Abstract
Objective:
To implement and evaluate a Frailty Care Bundle (FCB) targeting mobilisation, nutrition, and cognition in older trauma patients to reduce hospital associated decline.
Methods:
We used a two group, pretest-posttest design. The FCB intervention was delivered on two orthopaedic wards and two rehabilitation wards, guided by behaviour change theory (COM-B) to implement changes in ward routines (patient mobility goals, nurse assisted mobilisation, mealtimes, communication). Primary outcomes were patient participants’ return to pre-trauma functional capability (modified Barthel Index - mBI) at 6-8 weeks post-hospital discharge and average hospital daily step-count. Statistical analysis compared pre versus post FCB group differences using ordinal regression and log-linear models.
Results:
We recruited 120 patients (pre n=60 and post n=60), and 74 (pre n=43, post n=36) were retained at follow-up. Median age was 78 years and 83% were female. There was a non-significant trend for higher mBI scores (improved function) in the post compared to pre FCB group (OR 2.29, 95% CI 0.98-5.36), associated with an average 11% increase in step-count.
Conclusion:
It was feasible, during the Covid-19 pandemic, for multidisciplinary teams to implement elements of the FCB. Clinical facilitation supported teams to prioritise fundamental care above competing demands, but sustainability requires ongoing attention. ISRCTN registry: ISRCTN15145850 (https://doi.org/10.1186/ISRCTN15145850)
Keywords: Hospital associated decline, Mobility, Nutrition, Orthopaedic
Introduction
Hospital associated decline or deconditioning (HAD) is an under recognised but significant adverse event affecting up to 30-40% of older people during hospitalisation[1,2]. HAD is defined as the onset of a new or a deterioration in a disability during hospitalisation not present at hospital admission and is attributed to the interaction between a patient’s vulnerability (physical function, cognition, acute illness) and hospital processes, ward routines and environment, that compound these vulnerabilities[1,3,4]. People who sustain an acute trauma that impacts mobility and especially if already living with frailty are at high risk of HAD[2]. Older people experience a higher incidence (58%) of traumatic injury including hip fractures and is a leading cause of dependency, increased mortality and morbidity in this population[5]. Globally over 2,000,000 people a year sustain a hip fracture, and in Ireland this figure is 3600 people per year[6]. Recovery from such injury in older people is complicated by age-related physiological decline, higher incidents of long-term conditions, including frailty, and polypharmacy[2,6,7]. Compounding these vulnerabilities are hospital factors that can result in prolonged sedentary behaviour, inadequate nutrition and hydration and hospital acquired delirium[1-3,5-7]. In essence, these are modifiable risk factors (mobility, nutrition and cognitive engagement) for HAD that require proactive care planning and targeted intervention in acute care and rehabilitation settings to optimise older people’s immediate and longer-term recovery. Yet, the ability of nursing and multidisciplinary teams (MDTs) to prioritise these aspects of fundamental care above competing demands receives limited attention[8].
Background
Functional recovery to baseline (pre-injury) capability is one of the main therapeutic goals in trauma care and rehabilitation. Implementation of the National Hip Fracture pathway and audit standards[9] has improved outcomes for this patient group, but the management of older patients with other fractures is not as closely monitored. One-year mortality following a hip fracture has reduced from 30% to 23%[6], but morbidity in terms of reduced mobility, loss of independence in daily activities and poorer quality of life remains high, with 40% of people never regaining their pre-fracture level of mobility[10]. Frail older people experience even worse outcomes with higher levels of post-operative complications, longer hospital stays, and higher levels of institutionalisation[7,10,11].
Optimising recovery for this patient group requires a multilevel pre-perioperative and post-operative response with the consistent implementation of evidence-based practice at every stage of the patient pathway[9]. While improvements have been made in the pre- and perioperative part of the patient pathway, the post-operative phase receives less focus on HAD modifiable factors (mobility, nutrition and cognitive engagement). For example, while there is a hip fracture audit standard for early mobilisation, there is no standard for consistent and progressive mobility, and nutrition and cognition do not form part of the suite of standards. There is also concern that patients with other fractures may not receive the same level of time sensitive attention as hip fracture patients but have equally poor outcomes[12].
There are several factors that impact on the consistent prioritisation and delivery of fundamental care to reduce HAD risk including ward staffing levels, competing priorities, an increased focus on technological aspects of care, and lack of workforce gerontological capability that negatively impacts older patients’ experiences and outcomes[13,14]. There are also system barriers such as rigid mealtimes, lack of age-appropriate diets, and environmental factors (cluttered corridors, poor lighting, lack of equipment and social (non-clinical) spaces for patients) that increase the challenge of delivering fundamental care[15,16].
At a ward level, fundamental care activities that prioritise early and consistent mobilisation (in addition to scheduled physiotherapy), optimal nutrition and hydration, and non-pharmacological management of delirium are key modifiable factors in improving outcomes in older patients[17-19]. A systematic review of in-hospital mobility programs showed that early and consistent mobilisation of older adults improved health outcomes and reduced length of stay[20]. However, single interventions tend to oversimplify solutions for highly heterogeneous and complex populations[21-23]. Undernutrition and malnutrition in patients undergoing surgery are strongly related to poorer outcomes[24]. Yet, in 2019 only 50% of Irish hip fracture patients received a nutritional assessment during their admission[9]. Equally concerning, up to 40% of older patients eat less than half of the food provided thus they do not receive the recommended daily calorie and protein intake[25,26].
A coordinated multidisciplinary response is required to optimise the recovery of older patients following major trauma. A systematic review of interventions to impact hospital associated or functional decline identified 18 intervention studies that primarily involved nursing teams[8]. The majority of the studies reported improvements in patient mobility or nutritional intake, but only four studies targeted the major modifiable risk factors for HAD[8]. A common thread across the studies was the use of multicomponent implementation strategies, the need to tailor the intervention to the ward circumstances, and ward manager leadership[8]. One of the few randomised trials in this area, the CHERISH study implemented a ward-based age-friendly programme targeting mobilisation, nutrition and cognition in four hospitals[27]. There is an urgent need to expand the evidence base and identify pragmatic strategies to enable ward teams to prioritise fundamental care above competing demands, especially as health systems and front-line teams face the new challenge of living with COVID-19.
The study aimed to implement and evaluate a Frailty Care Bundle (FCB) for orthopaedic trauma patients in acute and rehabilitation settings to increase mobilisation, nutrition and cognitive engagement in order to accelerate functional recovery and reduce HAD risk.
The objectives were to:
a) co-produce the FCB intervention components in collaboration with the nursing and multidisciplinary teams,
b) apply evidence-based implementation strategies to embed the FCB into ward routines,
c) measure the effect of the FCB on patient, service and implementation outcomes.
The intervention was delivered over the two years of the COVID-19 pandemic, which forced unanticipated modifications from the original protocol[28].
The study hypothesis I: Using behaviour change strategies ward teams will be able to implement components of a frailty care bundle to increase attention on patient fundamental care (mobilisation, nutrition and cognitive engagement).
Hypothesis II: Consistent implementation of the FCB components by ward teams will increase patient in-hospital mobilisation and nutrition, and thus improve patients’ functional outcomes, defined as a return to pre-injury functional baseline at 6-8 weeks follow-up.
Materials and Methods
The study is reported as per Standards for Transparent Reporting of Non-randomised Designs[29] and Template for Intervention Description and Replication (TIDieR)[30].
We used a two-group pretest-posttest intervention design and a multiple-methods evaluation. The study was informed by the MRC Framework for developing and testing complex interventions across different settings and organisational contexts[31] and was informed by an implementation science approach measuring patient, service and implementation outcomes[32].
Setting
The intension was to deliver the FCB on both surgical and medical older adult wards, this paper reports on the surgical population. The intermittent closure and subsequent conversion of the medical older adult ward to COVID-19 ward and the higher than expected levels of cognitive impairment among the medical population prevented us from recruiting patients for the FCB post intervention evaluation.
The intervention was delivered in two hospitals, across four ward (two wards per hospital) providing care for orthopaedic trauma patients. In the region, patients requiring surgical intervention for orthopaedic trauma are managed in a tertiary referral centre for major trauma (Site 2). All patients are admitted through the emergency department, and following surgery and post-operative recovery, patients requiring orthopaedic rehabilitation are transferred to a nearby elective surgical and rehabilitation hospital (Site 1).
Site 2 has 800 beds and 50 dedicated surgical orthopaedic trauma beds. These dedicated beds are configured as two wards with 62 beds in total (each ward has 31 beds, laid out in four 5-beded rooms and the remainder as single or double rooms). Site 1 has 192 in-patient beds, including 33 dedicated rehabilitation beds across two wards of 15 beds and 18 beds (laid out as triple, double and single rooms).
