Abstract
Osteoarthritis (OA) is a chronic inflammatory joint disease characterized by progressive cartilage degeneration, synovitis, and osteoid formation. In order to effectively treat OA, it is important to block the harmful feedback caused by reactive oxygen species (ROS) produced during joint wear. To address this challenge, we have developed injectable nanocomposite hydrogels composed of polygallate-Mn (PGA-Mn) nanoparticles, oxidized sodium alginate, and gelatin. The inclusion of PGA-Mn not only enhances the mechanical strength of the biohydrogel through a Schiff base reaction with gelatin but also ensures efficient ROS scavenging ability. Importantly, the nanocomposite hydrogel exhibits excellent biocompatibility, allowing it to effectively remove ROS from chondrocytes and reduce the expression of inflammatory factors within the joint. Additionally, the hygroscopic properties of the hydrogel contribute to reduced intra-articular friction and promote the production of cartilage-related proteins, supporting cartilage synthesis. In vivo experiments involving the injection of nanocomposite hydrogels into rat knee joints with an OA model have demonstrated successful reduction of osteophyte formation and protection of cartilage from wear, highlighting the therapeutic potential of this approach for treating OA.
Keywords: Osteoarthritis, Anti-inflammatory, Extracellular, Matrix, Oxidized alginate, Gelatin
Graphical abstract
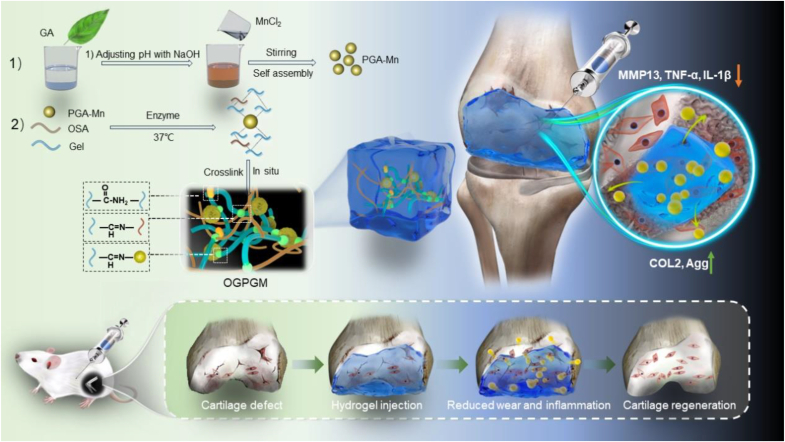
Preparation and action process of bio-based nanocomposite hydrogels.
1. Introduction
Osteoarthritis (OA) is the most prevalent degenerative disease, characterized by the gradual deterioration of the cartilage matrix, persistent inflammation of the synovium, and the development of subchondral bone sclerosis [1]. Anticipated to afflict more than 67 million people by 2030, this condition imposes a substantial economic burden on society [2]. Current treatments for OA primarily involve the administration of nonsteroidal drugs, either locally or systemically, and in some cases, resorting to total joint replacement [3]. The former approach is linked to the risk of severe adverse effects due to prolonged drug use, while the latter entails surgical complications and significant financial costs for patients [4,5]. As such, there exists an urgent need for the development of safe and efficacious therapeutic approaches to either alleviate or impede the progression of OA.
The chondrocytes, the primary cells in articular cartilage, are surrounded by an amorphous extracellular matrix in normal joints [6]. The matrix is rich in collagen and proteoglycans, which are essential for supporting the normal production of type II collagen (COL2) and aggrecan by chondrocytes [7]. However, current research has shown that chondrocytes can produce excessive reactive oxygen species (ROS) in the context of OA [8]. ROS are closely linked to inflammatory responses and cellular metabolism, and they can have a profound impact on chondrocyte viability, potentially leading to apoptotic cell death [9]. Furthermore, ROS can regulate the production of inflammatory cytokines and induce chondrocytes to secrete substantial amounts of matrix-degrading enzymes, such as matrix metalloproteinases (MMPs) and metalloproteinases with platelet-responsive protein motifs (ADAMTSs), consequently resulting in ECM degradation [10,11]. Chondrocyte apoptosis and ECM degradation greatly compromise cartilage integrity, exacerbating friction-induced wear and further accelerating the progression of OA [12,13]. Therefore, the development of biomaterials that can rapidly remove intra-articular ROS and provide lubrication to the articular surface is key to blocking malignant feedback in OA.
Research on antioxidants in OA has explored the use of small-molecule drugs like vitamin C [14], N-acetylcysteine [15], and nanoparticles [16], to counteract the effects of excessive ROS. Among them, gallic acid, a nature polyphenolic compound found in plants, possesses multiple hydroxyl groups that can reduce various ROS [17,18]. Additionally, the o-phenol structure of gallic acid can serve as a chelating site for binding with manganese ions, forming polygallocatem-manganese nanoparticles [19]. The coordination of polyphenol and metal ions serves to enhance their ability to clear ROS through the acceleration of electron transfer [20]. Additionally, the presence of polyphenols plays a role in promoting the synthesis of extracellular matrix metabolism [21,22]. However, the absorption rate of polyphenols in the body is low, and they are prone to oxidation [23]. The coordination of metal ions can enhance their stability and absorption in the body [24], thereby facilitating the synthesis of extracellular matrix. Therefore, the PGA-Mn nanoparticles, a combination of gallic acid and manganese, could potentially be used for treating OA, which can hopefully improve chondrocyte viability by reducing inflammation and maintain cartilage integrity by promoting ECM synthesis.
Intra-articular administration of nanomaterials is a more common method for treating OA compared to systemic delivery, mainly due to the absence of vascularized structures within the joints [[25], [26], [27]]. To circumvent the side effects associated with frequent injections, it is crucial to have an appropriate drug delivery system [[28], [29], [30]] Currently, a range of delivery systems, including hydrogels, microspheres, liposomes, and carbon nanotubes, have been developed [[31], [32], [33]]. However, these systems often come with limitations, such as inadequate sustained release, limited affinity for cartilage, an inability to offer sufficient anti-friction properties, and insufficient mechanical strength to withstand the high pressures and mechanical loads experienced within the joint [31,32,34]. Hence, the development of a delivery system with high strength and exceptional anti-friction properties is essential for the effective treatment of OA. Alginate and gelatin are bioactive substances that widely exist in nature and have good biodegradability [35], Alginate can be modified by cleaving the carbon-carbon (C2–C3) bond of the sugar ring using periodate, resulting in oxidized sodium alginate (OSA), which has enhanced biodegradability compared to its original form [36]. Gelatin has a similar chemical structure to that in the ECM and has been shown to promote cell proliferation and ECM deposition [37]. Therefore, suitable nanocomposite hydrogels based on the above biomaterials compatible with various features is expected to serve as a better quality treatment-delivery system for OA.
Herein, we developed the nanocomposite hydrogels (OGPGM) by incorporating PGA-Mn into OSA-Gelatin solution and then cross-linked by transglutaminase (TG) enzyme. In addition to being an antioxidant, PGA-Mn can also be used as a bioactive ingredient to promote the cross-linking of molecular chains between OSA and Schiff base reaction with gelatin after the above-mentioned substances are cross-linked together. Therefore, OGPGM can be used as a high-intensity platform for the controlled release of PGA-Mn. In addition, due to the abundant hydrophilic phenolic hydroxyl group in PGA-Mn, the hygroscopicity of OGPGM is greatly improved, thus giving OGPGM efficient lubrication ability. Consequently, OGPGM has the potential to mitigate intra-articular wear and protect cartilage by mitigating intra-articular ROS, positioning it as a promising non-surgical treatment for OA (Scheme 1).
Scheme 1.
Preparation and action process of nanocomposite hydrogels.
2. Materials and methods
2.1. Materials
Gallic acid (GA, 98%), diammonium salt (ABTS), potassium persulfate (K2S2O8), and dihydroethidium (DHE) were from Macklin Co., Ltd. (Shanghai, China). Sodium hydroxide, ethanol (99.7%), phosphate buffered saline (PBS), manganese chloride (MnCl2), Sodium dodecyl sulfate (SDS), and sodium alginate were purchased from Aladdin Reagent Co. (Shanghai, China). HE staining kit, Diaminobenzidine (DAB) developer, and Aqueous mounting medium containing DAPI and Fluoroshield were purchased from Solarbio Science & Technology (Beijing, China). Hydrogen peroxide (H2O2) and dimethylsulfoxide (DMSO) were purchased from Sigma-Aldrich (St Louis, MO, USA). Calcein/PI Cell Viability/Cytotoxicity Assay Kit/Total Superoxide Dismutase Assay Kit with WST-8/BCA protein assay kit were purchased from Beyotime Biotechnology (Shanghai, China). Hydroxyphenyl fluorescein (HPF) was from Shanghai Maokang Biotechnology Co., Ltd. (Beijing, China). Primary antibodies for MMP13, COL2, TNF-α, IL-1β, and GADPH were purchased from Abcam (Cambridge, UK). Cell counting kits were purchased from Meilunbio (Dalian, China). The reagents for cell culture were obtained from Gibco (Grand Island, NY, USA). All other reagents were purchased from Aladdin Reagent Co. (Shanghai, China) unless otherwise indicated.
2.2. Preparation of OSA
5 g of sodium alginate was taken with 450 mL of deionized H2O (ddH2O) and stirred at room temperature for 24 h. Subsequently, sodium periodate solution (50 mL ddH2O + 3.75 g of sodium periodate) was added and stirred for 8 h away from light. The reaction was terminated by adding 4 mL of ethylene glycol and stirring for 0.5 h The by-products of sodium periodate and ethylene glycol were completely removed by dialyzing for 3 days, changing ddH2O every 12 h. Finally lyophilized to a powder.
