Abstract
Background:
The vast majority of individuals who come to the emergency department (ED) for care after a motor vehicle collision (MVC) are diagnosed with musculoskeletal strain only and are discharged to home. A significant subset of this population will still develop persistent pain and posttraumatic psychological sequelae may play an important role in pain persistence.
Methods:
We conducted a multisite longitudinal cohort study of adverse post-traumatic neuropsychiatric sequelae among patients seeking ED treatment in the aftermath of a traumatic life experience. We report on a sub-group of patients (n = 666) presenting after an MVC, the most common type of trauma and we examine associations of socio-demographic and MVC characteristics, and persistent pain 8 weeks after MVC. We also examine the degree to which these associations are related to peritraumatic psychological symptoms and 2-week acute stress reactions using an applied approach.
Results:
Eight-week prevalence of persistent moderate or severe pain was high (67.4%) and positively associated with patient sex (female), older age, low socioeconomic status (education and income) and pain severity in the ED. Peritraumatic stress symptoms (distress and dissociation) appear to exert some influence on both acute pain and the transition from acute to persistent pain.
Discussion and Conclusions:
The early aftermath of an MVC may be an important time period for intervening to prevent and reduce persistent pain. Substantial variation in mediating pathways across predictors also suggests potential diverse and complex underlying biological and psychological pathogenic processes are at work in the early weeks following trauma.
Significance:
The first several days after trauma may dictate recovery trajectories. Persistent pain, pain lasting beyond the expected time of recovery, is associated with pain early in the recovery period, but also mediated through other pathways. Future work is needed to understand the complex neurobiological processes in involved in the development of persistent and acute post-traumatic pain.
1 |. INTRODUCTION
A substantial subset of individuals experiencing traumatic stress develop persistent adverse post-traumatic neuropsychiatric sequelae (APNS) (Koenen et al., 2017; Santiago et al., 2013). APNS include post-traumatic stress, post-concussive (somatic) symptoms, depressive symptoms and chronic pain (Kessler, 2000; Kessler et al., 1995; Roberts et al., 2011). As with the other types of APNS, the prevention of persistent and chronic pain after traumatic stress exposure is an important public health priority: Chronic pain afflicts more than 30% of US adults, (Grol-Prokopczyk, 2017; Johannes et al., 2010; McBeth & Jones, 2007) accounts for more than $260 billion in annual US health care costs, (Gaskin & Richard, 2012) and drives the majority of US opioid prescriptions (Ernst et al., 2015; Moshfegh et al., 2018).
Hundreds of millions of opportunities occur each year to screen and initiate interventions to prevent APNS, including chronic pain, however screening and intervention are rarely performed. For example, very few of the 40 million Americans who present to an emergency department (ED) each year in the immediate aftermath of trauma exposure receive any screening or interventions for APNS (Roberts et al., 2011). Efforts to develop such interventions are hampered by critical knowledge gaps, including knowledge gaps related to the pathogenesis of specific APNS such as chronic pain.
Motor vehicle collisions (MVCs) in particular are one of the most common life-threatening traumatic stress exposures and even minor MVCs can result in high morbidity from chronic pain and other APNS (Ravn et al., 2019, 2020). In the US there are over 4 million ED visits related to MVCs each year (Albert & McCaig, 2015). More than 90% of individuals who come to the ED for care after MVC are discharged home with a diagnosis of musculoskeletal strain only, (Bortsov et al., 2013). However, a substantial portion of this population develops persistent pain, that is pain that lasts beyond the expected time of normal healing (Hartling et al., 2001; McLean et al., 2014; Suissa et al., 2001) and ultimately persistent pain can become chronic (Nolet et al., 2020). Historically such pain was attributed to tissue injury, but more recently neurobiological stress systems have been implicated in both posttraumatic psychologic symptoms and pain persistence after physical trauma (McLean, 2016). Furthermore, the development of post-traumatic stress disorder and chronic pain are often co-morbid, suggesting potentially a shared neurobiological mechanism (Ravn et al., 2020). Targeting acute peritraumatic stress after trauma has also been shown to improve pain outcomes (Sterling et al., 2019) and other APNS (Gil-Jardine et al., 2018). Peritraumatic stress can be thought of as the acute psychological response to trauma and is often characterized by specific clusters of symptoms, particularly, distress and dissociation. Since the presence of peritraumatic stress symptoms (distress and dissociation) predict PTSD independent of trauma severity or sociodemographic characteristics, (Bryant, 2011) it reasons that peritraumatic distress and dissociation may also mediate pain persistence after a traumatic event. This is particularly important to understand as the early aftermath of trauma exposure may be the an optimal time for therapeutic intervention (Bryant, 2003).
In this analysis, we examine the association of known PTSD predictors (e.g. sex, race/ethnicity, MVC-related characteristics and peritraumatic stress symptoms, [Bryant, 2011]) with both acute and persistent pain development after an MVC. In addition, we explored whether associations between socio-demographic, MVC-related, or peritraumatic stress symptoms and persistent pain were due to their role in acute pain, rather than exerting influence on the transition from acute to persistent pain. We investigate these important issues of APNS dynamics in a large prospective cohort of ED patients involved in an MVC.
2 |. METHODS
2.1 |. Overview
Progress in improving our ability to help prevent and treat APNS is being conducted through programmatic research; the National Institute of Mental Health recently initiated a collaborative study known as the AURORA (Advancing Understanding of RecOvery afteR traumA) study designed to collect a combination of prospective genomic, neuroimaging, psychophysical, physiological, neurocognitive, digital phenotype and self-report data from an enriched sample of approximately 5,000 trauma survivors recruited from EDs in the early aftermath of trauma and to follow these patients closely for 1 year after trauma exposure. As described in more detail elsewhere (McLean et al., 2020), each of the four traditional APNS (post-traumatic stress, post-concussion syndrome, depression and pain) and their intermediate phenotypes are being characterized in AURORA with both self-reported scales and biomarkers from different Research Domain Criteria (RDoC) ‘units of analysis’ (https://bit.ly/2pudCZH) to assess data-driven multidimensional phenotypes based on the most frequent symptoms observed among trauma survivors. With supplemental support (see Acknowledgements), AURORA aims to produce a well-powered, multi-layered publicly available dataset capable of identifying APNS and studying risk and protective factors for APNS onset and course.
Initial AURORA analyses are focusing on patterns and associations of trauma experiences and peritraumatic symptoms with the separate traditional APNS in the first 8 weeks after trauma exposure. This timeframe was chosen as the early aftermath of trauma exposure is likely a critical intervention period and because pain trajectories are relatively stable 8 weeks after trauma (Hu et al., 2016; Sterling et al., 2011; Ulirsch, Weaver, et al., 2014). In this empirical report of AURORA findings, we focus on the development of persistent moderate to severe pain among participants involved in a MVC. We consider only the AURORA respondents who were drivers or passengers involved in an MVC, as this is by far the most common trauma encountered in EDs and makes up the vast majority of initial AURORA participants. We further consider the associations of socio-demographic and MVC characteristics with moderate/severe pain (MSP) as of the 8-week post-MVC assessment and the extent to which these associations are mediated through peritraumatic distress and dissociation (assessed in the immediate aftermath of the MVC in the participating EDs) and MSP measured at the 2-week post-MVC assessment.
2.2 |. Participants
AURORA enrolment began in September 2017. This analysis includes participants who completed the 8-week assessment (described below) enrolled through March 2019. Enrolment occurred at 23 urban EDs across the US. To qualify for participation, patients had to present to a study ED within 72 hr of exposure to a qualifying trauma (physical or sexual assault, MVC, other life-threatening traumatic events). As noted in the introduction, this investigation focuses on patients who were occupants of a vehicle involved in a MVC. In addition to having a MVC, AURORA participants were required to be 18- to 75-years-old, able to speak and read English, able to provide informed consent and to follow protocol at the time of enrolment, physically able to use a smart phone (i.e. not deaf, blind, or have physical impairment that prevented their use of one) (Figure 1). We excluded patients who had a solid organ injury American Association for the Surgery of Trauma (AAST) Grade >1, significant haemorrhage, required an operative intervention, or were likely to be admitted for >72 hr. Of the 867 patients enrolled at baseline, 666 were involved in an MVC trauma and completed both the 2- and 8-week assessments and were analysed as part of this investigation. A comparison of all those enrolled versus those included in the final analyses are presented in the appendix (Table A3).
FIGURE 1.
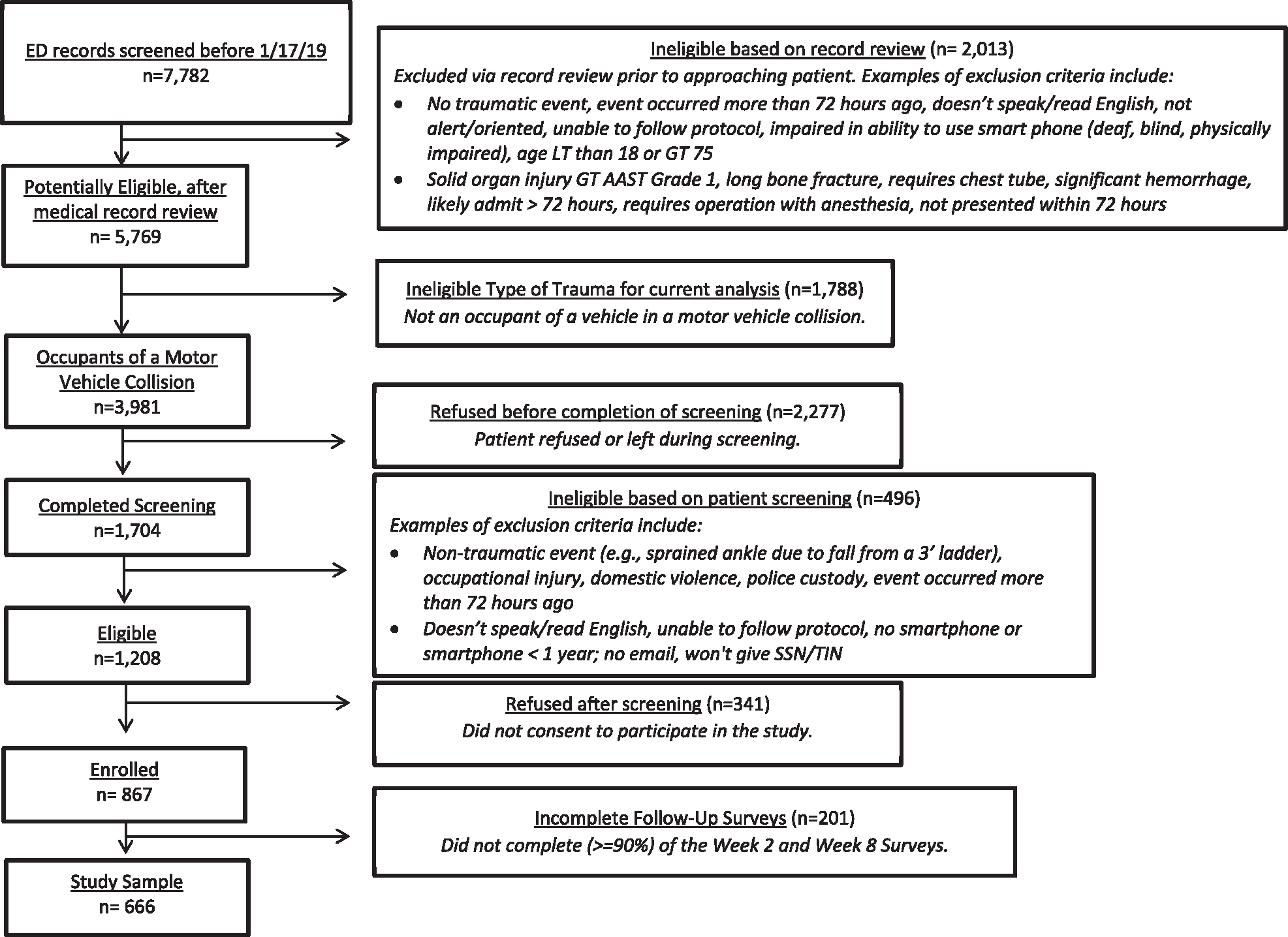
Flowchart of patients reviewed in records at the participating EDs as of 1/17/19
2.3 |. Measures
After providing written informed consent in the ED, each participant received an interviewer-administered assessment that included both self-report questions and biological sample collections described elsewhere (McLean et al., 2020). The self-report questions assessed characteristics of the trauma and peritraumatic symptoms described below. Subsequent 2- and 8-week web surveys were then sent by text or e-mail for self-completion but could be completed with the help of telephone interviewers when this was preferred by the participant. Each participant was reimbursed $60 for the ED assessment, $40 for the 2-week survey, and $40 for the 8-week survey. These procedures were approved on May 12, 2017 by the Biomedical IRB at UNC Chapel Hill through the Office of Human Research Ethics, which also served as the central IRB for all enrolling ED sites.
