Abstract
Efferocytosis is a process whereby apoptotic cells are cleared to maintain tissue homeostasis. In the lungs, efferocytosis has been implicated in several acute and chronic inflammatory diseases. A long-standing method to study efferocytosis in vivo is to instill apoptotic cells into the lungs to evaluate macrophage uptake. However, this approach provides nonphysiologic levels of cells to the airspaces, where there is preferential access to the alveolar macrophages. To circumvent this limitation, we developed a new method to study efferocytosis of damaged alveolar type 2 (AT2) epithelial cells in vivo. A reporter mouse that expresses TdTomato in AT2 epithelial cells was injured with influenza (strain PR8) to induce apoptosis of AT2 cells. We were able to identify macrophages that acquire red fluorescence after influenza injury, indicating efferocytosis of AT2 cells. Furthermore, evaluation of macrophage populations led to the surprising finding that lung interstitial macrophages were the primary efferocyte in vivo. In summary, we present a novel finding that the interstitial macrophage, not the alveolar macrophage, primarily mediates clearance of AT2 cells in the lungs after influenza infection. Our method of studying efferocytosis provides a more physiologic approach in evaluating the spatiotemporal dynamics of apoptotic cell clearance in vivo and opens new avenues to study the mechanisms by which efferocytosis regulates inflammation.
Keywords: efferocytosis, apoptosis, lung inflammation, influenza
With a large mucosal surface, the lungs have adapted to constant environmental pressures, both sterile and infectious, to contain inflammation and repair any damage (1). Damaged epithelial cells that cannot fully repair themselves after exposure to a noxious stimulus will initiate programmed cell death (2). Efferocytosis is a well-defined process by which apoptotic cells are silently cleared to maintain tissue homeostasis (3). If clearance of apoptotic cells is impaired, the dying cells undergo secondary necrosis, resulting in the release of highly inflammatory intracellular contents (4). As an epithelial cell becomes apoptotic, “find me” signals are presented on the cell surface that target phagocytes to the dying cell (5), and other factors act as “eat me” signals that promote engulfment (6). In contrast, apoptotic cells can also present surface molecules that function as “don’t eat me” signals, and these must be eliminated in some manner to promote efferocytosis (7). Moreover, efferocytosis is antiinflammatory by inducing the release of immunosuppressive cytokines, such as transforming growth factor-β1 and IL-10, from macrophages (8–13). To that end, it is unsurprising that dysregulated efferocytosis can acutely cause tissue inflammation and chronically promote aberrant tissue remodeling (7, 14).
In vivo studies of efferocytosis in the lungs have largely used a model in which apoptotic cells are instilled into the airspaces to determine the uptake by alveolar macrophages (AMs) (14–16). However, introduction of exogenous cells at nonphysiologic levels cannot accurately replicate the spatiotemporal dynamics of apoptotic cell clearance that occur in the lung microenvironment after acute injury. Moreover, in vitro studies using primary macrophages, macrophages derived from human peripheral blood monocytes, and murine bone marrow–derived macrophages have been used in studying mechanisms of efferocytosis (16–19). However, experiments using cultured macrophages need to be interpreted with caution because the macrophage phenotype is clearly dependent on the niche where it exists (20–23).
In this study, we aimed to develop a physiologic model to examine efferocytosis of damaged alveolar type 2 (AT2) epithelial cells in vivo using mice that conditionally express TdTomato that is restricted to AT2 cells. With this method, our results revealed a surprising finding that the interstitial macrophage (IM), and not the AM, played a predominant role in the clearance of apoptotic AT2 cells after influenza and bleomycin injury.