2.3. Preparation of PGA-Mn nanoparticles
First, 0.17 g of GA was dissolved in 25 mL of PBS (pH = 7), then 0.125 g of SDS was added and 20 mL of absolute ethanol was added. After the solution was completely dissolved, 0.0395g of manganese chloride was added and 2 mL NaOH (1 M) was added, stirring at a constant speed for 1 h, and finally PGA-Mn nanoparticles were collected by centrifugation (8000 rpm, 5 min), washed twice with 75% alcohol, and stored at 4 °C for further use.
2.4. Preparation of nanocomposite hydrogels
Gelatin (20% wt) was mixed with ddH2O and stirred at 50 °C until completely dissolved to obtain a gelatin stock solution. Then, OSA was dissolved in ddH2O to obtain 2% wt. The transglutaminase (TGase) powder was then dissolved in ddH2O to obtain a TGase solution (20 U mL−1). Subsequently, 800ul OSA (2% wt), 2 mL gelatin (20% wt), 800 μl TGase solution (20 U mL−1), and 400 μl PGA-Mn solution mix thoroughly and leave the mixture for 180 min to form covalent and non-covalent cross-linking networks. OGPGM (1:10) (the mass of PGA-Mn accounted for 1:10 of the total gel mass) and OGPGM (1:5) (the mass of PGA-Mn accounted for 1:5 of the total gel mass)
2.5. Hygroscopicity of hydrogel
3g of hydrogel OG, OGPGM (1:10), and OGPGM (1:5) containing different masses of PGA-Mn was synthesized in three portions per group as described above. The dry weight (W0) of the hydrogels was recorded after freeze-drying, followed by the addition of 10 mL of PBS to each sample. The PBS was incubated with the samples at 37 °C and the samples were weight-bearing (Wt) at intervals of 24 h. The swelling ratio was calculated by the formula [(Wt- W0)/W0] × 100%.
2.6. Degradability of nanocomposite hydrogel
The hydrogels were divided into two groups, one with or without PGA-Mn, with three identical samples in each group, and the initial weights were recorded (W0). The hydrogel was immersed in 15 mL of PBS and slowly shaken at room temperature. They were weighed every four days, the weight change was recorded (Wt), and finally, the degradation rate was calculated according to the formula Wt/W0 × 100%.
2.7. Release of PGA-Mn
OGPGM (1.0 g) was immersed in 10 mL PBS (pH = 7.4) and slowly shaken at 37 °C (n = 3). PBS was extracted at 2-day intervals and replaced with an equal volume of PBS. The amount of PGA-Mn in PBS was measured using a UV spectrophotometer.
2.8. Rheological studies
Hydrogel storage modulus and loss modulus were examined using parallel plates (8 mm diameter) in oscillating mode at 25 °C using a rheometer. Frequency scanning tests were performed in a predetermined linear viscoelastic region (0.1% strain) ranging from 0.01 to 10 Hz. To prevent water evaporation during the measurements, silicone oil was applied around the perimeter of the samples.
2.9. Scanning electron microscope
In order to characterize the microstructure of the hydrogel, the freeze-dried hydrogel was subjected to a scanning electron microscope (SEM) operated at a voltage of 3 kV and a current of 10.0 mA. Before seeing the observation, the samples were coated with platinum.
2.10. ABTS scavenging activity
ABTS (2,2′-Azino-bis(3-ethylbenzthiazoline-6-sulfonic acid)) radicals were generated by reacting a 7 mM ABTS stock solution with 2.45 mM K2S2O8 for 16 h in the dark, and then the ABTS radical solution was diluted with PBS to achieve the appropriate absorbance at 734 nm. Samples were mixed with 2 mL ABTS solution and placed in the dark for 10 min. The absorbance peak at 734 nm was then determined by UV–vis absorption spectroscopy.
2.11. H2O2 scavenging activity
The H2O2 solution (800 μM) was mixed with different concentrations of PGA-Mn. Then TiSO4 (1 mg/mL) was added and reacted for 5 min. The absorbance of the obtained solution was at 410 nm measured by UV–vis spectrophotometer.
2.12. ·OH scavenging activity of PGA-Mn
The scavenging activity of ·OH was determined by TMB chromogenic assay. ·OH can be produced by the Fenton reaction of Fe2+ and H2O2 and can oxidize TMB to oxTMB. The characteristic absorption peak of oxTMB was 652 nm. Therefore, the ·OH content can be determined by observing the 652 nm absorption peak. Working solutions of 250 μm TMB, 1 mM FeSO4, 2 mM H2O2, and different concentrations of PGA-Mn were first prepared in PBS buffer (pH = 5) for 5 min in the dark. Finally, the absorbance of the solution at 652 nm was measured by UV–vis spectrophotometer.
2.13. ·O2− scavenging activity of PGA-Mn
The scavenging activity of PGA-Mn against ·O2− was determined using the Total Superoxide Dismutase Assay Kit with WST-8. ·O2− radical test solutions were prepared according to the instructions provided by the reagent manufacturer and then reacted with different concentrations of PGA-Mn for 10 min at 37 °C. Finally, the absorbance peak of the 450 nm solution was monitored by UV–vis spectroscopy.
2.14. Cytocompatibility
The chondrocytes were seeded in the lower chamber of the transwell well plate, and the hydrogel was placed in the upper chamber. Cell proliferation was detected by live/dead staining and CCK8 after co-culture for 1, 2, and 3 days. For the live/dead staining assay, cells were incubated with calcein-AM/propidium iodide for 20 min, followed by fluorescence microscopy. For the CCK8 assay, the CCK8 working solution was prepared by mixing the CCK8 stock solution with F-12 medium 1:9 according to the manufacturer's instructions. After incubation with the cells for 2 h, the absorbance (450 nm) was measured using a microplate reader.
2.15. OA cell model
To mimic the environment in which excess ROS is present in OA, we seeded chondrocytes in the lower chambers of Transwell well plates and exposed the cells to 100 μM H2O2. Different materials were placed in the upper chamber to examine the therapeutic effect of different materials on the cells. Cells without any treatment were used as controls.
2.16. Live/dead staining assay
Chondrocytes were seeded in the lower chamber of the Transwell plate, and 100 μM H2O2 and different hydrogels were added to the upper chamber after the cells were completely attached to the wall. For the live/dead staining assay, cells were incubated with calcein-AM/propidium iodide for 20 min, followed by fluorescence microscopy.
2.17. Measurement of ROS levels
Chondrocytes were inoculated in 24-well plates and incubated overnight. After stimulation with H2O2 for 30 min and the addition of different materials for 24 h of co-culture, H2O2 was detected with 10 μM 7 ′-dichlorofluorescein diacetate (DCFH-DA), DHE was used for ·O2− detection, 10 μM HPF was used for ·OH, and finally, the cells were visualized by fluorescence microscopy. To quantify the level of ROS, cells were grown in 6-well plates, and cells were treated as described above. Cells were collected and fluorescence intensity was measured by flow cytometry (Beckman Coulter WM2016014, USA).
2.18. Rat model of OA
OA was modeled by anterior cruciate ligament transection and medial meniscectomy (ACLT + MMx), and the therapeutic effect of hydrogel was observed by injecting hydrogel one week after the operation (Fig. S7). It has been reported that 30 μL of solution can fill the rat knee joint cavity without overflow [38]. Therefore, an injection volume of 30 μL was chosen for this study. The modeled osteoarthritic rats were randomly divided into 4 groups (5 rats per group), sham group, PBS group, OG group, and OGPGM group. Among them, rats in the sham group received only anesthesia and skin incision. All rats were executed at week 8.
2.19. Radiographic evaluation
Eight weeks after surgery, six rats were randomly selected from each group, and the rats were subsequently anesthetized by intraperitoneal injection of 10% pentobarbital (40 mg/kg). X-ray images of the rat knee joints were obtained using a Faxitron X-ray machine (Kubtec model XPERT.8, USA) with a 10-s exposure at 32 kV. The JSW was measured in anterior-posterior and lateral views. For further observation, isolated knee joints were collected for micro CT analysis (Bruker 5000, Germany) after the rats were executed.
2.20. Evaluation of in vivo retention time
According to a previous study [39], retention time in rat knee joints was evaluated by doping fluorescently labeled cy5.5 PGA-Mn into OGPGM and injecting 30 μl of fluorescently labeled PGA-Mn and OGPGM into the knee joints of rats. The fluorescence intensity was measured by the IVIS spectroscopy system (Xenogen, USA) at different time points. The fluorescence value of the negative sample (PBS-injected rat knee joint) was used as the background fluorescence.
2.21. Western blotting (WB)
Cells were collected and added to RIPA lysate containing PMSF, a protease inhibitor, as well as other phosphatase inhibitors, and placed on ice for 1 h to fully lys. Protein concentration was measured using a BCA protein assay kit. Equal amounts of proteins were loaded onto a 10% SDS-PAGE gel, electrophoresed at 80 V running voltage, and then transferred to a polyvinylidene fluoride (PVDF) membrane at 220 mA current. After sealing with rapid sealing solution, the membrane was used with primary antibodies (COL2 (1:1000), MMP13 (1:1000), TNF-α (1:1000), IL-1β (1:1000), GADPH(1:1000)) overnight at 4 °C. After three washes with TBST, the membrane and horseradish peroxidase P-conjugated secondary antibody were incubated for 2 h at room temperature. Finally using ECL reagent was protein bands visualized. Protein expression was analyzed by ImageJ using GADPH for normalization.