The four wards received the intervention (no control wards). There was a phased approach to intervention role out and data collection starting with Site 1 (rehabilitation wards), patient level data was collected from September to November 2020, and post intervention data was collected between April to July 2021. On Site 2; pre data was collected between December 2020 and April 2021 and post data between July and November 2021. There was periodic disruption to data collection due to COVID-19.
Participants
The Pre-intervention primarily focused on nursing team (nurses and health care assistants (HCA)) behavior change. As the FCB was regarded as fundamental care, all staff and patients were eligible to receive the intervention. The outcomes were measured at the patient and nursing team level. Nurses and HCAs in permanent posts working on the participating wards were invited to complete pre-post staff survey. To measure patient outcomes, we recruited participants at baseline and following the FCB implementation on each of the wards.
The inclusion criteria were:
Age 60 years or older (age limit was reduced from 65 years in the protocol to increase the pool of patients for recruitment)
Medically stable and able to sit out of bed
Eligible to be mobilised by nursing staff based on physiotherapy assessment
Mobile prior to admission (able to walk across a medium-size room (e.g. 3-4 meters, +/-walking aid) in the two weeks prior to admission
Able to provide written informed consent (no significant cognitive impairment or delirium as measured by 4-AT or recorded in medical notes)
Exclusion criteria:
Unable to mobilise with assistance prior to admission
Can only be mobilised by a physiotherapist
Patients on end-of-life or palliative pathway
Patients who cannot provide informed consent to participate
Sample size
We aimed to recruit a sample size of 180 patients (pre n=90 and post n=90) as per the protocol sample size calculation[26]. We anticipated (prior to COVID-19) it would be possible to recruit this number to provide a between-patient effect size that would allowed us to detect a 40% improvement in average daily step count (using log-linear models) with 80% power.
Intervention
The intervention components and implementation of the FCB were strongly influenced by the ‘EAT, WALK, TALK’ intervention[22]. The FCB principles, outlined below, were tailored to each ward based on a detailed ward situational analysis and input from the nursing, MDT and local implementation group (LIG) (Table 1).
Table 1.
Summary of ward situational analysis.
| Topic | Observations | Actionable Issue |
|---|---|---|
| Mobilisation | Proportion of patients sat out of bed: 83% (min 71%, max 93%) | Mobilisation opportunities were ad-hoc and depended on patient and nurse/HCA. Patient passive – waiting for assistance. Low levels of mobility for exercise. Prolonged period of sitting in chair. |
| Proportion of patients walking 55% (min 44% max 77%) Median proportion time walking 8am-5pm: 8% (min 5%, max 16%) | ||
| Proportion of patients walking for exercise 55% (39%-77%) Median time walking for exercise: 6.5% (min 0, max 25%) | ||
| Median time sitting 8am-5pm: 44% (min 43%, max 62%) | ||
| Nutrition | Eating half or less of meal: 56 % (min 45%, max 68%) | High proportion of food waste. Quality of meals perceived as good, but portion sizes too big. Textured modified diet poorly tolerated. Patients generally received timely feeding assistance. No alternative or snack offered if low intake at mealtime. No snack/hydration rounds available on acute care wards. The nursing teams had limited influence over aspects of nutrition (scheduling of mealtimes, tailoring to patient food preferences, availability of ward-based snacks, drinks/snacks rounds). |
| % of meals where patients sitting out of bed: 64% (min 31%, max 100%) | ||
| % waiting >10 mins for assistance to eat: 1.5% (min 0%, max 2%) | ||
| Meal interruptions: 38% (min 15%, max 55%) | ||
| Cognition | 34% (0 min-83% max) of time resting (doing nothing, but not asleep) | The majority of day spent resting, or sleeping. Most frequent distraction activity was talking on or looking at phone. No visitors due to COVID-19. No non-pharmacological interventions for patients with distressed behaviour. |
| 27% (15-34%) of patients had cognitive impairment, dementia or episodes of distressed behaviour | ||
| Intra nursing & MDT Communication on fundamental care | No record of daily mobility, no mobility goals visible to patient or bedside nursing team. Food charts recorded for high risk patients. MDT board rounds. No nursing team huddles, no discussion on nutrition/mobilisation/cognition unless distressed behaviour disrupting ward activity. Paper based medical records, difficult to locate information/decipher hand writing. | Professional silos - minimal communication between nursing and therapist on individual patient goal setting or action plans. Variable attitude of nurses/HCA role on assisted patient mobility for exercise. Increase opportunities for direct communication between therapist and bedside nursing team |
| Staffing levels | Registered nurse to patient ratio: Site 1= 1: 3.3 (min 1:3. Max 1:4); Site 2 =1: 4.9 (min 1:4.8, max 1:5.2). Healthcare assistant 1-2 per ward. One physiotherapist per ward, six monthly rotations. Nursing staff perceived the main barrier was insufficient staffing levels to meet patient acuity and dependency. Therapists also perceived their service was understaffed. | The FCB intervention had no influence over staffing levels. Nursing staff levels were often unpredictable due COVID-19. Staff experienced fatigue and stress from uncertainty of pandemic, wearing PPE, post-viral fatigue. |
Key: FCB Frailty Care Bundle; MDT multi-disciplinary team; HCA Health Care Assistant; PPE Personal Protective Equipment.
FCB Principles
Early mobilisation:
Mobilisation assessment by physiotherapist within 24 hours of surgery or transfer to rehabilitation
Nurse assisted mobilisation in addition to scheduled physiotherapy sessions
Individualised Patient Mobility goal (aim for minimum of three times a day aligned to baseline function)
Provision of mobilisation assistance (as appropriate) and supervision by nursing and MDT
Enhanced nutrition:
Increase supervision and assistance at mealtimes
Reduce disruption at mealtimes
Nutrition screening and weekly re-appraisal
Increase availability of high protein and calorie food (e.g. enhanced drinks round and protein snacks)
Cognitive engagement:
Increase cognitive engagement activities among patients (talking, on-line games, reading, listening to radio)
Improvement in environment layout to promote orientation and patient mobilisation
Ward situational analysis
In order to tailor intervention components, each ward underwent a detailed situational analysis, involving structured observation audits of patient mobilisation and mealtimes using validated instruments[15,25], observation of interprofessional communication on fundamental care, staff surveys including their suggestions for change, and informal conversations with patients and staff. Data were collected over a six-week baseline period on each ward. Despite the different focus of the acute trauma and rehabilitation wards there were common patterns in the data (Table 1).
During this phase, each site was supported to establish a local implementation group (LIG) comprised of nurse managers from the participating wards, a lead geriatrician, physiotherapy and dietician representative, and catering manager. The group was co-chaired by an Assistant Director of Nursing (ADON) and the FCB facilitator. The ward baseline data was presented to the LIG to inform the FCB intervention and implementation plan. The role of the LIG was also to escalate barriers beyond the control of ward teams and to raise the profile of the project at executive level within each site.
Intervention Implementation
We used the COM-B (communication, opportunity, motivation) behaviour change theory and the Integrated Promoting Action on Research Implementation in Health Services (i-PHAISH) framework to guide implementation strategies and project management[33,34]. There are overlapping principles between these theories. COM-B focuses on behaviour change at the individual or team level[33]. The i-PARIHS framework describes facilitation (role and process) as the mechanism for change to align the innovation (intervention) with recipients’ capacity for change within local ward and organisational context and external health system context[34].
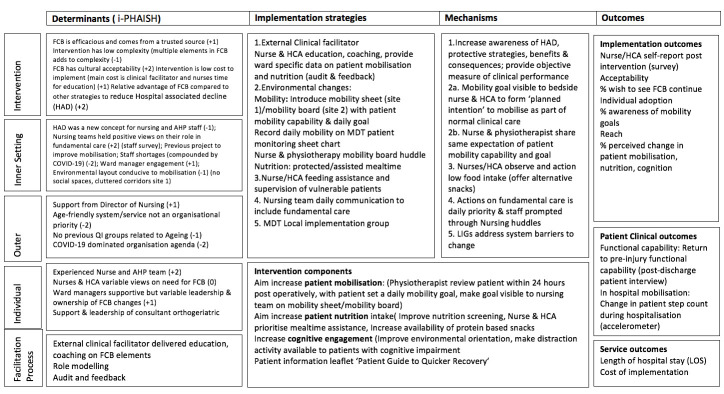
An updated project Logic model (Figure 1) summarises the determinates, intervention components, implementation strategies, mechanism of action and outcome measures[35].
Figure 1.
Implementation of Frailty Care Bundle logic model (Smith 2020). The context and determinates are rated on a 5 point scale (-2 to +2) as an indicator of the strength of the factors influence as a barrier (-) or facilitator (+). Key: FCB – Frailty Care Bundle; HAD – Hospital Associated Decline; AHP – Allied Health Professional; QI – Quality Improvement; MDT – Multidisciplinary Team; HCA – Health Care Assistant; LIGs -Local implementation group; LOS – Length of Stay.
