Abstract
Background: Chronic obstructive pulmonary disease is a chronic, often progressive disease, which in most patients is caused by tobacco smoking. Our study focuses on differences in COPD-related outcomes between never smokers, former smokers, and current smokers.
Methods: A nationwide, population-based cohort study utilizing Danish health registries. Clinical and socioeconomic variables including smoking status, comorbidities, and dyspnea were obtained. Poisson and Cox Regression were used to calculate the impact of these clinical parameters on the risk of moderate and severe exacerbations and mortality during 12 months of follow-up.
Results: A total of 49,826 patients ≥40 years of age, with a hospital diagnosis of COPD in 2008–2017, were identified (mean age 69.2 years, 52% females). A total of 2127 (4%) were never smokers, 29,854 (60%) were former smokers and 17,845 (36%) current smokers. Compared to former and current smokers, never smokers reported a lower modified Medical Research Council score and had a milder COPD stage according to the Global Initiative for Chronic Obstructive Lung Disease classification. During follow-up, never smokers had a significantly lower risk of severe exacerbations (hazard ratio 0.87, 95% confidence interval [CI] 0.78–0.97) and a lower rate of death (mortality ratio 0.75, 95% CI 0.70–0.81) compared to patients with a smoking history.
Discussion: Our nationwide study showed that COPD in never smokers is characterized by a lower level of dyspnea, milder lung function impairment, and a lower risk of exacerbations and death. At the same time, we found active smokers to have the highest risk. These findings highlight the need for campaigns to prevent smoking and may help general practitioners as well as other health care professionals to motivate patients with COPD to stop smoking.
Keywords: COPD, exacerbations, smoking, never smokers, prognosis
Introduction
This article contains supplemental material.
Chronic obstructive pulmonary disease (COPD) is characterized by an often progressive deterioration in lung function and increasing risk of hospitalization over time. Typical symptoms of COPD are dyspnea, fatigue, and reduced quality of life.1 In particular, in the Western world, cigarette smoking is the strongest risk factor for development and progression of the disease.2-4 However, some patients with COPD have never smoked. Most studies on COPD have been focusing on the effect of cigarette smoking and, therefore, little is known about never smokers with COPD.5-7 Fortunately, the prevalence of smoking in industrialized countries has declined and is projected to continue to do so in the future. Therefore, an increased interest in never smokers with low lung function, respiratory symptoms, and COPD has developed and several population-based studies on risk factors and pathogenesis have been published.7-9 In Denmark, all patients with a confirmed diagnosis of COPD are registered with their personal identification number (CPR-number) as well as multiple demographic and medical data. It is, therefore, possible to describe outcomes in relation to different factors, including smoking status, and to predict outcomes based on smoking status. In our present study, we aim to investigate the differences in COPD-related outcomes between never smokers, former smokers, and current smokers by combining information from several nationwide clinical registries containing data on all Danish patients with COPD treated in a hospital setting. We focus on the clinical characteristics of the patients and on the subsequent risk of moderate and severe COPD exacerbations and mortality.
Methods
Our study was a population-based cohort study using various data sources to investigate Danish patients with COPD in the period from 2008 to 2017. Data were obtained from the Danish National Health Registries including: (1) the National Hospital Registry, covering all inpatient, somatic hospitalizations in Denmark since 1977 and all outpatient visits since 1995, (2) the National COPD Registry, which contains data on all COPD patients followed in outpatient, hospital-based specialist clinics in Denmark10 since 2008, (3) the National Prescription Registry, including all prescriptions redeemed at pharmacies since 1995 using anatomical therapeutic chemical (ATC) codes, and (4) the Civil Registration System for information on vital status, marital status, and education level. Data from all registries were merged individually by using the unique 10-digit personal identification number assigned to all Danish residents at birth. The diagnosis of COPD was defined by a post-bronchodilator spirometry with a forced expiratory volume in 1 second (FEV1) to forced vital capacity (FVC) ratio < 0.7.
Population
From the National COPD Registry, we included all Danish patients ≥ 40 years with an International Classification of Diseases-10th Revision (ICD-10) code J44 (chronic obstructive pulmonary disease) as primary or secondary diagnosis and inpatient or outpatient contacts in the period 2008 to 2017. Patients with missing information on the modified Medical Research Council (mMRC) dyspnea scale11 or incomplete registrations of other clinical outcome measures were excluded. This resulted in a cohort of 49,826 patients with COPD between 40 and 99 years of age.
Data
Clinical variables of interest included gender, age, number of comorbidities, mMRC, FEV1, smoking status (active smokers, former smokers who stopped smoking > 6 months ago, or never smokers), body mass index (BMI), prior exacerbation history, and Charlson Comorbidity Index (CCI) score. Only patients with information on all parameters were included in the present analyses.
Definition of Exacerbations
Exacerbations prior to inclusion were self-reported. During follow-up, moderate exacerbations were defined as short-term use of minimum 25mg orally administered corticosteroids (OCSs) per day in 10 days, whereas severe exacerbations were defined as exacerbations leading to hospitalization.
Moderate exacerbations were captured on the basis of a search for prednisolone (ATC H02AB06) and prednisone (H02AB07) in the Danish Prescription Registry and defined as short-term use of a minimum 25mg of an OCS per day for 5 days for patients with a known diagnosis of COPD. Severe exacerbations were identified in the Hospital Registry and defined as hospitalization or emergency department visits with ICD-10 codes J44 as primary diagnosis or J13-18 (pneumonia) or J96 (acute respiratory failure) as primary diagnosis in combination with secondary diagnosis J44.
