Abstract
CD4+- and CD8+-T-cell death is a frequent immunological dysfunction associated with the development of human AIDS. We studied a murine model of AIDS, the CD4C/HIV transgenic (Tg) mouse model, to assess the importance of the apoptotic pathway in human immunodeficiency virus type 1 (HIV-1) pathogenesis. In these Tg mice, Nef is the major determinant of the disease and is expressed in immature and mature CD4+ T cells and in cells of the macrophage/myeloid lineage. We report here a novel AIDS-like phenotype: enhanced death, most likely by apoptosis (as assessed by 7-aminoactinomycin D and annexin V/propidium iodide staining), of Tg thymic and peripheral CD4+ and CD8+ T cells. The Tg CD4+ and CD8+ T cells were also more susceptible to cell death after activation in vitro in mixed lymph node (LN) cultures. However, activation-induced cell death was not higher in Tg than in non-Tg-purified CD4+ T cells. In addition, expression of Fas and FasL, assessed by flow cytometry, was increased in CD4+ and CD8+ T cells from Tg mice compared to that of non-Tg littermates. Despite the enhanced expression of Fas and FasL on Tg CD4+ and CD8+ T cells, Fas (lpr/lpr) and FasL (gld/gld) mutant CD4C/HIV Tg mice developed an AIDS-like disease indistinguishable from lpr/+ and gld/+ CD4C/HIV Tg mice, including loss of CD4+ T cells. Similarly, CD4C/HIV Tg mice homozygous for mutations of two other genes implicated in cell death (interleukin-1β-converting enzyme [ICE], tumor necrosis factor receptor 1 [TNFR-1]) developed similar AIDS-like disease as their respective heterozygous controls. Moreover, the double-Tg mice from a cross between the Bcl2/Wehi25 and CD4C/HIV Tg mice showed no major protection against disease. These results represent genetic evidence for the dispensable role of Fas, FasL, ICE, and TNFR-1 on the development of both T-cell loss and organ disease of these Tg mice. They also provide compelling evidence on the lack of protection by Bcl2 against Tg CD4+-T-cell death. In view of the high resemblance between numerous phenotypes observed in the CD4C/HIV Tg mice and in human AIDS, our findings are likely to be relevant for the human disease.
Human immunodeficiency virus type 1 (HIV-1) infection is characterized by a progressive loss of CD4+ lymphocytes (17, 75). The loss of thymic and peripheral CD4+ T cells seems to be related, at least in part, to the inappropriate induction of a programmed cell death (apoptosis) (for a review, see reference 6). The mechanisms of T-cell loss in AIDS are complex and involve loss of not only productively infected cells but predominantly bystander CD4+ and CD8+ T cells, either not productively infected or not infected (18). Indeed, although direct killing of infected CD4+ T cells by HIV-1 or simian immunodeficiency virus (SIV) gene products can be demonstrated in vitro, it does not appear to account for the magnitude of the loss of these cells in vivo (6, 56, 75).
In other tissues or organs which are affected in AIDS, apoptosis may also play a critical role. HIV-1 infection of human or brain cultures induces neuronal death, which appears to occur by apoptosis (61, 65). In the heart disease associated with HIV-1 infection, cardiomyocytes also show signs of enhanced apoptosis (36, 90). Similarly, kidney disease developing in HIV-1-infected individuals shows enhanced apoptosis (8). However, the underlying mechanisms of HIV-1-induced apoptosis of distinct cell types are not fully understood.
The Fas/FasL pathway of apoptosis has been studied after HIV-1 infection. Fas and FasL have been shown to be upregulated on the CD4+ and CD8+ T cells or peripheral blood mononuclear cells of HIV-1-infected patients or SIV-infected macaques or in T cells infected in vitro with HIV-1 (10, 20, 30, 32, 35, 53, 71, 82, 93), and these lymphocytes were shown to have increased susceptibility to Fas-mediated killing (14, 16, 31, 35, 82, 97). In particular, the induction of FasL or Fas expression and apoptotic cell death of CD4+ and CD8+ T cells by HIV-1 or SIV were found to be dependent on a functional nef gene (30, 32, 93, 94, 97). Also, the upregulation of FasL on HIV-1-infected macrophages has been reported to mediate the apoptosis of uninfected T cells (4, 5). However, despite these observations, a clear consensus on the involvement of Fas/FasL in HIV-1 pathogenesis has not emerged. Several investigators indeed found neither upregulation of Fas/FasL expression nor involvement of Fas and FasL in spontaneous and/or activation-induced T-cell death in HIV-1-infected patients (19, 20, 34, 39, 60, 68, 95; reviewed in reference 33).
The activity of interleukin-1β (IL-1β)-converting enzyme (ICE)/caspase 1 is required for the processing and release of mature IL-1β (47). Fas-induced apoptosis of thymocytes has been shown to be prevented in ICE/caspase-1-deficient mice by one group (41) but not other investigators (83). Moreover, the ICE/IL-1β pathway has been studied during HIV-1 infection. IL-1β levels were found to be elevated in infected cells in vitro or in serum or circulatory cells from HIV-1-infected individuals or in lymph nodes (LN) of SIV-infected macaques (45, 55, 72, 98). ICE itself was also found to be upregulated in established T cells infected with HIV-1 or in T cells of infected patients (80, 81). Moreover, ICE has been implicated in Fas-mediated apoptosis in HIV-1 infection (81).
Tumor necrosis factor receptor 1 (TNFR-1) belongs to the same family of death receptors as the Fas receptor and is the main receptor mediating the apoptotic effect of TNF (48). Moreover, levels of TNF and TNFRs have been shown to be elevated in serum and tissues of HIV-1-infected individuals or SIV-infected macaques (29, 70, 89, 96, 98). However, studies on the involvement of the TNF/TNFR-1 pathway in HIV-1-induced CD4+-T-cell death have been controversial (4, 11, 35, 64).
To determine the importance of the apoptotic pathways in HIV-1 pathogenesis in vivo, we used a murine model of AIDS, the CD4C/HIV transgenic (Tg) mouse model. These Tg mice develop a severe AIDS-like disease characterized by premature death, wasting, immune abnormalities (loss of thymocytes, low number of CD4+ T cells, B-cell activation, depletion of dendritic cells, failure to generate germinal centers), kidney disease (cystic dilatation, interstitial nephritis, segmental glomeruloslerosis), lung disease (lymphocytic interstitial pneumonitis), and cardiac disease (myocytolysis and myocarditis) (24, 25, 36, 67, 92). Since enhanced apoptosis in the heart (36), thymus, and T cells (see below) was documented in these Tg mice, we bred these Tg mice with mutant mice lacking genes involved in apoptosis (Fas, FasL, TNFR-1, ICE) or overexpressing the antiapoptotic Bcl2 molecule in order to determine the contribution of each of these apoptotic pathways in some or all of the various AIDS-like phenotypes induced in these Tg mice.
MATERIALS AND METHODS
Mice.
The CD4C/HIVNef (previously designated CD4C/HIVMutG) and CD4C/HIVMutA Tg mice expressing only nef or rev, env, and nef genes of HIV-1, respectively, have been described previously (25). C3H-lpr/lpr (91), C3H-gld/gld (49, 88) mutant, and Bcl2/Wehi25 Tg (86) mice which carry the human Bcl2 sequences under the control of 5′ Igh enhancer (Eμ) as a transgene were purchased from Jackson Laboratories (Bar Harbor, Maine). Mice lacking the ICE (47) or TNFR-1 (66) were obtained from Tara Sesbadri (BASF Bioresearch Corp.) and Tak Mak (Amgen; University of Toronto, Toronto, Ontario, Canada), respectively. All knockout and Tg mice, except the Fas and FasL mutants (already on the C3H background), were bred as heterozygotes on the C3H/HeN (Harland) background for at least 6 generations before being used for the present experiment. These gene-deficient mice were backcrossed with CD4C/HIVMutA (F21388) Tg mice to generate double-mutant (homozygote) and control (heterozygote) Tg mice as described previously (67, 92). The Bcl2/Wehi25 Tg mice were crossed with the CD4C/HIVMutA Tg mice to generate double-Tg mice. All mice used for this work were kept under specific-pathogen-free conditions, and all experiments were approved by the Institutional Animal Ethics Committee.
Antibodies and reagents.
Fluorescein isothiocyanate (FITC)-coupled anti-mouse CD8, FITC-coupled anti-mouse TCRαβ, biotin-conjugated anti-mouse TCRαβ, phycoerythrin (PE)-coupled anti-mouse CD4 and CD8, biotin-conjugated anti-mouse B220, and streptavidin PE-Cy5 were purchased from Cedarlane Laboratories with their appropriate isotype controls. Allophycocyanin (APC)-conjugated CD4 and CD8, FITC-coupled anti-mouse Fas (clone Jo2), biotin-conjugated anti-mouse FasL (clone MFL3), and their appropriate isotype controls were purchase from PharMingen. Annexin V-FITC and 7-aminoactinomycin D (7-AAD) were bought from Cedarlane, and propidium iodide (PI) was from Sigma. Cell lines L1210, L1210-Fas, and d11S were a gift from Ronald E. Gress (Experimental Immunology Branch, National Institutes of Health).
Purification of CD4+ T cells.
Peripheral (axillary, inguinal, cervical, and brachial) LN were collected in complete medium, counted, and resuspended in blocking buffer (1× phosphate-buffered saline [PBS]-20% heat-inactivated fetal bovine serum (FBS), azide free) for 20 min. Staining was performed with anti-major histocompatibility complex class II (MHC-II) PE, anti-CD8 PE, anti-B220 PE, and anti-TCRγδ PE on ice for 40 min. CD4+ T cells were sorted by negative selection using the MoFlo cell sorter by gating on MHC-II-, CD8-, and B220/TCRγδ-negative cells as previously described (92). The purity of CD4+ TCRαβ+ cells was 98% in non-Tg mice and 93% in Tg mice as detected by flow cytometry (FACScalibur or FACScan; Becton Dickinson).
Activation-induced cell death of CD4+ T cells.
Purified CD4+ T cells were washed and resuspended in ISCOVE complete medium supplemented with 10% heat-inactivated fetal bovine serum FBS, 2 mM l-glutamine, 50 μM β-mercaptoethanol, 100 U/ml penicillin, and 100 μg/ml streptomycin. The CD4+ T cells were cultured in anti-CD3 monoclonal antibody (MAb; 10 μg/ml)-coated 96-well plastic plates (105 cells/well) in 200 μl of the complete medium containing recombinant mouse IL-2 (50 U/ml; Roche Diagnostics GumbH) for 3 days. Unstimulated cells were incubated under similar conditions in the absence of anti-CD3 MAb and IL-2. The incubation of total LN cells was performed under the same conditions, except that 2 × 105 cells per well were used. After 3 days of incubation, the cells were harvested and stained with CD4-APC, CD8-FITC, and 7AAD for total LN cells or with CD4-APC and annexin V-FITC/PI for purified CD4+ T cells and analyzed with a fluorescence-activated cell sorter (FACS).
Induction of Fas or FasL expression in CD4+ T cells in culture.
Purified CD4+ T cells were cultured for 2 days, as described above, except that matrix metalloproteinase inhibitor KB8301 (PharMingen) was added at 10 μg/ml. After this period, the cells were harvested and stained with CD4-APC and anti-Fas-FITC or CD4-APC and FasL-biotin plus streptavidin-FITC and analyzed by FACS. Appropriate isotypic controls were used in every experiment. The L1210/Fas and L1210 lymphoblastoma cell lines were used as positive and negative controls, respectively, for Fas expression. The d11S cell line was used as positive control after phorbol myristate acetate (PMA; 10 ng/ml) and ionomycin (500 ng/ml) stimulation for FasL expression.
Flow cytometric analysis.
Cell suspensions were prepared from lymphoid organs (lymph nodes, spleen, thymus) of aged-matched littermates. Immunostaining was then performed on ice in FACS buffer (2% FBS, 1× PBS, 0.05% sodium azide) as described before (67, 92). Nonspecific binding was blocked by using the blocking buffer (20% FBS, 1× PBS, 0.05% sodium azide). FACScan and FACScalibur flow cytometer and Cellquest software (Becton Dickinson, San Jose, CA) were used for flow cytometric analyses.
