Abstract
Feline obesity continues to be a priority health and welfare issue. Most research surrounding obesity currently focuses on obesity treatment. However, treatment for feline obesity is slow, often unsuccessful and not without consequences. Identifying high-risk populations for obesity onset is crucial for developing and implementing preventive strategies. This review identifies post-gonadectomy kittens aged 5–12 months as the primary target population for obesity prevention in domestic cats and highlights dietary and feeding management strategies to be implemented for obesity prevention.
Keywords: Weight gain, nutrition, energy requirement, gonadectomy, feeding management, dietary intervention
Importance of obesity prevention
Feline obesity affects 11–63% of domestic cats1 –5 and is considered a health and welfare priority. 6 Obesity is characterized as excess adipose tissue accumulation that can result in negative health consequences.7,8 For cats, these consequences can include, but are not limited to, insulin resistance, diabetes mellitus, osteoarthritis and skin conditions.4,8 –10
Obesity treatment and management is a slow, often unsuccessful, process that can include energy restriction, veterinary therapeutic diets for weight loss, feeding management strategies and exercise for cats. 11 Various treatment and management plans as well as risks for obesity and weight loss plans for cats have been reviewed extensively elsewhere.11,12 Poor owner compliance can be a setback for weight loss, possibly resulting from financial constraints, obesity or body condition score (BCS) misperceptions, or the inconvenience of weight loss plans. In addition, concurrent disorders can occur during slow weight loss, such as consequences of obesity (ie, diabetes mellitus), or from too rapid weight loss (ie, feline hepatic lipidosis), and can complicate the weight loss plan. Moreover, calorie restriction without an appropriately formulated diet can lead to nutrient deficiencies, specifically selenium and choline. 13 Weight regain after treatment is all too common. 14 In cats, it was found that weight regain after successful weight loss to an ideal weight resulted in higher body fat mass than during the original obese phase in all cats within an average of 14 weeks. Further, after successful weight loss by calorie restriction, more than half the cats regained over half the weight they had lost in a long-term follow-up (median of 954 days). 14
While treatment has been a research priority for feline obesity, it is crucial that prevention strategies are communicated and implemented throughout a cat’s life span. Cat ownership is increasing steadily; thus, the number of cats requiring obesity management is likely to increase.15,16 As domestic cat populations continue to rise, a shift to focus on prevention becomes more critical.
The benefits of prevention are plentiful. In 2009, a report estimated that the first 12 months after a cat is diagnosed with obesity costs the pet owner over US$1000 in veterinary bills, without accounting for the additional costs of other veterinary care, such as clinical pathology charges and overnight hospital fees, or for any other health consequences.17,18 More recent reports state that owners of overweight or obese cats spend 36% more on diagnostic services and 53% more on surgical procedures than owners of normal-weight cats.18,19 Obesity is the most common nutritional disorder in cats within general clinical practice.7,12 Reducing the strain on the veterinary community can be achieved by reducing the number of cats that become obese and require this veterinary care. In addition, obesity affects welfare and quality of life.6,20 Due to the associated disorders that can arise from obesity,7,21 it is thought that obesity could also reduce the life span of cats, as has been found in dogs;22,23 however, this research does not yet exist for cats. To maintain and improve upon these variables, preventing obesity is a priority.
Major risk factors for feline obesity include age,24,25 sex,25,26 breed, 26 indoor confinement,26,27 feeding primarily a dry food diet,26 –28 free-feeding and feeding frequency, 29 overestimating food allotments,28,30 owner misperceptions and even the human–animal bond. 31 Interestingly, gonadectomy is consistently reported as a major risk factor for feline obesity (Table 1).32 –45
Table 1.
Summary of reported effects of gonadectomy on BW, FM, LSTM, EI, MER and EE in experiments with domestic cats
| Reference | n | Sex | Life stage | Feeding method | BW | FM | LSTM | EI | MER | EE |
|---|---|---|---|---|---|---|---|---|---|---|
| Allaway et al, 2017 36 | 16 | Male | Growth | Stable BCS | Increased | N/A | N/A | Increased | N/A | NC |
| Backus et al, 2007 32 | 24 | Mixed | Growth | Ad lib | Increased | Increased | NC | Increased | N/A | N/A |
| Belsito et al, 2009 39 | 8 | Female | Adult | Ad lib* | Increased | Increased | N/A | Increased | N/A | N/A |
| Fettman et al, 1997 33 | 12 | Mixed | Adult | Ad lib | Increased | Increased | N/A | Increased | N/A | N/A |
| Flynn et al, 1996 40 | 15 | Female | Adult | Stable BW | N/A | N/A | N/A | N/A | Reduced | N/A |
| Harper et al, 2001 41 | 49 | Female | Adult | Ad lib | Increased | Increased | N/A | Increased | N/A | N/A |
| Hoenig & Ferguson, 2002 34 | 20 | Mixed | Adult | Stable BW | N/A | N/A | N/A | N/A | Reduced | N/A |
| Kanchuck et al, 2002 38 | 32 | Male | Adult | Ad lib | Increased | Increased | NC | Increased | N/A | N/A |
| Kanchuck et al, 2003 37 | 16 | Male | Adult | Ad lib | Increased | Increased | NC | Increased | N/A | NC |
| Martin et al, 2001 42 | 42 | Mixed | Adult | Ad lib | Increased | Increased | N/A | N/A | N/A | Reduced |
| Mitsuhashi et al, 2011 43 | 22 | Female | Adult | Pre-neuter MER | Increased | N/A | N/A | N/A | Reduced | N/A |
| Nguyen et al, 2004 44 | 24 | Mixed | Adult | Ad lib | Increased | Increased | N/A | NC | N/A | NC |
| Vester et al, 2009 45 | 8 | Female | Adult | Ad lib | Increased | Increased | N/A | Increased | N/A | Reduced |
| Wei et al, 2014 35 | 9 | Male | Adult | Ad lib | Increased | Increased | NC | Increased | N/A | NC |
Ad libitum feeding began 12 weeks after spaying; during weeks 0–12, cats were fed to maintain BW
Ad lib = ad libitum; BCS = body condition score; BW = body weight; EE = energy expenditure; EI = energy intake; FM = fat mass; LSTM = lean soft tissue mass; MER = maintenance energy requirements; N/A = not analyzed; NC = no change
Role of gonadectomy in feline obesity
Previous reports estimate that over 80% of cats in North America and up to 92% of cats in the United Kingdom are gonadectomized.46–49 While gonadectomy increases the risk for obesity, there are also many benefits to this procedure, such as aiding in population control, reduced likelihood of abandonment, curbing negative behavioral patterns, and preventing certain diseases and reproductive disorders, such as mammary gland neoplasia, as previously described. 50
Although many benefits are associated with gonadectomy, its role in obesity onset is alarming. Cats after gonadectomy have increased food intake, resulting in rapid body weight (BW) gain largely driven by increased body fat mass (Table 1).32,33,35,37,45,51 Comparison of the growth curves of neutered kittens with those of intact kittens further confirms growth disturbances, characterized by greater BW and body fat mass, following gonadectomy, 51 and female kittens appear to be most affected. In addition, lower energy expenditure has been observed in cats after the gonadectomy procedure.42,45 Energy requirements can also be reduced by up to 30% after gonadectomy, 42 while energy intake after gonadectomy is reported to increase by up to 50%. 32 Several hypotheses for these findings have been proposed, such as reduced sexual hormone production affecting satiety hormones and growth hormones, and also a reduction in the energy required to produce and maintain sexual hormones and sex organs, though it is likely that this phenomenon is multifactorial, including a combination of hyperphagia, reduced physical activity and lowered energy requirements.32,35,39,42,52
The timing of neutering may influence this relationship in cats. The traditional age of neutering is often 6–9 months, whereas early-age neutering is considered at less than 5.5 months of age. Early-age neutering is still controversial; however, neutering and anesthesia procedures are considered safe for cats aged as young as 7 weeks. 53 Various organizations recommend that cats be neutered at 6–14 weeks of age, or before 5 months.53,54 Early- and traditional-age spaying of female kittens were both found to require subsequent energy restriction to maintain ideal BCS; 36 however, early-age spaying did not appear to induce acute hyperphagia, which was observed with traditional-age spaying. A recent investigation suggests that female kittens spayed early are more at risk of greater weight gain than female kittens spayed later in life. 51 Regardless of sex, gonadectomy at both early and traditional age appears to increase the risk of weight gain resulting in obese conditions. Therefore, this population should be considered a primary target for obesity prevention.