Recurrent exacerbations within 28 days were combined and considered as one exacerbation. Patients with both a moderate and a severe exacerbation within 28 days were counted as having a severe exacerbation with the start date of the exacerbation that occurred first.
Information on dyspnea was obtained by the treating nurse using the mMRC dyspnea scale11 and recorded in the National COPD Registry with a score ranging from 0 (not troubled by breathlessness except on strenuous exercise) to 4 (too breathless to leave the house, or breathless when dressing and undressing).
Charlson Comorbidity Index (CCI)12 was used as a measure of comorbidities and based on diagnoses (ICD-10 codes) recorded up to 12 months prior to index as part of inpatient and outpatient hospital care.
Statistics
Baseline characteristics were extracted at the date of the first mMRC measurement (index date). Extraction of follow-up data started from the index date between January 1, 2008, and December 31, 2016, and ended on the date of death, on the date of emigration, 12 months after index date, or at end of the study December 31, 2017, depending on which came first. Poisson regression was used to model the effect of clinical parameters and sociodemographic information on the number of exacerbations and hospitalization days in a 12-month follow-up period after index. Smoking status at index (never smoker, former smoker, current smoker) was included as the main explanatory variable together with other clinical variables such as mMRC, FEV1, BMI, CCI, and the sociodemographic variables such as age, gender, marital status, and education status at index. Cox proportional-hazards model was used to estimate the hazard ratio for death for the same explanatory variables. The Cox model was used to test and adjust for nonproportional hazards over time.13
Results
Baseline Characteristics
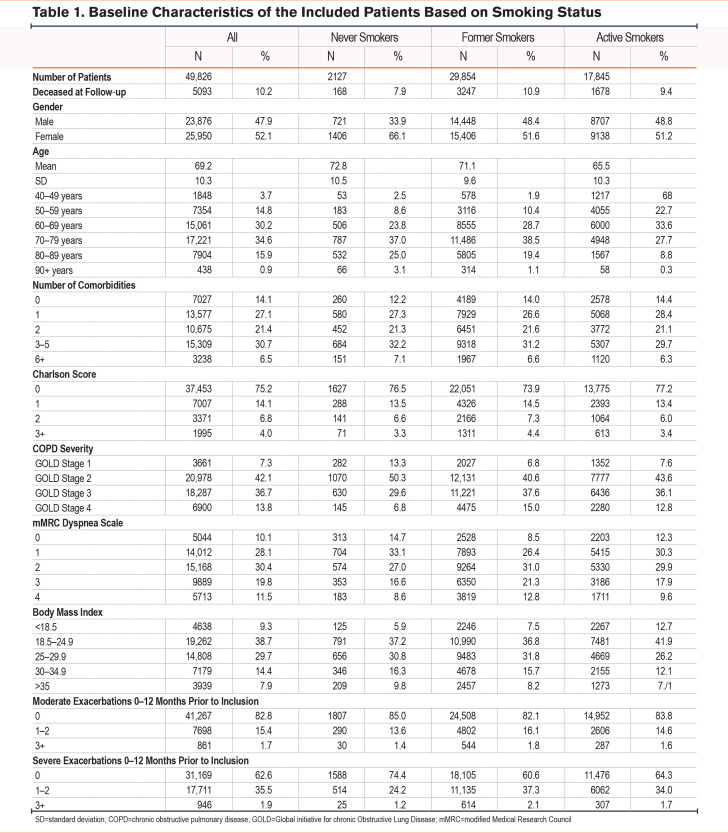
In total, 49,826 patients with COPD between 40 and 99 years of age were included in the study of whom 2127 (4%) were never smokers, 29,854 (60%) were former smokers, and 17,845 (35%) were present smokers. Never smokers reported milder symptoms based on mMRC scores and were classified with milder COPD stage according to Global initiative for chronic Obstructive Lung Disease14 (GOLD) 1–classification compared to patients with a smoking history. Table 1 shows baseline characteristics of the included patients based on smoking status. The majority of never smokers were women (66%) while the gender distribution was more equal among former smokers and active smokers. Mean age was 69.2 years with active smokers being younger (mean age 65 years). No differences were observed between never smokers, former smokers, and active smokers with respect to education. The minority of patients went to college, whereas most patients had junior high school as the highest achieved level of education.
Regarding dyspnea, 47.8% of never smokers reported an mMRC score of 0–1, while only 34.9% of former smokers and 42.6% of present smokers reported an mMRC of 0–1. In terms of very severe dyspnea, 8.6% of never smokers reported an mMRC of 4, compared to 12.8% of formers smokers and 9.6% of present smokers. With regard to FEV1 percentage of predicted value (%pred), 13% of never smokers classified as having mild COPD (GOLD Stage 1) based on lung function parameters as compared to 6.8% and 7.6% for former and current smokers, respectively. In comparison, 15% of former smokers and 12.8% of current smokers were classified as having very severe COPD (GOLD Stage 4) as compared to only 6.8% of never smokers.
No differences in number of moderate exacerbations during the last year prior to inclusion were found between the 3 smoking groups. However, severe exacerbations were more common among former and current smokers as compared to never smokers. Only 24.2% of never smokers had had 1–2 severe exacerbations one year prior to inclusion as compared to 37.3% of former smokers and 34% of current smokers.
