Abstract
Mammalian host factors required for efficient viral gene expression and propagation have been often recalcitrant to genetic analysis. A case in point is the function of cellular factors that trans-activate internal ribosomal entry site (IRES)-driven translation, which is operative in many positive-stranded RNA viruses, including all picornaviruses. These IRES trans-acting factors have been elegantly studied in vitro, but their in vivo importance for viral gene expression and propagation has not been widely confirmed experimentally. Here we use RNA interference to deplete mammalian cells of one such factor, the polypyrimidine tract binding protein, and test its requirement in picornavirus gene expression and propagation. Depletion of the polypyrimidine tract binding protein resulted in a marked delay of particle propagation and significantly decreased synthesis and accumulation of viral proteins of poliovirus and encephalomyocarditis virus. These effects could be partially restored by expression of an RNA interference-resistant exogenous polypyrimidine tract binding protein. These data indicate a critical role for the polypyrimidine tract binding protein in picornavirus gene expression and strongly suggest a requirement for efficient IRES-dependent translation.
Picornaviruses encode a single polyprotein in one plus-strand RNA that is missing a 5′ guanosine cap (36). The uncapped genomic RNA directs translation initiation via the internal ribosomal entry site (IRES), a highly structured cis-acting element in the 5′ nontranslated region of the viral genome (25, 37, 38, 48; reviewed in references 19, 24, and 44). Based on structural differences, picornavirus IRESs are either classified as type 1 (Enterovirus and Rhinovirus genera) or type 2 (Cardiovirus and Aphthovirus genera) (53). In addition to picornaviruses, other viral RNAs (e.g., hepatitis C virus [HCV]) (48) and some cellular mRNAs may also contain IRESs (reviewed in references 19 and 44). As its name suggests, the IRES recruits the 40S ribosomal subunits and conventional translation factors in a 5′ end-, cap-independent manner (38). IRESs also recruit other cellular proteins that have no known role in cap-dependent translation but activate IRES function in vitro (reviewed in reference 19). These activating proteins, or IRES trans-acting factors (ITAFs), have previously been identified as ITAF45 (which is also known as Mpp1) (41), the La autoantigen (32), poly(rC) binding protein 2 (5), unr and unr-interacting protein (22), the HCV NS1-associated protein (30), and the polypyrimidine tract binding protein (PTB) (2, 6, 20, 54).
PTB has been implicated in many and diverse RNA transactions (50, 51). In the nucleus, PTB is required for silencing alternative exons in FGFR2 and fibronectin and within its own transcripts and is likely involved in silencing many other regulated exons (8, 9, 31, 50-52, 56). Additionally, PTB has been implicated in several cytoplasmic events other than IRES-driven translation. PTB is important for the localization of the Vg-1 mRNA in Xenopus laevis oocytes (10) and for the control of insulin mRNA stability in mammalian cells (47). Although PTB has been implicated in the function of viral and cellular IRESs in vitro, a requirement for PTB in vivo remains controversial (23, 28, 34, 38, 40, 43). Depletion of PTB in vitro results in inhibition of IRES translation; however, the addition of purified PTB has sometimes failed to restore activity (20). Addition of PTB to in vitro translation systems enhances translation driven by an encephalomyocarditis virus (EMCV) IRES only twofold (23, 28), whereas PTB addition is required for translation driven by the foot-and-mouth disease virus IRES and Theiler′s murine encephalomyelitis virus (TMEV) IRESs (43). The variability of the effects observed, the difficulties with biochemical complementation, and inherent questions about in vitro systems in general underscore the need to resolve whether PTB is an ITAF in vivo. Some attempts have been made to extend the analysis in vivo. Gosert et al. (2000) showed that PTB overexpression leads to increased reporter expression when translation is driven by a picornavirus (poliovirus [PV] or hepatitis A virus) or a flavivirus (HCV) IRES (15). Pilipenko et al. (2001) showed that the capacity of variant TMEV IRESs to bind neural PTB, a homologue of PTB, correlates with the ability to promote viral replication, implying a role in vivo (42). A further attempt to demonstrate in vivo relevance employed the expression of RNA decoys selected to bind and sequester PTB. Although these decoys inhibited IRES-dependent translation in vivo, it was not possible to assess the specificity of their effect (3). Recently, PTB knockdown was achieved by an adenovirus-delivered siRNA and shown to inhibit HCV replicon replication in HCV-permissive cells (57). Although, this work demonstrated inhibition of viral replication, it did not properly control for off-target effects of siRNAs.
This study was initiated to address the requirement for PTB in IRES function, and more importantly in picornavirus propagation, in vivo. In this report we make this requirement evident by taking advantage of RNA interference (RNAi) methods, which can efficiently and specifically deplete PTB in vivo. PTB depletion using multiple siRNAs resulted in a marked delay and reduction of PV growth and in significant reduction in the synthesis and accumulation of viral proteins. PTB depletion had equally profound effects on gene expression and proliferation of EMCV, which has a different class of IRES than poliovirus. Furthermore, complementation of PTB-depleted cells with an RNAi-resistant PTB partially rescued viral protein production and particle propagation. RNAi-mediated PTB depletion did not alter gene expression of respiratory syncytial virus, a member of Paramyxoviridae, suggesting that RNA interference did not set up a pleiotropic antiviral state. We also show that PTB redistributes to the cytoplasm early after polioviral infection, which is consistent with a cytoplasmic function for a protein that is predominantly nuclear. These data indicate a critical role for PTB in picornavirus gene expression and suggest a requirement for efficient IRES-dependent translation.
MATERIALS AND METHODS
Viruses.