Apoptosis analysis.
Apoptosis/death of CD4+ and CD8+ T cells was evaluated with Cell Quest software using two different techniques. First, cells were stained with annexin V and PI to detect early-apoptotic cells (single positive [SP], annexin V+ PI−) and dead cells (double positive[DP], annexin V+ PI+). Alternatively, 7AAD staining (membrane-impermeable dye) was used to detect apoptotic and dead cells gating on intermediate and high 7AAD+ cells. In both techniques, fragments and necrotic cells were excluded by gating on high and intermediate foward scatter (FSC) excluding low FSC and low and high side scatter (SSC). Both techniques provided comparable data.
Statistics.
Comparison between groups was performed by using Student's t test. The data were expressed as means ± standard errors of the means. For statistical inference, a P value of <0.05 was considered statistically significant.
Histology.
The organs to be assessed were fixed in 3.7% formaldehyde, embedded in paraffin, sectioned, and stained with hematoxylin and eosin as described previously (25). Statistical analysis was performed using the Kruskal-Wallis test and the chi-square test.
ISH on DN B220+ T cells.
Total cells from the LN of lpr/lpr Tg and non-Tg mice were prepared and stained with PE-coupled anti-mouse CD4 and CD8, FITC-coupled anti-mouse TCRαβ, biotin-conjugated anti-mouse B220, and streptavidin-APC conjugate (PharMingen). About 350,000 double-negative (DN) B220+ T cells (purity, >95%) were sorted by gating on CD4− CD8− B220+ TCRαβ+ cells using a Coulter sorter or MoFlo cell sorter and were fixed in 1.9% formaldehyde. The sorted cells were cytospotted, and in situ hybridization (ISH) was performed with 35S-labeled sense and antisense riboprobes specific for HIV-1 as described previously (24). Quantitation was performed by counting the percentage of cells with ≥3 grains after hybridization with the antisense probe, since only a small percentage (3.9%) of cells hybridized with the sense probe had >3 grains. The experiment was carried out three times. At least 1,000 cells were counted in each group.
RESULTS
Enhanced death of CD4+ and CD8+ T cells from CD4C/HIV Tg mice.
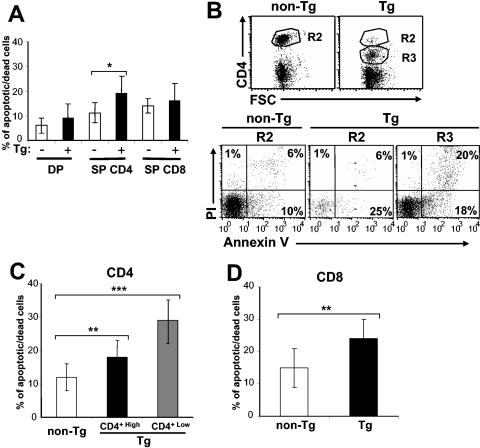
We previously reported that CD4C/HIVMutG Tg mice, expressing only HIV-1 Nef, show thymic atrophy and a lower number of peripheral CD4+ T cells (25, 92). These latter cells were previously reported to represent two subpopulations, CD4+Low and CD4+High. The CD4+Low T cells express Nef at ∼20- to 30-fold higher levels than the CD4+High T cells (92). As the disease progresses, both populations are lost. This is observed both in wild-type Tg mice and in Tg mice bred on different knockout backgrounds (see below). To determine whether the T-cell depletion phenotype was associated with cell death by apoptosis, we measured indices of apoptosis in cells of lymphoid organs using annexin V/PI or 7AAD staining. By FACS analysis, the proportions of 7AAD+ (apoptotic/dead cells) DP or SP CD8+ thymocytes were comparable in Tg and non-Tg mice (Fig. 1A). However, a significantly higher percentage of Tg SP CD4+ thymocytes were 7AAD positive than non-Tg SP CD4+ thymocytes (Fig. 1A). Similarly, in peripheral lymphoid organs, the proportion of CD4+ (Fig. 1B and C) and CD8+ (Fig. 1D) T cells positive for annexin V/PI or 7AAD staining was larger in Tg mice from two distinct founder lines than in non-Tg mice. This enhanced staining was observed in both the CD4+High and CD4+Low subpopulations, but to a larger extent in the CD4+Low T cells.
FIG. 1.
CD4+- and CD8+-T-cell death in CD4C/HIV Tg mice. All mice were 6 to 8 weeks old. (A) Quantitation of apoptotic/dead thymocytes. Thymocytes of Tg or control mice were analyzed by FACS analysis after staining with anti-CD4 and anti-CD8 MAbs and 7AAD. Histograms represent percentages of apoptotic/dead cells among the SP CD4+, SP CD8+, and DP CD4+ CD8+-T-cell subpopulations. Statistical analysis was performed with the Student′s t test, and data from non-Tg (n = 8) and Tg (n = 11) mice were pooled. *, P < 0.01. (B) FACS profiles of apoptotic/dead peripheral CD4+ T cells. pLN cells were stained with anti-CD4 and annexin V/PI and analyzed by FACS analysis by gating on CD4+High (R2) or CD4Low (R3) populations. The percentages of apoptotic/dead cells of different CD4+-T-cell populations are indicated. The figure shows a representative experiment out of 15. (C and D) Quantitation of apoptosis/death in peripheral T cells. Data from FACS analysis of annexin V/PI as shown in panel B or of 7AAD staining were pooled for non-Tg T cells (n = 24) (white bar) and Tg CD4High (n = 26) (black bar) and Tg CD4Low (n = 30) (grey bar) T cells (panel C) and for non-Tg (n = 16) (white bar) and Tg (n = 22) (black bar) CD8+ T cells (panel D). Statistical analysis was performed with Student's t test. **, P < 0.001; ***, P < 0.0001.
We have recently reported that a larger proportion of Tg CD4+ T cells show an “activated/memory” phenotype (CD25+, CD44+, CD45RBLow, CD62LLow, CD69+) (92). We measured the percentage of these “activated/memory” CD4+ T cells undergoing apoptosis/death, compared to naive cells, by gating on CD44High, CD69High, CD45RBLow, or CD62LLow cells and staining with 7AAD or annexin V/PI. This analysis showed that most Tg or non-Tg CD4+ T cells positive for annexin V/PI or 7AAD also express the markers of activation, while little death was detected in Tg and non-Tg largely naive (CD62LHigh, CD45RBHigh) CD4+ T cells (data not shown). These results suggest that death in Tg CD4+ T cells is primarily happening in the activated/effector-cell population. The larger proportion of Tg CD4+ T cells undergoing apoptosis/death most likely reflects the increased number of “activated” Tg CD4+ T cells relative to non-Tg cells.
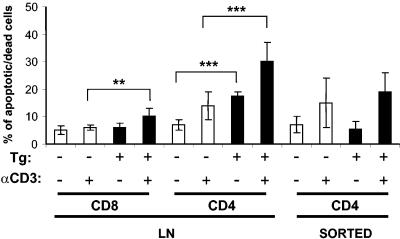
We next tested whether the Tg CD4+ or CD8+ T cells were more susceptible to activation-induced cell death. Upon incubation of LN cells in vitro for 3 days in the absence of stimulation, a larger proportion of Tg than non-Tg CD4+ LN cells was annexin V/PI or 7AAD positive (Fig. 2). Following a 3-day in vitro stimulation of total LN cells with anti-CD3 MAb, a large percentage of Tg CD4+ and CD8+ T cells was also found to be annexin V/PI or 7AAD positive compared to non-Tg T cells (Fig. 2). Similar results were obtained after a 1- or 2-day stimulation period (data not shown). However, when the same stimulation was carried out on purified CD4+ T cells, the percentage of apoptotic/dead T cells was not statistically different between Tg and non-Tg mice (Fig. 2).
FIG. 2.
Quantitation of apoptotic/dead CD4+ and CD8+ T cells following in vitro stimulation. Total pLN cells or purified (sorted) CD4+ T cells were cultured in 96-well plates coated or not coated with anti-CD3 MAb. After incubation for 3 days, the cells were harvested; stained with anti-CD4, anti-CD8, and anti-TCRαβ MAb and 7AAD or with annexin V/PI; and analyzed by FACS analysis to obtain apoptosis/death profiles following gating on lymphocytes. The histograms represent data pooled from 5, 8, and 11 experiments for LN CD8+, CD4+, and sorted CD4+ T cells, respectively. The numbers of mice (6 to 8 weeks old) studied (non-Tg, Tg) in each group were as follows, respectively: for CD8+, 5 and 8, for CD4+, 11 and 15, for LN cells and sorted CD4+ T cells, 16 and 18. Statistical analysis was performed with Student's t test. **, P < 0.001; ***, P < 0.0001.
These results show that a larger number of Nef-expressing Tg peripheral CD4+ and CD8+ T cells (the majority of the latter not expressing the transgene with these CD4C regulatory sequences [27]) die most likely by apoptosis, compared to non-Tg cells, both in vivo and in vitro when maintained in a complex cellular environment.
Increased expression of Fas and FasL on CD4+ T cells from CD4C/HIV Tg mice.
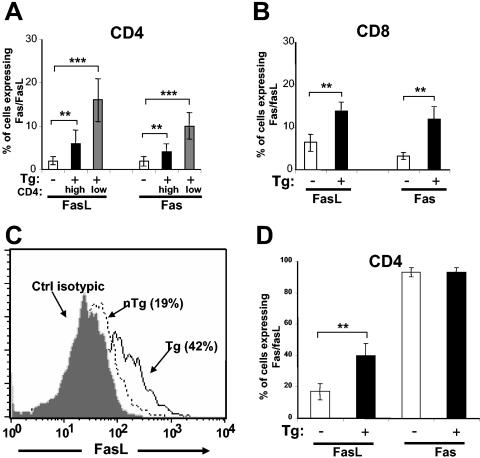
Apoptosis of activated T cells often involves signaling through the death receptor Fas or TNFR-1 (43). To determine whether the Fas/FasL pathway was activated in Tg mice, we first assessed the levels of expression of Fas and FasL on pLN CD8+ and CD4+ T cells, including on the CD4+High and CD4+Low subpopulations. Both Fas and FasL were significantly upregulated on Tg CD4+Low (Fig. 3A) and CD8+ (Fig. 3B) T cells, compared to levels on non-Tg or on Tg CD4+High T cells. In addition, after anti-CD3 stimulation in vitro, FasL was upregulated to a greater degree on CD4+ T cells of Tg mice than on non-Tg mice (Fig. 3C and D), while no difference in expression of Fas was noted between Tg and non-Tg CD4+ T cells (Fig. 3D). In addition, anti-CD3 stimulation also appeared to modify expression of FasL on CD8+ T cells (data not shown). The enhanced expression of Fas and FasL suggested that the Fas/FasL pathway may be an important mediator of apoptosis in Tg T cells.
FIG. 3.
Expression of Fas and FasL on CD4+ and CD8+ T cells of CD4C/HIV Tg mice. (A and B) pLN cells were stained with anti-CD4 (panel A) or anti-CD8 (panel B) with anti-Fas, anti-FasL, or isotypic controls and analyzed with FACS. Gating on CD4High and CD4Low T cells was done as shown in Fig. 1B. The histograms represent data pooled from non-Tg CD4+ (n = 12) (white bar), Tg CD4High (n = 13) (black bar), Tg CD4+Low (n = 14) (grey bar) T cells or from non-Tg (n = 5) (white bar) and Tg (n = 8) (black bar) CD8+ T cells. Statistical analysis was performed with Student's t test. **, P < 0.001; ***, P < 0.0001. (C) FACS profiles of FasL expression after in vitro stimulation of purified CD4+ T cells with anti-CD3 MAb are shown. Sorted CD4+ T cells from pLN were cultured in 96-well plates coated with anti-CD3 MAb. After 2 days of incubation, cells were harvested, stained as described above for panel A, and analyzed with FACS. The picture is for a representative experiment among six performed. In every experiment, an isotypic control MAb (grey) was used. (D) Quantitation of Fas and FasL expression after in vitro stimulation of purified CD4+ T cells with anti-CD3 MAb is shown. Cells were prepared and analyzed with FACS, as described above for panel C. The histograms represent data pooled from six experiments with 11 non-Tg (white bar) and 14 Tg (black bar) (6- to 8-week-old) mice. Statistical analysis was performed with Student′s t test. **, P < 0.001.