Risk of Moderate Exacerbations at Follow-Up
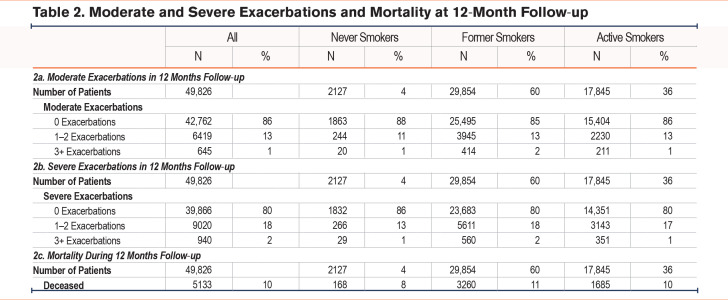
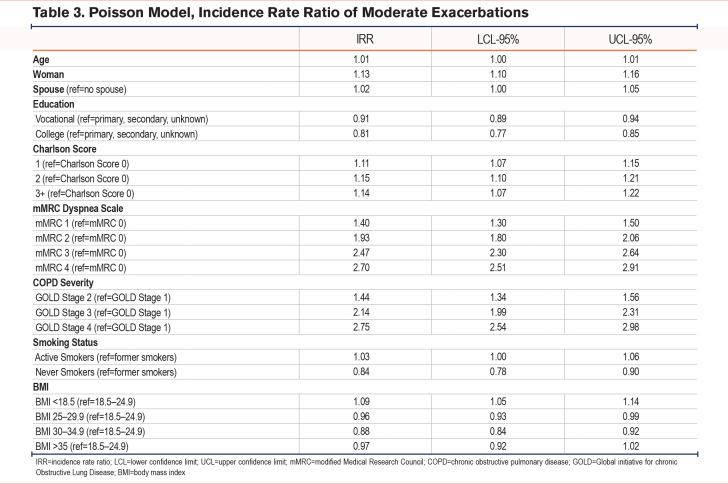
During the 12-month follow up, 7064 patients (14%) experienced 1 or more moderate exacerbations, of whom 264 (4%) were never smokers, 4359 (62%) were former smokers, while 2441 (34%) were current smokers (Table 2a). Based on Poisson regression models, never smokers had a significantly lower risk of having a moderate exacerbation compared to patients with a smoking history (incidence rate ratio [IRR] 0.87 [95% confidence interval (CI) 0.78–0.97]). Other risk factors were found to be female sex (IRR 1.23 [95% CI 1.18–1.28]) and level of education (Table 3).
The most pronounced risk factors for moderate exacerbations were the severity of dyspnea (mMRC) and the severity of airway obstruction measured as FEV1 %pred (Table 3). Having an mMRC of 4 increased the incident risk rate to 1.9 (95% CI 1.71–2.11) compared to mMRC 0 while FEV1 <30% increased the incident risk rate to 2.03 (1.81–2.27) times compared to FEV1 ≥ 80%.
The number of comorbidities were inversely correlated to risk of moderate exacerbations with an IRR of having ≥3 comorbidities at 0.63 (95%CI 0.55–0.71)
Risk of Severe Exacerbations at Follow-Up
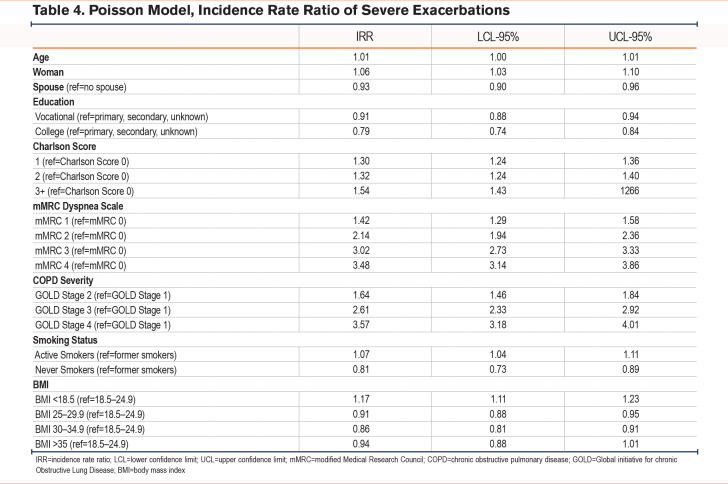
During the 12-month follow-up, 9960 patients (20%) experienced 1 or more severe exacerbations, of whom 295 (3%) were never smokers, 6171 (62%) were former smokers, while 3494 (35%) were current smokers (Table 2b). Based on Poisson regression models, never smokers had a significantly lower risk of having a severe exacerbation compared to patients with a smoking history (IRR 0.81 [0.73–0.89]) (Table 4). Having an mMRC of 4 increased the incident risk rate to 3.48 (3.14–3.86) compared to an mMRC of 0 while FEV1 <30% increased the incident risk rate to 3.57 (3.18–4.01) compared to FEV1 ≥ 80%. As opposed to risk of moderate exacerbations, number of comorbidities was associated with a higher risk of severe exacerbations with more comorbidities increasing the risk (IRRCharlson score 1 1.30 [1.24–1.36], IRRCharlson score 2 1.32 [1.24–1.40], IRRCharlson score 3+ 1.54 [1.43–1.66]) compared to no comorbidities.
Risk of severe exacerbations was highest among people with a BMI <18.5 compared to a BMI between 18.5–24.9 (IRR 1.17 [95% CI 1.11–1.23]) whereas having a BMI between 30 and 34.9 had a lower risk of severe exacerbations (IRR 0.94 [0.88–1.01]). However, this result was not found to be significant.