A poliovirus type 1 (Sabin) [PV1(S)] infectious cDNA clone was obtained from Akio Nomoto (University of Tokyo, Japan) and was used to derive and propagate virus by established procedures (16). A tissue culture adapted EMCV strain isolated from a chimpanzee (Florida, 1944) was obtained from the American Type Culture Collection (no. VR-129B). The chimeric virus PV1(ENPOS), featuring the EMCV IRES within a poliovirus background containing the coding region for the capsid proteins of PV1(S), has been described before (1, 16). PV1(ENPOS)-6A was derived from PV1(ENPOS) as follows: two contiguous PCR fragments encompassing the EMCV IRES were generated using primers 5′-CCGAATTCCCCCCCCCCTAA-3′ and 5′-CCTCGACTAAACACATGTAAAGC-3′ as well as 5′-CCTCGAGGTTAAAAAACGTCTAGG-3′ and 5′-CCTGAGCTCCCATATTATCATCGTG-3′. Ligation of both amplicons with the PV1(ENPOS) cDNA digested with EcoRI and SacI yielded PV1(ENPOS)-6A. In vitro transcription of viral infectious RNA, RNA transfection, and virus recovery were performed as described before (16).
Respiratory syncytial virus (RSV) was derived from a field isolate originally obtained from respiratory secretions from a patient with acute respiratory disease (Washington, D.C., 1952; obtained from American Type Culture Collection) and propagated and purified according to standard procedures (a kind gift of Robert Brazas in the Garcia-Blanco laboratory). For Western blot detection of RSV gene expression, we used hyperimmune polyclonal anti-RSV serum generated in goat (Fitzgerald Co.).
siRNA transfection procedures.
We employed oligoribonucleotides P9 (described in reference 52 as PTB siRNA duplex), P2 (described in reference 56 as PTB P2), P1 (described in reference 56 as PTB P1), C2 (described in reference (52 as nonspecific siRNA duplex), and luc (described in reference 12). siRNAs were created by annealing the sense to the antisense RNA oligonucleotides (all oligoribonucleotides were obtained from Dharmacon). siRNA transfections were performed using a modification of the protocol of Wagner and Garcia-Blanco, 2002 (52): cells were plated to a density of 1 × 105 cells per well of a 24-well plate and a day later transfected with 100 nM siRNA in Lipofectamine 2000 (Invitrogen). The following day, cells were trypsinized, replated into a single well of a six-well plate and allowed to grow overnight, and transfected with 25 nM siRNA. At 72 h posttransfection, the cells were counted using a hemocytometer and plated to a density of 5 × 105 cells per 35-mm well. After incubation overnight at 37°C, the cultures were infected with virus.
For the complementation experiment, cells were treated in an identical fashion with the following exception: following the second transfection of siRNA, cells were allowed to recover overnight. The following day, cells were transfected (Lipofectamine; Invitrogen) with 2 μg of pEGFP-N1 (Clontech), pCMV-SPORT-PTB1, or pCMV-SPORT-PTB4. Cells were then allowed to grow for 18 h preceding infection as described above.
One-step growth curves and analyses of viral gene expression.
Following treatment with siRNAs, respectively, HeLa R19 or HeLa S3 cells were infected at a multiplicity of infection (MOI) of 10 with either PV1(S), EMCV, PV1(ENPOS), or PV1(ENPOS)-6A. Infected cells were rocked for 30 min at room temperature (RT) and rinsed three times with 2 ml of serum-free Dulbecco's minimal essential medium. After adding 1.5 ml of Dulbecco's minimal essential medium containing 2% fetal bovine serum, the cells were incubated at 37°C. At the indicated time points, the cells were freeze thawed twice and the resulting lysate was resuspended through repeated pipetting and rigorously vortexed. A 50-μl aliquot was removed to determine the virus titer by plaque assay as described elsewhere (16). PV1(ENPOS), PV1(ENPOS)-6A, and PV1(S) were plaqued on HeLa R19 cells, whereas EMCV was plaqued on Vero cells. The results of the one-step growth curves indicated as PFU per ml were the averages of duplicate or triplicate samples. The remaining lysate was centrifuged at 13,200 rpm for 3 min. The pellet fractions were resuspended in 200 μl phosphate-buffered saline (PBS) containing 0.5% NP-40. After determination of the total protein concentration through Bradford assay (Bio-Rad), samples with equal total protein content were processed for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting essentially as described previously (11, 52). Poliovirus or EMCV gene expression was assayed by Western blotting using anti-poliovirus 2C monoclonal antibody (kindly provided by E. Wimmer, Stony Brook, NY) or anti-EMCV 3D monoclonal antibody (kindly provided by A. Palmenberg, Madison, WI), respectively.
Metabolic labeling of poliovirus-infected HeLa R19 cells.
After treatment with P9 or C2 siRNAs, HeLa monolayers were infected with PV1(S) at an MOI of 10 following procedures described before (16). At the desired intervals, the cells were overlaid with methionine-deficient medium for 40 min and pulsed with [35S]methionine (300 μCi/ml) added to the medium. After incubation for 10 min at 37°C in the presence of the label, the cells were rinsed thoroughly and lysed in SDS-PAGE loading buffer, and the resulting lysate was analyzed by SDS-PAGE as described elsewhere (35).
Luciferase-expressing HeLa cells.
HeLa R19 cells were stably transfected with pcDNA 6/V5-HisA (Invitrogen) containing the open reading frame for the pGL3 firefly luciferase (Promega) cloned into the multiple cloning site with HindIII and AgeI and selected for blasticidin resistance according to standard protocols. For siRNA transfection experiments, the cells were carried in the absence of blasticidin. Firefly luciferase activity assays were performed according to Promega′s instructions in a Berthold luminometer (no. LB9507).
Immunofluorescence.
