Abstract
Tax corresponds to a 40-kDa transforming protein from the pathogenic retrovirus human T-cell leukemia virus type 1 (HTLV-1) that activates nuclear expression of the NF-κB/Rel family of transcription factors by an unknown mechanism. Tax expression promotes N-terminal phosphorylation and degradation of IκBα, a principal cytoplasmic inhibitor of NF-κB. Our studies now demonstrate that HTLV-1 Tax activates the recently identified cellular kinases IκB kinase α (IKKα) and IKKβ, which normally phosphorylate IκBα on both of its N-terminal regulatory serines in response to tumor necrosis factor alpha (TNF-α) and interleukin-1 (IL-1) stimulation. In contrast, a mutant of Tax termed M22, which does not induce NF-κB, fails to activate either IKKα or IKKβ. Furthermore, endogenous IKK enzymatic activity was significantly elevated in HTLV-1-infected and Tax-expressing T-cell lines. Transfection of kinase-deficient mutants of IKKα and IKKβ into either human Jurkat T or 293 cells also inhibits NF-κB-dependent reporter gene expression induced by Tax. Similarly, a kinase-deficient mutant of NIK (NF-κB-inducing kinase), which represents an upstream kinase in the TNF-α and IL-1 signaling pathways leading to IKKα and IKKβ activation, blocks Tax induction of NF-κB. However, plasma membrane-proximal elements in these proinflammatory cytokine pathways are apparently not involved since dominant negative mutants of the TRAF2 and TRAF6 adaptors, which effectively block signaling through the cytoplasmic tails of the TNF-α and IL-1 receptors, respectively, do not inhibit Tax induction of NF-κB. Together, these studies demonstrate that HTLV-1 Tax exploits a distal part of the proinflammatory cytokine signaling cascade leading to induction of NF-κB. The pathological alteration of this cytokine pathway leading to NF-κB activation by Tax may play a central role in HTLV-1-mediated transformation of human T cells, clinically manifested as the adult T-cell leukemia.
Human T-cell leukemia virus type 1 (HTLV-1) represents the first pathogenic human retrovirus identified (49, 78). After a long latency period, approximately 0.05 to 0.1% of individuals infected with this retrovirus develop an often aggressive and fatal proliferation of activated CD3+ CD4+ T cells, termed adult T-cell leukemia (ATL) (66). HTLV-1 has also been etiologically linked with a progressive neurodegenerative syndrome termed HTLV-1-associated myelopathy/tropical spastic paraparesis (13, 46).
HTLV-1 encodes a 40-kDa regulatory protein termed Tax that appears to play a central role in cell transformation (18, 36, 50, 58, 64, 74). Although Tax does not bind directly to DNA, it alters the activity of several host transcription factors, notably cyclic AMP responsive element (CRE) binding protein (CREB)/activating transcription factor (ATF), serum response factor (SRF), and NF-κB/Rel (79). Tax-induced transactivation of the HTLV-1 long terminal repeat (LTR) is mediated through the CREB/ATF pathway (5, 15, 63). In this response, Tax participates in the formation of a ternary complex that includes the viral CREs and CREB, appears to enhance CREB dimerization, and stabilizes CREB binding to the CREs (3, 6, 48, 63, 69, 81). Additionally, the indirectly tethered Tax protein facilitates recruitment of the transcriptional coactivator CREB binding protein (CBP), through an interplay with the KIX domain of CBP (16, 28, 76).
Tax also modulates transcription of various cellular genes, some of which may be involved in T-cell transformation (19, 57, 77, 79). Many of these genes are regulated by members of the NF-κB/Rel family of transcription factors. For example, Tax induces various cytokines and the interleukin-2 (IL-2) receptor α-chain gene via the NF-κB pathway (19, 57, 77). Tax also induces the c-fos oncogene through SRF (11, 12). Of note, Tax induction of the CREB/ATF, SRF, and NF-κB/Rel pathways is selectively impaired by the introduction of site directed mutations into Tax (36, 55, 56, 74). For example, the M22 mutant of Tax (T130S L131A) retains CREB/ATF-stimulatory activity but is strongly impaired in its ability to activate NF-κB (56). These findings raise the possibility that different subregion domains of Tax mediate the activation of these various host transcription factor pathways. The precise role of each of these pathways in Tax-induced cellular transformation remains unresolved (36, 58, 74).
The prototypical NF-κB complex corresponds to a heterodimer of the p50 (NFKB1) and RelA (p65) members of the NF-κB/Rel family of transcription factors (1, 2, 68). Other factors in this family include RelB, c-Rel, and p52 (NFKB2). Prior to activation, NF-κB is sequestered in the cytoplasm by its physical interaction with a set of inhibitors termed the IκBs (IκBα, IκBβ, and IκBɛ), p105 (NFKB1), and p100 (NFKB2) (1, 2, 68). Stimulation of the cell with proinflammatory cytokines (e.g., tumor necrosis factor alpha [TNF-α] and interleukin-1 [IL-1]), phorbol esters, lipopolysaccharide, and several other activators leads to the phosphorylation of IκBα on two N-terminal serine residues located at positions 32 and 36. This posttranslational modification targets the inhibitor for rapid ubiquitination and degradation within the 26S proteasome. The proteolysis of cytoplasmic IκBα allows nuclear translocation of NF-κB (1, 2, 68). How cytoplasmic sequestration of NF-κB by the p100 and p105 inhibitors is relieved remains largely unknown.
The molecular basis for Tax induction of NF-κB has been a topic of extensive investigation. Tax has been reported to interact physically with many members of the NF-κB/Rel family (2, 21, 79) but appears to display highest affinity for the p100 product of the NFKB2 gene (4, 26, 31, 44). The amino terminus of p100, which contains p52 and its Rel homology domain, appears to mediate this interaction (4, 26, 44). Expression of Tax has also been reported to overcome the cytoplasmic sequestration property of p100 and p105, permitting the release and nuclear translocation of NF-κB (26, 43, 44).
However, a convincing series of studies has shown that Tax promotes the phosphorylation and degradation of both IκBα and IκBβ, suggesting that this HTLV-1 regulatory protein may induce nuclear translocation of NF-κB by acting prior to or at the level of IκB phosphorylation (7, 17, 24, 29, 39, 62). This notion is further strengthened by the finding that degradation-resistant IκBα mutants lacking the two N-terminal serine phosphorylation sites effectively block Tax-induced κB-dependent transcription (7, 25). Together, these findings suggest that Tax may coopt a signaling pathway that leads to IκB phosphorylation.
Three cellular kinases involved in TNF-α and IL-1 induction of NF-κB have recently been identified. These include the NF-κB-inducing kinase (NIK), a mitogen-activated protein kinase kinase kinase (MAP3K)-related kinase that associates with various TNF receptor (TNFR)-associated factors (TRAFs) and corresponds to a strong inducer of NF-κB. When rendered catalytically inactive, NIK functions as a dominant-negative inhibitor of TNF-α- and IL-1-induced NF-κB activation (35, 59). However, NIK does not directly phosphorylate the IκBs but instead associates with and activates (51) the recently recognized IκB kinase α (IKKα) (10, 40, 51), previously designated the conserved helix-loop-helix ubiquitous kinase (9). IKKα has been shown to phosphorylate both N-terminal serines of IκBα and preferentially serine 23 of IκBβ (10, 39, 50). A second related IκB kinase, termed IKKβ, has also been isolated and characterized (40, 72, 80). This enzyme phosphorylates the regulatory serines of both IκBα and IκBβ. These α and β IKKs can form hetero- and homodimers (40, 72, 80) via their leucine zipper domains, although heterodimers appear to be favored in vivo.
The studies described in this report were aimed at identifying the cellular signaling intermediates involved in HTLV-1 Tax induction of nuclear NF-κB expression. We demonstrate that Tax activates the enzymatic activity of ectopically expressed IKKα and IKKβ and that the enzymatic activity of endogenous IKKs is elevated in HTLV-1-infected and Tax-expressing T-cell lines. In addition, we report that kinase-deficient versions of NIK, IKKα, and IKKβ effectively block Tax action. In contrast, based on the use of dominant negative mutants, Tax induction of NF-κB does not appear to involve the TRAF2 and TRAF6 adaptor proteins which represent more proximal components of the TNFα and IL-1 signaling pathways. These findings suggest that distal components of the proinflammatory cytokine signaling pathway are selectively coopted by Tax, leading to the activation of NF-κB.
MATERIALS AND METHODS
Expression vectors, biological reagents, and cell lines.
Plasmids pCMV4-HA-IκBα(SS32/36AA), κB-TATA-luciferase, pCMV4Tax, and pCMV4TaxM22 have been previously described (4, 61). The HTLV-1 LTR luciferase reporter (pLuc HTLV-1 LTR) was generated by introducing the full-length HTLV-1 LTR into the pGL2-Basic vector (Promega, Madison, Wis.). The Rous sarcoma virus LTR-driven LacZ reporter construct (6RZ) was obtained from D. Pearce (University of California, San Francisco) and has been previously described (47). The wild-type FLAG-TRAF2 and dominant negative FLAG-TRAF2(87-501) expression plasmids were provided by D. V. Goeddel (Tularik Inc., South San Francisco, Calif.). The expression vector for the N-terminal truncated constitutively active MEKK1 was provided by G. L. Johnson (National Jewish Medical and Research Center, Denver, Colo.). The Myc-PAK1 K299R expression plasmid was provided by G. M. Bokoch and U. Knaus (Scripps Research Institute, La Jolla, Calif.). The FLAG-JNK1 expression vector was provided by M. Karin (University of California, San Diego).
