ABSTRACT
The ESKAPEE pathogen Pseudomonas aeruginosa is a common cause of chronic wound and cystic fibrosis lung infections, as well as acute burn and nosocomial infections. Many of these infections are recalcitrant to conventional antibiotic therapies due to both traditional antibiotic resistance mechanisms and antimicrobial tolerance. Recent successes with bacteriophage (phage) therapy to treat chronic human P. aeruginosa infections have led to a renewed interest in isolating and characterizing new P. aeruginosa phages. Here, we isolated and characterized a new lytic phage (termed PIP, pili-infecting phage) capable of infecting P. aeruginosa PA14. PIP is a tailed phage with an icosahedral head and flexible tail containing a genome that is 57,462 bp in length. Phylogenetic analysis reveals that PIP belongs to the subfamily Queuovirinae and genus Nipunavirus but is highly divergent in gene content from known Nipunaviruses. By isolating and characterizing a P. aeruginosa strain that spontaneously evolved resistance to PIP, we show that the receptor for PIP is Type IV pili. In summary, we isolated a new P. aeruginosa phage species with a unique genome, thus increasing the diversity of phages known to infect this important human pathogen.
IMPORTANCE
The opportunistic pathogen Pseudomonas aeruginosa causes both acute and chronic human infections. These infections are notoriously difficult to treat due to both antibiotic resistance and antibiotic tolerance. The increasing frequency of antibiotic failure in P. aeruginosa infections has led scientists to explore other treatment options, including bacteriophage (phage) therapy. To this end, there has been a significant effort to identify new Pseudomonas phages. Here, we isolated and characterized a bacteriophage (termed PIP, pili-infecting phage) that infects P. aeruginosa PA14. Examination of the PIP genome revealed that this phage represents a new species in the subclass Queuovirinae. The isolation and characterization of spontaneous PA14 mutants that are resistant to PIP infection revealed Type IV pili as the PIP receptor. Ultimately, this study characterizes a new species of Pseudomonas phage, thus enhancing the known diversity of phages that infect this important pathogen.
KEYWORDS: Pseudomonas aeruginosa, bacteriophage, Queuovirinae, pili
OBSERVATION
Bacteriophages (phages) are the most common biological entities on earth, found in any environment where bacteria exist. Yet despite their abundance, our knowledge about phages is far from extensive, and they have been deemed the “dark matter of the biological world” (1–3). Not only do gaps in knowledge exist in the sheer number of phage species on earth but also in the mechanisms they use to infect bacteria.
A renewed interest in the use of phages for treating infections (4–6) has led to a surge in the identification and characterization of new phages. This is particularly true for the ESKAPEE pathogen Pseudomonas aeruginosa, and phage therapy has shown promise in treating P. aeruginosa human chronic infections (4, 7, 8). P. aeruginosa is a common cause of chronic wound and cystic fibrosis lung infections, as well as acute nosocomial infections (9). Phages provide a potential therapeutic solution to address the fact that many of these infections are not cleared with conventional antibiotic therapies due to both antibiotic resistance and tolerance mechanisms. Here, we isolated and characterized a new species of phage (termed PIP, pili-infecting phage) capable of infecting P. aeruginosa strain PA14.
PIP was isolated from a stream in Decatur, GA, USA in 2021, located at latitude 33.76 and longitude −84.29. This is an urban area, and the stream had been contaminated by raw sewage numerous times prior to our collection. Water and sediment were collected from the stream, filtered through a 0.45 µm pore membrane, and then mixed with P. aeruginosa strain PA14 in soft agar to test for the presence of phage. Twelve plaques were observed after 24 hours. Plaques were 1–2 mm in diameter and mostly clear with observable halos. A single plaque was picked, the phage was amplified in PA14 planktonic cells overnight, and then the plaque was purified to yield a pure culture of PIP.