2.22. Immunofluorescence staining (IF)
Samples were fixed with 4% paraformaldehyde for 15 min, permeabilized with 0.1% Triton X-100 for 3 min, and closed with 5% (W/V) bovine serum albumin (BSA, Beyotime, China) for 2 h. The samples were incubated with the indicated primary antibodies (COL2 (1:200), MMP13 (1:200), TNF-α (1:200), IL-1β (1:200)) overnight at 4 °C. The next day, the samples were further visualized under a fluorescence microscope with Alexa 488 secondary antibody at room temperature.
2.23. Histological evaluation
Cartilage samples were fixed with 4% paraformaldehyde, followed by decalcification of the samples with 10% ethylenediaminetetraacetic acid decalcification (EDTA, Solarbio, China) for 1 month, and finally embedded into paraffin. Paraffin sections with a thickness of 5 μm were taken for histological analysis, and histological and immunohistochemical analyses were performed with hematoxylin-eosin (HE), safranin-O (SO), and fast green. The severity of OA lesions was assessed using the OARSI (Osteoarthritis Research Society International) score established by Pritzker et al. [40], which is the product of grade 6 (lesion depth) and stage 4 (extent of involvement) on a scale of 0 (normal) to 24 (severe OA). The modified Mankin histological score was used to score histological damage to articular cartilage [41], and the grading and scoring criteria are shown in Table S1.
2.24. TUNEL
Deparaffinise paraffin sections in xylene for 5–10 min. Replace with fresh xylene and dewax for another 5–10 min. Anhydrous ethanol for 5 min, 90% ethanol for 2 min, 70% ethanol for 2 min, and distilled water for 2 min. Dropwise addition of 20 μg/mL DNase-free proteinase K. Action at 37 °C for 20 min. PBS or HBSS was washed 3 times. Subsequently, the TUNEL working solution was added and incubated at 37 °C for 60 min protected from light. Finally, it was observed under a fluorescence microscope.
2.25. Immunohistochemistry (IHC)
Paraffin sections were deparaffinized as in the above procedure and used for antigen repair with sodium citrate buffer at 65 °C overnight. The sections were then incubated with the antibody at 4 °C overnight. Labeling was done with horseradish peroxidase (HRP) labeled secondary antibody. Finally, the sections were developed with diaminobenzidine (DAB).
2.26. Statistical analysis
All experiments were repeated at least three times and all data are expressed as mean +standard deviation. We analyzed and processed the experimental data by GraphPad Prism 9.0 software using one-way ANOVA and Tukey post hoc test. Values of P < 0.05 were considered statistically significant, with increased confidence levels shown as *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001, #p < 0.05, ##p < 0.01, ###p < 0.001, ####p < 0.0001.
3. Results and discussion
3.1. Preparation and characterization of nanocomposite hydrogels
The functional biological units of nanocomposite hydrogels mainly include PGA-Mn, OSA, and gelatin. After combining the component materials, OGPGM can undergo self-gelation and form a homogeneous, transparent, water-rich hydrogel with a soft, elastic texture at 37 °C. This unique property allows the hydrogel to undergo in situ gelation in vivo (Fig. S1A). Additionally, OGPGM can be used to draw various shapes and letters with an injector, demonstrating its ability to be injected into the joint cavity without causing blockage of the injector (Fig. S1B).
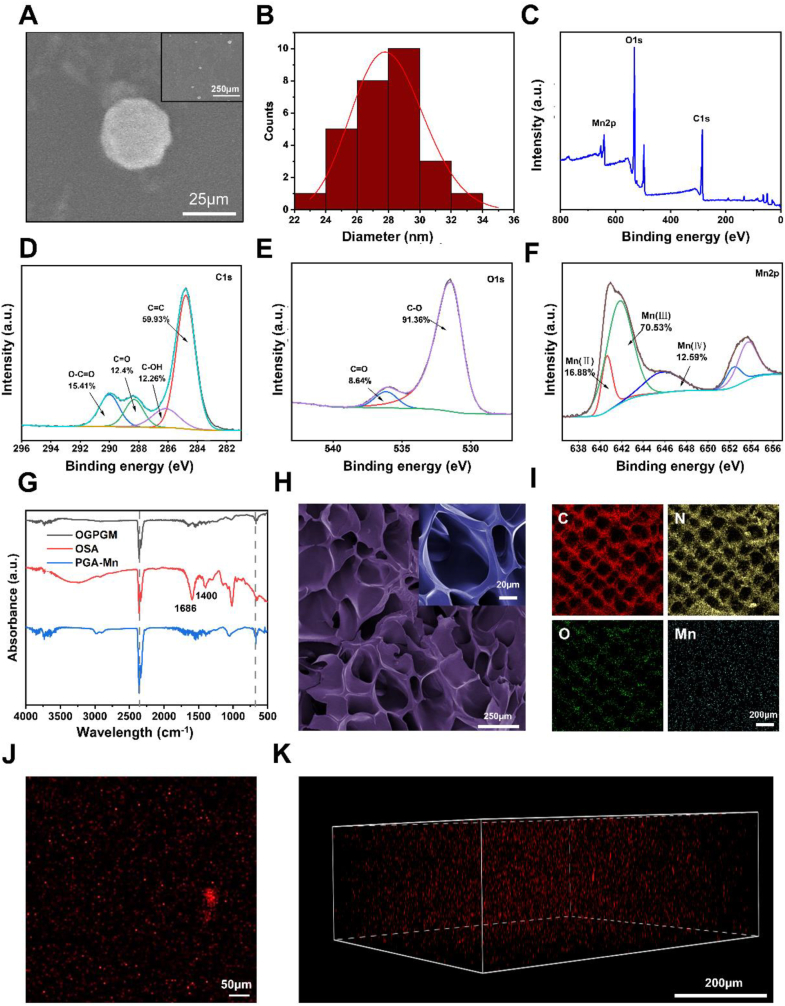
PGA-Mn was synthesized by polymerizing gallic acid with manganese chloride in an aqueous solution, followed by a self-assembly process (Fig. S2). SEM images in Fig. 1. A revealed that PGA-Mn nanoparticles are small, with an average size of approximately 27 nm, and display a uniform particle size distribution without noticeable aggregation. Dynamic light scattering (DLS) analysis showed a hydrodynamic particle size of 28.63 nm, with a particle size distribution conforming to a Gaussian normal distribution (Fig. 1B). Moreover, X-ray photoelectron spectroscopy (XPS) was used to analyze the surface composition of PGA-Mn. The full-scan XPS spectrum indicated that PGA-Mn primarily consists of carbon, oxygen, and manganese (Fig. 1C). High-resolution C1s XPS spectrum displayed four peaks at 284.6, 286.4, 288.3, and 282.7 eV, corresponding to C=C (59.93%), C–OH (12.26%), C=O (12.4%), and O–C=O (15.41%) bonds, respectively (Fig. 1D). The O1s XPS spectra showed two peaks at 531.8 eV and 536.8 eV, indicating the presence of C=O and C–O bonds (Fig. 1E). Gaussian curve fitting of the Mn 2p XPS spectrum revealed three peaks at 640.6 eV, 642.2 eV, and 646.1 eV, suggesting the existence of Mn2+-Mn4+ species in PGA-Mn, with a Mn3+/Mn2+ ratio of approximately 58.4:26.4 (Fig. 1F). To fully utilize PGA-Mn in joints, the selection of a functionalized hydrogel loaded with PGA-Mn is critical. In this study, high-strength OGPGM hydrogels were chosen for enzyme-catalyzed delivery of PGA-Mn. Transglutaminase facilitated cross-linking between or within gelatin molecules, while OSA promoted cross-linking through the formation of Schiff bases between its aldehyde group, the amino group of gelatins, and the phenolic hydroxyl group of PGA-Mn. This was confirmed by attenuated total reflectance Fourier-transform infrared spectroscopy (ATR-FTIR) analysis, characterizing the chemical structure of the nanocomposite hydrogels. In addition, the characteristic peaks at 1686 cm−1 and 1400 cm−1 in OSA were derived from the asymmetric and symmetric stretching vibrations of the –COO– group on the alginate backbone, respectively, which proved the carbonylation of alginate. (Fig. 1G). An efficient delivery platform also requires sufficient space to accommodate PGA-Mn. The stable multi-void multi-channel structure of the OG hydrogel provides structural contacts for more efficient loading of PGA-Mn and water (Fig. S3). As shown in Fig. 1H, after the incorporation of PGA-Mn, OGPGM also maintained the multi-hollow and multi-channel structure, and the number of pores was partially increased. Additionally, energy dispersive spectroscopy (EDS) and elemental analysis demonstrated the uniform distribution of elements (including C, O, N, and Mn) in OGPGM, suggesting the homogeneous distribution of PGA-Mn within OGPGM (Fig. 1I). The homogeneous distribution of red fluorescence from cy5.5-labeled PGA-Mn within the hydrogel further confirmed the successful incorporation of PGA-Mn (Fig. 1J and K).
Fig. 1.
Characterization of PGA-Mn and OGPGM. (A) SEM image of the PGA-Mn. (B) Size distribution liposomes. (C) Full scan XPS survey spectrum and the high-resolution spectra of PGA-Mn for (D) C1s (E) O1s (F) and Mn2p. (G) ATR-FTIR analysis of the chemical structures of OGPGM. (H) SEM images of OGPGM. (I) Elemental mapping images of OGPGM (J, K) The homogeneous distribution of red fluorescence from cy5.5-labeled PGA-Mn in the OGPGM.