Murine IKKα was amplified by PCR from a previously described vector (9) and subcloned either into pEV3S (37), in frame with a C-terminal T7 tag to generate pEV-IKKα-T7, or into pcDNA 3.1 (Invitrogen, Carlsbad, Calif.), in frame with a C-terminal hemagglutinin (HA) epitope tag to generate pcDNA-IKKα-HA. The human IKKβ cDNA was amplified by PCR using a 3′ oligonucleotide encoding a FLAG epitope tag and subcloned into pcDNA3.1 to generate pcDNA-IKKβ-FLAG. The TRAF6(289-522) and NIK cDNAs were generated by reverse transcription-PCR using Jurkat E6-1 mRNA; the TRAF2 cDNA was amplified by PCR from the vector described above. Each of these cDNAs was then subcloned in frame with an N-terminal Myc epitope tag in the pRK6 vector. The kinase-deficient K44M IKKα, K44A IKKβ, and KK429/430AA NIK mutants were generated by PCR.
Recombinant human TNF-α and IL-1β were purchased from Endogen (Cambridge, Mass.). The 293 human embryonic kidney and HeLa cell lines were maintained in Dulbecco modified Eagle medium supplemented with 10% heat-inactivated fetal bovine serum and antibiotics. Jurkat E6-1 cells as well as the HTLV-1-infected T-cell lines C8166 and HUT102 were maintained in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum and antibiotics. The previously described (70) Jurkat-Tax and Jurkat-anti-Tax cell lines were maintained in the same medium.
Transfections and reporter assays.
293 cells (7 × 105 cells per well) or HeLa cells (2 × 105 cells per well) were seeded into six-well (35-mm-diameter) plates and transfected the following day with 4 μg of DNA by the calcium phosphate precipitation method (53). Transfection of Jurkat E6-1 cells (2 × 106) was performed as follows. Cells were incubated with 4.25 μg of DNA and 8 μl of DMRIE-C (Gibco-BRL Life Technologies, Gaithersburg, Md.) for 4 h in serum-free medium (OPTIMEM; Gibco-BRL Life Technologies) in 35-mm-diameter wells. Cells were then suspended in RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum. Luciferase activity was determined 18 to 24 h later, using an enhanced luciferase assay kit and a Monolight 2010 luminometer (Analytical Luminescence Laboratory, Ann Arbor, Mich.). All transfections included the 6RZ plasmid to allow normalization for differences in gene transfer efficiency by assay of β-galactosidase activity.
Immune complex kinase assays.
For the IKK assays, HeLa cells were transfected by the calcium phosphate precipitation method and lysed 24 to 48 h posttransfection in a buffer containing 1% Nonidet P-40, 250 mM NaCl, 50 mM HEPES (pH 7.4), 1 mM EDTA, and the protease inhibitors phenylmethylsulfonyl fluoride (1 mM), antipain (5 μg/ml), aprotinin (5 μg/ml), leupeptin (5 μg/ml), pepstatin (0.5 μg/ml), bestatin (7.5 μg/ml), phosphoroamidon (4 μg/ml), and trypsin inhibitor (5 μg/ml). Lysates were immunoprecipitated with either anti-HA monoclonal antibody covalently linked to Sepharose (BABCO, Richmond, Calif.) or anti-FLAG M2 antibody covalently attached to agarose (Eastman Kodak Company, New Haven, Conn.). Immunoprecipitates were washed three times in lysis buffer and then once in kinase buffer containing 10 mM HEPES (pH 7.4), 1 mM MnCl2, 5 mM MgCl2, 12.5 mM β-glycero-2-phosphate, 50 μM Na3VO4, 2 mM NaF, 50 μM dithiothreitol, and 10 μM ATP. To assess endogenous IKKα/β enzymatic activity, 25 × 106 cells were lysed and immunoprecipitated with the anti-IKKα antibody H744 (Santa Cruz Biotechnology, Santa Cruz, Calif.), which cross-reacts with IKKβ. After suspension in 20 μl of kinase buffer, the immunoprecipitates were incubated with 5 μCi of [γ-32P]ATP (6,000 Ci/mmol) and 1 μg of recombinant glutathione S-transferase (GST)–IκBα(1-62) as an exogenous substrate for 30 min at 30°C. The kinase reactions were terminated by the addition of sodium dodecyl sulfate (SDS) sample buffer. The samples were analyzed by SDS-polyacrylamide gel electrophoresis (PAGE) followed by transfer to nitrocellulose membranes and exposure to Hyperfilm MP (Amersham Life Sciences). The membranes were subsequently probed with either anti-HA, anti-FLAG M2, or anti-IKKα H744 antibody to determine the amount of immunoprecipitated kinase. Cell lysates were immunoblotted with peptide-specific anti-Tax antibodies to assess the levels of Tax protein expression. The JNK1 kinase assays were performed in a similar fashion except that (i) 293 cells were transfected with FLAG-JNK1 and (ii) GST–c-Jun(1-79) (Santa Cruz Biotechnology) was used as an exogenous substrate in the kinase reaction.
RESULTS
Signaling pathways leading to JNK and NF-κB activation.
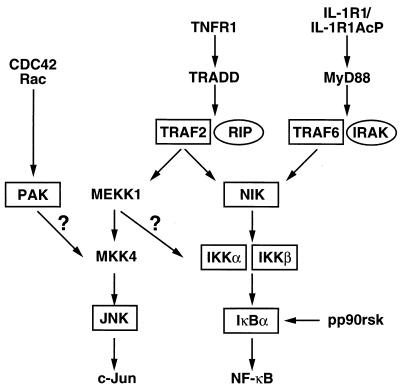
A summary of the recognized signaling pathways leading to NF-κB and c-Jun activation is schematically presented in Fig. 1. TRAF2 and TRAF6 represent proximal signaling intermediates that are recruited to the cytoplasmic tail of the type 1 TNFR (TNFR1) and the type 1 IL-1 receptor (IL-1R1), respectively, following ligand binding (8, 22). TRAF2 is recruited through an interaction with TRADD, while TRAF6 appears to associate with MyD88 and IRAK (IL-1R-associated kinase) (8, 22, 45, 71). Both of these TRAFs are capable of engaging NIK, a MAP3K-related kinase, which thus forms a nexus in these two proinflammatory cytokine signaling pathways leading to NF-κB activation (35, 59). In turn, NIK associates with and activates IKKα and -β (51, 72). The IKKs phosphorylate the IκBs on two N-terminal serine residues (10, 40, 51, 72, 80), leading to the ubiquitination and degradation of these inhibitors in the 26S proteasome (1, 2, 68). MEKK1 corresponds to a second MAP3K that forms a key signaling intermediate in the TNFR1 pathway leading to JNK activation. MEKK1 is also capable of activating NF-κB when ectopically expressed, but its role in TNF-α induction of this transcription factor remains controversial (20, 32, 34, 59). PAK1 corresponds to a kinase that is activated by small GTPases of the Rho family that has also been shown to activate JNK (27). The precise involvement of PAK1 in these signaling pathways is not yet clear. Finally, pp90rsk is a phorbol ester-activated kinase, positioned downstream of the extracellular response kinases (ERKs) (60), that has been shown to directly phosphorylate IκBα on serine 32 in vitro and to induce degradation of this inhibitor (14, 54). We have explored the participation of several of these signaling components in the HTLV-1 Tax response leading to NF-κB induction.
FIG. 1.
Overview of the identified signaling pathways leading to JNK and NF-κB activation. Question marks indicate points in these signaling cascades that require confirmation. Components of these pathways that are boxed were examined for their involvement in HTLV-1 Tax action. RIP, receptor-interacting protein.
The IκBα SS32/36AA mutant blocks HTLV-1 Tax induction of NF-κB in 293 cells.
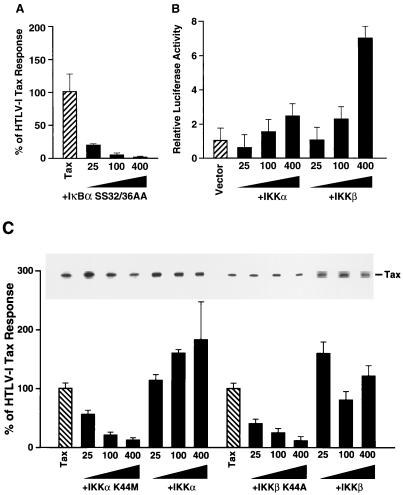
As a starting point in our studies, we verified that a constitutive repressor mutant of IκBα (the SS32/36AA mutant), in which serines 32 and 36 were substituted with alanines, effectively blocked Tax induction of κB-dependent luciferase activity in human 293 cells. These cells were cotransfected with a Tax expression vector and increasing amounts of the IκBα SS32/36AA mutant (25 to 400 ng of expression plasmid [Fig. 2A]). In these cells, the IκBα SS32/36AA mutant functioned as a potent inhibitor of Tax-induced NF-κB expression. This finding confirms for 293 cells the results of prior studies performed with different cell types (7, 25) and suggests that Tax activates NF-κB by entering a signaling pathway prior to or at the level of IκBα.
FIG. 2.
