Abstract
Meiotic development (sporulation) in Saccharomyces cerevisiae is characterized by an ordered pattern of gene expression, with sporulation-specific genes classified as early, middle, mid-late, or late depending on when they are expressed. SMK1 encodes a mitogen-activated protein kinase required for spore morphogenesis that is expressed as a middle sporulation-specific gene. Here, we identify the cis-acting DNA elements that regulate SMK1 transcription and characterize the phenotypes of mutants with altered expression patterns. The SMK1 promoter contains an upstream activating sequence (UASS) that specifically interacts with the transcriptional activator Abf1p. The Abf1p-binding sites from the early HOP1 and the middle SMK1 promoters are functionally interchangeable, demonstrating that these elements do not play a direct role in their differential transcriptional timing. Timing of SMK1 expression is determined by another cis-acting DNA sequence termed MSE (for middle sporulation element). The MSE is required not only for activation of SMK1 transcription during middle sporulation but also for its repression during vegetative growth and early meiosis. In addition, the SMK1 MSE can repress vegetative expression in the context of the HOP1 promoter and convert HOP1 from an early to a middle gene. SMK1 function is not contingent on its tight transcriptional regulation as a middle sporulation-specific gene. However, promoter mutants with different quantitative defects in SMK1 transcript levels during middle sporulation show distinct sporulation phenotypes.
The life cycle of the yeast Saccharomyces cerevisiae comprises a series of interconnected growth states and developmental programs that are under both genetic and environmental control. Meiotic development (sporulation) is induced when a diploid cell is starved for essential nutrients and a fermentable carbon source (21). Following induction, the cell withdraws from the mitotic cycle at G1 and enters meiotic prophase, during which a single round of DNA replication, synaptonemal complex formation, and recombination occur. Meiotic prophase is followed by the meiosis I reductional and meiosis II equational divisions. Spore wall morphogenesis initiates with the outgrowth of a double membranous structure (the prospore wall) from the outer plaques of each meiosis II spindle pole body which envelops each haploid meiotic product. Two layers that appear similar to the vegetative cell wall and two protective spore-specific layers are subsequently assembled from within and around the prospore wall. The end product of sporulation is the differentiated ascus which contains four dormant haploid spores. The tightly regulated sequence of cell cycle and morphogenetic events that occur during sporulation provides a model system for study of the mechanisms that regulate and coordinate development.
During sporulation, specific sets of genes that can be classified as early, middle, mid-late, or late are sequentially expressed. Early genes are expressed at the onset of sporulation and are involved in events including formation of the synaptonemal complex and the meiotic divisions. The transcriptional regulation of the early genes has been extensively studied, and regulatory cis-acting DNA sequences and proteins that bind to many of these sites have been identified (21, 26). One element commonly found in early promoters is recognized by the Abf1p DNA-binding protein, a general transcriptional activator (16). URS1, a second cis-acting element found in most and perhaps all early promoters, is recognized by the Ume6p DNA-binding protein (26, 29, 35, 37). During vegetative growth, Ume6p interacts with a complex of proteins which includes Sin3p and the Rpd3p histone deacetylase, to repress transcription (19). During early sporulation, changes in the Ume6p-complex occur that lead to transient derepression as well as transcriptional activation (4, 35). Gene products required for the developmentally regulated conversion from a repression to an activation complex include the Rim11p protein kinase, as well as Ime1p, which has transcriptional activation properties and may interact directly with Ume6p (3, 30).
In comparison to the early meiotic genes, less is known about transcriptional regulation of the later temporal classes of sporulation-specific genes. The cis-acting promoter sequences responsible for middle-transcriptional regulation have been identified in the SPS4 and SPR3 genes (17, 27, 28). The SPR3 promoter contains a consensus Abf1p binding site which is required for transcriptional activation. An additional site found in both SPS4 and SPR3, as well as in several other middle promoters, is the MSE (middle sporulation element), which has been shown to be required for the activation of middle gene expression and to be capable of activating heterologous promoters during middle sporulation (17, 28). Recently, Ndt80p has been shown to specifically interact with MSE DNA in vitro and to activate middle sporulation-specific gene expression in vivo (7). NDT80 is expressed predominantly as a middle gene, and it has been proposed that completion of a meiotic checkpoint is required for its full activity (7, 17a, 43). Mid-late and late genes appear to require distinct cis-acting elements. A negative regulatory element (NREDIT) that requires the Ssn6p and Tup1p corepressor complex regulates the divergently transcribed mid-late DIT1 and DIT2 genes (14). NREDIT derepression during sporulation requires complex interactions with at least two additional cis-acting elements. Thus, the different temporal classes of sporulation-specific promoters require multiple transcriptional activators that are expressed at different times during sporulation, as well as distinct transcriptional-repression pathways.
SMK1 encodes a middle sporulation-specific mitogen-activated protein (MAP) kinase homolog that is required for spore wall morphogenesis. In an smk1 null homozygous mutant, multiple abnormal spore wall assembly patterns are observed within a single ascus, with spore wall layers inverted or missing (20). Different smk1 missense mutants stall at distinct stages in spore morphogenesis. Furthermore, modest increases in dosage of certain smk1 alleles can restore wild-type spore wall morphogenesis, and different spore resistance phenotypes require distinct smk1 allelic thresholds (41, 42). These results show that SMK1 plays a central role in coordinating multiple events that are required for spore formation and suggest that different morphogenetic events can have distinct MAP kinase threshold requirements.
The transcriptional regulation of SMK1 raises a number of interesting questions that are now amenable to experimental investigation. What are the cis-acting promoter elements that regulate SMK1 expression? What is the functional significance of the tight control of SMK1 transcription during spore development? Can promoter mutants cause distinct phenotypes similar to those seen with the amino acid substitution mutants? Analysis of SMK1 transcriptional regulation should also provide insights on how the expression of different temporal classes of genes is regulated during development.
MATERIALS AND METHODS
Yeast strains and culture conditions.
The genotypes and sources of yeast strains used in this study are shown in Table 1. Vegetative cultures were propagated in either YEPD (1% yeast extract, 2% peptone, 2% glucose), YEPA (1% yeast extract, 2% peptone, 2% potassium acetate), SD (0.67% Difco yeast nitrogen base without amino acids, 2% glucose) or SA (0.67% yeast nitrogen base without amino acids, 1% potassium acetate, 1% phthalic acid [pH 5.5]) supplemented with nutrients essential for auxotrophic strains at the levels specified by Sherman et al. (31). For synchronous sporulation of strains containing plasmids, cells were pregrown in SA-uracil, and for strains lacking plasmids, cells were pregrown in YEPD. Logarithmically growing cultures were used to inoculate YEPA, the cultures were expanded overnight at 30°C to a density of 1 × 107 cells/ml (4 to 5 generations), and cells were collected by centrifugation, washed once in 2% potassium acetate, and resuspended at 4 × 107 cells/ml in SM (2% potassium acetate, 10 μg of adenine/ml, 5 μg of histidine/ml, 30 μg of leucine/ml, 7.5 μg of lysine/ml, 10 μg of tryptophan/ml, 5 μg of uracil/ml) prewarmed to 30°C. Under these conditions, the strains used in this study completed spore formation within 12 h. For testing the effects of ume6, sin3, and rpd3 mutations on SMK1 expression, the FT5-derived strains described by Kadosh and Struhl were used (19). The effects of ssn6 and tup1 mutations were tested by using the strains described by Friesen et al. (14).
TABLE 1.
