Abstract
Background
Digital interventions provided through smartphones or the internet that are guided by a coach have been proposed as promising solutions to support the self-management of chronic conditions. However, digital intervention for poststroke self-management is limited; we developed the interactive Self-Management Augmented by Rehabilitation Technologies (iSMART) intervention to address this gap.
Objective
This study aimed to examine the feasibility and initial effects of the iSMART intervention to improve self-management self-efficacy in people with stroke.
Methods
A parallel, 2-arm, nonblinded, randomized controlled trial of 12-week duration was conducted. A total of 24 participants with mild-to-moderate chronic stroke were randomized to receive either the iSMART intervention or a manual of stroke rehabilitation (attention control). iSMART was a coach-guided, technology-supported self-management intervention designed to support people managing chronic conditions and maintaining active participation in daily life after stroke. Feasibility measures included retention and engagement rates in the iSMART group. For both the iSMART intervention and active control groups, we used the Feasibility of Intervention Measure, Acceptability of Intervention Measure, and Intervention Appropriateness Measure to assess the feasibility, acceptability, and appropriateness, respectively. Health measures included the Participation Strategies Self-Efficacy Scale and the Patient-Reported Outcomes Measurement Information System’s Self-Efficacy for Managing Chronic Conditions.
Results
The retention rate was 82% (9/11), and the engagement (SMS text message response) rate was 78% for the iSMART group. Mean scores of the Feasibility of Intervention Measure, Acceptability of Intervention Measure, and Intervention Appropriateness Measure were 4.11 (SD 0.61), 4.44 (SD 0.73), and 4.36 (SD 0.70), respectively, which exceeded our benchmark (4 out of 5), suggesting high feasibility, acceptability, and appropriateness of iSMART. The iSMART group showed moderate-to-large effects in improving self-efficacy in managing emotions (r=0.494), symptoms (r=0.514), daily activities (r=0.593), and treatments and medications (r=0.870), but the control group showed negligible-to-small effects in decreasing self-efficacy in managing emotions (r=0.252), symptoms (r=0.262), daily activities (r=0.136), and treatments and medications (r=0.049). In addition, the iSMART group showed moderate-to-large effects of increasing the use of participation strategies for management in the home (r=0.554), work (r=0.633), community (r=0.673), and communication activities (r=0.476). In contrast, the control group showed small-to-large effects of decreasing the use of participation strategies for management in the home (r=0.567), work (r=0.342, community (r=0.215), and communication activities (r=0.379).
Conclusions
Our findings support the idea that iSMART was feasible to improve poststroke self-management self-efficacy. Our results also support using a low-cost solution, such as SMS text messaging, to supplement traditional therapeutic patient education interventions. Further evaluation with a larger sample of participants is still needed.
Trial Registration
ClinicalTrials.gov 202004137; https://clinicaltrials.gov/study/NCT04743037?id=202004137&rank=1
Keywords: digital intervention, feasibility, mobile health, participation, rehabilitation, self-efficacy, self-management, stroke, technology, telehealth, telemedicine, text messaging
Introduction
People receive limited inpatient rehabilitation services after a stroke, with an average rehabilitation stay of 18.6 days [1]. Those with no major motor impairments (eg, neurologically mild stroke) are often discharged from acute care without rehabilitation [2,3]. Stroke survivors are at risk for developing depression [4], experiencing reduced quality of life [5], and having an increased chance of stroke recurrence [6,7]. Moreover, restricted participation in home, community, work, and social activities following stroke is common [8,9] and can last over 6 months [10]. Stroke survivors often manifest chronic neuropsychiatric symptoms (eg, fatigue, depressed mood, and cognitive dysfunction), which can impact their stroke recovery and delay or prevent a return to prestroke social roles [11]. Thus, learning strategies to manage poststroke symptoms and cope with challenges after transitioning back to community living is essential in stroke rehabilitation [9]. Self-management programs, also known as therapeutic patient education interventions [12], could help stroke survivors improve health management and participation in home, work, and community activities [11,13]. Most stroke self-management programs use a self-efficacy–building approach to promote and maintain active participation in home and community activities poststroke [14]. Improving self-efficacy to manage symptoms and chronic conditions ultimately leads to enhanced participation [11,13]. A systematic review of 22 studies (N=1761) investigated the influence of interventions supporting self-management skills on poststroke outcomes. Given the heterogeneity of the findings, no meta-analysis was conducted. However, the results showed that self-management interventions based on self-efficacy principles could improve the quality of life, depression, daily activities, and physical functioning in stroke survivors [15]. Targeting self-efficacy in managing symptoms and behaviors becomes a critical behavioral approach to addressing the long-term consequences of stroke [15,16].
Self-management interventions are well suited to mobile health (mHealth) technologies [17,18] as mHealth delivery methods offer several advantages, including increased access for individuals who live in rural areas or have limited transportation options. Additionally, mHealth technologies provide the potential for real-time monitoring and feedback, the ability to tailor intervention components to individualized needs, and the ability to reduce administration costs [19,20]. A meta-analysis of 14 randomized controlled trials (N=1597) focused on examining what theories were applied to the development of technology-based self-management interventions and investigating their effectiveness in improving depression, anxiety, fatigue, and self-efficacy for people with neurological disorders. The results showed that cognitive-behavioral and social-cognitive theories are the 2 most common theories used to develop technology-based self-management interventions in individuals with neurological disorders. In addition, cognitive-behavioral theory–based interventions were effective in enhancing self-efficacy and reducing depression, anxiety, and fatigue. In contrast, social-cognitive theory–based interventions were effective in reducing depression only [21]. In particular, this review found large effects in enhancing self-efficacy and reducing anxiety and moderate effects in reducing depression and fatigue. Although this meta-analysis showed promising results for neurological disorders, the study populations in these 16 studies did not include people after a stroke. Thus, research is needed to verify that this evidence applies to people after a stroke. To harness the benefits of the mHealth delivery, we developed a technology-supported self-management intervention, the interactive Self-Management Augmented Rehabilitation Technologies (iSMART) intervention, adapted from the face-to-face, stroke-focused psychoeducation program Improving Participation after Stroke Self-Management (IPASS) [11,13]. iSMART simplified the original IPASS psychoeducation sessions and added text messaging and behavioral coaching components [22]. We integrated SMS text messaging into iSMART because it is easily customized to individual needs and accessible to anyone with a cell phone [23,24]. Live health coaches, based on behavioral activation [25], supplement psychoeducation sessions to support intervention uptake and promote effective collaboration, negotiation, and motivation while encouraging individuals to take responsibility for their recovery and wellness by fostering healthy behaviors [26].
To test this novel intervention’s feasibility and potential benefits, this study aimed to (1) evaluate the acceptability, appropriateness, and feasibility of iSMART in individuals with stroke and (2) establish the preliminary effect size of iSMART in improving self-management self-efficacy in individuals after stroke. We hypothesized that (1) iSMART would be feasible to deliver and be acceptable to people with stroke and (2) iSMART would result in a moderate effect for improving poststroke self-management self-efficacy.
Methods
Design and Recruitment
We conducted a parallel, 2-arm, nonblinded, randomized controlled trial of 12-week duration. Participants were recruited from a stroke registry at a university-affiliated acute care hospital between January and March 2021. Using a random number generator guided by a biostatistician, participants were randomized to receive either the iSMART intervention or a manual of stroke rehabilitation (attention control). All participants in both groups continued receiving standard-of-care rehabilitation services their treating physicians recommended.
Participants and Randomization
Potential participants (N=31) were recruited between January 2021 and March 2021 based on the following inclusion and exclusion criteria. Inclusion criteria were (1) mild-to-moderate stroke (National Institutes of Health Stroke Scale scores ≤13) [27], (2) ischemic or hemorrhagic stroke, (3) aged 18 years or older, (4) English-speaking, (5) ≥3 months after stroke, (6) self-identified as having ≥1 chronic condition, and (7) mobile phone ownership. Exclusion criteria were (1) preexisting neurologic or psychiatric disorder (eg, dementia or schizophrenia), (2) severe poststroke cognitive impairment (Short Blessed Test score ≥9), (3) history of functional problems (Premorbid Modified Ranking Scale score ≥2) before the stroke, (4) severe aphasia (Boston Naming Test <10) [28], and (5) visual problems that make reading words on the device difficult. Of the screened individuals who had a stroke, 24 were randomized (CONSORT [Consolidated Standards of Reporting Trials] diagram; Figure 1).
Figure 1.