HeLa cells were infected with PV1(S) as described above and fixed in 3.7% formaldehyde for 15 min at various time points postinfection (p.i.). All samples were blocked for 1 h at RT with PBS containing 5 mg/ml bovine serum albumin. Subsequently, the samples were overlaid with PBS containing either polyclonal anti-PTB antiserum (Intronn, Inc.) at a 1:1,000 dilution or anti-SC35 mouse monoclonal antibody (Santa Cruz) at a 1:3,000 dilution. The fixed cells were incubated for 1 h at RT with the primary antibody followed by 3 5-min washes in PBS containing 5 mg/ml bovine serum albumin and 0.4% Tween 20. Subsequently, the cells were incubated with anti-rabbit immunoglobulin G (conjugated with rhodamine) and anti-mouse immunoglobulin G (conjugated with fluorescein) secondary antibodies according to the manufacturer's protocol (Jackson Immunolabs). After three rinse cycles in washing medium, the cells were overlaid with mounting medium (ProLong Antifade kit; Molecular Probes), covered, and imaged using standard techniques.
RESULTS
PTB is required for efficient PV protein synthesis and propagation. To examine the role of PTB in picornavirus propagation, we significantly reduced PTB levels in HeLa R19 cells using P9, a PTB-targeted siRNA, as previously described (52) (Fig. 1A ). Control cultures were treated with C2, which does not target any human mRNA for RNAi (52) (Fig. 1A). To probe the effect of PTB depletion on PV1(S) translation, we detected [35S]methionine incorporation in P9 and C2 siRNA-treated PV1(S)-infected HeLa cells. This and all other infections described in this report were carried out at an MOI of 10. In mock-depleted cells, the synthesis of viral gene products became apparent by 4 h p.i. and was pronounced by 10 h p.i., when it was accompanied by a clear shutoff of host cell translation (Fig. 1B). RNAi-mediated PTB knockdown reduced the rate of viral protein synthesis throughout the course of the experiment and, importantly, prevented efficient shutoff of host cell translational activity. The latter observation is evidenced by the appearance of numerous labeled cellular polypeptides as late as 10 h p.i. in P9-treated cells, when mock-depleted cells solely produced viral gene products (Fig. 1B). Our findings suggest that PTB is required for efficient viral translation at a very early stage, delaying the accumulation of viral gene products from the onset. Since host cell protein synthesis shutoff is a by-product of the activity of viral gene products, reduced viral gene expression in early phases of the infectious cycle is likely to prevent this effect in PTB-depleted infected cells. This experiment also indicated that PTB depletion did not affect global host protein synthesis, which is consistent with the observations that cells transiently depleted of PTB continue to grow, eventually recovering PTB levels (52).
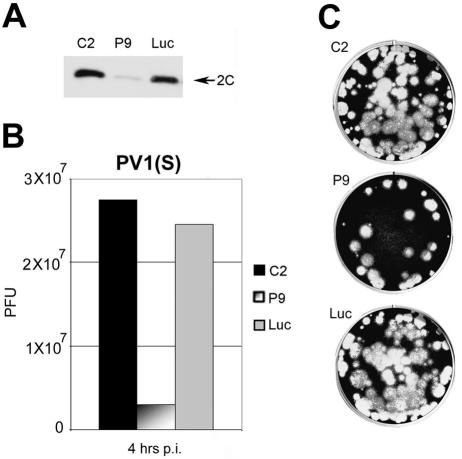
FIG. 1.
PTB knockdown reduces translation of PV gene products and prevents efficient shutoff of host cell protein synthesis. (A) Western blot analysis demonstrates knockdown of PTB in HeLa R19 cells transfected with P9 (PTB) siRNA compared to the C2 (control) siRNA in duplicate samples. Cross-reacting proteins are not affected by P9 treatment. (B) Metabolic labeling of PV1(S)-infected HeLa cells treated with either P9 or C2 siRNA was carried out at the indicated time points as described in Materials and Methods. The identities of the most abundant viral polypeptides are indicated on the right.
We then investigated the effect of PTB knockdown on PV propagation. PTB knockdown with the P9 siRNA resulted in a reduction in the cytopathic effect (CPE) (data not shown), a fall in viral 2C protein accumulation (Fig. 2A), and a dramatic decrease in the yield of PV1(S) progeny (Fig. 2B and C). In these experiments we assessed the dynamics of PV1(S) growth in HeLa R19 cells that ectopically express luciferase (luc) in order to use luc knockdowns as a specificity control. Whereas treatment with the luc siRNA led to a threefold decrease in luc activity relative to that observed with C2 siRNA (not shown), luc depletion did not affect PV1(S) gene expression (Fig. 2A) or particle propagation (Fig. 2B and C). These data indicated that activation of RNAi per se did not account for reduced PV1(S) growth after PTB knockdown and strongly suggested a role for PTB in PV propagation. We felt, however, that it was important to further evaluate the specificity of the RNA interference.
FIG. 2.
siRNA-mediated PTB knockdown inhibits PV propagation. (A) Knockdown of PTB with P9 siRNA reduced viral gene expression at 4 h p.i., while C2 siRNA- and luc siRNA-treated samples did not affect accumulation of PV1(S) 2C protein. (B) Viral yield 4 h p.i. after a one-step growth of PV-infected HeLa R19, luc-expressing cells treated with C2, P9, or luc siRNAs. The bars indicate range of duplicate samples. (C) PV1(S) progeny after infection of HeLa R-19 as determined by plaque assay from lysates shown in B.
Repression of viral propagation in PTB-depleted cells is rescued by PTB reconstitution.
To solidify our observations and exclude bias due to the specific sequence of the P9 PTB-targeting siRNA, we evaluated PV1(S) propagation phenotypes in cells treated with siRNAs targeting diverse regions of the PTB message. We tested three different siRNAs (P9, P2, and P1) targeting different regions of the PTB message (see Materials and Methods). HeLa R19 cells treated with C2 P9, P2, or P1 siRNAs, respectively, were infected with PV1(S) and assayed for viral gene expression and viral propagation at 4 h p.i. All PTB-targeting siRNAs led to a decrease in PTB levels, albeit to different degrees (Fig. 3A), and the effectiveness of the PTB knockdown corresponded directly to the decrease in PV1(S) propagation (Fig. 3B). These results constitute strong evidence that inhibition of PV propagation is not due to siRNA off-target or nonspecific effects but rather is a direct consequence of PTB depletion.
