Abstract
T lymphocytes undergo apoptosis in response to a variety of stimuli, including exposure to UV radiation and γ-irradiation. While the mechanism by which stress stimuli induce apoptosis is not well understood, we have previously shown that the induction of Fas ligand (FasL) gene expression by environmental stress stimuli is dependent on c-Jun N-terminal kinase (JNK) activation. Using inducible dominant-active (DA) JNK kinase kinase (MEKK1) expression in Jurkat cells, we map a specific MEKK1-regulated response element to positions −338 to −316 of the Fas ligand (FasL) promoter. Mutation of that response element abrogated MEKK1-mediated FasL promoter activation and interfered in stress-induced activation of that promoter. Using electrophoretic mobility shift assays, we demonstrate that activator protein 1 (AP-1) binding proteins, namely, activating transcription factor 2 (ATF2) and c-Jun, bind to the MEKK1 response element. Transient transfection of interfering c-Jun and ATF2 mutants, which lack the consensus JNK phosphorylation sites, abrogated the transcriptional activation of the FasL promoter, demonstrating the involvement of these transcription factors in the regulation of the FasL promoter. Taken together, our data indicate that MEKK1 and transcription factors regulated by the JNK pathway play a role in committing lymphocytes to undergo apoptosis by inducing FasL expression via a novel response element in the promoter of that gene.
In response to antigenic challenge, lymphocytes become activated, secrete cytokines, and proliferate. Yet to maintain homeostasis, once the antigen has been cleared, activated lymphocytes are removed by a process of apoptosis (6, 45, 52). While recent studies have provided considerable insight into the role of intracellular signaling pathways utilized by death-inducing receptors such as Fas and tumor necrosis factor alpha (TNF-α) type 1 receptor (TNFR1) (1, 13, 22, 23, 36, 45, 48), we still lack a clear understanding of the molecular pathways which regulate the commitment of lymphocytes to die. A number of signaling pathways and nuclear transcription factors play a role in regulating apoptosis (5, 14, 17, 40, 48, 54, 58). However, relatively little is known about the signaling pathways and transcription factors which tightly regulate the expression of Fas ligand (FasL). We have recently shown that T-cell receptor (TCR)-mediated signals leading to activation of nuclear factor of activated T cells (NF-AT) response elements in the FasL promoter are important for FasL expression in lymphocytes (28). Additionally, we have shown that FasL expression plays a role in stress-mediated apoptosis in T cells (14). In contrast to FasL inducing signals delivered through the TCR, stress signals leading to FasL expression, such as those resulting from exposure to UV radiation (UVR), γ-irradiation, or protein synthesis inhibitors, appear to be mediated via activation of the c-Jun N-terminal kinase (JNK) cascade (14).
The role of the JNK cascade in cellular apoptosis has been controversial, with evidence reported for pro- and antiapoptotic effects. Data which favor a proapoptotic role include (i) induction of apoptosis by dominant-active (DA) JNK kinase kinase (MEKK1) in Jurkat cells, fibroblasts, and PC12 cells (14, 26, 58), (ii) interference in apoptosis induction by ceramide or growth factor withdrawal by dominant-negative (DN) JNK cascade components, e.g., DN-SAP/ERK kinase (SEK1) or DN-MEKK1 (18, 55), (iii) induction of apoptosis in T lymphocytes during prolonged activation of the JNK cascade, e.g., γ-irradiation and DA-MEKK1 expression (7, 8, 14), and (iv) interference in apoptosis induction and JNK activation by a DN version of the Daxx effector, which interacts with the intracellular death domain of the Fas receptor (27a). In contrast, evidence against the involvement of the JNK cascade in promoting apoptosis includes the proapoptotic effect of homozygous SEK1 knockout in murine thymocytes (38). Moreover, TNFR1 still induces apoptosis when its JNK-inducing component, tumor necrosis factor receptor-associated factor (TRAF2), is mutagenized so that it no longer activates JNK (31). It has been suggested that JNK activation may follow activation of the caspase cascade (4, 29, 31, 37). These data suggest, therefore, that the JNK cascade may be redundant or may occur secondary to cellular damage associated with apoptosis. This notion explains why a cell nucleus and accompanying nuclear response elements are not obligatory requirements for the induction of apoptosis (2, 29, 50).
Our recent studies have shed new light on the paradoxical role of the JNK cascade in apoptosis. While DN-MEKK1 failed to interfere with TNFR1-induced apoptosis, prolonged activation of the JNK cascade by stress stimuli or DA-MEKK1 expression in Jurkat cells induced apoptosis through FasL expression (7, 8, 14, 31). This process was reversed by Fas-Fc fusion protein, which interferes in the Fas-FasL interaction (14). Moreover, several groups have shown that there is a decreased rate of apoptosis in MRL-lpr/lpr mice during exposure to environmental stress, e.g., UVR (9, 43). These findings have led us to propose that, instead of a direct role in the delivery of the death signal, the JNK cascade commits T cells to apoptosis through FasL expression. FasL expression may, therefore, act as a fail-safe mechanism to remove T cells which have been damaged by noxious stimuli. Further, this mechanism appears to operate only when there is prolonged JNK activation; evanescent JNK activation by TCR-CD28 coligation fails to induce apoptosis (14). We propose that TCR-mediated signals which regulate FasL expression differ from stress-induced signals.
In order to clarify the role of the JNK cascade in FasL expression, we asked whether there is a MEKK1 response element in the promoter of that gene. By transfecting 5′ serially deleted FasL promoter constructs into Jurkat cells with tetracycline-inducible DA-MEKK1 expression, we show that DA-MEKK1 expression induces the transcriptional activation of the −486 FasL promoter through a specific MEKK1 responsive element which was mapped between positions −336 and −318 upstream from the transcriptional start site. Mutation of that site abrogated FasL promoter activation by the JNK cascade and stress stimuli. Using electrophoretic mobility shift assays (EMSA), we find that activator protein 1 (AP-1) proteins, including activating transcription factor 2 (ATF2) and c-Jun, bind to the specific response element. Further, by transient transfection, we demonstrate that these proteins are involved in the transcriptional regulation of the FasL promoter.
MATERIALS AND METHODS
Reagents.
Anti-MEKK1 monoclonal antibody (MAb) as well as antibodies for supershift analysis, i.e., anti-c-Jun, anti-Fos, and anti-ATF2, were from Santa Cruz Biotechnology (Santa Cruz, Calif.). The horseradish peroxidase (HRP)-conjugated protein A was purchased from Amersham (Arlington Heights, Ill.). The FasL antibody was from PharMingen (San Diego, Calif.). The glutathione S-transferase (GST)–c-Jun construct was generously provided by J. Woodgett (Ontario Cancer Institute, Ontario, Canada). Phorbol myristate acetate (PMA) and ionomycin were purchased from Sigma (St. Louis, Mo.). The tetracycline-repressible system, including the pUHD15.1 and pUHD10.3 vectors, was a kind gift from H. Bujard (Heidelberg, Germany) (19). The pUHD15.1 plasmid encodes for the tetracycline-controlled transactivator (tTA), and the pUHD10.3 plasmid contains a tTA-dependent promoter upstream of a multiple cloning site (19). The cDNAs for DA-MEKK1 (MEKKΔ) and DN-MEKK (MEKKΔ K432M) were a gift from G. Johnson (National Jewish Center for Immunology and Research, Denver, Colo.) (33). DA-MEKK1 and DN-MEKK1 were subcloned into the multiple cloning site of pUHD10.3, respectively. DN JNK kinase (SEK1 and MKK4) was generously provided by Leonard Zon (Harvard Medical School, Boston, Mass.).
FasL constructs.
The −486 FasL promoter construct, consisting of the first 486 nucleotides upstream of the start site, has been described previously (28). Briefly, the −486-bp FasL reporter construct was created by cloning a HindIII-flanked 486-bp PCR product derived from genomic sequence upstream of the FasL translational start site into the Luc-Link reporter plasmid. The truncation mutants were created by cloning a BamHI/HindIII-flanked (or BglII/HindIII-flanked, for FasL−420) PCR product derived from the above construct into the Luc-Link plasmid. The reverse primer was the same as for the 486-bp product, while the forward primers were as follows: for FasL−420, CATAGATCTGAGCAGTTCACACTAACAGGGCT; for FasL−353, CATGGATCCGCATAGCCTACTAACCTGTTTGGG; for FasL−335, CATGGATCCTTTGGGTAGCACAGCGACAGCAA; for FasL−318, CAGGGATCCGCTCTGAGCTTCTTG; for FasL−258, CAGGGATCCCAGCAACTGAGGCCTTGAAGGC; for FasL−132, CATGGATCCCTCTATAAGAGAGATCCAGCTTGC; and for FasL−95, TACGGATCCAGCAGTCAGCAACAGGGTCCCG. The −369 construct was created by subcloning a BamHI-HindIII fragment from −486 into Luc-Link. The distal NF-AT binding site mutant and the −336/−318 mutant constructs were created by overlap extension PCR with the following forward (F) and reverse (R) end primers: F, 5′-GAACAAGCTTAATGTATAAAAAAGCATGCAATTATAATTC-3′; R, 5′-ACATAAGCTTGGCAGCTGGTGAGTCAGGCCA-3′. The following primers were used to incorporate the appropriate mutations: for the FasLδNFAT mutant, F, 5′-GTGGGAATCAACTTCCAGG-3′, and R, 5′-CCTGGAAGTTGATTCCCAC-3′, and for the FasLδ−336/−318 mutant, F, 5′-GATTCAGATCTCTTTGAAGCAACTGAGGCCTTGAAGGCT-3′, and R, 5′-TCAAAGAGATCTGAATCACAGGTTAGTAGGCTATGCTCACC-3′. Sequences of all PCR-derived constructs were confirmed by fluorescent automated sequencing (University of Iowa DNA facility, Iowa City, Iowa).