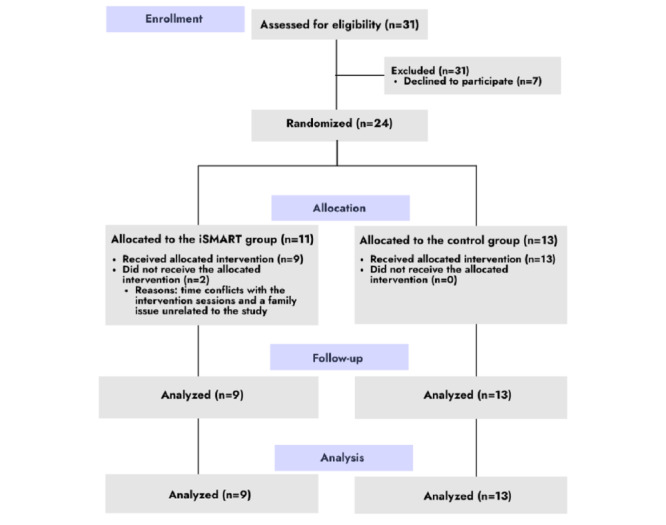
CONSORT (Consolidated Standards of Reporting Trials) flow diagram showing participant recruitment and completion. iSMART: interactive Self-Management Augmented by Rehabilitation Technologies.
Procedures
Overview
This study was a remote clinical trial, that is, a clinical trial performed remotely, including the interaction between the experimenter and participant and the assessment of outcomes [29]. Study staff contacted potential participants from a stroke research registry at a university-affiliated hospital in the Midwestern United States to explore their interest in the study. After that, study staff sent participants a secure link through email or SMS through the REDCap (Research Electronic Data Capture; Vanderbilt University) [30] and scheduled video or phone sessions to assist participants in completing the consent form and screening test for eligibility. Eligible participants were randomly allocated to the iSMART or control groups using a random sequence computer-generated program to ensure allocation concealment. Neither study staff nor participants were masked for randomization assignments. Following consent, participants underwent a remote enrollment, at which iSMART participants were oriented to technologies used in the study (ie, the videoconferencing platform and SMS) by study staff. Study staff also obtained the phone’s operating system (Android or iOS) and linked the phone number to the web-based iSMART platform used to send and receive text messages from participants. Participants in both groups started their allocated interventions after all participants completed baseline testing. The intervention lasted for 3 months. After completing their allocated interventions, all participants completed a postintervention assessment. Participants in both groups continued to receive health services as prescribed by their clinicians. Participants in the iSMART group were compensated US $300 for completing the allocated intervention and outcome measures and data plan coverage. Participants in the control group were compensated US $120 for completing the allocated intervention and outcome measures. No messages were sent to participants in the control arm, so they were not compensated for data usage. The trial ended in June 2021.
The iSMART Intervention
The iSMART was a 12-week, technology-supported, coach-guided, self-management intervention comprising 3 components: psychoeducation, behavioral coaching, and text messaging. A licensed occupational therapist served as the coach in this study. The psychoeducation component was built upon the Social Cognitive Theory [31] and the person-environment-occupation-performance model [32] and implemented through weekly, 2.5-hour sessions in a group videoconferencing format. These sessions focused on teaching participants self-management strategies, including problem-solving, decision-making, positive thinking, communication, and accommodation, for managing symptoms and supporting participation in home, work, community, and social activities.
The coaching component was built on behavioral activation theory and modified from the Revised Treatment Manual of the Brief Behavioral Activation Treatment for Depression [25]. It was implemented weekly in 0.5-hour sessions in a one-to-one videoconferencing format. Individual coaching sessions engaged participants in collaborative goal setting with the coach to identify values and select personal activity goals from 25 predefined goals. The coach then entered the selected goals into the web-based iSMART platform so participants could receive messages customized to their chosen goals. These goals target improving participation in different life areas, including daily responsibilities, relationships, interests and recreation, education and career, and mind, body, and spirituality derived from the behavioral activation manual [25].
The text messaging component was adapted from previous studies, with effectiveness demonstrated in hospital workers [33,34] and adults with severe mental illness [35]. We adapted and pretested text messages with the planning group members, intending to increase the uptake by individuals with stroke (details in the next paragraph). Text messages were sent following the predefined schedules, including goal reminders (delivered on Mondays), goal monitoring (Tuesdays), mood monitoring (daily), self-management tips (Thursdays to Saturdays), ecological needs assessment (Saturdays), and motivational messages (Sundays). Figure 2 provides snapshots of these messages.
Figure 2.
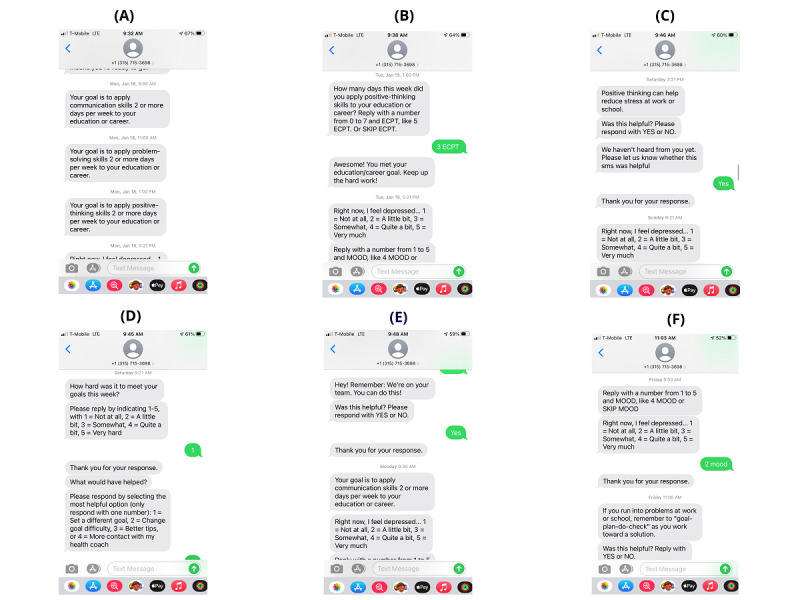
Screenshots of different types of messages. (A) goal reminder, (B) goal monitoring, (C) self-management tip, (D) ecological needs assessment, (E) general motivation, and (F) mood monitoring.
We formed a planning group, including 2 stroke rehabilitation clinicians, a stroke survivor, a technologist, and a self-management expert, to guide the intervention adaptation using a systematic intervention-mapping process [22,36]. During this adaptation process, we applied the behavior change wheel [37] and behavioral change technique taxonomy [38] to specify strategies that help individuals change self-management behaviors. Specifically, we identified 7 behavioral determinants most likely to affect the intervention goal and outcomes, including knowledge, behavioral regulation, skills, self-efficacy, motivation, negative and positive affect, and social and environmental support. We also identified the mechanisms of action (eg, beliefs about capabilities, values, knowledge, and motivation) most likely to affect the selected behavioral determinants. We then used the linkage table published by Carey et al [39] to match the behavioral change techniques (eg, information about health consequences, information about social and environmental consequences, instructions on how to perform the behavior, and feedback on behavior) to each of the mechanisms of action. Finally, to ensure iSMART should be applied to the selected behavioral change techniques, we developed a set of empirically supported strategies and integrated these strategies into different parts of the 3 treatment components. Details of the intervention development of iSMART, including the theoretical framework, mechanisms of action, behavioral change techniques, and the set of empirically supported strategies, are described elsewhere [22].
Control Intervention
Participants in the control group received a study-specific manual comprising stroke-specific information based on resources from the American Stroke Association and the Canadian Stroke Association. Manual content includes stroke overview, stroke prevention, rehabilitation, fatigue, weight management, fitness, medication, sleep, balance, healthy eating, emotional changes, social support, home modifications, and return to work or school. This study staff made telephone calls once a week to ask if participants had any problems while reading the manual and encouraged participants to read through the manual. The study staff did not deliver any iSMART content.
Outcome Measures
Feasibility Measures
Rates of retention and engagement were automatically recorded through the web-based iSMART platform. We defined retention as the rate at which participants completed or remained in the study and engagement as the rate at which participants responded to text messages. We defined retention and engagement rates as ≥80%, based on a previous technology intervention that showed participants who achieved these criteria demonstrated better outcomes [35]. The project found that participants who met the criteria would demonstrate better target health outcomes. Participants also completed three 4-item implementation measures postintervention: the Feasibility of Intervention Measure (FIM), the Acceptability of Intervention Measure (AIM), and the Intervention Appropriateness Measure (IAM) [40]. Weiner et al [40] found that these measures had strong structural validity with .89 for FIM, .85 for AIM, and 0.91 for IAM and test-retest reliability with .88 for FIM, .83 for AIM, and .87 for IAM. However, no discriminant validity of these measures was studied [40]. We defined the benchmark for high feasibility, acceptability, and appropriateness as the mean score of 4 (out of 5) on the FIM, AIM, and IAM.
