Abstract
It has been demonstrated previously that Pax-6, a paired domain (PD)/homeodomain (HD) transcription factor critical for eye development, contributes to the activation of the αB-, αA-, δ1-, and ζ-crystallin genes in the lens. Here we have examined the possibility that the inverse relationship between the expression of Pax-6 and β-crystallin genes within the developing chicken lens reflects a negative regulatory role of Pax-6. Cotransfection of a plasmid containing the βB1-crystallin promoter fused to the chloramphenicol acetyltransferase reporter gene and a plasmid containing the full-length mouse Pax-6 coding sequences into primary embryonic chicken lens epithelial cells or fibroblasts repressed the activity of this promoter by as much as 90%. Pax-6 constructs lacking the C-terminal activation domain repressed βB1-crystallin promoter activity as effectively as the full-length protein, but the PD alone or Pax-6 (5a), a splice variant with an altered PD affecting its DNA binding specificity, did not. DNase footprinting analysis revealed that truncated Pax-6 (PD+HD) binds to three regions (−183 to −152, −120 to −48, and −30 to +1) of the βB1-crystallin promoter. Earlier experiments showed that the βB1-crystallin promoter sequence from −120 to −48 contains a cis element (PL2 at −90 to −76) that stimulates the activity of a heterologous promoter in lens cells but not in fibroblasts. In the present study, we show by electrophoretic mobility shift assay and cotransfection that Pax-6 binds to PL2 and represses its ability to activate promoter activity; moreover, mutation of PL2 eliminated binding by Pax-6. Taken together, our data indicate that Pax-6 (via its PD and HD) represses the βB1-crystallin promoter by direct interaction with the PL2 element. We thus suggest that the relatively high concentration of Pax-6 contributes to the absence of βB1-crystallin gene expression in lens epithelial cells and that diminishing amounts of Pax-6 in lens fiber cells during development allow activation of this gene.
Pax proteins are multifunctional transcription factors that are critical for numerous developmental processes in animals (25). Pax-6, a member of this family, is required for early eye determination in animals as diverse as vertebrates, insects, and cephalopod mollusks (26, 39, 47). Vertebrates heterozygous for Pax-6 mutations exhibit a wide variety of eye defects, including aniridia, corneal opacification, and cataracts (6). Eye development in homozygotes is absent due to a failure of lens induction from the head ectoderm (21). Pax-6 was recently shown to be sufficient for lens induction from the head ectoderm when ectopic expression of Pax-6 in Xenopus animal caps resulted in ectopic lens induction in the absence of neural tissue (1). In the developing and adult lens, Pax-6 mRNA is found mainly in the cuboidal epithelium on the anterior surface of the lens and the proliferative zone located at the lens equator (29, 31, 40). In the embryonic lens, Pax-6 protein is still detectable in the nuclei of the postmitotic fiber cells after their generation by the proliferative zone but is then lost as lens fiber cell differentiation proceeds (29, 40).
The refractive properties of the lens are dependent on the accumulation of high concentrations of water-soluble proteins known collectively as crystallins (2, 48). The genes encoding crystallins are generally expressed either specifically or preferentially in the lens and are independently regulated at the transcriptional level (11, 38). While the roles that Pax-6 plays in eye development after the initial inductive events are not well understood, it has been demonstrated that Pax-6 contributes to the transcriptional activation of two crystallin genes initially expressed in the lens placode, chicken δ1-crystallin (10, 43) and mouse αB-crystallin (20, 22, 41), as well as three others expressed in the lens, guinea pig ζ-crystallin (40), and chicken and mouse αA-crystallin (8, 9). However, it is clear that crystallin genes are regulated by a complex network of transcription factors (11) and that Pax-6 is only one of the many proteins involved.
βB1-crystallin (β35 [23]), another lens-preferred protein, is initially transcribed later in lens development than the above proteins, with low levels of expression first detected in elongating, postmitotic primary fiber cells (4, 36). In chickens, βB1-crystallin mRNA levels increase substantially in lens fiber cells in the late embryonic period and are maintained at this level until at least 90 days of age (23). In the present study, we investigated the role that Pax-6 plays in chicken βB1-crystallin gene regulation and have demonstrated that Pax-6 represses promoter activity of this marker of lens terminal differentiation. These investigations demonstrate for the first time that Pax-6 can function as a repressor and support the idea that one of the many roles of Pax-6 is the regulation of spatial and temporal gene expression in the lens.
MATERIALS AND METHODS
Plasmid constructions.
The chicken βB1-crystallin promoter plasmids 434/+30/CAT (pB434 [17]), −126/+30/CAT (pβB1P126 [42]), and 3× PL2/CAT (18) and the chicken αA-crystallin (−162/+77)/CAT construct (8) were previously described.
The full-length Pax-6 cDNA cloned into pKW10 was previously described (13). The Pax-6 (5a) expression vector was generated by subcloning the NotI fragment for pCMV5a (19) into pKW10. The construction of plasmids expressing the Pax-6 paired domain (PD) alone, the Pax-6 PD and homeodomain (HD) together [Pax-6 (PD+HD)], and Pax-6 with 40 amino acids (aa) truncated from the C terminus are described elsewhere (12). The plasmids for expression of the PD and HD of Pax-6 as glutathione S-transferase (GST) fusions were provided by J. Epstein and R. L. Maas (Howard Hughes Medical Institute, Boston, Mass.). pCMVβGAL was purchased from Clontech (Palo Alto, Calif.).
Cell cultures and transfections.
Cultures of chicken primary lens epithelial cells (PLEs) and embryonic fibroblasts were prepared as previously described (3). PLEs were cultured from six lenses/60-mm-diameter dish and transfected 48 h later by the calcium phosphate precipitation method (42). Each dish received 10 μg of promoter/chloramphenicol acetyltransferase (CAT) plasmid, 1.2 μg of pCMV/βGAL, and various amounts of either the respective Pax-6 expression plasmid or the parental plasmid (pKW10). Cells were harvested 48 h after transfection and cellular extracts were prepared by multiple cycles of freeze/thaw. The extracts were assayed for CAT and β-galactosidase activity as previously described (42). All cotransfection experiments were performed at least twice in triplicate.
COP-8 cells were transfected with pCMV/Pax-6, and cellular extracts were prepared as previously described (14).
DNase I footprinting and EMSA.
