Abstract
Methicillin-resistant Staphylococcus aureus (MRSA) is an important human pathogen that has emerged through the horizontal acquisition of the staphylococcal cassette chromosome mec (SCCmec). Previously, we showed that SCCmec from heat-killed donors can be transferred via natural transformation in biofilms at frequencies of 10−8-10−7. Here, we show an improved transformation assay of SCCmec with frequencies up to 10−2 using co-cultured biofilms with living donor cells. The Ccr-attB system played an important role in SCCmec transfer, and the deletion of ccrAB recombinase genes reduced the frequency ∼30-fold. SCCmec could be transferred from either MRSA or methicillin-resistant coagulase-negative staphylococci to some methicillin-sensitive S. aureus recipients. In addition, the transformation of other plasmid or chromosomal genes is enhanced by using living donor cells. This study emphasizes the role of natural transformation as an evolutionary ability of S. aureus and in MRSA emergence.
Keywords: Staphylococcus aureus, Antimicrobial resistance, SCC, Natural transformation, Biofilm
1. Introduction
Staphylococcus aureus is a Gram-positive bacterium that commonly inhabits the skin and mucosal surfaces of animals and humans. As an opportunistic pathogen, it causes a wide variety of diseases, ranging from minor skin abscesses and food poisoning to endocarditis, toxic shock syndrome, and sepsis. S. aureus is notorious for its ability to acquire antibiotic resistance [1,2], and methicillin-resistant S. aureus (MRSA) has significant mortality and morbidity as the leading cause of nosocomial infections [3]. MRSA has also spread to the community (community-associated MRSA, CA-MRSA) and has been associated with the livestock (livestock-associated MRSA, LA-MRSA), posing a serious health and economic burden on a global scale [4,5].
MRSA emerges through the acquisition of a mobile genetic element known as staphylococcal cassette chromosome mec (SCCmec) carrying the methicillin-resistance gene mecA. SCCmec is currently classified into 13 types, with types I–V (20–60 kb) being the most prevalent and widely distributed [6]. The cassette chromosome recombinases (Ccr) encoded by SCCmec (ccrAB for types I-IV, ccrC for type V) mediate its excision and integration at a specific attachment site (attB) located at the 3′end of the orfX gene (rmlH) in S. aureus chromosome [7,8]. The origins of SCCmec are not fully clear, but ancestral fragments have been identified in coagulase-negative staphylococci (CoNS) species such as S. sciuri (recently reclassified to Mammaliicoccus sciuri), S. fleurettii, S. lentus, and S. vitulinus, in addition to Macrococcus caseolyticus [6,[9], [10], [11]], many of which are animal commensals [12]. This suggests that MRSA has likely acquired mecA from animal-derived CoNS species [9,13]. At least 20 independent acquisitions of SCCmec were predicted to have occurred in S. aureus [14]. In clinical settings, rare horizontal SCCmec transfer could have happened from MR-CoNS to MSSA, generating a new MRSA in the same patient [12,15]. One case is the MSSA strain WKZ-1 and the MRSA strain WKZ-2, which were recovered from the same neonate and are isogenic except for the presence of SCCmec IV in WKZ-2, which was also present in a CoNS strain isolated from the same neonate [[15], [16], [17]]. Despite evidence of dissemination by horizontal gene transfer (HGT), the major mechanism of SCCmec transmission has remained a mystery for several decades [18].
Bacteriophage-mediated transduction and conjugation have been suggested as possible mechanisms of SCCmec transfer, but only short or fragmented SCCmec could be transmitted by these mechanisms [19,20]. Natural competence for DNA transformation is another HGT mechanism enabling bacteria to incorporate extracellular DNA through the DNA-uptake machinery encoded by a series of competence genes [21]. This process in Gram-positive bacteria has been extensively studied in Bacillus subtilis and Streptococcus pneumonia. While the DNA-uptake machinery is highly conserved, the signalling and conditions regulating competence development are diverse among species, and finding the bona fide transformation conditions for a given specific species or strain is challenging [22]. In B. subtilis, the transcription factor ComK controls the expression of competence genes in a subpopulation. ComK is tightly regulated by environmental signals such as quorum sensing and nutrient limitation. In S. pneumonia, competence is activated by the SigX sigma factor (a.k.a. ComX) in the whole population upon induction by quorum sensing and stressors. In S. aureus, a subpopulation expresses competence genes when grown in a complete synthetic medium (CS2). The competence operons, comG and comE operons, are under the transcriptional control of SigH and ComK [[23], [24], [25]]. Recently, we showed that growth in biofilm conditions promotes natural transformation in S. aureus cells [26]. In these biofilm conditions, we observed cell-to-cell inter- and intraspecies transfer of SCCmec by natural transformation. However, the transformation frequencies of SCCmec were low (∼10−8-10−7).
In this study, we aimed to seek the better biofilm conditions required for the SCCmec transformation. Our results show that mixed biofilms with living donor cells can be the appropriate place for SCCmec as well as other genetic elements to be transferred efficiently.
