Abstract
Small extracellular vesicles (sEVs) are known to be secreted by a vast majority of cells. These sEVs, specifically exosomes, induce specific cell-to-cell interactions and can activate signaling pathways in recipient cells through fusion or interaction. These nanovesicles possess several desirable properties, making them ideal for regenerative medicine and nanomedicine applications. These properties include exceptional stability, biocompatibility, wide biodistribution, and minimal immunogenicity. However, the practical utilization of sEVs, particularly in clinical settings and at a large scale, is hindered by the expensive procedures required for their isolation, limited circulation lifetime, and suboptimal targeting capacity. Despite these challenges, sEVs have demonstrated a remarkable ability to accommodate various cargoes and have found extensive applications in the biomedical sciences. To overcome the limitations of sEVs and broaden their potential applications, researchers should strive to deepen their understanding of current isolation, loading, and characterization techniques. Additionally, acquiring fundamental knowledge about sEVs origins and employing state-of-the-art methodologies in nanomedicine and regenerative medicine can expand the sEVs research scope. This review provides a comprehensive overview of state-of-the-art exosome-based strategies in diverse nanomedicine domains, encompassing cancer therapy, immunotherapy, and biomarker applications. Furthermore, we emphasize the immense potential of exosomes in regenerative medicine.
Keywords: Extracellular vesicles, Exosomes, Regenerative medicine, Nanomedicine, Drug delivery
Graphical abstract
Highlights
-
•
Updated review on exosomes in recent nanomedicine and drug delivery applications.
-
•
Overview of the last decade's exosome applications in immunotherapy and biomarkers.
-
•
Recent advances in exosomes in regenerative medicine and tissue-specific applications.
-
•
Comprehensive classification for conventional and advanced exosome isolation methods.
-
•
Recent advances in exosome characterization and loading techniques.
1. Introduction
Exosomes and other small extracellular vesicles (sEVs) are particles that are enclosed by a phospholipids bilayer and can be secreted by various cells. These particles contain a variety of bioactive molecules, including proteins, lipids, and nucleic acids, and have been shown to play crucial roles in intercellular communication as well as the regulation of both physiological and pathological processes [[1], [2], [3]]. The International Society for Extracellular Vesicles (ISEV) has played a pivotal role in shaping EV research through its guideline called Minimal Information for Studies of Extracellular Vesicles (MISEV), introduced in 2014 [4] and updated in 2018 [5]. This guideline provides standards for studying diverse EV subtypes and addresses challenges that arise while working with EVs, highlighting ongoing efforts to improve reproducibility in EV measurements. Moreover, the European Cooperation in Science and Technology (COST) action, supported by the ISEV and EU Horizon 2020, provides recommendations for the pharmaceutical categorization of new EVs-based therapeutics and studies for procedures structured according to pharmaceutical quality requirements [6].
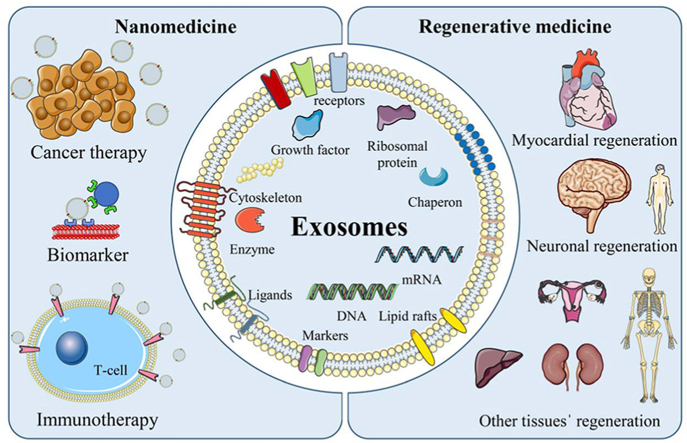
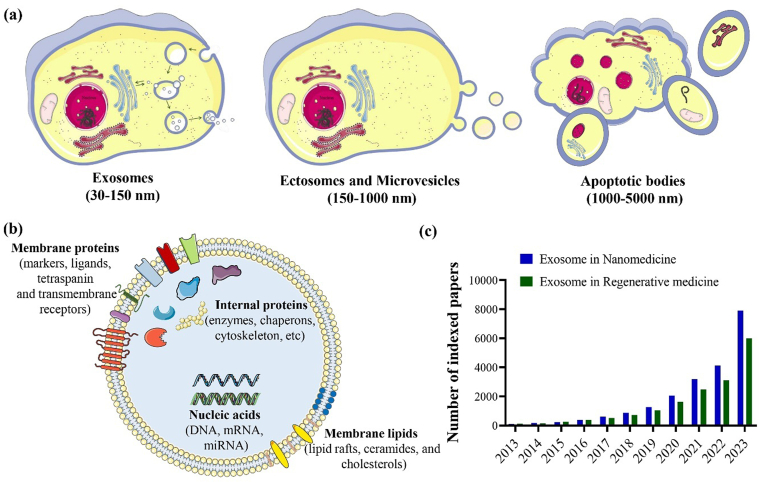
Depending on their size and biogenesis, EVs can be categorized into three main groups: (i) exosomes (30–150 nm), originated from endosomes; (ii) ectosomes and microvesicles (150–1000 nm), derived from plasma membrane; and (iii) apoptotic bodies (1000–5000 nm), which are formed through blebbing by cells undergoing apoptosis (Fig. 1a) [[7], [8], [9], [10], [11], [12]]. Other EVs besides exosomes, microvesicles, and apoptotic EVs are large tumor-derived vesicles (oncosomes; 1–10 μm) [13] and migrating cells-derived vesicles (migrasomes; 500–3000 nm) [14,15]. Historically, Trams et al. [16] used the word “exosome” to describe the tiny vesicles with a lipid bilayer released by a variety of cultivated cells. Exosomes can be secreted by almost all types of cells [17], and they are enriched with a variety of biological elements from their source cells, including proteins (e.g., adhesion molecules, cytoskeletons, cytokines, ribosomal proteins, growth factors, metabolic enzymes), lipids (e.g., cholesterol, lipid rafts, ceramides), and nucleic acids (e.g., DNA, mRNA, and miRNA) (Fig. 1b) [[18], [19], [20]]. The composition of the bigger exosomes was more similar to that of their parental cells [21]. Almost all forms of body fluids, including saliva [22,23], milk [24,25], amniotic fluid [26,27], serum or plasma [28,29], and urine [30,31], have been discovered to contain sEVs. Due to their intrinsic endogenous capabilities, including low toxicity, minimal immunogenicity, and capacity to transport cargo with high biocompatibility and stability, exosomes have gained an increasing interest in being employed in various biomedical applications and the attraction of the exosomesˈ application in nanomedicine and regenerative medicine has been progressing in the last decade (Fig. 1c) [[32], [33], [34]].
Fig. 1.
Schematic illustration of extracellular vesicles' classification based on their size and biogenesis (a), exosomes and their molecular composition, including proteins, lipids, and nucleic acids (b), and a graph representing the number of Scopus-indexed papers published in the last decade (2013–2023) regarding the use of exosomes in regenerative medicine and nanomedicine (c).
Nanomedicine has emerged as a rapidly growing field due to the increasing interest in using nanotechnology to diagnose, treat, and prevent diseases, alleviate pain, and enhance human health [[35], [36], [37]]. The distinctive physicochemical properties of exosomes and other types of nanoparticles used in nanomedicine have resulted in a vast research and development area. These unique properties distinguish them from bulk materials and often lead to the emergence of new properties [[38], [39], [40], [41]]. Exosomes generated by cancer cells have been found to transport tumor-promoting substances to normal cells, thereby altering the extracellular matrix and promoting immune evasion, which can facilitate tumor growth and spread [[42], [43], [44]]. Consequently, exosomes have been investigated as potential targeted delivery systems for cancer therapies and biomarkers for cancer diagnosis [[45], [46], [47], [48]]. Exosomes derived from immune cells can also act as pro- or anti-inflammatory agents by transporting miRNAs, immunomodulatory cytokines, or other mediators between immune cells and other cell populations [49].
Regenerative medicine, another interdisciplinary field, employing biology, engineering, and medicine, is significantly advanced by the crucial involvement of exosomes. With their unique ability to modulate cellular functions, exosomes stand as promising agents in personalized and targeted regenerative therapies, signifying a significant leap forward in the transformative impact of regenerative medicine on patient care [32,50]. Exosomes derived from mesenchymal stem cells (MSCs) are a promising tissue engineering strategy for promoting tissue regeneration [51]. MSCs have been extensively studied as a regenerative medicine therapy due to their ability to alter the microenvironment and secrete paracrine factors that promote tissue repair [52,53]. However, MSC transplantation has limitations such as immunological rejection, teratoma formation, and low regenerative efficiency [32]. MSC-derived exosomes can potentially overcome these limitations by delivering paracrine signals to surrounding cells. This review aims to explore state-of-the-art exosome-based approaches within multiple nanomedicine domains, encompassing cancer therapy, immunotherapy, and biomarker research. Additionally, we aim to underscore the potential of exosomes in the field of regenerative medicine.
2. Exosomes, as cell-derived nanovesicles
In recent years, nanovesicles have attracted significant attention due to their potential use in various applications, due to their various advantages, including the ability to load both hydrophobic and hydrophilic agents, be functionalized, prolong the time of blood circulation, and increase drug permeability into biological membranes [[54], [55], [56], [57], [58]]. Exosomes, as cell-derived nanovesicles, have been shown to possess remarkable similarities to their artificial counterparts, such as liposomes and niosomes, in terms of their ability to encapsulate and deliver cargo to target cells. Furthermore, compared to synthetic vesicles, utilizing exosomes may present a significant advantage in terms of immunogenicity reduction [59]. Exosomes share similarities with cells in terms of their deformable cytoskeleton and cytoplasmic core, which has a “gel-like” consistency. These biophysical characteristics enhance exosome structural integrity and stability during in vivo trafficking in the blood [59,60]. As exosomes continue to gain importance in research, their distribution among various cell types highlights their potential for a wide range of biological functions. Once considered mere cellular debris, exosomes emerged as critical players in intercellular communication with various and significant roles [[61], [62], [63]]. Exosomes are involved in maintaining cellular homeostasis by carrying diverse proteins, RNAs, and lipids that can vary across different organisms, cell types, and physiological and pathological conditions [64,65]. Nevertheless, some molecules are commonly found in exosomes, including certain CD markers, from the tetraspanin family, heat shock proteins (HSPs), and proteins that participate in exosome biogenesis and release [66,67].
2.1. Exosome biogenesis
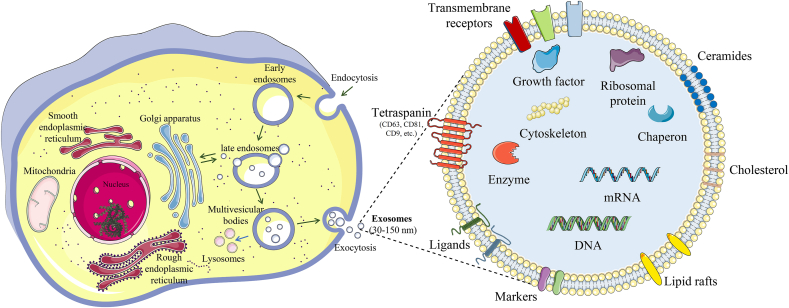
Biogenesis of exosomes has emerged as a subject of intense investigation in recent years. Notably, the role of proteins, such as members of the endosomal sorting complex required for transport, small GTPases, and glutaminase in exosome biogenesis, has been an important area of focus. To explore in-depth the involvement of these proteins in exosome biogenesis and the latest developments in the field, we recommend referring to the recent well-crafted review by Han et al. [68]. Generally, exosomes are formed inside endosomes, which are referred to as multivesicular bodies (MVB). The biogenesis of exosomes begins with the inward folding of the cell's plasma membrane, leading to the formation of an endosome. Although endosomes are known for their role in the autolysosomal degradation of cellular debris [69], the focus here is on exosome-related endosomes, which are essentially lipid vesicles. These endosomes undergo invaginations of their membrane, forming smaller nanovesicles (30–150 nm) [70], known as intraluminal vesicles (ILVs). The MVB containing these ILVs will fuse with the plasma membrane, allowing the release of the ILVs with specific cargo, which are called exosomes (Fig. 2) [71,72]. Exosomes then travel toward their target cell and fuse, releasing their contents, including cytosolic proteins, mRNA, miRNA, lncRNA, DNA, enzymes, transcription factors, and lipids [73,74].
Fig. 2.
Schematic representation of exosome biogenesis and detailed structure.
2.2. Exosome isolation
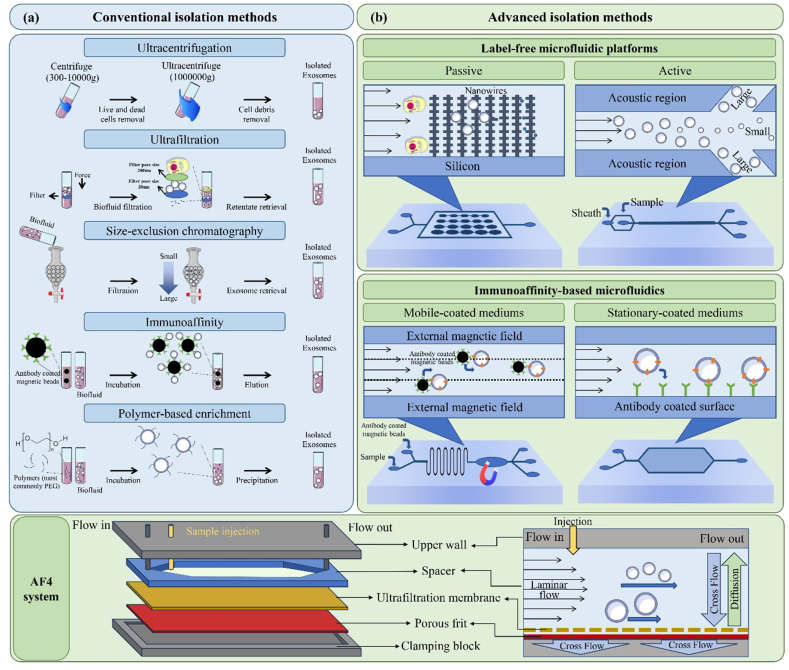
Isolating exosomes from biological sources is challenging due to their small size and heterogeneity. An ideal purification method should separate exosomes from interfering components like cellular debris and proteins. Currently, the clinical application of exosomes is mostly hindered by the lack of effective techniques for isolating exosomes from heterogeneous mixtures [75]. The exosome isolation techniques can be classified into two conventional and advanced groups (Table 1).
Table 1.
Conventional and advanced exosome isolation techniques and their advantages/disadvantages.
| Classification | Isolation techniques | Mechanism | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|---|
| Conventional | Ultracentrifugation | Size and density | Gold standard exosome isolation technique; absence of special reagents; suitable for large-scale production | Requires expensive equipment; nonspecific purification; decrease in biological activity; low yield; time-consuming | [6,76,77] |
| Ultrafiltration | Size and molecular weight | Suitable for large-scale production; easy and fast isolation; absence of special equipment and reagents | Filter membrane clogging and exosome loss; particle size heterogeneity | [78,79,80] | |
| SEC | Rapid, economic, and efficient isolation; high reproductivity and purity; availability of commercial kits | Require special equipment; co-isolation possibility of similarly sized proteins | [81,82] | ||
| Immunoaffinity | Antigen-antibody-specific reactions | High purity; time-saving technique; high specificity of exosome subtype isolation | Require purification step after antibody binding; not suitable for large-scale production | [83,84] | |
| Polymer-based enrichment | Solubility, dispersibility, and Surface charge | High yield; availability of commercial kits; simple; possibility to be used in large-scale production | Expensive; low specificity; possibility of free proteins and nucleic acid contamination | [6,81] | |
| Advanced | Label-free microfluidic platforms | Size, density, and acoustic | Time- and labor-saving technique; label-free; high efficiency; low sample volume consumption | Require method validation and standardization; not being studied widely; low sample volume | [85,86] |
| Immunoaffinity-based microfluidics | Antigen-antibody reactions and magnetic force | Rapid and efficient isolation; high purity; Ability to combine multiple functions; low sample volume consumption | Not suitable for large-scale production; expensive; requires method validation and standardization; exosomes attachment to the magnetic beads | [79,86] | |
| AF4 | Size, density, Brownian motion, and translational diffusion | High purity; Rapid; High reproducibility; Mimic physiological conditions | Not suitable for large-scale production | [87,88] |
Abbreviations: SEC, Size exclusion chromatography; AF4, Asymmetric-flow field-flow fractionation.
2.2.1. Conventional exosome isolation techniques
Conventional methods, such as ultracentrifugation, ultrafiltration, and size-exclusion chromatography (Fig. 3), have been widely employed for exosome isolation [[89], [90], [91]]. Ultracentrifugation is currently the most commonly used technique and operates on the principle that exosomes and contaminants in the sample have different densities and sizes [92]. However, this technique is time-consuming, results in low throughput, and necessitates specialized, and expensive equipment, i.e., an ultracentrifuge [76,77]. As two other simple exosome isolation techniques, ultrafiltration and size-exclusion chromatography use filtering membranes with different pore sizes to isolate exosomes based on molecular size differences [93]. However, ultrafiltration may present certain challenges regarding reducing membrane lifetime and isolation efficacy due to vesicular clogging and entrapment. As an ideal solution, the tangential flow filtration technique can minimize the potential clogging by flowing a feed stream parallel to the membrane [78]. Recently, Chen et al. [94] have introduced a novel ultrafast-isolation system (EXODUS), which utilizes a dual membrane filter configuration and periodic negative pressure and air pressure switching to generate periodic negative pressure oscillations on the nanoporous anodic aluminum oxide membrane. This allows small particles, such as proteins and nucleic acids, and fluids to pass through while retaining larger exosomes in the central chamber. The system also includes two pairs of oscillators that effectively limit fouling and particle aggregation by resuspending particles into the liquid via transverse waves and acoustofluidic streaming [94]. Size exclusion chromatography (SEC) is another method for exosome separation based on their size and molecular weight difference by the SEC column, which contains multiple holes and shafts. Despite its efficacy, SEC has limitations, as co-isolation of proteins with similar size and molecular weight is possible [81,82]. Another conventional method is immunoaffinity, which is based on antigen-antibody-specific reactions and can be used to separate and purify exosomes [83]. Although this method has a high specificity of exosome subtype isolation, it is not suitable for large-scale production and requires a purification step after antibody binding [83,84].
Fig. 3.
Schematic illustration of exosome-isolation methods; Conventional isolation methods (a), including ultracentrifugation, ultrafiltration, size-exclusion chromatography, immunoaffinity, and polymer-based enrichment, and Advanced isolation methods (b), including (I) label-free microfluidic-based isolation includes passive methods, such as nanowires-on-micropillar structure, in which exosomes can be trapped physically, or active methods, like acoustic-nanofilter device, that could separate the exosomes from microvesicles by acoustic radiation pressure, (II) immunoaffinity-based microfluidic isolation method includes an immunomagnetic isolation chip or mobile-coated mediums and the stationary-coated media, and (III) AF4 system, where elution occurs via laminar flow in a parabolic pattern and a cross-flow drives exosomes towards the membrane, countered by the exosomes' size-related diffusion properties.
2.2.2. Advanced exosome isolation techniques
Although widely employed, conventional methods for exosome isolation suffer from suboptimal efficiency due to several factors. These include the need for large volumes of samples, potential protein contamination, expensive instruments, low exosome recovery and purity, and extensive isolation procedures [79]. In recent years, the field of nanotechnologies and microfluidics has made significant advancements, leading to the development of novel exosome isolation methods that are less time- and labor-intensive, require low sample volumes, and produce exosomes with high purity [95]. Microfluidic-based techniques for exosome isolation, which rely on physical properties or immunoaffinity, are powerful tools for quick, precise, and effective exosome isolation [75]. These techniques can combine multiple functions, such as exosome separation, in situ detection, and sample pretreatment, into a single chip, reducing sample loss and providing a highly efficient analysis [96]. Physical-property-based microfluidic isolation techniques use label-free strategies, such as nanofilters, nanoporous membranes, and microvilli. This method can be classified into two groups depending on the presence of external forces: (i) passive isolation methods, which rely on complex channel structures or hydrodynamic properties in microfluidic devices, and (ii) active isolation methods, which use external electrical, centrifugal, and acoustical forces to achieve faster isolation [75]. For example, in acoustic-nanofilter devices, the acoustic radiation pressure transports vesicles from the acoustic region to the nodes of the acoustic pressure region. Because the acoustic force is related to the vesicle volume and Sheath flows are located at the node area, large vesicles are removed while tiny ones are retained, allowing larger vesicles to move more quickly [85]. Other advanced methods for exosome isolation include immunoaffinity-based microchips that use either antibody-coated magnetic nanoparticles or antibodies/aptamers modification, known as mobile-coated and stationary-coated mediums, respectively. Generally, magnetic nanomaterials in mobile-coated mediums provide higher surface area and maneuverability, improving the exosome collection/release efficiency and the downstream microfluidics processing. In contrast, the stationary-coated media is based on interactions between exosomes and antibodies or aptamers attached to the surface of microchannels, which could amplify capture affinity [79].
Recently, the efficiency in exosomal isolation could be improved by asymmetric-flow field-flow fractionation (AF4), which is a size-based isolation technique that offers programmable crossflow intensity. Briefly, this system is designed to transport samples from the inlet to the outlet of the chamber with the aid of a laminar flow. A perpendicular physical field is applied to facilitate the accumulation of samples at the bottom wall. It is noteworthy that the system exploits the Brownian motion phenomenon, whereby smaller particles exhibit higher mobility than larger particles or molecules in the flow. Consequently, smaller particles migrate further and faster than their larger counterparts in this system (Fig. 3) [87]. AF4 has demonstrated high reproducibility and purity in exosome separation, making it an attractive option for exosome isolation and purification [88]. However, this technique is subject to a limitation, as it can only accommodate small amounts of sample, usually between 40 and 100 μg. Consequently, it may not be deemed efficient for large-scale preparations [87].
2.3. Exosome characterization
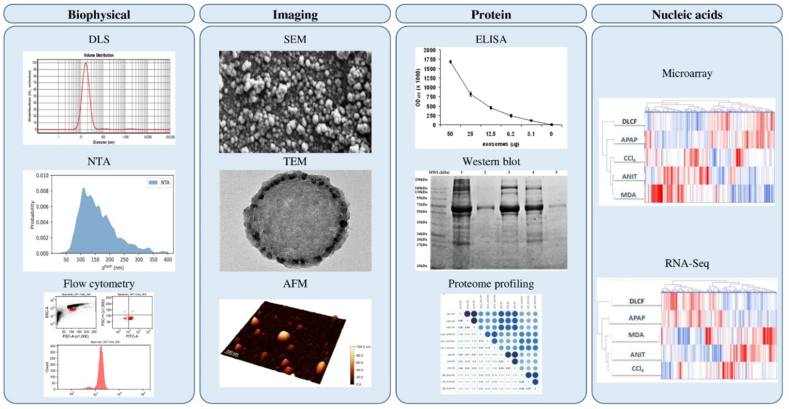
After isolation, exosomes should be carefully characterized using various techniques to verify the isolation process. Biophysical, imaging, protein, and nucleic acid characterizations are some of the most used methods for exosome characterization (Fig. 4).
Fig. 4.
Exosome characterization methods; (i) Biophysical characterization: DLS [97], NTA [98], and flow cytometry [99]; (ii) Imaging: SEM [97], TEM [100], and AFM height imaging [98]; (iii) Protein characterization: ELISA [101], western blotting [100], and proteome profiling [102]; and (iv) Nucleic acid characterization: RNA-Seq and microarray gene expression platforms, genes (columns) and samples (rows) are organized hierarchically with dendrograms and clusters, upregulation is indicated by red on the heatmap, whereas downregulation is indicated by blue [103].
2.3.1. Biophysical characterization
Dynamic light scattering (DLS) can estimate the size of nanoparticles in a suspension by analyzing the dynamic changes in the intensity of scattered light reflections of the particles under Brownian motion. DLS instruments, such as Malvern Zetasizer® and Wyatt DynaPro®, are commonly used for this purpose [104]. Another popular characterization tool that is often used in conjunction with DLS is the zeta potential, which measures the potential of a colloid particle moving in an electric field [105]. However, the limitation of DLS is its tendency to detect larger particles, which makes it unable to distinguish between combinations of microvesicles and exosomes [106]. Nanoparticle tracking analysis (NTA) is another physical technique that can be used to estimate particle concentration and size distribution by visualizing vesicles based on light scattering and tracking their Brownian motion [107]. Compared to DLS, NTA has higher peak resolution, but its measurements take more time, and multiple measurements are needed to obtain meaningful output [108]. The disadvantage of NTA is that it cannot detect the vesiclesˈ phenotype, and two populations can only be distinguished if their particle sizes differ by at least 1.5 times [106,107]. Additionally, the diluents during the preparation may cause contamination. Flow cytometry (FCM) is another standard biophysical characterization method for exosomes. It allows for quick analysis of individual cells or particles as they pass in front of one or more lasers while floating in a buffered salt solution [109]. With this technique, it is possible to study the exosome subpopulations using specific surface markers expressed on individual exosomes [110]. However, traditional FCM techniques often struggle to accurately differentiate submicron particles from background noise. Recently, nano- and imaging-FCM have emerged as powerful and effective methods for discriminating and analyzing single submicron EVs [111,112]. In addition, Raman spectroscopy is another biophysical characterization of exosomes. This label-free technique relies on the inelastic scattering of laser light caused by the interaction of photons with molecular vibrations. Interestingly, this method has also been used for the characterization [113].
2.3.2. Imaging
The morphological analysis and size quantification of exosomes can be studied using imaging characterization methods, including scanning and (SEM) transmission electron microscopy (TEM) and atomic force microscopy (AFM). Cryo-electron microscopy (cryo-EM) helps to preserve the exosomes’ structure and prevent the crystallization of the sample [114]. AFM allows assessment of the mechanical properties, particle height, and biomolecular load of exosomes, which is useful for characterizing plasma-derived exosomes with unknown origins and designing and developing separation protocols [115]. However, AFM has some disadvantages, such as resolution limit, scanner drift, and changes in particle height due to the drying process [116,117].
2.3.3. Protein characterization
The enzyme-linked immunosorbent assay (ELISA) and Weston blotting are two common methods for targeted protein analysis that have been widely used in various fields of molecular/biological sciences [118]. The foundation of ELISA is the establishment of antigen-specific antibodies and radioimmunoassay techniques, which allow for the indirect quantification of proteins by labeling them with antibodies [119]. The principle of Western blotting, on the other hand, is immunochromatography, where an antibody recognizes the target protein and the protein lysates can be separated by gel electrophoresis based on their molecular weight and isoelectric point [120]. While ELISA and Western blotting may only be able to determine the expression levels of a small number of specific proteins, various proteome profiling techniques such as mass spectrometry have emerged to evaluate complex protein mixtures more sensitively [121]. ELISA, Western blot, and mass spectroscopy are conventional protein analysis methods that have been used for almost 40 years. However, due to their high sample requirement, and extensive processing and purification procedures, these methods are not ideal for clinical applications [118,122]. To overcome the technical challenges of conventional protein quantification, numerous novel protein analysis techniques, including small particle flow cytometry [123], nanoplasmonic exosome sensor [124], integrated magnetic-electrochemical exosome sensor [125], and micro-nuclear magnetic resonance [126] are currently under development [118,127].
2.3.4. Nucleic acids characterization
Gene expression analysis is a widely used and effective technique for examining the transcriptional activity of biological systems, identifying disease-related cell states, and performing other functions [128]. Exosomes have been found to contain various nucleic acids, such as DNA, single-strand DNA, mitochondrial DNA, miRNA, messenger RNA, and non-coding RNA [129,130]. DNA microarrays and RNA sequencing are the most common technologies used for global gene expression analysis [128]. In recent years, microarrays have been the most cost-effective and popular technique for gene expression analysis, despite their initial quantitative limitations [131]. RNA sequencing is known to provide more comprehensive gene profiling as it can simultaneously identify total gene expression levels and the various RNA types [132]. However, RNA-seq normalization methods are currently under development, and better approaches are still needed to provide a strong technical normalizing across a wide dynamic range of datasets [133].
2.3.5. Single-EV analysis
Numerous techniques have been employed to analyze EVs, with earlier methods relying on bulk measurements requiring 102 to 106 EVs for a single measurement [134]. However, current research focuses on the development of single-EV analysis methods that are simple, sensitive, multiplexable, practical, and capable of measuring various parameters [135]. Some of these novel methods include multifluorescence single-EV analysis [134,136], single-particle interferometric reflectance imaging [137], microfluidic resistive pulse sensing [138], and nanoflow cytometry [139]. In the field of cancer diagnosis, single EV analysis is regarded as the most reliable strategy to determine specific molecular and phenotypic features of the disease, including physical, genetic, lipidic, proteomic, and metastatic variations. Single-EV analysis is considered the most robust approach for detecting specific features of the disease with high accuracy and precision [140,141]. Therefore, the single-EV methods have not only generated more precise and comprehensive data on EVs, facilitating a deeper understanding of their biological functions but have also improved biomedical applications with the potential for disease diagnosis. The ability to measure EVs individually has led to more accurate and detailed information, which can aid in the development of new therapies and diagnostic tools. Overall, the use of single-EV methods represents a significant advancement in the study of EVs and their potential clinical applications.
2.4. Exosome loading methods
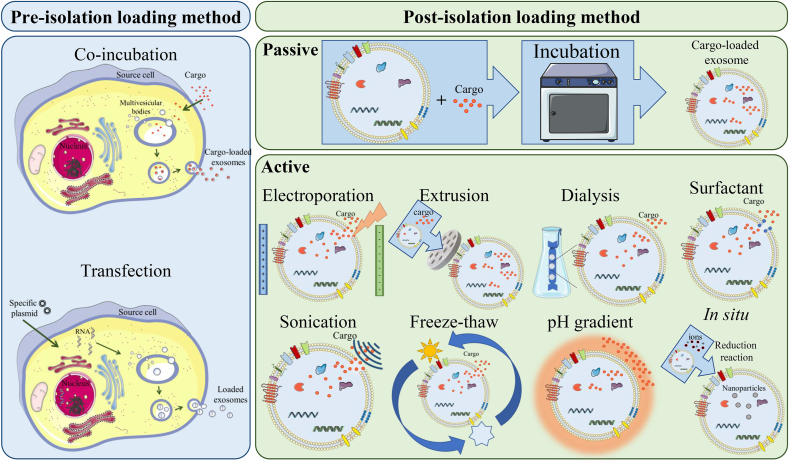
The structure of exosomes includes a bilayer lipid membrane with ligands and receptors from their source cell surrounding a hydrophilic center that can be loaded with various types of cargo, such as drugs, genes, vaccines, and bioactive compounds [142]. Depending on the goal of the exosomal approach, the agents in the core, membrane, and surface can either originate from the source cell or be loaded after the exosome isolation. Therefore, various strategies have been developed for loading materials into exosomes, which can be classified into two types: (i) pre-isolation loading method, where the cargo can be loaded during their biogenesis in the source cell, and (ii) post-isolation loading method, which can be performed after exosome isolation using passive or active encapsulation techniques (as shown in Fig. 5 and summarized in Table 2) [6,122,[143], [144], [145], [146]].
Fig. 5.
Schematic illustration of exosomesˈ cargo loading methods, including pre-and post-isolation loading techniques.
Table 2.
Classification of cargo loading techniques for exosomes.
| Loading strategies | Loading techniques | Principle | Advantages | Disadvantages | Example cargo | Ref. |
|---|---|---|---|---|---|---|
| Pre-isolation | Co-incubation | Membrane diffusion | Easy to use, exosome integrity preservation | Cargo cytotoxicity, low entrapment efficiency, size-dependent encapsulation | Drugs (doxorubicin), nanomaterials (gold nanoparticles, iron oxide nanoparticles) | [147,148,149] |
| Transfection | Gene edition | Overexpression of target molecules; suitable for peptide agents and nucleic acids | Epigenetic changes, low entrapment efficiency, the toxicity of transfection agents | Proteins (myostatin propeptide, T7 peptide), Nucleic acids (miRNA-497) | [150,151,152] | |
| Post-isolation (passive) | Incubation | Membrane diffusion | Easy to use, low-cost, exosome integrity preservation | Low entrapment efficiency | Drugs (gemcitabine), Proteins (catalase), Nucleic acids (miRNA-159), Nanomaterials (iron oxide magnetic nanoparticles) | [[153], [154], [155]] |
| Post-isolation (active) | Electroporation | Electric field-based pores creation | Easy to use | Exosome aggregation, low entrapment efficiency, require process optimization | Drugs (doxorubicin), Proteins (tyrosinase-related protein‐2), Nucleic acids (siRNA), Nanomaterials (gold nanoparticles) | [156,157] |
| Sonication | Shear force-based pores creation | High entrapment efficiency | Exosome aggregation, bilayer damage risk | Drugs (atorvastatin, paclitaxel), proteins, peptides, nanomaterials | [97,158] | |
| Extrusion | Membrane recombination | High entrapment efficiency, size homogeneity | Exosomal surface structure damage and recombine risk | Drugs (doxorubicin), Proteins (catalase) | [159] | |
| Freeze-thaw cycle | Membrane recombination | Easy to use, exosomes-mimetic particle generation, suitable for peptide agents | Exosome aggregation | Proteins (macrophages) | [97] | |
| Hypotonic dialysis | Concentration-based diffusion | High entrapment efficiency | Protein degradation risk, requires validation | Drugs (doxorubicin, porphyrins), Nucleic acids (miRNA-93-5p) | [160,161] | |
| pH gradient | pH-based diffusion | Easy to use | Exosome aggregation, protein degradation risk | Drug (piceatannol) | [145,162] | |
| Surfactant treatment | Active agent-based pores creation | High entrapment efficiency | Surfactant toxicity, cargo damage risk, require purification | Drugs (porphyrins), Proteins (catalase) | [163,164] | |
| Post-isolation | Direct transfection | Gene edition | Easy to use | Restricted to small RNAsˈ loading | Small RNAs (miRNA-497, miRNA-126, tetraspanin 2 siRNA) | [[150], [165], [166]] |
| In situ synthesis | Chemical reaction | Exosome integrity preservation, suitable for nanomaterials | Complex operation processes and technological barriers | Nanomaterials (gold nanopopcorn, palladium nanosheets) | [167,168] |
2.4.1. Pre-isolation loading method
In the pre-isolation loading method, also known as the in vivo or endogenous loading method, exosomes are loaded with the cargo during their biogenesis in the source cell before they are released into the extracellular environment [144]. Pre-isolation loading can be achieved by different methods, including the co-incubation method, which involves adding the specific cargo to the source cell medium for a set amount of time, and the transfection method, in which specific plasmids are transfected into cells to overexpress desired nucleic acids or peptide agents [143,169]. These techniques are recommended for encapsulating a variety of types of nucleic acids in exosomes, and there are numerous reports of successful and functional delivery of siRNA and miRNA to target cells [[170], [171], [172]]. However, the amount of cargo loaded using the endogenous method is typically low, and more importantly, the entrapment efficiency cannot be controlled [144]. Nanomaterials, including metallic nanoparticles for magnetic targeting and magnetic resonance imaging, can also be loaded into exosomes using the co-incubation technique, although they may trigger autophagy and be destroyed in lysosomes [143,147]. Additionally, the treatment of the source cell may affect its viability or cause epigenetic changes [6]. One drawback of the co-incubation technique is that it is unclear whether the cargo is loaded during biogenesis or after the secretion, making it difficult to classify the loading method as either endogenous or passive exogenous [169].
