Highlights
-
•
Pineapple by-products and whey protein were fermented by endophytic microbes.
-
•
Core microbial communities were dominated by Lactococcus, Weissella and others.
-
•
Core organisms were highly correlated with quality attributes of the product.
-
•
Core organisms played vital roles in improving nutrient, flavor and taste of product.
-
•
This study could provide new ideas for the utilization of fruit by-product resources.
Keywords: Pineapple by-products, Whey protein, Fermentation, Volatile compounds, Microbial community
Abstract
In this study, a new fermented food was developed using pineapple by-products and whey protein (2.6%) as raw materials through the co-fermentation of autochthonous lactic acid bacteria and yeast. To better understand the fermentation mechanism and the impact of microorganisms on the entire fermentation system, we tracked the changes in carbohydrate and amino acid profiles, organoleptic quality and microbial community during the fermentation process. Compared with unfermented samples, dietary fiber and free amino acids increased significantly as fermentation proceeded. The fermented samples were significantly lower in astringency and bitterness and significantly higher in sourness, umami and richness. The fermented products were richer in volatile compounds with floral, cheesy, fruity and other flavors. Relevant analyses showed that the core microbial community was highly correlated with the quality attributes of the fermented products. Microorganisms such as Lactococcus, Weissella, Hanseniaspora, Saccharomyces and Lachancea contributed significantly to the fermented products.
Introduction
Fruits are a crucial source of nutrients for human survival and serve as a fundamental raw material for the food industry’s growth and development. Because of the large scale and concentration of the fruit industry, the quantity of fruit is increased rapidly, leading to an increase in the amount of fruit by-products (Herrera et al., 2020). Pineapples (Ananas comosus L.) are rich in nutrients such as organic acids, amino acids, vitamins and minerals, and they have a sweet and fragrant flavor. As a result, pineapples are increasingly popular with consumers. According to Food and Agriculture Organization of the United Nations (FAO) estimates, global pineapple production will reach 35 million tons by 2023, with China being the major pineapple producer in Asia (Gansemans, Martens, D’Haese & Orbie, 2017). Apart from being consumed fresh, pineapples are processed into canned pineapple and pineapple juice. During pineapple processing, numerous processing by-products are generated due to outdated processing techniques and a limited portfolio. These by-products account for roughly 50 % of the total pineapple and have a similar nutritional composition as pineapple pulp (Herrera et al., 2020). To prevent negative environmental impacts, these by-products are commonly utilized for energy production or disposed of as waste in landfills. However, pineapple by-products contain valuable substances, such as volatile compounds, organic acids, pineapple proteases and phenolics, and they have the potential for high-value applications.
Microbial fermentation is an effective method of converting fruit processing by-products into value-added end-products (Casarotti, Borgonovi, Batista & Penna, 2018). During the fermentation process, microorganisms from the raw material and the environment can promote the release of active substances in the fermentation matrix and the production of flavor; microbial fermentation can extend the shelf life of food as well. Microbial fermentation can confer new flavors on a substrate. Chen et al. found that most of the flavor compounds during fermentation were mainly produced through the breakdown of lipids and proteins by microorganisms (Chen et al., 2021). Recently, many advantages have been found during fruit fermentation using autogenous starter cultures (Fonseca, Melo, Ramos, Dias & Schwan, 2021). In addition, the use of a mixed strain starter is more effective than a single strain. Hu et al. found that co-fermenting dough with Lactobacillus plantarum and yeast could produce more organic acids and reduce macromolecular proteins in the dough (Hu, Li, Huang, Du, Huang & Tao, 2023). Li et al. used Saccharomyces cerevisiae and Lactobacillus plantarum as fermenters for glucuronic acid-rich apple cider fermentation and found that the cider had good antioxidant properties (Li et al., 2021). Xing et al. (2018) used an ester-producing yeast strain isolated from Daqu to ferment Shanxi aged-vinegar. They found that using native yeast significantly improved the aroma profile of Shanxi aged-vinegar, giving the vinegar product special floral, fruity and a slightly sweet nutty flavor. They concluded that using the native yeast could improve the quality of the vinegar (Xing et al., 2018).
Whey protein is one of the most nutritious by-products of dairy processing, especially in the production of cheese or yogurt (Krolczyk, Dawidziuk, Janiszewska-Turak & Solowiej, 2016). It has been shown that the co-fermentation of whey protein together with fruit and vegetables not only provides a nitrogen source for the fermentation system, and they also provide better flavor and functional properties to the final product. It has been shown that co-fermentation of whey protein from goat milk together with strawberry juice could develop a functional beverage with a pleasant flavor and enriched with probiotics (Marcillo, Galarza, Mosquera, Valladares, Perez & Pais-Chanfrau, 2022). Wang et al. used Lactobacillus plantarum to ferment blueberries with whey protein and found that the addition of whey protein improved the stability of blueberry anthocyanins and phenolic acids (Wang et al., 2021, Wang et al., 2021, Wang et al., 2021). Therefore, it would be a good choice to add whey protein during the process of fermenting plant food. Pineapple processing by-products are rich in carbohydrates, yet their protein content is comparatively low compared with other fruit by-products. Co-fermentation of whey protein and pineapple by-products could provide a more complete and balanced nutritional profile to the end-product. It could also provide a good flavor to the end-product. To date, the research on using endophytic bacteria to ferment pineapple processing by-products and whey protein to develop a fermented food with good flavor and rich nutrition is still limited.
In this study, autotrophic lactic acid bacteria (Lactococcus lactis LA5) and yeast (Hanseniaspora opuntiae SA2) isolated from pineapple cores were used to ferment a mixture of pineapple processing by-products and whey protein. We aimed to investigate (1) the changes in carbohydrate and amino acid profiles in the fermentation system; (2) the changes in flavor and taste attributes in the fermentation system; and (3) the impact of microorganisms on the entire fermentation system.
Materials and methods
Chemicals
Whey protein was purchased from Yuan Ye Bio-Technology (purity 98 %, Shanghai, China). The MRS and YPD broths were obtained from Solarbio Science (Beijing, China). Amino acid standards were bought from Wako (Wako-Shi, Japan). All other reagents were analytical grade and purchased from Aladdin (Shanghai, China).
Microorganisms
The lactic acid bacteria (Lactococcus lactis LA5) and yeast (Hanseniaspora opuntiae SA2) were used as potential starter cultures of fermentation. These two strains were pineapple endophytic microorganisms isolated from pineapple cores in our previous experiment (Luo et al., 2023), and the procedures followed the methods described by Taha, Jaini, Saidi, Rahim, Shah & Hashim (2019). The second transfer of culture strains was reactivated following the next steps, lactic acid bacteria were cultured in MRS broth at 37 °C for 24 h, and yeasts were cultured in YPD broth at 30 °C for 24 h.
Sample preparation and collection
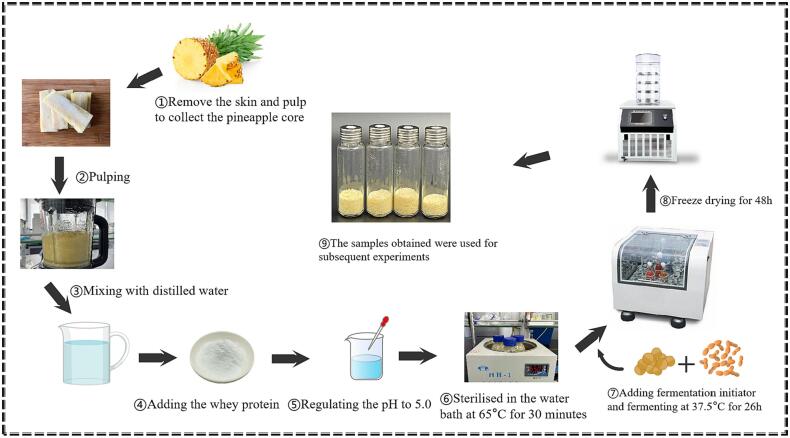
The samples were prepared with reference to our previous study (Luo et al., 2023), and the preparation process is shown in Fig. 1. The pineapples were thoroughly cleansed, with the removal of both peel and flesh, resulting in the collection of pineapple cores. The pineapple cores were pulped using a high-speed blender and mixed with distilled water at a mass ratio of 6:4 (homogenate:water), then whey protein (2.6 %, w/w) was incorporated into the mixture, followed by pH adjustment to 5.0. Afterward, the mixture of substrates was sterilized in a water bath at 65 °C for 30 min. The starter culture strain was inoculated into the fermentation substrate at a volume ratio of 6:1 of lactic acid bacteria and yeast, with a 1.6 % (w/w) inoculum of lactic acid bacteria. The concentration of both lactic acid bacteria and yeast was 1 × 106 CFU/mL. The mixture was then incubated at 37.5 °C for 26 h in the dark. The fermentation broth was collected at 0, 8, 14 and 26 h; at each fermentation time, the samples were freeze-dried for 48 h to obtain powdery products, and then stored at –80 °C for subsequent experiments.