2. Results
2.1. Living donor cells enhance transformation efficiency of SCCmec in biofilms
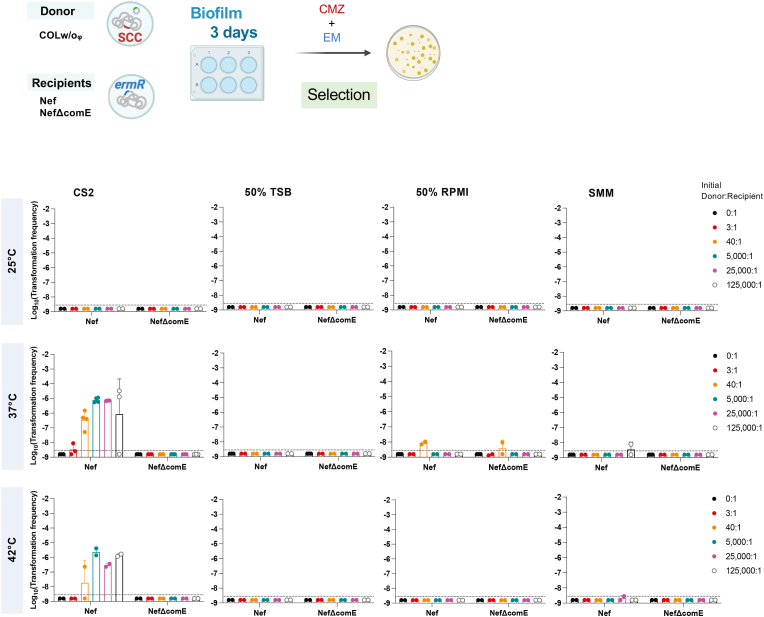
To explore better biofilm conditions for SCCmec transfer, we tested different culture media, temperatures, donor cell status (heat-killed or alive), and the ratio of the donor to recipient (Fig. S1, Fig. 1). The laboratory strain Nef (N315 derivative that lacks conjugative genes, a lysogenic phage, and SCCmec) was grown statically (biofilm growth conditions) with donor cells for 3 days in one medium: CS2 medium [23,26], diluted TSB (50% TSB), diluted RPMI (50% RPMI), or SMM [27,28] at different temperatures: 25 °C, 37 °C, and 42 °C. COLw/oφ (COL derivative that lacks conjugative genes and the lysogenized phage but carries SCCmec) was used as the donor. In Fig. S1, we used heat-killed donor cells as in previous reports [23,26], while in Fig. 1, living donor cells were tested. In all experiments, a fixed amount of donor cells (∼5 × 108 CFU per well equivalent) was used while the recipient cell number was changed, generating the series of donor-to-recipient ratios shown in each figure. SCCmec-transformants were selected using the β-lactam antibiotic cefmetazole. Erythromycin was added to eliminate the living donor (Fig. 1). The ability of emerged colonies to grow in the presence of cefmetazole was confirmed by replica [26]. The transformation frequencies were calculated as the ratio of the number of emerged transformants to the total CFU of the recipient at the end of the transformation assay and are shown in Fig. 1.
Fig. 1.
Transformation efficiency of SCCmec in the biofilms in different conditions using living donor.
The recipient cells (Nef or NefΔcomE) were statically grown in different media and temperatures for 3 days. An initial fixed amount of living COLw/oφ donor cells was used, generating the following donor-to-recipient ratios: 0:1, 3:1, 40:1, 5000:1, 25000:1, and 125000:1. Transformation efficiencies were determined after 3 days by selection with cefmetazole and erythromycin (EM).
The mean of at least n = 2 independent experiments is shown with SD. Data points represent independent experiments. The dotted lines represent the detection limit.
The condition of our previously reported protocol using heat-killed donor is marked by the red arrow (Fig. S1: CS2, 37 °C, 4:1), and the frequency was about 10−8. This culture condition (CS2 at 37 °C) was one of the best experimental settings for transformation that we have found in previous studies, and this study again indicates that no other combination of the tested culture medium and temperature is better for transformation (Fig. S1). Increasing the ratio of the heat-killed donor to the recipient (reducing the number of recipient cells) did not improve the transformation frequency. Strikingly, on the other hand, when we used living donor cells instead of heat-killed cells, increasing the donor-to-recipient ratio resulted in higher transformation frequencies of SCCmec (up to 10−5-10−4) (Fig. 1: CS2, 37 °C). This efficient transfer of SCCmec from living cells is via natural transformation because no transformants were generated when NefΔcomE was used as the recipient. In a 50% RPMI medium, three cefmetazole-resistant colonies were detected from NefΔcomE recipient in one experiment, but it was not reproducible, and the reason is not known.
Time course of transformation using the living donor cells showed the transformation frequencies of SCCmec increasing towards day 2, with a peak around 10−4 on average and the maximum being 10−3-10−2, while the CFU values of recipient cells were sustained (Fig. 2A, see 5000–125000:1). This generated up to over 104 transformants (5234 on average) per well including 107 CFU recipient (Table S1, Fig. S3A). The generated transformants had the same genetic backbone as the recipient (Fig. S3) and full-length SCCmec (Fig. 1), as confirmed by PCR. These frequencies are drastically (104-fold) higher than the frequencies reported in our previous study [26].
Fig. 2.
High donor-to-recipient ratio in 2-day biofilms enhances transformation efficiency of SCCmec.
(A) The recipient cells (Nef or NefΔcomE) were statically grown in CS2 at 37 °C for up to 3 days. An initial fixed amount of the living COLw/oφ donor cells was used, generating the following donor-to-recipient ratios: 0:1, 3:1, 40:1, 5000:1, 25000:1, and 125000:1. Transformation efficiencies (top) or CFU/mL (bottom) of recipient cells were determined after every 24 h. Transformants were selected by cefmetazole (CMZ) and erythromycin (EM).
The mean of at least n = 3 independent experiments is shown with SD. Data points represent independent experiments. The dotted lines represent the detection limit.