The triplicated −336/−318 construct was synthesized by annealing the following oligonucleotides containing overhanging XhoI compatible sites into a minimal interleukin 2 (IL-2) promoter luciferase construct: F, 5′-TCGAGTGTCGCTGTGCTACCCAAACAGTGTCGCTGTGCTACCCAAACAGTGTCGCTG TGCTACCCAAACAGC-3′, and R, 5′-TCGAGCTGTTTGGGTAGCACAGCGACACTGTTTGGGTAGCACAGCGACACTGTTTGGGTAGCACAGCGACAC-3′.
Transfection and generation of stable transfectants.
A subclone of Jurkat cells, BMS2, was transfected by electroporation with 10 μg of pUHD15.1 plasmid as previously described (15). A stable Jurkat tTA cell line was generated by selection in 2 mg of G418/ml and cloning by limiting dilution as previously described (15). Jurkat tTA cells were transfected with 30 μg of pUHD10.3 encoding DA-MEKK1. Cells were selected in 270 μg of hygromycin/ml for 4 weeks prior to the start of experiments.
Luciferase assays.
A total of 107 DA-MEKK1 cells were transiently transfected with 30 μg of FasL promoter-reporter construct. Duplicate samples were pooled and grown in the presence or absence of 0.1 μg of tetracycline/ml as indicated. The cells were either left unstimulated or treated with a combination of 100 nM PMA and 1 μg of ionomycin/ml for 7 h. The cells were washed and lysed in luciferase buffer (Analytical Luminescence, Ann Arbor, Mich.), and luciferase activity was measured by using 100 μg of protein in a Monolight 2010 luminometer (Analytical Luminescence) (15). Transfection efficiency was monitored by cotransfection of a β-galactosidase-encoding plasmid (CMV-β-Gal) and measurement of relative β-galactosidase activity. The same approach was used for transient transfection of Jurkat-tTA cells with DN-MEKK1 subcloned into the pUHD10.3 vector.
EMSA.
EMSA was performed as previously described (28). Briefly, the double-stranded oligonucleotide, 5′-TTGGGTAGCACAGCGA-3′, corresponding to positions −336 to −318 of the FasL promoter, was end labeled with Klenow fragment in the presence of [α-32P]dCTP (Dupont-NEN, Boston, Mass.). Nuclear extracts were prepared from unstimulated cells, and cells were treated with PMA plus ionomycin for 3 h as previously described (25). Ten micrograms of nuclear extract was incubated with 12 ng of radiolabeled probe for 20 min at room temperature and separated on 4.5% acrylamide gels. Gels were dried and autoradiographed. For supershift analysis, nuclear extracts were preincubated with 0.5 μg of each respective antibody for 20 min prior to the binding reaction.
Western blot analysis.
Tetracycline was withdrawn for 24 h from aliquots of 107 Jurkat tTA cells transfected with DA-MEKK1. The cells were washed and lysed as previously described (14). Cell lysates (100 μg) were separated by sodium dodecyl sulfate–10% polyacrylamide gel electrophoresis (SDS–10% PAGE) and transferred to Immobilon-P membranes. The membranes were probed with 0.2 μg of anti-MEKK1/ml, followed by a 1:3,000 dilution of HRP-coupled protein A (14). The blots were developed by enhanced chemiluminescence (ECL) according to the manufacturer’s instructions.
JNK kinase assays.
A total of 5 × 106 DA-MEKK1 Jurkat cells were grown in the presence (tet+) or absence (tet−) of tetracycline for 24 h. The cells were left untreated or were stimulated with a combination of 100 nM PMA and 1 μg of ionomycin/ml for 10 min. The cells were lysed, the supernatants were incubated with recombinant GST–c-Jun(1-79) bound to glutathione-coupled beads, and the complex was washed extensively in lysis buffer. Kinase assays were performed as previously described (15). The fold increase in kinase activity was determined by PhosphorImager analysis.
Measurement of apoptosis.
Trypan blue exclusion was used to determine cell viability as determined by two independent observers. Cell death by apoptosis was determined by 7-amino actinomycin D (7AAD) staining as previously described (14, 42). The effect of the recombinant Fas-Fc protein (27) on induction of apoptosis by DA-MEKK1 was determined by incubating the cells with 25 μg of Fas-Fc/ml in the culture medium for the indicated time periods.
FasL expression.
DA-MEKK1 cells were grown under off (tet+) or on (tet−) conditions for 24 h. For comparison, Jurkat-tTA cells were either left untreated or stimulated with 100 nM PMA plus 1 μg of ionomycin/ml for 12 h. Immunostaining for FasL expression was performed by incubating the cells with anti-FasL (NOK1) MAb, followed by fluorescein isothiocyanate (FITC)-coupled anti-mouse immunoglobulin. The cells were analyzed by flow cytometry, by using the Cell Quest program (Becton Dickinson).
RESULTS
Tetracycline-regulated expression of DA-MEKK1 induces FasL-dependent apoptosis in Jurkat T cells.
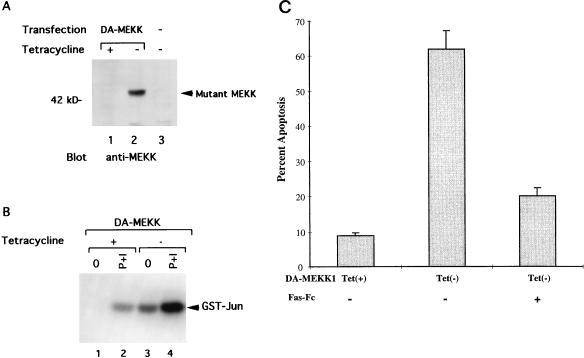
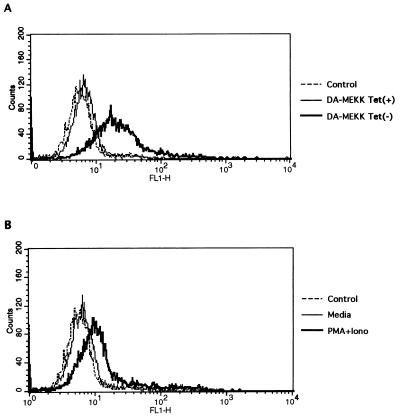
We have established a Jurkat cell line with stable, tetracycline-regulated DA-MEKK1 expression (Fig. 1) (15). Cells grown in the presence of tetracycline (tet+) do not express DA-MEKK1 (Fig. 1A, lane 1) and show basal levels of JNK activity, which is inducible by treatment with PMA plus ionomycin (Fig. 1B, lanes 1 and 2). In contrast, in the absence of tetracycline (tet−), an abundance of DA-MEKK1 is expressed (Fig. 1A, lane 2), which resulted in an 18-fold increase in JNK activation over unstimulated tet+ cells (Fig. 1B, lane 3). This JNK activity is further enhanced by treatment with PMA plus ionomycin (Fig. 1B, lane 4). Compared to tet+ cells, 62% of cells die when DA-MEKK1 is expressed for 72 h (Fig. 1C). This cell death is due to apoptosis, as determined by 7AAD staining and demonstration of DNA laddering (14). Moreover, addition of recombinant Fas-Fc protein, which interferes in Fas-FasL binding, reduced the rate of apoptosis in DA-MEKK1-expressing cells to near-background levels (Fig. 1C). As shown previously, we demonstrate by flow cytometry de novo FasL expression in DA-MEKK1-expressing cells as well as in cells activated by PMA plus ionomycin (Fig. 2). As expected, protein expression at the cell surface is accompanied by an increase in FasL message (data not shown) and correlates with FasL reporter activity (14). Taken together, these data confirm previous findings which indicate that prolonged MEKK1 activation leads to the induction of apoptosis in a FasL-dependent manner (14).
FIG. 1.
Inducible expression of DA-MEKK1 in Jurkat cells leads to constitutive JNK activation and induction of apoptosis. (A) Western blot showing the inducible expression of DA-MEKK1 in stably transfected Jurkat-tTA cells. Jurkat-tTA cells were transfected with 30 μg of cDNA encoding DA-MEKK1 in the pUHD10.3 vector (lanes 1 and 2). Cells in lane 3 were untransfected Jurkat-tTA cells. Following selection in 270 μg of hygromycin/ml for 4 weeks, the cells were grown in the presence (+) or absence (−) of 0.1 μg of tetracycline/ml for 24 h. Total cell lysates from 5 × 106 cells were separated by SDS–10% PAGE and transferred to an Immobilon-P membrane. The membrane was overlaid with 0.1 μg of anti-MEKK1 antibody/ml, followed by HRP-conjugated protein A, and was developed by ECL. (B) In vitro kinase assay showing the constitutive activation of JNK by DA-MEKK1. The transfected cells described above were either left untreated (lanes 1 and 3) or stimulated for 10 min with 100 nM PMA and 1 μg of ionomycin/ml (P+I) at 37°C (lanes 2 and 4), and JNK activity was measured as previously described (14). (C) Cell viability assay in DA-MEKK1-expressing cells and the effect of Fas-Fc fusion protein. DA-MEKK1 Jurkat cells were incubated in the presence or absence of 30 μg of Fas-Fc/ml and grown under tet+ or tet− conditions for 72 h. Cell viability was measured by trypan blue exclusion. Duplicate counts were performed by two independent observers. We have previously shown that in these cells, trypan blue uptake is accompanied by 7AAD uptake and DNA laddering (14). Similar results were obtained in three separate experiments.