Methods
Efferocytosis Model
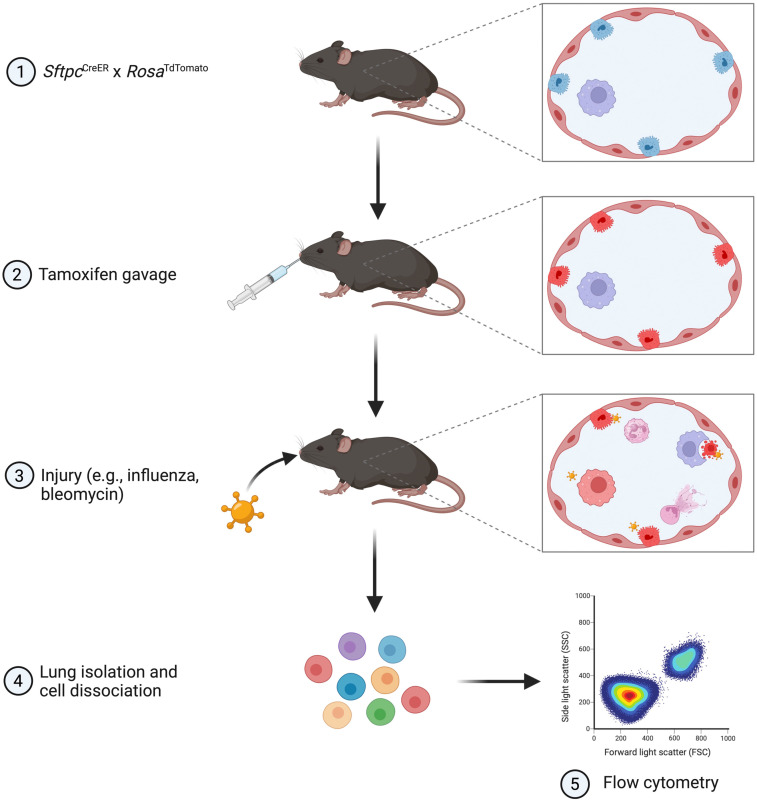
AT2TdTomato mice were generated by the crossing of SftpcCreER (The Jackson Laboratory, strain 028054) and RosaTdTomato (The Jackson Laboratory, strain 007909). Tamoxifen (T5648; Sigma-Aldrich) was dissolved in corn oil (20 mg/ml), and 200 mg/kg was administered by oral gavage every other day for four total doses to induce TdTomato expression in AT2 cells. One week after the last tamoxifen dose, mice were injured with either mouse-adapted H1N1 influenza virus A/Puerto Rico/8/34 (PR8) or bleomycin. For influenza injury, PR8 was intranasally inoculated (250 plaque-forming units in 25 μl of PBS) under inhalation anesthesia with isoflurane. For bleomycin injury, mice were intubated, and bleomycin (0.5 U/kg in 50 μl; APP Pharmaceuticals) was instilled intratracheally.
Flow Cytometry
Single-cell suspensions of mouse lungs were dissociated as follows. Lungs were instilled with elastase (Worthington Biochemical Corporation, LS002279; 4 U/ml in DMEM/F12), chopped, and incubated at 37°C in 10 ml Liberase (Sigma-Aldrich, 5401119001; 1× in DMEM/F12). After 30 minutes, the digestion was stopped by adding cold 2% FBS and 1 mM EDTA in Hanks’ balanced salt solution. To obtain a single-cell suspension, homogenized lungs were then passed through a 70-μm filter. Cells were pelleted and incubated with 1 ml 1× red blood cell lysis buffer (eBioscience, 00-4333-57) for 1 minute at room temperature. Cells were washed with cold flow buffer (PBS + 5% FBS), and the cell pellet was then resuspended in flow buffer and stained with a mixture of fluorochrome-conjugated antibodies for 30 minutes at 4°C. After incubation, cells were washed with flow buffer, fixed with 4% paraformaldehyde for 10 minutes at room temperature, and washed and stored in flow buffer until flow cytometric analysis. Data were acquired on BD LSRFortessa cell analyzer, and data were analyzed with FlowJo software.
All flow cytometry antibodies were from BioLegend and incubated with cells at a 1:100 dilution in flow buffer: CD45-Alexa Fluor 700 (clone 30-F11); CD11b-Brilliant Violet 510 (clone M1/70); Siglec F-allophycocyanin (clone S1700L); Ly6G-Brilliant Violet 421 (clone 1A8); Ly6C-allophycocyanin/cyanine 7 (clone HK1.4); CD11c-phycoerythrin/cyanine 7 (clone N418); CD64-FITC (clone X54-5/7.1); and F4/80-Brilliant Violet 650 (clone BM8).