Fig. 1.
Sample preparation procedure of this study.
Measurement of total soluble and reducing sugars
An equal mass of distilled water was added to the freeze-dried samples from different fermentation stages, mixed thoroughly for 1 min, and then centrifuged (3–30 KS, Sigma, Osterloh, Germany) at 10,956 g for 10 min to obtain the supernatant for the determination of total soluble and reducing sugars. Total soluble sugars were determined with slight modifications according to the method of Dreher et al. (2013). Briefly, 1 mL phenol (6 %) and 5 mL concentrated sulfuric acid were added to 2 mL of the sample, shaken well and cooled at ambient temperature for 30 min. Then the absorbance value was measured at 490 nm using a UV spectrophotometer (Evolution 200, Thermo, MA, USA). The total sugar content was calculated using the glucose standard curve. The determination of reducing sugars was slightly modified according to the method of Gómez Herrera et al. (2020). A sample of 1 mL was taken in a 25 mL stoppered cuvette, and 1 mL distilled water with 2 mL 3,5-dinitrosalicylic acid solution was added, heated in a boiling water bath for 5 min. This was cooled with running water and then increased to 25 mL with distilled water. The absorbance value was measured at 540 nm using a UV spectrophotometer (Evolution 200, Thermo, MA, USA). The total sugar content was calculated using the standard curve.
Measurement of dietary fiber
The determination of dietary fiber was slightly modified from the method of Martinez, Torres, Meneses, Figueroa, Perez-Alvarez & Viuda-Martos (2012). Adding 15 mL distilled water to 1 g freeze-dried sample and placed in a boiling water bath for 15 min. After the system had cooled to room temperature, amylase and glycosylase were added, followed by heating in a water bath at 60 °C for 60 min. Protease was added to the system and treated in a water bath at 40 °C for 90 min, then treated in a boiling water bath for 15 min to inactivate the enzyme. After being centrifuged (3–30 KS) at 10,956 g for 10 min, the precipitate was washed three times with hot water and freeze-dried to obtain the insoluble dietary fiber. After being centrifuged, the supernatant was spin-distilled to 1/4 of the original volume, and then four times volume of 95 % ethanol was added and left to stand overnight. The precipitate was obtained by vacuum filtration and freeze-dried to obtain the soluble dietary fiber, and the obtained dietary fiber was weighed and recorded.
Measurement of free amino acids
The free amino acids (FAAs) were determined following our previous report (Luo et al., 2023). First, 10 mL of 0.02 mol/L HCl was added to the sample. The C18 pretreatment column (75 mm × 250 mm, 1.9 µm particle size) was activated by adding 5 mL methanol and 5 mL water. Then, 2.5 mL of the sample and 1.5 mL HCl (0.02 mol/L) were added. After passing through the column, the volume of the sample was increased to 5 mL with 0.02 mol/L HCl and then passed through a 0.45 μm filter membrane for determination. Samples were measured using an amino acid analyzer (LA8080, Hitachi, Tokyo, Japan) equipped with a cation exchange column (2622PH, 4.6 mm × 60 mm, 3 μm) and UV detectors (1260 Infinity II, Agilent, CA, USA; 570 and 440 nm). The mobile phases included PH1, PH2, PH3, PH4, PH-RG, ninhydrin solution, ninhydrin buffer solution, 5 % ethanol and HPLC grade H2O.
Volatile components analysis using gas chromatography–Ion mobility spectrometry (GC–IMS)
The volatile compounds in the fermentation process were determined and analyzed using GC–IMS (FlavourSpec®, G.A.S., Dortmund, Germany) with slight modifications according to the method of Zhang et al. (2020). The sample (1 g) was weighed into a 20 mL headspace vial and incubated at 60 °C for 15 min at 500 rpm in the autosampling unit. It was then incubated for 15 min at 60 °C in the autosampling unit at 500 rpm. The GC was equipped with an FS-SE-54-CB-1 capillary column (15 m × 0.53 mm) and nitrogen gas carrier. Chromatographic separation was performed as follows: the initial flow rate was 2 mL/min and maintained for 2 min. Then the flow rate was increased to 10 mL/min within 8 min, followed by 100 mL/min within 10 min, and finally increased to 150 mL/min for 5 min. The IMS conditions were as follows: the column temperature was 40 °C, the drift gas was N2 and the flow rate was 150 mL/min. The qualitative analysis of volatile compounds was based on a comparison of the refractive index and drift time of detected compounds with those from the GC–IMS library (NIST and IMS databases, respectively). The relative quantitative analysis was based on the signal intensity of volatile compounds processed by the Laboratory Analytical Viewer. Each sample was determined in triplicate.
Measurement of taste changes during fermentation using an E-tongue
Measurement of the taste changes during fermentation was based on the method of Wang et al. (2022) with slight modifications using the E-tongue (SA-402B, Insent, Tokyo, Japan). The SA-402B E-tongue sensor system is equipped with eight basic taste indicators, namely bitterness, astringency, umami, saltiness, richness, sourness, aftertaste astringency (aftertaste-A) and aftertaste bitterness (aftertaste-B). After precisely weighing samples (1 g) from different fermentation stages, 50 mL of distilled water was added, followed by sonicating for 30 min and centrifuging at 5478 g for 5 min, finally filtered using filter paper (3–30 KS). The 35 mL of supernatant was poured into a tasting cup for determination. The sensors were cleaned using a clean solution and the reference solution to keep the E-tongue sensor stable.
Microbial community analysis during fermentation
Total genomic DNA was extracted from the samples, and the DNA concentration was monitored using a Qubit® dsDNA HS Assay Kit (Azenta Life Sciences, Inc., South Plainfield, NJ, USA). Amplification of bacterial 16 s rRNA genes V3 and V4 hypervariable regions with fungal biological ITS rRNA genes used forward primers (5′-ACTCCTACGGAGGCAGCAG-3′, 5′-CTTGGTCATTTAGAGAGGAAGTAA-3′) and reverse primers (5′-GGACTACHVGGGTWTCTAAT-3′, 5′-GCTGCGTTCTTCATCGATGC-3′). Next-generation sequencing was conducted on an Illumina Miseq/Novaseq Platform (Illumina, San Diego, CA, USA) at Azenta Life Sciences, Inc. (South Plainfield, NJ, USA). Then, the RDP classifier (Ribosomal Database Program) based on the Bayesian algorithm of OTU species taxonomy was used to analyze representative sequences, different species classification level statistics and the microbial community compositions of each sample (Wang et al., 2021, Wang et al., 2021, Wang et al., 2021).
Data analysis
All data analyses were performed in triplicate and the results of the experiments were expressed as mean ± SD. One-way analysis of variance tests and Tukey’s post hoc test were performed using SPSS Statistics software (IBM, Armonk, USA) to determine differences between samples. Significant differences between the two groups were indicated by different letters and determined at the alpha level of p < 0.05. The Circos graph was drawn using Circize in the R program and applying Gephi to visualize the network of interactions between the microbial community and each indicator.