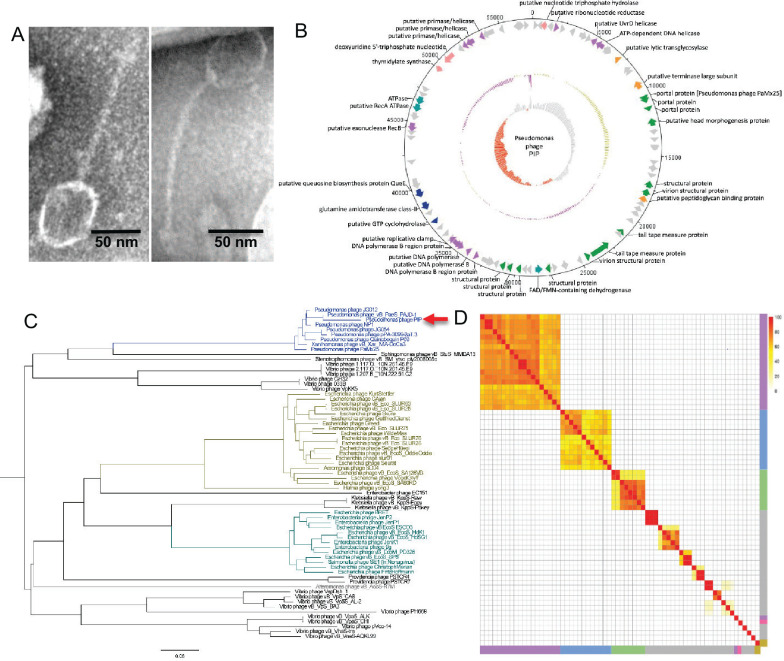
Examination of PIP using negative-stain transmission electron microscopy revealed a tailed phage with an icosahedral head and flexible tail (Fig. 1A). This physical structure, according to the guidelines set forth by the International Committee on Taxonomy of Viruses (ICTV), is characteristic of the Caudoviricetes class of phages. PIP has a capsid size of 59.3 ± 3.6 nm in diameter and a tail length of 120.2 ± 4.4 nm (Fig. 1A).
Fig 1.
Characterization of Pseudomonas phage PIP. (A) Negative stain TEM images of PIP. (B) PIP genome map. Open reading frame functions were sorted into eight classes: nucleotide metabolism and transport (pink), DNA replication and repair (purple), lysis (orange), structural (green), energy production and conversion (light blue), biosynthesis (dark blue), and hypothetical proteins (gray). (C) Phylogenetic relationships of all available Queuovirinae viruses constructed with VICTOR and visualized with FigTree. The red arrow points to PIP. (D) Heatmap of the percentage of proteins shared between Pseudomonas Queuovirinae phage genomes. The four genera of Queuovirinae were highlighted along with unclassified Queuovirinae including Seuratvirus (purple), Nonagvirus (blue), Nipunavirus (green), Amoyvirus (yellow), and unclassified (gray). PIP is highlighted in pink. The scale bar on the top right is the percentage of shared proteins between two phages. All Queuovirinae genomes for these analyses were downloaded on 6 June 2023.
We sequenced the genome of PIP using Nanopore long-read sequencing (970× coverage), yielding a double-stranded DNA genome 57,462 bp in length with a GC content of 57%, which is lower than that in P. aeruginosa PA14 (66%). Using Prokka (10), we identified 90 predicted open reading frames (ORFs) in the PIP genome (Fig. 1B). BLASTX was performed manually for each of the ORFs, revealing 37 PIP ORFs that had homology (E-value ≤ 1 × 10−5) to proteins with known function (Table S1). The remaining genes were designated as hypothetical. Most of the PIP ORFs had homology to other Pseudomonas phages belonging to the subfamily Queuovirinae and genus Nipunavirus. PIP is categorized as a lytic phage as genes commonly associated with lysogeny, including an integrase and an excisionase, were not identified on the genome.
A BLASTN search of the entire PIP sequence revealed that it had the highest sequence identity to the Nipunavirus Pseudomonas phage vB_PaeS_PAJD-1, a phage put forth as a candidate for phage therapy against P. aeruginosa mastitis infection (11). Similarity analysis showed that PIP had a percent identity of 80.35% and a query cover of 91% when compared to phage vB_PaeS_PAJD-1. To further assess the taxonomy of PIP, a phylogenetic tree of all available Queuovirinae viruses (downloaded on 6 June 2023) was constructed with VICTOR (12) and visualized with FigTree. PIP clustered with Nipunavirus phages, with the closest related phage being vB_PaeS_PAJD-1 at a branch distance of 0.0623 (Fig. 1C). Based on these results, and the fact that ICTV guidelines classify a phage as a new species if it has less than 95% sequence identity to another phage (13), we propose PIP as a new Pseudomonas phage species.
