Abstract
Platelet-rich plasma (PRP) is a concentrated platelet preparation known for its regenerative properties due to the various growth factors it contains. Its application in the medical field, including dentistry, gynecology, and plastic surgery, has surged. In obstetrics and gynecology, PRP has shown promise in improving low libido, vaginal rejuvenation, ovarian reserve, and endometrial receptivity. This study presents a 29-year-old woman experiencing primary infertility attributed to low levels of anti-Müllerian hormone alongside the presence of asthenozoospermia in her husband’s semen. After failed intrauterine insemination as well as in vitro fertilization (IVF), attempts at laparoscopic PRP treatment were administered before the second IVF cycle to enhance ovarian reserve and quality. The PRP treatment led to an increased follicle count, improved oocyte quality, and a successful pregnancy outcome in the second IVF cycle. PRP treatment promises to be effective in fertility treatments, potentially increasing ovarian reserve, improving oocyte quality, and enhancing successful pregnancy outcomes. This case report highlights its beneficial impact on a couple facing primary infertility, providing hope for patients with similar reproductive challenges.
Keywords: poor ovarian reserve, infertility, invitro-fertilization ivf, oocyte quality, platelet-rich plasma/ prp
Introduction
Anti-Müllerian hormone (AMH) is a glycoprotein produced by Sertoli cells in males and by granulosa cells of the ovary in females. AMH helps in the initial phases of follicular development and prevents premature activation of follicles. It also plays a role in recruiting and selecting follicles that possess the ability to mature during the menstrual cycle [1]. AMH is an important indicator for evaluating ovarian reserve in women. Other indicators for ovarian functions include the patient’s age, antral follicular count, and hormone levels such as follicle-stimulating hormone (FSH), estradiol, and luteinizing hormone (LH). A decrease in oocyte quantity and quality with advancing age is a normal physiologic phenomenon. Sometimes, a decline in ovarian reserve occurs very early in life and can be one of the significant causes of female infertility [2]. Treatment with platelet-rich plasma (PRP) has been shown to be effective in such patients. PRP is concentrated platelets obtained through centrifugation of the patient’s blood. Several growth factors are present in PRP, such as platelet-derived growth factor, vascular endothelial growth factor, insulin-like growth factor, epidermal growth factor, and fibroblast growth factor [3]. These factors stimulate the proliferation of cells, tissue repair, and regeneration; promote angiogenesis; stimulate the migration (chemotaxis) and proliferation (mitogenesis) of different cell types; regulate collagen synthesis; and stimulate immune cells to migrate toward the area of injury.
Nowadays, PRP is commonly employed in the medical field. It is used in orthopedics to treat ligament injuries and osteoarthritis for tissue healing and to reduce pain and inflammation, in dermatology for hair loss treatment and skin treatment, in ophthalmology to promote corneal healing, and in sports medicine to treat injuries [3]. Its use in obstetrics and gynecology has also been explored. PRP has the potential to enhance ovarian reserve and improve oocyte quality in women with low AMH levels [4]. Moreover, PRP is said to help treat female sexual dysfunction. It is believed to enhance sexual response by improving genital blood flow and tissue health. It may help improve vaginal tightness, lubrication, and overall tissue health. PRP has been considered as a potential treatment for stress urinary incontinence and pelvic floor disorders. Studies indicate that PRP enhances endometrial receptivity in women undergoing fertility treatments [5,6]. Due to its multiple functions and applications, PRP has become a valuable therapeutic option for treating infertility. This case report suggests that PRP may have a regenerative effect on ovarian tissue, stimulating cell proliferation and promoting the growth and development of healthy ovarian follicles. These effects could result in increasing the number of viable eggs and improving fertility outcomes in females struggling with diminished ovarian reserve.
Case presentation
This is a case report of a 29-year-old woman who presented with a complaint of primary infertility seven months ago. Her husband’s age was 31, and they had been married for two years. They had been attempting to conceive for one and a half years. Initially, they sought religious ways to cure infertility, then decided to consult a doctor. There was no significant history or family history. There was no history of previous surgeries. The woman’s age of menarche was 13 years, and she had regular cycles of 29 days with normal flow. The husband, a businessman, had been an active smoker for the past nine years and smoked nine to 10 cigarettes per day.