3.2. Hygroscopicity and lubricity
To explore how the amount of incorporated PGA-Mn affects the properties of these nanocomposite hydrogels, hydrogel samples with different mass ratios of PGA-Mn were created and subsequently characterized. The water absorbency of these hydrogels is a key factor in lubricating joint surfaces and mitigating inflammatory responses, which can impede the progression of OA.
As shown in Fig. 2A, upon immersion in PBS, the hydrogel samples underwent rapid volumetric expansion within 24 h. Specifically, the swelling ratios for OGPGM(1:5), OGPGM(1:10), and OG were measured at 550%, 479%, and 371%, respectively. Finally, these swelling ratios further increased to 1200%, 925%, and 791%, respectively. The hygroscopicity capacity of OGPGM hydrogels showed a direct correlation with the content of PGA-Mn. This improved hygroscopicity is attributed to molecular interactions between the phenolic hydroxyl groups of PGA-Mn and water molecules, facilitating hydrogen bonding and electrostatic interactions. Furthermore, the formation of Schiff base linkages between the phenolic hydroxyl groups, the amino group of gelatins, and the aldehyde group of OSA induced changes in the physical properties and pore structure of the hydrogel. As a result, a denser pore structure was formed, enhancing hygroscopicity capabilities.
Fig. 2.
The material properties of the OGPGM. (A) The hygroscopicity of OG, OGPGM(1:5) and OGPGM(1:10) (n = 3). (B) COF–time plots and (C) COF histograms for PBS, OG, OGPGM(1:5), and OGPGM(1:10) under the loading of 2 N (n = 3). (D, E) The storage modulus of Gelatin, OG, OGPGM(1:5), and OGPGM(1:10) (n = 3). (F) The loss modulus of Gelatin, OG, OGPGM(1:5), and OGPGM(1:10). (Data presented as mean ± SD, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, ns, not significant).
Healthy joints contain synovial fluids that consist of various macromolecules [42]. These fluids serve as effective lubricants and play a crucial role in reducing the coefficient of friction (COF) to a range of 0.001–0.01 [43]. However, in the progression of OA, the lubricating ability of the joints decreases, leading to inflammation caused by prolonged friction and the inflammation is a key factor in the degeneration of cartilage. Therefore, it is important to have sufficient biological lubrication in joints to facilitate normal joint movement and prevent wear of the articular cartilage. In accordance with the hydration lubrication mechanism, OGPGM, which possesses excellent hygroscopicity properties, can effectively reduce the coefficient of friction and provide lubrication. To examine the tribological characteristics of these hydrogel samples, frictional tests were conducted using a reciprocating UMT-3 apparatus. As shown in the COF-time plots and COF histograms (Fig. 2 B and C), it is evident that both hydrogels with and without PGA-Mn exhibit lower COF compared to PBS. In particular, OGPGM (1:5) demonstrated the most significant reduction in COF, with a sharp decrease from 0.03 to 0.012. These results suggested that the presence of PGA-Mn in hydrogels can enhance their lubrication properties, leading to a lower COF.
3.3. Mechanical characteristics
Given the substantial mechanical stress that knee joints endure, it is crucial for OGPGM hydrogels to exhibit exceptional mechanical strength to resist fracture under pressure [34]. Compressive experiments provided evidence that OGPGM(1:5) displayed the highest compression modulus, which increased from 168.07 to 264.89 kPa (Fig. S4). It is due to the cross-linked network that can be formed by PGA-Mn and OSA, which increases the consistency and structural stability of the hydrogel, thereby enhancing its compression resistance. Furthermore, oscillatory rheology analysis was employed to further investigate the mechanical properties of the hydrogel samples. As shown in Fig. 2 D and S5, the results consistently indicated that the storage modulus (G′) exceeded the loss modulus (G″) for all hydrogel samples, signifying their elastic behavior under applied strain. Notably, OGPGM(1:5) exhibited the highest G′ and G″ values, underscoring its superior mechanical properties. The inclusion of PGA-Mn in the OG hydrogel contributed to the improvement of their mechanical characteristics by facilitating covalent cross-linking and enabling energy dissipation through weak π-π stacking interactions within the hydrogel network. This promising finding holds significant potential for future biological applications.
3.4. Cell viability and cell cytotoxicity
The aforementioned experiments demonstrated that OGPGM(1:5) exhibited superior mechanical properties compared to OGPGM(1:10). Consequently, subsequent in vitro and in vivo experiments were conducted using OGPGM(1:5). The biocompatibility of OGPGM nanocomposite hydrogels was further evaluated through both in vivo and in vitro investigations. In the in vitro experiments, various hydrogels were generated in the upper chamber of transwell plates, while chondrocytes were co-cultured in the lower chamber over three days (Fig. 3A). To assess biocompatibility, we employed live/dead cell staining and CCK-8 assays. As shown in Fig. 3B, after live/dead staining, there were no dead cells in the chondrocytes co-cultured with OG and OGPGM hydrogels, and the density of viable cells continued to increase over time (Fig. 3C). Furthermore, the CCK-8 assay indicated no significant variation in cell proliferation among the three experimental groups (Fig. 3D). These findings robustly support the favorable biocompatibility of OG and OGPGM hydrogels. Given the local application of OG and OGPGM hydrogels within the joint, the limited presence of vascular tissue minimizes the likelihood of systemic dissemination. Histological examination, employing HE staining, was conducted on vital organs, including the liver, heart, kidney, spleen, and lung, within the OG and OGPGM groups. This examination revealed no evidence of inflammatory cell infiltration, cell death, or structural damage (Fig. 3E). These observations signify the excellent systemic biocompatibility of OG and OGPGM hydrogels, establishing their suitability and safety for OA treatment.
Fig. 3.
Biocompatibility and residency of OGPGM. (A) Schematic representation of OG and OGPGM co-cultured with chondrocytes (n = 3). (B) Live-dead staining of OG and OGPGM after 1, 3, and 5 days of co-culture with chondrocytes (n = 3). (C) Quantitative evaluation of live cells. (D) CCK8 detection of chondrocyte proliferation under co-culture with OG and OGPGM (n = 3). (E) HE staining images of various organs at 0, 4, and 8 weeks after local injection of OG and OGPGM (n = 3). (F). Release curve of PGA-Mn from OGPGM (n = 3). (G, H) In vivo imaging of cy5.5-labeled PGA-Mn in rat joints after local injection of PGA-Mn and OGPGM (n = 3). (Data presented as mean ± SD, *P < 0.05, ***P < 0.001, ****P < 0.0001, ns, not significant).
3.5. PGA-Mn release and retention
The sustained release of PGA-Mn from OGPGM hydrogels is essential for maintaining the functionality of PGA-Mn and avoiding the need for repeated injections. Therefore, the release rate of PGA-Mn in OGPGM hydrogel was observed. As shown in Fig. 3F, PGA-Mn release was relatively rapid during the first 10 days, followed by a gradual slowing of release. The overall release was sustained and stable, and the total release reached 80% within 40 days. The degradation properties of biomaterials administered through intra-articular injection play a crucial role in the treatment of OA. The hydrogels were subjected to immersion in PBS and subsequently agitated at 37 °C using a shaker. OG exhibited rapid degradation at a relatively consistent pace, with complete degradation observed by the eighth day. Conversely, OGPGM displayed a degradation rate of approximately half that of OG, taking 16 days for complete degradation. This duration is considered adequate for PGA-Mn to effectively combat chronic inflammation associated with OA, while still allowing for timely clearance without being excessively prolonged (Fig. S6). To assess the residency duration of PGA-Mn in the joint, cy5.5-labeled PGA-Mn was utilized, and in vivo, imaging of rats was conducted at various time points (Fig. 3G). The results showed that PGA-Mn disappeared completely within 2 weeks after direct injection, which may be attributed to factors such as circulation, metabolic enzymes, permeability, and joint movement [44]. Notably, when released from OGPGM hydrogels, PGA-Mn remained in the knee joint for a minimum of four weeks (Fig. 3H). This extended residency duration enables the optimal utilization of PGA-Mn's biological properties. Consequently, the utilization of OGPGM hydrogels facilitates the prolonged and controlled delivery of PGA-Mn within the joint, eliminating the requirement for frequent injections and ensuring effective treatment.
3.6. ROS scavenging activity of PGA-Mn and OGPGM
The imbalance between the production of ROS and the protective antioxidant mechanisms in chondrocytes plays a significant role in the advancement of OA [45]. To investigate the ROS scavenging capacity of PGA-Mn in OGPGM, we conducted colorimetric assays to assess PGA-Mn's ability to scavenge common ROS (·OH, ·O2−, H2O2). The results demonstrated that PGA-Mn exhibited high scavenging activity against several ROS species, including ·OH, ·O2−, and H2O2. The scavenging rate increased with increasing concentration of PGA-Mn. For instance, at a concentration of 100 μg/mL, PGA-Mn was able to scavenge 95.40% of ·OH, 96.21% of ·O2−, and 83.89% of H2O2 (Fig. 3A–C). We further evaluated the total antioxidant capacity of PGA-Mn using ABTS free radicals. The results showed that nearly all ABTS radicals were scavenged by PGA-Mn at a concentration of 100 μg/mL (Fig. 3D). Moreover, the characteristic absorption peaks of ·OH, ·O2−, H2O2, and ABTS radicals gradually decreased over time in the presence of PGA-Mn (Fig. 3E–H). These findings indicated that PGA-Mn exhibited robust ROS scavenging ability even at lower concentrations and within a short period. This suggested that PGA-Mn has the potential to effectively mitigate ROS-induced oxidative stress in chondrocytes implicated in the progression of OA.