Functional analysis of the roles of IKKα and IKKβ in HTLV-1 Tax induction of NF-κB. (A) IκBα mutant HA-IκBα SS32/36AA, which fails to undergo stimulus-coupled phosphorylation and degradation, blocks Tax activation of NF-κB in human 293 cells. Cells were transfected with 1 μg of Tax expression vector, 200 ng of κB-TATA-luciferase, 20 ng of 6RZ (β-galactosidase reporter), and 25, 100, or 400 ng of HA-IκBα SS32/36AA. The total DNA concentration was held constant at 4 μg by supplementation with the parental pCMV4 vector. To control for differences in transfection efficiency, luciferase activity was normalized to β-galactosidase activity. The results presented represent the means (± standard deviations) derived from at least three independent transfections. All values were first calculated as fold induction relative to cells transfected with reporter plasmids only. These values were then expressed as percentages of the response obtained with Tax alone. (B) Transfection of 293 cells with various amounts of wild-type IKKα-T7 and IKKβ-FLAG, 200 ng of κB-luciferase reporter plasmid, and 20 ng of 6RZ. Activity is expressed as arbitrary light units normalized to β-galactosidase activity. Error bars depict standard deviations obtained from triplicate determinations. (C) Functional effects of wild-type and kinase-deficient forms of IKKα-T7 and IKKβ-FLAG on HTLV-1 Tax activation of κB-luciferase activity. Human 293 cells were transfected with 1 μg of Tax expression vector, 200 ng of κB-TATA-luciferase plasmid, 20 ng of 6RZ, and 25, 100, or 400 ng of either the wild-type IKKs or the kinase-deficient mutant of IKKα-T7 (K44M) or IKKβ-FLAG (K44A). κB-dependent luciferase activity is depicted as a percentage of the response obtained in the presence of Tax alone as described for panel A. The inset depicts the level of Tax protein expressed in each of the cultures assessed by immunoblotting with an anti-Tax antibody.
Kinase-deficient mutants of IKKα and IKKβ block HTLV-1 Tax induction of NF-κB in 293 cells.
The effects of both wild-type and kinase-deficient mutants of IKKα (K44M) and IKKβ (K44A) on Tax-induced NF-κB expression were next assessed in 293 cells. Since the wild-type IKKs were included as controls for their kinase-deficient mutant counterparts, the functional effects of wild-type IKKα and IKKβ were first evaluated (Fig. 2B). IKKα produced only a twofold increase in κB-luciferase activity at the highest dose of expression vector (400 ng), while IKKβ mediated a sevenfold increase in luciferase activity at the same dose of expression vector (400 ng).
The addition of increasing amounts of either the IKKα (K44M) or IKKβ (K44A) kinase-deficient expression vector produced a dose-dependent suppression of the Tax response (Fig. 2C). In contrast, addition of increasing amounts of the wild-type IKKα and IKKβ expression vectors did not inhibit the Tax response and in some cases produced a slight amplification of the response. Immunoblotting of the cell lysates confirmed the presence of comparable levels of Tax protein expression in each of the transfection conditions (Fig. 2C, inset), eliminating suppressive effects of the IKKα and IKKβ kinase-deficient mutants on Tax expression as the cause of the observed inhibition.
Kinase-deficient IKKα and IKKβ mutants inhibit HTLV-1 Tax-mediated activation of NF-κB in Jurkat T cells.
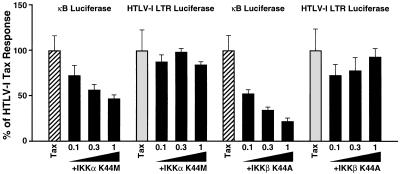
Since HTLV-1 preferentially infects human T cells, which in some cases leads to ATL, the effects of the kinase-deficient mutants of IKKα and IKKβ on Tax induction of κB-luciferase activity were evaluated in human Jurkat T cells (Fig. 3). As a control for these experiments, the effects of these IKK mutants on Tax-mediated activation of the HTLV-1 LTR, which involves the CREB/ATF rather than NF-κB/Rel family of transcription factors, was evaluated in parallel. Transfection of escalating doses (0.1, 0.3, and 1 μg) of the expression vectors encoding either IKKα (K44M) or IKKβ (K44A) resulted in dose-dependent inhibition of Tax activation of κB-luciferase activity (Fig. 3). In contrast, over these same doses, the kinase-deficient IKK mutants produced no significant inhibition of Tax-induced HTLV-1 LTR luciferase activity. These results suggest that Tax induction of NF-κB in human Jurkat T cells, as in 293 cells, involves the participation of IKKα and IKKβ.
FIG. 3.
Functional effects of kinase-deficient IKKα and IKKβ mutants on HTLV-1 Tax induction of NF-κB in human Jurkat T cells. Cells were transfected by using DMRIE-C with 350 ng of Tax, 0.1, 0.3, or 1 μg of the K44M mutant of IKKα-T7 or the K44A mutant of IKKβ-FLAG, and 500 ng of either the κB-luciferase or the HTLV-1 LTR luciferase reporter plasmid. Each transfection also contained 250 ng of 6RZ and was supplemented to 4.25 μg of DNA with a pCMV4-based CD8 expression vector. Luciferase results were normalized to β-galactosidase activity, expressed as fold induction relative to cells transfected with reporters only and finally as a percentage of the HTLV-1 Tax response.
HTLV-1 Tax activates IKKα and IKKβ enzymatic activity.
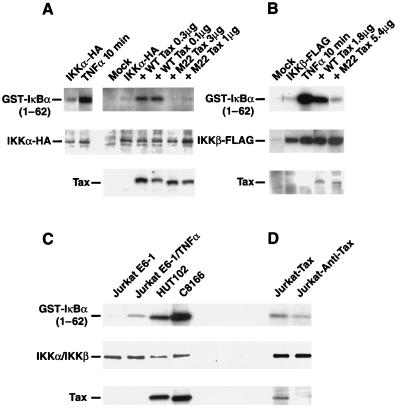
To independently assess whether HTLV-1 Tax induction of NF-κB proceeds through IKKα and IKKβ, we examined whether Tax, like TNF-α, stimulates the enzymatic activity of IKKα and IKKβ (Fig. 4). We chose to perform these experiments with HeLa cells because of a failure to observe induction of IKK enzymatic activity by either Tax or TNF-α in 293 cells. This result most likely reflects a high constitutive enzymatic activity due to overexpression of these kinases. Thus, HeLa cells were cotransfected with expression vectors encoding either IKKα or IKKβ and wild-type Tax or the M22 mutant of Tax. M22 Tax fails to induce NF-κB expression; however, it continues to activate the HTLV-1 LTR via the CREB/ATF pathway, indicating that it is not completely misfolded (55). As an additional control, selected cell aliquots were stimulated with TNF-α for 10 min. Kinase activity for IKKα (Fig. 4A) and IKKβ (Fig. 4B) was assessed in in vitro kinase assays involving immunoprecipitation of the kinases and the addition of recombinant GST-IκBα(1-62) as an exogenous substrate. As shown in Fig. 4A and B, wild-type Tax, like TNF-α, but not M22 Tax activated the enzymatic activity of the IKKα and IKKβ proteins. The levels of immunoprecipitated IKKα and IKKβ, as well as the levels of Tax in the cell lysates, in each transfection condition are also shown. Of note, the M22 Tax mutant was consistently expressed at lower levels than wild-type Tax; however, when comparable levels of protein expression were examined (for example, wild-type Tax at 0.1 μg and M22 Tax at 1 μg), wild-type Tax significantly activated the enzymatic activity of IKKα compared with the M22 Tax mutant (Fig. 4A). Similarly, when 1.8 μg of wild-type Tax was compared to 5.4 μg of M22 Tax, a significant induction in IKKβ enzymatic activity was observed in response to Tax expression (Fig. 4B). Utilization of a GST-IκBα substrate containing alanine substitutions for serine at positions 32 and 36 demonstrated that the measured Tax response involved phosphorylation at the two N-terminal serine residues of IκBα (data not shown). Together, these findings demonstrate that like the proinflammatory cytokine TNF-α, HTLV-1 Tax, but not M22 Tax, augments the enzymatic activity of both IKKα and IKKβ.
FIG. 4.
HTLV-1 Tax activates ectopically expressed and endogenous IKKα and IKKβ enzymatic activity. (A) Approximately 4 × 105 HeLa cells were transfected with 1 μg of IKKα-HA expression plasmid and various amounts of wild-type (WT) Tax (0.3 or 1 μg) or M22 Tax (3 or 1 μg) expression vector. As a control, cells transfected with IKKα-HA only were stimulated with medium or TNF-α (20 ng/ml) for 10 min. Cell lysates were then immunoprecipitated with anti-HA antibodies and subjected to in vitro kinase assay in the presence of GST-IκBα(1-62) as an exogenous substrate. Levels of immunoprecipitated IKKα-HA and levels of wild-type and M22 Tax present in the cell lysates are also shown. (B) Approximately 107 HeLa cells were transfected with 900 ng of IKKβ-FLAG expression plasmid and either 1.8 μg of wild-type Tax or 5.4 μg of M22 Tax expression vector. Cell lysates were then immunoprecipitated with anti-FLAG M2 antibody and subjected to in vitro kinase assay in the presence of GST-IκBα(1-62) as an exogenous substrate. Levels of immunoprecipitated IKKβ-FLAG and levels of wild-type and M22 Tax present in the lysates are also shown. In vitro kinase assays were performed on endogenous IKKα/β immunoprecipitated with antibody H744, which cross-reacts with both enzymes. GST-IκBα(1-62) fusion protein was added as an exogenous substrate, and activities were compared for unstimulated and TNF-α (20 ng/ml for 30 min)-induced Jurkat E6-1 cells and HTLV-1-infected HUT102 and C8166 T cells (C) or Jurkat T cells stably transfected with a sense (Jurkat-Tax) or antisense (Jurkat-anti-Tax) expression vector (D). Levels of H744-immunoprecipitated endogenous IKKα/β proteins and levels of Tax in the cell lysates are also shown.