The purpose of orthopaedic trauma care is to restore patient mobilisation and baseline functional capability, thus the priority for change was to increase patients’ opportunity to mobilise through the assistance of the nursing team as an addition to patients’ scheduled physiotherapy. The main nutrition change was to increase the opportunities for food intake through the provision of enhanced snack/hydration rounds (especially protein) combined with re-emphasising assisted mealtime principles. Cognitive engagement changes were introduced on the rehabilitation site, while delirium screening was a priority on the acute care wards and at this time was managed by the orthogeriatric team (not part of the FCB).
Clinical facilitation
The intervention was based on a clinical facilitation model. The facilitator, an experienced nurse funded through the FCB study, worked with ward nursing teams and the MDT to identify the intervention components and implementation strategies. The COM-B theory posits that behaviour change is dependent on the individual or team having the capability (psychological, physical), the opportunity (physical and social) and motivation (reflective and automatic) to change daily practice[33]. A summary of the intervention is provided using the TIDieR checklist (Table 2). The active implementation phase in site 1 was eight weeks, while the larger site 2, implementation was concentrated over 12 weeks, but there were episodic interruptions due to COVID-19.
Table 2.
TIDieR summary intervention components.
| Brief Name of the Intervention | Frailty Care Bundle (FCB) |
|---|---|
| Why |
Aim: To increase older patient mobilisation, nutrition intake and cognitive engagement following surgery for orthopaedic trauma. Proposed mechanism: To enhance nursing and MDT strategies to prioritise fundamental care above competing demands: a) Mobilisation- set patient daily mobility goal, share goal (make visible) to patient and bedside nursing team. b) Nutrition: increase nurse supervision & assistance during mealtimes, action low intake in real time by offering snacks (especially protein). c) Increase cognitive distraction, positive engagement for patients especially patients with cognitive impairment. Theory/Framework: COM-B behaviour change model. |
| What (Materials & procedure) | A Clinical facilitator worked with ward teams to introduce changes, provided feedback on individual ward baseline data and education (consequences of low mobility/undernutrition, practical strategies based on staff suggestions and literature). Mobility: Site 1: individual Patient Mobility Sheet (physiotherapist & patient agree & write daily mobility goal; patient/nurse record highest level of mobility each day). Modification: Site 2 Mobility board on wall inside the room of each six bedded bay. Physiotherapist & bedside nurse asked to agree patient mobility capability and mobility goal. Material: laminated easy clean sheets; white board & marker; Nutrition: Re-enforce principles of assisted mealtimes, emphasise on supervision during mealtimes, noticing & actioning low intake in real time (offer protein snacks). Site 1:catering staff promoted protein snack on hydration rounds. Material: Extra snacks: protein enriched soup at supper, fruit pots trialled. Modification: Site 2 - no hydration rounds- but increased range of snacks on ward (e.g high protein ice-cream). Material: ward freezer to store Ice-cream, high protein ice-cream ordered as ward stock plus additional snacks (custard & rice pots, soft cheese). Cognition: Site 1 only: education for HCA on dementia communication skills. Material: distraction resources, dementia orientation clocks, signage. Nursing communication: one ward manager trialled Team huddles, two wards used end of bed walk-round. Local Implementation group: agree FCB components, address organisational barriers Patients: patient information leaflet ‘Lets Eat Walk and Talk for Recovery’, provided to all patients |
| Who provided | Clinical facilitator was an experienced orthopaedic nurse with a post graduate diploma in gerontological nursing. Coaching was provided on QI & COM-B theory, weekly team de-briefings. Role modelling: consultant geriatrician mobilised patient during ward round, ward managers prompted bedside nurse/physio mobility board meetings. Patients: agreed mobility goals with physiotherapist, encouraged to ask nurse/HCA to assist with meeting goal. Modification: Families due to intermittent/restricted family visits, not involved in study as envisioned in protocol. |
| How is it delivered | Face-to-face ward based, small group education sessions nurses & HCA (40-50 mins). Physiotherapist demonstrated use of mobilisation equipment on request from individual nurse/HCA. HCA Dementia communication training X2 sessions was delivered in class room. |
| Where | Two Rehabilitation wards, two acute trauma wards. |
| When and How Much | Clinical facilitator was present 3-4 days a week for small group and bed-side coaching, prompting use of FCB resources. LIG monthly meetings during baseline and implementation period |
| Tailoring |
Mobility: Site 1: individual patient mobility sheet was used. Site 2: was too busy with a high patient turnover, so a centrally located mobility board was used to display patient mobility goals. Nutrition: Site 1 had more flexibility to introduce nutrition innovations, Site 2 had limited influence over nutrition routines, thus increasing availability of ward snacks was the main change. |
| Modifications | The clinical facilitator doubled the duration of time spent on each site to compensate for disruption due to COVID-19. The FCB had to conform to strict infection control guidance. Families were not involved in intervention. LIG meetings on site2 – reduced to 3 meetings |
| How well | It was feasible to implement FCB changes even with strict infection control measures. FCB was reasonably consistently implemented while the clinical facilitator remained in situ, but once this resource was withdrawn attention on the FCB gradually lessened. COVID-19 negatively impacted the FCB implementation: intermittent outbreaks and ward closures, staff fatigue, and both staff and their managers had limited capacity to engage with something new during this period. Organisational readiness for change: existing organisational structures and ways of working limited implementation & sustainability, AHP six-monthly rotations (especially physiotherapists), part-time and temporary consultant contracts, lack of established QI groups and weak mechanism to address organisational barriers impacted capacity for sustainable change. |
| See supplemental file for all FCB materials developed |
FCB – Frailty Care Bundle; AHP – Allied Health Professional; QI – Quality improvement.
The clinical facilitator, using plan-do-study-act (PDSA) cycles, worked with ward teams to increase patient mobilisation through patient goal setting and improved information exchange between nursing, physiotherapy and medical teams. Using either an individual patient mobilisation sheet (site 1) or mobility (white) board (site 2), patients’ mobility capability (level of assistance and equipment required) and daily mobility goal (e.g. Walk 10 metres, three times a day) was made visible to patients and the bedside nurse/HCA with the expectation that nurse/HCA assisted mobilisation would increase[36]. Nutrition changes focused on the nursing team providing assistance during mealtimes and actioning low food intake (<50% of meal eaten) in real time through offering protein-based snacks (e.g. high protein ice-cream). Where hydration/snack rounds were available, we encouraged catering staff to offer protein-based snacks to patients. These changes were in addition to ward standard practice on nutrition screening and referral to dietitians. Changes to improve positive cognitive engagement in site 1 were concentrated on patients with cognitive impairment and involved dementia communication skills training for HCAs and environmental changes.
Implementation strategies involved small group education, audit and feedback, role modelling by consultant ortho-geriatrician, and ward managers incorporating mobilisation, nutrition and cognition as part of routine nursing communication at nursing huddles or ‘end- of -bed rounds’.
The COVID-19 pandemic forced modifications to the FCB implementation, the duration of clinical facilitation had to be extended on each site to compensate for staff absence due to sickness or isolation, group teaching was reduced to 1-2 members of staff per session, ward managers and their nursing teams were focused on frequently changing infection control guidance, leaving less time and energy for FCB. During this period, visitors were largely absent from the hospitals, thus families were not involved as intended in supporting the FCB.
Data collection
A multiple-methods evaluation was carried out to compare patient outcomes pre and post the FCB implementation[28]. Patient participant data were collected at four time points: Time 1 (T1) (patient self-report activities prior to their injury, T2 on enrolment into the study (once medically stable following surgery), T3 at hospital discharge, and T4 at 6-8 week follow-up via telephone interview.
Outcome measures
A detailed patient demographic and health profile was obtained including the clinical frailty scale (CFS), a clinical frailty index based on 24 items adopted from the Irish Longitudinal Study of Ageing[37], and SARC-F a measure of sarcopenia[38].
The primary patient outcome was the proportion of patients returning to functional (pre-injury) baseline at 6-8 weeks post hospital discharge. Functional capability was measured using the modified Barthel Index (mBI)[39]. Patients’ functional baseline was defined as two weeks prior to admission (before their injury) (T1) and was compared to the mBI at post-discharge follow-up (T4). The main process outcome of interest was patients’ average daily step count (mobilisation) during hospitalisation, measured over 3-4 days on enrollment into the study. The Step Watch Activity Monitor (SAM) attached via a Velcro strap to the patient’s ankle was used as it is validated to capture slow gait speed[40,41].
Secondary outcomes included hand-grip strength, the simple nutrition assessment questionnaire (SNAQ), EQ-5D Visual Analogue Scale for quality of life and 4-metre gait speed[42-45].
In line with i-PARIHS, we measured implementation outcomes (e.g. acceptability, individual adoption, penetration and sustainability) in the nursing staff survey, using a 10-item bespoke questionnaire tailored to the intervention components[32]. Sustainability was examined in interviews with ward mangers and members of the MDT.