Growth as a target population for obesity prevention
Early development has previously been identified as a key life stage for preventing various diseases and disorders, such as obesity, in cats. 55 In humans, poor nutrition during fetal development and childhood overweight or obese condition are associated with health complications into adulthood, such as obesity and diabetes mellitus; this is known as the Barker hypothesis.56–60 This hypothesis identifies that preventing and treating childhood obesity is essential to reducing obesity risks and prevalence in adulthood. These studies were conducted in humans; however, similar results have been observed in cats,8,24,55,61 –63 such that rapid growth in kittens was a predictor of obesity in the adult life stage. 24
Feline growth can be broken down into five stages (Table 2).64–66 To avoid interfering with skeletal growth, prevention should begin in the sustained growth phase. However, diet and feeding management strategies can be implemented in the post-weaning phase, particularly when early-age neutering occurs.
Table 2.
| Growth phase | Age | Dietary habits | Expected growth rate | Description | ||
|---|---|---|---|---|---|---|
| Obesity prevention timeline and status |

|
Neonatal | 0–4 weeks | Rely on mother’s milk | More than 90% of time spent sleeping | |

|
Weaning | 4–8 weeks | Transition to solid food for growth | 10–30 g per week | Increased time spent interacting with littermates; skill and behavior development; growth increases; reaching up to 100 g/week | |

|
Post-weaning (rapid growth) | 2–4 months | Food for growth, transitioned fully | <100 g per week | Regular vaccinations; early-age neutering | |

|
Sustained growth | 5–12 months | Food for growth | Male: <20 g/day; Female: <11 g/day (80% of adult weight by the age of 8 months) | Energy requirements decrease; traditional-age neutering (6 months) | |
| Adulthood | 12+ months | Transition to adult maintenance food | Skeletal maturity reached by 10–12 months | Additional growth for maturation and muscle development may occur up to 15 months | ||
 Minimal risk: weight monitored weekly to ensure appropriate growth Minimal risk: weight monitored weekly to ensure appropriate growth | ||||||
 Minimal risk: weight monitored bi-weekly Minimal risk: weight monitored bi-weekly | ||||||
 Moderate risk: weight monitored bi-weekly; food allotments weighed to energy requirements Moderate risk: weight monitored bi-weekly; food allotments weighed to energy requirements | ||||||
 Critical risk: weight monitored weekly; implement and maintain obesity prevention strategies Critical risk: weight monitored weekly; implement and maintain obesity prevention strategies | ||||||
Obesity prevention strategies for kittens
For kittens, multiple evidence-based strategies can be implemented to prevent obesity. A successful prevention plan involves commitment and compliance from the owner, and guidance from the veterinary team.11,67 Although further research is necessary to understand the influence of different communication strategies on the health outcome of pets, effective communication between the owner and the veterinary team is likely to have a positive impact on feline health. Meaningful discussion surrounding weight management could also play a key role in ensuring the prevention of obesity during the growth phase, and thereafter into adulthood.68,69 Additional strategies that can be implemented by the owner, including regular monitoring of growth and appropriate feeding practices, can also aid in successful obesity prevention.
Effective veterinary–owner communication
The quality of communication can directly impact the strength of the veterinarian–pet owner bond, and, as a result, the standard of care the pet receives.70,71 By practicing effective communication skills, veterinarians can strengthen their bond with cat owners and improve client loyalty. This, in turn, can increase owners’ willingness to adhere to recommendations and, subsequently, improve upon clinical outcomes.71–74
Despite knowledge that communication is a key component in successful veterinary intervention, veterinary team members and pet owners inconsistently address the management and prevention of obesity during appointments.67,69,75,76 Reported barriers for veterinarians to discussing obesity prevention include concern that owners will be offended and/or non-compliant, fear of harming relationships with clients and of being seen as purely financially motivated, time constraints and lack of education on obesity management strategies.68,70,77,78 Barriers for pet owners include ineffective communication, in which the owner is made to feel judged or blamed and can result in defensiveness and less adherence to weight care. 78
In an observational study using veterinarian and pet owner focus groups, owners said they would be more willing to adhere to recommendations if veterinarians take into consideration their lifestyle and their pet’s lifestyle, provide a variety of strategies, such as different diet choices and exercise regimes, and explain all aspects in a clear and direct manner rather than make ambiguous suggestions. 78 Further, owners believed weight to be a vital part of their pet’s overall health, and that it should be measured and discussed at most appointments. 78 However, observational data suggest that obesity prevention is discussed by veterinarians in fewer than 25% of appointments, and the degree to which it is discussed varies. 69 For cat owners specifically, discussions surrounding a nutritional history of the cat, dietary recommendations, and ways to measure and maintain BCS are often incomplete or unclear. 69 Without clear guidance from veterinarians when assessing appropriate BCS and diet, owners lack the proper tools and knowledge to effectively manage their cat’s weight. This has been seen in previous studies, in which cat owners misuse BCS charts, and incorrectly estimate their overweight or obese pet to be at an appropriate weight.79,80
To the authors’ knowledge, research focusing on weight management or obesity prevention communication from veterinary staff to new kitten owners has yet to be conducted. Current guidelines from the American Animal Hospital Association (AAHA) and American Association of Feline Practitioners recommend discussions surrounding diet and feeding practices begin at the kitten stage (up to 1 year), and discussions surrounding obesity risks and prevention strategies begin at the young adult stage (1–6 years). 81 However, considering the high risk of obesity onset during growth, research investigating the impact of discussing obesity prevention with all kitten owners could prove beneficial.