Results and discussion
Changes in carbohydrates during fermentation
Changes in total soluble sugars and reducing sugars during fermentation
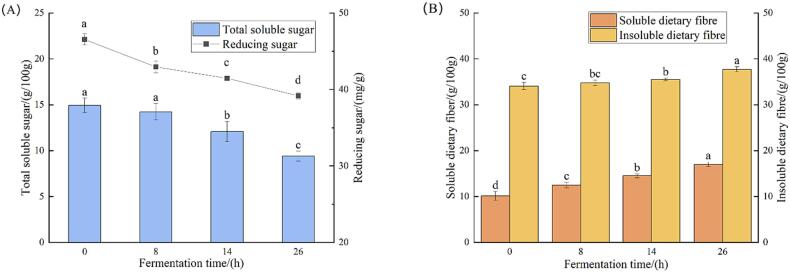
The changes in total soluble sugars and reducing sugars during the fermentation process are illustrated in Fig. 2(A). The total soluble sugar remained stable during the initial 8 h of fermentation but significantly decreased to 9.41 g/100 g by the end of the process. This suggests that microorganisms may use these sugars for cell growth and biotransformation, an observation that was consistent with the results of Gengatharan et al. (2021). The absence of significant changes in total soluble sugars at the beginning of fermentation may be attributed to the amylase activity of lactic acid bacteria during fermentation, resulting in sugar production comparable to microbial consumption. The trend of reducing sugars during fermentation was the same as that of total soluble sugars, which decreased from 46.54 mg/g to 39.17 mg/g at the end of fermentation, which was similar to the results reported by Olaitan, Obadina, Omemu, Atanda & Olotu (2015). The content of total soluble sugars and reducing sugars during the fermentation process can reflect the microorganisms’ utilization of carbon sources and their efficiency at different fermentation stages (Gengatharan et al., 2021). It can be seen that the fermentation microorganisms used in this study had good fermentation characteristics and a high capacity for carbohydrate utilization.
Fig. 2.
Changes in carbohydrates during fermentation. (A) Changes in total soluble sugars and reducing sugars during fermentation; (B) Changes in dietary fibre during fermentation. Different letters (a-d) indicate significant differences (P < 0.05).
Changes in dietary fiber during fermentation
Dietary fiber can be divided into soluble dietary fiber (SDF) and insoluble dietary fiber (IDF). Fig. 2(B) shows the changes in SDF and IDF during the fermentation of pineapple processing by-products. As shown in the figure, both SDF and IDF showed a significant increase at the end of fermentation compared with pre-fermentation. This is consistent with the results of fermented apple by-products using lactic acid bacteria (Cantatore et al., 2019). Dietary fiber is an important part of a healthy human diet and it is effective at preventing obesity and type 2 diabetes. The SDF has been shown to play an important role in reducing cholesterol and improving cardiovascular diseases, and it has potential prebiotic properties. IDF is important in promoting intestinal health and stool formation (Cantatore et al., 2019). Chen et al. found that fungi such as Saccharomyces cerevisiae could produce amylase, cellulase and other secondary metabolites during fermentation, which contributed to the release of dietary fiber (Chen et al., 2021). It was also found that lactic acid bacteria fermentation could modify and improve the production and activity of dietary fiber (Li et al., 2022).
Changes in FAAs during fermentation
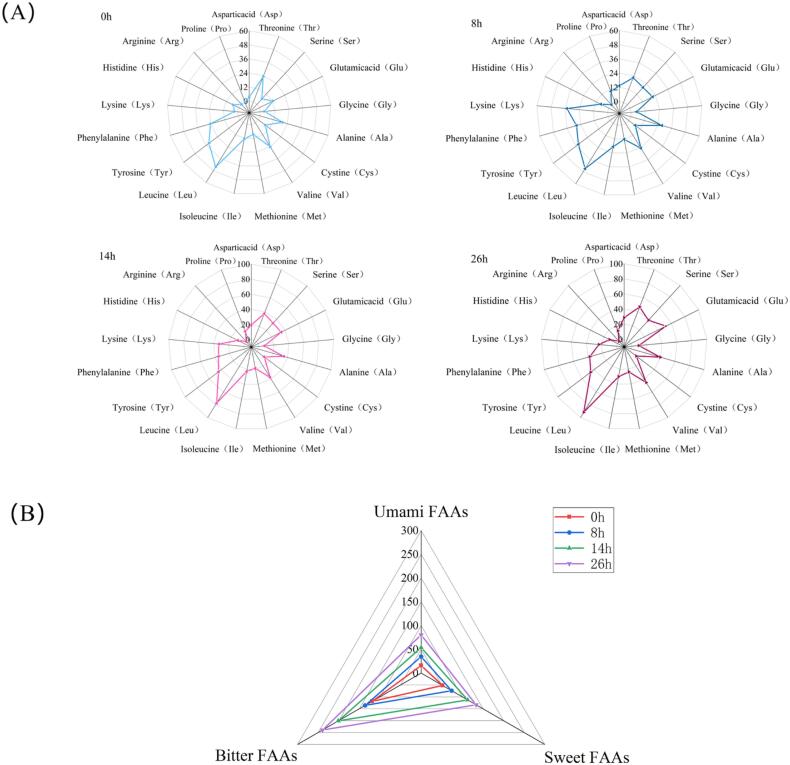
In this study, whey protein was added to the pineapple by-products for co-fermentation to provide better flavor and functional activity to the fermented product. Free fatty acids are an important factor in food flavor. During fermentation, microorganisms can decompose proteins into peptides and FAAs by producing proteases (Chen et al., 2019). A total of 16 kinds of FAAs were detected in the fermentation products, they were Asp, Thr, Ser, Glu, Gly, Ala, Cys, Val, Met, Ile, Leu, Tyr, Phe, Lys, His and Pro, whereas Arg was not detected in this study (Fig. 3(A)). The major FAAs in unfermented pineapple by-products were Leu (44.33 mg/g) and Phe (24.33 mg/g). At the end of fermentation (26 h), all detected FAAs were significantly increased in comparison to unfermented samples (p < 0.05), which agreed with the results of Chen et al. (2019). Different from the unfermented products, Leu (91.67 mg/g), Glu (51.67 mg/g), Thr (46.67 mg/g), Tyr (45.67 mg/g) and Val (45.33 mg/g) were the major FAAs in the fermentation products. As described before, the taste characteristics of FAAs can be classified as umami (Asp and Glu), sweet (Thr, Ser, Gly, Ala and Pro) and bitter (Tyr, Leu, Ile, Val, Phe, Lys, His, Arg and Met) (Chen et al., 2019). Fig. 3(B) shows that compared with unfermented samples, a significant increase in umami FAAs, sweet FAAs and bitter FAAs in fermented products was found. A similar trend was found in pickled Brassica napus leaf by Zhang et al. (2023). Compared with the unfermented samples, the amounts of umami FAAs, bitter FAAs and sweet FAAs in the fermented products were 5.1 times, 2.5 times and 2.5 times higher than unfermented samples. These results indicate that fermentation can improve the taste of fermentation products by improving FAAs composition and increasing the content of FAAs.
Fig. 3.
Free amino acid changes during fermentation. (A) Free amino acids content (mg/g) in substrates at different fermentation stages; (B) Total content of Free amino acids with taste characteristics in different fermentation stages.
