Abstract
Age-related changes in intestinal microbiome composition and function are increasingly recognized as pivotal in the pathophysiology of aging and are associated with the aging phenotype. Diet is a major determinant of gut-microbiota composition throughout the entire lifespan, and several of the benefits of a healthy diet in aging could be mediated by the microbiome. Mediterranean diet (MD) is a traditional dietary pattern regarded as the healthy diet paradigm, and a large number of studies have demonstrated its benefits in promoting healthy aging. MD has also a positive modulatory effect on intestinal microbiome, favoring bacterial taxa involved in the synthesis of several bioactive compounds, such as short-chain fatty acids (SCFAs), that counteract inflammation, anabolic resistance, and tissue degeneration. Intervention studies conducted in older populations have suggested that the individual response of older subjects to MD, in terms of reduction of frailty scores and amelioration of cognitive function, is significantly mediated by the gut-microbiota composition and functionality. In this context, the pathophysiology of intestinal microbiome in aging should be considered when designing MD-based interventions tailored to the needs of geriatric patients.
Keywords: Frailty, Dementia, Sarcopenia, Malnutrition, Healthy diet
Introduction
The intestinal microbiome is the ensemble of microorganisms, predominantly bacteria, living in the gastrointestinal lumen and establishing physiologic interactions with the human body, as well as their theater of activity including a whole set of molecules related to gut and host physiology [1]. In healthy adult subjects, an equilibrium between bacterial species with purported health-promoting properties and opportunistic pathogens is generally present, while significant differences in gut microbial communities can be detected among different subjects [1, 2]. This inter-individual variability depends on a large number of factors, including host genetics, environmental exposures, lifestyle and physiologic status [3, 4]. Diet is perhaps the most influential of these factors, as suggested by metagenome-wide association studies [4, 5].
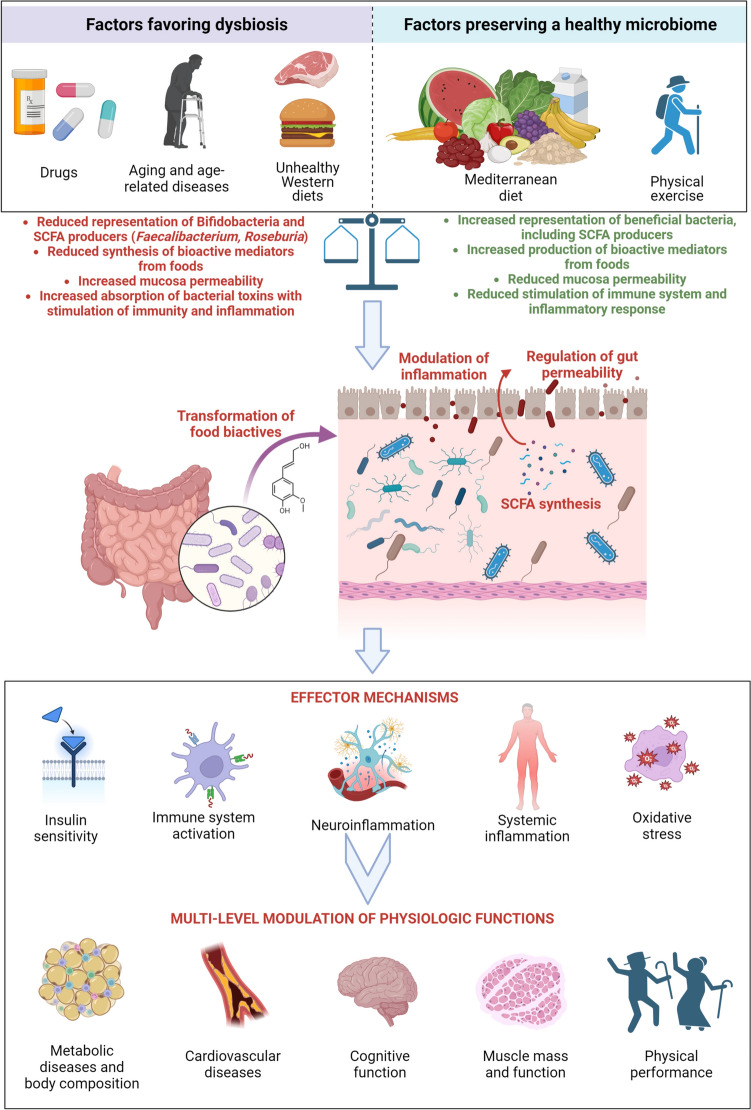
The process of aging implies a certain degree of disruption of the equilibrium between beneficial, neutral and potentially pathogenic bacteria [6], especially after 70 years old, partly as a consequence of aging of the gastrointestinal and immune system [7], and partly as the result of disease, exposure to drugs, and change of diet and mobility [8]. This imbalance of intestinal microbial communities can negatively influence several aspects of the host physiology and is defined as dysbiosis. Although dysbiosis can occur also in earlier stages of the human life, it is consistently involved in the pathogenesis of several age-related diseases and syndromes and thus increasingly regarded as one of the fundamental pathogenetic drivers of aging [9].
Dysbiosis does not only imply a change in the composition of the microbiome, with increased representation of opportunistic pathogens at the expense of taxa with purported health-promoting activity, but it is also associated with a different microbiome functionality, causing changes in the release of physiologically active compounds [10]. In the last decade, several experimental and clinical studies have suggested that age-related gut-microbiota alterations can negatively influence the pathogenesis of many diseases and conditions with high prevalence in geriatric patients [11], including dementia [12], sarcopenia [13], type 2 diabetes [14], hypertension, and other cardiovascular diseases [15].
In older subjects, inter-individual differences in intestinal microbiota composition are also emphasized in comparison with subjects under 70 years of age [16]. As such, fit individuals who reach extreme ages of life, such as centenarians and supercentenarians, may maintain an intestinal microbiome structure more similar to the one of adult subjects, with good representation of bacterial taxa with beneficial modulatory properties for the body functioning, such as anti-inflammatory and pro-anabolic action [17]. Conversely, the deepest levels of dysbiosis are generally observed in frail multimorbid subjects [18, 19].
These circumstances suggest that maintenance of a good equilibrium in intestinal microbiome should be a goal for promoting successful aging [20]. The administration of live bacteria (i.e., probiotics) or functional components (i.e., prebiotics) and foods has shown limited effectiveness on microbiome structure and clinical endpoints in older individuals, although few studies are available to date [21–23]. Dietary interventions, instead, have been emphasized as promising strategies to counteract dysbiosis by inducing generalized and durable rearrangements on microbiome composition and function [24]. Mediterranean diet (MD), in particular, has emerged as the healthy diet paradigm and has been associated with a wide range of beneficial effects on primary and secondary prevention of several non-communicable diseases [25, 26].
In this review, we summarize the current evidence on the interactions between MD and gut microbiome, and their importance for mitigating the pathophysiological processes associated with aging and some of the most relevant age-related conditions. First, we consider the effect of MD on the microbiome of adult subjects; then, we explore the impact of MD on older adults; and last, we comment on the main underlying mechanisms behind the anti-aging effects of MD on the gut microbiome.
Association between Mediterranean diet and microbiome in adult subjects
Observational studies
MD, acknowledged by UNESCO as Intangible Cultural Heritage of Humanity in 2010, is a traditional dietary pattern implying daily consumption of plant foods, including cereals, fruit, vegetables and legumes with olive oil as the main source of added fat, and moderate consumption of fish, seafood, eggs, poultry and milk or dairy products. Read meat and unprocessed sugars, which represent important components of modern Western diets, are generally present in limited amounts in MD [27], as well as parsimonious consumption of wine during mealtimes. Apart from its mere composition, MD also implies socialization and conviviality during meals, seasonality and biodiversity in dietary choices, frequent consumption of traditional and local food products and, overall, a healthy lifestyle with a good balance between physical activity and rest [27].
Adherence to MD pattern can be measured in each individual through specific scores validated by the scientific literature. These scores are generally based on the analysis of semi-quantitative food-frequency questionnaires and on the identification of the frequency of consumption of key foods for MD [28]. Despite the limitations of this approach, including the possibility of recall bias, population-based studies conducted in different countries have shown a correlation between MD adherence scores and fecal microbiome composition [29–35]. The results of these studies are summarized in Table 1.
Table 1.
Overview of the main findings of the observational studies investigating the relationship between Mediterranean diet and gut microbiome in adult subjects
| Main author, year, reference | Participants | Main microbial biomarkers of MD | Main microbial biomarkers of Western diet | Other key findings |
|---|---|---|---|---|
| Gutiérrez-Díaz 2016 [29] | Thirty-one adults not reporting chronic diseases |
Bacteroidetes Prevotellaceae Prevotella |
Firmicutes Lachnospiraceae |
MD adherence was associated with increased fecal propionate and butyrate |
| De Filippis 2016 [30] | One hundred and fifty-three adults habitually following omnivore, vegetarian or vegan diets |
Lachnospira Prevotella |
Ruminococcus Streptococcus |
MD adherence was associated with representation of fiber-degrading bacteria and increased fecal SCFA levels Omnivore diet implied higher levels of TMAO |
| Mitsou 2017 [31] | One hundred and sixteen adult volunteers in good health |
Bifidobacteria Candida albicans |
Bacteroides Escherichia coli |
MD adherence was associated with increased production of SCFAs and with lower burden of gastrointestinal symptoms |
| Gallé 2020 [32] | One hundred and forty university students |
Lachnospira Lactobacillus Lactococcus |
Ruminococcus Oscillospira Paraprevotella |
Microbiota composition was more influenced by habitual physical activity and BMI than MD adherence scores |
| Wang 2021 [33] | Three hundred and seven male health professionals aged 45 or older |
Faecalibacterium prausnitzii Eubacterium rectale Bacteroides cellulosilyticus |
Ruminococcus torques Clostridium leptum Collinsella |
MD adherence was associated with increased plant polysaccharide degradation potential and SCFA synthesis The association between MD adherence and blood biomarkers of cardiometabolic risk was mediated by Prevotella copri abundance |
| Turpin 2022 [34] | Two thousand two hundred and eighty nine healthy relatives of subjects with Chron’s disease |
Lachnospira Faecalibacterium Clostridium Veillonella |
Ruminococcus Dorea Actinomyces |
Clostridium, Parvimonas and Dialister were the main taxa mediating the anti-inflammatory effect of diet on the intestinal mucosa |
| Rosés 2021 [35] | Three hundred and sixty adults with different weight (normal, overweight or obese) |
Bifidobacterium animalis Roseburia faecis Flavonifractor plautii Ruminococcus bromii |
Eubacterium saphenum Succinivibrio dextrinosolvens Gordonibacter pamelaeae |
MD adherence was significantly associated with the abundance of several bacterial taxa able to synthetize SCFAs, particularly butyrate |
MD Mediterranean Diet; SCFA Short-Chain Fatty Acids; TMAO Trimethylamine-N-oxide; BMI Body Mass Index
Interestingly, in most studies, MD adherence was significantly associated neither with biodiversity, that is, species richness, nor with the overall composition of the intestinal microbial community [29, 33, 34]. Instead, MD seemed more associated with increased representation of bacterial taxa with purported health-promoting activities and metabolic function of bacteria, like Lachnospira, Prevotella, bifidobacteria, Faecalibacterium prausnitzii, Eubacterium rectale [29–35]. Conversely, Ruminococcus, Oscillospira, Escherichia coli and other members of Enterobacteriaceae were the main microbial taxa associated with Western-style diets [32, 34].