Motivated by the robust ROS scavenging capabilities of PGA-Mn demonstrated in tubes, we conducted comprehensive investigations to assess its impact on intracellular ROS scavenging within hydrogels co-cultured with cells. Following exposure to stimulation of 250 μM H2O2, the cells exhibited a remarkable upregulation of ROS production, sensitively detected through the application of 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA) for specific intracellular H2O2 detection (Fig. 4I1). Analysis via fluorescence microscopy and flow cytometry unveiled the peak fluorescence intensity within both the Blank group and the OG group, with no statistically significant disparity observed between these groups (Fig. 4I2, 4I3). Conversely, maintaining highly efficient H2O2 scavenging capability, the OGPGM group displayed fluorescence intensity similar to the control group. Notably, the detection of ·O2− and ·OH radicals was facilitated through the utilization of dihydroethidium (DHE) and hydroxyphenyl fluorescein (HPF), respectively, with resulting signals appearing in the red and green channels. By observing the extensive and high-intensity red fluorescence signal in the H2O2 group, it is evident that a significant amount of ·O2− is generated in most cells when exposed to H2O2 stimulation (Fig. 4J). Notably, only OGPGM loaded with PGA-Mn exhibits the capacity to scavenge ·O2−, whereas OG alone does not display significant ·O2− scavenging ability. This observation is further supported by Fig. 4K, where an abundance of green fluorescence signals is present in the H2O2 group; however, in comparison to the OG group, only the signal intensity in the OGPGM group is weakened. These findings suggested that the generation of ·OH induced by H2O2 can be reduced by OGPGM, whereas OG does not possess notable ·OH scavenging ability. The aforementioned findings demonstrate that the utilization of OGPGM loaded with PGA-Mn is highly effective in efficiently scavenging various forms of ROS.
Fig. 4.
ROS scavenging activity of PGA-Mn and OGPGM. (A–D) Clearance of ABTS, ·OH, H2O2, and ·O2− by PGA-Mn with increasing concentration (n = 3). (E–F) UV characteristic absorption of PGA-Mn ABTS, ·OH, H2O2, ·O2− over time (n = 3). (I–K) Fluorescence micrographs (1), flow cytometry (2), and intensity quantification (3) show the activities of OGPGM to scavenge different types of ROS in chondrocytes with H2O2 treatment (n = 3). (H) Live/dead Fluorescence micrographs of chondrocytes under different treatments (n = 3). (Data presented as mean ± SD, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, ns, not significant).
As ROS stands as a pivotal factor driving cell necrosis [46], we proceeded to investigate cellular bioactivity under varying treatment conditions. Employing live-dead staining alongside fluorescence microscopy, a distinct abundance of non-viable cells was visually discernible within the Blank and OG groups, while conversely, the OGPGM group exhibited only a limited extent of cellular death (Fig. 4L1, 4L2). Subsequent verification through CCK8 analysis consistently revealed diminished cell survival rates for both the Blank and OG groups, which amounted to a mere 51.0% and 48.6% of the control group, respectively. Remarkably, no significant disparity in cell survival rates emerged between the OGPGM group and the control group (Fig. 4L3). Collectively, the findings derived from live-dead staining and CCK8 analysis establish the remarkable capacity of OGPGM to fully shield cells from the deleterious effects conferred by 250 μM H2O2, a concentration substantially surpassing the pathological microenvironmental threshold commonly associated with OA.
3.7. OGPGM decreased inflammation, promoted ECM anabolic, and inhibited ECM catabolic
OA is a multifaceted disease influenced by various factors, and its pathogenesis and progression involve multiple mechanisms. ROS found in deteriorating joints can disrupt cellular processes and trigger the activation of pro-inflammatory factors, such as TNF-α and IL-1β [10]. These inflammatory factors contribute to the degradation of the ECM, which is a characteristic feature of OA [[47], [48], [49]]. Inflammatory conditions can lead to a decrease in the expression of COL2, a critical component of the ECM, while the expression of MMP13, a protease that is significantly upregulated during joint degeneration, is increased. Imbalanced expression of MMP13 and COL2 plays a role in degrading the ECM and accelerating the degradation of cartilage [50]. To mimic the inflammatory environment of OA, chondrocytes were induced using H2O2, and the effects of OGPGM were observed on inflammation levels and ECM metabolism using immunofluorescence and western blot techniques. These experiments aimed to assess the ability of OGPGM hydrogels to improve the inflammatory response and regulate ECM metabolism in chondrocytes.
In Fig. 5A, the results demonstrated that upon stimulation with H2O2, the fluorescence intensity of IL-1β and TNF-α significantly increased in the blank group, showing upregulation by 3.13 fold compared with the control group. The OG group exhibited no significant difference in fluorescence intensity compared to the blank group. Interestingly, the OGPGM group showed no significant difference in fluorescence intensity compared to the control group, indicating that the use of OGPGM loaded with PGA-Mn can effectively reduce the expression of inflammatory factors induced by H2O2 (Fig. 5C and D). As shown in Fig. 5B, the reduction of inflammatory factors produced a large promoting effect on ECM synthesis. In the blank group, the staining intensity of COL2 markedly weakened, accompanied by downregulation of its expression by 0.35 fold, while the expression of MMP13 increased by 3.13 fold compared with the control group. In the OG group, there were no significant differences observed in the expression of COL2 and MMP13 compared to the blank group. However, in the OGPGM group, the expression levels of COL2 and MMP13 were not noticeably altered, indicating that OGPGM could hinder the degradation of the ECM, promote its synthesis, and improve the denaturation process (Fig. 5E and F). Similarly, Western blot analysis revealed that the expression of IL-1β, TNF-α, MMP13, and COL2 in chondrocytes was consistent with the findings mentioned above, which suggested that OGPGM had a significant treatment effect on inflammation and ECM metabolic imbalance. (Fig. 5G–K). The findings revealed that chondrocytes exhibited heightened production of inflammatory factors, including TNF-α and IL-6, upon exposure to ROS, leading to ECM metabolism imbalance and a shift towards ECM degradation. Significantly, following co-culture with OGPGM loaded with PGA-Mn, the expression of ROS-induced inflammatory factors decreased, and ECM metabolism was restored. This suggested that OGPGM possessed the capability to diminish inflammatory factors in OA and upheld the metabolic equilibrium of cartilage tissue, as evidenced by in vivo experiments, thereby signifying its potential as a treatment for OA.
Fig. 5.
OGPGM alleviated chondrocyte inflammation and promoted ECM synthesis (A, B) Representative immunofluorescence staining images display the protein expression level of COL2, MMP13, TNF-α, and IL-1β in the H2O2 treated chondrocytes, which are co-cultured with the OG and OGPGM for 12 h. Green: COL2; Blue: cell nuclei; Red: cell F-actin. (n = 3) (C–F) Relative fluorescence quantitative analysis of TNF-α, IL-1β, MMP13, and COL2 in different groups. (G) Western blot analysis and semi quantification of TNF-α, IL-1β, MMP13, and COL2 proteins (n = 3). (I–K) Relative protein expression of TNF-α, IL-1β, MMP13 and COL2. (Data presented as mean ± SD, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, ns, not significant).
3.8. Therapeutic effect on OA in vivo
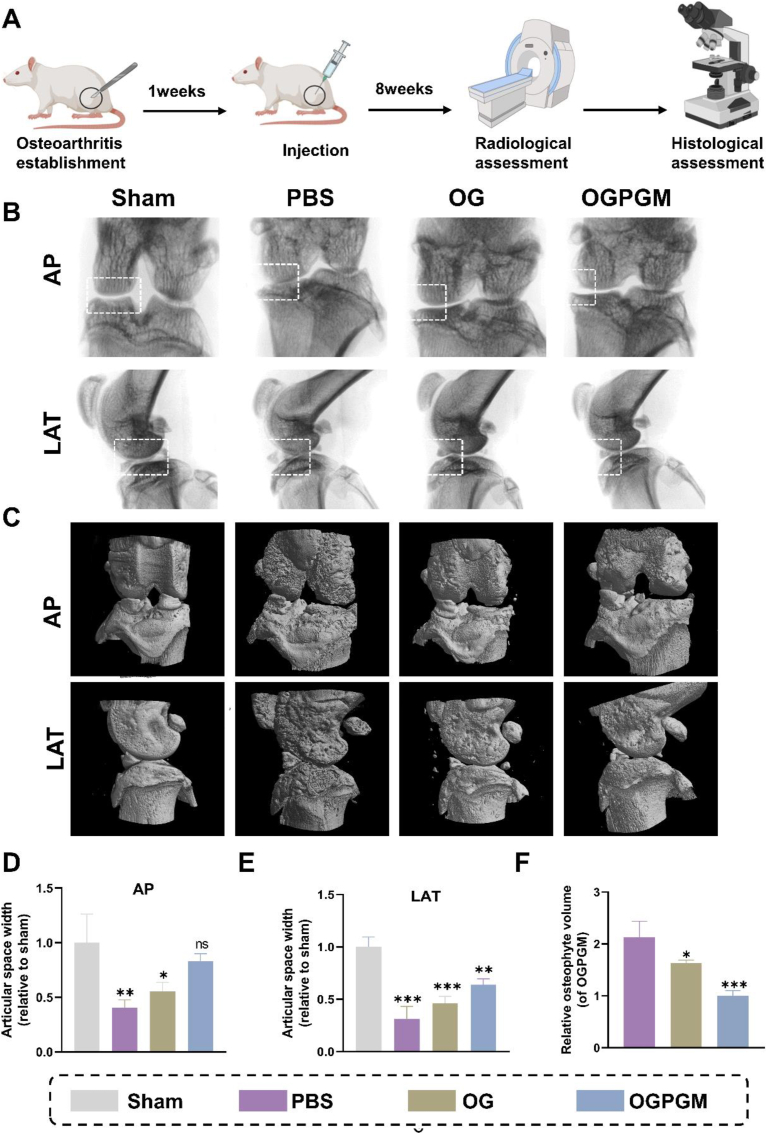
To evaluate the therapeutic impact of OGPGM on OA in rats in vivo, we established a rat OA model through ACL transection and medial meniscectomy, and conducted imaging and histological examinations (Fig. 6A). OA is typically characterized by joint space narrowing and the formation of osteophytes [[51], [52], [53]]. As shown in Fig. 6B–D, and E, the joint space widths (JSWs) were observed under x-ray and were significantly reduced in the PBS group compared to the sham group. The OG group, owing to the hygroscopicity and the component of OG, preserved the JSWs to some extent, albeit smaller than that in the sham group. Remarkably, the OGPGM group demonstrated significantly higher JSWs compared to the PBS group, with no significant difference observed when compared to the sham group. This suggested that OGPGM shows potential as a joint lubricant and drug carrier, effectively reducing joint wear and cartilage degeneration. Osteophytes, which serve as a compensatory response to OA injury, are another characteristic feature of the disease. As depicted in Fig. 6C, it can be observed that the sham group did not display any significant osteophyte formation, while the PBS group exhibited a substantial amount of osteophyte formation. Importantly, after intra-articular injection, the volume of osteophytes was significantly reduced by 53.1% and 23.47% in the OG and OGPGM groups, respectively, compared to the osteophyte volume observed in the PBS group. (Fig. 6F). Overall, these findings demonstrated that OGPGM possessed the potential to mitigate joint wear, cartilage degeneration, and osteophyte formation, potentially serving as an effective therapeutic intervention for OA in a rat model.
