Abstract
Objective:
Electronic cigarettes have increased in popularity globally. Vaping may be associated with oral symptoms and pathologies including dental and periodontal damage, both of which have an underlying microbial etiology. The primary aim of this pilot study, therefore, was to compare the oral microbiome of vapers and non-vapers.
Subjects and Methods:
This secondary data analysis had a cross-sectional comparative descriptive design and included data for 36 adults. Bacterial 16S rRNA genes were extracted and amplified from soft tissue oral swab specimens and taxonomically classified using the Human Oral Microbiome Database.
Results:
Data for 18 vapers and 18 non-vapers were included in this study. Almost 56% of the vapers also smoked conventional cigarettes. Beta diversity differences were identified between vapers and non-vapers. Vapers had a significantly higher relative abundance of an unclassified species of Veillonella compared with non-vapers. Dual users had higher alpha diversity compared with exclusive vapers. Beta diversity was also associated with dual use. Multiple OTUs were identified to be associated with dual use of e-cigarettes and conventional cigarettes.
Conclusions:
Vapers exhibit an altered oral microbiome. Dual use of electronic cigarettes and conventional cigarettes is associated with the presence of several known pathogenic microbes.
Keywords: e-cigarette, electronic cigarette, ENDS, oral microbiome, vape
1 ∣. INTRODUCTION
Electronic cigarettes (e-cigarettes) are battery-powered devices designed to heat and aerosolize liquids with constituents such as propylene glycol, glycerol as humectants, varied flavors, and in most cases, nicotine (McRobbie et al., 2015). Since coming on the market in the United States and Europe in 2006 (Rahman et al., 2014), e-cigarettes have increased in popularity. Globally, the number of vapers has grown from seven million in 2011 to 41 million in 2018, and is estimated to increase to 55 million by 2021 (Jones, 2019). The United States ranks as the number one market for vape products (Jones, 2019). The most current U.S. statistics indicate that in 2018, an estimated 8.1 million U.S. adults were current vapers and 14.9% of adults reported “ever use” of an e-cigarette (Villarroel et al., 2020).
The appeal of e-cigarettes is attributed to various reasons including: ease of accessibility, choice of flavors, sense of fashion, and the perception that they are a healthier alternative to conventional cigarettes (Sapru et al., 2020). While it is widely accepted that vaping is less harmful than smoking (CDC, 2020; Sapru et al., 2020), it is important to note that vaping is not harmless (CDC, 2020), and e-cigarettes expose users to several chemicals known to have adverse health effects including nicotine (Marques et al., 2021), volatile organic compounds, and carbonyl compounds (Ebersole et al., 2020). The formation of hazardous e-cigarette components is influenced by vaping behavior and e-cigarette constituents. Specifically, the puffing topography, power output, device design, coil composition, and e-liquid formula all influence the emission levels of toxic carbonyls such as formaldehyde, aldehyde, and acrolein. Formaldehyde and aldehyde have enhanced reactivity and water solubility leading to concerns regarding oronasal retention and potential adverse oral health effects (Samburova et al., 2018).
Emerging research on the oral health effects of e-cigarette use suggests that vaping may be associated with various oral symptoms and pathologies including dental, and periodontal damage (Yang et al., 2020). Recent data revealed vegetable glycerin used in e-liquids elicits a 4-fold increase in microbial adhesion to enamel and a 2-fold increase in biofilm formation leading to pathogenic bacterial invasion and eventual tooth decay (Kim et al., 2018). Because dental caries and periodontal disease are the two most common oral pathologies and because they both have an underlying microbial etiology, the oral microbiome is an appropriate target of research to begin exploring oral health outcomes of vaping.
The impact of e-cigarette use on the oral microbiome is not yet fully understood. Research is emerging, with studies identifying an association between vaping and an increased prevalence of candidiasis or growth/abundance of Candida albicans (Alanazi et al., 2019; Bardellini et al., 2018; Mokeem et al., 2019), as well as a higher abundance of Proteobacteria, and opportunistic pathogens such as Rothia and Haemophilus (Chopyk et al., 2021; Kumar et al., 2019). The viscosity of e-liquids may also contribute to the increased adhesiveness of caries-causing Streptococcal spp. (Kim et al., 2018).
Compared with conventional smoking, some studies show little to no impact of vaping on the oral microbiome (Cuadra et al., 2019; Stewart et al., 2018), while newer studies suggest that vapers have a distinct oral microbiome profile compared with that of both conventional cigarette users and non-vapers/non-smokers (Pushalkar et al., 2020). The literature to date is equivocal. Our study seeks to contribute to and clarify the emerging literature in this area. We hypothesize that there will be oral microbiome differences between vapers and non-vapers; and exclusive vapers and dual users. The primary aim of this pilot study is to compare the oral microbiome of vapers and non-vapers. Subgroup analyses addressed a secondary aim to compare exclusive vapers and dual users.
2 ∣. MATERIALS AND METHODS
2.1 ∣. Design
A cross-sectional comparative descriptive design was used for this secondary data analysis. Emory University Institutional Review Board approval was obtained prior to commencement of the parent study activities (IRB00103602; Initial approval 6/12/18). The study was performed in accordance with the Declaration of Helsinki. Written informed consent was obtained from each participant after receiving a detailed explanation of the study.
2.2 ∣. Setting and participants
This was a secondary data analysis of a study designed to assess the feasibility of saliva and exhaled breath condensate as non-invasive matrices useful for detecting passive e-cigarette vapor exposure in children. Participants were enrolled for the parent study using convenience sampling from the local community between July 2018 and February 2019. Eligibility criteria for the parent vapers in the original study included self-reported daily use of e-cigarette products in the presence of children within the home or car and ability to speak and read English to complete survey questionnaires. Given that this was a secondary data analysis and pilot exploration, a priori power or sample size analyses were not conducted.