FIG. 2.
Immunostaining showing enhanced FasL expression in cells expressing DA-MEKK1. DA-MEKK1 cells, grown under tet+ or tet− conditions for 36 h (A), were stained with anti-FasL (NOK1) MAb, followed by FITC-coupled anti-mouse immunoglobulin, and were analyzed by flow cytometry by using the Cell Quest program (Becton Dickinson). For comparison, Jurkat-tTA cells were either left untreated or stimulated with 100 nM PMA plus 1 μg of ionomycin (Iono)/ml for 12 h (B) and analyzed as above.
Stress-induced FasL promoter activation is dependent on MEKK1 activity.
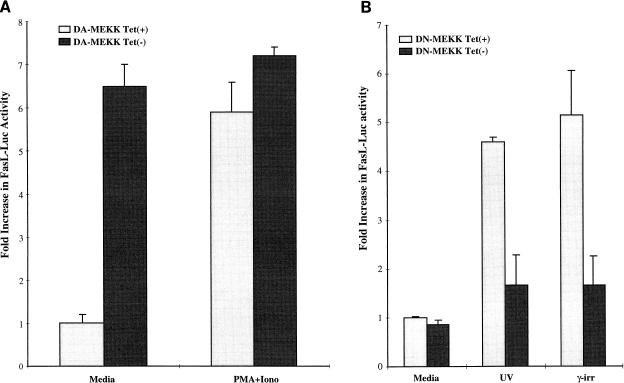
Under physiological and pathological conditions, the JNK cascade is induced by a range of stress stimuli, including UV light, γ-irradiation, DNA-damaging drugs, inflammatory cytokines, and protein synthesis inhibitors (21–23, 59). We have previously shown that UVR, γ-irradiation, and anisomycin induce FasL expression in T lymphocytes (14). Transient transfection of a FasL promoter-reporter construct (FasL−486), containing 486 bp immediately upstream of the transcriptional start site (28), into Jurkat DA-MEKK1 cells showed induction of luciferase activity in tet− as compared to tet+ cells (Fig. 3A). Further treatment of tet+ cells with PMA plus ionomycin, a potent stimulus for JNK in T cells, also leads to the induction of FasL reporter activity (Fig. 3A). These results show that constitutively active MEKK1 induces transcriptional activation of the FasL promoter in Jurkat T cells.
FIG. 3.
Regulation of the transcriptional activation of the FasL promoter by DA- and DN-MEKK1. (A) Luciferase assay showing the regulation of the FasL promoter by DA-MEKK1. DA-MEKK1 cells, transiently transfected with 30 μg of FasL-486 luciferase construct, were grown in the presence [Tet(+)] or absence [Tet(−)] of 0.1 μg of tetracycline/ml for 24 h. Cells were left unstimulated or were treated with 100 nM PMA plus 1 μg of ionomycin (Iono)/ml for 8 h. The cells were lysed, and 100 μg of cell lysates was analyzed for luciferase activity. The fold increase in luciferase activity was calculated over the value for unstimulated tet+ cells, which amounted to 6,576 relative light units. These data are representative of four experiments. (B) Luciferase activity showing the effect of DN-MEKK1 on the activation of the FasL promoter by stress stimuli. Jurkat tTA cells were transiently cotransfected with 30 μg of FasL-486 luciferase and 20 μg of DN-MEKK1 subcloned into the pUHD10.3 vector. The cells were grown in the presence or absence of 0.1 μg of tetracycline/ml for 24 h and were stimulated with 200 J of UVR/m2 or 3,300 rads of γ-irradiation in the presence of 30 μM Z-Val-Ala-Asp(OMe)-CH2F (Z-VAD). The cells were lysed 8 h later and analyzed for luciferase activity. Fold increase in luciferase activity was calculated over the value for unstimulated tet+ cells, which amounted to 7,380 relative light units. These data are representative of three experiments.
As previously mentioned, UVR and γ-irradiation induce activation of the FasL promoter-reporter (14). In order to determine whether MEKK1 plays a role in this induction, we used transient, tetracycline-regulated expression of DN-MEKK1 in Jurkat-tTA cells (Fig. 3B). In the absence of DN-MEKK1 expression, UVR and γ-irradiation induced a 4.8- or 5.2-fold increase in FasL promoter-luciferase activity (Fig. 3B). Expression of DN-MEKK1 markedly reduced the UVR- and γ-irradiation-induced FasL reporter activity (Fig. 3B). These results show that the transcriptional activation of the FasL promoter by environmental stress stimuli is dependent on kinases upstream of JNK.
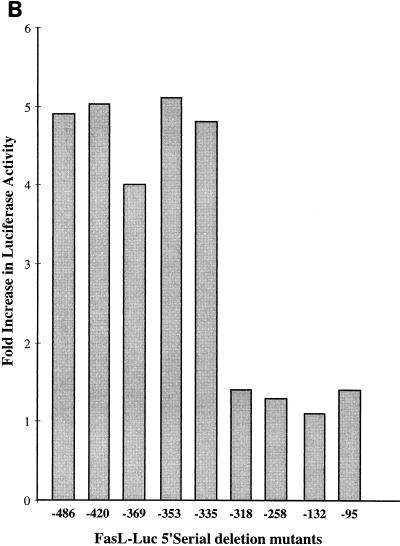
Sequential deletion of the FasL promoter maps the MEKK1 response element downstream of bp −335.
To identify the MEKK1-responsive site in the FasL promoter, we generated serial 5′ deletion mutants of the −486-bp promoter-reporter and inserted these into the Luc-Link reporter (Fig. 4A). We compared the promoter-reporter activity of these mutants to that of the wild-type promoter by transient transfection into Jurkat DA-MEKK1 cells (Fig. 4B). Compared to tet+ cells, DA-MEKK1 induced almost identical increases in luciferase activity in the FasL−486, −420, −369, and −353 reporters (Fig. 4B). In contrast, DA-MEKK1 failed to stimulate promoter-reporter activity in constructs downstream of bp −335. The loss in reporter gene activity in promoter constructs shorter than 335 bp was not due to differences in transfection efficiency, since this was corrected for by cotransfection of a β-galactosidase-expressing vector. Similar results were obtained with PMA–ionomycin stimulation (data not shown), suggesting that the MEKK1 response element is located downstream of bp −335.
FIG. 4.
The MEKK1 response element maps downstream of position −335 in the FasL promoter. (A) Schematic representation of the FasL promoter-reporter constructs showing the MEKK1- and NF-AT-responsive elements (RE) in the 486-bp promoter. Serial 5′ deletion mutants were generated as described in Materials and Methods. (B) Comparison of luciferase activity in the FasL promoter deletion mutants. DA-MEKK1 cells were transiently transfected with 30 μg of the 486-bp FasL luciferase construct or its 5′ deletion mutants. Luciferase activity was determined as described above. The fold increase in luciferase activity was calculated over the value of tet+ cells, which amounted to 10,142 relative light units. These data are representative of three experiments.
The JNK cascade induces mobility shift complexes with an oligonucleotide corresponding to positions −336 to −318 of the FasL promoter.
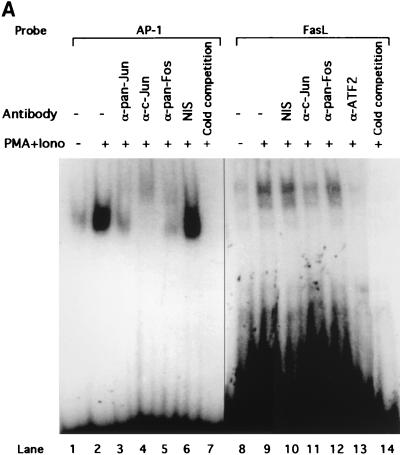
The precipitous decline of FasL promoter-reporter activity with promoter truncations 3′ of −335 suggests that this base pair may be at or in close proximity to the actual MEKK1 response element. We therefore constructed an oligonucleotide corresponding to bp −336 to −318 of the FasL promoter to probe for a MEKK1 response element. This sequence (5′-TTGGGTAGCACAGCGA-3′) does not contain homology to a recognized AP-1 binding site. In an EMSA, nuclear extracts from resting Jurkat cells displayed constitutive binding of two shift bands to this probe (Fig. 5A, lane 8). Both bands were abolished in the presence of a molar excess of unlabeled oligonucleotide, indicating binding specificity (Fig. 5A, lane 14). PMA plus ionomycin increased the abundance of both shift complexes (Fig. 5A, lane 9), which are larger than the shift complexes obtained with a consensus AP-1 oligonucleotide (Fig. 5A, lane 2).
