Abstract
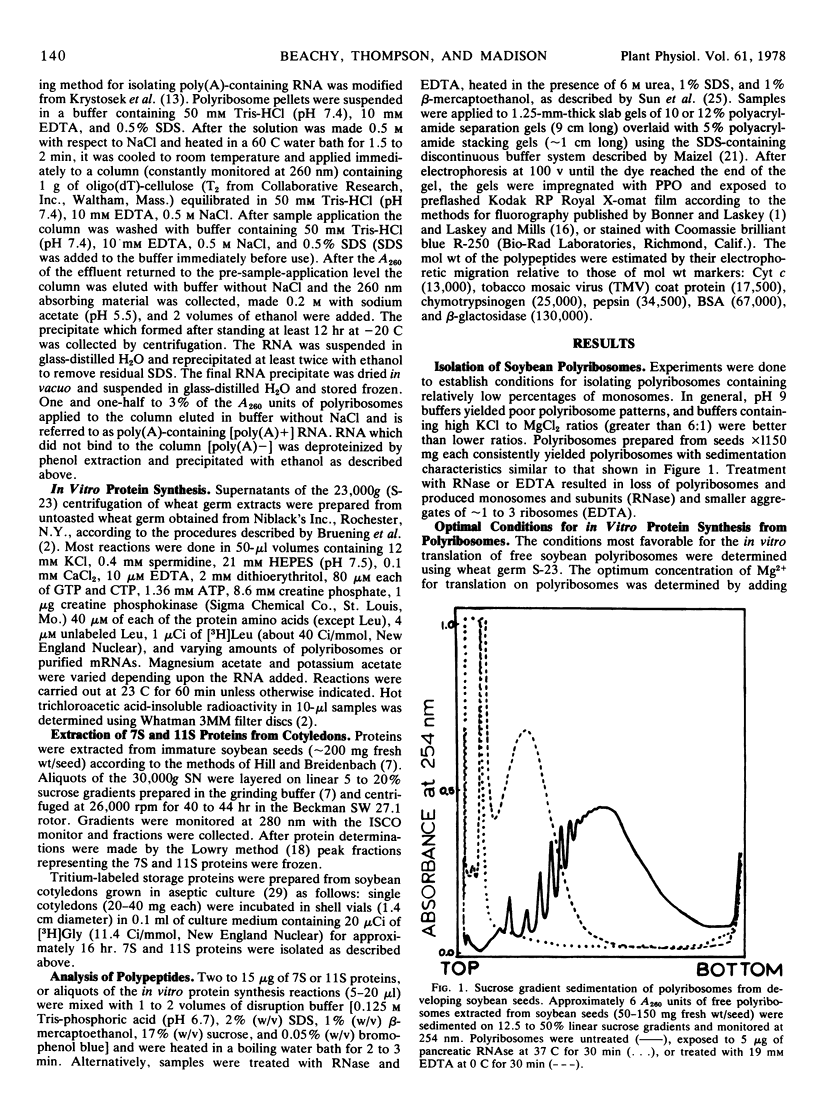
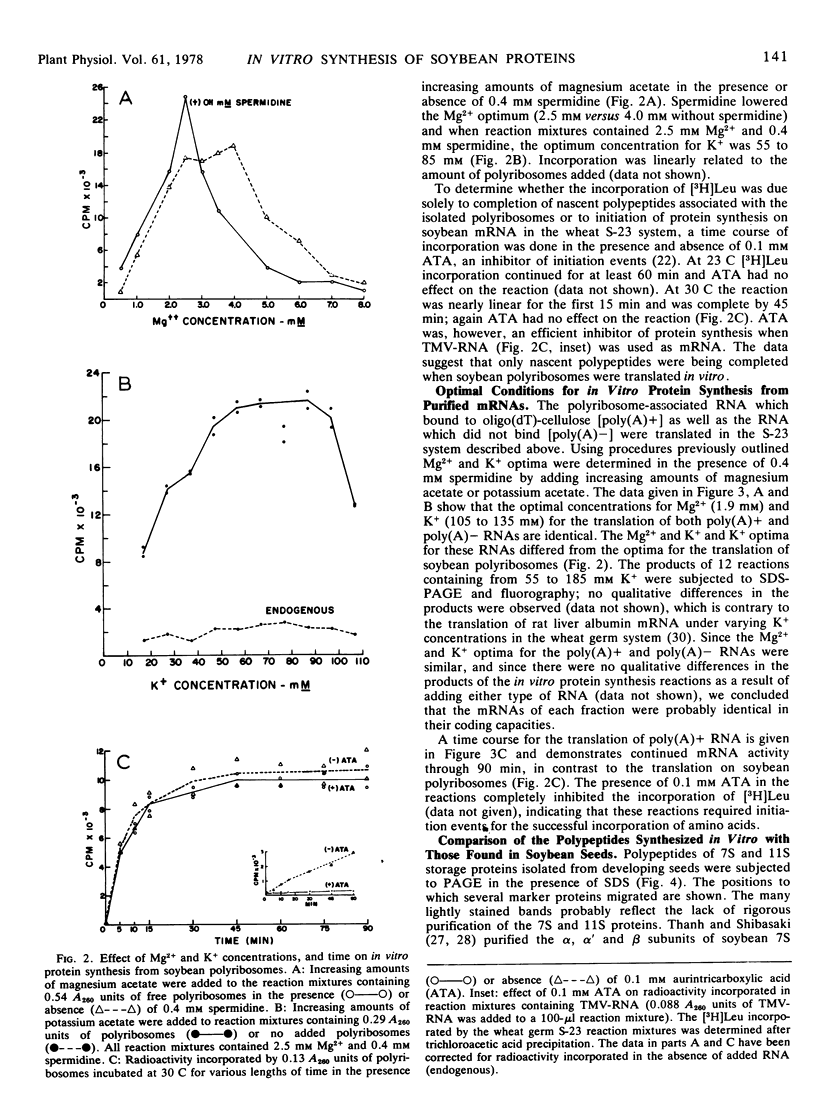
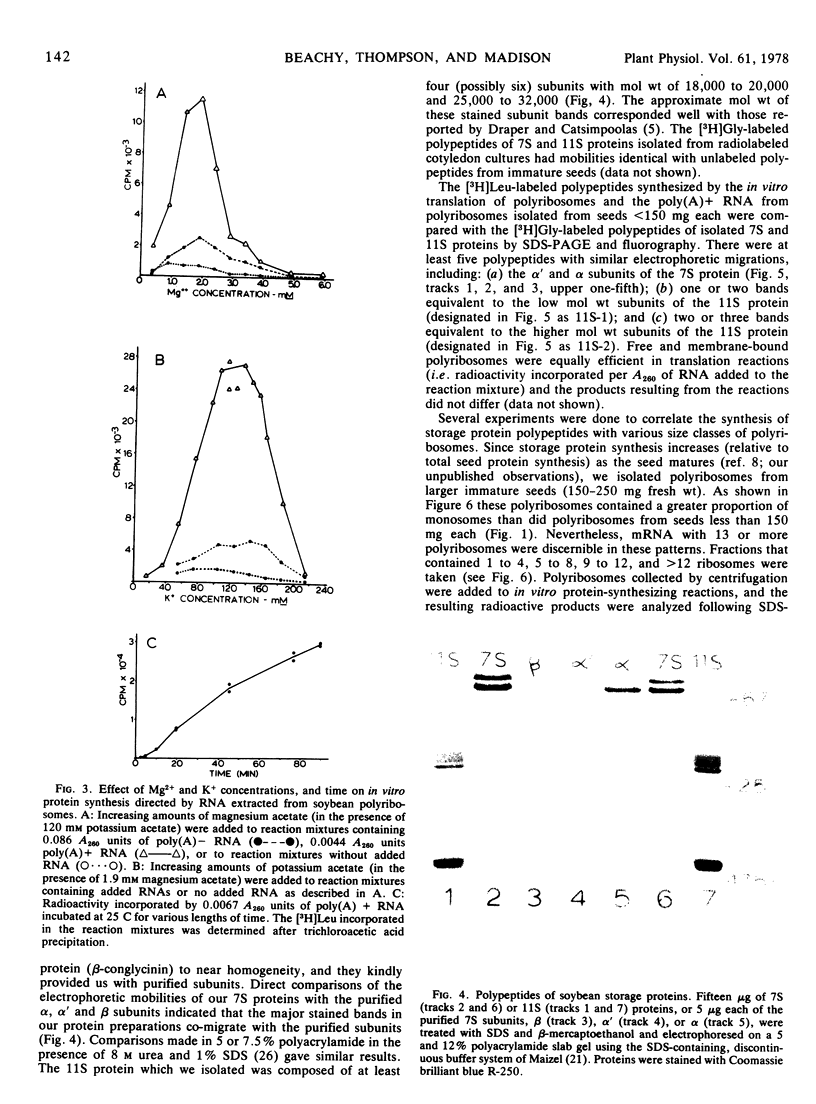
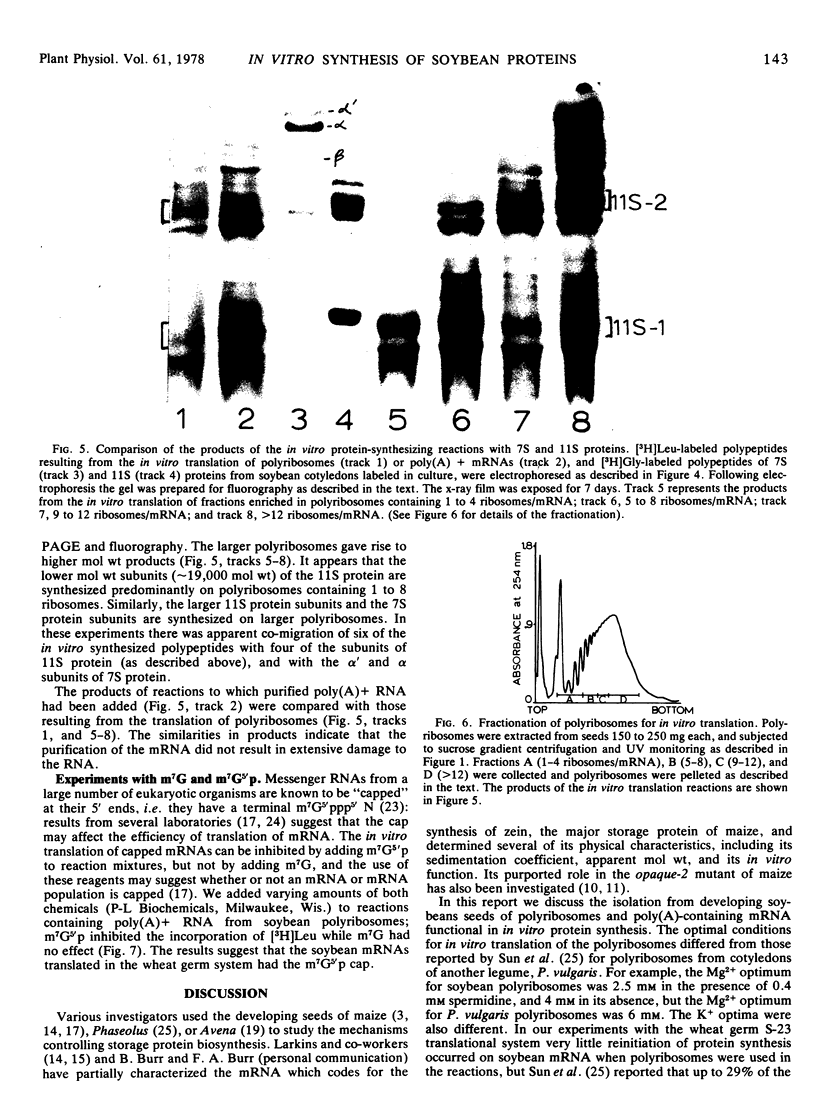
Polyribosome preparations containing low proportions of monosomes to polyribosomes have been isolated from developing seeds of Glycine max L. Merrill using a high pH-high KCl buffer. The polyribosomes were functional in in vitro protein synthesis reactions using wheat germ 23,000g supernatant preparations. Results of experiments using aurintricarboxylic acid indicated that most or all of the amino acid incorporation in vitro resulted from the completion of nascent polypeptides associated with the isolated polyribosmes. RNA purified from polyribosome preparations by affinity chromatography on oligo(dT)-cellulose was also active in vitro, and had different Mg and K requirements for translation than did the polyribosomes. Translation of oligo(dT)-cellulose-purified mRNA was inhibited by the addition of 7-methylguanosine 5′-phosphate, suggesting that soybean mRNAs are “capped” at their 5′ ends. Some, but not all, of the products of these reactions were identical in electrophoretic mobility to radioactive polypeptides of storage proteins produced in soybean cotyledons grown in culture.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Bruening G., Beachy R. N., Scalla R., Zaitlin M. In vitro and in vivo translation of the ribonucleic acids of a cowpea strain of tobacco mosaic virus. Virology. 1976 Jun;71(2):498–517. doi: 10.1016/0042-6822(76)90377-9. [DOI] [PubMed] [Google Scholar]
