Abstract
Allelopathy is the interaction between donor plants and receiver plants through allelochemicals. According to a great number of publications, allelopathy may be involved in several ecological aspects such as the formation of monospecific stands and sparse understory vegetation for certain plant species. Allelopathy also contributes to the naturalization of invasive plant species in introduced ranges. Autotoxicity is a particular type of allelopathy involving certain compounds. Many medicinal plants have been reported to show relatively high allelopathic activity. We selected plant species that show high allelopathic activity and isolated allelochemicals through the bioassay-guided purification process. More than 100 allelochemicals, including novel compounds have been identified in some medicinal and invasive plants, plants forming monospecific stands, plants with sparse understory vegetation, and plants showing autotoxicity. The allelopathic activity of benzoxazinones and related compounds was also determined.
Keywords: allelopathy, autotoxicity, invasive plant, medicinal plant, monospecific stand, phytotoxicity
Introduction
Allelopathy is the chemical interaction between donor plants and receiver plants, and the compounds involved in the allelopathy are defined as allelochemicals.1–3) The phenomenon of allelopathy can be found in several ecological aspects that have been documented in a great number of publications. Allelochemicals suppress the germination, growth, and regeneration process of the receiver plant species.4–6) Some allelochemicals such as cinnamic acid, benzoic acid, and their derivatives disturb cell membrane permeability, water balance, and stomatal functions, and suppress the enzyme activities involved in the photosynthesis, protein synthesis, respiration, and the metabolism of some secondary metabolites including plant hormones.7–9) The disruption of the metabolism and cell functions by allelochemicals may cause the inhibition of the germination, growth, and regeneration process of the receiver plant species.
Allelochemicals also disturb the mutualism of receiver plants with arbuscular mycorrhizal fungi and/or rhizobia.10–12) Rhizobium nodulation occurs in legumes, and enhances the host plant performance through the supply of ammonium and nitrogen to the host plants.13,14) Colonization of the arbuscular mycorrhizal fungi occurs in the most territorial plants, and enhances the host plant performance through the increasing the absorption of water and nutrients, photosynthesis of the host plants, and their defense functions against pathogen attacks and stress conditions.15–17) Therefore, the disturbance of these mutualism by allelochemicals may reduce the competitive ability of the receiver plant species, resulting in the relative elevation of the competitive ability of donor plants.
Allelochemicals are released from the donor plants into the surrounding environments including the rhizosphere soil through volatilization, rainfall leachates, and root exudation from the donor plants, and decomposition processes of the donor plant litter and residues. Some plants possessing allelopathic potential synthesize allelochemicals and stored them in certain plant tissues until they are released into the surrounding environments.1–3) Therefore, the extraction of the allelopathic plant species allows us the isolation and identification of the allelochemicals stored in the plant tissues. In addition, the extracts and residues of several plant species have shown significant weed control ability as soil additives and mulch because of the allelochemicals.3–6)
We selected plant species through the following three-step evaluation process: 1) the selection of possible plant species that dominate in natural conditions, 2) investigate plant species in publications and websites, and 3) evaluate the allelopathic activity of the crude extracts of the plant species. After the selection, we extracted the plant species, separated the extracts through the bioassay-guided purification process: evaluate the allelopathic activity of all fractions obtained after each separation step, and apply most active fraction to the next separation step. We extracted plant species that showed high allelopathic activity and isolated and identified more than 100 of allelochemicals including novel compounds. The objective of this review is to describe some of identified allelochemicals and their activities, and to discuss the possible involvement of the allelochemicals in some ecological aspects such as the formation of monospecific stands and the naturalization of invasive plant species. The allelopathic activity of benzoxazinones and related degradative products is also discussed.
1. Allelochemicals in medicinal plants
Many of medicinal plants have shown relatively high allelopathic activity.18,19) Therefore, we surveyed the allelopathic activity of several medicinal plants and isolated the allelochemicals from plants that showed high allelopathic activity.
1.1. Neem
Neem (Azadirachta indica), belonging to the Meliaceae family, is native to the Indian subcontinent and Indochina, and is used as a traditional medicinal plant in the native ranges and in the Ayurvedic medicine system. Local people in the native ranges have also been used various parts of the plant and its extracts to control weeds and insects.20–22) Extracts of the neem leaves showed high allelopathic activity, significantly suppressing the growth of Festuca myuros, Phleum pretense, Echinochloa crus-galli and Echinochloa colonum.23) The extracts were then separated and two allelochemicals were isolated and characterized as novel compounds, nimbolide B (1) and nimbic acid B (2) (Fig. 1).24) More than 200 natural compounds have been isolated and identified from neem, with some showing biological activity.25–28) However, compounds 1 and 2 were isolated from the most active fraction with bioassay-guided purification process. Compounds 1 and 2 inhibited the growth of Echinochloa crus-galli at concentrations greater than 0.3 µM and 1 µM, respectively. The concentration of compounds 1 and 2 required for 50% inhibition, defined as IC50, on the growth of E. crus-galli was 3.7 µM and 29 µM for compounds 1 and 2, respectively, suggesting the allelopathic activity of compound 1 is 7.8-fold greater than that of compound 2.
Fig. 1. Allelochemicals of neem.
1.2. Aglaia odorata
Aglaia odorata is also a member of the Meliaceae family, and has been used as a traditional herb in Southeast Asia.29–31) Leaf powder of the species and its ethanol extracts have shown allelopathic activity against the emergence and growth of the weed species, Digitaria adscendens and Trianthema portulacastrum under the field conditions,32,33) and against the growth of Phleum pretense, Lolium multiflorum and Echinochloa crus-galli under the laboratory conditions.34) The extracts were then separated, and the most active allelochemical was isolated and identified as rocaglaol (3) (Fig. 2).34) Although the biological activity of this compound against human and animal cells has been reported,35,36) the allelopathic effect of the compound has not been reported. The compound suppressed the growth of E. crus-galli at concentrations greater than 0.03 µM, and its IC50 value on the growth of E. crus-galli was 0.09 µM.
Fig. 2. Allelochemical of Aglaia odorata.
1.3. Ginkgo
Ginkgo (Ginkgo biloba) is one of the oldest tree species, and the only surviving species in the family of Ginkgoaceae that appeared in the Jurassic period.37) The species has been used as a traditional herb in China, and its leaf extracts of Ginkgo such as EGb761 are sold worldwide as dietary supplement and phytomedicine.38) Extract of the ginkgo leaves significantly suppressed the growth of Phleum pratense and Lolium multiflorum. The extracts were then separated, and a main allelochemical was isolated and characterized as a novel compound, 2-hydroxy-6-(10-hydroxypentadec-11-enyl)benzoic acid (4) (Fig. 3).39) The compound suppressed the growth of P. pretense at concentrations greater than 3 µM, and its IC50 value on the growth of P. pretense was 17 µM. The compound was also found in the litter under the ginkgo trees, and the concentration of the compound was greater in the litter collected near the ginkgo trees than that from farther away.40) Therefore, the growth inhibitory activity of the ginkgo litter layer against other plant species may be higher the closer the litter is to the ginkgo trees, which provides ginkgo with the advantage of increased acquisition of water and nutrients because the growth of other plants are suppressed. Its related compound, nonanoic acid (pelargonic acid) exists as esters in the oil of Pelargonium species. This compound suppressed the growth of P. pretense at concentrations greater than 30 µM, and its IC50 value on the growth of P. pretense was 265 µM.39) Therefore, the inhibitory activity of the newly identified compound 4 was 10- to 16-fold greater than that of nonanoic acid.
Fig. 3. Allelochemical of ginkgo.
1.4. Orthosiphon stamineus
Orthosiphon stamineus (syn. Orthosiphon aristatus), belonging to the Lamiaceae family, is known as Java tea, and is used widely in the herbal tea in Asia.41,42) Extracts of the species inhibited the growth of lettuce (Lactuca sativa) and Lepidium sativum, and a novel compound, 13-epi-orthosiphol N (5), was isolated from the extracts (Fig. 4).43) The compound inhibited the growth of lettuce and L. sativum at concentrations greater than 10 µM. The IC50 values of the compound 5 on the growth of lettuce and L. sativum were 41 µM and 39 µM, respectively.
Fig. 4. Allelochemical of Orthosiphon stamineus.
1.5. Marsdenia tenacissima
Marsdenia tenacissima, belonging to the Asclepiadaceae family, has been widely used as a traditional household remedy in South Asia.44,45) This plant exhibits many pharmacological activities such as anti-tumor, anti-diarrheal, anti-inflammatory and immunomodulatory activity.46,47) Leaf extracts suppressed the growth of Phleum pretense, Lolium multiflorum and Echinochloa crus-galli in a concentration dependent-manner.48,49) The extracts were then separated, and two novel steroidal glycosides, 5,6-dihydrogen-11α-O-acetyl-12β-O-tigloyl-17β-marsdenin (6) and 8-dehydroxy-11β-O-acetyl-12β-O-tigloyl-17β-marsdenin (7), and one known steroidal glycoside, 5,6-dihydrogen-11α,12β-di-O-tigloyl-17β-marsdenin (8), were isolated (Fig. 5).48,49) The relative configuration of the compound 6 was deduced from the NOESY spectrum. Yield of compound 7 was not sufficient for the NOEST analysis. The compounds suppressed the growth of Lepidium sativum at concentrations greater than 0.03 mM, 0.03 mM and 0.1 mM for compounds 6, 7 and 8, respectively. Thus, the growth inhibitory activity of compounds 6 and 7 was greater than that of compound 8.