Yeast strains
| Straina | Genotype | Source or reference |
|---|---|---|
| LNY150 | MATa/MATα leu2-hisG/leu2-hisG trp1-hisG/trp1-hisG lys2/lys2 his4-N/his4-G ura3/ura3 ho::LYS2/ho::LYS2 | L. Neigeborn |
| LNY3 | MATa/MATα leu2-hisG/leu2-hisG trp1-hisG/trp1-hisG lys2/lys2 ura3/ura3 ho::LYS2/ho::LYS2 | L. Neigeborn |
| MDPY10 | MATa/MATα leu2-hisG/leu2-hisG trp1-hisG/trp1-hisG lys2/lys2 his4-N/his4-G ura3/ura3 ho::LYS2/ho::LYS2 smk1::LEU2/smk1::LEU2 | 42 |
| YV16 | MATa/MATα trp1/TRP1 ura3-52/ura3-x can1/CAN1 cyh2/CYH2 ade2-R8/ade2-1 | 39 |
| LNY273 | MATa/MATα leu2-hisG/leu2-hisG trp1-hisG/trp1-hisG lys2/lys2 gal80::LEU2/gal80::LEU2 IME1-14-TRP1/IME4 ura3/ura3 ho::LYS2/ho::LYS2 | L. Neigeborn |
| MDPY1003 | MATa leu2-hisG trp1-hisG lys2 his4-N ura3 ho::LYS2 smk1::LEU2 | This study |
| MDPY1004 | MATα leu2-hisG trp1-hisG lys2 his4-N ura3 ho::LYS2 smk1::LEU2 | This study |
| MDPY34 | MDPY10 + URA3/pRS406/ura3 | This study |
| MDPY35 | MDPY10 + URA3::pMDP149/ura3 | This study |
| MDPY36 | MDPY10 + URA3::pMDP152/ura3 | This study |
| MDPY48 | MDPY10 + URA3::pRS406/URA3::pMDP199 | This study |
| MDPY51 | MDPY10 + URA3::pRS406/URA3::pMDP183 | This study |
| MDPY44 | MDPY10 + URA3::pRS406/URA3::pMDP185 | This study |
| MDPY46 | MDPY10 + URA3::pRS406/URA3::pMDP187 | This study |
All strains are SK1 background (1), with the exception of YV16.
Plasmids.
Plasmids, markers, and sources are detailed in Table 2. Mutations in the SMK1 promoter were introduced by using PCR-based strategies and confirmed by dideoxynucleotide sequence analysis. The SMK1-lacZ reporter plasmid pMDP83 was constructed by replacing the BglII-SalI fragment of pLAK42 (codons 183 to 388 of SMK1 and the 3′ noncoding region) with a 3,072-bp BglII-SalI fragment containing lacZ generated by PCR using pMC1871 as a template. The 5′ primer changed the first and last nucleotides of the BamHI site found in pMC1871 to A and T, respectively, to generate the BglII site, and the 3′ primer included the SalI site in pMC1871. Thus, pMDP83 contains 219 bp of the SMK1 promoter and 546 bp of the SMK1 coding region fused in frame to lacZ. SMK1 promoter deletion constructs pMDP86, -89, -126, -95, and -92 were generated by replacing the KpnI-BglII fragment of pMDP83 with KpnI-BglII fragments generated by PCR that lacked the indicated base pairs. The pseudo-urs1S mutation removed bp −84 to −90, and the mseS mutation changed the sequence TTTG at positions −79 to −76 to CCCA. Both of these mutations were introduced by using an XhoI site that was introduced into the SMK1 promoter by a T→C A→G double substitution at positions −68 and −65, respectively (see Fig. 1). In control experiments the XhoI site reduced the level of expression twofold but had no effect on vegetative repression or the timing or pattern of SMK1 expression. To test for mutant smk1 promoter phenotypes, an integrating SMK1 plasmid was constructed by subcloning the KpnI-XhoI fragment of SMK1 (containing the entire SMK1 gene and its promoter) into these sites in pRS406 to generate pMDP199. Promoter mutations were introduced into pMDP199 by replacing the KpnI/BglII fragment of pMDP199 with the KpnI/BglII fragments from the lacZ expression plasmids. The SMK1 promoter in pLAK42 was replaced with a 200-bp PCR fragment of the HOP1 promoter (measured from the ATG) to generate pJS1 by using the unique BstXI site located at codon 13 of the SMK1 gene and the unique KpnI site at the plasmid/insert junction.
TABLE 2.
Plasmids
| Plasmid | Markers | Source or reference |
|---|---|---|
| pRS316 | CEN URA3 | 33 |
| pLAK40 | pRS316 + SMK1 | 20 |
| YEp352 | 2μm URA3 | 18 |
| pMC1871 | lacZ | 6 |
| pLAK42 | YEp352 + SMK1 | 20 |
| pJS1 | YEp352 + HOP1/SMK1 | This study |
| pMDP83 | YEp352 + SMK1/lacZ (−219) | This study |
| pMDP86 | YEp352 + SMK1/lacZ (−181) | This study |
| pMDP89 | YEp352 + SMK1/lacZ (−139) | This study |
| pMDP126 | YEp352 + SMK1/lacZ (−124) | This study |
| pMDP95 | YEp352 + SMK1/lacZ (−96) | This study |
| pMDP92 | YEp352 + SMK1/lacZ (−71) | This study |
| pMDP119 | pMDP89 + pseudo-urs1S | This study |
| pMDP174 | pMDP89 + mseS | This study |
| pMDP176 | pMDP89 + pseudo-urs1S mseS | This study |
| pRS406 | URA3 | 33 |
| pMDP149 | pRS406 + SMK1 (−124) | This study |
| pMDP152 | pMDP149 + C118A | This study |
| pMDP199 | pRS406 + SMK1 (−139) | This study |
| pMDP183 | pMDP199 + pseudo-urs1S | This study |
| pMDP185 | pMDP199 + mseS | This study |
| pMDP187 | pMDP199 + pseudo-urs1S + mseS | This study |
| pMDP113 | pMDP89 + UASH | This study |
| pAV124 | 2μm URA3 HOP1/lacZ − URS1H | 39 |
| pCC51 | pAV124 + URS1H | 15 |
| pJX42 | pAV124 + pseudo-URSS | This study |
| pJX43 | pAV124 + MSES | This study |
| pAV130 | 2μm URA3 HOP1/lacZ − UASH | 39 |
| pCC83 | pAV130 + UASH | 16 |
| pJX33 | pAV130 + UASH | This study |
| pAV73 | 2μm URA3 CYC1/lacZ | 39 |
| pJX49 | pAV73 + MSES | This study |
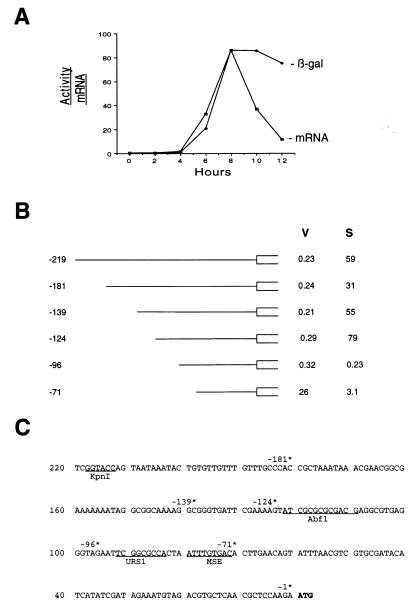
FIG. 1.
Analysis of the SMK1 promoter using SMK1-lacZ expression plasmids. (A) Comparison of relative SMK1 mRNA levels and SMK1-lacZ enzyme activities during sporulation. β-Galactosidase activity levels were measured at 2-h intervals postinduction by using strain LNY150 transformed with pMDP83. Activity is expressed as units of β-galactosidase per milligram of total protein. SMK1 mRNA levels were quantitated by Northern blot hybridization (20). (B) Deletion analysis of the SMK1 promoter. The indicated deletions were generated from the −219 promoter construct pMDP83 (see Table 2). β-Galactosidase activities were determined in vegetative cells (V) and 10 h postinduction (S) and are averages from at least two separate determinations. In each case the deletion point indicates the number of base pairs between the initiator ATG and the C that is the most 3′ base of the KpnI restriction endonuclease site of the parental plasmid. (C) Nucleotide sequence of the SMK1 promoter. Deletion endpoints diagrammed in panel B are indicated (*), and the initiator ATG of SMK1 is boldfaced. Consensus elements referred to in the text and the KpnI restriction enzyme site used in the construction of pMDP83 are underlined.
To construct HOP1-lacZ reporter promoters with the wild-type and mutant SMK1 Abf1p binding sites, synthetic oligonucleotides containing the top and bottom strands of each site were annealed and ligated into the BglII site of plasmid pAV130 as described previously (16). pAV130 contains a HOP1-lacZ fusion gene in which there is an 8-bp substitution in the Abf1p-binding site of the HOP1 promoter (39). pCC83 was derived from pAV130 by inserting a 19-mer double-stranded oligonucleotide containing the wild-type HOP1 Abf1p-binding site into the BglII site 10 bp upstream of the disrupted site (16). pJX33 was derived from pAV130 by inserting the wild-type SMK1 Abf1p-binding site 5′-gatcTATCGCGCGCGACGA-3′ (top-strand oligonucleotide with lowercase bases used for cloning into the restriction site) in the same manner.