2.3 ∣. Variables and groups
The outcome variable of this study was the oral microbiome. The exposure variable was e-cigarette use. Accordingly, our groups were defined by exposure. In our comparison of vapers and non-vapers, we accounted for conventional smoking status which is a known confounder of the oral microbiome as well as sex, age, and partner status which were identified to differentiate the exposure groups. The vaping group was further differentiated into sub-groups of exclusive vapers and dual users (e-cigarettes and conventional cigarettes).
2.4 ∣. Data sources and measurement
2.4.1 ∣. Oral microbiome samples
The parent study team reviewed with participants and oversaw the procedure for soft tissue oral swab collection of the dorsal tongue, hard palate, buccal mucosa, and keratinized gingiva, which were pooled and immediately placed in 750 μl of MoBio buffer contained in sterile MoBio bead tubes (MoBio laboratories, Inc.) using protocols based on the Human Microbiome Project (HMP) (McInnes & Cutting, 2010). Samples were collected at various times of day depending on participant availability. Samples were placed on ice and transported for storage at −80°C freezer until ready for analysis.
2.4.2 ∣. DNA isolation and 16S rRNA gene library preparation and sequencing
Specimens were sent to Microbiome Insights for extraction and sequencing where they were placed into a MoBio PowerMag Soil DNA Isolation Bead Plate. DNA was extracted following MoBio's instructions on a KingFisher robot. Bacterial 16S rRNA genes were PCR-amplified with dual-barcoded primers (fusion 515F + 806R 16S primers) targeting the V4 region, as per the protocol of Kozich et al. (2013). While using multiple hypervariable regions increases nucleotide heterogeneity, the single V4 region was chosen for this study since decreasing read length can increase the quality or accuracy of the reads (Bukin et al., 2019). Normalized library concentrations of 1–2 ng/μl (as per the specifications of the Sequal Prep normalization kit) were used. A final library concentration of 8 pM (10% phiX) was loaded onto the flow cell (as per Kozich et al., 2013). Amplicons were sequenced with an Illumina MiSeq using the 300-bp paired-end kit (v.3). Sequences were denoised, taxonomically classified using Human Oral Microbiome Database HOMD (v.15.1) as the reference database (Chen et al., 2010), and clustered into 97%-similarity operational taxonomic units (OTUs) with the mothur software package (v.1.39.5) (Schloss et al., 2009), following the recommended procedure (https://www.mothur.org/wiki/MiSeq_SOP; accessed Nov 2017).
2.4.3 ∣. Other data
Self-reported sociodemographic data and smoking status were obtained from survey data from the parent study. Sociodemographic data were dichotomized for chi-square analyses to identify covariates of oral microbiome differences between vaping and non-vaping groups.
2.5 ∣. Bias
The potential for contamination was addressed by co-sequencing DNA amplified from specimens and from four each of template-free controls and extraction kit reagents processed the same way as the specimens. Two positive controls, consisting of cloned SUP05 DNA, were also included (number of copies = 2 × 106). OTUs were considered putative contaminants (and were removed) if their mean abundance in controls reached or exceeded 25% of their mean abundance in specimens.
2.6 ∣. Statistical methods
Microbiome analyses were conducted between vaper and non-vaper groups. Sociodemographic factors were compared between groups using independent t-tests and chi-square tests as appropriate. We had no missing data in our data set. Demographic factors that were significantly different between vapers and non-vapers were dichotomized and controlled for in the microbiome analyses. In addition, because of smoking's known impact on the oral microbiome (Wu et al., 2016), smoking of conventional cigarettes was also controlled for in the analysis. Because a sizable portion of our vaping group were dual users (vaping + conventional smoking), the vaping group was divided into sub-groups and second tier analyses were conducted within the vaping group to compare exclusive vapers with dual users.
Microbiome analyses included analysis of alpha and beta diversities. Alpha diversity, a measure of species diversity within a particular ecosystem, was calculated using the Chao1 and Shannon indices using the Vegan R package (Oksanen, 2020) on the OTU count table. Chao1 describes richness or the total number of species present in a community, that is, the higher the Chao1 score, the greater the number of species present (Lemos et al., 2011). The Shannon index provides a measure of both richness and evenness (Magurran, 2013). Communities numerically dominated by one or a few species exhibit a low Shannon score, whereas communities in which abundance is distributed equally among species will exhibit high evenness. Chao1 was calculated at the log scale to have a similar range to Shannon and was based on the rarefied OTU table at a rarefaction depth of 15,887 reads. No rarefaction was performed for Shannon calculations. The alpha diversity differences were tested using the Wilcoxon rank-sum test.
Beta diversities, which measure similarity/dissimilarity between a pair of communities, were calculated using the Bray–Curtis (abundance-weighted) and Jaccard (presence/absence of detected OTUs) distances. The communities were visualized on a principal coordination analysis (PCoA) plot based on these distance matrices to assess any clustering by groups of interest. The significance of the cluster differences (i.e., variation in community structure in relationship to group status) was assessed using the permutational multivariate analysis of variance (PERMANOVA) controlling for confounders. Distance matrices were used to conduct permutational univariate and multivariate nonparametric analysis of dissimilarities using the R package vegan, and p-values were adjusted for multiple testing using the Bonferroni correction. To estimate beta diversity across samples, Bray–Curtis and Jaccard distance indices were computed and visualized on an ordination plot; the Jaccard distance indices were the expected values when the OTU table was repeatedly rarefied to 15,887 depth (Hu & Satten, 2020).
The Linear Decomposition Model (LDM) (Hu & Satten, 2020) was used to determine differences at the individual OTU level, both in terms of relative abundance and presence/absence, controlling for false discovery rate at a nominal level of 10%.
