Abstract
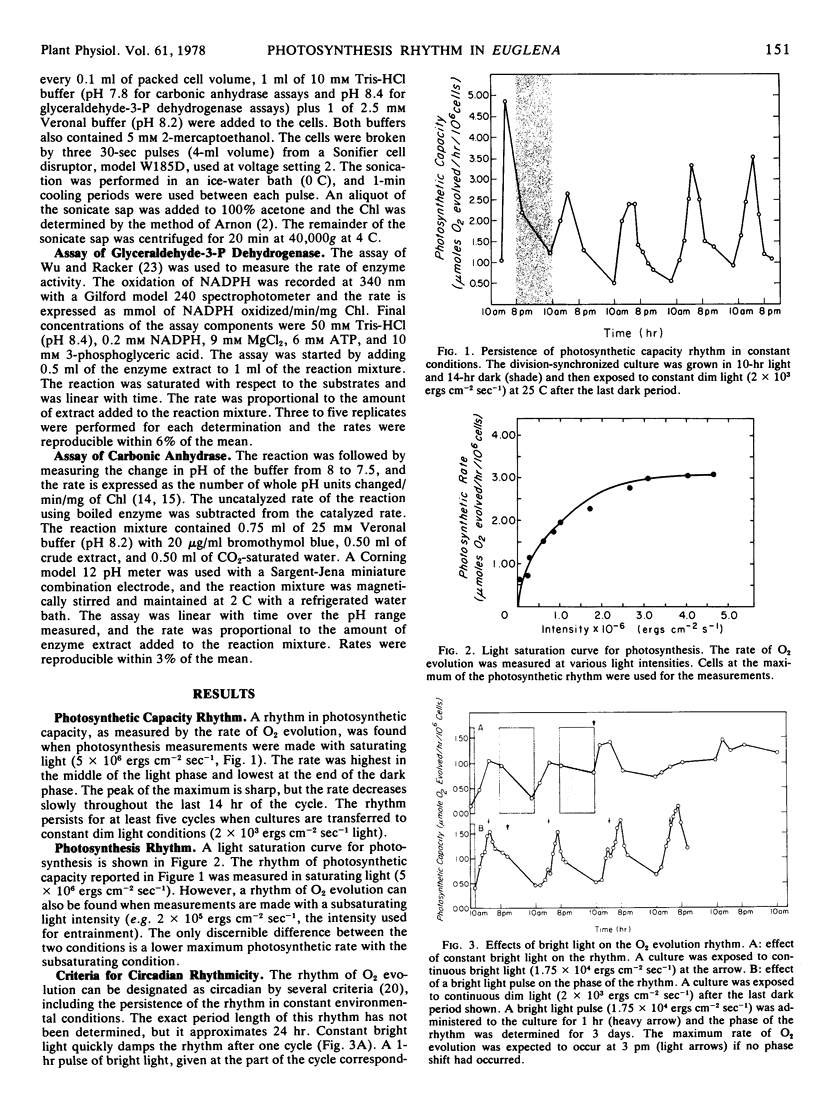
A circadian rhythm of O2 evolution has been found in Euglena gracilis, Klebs strain Z. The rhythm persists for at least 5 days in constant dim light and temperature, but damps out in constant bright light. The phase of this rhythm can be shifted by a pulse of bright light and the period length is not changed over a 10 C span of growth temperature.
The O2 evolution rhythm is found in both logarithmic and stationary phase cultures, but CO2 uptake is clearly rhythmic only in stationary phase cultures.
