Abstract
The aggregation of Alpha Synuclein (α-Syn) into fibrils is associated with the pathology of several neurodegenerative diseases. Pathologic aggregates of α-Syn adopt multiple fibril topologies and are known to be transferred between cells via templated seeding. Monomeric α-Syn is an intrinsically disordered protein with amphiphilic N-terminal, hydrophobic-central, and negatively charged C-terminal domains. Here, we review recent work elucidating the mechanism of α-Syn aggregation and identify the key and multifaceted roles played by the N- and C-terminal domains in the initiation and growth of aggregates as well as in templated seeding involved in cell-to-cell propagation. The charge content of the C-terminal domain, which is sensitive to environmental conditions like organelle pH, is a key regulator of intermolecular interactions involved in fibril growth and templated propagation. An appreciation of the complex and multifaceted roles played by the intrinsically disordered terminal domains suggests novel opportunities for the development of potent inhibitors against synucleinopathies.
Introduction
More than 50 human diseases, including neurodegenerative diseases such as Parkinson’s disease, Alzheimer’s disease, and Huntington’s disease, involve the deposition of fibrillar protein aggregates [1]. α-Syn is an intrinsically disordered protein (IDP) that is found in the brain, particularly in the presynaptic nerve termini. Due to its intrinsically disordered nature, α-Syn has multivalent, weak, and reversible interactions that allow it to participate in both functional and misfolding pathways [2]. α-Syn has been associated with various synucleinopathies including Parkinson’s disease (PD), dementia with Lewy body (DLB), and multiple system atrophy (MSA).
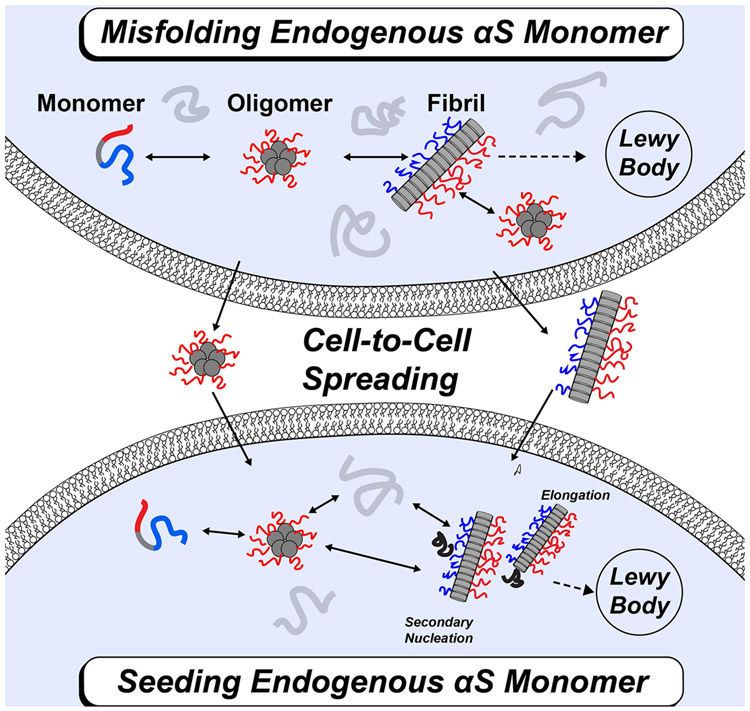
In disease, α-Syn can self-associate into multiple conformations including dimers and oligomers. These aggregates can go on to form amyloid fibrils that are incorporated into the Lewy bodies of patients with PD (Figure 1A) [3-5]. Additional disease progression is associated with a prion-like process of cell-to-cell transfer where fibril seeds are released from one neuron and taken up by a neighboring neuron [6-8]. This cellular uptake allows for endogenous α-Syn to interact with the fibril seed through elongation or secondary nucleation leading to further fibril growth (Figure 1B) [7,8]. It has been observed that α-Syn fibrils isolated from patients with different synucleinopathies are structurally distinct from each other [9]. These polymorphs also differ in their biological and pathological activity including seeding and cell-to-cell transfer.
FIGURE 1: Schematic of α-Syn protein aggregation and cell-to-cell spreading.
(a) Overview of α-Syn aggregation. Endogenous α-Syn monomers (grey) can self-associate into various aggregates including dimers and oligomers. These aggregates can further go on to form different polymorphic amyloid fibrils that can be found in the Lewy bodies of patients with PD. Fibril seeds from one neuron can be released and taken up by a neighboring neuron. This cell-to-cell spreading leads to additional disease progression. (b) Mechanism of α-Syn protein fibril growth and propagation. Endogenous α-Syn monomer can interact with exogenous fibril seeds through elongation and/or secondary nucleation to form mature fibrils.
α-Syn is frequently described as having three different domains: an amphiphilic N-terminal domain from residues 1-60 that contains imperfect KTKXGV repeats, a hydrophobic NAC domain from residues 61-95, and a negatively charged C-terminal domain from residues 96-140 [6]. α-Syn fibrils are characterized by the presence of parallel, in-register β-sheets, a common structural feature of amyloid fibrils. These fibril structures are stabilized via hydrogen bonding and “zippered” hydrophobic interactions formed by residues in the NAC domain [10].
In this review we discuss recent insights into the key and multifaceted roles played by the N- and C-terminal domains at all stages in the fibril aggregation process. We identify diverse intermolecular protein-protein interactions involving these domains that control early events in the aggregation process. Combining key results from recent studies, we propose that environment-dependent charge-charge interactions between monomer N- and fibril C-terminal domains are critical for fibril seeding and elongation. A molecular understanding of the intermolecular protein-protein interactions that arise during the fibril formation process provides a foundation for developing therapeutic strategies against aggregation in synucleinopathies.