FIG. 3.
PTB is required for efficient PV propagation. (A) Western blot analysis of lysates collected from HeLa R19 cells treated with either C2 or PTB (P9, P2, or P1) siRNAs and infected with PV1(S). Blots were probed with anti-PTB antiserum. The asterisk marks a cross-reacting protein. (B) One-step growth kinetics in PV1(S)-infected HeLa R19 cells treated with either controls (C2 or luc) or PTB (P9, P2, or P1) siRNA at 0 and 4 h p.i. The bars indicate the range of duplicate samples. (C) Western blot analysis of lysates collected from HeLa R19 cells treated with either C2 or P1 siRNAs, transfected with plasmids that express either empty vector alone, PTB1, or PTB4 and infected PV1(S). Blots were probed with anti-PTB antiserum. (D) One-step growth kinetics in PV1(S)-infected HeLa R19 cells treated with either C2 or P1 siRNAs and, where indicated, transfected with plasmids that express either empty vector, PTB1, or PTB4. The bars indicate standard deviation of triplicate samples. (E) Representative plaque assays (5 h p.i.) from the reconstitution experiment shown in (D).
Since P1 targets the 5′ untranslated region of the PTB message, depletion with P1 siRNA opened the possibility of reconstituting PTB expression from a cDNA featuring a heterologous 5′ untranslated region not targeted by P1 siRNA. Transfection of cDNAs encoding either PTB1 or PTB4 in HeLa cells treated with P1 siRNA replenished the levels of these PTB isoforms (Fig. 3C). Accordingly, PV1(S) infection responded to PTB reconstitution with a reproducible increase in particle propagation, with PTB4 providing slightly greater complementation than PTB1 (Fig. 3D and E). No such increase was observed when His-LacZ, enhanced green fluorescent protein, or HA-SLBP (an unrelated RNA binding protein) was overexpressed (not shown). Taken together, viral growth repression by multiple independent PTB siRNAs and its partial reversal by PTB reconstitution point to a specific effect of PTB on PV propagation.
RNAi-mediated PTB depletion does not lead to a general antiviral state.
To test the possibility that PTB depletion could lead to a generalized antiviral state, we asked whether RNAi-mediated PTB depletion affected gene expression of RSV, a minus-strand RNA virus of the Paramyxovirus family. RSV gene expression, which is mediated by cap-dependent initiation of translation, would be predicted to be insensitive to PTB knockdown. Indeed, we observed that the kinetics of RSV gene expression was not affected by RNAi-mediated depletion of PTB (Fig. 4). We concluded from these experiments that neither PTB depletion nor the activation of RNAi led to a generalized antiviral state.
FIG. 4.
siRNA-induced PTB depletion does not affect RSV gene expression. HeLa R19 cells treated with C2 (control) or P9 (PTB) siRNAs were subjected to synchronized infection with RSV. Infected-cell samples were treated with SDS loading buffer at the indicated time points and analyzed by SDS-PAGE and Western blotting for the presence and accumulation dynamics of RSV gene products. The identities of the most abundant viral polypeptides are indicated on the right.
PTB is required for gene expression and propagation of EMCV, which contains a type 2 IRES.
To evaluate the importance of PTB for type 2 IRES function, we tested the effect of PTB depletion on EMCV gene expression and propagation. HeLa-S3 cells were mock depleted with C2 siRNA or depleted of PTB with P9 siRNA, respectively (Fig. 5A, left panel). Subsequently, siRNA-treated cells were infected with EMCV. As observed with PV1(S), PTB knockdown caused a profound and enduring inhibition of 3Dpol accumulation (Fig. 5A, right panel) and EMCV propagation (Fig. 5B and C). Our observations suggest a requirement of PTB for gene expression and propagation of cardioviruses.
FIG. 5.
PTB knockdown inhibits accumulation of gene products and replication of EMCV. (A) Western blot analysis demonstrates knockdown of endogenous PTB in HeLa S3 cells transfected with C2 (control) siRNA compared to P9 (PTB) siRNA (left panel). A cross-reacting protein, which is seen in HeLa S3 cells, is not affected by P9 treatment. Western blot analysis of lysates collected from HeLa S3 cells treated with either C2 or P9 siRNAs and infected with EMCV. Blots were probed for the EMCV 3D protein; the asterisk marks a cross-reacting protein indicating equal loading (right panel). (B) EMCV progeny after infection of HeLa S3 as determined by plaque assay from lysates shown in A. (C) One-step growth kinetics in EMCV-infected HeLa S3 cells treated with either C2 or P9 siRNAs. It should be noted that while the results of the one-step growth kinetics represent the average of duplicate samples, the Western blot results show levels of EMCV 3D for only one of these samples. In the few cases where the samples had large variance (e.g., the 6-h p.i. samples), it is not possible to make direct comparisons between levels of 3D and viral progeny.
A widely used EMCV IRES employed in in vitro translation studies and expression vectors contains an insertion of a single adenosine residue in the “A-bulge” at the junction of stem-loop domains J and K (Fig. 6A), expanding the bulge to seven adenosines rather than six, as found in most EMCV field isolates (28). The additional adenosine makes the IRES PTB dependent in vitro, while a wild-type EMCV IRES was minimally PTB dependent (28).
FIG. 6.
The 6A and 7A forms of the EMCV IRES are equally dependent on PTB in vivo. (A) Schematic of the genomes of PV1(ENPOS)-6A and PV1(ENPOS)-7A. The heterologous EMCV IRES and the position of the “A-bulge” are indicated. (B) One-step growth curve of PV1(ENPOS)-6A and PV1(ENPOS)-7A in HeLa R19 cells treated with either control (C2) or PTB (P9) siRNA. The values are averages of triplicate samples, and the bars indicate standard deviation. (C) Progression of CPE in PV1(ENPOS)-6A-infected HeLa R19 cells after treatment with C2 and P9 siRNAs, respectively.
