Abstract
Dementia represents a growing public health burden with large social, racial, and ethnic disparities. The etiology of dementia is poorly understood, and the lack of robust biomarkers in diverse, population-representative samples is a barrier to moving dementia research forward. Existing biomarkers and other measures of pathology—derived from neuropathology, neuroimaging, and cerebrospinal fluid samples—are commonly collected from predominantly White and highly educated samples drawn from academic medical centers in urban settings. Blood-based biomarkers are noninvasive and less expensive, offering promise to expand our understanding of the pathophysiology of dementia, including in participants from historically excluded groups. Although largely not yet approved by the Food and Drug Administration or used in clinical settings, blood-based biomarkers are increasingly included in epidemiologic studies on dementia. Blood-based biomarkers in epidemiologic research may allow the field to more accurately understand the multifactorial etiology and sequence of events that characterize dementia-related pathophysiological changes. As blood-based dementia biomarkers continue to be developed and incorporated into research and practice, we outline considerations for using them in dementia epidemiology, and illustrate key concepts with Alzheimer’s Disease Neuroimaging Initiative (2003–present) data. We focus on measurement, including both validity and reliability, and on the use of dementia blood-based biomarkers to promote equity in dementia research and cognitive aging.
This article is part of a Special Collection on Mental Health.
Keywords: Alzheimer disease, biomarker, blood-based biomarker, dementia
Abbreviations
- Aβ
amyloid-beta
- AD
Alzheimer disease
- ADNI
Alzheimer’s Disease Neuroimaging Initiative
- CSF
cerebrospinal fluid
- MRI
magnetic resonance imaging
- NfL
neurofilament light
- PET
positron emission tomography
- p-tau
phosphorylated tau
Dementia, a clinical syndrome characterized by cognitive impairment that interferes with a person’s daily life and activities, is a large and growing public health burden (1–3). In addition, major social, racial, and ethnic inequalities in dementia make it a priority for health equity researchers (1). Dementia diagnosis does not imply a specific pathology but may reflect the functional consequences of several different pathological processes. Biomarkers (measures of substances in the body that are indicators of a disease or condition (4)) for pathologies contributing to dementia are not commonly used in clinical practice but are important research tools to advance the understanding of disease biology, prevention, and treatment. For example, biomarkers often precede clinical manifestations of disease, and thus can help establish temporal order between certain exposures and disease outcomes. Biomarkers play an important role in distinguishing different underlying pathologies, such as distinguishing dementia due to Alzheimer disease (AD) from dementia due to vascular disease. Development of disease-modifying treatments (i.e., therapies that slow disease progression) has been challenging, and improved understanding of etiology would likely accelerate treatment research (5–10). However, dementia biomarkers present significant challenges for study design, analysis, and interpretation.
Blood-based biomarkers for dementia, particularly AD pathologies, are a relatively easy-to-implement modality and area of rapid development in dementia research. Although largely not yet approved by the Food and Drug Administration for use in clinical settings (11), blood-based biomarkers are increasingly included in epidemiologic research studies on dementia, and will soon be available in other commonly used data sets, such as the Health and Retirement Study. In this article, we discuss the potential and limitations of blood-based biomarkers for epidemiologic dementia research. We start with an introduction to existing dementia biomarkers and pathology measures, and the relatively new modality of blood-based biomarkers, and then highlight some technical considerations for using and interpreting blood-based biomarkers in dementia epidemiology. Finally, we discuss the use of dementia blood-based biomarkers to promote equity in dementia research.
OVERVIEW OF PATHOLOGY MEASURES AND BIOMARKERS IN DEMENTIA RESEARCH
Dementia is a clinical syndrome that can result from a heterogeneous set of diseases, but the most common, known pathologies are AD (amyloid and tau) and cerebrovascular disease. Other pathologies include alpha synuclein (e.g., Lewy body disease), TAR DNA-binding protein 43 (frontotemporal lobar degeneration-TDP-43 and limbic-predominant age-related TDP-43 encephalopathy), and tau without the presence of significant amyloid (e.g., tau-positive frontotemporal dementia, chronic traumatic encephalopathy, and primary age-related tauopathy). Most older adults with dementia have multiple neuropathologies (1). Importantly, clinical signs and symptoms can only be attributed to pathologies that are measured, a particularly substantial concern in dementia given the prevalence of co-occurring pathologies and the likelihood that mixed pathologies are a major contributor to clinical disease. However, since measurement of AD pathology (amyloid and tau) has been a scientific focus in the field, much of our technical discussion focuses on these measures.
Historically, measures of dementia pathology have been difficult to obtain. Although neuropathological data are considered the gold standard, they are available only postmortem (12), making etiological analyses of cognitive change challenging. Not only is postmortem brain tissue rarely available, the temporality between the neuropathological measure and cognitive function is unclear. Commonly used in vivo measures of pathology in cognitive aging research include magnetic resonance imaging (MRI) measures (of brain structure, metabolism, and function) and positron emission tomography (PET) or cerebrospinal fluid (CSF) measures of amyloid and tau proteinopathies, key biomarkers in distinguishing AD from other dementias. Table 1 summarizes the most widely used measures in epidemiologic analyses. However, due to challenges associated with obtaining these measures, many dementia studies with robust pathology measures rely on small, selected samples of predominantly White and highly educated individuals (e.g., Alzheimer’s Disease Neuroimaging Initiative (ADNI) and National Institute on Aging Alzheimer’s Disease Research Center Cohorts). Many such studies recruit primarily from patients who attend memory clinics seeking care, so selection on the outcome of interest is built into the study design.