Fig. 6.
The radiological data of animal experiments. (A) Overview of animal experiments. (B) Representative x-ray (n = 5) and (C) micro-CT images in anterior-posterior (AP) and lateral (LAT) view of the knee joint (n = 5). (D) Relative knee joint gap widths on the LAT and (E) AP of the knee in different groups of X-rays (F) The relative osteophyte volume measured from micro-CT images. (Data presented as mean ± SD, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, ns, not significant).
In Fig. 7A, the gross observation of the knee surface of OG and OGPGM after 8 weeks of treatment is presented. In the sham group, a smooth articular surface was evident without any noticeable wear. In the PBS group, the articular surface exhibited roughness and unevenness, with the formation of osteophytes and a substantial amount of irregular white connective tissue wrapping around the area, possibly associated with long-term wear and delayed repair. Partial wear and osteophyte formation were also observed in the OG group. Importantly, in the OGPGM group, the smoothness of the articular surface was relatively preserved, and there was no significant osteophyte formation. Furthermore, histological evaluation of rat tissues was conducted to assess articular cartilage changes. HE and Safranin O-fast green staining were performed in Fig. 7B and C illustrates the joints in the sham group, which exhibited smooth articular surfaces, normal structural organization, regular cell counts, and strong Safranin O-fast green staining (the cartilage appears red). In contrast, the PBS group displayed severe articular surface wear, disorganized structure, significantly reduced cell count, and attenuated Safranin O-fast green staining. However, in the OG and OGPGM groups, degeneration was reduced, with the OGPGM group showing no significant degeneration. To systematically evaluate the quality of articular cartilage, we summarized the results of the OARSI score (24 points in total) and the Mankin score (14 points in total). The OGPGM group exhibited the lowest OARSI score (3.33) and was closest to the sham group compared to the PBS group (14.66) and the OG group (8.67) (Fig. 7D). Additionally, the Mankin score indicated that the repair effect in the OGPGM group (5 points) was superior to that in the PBS (4 points) and OG (6 points) groups (Fig. 7E). Furthermore, the protein levels of COL2 and MMP13 were evaluated using immunohistochemistry (Fig. 7F). PBS group exhibited significantly lower COL2 expression (0.27 fold) and elevated MMP13 (3.82 fold) expression compared to the sham group (1.0 fold). The OG group showed a partial decrease in COL2 expression (0.37 fold) and a partial increase in MMP13 expression (3.18 fold). Conversely, the OGPGM group displayed expression levels of COL2 (0.64 fold) and MMP13(1.59 fold) that were not significantly different from the sham group (Fig. 7I. J). These results histologically demonstrated that OGPGM can reduce joint wear and retard the progression of OA.
Fig. 7.
The histological data of animal experiments. (A) Macroscopic observation of the femur. (B) Representative images of HE staining and (C) Safranin O-fast green staining from each group at 8 weeks after surgery (n = 5). (D) IHC of COL2 and MMP13 at 8 weeks after surgery (n = 5). (E) OARSI score, (F) and Mankin score of knee joint tissue. (G) Apoptosis level of chondrocytes in the knee joint detected by TUNEL (n = 5). (H) TNF-α characterized by knee joint tissue immunofluorescent staining (n = 5). (I) Quantification of the relative expression of collagen II and (J)MMP -13 (n = 5). (K) Percentage of TUNEL-positive cell nuclei. (L) Relative fluorescence intensity of TNF-α. The white dotted lines point toward the surface of the articular cartilage. (Data presented as mean ± SD, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, ns, not significant).
To detect articular chondrocyte apoptosis, the TUNEL assay was performed (Fig. 7G). Sham group and OGPGM group exhibited weak fluorescent expression of TUNEL-positive chondrocytes, with no significant difference, yielding a positive rate of 10.07% and 20.04%, respectively. In contrast, the PBS and OG groups demonstrated greater expression of TUNEL-positive chondrocytes, with positive rates of 46.78% and 38.01%, respectively (Fig. 7K). This confirmed that OGPGM reduced apoptosis during the joint degenerative process. The presence of inflammation within the joint was further confirmed by immunofluorescence of TNF-α (Fig. 7H). The strong fluorescent expression observed in the PBS group compared to the sham group indicates significant inflammation during the degenerative process. Conversely, the fluorescence intensity in the OG and OGPGM groups was diminished, with the OGPGM group displaying fluorescence intensity only 45.4% of that in the PBS group (Fig. 7L). This suggested that OGPGM reduces the inflammatory response brought about by joint friction and can effectively protect chondrocytes in articular cartilage.
4. Conclusion
In this work, we have successfully developed a nanocomposite hydrogel with remarkable attributes, encompassing high-strength lubrication and potent anti-inflammatory effects. This achievement was made possible through the self-assembly of GA with Mn2+, followed by its incorporation into a nanocomposite hydrogel composed of OSA and gelatin. The integration of PGA-Mn into OGPGM provided not only exceptional capabilities for scavenging ROS but also a significant abundance of phenolic hydroxyl groups. These hydroxyl groups contributed to the improved hygroscopicity and lubrication properties of OGPGM. Furthermore, by crosslinking the molecular chains of OSA and gelatin, our nanocomposite hydrogels, OGPGM, exhibited robust mechanical properties and had the ability to release PGA-Mn steadily over an extended period within the joints. Through in vitro cellular experiments, we have established the biocompatibility of OGPGM, as it showed reduced inflammatory responses in chondrocytes while significantly enhancing protein levels associated with cartilage anabolism. Moreover, our in vitro experiments demonstrated that OGPGM effectively mitigated cartilage wear and protected chondrocytes from inflammatory interference, thereby preserving their vitality and overall health. Given these compelling findings, we hold an optimistic outlook regarding the potential of OGPGM to revolutionize the treatment landscape for chronic inflammatory disorders associated with friction, notably OA.
CRediT authorship contribution statement
Qizhu Chen: Writing – original draft, Writing – review & editing, Visualization, Data curation. Yuxin Jin: Methodology. Tao Chen: Formal analysis. Hao Zhou: Data curation. Xinzhou Wang: Methodology. Ouqiang Wu: Software. Linjie Chen: Investigation, Formal analysis. Zhiguang Zhang: Methodology. Zhengyu Guo: Software. Jin Sun: Methodology. Aimin Wu: Visualization, Investigation, Funding acquisition, Conceptualization. Qiuping Qian: Writing – original draft, Writing – review & editing, Project administration, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by Qiuping Qian from the Major Science and Technology Project of Wenzhou Science and Technology (ZG2022017) and Aimin Wu from the Wenzhou Major Scientific and Technological Innovation Project (ZY2022010)
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.mtbio.2024.100993.
Contributor Information
Aimin Wu, Email: aiminwu@wmu.edu.cn.
Qiuping Qian, Email: qianqp@ucas.ac.cn.
Appendix A. Supplementary data
The following is/are the supplementary data to this article.
Data availability
Data will be made available on request.