Multifaceted Role of N- and C-terminal Regions in the Early Stages of α-Syn Aggregation
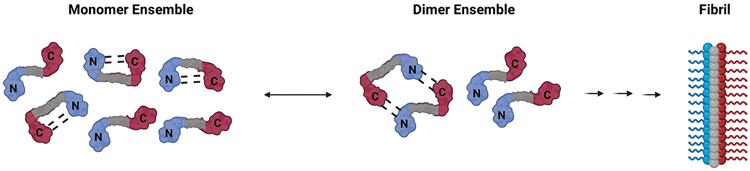
It has long been appreciated by many groups that the N-terminal domain plays a critical role in the aggregation propensity and kinetics of α-Syn [11]. α-Syn aggregation is very susceptible to genetic mutations and environmental factors including posttranslational modifications. In its functional state, α-Syn binds to membranes and forms α-helical structures [12]. These structures are in equilibrium with the monomeric state of α-Syn composed of an ensemble of intrinsically disordered monomers, with the transient formation of intra-chain interactions between the N- and C-terminal domains (Figure 2) [13,14]. Recent examples of experimental studies that highlight the complex role of the N-terminus include mutations to the N-terminus, deletions/alterations to the master regulator regions P1 (res 36-42) and P2 (res 45-57), post-translational modifications, and N-terminal acetylation [15-20]. Furthermore, it has been shown that various molecular chaperones interact with α-Syn through its N-terminal domain to delay aggregation [21,22].
FIGURE 2: Conformational equilibria involved in the early stages of α-Syn aggregation.
Soluble α-Syn monomers contain a disordered N-terminus (depicted in blue) and a disordered C-terminus (depicted in red). The soluble state monomer ensemble contains various conformations. Specifically, there exist monomers that are in a conformation where the N- and C-terminal domains are close enough to interact with each other. It has been shown in vitro that monomers are in equilibria with a small population of dimers. NMR PRE experiments performed on the monomer-dimer equilibrium revealed the existence of weak transient, but highly specific interactions between N- and C- terminal domains in dimers. Interactions between the termini are shown in dashed lines.
NMR paramagnetic relaxation experiments (PRE) and mass spectrometry (MS) approaches performed under monomer-dimer equilibrium conditions showed the existence of weak transient interchain dimer interactions between residues in the N- and C-terminal domains (Figure 2) [23,24]. Although weak, these highly selective N-N and N-C interactions may have an effect on the kinetics of further fibril aggregation. Due to the parallel arrangement of the beta strands in the fibril, it has been proposed that the in-parallel N-N interactions in the early stages may promote aggregation [24]. On the other hand, the anti-parallel N-C interactions may delay aggregation by providing a kinetic barrier (Figure 2) [24,25]. Further experiments on chimeras of alpha and beta synuclein (β-Syn) at physiological pH support the notion that a more negatively charged β-Syn C-terminal domain enhances N-C interactions in the dimer therefore delaying aggregation [25,26]. Computational studies using molecular dynamics (MD) simulations also support the conclusion that the N-terminal domain plays a critical role in the dimer formation of α-Syn [27,28].
N- and C-terminal Regions in Fibril Polymorph Structures are Disordered
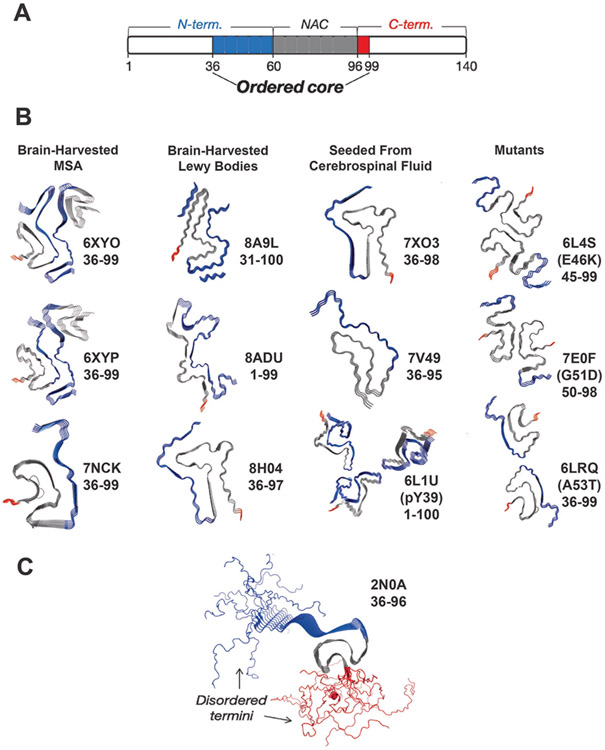
Structures of α-Syn amyloid fibrils determined from cryo-EM and solid-state NMR (ssNMR) indicate that fibrils have exposed disordered N- and C-terminal segments flanking a rigid Greek-key fibril core [11,29]. To date there are many cryo-EM and NMR structures of WT α-Syn, mutant α-Syn and brain-harvested fibril strains, examples of which are presented in Figure 3 (references for Fig. 3 are included in the figure legend). In general, fibril structures feature a typical cross-beta topology formed primarily by interchain self-association of residues in the NAC and, in some cases, N-terminal domain with parallel in-register beta strand topology [3,30-32]. Different fibril polymorphs determined to date show variable lengths of N-terminal domains participating in the core fibril structure, while the C-terminal domain remains disordered almost entirely, without exception (Figure 3A, B). The ordered core captured by the cryo-EM structures span approximately from residues 36-99, indicating that about 50% of the N-terminus as defined by residues 1-60 tend to be incorporated into the fibril core (depicted in blue, Figure 3) while the full C-terminus (depicted in red, Figure 3) is essentially never included in the core and is not resolved in the cryo-EM structures.