Table 1.
Summary of Traditional Pathology Measures in Cognitive Aging Research
| Modality | Measure | Target of Measurement | Limitations of Measure |
|---|---|---|---|
| PET measures | Amyloid-PET | Measures fibrillar beta-amyloid burden | Radiation exposure due to ligand; costly, time-consuming, and logistically complex (requires availability of both PET scanners and radiotracers); limited sensitivity in early stages of AD. PET analyses are more precise if accompanied by structural MRI or CT scan. FDG-PET is not specific to AD. |
| Tau-PET | Measures AD tau burden | ||
| Fluorodeoxy-glucose-PET | Measures regions of glucose hypometabolism that occur with increasing neurodegeneration | ||
| Structural MRI measures | Volumetric measures and cortical thickness measures (e.g., total brain volume, hippocampal volume, cortical thickness of AD-signature regions) | Measures of atrophy/neurodegeneration | Not specific to AD or other types of dementia; some changes extremely common in older adults and may be causes or consequences of other pathologies; cannot be used in certain patient populations (e.g., persons with cochlear implants) |
| White matter hyperintensity volumes, and other less commonly used structural MRI measures, including infarctions, microbleeds, and perivascular spaces, as well as other measures of white-matter integrity (diffusion-tensor imaging) | Measure of vascular burden | Although considered indicative of vascular dementia risk, some changes are common in older adults; cannot be used in certain patient populations (e.g., persons with cochlear implants) | |
| CSF biomarkers | Various amyloid and tau measures | Correlates of rates of accumulation of amyloid and tau versus overall cumulative measures (burden). | Invasive (requires lumbar puncture), risk of subsequent complications; requires consideration of storage and analytical conditions; NfL is not disease-specific |
| Neurofilament light | Neuronal scaffolding protein; correlated with rate of neurodegeneration |
Abbreviations: AD, Alzheimer’s disease; CSF, cerebrospinal fluid; CT, computed tomography; FDG, fluorodeoxy-glucose; MRI, magnetic resonance imaging; NfL, neurofilament light; PET, positron emission tomography.
Dementia biomarkers (including those measuring AD pathology) measured in blood may offer an avenue to incorporate biological measures into dementia research in broader samples (13). Because they are obtained from simple blood draws, blood-based biomarkers pose substantially less burden on participants than neuroimaging measures or CSF-based biomarkers: Unlike imaging, they do not require clinic visits; do not have any size or weight limits for collection; can be collected without risk in individuals with shrapnel, pacemakers, and cochlear implants; and do not involve exposure to ionizing radiation (unlike PET). Unlike CSF collection, blood-based biomarkers do not require an invasive procedure, and blood collection can be performed by a phlebotomist. The ease of administration and relatively low logistical burden and cost of blood-based biomarkers also make them well-suited for repeat/follow-up visits in large, population-representative, longitudinal cohort studies. Because these measures can be obtained from stored samples, they also allow for biomarker assessment in existing studies that have banked samples.
Table 2 summarizes commonly used blood-based dementia biomarkers. Consistent with the emphasis on AD pathology in the literature, we focus on amyloid-beta and tau, specifically the ratio of amyloid-beta (Aβ)-42 to Aβ-40 (Aβ-42/40) and phosphorylated tau (p-tau) measures. Like their cerebrospinal fluid counterparts, higher levels of the ratio (Aβ-42/40) in blood are correlated with less AD pathology since they are indicative of improved clearance of more pathogenic forms of amyloid, and, conversely, high levels of p-tau are associated with more AD pathology. We also consider a nonspecific biomarker of neurodegeneration, neurofilament light (NfL), which may be useful in the context of vascular and other neurodegenerative disease, in addition to AD, with higher levels indicative of more neurodegeneration. However, many of the technical concepts we highlight are relevant for blood-based biomarkers in epidemiologic research more broadly, including other less commonly available dementia biomarkers such as measures of vascular dementia (in development and early implementation (14–16)) and TDP-43 and alpha synuclein (yet to be developed). Given rapid developments in dementia blood-based biomarkers and changes in price, their availability in epidemiologic cohorts can be expected to change in the coming years.
Table 2.
Emerging Blood-Based Biomarkers in Cognitive Aging Researcha
| ATN Framework Target | Blood-Based Biomarker Measuresb | Target of Measurement |
|---|---|---|
| Amyloid | Aβ-40, Aβ-42, Aβ-42/40 ratio | Measures Aβ in blood; higher Aβ-42/40 ratios thought to be indicative of less brain accumulation (greater clearance of the more pathogenic form) |
| Tau | Various p-tau measures (e.g., p-tau 181, p-tau 217) | Measures p-tau in blood |
| Neurodegeneration | Neurofilament light | Measures quantity of a neuronal scaffolding protein in blood |
Abbreviations: Aβ, amyloid-beta; ATN, amyloid, tau, neurodegeneration; p-tau, phosphorylated tau.
a We focus on the most common and best-validated measures in our assessment. Other measures include glial fibrillary acidic protein measures, as well as vascular disease markers that are in the early stages of development.
b Number designations for amyloid (e.g., 40, 42) refer to the number of residues in the fragment; number designations for p-tau refer to residue of phosphorylation.
Issues related to validity and reliability of blood-based biomarker levels
Lack of clear gold standard.