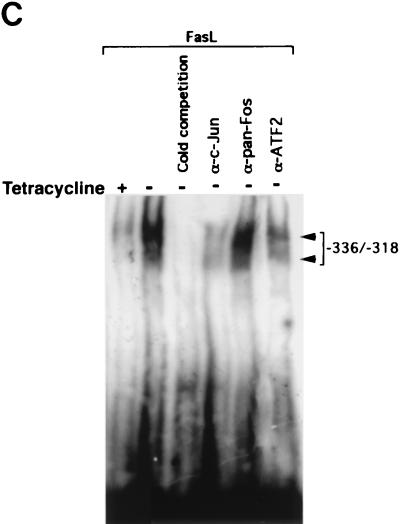
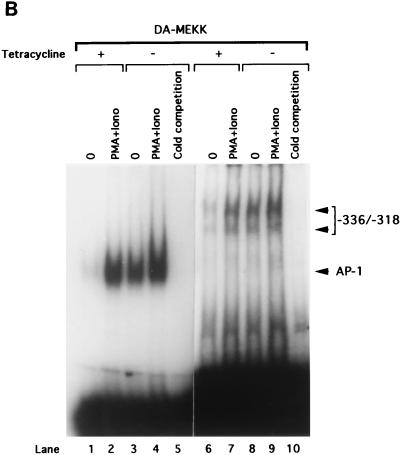
FIG. 5.
MEKK1 activation induces a mobility shift complex with an oligonucleotide corresponding to positions −336 to −318 of the FasL promoter. (A) EMSA showing the association of c-Jun and ATF2 with the MEKK1-responsive site of the FasL promoter. A total of 5 × 106 Jurkat-tTA cells were either left untreated or stimulated with 100 nM PMA plus 1 μg of ionomycin (Iono)/ml for 3 h. Nuclear extracts (10 μg) were incubated in buffer or pretreated with 0.5 μg of anti-pan-Jun (lane 3), anti-c-Jun (lanes 4 and 11), anti-pan-Fos (lanes 5 and 12), or anti-ATF2 (lane 13) polyclonal antibodies or with nonimmune serum (NIS) (lanes 6 and 10) for 40 min. EMSA was performed by using a 32P-labeled AP-1 oligonucleotide (lanes 1 through 7) or the oligonucleotide corresponding to positions −336 to −318 from the start site of the FasL promoter (lanes 8 through 14). The DNA-binding complexes were separated by 4.5% acrylamide gel electrophoresis. The gel was dried and visualized by autoradiography. (B) EMSA showing that DA-MEKK1 induces DNA shift complexes with the MEKK1-responsive site of the FasL promoter. A total of 5 × 106 Jurkat-tTA cells, stably transfected with DA-MEKK1, were grown in the presence (+) or absence (−) of tetracycline for 24 h. The cells were either left untreated or stimulated with 100 nM PMA plus 1 μg of ionomycin (Iono)/ml for 3 h. Nuclear extracts (10 μg) were incubated with 2 ng of 32P-labeled AP-1 oligonucleotide (lanes 1 through 5) or the oligonucleotide corresponding to the JNK response element (lanes 6 through 10). The DNA-binding complexes were analyzed as described above. (C) EMSA showing the effects of anti-c-Jun and anti-ATF2 on DA-MEKK1-induced shift complexes. EMSA was conducted by using the MEKK1-responsive oligonucleotide together with nuclear extracts from DA-MEKK1-expressing Jurkat cells as described for panel B. Anti-c-Jun, anti-ATF2, and anti-Fos antibodies were incubated together with the nuclear extracts as described above for panel A.
Based on the role of the JNK cascade in regulating the expression and transcriptional activation of AP-1 proteins (57, 58), we asked whether the −336-to-−318 response element in the FasL promoter associates with members of the AP-1 protein family. Nuclear extracts from cells treated with PMA plus ionomycin were preincubated with antibodies to c-Jun, ATF2, or pan-Fos and were analyzed by EMSA (Fig. 5A). While treatment with anti-c-Jun and anti-ATF2 interfered with the binding of both complexes to the FasL oligonucleotide (Fig. 5A, lanes 11 and 13), anti-pan-Fos had no effect on the protein-DNA interaction (Fig. 5A, lane 12). This indicates the presence of c-Jun and ATF2 but not Fos proteins in the FasL oligonucleotide complexes. In contrast, supershift analysis using the consensus AP-1 probe revealed the presence of both c-Jun and Fos family proteins in the complex (Fig. 5A, lanes 3 to 5). This suggests that although both complexes contain AP-1 proteins, there are differences in the makeup of these transcription factors.
To determine whether MEKK1 induces similar shift complexes, nuclear extracts were prepared from DA-MEKK1 expressing Jurkat cells and incubated together with the −336-to-−318 probe (Fig. 5B, lanes 6 to 10). DA-MEKK1 expression induced two gel shift complexes, both of which specifically associate with the FasL probe (Fig. 5B, lane 8) and are competed with a 50-fold excess of unlabeled probe (Fig. 5B, lane 10). Compared to tet+ cells, there was increased abundance of both complexes in tet− cells (Fig. 5B, lanes 6 and 8). Similarly, UVR and γ-irradiation induced two shift complexes with the −336-to-−318 probe, indicating that relevant stress stimuli target the same response element (data not shown).
In order to determine which AP-1 proteins are induced to bind to the −336-to-−318 probe by DA-MEKK1, we used the same antibodies shown in Fig. 5A to conduct supershift analysis using nuclear extracts from DA-MEKK1-expressing cells. The data showed decreased complex formation in the presence of anti-c-Jun and anti-ATF2 antibodies but did not show an effect for anti-pan-Fos antiserum (Fig. 5C). This is in agreement with the findings in Fig. 5A.
The MEKK1 response element in the FasL promoter functions independently to drive transcription in JNK-activated cells.
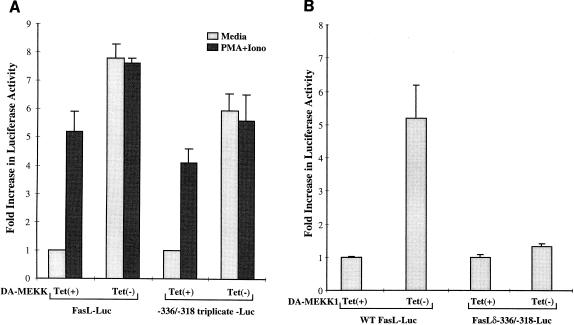
We next addressed whether the −336-to-−318 binding site in the FasL promoter can act independently as a MEKK1 response element. We inserted a triplicate repeat of the −336-to-−318 binding site upstream of a luciferase reporter. This construct was transiently transfected into DA-MEKK1 cells, and luciferase activity was measured in DA-MEKK1-expressing versus non-DA-MEKK1-expressing cells (Fig. 6A). In control nonexpressing tet+ cells, treatment with PMA plus ionomycin, induced a 4.6-fold increase in the activity of the triplicated MEKK1 response element reporter (Fig. 6A), similar to results seen with the 486-bp promoter. Further, the expression of DA-MEKK1 results in a 6.6-fold induction of the activity of the triplicated reporter luciferase, again similar to results seen with the full-length FasL reporter (Fig. 6A). UVR and γ-irradiation also induced activation of the triplicated MEKK1 response element, and this response could be inhibited by DN-MEKK1 (Table 1). These results indicate that, analogous to the intact FasL promoter, the triplicated individual response element at −336 to −318 is regulated through a pathway involving MEKK1.
FIG. 6.
The response element between positions −336 and −318 is critical for MEKK1-mediated FasL promoter activity. (A) Luciferase assay showing the effect of the DA-MEKK1 on the transcriptional activation of the MEKK1-responsive element. DA-MEKK1 cells were transiently transfected with 30 μg of luciferase constructs representing the 486-bp FasL promoter or the triplicated −336-to-−318 site. The cells were grown in the presence or absence of 0.1 μg of tetracycline/ml for 24 h. Cells were either left unstimulated or treated with 100 nM PMA plus 1 μg of ionomycin (Iono)/ml for 8 h. The cells were lysed and analyzed for luciferase activity as for Fig. 3. The fold increase in luciferase activity was calculated based on the value for tet+ cells. Transfection efficiency was monitored by cotransfection of a β-galactosidase-encoding plasmid (CMV-β-Gal). These data are representative of three experiments. (B) Luciferase assay showing the regulation of the FasL promoter by the MEKK1 response element. DA-MEKK1 cells were transiently transfected with 30 μg of the wild-type FasL-486 reporter or the FasLδ−336/−318 construct carrying a mutant MEKK1-responsive element. The cells were grown in the presence or absence of 0.1 μg of tetracycline/ml for 24 h, lysed, and analyzed for luciferase activity. The fold increase in luciferase activity was calculated based on the value for tet+ cells.
TABLE 1.
Luciferase reporter assay showing activation of the triplicated MEKK1 response element by UVR and γ-irradiationa
| Treatment of cells | Fold increase in reporter activity
|
|
|---|---|---|
| Without DN-MEKK1 | With DN-MEKK1 | |
| Unstimulated | 1.0 | 0.66 |
| UVR | 5.3 | 1.12 |
| γ-Irradiation | 4.9 | 1.47 |
Jurkat-tTA cells were transiently transfected with 30 μg of the triplicated −336/−318 Luc vector and 20 μg of DN-MEKK1, subcloned into the pUHD10.3 vector (15). Cells were grown in the absence or presence of tetracycline for 24 h and then were stimulated with 3,300 rads of γ-irradiation or 200 J of UVR/m2 for 8 h in the presence of 30 μM Z-VAD (14). Luciferase activity was determined as described for Fig. 6. The fold increase was calculated based on the value of unstimulated tet+ cells. Data are averages of duplicate measurements in which the standard error of the mean variation was <10%.