To construct HOP1-lacZ reporter promoters with the SMK1 pseudo-URS1S and MSES sites, synthetic oligonucleotides containing the top and bottom strands of each site were annealed and ligated into the XhoI site of plasmid pAV124, as described previously (15). pAV124 contains a 207-bp region of the HOP1 promoter and the region coding for the first 115 residues of the protein fused in frame to the lacZ gene. Five base pairs within the HOP1 URS1H site in the promoter have been mutated to create an XhoI site into which the pseudo-URS1S 5′-tcgacTAGAATTCGGCGCCACTAATc-3′ or MSES 5′-tcgacCCACTAATTTGTGACACTTGc-3′ (top strands) was cloned by using duplex oligonucleotides. pCC51 contains a wild-type HOP1 URS1H site that was inserted into the XhoI site of pAV124 and was used as a control for normal HOP1 expression (15). The ability of MSES to repress the CYC1 promoter was tested by subcloning the MSES oligonucleotides described above into the XhoI site of pAV73 (39) to generate pJX49.
Assays for spore wall assembly.
For light microscopy, cells were fixed in ethanol and stained with the DNA-specific dye 4′,6-diamidino-2-phenylindole (DAPI) (31). Samples were viewed and photographed as a wet mount under phase-contrast oil immersion optics by using a Nikon Optiphot equipped for epifluorescence. The procedure for the fluorescence assay has been described previously (42). Spore viability after heat shock (40 min at 55°C) or treatment with glusulase (1 h at 26°C) was determined as described by Briza et al. (5). Sensitivity of cells to ether exposure (3 min with constant gentle rocking) was assayed according to the method of Dawes and Hardie (8).
EMSA.
For electrophoretic mobility shift assays (EMSA), oligonucleotides were end labeled with [γ-32P]ATP by using polynucleotide kinase and were purified by Nensorb columns (NEN). The oligonucleotides were made double-stranded by mixing with a threefold excess of the matching strand, incubating at 90°C for 20 min, and slowly cooling to 25°C overnight. Binding reactions were carried out in a solution containing 10 mM Tris-HCl (pH 7.5), 40 mM NaCl, 4 mM MgCl2, 6% (wt/vol) glycerol, 10 μg of sonicated salmon sperm DNA/ml, and 32P-labeled oligonucleotide (10,000 cpm) in a total volume of 20 μl at room temperature for 20 min. Crude extracts and partially purified Abf1p were prepared as previously described (16). Protein dilutions were made in a solution containing 20 mM Tris-HCl (pH 8), 50 mM NaCl, 1 mM EDTA, 1 mg of bovine serum albumin (BSA)/ml, 5 mM β-mercaptoethanol, and 1 mM phenylmethylsulfonyl fluoride (PMSF). Samples were analyzed on a 6% polyacrylamide gel (run in 0.5× Tris-borate-EDTA [TBE] buffer for 60 min at 250 V). Gels were dried after electrophoresis, exposed to a phosphor screen, scanned, and quantitated on a Model 425E Molecular Dynamics PhosphorImager.
Miscellaneous methods.
Preparation of total RNA and Northern blot hybridization analysis were carried out as previously described (20). DNA probes from the coding regions of the indicated genes were isolated from preparative agarose gels, radiolabeled with 32P by random priming (2), and used in hybridization analysis at 106 dpm/ml. The DNA hybridization probe used for SMK1 consisted of the 0.8-kb StyI fragment of its coding region. This probe does not detect any transcripts that may be produced at the smk1::LEU2 locus, since it is internal to the boundaries of this deletion/insertion mutation (20). The DNA probe used for SPO12 was the 0.4-kb EcoRI-BamHI fragment of its coding region, and that for HOP1 was the BamHI-SacI restriction fragment of its coding region. The PC4/2 control probe for RNA loading has been described previously (22, 38). For β-galactosidase assays, cell pellets were frozen at −80°C and subsequently resuspended with an equal volume of glass beads and breaking buffer (100 mM Tris-HCl, 1 mM dithiothreitol, 20% glycerol, 1 mM PMSF). Cell lysis was achieved by vortexing for seven 1-min intervals separated by cooling on ice. Samples were centrifuged for 10 min at 13,000 × g and stored at −80°C. Protein concentrations were determined by the method of Bradford with BSA as a standard, and β-galactosidase was assayed by using o-nitrophenyl-β-d-galactopyranoside (ONPG). Oligonucleotides were synthesized on an Applied Biosystems 392-5 DNA synthesizer, and when appropriate, purified by C18 reverse-phase high-performance liquid chromatography (HPLC).
RESULTS
The SMK1 promoter contains distinct activating and repressing transcriptional control elements.
We have previously shown that SMK1 is expressed as a middle sporulation-specific gene (20). To assay the SMK1 promoter we constructed pMDP83, which contains 219 bp of the promoter and 546 bp of the coding region fused in frame to lacZ (Table 2). The SK1 strain background (LNY150; Table 1) was used for these studies because it can be induced to undergo sporulation in a relatively rapid and synchronous manner. pMDP83 diploid transformants were sporulated, cells were harvested at 2-h intervals, and β-galactosidase activities were assayed. Figure 1A compares the SMK1 mRNA expression profile directly determined by Northern blot hybridization analysis (20) to the lacZ expression profile. The levels of β-galactosidase and endogenous SMK1 mRNA were low in vegetative cells and remained low until around the time of completion of meiosis II (6 h). Both β-galactosidase and SMK1 mRNA levels peaked around the time when the major steps of spore wall morphogenesis were occurring (8 h). β-Galactosidase levels remained high and decayed relatively slowly thereafter. In contrast, SMK1 mRNA levels decayed rapidly. These results demonstrate that 219 bp of the SMK1 promoter are sufficient for the regulated expression of SMK1. In addition, these results show that SMK1-lacZ fusions can be used to assay the onset and magnitude of SMK1 transcription.
In order to further define the cis-acting sequences that regulate SMK1 transcription, a series of deletion constructs was generated from pMDP83, and the expression patterns were measured in vegetative cells and cells that had been sporulated for 10 h. As can be seen in Fig. 1B, successive deletions of the SMK1 promoter from −219 through −181, −139, and −124 had only modest (less than twofold) effects on the level of expression seen in both vegetative and sporulation cultures. In contrast, removal of an additional 28 bp (from −124 to −96) reduced the level of sporulation-specific expression over 200-fold while leaving the low level of vegetative expression unaffected. Thus the region between −124 and −96 is required for sporulation-specific expression. We refer to this upstream activating site in the SMK1 promoter as UASS. Removal of an additional 25 bp resulted in a 50-fold increase in vegetative expression. Thus, the region between −96 and −71 is required for repression of SMK1 in vegetative cells. Expression of the derepressed −71 deletion construct in vegetative cells appears to require the remaining SMK1 promoter sequences, since substitution mutations in this interval reduced promoter activity (data not shown). However, the −71 construct does not appear to direct any transcription during sporulation, because the low level of β-galactosidase in sporulated transformants can be accounted for by the stability of vegetative pools of the enzyme (see Fig. 1A, and compare Fig. 4 and 5).
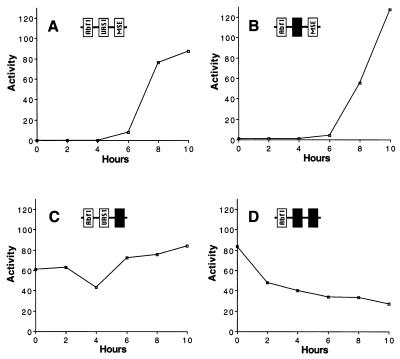
FIG. 4.
Expression of β-galactosidase by mseS and pseudo-urs1S SMK1 promoter plasmids during sporulation. Yeast strain LNY150 transformed with a plasmid expressing β-galactosidase under the control of the wild-type (A), pseudo-urs1S (B), mseS (C), or pseudo-urs1S mseS (D) −139 promoter (pMDP89, pMDP119, pMDP174, or pMDP176, respectively) was synchronously sporulated, and β-galactosidase levels (expressed as units per milligram of total protein) were determined at 2-h intervals. The experiment was performed at least twice for each promoter with similar results.
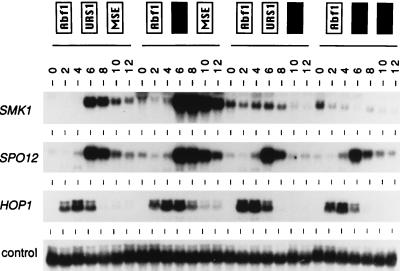
FIG. 5.