Investigations
Both underwent a comprehensive investigation. The husband's semen analysis indicated asthenozoospermia, with a sperm count of 20 million/ml and 25% motility. However, his hormonal evaluation, which included tests for testosterone, LH, and FSH, showed no abnormalities. The woman’s evaluation revealed low ovarian reserve, with low AMH levels measuring at 0.2 ng/dl and high FSH levels. The transvaginal ultrasound revealed the presence of three follicles on both the right and left ovaries before the first oocyte retrieval. However, hysterosalpingogram results indicated normal and open fallopian tubes, ruling out tubal factors as a cause of infertility.
Treatment procedure
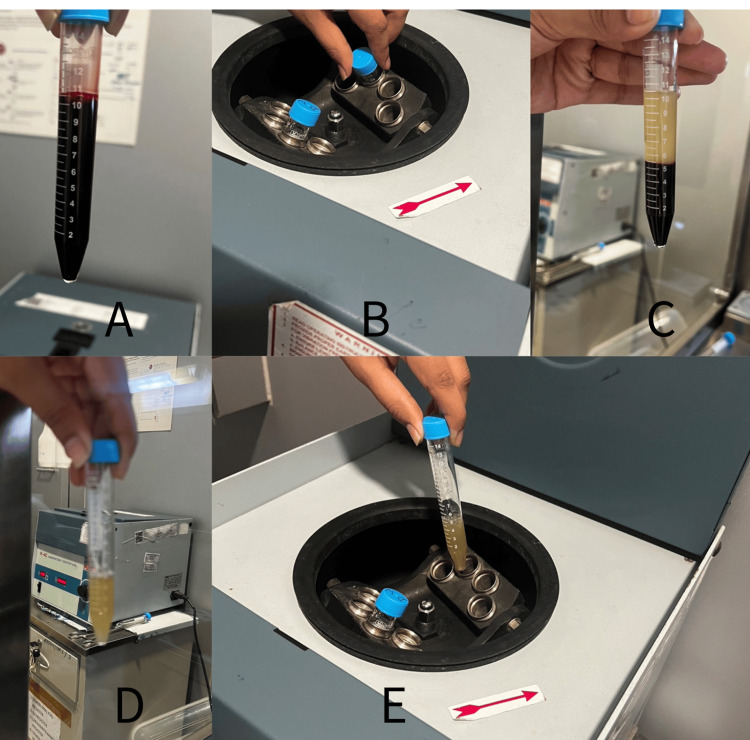
Intrauterine insemination (IUI) using the husband’s semen was attempted, but unfortunately, it was unsuccessful. Consequently, the couple opted for in vitro fertilization (IVF) as their next treatment option. After two months, the first cycle of IVF began after the female underwent controlled ovarian stimulation. Antral follicular count was assessed on ultrasound; three follicles were observed in the right ovary, while an additional three were noted in the left ovary. During ovum pick-up, four oocytes were retrieved: one germinal vesicle, two metaphase I (MI), and one MII. The quality of the MII oocyte was not good either. Intracytoplasmic sperm injection was done on the MII oocyte. It got fertilized but degenerated later, so no embryos were formed, and the IVF cycle failed. Two months later, after thorough counseling and consent, the decision was made to proceed with laparoscopic PRP treatment before the second IVF cycle to potentially improve the patient’s ovarian reserve and oocyte quality. PRP was prepared by double centrifugation (Figure 1). To start with, an 11-ml venous blood sample was collected from the woman’s arm in a sterile tube. Then, the blood sample underwent centrifugation at a speed of 1,200 rpm for 10 minutes. As a result, it was separated into three parts: the top had platelets, the middle part had platelets and white blood cells, and the bottom part had red blood cells. After that, the top and buffy coat were carefully separated into a sterile tube. The transferred plasma underwent a second centrifugation for 10 minutes at 2,000 rpm. The top two-thirds of the centrifuged plasma was discarded since it had a lower concentration of platelets. The pellets at the bottom of the tube were mixed and blended with the remaining plasma. At this point, the PRP was considered ready for use. Ultimately, 1.5-2 ml of PRP was instilled laparoscopically in each ovary 10 days after menstruation.
Figure 1. Preparation of PRP. (A) Collection of blood in a sterile tube. (B) Centrifugation of blood at 1,200 rpm for 10 minutes. (C) Separation of blood into three layers. (D) Extraction of the top layer and buffy coat into a separate tube. (E) Further centrifugation at 2,000 rpm for 10 minutes.