Fig. 5. Allelochemicals of Marsdenia tenacissima.

1.6. Garcinia xanthochymus
Garcinia xanthochymus, belonging to the Clusiaceae family, has been widely used in folk medicine to the treatments for diarrhea, dysentery, and bilious conditions.50) Extracts suppressed the growth of Echinochloa crus-galli, Vulpia myuros, Lolium multiflorum and Phleum pratense, and a novel compound, garcienone (9) was isolated (Fig. 6).51) The compound suppressed the growth of Lepidium sativum at concentrations greater than 10 µM, and its IC50 value on the growth of L. sativum was 120 µM.
Fig. 6. Allelochemical of Garcinia xanthochymus.
1.7. Wedelia chinensis
Wedelia chinensis (Asteraceae family) has been widely used as a traditional medicine to the treatments for jaundice, diarrhea, kidney dysfunction, fever, and wounds.52,53) Extracts of the above-ground parts of the species suppressed the growth of Vulpia myuros, Phleum pretense, Lolium multiflorum, and Echinochloa crus-galli. The extracts were separated and a novel compound, wedelienone (10), was isolated (Fig. 7).54) Wedelienone suppressed the growth of P. pretense at concentrations greater than 10 µM, and IC50 value of wedelienone on the growth of P. pretense was 85.1 µM.
Fig. 7. Allelochemical of Wedelia chinensis.
1.8. Albizia richardiana and Elaeocarpus floribundus
Two other medicinal plants, Albizia richardiana (Fabaceae family) and Elaeocarpus floribundus (Elaeocarpaceae family) showed allelopathic activity, and two novel compounds, 3-hydroxy-4-oxo-β-dehydroionol (11) and elaeocarpunone (12) were isolated, respectively (Fig. 8).55,56) These compounds suppressed the growth of Lepidium sativum at concentrations greater than 0.03 mM and 0.3 mM for compounds 11 and 12, respectively, and the IC50 values on the growth of L. sativum were 0.06 mM and 0.5 mM for compounds 11 and 12, respectively.
Fig. 8. Allelochemicals of Albizia richardiana and Elaeocarpus floribundus.
2. Allelochemicals involved in the formation of monospecific stands
Some plant species including moss and fern species outcompete other plant species because of high allelopathic activity, and form the monospecific stands, resulting in the elimination of other plant species from the areas.57–60) We determined the allelopathic activity of such dominant species in the natural ecosystems and identified their allelochemicals.
2.1. Hypnum plumaeforme
The moss species Hypnum plumaeforme (syn. Calohypnum plumiforme) belongs to the Hypnaceae family and thrives in sunny places from the uplands to lowlands including wet lands in East Asia. The species outcompetes other plant species, and forms large monospecific stands (mats) on soils and rocks.61,62) These observations suggest that the species possesses high allelopathic activity and releases allelochemicals into its rooting zones. The allelochemicals may suppress the growth of other plant species and contribute to the formation of the monospecific stands. Therefore, the species was selected, and the soil under the H. plumaeforme monospecific stands was collected for investigation. Extracts of the soil suppressed the growth of Phleum pratense, Lolium multiflorum, Digitaria sanguinalis and Echinochloa crus-galli in a concentration-dependent manner. The extracts were then separated, and two allelochemicals were isolated and identified as momilactone A (13) and momilactone B (14) (Fig. 9).63) Compounds 13 and 14 were also identified in the moss tissues and growth medium. The concentration of compounds 13 and 14 was 58.7 and 23.4 µg/g dry weight in the moss tissues respectively, and 4.3 and 6.4 µg/g dry weight in the growth medium, respectively.63) These results indicate the moss H. plumaeforme produces compounds 13 and 14, and releases them into the growth medium. Considering the inhibitory activity and concentration in the medium, compounds 13 and 14 may contribute to the formation of the monospecific stands of the moss as allelopathic agents.
Fig. 9. Allelochemicals of Hypnum plumaeforme.
Compounds 13 and 14 were first isolated from the husks of rice (Oryza sativa), and then identified in the rice straws, leaves, roots and root exudate.64–66) Both compounds have also been identified in several other Oryza species such as Oryza rufipogon, O, burthii, O. brachyatha, O. glumaepatula, O. glaberrima, O. meridionalis, and O. punctatas,67) and the moss species H. plumaeforme as described above. The compounds in rice plants showed defense functions against the fungal pathogen attacks, and allelopathy. Rice plants suppress the growth of neighboring plant species through the root exudation of the compounds into their rhizosphere. Pathogen attacks, and biotic and abiotic elicitors induced the production of momilactones through the jasmonic acid signaling pathway and jasmonic acid independent signaling pathway. Rice allelopathy was also induced by jasmonic acid, and the conditions of the nutrient competition with the neighboring plant species.66,67) Momilactone-deficient rice mutants showed considerable reduction in the tolerance to the pathogen attacks, and lost the allelopathic activity. Momilactones are synthesized form geranylgeranyl diphosphate through cyclization steps. The genes involved in the synthesis, OsCPS4, OsKSL4, CYP99A2, CYP99A3, OsMS1, and OsMS2 are located on chromosome 4 in plastids of rice cells.68,69)
Biotic and abiotic elicitors, and jasmonic acid also induced momilactones in the moss H. plumaeforme.70) In addition, momilactones are synthesized from geranylgeranyl diphosphate in the moss, and some of synthesis-related genes (CpMS, CpCYP970A14, CpDTC1/HpDTC1. and CpCYP964A1) are located on the same chromosome of the moss.71) Although momilactones have been found in taxonomically quite distinct species, H. plumaeforme and Oryza species, momilactones may play an important ecological role in the evolution of the moss and Oryza because of the presence of a dedicated biosynthetic gene cluster in their genomes.67,72)
2.2. Dicranopteris linearis
The fern species Dicranopteris linearis (Gleicheniaceae family) thrives in sunny places, and is one of the most distributed fern species from the temperature to tropical regions of Asia, Europe and Africa. This fern also outcompetes other plant species and often forms large monospecific stands.73) Extracts of the fern suppressed the growth of Echinochloa colonum and Avena fatua. The most active allelochemical was isolated from the extracts and identified as cinnamtannin B-1 (15) (Fig. 10).74) Compound 15 was also found in the soil under the monospecific stands of the fern. The concentration of the compound was 4.3 mM and 14.5 mM in the soil collected from the edge of the stands and the soil collected from under the stands, respectively.74) This finding suggests that the compound may be released into the soil either as an exudate from living plants or through the decomposition process of the plant residues. Compound 15 suppressed the growth of A. fatua and E. colonum at concentrations greater than 0.2 mM. The concentration of the compound in the soil exceeded the threshold, which caused the growth inhibition. Therefore, the compound may contribute to the formation of monospecific stands of the fern species.
Fig. 10. Allelochemical of Dicranopteris linearis.
2.3. Gleichenia japonica
The fern species, Gleichenia japonica, belonging to the Gleicheniaceae family, thrives in sunny places, and is one of the most distributed ferns from South to East Asia. The fern also forms large monospecific stands.75,76) Extracts of the fern suppressed the growth of Phleum pretense and Lolium multiflorum. Extracts of its litter obtained from the soil surface under the fern also suppressed the growth of L. multiflorum and Echinochloa crus-galli. The litter extract was then separated, and two novel compounds 3-O-β-allopyranosyl-13-O-β-fucopyranosyl-3β-hydroxymanool (16), and 13-O-β-fucopyranosyl-3β-hydroxymanool (17), and a known compound 18-O-β-L-rhamnopyranosyl-(1→2)-β-D-glucopyranosyl-13-epitorreferol (18) were isolated and identified (Fig. 11).77,78) The IC50 values againstthe growth of E. crus-galli were 0.72 mM, 0.09 mM and 0.97 mM for compounds 16, 17 and 18, respectively. The concentration of those compounds in the soil under the fern species was 4.9 mM, 0.8 mM and 5.7 mM for compounds 16, 17 and 18, respectively, indicating that the concentration of these three compounds in the soil exceeded their IC50 values.77,78) Therefore, these compounds may contribute to the allelopathy of G. japonica, and be involved in the formation of the monospecific stands of the species.
Fig. 11. Allelochemicals of Gleichenia japonica.
2.4. Schumannianthus dichotomus
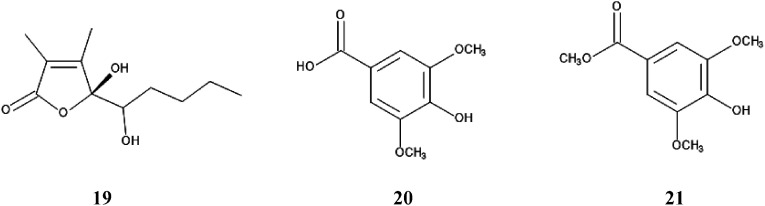
Schumannianthus dichotomus belongs to the Marantaceae family, and is known as cool mat. The plant is cultivated without the application of pesticides because of its significant ability to defend itself against insects and fungi, and is used for making traditional bed mats in Indian continental.79) Extracts of the species suppressed the growth of Lolium multiflorum, Phleum pretense, and Echinochloa crus-galli in a concentration-dependent manner. A novel compound; schumannione (19), and two known compounds; syringic acid (20) and methyl syringate (21) were isolated form the extracts (Fig. 12).80,81) These three compounds suppressed the growth of Lepidium sativum at concentrations greater than 10 µM, and the IC50 values on the growth of L. sativum were 109 µM, 61 µM and 32 µM for compounds 19, 20 and 21, respectively.