Expression of SMK1 mRNA by mseS and urs1S promoter mutants during sporulation. Diploid yeast containing a single integrated copy of wild-type SMK1 coding information under the control of the indicated mutant promoter was transferred to sporulation medium, and total RNA was prepared from cells harvested at the indicated times and assayed by Northern blot hybridization. The indicated smk1 promoter mutants were generated using the following integrating plasmids, from left to right: wild-type (pMDP199), pseudo-urs1S (pMDP183), mseS (pMDP185), and pseudo-urs1S mseS (pMDP187) (see Table 2). The same filter was probed with sequences specific for the SPO12 middle gene, the HOP1 early gene, and the pC4/2 constitutive expression control.
Abf1p interacts with UASS.
The Abf1p DNA-binding protein is required for the regulated expression of many diverse genes. It appears to play an important role in sporulation-specific gene expression, and Abf1p-binding sites are found in a high percentage of sporulation-specific promoters (16, 27, 28). High-affinity Abf1p binding in vitro has previously been shown to require the 13-bp sequence RTCRYBNNNNACG (where Y is pyrimidine, R is purine, B is G, C, or T, and N is any base) (10, 12, 13). Within the 28-bp region that is required for UASS activity, an element that conforms to this consensus is found starting at position −122 (Fig. 1C). We tested whether Abf1p would bind to this site in vitro. As a positive control, binding to the characterized Abf1p-binding site, which is required for transcriptional activation of the early sporulation HOP1 promoter (UASH), was also assayed. As shown in Fig. 2A, Abf1p specifically bound to a 13-bp oligonucleotide duplex from UASS. Furthermore, substitution of the CG base pair corresponding to position −118 with an AT base pair (referred to below as the −118A substitution) eliminated detectable Abf1p binding, consistent with previously described binding requirements. In contrast, substitution of the GC base pair at position −115, which is found in the degenerate core of the Abf1p-binding site, with an AT base pair had no effect on binding. Although Abf1p bound to the UASS site, it bound with lower affinity than to the UASH site. We were therefore interested to determine if the SMK1 Abf1p site would be functional in the context of the HOP1 promoter and vice versa. It has previously been shown that removal of UASH from the HOP1 promoter reduces the early sporulation-specific expression peak (Fig. 2B) (39). Introduction of the SMK1 Abf1p-binding site into this mutant HOP1 promoter restored activity. Furthermore, the early sporulation timing of the HOP1 promoter containing the SMK1 Abf1p site was indistinguishable from the timing of the HOP1 promoter reconstituted with the HOP1 Abf1p site. Similarly, replacement of the Abf1p-binding site in the SMK1 promoter with the Abf1p-binding site from HOP1 had no detectable effect on the timing or level of SMK1 expression (Fig. 2B). These results show that the Abf1p sites from these promoters are functionally interchangeable. Thus, both biochemical and functional assays indicate that Abf1p can activate the SMK1 promoter. These results also suggest that different Abf1p-binding sites do not play a direct role in setting the timing of these temporally distinct classes of promoters.
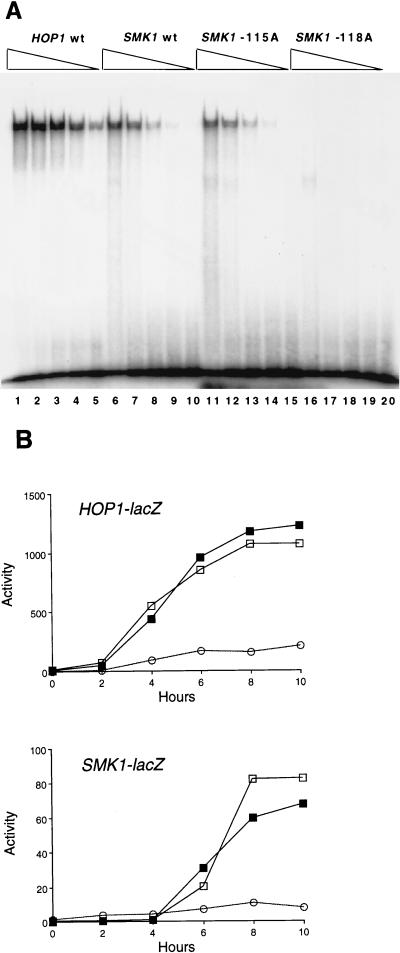
FIG. 2.
Comparison of UASS and UASH sites. (A) EMSA of Abf1p binding. Partially purified Abf1p was serially diluted fivefold and used in binding reactions to radiolabeled 13-bp oligonucleotide duplexes containing the HOP1 Abf1p-binding site (lanes 1 to 5), the wild-type SMK1 Abf1p-binding site (lanes 6 to 10), and the mutant SMK1 Abf1p sites containing the −115A (lanes 11 to 15) and −118A (lanes 16 to 20) substitutions. (B) Analysis of HOP1 and SMK1 promoters containing heterologous Abf1p-binding sites. The HOP1 promoter (upper panel) lacking its Abf1p-binding element (open circles), reconstituted with the HOP1 binding site (open squares), or reconstituted with the SMK1 Abf1 site (closed squares) was tested by using the HOP1-lacZ plasmid pAV130, pCC83, or pJX33, respectively. The SMK1 promoter (lower panel) lacking a functional Abf1p-binding site (−118A mutant; open circles), containing its normal Abf1p-binding site (open squares), or reconstituted with the Abf1p-binding site from HOP1 (closed squares) was tested for expression of β-galactosidase by using the SMK1-lacZ plasmid pMDP126-118A, pMDP126, or pMDP113, respectively, in yeast strain LNY150 as described in Materials and Methods. The experiment was performed independently three times with similar results.
We were interested in identifying mutations that reduce SMK1 promoter activity to different extents in order to test the functional consequences of reducing SMK1 expression by defined thresholds. Toward this goal, a series of substitution mutations were introduced in UASS and their effects on the expression of SMK1-lacZ were assayed. As shown in Fig. 3A, substitutions that were predicted to reduce Abf1p binding strongly, such as −118A and −117A (10, 13, 16), strongly reduced the level of expression. Also, as expected, mutations in bp −116, −115, −114, and −113, which correspond to the degenerate core of the site, caused only modest transcriptional effects. Surprisingly however, the double −110A −111A substitution, which changes two positions that are strongly conserved among Abf1p-binding sites, reduced the level of SMK1-lacZ expression only modestly. The effects of substitutions in positions that are adjacent to the consensus Abf1p-binding site (−108A, −107A, −106A, and −105A) were also tested. Some of these mutations strongly reduced SMK1 promoter activity. The most severe of these mutations (−106A) also strongly reduced the activity of the SMK1 promoter containing the Abf1p-binding site from HOP1. Surprisingly, we found that the transcriptional defect of the −106A substitution was partially suppressed by mutations in the center of the site (see the −115A −106A, −114A −106A, and −113A −106A double substitutions).
FIG. 3.
Mutational analysis of UASS. (A) Effects of UASS mutations on expression of SMK1 and binding to Abf1p. Strains with the indicated mutations in the −124 promoter SMK1-lacZ plasmid pMDP126 were assayed for β-galactosidase activity in vegetative (V) and sporulated cultures at 10 h postinduction (S). β-Galactosidase activities are averages from at least two separate comparisons to the wild-type plasmid (pMDP126). Both vegetative and sporulation values are shown as percentages of the wild-type sporulation value (79 ± 8 U/mg of total protein). Relative complex formation between oligonucleotide duplexes containing the indicated mutation and partially purified Abf1p (Binding) was quantitated from a single titration curve performed as shown in Fig. 2. The mutations are referred to by the numbers at the left. Sequence requirements for Abf1p binding (Abf1 con) are shown above for comparison. The HOP1-Abf1 mutation contains a 6-bp substitution to generate the HOP1 Abf1p-binding site, which reads 5′-ATCACTTCACACG-3′. (B) Hybridization analysis of UASS promoter mutants. Indicated promoter mutations in the context of the wild-type SMK1 gene were integrated at the ura3 locus, and RNA was prepared from vegetative (V) or sporulated (S) cultures 10 h postinduction. Hybridization analysis was performed on total RNA by Northern blot analysis using an SMK1-specific probe. The same filter was subsequently hybridized with the middle sporulation SPO12-specific probe as a normalization control. The SMK1-specific hybridization signal in sporulating samples (normalized for SPO12 hybridization) was reduced to 51, 48, 23, and 17% of the wild-type signal in the −116A, −115A, −115A −106A, −117A, and −118A mutant strains, respectively.