Escherichia coli extracts containing the Pax-6 (PD+HD)/GST fusion protein were prepared as recommended by the manufacturer (Pharmacia, Uppsala, Sweden). DNase I footprints were performed in the presence of 500 ng of poly(dA-dT) as previously described (20). The PstI/PvuII fragment (−392 to +30) of the chicken βB1-crystallin promoter was radiolabeled on the PvuII end. All electrophoretic mobility shift assays (EMSAs) were performed in a total volume of 12.5 μl containing 6 μl of A100 (20 mM HEPES [pH 7.9], 20% [vol/vol] glycerol, 100 mM KCl, 5 mM MgCl2, 0.2 mM EDTA, 0.5 mM dithiothreitol), 250 ng of poly(dA-dT), 2.5 μg of bovine serum albumin, and 20,000 Cerenkov counts of double-stranded DNA probe. Nonradioactive competitors were added at 100-fold molar excess. After a 20-min preincubation at room temperature, reactions were electrophoresed through 5% polyacrylamide gels, using 0.5× Tris-borate-EDTA as the buffer, at 4°C. The oligonucleotides used for EMSA (sense strands only shown) were Pax-6 consensus (19), βB1 −190/−139 (5′ AAA GGA AAG TGC TGG GTT CAG CGG CTG GGC ACA GGG CCG GGG AGA GGC TTC 3′), βB1 −126/−46 (5′ GCT TTG CAG GAT GTG ATG ACT GGG CGG CCG CAC AGA CAC TGA TGA GCT GGC ACT TCC ATT GTG TGC CCG CCC GCG CTC TG 3′), βB1 −126/−46 (M6a; mut PL1) (see Fig. 6), βB1 −126/−46 (M7; mut PL2) (see Fig. 6), βB1 −34/+2 (5′ TAT AAA GTG GGG GCC CCG CTG CAC CCC GAA ACA CAA 3′), βB1/PL1 (5′ GGA TGT GAT GAC TGG GCG GCC GCA 3′), βB1/PL2 (5′ AGA CAC TGA TGA GCT GGC ACT TCC 3′), 3XPL1 (5′ GAT CCG GAT GTG ATG ACT GGG ATG TGA TGA CTG GGA TGT GAT GAC TGG 3′), 3XPL2 (5′ TCG ACC ACA GAC ACT GAT GAG CTG GCA CAG ACA CTG ATG AGC TGG CAC AGA CAC TGA TGA GCT GGC AG 3′), and 3XPL2(M7) (5′ GAT CCA CAG ACA CTA GGC CTC TGG CAC AGA CAC TAG GCC TCT GGC ACA GAC ACT AGG CCT CTG 3′).
FIG. 6.
The PL2 element is essential for Pax-6 binding to the −120/−48 region of the chicken βB1-crystallin promoter. An oligonucleotide consisting of the −126/−46 sequence of βB1-crystallin was radiolabeled and used for EMSAs with the Pax-6 (PD+HD)/GST fusion protein. Pax-6 bound to the −126/−46 oligonucleotide and was competed by a 100-fold molar excess of nonradioactive oligonucleotides corresponding to self, self with a mutation in the PL1 element (M6A) and a Pax-6 consensus (con) binding site. By contrast, little to no competition was observed with a 100-fold molar excess of self oligonucleotide harboring a mutation in PL2 (M7).
Western blot analysis and immunocytochemistry.
All fertilized chicken eggs and posthatch chickens were obtained from Truslow Farms (Chestertown, Md.). Lenses were dissected from 6-day embryonic, newly hatched, and adult chickens and homogenized in 20 mM Tris-HCl–1 mM EDTA–1 mM EGTA (pH 7.5) to obtain water-soluble protein. For Coomassie blue staining, 10 μg of each protein preparation was subjected to polyacrylamide gel electrophoresis (PAGE) through a sodium dodecyl sulfate (SDS)–14% polyacrylamide gel, stained with colloidal Coomassie blue (Novex, San Diego, Calif.), and destained in water. For Western blots, 15 μg of each protein preparation was electrophoresed through SDS–12% polyacrylamide gels and electroblotted onto a nitrocellulose membrane. The membranes were blocked overnight in 10% powdered milk in phosphate-buffered saline with 0.1% Tween 20 and incubated with either a 1:1,000 dilution of an antibody raised against the C terminus of Pax-6 (15) or 1:1,000 dilution of an antibody raised against βB1-crystallin (4). The bound antibody was detected with the Vectastain ABC Elite kit (Vector Laboratories, Ingold, Calif.) and the SG substrate.
For immunocytochemistry, heads of 6-day embryonic chickens were fixed in 4% paraformaldehyde in phosphate-buffered saline overnight and embedded in paraffin, and 8-μm-thick sections prepared. These sections were deparaffinized, blocked with a 1:100 dilution of horse serum, and immunostained as described above for Western blotting except that the ABC-antibody complexes were detected with the VIP substrate kit (Vector Laboratories).
RESULTS
The expression patterns of Pax-6 and β-crystallin are inversely related in the embryonic lens.
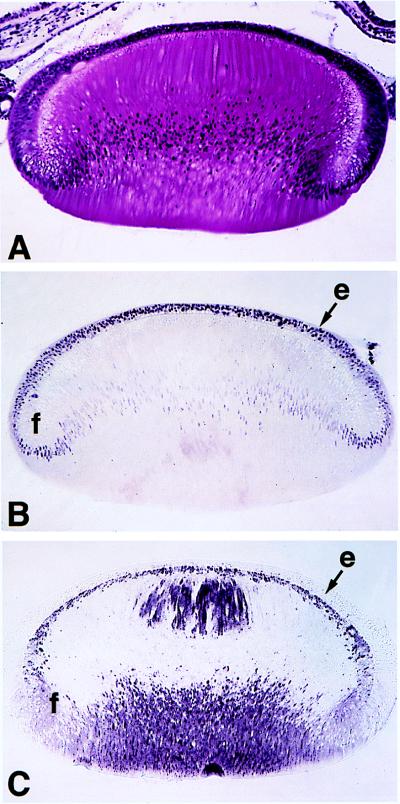
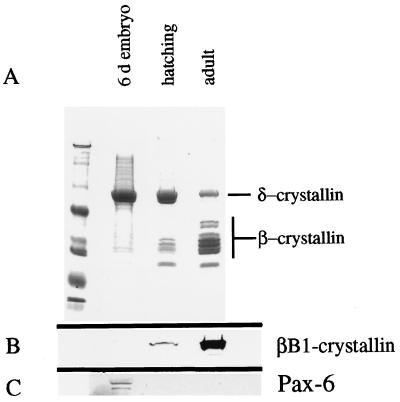
We confirm (29) that in 6-day embryonic lenses, Pax-6 levels are highest in lens epithelial cells, with much lower amounts persisting in postmitotic fiber cell nuclei (Fig. 1B). We also confirm (4, 23) that βB1-crystallin was detected only in postmitotic fiber cells (Fig. 1C). To investigate the relationship between Pax-6 and crystallin expression further, SDS-PAGE and Western immunoblotting were performed on lens fiber cell proteins obtained from embryonic day 6, posthatching day 1, and adult chickens. At embryonic day 6, δ-crystallin was the predominant lens protein and little to no β-crystallin was detected (Fig. 2A) (see reference 37 for a review). In adult chickens, the relative amount of δ-crystallin had decreased markedly, while β-crystallins became the predominant water-soluble proteins of the lens (Fig. 2A). Western blot analysis of βB1-crystallin (Fig. 2B) demonstrated that the amount of βB1-crystallin protein increases greatly in the lens after hatching. The amount of Pax-6 detectable in lens extracts by immunoblotting was much lower in older animals than in younger animals and was inversely related to the amount of β-crystallin (Fig. 2C). Thus, the upregulation of β-crystallin expression may be correlated with a downregulation of Pax-6 levels in the lens. Consequently, we investigated the possible role of Pax-6 in β-crystallin regulation.