If approached in a constructive and non-confrontational way, there is potential to have educational and effective conversations regarding obesity prevention between cat owners and veterinarians. When using effective communication strategies, obesity management can be developed into a long-term team effort, rather than an unsolicited or overwhelming intervention. Strategies to broach the topic of obesity prevention are summarized in Table 3.
Table 3.
Effective communication strategies for veterinary professionals when discussing obesity prevention with cat owners
| Strategies for effective communication | Ways to implement strategies | Justification |
|---|---|---|
| Utilize all members of the veterinary team | • Delegate aspects of communication to trained technicians or other veterinary support staff | Utilizing the knowledge and skill sets of all staff members in the practice can help provide pet owners with a variety of perspectives, suggestions and techniques for preventing obesity in their cat, and can maximize staff members’ time |
| Consider using the relationship-centered approach | • Treat relationships with pet owners as a partnership
• Recognize and understand the client’s perspectives, motivations and expertise 82 • Avoid authoritarian language, judgment and blame • Provide individualized, financially accessible, prevention strategies |
Including owners in the decision-making process and ensuring their opinions are valued can lead to increased adherence to care69,78 |
| Begin discussion at the first veterinary appointment for any cat owner, though specifically for owners of kittens, regardless of growth stage | • Provide/explain a BCS/growth chart
• Include obesity prevention strategies in a kitten package or other brochures • Introduce ways to increase physical activity and enrichment (interactive feeding toys, dedicated play times) |
Proper communication early on can promote obesity awareness to the cat owner and aid in preparations for prevention11,68,69,83,84 |
| Encourage regular veterinary checkups for cat owners | • Encouragement can include: kitten’s improved behavior, specifically when at the clinic; early detection of diseases or disorders (reducing long-term costs and improving overall welfare); maintaining appropriate vaccination status; and an improved relationship between the veterinary team and owner68,85,86 | Explaining the benefits of regular veterinary visits during the cat’s growth stage can encourage owners to schedule more frequent visits, allowing for greater maintenance of a weight management plan |
BCS = body condition score
Monitoring growth
One way to start the conversation in the consultation room is by use of kitten growth charts 66 as part of an obesity prevention plan. This discussion should also encourage easy access to and use of a scale so owners can regularly weigh their kitten at home and plot its weight on the growth chart. Growth charts allow the owner and veterinary team to monitor growth and to identify any growth disturbances. 84 Further, incorporating growth charts can foster the human–animal bond and owners may feel more involved in their kitten’s growth and development.
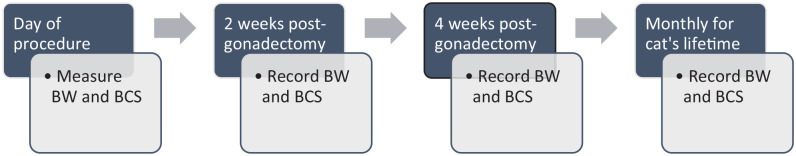
For gonadectomized kittens, growth charts can be especially important for identifying impacts on BW (Figure 1). Plotting weight change on a graph, and teaching owners to do this at home, allows for identification of rapid or inappropriate weight gain after neutering. BCS and muscle condition score (MCS) can also be documented as a guide for body fat percentage; however, it is important to note that BCS and MCS, while validated for adult cats,87,88 are not validated for growth. Regardless, teaching cat owners how to BCS and MCS early in life can be beneficial in obesity prevention as obesity diagnosis in clinical practice uses BW, BCS, MCS and other morphometric measures, such as girth circumference.11,85
Figure 1.
Recommended timeline for monitoring body weight (BW) and body condition score (BCS) during growth in kittens to prevent obesity after gonadectomy
If rapid weight gain occurs, adjustments to the obesity prevention plan should be made and BW reassessed after 2 weeks. 84 Further adjustments should be made until the rate of growth is back on track. Adjustments can also be made using feeding management strategies or nutritional interventions.
Feeding to energy requirements
During growth, energy restriction is not recommended – rather, the prevention of additional excess weight gain is encouraged; therefore, determining and feeding to a kitten’s energy requirement rather than free-feeding is critical as an obesity prevention strategy.11,84,89 The gold standard for determining energy requirements is via indirect calorimetry; however, this is not available in a clinical setting.90,91 Therefore, for pet owners, reliance on predictive equations – BW, BCS and MCS monitoring, growth curves and individual progress, diet history and energy intake – is required.
Many predictive equations are proposed and available (Table 4). With regard to growth energy requirements, there can be differences between both the rapid and sustained growth phases and the appropriate calculations. Gross et al 64 and AAHA 83 use the traditional resting energy requirement and multiply by an appropriate life stage factor to calculate the daily energy requirement (DER). Alternatively, the National Research Council recommends one equation throughout both growth stages that incorporates the current BW of the kitten and the expected BW at maturity. 92
Table 4.
Published predictive equations to determine daily energy requirements (kcal/day) for growth in cats
| Sex | Growth stage | Equation | Reference | Example* (kcal/d) |
|---|---|---|---|---|
| N/A | Rapid | DER (kcal/day) = (70 × BW0.75) × 3 | 64 | 353.20 |
| N/A | Sustained | DER (kcal/day) = (70 × BW0.75) × 2.5 | 64 | 294.30 |
| N/A | Rapid and sustained | DER (kcal/day) = (70 × BW0.75) × 2.5 | 11 | 294.30 |
| N/A | Rapid and sustained | DER (kcal/day) = 100 × BWa0.67 × 6.7 × [e(-0.189p)-0.66] † | 92 | 266.32 |
| Male | Rapid and sustained | MEI (kcal/kg BW0.67/day) = 176.27-0.037t, R2 = 0.79 ‡ | 93 | 241.80 |
| Female | Rapid and sustained | MEI (kcal/kg BW0.67/day) = 166.86-0.044t, R2 = 0.62‡ | 93 | 222.80 |
Example using a 4-month-old kitten weighing 2.0 kg, and expected mature weight of 4 kg
Where p = BWa/BWm, BWa/BWm; BW a = actual body weight; BW m = mature body weight; e = base of natural log ~2.718
Where t is age in months
BW = body weight; DER = daily energy requirements; MEI = metabolizable energy intake
Expected mature BW can be estimated using published data on average BW for adult cats or by using growth charts developed from clinical data available for sexually intact kittens.64,66,93 While these equations can provide an estimate for the energy required, they do not account for sex or age or body composition. Merenda et al 93 propose predictive equations for kittens that use age (in months) and sex to determine the energy requirements for growth (Table 4). These models account for both growth phases and are more specific to growth patterns in females and males, respectively. However, current equations and the proposed equations from Merenda et al 93 are derived from research in colony cat populations using domestic shorthair cats. Research is not available for other breeds such as Maine Coon, Ragdoll and Sphynx. Further, data using client-owned cats for determining energy requirements via gold-standard methodologies are not available due to various limitations, such as acclimation to equipment, procedures and accessibility. 94 Research methods for client-owned cats are limited to measuring the energy intake required to maintain stable BW or BCS, though this research can be valuable in making comparisons with colony cats and for determining models for energy requirements. More research can contribute to the understanding of cats’ energy requirements, specifically post-gonadectomy, as there are no equations currently available for cats after gonadectomy although research suggests a reduction in DER after gonadectomy. 42
Discussing energy requirements and the calculations for food allotments for the cat with the pet owner is an important component to the growth and obesity prevention plan and should include guidelines on when, and how, to adjust food allotments based on BW and BCS. Calculating the food allotment is a simple equation:
The most accurate way to measure the food allotment is by using a gram scale, and not by scoop or cup.30,95 Encouraging pet owners to use a gram scale and demonstrating how to properly use the scale can be an effective component in the prevention of obesity.