The ICE/caspase 1 gene is dispensable for the development of the AIDS-like disease in CD4C/HIV Tg mice.
Fas- but not dexamethasone- (41) or cytotoxic lymphocyte (CTL)- (83) induced apoptosis of T cells has been shown to be prevented in ICE/caspase-1-deficient mice (41), although this has remained controversial (83). We therefore first investigated whether the ICE/caspase 1 pathway was involved in T-cell loss in CD4C/HIV Tg mice by using ICE gene-deficient mice.
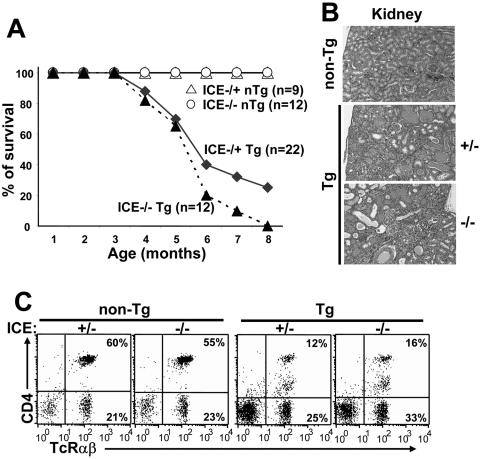
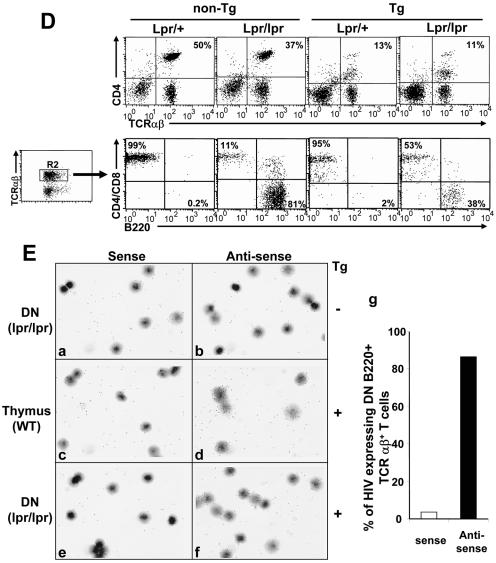
The CD4C/HIV Tg mice were bred on the ICE gene-deficient background to generate appropriate groups for studies. The total absence of the ICE gene product (ICE−/−) appeared to have some detrimental effect on the survival of Tg mice (Fig. 4A). This effect was most apparent in the late stage of the disease (>180 days). However, no significant differences in clinical appearance or pathological changes in lungs (data not shown) and kidneys (Fig. 4B; Table 1) or in FACS profiles (DP or SP thymocytes [results not shown], and LN CD4+ and CD8+ T cells, B cells, or macrophages [Fig. 4C; Table 2 ]) were observed between ICE+/− and ICE−/− CD4C/HIV Tg mice. An increase of CD4+-T-cell apoptosis (7AAD staining) could still be documented in ICE−/− CD4C/HIV Tg mice compared to that in ICE−/− non-Tg controls (data not shown). All these phenotypes were comparable to those previously reported for wild-type C3H Tg mice (24, 25). These results indicate that ICE/caspase 1 is not an essential mediator of the numerous phenotypes developing in CD4C/HIV Tg mice. However, its presence may have a protective effect in later stages of the disease.
FIG. 4.
CD4C/HIV Tg mice deficient for ICE/caspase 1 develop an AIDS-like disease. (A) Survival curve of homozygous ICE−/− (n = 12) or heterozygous ICE+/− (n = 22) CD4C/HIV Tg mice as well as ICE−/− (n = 12) and ICE+/− (n = 9) non-Tg mice. Mice were observed for up to 8 months. (B) Kidney histology of non-Tg and ICE−/− or ICE+/− Tg mice. Note the comparable changes (cystic dilatations, interstitial nephritis, and segmental glomerulosclerosis) in 3- to 6-month-old ICE−/− and ICE+/− Tg mice. (C) FACS profiles of CD4+ T cells from 3-month-old ICE−/− or ICE+/− Tg mice and their controls. pLN cells were stained with anti-CD4 and anti-TCRαβ MAbs. FACS analysis was done by gating on lymphocytes. The dot plot represents one representative experiment out of six.
TABLE 1.
Gross and microscopic evaluation of CD4C/HIV Tg mice bred on ICE+/− or ICE−/− deficient background
| Pathology | No. of animals with ICE phenotype/no. of animals studieda
|
|||
|---|---|---|---|---|
| Non-Tg | Tg | |||
| +/− | −/− | +/− | −/− | |
| Weight loss/hypoactivity | 0/11 (0) | 0/10 (0) | 6/8 (75) | 10/12 (83.3) |
| Edema | 0/11 (0) | 0/10 (0) | 1/8 (12.5) | 1/12 (8.3) |
| Thymus atrophy | 1/11 (10) | 1/10 (10) | 6/8 (75) | 11/12 (91.7) |
| Lymph node atrophyb | 0/11 (0) | 0/10 (0) | 5/8 (62.5) | 10/12 (83.3) |
| Kidney disease | 0/11 (0) | 0/10 (0) | 8/8 (100) | 12/12 (100) |
Mice were 3 to 6 months old. Numbers in parentheses are percentages.
Determined by only histological analysis.
TABLE 2.
Cell number in LN of control and CD4C/HIVMutA Tg mice bred on gene-deficient backgrounds
| Straina | Genotype | Total | CD4 | CD8 | B220 | DNb |
|---|---|---|---|---|---|---|
| lpr | ||||||
| Non-Tg | lpr/+ | 18.9 ± 12.8 | 8.7 ± 7.7 | 4.1 ± 3.0 | 3.8 ± 2.3 | 0.37 ± 0.31 |
| lpr/lpr | 359 ± 171.4 | 48.2 ± 17.9 | 20.7 ± 17.1 | 63.4 ± 31.6 | 198.7 ± 149.5 | |
| Tg | lpr/+ | 1.5 ± 0.69 | 0.12 ± 0.09 | 0.27 ± 0.10 | 0.69 ± 0.33 | 0.08 ± 0.07 |
| lpr/lpr | 5.8 ± 3.5 | 0.21 ± 0.18 | 0.68 ± 0.61 | 3.5 ± 2.1 | 0.59 ± 0.52 | |
| gld | ||||||
| Non-Tg | gld/+ | 12.4 ± 5.0 | 5.0 ± 3.3 | 2.3 ± 1.7 | 1.5 ± 0.45 | 0.84 ± 0.71 |
| gld/gld | 421 ± 222.6 | 57.3 ± 32.9 | 20.4 ± 11.0 | 65.3 ± 54.4 | 299.3 ± 188.4 | |
| Tg | gld/+ | 9.6 ± 3.5 | 1.2 ± 1.0 | 1.9 ± 0.74 | 5.0 ± 2.1 | 0.82 ± 0.68 |
| gld/gld | 14.5 ± 5.2 | 0.96 ± 0.59 | 1.7 ± 0.79 | 7.8 ± 5.5 | 2.2 ± 0.72 | |
| ICE | ||||||
| Non-Tg | +/− | 10.7 ± 3.0 | 5.0 ± 0.8 | 2.1 ± 0.79 | 2.0 ± 1.2 | N/A |
| −/− | 8.3 ± 2.1 | 5.0 ± 1.3 | 1.78 ± 0.76 | 1.2 ± 0.38 | N/A | |
| Tg | +/− | 1.9 ± 0.93 | 0.09 ± 0.05 | 0.42 ± 0.23 | 0.98 ± 0.60 | N/A |
| −/− | 1.9 ± 1.0 | 0.15 ± 0.10 | 0.45 ± 0.28 | 0.81 ± 0.54 | N/A | |
| TNFR-1 | ||||||
| Non-Tg | +/− | 10.6 ± 3.7 | 4.6 ± 1.8 | 2.1 ± 0.49 | 1.6 ± 1.0 | N/A |
| −/− | 8.5 ± 0.57 | 3.9 ± 0.62 | 1.6 ± 0.55 | 1.7 ± 0.99 | N/A | |
| Tg | +/− | 2.2 ± 2.0 | 0.22 ± 0.25 | 0.57 ± 0.59 | 1.1 ± 0.85 | N/A |
| −/− | 1.9 ± 1.2 | 0.16 ± 0.09 | 0.60 ± 0.40 | 0.62 ± 0.45 | N/A |
Mice that were 3 to 8 months old were used. Each group had six to eight mice. Non-Tg and Tg applies for the CD4C/HIV transgene.
N/A, not applicable.
The Fas and FasL genes are dispensable for the development of AIDS-like disease in CD4C/HIV Tg mice.
In view of the enhanced expression of Fas and FasL observed on CD4+ T cells of CD4C/HIV Tg mice, we next investigated whether activation of the Fas/FasL pathway was critical in vivo for the development of some of the phenotypes in CD4C/HIV Tg mice by breeding these Tg mice on Fas(lpr) and FasL(gld) gene-mutant backgrounds. As a result of impaired apoptosis (40), these mutant mice have been reported to develop a severe lymphadenopathy and splenomegaly, caused mainly by the accumulation of TCRαβ+ CD4+ and CD8+ T cells and of a new population of TCRαβ+ B220+ CD4− CD8− (DN) T cells (84).
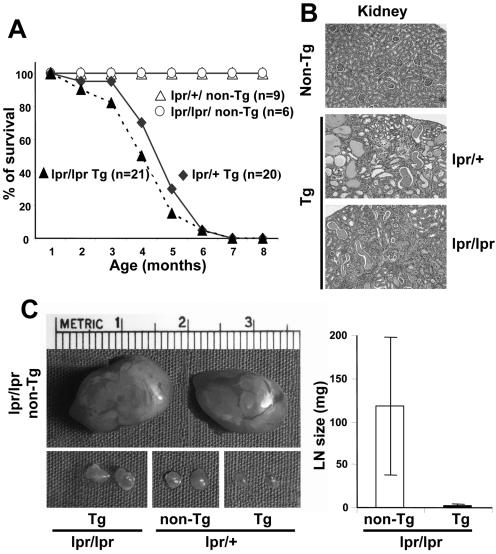
Groups of homozygote (lpr/lpr) and control heterozygote (lpr/+) CD4C/HIV Tg mice, along with lpr/lpr and lpr/+ non-Tg mice, were generated and observed for up to 8 months. No significant difference in mortality was observed between lpr/lpr and lpr/+ CD4C/HIV Tg mice (Fig. 5A). Both lpr/lpr and lpr/+ CD4C/HIV Tg mice developed numerous signs of the AIDS-like disease usually observed in these mice and previously reported weight loss, edema, hypoactivity, thymic atrophy, and lung (lymphocytic interstitial pneumonitis) and kidney (interstitial nephritis, segmental glomerulosclerosis, and cystic dilatations) disease (Fig. 5B; Table 3). However, a striking difference between homozygote lpr/lpr CD4C/HIV Tg and non-Tg mice was noticed in the peripheral lymphoid organs. While the lpr/lpr non-Tg mice developed massive lymphoproliferation and splenomegaly, the lpr/lpr CD4C/HIV Tg mice did not show this phenotype and their LN and spleen were close to normal size or only slightly enlarged (Fig. 5C and D).
FIG. 5.