FIG. 1.
Immunocytochemical localization of Pax-6 and βB1-crystallin proteins in the embryonic chicken lens. (A) Hematoxylin-and-eosin-stained transverse section through the lens of a 6-day chicken embryo. (B) Immunocytochemical detection of Pax-6 protein in the lens of a 6-day chicken embryo. Note that the highest levels of Pax-6 protein are found in the lens epithelium (e), with nuclear levels decreasing during fiber cell (f) differentiation. (C) Immunocytochemical detection of βB1-crystallin protein in the lens of a 6-day chicken embryo. Note that no βB1-crystallin was detected in the lens epithelium, while it was relatively abundant in the lens fibers. The significance of the restricted localization of βB1-crystallin within fiber cells is not clear (4) but may indicate an involvement in the establishment of the refractive index gradient (30) important for lens function. Magnification for all panels, ×89.
FIG. 2.
Developmental analysis of the water-soluble proteins of the chicken lens. (A) Coomassie blue-stained SDS-polyacrylamide gel of water-soluble proteins obtained from 6-day embryonic, newly hatched, and adult chicken lenses. Note that δ-crystallin is the predominant protein in the lens at embryonic day 6, while little to no β-crystallin is detectable. By the time of hatching, significant levels of β-crystallin are present with a relative decrease in δ-crystallin protein levels. By adulthood, β-crystallins as a class are the predominant proteins of the chicken lens. (B) Western blot analysis of βB1-crystallin during lens development. Note that the levels of βB1-crystallin increase between hatching and adulthood. (C) Western blot analysis of Pax-6 during lens development. Note that the levels of Pax-6 decrease during lens development.
Pax-6 represses βB1-crystallin expression.
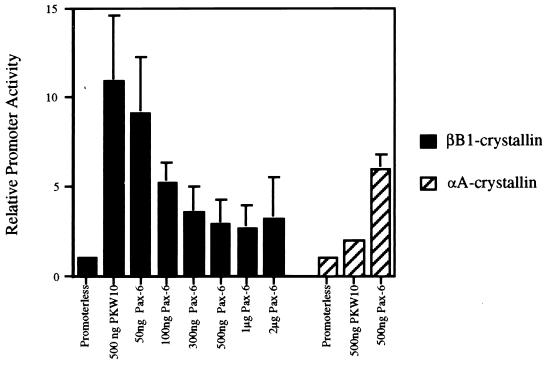
The possibility that Pax-6 plays a role in the expression of βB1-crystallin was examined by cotransfecting embryonic fibroblasts with increasing amounts of pKW10 expressing Pax-6 or the parental plasmid pKW10 alone with a plasmid containing the βB1-crystallin promoter driving cat gene expression. As little as 100 ng of the Pax-6 expression vector repressed the activity of the βB1-crystallin promoter by 50%, while cotransfection with the parental plasmid did not affect βB1-crystallin promoter activity (Fig. 3). Larger amounts of Pax-6 expression vector increased the efficiency of repression until it reached a plateau of 70% repression at 500 ng. In contrast, parallel cotransfection of a chicken αA-crystallin promoter (−162/+77)/CAT construct with 500 ng of Pax-6 expression vector resulted in a threefold increase in promoter activity (Fig. 3) as reported previously (8). These cotransfections were also performed in PLEs, and a similar dose response for transcriptional repression was obtained (data not shown).
FIG. 3.
Pax-6 represses the βB1-crystallin promoter, as determined by cotransfection of a plasmid harboring the chicken βB1-crystallin promoter (−434/+30 [18]) or the chicken αA-crystallin promoter (−162/+77 [8]) linked to the cat gene with either pKW10 expressing full-length Pax-6 protein or pKW10 alone into chicken embryonic fibroblasts. CAT activity is relative to the activity of the respective promoter in the absence of the Pax-6 expression vector, set at 1.
Pax-6 repression does not require the activation domain.
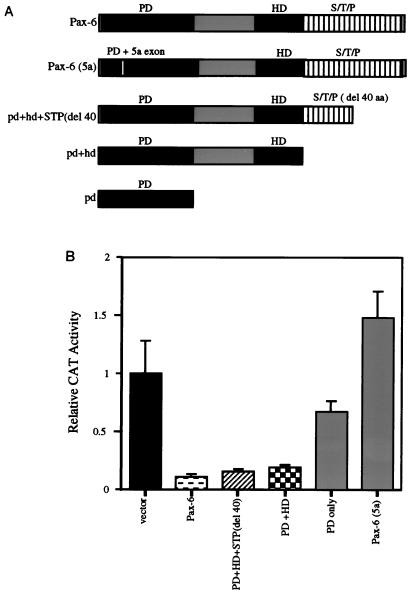
The portions of the Pax-6 protein required for the repression of βB1-crystallin promoter activity were investigated by cotransfection of 500 ng of the various truncated Pax-6 expression constructs with the chicken βB1-crystallin promoter/cat reporter gene construct into PLEs. Pax-6 lacking either 40 aa from the C terminus or the entire C-terminal transcriptional activation domain (Fig. 4A) repressed βB1-crystallin expression as efficiently as wild-type Pax-6 (Fig. 4B). Neither the Pax-6 PD alone nor the minor splice variant Pax-6 (5a) which has an alternate PD (19) was capable of mediating transcriptional repression (Fig. 4B). These data support the conclusion that both the HD and canonical PD of Pax-6 are important for the repression of the βB1-crystallin promoter and imply that sequence-specific binding of Pax-6 to the βB1-crystallin promoter is required for this repression since the alternatively spliced Pax-6 (5a), which has a PD with altered DNA binding specificity, did not mediate transcriptional repression.
FIG. 4.