Risk of Mortality at Follow-Up
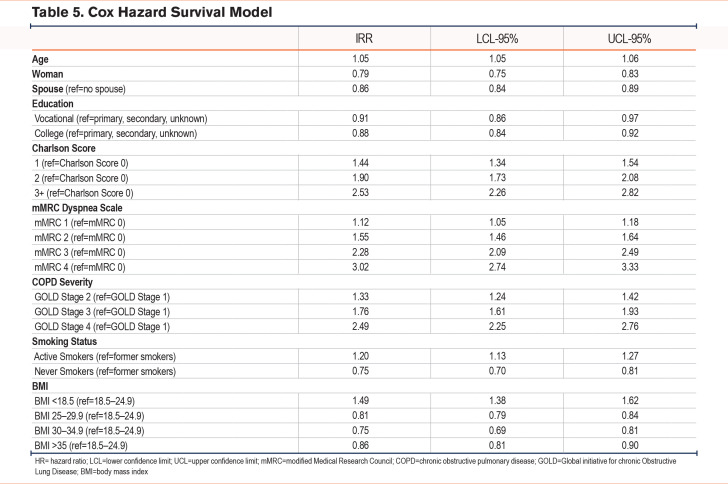
During the 12-month follow-up period, 5113 (10%) patients died. Among the patients who died during the 12 months follow-up, 168 (3%) were never smokers, 3260 (64%) were former smokers, while 1685 (33%) were current smokers (Table 2C). With the Cox hazard survival Model, current smokers had an increased hazard ratio for death of 1.20 (1.13–1.27) compared to former smokers, whereas never smokers had a significantly lower hazard ratio for death (0.75 [0.70–0.81]) compared to former smokers (Table 5).
Regarding the other variables of interest, we found that a higher mMRC score, a lower FEV1, more comorbidities, and a low BMI were associated with a higher risk of death. Having an mMRC of 4 increased the hazard ratio for death to 3.02 (2.74–3.33) compared to an mMRC of 0. An FEV1 < 30% increased the hazard ratio for death to 2.49 (2.25–2.76) compared to an FEV1 ≥ 80%. Having 3 or more comorbidities increased the hazard ratio for death to 2.53 (2.26–2.82) compared to no comorbidity. The hazard ratio for death was significantly higher for people with a BMI <18.5 (HR 1.48, 1.38–1.62) than for people with a BMI between 18.5 and 24.9, whereas having a BMI between 30 and 34.9 seemed to be protective (HR 0.75; 0.69–0.81) (Table 5).
Discussion
Results from our nationwide study show that the prognosis of never smokers with COPD is much more favorable than in individuals who are former or current smokers. Never smokers are at lower risk of both moderate and severe exacerbations and death. They report lower levels of dyspnea and are classified with a milder stage of COPD according to GOLD classification. This is in line with results from other studies showing a significantly better prognosis for never smokers with COPD compared to current smokers and former smokers with COPD.8,9,15 The pathogenesis of COPD in never smokers is not fully understood. However, potential mechanisms include inflammation, oxidative stress, airway remodeling, and accelerated lung aging.8
Even though smoking is still the most pronounced risk factor for developing COPD, smoking prevalence has declined by 27.2% for men since 1990 and by 37.9% for women.16
Several studies have found environmental exposures to be risk factors for COPD in never smokers, such as passive smoking, burning biomass, and occupational exposure to vapor, gas, dust, and organic dust as well as a history of asthma or respiratory infections during childhood.7,17,18 While smoking rates have declined worldwide, nicotine vaping has substantially increased. However, according to 2021 and 2022 data from the Danish National COPD Registry, only 2% of patients with COPD were active vapers, whereas 4% and 6% were former vapers in 2021 and 2022, respectively.19 Data on passive smoking, biomass burning, and exposure to gas, dust, and organic dust were not available in our study. Therefore, further studies in never smokers with COPD are needed to fully understand the pathogenesis and mechanisms of COPD in these individuals.
We also found that former smokers are at lower risk of both moderate and severe exacerbations and death compared to current smokers. There was a significant association between smoking-related symptoms such as severity of dyspnea, severity of airway obstruction, and number of comorbidities on both risk of moderate exacerbations, risk of severe exacerbations leading to hospitalizations, and risk of death. These data are in line with the results of other studies showing that the strongest modifiable risk factor for the prognosis of COPD is smoking.2,4,20-22 Furthermore, several studies have shown that severity of dyspnea and severity of airway obstruction are associated with smoking status and that smoking cessation improves both dyspnea and airway obstruction.23-26
Smoking is also a risk factor for other diseases such as cardiovascular diseases and diabetes.27-32 As results from our study show, individuals with a higher number of comorbidities are at higher risk of severe COPD exacerbations and death from COPD. This emphasizes that smoking cessation does positively affect both COPD outcomes as well as clinical outcomes for many other diseases, eventually resulting in a higher quality of life.
This knowledge is important as a motivational factor for doctors and other health care professionals to support smoking cessation for patients with COPD. Even though patients may not feel immediate effect, or even feel temporary worsening of dyspnea, after smoking cessation, the results from our study confirm the positive long-term effect of smoking cessation on COPD-related outcomes.
