroImage, 221, 117196. 10.1016/j.neuroimage.2020.117196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wecker, N. S. , Kramer, J. H. , Wisniewski, A. , Delis, D. C. , & Kaplan, E. (2000). Age effects on executive ability. Neuropsychology, 14(3), 409–414. 10.1037//0894-4105.14.3.409 [DOI] [PubMed] [Google Scholar]
- Wei, D. , Zhuang, K. , Ai, L. , Chen, Q. , Yang, W. , Liu, W. , Wang, K. , Sun, J. , & Qiu, J. (2018). Structural and functional brain scans from the cross‐sectional Southwest University adult lifespan dataset. Scientific Data, 5, 180134. 10.1038/sdata.2018.134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen, X. , Dong, L. , Chen, J. , Xiang, J. , Yang, J. , Li, H. , Liu, X. , Luo, C. , & Yao, D. (2020). Detecting the information of functional connectivity networks in normal aging using deep learning from a big data perspective. Frontiers in Neuroscience, 13, 1435. 10.3389/fnins.2019.01435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen, X. , He, H. , Dong, L. , Chen, J. , Yang, J. , Guo, H. , Luo, C. , & Yao, D. (2020). Alterations of local functional connectivity in lifespan: A resting‐state fMRI study. Brain and Behavior, 10(7), e01652. 10.1002/brb3.1652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- West, K. L. , Zuppichini, M. D. , Turner, M. P. , Sivakolundu, D. K. , Zhao, Y. , Abdelkarim, D. , Spence, J. S. , & Rypma, B. (2019). BOLD hemodynamic response function changes significantly with healthy aging. Neuroimage, 188, 198–207. 10.1016/j.neuroimage.2018.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wig, G. S. (2017). Segregated systems of human brain networks. Trends in Cognitive Sciences, 21(12), 981–996. 10.1016/j.tics.2017.09.006 [DOI] [PubMed] [Google Scholar]
- Wig, G. S. , Schlaggar, B. L. , & Petersen, S. E. (2011). Concepts and principles in the analysis of brain networks. Annals of the New York Academy of Sciences, 1224, 126–146. 10.1111/j.1749-6632.2010.05947.x [DOI] [PubMed] [Google Scholar]
- Wu, J. T. , Wu, H. Z. , Yan, C. G. , Chen, W. X. , Zhang, H. Y. , He, Y. , & Yang, H. S. (2011). Aging‐related changes in the default mode network and its anti‐correlated networks: A resting‐state fMRI study. Neuroscience Letters, 504(1), 62–67. 10.1016/j.neulet.2011.08.059 [DOI] [PubMed] [Google Scholar]
- Wu, K. , Taki, Y. , Sato, K. , Kinomura, S. , Goto, R. , Okada, K. , Kawashima, R. , He, Y. , Evans, A. C. , & Fukuda, H. (2012). Age‐related changes in topological organization of structural brain networks in healthy individuals. Human Brain Mapping, 33(3), 552–568. 10.1002/hbm.21232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, K. , Taki, Y. , Sato, K. , Qi, H. , Kawashima, R. , & Fukuda, H. (2013). A longitudinal study of structural brain network changes with normal aging. Frontiers in Human Neuroscience, 7, 113. 10.3389/fnhum.2013.00113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia, Y. , Chen, Q. , Shi, L. , Li, M. , Gong, W. , Chen, H. , & Qiu, J. (2019). Tracking the dynamic functional connectivity structure of the human brain across the adult lifespan. Human Brain Mapping, 40(3), 717–728. 10.1002/hbm.24385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie, W. , Peng, C. K. , Shen, J. , Lin, C. P. , Tsai, S. J. , Wang, S. , Chu, Q. , & Yang, A. C. (2020). Age‐related changes in the association of resting‐state fMRI signal variability and global functional connectivity in non‐demented healthy people. Psychiatry Research, 291, 113257. 10.1016/j.psychres.2020.113257 [DOI] [PubMed] [Google Scholar]
- Yabluchanskiy, A. , Nyul‐Toth, A. , Csiszar, A. , Gulej, R. , Saunders, D. , Towner, R. , Turner, M. , Zhao, Y. , Abdelkari, D. , Rypma, B. , & Tarantini, S. (2021). Age‐related alterations in the cerebrovasculature affect neurovascular coupling and BOLD fMRI responses: Insights from animal models of aging. Psychophysiology, 58(7), e13718. 10.1111/psyp.13718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan, C. G. , Craddock, R. C. , He, Y. , & Milham, M. P. (2013). Addressing head motion dependencies for small‐world topologies in functional connectomics. Frontiers in Human Neuroscience, 7, 910. 10.3389/fnhum.2013.00910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, A. C. , Tsai, S. J. , Lin, C. P. , Peng, C. K. , & Huang, N. E. (2018). Frequency and amplitude modulation of resting‐state fMRI signals and their functional relevance in normal aging. Neurobiology of Aging, 70, 59–69. 10.1016/j.neurobiolaging.2018.06.007 [DOI] [PubMed] [Google Scholar]