2.4.2. Post-isolation loading method
In the post-isolation loading method, also known as in vitro or exogenous loading technique, the efficiency of the loaded cargo is more controllable, allowing for manipulation and measurement of the amount of the encapsulated cargo, which can be defined as entrapment efficiency and loading capacity [173]. The post-isolation method has two subcategories: (i) passive loading method, where substances penetrate isolated exosomes and exosome-like nanoparticles by a gradient of concentration, without the need for an external force or energy source [174], and (ii) active loading process which involves a permeabilization procedure that can be achieved through various techniques [6]. Electroporation and sonication induce the formation of transient pores in the exosome's membrane. Electroporation achieves this by subjecting the exosomes to an electrical field, while sonication utilizes high-frequency sound waves for the same purpose [97,158]. Extrusion is another active loading strategy that in the sEVs and cargo molecules can be co-cultured and then extruded using a syringe-based lipid extruder [159]. Moreover, a sequence of freeze-thawing cycles results in the disruption of the sEVs' membranes, which allows for their loading [175,176]. Other active exogenous loading methods include hypotonic dialysis, pH gradient, and surfactant treatment. In hypotonic dialysis, which relies on osmotic pressure differences, the loading efficiency could be significantly enhanced by dialyzing exosomes and cargo through the mixing process within a dialysis membrane or tube [160]. The pH gradient method enhances cargo loading into exosomes by creating a pH differential between the exosome's internal pH of 9 and a cargo solution adjusted to pH 4.5, resulting in a threefold increase in loading efficiency [145,162]. The surface treatment method involves applying surfactants like saponin or Triton to disperse exosomal membrane molecules, leading to the creation of pores on the exosomal surface and an increase in membrane permeability to facilitate the loading process [163,164].
Nooshabadi et al. (2020) employed different types of post-isolation techniques for atorvastatin loading in exosomes isolated from endometrial stem cells, and the surfactant treatment method showed the highest entrapment efficiency, indicating that loading capacity is highly dependent on the loading method [97]. One transfection-based strategy to load small RNAs into exosomes is direct transfection [143]. In this method, exosomes may be chemically treated by commercial reagent kits, such as the ExoFectin® sRNA-into-Exosome Kit, to directly transfect them with nucleic acids [177]. Using the direct transfection method, miRNA-126 [165], miRNA-497 [150], and Tetraspanin 2 siRNA [166] have been loaded in breast cancer, embryonic kidney, and microglial cells-derived exosomes for cancer therapy applications, respectively. Although this technique facilitates control and characterization of loading efficiency, it is restricted to loading small RNAs.
In situ synthesis is another active exogenous strategy to load nanomaterials on the surface or in the core of exosomes without significantly impairing the exosome integrity. However, this approach is limited to loading noble metals and requires a complicated operating process [6,167,168].
2.5. Exosomes in clinical trials
However, this technique is time-consuming various fields such as diagnostics, drug delivery, and therapy, have garnered significant attention from the scientific community. The potential of exosomes for medical applications is reflected in the ongoing clinical trials, as presented in Table 3. These trials highlight the vast possibilities that exosomes offer in the medical domain. Thus, it is imperative to explore the potential of exosomes and their therapeutic applications in various fields of medicine. Despite exosomes attracting significant attention in recent years, their transition from laboratory to market is a complex process that presents several noteworthy challenges. Most importantly, the high cost of exosome isolation techniques limits their clinical use and restricts them to preclinical investigation. Therefore, developing rapid and cost-effective techniques that isolate exosomes with a high purity is needed for large-scale production [6]. Other technical and economic challenges are described in Section 7. Ensuring that these challenges are addressed effectively will be instrumental in successfully integrating exosomes into the marketplace.
Table 3.
Current clinical studies and trials involving exosomes, as reported by the National Library of Medicine in the USA.
| Exosomeˈs application | Pathology | Phase | Start year | Source of exosome | Sponsor | Clinical trial number |
|---|---|---|---|---|---|---|
| Drug delivery |
NSCLC | 2 | 2010 | Dendritic cells | Gustave Roussy, Cancer Campus, Grand Paris | NCT01159288 |
| Irritable Bowel Disease | NA | 2018 | Plant (ginger) | University of Louisville | NCT04879810 | |
| Inflammatory responses (several diseases) |
1 |
2023 |
Engineered exosome |
ILIAS Biologics Inc. |
NCT05843799 |
|
| Therapy |
Psoriasis | 1 | 2022 | MSCs | National University Hospital, Singapore | NCT05523011 |
| Degenerative Disc Disease | 1 | 2021 | Blood cells | Dr. Himanshu Bansal Foundation | NCT04849429 | |
| ARDS | 2 | 2020 | Bone Marrow | Direct Biologics, LLC | NCT04493242 | |
| Oral Mucositis | 1 | 2012 | Plant (grapes) | University of Louisville | NCT01668849 | |
| Severe Lung diseases | 1 | 2020 | Blood cells | Ruijin Hospital | NCT04313647 | |
| SARS-CoV-2 PNEUMONIA | 1 | 2020 | MSCs | Ruijin Hospital | NCT04276987 | |
| Covid19|SARS-CoV-2 pneumonia|COVID19 |
1|2 |
2020 |
MSCs |
State-Financed Health Facility “Samara Regional Medical Center Dinasty" |
NCT04491240 |
|
| Biomarker | Colorectal Cancer | NA | 2021 | Blood | CHU de Reims | NCT04394572 |
| Obstructive Sleep Apnea Syndromes | NA | 2019 | Plasma and serum | University Hospital, Angers | NCT03811600 | |
| Hypertension | NA | 2016 | Blood and urine | University Hospital Inselspital, Berne | NCT03034265 | |
| Lung Cancer (diagnosis) | NA | 2017 | Serum | Wuhan Union Hospital, China | NCT03830619 |
Abbreviations: Non-small cell lung cancer, NSCLC; Acute respiratory distress syndrome, ARDS; Mesenchymal stem cells, MSCs; Parkinson's Disease, PD; Not applicable, NA.
3. Exosome in nanomedicine and drug delivery
Exosomes, which are biologically active nanovesicles, have been found to play a critical role in the formation, development, progression, invasion, and metastasis in the cancer microenvironment [178,179]. As a result, exosomes have great potential as therapeutic targets, drug delivery systems for cancer therapy, and cancer biomarkers (Table 4) [180]. They not only aid in the treatment and prognosis of cancer and immunity conditions but also serve as cell-free vaccines for detecting and preventing illnesses [181,182]. Exosomes have several advantages, including excellent biocompatibility, high chemical stability, the capacity to cross biological barriers and permeate tissue structures, as well as the ability to target specific tissues and increase productivity [18,181].
Table 4.
Recent advances in exosome applications in nanomedicine.
| Disorder | Pathology | Exosome source | Loaded agent | Loading technique | Characterization techniques | Size (nm) | Exosome advantage | Ref. |
|---|---|---|---|---|---|---|---|---|
| Brain | Glioblastoma | Ginseng | vvi-miR-396b, ptc-miR-396g-5p, ptc-miR-396f |
Active post-isolation (Direct transfection) | TEM, NTA, DLS, RNA-seq, lipid profiling, proteomics | 151.6 | To enhance the targeting ability to the BBB | [183] |
| Human glioblastoma cell lines (U251, U87) | Doxorubicin | Active post-isolation (Sonication) | DLS, TEM, WB, RT-PCR | 151.9 | To allow targeted chemotherapy with a deep penetration into tumor parenchyma | [184] | ||
| Human endometrial stem cells | Atorvastatin | Active post-isolation (Sonication, freeze-thaw cycle, and surfactant treatment) | DLS, SEM, WB, RT-PCR | 30–150 | To increase intracellular uptake, induce glial cell death, and provide a sustainable atorvastatin delivery | [97] | ||
| Stable 293 T cell line | Antisense miRNA oligonucleotides against miRNA-21 | Pre-isolation (Transfection) | SEM, WB | 15–50 | To enhance the BBB penetration and bind the tumor transferrin receptor by the targeting ligand | [152] | ||
| Cerebrospinal fluid | miR-1298-5p | – | TEM, WB, RNA-seq | 30–100 | To knockdown hnRNPA2B1 targeting glioma cells to block the process | [185] | ||
| Human leukemia monocytic cell line (THP-1) | Temozolomide | Pre-isolation (Co-incubation) | NTA, WB | 50–240 | To increase BBB penetration ability and perfect GBM accumulation due to target ligands | [186] | ||
| Human brain neuronal glioblastoma-astrocytoma cells (U-87) | Paclitaxel | Passive (incubation) and active (sonication) post-isolation | DLS, SEM, TEM | 50–150 | To increase the anticancer drug efficiency in glioblastoma multiform treatment | [158] | ||
| bEnd.3 Brain-derived Endothelial cells | Doxorubicin | Active post-isolation (Sonication) | NTA, TEM, WB | 116 | To cross the BBB and target glioblastoma | [187] | ||
| Alzheimer | ASCs | Neprilysin (CD10) | Pre-isolation (Transfection) | DLS, TEM, WB, ELISA, RT-PCR, FCM | 110 ± 35 | To target the hippocampal area of the brain, reduce the production of the proinflammatory genes, and increase the anti-inflammatory gene | [188] | |
| Plasma | Quercetin | Active post-isolation (Surfactant treatment) | DLS, AFM, WB | ∼150 | To enhance the bioavailability of quercetin and promote its brain targeting, thereby inhibiting neurofibrillary tangle formation | [189] | ||
| PD | Human embryonic kidney cell line (HEK293T) | DNA aptamers | Pre-isolation (Transfection) | TEM, WB | ∼100 | To pass BBB and reduce the neuropathological and behavioral deficits in the mouse PD model | [190] | |
| Human MSCs | Catalase mRNA | Pre-isolation (Transfection) | NTA, RT-PCR, ELISA | ∼100 | To attenuate neurotoxicity and neuroinflammation in vitro and in vivo PD models | [191] | ||
| Huntington's disease | Human brain neuronal glioblastoma-astrocytoma cells (U-87) | siRNA | Passive post-isolation | NTA, TEM, WB | ∼140 | To promote the distribution of oligonucleotides and increase bilateral silencing of huntingtin mRNA | [192] | |
| Human embryonic kidney cells (HEK293) | miRNA-124 | Pre-isolation (Transfection) | WB, RT-PCR | – | To deliver miRNA-124 to the target gene in the striatum and produce a better therapeutic effect | [193] | ||
| CNS-TB | BMSCs | Rifampin | Active post-isolation (Electroporation) | NTA, TEM, WB | 50–150 | To increase brain targeting ability in vitro and vivo | [194] | |
| Neuroinflammation | BMSCs | miRNA-193b-3p | Active post-isolation (Electroporation) | TEM, WB, NGS, RT-PCR | ∼100 | To target the brain after subarachnoid hemorrhage and weaken neuroinflammation by inhibition of the HDAC3/NF-κB signal pathway | [195] | |
| HAND | HTHU microglia cells | Tetraspanin 2 siRNA | Active post-isolation (Direct transfection) | DLS, TEM | 93–218 | To increase the permeability rate, cross the BBB and can be used as an efficient delivery vehicle to the central nerve system | [166] | |
| Lung | Cancer | BMSCs | miR-30b-5p | Pre-isolation (Transfection) | NTA, TEM, WB, RNA-seq | 60–260 | To prevent NSCLC progression by inhibition of EZH2 expression and PI3K/AKT signaling pathway | [196] |
| Human breast cancer MDA-MB-231 cells | miRNA-126 | Active post-isolation (Direct transfection) | DLS, AFM, TEM, FCM | 30–120 | To escape from innate immune cells effectively and cause an inhibitory effect on proliferation and migration in lung cancer metastasis model in mice | [165] | ||
| Human bronchial epithelioid cells (HBE) and NSCLC cells (A549 and H460) | miRNA-126 | Active post-isolation (Direct transfection) | TEM, WB | – | To block the progression of NSCLC through the mediation of its target gene integrin alpha-6 by miRNA-126 overexpression | [177] | ||
| Human embryonic kidney 293T cells | miRNA-497 | Active post-isolation (Direct transfection) | NTA, TEM, WB, RT-PCR | 30–100 | To deliver miRNA-497 and cause inhibition in tumor growth, angiogenesis, and migration | [150] | ||
| Primary bone-marrow-derived macrophages | Paclitaxel | Active post-isolation (Sonication) | DLS, NTA, WB | 110.8 ± 4.1 | To increase the drug accumulation in cancer cells and prolong the blood circulation time | [197] | ||
| Raw cow milk | siRNA against KRAS | Pre-isolation (Transfection), and Active post-isolation (Electroporation) | – | – | To effectively deliver the siRNA and significantly inhibit the A549 tumor xenografts | [198] | ||
| Acute lung injury | ADSCs | miR-125b-5p inhibitor | Active post-isolation (Direct transfection) | NTA, TEM, WB | 117.5 | To alleviate the injury and decrease ferroptosis | [199] | |
| Inflammation | Mouse blood serum | miRNA-155 | – | NTA, TEM, WB | 40–150 | To promote macrophage proliferation and inflammation by targeting SHIP1 and SOCS1, respectively | [200] | |
| Allergic asthma | Bone marrow-derived macrophages | DNA methyl- transferase 3A | Active post-isolation (Surfactant treatment) | DLS, TEM, RT-PCR, WB | 108 ± 3.2 | To silence the Dnmt3aos, the key target gene for allergic asthma, and reduce airway inflammation | [201] | |
| Pulmonary fibrosis | Murine fibroblast cell line (L-929) | HSP70, CD9, and calnexin | – | DLS, AFM, WB | ∼120 | To combine with clodronate-loaded liposomes promote liposomal penetration and increase the delivery efficiency | [202] | |
| Liver | HCC | Human hepatocellular carcinoma cells (Hep3B) | miRNA-125 | – | NTA, WB, RT-PCR | 50–200 | As a potential biomarker for diagnosis and prognosis of hepatic cancer | [203] |
| Human HCC cell lines (MHCC97-H, SMMC-7721, Huh7), Human normal liver cell line (7702) | miRNA-320a | – | TEM, WB, RT-PCR | 30–100 | To transfer miRNA-320a which can suppress HCC progression as an antitumor miRNA by targeting PBX homeobox 3 | [204] | ||
| HCC patientsˈ serum | miRNA-718 | – | TEM, WB, RT-PCR | 25–75 | As a novel biomarker for predicting the recurrence and therapeutic targets of HCC | [205] | ||
| ASCs | miRNA-122 | Pre-isolation (Transfection) | WB, RT-PCR | – | To enhance HCC chemosensitivity | [206] | ||
| Mouse blood serum | Doxorubicin | Passive post-isolation | TEM, WB | 40–110 | To enhance cancer targeting by SMNC labeling and an external magnetic field | [155] | ||
| Alcoholic hepatitis | Human hepatocytes and hepatoma cell line | miRNA-122 | Pre-isolation (Transfection) | NTA, TEM, SEM, ELISA, WB, RT-PCR | 90 | To reprogram monocytes inducing sensitization to LPS, inhibit the heme oxygenase 1 pathway, and increase the levels of pro-inflammatory cytokines | [207] | |
| Fibrosis and injury | ADSCs | HGF | Pre-isolation (Transfection) | NTA, TEM, WB, RT-PCR | 40–100 | To alleviate liver fibrosis and restore liver function | [208] | |
| BMSCs | circCDK13 | Pre-isolation (Transfection) | NTA, TEM, WB, RT-PCR | 140.9 | To inhibit liver fibrosis by regulating the expression of MFGE8 | [209] | ||
| Hepatic stellate cells (LX-2) | Cas9 ribonucleoprotein | Active post-isolation (Electroporation) | DLS, TEM, WB | 50–200 | To facilitate cytosolic delivery of ribonucleoprotein in vitro and for specific liver tissue accumulation in vivo | [210] |
Abbreviations: Populus trichocarpa, ptc; Vitis vinifera, vvi; Central nervous system tuberculosis, CNS-TB; Meta-tetra(hydroxyphenyl)chlorine, mTHPC; Non-small cell lung cancer, NSCLC; Bone marrow mesenchymal stem cells, BMSCs; Adipose-derived stem cells, ASCs; heterogeneous nuclear ribonucleoprotein A2B1, hnRNPA2B1; Flow cytometry, FCM; nanoparticle tracking analysis, NTA; scanning electron microscopy, SEM; transmission electron microscopy, TEM; atomic force microscopy, AFM; enzyme-linked immunosorbent assay, ELISA; Western blot, WB, dynamic light scattering, DLS; Next-generation sequencing, NGS; Reverse transcription polymerase chain reaction, RT-PCR; mesenchymal stem cells, MSCs; histone deacetylase 3, HDAC3; HIV-1 associated neurocognitive disorders, HAND; Enhancer of zeste homolog 2, EZH2; Phosphoinositide 3-kinase/protein kinase B, PI3K/AKT; Adipose-derived stem cells, ADSCs; Hepatocyte growth factor, HGF; Milk fat globulin-EGF factor 8, MFGE8; Human telomerase reverse transcriptase immortalized human microglial cells, HTHU; Nuclear factor-κB, NF-κB; Kirsten rat sarcoma virus, KRAS; Parkinson's disease, PD; Heat shock proteins, HSP; Hepatocellular carcinoma, HCC; Superparamagnetic magnetite nanocrystal clusters, SMNC; lipopolysaccharides, LPS.
3.1. Exosome-based drug delivery for brain disorders
Efficient therapies for the central nervous system and brain drug delivery have been a challenging area in nanomedicine in recent decades due to the existence of selective permeability barriers, such as the blood-brain barrier (BBB) and the blood-cerebrospinal fluid barrier (BCSFB) [89]. These barriers prevent the passage of nearly all large-molecule biological therapeutics, including recombinant proteins, monoclonal antibodies, and gene-based drugs, as well as around 98% of small-molecule pharmaceuticals [211]. Exosomes have been suggested to penetrate the BBB/BCSFB owing to their unique lipid/protein composition and deliver functional cargoes from hematopoietic cells to the brain [212]. Additionally, recent findings have demonstrated that exosomes play a crucial role in communication between neuronal cells and neuroprotection, supporting synaptic plasticity [213] and preserving neuronal integrity [214]. Due to their neuroprotective properties, exosomes are potentially therapeutic agents for treating neurodegenerative disorders [215]. Glioblastoma, the most aggressive cancer type in the central nervous system, is challenging to treat due to drug resistance and the BBB presence [216]. However, it has been reported that transferrin-coated exosomes can target antisense miRNA oligonucleotides against miRNA-21, a promising therapeutic strategy for glioblastoma, and deliver them more effectively to the brain [152]. The exosome can be easily isolated from the serum of clinical glioblastoma patients and then be used to provide their exclusive drugs [217]. Despite its immense relevance, the mechanisms of exosomal entry into the brain are not yet fully understood [212].
3.2. Exosome-based drug delivery for lung disorders
Exosomal miRNAs and long noncoding RNAs (lncRNAs) may play important roles in the development of several respiratory illnesses, including asthma, lung cancer, and chronic obstructive pulmonary disease [218]. Lung cancer, responsible for approximately 350 deaths per day in 2022, is the most common cancer with the highest morbidity rates [219]. However, chemo- and radiotherapy resistance frequently contributes to treatment failure in lung cancer, and some studies have suggested that exosomes can transfer these resistances from donor cells to recipient cells [220]. This indicates that the turnover of certain miRNAs in exosomes and their target genes may be a potential treatment approach for lung cancer. Exosomal miRNAs act as significant moderators of drug resistance acquisition in lung cancer cells [218]. For instance, when lung cancer cells are exposed to X-rays, miRNA-23a is released and expressed more in their primary exosomes. Human umbilical vein endothelial cells can then take up this miRNA and use it to promote the proliferation and migration of recipient cells by inhibiting the expression of Phosphatase and tensin homolog (PTEN), which increases angiogenesis and radiotherapy resistance. Therefore, the exosomal miRNA-23a/PTEN pathway may be a potential therapeutic target for reducing lung cancer's radiation resistance [221].
Recent studies have shown that exosomes derived from breast cancer cells can interact with non-small cell lung cancer cells (NSCLC) in a specific manner through the surfactant protein C on the cancer cells and overexpressed integrin 4 on the exosomeˈs membrane, which may result in the internalization of the exosomes by the cancer cells [165]. Moreover, Furthermore, exosomes derived from raw cow milk and loaded with siRNA against Kirsten rat sarcoma virus (KRAS) and functionalized with folic acid demonstrated significant inhibition of the A549 tumor xenograft model. Mutant KRAS has been implicated in the development of several malignancies, including lung cancer [198]. Exosomes have demonstrated potential in treating a range of pulmonary disorders beyond lung cancer. These include inflammation, injury, fibrosis, and asthma (Table 4). As such, exosomes have emerged as a potentially viable treatment option for these conditions. Further research into their efficacy and safety is required, but the potential therapeutic benefits of exosomes in treating these lung disorders could represent a significant breakthrough in the field of pulmonary medicine.
3.3. Exosome-based drug delivery for liver disorders
Hepatocellular carcinoma (HCC), also known as primary liver cancer, is one of the most lethal tumors worldwide. In China, it is a prevalent malignant tumor that ranks second in terms of mortality and third in terms of morbidity [222]. Traditional treatments for HHC show insensitivity or high, but miRNA-122, the most often discovered and widely investigated miRNA in liver disorders, can inhibit HCC development. Knockdown of miRNA-122 increases the viability of HCC cells [223]. Lou et al. reported that exosomes containing miRNA-122 isolated from adipose-derived stem cells (ASCs) have the potential to be an effective strategy to promote the chemosensitivity of HCC cells [206]. In another study, it has been observed that alcohol-exposed hepatic cells produce exosomes with higher miRNA-122, which are then taken up by macrophages and make them more sensitive to lipopolysaccharide, thereby increasing in vitro cytokine secretion [207].
Moreover, as many liver-related disorders are caused by mutations in a single gene, gene editing has emerged as a promising treatment strategy for liver diseases [224], and the CRISPR-associated nuclease protein 9 (Cas9)-based technologies have been established as an effective tool for therapeutic genome editing [225]. In this regard, Wan et al. [210] reported the potential of Cas9 ribonucleoprotein (RNP)-loaded exosomes as tissue-specific gene therapy for liver diseases. In their study, they encapsulated RNP by electroporation of exosomes derived from hepatic stellate cells and assessed its CRISPR genome-editing therapeutic potential in liver diseases, including acute liver injury, chronic liver fibrosis, and hepatocellular carcinoma mouse models by targeting p53 up-regulated modulator of apoptosis (PUMA), cyclin E1 (CcnE1), and K (lysine) acetyltransferase 5 (KAT5), respectively [210].
Viral hepatitis is the most prevalent liver infectious disease, and exosomes play a crucial signaling role in the interaction between hepatocytes, viruses, and the immune system following virus invasion, making them an attractive target for future viral hepatitis therapy plans [226]. It is noteworthy that once the hepatocytes are infected by hepatitis viruses, the exosomes they produce play a critical role in both propagating the viruses and activating the body's immune functions to combat the infection. This highlights the potential of exosomes as a valuable tool for studying the pathogenesis of viral infections and developing new therapeutic strategies [227]. It has been reported that hepatocytes infected with hepatitis B virus can produce exosomes containing miR-21, miR-192, miR-215, miR-221, and miR-222. These exosomes have the potential to inhibit T cells from secreting IL-21, which is an important inflammatory molecule for hepatitis immunity. Consequently, this may hamper the immune system's ability to kill this virus [228]. This finding highlights the significance of further research into the role of exosomes in the pathogenesis of virus-associated liver disease. Such research could potentially provide important insights into the development of novel therapeutic interventions for this condition.
3.4. Exosome-based drug delivery for other disorders
Exosomes can be utilized as drug delivery systems for various types of cancer, not the brain, lung, and liver disorders. Doxorubicin, a commonly used drug for treating solid tumors [229], has been loaded into exosomes as a potential treatment for different cancer types, including osteosarcoma [175,230,231], colon cancer [232,233], ovarian cancer [234,235], breast cancer [[236], [237], [238]], and pancreatic cancer [239,240]. Hadla et al. [241] conducted a study on the therapeutic effect of doxorubicin-loaded exosomes on breast and ovarian cancers. The results from both in vitro and in vivo experiments showed that the approach had higher cytotoxicity against cancer cells and greater tumor volume reduction compared to free doxorubicin [241]. Moreover, exosomes have been used to deliver other chemotherapy agents for various therapeutic applications, such as paclitaxel for prostate and pancreatic cancer therapy [242,243], curcumin for inflammatory diseases [244], and cisplatin for ovarian cancer therapy [245]. Exosomes from raw milk can potentially overcome physicochemical and pharmacokinetic limitations in delivering anthocyanidins, which are known as potent anti-oxidant, anti-proliferative, apoptotic, and anti-inflammatory agents in the past two decades, and have shown effectiveness against multiple cancer types, including lung, breast, ovarian, colon, pancreas and prostate cancers [246]. Furthermore, by modifying exosomal surface proteins, it is possible to impart cell and tissue specificity, resulting in targeted delivery of therapeutic agents or diagnostic markers. This property positions exosomes as a promising platform for personalized medicine strategies. However, further research is needed to fully understand the implications and potential of engineered exosomes in the context of biomedical applications [247].
4. Exosome in immunotherapy
Exosome-based immunotherapy has drawn increasing attention in recent years as a promising method for cancer treatment [248]. Cancer immunotherapy is a therapeutic strategy aimed at regulating the immune system to overcome pathways that lead to tumor escape and to reactivate antitumor immune responses [249]. The goal of cancer immunotherapy is to enhance the activity of cytotoxic T lymphocytes within a tumor, prepare tumor-specific cytotoxic T lymphocytes for use in lymphoid organs, and establish efficient and long-lasting antitumor immunity. CD4+ T cells have also been shown to play a positive role in cancer immunotherapy [250]. Moreover, exosomes originated from CAR-T cells, NK cells, macrophage (M1), and tumor cells have been shown to improve immune responses and restrain tumor cells directly or indirectly [251].
Exosome-based cancer immunotherapy has highlighted the application of tumor-derived exosomes and dendritic cell-derived exosomes [252,253]. Tumor-associated exosomes were reported as a prospective antigen for immunotherapy based on dendritic cell vaccines. One study investigated the influence of tumor-associated exosomes on dendritic cells and demonstrated that they facilitated the maturation of these cells and improved histocompatibility complex cross-presentation more effectively than tumor cell lysates, thereby inducing a stronger cytotoxic T lymphocyte response to the specific tumor [254]. As a crucial type of antigen-presenting cell, dendritic cells play an important role in cancer immunotherapeutic strategies. Furthermore, exosomes secreted from dendritic cells have been identified to participate in antigen presentation in the process of anti-tumor immune responses [255].
Research has discovered that tumor-derived exosomes become less immunogenic due to TGF-β1. However, under this background, exosomes secreted from leukemia cells that have been silenced for TGF-β1 (LEXTGF-β1si) have demonstrated a better outcome in inducing a particular antitumor effect when compared to non-modified exosomes. The results have shown that LEXTGF-β1si can promote the proliferation of CD4+ T-cell and secretion of Th1 cytokine, more efficiently stimulating a particular response of cytotoxic lymphocytes and cytotoxicity of nature killer cells than non-modified LEX [256].
Currently, exosomes are primarily used as biological carriers for drug delivery in cancer immunotherapy and in the development of cancer vaccines [257]. An efficient cancer vaccine design should have the ability to induce both effective CD4+ and CD8+ T effector response and memory response. Although cancer vaccine designs are frequently suboptimal, they have shown promising results in clinical activity, especially in increasing overall survival [258]. A prophylactic vaccine was developed by using exosomes derived from murine ESCs (embryonic stem cells) to produce ESC-exo/GM-CSF (granulocyte-macrophage colony-stimulating factor), the studies showed that the advance of metastatic lung tumors was inhibited in mice vaccinated with ES-exo/GM-CSF vaccine [259]. In addition, vaccines based on exosomes originating from dendritic cells have been confirmed to be simpler in terms of cost-effectiveness and management compared to dendritic cell vaccines [260]. Exosomes derived from various types of cells, including immune and cancer cells, have been used as a vaccine for treating colorectal cancer due to their simplicity, affordability, and lack of toxicity compared to traditional vaccines [261].
A study reported that exosomes enriched with HSP70 produced from heat-treated tumors generated potent Th1 immune responses, resulting in the elimination of cancer cells in murine models [262]. Additionally, a tumor vaccine was developed based on the design of exosome-like nanovesicles derived from FAP gene-engineered tumor cells (eNVs-FAP). The eNVs-FAP vaccine induced efficient and robust cytotoxic T lymphocyte immune responses, inhibiting tumor growth [263].
Exosomes and exosome hybrids have also been used in drug delivery systems due to their biocompatibility, high bioactivity, and low toxicity [264]. Furthermore, exosomes have the prospect to transit medications through the BBB and go inside the targeted cells that have minimal immunogenicity [265]. In addition, one potential approach to improve the efficacy of immunotherapy is to selectively target tumor exosomes to reduce tumor-induced immunosuppression [235]. Such strategies may represent promising avenues for cancer research and treatment.
Major histocompatibility complex on exosomes was found to be associated with anticancer immune responses mediated by exosomes [266]. Tumor-derived re-assembled exosomes can be used as not only a drug delivery carrier but also an immunostimulatory agent. For instance, Chlorin e6-loaded R-Exo facilitated the secretion of cytokines from immune cells [267]. Synthetic multivalent antibody retargeted exosomes were developed for the treatment of breast cancer, and they exhibited efficient specific anti-tumor characteristics both in vitro and in vivo [268]. The study of the molecular mechanisms of exosomes is significant for their application in cancer immunotherapy and the prospects of clinical application.
5. Exosomes as a target in cancer therapy
The tumor microenvironment is quite distinct from that of normal tissue, which has the potential to be used in targeted therapy. There is a growing acknowledgment of the significant role played by the tumor microenvironment in tumor evolution and metastasis. This microenvironment is characterized by a multitude of pathological responses, including hypoxia, inflammation, and angiogenesis, and encompasses various cell types, including macrophages, dendritic cells, T cells, endothelial cells, and fibroblasts, along with extracellular matrix components, proteases, and cytokines [269,270]. Hypoxia plays a vital role in the complex mechanisms of cancer progression and metastasis. Understanding these mechanisms helps the development of more effective treatments to combat cancer. In cancer immunotherapy, exosomes can be utilized as potential targets to enhance the immune response against cancer cells. By targeting exosomes, it is possible to modulate the tumor microenvironment and enhance the efficacy of immunotherapy. For instance, exosomes can be engineered to carry tumor-specific antigens, which can stimulate an immune response against cancer cells. Additionally, targeting exosomes can also reduce the immunosuppressive effects of the tumor microenvironment, thereby promoting the activation and proliferation of immune cells [248]. Exosome-based immunotherapy that targets tumor-associated macrophages (TAMs) has been proven to be a promising treatment for glioma [271]. In addition, bioengineered exosomes have been utilized to deliver effective anti-tumor medications, such as chemotherapeutic compounds and siRNAs, preferentially to cancer cells [182]. As another therapeutic targeting modality, angiogenesis-targeted cancer therapy holds promise for managing cancer progression by modulating the delicate balance of angiogenic and anti-angiogenic factors carried by exosomes [272]. For example, the PTEN/PI3K/AKT signaling axis, operating via the proteasome, regulates hypoxia-induced factor 1 alpha (HIF-1a) to control tumor-induced angiogenesis [270]. Exploring the clinical potential of antiangiogenic therapy and directing attention to these proangiogenic exosomes could open up novel avenues for cancer treatment [269]. Further research on exosomal miRNAs, mRNAs, and proteins is crucial to understanding and enhancing the inhibition of angiogenesis in endothelial cells for more effective treatments.
6. Exosome in cancer biomarker
The assessment of biomarkers in patient samples, such as blood, tissue, urine, and cerebrospinal fluid, has numerous benefits in oncology, including risk assessment, screening, differential diagnosis, prognosis determination, and disease progression monitoring [271,273,274]. The signal molecules present in exosomes from different types of cancer cells are not the same due to their specific surface proteins and other exosomal contents. Furthermore, the expression levels of these molecules in exosomes from one type of tumor cell differ from that of the molecules in serum and source cells [275]. Therefore, exosomes have been considered potential therapeutic targets and cancer biomarkers that help early cancer diagnosis, prognosis improvement, and higher survival rates [276,277]. Moreover, malignant cells produce a significantly higher number of exosomes than healthy cells due to their enhanced cellular activity. Therefore, in addition to other specific cancer biomarkers, which may be more concentrated in exosomes than parental cells, the number of exosomes present in body fluids can serve as a diagnostic biomarker [278]. Exosomes are stable in biofluids and can be utilized for dynamic tracking [279]. Recent studies showed they could be employed as a promising perspective to develop novel biomarkers for various cancer types, such as Glioblastoma [280], gastric cancer [281], HCC [282,283], pancreatic cancer [284], melanoma [285], prostate cancer [286], and ovarian cancer [287]. Moreover, exosomes obtained from both urine and prostate cancer cell lines were subjected to RNA expression analysis, which revealed that known RNA markers for prostate cancer, such as the TMPRSS2:ERG fusion gene and prostate cancer antigen were detectable in exosomes using RT-PCR [288]. These findings suggest that exosomes may serve as a non-invasive diagnostic tool for prostate cancer, as they contain RNA markers commonly associated with the disease. Additionally, exosomes are useful biomarkers for a variety of other disorders, including neurodegenerative diseases such as Alzheimer's and Parkinson's [289], liver disorders such as fibrosis [290], and alcoholic liver disease [291,292], cardiovascular diseases [293,294], and infectious diseases such as COVID-19 [295], HIV [296], and tuberculosis [297]. Recently, Jia et al. [289] reported that the neuronal-derived exosomal proteins growth-associated protein 43, neurogranin, synaptosome-associated protein 25, and synaptotagmin 1 can identify preclinical Alzheimer's disease 5–7 years before cognitive impairment appears. Furthermore, neural-derived exosomes may serve as potential biomarkers for Parkinson's disease, as plasma levels of DJ-1 and α-synuclein, two mutated gene products at the early stage of the illness, have been reported to be present in exosomes [298]. Current research is underway to investigate the potential of exosomes as biomarkers for even more disorders, highlighting their versatility and potential in the field of medicine. To sum up, exosomes as multicomponent biomarker platforms play a significant role in the future of cancer biomarkers and further research is necessary to determine the clinical significance of these findings and to determine the potential utility of exosome-based diagnostics in cancer management.