At functional level, MD adherence was associated with different metabolic signatures in blood, urine and fecal samples [36]. In particular, it was associated with increased microbial synthesis of short-chain fatty acids (SCFAs), including acetate, propionate and butyrate, which exhibit an overall anti-inflammatory and pro-anabolic function favoring insulin sensitivity [29–31, 33]. Bacterial taxa with known capacity of synthetizing SCFAs, such as F. prausnitzii, Butyrivibrio, and Roseburia, seem to play an important role as mediators of the beneficial metabolic effects of MD [37]. High fiber intake, which is a key component of MD, is in fact able to stimulate the representation of these taxa in intestinal microbial communities, while fibers themselves represent the biochemical substrates for SCFA synthesis [38].
MD adherence was also associated with reduced synthesis of trimethylamine-N-oxide (TMAO) [30, 32], an emerging marker of cardiovascular risk synthetized with the contribution of the gut microbiota [39]. According to a study conducted on 307 male adults, Prevotella copri also resulted as a key mediator of the beneficial effects of MD adherence on biomarkers of cardiometabolic risk, including serum levels of C-reactive protein (CRP), total cholesterol, triglycerides, and glycated hemoglobin [33]. Microbiome mediation in the relationship between MD adherence and reduction of inflammation was also confirmed in groups of patients with chronic inflammatory conditions, such as human immunodeficiency virus (HIV) infection [40], or at risk of inflammatory bowel disease (IBD) [34].
Intervention studies
MD intervention is generally assumed to modify gut-microbiome composition and function not only in subjects with chronic diseases (Table 2), but also in healthy subjects [41–43]. The magnitude and the characteristics of these changes, however, are inconsistent across studies.
Table 2.
Summary of the main findings of randomized controlled trials testing the effects of Mediterranean diet on gut microbiome in subjects with chronic illness
| Reference | Participants | Study arm | Comparison arm | Duration of intervention | Effects on gut microbiome | Effects on other physiologic parameters | Other key findings |
|---|---|---|---|---|---|---|---|
| Haro 2016 [46] | Twenty obese subjects | MD | LFHCC diet | 1 year |
↑ Roseburia, Oscillospira, Parabacteroides distasonis ↓ Prevotella, Faecalibacterium prausnitzii |
↑ Insulin sensitivity index Different urinary metabolomic profile |
LFHCC diet also induced favorable changes in gut microbiome composition |
| Meslier 2020 [47] | Eighty-two overweight and obese subjects with low fruit and vegetable intake | MD | Habitual diet | 8 weeks |
↑ Faecalibacterium prausnitzii ↓ Ruminococcus gnavus ↑ representation of genes involved in carbohydrate metabolism and SCFA synthesis |
↓ plasma cholesterol ↑ Insulin sensitivity ↓ fecal bile acids |
MD resulted into increased levels of urinary urolithins, proportionate to plant food intake |
| Pagliai 2020 [48] | Twenty-three overweight subjects with low/moderate cardiovascular risk | Low-calorie MD | Vegetarian diet | 3 + 3 months (cross-over design) |
↑ Enterohabdus, Lachnoclostridium, Parabacteroides ↑ synthesis of propionic acid |
↓ inflammatory cytokines | No major changes in microbiome composition with either dietary pattern |
| Rinott 2022 [49] | Two hundred and forty-nine subjects with abdominal obesity/dyslipidemia | MD or Green MD plus walnuts | Standard guideline-based healthy diet | 6 months |
↑ Prevotella ↓ Bifidobacterium ↑ representation of genes involved in branched-chain amino acid metabolism |
↓ Body weight, blood pressure, blood lipids ↑ Insulin sensitivity |
The beneficial effects of MD are mediated by specific changes in gut microbiome, that are emphasized in green MD |
| Calabrese 2022 [50] | One hundred and nine patients with NAFLD | LGIMD with or without aerobic activity | Standard guideline-based healthy diet | 3 months |
Changes in overall composition ↑ Ruminococcus, Enterohabdus, Coprobacter, Lachnospiraceae |
↓ elastosonographic index of liver fibrosis | The effects of MD on gut microbiome are emphasized when MD is associated with physical activity |
| Ben-Yacov 2023 [53] | Two hundred adults with pre-diabetes | MD | PPT diet based on machine-learning | 6 months | ↑ Bifidobacterium adolescentis and other taxa |
↓ plasma cholesterol ↑ Insulin sensitivity |
PPT diet was more effective in driving changes in gut microbiome composition |
| Haro 2016 [54] | Two hundred and thirty-nine patients with CHD (with or without metabolic syndrome) | MD | Low-fat diet | 2 years | ↑ Parabacteroides distasonis, Bacteroides thetaiotamicron, Faecalibacterium prausnitzii, Bifidobacterium adolescentis in patients with metabolic syndrome |
↓ waist circumference ↓ glucose plasma levels ↓ triglycerides ↑ HDL |
Mediterranean diet-induced marked changes in fecal microbiome composition only in subjects with metabolic syndrome |
| Galié 2021 [55, 56] | Thirty-eight overweight patients with metabolic syndrome | MD | Free diet with 50 g/day nut supplement | 2 + 2 months (cross-over design) | ↑ Lachnospiraceae, Ruminococcaceae | ↓ glucose, insulin, HOMA-IR | MD-induced changes in microbiome were associated with positive changes in fecal acetate and with changes in 65 serum metabolites |
| Lewis 2021 [57] | One hundred and ninety-one patients with Crohn’s disease | MD | Carbohydrate diet tailored to Chron’s disease | 6 weeks | No major differences in microbiome composition | Improvement of parameters of inflammation in both diets | Two distinct clusters of microbiome response to diet were identified, one with increasing abundance of Bacteroides vulgatus and the other with decreasing abundance of Faecalibacterium prausnitzii |
MD Mediterranean Diet; LFHCC Low-Fat High-Complex Carbohydrate; LGIMD Low-Glycemic Index Mediterranean Diet; PPT Personalized Postprandial Targeting; CHD Coronary Heart Disease; HDL High-Density Lipoprotein; HOMA-IR Homeostatic Model Assessment of Insulin Resistance; NAFLD Non-Alcoholic Fatty Liver Disease
In a group of 20 healthy male volunteers, Barber et al. showed no major differences in gut-microbiome composition before and after a 2-week MD intervention, but they just demonstrated significant variations in a few bacterial taxa [41]. These findings were probably influenced by the high baseline inter-individual variability in the intestinal microbiome of participants, who responded to the dietary intervention in an individualized manner. However, metabolomic analyses revealed a different urinary metabolic profile after the intervention, with significant variations in the levels of several metabolites of bacterial origin [41]. In another study by Godny et al., a 4-week MD intervention associated with daily physical activity measurement resulted into increased representation of bacterial taxa involved in SCFA synthesis, including F. prausnitzii, Lachnospiraceae and Bifidobacterium spp., with reduced levels of inflammatory biomarkers such as fecal calprotectin [42]. Interestingly, similar results were obtained also by Rejeski et al. in a group of 10 healthy subjects, where a 2-week MD intervention also improved the overall microbiome species richness [43].
The modulatory effects of MD on gut microbiome are potentially useful for the prevention and treatment of several chronic conditions, including obesity, type 2 diabetes, and metabolic syndrome [44]. In 18 overweight subjects with body mass index (BMI) ≥ 25 kg/m2, a 3-month dietary intervention consisting of MD enriched with 40 g/day of high-quality extra-virgin olive oil was associated with improved fecal microbiome biodiversity, significant reduction of myeloperoxidase activity, oxidative stress markers and pro-inflammatory cytokines, and significant increase in adiponectin and plasma anti-inflammatory cytokines such as interleukin-10 (IL-10) [45].
Randomized controlled trials (RCTs) investigating the effects of MD and its variants on the gut microbiome of overweight and obese subjects have also shown beneficial effects, ranging from increased biodiversity to increased representation of SCFA-producing taxa or bacteria involved in branched-chain amino acid metabolism (Table 2) [46–49]. Interestingly, other healthy dietary patterns not falling within the MD definition, such as the vegetarian [48] or the low-fat high-complex carbohydrate (LFHCC) diet [46], also induced beneficial changes in gut-microbiome composition and function, but these changes were distinct from those induced by a traditional MD pattern. LHFCC diet, for example, determined a more pronounced increase in the representation of F. prausnitzii than MD [46], while vegetarian diet promoted growth of Anaerostipes, Streptococcus, Clostridium, and Odoribacter not observed in MD [48].
The effects of MD on the gut microbiome are emphasized by concomitant involvement in a structured physical exercise program. In a group of subjects with non-alcoholic fatty liver disease (NAFLD), a condition frequently overlapped with obesity, the abundance of Ruminococcaceae, Oscillospiraceae and Lachnospiraceae was much more influenced by the association between aerobic physical exercise and MD than MD alone [50].
The interactions between gut-microbiome modifications induced by MD and type 2 diabetes are less known. In subjects following a MD pattern, an intestinal microbiome signature consisting in high abundance of Prevotella, Saccharibacteria, and Betaproteobacteria was associated with increased risk of developing diabetes [51]. Conversely, a 4-week MD intervention in subjects with type 2 diabetes resulted into an improved bacterial richness of gut microbiota, which exhibited a negative correlation with insulin resistance [52]. However, in a recent RCT comparing the effects of MD with a personalized post-prandial targeting (PPT) diet in subjects with type 2 diabetes (Table 2), MD was associated with only minor modifications of fecal microbiome, such as increased abundance of Bifidobacterium adolescentis, and resulted less effective than PPT diet in inducing metabolically-favorable changes of intestinal microbial communities [53]. Nevertheless, it should be highlighted that here the authors compared two different dietary strategies (general vs. personalized dietary advice) and that further interventions with personalized MD intervention could be of interest.
In subjects with metabolic syndrome (Table 2), however, a long-lasting MD intervention was associated with increases in the fecal microbiome representation of Parabacteroides distasonis, Bacteroides thetaiotaomicron, F. prausnitzii, B. adolescentis and Bifidobacterium longum [54]. In two different analyses of the METADIET randomized, controlled, cross-over trial, Galié et al. showed that MD intervention was associated with increased levels of Lachnospiraceae and Ruminococcaceae and improved insulin sensitivity in patients with metabolic syndrome (Table 2) [55, 56].