3 ∣. RESULTS
3.1 ∣. Participant characteristics
Microbiome data for a total of 36 adults (18 vapers and 18 non-vapers) were used in this study. Within the vaping group, 55.6% were dual users, meaning that in addition to daily vaping, they also smoked conventional cigarettes some days or daily. The two groups differed on sex, race, and partner status (Table 1). Sex, race, and conventional smoking were all controlled for in subsequent microbiome analyses. Because there is no evidence to support partner status as a confounder of the oral microbiome, analyses were conducted both with and without partner status as a control variable. Results were similar confirming that partner status is not a significant confounder. The results and figures presented below, therefore, represent the analysis without partner status as a control variable.
TABLE 1.
Sociodemographic characteristics of participants (N = 36)
| Vaper | Non-Vaper | p | |
|---|---|---|---|
| Age | 34.97 ± 7.65 | 38.67 ± 7.99 | 0.163 |
| Sex | |||
| Female | 11 | 18 | 0.012 |
| Male | 7 | 0 | |
| Race | |||
| Non-White | 14 | 7 | 0.043 |
| Black or African American | 13 | 6 | |
| Asian | 1 | 0 | |
| More than one race | 0 | 1 | |
| White | 4 | 11 | |
| Partner status | |||
| Partnered | 11 | 3 | 0.017 |
| Non-partnered | 7 | 15 | |
| Education | |||
| <High school | 3 | 2 | 1.00 |
| >High School | 15 | 15 | |
| Income | |||
| <50K | 12 | 5 | 0.062 |
| ≥50K | 6 | 12 | |
| Employment | |||
| FT or PT | 4 | 4 | 1.00 |
| All else | 14 | 14 | |
| Insurance | |||
| Medicaid/Medicare | 6 | 2 | 1.446 |
| All else | 12 | 16 | |
3.2 ∣. ENDS use characteristics
Vapers used either disposable e-cigarettes or a refillable cartridge or tank system, with the tank system being the most popular. All vapers used e-liquid with nicotine and the most popular flavors were menthol and fruit (Table 2). Dual users reported a mean of 3.9 (SD 3.5, range 1–10) conventional cigarettes smoked per day.
TABLE 2.
Self-reported ENDS use behaviors and patterns (n = 18)
| n(%) | |
|---|---|
| Type of devicea | |
| Disposable | 3 (16.7) |
| Refillable cartridge | 7 (38.9) |
| Refillable tank system | 10 (55.6) |
| Nicotine concentration | |
| 1–6 mg | 11 (68.8) |
| 7–12 mg | 3 (16.7) |
| 13–18 mg | 1 (5.6) |
| 25 mg or more | 1 (5.6) |
| Don't know | 2 (11.1) |
| Flavors useda | |
| Menthol | 9 (50) |
| Fruit | 11 (68.8) |
| Chocolate | 1 (5.6) |
| Alcohol | 1 (5.6) |
Some participants reported using more than one type of device or flavor.
3.3 ∣. Description of microbiome data
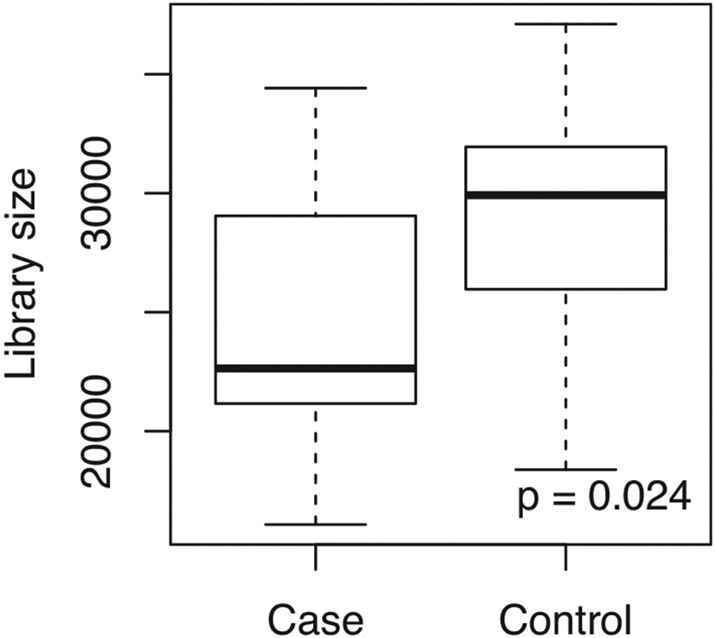
Bioinformatic processing of sequences yielded 3901 OTUs. In checking for batch effects, we determined that non-vapers (controls) had a significantly higher library size than vapers (cases; Figure 1). After filtering out 3641 rare OTUs and 154 contaminated OTUs, 241 remained for downstream analyses. Note that all analyses based on presence-absence data (Chao1 index, Jaccard distance matrix, presence-absence LDM) accounted for the variation in library size (Hu et al., 2020).
FIGURE 1.
Comparison of OTU library size between groups
3.4 ∣. Characterization of oral microbiome of vapers and non-vapers
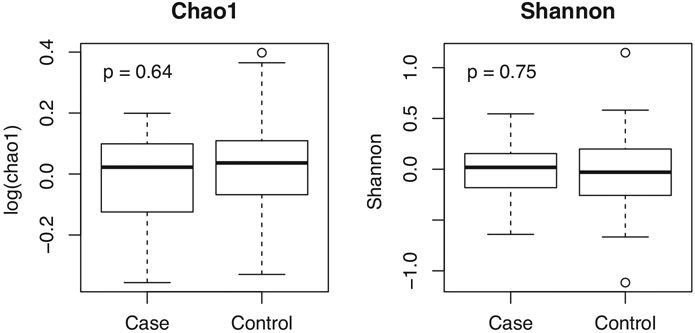
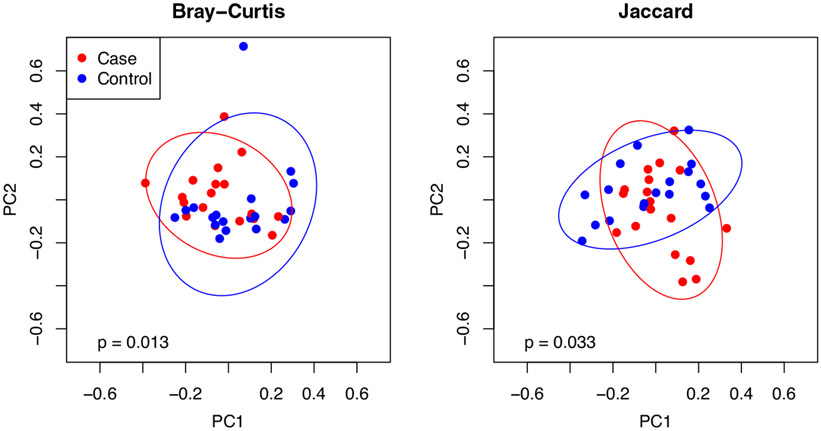
There was no difference in either Chao1 or Shannon diversity between groups controlling for race, sex, and smoking (Figure 2). PERMANOVA testing, controlling for the same three confounders, however, suggests a significant difference between the two groups in terms of both Bray–Curtis and Jaccard distances (Figure 3).