- Burr B., Burr F. A. Zein synthesis in maize endosperm by polyribosomes attached to protein bodies. Proc Natl Acad Sci U S A. 1976 Feb;73(2):515–519. doi: 10.1073/pnas.73.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray R. E., Cashmore A. R. RNA synthesis in plant leaf tissue: the characterization of messenger RNA species lacking and containing polyadenylic acid. J Mol Biol. 1976 Dec 15;108(3):595–608. doi: 10.1016/s0022-2836(76)80139-8. [DOI] [PubMed] [Google Scholar]
- Hill J. E., Breidenbach R. W. Proteins of Soybean Seeds: II. Accumulation of the Major Protein Components during Seed Development and Maturation. Plant Physiol. 1974 May;53(5):747–751. doi: 10.1104/pp.53.5.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill J. E., Breidenbach R. W. Proteins of soybean seeds: I. Isolation and characterization of the major components. Plant Physiol. 1974 May;53(5):742–746. doi: 10.1104/pp.53.5.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson A. O., Larkins B. A. Influence of Ionic Strength, pH, and Chelation of Divalent Metals on Isolation of Polyribosomes from Tobacco Leaves. Plant Physiol. 1976 Jan;57(1):5–10. doi: 10.1104/pp.57.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. A., Larkins B. A., Tsai C. Y. Storage Protein Synthesis in Maize: II. Reduced Synthesis of a Major Zein Component by the Opaque-2 Mutant of Maize. Plant Physiol. 1977 Apr;59(4):525–529. doi: 10.1104/pp.59.4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. A., Larkins B. A., Tsai C. Y. Storage Protein Synthesis in Maize: III. Developmental Changes in Membrane-bound Polyribosome Composition and in Vitro Protein Synthesis of Normal and Opaque-2 Maize. Plant Physiol. 1977 Apr;59(4):733–737. doi: 10.1104/pp.59.4.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Larkins B. A., Bracker C. E., Tsai C. Y. Storage Protein Synthesis in Maize: Isolation of Zein-synthesizing Polyribosomes. Plant Physiol. 1976 May;57(5):740–745. doi: 10.1104/pp.57.5.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkins B. A., Dalby A. In vitro synthesis of zein-like protein by maize polyribosomes. Biochem Biophys Res Commun. 1975 Oct 6;66(3):1048–1054. doi: 10.1016/0006-291x(75)90746-9. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Lodish H. F., Rose J. K. Relative importance of 7-methylguanosine in ribosome binding and translation of vesicular stomatitis virus mRNA in wheat germ and reticulocyte cell-free systems. J Biol Chem. 1977 Feb 25;252(4):1181–1188. [PubMed] [Google Scholar]