7. Exosomes in regenerative medicine: tissue-specific applications
Exosomes have revolutionized regenerative medicine with their multipotency and self-renewing properties. Exosomes reduce inflammation, apoptosis, while promoting proliferation and angiogenesis, making them a promising therapy for tissue regeneration in various organs (Table 5). Especially, MSCs-derived exosome, which have been shown to closely mimic the effects of the parent MSCs, transport various proteins, mRNA, and miRNAs to modulate the activity of recipient cells. Compared to MSCs, exosomes are more convenient to store and transport, and they may be a more effective and safer option than cell transplantation [[299], [300], [301]]. Moreover, exosomes have been incorporated into scaffolds that are specifically designed for tissue engineering applications, which could induce enhancing cellular responses, including proliferation, migration, and differentiation, leading to improved tissue regeneration and repair [50]. This approach has been explored extensively a wide range of tissues, including bone [302,303], cartilage [304,305], skin [300,306], heart [307,308], liver [309], endometrium [310], and kidney [311,312].
Table 5.
Recent advances in the applications of exosomes in regenerative medicine.
| Disorder | Exosome source | Aim of using exosomes | Regenerative medicine methodology | In vivo/In vitro | Ref. |
|---|---|---|---|---|---|
| Heart | Cardiosphere-derived cells | Improving cardiac functions after myocardial hypertrophy treatment | Enhancing accumulation of exosomes by expressing heart homing peptide, miRNA-148a delivery, and inhibition of β-MHC, BNP, GP130, p-STAT3, p-ERK1/2, and p-AKT | In vitro, in vivo | [313] |
| Enhancing endocytosis of exosomes by binding cardiomyocyte-specific peptide | Ligation of modified exosomes to cardiomyocyte-specific peptide | In vitro, in vivo | [314] | ||
| Modifying injured skeletal and cardiac muscle function | Transcriptome profile reversion and increasing cardio myogenesis | In vivo | [315] | ||
| Improving the cardiac functions in DMD patients | Reduction in collagen I and III levels, increase in cardiomyocyte proliferation and MYOD levels, restoration of dystrophin levels | In vivo | [316] | ||
| Cardiac regeneration | Derived exosomes enriched in miR-146a, enhancing cell survival and angiogenesis | In vitro, in vivo | [317] | ||
| Hypoxia-pretreated Cardiosphere-derived cells | Cardio-protection | Upregulation miR-210, miR-130a, and miR-126 and angiogenesis | In vitro | [318] | |
| MSCs | Cardio-protection after ischemic injury | HSF1 overexpressing MSCs and isolating miRNAs' enriched exosomes | In vivo | [319] | |
| Reduction of infarct size | Increasing ATP and NADH levels and phosphorylated-Akt and phosphorylated-GSK-3β, and decreasing oxidative stress and phosphorylated-c-JNK | In vivo | [320] | ||
| Human UCMSCs | Myocardial protection by preventing apoptosis of myocardial cells | Increasing in Bcl-2 expression | In vitro, in vivo | [321] | |
| Cardiac regeneration after acute myocardial infarction | Exosomal TGF-β3 could expand angiogenesis, diminish myocardial fibrosis, and preserve the heart function | In vitro, in vivo | [322] | ||
| Cardiac progenitor cells | Apoptosis inhibitor | Enriching in miRNAs that inhibit apoptosis or help the formation of the endothelial tube such as miR-210, miR-132, miR-146a-3p, and miR-181 | In vitro, in vivo | [323] | |
| Cardiomyocytes | Angiogenesis | HSP20 association with Akt and ERK signaling pathways and VEGFR2 activation | In vitro, in vivo | [324] | |
| HT1080 and cardiosphere-derived cells | Targeting exosomes by cardiac homing peptide | Target delivery of infracted heart, improve survival of neonatal rat cardiomyocytes, and vascularization | In vitro, in vivo | [325] | |
| Atorvastatin-pretreated MSCs | Cardio-protection | IL-6 and TNF-α inhibition, regulation of miR-675 expression, activation of vascular endothelial growth factor, improve lncRNA H19 expression | In vitro, in vivo | [326] | |
| Transduced MSCs with GATA-4 | The effects of GATA-4 transduction on levels of miRs | Increasing in miR-19a expression, decreasing in PTEN levels, activation of Akt and ERK signaling | In vitro, in vivo | [327] | |
| Blood | Cardio-protection | HSP70 and toll-like receptor 4 communication and HSP27 activation | Ex vivo | [328] | |
| Central nervous system | Rat multipotent MSCs | Improve hippocampal neurogenesis in rats of TBI | Exosomes carrying miRNA-124 are correlated with M2 polarization of microglia via the TLR4 pathway | In vivo | [329] |
| Stimulate neurite outgrowth after stroke | Exosomal transfer of miRNA-133b to neural cells | In vitro | [330] | ||
| Promote endogenous angiogenesis and neurogenesis and reduce neuroinflammation | Correlated with suppression of activated microglia and macrophages by exosomes | In vivo | [331] | ||
| Human BMSCs | Promote endogenous angiogenesis and neurogenesis and reduce neuroinflammation | Correlated with suppression of activated microglia and macrophages by exosomes | In vivo | [332] | |
| Promote retinal ganglion cells' survival and regeneration of their axons | Knockout of Argonaute-2, a key miRNA effector molecule | In vitro, in vivo | [333] | ||
| Human UCMSCs | Inhibition neural apoptosis, reduced inflammation and promoted neurological regeneration in rats after TBI. | Suppression of NF-kB signaling pathway | In vivo | [334] | |
| Peripheral nervous system | Rat ASCs | PNS regeneration, by reducing apoptosis | Upregulation the anti‐apoptotic Bcl‐2 mRNA expression and downregulating the pro‐apoptotic Bax mRNA expression | In vitro | [335] |
| Promote regeneration of the myelin sheath | Kpna2 downregulation via miR-25b | In vitro, in vivo | [336] | ||
| Murine ASCs | Enhancing nerve regeneration after nerve crush injury | Might be correlated with HDAC, APP and ITGB1, candidates involved in exosomes-mediated nerve regeneration | In vivo | [337] | |
| Modulate the microenvironment in neuro-inflammatory and neurodegenerative disorders. | Associated with inhibition of apoptotic cascade | In vitro | [338] | ||
| Human ASCs | Promote neural survival and proliferation | MALAT1 protein mediates the splicing of pkcδII, an anti-apoptotic protein | In vitro | [339] | |
| Rat BMSCs | Stimulate peripheral nerves' regeneration | Closely related to expression of VEGFA and S100b genes via a miRNA-mediated mechanism | In vitro, in vivo | [340] | |
| Dedifferentiated Schwann cells | Increased axonal regeneration in vitro and enhanced regeneration after sciatic nerve injury in rat | Inhibition of GTPase Rhoa activity, thereby inhibiting axonal elongation and promoting growth cone collapse after activation | In vitro, in vivo | [341] | |
| Endothelial cells | Boosting and maintaining the repair phenotypes of Schwann cells | Stimulation of PI3K/AKT/PTEN signaling pathway | In vitro, in vivo | [342] | |
| Gingiva-derived MSCs | Peripheral nerve regeneration | Closely related to activation of c-JUN activity, and upregulation of Notch1, GFAP and SOX2 | In vivo | [343] | |
| Pericytes | Promote angiogenesis and cavernous nerve regeneration under diabetic conditions | Might be correlated with Lcn2 which acts activating MAP kinase and PI3K/Akt and suppressing P53 signaling | In vivo | [344] | |
| Skin | ASCs | Molecular mechanism in skin wound healing | Long noncoding RNA H19 targets miR-15 b y and this, in turn, targets SOX9, which activates the Wnt/β-catenin pathway | In vitro, in vivo | [345] |
| Skin wound healing | miR-19b exosome regular the TFG-β pathway by CCL1 | In vitro, in vivo | [346] | ||
| Photoaging by UVB irradiation | Upregulate the expression of type I collagen mRNA and downregulate the expression of type III collagen, MMP-1, and MMP-3 mRNA | In vitro, in vivo | [347] | ||
| Inflammatory response and skin wound healing | Reduces the lipopolysaccharide-induced inflammatory mRNA and M1-type macrophage-specific marker expression and increases cytokines IL-10, VEGF, and TGF-β and M2-type macrophage marker Arg1 expression | In vitro, in vivo | [348] | ||
| Human platelet lysate | Skin aging | Reduces MMP-1 levels and, consequently, increases collagen levels | In vivo | [349] | |
| Bovine milk | Aging and skin hydration | Hydrating effect on keratinocytes through the increase of filaggrin and CD44 receptor. Hydrating effect on fibroblasts through the increase of HAS2. Prevents the decrease of type II and III collagen after exposure to UVB rays. |
In vitro, in vivo | [350] | |
| Bovine colostrum | Improves on aging and UV-induced damage to various skin cells | Antioxidant effect on keratinocytes by reducing intracellular ROS through the glutathione oxidation pathway. Effect on elasticity by decreasing MMP2 expression. | In vitro | [351] | |
| Solanum tuberosum | Photoaging by UVB irradiation | Inhibition action on MMP1, 2, and 9, as well as on the cytokines IL6 and TNF-α in keratinocytes. Antioxidant effect through the expression of glutathione S-transferase α 4 | In vitro | [352] | |
| Lactobacillus plantarum | Skin aging | Decrease MMP-1 mRNA expression and elastase activity and increase filaggrin mRNA expression and HAS2 protein expression. | In vitro, in vivo | [353] | |
| Apple | Skin aging and reparation | Negative effect on the activity of Toll-like Receptor 4 and NF-κB pro-inflammatory pathway | In vitro | [354] | |
| Human amniotic fluid-derived stem cells | Skin wound healing | Decreased secretion of inflammation-associated cytokines through CXCR4 | In vivo | [355] | |
| Human UCMSCs | Molecular mechanism in skin wound healing | PI3K/AKT pathway is regulated by phosphatase and tensin homolog | In vitro | [356] | |
| BMSCs | Photoaging by UVB irradiation | Suppressed the mRNA of MMP-2 | In vitro | [357] | |
| Hypoxia-pretreated ASCs | Regenerative effects in UVB-induced skin injury | circ-Ash 1l targets miR-700-5p and GPX4, and miR-700-5p target GPX4. | In vitro, in vivo | [358] | |
| Epidermal stem cells | Skin wound healing | May inhibit the differentiation of fibroblasts to myofibroblasts via suppressing TGF-β1 expression via miR-425-5p and miR-142-3p | In vivo | [359] | |
| Human dermal fibroblast | Photoaging by UVB irradiation | Increased procollagen type I expression and a decrease in MMP-1 expression, mainly through the downregulation of TNF-α and the upregulation of TGF-β | In vitro, in vivo | [360] | |
| Acellular gelatinous Wharton's jelly of the human umbilical cord | Mechanism of action in skin wound healing. | Could be related to the paracrine effects of alpha-2-macroglobulin | In vitro, in vivo | [361] | |
| Human pluripotent stem cells | Photoaging by UVB irradiation and natural senescence | Decreases mRNA expression of MMP-1 and MMP-3 Increases the expression of type I collagen mRNA. |
In vitro | [362] | |
| Muscle | ASCs | Regeneration of skeletal muscle defect | Enhancing myocyte proliferation as well as MYOG and MYOD genes | In vitro, in vivo | [363] |
| Muscle regeneration | Increasing the number of centrally located nuclei | In vivo | [364] | ||
| C2C12 myoblasts | Promoting the musculoskeletal repair and regeneration | Exosomes derived from mechanically strained C2C12 cells improved cell proliferation and differentiation | In vitro | [365] | |
| Reveal the mechanism of interactions between exosomes and muscle regeneration | Increasing the levels of Pax7; an increase on day 3 and decrease on day 5 of peroxisome proliferator-activated receptor γ (PPARγ) levels, α-SMA, Collagen-1 | In vivo | [366] | ||
| Detecting endocrine signal role of exosomes | Regulating miRNAs, which are important for cell differentiation and growth control as well as myogenesis | In vitro | [367] | ||
| M2 macrophages | The role of exosomes derived from M2 macrophage on muscle regeneration | Exosomes enriched in miR-501, targeting Yin Yang 1, and increasing the levels of MyHC and MyoG | In vitro, in vivo | [368] | |
| PRPs and MSCs | Functional recovery of injured muscle | Increase in the expression of MYOG in PRP-exosome group; reduction of TGF-β in MSC-exosomes group; no effects on MYOD and IL-1β levels | In vivo | [369] | |
| MSCs | Muscle regeneration | Exosomes enriched in miR-21, miR-1, miR-133, miR-206, and miR-494 enhance tube formation and improve vascularization; increase myofibers diameter and centrally located nuclei; decrease fibrotic area; increase MYOD and MYOG expressions | In vitro, in vivo | [370] | |
| Human skeletal myoblasts, differentiating to myotube | Muscle regeneration | Enrich in growth factors such as IGFs, VEGF, HGF, NT-3, FGF2, and PDGF-AA; upregulation of FGF2, TNF, MYOD1, DAG1, DES, MYH1/2, and TNNT1 | In vitro and in vivo | [371] | |
| Liver |
Hepatocyte-derived cells | Promoting liver regeneration after acute liver failure | The miR-183-5p uptake leads to the activation of FoxO1/Akt/GSK3β/β-catenin signaling | In vitro, in vivo | [372] |
| Hepatocytes | Hepatocyte proliferation and regeneration after acute hepatic injury | Exosomal transfer of SK2 to target hepatocytes | In vitro, in vivo | [373] | |
| Human UCMSCs | Antioxidant and antiapoptotic effects, rescuing liver from failure | Might be correlated with GPX1 | In vivo | [374] | |
| Human BMSCs | Promote anti-fibrosis by stimulating hepatocyte regeneration | Suppression of Wnt/β-catenin signaling components (PPARγ, Wnt3a, Wnt10b, β-catenin, WISP1, Cyclin D1) | In vivo | [375] | |
| ASCs | Liver regeneration after hepatic ischemia-reperfusion in rats | Activation of Wnt/β-catenin signaling | In vivo | [376] | |
| Human Placenta-derived MSCs |
Upregulate angiogenesis and liver regeneration | Might be related with Wnt/β-catenin signaling triggered by C-reactive protein | In vitro, in vivo | [377] | |
| Promote cell proliferation and liver regeneration after hepatectomy |
Related with the exosomal circ-RBM23 mopping miR-139-5p, activating eIF4G expression and AKT/mTOR pathway |
In vitro, in vivo |
[378] |
||
| Vessel |
Endothelial progenitor cells | Reendothelialization | elevating angiogenesis genes levels such as HIF-1a, VEGF family, eNOS, E-selectin, IL8, ANG1, CXCL family; down-regulation of MMP-9 and PDFGB | In vitro, in vivo | [379] |
| Investigating the mechanism of vascularization by derived exosomes | Delivering miR-21-5p and targeting angiogenesis inhibitor Thrombospondin-1 | In vitro, in vivo | [380] | ||
| Induced vascular progenitor cells | angiogenesis | Enhance endothelial tube formation, cell migration, and proliferation; enrich in miR-143-3p, miR-291b, miR-20b-5p, and IGF-binding protein | In vitro, ex vivo, and in vivo | [381] | |
| MSCs cultured on titanium | Vascular regeneration | Improve VEGFR2 level and cell migration; downregulation of miR-15b-5p, miR-16-5p, miR-155-5p, miR-24-3p, miR-32-5p, mir-125b-5p, miR-146a-5p, and miR-320a | In vitro | [382] | |
| MSCs | Identifying the angiogenesis factors of exosomes | Induce proangiogenic via PDGF, EGF, FGF, and NFkB pathways | In vitro | [383] | |
| ASCs | Improving angiogenesis properties of EVs by PDGF | Enrich in proangiogenic factors such as MMP, c-kit, and SCF; enhance endothelial tube formation | In vitro, in vivo | [384] | |
| Annulus fibrous cells | Investigation of vascularization mechanism | Enhance endothelial cells migration and increase IL-6, TNF-α, MMP-3, MMP-13 and VEGF expressions | In vitro | [385] | |
| hypoxia-resistant multiple myeloma cell line | Improving angiogenesis | Upregulation of miR-210 and miR-135b, which miR-135b targets inhibiting hypoxia-inducible factor-1 | In vitro, in vivo | [386] | |
| Leukemia cells in hypoxia | Endothelial tube formation | Elevation of several miRNAs such as miR-18b and miR-210 | In vitro | [387] | |
| Lung cancer cells in hypoxia | Reveal the exosomal communication of cancer cells and endothelial cells | Elevation of miR-23a, suppression of t prolyl hydroxylase 1 and 2, increase in hypoxia-inducible factor-1α (HIF-1α) level, and downregulation of tight junction protein ZO-1 | In vitro, in vivo | [388] | |
| Overexpressed miR-21 ASCs |
The role of miR-21 on vascularization |
Increasing in HIF-1α, VEGF, SDF-1, p-Akt, p-ERK1/2 and decreasing in PTEN levels |
In vitro |
[389] |
|
| Cartilage | MSCs | Enhance proliferation, attenuate apoptosis, and modulate immune reactivity | Activation of AKT and ERK signaling, and higher infiltration of CD163+ M2 macrophages | In vitro, in vivo | [390] |
| Synovial MSCs | Enhance proliferation and migration in vitro and prevent osteoarthritis | Exosomal transfer of miRNA-140-5p to target cells. Might also be related with Wnt signaling | In vitro, in vivo | [391] | |
| Human BMSCs | Inhibit inflammatory mediators, promoting cartilage regeneration in vitro | Abolishment of TNF-alpha-mediated upregulation of COX2 | In vitro | [392] | |
| Subcutaneous MSCs | Ameliorate the pathological severity degree of cartilage | Delivery of miR-199a-3p-mediates mTOR-autophagy pathway | In vitro, in vivo | [393] | |
| KGN-pre-treated BMSCs | Chondral matrix formation and cartilage repair | Could be related to targeting C-myc and further regulating the MAPK signaling pathway | In vitro, in vivo | [394] | |
| Ovary | Amniotic fluid stem cells | POI | miR-369-3p inhibition of expression YAF2 and PDCD5/p53 in OGCs | In vitro, in vivo | [395] |
| POI | Anti-apoptosis and proliferative effect in OGCs by PI3K/AKT/mTOR pathway | In vitro, in vivo | [396] | ||
| POI | Anti-apoptosis on OGCs through regulation of miR-146a and miR-10a pathway | In vitro, in vivo | [397] | ||
| BMSCs | POI | Anti-apoptosis effect on OGCs through miR-144-5p suppressing PTE gene expression and this in turn increases PI3K/AKT pathway | In vitro, in vivo | [398] | |
| POI | Anti-apoptosis effect on OGCs through miR-664-5p inhibiting p53 luciferase activity | In vitro, in vivo | [399] | ||
| Amniotic fluid | POI | Antifibrotic effect by increasing SMAD6, which in turn inhibits the TGF-β signaling pathway | In vivo | [400] | |
| Ovarian tissue | POI | Anti-apoptosis effect in OGC by regulating BCL9 expression by miR-122-5p inhibitor | In vitro, in vivo | [401] | |
| Menstrual blood-derived stromal cells | POI and mechanism ovulation | Follicular development through increased A-azoospermia Like, proliferation of OGCs through increased forkhead box L2 | In vitro, in vivo | [402] | |
| Human UCMSCs | Inhibited apoptosis, increased OR, and recoopered the function of POI | Anti-apoptosis and proliferation effect in OGC through miR-17-5p inhibiting SIRT7 and target gene (γH2AX, PARP1 and XRCC6) | In vitro, in vivo | [403] | |
| Human ASCs | POI | Ovarian function is regulated through the SMAD pathway, which in turn inhibits the expression of apoptosis genes (Fas, FasL, caspase-3, and caspase-8) | In vitro, in vivo | [404] | |
| Skeleton | MSCs | osteogenesis | Improve the bone formation and expressions of angiogenesis genes such as VEGF, ANG 1, ANG2, COL1, and ALP | In vitro, in vivo | [405] |
| Fabricating cell-free scaffold for bone regeneration | Increasing the levels of osteogenic factors such as osteopontin, ALP, Hsa-miR-146a-5p, HsamiR-503-5p, Hsa-miR-483-3p, Hsa-miR-129-5p; decreasing the levels of anti-osteogenic miRNAs such as Hsa-miR-32-5p, Hsa-miR-133a-3p, and Hsa-miR-204-5p; activation of PI3K/Akt and MAPK signaling pathways | In vitro | [406] | ||
| Improving bone healing | Enrich in miR-4532, -125b-5p, −4516, -338-3p, and −548aa | In vitro, in vivo | [407] | ||
| BMSCs | Osteogenesis and bone tissue targeting | Enhancing the osteogenic activity of BMSCs due to elevation of miR-26a, −29a, −218, −34a, and −3960; enhancing bone localization of exosomes due to conjugation with BMSC-specific aptamer | In vitro, in vivo | [408] | |
| Promoting angiogenesis and regulating of osteoclast-related activities during bone remodeling process | miR-26a can influence bone formation through the Tob gene, which acts as a negative regulator of the BMP/SMAD pathway | In vitro, in vivo | [409] | ||
| human UCMSCs | Reduction of cell apoptosis | Elevation of miR-186, miR-1304, miR144, miR-1263, and miR-302b levels; regulation of apoptosis by inhibition Hippo signaling pathway | In vitro, in vivo | [410] | |
| Fracture healing | Elevation of β-catenin, Wnt3a, Col1, OPN, and RUNX2 | In vivo | [411] | ||
| ASCs | Osteogenesis effects of cell-free scaffold incorporated with exosomes | Improve cell migration, RUNX2, ALP, COL1A1 expressions of MSCs cultured in the osteogenic medium; enhance new bone formation | In vitro, in vivo | [412] | |
| Noggin-suppressed MSCs | Increasing bone healing efficacy | Downregulation of noggin; upregulation of ALP, RUNX2, Osterix, and OCN; inhibition of miR-26 | In vitro, in vivo | [413] | |
| miR‐375‐overexpressing ASCs | Improving bone regeneration with miR-375 | Exosomes were enriched in miR-375; improved osteogenesis of BMSCs, increase ALP, COL1A1, and RUNX2 levels as well as AZR quantification | In vitro, in vivo | [414] | |
| miR-21 transfected Human Wharton's jelly of UCMSCs | Decreasing osteocytes apoptosis | Elevation of miR-21-PTEN-AKT signaling pathway | In vitro, in vivo | [415] | |
| HIF-α overexpressing BMSCs | Improving bone healing with HIF-α | Increasing the levels of HIF-α, ALP, and OCN | In vitro, in vivo | [416] | |
| MSCs derived from human induced pluripotent stem cells | Improving osteogenesis and angiogenesis | Improving osteogenic differentiation and vascularization; Increase in ALP, RUNX-2, and COL1A1 levels | In vitro, in vivo | [417] | |
| MSCs derived from human induced pluripotent stem cells | Angiogenesis | Increasing microvessels and cell necrosis inhibition; upregulation of PI3K/Akt signaling pathway | In vitro, in vivo | [418] | |
| TNF-α preconditioned ASCs | Improving bone regeneration efficacy of exosomes | Upregulation of Wnt3a, RUNX2, osteopontin, and bone sialoprotein | In vitro | [419] | |
| Endothelial progenitor cells | Bone healing via angiogenesis | Enrich in miR-126; downregulation of SPRED1; regulation of Raf/ERK signaling pathway | In vitro, in vivo | [420] |
Abbreviations: Adipose-derived stem cells, ASCs; Bone marrow mesenchymal stem cells, BMSCs; Mesenchymal stem cells, MSCs; Umbilical cord mesenchymal stem cells, UCMSCs; Traumatic brain injury, TBI; Chemokine CC motif ligand 1, CCL1; Duchenne muscular dystrophy, DMD; Hyaluronidase 2, HAS2; Interleukin 10, IL-10; Heat shock proteins, HSP; heat shock factor 1, HSF1; Matrix metalloproteinase-1, MMP1; Matrix metalloproteinase-2, MMP2; Matrix metalloproteinase-3, MMP3; insulin-like growth factors, IGFs; vascular endothelial growth factor, VEGF; hepatocyte growth factor, HGF; neurotrophin-3, NT-3; fibroblast growth factor-2, FGF2; Platelet-derived growth factor-AA, PDGF-AA; Reactive oxygen species, ROS; Transforming growth factor beta, TGF-β; Tumor necrosis factor-alpha, TNF-α; Interleukin-6, IL-6; Bone morphogenetic proteins, BMP; Ultraviolet radiation, UV; Ultraviolet B, UVB; vascular endothelial growth factor, VEGF; microARN, miARN; ovarian granulosa cells, OGCs; Premature ovarian insufficiency, POI; Platelet-Rich Plasma, PRP; mother against decapentaplegic-related proteins, SMADs; SMAD family member 6, SMAD6.
7.1. Myocardial regeneration
Regenerative medicine is a promising strategy in the regeneration, function restoration, or replacement of damaged myocardial tissue. Various types of sources of stem cells, including mesenchymal, cardiac, embryonic, hematopoietic, and cardio-sphere-derived stem cells, have been used to protect and regenerate cardiac tissue through the use of exosomes [421]. MSCs were the first type of stem cells used for myocardial regeneration [422]. Timmers et al. [423] reported that the conditioned medium obtained from MSCs was capable of reducing myocardial infarct size. Later, this group demonstrated that the conditioned medium consisted of exosomes with a diameter of 50–100 nm, which could be the reason for the reduction in myocardial injury [424]. Arslan et al. [320] created mice models of myocardial ischemia/reperfusion injury and demonstrated that exosomes obtained from MSCs conditioned medium could decrease the infarct size by decreasing oxidative stress, increasing ATP level, and activating the PI3K/Akt signaling pathway. In vivo studies have also shown that human umbilical cord MSCs conditioned media infused into AMI model rats can protect against apoptosis and promote endothelial tube formation. The groups containing exosomes derived from MSCs displayed a significant increase in Bcl-2 expression compared to the groups without exosomes in both normoxia and hypoxia [321].
Exosomes collected from rat myocytes release heat HSP20, which enhances angiogenesis by activating vascular endothelial growth factor receptor 2 [324]. On the other hand, exosomes from cardiomyocytes of type 2 diabetic rats exhibited anti-angiogenic properties due to containing more miR-320 and less miR-126 compared to the exosomes from non-diabetic rat cardiomyocytes [425]. These results emphasize the importance of the exosome source in determining its angiogenic effects.
Exosomes secreted from atorvastatin-pretreated MSCs show myocardial protection by inhibiting apoptosis, decreasing IL-6 and TNF-α levels related to inflammation, reducing Col1a1 and Col3a1 levels related to fibrosis, upregulating lncRNA H19 expression related to angiogenesis regulation and treating myocardial injury [326]. Pretreated MSCs can also be used to express exosomes for specific cardioprotection. For instance, Yu et al. reported that MSCs transfected with the GATA-4 gene could significantly decrease the levels of miR-15 families, which are associated with heart failure, as well as increase the levels of the anti-apoptotic factor Bcl-w [426] and miR-19a [327].
Exosomes derived from the blood of rats and humans express CD63, CD81, and HSP70. Ex vivo analysis of these exosomes revealed that cardio-protection results from the communication of HSP70 and toll-like receptor 4 and the activation of HSP27 [328], which enhances the myocardial response to oxidative stress [427]. Furthermore, extracellular vehicles present in the conditioned medium obtained from the culture of human cardiac progenitor cells demonstrate an apoptosis inhibitor role as well as enhancing endothelial tube formation, whereas EV-depleted medium does not show these results. Extracellular vehicles are positive for exosome markers (CD63, CD9, and CD81) as well as MSC/stromal markers (CD105, CD90, CD146, CD172, and NG2) [323]. Moreover, exosomal miRNAs secreted by hypoxic and normoxic cardiac progenitor cells indicate that 11 miRNAs increase in level in hypoxic conditions, and 6 of them are involved in cardiac function [428].
Exosomes from cardio-sphere-derived cells have also been used to regenerate the heart by transferring miR-146a [317], affecting nuclear factor κB phosphorylation and myogenesis in mdx mice [315], increasing Ki67-expressed cardiomyocytes and myoblast determination protein 1-expressed cells, and decreasing inflammation [316], upregulating pro-angiogenesis miRNAs (miR-210, miR-130a, and miR-126) and endothelial tube formation [318], and inhibiting apoptosis [429].
To target exosome delivery, Vandergriff et al. [325] conjugated exosomes obtained from either conditional media of cardio-sphere-derived cells or HT1080 with a cardiac homing peptide (CHP; CSTSMLKAC) to target infracted heart tissue and cells. The results indicated a high accumulation of exosomes in the infracted heart, a reduction of apoptosis, and an improvement of cardiac function and angiogenesis. Similarly, targeted exosomes ligated with a cardiomyocyte-specific peptide (CMP; WLSEAGPVVTVRALRGTGSW) were used to avoid exosome biodistribution and enhance exosome uptake by cardiomyocytes. To achieve this, plasmid cloning was employed to express Lamp2 on the external surface of exosomes, which were then bound to CMP [314]. Moreover, Mao et al. [313] demonstrated that exosomes conjugated with the heart homing peptide (HHP; CRPPR) reduced hypertrophy and improved heart function in a hypertrophy mice model. This effect was attributed to the improved accumulation of exosomes in the heart, delivery of miRNA-148a, and inhibition of β-MHC, BNP, GP130, p-STAT3, p-ERK1/2, and p-AKT [313]. Further exosome research sheds light on the precise process of myocardial regeneration and heart repair.
7.2. Neuronal regeneration
Exosomes derived from the nervous tissue are released by cells under both normal and pathological conditions, and they are considered to be clinical biomarkers for brain injuries [430]. However, it is well established that exosomes are also positively correlated with nerve and neuron regeneration. A feasibility study led by Ji et al. [431] suggested that serum exosomal miR-9 and miR-124 could be promising biomarkers for diagnosing and assessing the damage caused by an acute ischemic stroke. Yang et al. [329] showed that miR-124-enriched exosomes could also improve hippocampal neurogenesis after traumatic brain injury in rats by inhibiting the TLR4 pathway. Furthermore, Xin et al. [330] observed that miR-133b was transferred to rat neural cells via exosomes generated by MSCs, which stimulated neurite remodeling and functional recovery after stroke. This work revealed, for the first time, that MSCs can communicate with brain parenchymal cells via miRNA [330]. Zhang et al. [332] built upon Xin et al. [330] work and further demonstrated that exosomes generated from MSCs or human bone marrow MSCs increased angiogenesis and neurogenesis after traumatic brain injury in rats. Additionally, Zhang et al. [334] reported that exosomes derived from human umbilical cord MSCs could promote neuron regeneration after traumatic brain injury in rats while also inhibiting neuronal apoptosis and reducing proinflammatory cytokine expression by suppressing the NF-kB signaling.
Building on recent observations of MSCs neuroprotective potential, Mead and Tomarev [333] induced the knockdown of Argonaute-2 (a key protein involved in the RNA interference function of miRNA) in bone marrow MSCs. As result, the exosomes released from these cells exhibited a reduced number of miRNAs, leading to impaired survival of retinal ganglion cells and compromised axon regeneration in rats. This study reinforces the consistent finding across various studies, affirming the correlation between miRNA-loaded exosomes and neuroregeneration. Other studies have also shown that MSCs-derived exosomes carrying miRNAs, such as miR133b, miR126, miR21, and miR19b, promote apoptosis attenuation and recovery after spinal cord injury [[432], [433], [434]].
Peripheral nerve injury is a major public health burden. However, studies have claimed that exosomes can improve peripheral nerve injury recovery [[435], [436], [437]]. In a pioneering study, Lopez -Verrilli et al. [438] compared exosomes from different MSC sources and demonstrated that exosomes from menstrual, bone marrow, and umbilical cord MSCs could enhance neurite growth and survival in both the central and peripheral nervous system. Using a similar mechanism as Mead and Tomarev [333], Zhao et al. [340] revealed that bone marrow MSC-derived exosomes have significant neuroprotective and regenerative effects on the sciatic nerve, possibly through miRNAs related to vascular endothelial growth factor-A gen.
Recently, Rau et al. [337] reported that exosomes secreted by adipose-derived stem cells (ADSCs)enhance regeneration in a mouse model of sciatic nerve crush injury. Farinazzo et al. [338] suggested that the same exosomes improve nerve regeneration by increasing remyelination. ADSC-derived exosomes might promote nerve regeneration through two other mechanisms. Firstly, El Bassit et al. [339] demonstrated that ADSC-derived exosomes carry MALAT1, a protein that mediates the splicing of pkcδII, an anti-apoptotic protein, thereby increasing its expression and suppressing apoptosis. Secondly, Bucan et al. [439] observed sciatic nerve regeneration by stimulating the proliferation of Schwann cells. Recently, Liu et al. [335] suggested that the crosstalk between ADSCs and Schwann cells relies on both the modified expression of the apoptosis‐related genes Bcl‐2 and Bax [335] and the reduced autophagy of injured Schwann cells via downregulation of Kpna2 by miRNA-26b [336].
Lopez-Verrilli et al. [341] were the first to provide evidence that axonal regeneration in the peripheral nervous system is normally supported by exosomes derived from Schwann cells. More recently, Huang et al. [342] revealed that exosomes secreted by endothelial cells and carrying miR199-5p were internalized by Schwann cells, leading to the activation of the PI3K/AKT/PTEN signaling pathway, which maintained repair-related phenotypes of Schwann cells, and promoted axonal regeneration. Moreover, Mao et al. [343] found that gingiva-derived MSC-secreted exosomes promoted these Schwann cell phenotypes via c-JUN gene expression.
Finally, Anita et al. [344] demonstrated that exosomes derived from pericytes could promote angiogenesis and cavernous nerve regeneration in mice under diabetic conditions. The underlying mechanism may involve the intercellular transfer of Lipocalin 2 to target cells, which activates both MAP kinase and PI3/Akt and suppresses P53 signaling [344]. The comprehensive review of exosome-based therapy research underscores its efficacy in promoting neurorepair and neurogenesis. Notably, the intriguing prospect of augmenting these effects through the combination of exosomes with 3D scaffolds represents a promising avenue for further research and holds significant potential for advancing therapeutic approaches in the field of neurological regenerative medicine [440].
7.3. Cutaneous regeneration
Extracellular vesicles, particularly exosomes, derived from stem cells [[346], [348], [356], [358], [362], [441], [442], [443]], bacteria [353], tubers [352], and fruits [354], have been shown to have positive effects on skin regeneration. Exosomes can interact with different cell types, molecules, and extracellular matrix components in the skin, which accelerate healing and reverse natural or UV-irradiated aging.