The original picture was captured by the author of this article.
PRP, platelet-rich plasma
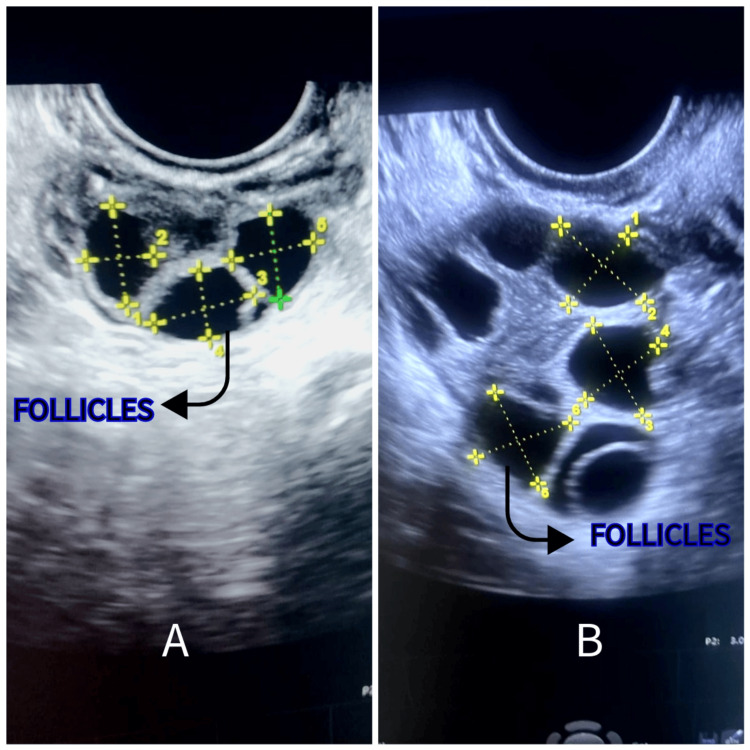
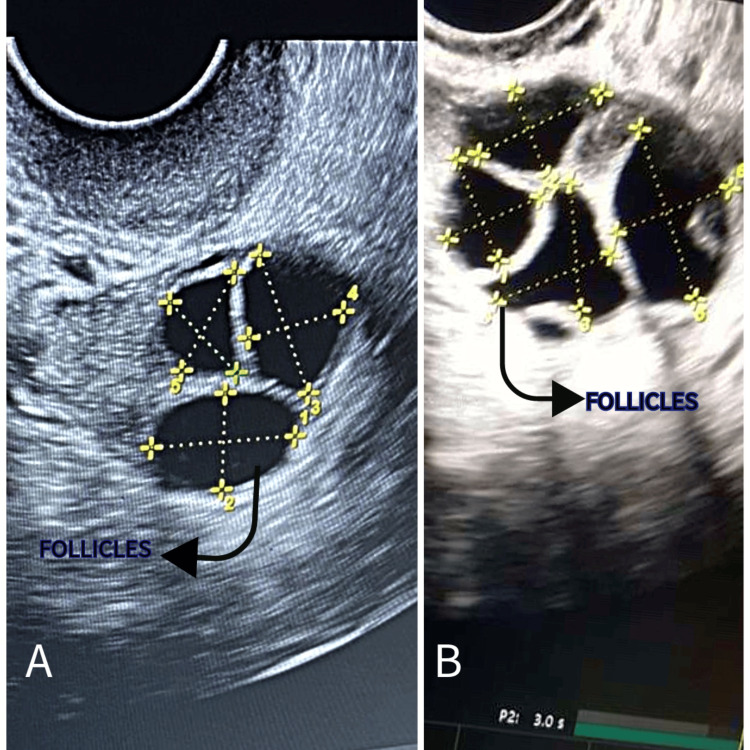
In the next menstrual cycle, the female partner received gonadotropin-releasing hormone antagonists. Subsequently, ovarian stimulation was initiated using gonadotropins. The follicular study showed six follicles on the right ovary (Figure 2) and four on the left ovary (Figure 3). On ovum pick-up, eight oocytes were retrieved: two MI and six MII. All six MII were fertilized, and five progressed to the blastocyst stage. Two embryos were transferred (fresh), and three were cryopreserved. After two weeks, the pregnancy test was positive. The couple was overjoyed with the successful outcome of the treatment and expressed their heartfelt gratitude. In this case report, PRP therapy improved ovarian reserve and oocyte quality, resulting in successful implantation and a positive pregnancy outcome.