FIGURE 2.
No difference in alpha diversity between vapers (cases) and non-vapers (controls)
FIGURE 3.
Beta diversity differences between vapers (cases) and non-vapers (controls)
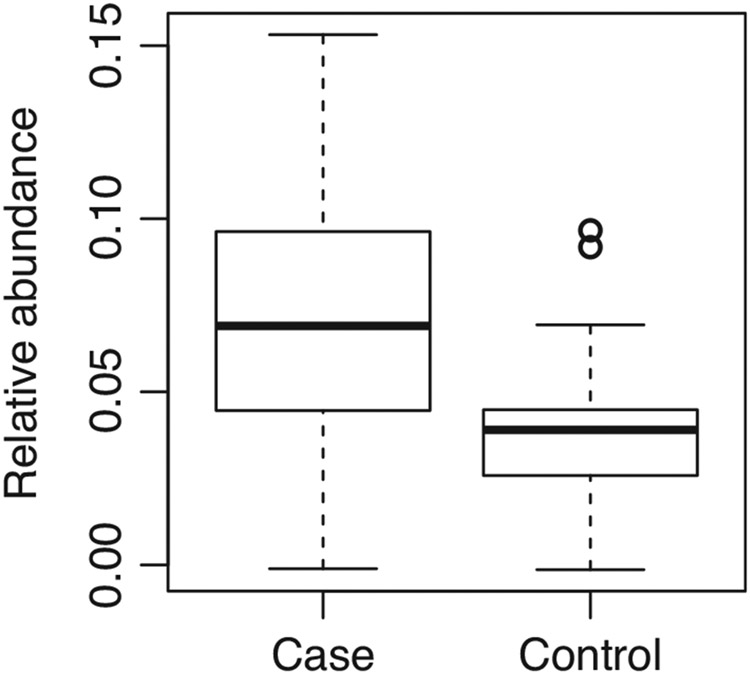
At the level of the individual OTU, the relative abundance of one OTU was detected by the LDM to be associated with group status. Vapers had a significantly higher relative abundance of an unclassified species of Veillonella (OTU0007) compared with non-vapers (adj. p = 0.04; Figure 4). In terms of presence/absence of taxa, however, no OTU, was detected to be associated with group status.
FIGURE 4.
Vapers demonstrated a higher relative abundance of Veillonella unclassified
3.5 ∣. Characterization of oral microbiome of exclusive vapers and dual users
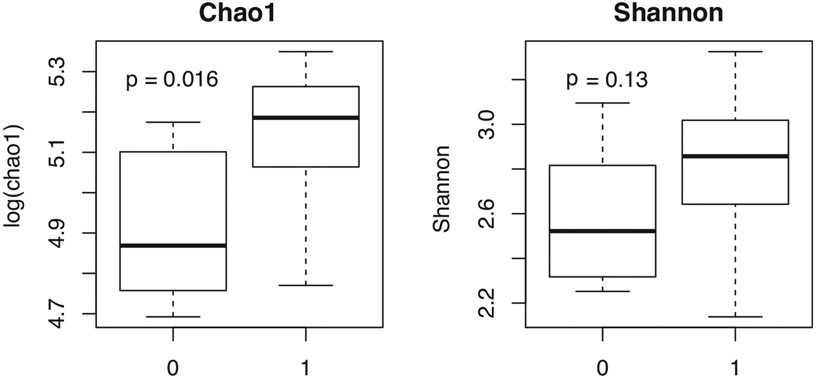
Dual users had higher alpha diversity compared with exclusive vapers with a significantly higher log Chao1 value and a Shannon score that also trended higher (Figure 5).
FIGURE 5.
Dual users (1) have a higher alpha diversity than exclusive vapers (0)
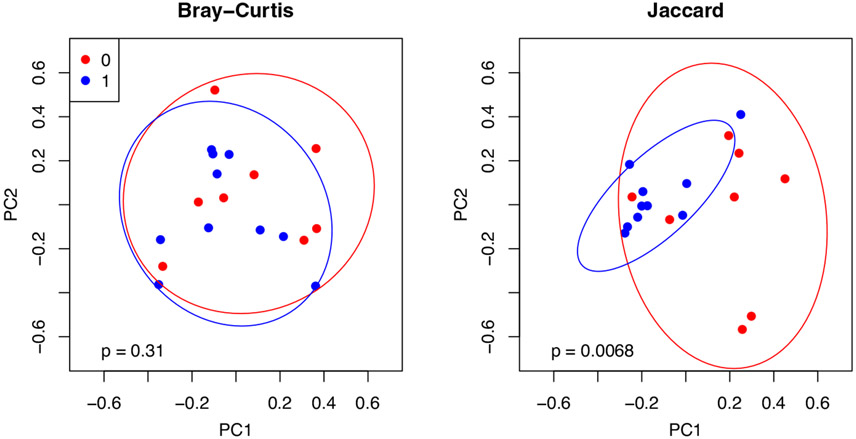
The beta diversity of these two sub-groups differed in terms of the Jaccard similarity measure (presence/absence of OTUs), but not the Bray–Curtis metric (abundance-weighted metric) (Figure 6).