Statistical Analysis
Categorical variables are described by their counts and percentages in each category, while continuous variables are presented using their medians and interquartile ranges (IQR). The average daily step count (across all days of observation) was calculated for each patient. Pre versus post differences in average daily step counts was then estimated using a log-linear regression model, adjusted for hospital. The resulting effect estimates are presented as percent changes in the geometric mean of average step counts among patients recruited during the post-intervention phase versus those recruited during the pre-intervention phase. Pre vs Post differences in all other outcomes (changes in mBI, grip strength, SNAQ scores, EQ5D VAS and 4m gait speed) are similarly estimated using ordinal regression models with a logit link (proportional odds models [454]), adjusted for hospital. Effect estimates from these ordinal models are reported as odds ratios (ORs), where values greater than 1.0 reflect a tendency of higher outcome values in the patients recruited in the post-intervention study phase versus those recruited in the pre-intervention phase. These models can be usefully viewed as covariate-adjusted rank-based non-parametric tests of between-group differences that don’t rely on distributional assumptions about the outcomes. All models are estimated on a complete case basis. Given the exploratory nature of the study, all estimates are reported alongside p-values without any adjustment for multiple comparisons.
All analyses were conducted using the R language for statistical computing (Version 4.0.3,) and RStudio (Version 1.4.1106)[45]. Ordinal regression models were estimated using the package rms[46], while tables and plots were respectively constructed using the gtsummary and ggplot2packages[47,48].
Results
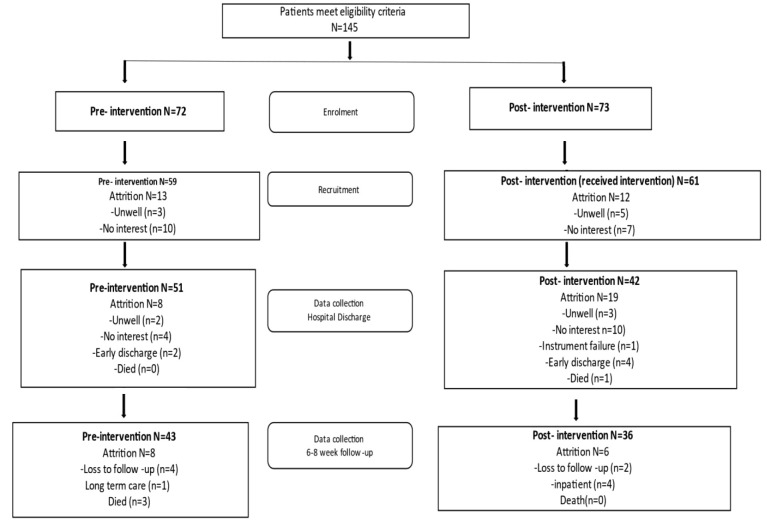
In total, 145 patients were approached, and 120 consented to participate (pre n=60, post n=60), at 6-8 week follow-up (T4) 79 participants remained in the study (pre n=43, post n=36) (Figure 2). The sample size allowed us to test the feasibility of patient recruitment and intervention implementation across different sites, but it was under-powered to detect statistically significant differences. There was a slightly higher attrition from the post-intervention group due to patients withdrawing consent, early discharge, becoming unwell, or loss to follow-up.
Figure 2.
FCB Patient flow diagram (recruitment and reasons for attrition).
The demographic and health profile indicated the pre- and post-intervention cohorts were largely similar, but there was a trend toward an older, frailer population in the post-intervention group (Table 3). The overall median age was 78 years (IQR 73,84), the majority were female (83%), and just over 40% lived alone. Hip fracture (67%) was the most frequent injury. The clinical frailty scale (CFS) indicated both groups were similar for baseline functional capability, but the clinical frailty index (includes chronic conditions, functional and sensory limitations) indicated the post-intervention group were more frail than the pre-intervention group and were prescribed more medication.
Table 3.
Patient demographic and health profile (n=120).
| Study Phase | |||||
|---|---|---|---|---|---|
| Characteristic | N | Overall, N = 120 | Pre, N = 59 | Post, N = 61 | p-value1 |
| Age (years), median (IQR) | 109 | 78 (73, 84) | 79 (73, 85) | 78 (73, 83) | 0.7 |
| Sex | 120 | 0.14 | |||
| Female, % | 83 (69%) | 45 (76%) | 38 (62%) | ||
| Male, % | 37 (31%) | 14 (24%) | 23 (38%) | ||
| Reason for admission | 118 | 0.4 | |||
| Hip fracture/repair, % | 67 (57%) | 37 (63%) | 30 (51%) | ||
| Lower limb fracture/injury, % | 25 (21%) | 12 (20%) | 13 (22%) | ||
| Wound infection/soft tissue injury, % | 15 (13%) | 4 (6.8%) | 11 (19%) | ||
| Pelvic fracture, % | 2 (1.7%) | 2 (3.4%) | 0 (0%) | ||
| Spinal fracture, % | 3 (2.5%) | 2 (3.4%) | 1 (1.7%) | ||
| Upper limb fracture, % | 2 (1.7%) | 1 (1.7%) | 1 (1.7%) | ||
| Other, % | 4 (3.2%) | 1 (1.7%) | 2 (3.4%) | ||
| Living situation | 120 | 0.4 | |||
| Lives alone, % | 50 (42%) | 23 (39%) | 27 (44%) | ||
| Number of chronic conditions, median (IQR) | 120 | 3.00 (2.00, 5.00) | 3.00 (2.00, 4.00) | 4.00 (2.00, 5.00) | 0.095 |
| Number of medications at admission, median (IQR) | 104 | 6.0 (3.0, 9.0) | 5.0 (3.0, 7.0) | 7.0 (5.0, 10.0) | <0.001 |
| SARC-F score, median (IQR) | 117 | 2.00 (1.00, 4.00) | 2.00 (1.00, 3.50) | 2.00 (1.00, 5.00) | 0.4 |
| Clinical Frailty Index (24 items) , median (IQR) | 120 | 0.25 (0.20, 0.33) | 0.23 (0.17, 0.31) | 0.28 (0.21, 0.35) | 0.025 |
| Pre-admission clinical frailty score (CFS) , median (IQR) | 101 | 3.00 (2.00, 5.00) | 3.00 (2.00, 4.00) | 4.00 (3.00, 5.00) | 0.089 |
Wilcoxon rank sum test; Pearson’s Chi-squared test.
Primary Outcome
At baseline, the majority of participants were independent for basic activities of daily living (ADLs) on the mBI, but it was slightly lower in the post-intervention group (Table 4). Post-intervention participants were more likely than pre-intervention participants to report higher mBI scores at T4 (follow-up) relative to pre-admission mBI (OR 2.29, 95% CI 0.98-5.36), indicating a return to pre-injury function, but this had border line statistical significance (p=0.056).
Table 4.
Analysis of modified Bartel Index (mBI) and hospital step count.
| Study Phase | ||||||
|---|---|---|---|---|---|---|
| Characteristic | N | Overall, N = 1201 | Pre, N = 591 | Post, N = 611 | Odds Ratio | p-value |
| mBI2 pre-admission | 110 | 100 [90, 100]; | 100 [92, 100] | 95 [90, 100] | 0.56 (0.27 - 1.16)3 | 0.12 |
| mBI2 on enrolment to study (T 1) | 108 | 60 [50, 70] | 65 [45, 70]; | 60 [50, 70] | 1.24 (0.64-2.39) 3 | 0.525 |
| mBI2 6-8 weeks post discharge(T 4 ) | 79 | 90 [80, 100] | 90 [80, 100] | 90 [89, 100] | 1.72 (0.78-3.82) 3 | 0.182 |
| Change in mBI pre-admission to post-discharge | 73 | -5 [-15, 0]; | -5 [-15, 0] | 0 [-5, 0] | 2.29 (0.98 -5.36) 3 | 0.056 |
| mBI recovery (proportion of patients returning to baseline function) | 73 | 36 (49%) | 16 (41%) | 20 (59%) | 2.03 (0.8-5.2) 3 | 0.138 |
| Step count during hospitalisation | 119 | 336 (174,568) | 324 (163, 529) | 371 (192, 816) | 1.11 (0.72-1.70)4 | 0.63 |
| Site 1 step count | 53 | 443 (221, 727) | 470 (368,555) | 295 (203,877) | 0.78 (0.49-1.25) 4 | 0.30 |
| Site 2 step count | 66 | 265 (133,523) | 190 (116,351) | 386 (186,742) | 1.47 (0.75-2.87) 4 | 0.26 |
Median [IQR]; (Range); n (%);
mBI - Modified Barthel Index;
Treatment effect estimated using ordinal regression, adjusted for hospital;
Treatment effect estimated using log-linear regression.