FIGURE 3: The C-terminal domain forms a fuzzy coat on the α-Syn fibril surface.
(a) Schematic of α-Syn domains and their involvement in the fibril core. Monomer domains are defined as shown at the top of the schematic while the fibril structures reveal an ordered core composed mainly of the residues 36-99 of the N–terminal (blue: 36-60) and the NAC region (grey; 60-96). A very small portion of the core is composed of residues from the C-terminal domain (red: 96-99). The rest of the residues (white) are considered disordered in the fibril structure. (b) Cryo-EM structures (PDB IDs provided) of the ordered cores brain-harvested α-Syn fibrils from MSA, brain-harvested α-Syn fibrils from Lewy bodies, α-Syn fibrils seeded from cerebrospinal fluid and mutant α-Syn [36-44] . The ordered cores as captured consist almost exclusively of residues in the N-terminal (blue) and NAC (grey) domains while the C-terminal (red) domain is rarely included. (c) The ssNMR structure of an α-Syn fibril as performed by Tuttle et al [33]. The N-terminal residues are depicted in blue, and the C-terminal residues are depicted in red. The calculated NMR structure reflects the disordered N- and C-termini.
In contrast, the ssNMR structure of the α-Syn fibril performed by Tuttle et al (Figure 3C) directly visualizes the disordered N- and C-termini [33]. The flanking intrinsically disordered regions (IDRs) are too conformationally heterogenous to visualize in cryo-EM or in cross-polarization based ssNMR experiments. However, recently, peaks corresponding to the IDR segments of α-Syn have been directly visualized via through-bond based ssNMR experiments. These IDR residues have been shown to correspond to the C-terminus of the fibril [34]. Furthermore, hydrogen-deuterium exchange experiments coupled with MS have shown that the C-terminal domain remains surface accessible in various fibril polymorphs while exposure of the N-terminal domain varies [35]. Taken together with the ssNMR experiments, these studies further demonstrate that the C-terminal domain remains entirely disordered in different fibril polymorphs while the N-terminal lengths incorporated within the fibril core vary. Overall, the molecular picture that emerges is that in fibrillar structures these disordered terminal segments form a “fuzzy-coat” composed of disordered C-termini (approximately residues 97-140) and N-termini (approximately residues 1-36); although some fibril polymorphs feature the participation of a longer tract of the N-terminal segment in the ordered fibril structure [36,37].
Electrostatic Effects Mediated by the C-terminal Domain of the Fibrils are the Master Regulators of Fibril Seeding
Recent mechanistic studies provide evidence that the fuzzy-coat structure, particularly that formed by the negatively charged C-terminal segment, is a critical element in the molecular mechanism of templated seeding. Baum and colleagues used relaxation-based solution NMR experiments on fibril seeds in equilibrium with α-Syn monomer to show that the first 11 N-terminal residues on the monomer constitute the binding interface to the fibril and suggest that the monomer interface interacts with fuzzy-coat on the fibril surface [45]. Riek, Eichmann, and colleagues used NMR and Electron Paramagnetic Resonance (EPR) spectroscopy to show that α-Syn monomer–fibril binding during secondary nucleation is primarily mediated by transient electrostatic interactions between the positively-charged monomer N-terminal domain and the negatively charged fibril C-terminal domain [46]. These monomer-fibril interactions lead both to an alignment of monomers on the fibril surface and an effective weakening of intramolecular N- and C-terminal domain interactions in the monomer that otherwise protect the aggregation-prone NAC domain from inter-chain contacts [46]. The net result is a high local concentration of aligned and NAC-exposing α-Syn monomers on the fibril surface, which forms a nucleus for templated aggregation.
The fuzzy-coat model of fibril seeding has potential implications for secondary nucleation-mediated fibril formation and cell-to-cell spreading of α-Syn aggregates. The kinetics of fibril elongation by addition of monomers at fibril ends is relatively insensitive to pH and is dominated by hydrophobic and hydrogen bonding interactions between residues in the NAC domain [47]. In contrast, fibril-catalyzed secondary nucleation is known to be highly susceptible to pH and other environmental conditions [48]. At pH values of <6, often present in organelles such as endosomes and lysosomes, secondary nucleation-mediated fibril growth dominates over elongation [49]. Linse, Pálmadóttir, and colleagues found that fibril formation leads to a significant uptake of protons corresponding to 0.9 pH units [50]. These protons are taken up by negatively charged residues in the C-terminal domain, whose pKas are significantly up-shifted to an even greater degree than in the monomeric state. These shifts may result from a high effective concentration in the fibril as well as potentially altered dielectric environment upon compaction - a lower dielectric constant is expected to accentuate electrostatic effects. It has further been shown that the secondary nucleation of α-Syn, enabled at mildly acidic pH using seeds formed at physiological pH, leads to fibrils that structurally resemble those formed de novo under mildly acidic pH conditions, rather than the seeds formed under high-pH conditions. This suggests that secondary nucleation is controlled by the properties of the C-terminal domain fuzzy coat rather than the NAC domain [48].