(B) SCCmec I amplification in the Nef transformants obtained from 2-day biofilm using the 5000:1 donor-to-recipient ratio. The schematic structure of the SCC and primer locations indicated by arrows are shown. The DNA of COLw/oφ donor and Nef recipient was used for positive and negative controls. Suffixes (1), (2), and (3) represent transformants obtained from three independent experiments. M: DNA marker, λ-HindIII.
2.2. The Ccr-attB system is important for SCCmec transfer
SCCmec carries the ccr genes which encode recombinases that allow its excision and integration at the attB attachment site in the recipient's chromosome. We tested whether SCCmec transfer is mediated by the Ccr-attB excision/integration system in the optimized transformation conditions using the living donor. Nef derivative strain that has a mutated attB site (attB*) showed a significant reduction (∼22-fold) in SCCmec transformation efficiency compared with Nef (Fig. 3A; at 5000:1 donor-to-recipient ratio in Fig. S2). Moreover, when the living donor lacking the ccr genes (COLw/oφ-Δccr) was used, the transformation frequency was reduced ∼29-fold compared with the parental donor strain COLw/oφ (P = 0.05) (Fig. 3A). Transformation frequencies of pT181 plasmid did not differ (Fig. 3B), suggesting that mutations in ccr and attB did not affect the efficiency of natural transformation itself.
Fig. 3.
The Ccr-attB system is important for SCCmec transformation.
(A-B) Living donor (COLw/oφ or COLw/oφ-Δccr) cells were added to recipient cells (Nef or attB*) in a 5000:1 ratio. The cells were statically grown in CS2 for 2 days at 37 °C. Transformants were selected by erythromycin (EM) and cefmetazole (CMZ) (A), or by erythromycin and tetracycline (TET) (B). The mean of at least n = 3 independent experiments is shown with SD. Data points represent independent experiments. The dotted lines represent the detection limit. Statistical significance was determined by 2-way ANOVA followed by Tuckey's multiple comparison tests from Fig. S2. *P < 0.05. P = 0.05 by Student's t-test.
2.3. SCCmec can be transferred to clinical MSSA isolates in mixed biofilms
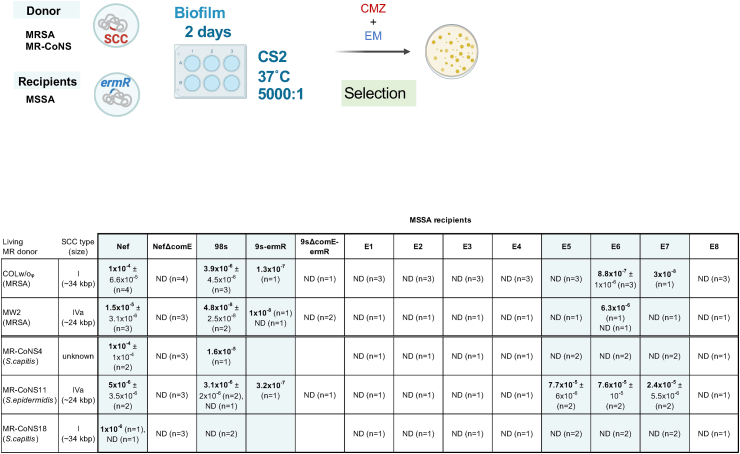
Previously, we showed that SCCmec can be transferred by natural transformation to clinical MSSA isolates in the biofilm using heat-killed donors [26]. We aimed to clarify whether the transformation using living donors can more efficiently transfer SCCmec to clinical MSSA isolates. We used 10 erythromycin-resistant MSSA (98s, 9s-ermR, E1-E8) and erythromycin-sensitive MRSA/MRCoNS donors (COLw/oφ, MW2, MR-CoNS4, 11, 18), to use erythromycin to eliminate donors after transformation (Fig. 4). The donors COLw/oφ and MR-CoNS18 carry SCCmec I (∼34 kbp), MW2 and MR-CoNS11 carry SCCmec IVa (∼24 kbp), while MR-CoNS4 carries an unknown type of SCCmec. Nef, NefΔcomE, and 9sΔcomE-ermR strains were used as positive or negative controls. Five of the 10 tested clinical MSSA strains could generate SCCmec-transformants, and some of them showed high frequencies up to 10−5 (Fig. 4), which is much higher than in our previous report (∼10−8) [26].
Fig. 4.
SCCmec can be transferred from living MR-CoNS donors in the biofilm.
A fixed amount of living donor (MRSA or MR-CoNS) cells was added to different amounts of MSSA recipient cells in a 5000:1 ratio. Here we selected donors based on their erythromycin susceptibility, and not by the other features such as species and SCC type. The cells were statically grown in CS2 for 2 days at 37 °C. Transformants were selected by erythromycin (EM) and cefmetazole (CMZ). The mean of n independent experiments ± SD are shown. ND, none-detected.