Feeding management strategies
Many feeding management strategies can be used and implemented as part of the obesity prevention plan for kittens. As stated previously, kittens should be fed a food allotment that meets their DER and not free-fed.11,85 In addition, for single-cat homes, individualized feeders could reduce food-seeking behavior. For cats in multiple-pet households, microchip feeders as well as separating pets at mealtimes can reduce competition for food and overconsumption.96 –98 A recent consensus statement recommends feeding cats multiple small meals per day to mitigate behavioral concerns and potentially improve welfare; 97 however, once-a-day feeding may be beneficial in promoting satiety and fatty acid oxidation in cats compared with multiple feedings. 29 To date, there is a dearth of data investigating the long-term physiological effects of feeding frequency in cats and its role in obesity prevention. Eliminating table scraps and minimizing treats to account for up to 10% of the DER is also an effective method to reduce excess calories being consumed.11,12,85,97
Food toys can increase physical activity and may have cognitive and enrichment benefits,11,85,97 though a recent pilot study found no effect of food toys on overall activity in adult neutered cats but found potential benefits for reducing stress and improving overall wellbeing. 99 Moving or hiding food around the house can increase animal movement and provide enrichment as a way to mimic hunting for prey. Increasing exercise is often overlooked in cats but should always be encouraged as part of a lifestyle or obesity prevention plan.11,84,85,89 Dedicating a daily minimum of 15 minutes to playing with toys or the use of electronic or interactive toys provides regular daily activity, improves the human–animal bond and provides additional enrichment.100,101 This time can be broken up into small intervals throughout the day based on the owner’s schedule and flexibility. Vertical space is another way to increase physical activity for cats. Vertical space can be improved by cat trees, hammocks or shelves that encourage climbing behaviors.
Nutritional interventions
Regardless of growth stage and BCS, kittens should be offered a life-stage-appropriate food. For kittens younger than 12 months, this should be a food labelled for growth or all life stages. Growth diets tend to have higher energy densities to meet the greater energy demands for growth, while also accounting for the small stomach capacity of kittens to prevent ‘gut-fill’.64,65,102 However, because of the high energy density, it is especially important to feed to DER, as a small increase in food amount can be a large increase in energy intake, resulting in excess weight gain.
If a kitten’s BCS is higher than ideal, owners should be discouraged from changing the diet to a weight management or weight loss option. Most weight management and weight loss foods do not meet the energy density demands for growth or the additional essential micronutrients required at higher levels for growth. Rather, veterinary-pet-owner communication is a priority to ensure emphasis on the importance of feeding to DER, adjusting as per individual needs. In addition, utilizing the feeding management practices detailed in this review should be encouraged.
Macronutrients
Dietary protein, fat and carbohydrates, but not dietary fiber, contribute to the energy density of food. Regardless, each macronutrient has a potential role in obesity prevention (Table 5). 92
Table 5.
Roles for macronutrients in the diet of kittens and the recommendations and dietary targets for these in obesity prevention11,64,85,92,103
| Role in growth | Recommendations for obesity prevention | Target | |
|---|---|---|---|
| Energy | Higher energy density for increased demand during growth; higher energy density reduces food amount required and accounts for limited expandability of kitten stomachs | Encourage feeding to DER; look for lower energy density growth diets | 4000–4500 kcal/kg ME |
| Protein | Delivers nitrogen and amino acids; growth and maintenance of muscle mass; immune system and function; enzyme and hormones; structural components | High-protein diets are beneficial for growth and muscle mass | 40–45% crude protein DM |
| Fat | Delivers essential fatty acids during growth (linoleic acid, arachidonic acid, a-linolenic acid, EPA and DHA); vehicle for absorption of fat-soluble vitamins; energy dense; improves palatability | Moderate fat levels for energy density and palatability; lower fat content will be found in lower energy density growth diets | 18–35% crude fat DM |
| Carbohydrates | No dietary requirement though glucose is physiologically essential; contributes to energy density; important for texture and structure of food | NFE estimates carbohydrates; minimize simple sugars; focus on complex carbohydrates | 12–37% DM* |
| Dietary fiber | No dietary requirement; beneficial for gut motility, building/feeding the microbiome and providing bulk in the gastrointestinal tract; crude fiber only accounts for insoluble fibers, not soluble fibers | Amount and types are relatively unknown for cats for obesity prevention | 5–8% DM |
Calculated based on lower and upper ranges of additional macronutrients
DER = daily energy requirement; DHA = docosahexaenoic acid; DM = dry matter; EPA = eicosapentaenoic acid; ME = metabolizable energy; NFE = nitrogen-free extract
Limited research has been conducted on the effects of macronutrient compositions after gonadectomy; however, consistent with previous reports in adult cats without gonadectomy as a factor,104,105 gonadectomized cats of both sexes experience a positive correlation between BW and fat mass with increasing dietary fat content.32,44 In both studies, cats were gonadectomized at 7–10 months of age, they were fed ad libitum and test diets were not formulated specifically for growth. Overall, the results of these studies suggest that high-fat diets may present a challenge for preventing obesity after gonadectomy, though more research is required. Currently, there are no studies, to the authors’ knowledge, assessing dietary macronutrients in obesity prevention during growth, specifically after gonadectomy.
Dietary fiber is often added to diets for the management of obesity due to its proposed role in diluting energy density, reducing overall energy intake and its effects on gastric emptying, satiety and promoting a healthy microbiome.106 –110 Regarding obesity prevention, the effects of fiber on satiety could be beneficial for cats when fed ad libitum (as commonly occurs with kittens). Fiber has shown benefits in weight loss, diabetes and gastrointestinal diseases in cats.109,111–115 Research on fiber for obesity prevention in cats is lacking, specifically after gonadectomy and during growth.
Diet format
Often overlooked is the format of the diet. Of particular interest, when fed ad libitum for 4 weeks after gonadectomy, cats consuming an extruded dry food were reported to have greater weight gain and increased BCS at weeks 5 and 6 after neutering compared with cats consuming a wet canned food. 116 These findings were likely attributed to the lower energy density of the wet food compared with the dry food. Further, it was also reported that adding 40% water to a dry food improved physical activity levels and weight gain was lower despite similar energy intake compared with a control group. 117 Thus, moisture content and proper hydration may have an important role in weight management. To the authors’ knowledge, research investigating different diet formats for obesity prevention after gonadectomy is lacking. This is particularly important due to the rapid growth of the pet food industry and the introduction of various food formats from extruded kibble and wet foods to gently cooked and raw meat-based diets, which can add another complexity to nutritional interventions. 118
Conclusions
Recognizing the significance of early life nutrition and growth, specifically after neutering in obesity prevention is paramount. The Barker hypothesis, supported by subsequent studies in humans and cats alike, underscores the lasting impact of early prevention of obesity on long-term health. For kittens, a comprehensive approach is essential, involving effective communication between veterinary teams and owners, vigilant and thorough growth monitoring and prevention plans, and optimal feeding management strategies that can include tailored diets, macronutrient balance and environmental enrichment. By prioritizing these measures, veterinary teams and owners can positively influence the lifelong health of cats, specifically the vital role of early interventions in curbing the long-term effects of obesity.