Mutational analysis of Abf1p-binding sites in the promoters of other genes has shown that in general, substitutions that eliminate UAS activity in vivo also eliminate Abf1p binding in vitro. We therefore tested several of the UASS point mutations for their effects on Abf1p-binding affinity (Fig. 3A). Based on the predicted consensus requirements of RTCRYBNNNNACG for Abf1p recognition, all of the point mutations that were predicted to reduce Abf1p binding did so (10, 13, 16). Interestingly, the −110A −111A substitution, which had a relatively modest effect on transcriptional activation in vivo, caused a large reduction in DNA-binding affinity. A similar difference in DNA-binding affinity and transcriptional activation has been observed in the mutational analysis of the Abf1p-binding site in the HOP1 promoter (16). The −105A, −106A, and −107A substitutions, which are outside the Abf1p-binding consensus, had no effect on Abf1p-binding affinity in vitro. Our results suggest that the Abf1p-UASS interaction is complex and that recognition by the protein may be influenced by additional factors in vivo (see Discussion).
SMK1 promoter sequences from SMK1-lacZ plasmids whose expression was reduced by varying extents were used to reconstitute the SMK1 gene in an integrating plasmid vector. The resulting SMK1 promoter mutants were integrated into the chromosome at the ura3 locus, and SMK1 mRNA levels were determined by Northern blot hybridization. The expression of the middle sporulation-specific SPO12 gene was determined in the same samples as a normalization control. Quantitation of the hybridization signals showed that mutations that reduced the level of SMK1-lacZ expression also reduced expression of SMK1 in the chromosomal context. This series of strains proved useful for assessing the functional significance of the level of SMK1 expression (see below).
The MSE is required for vegetative repression as well as sporulation-specific activation of the SMK1 promoter.
Within the −96-to-−71 deletion interval that derepressed vegetative SMK1 expression, there are two sequence elements of interest. The first element is similar to the previously described MSE. The MSEs of the SPS4 and SPR3 middle genes have been shown to activate middle expression in heterologous promoter constructs (17, 27). A consensus MSE sequence (GNCRCAAAA/T) (28) is found at positions −72 to −80 of the SMK1 promoter, and we refer to it hereafter as MSES. The second DNA element of interest is similar to the consensus URS1 element (TCGGCGGCT) and is found at positions −84 to −92 of the SMK1 promoter (Fig. 1). URS1 sites have been shown to interact with Ume6p, and in the context of early sporulation promoters, they function to repress expression during vegetative growth (29, 37, 39). URS1 sites have also been shown to function as meiosis-specific activator elements (3, 30). The transcriptional regulatory properties of the URS1 consensus in SMK1 differs in certain respects from the URS1s that have been characterized in early sporulation-specific genes (see below). We therefore refer to this element in SMK1 as pseudo-URS1S (or Ψ-URS1S) hereafter.
To address the significance of MSES and pseudo-URS1S in the SMK1 promoter, mutations were constructed in these sites in the context of the SMK1-lacZ reporter gene. Promoter mutants and wild-type controls were synchronously sporulated, and β-galactosidase activities were determined at 2-h intervals. An almost complete removal of pseudo-URS1S by deletion of bp −84 to −90 (pMDP119) had no effect on the timing of expression (compare Fig. 4A and B). In contrast, a 4-bp substitution in MSES (bp −73 to −76, which make up the core of MSE similarity; pMDP174) significantly derepressed the promoter in vegetative cells, and high levels of β-galactosidase were detected at all time points tested (Fig. 4C). In the double pseudo-urs1S mseS mutant promoter (pMDP176), the level of β-galactosidase in vegetative cells was indistinguishable from that in the single mseS mutant. However the double pseudo-urs1S mseS mutant showed lower β-galactosidase activities than the single mseS mutant during late sporulation (compare Fig. 4C and D). These data show that MSES is a strong transcriptional repressor site in vegetative cells and that it is also required for the sporulation-specific peak of SMK1 expression. These data also suggest that pseudo-URS1S can function in a positive fashion during sporulation, but this is only apparent in the mseS mutant promoter background.
To directly assay the effects of the promoter mutations on mRNA levels, the mutant promoters were used to replace the promoter in SMK1 on an integrating plasmid vector (to generate pMDP199, pMDP183, pMDP185, and pMDP187 [Table 2]). Wild-type and mutant promoters were integrated at the ura3 locus in a smk1::LEU2 strain, and diploid transformants were synchronously sporulated. Cultures were harvested at 2-h intervals, and RNA was prepared and analyzed by Northern blot hybridization with a SMK1 probe specific for the integrated promoter mutant (see Materials and Methods). Northern hybridization analysis was also carried out by using representative early, middle, and constitutively expressed gene sequences as controls. As can be seen in Fig. 5, the pseudo-urs1 mutation modestly increased the level of SMK1 expression (two- to threefold) at all time points, but it had no detectable effect on the overall timing of expression, consistent with the effect of this mutation in the lacZ expression assay system. In the mseS mutant samples, SMK1 mRNA levels were derepressed in vegetative samples and the middle peak of expression was undetectable. This result directly confirms that MSES is required for transcriptional repression in vegetative cells as well as for the peak of sporulation-specific expression. Interestingly, a new peak of expression that is significantly earlier than that seen in the wild-type control is observed in the mseS mutant. This early peak is absent in the mseS pseudo-urs1S double mutant. These results show that in an mseS mutant promoter, pseudo-URS1S can function positively during early meiotic development, consistent with the ability of URS1 elements to activate the expression of a variety of early sporulation promoters (4). Furthermore, the level of vegetative derepression in the double pseudo-urs1S mseS mutant is indistinguishable from that seen in the single mseS mutant. This result indicates that pseudo-URS1S is unable to repress the vegetative expression of SMK1.
To further characterize the activities of the MSES and pseudo-URS1S elements, these sites were used to replace the Ume6p-binding site (URS1H) in the early sporulation HOP1 promoter. It has previously been shown that a mutation in the URS1H site allows expression of HOP1 in vegetative cells and also leads to low-level constitutive expression throughout sporulation (39). Wild-type regulation can be restored by inserting the URS1H site back into this mutant promoter (Fig. 6) and (15). Pseudo-URS1S inserted into the mutant promoter repressed HOP1 expression to an extent similar to that seen in the URS1H-containing recombinant but only weakly activated HOP1 expression during sporulation. The URS1H in the HOP1 promoter was also replaced with a 22-bp fragment containing MSES. Strikingly, MSES fully repressed vegetative expression of the HOP1 promoter and converted this gene from an early to a tightly controlled middle sporulation-specific gene whose expression pattern is indistinguishable from that with the wild-type SMK1 promoter. Thus, MSES can set the developmental timing of middle gene activation and also repress expression in vegetative and early meiotic cells.
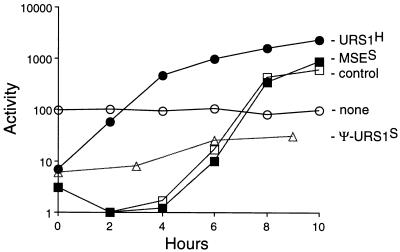
FIG. 6.
Reconstitution of the HOP1 promoter with MSES and URS1S. Yeast cells of strain LNY273 transformed with HOP1 promoter-lacZ plasmids containing the indicated cis-regulatory elements were synchronously sporulated, and β-galactosidase levels were measured. pAV124 contains a 5-bp substitution that destroys the naturally occurring URS1H and introduces an XhoI site (open circles); pCC51 contains URS1H (closed circles); pJX42 contains pseudo-URSS (open triangles); and pJX43 contains MSES (closed squares) in the XhoI site of pAV124. The control is pMDP89 (open squares), which is the SMK1 promoter-lacZ plasmid. Data are averages from three determinations.
The ability of MSES to repress vegetative expression was also tested by using the CYC1 promoter, which normally is expressed in vegetative cells and which, unlike the SMK1 and HOP1 promoters, does not contain an Abf1p-binding consensus element. For these experiments the MSES was inserted into the CYC1-lacZ fusion plasmid pAV73 to generate pJX49. The levels of β-galactosidase in pAV73 and pJX49 transformants were 102 ± 5.7 and 5.6 ± 0.7 U, respectively. Thus, MSES can repress the expression of heterologous promoters that are not normally expressed during sporulation, as well as those that are sporulation specific.