Patients with COPD have been shown to be more resistant to smoking cessation compared to healthy smokers, partly because they find symptom relief when smoking and because of old habits with friends and family.33 Studies have shown that only about 1% of smokers succeed in quitting smoking every year without help and that one year after an initial smoking cessation attempt, 80% of patients with COPD are still smoking.2,4,34
However, several studies have shown that smokers with COPD using approved smoking cessation pharmacologic aids, such as varenicline, bupropion, or nicotine replacement, plus at least short-term counseling, are, on average, as successful at quitting and maintaining abstinence for 1 year as smokers in general.35-37 This emphasizes the need for a prolonged effort preferably based on the combination of nicotine substitution treatment and behavioral counseling37 preferably based on interviewing with the purpose of increasing motivation and self-efficacy in the patient.38
In line with other studies,39,40 the majority of never smokers with COPD are shown to be women. World-wide, exposure to biomass smoke is thought to be the main risk factor for nonsmoking-related COPD, and mainly women cook in poorly ventilated homes in developing countries.41,42 However, this study is conducted in Denmark, one of the richest and most developed countries in the world, and thus, the reason for never-smoking women being more vulnerable to develop COPD must be found elsewhere. Gut-Gobert et al suggests that women might have a specific COPD-related phenotype,39 whereas Aryal et al mentions that people with asthma, which is more common in women, have a higher risk for developing COPD.41
Furthermore, we found that women were at higher risk for moderate as well as severe exacerbations, regardless of age and smoking status. This is in line with results from other studies43,44 underlining the need for appropriate identification and management of women with COPD.
Another finding from our study was a higher risk of both moderate and severe exacerbations in patients with only a junior high degree compared to patients with a college degree. The reason for this might be that well-educated people do not smoke as much and for as long a time as less educated people.45,46 Being married was shown to be protective, at least against severe exacerbations. The reason for this could be similar to educational influence, namely that married people tend to smoke less.47,48 Another explanation could be that a spouse can help look after the partner and thus, reduce the need for hospitalization.
Similar to other studies, our study found that obese people with a BMI between 30 and 34.9 had a lower risk of severe exacerbations and mortality than those with a BMI in the normal range.49-52 Putcha et al showed that while underweight COPD participants in the UPLIFT and TIOSPIR studies had a significantly higher risk of death and severe exacerbations versus normal weight participants, overweight and obese participants were at lower to no additional risk.52 In line with this, Spelta et al reported a significant protective effect of obesity in patients with COPD on all-cause mortality, and referred to this as the “obesity paradox.”50
A possible explanation could be that the lower FEV1 found in obese people may be linked to a restrictive defect rather than to an obstructive one. In line with this, obese COPD patients may have less hyperinflation of the lungs due to increased fat mass in the abdomen. Former studies have shown an association between hyperinflation and severe exacerbations and mortality.53,54
The study has several strengths. It is a large, nationwide study including more than 49,000 individuals. It is based on data from the Danish National Health Registries which are valid and updated, and because of the individual identification number (CPR-number), patients can be followed up regarding both hospitalizations, redeemed prescriptions, and death. It is, thereby, possible to predict risk of COPD outcomes based on smoking status nationwide. The population in this study is identical with White COPD patients from other countries, regarding age, education, severity of the disease, number of comorbidities, and BMI. Therefore, the study can be applied to other White populations.
Limitations of the current study include the short follow-up period, the absence of confirmation that the participants did indeed have COPD (despite their assignment by the treating physician to an ICD-10 code consistent with COPD) based on a post-bronchodilator FEV1/FVC below 80% or the lower limit of normal. Other limitations include the failure to take into account a history of asthma that could have led to a misdiagnosis of COPD or have affected COPD disease severity, as well as information on other risk factors for COPD in never smokers, including childhood respiratory infections, occupational exposures, and exposure to second-hand smoke.
Being epidemiological by nature, the study can only show associations but not give any information about causality. Furthermore, we cannot tell if the collected medication was actually taken. However, as moderate exacerbations were captured on the basis of redeemed prescriptions of prednisolone or prednisone in the Danish Prescription Registry and defined as short-term use of minimum 25 mg of an OCS per day for 5 days in patients with a known history of COPD, we must assume that the medication has been taken.
Given the observational nature of this study, limitations also include the difficulty of interpreting outcomes that could be related to treatment since COPD patients are usually on different treatment regimens that are prescribed at different times without details about their treatment duration. Regarding standard COPD medications such as long-acting beta2- agonists, long-acting muscarinic antagonists, and inhaled corticosteroids, we do not include any data on regular use and, thereby, the data cannot show any differences in medication among active smokers, former smokers, and never smokers. This is a limitation of the current study as the primary factors of interest are dyspnea, FEV1, exacerbations, and hospitalizations, all factors that could be impacted by medication use and not only by smoking status. However, with 49,826 patients included in the dataset, differences in medication must be expected to be equally distributed among the 3 groups.
Another limitation is that smoking status is recorded in the registries based on patient self-report and not verified by biomarkers like cotinine. It could be relevant to confirm smoking status with the above-mentioned methods, but these data were not available in the present study. However, only 4% of the included patients reported to be never smokers. This number is significantly lower than reported in other studies where up to 40% claim to be never smokers.24 One explanation for the very low rate of never smokers found in this study could be the fact that the study is conducted in Denmark where the general air pollution is low and the standard of living is very high meaning that the absolute highest risk factor for developing COPD is tobacco. However, this cannot be confirmed as the study does not include data on environmental risk factors (i.e., exposure to burning biomass, occupational exposures to vapor, gas, dust, and organic dust as well as a history of asthma or respiratory infections during childhood) for developing COPD as these data were not available.