Fig. 12. Allelochemicals of Schumannianthus dichotomus.
3. Allelochemicals involved in causing sparse understory vegetation
Some tree plant species are characterized by sparse understory vegetation because of their high allelopathic activity.82) We determined the allelopathic activity of Japanese red pine and Citrus junos, and identified the allelochemicals involved in causing the sparse understory vegetation.
3.1. Japanese red pine
Japanese red pine (Pinus densiflora), belonging to the Pinaceae family, is native to Eastern Asia. The species is characterized by sparse understory vegetation even though sunlight at the forest floor levels is sufficient for the growth of other understory plant species.1) This phenomenon was also written about in Daigaku-Wakumon by the Japanese Confucian Kumazawa Banzan in the 17 the century (the Edo period).83) Soil obtained from the Japanese red pine forest floor showed allelopathic activity against Lolium multiflorum, which exists at the margin of the pine forests but not in the forests. Two allelochemicals were isolated from the soil, and characterized as 7-oxodehydroabietic acid (22) and 15-hydroxy-7-oxodehydroabietate (23) (Fig. 13).84) These compounds may be formed by the degradation processes of abietic acid, which is abundant in the leaves of the species. The leaves were accumulated on the forest floor by the defoliation and decompose into the soil, where the compounds are generated. The species is evergreen, but drops its old leaves in early spring.85) The concentrations of compounds 22 and 23 in the soil under the pine trees was 397 µM and 312 µM, respectively. The IC50 values of compounds 22 and 23 against the growth of L. multiflorum were 34 µM and 137 µM, respectively. Therefore, the concentrations of both compounds in the soil on the pine forest floor were sufficient to cause growth inhibition. In addition, a mixture of both compounds showed a synergic effect on the growth inhibition. The compounds may contribute to the allelopathy of Japanese red pine and suppress the growth of undergrowth plant species of the pine forests, resulting in the establishment of sparse understory vegetation.
Fig. 13. Allelochemicals of Japanese red pine.
3.2. Citrus junos
Citrus junos, belonging to the Rutaceae family, is also characterized by sparse undergrowth vegetation compared with other Citrus species.86) When the fruit peel of C. junos was incorporated into the soil, the soil, the growth of Digitaria ciliaris, Alopecurus aequalis, and Sonchus oleraceus was supressed.86,87) The peel extracts also showed allelopathic activity.88,89) An allelochemical was isolated from the peel extracts, and characterized as an abscisic acid-β-D-glucopyranosyl ester (ABA-GE; 24) (Fig. 14).90) The compound suppressed the seedling growth of lettuce and Arabidopsis thaliana at concentrations greater than 0.3 µM, and its IC50 values on the growth of lettuce and A. thaliana were 1.4 µM and 1.8 µM, respectively.90,91) ABA-β-D-glucosidase was induced in A. thaliana seedlings after the application of ABA-GE, suggesting that exogenously applied ABA-GE may be hydrolyzed into abscisic acid (ABA) by apoplastic ABA-β-D-glucosidase in the root cortexes.91,92) Liberated ABA may be loaded into the xylem through the parenchyma, transported to the target cells, and induce the growth suppression. Soil obtained from several crop fields, pastures, and forests has been reported to contain a significant amount of ABA, which was thought to be released from the roots of the previously existing plant species in those areas.93)
Fig. 14. Allelochemical of Citrus junos.
4. Allelochemicals in the invasive plant species
Allelopathy also contributes to the naturalization of invasive plant species in the introduced ranges. The allelopathic activity of many invasive plant species is high, and some of the invasive plant species eliminate native plant species because of their allelochemicals.94–96) The allelochemicals of the invasive plant species enable to suppress the regeneration process of the native plant species by inhibiting germination and growth, resulting in the expansion of invasive plant species in the introduced ranges.97,98) We determined the allelopathic activity of some invasive plant species and identified the allelochemicals involved in the invasion.
4.1. Pueraria montana
Pueraria montana (syn. Pueraria lobata, Pueraria thunbergiana), belonging to the Fabaceae family, is a perennial climbing vine, and has been listed in the top 100 of the world’s worst invasive alien species.99,100) Sterilized quartz sand mixed with the leaf powder of P. montana suppressed the germination, and the growth of Lepidium sativum, Phleum pretense and Lolium multiflorum.101) Two allelochemicals were isolated from the leaves, and identified as cis,trans-xanthoxin (25) and trans,trans-xanthoxin (26)(Fig. 15).102) Compounds 25 and 26 suppressed the growth of L. sativum at concentrations greater than 0.3 µM and 3 µM, respectively. The IC50 values on the growth of L. sativum were 1.1 µM and 14 µM for compounds 25 and 26, respectively. Therefore, xanthoxins may contribute to the allelopathy, and play an important role in the invasiveness of P. montana. In addition, compound 24 has been converted to ABA in cell free systems.103–105)
Fig. 15. Allelochemicals of Pueraria montana.
4.2. Imperata cylindrica
Imperata cylindrica, belongs to the Poaceae family, and is a C4 perennial rhizomatous grass species that has also been listed in the top 100 of the world’s worst invasive alien species.106,107) Three allelochemicals, 5-methoxyflavone (27), 5,2ʹ-dimethoxyflavone (28), and methyl caffeate (29), were isolated from the rhizomes extracts of I. cylindrica (Fig. 16). These compounds suppressed the growth of Lepidium sativum at concentrations greater than 0.08 mM, 0.2 mM and 0.6 mM for compounds 27, 28 and 29, respectively, and the IC50 values on the growth of L. sativum were 0.08 mM, 1.1 mM and 0.23 mM for compounds 27, 28 and 29, respectively.108) These compounds may contribute to the invasiveness of I. cylindrica.
Fig. 16. Allelochemicals of Imperata cylindrica.
4.3. Polygonum chinense
Polygonum chinense (syn. Persicaria chinensis), belonging to the Polygonaceae family, is native to South and South East Asia, and forms dense monospecific stands, and is listed a noxious invasive plant species.109,110) Extracts of the plants suppressed the growth of Phleum pretense, Lolium multiflorum, and Echinochloa crus-galli, and two allelochemicals (3R)-3-hydroxy-β-ionone (30) and (3R)-3-hydroxy-7,8-dihydro-β-ionone (31), were isolated (Fig. 17).111) These compounds suppressed the growth of Lepidium sativum at concentrations of 0.01 mM and 1 mM for compounds 30 and 31, respectively, and the IC50 values on the growth of L. sativum were 0.05 mM and 0.42 mM for compounds 30 and 31, respectively. The allelopathic activity of compound 30 was 8-fold greater than that of compound 31.
Fig. 17. Allelochemicals of Polygonum chinense.
4.4. Tithonia diversifolia
Tithonia diversifolia belongs to the Asteraceae family, and aggressively expands its population and habitats. The species is known as a noxious weed in agricultural fields, and disturbs native plant communities as an invasive plant species.112,113) Extracts of T. diversifolia leaves suppressed the growth of Phleum pretense, Lolium multiflorum and Echinochloa crus-galli. Tagitinin C (32) was isolated from the extracts (Fig. 18), and inhibited the growth of P. pretense, L. multiflorum and E. crus-galli at concentrations greater than 0.1–0.3 mM in a concentration-dependent manner.114) The IC50 values of the compound were 0.13 mM, 0.22 mM and 0.13 mM for P. pretense, L. multiflorum and E. crus-galli, respectively. This compound may contribute to the invasiveness of the species.
Fig. 18. Allelochemical of Tithonia diversifolia.
5. Compounds involved in autotoxicity
Some crop plants cannot be successively cultivated for a long time in the same fields because of their autotoxicity. Autotoxicity is a particular type of allelopathy, in which the compounds released by these crop plants suppresses their own growth and productivity.1) We determined the autotoxic activity of asparagus and kiwifruit, and identified the compounds involved in the autotoxicity.
5.1. Asparagus
Asparagus (Asparagus officinalis; Asparagaceae family) is a widely cultivated perennial vegetable that can be harvested for more than 10 years. However, the yield and quality of asparagus decrease after several year’ cultivation, known as “asparagus decline”.115,116) Although the senescent asparagus plants were replaced with juvenile asparagus plants in the same fields, the quality and yield of the juvenile asparagus remained low, known as the “asparagus replant problem”.117,118) The “asparagus decline” and “asparagus replant problem” were reported to be caused by a combination of pathogen infection such as by Fusarium species and autotoxicity.119,120) However, the compounds involved in the autotoxicity of asparagus had not been reported.