The accelerometer mobility data, indicated there was a non-significant 11% increase in average inhospital daily step count in the post-intervention group compared to the pre-intervention group (Table 4). There was a hospital effect, the acute care wards (site 2) demonstrated a 47% increase in step count, whereas the rehabilitation wards (site 1) saw a reduction in median step count from the pre-intervention period. It should be noted that in site 1 post-intervention data were collected three months after active implementation had stopped (COVID-19 delayed data collection), while, in site 2 the data collection started 4 weeks after clinical facilitation had stopped. There was no significant difference seen in other outcome measures (supplemental file Table S1).
Implementation outcomes and sustainability
In the post intervention staff survey, we achieved a 42% (33/79) response rate. The response rate was likely impacted by COVID-19 outbreaks that resulted in periodic ward closure to research, staff fatigue, sick leave and turnover. Overall, 84% (28/33) of staff felt positive about the FCB, while 48% felt it required ongoing facilitation to sustain the changes (Table 5). The majority of respondents believed there was an increase in patient mobilisation, awareness of mobility goals, nutrition and patient education, but very little change in cognitive engagement activities.
Table 5.
FCB Implementation outcomes (n=33).
| Implementation outcome | Item | No Change, same as before the FCB | Small changes, (inconsistent, depends on who is on shift) | Moderate changes, (happens most of the time) | Significant changes, (nearly always happens) |
|---|---|---|---|---|---|
| N (%) | N (%) | N (%) | N (%) | ||
| Penetration (level of spread within setting) | Patient mobilisation (increase in walking or sit-to-stand) | 3 (2.2) | 15 (11.0) | 11 (8.0) | 3 (2.2) |
| Feasibility (actual fit or utility) | Patients have a daily mobility goal set | 3 (2.2) | 12 (8.8) | 13 (9.6) | 5 (3.7) |
| Individual Adoption (self-reported uptake) | As the nurse, I am informed of / know my patients’ mobility goal (how far & frequency) | 2 (1.5) | 10 (7.4) | 13 (9.6) | 8 (5.9) |
| Penetration (level of spread within setting) | Patient nutrition (increased supervision & monitoring at mealtimes) | 5 (3.7) | 9 (6.6) | 16 (11.8) | 3 (2.2) |
| Penetration (level of spread within setting) | Increase in protein snacks offered if low meal intake | 5 (3.7) | 10 (7.4) | 12 (8.8) | 6 (4.4) |
| Penetration | Patients encourages to keep their mind active (distraction activity) | 16 (11.8) | 3 (2.2) | 11 (8.1) | 3 (2.2) |
| Penetration | Patients with dementia/ delirium have time protected for distraction activities | 16 (11.8) | 5 (3.7) | 8 (5.9) | 4 (2.9) |
| Penetration (level of spread within setting) | Key message/ educate patients on protein intake & mobility for muscle strength & recovery | 7 (5.1) | 10 (7.4) | 12 (8.8) | 4 (2.9) |
| Penetration (level of spread within setting) | Nursing team huddle includes patient nutrition and mobilisation | 5 (3.7) | 3 (2.2) | 13 (9.6) | 8 (5.9) |
| No, it is not required | Not sure, not really a priority for us | Yes, we can sustain the progress on our own | Yes, but we need a dedicated resource to continue | ||
| Acceptability & Sustainability (maintenance, continuation) | Overall, I feel positive about the FCB and would like to see it continue | 1 (0.7) | 2 (1.5) | 12 (8.8) | 16 (11.8) |
The intervention was multicomponent and tailored to each ward context and available resources thus it was difficult to assess fidelity. The most consistent element across all settings was the staff education sessions which were delivered by the FCB clinical facilitator. The completion of the mobility sheet or mobility board was regularly audited with feedback provided to the nursing and physiotherapy team. We also repeated the ward situational analysis audits for mobilisation and mealtimes. The data showed no change in the proportion of food consumed at mealtimes, or the proportion of time spent walking /exercising, but there was a 17% (47% min-94% max) increase in proportion of patients observed mobilising.
While the clinical facilitator remained on the wards, there was a reasonably consistent uptake in the FCB changes, but once the facilitator withdrew, it was more difficult for teams to sustain the changes, in particular professional silo working tended to re-emerge. Some organisation barriers to sustainability were the six-month rotation of therapists, turnover in the nursing and medical teams, and limited influence of the LIGs to address organisational barriers (e.g. lack of hydration rounds, ward nutrition routines, staffing levels).
Discussion
The FCB was a multicomponent intervention designed to reduce the risk of HAD through increasing in-hospital mobilisation, nutrition, and cognitive well-being in older patients.
Hypothesis I: It was feasible for ward teams to introduce new ward practices for some, but not all of the FCB components. Clinical practice around mobilisation and nutrition were easier to influence that cognitive well-being. The FCB utilised pragmatic strategies (goal setting, assisted mealtimes, interprofessional communication) to enable nursing teams with the support of the wider MDT to prioritise fundamental care.
Hypothesis II: It was possible to increase patient average daily step count during hospitalisation in the acute care wards, which may be a critical factor in accelerating patients’ return to baseline functional capability. The post intervention group tended to be more frail than the pre-implementation group, perhaps attributed to deconditioning during the pandemic, thus increasing step count in this group may have required greater effort by patients and ward teams. While we achieved initial changes in practice, sustainability without ongoing clinical facilitation and organisation resources remained a challenge.
The findings in this study align with the growing evidence on fundamental care and its protective effects against HAD[8,50]. Brown et al[51] and Cohen et al[52] increased nurse-led patient mobilisation and significantly decrease the proportion of older patients experiencing persistent functional decline post-discharge. Similarly, Mudge et al demonstrated the effect of a ward-based facilitation model to promote mobilisation, nutrition and cognitive engagement in reducing risk of delirium and the potential to influence other hospital harms, as in our study sustainability remained a key concern in the face of staff turnover and competing organisation priorities[27].
Implications for practice
In hospital-based intervention studies there are recurring barriers to implementing and sustaining changes to evidence-based practice[53]. Staff resource is without doubt the most widely reported barrier and generally difficult to influence within the scope of a time limited research project. Another factor is middle manager (e.g. assistant director of nursing (ADON), ward manager, consultant clinician, therapist team lead) implementation and organisation change leadership[54,55]. In this study, ADON, ward manager and clinician role modelling were crucial in influencing attitudes and front-line nursing and therapists’ behaviour. This included providing structured opportunities, such as nursing huddles to include fundamental care, opportunities for therapists and bedside nurses to set patient goals, and role modelling changes as part of the MDT. An observation in this study is that while ward managers were held accountable for patient harms (falls, pressure ulcers, missed care) they often had very little autonomy to influence key contributing factors. These factors included AHP ward allocation and rotations, nurse staffing levels and skill mix, physical environment, and catering practices[56]. Grealish et al[16] emphasises the responsibility of senior nurse leadership in shifting organisational culture to value fundamental care as important nursing work and a refusal to accept ‘missed care’ and ‘rationed care’ as normalised culture. A misperception is that fundamental care is just the preserve of nursing. Delivering high quality fundamental care requires effective interdisciplinary working, collaborative partnerships with patients, and strong middle manager and executive leadership[57]. External system levers to prioritise fundamental care is likely to require explicit standards incorporated as part of the National Hip Fracture Audit and Standards in Care for Older People in Hospital with appropriate organisation self-monitoring and external accreditation to achieve age-attuned health systems[58,59].
An updated systematic review by Bridges et al[60], identified little improvement in the experiences of older people in acute care over the last 20 years. The review highlighted that ‘The physical and social environment of the hospital positioned many older patients as insignificant and powerless to influence the care they received’ including fundamental care[60]. Fundamental care combined with effective patient communication is at the heart of patient safety and improving outcomes for older people. We saw an increase in step count and improved mBI in acute care wards despite the effects of the COVID-19 pandemic and strict infection control measures which undoubtedly increased the risk of HAD and other adverse events[61]. Championing fundamental care at the micro ward, meso organisation and macro system level is more important than ever as hospitals deal with post-pandemic deconditioning[56,61].
Limitations
Our study was not powered to show a significant effect, and as a pre-post design it cannot establish causation, in addition data collectors were not blinded to the intervention. The data may not be generalisable beyond the current study context. While the intervention included all patients, our evaluation was restricted to patients who were deemed capable of providing informed consent. With ever-increasing numbers of patients with cognitive impairment in our services, we need better ways of capturing routine data on hospital outcomes on mobility, nutrition, continence etc., beyond falls and pressure ulcers. We also need more diverse study designs beyond randomised controlled trials with overly restricted patient recruitment and inflexible interventions to provide the evidence we need to bridge this practice theory gap.
Conclusion
Although COVID-19 and increased levels of cognitive impairment led to under-recruitment and inadequate power, we saw a trend toward a return to pre-injury physical function associated with an increased patient step count on some wards. At the micro ward level, initiating and sustaining change is likely to require ongoing clinical facilitation, implementation leadership capability and interdisciplinary work to innovate and test new ideas. At the meso level and macro level senior leadership and health system regulators need to champion fundamental care as valued work to improve outcomes and refuse to accept missed and rationed care as an acceptable compromise. The FCB requires further refinement and testing with particular attention paid to the third element of the bundle cognition as well as the role of middle and senior managers as change agents influencing age attunded ward and hospital culture.