Conclusion
Never smokers have milder symptoms and a better prognosis of COPD than both former and current smokers with COPD. However, it is not too late to quit smoking for patients with COPD, as former smokers have milder symptoms and a better prognosis compared to current smokers. As smoking is the most important modifiable risk factor for COPD, smoking cessation should be recommended to all patients with COPD and supported by motivation and behavioral interventions.
Abbreviations
Abbreviations: ATC=anatomical therapeutic codes; BMI=body mass index; CCI=Charlson Comorbidity Index; CI=confidence interval; COPD=chronic obstructive pulmonary disease; FEV1=forced expiratory volume in 1 second; FVC=forced vital capacity; GOLD=Global initiative for chronic Obstructive Lung Disease; HR=hazard ratio; IRR=incidence rate ratio; LCL=lower confidence limit; mMRC=modified Medical Research Council; OCS=orally-administered corticosteroids; SD=standard deviation; UCL=upper confidence limit
Funding Statement
No funding.
References
- 1.Nielsen AO,Hilberg O,Jensen JUS,et al. Withdrawal of inhaled corticosteroids in patients with COPD: a prospective observational study. Int J Chron Obstruct Pulmon Dis. 2021;16:807-815. doi: https://doi.org/10.2147/COPD.S294217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tashkin DP. Smoking cessation in chronic obstructive pulmonary disease. Semin Respir Crit Care Med. 2015;36(4):491-507. doi: https://doi.org/10.1055/s-0035-1555610 [DOI] [PubMed] [Google Scholar]
- 3.Warnier MJ,Van Riet EES,Rutten FH,De Bruin ML,Sachs APE. Smoking cessation strategies in patients with COPD. Eur Respir J. 2013;41:727-734. doi: https://doi.org/10.1183/09031936.00014012 [DOI] [PubMed] [Google Scholar]
- 4.Thabane M,Group CW. Smoking cessation for patients with chronic obstructive pulmonary disease (COPD): an evidence-based analysis. Ont Health Technol Assess Ser. 2012;12(4):1-50. [PMC free article] [PubMed] [Google Scholar]
- 5.Guan WJ,Zheng XY,Chung KF,Zhong NS. Impact of air pollution on the burden of chronic respiratory diseases in China: time for urgent action. Lancet. 2016;388(10054):1939-1951. doi: https://doi.org/10.1016/S0140-6736(16)31597-5 [DOI] [PubMed] [Google Scholar]
- 6.Doiron D,de Hoogh K,Probst-Hensch N,et al. Air pollution, lung function and COPD: results from the population-based UK Biobank study. Eur Respir J. 2019;54:1802140. doi: https://doi.org/10.1183/13993003.02140-2018 [DOI] [PubMed] [Google Scholar]
- 7.Pando-Sandoval A,Ruano-Ravina A,Candal-Pedreira C,et al. Risk factors for chronic obstructive pulmonary disease in never-smokers: a systematic review. Clin Respir J. 2022;16(4):261-275. doi: https://doi.org/10.1111/crj.13479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang IA,Jenkins CR,Salvi SS. Chronic obstructive pulmonary disease in never-smokers: risk factors, pathogenesis, and implications for prevention and treatment. Lancet Respir Med. 2022;10(5):497-511. doi: https://doi.org/10.1016/S2213-2600(21)00506-3 [DOI] [PubMed] [Google Scholar]
- 9.Lamprecht B,McBurnie MA,Vollmer WM,et al. COPD in never smokers: results from the population-based burden of obstructive lung disease study. Chest. 2011;139(4):752-763. doi: https://doi.org/10.1378/chest.10-1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lange P,Tøttenborg SS,Sorknæs AD,et al. Danish Register of chronic obstructive pulmonary disease. Clin Epidemiol. 2016;8:673-678. doi: https://doi.org/10.2147/CLEP.S99489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stenton C. The MRC breathlessness scale. Occup Med. 2008;58(3):226-227. doi: https://doi.org/10.1093/occmed/kqm162 [DOI] [PubMed] [Google Scholar]
- 12.Løkke A,Hilberg O,Lange P,et al. Disease trajectories and impact of one moderate exacerbation in GOLD B COPD patients. Int J Chron Obstruct Pulmon Dis. 2022;17:569-578. doi: https://doi.org/10.2147/COPD.S344669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borucka J. Extensions of Cox model for non-proportional hazards purpose. Econometrics. 2014;3(45):85-101. doi: https://doi.org/10.15611/ekt.2014.3.07 [Google Scholar]
- 14.Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease, 2023 report. GOLD website. Published 2023. Accessed June 2023. https://goldcopd.org/wp-content/uploads/2023/03/GOLD-2023-ver-1.3- 17Feb2023_WMV.pdf [Google Scholar]
- 15.Thomsen M,Nordestgaard BG,Vestbo J,Lange P. Characteristics and outcomes of chronic obstructive pulmonary disease in never smokers in Denmark: a prospective population study. Lancet Respir Med. 2013;1(7):543-550. doi: https://doi.org/10.1016/S2213-2600(13)70137-1 [DOI] [PubMed] [Google Scholar]
- 16.Dai X,Gakidou E,Lopez AD. Evolution of the global smoking epidemic over the past half century: strengthening the evidence base for policy action. Tob Control. 2022;31:129-137. doi: https://doi.org/10.1136/tobaccocontrol-2021-056535 [DOI] [PubMed] [Google Scholar]