Novel biomarkers need to be validated, but the appropriate standard against which to compare blood-based biomarkers for dementia is unclear. Neuropathology is typically considered the gold standard biomarker but has limited feasibility due to the cost, logistics (including brain donation willingness), and expertise required, as well as longer-running studies required to obtain sufficient sample sizes. Further, proteinopathies and vascular burden measures in neuropathological studies are categorized into discrete staging categories (i.e., 6 Thal phases, 7 Braak stages, and 4 Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) scores (17)), leading to standard issues with information loss with discretization when comparing with another measure (18–20). In addition, while interrater reliability is thought to be high for diagnosis of AD, reliability may be lower for individual staging categories and can appreciably differ across laboratories (21). Finally, neuropathology can only be obtained postmortem and does not yield information about the development and impact of pathology during the life course.
Neuroimaging measures have been used as an alternative gold standard for validating blood-based biomarkers (22–24) but have limitations of their own. Neuroimaging data often have sampling limitations similar to those in neuropathology studies, as well as additional measurement issues (25). For example, PET measurements are collected as continuous measures, but measurement error and comparability across scanners, tracers, and reference brain regions used are concerns, even within a single study. As a result, PET data are often dichotomized (e.g., amyloid-positive versus amyloid‑negative), but differences in PET levels within amyloid categories have been found to predict cognition (e.g., van der Kall et al. (26)), indicating potentially important information loss with dichotomization. Thus, neither continuous PET measures nor dichotomized positivity measures from PET imaging are optimal gold standards against which to validate blood-based biomarkers.
Finally, since the goal is to identify biomarkers that accurately predict functioning, cognitive measures and clinical diagnosis have been used instead of PET measures to validate blood-based measures (22). However, this also comes with pitfalls since cognitive test scores reflect many factors besides brain pathology, such as cognitive skills developed in early life, familiarity with cognitive tests, interviewer effects, medication use, sleep loss, and many other sources of short-term variability. As a result, the correlation between brain pathology and cognitive measures will never be perfect and may vary across populations for many reasons unrelated to the biomarker (e.g., educational attainment of the participants).
Stage of disease measured.
The physiological separation between blood and brain presents another challenge to interpretation of blood-based biomarkers. In fact, blood-based measures may be capturing proteins that are not bound up in the brain (i.e., proteins circulating in the bloodstream). Due to the blood-brain barrier, blood-based biomarkers also capture processes that are occurring unrelated to the brain. As a result, blood-based amyloid measures (such as Aβ-42/40) do not directly correspond with what is being measured by neuroimaging modalities such as amyloid-PET, nor do blood-based phosphorylated tau measures (p-tau 181, 217, and 231, depending on the phosphorylation residue) correspond precisely with tau-PET measures. To the contrary, it has been suggested that fluid (blood-based and CSF) measures of amyloid and tau could be measures of rate of accumulation rather than total burden of the protein being measured (27). However, there is not enough evidence to determine whether this is plausible across all disease stages, particularly late stages.
Evidence to date indicates that blood-based p-tau measures correlate best with neuroimaging-based measures of amyloid in later-accumulating brain regions (i.e., widespread cortical amyloid burden (27, 28)). These measures are also correlated with imaging-based measures of tau burden, although more weakly than for amyloid, and predict future imaging-based measures of tau accumulation (27). In fact, blood-based p-tau may be a more valid measure of cerebral amyloid burden than blood-based amyloid measures (29). This could be because p-tau measures correlate with rate of tau deposition, and, under the dominant (Jack et al. (30, 31)) model of Alzheimer disease progression, tau deposition is a consequence of amyloid burden. Figure 1A, using data from ADNI, shows that Aβ-42/40 (ratio of Aβ-42 to Aβ-40) is inversely correlated with amyloid-PET-positivity, but discrimination between amyloid-positive and amyloid‑negative individuals using this measure is poor (32).
Figure 1.
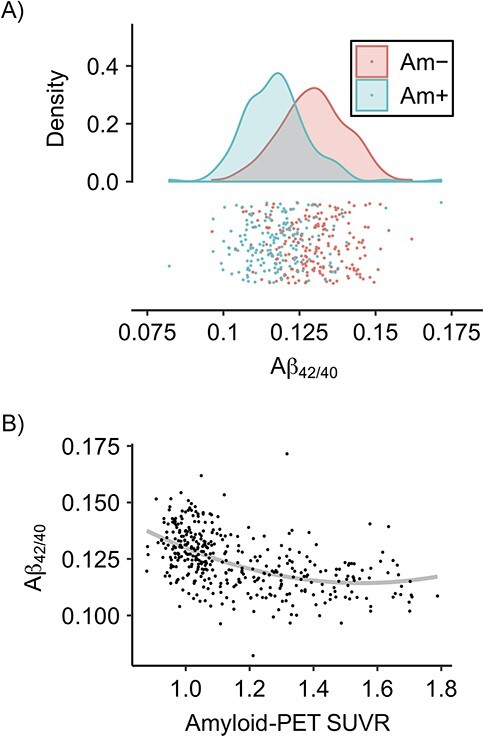
Measurement issues for blood-based biomarkers, illustrated with data from the Alzheimer’s Disease Neuroimaging Initiative (ADNI), United States, 2003 to present. A) Distribution of amyloid-beta (Aβ)-42 to Aβ-40 (Aβ-42/40) ratios by amyloid positivity (Am+) and amyloid negativity (Am−) as determined by amyloid-positron emission tomography (PET). While the means differ for these 2 groups, the distributions overlap considerably, indicating that the discriminatory ability of the blood-based biomarker for amyloid positivity is not high. B) Aβ-42/40 plotted against continuous global amyloid-PET standardized uptake value ratio (SUVR; florbetapir) using data from ADNI. The gray line gives the best quadratic fit. There is an association between plasma and PET measures, with high Aβ-42/40 indicative of lower global SUVR. However, the significant amount of noise or within-person variability, along with differences in dynamic range, make a one-to-one mapping between one measure and the other difficult. Data used in the preparation of this figure were obtained from the ADNI database (https://adni.loni.usc.edu/). The ADNI was launched in 2003 as a public-private partnership, led by Principal Investigator Michael W. Weiner, MD. The primary goal of ADNI has been to test whether serial magnetic resonance imaging (MRI), PET, other biological markers, and clinical and neuropsychological assessment can be combined to measure the progression of mild cognitive impairment and early Alzheimer disease. For up-to-date information, see https://adni.loni.usc.edu/. Aβ-42/40 data was obtained from the 2022 Bateman lab data (liquid chromatography-tandem mass spectrometry).