Self-Efficacy Measures
Participants completed the Participation Strategies Self-Efficacy Scale (PS-SES) and the Patient-Reported Outcomes Measurement Information System’s Self-Efficacy (PROMIS-SE) for managing chronic conditions at baseline and postintervention. PS-SES is a 35-item measure to assess self-efficacy in using participation strategies to manage home, work, community, and communication [41]. Lee et al [41] found that the Cronbach α coefficients of internal consistency of PE-SES were high (α=.884 to .926).
PROMIS-SE consists of five 4-item short forms to assess self-efficacy for managing daily activities, medications, treatment, symptoms, emotions, and social interactions [42]. Confirmatory factor analyses confirmed the multidimensional structure of the PROMIS-SE.
Data Analysis
Participants who completed the intervention were selected for data analyses, as we did not compute any missing values of outcomes for those who did not complete the study. Demographic characteristics between the 2 groups were evaluated using Fisher exact tests or Wilcoxon rank sum tests. Considering the small sample size of this study, we computed nonparametric analyses with median scores of FIM, AIM, and IAM and self-efficacy measures. We reported both mean and median scores for resolution purposes.
We compared retention and engagement rates and the FIM, AIM, and IAM scores of the iSMART intervention with the predefined benchmarks. We conducted Wilcoxon rank sum tests to evaluate any differences between the groups on FIM, AIM, and IAM scores. To establish the effect sizes for change in self-efficacy, we computed change scores from baseline to postintervention. We then compared the change scores between the 2 groups using Wilcoxon rank sum tests. Due to the small size, any demographic differences between groups at baseline may have artificially inflated the group difference in study outcomes. Thus, we also examined any significant changes for each group using Wilcoxon signed rank tests. We used effect sizes to interpret the intervention effect instead of statistical significance (ie, P≤.05) [43]. We defined small effects if 0.1≤r<0.3, moderate effects if 0.3≤r<0.5, and large effects if r≥0.5 [44]. We reported effect sizes as they were independent of sample size so that we could express the size of an intervention effect regardless of the size of the study [45].
Ethical Considerations
All participants provided informed consent. The ethics committees of Washington University (202004137) and Northwestern University (STU00215743) reviewed and approved this study. We registered the study at ClinicalTrials.gov (202004137). We reported this study adhering to the CONSORT statement [46,47].
Results
Participants
Participant flow is presented in Figure 1. A total of 31 participants were screened, 24 were randomized, and 22 (iSMART: n=13 and control: n=9) completed the study. Table 1 shows the baseline characteristics of the participants.
Table 1.
Clinical and demographic information of the participants.
| Variables | Overall (n=24) | Control (n=13) | iSMARTa (n=11) | P valueb | ||||||||||
| Age (years), mean (SD) | 59 (12) | 57 (12) | 62 (11) | .35 | ||||||||||
| Sex, n (%) | >.99 | |||||||||||||
|
|
Male | 14 (58) | 8 (62) | 6 (55) |
|
|||||||||
|
|
Female | 10 (42) | 5 (38) | 5 (45) |
|
|||||||||
| Marital status, n (%) | >.99 | |||||||||||||
|
|
Married or cohabitating | 13 (54) | 7 (54) | 6 (55) |
|
|||||||||
|
|
Separated, divorced, or widowed | 7 (29) | 4 (31) | 3 (27) |
|
|||||||||
|
|
Single | 4 (17) | 2 (15) | 2 (18) |
|
|||||||||
| Total household income (US $), n (%) | .18 | |||||||||||||
|
|
0 to 14,999 | 3 (12) | 0 (0) | 3 (27) |
|
|||||||||
|
|
15,000 to 34,999 | 5 (21) | 2 (15) | 3 (27) |
|
|||||||||
|
|
35,000 to 54,999 | 4 (17) | 4 (31) | 0 (0) |
|
|||||||||
|
|
55,000 to 74,999 | 3 (12) | 2 (15) | 1 (9.1) |
|
|||||||||
|
|
75,000 or more | 7 (29) | 4 (31) | 3 (27) |
|
|||||||||
|
|
Do not wish to answer | 2 (8.3) | 1 (7.7) | 1 (9.1) |
|
|||||||||
| Premorbid disability (Modified Rankin Scale), n (%) | .77 | |||||||||||||
|
|
No symptoms | 20 (83) | 11 (85) | 9 (82) |
|
|||||||||
|
|
No significant disability | 3 (12) | 2 (15) | 1 (9.1) |
|
|||||||||
|
|
Slight disability | 1 (4.2) | 0 (0) | 1 (9.1) |
|
|||||||||
| Stroke severity (NIHc Stroke Scale), mean (SD) | 3.5 (4.2) | 1.8 (3.1) | 5.5 (4.7) | .06 | ||||||||||
| Residential status, n (%) | .21 | |||||||||||||
|
|
Alone | 8 (33) | 6 (46) | 2 (18) |
|
|||||||||
|
|
With others | 16 (67) | 7 (54) | 9 (82) |
|
|||||||||
| Financial responsibilities, n (%) | .46 | |||||||||||||
|
|
Dependent | 23 (96) | 13 (100) | 10 (91) |
|
|||||||||
|
|
Primary or partial responsibility | 1 (4.2) | 0 (0) | 1 (9.1) |
|
|||||||||
| Race, n (%) | .68 | |||||||||||||
|
|
Black | 9 (38) | 4 (31) | 5 (45) |
|
|||||||||
|
|
White | 15 (62) | 9 (69) | 6 (55) |
|
|||||||||
| Stroke diagnosis, n (%) | >.99 | |||||||||||||
|
|
Hemorrhagic | 4 (17) | 2 (15) | 2 (18) |
|
|||||||||
|
|
Ischemic | 20 (83) | 11 (85) | 9 (82) |
|
|||||||||
| Stroke side, n (%) | .75 | |||||||||||||
|
|
Bilateral | 1 (4.2) | 0 (0) | 1 (9.1) |
|
|||||||||
|
|
Left | 7 (29) | 3 (23) | 4 (36) |
|
|||||||||
|
|
Right | 9 (38) | 6 (46) | 3 (27) |
|
|||||||||
|
|
Unknown | 7 (29) | 4 (31) | 3 (27) |
|
|||||||||
| Time since stroke (days), mean (SD) | 1245 (1079) | 957 (1059) | 1585 (1048) | .09 | ||||||||||
| Education (years), mean (SD) | 15 (3) | 14 (3) | 15 (3) | .55 | ||||||||||
| Number of the previous stroke, mean (SD) | 2 (4) | 2 (2) | 3 (5) | .18 | ||||||||||
aiSMART: interactive Self-Management Augmented by Rehabilitation Technologies
bWilcoxon rank sum test; Fisher exact test.
cNIH: National Institutes of Health.
Feasibility Measures
Retention and Engagement
A total of 2 participants in the iSMART group withdrew from the study, resulting in a retention rate of 82% (9/11) that exceeded the predefined benchmark. Reasons for withdrawal included (1) time conflicts with the group sessions and (2) a family issue unrelated to the intervention. The engagement (SMS text message response) rate across all participants was 76%, ranging from 22% to 96%. Although the overall engagement rate was slightly below the predefined benchmark, only 2 out of 9 participants had response rates less than 80% (ie, 22% and 49%).
Feasibility, Acceptability, and Appropriateness
The mean scores of FIM, AIM, and IAM for the iSMART participants were 4.11 (SD 0.61), 4.44 (SD 0.73), and 4.36 (SD 0.70), respectively, which met our benchmarks, suggesting high feasibility, acceptability, and appropriateness of the iSMART intervention (Table 2). Participants in the iSMART group rated higher FIM, AIM, and IAM scores than those in the control group, with a moderate effect for feasibility (r=0.449; P=.04) and large effects for acceptability (r=0.505; P=.02) and appropriateness (r=0.540; P=.01).
Table 2.