There are various mechanisms through which healing is promoted, such as (i) human umbilical cord-derived miRNA-150-5p exosomes activating the PI3K/AKT pathway [356], (ii) human adipose tissue-derived exosome activating the Wnt/β-catenin pathway [345], and (iii) miRNA-19b exosome activating the TFG-β pathway [346]. Exosomes can also positively or negatively regulate collagen production depending on the timing of skin wounding (early or late phase) [[362], [442], [443],[347], [355], [357], [359], [360], [361]]. For example, during the initial phase of wound healing, mature collagen fibers were observed with the treatment of exosomes derived from mesenchymal stem cells, in contrast to the control group, which exhibited similar outcomes during the intermediate phase of wound healing [442]. Similarly, a significant decrease in the percentage of collagen I mRNA was described in the late phase of wound healing in the group treated with exosomes derived from epidermal stem cells, due to the inhibition of TGF-β [359]. Furthermore, in the skin extracellular matrix, exosomes up-regulate matrix metalloproteinases genes (MMP1 and MMP2), preventing the degradation of interstitial collagen types I and III [362,353,357,[347], [351], [360]]. At the inflammatory level, they can reduce cytokine activity through the NF-κB pathway [354] and downregulate polysaccharide-induced inflammatory factor mRNA expression [348]. By utilizing the mechanisms mentioned above, exosomes can accelerate wound closure in a shorter time [441,442,443,345,355,361,359].
UV irradiation can cause various types of damage, but exosomes containing circ-Asch 1l and GPX4 have been found to reverse these effects [358]. In addition, exosomes can also act on melanocytes and reverse hyperpigmentation caused by UV irradiation or natural aging [353,351]. Moreover, exosomes have a positive impact on keratinocytes and fibroblasts, leading to increased skin hydration [350] and a reduction in the expression of collagenase, including MMP-1, and proinflammatory cytokines [350,349]. The presented findings are encouraging regarding exosome therapy for skin regeneration and its potential short-term application in human patients.
7.4. Muscle regeneration
Exosomes derived from ADSCs have shown significant improvement in muscle-skeletal regeneration by enhancing myocyte proliferation and myogenic differentiation, leading to an increase in the expression of MYOG and MYOD [363], as well as inhibiting atrophy and promoting myofiber regeneration [364]. Similarly, exosomes derived from platelet-rich plasma were observed to enhance MYOG levels in muscle defect treatment but did not affect MYOD expression. On the other hand, exosomes derived from MSCs were found to accelerate muscle recovery by increasing centrally nucleated fiber. However, a decrease in the level of TGF-β, an important factor for muscle regeneration, was also observed [369]. Exosomes derived from MSCs have been reported to affect muscle regeneration through various mechanisms, including miR-21 as an anti-apoptosis factor, miR-1, miR-133, miR-206, and miR-494 as miRNAs related to myogenesis and cell migration [370]. Interestingly, exosomes derived during the differentiation of human skeletal myoblasts contained transforming growth factor beta as well as different growth factors, such as hepatocyte growth factor, and upregulated mRNAs, such as MYOD1, which are necessary for muscle regeneration [371].
Exosomes originating from non-stem cells have also shown therapeutic effects on muscles. For instance, Zhou et al. [368] investigated exosomes derived from M2 macrophages and reported that these exosomes are enriched with miR-501, which suppressed Yin Yang 1 gene expression related to myoblast differentiation, and upregulates MyHC and MyoG. Similarly, exosomes derived from C2C12 cells have been found to promote muscle regeneration by upregulating Pax7 and affecting the expressions of MYOD, peroxisome proliferator-activated receptor γ, α-SMA, collagen-1, proliferating cell nuclear antigen [366]. These exosomes upregulate miR-181a, 146a, miR-145, miR-1, miR-24, miR-206, miR-133a, miR-133b, miR-378, let-7d, and let-7e and downregulating Sirtuin1 [367]. Additionally, the mechanical strain on C2C12 myoblasts shows promise for musculoskeletal repair and tissue regeneration, where the generated exosomes have enhanced efficacy for injury alleviation [365].
7.5. Vascular regeneration
Exosomes derived from endothelial and vascular progenitor cells have been shown to improve vascularization by enriching in various miRNAs, including miRNA-21-5p, -143-3p, −291b, and −20b-5p, which enhance the expression of angiogenesis factors, such as eNOS, HIF-1α, VEGFA, VEGFR-2, ANG-1, E-selectin, CXCL16, PDGFA, IGF-binding protein-3, and pentaxtrin-3. These exosomes also downregulate MMP-9 and PDGFB, improve reendothelialization, and promote the proliferation and migration of endothelial cells [[379], [380], [381]]. VEGFR2 levels were also shown to improve in cells cultured in a medium supplemented with exosomes derived from MSCs cultured in nanomodified titanium [382]. MSCs-derived exosomes are associated with different angiogenesis factors, such as platelet-derived growth factor (PDGF), epidermal growth factor (EGF), and fibroblast growth factor (FGF), as well as proteins contributing to nuclear factor-kappaB (NFkB) signaling pathway [383]. Additionally, PDGF can be used as a medium supplement to improve the angiogenic properties of exosomes. Lopatina et al. [384] showed that EVs derived from conditioned media supplemented with PDGF contained MMPs, c-kit, and SCF, which are important for recruiting endothelial progenitor cells, their migration, growth factors expression, and tube formation. In endothelial cells exposed to exosomes derived from annulus fibrosus cells, expressions of MMPs, along with IL-6, TNF-α, and VEGF were also increased [385].
Moreover, studies have indicated that cells cultured under hypoxic conditions express more exosomes that can enhance angiogenesis [386,444]. Hypoxia is one of the critical parameters in tumor development that promotes angiogenesis. Therefore, exosomes produced by tumor cells cultured under hypoxic conditions have been studied for their angiogenesis properties [445,388]. For instance, exosomes derived from glioma cells cultured in hypoxia have been found to possess greater angiogenic properties than cells cultured in normoxia. This is achieved via the activation of angiogenesis factors such as EGFR, VEGFR2, and PI3K/AKT pathways, which enhance tumor vascularization [446]. Upregulation of miR-210 has also been reported in the literature using exosomes derived from cell lines cultured in hypoxia for inducing vascularization [386,387]. Additionally, exosomes derived from tumor cells are associated with angiogenesis via the upregulation of miR-21 [447]. To further study the reason for vascularization due to the upregulation of miR-21, exosomes derived from ADSCs that were transfected to overexpress miR-21 were evaluated. The results showed enhanced angiogenesis by increasing the levels of HIF-1α, VEGF, SDF-1, p-Akt, and p-ERK1/2 and decreasing PTEN levels [389].
7.6. Chondral regeneration
Some studies have demonstrated the efficacy of different MSCs-derived exosomes in cartilage repair. Zhang et al. [448] showed for the first time that intra-articular administration of human embryonic MSC-derived exosomes promoted the regeneration of osteochondral defects in a rat model. This effect may be due to both proliferative and antiapoptotic responses, as well as immune reactivity modulation. These responses are related to the AKT and ERK pathways, and a higher infiltration of CD163+ regenerative M2 macrophages, with a concomitant reduction of pro-inflammatory cytokines, such as IL-1β and TNF-α [390]. Additionally, Vonk et al. [392] verified that exosomes secreted from human bone marrow mononuclear cells are taken up by chondrocytes in osteoarthritic joints, inhibiting the TNF-alpha-induced inflammatory response and promoting cartilage repair. Chen et al. [449] reported that exosomes secreted by chondrocytes had preferable cartilage regeneration and stability compared with bone marrow MSC-generated exosomes in a rabbit model. Moreover, some studies have shown that modifications of MSCs can improve the therapeutic potential of their exosomes. Liu et al. [394] increased the cartilage regeneration effects of bone marrow MSC-derived exosomes by pretreating these cells with kartogenin. Similarly, Shao et al. [450] found that infrapatellar fat pat MCS-derived exosomes, after pretreatment with kartogenin, had a potent ability to induce in vitro chondrogenic differentiation of stem cells and improve articular cartilage regeneration in a rabbit model. Likewise, Zhao et al. [393] reported that exosomes derived from subcutaneous fat MCS could also promote cartilage repair via miR-199a-3p delivery. Additionally, Tao et al. [391] compared MSC-generated exosomes with or without miR-140-5p overexpression and found that those with miR-140-5p overexpression enhanced cartilage regeneration after osteoarthritis in a rat model. They suggested that in vitro enhanced proliferation and migration of chondrocytes was related to the Wnt signaling pathway.
Furthermore, Zhu et al. [451] compared the efficiency of exosomes secreted by synovial membrane MSCs and by iPSC-MSC in the treatment of osteoarthritis. Although both types of MSCs secreting exosomes were efficient in attenuating osteoarthritis, iPSC-MSCs exhibited superior regenerative potential [451]. In summary, the research on MSC-derived exosomes for cartilage repair shows promise in addressing conditions like osteoarthritis. Yet, iPSC-MSC exosomes demonstrate superior regenerative capabilities in the treatment of osteoarthritis, contributing to the ongoing exploration of innovative cartilage-related disorder treatments.
7.7. Hepatic regeneration
According to Nojima et al. [373], exosomes secreted by healthy hepatocytes transfer exosomal sphingosine kinase (SK2) to target hepatocytes, supporting their proliferation and regeneration after acute hepatic injury. A proof-of-concept study guided by Tan et al. [452] investigated the effect of MCS-derived exosomes in carbon tetrachloride (CCl4)-injured mice and observed in vivo liver regeneration and activation of proliferative responses after toxicants-induced injury. Likewise, the positive consequences of exosomes secreted from different types of MSCs have been portrayed in several studies. Yan et al. [374] revealed that systemic administration of exosomes from allogenic human umbilical cord MSCs could rescue miceˈs liver after CCl4-induced hepatic failure due to glutathione peroxidade 1 antioxidative property. Rong et al. [375] reported that human bone marrow MSCs-derived exosomes exhibit hepatoprotection and anti-fibrotic effects by stimulating hepatocyte regeneration and suppressing Wnt/β-catenin signaling in rat CCl4-induced liver fibrosis. Conversely, Piao et al. [376] suggested that activation of Wnt/β-catenin signaling using ADSCs-derived exosomes promoted liver regeneration in hepatic ischemia-reperfusion in rats. Furthermore, placenta MSC-derived exosomes have been widely used. Jun et al. [377] reported that C-reactive protein in placenta MSCs-derived exosomes triggers hepatic regeneration via the Wnt signaling pathway. Moreover, Li et al. [378] suggested that human placenta PMSCs exosomal circ-RBM23, by sponging miR-139-5p, stimulate cell proliferation and liver regeneration after hepatectomy through the AKT/mTOR pathway. More recently, Chen et al. [372] showed that exosomes derived from hepatocyte-derived liver progenitor-like cells (HepLPCs), could enhance hepatic regeneration after acute liver failure in vivo via the FoxO1/Akt/GSK3β/β-catenin axis. These findings underscore the versatility of exosome-based therapies in supporting hepatic recovery and suggest a promising path for future clinical applications in the treatment of liver injuries and diseases. Nevertheless, further investigations are warranted to unravel the intricacies of exosomal mechanisms.
7.8. Ovary regeneration
Exosomes are being investigated as a therapeutic strategy for the treatment of premature ovarian insufficiency (POI). Exosomes derived from stem cells have shown anti-apoptotic effects, restoration of normal hormone production, and increased birth rate in animals with POI.
Exosomes prevent apoptosis in granulosa cells through various mechanisms of action. For example, exosomes derived from amniotic fluid stem cells expressing miR-369-3p inhibit apoptosis through the YAF2/PDCD5/p53 pathway [395]. Exosomes derived from umbilical cord MSCs expressing (i) miR-126-3p exosome inhibit apoptosis through inhibition of the PI3KR2 gene and activation of the PI3K/AKT/mTOR pathway [396] or (ii) miR-17-5p exosome through inhibition of SIRT7 and its target genes (γH2AX, PARP1, and XRCC6) [403]. Exosomes derived from bone marrow MSCs expressing (i) miR-144-5p exosome inhibit apoptosis through suppression of the PTE gene expression and upregulation of the PI3K/AKT pathway [398], or (ii) miR-664-5p through inhibition of p53 luciferase activity [399]. Furthermore, ovarian tissue produces miR-122-5p exosomes capable of inhibiting apoptosis through the BCL9 pathway [401]. Exosomes derived from menstrual blood-derived stromal cells significantly increase the production of collagen IV, FN1, and laminin at the level of the ovarian extracellular matrix [402].
In animal models of POI, exosome treatment has been shown to significantly increase E2 and AMH levels, and significantly decrease FSH levels compared to the untreated group [[395], [396], [398], [403],402,453]. Moreover, exosome treatment has led to an increase in the number of follicles [[395], [396], [398], [403],[402], [404], [453], [454]], and corpus luteum [399] compared to the POI group. Finally, exosome treatment has demonstrated encouraging therapeutic effects on POI, including a significant increase in the number of offspring [402,453,400,455]. However, it is worth noting that despite exosomes enabling offspring birth in POI patients, this effect does not persist in the long term [456]. In summary, research on exosomes for the treatment of POI suggests that they can alleviate ovarian lesions, restore ovarian functions, and enable offspring birth. However, further studies are still needed to ensure the long-term effectiveness of the treatment and its implementation in human patients.
7.9. Skeletal regeneration
Exosomes derived from ADSCs and MSCs have been shown to stimulate new bone formation and upregulate the levels of runt-related transcription factor 2 (RUNX2), alkaline phosphatase (ALP), collagen type I alpha 1 (COL1A1), vascular endothelial growth factor (VEGF), angiopoietin 1 (ANG1), angiopoietin 2 (ANG2), osteocalcin (OCN), osteopontin (OPN), miR-146a-5p, -503-5p, -483-3p, and -129-5p. They also downregulate -32-5p, −133a-3p, and -204-5p and activate the PI3K/Akt and MAPK signaling pathways [[405], [406], [412], [457], [458], [459], [460], [461]]. Exosomes derived from miR‐375‐overexpressing ADSCs also increase the levels of COL1A1, RUNX2, and ALP, indicating the positive effects of miR-375 on bone regeneration [414]. Moreover, Furuta et al. [407] found out that the bone regeneration capability of MSCs-derived exosomes is related to carrying miRNAs, such as miR-4532, -125b-5p, −4516, -338-3p, and −548aa. However, these exosomes contain low levels of monocyte chemotactic protein and stromal cell-derived factor, which are important for bone healing. It should be noted that the therapeutic potential of MSCs is age-dependent. Xu et al. [462] recently showed that exosomes from aged rat MSCs have an attenuated effect on bone healing due to their enrichment in miR-128-3P, which suppresses SMAD5.
Moreover, transfected MSCs have been employed to improve bone regeneration. For instance, exosomes derived from miR-21 transfected MSCs have been shown to inhibit osteocyte apoptosis by activating the miR-21-PTEN-AKT signaling pathway and subsequent inhibition of proteins such as BAD and caspase 3 [415]. Furthermore, exosomes derived from HIF-α transfected MSCs have been found to improve osteogenic differentiation and angiogenesis in defective bone tissue by increasing the levels of HIF-α, ALP, and OCN [416]. Exosomes derived from noggin-suppressed MSCs have also demonstrated improvement in bone healing as a result of miR-29 inhibition [413]. On the other hand, local targeting of bone tissue can be improved by conjugating bone marrow stromal cells-specific aptamers to exosomes from bone marrow MSCs, which improves osteogenesis of these cells and elevates the pro-osteogenic miRNAs of miR-26a, −29a, −218, −34a, and −3960 [408].
MSCs derived from induced pluripotent stem cells have also been used for secreting exosomes to improve bone healing due to the upregulation of ALP, RUNX-2, COL1A1, and decrease in cell necrosis by vascularization and activation of PI3K/Akt signaling pathways [[417], [418], [463]]. Exosomes from human umbilical cord MSCs have been shown to reduce cell apoptosis due to carrying miR-1263, which inhibits Mob1 [410], and these exosomes have also been found to improve bone healing as a result of upregulation of β-catenin, Wnt3a, Col1, OPN, RUNX2, VEGF, and HIF‐1α [411,464]. Additionally, Wnt3a level elevation was observed in exosomes derived from ADSCs preconditioned with TNF-α [419]. Furthermore, bone healing due to vascularization has been reported as a result of using exosomes derived from endothelial progenitor cells [420], from MSCs conditioned by dimethyloxaloylglycine [465], and from exosomes loaded with VEGF plasmid gene using the electroporation method [466]. Further investigations are required to elucidate the precise role and mechanism of exosomes derived from different cell sources in the process of bone regeneration.
8. Limitations and challenges
Despite its numerous advantages, including efficient transport of biological cargo across cells, low immunogenicity, high stability, and rapid recognition as a potential medication delivery mechanism, the use of exosomes may be limited by several disadvantages. The first challenging issue is the limitations of the current exosome isolation and detection methods, which hamper the widespread application of exosomes [218]. The conventional technique, ultracentrifugation, is labor- and time-intensive and may lead to nonspecific purification, thereby reducing biological activity [6]. Although commercially available reagents can simplify exosome extraction, their high cost limits their widespread clinical use, and they are mostly restricted to preclinical evaluations [218]. Therefore, researchers are exploring simpler and more cost-effective technologies for exosome isolation that have minimal detrimental effects on exosome integrity. Qi et al. [155] studied doxorubicin delivery through plasma exosomes isolated and modified with magnetic nanoparticles fused to transferrin. This strategy has several advantages, including magnetic field targeting and relatively simple exosome isolation without the use of expensive equipment.
The primary criterion for distinguishing among EVs is their intracellular origin. However, recent evidence indicates that multiple subpopulations may exist within these EV populations. This heterogeneity may have distinct roles in the intricate biological processes and introduces a layer of complexity to the study of EV biology and function [467]. Current limitations of isolation and characterization techniques remain a hindrance to effectively addressing the EV subpopulation issue. Nevertheless, the scientific community is actively seeking to develop new and improved techniques to address this issue and gain a better understanding of EV biology and function. In the last decade, developing novel exosome isolation and detection methods has been one the most challenging areas in exosome research. Currently, several cutting-edge nano-based methods are being developed, including (i) nano-deterministic lateral displacement (Nano-DLD), (ii) resistive pulse sensing (RPS), and (iii) surface plasmon resonance (SPR)-based nanosensors. Nano-DLD is a promising passive microfluidic technique and can isolate very small exosomes (20–100 nm) that earlier technologies cannot achieve [468]. However, it faces challenges in simulating 3D devices and optimizing practical design guidelines. Commercially, the technology demands cost-effective fabrication methods and improvements for mass production to reach full maturity [469]. As another novel nano-based method, RPS characterizes single particle properties including quantity, shape, charge, and size of particles in suspension [470]. This device involves in-plane nanochannels and nanopores, allowing for seamless integration of multiple elements within a single device without post-fabrication alignment [471]. On the other hand, SPR-based nanosensors exhibit substantial signal changes upon target-specific exosome binding on the array. The system is scalable, accommodating multiple sensing units on a single chip, and the assay is label-free, eliminating the necessity for secondary labeling with nanoparticles [472]. These devices are extremely useful for clinical diagnosis by providing the potential for exosome identification using multiple biomarkers [473].
While exosomes have many advantages as delivery mechanisms, their application can be hindered by a lack of cell-targeting specificity and quick removal from circulation [474]. Exosomes can inherit unique cell adhesion molecules, lipids, and ligands from their parent cells, which can be altered through suitable transfection to produce specific peptides and proteins that appear on the surface of the exosomes, thereby addressing the issue of specificity [475]. The application of targeted ligands is another method to alter exosome specificity. An example of how exosome specificity can be improved is by coating them with an octapeptide that binds to integrin α3β1, which is overexpressed in NSCLC. This targeting ligand has been shown to significantly reduce systemic toxicity in vitro and in vivo, while greatly increasing tumor accumulation and retention [476]. The conjugation of exosomes with polyethylene glycol (PEG) can extend their circulation time, and this effect is largely dependent on the PEG chain length and brush density, which can affect the degree of obfuscation [477]. In a study by Kooijmans et al. [474], intravenously-administrated uncoated exosomes were rapidly removed from the circulation within 10 min, while PEGylated exosomes were detectable in plasma for more than 60 min after injection, demonstrating the effectiveness of PEGylation in increasing exosome circulation time. Another approach to enhance exosome effectiveness is through structural modification. Exosome-liposome hybrid systems are a novel strategy that can create a new lipid profile for the exosome membrane, leading to decreased immunogenicity, increased colloidal stability, improved cellular uptake, and a longer half-life in blood [475,478].
The specific components of exosomes that promote tissue regeneration are not yet fully understood. However, if we can identify and understand these pro-regeneration effects, it could be therapeutically beneficial for patients who have limited or insufficient regeneration signals [32]. Evaluation of some critical characteristics of pre-isolation-loaded exosomes, such as the exosome/drug proportion and loading efficiency, can be challenging, and many studies do not report these parameters [145]. Future research should focus on optimizing and discussing this ratio as it may significantly affect the incorporation of loaded molecules into the exosomal structure. Despite the increasing interest in exosomes, little is known about their precise molecular mechanisms and roles in cancer development, progression, invasion, metastasis, diagnosis, and treatment [226]. Moreover, the mechanisms of exosomal uptake by cells and entry into biological membranes are still unclear [212]. Therefore, understanding the signaling routes and regulation mechanisms of cancer-related exosomes is crucial for developing future diagnostic and therapeutic approaches. Additionally, while exosomes have shown great potential as a cell-free therapy, their biological activities cannot be stored for a long time. Currently, freezing, freeze-drying, and spray-drying are among the most used techniques for protecting exosomes. However, it is far from ideal and necessary to develop exosome preservation technologies to protect their properties and facilitate their transportation and clinical application [479,480].
9. Concluding remarks
Exosomes are a specific and effective means of intercellular communication networks that can regulate a wide range of biological activities and are even more effective at crossing biological barriers than synthetic nanovesicles. Over the past few decades, exosomes have demonstrated promising potential for pharmaceuticals, biomarker investigation for disease prognosis and diagnosis, and tissue regeneration applications and have opened new opportunities for cell-free therapy and vaccine development in cancer immunotherapy by delivering antigens. Despite these advances, exosomes still face several challenges to become a clinically viable and successful strategy in nanomedicine and regenerative medicine. While numerous preparation techniques have been recently developed and improved for laboratory applications, large-scale production for clinical use remains limited by current isolation techniques and the need for purification procedures to remove unwanted byproducts and unloaded cargo. Most studies indicate that human exosomes, adhering to Good Manufacturing Practice (GMP) standards, can meet the requirements for use in clinical trials. An interdisciplinary collaboration among researchers, including clinicians, cell biologists, technology experts, and computer scientists, has the potential to bring significant advancements in the field. Although in vivo investigations have increased, exosomesˈ molecular mechanisms, essential properties, pharmaceutical aspects, and signaling pathways remain incompletely understood and require further study. We believe that the effectiveness of exosomes in disease management primarily hinges on technological advancements that enhance their discovery, sensitivity, and specificity in identifying distinct disease developments.
Ethics
This is a review article. Therefore, no patients or animals were involved in the preparation of this manuscript.
Ethics approval and consent to participate
There are no human and animal subjects in this review and informed consent is not applicable.
CRediT authorship contribution statement
Saeid Moghassemi: Writing – original draft, Supervision, Conceptualization. Arezoo Dadashzadeh: Writing – original draft. Maria João Sousa: Writing – original draft. Hanne Vlieghe: Writing – original draft. Jie Yang: Writing – original draft. Cecibel María León-Félix: Writing – original draft. Christiani A. Amorim: Writing – review & editing, Funding acquisition.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:Saeid Moghassemi reports financial support was provided by Louvain Foundation. Arezoo Dadashzadeh reports financial support was provided by Louvain Foundation. Christiani A. Amorim reports financial support was provided by Fund for Scientific Research. Maria Joao Tavares Sousa reports financial support was provided by Catholic University of Louvain. Jie Yang reports financial support was provided by China Scholarship Council.
Acknowledgment
This study was supported by grants from the Foundation Louvain (awarded to C.A.A., Ph.D. scholarship awarded to S.M., as part of a legacy from Mr. Frans Heyes, and Ph.D. scholarship awarded to A.D. as part of a legacy from Mrs. Ilse Schirmer), the Fonds National de la Recherche Scientifique de Belgique (FNRS-PDR Convention grant number T.0004.20 awarded to C.A.A., Ph.D. scholarship awarded to H.V.), the UCLouvain's Fonds spéciaux de recherche (IREC FSR22, awarded to C.A.A., Ph.D. scholarship awarded to M.J.S.), the Fonds National de la Recherche Scientifique de Belgique (FNRS-PINT-Multi/VA Convention grant number R.8002.21 awarded to C.A.A., Ph.D. scholarship awarded to C.M.L.F.), Erasmus+ Dimension internationale (convention grant number DS/VM/CAI45-1011 awarded to C.A.A., Ph.D. scholarship awarded to C.M.L.F.) and the China Scholarship Council (Ph.D. scholarship awarded to J.Y.). The figures were partly generated using Servier Medical Art, provided by Servier, licensed under a Creative Commons Attribution 3.0 unported license.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
References
- 1.Nam G.H., Choi Y., Kim G.B., Kim S., Kim S.A., Kim I.S. Emerging prospects of exosomes for cancer treatment: from conventional therapy to immunotherapy. Adv. Mater. 2020;32(51) doi: 10.1002/adma.202002440. [DOI] [PubMed] [Google Scholar]
- 2.Kar R., Dhar R., Mukherjee S., Nag S., Gorai S., Mukerjee N., Mukherjee D., Vatsa R., Chandrakanth Jadhav M., Ghosh A. Exosome-based smart drug delivery tool for cancer theranostics. ACS Biomater. Sci. Eng. 2023;9(2):577–594. doi: 10.1021/acsbiomaterials.2c01329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collino F., Pomatto M., Bruno S., Lindoso R.S., Tapparo M., Sicheng W., Quesenberry P., Camussi G. Exosome and microvesicle-enriched fractions isolated from mesenchymal stem cells by gradient separation showed different molecular signatures and functions on renal tubular epithelial cells. Stem cell reviews and reports. 2017;13(2):226–243. doi: 10.1007/s12015-016-9713-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lötvall J., Hill A.F., Hochberg F., Buzás E.I., Di Vizio D., Gardiner C., Gho Y.S., Kurochkin I.V., Mathivanan S., Quesenberry P. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. Taylor & Francis. 2014 doi: 10.3402/jev.v3.26913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Théry C., Witwer K.W., Aikawa E., Alcaraz M.J., Anderson J.D., Andriantsitohaina R., Antoniou A., Arab T., Archer F., Atkin‐Smith G.K. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles. 2018;7(1) doi: 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kimiz-Gebologlu I., Oncel S.S. Exosomes: large-scale production, isolation, drug loading efficiency, and biodistribution and uptake. J. Contr. Release. 2022;347:533–543. doi: 10.1016/j.jconrel.2022.05.027. [DOI] [PubMed] [Google Scholar]
- 7.Yáñez-Mó M., Siljander P.R.-M., Andreu Z., Bedina Zavec A., Borràs F.E., Buzas E.I., Buzas K., Casal E., Cappello F., Carvalho J. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles. 2015;4(1) doi: 10.3402/jev.v4.27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ståhl A.-l., Johansson K., Mossberg M., Kahn R., Karpman D. Exosomes and microvesicles in normal physiology, pathophysiology, and renal diseases. Pediatr. Nephrol. 2019;34(1):11–30. doi: 10.1007/s00467-017-3816-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grange C., Bussolati B. Extracellular vesicles in kidney disease. Nat. Rev. Nephrol. 2022;18(8):499–513. doi: 10.1038/s41581-022-00586-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jeppesen D.K., Zhang Q., Franklin J.L., Coffey R.J. Extracellular vesicles and nanoparticles: emerging complexities. Trends Cell Biol. 2023;33(8):667–681. doi: 10.1016/j.tcb.2023.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colombo M., Raposo G., Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 2014;30:255–289. doi: 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- 12.Gurung S., Perocheau D., Touramanidou L., Baruteau J. The exosome journey: from biogenesis to uptake and intracellular signalling. Cell Commun. Signal. 2021;19(1):1–19. doi: 10.1186/s12964-021-00730-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buzas E.I. The roles of extracellular vesicles in the immune system. Nat. Rev. Immunol. 2023;23(4):236–250. doi: 10.1038/s41577-022-00763-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rani S., Lai A., Nair S., Sharma S., Handberg A., Carrion F., Möller A., Salomon C. Extracellular vesicles as mediators of cell-cell communication in ovarian cancer and beyond–A lipids focus, ytokine Growth Factor Rev. 2023;73:52–68. doi: 10.1016/j.cytogfr.2023.06.004. [DOI] [PubMed] [Google Scholar]