To further examine the similarities between PIP and other related viruses, a pangenome was constructed for all Queuovirinae phages, yielding 2,062 genes. The pangenome had 0 core genes (genes included in 99%–100% of all Queuovirinae phages), 0 softcore genes (95%–99%), 115 shell genes (15%–95%), and 1,947 cloud genes (0%–15%). To examine the similarity in gene content between Queuovirinae, pairwise comparisons between all phage genomes were performed, and a heatmap was created showing the percentage of proteins shared between each pair of phages (Fig. 1D). This analysis revealed that an average of 17 genes were present in any two phages, and an average of 304 genes were present in only one of the phages in each pairwise comparison. Although most phages clustered by genus in the pairwise comparisons, both PIP and Hafnia phage yong3 did not. These data indicate that there are significant gene content differences in the Queuovirinae, and PIP is substantially different from other members of this subfamily and the Nipunavirus genus.
During our testing of the activity of our purified phage stocks, we observed PA14 colonies growing within the PIP lysis zone (Fig. 2A, white arrow). We isolated one of these colonies (termed PA14-PIPR) and confirmed that it was indeed resistant to PIP infection (Fig. 2B). We then sequenced PA14-PIPR using Nanopore long read sequencing (223× coverage) and compared this sequence to that of PA14 to identify potential mutations responsible for PIP resistance. Single nucleotide polymorphism (SNP) analysis revealed 95 SNPs in PA14-PIPR compared to our wild-type PA14 (Table S2), including an SNP at base 79 of the coding sequence of pilB, which resulted in a premature stop codon. This SNP results in a truncated PilB protein containing 26 amino acids instead of the normal 567 amino acids. PilB belongs to the secretion NTPase superfamily that powers the extension of Type IV pili in P. aeruginosa, and pilB mutants are unable to produce Type IV pili (14, 15). We confirmed a functional defect in Type IV pili in PA14-PIPR as this strain showed a defect in twitching motility (Fig. S1A). Since Type IV pili are common receptors for Pseudomonas phage (16, 17), we hypothesized that this SNP was responsible for PIP resistance. To test this, we constructed a plasmid containing PA14 pilB under the control of its native promoter (pUCP20-pilB), then introduced this plasmid or its parent plasmid pUCP20 into PA14-PIPR and tested for PIP susceptibility. As expected, PA14-PIPR carrying pUCP20 was not susceptible to PIP (Fig. 2C). However, PA14-PIPR carrying pUCP20-pilB rescued susceptibility to PIP (Fig. 2D), indicating that the mutation in pilB is responsible for the resistance of PA14-PIPR to PIP infection. As expected, pUCP20-pilB also rescued twitching motility in PA14-PIPR (Fig. S1A).
Fig 2.
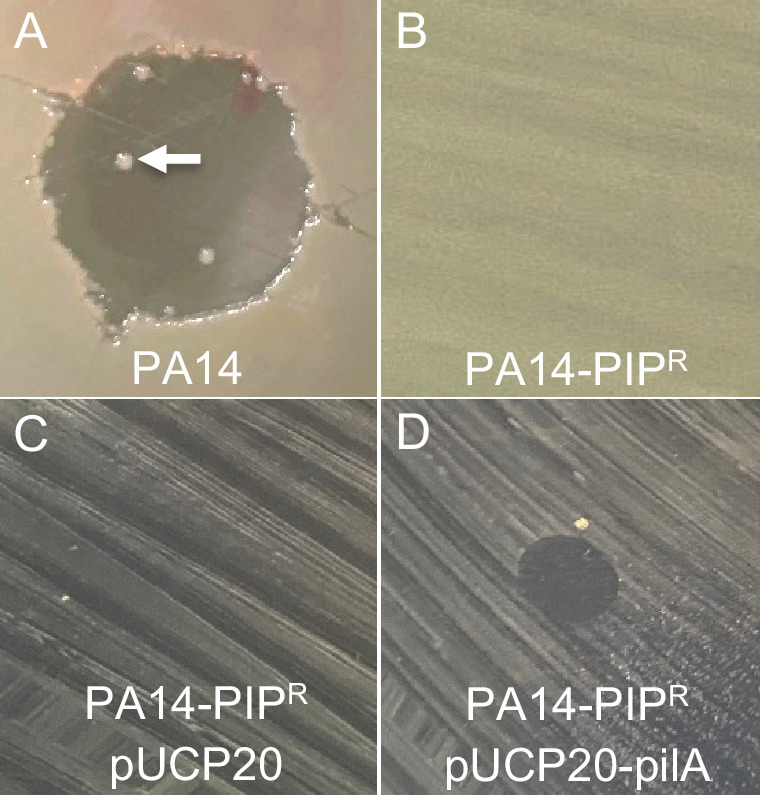
Type IV pili are required for PA14 PIP infection. (A) PIP zone of clearing on strain PA14 with resistant strains growing within the lysis zone (white arrow). (B) PIP causes no zone of clearing on the PA14 spontaneous resistance mutant PA14-PIPR. (C) PA14-PIPR carrying the plasmid pUCP20 is resistant to PIP. (D) Introduction of the pilB complementation plasmid pUCP20-pilB into PA14-PIPR restored sensitivity to PIP. Note: the size of the zones of clearing does not indicate relative susceptibilities, as the images in panels A and B were taken at a higher magnification to allow visualization of resistant colonies in panel A.