The MSES and URS1 elements appear to function in a similar fashion in several key respects: both can repress expression during vegetative growth, and both activate expression during sporulation (but during different temporal windows). URS1-dependent repression requires the UME6, SIN3, and RPD3 gene products, which encode a DNA-binding protein, a hypothesized coadapter, and a histone deacetylase, respectively (19, 29, 37, 40). DIT1 and DIT2 are mid-late sporulation-specific genes that are regulated by the SSN6 and TUP1 transcriptional corepressor complex (14). These observations prompted us to determine whether the UME6, SIN3, RPD3, SSN6, or TUP1 gene product is required for the vegetative repression of the SMK1 promoter. The wild-type SMK1 promoter/lacZ plasmid pMDP89 was transformed into mutant and wild-type control strains, and the level of β-galactosidase expression was determined in vegetative cultures (see Materials and Methods). For ume6, sin3, rpd3, ssn6, and tup1 mutants, the β-galactosidase levels were 1.2-, 1.5-, 1.3-, 0.4-, and 3.0-fold that seen in the corresponding congenic control strain (values are averages of two independent experiments carried out in triplicate). These small differences could easily be due to indirect effects. Similar results were obtained with the pseudo-urs1S promoter mutant (plasmid pMDP-119). These data show that MSE repression functions through a pathway distinct from the SIN3/RPD3 and SSN6/TUP1 pathways.
SMK1 function during spore morphogenesis is not contingent on its expression as a sporulation-specific middle gene.
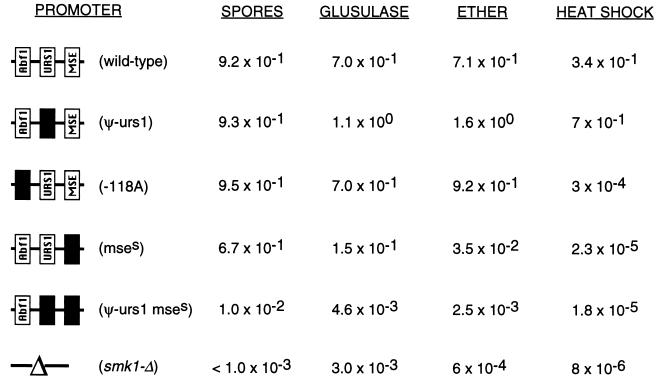
To assess the functional significance of the tight transcriptional regulation of the SMK1 MAP kinase, we examined the phenotypes of the collection of integrated SMK1 promoter mutants that directed the expression of wild-type Smk1p. smk1 promoter mutants were sporulated for 36 h, and the end stage products were assayed for the completion of meiosis by DAPI staining, for spore wall morphogenesis by phase-contrast microscopy, and for acquisition of resistance to glusulase, heat shock, and ether (Table 3). The −118A promoter mutant that expressed SMK1 mRNA as a middle gene, but to only 17% of the level seen in the wild-type promoter control (Fig. 3B), formed spores with near-wild-type efficiency, as judged by phase-contrast microscopy. Based on these results, and taking into account modest differences between the expression of SMK1 from its normal chromosomal locus and from the ura3::SMK1 locus (data not shown) and the fact that the smk1 promoter alleles in these experiments were present in only a single copy, we estimate that spore wall formation can occur when the level of SMK1 mRNA is reduced to 10% of the level seen in wild-type SMK1 homozygotes. However, while spore wall formation did occur in these backgrounds, the resistance phenotypes were not like the wild-type phenotypes. In other experiments, we have shown that SMK1 is required to complete multiple steps in spore morphogenesis (20, 41, 42). In addition, we have isolated a series of smk1 missense hypomorphic alleles that fail to complete distinct steps in sporulation (41). Some of these mutants are defective only in their resistance to heat shock, some are defective in heat shock and ether resistance, and others are defective in all of the resistance phenotypes tested. The −118A SMK1 promoter mutant was resistant to glusulase and ether treatments. However, this mutant is over 103-fold more sensitive to heat shock than the control strain (Table 3). The −117A mutant strain, which expresses levels of SMK1 mRNA only slightly higher than those of the −118A mutant (Fig. 3B), is indistinguishable from the wild type for all resistance phenotypes (data not shown). These results demonstrate that it is possible to reduce the magnitude of SMK1 expression by roughly 90% and still generate wild-type spores, but when expression is reduced below this level, there are threshold requirements for the acquisition of different resistance phenotypes. These data suggest that acquisition of heat shock resistance requires higher levels of SMK1 expression than either spore morphogenesis or the acquisition of glusulase or ether resistance.
TABLE 3.
Sporulation phenotypes of smk1 promoter mutantsa
Diploid smk1-Δ strains containing a single copy of the indicated smk1 promoter constructs were sporulated, and the fraction of cells that formed spores, as assayed by phase-contrast microscopy (n > 200), and fractional resistance to glusulase, ether, or heat shock treatment were determined as described in Materials and Methods. Completion of meiosis was greater than 84% in all samples, as monitored by fluorescence microscopy of DAPI-stained samples. The smk1 promoter mutants were generated by using the following integrating plasmids, from top to bottom: wild-type (pMDP149), pseudo-urs1S (pMDP183), −118A substitution (pMDP152), mseS (pMDP185), and pseudo-urs1S mseS (pMDP187). These strains are identical to those assayed by Northern blot analysis, for which results are shown in Fig. 3B and 5. Values are averages from at least two separate experiments.
The phenotypes of mutants that express SMK1 at inappropriate times were also characterized. In this respect it is worth noting that the derepressed smk1-mse promoter mutant grew at wild-type rates as a haploid or diploid. We have also expressed SMK1 using the strong GAL1,10 promoter and similarly found that this strain grows well in galactose or glucose media (data not shown). Thus, vegetative expression of SMK1 mRNA does not appear to be deleterious to vegetative growth. The derepressed smk1-mse mutant undergoes meiosis and generates spore walls with near-wild-type efficiency. Thus, vegetative expression and early misexpression of SMK1 do not adversely affect the execution of these events. While the glusulase resistance phenotype is only modestly reduced in the smk1-mse mutant (roughly fivefold compared to that in the wild type), the ether resistance phenotype is reduced by more than 2 orders of magnitude and heat shock resistance is reduced by more than over 4 orders of magnitude. These phenotypes are all recessive (strains are completely resistant when heterozygous over wild type). This indicates that these sensitivity phenotypes are not the consequence of Smk1p misexpression but of reduced Smk1p levels. The double smk1-mse pseudo-urs1 promoter mutant, which accumulates the lowest level of SMK1 mRNA during middle sporulation (Fig. 5), failed to form spores as assayed by phase-contrast microscopy and was fully sensitive to glusulase, heat shock, and ether.
The observation that the smk1-mse mutant forms spore walls while the smk1-mse pseudo-urs1 mutant does not, with the only difference between these promoters being the peak of early expression, suggests that SMK1 expressed as an early gene can complement certain aspects of spore wall morphogenesis. To determine whether SMK1 expressed as an early gene could fully complement all of the sporulation phenotypes, the SMK1 coding region was cloned downstream of the early HOP1 promoter in a 2μm high-copy-number vector. As shown in Table 4, expression of SMK1 as an early gene fully complements all of the sporulation defects of a smk1-Δ strain. These results strongly support the idea that the tight timing of SMK1 transcription functions solely to ensure that sufficient levels of the gene product are present during the developmental interval when it is needed. Thus, the function of the SMK1 pathway is not contingent on the graded accumulation of SMK1 mRNA during middle sporulation.
TABLE 4.
Sporulation phenotypes of strains expressing SMK1 as an early genea
| Plasmid | Fraction of cells forming spores | Fraction of cells with resistance to:
|
||
|---|---|---|---|---|
| Glusulase | Ether | Heat shock | ||
| 2μm | 0.00 | <1.0 × 10−3 | <1.0 × 10−3 | <1.0 × 10−3 |
| 2μm SMK1 | 0.63 | 1.0 | 0.31 | 0.72 |
| 2μm HOP1/SMK1 | 0.53 | 1.2 | 0.85 | 0.45 |
The smk1-Δ yeast strain MDPY10, harboring a 2μm control plasmid (YEp352), a 2μm plasmid containing the entire SMK1 gene (PLAK40), and a 2μm plasmid containing the HOP1 promoter directing the expression of SMK1 (pJS1) were sporulated, and the fractions of cells that completed sporulation or that were resistant to the indicated treatment were assayed as described for Table 3 and in Materials and Methods. Values are averages from at least two separate experiments.