Footnotes
Accepted: 4 January 2024
HG declares that they have participated in paid internships and engagements with pet food companies within Canada. SKA holds the Nestle Purina Professorship in Companion Animal Nutrition at the Ontario Veterinary College and is the owner of Sit, Stay, Speak Nutrition and provides nutrition consultation to industry partners. AV is the Royal Canin Veterinary Diets Endowed Chair in Canine and Feline Clinical Nutrition and declares that they serve on the Health and Nutrition Advisory Board for Vetdiet. AV has also received honoraria and research funding from various pet food manufacturers and ingredient suppliers. The authors declare that these do not conflict with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Ethical approval: This work did not involve the use of animals and therefore ethical approval was not specifically required for publication in JFMS.
Informed consent: This work did not involve the use of animals (including cadavers) and therefore informed consent was not required. No animals or people are identifiable within this publication, and therefore additional informed consent for publication was not required.
ORCID iD: Hannah Godfrey  https://orcid.org/0000-0002-2102-6926
https://orcid.org/0000-0002-2102-6926
References
- 1. Michel K, Scherk M. From problem to success: feline weight loss programs that work. J Feline Med Surg 2012; 14: 327–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Courcier EA, O’Higgins R, Mellor DJ, et al. Prevalence and risk factors for feline obesity in a first opinion practice in Glasgow, Scotland. J Feline Med Surg 2010; 12: 746–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Colliard L, Paragon BM, Lemuet B, et al. Prevalence and risk factors of obesity in an urban population of healthy cats. J Feline Med Surg 2009; 11: 135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tarkosova D, Story MM, Rand JS, et al. Feline obesity – prevalence, risk factors, pathogenesis, associated conditions and assessment: a review. Veterinární Medicína 2016; 61: 295–307. [Google Scholar]
- 5. Mendes-Junior AF, Passos CB, Gáleas MAV, et al. Prevalence and risk factors of feline obesity in Alegre, Espírito Santo, Brazil. Semina Ciênc Agrár 2013; 34: 1801–1806. [Google Scholar]
- 6. Rioja-Lang F, Bacon H, Connor M, et al. Determining priority welfare issues for cats in the United Kingdom using expert consensus. Vet Rec Open 2019; 6. DOI: 10.1136/vetreco-2019-000365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. German AJ. The growing problem of obesity in dogs and cats. J Nutr 2006; 136 Suppl 7: 1940–1946. [DOI] [PubMed] [Google Scholar]
- 8. Lund EM, Armstrong PJ, Kirk CA, et al. Prevalence and risk factors for obesity in adult cats from private US veterinary practices. Intern J Appl Res Vet Med 2005; 3: 88–96. [Google Scholar]
- 9. Rand JS, Marshall RD. Diabetes mellitus in cats. Vet Clin North Am Small Anim Pract 2005; 35: 211–224. [DOI] [PubMed] [Google Scholar]
- 10. Scarlett JM, Donoghue S. Associations between body condition and disease in cats. J Am Vet Med Assoc 1998; 212: 1725–1731. [PubMed] [Google Scholar]
- 11. Cline MG, Burns KM, Coe JB, et al. 2021 AAHA nutrition and weight management guidelines for dogs and cats. J Am Anim Hosp Assoc 2021; 57: 153–178. [DOI] [PubMed] [Google Scholar]
- 12. Laflamme DP. Understanding and managing obesity in dogs and cats. Vet Clin North Am Small Anim Pract 2006; 36: 1283–1295. [DOI] [PubMed] [Google Scholar]
- 13. Grant CE, Shoveller AK, Blois S, et al. Dietary intake of amino acids and vitamins compared to NRC requirements in obese cats undergoing energy restriction for weight loss. BMC Vet Res 2020; 16: 426. DOI: 10.1186/s12917-020-02649-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Deagle G, Holden SL, Biourge V, et al. Long-term follow-up after weight management in obese cats. J Nutr Sci 2014; 3: e25. DOI: 10.1017/jns.2014.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Agriculture Canada. Sector trend analysis – pet food trends in Canada. https://agriculture.canada.ca/en/international-trade/market-intelligence/reports/sector-trend-analysis-pet-food-trends-canada (2021, accessed 30 June 2023).
- 16. American Pet Products Association. Pet industry market size, trends & ownership statistics. https://www.americanpetproducts.org/ (accessed 07 April 2023).
- 17. Bartlett P, Van Buren J. Counting the cost of chronic disease. https://www.dvm360.com/view/counting-cost-chronic-disease (2009, accessed 30 June 2023).
- 18. Bomberg E, Birch L, Endenburg N, et al. The financial costs, behaviour and psychology of obesity: a one health analysis. J Comp Pathol 2017; 156: 310–325. [DOI] [PubMed] [Google Scholar]
- 19. Banfield Pet Hospital. State of pet health®. https://www.banfield.com/pet-health/State-of-pet- (accessed 07 April 2023).