FIGURE 6.
Exclusive vapers (0) and dual users (1) had significantly different beta diversity in terms of the Jaccard metric (presence/absence)
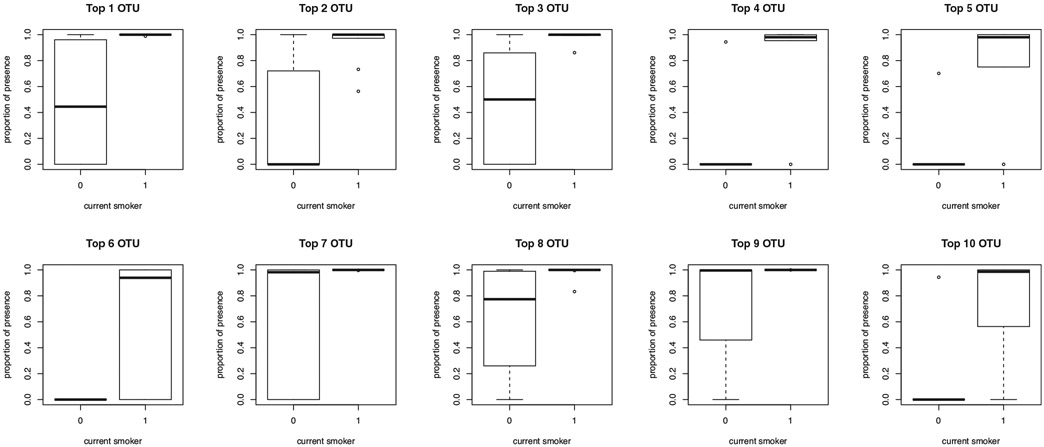
As suggested by the non-significant association between the Bray–Curtis indices and sub-group status, there were no OTUs detected, for which the relative abundance was associated with sub-group status after multiple testing correction. Multiple OTUs at the presence-absence level, however, were identified to be associated with sub-group status, such that several taxa were more likely to be present in dual users of both conventional cigarettes and e-cigarettes compared with exclusive vapers (top 10 OTUs—see Table 3 and Figure 7).
TABLE 3.
Top 10 OTUs associated with dual use of both e-cigarettes and conventional cigarettes
| Top 10 OTUs | Raw p-value |
Adj. p-value |
Proportion presence |
Direction | OTU name | OTU size | Taxonomy |
|---|---|---|---|---|---|---|---|
| 1 | 6.94e–05 | 0.01370 | 0.7665954 | + | OTU0109 | 263 | Alloprevotella rava |
| 2 | 7.64e–04 | 0.0478 | 0.6502819 | + | OTU0095 | 333 | Prevotella maculosa |
| 3 | 7.64e–04 | 0.0478 | 0.7537158 | + | OTU0132 | 150 | Tannerella forsythia |
| 4 | 1.87e–03 | 0.0706 | 0.4898704 | + | OTU0224 | 51 | Prevotella HMT 315 |
| 5 | 1.98e–03 | 0.0706 | 0.4546961 | + | OTU0145 | 119 | Treponema unclassified |
| 6 | 2.26e–03 | 0.0706 | 0.3773023 | + | OTU0218 | 54 | Prevotella HMT 526 |
| 7 | 2.88e–03 | 0.0773 | 0.8309653 | + | OTU0064 | 700 | Porphyromonas endodontalis |
| 8 | 3.58e–03 | 0.0839 | 0.8264414 | + | OTU0047 | 1642 | Prevotella oulorum |
| 9 | 4.96e–03 | 0.0985 | 0.8839027 | + | OTU0063 | 712 | Streptococcus anginosus |
| 10 | 5.24e–03 | 0.0985 | 0.4572339 | + | OTU0112 | 255 | Actinomyces HMT 897 |
FIGURE 7.
Exclusive vapers (0) and dual users (1) had significantly different (expected) proportion of presence (when the data were rarefied to 15,887 depth) at the 10 detected OTUs
4 ∣. DISCUSSION
The human oral cavity has a distinct microbiome integral to both the maintenance of oral health and overall health (Wade, 2013). A variety of factors influence the oral microbiome including dietary patterns, sugar consumption, antibiotic use, and oral hygiene. While smoking is known to alter the oral microbiome and contribute to oral disease, little is known about the impact of vaping on the oral microbiome. The aim of this study, therefore, was to compare the oral microbiome of vapers and non-vapers. Additionally, we compared the oral microbiome of exclusive vapers and dual users.
4.1 ∣. Vapers have a distinct oral microbiome
Alpha diversity (how many OTUs are present in a community and how evenly they are distributed) for the participants in our study did not differ between vapers and non-vapers when controlling for race, sex, and smoking status. This contrasts with recent studies which identified a higher alpha diversity in the oral microbiome of vapers compared with non-vapers (Chopyk et al., 2021; Ganesan et al., 2020; Pushalkar et al., 2020). The discrepancy may be related to the matrix sampled in our study, that is, soft tissue swabs. Studies which identified higher alpha diversities among vapers compared with non-vapers assessed the salivary microbiome (Chopyk et al., 2021; Pushalkar et al., 2020) or the subgingival microbiome (Ganesan et al., 2020). Recent evidence by Chopyk and colleagues suggests alpha diversity differences are not detectable with soft tissue swabs nor in buccal swab specimens. However, significant alpha diversity differences were observed in saliva specimens (Chopyk et al., 2021).