Changes in volatile compounds during fermentation
Volatile compounds and principal component analysis in the fermentation system
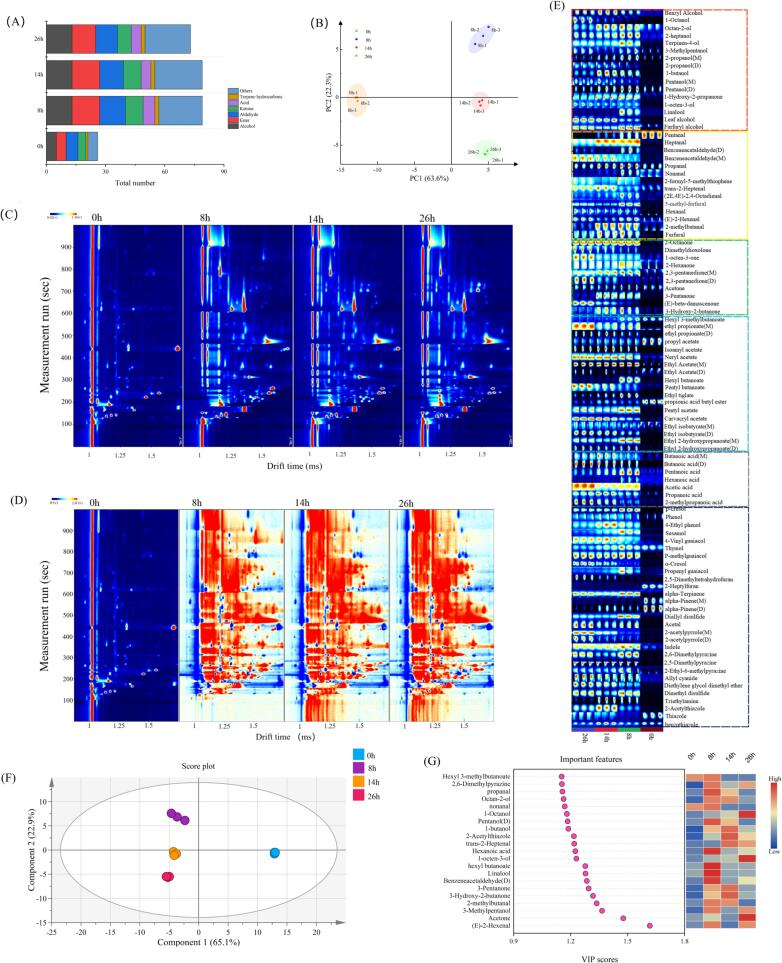
As shown in Fig. 4(A), a total of 94 kinds of volatile compounds were detected, they were 16 alcohols, 14 aldehydes, 10 ketones, 18 esters, 7 acids, 9 phenols, 4 olefins and 16 other compounds. Thirty-nine kinds of volatile compounds were detected in the unfermented pineapple by-products, and 88 kinds of volatile compounds were detected at the end of fermentation (26 h). As the fermentation proceeded, the variety of volatile compounds generated increased and significant differences in composition and quantity of volatile compounds were found before and after fermentation, which was consistent with the results reported by Liao, Luo, Huang & Xia (2023) conducted on pickled chilies.
Fig. 4.
GC-IMS analysis of different fermentation stages. (A) Types of volatile compounds at different stages of fermentation; (B) PCA analysis of GC-IMS; (C) 2D topographic maps; (D) The difference comparison topographic maps; (E) Gallery plot of the volatile compounds in different stages of fermentation; (F) PLS-DA score plot of samples. (G) VIP scores of volatile compounds in PLS-DA.
A principal component analysis (PCA) can evaluate the similarities and differences between samples by the contribution of PC factors (Wang et al., 2021, Wang et al., 2021, Wang et al., 2021). The PCA results for volatile compounds at different fermentation stages are shown in Fig. 5(B). In general, the PCA model for separate factors requires a contribution rate of at least 60 %. As shown in Fig. 4(B), the sum of the contributions of PC1 and PC2 reached 85.9 %, indicating the two principal components can characterize most of the information in volatile compounds. The fermentation products at different fermentation times were in different regions and they were well distinguished. The unfermented (0 h) was far from the early fermentation (8 h), middle fermentation (14 h) and end fermentation (26 h), indicating that PC1 could effectively distinguish the unfermented products from other fermentation stages, whereas PC2 could well distinguish between early fermentation (8 h), middle fermentation (14 h) and end fermentation (26 h). It can be concluded that the volatile compounds in different fermentation stages had significant variability, which was consistent with the results of Sun et al. (2023).
Fig. 5.
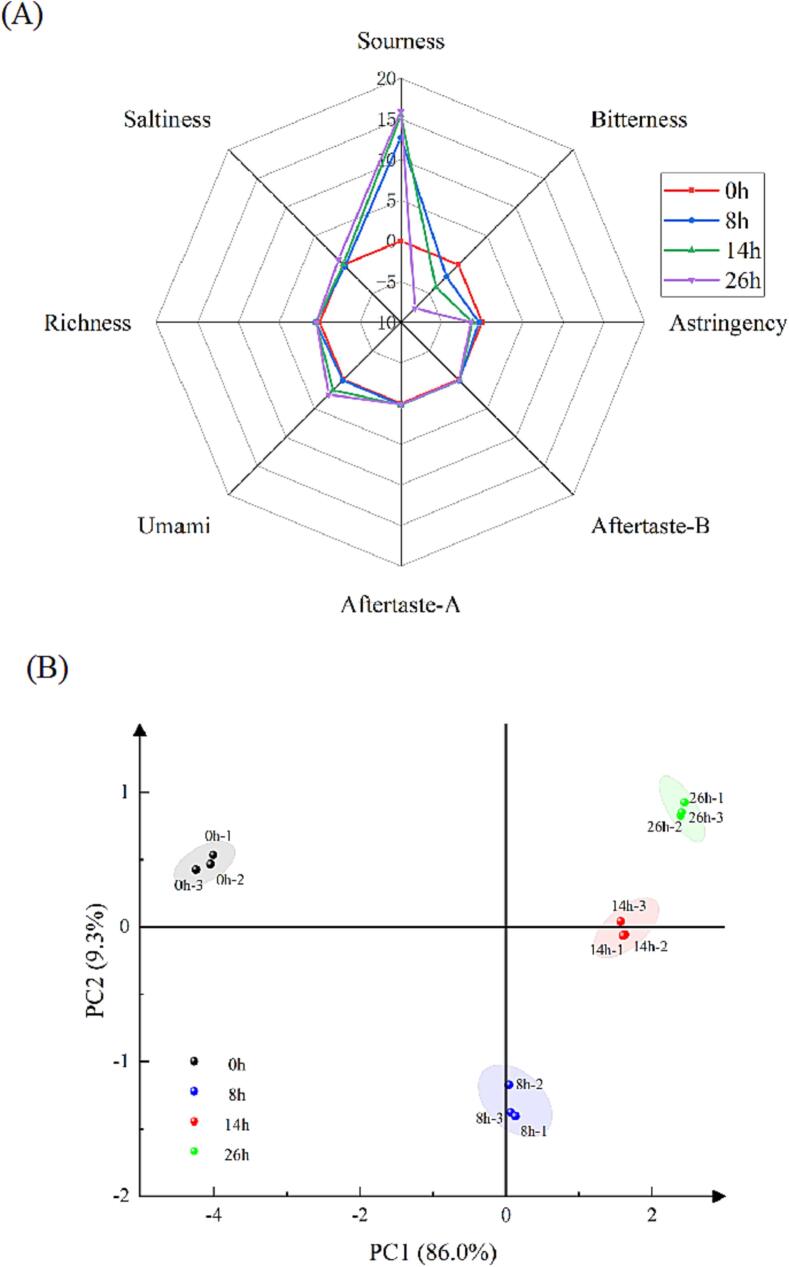
E-tongue analysis. (A) Radar chart of taste attributes of samples in different fermentation stages; (B) PCA of taste attributes of samples in different fermentation stages.
Fig. 4(C) and 4(D) show a 2D topographic visualization of volatile components by GC–IMS. In Fig. 4(C), the background of the GC–IMS spectrum is blue and the red vertical line at the horizontal coordinate 1.0 is the normalized reactive ion peak (RIP). Each point on either side of the RIP represents a volatile compound and the color reflects the concentration of the compound, with white indicating a lower concentration and red indicating a higher concentration. The intensity of the signal increases as the color deepens, with darker colors indicating higher concentrations of volatile compounds. As shown in Fig. 4(C), most of the signals appeared in the retention time of 100–800 s and the drift time of 1.0–2.0 ms. It can be observed that a large number of volatile compounds were formed during the fermentation process, which is consistent with the findings in fermented ginkgo rice wine reported by Chen et al., 2023, Chen et al., 2023. To visualize the differences in volatile compounds between different fermentation stages, a non-fermentation sample (0 h) was used as a control and the topographic maps of other fermentation stages were derived from it (Fig. 4(D)). White color indicates the same concentration of volatile compounds between samples. Red color indicates a higher volatile compound concentration than that in the control, and blue color indicates a lower compound concentration than that in the control. As show in Fig. 4(D), discernible variations were observed in both the composition and concentration of volatile compounds among samples at different stages of fermentation, implying that microbial fermentation could contribute to the formation of distinctive flavors in the substrate (Chen et al., 2023, Chen et al., 2023).