Immunofluorescence
Lungs were inflated with 10% neutral formalin and then placed in 70% alcohol after 24 hours. Formaldehyde-fixed, paraffin-embedded sections were further processed and histologically evaluated. The 7-mm-thick sections underwent 10 mM citrate buffer antigen retrieval, permeabilization with 10% methanol and 0.4 H2O2, and blocked with 5% BSA. Lung sections were stained with anti-F4/80 rat monoclonal antibody (1:100; Abcam, ab16911), anti-cleaved caspase-3 rabbit polyclonal antibody (1:200; Cell Signaling Technology, CST9661), anti-Sftpc rabbit polyclonal antibody (1:500; Invitrogen, pa5-71680), and anti–red fluorescent protein rabbit polyclonal antibody (1:200; Rockland, 600-401-379S) or anti–red fluorescent protein goat polyclonal antibody (1:500; SICGEN antibodies, AB8181-200). Alexa Fluor–coupled secondary antibodies (Invitrogen) were used at 1:500 dilution. All immunofluorescence images were obtained with a Zeiss LSM780 confocal microscope.
Statistics
Prism software (GraphPad Software) was used for all statistical analysis and generation of graphs. The Kolmogorov-Smirnov test was used to determine the normality of the data. One-way ANOVA was used for normally distributed data. For data not in a normal distribution, the Friedman test was used to evaluate the statistical significance of differences between groups. Outliers were not tested for, because no data were removed from the analyses.
Study Approval
All animal experiments were approved by the institutional animal care and use committee at Cedars-Sinai Medical Center (IACUC009018).
Results
Characterizing Efferocytosis of Apoptotic AT2 Cells after Influenza Infection
A RosaTdTomato reporter line crossed with an SftpcCreER transgenic mouse was created (herein called AT2TdTomato) to study in vivo efferocytosis (see Figure 1 and the Methods section for workflow). In an uninjured state, TdTomato expression is confined to AT2 cells (Figures 2A and 2B), which show no evidence of apoptosis in an uninjured state (Figure 2C). Influenza is tropic to the lung epithelium, where the virus replicates and causes apoptotic death of the infected epithelial cell (24). Therefore, AT2TdTomato mice were injured with a mouse-adapted H1N1 influenza virus (A/Puerto Rico/8/1934; also called PR8), which caused AT2 cell apoptosis (Figure 2D).
Figure 1.
Schematic of the workflow for the in vivo efferocytosis model. The SftpcCreER × RosaTdTomato mice were given tamoxifen to label alveolar type 2 (AT2) cells with red fluorescence. Mice were then injured with intranasal influenza or bleomycin to induce AT2 cell apoptosis. At the appropriate time point, murine lungs were isolated and dissociated into single-cell suspensions for immunostaining and analysis by flow cytometry. This figure was created with BioRender.com.
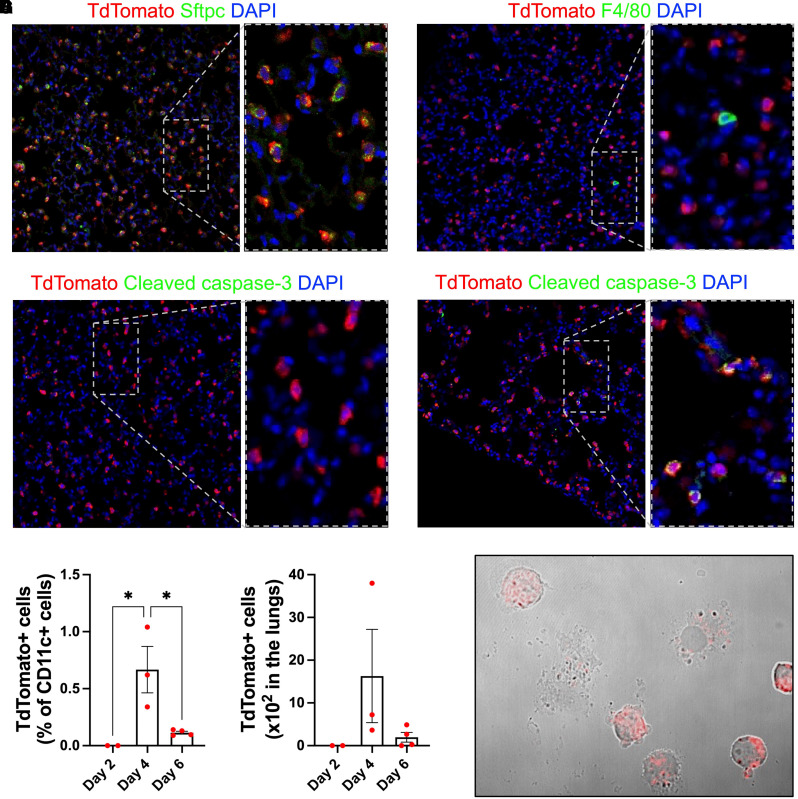
Figure 2.