The effects of MD interventions on the gut microbiome of adult patients with other chronic illnesses not included in the metabolic syndrome spectrum have been scarcely investigated (Table 2). In a RCT comparing MD with the specific carbohydrate diet in adults with Crohn’s disease, neither dietary approach was superior in reducing parameters of inflammation and disease activity after 6 weeks, and no major differences in post-intervention fecal microbiome composition were detected between study arms [57]. Therefore, the current knowledge on the physiologic effects of the interaction between MD and intestinal microbiome in subjects younger than 70 years old is basically limited to improvements in insulin resistance, glucose and lipid metabolism, and chronic subclinical inflammation related to obesity (Table 2).
Mediterranean diet and gut microbiome in older subjects
Observational studies
Despite the gut-microbiome composition of older individuals is less resilient to stressors and more influenced by a large number of (physio)pathologic conditions, diet remains a major driver of the inter-individual variability observed for this age group. In a cross-sectional analysis of data from the NU-AGE RCT, including 226 Dutch subjects aged between 65 and 79, BMI exhibited a much stronger statistically significant association with overall fecal microbiome composition than frailty phenotype [58]. The main dietary factors explaining microbiome inter-individual variability were, on the one side, consumption of fruits, nuts, grain products, and carbohydrates, which are fundamental parts of the MD pattern, and, on the other side, consumption of processed and red meats, which are consumed only occasionally in MD [58]. The main bacterial taxa showing positive associations with foods typically well represented in MD included F. prausnitzii, E. rectale and Eubacterium biforme, while animal protein-based diets were mainly associated with the abundance of taxa with purported pro-inflammatory action, such as Ruminococcus gnavus and Collinsella [58].
Adherence to MD was associated with increased representation of F. prausnitzii also in a group of 74 older Spanish volunteers, where Clostridium cluster XIVa was identified as another important marker of MD [59]. These features of gut microbiome were also associated with specific metabolic signatures, including increased fecal levels of SCFAs, benzoic and 3-hydroxyphenylacetic acids [59, 60]. These signatures are likely the result of the increased-intestinal microbial metabolism of fibers and (poly)phenols associated with MD, respectively [59, 60]. In a group of older Caribbean Latinos living in the United States, instead, adherence to MD was associated with a distinct cluster of fecal microbiome composition, characterized by different abundance of P. copri and co-occurring bacterial networks [61].
In a group of 17 centenarians and 29 nonagenarians from Sardinia (Italy), adherence to MD was associated with several bacterial taxa in fecal microbiome [62]. However, the taxa positively correlated with MD were different between centenarians (Lactobacillus, Clostridium, and Dorea) and nonagenarians (Bacteroides, Parabacteroides, and Pedobacter), with the only exception of Peptoniphilus [62]. Interestingly, MD was associated with depletion of bifidobacteria only in nonagenarians, but not in centenarians [62]. In fact, the microbial ecology of centenarians is characterized by the persistence of a subdominant fraction of bifidobacteria, which is usually depleted in older individuals who do not reach extreme ages [63, 64].
Intervention studies
The existing intervention studies with MD in older individuals have adapted the traditional MD pattern to the specific needs of aging, targeting, in particular, modulation of inflammation, weight loss, and cognition. Berendsen et al. elaborated a modified MD pattern based on recommended daily allowances (RDAs) of macro- and micronutrients for older subjects, with the aim of modulating inflammaging (i.e., the chronic subclinical activation of inflammatory pathways responsible for the pathogenesis of several chronic illnesses and geriatric syndromes typical of the older age) and maintaining a good balance in protein-energy metabolism [65, 66]. This dietary approach —the so-called NU-AGE diet— was the main intervention studied in the NU-AGE RCT, where 1141 pre-frail and fit subjects aged 65 to 79 years old from five European countries were randomized to receive MD tailored to older people (NU-AGE diet) or a control free diet [65]. It was demonstrated as feasible in pre-frail and non-frail older subjects in the long term, with good adherence scores after 1 year from the initiation of the intervention [67]. The gut-microbiota composition and function was among the endpoints of the NU-AGE study and fecal microbiome profiling of 612 participants showed significant changes in the abundance of several taxa [68]. F. prausnitzii, E. rectale, Roseburia, Blautia, Anaerostipes and Prevotella were the main taxa showing positive association with the NU-AGE dietary intervention, while the abundance of Collinsella, Ruminococcus, Dorea, Blautia and Coprococcus exhibited a negative association [68]. Interestingly, after 1-year intervention, those subjects with deeper degrees of gut-microbiome change, reflected in higher levels of the MD microbiome index, also exhibited significant improvement in measures of inflammation (CRP levels), frailty (Fried score and gait speed) and cognition (verbal fluency, Babcok memory score, and constructional praxis score). Conversely, the subjects in the intervention arm who exhibited reduced variations of fecal microbiome composition experienced less pronounced variations in clinical endpoints [68]. Therefore, the NU-AGE diet intervention modulated gut microbiota in a way that reflected negative associations with inflammation and frailty.
A MD-based intervention with energy restriction resulted into significant modulation of fecal microbiome even after only 15 days in a smaller study conducted in 20 obese older women [69]. However, the observed changes were partly different than those of the NU-AGE RCT, with increased representation of Coprococcus, Oscillospira, Bacteroides, and Akkermansia, while Collinsella was confirmed as negatively associated with the dietary intervention [69]. In the PREDIMED-Plus Study, traditional MD intervention was compared with an energy-restricted MD associated with promotion of physical activity in a group of 343 overweight and obese Spanish subjects aged between 55 and 75 years old [70]. Interestingly, the changes observed in gut microbiota after 1-year follow-up were similar between the two groups, but more pronounced in the energy-restricted MD arm, and included increased representation of SCFA producers from the Lachnospiraceae family [70].
The effects of MD intervention approaches on intestinal microbiome have been recently studied also in age-related neurological conditions. Rusch et al. showed significant changes in fecal microbiome composition, including in particular an increase in the SCFA producer Roseburia, after a 5-week MD intervention in a small group of patients with Parkinson’s disease [71]. These changes were associated with improvements in gastrointestinal symptoms (constipation scores), but no neurologic endpoints were measured in the study.
In the field of dementia research, ketogenic diets have shown a potential of slowing down cognitive impairment by improving cerebral metabolism [72]. Recently, some authors have proposed to combine the Mediterranean and ketogenic diet approaches into a modified Mediterranean-ketogenic diet (MMKD) [72], which has shown the capacity of improving body composition and reducing levels of cerebrospinal fluid biomarkers of amyloid deposition (Aβ42) and tau protein [73, 74]. In a randomized cross-over trial conducted on 17 subjects with and without mild cognitive impairment, 6-week MMKD intervention induced increased representation of Akkermansia and Slackia and reduced Bifidobacterium and Lachnobacterium, with the family Lachnospiraceae showing significant correlation with cerebrospinal fluid levels of Aβ42 [75]. Interestingly, changes in symbiont intestinal fungal species, the so-called mycobiome, were also observed [76]. At the functional level, MMKD induced changes in bacterial SCFA synthesis, with significant increases in fecal butyrate and propionate levels [75]. In a recent study conducted in 20 subjects with mild cognitive impairment, MMKD induced lower representation of Alistipes, a bacterial species known for its capacity of producing gamma-amino-butyric acid (GABA), and higher representation of Akkermansia, a taxon with GABA-regulating functionality [77]. GABA imbalance is involved in the gut-brain axis and in the pathogenesis of dementia. Therefore, MD diet could influence important aspects of cognitive function by inducing subtle modifications in gut microbiome.
Anti-Aging effects of mediterranean diet mediated by intestinal microbiome: main underlying mechanisms
Microbial synthesis of short-chain fatty acids (SCFAs)
The depletion of bacterial taxa with capacity to synthetize SCFAs, and particularly F. prausnitzii, Roseburia, Butyrivibrio, and Succinivibrio, is a keynote characteristic of the gut microbiome of older individuals with frailty [78–80], sarcopenia [81, 82] and cognitive decline [83]. Frailty is associated with reduced fecal levels of butyrate [80], and an inverse correlation has been demonstrated between gut microbial synthesis of butyrate and appendicular lean mass in subjects at risk for sarcopenia [82]. Conversely, fit centenarians generally exhibit higher fecal levels of SCFAs than subjects between 60 and 70 years old [84].
In this scenario, the capacity of MD to stimulate the growth of SCFA-synthetizing bacteria and improve SCFA levels can be extremely important in an anti-aging perspective. However, the functional capacity of gut bacteria to effectively release SCFAs does not depend only on the fiber content of diet, but it also relies on complex cross-feeding interactions among bacteria and on the interaction between bacteria and host [85, 86]. For example, an adequate butyrate production by F. prausnitzii is possible only in presence of a good representation of bifidobacteria in the gut environment [87, 88].
SCFAs, particularly butyrate, exhibit pleiotropic functions for the host [89]. First, they promote gut mucosal integrity and tropism, representing one of the main sources of energy for colonocytes [89]. Second, they exhibit marked capacity to modulate the inflammatory response, which is pivotal for controlling inflammaging and the pathogenesis of several age-related diseases and conditions, including frailty [90, 91]. In a group of Chinese older patients with cognitive impairment related to diabetes, reduced fecal butyrate levels were associated with higher circulating levels of pro-inflammatory cytokines and worse cognitive performance [92]. In animal models of dementia, butyrate administration is in fact able to improve indices of neuroinflammation and cognitive performance via the gut-brain axis [93, 94].
In systemic circulation, butyrate also improves insulin resistance and has an overall pro-anabolic function, which is pivotal in counteracting type 2 diabetes and obesity [95]. At the skeletal muscle level, these actions result into increased protein synthesis and reduced muscle wasting [96], and this is the main reason why butyrate is considered a powerful anti-sarcopenic mediator in the context of the so-called gut-muscle axis [82, 97]. In fact, butyrate also exhibits an inhibitory capacity at the histone deacetylase level, resulting into increased muscle mass in mouse models of cachexia [98].
Reduction of intestinal mucosa permeability
Aging, even with a healthy active pattern, is associated with increased-intestinal permeability, witnessed by elevated levels of the serum biomarker zonulin [99]. According to a recent meta-analysis of case–control studies investigating biomarkers of frailty, serum zonulin levels are in average higher in frail than in healthy older subjects, reflecting a progressive loss of the barrier function of the intestinal mucosa [100]. This condition is associated with loss of skeletal muscle strength, sarcopenia, and functional autonomy in older subjects, either healthy or with chronic conditions such as chronic obstructive pulmonary disease (COPD) and dementia [101–103].