- 17.Hagstad S,Bjerg A,Ekerljung L,et al. Passive smoking exposure is associated with increased risk of COPD in never-smokers. Chest. 2014;145(6):1298-1304. doi: https://doi.org/10.1378/chest.13-1349 [DOI] [PubMed] [Google Scholar]
- 18.Würtz ET,Schlünssen V,Malling TH,Hansen JG,Omland Ø. Occupational COPD among Danish never-smokers: a population-based study. Occup Environ Med. 2015;72(6):456-459. doi: https://doi.org/10.1136/oemed-2014-102589 [DOI] [PubMed] [Google Scholar]
- 19.Danish Registry for Chronic Obstructive Pulmonary Disease (DrCOL) [in Danish]. DrCOL website. Published 2023. Accessed August 10, 2023. https://www.rkkp.dk/kvalitetsdatabaser/databaser/dansk-register-for-kronisk-obstruktiv-lungesygdom/ [Google Scholar]
- 20.Vij N,Chandramani-Shivalingappa P,Van Westphal C,Hole R,Bodas M. Cigarette smoke-induced autophagy impairment accelerates lung aging, COPD-emphysema exacerbations and pathogenesis. Am J Physiol Cell Physiol. 2018;314(1):C73-C87. doi: https://doi.org/10.1152/ajpcell.00110.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laniado-Laborin R. Smoking and chronic obstructive pulmonary disease (COPD). Parallel epidemics of the 21st century. Int J Environ Res Public Health. 2009;6(1):209-224. doi: https://doi.org/10.3390/ijerph6010209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Josephs L,Culliford D,Johnson M,Thomas M. Improved outcomes in ex-smokers with COPD: a UK primary care observational cohort study. Eur Respir J. 2017;49:1602114. doi: https://doi.org/10.1183/13993003.02114-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Y,Pleasants RA,Croft JB,et al. Smoking duration, respiratory symptoms, and COPD in adults aged ≥45 years with a smoking history. Int J Chron Obstruct Pulmon Dis. 2015;10:1409-1416. doi: https://doi.org/10.2147/COPD.S82259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grnøseth R,Vollmer WM,Hardie JA,et al. Predictors of dyspnoea prevalence: results from the BOLD study. Eur Respir J. 2014;43:1610-1620. doi: https://doi.org/10.1183/09031936.00036813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lange P,Groth S,Nyboe J,et al. Effects of smoking and changes in smoking habits on the decline of FEV1. Eur Respir J. 1989;2:811-816. doi: https://doi.org/10.1183/09031936.93.02090811 [PubMed] [Google Scholar]
- 26.Xu X,Weiss ST,Rijcken B,Schouten JP. Smoking, changes in smoking habits, and rate of decline in FEV1: new insight into gender differences. Eur Respir J. 1994;7:1056-1061. doi: https://doi.org/10.1183/09031936.94.07061056 [PubMed] [Google Scholar]
- 27.Cavaillès A,Brinchault-Rabin G,Dixmier A,et al. Comorbidities of COPD. Eur Respir Rev. 2013;22:454-475. doi: https://doi.org/10.1183/09059180.00008612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zaher C,Halbert R,Dubois R,George D,Nonikov D. Smoking-related diseases: the importance of COPD. Int J Tuberc Lung Dis. 2004;8(12):1423-1428. https://www.ingentaconnect.com/contentone/iuatld/ijtld/2004/00000008/00000012/art00005 [PubMed] [Google Scholar]
- 29.Kondo T,Nakano Y,Adachi S,Murohara T. Effects of tobacco smoking on cardiovascular disease. Circ J. 2019;83(10):1980-1985. doi: https://doi.org/10.1253/circj.CJ-19-0323 [DOI] [PubMed] [Google Scholar]
- 30.Sliwinska-Mosson M,Milnerowicz H. The impact of smoking on the development of diabetes and its complications. Diab Vasc Dis Res. 2017;14(4):265-276. doi: https://doi.org/10.1177/1479164117701876 [DOI] [PubMed] [Google Scholar]
- 31.Ding L,Xiao X. Smoking and diabetes [in Chinese]. Chinese J Diabetes Mellit. 2020;12(9):736-739. doi: https://doi.org/10.3760/cma.j.cn115791-20200213-00070 [Google Scholar]
- 32.Villablanca AC,McDonald JM,Rutledge JC. Smoking and cardiovascular disease. Clin Chest Med. 2000;21(1):159-172. doi: https://doi.org/10.1016/S0272-5231(05)70015-0 [DOI] [PubMed] [Google Scholar]
- 33.Babb S,Malarcher A,Schauer G,Asman K,Jamal A. Quitting smoking among adults -United States, 2000-2015. MMWR Morb Mortal Wkly Rep. 2017;65(52):1457-1464. doi: https://doi.org/10.15585/mmwr.mm6552a1 [DOI] [PubMed] [Google Scholar]
- 34.Garcia-Gomez L,Hernández-Perez A,Noe-Diaz V,Antonio Riesco-Miranda J,Jimenez-Ruiz C. Smoking cessation treatments: current psychological and pharmacological options. Rev Invest Clin. 2019;71:7-16. doi: https://doi.org/10.24875/RIC.18002629 [DOI] [PubMed] [Google Scholar]
- 35.Tashkin DP,Rennard S,Hays JT,Ma W,Lawrence D,Lee TC. Effects of varenicline on smoking cessation in patients with mild to moderate COPD: a randomized controlled trial. Chest. 2011;139(3):591-599. doi: https://doi.org/10.1378/chest.10-0865 [DOI] [PubMed] [Google Scholar]