Extracts of 10-year-asparagus cultivated soils suppressed the growth of asparagus seedlings in a concentration-dependent manner. The extracts were then separated, and a main autotoxic substance was isolated and determined to be trans-cinnamic acid (33) (Fig. 19).121) The compound suppressed the growth of asparagus seedlings at concentrations greater than 10 µM, and the IC50 value of the compound on the growth of asparagus seedlings was 24.1 µM. The concentration of the compound in the 10-year-asparagus soils was 174 µM, which is enough to suppress the growth the asparagus. Compound 33 was also found in the shoots and roots of the asparagus and its growth medium.121) Therefore, the compound 33 may be synthesized in the plants, and released into the medium and soil. Compound 33 is isomerized to cis-cinnamic acid (34) by sunlight and UV-light,122,123) and the growth inhibitory activity of compound 34 was 10-fold greater than that of compound 33.122) Compound 34 inhibits growth because it disturbs cellular auxin transport and accumulation,123) and compound 33 suppresses proton transport through plasma membrane H+-ATPases.124) Compound 33 in asparagus soil may be isomerized to compound 34 by sunlight during farm work. Therefore, both cinnamic acids may be involved in the autotoxicity of asparagus, and may be in partly responsible for the “asparagus decline” and the “asparagus replant problem”.
Fig. 19. Autotoxic substances of asparagus.
5.2. Kiwifruit
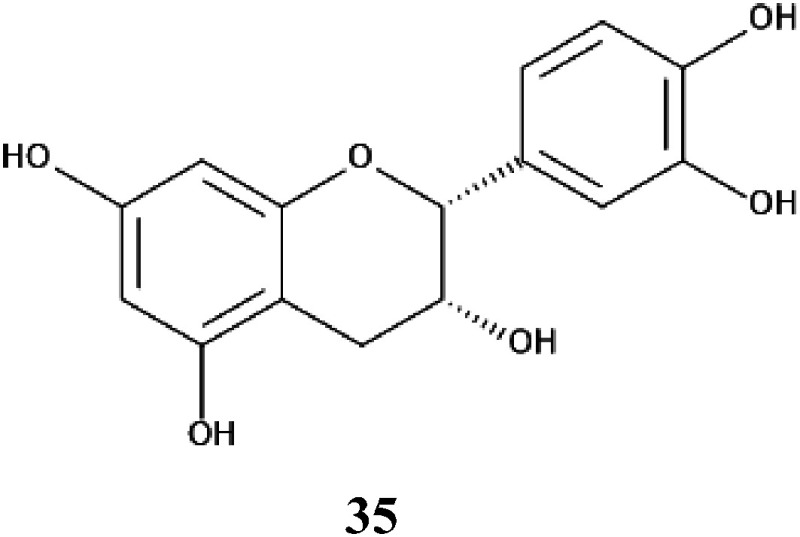
Kiwifruit (Actinidia deliciosa), belonging to the Actinidiaceae family, is cultivated worldwide. Reductions in the yield of kiwifruit occur in old orchards. When the old kiwifruit plants are replaced with juvenile plants in the same orchards, the growth and productivity of the juvenile plants are often lower than expected. This symptom is typical of the “replant problems” reported in several other fruit trees.1,125,126) Kiwifruit leaf extracts showed autotoxic activity against the growth of kiwifruit seedlings. The compound involved in the autotoxicity was isolated and identified as (−)-epicatechin (35) (Fig. 20).127) (−)-Epicatechin suppressed the growth of kiwifruit seedlings at concentrations greater than 3 mM, and the IC50 values of the compound on the growth of kiwifruit was 7.6 mM. Therefore, compound 35 may be involved in the autotoxicity of kiwifruit and be responsible for the “replant problems”.
Fig. 20. Autotoxic substance of kiwifruit.
6. Allelopathic activity of benzoxazinones and related compounds
Several benzoxazinones have been found in some Poaceae species such as wheat, rye, and maize.128) Benzoxazinones and their precursor hydroxamic acids showed plant defense functions against pathogenic fungi and herbivorous insects.128–131) These compounds and their degradation products are also involved in the allelopathy.131–135)
The main benzoxazinone of wheat and maize is 2,4-dihydroxy-7-methoxy-2H-1,4-benzoxazin-3(4H)-one (DIMBOA), while that of rye is the dimethoxylated analogue, 2,4-dihydroxy-2H-1,4-benzoxazin-3(4H)-one (DIBOA). DIMBOA and DIBOA exist in plant tissues as inactive glucoside esters, DIMBOA-β-O-D-glucopyranoside (DIMBOA-glucose) and DIBOA-β-O-D-glucopyranoside (DIBOA-glucose), respectively.128,136,137) These glucosides are rapidly converted to the aglycones, DIMBOA and DIBOA by β-glucosidase upon plant cell disruptions, and show defense functions against attacks by fungal pathogens and herbivorous insects. DIMBOA and DIBOA are further degraded to MBOA and BOA, respectively.128,137) These two degradation products, MBOA and BOA, have been found in the plant tissues and showed allelopathic activity.134–137) After harvesting, the straw and roots of the plants are sometimes left in the fields. MBOA and BOA are produced during the degradation process of these straw and roots, and released into the field soil.136–138) MBOA and BOA suppressed the germination and growth of several plant species, and the gibberellin-induced α-amylase activity.139–143) α-Amylase triggers starch degradation in seed reserves, and enabling the seeds to germinate and grow.144,145) Suppression of α-amylase induction by these compounds is one of the cause of the inhibition of germination and growth. Aminophenoxazinone (AMPO, AHPO and APO) were thought to be the final degradation products of DIMBOA and DIBOA because those compounds were found in the soil (Fig. 21).136,137)
Fig. 21. Benzoxazinones and related compounds.

Natural benzoxazinones (DIMBOA and DIBOA), degradation products (MBOA, BOA, APO, AHPO and AMPO), and synthetic compounds (D-HBOA, D-DIBOA, HBOA, D-HMBOA, D-DIMBOA and HMBOA) were evaluated for their growth inhibitory and α-amylase suppression activity (Fig. 21). The IC50 values against the growth of Lepidium sativum were 0.13 mM, 0.15 mM, 0.16 mM, 0.19 mM, 0.47 mM, 0.54 mM, 0.62 mM, 0.81 mM, and 0.82 mM for DIMBOA, D-DIMBOA, DIBOA, D-DIBOA, MBOA, APO, BOA, D-HMBOA, and D-HBOA, respectively. The IC50 values againstα-amylase suppression activity were 0.07 mM, 0.09 mM, 0.11 mM, 0.14 mM, 0.32 mM, 0.38 mM, 0.61 mM, 0.65 mM, and 0.75 mM for DIMBOA, D-DIMBOA, DIBOA, D-DIBOA, MBOA, APO, BOA, D-HMBOA, and D-HBOA, respectively. The IC50 values against the growth of L. sativum and α-amylase suppression activity of HBOA, HMBOA, AHPO, and AMPO could not be determined because of their low activity. The inhibitory activity of these compounds was classified into three groups, high active group (DIMBOA, D-DIMBOA, DIBOA, and D-DIBOA), moderate active group (MBOA, APO, BOA, D-HMBOA, and D-HBOA), and low active group (HBOA, HMBOA, AHPO, and AMPO).146) The structure–activity of these compounds suggests that the compounds with the benzoxazinone skeleton are the most active structure. A hydroxyl group at position N-4 on the benzoxazinone skeleton is essential for the inhibitory activity, whereas a hydroxyl group at position C-2 on the benzoxazinone skeleton may not affect the inhibitory activity.
Conclusion
The allelopathic activity of some medicinal plants was high, and several potent allelochemicals including novel compounds were isolated and identified. The allelopathic activity of the moss Hypnum plumaeforme, and the fern species Dicranopteris linearis and Gleichenia japonica, which form monospecific stands, were high, and the concentrations of the identified allelochemicals in the soil under their monospecific stands were sufficient levels to cause the growth inhibition. Therefore, those allelochemicals may contribute to forming the monospecific stands. Japanese red pine is characterized by sparse understory vegetation even though sunlight at the forest floor level is sufficient levels for the growth of other understory plant species. The allelochemicals found in the soil under the Japanese red pine trees may also contribute to causing the sparse understory vegetation. The invasive plants, Pueraria montana, Imperata cylindrica, Polygonum chinense and Tithonia diversifolia showed high allelopathic activity, and the identified allelochemicals may contribute to the invasiveness and naturalization of these species in the introduced ranges. Asparagus and kiwifruit show autotoxicity and cannot be successively cultivated for a long time in the same field soil. The identified compounds from the soil and/or plants may contribute to their autotoxicity. These investigations suggest the allelopathy and allelochemicals may be involved in several ecological aspects in the natural ecosystems.
References
- 1) E. L. Rice: “Allelopathy,” 2nd ed., Academic Press, Orlando, USA, 1984.