Feasibility, acceptability, and appropriateness measures between the interactive Self-Management Augmented by Rehabilitation Technologies (iSMART) and control groups.
| Measures | Control (n=13) | iSMART (n=9) | Wilcoxon statistic | Effect size | |||||
|
|
Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) |
|
|
|||
| FIMa | 3.48 (0.65) | 3 (3, 4) | 4.11 (0.61) | 4 (4, 4.25) | 28.5 | 0.449 | |||
| AIMb | 3.60 (0.66) | 3.5 (3, 4) | 4.44 (0.73) | 5 (4, 5) | 24.5 | 0.505 | |||
| IAMc | 3.54 (0.63) | 3.5 (3, 4) | 4.36 (0.70) | 4.25 (4, 5) | 22 | 0.540 | |||
aFIM: Feasibility of Intervention Measure.
bAIM: Acceptability of Intervention Measure.
cIAM: Intervention Appropriateness Measure.
Self-Efficacy Measures
Figures 3 and 4 show the PS-SES and PROMIS-SE change scores, illustrating significantly greater improvements in the iSMART group than in the control group. Table 3 shows the between-group effect sizes. All between-group effects were favorable to the iSMART group. PS-SES home management (r=0.571; P=.008), PS-SES community management (r=0.500; P=.02), and PROMIS-SE medications and treatments (r=0.506; P=.02) showed large effects. PS-SES work (r=0.464; P=.03), PS-SES communication management (r=0.478; P=.03), and PROMIS-SE emotions (r=0.313; P=.15) showed moderate effects.
Figure 3.
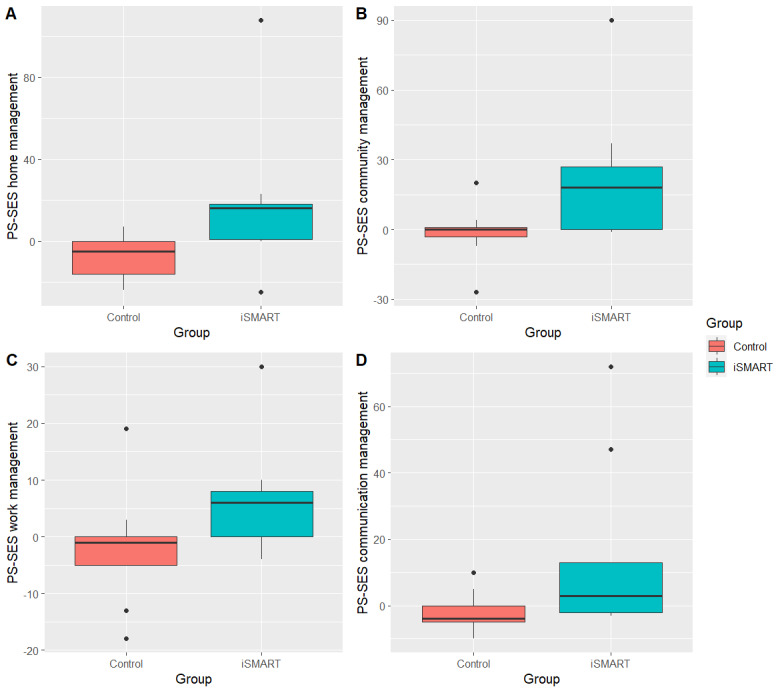
Changes in Participation Strategies Self-Efficacy Scale (PS-SES) scores after intervention. iSMART: interactive Self-Management Augmented by Rehabilitation Technologies.
Figure 4.
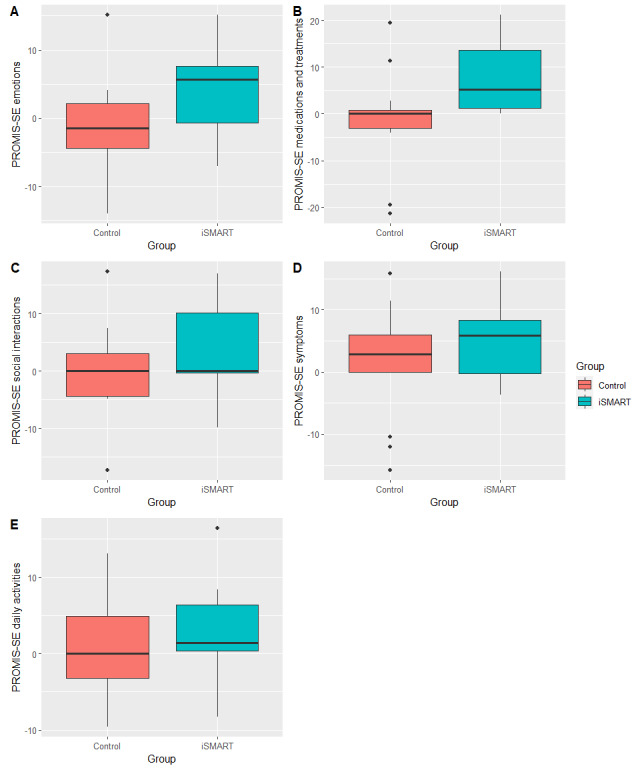
Changes in Patient-Reported Outcomes Measurement Information System’s Self-Efficacy (PROMIS-SE) scores after intervention. iSMART: interactive Self-Management Augmented by Rehabilitation Technologies.
Table 3.
Pre- and postintervention self-efficacy scores between the control and interactive Self-Management Augmented by Rehabilitation Technologies (iSMART) groups.
| Outcome measures | Control (n=13) | iSMART (n=9) | Between-group | ||||||||||||||
|
|
Pre, mean (SD) | Post, mean (SD) | Pre, median (IQR) | Post, median (IQR) | W | r | Pre, mean (SD) | Post, mean (SD) | Pre, median (IQR) | Post, median (IQR) | W | r | W | r | |||
| PS-SESa | |||||||||||||||||
|
|
Home management | 105 (12.6) | 97.5 (16.3) | 106 (96, 113) | 92 (82, 113) | 47.5 | .567 | 83.6 (38.5) | 102 (21.5) | 102 (57, 114) | 115 (86, 120) | 7 | .554 | 18.5 | .571 | ||
|
|
Community management | 82.8 (16.5) | 81.3 (17.8) | 82 (77, 100) | 80 (72, 95) | 35.5 | .215 | 60.7 (36) | 82.8 (20.8) | 43 (35, 99) | 98 (70, 100) | 3 | .673 | 23.5 | .500 | ||
|
|
Work management | 60.6 (10.8) | 58.8 (9.5) | 65 (56, 68) | 62 (51, 68) | 39 | .342 | 46.8 (19.1) | 53.2 (18.3) | 47 (36, 63) | 56 (38, 70) | 4 | .633 | 26 | .464 | ||
|
|
Communication management | 67.4 (15) | 65.5 (14.6) | 72 (66, 79) | 71 (56, 76) | 55.5 | .379 | 52.9 (26.7) | 67.9 (15.3) | 62 (30, 77) | 74 (60, 80) | 7 | .476 | 25 | .478 | ||
| PROMIS-SEb | |||||||||||||||||
|
|
Emotions | 47.1 (9.1) | 46.1 (8.6) | 49.6 (38.8, 53.2) | 46.1 (38.5, 51.6) | 58.5 | .252 | 49.1 (7.8) | 53.1 (7.6) | 49.6 (41.0, 53.9) | 51.5 (48.2, 55.3) | 10 | .494 | 36.5 | .313 | ||
|
|
Medications and treatments | 46.1 (8.7) | 45.1 (9.1) | 43.5 (40.4, 50.4) | 41.1 (38.8, 50.4) | 42.5 | .049 | 43.2 (9.9) | 51.5 (10) | 41.1 (37.1, 47.3) | 55.5 (44.0, 60.6) | 0 | .870 | 23 | .506 | ||
|
|
Social interactions | 44 (8.5) | 44.3 (9.4) | 42.5 (37.3, 48.4) | 42.5 (38.8, 53.0) | 36.5 | .049 | 49.9 (10.1) | 52.8 (7.5) | 49.7 (42.5, 59.8) | 52.9 (48.7, 59. 8) | 6 | .182 | 48.5 | .143 | ||
|
|
Symptoms | 49.4 (8.8) | 51.1 (8.1) | 49 (44.8, 52.8) | 47.7 (45.3, 57.2) | 28.5 | .262 | 49.7 (7.5) | 55.1 (6.9) | 48.8 (46.9, 53.7) | 54.6 (50.0, 63.5) | 6 | .514 | 50 | .121 | ||
|
|
Daily activities | 47.7 (8.2) | 49 (6.7) | 47.7 (42.7, 51.2) | 46 (43.4, 54.8) | 32 | .136 | 46.7 (9.7) | 49.9 (8.9) | 44.4 (37.8, 53.3) | 52.5 (42.1, 55.7) | 6 | .593 | 44.5 | .199 | ||
aPS-SES: Participation Strategies Self-Efficacy Scale.
bPROMIS-SE: Patient-Reported Outcome Measurement Information System’s Self-Efficacy.