Baseline surveys collected in the ED assessed socio-demographics, MVC characteristics, and injury information Socio-demographics included age (18–2 4, 25–34, 35–49, 50–75), sex, race-ethnicity (Non-Hispanic Black, Non-Hispanic White, Hispanic, Other), marital status (married/cohabitating, previously married, never married), education (less than high school graduate, high school graduate, some college, college graduate), family income before taxes (divided into approximate tertiles of less than $19K, $19–35K, more than $35K), and employment status (employed vs. others). A number of MVC and injury characteristics that were recorded and considered here include: whether the patient was the driver (with vs. without passengers) or passenger; whether the collision was with a moving vehicle or stationary object; the amount of damage to the patient’s vehicle (on a scale from ‘none’ to ‘severe’); severity of injuries sustained by people other than the patient (separately for passengers in the patient’s vehicle and others); transported to the ED by ambulance or some other mode (immediately or delayed); patient injuries (hit head, sustained a traumatic brain injury, defined as experiencing a head injury with either loss of consciousness, amnesia or disorientation (McLean et al., 2009); severity of injury as based on the Abbreviated Injury Scale (AIS; [Loftis et al., 2018]); admitted vs. discharged without an admission), and patient ratings in the ED of current pain and other symptoms compared to during the 30 days before the MVC.
Overall pain severity was assessed in the ED with a single question using a 0–10 response scale for each of the two time periods (i.e. currently and 30 days before the MVC), where 0 means ‘no pain or tenderness’ and 10 means ‘severe pain or tenderness’ (Farrar et al., 2001). The difference in participant-reported overall pain severity between these two time points was transformed into an overall pain severity score was then standardized to a within-s ample mean of 0 and variance of 1. Comparable pairs of questions were asked about the severity of 20 other somatic symptoms at the same time periods by asking ‘how much of a problem’ each symptom was on a 0–10 scale where 0 means ‘no problem’ and 10 means ‘a major problem’. The 20 symptoms included 12 adapted from the Pennebaker Inventory of Limbic Languidness scale (PILL; [Pennebaker & Watson, 1991]) and an additional 8 from the Rivermead Post-Concussion Symptoms Questionnaire (RPQ; [King et al., 1995]). Each individual-level difference score was standardized to a mean of 0 and a variance of 1. These 20 standardized difference scores were then summed into an overall scale (Cronbach’s α=0.85).
Peritraumatic stress symptoms, distress and dissociation, were assessed in the ED with a rationally-selected 8 items from the short form of the 13-item Peritraumatic Distress Inventory (PDI; [Brunet et al., 2001]) and the 5-item revised Michigan Critical Events Perception Scale (MCEPS; [Michaels et al., 1999]), both validated in MVC patients. The 8 PDI items were selected using part-whole correlation (r = .946) derived from data in an existing cohort of MVC patients (n = 1861) (Beaudoin et al., 2018). We modified the introduction to both question series to ask about frequency of feelings and experiences ‘during and immediately after’ the MVC and used a 0–4 response scale for the PDI and a 1–5 response scale for the MCEPS (‘none of the time’, ‘a little’, ‘some’, ‘most’, ‘all or almost all the time’). Scores were summed to create scales with these respective ranges, 0–32 for PDI and 5–25 for MCEPS scales. Cronbach’s α was 0.80 for the PDI and 0.77 for the MCEPS. Each score was subsequently standardized to a mean of 0 and variance of 1 to facilitate interpretation of associations with later MSP.
Pain outcomes were evaluated using both dichotomous and continuous measures at 2 and 8 weeks after the traumatic event in order to reflect ongoing acute and persistent pain, respectively. These time frames were chosen as the early post-traumatic period is of interest in terms of intervention strategies and by 8 weeks pain trajectories are relatively well established, meaning the likelihood of additional recovery is low (Hu et al., 2016; Sterling et al., 2011; Ulirsch, Weaver, et al., 2014) In the week 2 and week 8 surveys, pain was assessed using a Pain Intensity Numeric Rating Scale (PI-NRS), a single-item measure of pain intensity (Farrar et al., 2001). Patients were asked to report the ‘usual intensity’ of any and all physical pain in the past 2 weeks (2-week survey) or past 30 days (8-week survey) on a 0–10 scale, where 0 = ‘no pain’ and 10 = ‘the worst possible pain’. A score of 4 or more on this single item was used as the threshold to define MSP. For our continuous scale, we used a count of the number of body regions with clinically significant new or worsening pain, which compared 18 body region pain severity scores at 30 days before the MVC to the past 2 weeks (2-week survey) or past 30 days (8-week survey). Clinically significant new or worsening pain was defined as an increase in pain severity by 2 or more points (on a 0–10 response scale) from pre-trauma to post-trauma (Ulirsch, Ballina, et al., 2014). Cronbach’s α was 0.91 for 2-week clinically significant new or worsening pain and 0.95 for 8-week clinically significant new or worsening pain (Bortsov et al., 2013, 2014; McLean et al., 2014).
2.4 |. Analysis methods
We first examined bivariate associations between peritraumatic symptom scales and 2- and 8-week MSP outcomes. We also constructed logistic regression equations to estimate the separate, joint, and interactive associations between peritraumatic symptom scales and 8-week MSP. We examined whether or not the association of peritraumatic symptoms (distress and dissociation) on 8-week MSP was mediated by 2-week pain in a model adjusting for 2-week MSP (both the dichotomous yes/no MSP variable and continuous clinically significant new or worsening pain symptom scores). The sample size led us not to carry out a formal mediation analysis (Pan et al., 2018), but rather we explore possible mediating effects through determining associations (odds-ratios) with the 8-week outcome with and without controls for the peritraumatic symptoms and the 2-week outcomes. Future analyses of the larger AURORA sample will use more formal decomposition methods if we find evidence of important mediating processes based on preliminary analyses such as those reported here.
Linear regression models were then estimated for the associations of socio-demographic and MVC characteristics with peritraumatic distress and dissociation, followed by expanded logistic models for the associations of these same predictors with 8-week MSP. Estimates were generated for models both with and without controls for peritraumatic symptoms and 2-week MSP, which allowed us to examine the extent to which the gross associations of the predictors with 8-week MSP were mediated by peritraumatic symptoms and 2-week MSP. Logits and logits ±2 standard errors were exponentiated and are reported as odds-ratios (ORs) with 95% confidence intervals (CIs). Statistical significance was evaluated using α=0.05-level two-sided tests.
2.5 |. Missing data
Given the small amount of item-level missing data (<1% for any item, range of 0–5 missing responses per item) item missing values were imputed using simple mean/modal/median imputations. Data were assumed to missing at random. Complete information was collected on patient age, sex, MVC characteristics and most patient injury characteristics. The exception was that definitive information on whether the patient experienced a head injury was missing for 8.6% of patients and the presence of missingness was not associated with socio-demographics characteristics. Small numbers of patients were also missing ED information on pain severity (2 patients) and severity of other somatic symptoms 30-days before the MVC (1–2 items for 34 patients, all 20 items for 2 patients). Central tendency imputations were then used for the small amount of missing data on peritraumatic symptoms (mean imputation), education and income (median imputation), and race/ethnicity, marital status and employment status data (mode imputation). MSP for the small number of patients missing on the single overall pain item (n = 2 2-week and n = 4 8-week) were assigned using modal item-level imputations. More respondents were missing 1 or more of the 2-week continuous clinically significant new or worsening pain difference scores (n = 26), and 8-week continuous score (n = 29). Missing items were imputed to the mode of the missing item(s) to calculate the pain difference scores for each body region.
3 |. RESULTS
3.1 |. Prevalence of moderate to severe overall pain (MSP) in the aftermath of MVC
The overall prevalence (standard error, n) of MSP 2 and 8 weeks after MVC in the September 2017 – March 2019 AURORA MVC sample was 81.4% (1.5, n = 542) and 67.4% (1.8, n = 449), respectively. Among participants who reported moderate to severe pain at 2 weeks, 77.1% (1.8, n = 418) continued to have MSP at 8 weeks. A smaller but still sizeable proportion of participants who did not have MSP at 2 weeks, 25.0% (3.9, n = 31, reported MSP at 8-weeks, representing 6.9% of all 8-week participants.
3.2 |. Associations of socio-demographics, MVC, and injury characteristics with peritraumatic symptoms
Table 1 displays the socio-demographic characteristics of the study participants. A majority of participants were younger than age 35, women, Non-Hispanic Black, had completed at some education after high school, and were employed. Table 2 displays MVC and injury characteristics. Most participants had minor injuries such as isolated musculoskeletal strains or abrasions/contusions (AIS-M ax = 1) and were discharged home (>95%) after ED evaluation. Peritraumatic distress was significantly elevated among females (ß = 0.5), those with the lowest family incomes (ß = 0.2), and those not employed (ß = 0.2) (Table A1). A multivariate model with all socio-demographic characteristics significantly predicted distress (F15,650 = 3.0, p<.001) but not dissociation (F15,650 = 0.7, p=.80), a relationship explored in detail in a companion publication. The individual’s role in the MVC (i.e. passenger vs. driver), whether anyone other than in the patient’s vehicle was injured, and two indicators of patient injury (AIS-Max score, admitted vs. discharged) were unrelated to peritraumatic distress or dissociation. However, both were significantly and positively associated with the amount of damage sustained by the patient’s vehicle (ß = 0.6–0.4 for severe vs. no vehicle damage), the number of passengers with moderate-severe injuries in the vehicle (ß = 0.1), and transport to the ED by ambulance (b = 0.4–0.3). Peritraumatic distress and dissociation were both positively associated with self-reported severity of pain (ß = 0.2–0.1) and other somatic symptoms (ß = 0.2) (Table A2).
TABLE 1.