Comparison of volatile compound fingerprints and identification of key volatile compounds
The fingerprints of different fermentation stages were used to reveal the flavor characteristics and the mechanism of volatile compound changes, the results are shown in Fig. 4(E). As the fermentation progressed, there was a gradual increase in alcohol concentration, with the final signal intensity being 2.92 times higher than that of the unfermented sample (0 h). The highest abundance of alcohols was observed during the middle stages of fermentation (8–14 h), which was consistent with previous findings by Pittari, Moio & Piombino (2023). This could be attributed to yeast growth and metabolism leading to increased production of alcohols within the fermentation system. 3-Methylpentanol, which emits a whiskey-like aroma, was the typical volatile compound of fermentation products. Its signal intensity was significantly lower in unfermented samples (0 h) than in other samples. In addition, 1-butanol and (Z)-3-hexen-1-ol with fruity and green flavors were also present in the final fermentation product.
Because of the low odor threshold of aldehydes, they play an important role in the characteristic flavor of fermented foods (Wu et al., 2019). In this study, aldehydes were the main flavor compounds in the products. At the end of fermentation (26 h), the total signal intensity of aldehydes was 1.65-fold higher than in the unfermented samples. Wu et al. (2019) found that aldehydes were the main products of amino acid degradation. In this study, whey protein was added for co-fermenting with pineapple by-products, and the increase in aldehydes may be due to the microorganisms utilizing the proteins and amino acids present in the substrate during the fermentation process. Many kinds of aldehydes have sweet and fruity flavors. In this study, (E)-2-hexenal and 2-methylbutanal were the key aldehydes during fermentation and accumulated in the middle of fermentation (8–14 h). After fermentation, 5-methylfurfural, described as having toasted bread and almond aromas, showed a significant increase in signal intensity. It was the main aldehyde in the fermented products. The signal intensity of nonanal, which is responsible for aldehydic odor, decreased during the fermentation process. These data indicated that fermentation facilitated the removal of off flavors from pineapple by-products.
Esters are the most common volatile compounds in fermented foods, they are the main contributors to the fruity and sweet flavors of fermentation products (Hu et al., 2020). As shown in Fig. 4(E), the total signal intensity of esters was significantly increased, which may be due to the process of esterification of carboxylic acids with alcohols in the fermentation matrix (Hu et al., 2020). Ethyl acetate and butyl propanoate had the highest signal intensities among the esters of the unfermented samples, both of which had pineapple and tropical fruit aromas. The signal intensity of ethyl acetate, carvacryl acetate, isoamyl acetate and ethyl 2-hydroxypropanoate increased as the fermentation time was prolonged, in which ethyl 2-hydroxypropanoate carried the aroma of fermented dairy products, making the final fermented product more aromatic.
Ketones are produced through the oxidation or degradation of unsaturated fatty acids with amino acids (Shi et al., 2023, Shi et al., 2023). In this study, the total signal value of ketones was significantly increased, which may be due to the addition of whey protein for co-fermentation with pineapple by-products. 2-Octanone and (E)-beta-damascenone were the highest signal intensity ketones in the final fermentation products, and they provided a rich cheesy and creamy aroma to the fermented product.
The overall signal intensity of acids exhibited a significant increase during fermentation. This can be attributed to the presence of lactic acid bacteria as fermentation initiators that generated organic acids throughout the process (Shi et al., 2023, Shi et al., 2023). Butanoic and hexanoic acids are known as cheesy and fruity aromas respectively. Compared with earlier stages, they were identified as key components in the fermentation process with higher signal intensities observed in the final products.
To investigate the key volatile compounds that contribute to the distinction between different samples during the fermentation process, a partial least squares discriminant analysis (PLS–DA) model was established. The Q2 of this model was 0.989, and the validity of the model was proved by the permutation plot. As shown in Fig. 4(F), the samples were divided into four categories according to the similarity of volatile compounds: unfermented samples (0 h), pre-fermentation samples (8 h), mid-fermentation samples (14 h) and final fermentation products (26 h). The results showed that fermentation had a strong influence on the flavor of pineapple by-products, and it is found that some volatile compounds were formed in the middle of the fermentation stage and disappeared in the late fermentation period. We calculated variable importance in projection scores (Fig. 4(G)) for the volatile compounds of the samples and 21 volatile compounds with discriminatory properties were selected, of which the contribution of (E)-2-hexenal was the most significant and can be considered the most significant differentiation.
Changes in taste during fermentation
The basic taste indicators such as bitterness, astringency, umami, sourness and saltiness of pineapple by-products during fermentation were measured with unfermented samples as controls; the results are shown in Fig. 5. Analysis of the E-tongue showed that there were significant changes in bitterness, astringency, umami and sourness during the fermentation process, whereas no significant changes were observed in other tastes. As shown in Fig. 5(A), the sourness and umami significantly increased with fermentation, which was similar to the results of Chen et al., 2023, Chen et al., 2023. It has been confirmed in our previous study that microorganisms produced large amounts of organic acids, such as lactic acid, succinic acid and ascorbic acid, during the fermentation of pineapple by-products, which may be responsible for the sourness increase (Luo et al., 2023). The reason for the enhanced umami may be the microbial breakdown of proteins into peptides and FAAs during fermentation, these data corresponded to the changes in umami FAAs (Asp and Glu). Cincotta et al. found that during the preparation of yogurt, lactic acid bacteria broke down milk proteins into amino acids, which was a key factor in the formation of yogurt flavor (Cincotta et al., 2021). In this study, bitterness and astringency showed the opposite trend. The bitterness of the fermented samples was significantly reduced compared with the unfermented samples, which was consistent with the findings of Tran et al. (2020). Popescu et al. found that yeast could use the bitter substances for growth and metabolism during fermentation thereby reducing the bitterness of the fermented product (Popescu, Soceanu, Dobrinas & Stanciu, 2013). The change of astringency was consistent with the bitterness, which also decreased as fermentation proceeded. This may be because MDH (Methanol dehydrogenase) and NADP+ (Isocitrate dehydrogenase) are produced by yeast during fermentation. These products could convert malic acid into lactic acid and available carbon sources (Moriyama, Nishio & Mizushima, 2018). Malic acid is the main astringent substance in fruits. In traditional wine fermentation, non-winemaking yeasts are often used to consume malic acid, thus reducing the astringency of the wine (Vicente et al., 2023).
The PCA analysis was used to analyze the taste values of the products at different fermentation stages, and the results are shown in Fig. 5(B). The sum of the contribution of PC1 and PC2 reached 95.3 %, indicating that these two principal components can characterize most of the information on the taste value of fermented products. The different fermentation times were in different regions and distinguished well. Fermentation time 0 h was far away from 8 h, 14 h and 26 h, indicating that PC1 could effectively distinguish 0 h from the other three categories, whereas PC2 could well distinguish the other fermentation times. Taken together, the results showed a significant difference in taste values at different fermentation stages.