Data availability
The anonymised data that support the findings of this study are openly available in Zenodo at https://doi.org/10.5281/zenodo.7781454. Additional study documentation can be accessed through an Open Science Framework at https://doi.org/10.17605/OSF.IO/XP8CY.
Ethics approval
The study received ethical approval from the Clinical Research Ethics Committee of the Cork Teaching Hospitals (Ref ECM 3 (d) 12/11/2019).
Consent to participate
Informed consent was obtained from all participants for the evaluation of the FCB. For the nursing and HCA staff, the return of the survey indicated consent to participate. The FCB intervention was regarded as best practice in fundamental care and was thus exempt from individual patient or staff consent to receive the FCB components [31].
Funding
The research project was funded by the Health Research Board of Ireland (Ref APA-2019-009). Funding was also provided by the HSE South West Hospital Group as part of the HRB Applied Partnership funding award. The funders monitor the project progress but do not have any role in intervention implementation, data collection, analysis or interpretation.
Acknowledgements
The research team would like to acknowledge the time, commitment and engagement of patients and staff, especially ward managers for their participation in the study. We would also like to acknowledge members of the local implementation groups and the research steering group and Directors of Nursing for their time and advice.
Appendix
Supplementary files
Resources developed as part of FCB
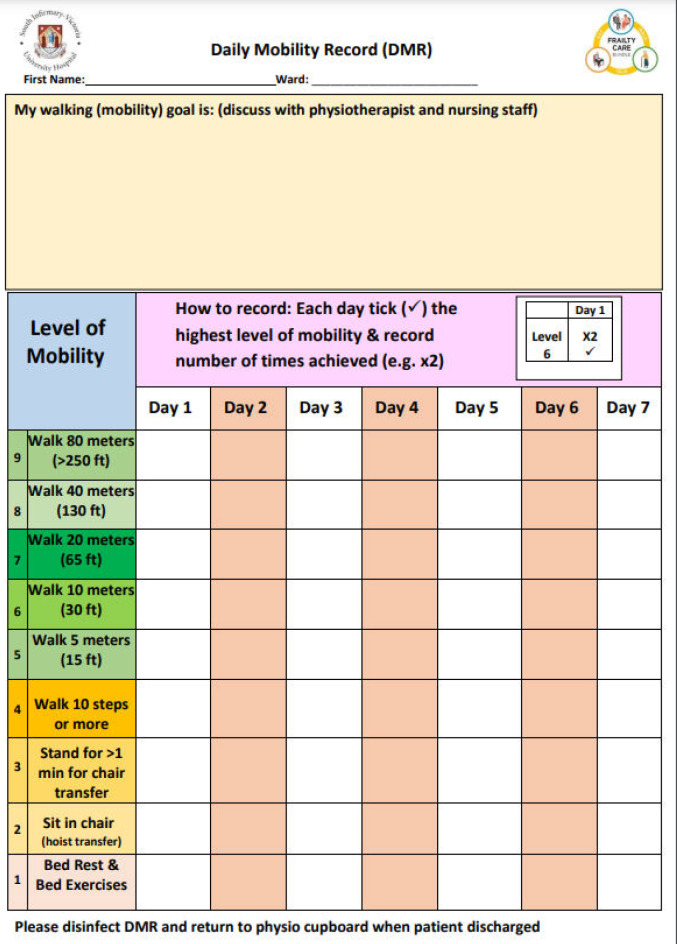
Mobilisation sheet used on Rehabilitation wards
Supplementary Figure 1.
Patient mobilisation sheet tested on rehabilitation ward (not sustained).
Supplementary Figure 2.
Guidance for nurse and physiotherapy teams on patient mobility goal setting.
Tool 2: Mobilisation Board used in Orthopaedic trauma ward
The mobilisation board (white board) contains four columns: Bed number, Current status/capability, daily Goals, completed. The board has been adopted for use on three medical/older adult wards on two sites.
See instructions below of completing board
Supplementary Figure 3.
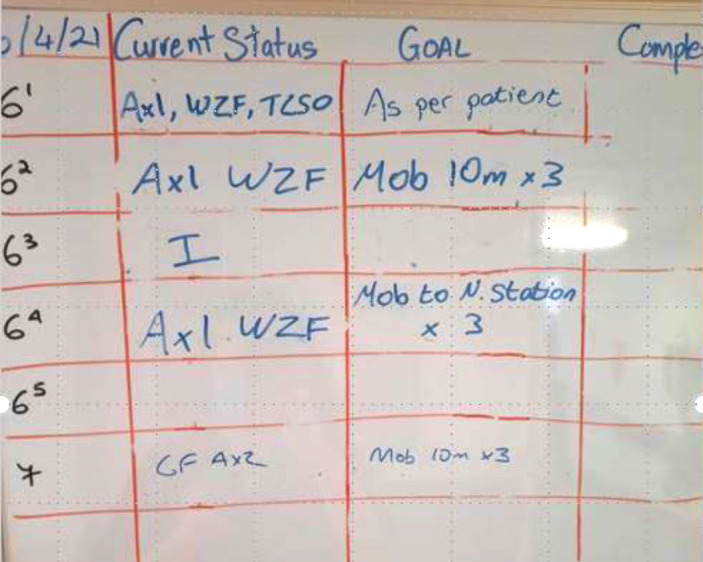
Patient mobility board tested and sustained on acute care wards.
Guidance instructions for completing the mobility board
These instructions are laminated and placed beside the board, ward teams are able to modify the board as required as long as the core information is displayed.
Patient Mobilisation + Goal Setting Mobility board
Assess every patient for mobility capability and assign a capability and any equipment required (e.g. assistance of 1 with Wheel Zimmer frame (ASS*1, WZF))
Every patient for active management should have a mobility/walking goal assigned within 48 hrs of admission or once clinically stable (see table 2 below)
The goal is set by the Nursing Team + Physiotherapist (for pts referred for physio)
The goal is based on the patient’s baseline mobility (2 weeks prior to admission) and in consultation with the patient
Keep the goal simple- see Table 2 for suggestions
The goal is reviewed and amended as required by the nursing team, but at least twice per week
At the end of each day shift, mark on mobility board whether goal competed (C ), not completed (NC) or partially completed (PC) + Date of shift
Supplementary Table 1.
Patient Mobility Assessment (withing 48 hrs of admission).
| Pt Mobility Staus/Capability Equipment | |
|---|---|
| Code | |
| Hoist | |
| STS | Sit to Stand |
| BB T/F | Banana board transfer |
| ASSx1 | Assist of 1 |
| Assx2 | Assist of 2 |
| S | Supervision of 1 or 2 |
| IND | Independent |
| WZF | Wheel Zimmer Frame |
| W/STICK | Walking Stick |
| 3WR | 3 wheel Rollator |
| E/C | Elbow Crutches |
| BB | Banana board |
Supplementary Table 2.
Patient Daily Mobility Goal (within 48 hrs of admission).
| Patient Goal | Example | Outcome | Date of shift | |
|---|---|---|---|---|
| SC | Sit in Chair | Hoist, SC for 2 hrs | Complete (C) | |
| STS | Sit to Stand | BB T/F, Ass*2, | ||
| STS X 2 times | Not Complete | |||
| MTT | Mobilise to toilet | ASS*1, W/Stick | ||
| MTT x 3 times | Partial complete (PC) | |||
| 5 m | Mobilise 5 metres | ASS*2 WZF, | ||
| 5m X 2 times | ||||
| 10 m | Mobilise 10 metres | S*1, 3WR, | ||
| 10m x 3 times | ||||
| Corridor | Mobilise length of corridor | IND, | ||
| Corridor x 3 times |
Supplementary Figure 4.
Patient information leaflet promoting Eat, Walk, Talk.
Footnotes
Edited by: Jagadish K. Chhetri
Authors’ contributions
Corina Naughton: study conceptualisation, methodology, article drafting. Marguerite de Foubert: article drafting, investigation. Helen Cummins: writing review and editing, study administration. Ruth McCullagh: study conceptualisation, methodology and writing review and editing. Teresa Will: writing review and editing. Dawn A. Skelton: methodology, supervision, writing review and editing. Denis O’Mahony: methodology, writing review and editing. Emer Ahern: writing review and editing. Salvatore Tedesco: data analysis. Bridie O Sullivan writing review and editing. Darren Dahly methodology, data analysis and curation. All authors read and approved the final version of the manuscript.
Disclaimer
Prof. Dawn Skelton is co-Editor-in-Chief of the Journal of Frailty, Sarcopenia and Falls. The manuscript underwent peer review process by independent experts.
