Abstract
The Saudi Initiative for Asthma 2024 (SINA-2024) is the sixth version of asthma guidelines for the diagnosis and management of asthma for adults and children that was developed by the SINA group, a subsidiary of the Saudi Thoracic Society. The main objective of the SINA is to have guidelines that are up-to-date, simple to understand, and easy to use by healthcare workers dealing with asthma patients. To facilitate achieving the goals of asthma management, the SINA Panel approach is mainly based on the assessment of symptom control and risk for both adults and children. The approach to asthma management is aligned for age groups: adults, adolescents, children aged 5–12 years, and children aged <5 years. SINA guidelines have focused more on personalized approaches reflecting a better understanding of disease heterogeneity with the integration of recommendations related to biologic agents, evidence-based updates on treatment, and the role of immunotherapy in management. The medication appendix has also been updated with the addition of recent evidence, new indications for existing medication, and new medications. The guidelines are constructed based on the available evidence, local literature, and the current situation at national and regional levels. There is also an emphasis on patient–doctor partnership in the management that also includes a self-management plan.
Keywords: Asthma, asthma control test, guidelines, Saudi Arabia
Section 1: Introduction
Asthma is a chronic heterogeneous inflammatory disease characterized by a history of respiratory symptoms such as wheezing, shortness of breath, chest tightness, and cough that vary over time and in intensity, together with variable expiratory airflow limitation.[1,2] Asthma is one of the most common chronic diseases in Saudi Arabia with increasing prevalence in the past decades.[3] It has a significant impact on patients, their families, the health-care system, and the community as a whole in terms of lost work and school days, poor quality of life, frequent Emergency Department visits, hospitalizations, and deaths.[4,5,6] Inadequate knowledge, lack of familiarity with new drugs, and awareness of the importance of disease control are common among primary care physicians who care for asthma patients in Saudi Arabia.[7,8] In addition to these key factors, there are other issues that influence the magnitude of the disease burden, such as socioeconomic status, number of siblings, knowledge of caregivers, and income.[9,10,11,12,13] Consequently, many asthma patients are uncontrolled and continue to be under-diagnosed, under-treated, and at risk of acute attacks.[14] This was also observed among pregnant women with asthma as one study from Saudi Arabia showed that almost half of pregnant women with asthma had the intention to stop asthma medications during pregnancy.[15] The increasing prevalence of asthma in the past three decades may be attributed to rapid lifestyle changes related to the modernization of Saudi society, changes in dietary habits, and exposure to environmental factors such as indoor allergens, dust, sandstorms, and tobacco. In addition, this high prevalence of asthma could be attributed to an increase in asthma awareness in the general population and among healthcare workers, allowing more individuals to be diagnosed.
As part of its long-term commitment to promote best practices in the field of respiratory diseases, the Saudi Thoracic Society (STS) launched the Saudi Initiative for Asthma (SINA) group in 2008. The SINA Panel is a group of Saudi experts with well-respected academic backgrounds and experience in the field of asthma. Sections related to asthma in children represent the views of a panel from the Saudi Pediatric Pulmonology Association, another subsidiary of the STS.
The SINA Panel aims to have updated guidelines, which are simple to understand and easy to use. It also aims toward enhancing the multidisciplinary care of asthma patients with special attention to non-asthma specialists, including primary care and general practice physicians and other healthcare workers.[16,17,18,19,20] The updated 2024 edition of SINA guidelines received a comprehensive update with an emphasis on personalized approaches reflecting a better understanding of disease heterogeneity with integration of recommendations related to new medications, approved biologic agents, evidence-based updates on treatment, especially on mild asthma, and update on the role of biologics in asthma management. The SINA Panel has also reviewed the stepwise approach to provide practical clinical practice guidelines based on the best available evidence and practices. Special attention was made to managing asthma during the time of emerging acute respiratory infections such as the recent coronavirus disease (COVID-19) caused by severe acute respiratory syndrome coronavirus 2. The SINA Panel stratified the guidelines based on the following age groups: adults (age above 18 years) and adolescents (age of 13–18 years); and children that were stratified into two groups: Ages of 5–12 years and ages below 5 years.[1,2]
Methods
The SINA Panel produced this clinical practice guideline for the diagnosis and management of asthma in adults and children based on the available evidence with special emphasis on local literature and the current setting in Saudi Arabia. The consensus among the SINA Panel was followed whenever there was inadequate or lack of evidence.[21] The following criteria are used to grade the evidence:
Evidence category A: Randomized controlled trials with a rich body of data
Evidence category B: Randomized controlled trials with a limited body of data
Evidence category C: Non-randomized trials and observational studies
Evidence category D: SINA Panel consensus judgment. This category is only used in cases where the provision of some guidance was deemed valuable, but the clinical literature addressing the subject was insufficient to justify placement in one of the other categories.
For this update, a similar approach to previous versions has been employed, whereby each section has been internally reviewed at least twice by SINA panel members. The SINA panel conducted frequent round-table discussions and virtual discussions. A panel of international experts reviewed the guidelines, and their recommendations were thoughtfully considered.
Section 2: Pathophysiology of Asthma
Asthma is a chronic inflammatory airway disease that results in a narrow airway lumen. The airway narrowing is caused by smooth muscle contraction, airway thickening, bronchospasm, and increased mucus secretion as well as bronchial wall thickening due to edema, smooth muscle hypertrophy, and subepithelial fibrosis. The pathophysiological mechanisms that underlie these changes are diverse and heterogeneous [Box 2.1]. They are driven by a variety of cell types including immune cells; mainly T-helper cells (Th2, Th17, Th1), B-cells, mast cells, eosinophils, dendritic cells, and neutrophils; as well as structural bronchial cells such as epithelial cells, myofibroblasts, and smooth muscle cells.[22] These mechanisms can be broadly classified into two major categories and further subdivided into subclasses (endotypes). Other classifications exist, but the classification that appears below is simpler and can be easily recognized by readily available biomarkers in any clinical setting. This classification is more pertinent to severe asthma and, therefore, has special implications for therapy with biologics.
Box 2.1.
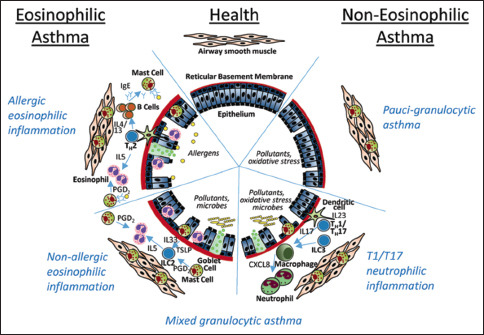
Immunopathology of asthma
Type 2 inflammation (T-helper 2 high) asthma
This is the most common type and includes 40%–70% of asthma patients. It is defined by sputum eosinophilia of ≥2% of leukocytes in a sample. Other ways to confirm the presence of type 2 inflammation are blood eosinophilia of ≥150\μl and Fractional exhaled nitric oxide (FeNO) ≥20 ppb. This eosinophil cutoff is way below the lower normal peripheral eosinophil count. The eosinophilic count may be reduced by high-dose inhaled corticosteroids (ICS) or maintenance systemic oral corticosteroids (OCS). Eosinophils secrete mediators such as major basic protein and eosinophil cationic protein that can cause bronchial epithelial damage and subepithelial fibrosis. Those patients usually respond well to ICS especially if they have mild or moderate disease. It is further subdivided into three phenotypes:
Early-onset allergic phenotype: This type usually starts in childhood, and it is associated with atopy. It can be triggered by allergen exposure. Allergens are taken up by dendritic cells and presented to naïve T-cells that develop into Th2 cells characterized by the secretion of type 2 cytokines: Interleukins (IL) namely, IL-4, IL-5, and IL-13. IL-4 and IL-13 are necessary for specific B-cell activation and switching into immunoglobulin E (IgE) producing cells. IgE binds to its high-affinity receptor on mast cells. Subsequent cross-linking of IgE molecules by the allergen will lead to mast cell degranulation and release of mediators such as histamine and tryptase as well as type 2 cytokines. In addition, IL-13 causes smooth muscle and goblet cell hyperplasia. This phenotype is characterized by positive allergy skin tests and increased serum-specific IgE. It usually responds to ICS and omalizumab, an anti-IgE therapy. Symptoms could also be triggered by similar triggers of the non-allergic type (see below)
Late-onset eosinophilic phenotype: This type usually starts during adulthood. Patients typically have no allergies, but usually have more severe airway limitation and hyperresponsiveness. It is usually less responsive to ICS compared to the previous phenotype. Patients with this phenotype may develop chronic rhinosinusitis with nasal polyps (CSwNP). It is triggered by microbes (bacteria and viruses), pollutants, and irritants. IL-5 is essential for eosinophil maturation, and survival and contributes with certain other chemokines to their recruitment to the bronchial airways.[23,24] An alternative mechanism of eosinophil recruitment originates from bronchial epithelial cells that in response to non-allergenic stimuli including viral infections release alarming such as IL-25, IL-33, and thymic stromal lymphopoietin (TSLP) that will stimulate innate lymphoid cells type 2 to release IL-5 and IL-13.[25] This phenotype is associated with a high level of blood/tissue eosinophil and/or high FeNO
Aspirin-exacerbated respiratory disease (AERD) phenotype: This is a subset of the late-onset eosinophilic phenotype, and it is characterized by asthma, nasal polyps, and cyclooxygenase-1 (COX-1) inhibitor-induced respiratory reactions. In this phenotype, prostaglandin E2 (PGE2) and its receptor EP2 baseline levels are both severely reduced. PGE2 is essential for preventing mast cells, and eosinophils from becoming activated. Loss of homeostatic PGE2 expression eliminates the 5-lipoxygenase (5-LOX) pathway’s negative feedback, which increases constitutive cysLT production. Strong COX-1 and COX-2 inhibitors include aspirin. When COX is inhibited, the 5-LOX pathway takes over as the mechanism for arachidonic acid metabolism. As a result of this inflammatory cascade, residual homeostatic PGE2 is suppressed, which leads to an excess of CysLT being produced by mast cells, eosinophils, and macrophages. Leukotriene C4 synthase (LTC4S) mediates this atypical cysLT synthesis. Most of the symptoms of AERD are caused by CysLTs, which are potent bronchoconstrictors and include leukotriene C4 (LTC4), LTD4, and LTE4.
Non-type 2 (T-helper 2 low) asthma: This can further be subdivided into 2 types
Neutrophilic phenotype: Variably defined as neutrophils of ≥40% of leukocytes in an induced sputum sample. It is less clearly characterized and involves the release of Th1 and Th17-related cytokines and IL8, granulocyte-macrophage colony-stimulating factor that attracts neutrophils to the airways. It is triggered by infections, irritants, and tobacco smoke and may be a manifestation of the use of steroids in patients with eosinophilic inflammation. Those patients are mostly adults and do not respond to ICS as well[26]
Pauci-granulocytic: In this form there is not as much inflammation. The airway limitation is supposedly driven by other mechanisms. It is the least common and patients usually have milder disease.[27]
Mixed type 2-high and type 2-low (granulocytic) asthma
This type has features of both eosinophilic and neutrophilic inflammation including their cytokines profile. It is less common than the two previous main types and tends to be more severe and more difficult to treat.[28]
Airway hyperresponsiveness
This is a major feature of all asthma endotypes. Its mechanisms and mediators are poorly understood. It worsens during and immediately after asthma attacks. It is usually worse in patients with severe asthma. However, it does not correlate well with markers of inflammation. Smooth muscle hypertrophy and neurohumoral factors may play a role in determining airway hyperresponsiveness (AHR).[29]
Airway remodeling
This is a major feature of asthma that starts early in the disease process and causes incomplete reversibility by bronchodilators. It is characterized by bronchial epithelial damage, thickening of the basement membrane, and muscle hypertrophy.[30,31] It is influenced by the ongoing airway inflammation and recurrent bronchoconstriction.[32]
Pathophysiology of acute asthma
The pathophysiology of acute asthma is less clear due to limited information. This is because of the difficulty in studying disease pathology and obtaining samples during exacerbations. The pathological manifestations generally depend on the trigger. At least 80% of cases of moderate to severe acute asthma are triggered by viruses, most commonly rhinovirus, but also respiratory syncytial and influenza viruses.[33] Viral infections can cause significant epithelial damage and symptoms tend to be more severe and last longer. On the other hand, allergen or irritant-triggered attacks tend to be milder and resolve more quickly. Recurrent attacks may lead to a progressive decline in lung function and increasing baseline asthma severity.[34,35,36]
Section 3: Diagnosis of Asthma in Adults and Adolescents
The diagnosis of asthma is based on clinical assessment by a detailed history and physical examination supported by spirometry with reversibility testing.
History
The symptoms of asthma are wheezing, cough, shortness of breath, and chest tightness but they are not specific to asthma and can be seen with other pulmonary diseases. However, the combination of these symptoms increases the probability of asthma. The pattern of symptoms is usually variable over time and the patient may be entirely asymptomatic between attacks.[37] Symptoms are usually worse at night and can be provoked by exercise or other triggering factors such as viral infections and smoke. Asthma diagnosis can be supported by taking a detailed history including the patient’s occupation, family history of asthma, other allergic disorders, smoking, and vaping. Box 3.1 lists the relevant questions that are commonly considered when taking a history where the diagnosis of asthma is under consideration. Asthma control may be worsened by coexisting symptomatic gastroesophageal reflux disease (GERD), rhinosinusitis, obesity, sleep disorders, or the use of some medications such as beta blockers and nonsteroidal anti-inflammatory drugs (NSAIDs) including aspirin.[38] Asthma and rhinosinusitis commonly coexist.[39,40]
Box 3.1.
Relevant questions in the diagnosis of asthma
| Does the patient or his/her family have a history of asthma or other atopic conditions, such as eczema or allergic rhinitis? |
| Does the patient have recurrent attacks of wheezing? |
| Does the patient have a troublesome cough at night? |
| Does the patient wheeze or cough after exercise? |
| Does the patient experience wheezing, chest tightness, or cough after exposure to pollens, dust, feathered or furry animals, exercise, viral infection, or environmental smoke (cigarettes, burning incense “Bukhoor,” or wood)? |
| Does the patient experience worsening symptoms after taking aspirin/nonsteroidal anti-inflammatory medication or use of |
| B-blockers? |
| Does the patient’s colds “go to the chest” or take>10 days to clear up? |
| Are symptoms improved by appropriate asthma treatment? |
| Are there any features suggestive of occupational asthma? |
Patients with asthma have different clinical phenotypes with specific clinical characteristics like allergic asthma which usually starts during childhood.[41] It could also be associated with CSwNP. The other phenotype is called non-allergic asthma which is not associated with a history of atopy or allergy. Other phenotypes include late-onset asthma, asthma with fixed airway obstruction, and asthma associated with obesity.
Physical examination
The physical examination of the chest may be normal in stable and controlled asthma but the presence of bilateral expiratory widespread, high-pitched, variable musical wheezing, are characteristic feature of asthma. This may be accompanied by shortness of breath or diminished oxygen saturation. The presence of wheezing indicates airway narrowing, but it is not correlated with asthma severity. Examination of the upper airways is important to look for evidence of allergic rhinitis, such as nasal mucosal swelling, nasal polyps, and postnasal dripping. Other allergic manifestations, such as atopic dermatitis, also support the diagnosis of allergic asthma.[42] The presence of a localized wheeze, crackles, stridor, clubbing, or heart murmurs should suggest alternative diagnoses.[43] Therefore, a careful consideration of any alternative diagnoses before commencing asthma treatment by a physician should be made.
Investigations
Spirometry is necessary to confirm airflow obstruction and demonstrates significant reversibility by performing a spirometry. The degree of significant reversibility is defined as an improvement in forced expiratory volume in 1 s (FEV1) ≥12% and ≥200 ml from the pre-bronchodilator value.[44] It may also help to identify other alternative diagnoses such as upper airway obstruction. However, normal spirometry or failure to show reversibility does not rule out the diagnosis of asthma, as it can be normal with the patient still being symptomatic.[45] Serial peak expiratory flow rate (PEF) measurements may be helpful in the diagnosis of asthma by showing the characteristic increased variability of <10% in twice daily PEF over 2 weeks and also for follow-up after starting treatment. A diagnostic therapeutic trial with significant reversibility after 4 weeks of ICS may be useful in confirming a diagnosis when it shows favorable reversibility.[45] Bronchoprovocation testing is another tool to rule out asthma with atypical presentation and normal spirometry, but it is not routinely required.
Chest X-ray (CXR) is not routinely recommended unless the diagnosis is in doubt when symptoms are not typical or suggest alternative diagnoses, when assessing a patient with severe asthma, or when asthma is associated with other medical conditions. Peripheral eosinophilia and elevated IgE levels are supportive of the diagnosis but are not routinely recommended unless dealing with moderate to severe asthma.[45] FeNO is an alternative method for detecting airway inflammation in eosinophilic asthma; however, it can be suppressed with the use of ICS in smokers.[46] Skin prick testing and radioallergosorbent test (RAST) are not routinely required to diagnosis asthma but may be helpful in identifying allergens to which the patient has been sensitized and in developing a strategy for avoiding allergen exposure.[47]
Section 4: Clinical Assessment in Adults and Adolescents
Principles of asthma assessment
The principles of optimal asthma management are recommended to initially consist of an assessment of asthma control.[48] Before commencing a patient on treatment, the SINA Panel recommends ensuring the following:
Assessment of asthma control
Assessment of risk factors for poor asthma control and fixed airway obstruction.
Performance of pulmonary function testing (PFT) with spirometry and/or PEF to assess airflow limitations and postbronchodilator reversibility
Documentation of current treatment and any issues related to adherence, inhaler technique, or side effects
Utilization of a written asthma action plan
Assessment of comorbidities such as rhinosinusitis, GERD, obesity, obstructive sleep apnea, anxiety, and exercise-induced laryngeal obstruction[49]
Close monitoring for patients with severe asthma and a history of asthma attacks.
Assessment of asthma symptoms control
In adults and adolescents, asthma control is based on assessing asthma symptoms, use of reliever medications, and impact on daily activities. Asthma control reflects the adequacy of management by describing the clinical status of a patient as controlled, partly controlled, or uncontrolled over the past 4 weeks. The control status may vary markedly over time and is recommended to entail frequent assessment of current asthma status, asthma burden, and medical management.[50] Focusing on asthma control may improve patient perceptions and expectations that improve symptom reporting and subsequently treatment decisions by clinicians.[51] Poor asthma control is associated with increased burden of the disease, asthma attacks, and mortality.[52] Therefore, symptom control assessment should be carried out during every clinical evaluation. The SINA Panel recommends the utilization of the asthma control test (ACT).
Asthma control test
The ACT is a commonly used tool to assess asthma control.[53] It is a short, validated, self-administered questionnaire to assess asthma control in the past 4 weeks.[54] It consists of five items including limitation of activity, shortness of breath, frequency of night symptoms, use of rescue medication, and the patient’s rating of overall control of asthma symptoms over the past 4 weeks.[55] The score ACT is the sum of the five questions where each is scored from 1 (worst) to 5 (best), leading to a maximum best score of 25. The SINA Panel recommends the utilization of asthma ACT to initiate asthma treatment in adults and adjust it at follow-up.[56,57,58] The clinically important significant change in ACT score is considered to be ≥3 units.[59] The level of asthma control is categorized into:
Controlled: An ACT score of ≥20 points
Partly controlled: An ACT score of 16–19 points
Uncontrolled: An ACT score of <16 points.
Fractional concentration of exhaled nitric oxide
Is closely associated with type 2 inflammation and commonly elevated in eosinophilic asthma. It is suggested that FeNO <25 ppb that indicates eosinophilia is unlikely, 25–49 ppb indicates possible eosinophilia, and FeNO >50 ppb indicates that eosinophilia is likely. However. FeNO can also be elevated in non-asthma conditions such as allergic rhinitis, atopy, eczema, and eosinophilic bronchitis. It can also be reduced in smokers and neutrophilic asthma. These considerations may restrict its application to asthma diagnosis. Nevertheless, in the right clinical context with the presence of variable respiratory symptoms, elevated FeNO >50 ppb allows a valid ruling-in of an asthma diagnosis.[60] A lower level of FeNO cannot rule out asthma. The presence of a high level of FeNO is also a risk factor for future severe and frequent asthma exacerbations and a decline in lung function. FeNO ≥20 ppb is indicative of type 2 inflammation, and it is a good predictor of response to the appropriate biologic. FeNO can also aid in determining steroid responsiveness and optimizing ICS doses. A higher level of FeNO can be used as an indication of poor adherence to ICS, for which the FeNO suppression test can be used to evaluate adherence in such patients.
Assessment of risk factors for future asthma attacks
The future risk of adverse outcomes should be assessed. This is achieved by assessing future risk of attacks, fixed airflow obstruction, and adverse effects of medications.[1,2] The SINA Panel recommends assessment of risk factors for poor asthma outcomes, especially in patients experiencing attacks by assessing risk factors for:
Independent risk factors for acute severe asthma attacks in the past 12 months or prior history of admission to an intensive care unit (ICU); especially if intubated[61,62]
Other modifiable risk factors are recommended to be addressed, such as high usage of relievers, frequent use of OCS, low FEV1, pregnancy, inadequate ICS, smoking and vaping, comorbidities, major psychological disorders, reduced socioeconomic status, presence of comorbidities[62]
Risk factors for fixed airway obstruction include inadequate ICS treatment, exposure to tobacco smoke or other noxious substances, low initial FEV1, or peripheral blood eosinophilia.[63]
Asthma severity assessment in clinical practice
There is a trend in clinical practice to retrospectively assess asthma severity based on the step of treatment required to control symptoms and attacks.[1,2,63,64,65] Before classifying asthma severity, it is essential to ensure that control is achieved and maintained while using a minimal level of medications over more than 3 months. Since asthma severity level could change over years or months, therefore, asthma level of severity can be classified as follows:
Mild asthma: Controlled asthma at step 1 or 2
Moderate asthma: Controlled asthma at step 3
Severe asthma: Asthma that requires treatment step 4 or 5.
Assessment when control is not achieved
If asthma control is not achieved at any step during therapy, the SINA panel recommends assessing the following:
Appropriateness of prescribed medications and doses
Patient adherence and correct technique in using devices
Selection of the appropriate device and appropriate spacer with a pressurized metered-dose inhaler (MDI) device
Obstacles in taking prescribed medications (e.g., cost, time, and patients’ concerns on lack of perceived need)
Environmental exposure to allergens at home
Assessment of comorbidities such as rhinosinusitis, CSwNP, GERD, obesity, obstructive sleep apnea, and anxiety
Future risk of attacks and fixed airflow obstruction.
Section 5: Non-pharmacological Management in Adults and Adolescents
By utilizing pharmacological and non-pharmacological measures, the long-term goals of asthma management aim toward maintaining asthma control, minimizing exacerbations, and avoiding asthma-related death [Box 5.1]. There has been a shift in asthma treatment concepts from symptom control toward clinical remission that aims toward sustained absence of symptoms and exacerbations, stable lung function, and no need for OCS.[66] The appropriate implementation of non-pharmacological measures also aims to the use of the least possible doses of asthma medications to minimize their side effects.
Box 5.1.
Long-term goals of asthma management
| Control asthma symptoms (cough, wheezing, and shortness of breath) |
| Infrequent and minimal use (≤2 days a week) of the reliever therapy |
| Maintain (near) normal pulmonary function |
| Maintain a normal level of exercise and physical activity |
| Prevent asthma exacerbations and minimize the need for emergency department visits or hospitalizations |
| Optimize asthma control to avoid oral corticosteroids |
| Achieving clinical remission of asthma |
| Improve quality of life and reduce the risk of adverse outcomes |
| Avoiding asthma-related mortality |
Developing a partnership with the patient
The development of a partnership between patients and healthcare professionals leads to the enhancement of knowledge, skills, and attitudes toward a better understanding of asthma and its management. Based on the agreed goals of management, a written self-management action plan is recommended to be offered to all patients. A wide variety of plans are available. This is expected to reflect positively on patient adherence, which is a major issue in management.
Asthma education
The goal of asthma education is to provide patients with adequate training to enhance their knowledge and skills to be able to adjust treatment according to a guided self-management plan.[67,68,69,70] To enhance the level of knowledge and skills among asthma patients, it is recommended to include knowledge about asthma and skills related to prescribed inhaler devices, as there may be misperceptions about the use of inhalers and the safety of ICS [Box 5.2].[70,71,72] Asthma education is recommended to be conducted by a well-trained healthcare worker, who has good communication skills and is able to create an interactive dialogue in a friendly environment. With the availability of appropriate information, patients are expected to continue on the management plan and be reassured about the control of their asthma.[73] It is essential to get feedback from the patient to maintain a bidirectional rapport. Reproducible evidence has shown that a well-structured asthma education program improves the quality of life, reduces cost, and decreases the utilization of healthcare resources.[74,75]
Box 5.2.
Outcomes of asthma education program
| Creation of patient-healthcare worker partnership |
| Understanding the clinical presentation of asthma and methods of diagnosis |
| Ability to differentiate between “reliever” and “controller” medications and their appropriate indications |
| Realizing the importance of persistence and adherence to asthma treatment |
| Recognition of potential side effects of medications and the appropriate action to minimize them |
| The ability to use inhaler devices correctly |
| Identification of symptoms and signs that suggest worsening of asthma control and the appropriate action to be taken |
| Understanding the approach for monitoring asthma control |
| Recognition of the situations that need urgent medical attention |
| Ability to use a written self-management plan |
Identify and reduce exposure to risk factors
Measures to prevent or reduce exposure to risk factors should be implemented wherever possible. There are different triggers leading to acute asthma exacerbations, which may include: allergens, viral infections, pollutants, drugs, and occupational agents. These factors can be classified as indoor or outdoor allergens and occupational sensitizers.
Indoor allergens and air pollutants: There is a wide spectrum of indoor allergens that includes dust mites, animals (mainly cats), cockroaches, and fungi (e.g., alternaria and aspergillus). Single-allergen interventions are likely to fail. However, multifaceted, tailored intensive interventions may help in improving asthma control. It may take a few months for the allergen level to become significantly lower from the implementation of the related control measures.[76] The most important indoor air pollutant is related to tobacco exposure. Measures to avoid tobacco exposure are expected to lead to better asthma control and avoidance of long-term lung function impairment
Outdoor allergens and dust: Outdoor allergens such as pollens and molds are difficult to avoid completely; however, exposure may be reduced by closing windows and doors and using air conditioning. It is recommended to avoid strenuous outdoor physical activities in cold weather, low humidity, or high air pollution. In a single-center study in Saudi Arabia, sandstorms were shown to worsen asthma symptoms but not hospital admission in children with asthma. It is advisable to avoid going out in the storm, especially for those with uncontrolled asthma[77]
Occupational exposures: Whenever an occupational sensitizer is identified, it is advisable to keep the affected person away from that environment.[78] The earlier the removal of this sensitizer takes place, the higher the chance of complete recovery from occupational asthma
Food and drugs: Food and food additives are uncommon triggers of asthma. Avoidance is not generally recommended until it is documented by a specialist.[79] However, certain drugs that could worsen asthma symptoms should be avoided (e.g., beta-blockers), whenever possible
Vaccination: Annual influenza vaccination is strongly recommended for individuals with asthma, especially those with severe asthma.[80,81,82] It usually becomes available early in the fall season. Pneumococcal vaccination and COVID-19 vaccination are also recommended as per the local guidelines.[83]
Section 6: Pharmacological Management in Adults and Adolescents
The SINA panel recommends asthma treatment to be based on the following phases:
Initiation of treatment
Adjustment of treatment
Maintenance of treatment.
At each phase, the patient is recommended to have a clinical assessment that includes symptoms assessment by ACT, a physiological measurement with spirometry and FeNO, a review of current medications and patients’ adherence and inhaler technique, a risk for exacerbations, and the response to treatment. Based on the clinical and physiological assessment, the patient is placed on the appropriate treatment step. The medication appendix contains more information about medications used in asthma treatment.
Saudi initiative for asthma strategies for asthma treatment in adolescents and adults
The SINA panel recommends the following strategies for asthma treatment:
ICS is recommended for all steps as it is the most effective controller and the cornerstone of asthma treatment (Evidence A).[84,85,86] Uncontrolled patients on ICS may require the addition of other controllers that include a long-acting β2 agonist (LABA), a long-acting anti-muscarinic agent (LAMA), leukotriene receptor antagonists (LTRA), or biologics
The combination of ICS/formoterol in a single inhaler is recommended to be used for regular maintenance dosing and when needed as well. This is called the maintenance and reliever therapy (MART) approach[87,88]
The combination of ICS/non-formoterol LABA in a single inhaler is recommended for proactive regular dosing with a short-acting bronchodilator (SABA) reliever on as needed basis (with or without ICS)[89]
A once-a-day fixed-dose combination of ICS and LABA is currently available that can be prescribed with SABA (with or without ICS) as a reliever. When compared to a twice-a-day combination, a once-a-day combination led to better adherence and a lower risk of discontinuing treatment.[90] Once-a-day single-inhaler triple therapy (SITT) is also available that contains ICS/LABA/LAMA
-
Relievers are fast-acting bronchodilator medications that must be available to patients at all steps. Increasing the use of reliever treatment should be considered as an early sign of worsening of asthma control (Evidence A).[91] The available relievers are:
- SABAs, such as salbutamol, are recommended to be taken as-needed to relieve symptoms. Using SABA alone without a controller was found to increase the risk of asthma exacerbations and asthma-related death. Patients who consumed more than three canisters of SABA per year were found to be at risk of increasing morbidity and mortality.[92] When compared with SABA alone as a reliever, fixed-dose combination (FDC) ICS/SABA led to better outcomes with a significant reduction in asthma exacerbations, hospitalization, and asthma-related death.[93] Whenever a fixed-dose ICS/SABA combination becomes available in the Saudi market, it is recommended to be prescribed on an as-needed basis to relieve symptoms (Evidence A).[94,95,96]
- Formoterol/ICS combination could be used as a reliever therapy on an as-needed basis for mild asthma and whenever the combination of formoterol/ICS is prescribed as maintenance therapy (MART approach) (Evidence A).[97,98,99] The maximum recommended dose of formoterol component is 72 μg. Exceeding this level for 2–3 days may be a warning sign of deterioration of asthma control that requires seeking medical advice.[98,99,100]
Regular assessment of the adequacy of treatment, proper technique, and adherence
Regular assessment for independent risk factors for acute asthma exacerbations in the past 12 months or prior history of admission to an ICU; especially if intubated.[61,101] Other modifiable risk factors are recommended to be addressed, such as pregnancy, inadequate ICS, smoking and vaping, comorbidities, and major psychological conditions
Regular assessment of risk factors for fixed airway obstruction that includes inadequate ICS treatment, exposure to tobacco smoke or other noxious substances, low initial FEV1, or peripheral blood eosinophilia[102]
Management of comorbidities with special attention to rhinosinusitis, GERD, and Obesity. Rhinosinusitis is a condition that affects asthma control where its treatment is expected to improve asthma outcome (Evidence A).[103] Treatment includes nasal saline washes, nasal steroids, LTRA, and antihistamines. Concomitant rhinosinusitis is recommended to be treated appropriately as well.
Adherence to treatment
It is challenging to objectively assess adherence to medications during routine busy practice. It has been found that self-reported adherence and clinicians’ judgment of adherence are often overestimated.[104] In one study, only 13% of patients who received two controllers for asthma management were considered to have optimum adherence.[105] Regular ICS dosing (with or without LABA) had better asthma symptom control, a greater degree of bronchoprotection, and still maintained low systemic activity for patients with mild asthma when compared to intermittent dosing.[86,106] Therefore, healthcare professionals are recommended to regularly advise patients to be adherent to their inhalers and objectively assess patterns of prescription of relievers and controller inhalers.[107,108] Factors leading to non-adherence may be related to poor inhaler technique, a regimen with multiple drugs or devices, concern regarding side effects from the drugs, and cost of medications.[105,109,110,111,112] Other factors include a lack of knowledge about asthma, lack of partnership in its management, inappropriate expectations, underestimation of asthma symptoms, use of unconventional therapy, and cultural issues.[112,113]
Initiation of asthma treatment
Patients with asthma often underestimate the presence of symptoms and tend to assume their asthma is under control even when this is not the case.[104,105] Therefore, the consensus among the SINA Panel is to simplify the approach and supplement the initiation of asthma therapy by utilizing an objective measurement with the ACT bases on the available evidence (Evidence B).[58] The following initial steps are recommended for treatment-naïve patients based on ACT score [Box 6.1].
Box 6.1.
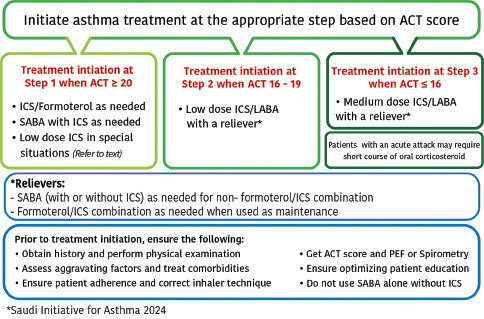
The SINA approach for asthma treatment dinitiation based on ACT score for adults an adolescents
Start at step 1 when the ACT score is 20 points or more:
It is recommended to use a fixed-dose combination of ICS/formoterol on an as-needed basis (Evidence A).[99,114]
An alternative option is to use SABA together with low-dose ICS on an as-needed basis in separate inhalers (Evidence B).[95,115,116] Whenever a fixed-dose combination of ICS/SABA becomes available in the Saudi market, it is recommended to be prescribed on an as-needed basis (Evidence A).[94,95,96]
Maintenance of daily low-dose ICS (with or without LABA) is recommended for patients with symptoms more than twice a week, risk factors for acute exacerbation (severe exacerbations in the past 12 months or prior history of admission to an ICU; especially if intubated) or evidence of fixed airway obstruction (Evidence B).[102,117,118,119,120] Early introduction of ICS leads to greater improvement of FEV1.[117]
Start at step 2 when the ACT score is between 16 and 19 points:
It is recommended to use a fixed-dose combination of low-dose ICS/LABA for patients when an ACT score of 16–19 points (Evidence A).[58] Physicians should ensure the adherence of patients to this regimen.[106] Reliever therapy is recommended as well in the form of SABA (with or without ICS) whenever a maintenance fixed-dose combination of ICS/non-formoterol LABA or of ICS/formoterol whenever a maintenance fixed-dose ICS/formoterol combination is prescribed (MART approach).
Start at step 3 when the ACT score is <16 points:
It is recommended to use a fixed-dose combination of medium-dose ICS/LABA as maintenance treatment for patients with an ACT score of <16 points (Evidence A).[58] Reliever therapy is recommended as well in the form of SABA (with or without ICS) whenever a maintenance fixed-dose combination of ICS/non-formoterol LABA or of ICS/formoterol whenever a maintenance fixed-dose ICS/formoterol combination is prescribed
For patients with ACT score <16 points presenting with early signs of asthma exacerbation, it is recommended to consider adding a short course of oral steroids (Oral prednisolone 30–60 mg for 3–5 days[121] Those patients need close follow-up to adjust treatment as appropriate to achieve asthma control.
Adjustment of treatment
After initiation of asthma treatment, it is recommended to assess the patient at 1–3 month intervals as appropriate (Evidence D).[122] The SINA Panel recommends the utilization of a step-wise approach of therapy to achieve asthma control. The stepwise approach consists of five steps as shown in Box 6.2 The SINA Panel also presents a simplified chart in Box 6.3 that includes the first option for asthma management at each step; this simplified chart should be supplemented by reviewing the detailed chart in Box 6.2 and the following narrative text.
Box 6.2.
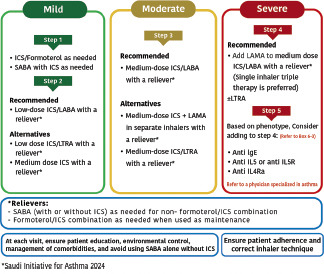
The SINA pproach for asthma treatment adjustment and maintenance for adults and adolescents
Box 6.3.
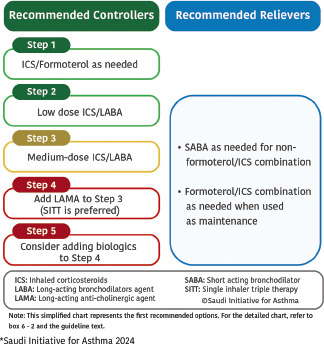
SINA 2024 simplified the approach for asthma treatment for adults and adolescents
The SINA panel recommends that the stepwise approach is not meant to be compartmental; it is rather a continuum of care based on patient engagement and close monitoring of the disease (Evidence D).[123] In clinical practice, asthma severity can be retrospectively assessed based on the step of treatment required to control symptoms:[63,64,65,124]
Mild asthma: controlled asthma at step 1 or 2
Moderate asthma: controlled asthma at step 3
Severe asthma: requires asthma management at step 4 or 5.
Reliever medications must be made available to patients at all steps. Increasing the use of reliever treatment is usually an early sign of asthma control worsening (Evidence A).[91,125] Approximately one in five patients with mild asthma who are not receiving appropriate treatment may develop at least one exacerbation in the following 12 months.[99,126,127] Reliever therapy is recommended as well in the form of SABA (with or without ICS) whenever a maintenance fixed-dose combination of ICS/non-formoterol LABA or of ICS/formoterol whenever a maintenance fixed-dose ICS/formoterol combination is prescribed. The following paragraphs will describe asthma treatment at each step.
Treatment at step 1
Symptoms are usually mild and infrequent (usually < twice a week) with an ACT score of ≥20 points and no risk factors for asthma exacerbations. At this step, SABA alone on an as-needed basis is not anymore recommended.
Recommended option: It is recommended to use ICS/formoterol on an as-needed basis (Evidence A)[98,99,100,114]
Alternative option: Use SABA together with low-dose ICS on an as-needed basis from separate inhalers (Evidence B).[95,115,116] Whenever a fixed-dose combination of ICS/SABA becomes available in the Saudi Market, it is recommended to be prescribed on an as-needed basis (Evidence A)[94,95,96]
Healthcare professionals are recommended to monitor the frequency of reliever usage to avoid a status of overreliance. Whenever this is observed, it is recommended to step up therapy (Evidence D)
Patients with seasonal asthma, who are symptomatic during the season, are recommended to be treated with low-dose ICS (with or without LABA) before the beginning of the season (Evidence D).