CD4C/HIV Tg mice deficient for Fas develop an AIDS like disease. (A) Survival curve of homozygous lpr/lpr (n = 21) or heterozygous lpr/+ (n = 20) CD4C/HIV Tg mice as well as lpr/lpr (n = 6) and lpr/+ (n = 9) non-Tg mice. Mice were observed for up to 8 months. (B) Kidney histology of non-Tg and lpr/lpr or lpr/+ Tg mice. Note comparable changes (cystic dilatations, interstitial nephritis, and segmental glomerulosclerosis) in 3- to 6-month-old lpr/lpr and lpr/+ Tg mice. (C) pLN size and weight. pLN hypertrophy observed in lpr/lpr non-Tg mice is much reduced in lpr/lpr Tg mice. (D) FACS profiles of CD4+/TCRαβ+ and DN B220+ TCRαβ+ T cells from 7-week-old control and lpr/lpr Tg mice. pLN cells were stained, and FACS analysis was performed as described in the legend to Fig. 4C. For B220+ TCRαβ+ DN cells, gating was first done on TCRαβ+ cells. The dot plot represents one representative experiment out of eight. (E) Detection of Nef expression in purified (sorted) DN B220+ TCRαβ+ cells by ISH. pLN Tg cells were stained with anti-CD4/CD8/TCRαβ/B220 MAbs, and the DN B220+ TCRαβ+ cells were purified by cell sorting. About 105 cells prepared by cytospotting were processed for ISH as described in Materials and Methods and hybridized with an antisense or control sense 35S-labeled HIV-1-specific riboprobe. Panels a and b are non-Tg negative control cells, panels c and d are Tg thymocytes (positive controls, wild type [WT]), and panels e and f are DN B220+ TCRαβ+ sorted cells (DN). Magnification, ×250. For quantitation (g), 1,410 cells (in 46 different fields) and 1,054 cells (in 53 fields) were examined after hybridization with the antisense and sense probes, respectively. The experiment was repeated three times with comparable results.
TABLE 3.
Gross and microscopic evaluation of CD4C/HIV Tg mice bred on an lpr/+ or lpr/lpr background
| Pathology | No. of animals with phenotype/no. of animals studieda
|
|||
|---|---|---|---|---|
| Non-Tg
|
Tg
|
|||
| lpr/+ | lpr/lpr | lpr/+ | lpr/lpr | |
| Weight loss/hypoactivity | 0/14 (0) | 0/16 (0) | 11/15 (73.3) | 23/28 (82.1) |
| Edema | 0/14 (0) | 0/16 (0) | 4/15 (26.6) | 8/28 (28.5) |
| Thymus atrophy | 0/14 (0) | 0/16 (0) | 10/15 (66.6) | 19/28 (67.9) |
| Typical lpr/lpr lymphadenopathyb | 0/14 (0) | 15/16 (93.7) | 0/15 (0) | 0/28 (0) |
| Kidney disease | 0/14 (0) | 0/16 (0) | 15/15 (100) | 28/28 (100) |
Mice were 3 to 6 months old. Numbers in parentheses are percentages.
Large LN as shown in Fig. 5C, upper panel.
FACS analysis in LN and spleen cells showed a similar decrease in percentage and in absolute cell numbers of CD4+ TCRαβ+ T cells in lpr/lpr and lpr/+ Tg mice compared to those in lpr/+ non-Tg mice (Fig. 5D; Table 2). In Tg mice of both lpr genotypes, downregulation of the cell surface CD4 molecule was observed, as previously reported in wild-type CD4C/HIV Tg mice (25). Increased percentages of B220+ TCRαβ− B cells and of Mac-1+ monocytes/macrophages/dendritic cells/neutrophils were sometimes observed in lpr/lpr Tg mice as in lpr/+ Tg mice (data not shown). An increase of CD4+-T-cell apoptosis (7AAD staining) could still be documented in lpr/lpr CD4C/HIV Tg mice compared to that in lpr/lpr non-Tg controls (data not shown). Assessment of the DN B220+ TCRαβ+ T-cell population revealed a marked decrease in percentage and in absolute cell numbers of this population in lpr/lpr CD4C/HIV Tg mice compared to those in lpr/lpr non-Tg mice (average, 10% versus 49%), although lpr/lpr Tg mice always had more DN B220+ T cells than lpr/+ non-Tg mice (Table 2). The decrease of this DN B220+ T-cell population in lpr/lpr Tg mice correlated with the reduced LN and spleen hypertrophy observed in these Tg mice compared to that in lpr/lpr non-Tg mice (Fig. 5C). These results indicated that Nef expression prevents the full accumulation of DN B220+ TCRαβ+ T cells in lpr/lpr Tg mice.
To determine whether the lower number of DN B220+ T cells in lpr/lpr CD4C/HIV Tg mice was related to the expression of the transgene in these cells, we measured Tg expression on sorted DN B220+ T cells from these mice by in situ hybridization. Most of these sorted cells scored positive in this assay, although a level of HIV-1 expression lower than that in Tg thymocytes was observed (Fig. 5E).
This genetic analysis was extended to the FasL(gld)-deficient CD4C/HIV Tg mice. These Tg mice were found to be phenocopies of the lpr/lpr CD4C/HIV Tg mice. The homozygote gld/gld CD4C/HIV Tg mice (n = 11) had comparable mortality rates and showed pathological changes similar to those of the heterozygote gld/+ CD4C/HIV Tg mice (n = 17) (Table 4; data not shown). Moreover, FACS profiles on thymocytes and pLN were comparable for gld/+ and gld/gld Tg mice and very similar to those obtained from lpr/lpr Tg mice (Table 2; data not shown). In particular, as in the lpr/lpr CD4C/HIV Tg mice, the absolute cell number of LN DN B220+ T cells was severely decreased in gld/gld CD4C/HIV Tg mice, compared to that in gld/gld non-Tg mice (Table 2). Together, these results show that the Fas or FasL gene products are not required for the Nef-induced phenotypes in CD4C/HIV Tg mice. Moreover, these results indicate that the unique DN B220+ T-cell population emerging in lpr/lpr or gld/gld mice remains susceptible to the action of Nef, either being prevented to differentiate or being killed by Nef.
TABLE 4.
Gross and microscopic evaluation of CD4C/HIV Tg mice bred on a gld/+ or gld/gld background
| Pathology | No. of animals with phenotype/no. of animals studieda
|
|||
|---|---|---|---|---|
| Non-Tg
|
Tg
|
|||
| gld/+ | gld/gld | gld/+ | gld/gld | |
| Weight loss/hypoactivity | 0/5 (0) | 1/7 (14) | 7/7 (100) | 5/5 (100) |
| Edema | 0/5 (0) | 0/7 (0) | 0/7 (0) | 0/5 (0) |
| Thymus atrophy | 0/5 (0) | 0/7 (0) | 7/7 (100) | 5/5 (100) |
| Thymus hypertrophy | 0/5 (0) | 6/7 (85.7) | 0/7 (0) | 0/5 (0) |
| Typical gld/gld lymphadenopathyb | 0/5 (0) | 7/7 (100) | 0/7 (0) | 0/5 (0) |
| Kidney disease | 0/5 (0) | 0/7 (0) | 7/7 (100) | 5/5 (100) |
Mice were 3 to 6 months old. Numbers in parentheses are percentages.
Large LN as shown in Fig. 5C, upper panel.
The TNFR-1 gene is dispensable for the development of the AIDS-like disease in CD4C/HIV Tg mice.
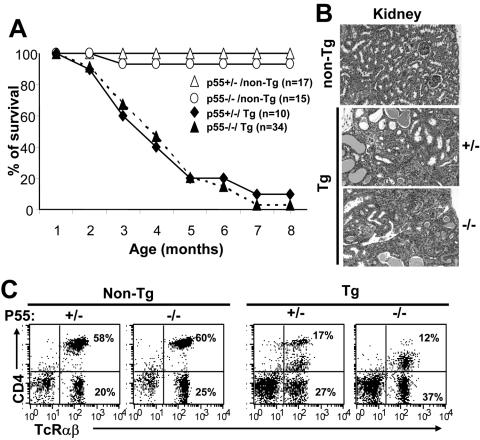
We have recently found that lipopolysaccharide-induced tumor necrosis factor alpha (TNF-α) production is higher in CD4C/HIV Tg macrophages than in non-Tg macrophages (D. V. Jovanovic, P. Vincent, E. Priceputu, Z. Hanna, and P. Jolicoeur, unpublished data). TNFR-1 is responsible for mediating the apoptotic effect of TNF-α. Moreover, TNFR-1 belongs to the same family of death receptors as the Fas receptor. To determine whether activation of TNFR-1 was involved in the development of some of the phenotypes in CD4C/HIV Tg mice, these Tg mice were bred on the TNFR-1 (p55) gene-deficient background (66). No significant difference in mortality was observed between the TNFR-1+/− and the TNFR-1−/− CD4C/HIV Tg mice (Fig. 6A). These two groups of mice developed indistinguishable AIDS-like diseases (weight loss, wasting) with pathological lesions in lungs (interstitial lymphocytic pneumonitis) and in kidneys (interstitial nephritis, segmental glomerulosclerosis, cystic dilatation) (Fig. 6B; Table 5) which closely resembled previously reported histopathology in wild-type CD4C/HIV Tg mice (24, 25). Thymic atrophy, the loss of CD4+ T cells (Fig. 6C; Table 2), and enhanced apoptosis (7AAD staining) relative to those in non-Tg mice (data not shown) were also observed in TNFR-1+/− and TNFR-1−/− Tg mice and were comparable to those previously observed in wild-type C3H Tg mice (24, 25).
FIG. 6.
CD4C/HIV Tg mice deficient for TNFR-1 (p55) develop an AIDS-like disease. (A) Survival curve of non-Tg (n = 17 and n = 15) and CD4C/HIV Tg mice bred on an homozygous p55−/− (n = 34) or heterozygous p55+/− (n = 10) background. Mice were observed for up to 8 months. (B) Kidney histology of non-Tg and p55−/− or p55+/− Tg mice. Note the comparable changes (cystic dilatations, interstitial nephritis, and segmental glomerulosclerosis) in p55−/− and p55+/− Tg mice. (C) FACS profiles of CD4+ T cells from 3- to 6-month-old p55−/− or p55+/− Tg mice and their controls. Mesenteric LN cells were stained, and FACS analysis was performed as described in the legend to Fig. 4C. The dot plot represents one representative experiment out of five.
TABLE 5.
Gross and microscopic evaluation of CD4C/HIV Tg mice bred on a TNFR-1+/− or TNFR-1−/− deficient background
| Pathology | No. of animals with phenotype/no. of animals studieda
|
|||
|---|---|---|---|---|
| Non-Tg
|
Tg
|
|||
| p55 | p55 | |||
| +/− | −/− | +/− | −/− | |
| Weight loss/hypoactivity | 0/10 (0) | 0/11 (0) | 5/5 (100) | 17/17 (100) |
| Edema | 0/10 (0) | 0/11 (0) | 2/5 (40) | 6/17 (35) |
| Thymus atrophy | 0/10 (0) | 0/11 (0) | 4/5 (80) | 14/17 (82.3) |
| Lymph node atrophyb | 0/5 (0) | 0/11 (0) | 4/5 (80) | 14/17 (82.3) |
| Kidney disease | 0/5 (0) | 0/11 (0) | 5/5 (100) | 17/17 (100) |
Mice were 3 to 6 months old. Numbers in parentheses are percentages.
Determined by only histological analysis.
These results indicated that activation of the TNFR-1 pathway is not essential for the development of numerous phenotypes observed in CD4C/HIV Tg mice.
Bcl2 overexpression in T cells does not prevent the loss of CD4+ T cells in CD4C/HIV Tg mice.
Since the above results (Fig. 1 through 3) suggested that T-cell apoptosis is an important characteristic of the immune system of these CD4C/HIV Tg mice, we attempted to prevent apoptosis by overexpressing Bcl2 in T cells. The Bcl2 gene has been shown to protect various cell types, including T cells, from apoptosis induced by several stimuli (59, 74, 77, 86). Overexpression of Bcl2 in thymocytes of Bcl2/Wehi25 Tg mice was indeed shown to protect thymocytes from apoptosis induced by a number of insults (gamma radiation, corticosteroids, calcium ionophores, sodium azide, PMA) (86).