We did identify a difference between vapers and non-vapers in terms of overall microbiome composition as indicated by significant associations between beta diversity indices and vaping group status, even when controlling for confounders such as sex, race, and smoking status (Figure 3). Both Bray–Curtis and Jaccard distance indices were significantly associated with vaping status, a strong indication that vaping alters the oral microbiome, in terms of relative abundance of taxa and presence/absence of taxa. Pushalkar et al. (2020) similarly found significantly altered salivary microbiome beta diversity among their vapers compared with smokers and never smokers, as did Chopyk et al. (2021) for their buccal swab samples, and Ganesan et al. (2020) for their subgingival samples. Our study, therefore, contributes to the emerging evidence that strongly suggests that vapers have a distinct oral microbiome.
Differential abundance testing suggested that an unclassified species of Veillonella contributed to this difference. Vapers had a higher relative abundance of this species of Veillonella compared with non-vapers. This finding mirrors that of Chopyk et al. (2021) and Ganesan et al. (2020) who also identified increased relative abundance of Veillonella in e-cigarette users compared with non-vapers. Veillonella contain species that are typically found in the oral cavity and are known to act as opportunistic pathogens, stimulate the growth of other pathogenic organisms (Knapp et al., 2017), and are enriched among conventional smokers (Al-Zyoud et al., 2019). Furthermore, species such as V. parvula are overrepresented in carious lesions compared with healthy tooth surfaces (Tanner et al., 2011). Veillonella, thus emerges as a genus target for future investigations of the oral microbiome among e-cigarette users. Specifically, research should be focused on identifying species, strains, and functional profiles of Veillonella that are enriched among vapers in order to understand the potential clinical manifestations of this microbiome shift.
4.2 ∣. The impact of dual use of e-cigarettes and conventional cigarettes on the oral microbiome
Our findings demonstrate that dual users have an oral microbiome that is clearly distinct from that of exclusive vapers in terms of both alpha and beta diversity. The dual users in our group reflected a higher alpha diversity similar to that of smokers (Mason et al., 2015). This finding departs from that of Ganesan et al. (2020) who found that the dual users clustered taxonomically with former smokers and with exclusive ENDS users. This difference in findings, again, may be a function of the different matrices that were sampled. Our study sampled soft tissue while Ganesan et al. sampled subgingival plaque. Differences may also be explained by variations in duration of dual use as well as variations in proportion and frequency of e-cigarette/conventional cigarette use.
We observed that the differential presence of several taxa was associated with dual use, which is not surprising given the known impact of conventional smoking on the oral microbiome (Wu et al., 2016). The beta diversity analyses in our study did not identify differences in abundance of taxa between exclusive vapers and dual users (Bray–Curtis). We did, however, identify multiple taxa that differentiated the two groups in terms of presence/absence. The taxa listed in Table 2 reflect the top 10 OTUs that significantly differed between exclusive vapers and dual users in terms of presence/absence (Jaccard distance). All were more likely to be present in dual users of both conventional cigarettes and e-cigarettes suggesting that the use of conventional smoking is the driving force of this enrichment.
Some of these organisms have strong associations with periodontitis, for example, T. forsythia and P. endodontalis (Lombardo Bedran et al., 2012). Other organisms are lesser known but may have periopathogenic potential. A. rava, for example has been isolated from subgingival plaque (Downes et al., 2013). This obligate anaerobe is closely related to Prevotella tannerae (Downes et al., 2013), an organism that has been associated with endodontic infection (Xia et al., 2000). Several Prevotella species differentiated our dual users from vapers. Named or unnamed, not much is known about these taxa; however, organisms belonging to the genus Prevotella are a normal part of the human oral flora and have been frequently isolated from oral infections such as periodontitis, dental caries, and abscesses (Downes et al., 2007). Actinomyces spp. are known to play an early role in gingivitis. S. anginosus is a gram-positive streptococcus native to the oral cavity and upper respiratory tract and has been associated with opportunistic infections throughout the body (Jiang et al., 2020). Smoking of conventional cigarettes is known to alter the oral microbiome by decreasing the commensal organisms and increasing pathogens (Al-Zyoud et al., 2019). Our findings lean toward this direction with dual users of both e-cigarettes and conventional cigarettes having an enrichment of potentially pathogenic species.
5 ∣. LIMITATIONS AND FUTURE RESEARCH CONSIDERATIONS
Several limitations of this study must be acknowledged. First, the small sample size and cross-sectional design limit our power to identify and determine the direction of the associations. Longitudinal investigation of the oral microbiome with a larger cohort is needed. Second, because this was a secondary data analysis, we were limited in our ability to collect data on important confounders such as clinical oral health status and oral hygiene variables. Our ability to draw species level conclusions was limited by marker gene sequencing. Future research should consider deeper sequencing methods such as shotgun metagenomic sequencing which would enable taxonomic identification down to the species and strain level. Finally, our oral soft tissue matrix may not have comprehensively captured the oral cavity, for example, dentine surfaces and subgingival spaces. Future research should consider the collection of multiple matrices to comprehensively understand the microbial ecosystem in the oral cavity. Saliva may also offer a global snapshot of the oral microbiome since saliva bathes the entire oral cavity collecting organisms from various habitats (Yamashita & Takeshita, 2017).
6 ∣. CONCLUSION
Despite these limitations, the findings of this pilot exploration suggest an association between exclusive vaping and variations within the oral microbiome. Likewise, the evaluation of dual usage consisting of e-cigarettes and conventional cigarettes revealed variances in several known and unknown pathogenic microbes. The increasing uptake of e-cigarettes, various e-cigarette formats, vaping behavior and dual use may play a role in oral microbiome alterations, which may elicit oral disease. The observations described herein indicate an intersection between pathogenic oral microbiota and vaping warranting further investigation. A deeper understanding of the function and diversity of microbes in the oral cavity will inform clinician assessment and patient education.