2.4. Transformation of plasmid and chromosomal genes are enhanced by using living donor in the biofilm
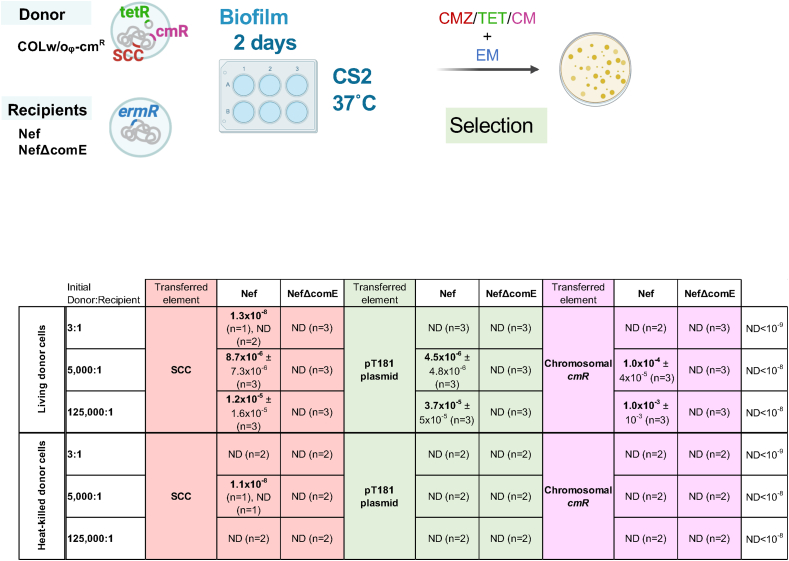
In the optimized mixed biofilm conditions, we observed the transformation of mecA in the absence of the Ccr system (Fig. 3A, Fig. S2). This implicates that the efficiency of the natural transformation of chromosomal genes would also be increased. Indeed, when we looked at the transformation frequencies of another chromosomal marker (the chloramphenicol resistance gene cmR in the donor COLw/oφΔcls1-cmR strain), it was about 10−4-10−3 in the mixed biofilm conditions, while its transfer was undetectable in the previous protocol using heat-killed donors. Similarly, the transformation of plasmid pT181 (that has a tetracycline-resistance gene) was only detectable in the living donor system and about 10−6-10−5 (Fig. 5).
Fig. 5.
Living donor enhances transformation frequencies of genetic elements.
A fixed amount of either living or heat-killed donor (COLw/oφΔcls1-cmR) cells was added to different amounts of recipient cells (Nef or NefΔcomE), generating the 3:1, 5000:1, or 125000:1 ratios. The cells were statically grown in CS2 for 2 days at 37 °C. Transformants were selected by erythromycin (EM) with cefmetazole (CMZ) for SCC, tetracycline (TET) for the pT181 plasmid, or chloramphenicol (CM) for the chromosomal cmR gene. The mean of n independent experiments ± SD are shown. ND, none-detected.
3. Discussion
SCCmec is a large mobile genetic element conveying methicillin resistance in Staphylococcus and Macrococcus species [6,29]. The mechanism by which SCCmec transfers horizontally among staphylococci has been extensively researched in the past few decades in efforts to clarify how MRSA strains emerge and disseminate globally. Previously, we showed that natural transformation can mediate inter- and intraspecies transfer of SCCmec at low frequencies when the cells are grown in biofilm conditions [26]. In this study, we present an enhanced transformation protocol that drastically improves the inter- and intraspecies transformation efficiency of SCCmec and other genetic elements in S. aureus biofilms. The high transformation frequencies detected in multiple strains and the dependency on the Ccr-attB system in SCCmec transfer support the importance of natural transformation in antibiotic resistance acquisition and MRSA emergence.
Heat-killed cells can serve as a donor in biofilm transformation assays, but physically disrupted cell lysate and purified genomic DNA cannot (Fig. S6) (mentioned in Ref. [26]). In heat-killed cells, the membrane is damaged but the DNA resides within a peptidoglycan cell wall that is not destroyed by heat (Fig. S4). It is conceivable that the cell wall structure reduces the DNA degradation by extracellular DNase (MNase or thermonuclease) that is known to be expressed in S. aureus biofilms [30]. Indeed, we previously confirmed that the heat-killed cells can partially block the DNase I access to the inside DNA (Fig. S15 in Ref. [26]). On the other hand, living cells can completely protect the DNA, suggesting that the intact membrane is critical for prolonged protection. The protection of donor DNA until its use by the competent cells would be one of the reasons for the present finding that living donor cells are better than heat-killed cells (Fig. 1, Fig. 5). In B. subtilis, the living donor cells have also been shown to enhance transformation efficiency and provide protection from DNase degradation [31]. Another point to consider is that extracellular DNA sequestered in biofilm [32,33] may not be able to serve as a donor for transformation.
This study did not address how the DNA is released from living donor cells and gets access to the recipient cells, but several mechanisms of DNA release have been suggested to occur in biofilms, including autolysis, prophage-mediated lysis, vesicles, and active secretion systems [[34], [35], [36], [37]]. In our transformation assay, the phage-mediated lysis is dispensable, as the combination of COLw/oφ donor and Nef recipient does not include any phage components, but it remains a strong candidate in nature as most S. aureus isolates have at least one prophage. It might be valuable to mention that growth in the CS2 medium causes unusual cell morphology [38], which may contribute to the DNA supply. It would also be valuable to mention here that about 10–20% of cells are PI-positive in our biofilm conditions at day 1.
The enhanced transformation in this study requires a very high initial donor-to-recipient ratio (5000:1 or more). This was not observed in B. subtilis transformation [31,39], and cannot be explained simply by the importance of cell-to-cell contact or proximity between donor and recipient cells. The expression of ccr genes is limited to a subpopulation [40], and this might explain the necessity of a high donor amount for SCCmec transformation. However, the transfer of other genetic elements (plasmids and chromosomal genes) that are not dependent on ccr was efficiently enhanced as well (Fig. 5). One speculation is that a part of the donor cells continuously undergoes lysis, releasing the transforming DNA. Donor cells may also serve as a nutrient source for the growing recipient in the biofilm. As mentioned above, about 10–20% of cells are PI-positive (membrane damaged) in 1 day biofilm. Moreover, 50–60% of cells are CTC-negative (metabolically inactive) (data not shown). Whether and how the changes in cell viability and metabolism including the presence of dormant or viable but non-culturable cells (VBNCs) influence the transformation remain elusive.