Table 3 further shows the within-group effect sizes. The iSMART showed moderate-to-large effects of increasing the use of participation strategies for management in the home (r=0.554; P=.14 [large]), work (r=0.633; P=.06 [large]), community (r=0.673; P=.04 [large]), and communication activities (r=0.476; P=.14 [moderate]). In contrast, the control group showed small-to-large effects of decreasing the use of participation strategies for management in the home (r=0.567; P=.05 [large]), work (r=0.342; P=.26 [moderate]), community (r=0.215; P=.44 [small]), and communication activities (r=0.379; P=.21 [moderate]).
In addition, the iSMART showed moderate-to-large effects of increasing self-efficacy in managing emotions (r=0.494; P=.16 [moderate]), symptoms (r=0.514; P=.11 [large]), daily activities (r=0.593; P=.11 [large]), and treatments and medications (r=0.870; P=.01 [large]), except a small effect of increasing self-efficacy in managing social interactions (r=0.182; P=.40). In contrast, the control group showed small effects of decreasing self-efficacy in managing emotions (r=0.252; P=.38), symptoms (r=0.262; P=.43), daily activities (r=0.136; P=.61), and treatments and medications (r=0.049; P=.81), except no change in self-efficacy in managing social interactions (r=0.049; P=.88).
Discussion
Principal Findings
This study evaluated the feasibility and established preliminary effect sizes of iSMART, an mHealth intervention for improving self-efficacy for chronic stroke management, in a group of community-dwelling stroke survivors. Our results showed that iSMART is feasible and acceptable for mild-to-moderate chronic stroke survivors. Participants also showed moderate improvements in most self-efficacy measures after completing the iSMART.
Previous Works and Study Implications
We observed sufficient retention (82%) and engagement (SMS text message response) rates (76%) in the iSMART group. In addition, the iSMART group showed greater ratings than the control group on all 3 implementation measures, suggesting that iSMART is a feasible self-management program for stroke survivors. The iSMART had a similar retention rate to those reported in mHealth interventions for pediatric weight management (78%) [48], antiretroviral therapy (85%) [49], and tuberculosis treatment (87%) [49]. The text message response rate was similar to other mHealth interventions targeting behavior changes in neuropsychiatric conditions. Suffoletto et al [50] reported 74% to 97% messaging response rates in an education and behavioral support intervention using text messages to assess daily symptoms and provide support to adults with mild traumatic brain injury. Although we found that a larger portion of the iSMART participants met the engagement criteria (>80%), 2 out of 9 participants had response rates less than 80% (ie, 22% and 49%). The wide range of engagement was commonly found in other technology-based interventions for stroke survivors. For example, Guidetti et al [51] developed a technology-supported intervention for stroke survivors in Sweden and Uganda and stated that participants responded to 44% to 100% (mean 78%) of the text messages they received. A recent study of mHealth weight management intervention in adults with mental illness from which the iSMART was derived found that participants who met the criteria (>80% of text responses) in the first month of intervention had greater weight loss than those who did not [35]. These results suggest that future technology-based interventions may enhance intervention responses and effectiveness by increasing participants’ engagement up to the criteria that may maximize health and rehabilitation outcomes. Future studies are needed to formally test the engagement criteria and examine their relationships with treatment responses and outcomes for iSMART in stroke survivors.
Our findings indicated that iSMART yielded moderate-to-large effects in improving self-efficacy in using participation strategies for home, work, community, and communication management. Future interventions in improving participation outcomes following a stroke should make it a key behavioral target, given its beneficial mediatory effect on mobility and participation [52]. Participants who completed the iSMART intervention showed moderate-to-large effects of increasing self-efficacy in managing emotions, symptoms, daily activities, and treatments and medications. In contrast, the control intervention only yielded small effects. The beneficial effects of the iSMART intervention are consistent with other technology-supported self-management interventions that were effective in increasing self-efficacy and perceived participation in everyday life among stroke survivors [51,53]. This study also observed that mHealth delivery might amplify treatment effects. Compared to a nontechnology-based self-management intervention (ie, IPASS) that the iSMART was derived from, the SMART showed superior effects than the IPASS [11]. Nevertheless, because this study had a small sample (N=22), interpretations of these results should be very cautious. A future study using a larger sample size and using the face-to-face self-management program as a control is warranted to test the additional benefit of mHealth delivery of self-management interventions.
Limitations and Future Directions
This study had several limitations. We did not conduct the intent-to-treat analysis in this pilot study. The intent-to-treat analysis has been considered the standard approach to randomized controlled trial analyses [54]. A future, definitive trial will complete this analysis to avoid biased estimates. In addition to the constraints associated with a small sample size, participants were recruited from a single institution, restraining the generalizability of the findings. We found a trend toward statistical significance for greater stroke severity and longer time since stroke in the iSMART group at baseline than the control group, which may have artificially inflated the difference between groups on study outcomes. For this feasibility study, we examined the intervention score changes using within-group models to avoid this potential bias and found results favoring the iSMART group. Nevertheless, future, and larger-scale studies are needed to examine if these factors were potential covariates affecting the treatment outcomes. We used 3 implementation measures to examine the treatment’s acceptability, appropriateness, and feasibility. Notably, these measures were fairly correlated, and their discriminant validity was not thoroughly tested. Thus, future research would benefit from further exploration of the discriminant validity of these constructs. This study did not collect information on how social support, built environment, technology access, and other environmental barriers impact intervention engagement in individuals with neuropsychiatric conditions, including stroke [55,56]. Future studies should examine whether these barriers mediate or modulate the impact of iSMART on poststroke outcomes.
Future research should consider the co-design approach when designing or adapting digital interventions to increase participant retention and engagement. Co-design is a process in which targeted end users and other relevant stakeholders’ partner with the research team to work together in all aspects of intervention development, testing, and dissemination [57]. Co-designed digital interventions are more effective than traditional approaches, where researchers and clinicians primarily design interventions [58]. This approach is particularly beneficial when collaborating with underrepresented and minority communities because the co-design allows for conceptual or tool redevelopments and refinements based on the social, linguistic, and cultural needs of partnership groups [59]. Future studies of iSMART will need to engage more stroke survivors and caregiver stakeholders in user-centered design activities, especially those from underserved communities, to identify which characteristics of the intervention, individual users, and the care environment best facilitate iSMART implementation and effectiveness [60].
This study only examined the effect of iSMART on self-efficacy over 12 weeks. Future studies are warranted to examine the long-term impact on self-efficacy and other disability outcomes, such as the reintegration of everyday living, quality of life, and perceived recovery in stroke survivors. iSMART included three intervention components. While considering all components together as a complex intervention, we found this intervention to have adequate feasibility and positive initial effects. A specific approach, the multiphase optimization strategy framework [61], has been used to test the performance of individual intervention components in the development of technology-supported interventions such as weight loss [62], palliative care [63], and physical activity promotion [64]. A future study is needed to identify the iSMART components (main effects or interactions) that contribute meaningfully to improvement in intervention engagement and health outcomes in people after stroke. Future research may test the multiphase optimization strategy approach to identify if all or some intervention components are needed to optimize the iSMART intervention.
Conclusions
This study provides preliminary evidence to support the feasibility of delivering iSMART, a technology-supported self-management intervention to help stroke survivors increase self-efficacy for managing chronic conditions and supporting home, work, and community participation. Our findings support using a low-cost solution, such as text messaging, to supplement traditional therapeutic patient education interventions. More research is needed to provide more robust efficacy data to support the benefits of the iSMART intervention.
Acknowledgments
The authors would like to thank graduate students, staff, and faculty members at Washington University and Shirley Ryan AbilityLab for their research assistantship, in-kind resource sharing, mentorship, and involvement in different aspects of the research. The contents of this publication and the writing effort of the last author were supported by grants from the American Occupational Therapy Foundation (AOTFIRG20Wong) and the National Center for Medical Rehabilitation Research (K01HD095388). The research reported in this publication was also supported by the National Institute of Mental Health (R34 MH118395) and the Washington University Mobile Health Research Core, part of the Institute of Clinical and Translational Sciences, funded by the National Center for Advancing Translational Sciences (UL1TR002345). The content is solely the authors’ responsibility and does not necessarily represent the official view of funding agencies.