- 36.Chen D,Wu LT. Smoking cessation interventions for adults aged 50 or older: a systematic review and meta-analysis. Drug Alcohol Depend. 2015;154:14-24. doi: https://doi.org/10.1016/j.drugalcdep.2015.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wei X,Guo K,Shang X,et al. Effects of different interventions on smoking cessation in chronic obstructive pulmonary disease patients: a systematic review and network meta-analysis. Int J Nurs Stud. 2022;136:104362. doi: https://doi.org/10.1016/j.ijnurstu.2022.104362 [DOI] [PubMed] [Google Scholar]
- 38.Lindson-Hawley N,Thompson TP,Begh R. Motivational interviewing for smoking cessation. Cochrane Database Syst Rev. 2015;3:CD006936. doi: https://doi.org/10.1002/14651858.CD006936.pub3 [DOI] [PubMed] [Google Scholar]
- 39.Gut-Gobert C,Cavaillès A,Dixmier A,et al. Women and COPD: do we need more evidence? Eur Respir Rev. 2019;28:180055. doi: https://doi.org/10.1183/16000617.0055-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wheaton AG,Liu Y,Croft JB,et al. Chronic obstructive pulmonary disease and smoking status. MMWR Morb Mortal Wkly Rep. 2019;68(24):533-538. doi: http://dx.doi.org/10.15585/mmwr.mm6824a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aryal S,Diaz-Guzman E,Mannino DM. Influence of sex on chronic obstructive pulmonary disease risk and treatment outcomes. Int J Chron Obstruct Pulmon Dis. 2014;9(1):1145-1154. doi: https://doi.org/10.2147/COPD.S54476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Salvi SS,Barnes PJ. Chronic obstructive pulmonary disease in non-smokers. Lancet. 2009;374(9691):733-743. doi: https://doi.org/10.1016/S0140-6736(09)61303-9 [DOI] [PubMed] [Google Scholar]
- 43.DeMeo DL,Ramagopalan S,Kavati A,et al. Women manifest more severe COPD symptoms across the life course. Int J Chron Obstruct Pulmon Dis. 2018;13:3021-3029. doi: https://doi.org/10.2147/COPD.S160270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stolz D,Kostikas K,Loefroth E,et al. Differences in COPD exacerbation risk between women and men: analysis from the UK clinical practice research datalink data. Chest. 2019;156(4):674-684. doi: https://doi.org/10.1016/j.chest.2019.04.107 [DOI] [PubMed] [Google Scholar]
- 45.Broms U,Silventoinen K,Lahelma E,Koskenvuo M,Kaprio J. Smoking cessation by socioeconomic status and marital status: the contribution of smoking behavior and family background. Nicotine Tob Res. 2004;6(3):447-455. doi: https://doi.org/10.1080/14622200410001696637 [DOI] [PubMed] [Google Scholar]
- 46.Giannico OV,Ambrosino I,Patano F,Germinario C,Quarto M,Moretti AM. Educational level, marital status and sex as social gender discharge determinants in chronic obstructive pulmonary disease exacerbations: a time-to-event analysis. Monaldi Arch Chest Dis. 2019;89(2). doi: https://doi.org/10.4081/monaldi.2019.1017 [DOI] [PubMed] [Google Scholar]
- 47.Ramsey MW,Chen-Sankey JC,Reese-Smith J,Choi K. Association between marital status and cigarette smoking: variation by race and ethnicity. Prev Med. 2019;119:48-51. doi: https://doi.org/10.1016/j.ypmed.2018.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lindström M. Social capital, economic conditions, marital status and daily smoking: a population-based study. Public Health. 2010;124(2):71-77. doi: https://doi.org/10.1016/j.puhe.2010.01.003 [DOI] [PubMed] [Google Scholar]
- 49.Zewari S,Vos P,van den Elshout F,Dekhuijzen R,Heijdra Y. Obesity in COPD: revealed and unrevealed issues. COPD. 2017;14(6):663-673. doi: https://doi.org/10.1080/15412555.2017.1383978 [DOI] [PubMed] [Google Scholar]
- 50.Spelta F,Pasini AMF,Cazzoletti L,Ferrari M. Body weight and mortality in COPD: focus on the obesity paradox. Eat Weight Disord. 2018;23:15-22. doi: https://doi.org/10.1007/s40519-017-0456-z [DOI] [PubMed] [Google Scholar]
- 51.Hanson C,Rutten EP,Wouters EFM,Rennard S. Influence of diet and obesity on COPD development and outcomes. Int J Chron Obstruct Pulmon Dis. 2014;9(1):723-733. doi: https://doi.org/10.2147/COPD.S50111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Putcha N,Anzueto AR,Calverley PMA,et al. Mortality and exacerbation risk by body mass index in patients with COPD in TIOSPIR and UPLIFT. Ann Am Thorac Soc. 2022;19(2):204-213. doi: https://doi.org/10.1513/AnnalsATS.202006-722OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim YW,Lee CH,Hwang HG,et al. Resting hyperinflation and emphysema on the clinical course of COPD. Sci Rep. 2019;9:3764. doi: https://doi.org/10.1038/s41598-019-40411-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Budweiser S,Harlacher M,Pfeifer M,Jörres RA. Co-morbidities and hyperinflation are independent risk factors of all-cause mortality in very severe COPD. COPD. 2014;11(4):388-400. doi: https://doi.org/10.3109/15412555.2013.836174 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This article contains supplemental material.