References
- 1.Zisberg A, Shadmi E, Gur-Yaish N, Tonkikh O, Sinoff G. Hospital-associated functional decline:the role of hospitalisation processes beyond individual risk factors. J Am Geriatr Soc. 2015;63(1):55–62. doi: 10.1111/jgs.13193. [DOI] [PubMed] [Google Scholar]
- 2.Ouellet JA, Ouellet GM, Romegialli AM, Hirsch M, Berardi L, Ramsey CM, Cooney LM, Jr, Walke LM. Functional Outcomes After Hip Fracture in Independent Community-Dwelling Patients. J Am Geriatr Soc. 2019;67(7):1386–1392. doi: 10.1111/jgs.15870. Epub 2019 Apr 9. Erratum in:J Am Geriatr Soc 2020;68(2)450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Grootven B, Jeuris A, Jonckers M, Devriendt E, Dierckx de Casterlé B, Dubois C, Fagard K, Herregods MC, Hornikx M, Meuris B, Rex S, Tournoy J, Milisen K, Flamaing J, Deschodt M. Predicting hospitalisation-associated functional decline in older patients admitted to a cardiac care unit with cardiovascular disease:a prospective cohort study. BMC Geriatr. 2020;20(1):112. doi: 10.1186/s12877-020-01510-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen Y, Almirall-Sánchez A, Mockler D, Adrion E, Domínguez-Vivero C, Romero-Ortuño R. Hospital-associated deconditioning:Not only physical, but also cognitive. Int J Geriatr Psychiatry. 2022;37(3):1–11. doi: 10.1002/gps.5687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Office of Clinical Audit (NOCA) 2019 Major Trauma Audit, Dublin Ireland. [Accessed 16/05/2023]. https://www.noca.ie/audits/major-trauma.
- 6.Downey C, Kelly M, Quinlan JF. Changing trends in the mortality rate at 1-year post hip fracture-a systematic review. World J Ortho. 2019;10(3):166. doi: 10.5312/wjo.v10.i3.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma Y, Wang A, Lou Y, Peng D, Jiang Z, Xia T. Effects of Frailty on Outcomes Following Surgery Among Patients With Hip Fractures:A Systematic Review and Meta-Analysis. Front Med (Lausanne) 2020;9:829762. doi: 10.3389/fmed.2022.829762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Foubert M, Cummins H, McCullagh R, Brueton V, Naughton C. Systematic review of interventions targeting fundamental care to reduce hospital-associated decline in older patients. J Adv Nurs. 2021;77(12):4661–78. doi: 10.1111/jan.14954. [DOI] [PubMed] [Google Scholar]
- 9.National Office of Clinical Audit 2019 Irish Hip Fracture Database National Report 2018. Dublin. [Accessed 12/11/2023]. https://www.noca.ie/audits/irish-hip-fracture-database.
- 10.Dyer SM, Crotty M, Fairhall N, Magaziner J, Beaupre LA, Cameron ID, Sherrington C. A critical review of the long-term disability outcomes following hip fracture. BMC Geriatrics. 2016;16(1):1–18. doi: 10.1186/s12877-016-0332-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen CL, Chen CM, Wang CY, Ko PW, Chen CH, Hsieh CP, Chiu HC. Frailty is associated with an increased risk of major adverse outcomes in elderly patients following surgical treatment of hip fracture. Scient Rep. 2019;9(1):1–9. doi: 10.1038/s41598-019-55459-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gosch M, Druml T, Nicholas JA, Hoffmann-Weltin Y, Roth T, Zegg M, Blauth M, Kammerlander C. Fragility non-hip fracture patients are at risk. Arch Orthop Trauma Surg. 2015;135(1):69–77. doi: 10.1007/s00402-014-2115-4. [DOI] [PubMed] [Google Scholar]
- 13.Barnicot K, Allen K, Hood C, Crawford M. Older adult experience of care and staffing on hospital and community wards:a cross-sectional study. BMC Health Serv Res. 2020;20(1):583. doi: 10.1186/s12913-020-05433-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schubert M, Ausserhofer D, Bragadóttir H, Rochefort CM, Bruyneel L, Stemmer R, et al. Interventions to prevent or reduce rationing or missed nursing care:A scoping review. J Advan Nurs. 2021;77(2):550–64. doi: 10.1111/jan.14596. [DOI] [PubMed] [Google Scholar]
- 15.Mudge AM, McRae P, McHugh K, Griffin L, Hitchen A, Walker J, et al. Poor mobility in hospitalised adults of all ages. J Hosp Med. 2016;11(4):289–91. doi: 10.1002/jhm.2536. [DOI] [PubMed] [Google Scholar]
- 16.Grealish L, Ranse K, Todd JA, Armit L, Billett S, Collier L, Bail K, Moyle W. Barriers and enablers to embedding fundamental nursing care for older patients-Implications of a mixed methods study for nursing leadership. J Adv Nurs. 2022;14:1162–1173. doi: 10.1111/jan.15194. [DOI] [PubMed] [Google Scholar]
- 17.Lafont C, Gérard S, Voisin T, Pahor M, Vellas B. Reducing “iatrogenic disability”in the hospitalised frail elderly. J Nutr Health Aging. 2011;15(8):645–60. doi: 10.1007/s12603-011-0335-7. [DOI] [PubMed] [Google Scholar]
- 18.Lafrenière S, Folch N, Dubois S, Bédard L, Ducharme F. Strategies used by older patients to prevent functional decline during hospitalisation. Clin Nurs Res. 2017;26(1):6–26. doi: 10.1177/1054773815601392. [DOI] [PubMed] [Google Scholar]
- 19.Burton JK, Craig LE, Yong SQ, Siddiqi N, Teale EA, Woodhouse R, et al. Non-pharmacological interventions for preventing delirium in hospitalised non-ICU patients. Cochrane Database Syst Rev. 2021;21(7):CD013307. doi: 10.1002/14651858.CD013307.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smart DA, Dermody G, Coronado ME, Wilson M. Mobility programs for the hospitalised older adult:A scoping review. Gerontol Geriatr Med. 2018;4:1–17. doi: 10.1177/2333721418808146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu SY, Li C, Zhang PX. Enhanced recovery after surgery for hip fractures:a systematic review and meta-analysis. Perioper Med (Lond) 2021;10(1):31. doi: 10.1186/s13741-021-00201-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mudge AM, Banks MD, Barnett AG, Blackberry I, Graves N, Green T, et al. CHERISH (collaboration for hospitalised elders reducing the impact of stays in hospital):Protocol for a multi-site improvement program to reduce geriatric syndromes in older inpatients. BMC Geriatrics. 2017;17(1):11. doi: 10.1186/s12877-016-0399-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCullagh R, O'Connell E, O'Meara S, Dahly D, O'Reilly E, O'Connor K, Horgan NF, Timmons S. Augmented exercise in hospital improves physical performance and reduces negative post hospitalisation events:a randomised controlled trial. BMC Geriatr. 2020;20(1):46. doi: 10.1186/s12877-020-1436-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mills SR, Wilcox CR, Ibrahim K, Roberts HC. Can fortified foods and snacks increase the energy and protein intake of hospitalised older patients?A systematic review. J Human Nutr Diet. 2018;31(3):379–89. doi: 10.1111/jhn.12529. [DOI] [PubMed] [Google Scholar]
- 25.Young A, Allia A, Jolliffe L, de Jersey S, Mudge A, McRae P, et al. Assisted or protected mealtimes?exploring the impact of hospital mealtime practices on meal intake. J Adv Nurs. 2016;72(7):1616–25. doi: 10.1111/jan.12940. [DOI] [PubMed] [Google Scholar]
- 26.Naughton C, Simon R, White TJ, de Foubert M, Cummins H, Dahly D. Mealtime and patient factors associated with meal completion in hospitalised older patients:An exploratory observation study. J Clin Nurs. 2021;30(19-20):2935–2947. doi: 10.1111/jocn.15800. [DOI] [PubMed] [Google Scholar]
- 27.Mudge AM, McRae P, Banks M, Blackberry I, Barrimore S, Endacott J, et al. Effect of a Ward-Based Program on Hospital-Associated Complications and Length of Stay for Older Inpatients:The Cluster Randomized CHERISH Trial. JAMA Intern Med. 2022;182(3):274–282. doi: 10.1001/jamainternmed.2021.7556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Naughton C, Cummins H, de Foubert M, et al. Implementation of the Frailty Care Bundle (FCB) to promote mobilisation, nutrition and cognitive engagement in older people in acute care settings:protocol for an implementation science study. HRB Open Res. 2022;5:3. https://doi.org/10.12688/hrbopenres.13473.1 Accessed 15/11/2023. [Google Scholar]
- 29.Center for Disease Control and Prevention. Transparent Reporting of Evaluations with Nonrandomized Designs (TREND) [Accessed 08/07/2023]. https://www.cdc.gov/trendstatement/index.html.