Figure 2. Ultrasonographic image of the right ovary showing follicles. (A) Before PRP therapy. (B) After PRP therapy.
The original picture was captured by the author of this article, and informed consent was obtained for publication from the patient.
PRP, platelet-rich plasma
Figure 3. Ultrasonographic image of the left ovary showing follicles. (A) Before PRP therapy. (B) After platelet-rich plasma therapy.
The original picture was captured by the author of this article, and informed consent was obtained for publication from the patient.
PRP, platelet-rich plasma
Follow-up
Following the successful treatment, the woman was advised to undergo regular antenatal check-ups and was prescribed essential prenatal vitamins. Her health and fetal growth were diligently monitored through routine assessments and examinations.
Discussion
This case report demonstrates that ovarian reserve and oocyte quality were enhanced after PRP treatment. PRP treatment for ovarian reserve is still considered an emerging area of research and is a subject of ongoing investigation. While there have been some studies and case reports suggesting the potential benefits of PRP in improving ovarian reserve and oocyte quality, the overall evidence is limited, and its widespread use is not yet common. A study by Sills and Wood showed increased serum AMH and retrieval of MII after intraovarian PRP treatment [7]. Sfakianoudis et al. reported a remarkable case of a successful pregnancy in a 35-year-old menopausal woman following an intraovarian PRP injection, but she had a miscarriage in the fifth week [8]. Hosseini et al. noticed a notable increase in the formation of early antral follicles when supplementing them with PRP culture for 10 days [9]. This finding led the researchers to conclude that PRP might be a promising and successful method for stimulating follicular development. According to Cakiroglu et al., PRP improves IVF success rates in women with primary ovarian insufficiency (POI) [10]. Aflatoonian et al. investigated the influence of intraovarian PRP injections in females who had POI and poor ovarian response (POR). A total of 47% conceived, and half of those led to live childbirth in women with POR [11]. In an article by Petryk and Petryk, it was found that injecting a concentrated PRP solution into the ovary using ultrasound guidance through the vagina had positive and enduring impacts on reproductive function [12]. In a case report by Hsu et al., the combination of PRP and gonadotropin treatment resulted in a successful pregnancy culminating in a live birth in a 37-year-old woman with POI who previously showed very low responsiveness to external gonadotropin treatment [13]. Similarly, a study by Parvanov et al. demonstrated a notable rise in the count of successfully retrieved MII oocytes as well as the number of blastocysts following PRP treatment. Additionally, they observed enhanced quality of oocytes and embryos in approximately 75% of patients who underwent PRP ovarian treatment [14].
Currently, PRP treatment for ovarian reserve is typically considered experimental, and its application in clinical practice may vary among fertility clinics and healthcare providers. The use of PRP in fertility treatments, including its impact on ovarian reserve, requires further rigorous research and clinical trials to establish its safety, effectiveness, and optimal protocols.
Conclusions
This case report highlights the role of PRP treatment in ovarian rejuvenation. The successful outcome of this 29-year-old woman’s journey to pregnancy after failed IUI and IVF attempts shows the potential benefits of PRP therapy. It is important to note that individual responses to treatment can vary widely, and this case report is based on a single patient, limiting conclusive interpretation of the findings. By increasing follicle count, improving oocyte quality, and ultimately leading to a successful pregnancy in the second IVF cycle, PRP has demonstrated its potential to enhance fertility treatments. This report offers hope for couples dealing with infertility due to poor ovarian reserve. It still requires further research and clinical applications of PRP in the fields of obstetrics and gynecology. PRP treatment represents an innovative procedure for improving outcomes in fertility treatments.
The authors have declared that no competing interests exist.
