Abstract
Objective
The global impact of osteoarthritis is growing. Currently no disease modifying osteoarthritis drugs/therapies exist, increasing the need for preventative strategies. Knee injuries have a high prevalence, distinct onset, and strong independent association with post-traumatic osteoarthritis (PTOA). Numerous groups are embarking upon research that will culminate in clinical trials to assess the effect of interventions to prevent knee PTOA despite challenges and lack of consensus about trial design in this population. Our objectives were to improve awareness of knee PTOA prevention trial design and discuss state-of-the art methods to address the unique opportunities and challenges of these studies.
Design
An international interdisciplinary group developed a workshop, hosted at the 2023 Osteoarthritis Research Society International Congress. Here we summarize the workshop content and outputs, with the goal of moving the field of PTOA prevention trial design forward.
Results
Workshop highlights included discussions about target population (considering risk, homogeneity, and possibility of modifying osteoarthritis outcome); target treatment (considering delivery, timing, feasibility and effectiveness); comparators (usual care, placebo), and primary symptomatic outcomes considering surrogates and the importance of knee function and symptoms other than pain to this population.
Conclusions
Opportunities to test multimodal PTOA prevention interventions across preclinical models and clinical trials exist. As improving symptomatic outcomes aligns with patient and regulator priorities, co-primary symptomatic (single or aggregate/multidimensional outcome considering function and symptoms beyond pain) and structural/physiological outcomes may be appropriate for these trials. To ensure PTOA prevention trials are relevant and acceptable to all stakeholders, future research should address critical knowledge gaps and challenges.
Keywords: Knee, Osteoarthritis, Post-traumatic osteoarthritis, Prevention, Randomised controlled trials, Trial design
1. Introduction
Osteoarthritis (OA) affects more than 14% of the world's population [[1], [2], [3]] and is a leading cause of pain, disability and socioeconomic costs [1,2]. The burden of OA is well established and expected to continue to grow [4]. There are no disease modifying drugs (DMOAD) or therapies (DMOAT) to combat OA [5]. This leaves prevention as the primary means available to curb the increasing global impact of OA [6]. The field of OA prevention is relatively young [7].
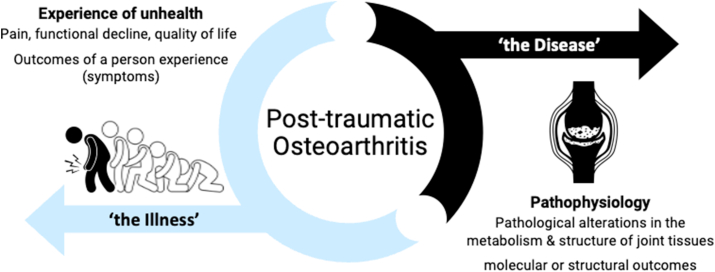
Traumatic knee injury has emerged as an attractive prevention target given it is highly prevalent, has a distinct onset and a strong independent association with future post-traumatic OA (PTOA) [15], which accounts for at least 12% of OA cases globally (i.e., 36 million people) [3]. PTOA can be viewed as both a disease (pathophysiology measured with molecular and structural outcomes) and an illness (experience of unhealth measured as symptoms including pain, functional decline and reduced quality of life; Fig. 1) [7].
Fig. 1.
Post-traumatic osteoarthritis, as both a disease and an illness.
Globally, scientific groups are embarking upon research programs that will culminate in prevention clinical trials targeting people at elevated risk of knee OA following knee injury (i.e., secondary prevention) [[8], [9], [10]]. Common examples of knee injuries include anterior cruciate ligament (ACL) ruptures and acute traumatic meniscal tears. However, there are significant challenges associated with the design of randomised controlled trials (RCTs) seeking to assess the effect of preventative interventions in these populations, and no consensus in the scientific community about how to approach these studies [7].
In an effort to improve awareness of PTOA prevention RCT design and the unique opportunities and challenges presented by seeking to prevent knee PTOA, an international organising group met over an 11-month period and developed a workshop entitled ‘Designing human intervention studies to prevent osteoarthritis after knee injury: an interdisciplinary workshop’. This workshop was hosted during the 2023 Osteoarthritis Research Society International (OARSI) Congress on March 17, 2023, Denver, Colorado, US. This paper synthesises the outputs of this process with the goal of moving the field of OA prevention trial design forward.
2. Purpose and learning objectives
The workshop's overarching aims were to increase awareness, present the current state-of-the-art on trial design, review challenges, and inform a research agenda for designing interventional trials preventing knee PTOA. The ‘a priori’ learning objectives for the workshop were purposefully multifaceted (Fig. 2).
Fig. 2.
Workshop learning objectives.
3. Organizing group and workshop scope
Experts from the knee injury, OA, and prevention fields who represented multiple disciplines and diverse perspectives were invited to join an organising group led by FW to provide insights into the potential challenges associated with knee PTOA prevention trials. This 18-person organising group (spanning biomedical, clinical and health services research) and diversity of age, career stage, gender, culture, and country developed the workshop program. A proposal was submitted to an open call by OARSI for pre-congress workshops. The speaker list and program were finalized by the organising group in conjunction with the OARSI board and congress program committee. The 150-min pre-congress workshop was open to all delegates attending the 2023 OARSI annual congress in-person.