An important question is whether the above MEKK1-inducible element plays a critical role in the activation of the 486-bp FasL promoter. We mutagenized that site in the full-length promoter sequence and transfected that mutant reporter gene into Jurkat DA-MEKK1 cells (Fig. 6B). Our data show that while DA-MEKK1 induced a 5.2-fold increase in the transcriptional activation of the wild-type FasL promoter, only a 1.3-fold increase could be achieved in cells transfected with the mutant promoter (Fig. 6B). Similarly, where stress stimuli such as UVR and γ-irradiation were used, the respective 9.8- and 9.2-fold increases in FasL promoter activity were abrogated by mutation of the MEKK1-responsive element (Table 2). Taken together, our results suggest that the MEKK1-inducible site located between −336 and −318 plays a critical role in stress-induced transcriptional activation of the FasL promoter.
TABLE 2.
Luciferase reporter assay showing lack of activation of the mutant FasL promoter by UVR and γ-irradiationa
| Treatment of cells | Fold increase in reporter activity in:
|
|
|---|---|---|
| Wild-type FasL-Luc | FasLδ−336/−318 Luc | |
| Unstimulated | 1.0 | 1.0 |
| UVR | 9.87 | 1.23 |
| γ-Irradiation | 9.46 | 1.30 |
Jurkat-tTA cells were transiently transfected with 30 μg of FasL −336/−318 luciferase construct as described for Fig. 7. After resting for 24 h, cells were stimulated with γ-irradiation and UVR in the presence of Z-VAD as described for Table 1. Luciferase activity and fold increase were determined as for Fig. 6. Data are averages of duplicate measurements in which the standard error of the mean varied <10%.
Distinct response elements mediate the activation of the FasL promoter by the TCR and stress stimuli.
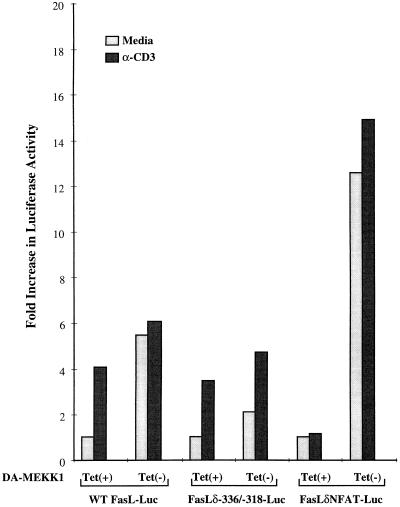
We have previously shown that ligation of the TCR induces the transcriptional activation of the FasL promoter by inducing the activation of the distal NF-AT site in that promoter (28). Mutation of the distal NF-AT site prevents the activation of the FasL promoter by anti-CD3 (Fig. 7), confirming our previous findings (28). Transient transfection of the FasL promoter construct lacking the distal NF-AT site (FasLδNFAT) into DA-MEKK1 cells showed that, in contrast to TCR-mediated activation, mutation of the distal NF-AT site did not interfere in the transcriptional activation of the FasL promoter by DA-MEKK1 (Fig. 7). In fact, the response of the FasLδNFAT-Luc reporter was increased compared to that of the wild-type promoter. Whether this reflects involvement of a repressor or a positional effect changing transcription factor interactions with the polymerase II initiation complex is unknown at present.
FIG. 7.
Distinct response elements mediate the activation of the FasL promoter by the TCR and DA-MEKK1. DA-MEKK1 cells, transiently transfected with 30 μg of FasL−486, FasLδ−336/−318, or FasLδNFAT luciferase constructs, were grown in the presence or absence of 0.1 μg of tetracycline/ml for 24 h. The cells were either left unstimulated or treated with 10 μg of anti-CD3 MAb/ml for 8 h. The cells were lysed and analyzed for luciferase activity. The fold increase in luciferase activity was calculated based on the value for tet+ cells. Transfection efficiency was monitored by cotransfection of a β-galactosidase-encoding plasmid. Similar results were obtained in two experiments.
In the reverse experiment, we investigated the effect of TCR ligation on the FasL promoter construct containing a mutant MEKK1 site (FasLδ−336/−318). As shown in Fig. 6, mutation of the −338-to-−318 site abrogated the responsiveness of the FasL promoter to DA-MEKK1 (Fig. 7). In contrast, anti-CD3 stimulation resulted in similar responses in wild-type FasL and mutant FasL promoters (Fig. 7). These results indicate that TCR ligation and the JNK cascade induce the expression of FasL by utilizing distinct response elements in the FasL promoter.
DN mutants of c-Jun and ATF2 interfere in the JNK-mediated activation of the FasL promoter.
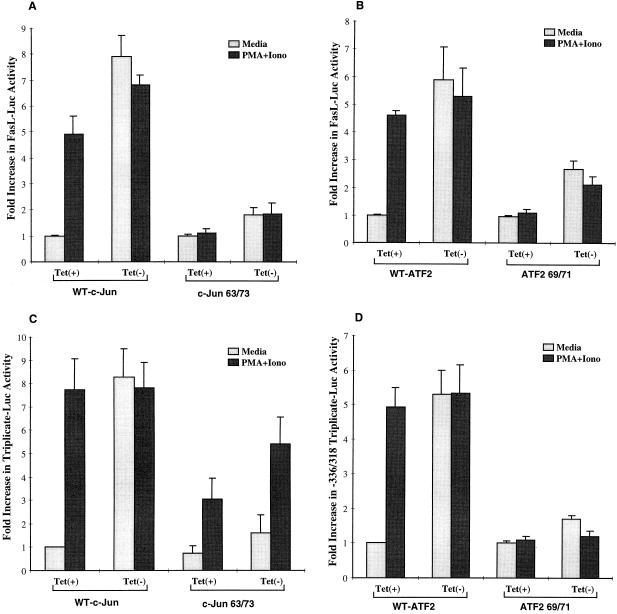
Data shown in Fig. 5A suggest that the MEKK1-inducible site associates with c-Jun and ATF2 proteins. To determine whether these transcription factors play an essential role in the transcriptional activation of the FasL promoter, we used mutant c-Jun (Jun 63/73) and mutant ATF2 (ATF2 69/71) (20, 41) constructs to determine their effects on the FasL promoter. While in the presence of wild-type c-Jun, DA-MEKK1 induced an eightfold increase in FasL promoter activity, mutant c-Jun (Fig. 8A) and mutant ATF2 (Fig. 8B) abrogated reporter activity by 75% (Fig. 8A) and 54% (Fig. 8B), respectively. Similarly, the mutant constructs reduced responsiveness to PMA plus ionomycin by 75% (Fig. 8A) and 50% (Fig. 8B), respectively, in tet+ and tet− cells. Moreover, mutant c-Jun (Jun 63/73) and mutant ATF2 (ATF2 69/71) exerted inhibitory effects on the individual response element used as a triplicate repeat of the JNK-inducible site in a luciferase vector (Fig. 8C and D). The proportionally smaller effect of DN c-Jun on the individual response element (Fig. 8C) compared to the wild-type promoter (Fig. 8A) may be due to differences in the level of mutant c-Jun expression in these experiments. Taken together, these data show that c-Jun and ATF2 play a critical role in the transcriptional activation of the FasL promoter and the MEKK-inducible site in that promoter. Since in the above experiments we used c-Jun and ATF2 mutants which lack consensus serine residues required for transcriptional activation by JNK (20, 41), our data suggest the involvement of JNK in the events downstream of MEKK1. Further confirmation of that notion was sought by looking at the effect of a dominant interfering mutant of the JNK kinase, SEK1, on transcriptional activation of the wild-type FasL-Luc reporter by UVR and PMA plus ionomycin. Our data demonstrate 6.5- and 5.2-fold stimulation of FasL-Luc by UVR and PMA plus ionomycin, respectively; in the presence of DN-SEK1, these responses were limited to 2.6- and 2.0-fold increases, respectively. This supports a role for JNK in the activation of the FasL promoter.
FIG. 8.
Involvement of c-Jun and ATF2 in the MEKK1-mediated activation of the FasL promoter. (A and C) Luciferase assay showing the effect of mutant c-Jun on the transcriptional activation of the FasL promoter (A) and the triplicated MEKK1-responsive element (C). DA-MEKK1 cells were transiently transfected with 30 μg of FasL−486 or triplicated JNK response element reporter constructs in the presence of 20 μg of either wild-type (WT) or mutant (63/73) c-Jun. The experiment was performed as described for Fig. 3. The intersample variation in transfection efficiency was adjusted by using CMV-β-Gal cotransfection. (B and D) Luciferase assay showing the effect of mutant ATF2 on the transcriptional activation of the FasL promoter (B) and the triplicated MEKK1-responsive element (D). DA-MEKK1 cells were transiently transfected with 30 μg of FasL−486 or triplicated JNK response element reporter constructs in the presence of 20 μg of either wild-type (WT) or mutant (69/71) ATF2. The experiment was performed as described above.
DISCUSSION
In this paper, we show that induction of FasL gene expression by environmental stress stimuli is dependent on MEKK1 activation. Using inducible DA-MEKK1 expression in Jurkat cells, we demonstrate the presence of a specific MEKK1- regulated element at positions −338 to −316 of the FasL promoter. Mutation of that response element abrogated MEKK1-mediated FasL promoter activation and interfered in stress-induced induction of this promoter. The effect of MEKK1 on the FasL promoter is mediated by c-Jun and ATF2, which associate with and transcriptionally activate the MEKK1 response element. Transfection of interfering c-Jun and ATF2 mutants, which lack the consensus JNK phosphorylation sites, abrogated the transcriptional activation of the FasL promoter in parallel with the triplicated JNK response element. Together, these data indicate that MEKK1 and downstream targets of the JNK cascade play a role in committing lymphocytes to apoptosis by inducing FasL expression via a novel response element in the promoter of that gene.