The Ccr recombinases enable the excision and integration of SCCmec at the attB site in the recipient's chromosome [7]. The excision of SCCmec happens in a subpopulation that may later serve as a donor for horizontal transmission [40]. SCCmec transfer was reduced but not abolished in the absence of the ccrAB genes on the donor side or in the absence of attB on the recipient side (Fig. 3). This minor Ccr-independent transfer would be through homologous recombination, as is observed for other chromosomal genes (Fig. 5). The importance of Ccr in the transformation observed in this study emphasizes the relevance of the natural transformation in SCC transmission among the HGT mechanisms. Whether Ccr is involved in other HGT mechanisms, such as phage transduction, is still elusive.
Polymicrobial biofilms are thought to be a hotspot for exchanging genetic material through HGT [41], and it is conceivable that staphylococcal biofilms also enhance HGT [18,26,42]. In the present study, we established that a mixed biofilm enables the efficient transfer of SCCmec, providing insights into the environment in which MRSA is born. Importantly, many of the antibiotic-resistant genes, including mec genes, are shared between animal and human Staphylococcus species [43]. This study did not test all types of SCC and CoNS species, but the established transformation protocol allows us to test them and it will be reported soon. The protocol established in this study is a valuable tool for studying drug resistance and virulence dissemination among Staphylococcus species, as well as the factors affecting SCCmec stability in S. aureus [26]. In addition, it would also help provide a better understanding of the interplay between natural transformation and the different HGT mechanisms that might have co-evolved in biofilms.
4. Materials and methods
4.1. Bacterial strains, plasmids, primers, and media
The bacterial strains and plasmids used in this study are shown in Table S3. The primers used in this study are shown in Table S4. Clinical staphylococcal samples (9 MSSA isolates and 4 MR-CoNS isolates) were collected from the Kanto area of Japan.
Staphylococci were grown in TSB for routine cultures. For biofilm formation and transformation assays, S. aureus was grown in CS2 (complete synthetic medium), diluted TSB, diluted BHI, diluted RPMI 1640, or SMM. E. coli strains were grown in LB. Where required for selection, the medium was supplemented with chloramphenicol (12.5 μg/mL), tetracycline (5 μg/mL), cefmetazole (4 μg/mL), erythromycin (16 μg/mL), or ampicillin (for E. coli, 100 μg/mL).
4.2. Genetic characterization of staphylococcal strains
Multiplex PCR was performed using the QIAGEN multiplex PCR kit. The primer set is based on the previously reported methods to determine the CC types [44], with the addition of primers for gyrA [26]. The clonal complex information shown in Table S3 is based on the amplification pattern of the target genes [44]. Long amplifications of SCCmec were performed using KOD One PCR master mix (TOYOBO).
4.3. Construction of mutants
Deletion mutants were constructed by double-crossover homologous recombination using the pMADcat vector [45]. Fragments flanking the upstream (primers A and B, Table S4) and downstream (primers C and D, Table S4) regions of the locus targeted for deletion were amplified by PCR using chromosomal DNA from COLw/oφ as a template. The PCR products (AB and CD fragments) were used as templates for the overlap extension PCR using primers A and D (Table S4). The product was cloned into the BamH I–Sal I site of pMADcat to generate the vector for ccrAB deletion (pMADcat-ΔccrAB, Table S3). The plasmids were purified from E. coli and introduced into COLw/oφ after passaging through RN4220. Mutants (chloramphenicol sensitive, β-galactosidase negative) were selected [45] and the absence of the target gene was confirmed by PCR using the primers E and F (Table S4). The vector for cls1 deletion (pMADcat1155, Table S3) was used as previously described [45] to generate the COLw/oφΔcls1-cmR strain.
9s-ermR and 9sΔcomE-ermR strains were generated by transduction of the erythromycin-resistance gene from the Nef strain.
4.4. Heat-killed donor preparation for natural transformation assays
Log-phase heat-killed donor cells were prepared as previously described [26]. Overnight cultures were diluted 20-fold in TSB and grown for 3 h at 37 °C with shaking. Cells were then harvested in PBS, boiled for 10 min, washed, and suspended in PBS (∼109 CFU equivalent/mL). The absence of viable cells was confirmed by plating on TSB plates, and by viability staining. For the SCCmec donor, COLw/oφ was used.
4.5. Natural transformation assays using heat-killed donor
Natural transformation assays were conducted in a polystyrene 6-well plate under biofilm growth conditions [26]. Briefly, 250 μL of log-phase heat-killed donor cells (∼2.5 × 108 CFU) were added to 750 μL of log-phase recipient cells (∼108 CFU/mL), and the total volume of growth medium was adjusted to 1.5 mL per well. To generate increasing donor-to-recipient ratios as indicated in Fig. S1, a fixed amount of donor cells (∼2.5 × 108 CFU) was added to different amounts of recipient cells in a total volume of 1.5 mL per well. The 6-well plates were incubated statically for 3 days at 37 °C and the medium was refreshed every 24 h. The biofilms were collected by pipetting and poured into BHI agar supplemented with cefmetazole (for SCCmec donors) for the selection of transformants. NefΔcomE strain was used as the negative control.