The PD and HD of Pax-6 are required for Pax-6-mediated transcriptional repression. (A) Schematic diagram illustrating the structures of the Pax-6 proteins produced from the expression vectors used for panel B. S, serine; T, threonine; P, proline. (B) Cotransfection into PLEs of a plasmid harboring the chicken βB1-crystallin promoter (−434/+30 [18]) linked to the cat gene with vectors expressing either the full-length Pax-6 protein, the Pax-6 (5a) splice variant, or truncated forms of canonical Pax-6. The vector was pKW10 lacking Pax-6 coding sequences, used as a control.
Pax-6 can interact directly with the βB1-crystallin promoter.
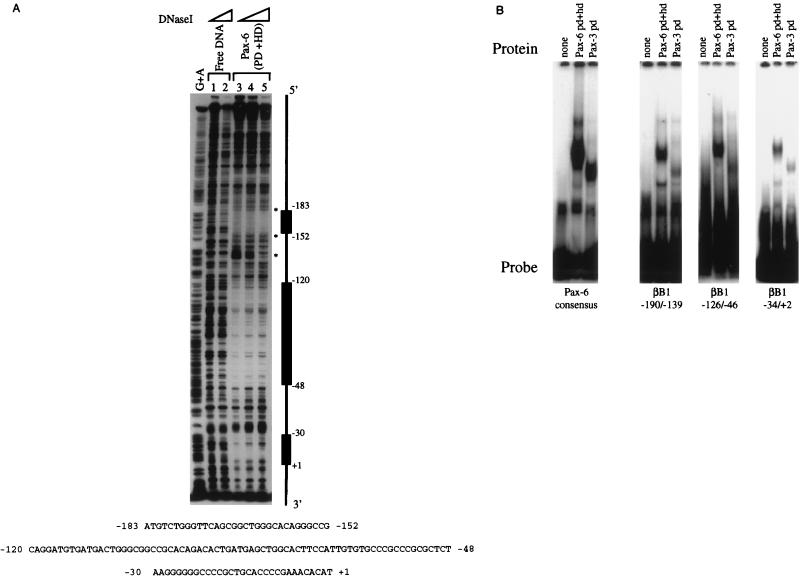
The ability of Pax-6 to bind to the βB1-crystallin promoter was assayed by DNase I footprinting. Pax-6 (PD+HD) was able to protect three regions of the βB1-crystallin promoter (−183 to −152, −120 to −48, and −30 to +1) (18, 42) from digestion with DNase I (Fig. 5A). The specificity of this interaction was investigated further by EMSA (Fig. 5B). All three footprinted sequences detected in the βB1-crystallin promoter interacted specifically with recombinant Pax-6, as judged by competition with nonradioactive nucleotides in EMSAs (data not shown). The interaction of these sites with the PD and HD of Pax-6 was generally weaker than that of a Pax-6 PD consensus binding site (19), as judged from the intensity of the radioactive band in the resulting complexes.
FIG. 5.
The chicken βB1-crystallin promoter can interact directly with the DNA binding domains of Pax-6. (A) DNase I footprinting analysis of the chicken βB1-crystallin promoter with a truncated recombinant Pax-6 protein consisting of the PD and HD fused to GST. Three regions of the proximal promoter which are protected from DNase I digestion (−185/−152, −120/−48, and −30/+1) are marked as solid boxes. (B) EMSA of the footprinted regions identified above with truncated recombinant Pax-6 consisting of the PD and HD.
Pax-6 binding at the PL2 element of chicken βB1-crystallin is involved in Pax-6 repression.
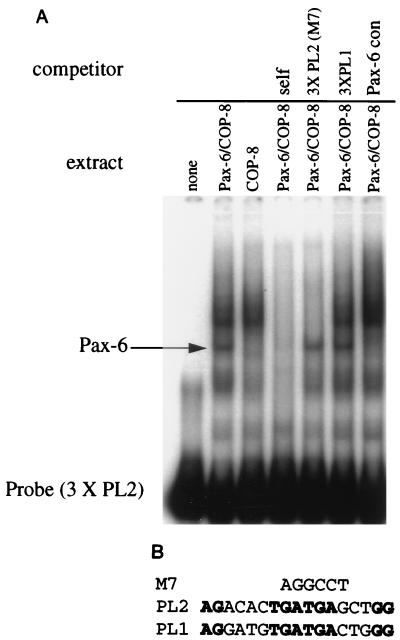
We have previously demonstrated that the −120 to −48 (−120/−48) region of the βB1-crystallin promoter footprinted by Pax-6 contains two cis elements (PL1 and PL2) which are required for full promoter activity in transfection experiments as well as in transgenic mice (18, 42). The role of these two elements in the interaction of Pax-6 with the −120/−48 sequence was tested by EMSA. The Pax-6 (PD+HD)/GST fusion protein binds to an oligonucleotide corresponding to the entire footprinted region (Fig. 6). The formation of a labeled complex was significantly reduced by competition with nonradioactive self oligonucleotide, a Pax-6 PD consensus oligonucleotide, and the −126/−46 sequence containing a functional mutation in PL1 (M6A [18, 42]) (Fig. 6). However, −126/−46 containing a functional mutation in PL2 (M7 [18, 42]) was unable to compete with radiolabeled wild-type oligonucleotide for binding. These data demonstrate that an intact PL2 element is necessary for Pax-6 binding to the −126/−46 region whereas an intact PL1 element is not, suggesting that Pax-6 makes direct contact with the PL2 element.
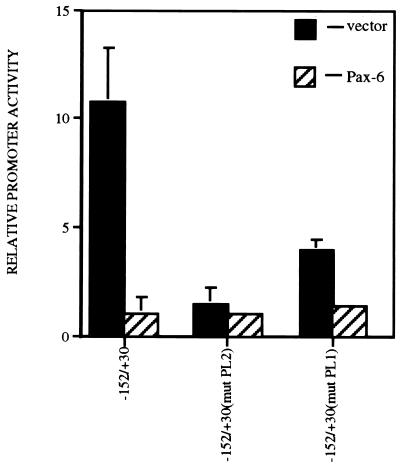
The functional role for Pax-6 interaction with the PL2 element was tested by cotransfection of 500 ng of either the Pax-6 expression vector or the parental plasmid pKW10 with promoter constructs consisting of the cat gene linked either to the wild-type chicken βB1-crystallin promoter (−152/+30), to a −152/+30 promoter harboring a mutation in PL1, or to a −152/+30 promoter harboring a mutation in PL2 (Fig. 7). As was described previously (18, 42), mutation of either the PL1 or the PL2 element significantly decreased activity of the βB1-crystallin promoter, demonstrating that both elements are important for its full transcriptional activity. Pax-6 was able to further repress the expression of a promoter harboring mutations in PL1 but not PL2, which suggests that Pax-6 interaction with PL2 is required to mediate transcriptional repression. However, it is possible that the βB1-crystallin promoter harboring mutations in PL2 is already fully repressed since it is not significantly more active than a promoterless vector. Thus, the role of Pax-6–PL2 interactions in transcriptional repression was explored further.