Treatment at step 2
Recommended options: It is recommended to use a daily fixed-dose combination of low-dose ICS/LABA with an as-needed reliever for symptom relief (Evidence A).[87,128,129,130] Reliever therapy is recommended in the form of SABA (with or without ICS) whenever a maintenance fixed-dose combination of ICS/non-formoterol LABA or in the form of ICS/formoterol whenever a maintenance fixed-dose ICS/formoterol combination is prescribed.[131,132] Physicians should ensure the adherence of patients to this regimen[106]
-
Alternative options:
Treatment at step 3
Recommended options: The fixed-dose combination of medium-dose ICS/LABA was found to improve asthma control and reduce asthma exacerbations for patients whose asthma is not controlled at step 2 (Evidence A).[87,128,129,137] Reliever therapy is recommended in the form of SABA (with or without ICS) whenever a maintenance fixed-dose combination of ICS/non-formoterol LABA or in the form of ICS/formoterol whenever a maintenance fixed-dose ICS/formoterol combination is prescribed. Physicians should ensure the adherence of patients to this regimen[106]
Alternative options: Tiotropium is a LAMA approved for the treatment of chronic obstructive pulmonary disease (COPD).[138,139,140] Evidence has shown that when tiotropium is added to an ICS delivered by multiple inhalers; it improves symptoms, reduces the risk of exacerbation, and improves lung function in patients with inadequately controlled asthma. This evidence supports that tiotropium can be combined with ICS whenever LABA cannot be used.[141] Its effect appears to be at least equivalent to LABA (Evidence A)[142,143,144]
Consultation with an asthma specialist is recommended whenever there is difficulty in achieving control at step 3 (Evidence D).
Available options for fixed-dose inhaled corticosteroids/long-acting β2 agonist combination at step 2 and 3
ICS in the form of beclomethasone propionate, budesonide, fluticasone propionate is available in combination with salmeterol. These are prescribed twice daily with SABA (with or without ICS) as a reliever
ICS combined with formoterol can be used as maintenance and reliever without adding SABA[95,97,115,116]
Once-a-day fixed-dose combination of ICS with vilanterol or indacaterol is also available. SABA (with or without ICS) should be utilized as a reliever. This combination leads to better adherence and a lower risk of discontinuing treatment when compared to twice a day combination[90]
Inhaled LABA or LAMA should never be used as monotherapy in asthma management but must always be accompanied by the use of ICS, preferably in one inhaler device for the combination of ICS/LABA.[145] Asthma patients taking inhaled LABA without inhaled ICS are at an increased risk of asthma exacerbations, hospitalizations, and death[146]
-
Twice a day combination:
- If salmeterol/ICS fixed-dose combination is selected, it can achieve asthma control in a majority of patients (Evidence A).[129] Salmeterol has a slow onset of action; therefore, it should only be used for maintenance treatment with SABA (with or without ICS) as a reliever
- If a formoterol/ICS fixed-dose combination is prescribed, it is recommended to be used as maintenance with one inhalation twice daily for step 2 or with two inhalations twice daily for step 3. Extra doses of up to 12 inhalations per day can be used as the reliever therapy from the same device (Evidence A).[95,97,115,116] Those patients who require such high doses for 2–3 days should seek medical advice to step up maintenance therapy (Evidence A).[97]
-
Once-a-day combination:
- The once-a-day combination of fluticasone furoate/vilanterol 100/25 μg in the Ellipta® device (Relvar®) can be prescribed for adults and children above 12 (Evidence A).[147,148] Vilanterol has the advantage of an onset of action within 15 min and a long half-life; however, it can only be used as a maintenance treatment while continue using SABA (with or without ICS) as a reliever
Treatment at step 4
-
Recommended options: If control is not achieved after escalation to medium-dose ICS/LABA at step 3, adding LAMA in a single inhaler is recommended (Evidence A).[152] This novel approach of SITT was found to be a safe and effective therapeutic approach.[153,154] Moreover, when compared to multiple inhalers triple therapy (MITT), SITT usage is cost-effective and is associated with better adherence.[152] The following SITT are recommended upon availability:
- Once-a-day SITT combination of fluticasone furoate/umeclidinium/vilanterol 200/62.5/25 μg (Trelegy Ellipta®) is a recommended option. Adding umeclidinium to the combination of ICS/LABA was found to be an effective treatment option with a favorable risk-benefit profile as it led to improved symptoms and FEV1, particularly in patients with raised biomarkers of type 2 airway inflammation (Evidence A).[155] Real-world data showed that this SITT has led to lower OCS use, asthma-related exacerbations, and lower SABA usage compared to pre-treatment data.[156] SITT with a lower dose of fluticasone furoate (fluticasone furoate/umeclidinium/vilanterol 100/62.5/25 μg) could be considered as an option for a patient with lower biomarkers for type 2 airway inflammation (Evidence B).[156] It can also be considered for stepping down once control is achieved upon using higher dose SITT. Use SABA (with or without ICS) as a reliever with this combination
- Once-a-day SITT combination of mometasone furoate/indacaterol/glycopyrronium 160/150/50 μg in the Breezhaler® device (Enerzair®) is a SITT option that improves symptoms and lung function (Evidence A).[151] Further data showed that this SITT improved lung function, reduced asthma exacerbations, and provided comparable asthma control to the ICS/LABA combination.[157] SITT with a lower dose of mometasone furoate (mometasone furoate/indacaterol/glycopyrronium 80/150/50 μg) could be considered as an option for a patient with lower biomarkers for type 2 airway inflammation (Evidence D). It can also be considered for step down once control is achieved upon using higher dose SITT. Use SABA (with or without ICS) as a reliever
- Twice-a-day SITT combination of beclomethasone dipropionate/formoterol fumarate/glycopyrronium 172/5/9 μg in an MDI device (Trimbow®) is a SITT option that improves lung function and reduces exacerbation (Evidence A).[158] SITT with a lower dose beclomethasone dipropionate (beclomethasone dipropionate/formoterol fumarate/glycopyrronium 87/5/9 μg) could be considered as an option for a patient with lower biomarkers for type 2 airway inflammation (Evidence D).[158] It can also be considered for step down once control is achieved upon using higher dose SITT. Use SABA (with or without ICS) as a reliever.
-
Other considerations:
- MITT by adding tiotropium in a separate inhaler to the combination of medium-dose ICS and LABA is another option as it significantly improves lung function in uncontrolled cases and modestly reduces asthma exacerbations (Evidence A).[159,160,161] However, SITT has the advantage of being cost-effective and of better adherence when compared with MITT[152]
- High-dose ICS/LABA may be considered in some patients who are uncontrolled on medium-dose ICS/LABA.[137,165] However, it is recommended to step down whenever possible to avoid potential side effects.[136,166] An additional controller is recommended to be introduced before considering the high-dose ICS
- If a patient is still uncontrolled at step 4, biologic therapy is recommended to be considered as described in step 5.
Treatment at step 5
Early consideration of biological therapy may lead to clinical remission and save the patient from frequent or chronic use of OCS and reduce asthma exacerbations.[66] This therapy is recommended based on appropriate indications and availability. When choosing a biological agent, several factors should be considered including the frequency of administration, cost, side effect profile, age of onset of asthma, presence of comorbid conditions such as nasal polyps, previous response, and physician experience with the treatment. Consultation with an asthma specialist is strongly recommended for patients requiring treatment at step 5 (Evidence D). The following biological agents are available for step 5. The medication Appendix contains the details of these biologic:
Anti-IgE therapy: Omalizumab
Anti-IL 5 therapy: Mepolizumab
An anti-IL-5 receptor therapy: Benralizumab
An anti-IL 4 receptor α antibody: Dupilumab
Anti-TSLP: Tezepelumab (Not yet locally available).
Approach to selecting appropriate biologic therapy
The SINA Panel recommends assessing patients requiring biologics thorough clinical evaluation, ACT, peripheral eosinophil count, FeNO, IgE level, and RAST. Box 6.4 shows the approach for a patient requiring biologics.
Box 6.4.
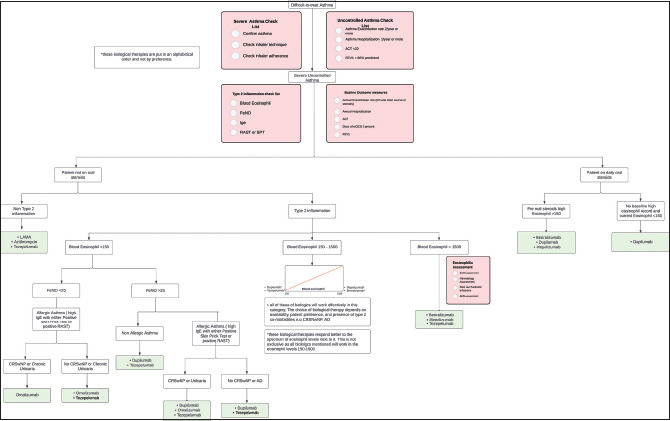
Algorithm for selection of appropriate biologics for severe asthma
Assessment of response to biologics
Biologics efficacy needs to be reassessed after 4–6 months after initiation. This can be made by comparing baseline and follow-up outcomes parameters such as ACT score, exacerbation rate, hospitalization, FEV1, and daily OCS dose. If there is no response to biologics based on these outcomes, assessment of asthma diagnosis and ruling out coexisting conditions are needed, ensuring adherence and compliance, then consideration of a switch to a different biologic.
Combining biological therapy was tried in real life and one retrospective study.[167] It appeared that the biologics combination was well tolerated with no signal of significant side effects. In other case reports,[167,168] a cyclical method was tried in combining dupilumab twice monthly and mepolizumab monthly was able to control a patient with severe asthma. A prospective study is needed to assess the effect of such an approach.
Patient at step 5 who is not a candidate for biologics
If the patient does not have any of the biologics phenotypes, or biologics are not available or not adequately controlling the disease, the alternative approach is to use the lowest possible dose of long-term OCS (Evidence D).[65] Other alternatives are mentioned in the severe asthma section, such as long-term macrolides.
For patients who require long-term OCS, the following are recommended to be considered:
Use the lowest possible dose to maintain control
Closely monitor the development of corticosteroid-related side effects
When asthma control is achieved, attempts to reduce the dose of systemic corticosteroids, preferably to every other day frequency, are recommended. Maintaining a high dose of ICS therapy may help to reduce the dose of systemic corticosteroid
Upward adjustment of the corticosteroid dose at the time of stress (e.g., infection, asthma exacerbations, surgery, etc.) is essential
Concurrent treatments with calcium supplements, vitamin D, and bone-sparing medications (e.g., bisphosphonates) in patients who have risk factors for osteoporosis or low bone mineral density are strongly recommended.
Maintaining asthma control
Regular follow-up by a healthcare worker is essential. Depending on the level of asthma control, it is recommended to have a follow-up at 1–3 months intervals after treatment initiation (Evidence D).[169,170] Follow-up is recommended to include monitoring and reviewing the patient’s written asthma action plan, medication adherence, and inhaler technique, patient’s behaviors, comorbidities, and possible side effects of the medications. Once asthma is controlled for at least 3 months, a step down in pharmacologic therapy is recommended at the minimum level that can maintain good control and minimize the side effects (Evidence D). The following are the general recommendations:
Reduction in therapy is recommended to be gradual and closely monitored based on clinical judgment of the individual patient’s response to therapy and ACT score (Evidence D)
If the patient is on ICS as monotherapy at Step 1, the dose of ICS may be reduced gradually every 3–6 months to the lowest dose possible that is required to maintain control (Evidence B),[67,171,172] and then changed to a single daily dose (Evidence A).[173] It is recommended to be clearly explained to the patient that asthma control may deteriorate if treatment is abruptly discontinued[174]
If the patient is on a combination of ICS/LABA at step 2 or 3, abrupt discontinuation of LABA is not recommended as it may lead to deterioration of the control. Therefore, initial gradual reduction of ICS to the lowest possible ICS dose before discontinuation of LABA is recommended[175]
If the patient is on a combination of ICS and LABA, LTRA, or other controllers; start by tapering ICS to the lowest possible dose (Evidence B).[176,177] If control is achieved, LTRA may be discontinued first (Evidence D)[176]
For significant oral side effects occur, consider a change in therapy, reduction in the dose or frequency of ICS (if possible), advise vigorous mouth washing after inhalation, use of spacer (concomitant with MDI devices), and/or use of appropriate local antifungal therapy for severe oral thrush[178]
For patients with well-controlled eosinophilic asthma treated with mepolizumab for at least 18 months, extending the dosage intervals gradually between the injections up to 6–8 weeks bears the potential to save costs for the health care system without compromising asthma control[179]
For patients with well-controlled asthma treated with omalizumab for at least 18 months, extending the dosage intervals gradually between the injections up to 3–4 weeks bears the potential to save costs for the health care system without compromising asthma control[180]
Patients should be informed that asthma control may deteriorate if treatment is completely discontinued.[174]
Referral to an asthma specialist
Situations that require referral to an asthma specialist for consultation or co-management include:
Uncertainty regarding the diagnosis
Difficulty achieving or maintaining asthma control
Immunotherapy or biological therapy is being considered
Difficulty to achieve asthma control at step 3 or higher
Acute asthma exacerbation requires hospitalization
Patient request for a second opinion or further advice.
Severe asthma
There are several terms used in practice for uncontrolled asthma where each points to an aspect of the disease such as chronic severe asthma, steroid-dependent asthma, and refractory asthma are some of these terminologies.[181,182] However; it is important to distinguish between severe asthma and uncontrolled asthma. Severe asthma is defined as “Uncontrolled asthma at SINA step 4 despite adequate adherence and after addressing all comorbidities.”[65] Severe asthma probably accounts for 5%–10% of adult asthma, but the healthcare cost is disproportionally high.[183] Morbidity and mortality are also higher compared to regular asthma patients because of increased side effects of treatment and more frequent exacerbations and/or hospitalizations.[184,185] Before a diagnosis of severe asthma is considered, patients must undergo a systematic assessment where the diagnosis of asthma is confirmed, and comorbidities are identified and treated.[186] Patients whose poor asthma control is related to other factors, such as poor adherence, inhaler use technique, or due to the presence of other diseases, are to be termed “difficult-to-treat asthma.”[187] There are common comorbidities that need to be assessed in severe asthma such as allergic rhinoconjunctivitis, CSwNP, COPD, dysfunctional breathing, vocal cord dysfunction, anxiety/depression, obstructive sleep apnea, GERD, bronchiectasis, and allergic bronchopulmonary aspergillosis (ABPA).[188] The following are recommended items for assessment of patients with severe asthma:[189,190,191,192,193,194]
Patient is adherent to all medications with a good inhalation technique
Other possible misdiagnoses where the problem is not bronchial asthma but other diseases that mimic asthma symptoms, e.g., bronchiectasis, endobronchial tumors, dysfunctional breathing, vocal cord dysfunction, ABPA, or eosinophilic granulomatosis with polyangiitis (Churg-Strauss syndrome)[192,195]
Comorbidities that can worsen bronchial asthma and make it difficult to manage (e. g., dysfunctional breathing, CSwNP, GERD, sleep apnoea syndrome, ABPA, obesity, and congestive heart failure[196]
Medication overuse or side effects
Any psychosocial contributing factors
Other confounding factors, e.g., presence of allergens at home or work, active or passive smoking and vaping, or psychosocial problems.[192]
As it may be difficult to achieve full control in many patients with severe asthma, the aim of treatment in this situation is to reach the best possible control.[197] After dealing with all comorbidities and other confounding factors that could have made asthma difficult to control, maximum therapy is recommended, which may include combination therapy of high-dose ICS/LABA, LTRA, or LAMA and the addition of one of the available biologics as appropriate.[198,199,200]
A significant percentage of patients with severe asthma do not respond adequately to high-dose ICS and other controller therapy, thus, they need frequent or continuous oral steroid therapy to achieve a reasonable response.[201] Such control may be lost when oral steroid is discontinued. Patients may differ in the degree of their responsiveness to OCS.[202] Some patients may fail to improve their FEV1 by more than 15% following treatment with OCS for 2 weeks, a condition called “corticosteroids-resistant asthma.[203,204] If OCS is necessary, then it is recommended to use the lowest possible dose and to shorten the duration as much as possible.[205] In this situation, osteoporosis prophylaxis is recommended.
For patients with severe asthma who do not qualify or respond to biologics, other modalities of treatment of severe asthma are recommended such as macrolides. Due to their role in reducing neutrophilic airway inflammation, they were shown to have a role in the management of severe asthma. A study has assessed the benefit of azithromycin at a dose of 250–500 mg 3 days/weeks as add-on therapy for 48 weeks for patients with persistent symptomatic asthma.[206] Azithromycin significantly reduced the experience of at ‘least one asthma exacerbation’ from 61% to 44% and improved asthma-related quality of life measures. Maintenance use of azithromycin reduces exacerbations in patients with eosinophilic, non-eosinophilic, and severe asthma.[207]
Allergen immunotherapy
The allergen immunotherapy (AIT) is a treatment modality to desensitize patients to specific allergens. It is considered for those with stable asthma and evidence of clinically relevant allergic sensitization at which the immunotherapy can be directed, especially if they have coexisting allergic rhinitis. Patients with poorly controlled asthma should not be started on immunotherapy.[208,209] Although there are insufficient data on the impact of AIT on asthma attacks and quality of life scores, it has specifically been shown to:
Improve asthma symptoms and stepping down asthma treatment (Evidence A)[210]
Improve AHR (Evidence B)[211]
Decrease the progression of allergic rhinitis to asthma (Evidence B)[212]
Decrease the chance of development of new sensitizations (Evidence B).[208]
AIT is likely to be cost-effective when appropriately used.[209] There are currently two types of AITs in clinical practice; subcutaneous immunotherapy (SCIT) and sublingual immunotherapy (SLIT). Most studies that compared SCIT to SLIT showed a better clinical efficacy of SCIT. However, SLIT has a better safety profile than SCIT as SCIT may rarely cause anaphylaxis.[210] Patients at risk are mainly those with asthma, especially if uncontrolled. A high level of caution should be taken in patients using beta-blockers due to the risk of more serious anaphylaxis that is resistant to treatment with epinephrine.[208] Data are limited in pediatrics, but AIT has been used safely in children over 5 years of age and was shown to reduce long-term asthma medication use and improve FEV1.[213] Although beneficial effects may be observed a few months from starting AIT, treatment with AIT needs the patient’s commitment for at least 3 years to have sustained desensitization after stopping the treatment. Furthermore, AIT can be continued, but not initiated, during pregnancy. The most studied allergens’ specific immunotherapy are dust mites, alternaria, grass pollens, ragweed, and cat. Anti-IgE therapy could improve tolerability to AIT in patients with moderate to severe asthma.[214] If the patient is considered a candidate for AIT, referral to an allergist is recommended to explore this option further.
Section 7: Management of Acute Asthma in Adults and Adolescents
An acute asthma attack is a challenging clinical scenario that requires a systemic approach to rapidly diagnose, evaluate the severity, and initiate therapy. The first step in managing acute asthma is early recognition to prevent the occurrence of attacks. Asthma in general has a low mortality rate compared with other chronic lung diseases.[33] Nevertheless, asthma death is still seen in clinical practice, especially among patients with poorly controlled asthma whose condition deteriorates over a period of days before the final fatal event.[215,216,217] The most specific marker associated with increased asthma mortality is a history of repeated hospital admissions, particularly if the patient requires intensive care treatment or ventilatory support.[218,219] Patients admitted with severe asthma attacks in Saudi Arabia were found to be younger and predominantly males and used less ICS/LABA combination.[3,220] This section includes an assessment of the patients presented with acute asthma, initial management, and follow-up after hospital discharge. Detailed information about medications used in acute asthma can be found in the medication appendix. Box 7.1 shows the summary of the key recommendations for acute asthma management.
Box 7.1.
Key recommendations of acute asthma management
| Assess the severity of the attack based on the degree of dyspnoea, pulse rate, respiratory rate, peak-expiratory flow rate, and oxygen saturation |
| Start treatment immediately by repeated administration of salbutamol, controlled oxygen concentration, and systemic steroid |
| Review response to treatment after 1 h of continuous therapy |
| Consider other therapies (ipratropium bromide and magnesium sulfate) in managing severe attacks |
| Do not request routine chest X-rays or arterial blood gases routinely unless indicated |
| Do not prescribe routine antibiotics or sedatives unless indicated |
| Evaluate the need for hospital admission based on response to therapy, history of previous admission, and the ability to manage at-home |
General assessment of acute asthma attack
The initial clinical assessment should rapidly determine whether the patient’s presenting symptoms are related to an acute asthma attack and exclude alternative diagnoses or complications, such as pneumonia, pneumothorax, or atelectasis [Box 7.2]. Although most acute asthma attacks develop over a period of days, patients with brittle asthma may present with a much more dramatic deterioration.[221,222] It is important to realize that most patients who die from an acute asthma attack had chronically uncontrolled asthma, had received suboptimal treatment with ICS and other controllers, and had inadequate monitoring of their asthma.[223] Management of acute asthma is the extreme spectrum of uncontrolled asthma and represents a failure to reach adequate asthma control. Poor prognostic features of acute asthma include previous history of near-fatal asthma or hospital admission in the last year, heavy usage of relievers, patients who are not on regular ICS, a history of psychiatric or psychosocial illness, and poor adherence to asthma medications and lack of asthma action plan.[224,225]
Box 7.2.
Levels of severity of acute asthma in adults
| Level | Characteristics |
|---|---|
| Acute moderate asthma attacks | Increasing symptoms |
| PEF >50%–75% best or predicted reading | |
| No features of acute severe asthma | |
| Acute severe asthma | Any one of the following |
| PEF 30%–50% best or predicted reading | |
| Respiratory rate ≥25/min | |
| Heart rate ≥120/min | |
| Inability to complete sentences in one breath | |
| Life-threatening asthma | Any one of the following in a patient with acute severe asthma |
| SpO2 <92% (PaO2 <60 mmHg) on high-flow FIO2 | |
| PEF <30% best or predicted | |
| Bradycardia | |
| Dysrhythmia | |
| Cyanosis | |
| Hypotension | |
| Normal or high PaCO2 | |
| Exhaustion | |
| Confusion | |
| Silent chest | |
| Coma | |
| Weak respiratory effort | |
| Near-fatal asthma | Raised PaCO2 and/or requiring mechanical ventilation |
| Brittle asthma | Type 1: Wide PEF variability (>40% diurnal variation for >50% of the time over a period of >3–6 months) despite intense therapy |
| Type 2: Sudden severe attacks on a background of apparently well-controlled asthma |
PaCO2=Partial pressure of carbon dioxide, PEF=Peak expiratory flow
The patient should be carefully assessed on presentation to determine the severity of the attacks [Box 7.3] and the type of treatment required [Box 7.4].[226] PEF and pulse oximetry measurements are complementary to history taking and physical examination. The major causes of death in acute asthma are cardiac arrhythmia, asphyxia, and cardiogenic shock. The risk of cardiac arrhythmia is theoretically increased by hypokalemia and QT interval prolongation related to the use of high-dose SABA or IV aminophylline.[186,227] However, in a series of patients with near-fatal attacks, only a few arrhythmias other than sinus tachycardia and bradycardia were reported.[101,215,216,228] Hence, a more likely cause for death is probably related to the development of severe auto-PEEP leading to reduced venous return and secondary increased intracranial pressure and low cardiac output. Severe asphyxia due to severe airflow obstruction and hypoxemia are other likely causes. This is supported by the pathologic evidence of extensive airway obstruction, mucous plugging, and dynamic hyperinflation found at autopsy in patients who died of acute severe asthma.[229]
Box 7.3.
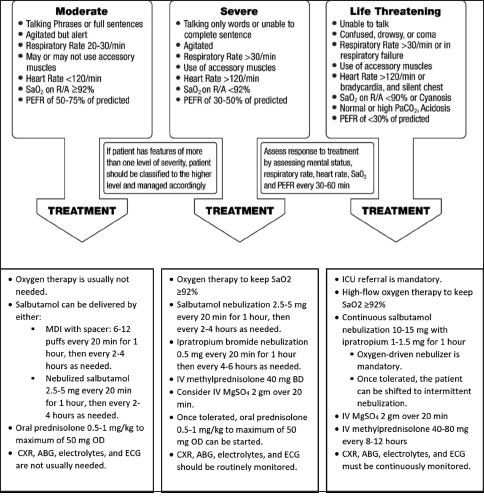
Initial management of acute asthma for adults and adolescents
Box 7.4.
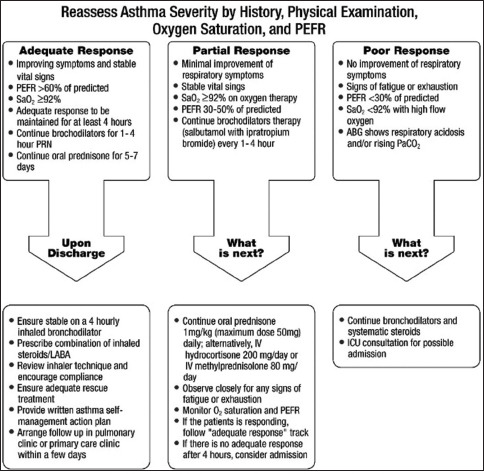
Adjustment of acute asthma treatment for adults and adolescent
SINA Panel recommends the following steps for the management of acute asthma:
Assess the severity of the attack
Initiate treatment to rapidly control the attack
Evaluate continuously the response to treatment.
The levels of acute asthma severity and the initial management are summarized in Boxes 7.3 and 7.4 respectively. It is recommended to adjust treatment intensity based on the severity of the attack and the following are the general guidelines for the treatment of acute asthma.
Assessment and management of mild-moderate acute asthma attack
Patients presenting with mild asthma attacks are usually treated in an outpatient setting by stepping up asthma management, including increasing the dose of ICS. However, some cases may require a short course of oral steroids and early outpatient department (OPD) referral. Patients with moderate acute asthma attacks are clinically stable. They are usually alert and oriented but may be agitated. They can communicate and talk in full sentences. They are tachypneic and may be using their respiratory accessory muscles. Heart rate is usually <120/min and blood pressure is normal. A prolonged expiratory wheeze is usually heard clearly over the lung fields, but examination of the chest may be relatively normal. Oxygen saturation is usually normal secondary to hyperventilation. The Peak expiratory flow meter (PEFR) is usually >50% of predicted or previously documented best. Measurement of arterial blood gases (ABGs) is not routinely required in this setting; however, if done, it shows widened alveolar–arterial oxygen gradient and low partial pressure of carbon dioxide (PaCO2), secondary to increased ventilation-perfusion mismatch and hyperventilation, respectively. CXR is not usually required for moderate asthma exacerbations unless pneumonia is suspected.
Management of moderate acute asthma attack include the following
Oxygen saturation is usually normal in a moderate acute asthma attack, and oxygen supplementation or continuous monitoring is not routinely needed
-
The standard therapy for initial care in the emergency department is inhaled SABA. It is recommended to be delivered by either:
- Nebulizer: 2.5–5 mg salbutamol every 20 min for 1 h, then every 2 h according to response (Evidence A).[232]
Assessment and management of severe acute asthma attack
Patients are usually agitated and unable to complete full sentences. Their respiratory rate is usually >30/min and usage of accessory muscles is common. Significant tachycardia (pulse rate >120/min) and hypoxia (SaO2 <92% on room air) are usually evident. Chest examination reveals prolonged wheeze secondary to severe airflow limitation and hyperinflation, more ominously the chest may be silent on auscultation in severe cases. CXR is required if complications are clinically suspected, such as pneumothorax or pneumonia. PEFR is usually in the range of 30%–50% predicted. ABG reveals significant hypoxemia and elevated alveolar–arterial oxygen gradient. PaCO2 may be normal in patients with severe acute asthma attacks. Such a finding is an alarming sign, as it indicates fatigue, inadequate ventilation, and pending respiratory failure. Unlike hypoxemia, PEFR measurement has shown a good correlation with hypercapnia and is considered a useful screening tool (e.g., PaCO2 >45 mmHg), making routine assessment of arterial blood gases unnecessary in most cases. In the absence of respiratory depressants, hypercapnia is rarely present when the PEF is ≥30% of normal or ≥150 L/min.[235] Thus, ABG measurements in acute severe asthma may be limited to the following situations:
Patients with persistent symptoms, whose PEF is <30% of normal or below 150 L/min despite initial bronchodilator therapy
Patients who are unable to perform a peak flow measurement
Patients whose respiratory status is deteriorating despite adequate intensive therapy
Patients who demonstrate signs or symptoms of hypercapnia, such as depressed consciousness, inappropriately slow respiratory rate, bradycardia, or myoclonus.
The management of severe acute asthma attacks include
Adjusted low-flow oxygen is recommended to maintain saturation ≥92%, as patients who received 28% oxygen did better than those who received 100% oxygen.[236,237] The ready availability of transcutaneous pulse oximetry allows noninvasive screening for hypoxemia among those patients. Despite the poor correlation between PEFR and oxygen saturation, it is recommended to use transcutaneous pulse oximetry among patients who are in severe distress, have a FEV1 or PEF <50% of baseline, or are unable to perform lung function measurements
Nebulized salbutamol (2.5–5 mg) is recommended to be administered back-to-back (repeated every 20 min for 1 h), then hourly according to response.[237] Oxygen-driven nebulizers are needed to avoid the risk of oxygen desaturation while using air-driven compressors (Evidence A)[233,234,238]
Ipratropium bromide is recommended to be added to salbutamol at a dose of 0.5 mg every 20 min for three doses by the nebulized route, then every 4–6 h as needed (Evidence B). Alternatively, ipratropium can be administered by MDI at a dose of 4–8 puffs every 20 min, then every 4–6 h as needed.[239,240,241,242] The efficacy of adding ipratropium to SABA to treat severe acute asthma attacks has been examined in a number of trials and systematic reviews.[243] It has been shown that patients who received the combination therapy of SABA and ipratropium were less likely to be admitted to the hospital than those treated with SABA alone. This benefit pertained only to those presented with severe acute asthma attacks and not to those with mild or moderate exacerbations[244]
Systemic glucocorticoid therapy is essential to treat the escalated airway inflammation that leads to asthma attacks, persistent airflow obstruction, and intraluminal mucus plugging.[243,245] Hence, systemic steroid is recommended to be started as soon as possible (Evidence A). The optimal dose for systemic glucocorticoids in acute asthma attacks remains unknown. SINA authors recommend starting IV methylprednisolone 40 mg twice daily in most patients present with acute severe asthma attacks. Higher doses are often chosen by some experts (60–80 mg every 6–12 h) for patients who are admitted to the ICU. If the patient can tolerate orally, oral prednisolone 0.5–1 mg/kg to a maximum of 60 mg daily is recommended[233,246]
If there is an inadequate response to previous measures, it is recommended to administer a single dose of IV magnesium sulfate at a dose of 1–2 g over 20 min (Evidence B).[247] Intravenous (IV) magnesium sulfate has a bronchodilator response in acute asthma, possibly due to inhibition of calcium influx into airway smooth muscle cells. The best evidence for effectiveness in acute asthma attacks comes from a systematic review, which found a decrease in hospitalization days with IV magnesium compared with placebo and mild improvement in lung function[248]
Continuous oxygen monitoring by transcutaneous pulse oximetry, blood gases (particularly in those patients with lung function <30% predicted), CXR, serum electrolytes, glucose, and 12-lead electrocardiography (ECG) should be ordered and checked.
Assessment and management of life-threatening acute asthma attack
Patients with life-threatening acute asthma attacks are severely breathless and unable to talk. They may present in extreme agitation, confusion, drowsiness, or even coma. The patient usually breath at a respiratory rate >30/min and uses their accessory muscle secondary to increased work of breathing. Heart rate is usually >120/min, but at a later stage, patients can be bradycardic. Patients may develop different types of arrhythmias secondary to hypoxia or acidemia, and hence, ECG monitoring is recommended. Oxygen saturation is usually low (<90%) and not easily corrected with oxygen. ABGs are mandatory in this group and usually reveal significant hypoxia and normal or high PaCO2. Respiratory and metabolic acidosis may be present secondary to inadequate ventilation and lactic acidosis, respectively. PEFR is usually very low (<30% of the predicted). CXR is mandatory in life-threatening asthma to rule out complications, such as pneumothorax or pneumomediastinum.
It is important to realize that some patients might have mixed features from more than one level of acute asthma severity. For the patients’ safety, they should be classified at a higher level of severity and managed accordingly.
The management of life-threatening acute asthma attacks includes
Patients in this category can progress rapidly to near-fatal asthma, respiratory failure, and death. Hence, an aggressive management approach and continuous monitoring are mandatory.
Consult ICU service. The intubation setting should be readily available
Adequate oxygen flow to keep saturation ≥92%.[237] Deliver continuous nebulized salbutamol at a dose of SABA 10–15 mg with Ipratropium bromide at a dose of 1.5 mg over 1 h (Evidence A).[249] Continuous treatment was found to be safe and well tolerated and led to better improvement in pulmonary functions and reduction in hospitalization when compared to intermittent delivery (Evidence A).[250] Oxygen-driven nebulizers are mandatory due to the risk of oxygen desaturation while using air-driven compressors (Evidence A)[238]
Once the patient showed adequate response to continuous nebulization, a shift to intermittent delivery is recommended (Evidence D)
Systemic steroid to be started as soon as possible at a dose of methylprednisolone 60–80 mg every 8–12 h (Evidence A)[251,252]
Single dose of IV magnesium sulfate at a dose of 2 g over 20 min (Evidence B)[246]
Frequent clinical evaluation, CXR, electrolytes, glucose, 12-lead ECG, and ABGs are essential.
Follow-up after initial treatment and discharge planning
Close evaluation of the treatment response is essential. This includes patient’s mental and physical status, respiratory rate, heart rate, blood pressure, oxygen saturation, and PEF. Response to treatment is defined as adequate, partial, or poor. Assessment of the treatment response, subsequent action, and discharge planning are illustrated in Box 7.4
Criteria for intensive care unit referral
ICU referral is recommended for patients who are:
Presenting or progressing to severe acute or life-threatening asthma
-
Failing to respond to initial therapy, as defined by:
- Requiring ventilatory support
- Deteriorating lung function (FEV1 and/or PEF)
- Persisting or worsening hypoxia
- Hypercapnia (either initially or subsequently)
- ABG analysis showing respiratory acidosis
- Exhaustion, shallow respiration, or drowsiness.