- 15.Salomon C., Das S., Erdbrügger U., Kalluri R., Kiang Lim S., Olefsky J.M., Rice G.E., Sahoo S., Andy Tao W., Vader P., Wang Q., Weaver A.M. Extracellular vesicles and their emerging roles as cellular messengers in endocrinology: an endocrine society scientific statement. Endocr. Rev. 2022;43(3):441–468. doi: 10.1210/endrev/bnac009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trams E.G., Lauter C.J., Salem J.N., Heine U. Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochim. Biophys. Acta Biomembr. 1981;645(1):63–70. doi: 10.1016/0005-2736(81)90512-5. [DOI] [PubMed] [Google Scholar]
- 17.Kalluri R., LeBleu V.S. The biology, function, and biomedical applications of exosomes. Science. 2020;367(6478) doi: 10.1126/science.aau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Y.-J., Wu J.-Y., Liu J., Xu W., Qiu X., Huang S., Hu X.-B., Xiang D.-X. Artificial exosomes for translational nanomedicine. J. Nanobiotechnol. 2021;19(1):1–20. doi: 10.1186/s12951-021-00986-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pegtel D.M., Gould S.J. Exosomes, Annual review of biochemistry. 2019;88:487–514. doi: 10.1146/annurev-biochem-013118-111902. [DOI] [PubMed] [Google Scholar]
- 20.Barile L., Gherghiceanu M., Popescu L.M., Moccetti T., Vassalli G. Ultrastructural evidence of exosome secretion by progenitor cells in adult mouse myocardium and adult human cardiospheres. J. Biomed. Biotechnol. 2012;2012 doi: 10.1155/2012/354605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scott R.E. Plasma membrane vesiculation: a new technique for isolation of plasma membranes. Science. 1976;194(4266):743–745. doi: 10.1126/science.982044. [DOI] [PubMed] [Google Scholar]
- 22.Li K., Lin Y., Luo Y., Xiong X., Wang L., Durante K., Li J., Zhou F., Guo Y., Chen S. A signature of saliva-derived exosomal small RNAs as predicting biomarker for esophageal carcinoma: a multicenter prospective study. Mol. Cancer. 2022;21(1):1–13. doi: 10.1186/s12943-022-01499-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mi B., Chen L., Xiong Y., Yan C., Xue H., Panayi A.C., Liu J., Hu L., Hu Y., Cao F. Saliva exosomes-derived UBE2O mRNA promotes angiogenesis in cutaneous wounds by targeting SMAD6. J. Nanobiotechnol. 2020;18(1):1–14. doi: 10.1186/s12951-020-00624-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zempleni J., Sukreet S., Zhou F., Wu D., Mutai E. 2019. Milk-derived Exosomes and Metabolic Regulation. [DOI] [PubMed] [Google Scholar]
- 25.del Pozo-Acebo L., López de las Hazas M., Tomé-Carneiro J., Gil-Cabrerizo P., San-Cristobal R., Busto R., García-Ruiz A., Dávalos A. Bovine milk-derived exosomes as a drug delivery vehicle for miRNA-based therapy. Int. J. Mol. Sci. 2021;22(3):1105. doi: 10.3390/ijms22031105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xiao G.-Y., Cheng C.-C., Chiang Y.-S., Cheng W.T.-K., Liu I., Wu S.-C. Exosomal miR-10a derived from amniotic fluid stem cells preserves ovarian follicles after chemotherapy. Sci. Rep. 2016;6(1):1–12. doi: 10.1038/srep23120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mobarak H., Heidarpour M., Rahbarghazi R., Nouri M., Mahdipour M. Amniotic fluid-derived exosomes improved spermatogenesis in a rat model of azoospermia. Life Sci. 2021;274 doi: 10.1016/j.lfs.2021.119336. [DOI] [PubMed] [Google Scholar]
- 28.An M., Lohse I., Tan Z., Zhu J., Wu J., Kurapati H., Morgan M.A., Lawrence T.S., Cuneo K.C., Lubman D.M. Quantitative proteomic analysis of serum exosomes from patients with locally advanced pancreatic cancer undergoing chemoradiotherapy. J. Proteome Res. 2017;16(4):1763–1772. doi: 10.1021/acs.jproteome.7b00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Domenyuk V., Zhong Z., Stark A., Xiao N., O'Neill H.A., Wei X., Wang J., Tinder T.T., Tonapi S., Duncan J. Plasma exosome profiling of cancer patients by a next generation systems biology approach. Sci. Rep. 2017;7(1):1–15. doi: 10.1038/srep42741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pan W., Xu X., Zhang M., Song X. Human urine-derived stem cell-derived exosomal miR-21-5p promotes neurogenesis to attenuate Rett syndrome via the EPha4/TEK axis. Lab. Invest. 2021;101(7):824–836. doi: 10.1038/s41374-021-00574-w. [DOI] [PubMed] [Google Scholar]
- 31.Wang C.-B., Chen S.-H., Zhao L., Jin X., Chen X., Ji J., Mo Z.-N., Wang F.-B. Urine-derived exosomal PSMA is a promising diagnostic biomarker for the detection of prostate cancer on initial biopsy. Clin. Transl. Oncol. 2022:1–10. doi: 10.1007/s12094-022-02983-9. [DOI] [PubMed] [Google Scholar]
- 32.Jing H., He X., Zheng J. Exosomes and regenerative medicine: state of the art and perspectives. Transl. Res. 2018;196:1–16. doi: 10.1016/j.trsl.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 33.Betzer O., Barnoy E., Sadan T., Elbaz I., Braverman C., Liu Z., Popovtzer R. Advances in imaging strategies for in vivo tracking of exosomes. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology. 2020;12(2) doi: 10.1002/wnan.1594. [DOI] [PubMed] [Google Scholar]
- 34.Aheget H., Tristán-Manzano M., Mazini L., Cortijo-Gutierrez M., Galindo-Moreno P., Herrera C., Martin F., Marchal J.A., Benabdellah K. Exosome: a new player in translational nanomedicine. J. Clin. Med. 2020;9(8):2380. doi: 10.3390/jcm9082380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Freitas R.A., Jr. What is nanomedicine? Nanomed. Nanotechnol. Biol. Med. 2005;1(1):2–9. [Google Scholar]
- 36.Jain K.K. Nanodiagnostics: application of nanotechnology in molecular diagnostics. Expert Rev. Mol. Diagn. 2003;3(2):153–161. doi: 10.1586/14737159.3.2.153. [DOI] [PubMed] [Google Scholar]
- 37.Moghassemi S., Dadashzadeh A., Azevedo R.B., Amorim C.A. Nanoemulsion applications in photodynamic therapy. J. Contr. Release. 2022;351:164–173. doi: 10.1016/j.jconrel.2022.09.035. [DOI] [PubMed] [Google Scholar]
- 38.Olivo M., Bhuvaneswari R., Lucky S.S., Dendukuri N., Soo-Ping Thong P. Targeted therapy of cancer using photodynamic therapy in combination with multi-faceted anti-tumor modalities. Pharmaceuticals. 2010;3(5):1507–1529. doi: 10.3390/ph3051507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chakraborty R., Basu T. Metallic copper nanoparticles induce apoptosis in a human skin melanoma A-375 cell line. Nanotechnology. 2017;28(10) doi: 10.1088/1361-6528/aa57b0. [DOI] [PubMed] [Google Scholar]
- 40.Gowda R., Kardos G., Sharma A., Singh S., Robertson G.P. Nanoparticle-based celecoxib and plumbagin for the synergistic treatment of melanoma. Mol. Cancer Therapeut. 2017;16(3):440–452. doi: 10.1158/1535-7163.MCT-16-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Valencia-Serna J., Aliabadi H.M., Manfrin A., Mohseni M., Jiang X., Uludag H. siRNA/lipopolymer nanoparticles to arrest growth of chronic myeloid leukemia cells in vitro and in vivo. Eur. J. Pharm. Biopharm. 2018;130:66–70. doi: 10.1016/j.ejpb.2018.06.018. [DOI] [PubMed] [Google Scholar]
- 42.Paskeh M.D.A., Entezari M., Mirzaei S., Zabolian A., Saleki H., Naghdi M.J., Sabet S., Khoshbakht M.A., Hashemi M., Hushmandi K. Emerging role of exosomes in cancer progression and tumor microenvironment remodeling. J. Hematol. Oncol. 2022;15(1):1–39. doi: 10.1186/s13045-022-01305-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Feng W., Dean D.C., Hornicek F.J., Shi H., Duan Z. Exosomes promote pre-metastatic niche formation in ovarian cancer. Mol. Cancer. 2019;18(1):1–11. doi: 10.1186/s12943-019-1049-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meng F., Yu W., Niu M., Tian X., Miao Y., Li X., Zhou Y., Ma L., Zhang X., Qian K. Ratiometric electrochemical OR gate assay for NSCLC-derived exosomes. J. Nanobiotechnol. 2023;21(1):1–15. doi: 10.1186/s12951-023-01833-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.LeBleu V.S., Kalluri R. Exosomes as a multicomponent biomarker platform in cancer. Trends in cancer. 2020;6(9):767–774. doi: 10.1016/j.trecan.2020.03.007. [DOI] [PubMed] [Google Scholar]
- 46.Kok V.C., Yu C.-C. Cancer-derived exosomes: their role in cancer biology and biomarker development. Int. J. Nanomed. 2020;15:8019. doi: 10.2147/IJN.S272378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yu W., Hurley J., Roberts D., Chakrabortty S., Enderle D., Noerholm M., Breakefield X., Skog J. Exosome-based liquid biopsies in cancer: opportunities and challenges. Ann. Oncol. 2021;32(4):466–477. doi: 10.1016/j.annonc.2021.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu J., Ostowari A., Gonda A., Mashayekhi K., Dayyani F., Hughes C.C., Senthil M. Exosomes as a source of biomarkers for gastrointestinal cancers. Cancers. 2023;15(4):1263. doi: 10.3390/cancers15041263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hazrati A., Soudi S., Malekpour K., Mahmoudi M., Rahimi A., Hashemi S.M., Varma R.S. Immune cells-derived exosomes function as a double-edged sword: role in disease progression and their therapeutic applications. Biomark. Res. 2022;10(1):1–25. doi: 10.1186/s40364-022-00374-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rahmati S., Khazaei M., Nadi A., Alizadeh M., Rezakhani L. Exosome-loaded scaffolds for regenerative medicine in hard tissues. Tissue Cell. 2023 doi: 10.1016/j.tice.2023.102102. [DOI] [PubMed] [Google Scholar]
- 51.Zhao X., Fu L., Zou H., He Y., Pan Y., Ye L., Huang Y., Fan W., Zhang J., Ma Y. Optogenetic engineered umbilical cord MSC-derived exosomes for remodeling of the immune microenvironment in diabetic wounds and the promotion of tissue repair. J. Nanobiotechnol. 2023;21(1):1–25. doi: 10.1186/s12951-023-01886-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Satija N.K., Singh V.K., Verma Y.K., Gupta P., Sharma S., Afrin F., Sharma M., Sharma P., Tripathi R., Gurudutta G. Mesenchymal stem cell‐based therapy: a new paradigm in regenerative medicine. J. Cell Mol. Med. 2009;13(11‐12):4385–4402. doi: 10.1111/j.1582-4934.2009.00857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Han Y., Li X., Zhang Y., Han Y., Chang F., Ding J. Mesenchymal stem cells for regenerative medicine. Cells. 2019;8(8):886. doi: 10.3390/cells8080886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dua J., Rana A., Bhandari A. Liposome: methods of preparation and applications. Int J Pharm Stud Res. 2012;3(2):14–20. [Google Scholar]
- 55.Nisini R., Poerio N., Mariotti S., De Santis F., Fraziano M. The multirole of liposomes in therapy and prevention of infectious diseases. Front. Immunol. 2018;9:155. doi: 10.3389/fimmu.2018.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moghassemi S., Dadashzadeh A., Camboni A., Feron O., Azevedo R.B., Amorim C.A. Photodynamic therapy using OR141-loaded nanovesicles for eradication of leukemic cells from ovarian tissue. Photodiagnosis Photodyn. Ther. 2022 doi: 10.1016/j.pdpdt.2022.103139. [DOI] [PubMed] [Google Scholar]
- 57.Moghassemi S., Dadashzadeh A., Jafari H., Ghaffari-Bohlouli P., Shavandi A., Amorim C.A. Liposomal oxygen-generating hydrogel for enhancing cell survival under hypoxia condition. Colloids Surf. B Biointerfaces. 2023 doi: 10.1016/j.colsurfb.2023.113562. [DOI] [PubMed] [Google Scholar]
- 58.Moghassemi S., Dadashzadeh A., Azevedo R.B., Feron O., Amorim C.A. Photodynamic cancer therapy using liposomes as an advanced vesicular photosensitizer delivery system. J. Contr. Release. 2021;339:75–90. doi: 10.1016/j.jconrel.2021.09.024. [DOI] [PubMed] [Google Scholar]
- 59.Li H., Jin H., Wan W., Wu C., Wei L. Cancer nanomedicine: mechanisms, obstacles and strategies. Nanomedicine. 2018;13(13):1639–1656. doi: 10.2217/nnm-2018-0007. [DOI] [PubMed] [Google Scholar]
- 60.Ferguson S.W., Nguyen J. Exosomes as therapeutics: the implications of molecular composition and exosomal heterogeneity. J. Contr. Release. 2016;228:179–190. doi: 10.1016/j.jconrel.2016.02.037. [DOI] [PubMed] [Google Scholar]
- 61.Mittelbrunn M., Gutiérrez-Vázquez C., Villarroya-Beltri C., González S., Sánchez-Cabo F., González M.Á., Bernad A., Sánchez-Madrid F. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat. Commun. 2011;2(1):282. doi: 10.1038/ncomms1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Greening D.W., Gopal S.K., Xu R., Simpson R.J., Chen W. Exosomes and their roles in immune regulation and cancer. Semin. Cell Dev. Biol. 2015;40:72–81. doi: 10.1016/j.semcdb.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 63.Gangoda L., Boukouris S., Liem M., Kalra H., Mathivanan S. Extracellular vesicles including exosomes are mediators of signal transduction: are they protective or pathogenic? Proteomics. 2015;15(2–3):260–271. doi: 10.1002/pmic.201400234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Valadi H., Ekström K., Bossios A., Sjöstrand M., Lee J.J., Lötvall J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007;9(6):654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 65.Simpson R.J., Lim J.W., Moritz R.L., Mathivanan S. Exosomes: proteomic insights and diagnostic potential. Expert Rev. Proteomics. 2009;6(3):267–283. doi: 10.1586/epr.09.17. [DOI] [PubMed] [Google Scholar]
- 66.Escola J.M., Kleijmeer M.J., Stoorvogel W., Griffith J.M., Yoshie O., Geuze H.J. Selective enrichment of tetraspan proteins on the internal vesicles of multivesicular endosomes and on exosomes secreted by human B-lymphocytes. J. Biol. Chem. 1998;273(32):20121–20127. doi: 10.1074/jbc.273.32.20121. [DOI] [PubMed] [Google Scholar]
- 67.Srivastava P. Interaction of heat shock proteins with peptides and antigen presenting cells: chaperoning of the innate and adaptive immune responses. Annu. Rev. Immunol. 2002;20:395–425. doi: 10.1146/annurev.immunol.20.100301.064801. [DOI] [PubMed] [Google Scholar]
- 68.Han Q.-F., Li W.-J., Hu K.-S., Gao J., Zhai W.-L., Yang J.-H., Zhang S.-J. Exosome biogenesis: machinery, regulation, and therapeutic implications in cancer. Mol. Cancer. 2022;21(1):1–26. doi: 10.1186/s12943-022-01671-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Naslavsky N., Caplan S. The enigmatic endosome – sorting the ins and outs of endocytic trafficking. J. Cell Sci. 2018;131(13):jcs216499. doi: 10.1242/jcs.216499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Trams E.G., Lauter C.J., Salem N., Jr., Heine U. Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochim. Biophys. Acta. 1981;645(1):63–70. doi: 10.1016/0005-2736(81)90512-5. [DOI] [PubMed] [Google Scholar]
- 71.Minciacchi V.R., Freeman M.R., Di Vizio D. Extracellular vesicles in cancer: exosomes, microvesicles and the emerging role of large oncosomes. Semin. Cell Dev. Biol. 2015;40:41–51. doi: 10.1016/j.semcdb.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sahu R., Kaushik S., Clement C.C., Cannizzo E.S., Scharf B., Follenzi A., Potolicchio I., Nieves E., Cuervo A.M., Santambrogio L. Microautophagy of cytosolic proteins by late endosomes. Dev. Cell. 2011;20(1):131–139. doi: 10.1016/j.devcel.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mathivanan S., Ji H., Simpson R.J. Exosomes: extracellular organelles important in intercellular communication. J. Proteonomics. 2010;73(10):1907–1920. doi: 10.1016/j.jprot.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 74.Krylova S.V., Feng D. The machinery of exosomes: biogenesis, release, and uptake. Int. J. Mol. Sci. 2023;24(2):1337. doi: 10.3390/ijms24021337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bai J., Wei X., Zhang X., Wu C., Wang Z., Chen M., Wang J. Microfluidic strategies for the isolation and profiling of exosomes. TrAC, Trends Anal. Chem. 2022 [Google Scholar]
- 76.Coughlan C., Bruce K.D., Burgy O., Boyd T.D., Michel C.R., Garcia‐Perez J.E., Adame V., Anton P., Bettcher B.M., Chial H.J. Exosome isolation by ultracentrifugation and precipitation and techniques for downstream analyses. Curr. Protoc. Cell Biol. 2020;88(1):e110. doi: 10.1002/cpcb.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Helwa I., Cai J., Drewry M.D., Zimmerman A., Dinkins M.B., Khaled M.L., Seremwe M., Dismuke W.M., Bieberich E., Stamer W.D. A comparative study of serum exosome isolation using differential ultracentrifugation and three commercial reagents. PLoS One. 2017;12(1) doi: 10.1371/journal.pone.0170628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yang D., Zhang W., Zhang H., Zhang F., Chen L., Ma L., Larcher L.M., Chen S., Liu N., Zhao Q. Progress, opportunity, and perspective on exosome isolation-efforts for efficient exosome-based theranostics. Theranostics. 2020;10(8):3684. doi: 10.7150/thno.41580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen J., Li P., Zhang T., Xu Z., Huang X., Wang R., Du L. Review on strategies and technologies for exosome isolation and purification. Front. Bioeng. Biotechnol. 2022;9 doi: 10.3389/fbioe.2021.811971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Akuma P., Okagu O.D., Udenigwe C.C. Naturally occurring exosome vesicles as potential delivery vehicle for bioactive compounds. Front. Sustain. Food Syst. 2019;3:23. [Google Scholar]
- 81.Abhange K., Makler A., Wen Y., Ramnauth N., Mao W., Asghar W., Wan Y. Small extracellular vesicles in cancer. Bioact. Mater. 2021;6(11):3705–3743. doi: 10.1016/j.bioactmat.2021.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Alameldin S., Costina V., Abdel-Baset H.A., Nitschke K., Nuhn P., Neumaier M., Hedtke M. Coupling size exclusion chromatography to ultracentrifugation improves detection of exosomal proteins from human plasma by LC-MS. Practical Laboratory Medicine. 2021;26 doi: 10.1016/j.plabm.2021.e00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yousif G., Qadri S., Parray A., Akhthar N., Shuaib A., Haik Y. Exosomes derived neuronal markers: immunoaffinity isolation and characterization. NeuroMolecular Med. 2021:1–13. doi: 10.1007/s12017-021-08696-6. [DOI] [PubMed] [Google Scholar]
- 84.Patil S.M., Sawant S.S., Kunda N.K. Exosomes as drug delivery systems: a brief overview and progress update. Eur. J. Pharm. Biopharm. 2020;154:259–269. doi: 10.1016/j.ejpb.2020.07.026. [DOI] [PubMed] [Google Scholar]
- 85.Lee K., Shao H., Weissleder R., Lee H. Acoustic purification of extracellular microvesicles. ACS Nano. 2015;9(3):2321–2327. doi: 10.1021/nn506538f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shirejini S.Z., Inci F. The Yin and Yang of exosome isolation methods: conventional practice, microfluidics, and commercial kits. Biotechnol. Adv. 2022;54 doi: 10.1016/j.biotechadv.2021.107814. [DOI] [PubMed] [Google Scholar]
- 87.Zhang H., Lyden D. Asymmetric-flow field-flow fractionation technology for exomere and small extracellular vesicle separation and characterization. Nat. Protoc. 2019;14(4):1027–1053. doi: 10.1038/s41596-019-0126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu W.-z., Ma Z.-j., Kang X.-w. Current status and outlook of advances in exosome isolation. Anal. Bioanal. Chem. 2022;414(24):7123–7141. doi: 10.1007/s00216-022-04253-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bashyal S., Thapa C., Lee S. Recent progresses in exosome-based systems for targeted drug delivery to the brain. J. Contr. Release. 2022;348:723–744. doi: 10.1016/j.jconrel.2022.06.011. [DOI] [PubMed] [Google Scholar]
- 90.Martins T.S., Vaz M., Henriques A.G. A review on comparative studies addressing exosome isolation methods from body fluids. Anal. Bioanal. Chem. 2023;415(7):1239–1263. doi: 10.1007/s00216-022-04174-5. [DOI] [PubMed] [Google Scholar]
- 91.Omrani M., Beyrampour-Basmenj H., Jahanban-Esfahlan R., Talebi M., Raeisi M., Serej Z.A., Akbar-Gharalari N., Khodakarimi S., Wu J., Ebrahimi-Kalan A. Global trend in exosome isolation and application: an update concept in management of diseases. Mol. Cell. Biochem. 2023:1–13. doi: 10.1007/s11010-023-04756-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zarovni N., Corrado A., Guazzi P., Zocco D., Lari E., Radano G., Muhhina J., Fondelli C., Gavrilova J., Chiesi A. Integrated isolation and quantitative analysis of exosome shuttled proteins and nucleic acids using immunocapture approaches. Methods. 2015;87:46–58. doi: 10.1016/j.ymeth.2015.05.028. [DOI] [PubMed] [Google Scholar]
- 93.Li P., Kaslan M., Lee S.H., Yao J., Gao Z. Progress in exosome isolation techniques. Theranostics. 2017;7(3):789. doi: 10.7150/thno.18133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chen Y., Zhu Q., Cheng L., Wang Y., Li M., Yang Q., Hu L., Lou D., Li J., Dong X. Exosome detection via the ultrafast-isolation system: exodus. Nat. Methods. 2021;18(2):212–218. doi: 10.1038/s41592-020-01034-x. [DOI] [PubMed] [Google Scholar]
- 95.Sidhom K., Obi P.O., Saleem A. A review of exosomal isolation methods: is size exclusion chromatography the best option? Int. J. Mol. Sci. 2020;21(18):6466. doi: 10.3390/ijms21186466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lin B., Lei Y., Wang J., Zhu L., Wu Y., Zhang H., Wu L., Zhang P., Yang C. Microfluidic‐based exosome analysis for liquid biopsy. Small Methods. 2021;5(3) doi: 10.1002/smtd.202001131. [DOI] [PubMed] [Google Scholar]
- 97.Nooshabadi V.T., Khanmohammadi M., Shafei S., Banafshe H.R., Malekshahi Z.V., Ebrahimi-Barough S., Ai J. Impact of atorvastatin loaded exosome as an anti-glioblastoma carrier to induce apoptosis of U87 cancer cells in 3D culture model. Biochemistry and biophysics reports. 2020;23 doi: 10.1016/j.bbrep.2020.100792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Życieńska K., Pszczółkowska B., Brzozowska B., Kamiński M., Lorenc T., Olejarz W., Sęk S., Ginter J. Brownian motion influence on AFM exosomes' size measurements. Int. J. Mol. Sci. 2022;23(17) doi: 10.3390/ijms231710074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rim K.-T., Kim S.-J. Quantitative analysis of exosomes from murine lung cancer cells by flow cytometry. Journal of cancer prevention. 2016;21(3):194. doi: 10.15430/JCP.2016.21.3.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dash M., Palaniyandi K., Ramalingam S., Sahabudeen S., Raja N. Exosomes isolated from two different cell lines using three different isolation techniques show variation in physical and molecular characteristics. Biochim. Biophys. Acta Biomembr. 2021;1863(2) doi: 10.1016/j.bbamem.2020.183490. [DOI] [PubMed] [Google Scholar]
- 101.Logozzi M., De Milito A., Lugini L., Borghi M., Calabro L., Spada M., Perdicchio M., Marino M.L., Federici C., Iessi E. High levels of exosomes expressing CD63 and caveolin-1 in plasma of melanoma patients. PLoS One. 2009;4(4) doi: 10.1371/journal.pone.0005219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Luo H.-T., Zheng Y.-Y., Tang J., Shao L.-J., Mao Y.-H., Yang W., Yang X.-F., Li Y., Tian R.-J., Li F.-R. Dissecting the multi-omics atlas of the exosomes released by human lung adenocarcinoma stem-like cells. NPJ Genomic Medicine. 2021;6(1):1–14. doi: 10.1038/s41525-021-00217-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rao M.S., Van Vleet T.R., Ciurlionis R., Buck W.R., Mittelstadt S.W., Blomme E.A., Liguori M.J. Comparison of RNA-Seq and microarray gene expression platforms for the toxicogenomic evaluation of liver from short-term rat toxicity studies. Front. Genet. 2019;9:636. doi: 10.3389/fgene.2018.00636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Stetefeld J., McKenna S.A., Patel T.R. Dynamic light scattering: a practical guide and applications in biomedical sciences. Biophysical reviews. 2016;8(4):409–427. doi: 10.1007/s12551-016-0218-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bhattacharjee S. DLS and zeta potential–what they are and what they are not? J. Contr. Release. 2016;235:337–351. doi: 10.1016/j.jconrel.2016.06.017. [DOI] [PubMed] [Google Scholar]
- 106.Dragovic R.A., Gardiner C., Brooks A.S., Tannetta D.S., Ferguson D.J., Hole P., Carr B., Redman C.W., Harris A.L., Dobson P.J. Sizing and phenotyping of cellular vesicles using Nanoparticle Tracking Analysis. Nanomed. Nanotechnol. Biol. Med. 2011;7(6):780–788. doi: 10.1016/j.nano.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sitar S., Kejžar A., Pahovnik D., Kogej K., Tušek-Žnidarič M., Lenassi M., Žagar E. Size characterization and quantification of exosomes by asymmetrical-flow field-flow fractionation. Anal. Chem. 2015;87(18):9225–9233. doi: 10.1021/acs.analchem.5b01636. [DOI] [PubMed] [Google Scholar]
- 108.McComiskey K.P., Tajber L. Comparison of particle size methodology and assessment of nanoparticle tracking analysis (NTA) as a tool for live monitoring of crystallisation pathways. Eur. J. Pharm. Biopharm. 2018;130:314–326. doi: 10.1016/j.ejpb.2018.07.012. [DOI] [PubMed] [Google Scholar]
- 109.McKinnon K.M. Flow cytometry: an overview. Curr. Protoc. Im. 2018;120(1):5.1. 1–5.1. 11. doi: 10.1002/cpim.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhang M., Jin K., Gao L., Zhang Z., Li F., Zhou F., Zhang L. Methods and technologies for exosome isolation and characterization. Small Methods. 2018;2(9) [Google Scholar]
- 111.Woud W.W., van der Pol E., Mul E., Hoogduijn M.J., Baan C.C., Boer K., Merino A. An imaging flow cytometry-based methodology for the analysis of single extracellular vesicles in unprocessed human plasma. Commun. Biol. 2022;5(1):633. doi: 10.1038/s42003-022-03569-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wu M., Chen Z., Xie Q., Xiao B., Zhou G., Chen G., Bian Z. One-step quantification of salivary exosomes based on combined aptamer recognition and quantum dot signal amplification. Biosens. Bioelectron. 2021;171 doi: 10.1016/j.bios.2020.112733. [DOI] [PubMed] [Google Scholar]
- 113.Stremersch S., Marro M., Pinchasik B.E., Baatsen P., Hendrix A., De Smedt S.C., Loza‐Alvarez P., Skirtach A.G., Raemdonck K., Braeckmans K. Identification of individual exosome‐like vesicles by surface enhanced Raman spectroscopy. Small. 2016;12(24):3292–3301. doi: 10.1002/smll.201600393. [DOI] [PubMed] [Google Scholar]
- 114.Renaud J.-P., Chari A., Ciferri C., Liu W.-t., Rémigy H.-W., Stark H., Wiesmann C. Cryo-EM in drug discovery: achievements, limitations and prospects. Nat. Rev. Drug Discov. 2018;17(7):471–492. doi: 10.1038/nrd.2018.77. [DOI] [PubMed] [Google Scholar]
- 115.Parisse P., Rago I., Ulloa Severino L., Perissinotto F., Ambrosetti E., Paoletti P., Ricci M., Beltrami A., Cesselli D., Casalis L. Atomic force microscopy analysis of extracellular vesicles. Eur. Biophys. J. 2017;46(8):813–820. doi: 10.1007/s00249-017-1252-4. [DOI] [PubMed] [Google Scholar]
- 116.Sun Y., Pang J.H. AFM image reconstruction for deformation measurements by digital image correlation. Nanotechnology. 2006;17(4):933. doi: 10.1088/0957-4484/17/4/016. [DOI] [PubMed] [Google Scholar]
- 117.Wang J., Chen D., Ho E.A. Challenges in the development and establishment of exosome-based drug delivery systems. J. Contr. Release. 2021;329:894–906. doi: 10.1016/j.jconrel.2020.10.020. [DOI] [PubMed] [Google Scholar]
- 118.Shao H., Im H., Castro C.M., Breakefield X., Weissleder R., Lee H. New technologies for analysis of extracellular vesicles. Chem. Rev. 2018;118(4):1917–1950. doi: 10.1021/acs.chemrev.7b00534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Konstantinou G.N. Food Allergens; Springer: 2017. Enzyme-linked Immunosorbent Assay (ELISA) pp. 79–94. [DOI] [PubMed] [Google Scholar]
- 120.Pillai-Kastoori L., Schutz-Geschwender A.R., Harford J.A. A systematic approach to quantitative Western blot analysis. Anal. Biochem. 2020;593 doi: 10.1016/j.ab.2020.113608. [DOI] [PubMed] [Google Scholar]
- 121.Aslam B., Basit M., Nisar M.A., Khurshid M., Rasool M.H. Proteomics: technologies and their applications. J. Chromatogr. Sci. 2017;55(2):182–196. doi: 10.1093/chromsci/bmw167. [DOI] [PubMed] [Google Scholar]
- 122.Li C., Qin S., Wen Y., Zhao W., Huang Y., Liu J. Overcoming the blood-brain barrier: exosomes as theranostic nanocarriers for precision neuroimaging. J. Contr. Release. 2022;349:902–916. doi: 10.1016/j.jconrel.2022.08.002. [DOI] [PubMed] [Google Scholar]
- 123.Van Der Pol E., Van Gemert M., Sturk A., Nieuwland R., Van Leeuwen T. Single vs. swarm detection of microparticles and exosomes by flow cytometry. J. Thromb. Haemostasis. 2012;10(5):919–930. doi: 10.1111/j.1538-7836.2012.04683.x. [DOI] [PubMed] [Google Scholar]
- 124.Brolo A.G. Plasmonics for future biosensors. Nat. Photonics. 2012;6(11):709–713. [Google Scholar]
- 125.Jeong S., Park J., Pathania D., Castro C.M., Weissleder R., Lee H. Integrated magneto–electrochemical sensor for exosome analysis. ACS Nano. 2016;10(2):1802–1809. doi: 10.1021/acsnano.5b07584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Shao H., Min C., Issadore D., Liong M., Yoon T.-J., Weissleder R., Lee H. Magnetic nanoparticles and microNMR for diagnostic applications. Theranostics. 2012;2(1):55. doi: 10.7150/thno.3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hasan M., Ahommed M., Daizy M., Bacchu M., Ali M., Al-Mamun M., Aly M.A.S., Khan M., Hossain S.I. Recent development in electrochemical biosensors for cancer biomarkers detection. Biosens. Bioelectron. X. 2021;8 [Google Scholar]
- 128.Lovén J., Orlando D.A., Sigova A.A., Lin C.Y., Rahl P.B., Burge C.B., Levens D.L., Lee T.I., Young R.A. Revisiting global gene expression analysis. Cell. 2012;151(3):476–482. doi: 10.1016/j.cell.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Guescini M., Genedani S., Stocchi V., Agnati L.F. Astrocytes and Glioblastoma cells release exosomes carrying mtDNA. J. Neural. Transm. 2010;117(1):1–4. doi: 10.1007/s00702-009-0288-8. [DOI] [PubMed] [Google Scholar]
- 130.Yang X.-X., Sun C., Wang L., Guo X.-L. New insight into isolation, identification techniques and medical applications of exosomes. J. Contr. Release. 2019;308:119–129. doi: 10.1016/j.jconrel.2019.07.021. [DOI] [PubMed] [Google Scholar]
- 131.DeRisi J., Penland L., Bittner M., Meltzer P., Ray M., Chen Y., Su Y., Trent J. Use of a cDNA microarray to analyse gene expression. Nat. Genet. 1996;14:457–460. doi: 10.1038/ng1296-457. [DOI] [PubMed] [Google Scholar]
- 132.Casamassimi A., Federico A., Rienzo M., Esposito S., Ciccodicola A. Transcriptome profiling in human diseases: new advances and perspectives. Int. J. Mol. Sci. 2017;18(8):1652. doi: 10.3390/ijms18081652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Black M.B., Parks B.B., Pluta L., Chu T.-M., Allen B.C., Wolfinger R.D., Thomas R.S. Comparison of microarrays and RNA-seq for gene expression analyses of dose-response experiments. Toxicol. Sci. 2014;137(2):385–403. doi: 10.1093/toxsci/kft249. [DOI] [PubMed] [Google Scholar]
- 134.Ferguson S., Yang K.S., Zelga P., Liss A.S., Carlson J.C., Del Castillo C.F., Weissleder R. Single-EV analysis (sEVA) of mutated proteins allows detection of stage 1 pancreatic cancer. Sci. Adv. 2022;8(16) doi: 10.1126/sciadv.abm3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lee K., Fraser K., Ghaddar B., Yang K., Kim E., Balaj L., Chiocca E.A., Breakefield X.O., Lee H., Weissleder R. Multiplexed profiling of single extracellular vesicles. ACS Nano. 2018;12(1):494–503. doi: 10.1021/acsnano.7b07060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Cho S., Yi J., Kwon Y., Kang H., Han C., Park J. Multifluorescence single extracellular vesicle analysis by time-sequential illumination and tracking. ACS Nano. 2021;15(7):11753–11761. doi: 10.1021/acsnano.1c02556. [DOI] [PubMed] [Google Scholar]
- 137.Deng F., Ratri A., Deighan C., Daaboul G., Geiger P.C., Christenson L.K. Single-particle interferometric reflectance imaging characterization of individual extracellular vesicles and population dynamics. JoVE. 2022;179 doi: 10.3791/62988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ortega-Sanchez F.G., Teresa V., Widmann T., Regiart M., Jerez-Salcedo M.T., Fernández-Baldo M.A., de Miguel-Perez D. Microfluidic systems in extracellular vesicles single analysis. A systematic review. TrAC, Trends Anal. Chem. 2023 [Google Scholar]
- 139.Liu H., Tian Y., Xue C., Niu Q., Chen C., Yan X. Analysis of extracellular vesicle DNA at the single‐vesicle level by nano‐flow cytometry. J. Extracell. Vesicles. 2022;11(4) doi: 10.1002/jev2.12206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wang T., Xing Y., Cheng Z., Yu F. Analysis of single extracellular vesicles for biomedical applications with especial emphasis on cancer investigations. TrAC, Trends Anal. Chem. 2022;152 [Google Scholar]
- 141.Liu C., Xu X., Li B., Situ B., Pan W., Hu Y., An T., Yao S., Zheng L. Single-exosome-counting immunoassays for cancer diagnostics. Nano Lett. 2018;18(7):4226–4232. doi: 10.1021/acs.nanolett.8b01184. [DOI] [PubMed] [Google Scholar]
- 142.Chiang C.-L., Cheng M.-H., Lin C.-H. From nanoparticles to cancer nanomedicine: old problems with new solutions. Nanomaterials. 2021;11(7):1727. doi: 10.3390/nano11071727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Fu S., Wang Y., Xia X., Zheng J.C. Exosome engineering: current progress in cargo loading and targeted delivery. NanoImpact. 2020;20 [Google Scholar]