Previous studies have linked JNK activation with apoptosis. While the role of the JNK cascade in apoptosis has been controversial, our studies suggest that the role of MEKK1 is the commitment of T cells to apoptosis rather than the actual delivery of the death signal. In favor of this hypothesis are the findings that neither DN-MEKK1 nor DN-SEK1 interferes in Fas-induced apoptosis (18, 55), while DA-MEKK1 is able to induce apoptosis (14, 26, 58). Moreover, DA-MEKK1-mediated apoptosis can be inhibited by interfering with Fas-FasL interactions (Fig. 1). We propose, therefore, that FasL expression represents a mechanism through which MEKK1 commits T cells to apoptosis. The kinetics of MEKK1 activation appear to be important in this process, since prolonged, but not transient, activation leads to induction of apoptosis (7, 8, 14). The kinetics of MEKK1 activation may set a threshold which influences the decision of T cells to die when they are irreversibly damaged by environmental stress. Instead of directly inducing apoptosis, the JNK cascade regulates the expression of FasL, which subsequently delivers the death signal through Fas (Fig. 3). The JNK cascade may therefore function as a fail-safe mechanism that induces cell death when ligands for death effector domain receptors are not readily available. Another ligand which may play a role in MEKK1-induced apoptosis is the cytokine which binds to TNFR1, namely, TNF-α. In this regard, DA-MEKK1 has been demonstrated to induce TNF-α secretion in mast cells in parallel with transcriptional activation of the TNF-α promoter (24). TNF-α secretion may serve as an alternative or complementary mechanism which contributes to stress-induced apoptosis (11). The use of one or both mechanisms may differ from tissue to tissue or stimulus to stimulus. For instance, UVR has been shown to induce FasL expression in T cells and keratinocytes while inducing TNF-α in keratinocytes but not in peripheral-blood T lymphocytes (30, 51). It is important to mention that UVR- and γ-irradiation-induced apoptosis may involve mechanisms other than FasL expression, since Fas-Fc protein, which interferes in Fas-FasL binding, only partially inhibits induction of cell death by these stress stimuli (14). Possible mechanisms include expression of additional apoptotic receptors or their ligands, e.g., TNF-α, as well as activation of further signaling pathways, e.g., p38 mitogen-activated protein kinase (MAPK) and NF-κB kinases. In this regard, putative AP-1 and NF-κB response elements have been defined in the FasL promoter >900 bp upstream of the start site (27a). The latter study also confirmed the involvement of MEKK1 in a stress-induced apoptosis in Jurkat cells with a tetracycline-regulated vector system (27a). Our data differ from the findings of this study, however, in that we could not show significant JNK activation in Jurkat cells by the genotoxic agents, etoposide and tenoposide, used in that study (27a). We do not understand the reason for these differences. It is also important to consider the dose of UVR and γ-irradiation in FasL-mediated apoptosis, because a decrease in the dose of γ-irradiation from 3,300 to 1,500 rads induced apoptosis which could be blocked >40% by Fas-Fc protein (14). Moreover, the dose of Fas-Fc protein may be important in demonstrating the role of FasL, since a higher dose of Fas-Fc protein has been demonstrated to inhibit UVR-induced apoptosis in Jurkat cells by >50% (27a). While we clearly need to learn more about UVR- and γ-irradiation-induced apoptosis pathways, our published data demonstrate that these stress stimuli do induce FasL expression, which contributes to apoptosis (14). As a cautionary note, we also want to emphasize that our study does not rule out a role for the JNK cascade in the execution of apoptosis in nonlymphoid cells. Indeed, recent studies have shown that the Fas binding protein Daxx plays a role in apoptosis in fibroblasts through activation of the JNK cascade (59). The mechanism of action of JNK in the apoptotic machinery of these cells needs to be elucidated.
Our data suggest that the mechanism by which MEKK1 regulates the FasL promoter is through the transcriptional activation of c-Jun and ATF2, which interact with the −336-to-−318 element (Fig. 5). This is in keeping with the well-characterized effect of JNK on the transcriptional activation and expression of AP-1 proteins (34, 47, 56, 57). The transcriptional activation of c-Jun is dependent on JNK-induced phosphorylation of serine residues, S63 and S73, in its N-terminal domain (12, 47). Moreover, expression of c-Jun is dependent on transcriptional activation of a modified AP-1 site in the c-Jun promoter known as the cyclic AMP response element binding protein (CREB)/ATF2 site, which binds to a c-Jun–ATF2 heterodimer (34, 47). It is interesting, therefore, that ATF2 transcriptional activation is also dependent on JNK phosphorylation (20). Further evidence for the involvement of JNK in the events downstream of MEKK1 was provided by the finding that DN-SEK1 (JNK kinase) could reduce the activation of the wild-type FasL promoter by 60 and 62%, respectively, upon stimulation with UVR and PMA plus ionomycin. Our finding that the MEKK1 response element in the FasL promoter interacts with c-Jun and ATF2 (Fig. 5) prompted us to compare the −336-to-−318 sequence of the FasL promoter to AP-1 and AP-1-like sequences which are known to play a role in the transcriptional activation of genes in the immune system (Table 3). As demonstrated in Table 3, our sequence did not match any of the AP-1, AP-1-like, or CREB/ATF2 sequences published to date (10, 16, 32, 35, 36, 53). However, this sequence did show short homologous sequences (ACA or GGTA) which appear in some modified AP-1 sites. Neither a BLAST homology search nor use of the TESS database revealed any sequences in the mammalian genome which exactly match the FasL promoter response element. We suggest, therefore, that our sequence represents a novel CREB/AP-1 binding site. This notion is further supported by the fact that mutant c-Jun and ATF2, lacking JNK phosphorylation sites, prevent the transcriptional activation of the FasL promoter and the triplicated JNK response element in that promoter (Fig. 8).
TABLE 3.
Diversity in the sequences of known AP-1 and CREB/ATF2 sites in cytokine promoters
| Promoter and cytokinea | Site | Sequence | Reference(s) |
|---|---|---|---|
| FasL | −336 | TTGGGTAGCACAGCGA | |
| Consensus AP-1 | TGAGTCA | ||
| IL-2 | −280 | TGTTTCA | 16, 32 |
| −135 | GAAGGTA | 16, 32 | |
| IL-4 | −84 | TCGTTACA | 44 |
| IFN-γ | −96 | TGTCACCA | 10 |
| −66 | TACGTAA | 10 | |
| RANTES | −342 | AGACTCA | 35 |
| −334 | TGACTTC | 35 | |
| Consensus CREB/ATF2 | TGACGTCA | ||
| IL-8 | −81 | TGACATAA | 39 |
| TNF-α | −106 | TGAGCTCA | 53 |
IFN-γ, gamma interferon.
In light of the multitude of stimuli which induce FasL expression, it is likely that, in addition to the MEKK1 response element located at −336 to −318, other response elements in the FasL promoter may play a role in the regulation of that gene. For instance, ligation of the TCR induces the apoptosis of T lymphocytes by activation-induced cell death, a process dependent on FasL expression (52, 54). We have shown that this response is mediated through an NF-AT site at position −276 of the FasL promoter (28). As expected from an NF-AT-dependent response, transcriptional activation of the FasL promoter by the TCR occurred via a Ca2+-dependent pathway inhibitable by cyclosporin A (28). We have previously shown that mutation of this NF-AT response element decreases the responsiveness of the FasL promoter to TCR ligation (28). Although the distal NF-AT site is critical for FasL expression following TCR ligation, mutation of that site in the FasL promoter had no inhibitory effect on the transcriptional activation of the mutant promoter by DA-MEKK1 (data not shown). This suggests that TCR engagement and environmental stress utilize different mechanisms for the induction of the FasL promoter. Stress stimuli function by inducing the prolonged activation of the JNK cascade (7, 8), resulting in the transcriptional activation of c-Jun and ATF2 (56, 57). In contrast, ligation of the TCR without CD28 costimulation fails to induce JNK activation (15, 49). Therefore, while TCR functions by inducing a Ca2+-dependent pathway resulting in NF-AT activation, stress stimuli induce FasL expression by way of the JNK cascade. Although TCR and stress stimuli induce FasL gene expression by two different signaling pathways, these processes are not mutually exclusive. The JNK cascade may synergize with TCR-mediated events in order to remove damaged or excess cells. This may be especially true in cases of inflammatory diseases.
Taken together, our data support a model in which prolonged MEKK1 activation by stress stimuli commit the cells to undergo apoptosis by inducing the expression of FasL on the cell surface. This method of cell death seems to play an essential role in the immune system, as demonstrated by the inability of MRL-lpr/lpr mice to eliminate excess lymphocytes in response to irradiation (9, 43). Although the role of the JNK cascade in the induction of apoptosis is not limited to lymphoid cells, the extent of cell death mediated by the JNK cascade may be cell type specific. While T lymphocytes respond to prolonged JNK activation by expressing FasL and undergoing apoptosis, other cell types, such as small-cell lung cancer cells and prostate cells, may require alternative means of inducing cell death (3).
ACKNOWLEDGMENTS
This work was supported by United States Public Health Service grants AG14763, AG14992, and AI52735. A.N. is supported by the Southern California Chapter of the Arthritis Foundation. G.A.K. is also supported by the Arthritis Foundation. K.M.L. is supported by the University of Iowa Medical Scientist Training Program, NIH grant T326M07337.