- 2) H. P. Bais, T. L. Weir, L. G. Perry, S. Gilroy and J. M. Vivanco: The role of root exudates in rhizosphere interactions with plants and other organisms. Annu. Rev. Plant Biol. 57, 233–266 (2006). [DOI] [PubMed] [Google Scholar]
- 3) R. G. Belz: Allelopathy in crop/weed interactions: An update. Pest Manag. Sci. 63, 308–326 (2007). [DOI] [PubMed] [Google Scholar]
- 4) H. Kato-Noguchi and D. Kurniadie: Allelopathy and allelopathic substances of mango (Mangifera indica L.). Weed Biol. Manage. 20, 131–138 (2020). [Google Scholar]
- 5) H. Kato-Noguchi: Phytotoxic substances involved in teak allelopathy and agroforestry. Appl. Sci. (Basel) 11, 3314 (2021). [Google Scholar]
- 6) H. Kato-Noguchi and M. Kato: Evolution of the secondary metabolites in invasive plant species Chromolaena odorata for the defense and allelopathic functions. Plants 12, 521 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7) B. R. Dalton: The occurrence and behavior of plant phenolic acids in soil environments and their potential involvement in allelochemical interference interactions: Methodological limitations in establishing conclusive proof of allelopathy. In “Principals and Practices in Plant Ecology: Allelochemical Interactions,” ed by K. M. Inderjit, M. Dakshini and C. L. Foy, CRC Press: Boca Raton, Florida USA, pp. 57–74, 1999.
- 8) R. R. Barkosky, F. A. Einhellig and J. L. Butler: Caffeic acid-induced changes in plant-water relationships and photosynthesis in leafy spurge Euphorbia esula. J. Chem. Ecol. 26, 2095–2109 (2000). [Google Scholar]
- 9) F. A. Einhellig: Mode of action of allelochemical action of phenolic compounds. In “Chemistry and Mode of Action of Allelochemicals,” ed by F. A. Macías, J. C. G. Galindo, J. M. G. Molino and H. G. Cutler, CRC Press, Boca Raton, London, New York, Washington D.C., pp. 217–238, 2004.
- 10) H. Kato-Noguchi: Allelopathy of knotweeds as invasive plants. Plants 11, 3 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11) H. Kato-Noguchi and M. Kato: Allelopathy and allelochemicals of Solidago canadensis L. and S. altissima L. for their naturalization. Plants 11, 3235 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12) H. Kato-Noguchi: Invasive mechanisms of one of the world’s worst alien plant species Mimosa pigra and its management. Plants 12, 1960 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13) A. V. Tsyganova, N. J. Brewin and V. E. Tsyganov: Structure and development of the legume-rhizobial symbiotic interface in infection threads. Cells 10, 1050 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14) U. Mathesius: Are legumes different? Origins and consequences of evolving nitrogen fixing symbioses. J. Plant Physiol. 276, 153765 (2022). [DOI] [PubMed] [Google Scholar]
- 15) S. E. Smith and D. J. Read: “Mycorrhizal Symbiosis,” 3rd ed., Academic Press, London, UK, 2008.
- 16) N. Diagne, M. Ngom, P. I. Djighaly, D. Fall, V. Hocher and S. Svistoonoff: Roles of arbuscular mycorrhizal fungi on plant growth and performance: Importance in biotic and abiotic stressed regulation. Diversity (Basel) 12, 370 (2020). [Google Scholar]
- 17) H. Tang, M. U. Hassan, L. Feng, M. Nawaz, A. N. Shah, S. H. Qari, Y. Liu and J. Miao: The critical role of arbuscular mycorrhizal fungi to improve drought tolerance and nitrogen use efficiency in crops. Front. Plant Sci. 13, 919166 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18) Y. Fujii, T. Shibuya and T. Yasuda: Survey of Japanese weed and crops for the detection of water-extractable allelopathic chemicals using Richards’ function fitted to lettuce germination test. Weed Res. Jpn. 35, 362–370 (1990) (in Japanese). [Google Scholar]
- 19) Y. Fujii, S. S. Parvez, M. M. Parvez, Y. Ohmae and O. Iida: Screening of 239 medicinal plant species for allelopathic activity using the sandwich method. Weed Biol. Manage. 3, 233–241 (2003). [Google Scholar]
- 20) J. F. Islas, E. Acosta, Z. G-Buentello, J. L. Delgado-Gallegos, M. G. Moreno-Treviño, B. Escalante and J. E. Moreno-Cuevas: An overview of neem (Azadirachta indica) and its potential impact on health. J. Funct. Foods 74, 104171 (2020). [Google Scholar]
- 21) S. C. Gupta, S. Prasad, A. K. Tyagi, A. B. Kunnumakkara and B. B. Aggarwal: Neem (Azadirachta indica): An Indian traditional panacea with modern molecular basis. Phytomedicine 34, 14–20 (2017). [DOI] [PubMed] [Google Scholar]
- 22) Z. S. S. Al-Hashemi and M. A. Hossain: Biological activities of different neem leaf crude extracts used locally in Ayurvedic medicine. Pac. Sci. Rev. A: Nat. Sci. Eng. 18, 128–131 (2016). [Google Scholar]
- 23) M. A. Salam and H. Kato-Noguchi: H. Evaluation of allelopathic potential of neem (Azadirachta indica. A. Juss) against seed germination and seedling growth of different test plant species. Int. J. Sustain. Agric. 2, 20–25 (2010). [Google Scholar]
- 24) H. Kato-Noguchi, M. A. Salam, O. Ohno and K. Suenaga: K. Nimbolide B and nimbic acid B, phytotoxic substances in neem leaves with allelopathic activity. Molecules 19, 6929–6940 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25) National Research Council: “Neem, A Tree for Solving Global Problems,” National Academy Press, Washington D.C. USA, 1992. [PubMed]
- 26) G. Brahmachari: Neem. An omnipotent plant: A retrospection. ChemBioChem 5, 408–421 (2004). [DOI] [PubMed] [Google Scholar]
- 27) R. Subapriya and S. Nagini: Medicinal properties of neem leaves: A review. Curr. Med. Chem. Anticancer Agents 5, 149–156 (2005). [DOI] [PubMed] [Google Scholar]
- 28) I. P. Ogbuewu, V. U. Odoemenam, H. O. Obikaonu, M. N. Opara, O. O. Emenalom, M. C. Uchegbu, I. C. Okoli, B. O. Esonu and M. U. Iloeje: The growing importance of neem (Azadirachta indica A. Juss) in agriculture, industry, medicine and environment: A review. Res. J. Med. Plant 5, 230–245 (2011). [Google Scholar]
- 29) N. Bunyapraphatsara: “Medicinal plants indigenous to Thailand,” Vol. 2, Mahidol University, Bangkok Prachachon, Bangkok, 2000.
- 30) Y. Bernard, N. Ribeiro, F. Thuaud, G. Tűrkeri, R. Dirr, M. Boulberdaa, C. G. Nebigil and L. Désaubry: Flavaglines alleviate doxorubicin cardiotoxicity: Implication of Hsp27. PLoS One 6, e25302 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31) N. Ribeiro, F. Thuaud, C. Nebigil and L. Désaubry: Recent advances in the biology and chemistry of the flavaglines. Bioorg. Med. Chem. 20, 1857–1864 (2012). [DOI] [PubMed] [Google Scholar]
- 32) C. Laosinwattana, T. Poonpaiboonpipat, M. Teerarak, W. Phuwiwat, T. Mongkolaussavaratana and P. Charoenying: Allelopathic potential of Chinese rice flower (Aglaia odorata Lour.) as organic herbicide. Allelopathy J. 24, 45–54 (2009). [Google Scholar]
- 33) C. Laosinwattana, M. Teerarak and P. Charoenying: Effects of Aglaia odorata granules on the seedling growth of major maize weeds and the influence of soil type on the granule residue’s efficacy. Weed Biol. Manage. 12, 117–122 (2012). [Google Scholar]
- 34) H. Kato-Noguchi, M. Suzuki, K. Noguchi, O. Ohno, K. Suenaga and C. Laosinwattana: A potent phytotoxic substance in Aglaia odorata Lour. Chem. Biodivers. 13, 549–554 (2016). [DOI] [PubMed] [Google Scholar]
- 35) O. Yodsaoue, J. Sonprasit, C. Karalai, C. Ponglimanont, S. Tewtrakul and S. Chantrapromma: Diterpenoids and triterpenoids with potential anti-inflammatory activity from the leaves of Aglaia odorata. Phytochemistry 76, 83–91 (2012). [DOI] [PubMed] [Google Scholar]
- 36) L. Pan, U. M. Acuña, J. Li, N. Jena, T. N. Ninh, C. M. Pannell, H. Chai, J. R. Fuchs, E. J. C. de Blanco, D. D. Soejarto and A. D. Kinghorn: Bioactive flavaglines and other constituents isolated from Aglaia perviridis. J. Nat. Prod. 76, 394–404 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37) D. L. Royer, L. J. Hickey and S. L. Wing: Ecological conservatism in the living fossil’ Ginkgo. Paleobiology 29, 84–104 (2003). [Google Scholar]
- 38) D. J. McKenna, K. Jones and K. Hughes: Efficacy, safety, and use of Ginkgo biloga in clinical and preclinical applications. Altern. Ther. Health Med. 7, 70–86, 88–90 (2001). [PubMed] [Google Scholar]
- 39) H. Kato-Noguchi, S. Takeshita, F. Kimura, O. Ohno and K. Suenaga: A novel substance with allelopathic activity in Ginkgo biloba. J. Plant Physiol. 170, 1595–1599 (2013). [DOI] [PubMed] [Google Scholar]
- 40) H. Kato-Noguchi and S. Takeshita: Contribution of a phytotoxic compound to the allelopathy of Ginkgo biloba. Plant Signal. Behav. 8, e26999 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41) K. Ashraf, S. Sultan and A. Adam: Orthosiphon stamineus Benth. is an outstanding food medicine: Review of phytochemical and pharmacological activities. J. Pharm. Bioallied Sci. 10, 109–118 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42) O. Z. Ameer, I. M. Salman, M. Z. Asmawi, Z. O. Ibraheem and M. F. Yam: Orthosiphon stamineus: Traditional uses, phytochemistry, pharmacology, and toxicology. J. Med. Food 15, 678–690 (2012). [DOI] [PubMed] [Google Scholar]
- 43) H. Kato-Noguchi, N. Hamada, M. Morita and K. Suenaga: A novel allelopathic substance, 13-epi-orthosiphol N, in Orthosiphon stamineus. J. Plant Physiol. 170, 1–5 (2013). [DOI] [PubMed] [Google Scholar]