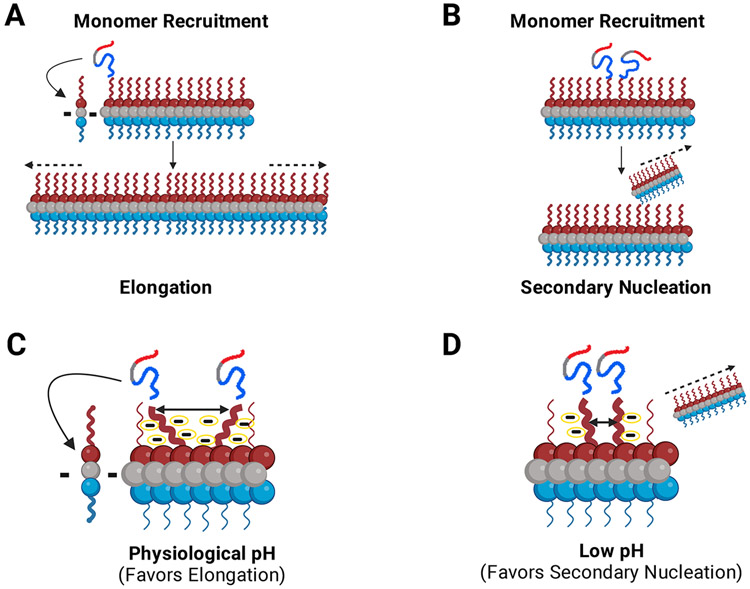
In this review, we propose a mechanistic model for fibril seeding in which the negatively charged C-terminal domain acts as a pH-dependent “master regulator” of the templated seeding process via modulation of interchain N-terminal monomer to C-terminal fibril interactions (Figure 4C, D). We suggest that at physiological pH, the high negative charge in the C-terminal domain of the fibril leads to an expanded volume in the fuzzy coat. Incoming monomers that interact via their N-terminal segments with individual C-terminal domains are thus, on average, sufficiently distant from each other such that fibril surface-mediated nucleation is disfavored compared to NAC domain-dominated elongation at fibril ends (Figure 4C). At low pH, however, because of the increased pKas of negatively charged residues in the fibril, the inter-chain repulsion in the fuzzy coat is dramatically lowered. This leads to the greater proximity and aggregation-promoting alignment of transiently bound incoming monomers (Figure 4D). The higher effective concentration and parallel alignment of surface-bound monomers decreases the barrier to nucleation, leading to as much as a four orders of magnitude increase in secondary nucleation-mediated fibril formation compared to high-pH conditions [49]. Thus, the negatively charged C-terminal domain acts as a master regulator of both de novo fibril formation and, crucially, secondary nucleation-mediated propagation of α-Syn aggregates.
FIGURE 4: Monomers are recruited via the fuzzy coat C-terminal domain for fibril growth and propagation.
The negatively charged C-terminal domain acts as a pH dependent master regulator of the seeding process via modulation of interchain N-terminal monomer to C-terminal fibril interactions. (a, b). α-Syn monomers are recruited to the fibril via interactions between the monomer N-terminal domain and the fuzzy-coat C-terminal domain of fibrils. Incoming monomers can be integrated into the fibril through two different aggregation pathways: (a) Depiction of fibril elongation. Monomers are recruited and added onto either end of the fibril leading to elongation of the fibril. (b) Depiction of fibril secondary nucleation. Recruited monomers use the fuzzy-coat C-terminus on existing fibrils as a template for secondary nucleation. (c, d). The proposed model for the role of C-terminal charge on fibril growth. We postulate that elongation and secondary nucleation are in a constant dynamic balance. This balance can shift depending on numerous factors including pH and environmental conditions. (c) At physiological pH (~7.4), the C–terminal regions of the fibril are highly charged leading them to repel one another. Since the C-terminal regions repel each other, the incoming recruited monomers are relatively dispersed on the fibril surface and elongation is the favored mechanism of aggregation at physiological pH. (d) At low pH (~5.5-6), the charge content and distribution of the C-terminal region is changed due to the altered pKas of negatively charged residues. On average, the termini are less negatively charged and therefore, lower repulsion leads to the monomers being more proximal, productively aligned and, thus, more likely to nucleate a new fibril. The more compact nature of the complex gives rise to secondary nucleation as the dominant mechanism of aggregation at low pH. Dashes (c, d) indicate schematic representation of negative charges around the fibril C-terminal region.
As aggregation is an inherently multi-body phenomenon, this model suggests that even small reductions in inter-chain charge-charge repulsion can substantially alter the stability of aggregated species leading to potentially dramatic effects on aggregation propensity. The homologous IDP β-Syn has a shorter and less hydrophobic (therefore, less aggregation prone) NAC domain and features a significantly more negatively charged C-terminal domain [26]. As expected from the mechanistic scenario described above, Moriarty et al. found that β-Syn forms fibrils at mildly acidic pH but not physiological pH [51]. However, a single negative-charge-decreasing amino acid substitution (E61A) leads to rapid fibril formation by β-Syn at both physiological and mildly acidic pH. Removal of negative charges by the deletion of α-Syn C-terminal residues also results in enhanced aggregation [52-54]. Taken together, recent studies are consistent with our model that the negatively charged residues in both α-Syn and β-Syn act as gatekeepers against aggregation. Thus, effective charge reduction induced by environmental factors, e.g., pH, dielectric changes due to conformational compaction, and/or genetic mutations can lead to large increases in aggregation propensity. Age-dependent or stochastic fluctuations in the cellular environment combined with the high sensitivity of α-Syn aggregation to these effects may, thus, underlie pathological aggregation and promote prion-like propagation [55].