Macrophage efferocytosis of apoptotic AT2 cells during influenza infection. (A–C) Immunofluorescence of uninjured AT2TdTomato mice. (A) Sftpc and TdTomato staining shows that TdTomato signal colocalizes with AT2 cells. (B) F4/80 and TdTomato staining shows macrophages lack TdTomato signal. (C) Cleaved caspase-3 and TdTomato staining does not identify any apoptotic cells in an uninjured state. (D–F) AT2TdTomato mice were injured with PR8 (250 plaque-forming units). (D) Immunofluorescence of injured lungs for cleaved caspase-3 and TdTomato shows AT2 apoptosis on Day 4 after influenza infection. (E–G) Lungs were processed for flow cytometry of CD11c+/TdTomato+ cells. To determine the TdTomato gating strategy, we used a wild-type C57BL/6 control mouse infected with PR8 to determine the cutoff for a TdTomato-negative population. (E) Frequency and (F) total number of CD11c+ cells that had TdTomato positivity in the lungs at Days 2, 4, and 6 after influenza infection. *P < 0.05 by one-way ANOVA. Each dot represents an individual mouse. (G) Cytospin of CD11c+/TdTomato+ cells captured by FACS (BD Influx System).
TdTomato was largely absent in macrophages on Day 2 after infection (Figures 2E and 2F), which is early in the disease course and before the development of lung inflammation (24). However, CD11c+ cells acquired the TdTomato signal at Day 4 and less so at Day 6 after influenza infection, thereby establishing our model as a feasible method to study efferocytosis of AT2 cells after injury (Figures 2E–2G). Because Sftpc is exclusively expressed by AT2 cells (Figure 2A), CD11c+, TdTomato+ cells identified after influenza injury indicate immune cell engulfment of AT2 cells, which is conceptually similar to our previous study where we evaluated efferocytosis by peritoneal macrophages in bone marrow chimeras with DsRed-labeled circulating leukocytes (25).
The IM Primarily Mediates Efferocytosis of Apoptotic AT2 Cells after Injury
The literature suggests the AM is the primary efferocyte in the lungs (14, 26). Data supporting this conclusion are based on studies in which apoptotic cells are intranasally instilled into the lungs and AMs are recovered by BAL to identify the uptake of apoptotic cells (14, 15). However, methods to study the clearance of apoptotic epithelial cells in vivo under physiologic conditions are lacking. Therefore, we used the AT2TdTomato mice to evaluate the relative contributions of various macrophage populations in clearing apoptotic AT2 cells (Figure 3A).
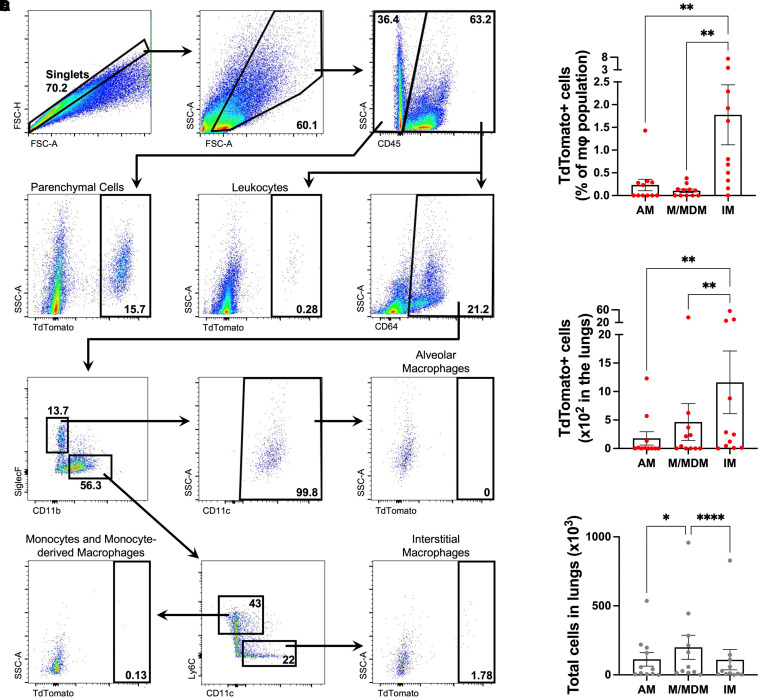
Figure 3.