Abbreviations
- AIM
Acceptability of Intervention Measure
- CONSORT
Consolidated Standards of Reporting Trials
- FIM
Feasibility of Intervention Measure
- IAM
Intervention Appropriateness Measure
- IPASS
Improving Participation after Stroke Self-Management
- iSMART
interactive Self-Management Augmented by Rehabilitation Technologies
- mHealth
mobile health
- NIH
National Institutes of Health
- PROMIS-SE
Patient-Reported Outcomes Measurement Information System’s Self-Efficacy
- PS-SES
Participation Strategies Self-Efficacy Scale
- REDCap
Research Electronic Data Capture
CONSORT-eHEALTH (V 1.6.1).
Data Availability
The data generated and analyzed during this study cannot be sufficiently deidentified and, therefore, cannot be made publicly available due to ethical considerations. Deidentified data can be made available for further research from the corresponding author on reasonable request.
Footnotes
Authors' Contributions: AWKW, GEN, and DCM contributed to the study’s conception and design. OD and AWKW contributed to the preparation of the materials. QB contributed to data management and analysis. ZL, YL, and AWKW wrote the first draft of the manuscript. All the authors commented on previous versions and read and approved the final manuscript.
Conflicts of Interest: GEN has received research support from the National Institutes of Health (NIH), the Health Resources and Services Administration, the Barnes Jewish Hospital Foundation, the Washington University McDonnell Center for Systems Neuroscience, the Mallinckrodt Institute of Radiology, and the Usona Institute (drug only) and has served as a consultant for Alkermes, Inc, CarelonRx, Otsuka, and Sunovion. DCM reported research support from the NIH. He has served as a consultant for Otsuka Pharmaceuticals, Optum Behavioral Health, the Centerstone Research Institute, and the OneMind Foundation. He receives royalties from Oxford Press and has an ownership interest in Adaptive Health. SIL reported on research support from the NIH. MWMF has served as an independent contractor for Isaac Ray Forensic Group and Michigan Avenue Neuropsychologists. CLM has an ownership interest in Infinite Arms. He reported on subcontracts from the NIH and VA Headache Centers of Excellence. AWKW reported on research support from the NIH, the National Institute on Disability, Independence, and Rehabilitation Research, and the Craig H Neilsen Foundation. No other disclosures were reported.
References
- 1.Gassaway J, Horn SD, DeJong G, Smout RJ, Clark C, James R. Applying the clinical practice improvement approach to stroke rehabilitation: methods used and baseline results. Arch Phys Med Rehabil. 2005 Dec;86(12 Suppl 2):S16–S33. doi: 10.1016/j.apmr.2005.08.114.S0003-9993(05)01147-0 [DOI] [PubMed] [Google Scholar]
- 2.Rochette A, Korner-Bitensky N, Bishop D, Teasell R, White CL, Bravo G, Côté R, Green T, Lebrun LH, Lanthier S, Kapral M, Bayley M. The YOU CALL-WE CALL randomized clinical trial: impact of a multimodal support intervention after a mild stroke. Circ Cardiovasc Qual Outcomes. 2013;6(6):674–679. doi: 10.1161/CIRCOUTCOMES.113.000375. https://www.ahajournals.org/doi/10.1161/CIRCOUTCOMES.113.000375 .CIRCOUTCOMES.113.000375 [DOI] [PubMed] [Google Scholar]
- 3.Wolf TJ, Baum C, Conner LT. Changing face of stroke: implications for occupational therapy practice. Am J Occup Ther. 2009;63(5):621–625. doi: 10.5014/ajot.63.5.621. https://europepmc.org/abstract/MED/19785261 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Altieri M, Maestrini I, Mercurio A, Troisi P, Sgarlata E, Rea V, Di Piero V, Lenzi GL. Depression after minor stroke: prevalence and predictors. Eur J Neurol. 2012;19(3):517–521. doi: 10.1111/j.1468-1331.2011.03583.x. [DOI] [PubMed] [Google Scholar]
- 5.Xie J, Wu EQ, Zheng ZJ, Croft JB, Greenlund KJ, Mensah GA, Labarthe DR. Impact of stroke on health-related quality of life in the noninstitutionalized population in the United States. Stroke. 2006;37(10):2567–2572. doi: 10.1161/01.STR.0000240506.34616.10. https://www.ahajournals.org/doi/10.1161/01.STR.0000240506.34616.10 .01.STR.0000240506.34616.10 [DOI] [PubMed] [Google Scholar]
- 6.Rothwell PM, Giles MF, Chandratheva A, Marquardt L, Geraghty O, Redgrave JNE, Lovelock CE, Binney LE, Bull LM, Cuthbertson FC, Welch SJV, Bosch S, Alexander FC, Silver LE, Gutnikov SA, Mehta Z. Effect of urgent treatment of transient ischaemic attack and minor stroke on early recurrent stroke (EXPRESS study): a prospective population-based sequential comparison. Lancet. 2007;370(9596):1432–1442. doi: 10.1016/S0140-6736(07)61448-2.S0140-6736(07)61448-2 [DOI] [PubMed] [Google Scholar]
- 7.Mohan KM, Wolfe CDA, Rudd AG, Heuschmann PU, Kolominsky-Rabas PL, Grieve AP. Risk and cumulative risk of stroke recurrence: a systematic review and meta-analysis. Stroke. 2011;42(5):1489–1494. doi: 10.1161/STROKEAHA.110.602615. https://www.ahajournals.org/doi/10.1161/STROKEAHA.110.602615 .STROKEAHA.110.602615 [DOI] [PubMed] [Google Scholar]
- 8.Woodman P, Riazi A, Pereira C, Jones F. Social participation post stroke: a meta-ethnographic review of the experiences and views of community-dwelling stroke survivors. Disabil Rehabil. 2014;36(24):2031–2043. doi: 10.3109/09638288.2014.887796. [DOI] [PubMed] [Google Scholar]
- 9.Mountain A, Lindsay MP, Teasell R, Salbach NM, de Jong A, Foley N, Bhogal S, Bains N, Bowes R, Cheung D, Corriveau H, Joseph L, Lesko D, Millar A, Parappilly B, Pikula A, Scarfone D, Rochette A, Taylor T, Vallentin T, Dowlatshahi D, Gubitz G, Casaubon LK, Cameron JI. Canadian stroke best practice recommendations: rehabilitation, recovery, and community participation following stroke. Part two: transitions and community participation following stroke. Int J Stroke. 2020;15(7):789–806. doi: 10.1177/1747493019897847. https://journals.sagepub.com/doi/10.1177/1747493019897847 . [DOI] [PubMed] [Google Scholar]
- 10.Rochette A, Desrosiers J, Bravo G, St-Cyr-Tribble D, Bourget A. Changes in participation after a mild stroke: quantitative and qualitative perspectives. Top Stroke Rehabil. 2007;14(3):59–68. doi: 10.1310/tsr1403-59. [DOI] [PubMed] [Google Scholar]
- 11.Wolf TJ, Baum CM, Lee D, Hammel J. The development of the Improving Participation after Stroke Self-management program (IPASS): an exploratory randomized clinical study. Top Stroke Rehabil. 2016;23(4):284–292. doi: 10.1080/10749357.2016.1155278. https://europepmc.org/abstract/MED/27077987 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Correia JC, Waqas A, Assal JP, Davies MJ, Somers F, Golay A, Pataky Z. Effectiveness of therapeutic patient education interventions for chronic diseases: a systematic review and meta-analyses of randomized controlled trials. Front Med (Lausanne) 2022;9:996528. doi: 10.3389/fmed.2022.996528. https://europepmc.org/abstract/MED/36760883 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee D, Fischer H, Zera S, Robertson R, Hammel J. Examining a participation-focused stroke self-management intervention in a day rehabilitation setting: a quasi-experimental pilot study. Top Stroke Rehabil. 2017;24(8):601–607. doi: 10.1080/10749357.2017.1375222. [DOI] [PubMed] [Google Scholar]
- 14.Damush TM, Ofner S, Yu Z, Plue L, Nicholas G, Williams LS. Implementation of a stroke self-management program: a randomized controlled pilot study of veterans with stroke. Transl Behav Med. 2011;1(4):561–572. doi: 10.1007/s13142-011-0070-y. https://europepmc.org/abstract/MED/24073080 .70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones F, Riazi A. Self-efficacy and self-management after stroke: a systematic review. Disabil Rehabil. 2011;33(10):797–810. doi: 10.3109/09638288.2010.511415. [DOI] [PubMed] [Google Scholar]
- 16.Marks R, Allegrante JP, Lorig K. A review and synthesis of research evidence for self-efficacy-enhancing interventions for reducing chronic disability: implications for health education practice (part II) Health Promot Pract. 2005;6(2):148–156. doi: 10.1177/1524839904266792.6/2/148 [DOI] [PubMed] [Google Scholar]