FIG. 7.
Cotransfection analysis of various chicken βB1-crystallin promoter constructs with a vector expressing full-length Pax-6. Note that promoters consisting of the −152/+30 region as well as the −152/+30 region harboring a mutation in the PL1 element were efficiently repressed by Pax-6, while the activity of a −152/+30 promoter harboring a mutation in PL2 was not. Promoter activity is relative to the activity of the promoterless vector, set at 1.
Surprisingly, oligonucleotides containing only a single copy of PL1 (βB1/PL1) or PL2 (βB1/PL2) (46) were unable to interact with Pax-6 (data not shown), indicating that the entire −120/−48 region is required for binding. Since it has been reported that Pax-6 can induce conformational changes in DNA (5), the ability of Pax-6 to induce DNA bending in the −126/−46 sequence was tested by circular permutation EMSAs (28). Our results showed that the migration of the DNA-protein complexes was not dependent on the position of the −126/−46 sequence in a larger DNA fragment (data not shown). Taken together, these data suggest that Pax-6 contacts the −120/−48 footprint directly but does not bend the DNA.
Pax-6 repression of PL2 activity was investigated further by EMSA with cellular extracts prepared from COP-8 cells or from COP-8 cells transfected with a plasmid expressing the full-length Pax-6 protein (13). The multimerized PL2 element formed a complex with Pax-6 as well as with a number of COP-8 cell proteins (Fig. 8A). The formation of these labeled complexes was virtually eliminated by competition with a 100-fold excess of nonradioactive self oligonucleotide but not by competition with the multimerized PL1 element. An oligonucleotide containing the multimerized PL2 element with the M7 mutation was unable to compete for Pax-6 binding, but it did compete for binding with the COP-8 proteins (Fig. 8A). Conversely, an oligonucleotide comprising a consensus binding site for Pax-6 competed with the labeled 3XPL2 oligonucleotide for binding to Pax-6 but did not diminish the intensity of the COP-8 protein complexes. Together, these data demonstrate that full-length Pax-6 interacts with PL2 at positions that are required for the maximal transcriptional activity of the βB1-crystallin promoter.
FIG. 8.
(A) The full-length Pax-6 protein interacts specifically with the multimerized PL2 element. (A) EMSA demonstrates that the trimerized PL2 element interacts specifically with full-length Pax-6 protein as well as proteins expressed by COP-8 fibroblasts. Note that the 3XPL2 oligonucleotide harboring the M7 mutation competes for binding with COP-8 proteins but not Pax-6, while the Pax-6 consensus (con) oligonucleotide competes only for the binding of Pax-6. (B) Alignment of the nucleotide sequences of PL1 and PL2. The nucleotides altered in the M7 functional PL2 mutation (18, 42) are noted above.
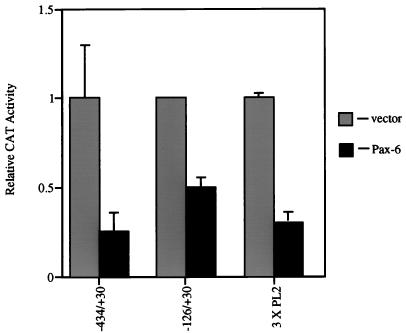
The functional significance of Pax-6 interaction with the PL2 element was further tested by cotransfecting the Pax-6 expression vector with a plasmid containing the cat reporter gene driven by either the minimal promoter of βB1-crystallin (−126/+30 [42]) or an artificial promoter composed of three copies of the PL2 element placed upstream of the β-actin basal promoter (18). The minimal promoter was repressed by Pax-6 (Fig. 9), demonstrating that the interaction at −183 to −152 is not essential for repression. The 3XPL2/β-actin promoter was also repressed (Fig. 8B), consistent with the idea that Pax-6 interaction with PL2 is involved in Pax-6-mediated transcriptional repression. We attempted to confirm that the nucleotides altered in the M7 mutation of the PL2 element were critical for Pax-6 repression by cotransfecting a 3XPL2 (mut M7)/β-actin CAT construct with the Pax-6 expression vector as shown in Fig. 9 [see Fig. 8B for a description of 3XPL2 (mut M7)]. However, this attempt was unsuccessful since the 3XPL2 (mut M7)/β-actin CAT construct was transcriptionally inactive when transfected into PLEs (data not shown).
FIG. 9.
Cotransfection of plasmids harboring various chicken βB1-crystallin promoter fragments linked to cat and either pKW10 expressing full-length Pax-6 protein or pKW10 alone into PLEs. Note that the minimal promoter (−126/+30) as well as an artificial construct driven by a multimerized PL2 element (3XPL2) are efficiently repressed by Pax-6. CAT activity is relative to the activity of the respective promoter in the absence of the Pax-6 expression vector, set at 1.
DISCUSSION
Pax-6 is essential for eye development in vertebrates (6, 21, 26). However, like Six-3 and Otx-2 (other proteins critical for lens development [32, 34, 35, 44]), the expression of Pax-6 persists in the eye after its determination is complete (29, 31, 40). While Pax-6 does transcriptionally activate some crystallin genes expressed throughout lens differentiation (8–10, 20, 40), the function of Pax-6 during maturation of the eye has not been investigated in depth. The present study suggests that Pax-6 transcriptionally represses βB1-crystallin gene expression. We believe that this contributes to the absence of expression of this crystallin in the embryonic lens epithelia of chicken and mice, where Pax-6 is prevalent, and to the apparent inverse relationship between the amount of Pax-6 and βB1-crystallin promoter activity in the lens fiber cells of embryonic chickens (4, 23, 31).
Pax-6 as a transcriptional repressor.
During mouse and chicken lens development, the expression of βB1-crystallin commences when cells in the posterior of the lens vesicle leave the cell cycle, elongate, and differentiate into primary lens fiber cells (4). In chicken lens, the expression levels of this gene remain low compared to δ-crystallin until embryonic day 19, when βB1-crystallin transcription increases (23).
The expression pattern of Pax-6 appears to be inversely correlated with that of βB1-crystallin. Throughout development, Pax-6 is found at high levels in lens epithelial cells (Fig. 1) (29, 31, 40), which express δ-crystallin (23, 49) but do not express βB1-crystallin (Fig. 2) (4, 23). As Pax-6 protein levels decrease in differentiating lens fiber cells, the transcription of β-crystallins appears to increase. In the adult chicken lens, which expresses high levels of βB1-crystallin (23), little to no Pax-6 protein is detectable in the lens fiber cells. These observations are consistent with the proposition that Pax-6 represses βB1-crystallin transcription in the lens.