Section 8: Asthma in Special Situations
Gastro-esophageal reflux disease
GERD is more prevalent in patients with asthma, compared to the general population.[253] The mechanisms by which GERD worsens asthma include vagal mediated reflex and also reflux secondary to micro-aspiration of gastric contents into the upper and lower airways.[254] GERD induced asthma symptoms are mainly dry cough at night but can lead to asthma symptoms and it is more common with severe asthma than mild asthma. Patients with asthma should be questioned about symptoms of GERD. If GERD symptoms are present, a trial of anti-GERD measures, including a proton pump inhibitor and lifestyle modifications, is recommended for 6–8 weeks. However, if symptoms are not resolved, a referral to a gastroenterologist for further investigations like endoscopy or pH monitoring is warranted. The benefit of proton pump inhibitors is limited to patients with symptomatic GERD and night-time respiratory symptoms. On the contrary, patients with uncontrolled asthma and asymptomatic GERD are not likely to benefit from empiric GERD therapy.[255] Medical treatment for GERD for patients with asthma may provide small benefit for asthma related symptoms, modest improvement in lung function measures, and reduction of rescue medications for asthma control.[256] A recent meta-analysis does not support a recommendation for PPIs therapy as empirical treatment in asthma patients with GERD.[257]
Rhinosinusitis and nasal polyp
Most asthma patients have coexisting rhinitis and/or sinusitis and around 40% of patients with rhinitis have asthma.[258] CRSwNP is usually associated with severe and uncontrolled asthma. Asking patients about rhinitis symptoms (nasal blockage and/or nasal discharge, facial pain, or pressure and/or smell loss) and examination of the upper airways is recommended to be part of the routine management of asthma. Treatment with intranasal corticosteroids has been associated with a decrease in asthma hospitalization and emergency department visit.[259] Biologic therapy is recommended for patients with CRSwNP and severe asthma that is not controlled with the standard therapy.[260,261]
Obesity and asthma
Asthma is more common in obese than non-obese patients.[262] Studies has shown that obese asthmatics have a different pattern of inflammation when compared to non-obese asthmatics.[263] Therefore, Obese asthma patients have more symptoms, more frequent and severe exacerbations, reduced response to asthma medications, poor quality of life and more difficult to control asthma.[264] This could be in part related to reduced lung volumes, lack of fitness, and associated sleep apnea and GERD.[265] Treatment of obese asthma patients is recommended to follow same stepwise approach for asthma management; however, weight reduction, exercise and diet control are essential part of their management.[266] Patient with symptoms related to obstructive sleep apnea are recommended to visit sleep medicine specialist. For morbidly obese patient with asthma, it is recommended to discuss the risks and benefits of bariatric surgery when other measures are failed.[267,268]
Cough-variant asthma
Patients with cough-variant asthma may have chronic cough as their main or only symptom especially at night.[269,270] Perhaps cough-dominant asthma is a better term as variant implies different pathophysiology. Studies of airway inflammation in Cough variant asthma (CVA) are similar to those of asthma presenting with different combinations of symptoms. Other diagnoses to be considered are drug-induced cough caused by angiotensin-converting enzyme inhibitors, GERD, chronic upper airway cough syndrome manifesting as postnasal drip, eosinophilic bronchitis, and chronic sinusitis. Once the diagnosis is established, treatment is recommended at Step 2 or higher as appropriate.[271,272] This condition may be confused with eosinophilic bronchitis which is characterized by cough and sputum eosinophilia with normal spirometry and airway responsiveness.[273,274]
Exercise-induced bronchoconstriction
Exercise-induced bronchoconstriction (EIB) is common in inadequately controlled asthma patients. However, asthma-like symptoms can sometimes be triggered only by physical activities. Normally, bronchodilation occurs during exercise and lasts for a few minutes.[275,276] In patients with EIB, the initial bronchodilation is followed by bronchoconstriction that generally peaks within 10–15 min after completing the exercise and resolves within 60 min. EIB can be prevented by using SABA or ICS/formoterol a few minutes before exercise.[277,278] A warm-up period before exercise may also reduce EIB symptoms. If this approach does not control the symptoms, the patient is recommended to have maintenance therapy at Step 2 or higher as appropriate.[135,277] Regular use of LTRA may also help in this condition, especially in children.[135,275,277]
Aspirin-exacerbated respiratory disease
AERD is a special phenotype characterized by a triad of asthma, chronic rhinosinusitis with nasal polyposis, and respiratory reactions to aspirin.[279] About 7% of adult asthma patients and 15% in those with severe asthma suffer from attacks in response to ASA or NSAIDs that inhibit COX-1.[280,281] The majority of patients experience first symptoms during their third or fourth decade of life. Once ASA or NSAID hypersensitivity develops, it persists for life. Characteristically, within minutes to 2 h following ingestion of ASA, an acute severe asthma attack develops. It is usually accompanied by rhinorrhea, nasal obstruction, conjunctival irritation, and scarlet flush of the head and neck and even severe bronchospasm and death.[282] A typical history of upper and lower respiratory reaction to aspirin or NSAIDs is very suggestive for the diagnosis, which is confirmed by aspirin challenge which is not done routinely but if indicated, it be recommended to be done in a specialized allergy center.[283] Patients known to have aspirin-induced asthma should avoid all aspirin-containing products and NSAIDs. Where an NSAID is indicated, COX-2 inhibitors or alternative analgesics such as paracetamol are recommended.[284,285] Prophylactic low-dose aspirin should also be avoided. However, referral to an allergy specialist for ASA desensitization is recommended in patients for whom aspirin is required as anti-platelet therapy.[286] Montelukast may help in the treatment of this type of asthma in some patients.[287]
Pregnancy
Almost half of the pregnant women had the desire to stop asthma medications during pregnancy as they believed that asthma medications would harm them and their babies more than asthma itself.[15] As such, a great effort should be directed towards the education of asthma in pregnancy in order to correct this misbelief. The course of asthma during pregnancy is unpredictable; however, one-third of pregnant asthmatics may have a worsening of their asthma control.[288,289] Maintaining adequate control of asthma during pregnancy is essential for the health and wellbeing of both the mother and her baby. Poor asthma control during pregnancy increases the risk of poor outcomes for the baby such as pre-term delivery, low birth weight, and increased perinatal mortality.[290] It also increases the risk for the mother and occurrence of poor asthma control during the first trimester of pregnancy that significantly increase the risk of a congenital malformation.[291] Identifying and avoiding the indoor and outdoor triggers and providing educational resources for the patient are recommended as the first step of therapy for asthma during pregnancy. Asthma treatment is recommended to take the same stepwise approach as in the non-pregnant patient with monthly follow-up and assessment. Salbutamol is the preferred reliever due to its excellent safety profile. An ICS based regimen is the preferred treatment for long-term control.[292] Asthma medications, including ICS, SABA, LABA, and LTRA are generally safe and have not shown to increase the risk of fetal abnormalities.[293,294,295] However, prolonged use of OCS may be associated with pregnancy-related complications, especially in the first trimester.[289] Data about safety of biological therapy during pregnancy is lacking, however, based on SINA Panel consensus, we recommend not to initiate biologics for new patient during pregnancy. For patients already on biologics before pregnancy, counselling patient is recommended for biologics benefit/risk ratio.[296,297] For acute asthma exacerbations, it is recommended to follow the same drug treatment guidelines as non-pregnant patients including systemic steroids if indicated.[298,299,300] Fetal monitoring is recommended in severe asthma attack. During labor and delivery, usual controller medications should be continued and if anesthesia is required during labor, regional anesthesia is recommended whenever possible.[301] The use of prostaglandin F2α may be associated with severe bronchospasm and should be avoided if possible.[302] If asthma is well controlled during pregnancy, acute asthma is rare during labor. Pregnant asthma patients should be encouraged to breastfeed after delivery and to continue their usual asthma medications during lactation.[303]
Occupational asthma
Patients with asthma should be asked about their occupational history and exposures for possible occupation-related allergens. A simple screening test is to ask the patient if their symptoms improve when they are away from work.[304] Once identified, early detection and elimination of occupational sensitizers and removal of patients from further exposure are an essential aspect of the management. Referral to an asthma specialist for assessment and advice is recommended for patients with suspected or confirmed occupational asthma because of the legal implications of the diagnosis.[305,306]
Asthma-chronic obstructive pulmonary disease overlap
Asthma-chronic obstructive pulmonary disease overlap (ACO) is a unique complex descriptive entity sharing features of both asthma and COPD. At this stage, there is no formal definition of ACO as there is inadequate data to describe its features, characteristics, and its optimal therapeutic intervention.[302] ACO is a term used to describe patients who have persistent airflow limitation together with clinical features that are consistent with both asthma and COPD. This is not a definition of a single disease entity, but a descriptive term for clinical use that includes several different clinical phenotypes, different inflammatory patterns, and different underlying mechanisms.[307,308] Patients with features of both asthma and COPD have a greater burden of symptoms, experience frequent exacerbations, have poor quality of life, a more rapid decline in lung function, higher mortality, and greater use of healthcare resources, compared with patients with asthma or COPD alone.[309] Spirometry is required to confirm the diagnosis of chronic airflow limitation and document persistent airflow limitation, variability and reversibility. If the initial assessment suggests the diagnosis of asthma or ACO, or there is uncertainty about the diagnosis of COPD, it is prudent to treat as asthma by starting at Step 2 or higher as appropriate and to avoid using LABA and/or LAMA as the only therapeutic option. Patients having asthma with COPD had lower morbidity and hospitalizations if they received ICS treatment; a similar benefit was seen in those with COPD plus concurrent asthma. For ACO patients, non-pharmacological measures including smoking cessation, pulmonary rehabilitation, vaccinations, and treatment of comorbidities as additive therapeutic strategies.[310]
Asthma in elderly
Most elderly patients with asthma had been previously diagnosed with asthma during childhood or early adulthood. New onset asthma above the age of 65 is uncommon and estimated to be 4%–8%. Elderly patients with asthma perceive symptoms of asthma differently than younger patients, often have comorbid conditions with similar symptoms, and have late presentation with more severe airway obstruction. Approximately 50% of deaths from asthma occur in the elderly.[311,312] The diagnosis of asthma in elderly is challenging due to the high prevalence of smoking among elderly patients and because symptoms of asthma overlap with other diseases like COPD and bronchiectasis, and congestive heart failure. Elderly patients with asthma underestimate asthma symptoms as they may contribute it to age process or associated comorbidities such as cardiovascular disease, smoking related diseases, or medications.[312] Confirmation of asthma diagnosis in elderly is based on the presence of respiratory symptoms suggestive of asthma and the demonstration of reversible expiratory airflow obstruction on PFT testing in the absence of alternative diagnoses. The Management of asthma in elderly patients is recommended to follow the same management guidelines in adults and adolescents. Difficulties in learning inhaler technique is usually common in elderly and it is attributed to cognitive impairment, muscle weakness, arthritis or impaired vision and it is an important consideration in this patient’s population when choosing the inhaler device. Multiple inhaler devices are not recommended.[313,314] MDI with holding chamber, a breath-actuated dry powder inhaler (DPI), or a nebulizer are alternative options which may improve medications delivery.[315] Elderly patients have lower inspiratory flow rates and may not be able to achieve the higher inspiratory flow rates required for some DPIs. Adverse effect of ICS are common in elderly patients such as skin bruising, risk of osteoporosis, and cataracts.[316]
Section 9: Asthma in Children
Asthma represents one of the commonest chronic illnesses of childhood with significant economic impact.[317] It is also a leading cause of childhood morbidity as measured by school absences, Emergency Department visits and hospitalizations.[318] From the perspective of both patient and society, the cost of not treating asthma is higher than the cost of asthma treatment.[319,320] This section aims toward enhancing the multidisciplinary care of asthma in children with special attention to non-asthma specialists, including primary care and general practice physicians and other healthcare workers.
Diagnosis of asthma in children
Accurate diagnosis of asthma in children is crucial to prevent inappropriate management and reducing morbidity and mortality due to under-or over-diagnosis.[321,322] Asthma diagnosis in children continues to be a clinical diagnosis. However, clinical diagnosis is notoriously inaccurate, and, although there is no one diagnostic asthma “test”, every effort should be made to obtain objective evidence for the diagnosis. The more tests that are negative, the less likely is the diagnosis to be correct. Careful clinical evaluation should focus on the presence of recurrent or chronic symptoms related to airway obstruction such as physician-diagnosed wheezing, coughing, night symptoms, activity limitation, and shortness of breath. These symptoms result from pathological small airway hyperreactivity due to various stimuli, and typically would be reversible either spontaneously or after receiving an asthma therapy. The diagnosis can be further supported by the presence of atopy, early sensitization, and a family history of atopy. Spirometry is recommended to be performed for capable children to quantify airflow limitation and to show post-bronchodilator reversibility of airway obstruction.[317] Spirometry can be performed in children aged 5 years and older. It is preferably planned when the initial diagnosis is made and after 3 months of controller therapy initiation and with subsequent follow-up assessments. Box 9.1 presents a summary of findings suggestive of the diagnosis of asthma in children.
Box 9.1.
Findings suggestive of the diagnosis of asthma in children
| Findings | Remarks |
|---|---|
| History of multiple attacks of SOB or wheezing in a season | >3 attacks/season, recurrent, and worse during sleep with triggers such as physical activity, laughing, crying, upper respiratory tract infections or exposure to tobacco smoke, or air pollution. Ideally, wheezing should be confirmed by a doctor |
| Coughing | >2 weeks, not related to URTI, recurrent, and worse during sleep with triggers such as activity, laughing, crying or exposure to tobacco smoke or air pollution. Though isolated cough, without wheeze or breathlessness, is unlikely to be due to asthma |
| Reduced activity | Not able to run, play, or laugh at the same intensity as other children and tires earlier during walks (wants to be carried) |
| Family history | Atopy (allergic rhinitis, atopic dermatitis, food allergy) and asthma in first-degree relatives |
| Atopy | Eczema, aeroallergen and food sensitization |
| Wheezing | Equal bilaterally, during expiratory phase, especially on forced expiration |
| Breath sounds | Prolonged expiratory phase |
| Therapeutic trial | Trial of inhaled corticosteroid therapy. However, many respiratory symptoms in childhood improve spontaneously that should not be confused with therapeutic response. A three-stage trial is suggested 1. Commence a trial of ICS for 6 weeks 2. Stop ICS at 6 weeks and review response. If there was no improvement, ICS-responsive asthma is unlikely that recures further evaluation 3. If symptoms recur upon stopping therapy, reintroduce ICS and adjust dose accordingly |
| Spirometry | Typically, in children >5 years with bronchodilator response assessment |
| Chest X-ray | May be considered in infants to rule out other causes |
| Tests for hypersensitivity | Both skin testing and/or allergen specific IgE blood testing and eosinophils count |
IgE=Immunoglobulin E, SOB=Shortness of breath, URTI=Upper respiratory tract infection, ICS=Inhaled corticosteroid
Asthma mimickers should be suspected when any of the atypical feature is present [Box 9.2].[323] Clinical suspicion of asthma mimickers is an acceptable indication for CXR, otherwise, a routine CXR is not recommended to be part of the initial routine work up of asthma in children.[324,325]
Box 9.2.
Features suggest asthma mimickers
| Failure to thrive |
| Onset of symptoms during infancy |
| Very sudden onset symptoms (suggests inhaled foreign body) |
| Vomiting associated with respiratory symptoms |
| Continuous (biphasic) wheezing |
| Failure to respond to asthma controller medication |
| Clubbing |
| Focal auscultation signs |
| Symptoms that are not associated with typical triggers |
| Chronic sputum production |
| Prominent upper airway symptoms |
Wheezing in preschool children
Wheezing and shortness of breath in preschool children are among the most common presenting symptoms in pediatric practice. Approximately one third of children have at least one episode of wheeze before their third birthday.[326,327] Uncontrolled asthma in preschool children can lead to developmental disadvantages due to the negative impact of uncontrolled asthma on their social interaction and sleep. In preschool children, asthma diagnosis and management differ from that of older children and adolescents in many ways. Early childhood wheezing can evolve to different asthma phenotypes that can have variable response to standard therapy.[328] Box 9.3 shows the differential diagnosis of wheezing in preschool children. In this age subgroup, asthma diagnosis represents a challenging clinical judgment due to the lack of objective assessment such as PFT or inflammatory biomarkers (FeNo and eosinophils count).[327] The use of the term “reactive airway disease” is discouraged as it can restrain full clinical assessment and proper management of asthmatic children in this age group.[329,330,331]
Box 9.3.
Common differential of asthma in preschool children
| Congenital cardiovascular defects |
| Congenital structural aerodigestive defects |
| Functional aerodigestive disorders |
| Infections especially viral bronchiolitis |
| Obliterative bronchiolitis |
| Aspiration syndromes including gastroesophageal reflux, unsafe |
| swallow, H-type fistula |
| Bronchopulmonary dysplasia |
| Cystic fibrosis |
| Primary ciliary dyskinesia |
| Foreign body inhalation |
| Tracheobronchomalacia |
Phenotypes and endotypes of wheezing in children
Phenotype is defined as the observable characteristic or trait of a disease, such as developmental, clinical or epidemiological properties, whereas endotype describes its distinct pathophysiologic mechanisms at a cellular and molecular level.[332] Based on several longitudinal studies, wheezing in children has been categorized epidemiologically into transient and persistent wheeze phenotypes. It is also categorized based on symptoms into episodic/viral induced and multi-trigger wheeze phenotypes.[327,333] Major factors that may predict persistent symptoms are allergic disease, reduced lung function, viral respiratory infection, and bacterial colonization in infancy. On the other hand, advances in biomolecular and cellular techniques enabled further analysis of pooled large datasets of asthmatics which in turn resulted in further classification of asthma endotypes.[334] Two major endotype subgroups have been proposed: Th2 high and Th2 low (or non-Th2). More sophisticated clustering using combination of clinical, physiological, and biomarker parameters is an ongoing area of research.[335] Different responses to treatment and variable outcomes have been attributed to phenotype-endotype heterogeneity, overlap and instability over time. The allocation of children into these categories still remains a subject of debate, as their clinical usefulness is still under investigation.[336] However, applying the evolving understanding of asthma phenotype-endotype is believed to enhance precision medicine practice in asthma management.[337] Box 9.4 summarizes wheeze phenotype-endotype in children.[41,327,337,338,339]
Box 9.4.
Wheezing endotypes in young children
| Endo type | Characteristics |
|---|---|
| Th2-high | |
| Atopic, early onset | Blood/sputum eosinophilia, serum specific allergen IgE, high FeNO, serum periostin, high total IgE |
| Atopic, late onset, more severe | |
| Non-Th2 | |
| Nonatopic | Paucigranulocytic or neutrophilic sputum, MMP-9 in BAL |
| Obesity related | Oxidative stress, neutrophils, increased innate immune activation; more in females; dysynaptic airway growth |
FeNO=Fractional exhaled nitric oxide, MMP-9=Metalloproteinase 9, Th2=T-helper lymphocyte type-2, IgE=Immunoglobulin E, BAL=Bronchoalveolar lavage
Prediction of asthma in preschool children
SINA expert panel recommends the utilization of the modified asthma predictive index (API) for early identification of the risk for persistent asthma among preschool children. This tool is a clinical scoring instrument that can be used to predict whether a child with intermittent wheezing before the age of 3 years will develop persistent asthma pattern during school-age years [Box 9.5].[340,341] Preschool children with positive modified-API are at a 4–10-fold increase in the risk of having asthma later in their childhood. On other side, children with negative modified-API will have 95% chance of outgrowing their asthma later on life.[342]
Box 9.5.
Positive modified asthma predictive index
| History of ≥4 wheezing episodes before the age of 3 years (at least one physician diagnosed) and either | ||
|
| ||
| ≥1 of the major criteria | or | ≥2 of the minor criteria |
|
| ||
| Parental history of asthma | Eosinophilia (≥4%) | |
| Skin test positive to aeroallergens | Wheezing unrelated to colds | |
| Eczema (physician-diagnosed atopic dermatitis) | Allergic sensitization to milk, egg, or peanuts | |
Assessment of asthma in children
The long-term goals of asthma management in children are not different from those of adults [Box 5.1].[343] Asthma management requires effective partnership between patients/caregivers and their healthcare providers.[344] Once established and strengthened, this relationship will positively impact asthma management. Asthma control shall be assessed routinely by physician during every clinic visit. Assessment of a child with asthma is recommended to cover two important aspects: future risk of adverse outcomes, and symptom control.
Assessing future risk of adverse outcomes
This is achieved by assessing future risk of attacks, fixed airflow obstruction, and adverse effects of medications [Box 9.6].
Box 9.6.
Assessment of future risk of adverse outcomes of asthma in children
| Risk factors | Assessment |
|---|---|
| Asthma attacks within the next few months | Uncontrolled asthma symptoms |
| One or more severe asthma attacks in the previous year | |
| The start of the child’s usual exacerbations season (especially if autumn/fall) | |
| Exposures to tobacco smoke; indoor or outdoor air pollution; indoor allergens, especially in combination with viral infection | |
| Major psychological or socioeconomic problems for a child or family | |
| Poor adherence manifested as underuse of ICS and/or over-use of SABAs | |
| Failure to attend regular follow up appointments | |
| Fixed airflow limitation | Persistent low FEV1 in patients with |
| Severe asthma with several hospitalizations | |
| History of bronchiolitis | |
| Medication side-effects | Systemic: Frequent courses of oral corticosteroids or high-dose ICS; neuropsychiatric adverse reactions after the initiation of LTRA |
| Local: Moderate/high-dose or potent ICS; incorrect inhaler technique; failure to protect skin or eyes when using ICS by nebulizer or spacer with face mask. Failure to use a spacer with | |
| MDI (common in teenagers) |
ICS=Inhaled corticosteroid, SABAs=Short-acting bronchodilators, FEV1=Forced expiratory volume in 1 s, LTRA=Leukotriene receptor antagonist, MDI=Metered-dose inhaler
Assessing symptom control
This implies a periodical assessment of asthma control combined with adjustments (if needed) of treatment based on the level of control. It is strongly recommended to use asthma treatment in a stepwise approach with the ultimate goal of achieving “optimal” control with “minimal” amount of medications and dosage.[345] Adherence to the prescribed medications and the proper use of their devices are recommended to be addressed before any modification of the treatment plan. Asthma control reflects the adequacy of management by describing the clinical status of a child as controlled, partially controlled, or uncontrolled. Focusing on asthma control may improve patient perceptions and expectations that improve symptoms reporting by children and their caregivers and subsequently treatment decisions by clinicians.[346] Assessment of asthma control is recommended to cover the domains of both physician and patient/caregiver inputs.
Patient/caregiver assessment of control
Different numerical tools have been developed and validated to objectively assess asthma control utilizing patients and their caregiver perception. However, they are recommended to be used as a complimentary tool rather than replacing physician assessment as these tools have some limitations.[347] The SINA expert panel recommends utilizing the childhood-ACT (C-ACT) [Box 9.10] for children aged 5–12 years and the respiratory and asthma control in kids (TRACK) [Box 9.13] for children younger than 5 years of age. These questionnaires are completed by patients and/or their caregivers prior to physician evaluation based on the age of the child.
Box 9.10.

The childhood asthma control test
Box 9.13.

The Test for Respiratory and Asthma Control in Kids (TRACK)
Non-pharmacological management of asthma in children
Patient education: Patient education is recommended to be an integral part of asthma management strategy in children. It is recommended to involve the basic knowledge of the disease pathophysiology, identifying and avoiding triggering factors, environmental controls (especially cigarette smoke exposures), proper use of treatment devices, and recognition of worsening asthma symptoms and the optimal time to seek advice.[348,349] Caregivers of preschool children are recommended to be educated that asthma control is an achievable target and affected children should not be prevented from engagement in age-appropriate activities. Proper asthma education can lead to a significant reduction in Emergency Department visits and hospitalizations, improve self-management of asthma attacks, and an overall reduction in the cost of asthma care [Box 9.7].[350]
Box 9.7.
Patient asthma-education goals and objectives
| Goals | Outcome |
|---|---|
| Developing partnership and common goals | Understand asthma risk by explaining in a simplified pathophysiology term |
| Sharing understandable information | Acknowledge role of prevention, environmental control and, importance of avoiding triggers |
| Addressing relevant concerns and expectations | Recognize difference between “controller” and “reliever” treatment |
| Continuity and consistency in providing education | Address adverse events of medications |
| Explain devices use and importance of compliance with treatment plans | |
| Recognize worsening asthma and explain action plan | |
| Define comorbid conditions and provide recommendations |
Setting asthma action-plans
An action plan that documents medications, doses, and device technique is recommended to be provided to patients and their caregivers. The action plan is also recommended to include information for patients and caregivers on how to recognize worsening of asthma symptoms and advice of treatment modification in these situations [Box 9.8].
Box 9.8.
Components of asthma management action plan
| Item | Description |
|---|---|
| Patient identification | Name, medical record number, age, and weight |
| List of patient’s medications | Dosage, frequency, controller versus rescuer medications |
| Recognition of asthma control status | In simple terms and color coded |
| Suggested action based on asthma control status | |
| How to use inhalational devices | Use illustrations |
| When and how to seek medical advice | Access to emergency care or call center |
| Others | How to clean inhalers and spacer and advice on environmental control and mouth washing after ICS use |
ICS=Inhaled corticosteroid
Prevention
Asthma attacks can be triggered by a variety of factors including allergens, viral infection, pollutants, and drugs. Eliminating these exposures improves the control of asthma and reduces medications need. Parents/caregivers of children with asthma should be strictly advised not to smoke or vape at home at all.[343] Breast-feeding and vitamin D supplementation may decrease the chance of developing early wheezing episodes, while probiotics benefit is still doubtful in preventing allergic disease.[351,352,353,354,353] A study on early-life use of probiotic supplementation did not show significant impact to prevent asthma or eczema at the age of 2 years for children at high risk.[354] School-based programs that target asthma self-management are associated with improved asthma outcomes, fewer Emergency Department visits, fewer hospitalizations, fewer days of reduced activity.[355]
Precautions during viral pandemics
During the occurrence of viral infection pandemics, precautions related to infection control measures that include handwashing, social distancing, optimizing asthma control, and minimizing use of nebulization as the mean of drug delivery to reduce the risk of droplet generation are of extreme importance. Referral to specific guidelines by local and international experts on this task is recommended. SINA expert panel has released specific advices on asthma management of adult and children during the COVID-19 pandemic.[356,357] SINA expert panel advice to continue routine care for patient with acute exacerbation of bronchial asthma and to follow the regular clinical guideline in the occasion of pandemics.
Concepts of pharmacological management of asthma in children
The SINA expert panel have the following strategies for reliever and controller therapy:
Reliever therapy
Reliever therapy is a group of medications used to rescue asthmatic patients during exacerbation to relief acute symptoms of asthma. The following are considerations for this category:
Controller therapy
This is a group of medications used on a regular basis to keep asthma under control and prevents future risk of adverse outcomes. The following are considerations for this category:
ICS are considered the most effective first-line maintenance monotherapy for childhood asthma (Evidence A)[360,361]
For significant local side effects of ICS, enforce use of MDI with spacer, consider a change in therapy, reduction in the dose or frequency of ICS (if possible), advice for a vigorous mouth washing after inhalation, and/or use of appropriate local antifungal therapy for severe oral thrush[178]
The combination of ICS/LABA is recommended to be used regularly at Step 3 and higher
The combination of ICS/formoterol is specified because formoterol has a fast-acting component. Hence, it is the base of MART which is an acceptable approach for Step 3 and higher in children ≥5 years
For children ≥5 years, MART approach is recommended to be administered as maintenance (1–2 puffs once to twice daily) and as needed for asthma symptoms (1–2 puffs every 4–6 h). The maximum number of puffs per day is 8 (total of 36 μg based on 4.5 μg/puff formoterol formulation). Children who need to use more than these amounts should be advised (and their caregivers) to contact their physician or to seek emergency department advice. MART approach was demonstrated to be associated with lower risk of acute exacerbation and to be more cost effective (Evidence B).[362,363,364] However, attention should be paid to the maximum daily dose allowance used with this approach
There are insufficient data to recommend short courses of high-dose ICS in children with mild intermittent asthma attacks (Evidence B).[365] The safety of this approach has not been established
Children with frequent or severe asthma attacks are recommended to receive regular treatment with ICS (Evidence A).[366] Doubling the dose or quintupling it at the early stages of loss of control did not result in reduction of asthma attacks or improvement in other outcomes.[243] It recommended to refer to the stepwise approach [Box 9.12 and Box 9.15]
Chronic use of ICS for more than 3 months in pre-pubertal-aged children can suppress growth velocity which is dose dependent. However, asthmatic children when treated with low-dose ICS attain normal adult height but at a later age (Evidence A).[367,368] Any potential adverse effects of ICS needs to be weighed against the well-established benefit to control persistent asthma. More details of the use of ICS in children are available in the medication appendix
Children commenced on LTRA should be followed during the early phase of initiation for the development of neuropsychiatric adverse drug reactions (like anxiety, irritability, aggressiveness, and sleep disturbances) which warrant cessation of therapy in almost 1 out of 6 children. Patient and/or caregiver should be educated on this potential.[369,370,371]
Box 9.12.
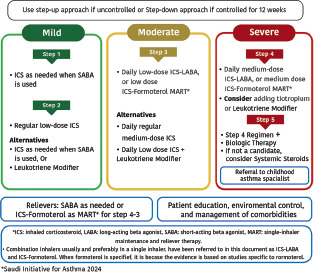
The SINA Approach for Outpatient management of Asthma for childern aged 5 to 12 years
Box 9.15.
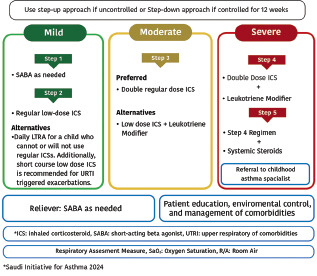
The Saudi Initiative for Asthma approach for outpatient management of asthma for children aged <5 years
Devices
It is important to select the best device for optimal treatment delivery [Box 9.9].
Box 9.9.
Choosing an inhaler device for children
| Age (years) | Preferred device | Alternative device |
|---|---|---|
| <4 | MDI + spacer with face mask | Nebulizer with face mask |
| 4–6 | MDI + spacer with mouthpiece | Nebulizer with mouthpiece |
| >6 | Dry powder inhaler, breath actuated pressurized MDI, MDI + spacer with mouthpiece | Nebulizer with mouthpiece |
MDI=Metered-dose inhaler
Use of valved-holding spacer, with mouthpiece when possible, is recommended when an MDI is prescribed (Evidence B).[372] Breath-actuated devices (e.g., DPIs) represent an effective and simpler option for maintenance therapy in children 5–12 years of age (Evidence C).[373,374] For more information about medications, refer to the medications appendix[372,373,374]
Nebulizers are not superior to MDI delivered by spacer in both acute and chronic asthma management (Evidence A).[375]
Strategies for outpatient management of asthma in children
Management of asthma is recommended to be adjusted continuously based on asthma control. If current treatment did not achieve control, step up is recommended until control is achieved. Whenever control is maintained for at least 3 months, then treatment can be stepped down. This stepwise approach is essential to maintain optimum control with the lowest step to maximize safety and minimize cost. SINA expert panel recommends ensuring consistency in the approach of asthma in adults, adolescents, and children. Therefore, outpatient treatment will be stratified based on age groups: 5–12 years and <5 years. It is further described in three phases: initiation, adjustment, and maintenance.
Initiation of asthma treatment in children
Prior to initiating asthma treatment in children, it is recommended to ensure availability of initial clinical assessment data, such as the status of asthma control and assessing for risk factors. It is also recommended to provide teaching of inhalers technique, and action plan.
Adjustment of asthma treatment in children
Before treatment adjustments, it is recommend to assess adherence to treatment, proper device use, control of environment and confirmation of the diagnosis especially if there is a failure to respond to therapy.[376] Adjustment of therapy is recommended after 1–3 months depending on the level of asthma control upon presentation and the C-ACT score for children aged 5–12 years or TRACK score for children aged <5 years. Based on clinical assessment and the level of asthma control, the treatment is recommended to be adjusted. If asthma control is achieved, treatment is recommended to be maintained at the same step; however, stepping down may be considered during low seasons for asthma attacks. The need for continuation of ICS should be regularly assessed as wheeze improves in a significant portion of children.[377] For a child with uncontrolled asthma, escalation of treatment to at least the next step is recommended.
Maintenance of asthma treatment in children
It is recommended to perform a full clinical assessment including asthma control status. The child is recommended to be clinically assessed regarding medications and doses, compliance with treatment, accuracy of inhalers technique, and any related environmental factors. Based on clinical assessment and asthma control status, SINA panel recommends the following:
Step up treatment for children who are uncontrolled based on physician assessment and complemented by a C-ACT score of ≤19 for a child aged 5–12 years or TRACK score of ≤80 for a child aged <5 years. It is recommended to rule out any modifiable factors preventing reaching optimal asthma control
Maintain treatment for children who reached controlled status based on physician assessment complemented by a C-ACT score of ≥20 for a child aged 5–12 years or TRACK score of >80 for a child aged <5 years
Consider stepping down treatment for children who are controlled for at least 3 months.
Reduction in therapy is recommended to be gradual and closely monitored based on clinical judgment complemented by either C-ACT score or TRACK score. Furthermore, close monitoring upon treatment stepping down is recommended for patients who have risk of asthma attack especially during seasonal variation or for those with prior acute asthma attack in the past year or history of ICU admission.
Referral to an asthma specialist
Referral to a pediatric asthma specialist for consultation or co-management is recommended in the following situations:
Uncertainty regarding the diagnosis
Difficulty achieving or maintaining control of asthma
Biological therapy or Immunotherapy are being considered
The patient requires step 4 care or higher
The patient has had an asthma attack requiring hospitalization or 2 or more OCS in the past 12 months.
Outpatient management of asthma in children aged 5–12 years
In addition to physician evaluation of asthma control, it is recommended that child/caregiver obtain the C-ACT score which is a validated test for children 5–12 years [Box 9.10]. C-ACT is a two-part questionnaire with a total of seven questions. The first part is to be answered by the patient and the second part by the caregiver. The final C-ACT score is made up of the sum of the scores of the two parts, ranging from 0 (poorest asthma control) to 27 (optimal asthma control). A score of ≤19 points suggests that a child’s asthma is not adequately controlled.[378]
This section describes treatment initiation, adjustment, maintenance, and step down which is mainly based on physician evaluation and assessment of disease control by obtaining C-ACT score.
Treatment initiation
It is recommended to assess asthma control status by physician assessment and/or C-CAT score for children aged ≥5 years [Box 9.11]. The following are recommended options based on asthma control level and/or C-ACT score.
Box 9.11.
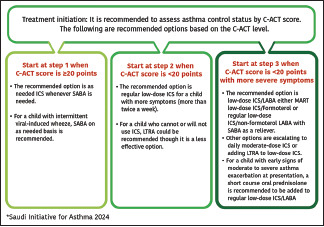
The SINA Approach for Asthma Treatment Initiation for children aged 5 to 12 years
-
Start at Step 1 when asthma is controlled by physician assessment and/or C-ACT score is ≥20 points:
-
Start at Step 2 when asthma is uncontrolled/partially controlled by physician assessment and/or C-ACT score is <20 points:
-
Start at Step 3 when asthma is uncontrolled/partially controlled by physician assessment and/or C-ACT score is < 20 points with more severe symptoms:
Adjustment and maintenance of treatment
It is recommended to perform a full clinical assessment including asthma control status and obtaining C-ACT score. Patient is recommended to be clinically assessed regarding medications and doses, compliance to treatment, accuracy of inhalers technique, and any related environmental factors. Based on clinical assessment and asthma control status, SINA expert panel recommends stepping up treatment for children who are uncontrolled based on physician assessment and complemented by a C-ACT score of ≤19. It is also recommended to rule out any modifiable factors that affect achieving asthma control. For patients who achieved control status, treatment based on physician assessment is recommended to be complemented by a C-ACT score of ≥20. The following are the five recommended five steps for children aged 5–12 years which is highlighted in Box 9.12
Step 1
Step 2
The recommended option is low dose-ICS (step 2) (Evidence A)[360,361]
Alternatively, either use of ICS whenever SABA is required, or daily LTRA that are less effective option at this step[115,400,401,402]
For a child with intermittent viral-induced wheeze daily low-dose ICS is recommended.[383]
Step 3
The recommended option is low-dose ICS/LABA either MART of ICS/Formoterol or regular ICS/non-formoterol LABA with SABA as a reliever[393,394,395,396,397,398,399,405,406,407]
Other options are escalating to daily moderate-dose ICS or adding LTRA to low-dose ICS (Evidence A)[115,400,401,402]
Whenever there is difficulty controlling asthma at Step 3, it is recommended to refer the child to a physician specialized in asthma for further evaluation for step 4–5 care.
Step 4
The recommended option is medium-dose ICS/LABA either MART of ICS/Formoterol or daily of ICS/non-formoterol LABA inhaler with SABA as a reliever[393,394,395,396,397,398,399]
Tiotropium or LTRA may be added to this combination if control is not achieved[408,409,410]
Whenever there is difficulty controlling asthma at Step 4, it is strongly recommended to refer the child to a physician specialized in asthma for further evaluation.
Step 5
It is recommended to refer the child to a physician specialized in asthma as there are growing evidence to support biologics for children with uncontrolled asthma at Step 4. The following are SINA expert panel recommendations for biological therapy at step 5 for this age group:
Anti-IgE therapy is a well-established therapy in children aged ≥6 years with uncontrolled asthma at Step 4 who fulfil the following criteria: severe persistent allergic asthma with frequent daytime symptoms or night-time awakenings, and who have multiple documented severe asthma attacks despite treatment at Step 4 (Evidence A)[411,412,413,414]
Mepolizumab is an anti-IL-5 agent approved for children aged ≥6 years (Evidence A)[415,416] that is indicated when eosinophil level is ≥150 cells/uL at treatment initiation or ≥300 cells/uL at any time in the prior 12 months. The dose is 40 mg for patients 6–11 years of age subcutaneously every 4 weeks
Dupilumab is a recombinant anti-IL-4 receptor, inhibiting the biological effects of both IL-4 and IL-13, and has been approved for the treatment of asthma in patients 6-12 years of age. Dupilumab reduces the rate of severe asthma exacerbations, improves lung function, and enhances asthma control in children with uncontrolled, moderate-to-severe asthma with evidence of type 2 inflammation as identified by blood eosinophils ≥150cells/uL or FeNO ≥20 ppb) (Evidence A).[417,418,419,420] The dose is 100 mg subcutaneously every 2 weeks or 300 mg subcutaneously every 4 weeks for children <30 Kg and 200 mg subcutaneously every 2 weeks for children ≥30 Kg
There are other biologics that have been approved for adult asthmatic population, which is currently investigated for expanded applications in children, readers discretion advised. This line of therapeutics should be initiated by an asthma and allergy specialist.
Specific immunotherapy in pediatrics
They are limited data, but it can be used for children >5 years of age and was shown to reduce long-term asthma medication use and improve FEV1 as detailed in immunotherapy subsection.[213] It should be initiated by an asthma and allergy specialist.
Recommendations for treatment step down
SINA expert panel recommends the following concepts for stepping down treatment:
If the patient is on ICS as monotherapy, the dose of ICS may be reduced by 25%–50% every 3 months to the lowest possible dose that is required to maintain control (Evidence B).[171,172,173] It should be clearly explained to the patient and/or caregiver that asthma control may deteriorate if treatment is abruptly discontinued.[174] In such a situation, an action plan that contains instruction on resuming controller therapy if asthma symptoms recurred is recommended to be provided to patients and their caregiver
If the patient is on combination of ICS/LABA at Step 3 or 4, abrupt discontinuation of LABA may lead to deterioration of asthma control[175]
If the patient is on a combination of ICS with LABA or LTRA, taper ICS to the lowest possible dose (Evidence B).[176,177] If control is maintained, LABA or LTRA may then be discontinued while patient on ICS (Evidence D)[176]
For patients on long-term oral steroids, the dose is recommended to be tapered to the lowest dose and preferably to every other day (Evidence D). It is recommended to refer the child to a specialized physician in asthma management.
Outpatient management of asthma in children aged <5 years
In addition to physician evaluation, it is recommended that caregiver obtain the TRACK score which is a validated ACT for children <5 years [Box 9.13]. It is a 5-item standardized questionnaire, with four questions that address the impairment domain and one question that address the risk domain of asthma control. Each item is scored from 0 to 20 points on a 5-point Likert-type scale for a total score ranging from 0 to 100. Higher scores would indicate better respiratory and asthma control; a score of <80 points suggests that a child’s asthma is not controlled.[421]
This section describes treatment initiation, adjustment, maintenance, and step down which is mainly based on physician evaluation and assessment of disease control by obtaining TRACK score.
Treatment initiation
It is recommended to assess asthma control status by TRACK score for children aged <5 years [Box 9.14]. The following are recommended options based on physician assessment and TRACK score.
Box 9.14.
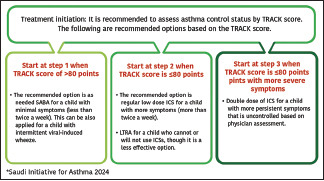
The SINA Approach for Asthma Treatment Initiation for children aged less than 5 years
-
Start at Step 1 when asthma is controlled by physician assessment and/or TRACK score of >80 points:
-
Start at Step 2 when asthma is uncontrolled/partially controlled by physician assessment and/or TRACK score is ≤80 points with mild symptoms:
-
Start at Step 3 when asthma is uncontrolled/partially controlled by physician assessment and/or TRACK score is ≤80 points pints with more severe symptoms:
- The recommended option is regular use of double “low-dose” of ICS for a child with more persistent symptoms that is uncontrolled based on physician assessment.[424]
Adjustment and maintenance of treatment
It is recommended to perform a full clinical assessment including asthma control status and obtaining TRACK score. Patients are recommended to be clinically assessed regarding medications with doses, compliance to treatment, accuracy of inhalers technique, and any related environmental factors. Based on clinical assessment and asthma control status, SINA expert panel recommends stepping up treatment for children who are uncontrolled based on physician assessment and complemented by a TRACK score of ≤80. It is also recommended to rule out any modifiable factors preventing reaching optimal asthma control. For children achieved control status, maintain treatment based on physician assessment complemented by a TRACK score of >80.
The following are the five recommended five steps for children aged <5 years which is highlighted in Box 9.15
Step 1
The recommended option is as needed SABA for a child with minimal symptoms (less than twice a week). This can be also applied for a child with intermittent viral-induced wheeze.[384,385]
Step 2
The recommended option is regular low-dose ICS (Evidence A)[383]
Alternatively, daily LTRA for a child who cannot or will not use regular ICSs, though it is a less effective option (Evidence B).[400,401,402] Additionally, short course low dose ICS is recommended for URTI triggered exacerbations if regular use of ICS not applied.[360,361]
Step 3
The recommended option is regular double the “low-dose” of ICS (Evidence A)[406]
Alternatively, adding LTRA to low dose ICS is a less effective option[405,424]
It is recommended to refer the patient to a physician specialized in asthma for further evaluation and options whenever management is escalated beyond step 3.
Step 4
The recommended option is adding regular LTRA to the daily double “low-dose” ICS (Evidence B)[425,426]
There is no evidence to support the use of LABA for patients younger than 5 years
It is strongly recommended to refer the patient to a physician specialized in asthma for further evaluation and options whenever management is escalated beyond step 3.
Step 5
At this Step, regular use of systemic steroids shall be added to step 4 regimen, and the patient should be referred to an asthma specialist
There is no evidence to support the use of either LABA or biologics for children <5 years.