Distributions and univariate associations between socio- demographic characteristics and self-reported pain with and without controls for peritraumatic distress and dissociation in the Freeze 1 AURORA MVC sample (n = 666)
| 8- week |
2- week |
8- week controlling 2- week |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 3a |
Model 4b |
Model 1a |
Model 2b |
Model 5c |
Model 6d |
|||||||
| OR | (95% CI) | OR | (95% CI) | OR | (95% CI) | OR | (95% CI) | OR | (95% CI) | OR | (95% CI) | |
| Age | ||||||||||||
| 50+ | 2.6* | (1.5, 4.5) | 2.7* | (1.5, 4.6) | 1.5 | (0.8, 2.8) | 1.5 | (0.8, 2.9) | 2.8* | (1.5, 5.1) | 2.8* | (1.5, 5.2) |
| 35–49 | 1.9* | (1.2, 3.0) | 1.9* | (1.2, 3.1) | 1.5 | (0.9, 2.6) | 1.6 | (0.9, 2.7) | 1.8* | (1.1, 3.1) | 1.9* | (1.1, 3.1) |
| 25–34 | 1.2 | (0.8, 1.8) | 1.2 | (0.8, 1.9) | 1.2 | (0.7, 2.1) | 1.3 | (0.8, 2.2) | 1.1 | (0.7, 1.8) | 1.2 | (0.7, 1.9) |
| 18–24 | Ref | – | Ref | – | Ref | – | Ref | – | Ref | – | Ref | – |
| χ23* | 17.1* | 16.7* | 2.8 | 3.1 | 14.7* | 14.3* | ||||||
| Sex (female) | 1.8* | (1.2, 2.5) | 1.6* | (1.1, 2.3) | 2.2* | (1.5, 3.4) | 2.2* | (1.4, 3.4) | 1.4 | (0.9, 2.1) | 1.2 | (0.8, 1.9) |
| Race/ethnicity | ||||||||||||
| Non- Hispanic Black | 1.1 | (0.8, 1.6) | 1.1 | (0.8, 1.6) | 0.8 | (0.5, 1.3) | 0.8 | (0.5, 1.3) | 1.1 | (0.7, 1.7) | 1.2 | (0.8, 1.7) |
| Non- Hispanic White | Ref | – | Ref | – | Ref | – | Ref | – | Ref | – | Ref | – |
| Hispanic | 1.4 | (0.8, 2.5) | 1.4 | (0.7, 2.5) | 0.9 | (0.4, 1.8) | 0.8 | (0.4, 1.7) | 1.4 | (0.7, 2.8) | 1.4 | (0.7, 2.9) |
| Other | 0.7 | (0.3, 1.7) | 0.7 | (0.3, 1.8) | 0.4 | (0.1, 1.1) | 0.4 | (0.1, 1.0) | 0.9 | (0.3, 2.7) | 1.0 | (0.3, 2.9) |
| χ23* | 2.1 | 2.0 | 3.4 | 3.6 | 1.2 | 1.3 | ||||||
| Marital status | ||||||||||||
| Married/ cohabitating | Ref | – | Ref | – | Ref | – | Ref | – | Ref | – | Ref | – |
| Previously marriede | 1.2 | (0.7, 2.0) | 1.1 | (0.7, 1.9) | 0.9 | (0.5, 1.6) | 0.8 | (0.5, 1.6) | 1.3 | (0.7, 2.4) | 1.3 | (0.7, 2.3) |
| Never married | 0.8 | (0.6, 1.2) | 0.8 | (0.6, 1.2) | 0.7 | (0.5, 1.1) | 0.7 | (0.5, 1.1) | 0.9 | (0.6, 1.3) | 0.9 | (0.6, 1.3) |
| χ22* | 2.3 | 2.0 | 2.2 | 2.1 | 2.1 | 1.7 | ||||||
| Education | ||||||||||||
| College graduate | Ref | – | Ref | – | Ref | – | Ref | – | Ref | – | Ref | – |
| Some college | 1.2 | (0.8, 1.9) | 1.2 | (0.8, 1.8) | 1.0 | (0.6, 1.8) | 1.0 | (0.6, 1.7) | 1.2 | (0.8, 2.0) | 1.2 | (0.7, 1.9) |
| High school | 0.9 | (0.5, 1.4) | 0.8 | (0.5, 1.4) | 0.7 | (0.4, 1.3) | 0.7 | (0.4, 1.3) | 1.0 | (0.6, 1.7) | 1.0 | (0.6, 1.6) |
| graduate | ||||||||||||
| Less than high | 2.2* | (1.1, 4.5) | 2.1* | (1.0, 4.2) | 1.0 | (0.5, 2.1) | 0.9 | (0.4, 1.9) | 2.6* | (1.2, 5.6) | 2.4* | (1.1, 5.2) |
| school | ||||||||||||
| χ23* | 8.5* | 7.0 | 2.1 | 1.8 | 6.6 | 5.7 | ||||||
| Incomef | ||||||||||||
| More than $35K | Ref | – | Ref | – | Ref | – | Ref | – | Ref | – | Ref | – |
| $19–35K | 1.3 | (0.9, 1.9) | 1.2 | (0.8, 1.7) | 1.6 | (1.0, 2.6) | 1.5 | (0.9, 2.4) | 1.1 | (0.7, 1.7) | 1.0 | (0.7, 1.6) |
| Less than $19K | 1.5* | (1.0, 2.2) | 1.4 | (1.0, 2.1) | 1.3 | (0.8, 2.1) | 1.2 | (0.8, 2.0) | 1.4 | (0.9, 2.2) | 1.4 | (0.9, 2.1) |
| χ22* | 4.3 | 3.0 | 3.6 | 2.3 | 2.5 | 2.1 | ||||||
| Employed (yes vs. no) | 0.7 | (0.4, 1.0) | 0.7 | (0.5, 1.1) | 0.8 | (0.5, 1.3) | 0.8 | (0.5, 1.4) | 0.7 | (0.5, 1.1) | 0.8 | (0.5, 1.2) |
Abbreviations: CI, confidence interval; MVC, motor vehicle collision; OR, odds ratio.
Without controls for peritraumatic distress/dissociation.
With controls for peritraumatic distress/dissociation.
With controls for 2- week pain (both continuous and categorical) but not peritraumatic distress/dissociation.
With controls for 2- week pain (both continuous and categorical) and peritraumatic distress/dissociation.
Separated, widowed, or divorce.
Family income before taxes.
Significant at the 0.05 level, two- sided test.
TABLE 2.
Distributions and net univariate associations of MVC and injury characteristics after controlling socio- demographics with self- reported pain with and without controls for peritraumatic distress and dissociation in the Freeze 1 A
| 8- week |
2- week |
8- week controlling 2- week |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 3a |
Model 4b |
Model 1a |
Model 2b |
Model 5c |
Model 6d |
|||||||
| OR | (95% CI) | OR | (95% CI) | OR | (95% CI) | OR | (95% CI) | OR | (95% CI) | OR | (95% CI) | |
| Role in MVC | ||||||||||||
| Passenger | 1.0 | (0.6, 1.5) | 1.0 | (0.6, 1.5) | 1.2 | (0.7, 2.0) | 1.2 | (0.7, 2.1) | 0.9 | (0.6, 1.4) | 0.9 | (0.6, 1.5) |
| Driver with others | 1.1 | (0.7, 1.7) | 1.0 | (0.6, 1.6) | 0.8 | (0.5, 1.4) | 0.8 | (0.4, 1.3) | 1.2 | (0.7, 2.0) | 1.1 | (0.7, 1.9) |
| Driver alone | Ref | – | Ref | – | Ref | – | Ref | – | Ref | – | Ref | – |
| χ22* | 0.2 | 0.0 | 1.3 | 1.8 | 0.8 | 0.5 | ||||||
| Your vehicle collided with | ||||||||||||
| Other moving vehicle | 0.9 | (0.5, 1.4) | 0.8 | (0.5, 1.3) | 0.6 | (0.3, 1.1) | 0.5 | (0.3, 1.0) | 1.0 | (0.6, 1.8) | 1.0 | (0.6, 1.7) |
| Stationary object | 0.7 | (0.4, 1.3) | 0.6 | (0.3, 1.2) | 0.5 | (0.2, 1.1) | 0.5 | (0.2, 1.1) | 0.8 | (0.4, 1.6) | 0.8 | (0.4, 1.5) |
| Othere | Ref | – | Ref | – | Ref | – | Ref | – | Ref | – | Ref | – |
| χ22* | 1.6 | 1.9 | 3.5 | 4.0 | 0.8 | 0.8 | ||||||
| Damage to your vehicle | ||||||||||||
| Severe | 1.9 | (0.9, 3.7) | 1.5 | (0.7, 3.0) | 1.4 | (0.7, 3.2) | 1.2 | (0.5, 2.7) | 1.8 | (0.8, 3.8) | 1.5 | (0.7, 3.2) |
| Moderate | 1.2 | (0.6, 2.5) | 1.1 | (0.5, 2.4) | 1.0 | (0.4, 2.2) | 1.0 | (0.4, 2.2) | 1.3 | (0.6, 2.9) | 1.3 | (0.6, 2.8) |
| Minor | 1.9 | (0.8, 4.6) | 2.0 | (0.8, 4.8) | 2.3 | (0.8, 6.9) | 2.6 | (0.9, 8.0) | 1.5 | (0.6, 3.9) | 1.6 | (0.6, 4.0) |
| Otherf | Ref | – | Ref | – | Ref | – | Ref | – | Ref | – | Ref | – |
| χ23* | 6.7 | 3.9 | 5.5 | 5.0 | 3.1 | 1.5 | ||||||
| Passengers with injuries (0–4 standardized)g | 1.1 | (0.9, 1.3) | 1.1 | (0.9, 1.3) | 1.1 | (0.9, 1.4) | 1.1 | (0.9, 1.4) | 1.1 | (0.9, 1.3) | 1.0 | (0.9, 1.3) |
| Others with injuries (any vs. none)g | 0.9 | (0.5, 1.5) | 0.8 | (0.5, 1.4) | 1.7 | (0.8, 3.6) | 1.5 | (0.7, 3.3) | 0.6 | (0.4, 1.2) | 0.6 | (0.3, 1.1) |
| Transportation to ED | ||||||||||||
| Ambulance | 1.2 | (0.8, 1.8) | 1.1 | (0.7, 1.6) | 1.5 | (0.9, 2.3) | 1.2 | (0.8, 2.0) | 1.0 | (0.7, 1.6) | 0.9 | (0.6, 1.5) |
| Other immediately | 0.6 | (0.4, 1.1) | 0.6 | (0.4, 1.1) | 0.8 | (0.4, 1.5) | 0.8 | (0.4, 1.4) | 0.6 | (0.3, 1.0) | 0.6 | (0.3, 1.0) |
| Other delay | Ref | – | Ref | – | Ref | – | Ref | – | Ref | – | Ref | – |
| χ22* | 7.3* | 4.9 | 5.2 | 3.0 | 5.1 | 4.2 | ||||||
| Personal injury | ||||||||||||
| Hit head (yes vs. no) | 1.2 | (0.9, 1.7) | 1.1 | (0.8, 1.5) | 1.1 | (0.7, 1.6) | 0.9 | (0.6, 1.4) | 1.1 | (0.8, 1.7) | 1.1 | (0.7, 1.6) |
| TBI (yes vs. no) | 1.0 | (0.7, 1.4) | 0.7 | (0.5, 1.1) | 1.1 | (0.7, 1.7) | 0.8 | (0.5, 1.2) | 0.9 | (0.6, 1.3) | 0.7 | (0.5, 1.1) |
| AIS- Maxh (2+ vs. 1) | 1.4 | (0.8, 2.4) | 1.4 | (0.8, 2.3) | 1.8 | (0.9, 3.6) | 1.7 | (0.8, 3.3) | 1.3 | (0.7, 2.3) | 1.2 | (0.7, 2.2) |
| Admitted (yes vs. no) | 0.7 | (0.3, 1.7) | 0.6 | (0.3, 1.5) | 0.7 | (0.3, 1.7) | 0.6 | (0.2, 1.4) | 0.7 | (0.3, 1.9) | 0.7 | (0.3, 1.8) |
| Severity of paini | 1.5* | (1.3, 10.8) | 1.4* | (1.2, 1.7) | 1.8* | (1.4, 2.2) | 1.7* | (1.4, 2.1) | 1.3* | (1.0, 1.5) | 1.2 | (1.0, 1.5) |
| Severity of other somatic symptomj | 1.2 | (1.0, 1.4) | 1.1 | (0.9, 1.3) | 1.3* | (1.0, 1.6) | 1.2 | (1.0, 1.5) | 1.0 | (0.8, 1.2) | 1.0 | (0.8, 1.2) |
Abbreviations: AIS, Abbreviated Injury Scale; CI, confidence interval; ED, emergency department; MVC, motor vehicle collision; OR, odds ratio; TBI, traumatic brain injury.
Without controls for peritraumatic distress/dissociation.
With controls for peritraumatic distress/dissociation.
With controls for 2- week pain (both continuous and categorical) but not peritraumatic distress/dissociation.
With controls for 2- week pain (both continuous and categorical) and peritraumatic distress/dissociation.
No collision (n = 81), “other” (n = 8), and “don’t know” (n = 8).
None (n = 12) and “don’t know” (n = 31).