Changes in core microflora during fermentation and their correlation with nutrients and sensory quality
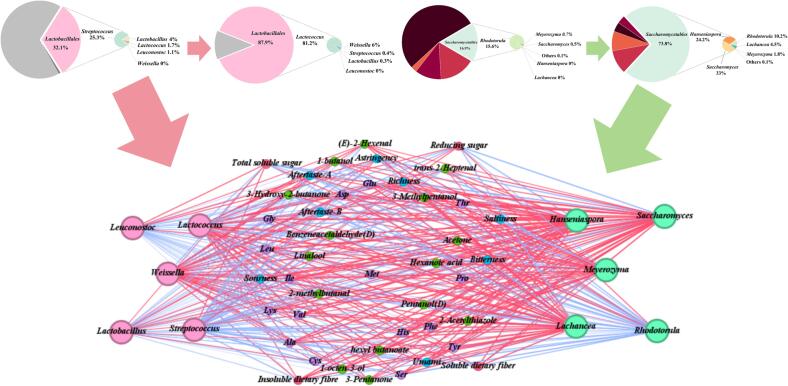
Changes in nutrients and the production of volatile substances during fermentation are closely related to the structural changes of the microbial community in the fermentation system (Zhang, Zhang, Xin, Liu & Zhang, 2021). Therefore, it is important to determine the core microbial community that plays a major role in the fermentation system. In this study, Lactococcus lactis and Hanseniaspora opuntiae were added to the substrate as fermentation initiators. Therefore, we focused on the flora changes of Lactobacillales and Saccharomycetales during the fermentation process. The relationship between nutrients and sensory quality was calculated using Spearman’s correlation method and visualized using Gephi. As shown in Fig. 6, the results showed that Lactobacillales accounted for about 32.1 % of the total bacterial community in the unfermented samples. Among them, Streptococcus was the main part of Lactobacillales of unfermented samples, and the abundance of Lactococcus was only 1.7 %. At the end of fermentation, Lactobacillales accounted for 87.9 % of the bacterial community and was the dominant bacterium in the fermented product. Meanwhile, the composition of Lactobacillales changed with Lactococcus and Weissella increasing, and they became the dominant bacteria in Lactobacillales. Weissella is widely present in food, and it is an essential component of the human intestinal flora. Kim et al. reported that Weissella had a promising application as a probiotic in a variety of fermented foods, contributing to the enhancement of flavor and texture of fermented milks and vegetables (Kim, Yang & Kim, 2023). There were significant changes in the fungal community as well. In the initial stages of fermentation, Saccharomycetales comprised only 16.9 % of the fungal community, and Rhodotorula was the dominant fungal genus in the Saccharomycetales. As the fermentation progressed, the relative abundance of Saccharomycetales increased. Meanwhile, Hanseniaspora and Saccharomyces increased in abundance with fermentation and became the major fungal genera in the final fermentation products. Saccharomyces is one of the most widely utilized microorganisms by humans, mostly in bread and wine making. Hirst & Richter (2016) found that the metabolism of Saccharomyces is divided into two phases, namely primary metabolism and secondary metabolism. They reported that primary metabolism produces ethanol and glycerol, and secondary metabolism produces a large number of small molecules, including esters, terpenes and other compounds. These secondary metabolites contribute to the good organoleptic properties of food and beverage products (Hirst & Richter, 2016).
Fig. 6.
Changes in core microbial communities during fermentation and their correlation with physicochemical indicators and sensory characteristics. Note: The red line connecting the circles indicates a positive correlation and the blue line indicates a negative correlation between microbiota and indicators. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
The changes in carbohydrates in the fermentation substrate are closely related to the growth and metabolism of microorganisms. In this study, Lactococcus, Weissella, Hanseniaspora, Saccharomyces, Meyerozyma and Lachancea were negatively correlated with the content of total soluble and reducing sugars during fermentation. These data indicated that microorganisms could use available carbon sources to maintain their growth and metabolism during fermentation. These findings were similar to the results reported by Chen et al., 2023, Chen et al., 2023. Hanseniaspora, Saccharomyces, Meyerozyma and Lachancea were positively correlated with the content of dietary fiber during fermentation. Chen et al. (2020) also found that the fermentation of tea pomace by fungi such as Trichoderma viride could significantly enhance the dietary fiber content. Lactococcus also had a positive correlation with the increase in dietary fiber. Similar findings were reported by Tu, Chen, Wang, Ruan, Zhang & Kou (2014) who found that fermentation by lactic acid bacteria could significantly enhance the SDF in soybean residue.
The sensory properties of fermented products are inextricably linked to the action of microorganisms (García-Cayuela et al., 2012). As for bacteria, lactic acid bacteria are usually considered key strains in the microbial fermentation process, which have an important impact on the flavor characteristics of fermented products (Luan, Zhang, Devahastin & Liu, 2021). In this study, Lactococcus as the main bacterium in the fermentation process was highly correlated with most of the sensory properties of the fermentation products. In terms of flavor attributes, Lactococcus was positively correlated with alcohols (3-methylpentan-1-ol and 1-butanol), esters (hexyl butanoate) and acids (hexanoic acid). This indicated that Lactococcus might bring fruity, sweet, floral and green flavors to the fermentation products. Wu et al. found that lactic acid bacteria could produce abundant volatile compounds through multiple metabolic pathways (Wu et al., 2018). In terms of taste, Lactococcus was positively correlated with umami FAAs, sourness and richness. The increase of umami FAAs and richness may be due to the growth and metabolism of Lactococcus using the proteins in the fermentation substrate, resulting in producing a large number of FAAs (García-Cayuela et al., 2012). Organic acids are the main metabolites of Lactococcus, which may be the reason for the increase in sourness in fermentation products (Chen et al., 2020). Moreover, Lactococcus was negatively correlated with bitterness and astringency. Similar results were found by Chagas Junior, Ferreira & Lopes (2021), who found that the lactic acid bacteria reduced the bitterness of cocoa during the fermentation process. In the current study, Weissella was also the core microorganism in the fermentation process, as shown in Fig. 6, Weissella was highly positively correlated with ketones (3-hydroxy-2-butanone and 3-pentanone), which indicated that Weissella may provide creamy and cheesy flavors to the fermented products. These findings were similar to the report by Choi et al. (2019). In contrast, Streptococcus was negatively correlated with most of the sensory parameters, the same phenomenon was found by Shi et al., 2023, Shi et al., 2023. This may be because, as the main microbial community, lactic acid bacteria produced a large number of organic acids during the fermentation process and led to a decrease in pH in the fermentation system, thus inhibiting the growth of microorganisms such as Streptococcus.
Fungus also plays an important role in fermentation. In the current study, as shown in Fig. 6, Hanseniaspora and Saccharomyces were the main fungi in the fermentation products, and they were highly associated with several sensory properties. Hanseniaspora is one of the major contributors of volatile compounds, it is highly positively correlated with volatile compounds such as 2-acetylthiazole, benzeneacetaldehyde and 1-octen-3-ol. These substances could provide whiskey and floral flavors to the fermented products. As described above, Hanseniaspora could produce volatile compounds associated with floral and fruity flavors during the traditional winemaking process (Shiguo et al., 2022). In terms of taste attributes, Hanseniaspora was highly negatively correlated with bitterness and astringency. Shiguo et al. found that co-fermentation of Tilapia fish protein hydrolysates by Hanseniaspora and Lactobacillus could reduce its bitterness (Shiguo et al., 2022). It is believed that Saccharomyces is the most common microorganism involved in the fermentation of wine. Stribny, Gamero, Pérez-Torrado & Querol (2015) found that Saccharomyces could enhance the higher alcohols in alcoholic beverages. In this study, Saccharomyces was highly correlated with various volatile compounds, and highly positively correlated with umami FAAs, sweet FAAs and bitter FAAs during fermentation. These data suggest that Saccharomyces could improve the sensory properties of fermented pineapple by-products. And our findings are consistent with those of Luan et al. (2021).
Conclusion
This work investigated the dynamics of nutrients, sensory quality and microbial communities and their interactions during the co-fermentation of pineapple by-products and whey protein using lactic acid bacteria and yeast. The results showed that the content of total soluble and reducing sugars decreased with increasing fermentation time. Compared with unfermented samples, dietary fiber and FAAs increased significantly as fermentation proceeded. Compared with the unfermented samples, the fermented samples were significantly lower in astringency and bitterness and significantly higher in sourness, umami and richness. The fermented samples were richer in volatile compounds with floral, cheesy, fruity and other flavors. The core microbial community was highly correlated with the quality attributes of the fermented pineapple by-products. Microorganisms such as Lactococcus, Weissella, Hanseniaspora, Saccharomyces and Lachancea contributed much to the fermented products. The results showed that the nutrients and sensory quality were highly correlated with the succession of microbial communities during the fermentation of pineapple by-product and whey protein. The results of this study contribute to the high-value utilization of pineapple by-products. Our study also provides theoretical support and new ideas for the comprehensive utilization of fruit by-product resources.