- 20. Hanford R, Linder DE. Impact of obesity on quality of life and owner’s perception of weight loss programs in cats. Vet Sci 2021; 8. DOI: 10.3390/vetsci8020032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sallander M, Eliasson J, Hedhammar Å. Prevalence and risk factors for the development of diabetes mellitus in Swedish cats. Acta Vet Scand 2012; 54. DOI: 10.1186/1751-0147-54-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kealy RD, Lawler DF, Ballam JM, et al. Effects of diet restriction on life span and age-related changes in dogs. J Am Vet Med Assoc 2002; 220: 1315–1320. [DOI] [PubMed] [Google Scholar]
- 23. Salt C, Morris PJ, Wilson D, et al. Association between life span and body condition in neutered client-owned dogs. J Vet Intern Med 2019; 33: 89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Serisier S, Feugier A, Venet C, et al. Faster growth rate in ad libitum-fed cats: a risk factor predicting the likelihood of becoming overweight during adulthood. J Nutr Sci 2013; 2: e11. DOI: 10.1017/jns.2013.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Courcier EA, Mellor DJ, Pendlebury E, et al. An investigation into the epidemiology of feline obesity in Great Britain: results of a cross-sectional study of 47 companion animal practises. Vet Rec 2012; 171: 560. DOI: 10.1136/vr.100953. [DOI] [PubMed] [Google Scholar]
- 26. Teng KT, McGreevy PD, Toribio JLML, et al. Risk factors for underweight and overweight in cats in metropolitan Sydney, Australia. Prev Vet Med 2017; 144: 102–111. [DOI] [PubMed] [Google Scholar]
- 27. Rowe E, Browne W, Casey R, et al. Risk factors identified for owner-reported feline obesity at around one year of age: dry diet and indoor lifestyle. Prev Vet Med 2015; 121: 273–281. [DOI] [PubMed] [Google Scholar]
- 28. Rowe EC, Browne WJ, Casey RA, et al. Early-life risk factors identified for owner-reported feline overweight and obesity at around two years of age. Prev Vet Med 2017; 143: 39–48. [DOI] [PubMed] [Google Scholar]
- 29. Camara A, Verbrugghe A, Cargo-Froom C, et al. The daytime feeding frequency affects appetite-regulating hormones, amino acids, physical activity, and respiratory quotient, but not energy expenditure, in adult cats fed regimens for 21 days. PLoS One 2020; 15. DOI: 10.1371/journal.pone.0238522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. German AJ, Holden SL, Mason SL, et al. Imprecision when using measuring cups to weigh out extruded dry kibbled food. J Anim Physiol Anim Nutr 2011; 95: 368–373. [DOI] [PubMed] [Google Scholar]
- 31. Kienzle E, Bergler R, Mandernach A. A comparison of the feeding behavior and the human–animal relationship in owners of normal and obese dogs. J Nutr 1998; 128: 2779S–2782S. [DOI] [PubMed] [Google Scholar]
- 32. Backus RC, Cave NJ, Keisler DH. Gonadectomy and high dietary fat but not high dietary carbohydrate induce gains in body weight and fat of domestic cats. Br J Nutr 2007; 98: 641–650. [DOI] [PubMed] [Google Scholar]
- 33. Fettman MJ, Stanton CA, Banks LL, et al. Effects of neutering on bodyweight, metabolic rate and glucose tolerance of domestic cats. Res Vet Sci 1997; 62: 131–136. [DOI] [PubMed] [Google Scholar]
- 34. Hoenig M, Ferguson DC. Effects of neutering on hormonal concentrations and energy requirements in male and female cats. Am J Vet Res 2002; 63: 634–639. [DOI] [PubMed] [Google Scholar]
- 35. Wei A, Fascetti AJ, Kim K, et al. Early effects of neutering on expenditure in adult male cats. PLoS One 2014; 9. DOI: 10.1371/journal.pone.0089557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Allaway D, Gilham M, Colyer A, et al. The impact of time of neutering on weight gain and energy intake in female kittens. J Nutr Sci 2017; 6: e19. DOI: 10.1017/jns.2017.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kanchuk ML, Backus RC, Calvert CC. Weight gain in normal and lipase–deficient male domestic cats results from increased food intake and not decreased energy expenditure. J Nutr 2003; 133: 1866–1874. [DOI] [PubMed] [Google Scholar]
- 38. Kanchuk ML, Backus RC, Calvert CC, et al. Neutering induces changes in food intake, body weight, plasma insulin and leptin concentrations in normal and lipoprotein lipase-deficient male cats. J Nutr 2002; 132: 1730–1732. [DOI] [PubMed] [Google Scholar]
- 39. Belsito KR, Vester BM, Keel T, et al. Impact of ovariohysterectomy and food intake on body composition, physical activity, and adipose gene expression in cats. J Anim Sci 2009; 87: 594–602. [DOI] [PubMed] [Google Scholar]
- 40. Flynn MF, Hardie EM, Armstrong PJ. Effect of ovariohysterectomy on maintenance energy requirement in cats. J Am Vet Med Assoc 1996; 209: 1572–1581. [PubMed] [Google Scholar]
- 41. Harper EJ, Stack DM, Watson TD, et al. Effects of feeding regimens on bodyweight, composition and condition score in cats following ovariohysterectomy. J Small Anim Pract 2001; 42: 433–438. [DOI] [PubMed] [Google Scholar]
- 42. Martin L, Siliart B, Dumon H, et al. Leptin, body fat content and energy expenditure in intact and gonadectomized adult cats: a preliminary study. J Anim Physiol Anim Nutr 2001; 85: 195–199. [DOI] [PubMed] [Google Scholar]
- 43. Mitsuhashi Y, Chamberlin AJ, Bigley KE, et al. Maintenance energy requirement determination of cats after spaying. Br J Nutr 2011; 106 Suppl 1: S135–138. [DOI] [PubMed] [Google Scholar]
- 44. Nguyen PG, Dumon HJ, Siliart BS, et al. Effects of dietary fat and energy on body weight and composition after gonadectomy in cats. Am J Vet Res 2004; 65: 1708–1713. [DOI] [PubMed] [Google Scholar]
- 45. Vester BM, Sutter SM, Keel TL, et al. Ovariohysterectomy alters body composition and adipose and skeletal muscle gene expression in cats fed a high-protein or moderate-protein diet. Animal 2009; 3: 1287–1298. [DOI] [PubMed] [Google Scholar]
- 46. Perrin T. The Business of Urban Animals Survey: the facts and statistics on companion animals in Canada. Can Vet J 2009; 50: 48–52. [PMC free article] [PubMed] [Google Scholar]
- 47. Chu K, Anderson WM, Rieser MY. Population characteristics and neuter status of cats living in households in the United States. J Am Vet Med Assoc 2009; 234: 1023–1030. [DOI] [PubMed] [Google Scholar]
- 48. Trevejo R, Yang M, Lund EM. Epidemiology of surgical castration of dogs and cats in the United States. J Am Vet Med Assoc 2011; 238: 898–904. [DOI] [PubMed] [Google Scholar]
- 49. Murray JK, Roberts MA, Whitmars A, et al. Survey of the characteristics of cats owned by households in the UK and factors affecting their neutered status. Vet Rec 2009; 164: 137–141. [DOI] [PubMed] [Google Scholar]
- 50. Reichler IM. Gonadectomy in cats and dogs: a review of risks and benefits. Reprod Domest Anim Zuchthyg 2009; 44 Suppl 2: 29–35. [DOI] [PubMed] [Google Scholar]
- 51. Salt C, Butterwick R, Henzel K, et al. Comparison of growth in neutered domestic shorthair kittens with growth in sexually-intact cats. PLoS One 2023; 18. DOI: 10.1371/journal.pone.0283016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Vendramini THA, Amaral AR, Pedrinelli V, et al. Neutering in dogs and cats: current scientific evidence and importance of adequate nutritional management. Nutr Res Rev 2020; 33: 134–144. [DOI] [PubMed] [Google Scholar]
- 53. AAFP Position Statement: pediatric sterilization in cats. J Feline Med Surg 2020; 22: 870. DOI: 10.1177/1098612X20948325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Federation of Veterinarians of Europe. Early neutering of kittens. https://fve.org/publications/early-neutering-of-kittens/ (2019, accessed 28 August 2023).