Moderate or severe injuries.
Max score of the nine AIS regions.
Self- reported 0– 10 scale on pain intensity right now, standardized to mean = 0 and standard deviation = 1.
Sum of all differences in each somatic symptom between 30- day (self-reported 0– 10 scale) and right now (self- reported 0– 10 scale), standardized to mean = 0 and standard deviation = 1.
Significant at the 0.05 level, two- sided test.
3.3 |. Associations of socio-demographics, MVC, and injury characteristics with 2- and 8-week moderate to severe pain
Women were significantly more likely than men to report MSP in the 2 weeks after the MVC not only in the crude model (OR = 2.2) but also after adjusting for peritraumatic symptoms (OR = 2.2) (Table 1). This means that women were significantly more likely than men with the same peritraumatic symptoms to develop MSP early in the course of trauma recovery. The gross odds of having MSP at 8 weeks Table 3, M3) was significantly elevated among individuals in the two highest age categories (OR 1.9–2.6), women OR = 1.8), individuals with less than a high school education (OR = 2.2), and individuals in the lowest income categories (OR = 1.5). Income was no longer significant when peri-traumatic distress and dissociation were controlled for Table 3, M4). Notably, there was no significant sex difference in 8-week MSP once 2-week pain symptoms were controlled for (OR = 1.2–1.4), indicating that MSP persistence was not higher among women than men who had the same pain at 2 weeks. In contrast, after controlling for peritraumatic distress and dissociation, as well as 2-week MSP, patients in the two highest age categories (ages 35–49, 55+) and those with less than a high school education had greater odds of MSP persistence at 8-weeks. In summary, sex differences influence persistent pain via their influence on peritraumatic psychological and pain outcomes in the early-post trauma period, whereas older age and lower education continue to influence worse pain outcomes throughout the first 2 months after trauma, in addition to any association on peritraumatic psychological and pain outcomes.
TABLE 3.
Associations of peritraumatic distress and dissociation with week 8 self- reported paina in the Freeze 1 AURORA MVC sample (n = 666)
| 8- week |
2- week |
8- week controlling 2- weekb |
||||
|---|---|---|---|---|---|---|
| OR | (95% CI) | OR | (95% CI) | OR | (95% CI) | |
| Peritraumatic distress | 1.05* | (1.02, 1.08) | 1.03 | (0.99, 1.06) | 1.05* | (1.02, 1.08) |
| Peritraumatic dissociation | 1.02 | (0.98, 1.06) | 1.08* | (1.03, 1.14) | 0.99 | (0.95, 1.03) |
| χ22* | 25.0* | 22.0* | 10.8* | |||
The peritraumatic distress and dissociation scales were both standardized to mean 0 and variance 1, allowing the ORs to be interpreted as the relative odds of pain associated with a one standard deviation difference in peritraumatic symptom scores.
Controlling for the dichotomous measure of 2- week pain and the continuous 2- week pain symptom scale score.
Significant at the 0.05 level, two- sided test.
The majority of MVC characteristics (e.g. collision type, extent of vehicle damage) and patient injury characteristics (e.g. whether the patient’s head was hit, injury severity score, whether the individual was admitted vs. discharged) were unrelated to 2- or 8-week MSP (Table 2). Pain severity in the ED predicted both 2- and 8-week MSP, in both crude models and in models that adjusted for socio-demographics, and peri-traumatic distress and dissociation. However, when 2-week pain was also controlled, pain severity in the ED was no longer predictive of 8-week MSP. In other words, the association of pain severity in the ED on 8-week MSP was mediated by 2-week MSP.
3.4 |. Associations of peritraumatic symptoms with 2- and 8-week moderate to severe pain
Both peritraumatic distress and dissociation had small but significant positive associations in an additive model predicting 8-week MSP (peritraumatic distress, OR = 1.05; peritraumatic dissociation, OR = 1.02) (Table 3). In addition, both peritraumatic symptom scales significantly predicted 2-week MSP (distress OR = 1.03, dissociation OR = 1.08). However, when 8-week MSP was evaluated while controlling 2-week MSP, only distress was significant (OR = 1.05). In other words, distress and dissociation both predicted early MSP pain symptoms, but only peritraumatic distress predicted 8-week MSP independent of its effect on 2-week MSP. The best-fitting multivariate model for the joint associations of peritraumatic distress and dissociation symptoms with 8 week MSP was an additive model that included linear effects of both predictors. An additive model was also most appropriate as peritraumatic distress and dissociation were correlated with one another (r = .57; Pearson correlation) but only weakly correlated with 2-week MSP (rpb = 0.15–0.18) and with 8-week MSP (rpb = 0.14–0.19). Quadratic terms and an interaction term between peritraumatic distress and dissociation were non-significant and consequently were not included in the final model. These associations held both in crude models and models adjusted for in for socio-demographic, MVC and injury characteristics.
4 |. DISCUSSION
Approximately two-thirds of individuals in the present sample experienced moderate or severe MSP 8 weeks after the MVC. This incidence is similar to the results of previous large US ED-based cohort studies (e.g. Feinberg et al., 2017; Linnstaedt et al., 2018), and is consistent with a previously published reports that demonstrated worse pain outcomes after MVC among African Americans versus non-Hispanic white Americans and among those with less educational opportunity/attainment (Beaudoin et al., 2018; McLean et al., 2014). The high incidence of persistent pain in this study suggests that those who come to ED for evaluation after MVC are a group at markedly elevated risk for chronic pain and other APNS. Despite this fact, no screening tools are currently available that could help emergency care providers identify individuals at high risk for APNS; secondary preventive interventions are currently unavailable; and research is lacking on evaluation of candidate interventions to prevent chronic pain development after MVC has been performed. The development of such tools and the identification of treatment targets for future intervention studies is an important goal of the AURORA Study.
The results of this analysis provide important new information regarding the timing of mediating processes for persistent pain development after MVC. Consistent with previous studies, distress and dissociation were associated with acute pain symptoms in the ED e.g., (Beaudoin et al., 2018; Bortsov et al., 2013), as well as persistent pain development (McLean et al., 2014). Interestingly, the association of dissociation with pain persistence appears to be mediated via pain in the early aftermath of trauma, whereas the association between distress on pain persistence occurred between the 2 and 8 weeks periods. These data are consistent with evidence that increased peritraumatic distress is associated with persistent post-raumatic hyperarousal symptoms, and that such hyperarousal symptoms promote the transition from acute to chronic pain during the initial weeks after trauma (Feinberg et al., 2017; Kimerling et al., 2000; Liedl et al., 2010). These data are also consistent with evidence that hyperarousal symptoms, and associated biases towards pain and aversive stimuli, may directly augment pain awareness and experiences (Baum et al., 2011; Liedl & Knaevelsrud, 2008; Sharp & Harvey, 2001). This information may support investigation of interventions that target such things as hyperarousal and their underlying neurobiological systems (e.g. noradrenergic).
The increased incidence of persistent pain among women versus men experiencing MVC observed in this study is consistent with data from many previous studies (Holm et al., 2009). Our finding that this increased pain vulnerability persists after adjustment for peritraumatic symptoms is consonant with evidence that biological factors (e.g. escape from X-chromosome inactivation [Linnstaedt et al., 2015]), rather than differences in pain reporting alone, play a central role in mediating these differences. The finding in this study that women do not have increased incidence of pain at 2 months, after pain outcome at 2 weeks is adjusted for, is consistent with previous evidence that women on average have better pain coping over time than men (Bortsov et al., 2014).
The fact that older adult MVC survivors have worse pain outcomes over time, even after adjustment for peritraumatic symptoms and pain severity at 2 weeks, is consistent with evidence that older adults have worse outcomes after ED discharge across a broad range of conditions, (LaMantia & Platts-Mills, 2017) including worse pain and functional decline after MVC (Platts-Mills, Flannigan, et al., 2016). Older adults may continue to accrue worse pain outcomes over time in part because they have less access to, or more side effects from, medications that reduce initial pain and help with mobilization (Hunold et al., 2013; Platts-M ills et al., 2018). Older adults also have greater and more persistent reductions in activity after MVC versus younger adults, contributing to worse pain outcomes after the initial post-trauma period (Platts-M ills, Nicholson, et al., 2016). Specific mechanisms mediating worse pain outcomes in older adults remain poorly understood and are an important area of study, given the fact that by 2025, people 65 and older will account for 25% of U.S. drivers (McCracken & Gross, 1998), and that by 2030 over 2 million police reported collisions are projected to involve drivers ≥65 years of age (Herndon et al., 2001).
Another important area of study is mechanisms by which lower educational attainment relates to worse pain outcomes, and why the relationship with lower educational attainment, like older age, continues after a first weeks after traumatic stress exposure. Interestingly, lower educational attainment has been found to have a greater relationship to pain outcomes after MVC in some studies than lower income (McLean et al., 2014; Platts-M ills et al., 2012). Lower educational status may influence the transition from acute to persistent pain in a number of ways, including via reducing expectations of recovery, increasing negative cognitions regarding pain (Kim et al., 2014), and altering medication use (Platts-Mills et al., 2012). Further research to understand the specific beliefs, cognitions, and behaviors mediating the influence of lower educational attainment on pain outcomes is important, in order to design optimal preventive interventions. Finally, the fact that ED pain severity, with and without adjustment for sociodemographic factors, predicts persistent pain severity is consistent with the fact that previous studies have found that acute pain severity is the strongest predictor of transition to chronic pain (Carroll et al., 2008; McLean et al., 2014; Sterling et al., 2005).
In aggregate this information helps us determine which subpopulations (e.g. women) might be important targets for intervention, as well as which factors might be modifiable. Both peritraumatic stress symptoms and acute pain in the ED are potential targets for intervention and have been studied in previous ED-based interventional studies in trauma patients (Gil-J ardine et al., 2018; Sterling et al., 2019). However, it is worth noting that although we detected statistical significant associations, many of these were weak (ORs ranging from 1.02 to 1.08). This implies that even if these targets were modifiable, they may not greatly reduce the burden of the outcome and suggest that other factors may be stronger contributors. In this context is worth noting that our narrow focus on MVCs might not generalize to other types of trauma. It is possible that distress and dissociation may play a different role in other traumatic events, for instance those with an interpersonal component (e.g. physical or sexual assault).
4.1 |. Limitations
Several limitations should be considered when interpreting the above results. Fewer than half of the participants approached in the ED enrolled in the study. While this is understandable given the relatively intensive nature of this year-long deep phenotyping study, the effect of selection bias on generalizability is unknown. However, previous large-scale ED-based studies of pain outcomes after MVC, with much higher consent rates, have found very similar rates of persistent pain 6 to 8 weeks after MVC as the present cohort (Bortsov et al., 2014; Feinberg et al., 2017; Linnstaedt et al., 2018). Second, we evaluated pain persistence at 8 weeks, rather than chronic pain at a later timepoint. While previous studies have shown that pain trajectories and levels of pain are generally established relatively early in the post-trauma period, within the 6–8 week timeframe (Hu et al., 2016; Sterling et al., 2011; Ulirsch, Weaver, et al., 2014), future studies could focus on more distal timepoints to better understand this. Third, this report is a relatively coarse look at pain in the first 8 weeks after MVC, given that survey data were evaluated from only three timepoints (ED, 2 weeks, and 8 weeks). More frequent pain assessments (e.g. several times per week) over a longer follow-up period would likely provide additional insights. As described in detail elsewhere (McLean et al., 2020), AURORA participants completed short daily ‘flash’ surveys using smartphone-based assessments that included periodic marker questions on pain. These data are currently being examined to provide a more textured characterization of changes in pain as part of larger multivariate APNS symptom profiles and trajectories for future analyses. This additional data collection can also help to augment self-reported standard assessments which can be at risk reporting bias. Lastly, we focus on measures of association, but we have not developed a predictive model, nor have we carried out a statistical mediation analyses. Additional analyses would need to be carried out in order to determine the set of predictors that identify individuals at risk of APNS, including those studied here.