- 44) G. Kumar, G. S. Banu, A. G. Murugesan and M. R. Pandian: Effect of Helicteres isora bark extract on protein metabolism and marker enzymes in streptozotocin induced diabetic rats. Iran. J. Pharm. Sci. 6, 123–129 (2007). [Google Scholar]
- 45) H. T. Li, L. P. Kang, B. L. Guo, Z. L. Zhang, Y. H. Guan, X. Pang, C.-Z. Peng, B. Ma and L.-X. Zhang: Original plant identification of Dai nationality herb “Daibaijie”. Zhongguo Zhongyao Zazhi 39, 1525–1529 (2014). [PubMed] [Google Scholar]
- 46) W. Zhang, Z. F. Wang, J. Wang, J. Yang and Y. Min: Chemical constituents from Marsdenia Tenacissima and their anti-tumor activities. Zhongchengyao 39, 334–338 (2017). [Google Scholar]
- 47) V. Tiwari, A. Singh and A. Tiwari: Phytopharmacological overview on controversial drug: Murva. Tradi. Folk Herb. Med. Recent Res. 2, 475–526 (2018). [Google Scholar]
- 48) S. M. Moh, A. Iwasaki, K. Suenaga and H. Kato-Noguchi: Allelopathic activity of a novel compound, 5,6-dihydrogen-11α-O-acetyl-12β-O-tigloyl-17β-marsdenin, and a known steroidal glycoside from the leaves of Marsdenia tenacissima (Roxb.) Moon. Agronomy (Basel) 12, 1536 (2022). [Google Scholar]
- 49) S. M. Moh, N. Kurisawa, K. Suenaga and H. Kato-Noguchi: Allelopathic potential of Marsdenia tenacissima (Roxb.) Moon against four test plants and the biological activity of its allelopathic novel compound, 8-dehydroxy-11β-O-acetyl-12β-O-Tigloyl-17β-marsdenin. Plants 12, 1663 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50) C. N. Nguyen, B. T. Trinh, T. B. Tran, L. T. T. Nguyen, A. K. Jäger and L. H. D. Nguyen: Anti-diabetic xanthones from the bark of Garcinia xanthochymus. Bioorg. Med. Chem. Lett. 27, 3301–3304 (2017). [DOI] [PubMed] [Google Scholar]
- 51) M. M. Rob, A. Iwasaki, R. Suzuki, K. Suenaga and H. Kato-Noguchi: Garcienone, a novel compound involved in allelopathic activity of Garcinia xanthochymus Hook. Plants 8, 301 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52) Y. T. Huang, C. C. Wen, Y. H. Chen, W. C. Huang, L. T. Huang, W. C. Lin, P. Arulselvan, J. W. Liao, S. H. Lin, P. W. Hsiao, S. C. Kuo and N. S. Yang: Dietary uptake of Wedelia chinensis extract attenuates dextran sulfate sodium-induced colitis in mice. PLoS One 8, e64152 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53) Y. L. Zhong, Y. B. Zhang, D. Luo, Q. W. Niu, J. Qin, L. J. He, Y. L. Li and G. C. Wang: Two new compounds from Wedelia chinensis and their anti-inflammatory activities. Chem. Select 3, 3459–3462 (2018). [Google Scholar]
- 54) K. R. Das, A. Iwasaki, K. Suenaga and H. Kato-Noguchi: A kaurene-type novel phytotoxic substance in Wedelia chinensis. Tetrahedron Lett. 61, 151600 (2020). [Google Scholar]
- 55) K. Hossen, A. Iwasaki, K. Suenaga and H. Kato-Noguchi: Phytotoxicity of the novel compound 3-hydroxy-4-oxo-β-dehydroionol and compound 3-oxo-α-ionone from Albizia richardiana (Voigt.) King & Prain. Environ. Technol. Innov. 23, 101779 (2021). [Google Scholar]
- 56) K. Hossen, Y. Asato, T. Teruya and H. Kato-Noguchi: Identification of four allelopathic compounds including a novel compound from Elaeocarpus floribundus Blume and determination of their allelopathic activity. J. Environ. Manage. 326(Pt B), 116728 (2023). [DOI] [PubMed] [Google Scholar]
- 57) J. M. Blime: The role of bryophytes in temperate forest ecosystems. Hikobia 13, 267–289 (2001). [Google Scholar]
- 58) H. Kato-Noguchi, T. Seki and H. Shigemori: Allelopathy and allelopathic substance in the moss Rhynchostegium pallidifolium. J. Plant Physiol. 167, 468–471 (2010). [DOI] [PubMed] [Google Scholar]
- 59) H. Kato-Noguchi: Involvement of allelopathy in the formation of monospecific colonies of ferns. Nat. Prod. Commun. 10, 811–814 (2015). [PubMed] [Google Scholar]
- 60) H. Kato-Noguchi: Allelopathic chemical interaction of bryophytes with vascular plants. Mini Rev. Org. Chem. 13, 422–429 (2016). [Google Scholar]
- 61) H. Ando and A. Matsuo: Applied bryology. In “Advance in Bryology, Vol. 2,” ed., by W. Schultze-Motel, International Association of Bryologists, Vaduz, Liechtenstein, pp. 133–224, 1984.
- 62) H. Tsubota, A. Kuroda, H. Masuzaki, M. Nakahara and H. Deguchi: Preliminary study on allelopathic activity of bryophytes under laboratory conditions using the sandwich method. J. Hattori Bot. Lab. 100, 517–525 (2006). [Google Scholar]
- 63) H. Kato-Noguchi, K. Kobayashi and H. Shigemori: Allelopathy of the moss Hypnum plumaeforme by the production of momilactone A and B. Weed Res. 49, 621–627 (2009). [Google Scholar]
- 64) H. Kato-Noguchi, T. Ino, N. Sata and S. Yamamura: Isolation and identification of a potent allelopathic substance in rice root exudates. Physiol. Plant. 115, 401–405 (2002). [DOI] [PubMed] [Google Scholar]
- 65) H. Kato-Noguchi: Allelopathic substance in rice root exudates: Rediscovery of momilactone B as an allelochemical. J. Plant Physiol. 161, 271–276 (2004). [DOI] [PubMed] [Google Scholar]
- 66) H. Kato-Noguchi and R. J. Peters: The role of momilactones in rice allelopathy. J. Chem. Ecol. 39, 175–185 (2013). [DOI] [PubMed] [Google Scholar]
- 67) H. Kato-Noguchi: Defensive molecules momilactones A and B: Function, biosynthesis, induction and occurrence. Toxins (Basel) 15, 241 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68) J. Zhang and R. J. Peters: Why are momilactones always associated with biosynthetic gene clusters in plants? Proc. Natl. Acad. Sci. U.S.A. 117, 13867–13869 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69) R. Li, J. Zhang, Z. Li, R. J. Peters and B. Yang: Dissecting the labdane-related diterpenoid biosynthetic gene clusters in rice reveals directional cross-cluster phytotoxicity. New Phytol. 233, 878–889 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70) H. Kato-Noguchi and K. Kobayashi: Jasmonic acid, protein phosphatase inhibitor, metals and UV-irradiation increased momilactone A and B concentrations in the moss Hypnum plumaeforme. J. Plant Physiol. 166, 1118–1122 (2009). [DOI] [PubMed] [Google Scholar]
- 71) L. Mao, H. Kawaide, T. Higuchi, M. Chen, K. Miyamoto, Y. Hirata, H. Kimura, S. Miyazaki, M. Teruya, F. Fujiwara, K. Tomita, H. Yamane, K. Hayashi, H. Nojiri, L. Jia, J. Qiu, C. Ye, M. P. Timko, L. Fan and K. Okada: Genomic evidence for convergent evolution of gene clusters for momilactone biosynthesis in land plants. Proc. Natl. Acad. Sci. U.S.A. 117, 12472–12480 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72) H. Kato-Noguchi: Convergent or parallel molecular evolution of momilactone A and B: Potent allelochemicals, momilactones have been found only in rice and the moss Hypnum plumaeforme. J. Plant Physiol. 168, 1511–1516 (2011). [DOI] [PubMed] [Google Scholar]
- 73) A. E. Russell, J. W. Raich and P. M. Vitousek: The ecology of the climbing fern Dicranopteris linearis on windward Mauna Loa, Hawaii. J. Ecol. 86, 765–779 (1998). [Google Scholar]
- 74) H. Kato-Noguchi, Y. Saito and K. Suenaga: Involvement of allelopathy in the establishment of pure colony of Dicranopteris linearis. Plant Ecol. 213, 1937–1944 (2012). [Google Scholar]
- 75) K. Shiojima, M. Suzuki, H. Aoki and H. Ageta: Fern constitutes: four new diterpenoid glycosides from fresh leaves of Gleichenia japonica. Chem. Pharm. Bull. 43, 5–8 (1995). [Google Scholar]
- 76) A. Kupoda, S. Mukai and G. Toyohara: Floristic composition and community structure of dense undergrowth vegetation formed by evergreen perennial ferns, Dicranopteris linearis and Gleichenia japonica (Gleicheniaceae). Veg. Sci. 23, 25–36 (2006). [Google Scholar]
- 77) H. Kato-Noguchi, Y. Saito, O. Ohno and K. Suenaga: Allelopathy is involved in the formation of pure colonies of the fern Gleichenia japonica. J. Plant Physiol. 170, 577–582 (2013). [DOI] [PubMed] [Google Scholar]
- 78) H. Kato-Noguchi, Y. Saito, O. Ohno and K. Suenaga: A phytotoxic active substance in the decomposing litter of the fern Gleichenia japonica. J. Plant Physiol. 176, 55–60 (2015). [DOI] [PubMed] [Google Scholar]
- 79) R. N. Mandal, R. Bar and P. P. Chakrabarti: ‘Pati bet,’ Schumannianthus dichotomus (Roxb.) Gagnep.—A raw material for preparation of livelihood supporting handicrafts. Indian J. Nat. Prod. Resour. 5, 365–370 (2014). [Google Scholar]
- 80) M. M. Rob, K. Ozaki, T. Teruya and H. Kato-Noguchi: Schumannione, a new butenolide derivative isolated from Schumannianthus dichotomus as a potential phytotoxic agent. Tetrahedron Lett. 61, 152168 (2020). [Google Scholar]
- 81) M. M. Rob, K. Hossen, A. Iwasaki, K. Suenaga and H. Kato-Noguchi: Phytotoxic activity and identification of phytotoxic substances from Schumannianthus dichotomus. Plants 9, 102 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82) H. Kato-Noguchi: Bioactive compounds involved in the formation of the sparse understory vegetation in pine forests. Curr. Org. Chem. 25, 1731–1738 (2021). [Google Scholar]
- 83) Y. Fujii: “Allelopathy, Mode of Action and Utilization of Allelochemicals,” Rural Culture Association Japan, Tokyo, 2000. (in Japanese).