Conclusions and Perspectives
Recent studies investigating the mechanism of aggregation of α-Syn compellingly show that beyond the NAC domain, the intrinsically disordered N- and C-terminal domains play critical regulatory key roles at all stages in the aggregation of α-Syn. Interactions mediated by the N-terminal domain control aggregation kinetics and approximately half (or more) of the N-terminal domain is structured in the core structure of all fibril polymorphs. The C-terminal domain retains its intrinsically disordered character in fibrils and forms a “fuzzy coat” on the fibril surface that plays the role of a “master regulator” in fibril growth. The charge content of the C-terminal domain is sensitive to environmental changes, e.g., pH and ionic strength. Charge-charge interactions mediated by the C-terminal domain are critical regulators of the balance between early stages of aggregation, fibril elongation and fibril surface-mediated secondary nucleation. In the early stages the C-terminal domain is protective against aggregation by forming transient intra-molecular N-C- terminal interactions in the monomer and N-C anti-parallel interactions in the dimer [24,53]. In contrast, at later stages of aggregation, the disordered C-terminal domain in the fibril acts to recruit monomers to the fibril via interactions with the monomer N-terminal domain [45,46].
Despite much effort, no inhibitors of α-Syn aggregation are currently approved for clinical use to combat the growing burden of synucleinopathies. Detailed mechanistic studies and an increasing appreciation of the role played by the interactions mediated by disordered terminal segments in fibril elongation and seeding highlight the challenges and suggest novel pathways for the development of inhibitors. As α-Syn aggregation mechanisms involve a number of inter-domain multivalent interactions between the N-, C-, and NAC domains, introducing multivalency in designing inhibitors is likely to be an effective strategy. Recently, Yushchenko, Shvadchak and colleagues constructed fusion proteins composed of α-Syn monomer or crosslinked dimers and a bulky globular protein [56,57]. These inhibitors likely efficiently bind to the fibril via the α-Syn protein and the bulky globular protein blocks access to the fibril ends for further monomeric α-Syn molecules. As our understanding of the polymorphic structure of fibrils via their disordered termini and their correlation with diseases matures, blocking fibril ends, particularly using multivalent modalities offers a promising route for the development of effective inhibitors.
Given the importance of the intrinsically disordered domains, experimental methods that can probe the structure of these regions in fibrils are needed as their conformations are too heterogeneous for resolution using cryo-EM. Elucidation of the structure of disordered regions using ssNMR is likely to provide insights into the roles of disordered regions in fibrils. Recent ssNMR-based elucidation of the structure of disordered regions in tau and huntingtin fibrils provide an exciting example of the experimental clarification of the critical role played by disorder in the growth and propagation of ordered fibrillar structures [58,59].
The overall picture that emerges is that interchain interactions between the NAC domain provide structure and stability for fibrils, whereas the terminal domains play multifaceted roles in the aggregation process via weak, transient, multi-valent, and environmentally sensitive charge-charge interactions. These regulatory effects of the terminal domains are critical for fibril polymorphism, aggregation kinetics, mechanisms of fibril growth, and cell-to-cell spread. A fuller understanding of the roles of the terminal domains would provide a basis for the design of novel multi-valent therapeutic interventions against cytotoxicity.
Highlights.
Intrinsically disordered domains play key roles in α-Syn aggregation
Interactions of the N-terminal region (residues ~1-60) control fibril formation kinetics.
The C-terminal region (residues ~100-140) of fibril interacts with the monomer N-terminal region during fibril growth
Mechanistic studies provide insight into the role of charge-charge interactions involving the C-terminal region involved in monomer-monomer and monomer-fibril interactions
Multivalent inhibition targeting multiple regions of α-Syn may be valuable for inhibitor development
Acknowledgements
SDK acknowledges funding from NSF CHE2226816, and JB acknowledges funding from NIH GM136431.
References
- 1.Sinnige T: Molecular mechanisms of amyloid formation in living systems. Chemical Science 2022, 13:7080–7097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Longhena F, Faustini G, Spillantini MG, Bellucci A: Living in Promiscuity: The Multiple Partners of Alpha-Synuclein at the Synapse in Physiology and Pathology. Int J Mol Sci 2019, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **3. Mehra S, Gadhe L, Bera R, Sawner AS, Maji SK: Structural and Functional Insights into α-Synuclein Fibril Polymorphism. Biomolecules 2021, 11. Insightful review article discusses how the intrinsically disordered alpha synuclein protein can adopt various structures and conformations. Different polymorphs exhibit different biochemical and pathological properties, and different synucleinopathies have their own unique strain of alpha synuclein.
- 4.Iadanza MG, Jackson MP, Hewitt EW, Ranson NA, Radford SE: A new era for understanding amyloid structures and disease. Nature Reviews Molecular Cell Biology 2018, 19:755–773. [DOI] [PubMed] [Google Scholar]
- 5.Buell AK: The growth of amyloid fibrils: rates and mechanisms. Biochemical Journal 2019, 476:2677–2703. [DOI] [PubMed] [Google Scholar]
- 6.Gadhe L, Sakunthala A, Mukherjee S, Gahlot N, Bera R, Sawner AS, Kadu P, Maji SK: Intermediates of α-synuclein aggregation: Implications in Parkinson's disease pathogenesis. Biophysical Chemistry 2022, 281:106736. [DOI] [PubMed] [Google Scholar]
- 7.Marotta NP, Lee VM: Modeling the cellular fate of alpha-synuclein aggregates: A pathway to pathology. Curr Opin Neurobiol 2022, 72:171–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oliveira LMA, Gasser T, Edwards R, Zweckstetter M, Melki R, Stefanis L, Lashuel HA, Sulzer D, Vekrellis K, Halliday GM, et al. : Alpha-synuclein research: defining strategic moves in the battle against Parkinson’s disease. npj Parkinson's Disease 2021, 7:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marotta NP, Ara J, Uemura N, Lougee MG, Meymand ES, Zhang B, Petersson EJ, Trojanowski JQ, Lee VMY: Alpha-synuclein from patient Lewy bodies exhibits distinct pathological activity that can be propagated in vitro. Acta Neuropathologica Communications 2021, 9:188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodriguez JA, Ivanova MI, Sawaya MR, Cascio D, Reyes FE, Shi D, Sangwan S, Guenther EL, Johnson LM, Zhang M, et al. : Structure of the toxic core of α-synuclein from invisible crystals. Nature 2015, 525:486–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **11. Ulamec SM, Brockwell DJ, Radford SE: Looking Beyond the Core: The Role of Flanking Regions in the Aggregation of Amyloidogenic Peptides and Proteins. Frontiers in Neuroscience 2020, 14. Informative review on the properties of the unstructured flanking regions around the amyloid core in several amyloid-forming proteins. These flanking regions are crucial for aggregation, fibril formation, and cellular function. Additionally, these flanking regions are crucial for the interactions with chaperones, RNA, and other amyloid-forming proteins.