Differences in the dynamic range of blood-based and PET measures provide additional evidence that disease stages measured by each may differ. Dynamic range refers to the range of values a measure can take on. The dynamic range of a biomarker determines the range of disease stages at which the biomarker is sensitive to changes (analogous to item difficulty in the measurement literature) (30). PET measures for amyloid and tau may have different dynamic ranges compared with their blood-based biomarker analogues (33). This means that the 2 biomarkers may be sensitive to very different stages of pathology accumulation. The dynamic range for blood-based biomarker levels (i.e., the region where a change the biomarker level indicates a change in burden of pathology) may fall below PET levels that would be considered amyloid- and tau-positive (Figure 1B and, more compellingly, CSF-amyloid examples in Pichet Binette et al. (34)). That is, a blood-based biomarker might be able to distinguish between someone with no disease versus early disease but not be able to distinguish someone with early versus late disease, whereas a PET can distinguish someone with early versus late disease, but not necessarily detect transition into the earliest disease stages. This also means that blood-based measures may be more highly correlated with PET measures in specific ranges (e.g., near the median), potentially yielding differential measurement error based on disease stage when defining PET measures as the gold standard. These issues are likely most relevant to Aβ-42/40 measures, and more work remains to be done in this area to understand the implications of different dynamic range for the use of blood-based biomarkers in epidemiologic studies.
Complementary information from blood-based and PET measures may be valuable. However, this is a limitation if the intent is to use blood-based measures in lieu of PET measures, particularly in populations with higher levels of pathology, such as individuals with dementia. Additional blood-based biomarkers indicative of later-stage diseases, such as NfL, discussed below, might remedy this (34, 35).
Nonspecificity of neurodegeneration measures.
NfL is an axonal scaffolding protein and as neurons die, their axons degrade and release NfL into the extracellular space. Thus, the presence of NfL in CSF, and subsequently peripheral blood, is indicative of neurodegeneration (36). NfL is a useful measure for later-stage disease, while earlier-stage disease is better captured by blood-based amyloid and tau (37). Blood-based NfL correlates with MRI measures of brain atrophy, as well as amyloid and tau burden (37, 38). However, since NfL is a nonspecific marker, it is elevated in diseases other than Alzheimer disease that result in neuronal death. Most relevantly, NfL is elevated in vascular (39, 40) and frontotemporal (40) dementia, as well as in diseases that occur outside of the context of cognitive aging, such as multiple sclerosis (41). To complicate matters, NfL levels may increase with age independent of pathology (37).
Differences in molecular targets and laboratory techniques.
Blood-based measures may present challenges for harmonization across data sets if assays measuring different protein targets are used. For example, p-tau 181 and p-tau 217 refer to tau phosphorylated at the 181st and 217th amino acid residues, respectively. Both measures have been separately validated, with some evidence of better performance of p-tau 217 than p-tau 181 when compared with tau-PET (42). The 2 measures have different slopes when plotted against PET measures of amyloid and tau burden (43).
More generally, inconsistency across laboratories exacerbates heterogeneity. Different assays (e.g., using different antibodies), and a specific assay versus mass spectrometry measuring the same form of the same biomarker (e.g., Aβ-42 or p-tau 181), perform differently and are often not co-calibrated (32, 39–41). This presents a similar challenge to harmonization of imaging data using different PET tracers, scanners, or reference regions (a brain region with very little amyloid accumulation used to scale PET signals in brain regions of interest). Standardized calibration approaches in the future may alleviate concerns related to laboratory techniques impacting measures, but researchers must recognize that such standardization protocols are not yet consistently adopted (44).
Test-retest reliability and noise.
Blood-based measures may have low test-retest reliability and vary between individuals due to individual factors not directly measuring brain disease, such as kidney and liver function (33, 34, 37, 38, 45–48) or fasting status (49). Unreliability may be due to a variety of factors related to sample processing and storage, the assay used, and endogenous variability in biomarker levels (45). Low reliability offsets the benefit of the larger sample sizes achievable with blood-based measures. Because of the random variability in blood-based measures, larger sample sizes may be needed to obtain precise effect estimates (32). In contrast, amyloid- and tau-PET have high test-retest reliability (50–53). Such limitations can be addressed with increased sample size or potentially by using multiple measures to make inferences about disease states, such as using both Aβ-42/40 ratios and p-tau measures to make inferences about disease burden. Other sources of noise may also contribute to poor discrimination between amyloid-positive and amyloid‑negative individuals using this measure (Figure 1A).