Endogenous IKKs are enzymatically more active in HTLV-1-infected T-cell lines and Jurkat T cells expressing Tax.
To assess whether Tax also activates the endogenous IKKα and IKKβ enzymes, we analyzed the HTLV-1-infected HUT102 or C8166 T-cell lines and Jurkat T cells engineered to stably express Tax or antisense Tax (70). Endogenous IKKα and IKKβ were immunoprecipitated from these cells with antibody H744, which cross-reacts with both enzymes, and analyzed in an in vitro kinase assay (Fig. 4C). Although somewhat less IKKα/β was immunoprecipitated from HUT102 and C8166 cells than from Jurkat E6-1 cells, both HTLV-1-infected cell lines displayed significantly higher levels of endogenous IKKα/β enzymatic activity. Indeed these levels of activity exceeded that obtained in Jurkat E6-1 cells stimulated with TNF-α. While the HUT102 and C8166 cells expressed large amounts of Tax (Fig. 4C), it was possible that other differences in these cells from Jurkat T cells were responsible for the altered IKKα/β activity. Accordingly, we compared Jurkat-Tax and Jurkat-anti-Tax cell lines, which are identical except for the presence of Tax (70). Although expressing comparable amounts of IKKα/β, the Jurkat-Tax cells displayed moderately increased IKKα/β enzymatic activity relative to the Jurkat-anti-Tax cell line (Fig. 4D). The small increase in IKK activity paralleled the relatively low level of Tax expression observed in the Jurkat-Tax cells (Fig. 4D). These results demonstrate that IKKα/β enzymatic activity is constitutively elevated in T-cell lines expressing HTLV-1 Tax.
Kinase-deficient mutants of NIK, but not PAK1, inhibit HTLV-1 Tax induction of NF-κB.
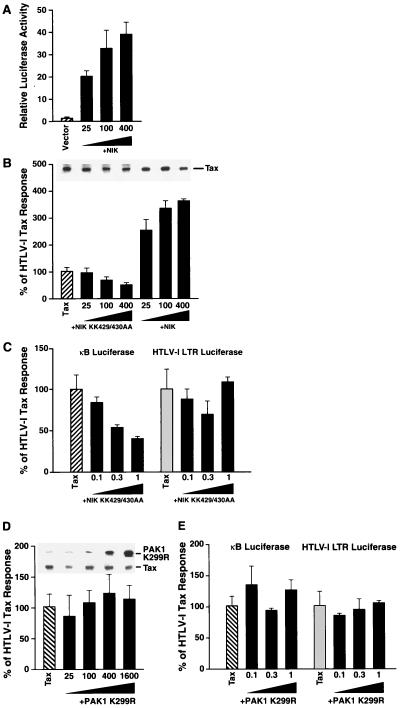
The potential involvement of NIK in Tax induction of NF-κB was next evaluated. As noted, NIK represents the next upstream component of the TNF-α and IL-1 signaling pathways (Fig. 1). When introduced into 293 cells alone, NIK functioned as a potent activator of κB-luciferase activity (Fig. 5A) as previously reported (35). Cotransfection of 293 cells with Tax and increasing amounts of the kinase-deficient NIK mutant (KK429/430AA) produced a dose-dependent inhibition of κB-luciferase reporter activity in these cells (Fig. 5B). In contrast, cotransfection of matching amounts of the wild-type NIK expression vector led to enhancement rather than inhibition of the Tax response. Immunoblotting of the cell lysates verified the presence of comparable levels of Tax in the presence of the NIK KK429/430AA mutant (Fig. 5B, inset).
FIG. 5.
Functional effects of wild-type and kinase-deficient NIK on HTLV-1 Tax-induced NF-κB expression. (A) 293 cells were transfected with various amounts of wild-type Myc-NIK expression vector, 200 ng of κB-luciferase reporter, and 20 ng of the 6RZ plasmid; 18 to 24 h later, cell lysates were prepared and κB-luciferase activity was determined. Results are presented as relative light units normalized to β-galactosidase activity. Error bars depict standard deviations obtained for triplicate transfections. (B) 293 cells were transfected with 1 μg of Tax expression vector, 200 ng of κB-TATA-luciferase, 20 ng of 6RZ, and 25, 100, or 400 ng of the kinase-deficient KK429/430AA mutant of Myc-NIK or wild-type Myc-NIK. Results are presented relative to the response obtained with Tax alone as described in the legend to Fig. 2. The inset shows the level of Tax expression in each of the cultures determined by immunoblotting. (C) Functional effects of a kinase-deficient NIK mutant on Tax induction of NF-κB expression in Jurkat T cells. Jurkat T cells were transfected as described in the legend to Fig. 3 with various amounts (0.1, 0.3, or 1 μg) of the KK429/430AA mutant of Myc-NIK and 350 ng of Tax and 500 ng of the κB-luciferase or HTLV-1 LTR luciferase reporter plasmid. Results are expressed as a percentage of the response obtained in the presence of Tax alone. (D) The same experiment as described for panel B was performed except that 293 cells were transfected with the K299R kinase-deficient mutant of Myc-PAK1. Levels of Tax and Myc-PAK1 K299R expressed in these cultures are shown in the inset. (E) The same experiment as described for panel C was performed except that Jurkat T cells were transfected with the kinase-deficient mutant Myc-PAK1 K299R.
The potential role of PAK1 in the Tax response was similarly evaluated. PAK1 is activated by certain small GTPases of the Rho family (Rac and CDC42) (67). PAK1, like the small GTPases, activates JNK, suggesting that it may be a downstream effector of the Rho GTPases (27). However, the role of PAK1 in NF-κB activation is unknown. Cotransfection of increasing amounts of a kinase-deficient mutant (K299R) of PAK1 had no effect on Tax induction of NF-κB (Fig. 5D). Blotting of the cellular lysates confirmed dose-related expression of PAK1 and comparable levels of Tax expression (Fig. 5D, inset).
The effects of the kinase-deficient NIK and PAK1 mutants on Tax induced NF-κB expression were also assessed in Jurkat T cells. The NIK KK429/430AA mutant produced dose-dependent inhibition of Tax-induced κB-luciferase activity but had essentially no effect on Tax induction of HTLV-1 LTR luciferase activity, which occurs independently of NF-κB (Fig. 5C). As observed in 293 cells, the kinase-deficient PAK1 K299R mutant did not inhibit κB-luciferase activity induced by Tax, nor did it alter the Tax induction of the HTLV-1 LTR (Fig. 5E).
The lack of effect of the kinase-deficient PAK1 mutant further supports a specific effect for the kinase-deficient NIK mutant. Together, these findings raise the possibility that Tax action proceeds through the activation of NIK enzymatic activity. However, the observed inhibitory effects of the NIK KK429/430AA mutant observed in both 293 and Jurkat T cells could also result from its binding to and inactivation of IKKs, making these latter kinases unresponsive to Tax. These two possibilities could be distinguished by assessing the effects of Tax on NIK enzymatic activity. However, our studies indicate that ectopically expressed NIK is constitutively active and not induced further by TNF-α, TRAF2, or Tax (data not shown). Resolution of this issue thus awaits the development of an immunoprecipitating anti-NIK antibody that will allow assessment of endogenous NIK activity in the presence and absence of Tax.
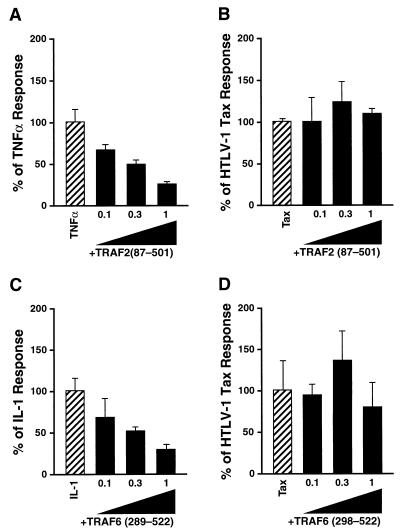
Dominant negative mutants of TRAF2 and TRAF6 fail to inhibit HTLV-1 Tax induction of NF-κB.
TRAF2 interacts with TRADD and activates NF-κB presumably through its interaction with NIK (35, 52). An amino-terminal deletion mutant of TRAF2 [TRAF2(87-501)], lacking its RING finger domain, functions as a dominant negative inhibitor in the TNF-α/TNFR1 signaling pathway, blocking both NF-κB and JNK activation (22, 34, 59). When cotransfected with Tax, the dominant negative TRAF2 mutant did not alter Tax activation of NF-κB (Fig. 6B). However, in agreement with prior studies (22), this same mutant significantly inhibited the TNF-α-induced NF-κB response (Fig. 6A). This result suggests that Tax enters the TNF-α signaling pathway downstream of TRAF2 or perhaps through a different TRAF. In this regard, TRAF6 is recruited to IL-1R1 by the MyD88 adaptor and a serine/threonine kinase termed IRAK (8, 45, 71). To rule out that Tax activates NF-κB via TRAF6, the amino-terminal deletion mutant TRAF6(289-522) was cotransfected with Tax. While the TRAF6 dominant negative mutant effectively blocked IL-1 signaling (Fig. 6C) (8), no significant inhibitory effects were obtained for Tax induction of NF-κB (Fig. 6D). These results indicate that Tax induction of NF-κB involves neither TRAF2 nor TRAF6 and suggest that Tax enters this proinflammatory cytokine signaling pathway downstream of the site of action of these TRAFs.