To examine whether PIP also infects other P. aeruginosa strains that produce Type IV pili, we tested the susceptibility of the laboratory strains PAO1, PAK, and 19SJ (18–20). While 19SJ was susceptible to PIP infection, both PAO1 and PAK were resistant (Fig. S1B), indicating that not all P. aeruginosa strains that produce Type IV pili are susceptible to PIP. This is likely due to either differences in the amino acid sequence of the pilin protein or differential pilin glycosylation.
In summary, we have isolated and characterized a new phage species for Pseudomonas aeruginosa with unique genomic content, thus increasing the known diversity of Pseudomonas phage.
ACKNOWLEDGMENTS
We thank members of the Whiteley lab and CMDI for valuable discussions, particularly Dr. Ellinor Alseth.
This study was supported by grants from the Cystic Fibrosis Foundation (WHITEL20A0 and WHITEL22G0 to M.W.).
Contributor Information
Marvin Whiteley, Email: mwhiteley3@gatech.edu.
Ethel Bayer-Santos, University of Sao Paulo, Sao Paulo, Brazil.
DATA AVAILABILITY
The raw sequencing files from this study can be found at NCBI Sequence Read Archive (SRA) under the accession number OR687155 for the phage genome and CP136842 for the PA14 pilB mutant.
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/spectrum.03719-23.
Fig. S1 and all experimental details.
PIP genes with putative functions.
SNPs identified in PA14-PIPR using medaka.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Benler S, Yutin N, Antipov D, Rayko M, Shmakov S, Gussow AB, Pevzner P, Koonin EV. 2021. Thousands of previously unknown phages discovered in whole-community human gut metagenomes. Microbiome 9:78. doi: 10.1186/s40168-021-01017-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hatfull GF. 2015. Dark matter of the biosphere: the amazing world of bacteriophage diversity. J Virol 89:8107–8110. doi: 10.1128/JVI.01340-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pedulla ML, Ford ME, Houtz JM, Karthikeyan T, Wadsworth C, Lewis JA, Jacobs-Sera D, Falbo J, Gross J, Pannunzio NR, Brucker W, Kumar V, Kandasamy J, Keenan L, Bardarov S, Kriakov J, Lawrence JG, Jacobs WR, Hendrix RW, Hatfull GF. 2003. Origins of highly mosaic mycobacteriophage genomes. Cell 113:171–182. doi: 10.1016/s0092-8674(03)00233-2 [DOI] [PubMed] [Google Scholar]
- 4. Chan BK, Turner PE, Kim S, Mojibian HR, Elefteriades JA, Narayan D. 2018. Phage treatment of an aortic graft infected with Pseudomonas aeruginosa. Evol Med Public Health 2018:60–66. doi: 10.1093/emph/eoy005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dedrick RM, Smith BE, Cristinziano M, Freeman KG, Jacobs-Sera D, Belessis Y, Whitney Brown A, Cohen KA, Davidson RM, van Duin D, et al. 2023. Phage therapy of Mycobacterium infections: compassionate use of phages in 20 patients with drug-resistant mycobacterial disease. Clin Infect Dis 76:103–112. doi: 10.1093/cid/ciac453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schooley RT, Biswas B, Gill JJ, Hernandez-Morales A, Lancaster J, Lessor L, Barr JJ, Reed SL, Rohwer F, Benler S, et al. 2017. Development and use of personalized bacteriophage-based therapeutic cocktails to treat a patient with a disseminated resistant Acinetobacter baumannii infection. Antimicrob Agents Chemother 61:e00954-17. doi: 10.1128/AAC.00954-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ferry T, Kolenda C, Laurent F, Leboucher G, Merabischvilli M, Djebara S, Gustave CA, Perpoint T, Barrey C, Pirnay JP, Resch G. 2022. Personalized bacteriophage therapy to treat pandrug-resistant spinal Pseudomonas aeruginosa infection. Nat Commun 13:4239. doi: 10.1038/s41467-022-31837-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Köhler