Increased intestinal mucosa permeability is associated with increased serum levels of bacterial toxins, including lipopolysaccharide (LPS), and increased presence of bacterial components into systemic circulation [104, 105]. These compounds provide activation of the innate immune response and antigenic stimulation of adaptive immunity, that ultimately result into persistent subclinical inflammation typical of aging with frailty, the so-called inflammaging [106]. Increased LPS toxinemia plays a pivotal role in the pathophysiology of age-related cognitive decline and Alzheimer’s disease, and is believed to represent one of the mainstays of the gut-brain axis dysregulation [107–109]. Age-related gut-microbiome alterations are deeply involved in this pathophysiological cascade, since germ-free mice show no development of chronic inflammation during aging and no increased LPS levels, while old mice with dysbiosis exhibit increased circulating levels of pro-inflammatory cytokines and macrophage dysfunction [110].
Higher adherence to MD is inversely associated with biomarkers of gastrointestinal mucosa permeability and with circulating LPS levels, in both adult subjects with chronic illnesses and older individuals [105, 111, 112]. These effects are emphasized with dietary interventions consisting in increased intake of (poly)phenol-rich foods, which are important components of MD [104, 113, 114]. In particular, the MaPLE randomized controlled trial showed that a (poly)phenol-rich food intervention in older subjects caused reduction in the serum levels of zonulin, associated with favorable changes in the gut microbiome including the increase of the relative abundance of F. prausnitzii [113]. Interestingly, these effects were less pronounced in those subjects with higher disruption of gut microbial community structure and with increased-intestinal permeability at baseline [104, 114]. Dysbiosis, in fact, does not only impair the integrity of the intestinal mucosa, but it can also limit the bioavailability of food bioactives contained in MD, especially (poly)phenolic compounds [115], making dietary interventions probably less effective in subjects with higher burden of frailty and associated gut-microbiome alterations.
The precise interactions among components of MD, gut microbiome, and host cells regulating intestinal mucosa permeability have not been elucidated yet. However, recent studies suggest that SCFAs and the bacterial taxa able to synthetize them from dietary fibers may play a central role [116, 117]. In the LIBRE RCT, for example, the baseline levels of SCFAs were independent predictors of the response to MD intervention in terms of intestinal permeability [117]. Therefore, the individual responses to MD diet may be consistently mediated by the preexisting composition and functionality of gut microbiome.
Biotransformation of food bioactives
(Poly)phenols or phenolic compounds are generally abundant in MD, where the intake of fruit and vegetables, wholegrain cereals, nuts, legumes, and extra virgin olive oil is recommended in large amounts [118]. The interaction between dietary (poly)phenols and the gut microbiome is able to generate several bioactive metabolites exhibiting anti-aging effects, especially at the skeletal muscle and central nervous system level [86, 119, 120]. However, the microbiome response to dietary interventions shows a consistent inter-individual variability in terms of production of bioactive compounds [121, 122]. In the case of some polyphenolic subclasses, including ellagitannins, flavanones, isoflavones, flavan-3-ols, prenylflavonoids, avenanthramides, resveratrol, and lignans, some microbiota-related metabotypes have been identified, so that the beneficial effects of these dietary components might be observed only in presence of a particular microbiome composition and functionality [121, 122].
For example, urolithin A, isourolithin A, and urolithin B are metabolites released by the gut microbiome after ingestion of ellagic acid and ellagitannins, polyphenols frequently found in walnuts, pomegranate, and strawberries. Not all the individuals are able to produce these metabolites, and subjects can be classified into the Uro-A or Uro-B metabotype according to their capability to not produce or produce, respectively, urolithin B or isourolithin A, in addition to urolithin A [123]. Conversely, subjects with the Uro-0 metabotype do not show production of (iso)urolithins A or B by the gut microbiome, even after a dietary challenge with foods with high ellagitannin content, and cannot benefit from the biologic functions of urolithins [123]. While isourolithin A and urolithin B have been associated to gut dysbiosis and the prevalence of the Uro-B metabotype increases with aging, urolithin A production and the Uro-A metabotype has been related to a healthier and younger profile [122]. The potential anti-aging effects of urolithin A include improvement of muscle strength and exercise endurance [124, 125], modulation of neuroinflammation and cell apoptosis with improvement in cognitive function [126–128], promotion of insulin sensitivity, modulation of lipid metabolism and inflammatory response [129]. Interestingly, in a recent RCT testing the effects of MD in obese subjects, MD was associated with an average increase in the urinary excretion of urolithins, even if the analyses did not consider metabotypes [47]. Similarly, urolithin urinary excretion was significantly associated with visceral adiposity reduction [130] and magnetic resonance-measured hippocampal occupancy score [131] in two distinct RCTs testing the effects of a long MD intervention.
Gut microbiota-derived metabotypes are less known for polyphenol subclasses other than ellagitannins. Hesperidin high-excretors and low-excretors have been identified after dietary intake of flavanones, a polyphenol subclass particularly represented in citrus [121, 122]. Hesperidin exhibits antioxidant, anti-inflammatory and pro-anabolic actions, promoting muscle protein synthesis [132, 133] and reducing amyloid deposition and neuroinflammation in animal models of Alzheimer’s disease [134, 135]. In an intervention study testing the effects of MD in subjects with type 2 diabetes, increased plasma levels of hesperidin and other flavanone derivatives were detected after 12 weeks, with significant reductions in inflammatory biomarkers [136]. Similarly, equol is a bioactive compound released after intestinal biotransformation of soy isoflavone daidzein, but it is produced only by a part of the population harboring a specific microbial profile. Equol exhibited neuroprotective actions against the onset of dementia in vitro [137–139], but it was associated in vivo with better cognitive performance only in presence of an equol-producer microbiome metabotype [140].
The role of dietary proteins and exercise in older age: a gut microbiome perspective
Nutritional guidelines and clinical recommendations against age-related physical frailty and sarcopenia generally emphasize the importance of increasing protein intake to overcome anabolic resistance [141, 142]. However, intervention studies have shown only mild improvements in muscle mass and strength after increases of daily protein intake up to 1.6 g/kg/day [143]. High-protein diets, especially rich of processed foods of animal origin, are also associated with deleterious consequences for the gut-microbiome composition and functionality in both human and animal experiments [144]. A recent systematic review of RCTs conducted in human beings has shown that higher meat intake is associated with marked reduction of Anaerostipes and Faecalibacterium, two of the main taxa producing SCFAs [145]. In this perspective, ad libitum consumption of animal proteins in the older age may produce conflicting effects on the pathophysiological mechanisms of physical frailty and sarcopenia, resulting in modest clinical benefits [146, 147].
The benefits of high-protein diets on frailty and inflammation, instead, may be more pronounced when the dietary intervention consists in consumption of proteins with high biologic value, such as whey protein [148, 149], or when dietary intervention is associated with regular exercise [150]. In a double-blind placebo-controlled cross-over study, Ford and colleagues demonstrated that a balanced high-protein diet, either in combination with probiotics/synbiotics or alone, was associated with generally favorable changes in the gut-microbiota composition of a group of healthy older women, although the representation of the SCFA producers Roseburia and Anaerostipes was reduced [151]. In healthy young subjects, the increase of lean red meat consumption under controlled conditions was not associated with detrimental consequences for the microbiome structure as well [152].
Protein consumption in MD is balanced and mainly includes vegetal proteins of high biologic value, such as those from legumes and lean meat, rather than proteins from processed red meats. Thus, increasing protein intake maintaining a Mediterranean dietary style may represent an optimal compromise from a gut-microbiota perspective [146, 147].
Furthermore, regular exercise seems to act as an enhancer of the beneficial effects of MD-style diets, both on the gut-microbiome composition and on clinical markers of aging. Multicomponent interventions consisting in regular exercise and tailored nutritional counseling have proven effective in reducing the burden of disability in physically frail older individuals [153, 154]. Exercise represents a powerful beneficial modulator of gut microbiome and can prevent dysbiosis in both adult and older age [155, 156]. However, no comprehensive investigation of the effects of exercise and its interaction with dietary patterns has been conducted in older individuals to date. Interestingly, in the PREDIMED-Plus Study the effects of energy-restricted MD dietary interventions on the gut microbiome of subjects aged 55–75 years old were more pronounced when diet was associated with regular exercise programs [70]. Therefore, multicomponent interventions combining MD or its variants with exercise programs should represent the best alternatives to counteract age-related frailty also from a gut microbiome perspective. Future studies should include also gut-microbiome parameters as endpoints of clinical interventions against frailty.
Conclusion and perspectives
A healthy diet can influence several pathophysiological aspects of aging through mediation of the intestinal microbiome, while Western-style diets may be associated with a tendency toward dysbiosis favoring the pathophysiological processes leading to frailty (Fig. 1). In this context, promoting MD in older individuals can represent an effective strategy to counteract the age-related drift of gut-microbiome composition and function toward dysbiosis and its detrimental consequences. In both adult subjects and older individuals, adherence to the MD pattern is associated with maintenance of a healthy microbiome able to modulate inflammation, anabolic resistance, oxidative stress, and neurodegeneration in a favorable way. Intervention studies have confirmed this interaction among diet, gut microbiome, and host (patho)physiology, but few studies have specifically targeted older individuals and outcomes of geriatric interest. In this regard, the results from the NU-AGE study, suggesting that older individuals respond to MD intervention in an individualized manner by mediation of the intestinal microbiome, are of paramount importance to understand the complex underlying mechanisms linking diet, aging, and its phenotype. Future studies should further investigate the role of MD and its variants in counteracting physical and cognitive decline in the older age, accounting also for the role of the microbiome from a multi-omics perspective.
Fig. 1.
Overview of the mechanisms linking dietary habits, and particularly Mediterranean diet, to gut-microbiome and pathophysiological aspects of aging (created with Biorender.com)
Author contributions
Andrea Ticinesi contributed to concept and design of the review, literature search and analysis, interpretation, and preparation of the manuscript. Antonio Nouvenne contributed to concept and design of the review, and manuscript draft. Nicoletta Cerundolo contributed to literature search and analysis. Alberto Parise contributed to literature search and analysis. Pedro Mena contributed to concept and design of the review and manuscript draft. Tiziana Meschi provided supervision and contributed to study concept and design.
Funding
Open access funding provided by Università degli Studi di Parma within the CRUI-CARE Agreement. Open access funding provided by Università degli Studi di Parma within the CRUI-CARE Agreement.
Data availability
Data sharing not applicable to this article as no datasets were generated or analyzed in the present work.
Declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose in relation with the present manuscript.
Ethical approval
Not applicable—this work does not report original research involving human subjects.