Recommendations for treatment step down
SINA expert panel recommends the following concepts for stepping down treatment:
If the patient is on ICS as monotherapy, the dose of ICS may be reduced by 25%–50% every 3 months to the lowest possible dose that is required to maintain control (Evidence B).[171,172] It is recommended to be clearly explained to the caregiver that asthma control may deteriorate if treatment is abruptly discontinued.[174] If asthma symptom is recurred, an action plan that contains instruction on resuming controller therapy is recommended to be provided to patients and their caregiver. Consultation with a healthcare provider is recommended if control is not achieved
For significant side effects, consider a change in therapy, reduction in the dose or frequency of ICS (if possible), advice for a mouth washing after inhalation if possible, enforce use of MDI with spacer, and/or use of appropriate local antifungal therapy for severe oral thrush.[178]
Section 10: Management of Acute Asthma in Children
Early recognition of acute asthma
Recognition of early signs of acute asthma is essential especially for those <5 years. Early symptoms of acute asthma include (Evidence D):
An attack of shortness of breath with wheeze or increase of shortness of breath with wheeze
A new cough, especially at night although this is non-specific
Impairment of daily activity
An increased need for or poor response to SABA
For a child <2 years, the presence of lethargy and poor feeding should raise the suspicion of acute asthma attack. However, viral bronchiolitis is a common differential diagnosis in this age group during winter season.
In a child aged 2–5 years, the combination of the above features can predict approximately 70% of acute asthma attacks with low false positive rate.[427] Moreover, URTI may frequently precede acute asthma attack in children. Clinical assessment is essential in children as the utilization of objective measures such as PFT is problematic, especially in the younger age groups.
Initial management of acute asthma at home
The SINA panel recommends management of a child with asthma to include an action plan that enables the caregiver to recognize worsening of asthma and the advice for initial treatment. The action plan is recommended to include features that mandate the need for urgent medical care that includes acute distress of the child, difficulty to complete few words in one breath, and poor response to SABA treatment at home.
In the case of acute attack, initial management at home by the caregiver is recommended to be started with salbutamol inhaler 2–4 puffs by a spacer that may be repeated every 20 min for a total of three doses. If the child improves, asthma therapy is recommended to be stepped up as per instructions in the action plan and medical advice should be sought as soon as possible.
Immediate transfer to hospital
If the child does not adequately improve within or after the initial period, urgent medical care is recommended. Box 10.1 shows the indications for immediate transfer to hospital for children with acute asthma exacerbation.
Box 10.1.
Description of indications for immediate transfer to hospital for children with acute asthma exacerbation
| Indications | Description |
|---|---|
| Features of severe symptoms | Unable to speak and/or risk to choke with food or drink |
| Bluish discoloration of lips and tongue (cyanosis) | |
| Rapid breathing >40/min for children <5 years of age | |
| Silent chest with evidence of increased work of breathing | |
| Lack of response to rescuer treatment | Lack of response after 1–2 h of proper technique and dosage |
| Limitation to deliver acute treatment | Social, family, or environmental reasons |
During the transfer to hospital, inhaled SABA should be provided as well as oxygen supply (to maintain saturation >94%). In addition to consideration to give the first dose of systemic corticosteroid, instant communication with specialized call center is recommended to provide the required consultations.
Assessment of asthma severity in the emergency department
Assessment of acute asthma severity in children has an important role in various components of acute asthma management such as: pharmacological interventions, need for hospitalization, and need for ICU admission. The assessment of acute asthma severity in young children is also important for clinical decision-making and evaluation of treatment effectiveness.[50,428,429,430,431] This is supported by the fact that PFT measurement is not feasible as more than half of asthma attacks in children presented to emergency departments for children <5 years.[432]
The Pediatric Respiratory Assessment Measure (PRAM) has been found to be feasible, valid, responsive and reliable tool to determine acute asthma severity in children aged 2–17 years.[432,433] The PRAM represents a useful means to record clinical signs in a standardized fashion [Box 10.2].[50] The PRAM score is a 12-point score consisting of oxygen saturation, suprasternal retractions, scalene muscle contraction, air entry, and wheezing.[430]
Box 10.2.
The pediatric respiratory assessment measure score
| Sign | 0 | 1 | 2 | 3 |
|---|---|---|---|---|
| On | Absent | Present | ||
| Scalene muscle contraction | Absent | Present | ||
| Air entry | Normal | Decreased at bases | Widespread decreased | Absent/minimal |
| Wheezing | Absent | Expiratory only | Inspiratory and expiratory | Audible wheeze/silent chest with minimal air entry |
| O2 saturation | ≥95% | 92%–94% | <92% |
Clinical pathways based on PRAM for inpatient asthma management has been shown to decrease the length of stay and bronchodilator use with no adverse outcomes or increased acute care encounters.[434,435,436] The SINA panel recommends measuring PRAM score for asthmatic patients in emergency as it can categorize the risk of hospitalization:
Total score of 1–3: Low risk with a chance of <10% for hospital admission
Total score of 4–7: Moderate risk with a chance of 10%–50% for hospital admission
Total score of 8–12: High risk with a chance of >50% for hospital admission.
Management of acute asthma in the emergency department
Management of acute asthma exacerbation in the Emergency Department should target the following goals:[437,438]
Rapid reversal of bronchospasm
Correction of hypoxemia if present
Reducing the need for hospitalization
Preventing recurrence of the attack after discharge.
After performing the necessary clinical assessment, the SINA expert recommends the utilization of PRAM as a tool to assess patients in the Emergency Department and guide further management as well. The PRAM score should be obtained at the initial assessment and after initiation of treatment as well. After initial clinical assessment and starting initial appropriate therapy, managing physician is recommended to focus on obtained history to identify risk factors for ICU admission, including:[439]
Previous life-threatening asthma attack
Previous ICU admission
Previous intubation
Deterioration while already on systemic steroid.
In addition, managing physician is recommended to be aware of the following clinical features of severe or life-threatening asthma that require immediate medical attention:
Child is unable to speak or drink
Central cyanosis
Confusion or drowsiness
Significant subcostal or subglottic retraction
Oxygen saturation <92%
Silent chest on auscultation
Tachycardia (heart rate >100 beats/min).
Implementation of clinical pathway that utilizes PRAM score for acute asthma management in children with moderate to severe asthma attacks markedly decrease the rate of hospitalization without increasing the rate of return to emergency care (Evidence B) [Box 10.3].[435,440,441,442] This has been supported by a study showing that PRAM score after 3 h of initial management was associated with a significant improvement in the prediction of admission rate compared to pure clinical judgment at triage.[433] Ancillary investigation that includes CXR and ABG are not routinely recommended.[439] Capillary blood gases is indicated in severe bronchial asthma that failed to respond to maximum therapy and required ICU admission. CXR is recommended in the following conditions:
Box 10.3.
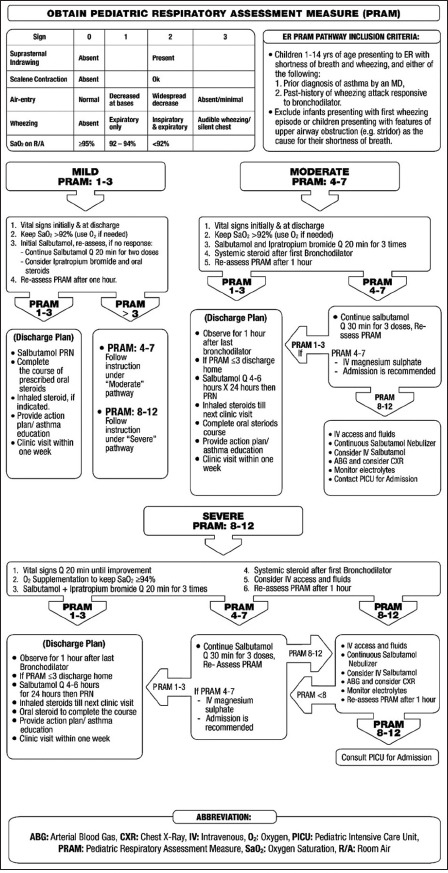
Assessment and treatment of acute asthma in children
Suspected bacterial pneumonia that presents with fever >39°C and presence of focal finding of decreased breath sound and crackles
To rule out bronchial asthma complications such as pneumothorax
Severe disease that does not respond to maximum treatment
Uncertainty about the diagnosis
Hypoxemia apparently disproportionate to the attack severity.
Viral infection is the usual cause of asthma attacks in children and thus routine use of antibiotics is strongly discouraged.[443] Antibiotics are recommended when bacterial pneumonia is clinically suspected.[440,441]
Acute asthma management based on Pediatric Respiratory Assessment Measure
The SINA panel recommends managing asthma based on PRAM score obtained at initial assessment:
-
(i)
Mild-PRAM score of 1–3: The initial management includes:
- Obtain vital signs initially and at discharge
- Prescribe appropriate oxygen dose to keep saturation ≥92%
- Less than 20 kg: 5 puffs by MDI/spacer or 2.5 mg by nebulizer
- 20 kg or more: 10 puffs by MDI/spacer or 5 mg by nebulizer-titrate MDI dose based on response)
- In mild cases, SABA with spacer is not inferior to nebulized SABA.
Ipratropium bromide may be considered at a dose of 4 puffs by MDI with spacer or 250 μg by nebulizer every 20 min for the 1st h only[445]
Oxygen flow via facemask during the administration of nebulized drugs should not go below 6 L/min[446]
Consider oral steroid if there is no response to the first dose of salbutamol. Prednisolone dose is 1–2 mg/kg up to a maximum dose based on age. The maximum dose is 20 mg for children <2 years, 30 mg for children 2–5 years, and 60 mg for children 5–12 years. Dexamethasone dose of 0.6 mg/kg up to maximum dose of 16 mg[447]
Reassess PRAM after 1 h.
-
Management after initial treatment based on PRAM score:
-
PRAM score is 1–3: The management includes:
- The child may be discharged on salbutamol inhaler and ICS inhaler with a spacer
- If an oral steroid course is given initially, dexamethasone is recommended for extra 1 day and prednisolone for total of 3–5 days
- It is recommended to offer the child an action plan, education on inhalers technique, and a follow-up visit within 1 week to the appropriate clinic
- PRAM score is 4–7: Treat as a moderate asthma attack (see below)
- PRAM score is 8–12: Treat as a severe asthma attack (see below).
-
-
(ii)
Moderate-PRAM score of 4–7: The management includes:
- Obtain vital signs
- Prescribe appropriate oxygen dose to keep Saturation ≥92%
- The combination of salbutamol and ipratropium bromide has been shown to be effective in this situation (Evidence B)[444]
- Re-assess PRAM after 1 h
- If PRAM score after 1 h is 1–3, observe for another hour.
-
Management after initial treatment based on PRAM score:
-
PRAM score is 1–3:
- The child may be discharged on salbutamol inhaler with a spacer and ICS if the patient is not already on controller treatment
- Complete the course of oral steroids. Dexamethasone is recommended for extra 1 day and prednisolone for a total of 3–5 days; both as once daily dose
- It is recommended to offer the child an action plan, education on inhalers techniques, and a follow-up visit within 1 week to the appropriate clinic.
-
PRAM score is 4–7: It is recommended to continue treatment with salbutamol every 30 min for three doses and to assess PRAM score every 30 min. Further evaluation is based on PRAM re-assessment:
- If PRAM score improves to 1–3, the child can be managed as above
- PRAM score is 8-12: treat as severe asthma attacks (see below).
-
-
(iii)
Severe-PRAM score of 8–12: The management includes:
- Obtain vital signs every 20 min until improvement
- Prescribe appropriate oxygen dose to keep saturation ≥92%
- Reassess PRAM after 1 h
- Consider IV access and appropriate IV fluids
- If PRAM score after 1 h is 1–3, Observe for another hour.
-
Management after initial treatment based on PRAM score:
-
PRAM score is 1–3:
- The child may be discharged on salbutamol inhaler with a spacer and ICS if the patient is not already on controller treatment
- It is recommended to offer the child/care giver an action plan, education on inhalers technique, and a follow-up visit within 1 week to the appropriate clinic.
-
PRAM score is 4–7: It is recommended to continue treatment with salbutamol every 30 min for three doses and to assess PRAM score every 30 min. Further evaluation is based on PRAM re-assessment:
- If PRAM score improves to 1–3, the child can be managed as above
-
PRAM score is 8–12: Deterioration of clinical status despite adequate treatment in the initial period warrants special care and attention. It is recommended to:
- Establish IV access and start on appropriate IV fluids
- If PRAM score does not improve, IV Magnesium sulphate is recommended as a single dose of 40–50 mg/kg to a maximum of 2 g by slow IV infusion over 20–30 min
- ABG, CXR and electrolyte are recommended to be obtained and the pediatrics critical care or equivalent service must be consulted.
-
Appendix: Medications Used in Asthma Treatment
Medications used to treat asthma can be classified as controllers or relievers. Controllers are medications taken daily on a long-term basis to keep asthma under clinical control.[456] Relievers are medications used on an “as-needed basis” that act quickly to reverse bronchoconstriction and relieve symptoms. Multiple combinations of controllers are available.
Reliever medications
Relievers are medications used on, an “as-needed basis”, and act quickly to reverse bronchoconstriction and relieve symptoms.
Fast onset inhaled β2-agonists
SABAs, such as salbutamol, have been traditionally used for relief of symptoms of acute attacks of asthma and for the pre-treatment of EIB. Use of MDI with a chamber (Spacer) is as effective as the nebulized route in treatment of acute episodes of wheeze in children.[230] Regular long-term use of SABA alone is not recommended. Formoterol is a LABA with fast-acting component that is combined with an ICS to be uses as an anti-inflammatory reliever that can be used alone on as needed basis in patients with mild asthma or as MART in more symptomatic patients.[97,98,99] Vilanterol is another LABA used once a day that has a fast onset of action within 15 min and long half-life; hence, the patient should be advised to only use it once a day on a regular basis and not a rescue medication.[147,148] In acute asthma, inhaled SABA is the preferred choice.[232,457] Repeated doses are recommended to be given at 15–20 min intervals. Alternatively, continuous nebulization (salbutamol at 5–10 mg/h) could be used for 1 h if there is an inadequate response to initial treatment. However, a meta-analysis of randomized controlled trials of adults with acute asthma found no significant differences between the continuous or intermittent methods in terms of pulmonary function or hospital admission; nevertheless, patients treated by continuous nebulization had fewer side effects.[458] In patients who are able to use the inhaler devices, 6–12 puffs of MDI with a spacer are equivalent to 5 mg of salbutamol by nebulizer. As the inhaled route has a faster onset of action and fewer adverse effects, the use of IV SABA in the initial treatment of patients with acute severe asthma is not generally recommended.[459] IV therapy should not be considered routinely and only used cautiously if the response to the inhaled drug is poor or if the patient cannot tolerate the inhaled route.
In patients with mild asthma as needed use of ICS and SABA in a single inhaler was as effective as regular use of ICS with a lower 6-month cumulative dose of the ICS.[95] In patients with uncontrolled moderate-to-severe asthma on various inhaled ICS-containing maintenance therapies, the risk of severe asthma exacerbation was significantly lower with as-needed use ICS/SABA and ICS fixed dose inhaler than with as-needed use of SABA alone.[94]
Anticholinergics
Anticholinergics are less effective than SABA in asthma. However, when used in combination with SABA in acute asthma, they provide an additional benefit.[444] They can also be an alternative bronchodilator for patients who experience adverse effects such as tachycardia, arrhythmia, and tremor from SABA. Their side effects include dryness of the mouth and a bitter taste.
In moderate to severe acute asthma, combining ipratropium bromide with salbutamol was shown to have additional bronchodilation effect and faster improvement in lung function, compared to salbutamol alone.[239,242] A systematic review showed the combination therapy has an added benefit in reducing hospitalizations.[241] Combining both agents led to reduction in hospital admission rates by 38%–57%, improvement in lung function, and substantial cost saving.[242,460,461] No evidence of benefit for length of hospital stay and other markers of response when inhaled anticholinergics are added to SABA in hospitalized asthmatic children with acute attacks.[462] The adult dosing of nebulized ipratropium bromide is 500 μg every 20 min for three doses, then as needed. Alternatively, ipratropium bromide can be administered by MDI at a dose of 4–8 puffs (80–160 μg) every 20 min, then as needed for up to 3 h.
Intravenous magnesium sulphate
In a systematic review, magnesium sulphate was shown to reduce hospitalizations in patients with severe or life-threatening asthma attacks that failed to respond to initial treatment.[463] A single dose of IV magnesium sulphate at a dose of 1–2 g over 20 min is safe and effective in acute severe asthma.[247]
Anti-inflammatory controller medications
Inhaled corticosteroids
ICS are currently the most effective anti-inflammatory medications for the treatment of asthma.[33,119,464] The available ICSs are beclomethasone dipropionate, budesonide, ciclesonide, fluticasone propionate, fluticasone furoate, mometasone furoate. They reduce symptoms, improve quality of life, improve lung function, decrease airway hyper reactivity, control airway inflammation, reduce frequency and severity of asthma attacks, and reduce asthma mortality. Early initiation of low dose ICS in asthma leads to improvement in lung functions.[117] When they are discontinued prematurely or abruptly, deterioration of clinical control follows within weeks to months in most patients. ICS differ in their potency and bioavailability. Most of the benefits from ICS are achieved in adults and children at relatively low doses. Exposure to tobacco smoking or vaping, including secondary and tertiary, reduces the responsiveness to ICS. To reach control, add-on therapy with another class of controller is preferred to increase the dose of ICS.[136,465]
Local adverse effects can occur and include oropharyngeal candidiasis and dysphonia; with metered dose inhalers (MDI), these effects are reduced by using a spacer device. Mouth and throat washing after inhalation may reduce oral candidiasis. The small risk of adverse events from the use of ICS is well balanced by their efficacy.[466] Therefore, low-medium dose of ICS is generally safe and well tolerated in adults and children. Systemic side effects are occasionally reported with high doses and long-term treatment.
Special considerations for use of inhaled corticosteroids in children
Growth retardation may be seen with all ICS when a high dose ICS is chronically used. Systematic reviews showed a reduction may affect height velocity in pre-pubertal children over 12 months use of low-to-medium dose of ICS, especially during the 1st year of life.[467] Though this effect was statistically significant and sustained during adult life, it is not clear if that will be of significant clinical impact.[468,469] For instance, use of moderate-dose ICS resulted in 1.2 cm reduction in the final adult height after more than 4 years use.[470] Moreover, more studies demonstrated the negative impact of medium-to-high doses ICS on bone mineralization.[471,472,473] However, it is crucial to remember that long-term use of ICS is safer than frequent bursts of OCS on bone mineralization. Adequate nutrition with sufficient intake of calcium and vitamin D can blunt these effects.[474] In summary, the potential adverse effects of ICS need to be weighed against the well-established benefit to control persistent asthma. Therefore, it is important to target the lowest possible ICS dose that maintains adequate asthma control.
Leukotriene modifiers
Leukotriene modifying agents reduce airway inflammation and improve asthma symptoms and lung function, but with individual variation in response and a less consistent effect in reducing the frequency of asthma attacks, especially when compared to ICS. Currently, montelukast is available locally that may be used as an alternative treatment to ICS for patients with mild asthma, especially in those who have clinical rhinitis. Some patients with aspirin-sensitive asthma respond well to the LTRA. However, when used alone as a controller, their effects are generally less than that of low-dose ICS. When added to ICS, LTRA may reduce the dose of ICS required by patients with uncontrolled asthma and may improve asthma control.[475,476] LTRA are generally well tolerated, however; it is recommended to be aware of the Food and Drug Administration warning about serious behavior and mood-related changes with montelukast.[164] In children, studies have shown that LTRA may be useful for reducing the number of asthma attacks induced by viruses and for reducing bronchial inflammation in atopic children.[477,478,479,480] There are no clinical data to support their use under the age of 6 months.[164]
Other controller medications
Long-acting inhaled β2-agonists
The commonly used LABA are formoterol and salmeterol that are used twice daily. Vilanterol and indacaterol are LABA agents with a 24-h or longer duration of action.[481,482,483,484,485,486,487] Due to lack of anti-inflammatory effect, LABA should not be used alone as monotherapy in asthma as this can lead to increased mortality, and should be used in combination in the same device with ICS when prescribed for asthma. When used in combination with ICS, there is an improvement in symptoms, decreased nocturnal asthma, improved lung function, decreased use of SABA, reduced number of asthma attacks and better control at a lower dose of ICS. LABA provides longer protection to prevent exercise-induced bronchospasm than SABA.[488] Their side effects include tachycardia, tremor, headaches, muscle cramps, and sometimes hypokalemia. Regular use of LABA combined with ICS may lead to a reduction in their side effects. The effect of LABA has not been adequately studied in children of <5 years.
Long-acting anti-muscarinic agents
LAMA inhibits the effect of acetylcholine on muscarinic receptors, thus producing bronchodilation. LAMA agents are classically used for the treatment of COPD patients. Tiotropium bromide was the first LAMA extended for use in asthma. Its bronchodilatation duration of action of more than 24 h allows for single daily dosing.[489,490] The earlier studies on tiotropium were conducted using the Handihaler© device, while the more recent studies were conducted using the new Respimat device. To date, tiotropium is mainly available in the Saudi Market in the Handihaler device in an 18-μg capsule format while the Respimat device is not widely available. Tiotropium was shown to be an effective stepping up strategy when added to a combination of ICS/LABA, and not inferior to LABA as an add on to a medium dose ICS.[139,140,160,491,492] Adding LAMA can significantly improve lung function in uncontrolled cases and reduce attacks (Evidence A).[138,159,160] Triple therapy of ICS/LABA/LAMA in one device is available for uncontrolled asthma treatment in once and twice a day combinations (see the section on single inhaler triple therapy below). The main side effect of LAMA is dryness of mouth, although mild prostatic symptoms in men have been reported.[493]
Theophylline
Theophylline is a weak bronchodilator with modest anti-inflammatory properties. It may provide benefits as an add-on therapy in patients who do not achieve control with ICS alone but is less effective than LABA or LTRA. Theophylline is not recommended for use as monotherapy in asthma treatment. Low-dose theophylline (150 mg twice daily) may have a role in improving steroid resistance in patients with severe asthma requiring high-dose ICS.[494,495] Side effects include gastrointestinal symptoms, cardiac arrhythmias, seizures, and even death. Nausea and vomiting are the early symptoms of toxicity. Liver disease and congestive heart failure may increase the risk of toxicity. The use of lower doses may decrease these side effects. Theophylline has a narrow therapeutic window, multiple drug interactions and a risk of toxicity that limits its use in treating asthma.
Oral short-acting bronchodilator
The side effect profile is much higher than that of inhaled SABA. Therefore, their use is highly discouraged in asthma management. The oral route is prohibited for children as well.
Combination therapy with inhaled corticosteroids and long-acting β2 agonist
Fixed combinations of ICS and LABA are considered more convenient for patients. Combination therapy is generally safe and results in significantly fewer asthma attacks.[132,496,497,498,499] They increase adherence and ensure that LABA is always accompanied by ICS. Although salmeterol and formoterol provide a similar duration of bronchodilation and protection against bronchoconstriction, formoterol has a faster onset of action than salmeterol. Therefore, combination inhalers containing formoterol may be used for both rescue and maintenance of control.[97,132] In mild asthmatics, studies compared as-needed ICS/formoterol combination with as-needed SABA alone or in combination with daily maintenance ICS showed that as-needed ICS/formoterol was non-inferior to budesonide maintenance therapy for severe exacerbations, but was superior in real-life studies and meta-analyses, and reduced urgent healthcare utilizations and hospitalizations in mild asthma. The dose of ICS in the as-needed ICS/formoterol approach was 17% to 25% of the dose of ICS in the maintenance therapy at the expense of a small, but significant, change in asthma control score in favor of budesonide maintenance therapy. ICS are now recommended to start from Step 1 either as ICS/formoterol combination on as-needed basis or SABA with ICS in a separate inhaler every time rescue treatment is needed.[98,99,496,497,498,499,500] Once a day dry powder combination of ICS/LABA with fluticasone furoate and vilanterol (Relvar) is available in Ellipta® device in two strengths of 100/25 and 200/25 μg with dispensed equivalent dose of 92/22 and 184/22 μg, respectively.[147,148] The dose of fluticasone furoate of 100 μg is approximately equivalent to fluticasone propionate of 250 μg.[501] Indacaterol combination with mometasone furoate 150/160 μg is available in Breezhaler device®.[150] Such combinations have a potential adherence advantage while maintaining the same safety.
Single Inhaler Triple therapy with Inhaled Corticosteroids, Long-acting β2 Agonist, and Long-acting Anti-muscarinic Agent
Fixed combination inhalation devices are considered more convenient for patients.[502] Triple therapy of ICS/LABA/LAMA was first introduced for COPD treatment. Available triple therapy combinations for asthma management are fluticasone furoate/umeclidinium/vilanterol (Trelegy®), Beclomethasone dipropionate/Formoterol/glycopyrronium (Trimbow®), and mometasone furoate/glycopyrronium/indacaterol (Enerzair Breezhaler®).[151,155,156,157,158] In a systematic review and meta-analysis, among children (aged 6–18 years) and adults with moderate to severe asthma, triple therapy, compared with dual therapy of medium to high dose ICS/LABA, was significantly associated with 17% fewer severe asthma exacerbations and modest improvements in asthma control without significant differences in quality of life or mortality.[503] In a meta-analysis of Phase III studies, triple combination therapies with a high-dose ICS/LABA/LAMA administered were more effective than medium-dose FDC against moderate to severe exacerbation and increasing trough FEV1.[154] Using biomarkers of type 2 airway inflammation measurement in one study showed that a higher dose of ICS reduced the rate of exacerbations.[65]
Systemic corticosteroids
Long-term oral steroid therapy (excluding short courses for acute attacks of asthma for a period of 1–2 weeks) may be required to control difficult-to-treat asthma despite maximum standard therapy when biological agents cannot be used. The dose should be reduced to the lowest possible and other controllers are recommended to be maximized to minimize the side effects from the OCS. Its use is limited by the risk of significant adverse effects. The use of intramuscular long-acting steroids is highly discouraged because of the increased risk of side effects. Side effects include osteoporosis, hypertension, diabetes, adrenal insufficiency, obesity, cataracts, glaucoma, skin thinning, and muscle weakness. Sudden withdrawal can elicit adrenal failure, therefore gradual withdrawal is recommended. In patients prescribed long-term systemic corticosteroids, prophylactic treatment for osteoporosis is recommended. If available, biologics should be used in these patients to decrease the corticosteroid burden.
Inhalation devices used in asthma
Medication aerosol can be delivered using three devices:
Small-volume nebulizer (SVN)
It is the most popular for patients and clinicians with acute asthma. Small-volume nebulizers (SVNs) are predominately powered by a compressed gas (air or oxygen) to convert one or more drug solutions or suspensions at any concentrations and dose into aerosols. SVN has the advantage of the need for minimal patient cooperation and is, therefore, suitable for all ages, with normal breathing and no inspiratory pause required. One of its main disadvantages is importability, short time to deliver the medication and potential contamination.
Pressurized metered-dose inhaler
It is a pre-pressurized inhaler with medication and a propellant, which when actuated will give one dose of the drug for a single inspiration. An MDI typically requires slow inspiratory flow (≤30 L/min). One of its main advantages is that it is pre-mixed and has the ability to provide multiple doses in a short period of time. It is also small and portable with limited contamination. It is the preferred method in children if used with a large-volume spacer device. Disadvantages include the need of patient training to coordinate inhalation with actuation, and if this is not done properly, there is a potential of high deposition of drug in the oropharynx and poor drug delivery. Also, because it does not have a dose counter, it is difficult to determine the dose remaining in the canister.
Dry powder inhaler
It is not pressurized (no propellant), and therefore requires high inspiratory flows (60–90 L/min) to disperse a full dose. In addition to its portability, advantages include an easier inhaler technique and a built-in dose counter. Disadvantages include the need for adequate inspiratory flow to disperse a full dose. If not used properly, high oropharyngeal impaction may occur, and exhaled humidity into the mouthpiece might affect the function of some devices. Therefore, it may not be suitable for very young or very old patients. The commonly available device in Saudi Arabia includes Turbohaler, Diskus, Handihaler, Easi-Breathe, Ellipta, Easyhaler, and Nexthaler devices.
Breath-actuated inhalers
These inhalers automatically release a spray of medication when the person begins to inhale. They are easy to use and improve asthma control and compliance with medications.[504,505,506,507]
Biologics in asthma treatment
The recent progress in biologics in asthma has made a step forward toward the practice of precision medicine for asthma patients. To date, there are no head-to-head studies to compare the available biologics with each other, neither between those with different targets nor between anti-IL5 therapies themselves. In addition, due to the wide variability of the published clinical trials’ inclusion criteria and analysis methodologies, it is also difficult to compare the efficacy of these therapies based on existing studies. Box 11.1 shows comparative descriptions and effects of different approved biological therapies for severe asthma.[508]
Box 11.1.
Comparative descriptions and effects of approved biological therapies for severe asthma
| Anti-IgE Omalizumab[514,515,516,517,518,519,520] |
Anti-IL5[200,509,510,511,512,513] | Anti-IL4Rα Dupilumab[420,529,530,531] |
Anti-TSLP Tezepelumab[532,533,534,535,536,537] |
||
|---|---|---|---|---|---|
|
| |||||
| Mepolizumab[511,521,522,523] | Benralizumab[520,524,525,526,527,528] | ||||
| Mechanism of action | Binds to IgE and prevents it from binding to mast cells, reducing inflammation | Blocks IL-5, reducing eosinophil production and inflammation | Binds to IL-5 receptor, leading to eosinophil apoptosis, and reducing inflammation | Blocks IL-4Rα, reducing inflammation and improving airway hyperresponsiveness | Binds to TSLP and prevents it from activating immune cells such as dendritic cells, T cells, and mast cells, which can lead to a reduction in inflammation and airway hyperresponsiveness |
| Approved indications | Moderate to severe persistent asthma with a positive skin test or in vitro reactivity to a perennial aeroallergen Nasal polyps Chronic idiopathic urticaria | Severe eosinophilic asthma Rhinosinusitis with nasal polyps EGPA Hypereosinophilic syndrome | Severe eosinophilic asthma | Atopic dermatitis Severe eosinophilic asthma or with oral corticosteroid dependent asthma Chronic rhinosinusitis with nasal polyposis EoE Prurigo nodularis | Severe asthma |
| Asthma eligibility criteria | Severe allergic asthma (at least one positive aeroallergen on skin prick testing or an elevated specific aeroallergen IgE level) uncontrolled on high-dose ICS combined with LABA and other controllers and who have an IgE level of within therapeutic range | Severe eosinophilic asthma (blood eosinophils should be >150/µL within the last 6 weeks or >300/µL within the last 12 months before starting) | Severe eosinophilic asthma (has blood eosinophil count ≥300 cells/µL in the last 12 months) | Severe eosinophilic asthma (blood eosinophils ≥150/µL or FeNO ≥25 ppb) and oral steroids dependent severe asthma regardless of blood eosinophils count | Severe exacerbations in the last year |
| Age of use in asthma | 6 years and above | 6 years and above | 12 years and above | 6 years and above | 12 years and above |
| Dose | 75–375 mg based on IgE and patient weight every 2–4 weeks | 12 years and older: 100 mg administered subcutaneously once every 4 weeks Aged 6–11 years: 40 mg administered subcutaneously once every 4 weeks | 30 mg subcutaneously once every 4 weeks for the first 3 doses Then once every 8 weeks thereafter | 600 mg SC once, then 300 mg q2 weeks | 210 mg SC every 4 weeks |
| Outcomes based on exacerbation rates (AER) | AER reduction: 0.56/100 person-years (95% CI: 0.4–0.77) | Relative reduction in AER: 53% (95% CI: 36–65) | Relative reduction of AER (0.49, 0.37–0.64; P<0.0001) | AER reduction: 47.7% with dupilumab than with placebo (P<0.001) | AER reduction: 0.44; 95% CI: 0.37–0.53; P<0.001 |
| Outcomes based on oral corticosteroids use | The median percentage reduction from baseline in the glucocorticoid dose was 50% (P=0.007) | Reduced the median final oral glucocorticoid doses from baseline by 75%, (P<0.001) | The percentage change in the glucocorticoid dose was−70.1% in the dupilumab group, (P<0.001) | Similar to placebo OR: 1.28; 95% CI: 0.69–2.35; P=0.43 | |
| Outcomes based on asthma symptoms | Decreased mean asthma symptom score (TASS score) (−0.26 [CI: −0.42–−0.10]) compared with placebo | Improvement in mean ACQ5 difference 0.44 points compared to placebo | Improvement in mean ACQ6 difference 0·25, 95% CI: 0·45–−0·06 compared to placebo | Improvement in mean ACQ5 difference 0.47 (95% CI: −0.76–−0.18) | Improvement in mean ACQ6 difference 0.33; 95% CI: −0.46–−0.20; P<0.001 |
| Outcomes based on QOL | Improvement in AQLQ difference, (0.29 point [CI: 0.15–0.43]) | Improvement in SGRQ difference 7.0 points compared to placebo | Improvement in AQLQ difference +0.1 (−0.08–+0.28) compared with placebo | Improvement in AQLQ difference, 0.34; 95% CI: 0.20–0.47; P<0.001 | |
| Outcomes based on FEV1 | Increase in FEV1 by 120.9 mL (30.6–211.2 mL; P=0.009) compared to placebo | Increase in FEV1 By 98 mL (P=0.03) compared to placebo | Increase in FEV1 by 159 mL, 0.068–0.249. Compared with placebo | Increase in FEV1 by 140 mL; P<0.001 compared to placebo | Increase in FEV1 by 130 mL; 95% CI: 0.08–0.18; P<0.001 compared to placebo |
| Predication of response | Higher blood eosinophil FeNO | Higher blood eosinophil Higher exacerbations history Adult-onset asthma Nasal polyps | Higher blood eosinophil OCS use nasal polyposis FVC <65% | Higher blood eosinophil Higher FeNO | Higher blood eosinophil Higher FeNO |
| Side effects | Serum sickness Unmasking hypereosinophilic conditions (EGPA) Black box warning Cardiovascular risk Anaphylaxis risk | Helminthic infection Hypersensitivity reaction Herpes zoster (rare) Headache Sore throat | Helminthic infection Hypersensitivity reaction Herpes zoster (rare) Headache Sore throat Arthralgia | Helminthic infection Hypersensitivity reaction Unmasking Hypereosinophilic conditions (EGPA) Conjunctivitis Consider completing all age-appropriate vaccinations as recommended by current immunization guidelines before initiating treatment | Pharyngitis Arthralgia Back pain Injection-site reactions |
| Pregnancy and breast-feeding precautions | US FDA: Not assigned There is availability of published registry which showed similar major congenital risk, live birth and lower birth weight as placebo | US FDA: Not assigned | US FDA: not assigned | US FDA: Not assigned | US FDA: Not assigned |
EoE=Eosinophilic esophagitis, OR=Odds ratio, CI=Confidence interval, FDA=Food and Drug Administration, EGPA=Eosinophilic granulomatosis with polyangiitis, FeNO=Fractional exhaled nitric oxide, FVC=Forced vital capacity, OCS=Oral corticosteroid, QOL=Quality of life, AQLQ=Asthma QOL Questionnaire, SGRQ=St George’s Respiratory Questionnaire, TASS=Total asthma symptom score, ACQ=Asthma Control Questionnaire, AER=Asthma exacerbation rate, LABA=Long-acting β2 agonist, TSLP=Thymic stromal lymphopoietin, IgE=Immunoglobulin E, ICS=Inhaled corticosteroid, FEV1=Forced expiratory volume in 1 s, IL-5=Interleukin-5, IL4Rα=Interleukin 4 receptor α, SC=Subcutaneous
Financial support and sponsorship
The SINA received financial and logistic support from the STS.
Conflicts of interest
The SINA is fully sponsored by the Saudi Thoracic Society.
Acknowledgment
The SINA Panel would like to thank Prof. Eric Bateman from the University of Cape Town Lung Institute, Cape Town, South Africa and Prof. Andrew Bus from Imperial College, National Heart and Lung Institute, London, United Kingdom for reviewing the SINA 2024. The Panel would also like to thank the following consultant reviewers for previous versions (2009, 2012, 2016, 2019, and 2021 versions) for their valuable reviews: Prof. Mark FitzGerald and Prof. Sheldon Spier from the University of British Columbia, Vancouver, British Columbia, Canada; Prof. Qutayba Hamid and Prof. Ronald Olivenstein from the Meakins-Christie Laboratories, McGill University, Montreal, Quebec, Canada; Prof. Eric Bateman from the University of Cape Town Lung Institute, Cape Town, South Africa; and Prof. Andrew Bush, Imperial College, National Heart and Lung Institute, London, United Kingdom.