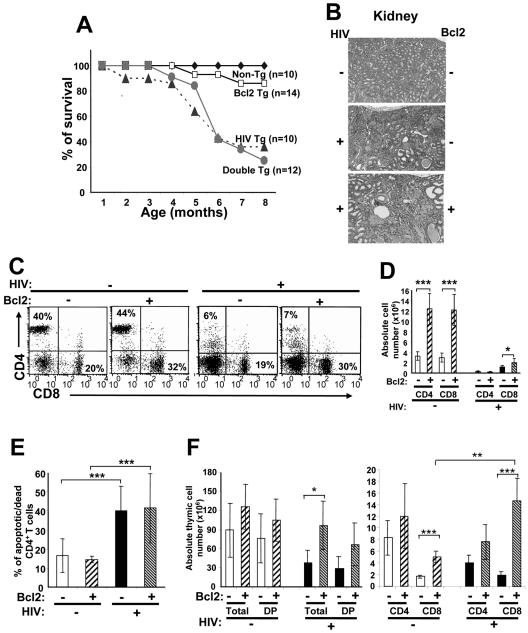
We used these Bcl2/Wehi25 Tg mice to determine whether enhanced expression of Bcl2 in T cells would affect the fate of T cells in CD4C/HIV Tg mice. Groups of (CD4C/HIV X Bcl2/Wehi25) double-Tg C3H mice were generated and compared to their single-Tg littermates. Levels of Tg Bcl2 protein were not significantly affected by the expression of Nef (data not shown). Survival curves of Tg mice of both genotypes (Nef+ Bcl2+, Nef+ Bcl2−) were comparable (Fig. 7A). This analysis also showed that the clinical and pathological organ phenotypes in lung (data not shown) and in kidney (Fig. 7B; Table 6), as well as the FACS profiles, except those for SP CD8+ T cells (Fig. 7C), were indistinguishable in single-CD4C/HIV and double-Tg mice. Loss of peripheral CD4+ T cells occurred to the same extent in both single-CD4C/HIV or double-Tg mice (Fig. 7D), indicating that Bcl2 overexpression in T cells could not prevent the appearance of this phenotype. Consistent with this result, the apoptosis/death of Nef-expressing CD4+ T cells was not changed by the expression of Bcl2 and was still enhanced compared to that of CD4+ T cells not expressing Nef (Fig. 7E). However, the number of CD8+ T cells from pLN was slightly higher in (CD4C/HIV X Bcl2/Wehi25) double-Tg mice than in CD4C/HIV single-Tg mice (Fig. 7D), although we could not document a significant difference in their survival directly ex vivo by staining with 7AAD (data not shown).
FIG. 7.
CD4C/HIV Tg mice overexpressing Bcl2 in T cells are not protected from loss of CD4+ T cells. (A) Survival curve of single-CD4C/HIV (n = 10) or double- (CD4C/HIV X Bcl2/Wehi25) (n = 12) Tg mice and their non-Tg (n = 10) or single-Bcl2/Wehi25 Tg (n = 14) controls. Mice were observed for up to 8 months. (B) Kidney histology of non-Tg and single- or double- (CD4C/HIV X Bcl2/Wehi25) Tg mice. Note the comparable changes (cystic dilatations, interstitial nephritis, and segmental glomerulosclerosis) in single-CD4C/HIV and double- (CD4C/HIV X Bcl2/Wehi25) Tg mice. (C) FACS profiles of CD4+ T cells from single-CD4C/HIV and double- (CD4C/HIV X Bcl2/Wehi25) Tg mice and their non-Tg controls. pLN cells were stained, and FACS analysis was performed as described in the legend to Fig. 4C. The dot plot represents one representative experiment out of six. (D) Quantitation of CD4+ and CD8+ T cells in pLN of single- or double- (CD4C/HIV X Bcl2/Wehi25) Tg mice. Data from FACS analysis as shown in panel C were pooled for CD4+ and CD8+ T cells from 7- to 9-month-old non-Tg (n = 7), single-Bcl2/Wehi25 (n = 7), CD4C/HIV (n = 6), and double- (CD4C/HIV X Bcl2/Wehi25 [n = 7]) Tg mice. Statistical analysis was performed with Student's t test. *, P = 0.04; ***, P < 0.0001. (E) Quantitation of apoptosis/death of CD4+ T cells in single-CD4C/HIV or double- (CD4C/HIV X Bcl2/Wehi25) Tg mice. pLN cells, from the same mice presented in panel D were stained with anti-CD4 and 7AAD and analyzed with FACS to obtain apoptosis/death profiles. The percentages of apoptotic/dead CD4+ T cells are indicated. The figure represents pooled data from six experiments. Statistical analysis was performed with Student's t test. ***, P < 0.0001. (F) Quantitation of thymocytes in single- or double- (CD4C/HIV X Bcl2/Wehi25) Tg mice. Data were obtained from 6- to 10-week-old non-Tg (n = 6), single-Bcl2/Wehi25 (n = 6), CD4C/HIV (n = 6), and double- (CD4C/HIV X Bcl2/Wehi25) (n = 6) Tg mice. Statistical analysis was performed with Student's t test. *, P = 0.05; **, P < 0.001; ***, P < 0.0001.
TABLE 6.
Gross and microscopic evaluation of CD4C/HIV Tg mice bred with Bcl2 Wehi25 Tg mice
| Pathology | No. of animals with phenotype/no. of animals studieda
|
|||
|---|---|---|---|---|
| HIV−
|
HIV+
|
|||
| Bcl2− | Bcl2+ | Bcl2− | Bcl2+ | |
| Weight loss/hypoactivity | 0/7 (0) | 0/8 (0) | 4/7 (57) | 0/7 (0) |
| Edema | 0/7 (0) | 0/8 (0) | 0/7 (0) | 0/7 (0) |
| Thymus atrophy | 0/7 (0) | 0/8 (0) | 5/7 (71) | 0/7 (0) |
| Thymus normal | 7/7 (100) | 8/8 (100) | 2/7 (28) | 7/7 (100) |
| Lymph node atrophyb | 0/7 (0) | 08 (0) | 7/7 (100) | 7/7 (100) |
| Kidney disease | 0/7 (0) | 0/8 (0) | 7/7 (100) | 6/7 (87) |
Mice were 7 to 9 months old. Numbers in parentheses are percentages.
Determined by only histological analysis.
Analysis of the thymus of young (6- to 8-week-old) double-Tg mice showed that Bcl2 significantly prevents the loss of thymocytes induced by HIV-1 Nef (Fig. 7F). Also, higher numbers of DP and SP CD4+ and CD8+ thymocytes were present in CD4C/HIV Tg mice expressing Bcl2 than in those not expressing it (Fig. 7F). This was especially evident for the SP CD8+-T-cell population whose number was even higher than that in mice expressing Bcl2 but not HIV-1. This increase of SP CD8+ thymocytes in double-Tg mice may be associated with the partial protection of peripheral CD8+ T cells also observed in these Tg mice (Fig. 7D).
Together, these data show that Bcl2 has a protective role against the Nef-mediated death of SP CD8+ thymocytes and, to a much lesser extent, of peripheral CD8+ T cells. However, Bcl2 cannot protect peripheral CD4+ T cells from CD4C/HIV Tg mice against death. These results suggest that the loss of CD4+ and CD8+ T cells may occur partially by distinct pathways in this model.
DISCUSSION
The CD4C/HIV Tg mice develop a severe AIDS-like disease with many phenotypes very similar to those found in HIV-1-infected individuals, in particular, in pediatric patients. These phenotypes include defects of the immune system (thymic atrophy, preferential loss of CD4+ T cells, loss of CD4+ helper function with impaired germinal center formation, T- and B-cell activation, production of autoantibodies) as well as organ diseases in lungs (lymphocytic interstitial pneumonitis), kidneys (tubulointerstitial nephritis, segmental glomerulosclerosis, cystic dilatation), and heart (24, 25, 36, 67, 92). The results shown here describe a novel T-cell phenotype of these Tg mice: enhanced cell death of thymocytes and CD4+ and CD8+ T cells relative to those of the non-Tg mice. A similar enhanced cell death has been observed in SIV- and HIV-infected thymocytes in CD4+ and CD8+ T cells from macaques and humans, respectively (2, 9, 12, 15, 18, 22, 23, 44, 46, 56, 58, 71, 73). In our study, this increased cell death was more evident in the Tg CD4+Low peripheral T-cell subpopulation than in the CD4+High peripheral T-cell subpopulation. The CD4+Low Tg cell population was also preferentially found to exhibit an activated/effector cell surface phenotype (92), suggesting a possible link between Nef-mediated T-cell activation and cell death. The enhanced cell death and activation observed preferentially on CD4+Low T cells suggested a cell-autonomous impairment of survival and direct action of Nef, since the CD4+Low T cells express higher levels of Nef (92). Consistent with a CD4+-T-cell autonomous defect, analysis of CD4+ T cells from CD68/HIV Tg mice revealed no enhanced apoptosis (data not shown). In these Tg mice, HIV-1 Nef is expressed in macrophages, but not in T cells, under the regulatory sequences of the CD68 gene (Z. Hanna, D. G. Kay, C. Hu, P. Brochu, and P. Jolicoeur, manuscript in preparation). Although these results indicate that Nef-expressing macrophages are not sufficient to induce CD4+-T-cell death in vivo, further investigation is needed to establish in which cell type the expression of Nef is necessary and sufficient to induce CD4+-T-cell apoptosis and loss in Tg mice.
In addition to increase cell death, enhanced expression of Fas and FasL was also documented on both CD4+ and CD8+ T cells from the CD4C/HIV Tg mice. This finding also parallels some reports showing similar increased expression of Fas or FasL in CD4+ or CD8+ T cells or the peripheral blood mononuclear cells of SIV-infected macaques or HIV-infected patients or in T cells infected in vitro with HIV-1 (10, 20, 30, 32, 35, 53, 71, 82, 93), although other studies came to opposite conclusions (19, 31, 60, 76). Attempts to assess the Fas pathway in HIV-1-infected cells or in cells from SIV-infected macaques or HIV-infected individuals also led to controversial results, some investigators finding enhanced Fas-mediated apoptosis (14, 16, 31, 35, 82, 97), while others did not (19, 20, 34, 39, 60, 68, 95). Therefore, strong evidence that enhanced activation of the Fas/FasL pathway is primarily responsible for CD4+-T-cell loss in HIV-1-infected individuals appears to be lacking (33).
Despite the enhanced expression of Fas and FasL on CD4+ and CD8+ T cells, we found that the genes coding for these molecules were totally dispensable for the development of the AIDS-like disease, both in the immune system and in the organs of CD4C/HIV Tg mice. In addition, the development of the severe lymphadenopathy (mainly caused by the accumulation of DN B220+ T cells), associated with the loss of Fas or FasL (84), was almost completely abrogated in lpr/lpr and gld/gld CD4C/HIV Tg mice. Since expression of HIV-1 could be documented in these DN B220+ T cells, this suggests that these cells may be derived either from thymic CD4+ precursors or from more mature CD4+ T cells, but possibly not from CD8+ T cells in which the CD4C promoter (driving expression of the HIV-1 transgene) is poorly active (26, 27). This would be consistent with the observation that their accumulation appears to be thymus dependent, since neonatal thymectomy has been reported to abrogate their accumulation (85). The use of the CD4C human/mouse chimeric regulatory sequences, already known to be quite specific for CD4+ T cells and cells of the macrophage/myeloid lineage (24-27), may indeed unravel a CD4+-specific gene program still active in DN B220+ T cells. However, such a hypothesis is in apparent contrast with reports showing that DN B220+ T cells originate from CD8+ T cells (21, 28, 50, 54, 57, 62, 79). This latter scenario would not be necessarily incompatible with our data, since the CD4C regulatory sequences are active in a small percentage of CD8+ T cells (27), which may comprise the DN precursors. Whatever the origin of these DN B220+ T cells, it appears that expression of Nef in these cells either prevents their expansion or accelerates their death by a Fas/FasL-independent pathway. A similar failure of DN B220+ T cells to accumulate has also been observed in lpr/lpr mice made deficient for fyn (7), MHC-I (50, 62), or β2 microglobulin (57). This requirement of fyn for the accumulation of DN T cells is interesting and suggests that Nef may disrupt the same pathway in these cells, since Nef has been shown to bind to members of the src family of tyrosine kinases (69), including fyn (3). We cannot rule out, however, a more indirect mechanism(s).