4.6. Natural transformation assays using living donor
To detect natural transformation using living donors in the biofilm, 75 μL of donor overnight culture (∼109 CFU/mL) were washed in the appropriate medium and added to different amounts of recipient cells from overnight cultures that were washed in the appropriate medium, generating the ratios indicated in Fig. 1, Fig. 2, Fig. 3, Fig. 4, Fig. 5. The cells were added to the polystyrene 6-well plate, and the total volume of the growth medium was adjusted to 1.5 mL per well. The plate was incubated for the appropriate time, and the medium was refreshed every 24 h. The collected biofilms were poured into melted BHI agar (precooled to 50 °C) supplemented with appropriate antibiotics and solidified at room temperature. A high concentration of erythromycin (16 μg/mL) was used to select cells derived from the erythromycin-resistant recipient, and this concentration can prohibit the growth of spontaneous mutants. The mecA-, tetR-, and cmR-transformants were selected by cefmetazole (4 μg/mL), tetracycline (5 μg/mL), and chloramphenicol (12.5 μg/mL). The transformants were confirmed by their ability to grow on replica and were verified by PCR (some examples are shown in Fig. S3). NefΔcomE strain was used as the negative control. In our experience, PCR testing is essential for chloramphenicol selection to eliminate spontaneous mutants, while spontaneous mutants for cefmetazole and tetracycline did not emerge. In the case of mecA transformants, it is known that some are unstable depending on the combination of donor and recipient [26], but this study did not distinguish them to calculate the transformation frequency.
4.7. Calculation of transformation frequency
Transformation frequency was calculated as the ratio of the number of transformants to the total CFU of the recipient after transformation. The dead or viable non-culturable recipient cells that may exist in the biofilm were not counted in this study. None detected values were assigned half the value of the detection limit for the calculation of mean values and statistical analyses.
4.8. Viability staining of donor cells
The living cells (overnight culture) and heat-killed donor cells (log-phase) were grown/prepared as described above; Cells were washed with PBS before the staining. They were stained by the Bacterial Viability Detection Kit PI-DAPI (BS08, Dojindo, Japan) or CTC-DAPI (BS09, Dojindo, Japan) following the manufacturer's instructions (Figs. S4 and S5). BODIPY FL conjugate of vancomycin (Van-BODIPY, Invitrogen) was added to the PI-DAPI-stained cells at a final concentration of 1 μg/mL and the cells were incubated for additional 5 min at room temperature. The cells were visualized using a fluorescence microscope (BZ-X710, Keyence).
4.9. Statistics
Statistical analyses were performed by GraphPad Prism (GraphPad Software, version 8.4.3) on data from three or more independent experiments. Error bars indicate SD. The difference among groups was analysed by two-way ANOVA followed by Tukey's multiple-comparison test or by two tailed Student's t-test, as indicated in figure legends. The log values of natural transformation frequencies were analysed statistically. *P < 0.05 was considered statistically significant.
CRediT authorship contribution statement
Mais Maree: Writing – review & editing, Writing – original draft, Visualization, Funding acquisition, Data curation. Yuri Ushijima: Writing – review & editing, Supervision, Conceptualization. Pedro B. Fernandes: Writing – review & editing, Visualization, Methodology. Masato Higashide: Writing – review & editing, Resources, Methodology. Kazuya Morikawa: Writing – review & editing, Writing – original draft, Supervision, Funding acquisition, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank our laboratory members for the discussions. This research was supported by AMED Grant Number JP23fk0108630, JSPS Bilateral Program Grant Number JPJSBP120229908, JSPS KAKENHI Grant Number 22H02863 (to KM), JSPS KAKENHI Grant Number 23K14515 (to MM). PBF is a JSPS International Research Fellow.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bioflm.2024.100184.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
Data availability
No data was used for the research described in the article.
References
- 1.Lowy F.D. Antimicrobial resistance: the example of Staphylococcus aureus. J Clin Investig. 2003;111(9):1265–1273. doi: 10.1172/JCI18535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chambers H.F., DeLeo F.R. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat Rev Microbiol. 2009;7(9):629–641. doi: 10.1038/nrmicro2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.C.f.D. Control, Prevention . US Department of Health and Human Services, Centres for Disease Control and; 2019. Antibiotic resistance threats in the United States, 2019. [Google Scholar]
- 4.Ito T., Okuma K., Ma X.X., Yuzawa H., Hiramatsu K. Insights on antibiotic resistance of Staphylococcus aureus from its whole genome: genomic island SCC. Drug Resist Updates. 2003;6(1):41–52. doi: 10.1016/s1368-7646(03)00003-7. [DOI] [PubMed] [Google Scholar]
- 5.Lee A.S., de Lencastre H., Garau J., Kluytmans J., Malhotra-Kumar S., Peschel A., Harbarth S. Methicillin-resistant Staphylococcus aureus. Nat Rev Dis Prim. 2018;4(1) doi: 10.1038/nrdp.2018.33. [DOI] [PubMed] [Google Scholar]