5 |. CONCLUSION
This study evaluated the timing of influence of sociodemographic and crash-related factors on pain persistence/severity 8 weeks after MVC, and the degree to which the influence of these factors was mediated by peritraumatic stress symptoms (distress, dissociation) or pain severity at 2 weeks. Peritraumatic stress symptoms appear to exert some influence on both acute pain and the transition from acute to persistent pain, underscoring the interwoven nature of posttraumatic pain and psychological responses and the fact that the early aftermath of an MVC may be an important time period for intervening to prevent/reduce APNS. In addition, results of these analyses suggest important lines of future research regarding the timing and nature of potential mechanisms of pain persistence. These lines of research include investigation of mechanisms by which lower education and older age continue to shape worse pain outcomes after the peritraumatic period, and mechanisms mediating the vulnerability of women to much more severe acute pain responses to traumatic stress, establishing a challenging setpoint for recovery.
ACKNOWLEDGEMENTS
The investigators wish to thank the trauma survivors participating in the AURORA Study. Their time and effort during a challenging period of their lives make our efforts to improve recovery for future trauma survivors possible.
Funding information
AURORA is supported by NIMH U01MH110925, the US Army Medical Research and Material Command, The One Mind Foundation, and The Mayday Fund. Verily Life Sciences and Mindstrong Health provide some of the hardware and software used to perform study assessments.
APPENDIX A
TABLE A1.
Univariate and multivariate associations of socio- demographic characteristics with self- reported peritraumatic distress and dissociation in the Freeze 1 AURORA MVC sample (n = 666)
| Univariate associations |
Multivariate associations |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Distress |
Dissociation |
Distress |
Dissociation |
|||||||
| % | (SE) | b 1 | (95% CI) | b 1 | (95% CI) | b 1 | (95% CI) | b 1 | (95% CI) | |
| Age | ||||||||||
| 50+ | 18.3 | (1.5) | 0.1 | (−0.2, 0.3) | −0.1 | (−0.3, 0.2) | 0.0 | (−0.2, 0.3) | −0.1 | (−0.4, 0.2) |
| 35–49 | 28.7 | (1.8) | 0.1 | (−0.2, 0.3) | −0.1 | (−0.3, 0.1) | 0.1 | (−0.1, 0.3) | −0.1 | (−0.4, 0.1) |
| 25–34 | 30.5 | (1.8) | −0.1 | (−0.3, 0.1) | −0.1 | (−0.3, 0.1) | −0.0 | (−0.2, 0.2) | −0.1 | (−0.3, 0.1) |
| 18–24 | 22.5 | (1.6) | Ref | – | Ref | – | Ref | – | Ref | – |
| F3,660 | 0.7 | 0.2 | 0.4 | 0.4 | ||||||
| Sex (female) | 73.0 | (1.7) | 0.5* | (0.3, 0.6) | 0.1 | (−0.1, 0.2) | 0.4* | (0.3, 0.6) | 0.1 | (−0.1, 0.2) |
| Race/ethnicity | ||||||||||
| Non- Hispanic Black | 56.3 | (1.9) | −0.0 | (−0.2, 0.1) | 0.0 | (−0.1, 0.2) | −0.1 | (−0.3, 0.1) | 0.0 | (−0.1, 0.2) |
| Non- Hispanic White | 30.0 | (1.8) | Ref | – | Ref | – | Ref | – | Ref | – |
| Hispanic | 10.5 | (1.2) | 0.0 | (−0.2, 0.3) | 0.0 | (−0.3, 0.3) | −0.0 | (−0.3, 0.3) | 0.0 | (−0.3, 0.3) |
| Other | 3.2 | (0.7) | −0.1 | (−0.6, 0.3) | 0.1 | (−0.4, 0.5) | −0.1 | (−0.6, 0.3) | 0.1 | (−0.3, 0.6) |
| F3,663 | 0.2 | 0.0 | 0.7 | 0.1 | ||||||
| Marital status | ||||||||||
| Married/cohabitating | 42.5 | (1.9) | Ref | – | Ref | – | Ref | – | Ref | – |
| Previously married2 | 14.0 | (1.3) | 0.1 | (−0.1, 0.4) | −0.1 | (−0.3, 0.2) | 0.0 | (−0.2, 0.3) | −0.1 | (−0.4, 0.1) |
| Never married | 43.5 | (1.9) | −0.0 | (−0.2, 0.2) | −0.0 | (−0.2, 0.1) | −0.0 | (−0.2, 0.1) | −0.1 | (−0.3, 0.1) |
| F2,663 | 0.6 | 0.2 | 0.1 | 0.8 | ||||||
| Education | ||||||||||
| College graduate | 22.1 | (1.6) | Ref | – | Ref | – | Ref | – | Ref | – |
| Some college | 44.0 | (1.9) | 0.1 | (−0.1, 0.3) | 0.1 | (−0.1, 0.3) | 0.1 | (−0.1, 0.3) | 0.1 | (−0.1, 0.3) |
| High school graduate | 24.0 | (1.7) | 0.0 | (−0.2, 0.3) | 0.0 | (−0.2, 0.3) | 0.1 | (−0.2, 0.3) | 0.0 | (−0.2, 0.3) |
| Less than high school | 9.9 | (1.2) | 0.3 | (−0.0, 0.5) | 0.1 | (−0.2, 0.4) | 0.1 | (−0.2, 0.5) | 0.1 | (−0.2, 0.4) |
| F3,662 | 1.3 | 0.7 | 0.3 | 0.5 | ||||||
| Income3 | ||||||||||
| More than $35K | 33.6 | (1.8) | Ref | – | Ref | – | Ref | – | Ref | – |
| $19–35K | 31.5 | (1.8) | 0.2* | (0.1, 0.4) | 0.2 | (−0.0, 0.4) | 0.2* | (0.0, 0.4) | 0.2 | (−0.0, 0.4) |
| Less than $19K | 34.8 | (1.8) | 0.2* | (0.0, 0.4) | 0.0 | (−0.2, 0.2) | 0.2 | (−0.1, 0.4) | −0.0 | (−0.2, 0.2) |
| F2,363 | 3.8* | 2.2 | 2.6 | 2.5 | ||||||
| Employed (yes vs. no) | 77.0 | (1.6) | −0.2* | (−0.4, −0.1) | −0.1 | (−0.3, 0.1) | −0.2 | (−0.4, 0.0) | −0.1 | (−0.3, 0.1) |
Standardized to mean = 0 and standard deviation = 1.
Separated, divorced, or widowed.
Family income before taxes.
Significant at the 0.05 level, two- sided test.
TABLE A2.
Univariate and multivariate associations of MVC and injury characteristics with self- reported peritraumatic distress and dissociation in the Freeze 1 AURORA MVC sample (n = 666)
| Univariate associations |
Multivariate associations |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Distress |
Dissociation |
Distress |
Dissociation |
|||||||
| %/Mean | (SE) | b 1 | (95% CI) | b 1 | (95% CI) | b 1 | (95% CI) | b 1 | (95% CI) | |
| Role in MVC | ||||||||||
| Passenger | 23.3 | (1.6) | 0.1 | (−0.1, 0.3) | 0.1 | (−0.1, 0.3) | 0.0 | (−0.2, 0.2) | −0.0 | (−0.2, 0.2) |
| Driver with others | 19.2 | (1.5) | 0.2 | (−0.0, 0.4) | 0.2 | (−0.0, 0.4) | 0.1 | (−0.1, 0.3) | 0.1 | (−0.1, 0.3) |
| Driver alone | 57.5 | (1.9) | Ref | – | Ref | – | Ref | – | Ref | – |
| F2,663 | 1.8 | 1.3 | 0.6 | 0.3 | ||||||
| Your vehicle collided with | ||||||||||
| Other moving vehicle | 68.2 | (1.8) | 0.3* | (0.1, 0.5) | 0.1 | (−0.1, 0.3) | 0.2 | (−0.0, 0.4) | 0.0 | (−0.2, 0.2) |
| Stationary object | 17.9 | (1.5) | 0.3 | (−0.0, 0.5) | 0.0 | (−0.3, 0.3) | 0.1 | (−0.1, 0.4) | −0.1 | (−0.4, 0.1) |
| Other2 | 14.0 | (1.3) | Ref | – | Ref | – | Ref | – | Ref | – |
| F2,663 | 3.1* | 0.4 | 1.9 | 1.3 | ||||||
| Damage to your vehicle | ||||||||||
| Severe | 58.3 | (1.9) | 0.6* | (0.3, 1.0) | 0.4* | (0.1, 0.7) | 0.6* | (0.3, 0.9)* | 0.3* | (0.0, 0.6) |
| Moderate | 26.4 | (1.7) | 0.2 | (−0.1, 0.5) | 0.1 | (−0.3, 0.4) | 0.2 | (−0.1, 0.6) | 0.1 | (−0.2, 0.4) |
| Minor | 8.9 | (1.1) | −0.1 | (−0.4, 0.3) | −0.1 | (−0.5, 0.3) | 0.0 | (−0.3, 0.4) | −0.0 | (−0.4, 0.4) |
| Other3 | 6.5 | (1.0) | Ref | – | Ref | – | Ref | – | Ref | – |
| F3,662 | 17.0* | 9.1* | 10.5* | 4.3* | ||||||
| Passengers with injuries (0–4 standardized)4 | 0.0 | (0.0) | 0.1* | (0.0, 0.2) | 0.1* | (0.0, 0.2) | 0.0 | (−0.1, 0.1) | 0.1 | (−0.0, 0.2) |
| Others with injuries (any vs. none)4 | 10.2 | (1.2) | 0.2 | (−0.1, 0.4) | 0.2 | (−0.1, 0.4) | −0.0 | (−0.3, 0.2) | −0.0 | (−0.3, 0.2) |
| Transportation to ED | ||||||||||
| Ambulance | 58.0 | (1.9) | 0.4* | (0.2, 0.6) | 0.3* | (0.2, 0.5) | 0.3* | (0.1, 0.5) | 0.2* | (0.0, 0.4) |
| Other immediately | 14.7 | (1.4) | 0.0 | (−0.2, 0.3) | 0.1 | (−0.1, 0.4) | 0.1 | (−0.1, 0.3) | 0.2 | (−0.1, 0.4) |
| Other delay | 27.3 | (1.7) | Ref | – | Ref | – | Ref | – | Ref | – |
| F2,663 | 13.6* | 7.5* | 6.8* | 2.9 | ||||||
| Personal injury | ||||||||||
| Hit head (yes vs. no) | 57.4 | (1.9) | 0.3* | (0.1, 0.4) | 0.4* | (0.2, 0.5) | −0.0 | (−0.2, 0.1) | 0.1 | (−0.1, 0.2) |
| TBI (yes vs. no) | 27.5 | (1.7) | 0.5* | (0.3, 0.6) | 0.6* | (0.4, 0.8) | 0.3* | (0.1, 0.5) | 0.4* | (0.2, 0.6) |
| AIS- Max5 (2+ vs. 1) | 13.1 | (1.3) | −0.0 | (−0.3, 0.2) | 0.1 | (−0.1, 0.4) | −0.1 | (−0.3, 0.1) | 0.0 | (−0.2, 0.3) |
| Admitted (yes vs. no) | 4.1 | (0.8) | 0.1 | (−0.3, 0.5) | 0.2 | (−0.1, 0.6) | −0.0 | (−0.4, 0.4) | 0.1 | (−0.2, 0.5) |
| Severity of pain (mean)6 | 0.0 | (0.0) | 0.2* | (0.1, 0.3) | 0.1* | (0.0, 0.2) | 0.2* | (0.1, 0.2) | 0.1 | (−0.0, 0.1) |
| Severity of other somatic symptoms (mean)7 | 0.0 | (0.0) | 0.2* | (0.1, 0.3) | 0.2* | (0.1, 0.2) | 0.1* | (0.0, 0.2) | 0.1* | (0.0, 0.2) |
Standardized to mean = 0 and standard deviation = 1.
No collision (n = 81), “other” (n = 8), and “don’t know” (n = 8).