Funding statement
This work was supported by the Dongguan Institute of Science and Technology High-Level Talent Research Start Project (GC300501-139), the Guangdong Basic and Applied Basic Research Foundation (2020A1515110211) and a Project of Educational Commission of Guangdong Province of China (2021KTSCX132).
CRediT authorship contribution statement
Jia-wei Luo: Writing – review & editing, Writing – original draft, Validation, Resources, Methodology, Investigation, Conceptualization. Shan Xiao: Writing – review & editing, Validation, Supervision, Resources, Methodology, Formal analysis, Data curation, Conceptualization. Hao Suo: Writing – review & editing, Data curation. Bo Wang: Validation, Data curation. Yan-xue Cai: Validation, Formal analysis, Data curation. Ji-hui Wang: Writing – review & editing, Resources, Project administration, Methodology, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2024.101254.
Contributor Information
Shan Xiao, Email: xiaoshan@dgut.edu.cn.
Ji-hui Wang, Email: wangjihui@dgut.edu.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Cantatore V., Filannino P., Gambacorta G., De Pasquale I., Pan S., Gobbetti M., Di Cagno R. Lactic acid fermentation to re-cycle apple by-products for wheat bread fortification. Frontiers in Microbiology. 2019;10 doi: 10.3389/fmicb.2019.02574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casarotti S.N., Borgonovi T.F., Batista C., Penna A. Guava, orange and passion fruit by-products: Characterization and its impacts on kinetics of acidification and properties of probiotic fermented products. LWT-Food Science and Technology. 2018;98:69–76. [Google Scholar]
- Chagas Junior G.C.A., Ferreira N.R., Lopes A.S. The microbiota diversity identified during the cocoa fermentation and the benefits of the starter cultures use: An overview. International Journal of Food Science & Technology. 2021;56(2):544–552. [Google Scholar]
- Chen H., Xiao G., Xu Y., Yu Y., Wu J., Zou B. High hydrostatic pressure and co-fermentation by lactobacillus rhamnosus and gluconacetobacter xylinus improve flavor of yacon-litchi-longan juice. Foods. 2019;8(8) doi: 10.3390/foods8080308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Huang H., Chen Y., Xie J., Song Y., Chang X., Liu S., Wang Z., Hu X., Yu Q. Effects of fermentation on the structural characteristics and in vitro binding capacity of soluble dietary fiber from tea residues. LWT. 2020;131 [Google Scholar]
- Chen L., Liu B., Li D., Wang S., Ma X., Zhang Y. Effects of fermentation on flavor and antioxidant activity in ginkgo rice wine. Food Bioscience. 2023;53 [Google Scholar]
- Chen X., Gui R., Li N., Wu Y., Chen J., Wu X., Qin Z., Yang S., Li X. Production of soluble dietary fibers and red pigments from potato pomace in submerged fermentation by Monascus purpureus. Process Biochemistry. 2021;111:159–166. [Google Scholar]
- Chen Y., Chen L., Liu L., Bi X., Liu X. Characteristics of microbial communities in fermentation of pickled ginger and their correlation with its volatile flavors. Food Bioscience. 2023;53 [Google Scholar]
- Choi Y., Yong S., Lee M.J., Park S.J., Yun Y., Park S., Lee M. Changes in volatile and non-volatile compounds of model kimchi through fermentation by lactic acid bacteria. LWT. 2019;105:118–126. [Google Scholar]
- Cincotta F., Condurso C., Tripodi G., Merlino M., Prestia O., Stanton C., Verzera A. Comparison of lactose free and traditional mozzarella cheese during shelf-life by aroma compounds and sensory analysis. LWT. 2021;140 [Google Scholar]
- Dreher M.L., Davenport A.J. Hass avocado composition and potential health effects. Critical Reviews in Food Science and Nutrition. 2013;53(7/9):738–750. doi: 10.1080/10408398.2011.556759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca H.C., Melo D.D., Ramos C.L., Dias D.R., Schwan R.F. Lactiplantibacillus plantarum CCMA 0743 and lacticaseibacillus paracasei subsp. paracasei LBC-81 metabolism during the single and mixed fermentation of tropical fruit juices. Brazilian Journal of Microbiology. 2021;52(4):2307–2317. doi: 10.1007/s42770-021-00628-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gansemans A., Martens D., D’Haese M., Orbie J. Do labour rights matter for export? a qualitative comparative analysis of pineapple trade to the EU. Politics and Governance. 2017;5(4):93–105. [Google Scholar]
- García-Cayuela T., Gómez De Cadiñanos L.P., Peláez C., Requena T. Expression in lactococcus lactis of functional genes related to amino acid catabolism and cheese aroma formation is influenced by branched chain amino acids. International Journal of Food Microbiology. 2012;159(3):207–213. doi: 10.1016/j.ijfoodmicro.2012.09.002. [DOI] [PubMed] [Google Scholar]
- Gengatharan A., Dykes G.A., Choo W.S. Fermentation of red pitahaya extracts using lactobacillus spp. and Saccharomyces cerevisiae for reduction of sugar content and concentration of betacyanin content. Journal of Food Science and Technology-Mysore. 2021;58(9):3611–3621. doi: 10.1007/s13197-021-05116-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez Herrera M.D., Alayon Luaces P., Avanza M.V. Organic compounds determined at different levels of ripening of the pineapple (Ananas comosus L. merr.) cv cayenne in two cultivation systems under subtropical conditions. International Journal of Fruit Science. 2020;20(3):371–384. [Google Scholar]
- Hirst M.B., Richter C.L. Review of aroma formation through metabolic pathways of Saccharomyces cerevisiae in beverage fermentations. American Journal of Enology and Viticulture. 2016;67(4):361–370. [Google Scholar]
- Hu L.P., Li Y., Huang X., Du C.D., Huang D.J., Tao X.M. The effect of co-fermentation with lactobacillus plantarum HLJ29L2 and yeast on wheat protein characteristics in sourdough and crackers. Foods. 2023;12(3) doi: 10.3390/foods12030555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y., Zhang L., Zhang H., Wang Y., Chen Q., Kong B. Physicochemical properties and flavour profile of fermented dry sausages with a reduction of sodium chloride. LWT. 2020;124 [Google Scholar]
- Kim E., Yang S.M., Kim H.Y. Weissella and the two janus faces of the genus. Applied Microbiology and Biotechnology. 2023;107(4):1119–1127. doi: 10.1007/s00253-023-12387-6. [DOI] [PubMed] [Google Scholar]
- Krolczyk J.B., Dawidziuk T., Janiszewska-Turak E., Solowiej B. Use of whey and whey preparations in the food industry - a review. Polish Journal of Food and Nutrition Sciences. 2016;66(3):157–165. [Google Scholar]
- Li Y., Nguyen T., Jin J.H., Lim J., Lee J., Piao M.Z., Mok I., Kim D. Brewing of glucuronic acid-enriched apple cider with enhanced antioxidant activities through the co-fermentation of yeast (Saccharomyces cerevisiae and pichia kudriavzevii) and bacteria (lactobacillus plantarum) Food Science and Biotechnology. 2021;30(4):555–564. doi: 10.1007/s10068-021-00883-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Niu L., Guo Q., Shi L., Deng X., Liu X., Xiao C. Effects of fermentation with lactic bacteria on the structural characteristics and physicochemical and functional properties of soluble dietary fiber from prosomillet bran. LWT. 2022;154 [Google Scholar]
- Liao H., Luo Y., Huang X., Xia X. Dynamics of quality attributes, flavor compounds, and microbial communities during multi-driven-levels chili fermentation: Interactions between the metabolome and microbiome. Food Chemistry. 2023;405 doi: 10.1016/j.foodchem.2022.134936. [DOI] [PubMed] [Google Scholar]