In order to directly investigate this in vivo, we decided to test the two “A-bulge” variants in the same genetic context. To this end we infected cells with the chimeric viruses PV1(ENPOS)-7A and PV1(ENPOS)-6A, where the cognate poliovirus IRES had been replaced with a seven-adenosine and a six-adenosine EMCV IRES, respectively (Fig. 6A) (the construction and characterization of PV1(ENPOS) is described in reference 16). In cells treated with C2 siRNA, vigorous propagation of both PV1(ENPOS)-7A and PV1(ENPOS)-6A occurred (Fig. 6B). Viral growth of PV1(ENPOS)-6A was consistently accelerated compared with the 7A variant (Fig. 6B). In PTB-depleted cells (P9), in step with the inhibition of viral growth, the progression of CPE was profoundly diminished regardless of the A-bulge structure of the chimeric virus used [Fig. 6C; only results for PV1(ENPOS)-6A are shown]. Our observations demonstrate that in vivo, in an identical genetic context, the six- and the seven-adenosine A-bulge EMCV IRESs appear equally sensitive to PTB depletion.
Cytoplasmic levels of PTB dramatically increase early after PV infection.
Although PTB has been found to shuttle between the nucleus and the cytoplasm (27), its localization is predominantly nuclear (21). Since very low levels of cytoplasmic PTB may be incompatible with vigorous IRES function, we predicted that it would be recruited to the cytoplasm early during picornavirus infection. Others have reported relocation of nuclear factors following PV infection (45), possibly due to viral protease-mediated degradation of nuclear pore complex components (17, 18). Previously, Back et al. (2002) showed that PTB is localized in the cytoplasm by 6 h after poliovirus infection (4). By that time, however, PTB is cleaved by the viral protease 3Cpro, and most of the cytoplasmic PTB must be proteolysed (4). In order to evaluate PTB localization early after PV1(S) infection, we performed indirect immunofluorescence. Whereas in uninfected cells PTB was almost exclusively nuclear, PV infection led to the appearance of PTB in the cytoplasm, which could be seen as early as 3 h p.i. (Fig. 7A). The cytoplasmic relocation of PTB is not due to a global loss of nuclear integrity, since the localization of splicing factor SC35 is unaffected at 3 h p.i. (Fig. 7A) (17). At this early time p.i., there is no evidence of PTB cleavage (Fig. 7B). These data led us to conclude that intact PTB accumulates in the cytoplasm early after PV infection, which is consistent with a requirement for PTB in PV IRES function.
FIG. 7.
PTB relocates to the cytoplasm early after PV infection and prior to cleavage by the PV 3Cpro protease. (A) HeLa R19 cells infected with PV1(S) were fixed at the indicated time points p.i. The subcellular localization of PTB or SC35 at the indicated time points was determined by indirect immunofluorescence; the nuclei were stained with 4′,6-diamidino-2-phenylindole. (B) The integrity of PTB was assayed by Western blot analysis of lysates collected at the indicated times p.i.
DISCUSSION
Well-controlled RNAi-mediated PTB depletion in vivo provides the first direct and conclusive evidence that PTB is required for efficient picornavirus gene expression and propagation. PTB binds pyrimidine-rich sequences in IRESs (19, 39, 49) and is likely to exert its function by modulating RNA structure and/or by interacting with other ITAFs or canonical translation factors (34). Multiple PTB binding sites appear to be required for function of the TMEV IRES, suggesting that PTB may act as an RNA chaperone maintaining proper IRES structure (44).
Recent empirical evidence suggests that exogenous RNAi activation may induce nonspecific responses, which may alter cellular susceptibility to virus infection (7, 46). Such responses could result from the cellular response to administration of siRNA, “leaky” targeting of unintended messages, or off-target effects of RNAi activation. We designed our approach to rule out unintended nonspecific effects of RNAi induction. Administration of siRNAs used in our studies did not set up a pleiotropic antiviral state. Treatment with the nontargeting C2 control siRNA and the targeting luc siRNA failed to inhibit PV1(S) replication, and PTB depletion with P9 did not affect RSV gene expression, albeit RSV is known to be relatively insensitive to interferon. The effect of PTB depletion on PV1(S) growth occurred with three different siRNAs targeting diverse regions of the PTB message. Thus, inhibition of viral gene expression and propagation is unlikely to result from depletion of unintended targets. Last, siRNA depletion of luc in luc-expressing HeLa cells did not alter the kinetics of PV1(S) propagation, demonstrating that the activation of RNAi on its own is not responsible for the observed phenomenon.
Complementation of the RNAi effects provides the most straightforward means to exclude unintended consequences of the exogenous activation of RNAi. Although we were unable to fully restore competent virus propagation through reconstitution of PTB in siRNA-treated cells, we did achieve a significant increase in viral gene expression and particle propagation. Although complementation does not formally rule out all potential artifacts, it provides compelling evidence that PTB is required for efficient picornavirus propagation.
Cells containing low levels of PTB are poor hosts for both EMCV and PV propagation, indicating that PTB is an essential host factor for efficient growth of picornaviruses. Our observation of an early delay of PV IRES-driven gene expression and host cell protein shutoff by PTB depletion points towards a role of PTB in early phases of the infectious cycle. The conditions for translation initiation in the PV-infected cell dramatically change with the progression of the infectious process. Early on, a small number of recently uncoated viral genomes have to compete with the cap-dependent translation machinery in the intact host cell. In later phases, effective competition with host cell protein synthesis through virus-induced cleavage of conventional translation factors [the eukaryotic initiation factor 4G (13) and poly(A) binding protein (29)] and vast amounts of replicating template generate an environment greatly favoring IRES-mediated translation initiation. Our findings suggest that the ITAF role played by PTB may be less relevant during late stages of the PV life cycle, an assumption that is supported by efficient IRES function despite virus-induced PTB cleavage in later phases of the infection.