DISCUSSION
This study demonstrates that the transcriptional regulation of SMK1 as a middle sporulation-specific gene requires two cis-acting DNA elements, UASS and MSES. UASS functions to activate expression of SMK1. MSES plays a dual role in regulating SMK1 expression. It is required for activation of SMK1 as a middle sporulation gene and also for repression during vegetative growth and early sporulation. Promoter mutants that express SMK1 during vegetative growth or early in meiosis show no overt phenotypes and are still able to complete spore wall morphogenesis as long as sufficient levels of SMK1 mRNA are present during the middle sporulation period. Mutants that reduce the level of SMK1 expression during middle sporulation to different extents show distinct sporulation defects. The implications of these results for transcriptional control mechanisms and the regulation of development by MAP kinases are discussed below.
Abf1 and transcriptional activation by UASS.
Our results demonstrate that UASS specifically interacts with the Abf1p transcription factor in vitro. It has previously been shown that an Abf1p-binding element activates expression of the early sporulation-specific HOP1 and middle sporulation-specific SPR3 promoters. Furthermore, many other sporulation-specific promoters contain Abf1p-binding sites (16, 27). We have shown that the Abf1p-binding sites from the early HOP1 and middle SMK1 promoters are functionally interchangeable. In addition, the early HOP1 promoter can be converted to a middle gene when its URS1 is replaced with MSES. These results indicate that Abf1p-binding sites have a similar role in early and middle sporulation promoters and that they function to regulate the magnitude of transcription and not the timing.
The sequence requirements for Abf1p binding in vitro and transcriptional activation in vivo have been characterized in several different promoters, and in general they are closely correlated (9, 11, 16, 25). While it is clear that an Abf1p-binding element can function to activate SMK1 expression, the sequence requirements for UASS function are more complex than would be predicted for Abf1p binding alone. There are two possible explanations for these results. One possibility is that there exists a transcription factor whose sequence requirements for binding are similar but not identical to those for Abf1p. The second possibility is that there may be base pairs in UASS which are not essential for sequence-specific recognition by Abf1p but are required for its function in vivo. For example, it has been shown that Abf1p causes a significant bend in DNA when it binds to its site and that it makes a number of phosphate contacts with the DNA outside the conserved recognition sequence (23, 24). DNA bending by Abf1p may be important to the mechanism by which it promotes transcription. It is possible that the mutant DNA sites that we have constructed have altered bending properties that affect the ability of Abf1p to promote transcription. Alternatively, the transcriptional activation properties of the collection of UASS mutants may reflect the presence of a factor that functions positively in concert with Abf1p. The suppression of the transcriptional defect of a mutation (−106A) that was outside the Abf1p-binding site consensus by substitutions within the degenerate core (−116A, −115A, and −114A) is consistent with this possibility. Abf1p is a multifunctional protein that is required for a variety of cellular processes which, in addition to transcriptional activation, include silencing of chromatin and DNA replication (11). Alterations in the properties of Abf1p-DNA complexes by accessory factors could provide additional levels of regulation for this general transcription factor. The further characterization of UASS may provide additional insight into the role of this ubiquitous factor.
MSE-dependent transcriptional activation and repression.
The MSE has previously been shown by others to be capable of activating the transcription of heterologous promoters during middle sporulation (7, 28). Our results show that MSES, in addition to activating transcription during middle sporulation, can also repress transcription in vegetative cells. Hepworth et al. previously tested whether the MSE found in the SPS4 middle sporulation promoter could repress vegetative transcription (17). Based on reconstitution experiments using the CYC1 promoter, it was concluded that although the SPS4 MSE specifically binds to a factor in vegetative cells, this element is not a direct repressor of transcription. Our results show that MSES can repress the vegetative expression of the CYC1 as well as the SMK1 and HOP1 promoters. Taken together, these data suggest that there are two different classes of MSE. The first class (the SPS4 MSE being the founding member) activates expression during middle sporulation but does not repress vegetative expression. The second class (the SMK1 MSES characterized here) activates middle sporulation-specific expression and also represses promoter activity in vegetative cells. Recently, Ndt80p has been shown to activate transcription of middle sporulation-specific genes in an MSE-dependent fashion and to specifically interact with MSE DNA in vitro (7). NDT80 is itself expressed at high levels as a middle sporulation-specific gene. As expected based on its expression pattern, an ndt80-Δ mutation does not derepress SMK1 expression in vegetative cells. These results show that MSES repression does not require Ndt80p and indicate that there is a distinct factor expressed in vegetative cells with an overlapping DNA-binding specificity.
MSES can function in a fashion strikingly similar to that of URS1 in early genes (4, 39). Both elements repress vegetative expression and lead to transient derepression as well as activation at precise intervals during sporulation. While these similarities suggest that URS1 and MSES could share certain components required for repression, mutations which affect URS1-dependent repression (ume6, sin3, or rpd3) in vegetative cells had no significant effects on MSE-dependent repression. Thus, URS1 and MSES appear to function through distinct repression pathways. In addition, we have shown that the Ssn6p-Tup1p corepression complex, which is required for repression of the DIT1/DIT2 divergently transcribed mid-late gene pair (14), does not affect SMK1 repression by MSES. Thus, MSES repression appears to function through a mechanism that is distinct from those previously described. The identification of genes required for MSES-dependent repression will shed new light on the mechanisms that regulate gene expression during development.
Context-dependent URS1 effects on transcription.
The presence of an early regulatory URS1 consensus site in the middle SMK1 promoter initially presented a paradox. URS1 has been shown to only transiently derepress expression during early sporulation, and we have shown that MSES is required for transient derepression during middle sporulation. If both of these elements were fully functional in a single promoter, it would be inactive throughout the entire sporulation program. This paradox is resolved by the observation that pseudo-URS1S only weakly represses the SMK1 promoter. This element has a stronger repression activity in the context of the early HOP1 promoter (Fig. 6), suggesting either that the HOP1 promoter contains sequences that are required for full pseudo-URS1S repression or that the SMK1 promoter contains sequences that antagonize the repression activity. In this respect, we have shown that pseudo-URS1S only weakly represses vegetative expression of a heterologous SMK1 promoter that contains the HOP1 Abf1p-binding site (data not shown). In contrast, the MSE is fully able to repress this promoter. Thus, the inability of pseudo-URS1S to function efficiently as a vegetative repressor of SMK1 is not dependent on the Abf1p element.
What is the function of pseudo-URS1S in the SMK1 promoter? The fact that SMK1 expressed from the early HOP1 promoter can complement a smk1-Δ mutant is consistent with the possibility that during its evolutionary history SMK1 was once regulated as an early sporulation gene. Thus, the pseudo-URS1S in SMK1 may be vestigial. However, analysis of NDT80 suggests a function for a consensus URS1 in middle promoters. While NDT80 is expressed predominantly as a middle gene, it also has a function early in sporulation, where it has been proposed to respond to meiotic recombination checkpoint controls (7, 17a, 43). NDT80 expression is dependent on IME1 (which positively regulates early transcription through the URS1 element). In the case of the NDT80 promoter, it is possible that a low level of early URS1-dependent NDT80 expression is required to initiate a positive MSE-dependent autoregulatory loop that leads to full expression of middle genes. In an ndt80 strain, SMK1 transcription is activated early in sporulation (7). This observation is consistent with the early peak of SMK1 expression seen in the mseS promoter mutant and suggests that pseudo-urs1S can function when Ndt80 activity is low.
Functional significance of SMK1 transcriptional regulation.
Our previous results demonstrate that SMK1 is required for coordination of spore morphogenesis and that regulated increases in SMK1 activity during spore development play a role in sequentially activating distinct steps required for spore morphogenesis (20, 41, 42). The tightly regulated increase in SMK1 transcription that occurs during spore morphogenesis raised the question of whether SMK1 transcriptional regulation is required to coordinate the morphogenetic program. Our results show that the constitutive expression of SMK1 does not affect vegetative growth, nor does it interfere with meiotic development or spore morphogenesis. In addition, promoter mutations which advance the timing of SMK1 expression to early sporulation make spores which are indistinguishable from wild-type spores. Thus, the developmental function of SMK1 is not contingent on its expression as a sporulation-specific middle gene. These results imply that mechanisms in addition to transcription regulate SMK1 activity during sporulation.
