Abstract
Among the environmental factors associated with type 1 diabetes (T1D), viral infections of the gut and pancreas has been investigated most intensely, identifying enterovirus infections as the prime candidate trigger of islet autoimmunity (IA) and T1D development. However, the association between respiratory tract infections (RTI) and IA/T1D is comparatively less known. While there are significant amounts of epidemiological evidence supporting the role of respiratory infections in T1D, there remains a paucity of data characterising infectious agents at the molecular level. This gap in the literature precludes the identification of the specific infectious agents driving the association between RTI and T1D. Furthermore, the effect of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infections on the development of IA/T1D remains undeciphered. Here, we provide a comprehensive overview of the evidence to date, implicating RTIs (viral and non‐viral) as potential risk factors for IA/T1D.
Keywords: autoimmunity, respiratory infection, type 1 diabetes, virome, virus
Abbreviations
- ABIS
all babies in Southeast Sweden study
- CDC
centres for diseases control and prevention
- CI
confidence interval
- COVID‐19
coronavirus disease 2019
- DIPP‐novum
type 1 diabetes prediction and prevention study
- DKA
diabetes ketoacidosis
- ECHO
enteric cytopathic human orphan virus
- ENDIA
environmental determinants of islet autoimmunity study
- EV
enterovirus
- EV‐B
Enterovirus B
- GADA
glutamic acid decarboxylase 6 antibodies
- IA
islet autoimmunity
- IAA
insulin autoantibodies
- HLA
human leucocyte antigen
- MIDIA
Norwegian environmental triggers of type 1 diabetes study
- NGS
next‐generation sequencing
- OR
odds ratio
- P
p‐value
- RTI
respiratory tract infection
- SARS‐CoV‐2
severe acute respiratory syndrome related coronavirus 2
- T1D
type 1 diabetes
- TEDDY
the environmental determinants of diabetes in the young study
- TRIGR
Trial to reduce insulin‐dependent diabetes mellitus
- VirCapSeq
virome capture sequencing
1. INTRODUCTION
Type 1 diabetes (T1D) is a chronic autoimmune condition affecting over nine million worldwide, 1 characterised by the loss of functional pancreatic islet β‐cells. This ultimately results in the lifelong dependency on exogenous insulin. 2 , 3 , 4 Although the pathophysiology of T1D is well characterised and understood, its aetiology remains unclear. However, it is well established that the mechanisms underlying the development of T1D is multifaceted and likely involves the complex interplay between genetic and environmental factors. 2 , 5 , 6 Among the environmental factors associated with T1D, infections with viruses are identified as prime candidate triggers of islet autoimmunity (IA) which precedes most clinical onset of T1D.
2. VIRAL AETIOLOGY OF TYPE 1 DIABETES
The reduced prevalence of T1D‐associated high‐risk human leucocyte antigen (HLA) genotypes among newly diagnosed individuals, increasing global incidence of T1D, 6 , 7 , 8 , 9 seasonal variations 6 , 10 and geographical differences 6 , 11 in genetically similar individuals as well as the convergence of IA/T1D incidence of migrants to their new country of residence 12 , 13 all strongly support the growing contribution of environmental factors in the pathogenesis of T1D.
Several hypotheses have been proposed on how environmental factors may influence the progression of T1D. The ‘β‐cell overload’ hypothesis postulates that factors increasing insulin demand such as infection, growth, trauma and other physiological stresses may result in β‐cell dysfunction and insulin resistance, instigating and accelerating the development of IA/T1D. 6 , 14 , 15 , 16 The ‘hygiene hypothesis’ conversely states that a decrease in childhood infections due to improved hygiene may increase the incidence of autoimmune diseases like T1D. 6 , 9 The hygiene hypothesis proposes that a lack of childhood infections can limit immune system's exposure to various microorganisms and stunt its development, leading to an inappropriate response to future infections that may cause T1D. 9 Another hypothesis, the ‘polio hypothesis’, suggests that the decreasing incidence of certain virus infections over time (such as enterovirus or poliovirus infections) has increased the proportion of infants who become infected in the absence of maternal antibodies that could protect against that virus, increasing the risk of complications such as β‐cell damage and T1D. 17 , 18
Among the environmental factors associated with T1D to date, viral infection has been investigated most thoroughly and hypothesised as the prime trigger of IA and progression to T1D, especially in utero and during childhood. 2 , 13 This is supported by a large body of molecular 6 , 19 , 20 and epidemiological 21 , 22 , 23 , 24 , 25 evidence, and multiple non‐mutually exclusive mechanisms have been proposed to explain how viral infections can induce and/or accelerate the development of IA/T1D. 26 , 27 , 28 , 29
To date, multiple viruses have been associated with T1D. Of the viruses investigated, enteroviruses (EV) have been the most deeply studied and now widely accepted as the prime candidate trigger of IA/T1D. 2 , 29 , 30 , 31 In total, over 26 different EV types have been associated with IA/T1D, mostly comprised of Enterovirus B (EV‐B) species members within the coxsackievirus B and enteric cytopathic human orphan virus (ECHO virus) groups. 2 , 32 EVs have been detected more frequently in the blood, 22 gut 33 , 34 and pancreas 24 , 35 , 36 of individuals with T1D compared to without, and are associated with an increased risk of T1D in prospective studies. 37 , 38 , 39
3. RESPIRATORY TRACT INFECTIONS AND ISLET AUTOIMMUNITY/TYPE 1 DIABETES
Although most research to date on the infectious aetiology of IA/T1D have focussed heavily on viral infections in the gut and pancreas, 2 , 5 , 30 respiratory tract infections (RTI), particularly within the first 12 months after birth, 26 , 40 , 41 , 42 have also been investigated as a potential risk factor for childhood T1D. Both lower RTIs (including pneumonia, bronchitis and bronchiolitis) and upper RTIs (including rhinitis, pharyngitis and laryngitis) have been examined by at least 19 observational studies as potential triggers for IA/T1D development (Figure 1, Table 1).
FIGURE 1.
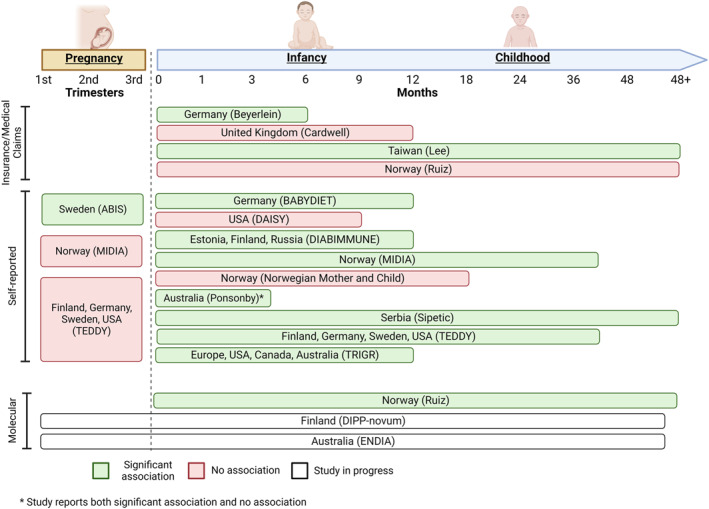
Graphic summary of studies reporting association between RTIs and islet autoimmunity (IA)/type 1 diabetes (T1D) grouped by participant age during period of infection. 26 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 59 , 68 Measures of exposure are categorised by insurance/medical claims data, self‐reported data and molecular data. Each study is represented by the region(s) of the study setting, with the study group or first author in brackets. ABIS = All Babies in Southeast Sweden study; DIPP = Type 1 Diabetes Prediction and Prevention study; MIDIA = Norwegian Environmental Triggers of Type 1 Diabetes study; TEDDY = the Environmental Determinants of Diabetes in the Young study; TRIGR = Trial to Reduce Insulin‐Dependent Diabetes Mellitus.