References
- 1.Levy ML, Bacharier LB, Bateman E, Boulet LP, Brightling C, Buhl R, et al. Key recommendations for primary care from the 2022 Global Initiative for Asthma (GINA) update. NPJ Prim Care Respir Med. 2023;33:7. doi: 10.1038/s41533-023-00330-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Venkatesan P. 2023 GINA report for asthma. Lancet Respir Med. 2023;11:589. doi: 10.1016/S2213-2600(23)00230-8. [DOI] [PubMed] [Google Scholar]
- 3.Al Ghobain MO, Algazlan SS, Oreibi TM. Asthma prevalence among adults in Saudi Arabia. Saudi Med J. 2018;39:179–84. doi: 10.15537/smj.2018.2.20974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mohamed Hussain S, Ayesha Farhana S, Mohammed Alnasser S. Time trends and regional variation in prevalence of asthma and associated factors in Saudi Arabia: A systematic review and meta-analysis. Biomed Res Int. 2018;2018:1–9. doi: 10.1155/2018/8102527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alatawi A, Alanazi M. Barriers of asthma care among asthmatic children in Saudi Arabia: Maternal perspectives. Open J Pediatr. 2020;10:302. [Google Scholar]
- 6.Taminskiene V, Alasevicius T, Valiulis A, Vaitkaitiene E, Stukas R, Hadjipanayis A, et al. Quality of life of the family of children with asthma is not related to asthma severity. Eur J Pediatr. 2019;178:369–76. doi: 10.1007/s00431-018-3306-8. [DOI] [PubMed] [Google Scholar]
- 7.Al-Harbi S, Al-Harbi AS, Al-Khorayyef A, Al-Qwaiee M, Al-Shamarani A, Al-Aslani W, et al. Awareness regarding childhood asthma in Saudi Arabia. Ann Thorac Med. 2016;11:60–5. doi: 10.4103/1817-1737.173194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hammad MA, Alakhali KM, Manal Hattan M, Noor DA, Sulaiman SS, Kharshid AM, et al. Asthma in Saudi Arabia: Risk factors and pharmacotherapy. Indo Am J Pharm Res. 2016;6:6814–21. [Google Scholar]
- 9.BinSaeed AA. Caregiver knowledge and its relationship to asthma control among children in Saudi Arabia. J Asthma. 2014;51:870–5. doi: 10.3109/02770903.2014.906608. [DOI] [PubMed] [Google Scholar]
- 10.BinSaeed AA, Torchyan AA, Alsadhan AA, Almidani GM, Alsubaie AA, Aldakhail AA, et al. Determinants of asthma control among children in Saudi Arabia. J Asthma. 2014;51:435–9. doi: 10.3109/02770903.2013.876649. [DOI] [PubMed] [Google Scholar]
- 11.Ahmed AE, Al-Jahdali H, Al-Harbi A, Khan M, Ali Y, Al Shimemeri A, et al. Factors associated with poor asthma control among asthmatic patient visiting emergency department. Clin Respir J. 2014;8:431–6. doi: 10.1111/crj.12090. [DOI] [PubMed] [Google Scholar]
- 12.Al-Jahdali H, Anwar A, Al-Harbi A, Baharoon S, Halwani R, Al Shimemeri A, et al. Factors associated with patient visits to the emergency department for asthma therapy. BMC Pulm Med. 2012;12:80. doi: 10.1186/1471-2466-12-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al-Jahdali H, Ahmed A, Al-Harbi A, Khan A, ALGamedi M, Alyami S, et al. The most common pulmonary diseases length of stay, and characteristics of patients admitted to pulmonary service. Ann Thorac Med. 2023;18:124–31. doi: 10.4103/atm.atm_348_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al-Zahrani JM, Ahmad A, Al-Harbi A, Khan AM, Al-Bader B, Baharoon S, et al. Factors associated with poor asthma control in the outpatient clinic setting. Ann Thorac Med. 2015;10:100–4. doi: 10.4103/1817-1737.152450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al Ghobain MO, AlNemer M, Khan M. Assessment of knowledge and education relating to asthma during pregnancy among women of childbearing age. Asthma Res Pract. 2018;4:2. doi: 10.1186/s40733-017-0038-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al-Moamary MS, Al-Hajjaj MS, Idrees MM, Zeitouni MO, Alanezi MO, Al-Jahdali HH, et al. The Saudi initiative for asthma. Ann Thorac Med. 2009;4:216–33. doi: 10.4103/1817-1737.56001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Al-Moamary MS, Alhaider SA, Al-Hajjaj MS, Al-Ghobain MO, Idrees MM, Zeitouni MO, et al. The Saudi initiative for asthma –2012 update: Guidelines for the diagnosis and management of asthma in adults and children. Ann Thorac Med. 2012;7:175–204. doi: 10.4103/1817-1737.102166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al-Moamary MS, Alhaider SA, Idrees MM, Al Ghobain MO, Zeitouni MO, Al-Harbi AS, et al. The Saudi initiative for asthma –2016 update: Guidelines for the diagnosis and management of asthma in adults and children. Ann Thorac Med. 2016;11:3–42. doi: 10.4103/1817-1737.173196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Al-Moamary MS, Alhaider SA, Alangari AA, Al Ghobain MO, Zeitouni MO, Idrees MM, et al. The Saudi initiative for asthma –2019 update: Guidelines for the diagnosis and management of asthma in adults and children. Ann Thorac Med. 2019;14:3–48. doi: 10.4103/atm.ATM_327_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Al-Moamary MS, Alhaider SA, Alangari AA, Idrees MM, Zeitouni MO, Al Ghobain MO, et al. The Saudi initiative for asthma –2021 update: Guidelines for the diagnosis and management of asthma in adults and children. Ann Thorac Med. 2021;16:4–56. doi: 10.4103/atm.ATM_697_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jadad AR, Moher M, Browman GP, Booker L, Sigouin C, Fuentes M, et al. Systematic reviews and meta-analyses on treatment of asthma: Critical evaluation. BMJ. 2000;320:537–40. doi: 10.1136/bmj.320.7234.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pavord ID, Beasley R, Agusti A, Anderson GP, Bel E, Brusselle G, et al. After asthma: Redefining airways diseases. Lancet. 2018;391:350–400. doi: 10.1016/S0140-6736(17)30879-6. [DOI] [PubMed] [Google Scholar]
- 23.Del Giacco SR, Bakirtas A, Bel E, Custovic A, Diamant Z, Hamelmann E, et al. Allergy in severe asthma. Allergy. 2017;72:207–20. doi: 10.1111/all.13072. [DOI] [PubMed] [Google Scholar]
- 24.Lambrecht BN, Hammad H. The immunology of asthma. Nat Immunol. 2015;16:45–56. doi: 10.1038/ni.3049. [DOI] [PubMed] [Google Scholar]
- 25.Brusselle GG, Maes T, Bracke KR. Eosinophils in the spotlight: Eosinophilic airway inflammation in nonallergic asthma. Nat Med. 2013;19:977–9. doi: 10.1038/nm.3300. [DOI] [PubMed] [Google Scholar]
- 26.Amelink M, de Nijs SB, de Groot JC, van Tilburg PM, van Spiegel PI, Krouwels FH, et al. Three phenotypes of adult-onset asthma. Allergy. 2013;68:674–80. doi: 10.1111/all.12136. [DOI] [PubMed] [Google Scholar]
- 27.Israel E, Reddel HK. Severe and difficult-to-treat asthma in adults. N Engl J Med. 2017;377:965–76. doi: 10.1056/NEJMra1608969. [DOI] [PubMed] [Google Scholar]
- 28.Papi A, Brightling C, Pedersen SE, Reddel HK. Asthma. Lancet. 2018;391:783–800. doi: 10.1016/S0140-6736(17)33311-1. [DOI] [PubMed] [Google Scholar]
- 29.Park JA, Sharif AS, Tschumperlin DJ, Lau L, Limbrey R, Howarth P, et al. Tissue factor-bearing exosome secretion from human mechanically stimulated bronchial epithelial cells in vitro and in vivo. J Allergy Clin Immunol. 2012;130:1375–83. doi: 10.1016/j.jaci.2012.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brightling CE, Gupta S, Gonem S, Siddiqui S. Lung damage and airway remodelling in severe asthma. Clin Exp Allergy. 2012;42:638–49. doi: 10.1111/j.1365-2222.2011.03917.x. [DOI] [PubMed] [Google Scholar]
- 31.Bergeron C, Al-Ramli W, Hamid Q. Remodeling in asthma. Proc Am Thorac Soc. 2009;6:301–5. doi: 10.1513/pats.200808-089RM. [DOI] [PubMed] [Google Scholar]
- 32.Grainge CL, Lau LC, Ward JA, Dulay V, Lahiff G, Wilson S, et al. Effect of bronchoconstriction on airway remodeling in asthma. N Engl J Med. 2011;364:2006–15. doi: 10.1056/NEJMoa1014350. [DOI] [PubMed] [Google Scholar]
- 33.Alangari AA. Corticosteroids in the treatment of acute asthma. Ann Thorac Med. 2014;9:187–92. doi: 10.4103/1817-1737.140120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O’Byrne PM, Pedersen S, Lamm CJ, Tan WC, Busse WW. START Investigators Group. Severe exacerbations and decline in lung function in asthma. Am J Respir Crit Care Med. 2009;179:19–24. doi: 10.1164/rccm.200807-1126OC. [DOI] [PubMed] [Google Scholar]
- 35.O’Brian AL, Lemanske RF, Jr, Evans MD, Gangnon RE, Gern JE, Jackson DJ. Recurrent severe exacerbations in early life and reduced lung function at school age. J Allergy Clin Immunol. 2012;129:1162–4. doi: 10.1016/j.jaci.2011.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Russell RJ, Brightling C. Pathogenesis of asthma: Implications for precision medicine. Clin Sci (Lond) 2017;131:1723–35. doi: 10.1042/CS20160253. [DOI] [PubMed] [Google Scholar]
- 37.Bloom CI, Palmer T, Feary J, Quint JK, Cullinan P. Exacerbation patterns in adults with asthma in England. A population-based study. Am J Respir Crit Care Med. 2019;199:446–53. doi: 10.1164/rccm.201808-1516OC. [DOI] [PubMed] [Google Scholar]
- 38.Bonsignore MR, Baiamonte P, Mazzuca E, Castrogiovanni A, Marrone O. Obstructive sleep apnea and comorbidities: A dangerous liaison. Multidiscip Respir Med. 2019;14:8. doi: 10.1186/s40248-019-0172-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Price D. Asthma and allergic rhinitis: Linked in treatment and outcomes. Ann Thorac Med. 2010;5:63–4. doi: 10.4103/1817-1737.62467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rogliani P, Sforza M, Calzetta L. The impact of comorbidities on severe asthma. Curr Opin Pulm Med. 2020;26:47–55. doi: 10.1097/MCP.0000000000000640. [DOI] [PubMed] [Google Scholar]
- 41.Kuruvilla ME, Lee FE, Lee GB. Understanding asthma phenotypes, endotypes, and mechanisms of disease. Clin Rev Allergy Immunol. 2019;56:219–33. doi: 10.1007/s12016-018-8712-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Naclerio R, Ansotegui IJ, Bousquet J, Canonica GW, D’Amato G, Rosario N, et al. International expert consensus on the management of allergic rhinitis (AR) aggravated by air pollutants: Impact of air pollution on patients with AR: Current knowledge and future strategies. World Allergy Organ J. 2020;13:100106. doi: 10.1016/j.waojou.2020.100106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Al-Mobeireek AF, Al-Sarhani A, Al-Amri S, Bamgboye E, Ahmed SS. Chronic cough at a non-teaching hospital: Are extrapulmonary causes overlooked? Respirology. 2002;7:141–6. doi: 10.1046/j.1440-1843.2002.00378.x. [DOI] [PubMed] [Google Scholar]
- 44.Graham BL, Steenbruggen I, Miller MR, Barjaktarevic IZ, Cooper BG, Hall GL, et al. Standardization of spirometry 2019 update. An official American thoracic society and European respiratory society technical statement. American journal of respiratory and critical care medicine. 2019;200:e70–e88. doi: 10.1164/rccm.201908-1590ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yi F, Han L, Liu B, Zhang X, Xue Y, Luo W, et al. Determinants of response to bronchodilator in patients with cough variant asthma- a randomized, single-blinded, placebo-controlled study. Pulm Pharmacol Ther. 2020;61:101903. doi: 10.1016/j.pupt.2020.101903. [DOI] [PubMed] [Google Scholar]
- 46.Song WJ, Kim HJ, Shim JS, Won HK, Kang SY, Sohn KH, et al. Diagnostic accuracy of fractional exhaled nitric oxide measurement in predicting cough-variant asthma and eosinophilic bronchitis in adults with chronic cough: A systematic review and meta-analysis. J Allergy Clin Immunol. 2017;140:701–9. doi: 10.1016/j.jaci.2016.11.037. [DOI] [PubMed] [Google Scholar]
- 47.Lux H, Lenz K, Budnik LT, Baur X. Performance of specific immunoglobulin E tests for diagnosing occupational asthma: A systematic review and meta-analysis. Occup Environ Med. 2019;76:269–78. doi: 10.1136/oemed-2018-105434. [DOI] [PubMed] [Google Scholar]
- 48.Humbert M, Holgate S, Boulet LP, Bousquet J. Asthma control or severity: That is the question. Allergy. 2007;62:95–101. doi: 10.1111/j.1398-9995.2006.01308.x. [DOI] [PubMed] [Google Scholar]
- 49.Halvorsen T, Walsted ES, Bucca C, Bush A, Cantarella G, Friedrich G, et al. Inducible laryngeal obstruction: An official joint European Respiratory Society and European Laryngological Society statement. Eur Respir J. 2017;50:1–15. doi: 10.1183/13993003.02221-2016. [DOI] [PubMed] [Google Scholar]
- 50.Cope SF, Ungar WJ, Glazier RH. International differences in asthma guidelines for children. Int Arch Allergy Immunol. 2009;148:265–78. doi: 10.1159/000170380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Apps LD, Chantrell S, Majd S, Eglinton E, Singh SJ, Murphy AC, et al. Patient perceptions of living with severe asthma: Challenges to effective management. J Allergy Clin Immunol Pract. 2019;7:2613–21.e1. doi: 10.1016/j.jaip.2019.04.026. [DOI] [PubMed] [Google Scholar]
- 52.Neffen H, Chahuàn M, Hernández DD, Vallejo-Perez E, Bolivar F, Sánchez MH, et al. Key factors associated with uncontrolled asthma –The asthma control in Latin America study. J Asthma. 2020;57:113–22. doi: 10.1080/02770903.2018.1553050. [DOI] [PubMed] [Google Scholar]
- 53.Vermeulen F, de Meulder I, Paesmans M, Muylle I, Bruyneel M, Ninane V. Asthma control measurement using five different questionnaires: A prospective study. Respir Med. 2013;107:1314–21. doi: 10.1016/j.rmed.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 54.Schatz M, Sorkness CA, Li JT, Marcus P, Murray JJ, Nathan RA, et al. Asthma control test: Reliability, validity, and responsiveness in patients not previously followed by asthma specialists. J Allergy Clin Immunol. 2006;117:549–56. doi: 10.1016/j.jaci.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 55.Nathan RA, Sorkness CA, Kosinski M, Schatz M, Li JT, Marcus P, et al. Development of the asthma control test: A survey for assessing asthma control. J Allergy Clin Immunol. 2004;113:59–65. doi: 10.1016/j.jaci.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 56.Lababidi H, Hijaoui A, Zarzour M. Validation of the Arabic version of the asthma control test. Ann Thorac Med. 2008;3:44–7. doi: 10.4103/1817-1737.39635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alanezi M, Al-Jahdali HH, Al-Hajjaj MS, Zeitoni MO, Al-Tasan TH. Levels of acceptance of asthma control test questionnaire among Saudi patients attending 5 tertiary care hospitals in Saudi Arabia. Saudi Med J. 2009;30:546–9. [PubMed] [Google Scholar]
- 58.Al Moamary MS, Al-Kordi AG, Al Ghobain MO, Tamim HM. Utilization and responsiveness of the asthma control test (ACT) at the initiation of therapy for patients with asthma: A randomized controlled trial. BMC Pulm Med. 2012;12:14. doi: 10.1186/1471-2466-12-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schatz M, Kosinski M, Yarlas AS, Hanlon J, Watson ME, Jhingran P. The minimally important difference of the asthma control test. J Allergy Clin Immunol. 2009;124:719–23.e1. doi: 10.1016/j.jaci.2009.06.053. [DOI] [PubMed] [Google Scholar]
- 60.Schneider A, Brunn B, Hapfelmeier A, Schultz K, Kellerer C, Jörres RA. Diagnostic accuracy of FeNO in asthma and predictive value for inhaled corticosteroid responsiveness: A prospective, multicentre study. EClinicalMedicine. 2022;50:101533. doi: 10.1016/j.eclinm.2022.101533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Miller MK, Lee JH, Miller DP, Wenzel SE. TENOR Study Group. Recent asthma exacerbations: A key predictor of future exacerbations. Respir Med. 2007;101:481–9. doi: 10.1016/j.rmed.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 62.Amnuaypattanapon K, Limjindaporn C, Srivilaithon W, Dasanadeba I. Characteristics and outcomes of treatment in status asthmaticus patients at emergency department. Asian Pac J Allergy Immunol. 2019;37:87–93. doi: 10.12932/AP-261217-0224. [DOI] [PubMed] [Google Scholar]
- 63.Reddel HK, Taylor DR, Bateman ED, Boulet LP, Boushey HA, Busse WW, et al. An official American Thoracic Society/European Respiratory Society statement: Asthma control and exacerbations: Standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med. 2009;180:59–99. doi: 10.1164/rccm.200801-060ST. [DOI] [PubMed] [Google Scholar]
- 64.Taylor DR, Bateman ED, Boulet LP, Boushey HA, Busse WW, Casale TB, et al. A new perspective on concepts of asthma severity and control. Eur Respir J. 2008;32:545–54. doi: 10.1183/09031936.00155307. [DOI] [PubMed] [Google Scholar]
- 65.Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43:343–73. doi: 10.1183/09031936.00202013. [DOI] [PubMed] [Google Scholar]
- 66.Lommatzsch M, Brusselle GG, Canonica GW, Jackson DJ, Nair P, Buhl R, et al. Disease-modifying anti-asthmatic drugs. Lancet. 2022;399:1664–8. doi: 10.1016/S0140-6736(22)00331-2. [DOI] [PubMed] [Google Scholar]
- 67.Gibson PG, Powell H. Written action plans for asthma: An evidence-based review of the key components. Thorax. 2004;59:94–9. doi: 10.1136/thorax.2003.011858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dailah H. UK: University of Salford; 2020. A Self-Management Program for Adults with Asthma in Saudi Arabia. [Google Scholar]
- 69.Richmond RS, Connolly M. A delineation of self-management and associated concepts. International Journal of Healthcare Management. 2021;14:1576–88. [Google Scholar]
- 70.Elsherif OE, Al-Abdullaah AA, Aljahdali HH, Jokhdar HA, Alqahtani SH, Nahhas MA, et al. The burden of asthma among children and adolescents in Saudi Arabia: A national cross-sectional survey. Allergy. 2022;2772:8293. doi: 10.1016/j.jacig.2022.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Al-Yami SM, Mohajer KA, Al-Jeraisy MI, Batarfi AM, Abolfotouh MA. Recurrent visits and admissions of children with asthma in central Saudi Arabia. Saudi Med J. 2010;31:921–4. [PubMed] [Google Scholar]
- 72.Basyouni MH, BinDhim NF, Saini B, Williams KA. Online health information needs for patients with asthma in Saudi Arabia. J Consum Health Internet. 2015;19:13–24. [Google Scholar]
- 73.Beydon N, Taillé C, Corvol H, Valcke J, Portal JJ, Plantier L, et al. Digital action plan (web app) for managing asthma exacerbations: Randomized controlled trial. J Med Internet Res. 2023;25:e41490. doi: 10.2196/41490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Khdour MR, Elyan SO, Hallak HO, Jarab AS, Mukattash TL, Astal A. Pharmaceutical care for adult asthma patients: A controlled intervention one-year follow-up study. Basic Clin Pharmacol Toxicol. 2020;126:332–40. doi: 10.1111/bcpt.13344. [DOI] [PubMed] [Google Scholar]
- 75.Dardouri M, Sahli J, Ajmi T, Mtiraoui A, Bouguila J, Mallouli M. Factors associated with acute health care use in children and adolescents with asthma. Compr Child Adolesc Nurs. 2021;44:122–33. doi: 10.1080/24694193.2020.1742249. [DOI] [PubMed] [Google Scholar]
- 76.Gold DR, Adamkiewicz G, Arshad SH, Celedón JC, Chapman MD, Chew GL, et al. NIAID, NIEHS, NHLBI, and MCAN workshop report: The indoor environment and childhood asthma-implications for home environmental intervention in asthma prevention and management. J Allergy Clin Immunol. 2017;140:933–49. doi: 10.1016/j.jaci.2017.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Alangari AA, Riaz M, Mahjoub MO, Malhis N, Al-Tamimi S, Al-Modaihsh A. The effect of sand storms on acute asthma in Riyadh, Saudi Arabia. Ann Thorac Med. 2015;10:29–33. doi: 10.4103/1817-1737.146857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ronsmans S, Le Moual N, Dumas O. Update on irritant-induced occupational asthma. Curr Opin Allergy Clin Immunol. 2023;23:63–9. doi: 10.1097/ACI.0000000000000884. [DOI] [PubMed] [Google Scholar]
- 79.Goyal S, Gupta M, Sharma P, Beniwal V. Microbes for Natural Food Additives. USA: Springer; 2023. Hypersensitivity associated with food additives; pp. 205–27. [Google Scholar]
- 80.Zeitouni MO, Al Barrak AM, Al-Moamary MS, Alharbi NS, Idrees MM, Al Shimemeri AA, et al. The Saudi Thoracic Society guidelines for influenza vaccinations. Ann Thorac Med. 2015;10:223–30. doi: 10.4103/1817-1737.167065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kyung Y, Choi MH, Lee JS, Lee JH, Jo SH, Kim SH. Influencing factors for influenza vaccination among South Korean adolescents with asthma based on a nationwide cross-sectional study. Int Arch Allergy Immunol. 2020;181:434–45. doi: 10.1159/000506336. [DOI] [PubMed] [Google Scholar]
- 82.Grohskopf LA, Alyanak E, Broder KR, Walter EB, Fry AM, Jernigan DB. Prevention and control of seasonal influenza with vaccines: Recommendations of the advisory committee on immunization practices –United States, 2019-20 influenza season. MMWR Recomm Rep. 2019;68:1–21. doi: 10.15585/mmwr.rr6803a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Alharbi NS, Al-Barrak AM, Al-Moamary MS, Zeitouni MO, Idrees MM, Al-Ghobain MO, et al. The Saudi Thoracic Society pneumococcal vaccination guidelines-2016. Ann Thorac Med. 2016;11:93–102. doi: 10.4103/1817-1737.177470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Penkalski MR, Reynaldo RF, Patterson K. A paradigm shift in pharmacotherapeutic management of asthma. Medsurg Nurs. 2023;32:145–54. [Google Scholar]
- 85.Domingo C, Garcia G, Gemicioglu B, Van GV, Larenas-Linnemann D, Neffen H, et al. Consensus on mild asthma management: Results of a modified Delphi study. J Asthma. 2023;60:145–57. doi: 10.1080/02770903.2022.2034850. [DOI] [PubMed] [Google Scholar]
- 86.O’Byrne PM, FitzGerald JM, Bateman ED, Barnes PJ, Zheng J, Gustafson P, et al. Effect of a single day of increased as-needed budesonide-formoterol use on short-term risk of severe exacerbations in patients with mild asthma: A post-hoc analysis of the SYGMA 1 study. Lancet Respir Med. 2021;9:149–58. doi: 10.1016/S2213-2600(20)30416-1. [DOI] [PubMed] [Google Scholar]
- 87.Reddel HK, Yan KY. Single maintenance and reliever therapy (SMART) of asthma. Thorax. 2011;66:86–7. doi: 10.1136/thx.2010.149773. [DOI] [PubMed] [Google Scholar]
- 88.Chapman KR, Barnes NC, Greening AP, Jones PW, Pedersen S. Single maintenance and reliever therapy (SMART) of asthma: A critical appraisal. Thorax. 2010;65:747–52. doi: 10.1136/thx.2009.128504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Scullion J. A proactive approach to asthma. Nurs Stand. 2005;20:57–65. doi: 10.7748/ns2005.11.20.9.57.c4001. [DOI] [PubMed] [Google Scholar]
- 90.Averell CM, Stanford RH, Laliberté F, Wu JW, Germain G, Duh MS. Medication adherence in patients with asthma using once-daily versus twice-daily ICS/LABAs. J Asthma. 2021;58:102–11. doi: 10.1080/02770903.2019.1663429. [DOI] [PubMed] [Google Scholar]
- 91.Berger WE. Levalbuterol: Pharmacologic properties and use in the treatment of pediatric and adult asthma. Ann Allergy Asthma Immunol. 2003;90:583–91. doi: 10.1016/S1081-1206(10)61859-5. [DOI] [PubMed] [Google Scholar]
- 92.Nwaru BI, Ekström M, Hasvold P, Wiklund F, Telg G, Janson C. Overuse of short-acting β(2)-agonists in asthma is associated with increased risk of exacerbation and mortality: A nationwide cohort study of the global SABINA programme. Eur Respir J. 2020;55:1–11. doi: 10.1183/13993003.01872-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Crossingham I, Turner S, Ramakrishnan S, Fries A, Gowell M, Yasmin F, et al. Combination fixed-dose beta agonist and steroid inhaler as required for adults or children with mild asthma. Cochrane Database Syst Rev. 2021;5:CD013518. doi: 10.1002/14651858.CD013518.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Papi A, Chipps BE, Beasley R, Panettieri RA, Jr, Israel E, Cooper M, et al. Albuterol-budesonide fixed-dose combination rescue inhaler for asthma. N Engl J Med. 2022;386:2071–83. doi: 10.1056/NEJMoa2203163. [DOI] [PubMed] [Google Scholar]
- 95.Papi A, Canonica GW, Maestrelli P, Paggiaro P, Olivieri D, Pozzi E, et al. Rescue use of beclomethasone and albuterol in a single inhaler for mild asthma. N Engl J Med. 2007;356:2040–52. doi: 10.1056/NEJMoa063861. [DOI] [PubMed] [Google Scholar]
- 96.Israel E, Cardet JC, Carroll JK, Fuhlbrigge AL, She L, Rockhold FW, et al. Reliever-triggered inhaled glucocorticoid in black and Latinx adults with asthma. N Engl J Med. 2022;386:1505–18. doi: 10.1056/NEJMoa2118813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.O'Byrne PM, Bisgaard H, Godard PP, Pistolesi M, Palmqvist M, Zhu Y, et al. Budesonide/formoterol combination therapy as both maintenance and reliever medication in asthma. Am J Respir Crit Care Med. 2005;171:129–36. doi: 10.1164/rccm.200407-884OC. [DOI] [PubMed] [Google Scholar]
- 98.Bateman ED, Reddel HK, O'Byrne PM, Barnes PJ, Zhong N, Keen C, et al. As-needed budesonide-formoterol versus maintenance budesonide in mild asthma. N Engl J Med. 2018;378:1877–87. doi: 10.1056/NEJMoa1715275. [DOI] [PubMed] [Google Scholar]
- 99.O’Byrne PM, FitzGerald JM, Bateman ED, Barnes PJ, Zhong N, Keen C, et al. Inhaled combined budesonide-formoterol as needed in mild asthma. N Engl J Med. 2018;378:1865–76. doi: 10.1056/NEJMoa1715274. [DOI] [PubMed] [Google Scholar]
- 100.Beasley R, Holliday M, Reddel HK, Braithwaite I, Ebmeier S, Hancox RJ, et al. Controlled trial of budesonide-formoterol as needed for mild asthma. N Engl J Med. 2019;380:2020–30. doi: 10.1056/NEJMoa1901963. [DOI] [PubMed] [Google Scholar]
- 101.Turner MO, Noertjojo K, Vedal S, Bai T, Crump S, Fitzgerald JM. Risk factors for near-fatal asthma. A case-control study in hospitalized patients with asthma. Am J Respir Crit Care Med. 1998;157:1804–9. doi: 10.1164/ajrccm.157.6.9708092. [DOI] [PubMed] [Google Scholar]
- 102.Graff S, Demarche S, Henket M, Paulus V, Louis R, Schleich F. Increase in blood eosinophils during follow-up is associated with lung function decline in adult asthma. Respir Med. 2019;152:60–6. doi: 10.1016/j.rmed.2019.04.020. [DOI] [PubMed] [Google Scholar]
- 103.Pols DH, Wartna JB, Moed H, van Alphen EI, Bohnen AM, Bindels PJ. Atopic dermatitis, asthma and allergic rhinitis in general practice and the open population: A systematic review. Scand J Prim Health Care. 2016;34:143–50. doi: 10.3109/02813432.2016.1160629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lee J, Tay TR, Radhakrishna N, Hore-Lacy F, Mackay A, Hoy R, et al. Nonadherence in the era of severe asthma biologics and thermoplasty. Eur Respir J. 2018;51:1–10. doi: 10.1183/13993003.01836-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.van Boven JF, Koponen M, Lalic S, George J, Bell JS, Hew M, et al. Trajectory analyses of adherence patterns in a real-life moderate to severe asthma population. J Allergy Clin Immunol Pract. 2020;8:1961–9.e6. doi: 10.1016/j.jaip.2019.12.002. [DOI] [PubMed] [Google Scholar]
- 106.Singh D, Garcia G, Maneechotesuwan K, Daley-Yates P, Irusen E, Aggarwal B, et al. New versus old: The impact of changing patterns of inhaled corticosteroid prescribing and dosing regimens in asthma management. Adv Ther. 2022;39:1895–914. doi: 10.1007/s12325-022-02092-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cloutier MM, Salo PM, Akinbami LJ, Cohn RD, Wilkerson JC, Diette GB, et al. Clinician agreement, self-efficacy, and adherence with the guidelines for the diagnosis and management of asthma. J Allergy Clin Immunol Pract. 2018;6:886–94.e4. doi: 10.1016/j.jaip.2018.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Stanford RH, Averell C, Parker ED, Blauer-Peterson C, Reinsch TK, Buikema AR. Assessment of adherence and asthma medication ratio for a once-daily and twice-daily inhaled corticosteroid/long-acting β-agonist for asthma. J Allergy Clin Immunol Pract. 2019;7:1488–96.e7. doi: 10.1016/j.jaip.2018.12.021. [DOI] [PubMed] [Google Scholar]
- 109.Noibi S, Mohy A, Gouhar R, Shaker F, Lukic T, Al-Jahdali H. Asthma control factors in the Gulf Cooperation Council (GCC) countries and the effectiveness of ICS/LABA fixed dose combinations: A dual rapid literature review. BMC Public Health. 2020;20:1211. doi: 10.1186/s12889-020-09259-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Janežič A, Locatelli I, Kos M. Inhalation technique and asthma outcomes with different corticosteroid-containing inhaler devices. J Asthma. 2020;57:654–62. doi: 10.1080/02770903.2019.1591442. [DOI] [PubMed] [Google Scholar]
- 111.Hussain FN, Paravattil B. Assessment of educational inhaler technique interventions among community pharmacists: A systematic review. Integr Pharm Res Pract. 2020;9:23–31. doi: 10.2147/IPRP.S239215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Al-Jahdali H, Wali S, Salem G, Al-Hameed F, Almotair A, Zeitouni M, et al. Asthma control and predictive factors among adults in Saudi Arabia: Results from the epidemiological study on the management of asthma in asthmatic Middle East adult population study. Ann Thorac Med. 2019;14:148–54. doi: 10.4103/atm.ATM_348_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Musharrafieh U, Tamim H, Houry R, AlBuhairan F. A nationwide study of asthma correlates among adolescents in Saudi Arabia. Asthma Res Pract. 2020;6:3. doi: 10.1186/s40733-020-00056-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hardy J, Baggott C, Fingleton J, Reddel HK, Hancox RJ, Harwood M, et al. Budesonide-formoterol reliever therapy versus maintenance budesonide plus terbutaline reliever therapy in adults with mild to moderate asthma (PRACTICAL): A 52-week, open-label, multicentre, superiority, randomised controlled trial. Lancet. 2019;394:919–28. doi: 10.1016/S0140-6736(19)31948-8. [DOI] [PubMed] [Google Scholar]
- 115.Martinez FD, Chinchilli VM, Morgan WJ, Boehmer SJ, Lemanske RF, Jr, Mauger DT, et al. Use of beclomethasone dipropionate as rescue treatment for children with mild persistent asthma (TREXA): A randomised, double-blind, placebo-controlled trial. Lancet. 2011;377:650–7. doi: 10.1016/S0140-6736(10)62145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sumino K, Bacharier LB, Taylor J, Chadwick-Mansker K, Curtis V, Nash A, et al. A pragmatic trial of symptom-based inhaled corticosteroid use in African-American children with mild asthma. J Allergy Clin Immunol Pract. 2020;8:176–85.e2. doi: 10.1016/j.jaip.2019.06.030. [DOI] [PubMed] [Google Scholar]
- 117.Selroos O. Effect of disease duration on dose-response of inhaled budesonide in asthma. Respir Med. 2008;102:1065–72. doi: 10.1016/j.rmed.2007.12.029. [DOI] [PubMed] [Google Scholar]
- 118.Qazi A, Armour C, Saini B. Perspectives of general practitioners about a collaborative asthma care model in primary care. J Asthma. 2021;58:1648–60. doi: 10.1080/02770903.2020.1823408. [DOI] [PubMed] [Google Scholar]
- 119.Pauwels RA, Pedersen S, Busse WW, Tan WC, Chen YZ, Ohlsson SV, et al. Early intervention with budesonide in mild persistent asthma: A randomised, double-blind trial. Lancet. 2003;361:1071–6. doi: 10.1016/S0140-6736(03)12891-7. [DOI] [PubMed] [Google Scholar]
- 120.Powell H, Gibson PG. Initial starting dose of inhaled corticosteroids in adults with asthma: A systematic review. Thorax. 2004;59:1041–5. doi: 10.1136/thx.2004.023754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sharma S, Harish R, Dutt N, Digra KK. To evaluate the efficacy of nebulized budesonide compared to oral prednisolone in the management of moderate exacerbation of acute asthma. Int J Contemp Pediatr. 2017;4:1278–83. [Google Scholar]
- 122.Sont JK. How do we monitor asthma control? Allergy. 1999;54(Suppl 49):68–73. doi: 10.1111/j.1398-9995.1999.tb04391.x. [DOI] [PubMed] [Google Scholar]
- 123.O'Byrne PM, Jenkins C, Bateman ED. The paradoxes of asthma management: Time for a new approach? Eur Respir J. 2017;50:1–12. doi: 10.1183/13993003.01103-2017. [DOI] [PubMed] [Google Scholar]
- 124.Asthma GIF. The Global Strategy for Asthma Management and Prevention. 2020. [[Last accessed on 2023 16 Sep]]. Available from: www.ginasthma.org .