- 144.Lener T., Gimona M., Aigner L., Börger V., Buzas E., Camussi G., Chaput N., Chatterjee D., Court F.A., Portillo H.A.d. Applying extracellular vesicles based therapeutics in clinical trials–an ISEV position paper. J. Extracell. Vesicles. 2015;4(1) doi: 10.3402/jev.v4.30087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mehryab F., Rabbani S., Shahhosseini S., Shekari F., Fatahi Y., Baharvand H., Haeri A. Exosomes as a next-generation drug delivery system: an update on drug loading approaches, characterization, and clinical application challenges. Acta Biomater. 2020;113:42–62. doi: 10.1016/j.actbio.2020.06.036. [DOI] [PubMed] [Google Scholar]
- 146.Liao W., Du Y., Zhang C., Pan F., Yao Y., Zhang T., Peng Q. Exosomes: the next generation of endogenous nanomaterials for advanced drug delivery and therapy. Acta Biomater. 2019;86:1–14. doi: 10.1016/j.actbio.2018.12.045. [DOI] [PubMed] [Google Scholar]
- 147.Silva A.K., Luciani N., Gazeau F., Aubertin K., Bonneau S., Chauvierre C., Letourneur D., Wilhelm C. Combining magnetic nanoparticles with cell derived microvesicles for drug loading and targeting. Nanomed. Nanotechnol. Biol. Med. 2015;11(3):645–655. doi: 10.1016/j.nano.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 148.Lara P., Palma-Florez S., Salas-Huenuleo E., Polakovicova I., Guerrero S., Lobos-Gonzalez L., Campos A., Muñoz L., Jorquera-Cordero C., Varas-Godoy M. Gold nanoparticle based double-labeling of melanoma extracellular vesicles to determine the specificity of uptake by cells and preferential accumulation in small metastatic lung tumors. J. Nanobiotechnol. 2020;18(1):1–17. doi: 10.1186/s12951-020-0573-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kanchanapally R., Deshmukh S.K., Chavva S.R., Tyagi N., Srivastava S.K., Patel G.K., Singh A.P., Singh S. Drug-loaded exosomal preparations from different cell types exhibit distinctive loading capability, yield, and antitumor efficacies: a comparative analysis. Int. J. Nanomed. 2019;14:531. doi: 10.2147/IJN.S191313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Jeong K., Yu Y.J., You J.Y., Rhee W.J., Kim J.A. Exosome-mediated microRNA-497 delivery for anti-cancer therapy in a microfluidic 3D lung cancer model. Lab Chip. 2020;20(3):548–557. doi: 10.1039/c9lc00958b. [DOI] [PubMed] [Google Scholar]
- 151.Ran N., Gao X., Dong X., Li J., Lin C., Geng M., Yin H. Effects of exosome-mediated delivery of myostatin propeptide on functional recovery of mdx mice. Biomaterials. 2020;236 doi: 10.1016/j.biomaterials.2020.119826. [DOI] [PubMed] [Google Scholar]
- 152.Kim G., Kim M., Lee Y., Byun J.W., Lee M. Systemic delivery of microRNA-21 antisense oligonucleotides to the brain using T7-peptide decorated exosomes. J. Contr. Release. 2020;317:273–281. doi: 10.1016/j.jconrel.2019.11.009. [DOI] [PubMed] [Google Scholar]
- 153.Li Y.-J., Wu J.-Y., Wang J.-M., Hu X.-B., Cai J.-X., Xiang D.-X. Gemcitabine loaded autologous exosomes for effective and safe chemotherapy of pancreatic cancer. Acta Biomater. 2020;101:519–530. doi: 10.1016/j.actbio.2019.10.022. [DOI] [PubMed] [Google Scholar]
- 154.Gong C., Tian J., Wang Z., Gao Y., Wu X., Ding X., Qiang L., Li G., Han Z., Yuan Y. Functional exosome-mediated co-delivery of doxorubicin and hydrophobically modified microRNA 159 for triple-negative breast cancer therapy. J. Nanobiotechnol. 2019;17(1):1–18. doi: 10.1186/s12951-019-0526-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Qi H., Liu C., Long L., Ren Y., Zhang S., Chang X., Qian X., Jia H., Zhao J., Sun J. Blood exosomes endowed with magnetic and targeting properties for cancer therapy. ACS Nano. 2016;10(3):3323–3333. doi: 10.1021/acsnano.5b06939. [DOI] [PubMed] [Google Scholar]
- 156.Yan C., Chen J., Wang C., Yuan M., Kang Y., Wu Z., Li W., Zhang G., Machens H.-G., Rinkevich Y. Milk exosomes-mediated miR-31-5p delivery accelerates diabetic wound healing through promoting angiogenesis. Drug Deliv. 2022;29(1):214–228. doi: 10.1080/10717544.2021.2023699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lv Q., Deng J., Chen Y., Wang Y., Liu B., Liu J. Engineered human adipose stem-cell-derived exosomes loaded with miR-21-5p to promote diabetic cutaneous wound healing. Mol. Pharm. 2020;17(5):1723–1733. doi: 10.1021/acs.molpharmaceut.0c00177. [DOI] [PubMed] [Google Scholar]
- 158.Salarpour S., Forootanfar H., Pournamdari M., Ahmadi-Zeidabadi M., Esmaeeli M., Pardakhty A. Paclitaxel incorporated exosomes derived from glioblastoma cells: comparative study of two loading techniques. Daru. 2019;27(2):533–539. doi: 10.1007/s40199-019-00280-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Haney M.J., Klyachko N.L., Zhao Y., Gupta R., Plotnikova E.G., He Z., Patel T., Piroyan A., Sokolsky M., Kabanov A.V. Exosomes as drug delivery vehicles for Parkinson's disease therapy. J. Contr. Release. 2015;207:18–30. doi: 10.1016/j.jconrel.2015.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Fuhrmann G., Serio A., Mazo M., Nair R., Stevens M.M. Active loading into extracellular vesicles significantly improves the cellular uptake and photodynamic effect of porphyrins. J. Contr. Release. 2015;205:35–44. doi: 10.1016/j.jconrel.2014.11.029. [DOI] [PubMed] [Google Scholar]
- 161.Wei H., Chen J., Wang S., Fu F., Zhu X., Wu C., Liu Z., Zhong G., Lin J. A nanodrug consisting of doxorubicin and exosome derived from mesenchymal stem cells for osteosarcoma treatment in vitro. Int. J. Nanomed. 2019:8603–8610. doi: 10.2147/IJN.S218988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Gao J., Wang S., Wang Z. High yield, scalable and remotely drug-loaded neutrophil-derived extracellular vesicles (EVs) for anti-inflammation therapy. Biomaterials. 2017;135:62–73. doi: 10.1016/j.biomaterials.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Thakur A., Sidu R.K., Zou H., Alam M.K., Yang M., Lee Y. Inhibition of glioma cells' proliferation by doxorubicin-loaded exosomes via microfluidics. Int. J. Nanomed. 2020:8331–8343. doi: 10.2147/IJN.S263956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Huang L., Wu E., Liao J., Wei Z., Wang J., Chen Z. Research advances of engineered exosomes as drug delivery carrier. ACS Omega. 2023;8(46):43374–43387. doi: 10.1021/acsomega.3c04479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Nie H., Xie X., Zhang D., Zhou Y., Li B., Li F., Li F., Cheng Y., Mei H., Meng H. Use of lung-specific exosomes for miRNA-126 delivery in non-small cell lung cancer. Nanoscale. 2020;12(2):877–887. doi: 10.1039/c9nr09011h. [DOI] [PubMed] [Google Scholar]
- 166.Reynolds J.L., Mahajan S.D. Transmigration of tetraspanin 2 (Tspan2) siRNA via microglia derived exosomes across the blood brain barrier modifies the production of immune mediators by microglia cells. J. Neuroimmune Pharmacol. 2020;15(3):554–563. doi: 10.1007/s11481-019-09895-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Sancho-Albero M., Rubio-Ruiz B., Pérez-López A.M., Sebastián V., Martín-Duque P., Arruebo M., Santamaría J., Unciti-Broceta A. Cancer-derived exosomes loaded with ultrathin palladium nanosheets for targeted bioorthogonal catalysis. Nat. Catal. 2019;2(10):864–872. doi: 10.1038/s41929-019-0333-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zhang D., Qin X., Wu T., Qiao Q., Song Q., Zhang Z. Extracellular vesicles based self-grown gold nanopopcorn for combinatorial chemo-photothermal therapy. Biomaterials. 2019;197:220–228. doi: 10.1016/j.biomaterials.2019.01.024. [DOI] [PubMed] [Google Scholar]
- 169.Tarasov V.V., Svistunov A.A., Chubarev V.N., Dostdar S.A., Sokolov A.V., Brzecka A., Sukocheva O., Neganova M.E., Klochkov S.G., Somasundaram S.G. Elsevier; 2021. Extracellular Vesicles in Cancer Nanomedicine, Seminars in Cancer Biology; pp. 212–225. [DOI] [PubMed] [Google Scholar]
- 170.Ohno S.-i., Takanashi M., Sudo K., Ueda S., Ishikawa A., Matsuyama N., Fujita K., Mizutani T., Ohgi T., Ochiya T. Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol. Ther. 2013;21(1):185–191. doi: 10.1038/mt.2012.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Elewaily M.I., Elsergany A.R. Emerging role of exosomes and exosomal microRNA in cancer: pathophysiology and clinical potential. J. Cancer Res. Clin. Oncol. 2021;147(3):637–648. doi: 10.1007/s00432-021-03534-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Faruqu F.N., Xu L., Al-Jamal K.T. Preparation of exosomes for siRNA delivery to cancer cells, JoVE. JoVE. 2018;(142) doi: 10.3791/58814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Aqil F., Munagala R., Jeyabalan J., Agrawal A.K., Gupta R. Exosomes for the enhanced tissue bioavailability and efficacy of curcumin. AAPS J. 2017;19(6):1691–1702. doi: 10.1208/s12248-017-0154-9. [DOI] [PubMed] [Google Scholar]
- 174.Li D.-f., Yang M.-f., Xu J., Xu H.-m., Zhu M.-z., Liang Y.-j., Zhang Y., Tian C.-m., Nie Y.-q., Shi R.-y. Extracellular vesicles: the next generation theranostic nanomedicine for inflammatory bowel disease. Int. J. Nanomed. 2022:3893–3911. doi: 10.2147/IJN.S370784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wei H., Chen J., Wang S., Fu F., Zhu X., Wu C., Liu Z., Zhong G., Lin J. A nanodrug consisting of doxorubicin and exosome derived from mesenchymal stem cells for osteosarcoma treatment in vitro. Int. J. Nanomed. 2019;14:8603. doi: 10.2147/IJN.S218988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Jeyaram A., Lamichhane T.N., Wang S., Zou L., Dahal E., Kronstadt S.M., Levy D., Parajuli B., Knudsen D.R., Chao W. Enhanced loading of functional miRNA cargo via pH gradient modification of extracellular vesicles. Mol. Ther. 2020;28(3):975–985. doi: 10.1016/j.ymthe.2019.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Li M., Wang Q., Zhang X., Yan N., Li X. Exosomal miR-126 blocks the development of non-small cell lung cancer through the inhibition of ITGA6. Cancer Cell Int. 2020;20(1):1–11. doi: 10.1186/s12935-020-01653-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ansari M.A., Thiruvengadam M., Venkidasamy B., Alomary M.N., Alamoudi A.J., Chung I.-M., Shariati M.A., Rebezov M. Exosome-based nanomedicine for cancer treatment by targeting inflammatory pathways: current status and future perspectives. Semin. Cancer Biol. 2022;856(Pt 2):678–696. doi: 10.1016/j.semcancer.2022.04.005. [DOI] [PubMed] [Google Scholar]
- 179.Abudoureyimu M., Zhou H., Zhi Y., Wang T., Feng B., Wang R., Chu X. Recent progress in the emerging role of exosome in hepatocellular carcinoma. Cell Prolif. 2019;52(2) doi: 10.1111/cpr.12541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Lin J., Li J., Huang B., Liu J., Chen X., Chen X.-M., Xu Y.-M., Huang L.-F., Wang X.-Z. Exosomes: novel biomarkers for clinical diagnosis. Sci. World J. 2015;2015 doi: 10.1155/2015/657086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Wei W., Ao Q., Wang X., Cao Y., Liu Y., Zheng S.G., Tian X. Mesenchymal stem cell–derived exosomes: a promising biological tool in nanomedicine. Front. Pharmacol. 2021;11 doi: 10.3389/fphar.2020.590470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Syn N.L., Wang L., Chow E.K.-H., Lim C.T., Goh B.-C. Exosomes in cancer nanomedicine and immunotherapy: prospects and challenges. Trends Biotechnol. 2017;35(7):665–676. doi: 10.1016/j.tibtech.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 183.Kim J., Zhu Y., Chen S., Wang D., Zhang S., Xia J., Li S., Qiu Q., Lee H., Wang J. Anti-glioma effect of ginseng-derived exosomes-like nanoparticles by active blood–brain-barrier penetration and tumor microenvironment modulation. J. Nanobiotechnol. 2023;21(1):1–19. doi: 10.1186/s12951-023-02006-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Zhou Y., Wang L., Chen L., Wu W., Yang Z., Wang Y., Wang A., Jiang S., Qin X., Ye Z. Glioblastoma cell-derived exosomes functionalized with peptides as efficient nanocarriers for synergistic chemotherapy of glioblastoma with improved biosafety. Nano Res. 2023:1–11. [Google Scholar]
- 185.Qi Y., Jin C., Qiu W., Zhao R., Wang S., Li B., Zhang Z., Guo Q., Zhang S., Gao Z. The dual role of glioma exosomal microRNAs: glioma eliminates tumor suppressor miR-1298-5p via exosomes to promote immunosuppressive effects of MDSCs. Cell Death Dis. 2022;13(5):426. doi: 10.1038/s41419-022-04872-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Liang S., Xu H., Ye B.-C. Membrane-decorated exosomes for combination drug delivery and improved glioma therapy. Langmuir. 2021;38(1):299–308. doi: 10.1021/acs.langmuir.1c02500. [DOI] [PubMed] [Google Scholar]
- 187.Wu J.-Y., Li Y.-J., Hu X.-B., Huang S., Luo S., Tang T., Xiang D.-X. Exosomes and biomimetic nanovesicles-mediated anti-glioblastoma therapy: a head-to-head comparison. J. Contr. Release. 2021;336:510–521. doi: 10.1016/j.jconrel.2021.07.004. [DOI] [PubMed] [Google Scholar]
- 188.Yu Y., Li W., Mao L., Peng W., Long D., Li D., Zhou R., Dang X. Genetically engineered exosomes display RVG peptide and selectively enrich a neprilysin variant: a potential formulation for the treatment of Alzheimer's disease. J. Drug Target. 2021;29(10):1128–1138. doi: 10.1080/1061186X.2021.1929257. [DOI] [PubMed] [Google Scholar]
- 189.Qi Y., Guo L., Jiang Y., Shi Y., Sui H., Zhao L. Brain delivery of quercetin-loaded exosomes improved cognitive function in AD mice by inhibiting phosphorylated tau-mediated neurofibrillary tangles. Drug Deliv. 2020;27(1):745–755. doi: 10.1080/10717544.2020.1762262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Ren X., Zhao Y., Xue F., Zheng Y., Huang H., Wang W., Chang Y., Yang H., Zhang J. Exosomal DNA aptamer targeting α-synuclein aggregates reduced neuropathological deficits in a mouse Parkinson's disease model. Mol. Ther. Nucleic Acids. 2019;17:726–740. doi: 10.1016/j.omtn.2019.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Kojima R., Bojar D., Rizzi G., Hamri G.C.-E., El-Baba M.D., Saxena P., Ausländer S., Tan K.R., Fussenegger M. Designer exosomes produced by implanted cells intracerebrally deliver therapeutic cargo for Parkinson's disease treatment. Nat. Commun. 2018;9(1):1–10. doi: 10.1038/s41467-018-03733-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Didiot M.-C., Hall L.M., Coles A.H., Haraszti R.A., Godinho B.M., Chase K., Sapp E., Ly S., Alterman J.F., Hassler M.R. Exosome-mediated delivery of hydrophobically modified siRNA for huntingtin mRNA silencing. Mol. Ther. 2016;24(10):1836–1847. doi: 10.1038/mt.2016.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Lee S.-T., Im W., Ban J.-J., Lee M., Jung K.-H., Lee S.K., Chu K., Kim M. Exosome-based delivery of miR-124 in a Huntington's disease model. Journal of movement disorders. 2017;10(1):45. doi: 10.14802/jmd.16054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Li H., Ding Y., Huang J., Zhao Y., Chen W., Tang Q., An Y., Chen R., Hu C. Angiopep-2 modified exosomes load rifampicin with potential for treating central nervous system tuberculosis. Int. J. Nanomed. 2023:489–503. doi: 10.2147/IJN.S395246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Lai N., Wu D., Liang T., Pan P., Yuan G., Li X., Li H., Shen H., Wang Z., Chen G. Systemic exosomal miR-193b-3p delivery attenuates neuroinflammation in early brain injury after subarachnoid hemorrhage in mice. J. Neuroinflammation. 2020;17(1):1–13. doi: 10.1186/s12974-020-01745-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Wu T., Tian Q., Liu R., Xu K., Shi S., Zhang X., Gao L., Yin X., Xu S., Wang P. Inhibitory role of bone marrow mesenchymal stem cells‐derived exosome in non‐small‐cell lung cancer: microRNA‐30b‐5p, EZH2 and PI3K/AKT pathway. J. Cell Mol. Med. 2023;27(22):3526–3538. doi: 10.1111/jcmm.17933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Kim M.S., Haney M.J., Zhao Y., Yuan D., Deygen I., Klyachko N.L., Kabanov A.V., Batrakova E.V. Engineering macrophage-derived exosomes for targeted paclitaxel delivery to pulmonary metastases: in vitro and in vivo evaluations. Nanomed. Nanotechnol. Biol. Med. 2018;14(1):195–204. doi: 10.1016/j.nano.2017.09.011. [DOI] [PubMed] [Google Scholar]
- 198.Aqil F., Munagala R., Jeyabalan J., Agrawal A.K., Kyakulaga A.-H., Wilcher S.A., Gupta R.C. Milk exosomes-Natural nanoparticles for siRNA delivery. Cancer Lett. 2019;449:186–195. doi: 10.1016/j.canlet.2019.02.011. [DOI] [PubMed] [Google Scholar]
- 199.Shen K., Wang X., Wang Y., Jia Y., Zhang Y., Wang K., Luo L., Cai W., Li J., Li S. miR-125b-5p in adipose derived stem cells exosome alleviates pulmonary microvascular endothelial cells ferroptosis via Keap1/Nrf2/GPX4 in sepsis lung injury. Redox Biol. 2023;62 doi: 10.1016/j.redox.2023.102655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Jiang K., Yang J., Guo S., Zhao G., Wu H., Deng G. Peripheral circulating exosome-mediated delivery of miR-155 as a novel mechanism for acute lung inflammation. Mol. Ther. 2019;27(10):1758–1771. doi: 10.1016/j.ymthe.2019.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Pei W., Li X., Bi R., Zhang X., Zhong M., Yang H., Zhang Y., Lv K. Exosome membrane-modified M2 macrophages targeted nanomedicine: treatment for allergic asthma. J. Contr. Release. 2021;338:253–267. doi: 10.1016/j.jconrel.2021.08.024. [DOI] [PubMed] [Google Scholar]
- 202.Sun L., Fan M., Huang D., Li B., Xu R., Gao F., Chen Y. Clodronate-loaded liposomal and fibroblast-derived exosomal hybrid system for enhanced drug delivery to pulmonary fibrosis. Biomaterials. 2021;271 doi: 10.1016/j.biomaterials.2021.120761. [DOI] [PubMed] [Google Scholar]
- 203.Lin Q., Zhou C.-R., Bai M.-J., Zhu D., Chen J.-W., Wang H.-F., Li M.-A., Wu C., Li Z.-R., Huang M.-S. Exosome-mediated miRNA delivery promotes liver cancer EMT and metastasis. Am. J. Tourism Res. 2020;12(3):1080. [PMC free article] [PubMed] [Google Scholar]
- 204.Zhang Z., Li X., Sun W., Yue S., Yang J., Li J., Ma B., Wang J., Yang X., Pu M. Loss of exosomal miR-320a from cancer-associated fibroblasts contributes to HCC proliferation and metastasis. Cancer Lett. 2017;397:33–42. doi: 10.1016/j.canlet.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 205.Sugimachi K., Matsumura T., Hirata H., Uchi R., Ueda M., Ueo H., Shinden Y., Iguchi T., Eguchi H., Shirabe K. Identification of a bona fide microRNA biomarker in serum exosomes that predicts hepatocellular carcinoma recurrence after liver transplantation. Br. J. Cancer. 2015;112(3):532–538. doi: 10.1038/bjc.2014.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Lou G., Song X., Yang F., Wu S., Wang J., Chen Z., Liu Y. Exosomes derived from miR-122-modified adipose tissue-derived MSCs increase chemosensitivity of hepatocellular carcinoma. J. Hematol. Oncol. 2015;8(1):1–11. doi: 10.1186/s13045-015-0220-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Momen-Heravi F., Bala S., Kodys K., Szabo G. Exosomes derived from alcohol-treated hepatocytes horizontally transfer liver specific miRNA-122 and sensitize monocytes to LPS. Sci. Rep. 2015;5(1):1–16. doi: 10.1038/srep09991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Yu L., Xue J., Wu Y., Zhou H. Therapeutic effect of exosomes derived from hepatocyte-growth-factor-overexpressing adipose mesenchymal stem cells on liver injury. Folia Histochem. Cytobiol. 2023 doi: 10.5603/fhc.95291. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 209.Ma J., Li Y., Chen M., Wang W., Zhao Q., He B., Zhang M., Jiang Y. hMSCs-derived exosome circCDK13 inhibits liver fibrosis by regulating the expression of MFGE8 through miR-17-5p/KAT2B. Cell Biol. Toxicol. 2023;39(2):1–22. doi: 10.1007/s10565-022-09714-4. [DOI] [PubMed] [Google Scholar]
- 210.Wan T., Zhong J., Pan Q., Zhou T., Ping Y., Liu X. Exosome-mediated delivery of Cas9 ribonucleoprotein complexes for tissue-specific gene therapy of liver diseases. Sci. Adv. 2022;8(37) doi: 10.1126/sciadv.abp9435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Chen C.C., Liu L., Ma F., Wong C.W., Guo X.E., Chacko J.V., Farhoodi H.P., Zhang S.X., Zimak J., Ségaliny A. Elucidation of exosome migration across the blood–brain barrier model in vitro. Cell. Mol. Bioeng. 2016;9(4):509–529. doi: 10.1007/s12195-016-0458-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Jarmalavičiūtė A., Pivoriūnas A. Exosomes as a potential novel therapeutic tools against neurodegenerative diseases. Pharmacol. Res. 2016;113:816–822. doi: 10.1016/j.phrs.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 213.Smalheiser N.R. Exosomal transfer of proteins and RNAs at synapses in the nervous system. Biol. Direct. 2007;2(1):1–15. doi: 10.1186/1745-6150-2-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Frühbeis C., Kuo-Elsner W.P., Müller C., Barth K., Peris L., Tenzer S., Möbius W., Werner H.B., Nave K.-A., Fröhlich D. Oligodendrocytes support axonal transport and maintenance via exosome secretion. PLoS Biol. 2020;18(12) doi: 10.1371/journal.pbio.3000621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Staff N.P., Jones D.T., Singer W. Mayo Clinic Proceedings, Elsevier; 2019. Mesenchymal Stromal Cell Therapies for Neurodegenerative Diseases; pp. 892–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Salarpour S., Barani M., Pardakhty A., Khatami M., Chauhan N.P.S. The application of exosomes and exosome-nanoparticle in treating brain disorders. J. Mol. Liq. 2022 [Google Scholar]
- 217.Cui J., Wang X., Li J., Zhu A., Du Y., Zeng W., Guo Y., Di L., Wang R. Immune exosomes loading self-assembled nanomicelles traverse the blood–brain barrier for chemo-immunotherapy against glioblastoma. ACS Nano. 2023;17(2):1464–1484. doi: 10.1021/acsnano.2c10219. [DOI] [PubMed] [Google Scholar]
- 218.Li Y., Yin Z., Fan J., Zhang S., Yang W. The roles of exosomal miRNAs and lncRNAs in lung diseases. Signal Transduct. Targeted Ther. 2019;4(1):1–12. doi: 10.1038/s41392-019-0080-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics. CA Cancer J Clin. 2022;72(1):7–33. doi: 10.3322/caac.21708. 2022. [DOI] [PubMed] [Google Scholar]
- 220.Bach D.H., Hong J.Y., Park H.J., Lee S.K. The role of exosomes and miRNAs in drug‐resistance of cancer cells. Int. J. Cancer. 2017;141(2):220–230. doi: 10.1002/ijc.30669. [DOI] [PubMed] [Google Scholar]
- 221.Zheng Y., Liu L., Chen C., Ming P., Huang Q., Li C., Cao D., Xu X., Ge W. The extracellular vesicles secreted by lung cancer cells in radiation therapy promote endothelial cell angiogenesis by transferring miR-23a. PeerJ. 2017;5:e3627. doi: 10.7717/peerj.3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Zhu J.Q., Wu H., Li Z.L., Xu X.F., Xing H., Wang M.D., Jia H.D., Liang L., Li C., Sun L.Y. Responsive hydrogels based on triggered click reactions for liver cancer. Adv. Mater. 2022;34(38) doi: 10.1002/adma.202201651. [DOI] [PubMed] [Google Scholar]
- 223.Sato K., Meng F., Glaser S., Alpini G. Exosomes in liver pathology. J. Hepatol. 2016;65(1):213–221. doi: 10.1016/j.jhep.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Aravalli R.N., Steer C.J. Gene editing technology as an approach to the treatment of liver diseases. Expet Opin. Biol. Ther. 2016;16(5):595–608. doi: 10.1517/14712598.2016.1158808. [DOI] [PubMed] [Google Scholar]
- 225.Doudna J.A. The promise and challenge of therapeutic genome editing. Nature. 2020;578(7794):229–236. doi: 10.1038/s41586-020-1978-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Wang H., Lu Z., Zhao X. Tumorigenesis, diagnosis, and therapeutic potential of exosomes in liver cancer. J. Hematol. Oncol. 2019;12(1):1–21. doi: 10.1186/s13045-019-0806-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Kouwaki T., Fukushima Y., Daito T., Sanada T., Yamamoto N., Mifsud E.J., Leong C.R., Tsukiyama-Kohara K., Kohara M., Matsumoto M. Extracellular vesicles including exosomes regulate innate immune responses to hepatitis B virus infection. Front. Immunol. 2016;7:335. doi: 10.3389/fimmu.2016.00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Enomoto Y., Takagi R., Naito Y., Kiniwa T., Tanaka Y., Hamada-Tsutsumi S., Kawano M., Matsushita S., Ochiya T., Miyajima A. Identification of the novel 3′ UTR sequences of human IL-21 mRNA as potential targets of miRNAs. Sci. Rep. 2017;7(1):7780. doi: 10.1038/s41598-017-07853-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Johnson-Arbor K., Dubey R. StatPearls Publishing; 2022. Doxorubicin, StatPearls. [Internet] [PubMed] [Google Scholar]
- 230.Wei H., Chen F., Chen J., Lin H., Wang S., Wang Y., Wu C., Lin J., Zhong G. Mesenchymal stem cell derived exosomes as nanodrug carrier of doxorubicin for targeted osteosarcoma therapy via SDF1-CXCR4 Axis. Int. J. Nanomed. 2022:3483–3495. doi: 10.2147/IJN.S372851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Jiang M., Jike Y., Liu K., Gan F., Zhang K., Xie M., Zhang J., Chen C., Zou X., Jiang X. Exosome-mediated miR-144-3p promotes ferroptosis to inhibit osteosarcoma proliferation, migration, and invasion through regulating ZEB1. Mol. Cancer. 2023;22(1):113. doi: 10.1186/s12943-023-01804-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Jang S.C., Kim O.Y., Yoon C.M., Choi D.-S., Roh T.-Y., Park J., Nilsson J., Lotvall J., Kim Y.-K., Gho Y.S. Bioinspired exosome-mimetic nanovesicles for targeted delivery of chemotherapeutics to malignant tumors. ACS Nano. 2013;7(9):7698–7710. doi: 10.1021/nn402232g. [DOI] [PubMed] [Google Scholar]
- 233.Shekh R., Ahmad A., Tiwari R.K., Saeed M., Shukla R., Al‐Thubiani W.S., Ansari I.A., Ashfaque M., Bajpai P. High therapeutic efficacy of 5‐Fluorouracil‐loaded exosomes against colon cancer cells. Chem. Biol. Drug Des. 2023;101(4):962–976. doi: 10.1111/cbdd.14205. [DOI] [PubMed] [Google Scholar]
- 234.Li Q., Song Q., Zhao Z., Lin Y., Cheng Y., Karin N., Luan Y. Genetically engineered artificial exosome-constructed hydrogel for ovarian cancer therapy. ACS Nano. 2023;17(11):10376–10392. doi: 10.1021/acsnano.3c00804. [DOI] [PubMed] [Google Scholar]
- 235.Gong X., Chi H., Strohmer D.F., Teichmann A.T., Xia Z., Wang Q. Exosomes: a potential tool for immunotherapy of ovarian cancer. Front. Immunol. 2023;13 doi: 10.3389/fimmu.2022.1089410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Shafei A., El-Bakly W., Sobhy A., Wagdy O., Reda A., Aboelenin O., Marzouk A., El Habak K., Mostafa R., Ali M.A. A review on the efficacy and toxicity of different doxorubicin nanoparticles for targeted therapy in metastatic breast cancer. Biomed. Pharmacother. 2017;95:1209–1218. doi: 10.1016/j.biopha.2017.09.059. [DOI] [PubMed] [Google Scholar]
- 237.Yang Y., Chen Y., Zhang F., Zhao Q., Zhong H. Increased anti-tumour activity by exosomes derived from doxorubicin-treated tumour cells via heat stress. Int. J. Hyperther. 2015;31(5):498–506. doi: 10.3109/02656736.2015.1036384. [DOI] [PubMed] [Google Scholar]
- 238.Chen G., Jiang D., Ding S., Huang C., Zhu D., Jiang H. A tumor cell exosome-mimicking multifunctional nanozyme for targeted breast cancer radiotherapy. Nanoscale. 2023;15(36):14949–14957. doi: 10.1039/d3nr03065b. [DOI] [PubMed] [Google Scholar]
- 239.El-Tanani M., Nsairat H., Matalka I.I., Aljabali A.A., Mishra V., Mishra Y., Naikoo G.A., Chava S.R., Charbe N.B., Tambuwala M.M. Impact of exosome therapy on pancreatic cancer and its progression. Med. Oncol. 2023;40(8):225. doi: 10.1007/s12032-023-02101-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Zhang S., Li D., Liu Y., Qin C., Tong L., Xu L. Multifunctional exosome-driven pancreatic cancer diagnostics and therapeutics. Extracellular Vesicle. 2023;2 [Google Scholar]
- 241.Hadla M., Palazzolo S., Corona G., Caligiuri I., Canzonieri V., Toffoli G., Rizzolio F. Exosomes increase the therapeutic index of doxorubicin in breast and ovarian cancer mouse models. Nanomedicine. 2016;11(18):2431–2441. doi: 10.2217/nnm-2016-0154. [DOI] [PubMed] [Google Scholar]
- 242.Saari H., Lázaro-Ibáñez E., Viitala T., Vuorimaa-Laukkanen E., Siljander P., Yliperttula M. Microvesicle-and exosome-mediated drug delivery enhances the cytotoxicity of Paclitaxel in autologous prostate cancer cells. J. Contr. Release. 2015;220:727–737. doi: 10.1016/j.jconrel.2015.09.031. [DOI] [PubMed] [Google Scholar]
- 243.Pascucci L., Coccè V., Bonomi A., Ami D., Ceccarelli P., Ciusani E., Viganò L., Locatelli A., Sisto F., Doglia S.M. Paclitaxel is incorporated by mesenchymal stromal cells and released in exosomes that inhibit in vitro tumor growth: a new approach for drug delivery. J. Contr. Release. 2014;192:262–270. doi: 10.1016/j.jconrel.2014.07.042. [DOI] [PubMed] [Google Scholar]
- 244.Sun D., Zhuang X., Xiang X., Liu Y., Zhang S., Liu C., Barnes S., Grizzle W., Miller D., Zhang H.-G. A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol. Ther. 2010;18(9):1606–1614. doi: 10.1038/mt.2010.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Zhang X., Liu L., Tang M., Li H., Guo X., Yang X. The effects of umbilical cord-derived macrophage exosomes loaded with cisplatin on the growth and drug resistance of ovarian cancer cells. Drug Dev. Ind. Pharm. 2020;46(7):1150–1162. doi: 10.1080/03639045.2020.1776320. [DOI] [PubMed] [Google Scholar]
- 246.Munagala R., Aqil F., Jeyabalan J., Agrawal A.K., Mudd A.M., Kyakulaga A.H., Singh I.P., Vadhanam M.V., Gupta R.C. Exosomal formulation of anthocyanidins against multiple cancer types. Cancer Lett. 2017;393:94–102. doi: 10.1016/j.canlet.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Liang Y., Duan L., Lu J., Xia J. Engineering exosomes for targeted drug delivery. Theranostics. 2021;11(7):3183. doi: 10.7150/thno.52570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Xu Z., Zeng S., Gong Z., Yan Y. Exosome-based immunotherapy: a promising approach for cancer treatment. Mol. Cancer. 2020;19(1):1–16. doi: 10.1186/s12943-020-01278-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Kennedy L.B., Salama A.K. A review of cancer immunotherapy toxicity. CA A Cancer J. Clin. 2020;70(2):86–104. doi: 10.3322/caac.21596. [DOI] [PubMed] [Google Scholar]
- 250.Borst J., Ahrends T., Bąbała N., Melief C.J., Kastenmüller W. CD4+ T cell help in cancer immunology and immunotherapy. Nat. Rev. Immunol. 2018;18(10):635–647. doi: 10.1038/s41577-018-0044-0. [DOI] [PubMed] [Google Scholar]
- 251.Wang X., Xia J., Yang L., Dai J., He L. Recent progress in exosome research: isolation, characterization and clinical applications. Cancer Gene Ther. 2023:1–15. doi: 10.1038/s41417-023-00617-y. [DOI] [PubMed] [Google Scholar]
- 252.Wolfers J., Lozier A., Raposo G., Regnault A., Théry C., Masurier C., Flament C., Pouzieux S., Faure F., Tursz T. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat. Med. 2001;7(3):297–303. doi: 10.1038/85438. [DOI] [PubMed] [Google Scholar]
- 253.Zitvogel L., Regnault A., Lozier A., Wolfers J., Flament C., Tenza D., Ricciardi-Castagnoli P., Raposo G., Amigorena S. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell derived exosomes. Nat. Med. 1998;4(5):594–600. doi: 10.1038/nm0598-594. [DOI] [PubMed] [Google Scholar]
- 254.Wang C., Huang X., Wu Y., Wang J., Li F., Guo G. Tumor cell-associated exosomes robustly elicit anti-tumor immune responses through modulating dendritic cell vaccines in lung tumor. Int. J. Biol. Sci. 2020;16(4):633. doi: 10.7150/ijbs.38414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Wang Y., Xiang Y., Xin V.W., Wang X.-W., Peng X.-C., Liu X.-Q., Wang D., Li N., Cheng J.-T., Lyv Y.-N. Dendritic cell biology and its role in tumor immunotherapy. J. Hematol. Oncol. 2020;13(1):1–18. doi: 10.1186/s13045-020-00939-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Huang F., Wan J., Hu W., Hao S. Enhancement of anti-leukemia immunity by leukemia–derived exosomes via downregulation of TGF-β1 expression. Cell. Physiol. Biochem. 2017;44(1):240–254. doi: 10.1159/000484677. [DOI] [PubMed] [Google Scholar]
- 257.Zhao Y., Liu L., Sun R., Cui G., Guo S., Han S., Li Z., Bai T., Teng L. Asian Journal of Pharmaceutical Sciences; 2022. Exosomes in Cancer Immunoediting and Immunotherapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Melief C.J., van Hall T., Arens R., Ossendorp F., van der Burg S.H. Therapeutic cancer vaccines. J. Clin. Invest. 2015;125(9):3401–3412. doi: 10.1172/JCI80009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Meng S., Whitt A.G., Stamp B.F., Eaton J.W., Li C., Yaddanapudi K. Exosome-based cancer vaccine for prevention of lung cancer. Stem Cell Invest. 2023;10 doi: 10.21037/sci-2022-030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Santos P., Almeida F. Exosome-based vaccines: history, current state, and clinical trials. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.711565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Sarvizadeh M., Ghasemi F., Tavakoli F., Sadat Khatami S., Razi E., Sharifi H., Biouki N.M., Taghizadeh M. Vaccines for colorectal cancer: an update. J. Cell. Biochem. 2019;120(6):8815–8828. doi: 10.1002/jcb.28179. [DOI] [PubMed] [Google Scholar]