Alveolar macrophage (AM), monocyte and monocyte-derived macrophage (M/MDM), and interstitial macrophage (IM) efferocytosis of apoptotic AT2 cells during influenza infection. AT2TdTomato mice were injured with PR8 (250 plaque-forming units), and on Day 4 after injury, lungs were processed for flow cytometry. To determine the TdTomato gating strategy, we used a wild-type C57BL/6 control mouse infected with PR8 to determine the cutoff for a TdTomato-negative population. (A) Gating strategy showing the TdTomato+ population in AMs, M/MDMs, and IMs. Bolded number denotes the percentage within the gated population. (B) Frequency and (C) total number of AMs, M/MDMs, and IMs that had TdTomato positivity. (D) Total number of AMs, M/MDMs, and IMs in the lungs of the mice. Each dot represents an individual mouse. Values are the mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.0001 by the Friedman test.
To our surprise, IMs had the most clearance of AT2 cells in vivo after influenza infection that exceeded the ability of AMs and a combined population of monocytes and monocyte-derived macrophages (M/MDMs) at Day 4 after PR8 infection (Figures 3B and 3C). Indeed, IMs compared with AMs and M/MDMs had significantly higher frequencies of TdTomato positivity (1.78 ± 0.66% vs. 0.23 ± 0.13% vs. 0.10 ± 0.04%, respectively) and total cell counts in the lungs (1,161 ± 550.4 vs. 177.7 ± 117.1 vs. 465.6 ± 323.2, respectively).
Over 6.5 times as many IMs had engulfed AT2 cells compared with AMs, whereas the abundance of AMs and IMs was similar (Figure 3D). Therefore, the finding of IMs having more efferocytosis of AT2 cells than AMs was not a result of shifts in relative numbers of macrophage subpopulations.
Next, we evaluated a single-cell RNA-sequencing dataset of lungs from wild-type mice after PR8 infection to determine the expression of genes that facilitate efferocytosis (see Figure E2 in the data supplement). The efferocytosis module score was similar between AMs, IMs, and M/MDMs, suggesting that the increased ability of IMs to engulf apoptotic AT2 cells in vivo was not due to inherent differences in the expression of efferocytosis receptors. In addition, mice were injured with bleomycin to evaluate the applicability of the in vivo efferocytosis model during noninfectious inflammation, and, once again, we found that IMs predominantly mediated the clearance of apoptotic AT2 cells (Figure E3).
Discussion
In this study, we describe an in vivo model to study efferocytosis in the lungs. By using transgenic lineage tracing to label AT2 cells with TdTomato, we were able to follow the acquisition of red fluorescence in macrophages as a method to quantify efferocytosis in the lungs after injury. We also present a novel finding that the IM primarily mediates clearance of AT2 cells in the lungs after influenza and bleomycin injury, which counters the prevailing concept that AMs are the primary efferocyte that clears apoptotic cells in the lungs.
Our results show a higher rate of red fluorescence in the IM compared with the AM. This finding could occur if IMs digested apoptotic cells more slowly or were in closer proximity to AT2 cells than AMs and MDMs. However, the more plausible mechanism is that IMs either have an increased phagocytic (“eat me”) or chemotactic (“find me”) ability for apoptotic cells compared with other macrophage populations in the lungs. Our results also do not exclude the potential for AMs being more effective in clearance of other target apoptotic cells (e.g., neutrophils, fibroblasts). However, this possibility would require target cell–specific receptor–ligand interactions, which is less likely, considering phosphatidylserine is the best characterized surface marker that stimulates efferocytosis and is ubiquitously expressed on all cell types undergoing apoptosis (3, 14). In addition, we did not find any differences in expression of efferocytosis receptors between AMs, IMs, and M/MDMs.
Interestingly, the AM has less efferocytosis ability than peritoneal macrophages (15). Hence, it remains to be seen which macrophage population serves as the primary efferocyte in other organs. However, this method is adaptable to study in vivo efferocytosis in any organ system and disease model because it only requires creating the appropriate cell type–specific reporter mouse. We used TdTomato as the fluorophore, which has ideal characteristics for these studies because it is relatively resistant to quenching in the low pH environment of the phagosome (27). Many lineage tracing models have been created (28) and, in theory, can be used to study efferocytosis. However, one should take caution in choosing the appropriate fluorescent proteins because this approach may be limited with certain fluorophores, such as GFP, that are sensitive to low pH environments, which cause a rapid attenuation of signal after engulfment.