ACKNOWLEDGMENTS
We are grateful to the Hercules Exposome Research Center (NIEHS: P30 ES019776) and the Center for Children’s Health, the Environment, the Microbiome, and Metabolomics (P50ES026071) for funding that supported this study.
Footnotes
CONFLICT OF INTEREST
None to declare.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Alanazi H, Semlali A, Chmielewski W, & Rouabhia M (2019). E-cigarettes increase Candida albicans growth and modulate its interaction with gingival epithelial cells. International Journal of Environmental Research and Public Health, 16(2), 294. 10.3390/ijerph16020294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Zyoud W, Hajjo R, Abu-Siniyeh A, & Hajjaj S (2019). Salivary microbiome and cigarette smoking: A first of its kind investigation in Jordan. International Journal of Environmental Research and Public Health, 17(1), 256. 10.3390/ijerph17010256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardellini E, Amadori F, Conti G, & Majorana A (2018). Oral mucosal lesions in electronic cigarettes consumers versus former smokers. Acta Odontologica Scandinavica, 76(3), 226–228. 10.1080/00016357.2017.1406613 [DOI] [PubMed] [Google Scholar]
- Bukin YS, Galachyants YP, Morozov IV, Bukin SV, Zakharenko AS, & Zemskaya TI (2019). The effect of 16S rRNA region choice on bacterial community metabarcoding results. Scientific Data, 6, 190007. 10.1038/sdata.2019.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. (2020). About electronic cigarettes (E-cigarettes). https://www.cdc.gov/tobacco/basicjnformation/e-cigarettes/about-e-cigarettes.html [Google Scholar]
- Chen T, Yu W-H, Izard J, Baranova OV, Lakshmanan A, & Dewhirst FE (2010). The Human Oral Microbiome Database: A web accessible resource for investigating oral microbe taxonomic and genomic information. Database, 2010, baq013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopyk J, Bojanowski CM, Shin J, Moshensky A, Fuentes AL, Bonde SS, Chuki D, Pride DT, & Crotty Alexander LE (2021). Compositional differences in the oral microbiome of E-cigarette users. Frontiers in Microbiology, 12, 599664. 10.3389/fmicb.2021.599664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuadra GA, Smith MT, Nelson JM, Loh EK, & Palazzolo DL (2019). A comparison of flavorless electronic cigarette-generated aerosol and conventional cigarette smoke on the survival and growth of common oral commensal. International Journal of Environmental Research and Public Health, 16(10), 1669. 10.3390/ijerph16101669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes J, Dewhirst FE, Tanner ACR, & Wade W (2013). Description of Alloprevotella rava gen. nov., sp. nov., isolated from the human oral cavity, and reclassification of Prevotella tannerae Moore et al 1994 as Alloprevotella tannerae gen. nov., comb. nov. International Journal of Systematic and Evolutionary Microbiology, 63(Pt 4), 1214–1218. 10.1099/ijs.0.041376-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes J, Sutcliffe IC, Booth V, & Wade WG (2007). Prevotella maculosa sp. nov., isolated from the human oral cavity. International Journal of Systematic and Evolutionary Microbiology, 57(Pt 12), 2936–2939. 10.1099/ijs.0.65281-0 [DOI] [PubMed] [Google Scholar]
- Ebersole J, Samburova V, Son Y, Cappelli D, Demopoulos C, Capurro A, Pinto A, Chrzan B, Kingsley K, Howard K, Clark N, & Khlystov A (2020). Harmful chemicals emitted from electronic cigarettes and potential deleterious effects in the oral cavity. Tobacco Induced Diseases, 18, 41. 10.18332/tid/116988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesan SM, Dabdoub SM, Nagaraja HN, Scott ML, Pamulapati S, Berman ML, Shields PG, Wewers ME, & Kumar PS (2020). Adverse effects of electronic cigarettes on the disease-naive oral microbiome. Science Advances, 6(22), eaaz0108. 10.1126/sciadv.aaz0108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu YJ, Lane A, & Satten GA (2020). Testing presence-absence associations in the microbiome using the LDM and PERMANOVA. bioRxiv. [Google Scholar]
- Hu YJ, & Satten GA (2020). Testing hypotheses about the microbiome using the linear decomposition model (LDM). Bioinformatics, 36(14), 4106–4115. 10.1093/bioinformatics/btaa260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang S, Li M, Fu T, Shan F, Jiang L, & Shao Z (2020). Clinical characteristics of infections caused by Streptococcus anginosus group. Scientific Reports, 10(1), 9032. 10.1038/s41598-020-65977-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones L. (2019). Vaping: How popular are e-cigarettes? BBC News. https://www.bbc.com/news/business-44295336 [Google Scholar]
- Kim SA, Smith S, Beauchamp C, Song Y, Chiang M, Giuseppetti A, Frukhtbeyn S, Shaffer I, Wilhide J, Routkevitch D, Ondov JM, & Kim JJ (2018). Cariogenic potential of sweet flavors in electronic-cigarette liquids. PLoS One, 13(9), e0203717. 10.1371/journal.pone.0203717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp S, Brodal C, Peterson J, Qi F, Kreth J, & Merritt J (2017). Natural competence is common among clinical isolates of Veillonella parvula and is useful for genetic manipulation of this key member of the oral microbiome. Frontiers in Cellular and Infection Microbiology, 7, 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozich JJ, Westcott SL, Baxter NT, Highlander SK, & Schloss PD (2013). Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Applied and Environment Microbiology, 79(17), 5112–5120. 10.1128/AEM.01043-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P, Clark P, Brinkman M, & Saxena D (2019). Novel nicotine delivery systems. Advances in Dental Research, 30(1), 11–15. 10.1177/0022034519872475 [DOI] [PubMed] [Google Scholar]