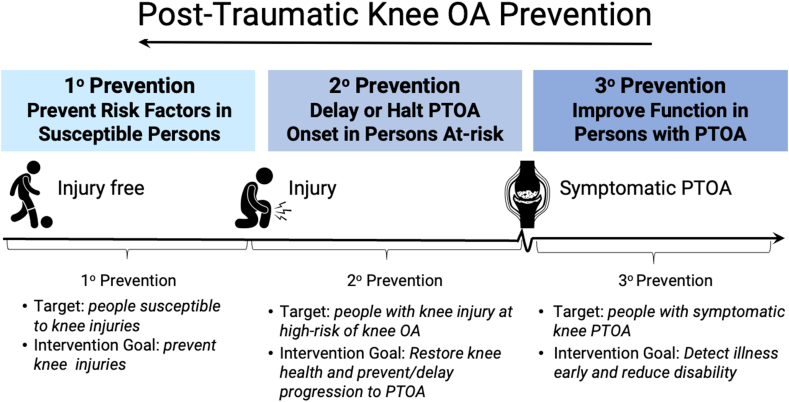
The workshop focused on human studies aiming for secondary prevention (halting, delaying, or reducing symptomatic OA severity after risk factor exposure) of OA after knee injury (Fig. 3). This focus acknowledged that learnings can be taken from pre-clinical and translational models and that knee joint injuries account for the bulk of evidence and majority of the health burden of PTOA (∼83%) [11]. The program consisted of three themed sessions identified by the organising group. Each session included a clarifying question period, with a longer moderated (FW, ME) discussion at the end of the workshop. No pre-workshop assignment or materials were provided. The workshop was not recorded but detailed notes were taken, compiled and reviewed by the speakers and organising group which directly informed this report.
Fig. 3.
Levels of post-traumatic knee osteoarthritis (PTOA) prevention. This workshop was focussed on trials intervening to achieve secondary prevention of PTOA, i.e., at the time of, or after the knee injury occurring.
4. Workshop program
The co-chairs (FW, ME) welcomed attendees and reviewed the workshops' priorities and learning objectives. The ethos for the event was introduced, centered around disciplines and attendees: (1) learning from each other's experience, (2) recognizing the possibility of more rigorous and impactful studies by harmonizing study design and reporting (accepting it is unlikely that one size fits all), and (3) working together to overcome key challenges, barriers, and knowledge gaps for the design and delivery of prevention RCTs.
4.1. Workshop sessions
The first session ‘Preventing post-traumatic OA illness and disease: vision and challenges’ consisted of two presentations. The first, entitled ‘What is PTOA, who develops it and what is the goal of an intervention?’ (JW) addressed the definition of PTOA or what are we trying to prevent; what prevention entails or what we are trying to do, and; who develops knee PTOA or who should we target with prevention trials. The second presentation, entitled ‘Defining the best outcome measures, their timing and relationships: symptoms, structure and molecules’ (SL) focused on candidate trial outcome(s) or endpoint(s) as they relate to measuring OA illness and disease, including surrogate outcome(s) or endpoint(s). See Table 1 for an overview of the speaker and key presentation points for this session.
Table 1.
Preventing post-traumatic osteoarthritis illness and disease: vision and challenges presentation topics, speakers, and key points.
| Topic and speaker | Overview of key presentation points |
|---|---|
|
What Is PTOA, who develops it and what is the goal of an intervention? Dr. Jackie Whittaker, PT, PhD, Associate Professor, University of British Columbia, Canada |
What is PTOA?
|
|
Defining the best outcome measures, their timing and relationships: symptoms, structure and molecules Dr. L. Stefan Lohmander, MD (Orthopaedic Surgery), PhD, Professor Emeritus, Lund University, Lund, Sweden |
Clinical Trial End Points/Outcomes
|
ACL (Anterior cruciate ligament), ACLR (ACL reconstruction), CT (Computerized Tomography), EMA (European Medicines Agency), FDA (Food and Drug Administration), ICOAP (Intermittent and constant OA pain score), IKDC (International knee documentation committee), KOOS4 (weighted average of four Knee injury and osteoarthritis outcome score subscales; pain, symptoms, function in sport and recreation, knee-related quality of life), MRI (magnetic resonance imaging), NRS (numerical rating scale), OA (osteoarthritis), PF (Patellofemoral), TF (Tibiofibular), PRO (patient-reported outcomes), PTOA (post-traumatic osteoarthritis), SF-36 (36-Item Short Form Survey), SF-12 (12-Item Short Form Survey), USI (ultrasound imaging), WOMET (Western Ontario Meniscal Evaluation Tool).
The second session entitled ‘Bridging the gap to clinical trials’ also consisted of two presentations. The first, entitled ‘Considerations for PTOA: A regulatory perspective’ (LS) addressed how therapeutics are approved in the USA including concept endpoints for confirmatory clinical trials in OA, the difference between real-world data and real-world effect, and considerations for prevention trial outcome selection considering current regulatory models. The second presentation, entitled ‘Can pre-clinical models and experimental medicine studies help intervention selection and trial design?’ (NG), covered the most commonly used pre-clinical models in PTOA, current understanding of the pathomechanisms underlying PTOA based on pre-clinical models, strengths and limitations of these models, and future opportunities for experimental medicine and early phase human clinical trials. See Table 2 for an overview of the speaker and key presentation points for this session.
Table 2.
Bridging the gap to clinical trials presentation topics, speakers, and key points.
| Topic and speaker | Overview of key presentation points |
|---|---|
|
Considerations for PTOA: a regulatory perspective Dr. Lee Simon, MD (Rheumatology), Former Division Director of the FDA Analgesic, Anti-inflammatory, Ophthalmologic Drug Products Division, Cambridge Massachusetts, USA |
DMOAD/DMOAT Approval
|
|
Can Pre-clinical Models and Experimental Medicine Studies Help Intervention Selection and Trial Design? Dr. Nicole Gerwin, PhD, Director, Immunology Disease Area at Novartis BioMedical Research, Basel, Switzerland |
Using Pre-clinical Models to Inform Intervention Selection and Trial Design
|
ACL (anterior cruciate ligament), ADAMTS (a disintegrin and metalloproteinase with thrombospondin motifs), DMOAD (disease modifying OA drug), DMOAT (disease modifying OA therapy), ECM (Extracellular Matrix), FDA (Food and Drug Administration), IL1Ra (Interleukin-1 receptor antagonist protein), IL1 (Interleukin-1), IL-10 (Interleukin 10), LNA043 (modified, recombinant version of the human angiopoietin-like 3), MMP (Matrix metalloproteinases), NLRP (Nod-like receptor protein), OA (Osteoarthritis), PTOA (post-traumatic osteoarthritis), RWD (Real World Data), RWE (Real World Evidence), TKA (Total Knee Arthroplasty).