The model we present here is meant to complement and not supplant more traditional methods of studying efferocytosis (e.g., instillation of cells into the airspaces, macrophage cultures). Indeed, many of the fundamental discoveries regarding the mechanisms of efferocytosis have been identified through use of these methods (17–19). However, our finding that the IM is the primary efferocyte was surprising and highlights how this model can provide novel insights in efferocytosis mechanisms, particularly when considering the kinetics of macrophage subpopulations in clearing apoptotic cells.
In conclusion, IMs, not AMs, primarily mediate clearance of dying AT2 cells in the lungs after influenza infection. Our physiologic model of studying efferocytosis can easily be modified to study the in vivo kinetics of efferocytosis of all cell types in the lungs and in other organs because it only requires a cell type–specific driver for Cre recombinase expression crossed with an appropriate lineage tracing transgenic, such as the RosaTdTomato reporter line. Accordingly, using this method in studying efferocytosis offers new opportunities for determining mechanisms of apoptotic cell clearance in vivo.
Footnotes
Supported by the Center for Scientific Review (grants K08HL141590, R01HL130938, R01HL155759, R01HL159953, R35HL140039).
Author Contributions: Conception and design: M.S.S.R.Z. and P.C. Data acquisition and analysis: T.P., C.Y., B.R.S., Y.W., C.M.S., Z.O., X.L., and W.J.J. Drafting and editing the manuscript: M.S.S.R.Z., T.P., W.J.J., and P.C.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1165/rcmb.2023-0217MA on January 11, 2024
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Sanches Santos Rizzo Zuttion M, Moore SKL, Chen P, Beppu AK, Hook JL. New insights into the alveolar epithelium as a driver of acute respiratory distress syndrome. Biomolecules . 2022;12:1273. doi: 10.3390/biom12091273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kuwano K. Epithelial cell apoptosis and lung remodeling. Cell Mol Immunol . 2007;4:419–429. [PubMed] [Google Scholar]
- 3. Henson PM. Cell removal: efferocytosis. Annu Rev Cell Dev Biol . 2017;33:127–144. doi: 10.1146/annurev-cellbio-111315-125315. [DOI] [PubMed] [Google Scholar]
- 4. Silva MT. Secondary necrosis: the natural outcome of the complete apoptotic program. FEBS Lett . 2010;584:4491–4499. doi: 10.1016/j.febslet.2010.10.046. [DOI] [PubMed] [Google Scholar]
- 5. Arandjelovic S, Ravichandran KS. Phagocytosis of apoptotic cells in homeostasis. Nat Immunol . 2015;16:907–917. doi: 10.1038/ni.3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Poon IK, Hulett MD, Parish CR. Molecular mechanisms of late apoptotic/necrotic cell clearance. Cell Death Differ . 2010;17:381–397. doi: 10.1038/cdd.2009.195. [DOI] [PubMed] [Google Scholar]
- 7. Grabiec AM, Hussell T. The role of airway macrophages in apoptotic cell clearance following acute and chronic lung inflammation. Semin Immunopathol . 2016;38:409–423. doi: 10.1007/s00281-016-0555-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mukherjee S, Subramaniam R, Chen H, Smith A, Keshava S, Shams H. Boosting efferocytosis in alveolar space using BCG vaccine to protect host against influenza pneumonia. PLoS One . 2017;12:e0180143. doi: 10.1371/journal.pone.0180143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lee YJ, Lee SH, Youn YS, Choi JY, Song KS, Cho MS, et al. Preventing cleavage of Mer promotes efferocytosis and suppresses acute lung injury in bleomycin treated mice. Toxicol Appl Pharmacol . 2012;263:61–72. doi: 10.1016/j.taap.2012.05.024. [DOI] [PubMed] [Google Scholar]
- 10. Lee YJ, Moon C, Lee SH, Park HJ, Seoh JY, Cho MS, et al. Apoptotic cell instillation after bleomycin attenuates lung injury through hepatocyte growth factor induction. Eur Respir J . 2012;40:424–435. doi: 10.1183/09031936.00096711. [DOI] [PubMed] [Google Scholar]