Equity considerations for blood-based biomarkers
Despite some of the technical challenges to overcome, a key promise of blood-based biomarkers in epidemiologic dementia research is to increase representation of Black, Latino, and other historically excluded populations in cognitive aging research by decreasing barriers to study participation and costs. Availability of biomarkers in population-based studies may help to reveal drivers of dementia and racial and ethnic disparities in dementia, by facilitating the study of sociocultural exposures, structural racism, and other social determinants of health measures with dementia outcomes, including mechanisms more closely tied to specific pathophysiological processes. Specifically, biomarkers in diverse samples can help investigators understand how social determinants of health, including life-course sociocultural and structural exposures, “get under the skin” and contribute to pathophysiological changes related to dementia, and inform our understanding of embodiment of social exposures and of shared biological processes in diverse groups of people.
However, to fulfill this promise and avoid bias, blood-based biomarkers must have similar validity and reliability across groups to avoid differential measurement error. Research on health disparities relies on correctly characterizing true differences and not conflating differential measurement performance with population-level differences. This is even more critical if blood-based biomarkers are to be incorporated into clinical care—a stated goal of many advocates of blood-based biomarkers. Differential accuracy across racial and ethnic groups could affect access to care and exacerbate health disparities in dementia, as it has in other domains (54–56). Biomarkers may perform differently across socially constructed racial groups due to the life-course embodiment of racially stratified social experiences, such as encounters with interpersonal and structural racism (e.g., comorbidity prevalence, discussed in detail below). On a more prosaic level, biomarkers in many disease areas have been developed and optimized using data from predominantly White populations, and any systematic phenotypic differences—even as seemingly unrelated to the biology of dementia (57)—may compromise the performance of biomarkers in unanticipated ways (e.g., Sjoding et al. (56)).
Unequal performance of blood-based biomarkers across racial groups is anticipated due to racially patterned comorbidity prevalence (58) and vascular burden of disease (59, 60), themselves driven by upstream social determinants of health such as structural racism. Studies evaluating the performance of blood-based biomarkers across racial/ethnic groups are currently very limited, yet there is reason for concern. Recent studies show that impairments in hepatic and renal function are associated with higher concentrations of blood-based biomarkers, including p-tau and NfL (34, 46–48). Other work emphasizes the role of body mass index and other comorbidities in concentration of blood-based biomarkers (34, 47). Taken together, this nascent literature suggests that analyses of blood-based biomarker measures must correct for these and other factors. Given racial disparities in the burden of these conditions (61–63), equal performance of these biomarkers across racial groups is not guaranteed. Corrections must be considered carefully to ensure they neither serve to obscure nor inflate measured racial and social inequalities. Finally, there is a greater burden of vascular disease among Black populations in the United States (60) but no widely used blood-based biomarkers specific for vascular dementia (64, 65). Existing biomarkers can overlap with AD, other neurodegenerative disease, and cerebrovascular disease biomarkers (16). Additional biomarkers are in development (14–16, 64).
Existing efforts to examine and validate blood-based biomarkers across racial and ethnic groups have substantial limitations; the 4 studies comparing blood-based biomarkers across racial and ethnic groups have shown mixed results. An analysis of a small sample (n = 360) of ADNI data found some racial differences in the level of blood-based biomarkers (66), but a simple comparison of biomarker levels across racial groups does not validate (or invalidate) the measures, given differences in dementia risk and prevalence. The other studies have estimated associations between blood-based biomarkers and other outcomes, including CSF, PET imaging, neuropathological data, and cognitive outcomes. Evidence from the community-based Washington Heights-Inwood Columbia Aging Project suggests that blood-based biomarkers, particularly p-tau, perform at least as well in predicting dementia in historically excluded racial and ethnic groups as in White participants, although performance was better for autopsy outcomes than clinical outcomes (22, 24). Although not explicitly a validation study, results from the population-based Chicago Health and Aging Project showed stronger associations between blood-based tau measures and clinical AD in Black populations than in White populations (67). A final study of research participants at an Alzheimer’s Disease Research Center in St. Louis noted differences by race in predictive performance of p-tau and NfL blood-based biomarkers, but outcomes were limited to CSF and PET measures of amyloid. That is, the associations between blood-based biomarkers and clinical diagnoses, cognition, or neuropathology outcomes were not evaluated (68).
While blood-based biomarkers have the potential to increase inclusion in dementia research, they do not negate the need for more representative neuroimaging samples. With the exception of the studies noted above, most studies incorporating and validating blood-based biomarkers use predominantly White clinical samples (22, 69). In studies with more racial and ethnic diversity, authors consistently note the need for larger and more diverse samples to understand the potential moderating effects of race and ethnicity and other demographic factors on blood-based biomarker concentrations and associations with neuropathological, PET, and cognitive outcomes. Ongoing efforts to evaluate biomarker performance may need to prioritize increasing racial and ethnic diversity in tau- and amyloid-PET samples to aid in evaluating the validity of blood-based biomarkers.