We also show that soon after picornavirus infection, PTB is summoned out of the nucleus, an event that may facilitate IRES-driven translation and, consequently, virus propagation. As infection progresses, PTB is cleaved, possibly favoring a switch from viral gene expression to viral genome replication and packaging of template RNA genomes (4). The cleavage appears selective, since the PTB1 isoform is more resistant to virus-induced proteolysis than PTB4. The physiological significance of this is not clear. Whereas the PTB1 isoform is more potent as an ITAF than PTB4 in vitro (55), our data do not fully support this conclusion in vivo.
RNAi-mediated depletion can be a powerful tool for studying the role of host factors required for virus propagation and consequently in the discovery and validation of antiviral drug targets. PTB depletion represses viral growth, suggesting that an early event in the viral life cycle, i.e., IRES-mediated translation, is critically dependent on PTB. A similar delay in viral replication was observed when siRNAs targeted PV RNA directly (14). It is reasonable to assume that antiviral agents targeting the PTB-IRES interaction would affect viral replication similarly to PTB depletion. This has implications not only for all picornavirus IRESs but also for the HCV IRES, which has been singled out as a target for antiviral therapy (26). The disruption of an essential ITAF achieved by RNAi demonstrates the potential for identifying molecular targets for antiviral chemotherapy in vivo. Furthermore, our experimental approach may be useful for the study of cellular processes that involve IRES-dependent translation of eukaryotic messages (33, 34, 40).
Acknowledgments
We thank E. Wimmer (SUNY Stony Brook) for the anti-PV 2C and A. Palmenberg (U. of Wisconsin, Madison) for the anti-EMCV 3D monoclonal antibody. We thank Robert Brazas for RSV and C. Smith (Cambridge, United Kingdom) for PTB expression plasmids. We thank Shelton Bradrick, Glen Coburn, Matt Wollerton, and Robert Brazas for critical reading of the manuscript and members of the Garcia-Blanco and Gromeier laboratories for helpful discussions. We thank Douglas Black for helpful discussions and for sharing data before publication. We also thank Annette Kennett for her assistance in the preparation of the manuscript.
The research was supported by PHS grants to M.A.G.-B. (GM63090) and to M.G. (CA87537) and by the ABC2 Foundation (to M.G.). M.G. is a recipient of a Burroughs Wellcome Career Award in the Biomedical Sciences. E.J.W. acknowledges the support of a Department of Defense predoctoral fellowship. P.F. acknowledges the support of research supplement for underrepresented minorities (NIH).
REFERENCES
- 1.Alexander, L., H. H. Lu, and E. Wimmer. 1994. Polioviruses containing picornavirus type 1 and/or type 2 internal ribosomal entry site elements: genetic hybrids and the expression of a foreign gene. Proc. Natl. Acad. Sci. USA 91:1406-1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ali, N., and A. Siddiqui. 1995. Interaction of polypyrimidine tract-binding protein with the 5′ noncoding region of the hepatitis C virus RNA genome and its functional requirement in internal initiation of translation. J. Virol. 69:6367-6375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anwar, A., N. Ali, R. Tanveer, and A. Siddiqui. 2000. Demonstration of functional requirement of polypyrimidine tract-binding protein by SELEX RNA during hepatitis C virus internal ribosome entry site-mediated translation initiation. J. Biol. Chem. 275:34231-34235. [DOI] [PubMed] [Google Scholar]
- 4.Back, S. H., Y. K. Kim, W. J. Kim, S. Cho, H. R. Oh, J.-E. Kim, and S. K. Jang. 2002. Translation of polioviral mRNA is inhibited by cleavage of polypyrimidine tract-binding proteins executed by polioviral 3Cpro. J. Virol. 76:2529-2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blyn, L. B., K. M. Swiderek, O. Richards, D. C. Stahl, B. L. Semler, and E. Ehrenfeld. 1996. Poly(rC) binding protein 2 binds to stem-loop IV of the poliovirus RNA 5′ noncoding region: identification by automated liquid chromatography-tandem mass spectrometry. Proc. Natl. Acad. Sci. USA 93:11115-11120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borman, A., M. T. Howell, J. G. Patton, and R. J. Jackson. 1993. The involvement of a spliceosome component in internal initiation of human rhinovirus RNA translation. J. Gen. Virol. 74:1775-1788. [DOI] [PubMed] [Google Scholar]
- 7.Bridge, A. J., S. Pebernard, A. Ducraux, A. L. Nicoulaz, and R. Iggo. 2003. Induction of an interferon response by RNAi vectors in mammalian cells. Nat. Genet. 34:263-264. [DOI] [PubMed] [Google Scholar]
- 8.Charlet, B. N., P. Logan, G. Singh, and T. A. Cooper. 2002. Dynamic antagonism between ETR-3 and PTB regulates cell type-specific alternative splicing. Mol. Cell 9:649-658. [DOI] [PubMed] [Google Scholar]
- 9.Chou, M. Y., J. G. Underwood, J. Nikolic, M. H. Luu, and D. L. Black. 2000. Multisite RNA binding and release of polypyrimidine tract binding protein during the regulation of c-src neural-specific splicing. Mol. Cell 5:949-957. [DOI] [PubMed] [Google Scholar]
- 10.Cote, C. A., D. Gautreau, J. M. Denegre, T. L. Kress, N. A. Terry, and K. L. Mowry. 1999. A Xenopus protein related to hnRNP I has a role in cytoplasmic RNA localization. Mol. Cell 4:431-437. [DOI] [PubMed] [Google Scholar]
- 11.Dobrikova, E. Y., P. Florez, and M. Gromeier. 2003. Structural determinants of insert retention of poliovirus expression vectors with recombinant IRES elements. Virology 311:241-253. [DOI] [PubMed] [Google Scholar]