- 17.Free C, Phillips G, Galli L, Watson L, Felix L, Edwards P, Patel V, Haines A. The effectiveness of mobile-health technology-based health behaviour change or disease management interventions for health care consumers: a systematic review. PLoS Med. 2013;10(1):e1001362. doi: 10.1371/journal.pmed.1001362. https://journals.plos.org/plosmedicine/article?id=10.1371/journal.pmed.1001362 .PMEDICINE-D-12-00520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buhi ER, Trudnak TE, Martinasek MP, Oberne AB, Fuhrmann HJ, McDermott RJ. Mobile phone-based behavioural interventions for health: a systematic review. Health Educ J. 2012;72(5):564–583. doi: 10.1177/0017896912452071. [DOI] [Google Scholar]
- 19.Lycett HJ, Raebel EM, Wildman EK, Guitart J, Kenny T, Sherlock JP, Cooper V. Theory-based digital interventions to improve asthma self-management outcomes: systematic review. J Med Internet Res. 2018;20(12):e293. doi: 10.2196/jmir.9666. https://www.jmir.org/2018/12/e293/ v20i12e293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nahum-Shani I, Smith SN, Spring BJ, Collins LM, Witkiewitz K, Tewari A, Murphy SA. Just-in-Time Adaptive Interventions (JITAIs) in mobile health: key components and design principles for ongoing health behavior support. Ann Behav Med. 2018;52(6):446–462. doi: 10.1007/s12160-016-9830-8. https://europepmc.org/abstract/MED/27663578 .10.1007/s12160-016-9830-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lau SC, Bhattacharjya S, Fong MW, Nicol GE, Lenze EJ, Baum C, Hardi A, Wong AW. Effectiveness of theory-based digital self-management interventions for improving depression, anxiety, fatigue and self-efficacy in people with neurological disorders: a systematic review and meta-analysis. J Telemed Telecare. 2022;28(8):547–558. doi: 10.1177/1357633X20955122. https://europepmc.org/abstract/MED/32954920 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong AWK, Fong MWM, Munsell EGS, Metts CL, Lee SI, Nicol GE, DePaul O, Tomazin SE, Kaufman KJ, Mohr DC. Using intervention mapping and behavior change techniques to develop a digital intervention for self-management in stroke: development study. JMIR Hum Factors. 2023;10:e45099. doi: 10.2196/45099. https://humanfactors.jmir.org/2023//e45099/ v10i1e45099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brennan DM, Mawson S, Brownsell S. Telerehabilitation: enabling the remote delivery of healthcare, rehabilitation, and self management. Stud Health Technol Inform. 2009;145:231–248. [PubMed] [Google Scholar]
- 24.Hall AK, Cole-Lewis H, Bernhardt JM. Mobile text messaging for health: a systematic review of reviews. Annu Rev Public Health. 2015;36:393–415. doi: 10.1146/annurev-publhealth-031914-122855. https://europepmc.org/abstract/MED/25785892 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lejuez CW, Hopko DR, Acierno R, Daughters SB, Pagoto SL. Ten year revision of the brief behavioral activation treatment for depression: revised treatment manual. Behav Modif. 2011;35(2):111–161. doi: 10.1177/0145445510390929.35/2/111 [DOI] [PubMed] [Google Scholar]
- 26.Frates EP, Moore MA, Lopez CN, McMahon GT. Coaching for behavior change in physiatry. Am J Phys Med Rehabil. 2011;90(12):1074–1082. doi: 10.1097/PHM.0b013e31822dea9a. [DOI] [PubMed] [Google Scholar]
- 27.Brott T, Adams HP, Olinger CP, Marler JR, Barsan WG, Biller J, Spilker J, Holleran R, Eberle R, Hertzberg V, Rorick M, Moomaw CJ, Walker M. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. 1989;20(7):864–870. doi: 10.1161/01.str.20.7.864. https://www.ahajournals.org/doi/10.1161/01.STR.20.7.864 . [DOI] [PubMed] [Google Scholar]
- 28.Kaplan E, Goodglass H, Weintraub S. Boston Naming Test. Austin, TX: Pro-ed; 2001. [Google Scholar]
- 29.Chiamulera C, Mantovani E, Tamburin S. Remote clinical trials: a timely opportunity for a virtual reality approach and its potential application in neurology. Br J Clin Pharmacol. 2021;87(10):3639–3642. doi: 10.1111/bcp.14922. doi: 10.1111/bcp.14922. [DOI] [PubMed] [Google Scholar]
- 30.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research Electronic Data Capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. https://linkinghub.elsevier.com/retrieve/pii/S1532-0464(08)00122-6 .S1532-0464(08)00122-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bandura A. Social cognitive theory: an agentic perspective. Annu Rev Psychol. 2001;52:1–26. doi: 10.1146/annurev.psych.52.1.1.52/1/1 [DOI] [PubMed] [Google Scholar]
- 32.Baum C, Christiansen CH, Bass JD. Occupational Therapy: Performance, Participation, and Well-being, 4th Edition. Thorofare, NJ: SLACK Incorporated; 2014. The Person-Environment-Occupaion-Performance (PEOP) model; pp. 49–55. [Google Scholar]
- 33.Tabak RG, Strickland JR, Stein RI, Dart H, Colditz GA, Kirk B, Dale AM, Evanoff BA. Development of a scalable weight loss intervention for low-income workers through adaptation of interactive Obesity Treatment Approach (iOTA) BMC Public Health. 2018;18(1):1265. doi: 10.1186/s12889-018-6176-0. https://bmcpublichealth.biomedcentral.com/articles/10.1186/s12889-018-6176-0 .10.1186/s12889-018-6176-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tabak RG, Strickland JR, Kirk B, Colvin R, Stein RI, Dart H, Colditz GA, Dale AM, Evanoff BA. Pilot test of an interactive obesity treatment approach among employed adults in a university medical billing office. Pilot Feasibility Stud. 2020;6:57. doi: 10.1186/s40814-020-00599-w. https://pilotfeasibilitystudies.biomedcentral.com/articles/10.1186/s40814-020-00599-w .599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nicol G, Jansen M, Haddad R, Ricchio A, Yingling MD, Schweiger JA, Keenoy K, Evanoff BA, Newcomer JW. Use of an interactive obesity treatment approach in individuals with severe mental illness: feasibility, acceptability, and proposed engagement criteria. JMIR Form Res. 2022;6(12):e38496. doi: 10.2196/38496. https://formative.jmir.org/2022/12/e38496/ v6i12e38496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bartholomew LK, Parcel GS, Kok G. Intervention mapping: a process for developing theory- and evidence-based health education programs. Health Educ Behav. 1998;25(5):545–563. doi: 10.1177/109019819802500502. [DOI] [PubMed] [Google Scholar]
- 37.Michie S, van Stralen MM, West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implement Sci. 2011;6:42. doi: 10.1186/1748-5908-6-42. https://implementationscience.biomedcentral.com/articles/10.1186/1748-5908-6-42 .1748-5908-6-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Michie S, Richardson M, Johnston M, Abraham C, Francis J, Hardeman W, Eccles MP, Cane J, Wood CE. The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: building an international consensus for the reporting of behavior change interventions. Ann Behav Med. 2013;46(1):81–95. doi: 10.1007/s12160-013-9486-6. https://core.ac.uk/reader/191129821?utm_source=linkout . [DOI] [PubMed] [Google Scholar]
- 39.Carey RN, Connell LE, Johnston M, Rothman AJ, de Bruin M, Kelly MP, Michie S. Behavior change techniques and their mechanisms of action: a synthesis of links described in published intervention literature. Ann Behav Med. 2019;53(8):693–707. doi: 10.1093/abm/kay078. https://europepmc.org/abstract/MED/30304386 .5126198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weiner BJ, Lewis CC, Stanick C, Powell BJ, Dorsey CN, Clary AS, Boynton MH, Halko H. Psychometric assessment of three newly developed implementation outcome measures. Implement Sci. 2017;12(1):108. doi: 10.1186/s13012-017-0635-3. https://implementationscience.biomedcentral.com/articles/10.1186/s13012-017-0635-3 .10.1186/s13012-017-0635-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee D, Fogg L, Baum CM, Wolf TJ, Hammel J. Validation of the Participation Strategies Self-Efficacy Scale (PS-SES) Disabil Rehabil. 2018;40(1):110–115. doi: 10.1080/09638288.2016.1242172. [DOI] [PubMed] [Google Scholar]
- 42.Gruber-Baldini AL, Velozo C, Romero S, Shulman LM. Validation of the PROMIS® measures of self-efficacy for managing chronic conditions. Qual Life Res. 2017;26(7):1915–1924. doi: 10.1007/s11136-017-1527-3. https://europepmc.org/abstract/MED/28239781 .10.1007/s11136-017-1527-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cohen J. Statistical Power Analysis for the Behavioral Sciences. Hillsdale, NJ: L. Erlbaum Associates; 1988. [Google Scholar]
- 44.Kassambara A. Wilcoxon effect size. rstatix. 2019. [2023-11-05]. https://rpkgs.datanovia.com/rstatix/reference/wilcox_effsize.html .