Finally, the third session entitled ‘From around the real world: Current examples of trials, their interventions, and comparators’ consisted of three presentations by speakers who gave examples of current prevention trials purposefully spanning, exercise-based, surgical and pharmaceutical interventions. The first, entitled ‘Exercise/Physical Therapy (SUPER-Knee)’ (AC), presented a rationale for exercise as a therapeutic for knee PTOA prevention and overviewed the ongoing SUpervised exercise-therapy and Patient Education Rehabilitation (SUPER)-Knee trial [10]. The second presentation, entitled ‘Surgical (ROTATE-Trial, COMPARE) (DM) discussed the basis for choosing orthopaedic surgery or exercise-based rehabilitation as therapeutics for knee PTOA prevention. This was followed by an overview of the Study of Traumatic meniscal tears: Arthroscopic Resection vs Rehabilitation (STARR) [12], Conservative vs Operative Methods for Patients with ACL Rupture Evaluation (COMPARE) [13], and Rupture Of The Anterior cruciaTe ligamEnt - an algorithm study (ROTATE) [14] trials. Finally, the third presentation, entitled ‘Pharmacological (OACTN Initiative)’ (DF) proposed a method of participant selection for testing pharmacology agents for knee PTOA prevention [15], and introduced the Arthritis Foundation's Osteoarthritis Clinical Trials Network (OACTN) Initiative. See Table 3 for an overview of the speaker and key presentation points for this session.
Table 3.
From around the real world: Current examples of trials, their interventions and comparators presentation topics, speakers, and key points.
| Topic and speaker | Overview of key presentation points |
|---|---|
|
Exercise/Physical Therapy (SUPER-Knee) Dr. Adam Culvenor PT, PhD, Senior Research Fellow, La Trobe Sport and Exercise Medicine Research Centre, La Trobe University, Melbourne, Australia |
Rationale for exercise-based Interventions
|
|
Surgical (STARR, COMPARE and ROTATE-Trials) Dr. Duncan Mueffels, MD (orthopaedic surgery), PhD |
Rationale for Comparing Surgical and Exercise-based interventions
|
|
Pharmacological (OACTN Initiative) Dr. David Felson MD (rheumatology), MPH, Professor, School of Medicine, Boston University, Boston, Mass, USA |
Rationale for Pharmacological Interventions
|
ACL (Anterior cruciate ligament), ACLR (ACL reconstruction), ACSM (American College of Sports Medicine, AF OACTN (Arthritis Foundation Osteoarthritis Clinical Trials Network), COMPARE (The Conservative versus Operative Methods for Patients with ACL Rupture Evaluation), DMOAD (Disease modifying Osteoarthritis Drugs), DM-Q-9 (Shared decision making questionnaire), EQ-5D-5L (5-level EQ-5D version quality of life questionnaire), GROC (Global Rating of Change Score), IKDC (International Knee Documentation Committee), HRQoL (Health-related quality of life), iMCQ (Medical Consumption Questionnaire), iPCO (Production Consumption Questionnaire), KOOS4 (weighted average of four Knee injury and osteoarthritis outcome score subscales; pain, symptoms function in sport and recreation, knee-related quality of life), MOAKS (MRI OA Knee Score), MOON (Multicenter Orthopedic Outcomes Network), MRI (magnetic resonance imaging), NRS (Numerical rating pain scale), OA (Osteoarthritis), PRO (patient-reported outcomes), PIKASO (Preventing Injured Knees from osteoArthritis Severity Outcomes), PTOA (Post-traumatic osteoarthritis), QALY (Quality-adjusted life year), RCT (Randomized controlled trial), ROM (Routine Outcome Measures questionnaire), ROTATE (Rupture Of The Anterior cruciaTe ligamEnt - an algorithm study), STARR (Study of Traumatic meniscal tears: Arthroscopic Resection vs Rehabilitation), TAM (technology acceptance model), TSK (Tampa Scale of Kinesiophobia questionnaire), VAS (Visual Analogue Scale), WOMET (Western Ontario Meniscal Evaluation Tool).
4.2. Workshop discussions
A summary of the key discussion points for individual sessions, and the overall workshop follow below.
4.2.1. Preventing post-traumatic OA illness and disease: vision and challenges
The questions and discussion that followed the first session's presentations were related to the concept of PTOA illness (i.e., symptomatic OA) and if this is sufficient as a stand-alone outcome; what features might help to identify people most likely to progress to knee PTOA (target trial population); and the need for outcomes specific to the period between injury and OA onset. During the discussion several important concepts arose. First, that pain is not a primary complaint for people who have recovered from a traumatic knee injury, so may not be a responsive outcome. Alternatively, there are other symptoms (e.g., functional loss or lack of confidence in the knee) that are self-reported to be more important [16]. Second, injury and/or surgery type, health-seeking behaviours, and functional status are constructs that might be useful for identifying target trial populations. Current evidence [17] supports that people at greatest risk for progression of symptomatic and structural OA are those with ACL ruptures treated with reconstruction surgery (ACLR) who have concomitant chondral and meniscal lesions, or additional meniscal surgery. Less is known about the relationship between health-seeking behaviours or functional status and progression to knee PTOA. Third, that knee PTOA prevention trial outcomes need to be specific and sensitive to the period between injury and OA onset, as opposed to the period beyond OA diagnosis. This may require the development and testing of new outcomes and/or use of existing outcomes (i.e., IKDC, KOOS) currently accepted by regulators. These existing outcomes have excellent measurement properties in populations that span people living with various traumatic knee injuries, OA and total joint arthroplasty [18,19]. Finally, the discussion emphasized that an intervention's effect on outcomes of OA illness (e.g., how a patient feels, functions and survives) should be the focus of prevention RCTs but that outcomes of OA disease (e.g., structure, pathophysiology) may be important secondary outcomes or in some cases could be considered as a co-primary outcome, depending on the nature of the intervention. Table 1 in the Supplementary File provides a detailed summary of the questions and responses for Session 1.