M. Faris and K. M. Latinis contributed equally to this study.
REFERENCES
- 1.Boldin M P, Goncharov T M, Goltsev Y Y, Wallach D. Involvement of MACH, a novel MORT1/FADD-interacting protease, in Fas/APO-1- and TNF receptor-induced cell death. Cell. 1996;85:803–815. doi: 10.1016/s0092-8674(00)81265-9. [DOI] [PubMed] [Google Scholar]
- 2.Brenner C, Hirsch T, Petit P X, Geuskens M, Kroemer G. A cytofluorometric assay of nuclear apoptosis induced in a cell-free system: application to ceramide-induced apoptosis. Exp Cell Res. 1997;236:397–403. doi: 10.1006/excr.1997.3733. [DOI] [PubMed] [Google Scholar]
- 3.Butterfield L, Storey B, Maas L, Heasley L E. c-Jun NH2-terminal kinase regulation of the apoptotic response of small cell lung cancer cells to ultraviolet radiation. J Biol Chem. 1997;272:10110–10116. doi: 10.1074/jbc.272.15.10110. [DOI] [PubMed] [Google Scholar]
- 4.Cahill M A, Peter M E, Kischkel F C, Chinnaiyan A M, Dixit V M, Krammer P H, Nordheim A. CD95 (APO-1/Fas) induces activation of SAP kinases downstream of ICE-like proteases. Oncogene. 1996;13:2087–2096. [PubMed] [Google Scholar]
- 5.Calnan B J, Szychowski S, Chan F K, Cado D, Winoto A. A role for the orphan steroid receptor Nur77 in apoptosis accompanying antigen-induced negative selection. Immunity. 1995;3:273–282. doi: 10.1016/1074-7613(95)90113-2. [DOI] [PubMed] [Google Scholar]
- 6.Castro J E, Listman J A, Jacobson B A, Wang Y, Lopez P A, Ju S, Finn P W, Perkins D L. Fas modulation of apoptosis during negative selection of thymocytes. Immunity. 1996;5:617–627. doi: 10.1016/s1074-7613(00)80275-7. [DOI] [PubMed] [Google Scholar]
- 7.Chen Y R, Meyer C F, Tan T-H. Persistent activation of c-Jun N-terminal kinase 1 (JNK1) in gamma radiation-induced apoptosis. J Biol Chem. 1996;271:631. doi: 10.1074/jbc.271.2.631. [DOI] [PubMed] [Google Scholar]
- 8.Chen Y R, Wang X, Templeton D, Davis R J, Tan T-H. The role of c-Jun N-terminal kinase (JNK) in apoptosis induced by ultraviolet C and gamma radiation. Duration of JNK activation may determine cell death and proliferation. J Biol Chem. 1996;271:31929–31936. doi: 10.1074/jbc.271.50.31929. [DOI] [PubMed] [Google Scholar]
- 9.Cheng J, Liu C, Yang P, Zhou T, Mountz J D. Increased lymphocyte apoptosis in Fas ligand transgenic mice. J Immunol. 1997;15:674–684. [PubMed] [Google Scholar]
- 10.Cippitelli M, Sica A, Viggiano V, Ye J, Ghosh P, Birrer M J, Young H A. Negative transcriptional regulation of the interferon-gamma promoter by glucocorticoids and dominant negative mutants of c-Jun. J Biol Chem. 1995;270:12548–12556. doi: 10.1074/jbc.270.21.12548. [DOI] [PubMed] [Google Scholar]
- 11.de Kossodo S, Cruz P D, Jr, Dougherty I, Thompson P, Silva-Valdez M, Beutler B. Expression of the tumor necrosis factor gene by dermal fibroblasts in response to ultraviolet irradiation or lipopolysaccharide. J Investig Dermatol. 1995;104:318–322. doi: 10.1111/1523-1747.ep12665361. [DOI] [PubMed] [Google Scholar]
- 12.Derijard B, Hibi M, Wu I H, Barrett T, Su B, Deng T, Karin M, Davis R J. JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell. 1994;76:1025–1037. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- 13.Enari M, Talanian R V, Wong W W, Nagata S. Sequential activation of ICE-like and CPP32-like proteases during Fas-mediated apoptosis. Nature. 1996;380:723–726. doi: 10.1038/380723a0. [DOI] [PubMed] [Google Scholar]
- 14.Faris M, Kokot N, Latinis K, Kasibhatla S, Green D R, Koretzky G A, Nel A. The c-Jun N-terminal kinase cascade plays a role in stress-induced apoptosis in Jurkat cells by up-regulating Fas ligand expression. J Immunol. 1998;160:134–144. [PubMed] [Google Scholar]
- 15.Faris M, Kokot N, Lee L, Nel A E. Regulation of interleukin-2 transcription by inducible stable expression of dominant negative and dominant active mitogen-activated protein kinase kinase kinase in Jurkat T cells. Evidence for the importance of Ras in a pathway that is controlled by dual receptor stimulation. J Biol Chem. 1996;271:27366–27373. doi: 10.1074/jbc.271.44.27366. [DOI] [PubMed] [Google Scholar]
- 16.Fraser J D, Irving B A, Crabtree G R, Weiss A. Regulation of interleukin-2 gene enhancer activity by the T-cell accessory molecule CD28. Science. 1991;251:313–316. doi: 10.1126/science.1846244. [DOI] [PubMed] [Google Scholar]
- 17.Gajewski T F, Thompson C B. Apoptosis meets signal transduction: elimination of a BAD influence. Cell. 1996;87:589–592. doi: 10.1016/s0092-8674(00)81377-x. . (Comment.) [DOI] [PubMed] [Google Scholar]
- 18.Goillot E, Raingeaud J, Ranger A, Tepper R I, Davis R J, Harlow E, Sanchez I. Mitogen-activated protein kinase-mediated Fas apoptotic signaling pathway. Proc Natl Acad Sci USA. 1997;94:3302–3307. doi: 10.1073/pnas.94.7.3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gossen M, Freundlieb S, Bender G, Muler G, Hillen W, Bujard H. Transcriptional activation by tetracyclines in mammalian cells. Science. 1995;68:1766–1769. doi: 10.1126/science.7792603. [DOI] [PubMed] [Google Scholar]
- 20.Gupta S, Campbell D, Derijard B, Davis R J. Transcription factor ATF2 regulation by the JNK signal transduction pathway. Science. 1995;267:389–393. doi: 10.1126/science.7824938. [DOI] [PubMed] [Google Scholar]
- 21.Haimovitz-Friedman A, Kan C C, Ehleiter D, Persaud R S, McLoughlin M, Fuks Z, Kolesnick R N. Ionizing radiation acts on cellular membranes to generate ceramide and initiate apoptosis. J Exp Med. 1994;180:525–535. doi: 10.1084/jem.180.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hsu H, Huang J, Shu H B, Baichwal V, Goeddel D V. TNF-dependent recruitment of the protein kinase RIP to the TNF receptor-1 signaling complex. Immunity. 1996;4:387–396. doi: 10.1016/s1074-7613(00)80252-6. [DOI] [PubMed] [Google Scholar]
- 23.Hsu H, Shu H B, Pan M G, Goeddel D V. TRADD-TRAF2 and TRADD-FADD interactions define two distinct TNF receptor 1 signal transduction pathways. Cell. 1996;84:299–308. doi: 10.1016/s0092-8674(00)80984-8. [DOI] [PubMed] [Google Scholar]
- 24.Ishizuka T, Terada N, Gerwins P, Hamelmann E, Oshiba A, Fanger G R, Johnson G L, Gelfand E W. Mast cell tumor necrosis factor alpha production is regulated by MEK kinases. Proc Natl Acad Sci USA. 1997;94:6358–6363. doi: 10.1073/pnas.94.12.6358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jamieson C, McCaffrey P G, Rao A, Sen R. Physiologic activation of T-cells via the T-cell receptors induces NF-kappa-B. J Immunol. 1991;147:416–420. [PubMed] [Google Scholar]
- 26.Johnson N L, Gardner A M, Diener K M, Lange-Carter C A, Gleavy J, Jarpe M B, Minden A, Karin M, Zon L I, Johnson G L. Signal transduction pathways regulated by mitogen-activated/extracellular response kinase kinase kinase induce cell death. J Biol Chem. 1996;271:3229–3237. doi: 10.1074/jbc.271.6.3229. [DOI] [PubMed] [Google Scholar]
- 27.Ju S T, Panka D J, Cui H, Ettinger R, el-Khatib M, Sherr D H, Stanger B Z, Marshak-Rothstein A. Fas(CD95)/FasL interactions required for programmed cell death after T-cell activation. Nature. 1995;373:444–448. doi: 10.1038/373444a0. [DOI] [PubMed] [Google Scholar]
- 27a.Kasibhatla S, Brunner T, Genestier L, Echeverria F, Mahboubi A, Green D R. DNA damaging agents induce expression of Fas ligand and subsequent apoptosis in T lymphocytes via the activation of NF-κB and AP-1. Mol Cell. 1998;1:543–551. doi: 10.1016/s1097-2765(00)80054-4. [DOI] [PubMed] [Google Scholar]
- 28.Latinis K M, Carr L L, Peterson E J, Norian L A, Eliason S L, Koretzky G A. Regulation of CD95 (Fas) ligand expression by TCR-mediated signaling events. J Immunol. 1997;158:4602–4611. [PubMed] [Google Scholar]