- 125.Fingleton J, Hardy J, Baggott C, Pilcher J, Corin A, Hancox RJ, et al. Description of the protocol for the PRACTICAL study: A randomised controlled trial of the efficacy and safety of ICS/LABA reliever therapy in asthma. BMJ Open Respir Res. 2017;4:e000217. doi: 10.1136/bmjresp-2017-000217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Reddel HK, Busse WW, Pedersen S, Tan WC, Chen YZ, Jorup C, et al. Should recommendations about starting inhaled corticosteroid treatment for mild asthma be based on symptom frequency: A post-hoc efficacy analysis of the START study. Lancet. 2017;389:157–66. doi: 10.1016/S0140-6736(16)31399-X. [DOI] [PubMed] [Google Scholar]
- 127.Dusser D, Montani D, Chanez P, de Blic J, Delacourt C, Deschildre A, et al. Mild asthma: An expert review on epidemiology, clinical characteristics and treatment recommendations. Allergy. 2007;62:591–604. doi: 10.1111/j.1398-9995.2007.01394.x. [DOI] [PubMed] [Google Scholar]
- 128.Pauwels RA, Löfdahl CG, Postma DS, Tattersfield AE, O’Byrne P, Barnes PJ, et al. Effect of inhaled formoterol and budesonide on exacerbations of asthma. Formoterol and Corticosteroids Establishing Therapy (FACET) International Study Group. N Engl J Med. 1997;337:1405–11. doi: 10.1056/NEJM199711133372001. [DOI] [PubMed] [Google Scholar]
- 129.Bateman ED, Bousquet J, Keech ML, Busse WW, Clark TJ, Pedersen SE. The correlation between asthma control and health status: The GOAL study. Eur Respir J. 2007;29:56–62. doi: 10.1183/09031936.00128505. [DOI] [PubMed] [Google Scholar]
- 130.Singh D, Oosterholt S, Pavord I, Garcia G, Abhijith Pg, Della Pasqua O. Understanding the clinical implications of individual patient characteristics and treatment choice on the risk of exacerbation in asthma patients with moderate-severe symptoms. Adv Ther. 2023;40:4606–25. doi: 10.1007/s12325-023-02590-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cates CJ, Schmidt S, Ferrer M, Sayer B, Waterson S. Inhaled steroids with and without regular salmeterol for asthma: Serious adverse events. Cochrane Database Syst Rev. 2018;12:CD006922. doi: 10.1002/14651858.CD006922.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Busse WW, Bateman ED, Caplan AL, Kelly HW, O’Byrne PM, Rabe KF, et al. Combined analysis of asthma safety trials of long-acting β(2)-agonists. N Engl J Med. 2018;378:2497–505. doi: 10.1056/NEJMoa1716868. [DOI] [PubMed] [Google Scholar]
- 133.Zeiger RS, Szefler SJ, Phillips BR, Schatz M, Martinez FD, Chinchilli VM, et al. Response profiles to fluticasone and montelukast in mild-to-moderate persistent childhood asthma. J Allergy Clin Immunol. 2006;117:45–52. doi: 10.1016/j.jaci.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 134.Vaquerizo MJ, Casan P, Castillo J, Perpiña M, Sanchis J, Sobradillo V, et al. Effect of montelukast added to inhaled budesonide on control of mild to moderate asthma. Thorax. 2003;58:204–10. doi: 10.1136/thorax.58.3.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Joos S, Miksch A, Szecsenyi J, Wieseler B, Grouven U, Kaiser T, et al. Montelukast as add-on therapy to inhaled corticosteroids in the treatment of mild to moderate asthma: A systematic review. Thorax. 2008;63:453–62. doi: 10.1136/thx.2007.081596. [DOI] [PubMed] [Google Scholar]
- 136.Szefler SJ, Martin RJ, King TS, Boushey HA, Cherniack RM, Chinchilli VM, et al. Significant variability in response to inhaled corticosteroids for persistent asthma. J Allergy Clin Immunol. 2002;109:410–8. doi: 10.1067/mai.2002.122635. [DOI] [PubMed] [Google Scholar]
- 137.Powell H, Gibson PG. Inhaled corticosteroid doses in asthma: An evidence-based approach. Med J Aust. 2003;178:223–5. doi: 10.5694/j.1326-5377.2003.tb05167.x. [DOI] [PubMed] [Google Scholar]
- 138.Kerstjens HA, van den Berge M. Regular treatment for moderate asthma: Guidelines hold true. Lancet Respir Med. 2015;3:88–9. doi: 10.1016/S2213-2600(14)70297-8. [DOI] [PubMed] [Google Scholar]
- 139.Beeh KM, Moroni-Zentgraf P, Ablinger O, Hollaenderova Z, Unseld A, Engel M, et al. Tiotropium Respimat®in asthma: A double-blind, randomised, dose-ranging study in adult patients with moderate asthma. Respir Res. 2014;15:61. doi: 10.1186/1465-9921-15-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tian JW, Chen JW, Chen R, Chen X. Tiotropium versus placebo for inadequately controlled asthma: A meta-analysis. Respir Care. 2014;59:654–66. doi: 10.4187/respcare.02703. [DOI] [PubMed] [Google Scholar]
- 141.Sobieraj DM, Baker WL, Nguyen E, Weeda ER, Coleman CI, White CM, et al. Association of inhaled corticosteroids and long-acting muscarinic antagonists with asthma control in patients with uncontrolled, persistent asthma: A systematic review and meta-analysis. JAMA. 2018;319:1473–84. doi: 10.1001/jama.2018.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Peters SP, Kunselman SJ, Icitovic N, Moore WC, Pascual R, Ameredes BT, et al. Tiotropium bromide step-up therapy for adults with uncontrolled asthma. N Engl J Med. 2010;363:1715–26. doi: 10.1056/NEJMoa1008770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Bateman ED, Kornmann O, Schmidt P, Pivovarova A, Engel M, Fabbri LM. Tiotropium is noninferior to salmeterol in maintaining improved lung function in B16-Arg/Arg patients with asthma. J Allergy Clin Immunol. 2011;128:315–22. doi: 10.1016/j.jaci.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 144.Smith LJ. Anticholinergics for patients with asthma? N Engl J Med. 2010;363:1764–5. doi: 10.1056/NEJMe1009429. [DOI] [PubMed] [Google Scholar]
- 145.Chowdhury BA, Dal Pan G. The FDA and safe use of long-acting beta-agonists in the treatment of asthma. N Engl J Med. 2010;362:1169–71. doi: 10.1056/NEJMp1002074. [DOI] [PubMed] [Google Scholar]
- 146.Koshak EA. New FDA safety warnings for LABAs: A call for asthma guidelines revisit for solo beta agonist. Ann Thorac Med. 2010;5:65–6. doi: 10.4103/1817-1737.62468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.O’Byrne PM, Bleecker ER, Bateman ED, Busse WW, Woodcock A, Forth R, et al. Once-daily fluticasone furoate alone or combined with vilanterol in persistent asthma. Eur Respir J. 2014;43:773–82. doi: 10.1183/09031936.00064513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Woodcock A, Bleecker ER, Lötvall J, O’Byrne PM, Bateman ED, Medley H, et al. Efficacy and safety of fluticasone furoate/vilanterol compared with fluticasone propionate/salmeterol combination in adult and adolescent patients with persistent asthma: A randomized trial. Chest. 2013;144:1222–9. doi: 10.1378/chest.13-0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gon Y, Ishii T, Lawrence D, Nikolaev I, Wang D, Sumi K, et al. Once-daily, single-inhaler indacaterol/mometasone versus twice-daily salmeterol/fluticasone in Asian patients with inadequately controlled asthma:Post hoc pooled analysis from PALLADIUM and IRIDIUM studies. J Asthma. 2022;59:1627–37. doi: 10.1080/02770903.2021.1962342. [DOI] [PubMed] [Google Scholar]
- 150.van Zyl-Smit RN, Krüll M, Gessner C, Gon Y, Noga O, Richard A, et al. Once-daily mometasone plus indacaterol versus mometasone or twice-daily fluticasone plus salmeterol in patients with inadequately controlled asthma (PALLADIUM): A randomised, double-blind, triple-dummy, controlled phase 3 study. Lancet Respir Med. 2020;8:987–99. doi: 10.1016/S2213-2600(20)30178-8. [DOI] [PubMed] [Google Scholar]
- 151.Kerstjens HAM, Maspero J, Chapman KR, van Zyl-Smit RN, Hosoe M, Tanase AM, et al. Once-daily, single-inhaler mometasone-indacaterol-glycopyrronium versus mometasone-indacaterol or twice-daily fluticasone-salmeterol in patients with inadequately controlled asthma (IRIDIUM): A randomised, double-blind, controlled phase 3 study. Lancet Respir Med. 2020;8:1000–12. doi: 10.1016/S2213-2600(20)30190-9. [DOI] [PubMed] [Google Scholar]
- 152.Al-Moamary MS, Al-Lehebi R, Idrees MM, Zeitouni MO. When single-inhaler triple therapy is a preferred option in asthma management? Ann Thorac Med. 2022;17:185–8. doi: 10.4103/atm.atm_341_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Agusti A, Fabbri L, Lahousse L, Singh D, Papi A. Single inhaler triple therapy (SITT) in asthma: Systematic review and practice implications. Allergy. 2022;77:1105–13. doi: 10.1111/all.15076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Rogliani P, Ritondo BL, Calzetta L. Triple therapy in uncontrolled asthma: A network meta-analysis of phase III studies. Eur Respir J. 2021;58:2004233. doi: 10.1183/13993003.04233-2020. [DOI] [PubMed] [Google Scholar]
- 155.Lee LA, Bailes Z, Barnes N, Boulet LP, Edwards D, Fowler A, et al. Efficacy and safety of once-daily single-inhaler triple therapy (FF/UMEC/VI) versus FF/VI in patients with inadequately controlled asthma (CAPTAIN): A double-blind, randomised, phase 3A trial. Lancet Respir Med. 2021;9:69–84. doi: 10.1016/S2213-2600(20)30389-1. [DOI] [PubMed] [Google Scholar]
- 156.Bogart M, Germain G, Laliberté F, Mahendran M, Duh MS. Real-world impact of triple therapy with fluticasone furoate, umeclidinium, and vilanterol on asthma control among US patients with asthma. Chest. 2022;162:A1930. [Google Scholar]
- 157.Sagara H, Barbier N, Ishii T, Yoshisue H, Nikolaev I, Hosoe M, et al. Efficacy of one time per day, single-inhaler indacaterol/glycopyrronium/mometasone in patients with inadequately controlled asthma:Post hoc analysis of IRIDIUM study in Asian population. BMJ Open Respir Res. 2021;8:e000856. doi: 10.1136/bmjresp-2020-000856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Virchow JC, Kuna P, Paggiaro P, Papi A, Singh D, Corre S, et al. Single inhaler extrafine triple therapy in uncontrolled asthma (TRIMARAN and TRIGGER): Two double-blind, parallel-group, randomised, controlled phase 3 trials. Lancet. 2019;394:1737–49. doi: 10.1016/S0140-6736(19)32215-9. [DOI] [PubMed] [Google Scholar]
- 159.Kerstjens HA, Disse B, Schröder-Babo W, Bantje TA, Gahlemann M, Sigmund R, et al. Tiotropium improves lung function in patients with severe uncontrolled asthma: A randomized controlled trial. J Allergy Clin Immunol. 2011;128:308–14. doi: 10.1016/j.jaci.2011.04.039. [DOI] [PubMed] [Google Scholar]
- 160.Kerstjens HA, Engel M, Dahl R, Paggiaro P, Beck E, Vandewalker M, et al. Tiotropium in asthma poorly controlled with standard combination therapy. N Engl J Med. 2012;367:1198–207. doi: 10.1056/NEJMoa1208606. [DOI] [PubMed] [Google Scholar]
- 161.Rodrigo GJ, Neffen H. Efficacy and safety of tiotropium in school-age children with moderate-to-severe symptomatic asthma: A systematic review. Pediatr Allergy Immunol. 2017;28:573–8. doi: 10.1111/pai.12759. [DOI] [PubMed] [Google Scholar]
- 162.American Lung Association Asthma Clinical Research Centers. Clinical trial of low-dose theophylline and montelukast in patients with poorly controlled asthma. Am J Respir Crit Care Med. 2007;175:235–42. doi: 10.1164/rccm.200603-416OC. [DOI] [PubMed] [Google Scholar]
- 163.Price DB, Hernandez D, Magyar P, Fiterman J, Beeh KM, James IG, et al. Randomised controlled trial of montelukast plus inhaled budesonide versus double dose inhaled budesonide in adult patients with asthma. Thorax. 2003;58:211–6. doi: 10.1136/thorax.58.3.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.FDA U. FDA Requires Boxed Warning about Serious Mental Health Side Effects for Asthma and Allergy Drug Montelukast (Singulair);Advises Restricting Use for Allergic Rhinitis. 2020. [[Last accessed on 2023 Aug 29]]. Available from: https://www.fda.gov/drugs/drug-safety-and-availability/fda-requires-boxed-warning-about-serious-mental-health-side-effects-asthma-and-allergy-drug#:~:text=We%20are%20requiring%20a%20Boxed,treated%20effectively%20with%20or%20cannot .
- 165.Kearns N, Maijers I, Harper J, Beasley R, Weatherall M. inhaled corticosteroids in acute asthma: A systemic review and meta-analysis. J Allergy Clin Immunol Pract. 2020;8:605–17.e6. doi: 10.1016/j.jaip.2019.08.051. [DOI] [PubMed] [Google Scholar]
- 166.Bateman ED, Boushey HA, Bousquet J, Busse WW, Clark TJ, Pauwels RA, et al. Can guideline-defined asthma control be achieved?The gaining optimal asthma control study. Am J Respir Crit Care Med. 2004;170:836–44. doi: 10.1164/rccm.200401-033OC. [DOI] [PubMed] [Google Scholar]
- 167.Pitlick MM, Pongdee T. Combining biologics targeting eosinophils (IL-5/IL-5R), IgE, and IL-4/IL-13 in allergic and inflammatory diseases. World Allergy Organ J. 2022;15:100707. doi: 10.1016/j.waojou.2022.100707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hamada S, Ogino E, Yasuba H. Cycling biologic therapy for severe asthma. Pulmonology. 2022;28:65–7. doi: 10.1016/j.pulmoe.2021.07.009. [DOI] [PubMed] [Google Scholar]
- 169.Al-Shimemeri A, Al-Ghadeer H, Giridhar H, Al-Jahdali M, Al-Moamary M, Khan J. Impact of an extensive Asthma education campaign for physicians on their drug prescription practices. Ann Thorac Med. 2006;1:20–5. [Google Scholar]
- 170.Ignacio-Garcia JM, Gonzalez-Santos P. Asthma self-management education program by home monitoring of peak expiratory flow. Am J Respir Crit Care Med. 1995;151:353–9. doi: 10.1164/ajrccm.151.2.7842191. [DOI] [PubMed] [Google Scholar]
- 171.Hawkins G, McMahon AD, Twaddle S, Wood SF, Ford I, Thomson NC. Stepping down inhaled corticosteroids in asthma: Randomised controlled trial. BMJ. 2003;326:1115. doi: 10.1136/bmj.326.7399.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Powell H, Gibson PG. High dose versus low dose inhaled corticosteroid as initial starting dose for asthma in adults and children. Cochrane Database Syst Rev. 2004;2004:CD004109. doi: 10.1002/14651858.CD004109.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Boulet LP, Drollmann A, Magyar P, Timar M, Knight A, Engelstätter R, et al. Comparative efficacy of once-daily ciclesonide and budesonide in the treatment of persistent asthma. Respir Med. 2006;100:785–94. doi: 10.1016/j.rmed.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 174.Rank MA, Hagan JB, Park MA, Podjasek JC, Samant SA, Volcheck GW, et al. The risk of asthma exacerbation after stopping low-dose inhaled corticosteroids: A systematic review and meta-analysis of randomized controlled trials. J Allergy Clin Immunol. 2013;131:724–9. doi: 10.1016/j.jaci.2012.11.038. [DOI] [PubMed] [Google Scholar]
- 175.Brozek JL, Kraft M, Krishnan JA, Cloutier MM, Lazarus SC, Li JT, et al. Long-acting β2-agonist step-off in patients with controlled asthma. Arch Intern Med. 2012;172:1365–75. doi: 10.1001/archinternmed.2012.3250. [DOI] [PubMed] [Google Scholar]
- 176.Masoli M, Weatherall M, Holt S, Beasley R. Budesonide once versus twice-daily administration: Meta-analysis. Respirology. 2004;9:528–34. doi: 10.1111/j.1440-1843.2004.00635.x. [DOI] [PubMed] [Google Scholar]
- 177.Bateman ED, Fairall L, Lombardi DM, English R. Budesonide/formoterol and formoterol provide similar rapid relief in patients with acute asthma showing refractoriness to salbutamol. Respir Res. 2006;7:13. doi: 10.1186/1465-9921-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Randell TL, Donaghue KC, Ambler GR, Cowell CT, Fitzgerald DA, van Asperen PP. Safety of the newer inhaled corticosteroids in childhood asthma. Paediatr Drugs. 2003;5:481–504. doi: 10.2165/00128072-200305070-00005. [DOI] [PubMed] [Google Scholar]
- 179.Bölke G, Tong X, Zuberbier T, Bousquet J, Bergmann KC. Extension of mepolizumab injection intervals as potential of saving costs in well controlled patients with severe eosinophilic asthma. World Allergy Organ J. 2022;15:100703. doi: 10.1016/j.waojou.2022.100703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Domingo C, Pomares X, Navarro A, Amengual MJ, Montón C, Sogo A, et al. A step-down protocol for omalizumab treatment in oral corticosteroid-dependent allergic asthma patients. Br J Clin Pharmacol. 2018;84:339–48. doi: 10.1111/bcp.13453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Currie GP, Douglas JG, Heaney LG. Difficult to treat asthma in adults. BMJ. 2009;338:b494. doi: 10.1136/bmj.b494. [DOI] [PubMed] [Google Scholar]
- 182.Brożek JL, Bousquet J, Agache I, Agarwal A, Bachert C, Bosnic-Anticevich S, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines-2016 revision. J Allergy Clin Immunol. 2017;140:950–8. doi: 10.1016/j.jaci.2017.03.050. [DOI] [PubMed] [Google Scholar]
- 183.Sullivan SD, Rasouliyan L, Russo PA, Kamath T, Chipps BE. TENOR Study Group. Extent, patterns, and burden of uncontrolled disease in severe or difficult-to-treat asthma. Allergy. 2007;62:126–33. doi: 10.1111/j.1398-9995.2006.01254.x. [DOI] [PubMed] [Google Scholar]
- 184.Serra-Batlles J, Plaza V, Morejón E, Comella A, Brugués J. Costs of asthma according to the degree of severity. Eur Respir J. 1998;12:1322–6. doi: 10.1183/09031936.98.12061322. [DOI] [PubMed] [Google Scholar]
- 185.Chen H, Blanc PD, Hayden ML, Bleecker ER, Chawla A, Lee JH, et al. Assessing productivity loss and activity impairment in severe or difficult-to-treat asthma. Value Health. 2008;11:231–9. doi: 10.1111/j.1524-4733.2007.00229.x. [DOI] [PubMed] [Google Scholar]
- 186.Tiwari A, Guglani V, Jat KR. Ketamine versus aminophylline for acute asthma in children: A randomized, controlled trial. Ann Thorac Med. 2016;11:283–8. doi: 10.4103/1817-1737.191874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Asthma GIF. The Global Strategy for Asthma Management and Prevention. 2020 [Google Scholar]
- 188.Porsbjerg C, Ulrik C, Skjold T, Backer V, Laerum B, Lehman S, et al. Nordic consensus statement on the systematic assessment and management of possible severe asthma in adults. Eur Clin Respir J. 2018;5:1–13. doi: 10.1080/20018525.2018.1440868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Lee JH, Haselkorn T, Borish L, Rasouliyan L, Chipps BE, Wenzel SE. Risk factors associated with persistent airflow limitation in severe or difficult-to-treat asthma: Insights from the TENOR study. Chest. 2007;132:1882–9. doi: 10.1378/chest.07-0713. [DOI] [PubMed] [Google Scholar]
- 190.Sturdy PM, Victor CR, Anderson HR, Bland JM, Butland BK, Harrison BD, et al. Psychological, social and health behaviour risk factors for deaths certified as asthma: A national case-control study. Thorax. 2002;57:1034–9. doi: 10.1136/thorax.57.12.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Butler C, Heaney LG. Risk factors of frequent exacerbations in difficult-to-treat asthma. Eur Respir J. 2006;27:1324–5. doi: 10.1183/09031936.06.00022606. [DOI] [PubMed] [Google Scholar]
- 192.Gaga M, Papageorgiou N, Yiourgioti G, Karydi P, Liapikou A, Bitsakou H, et al. Risk factors and characteristics associated with severe and difficult to treat asthma phenotype: An analysis of the ENFUMOSA group of patients based on the ECRHS questionnaire. Clin Exp Allergy. 2005;35:954–9. doi: 10.1111/j.1365-2222.2005.02281.x. [DOI] [PubMed] [Google Scholar]
- 193.Sullivan SD, Wenzel SE, Bresnahan BW, Zheng B, Lee JH, Pritchard M, et al. Association of control and risk of severe asthma-related events in severe or difficult-to-treat asthma patients. Allergy. 2007;62:655–60. doi: 10.1111/j.1398-9995.2007.01383.x. [DOI] [PubMed] [Google Scholar]
- 194.Robinson DS, Campbell DA, Durham SR, Pfeffer J, Barnes PJ, Chung KF, et al. Systematic assessment of difficult-to-treat asthma. Eur Respir J. 2003;22:478–83. doi: 10.1183/09031936.03.00017003. [DOI] [PubMed] [Google Scholar]
- 195.Natarajan S, Subramanian P. Allergic bronchopulmonary aspergillosis: A clinical review of 24 patients: Are we right in frequent serologic monitoring? Ann Thorac Med. 2014;9:216–20. doi: 10.4103/1817-1737.140130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Jani AL, Hamilos DL. Current thinking on the relationship between rhinosinusitis and asthma. J Asthma. 2005;42:1–7. doi: 10.1081/jas-200044744. [DOI] [PubMed] [Google Scholar]
- 197.Koczulla AR, Vogelmeier CF, Garn H, Renz H. New concepts in asthma: Clinical phenotypes and pathophysiological mechanisms. Drug Discov Today. 2017;22:388–96. doi: 10.1016/j.drudis.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 198.Saji J, Arai M, Yamamoto T, Mineshita M, Miyazawa T. Efficacy of omalizumab in patients with severe asthma using the asthma health questionnaire and asthma control test. Arerugi. 2014;63:1338–47. [PubMed] [Google Scholar]
- 199.Storms W, Bowdish MS, Farrar JR. Omalizumab and asthma control in patients with moderate-to-severe allergic asthma: A 6-year pragmatic data review. Allergy Asthma Proc. 2012;33:172–7. doi: 10.2500/aap.2012.33.3527. [DOI] [PubMed] [Google Scholar]
- 200.Bousquet J, Brusselle G, Buhl R, Busse WW, Cruz AA, Djukanovic R, et al. Care pathways for the selection of a biologic in severe asthma. Eur Respir J. 2017;50:1–9. doi: 10.1183/13993003.01782-2017. [DOI] [PubMed] [Google Scholar]
- 201.Heaney LG, Brightling CE, Menzies-Gow A, Stevenson M, Niven RM. British Thoracic Society Difficult Asthma Network. Refractory asthma in the UK: Cross-sectional findings from a UK multicentre registry. Thorax. 2010;65:787–94. doi: 10.1136/thx.2010.137414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Adcock IM, Lane SJ, Brown CR, Peters MJ, Lee TH, Barnes PJ. Differences in binding of glucocorticoid receptor to DNA in steroid-resistant asthma. J Immunol. 1995;154:3500–5. [PubMed] [Google Scholar]
- 203.Barnes PJ. Corticosteroid resistance in airway disease. Proc Am Thorac Soc. 2004;1:264–8. doi: 10.1513/pats.200402-014MS. [DOI] [PubMed] [Google Scholar]
- 204.Ayres JG. Pseudo-steroid resistant asthma. Thorax. 1999;54:956. doi: 10.1136/thx.54.10.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Campbell JD, Blough DK, Sullivan SD. Comparison of guideline-based control definitions and associations with outcomes in severe or difficult-to-treat asthma. Ann Allergy Asthma Immunol. 2008;101:474–81. doi: 10.1016/S1081-1206(10)60285-2. [DOI] [PubMed] [Google Scholar]
- 206.Gibson PG, Yang IA, Upham JW, Reynolds PN, Hodge S, James AL, et al. Effect of azithromycin on asthma exacerbations and quality of life in adults with persistent uncontrolled asthma (AMAZES): A randomised, double-blind, placebo-controlled trial. Lancet. 2017;390:659–68. doi: 10.1016/S0140-6736(17)31281-3. [DOI] [PubMed] [Google Scholar]
- 207.Hiles SA, McDonald VM, Guilhermino M, Brusselle GG, Gibson PG. Does maintenance azithromycin reduce asthma exacerbations?An individual participant data meta-analysis. Eur Respir J. 2019;54:1901381. doi: 10.1183/13993003.01381-2019. [DOI] [PubMed] [Google Scholar]
- 208.Cox L, Nelson H, Lockey R, Calabria C, Chacko T, Finegold I, et al. Allergen immunotherapy: A practice parameter third update. J Allergy Clin Immunol. 2011;127:S1–55. doi: 10.1016/j.jaci.2010.09.034. [DOI] [PubMed] [Google Scholar]
- 209.Dhami S, Kakourou A, Asamoah F, Agache I, Lau S, Jutel M, et al. Allergen immunotherapy for allergic asthma: A systematic review and meta-analysis. Allergy. 2017;72:1825–48. doi: 10.1111/all.13208. [DOI] [PubMed] [Google Scholar]
- 210.Dominguez-Ortega J, Delgado J, Blanco C, Prieto L, Arroabarren E, Cimarra M, et al. Specific allergen immunotherapy for the treatment of allergic asthma: A review of current evidence. J Investig Allergol Clin Immunol. 2017;27:1–35. doi: 10.18176/jiaci.0149. [DOI] [PubMed] [Google Scholar]
- 211.Demoly P, Makatsori M, Casale TB, Calderon MA. The potential role of allergen immunotherapy in stepping down asthma treatment. J Allergy Clin Immunol Pract. 2017;5:640–8. doi: 10.1016/j.jaip.2016.11.024. [DOI] [PubMed] [Google Scholar]
- 212.Jacobsen L, Niggemann B, Dreborg S, Ferdousi HA, Halken S, Høst A, et al. Specific immunotherapy has long-term preventive effect of seasonal and perennial asthma:10-year follow-up on the PAT study. Allergy. 2007;62:943–8. doi: 10.1111/j.1398-9995.2007.01451.x. [DOI] [PubMed] [Google Scholar]
- 213.Rice JL, Diette GB, Suarez-Cuervo C, Brigham EP, Lin SY, Ramanathan M, et al. Allergen-specific immunotherapy in the treatment of pediatric asthma: A systematic review. Pediatrics. 2018;141((s5)) doi: 10.1542/peds.2017-3833. [DOI] [PubMed] [Google Scholar]
- 214.Elliott J, Kelly SE, Johnston A, Skidmore B, Gomes T, Wells GA. Allergen immunotherapy for the treatment of allergic rhinitis and/or asthma: An umbrella review. CMAJ Open. 2017;5:E373–85. doi: 10.9778/cmajo.20160066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Abramson MJ, Bailey MJ, Couper FJ, Driver JS, Drummer OH, Forbes AB, et al. Are asthma medications and management related to deaths from asthma? Am J Respir Crit Care Med. 2001;163:12–8. doi: 10.1164/ajrccm.163.1.9910042. [DOI] [PubMed] [Google Scholar]
- 216.Omachi TA, Iribarren C, Sarkar U, Tolstykh I, Yelin EH, Katz PP, et al. Risk factors for death in adults with severe asthma. Ann Allergy Asthma Immunol. 2008;101:130–6. doi: 10.1016/S1081-1206(10)60200-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Plaza V, Serrano J, Picado C, Sanchis J. High Risk Asthma Research Group. Frequency and clinical characteristics of rapid-onset fatal and near-fatal asthma. Eur Respir J. 2002;19:846–52. doi: 10.1183/09031936.02.00241502. [DOI] [PubMed] [Google Scholar]
- 218.Dharmage SC, Perret JL, Custovic A. Epidemiology of asthma in children and adults. Front Pediatr. 2019;7:246. doi: 10.3389/fped.2019.00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Suruki RY, Daugherty JB, Boudiaf N, Albers FC. The frequency of asthma exacerbations and healthcare utilization in patients with asthma from the UK and USA. BMC Pulm Med. 2017;17:74. doi: 10.1186/s12890-017-0409-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Alahmadi TS, Banjari MA, Alharbi AS. The prevalence of childhood asthma in Saudi Arabia. Int J Pediatr Adolesc Med. 2019;6:74–7. doi: 10.1016/j.ijpam.2019.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Ali F, Behbehani N, Alomair N, Taher A. Fatal and near-fatal thunderstorm asthma epidemic in a desert country. Ann Thorac Med. 2019;14:155–60. doi: 10.4103/atm.ATM_258_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Serrano-Pariente J, Plaza V. Near-fatal asthma: A heterogeneous clinical entity. Curr Opin Allergy Clin Immunol. 2017;17:28–35. doi: 10.1097/ACI.0000000000000333. [DOI] [PubMed] [Google Scholar]
- 223.D’Amato G, Vitale C, Lanza M, Sanduzzi A, Molino A, Mormile M, et al. Near fatal asthma:treatment and prevention. Eur Ann Allergy Clin Immunol. 2016;48:116–22. [PubMed] [Google Scholar]
- 224.Al-Dorzi HM, Al-Shammary HA, Al-Shareef SY, Tamim HM, Shammout K, Al Dawood A, et al. Risk factors, management and outcomes of patients admitted with near fatal asthma to a tertiary care hospital in Riyadh. Ann Thorac Med. 2014;9:33–8. doi: 10.4103/1817-1737.124441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Fergeson JE, Patel SS, Lockey RF. Acute asthma, prognosis, and treatment. J Allergy Clin Immunol. 2017;139:438–47. doi: 10.1016/j.jaci.2016.06.054. [DOI] [PubMed] [Google Scholar]
- 226.Al-Dawood KM. Pattern and risk factors associated with hospital emergency visits among schoolboys with bronchial asthma in Al-Khobar. Ann Saudi Med. 2002;22:29–33. doi: 10.5144/0256-4947.2002.29. [DOI] [PubMed] [Google Scholar]
- 227.Crane J, Burgess CD, Graham AN, Maling TJ. Hypokalaemic and electrocardiographic effects of aminophylline and salbutamol in obstructive airways disease. N Z Med J. 1987;100:309–11. [PubMed] [Google Scholar]
- 228.Hessel PA, Mitchell I, Tough S, Green FH, Cockcroft D, Kepron W, et al. Risk factors for death from asthma. Prairie provinces asthma study group. Ann Allergy Asthma Immunol. 1999;83:362–8. doi: 10.1016/s1081-1206(10)62832-3. [DOI] [PubMed] [Google Scholar]
- 229.Bai TR, Cooper J, Koelmeyer T, Paré PD, Weir TD. The effect of age and duration of disease on airway structure in fatal asthma. Am J Respir Crit Care Med. 2000;162:663–9. doi: 10.1164/ajrccm.162.2.9907151. [DOI] [PubMed] [Google Scholar]
- 230.Cates CJ, Crilly JA, Rowe BH. Holding chambers (spacers) versus nebulisers for beta-agonist treatment of acute asthma. Cochrane Database Syst Rev. 2006:CD000052. doi: 10.1002/14651858.CD000052.pub2. https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858. CD000052.pub3/full#. [DOI] [PubMed] [Google Scholar]
- 231.Gates GG, Bara A, Grilly AJ, Rowe BH. Holding chamber versus nebulisers for beta-agonist treatment of acute asthma (Cochrane Review) The Cochrane Library. 2003;(3) doi: 10.1002/14651858.CD000052. [DOI] [PubMed] [Google Scholar]
- 232.Rossing TH, Fanta CH, Goldstein DH, Snapper JR, McFadden ER., Jr Emergency therapy of asthma: Comparison of the acute effects of parenteral and inhaled sympathomimetics and infused aminophylline. Am Rev Respir Dis. 1980;122:365–71. doi: 10.1164/arrd.1980.122.3.365. [DOI] [PubMed] [Google Scholar]
- 233.Rowe BH, Spooner C, Ducharme FM, Bretzlaff JA, Bota GW. Early emergency department treatment of acute asthma with systemic corticosteroids. Cochrane Database Syst Rev. 2001:CD002178. doi: 10.1002/14651858.CD002178. https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858. CD002178/abstract. [DOI] [PubMed] [Google Scholar]
- 234.Rowe BH, Spooner C, Ducharme F, Bretzlaff J, Bota G, Group CA. Early emergency department treatment of acute asthma with systemic corticosteroids. Cochrane Database of Systematic Reviews. 1996;2010 [Google Scholar]
- 235.Vinayak AG, Kress J, Hall J. Pharmacotherapy in the emergency department, hospital floor, and intensive care unit. Lung Biol Health Dis. 2005;212:351. [Google Scholar]
- 236.O’Driscoll BR, Howard LS, Davison AG. British Thoracic Society. BTS guideline for emergency oxygen use in adult patients. Thorax. 2008;63(Suppl 6):i1–68. doi: 10.1136/thx.2008.102947. [DOI] [PubMed] [Google Scholar]
- 237.Chien JW, Ciufo R, Novak R, Skowronski M, Nelson J, Coreno A, et al. Uncontrolled oxygen administration and respiratory failure in acute asthma. Chest. 2000;117:728–33. doi: 10.1378/chest.117.3.728. [DOI] [PubMed] [Google Scholar]
- 238.Douglas JG, Rafferty P, Fergusson RJ, Prescott RJ, Crompton GK, Grant IW. Nebulised salbutamol without oxygen in severe acute asthma: How effective and how safe? Thorax. 1985;40:180–3. doi: 10.1136/thx.40.3.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Lanes SF, Garrett JE, Wentworth CE, 3rd, Fitzgerald JM, Karpel JP. The effect of adding ipratropium bromide to salbutamol in the treatment of acute asthma: A pooled analysis of three trials. Chest. 1998;114:365–72. doi: 10.1378/chest.114.2.365. [DOI] [PubMed] [Google Scholar]
- 240.Chassany O, Fullerton S. Meta-analysis of the effects of ipratropium bromide in adults with acute asthma. Am J Med. 2000;108:596–7. doi: 10.1016/s0002-9343(00)00298-9. [DOI] [PubMed] [Google Scholar]
- 241.Rodrigo G, Rodrigo C, Burschtin O. A meta-analysis of the effects of ipratropium bromide in adults with acute asthma. Am J Med. 1999;107:363–70. doi: 10.1016/s0002-9343(99)00243-0. [DOI] [PubMed] [Google Scholar]
- 242.Stoodley RG, Aaron SD, Dales RE. The role of ipratropium bromide in the emergency management of acute asthma exacerbation: A metaanalysis of randomized clinical trials. Ann Emerg Med. 1999;34:8–18. doi: 10.1016/s0196-0644(99)70266-0. [DOI] [PubMed] [Google Scholar]
- 243.Jackson DJ, Bacharier LB, Mauger DT, Boehmer S, Beigelman A, Chmiel JF, et al. Quintupling inhaled glucocorticoids to prevent childhood asthma exacerbations. N Engl J Med. 2018;378:891–901. doi: 10.1056/NEJMoa1710988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Kirkland SW, Vandenberghe C, Voaklander B, Nikel T, Campbell S, Rowe BH. Combined inhaled beta-agonist and anticholinergic agents for emergency management in adults with asthma. Cochrane Database Syst Rev. 2017;1:CD001284. doi: 10.1002/14651858.CD001284.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Menzies-Gow A, Busse WW, Castro M, Jackson DJ. Prevention and treatment of asthma exacerbations in adults. J Allergy Clin Immunol Pract. 2021;9:2578–86. doi: 10.1016/j.jaip.2021.05.016. [DOI] [PubMed] [Google Scholar]
- 246.Rowe BH, Spooner CH, Ducharme FM, Bretzlaff JA, Bota GW. Corticosteroids for preventing relapse following acute exacerbations of asthma. Cochrane Database Syst Rev. 2007:CD000195. doi: 10.1002/14651858.CD000195.pub2. [DOI] [PubMed] [Google Scholar]
- 247.Camargo C, Rowe B, Bourdon C, Blitz S, Bretzlaff J, Bota G. Magnesium sulfate for treating exacerbations of acute asthma in the emergency department. Cochrane Database Syst Rev. 2000;2000:CD001490. doi: 10.1002/14651858.CD001490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Kew KM, Kirtchuk L, Michell CI. Intravenous magnesium sulfate for treating adults with acute asthma in the emergency department. Cochrane Database Syst Rev. 2014:CD010909. doi: 10.1002/14651858.CD010909.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Shrestha M, Bidadi K, Gourlay S, Hayes J. Continuous versus intermittent albuterol, at high and low doses, in the treatment of severe acute asthma in adults. Chest. 1996;110:42–7. doi: 10.1378/chest.110.1.42. [DOI] [PubMed] [Google Scholar]
- 250.Camargo CA, Jr, Rachelefsky G, Schatz M. Managing asthma exacerbations in the emergency department: Summary of the National Asthma Education and Prevention Program Expert Panel Report 3 guidelines for the management of asthma exacerbations. Proc Am Thorac Soc. 2009;6:357–66. doi: 10.1513/pats.P09ST2. [DOI] [PubMed] [Google Scholar]
- 251.Rowe BH, Spooner C, Ducharme F, Bretzlaff J, Bota G, Group CA. Early emergency department treatment of acute asthma with systemic corticosteroids. Cochrane Database Syst Rev. 1996;2010 [Google Scholar]
- 252.Tokuda Y, Miyagi S. Oxygen treatment for acute severe asthma. Home oxygenation would be more effective. BMJ. 2001;323:1069. [PMC free article] [PubMed] [Google Scholar]
- 253.Havemann BD, Henderson CA, El-Serag HB. The association between gastro-oesophageal reflux disease and asthma: A systematic review. Gut. 2007;56:1654–64. doi: 10.1136/gut.2007.122465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Houghton LA, Lee AS, Badri H, DeVault KR, Smith JA. Respiratory disease and the oesophagus: Reflux, reflexes and microaspiration. Nat Rev Gastroenterol Hepatol. 2016;13:445–60. doi: 10.1038/nrgastro.2016.91. [DOI] [PubMed] [Google Scholar]
- 255.Asano K, Suzuki H. Silent acid reflux and asthma control. N Engl J Med. 2009;360:1551–3. doi: 10.1056/NEJMe0900117. [DOI] [PubMed] [Google Scholar]
- 256.Kopsaftis Z, Yap HS, Tin KS, Hnin K, Carson-Chahhoud KV. Pharmacological and surgical interventions for the treatment of gastro-oesophageal reflux in adults and children with asthma. Cochrane Database Syst Rev. 2021;5:CD001496. doi: 10.1002/14651858.CD001496.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Zheng Z, Luo Y, Li J, Gao J. Randomised trials of proton pump inhibitors for gastro-oesophageal reflux disease in patients with asthma: An updated systematic review and meta-analysis. BMJ Open. 2021;11:e043860. doi: 10.1136/bmjopen-2020-043860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Cruz AA, Popov T, Pawankar R, Annesi-Maesano I, Fokkens W, Kemp J, et al. Common characteristics of upper and lower airways in rhinitis and asthma: ARIA update, in collaboration with GA(2)LEN. Allergy. 2007;62(Suppl 84):1–41. doi: 10.1111/j.1398-9995.2007.01551.x. [DOI] [PubMed] [Google Scholar]
- 259.Corren J, Manning BE, Thompson SF, Hennessy S, Strom BL. Rhinitis therapy and the prevention of hospital care for asthma: A case-control study. J Allergy Clin Immunol. 2004;113:415–9. doi: 10.1016/j.jaci.2003.11.034. [DOI] [PubMed] [Google Scholar]
- 260.American Lung Association–Asthma Clinical Research Centers'Writing Committee. aDixon AE, Castro M, Cohen RI, Gerald LB, Holbrook JT, et al. Efficacy of nasal mometasone for the treatment of chronic sinonasal disease in patients with inadequately controlled asthma. J Allergy Clin Immunol. 2015;135:701–9.e5. doi: 10.1016/j.jaci.2014.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Gill AS, Alt JA, Detwiller KY, Rowan NR, Gray ST, Hellings PW, et al. Management paradigms for chronic rhinosinusitis in individuals with asthma: An evidence-based review with recommendations. Int Forum Allergy Rhinol. 2023;13:1758–82. doi: 10.1002/alr.23130. [DOI] [PubMed] [Google Scholar]