FIG. 6.
Effects of dominant negative mutants of TRAF2 and TRAF6 on Tax induction of κB-luciferase activity. (A) 293 cells were transfected with 200 ng of κB-TATA-luciferase, 20 ng of 6RZ, and 0.1, 0.3, or 1 μg of a dominant negative FLAG-TRAF2(87-501) mutant. Approximately 24 h after transfection, the cells were stimulated with TNF-α (10 ng/ml) for 6 h. (B) 293 cells were transfected with 1 μg of the Tax expression vector, 200 ng of κB-TATA-luciferase, 20 ng of 6RZ, and 0.1, 0.3, or 1 μg of FLAG-TRAF2(87-501). Luciferase activity was determined approximately 30 h later and normalized to β-galactosidase activity. (C) HeLa cells were transfected with 200 ng of κB-TATA-luciferase, 20 ng of 6RZ, and 0.1, 0.3, or 1 μg of a dominant negative Myc-TRAF6(289-522) mutant. At approximately 24 h posttransfection, the cells were stimulated with IL-1β (10 ng/ml) for 6 h. (D) HeLa cells were transfected with 1 μg of Tax expression vector, 200 ng of κB-TATA-luciferase, 20 ng of 6RZ, and 0.1, 0.3, or 1 μg of Myc-TRAF6(289-522). Luciferase activity was determined at approximately 30 h posttransfection. The total amount of DNA in each transfection was kept constant at 4 μg by supplementation with pCMV4. Luciferase activity was normalized to β-galactosidase activity, and the values represent the means (± standard deviations) of three independent transfections. The values were first expressed as fold induction relative to cells transfected with reporters only. Final values were expressed as percentages of the TNF-α, IL-1, or Tax response.
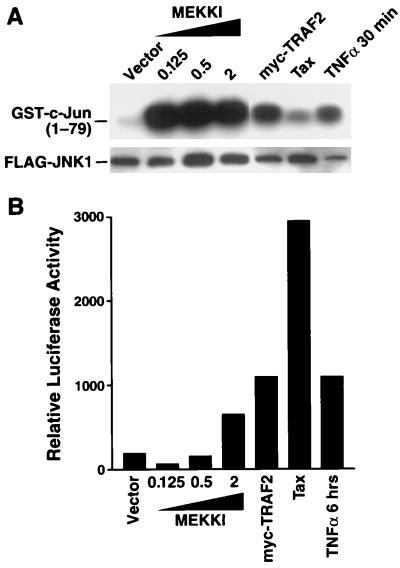
HTLV-1 Tax is a weak inducer of JNK1 enzymatic activity in 293 cells.
MEKK1 represents another kinase of the MAP3K level that acts immediately downstream of TRAF2 (34, 59). MEKK1 acting through the stimulation of MKK4 is a critical signaling intermediate for TNF-α activation of JNK, which in turn phosphorylates the transcription factor c-Jun (41, 75). MEKK1 has also been reported to activate NF-κB when ectopically expressed, but its role in the NF-κB arm of the TNF-α signaling pathway (Fig. 1) is controversial (20, 32, 34, 59). Having established that Tax enters the TNF-α/TNFR1 pathway downstream of TRAF2 and perhaps at the level of NIK, we next explored whether the MEKK1-MKK4-JNK axis (Fig. 1) was activated by Tax. For these studies, we examined whether HTLV-1 Tax activated JNK1 enzymatic activity. 293 cells were transfected with both κB-luciferase and FLAG-JNK. Cotransfection of an N-terminally truncated MEKK1 mutant (30) which is constitutively active led to substantial activation of JNK1 as assessed by phosphorylation of GST–c-Jun(1-79) substrate in vitro (Fig. 7A). At the highest dose of MEKK1 expression vector (2 μg), NF-κB was also activated (Fig. 7B). Cotransfection of Myc-TRAF2 or treatment of cells with TNF-α for 30 min also led to enhanced JNK1 activity (Fig. 7A). Myc-TRAF2 expression as well as treatment of cells with TNF-α for 6 h also led to NF-κB activation (Fig. 7B). In contrast, cotransfection of Tax caused only a modest induction of JNK1 activation compared to the other agonists despite causing substantial NF-κB activation in these same transfected cells (Fig. 7). This finding is consistent with a previous report demonstrating a similar modest induction of JNK1 enzymatic activity by Tax in other cell types (23). These results suggest that in contrast to its ability to activate NF-κB, Tax does not significantly activate the MEKK1-MKK4-JNK1 pathway.
FIG. 7.
Effect of Tax on JNK1 enzymatic activity. (A) 293 cells were transfected with 500 ng of κB-TATA-luciferase, 500 ng of FLAG-JNK1, and 50 ng of 6RZ and either 0.125, 0.5, or 2 μg of an N-terminally truncated but constitutively active form of MEKK1 or 1 μg of either Myc-TRAF2 or HTLV-1 Tax. Finally, 293 cells transfected with reporters only were treated for 30 min and 6 h with 100 ng of TNF-α per ml. Cell lysates were prepared to determine luciferase activity and β-galactosidase activity and were used for immunoprecipitation with anti-FLAG M2 antibody. Anti-FLAG M2 immunoprecipitates from each transfection were subjected to in vitro kinase assays in the presence of GST–c-Jun(1-79) as an exogenous substrate. In vitro kinase reactions were subjected to SDS-PAGE and transferred to nitrocellulose. Membranes were subsequently probed with anti-FLAG M2 antibody to establish levels of immunoprecipitated FLAG-JNK1. (B) κB-luciferase activity was measured in the same cell lysates as used for panel A and normalized to β-galactosidase activity to control for differences in transfection efficiency. The data are presented as relative luciferase activity based on arbitrary light units.
DISCUSSION
To identify the molecular mechanism(s) underlying HTLV-1 Tax induction of NF-κB, we have explored the potential involvement of several components of the TNF-α and IL-1 signaling pathways. These studies have revealed that kinase-deficient forms of IKKα, IKKβ, and NIK, but not PAK1, effectively inhibit Tax induction of NF-κB in both human 293 and Jurkat T cells. Further, these investigations have demonstrated that wild-type Tax enhances both IKKα and IKKβ enzymatic activities. In contrast, the M22 Tax mutant, which manifests a specific defect in NF-κB activation, does not significantly stimulate these kinases. Additionally, we have found that the endogenous IKKα and IKKβ enzymes present in HTLV-1-infected and Tax-expressing T-cell lines are quite active. Further, we have detected Tax activation of endogenous IKKα/β in Jurkat T cells stably expressing Tax compared with matched Jurkat anti-Tax controls. These results thus significantly extend our prior study in which we demonstrated the involvement of pp90rsk in phorbol ester- but not Tax-induced phosphorylation and degradation of IκBα (14). These findings indicate that the HTLV-1 transactivator protein activates only a subset of the cellular kinases capable of phosphorylating IκBα. The ability of Tax to activate the IKKs appears to be quite specific since a parallel pathway which is initiated by TRAF2 and involves MEKK1-MKK4 activation of JNK1 does not appear to be significantly induced by Tax. Thus, although MEKK1 has been proposed as an inducer of NF-κB, it seems unlikely that Tax activation of NF-κB proceeds through MEKK1. For this to occur, Tax would have to activate MEKK1 in a manner not leading to standard JNK1 activation.
The ability of a kinase-deficient form of NIK to block Tax induction of NF-κB raised the distinct possibility that Tax action involved this component of the TNF-α and IL-1 signaling cascade. However, the high constitutive activity of this enzyme obtained following its ectopic expression coupled with a lack of response of the kinase to TNF-α, TRAF2, or Tax precluded firm assignment of this enzyme to the intracellular pathway utilized by Tax. The involvement of NIK in this reaction could be evaluated if an active inducible form of endogenous NIK could be immunoprecipitated from cells expressing Tax. Unfortunately, reagents to perform such an experiment are not available at the present time. In addition, we cannot completely exclude the involvement of yet another MAP3K in the Tax response whose activity is inhibited by expression of kinase-deficient NIK. However, it is interesting that both Tax and NIK possess similar properties with regard to activation of IKKs and JNK. Specifically, Tax (Fig. 4), like NIK (51, 72), activates both IKKs, while neither of these proteins significantly activates JNK1 (23, 59, 73) (Fig. 7).
While Tax utilizes the IKKα, IKKβ, and perhaps NIK components of the TNF-α and IL-1 signaling pathways, more proximal components of these pathways, specifically TRAF2 and TRAF6, do not appear to be involved in Tax induction of NF-κB. This conclusion is based on the fact that dominant negative mutants of TRAF2 and TRAF6, which effectively block TNF-α and IL-1 signaling, respectively, fail to inhibit Tax induction of NF-κB. These findings further suggest that Tax does not activate NF-κB via the induced transcription and autocrine action of TNF-α or IL-1. However, our results do not formally exclude the possibility that Tax activates the expression of another cytokine that is secreted and binds to a receptor that functions independently of TRAF2 and TRAF6, leading to the stimulation of NIK and IKKs.