References
- 1.Goldring S.R., Goldring M.B. Changes in the osteochondral unit during osteoarthritis: structure, function and cartilage-bone crosstalk. Nat. Rev. Rheumatol. 2016;12:632–644. doi: 10.1038/nrrheum.2016.148. https://10.1038/nrrheum.2016.148 [DOI] [PubMed] [Google Scholar]
- 2.Hootman J.M., Helmick C.G. Projections of US prevalence of arthritis and associated activity limitations. Arthritis Rheum. 2006;54:226–229. doi: 10.1002/art.21562. https://10.1002/art.21562 [DOI] [PubMed] [Google Scholar]
- 3.Hunter D.J., Bierma-Zeinstra S. Osteoarthritis. Lancet. 2019;393:1745–1759. doi: 10.1016/S0140-6736(19)30417-9. https://10.1016/s0140-6736(19)30417-9 [DOI] [PubMed] [Google Scholar]
- 4.Lanas A., Tornero J., Zamorano J.L. Assessment of gastrointestinal and cardiovascular risk in patients with osteoarthritis who require NSAIDs: the LOGICA study. Ann. Rheum. Dis. 2010;69:1453–1458. doi: 10.1136/ard.2009.123166. https://10.1136/ard.2009.123166 [DOI] [PubMed] [Google Scholar]
- 5.Zhu C., Han S., Zeng X., Zhu C., Pu Y., Sun Y. Multifunctional thermo-sensitive hydrogel for modulating the microenvironment in Osteoarthritis by polarizing macrophages and scavenging RONS. J Nanobiotechnology. 2022;20:221. doi: 10.1186/s12951-022-01422-9. https://10.1186/s12951-022-01422-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rahmati M., Nalesso G., Mobasheri A., Mozafari M. Aging and osteoarthritis: Central role of the extracellular matrix. Ageing Res. Rev. 2017;40:20–30. doi: 10.1016/j.arr.2017.07.004. https://10.1016/j.arr.2017.07.004 [DOI] [PubMed] [Google Scholar]
- 7.Ni R., Guo X.E., Yan C., Wen C. Hemodynamic stress shapes subchondral bone in osteoarthritis: an emerging hypothesis. J Orthop Translat. 2022;32:85–90. doi: 10.1016/j.jot.2021.11.007. https://10.1016/j.jot.2021.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henrotin Y.E., Bruckner P., Pujol J.P. The role of reactive oxygen species in homeostasis and degradation of cartilage. Osteoarthritis Cartilage. 2003;11:747–755. doi: 10.1016/s1063-4584(03)00150-x. https://10.1016/s1063-4584(03)00150-x [DOI] [PubMed] [Google Scholar]
- 9.Bolduc J.A., Collins J.A., Loeser R.F. Reactive oxygen species, aging and articular cartilage homeostasis. Free Radic. Biol. Med. 2019;132:73–82. doi: 10.1016/j.freeradbiomed.2018.08.038. https://10.1016/j.freeradbiomed.2018.08.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forrester S.J., Kikuchi D.S., Hernandes M.S., Xu Q., Griendling K.K. Reactive oxygen species in metabolic and inflammatory signaling. Circ. Res. 2018;122:877–902. doi: 10.1161/CIRCRESAHA.117.311401. https://10.1161/circresaha.117.311401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li X., Li X., Yang J., Lin J., Zhu Y., Xu X., Cui W. Living and injectable porous hydrogel microsphere with paracrine activity for cartilage regeneration. Small. 2023;19 doi: 10.1002/smll.202207211. https://10.1002/smll.202207211 [DOI] [PubMed] [Google Scholar]
- 12.Grogan S.P., Chen X., Sovani S., Taniguchi N., Colwell C.W., Jr., Lotz M.K., D'Lima D.D. Influence of cartilage extracellular matrix molecules on cell phenotype and neocartilage formation. Tissue Eng Part A. 2014;20:264–274. doi: 10.1089/ten.tea.2012.0618. https://10.1089/ten.TEA.2012.0618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hodgkinson T., Kelly D.C., Curtin C.M., O'Brien F.J. Mechanosignalling in cartilage: an emerging target for the treatment of osteoarthritis. Nat. Rev. Rheumatol. 2022;18:67–84. doi: 10.1038/s41584-021-00724-w. https://10.1038/s41584-021-00724-w [DOI] [PubMed] [Google Scholar]
- 14.Hercberg S., Galan P., Preziosi P., Bertrais S., Mennen L., Malvy D., Roussel A.M., Favier A., Briançon S. The SU.VI.MAX Study: a randomized, placebo-controlled trial of the health effects of antioxidant vitamins and minerals. Arch. Intern. Med. 2004;164:2335–2342. doi: 10.1001/archinte.164.21.2335. https://10.1001/archinte.164.21.2335 [DOI] [PubMed] [Google Scholar]
- 15.Lee W.M., Kaplowitz N. Alcohol, fasting, and therapeutic dosing of acetaminophen: a perfect storm. Hepatology. 2021;73:1634–1636. doi: 10.1002/hep.31747. https://10.1002/hep.31747 [DOI] [PubMed] [Google Scholar]
- 16.Zhong G., Yang X., Jiang X., Kumar A., Long H., Xie J., Zheng L., Zhao J. Dopamine-melanin nanoparticles scavenge reactive oxygen and nitrogen species and activate autophagy for osteoarthritis therapy. Nanoscale. 2019;11:11605–11616. doi: 10.1039/c9nr03060c. https://10.1039/c9nr03060c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hassan Rasouli, Farzaei Mohammad Hosein, Khodarahmi Reza. Polyphenols and their benefits: a review. Int. J. Food Prop. 2017;20:1700–1741. https://10.1080/10942912.2017.1354017 [Google Scholar]
- 18.Li G., Liu S., Chen Y., Zhao J., Xu H., Weng J., Yu F., Xiong A., Udduttula A., Wang D., Liu P., Chen Y., Zeng H. An injectable liposome-anchored teriparatide incorporated gallic acid-grafted gelatin hydrogel for osteoarthritis treatment. Nat. Commun. 2023;14:3159. doi: 10.1038/s41467-023-38597-0. https://10.1038/s41467-023-38597-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo Y., Sun Q., Wu F.G., Dai Y., Chen X. Polyphenol-containing nanoparticles: synthesis, properties, and therapeutic delivery. Adv Mater. 2021;33 doi: 10.1002/adma.202007356. https://10.1002/adma.202007356 [DOI] [PubMed] [Google Scholar]
- 20.Wei Z., Peng G., Zhao Y., Chen S., Wang R., Mao H., Xie Y., Zhao C. Engineering antioxidative cascade metal-phenolic nanozymes for alleviating oxidative stress during extracorporeal blood purification. ACS Nano. 2022;16:18329–18343. doi: 10.1021/acsnano.2c06186. https://10.1021/acsnano.2c06186 [DOI] [PubMed] [Google Scholar]
- 21.Li Y., Shen B., Lv C., Zhu X., Naren Q., Xu D., Chen H., Wu F. Methyl gallate prevents oxidative stress induced apoptosis and ECM degradation in chondrocytes via restoring Sirt3 mediated autophagy and ameliorates osteoarthritis progression. Int Immunopharmacol. 2023;114 doi: 10.1016/j.intimp.2022.109489. https://10.1016/j.intimp.2022.109489 [DOI] [PubMed] [Google Scholar]
- 22.Natarajan V., Madhan B., Tiku M.L. Intra-articular injections of polyphenols protect articular cartilage from inflammation-induced degradation: suggesting a potential role in cartilage therapeutics. PLoS One. 2015;10 doi: 10.1371/journal.pone.0127165. https://10.1371/journal.pone.0127165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vaidyanathan J.B., Walle T. Glucuronidation and sulfation of the tea flavonoid (-)-epicatechin by the human and rat enzymes. Drug Metab. Dispos. 2002;30:897–903. doi: 10.1124/dmd.30.8.897. https://10.1124/dmd.30.8.897 [DOI] [PubMed] [Google Scholar]
- 24.Wei H., Qin J., Huang Q., Jin Z., Zheng L., Zhao J., Qin Z. Epigallocatechin-3-gallate (EGCG) based metal-polyphenol nanoformulations alleviates chondrocytes inflammation by modulating synovial macrophages polarization. Biomed. Pharmacother. 2023;161 doi: 10.1016/j.biopha.2023.114366. https://10.1016/j.biopha.2023.114366 [DOI] [PubMed] [Google Scholar]
- 25.Yao H., Xu J., Wang J., Zhang Y., Zheng N., Yue J., Mi J., Zheng L., Dai B., Huang W., Yung S., Hu P., Ruan Y., Xue Q., Ho K., Qin L. Combination of magnesium ions and vitamin C alleviates synovitis and osteophyte formation in osteoarthritis of mice. Bioact. Mater. 2021;6:1341–1352. doi: 10.1016/j.bioactmat.2020.10.016. https://10.1016/j.bioactmat.2020.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh H., Knapik D.M., Polce E.M., Eikani C.K., Bjornstad A.H., Gursoy S., Perry A.K., Westrick J.C., Yanke A.B., Verma N.N., Cole B.J., Chahla J.A. Relative efficacy of intra-articular injections in the treatment of knee osteoarthritis: a systematic review and network meta-analysis. Am. J. Sports Med. 2022;50:3140–3148. doi: 10.1177/03635465211029659. https://10.1177/03635465211029659 [DOI] [PubMed] [Google Scholar]
- 27.Kou L., Huang H., Tang Y., Sun M., Li Y., Wu J., Zheng S., Zhao X., Chen D., Luo Z., Zhang X., Yao Q., Chen R. Opsonized nanoparticles target and regulate macrophage polarization for osteoarthritis therapy: a trapping strategy. J Control Release. 2022;347:237–255. doi: 10.1016/j.jconrel.2022.04.037. https://10.1016/j.jconrel.2022.04.037 [DOI] [PubMed] [Google Scholar]