TABLE 1.
Studies that investigated the relationship between respiratory tract infections (RTI) and islet autoimmunity (IA)/type 1 diabetes (T1D).
| Study (year/region) | Cases (controls) | Outcome | Exposure | OR (95% CI) | p‐value |
|---|---|---|---|---|---|
| Insurance/Medical claims | |||||
| Beyerlein (2016/Germany) 26 | 720 (294424) | T1D | ≥1 RTI in the first 6 months of life | 1.17 (1.00‐1.37) | <0.05 |
| Cardwell (2008/United Kingdom) 45 | 367 (4579) | T1D | Medical consultations in first year of life for | ||
| Upper RTI | 0.84 (0.67‐1.04) | 0.11 | |||
| Lower RTI | 0.81 (0.55‐1.20) | 0.28 | |||
| Lee (2015/Taiwan) 43 | 632 (6320) | T1D | ≥1 acute RTI | 1.74 (1.30‐2.33) | <0.05 |
| ≥1 episode of pneumonia or influenza | 1.80 (1.35‐2.41) | <0.05 | |||
| Ruiz (2018/Norway) 44 | 2376 (2284274) | T1D | Pandemic influenza infection | 1.19 (0.97‐1.46) | >0.05 |
| Laboratory confirmed pandemic influenza infection | 2.26 (1.51‐3.38) | <0.05 | |||
| Self‐reported (postnatal) | |||||
| BABYDIET (2013/Germany) 40 | 26 (122) | IA | RTI in the first 6 months of life | 2.27 (1.32‐3.91) | <0.05 |
| RTI in 6–12 months of life | 1.32 (1.08‐1.61) | <0.05 | |||
| Upper RTI in 6 months prior to seroconversion | 1.57 (1.26‐1.95) | <0.05 | |||
| Lower RTI in 6 months prior to seroconversion | 1.28 (0.51‐3.17) | >0.05 | |||
| DAISY (2012/USA) 50 | 109 (1620) | IA | Upper respiratory symptoms (cough, cold, runny nose, stuffy nose, sinus infection, ear infection) in first 9 months of life | 1.00 (0.98‐1.01) | 0.65 |
| Respiratory disease (croup, pneumonia, bronchitis) in first 9 months of life | 0.99 (0.67‐1.74) | 0.98 | |||
| Diabimmune (2018/Estonia, Finland, Russia) 41 | 46 (744) | IA & T1D | Number of respiratory infections per child in the first year of life (IA cases vs. controls) | 0.003 | |
| Number of respiratory infections per child in the first year of life (T1D cases vs. controls) | 0.002 | ||||
| MIDIA (2011/Norway) 46 | 42 (843) | IA | ≥1 lower RTI by 4 years of life | 3.4 (1.6‐7.1) | 0.001 |
| Norwegian Mother and child (2018/Norway) 49 | 286 (70154) | IA | Upper RTI in first 18 months of life | ||
| 0‐3 | 1.00 (reference) | ||||
| 4‐5 | 0.97 (0.69‐1.38) | 0.88 | |||
| 6‐7 | 0.99 (0.69‐1.42) | 0.97 | |||
| ≥8 | 1.96 (0.77‐1.45) | 0.50 | |||
| ≥1 lower RTI in first 18 months of life | 0.85 (0.59‐1.21) | 0.36 | |||
| Ponsonby (2011/Australia) 42 | 26 (10602) | T1D | ≥1 upper RTI by 5 weeks of life | 2.74 (1.19‐6.32) | 0.02 |
| ≥1 upper RTI by 12 weeks of life | 1.55 (0.65‐3.69) | 0.32 | |||
| Sipetic (2003/Serbia) 59 | 105 (210) | T1D | Frequent (≥3 infections per year) RTI | 2.65 (1.37‐5.11) | <0.01 |
| TEDDY (2003/USA) 52 | 52 (1210) | IA | ≥1 episode of RTI symptoms during pregnancy | 0.66 (0.38‐1.15) | >0.05 |
| TEDDY (2017/Finland, Germany, Sweden, USA) 47 | 454 (7415) | IA | Respiratory infectious episodes during winter | 1.43 (1.17‐1.75) | 0.0005 |
| Common cold | 1.38 (1.11‐1.71) | 0.004 | |||
| Influenza‐like illness | 2.37 (1.35‐4.15) | 0.003 | |||
| Sinusitis | 2.63 (1.22‐5.67) | 0.01 | |||
| Laryngitis/tracheitis | 1.76 (1.04‐2.98) | 0.04 | |||
| TRIGR (2022/Europe, USA, Canada, Australia) 48 | 842 (1175) | IA & T1D | Upper RTI in first 12 months of life (IA as outcome) | 1.20 (1.00‐1.44) | 0.044 |
| Upper RTI in first 12 months of life (T1D as outcome) | 1.05 (0.73‐1.50) | 0.797 | |||
| Self‐reported (pregnancy) | |||||
| ABIS (2022/Sweden) 53 | 137 (16155) | T1D | ≥1 RTI during pregnancy | 1.49 (1.01‐2.22) | 0.04 |
| ≥1 RTI during first trimester | 2.31 (1.32‐4.04) | 0.002 | |||
| ≥1 RTI during second trimester | 1.10 (0.59‐2.04) | 0.77 | |||
| ≥1 RTI during third trimester | 1.15 (0.56‐2.35) | 0.71 | |||
| MIDIA (2011/Norway) 46 | 42 (843) | IA | RTI during pregnancy | ||
| 1 | 1.23 (0.96‐1.58) | 0.09 | |||
| ≥2 | 0.98 (0.74‐1.30) | 0.87 | |||
| TEDDY (2018/Finland, Germany, Sweden, USA) 51 | 438 (7034) | IA | Gestational RTI (IAA as outcome) | 0.88 (0.67‐1.15) | 0.35 |
| Gestational RTI (GADA as outcome) | 0.95 (0.73‐1.25) | 0.73 | |||
| Molecular | |||||
| Ruiz (2018/Norway) 44 | 2376 (2284274) | T1D | Laboratory confirmed pandemic influenza infection | 2.26 (1.51‐3.38) | <0.05 |
Abbreviations: ABIS, All Babies in Southeast Sweden study; CI, confidence interval; GADA, glutamic acid decarboxylase 6 antibodies; IA, islet autoimmunity; IAA, insulin autoantibodies; MIDIA, Norwegian Environmental Triggers of Type 1 Diabetes study; OR, odds ratio; RTI, respiratory tract infection; T1D, type 1 diabetes; TEDDY, the Environmental Determinants of Diabetes in the Young study; TRIGR, Trial to Reduce Insulin‐Dependent Diabetes Mellitus.