It remains unknown whether Tax subverts the TNF-α/IL-1 signaling pathway by directly interacting with and activating NIK and/or IKKα or IKKβ or by indirectly altering the expression levels of various components of this pathway. However, enhanced levels of IKKs in HTLV-1 Tax-expressing cell lines is unlikely to be the cause of increased NF-κB activity since the levels of endogenous IKKs were comparable to levels in cells that did not express Tax (Fig. 4C and D). A prior report indicating that Tax induces nuclear NF-κB expression in the absence of de novo protein synthesis further argues against an indirect effect involving new gene transcription (33). Although Tax has been reported to be principally a nuclear protein, it is also present in the cytoplasm (4, 26, 43). Indeed, Tax avidly binds to the p100 product of the NF-κB2 gene, an exclusively cytoplasmic member of the IκB family of proteins (4, 26, 31, 44). This interaction may thus ensure a cytoplasmic pool of Tax to mediate subsequent activation of IKKα, IKKβ, and perhaps NIK.
If Tax does in fact act in a direct manner at the level of NIK, our findings raise the intriguing possibility that Tax functions as a TRAF-like molecule. The TRAFs may act by producing oligomerization of the downstream kinase, leading to altered enzymatic activity. In this regard, the M22 mutant of Tax, which fails to activate IKKα and IKKβ catalytic activities, also fails to dimerize (65). Alternatively, Tax may function through an as yet unidentified TRAF to promote aggregation of NIK. Indeed, biological precedence exists for such a mechanism. Specifically, the cytoplasmic domain of the transforming LMP1 protein encoded by Epstein-Barr virus has been shown to associate with TRAFs and to induce NF-κB (42). It has been suggested that LMP1 causes aggregation of TRAFs and may mimic the effects of TNF binding to its trimeric receptor (42).
It is also possible that Tax induces nuclear NF-κB expression by altering the levels of endogenous NIK. In this regard, NIK expression levels have been shown to correlate with NF-κB activation (35) (Fig. 5A). Finally, it is possible that Tax induces the expression of an unknown activator of the NIK or IKK enzymes or perhaps down-regulates the expression or activity of an inhibitor of these kinases such as protein phosphatase 2A (10). Future studies will help delineate which of these mechanisms of action is utilized by Tax.
In terms of the biology of HTLV-1 infection, Tax induction of NF-κB in virally infected T cells may play a central role in cellular transformation leading to the ATL. One possible role for Tax-induced NF-κB expression would be to inhibit apoptosis in infected T cells. In this regard, it has recently been shown that NF-κB expression is induced by transforming Ras mutants and that NF-κB production inhibits a p53-independent pathway of apoptosis in these cells (38).
In conclusion, our studies reveal how HTLV-1 Tax subverts specific plasma membrane-distal components of the physiological proinflammatory signaling pathway leading to the pathologically sustained induction of NF-κB. These findings thus provide new insights into the molecular basis of Tax action, emphasizing its ability to directly or indirectly alter the activity of cytoplasmic kinases pivotally involved in signal-coupled degradation of IκB. Should the abnormal expression of NF-κB play a central role in HTLV-1-associated leukemogenesis, the development of inhibitors of NIK or IKKα and IKKβ might serve not only as anti-inflammatory agents but also as novel chemotherapeutics potentially active against HTLV-1-induced ATL.
ACKNOWLEDGMENTS
R.G. is supported by a Centennial Fellowship from the Medical Research Council of Canada. E.T.C. is supported by NIH grant KO8-EY00352. This work was supported by grants from the UCSF Center for AIDS Research (P30A127763) and from Pfizer.
R.G., S.F., and X.L. contributed equally to this work.
We thank G. Bokoch and U. Knaus for providing the PAK1 K299R expression vector, D. Goeddel for the TRAF2 and TRAF2(87-501) vectors, G. Johnson for the MEKK1 vector, D. Pearce for the 6RZ reporter construct, M. Karin for the FLAG-JNK1 construct, Christophe Beraud for helpful discussions, Weiduan Xu for technical support, and John Carroll, Neile Shea, Stephen Gonzales, and Chris Goodfellow for preparation of the figures.
REFERENCES
- 1.Baeuerle P A, Baltimore D. NF-κB: ten years after. Cell. 1996;87:13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- 2.Baldwin A J. The NF-κB and I κB proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 3.Baranger A M, Palmer C R, Hamm M K, Giebler H A, Brauweiler A, Nyborg J K, Schepartz A. Mechanism of DNA-binding enhancement by the human T-cell leukaemia virus transactivator Tax. Nature. 1995;376:606–608. doi: 10.1038/376606a0. [DOI] [PubMed] [Google Scholar]
- 4.Beraud C, Sun S C, Ganchi P, Ballard D W, Greene W C. Human T-cell leukemia virus type I Tax associates with and is negatively regulated by the NF-κB2 p100 gene product: implications for viral latency. Mol Cell Biol. 1994;14:1374–1382. doi: 10.1128/mcb.14.2.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brady J, Jeang K T, Duvall J, Khoury G. Identification of p40x-responsive regulatory sequences within the human T-cell leukemia virus type I long terminal repeat. J Virol. 1987;61:2175–2181. doi: 10.1128/jvi.61.7.2175-2181.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brauweiler A, Garl P, Franklin A A, Giebler H A, Nyborg J K. A molecular mechanism for human T-cell leukemia virus latency and Tax transactivation. J Biol Chem. 1995;270:12814–12822. doi: 10.1074/jbc.270.21.12814. [DOI] [PubMed] [Google Scholar]
- 7.Brockman J A, Scherer D C, McKinsey T A, Hall S M, Qi X, Lee W Y, Ballard D W. Coupling of a signal response domain in IκBα to multiple pathways for NF-κB activation. Mol Cell Biol. 1995;15:2809–2818. doi: 10.1128/mcb.15.5.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao Z, Xiong J, Takeuchi M, Kurama T, Goeddel D V. TRAF6 is a signal transducer for interleukin-1. Nature. 1996;383:443–446. doi: 10.1038/383443a0. [DOI] [PubMed] [Google Scholar]
- 9.Connelly M A, Marcu K B. CHUK, a new member of the helix-loop-helix and leucine zipper families of interacting proteins, contains a serine-threonine kinase catalytic domain. Cell Mol Biol Res. 1995;41:537–549. [PubMed] [Google Scholar]
- 10.DiDonato J A, Hayakawa M, Rothwarf D M, Zandi E, Karin M. A cytokine-responsive IκB kinase that activates the transcription factor NF-κB. Nature. 1997;388:548–554. doi: 10.1038/41493. [DOI] [PubMed] [Google Scholar]
- 11.Fujii M, Sassone C P, Verma I M. c-fos promoter trans-activation by the tax1 protein of human T-cell leukemia virus type I. Proc Natl Acad Sci USA. 1988;85:8526–8530. doi: 10.1073/pnas.85.22.8526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fujii M, Tsuchiya H, Chuhjo T, Akizawa T, Seiki M. Interaction of HTLV-1 Tax1 with p67SRF causes the aberrant induction of cellular immediate early genes through CArG boxes. Genes Dev. 1992;6:2066–2076. doi: 10.1101/gad.6.11.2066. [DOI] [PubMed] [Google Scholar]
- 13.Gessain A, Barin F, Vernant J C, Gout O, Maurs L, Calender A, de The G. Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet. 1985;ii:407–410. doi: 10.1016/s0140-6736(85)92734-5. [DOI] [PubMed] [Google Scholar]
- 14.Ghoda L, Lin X, Greene W C. The 90-kDa ribosomal S6 kinase (pp90rsk) phosphorylates the N-terminal regulatory domain of IκBα and stimulates its degradation in vitro. J Biol Chem. 1997;272:21281–21288. doi: 10.1074/jbc.272.34.21281. [DOI] [PubMed] [Google Scholar]
- 15.Giam C Z, Xu Y L. HTLV-1 tax gene product activates transcription via pre-existing cellular factors and cAMP responsive element. J Biol Chem. 1989;264:15236–15241. [PubMed] [Google Scholar]