4.2.2. Bridging the gap to clinical trials
The questions and discussion that followed the second session's presentations were related to the challenge of assessing pain (illness) in animal (rodent) models. More specifically, that validated PTOA animal models tend to focus on structural (histology) outcomes while reliable and accurate pain or functional outcomes for animals that translate directly to human illness (e.g., nociceptive, nociplastic and neuropathic pain) are not straight forward. It was pointed out that structural indicators that correlate with pain such as histological inflammation scores, joint swelling and bone remodelling may usefully inform effects of interventions on human illness [20,21]. It was also highlighted that the field is rapidly evolving and there are emerging behavioral pain assessments and direct assays for pain responses and pathways (electrophysiology, anatomy, omics) [22]. Further, functional MRI can inform us about how the central nervous system is activated. Another key discussion point was that some preclinical research purporting to test the effect of a knee OA intervention, is actually testing PTOA prevention (i.e., the intervention is administered prior to, or around the time of injury) so may be highly relevant to the field [23,24]. Table 2 in Supplementary File provides a detailed summary of the questions and responses for Session 2.
4.2.3. From around the real world: current examples of trials, their interventions and comparators
The questions and discussion that followed the third session's presentations related to challenges encountered when conducting rigorous RCTs in real world settings; the importance of monitoring adiposity after injury; the goal(s) of prevention interventions, and; similarities (or differences) between fast progressing traumatic and non-traumatic OA. Real world challenges discussed included the methods and feasibility of selecting and recruiting a homogenous yet representative sample of people that are likely to progress to PTOA, and screening for structural injury status (e.g., presence or absence of concurrent meniscal and osteochondral injury) prior to enrollment. It was recognized that body mass, and perhaps more specifically fat mass, are important considerations in this population, as injury and prolonged recovery can impact activity levels and result in a vicious cycle of weight gain. With that said, accurate assessment of fat mass is instrument-based (e.g., dual X-ray absorptiometry) which can be costly and increase participation burden which is why it is often not included. One key concept discussed was that the goal (and related design) of prevention intervention trials might not necessarily be to ‘halt’ the onset of PTOA, but rather to ‘flatten the slope’ or ‘delay’ and ‘reduce’ the severity of PTOA so that patients experience as few symptoms and disabilities as possible for as long as possible (decrease the number of ‘young people with old knees’) [25]. Finally, the differences between people with rapidly developing traumatic and rapidly progressing non-traumatic OA were highlighted, with suggestions that they may be fundamentally different sub-groups with different underlying processes at play (definitive evidence is lacking). Table 3 in the Supplementary File provides a detailed summary of the questions and responses for Session 3.
4.2.4. Final group discussion
After the last presentation session, all speakers participated in a broader panel question and answer session, with members of the convening group and audience asked to contribute. The questions that arose during the overall discussion were related to the optimal timing of pharmaceutical interventions after injury, including those that are targeting inflammation, and the best outcomes to measure OA illness in persons with a past ACL rupture, including performance-based and muscle function outcomes. The discussants acknowledged that while ACLR may benefit some, it induces a second insult to the knee joint [26], and it could be beneficial to target aspects of the injury response such as inflammation prior to surgery as part of a complex intervention design. It was also highlighted that orthopaedic surgeons would welcome discovery of new methods to reduce the trauma associated with an ACLR.
Another key theme in the conversation was the importance of selecting a clinically meaningful endpoint for prevention trials that is relevant to participants and clinicians (i.e., OA illness endpoint). In the post-ACLR population this may need to extend beyond pain to include constructs such as knee-related quality of life, the ability to participate in sport and recreational activities, social consequences of injury, fear of re-injury and overall knee satisfaction. It could also be appropriate to measure performance-based and knee muscle (e.g., strength) function, even though regulators have not historically been supportive of function as a primary outcome. It was highlighted that muscle function testing can be resource-intensive as it requires in-person data collection, instrumentation and skilled assessors. Also discussed was the lack of an agreed upon muscle function outcome [27], and limitations of comparing between legs (central changes) or to an often-unknown pre-injury value that may have been suboptimal and predisposed to the injury occurrence. Table 4 in the Supplementary File provides a detailed summary of the questions and responses for the overall discussion.
5. Discussion
5.1. Workshop themes and future directions
Across the presentations and discussion during the workshop, several high-level themes emerged relating to knee PTOA prevention RCT endpoints, target population, and intervention design.
The most consistent and emphasized theme was that prevention trials must prioritize a clinically important symptomatic endpoint, which is demonstrated to be a robust surrogate for OA illness. Stated another way, an outcome that a patient (and regulator) will perceive as meaningful (i.e., the effect of the intervention on how the patient feels, functions and survives). This means that OA illness should be the target of the prevention intervention [7,28]. It was also acknowledged that understanding and measuring the underlying pathophysiology (OA disease) is important. A note of caution was that investigators in preclinical, translational and clinical fields should avoid assuming that modifying pathophysiology or symptoms alone would be the only thing that drives a person's experience. Practically, this might lead to a primary illness outcome and either a co-primary or secondary disease outcome. With respect to what the ideal illness outcome is for this population, it was highlighted on multiple occasions that pain is not a prominent complaint for people in the years following a traumatic knee injury and that it will be important to consider other symptoms, function and knee-related quality of life [16]. This suggests that a single aggregate/multidimensional patient-reported outcome (e.g., KOOS4, IKDC, WOMET) may be most relevant. There may also be relevant pathophysiology outcomes for this population such as structural changes identified through imaging or molecular biomarkers, including those in development. Translational research bridging animal models and human studies may have an important role in further identifying markers of pathophysiology.
Efforts to develop a robust clinical definition of ‘early-stage symptomatic knee OA’ for application to clinical trials are underway [29]. It was noted that a ‘early-stage symptomatic knee OA’ definition must include ‘early-stage symptomatic PTOA’ as there is currently no evidence that PTOA and ‘non-traumatic’ OA differ, other than in diagnostic considerations on MRI interpretation at early points after an injury. This would entail not including age as a diagnostic criterion as seen with OA classification criteria [30] given that knee PTOA commonly presents at a relatively young age. Having an agreed ‘early-stage OA’ outcome (or surrogate), that is valid for individuals being enrolled into PTOA prevention trials is critical for success. Preclinical models where pathophysiology can be followed from injury to established PTOA may help to identify early molecular indicators or predictive biomarkers of PTOA.