Three retrospective case‐control and cohort studies reported a significant association between RTIs and T1D, 26 , 43 , 44 while two reported no association. 44 , 45 Limited sampling methods and heterogeneity in study design between studies may have contributed to inconsistent results. These studies relied on insurance claims or medical consultation data to ascertain RTI exposure, which only capture clinically overt symptomatic infections. Hence, such studies are likely to have underestimated the cumulative exposure to RTIs. Only one retrospective study included molecular testing to confirm the infectious agent, reporting a significant association between laboratory confirmed pandemic influenza A (H1N1) and T1D, but not between clinically diagnosed H1N1 and T1D. 44 All these studies lacked IA testing, precluding the examination of IA as an outcome associated with RTIs.
Prospective birth cohort studies investigating IA as an outcome have reported that early‐life RTIs increased the risk of IA. 40 , 41 , 46 , 47 , 48 These studies followed genetically at‐risk children from birth (as determined by HLA genotype and/or family history of T1D), prospectively collecting data on RTIs through questionnaires and health event logs, and performing regular blood tests to monitor the timing of seroconversion to IA. Norwegian and German studies reported a higher prevalence of IA in children with ≥1 RTI in the first 4 years of life [odds ratio (OR) 3.4, 95% confidence interval (CI) 1.6–7.1, p‐value (p) = 0.001] 46 and first 6 months of life (OR 2.27, 95% CI 1.32–3.91, p < 0.05). 40 These findings were supported by two large‐scale American/European birth cohort studies, the Environmental Determinants of Diabetes in the Young, which reported the risk of IA increased by 5.6% for every RTI recorded in children up to 4 years of age, 47 and the Trial to Reduce Insulin‐Dependent Diabetes Mellitus, which reported that upper RTIs in the first 12 months of life was associated with IA (OR 1.20, 95% CI 1.00–1.44, p = 0.04). 48 In contrast, other large European 49 and American 50 studies found no significant association between early‐life RTIs and IA. These conflicting results may be partly due to the limitations of analysing subjective data types, necessitating further research using molecular methods to definitively confirm and characterise infections and any viruses causing these infections.
Viral exposures in utero have been hypothesised as possible causes of IA/T1D. While most studies did not find an association between gestational RTI and IA/T1D, 46 , 51 , 52 a recent report from the All Babies in Southeast Sweden Study showed that gestational RTIs during the first trimester were associated with higher risk of T1D in offspring (OR 2.31, CI 1.32–4.04, p = 0.002). 53 A plausible explanation is that since the first trimester coincides with the embryological development of the pancreas, a congenital infection during early pregnancy may prime the offspring's immune system and pancreas to produce islet autoantibodies during a second infection postnatally, whereas a more developed pancreas would be less susceptible. 53 However, as no other studies have replicated these results, external validation in other prospective cohorts with maternal data and respiratory samples collected longitudinally during pregnancy is needed.
Specific respiratory viruses including parechoviruses and influenza virus have been associated with T1D in retrospective studies and animal studies. One mouse study found an association between a strain of parechovirus (Ljungan virus) and T1D. 54 While one Japanese retrospective cohort study reported an increased risk of T1D after the diagnosis of influenza, 55 and an Italian study found increased incidence of T1D diagnoses during the 2009 H1N1 pandemic, 56 most observational studies did not find an association between influenza 57 , 58 , 59 , 60 or parechoviruses 61 and T1D in humans. In addition, many EV species replicate in the respiratory tract, and the most common manifestation of EV infection is a common cold‐type disease. These EV species include rhinoviruses which are responsible for over 50% of all RTIs, 2 EV‐B, 62 and members belonging to Enterovirus C 63 and D, 64 that replicate primarily in the respiratory tract. Despite this, no epidemiological studies have examined EVs from respiratory samples in the context of IA/T1D.
The lack of molecular data in most retrospective and prospective studies is a key limitation to the identification of specific infectious agents (viral or non‐viral) that may be driving the association between RTIs and T1D. Molecular characterisation of infectious agents using comprehensive next‐generation sequencing (NGS) methods such as virome capture sequencing (VirCapSeq) can overcome this limitation by enabling sensitive characterisation of all viruses in a given specimen, with minimal investigation bias. 2 , 7 Despite this, there remains no comprehensive molecular study to date that has investigated the respiratory virome in at‐risk individuals. 65 , 66 , 67 Hence, large‐scale molecular research involving NGS that focuses on the association between RTI and IA/T1D is needed to support existing epidemiological studies. Current birth cohort studies including the Environmental Determinants of Islet Autoimmunity (ENDIA) 68 and Diabetes Prediction and Prevention novum (DIPP‐novum) 69 study are in progress that prospectively follow participants from in utero throughout childhood with molecular testing of the respiratory virome, which may shed further information on the relationship between RTI and T1D.
Recently, a machine learning approach was used to rank tissue‐specific transcription regulatory effects for single‐nucleotide polymorphisms in T1D associated genes, estimating their relative contributions to the development of T1D by integrating T1D case and autoantibody‐negative control genotypes with tissue‐specific quantitative trait loci (eQTL) data. 70 The investigators found that the largest gene regulatory contribution to the risk of T1D development was made by the rs6679677 eQTL, which is associated with changes to AP4B1‐AS1 transcript levels in lung tissues. Therefore, the strongest tissue‐specific eQTL effects associated with T1D risk occurred in the lung, supporting the potential contribution of respiratory infections on the development of IA/T1D.
4. CORONAVIRUS INFECTION AND TYPE 1 DIABETES
Severe acute respiratory syndrome related coronavirus 2 (SARS‐CoV‐2) infection and its related disease, coronavirus disease (COVID‐19), has an unclear relationship with T1D. Although several recent studies have reported possible associations between SARS‐CoV‐2 infection and IA/T1D, 71 , 72 , 73 , 74 it remains too early to draw any meaningful conclusions. Like other viruses, SARS‐CoV‐2 infections can induce a stress response that may diminish insulin secretion, release counter‐regulatory hormones like cortisol and adrenaline, induce excessive gluconeogenesis and impair glucose disposal, thereby causing transient hyperglycaemia. However, these mechanisms may not necessarily cause diabetes. 75 , 76 , 77
The mechanism of how SARS‐CoV‐2 may cause T1D has been explored within in vitro and ex vivo studies. The detection of SARS‐CoV‐2 in post‐mortem pancreatic samples 78 , 79 , 80 and reduced pancreatic function in people with COVID‐19 81 suggests SARS‐CoV‐2 and its related virus SARS‐CoV‐1 may damage pancreatic β‐cell and cause new‐onset diabetes via direct infection and the subsequent inflammatory response and interactions with the renin‐angiotensin system. 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 Nevertheless, whether the infection of pancreatic β‐cells in tissue samples accurately mimics in vivo infection remains unclear.