Recently, Altmann et al. (1) have demonstrated that the injection of ectopic Pax-6 mRNA into either Xenopus laevis animal caps or the animal pole of two- to four-cell-stage Xenopus embryos was sufficient to induce ectopic lens formation as assayed by both morphology and the induction of βB1-crystallin gene expression. While this experiment may at first seem to contradict the idea that Pax-6 represses βB1-crystallin gene expression, it actually may demonstrate that the injection of ectopic Pax-6 mRNA causes a faithful recapitulation of normal lens development. Since it is known that the determination of the lens placode from the head ectoderm requires the cell autonomous expression of Pax-6 (21), it is apparent that βB1-crystallin-expressing fiber cells (4, 18) can develop only when their mitotic precursors express Pax-6. Altmann et al. (1) demonstrated that the βB1-crystallin-expressing cells no longer have detectable amounts of the FLAG-tagged ectopic Pax-6 protein, which suggests that βB1-crystallin gene expression commences only after a reduction in the amount of ectopic protein. While their data also show that expression of the endogenous Pax-6 gene is induced in these embryos, the known expression pattern of Pax-6 in the lens (31) indicates that the increased expression is in the epithelial component of the ectopic lenses.
In the present study, the introduction of a Pax-6 expression vector into lens cells or embryonic fibroblasts cultured in vitro significantly decreased transcriptional activity of the βB1-crystallin promoter, suggesting that Pax-6 can function as a transcriptional repressor as well as an activator. Cotransfection experiments performed with constructs expressing truncated Pax-6 proteins demonstrated that the C terminus of Pax-6, including its entire activation domain (6, 14, 19), is not necessary for its transcriptional repression activity. In addition, neither the PD alone nor Pax-6 (5a) (a splice variant with an altered PD [19]) repressed βB1-crystallin transcription. These data demonstrate that the canonical PD and HD are necessary to mediate transcriptional repression and suggest that the mechanism requires DNA binding (13, 14).
DNase I footprinting and EMSAs have demonstrated that the PD and HD of Pax-6 bind directly to the βB1-crystallin promoter, further supporting the idea that transcriptional repression by Pax-6 is mediated through direct DNA-protein contacts. It is unlikely that this DNA-protein interaction results in transcriptional repression due to distortion of the DNA since circular permutation analysis (28, 50) indicated that Pax-6 bends the βB1-crystallin promoter only slightly if at all. Notably, one of the Pax-6 footprints on the βB1-crystallin promoter (−120/−48) encompasses two promoter elements (PL1 and PL2), which have been demonstrated by mutation analysis to be important for the full transcriptional activity of the βB1-crystallin promoter (18, 42). These elements have been shown to bind to a number of different proteins in qualitative EMSAs (42, 46), suggesting complex regulation mechanisms. In this study, EMSAs have demonstrated that the same mutations in PL2 which significantly decrease activity of the βB1-crystallin promoter (18, 42) also abolish the ability of Pax-6 to bind the −120/−48 region. Cotransfection experiments have demonstrated that Pax-6 can repress the transcriptional activity of an artificial promoter constructed from the β-actin basal promoter fused to a trimerized PL2 element (18), and EMSAs have demonstrated that Pax-6 can bind to the trimerized element in a sequence-specific manner. These data taken together strongly suggest that Pax-6 represses βB1-crystallin transcription by direct interaction with the PL2 element. However, it is clear that the mechanism of Pax-6 binding to the chicken βB1-crystallin promoter is complex and requires the entire footprinted region from −120/−48 since the PL2 element in isolation does not bind to Pax-6.
Notably, Pax-6 also binds to the chicken βA3/A1-crystallin promoter at a site (−95/−58) critical for promoter activity, as determined by deletion analysis, and represses the activity of the minimal promoter (unpublished data). While the nucleotides critical for the function of the chicken βA3/A1-crystallin minimal promoter have not been elucidated, this Pax-6 footprint does overlap with two regions of the promoter (−116/−79 and −66/−45) known to bind lens nuclear factors (33).
The Pax-6 binding sites that we have identified in the βB1-crystallin promoter differ considerably from the consensus Pax-6 PD binding sites described previously (14, 19). This probably reflects the fact that the repression of β-crystallin expression by Pax-6 requires both the PD and HD. It has been proposed that PD and HD proteins can recognize different target sequences by utilizing various combinations of their DNA binding domains (27). Unlike the consensus PD binding site (14), the naturally occurring sites found in the βB1-crystallin promoter bind Pax-6 with higher affinity than the PD of Pax-3 (data not shown). Thus, the naturally occurring sites appear to have a preferential affinity for Pax-6.
Comparison with other Pax proteins.
Other Pax family members have also been shown to function as both transcriptional activators and repressors. The transcriptional repression of the p53 gene by Pax-2, -5, and -8 requires both binding to the p53 promoter as well as the presence of a transcriptional inhibitory domain at the extreme C terminus of the Pax proteins (16, 45). Pax-3 inhibits the transcription of the gene for N-CAM in cotransfection tests; however, direct binding to the N-CAM promoter was not detected (5). The mechanism that Pax-3 uses to repress the N-CAM promoter is not clear. However, when the first 90 aa of Pax-3 (including a portion of the PD) are fused to the GAL-4 DNA binding domain, a transcriptional repressor is created (5). Thus, Pax-2, -3, -5, and -8 appear to contain a transcriptional repression domain (5, 16) that actively disrupts transcriptional activation. In contrast, Pax-6 seems to repress the transcription of the βB1- and βA3/A1-crystallin promoters by competition with transcriptional activators for promoter occupancy (7, 24).
Conclusions.
Previous experiments have established that Pax-6 can activate a number of crystallin genes (8–10, 20, 40). The present work demonstrates that Pax-6 can function as a transcriptional repressor as well. Our data suggest that this repression occurs by Pax-6 binding to positive acting cis elements. If this mechanism is correct, the transcriptional repression would be relieved as the levels of Pax-6 decrease in differentiating lens fiber cells, consistent with the observed lens fiber cell-specific expression of the βB1-crystallin gene.
ACKNOWLEDGMENTS
We thank Nicole Newman for preparing paraffin sections, J. A. Davis and R. R. Reed for the Pax-6 antibody, S. K. Brahma for the βB1-crystallin antibody, K. Yasuda for 3XPL2/CAT, and J. Epstein and R. L. Maas for the Pax-6/GST fusion construct.