Another important theme that arose during the workshop was that the leading target population for prevention trials are people who have experienced an ACL rupture, considering the relative prevalence, homogeneity of injury type, ease of identification and healthcare impact. Based on the existing evidence [17] this could be further narrowed down to individuals with ACL rupture and a concomitant injury (i.e., meniscal or osteo-chondral lesion) or those treated with an ACLR and/or other surgery (i.e., partial or total meniscectomy). It is also possible, although not yet supported by causal evidence, that those who have persistent knee symptoms, a higher body weight or fat mass (either at the time of injury, or in response to the injury), poorer knee function, or who are less physically active (either at the time of injury, or in the longer term) may progress to PTOA more rapidly. These points are relevant for trial inclusion criteria, but also as important factors which could confound outcomes if not considered carefully in trial design.
The heterogeneity of the ACL rupture population was also discussed. There were a number of calls during the workshop for methods to increase homogeneity in trial populations, based on either a specific injury type and/or other features associated with a high knee PTOA risk, balanced against the potential for reversible PTOA disease or illness (i.e., PTOA is not ‘inevitable’) and generalizability to a broader at-risk population. These considerations have direct implications for recruitment (i.e., feasibility) and the scientific and commercial case for intervention development in specific populations, particularly of relevance to pharmacological trials.
Two consistent messages that surfaced from the sharing of novel real-world approaches, were that PTOA prevention RCT design is challenging, not only because there is a considerable time lag between injury and OA onset, but because individual OA risk varies considerably and treating clinicians and patients often have strong beliefs about what the best intervention is. These factors can interfere with recruitment and treatment fidelity. There is emerging evidence that not all people with injury benefit from any one type of intervention (e.g., exercise-based, surgery, pharmaceuticals) [31], and that in the real world several intervention types are typically combined (initially and over time). It is important to not only identify who is most likely to benefit from a particular intervention to facilitate individualized care (personalised medicine), but consider combinations of interventions in trial design (or at least take into account and control for aspects of usual care).
5.2. Knowledge gaps and areas for future research
The organising group noted a number of critical gaps in our current knowledge. These gaps need to be addressed to enable secondary knee PTOA prevention RCTs at scale as an active area of OA research, rather than a niche, high-risk activity which is not recognized by pharma industry or regulators. These gaps were compiled following iterative review by the organising group after the workshop and are outlined in Table 4. Though not exhaustive, this list represents key areas where we believe further evidence and guidance is needed. This knowledge is critical for many aspects of study methodology and would directly benefit efforts to design high quality RCTs with maximum chance of success (where success is a new intervention with regulatory or other relevant approvals, allowing implementation and adoption into routine practice internationally).
Table 4.
Knowledge gaps that impede post-traumatic knee osteoarthritis prevention trials design and delivery.
| Aspect of triala design | Area | Gap |
|---|---|---|
| Population | Eligibility | What participant characteristics/features/markers are best to consider when including participants in trials? |
| Stratification | Should participants be stratified? Should stratification be done at enrollment or after? Should stratification be by participant characteristics (e.g., sex) or factors related to risk of OA, or intervention responsiveness? |
|
| Intervention | Timing | What is the best approach to define the window of opportunity for any given intervention? |
| Delivery | How is the acceptability (participants and providers) of an intervention (including dose and delivery mode) determined? | |
| Underlying mechanism | What are the mechanisms underlying the beneficial effect of interventions (e.g., exercise and lifestyle change, surgery, pharmaceutical) on pain, symptoms and function in persons at increased risk of knee PTOA? | |
| Comparator | Usual care | What is the definition of usual care for people at risk of OA after a traumatic knee injury? How does usual care vary internationally (and what is the best way to cater to this)? |
| Placebo | What is a credible placebo condition (controls for natural history, regression to the mean and contextual effects including attention, without offering a treatment effect) for education and exercise-based interventions? | |
| Other confounders | What is the best way to control for other factors that confound the intervention and outcome relationship (e.g., participant characteristics that change over time, other interventions)? | |
| Outcome | Primary symptomatic outcome | What is the optimum single symptomatic outcome (including composite outcomes) for use in trials that is reliable, valid, sensitive to change, meaningful to patients and clinicians, and acceptable to regulators? |
| Surrogate outcomes | What is the optimal method to define the transition from joint injury to early OA (relevant to eligibility criteria, end-point(s)) to ensure surrogate outcome(s) correlate with the stages of this transition appropriately? |
OA (osteoarthritis), PTOA (post-traumatic osteoarthritis).
The term trial throughout this table refers to secondary PTOA prevention trials.
On reflection it is also important to highlight that some noteworthy topics were not discussed during the workshop. This included the involvement of people with lived experience of knee injury and/or knee PTOA in prevention trial design, to maximise study feasibility and acceptability. Similarly, the role of feasibility [32], proof-of-concept [33] or experimental medicine studies to aid definitive effectiveness trial design (or hybrid effectiveness and implementation designs [34]) to reduce the ‘evidence to practice gap’ were not explored. To overcome the disproportionate impact of knee OA experienced by some social groups it will be important to better understand the role of social determinants of health, including sex and gender, as they relate to risk and outcomes for knee joint injury [35,36] and PTOA [37] so that targeted interventions can be developed. Other important topics that did not garner much detailed discussion were novel imaging, biomechanical or molecular-based assessments, either to identify trial target populations or as early (surrogate) outcomes.
6. Summary
The workshop was met with a great deal of optimism by the audience, speakers and organising group regarding the potential for design and delivery of PTOA prevention trials. There was widespread recognition of the significant opportunities associated with preventing knee PTOA particularly the ability to identify and test interventions in preclinical models, creating opportunities for true bench-to-bedside translation.