- 12.Elbashir, S. M., J. Harborth, W. Lendeckel, A. Yalcin, K. Weber, and T. Tuschl. 2001. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 411:494-498. [DOI] [PubMed] [Google Scholar]
- 13.Etchison, D., S. C. Milburn, I. Edery, N. Sonenberg, and J. W. Hershey. 1982. Inhibition of HeLa cell protein synthesis following poliovirus infection correlates with the proteolysis of a 220,000-dalton polypeptide associated with eucaryotic initiation factor 3 and a cap binding protein complex. J. Biol. Chem. 257:14806-14810. [PubMed] [Google Scholar]
- 14.Gitlin, L., S. Karelsky, and R. Andino. 2002. Short interfering RNA confers intracellular antiviral immunity in human cells. Nature 418:430-434. [DOI] [PubMed] [Google Scholar]
- 15.Gosert, R., K. H. Chang, R. Rijnbrand, M. Yi, D. V. Sangar, and S. M. Lemon. 2000. Transient expression of cellular polypyrimidine-tract binding protein stimulates cap-independent translation directed by both picornaviral and flaviviral internal ribosome entry sites in vivo. Mol. Cell. Biol. 20:1583-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gromeier, M., L. Alexander, and E. Wimmer. 1996. Internal ribosomal entry site substitution eliminates neurovirulence in intergeneric poliovirus recombinants. Proc. Natl. Acad. Sci. USA 93:2370-2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gustin, K. E., and P. Sarnow. 2001. Effects of poliovirus infection on nucleo-cytoplasmic trafficking and nuclear pore complex composition. EMBO J. 20:240-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gustin, K. E., and P. Sarnow. 2002. Inhibition of nuclear import and alteration of nuclear pore complex composition by rhinovirus. J. Virol. 76:8787-8796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hellen, C. U., and P. Sarnow. 2001. Internal ribosome entry sites in eukaryotic mRNA molecules. Genes Dev. 15:1593-1612. [DOI] [PubMed] [Google Scholar]
- 20.Hellen, C. U., G. W. Witherell, M. Schmid, S. H. Shin, T. V. Pestova, A. Gil, and E. Wimmer. 1993. A cytoplasmic 57-kDa protein that is required for translation of picornavirus RNA by internal ribosomal entry is identical to the nuclear pyrimidine tract-binding protein. Proc. Natl. Acad. Sci. USA 90:7642-7646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang, S., T. J. Deerinck, M. H. Ellisman, and D. L. Spector. 1997. The dynamic organization of the perinucleolar compartment in the cell nucleus. J. Cell Biol. 137:965-974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hunt, S. L., J. J. Hsuan, N. Totty, and R. J. Jackson. 1999. unr, a cellular cytoplasmic RNA-binding protein with five cold-shock domains, is required for internal initiation of translation of human rhinovirus RNA. Genes Dev. 13:437-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hunt, S. L., and R. J. Jackson. 1999. Polypyrimidine-tract binding protein (PTB) is necessary, but not sufficient, for efficient internal initiation of translation of human rhinovirus-2 RNA. RNA 5:344-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jackson, R. J. 2000. A comparative view of initiation site mechanisms, p. 127-183. In N. Sonenberg, J. W. B. Hershey, and M. B. Mathews (ed.), Translational control of gene expression. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 25.Jang, S. K., H. G. Krausslich, M. J. Nicklin, G. M. Duke, A. C. Palmenberg, and E. Wimmer. 1988. A segment of the 5′ nontranslated region of encephalomyocarditis virus RNA directs internal entry of ribosomes during in vitro translation. J. Virol. 62:2636-2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jubin, R. 2001. Hepatitis C IRES: translating translation into a therapeutic target. Curr. Opin. Mol. Ther. 3:278-287. [PubMed] [Google Scholar]
- 27.Kamath, R. V., D. J. Leary, and S. Huang. 2001. Nucleocytoplasmic shuttling of polypyrimidine tract-binding protein is uncoupled from RNA export. Mol. Biol. Cell 12:3808-3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaminski, A., and R. J. Jackson. 1998. The polypyrimidine tract binding protein (PTB) requirement for internal initiation of translation of cardiovirus RNAs is conditional rather than absolute. RNA 4:626-638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kerekatte, V., B. D. Keiper, C. Badorff, A. Cai, K. U. Knowlton, and R. E. Rhoads. 1999. Cleavage of poly(A)-binding protein by coxsackievirus 2A protease in vitro and in vivo: another mechanism for host protein synthesis shutoff? J. Virol. 73:709-717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim, J. H., K. Y. Paek, S. H. Ha, S. Cho, K. Choi, C. S. Kim, S. H. Ryu, and S. K. Jang. 2004. A cellular RNA-binding protein enhances internal ribosomal entry site-dependent translation through an interaction downstream of the hepatitis C virus polyprotein initiation codon. Mol. Cell. Biol. 24:7878-7890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu, H., W. Zhang, R. B. Reed, W. Liu, and P. J. Grabowski. 2002. Mutations in RRM4 uncouple the splicing repression and RNA-binding activities of polypyrimidine tract binding protein. RNA 8:137-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meerovitch, K., Y. V. Svitkin, H. S. Lee, F. Lejbkowicz, D. J. Kenan, E. K. Chan, V. I. Agol, J. D. Keene, and N. Sonenberg. 1993. La autoantigen enhances and corrects aberrant translation of poliovirus RNA in reticulocyte lysate. J. Virol. 67:3798-3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mitchell, S. A., E. C. Brown, M. J. Coldwell, R. J. Jackson, and A. E. Willis. 2001. Protein factor requirements of the Apaf-1 internal ribosome entry segment: roles of polypyrimidine tract binding protein and upstream of N-ras. Mol. Cell. Biol 21:3364-3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mitchell, S. A., K. A. Spriggs, M. J. Coldwell, R. J. Jackson, and A. E. Willis. 2003. The Apaf-1 internal ribosome entry segment attains the correct structural conformation for function via interactions with PTB and unr. Mol. Cell 11:757-771. [DOI] [PubMed] [Google Scholar]