- 6.Lakhundi S., Zhang K. Methicillin-resistant Staphylococcus aureus: molecular characterization, evolution, and epidemiology. Clin Microbiol Rev. 2018;31(4) doi: 10.1128/CMR.00020-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katayama Y., Ito T., Hiramatsu K. A new class of genetic element, Staphylococcus cassette chromosome mec, encodes methicillin resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 2000;44(6):1549–1555. doi: 10.1128/aac.44.6.1549-1555.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ito T., Ma Xiao X., Takeuchi F., Okuma K., Yuzawa H., Hiramatsu K. Novel type V staphylococcal cassette chromosome mec driven by a novel cassette chromosome recombinase, ccrC. Antimicrob Agents Chemother. 2004;48(7):2637–2651. doi: 10.1128/AAC.48.7.2637-2651.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsubakishita S., Kuwahara-Arai K., Sasaki T., Hiramatsu K. Origin and molecular evolution of the determinant of methicillin resistance in staphylococci. Antimicrob Agents Chemother. 2010;54(10):4352–4359. doi: 10.1128/AAC.00356-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou Y., Antignac A., Wu Shang W., Tomasz A. Penicillin-binding proteins and cell wall composition in β-lactam-sensitive and -resistant strains of Staphylococcus sciuri. J Bacteriol. 2008;190(2):508–514. doi: 10.1128/JB.01549-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Couto I., de Lencastre H., Severina E., Kloos W., Webster J.A., Hubner R.J., Sanches I.S., Tomasz A. Ubiquitous presence of a mecA homologue in natural isolates of Staphylococcus sciuri. Microb Drug Resist. 1996;2(4):377–391. doi: 10.1089/mdr.1996.2.377. [DOI] [PubMed] [Google Scholar]
- 12.Lade H., Kim J.-S. Molecular determinants of β-lactam resistance in methicillin-resistant Staphylococcus aureus (MRSA): an updated review. Antibiotics. 2023;12(9):1362. doi: 10.3390/antibiotics12091362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanssen A.-M., Ericson Sollid J.U. SCCmec in staphylococci: genes on the move. FEMS Immunol Med Microbiol. 2006;46(1):8–20. doi: 10.1111/j.1574-695X.2005.00009.x. [DOI] [PubMed] [Google Scholar]
- 14.Robinson D.A., Enright Mark C. Evolutionary models of the emergence of methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2003;47(12):3926–3934. doi: 10.1128/AAC.47.12.3926-3934.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jansen W.T.M., Beitsma M.M., Koeman C.J., Wamel W.J.B.v., Verhoef J., Fluit A.C. Novel mobile variants of staphylococcal cassette chromosome mec in Staphylococcus aureus. Antimicrob Agents Chemother. 2006;50(6):2072–2078. doi: 10.1128/AAC.01539-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wielders C.L., Vriens M.R., Brisse S., de Graaf-Miltenburg L.A., Troelstra A., Fleer A., Schmitz F.J., Verhoef J., Fluit A.C. In-vivo transfer of mecA DNA to Staphylococcus aureus [corrected] Lancet. 2001;357(9269):1674–1675. doi: 10.1016/s0140-6736(00)04832-7. [DOI] [PubMed] [Google Scholar]
- 17.Bloemendaal A.L.A., Brouwer E.C., Fluit A.C. Methicillin resistance transfer from Staphylocccus epidermidis to methicillin-susceptible Staphylococcus aureus in a patient during antibiotic therapy. PLoS One. 2010;5(7) doi: 10.1371/journal.pone.0011841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haaber J., Penadés J.R., Ingmer H. Transfer of antibiotic resistance in Staphylococcus aureus. Trends Microbiol. 2017;25(11):893–905. doi: 10.1016/j.tim.2017.05.011. [DOI] [PubMed] [Google Scholar]
- 19.Scharn Caitlyn R., Tenover Fred C., Goering Richard V. Transduction of staphylococcal cassette chromosome mec elements between strains of Staphylococcus aureus. Antimicrob Agents Chemother. 2013;57(11):5233–5238. doi: 10.1128/AAC.01058-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ray M.D., Boundy S., Archer G.L. Transfer of the methicillin resistance genomic island among staphylococci by conjugation. Mol Microbiol. 2016;100(4):675–685. doi: 10.1111/mmi.13340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Claverys J.-P., Martin B., Polard P. The genetic transformation machinery: composition, localization, and mechanism. FEMS (Fed Eur Microbiol Soc) Microbiol Rev. 2009;33(3):643–656. doi: 10.1111/j.1574-6976.2009.00164.x. [DOI] [PubMed] [Google Scholar]
- 22.Johnston C., Martin B., Fichant G., Polard P., Claverys J.P. Bacterial transformation: distribution, shared mechanisms and divergent control. Nat Rev Microbiol. 2014;12(3):181–196. doi: 10.1038/nrmicro3199. [DOI] [PubMed] [Google Scholar]
- 23.Morikawa K., Takemura A.J., Inose Y., Tsai M., Nguyen Thi le T., Ohta T., Msadek T. Expression of a cryptic secondary sigma factor gene unveils natural competence for DNA transformation in Staphylococcus aureus. PLoS Pathog. 2012;8(11) doi: 10.1371/journal.ppat.1003003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morikawa K., Inose Y., Okamura H., Maruyama A., Hayashi H., Takeyasu K., Ohta T. A new staphylococcal sigma factor in the conserved gene cassette: functional significance and implication for the evolutionary processes. Gene Cell. 2003;8(8):699–712. doi: 10.1046/j.1365-2443.2003.00668.x. [DOI] [PubMed] [Google Scholar]
- 25.Fagerlund A., Granum P.E., Håvarstein L.S. Staphylococcus aureus competence genes: mapping of the SigH, ComK1 and ComK2 regulons by transcriptome sequencing. Mol Microbiol. 2014;94(3):557–579. doi: 10.1111/mmi.12767. [DOI] [PubMed] [Google Scholar]