Studies investigating associations between SARS‐CoV‐2 and T1D have been steadily increasing across the last 3 years. Cross‐sectional studies 82 , 83 , 84 , 85 , 86 , 87 have reported an increase in incidence of T1D and diabetic ketoacidosis (DKA) during the pandemic, and there are case reports 89 , 90 , 91 , 92 , 93 , 94 of individuals with recent SARS‐CoV‐2 infection presenting to hospital with new‐onset T1D and DKA, which suggest that SARS‐CoV‐2 infection may accelerate T1D development or increase the risk of its metabolic complications. However, the increased incidence of DKA and T1D during the pandemic may be confounded by reduced access or hesitancy to use healthcare services, leading to delayed presentations of T1D and higher incidence of DKA, 95 , 96 and individuals presenting to hospital with COVID‐19 may have pre‐existing undiagnosed T1D.
National retrospective cohort studies based on medical claims databases have reported mixed results regarding the incidence of T1D following SARS‐CoV‐2 infections. A US Centres for Disease Control and Prevention (CDC) paper 97 using two US medical claims databases reported a significantly higher risk of new‐onset diabetes 30 days or more after SARS‐CoV‐2 infection in persons under 18 years. While the CDC report included all types of diabetes which lowers specificity, another national retrospective cohort in the US found higher risk of new‐onset T1D and DKA in individuals with previous SARS‐CoV‐2 infection. 98 A similar retrospective cohort in Scotland also reported an association between SARS‐CoV‐2 infection and T1D, but only for infection within the 30 days of T1D onset. 99 It is plausible that SARS‐CoV‐2 infection may acutely contribute to the accelerated progression of symptomatic T1D and diagnosis in at risk individuals, which aligns with the role of other viruses, such as enteroviruses, in the progression to clinical T1D. 100 However, since transient hyperglycaemia is associated with SARS‐CoV‐2 infection, 76 T1D may have been misdiagnosed during the acute stages of SARS‐CoV‐2 infection. Furthermore, higher opportunistic testing rates around the time of presentation of either SARS‐CoV‐2 or T1D may have also contributed to incidental diagnosis of the secondary condition, and SARS‐CoV‐2 may trigger metabolic decompensation that precipitates diagnosis of nascent T1D, 97 , 99 limiting the strength of these associations.
A meta‐analysis of eight retrospective cohort studies comprising 3700 hospitalised COVID‐19 patients found 14.4% had new‐onset T1D. 101 However, the meta‐analysis 101 of retrospective studies included individuals ranging from 47.0 to 64.9 years in age, outside of the typical age range when T1D is diagnosed, which may suggest an alternative pathogenesis. Indeed, several case reports feature individuals with new‐onset autoantibody‐negative T1D on a background of COVID‐19. 90 , 93 A prospective study that followed people with DKA and autoantibody‐negative T1D after COVID‐19 reported that most individuals achieved β‐cell recovery and insulin independence, suggesting an autoantibody‐negative T1D in contrast with the IA pathway classically seen in T1D. 102 Nevertheless, additional mechanistic studies are needed to validate this pathogenic hypothesis.
The relationship between COVID‐19 and T1D remains a poorly understood and rapidly evolving area of research, with its long‐term diabetogenic effects likely to be unknown until after many years of extensive research. To this end, a global registry (CoviDiab) was established to investigate their interaction. 103 Long‐term prospective analysis is needed to decipher any relationship between COVID‐19 and T1D.
5. CONCLUSION
There is an enormous body of accumulated evidence, both molecular and epidemiological, that support the hypothesised role of viral infections in the development of IA and T1D. By comparison, there remains a major gap in understanding and paucity of data, especially molecular data where infectious agents are characterised at the nucleic acid or protein level, that elucidates the relationship between RTI and IA/T1D. To address this gap, the use of comprehensive metage detection methods, and the prospective collection of respiratory samples and IA testing during pregnancy and early life in large prospective cohorts such as the ENDIA, 68 TEDDY 47 and DIPP‐novum 39 will be important. If a clinically significant association between specific respiratory viruses and T1D are established in the future, primary prevention of T1D may be possible through antiviral vaccines.
AUTHOR CONTRIBUTIONS
Roy Wu: writing—original draft; editing. Mohsin Mumtaz, Anna J. Maxwell, Sonia R. Isaacs, Jutta E. Laiho, William D. Rawlinson, Heikki Hyöty: reviewing, editing. Maria E. Craig, Ki Wook Kim: conceptualisation; preparation; writing; editing.
CONFLICT OF INTEREST STATEMENT
None of the authors have any conflicts of interest to declare.
ACKNOWLEDGEMENTS
Ki Wook Kim was supported by the Juvenile Diabetes Research Foundation (JDRF) International Postdoctoral Fellowship 3‐PDF‐2020‐940‐A‐N, the ENDIA Science Continuation Grant funded by the Leona M. and Harry B. Helmsley Charitable Trust (HCT), and the ENDIA Early‐Mid Career Science Accelerator Award (Grant # 2‐SRA‐2021‐1083‐M‐B) funded by HCT and JDRF Australian Type 1 Diabetes Clinical Research Network the recipient of Australian Government Department of Health funding through the Emerging Priorities and Consumer‐Driven Research Initiative, part of the Medical Research Future Fund. M.E. Craig is supported by a National Health and Medical Research Council Practitioner fellowship APP1136735. S. Isaacs is supported by Australian Government Research Training Program Scholarship and the Environmental Determinants of Islet Autoimmunity (ENDIA) PhD top‐up scholarship.
Open access publishing facilitated by University of New South Wales, as part of the Wiley ‐ University of New South Wales agreement via the Council of Australian University Librarians.
Wu R, Mumtaz M, Maxwell AJ, et al. Respiratory infections and type 1 diabetes: potential roles in pathogenesis. Rev Med Virol. 2023;33(2):e2429. 10.1002/rmv.2429
Maria E. Craig and Ki Wook Kim are joint senior.
DATA AVAILABILITY STATEMENT
All data used are available in this review.