None (n = 12) and “don’t know” (n = 31).
Moderate or severe injuries.
Max score of the nine AIS regions.
Self- reported 0– 10 scale on pain intensity right now, standardized to mean = 0 and standard deviation = 1.
Sum of all differences in each somatic symptom between 30- day (self-reported 0– 10 scale) and right now (self- reported 0– 10 scale), standardized to mean = 0 and standard deviation = 1.
Significant at the 0.05 level, two- sided test.
TABLE A3.
Associations of baseline, 2-, and 8- week measures with patterns of sample attrition among respondents who completed the baseline assessment
| All |
ED Only |
ED + WK2 Only |
ED + WK8 Only |
ED + WK2 + WK8 Only |
χ2/F- test | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Esta | SE | Esta | SE | Esta | SE | Esta | SE | Esta | SE | ||
| I. Demographics | |||||||||||
| Male (%) | 31.6 | (1.5) | 41.0 | (3.5) | 40.0 | (4.5) | 35.7 | (7.4) | 27.0 | (1.7) | 18.9* |
| Age (%) | 36.2* | ||||||||||
| 50+ | 15.5 | (1.1) | 12.5 | (2.3) | 3.3 | (1.6) | 19.0 | (6.1) | 18.3 | (1.5) | 19.4* |
| 35–49 | 26.2 | (1.4) | 19.0 | (2.8) | 23.3 | (3.9) | 28.6 | (7.0) | 28.7 | (1.8) | 8.1* |
| 25–34 | 33.0 | (1.5) | 38.5 | (3.4) | 41.7 | (4.5) | 21.4 | (6.3) | 30.5 | (1.8) | 11.3* |
| 18–24 | 25.4 | (1.4) | 30.0 | (3.2) | 31.7 | (4.2) | 31.0 | (7.1) | 22.5 | (1.6) | 8.3* |
| Race- ethnicity (%) | 13.9 | ||||||||||
| Non- Hispanic Black | 55.3 | (1.6) | 52.0 | (3.5) | 52.5 | (4.6) | 69.0 | (7.1) | 55.9 | (1.9) | 4.6 |
| Non- Hispanic White | 29.1 | (1.4) | 28.5 | (3.2) | 30.0 | (4.2) | 14.3 | (5.4) | 30.0 | (1.8) | 4.8 |
| Hispanic | 12.2 | (1.0) | 14.5 | (2.5) | 16.7 | (3.4) | 14.3 | (5.4) | 10.5 | (1.2) | 5.2 |
| Other | 3.5 | (0.6) | 5.0 | (1.5) | 0.8 | (0.8) | 2.4 | (2.4) | 3.6 | (0.7) | 4.0 |
| Marital status (%) | 13.5* | ||||||||||
| Married/cohabitating | 41.1 | (1.5) | 39.5 | (3.5) | 36.7 | (4.4) | 40.5 | (7.6) | 42.5 | (1.9) | 1.7 |
| Previously married | 12.0 | (1.0) | 9.5 | (2.1) | 5.8 | (2.1) | 9.5 | (4.5) | 14.0 | (1.3) | 8.2* |
| Never married | 46.9 | (1.6) | 51.0 | (3.5) | 57.5 | (4.5) | 50.0 | (7.7) | 43.5 | (1.9) | 9.9* |
| Education (%) | 31.6* | ||||||||||
| College graduate or more | 18.4 | (1.2) | 13.5 | (2.4) | 10.0 | (2.7) | 7.1 | (4.0) | 22.1 | (1.6) | 18.4* |
| Some college | 43.2 | (1.5) | 38.5 | (3.4) | 46.7 | (4.6) | 42.9 | (7.6) | 44.0 | (1.9) | 2.6 |
| High school graduate | 26.7 | (1.4) | 30.0 | (3.2) | 30.8 | (4.2) | 40.5 | (7.6) | 24.0 | (1.7) | 8.7* |
| Not high school graduate | 11.8 | (1.0) | 18.0 | (2.7) | 12.5 | (3.0) | 9.5 | (4.5) | 9.9 | (1.2) | 10.0* |
| Income (%) | 0.8 | ||||||||||
| More than $35k | 34.1 | (1.7) | – | – | 32.5 | (4.3) | – | – | 34.4 | (1.8) | 0.2 |
| $19k to $35k | 31.4 | (1.7) | – | – | 35.0 | (4.4) | – | – | 30.8 | (1.8) | 0.8 |
| Less than $19k | 34.5 | (1.7) | – | – | 32.5 | (4.3) | – | – | 34.8 | (1.8) | 0.2 |
| Employed (%) | 77.6 | (1.5) | – | – | 80.8 | (3.6) | – | – | 77.0 | (1.6) | 0.8 |
| II. Pre- MVC APNS | |||||||||||
| Global pain diagnosis (%) | 31.5 | (1.4) | 32.0 | (3.3) | 33.3 | (4.3) | 40.5 | (7.6) | 30.5 | (1.8) | 2.1 |
| Global pain severity (M) | −0.0 | (0.0) | −0.0 | (0.1) | 0.0 | (0.1) | 0.3 | (0.2) | −0.0 | (0.0) | 1.6 |
| Body region pain severity (M) | −0.0 | (0.0) | −0.1 | (0.1) | −0.1 | (0.1) | 0.5 | (0.2) | −0.0 | (0.0) | 3.8* |
| Somatic symptom severity (M) | −0.0 | (0.0) | 0.1 | (0.1) | −0.0 | (0.1) | 0.5 | (0.2) | −0.0 | (0.0) | 4.4* |
| III. MVC characteristics | |||||||||||
| Role in vehicle (%) | 7.6 | ||||||||||
| Passenger | 24.6 | (1.3) | 28.5 | (3.2) | 25.0 | (4.0) | 26.2 | (6.8) | 23.3 | (1.6) | 2.3 |
| Driver, alone | 55.1 | (1.6) | 51.5 | (3.5) | 51.7 | (4.6) | 42.9 | (7.6) | 57.5 | (1.9) | 5.7 |
| Driver, with others | 20.3 | (1.3) | 20.0 | (2.8) | 23.3 | (3.9) | 31.0 | (7.1) | 19.2 | (1.5) | 4.1 |
| Severity of vehicular damage (%) | 6.2 | ||||||||||
| Severe | 58.8 | (1.5) | 59.0 | (3.5) | 60.0 | (4.5) | 61.9 | (7.5) | 58.3 | (1.9) | 0.3 |
| Moderate | 26.3 | (1.4) | 26.5 | (3.1) | 28.3 | (4.1) | 16.7 | (5.8) | 26.4 | (1.7) | 2.3 |
| Minor | 9.1 | (0.9) | 9.5 | (2.1) | 9.2 | (2.6) | 11.9 | (5.0) | 8.9 | (1.1) | 0.5 |
| No damage/missing | 5.8 | (0.7) | 5.0 | (1.5) | 2.5 | (1.4) | 9.5 | (4.5) | 6.5 | (1.0) | 4.2 |
| Transportation to ED | 6.0 | ||||||||||
| Not direct | 28.4 | (1.4) | 27.0 | (3.1) | 34.2 | (4.3) | 35.7 | (7.4) | 27.3 | (1.7) | 3.6 |
| Other direct | 14.8 | (1.1) | 17.5 | (2.7) | 13.3 | (3.1) | 7.1 | (4.0) | 14.7 | (1.4) | 3.3 |
| Ambulance | 56.8 | (1.5) | 55.5 | (3.5) | 52.5 | (4.6) | 57.1 | (7.6) | 58.0 | (1.9) | 1.4 |
| Personal injuries | |||||||||||
| Hit Head (self- reported) | 49.0 | (1.6) | 49.5 | (3.5) | 50.0 | (4.6) | 47.6 | (7.7) | 48.8 | (1.9) | 1.7 |
| Inpatient admission versus discharge | 4.0 | (0.6) | 2.0 | (1.0) | 6.7 | (2.3) | 4.8 | (3.3) | 4.1 | (0.8) | 4.4 |
| Injuries of others | |||||||||||
| Number injured in vehicle (M) | −0.0 | (0.0) | 0.0 | (0.1) | −0.0 | (0.1) | 0.3 | (0.2) | −0.0 | (0.0) | 1.3 |
| Injuries outside vehicle | 9.6 | (0.9) | 9.0 | (2.0) | 7.5 | (2.4) | 9.5 | (4.5) | 10.2 | (1.2) | 1.0 |
| IV. Peritraumatic APNS | |||||||||||
| Peritraumatic distress (M) | −0.0 | (0.0) | −0.2 | (0.1) | −0.0 | (0.1) | 0.2 | (0.2) | 0.0 | (0.0) | 3.2* |
| Peritraumatic dissociation (M) | −0.0 | (0.0) | −0.1 | (0.1) | 0.0 | (0.1) | 0.2 | (0.2) | 0.0 | (0.0) | 1.3 |
| Global pain diagnosis (%) | 88.0 | (1.0) | 89.5 | (2.2) | 86.7 | (3.1) | 92.9 | (4.0) | 87.5 | (1.3) | 1.7 |
| Global pain severity (M) | −0.0 | (0.0) | 0.0 | (0.1) | −0.1 | (0.1) | 0.3 | (0.2) | −0.0 | (0.0) | 1.3 |
| Body region pain severity (M) | −0.0 | (0.0) | −0.0 | (0.1) | −0.0 | (0.1) | 0.2 | (0.2) | −0.0 | (0.0) | 0.9 |
| Count of CSNW body region pain (M) | −0.0 | (0.0) | 0.0 | (0.1) | 0.0 | (0.1) | −0.1 | (0.1) | −0.0 | (0.0) | 0.1 |
| Somatic symptom severity (M) | −0.0 | (0.0) | 0.0 | (0.1) | 0.0 | (0.1) | 0.4 | (0.2) | −0.0 | (0.0) | 2.4 |
| V. Two- week APNS | |||||||||||
| Global pain diagnosis (%) | 80.7 | (1.4) | – | – | 76.7 | (3.9) | – | – | 81.4 | (1.5) | 1.4 |
| Global pain severity (M) | −0.0 | (0.0) | – | – | −0.1 | (0.1) | – | – | 0.0 | (0.0) | 1.2 |
| Count of CSNW body region pain (M) | −0.0 | (0.0) | – | – | −0.1 | (0.1) | – | – | 0.0 | (0.0) | 1.4 |
| VI. Eight- week APNS | |||||||||||
| Global pain diagnosis | 67.1 | (1.8) | – | – | – | – | 61.9 | (7.5) | 67.4 | (1.8) | 0.5 |
| (n) | (1,028) | (200) | (120) | (42) | (666) | ||||||
Abbreviation: CSNW, clinically significant, new, or worsening.
Entries are either percentages (%) or standardized means (M).
Significant difference across subgroups the 0.05 level, two- sided test.