REFERENCES
- 1.Altmann C R, Chow R L, Lang R A, Hemmati-Brivanlou A. Lens induction by Pax-6 in Xenopus laevis. Dev Biol. 1997;185:119–123. doi: 10.1006/dbio.1997.8573. [DOI] [PubMed] [Google Scholar]
- 2.Bloemendal H, de Jong W W. Lens proteins and their genes. Prog Nucleic Acid Res. 1991;41:259–281. doi: 10.1016/s0079-6603(08)60012-4. [DOI] [PubMed] [Google Scholar]
- 3.Borras T, Peterson C A, Piatigorsky J. Evidence for positive and negative regulation in the promoter of the chicken delta 1-crystallin gene. Dev Biol. 1988;127:209–219. doi: 10.1016/0012-1606(88)90202-3. [DOI] [PubMed] [Google Scholar]
- 4.Brahma S K. Ontogeny of βB1-crystallin polypeptides during chicken lens development. Exp Eye Res. 1988;47:507–519. doi: 10.1016/0014-4835(88)90060-7. [DOI] [PubMed] [Google Scholar]
- 5.Chalepakis G, Jones F S, Edelman G M, Gruss P. Pax-3 contains domains for transcription activation and transcription inhibition. Proc Natl Acad Sci USA. 1994;91:12745–12749. doi: 10.1073/pnas.91.26.12745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Churchill A, Booth A. Genetics of aniridia and anterior segment dysgenesis. Br J Ophthalmol. 1996;80:669–673. doi: 10.1136/bjo.80.7.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cowell I G. Repression versus activation in the control of gene transcription. Trends Biochem Sci. 1994;19:38–42. doi: 10.1016/0968-0004(94)90172-4. [DOI] [PubMed] [Google Scholar]
- 8.Cvekl A, Sax C M, Bresnick E H, Piatigorsky J. A complex array of positive and negative elements regulates the chicken αA-crystallin gene: involvement of Pax-6, USF, CREB and/or CREM, and AP-1 proteins. Mol Cell Biol. 1994;14:7363–7376. doi: 10.1128/mcb.14.11.7363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cvekl A, Kashanchi F, Sax C M, Brady J N, Piatigorsky J. Transcriptional activation of the mouse αA-crystallin gene: activation dependent on a cyclic AMP-responsive element (DE1/CRE) and a Pax-6 binding site. Mol Cell Biol. 1995;15:563–660. doi: 10.1128/mcb.15.2.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cvekl A, Sax C M, Li X, McDermott J B, Piatigorsky J. Pax-6 and lens-specific expression of the chicken δ1-crystallin gene. Proc Natl Acad Sci USA. 1995;92:4681–4685. doi: 10.1073/pnas.92.10.4681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cvekl A, Piatigorsky J. Lens development and crystallin gene expression: many roles for Pax-6. Bioessays. 1996;18:621–630. doi: 10.1002/bies.950180805. [DOI] [PubMed] [Google Scholar]
- 12.Cvekl, A., et al. Submitted for publication.
- 13.Czerny T, Schaffner G, Busslinger M. DNA sequence recognition by Pax proteins: bipartite structure of the paired domain and its binding site. Genes Dev. 1993;7:2048–2061. doi: 10.1101/gad.7.10.2048. [DOI] [PubMed] [Google Scholar]
- 14.Czerny T, Busslinger M. DNA-binding and transactivation properties of Pax-6: three amino acids in the paired domain are responsible for the different sequence recognition of Pax-6 and BSAP (Pax-5) Mol Cell Biol. 1995;15:2858–2871. doi: 10.1128/mcb.15.5.2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davis J A, Reed R R. Role of Olf-1 and Pax-6 transcription factors in neurodevelopment. J Neurosci. 1996;16:5082–5094. doi: 10.1523/JNEUROSCI.16-16-05082.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dorfler P, Busslinger M. C-terminal activating and inhibitory domains determine the transactivation potential of BSAP (Pax-5), Pax-2 and Pax-8. EMBO J. 1996;15:1971–1982. [PMC free article] [PubMed] [Google Scholar]
- 17.Duncan M K, Roth H J, Thompson M, Kantorow M, Piatigorsky J. Chicken βB1-crystallin: gene sequence and evidence for functional conservation of promoter activity between chicken and mouse. Biochim Biophys Acta. 1995;1261:68–76. doi: 10.1016/0167-4781(94)00223-p. [DOI] [PubMed] [Google Scholar]
- 18.Duncan M K, Li X, Ogino H, Yasuda K, Piatigorsky J. Developmental regulation of the chicken βB1-crystallin promoter in transgenic mice. Mech Dev. 1996;57:79–89. doi: 10.1016/0925-4773(96)00533-3. [DOI] [PubMed] [Google Scholar]
- 19.Epstein J A, Glaser T, Cai J, Jepeal L, Walton D S, Maas R L. Two independent and interactive DNA-binding subdomains of the Pax-6 paired domain are regulated by alternative splicing. Genes Dev. 1994;8:2022–2034. doi: 10.1101/gad.8.17.2022. [DOI] [PubMed] [Google Scholar]
- 20.Gopal-Srivastava R, Cvekl A, Piatigorsky J. Pax-6 and αB-crystallin/small heat shock protein gene regulation in the murine lens. J Biol Chem. 1996;271:23029–23036. doi: 10.1074/jbc.271.38.23029. [DOI] [PubMed] [Google Scholar]
- 21.Grindley J C, Davidson D R, Hill R E. The role of Pax-6 in eye and nasal development. Development. 1995;121:1433–1442. doi: 10.1242/dev.121.5.1433. [DOI] [PubMed] [Google Scholar]
- 22.Haynes J I, Duncan M K, Piatigorsky J. Spatial and temporal activity of the αB-crystallin/small heat shock protein gene promoter in transgenic mice. Dev Dyn. 1996;207:75–88. doi: 10.1002/(SICI)1097-0177(199609)207:1<75::AID-AJA8>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 23.Hejtmancik J F, Beebe D C, Ostrer H, Piatigorsky J. δ- and β-crystallin mRNA levels in the embryonic and posthatched chicken lens: temporal and spatial changes during development. Dev Biol. 1985;109:72–81. doi: 10.1016/0012-1606(85)90347-1. [DOI] [PubMed] [Google Scholar]
- 24.Herschbach B M, Johnson A D. Transcriptional repression in eukaryotes. Annu Rev Cell Biol. 1993;9:479–509. doi: 10.1146/annurev.cb.09.110193.002403. [DOI] [PubMed] [Google Scholar]
- 25.Hill R E, Hanson I M. Molecular genetics of the Pax gene family. Curr Opin Cell Biol. 1992;4:967–972. doi: 10.1016/0955-0674(92)90126-w. [DOI] [PubMed] [Google Scholar]