Author Contributions
Concept and design: Jarul Shrivastava, Virul Shrivastava, Namrata Choudhary
Acquisition, analysis, or interpretation of data: Jarul Shrivastava, Akash More, Deepti Shrivastava
Drafting of the manuscript: Jarul Shrivastava
Critical review of the manuscript for important intellectual content: Jarul Shrivastava, Akash More, Virul Shrivastava, Namrata Choudhary, Deepti Shrivastava
Supervision: Akash More, Virul Shrivastava, Namrata Choudhary, Deepti Shrivastava
Human Ethics
Consent was obtained or waived by all participants in this study. Institutional Ethics Committee, Datta Meghe Institute of Higher Education & Research issued approval DMIHER(DU)/IEC/2023/576
References
- 1.Assessing ovarian response: antral follicle count versus anti-Müllerian hormone. Fleming R, Seifer DB, Frattarelli JL, Ruman J. Reprod Biomed Online. 2015;31:486–496. doi: 10.1016/j.rbmo.2015.06.015. [DOI] [PubMed] [Google Scholar]
- 2.AMH in women with diminished ovarian reserve: potential differences by FMR1 CGG repeat level. Pastore LM, McMurry TL, Williams CD, Baker VL, Young SL. J Assist Reprod Genet. 2014;31:1295–1301. doi: 10.1007/s10815-014-0276-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Platelet-rich plasma therapy - future or trend? Dhillon RS, Schwarz EM, Maloney MD. Arthritis Res Ther. 2012;14:219. doi: 10.1186/ar3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Evaluation of intra-ovarian platelet-rich plasma administration on oocytes-dependent variables in patients with poor ovarian response: a retrospective study according to the POSEIDON criteria. Farimani M, Nazari A, Mohammadi S, Anvari Aliabad R. https://doi.org/10.1186/s12958-021-00826-w. Reprod Biol Endocrinol. 2021;19:137. doi: 10.1186/s12958-021-00826-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Current clinical applications of platelet-rich plasma in various gynecological disorders: an appraisal of theory and practice. Dawood AS, Salem HA. Clin Exp Reprod Med. 2018;45:67–74. doi: 10.5653/cerm.2018.45.2.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.First data on in vitro fertilization and blastocyst formation after intraovarian injection of calcium gluconate-activated autologous platelet rich plasma. Sills ES, Rickers NS, Li X, Palermo GD. https://doi.org/10.1080/09513590.2018.1445219. Gynecol Endocrinol. 2018;34:756–760. doi: 10.1080/09513590.2018.1445219. [DOI] [PubMed] [Google Scholar]
- 7.Autologous activated platelet-rich plasma injection into adult human ovary tissue: molecular mechanism, analysis, and discussion of reproductive response. Sills ES, Wood SH. Biosci Rep. 2019;39 doi: 10.1042/BSR20190805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Autologous platelet-rich plasma treatment enables pregnancy for a woman in premature menopause. Sfakianoudis K, Simopoulou M, Nitsos N, et al. J Clin Med. 2018;8:1. doi: 10.3390/jcm8010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Platelet-rich plasma promotes the development of isolated human primordial and primary follicles to the preantral stage. Hosseini L, Shirazi A, Naderi MM, et al. https://doi.org/10.1016/j.rbmo.2017.04.007. Reprod Biomed Online. 2017;35:343–350. doi: 10.1016/j.rbmo.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 10.Effects of intraovarian injection of autologous platelet rich plasma on ovarian reserve and IVF outcome parameters in women with primary ovarian insufficiency. Cakiroglu Y, Saltik A, Yuceturk A, et al. Aging (Albany NY) 2020;12:10211–10222. doi: 10.18632/aging.103403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Effects of intraovarian injection of autologous platelet-rich plasma on ovarian rejuvenation in poor responders and women with primary ovarian insufficiency. Aflatoonian A, Lotfi M, Saeed L, Tabibnejad N. Reprod Sci. 2021;28:2050–2059. doi: 10.1007/s43032-021-00483-9. [DOI] [PubMed] [Google Scholar]
- 12.Ovarian rejuvenation through platelet-rich autologous plasma (PRP)—a chance to have a baby without donor eggs, improving the life quality of women suffering from early menopause without synthetic hormonal treatment. Petryk N, Petryk M. Reprod Sci. 2020;27:1975–1982. doi: 10.1007/s43032-020-00266-8. [DOI] [PubMed] [Google Scholar]
- 13.Live birth in woman with premature ovarian insufficiency receiving ovarian administration of platelet-rich plasma (PRP) in combination with gonadotropin: a case report. Hsu CC, Hsu L, Hsu I, Chiu YJ, Dorjee S. Front Endocrinol (Lausanne) 2020;11:50. doi: 10.3389/fendo.2020.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Autologous ovarian platelet rich plasma treatment improves oocyte and embryo quality: a before-after prospective study. Parvanov D, Ganeva R, Vidolova N, et al. Biotechnol Biotechnol Equip. 2022;36:425–432. [Google Scholar]