PATH FORWARD AND CONCLUSIONS
We have reviewed blood-based biomarkers for dementia, with an emphasis on AD pathology, including some technical challenges with their application. Despite these technical limitations, blood-based biomarkers offer great promise for expanding our understanding of the pathophysiology of dementia more broadly, including in historically excluded populations in dementia biomarker research. However, concerns about validity, reliability, generalizability, and equity remain, and the use of blood-based biomarkers in epidemiologic research requires thoughtful attention to these issues. Given the state of the science, there is a need to incorporate blood-based biomarkers into longitudinal epidemiologic studies with robust data on cognition and other measures of pathology (e.g., neuroimaging and neuropathology data). Such studies are needed to inform validity, choice of biomarker and assay, best practices, and optimal adjustment for comorbidities for use of blood-based biomarkers in dementia research. Studies are especially needed in racially and ethnically diverse samples and in groups with comorbidities that may affect blood-based biomarker performance. In the long term, we anticipate that large population-based studies, where neuroimaging measures are difficult to obtain, especially longitudinally, represent a key opportunity for blood-based biomarkers to advance the field. We are excited about the potential for blood-based biomarkers to move the field of dementia epidemiology forward and look forward to their continued development and incorporation into research and practice.
ACKNOWLEDGMENTS
Author affiliations: Department of Epidemiology, Fielding School of Public Health, University of California, Los Angeles, Los Angeles, California, United States (Eleanor Hayes-Larson, Elizabeth Rose Mayeda); Department of Epidemiology, Boston University School of Public Health, Boston, Massachusetts, United States (Sarah F. Ackley, M. Maria Glymour); Taub Institute for Research on Alzheimer’s Disease and the Aging Brain, Department of Neurology, Columbia University Irving Medical Center, New York, New York, United States (Indira C. Turney); and Memory and Aging Center, Department of Neurology, University of California, San Francisco, San Francisco, California, United States (Renaud La Joie).
E.H.L. and S.F.A. contributed equally as first authors.
This work was supported by the National Institute on Aging (award numbers R01AG057869, R56AG069126, K99AG065501, K99AG076975, K99AG073454, and K99AG075317). Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health grant U01 AG024904) and Department of Defense ADNI (DOD award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging and the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd. and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
Data used in preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (https://adni.loni.usc.edu/). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. A complete listing of ADNI investigators can be found at: https://adni.loni.usc.edu/wp-content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf.
All ADNI data are shared without embargo through the LONI Image and Data Archive (IDA), a secure research data repository. Interested scientists may obtain access to ADNI imaging, clinical, genomic, and biomarker data for the purposes of scientific investigation, teaching, or planning clinical research studies. Access is contingent on adherence to the ADNI Data Use Agreement.
A preprint of this article has been published online. Hayes-Larson E, Ackley S, Turney I, La Joie R, Glymour M. Considerations for use of blood-based biomarkers in epidemiologic dementia research. OSF Preprints. 2023. (doi:10.31219/osf.io/g2yht).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of interest: none declared.
REFERENCES
- 1. Alzheimer’s Association . 2022 Alzheimer’s disease facts and figures. Alzheimers Dement. 2022;18(4):700–789. [DOI] [PubMed] [Google Scholar]
- 2. McKeith I, Cummings J. Behavioural changes and psychological symptoms in dementia disorders. Lancet Neurol. 2005;4(11):735–742. [DOI] [PubMed] [Google Scholar]
- 3. Karantzoulis S, Galvin JE. Distinguishing Alzheimer’s disease from other major forms of dementia. Expert Rev Neurother. 2011;11(11):1579–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Califf RM. Biomarker definitions and their applications. Exp Biol Med (Maywood). 2018;243(3):213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Thambisetty M, Howard R. Lecanemab trial in AD brings hope but requires greater clarity. Nat Rev Neurol. 2023;19(3):132–133. [DOI] [PubMed] [Google Scholar]
- 6. Cummings JL, Doody R, Clark C. Disease-modifying therapies for Alzheimer disease: challenges to early intervention. Neurology. 2007;69(16):1622–1634. [DOI] [PubMed] [Google Scholar]
- 7. Cole MA, Seabrook GR. On the horizon—the value and promise of the global pipeline of Alzheimer’s disease therapeutics. Alzheimers Dement (N Y). 2020;6(1):e12009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cummings JL. Biomarkers in Alzheimer’s disease drug development. Alzheimers Dement. 2011;7(3):e13–e44. [DOI] [PubMed] [Google Scholar]
- 9. Pleen J, Townley R. Alzheimer’s disease clinical trial update 2019–2021. J Neurol. 2022;269(2):1038–1051. [DOI] [PubMed] [Google Scholar]
- 10. Mauricio R, Benn C, Davis J, et al. Tackling gaps in developing life-changing treatments for dementia. Alzheimers Dement (N Y). 2019;5:241–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Paczynski MM, Day GS. Alzheimer disease biomarkers in clinical practice: a blood-based diagnostic revolution. J Prim Care Community Health. 2022;13:21501319221141176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging–Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Delaby C, Hirtz C, Lehmann S. Overview of the blood biomarkers in Alzheimer’s disease: promises and challenges. Rev Neurol. 2023;179(3):161–172. [DOI] [PubMed] [Google Scholar]