- 35.Molla, A., A. V. Paul, and E. Wimmer. 1991. Cell-free, de novo synthesis of poliovirus. Science 254:1647-1651. [DOI] [PubMed] [Google Scholar]
- 36.Nomoto, A., Y. F. Lee, and E. Wimmer. 1976. The 5′ end of poliovirus mRNA is not capped with m7G(5′)ppp(5′)Np. Proc. Natl. Acad. Sci. USA 73:375-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pelletier, J., and N. Sonenberg. 1988. Internal initiation of translation of eukaryotic mRNA directed by a sequence derived from poliovirus RNA. Nature 334:320-325. [DOI] [PubMed] [Google Scholar]
- 38.Pestova, T. V., C. U. Hellen, and I. N. Shatsky. 1996. Canonical eukaryotic initiation factors determine initiation of translation by internal ribosomal entry. Mol. Cell. Biol. 16:6859-6869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pestova, T. V., V. G. Kolupaeva, I. B. Lomakin, E. V. Pilipenko, I. N. Shatsky, V. I. Agol, and C. U. Hellen. 2001. Molecular mechanisms of translation initiation in eukaryotes. Proc. Natl. Acad. Sci. USA 98:7029-7036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pickering, B. M., S. A. Mitchell, K. A. Spriggs, M. Stoneley, and A. E. Willis. 2004. Bag-1 internal ribosome entry segment activity is promoted by structural changes mediated by poly(rC) binding protein 1 and recruitment of polypyrimidine tract binding protein 1. Mol. Cell. Biol. 24:5595-5605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pilipenko, E. V., T. V. Pestova, V. G. Kolupaeva, E. V. Khitrina, A. N. Poperechnaya, V. I. Agol, and C. U. Hellen. 2000. A cell cycle-dependent protein serves as a template-specific translation initiation factor. Genes Dev. 14:2028-2045. [PMC free article] [PubMed] [Google Scholar]
- 42.Pilipenko, E. V., E. G. Viktorova, S. T. Guest, V. I. Agol, and R. P. Roos. 2001. Cell-specific proteins regulate viral RNA translation and virus-induced disease. EMBO J. 20:6899-6908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pilipenko, E. V., E. G. Viktorova, E. V. Khitrina, S. V. Maslova, N. Jarousse, M. Brahic, and V. I. Agol. 1999. Distinct attenuation phenotypes caused by mutations in the translational starting window of Theiler's murine encephalomyelitis virus. J. Virol. 73:3190-3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sarnow, P. 2003. Viral internal ribosome entry site elements: novel ribosome-RNA complexes and roles in viral pathogenesis. J. Virol. 77:2801-2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shiroki, K., T. Isoyama, S. Kuge, T. Ishii, S. Ohmi, S. Hata, K. Suzuki, Y. Takasaki, and A. Nomoto. 1999. Intracellular redistribution of truncated La protein produced by poliovirus 3Cpro-mediated cleavage. J. Virol. 73:2193-2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sledz, C. A., M. Holko, M. J. de Veer, R. H. Silverman, and B. R. Williams. 2003. Activation of the interferon system by short-interfering RNAs. Nat. Cell Biol. 5:834-839. [DOI] [PubMed] [Google Scholar]
- 47.Tillmar, L., C. Carlsson, and N. Welsh. 2002. Control of insulin mRNA stability in rat pancreatic islets. Regulatory role of a 3′-untranslated region pyrimidine-rich sequence. J. Biol. Chem. 277:1099-1106. [DOI] [PubMed] [Google Scholar]
- 48.Tsukiyama-Kohara, K., N. Iizuka, M. Kohara, and A. Nomoto. 1992. Internal ribosome entry site within hepatitis C virus RNA. J. Virol. 66:1476-1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vagner, S., B. Galy, and S. Pyronnet. 2001. Irresistible IRES. Attracting the translation machinery to internal ribosome entry sites. EMBO Rep. 2:893-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Valcarcel, J., and F. Gebauer. 1997. Post-transcriptional regulation: the dawn of PTB. Curr. Biol. 7:R705-R708. [DOI] [PubMed] [Google Scholar]
- 51.Wagner, E. J., and M. A. Garcia-Blanco. 2001. Polypyrimidine tract binding protein antagonizes exon definition. Mol. Cell. Biol. 21:3281-3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wagner, E. J., and M. A. Garcia-Blanco. 2002. RNAi-mediated depletion of PTB leads to enhanced exon definition. Mol. Cell 10:943-949. [DOI] [PubMed] [Google Scholar]
- 53.Wimmer, E., C. U. Hellen, and X. Cao. 1993. Genetics of poliovirus. Annu. Rev. Genet. 27:353-436. [DOI] [PubMed] [Google Scholar]
- 54.Witherell, G. W., A. Gil, and E. Wimmer. 1993. Interaction of polypyrimidine tract binding protein with the encephalomyocarditis virus mRNA internal ribosomal entry site. Biochemistry 32:8268-8275. [DOI] [PubMed] [Google Scholar]
- 55.Wollerton, M. C., C. Gooding, F. Robinson, E. C. Brown, R. J. Jackson, and C. W. Smith. 2001. Differential alternative splicing activity of isoforms of polypyrimidine tract binding protein (PTB). RNA 7:819-832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wollerton, M. C., C. Gooding, E. J. Wagner, M. A. Garcia-Blanco, and C. W. Smith. 2004. Autoregulation of polypyrimidine tract binding protein by alternative splicing leading to nonsense-mediated decay. Mol. Cell 13:91-100. [DOI] [PubMed] [Google Scholar]
- 57.Zhang, J., O. Yamada, T. Sakamoto, H. Yoshida, T. Iwai, Y. Matsushita, H. Shimamura, H. Araki, and K. Shimotohno. 2004. Down-regulation of viral replication by adenoviral-mediated expression of siRNA against cellular cofactors for hepatitis C virus. Virology 320:135-143. [DOI] [PubMed] [Google Scholar]