- 45.Schäfer T, Schwarz MA. The meaningfulness of effect sizes in psychological research: differences between sub-disciplines and the impact of potential biases. Front Psychol. 2019;10:813. doi: 10.3389/fpsyg.2019.00813. https://europepmc.org/abstract/MED/31031679 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eldridge SM, Chan CL, Campbell MJ, Bond CM, Hopewell S, Thabane L, Lancaster GA. CONSORT 2010 statement: extension to randomised pilot and feasibility trials. Pilot Feasibility Stud. 2016;2:64. doi: 10.1186/s40814-016-0105-8. https://pilotfeasibilitystudies.biomedcentral.com/articles/10.1186/s40814-016-0105-8 .105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cuschieri S. The CONSORT statement. Saudi J Anaesth. 2019;13(Suppl 1):S27–S30. doi: 10.4103/sja.SJA_559_18. http://www.saudija.org/article.asp?issn=1658-354X;year=2019;volume=13;issue=5;spage=27;epage=30;aulast=Cuschieri .SJA-13-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chai LK, Collins CE, May C, Ashman A, Holder C, Brown LJ, Burrows TL. Feasibility and efficacy of a web-based family telehealth nutrition intervention to improve child weight status and dietary intake: a pilot randomised controlled trial. J Telemed Telecare. 2021;27(3):146–158. doi: 10.1177/1357633X19865855. [DOI] [PubMed] [Google Scholar]
- 49.Nhavoto JA, Grönlund Å, Klein GO. Mobile health treatment support intervention for HIV and tuberculosis in Mozambique: perspectives of patients and healthcare workers. PLoS One. 2017;12(4):e0176051. doi: 10.1371/journal.pone.0176051. https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0176051 .PONE-D-16-39057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Suffoletto B, Wagner AK, Arenth PM, Calabria J, Kingsley E, Kristan J, Callaway CW. Mobile phone text messaging to assess symptoms after mild traumatic brain injury and provide self-care support: a pilot study. J Head Trauma Rehabil. 2013;28(4):302–312. doi: 10.1097/HTR.0b013e3182847468. [DOI] [PubMed] [Google Scholar]
- 51.Guidetti S, Gustavsson M, Tham K, Andersson M, Fors U, Ytterberg C. F@ce: a team-based, person-centred intervention for rehabilitation after stroke supported by information and communication technology—a feasibility study. BMC Neurol. 2020;20(1):387. doi: 10.1186/s12883-020-01968-x. https://bmcneurol.biomedcentral.com/articles/10.1186/s12883-020-01968-x .10.1186/s12883-020-01968-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chau JPC, Lo SHS, Choi KC, Butt L, Zhao J, Thompson DR. Participation self-efficacy plays a mediation role in the association between mobility and social participation among stroke survivors. Heart Lung. 2021;50(6):857–862. doi: 10.1016/j.hrtlng.2021.07.002.S0147-9563(21)00221-1 [DOI] [PubMed] [Google Scholar]
- 53.Kamwesiga JT, Eriksson GM, Tham K, Fors U, Ndiwalana A, von Koch L, Guidetti S. A feasibility study of a mobile phone supported family-centred ADL intervention, F@ce, after stroke in Uganda. Global Health. 2018;14(1):82. doi: 10.1186/s12992-018-0400-7. https://globalizationandhealth.biomedcentral.com/articles/10.1186/s12992-018-0400-7 .10.1186/s12992-018-0400-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gupta SK. Intention-to-treat concept: a review. Perspect Clin Res. 2011;2(3):109–112. doi: 10.4103/2229-3485.83221. http://www.picronline.org/article.asp?issn=2229-3485;year=2011;volume=2;issue=3;spage=109;epage=112;aulast=Gupta .PCR-2-109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Magasi S, Wong A, Gray DB, Hammel J, Baum C, Wang CC, Heinemann AW. Theoretical foundations for the measurement of environmental factors and their impact on participation among people with disabilities. Arch Phys Med Rehabil. 2015;96(4):569–577. doi: 10.1016/j.apmr.2014.12.002.S0003-9993(14)01308-2 [DOI] [PubMed] [Google Scholar]
- 56.Wong AWK, Ng S, Dashner J, Baum MC, Hammel J, Magasi S, Lai JS, Carlozzi NE, Tulsky DS, Miskovic A, Goldsmith A, Heinemann AW. Relationships between environmental factors and participation in adults with traumatic brain injury, stroke, and spinal cord injury: a cross-sectional multi-center study. Qual Life Res. 2017;26(10):2633–2645. doi: 10.1007/s11136-017-1586-5.10.1007/s11136-017-1586-5 [DOI] [PubMed] [Google Scholar]
- 57.Boyd H, McKernon S, Mullin B, Old A. Improving healthcare through the use of co-design. N Z Med J. 2012;125(1357):76–87. [PubMed] [Google Scholar]
- 58.Sanz MF, Acha BV, García MF. Co-design for people-centred care digital solutions: a literature review. Int J Integr Care. 2021;21(2):16. doi: 10.5334/ijic.5573. https://europepmc.org/abstract/MED/33981193 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Syme SL. Social determinants of health: the community as an empowered partner. Prev Chronic Dis. 2004;1(1):A02. https://europepmc.org/abstract/MED/15634364 .A02 [PMC free article] [PubMed] [Google Scholar]
- 60.Farao J, Malila B, Conrad N, Mutsvangwa T, Rangaka MX, Douglas TS. A user-centred design framework for mHealth. PLoS One. 2020;15(8):e0237910. doi: 10.1371/journal.pone.0237910. https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0237910 .PONE-D-20-01209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Collins LM, Murphy SA, Strecher V. The Multiphase Optimization Strategy (MOST) and the Sequential Multiple Assignment Randomized Trial (SMART): new methods for more potent eHealth interventions. Am J Prev Med. 2007;32(5 Suppl):S112–S118. doi: 10.1016/j.amepre.2007.01.022. https://europepmc.org/abstract/MED/17466815 .S0749-3797(07)00051-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Spring B, Pfammatter AF, Marchese SH, Stump T, Pellegrini C, McFadden HG, Hedeker D, Siddique J, Jordan N, Collins LM. A factorial experiment to optimize remotely delivered behavioral treatment for obesity: results of the Opt-IN study. Obesity (Silver Spring) 2020;28(9):1652–1662. doi: 10.1002/oby.22915. https://europepmc.org/abstract/MED/32656994 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dionne-Odom JN, Wells RD, Guastaferro K, Azuero A, Hendricks BA, Currie ER, Bechthold A, Dosse C, Taylor R, Reed RD, Harrell ER, Gazaway S, Engler S, McKie P, Williams GR, Sudore R, Rini C, Rosenberg AR, Bakitas MA. An early palliative care telehealth coaching intervention to enhance advanced cancer family caregivers' decision support skills: the CASCADE pilot factorial trial. J Pain Symptom Manage. 2022;63(1):11–22. doi: 10.1016/j.jpainsymman.2021.07.023. https://europepmc.org/abstract/MED/34343621 .S0885-3924(21)00478-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Phillips SM, Penedo FJ, Collins LM, Solk P, Siddique J, Song J, Cella D, Courneya KS, Ackermann RT, Welch WA, Auster-Gussman LA, Whitaker M, Cullather E, Izenman E, Spring B. Optimization of a technology-supported physical activity promotion intervention for breast cancer survivors: results from Fit2Thrive. Cancer. 2022;128(5):1122–1132. doi: 10.1002/cncr.34012. https://europepmc.org/abstract/MED/34812521 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
CONSORT-eHEALTH (V 1.6.1).
Data Availability Statement
The data generated and analyzed during this study cannot be sufficiently deidentified and, therefore, cannot be made publicly available due to ethical considerations. Deidentified data can be made available for further research from the corresponding author on reasonable request.