REFERENCES
- 1. Green A, Hede SM, Patterson CC, et al. Type 1 diabetes in 2017: global estimates of incident and prevalent cases in children and adults. Diabetologia. 2021/12/01 2021;64(12):2741‐2750. 10.1007/s00125-021-05571-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Isaacs SR, Foskett DB, Maxwell AJ, et al. Viruses and type 1 diabetes: from enteroviruses to the virome. Microorganisms. 2021;9(7):1519. 10.3390/microorganisms9071519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Knip M, Luopajarvi K, Harkonen T. Early life origin of type 1 diabetes. Semin Immunopathol. 2017;39(6):653‐667. 10.1007/s00281-017-0665-6 [DOI] [PubMed] [Google Scholar]
- 4. Mayer‐Davis EJ, Kahkoska AR, Jefferies C, et al. ISPAD clinical practice consensus guidelines 2018: definition, epidemiology, and classification of diabetes in children and adolescents. Pediatr Diabetes. 2018;19(27):7‐19. 10.1111/pedi.12773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Faulkner CL, Luo YX, Isaacs S, Rawlinson WD, Craig ME, Kim KW. The virome in early life and childhood and development of islet autoimmunity and type 1 diabetes: a systematic review and meta‐analysis of observational studies. Rev Med Virol. 2021;31(5):2209‐2214. 10.1002/rmv.2209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rewers M, Ludvigsson J. Environmental risk factors for type 1 diabetes. Lancet. 2016;387(10035):2340‐2348. 10.1016/S0140-6736(16)30507-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Craig ME, Nair S, Stein H, Rawlinson WD. Viruses and type 1 diabetes: a new look at an old story. Pediatr Diabetes. 2013;14(3):149‐158. 10.1111/pedi.12033 [DOI] [PubMed] [Google Scholar]
- 8. Mobasseri M, Shirmohammadi M, Amiri T, Vahed N, Hosseini Fard H, Ghojazadeh M. Prevalence and incidence of type 1 diabetes in the world: a systematic review and meta‐analysis. Health Promot Perspect. 2020;10(2):98‐115. 10.34172/hpp.2020.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Morgan E, Halliday SR, Campbell GR, Cardwell CR, Patterson CC. Vaccinations and childhood type 1 diabetes mellitus: a meta‐analysis of observational studies. Diabetologia. 2016;59(2):237‐243. 10.1007/s00125-015-3800-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rewers M, Hyöty H, Lernmark Å, et al. The environmental determinants of diabetes in the young (TEDDY) Study: 2018 update. Curr Diabetes Rep. 2018;18(12):136. 10.1007/s11892-018-1113-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kondrashova A, Reunanen A, Romanov A, et al. A six‐fold gradient in the incidence of type 1 diabetes at the eastern border of Finland. Ann Med. 2005;37(1):67‐72. 10.1080/07853890410018952 [DOI] [PubMed] [Google Scholar]
- 12. Oilinki T, Otonkoski T, Ilonen J, Knip M, Miettinen P. Prevalence and characteristics of diabetes among Somali children and adolescents living in Helsinki, Finland. Pediatr Diabetes. 2012;13(2):176‐180. 10.1111/j.1399-5448.2011.00783.x [DOI] [PubMed] [Google Scholar]
- 13. Söderström U, Åman J, Hjern A. Being born in Sweden increases the risk for type 1 diabetes – a study of migration of children to Sweden as a natural experiment. Acta Paediatr. 2012;101(1):73‐77. 10.1111/j.1651-2227.2011.02410.x [DOI] [PubMed] [Google Scholar]
- 14. Samuelsson U, Oikarinen S, Hyöty H, Ludvigsson J. Low zinc in drinking water is associated with the risk of type 1 diabetes in children. Pediatr Diabetes. 2011;12(3):156‐164. 10.1111/j.1399-5448.2010.00678.x [DOI] [PubMed] [Google Scholar]
- 15. Wilkin TJ. The accelerator hypothesis: weight gain as the missing link between type I and type II diabetes. Diabetologia. 2001;44(7):914‐922. 10.1007/s001250100548 [DOI] [PubMed] [Google Scholar]
- 16. Quinn LM, Wong FS, Narendran P. Environmental determinants of type 1 diabetes: from association to proving causality. Front Immunol. 2021;12. 10.3389/fimmu.2021.737964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Viskari HR, Koskela P, Lönnrot M, et al. Can enterovirus infections explain the increasing incidence of type 1 diabetes? Diabetes Care. 2000;23(3):414‐416. 10.2337/diacare.23.3.414 [DOI] [PubMed] [Google Scholar]
- 18. Viskari H, Ludvigsson J, Uibo R, et al. Relationship between the incidence of type 1 diabetes and maternal enterovirus antibodies: time trends and geographical variation. Diabetologia. 2005;48(7):1280‐1287. 10.1007/s00125-005-1780-9 [DOI] [PubMed] [Google Scholar]
- 19. Gamble DR, Kinsley ML, FitzGerald MG, Bolton R, Taylor KW. Viral antibodies in diabetes mellitus. BMJ. 1969;3(5671):627‐630. 10.1136/bmj.3.5671.627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gamble DR, Taylor KW, Cumming H. Coxsackie viruses and diabetes mellitus. BMJ. 1973;4(5887):260‐262. 10.1136/bmj.4.5887.260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gundersen E. Is diabetes of infectious origin? J Infect Dis. 1927;41(3):197‐202. 10.1093/infdis/41.3.197 [DOI] [Google Scholar]
- 22. Cinek O, Stene LC, Kramna L, et al. Enterovirus RNA in longitudinal blood samples and risk of islet autoimmunity in children with a high genetic risk of type 1 diabetes: the MIDIA study. Diabetologia. 2014;57(10):2193‐2200. 10.1007/s00125-014-3327-4 [DOI] [PubMed] [Google Scholar]
- 23. Kim KW, Horton JL, Pang CNI, et al. Higher abundance of enterovirus A species in the gut of children with islet autoimmunity. Sci Rep. 2019/02/11 2019;9(1):1749. 10.1038/s41598-018-38368-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Oikarinen M, Laiho JE, Oikarinen S, et al. Detection of enterovirus protein and RNA in multiple tissues from nPOD organ donors with type 1 diabetes. BioRxiv. 2018. 10.1101/459347 [DOI] [Google Scholar]
- 25. Sioofy‐Khojine AB, Lehtonen J, Nurminen N, et al. Coxsackievirus B1 infections are associated with the initiation of insulin‐driven autoimmunity that progresses to type 1 diabetes. Diabetologia. 2018;61(5):1193‐1202. 10.1007/s00125-018-4561-y [DOI] [PubMed] [Google Scholar]
- 26. Beyerlein A, Donnachie E, Jergens S, Ziegler AG. Infections in early life and development of type 1 diabetes. JAMA. 2016;315(17):1899‐1901. 10.1001/jama.2016.2181 [DOI] [PubMed] [Google Scholar]
- 27. Engelmann I, Alidjinou EK, Bertin A, et al. Persistent coxsackievirus B4 infection induces microRNA dysregulation in human pancreatic cells. Cell Mol Life Sci. 2017;74(20):3851‐3861. 10.1007/s00018-017-2567-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Horwitz MS, Bradley LM, Harbertson J, Krahl T, Lee J, Sarvennick N. Diabetes induced by Coxsackie virus: initiation by bystander damage and not molecular mimicry. Nat Med. 1998/07/01 1998;4(7):781‐785. 10.1038/nm0798-781 [DOI] [PubMed] [Google Scholar]