- 84) H. Kato-Noguchi, F. Kimura, O. Ohno and K. Suenaga: Involvement of allelopathy in inhibition of understory growth in red pine forests. J. Plant Physiol. 218, 66–73 (2017). [DOI] [PubMed] [Google Scholar]
- 85) W. M. Lonsdale: Predicting the amount of litterfall in forests of the world. Ann. Bot. 61, 319–324 (1988). [Google Scholar]
- 86) S. Fujihara and T. Shimizu: Growth inhibitory effect of peel extract from Citrus junos. Plant Growth Regul. 39, 223–233 (2003). [Google Scholar]
- 87) S. Fujihara, T. Shimizu and H. Kato-Noguchi: Yuzu peel contains a substance for effective weed suppression. J. Agric. Sci. 59, 414 (2004) (in Japanese). [Google Scholar]
- 88) H. Kato-Noguchi and Y. Tanaka: Allelopathic potential of Citrus junos fruit waste from food processing industry. Bioresour. Technol. 94, 211–214 (2004). [DOI] [PubMed] [Google Scholar]
- 89) H. Kato-Noguchi and Y. Tanaka: Potential of Ctrus junos fruit waste from the food processing industry for weed management. HortScience 41, 1516–1517 (2006). [Google Scholar]
- 90) H. Kato-Noguchi, Y. Tanaka, T. Murakami, S. Yamamura and S. Fujihara: Isolation and identification of an allelopathic substance from peel of Citrus junos. Phytochemistry 61, 849–853 (2002). [DOI] [PubMed] [Google Scholar]
- 91) H. Kato-Noguchi and Y. Tanaka: Effect of ABA-β-D-glucopyranosyl ester and activity of ABA-β-D-glucosidase in Arabidopsis thaliana. J. Plant Physiol. 165, 788–790 (2008). [DOI] [PubMed] [Google Scholar]
- 92) H. Kato-Noguchi and Y. Tanaka: Growth inhibitory activity of ABA-β-D-glucopyranosyl ester and ABA-β-D-glucosidase. Acta Physiol. Plant. 31, 407–409 (2009). [Google Scholar]
- 93) W. Hartung, A. Sauter, N. C. Turner, I. Fillery and H. Heilmeier: Abscisic acid in soils: What is its function and which factors and mechanisms influence its concentration? Plant Soil 184, 105–110 (1996). [Google Scholar]
- 94) R. M. Callaway and E. T. Aschehoug: Invasive plants versus their new and old neighbors: A mechanism for exotic invasion. Science 290, 521–523 (2000). [DOI] [PubMed] [Google Scholar]
- 95) N. Cappuccino and J. T. Arnason: Novel chemistry of invasive exotic plants. Biol. Lett. 2, 189–193 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96) S. J. Meiners, C. H. Kong, L. M. Ladwig, N. L. Pisula and K. A. Lang: Developing an ecological context for allelopathy. Plant Ecol. 213, 1861–1867 (2012). [Google Scholar]
- 97) W. Chengxu, Z. Mingxing, C. Xuhui and Q. Bo: Review on allelopathy of exotic invasive plants. Procedia Eng. 18, 240–246 (2011). [Google Scholar]
- 98) H. Kato-Noguchi and D. Kurniadie: Allelopathy of Lantana camara as an invasive plant. Plants 10, 1028 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99) H. Kato-Noguchi: The impact and invasive mechanisms of Pueraria montana var. lobata, one of the world’s worst alien species. Plants 12, 3066 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100) Global Invasive Species Database: Pueraria montana var. lobata. http://www.iucngisd.org/gisd/speciesname/Pueraria+montana+var.+lobata (Accessed on 14 August 2023)
- 101) H. Kato-Noguchi: Allelopathic potential of Pueraria thunbergiana. Biol. Plant. 46, 471–473 (2003). [Google Scholar]
- 102) H. Kato-Noguchi: Allelopathic substances in Pueraria thunbergiana. Phytochemistry 63, 577–580 (2003). [DOI] [PubMed] [Google Scholar]
- 103) A. K. Cowan and G. R. Richardson: Carotenogenic and abscisic acid biosynthesizing activity in a cell-free system. Physiol. Plant. 99, 371–378 (1997). [Google Scholar]
- 104) M. Seo and T. Koshiba: Complex regulation of ABA biosynthesis in plants. Trends Plant Sci. 7, 41–48 (2002). [DOI] [PubMed] [Google Scholar]
- 105) K. P. Jia, J. Mi, S. Ali, H. Ohyanagi, J. C. Moreno, A. Ablazov, A. Balakrishna, L. Berqdar, A. Fiore, G. Diretto, C. Martínez, A. R. de Lera, T. Gojobori and S. Al-Babili: An alternative, zeaxanthin epoxidase-independent abscisic acid biosynthetic pathway in plants. Mol. Plant 15, 151–166 (2022). [DOI] [PubMed] [Google Scholar]
- 106) H. Kato-Noguchi: Allelopathy and allelochemicals of Imperata cylindrica as an invasive plant species. Plants 11, 2551 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107) Global Invasive Species Database: Species profile: Imperata cylindrica. http://www.iucngisd.org/gisd/speciesname/Imperata+cylindrica (Accessed on 14 August 2023)
- 108) M. Suzuki, T. Tominaga, O. Ohno, A. Iwasaki, K. Suenaga and H. Kato-Noguchi: Plant growth inhibitory activity and active substances with allelopathic potential of cogongrass (Imperata cylindrica) rhizome. Weed Biol. Manage. 18, 92–98 (2018). [Google Scholar]
- 109) C. A. B. I. Compendium: Persicaria chinensis (Chinese knotweed) https://www.cabidigitallibrary.org/doi/full/10.1079/cabicompendium.118915 (Accessed on 14 August 2023)
- 110) United State Department of Agriculture: Weed risk assessment for Persicaria chinensis (L). H. Gross (Polygonaceae) Chinese knotweed. https://www.aphis.usda.gov/plant_health/plant_pest_info/weeds/downloads/wra/Persicaria_chinensis_WRA.pdf (Accessed on 14 August 2023)
- 111) T. L. Lun, A. Iwasaki, K. Suenaga and H. Kato-Noguchi: Isolation and identification of plant-growth inhibitory constituents from Polygonum chinense Linn and evaluation of their bioherbicidal potential. Plants 12, 1577 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112) Global Invasive Species Database: Tithonia diversifolia. http://www.iucngisd.org/gisd/speciesname/Tithonia+diversifolia (Accessed on 14 August 2023)
- 113) H. Kato-Noguchi: Involvement of allelopathy in the invasive potential of Tithonia diversifolia. Plants 9, 766 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114) M. Suzuki, A. Iwasaki, K. Suenaga and H. Kato-Noguchi: Phytotoxic property of the invasive plant Tithonia diversifolia and a phytotoxic substance. Acta Biol. Hung. 68, 187–195 (2017). [DOI] [PubMed] [Google Scholar]
- 115) Y. Matsubara, T. Okada and A. S. M. Nahiyan: Tolerance to allelopathy and fusarium disease, changes in antioxidative substances in mycorrhizal asparagus plants raised in decline soil. Acta Hortic., 417–424 (2010). [Google Scholar]
- 116) R. Yeasmin, K. Nakamatsu, H. Matsumoto, S. Motoki, E. Nishihara and S. Yamamoto: Inference of allelopathy and autotoxicity to varietal resistance of asparagus (Asparagus officinalis L.). Aust. J. Crop Sci. 8, 251–256 (2014). [Google Scholar]
- 117) E. Yergeau, V. Vujanovic and M. St-Arnaud: Changes in communities of Fusarium and arbuscular mycorrhizal fungi as related to different asparagus cultural factors. Microb. Ecol. 52, 104–113 (2006). [DOI] [PubMed] [Google Scholar]