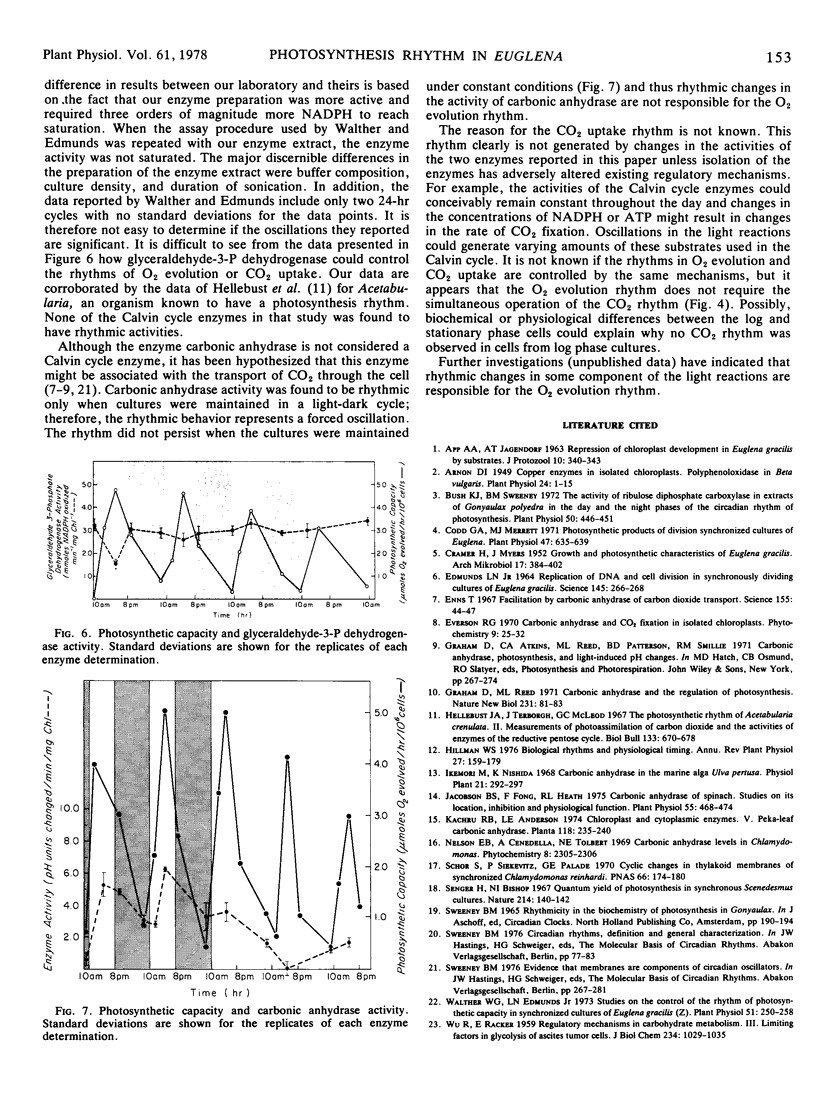
The activity of glyceraldehyde-3-phosphate dehydrogenase was not rhythmic as previously reported (Walther and Edmunds [1973] Plant Physiol. 51: 250-258). Carbonic anhydrase activity was rhythmic when the cultures were maintained under a light-dark cycle with the highest enzyme activity coinciding with the fastest rate of O2 evolution. However, the rhythm in carbonic anhydrase activity disappeared under constant conditions. Changes in the activities of these two enzymes are therefore not responsible for the rhythmic changes in photosynthetic capacity.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush K. J., Sweeney B. M. The Activity of Ribulose Diphosphate Carboxylase in Extracts of Gonyaulax polyedra in the Day and the Night Phases of the Circadian Rhythm of Photosynthesis. Plant Physiol. 1972 Oct;50(4):446–451. doi: 10.1104/pp.50.4.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codd G. A., Merrett M. J. Photosynthetic products of division synchronized cultures of euglena. Plant Physiol. 1971 May;47(5):635–639. doi: 10.1104/pp.47.5.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDMUNDS L. N., Jr REPLICATION OF DNA AND CELL DIVISION IN SYNCHRONOUSLY DIVIDING CULTURES OF EUGLENA GRACILIS. Science. 1964 Jul 17;145(3629):266–268. doi: 10.1126/science.145.3629.266. [DOI] [PubMed] [Google Scholar]
- Enns T. Facilitation by carbonic anhydrase of carbon dioxide transport. Science. 1967 Jan 6;155(3758):44–47. doi: 10.1126/science.155.3758.44. [DOI] [PubMed] [Google Scholar]
- Graham D., Reed M. L. Carbonic anhydrase and the regulation of photosynthesis. Nat New Biol. 1971 May 19;231(20):81–83. doi: 10.1038/newbio231081a0. [DOI] [PubMed] [Google Scholar]
- Jacobson B. S., Fong F., Heath R. L. Carbonic anhydrase of spinach: studies on its location, inhibition, and physiological function. Plant Physiol. 1975 Mar;55(3):468–474. doi: 10.1104/pp.55.3.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schor S., Siekevitz P., Palade G. E. Cyclic Changes in Thylakoid Membranes of Synchronized Chlamydomonas reinhardi. Proc Natl Acad Sci U S A. 1970 May;66(1):174–180. doi: 10.1073/pnas.66.1.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senger H., Bishop N. I. Quantum yield of photosynthesis in synchronous Scenedesmus cultures. Nature. 1967 Apr 8;214(5084):140–142. doi: 10.1038/214140a0. [DOI] [PubMed] [Google Scholar]
- WU R., RACKER E. Regulatory mechanisms in carbohydrate metabolism. III. Limiting factors in glycolysis of ascites tumor cells. J Biol Chem. 1959 May;234(5):1029–1035. [PubMed] [Google Scholar]
- Walther W. G., Edmunds L. N. Studies on the Control of the Rhythm of Photosynthetic Capacity in Synchronized Cultures of Euglena gracilis (Z). Plant Physiol. 1973 Feb;51(2):250–258. doi: 10.1104/pp.51.2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]