- 29. Op de Beeck A, Eizirik DL. Viral infections in type 1 diabetes mellitus‐‐why the β cells? Nat Rev Endocrinol. 2016;12(5):263‐273. 10.1038/nrendo.2016.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lloyd RE, Tamhankar M, Lernmark Å. Enteroviruses and type 1 diabetes: multiple mechanisms and factors? Annu Rev Med. 2022;73:483‐499. 10.1146/annurev-med-042320-015952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Simonen‐Tikka ML, Pflueger M, Klemola P, et al. Human enterovirus infections in children at increased risk for type 1 diabetes: the Baby diet study. Diabetologia. 2011;54(12):2995‐3002. 10.1007/s00125-011-2305-3 [DOI] [PubMed] [Google Scholar]
- 32. Watad A, Azrielant S, Bragazzi NL, et al. Seasonality and autoimmune diseases: the contribution of the four seasons to the mosaic of autoimmunity. J Autoimmun. 2017;82:13‐30. 10.1016/j.jaut.2017.06.001 [DOI] [PubMed] [Google Scholar]
- 33. Oikarinen M, Tauriainen S, Honkanen T, et al. Detection of enteroviruses in the intestine of type 1 diabetic patients. Clin Exp Immunol. 2008;151(1):71‐75. 10.1111/j.1365-2249.2007.03529.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Oikarinen M, Tauriainen S, Oikarinen S, et al. Type 1 diabetes is associated with enterovirus infection in gut mucosa. Diabetes. 2012;61(3):687‐691. 10.2337/db11-1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Krogvold L, Edwin B, Buanes T, et al. Detection of a low‐grade enteroviral infection in the islets of Langerhans of living patients newly diagnosed with type 1 diabetes. Diabetes. 2015;64(5):1682‐1687. 10.2337/db14-1370 [DOI] [PubMed] [Google Scholar]
- 36. Richardson SJ, Willcox A, Bone AJ, Foulis AK, Morgan NG. The prevalence of enteroviral capsid protein vp1 immunostaining in pancreatic islets in human type 1 diabetes. Diabetologia. 2009;52(6):1143‐1151. 10.1007/s00125-009-1276-0 [DOI] [PubMed] [Google Scholar]
- 37. Oikarinen S, Tauriainen S, Hober D, et al. Virus antibody survey in different European populations indicates risk association between coxsackievirus B1 and type 1 diabetes. Diabetes. 2014;63(2):655‐662. 10.2337/db13-0620 [DOI] [PubMed] [Google Scholar]
- 38. Rešić Lindehammer S, Honkanen H, Nix WA, et al. Seroconversion to islet autoantibodies after enterovirus infection in early pregnancy. Viral Immunol. 2012;25(4):254‐261. 10.1089/vim.2012.0022 [DOI] [PubMed] [Google Scholar]
- 39. Salminen K, Sadeharju K, Lönnrot M, et al. Enterovirus infections are associated with the induction of beta‐cell autoimmunity in a prospective birth cohort study. J Med Virol. 2003;69(1):91‐98. 10.1002/jmv.10260 [DOI] [PubMed] [Google Scholar]
- 40. Beyerlein A, Wehweck F, Ziegler AG, Pflueger M. Respiratory infections in early life and the development of islet autoimmunity in children at increased type 1 diabetes risk: evidence from the BABYDIET study. JAMA Pediatr. 2013;167(9):800‐807. 10.1001/jamapediatrics.2013.158 [DOI] [PubMed] [Google Scholar]
- 41. Mustonen N, Siljander H, Peet A, et al. Early childhood infections precede development of beta‐cell autoimmunity and type 1 diabetes in children with HLA‐conferred disease risk. Pediatr Diabetes. 2018;19(2):293‐299. 10.1111/pedi.12547 [DOI] [PubMed] [Google Scholar]
- 42. Ponsonby AL, Pezic A, Cochrane J, et al. Infant anthropometry, early life infection, and subsequent risk of Type 1 diabetes mellitus: a prospective birth cohort study. Pediatr Diabetes. 2011;12(4):313‐321. 10.1111/j.1399-5448.2010.00693.x [DOI] [PubMed] [Google Scholar]
- 43. Lee HY, Lu CL, Chen HF, Su HF, Li CY. Perinatal and childhood risk factors for early‐onset type 1 diabetes: a population‐based case‐control study in Taiwan. Eur J Publ Health. 2015;25(6):1024‐1029. 10.1093/eurpub/ckv059 [DOI] [PubMed] [Google Scholar]
- 44. Ruiz PLD, Tapia G, Bakken IJ, et al. Pandemic influenza and subsequent risk of type 1 diabetes: a nationwide cohort study. Diabetologia. 2018;61(9):1996‐2004. 10.1007/s00125-018-4662-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cardwell CR, Carson DJ, Patterson CC. No association between routinely recorded infections in early life and subsequent risk of childhood‐onset Type 1 diabetes: a matched case‐control study using the UK general practice research database. Diabet Med. 2008;25(3):261‐267. 10.1111/j.1464-5491.2007.02351.x [DOI] [PubMed] [Google Scholar]
- 46. Rasmussen T, Witso E, Tapia G, Stene LC, Ronningen KS. Self‐reported lower respiratory tract infections and development of islet autoimmunity in children with the type 1 diabetes high‐risk HLA genotype: the MIDIA study. Diabetes Metab Res Rev. 2011;27(8):834‐837. 10.1002/dmrr.1258 [DOI] [PubMed] [Google Scholar]
- 47. Lonnrot M, Lynch KF, Elding Larsson H, et al. Respiratory infections are temporally associated with initiation of type 1 diabetes autoimmunity: the TEDDY study. Diabetologia. 2017;60(10):1931‐1940. 10.1007/s00125-017-4365-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kordonouri O, Cuthbertson D, Belteky M, et al. Infections in the first year of life and development of beta cell autoimmunity and clinical type 1 diabetes in high‐risk individuals: the TRIGR cohort. Diabetologia. 2022;65(12):2098‐2107. 10.1007/s00125-022-05786-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tapia G, Størdal K, Mårild K, et al. Antibiotics, acetaminophen and infections during prenatal and early life in relation to type 1 diabetes. Int J Epidemiol. 2018;47(5):1538‐1548. 10.1093/ije/dyy092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Snell‐Bergeon JK, Smith J, Dong F, et al. Early childhood infections and the risk of islet autoimmunity: the diabetes autoimmunity study in the young (DAISY). Diabetes Care. 2012;35(12):2553‐2558. 10.2337/dc12-0423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lynch KF, Lee HS, Törn C, et al. Gestational respiratory infections interacting with offspring HLA and CTLA‐4 modifies incident β‐cell autoantibodies. J Autoimmun. 2018;86:93‐103. 10.1016/j.jaut.2017.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Stene LC, Barriga K, Norris JM, et al. Symptoms of common maternal infections in pregnancy and risk of islet autoimmunity in early childhood. Diabetes Care. 2003;26(11):3136‐3141. 10.2337/diacare.26.11.3136 [DOI] [PubMed] [Google Scholar]