- 118) M. Asaduzzaman, M. F. Mondal, T. Ban and T. Asao: Selection of ideal succeeding crops after asparagus, taro and beans replanting field in seedling growth bioassay. Allelopathy J. 32, 1–22 (2013). [Google Scholar]
- 119) W. H. Elmer and J. J. Pignatello: Effect of biochar amendments on mycorrhizal associations and Fusarium crown and root rot of asparagus in replant soils. Plant Dis. 95, 960–966 (2011). [DOI] [PubMed] [Google Scholar]
- 120) L. Molinero-Ruiz, E. Rubio-Pérez, E. González-Domínguez and M. J. Basallote-Ureba: Alternative hosts for Fusarium spp. causing crown and root rot of asparagus in Spain. J. Phytopathol. 159, 114–116 (2011). [Google Scholar]
- 121) H. Kato-Noguchi, K. Nakamura and N. Okuda: Involvement of an autotoxic compound in asparagus decline. J. Plant Physiol. 224-225, 49–55 (2018). [DOI] [PubMed] [Google Scholar]
- 122) W. S. Wong, D. Guo, X. L. Wang, Z. Q. Yin, B. Xia and N. Li: Study of cis-cinnamic acid in Arabidopsis thaliana. Plant Physiol. Biochem. 43, 929–937 (2005). [DOI] [PubMed] [Google Scholar]
- 123) W. Steenackers, P. Klíma, M. Quareshy, I. Cesarino, R. P. Kumpf, S. Corneillie, P. Araújo, T. Viaene, G. Goeminne, M. K. Nowack, K. Ljung, J. Friml, J. J. Blakeslee, O. Novák, E. Zažímalová, R. Napier, W. Boerjan and B. Vanholme: cis-Cinnamic acid is a novel, natural auxin efflux inhibitor that promotes lateral root formation. Plant Physiol. 173, 552–565 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124) K. I. Fujita and I. Kubo: Synergism of polygodial and trans-cinnamic acid on inhibition of root elongation in lettuce seedling growth bioassays. J. Chem. Ecol. 29, 2253–2262 (2003). [DOI] [PubMed] [Google Scholar]
- 125) F. Mizutani, H. Itamura, A. Sugiura and T. Tomana: Studies on the soil sickness problem for peach trees. II. Condensed tannins as growth inhibitors from peach roots. J. Jpn. Soc. Hortic. Sci. 48, 279–287 (1979) (in Japanese). [Google Scholar]
- 126) J. L. Henfrey, G. Baab and M. Schmitz: Physiological stress responses in apple under replant conditions. Sci. Hortic. (Amsterdam) 194, 111–117 (2015). [Google Scholar]
- 127) S. Okada, A. Iwasaki, I. Kataoka, K. Suenaga and H. Kato-Noguchi: Phytotoxic activity of kiwifruit leaves and isolation of a phytotoxic substance. Sci. Hortic. (Amsterdam) 250, 243–248 (2019). [Google Scholar]
- 128) H. M. Niemeyer: Hydroxamic acids (4-hydroxy-1,4-benzoxazin-3-ones), defense chemicals in the Gramineae. Phytochemistry 27, 3349–3358 (1988). [Google Scholar]
- 129) M. Frey, P. Chomet, E. Glawischnig, C. Stettner, S. Grün, A. Winklmair, W. Eisenreich, A. Bacher, R. B. Meeley, S. P. Briggs, K. Simcox and A. Gierl: Analysis of a chemical plant defense mechanism in grasses. Science 277, 696–699 (1997). [DOI] [PubMed] [Google Scholar]
- 130) H. R. Bravo and S. V. Copaja: Contents and morphological distribution of 2,4-dihydroxy-1,4-benzoxazin-3-one and 2-benzoxazolinone in Acanthus mollis in relation to protection from larvae of Pseudaletia impuncta. Ann. Appl. Biol. 140, 129–132 (2002). [Google Scholar]
- 131) A. E. Glenn, S. E. Gold and C. W. Bacon: Fdb1 and Fdb2, Fusarium verticillioides loci necessary for detoxification of preformed antimicrobials from cone. Mol. Plant Microbe Interact. 15, 91–101 (2002). [DOI] [PubMed] [Google Scholar]
- 132) Inderjit and S. O. Duke: Ecophysiological aspects of Allelopathy. Planta 217, 529–539 (2003). [DOI] [PubMed] [Google Scholar]
- 133) R. G. Belz and K. Hurle: A novel laboratory screening bioassay for crop seedling allelopathy. J. Chem. Ecol. 30, 175–198 (2004). [DOI] [PubMed] [Google Scholar]
- 134) H. Kato-Noguchi, S. Kosemura and S. Yamamura: Allelopathic potential of 5-chloro-6-methoxy-2-benzoxazolinone. Phytochemistry 48, 433–435 (1998). [Google Scholar]
- 135) H. Kato-Noguchi: Allelopathy in Maize II.: Allelopathic potential of a new benzoxazolinone, 5-chloro-6-methoxy-2-benzoxazolinone and its analogue. Plant Prod. Sci. 3, 47–50 (2000). [Google Scholar]
- 136) F. A. Macías, A. Oliveros-Bastidas, D. Marín, D. Castellano, A. M. Simonet and J. M. G. Molinillo: Degradation studies on benzoxazinoids. soil degradation dynamics of 2,4-dhydroxy-7-methoxy-(2H)-1,4-benzoxazin-3(4H)-one (DIMBOA) and its degradation products, phytotoxic allelochemicals from Gramineae. J. Agric. Food Chem. 52, 6402–6413 (2004). [DOI] [PubMed] [Google Scholar]
- 137) F. A. Macías, D. Marín, A. Oliveros-Bastidas, D. Castellano, A. M. Simonet and J. M. G. Molinillo: Structure–activity relationships (SAR) studies of benzoxazinoids, their degradation products and analogues. Phytotoxicity on standard target species (STS). J. Agric. Food Chem. 53, 538–548 (2005). [DOI] [PubMed] [Google Scholar]
- 138) Y. Hashimoto and K. Shudo: Chemistry of biologically active benzoxazinoids. Phytochemisrty 43, 551–559 (1996). [DOI] [PubMed] [Google Scholar]
- 139) H. Kato-Noguchi and F. A. Macías: Possible mechanism of inhibition of 6-methoxy-benzoxazolin-2(3H)-one on germination of cress (Lepidium sativum L.). J. Chem. Ecol. 32, 1101–1109 (2006). [DOI] [PubMed] [Google Scholar]
- 140) H. Kato-Noguchi and F. A. Macías: Effects of 6-methoxy-2-benzoxazolinone on the germination and α-amylase activity in lettuce seeds. J. Plant Physiol. 162, 1304–1307 (2005). [DOI] [PubMed] [Google Scholar]
- 141) H. Kato-Noguchi: Effects of four benzoxazinoids on gibberellin-induced α-amylase activity in barley seeds. J. Plant Physiol. 165, 1889–1894 (2008). [DOI] [PubMed] [Google Scholar]
- 142) H. Kato-Noguchi and F. A. Macías: Inhibition of germination and α-amylase induction by 6-methoxy-2-benzoxazolinone in twelve plant species. Biol. Plant. 52, 351–354 (2008). [Google Scholar]
- 143) Y. Ozaki and H. Kato-Noguchi: Effects of benzoxazinoids in wheat residues may inhibit the germination, growth and gibberellin-induced α-amylase activity in rice. Acta Physiol. Plant. 38, 24 (2016). [Google Scholar]
- 144) P. Perata, L. Guglielminetti and A. Alpi: Mobilization of endosperm reserves in cereal seeds under anoxia. Ann. Bot. 79(Suppl), 49–56 (1997). [Google Scholar]
- 145) B. B. Vartapetian and M. B. Jackson: Plant adaptations to anaerobic stress. Ann. Bot. 79(Suppl), 3–20 (1997). [Google Scholar]
- 146) H. Kato-Noguchi, F. A. Macías and J. M. G. Molinillo: Structure–activity relationship of benzoxazinones and related compounds with respect to the growth inhibition and α-amylase activity in cress seedlings. J. Plant Physiol. 167, 1221–1225 (2010). [DOI] [PubMed] [Google Scholar]