Human and animal rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study, formal consent is not required.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Berg G, Rybakova D, Fischer D, et al. Microbiome definition re-visited: old concepts and new challenges. Microbiome. 2020;8:103. doi: 10.1186/s40168-020-00875-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fassarella M, Blaak EE, Penders J, Nauta A, Smidt H, Zoetendal EG. Gut microbiome stability and resilience: elucidating the response to perturbations in order to modulate gut health. Gut. 2021;70:595–605. doi: 10.1136/gutjnl-2020-321747. [DOI] [PubMed] [Google Scholar]
- 3.Kurilshikov A, Medina-Gomez C, Bacigalupe R, et al. Large-scale association analyses identify host factors influencing human gut microbiome composition. Nat Genet. 2021;53:156–165. doi: 10.1038/s41588-020-00763-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhernakova A, Kurilshikov A, Bonder MJ, et al. Population-based metagenomics analysis reveals markers for gut microbiome composition and diversity. Science. 2016;352:565–569. doi: 10.1126/science.aad3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rotschild D, Weissbrod O, Barkan E, et al. Environment dominates over host genetics in shaping human gut microbiota. Nature. 2018;555:210–215. doi: 10.1038/nature25973. [DOI] [PubMed] [Google Scholar]
- 6.Ling Z, Liu X, Cheng Y, Yan X, Wu S. Gut microbiota and aging. Crit Rev Food Sci Nutr. 2022;62:3509–3534. doi: 10.1080/10408398.2020.1867054. [DOI] [PubMed] [Google Scholar]
- 7.Hohman LS, Osborne LC. A gut-centric view of aging: do intestinal epithelial cells contribute to age-associated microbiota changes, inflammaging, and immunosenescence? Aging Cell. 2022;21:e13700. doi: 10.1111/acel.13700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strasser B, Wolters M, Weyh C, Krüger K, Ticinesi A. The effects of lifestyle and diet on gut microbiota composition, inflammation, and muscle performance in our ageing society. Nutrients. 2021;13:2045. doi: 10.3390/nu13062045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. Hallmarks of aging: an expanding universe. Cell. 2023;186:243–268. doi: 10.1016/j.cell.2022.11.001. [DOI] [PubMed] [Google Scholar]
- 10.Haran JP, McCormick BA. Aging, frailty, and the microbiome-How dysbiosis influences human aging and disease. Gastroenterology. 2021;160:507–523. doi: 10.1053/j.gastro.2020.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strasser B, Ticinesi A. Intestinal microbiome in normal ageing, frailty and cognition decline. Curr Opin Clin Nutr Metab Care. 2023;26:8–16. doi: 10.1097/MCO.0000000000000878. [DOI] [PubMed] [Google Scholar]
- 12.Ticinesi A, Tana C, Nouvenne A, Prati B, Lauretani F, Meschi T. Gut microbiota, cognitive frailty and dementia in older individuals: a systematic review. Clin Interv Aging. 2018;13:1497–1511. doi: 10.2147/CIA.S139163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu C, Cheung WH, Li J, et al. Understanding the gut microbiota and sarcopenia: a systematic review. J Cachexia Sarcopenia Muscle. 2021;12:1393–1407. doi: 10.1002/jcsm.12784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gurung M, Li Z, You H, et al. Role of gut microbiota in type 2 diabetes pathophysiology. EBioMedicine. 2020;51:102590. doi: 10.1016/j.ebiom.2019.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nesci A, Carnuccio C, Ruggieri V, et al. Gut microbiota and cardiovascular disease: evidence on the metabolic and inflammatory background of a complex relationship. Int J Mol Sci. 2023;24:9087. doi: 10.3390/ijms24109087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghosh TS, Shanhan F, O’Toole PW. Toward an improved definition of a healthy microbiome for healthy aging. Nat Aging. 2022;2:1054–1059. doi: 10.1038/s43587-022-00306-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santoro A, Ostan R, Candela M, et al. Gut microbiota changes in the extreme decades of human life: a focus on centenarians. Cell Mol Life Sci. 2018;75:129–148. doi: 10.1007/s00018-017-2674-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao L, Zhang Y, Wang Y, et al. Gut microbiota diversity of hospitalized older adult patients with and without antibiotic-associated diarrhea. Aging Clin Exp Res. 2023;35:1541–1555. doi: 10.1007/s40520-023-02436-5. [DOI] [PubMed] [Google Scholar]
- 19.Milani C, Ticinesi A, Gerritsen J, et al. Gut microbiota composition and Clostridium difficile infection in hospitalized elderly individuals: a metagenomic study. Sci Rep. 2016;6:25945. doi: 10.1038/srep25945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vaiserman AM, Koliada AK, Marotta F. Gut microbiota: a player in aging and a target for anti-aging intervention. Ageing Res Rev. 2017;35:36–45. doi: 10.1016/j.arr.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 21.Prokopidis K, Giannos P, Kirwan R, et al. Impact of probiotics on muscle mass, muscle strength and lean mass: a systematic review and meta-analysis of randomized controlled trials. J Cachexia Sarcopenia Muscle. 2023;14:30–44. doi: 10.1002/jcsm.13132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krüger JF, Hillesheim E, Pereira ACSN, et al. Probiotics for dementia: a systematic review and meta-analysis of randomized controlled trials. Nutr Rev. 2021;79:160–170. doi: 10.1093/nutrit/nuaa037. [DOI] [PubMed] [Google Scholar]
- 23.de la Sánchez y Sánchez BB, Martínez Carrillo BE, Aguirre Garrido JF, et al. Emerging evidence on the use of probiotics and prebiotics to improve the gut microbiota of older adults with frailty syndrome: a narrative review. J Nutr Health Aging. 2022;26:926–935. doi: 10.1007/s12603-022-1842-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wolter M, Grant ET, Boudaud M, et al. Leveraging diet to engineer the gut microbiome. Nat Rev Gatroenterol Hepatol. 2021;18:885–902. doi: 10.1038/s41575-021-00512-7. [DOI] [PubMed] [Google Scholar]
- 25.Sofi F, Cesari F, Abbate R, Gensini GF, Casini A. Adherence to Mediterranean diet and health status: meta-analysis. BMJ. 2008;337:a1344. doi: 10.1136/bmj.a1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mazza E, Ferro Y, Pujia R, et al. Mediterranean diet in healthy aging. J Nutr Health Aging. 2021;25:1076–1083. doi: 10.1007/s12603-021-1675-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bach-Faig A, Berry EM, Lairon D, et al. Mediterranean diet pyramid today. Science and cultural updates. Public Health Nutr. 2011;14:2274–2284. doi: 10.1017/S1368980011002515. [DOI] [PubMed] [Google Scholar]
- 28.Zaragoza-Martí A, Cabañero-Martínez MJ, Hurtado-Sánchez JA, Laguna-Pérez A, Ferrer-Cascales R. Evaluation of Mediterranean diet adherence scores: a systematic review. BMJ Open. 2018;8:e019033. doi: 10.1136/bmjopen-2017-019033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gutiérrez-Díaz I, Fernández-Navarro T, Sánchez B, Margolles A, González S. Mediterranean diet and faecal microbiota: a transversal study. Food Funct. 2016;7:2347–2356. doi: 10.1039/c6fo00105j. [DOI] [PubMed] [Google Scholar]
- 30.De Filippis F, Pellegrini N, Vannini L, et al. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut. 2016;65:1812–1821. doi: 10.1136/gutjnl-2015-309957. [DOI] [PubMed] [Google Scholar]
- 31.Mitsou EK, Kakali A, Antonopoulou S, et al. Adherence to the Mediterranean diet is associated with the gut microbiota pattern and gastrointestinal characteristics in an adult population. Br J Nutr. 2017;117:1645–1655. doi: 10.1017/S0007114517001593. [DOI] [PubMed] [Google Scholar]
- 32.Gallé F, Valeriani F, Cattaruzza MS, et al. Mediterranean diet, physical activity and gut microbiome composition: a cross-sectional study among healthy young Italian adults. Nutrients. 2020;12:2164. doi: 10.3390/nu12072164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang DD, Nguyen LH, Li Y, et al. The gut microbiome modulates the protective association between a Mediterranean diet and cardiometabolic disease risk. Nat Med. 2021;27:333–343. doi: 10.1038/s41591-020-01223-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Turpin W, Dong M, Sasson G, et al. Mediterranean-like pattern associations with gut microbiome composition and subclinical gastrointestinal inflammation. Gastroenterology. 2022;163:685–698. doi: 10.1053/j.gastro.2022.05.037. [DOI] [PubMed] [Google Scholar]
- 35.Rosés C, Cuevas-Sierra A, Quintana S, et al. Gut microbiota bacterial species associated with Mediterranean diet-related food groups in a Northern Spanish population. Nutrients. 2021;13:636. doi: 10.3390/nu13020636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Almanza-Aguilera E, Urpi-Sarda M, Llorach R, et al. Microbial metabolites are associated with high adherence to a Mediterranean diet pattern using a 1H-NMR-based untargeted metabolomics approach. J Nutr Biochem. 2017;48:36–43. doi: 10.1016/j.jnutbio.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 37.Vitale M, Giacco R, Laiola M, et al. Acute and chronic improvement in postprandial glucose metabolism by a diet resembling the traditional Mediterranean dietary pattern: Can SCFAs play a role? Clin Nutr. 2021;40:428–437. doi: 10.1016/j.clnu.2020.05.025. [DOI] [PubMed] [Google Scholar]
- 38.Oliver A, Chase AB, Weihe C, et al. High-fiber, whole-food dietary intervention alters the human gut microbiome but not fecal short-chain fatty acids. mSystems. 2021;6:e00115–e121. doi: 10.1128/mSystems.00115-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tang WHW, Wang Z, Levison BS, et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368:1575–1784. doi: 10.1056/NEJMoa1109400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Manzano M, Talavera-Rodríguez A, Moreno E, et al. Relationship of diet to gut microbiota and inflammatory biomarkers in people with HIV. Nutrients. 2022;14:1221. doi: 10.3390/nu14061221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barber C, Mego M, Sabater C, et al. Differential effects of Western and Mediterranean-type diets on gut microbiota: a metagenomics and metabolomics approach. Nutrients. 2021;13:2638. doi: 10.3390/nu13082638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Godny L, Reshef L, Sharar Fischler T, et al. Increasing adherence to the Mediterranean diet and lifestyle is associated with reduced fecal calprotectin and intra-individual changes in microbial composition of healthy subjects. Gut Microbes. 2022;14:e2120749. doi: 10.1080/19490976.2022.2120749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rejeski JJ, Wilson FM, Nagpal R, Yadav H, Weinberg RB. The impact of a Mediterranean diet on the gut microbiome in healthy human subjects: a pilot study. Digestion. 2022;103:133–140. doi: 10.1159/000519445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dabke K, Hendrick G, Devkota S. The gut microbiome and metabolic syndrome. J Clin Invest. 2019;129:4050–4057. doi: 10.1172/JCI129194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luisi MLE, Lucarini L, Biffi B, et al. Effect of Mediterranean diet enriched in high quality extra virgin olive oil on oxidative stress, inflammation and gut microbiota in obese and normal weight adult subjects. Front Pharmacol. 2019;10:1366. doi: 10.3389/fphar.2019.01366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Haro C, Montes-Borrego M, Rangel-Zúñiga OA, et al. Two healthy diets modulate gut microbial community improving insulin sensitivity in a human obese population. J Clin Endocrinol Metab. 2016;101:233–242. doi: 10.1210/jc.2015-3351. [DOI] [PubMed] [Google Scholar]