- Luthe D. S., Peterson D. M. Cell-free Synthesis of Globulin by Developing Oat (Avena sativa L.) Seeds. Plant Physiol. 1977 May;59(5):836–841. doi: 10.1104/pp.59.5.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus A., Bewley J. D., Weeks D. P. Aurintricarboxylic acid and initiation factors of wheat embryo. Science. 1970 Mar 27;167(3926):1735–1736. doi: 10.1126/science.167.3926.1735. [DOI] [PubMed] [Google Scholar]
- Shih D. S., Dasgupta R., Kaesberg P. 7-Methyl-guanosine and efficiency of RNA translation. J Virol. 1976 Aug;19(2):637–642. doi: 10.1128/jvi.19.2.637-642.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swank R. T., Munkres K. D. Molecular weight analysis of oligopeptides by electrophoresis in polyacrylamide gel with sodium dodecyl sulfate. Anal Biochem. 1971 Feb;39(2):462–477. doi: 10.1016/0003-2697(71)90436-2. [DOI] [PubMed] [Google Scholar]
- Thanh V. H., Shibasaki K. Beta-conglycinin from soybean proteins. Isolation and immunological and physicochemical properties of the monomeric forms. Biochim Biophys Acta. 1977 Feb 22;490(2):370–384. doi: 10.1016/0005-2795(77)90012-5. [DOI] [PubMed] [Google Scholar]
- Thanh V. H., Shibasaki K. Heterogeneity of beta-conglycinin. Biochim Biophys Acta. 1976 Aug 9;439(2):326–338. doi: 10.1016/0005-2795(76)90068-4. [DOI] [PubMed] [Google Scholar]
- Tse T. P., Taylor J. M. Translation of albumin messenger RNA in a cell-free protein-synthesizing system derived from wheat germ. J Biol Chem. 1977 Feb 25;252(4):1272–1278. [PubMed] [Google Scholar]