- 30.Campbell M, Katikireddi SV, Hoffmann T, Armstrong R, Waters E, Craig P. TIDieR-PHP:a reporting guideline for population health and policy interventions, explanation and elaboration. BMJ. 2018;360:k1079. doi: 10.1136/bmj.k1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O'Cathain A, Croot L, Duncan E, Rousseau N, Sworn K, Turner KM, et al. Guidance on how to develop complex interventions to improve health and healthcare. BMJ Open. 2019;9(8):e029954. doi: 10.1136/bmjopen-2019-029954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Proctor E, Silmere H, Raghavan R, Hovmand P, Aarons G, Bunger A, et al. Outcomes for implementation research:conceptual distinctions, measurement challenges, and research agenda. Adm Policy Ment Health. 2011;38(2):65–76. doi: 10.1007/s10488-010-0319-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Michie S, van Stralen MM, West R. The behaviour change wheel:a new method for characterising and designing behaviour change interventions. Implement Sci. 2011;6:42. doi: 10.1186/1748-5908-6-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harvey G, Kitson A. PARIHS revisited:from heuristic to integrated framework for the successful implementation of knowledge into practice. Implement Sci. 2016;11:33. doi: 10.1186/s13012-016-0398-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith JD, Li DH, Rafferty MR. The Implementation Research Logic Model:a method for planning, executing, reporting, and synthesizing implementation projects. Implement Sci. 2020;15(1):84. doi: 10.1186/s13012-020-01041-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hoyer EH, Friedman M, Lavezza A, Wagner-Kosmakos K, Lewis-Cherry R, Skolnik JL, et al. Promoting mobility and reducing length of stay in hospitalized general medicine patients:A quality-improvement project. J Hosp Med. 2016;11(5):341–7. doi: 10.1002/jhm.2546. [DOI] [PubMed] [Google Scholar]
- 37.O'Halloran A, O'Shea M. Frailty 2016 Frailty. The longitudinal Study on Ageing. [Accessed 20/05/2023]. https://tilda.tcd.ie/publications/reports/pdf/w4-key-findings-report/Chapter%207.pdf.
- 38.Malmstrom TK, Morley JE. SARC-F:a simple questionnaire to rapidly diagnose sarcopenia. JAMDA. 2013;14(8):531–532. doi: 10.1016/j.jamda.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 39.Shah S, Vanclay F, Cooper B. Improving the sensitivity of the Barthel Index for stroke rehabilitation. J Clin Epidemiol. 1989;42(8):703–709. doi: 10.1016/0895-4356(89)90065-6. [DOI] [PubMed] [Google Scholar]
- 40.Resnick B, Nahm ES, Orwig D, Zimmerman SS, Magaziner J. Measurement of activity in older adults:reliability and validity of the Step Activity Monitor. J Nurs Meas. 2001;9(3):275–90. Winter. [PubMed] [Google Scholar]
- 41.McCullagh R, Dillon C, O'Connell AM, Horgan NF, Timmons S. Step-count accuracy of 3 motion sensors for older and frail medical inpatients. Arch Phys Med Rehabil. 2017;98(2):295–302. doi: 10.1016/j.apmr.2016.08.476. [DOI] [PubMed] [Google Scholar]
- 42.Wilson MM, Thomas DR, Rubenstein LZ, Chibnall JT, Anderson S, Baxi A, et al. Appetite assessment:simple appetite questionnaire predicts weight loss in community-dwelling adults and nursing home residents. Am J Clin Nutr. 2005;82(5):1074–81. doi: 10.1093/ajcn/82.5.1074. [DOI] [PubMed] [Google Scholar]
- 43.Dent E, Hoogendijk EO, Visvanathan R, Wright ORL. Malnutrition Screening and Assessment in Hospitalized Older People:a Review. J Nutr Health Aging. 2019;23(5):431–441. doi: 10.1007/s12603-019-1176-z. [DOI] [PubMed] [Google Scholar]
- 44.EuroQol group EQ-5D. [Assessed 15/11/2023]. https://euroqol.org/eq-5d-instruments/eq-5d-5l-about/
- 45.Liu Q, Shepherd BE, Li C, Harrell FE., Jr Modeling continuous response variables using ordinal regression. Stat Med. 2017;36(27):4316–4335. doi: 10.1002/sim.7433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.R Core Team 2020. R:A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. [Accessed 15/11/2023]. URL https://www. R-project.org/
- 47.Harrell F., Jr rms:Regression Modeling Strategies. R package version 6.2-0. 2021. [Accessed 15/11/2023]. https://CRAN. R-project.org/package=rms.
- 48.Sjoberg DD, Whiting K, Curry M, Lavery JA, Larmarange J. Reproducible summary tables with the gtsummary package. The R Journal. 2021;13:570–80. [Google Scholar]
- 49.Wickham H. ggplot2:Elegant Graphics for Data Analysis. Springer-Verlag New York, 2016 [Google Scholar]
- 50.Ley L, Khaw D, Duke M, Botti M. The dose of physical activity to minimise functional decline in older general medical patients receiving 24-hr acute care:A systematic scoping review. J Clin Nurs. 2019;28(17-18):3049–3064. doi: 10.1111/jocn.14872. [DOI] [PubMed] [Google Scholar]
- 51.Brown CJ, Foley KT, Lowman JD, Jr, MacLennan PA, Razjouyan J, Najafi B, et al. Comparison of Posthospitalization Function and Community Mobility in Hospital Mobility Program and Usual Care Patients:A Randomised Clinical Trial. JAMA Intern Med. 2016;176(7):921–7. doi: 10.1001/jamainternmed.2016.1870. [DOI] [PubMed] [Google Scholar]
- 52.Cohen Y, Zisberg A, Chayat Y, Gur-Yaish N, Gil E, Levin C, et al. M. Walking for Better Outcomes and Recovery:The Effect of WALK-FOR in Preventing Hospital-Associated Functional Decline Among Older Adults. J Gerontol A Biol Sci Med Sci. 2019;74(10):1664–1670. doi: 10.1093/gerona/glz025. [DOI] [PubMed] [Google Scholar]
- 53.Cowie J, Nicoll A, Dimova ED, Campbell P, Duncan EA. The barriers and facilitators influencing the sustainability of hospital-based interventions:a systematic review. BMC Health Serv Res. 2020;20(1):588. doi: 10.1186/s12913-020-05434-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Borge RH, Egeland KM, Aarons GA, Ehrhart MG, Sklar M, Skar AS. “Change Doesn't Happen by Itself”:A Thematic Analysis of First-Level Leaders'Experiences Participating in the Leadership and Organizational Change for Implementation (LOCI) Strategy. Adm Policy Ment Health. 2022;49(5):785–797. doi: 10.1007/s10488-022-01199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Birken SA, Currie G. Using organization theory to position middle-level managers as agents of evidence-based practice implementation [published correction appears in Implement Sci 2021 Apr 21;16(1):43] Implement Sci. 2021;16(1):37. doi: 10.1186/s13012-021-01106-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kitson A, Carr D, Conroy T, Feo R, Grønkjær M, Huisman-de Waal G, et al. Speaking Up for Fundamental Care:the ILC Aalborg Statement. BMJ Open. 2019;9(12):e033077. doi: 10.1136/bmjopen-2019-033077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pentecost C, Frost J, Sugg HVR, Hilli A, Goodwin VA, Richards DA. Patients'and nurses'experiences of fundamental nursing care:A systematic review and qualitative synthesis. J Clin Nurs. 2020;29(11-12):1858–1882. doi: 10.1111/jocn.15082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fulmer T, Mate KS, Berman A. The Age-Friendly Health System Imperative. J Am Geriatr Soc. 2018;66(1):22–24. doi: 10.1111/jgs.15076. [DOI] [PubMed] [Google Scholar]
- 59.Health improvement Scotland Ageing and frailty standards for the care of older people. [Accessed 05/12/2023]. https://www.healthcareimprovementscotland.org/our_work/standards_and_guidelines/stnds/ageing_and_frailty_standards.aspx.
- 60.Bridges J, Collins P, Flatley M, Hope J, Young A. Older people's experiences in acute care settings:Systematic review and synthesis of qualitative studies. Int J Nurs Stud. 2020;102:103469. doi: 10.1016/j.ijnurstu.2019.103469. [DOI] [PubMed] [Google Scholar]
- 61.Hoffman GJ, Malani PN, Solway E, Kirch M, Singer DC, Kullgren JT. Changes in activity levels, physical functioning, and fall risk during the COVID-19 pandemic. J Am Geriatr Soc. 2022;70(1):49–59. doi: 10.1111/jgs.17477. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The anonymised data that support the findings of this study are openly available in Zenodo at https://doi.org/10.5281/zenodo.7781454. Additional study documentation can be accessed through an Open Science Framework at https://doi.org/10.17605/OSF.IO/XP8CY.