- 26.Maree M., Thi Nguyen L.T., Ohniwa R.L., Higashide M., Msadek T., Morikawa K. Natural transformation allows transfer of SCCmec-mediated methicillin resistance in Staphylococcus aureus biofilms. Nat Commun. 2022;13(1):2477. doi: 10.1038/s41467-022-29877-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Machado H., Weng L.L., Dillon N., Seif Y., Holland M., Pekar J.E., Monk J.M., Nizet V., Palsson B.O., Feist A.M. Strain-specific metabolic requirements revealed by a defined minimal medium for systems analyses of Staphylococcus aureus. Appl Environ Microbiol. 2019;85(21) doi: 10.1128/AEM.01773-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cordero M., García-Fernández J., Acosta I.C., Yepes A., Avendano-Ortiz J., Lisowski C., Oesterreicht B., Ohlsen K., Lopez-Collazo E., Förstner K.U., Eulalio A., Lopez D. The induction of natural competence adapts staphylococcal metabolism to infection. Nat Commun. 2022;13(1):1525. doi: 10.1038/s41467-022-29206-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsubakishita S., Kuwahara-Arai K., Baba T., Hiramatsu K. Staphylococcal cassette chromosome mec-like element in Macrococcus caseolyticus. Antimicrob Agents Chemother. 2010;54(4):1469–1475. doi: 10.1128/AAC.00575-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moormeier Derek E., Bose Jeffrey L., Horswill Alexander R., Bayles Kenneth W. Temporal and stochastic control of Staphylococcus aureus biofilm development. mBio. 2014;5(5) doi: 10.1128/mBio.01341-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang X., Jin T., Deng L., Wang C., Zhang Y., Chen X. Stress-induced, highly efficient, donor cell-dependent cell-to-cell natural transformation in Bacillus subtilis. J Bacteriol. 2018;200(17) doi: 10.1128/JB.00267-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moormeier D.E., Bayles K.W. Staphylococcus aureus biofilm: a complex developmental organism. Mol Microbiol. 2017;104(3):365–376. doi: 10.1111/mmi.13634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kavanaugh J.S., Flack C.E., Lister J., Ricker E.B., Ibberson C.B., Jenul C., Moormeier D.E., Delmain E.A., Bayles K.W., Horswill A.R. Identification of extracellular DNA-binding proteins in the biofilm matrix. mBio. 2019;10(3) doi: 10.1128/mBio.01137-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Webb J.S., Thompson L.S., James S., Charlton T., Tolker-Nielsen T., Koch B., Givskov M., Kjelleberg S. Cell death in Pseudomonas aeruginosa biofilm development. J Bacteriol. 2003;185(15):4585–4592. doi: 10.1128/JB.185.15.4585-4592.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salgado-Pabón W., Du Y., Hackett K.T., Lyons K.M., Arvidson C.G., Dillard J.P. Increased expression of the type IV secretion system in piliated Neisseria gonorrhoeae variants. J Bacteriol. 2010;192(7):1912–1920. doi: 10.1128/JB.01357-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.DeFrancesco A.S., Masloboeva N., Syed A.K., DeLoughery A., Bradshaw N., Li G.W., Gilmore M.S., Walker S., Losick R. Genome-wide screen for genes involved in eDNA release during biofilm formation by Staphylococcus aureus. Proc Natl Acad Sci U S A. 2017;114(29):E5969–e5978. doi: 10.1073/pnas.1704544114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rice K.C., Mann E.E., Endres J.L., Weiss E.C., Cassat J.E., Smeltzer M.S., Bayles K.W. The cidA murein hydrolase regulator contributes to DNA release and biofilm development in Staphylococcus aureus. Proc Natl Acad Sci U S A. 2007;104(19):8113–8118. doi: 10.1073/pnas.0610226104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nguyen L.T.T., Takemura A.J., Ohniwa R.L., Saito S., Morikawa K. Sodium polyanethol sulfonate modulates natural transformation of SigH-expressing Staphylococcus aureus. Curr Microbiol. 2018;75(4):499–504. doi: 10.1007/s00284-017-1409-5. [DOI] [PubMed] [Google Scholar]
- 39.Cheng Y.-Y., Zhou Z., Papadopoulos J.M., Zuke J.D., Falbel T.G., Anantharaman K., Burton B.M., Venturelli O.S. Efficient plasmid transfer via natural competence in a microbial co-culture. Mol Syst Biol. 2023;19(3) doi: 10.15252/msb.202211406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stojanov M., Sakwinska O., Moreillon P. Expression of SCCmec cassette chromosome recombinases in methicillin-resistant Staphylococcus aureus and Staphylococcus epidermidis. J Antimicrob Chemother. 2013;68(4):749–757. doi: 10.1093/jac/dks494. [DOI] [PubMed] [Google Scholar]
- 41.Michaelis C., Grohmann E. Horizontal gene transfer of antibiotic resistance genes in biofilms. Antibiotics (Basel) 2023;12(2) doi: 10.3390/antibiotics12020328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Savage V.J., Chopra I., O'Neill A.J. Staphylococcus aureus biofilms promote horizontal transfer of antibiotic resistance. Antimicrob Agents Chemother. 2013;57(4):1968–1970. doi: 10.1128/AAC.02008-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wendlandt S., Shen J., Kadlec K., Wang Y., Li B., Zhang W.J., Feßler A.T., Wu C., Schwarz S. Multidrug resistance genes in staphylococci from animals that confer resistance to critically and highly important antimicrobial agents in human medicine. Trends Microbiol. 2015;23(1):44–54. doi: 10.1016/j.tim.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 44.Schwalm N.D., Verghese B., Knabel S.J. A novel multiplex PCR method for detecting the major clonal complexes of MRSA in nasal isolates from a Pennsylvania hospital. J Microbiol Methods. 2011;86(3):379–382. doi: 10.1016/j.mimet.2011.05.024. [DOI] [PubMed] [Google Scholar]
- 45.Tsai M., Ohniwa R.L., Kato Y., Takeshita S.L., Ohta T., Saito S., Hayashi H., Morikawa K. Staphylococcus aureus requires cardiolipin for survival under conditions of high salinity. BMC Microbiol. 2011;11(1):13. doi: 10.1186/1471-2180-11-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data was used for the research described in the article.