- 55. Gaillard V, Chastant S, England G, et al. Environmental risk factors in puppies and kittens for developing chronic disorders in adulthood: a call for research on developmental programming. Front Vet Sci 2022; 9. DOI: 10.3389/fvets.2022.944821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Reilly JJ, Armstrong J, Dorosty AR, et al. Early life risk factors for obesity in childhood: cohort study. BMJ 2005; 330: 1357. DOI: 10.1136/bmj.38470.670903.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Barker DJP, Eriksson JG, Forsén T, et al. Fetal origins of adult disease: strength of effects and biological basis. Int J Epidemiol 2002; 31: 1235–1239. [DOI] [PubMed] [Google Scholar]
- 58. Sun SS, Liang R, Huang TTK, et al. Childhood obesity predicts adult metabolic syndrome: the Fels Longitudinal Study. J Pediatr 2008; 152: 191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Liang Y, Hou D, Zhao X, et al. Childhood obesity affects adult metabolic syndrome and diabetes. Endocrine 2015; 50: 87–92. [DOI] [PubMed] [Google Scholar]
- 60. Umer A, Kelley GA, Cottrell LE, et al. Childhood obesity and adult cardiovascular disease risk factors: a systematic review with meta-analysis. BMC Public Health 2017; 17: 683. DOI: 10.1186/s12889-017-4691-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Linder D, Mueller M. Pet obesity management: beyond nutrition. Vet Clin North Am Small Anim Pract 2014; 44: 789–806. [DOI] [PubMed] [Google Scholar]
- 62. Zoran DL, Rand JS. The role of diet in the prevention and management of feline diabetes. Vet Clin North Am Small Anim Pract 2013; 43: 233–243. [DOI] [PubMed] [Google Scholar]
- 63. Cave NJ, Bridges JP, Weidgraaf K, et al. Nonlinear mixed models of growth curves from domestic shorthair cats in a breeding colony, housed in a seasonal facility to predict obesity. J Anim Physiol Anim Nutr 2018; 102: 1390–1400. [DOI] [PubMed] [Google Scholar]
- 64. Gross KL, Becvarova I, Debraekeleer J. Feeding growing kittens: postweaning to adulthood. In: Hand MS, Lewis LD. (eds). Small animal clinical nutrition. 5th ed. Topeka: Mark Morris Institute, 2010, pp 429–436. [Google Scholar]
- 65. Case LP, Daristotle L, Hayek MG, et al. Canine and feline nutrition: a resource for companion animal professionals. 3rd ed. Toronto: Elsevier, 2010. [Google Scholar]
- 66. Salt C, German AJ, Henzel KS, et al. Growth standard charts for monitoring bodyweight in intact domestic shorthair kittens from the USA. PLoS One 2022; 17. DOI: 10.1371/journal.pone.0277531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Sutherland KA, Coe JB, O’Sullivan TL. Exploring veterinary professionals’ perceptions of pet weight-related communication in companion animal veterinary practice. Vet Rec 2023; 192. DOI: 10.1002/vetr.1973. [DOI] [PubMed] [Google Scholar]
- 68. Churchill J, Ward E. Communicating with pet owners about obesity. Vet Clin North Am Small Anim Pract 2016; 46: 899–911. [DOI] [PubMed] [Google Scholar]
- 69. Phillips AM, Coe JB, Rock MJ, et al. Feline obesity in veterinary medicine: insights from a thematic analysis of communication in practice. Front Vet Sci 2017; 4. DOI: 10.3389/fvets.2017.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Lue TW, Pantenburg DP, Crawford PM. Impact of the owner-pet and client-veterinarian bond on the care that pets receive. J Am Vet Med Assoc 2008; 232: 531–540. [DOI] [PubMed] [Google Scholar]
- 71. Abood SK. Effectively communicating with your clients. Top Companion Anim Med 2008; 23: 143–147. [DOI] [PubMed] [Google Scholar]
- 72. Coe JB, Adams CL, Eva K, et al. Development and validation of an instrument for measuring appointment-specific client satisfaction in companion-animal practice. Prev Vet Med 2010; 93: 201–210. [DOI] [PubMed] [Google Scholar]
- 73. Dysart LMA, Coe JB, Adams CL. Analysis of solicitation of client concerns in companion animal practice. J Am Vet Med Assoc 2011; 238: 1609–1615. [DOI] [PubMed] [Google Scholar]
- 74. Kanji N, Coe JB, Adams CL, et al. Effect of veterinarian-client-patient interactions on client adherence to dentistry and surgery recommendations in companion-animal practice. J Am Vet Med Assoc 2012; 240: 427–436. [DOI] [PubMed] [Google Scholar]
- 75. Sutherland KA, Coe JB, O’Sullivan TL. Assessing owners’ readiness to change their behaviour to address their companion animal’s obesity. Vet Rec 2023; 192. DOI: 10.1002/vetr.1979. [DOI] [PubMed] [Google Scholar]
- 76. Kipperman BS, German AJ. The responsibility of veterinarians to address companion animal obesity. Animals 2018; 8. DOI: 10.3390/ani8090143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Bartges J, Kushner RF, Michel KE, et al. One health solutions to obesity in people and their pets. J Comp Pathol 2017; 156: 326–333. [DOI] [PubMed] [Google Scholar]
- 78. Sutherland KA, Coe JB, Janke N, et al. Pet owners’ and companion animal veterinarians’ perceptions of weight-related veterinarian-client communication. J Am Vet Med Assoc 2022; 260: 1697–1703. [DOI] [PubMed] [Google Scholar]
- 79. Peron L, Rahal SC, Castilho MS, et al. Owner’s perception for detecting feline body condition based on questionnaire and scores. Top Companion Anim Med 2016; 31: 122–124. [DOI] [PubMed] [Google Scholar]
- 80. Teixeira FA, Queiroz MR, Oba PM, et al. Brazilian owners perception of the body condition score of dogs and cats. BMC Vet Res 2020; 16: 463. DOI: 10.1186/s12917-020-02679-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Quimby J, Gowland S, Carney HC, et al. 2021 AAHA/AAFP feline life stage guidelines. J Feline Med Surg 2021; 23: 211–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Shaw JR, Bonnett BN, Adams CL, et al. Veterinarian-client-patient communication patterns used during clinical appointments in companion animal practice. J Am Vet Med Assoc 2006; 228: 714–721. [DOI] [PubMed] [Google Scholar]
- 83. Baldwin K, Bartges J, Buffington T, et al. AAHA nutritional assessment guidelines for dogs and cats. J Am Anim Hosp Assoc 2010; 46: 285–296. [DOI] [PubMed] [Google Scholar]
- 84. Shepherd M. Canine and feline obesity management. Vet Clin North Am Small Anim Pract 2021; 51: 653–667. [DOI] [PubMed] [Google Scholar]
- 85. Freeman L, Becvarova I, Cave N, et al. WSAVA nutritional assessment guidelines. J Small Anim Pract 2011; 52: 385–396. [DOI] [PubMed] [Google Scholar]
- 86. American Animal Hospital Association-American Veterinary Medical Association Preventive Healthcare Guidelines Task Force. Development of new canine and feline preventive healthcare guidelines designed to improve pet health. J Am Anim Hosp Assoc 2011; 47: 306–311. [DOI] [PubMed] [Google Scholar]