- Lemos LN, Fulthorpe RR, Triplett EW, & Roesch LF (2011). Rethinking microbial diversity analysis in the high throughput sequencing era. Journal of Microbiol Methods, 86(1), 42–51. 10.1016/j.mimet.2011.03.014 [DOI] [PubMed] [Google Scholar]
- Lombardo Bedran TB, Marcantonio RA, Spin Neto R, Alves Mayer MP, Grenier D, Spolidorio LC, & Spolidorio DP (2012). Porphyromonas endodontalis in chronic periodontitis: A clinical and microbiological cross-sectional study. Journal of Oral Microbiology, 4(1), 10123. 10.3402/jom.v4i0.10123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magurran AE (2013). Measuring biological diversity. Wiley-Blackwell. [DOI] [PubMed] [Google Scholar]
- Marques P, Piqueras L, & Sanz MJ (2021). An updated overview of e-cigarette impact on human health. Respiratory Research, 22(1), 151. 10.1186/s12931-021-01737-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason MR, Preshaw PM, Nagaraja HN, Dabdoub SM, Rahman A, & Kumar PS (2015). The subgingival microbiome of clinically healthy current and never smokers. The ISME Journal, 9(1), 268. 10.1038/ismej.2014.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McInnes P, & Cutting M (2010). Manual of procedures for Human Microbiome Project: Core microbiome sampling Protocol A, HMP Protocol# 07-001, version number: 12.0. hmpdacc.org/doc/sops_2/manual_of_procedures_v11.pdf [Google Scholar]
- McRobbie H, Phillips A, Goniewicz ML, Smith KM, Knight-West O, Przulj D, & Hajek P (2015). Effects of switching to electronic cigarettes with and without concurrent smoking on exposure to nicotine, carbon monoxide, and acrolein. Cancer Prevention Research, 8(9), 873–878. 10.1158/1940-6207.CAPR-15-0058 [DOI] [PubMed] [Google Scholar]
- Mokeem S, Abduljabbar T, Al-Kheraif A, Alasqah M, Michelogiannakis D, Samaranayake L, & Javed F (2019). Oral Candida carriage among cigarette-and waterpipe-smokers, and electronic-cigarette users. Oral Diseases, 25(1), 319–326. [DOI] [PubMed] [Google Scholar]
- Oksanen J. (2020). vegan: An introduction to ordination. https://cran.r-project.org/web/packages/vegan/vignettes/intro-vegan.pdf [Google Scholar]
- Pushalkar S, Paul B, Li Q, Yang J, Vasconcelos R, Makwana S, González JM, Shah S, Xie C, Janal MN, Queiroz E, Bederoff M, Leinwand J, Solarewicz J, Xu F, Aboseria E, Guo Y, Aguallo D, Gomez C, … Saxena D (2020). Electronic cigarette aerosol modulates the oral microbiome and increases risk of infection. Iscience, 23, 100884. 10.1016/j.isci.2020.100884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman MA, Hann N, Wilson A, & Worrall-Carter L (2014). Electronic cigarettes: Patterns of use, health effects, use in smoking cessation and regulatory issues. Tobacco Induced Diseases, 12(1), 21. 10.1186/1617-9625-12-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samburova V, Bhattarai C, Strickland M, Darrow L, Angermann J, Son Y, & Khlystov A (2018). Aldehydes in exhaled breath during E-cigarette vaping: Pilot study results. Toxics, 6(3), 46. 10.3390/toxics6030046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapru S, Vardhan M, Li Q, Guo Y, Li X, & Saxena D (2020). E-cigarettes use in the United States: Reasons for use, perceptions, and effects on health. BMC Public Health, 20(1), 1518. 10.1186/s12889-020-09572-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ, Sahl JW, Stres B, Thallinger GG, Van Horn DJ, & Weber CF (2009). Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Applied and Environment Microbiology, 75(23), 7537–7541. 10.1128/AEM.01541-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart CJ, Auchtung TA, Ajami NJ, Velasquez K, Smith DP, De La Garza R II, Salas R, & Petrosino JF (2018). Effects of tobacco smoke and electronic cigarette vapor exposure on the oral and gut microbiota in humans: A pilot study. PeerJ, 6, e4693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner ACR, Mathney JMJ, Kent RL, Chalmers NI, Hughes CV, Loo CY, Pradhan N, Kanasi E, Hwang J, Dahlan MA, Papadopolou E, & Dewhirst FE (2011). Cultivable anaerobic microbiota of severe early childhood caries. Journal of Clinical Microbiology, 49(4), 1464–1474. 10.1128/JCM.02427-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarroel MA, Cha AE, & Vahratian A (2020). Electronic cigarette use among U.S. adults, 2018. NCHS Data Brief, 365, 1–8. [PubMed] [Google Scholar]
- Wade WG (2013). The oral microbiome in health and disease. Pharmacological Research, 69(1), 137–143. 10.1016/j.phrs.2012.11.006 [DOI] [PubMed] [Google Scholar]
- Wu J, Peters BA, Dominianni C, Zhang Y, Pei Z, Yang L, Ma Y, Purdue MP, Jacobs EJ, Gapstur SM, Li H, Alekseyenko AV, Hayes RB, & Ahn J (2016). Cigarette smoking and the oral microbiome in a large study of American adults. The ISME Journal, 10(10), 2435–2446. 10.1038/ismej.2016.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia T, Baumgartner JC, & David LL (2000). Isolation and identification of Prevotella tannerae from endodontic infections. Oral Microbiology and Immunology, 15(4), 273–275. 10.1034/j.1399-302x.2000.150411.x [DOI] [PubMed] [Google Scholar]
- Yamashita Y, & Takeshita T (2017). The oral microbiome and human health. Journal of Oral Science, 59(2), 201–206. 10.2334/josnusd.16-0856 [DOI] [PubMed] [Google Scholar]
- Yang I, Sandeep S, & Rodriguez J (2020). The oral health impact of electronic cigarette use: A systematic review. Critical Reviews in Toxicology, 50(2), 97–127. 10.1080/10408444.2020.1713726 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.