- 12.Ulmer TS, Bax A, Cole NB, Nussbaum RL: Structure and dynamics of micelle-bound human alpha-synuclein. J Biol Chem 2005, 280:9595–9603. [DOI] [PubMed] [Google Scholar]
- 13.Dedmon MM, Lindorff-Larsen K, Christodoulou J, Vendruscolo M, Dobson CM: Mapping Long-Range Interactions in α-Synuclein using Spin-Label NMR and Ensemble Molecular Dynamics Simulations. Journal of the American Chemical Society 2005, 127:476–477. [DOI] [PubMed] [Google Scholar]
- 14.Bernadó P, Bertoncini CW, Griesinger C, Zweckstetter M, Blackledge M: Defining Long-Range Order and Local Disorder in Native α-Synuclein Using Residual Dipolar Couplings. Journal of the American Chemical Society 2005, 127:17968–17969. [DOI] [PubMed] [Google Scholar]
- 15.Watson MD, Lee JC: N-Terminal Acetylation Affects α-Synuclein Fibril Polymorphism. Biochemistry 2019, 58:3630–3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stephens AD, Zacharopoulou M, Moons R, Fusco G, Seetaloo N, Chiki A, Woodhams PJ, Mela I, Lashuel HA, Phillips JJ, et al. : Extent of N-terminus exposure of monomeric alpha-synuclein determines its aggregation propensity. Nature Communications 2020, 11:2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buratti FA, Boeffinger N, Garro HA, Flores JS, Hita FJ, Gonçalves PDC, Copello FDR, Lizarraga L, Rossetti G, Carloni P, et al. : Aromaticity at position 39 in α-synuclein: A modulator of amyloid fibril assembly and membrane-bound conformations. Protein Sci 2022, 31:e4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bell R, Castellana-Cruz M, Nene A, Thrush RJ, Xu CK, Kumita JR, Vendruscolo M: Effects of N-terminal Acetylation on the Aggregation of Disease-related α-synuclein Variants. Journal of Molecular Biology 2023, 435:167825. [DOI] [PubMed] [Google Scholar]
- 19.Doherty CPA, Ulamec SM, Maya-Martinez R, Good SC, Makepeace J, Khan GN, van Oosten-Hawle P, Radford SE, Brockwell DJ: A short motif in the N-terminal region of α-synuclein is critical for both aggregation and function. Nature Structural & Molecular Biology 2020, 27:249–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **20. Ulamec SM, Maya-Martinez R, Byrd EJ, Dewison KM, Xu Y, Willis LF, Sobott F, Heath GR, van Oosten Hawle P, Buchman VL, et al. : Single residue modulators of amyloid formation in the N-terminal P1-region of α-synuclein. Nature Communications 2022, 13:4986. Article elucidating the role of the P1 (residues 36-42) region in alpha synuclein aggregation. Mutations in this region have highly specific effects on cytotoxicity and on the kinetics of fibril formation. The findings of this research demonstrate that residues in the N-terminus are essential for aggregation.
- 21.Burmann BM, Gerez JA, Matečko-Burmann I, Campioni S, Kumari P, Ghosh D, Mazur A, Aspholm EE, Šulskis D, Wawrzyniuk M, et al. : Regulation of α-synuclein by chaperones in mammalian cells. Nature 2020, 577:127–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aspholm EE, Matečko-Burmann I, Burmann BM: Keeping α-Synuclein at Bay: A More Active Role of Molecular Chaperones in Preventing Mitochondrial Interactions and Transition to Pathological States? Life (Basel) 2020, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu K-P, Baum J: Detection of Transient Interchain Interactions in the Intrinsically Disordered Protein α-Synuclein by NMR Paramagnetic Relaxation Enhancement. Journal of the American Chemical Society 2010, 132:5546–5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Janowska MK, Wu KP, Baum J: Unveiling transient protein-protein interactions that modulate inhibition of alpha-synuclein aggregation by beta-synuclein, a pre-synaptic protein that co-localizes with alpha-synuclein. Sci Rep 2015, 5:15164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Williams JK, Yang X, Atieh TB, Olson MP, Khare SD, Baum J: Multi-Pronged Interactions Underlie Inhibition of α-Synuclein Aggregation by β-Synuclein. J Mol Biol 2018, 430:2360–2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williams JK, Yang X, Baum J: Interactions between the Intrinsically Disordered Proteins β-Synuclein and α-Synuclein. Proteomics 2018, 18:e1800109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *27. Lan-Mark S, Miller Y: Insights into the Interactions that Trigger the Primary Nucleation of Polymorphic α-Synuclein Dimers. ACS Chemical Neuroscience 2022, 13:370–378. The importance and role of the N-terminus in the dimerization of alpha synuclein monomers is highlighted. Experiments conducted show that residues in the N-terminus are able to initiate dimer nucleation through activation of residues in the NAC region. Additionally, it proposes that alpha helices in the N-terminus might impede contact between monomers and thus prevent dimerization.