- 28.Sandker M.J., Petit A., Redout E.M., Siebelt M., Müller B., Bruin P., Meyboom R., Vermonden T., Hennink W.E., Weinans H. In situ forming acyl-capped PCLA-PEG-PCLA triblock copolymer based hydrogels. Biomaterials. 2013;34:8002–8011. doi: 10.1016/j.biomaterials.2013.07.046. https://10.1016/j.biomaterials.2013.07.046 [DOI] [PubMed] [Google Scholar]
- 29.Graf D.N., Thallinger A., Zubler V., Sutter R. Intraarticular steroid injection in hip and knee with fluoroscopic Guidance: reassessing safety. Radiology. 2022;304:363–369. doi: 10.1148/radiol.210668. https://10.1148/radiol.210668 [DOI] [PubMed] [Google Scholar]
- 30.Nowaczyk A., Szwedowski D., Dallo I., Nowaczyk J. Overview of first-line and second-line pharmacotherapies for osteoarthritis with special focus on intra-articular treatment. Int. J. Mol. Sci. 2022;23 doi: 10.3390/ijms23031566. https://10.3390/ijms23031566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xue Xu, Hu Yan, Deng Yonghui, Su Jiacan. Recent advances in design of functional biocompatible hydrogels for bone tissue engineering. Adv. Funct. Mater. 2021;31 doi: 10.1002/adfm.202009432. [DOI] [Google Scholar]
- 32.Bian J., Cai F., Chen H., Tang Z., Xi K., Tang J., Wu L., Xu Y., Deng L., Gu Y., Cui W., Chen L. Modulation of local overactive inflammation via injectable hydrogel microspheres. Nano Lett. 2021;21:2690–2698. doi: 10.1021/acs.nanolett.0c04713. https://10.1021/acs.nanolett.0c04713 [DOI] [PubMed] [Google Scholar]
- 33.Large D.E., Abdelmessih R.G., Fink E.A., Auguste D.T. Liposome composition in drug delivery design, synthesis, characterization, and clinical application. Adv. Drug Deliv. Rev. 2021;176 doi: 10.1016/j.addr.2021.113851. https://10.1016/j.addr.2021.113851 [DOI] [PubMed] [Google Scholar]
- 34.Colella F., Garcia J.P., Sorbona M., Lolli A., Antunes B., D'Atri D., Barré F.P.Y., Oieni J., Vainieri M.L., Zerrillo L., Capar S., Häckel S., Cai Y., Creemers L.B. Drug delivery in intervertebral disc degeneration and osteoarthritis: selecting the optimal platform for the delivery of disease-modifying agents. J Control Release. 2020;328:985–999. doi: 10.1016/j.jconrel.2020.08.041. https://10.1016/j.jconrel.2020.08.041 [DOI] [PubMed] [Google Scholar]
- 35.Chen T., Qian Q., Makvandi P., Zare E.N., Chen Q., Chen L., Zhang Z., Zhou H., Zhou W., Wang H., Wang X., Chen Y., Zhou Y., Wu A. Engineered high-strength biohydrogel as a multifunctional platform to deliver nucleic acid for ameliorating intervertebral disc degeneration. Bioact. Mater. 2023;25:107–121. doi: 10.1016/j.bioactmat.2023.01.010. https://10.1016/j.bioactmat.2023.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Balakrishnan B., Lesieur S., Labarre D., Jayakrishnan A. Periodate oxidation of sodium alginate in water and in ethanol-water mixture: a comparative study. Carbohydr. Res. 2005;340:1425–1429. doi: 10.1016/j.carres.2005.02.028. https://10.1016/j.carres.2005.02.028 [DOI] [PubMed] [Google Scholar]
- 37.Murphy M.P., Koepke L.S., Lopez M.T., Tong X., Ambrosi T.H., Gulati G.S., Marecic O., Wang Y., Ransom R.C., Hoover M.Y., Steininger H., Zhao L., Walkiewicz M.P., Quarto N., Levi B., Wan D.C., Weissman I.L., Goodman S.B., Yang F., Longaker M.T., Chan C.K.F. Articular cartilage regeneration by activated skeletal stem cells. Nat Med. 2020;26:1583–1592. doi: 10.1038/s41591-020-1013-2. https://10.1038/s41591-020-1013-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aytekin K., Uysal M., Şahiner G.G., Danışman M., Baş O., Takır S., Coşkun Z.Ü., Akdeniz E., Esenyel C.Z. Evaluation of different intraarticular injection volumes to assess optimum efficient amount; an experimental study in rat knee joints. J. Pharmacol. Toxicol. Methods. 2020;101 doi: 10.1016/j.vascn.2019.106658. https://10.1016/j.vascn.2019.106658 [DOI] [PubMed] [Google Scholar]
- 39.Xavier M., García-Hevia L., Amado I.R., Pastrana L., Gonçalves C. In vitro intestinal uptake and permeability of fluorescently-labelled hyaluronic acid nanogels. Int J Nanomedicine. 2019;14:9077–9088. doi: 10.2147/IJN.S224255. https://10.2147/ijn.S224255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pritzker K.P., Gay S., Jimenez S.A., Ostergaard K., Pelletier J.P., Revell P.A., Salter D., van den Berg W.B. Osteoarthritis cartilage histopathology: grading and staging. Osteoarthritis Cartilage. 2006;14:13–29. doi: 10.1016/j.joca.2005.07.014. https://10.1016/j.joca.2005.07.014 [DOI] [PubMed] [Google Scholar]
- 41.Moon S.J., Woo Y.J., Jeong J.H., Park M.K., Oh H.J., Park J.S., Kim E.K., Cho M.L., Park S.H., Kim H.Y., Min J.K. Rebamipide attenuates pain severity and cartilage degeneration in a rat model of osteoarthritis by downregulating oxidative damage and catabolic activity in chondrocytes. Osteoarthritis Cartilage. 2012;20:1426–1438. doi: 10.1016/j.joca.2012.08.002. https://10.1016/j.joca.2012.08.002 [DOI] [PubMed] [Google Scholar]
- 42.Seror J., Zhu L., Goldberg R., Day A.J., Klein J. Supramolecular synergy in the boundary lubrication of synovial joints. Nat. Commun. 2015;6:6497. doi: 10.1038/ncomms7497. https://10.1038/ncomms7497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu G., Liu Z., Li N., Wang X., Zhou F., Liu W. Hairy polyelectrolyte brushes-grafted thermosensitive microgels as artificial synovial fluid for simultaneous biomimetic lubrication and arthritis treatment. ACS Appl. Mater. Interfaces. 2014;6:20452–20463. doi: 10.1021/am506026e. https://10.1021/am506026e [DOI] [PubMed] [Google Scholar]
- 44.Evans C.H., Kraus V.B., Setton L.A. Progress in intra-articular therapy. Nat. Rev. Rheumatol. 2014;10:11–22. doi: 10.1038/nrrheum.2013.159. https://10.1038/nrrheum.2013.159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ansari M.Y., Ahmad N., Haqqi T.M. Oxidative stress and inflammation in osteoarthritis pathogenesis: role of polyphenols. Biomed. Pharmacother. 2020;129 doi: 10.1016/j.biopha.2020.110452. https://10.1016/j.biopha.2020.110452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaminskyy V.O., Zhivotovsky B. Free radicals in cross talk between autophagy and apoptosis. Antioxid Redox Signal. 2014;21:86–102. doi: 10.1089/ars.2013.5746. https://10.1089/ars.2013.5746 [DOI] [PubMed] [Google Scholar]
- 47.Graham J.M., Ayati B.P., Ding L., Ramakrishnan P.S., Martin J.A. Reaction-diffusion-delay model for EPO/TNF-α interaction in articular cartilage lesion abatement. Biol. Direct. 2012;7:9. doi: 10.1186/1745-6150-7-9. https://10.1186/1745-6150-7-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kapoor M., Martel-Pelletier J., Lajeunesse D., Pelletier J.P., Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat. Rev. Rheumatol. 2011;7:33–42. doi: 10.1038/nrrheum.2010.196. https://10.1038/nrrheum.2010.196 [DOI] [PubMed] [Google Scholar]
- 49.Kang L.J., Yoon J., Rho J.G., Han H.S., Lee S., Oh Y.S., Kim H., Kim E., Kim S.J., Lim Y.T., Park J.H., Song W.K., Yang S., Kim W. Self-assembled hyaluronic acid nanoparticles for osteoarthritis treatment. Biomaterials. 2021;275 doi: 10.1016/j.biomaterials.2021.120967. https://10.1016/j.biomaterials.2021.120967 [DOI] [PubMed] [Google Scholar]
- 50.Hu Q., Ecker M. Overview of MMP-13 as a promising target for the treatment of osteoarthritis. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms22041742. https://10.3390/ijms22041742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nieminen M.T., Casula V., Nevalainen M.T., Saarakkala S. Osteoarthritis year in review 2018: imaging. Osteoarthritis Cartilage. 2019;27:401–411. doi: 10.1016/j.joca.2018.12.009. https://10.1016/j.joca.2018.12.009 [DOI] [PubMed] [Google Scholar]
- 52.Paixao T., DiFranco M.D., Ljuhar R., Ljuhar D., Goetz C., Bertalan Z., Dimai H.P., Nehrer S. A novel quantitative metric for joint space width: data from the Osteoarthritis Initiative (OAI) Osteoarthritis Cartilage. 2020;28:1055–1061. doi: 10.1016/j.joca.2020.04.003. https://10.1016/j.joca.2020.04.003 [DOI] [PubMed] [Google Scholar]
- 53.Lieben L. Osteoarthritis: osteophyte formation involves PAR2. Nat. Rev. Rheumatol. 2016;12:70–71. doi: 10.1038/nrrheum.2016.6. https://10.1038/nrrheum.2016.6 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.