- 47.Meslier V, Laiola M, Roager HM, et al. Mediterranean diet intervention in overweight and obese subjects lowers plasma cholesterol and causes changes in the gut microbiome and metabolome independently of energy intake. Gut. 2020;69:1258–1268. doi: 10.1136/gutjnl-2019-320438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pagliai G, Russo E, Niccolai E, et al. Influence of a 3-month low-calorie Mediterranean diet compared to the vegetarian diet on human gut microbiota and SCFA: the CARDIVEG Study. Eur J Nutr. 2020;59:2011–2024. doi: 10.1007/s00394-019-02050-0. [DOI] [PubMed] [Google Scholar]
- 49.Rinott E, Meir EY, Tsaban G, et al. The effects of the Green-Mediterranean diet on cardiometabolic health are linked to gut microbiome modifications: a randomized controlled trial. Genome Med. 2022;14:29. doi: 10.1186/s13073-022-01015-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Calabrese FM, Disciglio V, Franco I, et al. A low glycemic index Mediterranean diet combined with aerobic physical activity rearranges the gut microbiota signature in NAFLD patients. Nutrients. 2022;14:1773. doi: 10.3390/nu1409177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Camargo A, Vals-Delgado C, Alcala-Diaz JF, et al. A diet-dependent microbiota profile associated with incident type 2 diabetes: from the CORDIOPREV Study. Mol Nutr Food Res. 2020;64:2000730. doi: 10.1002/mnfr.202000730. [DOI] [PubMed] [Google Scholar]
- 52.Ismael S, Silvestre MP, Vasques M, et al. A pilot study on the metabolic impact of a Mediterranean diet in type 2 diabetes: is gut microbiota the key? Nutrients. 2021;13:1228. doi: 10.3390/nu13041228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ben-Yacov O, Godneva A, Rein M, et al. Gut microbiome modulates the effects of a personalised postprandial-targeting (PPT) diet on cardiometabolic markers: a diet intervention in pre-diabetes. Gut. 2023;72:1486–1496. doi: 10.1136/gutjnl-2022-329201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Haro C, Garcia-Carpintero S, Alcala-Diaz JF, et al. The gut microbial community in metabolic syndrome is modified by diet. J Nutr Biochem. 2016;27:27–31. doi: 10.1016/j.jnutbio.2015.08.011. [DOI] [PubMed] [Google Scholar]
- 55.Galié S, García-Gavilán J, Camacho-Barcía L, et al. Effects of the Mediterranean diet or nut consumption on gut microbiota composition and fecal metabolites and their relationship with cardiometabolic risk factors. Mol Nutr Food Res. 2021;65:2000982. doi: 10.1002/mnfr.202000982. [DOI] [PubMed] [Google Scholar]
- 56.Galié S, García-Gavilán J, Papandreou C, et al. Effects of Mediterranean diet on plasma metabolites and their relationship with insulin resistance and gut microbiota in a crossover randomized clinical trial. Clin Nutr. 2021;40:3798–3806. doi: 10.1016/j.clnu.2021.04.028. [DOI] [PubMed] [Google Scholar]
- 57.Lewis JD, Sandler RS, Brotherton C, et al. A randomized trial comparing the specific carbohydrate diet to a Mediterranean diet in adults with Chron’s disease. Gastroenterology. 2021;161:837–852. doi: 10.1053/j.gastro.2021.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Van Soest APM, Hermes GDA, Berendsen AM, et al. Associations between pro- and anti-inflammatory gastro-intestinal microbiota, diet, and cognitive functioning in Dutch healthy older adults: the NU-AGE study. Nutrients. 2020;12:3471. doi: 10.3390/nu12113471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gutiérrez-Díaz I, Fernández-Navarro T, Salazar N, et al. Adherence to a Mediterranean diet influences the fecal metabolic profile of microbial-derived phenolics in a Spanish cohort of middle-age and older people. J Agric Food Chem. 2017;65:586–595. doi: 10.1021/acs.jafc.6b04408. [DOI] [PubMed] [Google Scholar]
- 60.Ruiz-Saavedra S, Salazar N, Suárez A, de los Reyes-Gavilán CG, Gueimonde M, González S. Comparison of different dietary indices as predictors of inflammation, oxidative stress and intestinal microbiota in middle-aged and elderly subjects. Nutrients. 2020;12:3828. doi: 10.3390/nu12123828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Maldonado-Contreras A, Noel SE, Ward DV, Velez M, Mangano KM. Associations between diet, the gut microbiome, and short-chain fatty acid production among older Caribbean Latino adults. J Acad Nutr Diet. 2020;120:2047–2060. doi: 10.1016/j.jand.2020.04.018. [DOI] [PubMed] [Google Scholar]
- 62.Palmas V, Pisanu S, Madau V, et al. Gut microbiota markers and dietary habits associated with extreme longevity in healthy Sardinian centenarians. Nutrients. 2022;14:2436. doi: 10.3390/nu14122436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Biagi E, Franceschi C, Rampelli S, et al. Gut microbiota and extreme longevity. Curr Biol. 2016;26:1480–1485. doi: 10.1016/j.cub.2016.04.016. [DOI] [PubMed] [Google Scholar]
- 64.Wu L, Zeng T, Zinellu A, et al. A Cross-sectional study of compositional and functional profiles of gut microbiota in Sardinian centenarians. mSystems. 2019;4:e00325-19. doi: 10.1128/mSystems.00325-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Berendsen A, Santoro A, Pini E, et al. A parallel randomized trial on the effect of a heathful diet on inflammageing and its consequences in European elderly people: design of the NU-AGE dietary intervention study. Mech Ageing Dev. 2013;134:523–530. doi: 10.1016/j.mad.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 66.Santoro A, Pini E, Scurti M, et al. Comating inflammaging through a Mediterranean whole diet approach: the NU-AGE project’s conceptual framework and design. Mech Ageing Dev. 2014;136–137:3–13. doi: 10.1016/j.mad.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 67.Berendsen AAM, van de Rest O, Feskens EJM, et al. Changes in dietary intake and adherence to the NU-AGE diet following a one-year dietary intervention among European older adults-results of the NU-AGE randomized trial. Nutrients. 2018;10:1905. doi: 10.3390/nu10121905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ghosh TS, Rampelli S, Jeffery IB, et al. Mediterranean diet intervention alters the gut microbiome in older people reducing frailty and improving health status: the NU-AGE 1-year dietary intervention across five European countries. Gut. 2020;69:1218–1228. doi: 10.1136/gutjnl-2019-319654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cancello R, Turroni S, Rampelli S, et al. Effect of short-term dietary intervention and probiotic mix supplementation on the gut microbiota of elderly obese women. Nutrients. 2019;11:3011. doi: 10.3390/nu11123011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Muralidharan J, Moreno-Indias I, Bulló M, et al. Effects on gut microbiota of a 1-y lifestyle intervention with Mediterranean diet compared with energy-reduced Mediterranean diet and physical activity promotion: the PREDIMED-plus study. Am J Clin Nutr. 2021;114:1148–1158. doi: 10.1093/ajcn/nqab150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rusch C, Neke M, Tucciarone L, et al. Mediterranean diet adherence in people with Parkinson’s disease reduces constipation symptoms and changes fecal microbiota after a 5-week single-arm pilot study. Front Neurol. 2021;12:794640. doi: 10.3389/fneur.2021.794640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ellouze I, Sheffler J, Nagpal R, Arjmandi B. Dietary patterns and Alzheimer’s disease: an updated review linking nutrition to neuroscience. Nutrients. 2023;15:3204. doi: 10.3390/nu15143204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Neth BJ, Mintz A, Whitlow C, et al. Modified ketogenic diet is associated with improved cerebrospinal fluid biomarker profile, cerebral perfusion, and cerebral ketone body uptake in older adults at risk for Alzheimer’s disease: a pilot study. Neurobiol Aging. 2020;86:54–63. doi: 10.1016/j.neurobiolaging.2019.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brinkley TE, Leng I, Register TC, et al. Changes in adiposity and cerebrospinal fluid biomarkers following a modified mediterranean ketogenic diet in older adults at risk for Alzheimer’s disease. Front Neurosci. 2022;16:906539. doi: 10.3389/fnins.2022.906539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nagpal R, Neth RJ, Wang S, Craft S, Yadav H. Modified Mediterranean-ketogenic diet modulates gut microbiome and short-chain fatty acids in association with Alzheimer’s disease markers in subjects with mild cognitive impairment. EBioMedicine. 2019;47:529–542. doi: 10.1016/j.ebiom.2019.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nagpal R, Neth BJ, Wang S, Mishra SP, Craft S, Yadav H. Gut mycobiome and its interaction with diet, gut bacteria and Alzheimer’s disease markers in subjects with mild cognitive impairment: a pilot study. EBioMedicine. 2020;59:102950. doi: 10.1016/j.ebiom.2020.102950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dilmore AH, Martino C, Neth BJ, et al. Effect of a ketogenic and low-fat diet on the human metabolome, microbiome, and foodome in adults at risk for Alzheimer’s disease. Alzheimers Dement. 2023 doi: 10.1002/alz.13007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jackson MA, Jeffery IB, Beaumont M, et al. Signatures of early frailty in the gut microbiota. Genome Med. 2016;8:8. doi: 10.1186/s13073-016-0262-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xu Y, Wang Y, Li H, et al. Altered fecal microbiota composition in older adults with frailty. Front Cell Infect Microbiol. 2021;11:696186. doi: 10.3389/fcimb.2021.696186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Guo Y, Zhu F, Wang F, et al. Distinct serum and fecal metabolite profiles linking with gut microbiome in older adults with frailty. Front Med. 2022;9:827174. doi: 10.3389/fmed.2022.827174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ticinesi A, Mancabelli L, Tagliaferri S, et al. The gut-muscle axis in older subjects with low muscle mass and performance: a proof of concept study exploring fecal microbiota composition and function with shotgun metagenomics sequencing. Int J Mol Sci. 2020;21:8946. doi: 10.3390/ijms21238946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lv WQ, Lin X, Shen H, et al. Human gut microbiome impacts skeletal muscle mass via gut microbial synthesis of the short-chain fatty acid butyrate among healthy postmenopausal women. J Cachexia Sarcopenia Muscle. 2021;12:1860–1870. doi: 10.1002/jcsm.12788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cattaneo A, Cattane A, Galluzzi S, et al. Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol Aging. 2017;49:60–68. doi: 10.1016/j.neurobiolaging.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 84.Cai D, Zhao Z, Zhao L, et al. The age-accompanied and diet-associated remodeling of the phospholipid, amino acid, and SCFA metabolism of healthy centenarians from a Chinese longevous region: a window into exceptional longevity. Nutrients. 2022;14:4420. doi: 10.3390/nu14204420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang M, Li RW, Yang H, Tan Z, Liu F. Recent advances in developing butyrogenic foods to promote gut health. Crit Rev Food Sci Nutr. 2022 doi: 10.1080/10408398.2022.2142194. [DOI] [PubMed] [Google Scholar]