- 53. Bélteky M, Wahlberg J, Ludvigsson J. Maternal respiratory infections in early pregnancy increases the risk of type 1 diabetes. Pediatr Diabetes. 2020;21(7):1193‐1201. 10.1111/pedi.13075 [DOI] [PubMed] [Google Scholar]
- 54. Niklasson B, Heller KE, Schønecker B, et al. Development of type 1 diabetes in wild bank voles associated with islet autoantibodies and the novel ljungan virus. Int J Exp Diabetes Res. 2003;4(1):35‐44. 10.1080/15438600303733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Nishioka Y, Noda T, Okada S, et al. Association between influenza and the incidence rate of new‐onset type 1 diabetes in Japan. J Diabetes Investig. 2021;12(10):1797‐1804. 10.1111/jdi.13540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Nenna R, Papoff P, Moretti C, et al. Detection of respiratory viruses in the 2009 winter season in Rome: 2009 influenza A (H1N1) complications in children and concomitant type 1 diabetes onset. Int J Immunopathol Pharmacol. 2011;24(3):651‐659. 10.1177/039463201102400311 [DOI] [PubMed] [Google Scholar]
- 57. Group TESS. Infections and vaccinations as risk factors for childhood type I (insulin‐dependent) diabetes mellitus: a multicentre case‐control investigation. EURODIAB Substudy 2 Study Group. Diabetologia. 2000;43(1):47‐53. 10.1007/s001250050006 [DOI] [PubMed] [Google Scholar]
- 58. Verge CF, Howard JN, Irwig L, Simpson JM, Mackerras D, Silink M. Enviromental factors in childhood IDDM: a population‐based, case‐control study. Diabetes Care. 1994;12:1381‐1389. 10.2337/diacare.17.12.1381 [DOI] [PubMed] [Google Scholar]
- 59. Sipetic S, Vlajinac H, Kocev N, Radmanovic S. The Belgrade childhood diabetes study: association of infections and vaccinations on diabetes in childhood. Ann Epidemiol. 2003;13(9):645‐651. 10.1016/S1047-2797(03)00065-6 [DOI] [PubMed] [Google Scholar]
- 60. Visalli N, Sebastiani L, Adorisio E, et al. Environmental risk factors for type 1 diabetes in Rome and province. Arch Dis Child. 2003;88(8):695‐698. 10.1136/adc.88.8.695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kolehmainen P, Koskiniemi M, Oikarinen S, et al. Human parechovirus and the risk of type 1 diabetes. J Med Virol. 2013;85(9):1619‐1623. 10.1002/jmv.23659 [DOI] [PubMed] [Google Scholar]
- 62. Kim KW, Ho A, Alshabee‐Akil A, et al. Coxsackievirus B5 infection induces dysregulation of microRNAs predicted to target known type 1 diabetes risk genes in human pancreatic islets. Diabetes. 2016;65(4):996‐1003. 10.2337/db15-0956 [DOI] [PubMed] [Google Scholar]
- 63. Van Leer‐Buter CC, Poelman R, Borger R, Niesters HG. Newly identified enterovirus C genotypes, identified in The Netherlands through routine sequencing of all enteroviruses detected in clinical materials from 2008 to 2015. J Clin Microbiol. 2016;54(9):2306‐2314. 10.1128/jcm.00207-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Royston L, Tapparel C. Rhinoviruses and respiratory enteroviruses: not as simple as ABC. Viruses. 2016;8(1):16. 10.3390/v8010016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ferreira RC, Guo H, Coulson RM, et al. A type I interferon transcriptional signature precedes autoimmunity in children genetically at risk for type 1 diabetes. Diabetes. 2014;63(7):2538‐2550. 10.2337/db13-1777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hippich M, Oleynik A, Jain K, et al. Searching peripheral blood mononuclear cells of children with viral respiratory tract infections preceding islet autoimmunity for viruses by high‐throughput sequencing. Acta Diabetol. 2018;55(8):881‐884. 10.1007/s00592-018-1138-7 [DOI] [PubMed] [Google Scholar]
- 67. Tang JW, Lam TT, Zaraket H, et al. Global epidemiology of non‐influenza RNA respiratory viruses: data gaps and a growing need for surveillance. Lancet Infect Dis. 2017;17(10):e320‐e326. 10.1016/s1473-3099(17)30238-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Penno MAS, Couper JJ, Craig ME, et al. Environmental determinants of islet autoimmunity (ENDIA): a pregnancy to early life cohort study in children at‐risk of type 1 diabetes. BMC Pediatr. 2013/08/14 2013;13(1):124. 10.1186/1471-2431-13-124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Haller MJ, Schatz DA. The DIPP project: 20 years of discovery in type 1 diabetes. Pediatr Diabetes. 2016;17(Suppl 22):5‐7. 10.1111/pedi.12398 [DOI] [PubMed] [Google Scholar]
- 70. Ho D, Nyaga DM, Schierding W, et al. Identifying the lungs as a susceptible site for allele‐specific regulatory changes associated with type 1 diabetes risk. Commun Biology. 2021/09/14 2021;4(1):1072. 10.1038/s42003-021-02594-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Denina M, Trada M, Tinti D, et al. Increase in newly diagnosed type 1 diabetes and serological evidence of recent SARS‐CoV‐2 infection: is there a connection? Front Med. 2022;9:927099. 10.3389/fmed.2022.927099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Gulseth HL, Ruiz PLD, Størdal K, et al. SARS‐CoV‐2 infection and subsequent risk of type 1 diabetes in 1.2 million children. EASD. 2022:2022. [Google Scholar]
- 73. Kendall EK, Olaker VR, Kaelber DC, Xu R, Davis PB. Association of SARS‐CoV‐2 Infection with new‐onset type 1 diabetes among pediatric patients from 2020 to 2021. JAMA Netw Open. 2022;5(9):e2233014. 10.1001/jamanetworkopen.2022.33014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Xie Y, Al‐Aly Z. Risks and burdens of incident diabetes in long COVID: a cohort study. Lancet Diabetes Endocrinol. 2022;10(5):311‐321. 10.1016/S2213-8587(22)00044-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Atkinson MA, Powers AC. Distinguishing the real from the hyperglycaemia: does COVID‐19 induce diabetes? Lancet Diabetes Endocrinol. 2021;9(6):328‐329. 10.1016/s2213-8587(21)00087-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Chen J, Wu C, Wang X, Yu J, Sun Z. The impact of COVID‐19 on blood glucose: a systematic review and meta‐analysis. Review. Front Endocrinol. 2020;11:11574541. 10.3389/fendo.2020.574541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Laurenzi A, Caretto A, Molinari C, et al. No evidence of long‐term disruption of glycometabolic control after SARS‐CoV‐2 infection. J Clin Endocrinol Metab. 2022;107(3):e1009‐e1019. 10.1210/clinem/dgab792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Menezes RG, Rizwan T, Saad Ali S, et al. Postmortem findings in COVID‐19 fatalities: a systematic review of current evidence. Leg Med. 2022;54:102001. 10.1016/j.legalmed.2021.102001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Müller JA, Groß R, Conzelmann C, et al. SARS‐CoV‐2 infects and replicates in cells of the human endocrine and exocrine pancreas. Nature Metabolism. 2021/02/01 2021;3(2):149‐165. 10.1038/s42255-021-00347-1 [DOI] [PubMed] [Google Scholar]