- 16.Giebler H A, Loring J E, van Orden K, Colgin M A, Garrus J E, Escudero K W, Brauweiler A, Nyborg J K. Anchoring of CREB binding protein to the human T-cell leukemia virus type 1 promoter: a molecular mechanism of Tax transactivation. Mol Cell Biol. 1997;17:5156–5164. doi: 10.1128/mcb.17.9.5156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Good L, Sun S C. Persistent activation of NF-κB/Rel by human T-cell leukemia virus type 1 Tax involves degradation of IκBβ. J Virol. 1996;70:2730–2735. doi: 10.1128/jvi.70.5.2730-2735.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grassmann R, Dengler C, Muller F I, Fleckenstein B, McGuire K, Dokhelar M C, Sodroski J G, Haseltine W A. Transformation to continuous growth of primary human T lymphocytes by human T-cell leukemia virus type I X-region genes transduced by a herpesvirus saimiri vector. Proc Natl Acad Sci USA. 1989;86:3351–3355. doi: 10.1073/pnas.86.9.3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greene W C, Bohnlein E, Ballard D W. HIV-1, HTLV-1 and normal T-cell growth: transcriptional strategies and surprises. Immunol Today. 1989;10:272–278. doi: 10.1016/0167-5699(89)90141-2. [DOI] [PubMed] [Google Scholar]
- 20.Hirano M, Osada S, Aoki T, Hirai S, Hosaka M, Inoue J, Ohno S. MEK kinase is involved in tumor necrosis factor alpha-induced NF-κB activation and degradation of IκBα. J Biol Chem. 1996;271:13234–13238. doi: 10.1074/jbc.271.22.13234. [DOI] [PubMed] [Google Scholar]
- 21.Hiscott J, Petropoulos L, Lacoste J. Molecular interactions between HTLV-1 Tax protein and the NF-κB/IκB transcription complex. Virology. 1995;214:3–11. doi: 10.1006/viro.1995.9960. [DOI] [PubMed] [Google Scholar]
- 22.Hsu H, Shu H B, Pan M G, Goeddel D V. TRADD-TRAF2 and TRADD-FADD interactions define two distinct TNF receptor 1 signal transduction pathways. Cell. 1996;84:299–308. doi: 10.1016/s0092-8674(00)80984-8. [DOI] [PubMed] [Google Scholar]
- 23.Jin D Y, Teramoto H, Giam C Z, Chun R F, Gutkind J S, Jeang K T. A human suppressor of c-Jun N-terminal kinase 1 activation by tumor necrosis factor alpha. J Biol Chem. 1997;272:25816–25823. doi: 10.1074/jbc.272.41.25816. [DOI] [PubMed] [Google Scholar]
- 24.Kanno T, Brown K, Franzoso G, Siebenlist U. Kinetic analysis of human T-cell leukemia virus type 1 Tax-mediated activation of NF-κB. Mol Cell Biol. 1994;14:6443–6451. doi: 10.1128/mcb.14.10.6443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kanno T, Brown K, Siebenlist U. Evidence in support of a role for human T-cell leukemia virus type I Tax in activating NF-κB via stimulation of signaling pathways. J Biol Chem. 1995;270:11745–11748. doi: 10.1074/jbc.270.20.11745. [DOI] [PubMed] [Google Scholar]
- 26.Kanno T, Franzoso G, Siebenlist U. Human T-cell leukemia virus type I Tax-protein-mediated activation of NF-κB from p100 (NF-κB2)-inhibited cytoplasmic reservoirs. Proc Natl Acad Sci USA. 1994;91:12634–12638. doi: 10.1073/pnas.91.26.12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Knaus U G, Morris S, Dong H J, Chernoff J, Bokoch G M. Regulation of human leukocyte p21-activated kinases through G protein-coupled receptors. Science. 1995;269:221–223. doi: 10.1126/science.7618083. [DOI] [PubMed] [Google Scholar]
- 28.Kwok R P, Laurance M E, Lundblad J R, Goldman P S, Shih H, Connor L M, Marriott S J, Goodman R H. Control of cAMP-regulated enhancers by the viral transactivator Tax through CREB and the co-activator CBP. Nature. 1996;380:642–646. doi: 10.1038/380642a0. [DOI] [PubMed] [Google Scholar]
- 29.Lacoste J, Petropoulos L, Pepin N, Hiscott J. Constitutive phosphorylation and turnover of IκBα in human T-cell leukemia virus type I-infected and Tax-expressing T cells. J Virol. 1995;69:564–569. doi: 10.1128/jvi.69.1.564-569.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lange C C, Pleiman C M, Gardner A M, Blumer K J, Johnson G L. A divergence in the MAP kinase regulatory network defined by MEK kinase and Raf. Science. 1993;260:315–319. doi: 10.1126/science.8385802. [DOI] [PubMed] [Google Scholar]
- 31.Lanoix J, Lacoste J, Pepin N, Rice N, Hiscott J. Overproduction of NFKB2 (lyt-10) and c-Rel: a mechanism for HTLV-I Tax-mediated trans-activation via the NF-κB signalling pathway. Oncogene. 1994;9:841–852. [PubMed] [Google Scholar]
- 32.Lee F S, Hagler J, Chen Z J, Maniatis T. Activation of the IκBα kinase complex by MEKK1, a kinase of the JNK pathway. Cell. 1997;88:213–222. doi: 10.1016/s0092-8674(00)81842-5. [DOI] [PubMed] [Google Scholar]
- 33.Lindholm P F, Reid R L, Brady J N. Extracellular Tax1 protein stimulates tumor necrosis factor beta and immunoglobulin kappa light chain expression in lymphoid cells. J Virol. 1992;66:1294–1302. doi: 10.1128/jvi.66.3.1294-1302.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu Z G, Hsu H, Goeddel D V, Karin M. Dissection of TNF receptor 1 effector functions: JNK activation is not linked to apoptosis while NF-κB activation prevents cell death. Cell. 1996;87:565–576. doi: 10.1016/s0092-8674(00)81375-6. [DOI] [PubMed] [Google Scholar]
- 35.Malinin N L, Boldin M P, Kovalenko A V, Wallach D. MAP3K-related kinase involved in NF-κB induction by TNF, CD95 and IL-1. Nature. 1997;385:540–544. doi: 10.1038/385540a0. [DOI] [PubMed] [Google Scholar]
- 36.Matsumoto K, Shibata H, Fujisawa J I, Inoue H, Hakura A, Tsukahara T, Fujii M. Human T-cell leukemia virus type 1 Tax protein transforms rat fibroblasts via two distinct pathways. J Virol. 1997;71:4445–4451. doi: 10.1128/jvi.71.6.4445-4451.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matthias P, Muller M M, Schreiber E, Rusconi S, Schaffner W. Eukaryotic expression vectors for the analysis of mutant proteins. Nucleic Acids Res. 1989;17:6418. doi: 10.1093/nar/17.15.6418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mayo M W, Wang C-Y, Cogswell P C, Rogers-Graham K S, Lowe S W, Der C J, Baldwin A S., Jr Requirement of NF-κB activation to suppress p53-independent apoptosis induced by oncogenic Ras. Science. 1997;278:1812–1815. doi: 10.1126/science.278.5344.1812. [DOI] [PubMed] [Google Scholar]
- 39.McKinsey T A, Brockman J A, Scherer D C, Al M S, Green P L, Ballard D W. Inactivation of IκBβ by the Tax protein of human T-cell leukemia virus type 1: a potential mechanism for constitutive induction of NF-κB. Mol Cell Biol. 1996;16:2083–2090. doi: 10.1128/mcb.16.5.2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mercurio F, Zhu H, Murray B W, Shevchenko A, Bennett B L, Li J, Young D B, Barbosa M, Mann M, Manning A, Rao A. IKK-1 and IKK-2: cytokine-activated IκB kinases essential for NF-κB activation. Science. 1997;278:860–866. doi: 10.1126/science.278.5339.860. [DOI] [PubMed] [Google Scholar]
- 41.Minden A, Lin A, McMahon M, Lange C C, Derijard B, Davis R J, Johnson G L, Karin M. Differential activation of ERK and JNK mitogen-activated protein kinases by Raf-1 and MEKK. Science. 1994;266:1719–1723. doi: 10.1126/science.7992057. [DOI] [PubMed] [Google Scholar]
- 42.Mosialos G, Birkenbach M, Yalamanchili R, VanArsdale T, Ware C, Kieff E. The Epstein-Barr virus transforming protein LMP1 engages signaling proteins for the tumor necrosis factor receptor family. Cell. 1995;80:389–399. doi: 10.1016/0092-8674(95)90489-1. [DOI] [PubMed] [Google Scholar]
- 43.Munoz E, Courtois G, Veschambre P, Jalinot P, Israel A. Tax induces nuclear translocation of NF-κB through dissociation of cytoplasmic complexes containing p105 or p100 but does not induce degradation of IκBα/MAD3. J Virol. 1994;68:8035–8044. doi: 10.1128/jvi.68.12.8035-8044.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murakami T, Hirai H, Suzuki T, Fujisawa J, Yoshida M. HTLV-1 Tax enhances NF-κB2 expression and binds to the products p52 and p100, but does not suppress the inhibitory function of p100. Virology. 1995;206:1066–1074. doi: 10.1006/viro.1995.1029. [DOI] [PubMed] [Google Scholar]
- 45.Muzio M, Ni J, Feng P, Dixit V M. IRAK (Pelle) family member IRAK-2 and MyD88 as proximal mediators of IL-1 signaling. Science. 1997;278:1612–1615. doi: 10.1126/science.278.5343.1612. [DOI] [PubMed] [Google Scholar]
- 46.Osame M, Usuku K, Izumo S, Ijichi N, Amitani H, Igata A, Matsumoto M, Tara M. HTLV-I associated myelopathy, a new clinical entity. Lancet. 1986;i:1031–1032. doi: 10.1016/s0140-6736(86)91298-5. [DOI] [PubMed] [Google Scholar]
- 47.Pearce D, Yamamoto K R. Mineralocorticoid and glucocorticoid receptor activities distinguished by nonreceptor factors at a composite response element. Science. 1993;259:1161–1165. doi: 10.1126/science.8382376. [DOI] [PubMed] [Google Scholar]