- 86.Ticinesi A, Guerra A, Nouvenne A, Meschi T, Maggi S. Disentangling the complexity of nutrition, frailty and gut microbial pathways during aging: a focus on hippuric acid. Nutrients. 2023;15:1138. doi: 10.3390/nu15051138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rios-Covian D, Gueimonde M, Duncan SH, Flint HJ, de los Reyes-Gavilan CG. Enhanced butyrate formation by cross-feeding between Faecalibacterium prausnitzii and Bifidobacterium adolescentis. FEMS Microbiol Lett. 2015;362:fnv176. doi: 10.1093/femsle/fnv176. [DOI] [PubMed] [Google Scholar]
- 88.Kim H, Yeong Y, Kang S, You HJ, Ji GE. Co-culture with bifidobacterium catenulatum improves the growth, gut colonization, and butyrate production of Faecalibacterium prausnitzii: in vitro and in vivo studies. Microorganisms. 2020;8:788. doi: 10.3390/microorganisms8050788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Blaak EE, Canfora EE, Theis S, et al. Short chain fatty acids in human gut and metabolic health. Benef Microbes. 2020;11:411–455. doi: 10.3920/BM2020.0057. [DOI] [PubMed] [Google Scholar]
- 90.Hu T, Wu Q, Yao Q, Jiang K, Yu J, Tang Q. Short-chain fatty acid metabolism and multiple effects on cardiovascular diseases. Ageing Res Rev. 2022;81:101706. doi: 10.1016/j.arr.2022.101706. [DOI] [PubMed] [Google Scholar]
- 91.Hildebrand CB, Lichatz R, Pich A, et al. Short-chain fatty acids improve inflamm-aging and acute lung injury in old mice. Am J Physiol Cell Mol Physiol. 2023;324:L480–L492. doi: 10.1152/ajplung.00296.2022. [DOI] [PubMed] [Google Scholar]
- 92.Du Y, Li X, An Y, Song Y, Lu Y. Association of gut microbiota with short-chain fatty acids and inflammatory cytokines in diabetic patients with cognitive impairment: a cross-sectional, non-controlled study. Front Nutr. 2022;9:930626. doi: 10.3389/fnut.2022.930626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Matt SM, Allen JM, Lawson MA, Mailing LJ, Woods JA, Johnson RW. Butyrate and dietary soluble fiber improve neuroinflammation associated with aging in mice. Front Immunol. 2018;9:1832. doi: 10.3389/fimmu.2018.01832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhou Y, Xie L, Schröder J, et al. Dietary fiber and microbiota metabolite receptors enhance cognition and alleviate disease in the 5xFAD mouse model of Alzheimer’s disease. J Neurosci. 2023;43:6460–6475. doi: 10.1523/JNEUROSCI.0724-23.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Canfora EE, Jocken JW, Blaak EE. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat Rev Endocrinol. 2015;11:577–591. doi: 10.1038/nrendo.2015.128. [DOI] [PubMed] [Google Scholar]
- 96.Prokopidis K, Chambers E, Ni Lochlainn M, Witard OC. Mechanisms linking the gut-muscle axis with muscle protein metabolism and anabolic resistance: implications for older adults at risk of sarcopenia. Front Physiol. 2021;12:770455. doi: 10.3389/fphys.2021.770455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ticinesi A, Lauretani F, Milani C, et al. Aging Gut microbiota at the cross-road between nutrition, physical frailty, and sarcopenia: is there a gut-muscle axis? Nutrients. 2017;9:1303. doi: 10.3390/nu9121303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Walsch ME, Bhattacharya A, Sataranatarajan K, et al. The histone deacetylase inhibitor butyrate improves metabolism and reduces muscle atrophy during aging. Aging Cell. 2015;14:957–970. doi: 10.1111/acel.12387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Qi Y, Goel R, Kim S, et al. Intestinal permeabolity biomarker zonulin is elevated in healthy aging. J Am Med Dir Assoc. 2017;18:810.e1–810.e4. doi: 10.1016/j.jamda.2017.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rashidah NH, Lim SM, Neoh CF, et al. Differential gut microbiota and intestinal permeability between frail and healthy older adults: a systematic review. Ageing Res Rev. 2022;82:101744. doi: 10.1016/j.arr.2022.101744. [DOI] [PubMed] [Google Scholar]
- 101.Li C, Li Y, Wang N, et al. Intestinal permeability associated with the loss of skeletal muscle strength in middle-aged and older adults in rural area of Beijing. China Healthcare. 2022;10:1100. doi: 10.3390/healthcare10061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Karim A, Muhammad T, Ustrana S, Qaisar R. Intestinal permeability marker zonulin as a predictor of sarcopenia in chronic obstructive pulmonary disease. Respir Med. 2021;189:106662. doi: 10.1016/j.rmed.2021.106662. [DOI] [PubMed] [Google Scholar]
- 103.Karim A, Iqbal MS, Muhammad T, Ahmad Qaisar F. Elevated plasma zonulin and CAF22 are correlated with sarcopenia and functional dependency at various stages of Alzheimer’s disease. Neurosci Res. 2022;184:47–53. doi: 10.1016/j.neures.2022.08.004. [DOI] [PubMed] [Google Scholar]
- 104.Gargari G, Taverniti V, Del Bò C, et al. Higher bacterial DNAemia can affest the impact of a polyphenol-rich dietary pattern on biomarkers of intestinal permeability and cardiovascular risk in older subjects. Eur J Nutr. 2022;61:1209–1220. doi: 10.1007/s00394-021-02680-3. [DOI] [PubMed] [Google Scholar]
- 105.Pastori D, Carnevale R, Nocella C, et al. Gut-derived serum lipopolysaccharide is associated with enhanced risk of major adverse cardiovascular events in atrial fibrillation: effect of adherence to Mediterranean diet. J Am Heart Assoc. 2017;6:e005784. doi: 10.1161/JAHA.117.005784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Di Giosia P, Stamerra CA, Giorgini P, Jamialahamdi T, Butler AE, Sahebkar A. The role of nutrition in inflammaging. Ageing Res Rev. 2022;77:101596. doi: 10.1016/j.arr.2022.101596. [DOI] [PubMed] [Google Scholar]
- 107.Qu L, Li Y, Liu F, et al. Microbiota-gut-brain axis dysregulation in Alzheimer’s disease: multi-pathway effects and therapeutical potential. Aging Dis. 2023 doi: 10.14336/AD.2023.0823-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Inaba T, Yamashiro K, Kurita N, et al. Microbial lipopolysaccharide-induced inflammation contributes to cognitive impairment and white matter lesion progression in diet-induced obese mice with chronic cerebral hypoperfusion. CNS Neurosci Ther. 2023;29:200–212. doi: 10.1111/cns.14301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kalyan M, Tousif AH, Sonali S, et al. Role of endogenous lipopolysaccharides in neurological disorders. Cells. 2022;11:4308. doi: 10.3390/cells11244038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Thevaranjan N, Puchta A, Schulz C, et al. Age-associated microbial dysbiosis promotes intestinal permeability, systemic inflammation, and macrophage dysfunction. Cell Host Microbe. 2017;21:455–466. doi: 10.1016/j.chom.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Baratta F, Pastori D, Bartimoccia S, et al. Poor adherence to Mediterranean diet and serum polysaccharide are associated with oxidative stress in patients with non-alcoholic fatty liver disease. Nutrients. 2020;12:1732. doi: 10.3390/nu12061732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.André P, Pais de Barros JP, Merle BMJ, et al. Mediterranean diet and prudent diet are both associated with low circulating esterified 3-hydroxy fatty acids, a proxy of LPS burden, among older adults. Am J Clin Nutr. 2021;114:1080–1091. doi: 10.1093/ajcn/nqab126. [DOI] [PubMed] [Google Scholar]
- 113.Del Bò C, Bernardi S, Cherubini A, et al. A polyphenol-rich dietary pattern improves intestinal permeability, evaluated as serum zonulin levels, in older subjects: the MaPLE randomised controlled trial. Clin Nutr. 2021;40:3006–3018. doi: 10.1016/j.clnu.2020.12.014. [DOI] [PubMed] [Google Scholar]
- 114.Peron G, Gargari G, Meroño T, et al. Crosstalk among intestinal barrier, gut microbiota and serum metabolome after a polyphenol-rich diet in older subjects with “leaky gut”: the MaPLE trial. Clin Nutr. 2021;40:5288–5297. doi: 10.1016/j.clnu.2021.08.027. [DOI] [PubMed] [Google Scholar]
- 115.Hidalgo-Liberona N, González-Domínguez R, Vegas E, et al. Increased intestinal permeability in older subjects impacts the beneficial effects of dietary polyphenols by modulating their bioavailability. J Agric Food Chem. 2020;68:12476–12484. doi: 10.1021/acs.jafc.0c04976. [DOI] [PubMed] [Google Scholar]
- 116.Mörkl S, Lackner S, Meinitzer A, et al. Gut microbiota, dietary intakes and intestinal permeability reflected by serum zonulin in women. Eur J Nutr. 2018;57:2985–2997. doi: 10.1007/s00394-018-1784-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Seethaler B, Nguyen NK, Basrai M, et al. Short-chain fatty acids are key mediators of the favorable effects of the Mediterranean diet on intestinal barrier integrity: data from the randomized controlled LIBRE trial. Am J Clin Nutr. 2021;116:928–942. doi: 10.1093/ajcn/nqac175. [DOI] [PubMed] [Google Scholar]
- 118.Castro-Barquero S, Lamuela-Raventós RM, Doménech M, Estruch M. Relationship between Mediterranean dietary polyphenol intake and obesity. Nutrients. 2018;10:1523. doi: 10.3390/nu10101523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ticinesi A, Mancabelli L, Carnevali L, et al. Interaction between diet and microbiota in the pathophysiology of Alzheimer’s disease: focus on polyphenols and dietary fibers. J Alzheimers Dis. 2022;86:961–982. doi: 10.3233/JAD-215493. [DOI] [PubMed] [Google Scholar]