The AURORA STUDY Group includes J. S. Stevens1, D. Zeng2, T. C. Neylan3, G. D. Clifford,4,5, S. D. Linnstaedt6, S. L. Rauch7, S. L. House8, C. Lewandowski9, P. L. Hendry10, S. Sheikh10, P. I. Musey Jr.11, A. B. Storrow12, J. P. Haran13, C. W. Jones14, B. E. Punches15,16, M. S. Lyons15, R. A. Swor17, M. E. McGrath18, K. Mohiuddin19, A. M. Chang20, D. A. Peak21, C. Pearson22, R. M. Domeier23, R. C. Merchant24, L. D. Sanchez25,26, N. K. Rathlev27, S. E. Bruce28, R. H. Pietrzak29,30, J. Joormann31, D. M. Barch32, D. A. Pizzagalli33, J. F. Sheridan34,35, S. E. Harte36, J. M. Elliott37,38
1Department of Psychiatry and Behavioral Sciences, Emory University School of Medicine, Atlanta, GA, USA; 2Department of Biostatistics, Gillings School of Global Public Health, University of North Carolina, Chapel Hill, NC, USA; 3San Francisco VA Healthcare System and Departments of Psychiatry and Neurology, University of California, San Francisco, CA, USA; 4Department of Biomedical Informatics, Emory University School of Medicine, Atlanta, GA, USA; 5Department of Biomedical Engineering, Georgia Institute of Technology and Emory University School of Medicine, Atlanta, GA, USA; 6Institute for Trauma Recovery, Department of Anesthesiology, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA; 7Department of Psychiatry, McLean Hospital, Belmont, MA, USA; 8Department of Emergency Medicine, Washington University School of Medicine, St. Louis, MO, USA; 9Department of Emergency Medicine, Henry Ford Health System, Detroit, MI, USA; 10Department of Emergency Medicine, University of Florida College of Medicine -J acksonville, Jacksonville, FL, USA; 11Department of Emergency Medicine, Indiana University School of Medicine, Indianapolis, IN, USA; 12Department of Emergency Medicine, Vanderbilt University Medical Center, Nashville, TN, USA; 13Department of Emergency Medicine, University of Massachusetts Medical School, Worcester, MA, USA; 14Department of Emergency Medicine, Cooper Medical School of Rowan University, Camden, NJ, USA; 15Department of Emergency Medicine, University of Cincinnati College of Medicine, Cincinnati, OH, USA; 16Department of Emergency Medicine, University of Cincinnati College of Nursing, Cincinnati, OH, USA; 17Department of Emergency Medicine, Oakland University William Beaumont School of Medicine, Rochester Hills, MI, USA; 18Department of Emergency Medicine, Boston Medical Center, Boston, MA, USA; 19Department of Emergency Medicine/Internal Medicine, Einstein Medical Center, Philadelphia, PA, USA; 20Department of Emergency Medicine, Jefferson University Hospitals, Philadelphia, PA, USA; 21Department of Emergency Medicine, Massachusetts General Hospital, Boston, MA, USA; 22Wayne State University Department of Emergency Medicine, Ascension St. John Hospital, Detroit, MI, USA; 23Department of Emergency Medicine, Saint Joseph Mercy Hospital, Ann Arbor, MI, USA; 24Department of Emergency Medicine, Brigham and Women’s Hospital, Boston, MA, USA; 25Department of Emergency Medicine, Beth Israel Deaconess Medical Center, Boston, MA, USA; 26Department of Emergency Medicine, Harvard Medical School, Boston, MA, USA; 27Department of Emergency Medicine, University of Massachusetts Medical School-Baystate, Springfield, MA, USA; 28Department of Psychological Sciences, University of Missouri - St. Louis, St. Louis, MO, USA; 29Department of Psychiatry, Yale School of Medicine, West Haven, CT, USA; 30Department of Social and Behavioral Sciences, Yale School of Public Health, West Haven, CT, USA; 31Department of Psychology, Yale University, New Haven, CT, USA; 32Department of Psychological & Brain Sciences, College of Arts & Sciences, Washington University in St. Louis, St. Louis, MO, USA; 33Department of Psychiatry, Harvard Medical School, Boston, MA, USA; 34Department of Biosciences and Neuroscience, OSU Wexner Medical Center, Columbus, Ohio, USA; 35Institute for Behavioral Medicine Research, OSU Wexner Medical Center, Columbus, Ohio, USA; 36Chronic Pain and Fatigue Research Center, Departments of Anesthesiology and Internal Medicine-Rheumatology, University of Michigan Medical School, Ann Arbor, MI, USA; 37The Kolling Institute and Faculty of Health Sciences, The University of Sydney St Leonards, New South Wales, Australia; 38Physical Therapy & Human Movement Sciences - Feinberg School of Medicine, Northwestern University, Chicago, IL, USA.
Footnotes
Disclosures
No authors report any conflicts with the findings presented in this manuscript. DAP has received consulting fees from Akili Interactive Labs, BlackThorn Therapeutics, Boehringer Ingelheim, Posit Science and Takeda Pharmaceuticals, as well as an honorarium from Alkermes for activities unrelated to the current project. RCK received support for his epidemiological studies from Sanofi Aventis and was a consultant for Datastat, Inc., Sage Pharmaceuticals and Takeda.
Members of the AURORA STUDY Group at the end of the article.
REFERENCES
- Albert M, & McCaig LF (2015). Emergency department visits for motor vehicle traffic injuries. United States, 2010–2011. NCHS Data Brief, (185), 1–8. [PubMed] [Google Scholar]
- Baum C, Huber C, Schneider R, & Lautenbacher S (2011). Prediction of experimental pain sensitivity by attention to pain-related stimuli in healthy individuals. Perceptual and Motor Skills, 112(3), 926–946. 10.2466/02.09.22.PMS.112.3.926-946 [DOI] [PubMed] [Google Scholar]
- Beaudoin FL, Gutman R, Zhai W, Merchant RC, Clark MA, Bollen KA, Hendry P, Kurz MC, Lewandowski C, Pearson C, O’Neil B, Datner E, Mitchell P, Domeier R, & McLean SA (2018). Racial differences in presentations and predictors of acute pain after motor vehicle collision. Pain, 159(6), 1056–1063. 10.1097/j.pain.0000000000001186 [DOI] [PubMed] [Google Scholar]
- Bortsov AV, Platts-M ills TF, Peak DA, Jones JS, Swor RA, Domeier RM, Lee DC, Rathlev NK, Hendry PL, Fillingim RB, & McLean SA (2013). Pain distribution and predictors of widespread pain in the immediate aftermath of motor vehicle collision. European Journal of Pain, 17(8), 1243–1251. 10.1002/j.1532-2149.2013.00285.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortsov AV, Platts-M ills TF, Peak DA, Jones JS, Swor RA, Domeier RM, Lee DC, Rathlev NK, Hendry PL, Fillingim RB, & McLean SA (2014). Effect of pain location and duration on life function in the year after motor vehicle collision. Pain, 155(9), 1836–1845. 10.1016/j.pain.2014.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet A, Weiss DS, Metzler TJ, Best SR, Neylan TC, Rogers C, Fagan J, & Marmar CR (2001). The peritraumatic distress inventory: A proposed measure of PTSD criterion A2. American Journal of Psychiatry, 158(9), 1480–1485. 10.1176/appi.ajp.158.9.1480 [DOI] [PubMed] [Google Scholar]
- Bryant RA (2003). Early predictors of posttraumatic stress disorder. Biological Psychiatry, 53(9), 789–795. 10.1016/s0006-3223(02)01895-4 [DOI] [PubMed] [Google Scholar]
- Bryant RA (2011). Acute stress disorder as a predictor of posttraumatic stress disorder: A systematic review. Journal of Clinical Psychiatry, 72(2), 233–239. 10.4088/JCP.09r05072blu [DOI] [PubMed] [Google Scholar]
- Carroll LJ, Holm LW, Hogg-Johnson S, Côté P, Cassidy JD, Haldeman S, Nordin M, Hurwitz EL, Carragee EJ, van der Velde G, Peloso PM, & Guzman J (2008). Course and prognostic factors for neck pain in whiplash-associated disorders (WAD): Results of the bone and joint decade 2000–2010 Task Force on neck pain and its associated disorders. Spine, 33(4 Suppl), S83–S92. 10.1097/BRS.0b013e3181643eb8 [DOI] [PubMed] [Google Scholar]
- Ernst FR, Mills JR, Berner T, House J, & Herndon C (2015). Opioid medication practices observed in chronic pain patients presenting for all-causes to emergency departments: Prevalence and impact on health care outcomes. Journal of Managed Care & Specialty Pharmacy, 21(10), 925–936. 10.18553/jmcp.2015.21.10.925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrar JT, Young JP Jr, LaMoreaux L, Werth JL, & Poole RM (2001). Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain, 94(2), 149–158. 10.1016/s0304-3959(01)00349-9 [DOI] [PubMed] [Google Scholar]
- Feinberg RK, Hu JM, Weaver MA, Fillingim RB, Swor RA, Peak DA, Jones JS, Rathlev NK, Lee DC, Domeier RM, Hendry PL, Liberzon I, & McLean SA (2017). Stress-related psychological symptoms contribute to axial pain persistence after motor vehicle collision: Path analysis results from a prospective longitudinal study. Pain, 158(4), 682–690. 10.1097/j.pain.0000000000000818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskin DJ, & Richard P (2012). The economic costs of pain in the United States. The Journal of Pain, 13(8), 715–724. 10.1016/j.jpain.2012.03.009 [DOI] [PubMed] [Google Scholar]
- Gil-Jardiné C, Evrard G, Al Joboory S, Tortes Saint Jammes J, Masson F, Ribéreau-Gayon R, Galinski M, Salmi L-R, Revel P, Régis CA, Valdenaire G, & Lagarde E (2018). Emergency room intervention to prevent post concussion-like symptoms and post-traumatic stress disorder. A pilot randomized controlled study of a brief eye movement desensitization and reprocessing intervention versus reassurance or usual care. Journal of Psychiatric Research, 103, 229–236. 10.1016/j.jpsychires.2018.05.024 [DOI] [PubMed] [Google Scholar]
- Grol-Prokopczyk H (2017). Sociodemographic disparities in chronic pain, based on 12-year longitudinal data. Pain, 158(2), 313–322. 10.1097/j.pain.0000000000000762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartling L, Brison RJ, Ardern C, & Pickett W (2001). Prognostic value of the Quebec classification of whiplash-associated disorders. Spine, 26(1), 36–41. 10.1097/00007632-200101010-00008 [DOI] [PubMed] [Google Scholar]
- Herndon DN, Hart DW, Wolf SE, Chinkes DL, & Wolfe RR (2001). Reversal of catabolism by beta-blockade after severe burns. New England Journal of Medicine, 345(17), 1223–1229. 10.1056/NEJMoa010342 [DOI] [PubMed] [Google Scholar]
- Holm LW, Carroll LJ, Cassidy JD, Hogg-Johnson S, Côté P, Guzman J, Peloso P, Nordin M, Hurwitz E, van der Velde G, Carragee E, & Haldeman S (2009). The burden and determinants of neck pain in whiplash-associated disorders after traffic collisions: Results of the bone and joint decade 2000–2010 Task Force on neck pain and its associated disorders. Journal of Manipulative and Physiological Therapeutics, 32(2 Suppl), S61–S69. 10.1016/j.jmpt.2008.11.011 [DOI] [PubMed] [Google Scholar]
- Hu J, Bortsov AV, Ballina LE, Orrey DC, Swor RA, Peak DA, & McLean SA (2016). Chronic widespread pain after motor vehicle collision typically occurs via immediate development and non-recovery: Results of an emergency department-based cohort study. Pain, 157, 438–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunold KM, Esserman DA, Isaacs CG, Dickey RM, Pereira GF, Fillingim RB, Sloane PD, McLean SA, & Platts-Mills TF (2013). Side effects from oral opioids in older adults during the first week of treatment for acute musculoskeletal pain. Academic Emergency Medicine, 20(9), 872–879. 10.1111/acem.12212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannes CB, Le TK, Zhou X, Johnston JA, & Dworkin RH (2010). The prevalence of chronic pain in United States adults: Results of an Internet-based survey. The Journal of Pain, 11(11), 1230–1239. 10.1016/j.jpain.2010.07.002 [DOI] [PubMed] [Google Scholar]
- Kessler RC (2000). Posttraumatic stress disorder: The burden to the individual and to society. Journal of Clinical Psychiatry, 61(Suppl 5), 4–12; discussion 13–14. [PubMed] [Google Scholar]