Since it has been reported that Fas-induced apoptosis of thymocytes is prevented in ICE/caspase-1-deficient mice (41), we investigated the possible contribution of ICE/caspase 1 in the disease process. We found that ICE/caspase 1 was not required for the development of the AIDS-like disease in these Tg mice but appeared to have a protective role at later stages of the disease. The cellular and molecular basis for this protection remains unclear. Since late-stage disease is characterized by severe renal disease, the apparent protection of ICE/caspase 1 at late stages of the disease may involve its action in the kidney.
We also studied another member of the death receptor family, TNFR-1. Enhanced production of TNF and TNFRs during HIV-1 infection has been observed by several groups (29, 70, 89, 96, 98; for reviews, see references 37 and 52), but controversial results were obtained about the involvement of the TNF/TNFR-1 pathway in CD4+-T-cell death. Macrophage-induced apoptosis of CD4+ T cells from HIV-1-infected individuals has been reported to be mediated by TNF (4). Also, T lymphocytes from HIV-1-infected individuals were found to show an increased susceptibility to TNFR-1-mediated apoptosis (11). However, others have reported that apoptosis of primary CD4+ T cells was unaffected by TNFR-1 decoy (64) or by ligation with antibodies against TNFR-1 or TNFR-2 (35). We found that TNFR-1 was dispensable for the development of the disease in CD4C/HIV Tg mice. This suggests that the enhanced production of TNF-α found in macrophages of these Tg mice relative to their controls (Jovanovic et al., manuscript in preparation) either has a minor impact on disease development or acts through an alternative receptor, possibly the other known TNF receptor (TNFR-2).
Finally, we attempted to prevent the loss of T cells of CD4C/HIV Tg mice by overexpressing Bcl2 in these cells. We found that Tg overexpression of Bcl2 did not protect the CD4C/HIV Tg mice against loss of peripheral CD4+ T cells. However, it appeared to partially prevent some loss of peripheral CD8+ T cells and to prevent loss of SP CD8+ thymocytes completely. Bcl2 overexpression in T cells has been found to rescue T-lymphocyte development in IL-7R-deficient mice (1, 51) and to prevent cell death of thymocytes and T cells induced by a number of treatments (corticosteroids [74, 77, 86], calcium ionophores [77, 86], gamma radiation [59, 74, 86], anticancer drugs [59], anti-CD3 stimulation [74, 86], sodium azide [86], PMA [86]), but not by stimulation of Fas (59, 87) or of Thy-1 (86) receptors, nor by negative selection (74, 86). Therefore, the fact that death of peripheral CD4+ T cells is not prevented by overexpression of Bcl2 in the CD4C/HIV Tg mice implies that the pathway(s) of T-cell death used by HIV Nef in this model may be distinct from all those utilized by the death-inducing agents listed above. Conversely, the fact that the Nef-mediated loss of SP CD8+ thymocytes is prevented by Bcl2 would suggest that Nef may share some of the pathways used by the death-inducing treatments listed above. Intriguingly, the numbers of SP CD8+ thymocytes were significantly higher in Tg mice expressing both HIV-1 Nef and Bcl2 than in single-Bcl2 or HIV Tg mice, suggesting an enhanced CD8+ thymocyte differentiation, sometimes observed with defective CD4+ thymocyte selection/lineage commitment (38, 78). The findings in human cells infected with HIV-1 are consistent with our data. Although susceptibility to activation-induced apoptosis was found to correlate with Bcl2 expression in human T cells (42), death of HIV-1-infected T cells was found by other groups to be independent of Bcl2 (13, 39, 63).
In conclusion, the present work represents the first in vivo study of an animal model of AIDS on the contribution of a number of important genes of the apoptotic cell death pathway in the development of an AIDS-like disease. Our genetic data clearly show that the development of both the immune and organ components of the AIDS-like disease of these Tg mice occurs independently of Fas/FasL, ICE/caspase 1, and TNFR-1 and is not prevented by overexpression of Bcl2 in T cells. These results rule out a number of pathways previously implicated in T-cell death by HIV-1. In view of the high resemblance between numerous phenotypes observed in the CD4C/HIV Tg mice and in human AIDS, our findings on the dispensable role of Fas/FasL, ICE, and TNFR-1 and on the lack of protection by Bcl2 in these Tg mice are likely to be relevant for the human disease.
Acknowledgments
This work was supported by grants to P.J. from the Canadian Institute of Health Research (CIHR) and from the National Heart, Lung, and Blood Institute, NIH (no. HL63635).
We thank Tara Sesbadri and Tariq Ghayurt (formerly of BASF Bioresearch Corp. and now of Abbott) for providing the ICE gene-deficient mice. We are grateful to Eve-Lyne Thivierge and Benoît Laganière for excellent technical assistance. We thank Eric Massicotte and Martine Dupuis of the Cytofluorometry Core for their support and Claire Crevier of the Histopathology Core Facility for excellent work. We are grateful to Rita Gingras and Monique Villani for preparing the manuscript.
REFERENCES
- 1.Akashi, K., M. Kondo, U. von Freeden-Jeffry, R. Murray, and I. L. Weissman. 1997. Bcl-2 rescues T lymphopoiesis in interleukin-7 receptor-deficient mice. Cell 89:1033-1041. [DOI] [PubMed] [Google Scholar]
- 2.Amendola, A., M. L. Gougeon, F. Poccia, A. Bondurand, L. Fesus, and M. Piacentini. 1996. Induction of “tissue” transglutaminase in HIV pathogenesis: evidence for high rate of apoptosis of CD4+ T lymphocytes and accessory cells in lymphoid tissues. Proc. Natl. Acad. Sci. USA 93:11057-11062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arold, S., P. Franken, M. P. Strub, F. Hoh, S. Benichou, R. Benarous, and C. Dumas. 1997. The crystal structure of HIV-1 Nef protein bound to the Fyn kinase SH3 domain suggests a role for this complex in altered T cell receptor signaling. Structure 5:1361-1372. [DOI] [PubMed] [Google Scholar]
- 4.Badley, A. D., D. Dockrell, M. Simpson, R. Schut, D. H. Lynch, P. Leibson, and C. V. Paya. 1997. Macrophage-dependent apoptosis of CD4+ T lymphocytes from HIV- infected individuals is mediated by FasL and tumor necrosis factor. J. Exp. Med. 185:55-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Badley, A. D., J. A. McElhinny, P. J. Leibson, D. H. Lynch, M. R. Alderson, and C. V. Paya. 1996. Upregulation of Fas ligand expression by human immunodeficiency virus in human macrophages mediates apoptosis of uninfected T lymphocytes. J. Virol. 70:199-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Badley, A. D., A. A. Pilon, A. Landay, and D. H. Lynch. 2000. Mechanisms of HIV-associated lymphocyte apoptosis. Blood 96:2951-2964. [PubMed] [Google Scholar]
- 7.Balomenos, D., R. Rumold, and A. N. Theofilopoulos. 1997. The proliferative in vivo activities of lpr double-negative T cells and the primary role of p59fyn in their activation and expansion. J. Immunol. 159:2265-2273. [PubMed] [Google Scholar]
- 8.Bodi, I., A. A. Abraham, and P. L. Kimmel. 1995. Apoptosis in human immunodeficiency virus-associated nephropathy. Am. J. Kidney Dis. 26:286-291. [DOI] [PubMed] [Google Scholar]
- 9.Bonyhadi, M. L., L. Su, J. Auten, J. M. McCune, and H. Kaneshima. 1995. Development of a human thymic organ culture model for the study of HIV pathogenesis. AIDS Res. Hum. Retrovir. 11:1073-1080. [DOI] [PubMed] [Google Scholar]
- 10.Debatin, K. M., A. Fahrig-Faissner, S. Enenkel-Stoodt, W. Kreuz, A. Benner, and P. H. Krammer. 1994. High expression of APO-1 (CD95) on T lymphocytes from human immunodeficiency virus-1-infected children. Blood 83:3101-3103. [PubMed] [Google Scholar]
- 11.de Oliveira Pinto, L. M., S. Garcia, H. Lecoeur, C. Rapp, and M. L. Gougeon. 2002. Increased sensitivity of T lymphocytes to tumor necrosis factor receptor 1 (TNFR1)- and TNFR2-mediated apoptosis in HIV infection: relation to expression of Bcl-2 and active caspase-8 and caspase-3. Blood 99:1666-1675. [DOI] [PubMed] [Google Scholar]
- 12.Dittmer, U., H. Petry, C. Stahl-Hennig, T. Nisslein, M. Spring, W. Luke, W. Bodemer, F. J. Kaup, and G. Hunsmann. 1996. T cell apoptosis in human immunodeficiency virus type 2- and simian immunodeficiency virus-infected macaques. J. Gen. Virol. 77:2433-2436. [DOI] [PubMed] [Google Scholar]
- 13.Dobmeyer, T. S., S. A. Klein, J. M. Dobmeyer, B. Raffel, S. Findhammer, D. Hoelzer, E. B. Helm, R. Rossol, and D. Kabelitz. 1998. Differential expression of bcl-2 and susceptibility to programmed cell death in lymphocytes of HIV-1-infected individuals. Clin. Immunol. Immunopathol. 87:230-239. [DOI] [PubMed] [Google Scholar]
- 14.Dockrell, D. H., A. D. Badley, A. Algeciras-Schimnich, M. Simpson, R. Schut, D. H. Lynch, and C. V. Paya. 1999. Activation-induced CD4+ T cell death in HIV-positive individuals correlates with Fas susceptibility, CD4+ T cell count, and HIV plasma viral copy number. AIDS Res. Hum. Retrovir. 15:1509-1518. [DOI] [PubMed] [Google Scholar]
- 15.Economides, A., I. Schmid, D. J. Anisman-Posner, S. Plaeger, Y. J. Bryson, and C. H. Uittenbogaart. 1998. Apoptosis in cord blood T lymphocytes from infants of human immunodeficiency virus-infected mothers. Clin. Diagn. Lab. Immunol. 5:230-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Estaquier, J., M. Tanaka, T. Suda, S. Nagata, P. Golstein, and J. C. Ameisen. 1996. Fas-mediated apoptosis of CD4+ and CD8+ T cells from human immunodeficiency virus-infected persons: differential in vitro preventive effect of cytokines and protease antagonists. Blood 87:4959-4966. [PubMed] [Google Scholar]
- 17.Fauci, A. S., G. Pantaleo, S. Stanley, and D. Weissman. 1996. Immunopathogenic mechanisms of HIV infection. Ann. Int. Med. 124:654-663. [DOI] [PubMed] [Google Scholar]
- 18.Finkel, T. H., G. Tudor-Williams, N. K. Banda, M. F. Cotton, T. Curiel, C. Monks, T. W. Baba, R. M. Ruprecht, and A. Kupfer. 1995. Apoptosis occurs predominantly in bystander cells and not in productively infected cells of HIV- and SIV-infected lymph nodes. Nat. Med. 1:129-134. [DOI] [PubMed] [Google Scholar]