- 262.Peters U, Dixon AE, Forno E. Obesity and asthma. J Allergy Clin Immunol. 2018;141:1169–79. doi: 10.1016/j.jaci.2018.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Scott HA, Ng SH, McLoughlin RF, Valkenborghs SR, Nair P, Brown AC, et al. Effect of obesity on airway and systemic inflammation in adults with asthma: A systematic review and meta-analysis. Thorax. 2023;78:957–65. doi: 10.1136/thorax-2022-219268. [DOI] [PubMed] [Google Scholar]
- 264.Saint-Pierre P, Bourdin A, Chanez P, Daures JP, Godard P. Are overweight asthmatics more difficult to control? Allergy. 2006;61:79–84. doi: 10.1111/j.1398-9995.2005.00953.x. [DOI] [PubMed] [Google Scholar]
- 265.Desai AG, Togias A, Schechter C, Fisher B, Parow A, Skloot G. Peripheral airways dysfunction in obesity reflects increased bronchomotor tone. J Allergy Clin Immunol. 2015;135:820–2. doi: 10.1016/j.jaci.2014.09.016. [DOI] [PubMed] [Google Scholar]
- 266.Scott HA, Gibson PG, Garg ML, Pretto JJ, Morgan PJ, Callister R, et al. Dietary restriction and exercise improve airway inflammation and clinical outcomes in overweight and obese asthma: A randomized trial. Clin Exp Allergy. 2013;43:36–49. doi: 10.1111/cea.12004. [DOI] [PubMed] [Google Scholar]
- 267.Moreira A, Bonini M, Garcia-Larsen V, Bonini S, Del Giacco SR, Agache I, et al. Weight loss interventions in asthma: EAACI evidence-based clinical practice guideline (part I) Allergy. 2013;68:425–39. doi: 10.1111/all.12106. [DOI] [PubMed] [Google Scholar]
- 268.Boulet LP, Turcotte H, Martin J, Poirier P. Effect of bariatric surgery on airway response and lung function in obese subjects with asthma. Respir Med. 2012;106:651–60. doi: 10.1016/j.rmed.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 269.Cao Y, Lin SH, Zhu D, Xu F, Chen ZH, Shen HH, et al. WeChat public account use improves clinical control of cough-variant asthma: A randomized controlled trial. Med Sci Monit. 2018;24:1524–32. doi: 10.12659/MSM.907284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Achilleos A. Evidence-based evaluation and management of chronic cough. Med Clin North Am. 2016;100:1033–45. doi: 10.1016/j.mcna.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 271.Morice AH, McGarvey L, Pavord I. British Thoracic Society Cough Guideline Group. Recommendations for the management of cough in adults. Thorax. 2006;61(Suppl 1):i1–24. doi: 10.1136/thx.2006.065144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Spector SL, Tan RA. Effectiveness of montelukast in the treatment of cough variant asthma. Ann Allergy Asthma Immunol. 2004;93:232–6. doi: 10.1016/S1081-1206(10)61493-7. [DOI] [PubMed] [Google Scholar]
- 273.Desai D, Brightling C. Cough due to asthma, cough-variant asthma and non-asthmatic eosinophilic bronchitis. Otolaryngol Clin North Am. 2010;43:123–30. doi: 10.1016/j.otc.2009.11.006. x. [DOI] [PubMed] [Google Scholar]
- 274.Diver S, Russell RJ, Brightling CE. Cough and eosinophilia. J Allergy Clin Immunol Pract. 2019;7:1740–7. doi: 10.1016/j.jaip.2019.04.048. [DOI] [PubMed] [Google Scholar]
- 275.Weiler JM, Brannan JD, Randolph CC, Hallstrand TS, Parsons J, Silvers W, et al. Exercise-induced bronchoconstriction update-2016. J Allergy Clin Immunol. 2016;138:1292–5.e36. doi: 10.1016/j.jaci.2016.05.029. [DOI] [PubMed] [Google Scholar]
- 276.Dreßler M, Friedrich T, Lasowski N, Herrmann E, Zielen S, Schulze J. Predictors and reproducibility of exercise-induced bronchoconstriction in cold air. BMC Pulm Med. 2019;19:94. doi: 10.1186/s12890-019-0845-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Parsons JP, Hallstrand TS, Mastronarde JG, Kaminsky DA, Rundell KW, Hull JH, et al. An official American Thoracic Society clinical practice guideline: Exercise-induced bronchoconstriction. Am J Respir Crit Care Med. 2013;187:1016–27. doi: 10.1164/rccm.201303-0437ST. [DOI] [PubMed] [Google Scholar]
- 278.Lazarinis N, Jørgensen L, Ekström T, Bjermer L, Dahlén B, Pullerits T, et al. Combination of budesonide/formoterol on demand improves asthma control by reducing exercise-induced bronchoconstriction. Thorax. 2014;69:130–6. doi: 10.1136/thoraxjnl-2013-203557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Laidlaw TM, Boyce JA. Aspirin-exacerbated respiratory disease –New prime suspects. N Engl J Med. 2016;374:484–8. doi: 10.1056/NEJMcibr1514013. [DOI] [PubMed] [Google Scholar]
- 280.Rajan JP, Wineinger NE, Stevenson DD, White AA. Prevalence of aspirin-exacerbated respiratory disease among asthmatic patients: A meta-analysis of the literature. J Allergy Clin Immunol. 2015;135:676–81.e1. doi: 10.1016/j.jaci.2014.08.020. [DOI] [PubMed] [Google Scholar]
- 281.Morales DR, Guthrie B, Lipworth BJ, Jackson C, Donnan PT, Santiago VH. NSAID-exacerbated respiratory disease: A meta-analysis evaluating prevalence, mean provocative dose of aspirin and increased asthma morbidity. Allergy. 2015;70:828–35. doi: 10.1111/all.12629. [DOI] [PubMed] [Google Scholar]
- 282.Szczeklik A, Stevenson DD. Aspirin-induced asthma: Advances in pathogenesis, diagnosis, and management. J Allergy Clin Immunol. 2003;111:913–21. doi: 10.1067/mai.2003.1487. [DOI] [PubMed] [Google Scholar]
- 283.White AA, Stevenson DD. Aspirin-exacerbated respiratory disease. N Engl J Med. 2018;379:1060–70. doi: 10.1056/NEJMra1712125. [DOI] [PubMed] [Google Scholar]
- 284.Woessner KM, Simon RA, Stevenson DD. The safety of celecoxib in patients with aspirin-sensitive asthma. Arthritis Rheum. 2002;46:2201–6. doi: 10.1002/art.10426. [DOI] [PubMed] [Google Scholar]
- 285.Morales DR, Lipworth BJ, Guthrie B, Jackson C, Donnan PT, Santiago VH. Safety risks for patients with aspirin-exacerbated respiratory disease after acute exposure to selective nonsteroidal anti-inflammatory drugs and COX-2 inhibitors: Meta-analysis of controlled clinical trials. J Allergy Clin Immunol. 2014;134:40–5. doi: 10.1016/j.jaci.2013.10.057. [DOI] [PubMed] [Google Scholar]
- 286.Cahill KN, Boyce JA. Aspirin-exacerbated respiratory disease: Mediators and mechanisms of a clinical disease. J Allergy Clin Immunol. 2017;139:764–6. doi: 10.1016/j.jaci.2016.09.025. [DOI] [PubMed] [Google Scholar]
- 287.Park JS, Jang AS, Park SW, Lee YM, Uh ST, Kim YH, et al. Protection of leukotriene receptor antagonist against aspirin-induced bronchospasm in asthmatics. Allergy Asthma Immunol Res. 2010;2:48–54. doi: 10.4168/aair.2010.2.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Cohen JM, Bateman BT, Huybrechts KF, Mogun H, Yland J, Schatz M, et al. Poorly controlled asthma during pregnancy remains common in the United States. J Allergy Clin Immunol Pract. 2019;7:2672–80.e10. doi: 10.1016/j.jaip.2019.05.043. [DOI] [PubMed] [Google Scholar]
- 289.Bonham CA, Patterson KC, Strek ME. Asthma outcomes and management during pregnancy. Chest. 2018;153:515–27. doi: 10.1016/j.chest.2017.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Murphy VE, Clifton VL, Gibson PG. Asthma exacerbations during pregnancy: Incidence and association with adverse pregnancy outcomes. Thorax. 2006;61:169–76. doi: 10.1136/thx.2005.049718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Blais L, Kettani FZ, Forget A, Beauchesne MF, Lemière C. Asthma exacerbations during the first trimester of pregnancy and congenital malformations: Revisiting the association in a large representative cohort. Thorax. 2015;70:647–52. doi: 10.1136/thoraxjnl-2014-206634. [DOI] [PubMed] [Google Scholar]
- 292.Murphy VE, Gibson PG, Smith R, Clifton VL. Asthma during pregnancy: Mechanisms and treatment implications. Eur Respir J. 2005;25:731–50. doi: 10.1183/09031936.05.00085704. [DOI] [PubMed] [Google Scholar]
- 293.Holland SM, Thomson KD. Acute severe asthma presenting in late pregnancy. Int J Obstet Anesth. 2006;15:75–8. doi: 10.1016/j.ijoa.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 294.Hanania NA, Belfort MA. Acute asthma in pregnancy. Crit Care Med. 2005;33:S319–24. doi: 10.1097/01.ccm.0000182789.14710.a1. [DOI] [PubMed] [Google Scholar]
- 295.Lim A, Stewart K, König K, George J. Systematic review of the safety of regular preventive asthma medications during pregnancy. Ann Pharmacother. 2011;45:931–45. doi: 10.1345/aph.1P764. [DOI] [PubMed] [Google Scholar]
- 296.Pfaller B, JoséYepes-Nuñez J, Agache I, Akdis CA, Alsalamah M, Bavbek S, et al. Biologicals in atopic disease in pregnancy: An EAACI position paper. Allergy. 2021;76:71–89. doi: 10.1111/all.14282. [DOI] [PubMed] [Google Scholar]
- 297.Namazy J, Cabana MD, Scheuerle AE, Thorp JM, Jr, Chen H, Carrigan G, et al. The xolair pregnancy registry (EXPECT): The safety of omalizumab use during pregnancy. J Allergy Clin Immunol. 2015;135:407–12. doi: 10.1016/j.jaci.2014.08.025. [DOI] [PubMed] [Google Scholar]
- 298.Smy L, Chan AC, Bozzo P, Koren G. Is it safe to use inhaled corticosteroids in pregnancy? Can Fam Physician. 2014;60:809–12. e433-5. [PMC free article] [PubMed] [Google Scholar]
- 299.Rahimi R, Nikfar S, Abdollahi M. Meta-analysis finds use of inhaled corticosteroids during pregnancy safe: A systematic meta-analysis review. Hum Exp Toxicol. 2006;25:447–52. doi: 10.1191/0960327106het647oa. [DOI] [PubMed] [Google Scholar]
- 300.Gluck JC, Gluck PA. The effect of pregnancy on the course of asthma. Immunol Allergy Clin North Am. 2006;26:63–80. doi: 10.1016/j.iac.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 301.Sevelsted A, Stokholm J, Bisgaard H. Risk of asthma from cesarean delivery depends on membrane rupture. J Pediatr. 2016;171:38–42.e1. doi: 10.1016/j.jpeds.2015.12.066. [DOI] [PubMed] [Google Scholar]
- 302.Vallera C, Choi LO, Cha CM, Hong RW. Uterotonic medications: Oxytocin, methylergonovine, carboprost, misoprostol. Anesthesiol Clin. 2017;35:207–19. doi: 10.1016/j.anclin.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 303.Arora N, Mahajan K, Jana N, Maiti TK, Mandal D, Pandey R. Successful pregnancy outcome among women with end-stage renal disease requiring haemodialysis. J Indian Med Assoc. 2009;107:237–8. [PubMed] [Google Scholar]
- 304.Levy ML, Nicholson PJ. Occupational asthma case finding: A role for primary care. Br J Gen Pract. 2004;54:731–3. [PMC free article] [PubMed] [Google Scholar]
- 305.Baur X, Aasen TB, Burge PS, Heederik D, Henneberger PK, Maestrelli P, et al. The management of work-related asthma guidelines: A broader perspective. Eur Respir Rev. 2012;21:125–39. doi: 10.1183/09059180.00004711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Legiest B, Nemery B. Management of work-related asthma: Guidelines and challenges. Eur Respir Rev. 2012;21:79–81. doi: 10.1183/09059180.00002912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Chang C, Sun Y. Global strategy for asthma management and prevention: Interpretation of the updates in 2022. Chin Gen Pract. 2022;25:4355. [Google Scholar]
- 308.Barrecheguren M, Pinto L, Mostafavi-Pour-Manshadi SM, Tan WC, Li PZ, Aaron SD, et al. Identification and definition of asthma-COPD overlap: The CanCOLD study. Respirology. 2020;25:836–49. doi: 10.1111/resp.13780. [DOI] [PubMed] [Google Scholar]
- 309.Andersén H, Lampela P, Nevanlinna A, Säynäjäkangas O, Keistinen T. High hospital burden in overlap syndrome of asthma and COPD. Clin Respir J. 2013;7:342–6. doi: 10.1111/crj.12013. [DOI] [PubMed] [Google Scholar]
- 310.Kendzerska T, Aaron SD, To T, Licskai C, Stanbrook M, Vozoris NT, et al. Effectiveness and safety of inhaled corticosteroids in older individuals with chronic obstructive pulmonary disease and/or asthma. A population study. Ann Am Thorac Soc. 2019;16:1252–62. doi: 10.1513/AnnalsATS.201902-126OC. [DOI] [PubMed] [Google Scholar]
- 311.Stupka E, deShazo R. Asthma in seniors: Part 1. Evidence for underdiagnosis, undertreatment, and increasing morbidity and mortality. Am J Med. 2009;122:6–11. doi: 10.1016/j.amjmed.2008.09.022. [DOI] [PubMed] [Google Scholar]
- 312.Oraka E, Kim HJ, King ME, Callahan DB. Asthma prevalence among US elderly by age groups: Age still matters. J Asthma. 2012;49:593–9. doi: 10.3109/02770903.2012.684252. [DOI] [PubMed] [Google Scholar]
- 313.Gibson PG, McDonald VM, Marks GB. Asthma in older adults. Lancet. 2010;376:803–13. doi: 10.1016/S0140-6736(10)61087-2. [DOI] [PubMed] [Google Scholar]
- 314.Slavin RG, Haselkorn T, Lee JH, Zheng B, Deniz Y, Wenzel SE, et al. Asthma in older adults: Observations from the epidemiology and natural history of asthma: Outcomes and treatment regimens (TENOR) study. Ann Allergy Asthma Immunol. 2006;96:406–14. doi: 10.1016/S1081-1206(10)60907-6. [DOI] [PubMed] [Google Scholar]
- 315.Marcus P, Oppenheimer EA, Patel PA, Katz LM, Doyle JJ. Use of nebulized inhaled corticosteroids among older adult patients: An assessment of outcomes. Ann Allergy Asthma Immunol. 2006;96:736–43. doi: 10.1016/S1081-1206(10)61074-5. [DOI] [PubMed] [Google Scholar]
- 316.Reed CE. Asthma in the elderly: Diagnosis and management. J Allergy Clin Immunol. 2010;126:681–7. doi: 10.1016/j.jaci.2010.05.035. [DOI] [PubMed] [Google Scholar]
- 317.Graham BL, Steenbruggen I, Miller MR, Barjaktarevic IZ, Cooper BG, Hall GL, et al. Standardization of spirometry 2019 update. An official American Thoracic Society and European Respiratory Society technical statement. Am J Respir Crit Care Med. 2019;200:e70–88. doi: 10.1164/rccm.201908-1590ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Naja AS, Permaul P, Phipatanakul W. Taming asthma in school-aged children: A comprehensive review. J Allergy Clin Immunol Pract. 2018;6:726–35. doi: 10.1016/j.jaip.2018.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Denlinger LC, Sorkness CA, Chinchilli VM, Lemanske RF., Jr Guideline-defining asthma clinical trials of the National Heart, Lung, and Blood institute's asthma clinical research network and childhood asthma research and education network. J Allergy Clin Immunol. 2007;119:3–11. doi: 10.1016/j.jaci.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Ferrante G, La Grutta S. The Burden of pediatric asthma. Front Pediatr. 2018;6:186. doi: 10.3389/fped.2018.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Yang CL, Simons E, Foty RG, Subbarao P, To T, Dell SD. Misdiagnosis of asthma in schoolchildren. Pediatr Pulmonol. 2017;52:293–302. doi: 10.1002/ppul.23541. [DOI] [PubMed] [Google Scholar]
- 322.Danvers L, Lo DK, Gaillard EA. The role of objective tests to support a diagnosis of asthma in children. Paediatr Respir Rev. 2020;33:52–7. doi: 10.1016/j.prrv.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 323.Kann K, Long B, Koyfman A. Clinical mimics: An emergency medicine-focused review of asthma mimics. J Emerg Med. 2017;53:195–201. doi: 10.1016/j.jemermed.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 324.Hederos CA, Janson S, Andersson H, Hedlin G. Chest X-ray investigation in newly discovered asthma. Pediatr Allergy Immunol. 2004;15:163–5. doi: 10.1046/j.1399-3038.2003.00098.x. [DOI] [PubMed] [Google Scholar]
- 325.Gauthier MC, Fajt ML. Is it asthma?Recognizing asthma mimics. Difficult To Treat Asthma. USA: Springer; 2020. pp. 25–38. [Google Scholar]
- 326.Alfonso J, Pérez S, Bou R, Amat A, Ruiz I, Mora A, et al. Asthma prevalence and risk factors in school children: The RESPIR longitudinal study. Allergol Immunopathol (Madr) 2020;48:223–31. doi: 10.1016/j.aller.2019.06.003. [DOI] [PubMed] [Google Scholar]
- 327.Al-Shamrani A, Bagais K, Alenazi A, Alqwaiee M, Al-Harbi AS. Wheezing in children: Approaches to diagnosis and management. Int J Pediatr Adolesc Med. 2019;6:68–73. doi: 10.1016/j.ijpam.2019.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Chipps BE, Bacharier LB, Harder JM. Phenotypic expressions of childhood wheezing and asthma: Implications for therapy. J Pediatr. 2011;158:878–84.e1. doi: 10.1016/j.jpeds.2011.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Fahy JV, O’Byrne PM. “Reactive airways disease”. A lazy term of uncertain meaning that should be abandoned. Am J Respir Crit Care Med. 2001;163:822–3. doi: 10.1164/ajrccm.163.4.2005049. [DOI] [PubMed] [Google Scholar]
- 330.Weinberger MI, Sirey JA, Bruce ML, Heo M, Papademetriou E, Meyers BS. Predictors of major depression six months after admission for outpatient treatment. Psychiatr Serv. 2008;59:1211–5. doi: 10.1176/appi.ps.59.10.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Weinberger M. Pediatric asthma and related allergic and nonallergic diseases: Patient-oriented evidence-based essentials that matter. Pediatr Health. 2008;2:631–50. [Google Scholar]
- 332.Akdis CA, Bachert C, Cingi C, Dykewicz MS, Hellings PW, Naclerio RM, et al. Endotypes and phenotypes of chronic rhinosinusitis: A PRACTALL document of the European Academy of Allergy and Clinical Immunology and the American Academy of Allergy, Asthma &Immunology. J Allergy Clin Immunol. 2013;131:1479–90. doi: 10.1016/j.jaci.2013.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Bubshait DK, Albuali WH, Yousef AA, Obeid OE, Alkharsah KR, Hassan MI, et al. Clinical description of human bocavirus viremia in children with LRTI, Eastern Province, Saudi Arabia. Ann Thorac Med. 2015;10:146–9. doi: 10.4103/1817-1737.151437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Conrad LA, Cabana MD, Rastogi D. Defining pediatric asthma: Phenotypes to endotypes and beyond. Pediatr Res. 2021;90:45–51. doi: 10.1038/s41390-020-01231-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.McIntyre AP, Viswanathan RK. Phenotypes and endotypes in asthma. Precision Approaches to Heterogeneity in Asthma. USA: Springer; 2023. pp. 119–42. [DOI] [PubMed] [Google Scholar]
- 336.Savenije OE, Kerkhof M, Koppelman GH, Postma DS. Predicting who will have asthma at school age among preschool children. J Allergy Clin Immunol. 2012;130:325–31. doi: 10.1016/j.jaci.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 337.Guilbert TW, Biagini JM, Ramsey RR, Keidel K, Curtsinger K, Kroner JW, et al. Treatment by biomarker-informed endotype vs guideline care in children with difficult-to-treat asthma. Ann Allergy Asthma Immunol. 2022;128:535–43.e6. doi: 10.1016/j.anai.2022.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Wan XC, Woodruff PG. Biomarkers in severe asthma. Immunol Allergy Clin North Am. 2016;36:547–57. doi: 10.1016/j.iac.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Just J, Gouvis-Echraghi R, Couderc R, Guillemot-Lambert N, Saint-Pierre P. Novel severe wheezy young children phenotypes: Boys atopic multiple-trigger and girls nonatopic uncontrolled wheeze. J Allergy Clin Immunol. 2012;130:103–10.e8. doi: 10.1016/j.jaci.2012.02.041. [DOI] [PubMed] [Google Scholar]
- 340.Guilbert TW, Morgan WJ, Zeiger RS, Bacharier LB, Boehmer SJ, Krawiec M, et al. Atopic characteristics of children with recurrent wheezing at high risk for the development of childhood asthma. J Allergy Clin Immunol. 2004;114:1282–7. doi: 10.1016/j.jaci.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 341.Castro-Rodriguez JA. The asthma predictive index: A very useful tool for predicting asthma in young children. J Allergy Clin Immunol. 2010;126:212–6. doi: 10.1016/j.jaci.2010.06.032. [DOI] [PubMed] [Google Scholar]
- 342.Chang TS, Lemanske RF, Jr, Guilbert TW, Gern JE, Coen MH, Evans MD, et al. Evaluation of the modified asthma predictive index in high-risk preschool children. J Allergy Clin Immunol Pract. 2013;1:152–6. doi: 10.1016/j.jaip.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Pedersen SE, Hurd SS, Lemanske RF, Jr, Becker A, Zar HJ, Sly PD, et al. Global strategy for the diagnosis and management of asthma in children 5 years and younger. Pediatr Pulmonol. 2011;46:1–17. doi: 10.1002/ppul.21321. [DOI] [PubMed] [Google Scholar]
- 344.Yousef AA, Al-Shamrani AS, Al-Haider SA, Said YS, Al Harbi S, Al-Harbi AS. Pediatric pulmonary services in Saudi Arabia. Ann Thorac Med. 2013;8:224–8. doi: 10.4103/1817-1737.118502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Yawn BP, Brenneman SK, Allen-Ramey FC, Cabana MD, Markson LE. Assessment of asthma severity and asthma control in children. Pediatrics. 2006;118:322–9. doi: 10.1542/peds.2005-2576. [DOI] [PubMed] [Google Scholar]
- 346.Bakel LA, Hamid J, Ewusie J, Liu K, Mussa J, Straus S, et al. International variation in asthma and bronchiolitis guidelines. Pediatrics. 2017;140:e20170092. doi: 10.1542/peds.2017-0092. [DOI] [PubMed] [Google Scholar]
- 347.Liu AH, Zeiger R, Sorkness C, Mahr T, Ostrom N, Burgess S, et al. Development and cross-sectional validation of the childhood asthma control test. J Allergy Clin Immunol. 2007;119:817–25. doi: 10.1016/j.jaci.2006.12.662. [DOI] [PubMed] [Google Scholar]
- 348.Welsh EJ, Hasan M, Li P. Home-based educational interventions for children with asthma. Cochrane Database Syst Rev. 2011;2011:CD008469. doi: 10.1002/14651858.CD008469.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Wolf FM, Guevara JP, Grum CM, Clark NM, Cates CJ. Educational interventions for asthma in children. Cochrane Database Syst Rev. 2003:CD000326. doi: 10.1002/14651858.CD000326. [DOI] [PubMed] [Google Scholar]
- 350.Wood MR, Bolyard D. Making education count: The nurse's role in asthma education using a medical home model of care. J Pediatr Nurs. 2011;26:552–8. doi: 10.1016/j.pedn.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 351.Nurmatov U, Devereux G, Sheikh A. Nutrients and foods for the primary prevention of asthma and allergy: Systematic review and meta-analysis. J Allergy Clin Immunol. 2011;127:724–33.e1. doi: 10.1016/j.jaci.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 352.Pelucchi C, Chatenoud L, Turati F, Galeone C, Moja L, Bach JF, et al. Probiotics supplementation during pregnancy or infancy for the prevention of atopic dermatitis: A meta-analysis. Epidemiology. 2012;23:402–14. doi: 10.1097/EDE.0b013e31824d5da2. [DOI] [PubMed] [Google Scholar]
- 353.Kozyrskyj AL, Pawlowski AN. Maternal distress and childhood wheeze: Mechanisms and context. Am J Respir Crit Care Med. 2013;187:1160–2. doi: 10.1164/rccm.201304-0649ED. [DOI] [PubMed] [Google Scholar]
- 354.Cabana MD, McKean M, Caughey AB, Fong L, Lynch S, Wong A, et al. Early probiotic supplementation for eczema and asthma prevention: A randomized controlled trial. Pediatrics. 2017;140:e20163000. doi: 10.1542/peds.2016-3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Kneale D, Harris K, McDonald VM, Thomas J, Grigg J. Effectiveness of school-based self-management interventions for asthma among children and adolescents: Findings from a Cochrane systematic review and meta-analysis. Thorax. 2019;74:432–8. doi: 10.1136/thoraxjnl-2018-211909. [DOI] [PubMed] [Google Scholar]
- 356.Panel SIfA. Advisory from the Saudi Initiative for Asthma during COVID-19 Riyadh. Saudi Arabia: Saudi Thoracic Society; 2020. [[Last cited on 2023 Aug 29]]. Available from: https://saudithoracicsociety.org/wp-content/uploads/2020/04/Advisory-from-the-Saudi-Initiative-for-Asthma.pdf . [Google Scholar]
- 357.Al-Shamrani A, Al-Harbi AS, Alhaider SA, Alharbi S, Al-Harbi NS, Alanazi A, et al. Approach to childhood asthma in the era of COVID-19: The official statement endorsed by the Saudi Pediatric Pulmonology Association (SPPA) Int J Pediatr Adolesc Med. 2020;7:103–6. doi: 10.1016/j.ijpam.2020.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Bonner S, Matte T, Rubin M, Fagan JK, Ahern J, Evans D. Oral beta2-agonist use by preschool children with asthma in East and Central Harlem, New York. J Asthma. 2006;43:31–5. doi: 10.1080/02770900500446989. [DOI] [PubMed] [Google Scholar]
- 359.Lazarus SC, Boushey HA, Fahy JV, Chinchilli VM, Lemanske RF, Jr, Sorkness CA, et al. Long-acting beta2-agonist monotherapy versus continued therapy with inhaled corticosteroids in patients with persistent asthma: A randomized controlled trial. JAMA. 2001;285:2583–93. doi: 10.1001/jama.285.20.2583. [DOI] [PubMed] [Google Scholar]
- 360.Bisgaard H, Allen DB, Milanowski J, Kalev I, Willits L, Davies P. Twelve-month safety and efficacy of inhaled fluticasone propionate in children aged 1 to 3 years with recurrent wheezing. Pediatrics. 2004;113:e87–94. doi: 10.1542/peds.113.2.e87. [DOI] [PubMed] [Google Scholar]
- 361.Leflein JG, Szefler SJ, Murphy KR, Fitzpatrick S, Cruz-Rivera M, Miller CJ, et al. Nebulized budesonide inhalation suspension compared with cromolyn sodium nebulizer solution for asthma in young children: Results of a randomized outcomes trial. Pediatrics. 2002;109:866–72. doi: 10.1542/peds.109.5.866. [DOI] [PubMed] [Google Scholar]
- 362.Goldsobel A. Association of inhaled corticosteroids and long-acting β-agonists as controller and quick relief therapy with exacerbations and symptom control in persistent asthma: A systematic review and meta-analysis. Pediatrics. 2018;142(Suppl 4):S266–7. doi: 10.1001/jama.2018.2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Sobieraj DM, Weeda ER, Nguyen E, Coleman CI, White CM, Lazarus SC, et al. Association of inhaled corticosteroids and long-acting β-agonists as controller and quick relief therapy with exacerbations and symptom control in persistent asthma: A Systematic review and meta-analysis. JAMA. 2018;319:1485–96. doi: 10.1001/jama.2018.2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Cardet JC, Papi A, Reddel HK. “As-needed”inhaled corticosteroids for patients with asthma. J Allergy Clin Immunol Pract. 2023;11:726–34. doi: 10.1016/j.jaip.2023.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Dufour V, Millon L, Faucher JF, Bard E, Robinet E, Piarroux R, et al. Effects of a short-course of amoxicillin/clavulanic acid on systemic and mucosal immunity in healthy adult humans. Int Immunopharmacol. 2005;5:917–28. doi: 10.1016/j.intimp.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 366.Adams NP, Bestall JB, Jones PW. Inhaled beclomethasone versus placebo for chronic asthma. Cochrane Database Syst Rev. 2000:CD002738. doi: 10.1002/14651858.CD002738. [DOI] [PubMed] [Google Scholar]
- 367.Zhang L, Pruteanu AI, Prietsch SO, Chauhan BF, Ducharme FM. Cochrane in context: Inhaled corticosteroids in children with persistent asthma: Effects on growth and dose-response effects on growth. Evid Based Child Health. 2014;9:1047–51. doi: 10.1002/ebch.1984. [DOI] [PubMed] [Google Scholar]
- 368.Pruteanu AI, Chauhan BF, Zhang L, Prietsch SO, Ducharme FM. Inhaled corticosteroids in children with persistent asthma: Dose-response effects on growth. Evid Based Child Health. 2014;9:931–1046. doi: 10.1002/ebch.1989. [DOI] [PubMed] [Google Scholar]
- 369.Benard B, Bastien V, Vinet B, Yang R, Krajinovic M, Ducharme FM. Neuropsychiatric adverse drug reactions in children initiated on montelukast in real-life practice. Eur Respir J. 2017;50:1–11. doi: 10.1183/13993003.00148-2017. [DOI] [PubMed] [Google Scholar]
- 370.Urdaneta E. Neuropsychiatric adverse drug reactions in children initiated on montelukast in real-life practice. Eur Respir J. 2017;50:1–10. doi: 10.1183/13993003.01984-2017. [DOI] [PubMed] [Google Scholar]
- 371.Glockler-Lauf SD, Finkelstein Y, Zhu J, Feldman LY, To T. Montelukast and neuropsychiatric events in children with asthma: A nested case-control study. J Pediatr. 2019;209:176–82.e4. doi: 10.1016/j.jpeds.2019.02.009. [DOI] [PubMed] [Google Scholar]
- 372.Deerojanawong J, Manuyakorn W, Prapphal N, Harnruthakorn C, Sritippayawan S, Samransamruajkit R. Randomized controlled trial of salbutamol aerosol therapy via metered dose inhaler-spacer versus. Jet nebulizer in young children with wheezing. Pediatr Pulmonol. 2005;39:466–72. doi: 10.1002/ppul.20204. [DOI] [PubMed] [Google Scholar]
- 373.Drblik S, Lapierre G, Thivierge R, Turgeon J, Gaudreault P, Cummins-McManus B, et al. Comparative efficacy of terbutaline sulphate delivered by turbuhaler dry powder inhaler or pressurised metered dose inhaler with nebuhaler spacer in children during an acute asthmatic episode. Arch Dis Child. 2003;88:319–23. doi: 10.1136/adc.88.4.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Vincken W, Levy ML, Scullion J, Usmani OS, Dekhuijzen PN, Corrigan CJ. Spacer devices for inhaled therapy: Why use them, and how? ERJ Open Res. 2018;4:1–9. doi: 10.1183/23120541.00065-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Castro-Rodriguez JA, Rodrigo GJ. beta-agonists through metered-dose inhaler with valved holding chamber versus nebulizer for acute exacerbation of wheezing or asthma in children under 5 years of age: A systematic review with meta-analysis. J Pediatr. 2004;145:172–7. doi: 10.1016/j.jpeds.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 376.Halwani R, Vazquez-Tello A, Horanieh N, Dulgom S, Al-Aseri Z, Al-Khamis N, et al. Risk factors hindering asthma symptom control in Saudi children and adolescents. Pediatr Int. 2017;59:661–8. doi: 10.1111/ped.13268. [DOI] [PubMed] [Google Scholar]
- 377.Goksör E, Amark M, Alm B, Gustafsson PM, Wennergren G. Asthma symptoms in early childhood –What happens then? Acta Paediatr. 2006;95:471–8. doi: 10.1080/08035250500499440. [DOI] [PubMed] [Google Scholar]
- 378.Liu AH, Zeiger RS, Sorkness CA, Ostrom NK, Chipps BE, Rosa K, et al. The childhood asthma control test: Retrospective determination and clinical validation of a cut point to identify children with very poorly controlled asthma. J Allergy Clin Immunol. 2010;126:267–73.e1. doi: 10.1016/j.jaci.2010.05.031. [DOI] [PubMed] [Google Scholar]
- 379.Simons E. Use of beclomethasone dipropionate as rescue treatment for children with mild persistent asthma (TREXA): A randomized, double-blind, placebo-controlled trial. Pediatrics. 2011;128(Suppl 3):S131–2. doi: 10.1016/S0140-6736(10)62145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Jackson DJ, Bacharier LB. Inhaled corticosteroids for the prevention of asthma exacerbations. Ann Allergy Asthma Immunol. 2021;127:524–9. doi: 10.1016/j.anai.2021.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Rodríguez-Martínez CE, Sossa-Briceño MP, Buendia JA. As-needed use of short-Acting β(2)-agonists alone versus as-needed use of short-acting β(2)-agonists plus inhaled corticosteroids in pediatric patients with mild intermittent (step 1) asthma: A cost-effectiveness analysis. J Allergy Clin Immunol Pract. 2022;10:1562–8. doi: 10.1016/j.jaip.2022.02.022. [DOI] [PubMed] [Google Scholar]
- 382.Rodriguez-Martinez CE, Sossa-Briceño MP, Garcia-Marcos L. Use of inhaled corticosteroids on an intermittent or as-needed basis in pediatric asthma: A systematic review of the literature. J Asthma. 2022;59:2189–200. doi: 10.1080/02770903.2021.2008430. [DOI] [PubMed] [Google Scholar]
- 383.Ducharme FM, Lemire C, Noya FJ, Davis GM, Alos N, Leblond H, et al. Preemptive use of high-dose fluticasone for virus-induced wheezing in young children. N Engl J Med. 2009;360:339–53. doi: 10.1056/NEJMoa0808907. [DOI] [PubMed] [Google Scholar]
- 384.Bisgaard H, Hermansen MN, Loland L, Halkjaer LB, Buchvald F. Intermittent inhaled corticosteroids in infants with episodic wheezing. N Engl J Med. 2006;354:1998–2005. doi: 10.1056/NEJMoa054692. [DOI] [PubMed] [Google Scholar]
- 385.Papi A, Nicolini G, Baraldi E, Boner AL, Cutrera R, Rossi GA, et al. Regular versus prn nebulized treatment in wheeze preschool children. Allergy. 2009;64:1463–71. doi: 10.1111/j.1398-9995.2009.02134.x. [DOI] [PubMed] [Google Scholar]
- 386.Fitzpatrick AM, Jackson DJ, Mauger DT, Boehmer SJ, Phipatanakul W, Sheehan WJ, et al. Individualized therapy for persistent asthma in young children. J Allergy Clin Immunol. 2016;138:1608–18.e12. doi: 10.1016/j.jaci.2016.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Guilbert TW, Morgan WJ, Zeiger RS, Mauger DT, Boehmer SJ, Szefler SJ, et al. Long-term inhaled corticosteroids in preschool children at high risk for asthma. N Engl J Med. 2006;354:1985–97. doi: 10.1056/NEJMoa051378. [DOI] [PubMed] [Google Scholar]
- 388.Nielsen KG, Bisgaard H. The effect of inhaled budesonide on symptoms, lung function, and cold air and methacholine responsiveness in 2- to 5-year-old asthmatic children. Am J Respir Crit Care Med. 2000;162:1500–6. doi: 10.1164/ajrccm.162.4.2002019. [DOI] [PubMed] [Google Scholar]
- 389.Szefler SJ, Baker JW, Uryniak T, Goldman M, Silkoff PE. Comparative study of budesonide inhalation suspension and montelukast in young children with mild persistent asthma. J Allergy Clin Immunol. 2007;120:1043–50. doi: 10.1016/j.jaci.2007.08.063. [DOI] [PubMed] [Google Scholar]
- 390.Knorr B, Franchi LM, Bisgaard H, Vermeulen JH, LeSouef P, Santanello N, et al. Montelukast, a leukotriene receptor antagonist, for the treatment of persistent asthma in children aged 2 to 5 years. Pediatrics. 2001;108:E48. doi: 10.1542/peds.108.3.e48. [DOI] [PubMed] [Google Scholar]
- 391.Bisgaard H. Leukotriene modifiers in pediatric asthma management. Pediatrics. 2001;107:381–90. doi: 10.1542/peds.107.2.381. [DOI] [PubMed] [Google Scholar]
- 392.Ducharme FM, Hicks GC. Anti-leukotriene agents compared to inhaled corticosteroids in the management of recurrent and/or chronic asthma in adults and children. Cochrane Database Syst Rev. 2002:CD002314. doi: 10.1002/14651858.CD002314. [DOI] [PubMed] [Google Scholar]
- 393.O’Byrne PM, Barnes PJ, Rodriguez-Roisin R, Runnerstrom E, Sandstrom T, Svensson K, et al. Low dose inhaled budesonide and formoterol in mild persistent asthma: The OPTIMA randomized trial. Am J Respir Crit Care Med. 2001;164:1392–7. doi: 10.1164/ajrccm.164.8.2104102. [DOI] [PubMed] [Google Scholar]
- 394.Shrewsbury S, Pyke S, Britton M. Meta-analysis of increased dose of inhaled steroid or addition of salmeterol in symptomatic asthma (MIASMA) BMJ. 2000;320:1368–73. doi: 10.1136/bmj.320.7246.1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Matz J, Emmett A, Rickard K, Kalberg C. Addition of salmeterol to low-dose fluticasone versus higher-dose fluticasone: An analysis of asthma exacerbations. J Allergy Clin Immunol. 2001;107:783–9. doi: 10.1067/mai.2001.114709. [DOI] [PubMed] [Google Scholar]
- 396.Vaessen-Verberne AA, van den Berg NJ, van Nierop JC, Brackel HJ, Gerrits GP, Hop WC, et al. Combination therapy salmeterol/fluticasone versus doubling dose of fluticasone in children with asthma. Am J Respir Crit Care Med. 2010;182:1221–7. doi: 10.1164/rccm.201002-0193OC. [DOI] [PubMed] [Google Scholar]
- 397.Verberne AA, Frost C, Duiverman EJ, Grol MH, Kerrebijn KF. Addition of salmeterol versus doubling the dose of beclomethasone in children with asthma. The Dutch Asthma Study Group. Am J Respir Crit Care Med. 1998;158:213–9. doi: 10.1164/ajrccm.158.1.9706048. [DOI] [PubMed] [Google Scholar]
- 398.Ducharme FM, Ni Chroinin M, Greenstone I, Lasserson TJ. Addition of long-acting beta2-agonists to inhaled corticosteroids versus same dose inhaled corticosteroids for chronic asthma in adults and children. Cochrane Database Syst Rev. 2010:CD005535. doi: 10.1002/14651858.CD005535.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Ducharme FM, Ni Chroinin M, Greenstone I, Lasserson TJ. Addition of long-acting beta2-agonists to inhaled steroids versus higher dose inhaled steroids in adults and children with persistent asthma. Cochrane Database Syst Rev. 2010:CD005533. doi: 10.1002/14651858.CD005533.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Knorr B, Matz J, Bernstein JA, Nguyen H, Seidenberg BC, Reiss TF, et al. Montelukast for chronic asthma in 6- to 14-year-old children: A randomized, double-blind trial. Pediatric montelukast study group. JAMA. 1998;279:1181–6. doi: 10.1001/jama.279.15.1181. [DOI] [PubMed] [Google Scholar]
- 401.Zhou XJ, Qin Z, Lu J, Hong JG. Efficacy and safety of salmeterol/fluticasone compared with montelukast alone (or add-on therapy to fluticasone) in the treatment of bronchial asthma in children and adolescents: A systematic review and meta-analysis. Chin Med J (Engl) 2021;134:2954–61. doi: 10.1097/CM9.0000000000001853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Dixon EG, King C, Lilley A, Sinha IP, Hawcutt DB. Deprescribing montelukast in children with asthma: A systematic review. BMJ Open. 2022;12:e053112. doi: 10.1136/bmjopen-2021-053112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Harding T, Harding A. Multiple short courses of corticosteroids in children. Aust J Gen Pract. 2021;50:151–6. doi: 10.31128/AJGP-11-19-5164. [DOI] [PubMed] [Google Scholar]
- 404.Rodriguez-Martinez CE, Sossa-Briceño MP, Castro-Rodriguez JA. Dexamethasone or prednisolone for asthma exacerbations in children: A cost-effectiveness analysis. Pediatr Pulmonol. 2020;55:1617–23. doi: 10.1002/ppul.24817. [DOI] [PubMed] [Google Scholar]