- 11. Huynh ML, Fadok VA, Henson PM. Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-beta1 secretion and the resolution of inflammation. J Clin Invest . 2002;109:41–50. doi: 10.1172/JCI11638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Proto JD, Doran AC, Gusarova G, Yurdagul A, Jr, Sozen E, Subramanian M, et al. Regulatory T cells promote macrophage efferocytosis during inflammation resolution. Immunity . 2018;49:666–677, e6. doi: 10.1016/j.immuni.2018.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Das A, Ganesh K, Khanna S, Sen CK, Roy S. Engulfment of apoptotic cells by macrophages: a role of microRNA-21 in the resolution of wound inflammation. J Immunol . 2014;192:1120–1129. doi: 10.4049/jimmunol.1300613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McCubbrey AL, Curtis JL. Efferocytosis and lung disease. Chest . 2013;143:1750–1757. doi: 10.1378/chest.12-2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hu B, Sonstein J, Christensen PJ, Punturieri A, Curtis JL. Deficient in vitro and in vivo phagocytosis of apoptotic T cells by resident murine alveolar macrophages. J Immunol . 2000;165:2124–2133. doi: 10.4049/jimmunol.165.4.2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Morimoto K, Janssen WJ, Fessler MB, McPhillips KA, Borges VM, Bowler RP, et al. Lovastatin enhances clearance of apoptotic cells (efferocytosis) with implications for chronic obstructive pulmonary disease. J Immunol . 2006;176:7657–7665. doi: 10.4049/jimmunol.176.12.7657. [DOI] [PubMed] [Google Scholar]
- 17. Savill JS, Wyllie AH, Henson JE, Walport MJ, Henson PM, Haslett C. Macrophage phagocytosis of aging neutrophils in inflammation. Programmed cell death in the neutrophil leads to its recognition by macrophages. J Clin Invest . 1989;83:865–875. doi: 10.1172/JCI113970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Savill J, Dransfield I, Hogg N, Haslett C. Vitronectin receptor-mediated phagocytosis of cells undergoing apoptosis. Nature . 1990;343:170–173. doi: 10.1038/343170a0. [DOI] [PubMed] [Google Scholar]
- 19. Savill JS, Henson PM, Haslett C. Phagocytosis of aged human neutrophils by macrophages is mediated by a novel “charge-sensitive” recognition mechanism. J Clin Invest . 1989;84:1518–1527. doi: 10.1172/JCI114328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McQuattie-Pimentel AC, Ren Z, Joshi N, Watanabe S, Stoeger T, Chi M, et al. The lung microenvironment shapes a dysfunctional response of alveolar macrophages in aging. J Clin Invest . 2021;131:e140299. doi: 10.1172/JCI140299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lavin Y, Winter D, Blecher-Gonen R, David E, Keren-Shaul H, Merad M, et al. Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell . 2014;159:1312–1326. doi: 10.1016/j.cell.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gosselin D, Link VM, Romanoski CE, Fonseca GJ, Eichenfield DZ, Spann NJ, et al. Environment drives selection and function of enhancers controlling tissue-specific macrophage identities. Cell . 2014;159:1327–1340. doi: 10.1016/j.cell.2014.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Guth AM, Janssen WJ, Bosio CM, Crouch EC, Henson PM, Dow SW. Lung environment determines unique phenotype of alveolar macrophages. Am J Physiol Lung Cell Mol Physiol . 2009;296:L936–L946. doi: 10.1152/ajplung.90625.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Brauer R, Ge L, Schlesinger SY, Birkland TP, Huang Y, Parimon T, et al. Syndecan-1 attenuates lung injury during influenza infection by potentiating c-met signaling to suppress epithelial apoptosis. Am J Respir Crit Care Med . 2016;194:333–344. doi: 10.1164/rccm.201509-1878OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McCubbrey AL, McManus SA, McClendon JD, Thomas SM, Chatwin HB, Reisz JA, et al. Polyamine import and accumulation causes immunomodulation in macrophages engulfing apoptotic cells. Cell Rep . 2022;38:110222. doi: 10.1016/j.celrep.2021.110222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Aegerter H, Lambrecht BN, Jakubzick CV. Biology of lung macrophages in health and disease. Immunity . 2022;55:1564–1580. doi: 10.1016/j.immuni.2022.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gandasi NR, Vestö K, Helou M, Yin P, Saras J, Barg S. Survey of red fluorescence proteins as markers for secretory granule exocytosis. PLoS One . 2015;10:e0127801. doi: 10.1371/journal.pone.0127801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kretzschmar K, Watt FM. Lineage tracing. Cell . 2012;148:33–45. doi: 10.1016/j.cell.2012.01.002. [DOI] [PubMed] [Google Scholar]