- 19.Gandhi, R. T., B. K. Chen, S. E. Straus, J. K. Dale, M. J. Lenardo, and D. Baltimore. 1998. HIV-1 directly kills CD4+ T cells by a Fas-independent mechanism. J. Exp. Med. 187:1113-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gehri, R., S. Hahn, M. Rothen, M. Steuerwald, R. Nuesch, and P. Erb. 1996. The Fas receptor in HIV infection: expression on peripheral blood lymphocytes and role in the depletion of T cells. AIDS 10:9-16. [PubMed] [Google Scholar]
- 21.Giese, T., and W. F. Davidson. 1994. Chronic treatment of C3H-lpr/lpr and C3H-gld/gld mice with anti-CD8 monoclonal antibody prevents the accumulation of double negative T cells but not autoantibody production. J. Immunol. 152:2000-2010. [PubMed] [Google Scholar]
- 22.Gougeon, M. L., S. Garcia, J. Heeney, R. Tschopp, H. Lecoeur, D. Guetard, V. Rame, C. Dauguet, and L. Montagnier. 1993. Programmed cell death in AIDS-related HIV and SIV infections. AIDS Res. Hum. Retrovir. 9:553-563. [DOI] [PubMed] [Google Scholar]
- 23.Groux, H., G. Torpier, D. Monte, Y. Mouton, A. Capron, and J. C. Ameisen. 1992. Activation-induced death by apoptosis in CD4+ T cells from human immunodeficiency virus-infected asymptomatic individuals. J. Exp. Med. 175:331-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hanna, Z., D. G. Kay, M. Cool, S. Jothy, N. Rebai, and P. Jolicoeur. 1998. Transgenic mice expressing human immunodeficiency virus type 1 in immune cells develop a severe AIDS-like disease. J. Virol. 72:121-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hanna, Z., D. G. Kay, N. Rebai, A. Guimond, S. Jothy, and P. Jolicoeur. 1998. Nef harbors a major determinant of pathogenicity for an AIDS-like disease induced by HIV-1 in transgenic mice. Cell 95:163-175. [DOI] [PubMed] [Google Scholar]
- 26.Hanna, Z., N. Rebai, J. Poudrier, and P. Jolicoeur. 2001. Distinct regulatory elements are required for faithful expression of human CD4 in T cells, macrophages, and dendritic cells of transgenic mice. Blood 98:2275-2278. [DOI] [PubMed] [Google Scholar]
- 27.Hanna, Z., C. Simard, A. Laperrière, and P. Jolicoeur. 1994. Specific expression of the human CD4 gene in mature CD4+ CD8− and immature CD4+ CD8+ T cells and in macrophages of transgenic mice. Mol. Cell. Biol. 14:1084-1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herron, L. R., R. A. Eisenberg, E. Roper, V. N. Kakkanaiah, P. L. Cohen, and B. L. Kotzin. 1993. Selection of the T cell receptor repertoire in Lpr mice. J. Immunol. 151:3450-3459. [PubMed] [Google Scholar]
- 29.Hittinger, G., C. Poggi, E. Delbeke, N. Profizi, and A. Lafeuillade. 1998. Correlation between plasma levels of cytokines and HIV-1 RNA copy number in HIV-infected patients. Infection 26:100-103. [DOI] [PubMed] [Google Scholar]
- 30.Hodge, S., F. J. Novembre, L. Whetter, H. A. Gelbard, and S. Dewhurst. 1998. Induction of fas ligand expression by an acutely lethal simian immunodeficiency virus, SIVsmmPBj14. Virology 252:354-363. [DOI] [PubMed] [Google Scholar]
- 31.Hosaka, N., N. Oyaizu, M. H. Kaplan, H. Yagita, and S. Pahwa. 1998. Membrane and soluble forms of Fas (CD95) and Fas ligand in peripheral blood mononuclear cells and in plasma from human immunodeficiency virus-infected persons. J. Infect. Dis. 178:1030-1039. [DOI] [PubMed] [Google Scholar]
- 32.Johnson, R. P. 1997. Upregulation of Fas ligand by simian immunodeficiency virus—a nef-arious mechanism of immune evasion? J. Exp. Med. 186:1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaplan, D., and S. Sieg. 1998. Role of the Fas/Fas ligand apoptotic pathway in human immunodeficiency virus type 1 disease. J. Virol. 72:6279-6282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Katsikis, P. D., M. E. Garcia-Ojeda, E. S. Wunderlich, C. A. Smith, H. Yagita, K. Okumura, N. Kayagaki, M. Alderson, L. A. Herzenberg, and L. A. Herzenberg. 1996. Activation-induced peripheral blood T cell apoptosis is Fas independent in HIV-infected individuals. Int. Immunol. 8:1311-1317. [DOI] [PubMed] [Google Scholar]
- 35.Katsikis, P. D., E. S. Wunderlich, C. A. Smith, L. A. Herzenberg, and L. A. Herzenberg. 1995. Fas antigen stimulation induces marked apoptosis of T lymphocytes in human immunodeficiency virus-infected individuals. J. Exp. Med. 181:2029-2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kay, D. G., P. Yue, Z. Hanna, S. Jothy, E. Tremblay, and P. Jolicoeur. 2002. Cardiac disease in transgenic mice expressing human immunodeficiency virus-1 Nef in cells of the immune system. Am. J. Pathol. 161:321-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kedzierska, K., and S. M. Crowe. 2001. Cytokines and HIV-1: interactions and clinical implications. Antivir. Chem. Chemother. 12:133-150. [DOI] [PubMed] [Google Scholar]
- 38.Keefe, R., V. Dave, D. Allman, D. Wiest, and D. J. Kappes. 1999. Regulation of lineage commitment distinct from positive selection. Science 286:1149-1153. [DOI] [PubMed] [Google Scholar]
- 39.Kolesnitchenko, V., L. King, A. Riva, Y. Tani, S. J. Korsmeyer, and D. I. Cohen. 1997. A major human immunodeficiency virus type 1-initiated killing pathway distinct from apoptosis. J. Virol. 71:9753-9763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krammer, P. H. 2000. CD95's deadly mission in the immune system. Nature 407:789-795. [DOI] [PubMed] [Google Scholar]
- 41.Kuida, K., J. A. Lippke, G. Ku, M. W. Harding, D. J. Livingston, M. S. Su, and R. A. Flavell. 1995. Altered cytokine export and apoptosis in mice deficient in interleukin-1 beta converting enzyme. Science 267:2000-2003. [DOI] [PubMed] [Google Scholar]
- 42.Ledru, E., H. Lecoeur, S. Garcia, T. Debord, and M. L. Gougeon. 1998. Differential susceptibility to activation-induced apoptosis among peripheral Th1 subsets: correlation with Bcl-2 expression and consequences for AIDS pathogenesis. J. Immunol. 160:3194-3206. [PubMed] [Google Scholar]
- 43.Lenardo, M., K. M. Chan, F. Hornung, H. McFarland, R. Siegel, J. Wang, and L. Zheng. 1999. Mature T lymphocyte apoptosis—immune regulation in a dynamic and unpredictable antigenic environment. Annu. Rev. Immunol. 17:221-253. [DOI] [PubMed] [Google Scholar]
- 44.Lenardo, M. J., S. B. Angleman, V. Bounkeua, J. Dimas, M. G. Duvall, M. B. Graubard, F. Hornung, M. C. Selkirk, C. K. Speirs, C. Trageser, J. O. Orenstein, and D. L. Bolton. 2002. Cytopathic killing of peripheral blood CD4+ T lymphocytes by human immunodeficiency virus type 1 appears necrotic rather than apoptotic and does not require env. J. Virol. 76:5082-5093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lepe-Zuniga, J. L., P. W. Mansell, and E. M. Hersh. 1987. Idiopathic production of interleukin-1 in acquired immune deficiency syndrome. J. Clin. Microbiol. 25:1695-1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lewis, D. E., D. S. Tang, A. Adu-Oppong, W. Schober, and J. R. Rodgers. 1994. Anergy and apoptosis in CD8+ T cells from HIV-infected persons. J. Immunol. 153:412-420. [PubMed] [Google Scholar]
- 47.Li, P., H. Allen, S. Banerjee, S. Franklin, L. Herzog, C. Johnston, J. McDowell, M. Paskind, L. Rodman, J. Salfeld, et al. 1995. Mice deficient in IL-1 beta-converting enzyme are defective in production of mature IL-1 beta and resistant to endotoxic shock. Cell 80:401-411. [DOI] [PubMed] [Google Scholar]
- 48.Locksley, R. M., N. Killeen, and M. J. Lenardo. 2001. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell 104:487-501. [DOI] [PubMed] [Google Scholar]
- 49.Lynch, D. H., M. L. Watson, M. R. Alderson, P. R. Baum, R. E. Miller, T. Tough, M. Gibson, T. Davis-Smith, C. A. Smith, K. Hunter, et al. 1994. The mouse Fas-ligand gene is mutated in gld mice and is part of a TNF family gene cluster. Immunity 1:131-136. [DOI] [PubMed] [Google Scholar]
- 50.Maldonado, M. A., R. A. Eisenberg, E. Roper, P. L. Cohen, and B. L. Kotzin. 1995. Greatly reduced lymphoproliferation in lpr mice lacking major histocompatibility complex class I. J. Exp. Med. 181:641-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maraskovsky, E., L. A. O'Reilly, M. Teepe, L. M. Corcoran, J. J. Peschon, and A. Strasser. 1997. Bcl-2 can rescue T lymphocyte development in interleukin-7 receptor-deficient mice but not in mutant rag-1-/- mice. Cell 89:1011-1019. [DOI] [PubMed] [Google Scholar]
- 52.Matsuyama, T., N. Kobayashi, and N. Yamamoto. 1991. Cytokines and HIV infection: is AIDS a tumor necrosis factor disease? AIDS 5:1405-1417. [DOI] [PubMed] [Google Scholar]
- 53.McCloskey, T. W., N. Oyaizu, S. Bakshi, R. Kowalski, N. Kohn, and S. Pahwa. 1998. CD95 expression and apoptosis during pediatric HIV infection: early upregulation of CD95 expression. Clin. Immunol. Immunopathol. 87:33-41. [DOI] [PubMed] [Google Scholar]
- 54.Mehal, W. Z., and I. N. Crispe. 1998. TCR ligation on CD8+ T cells creates double-negative cells in vivo. J. Immunol. 161:1686-1693. [PubMed] [Google Scholar]
- 55.Merrill, J. E., Y. Koyanagi, and I. S. Chen. 1989. Interleukin-1 and tumor necrosis factor alpha can be induced from mononuclear phagocytes by human immunodeficiency virus type 1 binding to the CD4 receptor. J. Virol. 63:4404-4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Meyaard, L., S. A. Otto, R. R. Jonker, M. J. Mijnster, R. P. Keet, and F. Miedema. 1992. Programmed death of T cells in HIV-1 infection. Science 257:217-219. [DOI] [PubMed] [Google Scholar]
- 57.Mixter, P. F., J. Q. Russell, F. H. Durie, and R. C. Budd. 1995. Decreased CD4-CD8- TCR-alpha beta + cells in lpr/lpr mice lacking beta 2-microglobulin. J. Immunol. 154:2063-2074. [PubMed] [Google Scholar]
- 58.Monceaux, V., J. Estaquier, M. Fevrier, M. C. Cumont, Y. Riviere, A. M. Aubertin, J. C. Ameisen, and B. Hurtrel. 2003. Extensive apoptosis in lymphoid organs during primary SIV infection predicts rapid progression towards AIDS. AIDS 17:1585-1596. [DOI] [PubMed] [Google Scholar]
- 59.Newton, K., and A. Strasser. 2000. Ionizing radiation and chemotherapeutic drugs induce apoptosis in lymphocytes in the absence of Fas or FADD/MORT1 signaling. Implications for cancer therapy. J. Exp. Med. 191:195-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Noraz, N., J. Gozlan, J. Corbeil, T. Brunner, and S. A. Spector. 1997. HIV-induced apoptosis of activated primary CD4+ T lymphocytes is not mediated by Fas-Fas ligand. AIDS 11:1671-1680. [DOI] [PubMed] [Google Scholar]