- 87. Laflamme DP. Development and validation of a body condition score system for cats: a clinical tool. Feline Practice 1997; 25: 13–18. [Google Scholar]
- 88. Michel KE, Anderson W, Cupp C, et al. Correlation of a feline muscle mass score with body composition determined by dual-energy X-ray absorptiometry. Br J Nutr 2011; 106: S57–59. [DOI] [PubMed] [Google Scholar]
- 89. Cline MG, Murphy M. Obesity in the dog and cat. 1st ed. Boca Raton: CRC Press, 2019. [Google Scholar]
- 90. Levine JA. Measurement of energy expenditure. Public Health Nutr 2005; 8: 1123–1132. [DOI] [PubMed] [Google Scholar]
- 91. Schoeller DA, Cook CM, Raman A. Energy expenditure: indirect calorimetry. In: Caballero B. (ed). Encyclopedia of human nutrition. 3rd ed. Oxford: Elsevier, 2012, pp 170–176. [Google Scholar]
- 92. National Research Council. Nutrient requirements of dogs and cats. Washington DC: The National Academies Press, 2006, pp 354–370. [Google Scholar]
- 93. Merenda MEZ, Sato J, Scheibel S, et al. Growth curve and energy intake in male and female cats. Top Companion Anim Med 2021; 44. DOI: 10.1016/j.tcam.2021.100518. [DOI] [PubMed] [Google Scholar]
- 94. Gooding MA, Duncan IJH, Atkinson JL, et al. Development and validation of a behavioral acclimation protocol for cats to respiration chambers used for indirect calorimetry studies. J Appl Anim Welf Sci 2012; 15: 144–162. [DOI] [PubMed] [Google Scholar]
- 95. Coe JB, Rankovic A, Edwards TR, et al. Dog owner’s accuracy measuring different volumes of dry dog food using three different measuring devices. Vet Rec 2019; 185: 599. DOI: 10.1136/vr.105319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Witzel-Rollins A, Murphy M, Springer CM, et al. Evaluation of a pet-separating automatic feeder and high-frequency meal feeding for weight loss in multi-cat households. J Feline Med Surg 2022; 24: e281–e288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Sadek T, Hamper B, Horwitz D, et al. Feline feeding programs: addressing behavioural needs to improve feline health and wellbeing. J Feline Med Surg 2018; 20: 1049–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Hadar BN, Lambrecht KJ, Poljak Z, et al. Technology-enhanced weight-loss program in multiple-cat households: a randomized controlled trial. J Feline Med Surg 2022; 24: 726–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Naik R, Witzel A, Albright JD, et al. Pilot study evaluating the effect of feeding method on overall activity of neutered indoor pet cats. J Vet Behav 2018; 25: 9–13. [Google Scholar]
- 100. Kienzle E, Bergler R. Human-animal relationship of owners of normal and overweight cats. J Nutr 2006; 136: 1947S–1950S. [DOI] [PubMed] [Google Scholar]
- 101. Henning JSL, Nielsen T, Fernandez E, et al. Factors associated with play behavior in human-cat dyads. J Vet Behav 2022; 52–53: 21–30. [Google Scholar]
- 102. Lawler DF. Neonatal and pediatric care of the puppy and kitten. Theriogenology 2008; 70: 384–392. [DOI] [PubMed] [Google Scholar]
- 103. Ha MA, Jarvis MC, Mann JI. A definition for dietary fibre. Eur J Clin Nutr 2000; 54: 861–864. [DOI] [PubMed] [Google Scholar]
- 104. Wei A, Fascetti AJ, Liu KJ, et al. Influence of a high-protein diet on energy balance in obese cats allowed ad libitum access to food. J Anim Physiol Anim Nutr 2011; 95: 359–367. [DOI] [PubMed] [Google Scholar]
- 105. Gooding MA, Atkinson JL, Duncan IJH, et al. Dietary fat and carbohydrate have different effects on body weight, energy expenditure, glucose homeostasis and behaviour in adult cats fed to energy requirement. J Nutr Sci 2015; 4: e2. DOI: 10.1017/jns.2014.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Anderson JW, Akanji AO. Dietary fiber – an overview. Diabetes Care 1991; 14: 1126–1131. [DOI] [PubMed] [Google Scholar]
- 107. Butowski CF, Thomas DG, Cave NJ, et al. In vitro assessment of hydrolysed collagen fermentation using domestic cat (Felis catus) faecal inocula. Animals 2022; 12. DOI: 10.3390/ani12040498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Butowski CF, Thomas DG, Young W, et al. Addition of plant dietary fibre to a raw red meat high protein, high fat diet, alters the faecal bacteriome and organic acid profiles of the domestic cat (Felis catus). PLoS One 2019; 14. DOI: 10.1371/journal.pone.0216072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Fischer MM, Kessler AM, de Sá LRM, et al. Fiber fermentability effects on energy and macronutrient digestibility, fecal traits, postprandial metabolite responses, and colon histology of overweight cats. J Anim Sci 2012; 90: 2233–2245. [DOI] [PubMed] [Google Scholar]
- 110. Cline M, Witzel AL, Moyers T, et al. Comparison of high fiber and low carbohydrate diets on owner-perceived satiety of cats during weight loss. Am J Anim Vet Sci 2012; 7: 218–225. [Google Scholar]
- 111. Bissot T, Servet E, Vidal S, et al. Novel dietary strategies can improve the outcome of weight loss programmes in obese client-owned cats. J Feline Med Surg 2010; 12: 104–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Bennett N, Greco DS, Peterson ME, et al. Comparison of a low carbohydrate–low fiber diet and a moderate carbohydrate–high fiber diet in the management of feline diabetes mellitus. J Feline Med Surg 2006; 8: 73–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Moreno AA, Parker VJ, Winston JA, et al. Dietary fiber aids in the management of canine and feline gastrointestinal disease. J Am Vet Med Assoc 2022; 260: S33–S45. [DOI] [PubMed] [Google Scholar]
- 114. Nelson RW, Scott-Moncrieff JC, Feldman EC, et al. Effect of dietary insoluble fiber on control of glycemia in cats with naturally acquired diabetes mellitus. J Am Vet Med Assoc 2000; 216: 1082–1088. [DOI] [PubMed] [Google Scholar]
- 115. Pallotto MR, Godoy MRC de, Holscher HD, et al. Effects of weight loss with a moderate-protein, high-fiber diet on body composition, voluntary physical activity, and fecal microbiota of obese cats. Am J Vet Res 2018; 79: 181–190. [DOI] [PubMed] [Google Scholar]
- 116. Bian Z, Jian X, Liu G, et al. Wet-food diet promotes the recovery from surgery of castration and control of body weight in adult young cats. J Anim Sci 2023; 101. DOI: 10.1093/jas/skad039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Cameron KM, Morris PJ, Hackett RM, et al. The effects of increasing water content to reduce the energy density of the diet on body mass changes following caloric restriction in domestic cats. J Anim Physiol Anim Nutr 2011; 95: 399–408. [DOI] [PubMed] [Google Scholar]
- 118. Schleicher M, Cash SB, Freeman LM. Determinants of pet food purchasing decisions. Can Vet J 2019; 60: 644–650. [PMC free article] [PubMed] [Google Scholar]