- 80. Steenblock C, Richter S, Berger I, et al. Viral infiltration of pancreatic islets in patients with COVID‐19. Nat Commun. 2021;12(1):3534. 10.1038/s41467-021-23886-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Ben Nasr M, D'Addio F, Montefusco L, et al. Indirect and direct effects of SARS‐CoV‐2 on human pancreatic islets. Diabetes. 2022;71(7):1579‐1590. 10.2337/db21-0926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Alexandre MI, Henriques AR, Cavaco D, et al. New‐onset type 1 diabetes in children and COVID‐19. Diabetes Tipo 1 Inaugural em Idade Pediatrica e COVID‐19. Letter. Acta Med Port. 2021;34(9):630‐645. 10.20344/amp.16412 [DOI] [PubMed] [Google Scholar]
- 83. Dilek S, Gürbüz F, Turan İ, Celiloğlu C, Yüksel B. Changes in the presentation of newly diagnosed type 1 diabetes in children during the COVID‐19 pandemic in a tertiary center in Southern Turkey. J Pediatr Endocrinol Metab. 2021;34(10):1303‐1309. 10.1515/jpem-2021-0287 [DOI] [PubMed] [Google Scholar]
- 84. Goldman N, Fink D, Cai J, Lee Y.‐N, Davies Z. High prevalence of COVID‐19‐associated diabetic ketoacidosis in UK secondary care. Diabetes Res Clin Pract. 2020/08/01/ 2020;166:108291. 10.1016/j.diabres.2020.108291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Li J, Wang X, Chen J, Zuo X, Zhang H, Deng A. COVID‐19 infection may cause ketosis and ketoacidosis. Diabetes Obes Metabol. 2020;22(10):1935‐1941. 10.1111/dom.14057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Marks BE, Khilnani A, Meyers A, et al. Increase in the diagnosis and severity of presentation of pediatric Type 1 and Type 2 diabetes during the COVID‐19 pandemic. Horm Res Paediatr. 2021;94(7‐8):275‐284. 10.1159/000519797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. McGlacken‐Byrne SM, Drew SEV, Turner K, Peters C, Amin R. The SARS‐CoV‐2 pandemic is associated with increased severity of presentation of childhood onset type 1 diabetes mellitus: a multi‐centre study of the first COVID‐19 wave. Diabet Med. 2021;38(9):e14640. 10.1111/dme.14640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. van der Heide V, Jangra S, Cohen P, et al. Limited extent and consequences of pancreatic SARS‐CoV‐2 infection. Cell Rep. 2022;38(11):110508. 10.1016/j.celrep.2022.110508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Chee YJ, Ng SJH, Yeoh E. Diabetic ketoacidosis precipitated by COVID‐19 in a patient with newly diagnosed diabetes mellitus. Diabetes Res Clin Pract. 2020;164:e108166. 10.1016/j.diabres.2020.108166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Hollstein T, Schulte DM, Schulz J, et al. Autoantibody‐negative insulin‐dependent diabetes mellitus after SARS‐CoV‐2 infection: a case report. Nat Metab. 2020;2(10):1021‐1024. 10.1038/s42255-020-00281-8 [DOI] [PubMed] [Google Scholar]
- 91. Ishii K, Suwanai H, Saito T, et al. A case of diabetic ketoacidosis in a patient with COVID‐19 and newly diagnosed type 1 diabetes. Clin Case Rep. 2021;9(9):e04881. 10.1002/ccr3.4881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Marchand L, Pecquet M, Luyton C. Type 1 diabetes onset triggered by COVID‐19. Acta Diabetol. 2020;57(10):1265‐1266. 10.1007/s00592-020-01570-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Seow CJ, Wei Choon Koh A, Lian JX, Dalan R, Boehm BO. Non autoimmune type 1B diabetes after mild COVID‐19: report of three cases. Letter Diabetes Metab Res Rev. 2021;37(5):e3438. 10.1002/dmrr.3438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Venkatesh N, Astbury N, Thomas MC, et al. Severe acute respiratory syndrome coronavirus 2 as a potential cause of type 1 diabetes facilitated by spike protein receptor binding domain attachment to human islet cells: an illustrative case study and experimental data. Diabet Med. 2021;38(11):e14608. 10.1111/dme.14608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Boddu SK, Aurangabadkar G, Kuchay MS. New onset diabetes, type 1 diabetes and COVID‐19. Review. Diabetes Metabol Syndr. 2020;14(6):2211‐2217. 10.1016/j.dsx.2020.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Steenblock C, Schwarz PEH, Ludwig B, et al. COVID‐19 and metabolic disease: mechanisms and clinical management. Lancet Diabetes Endocrinol. 2021;9(11):786‐798. 10.1016/s2213-8587(21)00244-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Barrett CE, Koyama AK, Alvarez P, et al. Risk for newly diagnosed diabetes >30 days after SARS‐CoV‐2 infection among persons aged <18 Years – United States, March 1, 2020‐June 28, 2021. MMWR (Morb Mortal Wkly Rep). 2022;71(2):59‐65. 10.15585/mmwr.mm7102e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Qeadan F, Tingey B, Egbert J, et al. The associations between COVID‐19 diagnosis, type 1 diabetes, and the risk of diabetic ketoacidosis: a nationwide cohort from the US using the Cerner Real‐World Data. PLOS One. 2022;17(4):e0266809. 10.1371/journal.pone.0266809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. McKeigue PM, McGurnaghan S, Blackbourn L, et al. Relation of incident type 1 diabetes to recent COVID‐19 Infection: cohort study using e‐health record linkage in Scotland. Diabetes Care. 2022. 10.2337/dc22-0385 [DOI] [PubMed] [Google Scholar]
- 100. Yeung WC, Rawlinson WD, Craig ME. Enterovirus infection and type 1 diabetes mellitus: systematic review and meta‐analysis of observational molecular studies. BMJ. 2011;342(feb03 1):d35. 10.1136/bmj.d35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Sathish T, Kapoor N, Cao Y, Tapp RJ, Zimmet P. Proportion of newly diagnosed diabetes in COVID‐19 patients: a systematic review and meta‐analysis. Diabetes Obes Metabol. 2021;23(3):870‐874. 10.1111/dom.14269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Gupta RD, Atri A, Mondal S, et al. Characterizing progressive beta‐cell recovery after new‐onset DKA in COVID‐19 provoked A‐β+ KPD (ketosis‐prone diabetes): a prospective study from eastern India. J Diabetes Complicat. 2022/03/01/ 2022;36(3):108100. 10.1016/j.jdiacomp.2021.108100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Rubino F, Amiel SA, Zimmet P, et al. New‐onset diabetes in COVID‐19. N Engl J Med. 2020;383(8):789‐790. 10.1056/NEJMc2018688 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data used are available in this review.