- 14. Prabhakar P, Chandra SR, Christopher R. Circulating microRNAs as potential biomarkers for the identification of vascular dementia due to cerebral small vessel disease. Age Ageing. 2017;46(5):861–864. [DOI] [PubMed] [Google Scholar]
- 15. Liu Y, Chan DKY, Thalamuthu A, et al. Plasma lipidomic biomarker analysis reveals distinct lipid changes in vascular dementia. Comput Struct Biotechnol J. 2020;18:1613–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jagtap A, Gawande S, Sharma S. Biomarkers in vascular dementia: a recent update. Biomark Genom Med. 2015;7(2):43–56. [Google Scholar]
- 17. Serrano-Pozo A, Qian J, Muzikansky A, et al. Thal amyloid stages do not significantly impact the correlation between neuropathological change and cognition in the Alzheimer disease continuum. J Neuropathol Exp Neurol. 2016;75(6):516–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vandenbroucke JP, Elm E, Altman DG, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Ann Intern Med. 2007;147(8):W163–W194. [DOI] [PubMed] [Google Scholar]
- 19. MacCallum RC, Zhang S, Preacher KJ, et al. On the practice of dichotomization of quantitative variables. Psychol Methods. 2002;7(1):19–40. [DOI] [PubMed] [Google Scholar]
- 20. Cohen J. The cost of dichotomization. Appl Psychol Measur. 1983;7(3):249–253. [Google Scholar]
- 21. Scheltens P, Rockwood K. How golden is the gold standard of neuropathology in dementia? Alzheimers Dement. 2011;7(4):486–489. [DOI] [PubMed] [Google Scholar]
- 22. Brickman AM, Manly JJ, Honig LS, et al. Plasma p-tau181, p-tau217, and other blood-based Alzheimer’s disease biomarkers in a multi-ethnic, community study. Alzheimers Dement. 2021;17(8):1353–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Planche V, Bouteloup V, Pellegrin I, et al. Validity and performance of blood biomarkers for Alzheimer disease to predict dementia risk in a large clinic-based cohort. Neurology. 2023;100(5):e473–e484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Brickman AM, Manly JJ, Honig LS, et al. Correlation of plasma and neuroimaging biomarkers in Alzheimer’s disease. Ann Clin Transl Neurol. 2022;9(5):756–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ganguli M, Lee CW, Hughes T, et al. Who wants a free brain scan? Assessing and correcting for recruitment biases in a population-based sMRI pilot study. Brain Imaging Behav. 2015;9(2):204–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kall LM, Truong T, Burnham SC, et al. Association of β-amyloid level, clinical progression, and longitudinal cognitive change in Normal older individuals. Neurology. 2021;96(5):e662–e670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Moscoso A, Grothe MJ, Ashton NJ, et al. Time course of phosphorylated-tau181 in blood across the Alzheimer’s disease spectrum. Brain. 2021;144(1):325–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Therriault J, Vermeiren M, Servaes S, et al. Association of phosphorylated tau biomarkers with amyloid positron emission tomography vs tau positron emission tomography. JAMA Neurol. 2022;80(2):188–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Janelidze S, Mattsson N, Palmqvist S, et al. Plasma p-tau181 in Alzheimer’s disease: relationship to other biomarkers, differential diagnosis, neuropathology and longitudinal progression to Alzheimer’s dementia. Nat Med. 2020;26(3):379–386. [DOI] [PubMed] [Google Scholar]
- 30. Jack CR, Knopman DS, Jagust WJ, et al. Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12(2):207–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jack CR, Knopman DS, Jagust WJ, et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010;9(1):119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Brand AL, Lawler PE, Bollinger JG, et al. The performance of plasma amyloid beta measurements in identifying amyloid plaques in Alzheimer’s disease: a literature review. Alzheimers Res Ther. 2022;14(1):195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Palmqvist S, Insel PS, Stomrud E, et al. Cerebrospinal fluid and plasma biomarker trajectories with increasing amyloid deposition in Alzheimer’s disease. EMBO Mol Med. 2019;11(12):e11170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pichet Binette A, Janelidze S, Cullen N, et al. Confounding factors of Alzheimer’s disease plasma biomarkers and their impact on clinical performance. Alzheimers Dement. 2023;19(4):1403–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jack CR, Wiste HJ, Algeciras-Schimnich A, et al. Predicting amyloid PET and tau PET stages with plasma biomarkers. Brain. 2023;146(5):2029–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Giovannoni G. Peripheral blood neurofilament light chain levels: the neurologist’s C-reactive protein? Brain. 2018;141(8):2235–2237. [DOI] [PubMed] [Google Scholar]
- 37. Teunissen CE, Verberk IMW, Thijssen EH, et al. Blood-based biomarkers for Alzheimer’s disease: towards clinical implementation. Lancet Neurol. 2022;21(1):66–77. [DOI] [PubMed] [Google Scholar]
- 38. Pereira JB, Janelidze S, Stomrud E, et al. Plasma markers predict changes in amyloid, tau, atrophy and cognition in non-demented subjects. Brain. 2021;144(9):2826–2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ma W, Zhang J, Xu J, et al. Elevated levels of serum Neurofilament light chain associated with cognitive impairment in vascular dementia. Dis Markers. 2020;2020:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wolf F, Ghanbari M, Licher S, et al. Plasma tau, neurofilament light chain and amyloid-β levels and risk of dementia; a population-based cohort study. Brain. 2020;143(4):1220–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Novakova L, Zetterberg H, Sundström P, et al. Monitoring disease activity in multiple sclerosis using serum neurofilament light protein. Neurology. 