- Luan C., Zhang M., Devahastin S., Liu Y. Effect of two-step fermentation with lactic acid bacteria and Saccharomyces cerevisiae on key chemical properties, molecular structure and flavor characteristics of horseradish sauce. LWT. 2021;147 [Google Scholar]
- Luo J.W., Xiao S., Wang J.H., Wang B., Cai Y.X., Hu W.F. The metabolite profiling and microbial community dynamics during pineapple by-product fermentation using co-inoculation of lactic acid bacteria and yeast. Fermentation-Basel. 2023;9(2):79. [Google Scholar]
- Marcillo D., Galarza V.O., Mosquera N., Valladares R., Perez J.N., Pais-Chanfrau J.M. Multi-objective optimization of beverage based on lactic fermentation of goat’s milk whey and fruit juice mixes by kefir granules. Fermentation-Basel. 2022;8(10) [Google Scholar]
- Martinez R., Torres P., Meneses M.A., Figueroa J.G., Perez-Alvarez J.A., Viuda-Martos M. Chemical, technological and in vitro antioxidant properties of mango, guava, pineapple and passion fruit dietary fibre concentrate. Food Chemistry. 2012;135(3):1520–1526. doi: 10.1016/j.foodchem.2012.05.057. [DOI] [PubMed] [Google Scholar]
- Moriyama S., Nishio K., Mizushima T. Structure of glyoxysomal malate dehydrogenase (MDH3) from Saccharomyces cerevisiae. Acta Crystallographica Section F Structural Biology Communications. 2018;74(10):617–624. doi: 10.1107/S2053230X18011895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olaitan O.O., Obadina O.A., Omemu A.M., Atanda O.O., Olotu I.O. Nutritional quality and physico-chemical changes during breadfruit (Artocarpus altilis) natural fermentation. Quality Assurance and Safety of Crops & Foods. 2015;7(4):441–447. [Google Scholar]
- Pittari E., Moio L., Piombino P. Screening of the volatile composition and olfactory properties of aglianico and primitivo, two southern italian red wines. Applied Sciences. 2023;13(4):2165. [Google Scholar]
- Popescu V., Soceanu A., Dobrinas S., Stanciu G. A study of beer bitterness loss during the various stages of the romanian beer production process. Journal of the Institute of Brewing. 2013;119(3):111–115. [Google Scholar]
- Shi F., Wang L., Li S. Enhancement in the physicochemical properties, antioxidant activity, volatile compounds, and non-volatile compounds of watermelon juices through lactobacillus plantarum JHT78 fermentation. Food Chemistry. 2023;420 doi: 10.1016/j.foodchem.2023.136146. [DOI] [PubMed] [Google Scholar]
- Shi Q., Tang H., Mei Y., Chen J., Wang X., Liu B., Cai Y., Zhao N., Yang M., Li H. Effects of endogenous capsaicin stress and fermentation time on the microbial succession and flavor compounds of chili paste (a chinese fermented chili pepper) Food Research International. 2023;168 doi: 10.1016/j.foodres.2023.112763. [DOI] [PubMed] [Google Scholar]
- Shiguo L., Qiao Z., Qin X., Lirui D., Zhisheng P., Yongcheng L. Hanseniaspora pseudoguilliermondii improves the flavor of tilapia fish protein hydrolysates. Journal of Aquatic Food Product Technology. 2022;31(1/5):297–310. [Google Scholar]
- Stribny J., Gamero A., Pérez-Torrado R., Querol A. Saccharomyces。kudriavzevii and Saccharomyces uvarum differ from Saccharomyces cerevisiae during the production of aroma-active higher alcohols and acetate esters using their amino acidic precursors. International Journal of Food Microbiology. 2015;205:41–46. doi: 10.1016/j.ijfoodmicro.2015.04.003. [DOI] [PubMed] [Google Scholar]
- Sun F., Wang H., Liu Q., Xia X., Chen Q., Kong B. Proteolysis and quality characteristics of Harbin dry sausages caused by the addition of staphylococcus xylosus protease. Food Chemistry. 2023;404 doi: 10.1016/j.foodchem.2022.134692. [DOI] [PubMed] [Google Scholar]
- Tran A.M., Nguyen T.B., Nguyen V.D., Bujna E., Dam M.S., Nguyen Q.D. Changes in bitterness, antioxidant activity and total phenolic content of grapefruit juice fermented by lactobacillus and bifidobacterium strains. Acta Alimentaria. 2020;49(1):103–110. [Google Scholar]
- Tu Z., Chen L., Wang H., Ruan C., Zhang L., Kou Y. Effect of fermentation and dynamic high pressure microfluidization on dietary fibre of soybean residue. Journal of Food Science and Technology-mysore. 2014;51(11):3285–3292. doi: 10.1007/s13197-012-0838-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicente J., Kelanne N., Navascués E., Calderón F., Santos A., Marquina D., Yang B., Benito S. Combined use of schizosaccharomyces pombe and a lachancea thermotolerans strain with a high malic acid consumption ability for wine production. Fermentation-Basel. 2023;9(2):165. [Google Scholar]
- Wang D., Zhang J., Zhu Z., Lei Y., Huang S., Huang M. Effect of ageing time on the flavour compounds in Nanjing water-boiled salted duck detected by HS-GC-IMS. LWT. 2022;155 [Google Scholar]
- Wang F., Gao Y., Wang H., Xi B., He X., Yang X., Li W. Analysis of volatile compounds and flavor fingerprint in jingyuan lamb of different ages using gas chromatography–ion mobility spectrometry (GC–IMS) Meat Science. 2021;175 doi: 10.1016/j.meatsci.2021.108449. [DOI] [PubMed] [Google Scholar]
- Wang H., Huang Y., Huang Y. Microbiome diversity and evolution in stacking fermentation during different rounds of jiang-flavoured baijiu brewing. LWT. 2021;143 [Google Scholar]
- Wang W.Q., Zhang J.L., Yu Q., Zhou J.Y., Lu M.L., Gu R.X., Huang Y.J. Structural and compositional changes of whey protein and blueberry juice fermented using lactobacillus plantarum or lactobacillus casei during fermentation. RSC Advances. 2021;11(42):26291–26302. doi: 10.1039/d1ra04140a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Tian T., Liu Y., Shi Y., Tao D., Wu R., Yue X. The dynamic changes of chemical components and microbiota during the natural fermentation process in da-jiang, a chinese popular traditional fermented condiment. Food Research International. 2018;112:457–467. doi: 10.1016/j.foodres.2018.06.021. [DOI] [PubMed] [Google Scholar]
- Wu N., Wang X. Identification of important odorants derived from phosphatidylethanolamine species in steamed male Eriocheir sinensis hepatopancreas in model systems. Food Chemistry. 2019;286:491–499. doi: 10.1016/j.foodchem.2019.01.201. [DOI] [PubMed] [Google Scholar]
- Xing X., Wang Y., Huo N., Wang R., College O.A.S., College O.H., Shanxi A.U., Shanxi F.Q.S.S., College O.F.S.E., Department O.P.H.A. Candida ethanolica strain Y18 enhances aroma of Shanxi aged-vinegar. Food Science and Technology Research. 2018;24(6):1069–1081. [Google Scholar]
- Zhang J., Zhang C., Xin X., Liu D., Zhang W. Comparative analysis of traditional and modern fermentation for xuecai and correlations between volatile flavor compounds and bacterial community. Frontiers in Microbiology. 2021;12 doi: 10.3389/fmicb.2021.631054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Ding Y., Gu S., Zhu S., Zhou X., Ding Y. Identification of changes in volatile compounds in dry-cured fish during storage using HS-GC-IMS. Food Research International. 2020;137(Nov.):109331–109339. doi: 10.1016/j.foodres.2020.109339. [DOI] [PubMed] [Google Scholar]
- Zhang S., Li C., Wu J., Peng S., Mao H., Wu W., Liao L. Effect of salt concentration on flavor characteristics and physicochemical quality of pickled brassica napus. Fermentation-Basel. 2023;9(3):275. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.