- 262.Cho J.-a., Lee Y.-S., Kim S.-H., Ko J.-K., Kim C.-W. MHC independent anti-tumor immune responses induced by Hsp70-enriched exosomes generate tumor regression in murine models. Cancer Lett. 2009;275(2):256–265. doi: 10.1016/j.canlet.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 263.Hu S., Ma J., Su C., Chen Y., Shu Y., Qi Z., Zhang B., Shi G., Zhang Y., Zhang Y. Engineered exosome-like nanovesicles suppress tumor growth by reprogramming tumor microenvironment and promoting tumor ferroptosis. Acta Biomater. 2021;135:567–581. doi: 10.1016/j.actbio.2021.09.003. [DOI] [PubMed] [Google Scholar]
- 264.Lee J., Lee J.-H., Chakraborty K., Hwang J., Lee Y.-K. Exosome-based drug delivery systems and their therapeutic applications. RSC Adv. 2022;12(29):18475–18492. doi: 10.1039/d2ra02351b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Abdelsalam M., Ahmed M., Osaid Z., Hamoudi R., Harati R. Insights into exosome transport through the blood–brain barrier and the potential therapeutical applications in brain diseases. Pharmaceuticals. 2023;16(4):571. doi: 10.3390/ph16040571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Fang Z., Ding Y., Xue Z., Li P., Li J., Li F. Roles of exosomes as drug delivery systems in cancer immunotherapy: a mini-review. Discover Oncology. 2022;13(1):1–17. doi: 10.1007/s12672-022-00539-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Jang Y., Kim H., Yoon S., Lee H., Hwang J., Jung J., Chang J.H., Choi J., Kim H. Exosome-based photoacoustic imaging guided photodynamic and immunotherapy for the treatment of pancreatic cancer. J. Contr. Release. 2021;330:293–304. doi: 10.1016/j.jconrel.2020.12.039. [DOI] [PubMed] [Google Scholar]
- 268.Shi X., Cheng Q., Hou T., Han M., Smbatyan G., Lang J.E., Epstein A.L., Lenz H.-J., Zhang Y. Genetically engineered cell-derived nanoparticles for targeted breast cancer immunotherapy. Mol. Ther. 2020;28(2):536–547. doi: 10.1016/j.ymthe.2019.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.He G., Peng X., Wei S., Yang S., Li X., Huang M., Tang S., Jin H., Liu J., Zhang S. Exosomes in the hypoxic TME: from release, uptake and biofunctions to clinical applications. Mol. Cancer. 2022;21(1):1–22. doi: 10.1186/s12943-021-01440-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Hu C., Chen M., Jiang R., Guo Y., Wu M., Zhang X. Exosome-related tumor microenvironment. J. Cancer. 2018;9(17):3084. doi: 10.7150/jca.26422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Luo H., Zhang H., Mao J., Cao H., Tao Y., Zhao G., Zhang Z., Zhang N., Liu Z., Zhang J. Exosome-based nanoimmunotherapy targeting TAMs, a promising strategy for glioma. Cell Death Dis. 2023;14(4):235. doi: 10.1038/s41419-023-05753-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Olejarz W., Kubiak-Tomaszewska G., Chrzanowska A., Lorenc T. Exosomes in angiogenesis and anti-angiogenic therapy in cancers. Int. J. Mol. Sci. 2020;21(16):5840. doi: 10.3390/ijms21165840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Henry N.L., Hayes D.F. Cancer biomarkers. Mol. Oncol. 2012;6(2):140–146. doi: 10.1016/j.molonc.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Hartwell L., Mankoff D., Paulovich A., Ramsey S., Swisher E. Cancer biomarkers: a systems approach. Nat. Biotechnol. 2006;24(8):905–908. doi: 10.1038/nbt0806-905. [DOI] [PubMed] [Google Scholar]
- 275.Kogure T., Lin W.L., Yan I.K., Braconi C., Patel T. Intercellular nanovesicle‐mediated microRNA transfer: a mechanism of environmental modulation of hepatocellular cancer cell growth. Hepatology. 2011;54(4):1237–1248. doi: 10.1002/hep.24504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Li X., Corbett A.L., Taatizadeh E., Tasnim N., Little J.P., Garnis C., Daugaard M., Guns E., Hoorfar M., Li I.T. Challenges and opportunities in exosome research—perspectives from biology, engineering, and cancer therapy. APL Bioeng. 2019;3(1) doi: 10.1063/1.5087122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Zhou M., Weber S.R., Zhao Y., Chen H., Sundstrom J.M. Exosomes; 2020. Methods for Exosome Isolation and Characterization; pp. 23–38. [Google Scholar]
- 278.Lorencova L., Bertok T., Bertokova A., Gajdosova V., Hroncekova S., Vikartovska A., Kasak P., Tkac J. Exosomes as a source of cancer biomarkers: advances in electrochemical biosensing of exosomes. Chemelectrochem. 2020;7(9):1956–1973. [Google Scholar]
- 279.Mohan P.B., Rajpurohit S., Musunuri B., Bhat G., Lochan R., Shetty S. Exosomes in chronic liver disease. Clin. Chim. Acta. 2023 doi: 10.1016/j.cca.2022.117215. [DOI] [PubMed] [Google Scholar]
- 280.Li Z., Ye L., Wang L., Quan R., Zhou Y., Li X. Identification of miRNA signatures in serum exosomes as a potential biomarker after radiotherapy treatment in glioma patients. Ann. Diagn. Pathol. 2020;44 doi: 10.1016/j.anndiagpath.2019.151436. [DOI] [PubMed] [Google Scholar]
- 281.Shao Y., Tao X., Lu R., Zhang H., Ge J., Xiao B., Ye G., Guo J. Hsa_circ_0065149 is an indicator for early gastric cancer screening and prognosis prediction. Pathol. Oncol. Res. 2020;26:1475–1482. doi: 10.1007/s12253-019-00716-y. [DOI] [PubMed] [Google Scholar]
- 282.Yuan D., Chen Y., Li X., Li J., Zhao Y., Shen J., Du F., Kaboli P.J., Li M., Wu X. Long non-coding RNAs: potential biomarkers and targets for hepatocellular carcinoma therapy and diagnosis. Int. J. Biol. Sci. 2021;17(1):220. doi: 10.7150/ijbs.50730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Yang Q., Liang Y., Shi Y., Shang J., Huang X. The ALKBH5/SOX4 axis promotes liver cancer stem cell properties via activating the SHH signaling pathway. J. Cancer Res. Clin. Oncol. 2023:1–12. doi: 10.1007/s00432-023-05309-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Melo S.A., Luecke L.B., Kahlert C., Fernandez A.F., Gammon S.T., Kaye J., LeBleu V.S., Mittendorf E.A., Weitz J., Rahbari N. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature. 2015;523(7559):177–182. doi: 10.1038/nature14581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Shen Y., Li M., Liao L., Gao S., Wang Y. Plasma exosome-derived connexin43 as a promising biomarker for melanoma patients. BMC Cancer. 2023;23(1):242. doi: 10.1186/s12885-023-10705-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Shin S., Park Y.H., Jung S.-H., Jang S.-H., Kim M.Y., Lee J.Y., Chung Y.J. Urinary exosome microRNA signatures as a noninvasive prognostic biomarker for prostate cancer. NPJ Genomic Medicine. 2021;6(1):45. doi: 10.1038/s41525-021-00212-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Chen Z., Liang Q., Zeng H., Zhao Q., Guo Z., Zhong R., Xie M., Cai X., Su J., He Z. Exosomal CA125 as a promising biomarker for ovarian cancer diagnosis. J. Cancer. 2020;11(21):6445. doi: 10.7150/jca.48531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Duijvesz D., Luider T., Bangma C.H., Jenster G. Exosomes as biomarker treasure chests for prostate cancer. Eur. Urol. 2011;59(5):823–831. doi: 10.1016/j.eururo.2010.12.031. [DOI] [PubMed] [Google Scholar]
- 289.Jia L., Zhu M., Kong C., Pang Y., Zhang H., Qiu Q., Wei C., Tang Y., Wang Q., Li Y. Blood neuro‐exosomal synaptic proteins predict Alzheimer's disease at the asymptomatic stage. Alzheimer's Dementia. 2021;17(1):49–60. doi: 10.1002/alz.12166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Zhang W., Zhang J., Shi H., Liu F., Yu H., Shi H. Exosome GLUT1 derived from hepatocyte identifies the risk of non-alcoholic steatohepatitis and fibrosis. Hepatology International. 2023:1–12. doi: 10.1007/s12072-023-10520-1. [DOI] [PubMed] [Google Scholar]
- 291.Lee J.H., Shim Y.R., Seo W., Kim M.H., Choi W.M., Kim H.H., Kim Y.E., Yang K., Ryu T., Jeong J.M. Mitochondrial double‐stranded RNA in exosome promotes Interleukin‐17 production through toll‐like receptor 3 in alcohol‐associated liver injury. Hepatology. 2020;72(2):609–625. doi: 10.1002/hep.31041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Bala S., Babuta M., Catalano D., Saiju A., Szabo G. Alcohol promotes exosome biogenesis and release via modulating Rabs and miR-192 expression in human hepatocytes. Front. Cell Dev. Biol. 2022;9 doi: 10.3389/fcell.2021.787356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Guo M., Li R., Yang L., Zhu Q., Han M., Chen Z., Ruan F., Yuan Y., Liu Z., Huang B. Evaluation of exosomal miRNAs as potential diagnostic biomarkers for acute myocardial infarction using next-generation sequencing. Ann. Transl. Med. 2021;9(3) doi: 10.21037/atm-20-2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Wu T., Chen Y., Du Y., Tao J., Zhou Z., Yang Z. Serum exosomal MiR-92b-5p as a potential biomarker for acute heart failure caused by dilated cardiomyopathy. Cell. Physiol. Biochem. 2018;46(5):1939–1950. doi: 10.1159/000489383. [DOI] [PubMed] [Google Scholar]
- 295.Mimmi S., Zimbo A.M., Rotundo S., Cione E., Nisticò N., Aloisio A., Maisano D., Tolomeo A.M., Dattilo V., Lionello R. SARS CoV-2 spike protein-guided exosome isolation facilitates detection of potential miRNA biomarkers in COVID-19 infections. Clin. Chem. Lab. Med. 2023;(0) doi: 10.1515/cclm-2022-1286. [DOI] [PubMed] [Google Scholar]
- 296.Caobi A., Werne R., Gomez M., Andre M., Thomas C., Yndart A., Lima-Hernandez F., Nair M., Raymond A.D. Protein cargo of Nef-containing exosomal extracellular vesicles may predict HIV-associated Neurocognitive Impairment status. Res. Square. 2023;3 [Google Scholar]
- 297.Mirzaei R., Babakhani S., Ajorloo P., Ahmadi R.H., Hosseini-Fard S.R., Keyvani H., Ahmadyousefi Y., Teimoori A., Zamani F., Karampoor S. The emerging role of exosomal miRNAs as a diagnostic and therapeutic biomarker in Mycobacterium tuberculosis infection. Mol. Med. 2021;27:1–31. doi: 10.1186/s10020-021-00296-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Zhao Z.-H., Chen Z.-T., Zhou R.-L., Zhang X., Ye Q.-Y., Wang Y.-Z. Increased DJ-1 and α-synuclein in plasma neural-derived exosomes as potential markers for Parkinson's disease. Front. Aging Neurosci. 2019;10:438. doi: 10.3389/fnagi.2018.00438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Lu Y., Mai Z., Cui L., Zhao X. Engineering exosomes and biomaterial-assisted exosomes as therapeutic carriers for bone regeneration. Stem Cell Res. Ther. 2023;14(1):1–19. doi: 10.1186/s13287-023-03275-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Zhao H., Li Z., Wang Y., Zhou K., Li H., Bi S., Wang Y., Wu W., Huang Y., Peng B. Bioengineered MSC-derived exosomes in skin wound repair and regeneration. Front. Cell Dev. Biol. 2023;11 doi: 10.3389/fcell.2023.1029671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Wu P., Zhang B., Shi H., Qian H., Xu W. MSC-exosome: a novel cell-free therapy for cutaneous regeneration. Cytotherapy. 2018;20(3):291–301. doi: 10.1016/j.jcyt.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 302.Youseflee P., Ranjbar F.E., Bahraminasab M., Ghanbari A., Faradonbeh D.R., Arab S., Alizadeh A., Nooshabadi V.T. Exosome loaded hydroxyapatite (HA) scaffold promotes bone regeneration in calvarial defect: an in vivo study. Cell Tissue Bank. 2023;24(2):389–400. doi: 10.1007/s10561-022-10042-4. [DOI] [PubMed] [Google Scholar]
- 303.Zhou Y., Deng G., She H., Bai F., Xiang B., Zhou J., Zhang S. Polydopamine-coated biomimetic bone scaffolds loaded with exosomes promote osteogenic differentiation of BMSC and bone regeneration. Regenerative Therapy. 2023;23:25–36. doi: 10.1016/j.reth.2023.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Yan Z., Yin H., Wu J., Tian G., Li M., Liao Z., He S., Deng H., Ning C., Ding Z. Engineering exosomes by three-dimensional porous scaffold culture of human umbilical cord mesenchymal stem cells promote osteochondral repair. Materials Today Bio. 2023;19 doi: 10.1016/j.mtbio.2023.100549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Cai J., Xu J., Ye Z., Wang L., Zheng T., Zhang T., Li Y., Jiang J., Zhao J. Exosomes derived from kartogenin-preconditioned mesenchymal stem cells promote cartilage formation and collagen maturation for enthesis regeneration in a rat model of chronic rotator cuff tear. Am. J. Sports Med. 2023;51(5):1267–1276. doi: 10.1177/03635465231155927. [DOI] [PubMed] [Google Scholar]
- 306.Joorabloo A., Liu T. Engineering exosome-based biomimetic nanovehicles for wound healing. J. Contr. Release. 2023;356:463–480. doi: 10.1016/j.jconrel.2023.03.013. [DOI] [PubMed] [Google Scholar]
- 307.Sareen N., Srivastava A., Alagarsamy K.N., Lionetti V., Dhingra S. Stem cells derived exosomes and biomaterials to modulate autophagy and mend broken hearts. Biochim. Biophys. Acta (BBA) - Mol. Basis Dis. 2023 doi: 10.1016/j.bbadis.2023.166806. [DOI] [PubMed] [Google Scholar]
- 308.Wu R., Hu X., Wang J.a. Current optimized strategies for stem cell-derived extracellular vesicle/exosomes in cardiac repair. J. Mol. Cell. Cardiol. 2023;184:13–25. doi: 10.1016/j.yjmcc.2023.09.006. [DOI] [PubMed] [Google Scholar]
- 309.Liu X., Zhang L., Xu Z., Xiong X., Yu Y., Wu H., Qiao H., Zhong J., Zhao Z., Dai J. A functionalized collagen-I scaffold delivers microRNA 21-loaded exosomes for spinal cord injury repair. Acta Biomater. 2022;154:385–400. doi: 10.1016/j.actbio.2022.10.027. [DOI] [PubMed] [Google Scholar]
- 310.Xin L., Lin X., Zhou F., Li C., Wang X., Yu H., Pan Y., Fei H., Ma L., Zhang S. A scaffold laden with mesenchymal stem cell-derived exosomes for promoting endometrium regeneration and fertility restoration through macrophage immunomodulation. Acta Biomater. 2020;113:252–266. doi: 10.1016/j.actbio.2020.06.029. [DOI] [PubMed] [Google Scholar]
- 311.Ko K.-W., Park S.-Y., Lee E.H., Yoo Y.-I., Kim D.-S., Kim J.Y., Kwon T.G., Han D.K. Integrated bioactive scaffold with polydeoxyribonucleotide and stem-cell-derived extracellular vesicles for kidney regeneration. ACS Nano. 2021;15(4):7575–7585. doi: 10.1021/acsnano.1c01098. [DOI] [PubMed] [Google Scholar]
- 312.Cao J., Wang B., Tang T., Lv L., Ding Z., Li Z., Hu R., Wei Q., Shen A., Fu Y. Three-dimensional culture of MSCs produces exosomes with improved yield and enhanced therapeutic efficacy for cisplatin-induced acute kidney injury. Stem Cell Res. Ther. 2020;11:1–13. doi: 10.1186/s13287-020-01719-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Mao L., Li Y.-D., Chen R.-L., Li G., Zhou X.-X., Song F., Wu C., Hu Y., Hong Y.-X., Dang X. Heart-targeting exosomes from human cardiosphere-derived cells improve the therapeutic effect on cardiac hypertrophy. J. Nanobiotechnol. 2022;20(1):1–14. doi: 10.1186/s12951-022-01630-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Mentkowski K.I., Lang J.K. Exosomes engineered to express a cardiomyocyte binding peptide demonstrate improved cardiac retention in vivo. Sci. Rep. 2019;9(1):1–13. doi: 10.1038/s41598-019-46407-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Rogers R.G., Fournier M., Sanchez L., Ibrahim A.G., Aminzadeh M.A., Lewis M.I., Marbán E. Disease-modifying bioactivity of intravenous cardiosphere-derived cells and exosomes in mdx mice. JCI insight. 2019;4(7) doi: 10.1172/jci.insight.125754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Aminzadeh M.A., Rogers R.G., Fournier M., Tobin R.E., Guan X., Childers M.K., Andres A.M., Taylor D.J., Ibrahim A., Ding X. Exosome-mediated benefits of cell therapy in mouse and human models of Duchenne muscular dystrophy. Stem Cell Rep. 2018;10(3):942–955. doi: 10.1016/j.stemcr.2018.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Ibrahim A.G.-E., Cheng K., Marbán E. Exosomes as critical agents of cardiac regeneration triggered by cell therapy. Stem Cell Rep. 2014;2(5):606–619. doi: 10.1016/j.stemcr.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Namazi H., Mohit E., Namazi I., Rajabi S., Samadian A., Hajizadeh‐Saffar E., Aghdami N., Baharvand H. Exosomes secreted by hypoxic cardiosphere‐derived cells enhance tube formation and increase pro‐angiogenic miRNA. J. Cell. Biochem. 2018;119(5):4150–4160. doi: 10.1002/jcb.26621. [DOI] [PubMed] [Google Scholar]
- 319.Guru S.A., Saha P., Chen L., Tulshyan A., Ge Z.-D., Baily J., Simons L., Stefanowicz A., Bilewska A., Mehta V. HSF-1 enhances cardioprotective potential of stem cells via exosome biogenesis and their miRNA cargo enrichment. Stem Cell Reviews and Reports. 2023:1–14. doi: 10.1007/s12015-023-10565-7. [DOI] [PubMed] [Google Scholar]
- 320.Arslan F., Lai R.C., Smeets M.B., Akeroyd L., Choo A., Aguor E.N., Timmers L., van Rijen H.V., Doevendans P.A., Pasterkamp G. Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res. 2013;10(3):301–312. doi: 10.1016/j.scr.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 321.Zhao Y., Sun X., Cao W., Ma J., Sun L., Qian H., Zhu W., Xu W. Exosomes derived from human umbilical cord mesenchymal stem cells relieve acute myocardial ischemic injury. Stem Cell. Int. 2015;2015 doi: 10.1155/2015/761643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Ping P., Guan S., Ning C., Yang T., Zhao Y., Zhang P., Gao Z., Fu S. Fabrication of blended nanofibrous cardiac patch transplanted with TGF-β3 and human umbilical cord MSCs-derived exosomes for potential cardiac regeneration after acute myocardial infarction. Nanomed. Nanotechnol. Biol. Med. 2023 doi: 10.1016/j.nano.2023.102708. [DOI] [PubMed] [Google Scholar]
- 323.Barile L., Lionetti V., Cervio E., Matteucci M., Gherghiceanu M., Popescu L.M., Torre T., Siclari F., Moccetti T., Vassalli G. Extracellular vesicles from human cardiac progenitor cells inhibit cardiomyocyte apoptosis and improve cardiac function after myocardial infarction. Cardiovasc. Res. 2014;103(4):530–541. doi: 10.1093/cvr/cvu167. [DOI] [PubMed] [Google Scholar]
- 324.Zhang X., Wang X., Zhu H., Kranias E.G., Tang Y., Peng T., Chang J., Fan G.-C. Hsp20 functions as a novel cardiokine in promoting angiogenesis via activation of VEGFR2. PLoS One. 2012;7(3) doi: 10.1371/journal.pone.0032765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Vandergriff A., Huang K., Shen D., Hu S., Hensley M.T., Caranasos T.G., Qian L., Cheng K. Targeting regenerative exosomes to myocardial infarction using cardiac homing peptide. Theranostics. 2018;8(7):1869. doi: 10.7150/thno.20524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Huang P., Wang L., Li Q., Tian X., Xu J., Xu J., Xiong Y., Chen G., Qian H., Jin C. Atorvastatin enhances the therapeutic efficacy of mesenchymal stem cells-derived exosomes in acute myocardial infarction via up-regulating long non-coding RNA H19. Cardiovasc. Res. 2020;116(2):353–367. doi: 10.1093/cvr/cvz139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Yu B., Kim H.W., Gong M., Wang J., Millard R.W., Wang Y., Ashraf M., Xu M. Exosomes secreted from GATA-4 overexpressing mesenchymal stem cells serve as a reservoir of anti-apoptotic microRNAs for cardioprotection. Int. J. Cardiol. 2015;182:349–360. doi: 10.1016/j.ijcard.2014.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Vicencio J.M., Yellon D.M., Sivaraman V., Das D., Boi-Doku C., Arjun S., Zheng Y., Riquelme J.A., Kearney J., Sharma V. Plasma exosomes protect the myocardium from ischemia-reperfusion injury. J. Am. Coll. Cardiol. 2015;65(15):1525–1536. doi: 10.1016/j.jacc.2015.02.026. [DOI] [PubMed] [Google Scholar]
- 329.Yang Y., Ye Y., Kong C., Su X., Zhang X., Bai W., He X. MiR-124 enriched exosomes promoted the M2 polarization of microglia and enhanced Hippocampus neurogenesis after traumatic brain injury by inhibiting TLR4 pathway. Neurochem. Res. 2019;44(4):811–828. doi: 10.1007/s11064-018-02714-z. [DOI] [PubMed] [Google Scholar]
- 330.Xin H., Li Y., Buller B., Katakowski M., Zhang Y., Wang X., Shang X., Zhang Z.G., Chopp M. Exosome-mediated transfer of miR-133b from multipotent mesenchymal stromal cells to neural cells contributes to neurite outgrowth. Stem Cell. 2012;30(7):1556–1564. doi: 10.1002/stem.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Zhang Y., Chopp M., Meng Y., Katakowski M., Xin H., Mahmood A., Xiong Y. Effect of exosomes derived from multipluripotent mesenchymal stromal cells on functional recovery and neurovascular plasticity in rats after traumatic brain injury. J. Neurosurg. 2015;122(4):856–867. doi: 10.3171/2014.11.JNS14770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Zhang Y., Chopp M., Zhang Z.G., Katakowski M., Xin H., Qu C., Ali M., Mahmood A., Xiong Y. Systemic administration of cell-free exosomes generated by human bone marrow derived mesenchymal stem cells cultured under 2D and 3D conditions improves functional recovery in rats after traumatic brain injury. Neurochem. Int. 2017;111:69–81. doi: 10.1016/j.neuint.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Mead B., Tomarev S. Bone marrow-derived mesenchymal stem cells-derived exosomes promote survival of retinal ganglion cells through miRNA-dependent mechanisms. Stem Cells Transl Med. 2017;6(4):1273–1285. doi: 10.1002/sctm.16-0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Zhang Z.W., Wei P., Zhang G.J., Yan J.X., Zhang S., Liang J., Wang X.L. Intravenous infusion of the exosomes derived from human umbilical cord mesenchymal stem cells enhance neurological recovery after traumatic brain injury via suppressing the NF-κB pathway. Open Life Sci. 2022;17(1):189–201. doi: 10.1515/biol-2022-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Liu C.Y., Yin G., Sun Y.D., Lin Y.F., Xie Z., English A.W., Li Q.F., Lin H.D. Effect of exosomes from adipose-derived stem cells on the apoptosis of Schwann cells in peripheral nerve injury. CNS Neurosci. Ther. 2020;26(2):189–196. doi: 10.1111/cns.13187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Yin G., Yu B., Liu C., Lin Y., Xie Z., Hu Y., Lin H. Exosomes produced by adipose-derived stem cells inhibit schwann cells autophagy and promote the regeneration of the myelin sheath. Int. J. Biochem. Cell Biol. 2021;132 doi: 10.1016/j.biocel.2021.105921. [DOI] [PubMed] [Google Scholar]
- 337.Rau C.S., Kuo P.J., Wu S.C., Huang L.H., Lu T.H., Wu Y.C., Wu C.J., Lin C.W., Tsai C.W., Hsieh C.H. Enhanced nerve regeneration by exosomes secreted by adipose-derived stem cells with or without FK506 stimulation. Int. J. Mol. Sci. 2021;22(16) doi: 10.3390/ijms22168545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Farinazzo A., Turano E., Marconi S., Bistaffa E., Bazzoli E., Bonetti B. Murine adipose-derived mesenchymal stromal cell vesicles: in vitro clues for neuroprotective and neuroregenerative approaches. Cytotherapy. 2015;17(5):571–578. doi: 10.1016/j.jcyt.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 339.El Bassit G., Patel R.S., Carter G., Shibu V., Patel A.A., Song S., Murr M., Cooper D.R., Bickford P.C., Patel N.A. MALAT1 in human adipose stem cells modulates survival and alternative splicing of PKCδII in HT22 cells. Endocrinology. 2017;158(1):183–195. doi: 10.1210/en.2016-1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Zhao J., Ding Y., He R., Huang K., Liu L., Jiang C., Liu Z., Wang Y., Yan X., Cao F., Huang X., Peng Y., Ren R., He Y., Cui T., Zhang Q., Zhang X., Liu Q., Li Y., Ma Z., Yi X. Dose-effect relationship and molecular mechanism by which BMSC-derived exosomes promote peripheral nerve regeneration after crush injury. Stem Cell Res. Ther. 2020;11(1):360. doi: 10.1186/s13287-020-01872-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Lopez-Verrilli M.A., Picou F., Court F.A. Schwann cell-derived exosomes enhance axonal regeneration in the peripheral nervous system. Glia. 2013;61(11):1795–1806. doi: 10.1002/glia.22558. [DOI] [PubMed] [Google Scholar]
- 342.Huang J., Zhang G., Li S., Li J., Wang W., Xue J., Wang Y., Fang M., Zhou N. Endothelial cell-derived exosomes boost and maintain repair-related phenotypes of Schwann cells via miR199-5p to promote nerve regeneration. J. Nanobiotechnol. 2023;21(1):10. doi: 10.1186/s12951-023-01767-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Mao Q., Nguyen P.D., Shanti R.M., Shi S., Shakoori P., Zhang Q., Le A.D. Gingiva-derived mesenchymal stem cell-extracellular vesicles activate schwann cell repair phenotype and promote nerve regeneration. Tissue Eng. 2019;25(11–12):887–900. doi: 10.1089/ten.TEA.2018.0176. [DOI] [PubMed] [Google Scholar]
- 344.Anita L., Yin G.N., Hong S.S., Kang J.H., Gho Y.S., Suh J.K., Ryu J.K. Pericyte-derived extracellular vesicle-mimetic nanovesicles ameliorate erectile dysfunction via lipocalin 2 in diabetic mice. Int. J. Biol. Sci. 2022;18(9):3653–3667. doi: 10.7150/ijbs.72243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Qian L., Pi L., Fang B.R., Meng X.X. Adipose mesenchymal stem cell-derived exosomes accelerate skin wound healing via the lncRNA H19/miR-19b/SOX9 axis. Lab. Invest. 2021;101(9):1254–1266. doi: 10.1038/s41374-021-00611-8. [DOI] [PubMed] [Google Scholar]
- 346.Cao G., Chen B., Zhang X., Chen H. Human adipose-derived mesenchymal stem cells-derived exosomal microRNA-19b promotes the healing of skin wounds through modulation of the CCL1/TGF-β signaling Axis. Clin. Cosmet. Invest. Dermatol. 2020;13:957–971. doi: 10.2147/CCID.S274370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Liang J.X., Liao X., Li S.H., Jiang X., Li Z.H., Wu Y.D., Xiao L.L., Xie G.H., Song J.X., Liu H.W. Antiaging properties of exosomes from adipose-derived mesenchymal stem cells in photoaged rat skin. BioMed Res. Int. 2020;2020 doi: 10.1155/2020/6406395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Shen K., Wang X.J., Liu K.T., Li S.H., Li J., Zhang J.X., Wang H.T., Hu D.H. [Effects of exosomes from human adipose-derived mesenchymal stem cells on inflammatory response of mouse RAW264.7 cells and wound healing of full-thickness skin defects in mice] Zhonghua Shaoshang Zazhi. 2022;38(3):215–226. doi: 10.3760/cma.j.cn501120-20201116-00477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Rosmarwati E., Ellistasari E.Y., Kusumawardani A., Julianto I., Widhiati S., Setyawan N.A., Yanuar F. Human platelet lysate-derived exosomes are superior to the lysate at increasing collagen deposition in a rat model of intrinsic aging. J. Appl. Pharmaceut. Sci. 2023:211–216. [Google Scholar]
- 350.Ge X., Lu L., Bai W., Wang M., Han C., Du H., Wang N., Gao M., Li D., Dong F. The novel roles of bovine milk-derived exosomes on skin anti-aging. bioRxiv. 2023 doi: 10.1111/jocd.16112. 2023.03. 23.532505. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 351.Han G., Kim H., Kim D.E., Ahn Y., Kim J., Jang Y.J., Kim K., Yang Y., Kim S.H. The potential of bovine colostrum-derived exosomes to repair aged and damaged skin cells. Pharmaceutics. 2022;14(2) doi: 10.3390/pharmaceutics14020307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Lee Y., Jeong D.-Y., Jeun Y.C., Choe H., Yang S. Preventive and ameliorative effects of potato exosomes on UVB-induced photodamage in keratinocyte HaCaT cells. Mol. Med. Rep. 2023;28(3):1–10. doi: 10.3892/mmr.2023.13054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Jo C.S., Myung C.H., Yoon Y.C., Ahn B.H., Min J.W., Seo W.S., Lee D.H., Kang H.C., Heo Y.H., Choi H., Hong I.K., Hwang J.S. The effect of. Curr. Issues Mol. Biol. 2022;44(2):526–540. doi: 10.3390/cimb44020036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Trentini M., Zanolla I., Zanotti F., Tiengo E., Licastro D., Dal Monego S., Lovatti L., Zavan B. Apple derived exosomes improve collagen type I production and decrease MMPs during aging of the skin through downregulation of the NF-κB pathway as mode of action. Cells. 2022;11(24) doi: 10.3390/cells11243950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Wgealla M.M.A.M., Liang H., Chen R., Xie Y., Li F., Qin M., Zhang X. Amniotic fluid derived stem cells promote skin regeneration and alleviate scar formation through exosomal miRNA-146a-5p via targeting CXCR4. J. Cosmet. Dermatol. 2022;21(10):5026–5036. doi: 10.1111/jocd.14956. [DOI] [PubMed] [Google Scholar]
- 356.Xiu C., Zheng H., Jiang M., Li J., Zhou Y., Mu L., Liu W. MSCs-derived miR-150-5p-expressing exosomes promote skin wound healing by activating PI3K/AKT pathway through PTEN. Int J Stem Cells. 2022;15(4):359–371. doi: 10.15283/ijsc21135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Yan T., Huang L., Yan Y., Zhong Y., Xie H., Wang X. Bone marrow mesenchymal stem cell-derived exosome miR-29b-3p alleviates UV irradiation-induced photoaging in skin fibroblast. Photodermatol. Photoimmunol. Photomed. 2022;39(3):235–245. doi: 10.1111/phpp.12827. [DOI] [PubMed] [Google Scholar]
- 358.Zha J., Pan Y., Liu X., Zhu H., Liu Y., Zeng W. Exosomes from hypoxia-pretreated adipose-derived stem cells attenuate ultraviolet light-induced skin injury via delivery of circ-Ash1l. Photodermatol. Photoimmunol. Photomed. 2022;39(2):107–115. doi: 10.1111/phpp.12857. [DOI] [PubMed] [Google Scholar]
- 359.Duan M., Zhang Y., Zhang H., Meng Y., Qian M., Zhang G. Epidermal stem cell-derived exosomes promote skin regeneration by downregulating transforming growth factor-β1 in wound healing. Stem Cell Res. Ther. 2020;11(1):452. doi: 10.1186/s13287-020-01971-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Hu S., Li Z., Cores J., Huang K., Su T., Dinh P.U., Cheng K. Needle-free injection of exosomes derived from human dermal fibroblast spheroids ameliorates skin photoaging. ACS Nano. 2019;13(10):11273–11282. doi: 10.1021/acsnano.9b04384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Bakhtyar N., Jeschke M.G., Herer E., Sheikholeslam M., Amini-Nik S. Exosomes from acellular Wharton's jelly of the human umbilical cord promotes skin wound healing. Stem Cell Res. Ther. 2018;9(1):193. doi: 10.1186/s13287-018-0921-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Oh M., Lee J., Kim Y.J., Rhee W.J., Park J.H. Exosomes derived from human induced pluripotent stem cells ameliorate the aging of skin fibroblasts. Int. J. Mol. Sci. 2018;19(6) doi: 10.3390/ijms19061715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Byun S.-E., Sim C., Chung Y., Kim H.K., Park S., Kim D.K., Cho S., Lee S. Skeletal muscle regeneration by the exosomes of adipose tissue-derived mesenchymal stem cells. Curr. Issues Mol. Biol. 2021;43(3):1473–1488. doi: 10.3390/cimb43030104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Wang C., Song W., Chen B., Liu X., He Y. Exosomes isolated from adipose-derived stem cells: a new cell-free approach to prevent the muscle degeneration associated with torn rotator cuffs. Am. J. Sports Med. 2019;47(13):3247–3255. doi: 10.1177/0363546519876323. [DOI] [PubMed] [Google Scholar]
- 365.Mullen M., Williams K., LaRocca T., Duke V., Hambright W.S., Ravuri S.K., Bahney C.S., Ehrhart N., Huard J. Mechanical strain drives exosome production, function, and miRNA cargo in C2C12 muscle progenitor cells. J. Orthop. Res. 2023;41(6):1186–1197. doi: 10.1002/jor.25467. [DOI] [PubMed] [Google Scholar]
- 366.Ji S., Ma P., Cao X., Wang J., Yu X., Luo X., Lu J., Hou W., Zhang Z., Yan Y. Myoblast‐derived exosomes promote the repair and regeneration of injured skeletal muscle in mice. FEBS Open bio. 2022;12(12):2213–2226. doi: 10.1002/2211-5463.13504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Forterre A., Jalabert A., Chikh K., Pesenti S., Euthine V., Granjon A., Errazuriz E., Lefai E., Vidal H., Rome S. Myotube-derived exosomal miRNAs downregulate Sirtuin1 in myoblasts during muscle cell differentiation. Cell Cycle. 2014;13(1):78–89. doi: 10.4161/cc.26808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Zhou M., Li B., Liu C., Hu M., Tang J., Min J., Cheng J., Hong L. M2 Macrophage-derived exosomal miR-501 contributes to pubococcygeal muscle regeneration. Int. Immunopharm. 2021;101 doi: 10.1016/j.intimp.2021.108223. [DOI] [PubMed] [Google Scholar]
- 369.Iyer S.R., Scheiber A.L., Yarowsky P., Henn R.F., III, Otsuru S., Lovering R.M. Exosomes isolated from platelet-rich plasma and mesenchymal stem cells promote recovery of function after muscle injury. Am. J. Sports Med. 2020;48(9):2277–2286. doi: 10.1177/0363546520926462. [DOI] [PubMed] [Google Scholar]
- 370.Nakamura Y., Miyaki S., Ishitobi H., Matsuyama S., Nakasa T., Kamei N., Akimoto T., Higashi Y., Ochi M. Mesenchymal‐stem‐cell‐derived exosomes accelerate skeletal muscle regeneration. FEBS Lett. 2015;589(11):1257–1265. doi: 10.1016/j.febslet.2015.03.031. [DOI] [PubMed] [Google Scholar]
- 371.Choi J.S., Yoon H.I., Lee K.S., Choi Y.C., Yang S.H., Kim I.-S., Cho Y.W. Exosomes from differentiating human skeletal muscle cells trigger myogenesis of stem cells and provide biochemical cues for skeletal muscle regeneration. J. Contr. Release. 2016;222:107–115. doi: 10.1016/j.jconrel.2015.12.018. [DOI] [PubMed] [Google Scholar]
- 372.Chen Y., Wu Y.-l., Sun H., Zhang H., Tang D., Yuan T., Chen C., Huang W., Zhou X., Wu H.-P. 2023. Exosomal miR-183-5p from Human Hepatocyte-Derived Liver Progenitor-like Cells Promotes Liver Regeneration during Acute Liver Failure. [Google Scholar]