- 61.Ohagen, A., S. Ghosh, J. He, K. Huang, Y. Chen, M. Yuan, R. Osathanondh, S. Gartner, B. Shi, G. Shaw, and D. Gabuzda. 1999. Apoptosis induced by infection of primary brain cultures with diverse human immunodeficiency virus type 1 isolates: evidence for a role of the envelope. J. Virol. 73:897-906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ohteki, T., M. Iwamoto, S. Izui, and H. R. MacDonald. 1995. Reduced development of CD4-8-B220+ T cells but normal autoantibody production in lpr/lpr mice lacking major histocompatibility complex class I molecules. Eur. J. Immunol. 25:37-41. [DOI] [PubMed] [Google Scholar]
- 63.Park, I. W., E. Kondo, L. Bergeron, J. Park, and J. Sodroski. 1996. Effects of human immunodeficiency virus type 1 infection on programmed cell death in the presence or absence of Bcl-2. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 12:321-328. [DOI] [PubMed] [Google Scholar]
- 64.Petit, F., D. Arnoult, J. D. Lelievre, L. Moutouh-de Parseval, A. J. Hance, P. Schneider, J. Corbeil, J. C. Ameisen, and J. Estaquier. 2002. Productive HIV-1 infection of primary CD4+ T cells induces mitochondrial membrane permeabilization leading to a caspase-independent cell death. J. Biol. Chem. 277:1477-1487. [DOI] [PubMed] [Google Scholar]
- 65.Petito, C. K., and B. Roberts. 1995. Evidence of apoptotic cell death in HIV encephalitis. Am. J. Pathol. 146:1121-1130. [PMC free article] [PubMed] [Google Scholar]
- 66.Pfeffer, K., T. Matsuyama, T. M. Kundig, A. Wakeham, K. Kishihara, A. Shahinian, K. Wiegmann, P. S. Ohashi, M. Kronke, and T. W. Mak. 1993. Mice deficient for the 55 kd tumor necrosis factor receptor are resistant to endotoxic shock, yet succumb to L. monocytogenes infection. Cell 73:457-467. [DOI] [PubMed] [Google Scholar]
- 67.Poudrier, J., X. Weng, D. G. Kay, G. Paré, E. L. Calvo, Z. Hanna, M. H. Kosco-Vilbois, and P. Jolicoeur. 2001. The AIDS disease of CD4C/HIV transgenic mice shows impaired germinal centers and autoantibodies and develops in the absence of IFN-γ and IL-6. Immunity 15:173-185. [DOI] [PubMed] [Google Scholar]
- 68.Rasola, A., D. Gramaglia, C. Boccaccio, and P. M. Comoglio. 2001. Apoptosis enhancement by the HIV-1 Nef protein. J. Immunol. 166:81-88. [DOI] [PubMed] [Google Scholar]
- 69.Renkema, G. H., and K. Saksela. 2000. Interactions of HIV-1 Nef with cellular signal transducing proteins. Front. Biosci. 5:D268-D283. [DOI] [PubMed] [Google Scholar]
- 70.Rizzardi, G. P., W. Barcellini, G. Tambussi, F. Lillo, M. Malnati, L. Perrin, and A. Lazzarin. 1996. Plasma levels of soluble CD30, tumour necrosis factor (TNF)-alpha and TNF receptors during primary HIV-1 infection: correlation with HIV-1 RNA and the clinical outcome. AIDS 10:F45-F50. [DOI] [PubMed] [Google Scholar]
- 71.Rosenzweig, M., M. Connole, A. Forand-Barabasz, M. P. Tremblay, R. P. Johnson, and A. A. Lackner. 2000. Mechanisms associated with thymocyte apoptosis induced by simian immunodeficiency virus. J. Immunol. 165:3461-3468. [DOI] [PubMed] [Google Scholar]
- 72.Roux-Lombard, P., C. Modoux, A. Cruchaud, and J. M. Dayer. 1989. Purified blood monocytes from HIV 1-infected patients produce high levels of TNF alpha and IL-1. Clin. Immunol. Immunopathol. 50:374-384. [DOI] [PubMed] [Google Scholar]
- 73.Samuelsson, A., C. Brostrom, N. van Dijk, A. Sonnerborg, and F. Chiodi. 1997. Apoptosis of CD4+ and CD19+ cells during human immunodeficiency virus type 1 infection—correlation with clinical progression, viral load, and loss of humoral immunity. Virology 238:180-188. [DOI] [PubMed] [Google Scholar]
- 74.Sentman, C. L., J. R. Shutter, D. Hockenbery, O. Kanagawa, and S. J. Korsmeyer. 1991. bcl-2 inhibits multiple forms of apoptosis but not negative selection in thymocytes. Cell 67:879-888. [DOI] [PubMed] [Google Scholar]
- 75.Shearer, G. M. 1998. HIV-induced immunopathogenesis. Immunity 9:587-593. [DOI] [PubMed] [Google Scholar]
- 76.Sieg, S., D. Smith, Z. Yildirim, and D. Kaplan. 1997. Fas ligand deficiency in HIV disease. Proc. Natl. Acad. Sci. USA 94:5860-5865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Siegel, R. M., M. Katsumata, T. Miyashita, D. C. Louie, M. I. Greene, and J. C. Reed. 1992. Inhibition of thymocyte apoptosis and negative antigenic selection in bcl-2 transgenic mice. Proc. Natl. Acad. Sci. USA 89:7003-7007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Singer, A. 2002. New perspectives on a developmental dilemma: the kinetic signaling model and the importance of signal duration for the CD4/CD8 lineage decision. Curr. Opin. Immunol. 14:207-215. [DOI] [PubMed] [Google Scholar]
- 79.Singer, G. G., and A. K. Abbas. 1994. The fas antigen is involved in peripheral but not thymic deletion of T lymphocytes in T cell receptor transgenic mice. Immunity 1:365-371. [DOI] [PubMed] [Google Scholar]
- 80.Sloand, E. M., P. N. Kumar, S. Kim, A. Chaudhuri, F. F. Weichold, and N. S. Young. 1999. Human immunodeficiency virus type 1 protease inhibitor modulates activation of peripheral blood CD4(+) T cells and decreases their susceptibility to apoptosis in vitro and in vivo. Blood 94:1021-1027. [PubMed] [Google Scholar]
- 81.Sloand, E. M., J. P. Maciejewski, T. Sato, J. Bruny, P. Kumar, S. Kim, F. F. Weichold, and N. S. Young. 1998. The role of interleukin-converting enzyme in Fas-mediated apoptosis in HIV-1 infection. J. Clin. Investig. 101:195-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sloand, E. M., N. S. Young, P. Kumar, F. F. Weichold, T. Sato, and J. P. Maciejewski. 1997. Role of Fas ligand and receptor in the mechanism of T-cell depletion in acquired immunodeficiency syndrome: effect on CD4+ lymphocyte depletion and human immunodeficiency virus replication. Blood 89:1357-1363. [PubMed] [Google Scholar]
- 83.Smith, D. J., M. J. McGuire, M. J. Tocci, and D. L. Thiele. 1997. IL-1 beta convertase (ICE) does not play a requisite role in apoptosis induced in T lymphoblasts by Fas-dependent or Fas-independent CTL effector mechanisms. J. Immunol. 158:163-170. [PubMed] [Google Scholar]
- 84.Steinberg, A. D. 1994. MRL-lpr/lpr disease: theories meet Fas. Semin. Immunol. 6:55-69. [DOI] [PubMed] [Google Scholar]
- 85.Steinberg, A. D., J. B. Roths, E. D. Murphy, R. T. Steinberg, and E. S. Raveche. 1980. Effects of thymectomy or androgen administration upon the autoimmune disease of MRL/Mp-lpr/lpr mice. J. Immunol. 125:871-873. [PubMed] [Google Scholar]
- 86.Strasser, A., A. W. Harris, and S. Cory. 1991. bcl-2 transgene inhibits T cell death and perturbs thymic self-censorship. Cell 67:889-899. [DOI] [PubMed] [Google Scholar]
- 87.Strasser, A., A. W. Harris, D. C. Huang, P. H. Krammer, and S. Cory. 1995. Bcl-2 and Fas/APO-1 regulate distinct pathways to lymphocyte apoptosis. EMBO J. 14:6136-6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Takahashi, T., M. Tanaka, C. I. Brannan, N. A. Jenkins, N. G. Copeland, T. Suda, and S. Nagata. 1994. Generalized lymphoproliferative disease in mice, caused by a point mutation in the Fas ligand. Cell 76:969-976. [DOI] [PubMed] [Google Scholar]
- 89.Than, S., R. Hu, N. Oyaizu, J. Romano, X. Wang, S. Sheikh, and S. Pahwa. 1997. Cytokine pattern in relation to disease progression in human immunodeficiency virus-infected children. J. Infect. Dis. 175:47-56. [DOI] [PubMed] [Google Scholar]
- 90.Twu, C., N. Q. Liu, W. Popik, M. Bukrinsky, J. Sayre, J. Roberts, S. Rania, V. Bramhandam, K. P. Roos, W. R. MacLellan, and M. Fiala. 2002. Cardiomyocytes undergo apoptosis in human immunodeficiency virus cardiomyopathy through mitochondrion- and death receptor-controlled pathways. Proc. Natl. Acad. Sci. USA 99:14386-14391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Watanabe-Fukunaga, R., C. I. Brannan, N. G. Copeland, N. A. Jenkins, and S. Nagata. 1992. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature 356:314-317. [DOI] [PubMed] [Google Scholar]
- 92.Weng, X., E. Priceputu, P. Chrobak, J. Poudrier, D. G. Kay, Z. Hanna, T. W. Mak, and P. Jolicoeur. 2004. CD4+ T cells from CD4C/HIVNef transgenic mice show enhanced activation in vivo with impaired proliferation in vitro but are dispensable for the development of a severe AIDS-like organ disease. J. Virol. 78:5244-5257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Xu, X. N., B. Laffert, G. R. Screaton, M. Kraft, D. Wolf, W. Kolanus, J. Mongkolsapay, A. J. McMichael, and A. S. Baur. 1999. Induction of Fas ligand expression by HIV involves the interaction of Nef with the T cell receptor zeta chain. J. Exp. Med. 189:1489-1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Xu, X. N., G. R. Screaton, F. M. Gotch, T. Dong, R. Tan, N. Almond, B. Walker, R. Stebbings, K. Kent, S. Nagata, J. E. Stott, and A. J. McMichael. 1997. Evasion of cytotoxic T lymphocyte (CTL) responses by nef- dependent induction of Fas ligand (CD95L) expression on simian immunodeficiency virus-infected cells. J. Exp. Med. 186:7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yagi, T., A. Sugimoto, M. Tanaka, S. Nagata, S. Yasuda, H. Yagita, T. Kuriyama, T. Takemori, and Y. Tsunetsugu-Yokota. 1998. Fas/FasL interaction is not involved in apoptosis of activated CD4+ T cells upon HIV-1 infection in vitro. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 18:307-315. [DOI] [PubMed] [Google Scholar]
- 96.Zangerle, R., H. Gallati, M. Sarcletti, H. Wachter, and D. Fuchs. 1994. Tumor necrosis factor alpha and soluble tumor necrosis factor receptors in individuals with human immunodeficiency virus infection. Immunol. Lett. 41:229-234. [DOI] [PubMed] [Google Scholar]
- 97.Zauli, G., D. Gibellini, P. Secchiero, H. Dutartre, D. Olive, S. Capitani, and Y. Collette. 1999. Human immunodeficiency virus type 1 Nef protein sensitizes CD4(+) T lymphoid cells to apoptosis via functional upregulation of the CD95/CD95 ligand pathway. Blood 93:1000-1010. [PubMed] [Google Scholar]
- 98.Zou, W., A. A. Lackner, M. Simon, I. Durand-Gasselin, P. Galanaud, R. C. Desrosiers, and D. Emilie. 1997. Early cytokine and chemokine gene expression in lymph nodes of macaques infected with simian immunodeficiency virus is predictive of disease outcome and vaccine efficacy. J. Virol. 71:1227-1236. [DOI] [PMC free article] [PubMed] [Google Scholar]