- 120.Ticinesi A, Nouvenne A, Cerundolo N, Parise A, Meschi T. Accounting gut microbiota as the mediator of dietary (poly)phenols on skeletal muscle in aging. Nutrients. 2023;15:2367. doi: 10.3390/nu15102367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Narduzzi L, Agulló V, Favari C, et al. (Poly)phenolic compounds and gut microbiome: new opportunities for personalized nutrition. Microbiome Res Rep. 2022;1:16. doi: 10.20517/mrr.2022.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cortés-Martín A, Selma MV, Tomás-Barberán FA, González-Sarrías A, Espín JC. Where to look into the puzzle of polyphenols and health? The postbiotics and gut microbiota associated with human metabotypes. Mol Nutr Food Res. 2020;64:e1900952. doi: 10.1002/mnfr.201900952. [DOI] [PubMed] [Google Scholar]
- 123.García-Villalba R, Giménez-Bastida JA, Cortés-Martín A, et al. Urolithins : a comprehensive update on their metabolism, bioactivity, and associated gut microbiota. Mol Nutr Food Res. 2022;66:e2101019. doi: 10.1002/mnfr.202101019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Liu S, D’Amico D, Shankland E, et al. Effect of urolithin A supplementation on muscle endurance and mitochondrial health in older adults: a randomized clinical trial. JAMA Netw Open. 2022;5:e2144279. doi: 10.1001/jamanetworkopen.2021.44279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Singh A, D’Amico D, Andreux PA, et al. Urolithin A improves muscle strength, exercise performance, and biomarkers of mitochondrial health in a randomized trial in moddle-aged adults. Cell Rep Med. 2022;3:100633. doi: 10.1016/j.xcrm.2022.100633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kujawska M, Jourdes M, Kurpik M, et al. Neuroprotective effects of pomegranate juice against Parkinson’s disease and presence of ellagitannins-derived metabolite-urolithin A-in the brain. Int J Mol Sci. 2019;21:202. doi: 10.3390/ijms21010202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.DaSilva NA, Nahar PP, Ma H, et al. Pomegranate ellagitannin-gut microbial-derived metabolites, urolithins, inhibit neuroinflammation in vitro. Nutr Neurosci. 2019;22:185–195. doi: 10.1080/1028415X.2017.1360558. [DOI] [PubMed] [Google Scholar]
- 128.Gong Z, Huang J, Xu B, et al. Urolithin A attenuates memory impairment and neuroinflammation in APP/PS1 mice. J Neuroinflamm. 2019;16:62. doi: 10.1186/s12974-019-1450-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.D’Amico DD, Andreux PA, Valdés P, Singh A, Rinsch C, Auwerx J. Impact of the natural compound urolithin A on health, disease, and aging. Trends Mol Med. 2021;27:687–699. doi: 10.1016/j.molmed.2021.04.009. [DOI] [PubMed] [Google Scholar]
- 130.Zelicha H, Kloting N, Kaplan A, et al. The effect of high-polyphenol Mediterranean diet on visceral adiposity: the DIRECT PLUS randomized controlled trial. BMC Med. 2022;20:327. doi: 10.1186/s12916-022-02525-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kaplan A, Zelicha H, Meir AY, et al. The effect of a high-polyphenol Mediterranean diet (Green-MED) combined with physical activity on age-related brain atrophy: the Dietary Intervention Randomized controlled trial polyphenols unprocessed study (DIRECT PLUS) Am J Clin Nutr. 2022;115:1270–1281. doi: 10.1093/ajcn/nqac001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Imperatrice M, Cuijpers I, Troost FJ, Sthijns MMJPE. Hesperidin function as an ergogenic aid by increasing endothelial function and decreasing exercise-induced oxidative stress and inflammation, thereby contributing to improved exercise performance. Nutrients. 2022;14:2955. doi: 10.3390/nu14142955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Martínez Noguera FJ, Alcaraz PE, Vivas JC, Chung LH, Cascales EM, Pagán CM. 8 weeks of 2S-hesperidin supplementation improves muscle mass and reduces fat in amateur competitive cyclists: randomized controlled trial. Food Funct. 2021;12:3872–3883. doi: 10.1039/d0fo03456h. [DOI] [PubMed] [Google Scholar]
- 134.Thenmozhi AJ, Raja TRW, Janakiraman U, Manivasagam T. Neuroprotective effects of hesperidin on aluminium chloride induced Alzheimer’s disease in Wistar rats. Neurochem Res. 2015;40:767–776. doi: 10.1007/s11064-015-1525-1. [DOI] [PubMed] [Google Scholar]
- 135.Javed H, Vaibhav K, Ahmed ME, et al. Effect of hesperidin on neurobehavioral, neuroinflammation, oxidative stress and lipid alteration in intracerebroventricular streptozotocin induced cognitive impairment in mice. J Neurol Sci. 2015;348:51–59. doi: 10.1016/j.jns.2014.10.044. [DOI] [PubMed] [Google Scholar]
- 136.Al-Aubaidy HA, Dayan A, Deseo MA, et al. Twelve-week mediterranean diet intervention increases citrus bioflavonoid levels and reduces inflammation in people with type 2 diabetes mellitus. Nutrients. 2021;13:1133. doi: 10.3390/nu13041133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mayo B, Vázquez L, Flórez AB. Equol: a bacterial metabolite from the daidzein isoflavone and its presumed beneficial health effects. Nutrients. 2019;11:2231. doi: 10.3390/nu11092231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sekikawa A, Wharton W, Butts B, et al. Potential protective mechanisms of S-equol, a metabolite of soy isoflavone by the gut microbiome, on cognitive decline and dementia. Int J Mol Sci. 2022;23:11921. doi: 10.3390/ijms231911921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Johnson SL, Kirk RD, DaSilva NA, Ma H, Seeram NP, Bertin MJ. Polyphenol microbial metabolites exhibit gut and blood-brain barrier permeability and protect murine microglia against LPS-induced inflammation. Metabolites. 2019;9:78. doi: 10.3390/metabo9040078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Igase M, Igase K, Tabara Y, Ohyagi Y, Kohara K. Cross-sectional study of equol producer status and cognitive impairment in older adults. Geriatr Gerontol Int. 2017;17:2103–2108. doi: 10.1111/ggi.13029. [DOI] [PubMed] [Google Scholar]
- 141.Dent E, Morley JE, Cruz-Jentoft AJ, et al. International clinical practice guidelines for sarcopenia (ICFSR): screening, diagnosis and management. J Nutr Health Aging. 2018;22:1148–1161. doi: 10.1007/s12603-018-1139-9. [DOI] [PubMed] [Google Scholar]
- 142.Chen LK, Arai H, Assantachai P, et al. Roles of nutrition in muscle health of community-dwelling older adults: evidence-based expert consensus from Asian working group for sarcopenia. J Cachexia Sarcopenia Muscle. 2022;13:165–1672. doi: 10.1002/jcsm.12981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Nunes EA, Colenso-Semple L, McKellar SR, et al. Systematic review and meta-analysis of protein intake to support muscle mass and function in healthy adults. J Cachexia Sarcopenia Muscle. 2022;13:795–810. doi: 10.1002/jcsm.12922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mervant L, Tremblay-Franco M, Olier M, et al. Urinary metabolome analysis reveals potential microbiota alteration and electrophilic burden induced by high red meat diet: results from the French NutriNet-Santé cohort and an in vivo intervention study in rats. Mol Nutr Food Res. 2023;67:e2200432. doi: 10.1002/mnfr.202200432. [DOI] [PubMed] [Google Scholar]
- 145.Wang Y, Uffelman CN, Bergia RE, et al. Meat consumption and gut microbiota: a scoping review of literature and systematic review of randomized controlled trials in adults. Adv Nutr. 2023;14:215–237. doi: 10.1016/j.advnut.2022.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Prokopidis K, Cervo MM, Grandham A, Scott D. Impact of protein intake in older adults with sarcopenia and obesity: a gut microbiota perspective. Nutrients. 2020;12:2285. doi: 10.3390/nu12082285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ni Lochlainn M, Bowyer RCE, Steves CJ. Dietary protein and muscle in aging people: the potential role of gut microbiome. Nutrients. 2018;10:929. doi: 10.3390/nu10070929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lin CC, Shih MH, Chen CD, Yeh SL. Effects of adequate dietary protein with whey protein, leucine, and vitamin D supplementation on sarcopenia in older adults: an open-label, parallel-group study. Clin Nutr. 2021;40:1323–1329. doi: 10.1016/j.clnu.2020.08.017. [DOI] [PubMed] [Google Scholar]
- 149.Ticinesi A, Meschi T, Lauretani F, et al. Nutrition and inflammation in older individuals: focus on vitamin D, n-3 polyunsaturated fatty acids and whey proteins. Nutrients. 2016;8:186. doi: 10.3390/nu8040186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Negm AM, Lee J, Hamidian R, Jones CA, Khadaroo RG. Management of sarcopenia: a network meta-analysis of randomized controlled trials. J Am Med Dir Assoc. 2022;23:707–714. doi: 10.1016/j.jamda.2022.01.057. [DOI] [PubMed] [Google Scholar]
- 151.Ford AL, Nagulesapillai V, Piano A, et al. Microbiota stability and gastrointestinal tolerance in response to a high-protein diet with and without a prebiotic, probiotic, and synbiotic: a randomized, double-blind, placebo-controlled trial in older women. J Acad Nutr Diet. 2020;120:500–516.e10. doi: 10.1016/j.jand.2019.12.009. [DOI] [PubMed] [Google Scholar]
- 152.Wang Y, Lindemann SR, Cross TWL, et al. Effects of adding lean read meat to a U.S.-style healthy vegetarian dietary pattern on gut microbiota and cardiovascular risk factors in young adults: a crossover randomized controlled trial. J Nutr. 2023;153:1439–1452. doi: 10.1016/j.tjnut.2023.03.013. [DOI] [PubMed] [Google Scholar]
- 153.Pahor M, Guralnik JM, Anton SD, et al. Impact and lessons from the lifestyle interventions and independence for elders (LIFE) clinical trials of physical activity to prevent mobility disability. J Am Geriatr Soc. 2020;68:872–881. doi: 10.1111/jgs.16365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bernabei R, Landi F, Calvani R, et al. Multicomponent intervention to prevent mobility disability in frail older adults: andomized controlled trial (SPRINTT project) BMJ. 2022;377:e068788. doi: 10.1136/bmj-2021-068788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ticinesi A, Lauretani F, Tana C, Nouvenne A, Ridolo E, Meschi T. Exercise and immune system as modulators of intestinal microbiome: implications for the gut-muscle axis hypothesis. Exerc Immunol Rev. 2019;25:84–95. [PubMed] [Google Scholar]
- 156.Mailing LJ, Allen JM, Buford TW, Fields CJ, Woods JA. Exercise and the gut microbiome: a review of the evidence, potential mechanisms, and implications for human health. Exerc Sports Sci Rev. 2019;47:75–85. doi: 10.1249/JES.0000000000000183. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analyzed in the present work.