- 373.Nojima H., Freeman C.M., Schuster R.M., Japtok L., Kleuser B., Edwards M.J., Gulbins E., Lentsch A.B. Hepatocyte exosomes mediate liver repair and regeneration via sphingosine-1-phosphate. J. Hepatol. 2016;64(1):60–68. doi: 10.1016/j.jhep.2015.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Yan Y., Jiang W., Tan Y., Zou S., Zhang H., Mao F., Gong A., Qian H., Xu W. hucMSC exosome-derived GPX1 is required for the recovery of hepatic oxidant injury. Mol. Ther. 2017;25(2):465–479. doi: 10.1016/j.ymthe.2016.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Rong X., Liu J., Yao X., Jiang T., Wang Y., Xie F. Human bone marrow mesenchymal stem cells-derived exosomes alleviate liver fibrosis through the Wnt/β-catenin pathway. Stem Cell Res. Ther. 2019;10(1):98. doi: 10.1186/s13287-019-1204-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Piao C., Sang J., Kou Z., Wang Y., Liu T., Lu X., Jiao Z., Wang H. Effects of exosomes derived from adipose-derived mesenchymal stem cells on pyroptosis and regeneration of injured liver. Int. J. Mol. Sci. 2022;23(20) doi: 10.3390/ijms232012065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Jun J.H., Kim J.Y., Choi J.H., Lim J.Y., Kim K., Kim G.J. Exosomes from placenta-derived mesenchymal stem cells are involved in liver regeneration in hepatic failure induced by bile duct ligation. Stem Cell. Int. 2020;2020 doi: 10.1155/2020/5485738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Li T., Fu Y., Guo Z., Zhu H., Liao H., Niu X., Zhou L., Fu S., Li Y., Li S., Wang L., Zheng Y., Feng L., Gao Y., He G. A new cell-free therapeutic strategy for liver regeneration: human placental mesenchymal stem cell-derived extracellular vesicles. J. Tissue Eng. 2022;13 doi: 10.1177/20417314221132093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Li X., Chen C., Wei L., Li Q., Niu X., Xu Y., Wang Y., Zhao J. Exosomes derived from endothelial progenitor cells attenuate vascular repair and accelerate reendothelialization by enhancing endothelial function. Cytotherapy. 2016;18(2):253–262. doi: 10.1016/j.jcyt.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 380.Hu H., Wang B., Jiang C., Li R., Zhao J. Endothelial progenitor cell-derived exosomes facilitate vascular endothelial cell repair through shuttling miR-21-5p to modulate Thrombospondin-1 expression. Clin. Sci. 2019;133(14):1629–1644. doi: 10.1042/CS20190188. [DOI] [PubMed] [Google Scholar]
- 381.Johnson T.K., Zhao L., Zhu D., Wang Y., Xiao Y., Oguljahan B., Zhao X., Kirlin W.G., Yin L., Chilian W.M. Exosomes derived from induced vascular progenitor cells promote angiogenesis in vitro and in an in vivo rat hindlimb ischemia model. Am. J. Physiol. Heart Circ. Physiol. 2019;317(4):H765–H776. doi: 10.1152/ajpheart.00247.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Gardin C., Ferroni L., Erdoğan Y.K., Zanotti F., De Francesco F., Trentini M., Brunello G., Ercan B., Zavan B. Nanostructured modifications of titanium surfaces improve vascular regenerative properties of exosomes derived from mesenchymal stem cells: preliminary in vitro results. Nanomaterials. 2021;11(12):3452. doi: 10.3390/nano11123452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Anderson J.D., Johansson H.J., Graham C.S., Vesterlund M., Pham M.T., Bramlett C.S., Montgomery E.N., Mellema M.S., Bardini R.L., Contreras Z. Comprehensive proteomic analysis of mesenchymal stem cell exosomes reveals modulation of angiogenesis via nuclear factor-kappaB signaling. Stem Cell. 2016;34(3):601–613. doi: 10.1002/stem.2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Lopatina T., Bruno S., Tetta C., Kalinina N., Porta M., Camussi G. Platelet-derived growth factor regulates the secretion of extracellular vesicles by adipose mesenchymal stem cells and enhances their angiogenic potential. Cell Commun. Signal. 2014;12(1):1–12. doi: 10.1186/1478-811X-12-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Sun Z., Zhao H., Liu B., Gao Y., Tang W.-H., Liu Z.-H., Luo Z.-J. AF cell derived exosomes regulate endothelial cell migration and inflammation: implications for vascularization in intervertebral disc degeneration. Life Sci. 2021;265 doi: 10.1016/j.lfs.2020.118778. [DOI] [PubMed] [Google Scholar]
- 386.Umezu T., Tadokoro H., Azuma K., Yoshizawa S., Ohyashiki K., Ohyashiki J.H. Exosomal miR-135b shed from hypoxic multiple myeloma cells enhances angiogenesis by targeting factor-inhibiting HIF-1, Blood. The Journal of the American Society of Hematology. 2014;124(25):3748–3757. doi: 10.1182/blood-2014-05-576116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Tadokoro H., Umezu T., Ohyashiki K., Hirano T., Ohyashiki J.H. Exosomes derived from hypoxic leukemia cells enhance tube formation in endothelial cells. J. Biol. Chem. 2013;288(48):34343–34351. doi: 10.1074/jbc.M113.480822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Hsu Y., Hung J., Chang W., Lin Y., Pan Y., Tsai P., Wu C., Kuo P. Hypoxic lung cancer-secreted exosomal miR-23a increased angiogenesis and vascular permeability by targeting prolyl hydroxylase and tight junction protein ZO-1. Oncogene. 2017;36(34):4929–4942. doi: 10.1038/onc.2017.105. [DOI] [PubMed] [Google Scholar]
- 389.An Y., Zhao J., Nie F., Qin Z., Xue H., Wang G., Li D. Exosomes from adipose-derived stem cells (ADSCs) overexpressing miR-21 promote vascularization of endothelial cells. Sci. Rep. 2019;9(1) doi: 10.1038/s41598-019-49339-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Zhang S., Chuah S.J., Lai R.C., Hui J.H.P., Lim S.K., Toh W.S. MSC exosomes mediate cartilage repair by enhancing proliferation, attenuating apoptosis and modulating immune reactivity. Biomaterials. 2018;156:16–27. doi: 10.1016/j.biomaterials.2017.11.028. [DOI] [PubMed] [Google Scholar]
- 391.Tao S.C., Yuan T., Zhang Y.L., Yin W.J., Guo S.C., Zhang C.Q. Exosomes derived from miR-140-5p-overexpressing human synovial mesenchymal stem cells enhance cartilage tissue regeneration and prevent osteoarthritis of the knee in a rat model. Theranostics. 2017;7(1):180–195. doi: 10.7150/thno.17133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Vonk L.A., van Dooremalen S.F.J., Liv N., Klumperman J., Coffer P.J., Saris D.B.F., Lorenowicz M.J. Mesenchymal stromal/stem cell-derived extracellular vesicles promote human cartilage regeneration in vitro. Theranostics. 2018;8(4):906–920. doi: 10.7150/thno.20746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Zhao S., Xiu G., Wang J., Wen Y., Lu J., Wu B., Wang G., Yang D., Ling B., Du D. Engineering exosomes derived from subcutaneous fat MSCs specially promote cartilage repair as miR-199a-3p delivery vehicles in Osteoarthritis. J. Nanobiotechnol. 2023;21(1):1–26. doi: 10.1186/s12951-023-02086-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Liu C., Li Y., Yang Z., Zhou Z., Lou Z., Zhang Q. Kartogenin enhances the therapeutic effect of bone marrow mesenchymal stem cells derived exosomes in cartilage repair. Nanomedicine (Lond) 2020;15(3):273–288. doi: 10.2217/nnm-2019-0208. [DOI] [PubMed] [Google Scholar]
- 395.Geng Z., Chen H., Zou G., Yuan L., Liu P., Li B., Zhang K., Jing F., Nie X., Liu T., Zhang B. Human amniotic fluid mesenchymal stem cell-derived exosomes inhibit apoptosis in ovarian granulosa cell via miR-369-3p/YAF2/PDCD5/p53 pathway. Oxid. Med. Cell. Longev. 2022;2022 doi: 10.1155/2022/3695848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Qu Q., Liu L., Cui Y., Liu H., Yi J., Bing W., Liu C., Jiang D., Bi Y. miR-126-3p containing exosomes derived from human umbilical cord mesenchymal stem cells promote angiogenesis and attenuate ovarian granulosa cell apoptosis in a preclinical rat model of premature ovarian failure. Stem Cell Res. Ther. 2022;13(1):352. doi: 10.1186/s13287-022-03056-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Xiao G.Y., Cheng C.C., Chiang Y.S., Cheng W.T., Liu I.H., Wu S.C. Exosomal miR-10a derived from amniotic fluid stem cells preserves ovarian follicles after chemotherapy. Sci. Rep. 2016;6 doi: 10.1038/srep23120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Yang M., Lin L., Sha C., Li T., Zhao D., Wei H., Chen Q., Liu Y., Chen X., Xu W., Li Y., Zhu X. Bone marrow mesenchymal stem cell-derived exosomal miR-144-5p improves rat ovarian function after chemotherapy-induced ovarian failure by targeting PTEN. Lab. Invest. 2020;100(3):342–352. doi: 10.1038/s41374-019-0321-y. [DOI] [PubMed] [Google Scholar]
- 399.Sun B., Ma Y., Wang F., Hu L., Sun Y. miR-644-5p carried by bone mesenchymal stem cell-derived exosomes targets regulation of p53 to inhibit ovarian granulosa cell apoptosis. Stem Cell Res. Ther. 2019;10(1):360. doi: 10.1186/s13287-019-1442-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Nazdikbin Yamchi N., Ahmadian S., Mobarak H., Amjadi F., Beheshti R., Tamadon A., Rahbarghazi R., Mahdipour M. Amniotic fluid-derived exosomes attenuated fibrotic changes in POI rats through modulation of the TGF-β/Smads signaling pathway. J. Ovarian Res. 2023;16(1):118. doi: 10.1186/s13048-023-01214-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Zhang X., Zhang R., Hao J., Huang X., Liu M., Lv M., Su C., Mu Y.L. miRNA-122-5p in POI ovarian-derived exosomes promotes granulosa cell apoptosis by regulating BCL9. Cancer Med. 2022;11(12):2414–2426. doi: 10.1002/cam4.4615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Zhang S., Huang B., Su P., Chang Q., Li P., Song A., Zhao X., Yuan Z., Tan J. Concentrated exosomes from menstrual blood-derived stromal cells improves ovarian activity in a rat model of premature ovarian insufficiency. Stem Cell Res. Ther. 2021;12(1):178. doi: 10.1186/s13287-021-02255-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Ding C., Zhu L., Shen H., Lu J., Zou Q., Huang C., Li H., Huang B. Exosomal miRNA-17-5p derived from human umbilical cord mesenchymal stem cells improves ovarian function in premature ovarian insufficiency by regulating SIRT7. Stem Cell. 2020;38(9):1137–1148. doi: 10.1002/stem.3204. [DOI] [PubMed] [Google Scholar]
- 404.Huang B., Lu J., Ding C., Zou Q., Wang W., Li H. Exosomes derived from human adipose mesenchymal stem cells improve ovary function of premature ovarian insufficiency by targeting SMAD. Stem Cell Res. Ther. 2018;9(1):216. doi: 10.1186/s13287-018-0953-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Takeuchi R., Katagiri W., Endo S., Kobayashi T. Exosomes from conditioned media of bone marrow-derived mesenchymal stem cells promote bone regeneration by enhancing angiogenesis. PLoS One. 2019;14(11) doi: 10.1371/journal.pone.0225472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Zhai M., Zhu Y., Yang M., Mao C. Human mesenchymal stem cell derived exosomes enhance cell‐free bone regeneration by altering their miRNAs profiles. Adv. Sci. 2020;7(19) doi: 10.1002/advs.202001334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Furuta T., Miyaki S., Ishitobi H., Ogura T., Kato Y., Kamei N., Miyado K., Higashi Y., Ochi M. Mesenchymal stem cell-derived exosomes promote fracture healing in a mouse model. Stem cells translational medicine. 2016;5(12):1620–1630. doi: 10.5966/sctm.2015-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Luo Z.-W., Liu Y.-W., Rao S.-S., Yin H., Huang J., Chen C.-Y., Hu Y., Zhang Y., Tan Y.-J., Yuan L.-Q. Aptamer-functionalized exosomes from bone marrow stromal cells target bone to promote bone regeneration. Nanoscale. 2019;11(43):20884–20892. doi: 10.1039/c9nr02791b. [DOI] [PubMed] [Google Scholar]
- 409.Kuang H., Ma J., Chi X., Fu Q., Zhu Q., Cao W., Zhang P., Xie X. Integrated osteoinductive Factors─ exosome@ MicroRNA-26a hydrogel enhances bone regeneration. ACS Appl. Mater. Interfaces. 2023;15(19):22805–22816. doi: 10.1021/acsami.2c21933. [DOI] [PubMed] [Google Scholar]
- 410.Yang B.-c., Kuang M.-j., Kang J.-y., Zhao J., Ma J.-x., Ma X.-l. Human umbilical cord mesenchymal stem cell-derived exosomes act via the miR-1263/Mob1/Hippo signaling pathway to prevent apoptosis in disuse osteoporosis. Biochem. Biophys. Res. Commun. 2020;524(4):883–889. doi: 10.1016/j.bbrc.2020.02.001. [DOI] [PubMed] [Google Scholar]
- 411.Zhou J., Liu H., Li S., Gong Y., Zhou M., Zhang J., Zhu G. Effects of human umbilical cord mesenchymal stem cells-derived exosomes on fracture healing in rats through the Wnt signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2019;23(11):4954–4960. doi: 10.26355/eurrev_201906_18086. [DOI] [PubMed] [Google Scholar]
- 412.Li W., Liu Y., Zhang P., Tang Y., Zhou M., Jiang W., Zhang X., Wu G., Zhou Y. Tissue-engineered bone immobilized with human adipose stem cells-derived exosomes promotes bone regeneration. ACS Appl. Mater. Interfaces. 2018;10(6):5240–5254. doi: 10.1021/acsami.7b17620. [DOI] [PubMed] [Google Scholar]
- 413.Fan J., Lee C.-S., Kim S., Chen C., Aghaloo T., Lee M. Generation of small RNA-modulated exosome mimetics for bone regeneration. ACS Nano. 2020;14(9):11973–11984. doi: 10.1021/acsnano.0c05122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Chen S., Tang Y., Liu Y., Zhang P., Lv L., Zhang X., Jia L., Zhou Y. Exosomes derived from miR‐375‐overexpressing human adipose mesenchymal stem cells promote bone regeneration. Cell Prolif. 2019;52(5) doi: 10.1111/cpr.12669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Kuang M.-j., Huang Y., Zhao X.-G., Zhang R., Ma J.-x., Wang D.-c., Ma X.-l. Exosomes derived from Wharton's jelly of human umbilical cord mesenchymal stem cells reduce osteocyte apoptosis in glucocorticoid-induced osteonecrosis of the femoral head in rats via the miR-21-PTEN-AKT signalling pathway. Int. J. Biol. Sci. 2019;15(9):1861. doi: 10.7150/ijbs.32262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Li H., Liu D., Li C., Zhou S., Tian D., Xiao D., Zhang H., Gao F., Huang J. Exosomes secreted from mutant‐HIF‐1α‐modified bone‐marrow‐derived mesenchymal stem cells attenuate early steroid‐induced avascular necrosis of femoral head in rabbit. Cell Biol. Int. 2017;41(12):1379–1390. doi: 10.1002/cbin.10869. [DOI] [PubMed] [Google Scholar]
- 417.Qi X., Zhang J., Yuan H., Xu Z., Li Q., Niu X., Hu B., Wang Y., Li X. Exosomes secreted by human-induced pluripotent stem cell-derived mesenchymal stem cells repair critical-sized bone defects through enhanced angiogenesis and osteogenesis in osteoporotic rats. Int. J. Biol. Sci. 2016;12(7):836. doi: 10.7150/ijbs.14809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Liu X., Li Q., Niu X., Hu B., Chen S., Song W., Ding J., Zhang C., Wang Y. Exosomes secreted from human-induced pluripotent stem cell-derived mesenchymal stem cells prevent osteonecrosis of the femoral head by promoting angiogenesis. Int. J. Biol. Sci. 2017;13(2):232. doi: 10.7150/ijbs.16951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Lu Z., Chen Y., Dunstan C., Roohani-Esfahani S., Zreiqat H. Priming adipose stem cells with tumor necrosis factor-alpha preconditioning potentiates their exosome efficacy for bone regeneration. Tissue Eng. 2017;23(21–22):1212–1220. doi: 10.1089/ten.tea.2016.0548. [DOI] [PubMed] [Google Scholar]
- 420.Jia Y., Zhu Y., Qiu S., Xu J., Chai Y. Exosomes secreted by endothelial progenitor cells accelerate bone regeneration during distraction osteogenesis by stimulating angiogenesis. Stem Cell Res. Ther. 2019;10:1–13. doi: 10.1186/s13287-018-1115-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Lazar E., Benedek T., Korodi S., Rat N., Lo J., Benedek I. Stem cell-derived exosomes-an emerging tool for myocardial regeneration. World J. Stem Cell. 2018;10(8):106. doi: 10.4252/wjsc.v10.i8.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Maring J.A., Beez C.M., Falk V., Seifert M., Stamm C. Myocardial regeneration via progenitor cell-derived exosomes. Stem Cell. Int. 2017;2017 doi: 10.1155/2017/7849851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Timmers L., Lim S.K., Arslan F., Armstrong J.S., Hoefer I.E., Doevendans P.A., Piek J.J., El Oakley R.M., Choo A., Lee C.N. Reduction of myocardial infarct size by human mesenchymal stem cell conditioned medium. Stem Cell Res. 2008;1(2):129–137. doi: 10.1016/j.scr.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 424.Lai R.C., Arslan F., Lee M.M., Sze N.S.K., Choo A., Chen T.S., Salto-Tellez M., Timmers L., Lee C.N., El Oakley R.M. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010;4(3):214–222. doi: 10.1016/j.scr.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 425.Wang X., Huang W., Liu G., Cai W., Millard R.W., Wang Y., Chang J., Peng T., Fan G.-C. Cardiomyocytes mediate anti-angiogenesis in type 2 diabetic rats through the exosomal transfer of miR-320 into endothelial cells. J. Mol. Cell. Cardiol. 2014;74:139–150. doi: 10.1016/j.yjmcc.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Yu B., Gong M., He Z., Wang Y.-G., Millard R.W., Ashraf M., Xu M. Enhanced mesenchymal stem cell survival induced by GATA-4 overexpression is partially mediated by regulation of the miR-15 family. Int. J. Biochem. Cell Biol. 2013;45(12):2724–2735. doi: 10.1016/j.biocel.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Martin J.L., Mestril R., Hilal-Dandan R., Brunton L.L., Dillmann W.H. Small heat shock proteins and protection against ischemic injury in cardiac myocytes. Circulation. 1997;96(12):4343–4348. doi: 10.1161/01.cir.96.12.4343. [DOI] [PubMed] [Google Scholar]
- 428.Gray W.D., French K.M., Ghosh-Choudhary S., Maxwell J.T., Brown M.E., Platt M.O., Searles C.D., Davis M.E. Identification of therapeutic covariant microRNA clusters in hypoxia-treated cardiac progenitor cell exosomes using systems biology. Circ. Res. 2015;116(2):255–263. doi: 10.1161/CIRCRESAHA.116.304360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Lang J.K., Young R.F., Ashraf H., Canty J.M., Jr. Inhibiting extracellular vesicle release from human cardiosphere derived cells with lentiviral knockdown of nSMase2 differentially effects proliferation and apoptosis in cardiomyocytes, fibroblasts and endothelial cells in vitro. PLoS One. 2016;11(11) doi: 10.1371/journal.pone.0165926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Vaughn M.N., Winston C.N., Levin N., Rissman R.A., Risbrough V.B. Developing biomarkers of mild traumatic brain injury: promise and progress of CNS-derived exosomes. Front. Neurol. 2021;12 doi: 10.3389/fneur.2021.698206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Ji Q., Ji Y., Peng J., Zhou X., Chen X., Zhao H., Xu T., Chen L., Xu Y. Increased brain-specific MiR-9 and MiR-124 in the serum exosomes of acute ischemic stroke patients. PLoS One. 2016;11(9) doi: 10.1371/journal.pone.0163645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Li D., Zhang P., Yao X., Li H., Shen H., Li X., Wu J., Lu X. Exosomes derived from miR-133b-modified mesenchymal stem cells promote recovery after spinal cord injury. Front. Neurosci. 2018;12 doi: 10.3389/fnins.2018.00845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Huang J.H., Xu Y., Yin X.M., Lin F.Y. Exosomes derived from miR-126-modified MSCs promote angiogenesis and neurogenesis and attenuate apoptosis after spinal cord injury in rats. Neuroscience. 2020;424:133–145. doi: 10.1016/j.neuroscience.2019.10.043. [DOI] [PubMed] [Google Scholar]
- 434.Xu G., Ao R., Zhi Z., Jia J., Yu B. miR-21 and miR-19b delivered by hMSC-derived EVs regulate the apoptosis and differentiation of neurons in patients with spinal cord injury. J. Cell. Physiol. 2019;234(7):10205–10217. doi: 10.1002/jcp.27690. [DOI] [PubMed] [Google Scholar]
- 435.Hercher D., Nguyen M.Q., Dworak H. Extracellular vesicles and their role in peripheral nerve regeneration. Exp. Neurol. 2022;350 doi: 10.1016/j.expneurol.2021.113968. [DOI] [PubMed] [Google Scholar]
- 436.Galieva L.R., James V., Mukhamedshina Y.O., Rizvanov A.A. Therapeutic potential of extracellular vesicles for the treatment of nerve disorders. Front. Neurosci. 2019;13:163. doi: 10.3389/fnins.2019.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Qing L., Chen H., Tang J., Jia X. Exosomes and their MicroRNA cargo: new players in peripheral nerve regeneration. Neurorehabilitation Neural Repair. 2018;32(9):765–776. doi: 10.1177/1545968318798955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Lopez-Verrilli M.A., Caviedes A., Cabrera A., Sandoval S., Wyneken U., Khoury M. Mesenchymal stem cell-derived exosomes from different sources selectively promote neuritic outgrowth. Neuroscience. 2016;320:129–139. doi: 10.1016/j.neuroscience.2016.01.061. [DOI] [PubMed] [Google Scholar]
- 439.Bucan V., Vaslaitis D., Peck C.T., Strauß S., Vogt P.M., Radtke C. Effect of exosomes from rat adipose-derived mesenchymal stem cells on neurite outgrowth and sciatic nerve regeneration after crush injury. Mol. Neurobiol. 2019;56(3):1812–1824. doi: 10.1007/s12035-018-1172-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Hajinejad M., Ebrahimzadeh M.H., Ebrahimzadeh-bideskan A., Rajabian A., Gorji A., Sahab Negah S. Exosomes and nano-SDF scaffold as a cell-free-based treatment strategy improve traumatic brain injury mechanisms by decreasing oxidative stress, neuroinflammation, and increasing neurogenesis. Stem Cell Reviews and Reports. 2023:1–18. doi: 10.1007/s12015-022-10483-0. [DOI] [PubMed] [Google Scholar]
- 441.Kim M.H., Chung C., Oh M.H., Jun J.H., Ko Y., Lee J.H. Extracellular vesicles from a three-dimensional culture of perivascular cells accelerate skin wound healing in a rat. Aesthetic Plast. Surg. 2021;45(5):2437–2446. doi: 10.1007/s00266-021-02254-y. [DOI] [PubMed] [Google Scholar]
- 442.El-Tookhy O.S., Shamaa A.A., Shehab G.G., Abdallah A.N., Azzam O.M. Histological evaluation of experimentally induced critical size defect skin wounds using exosomal solution of mesenchymal stem cells derived microvesicles. Int J Stem Cells. 2017;10(2):144–153. doi: 10.15283/ijsc17043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Zhao B., Wu G.F., Zhang Y.J., Zhang W., Yang F.F., Xiao D., Zeng K.X., Shi J.H., Su L.L., Hu D.H. [Effects of human amniotic epithelial stem cells-derived exosomes on healing of wound with full-thickness skin defect in rats] Zhonghua Shaoshang Zazhi. 2017;33(1):18–23. doi: 10.3760/cma.j.issn.1009-2587.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 444.King H.W., Michael M.Z., Gleadle J.M. Hypoxic enhancement of exosome release by breast cancer cells. BMC Cancer. 2012;12:1–10. doi: 10.1186/1471-2407-12-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Ludwig N., Whiteside T.L. Potential roles of tumor-derived exosomes in angiogenesis. Expert Opin. Ther. Targets. 2018;22(5):409–417. doi: 10.1080/14728222.2018.1464141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Kucharzewska P., Christianson H.C., Welch J.E., Svensson K.J., Fredlund E., Ringnér M., Mörgelin M., Bourseau-Guilmain E., Bengzon J., Belting M. Exosomes reflect the hypoxic status of glioma cells and mediate hypoxia-dependent activation of vascular cells during tumor development. Proc. Natl. Acad. Sci. USA. 2013;110(18):7312–7317. doi: 10.1073/pnas.1220998110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Liu Y., Luo F., Wang B., Li H., Xu Y., Liu X., Shi L., Lu X., Xu W., Lu L. STAT3-regulated exosomal miR-21 promotes angiogenesis and is involved in neoplastic processes of transformed human bronchial epithelial cells. Cancer Lett. 2016;370(1):125–135. doi: 10.1016/j.canlet.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 448.Zhang S., Chu W.C., Lai R.C., Lim S.K., Hui J.H., Toh W.S. Exosomes derived from human embryonic mesenchymal stem cells promote osteochondral regeneration. Osteoarthritis Cartilage. 2016;24(12):2135–2140. doi: 10.1016/j.joca.2016.06.022. [DOI] [PubMed] [Google Scholar]
- 449.Chen Y., Xue K., Zhang X., Zheng Z., Liu K. Exosomes derived from mature chondrocytes facilitate subcutaneous stable ectopic chondrogenesis of cartilage progenitor cells. Stem Cell Res. Ther. 2018;9(1):318. doi: 10.1186/s13287-018-1047-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Shao J., Zhu J., Chen Y., Fu Q., Li L., Ding Z., Wu J., Han Y., Li H., Qian Q., Zhou Y. Exosomes from kartogenin-pretreated infrapatellar fat pad mesenchymal stem cells enhance chondrocyte anabolism and articular cartilage regeneration. Stem Cell. Int. 2021;2021 doi: 10.1155/2021/6624874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Zhu Y., Wang Y., Zhao B., Niu X., Hu B., Li Q., Zhang J., Ding J., Chen Y., Wang Y. Comparison of exosomes secreted by induced pluripotent stem cell-derived mesenchymal stem cells and synovial membrane-derived mesenchymal stem cells for the treatment of osteoarthritis. Stem Cell Res. Ther. 2017;8(1):64. doi: 10.1186/s13287-017-0510-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Tan C.Y., Lai R.C., Wong W., Dan Y.Y., Lim S.K., Ho H.K. Mesenchymal stem cell-derived exosomes promote hepatic regeneration in drug-induced liver injury models. Stem Cell Res. Ther. 2014;5(3):76. doi: 10.1186/scrt465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Li Z., Zhang M., Zheng J., Tian Y., Zhang H., Tan Y., Li Q., Zhang J., Huang X. Human umbilical cord mesenchymal stem cell-derived exosomes improve ovarian function and proliferation of premature ovarian insufficiency by regulating the hippo signaling pathway. Front. Endocrinol. 2021;12 doi: 10.3389/fendo.2021.711902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Zhang Q., Sun J., Huang Y., Bu S., Guo Y., Gu T., Li B., Wang C., Lai D. Human amniotic epithelial cell-derived exosomes restore ovarian function by transferring MicroRNAs against apoptosis. Mol. Ther. Nucleic Acids. 2019;16:407–418. doi: 10.1016/j.omtn.2019.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Pu X., Zhang L., Zhang P., Xu Y., Wang J., Zhao X., Dai Z., Zhou H., Zhao S., Fan A. Human UC-MSC-derived exosomes facilitate ovarian renovation in rats with chemotherapy-induced premature ovarian insufficiency. Front. Endocrinol. 2023;14 doi: 10.3389/fendo.2023.1205901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Park H.-s., Chugh R.M., Seok J., Cetin E., Mohammed H., Siblini H., Liakath Ali F., Ghasroldasht M.M., Alkelani H., Elsharoud A. Comparison of the therapeutic effects between stem cells and exosomes in primary ovarian insufficiency: as promising as cells but different persistency and dosage. Stem Cell Res. Ther. 2023;14(1):1–16. doi: 10.1186/s13287-023-03397-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Xu C., Kang Y., Dong X., Jiang D., Qi M. Integration exosomes with MOF-modified multifunctional scaffold for accelerating vascularized bone regeneration. Chin. Chem. Lett. 2023;34(2) [Google Scholar]
- 458.Ma S., Wu J., Hu H., Mu Y., Zhang L., Zhao Y., Bian X., Jing W., Wei P., Zhao B. Novel fusion peptides deliver exosomes to modify injectable thermo-sensitive hydrogels for bone regeneration. Materials Today Bio. 2022;13 doi: 10.1016/j.mtbio.2021.100195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Yu W., Li S., Guan X., Zhang N., Xie X., Zhang K., Bai Y. Higher yield and enhanced therapeutic effects of exosomes derived from MSCs in hydrogel-assisted 3D culture system for bone regeneration. Biomater. Adv. 2022;133 doi: 10.1016/j.msec.2022.112646. [DOI] [PubMed] [Google Scholar]
- 460.Swanson W.B., Zhang Z., Xiu K., Gong T., Eberle M., Wang Z., Ma P.X. Scaffolds with controlled release of pro-mineralization exosomes to promote craniofacial bone healing without cell transplantation. Acta Biomater. 2020;118:215–232. doi: 10.1016/j.actbio.2020.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Guo L., Quan M., Pang W., Yin Y., Li F. Cytokines and exosomal miRNAs in skeletal muscle–adipose crosstalk. Trends Endocrinol. Metabol. 2023;34(10):666–681. doi: 10.1016/j.tem.2023.07.006. [DOI] [PubMed] [Google Scholar]
- 462.Xu T., Luo Y., Wang J., Zhang N., Gu C., Li L., Qian D., Cai W., Fan J., Yin G. Exosomal miRNA-128-3p from mesenchymal stem cells of aged rats regulates osteogenesis and bone fracture healing by targeting Smad5. J. Nanobiotechnol. 2020;18:1–18. doi: 10.1186/s12951-020-00601-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Zhang J., Liu X., Li H., Chen C., Hu B., Niu X., Li Q., Zhao B., Xie Z., Wang Y. Exosomes/tricalcium phosphate combination scaffolds can enhance bone regeneration by activating the PI3K/Akt signaling pathway. Stem Cell Res. Ther. 2016;7(1):1–14. doi: 10.1186/s13287-016-0391-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Zhang Y., Hao Z., Wang P., Xia Y., Wu J., Xia D., Fang S., Xu S. Exosomes from human umbilical cord mesenchymal stem cells enhance fracture healing through HIF‐1α‐mediated promotion of angiogenesis in a rat model of stabilized fracture. Cell Prolif. 2019;52(2) doi: 10.1111/cpr.12570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Liang B., Liang J.-M., Ding J.-N., Xu J., Xu J.-G., Chai Y.-M. Dimethyloxaloylglycine-stimulated human bone marrow mesenchymal stem cell-derived exosomes enhance bone regeneration through angiogenesis by targeting the AKT/mTOR pathway. Stem Cell Res. Ther. 2019;10:1–11. doi: 10.1186/s13287-019-1410-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466.Zha Y., Li Y., Lin T., Chen J., Zhang S., Wang J. Progenitor cell-derived exosomes endowed with VEGF plasmids enhance osteogenic induction and vascular remodeling in large segmental bone defects. Theranostics. 2021;11(1):397. doi: 10.7150/thno.50741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Willms E., Cabañas C., Mäger I., Wood M.J., Vader P. Extracellular vesicle heterogeneity: subpopulations, isolation techniques, and diverse functions in cancer progression. Front. Immunol. 2018;9:738. doi: 10.3389/fimmu.2018.00738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Wunsch B.H., Smith J.T., Gifford S.M., Wang C., Brink M., Bruce R.L., Austin R.H., Stolovitzky G., Astier Y. Nanoscale lateral displacement arrays for the separation of exosomes and colloids down to 20 nm. Nat. Nanotechnol. 2016;11(11):936–940. doi: 10.1038/nnano.2016.134. [DOI] [PubMed] [Google Scholar]
- 469.Hochstetter A., Vernekar R., Austin R.H., Becker H., Beech J.P., Fedosov D.A., Gompper G., Kim S.-C., Smith J.T., Stolovitzky G. Deterministic lateral displacement: challenges and perspectives. ACS Nano. 2020;14(9):10784–10795. doi: 10.1021/acsnano.0c05186. [DOI] [PubMed] [Google Scholar]
- 470.Platt M., Willmott G.R., Lee G.U. Resistive pulse sensing of analyte‐induced multicomponent rod aggregation using tunable pores. Small. 2012;8(15):2436–2444. doi: 10.1002/smll.201200058. [DOI] [PubMed] [Google Scholar]
- 471.Young T.W., Kappler M.P., Hockaden N.M., Carpenter R.L., Jacobson S.C. Characterization of extracellular vesicles by resistive-pulse sensing on in-plane multipore nanofluidic devices. Anal. Chem. 2023;95(45):16710–16716. doi: 10.1021/acs.analchem.3c03546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472.Im H., Shao H., Weissleder R., Castro C.M., Lee H. Nano-plasmonic exosome diagnostics. Expert Rev. Mol. Diagn. 2015;15(6):725–733. doi: 10.1586/14737159.2015.1041378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.Im H., Shao H., Park Y.I., Peterson V.M., Castro C.M., Weissleder R., Lee H. Label-free detection and molecular profiling of exosomes with a nano-plasmonic sensor. Nat. Biotechnol. 2014;32(5):490–495. doi: 10.1038/nbt.2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474.Kooijmans S., Fliervoet L., Van Der Meel R., Fens M., Heijnen H., en Henegouwen P.v.B., Vader P., Schiffelers R. PEGylated and targeted extracellular vesicles display enhanced cell specificity and circulation time. J. Contr. Release. 2016;224:77–85. doi: 10.1016/j.jconrel.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 475.Malekian F., Shamsian A., Kodam S.P., Ullah M. Exosome engineering for efficient and targeted drug delivery: current status and future perspective. J. Physiol. 2022;601(22):4853–4872. doi: 10.1113/JP282799. [DOI] [PubMed] [Google Scholar]
- 476.Lin Q., Qu M., Zhou B., Patra H.K., Sun Z., Luo Q., Yang W., Wu Y., Zhang Y., Li L. Exosome-like nanoplatform modified with targeting ligand improves anti-cancer and anti-inflammation effects of imperialine. J. Contr. Release. 2019;311:104–116. doi: 10.1016/j.jconrel.2019.08.037. [DOI] [PubMed] [Google Scholar]
- 477.Photos P.J., Bacakova L., Discher B., Bates F.S., Discher D.E. Polymer vesicles in vivo: correlations with PEG molecular weight. J. Contr. Release. 2003;90(3):323–334. doi: 10.1016/s0168-3659(03)00201-3. [DOI] [PubMed] [Google Scholar]
- 478.Sato Y.T., Umezaki K., Sawada S., Mukai S.-a., Sasaki Y., Harada N., Shiku H., Akiyoshi K. Engineering hybrid exosomes by membrane fusion with liposomes. Sci. Rep. 2016;6(1):1–11. doi: 10.1038/srep21933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479.Zhang Y., Bi J., Huang J., Tang Y., Du S., Li P. Exosome: a review of its classification, isolation techniques, storage, diagnostic and targeted therapy applications. Int. J. Nanomed. 2020:6917–6934. doi: 10.2147/IJN.S264498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480.Charoenviriyakul C., Takahashi Y., Nishikawa M., Takakura Y. Preservation of exosomes at room temperature using lyophilization. Int. J. Pharm. 2018;553(1–2):1–7. doi: 10.1016/j.ijpharm.2018.10.032. [DOI] [PubMed] [Google Scholar]