Joint pain may not be the best symptomatic primary-endpoint/outcome for knee PTOA prevention RCTs and it will be important to consider other symptoms and functional outcomes. Aggregate or multidimensional outcomes may offer advantages. Targeting and measuring symptomatic PTOA outcomes would align with patient priorities and regulator needs based on experience of successful labels for OA and other aligned conditions. But this should not ignore the potential positive (or negative) effects on underlying disease process outcomes. An interdisciplinary approach was considered essential in developing the field successfully.
Author contributions
FW conceived the idea of the workshop and worked with others in the organising group to develop the bid, agenda and workshop. JW, RK and FW drafted the manuscript. All authors (organising group members and invited speakers) reviewed the minutes from the workshop and contributed to and reviewed the manuscript prior to submission.
Funding
FW is a recipient of a fellowship from UK Research & Innovation, a UK government supported research which includes the Medical Research Council (MR/S016538/1, MR/S016538/2 and MR/Y003470/1). This fellowship directly supported FW and RK in their time in coordinating all aspects of this work and in the writing of the manuscript. JW is supported by an Arthritis Society (Canada) STARS career development award (STAR 19–0493) and Michael Smith Health Research BC Scholar award (SCH-2020-0403). AC is a recipient of a National Health and Medical Research Council (NHMRC) of Australia Investigator Grant (GNT2008523). PC is supported in part by the United Kingdom National Institute for Health and Care Research (NIHR) Leeds Biomedical Research Centre. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health. The Centre for Sport, Exercise and Osteoarthritis Research Versus Arthritis (funded by Versus Arthritis, 21595) covered limited speaker travel costs associated with the workshop.
Funding sources had no role in the review or contents of this manuscript.
Organising/convening group affiliations
James Bilzon (representing CSEOA Research Versus Arthritis).
Philip Conaghan (PC) (representing ARUK∗ OA CSG expert working group).
Kay Crossley (KC) (representing OPTIKNEE, SEPA).
Adam CulvenorΨ (AC) (representing OPTIKNEE, SEPA), George Dodge (GD) (representing OARSI Strategic Alliance Committee).
Martin Englund (ME) (representing OARSI and ORS and member of ARUK∗ OA CSG expert working group).
David Felson (DF) (representing AF OACTN, ARUK∗ OA CSG expert working group).
Nicole Gerwin (Novartis).
Alan Getgood (AG) (representing AOSSM, ISAKOS, ACL study group).
Stefan Lohmander (representing OARSI Classification of Early OA taskforce; OPTIKNEE; ARUK∗CSG expert working group, CSEOA Scientific Advisory Board).
Xiaojuan Li (XL) (representing AF OACTN).
Elena Losina (EL) (representing AF OACTN).
Deborah Mason (DJM) (representing BORS pre-clinical science; ICORS; ARUK∗ OA CSG expert working group; Versus Arthritis Research Advisory Group).
Duncan Meuffels (DM) (representing Dutch Sport Orthopaedic Association and the Dutch Arthroscopic Society; ISAKOS).
Brian Pietrosimone (BP) (representing AF OACTN).
May Arna Risberg (MR) (representing OPTIKNEE, SEPA; CSEOA Scientific Advisory Board).
Frank Roemer (FR) (representing ARUK∗ OA CSG expert working group).
Lee Simon (representing SDG LLC).
Fiona Watt (FW) (representing NIHR MSK TRC, ARUK∗ OA CSG expert working group; Centre for OA Pathogenesis Versus Arthritis).
Jackie Whittaker (representing OPTIKNEE, SEPA, and Arthritis Research Canada).
AF OACTN (Arthritis Foundation Osteoarthritis Clinical Trials Network); AOSSM (American Orthopaedic Society for Sports Medicine); ARUK OA CSG (Arthritis Research UK (now known as Versus Arthritis) OA and crystal diseases Clinical Study Group); BORS (British Orthopaedic Research Society); CSEOA (Centre for Sports Exercise & Osteoarthritis); ICORS (International Combined Orthopaedic Research Society); ISAKOS (International Society of Arthroscopy, Knee Surgery and Orthopaedic Sports Medicine); OARSI (Osteoarthritis Research Society International); OPTIKNEE (International consensus group for optimizing knee health after injury to prevent knee osteoarthritis); ORS (Orthopaedic Research Society); SEPA (OARSI special interest group for osteoarthritis prevention related to Sport, Exercise, and Physical Activity).
∗ARUK, Arthritis Research UK, a UK Musculoskeletal charity now known as Versus Arthritis
ΨAC wishes to highlight that La Trobe University's SUPER-Knee program is not to be confused with the University of Newcastle's SuPeR Knee. Support. Predict. Recover® program (www.centrerehabinnovations.com.au).
Declaration of competing interest
JW is a senior editor with the Journal of Orthopaedic and Sports Physical Therapy and associate editor with the British Journal of Sports Medicine.
AC is an associate editor of British Journal of Sports Medicine and Osteoarthritis and Cartilage.
FW is an associate editor of Osteoarthritis & Cartilage. In the last 3 years she has received consulting fees from Pfizer. FW is chair of the PARIS Trial Oversight Committee (unremunerated). FW is Co-lead, UK NIHR Translational Research Collaboration, Common MSK Conditions workstream (unremunerated).
FR is shareholder of Boston Imaging Core Lab (BICL), LLC. and consultant to Grünenthal. He is Editor in Chief of Osteoarthritis Imaging and Associate Editor of Radiology.
ME has received consulting fees from Cellcolabs AB.
NG is an employee and shareholder of Novartis Pharma AG.
DJM has patents granted for the use of drugs to prevent PTOA, has received research funding from Orphelion and tested drugs supplied by Orphelion and Novartis.
MAR is a Scientific Advisory Board member for Centre for Sport, Exercise & Osteoarthritis Research Versus Arthritis and a Board member of a non-profit Hospital in Oslo; Norway: Lovisenberg Rehabilitering.
PC has received consulting fees from AbbVie, AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Galapagos, Genascence, GlaxoSmithKline, Janssen, Levicept, Novartis, Pfizer, Stryker and UCB.
GD has received consulting fees from Sanofi and Pacira and is owner/shareholder of Mechano Therapeutics LLC.