- 26.Hill R I, Favor J, Hogan B L M, Ton C C T, Saunders G F, Hanson I M, Prosser J, Jordan T, Hastie N D, van Heyningen V. Mouse small eye results from mutations in a paired-like homeobox-containing gene. Nature. 1991;354:522–525. doi: 10.1038/354522a0. [DOI] [PubMed] [Google Scholar]
- 27.Jun S, Desplan C. Cooperative interactions between paired domain and HD. Development. 1996;122:2639–2650. doi: 10.1242/dev.122.9.2639. [DOI] [PubMed] [Google Scholar]
- 28.Kim J, Zwieb C, Wu C, Adhya S. Bending of DNA by gene-regulatory proteins: construction and use of a DNA bending vector. Gene. 1989;85:15–23. doi: 10.1016/0378-1119(89)90459-9. [DOI] [PubMed] [Google Scholar]
- 29.Koroma B M, Yang J-M, Sundin O H. The Pax-6 homeobox gene is expressed throughout the corneal and conjunctival epithelia. Invest Ophthalmol Visual Sci. 1997;38:108–120. [PubMed] [Google Scholar]
- 30.Land M F, Fernald R D. The evolution of eyes. Annu Rev Neurosci. 1992;15:1–29. doi: 10.1146/annurev.ne.15.030192.000245. [DOI] [PubMed] [Google Scholar]
- 31.Li H-S, Yang J-M, Jacobson R D, Pasko D, Sundin O. Pax-6 is first expressed in a region of ectoderm anterior to the early neural plate: implications for stepwise determination of the lens. Dev Biol. 1994;162:181–194. doi: 10.1006/dbio.1994.1077. [DOI] [PubMed] [Google Scholar]
- 32.Matsuo I, Kuratani S, Kimura C, Takeda N, Aizawa S. Mouse Otx-2 functions in the formation and patterning of the rostral head. Genes Dev. 1995;9:2646–2658. doi: 10.1101/gad.9.21.2646. [DOI] [PubMed] [Google Scholar]
- 33.McDermott J B, Cvekl A, Piatigorsky J. Lens-specific expression of a chicken βA3/A1-crystallin promoter fragment in transgenic mice. Biochem Biophys Res Commun. 1996;221:559–564. doi: 10.1006/bbrc.1996.0635. [DOI] [PubMed] [Google Scholar]
- 34.Oliver G, Mailhos A, Wehr R, Copeland N G, Jenkins N A, Gruss P. Six-3, a murine homologue of the sine oculis gene, demarcates the most anterior border of the developing neural plate and is expressed during eye development. Development. 1995;121:4045–4055. doi: 10.1242/dev.121.12.4045. [DOI] [PubMed] [Google Scholar]
- 35.Oliver G, Loosli F, Koster R, Wittbrodt J, Gruss P. Ectopic lens induction in fish in response to the murine homeobox gene Six3. Mech Dev. 1996;60:233–239. doi: 10.1016/s0925-4773(96)00632-6. [DOI] [PubMed] [Google Scholar]
- 36.Ostrer H, Beebe D C, Piatigorsky J. β-Crystallin mRNAs: differential distribution in the developing chicken lens. Dev Biol. 1981;86:403–408. doi: 10.1016/0012-1606(81)90198-6. [DOI] [PubMed] [Google Scholar]
- 37.Piatigorsky J. Lens differentiation in vertebrates. A review of cellular and molecular features. Differentiation. 1981;19:134–53. doi: 10.1111/j.1432-0436.1981.tb01141.x. [DOI] [PubMed] [Google Scholar]
- 38.Piatigorsky, J. Gene sharing in lens and cornea: facts and implications. Prog. Retin. Eye Res., in press. [DOI] [PubMed]
- 39.Quiring R, Walldorf U, Kloter U, Gehring W. Homology of the eyeless gene of Drosophila to the small eye gene in mice and aniridia in humans. Science. 1994;265:785–789. doi: 10.1126/science.7914031. [DOI] [PubMed] [Google Scholar]
- 40.Richardson J, Cvekl A, Wistow G. Pax-6 is essential for lens-specific expression of ζ-crystallin. Proc Natl Acad Sci USA. 1995;92:4676–4680. doi: 10.1073/pnas.92.10.4676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robinson M L, Overbeek P A. Differential expression of αA- and αB-crystallin during murine ocular development. Invest Ophthalmol Visual Sci. 1996;37:2276–2284. [PubMed] [Google Scholar]
- 42.Roth H J, Das G C, Piatigorsky J. Chicken βB1-crystallin gene expression: presence of conserved functional polyomavirus enhancer-like and octamer binding-like promoter elements found in non-lens genes. Mol Cell Biol. 1991;11:1488–1499. doi: 10.1128/mcb.11.3.1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shinohara T, Piatigorsky J. Quantitation of delta-crystallin messenger RNA during lens induction in chick embryos. Proc Natl Acad Sci USA. 1976;73:2808–2812. doi: 10.1073/pnas.73.8.2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Simeone A, Acampora D, Mallamaci A, Stornaiuolo A, D’Apice M R, Nigro V, Boncinelli E. A vertebrate gene related to orthodenticle contains a homeodomain of the bicoid class and demarcates anterior neuroectoderm in the gastrulating mouse embryo. EMBO J. 1993;12:2735–2747. doi: 10.1002/j.1460-2075.1993.tb05935.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stuart E T, Haffner R, Oren M, Gruss P. Loss of p53 function through PAX-mediated transcriptional repression. EMBO J. 1995;14:5638–5645. doi: 10.1002/j.1460-2075.1995.tb00251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tomarev S I, Duncan M K, Roth H J, Cvekl A, Piatigorsky J. Convergent evolution of crystallin gene regulation in squid and chicken: the AP-1/ARE connection. J Mol Evol. 1994;39:134–143. doi: 10.1007/BF00163802. [DOI] [PubMed] [Google Scholar]
- 47.Tomarev S I, Callaerts P, Kos L, Zinovieva R, Halder G, Gehring W, Piatigorsky J. Squid Pax-6 and eye development. Proc Natl Acad Sci USA. 1997;94:2421–2426. doi: 10.1073/pnas.94.6.2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wistow G, Piatigorsky J. Lens crystallins: evolution and expression of proteins for a highly specialized tissue. Annu Rev Biochem. 1988;57:479–504. doi: 10.1146/annurev.bi.57.070188.002403. [DOI] [PubMed] [Google Scholar]
- 49.Zwaan J, Ikeda A. Macromolecular events during differentiation of the chicken lens. Exp Eye Res. 1968;7:301–311. doi: 10.1016/s0014-4835(68)80081-8. [DOI] [PubMed] [Google Scholar]
- 50.Zwieb C, Kim J, Adhya S. DNA bending by negative regulatory proteins: Gal and Lac repressors. Genes Dev. 1989;3:606–611. doi: 10.1101/gad.3.5.606. [DOI] [PubMed] [Google Scholar]