2017;89(22):2230–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Barthélemy NR, Horie K, Sato C, et al. Blood plasma phosphorylated-tau isoforms track CNS change in Alzheimer’s disease. J Exp Med. 2020;217(11):e20200861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Thijssen EH, La Joie R, Strom A, et al. Plasma phosphorylated tau 217 and phosphorylated tau 181 as biomarkers in Alzheimer’s disease and frontotemporal lobar degeneration: a retrospective diagnostic performance study. Lancet Neurol. 2021;20(9):739–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Balogun WG, Zetterberg H, Blennow K, et al. Plasma biomarkers for neurodegenerative disorders: ready for prime time? Curr Opin Psychiatry. 2023;36(2):112–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cullen NC, Janelidze S, Mattsson-Carlgren N, et al. Test-retest variability of plasma biomarkers in Alzheimer’s disease and its effects on clinical prediction models. Alzheimers Dement. 2022;19(3):797–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Berry K, Asken BM, Grab JD, et al. Hepatic and renal function impact concentrations of plasma biomarkers of neuropathology. Alzheimers Dement (Amst). 2022;14(1):e12321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Syrjanen JA, Campbell MR, Algeciras-Schimnich A, et al. Associations of amyloid and neurodegeneration plasma biomarkers with comorbidities. Alzheimers Dement. 2022;18(6):1128–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hall JR, Petersen M, Johnson LA, et al. Kidney function impacts plasma Alzheimer’s biomarkers in a cognitively Normal multi-ethnic cohort. Arch Nephrol Urol. 2023;6:23–30. [Google Scholar]
- 49. Huber H, Ashton NJ, Schieren A, et al. Levels of Alzheimer’s disease blood biomarkers are altered after food intake-a pilot intervention study in healthy adults [published online ahead of print May 27, 2023]. Alzheimers Dement. 2023. 10.1002/alz.13163. [DOI] [PubMed] [Google Scholar]
- 50. Tolboom N, Yaqub M, Boellaard R, et al. Test-retest variability of quantitative [11C]PIB studies in Alzheimer’s disease. Eur J Nucl Med Mol Imaging. 2009;36(10):1629–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Devous MD, Joshi AD, Navitsky M, et al. Test–retest reproducibility for the tau PET imaging agent Flortaucipir F 18. J Nucl Med. 2018;59(6):937–943. [DOI] [PubMed] [Google Scholar]
- 52. Timmers T, Ossenkoppele R, Visser D, et al. Test–retest repeatability of [18F]flortaucipir PET in Alzheimer’s disease and cognitively normal individuals. J Cereb Blood Flow Metab. 2020;40(12):2464–2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Joshi AD, Pontecorvo MJ, Clark CM, et al. Performance characteristics of amyloid PET with florbetapir F 18 in patients with Alzheimer’s disease and cognitively Normal subjects. J Nucl Med. 2012;53(3):378–384. [DOI] [PubMed] [Google Scholar]
- 54. Wilkins CH, Schindler SE, Morris JC. Addressing health disparities among minority populations: why clinical trial recruitment is not enough. JAMA Neurol. 2020;77(9):1063–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. McClure ES, Vasudevan P, Bailey Z, et al. Racial capitalism within public health-how occupational settings drive COVID-19 disparities. Am J Epidemiol. 2020;189(11):1244–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sjoding MW, Ansari S, Valley TS. Origins of racial and ethnic bias in pulmonary technologies. Annu Rev Med. 2023;74:401–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lee CM, Jacobs HIL, Marquié M, et al. 18F-flortaucipir binding in choroid plexus: related to race and hippocampus signal. J Alzheimers Dis. 2018;62(4):1691–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Crews DC, Liu Y, Boulware LE. Disparities in the burden, outcomes and care of chronic kidney disease. Curr Opin Nephrol Hypertens. 2014;23(3):298–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Babulal GM, Quiroz YT, Albensi BC, et al. Perspectives on ethnic and racial disparities in Alzheimer’s disease and related dementias: update and areas of immediate need. Alzheimers Dement. 2019;15(2):292–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Levine DA, Springer MV, Brodtmann A. Blood pressure and vascular cognitive impairment. Stroke. 2022;53(4):1104–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Assari S. Racial disparities in chronic kidney diseases in the United States; a pressing public health challenge with social, behavioral and medical causes. J Nephropharmacol. 2016;5(1):4–6. [PMC free article] [PubMed] [Google Scholar]
- 62. Kibria GMA, Crispen R. Prevalence and trends of chronic kidney disease and its risk factors among US adults: an analysis of NHANES 2003–18. Prev Med Rep. 2020;20:101193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Petersen R, Pan L, Blanck HM. Racial and ethnic disparities in adult obesity in the United States: CDC’s tracking to inform state and local action. Prev Chronic Dis. 2019;16:180579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Maclin JMA, Wang T, Xiao S. Biomarkers for the diagnosis of Alzheimer’s disease, dementia Lewy body, frontotemporal dementia and vascular dementia. Gen Psychiatr. 2019;32(1):e100054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Gorelick PB. Blood and cerebrospinal fluid biomarkers in vascular dementia and Alzheimer’s disease: a brief review. Clin Geriatr Med. 2023;39(1):67–76. [DOI] [PubMed] [Google Scholar]
- 66. Windon C, Iaccarino L, Mundada N, et al. Comparison of plasma and CSF biomarkers across ethnoracial groups in the ADNI. Alzheimers Dement (Amst). 2022;14(1):e12315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Rajan KB, Aggarwal NT, McAninch EA, et al. Remote blood biomarkers of longitudinal cognitive outcomes in a population study. Ann Neurol. 2020;88(6):1065–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Schindler SE, Karikari TK, Ashton NJ, et al. Effect of race on prediction of brain amyloidosis by plasma Aβ42/Aβ40, phosphorylated tau, and neurofilament light. Neurology. 2022;99(3):e245–e257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Barnes LL. Alzheimer disease in African American individuals: increased incidence or not enough data? Nat Rev Neurol. 2022;18(1):56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]