- Yao, Y. , Lu, W. L. , Xu, B. , Li, C. B. , Lin, C. P. , Waxman, D. , & Feng, J. F. (2013). The increase of the functional entropy of the human brain with age. Scientific Reports, 3, 2853. 10.1038/srep02853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellen, G. (2018). Fueling thought: Management of glycolysis and oxidative phosphorylation in neuronal metabolism. The Journal of Cell Biology, 217(7), 2235–2246. 10.1083/jcb.201803152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizawa, H. , Gazes, Y. , Stern, Y. , Miyata, Y. , & Uchiyama, S. (2014). Characterizing the normative profile of 18F‐FDG PET brain imaging: Sex difference, aging effect, and cognitive reserve. Psychiatry Research, 221(1), 78–85. 10.1016/j.pscychresns.2013.10.009 [DOI] [PubMed] [Google Scholar]
- Zalesky, A. , Fornito, A. , Harding, I. H. , Cocchi, L. , Yücel, M. , Pantelis, C. , & Bullmore, E. T. (2010). Whole‐brain anatomical networks: Does the choice of nodes matter? NeuroImage, 50(3), 970–983. 10.1016/j.neuroimage.2009.12.027 [DOI] [PubMed] [Google Scholar]
- Zhai, J. , & Li, K. (2019). Predicting brain age based on spatial and temporal features of human brain functional networks. Frontiers in Human Neuroscience, 13, 62. 10.3389/fnhum.2019.00062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, H. Y. , Chen, W. X. , Jiao, Y. , Xu, Y. , Zhang, X. R. , & Wu, J. T. (2014). Selective vulnerability related to aging in large‐scale resting brain networks. PLoS One, 9(10), e108807. 10.1371/journal.pone.0108807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, H. , Lee, A. , & Qiu, A. (2017). A posterior‐to‐anterior shift of brain functional dynamics in aging. Brain Structure & Function, 222(8), 3665–3676. 10.1007/s00429-017-1425-z [DOI] [PubMed] [Google Scholar]
- Zhu, W. , Wen, W. , He, Y. , Xia, A. , Anstey, K. J. , & Sachdev, P. (2012). Changing topological patterns in normal aging using large‐scale structural networks. Neurobiology of Aging, 33(5), 899–913. 10.1016/j.neurobiolaging.2010.06.022 [DOI] [PubMed] [Google Scholar]
- Zhu, Z. , Johnson, N. F. , Kim, C. , & Gold, B. T. (2015). Reduced frontal cortex efficiency is associated with lower white matter integrity in aging. Cerebral Cortex, 25(1), 138–146. 10.1093/cercor/bht212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman, B. , Rypma, B. , Gratton, G. , & Fabiani, M. (2021). Age‐related changes in cerebrovascular health and their effects on neural function and cognition: A comprehensive review. Psychophysiology, 58(7), e13796. 10.1111/psyp.13796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann, J. , Ritter, P. , Shen, K. , Rothmeier, S. , Schirner, M. , & McIntosh, A. R. (2016). Structural architecture supports functional organization in the human aging brain at a regionwise and network level. Human Brain Mapping, 37(7), 2645–2661. 10.1002/hbm.23200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zonneveld, H. I. , Pruim, R. H. , Bos, D. , Vrooman, H. A. , Muetzel, R. L. , Hofman, A. , Rombouts, S. A. , van der Lugt, A. , Niessen, W. J. , Ikram, M. A. , & Vernooij, M. W. (2019). Patterns of functional connectivity in an aging population: The Rotterdam study. NeuroImage, 189, 432–444. 10.1016/j.neuroimage.2019.01.041 [DOI] [PubMed] [Google Scholar]
- Zuendorf, G. , Kerrouche, N. , Herholz, K. , & Baron, J. C. (2003). Efficient principal component analysis for multivariate 3D voxel‐based mapping of brain functional imaging data sets as applied to FDG‐PET and normal aging. Human Brain Mapping, 18(1), 13–21. 10.1002/hbm.10069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo, X. N. , Kelly, C. , di Martino, A. , Mennes, M. , Margulies, D. S. , Bangaru, S. , Grzadzinski, R. , Evans, A. C. , Zang, Y. F. , Castellanos, F. X. , & Milham, M. P. (2010). Growing together and growing apart: Regional and sex differences in the lifespan developmental trajectories of functional homotopy. The Journal of Neuroscience, 30(45), 15034–15043. 10.1523/JNEUROSCI.2612-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supporting information
Data Availability Statement
The data used in this paper are publicly available from published journals articles.