AG has received consulting fees from Smith and Nephew and owns shares/stock in Precison OS, Osteosys Robotics and LinkX Robotics.
SL has received consulting fees from Joint Academy and is member clinical trial DSMB, Astra Zeneca.
EL is on the OARSI board of directors and is the deputy editor of The Journal of Bone and Joint Surgery.
Acknowledgments
We thank the workshop speakers, convening/organising group and coordinator (RK), OARSI coordinator (Summer Culkin) and the OARSI organization for making the workshop possible, and the approximately 300 workshop attendees.
Handling Editor: Professor H Madry
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ocarto.2024.100449.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Cross M., et al. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann. Rheum. Dis. 2014;73:1323–1330. doi: 10.1136/annrheumdis-2013-204763. [DOI] [PubMed] [Google Scholar]
- 2.Safiri S., et al. Global, regional and national burden of osteoarthritis 1990-2017: a systematic analysis of the Global Burden of Disease Study 2017. Ann. Rheum. Dis. 2020;79:819–828. doi: 10.1136/annrheumdis-2019-216515. [DOI] [PubMed] [Google Scholar]
- 3.Yu D., et al. Population trends in the incidence and initial management of osteoarthritis: age-period-cohort analysis of the Clinical Practice Research Datalink, 1992-2013. Rheumatology. 2017;56:1902–1917. doi: 10.1093/rheumatology/kex270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steinmetz J.D., et al. Global, regional, and national burden of osteoarthritis, 1990–2020 and projections to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet Rheumatol. 2023;5:e508–e522. doi: 10.1016/s2665-9913(23)00163-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Y., Jordan J.M. Epidemiology of osteoarthritis. Clin. Geriatr. Med. 2010;26:355–369. doi: 10.1016/j.cger.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roos E.M., Arden N.K. Strategies for the prevention of knee osteoarthritis. Nat. Rev. Rheumatol. 2016;12:92–101. doi: 10.1038/nrrheum.2015.135. [DOI] [PubMed] [Google Scholar]
- 7.Whittaker J.L., Runhaar J., Bierma-Zeinstra S., Roos E.M. A lifespan approach to osteoarthritis prevention. Osteo. Cart. 2021;29:1638–1653. doi: 10.1016/j.joca.2021.06.015. [DOI] [PubMed] [Google Scholar]
- 8.Whittaker J.L., et al. Efficacy of the SOAR knee health program: protocol for a two-arm stepped-wedge randomized delayed-controlled trial. BMC Muscoskel. Disord. 2022;23:85. doi: 10.1186/s12891-022-05019-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patterson B.E., Barton C.J., Culvenor A.G., Cooper R.L., Crossley K.M. Exercise-therapy and education for individuals one year after anterior cruciate ligament reconstruction: a pilot randomised controlled trial. BMC Muscoskel. Disord. 2021;22:64. doi: 10.1186/s12891-020-03919-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Culvenor A.G., et al. SUpervised exercise-therapy and Patient Education Rehabilitation (SUPER) versus minimal intervention for young adults at risk of knee osteoarthritis after ACL reconstruction: SUPER-Knee randomised controlled trial protocol. BMJ Open. 2023;13 doi: 10.1136/bmjopen-2022-068279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vos T., et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2163–2196. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van der Graaff S.J.A., et al. Arthroscopic partial meniscectomy versus physical therapy for traumatic meniscal tears in a young study population: a randomised controlled trial. Br. J. Sports Med. 2022;56:870–876. doi: 10.1136/bjsports-2021-105059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reijman M., et al. Early surgical reconstruction versus rehabilitation with elective delayed reconstruction for patients with anterior cruciate ligament rupture: COMPARE randomised controlled trial. Bmj. 2021;372:n375. doi: 10.1136/bmj.n375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Vos F.H., et al. Study protocol ROTATE-trial: anterior cruciate ligament rupture, the influence of a treatment algorithm and shared decision making on clinical outcome- a cluster randomized controlled trial. BMC Muscoskel. Disord. 2022;23:117. doi: 10.1186/s12891-021-04867-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Felson D., et al. New approach to testing treatments for osteoarthritis: FastOA. Ann. Rheum. Dis. 2023 doi: 10.1136/ard-2023-224675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whittaker J.L., et al. Health-related outcomes following a youth sport-related knee injury. Med. Sci. Sports Exerc. 2018;51:255–263. doi: 10.1249/MSS.0000000000001787. [DOI] [PubMed] [Google Scholar]
- 17.Whittaker J.L., et al. Risk factors for knee osteoarthritis after traumatic knee injury: a systematic review and meta-analysis of randomised controlled trials and cohort studies for the OPTIKNEE Consensus. Br. J. Sports Med. 2022;56:1406–1421. doi: 10.1136/bjsports-2022-105496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Collins N.J., Misra D., Felson D.T., Crossley K.M., Roos E.M. Measures of knee function: international knee documentation committee (IKDC) subjective knee evaluation Form, knee injury and osteoarthritis outcome score (KOOS), knee injury and osteoarthritis outcome score physical function Short Form (KOOS-PS), knee outcome Survey activities of daily living scale (KOS-ADL), Lysholm knee scoring scale, Oxford knee score (OKS), Western Ontario and McMaster universities osteoarthritis index (WOMAC), activity rating scale (ARS), and tegner activity score (TAS) Arthritis Care Res. 2011;63(Suppl 11):S208–S228. doi: 10.1002/acr.20632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Collins N.J., et al. Knee Injury and Osteoarthritis Outcome Score (KOOS): systematic review and meta-analysis of measurement properties. Osteo. Cart. 2016;24:1317–1329. doi: 10.1016/j.joca.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 20.Zhu S., et al. Subchondral bone osteoclasts induce sensory innervation and osteoarthritis pain. J. Clin. Invest. 2019;129:1076–1093. doi: 10.1172/JCI121561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adaes S., et al. Intra-articular injection of collagenase in the knee of rats as an alternative model to study nociception associated with osteoarthritis. Arthritis Res. Ther. 2014;16:R10. doi: 10.1186/ar4436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller R.E., Malfait A.M. Osteoarthritis pain: what are we learning from animal models? Best Pract. Res. Clin. Rheumatol. 2017;31:676–687. doi: 10.1016/j.berh.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lattermann C., et al. A multicenter study of early anti-inflammatory treatment in patients with acute anterior cruciate ligament tear. Am. J. Sports Med. 2017;45:325–333. doi: 10.1177/0363546516666818. [DOI] [PubMed] [Google Scholar]