- 28.Zhang Y, Wang Y, Liu Y, Wei G, Ding F, Sun Y: Molecular Insights into the Misfolding and Dimerization Dynamics of the Full-Length α-Synuclein from Atomistic Discrete Molecular Dynamics Simulations. ACS Chemical Neuroscience 2022, 13:3126–3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tompa P: Structural disorder in amyloid fibrils: its implication in dynamic interactions of proteins. The FEBS Journal 2009, 276:5406–5415. [DOI] [PubMed] [Google Scholar]
- 30.Gallardo R, Ranson NA, Radford SE: Amyloid structures: much more than just a cross-β fold. Curr Opin Struct Biol 2020, 60:7–16. [DOI] [PubMed] [Google Scholar]
- 31.Sawaya MR, Hughes MP, Rodriguez JA, Riek R, Eisenberg DS: The expanding amyloid family: Structure, stability, function, and pathogenesis. Cell 2021, 184:4857–4873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guerrero-Ferreira R, Kovacik L, Ni D, Stahlberg H: New insights on the structure of alpha-synuclein fibrils using cryo-electron microscopy. Curr Opin Neurobiol 2020, 61:89–95. [DOI] [PubMed] [Google Scholar]
- 33.Tuttle MD, Comellas G, Nieuwkoop AJ, Covell DJ, Berthold DA, Kloepper KD, Courtney JM, Kim JK, Barclay AM, Kendall A, et al. : Solid-state NMR structure of a pathogenic fibril of full-length human α-synuclein. Nat Struct Mol Biol 2016, 23:409–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *34. Medeiros J, Bamm VV, Jany C, Coackley C, Ward ME, Harauz G, Ryan SD, Ladizhansky V: Partial magic angle spinning NMR (1)H, (13)C, (15)N resonance assignments of the flexible regions of a monomeric alpha-synuclein: conformation of C-terminus in the lipid-bound and amyloid fibril states. Biomol NMR Assign 2021, 15:297–303. Through bond magic angle spinning solid-state NMR experiments (13C-13C INEPT TOBSY experiments) on α-Syn bound to DOPA:DOPC lipid vesicles and on α-Syn fibrils demonstrate that the C-terminus is unstructured and flexible in both contexts. NMR assignments of the flexible C terminal residues are obtained via 3D ssNMR experiments.
- **35. Landureau M, Redeker V, Bellande T, Eyquem S, Melki R: The differential solvent exposure of N-terminal residues provides "fingerprints" of alpha-synuclein fibrillar polymorphs. J Biol Chem 2021, 296:100737. Hydrogen-deuterium exchange experiments are coupled with MALDI Mass Spectrometry to map the solvent exposure of three different fibril polymorph structures: fibrils, ribbons, and fibrils 91. Results showed that while solvent accessibility to the C-terminal domain remains similar between polymorphs, the N-terminal exposure varies. It is proposed that these differences in surface accessibility may give rise to distinct binding properties of different polymorphs.
- 36.Lövestam S, Schweighauser M, Matsubara T, Murayama S, Tomita T, Ando T, Hasegawa K, Yoshida M, Tarutani A, Hasegawa M, et al. : Seeded assembly in vitro does not replicate the structures of α-synuclein filaments from multiple system atrophy. FEBS Open Bio 2021, 11:999–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fan Y, Sun Y, Yu W, Tao Y, Xia W, Liu Y, Zhao Q, Tang Y, Sun Y, Liu F, et al. : Conformational change of α-synuclein fibrils in cerebrospinal fluid from different clinical phases of Parkinson's disease. Structure 2022, 10.1016/j.str.2022.11.013. [DOI] [PubMed] [Google Scholar]
- 38.Schweighauser M, Shi Y, Tarutani A, Kametani F, Murzin AG, Ghetti B, Matsubara T, Tomita T, Ando T, Hasegawa K, et al. : Structures of α-synuclein filaments from multiple system atrophy. Nature 2020, 585:464–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang Y, Shi Y, Schweighauser M, Zhang X, Kotecha A, Murzin AG, Garringer HJ, Cullinane PW, Saito Y, Foroud T, et al. : Structures of α-synuclein filaments from human brains with Lewy pathology. Nature 2022, 610:791–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao K, Li Y, Liu Z, Long H, Zhao C, Luo F, Sun Y, Tao Y, Su XD, Li D, et al. : Parkinson's disease associated mutation E46K of α-synuclein triggers the formation of a distinct fibril structure. Nat Commun 2020, 11:2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao K, Lim YJ, Liu Z, Long H, Sun Y, Hu JJ, Zhao C, Tao Y, Zhang X, Li D, et al. : Parkinson's disease-related phosphorylation at Tyr39 rearranges α-synuclein amyloid fibril structure revealed by cryo-EM. Proc Natl Acad Sci U S A 2020, 117:20305–20315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun Y, Long H, Xia W, Wang K, Zhang X, Sun B, Cao Q, Zhang Y, Dai B, Li D, et al. : The hereditary mutation G51D unlocks a distinct fibril strain transmissible to wild-type α-synuclein. Nat Commun 2021, 12:6252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun Y, Hou S, Zhao K, Long H, Liu Z, Gao J, Zhang Y, Su XD, Li D, Liu C: Cryo-EM structure of full-length α-synuclein amyloid fibril with Parkinson's disease familial A53T mutation. Cell Res 2020, 30:360–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frieg B, Antonschmidt L, Dienemann C, Geraets JA, Najbauer EE, Matthes D, de Groot BL, Andreas LB, Becker S, Griesinger C, et al. : The 3D structure of lipidic fibrils of α-synuclein. Nat Commun 2022, 13:6810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **45. Yang X, Wang B, Hoop CL, Williams JK, Baum J: NMR unveils an N-terminal interaction interface on acetylated-α-synuclein monomers for recruitment to fibrils. Proceedings of the National Academy of Sciences 2021, 118:e2017452118. Using relaxation based solution-state NMR experiments, this paper investigates interactions between α-Syn monomers and fibrils, and demonstrates that the extreme N-terminus of the intrinsically disordered α-Syn monomer constitutes the binding interface to α-Syn fibril seeds. Based on the common interaction site of the α-Syn monomer with pre-formed fibrils and with pre formed oligomers, the authors propose a model in which the monomer N-terminus interacts transiently with the disordered C-terminal flanking region of the α-Syn fibril to promote monomer recruitment to the fibril for further fibril growth and propagation. Furthermore, this paper sheds light on how off-pathway oligomers may act as an inhibitor against fibril elongation.