- Kessler RC, Sonnega A, Bromet E, Hughes M, & Nelson CB (1995). Posttraumatic stress disorder in the National Comorbidity Survey. Archives of General Psychiatry, 52(12), 1048–1060. 10.1001/archpsyc.1995.03950240066012 [DOI] [PubMed] [Google Scholar]
- Kim HJ, Kim SC, Kang KT, Chang BS, Lee CK, & Yeom JS (2014). Influence of educational attainment on pain intensity and disability in patients with lumbar spinal stenosis: Mediation effect of pain catastrophizing. Spine, 39(10), E637–E644. 10.1097/BRS.0000000000000267 [DOI] [PubMed] [Google Scholar]
- Kimerling R, Clum GA, & Wolfe J (2000). Relationships among trauma exposure, chronic posttraumatic stress disorder symptoms, and self-reported health in women: Replication and extension. Journal of Traumatic Stress, 13(1), 115–128. 10.1023/A:1007729116133 [DOI] [PubMed] [Google Scholar]
- King NS, Crawford S, Wenden FJ, Moss NE, & Wade DT (1995). The Rivermead Post Concussion Symptoms Questionnaire: A measure of symptoms commonly experienced after head injury and its reliability. Journal of Neurology, 242(9), 587–592. 10.1007/BF00868811 [DOI] [PubMed] [Google Scholar]
- Koenen KC, Ratanatharathorn A, Ng L, McLaughlin KA, Bromet EJ, Stein DJ, Karam EG, Meron Ruscio A, Benjet C, Scott K, Atwoli L, Petukhova M, Lim CC, Aguilar-Gaxiola S, Al-Hamzawi A, Alonso J, Bunting B, Ciutan M, de Girolamo G, … Kessler RC (2017). Posttraumatic stress disorder in the World Mental Health Surveys. Psychological Medicine, 47(13), 2260–2274. 10.1017/s0033291717000708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMantia MA, & Platts-Mills TF (2017). Bending the curve of health trajectories for older adults discharged from the emergency department. Annals of Emergency Medicine, 69(4), 434–436. 10.1016/j.annemergmed.2016.10.030 [DOI] [PubMed] [Google Scholar]
- Liedl A, & Knaevelsrud C (2008). Chronic pain and PTSD: The Perpetual Avoidance Model and its treatment implications. Torture, 18(2), 69–76. [PubMed] [Google Scholar]
- Liedl A, O’Donnell M, Creamer M, Silove D, McFarlane A, Knaevelsrud C, & Bryant RA (2010). Support for the mutual maintenance of pain and post-traumatic stress disorder symptoms. Psychological Medicine, 40(7), 1215–1223. 10.1017/S0033291709991310 [DOI] [PubMed] [Google Scholar]
- Linnstaedt SD, Riker KD, Rueckeis CA, Kutchko KM, Lackey L, McCarthy KR, & McLean SA (2018). A functional riboS-Nitch in the 3’ untranslated region of FKBP5 alters MicroRNA-320a binding efficiency and mediates vulnerability to chronic post-traumatic pain. Journal of Neuroscience, 38(39), 8407–8420. 10.1523/JNEUROSCI.3458-17.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnstaedt SD, Walker MG, Parker JS, Yeh E, Sons RL, Zimny E, Lewandowski C, Hendry PL, Damiron K, Pearson C, Velilla M-A, O’Neil BJ, Jones J, Swor R, Domeier R, Hammond S, & McLean SA (2015). MicroRNA circulating in the early aftermath of motor vehicle collision predict persistent pain development and suggest a role for microRNA in sex-s pecific pain differences. Molecular Pain, 11, 66. 10.1186/s12990-015-0069-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftis KL, Price J, & Gillich PJ (2018). Evolution of the abbreviated injury scale: 1990–2015. Traffic Injury Prevention, 19(sup2), S109–S113. 10.1080/15389588.2018.1512747 [DOI] [PubMed] [Google Scholar]
- McBeth J, & Jones K (2007). Epidemiology of chronic musculoskeletal pain. Best Practice & Research Clinical Rheumatology, 21(3), 403–425. 10.1016/j.berh.2007.03.003 [DOI] [PubMed] [Google Scholar]
- McCracken LM, & Gross RT (1998). The role of pain-related anxiety reduction in the outcome of multidisciplinary treatment for chronic low back pain: Preliminary results. Journal of Occupational Rehabilitation, (8), 179–189. [Google Scholar]
- McLean SA (2016). Neurobiologic mechanisms of whiplash. In Turk DC, Jensen TS, & Kasch H (Eds.), Whiplash injury: Perspectives on the development of chronic pain (1st ed., pp. 155–169). IASP Press. [Google Scholar]
- McLean SA, Kirsch NL, Tan-Schriner CU, Sen A, Frederiksen S, Harris RE, Maixner W, & Maio RF (2009). Health status, not head injury, predicts concussion symptoms after minor injury. American Journal of Emergency Medicine, 27(2), 182–190.S0735–6757(08)00142–3 [pii] 10.1016/j.ajem.2008.01.054 [DOI] [PubMed] [Google Scholar]
- McLean SA, Ressler K, Koenen KC, Neylan T, Germine L, Jovanovic T, Clifford GD, Zeng D, An X, Linnstaedt S, Beaudoin F, House S, Bollen KA, Musey P, Hendry P, Jones CW, Lewandowski C, Swor R, Datner E, … Kessler R (2020). The AURORA Study: A longitudinal, multimodal library of brain biology and function after traumatic stress exposure. Molecular Psychiatry, 25(2), 283–296. 10.1038/s41380-019-0581-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean SA, Ulirsch JC, Slade GD, Soward AC, Swor RA, Peak DA, Jones JS, Rathlev NK, Lee DC, Domeier RM, Hendry PL, Bortsov AV, & Bair E (2014). Incidence and predictors of neck and widespread pain after motor vehicle collision among US litigants and nonlitigants. Pain, 155(2), 309–321. 10.1016/j.pain.2013.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaels AJ, Michaels CE, Moon CH, Smith JS, Zimmerman MA, Taheri PA, & Peterson C (1999). Posttraumatic stress disorder after injury: Impact on general health outcome and early risk assessment. The Journal of Trauma: Injury, Infection, and Critical Care, 47(3), 460–467. 10.1097/00005373-199909000-00005 [DOI] [PubMed] [Google Scholar]
- Moshfegh J, George SZ, & Sun E (2018). Risk and risk factors for chronic opioid use among opioid-naive patients with newly diagnosed musculoskeletal pain in the neck, shoulder, knee, or low back. Annals of Internal Medicine, 170(7), 504–505. 10.7326/M18-2261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolet PS, Emary PC, Kristman VL, Murnaghan K, Zeegers MP, & Freeman MD (2020). Exposure to a motor vehicle collision and the risk of future back pain: A systematic review and meta-analysis. Accident Analysis and Prevention, 142, 105546. 10.1016/j.aap.2020.105546 [DOI] [PubMed] [Google Scholar]
- Pan H, Liu S, Miao D, & Yuan Y (2018). Sample size determination for mediation analysis of longitudinal data. BMC Medical Research Methodology, 18(1), 32. 10.1186/s12874-018-0473-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennebaker JW, & Watson D (1991). The psychology of somatic symptoms. In Kirmayer LJ & Robbins JM (Eds.), Current concepts of somatization: Research and clinical perspectives (p. 21). American Psychiatric Association. [Google Scholar]
- Platts-Mills TF, Flannigan SA, Bortsov AV, Smith S, Domeier RM, Swor RA, Hendry PL, Peak DA, Rathlev NK, Jones JS, Lee DC, Keefe FJ, Sloane PD, & McLean SA (2016). Persistent pain among older adults discharged home from the emergency department after motor vehicle crash: A prospective cohort study. Annals of Emergency Medicine, 67(2), 166–176.e161. 10.1016/j.annemergmed.2015.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platts-Mills TF, Hollowell AG, Burke GF, Zimmerman S, Dayaa JA, Quigley BR, Bush M, Weinberger M, & Weaver MA (2018). Randomized controlled pilot study of an educational video plus telecare for the early outpatient management of musculoskeletal pain among older emergency department patients. Trials, 19(1), 10. 10.1186/s13063-017-2403-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platts-Mills TF, Hunold KM, Bortsov AV, Soward AC, Peak DA, Jones JS, Swor RA, Lee DC, Domeier RM, Hendry PL, Rathlev NK, & McLean SA (2012). More educated emergency department patients are less likely to receive opioids for acute pain. Pain, 153(5), 967–973. 10.1016/j.pain.2012.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platts-Mills TF, Nicholson RJ, Richmond NL, Patel KV, Simonsick EM, Domeier RM, Swor RA, Hendry PL, Peak DA, Rathlev NK, Jones JS, Lee DC, Weaver MA, Keefe FJ, & McLean SA (2016). Restricted activity and persistent pain following motor vehicle collision among older adults: A multicenter prospective cohort study. BMC Geriatrics, 16, 86. 10.1186/s12877-016-0260-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravn SL, Eskildsen NB, Johnsen AT, Sterling M, & Andersen TE (2020). There’s Nothing Broken. You’ve Had a Whiplash, That’s It: A qualitative study of comorbid posttraumatic stress disorder and whiplash associated disorders. Pain Medicine, 21(8), 1676–1689. 10.1093/pm/pnz369 [DOI] [PubMed] [Google Scholar]
- Ravn SL, Karstoft KI, Sterling M, & Andersen TE (2019). Trajectories of posttraumatic stress symptoms after whiplash: A prospective cohort study. European Journal of Pain, 23(3), 515–525. 10.1002/ejp.1325 [DOI] [PubMed] [Google Scholar]
- Roberts AL, Gilman SE, Breslau J, Breslau N, & Koenen KC (2011). Race/ethnic differences in exposure to traumatic events, development of post-t raumatic stress disorder, and treatment-seeking for post-traumatic stress disorder in the United States. Psychological Medicine, 41(1), 71–83. 10.1017/S0033291710000401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago PN, Ursano RJ, Gray CL, Pynoos RS, Spiegel D, Lewis-Fernandez R, Friedman MJ, & Fullerton CS (2013). A systematic review of PTSD prevalence and trajectories in DSM-5 defined trauma exposed populations: Intentional and non-intentional traumatic events. PLoS One, 8(4), e59236. 10.1371/journal.pone.0059236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp TJ, & Harvey AG (2001). Chronic pain and posttraumatic stress disorder: Mutual maintenance? Clinical Psychology Review, 21(6), 857–877. 10.1016/S0272-7358(00)00071-4 [DOI] [PubMed] [Google Scholar]
- Sterling M, Hendrikz J, & Kenardy J (2011). Similar factors predict disability and posttraumatic stress disorder trajectories after whiplash injury. Pain, 152(6), 1272–1278.S0304–3959(11)00094–7 [pii] 10.1016/j.pain.2011.01.056 [DOI] [PubMed] [Google Scholar]
- Sterling M, Jull G, Vicenzino B, Kenardy J, & Darnell R (2005). Physical and psychological factors predict outcome following whiplash injury. Pain, 114(1–2), 141–148. 10.1016/j.pain.2004.12.005 [DOI] [PubMed] [Google Scholar]
- Sterling M, Smeets R, Keijzers G, Warren J, & Kenardy J (2019). Physiotherapist-delivered stress inoculation training integrated with exercise versus physiotherapy exercise alone for acute whiplash-associated disorder (StressModex): A randomised controlled trial of a combined psychological/physical intervention. British Journal of Sports Medicine, 53(19), 1240–1247. 10.1136/bjsports-2018-100139 [DOI] [PubMed] [Google Scholar]
- Suissa S, Harder S, & Veilleux M (2001). The relation between initial symptoms and signs and the prognosis of whiplash. European Spine Journal, 10(1), 44–49. 10.1007/s005860000220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulirsch JC, Ballina LE, Soward AC, Rossi C, Hauda W, Holbrook D, Wheeler R, Foley KA, Batts J, Collette R, Goodman E, & McLean SA (2014). Pain and somatic symptoms are sequelae of sexual assault: Results of a prospective longitudinal study. European Journal of Pain, 18(4), 559–566. 10.1002/j.1532-2149.2013.00395.x [DOI] [PubMed] [Google Scholar]
- Ulirsch JC, Weaver MA, Bortsov AV, Soward AC, Swor RA, Peak DA, Jones JS, Rathlev NK, Lee DC, Domeier RM, Hendry PL, & McLean SA (2014). No man is an island: Living in a disadvantaged neighborhood influences chronic pain development after motor vehicle collision. Pain, 155(10), 2116–2123. 10.1016/j.pain.2014.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]