T, Luscher A, Falconnet L, Resch G, McBride R, Mai Q-A, Simonin JL, Chanson M, Maco B, Galiotto R, Riat A, Civic N, Docquier M, McCallin S, Chan B, van Delden C. 2023. Personalized aerosolised bacteriophage treatment of a chronic lung infection due to multidrug-resistant Pseudomonas aeruginosa. Nat Commun 14:3629. doi: 10.1038/s41467-023-39370-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Diggle SP, Whiteley M. 2020. Microbe profile: Pseudomonas aeruginosa: opportunistic pathogen and lab rat. Microbiology (Reading) 166:30–33. doi: 10.1099/mic.0.000860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Seemann T. 2014. Prokka: rapid prokaryotic genome annotation. Bioinformatics 30:2068–2069. doi: 10.1093/bioinformatics/btu153 [DOI] [PubMed] [Google Scholar]
- 11. Wang Z, Xue Y, Gao Y, Guo M, Liu Y, Zou X, Cheng Y, Ma J, Wang H, Sun J, Yan Y. 2021. Phage VB_PaeS-PAJD-1 rescues murine mastitis infected with multidrug-resistant Pseudomonas aeruginosa. Front Cell Infect Microbiol 11:689770. doi: 10.3389/fcimb.2021.689770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Meier-Kolthoff JP, Göker M. 2017. VICTOR: genome-based phylogeny and classification of prokaryotic viruses. Bioinformatics 33:3396–3404. doi: 10.1093/bioinformatics/btx440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Adriaenssens E, Brister JR. 2017. How to name and classify your phage: an informal guide. Viruses 9:70. doi: 10.3390/v9040070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nunn D, Bergman S, Lory S. 1990. Products of three accessory genes, pilB, pilC, and pilD, are required for biogenesis of Pseudomonas aeruginosa pili. J Bacteriol 172:2911–2919. doi: 10.1128/jb.172.6.2911-2919.1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Turner LR, Lara JC, Nunn DN, Lory S. 1993. Mutations in the consensus ATP-binding sites of XcpR and PilB eliminate extracellular protein secretion and pilus biogenesis in Pseudomonas aeruginosa. J Bacteriol 175:4962–4969. doi: 10.1128/jb.175.16.4962-4969.1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bradley DE, Pitt TL. 1974. Pilus-dependence of four Pseudomonas aeruginosa bacteriophages with non-contractile tails. J Gen Virol 24:1–15. doi: 10.1099/0022-1317-24-1-1 [DOI] [PubMed] [Google Scholar]
- 17. Harvey H, Bondy-Denomy J, Marquis H, Sztanko KM, Davidson AR, Burrows LL. 2018. Pseudomonas aeruginosa defends against phages through type IV pilus glycosylation. Nat Microbiol 3:47–52. doi: 10.1038/s41564-017-0061-y [DOI] [PubMed] [Google Scholar]
- 18. Deziel E, Paquette G, Villemur R, Lepine F, Bisaillon J. 1996. Biosurfactant production by a soil Pseudomonas strain growing on polycyclic aromatic hydrocarbons. Appl Environ Microbiol 62:1908–1912. doi: 10.1128/aem.62.6.1908-1912.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jacobs MA, Alwood A, Thaipisuttikul I, Spencer D, Haugen E, Ernst S, Will O, Kaul R, Raymond C, Levy R, Chun-Rong L, Guenthner D, Bovee D, Olson MV, Manoil C. 2003. Comprehensive transposon mutant library of Pseudomonas aeruginosa. Proc Natl Acad Sci U S A 100:14339–14344. doi: 10.1073/pnas.2036282100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Takeya K, Amako K. 1966. A rod-shaped Pseudomonas phage. Virology 28:163–165. doi: 10.1016/0042-6822(66)90317-5 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 and all experimental details.
PIP genes with putative functions.
SNPs identified in PA14-PIPR using medaka.
Data Availability Statement
The raw sequencing files from this study can be found at NCBI Sequence Read Archive (SRA) under the accession number OR687155 for the phage genome and CP136842 for the PA14 pilB mutant.