- **46. Kumari P, Ghosh D, Vanas A, Fleischmann Y, Wiegand T, Jeschke G, Riek R, Eichmann C: Structural insights into α-synuclein monomer–fibril interactions. Proceedings of the National Academy of Sciences 2021, 118:e2012171118. Using NMR and EPR spectroscopy to study Asyn monomer-fibril interactions, this paper demonstrates intermolecular transient interactions between the positively charged N-terminus of α-Syn monomers with the negatively charged C-terminus of α-Syn fibrils. Theses electrostatic interactions lead to the unfolding of α-Syn monomers, exposing the aggregation-prone NAC region. Unfolded monomers are then able to be dynamically aligned to the fibril surface, favoring the process of secondary nucleation.
- 47.Thacker D, Sanagavarapu K, Frohm B, Meisl G, Knowles TPJ, Linse S: The role of fibril structure and surface hydrophobicity in secondary nucleation of amyloid fibrils. Proceedings of the National Academy of Sciences 2020, 117:25272–25283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **48. Peduzzo A, Linse S, Buell AK: The Properties of α-Synuclein Secondary Nuclei Are Dominated by the Solution Conditions Rather than the Seed Fibril Strain. ACS Chemical Neuroscience 2020, 11:909–918. This paper demonstrates that the properties of fibrils produced by secondary nucleation are dictated by solution conditions and not the parent seeds. The findings in this study also provide evidence that at low pH, fibrils are propagated through secondary nucleation instead of elongation of existing fibrils.
- 49.Buell AK, Galvagnion C, Gaspar R, Sparr E, Vendruscolo M, Knowles TPJ, Linse S, Dobson CM: Solution conditions determine the relative importance of nucleation and growth processes in α-synuclein aggregation. Proceedings of the National Academy of Sciences 2014, 111:7671–7676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **50. Pálmadóttir T, Malmendal A, Leiding T, Lund M, Linse S: Charge Regulation during Amyloid Formation of α-Synuclein. Journal of the American Chemical Society 2021, 143:7777–7791. These studies highlight the importance of electrostatic interactions in the amyloid formation of alpha synuclein through the monitoring of pKa values. The experiments conducted reveal that the change in pH during fibril formation is correlated to the high presence of acidic residues in the C terminus tail.
- 51.Moriarty GM, Olson MP, Atieh TB, Janowska MK, Khare SD, Baum J: A pH-dependent switch promotes β-synuclein fibril formation via glutamate residues. J Biol Chem 2017, 292:16368–16379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ni X, McGlinchey RP, Jiang J, Lee JC: Structural Insights into α-Synuclein Fibril Polymorphism: Effects of Parkinson's Disease-Related C-Terminal Truncations. J Mol Biol 2019, 431:3913–3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Farzadfard A, Pedersen JN, Meisl G, Somavarapu AK, Alam P, Goksøyr L, Nielsen MA, Sander AF, Knowles TPJ, Pedersen JS, et al. : The C-terminal tail of α-synuclein protects against aggregate replication but is critical for oligomerization. Communications Biology 2022, 5:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van der Wateren IM, Knowles TPJ, Buell AK, Dobson CM, Galvagnion C: C-terminal truncation of α-synuclein promotes amyloid fibril amplification at physiological pH. Chemical Science 2018, 9:5506–5516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dunning CJ, George S, Brundin P: What's to like about the prion-like hypothesis for the spreading of aggregated α-synuclein in Parkinson disease? Prion 2013, 7:92–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kyriukha YA, Afitska K, Kurochka AS, Sachan S, Galkin M, Yushchenko DA, Shvadchak VV: α-Synuclein Dimers as Potent Inhibitors of Fibrillization. Journal of Medicinal Chemistry 2019, 62:10342–10351. [DOI] [PubMed] [Google Scholar]
- 57.Shvadchak VV, Afitska K, Yushchenko DA: Inhibition of α-Synuclein Amyloid Fibril Elongation by Blocking Fibril Ends. Angew Chem Int Ed Engl 2018, 57:5690–5694. [DOI] [PubMed] [Google Scholar]
- 58.Matlahov I, Boatz JC, van der Wel PCA: Selective observation of semi-rigid non-core residues in dynamically complex mutant huntingtin protein fibrils. Journal of Structural Biology: X 2022, 6:100077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dregni AJ, Mandala VS, Wu H, Elkins MR, Wang HK, Hung I, DeGrado WF, Hong M: In vitro 0N4R tau fibrils contain a monomorphic β-sheet core enclosed by dynamically heterogeneous fuzzy coat segments. Proceedings of the National Academy of Sciences 2019, 116:16357–16366. [DOI] [PMC free article] [PubMed] [Google Scholar]