- 24.van der Kraan P.M. Factors that influence outcome in experimental osteoarthritis. Osteoarthritis Cartilage. 2017;25:369–375. doi: 10.1016/j.joca.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 25.Lohmander L.S., Englund P.M., Dahl L.L., Roos E.M. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am. J. Sports Med. 2007;35:1756–1769. doi: 10.1177/0363546507307396. [DOI] [PubMed] [Google Scholar]
- 26.Watt F.E., et al. Towards prevention of post-traumatic osteoarthritis: report from an international expert working group on considerations for the design and conduct of interventional studies following acute knee injury. Osteo. Cart. 2019;27:23–33. doi: 10.1016/j.joca.2018.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Urhausen A.P., et al. Measurement properties for muscle strength tests following anterior cruciate ligament and/or meniscus injury: what tests to use and where do we need to go? A systematic review with meta-analyses for the OPTIKNEE consensus. Br. J. Sports Med. 2022;56:1422–1431. doi: 10.1136/bjsports-2022-105498. [DOI] [PubMed] [Google Scholar]
- 28.Whittaker J.L., et al. Optiknee 2022: consensus recommendations to optimise knee health after traumatic knee injury to prevent osteoarthritis. Br. J. Sports Med. 2022;56:1393–1405. doi: 10.1136/bjsports-2022-106299. [DOI] [PubMed] [Google Scholar]
- 29.Liew J.W., et al. A scoping review of how early-stage knee osteoarthritis has been defined. Osteo. Cart. 2023;31:1234–1241. doi: 10.1016/j.joca.2023.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Skou S.T., Koes B.W., Gronne D.T., Young J., Roos E.M. Comparison of three sets of clinical classification criteria for knee osteoarthritis: a cross-sectional study of 13,459 patients treated in primary care. Osteo. Cart. 2020;28:167–172. doi: 10.1016/j.joca.2019.09.003. [DOI] [PubMed] [Google Scholar]
- 31.Lohmander L.S., Roemer F.W., Frobell R.B., Roos E.M. Treatment for acute anterior cruciate ligament tear in young active adults. NEJM Evidence. 2023;2 doi: 10.1056/evidoa2200287. [DOI] [PubMed] [Google Scholar]
- 32.Bowen D.J., et al. How we design feasibility studies. Am. J. Prev. Med. 2009;36:452–457. doi: 10.1016/j.amepre.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thabane L., et al. A tutorial on pilot studies: the what, why and how. BMC Med. Res. Methodol. 2010;10:1. doi: 10.1186/1471-2288-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Curran G.M., Bauer M., Mittman B., Pyne J.M., Stetler C. Effectiveness-implementation hybrid designs: combining elements of clinical effectiveness and implementation research to enhance public health impact. Med Care. 2012;50:217–226. doi: 10.1097/MLR.0b013e3182408812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parsons J.L., Coen S.E., S B. Anterior cruciate ligament injury: towards a gendered environmental approach. Br J Sports Med Online First: 10 March 2021. 2020 doi: 10.1136/bjsports-2020-103173. [DOI] [PubMed] [Google Scholar]
- 36.Bruder A.M., et al. Let's talk about sex (and gender) after ACL injury: a systematic review and meta-analysis of self-reported activity and knee-related outcomes. Br. J. Sports Med. 2023;57:602–610. doi: 10.1136/bjsports-2022-106099. [DOI] [PubMed] [Google Scholar]
- 37.Gulati M., Dursun E., Vincent K., Watt F.E. The influence of sex hormones on musculoskeletal pain and osteoarthritis. Lancet Rheumatol. 2023;5:e225–e238. doi: 10.1016/S2665-9913(23)00060-7. [DOI] [PubMed] [Google Scholar]
- 38.Berg B., et al. What tests should be used to assess functional performance in youth and young adults following anterior cruciate ligament or meniscal injury? A systematic review of measurement properties for the OPTIKNEE consensus. Br. J. Sports Med. 2022;56:1454–1464. doi: 10.1136/bjsports-2022-105510. [DOI] [PubMed] [Google Scholar]
- 39.Culvenor A.G., et al. Rehabilitation after anterior cruciate ligament and meniscal injuries: a best-evidence synthesis of systematic reviews for the OPTIKNEE consensus. Br. J. Sports Med. 2022;56:1445–1453. doi: 10.1136/bjsports-2022-105495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Filbay S.R., et al. Long-term quality of life, work limitation, physical activity, economic cost and disease burden following ACL and meniscal injury: a systematic review and meta-analysis for the OPTIKNEE consensus. Br. J. Sports Med. 2022;56:1465–1474. doi: 10.1136/bjsports-2022-105626. [DOI] [PubMed] [Google Scholar]
- 41.Holm P.M., et al. The effects of different management strategies or rehabilitation approaches on knee joint structural and molecular biomarkers following traumatic knee injury - a systematic review of randomized controlled trials for the OPTIKNEE consensus. J. Orthop. Sports Phys. Ther. 2023;1–27 doi: 10.2519/jospt.2023.11576. [DOI] [PubMed] [Google Scholar]
- 42.Macri E.M., et al. Meaningful thresholds for patient-reported outcomes following interventions for anterior cruciate ligament tear or traumatic meniscus injury: a systematic review for the OPTIKNEE consensus. Br. J. Sports Med. 2022;56:1432–1444. doi: 10.1136/bjsports-2022-105497. [DOI] [PubMed] [Google Scholar]
- 43.Meuffels D.E., et al. Guideline on anterior cruciate ligament injury. Acta Orthop. 2012;83:379–386. doi: 10.3109/17453674.2012.704563. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.