- 29.Lenczowski J M, Dominguez L, Eder A M, King L B, Zacharchuk C M, Ashwell J D. Lack of a role for Jun kinase and AP-1 in Fas-induced apoptosis. Mol Cell Biol. 1997;17:170–181. doi: 10.1128/mcb.17.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leverkus M, Yaar M, Gilchrest B A. Fas/Fas ligand interaction contributes to UV-induced apoptosis in human keratinocytes. Exp Cell Res. 1997;232:255–262. doi: 10.1006/excr.1997.3514. [DOI] [PubMed] [Google Scholar]
- 31.Liu Z G, Hsu H, Goeddel D V, Karin M. Dissection of TNF receptor 1 effector functions: JNK activation is not linked to apoptosis while NF-kappa B activation prevents cell death. Cell. 1996;87:565–5676. doi: 10.1016/s0092-8674(00)81375-6. [DOI] [PubMed] [Google Scholar]
- 32.Mattila P S, Ullman K S, Fierling S, Emmel E A, et al. The actions of cyclosporin-A and FK506 suggest a novel step in the activation of T-lymphocytes. EMBO. 1990;9:4425–4433. doi: 10.1002/j.1460-2075.1990.tb07893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Minden A, Lin A, McMahon M, Lange-Carter C, Derijard B, Davis R J, Johnson G L, Karin M. Differential activation of ERK and JNK mitogen-activated protein kinases by Raf-1 and MEKK. Science. 1994;266:1719–1723. doi: 10.1126/science.7992057. [DOI] [PubMed] [Google Scholar]
- 34.Minden A, Lin A, Smeal T, Derijard B, Cobb M, Davis R, Karin M. c-Jun N-terminal phosphorylation correlates with activation of the JNK subgroup but not the ERK subgroup of mitogen-activated protein kinases. Mol Cell Biol. 1994;14:6683–6688. doi: 10.1128/mcb.14.10.6683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moriuchi H, Moriuchi M, Fauci A S. Nuclear factor-kappa B potently up-regulates the promoter activity of RANTES, a chemokine that blocks HIV infection. J Immunol. 1997;158:3483–3491. [PubMed] [Google Scholar]
- 36.Muzio M, Chinnaiyan A M, Kischkel F C, O’Rourke K, Shevchenko A, Ni J, Scaffidi C, Bretz J D, Zhang M, Gentz R, Mann M, Krammer P, Peter M E, Dixit V M. FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death-inducing signaling complex. Cell. 1996;85:817–827. doi: 10.1016/s0092-8674(00)81266-0. [DOI] [PubMed] [Google Scholar]
- 37.Nagata S. Apoptosis by death factor. Cell. 1997;88:355–365. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- 38.Nishina H, Fischer K D, Radvanyi L, Shahinian A, Hakem R, Rubie E A, Bernstein A, Mak T W, Woodgett J R, Penninger J M. Stress-signalling kinase Sek1 protects thymocytes from apoptosis mediated by CD95 and CD3. Nature. 1997;385:350–353. doi: 10.1038/385350a0. [DOI] [PubMed] [Google Scholar]
- 39.Okamoto S I, Mukaida N, Yasumoto K, Rice N, Ishikawa Y, Horiguchi H, Murakami S, Matsushima K. The interleukin-8 AP-1 and κB-like sites are genetic end targets of FK506-sensitive pathway accompanied by calcium mobilization. J Biol Chem. 1994;269:8582–8589. [PubMed] [Google Scholar]
- 40.Pandey S, Wang E. Cells en route to apoptosis are characterized by the upregulation of c-fos, c-myc, c-jun, cdc2, and RB phosphorylation, resembling events of early cell-cycle traverse. J Cell Biochem. 1995;58:135–150. doi: 10.1002/jcb.240580203. [DOI] [PubMed] [Google Scholar]
- 41.Petrak D, Memon S A, Birrer M J, Ashwell J D, Zacharchuk C M. Dominant negative mutant of c-Jun inhibits NF-AT transcriptional activity and prevents IL-2 gene transcription. J Immunol. 1994;153:2046–2051. [PubMed] [Google Scholar]
- 42.Philpott N J, Turner A J C, Scopes J, Westby M, Marsh J C W, Gordon-Smith E C, Dalgleish A G, Gibson F M. The use of 7-amino actinomycin D in identifying apoptosis: simplicity of use and broad spectrum application compared with other techniques. Blood. 1996;87:2244–2251. [PubMed] [Google Scholar]
- 43.Reap E A, Roof K, Maynor K, Borrero M, Booker J, Cohen P L. Radiation and stress-induced apoptosis: a role for Fas/Fas ligand interactions. Proc Natl Acad Sci USA. 1997;94:5750–5755. doi: 10.1073/pnas.94.11.5750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rooney J W, Hoey T, Glimcher L H. Coordinate and cooperative roles for NF-AT and AP-1 in the regulation of the murine IL-4 gene. Immunity. 1995;2:473–483. doi: 10.1016/1074-7613(95)90028-4. [DOI] [PubMed] [Google Scholar]
- 45.Russell H. Activation-induced death of mature T cells in the regulation of immune responses. Curr Opin Immunol. 1995;7:382–388. doi: 10.1016/0952-7915(95)80114-6. [DOI] [PubMed] [Google Scholar]
- 46.Salvesen G S, Dixit V M. Caspases: intracellular signaling by proteolysis. Cell. 1997;91:443–446. doi: 10.1016/s0092-8674(00)80430-4. [DOI] [PubMed] [Google Scholar]
- 47.Sanchez I, Hughes R T, Mayer B J, Yee K, Woodgett J R, Avruch J, Kyriakis J M, Zon L I. Role of SAPK/ERK kinase-1 in the stress-activated pathway regulating transcription factor c-Jun. Nature. 1994;372:794–798. doi: 10.1038/372794a0. [DOI] [PubMed] [Google Scholar]
- 48.Srinivasula S M, Ahmad M, Ottilie S, Bullrich F, Banks S, Wang Y, Fernandes-Alnemri T, Croce C M, Litwack G, Tomaselli K J, Armstrong R C, Alnemri E S. FLAME-1, a novel FADD-like anti-apoptotic molecule that regulates Fas/TNFR1-induced apoptosis. J Biol Chem. 1997;272:18542–18545. doi: 10.1074/jbc.272.30.18542. [DOI] [PubMed] [Google Scholar]
- 49.Su B, Jacinto E, Hibi M, Kallunki T, Karin M, Ben-Neriah Y. JNK is involved in signal integration during costimulation of T lymphocytes. Cell. 1994;77:727–736. doi: 10.1016/0092-8674(94)90056-6. [DOI] [PubMed] [Google Scholar]
- 50.Susin S A, Zamzami N, Larochette N, Dallaporta B, Marzo I, Brenner C, Hirsch T, Petit P X, Geuskens M, Kroemer G. A cytofluorometric assay of nuclear apoptosis induced in a cell-free system: application to ceramide-induced apoptosis. Exp Cell Res. 1997;236:397–403. doi: 10.1006/excr.1997.3733. [DOI] [PubMed] [Google Scholar]
- 51.Teunissen M B, Sylva-Steenland R M, Bos J D. Effect of low-dose ultraviolet-B radiation on the function of human T lymphocytes in vitro. Clin Exp Immunol. 1993;94:208–213. doi: 10.1111/j.1365-2249.1993.tb06002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thompson C B. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 53.Tsai E Y, Jain J, Pesavento P A, Rao A, Goldfeld A E. Tumor necrosis factor alpha gene regulation in activated T cells involves ATF-2/Jun and NFATp. Mol Cell Biol. 1996;16:459–467. doi: 10.1128/mcb.16.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Van Parijs L, Ibraghimov A, Abbas A K. The roles of costimulation and Fas in T cell apoptosis and peripheral tolerance. Immunity. 1996;4:321–328. doi: 10.1016/s1074-7613(00)80440-9. [DOI] [PubMed] [Google Scholar]
- 55.Verheij M, Bose R, Lin X H, Yao B, Jarvis W D, Grant S, Birrer M J, Szabo E, Zon L I, Kyriakis J M, Halmovitz-Friedman A, Fuks Z, Kolesnick R N. Requirement for ceramide-initiated SAPK/JNK signalling in stress-induced apoptosis. Nature. 1996;380:75–79. doi: 10.1038/380075a0. [DOI] [PubMed] [Google Scholar]
- 56.Westwick J K, Cox A D, Der C J, Cobb M H, Hibi M, Karin M, Brenner D A. Oncogenic Ras activates c-Jun via a separate pathway from the activation of extracellular signal-regulated kinases. Proc Natl Acad Sci USA. 1994;91:6030–6034. doi: 10.1073/pnas.91.13.6030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Whitmarsh A J, Davis R J. Transcription factor AP-1 regulation by mitogen-activated protein kinase signal transduction pathways. J Mol Med. 1996;74:589–607. doi: 10.1007/s001090050063. [DOI] [PubMed] [Google Scholar]
- 58.Xia Z, Dickens M, Raingeaud J, Davis R J, Greenberg M. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- 59.Yang X, Khosravi-Far R, Chang H Y, Baltimore D. Daxx, a novel Fas-binding protein that activates JNK and apoptosis. Cell. 1997;89:1067–1076. doi: 10.1016/s0092-8674(00)80294-9. [DOI] [PMC free article] [PubMed] [Google Scholar]