- 48.Perini G, Wagner S, Green M R. Recognition of bZIP proteins by the human T-cell leukaemia virus transactivator Tax. Nature. 1995;376:602–605. doi: 10.1038/376602a0. [DOI] [PubMed] [Google Scholar]
- 49.Poiesz B J, Ruscetti F W, Gazdar A F, Bunn P A, Minna J D, Gallo R C. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci USA. 1980;77:7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pozzatti R, Vogel J, Jay G. The human T-lymphotropic virus type I tax gene can cooperate with the ras oncogene to induce neoplastic transformation of cells. Mol Cell Biol. 1990;10:413–417. doi: 10.1128/mcb.10.1.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Regnier C H, Song H Y, Gao X, Goeddel D V, Cao Z, Rothe M. Identification and characterization of an IκB kinase. Cell. 1997;90:373–383. doi: 10.1016/s0092-8674(00)80344-x. [DOI] [PubMed] [Google Scholar]
- 52.Rothe M, Wong S C, Henzel W J, Goeddel D V. A novel family of putative signal transducers associated with the cytoplasmic domain of the 75 kDa tumor necrosis factor receptor. Cell. 1994;78:681–692. doi: 10.1016/0092-8674(94)90532-0. [DOI] [PubMed] [Google Scholar]
- 53.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 54.Schouten G J, Vertegaal A C, Whiteside S T, Israel A, Toebes M, Dorsman J C, van der Eb A J, Zantema A. IκBα is a target for the mitogen-activated 90 kDa ribosomal S6 kinase. EMBO J. 1997;16:3133–3144. doi: 10.1093/emboj/16.11.3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Semmes O J, Jeang K T. Mutational analysis of human T-cell leukemia virus type I Tax: regions necessary for function determined with 47 mutant proteins. J Virol. 1992;66:7183–7192. doi: 10.1128/jvi.66.12.7183-7192.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Smith M R, Greene W C. Identification of HTLV-I tax trans-activator mutants exhibiting novel transcriptional phenotypes. Genes Dev. 1990;4:1875–1885. doi: 10.1101/gad.4.11.1875. [DOI] [PubMed] [Google Scholar]
- 57.Smith M R, Greene W C. Molecular biology of the type I human T-cell leukemia virus (HTLV-I) and adult T-cell leukemia. J Clin Investig. 1991;87:761–766. doi: 10.1172/JCI115078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Smith M R, Greene W C. Type I human T cell leukemia virus tax protein transforms rat fibroblasts through the cyclic adenosine monophosphate response element binding protein/activating transcription factor pathway. J Clin Investig. 1991;88:1038–1042. doi: 10.1172/JCI115364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Song H Y, Regnier C H, Kirschning C J, Goeddel D V, Rothe M. Tumor necrosis factor (TNF)-mediated kinase cascades: bifurcation of nuclear factor-κB and c-jun N-terminal kinase (JNK/SAPK) pathways at TNF receptor-associated factor 2. Proc Natl Acad Sci USA. 1997;94:9792–9796. doi: 10.1073/pnas.94.18.9792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sturgill T W, Ray L B, Erikson E, Maller J L. Insulin-stimulated MAP-2 kinase phosphorylates and activates ribosomal protein S6 kinase II. Nature. 1988;334:715–718. doi: 10.1038/334715a0. [DOI] [PubMed] [Google Scholar]
- 61.Sun S, Elwood J, Greene W C. Both amino- and carboxyl-terminal sequences within IκBα regulate its inducible degradation. Mol Cell Biol. 1996;16:1058–1065. doi: 10.1128/mcb.16.3.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sun S C, Elwood J, Beraud C, Greene W C. Human T-cell leukemia virus type I Tax activation of NF-κB/Rel involves phosphorylation and degradation of IκBα and RelA (p65)-mediated induction of the c-rel gene. Mol Cell Biol. 1994;14:7377–7384. doi: 10.1128/mcb.14.11.7377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Suzuki T, Fujisawa J I, Toita M, Yoshida M. The trans-activator tax of human T-cell leukemia virus type 1 (HTLV-1) interacts with cAMP-responsive element (CRE) binding and CRE modulator proteins that bind to the 21-base-pair enhancer of HTLV-1. Proc Natl Acad Sci USA. 1993;90:610–614. doi: 10.1073/pnas.90.2.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tanaka A, Takahashi C, Yamaoka S, Nosaka T, Maki M, Hatanaka M. Oncogenic transformation by the tax gene of human T-cell leukemia virus type I in vitro. Proc Natl Acad Sci USA. 1990;87:1071–1075. doi: 10.1073/pnas.87.3.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tie F, Adya N, Greene W C, Giam C Z. Interaction of the human T-lymphotropic virus type 1 Tax dimer with CREB and the viral 21-base-pair repeat. J Virol. 1996;70:8368–8374. doi: 10.1128/jvi.70.12.8368-8374.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Uchiyama T. Human T cell leukemia virus type I (HTLV-I) and human diseases. Annu Rev Immunol. 1997;15:15–37. doi: 10.1146/annurev.immunol.15.1.15. [DOI] [PubMed] [Google Scholar]
- 67.Van Aelst L, D’Souza-Schorey C. Rho GTPases and signaling networks. Genes Dev. 1997;11:2295–2322. doi: 10.1101/gad.11.18.2295. [DOI] [PubMed] [Google Scholar]
- 68.Verma I M, Stevenson J K, Schwarz E M, Van A D, Miyamoto S. Rel/NF-κB/IκB family: intimate tales of association and dissociation. Genes Dev. 1995;9:2723–2735. doi: 10.1101/gad.9.22.2723. [DOI] [PubMed] [Google Scholar]
- 69.Wagner S, Green M R. HTLV-1 Tax protein stimulation of DNA binding of bZIP proteins by enhancing dimerization. Science. 1993;262:395–399. doi: 10.1126/science.8211160. [DOI] [PubMed] [Google Scholar]
- 70.Wano Y, Feinberg M, Hosking J B, Bogerd H, Greene W C. Stable expression of the tax gene of type I human T-cell leukemia virus in human T cells activates specific cellular genes involved in growth. Proc Natl Acad Sci USA. 1988;85:9733–9737. doi: 10.1073/pnas.85.24.9733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wesche H, Henzel W J, Shillinglaw W, Li S, Cao Z. MyD88: an adapter that recruits IRAK to the IL-1 receptor complex. Immunity. 1997;7:837–847. doi: 10.1016/s1074-7613(00)80402-1. [DOI] [PubMed] [Google Scholar]
- 72.Woronicz J D, Gao X, Cao Z, Rothe M, Goeddel D V. IκB kinase-β: NF-κB activation and complex formation with IκB kinase-α and NIK. Science. 1997;278:866–869. doi: 10.1126/science.278.5339.866. [DOI] [PubMed] [Google Scholar]
- 73.Xu X, Heidenreich O, Kitajima I, McGuire K, Li Q, Su B, Nerenberg M. Constitutively activated JNK is associated with HTLV-1 mediated tumorigenesis. Oncogene. 1996;13:135–142. [PubMed] [Google Scholar]
- 74.Yamaoka S, Inoue H, Sakurai M, Sugiyama T, Hazama M, Yamada T, Hatanaka M. Constitutive activation of NF-κB is essential for transformation of rat fibroblasts by the human T-cell leukemia virus type I Tax protein. EMBO J. 1996;15:873–877. [PMC free article] [PubMed] [Google Scholar]
- 75.Yan M, Dai T, Deak J C, Kyriakis J M, Zon L I, Woodgett J R, Templeton D J. Activation of stress-activated protein kinase by MEKK1 phosphorylation of its activator SEK1. Nature. 1994;372:798–800. doi: 10.1038/372798a0. [DOI] [PubMed] [Google Scholar]
- 76.Yin M J, Gaynor R B. HTLV-1 21 bp repeat sequences facilitate stable association between Tax and CREB to increase CREB binding affinity. J Mol Biol. 1996;264:20–31. doi: 10.1006/jmbi.1996.0620. [DOI] [PubMed] [Google Scholar]
- 77.Yoshida M. HTLV-1 Tax: regulation of gene expression and disease. Trends Microbiol. 1993;1:131–135. doi: 10.1016/0966-842x(93)90127-d. [DOI] [PubMed] [Google Scholar]
- 78.Yoshida M, Miyoshi I, Hinuma Y. Isolation and characterization of retrovirus from cell lines of human adult T-cell leukemia and its implication in the disease. Proc Natl Acad Sci USA. 1982;79:2031–2035. doi: 10.1073/pnas.79.6.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yoshida M, Suzuki T, Fujisawa J, Hirai H. HTLV-1 oncoprotein tax and cellular transcription factors. Curr Top Microbiol Immunol. 1995;193:79–89. doi: 10.1007/978-3-642-78929-8_4. [DOI] [PubMed] [Google Scholar]
- 80.Zandi E, Rothwarf D M, Delhase M, Hayakawa M, Karin M. The IκB kinase complex (IKK) contains two kinase subunits, IKKα and IKKβ, necessary for IκB phosphorylation and NF-κB activation. Cell. 1997;91:243–252. doi: 10.1016/s0092-8674(00)80406-7. [DOI] [PubMed] [Google Scholar]
- 81.Zhao L J, Giam C Z. Human T-cell lymphotropic virus type I (HTLV-1) transcriptional activator, Tax, enhances CREB binding to HTLV-I 21-base-pair repeats by protein-protein interaction. Proc Natl Acad Sci USA. 1992;89:7070–7074. doi: 10.1073/pnas.89.15.7070. [DOI] [PMC free article] [PubMed] [Google Scholar]