- 405.Chauhan BF, Ducharme FM. Addition to inhaled corticosteroids of long-acting beta2-agonists versus anti-leukotrienes for chronic asthma. Cochrane Database Syst Rev. 2014;2014:CD003137. doi: 10.1002/14651858.CD003137.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Ni Chroinin M, Greenstone I, Lasserson TJ, Ducharme FM. Addition of inhaled long-acting beta2-agonists to inhaled steroids as first line therapy for persistent asthma in steroid-naive adults and children. Cochrane Database Syst Rev. 2009:CD005307. doi: 10.1002/14651858.CD005307.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Ni Chroinin M, Lasserson TJ, Greenstone I, Ducharme FM. Addition of long-acting beta-agonists to inhaled corticosteroids for chronic asthma in children. Cochrane Database Syst Rev. 2009:CD007949. doi: 10.1002/14651858.CD007949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Santamaria F, Ziello C, Lorello P, Bouchè C, Borrelli M. Update on long-acting anticholinergics in children and adolescents with difficult and severe asthma. Front Pediatr. 2022;10:896865. doi: 10.3389/fped.2022.896865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Aierken A, Yu Su Fu BW, Xu P. Tiotropium as an add-on treatment to inhaled corticosteroids in children with severe and mild symptomatic asthma: Multi-center observational study for efficacy and safety analysis. Exp Ther Med. 2022;24:577. doi: 10.3892/etm.2022.11514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Antonio Buendía J, Patiño DG. Tiotropium for children and adolescents with severe asthma. J Asthma. 2023;60:1009–15. doi: 10.1080/02770903.2022.2120403. [DOI] [PubMed] [Google Scholar]
- 411.Kulus M, Hébert J, Garcia E, Fowler Taylor A, Fernandez Vidaurre C, Blogg M. Omalizumab in children with inadequately controlled severe allergic (IgE-mediated) asthma. Curr Med Res Opin. 2010;26:1285–93. doi: 10.1185/03007991003771338. [DOI] [PubMed] [Google Scholar]
- 412.Lanier B, Bridges T, Kulus M, Taylor AF, Berhane I, Vidaurre CF. Omalizumab for the treatment of exacerbations in children with inadequately controlled allergic (IgE-mediated) asthma. J Allergy Clin Immunol. 2009;124:1210–6. doi: 10.1016/j.jaci.2009.09.021. [DOI] [PubMed] [Google Scholar]
- 413.Deschildre A, Marguet C, Langlois C, Pin I, Rittié JL, Derelle J, et al. Real-life long-term omalizumab therapy in children with severe allergic asthma. Eur Respir J. 2015;46:856–9. doi: 10.1183/09031936.00008115. [DOI] [PubMed] [Google Scholar]
- 414.Licari A, Marseglia A, Caimmi S, Castagnoli R, Foiadelli T, Barberi S, et al. Omalizumab in children. Paediatr Drugs. 2014;16:491–502. doi: 10.1007/s40272-014-0107-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Gupta A, Pouliquen I, Austin D, Price RG, Kempsford R, Steinfeld J, et al. Subcutaneous mepolizumab in children aged 6 to 11 years with severe eosinophilic asthma. Pediatr Pulmonol. 2019;54:1957–67. doi: 10.1002/ppul.24508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Gupta A, Ikeda M, Geng B, Azmi J, Price RG, Bradford ES, et al. Long-term safety and pharmacodynamics of mepolizumab in children with severe asthma with an eosinophilic phenotype. J Allergy Clin Immunol. 2019;144:1336–42.e7. doi: 10.1016/j.jaci.2019.08.005. [DOI] [PubMed] [Google Scholar]
- 417.Licari A, Castagnoli R, Marseglia A, Olivero F, Votto M, Ciprandi G, et al. Dupilumab to treat type 2 inflammatory diseases in children and adolescents. Paediatr Drugs. 2020;22:295–310. doi: 10.1007/s40272-020-00387-2. [DOI] [PubMed] [Google Scholar]
- 418.Bacharier LB, Maspero JF, Katelaris CH, Fiocchi AG, Gagnon R, de Mir I, et al. Dupilumab in children with uncontrolled moderate-to-severe asthma. N Engl J Med. 2021;385:2230–40. doi: 10.1056/NEJMoa2106567. [DOI] [PubMed] [Google Scholar]
- 419.Jackson DJ, Bacharier LB, Phipatanakul W, Sher L, Domingo C, Papadopoulos N, et al. Dupilumab pharmacokinetics and effect on type 2 biomarkers in children with moderate-to-severe asthma. Ann Allergy Asthma Immunol. 2023;131:44–51.e4. doi: 10.1016/j.anai.2023.03.014. [DOI] [PubMed] [Google Scholar]
- 420.Castro M, Corren J, Pavord ID, Maspero J, Wenzel S, Rabe KF, et al. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. N Engl J Med. 2018;378:2486–96. doi: 10.1056/NEJMoa1804092. [DOI] [PubMed] [Google Scholar]
- 421.Zeiger RS, Mellon M, Chipps B, Murphy KR, Schatz M, Kosinski M, et al. Test for respiratory and asthma control in kids (TRACK): Clinically meaningful changes in score. J Allergy Clin Immunol. 2011;128:983–8. doi: 10.1016/j.jaci.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 422.O’Byrne P, Fabbri LM, Pavord ID, Papi A, Petruzzelli S, Lange P. Asthma progression and mortality: The role of inhaled corticosteroids. Eur Respir J. 2019;54:1–11. doi: 10.1183/13993003.00491-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Zeiger RS, Mauger D, Bacharier LB, Guilbert TW, Martinez FD, Lemanske RF, Jr, et al. Daily or intermittent budesonide in preschool children with recurrent wheezing. N Engl J Med. 2011;365:1990–2001. doi: 10.1056/NEJMoa1104647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Chong JK, Chauhan BF. Addition of antileukotriene agents to inhaled corticosteroids in children with persistent asthma. Paediatr Child Health. 2014;19:473–4. doi: 10.1093/pch/19.9.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Ducharme FM. Anti-leukotrienes as add-on therapy to inhaled glucocorticoids in patients with asthma: Systematic review of current evidence. BMJ. 2002;324:1545. doi: 10.1136/bmj.324.7353.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Simons FE, Villa JR, Lee BW, Teper AM, Lyttle B, Aristizabal G, et al. Montelukast added to budesonide in children with persistent asthma: A randomized, double-blind, crossover study. J Pediatr. 2001;138:694–8. doi: 10.1067/mpd.2001.112899. [DOI] [PubMed] [Google Scholar]
- 427.Swern AS, Tozzi CA, Knorr B, Bisgaard H. Predicting an asthma exacerbation in children 2 to 5 years of age. Ann Allergy Asthma Immunol. 2008;101:626–30. doi: 10.1016/S1081-1206(10)60226-8. [DOI] [PubMed] [Google Scholar]
- 428.Ducharme FM, Chalut D, Plotnick L, Savdie C, Kudirka D, Zhang X, et al. The pediatric respiratory assessment measure: A valid clinical score for assessing acute asthma severity from toddlers to teenagers. J Pediatr. 2008;152:476–80.e1. doi: 10.1016/j.jpeds.2007.08.034. [DOI] [PubMed] [Google Scholar]
- 429.Parkin PC, Macarthur C, Saunders NR, Diamond SA, Winders PM. Development of a clinical asthma score for use in hospitalized children between 1 and 5 years of age. J Clin Epidemiol. 1996;49:821–5. doi: 10.1016/0895-4356(96)00027-3. [DOI] [PubMed] [Google Scholar]
- 430.Chalut DS, Ducharme FM, Davis GM. The preschool respiratory assessment measure (PRAM): A responsive index of acute asthma severity. J Pediatr. 2000;137:762–8. doi: 10.1067/mpd.2000.110121. [DOI] [PubMed] [Google Scholar]
- 431.Al-Muhsen S, Horanieh N, Dulgom S, Aseri ZA, Vazquez-Tello A, Halwani R, et al. Poor asthma education and medication compliance are associated with increased emergency department visits by asthmatic children. Ann Thorac Med. 2015;10:123–31. doi: 10.4103/1817-1737.150735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Birken CS, Parkin PC, Macarthur C. Asthma severity scores for preschoolers displayed weaknesses in reliability, validity, and responsiveness. J Clin Epidemiol. 2004;57:1177–81. doi: 10.1016/j.jclinepi.2004.02.016. [DOI] [PubMed] [Google Scholar]
- 433.Alnaji F, Zemek R, Barrowman N, Plint A. PRAM score as predictor of pediatric asthma hospitalization. Acad Emerg Med. 2014;21:872–8. doi: 10.1111/acem.12422. [DOI] [PubMed] [Google Scholar]
- 434.Jarvis SW, Kovacs C, Badriyah T, Briggs J, Mohammed MA, Meredith P, et al. Development and validation of a decision tree early warning score based on routine laboratory test results for the discrimination of hospital mortality in emergency medical admissions. Resuscitation. 2013;84:1494–9. doi: 10.1016/j.resuscitation.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 435.Johnson KB, Blaisdell CJ, Walker A, Eggleston P. Effectiveness of a clinical pathway for inpatient asthma management. Pediatrics. 2000;106:1006–12. [PubMed] [Google Scholar]
- 436.Chacko J, King C, Harkness D, Messahel S, Grice J, Roe J, et al. Pediatric acute asthma scoring systems: A systematic review and survey of UK practice. J Am Coll Emerg Physicians Open. 2020;1:1000–8. doi: 10.1002/emp2.12083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Banasiak NC. Childhood asthma practice guideline part three: Update of the 2007 national guidelines for the diagnosis and treatment of asthma. The national asthma education and prevention program. J Pediatr Health Care. 2009;23:59–61. doi: 10.1016/j.pedhc.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 438.Al-Shamrani A, Al-Harbi AS, Bagais K, Alenazi A, Alqwaiee M. Management of asthma exacerbation in the emergency departments. Int J Pediatr Adolesc Med. 2019;6:61–7. doi: 10.1016/j.ijpam.2019.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Ortiz-Alvarez O, Mikrogianakis A. Canadian Paediatric Society Acute Care Committee. Managing the paediatric patient with an acute asthma exacerbation. Paediatr Child Health. 2012;17:251–62. doi: 10.1093/pch/17.5.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Norton SP, Pusic MV, Taha F, Heathcote S, Carleton BC. Effect of a clinical pathway on the hospitalisation rates of children with asthma: A prospective study. Arch Dis Child. 2007;92:60–6. doi: 10.1136/adc.2006.097287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Lougheed MD, Olajos-Clow JG. Asthma care pathways in the emergency department. Curr Opin Allergy Clin Immunol. 2010;10:181–7. doi: 10.1097/ACI.0b013e328339731d. [DOI] [PubMed] [Google Scholar]
- 442.Browne GJ, Giles H, McCaskill ME, Fasher BJ, Lam LT. The benefits of using clinical pathways for managing acute paediatric illness in an emergency department. J Qual Clin Pract. 2001;21:50–5. doi: 10.1046/j.1440-1762.2001.00405.x. [DOI] [PubMed] [Google Scholar]
- 443.Graham VA, Milton AF, Knowles GK, Davies RJ. Routine antibiotics in hospital management of acute asthma. Lancet. 1982;1:418–20. doi: 10.1016/s0140-6736(82)91619-1. [DOI] [PubMed] [Google Scholar]
- 444.Everard ML, Bara A, Kurian M, Elliott TM, Ducharme F, Mayowe V. Anticholinergic drugs for wheeze in children under the age of two years. Cochrane Database Syst Rev. 2005;2005:CD001279. doi: 10.1002/14651858.CD001279.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Griffiths B, Ducharme FM. Combined inhaled anticholinergics and short-acting beta2-agonists for initial treatment of acute asthma in children. Cochrane Database Syst Rev. 2013:CD000060. doi: 10.1002/14651858.CD000060.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Chinese College of Emergency Physicians (CCEP), Emergency Committee of PLA, Beijing Society for Emergency Medicine, Chinese Emergency Medicine. Expert consensus on nebulization therapy in pre-hospital and in-hospital emergency care. Ann Transl Med. 2019;7:487. doi: 10.21037/atm.2019.09.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Wei J, Lu Y, Han F, Zhang J, Liu L, Chen Q. Oral dexamethasone versus oral prednisone for children with acute asthma exacerbations: A systematic review and meta-analysis. Front Pediatr. 2019;7:503. doi: 10.3389/fped.2019.00503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Iramain R, Castro-Rodriguez JA, Jara A, Cardozo L, Bogado N, Morinigo R, et al. Salbutamol and ipratropium by inhaler is superior to nebulizer in children with severe acute asthma exacerbation: Randomized clinical trial. Pediatr Pulmonol. 2019;54:372–7. doi: 10.1002/ppul.24244. [DOI] [PubMed] [Google Scholar]
- 449.Griffiths B, Ducharme FM. Combined inhaled anticholinergics and short-acting beta2-agonists for initial treatment of acute asthma in children. Paediatr Respir Rev. 2013;14:234–5. doi: 10.1016/j.prrv.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 450.Rowe BH, Spooner CH, Ducharme FM, Bretzlaff JA, Bota GW. Corticosteroids for preventing relapse following acute exacerbations of asthma. Cochrane Database Syst Rev. 2001:CD000195. doi: 10.1002/14651858.CD000195. [DOI] [PubMed] [Google Scholar]
- 451.Cheuk DK, Chau TC, Lee SL. A meta-analysis on intravenous magnesium sulphate for treating acute asthma. Arch Dis Child. 2005;90:74–7. doi: 10.1136/adc.2004.050005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Powell CV, Kolamunnage-Dona R, Lowe J, Boland A, Petrou S, Doull I, et al. MAGNEsium trial in children (MAGNETIC): A randomised, placebo-controlled trial and economic evaluation of nebulised magnesium sulphate in acute severe asthma in children. Health Technol Assess. 2013;17:i–216. doi: 10.3310/hta17450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Powell C, Kolamunnage-Dona R, Lowe J, Boland A, Petrou S, Doull I, et al. Magnesium sulphate in acute severe asthma in children (MAGNETIC): A randomised, placebo-controlled trial. Lancet Respir Med. 2013;1:301–8. doi: 10.1016/S2213-2600(13)70037-7. [DOI] [PubMed] [Google Scholar]
- 454.Camargo CA, Jr, Spooner CH, Rowe BH. Continuous versus intermittent beta-agonists in the treatment of acute asthma. Cochrane Database Syst Rev. 2003;2003:CD001115. doi: 10.1002/14651858.CD001115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Scarfone RJ, Fuchs SM, Nager AL, Shane SA. Controlled trial of oral prednisone in the emergency department treatment of children with acute asthma. Pediatrics. 1993;92:513–8. [PubMed] [Google Scholar]
- 456.Creticos PS. Treatment options for initial maintenance therapy of persistent asthma: A review of inhaled corticosteroids and leukotriene receptor antagonists. Drugs. 2003;63(Suppl 2):1–20. doi: 10.2165/00003495-200363002-00002. [DOI] [PubMed] [Google Scholar]
- 457.McFadden ER., Jr Critical appraisal of the therapy of asthma –An idea whose time has come. Am Rev Respir Dis. 1986;133:723–4. doi: 10.1164/arrd.1986.133.5.723. [DOI] [PubMed] [Google Scholar]
- 458.Rodrigo GJ, Rodrigo C. Continuous versus intermittent beta-agonists in the treatment of acute adult asthma: A systematic review with meta-analysis. Chest. 2002;122:160–5. doi: 10.1378/chest.122.1.160. [DOI] [PubMed] [Google Scholar]
- 459.Travers AH, Rowe BH, Barker S, Jones A, Camargo CA., Jr The effectiveness of IV beta-agonists in treating patients with acute asthma in the emergency department: A meta-analysis. Chest. 2002;122:1200–7. doi: 10.1378/chest.122.4.1200. [DOI] [PubMed] [Google Scholar]
- 460.Rodrigo GJ, Rodrigo C. First-line therapy for adult patients with acute asthma receiving a multiple-dose protocol of ipratropium bromide plus albuterol in the emergency department. Am J Respir Crit Care Med. 2000;161:1862–8. doi: 10.1164/ajrccm.161.6.9908115. [DOI] [PubMed] [Google Scholar]
- 461.Rodrigo GJ, Rodrigo C. The role of anticholinergics in acute asthma treatment: An evidence-based evaluation. Chest. 2002;121:1977–87. doi: 10.1378/chest.121.6.1977. [DOI] [PubMed] [Google Scholar]
- 462.Vézina K, Chauhan BF, Ducharme FM. Inhaled anticholinergics and short-acting beta(2)-agonists versus short-acting beta2-agonists alone for children with acute asthma in hospital. Cochrane Database Syst Rev. 2014:CD010283. doi: 10.1002/14651858.CD010283.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Silverman RA, Osborn H, Runge J, Gallagher EJ, Chiang W, Feldman J, et al. IV magnesium sulfate in the treatment of acute severe asthma: A multicenter randomized controlled trial. Chest. 2002;122:489–97. doi: 10.1378/chest.122.2.489. [DOI] [PubMed] [Google Scholar]
- 464.Lemanske RF, Jr, Sorkness CA, Mauger EA, Lazarus SC, Boushey HA, Fahy JV, et al. Inhaled corticosteroid reduction and elimination in patients with persistent asthma receiving salmeterol: A randomized controlled trial. JAMA. 2001;285:2594–603. doi: 10.1001/jama.285.20.2594. [DOI] [PubMed] [Google Scholar]
- 465.Masoli M, Weatherall M, Holt S, Beasley R. Moderate dose inhaled corticosteroids plus salmeterol versus higher doses of inhaled corticosteroids in symptomatic asthma. Thorax. 2005;60:730–4. doi: 10.1136/thx.2004.039180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466.Fouda MA, Al-Kassimi FA. Budesonide and fluticasone and adrenal suppression. Ann Thorac Med. 2012;7:253. doi: 10.4103/1817-1737.102188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Pruteanu AI, Chauhan BF, Zhang L, Prietsch SO, Ducharme FM. Inhaled corticosteroids in children with persistent asthma: Dose-response effects on growth. Cochrane Database Syst Rev. 2014;2014:CD009878. doi: 10.1002/14651858.CD009878.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Raissy HH, Blake K. Does use of inhaled corticosteroid for management of asthma in children make them shorter adults? Pediatr Allergy Immunol Pulmonol. 2013;26:99–101. doi: 10.1089/ped.2013.0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469.Hossny E, Rosario N, Lee BW, Singh M, El-Ghoneimy D, Soh JY, et al. The use of inhaled corticosteroids in pediatric asthma: Update. World Allergy Organ J. 2016;9:26. doi: 10.1186/s40413-016-0117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 470.Hoover RM, Erramouspe J, Bell EA, Cleveland KW. Effect of inhaled corticosteroids on long-term growth in pediatric patients with asthma and allergic rhinitis. Ann Pharmacother. 2013;47:1175–81. doi: 10.1177/1060028013503125. [DOI] [PubMed] [Google Scholar]
- 471.Kelly HW, Van Natta ML, Covar RA, Tonascia J, Green RP, Strunk RC, et al. Effect of long-term corticosteroid use on bone mineral density in children: A prospective longitudinal assessment in the childhood asthma management program (CAMP) study. Pediatrics. 2008;122:e53–61. doi: 10.1542/peds.2007-3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472.Sidoroff VH, Ylinen MK, Kröger LM, Kröger HP, Korppi MO. Inhaled corticosteroids and bone mineral density at school age: A follow-up study after early childhood wheezing. Pediatr Pulmonol. 2015;50:1–7. doi: 10.1002/ppul.22968. [DOI] [PubMed] [Google Scholar]
- 473.Stelmach I, Olszowiec-Chlebna M, Jerzynska J, Grzelewski T, Stelmach W, Majak P. Inhaled corticosteroids may have a beneficial effect on bone metabolism in newly diagnosed asthmatic children. Pulm Pharmacol Ther. 2011;24:414–20. doi: 10.1016/j.pupt.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 474.Altintas DU, Karakoc GB, Can S, Yilmaz M, Kendirli SG. The effects of long term use of inhaled corticosteroids on linear growth, adrenal function and bone mineral density in children. Allergol Immunopathol (Madr) 2005;33:204–9. doi: 10.1157/13077744. [DOI] [PubMed] [Google Scholar]
- 475.Bjermer L, Diamant Z. The use of leukotriene receptor antagonists (LTRAs) as complementary therapy in asthma. Monaldi Arch Chest Dis. 2002;57:76–83. [PubMed] [Google Scholar]
- 476.Idrees MM, Al Moamary MS. Blocking leukotrienes optimize asthma control: The BLOC survey. Ann Thorac Med. 2007;2:99–102. doi: 10.4103/1817-1737.33696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477.Bisgaard H. Study Group on Montelukast and Respiratory Syncytial Virus. A randomized trial of montelukast in respiratory syncytial virus postbronchiolitis. Am J Respir Crit Care Med. 2003;167:379–83. doi: 10.1164/rccm.200207-747OC. [DOI] [PubMed] [Google Scholar]
- 478.Straub DA, Minocchieri S, Moeller A, Hamacher J, Wildhaber JH. The effect of montelukast on exhaled nitric oxide and lung function in asthmatic children 2 to 5 years old. Chest. 2005;127:509–14. doi: 10.1378/chest.127.2.509. [DOI] [PubMed] [Google Scholar]
- 479.Straub DA, Moeller A, Minocchieri S, Hamacher J, Sennhauser FH, Hall GL, et al. The effect of montelukast on lung function and exhaled nitric oxide in infants with early childhood asthma. Eur Respir J. 2005;25:289–94. doi: 10.1183/09031936.05.00031904. [DOI] [PubMed] [Google Scholar]
- 480.Bisgaard H, Zielen S, Garcia-Garcia ML, Johnston SL, Gilles L, Menten J, et al. Montelukast reduces asthma exacerbations in 2- to 5-year-old children with intermittent asthma. Am J Respir Crit Care Med. 2005;171:315–22. doi: 10.1164/rccm.200407-894OC. [DOI] [PubMed] [Google Scholar]
- 481.Lötvall J, Bateman ED, Bleecker ER, Busse WW, Woodcock A, Follows R, et al. 24-h duration of the novel LABA vilanterol trifenatate in asthma patients treated with inhaled corticosteroids. Eur Respir J. 2012;40:570–9. doi: 10.1183/09031936.00121411. [DOI] [PubMed] [Google Scholar]
- 482.Casarosa P, Kollak I, Kiechle T, Ostermann A, Schnapp A, Kiesling R, et al. Functional and biochemical rationales for the 24-hour-long duration of action of olodaterol. J Pharmacol Exp Ther. 2011;337:600–9. doi: 10.1124/jpet.111.179259. [DOI] [PubMed] [Google Scholar]
- 483.Pearlman DS, Greos L, LaForce C, Orevillo CJ, Owen R, Higgins M. Bronchodilator efficacy of indacaterol, a novel once-daily beta2-agonist, in patients with persistent asthma. Ann Allergy Asthma Immunol. 2008;101:90–5. doi: 10.1016/S1081-1206(10)60840-X. [DOI] [PubMed] [Google Scholar]
- 484.Sugihara N, Kanada S, Haida M, Ichinose M, Adachi M, Hosoe M, et al. 24-h bronchodilator efficacy of single doses of indacaterol in Japanese patients with asthma: A comparison with placebo and salmeterol. Respir Med. 2010;104:1629–37. doi: 10.1016/j.rmed.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 485.Cazzola M, Segreti A, Matera MG. Novel bronchodilators in asthma. Curr Opin Pulm Med. 2010;16:6–12. doi: 10.1097/MCP.0b013e32833303d2. [DOI] [PubMed] [Google Scholar]
- 486.Cazzola M, Matera MG. Novel long-acting bronchodilators for COPD and asthma. Br J Pharmacol. 2008;155:291–9. doi: 10.1038/bjp.2008.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487.LaForce C, Korenblat P, Osborne P, Dong F, Higgins M. 24-hour bronchodilator efficacy of single doses of indacaterol in patients with persistent asthma: Comparison with placebo and formoterol. Curr Med Res Opin. 2009;25:2353–9. doi: 10.1185/03007990903143143. [DOI] [PubMed] [Google Scholar]
- 488.Cates CJ, Cates MJ. Regular treatment with formoterol for chronic asthma: Serious adverse events. Cochrane Database Syst Rev. 2012;4:CD006923. doi: 10.1002/14651858.CD006923.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 489.van Noord JA, Bantje TA, Eland ME, Korducki L, Cornelissen PJ. A randomised controlled comparison of tiotropium nd ipratropium in the treatment of chronic obstructive pulmonary disease. The Dutch Tiotropium Study Group. Thorax. 2000;55:289–94. doi: 10.1136/thorax.55.4.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 490.Maesen FP, Smeets JJ, Sledsens TJ, Wald FD, Cornelissen PJ. Tiotropium bromide, a new long-acting antimuscarinic bronchodilator: A pharmacodynamic study in patients with chronic obstructive pulmonary disease (COPD). Dutch Study Group. Eur Respir J. 1995;8:1506–13. [PubMed] [Google Scholar]
- 491.Rodrigo GJ, Castro-Rodríguez JA. What is the role of tiotropium in asthma?A systematic review with meta-analysis. Chest. 2015;147:388–96. doi: 10.1378/chest.14-1698. [DOI] [PubMed] [Google Scholar]
- 492.Fardon T, Haggart K, Lee DK, Lipworth BJ. A proof of concept study to evaluate stepping down the dose of fluticasone in combination with salmeterol and tiotropium in severe persistent asthma. Respir Med. 2007;101:1218–28. doi: 10.1016/j.rmed.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 493.Gessner C, Kornmann O, Maspero J, van Zyl-Smit R, Krüll M, Salina A, et al. Fixed-dose combination of indacaterol/glycopyrronium/mometasone furoate once-daily versus salmeterol/fluticasone twice-daily plus tiotropium once-daily in patients with uncontrolled asthma: A randomised, Phase IIIb, non-inferiority study (ARGON) Respir Med. 2020;170:106021. doi: 10.1016/j.rmed.2020.106021. [DOI] [PubMed] [Google Scholar]
- 494.Elias JA, Lee CG, Zheng T, Ma B, Homer RJ, Zhu Z. New insights into the pathogenesis of asthma. J Clin Invest. 2003;111:291–7. doi: 10.1172/JCI17748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 495.Hamid Q, Tulic MK. New insights into the pathophysiology of the small airways in asthma. Ann Thorac Med. 2007;2:28–33. doi: 10.4103/1817-1737.30361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 496.Huchon G, Magnussen H, Chuchalin A, Dymek L, Gonod FB, Bousquet J. Lung function and asthma control with beclomethasone and formoterol in a single inhaler. Respir Med. 2009;103:41–9. doi: 10.1016/j.rmed.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 497.Nolte H, Pavord I, Backer V, Spector S, Shekar T, Gates D, et al. Dose-dependent anti-inflammatory effect of inhaled mometasone furoate/formoterol in subjects with asthma. Respir Med. 2013;107:656–64. doi: 10.1016/j.rmed.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 498.Grekas N, Athanassiou K, Papataxiarchou K, Rizea Savu S, Silvestro L. Pharmacokinetic study for the establishment of bioequivalence of two inhalation treatments containing budesonide plus formoterol. J Pharm Pharmacol. 2014;66:1677–85. doi: 10.1111/jphp.12303. [DOI] [PubMed] [Google Scholar]
- 499.Bodzenta-Lukaszyk A, Pulka G, Dymek A, Bumbacea D, McIver T, Schwab B, et al. Efficacy and safety of fluticasone and formoterol in a single pressurized metered dose inhaler. Respir Med. 2011;105:674–82. doi: 10.1016/j.rmed.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 500.O’Byrne PM, FitzGerald JM, Zhong N, Bateman E, Barnes PJ, Keen C, et al. The SYGMA programme of phase 3 trials to evaluate the efficacy and safety of budesonide/formoterol given 'as needed'in mild asthma: Study protocols for two randomised controlled trials. Trials. 2017;18:12. doi: 10.1186/s13063-016-1731-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 501.Bateman ED, O’Byrne PM, Busse WW, Lötvall J, Bleecker ER, Andersen L, et al. Once-daily fluticasone furoate (FF)/vilanterol reduces risk of severe exacerbations in asthma versus FF alone. Thorax. 2014;69:312–9. doi: 10.1136/thoraxjnl-2013-203600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 502.Jabbal S, Kuo CR, Lipworth B. Randomized controlled trial of triple versus dual inhaler therapy on small airways in smoking asthmatics. Clin Exp Allergy. 2020;50:1140–7. doi: 10.1111/cea.13702. [DOI] [PubMed] [Google Scholar]
- 503.Kim LH, Saleh C, Whalen-Browne A, O’Byrne PM, Chu DK. Triple versus dual inhaler therapy and asthma outcomes in moderate to severe asthma: A systematic review and meta-analysis. JAMA. 2021;325:2466–79. doi: 10.1001/jama.2021.7872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 504.Nair A, Menzies D, Barnes M, Burns P, McFarlane L, Lipworth BJ. Respirable dose delivery of fluticasone propionate from a small valved holding chamber, a compact breath actuated integrated vortex device and a metered dose inhaler. Br J Clin Pharmacol. 2008;66:20–6. doi: 10.1111/j.1365-2125.2008.03153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 505.Giraud V, Allaert FA, Roche N. Inhaler technique and asthma: Feasability and acceptability of training by pharmacists. Respir Med. 2011;105:1815–22. doi: 10.1016/j.rmed.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 506.Giraud V, Allaert FA. Improved asthma control with breath-actuated pressurized metered dose inhaler (pMDI): The SYSTER survey. Eur Rev Med Pharmacol Sci. 2009;13:323–30. [PubMed] [Google Scholar]
- 507.Donnell D. Inhaled corticosteroid delivery systems: Clinical role of a breath-actuated device. Eur Rev Med Pharmacol Sci. 2001;5:7–16. [PubMed] [Google Scholar]
- 508.Drazen JM, Harrington D. New biologics for asthma. N Engl J Med. 2018;378:2533–4. doi: 10.1056/NEJMe1806037. [DOI] [PubMed] [Google Scholar]
- 509.Pavord ID, Korn S, Howarth P, Bleecker ER, Buhl R, Keene ON, et al. Mepolizumab for severe eosinophilic asthma (DREAM): A multicentre, double-blind, placebo-controlled trial. Lancet. 2012;380:651–9. doi: 10.1016/S0140-6736(12)60988-X. [DOI] [PubMed] [Google Scholar]
- 510.Ortega HG, Yancey SW, Mayer B, Gunsoy NB, Keene ON, Bleecker ER, et al. Severe eosinophilic asthma treated with mepolizumab stratified by baseline eosinophil thresholds: A secondary analysis of the DREAM and MENSA studies. Lancet Respir Med. 2016;4:549–56. doi: 10.1016/S2213-2600(16)30031-5. [DOI] [PubMed] [Google Scholar]
- 511.Bel EH, Wenzel SE, Thompson PJ, Prazma CM, Keene ON, Yancey SW, et al. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014;371:1189–97. doi: 10.1056/NEJMoa1403291. [DOI] [PubMed] [Google Scholar]
- 512.FitzGerald JM, Bleecker ER, Menzies-Gow A, Zangrilli JG, Hirsch I, Metcalfe P, et al. Predictors of enhanced response with benralizumab for patients with severe asthma: Pooled analysis of the SIROCCO and CALIMA studies. Lancet Respir Med. 2018;6:51–64. doi: 10.1016/S2213-2600(17)30344-2. [DOI] [PubMed] [Google Scholar]
- 513.Chipps BE, Newbold P, Hirsch I, Trudo F, Goldman M. Benralizumab efficacy by atopy status and serum immunoglobulin E for patients with severe, uncontrolled asthma. Ann Allergy Asthma Immunol. 2018;120:504–11.e4. doi: 10.1016/j.anai.2018.01.030. [DOI] [PubMed] [Google Scholar]
- 514.Hanania NA, Wenzel S, Rosén K, Hsieh HJ, Mosesova S, Choy DF, et al. Exploring the effects of omalizumab in allergic asthma: An analysis of biomarkers in the EXTRA study. Am J Respir Crit Care Med. 2013;187:804–11. doi: 10.1164/rccm.201208-1414OC. [DOI] [PubMed] [Google Scholar]
- 515.Normansell R, Walker S, Milan SJ, Walters EH, Nair P. Omalizumab for asthma in adults and children. Cochrane Database Syst Rev. 2014:CD003559. doi: 10.1002/14651858.CD003559.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 516.Lowe PJ, Georgiou P, Canvin J. Revision of omalizumab dosing table for dosing every 4 instead of 2 weeks for specific ranges of bodyweight and baseline IgE. Regul Toxicol Pharmacol. 2015;71:68–77. doi: 10.1016/j.yrtph.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 517.Agache I, Beltran J, Akdis C, Akdis M, Canelo-Aybar C, Canonica GW, et al. Efficacy and safety of treatment with biologicals (benralizumab, dupilumab, mepolizumab, omalizumab and reslizumab) for severe eosinophilic asthma. A systematic review for the EAACI guidelines –Recommendations on the use of biologicals in severe asthma. Allergy. 2020;75:1023–42. doi: 10.1111/all.14221. [DOI] [PubMed] [Google Scholar]
- 518.Busse WW, Humbert M, Haselkorn T, Ortiz B, Trzaskoma BL, Stephenson P, et al. Effect of omalizumab on lung function and eosinophil levels in adolescents with moderate-to-severe allergic asthma. Ann Allergy Asthma Immunol. 2020;124:190–6. doi: 10.1016/j.anai.2019.11.016. [DOI] [PubMed] [Google Scholar]
- 519.Hanania NA, Alpan O, Hamilos DL, Condemi JJ, Reyes-Rivera I, Zhu J, et al. Omalizumab in severe allergic asthma inadequately controlled with standard therapy: A randomized trial. Ann Intern Med. 2011;154:573–82. doi: 10.7326/0003-4819-154-9-201105030-00002. [DOI] [PubMed] [Google Scholar]
- 520.Agache I, Rocha C, Beltran J, Song Y, Posso M, Solà I, et al. Efficacy and safety of treatment with biologicals (benralizumab, dupilumab and omalizumab) for severe allergic asthma: A systematic review for the EAACI Guidelines –Recommendations on the use of biologicals in severe asthma. Allergy. 2020;75:1043–57. doi: 10.1111/all.14235. [DOI] [PubMed] [Google Scholar]
- 521.Castro M, Zangrilli J, Wechsler ME, Bateman ED, Brusselle GG, Bardin P, et al. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: Results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respir Med. 2015;3:355–66. doi: 10.1016/S2213-2600(15)00042-9. [DOI] [PubMed] [Google Scholar]
- 522.Mukherjee M, Aleman Paramo F, Kjarsgaard M, Salter B, Nair G, LaVigne N, et al. Weight-adjusted intravenous reslizumab in severe asthma with inadequate response to fixed-dose subcutaneous mepolizumab. Am J Respir Crit Care Med. 2018;197:38–46. doi: 10.1164/rccm.201707-1323OC. [DOI] [PubMed] [Google Scholar]
- 523.Ortega HG, Liu MC, Pavord ID, Brusselle GG, FitzGerald JM, Chetta A, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371:1198–207. doi: 10.1056/NEJMoa1403290. [DOI] [PubMed] [Google Scholar]
- 524.Nair P, Wenzel S, Rabe KF, Bourdin A, Lugogo NL, Kuna P, et al. Oral glucocorticoid-sparing effect of benralizumab in severe asthma. N Engl J Med. 2017;376:2448–58. doi: 10.1056/NEJMoa1703501. [DOI] [PubMed] [Google Scholar]
- 525.Busse WW, Bleecker ER, FitzGerald JM, Ferguson GT, Barker P, Sproule S, et al. Long-term safety and efficacy of benralizumab in patients with severe, uncontrolled asthma:1-year results from the BORA phase 3 extension trial. Lancet Respir Med. 2019;7:46–59. doi: 10.1016/S2213-2600(18)30406-5. [DOI] [PubMed] [Google Scholar]
- 526.Chupp G, Lugogo NL, Kline JN, Ferguson GT, Hirsch I, Goldman M, et al. Rapid onset of effect of benralizumab on morning peak expiratory flow in severe, uncontrolled asthma. Ann Allergy Asthma Immunol. 2019;122:478–85. doi: 10.1016/j.anai.2019.02.016. [DOI] [PubMed] [Google Scholar]
- 527.Bleecker ER, FitzGerald JM, Chanez P, Papi A, Weinstein SF, Barker P, et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting β(2)-agonists (SIROCCO): A randomised, multicentre, placebo-controlled phase 3 trial. Lancet. 2016;388:2115–27. doi: 10.1016/S0140-6736(16)31324-1. [DOI] [PubMed] [Google Scholar]
- 528.Bleecker ER, Wechsler ME, FitzGerald JM, Menzies-Gow A, Wu Y, Hirsch I, et al. Baseline patient factors impact on the clinical efficacy of benralizumab for severe asthma. Eur Respir J. 2018;52:1–13. doi: 10.1183/13993003.00936-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 529.Cockcroft DW. Asthma. Can J Respir Crit Care Sleep Med. 2020;4(Suppl 1):S18–20. [Google Scholar]
- 530.Peters MC, Wenzel SE. Intersection of biology and therapeutics: Type 2 targeted therapeutics for adult asthma. Lancet. 2020;395:371–83. doi: 10.1016/S0140-6736(19)33005-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 531.Rabe KF, Nair P, Brusselle G, Maspero JF, Castro M, Sher L, et al. Efficacy and safety of dupilumab in glucocorticoid-dependent severe asthma. N Engl J Med. 2018;378:2475–85. doi: 10.1056/NEJMoa1804093. [DOI] [PubMed] [Google Scholar]
- 532.Corren J, Parnes JR, Wang L, Mo M, Roseti SL, Griffiths JM, et al. Tezepelumab in adults with uncontrolled asthma. N Engl J Med. 2017;377:936–46. doi: 10.1056/NEJMoa1704064. [DOI] [PubMed] [Google Scholar]
- 533.Simpson EL, Parnes JR, She D, Crouch S, Rees W, Mo M, et al. Tezepelumab, an anti-thymic stromal lymphopoietin monoclonal antibody, in the treatment of moderate to severe atopic dermatitis: A randomized phase 2a clinical trial. J Am Acad Dermatol. 2019;80:1013–21. doi: 10.1016/j.jaad.2018.11.059. [DOI] [PubMed] [Google Scholar]
- 534.Wechsler ME, Colice G, Griffiths JM, Almqvist G, Skärby T, Piechowiak T, et al. SOURCE: A phase 3, multicentre, randomized, double-blind, placebo-controlled, parallel group trial to evaluate the efficacy and safety of tezepelumab in reducing oral corticosteroid use in adults with oral corticosteroid dependent asthma. Respir Res. 2020;21:264. doi: 10.1186/s12931-020-01503-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 535.Menzies-Gow A, Colice G, Griffiths JM, Almqvist G, Ponnarambil S, Kaur P, et al. NAVIGATOR: A phase 3 multicentre, randomized, double-blind, placebo-controlled, parallel-group trial to evaluate the efficacy and safety of tezepelumab in adults and adolescents with severe, uncontrolled asthma. Respir Res. 2020;21:266. doi: 10.1186/s12931-020-01526-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 536.Tezepelumab K. NAVIGATOR Phase III Trial Met Primary Endpoint of a Statistically Significant and Clinically Meaningful Reduction in Exacerbations in a Broad Population of Patients with Severe Asthma: AstraZeneca. 2020. [[Last accessed on 2023 Sep 19]]. Available from: https://www.astrazeneca.com/media-centre/press-releases/2020/tezepelumab-navigator-phase-iii-trial-met-primary-endpoint.html .
- 537.Menzies-Gow A, Corren J, Bourdin A, Chupp G, Israel E, Wechsler ME, et al. Tezepelumab in adults and adolescents with severe, uncontrolled asthma. N Engl J Med. 2021;384:1800–9. doi: 10.1056/NEJMoa2034975. [DOI] [PubMed] [Google Scholar]