Phenotypic analysis of the smk1 promoter mutants show that different quantitative defects can lead to different qualitative end stage phenotypes. The UASS point mutants show that it is possible to reduce expression of SMK1 by 90% with no detectable sporulation phenotype. Thus, under optimal sporulation conditions, the level of SMK1 activity exceeds the requirements. However, when the level of SMK1 expression is reduced below a critical threshold (roughly 10% of the level expressed by wild-type cells), multiple distinct sporulation phenotypes that correlate with the degree to which SMK1 is expressed are observed. These results show that the SMK1 MAP kinase is required for the execution of multiple events during spore development and that the execution of distinct steps can require different levels of SMK1 activity.
ACKNOWLEDGMENTS
This work was supported by grants RPG-93-027-05-MG0 (to A.K.V.) and RPG-98-071-01-DDC (to E.W.) from the American Cancer Society, MCB-9630656 from the National Science Foundation (to E.W.), and a Busch Postdoctoral Fellowship (to V.G.-D.).
We thank Jacqueline Segall and Helena Friesen for sharing unpublished results and for helpful discussions.
REFERENCES
- 1.Alani E, Padmore R, Kleckner N. Analysis of wild-type and rad50 mutants of yeast suggest an intimate relationship between meiotic chromosome synapsis and recombination. Cell. 1990;61:419–436. doi: 10.1016/0092-8674(90)90524-i. [DOI] [PubMed] [Google Scholar]
- 2.Ausubel F M, Brent R, Kingston R E, Moore D D, Smith J A, Seidmann J G, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons; 1987. [Google Scholar]
- 3.Bowdish K S, Yuan H E, Mitchell A P. Analysis of RIM11, a yeast protein kinase that phosphorylates the meiotic activator IME1. Mol Cell Biol. 1994;14:7909–7919. doi: 10.1128/mcb.14.12.7909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bowdish K S, Yuan H E, Mitchell A P. Positive control of yeast meiotic genes by the negative regulator UME6. Mol Cell Biol. 1995;15:2955–2961. doi: 10.1128/mcb.15.6.2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Briza P, Breitenbach M, Ellinger A, Segall J. Isolation of two developmentally regulated genes involved in spore wall maturation in Saccharomyces cerevisiae. Genes Dev. 1990;4:1775–1789. doi: 10.1101/gad.4.10.1775. [DOI] [PubMed] [Google Scholar]
- 6.Casadaban M J, Martinez-Arias A, Shapira S K, Chou J. Beta-galactosidase gene fusions for analyzing gene expression in Escherichia coli and yeast. Methods Enzymol. 1983;100:293–308. doi: 10.1016/0076-6879(83)00063-4. [DOI] [PubMed] [Google Scholar]
- 7.Chu S, Herskowitz I. Gametogenesis in yeast is regulated by a transcriptional cascade dependent on Ndt80. Mol Cell. 1998;1:685–696. doi: 10.1016/s1097-2765(00)80068-4. [DOI] [PubMed] [Google Scholar]
- 8.Dawes I W, Hardie I D. Selective killing of vegetative cells in sporulated yeast cultures by exposure to diethyl ether. Mol Gen Genet. 1974;131:281–289. doi: 10.1007/BF00264859. [DOI] [PubMed] [Google Scholar]
- 9.Della Seta F, Ciafre S A, Marck C, Santoro B, Presutti C, Sentenac A, Bozzoni I. The ABF1 factor is the transcriptional activator of the L2 ribosomal protein genes in Saccharomyces cerevisiae. Mol Cell Biol. 1990;10:2437–2441. doi: 10.1128/mcb.10.5.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Della Seta F, Treich I, Buhler J M, Sentenac A. ABF1 binding sites in yeast RNA polymerase genes. J Biol Chem. 1990;265:15168–15175. [PubMed] [Google Scholar]
- 11.Diffley J F, Stillman B. Similarity between the transcriptional silencer binding proteins ABF1 and RAP1. Science. 1989;246:1034–1038. doi: 10.1126/science.2511628. [DOI] [PubMed] [Google Scholar]
- 12.Dorsman J C, Doorenbosch M M, Maurer C T, de Winde J H, Mager W H, Planta R J, Grivell L A. An ARS/silencer binding factor also activates two ribosomal protein genes in yeast. Nucleic Acids Res. 1989;17:4917–4923. doi: 10.1093/nar/17.13.4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dorsman J C, van Heeswijk W C, Grivell L A. Yeast general transcription factor GFI: sequence requirements for binding to DNA and evolutionary conservation. Nucleic Acids Res. 1990;18:2769–2776. doi: 10.1093/nar/18.9.2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friesen H, Hepworth S R, Segall J. An Ssn6-Tup1-dependent negative regulatory element controls sporulation-specific expression of DIT1 and DIT2 in Saccharomyces cerevisiae. Mol Cell Biol. 1997;17:123–134. doi: 10.1128/mcb.17.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gailus-Durner V, Chintamaneni C, Wilson R, Brill S J, Vershon A K. Analysis of a meiosis-specific URS1 site: sequence requirements and involvement of replication protein A. Mol Cell Biol. 1997;17:3536–3546. doi: 10.1128/mcb.17.7.3536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gailus-Durner V, Xie J, Chintamaneni C, Vershon A K. Participation of the yeast activator Abf1 in meiosis-specific expression of the HOP1 gene. Mol Cell Biol. 1996;16:2777–2786. doi: 10.1128/mcb.16.6.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hepworth S R, Ebisuzaki L K, Segall J. A 15-base-pair element activates the SPS4 gene midway through sporulation in Saccharomyces cerevisiae. Mol Cell Biol. 1995;15:3934–3944. doi: 10.1128/mcb.15.7.3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17a.Hepworth S R, Friesen H, Segall J. NDT80 and the meiotic recombination, checkpoint regulate expression of middle sporulation-specific genes in Saccharomyces cerevisiae. Mol Cell Biol. 1998;18:5750–5761. doi: 10.1128/mcb.18.10.5750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hill J E, Myers A M, Koerner T J, Tzagoloff A. Yeast E. coli shuttle vectors with multiple unique restriction sites. Yeast. 1986;2:163–167. doi: 10.1002/yea.320020304. [DOI] [PubMed] [Google Scholar]
- 19.Kadosh D, Struhl K. Repression by Ume6 involves recruitment of a complex containing Sin3 corepressor and Rpd3 histone deacetylase to target promoters. Cell. 1997;89:365–371. doi: 10.1016/s0092-8674(00)80217-2. [DOI] [PubMed] [Google Scholar]
- 20.Krisak L, Strich R, Winters R S, Hall J P, Mallory M J, Kreitzer D, Tuan R S, Winter E. SMK1, a developmentally regulated MAP kinase, is required for spore wall assembly in Saccharomyces cerevisiae. Genes Dev. 1994;8:2151–2161. doi: 10.1101/gad.8.18.2151. [DOI] [PubMed] [Google Scholar]
- 21.Kupiec M, Byers B, Esposito R, Mitchell A. Meiosis and sporulation in Saccharomyces cerevisiae. In: Pringle J, Broach J, Jones E, editors. The molecular and cellular biology of the yeast Saccharomyces. Cold Spring Harbor, N.Y: Cold Spring Harbor Press; 1997. pp. 889–1036. [Google Scholar]
- 22.Law D T, Segall J. The SPS100 gene of Saccharomyces cerevisiae is activated late in the sporulation process and contributes to spore wall maturation. Mol Cell Biol. 1988;8:912–922. doi: 10.1128/mcb.8.2.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McBroom L D, Sadowski P D. Contacts of the ABF1 protein of Saccharomyces cerevisiae with a DNA binding site at MATa. J Biol Chem. 1994;269:16455–16460. [PubMed] [Google Scholar]
- 24.McBroom L D, Sadowski P D. DNA bending by Saccharomyces cerevisiae ABF1 and its proteolytic fragments. J Biol Chem. 1994;269:16461–16468. [PubMed] [Google Scholar]
- 25.McBroom L D, Sadowski P D. Functional analysis of the ABF1-binding sites within the Ya regions of the MATa and HMRa loci of Saccharomyces cerevisiae. Curr Genet. 1995;28:1–11. doi: 10.1007/BF00311875. [DOI] [PubMed] [Google Scholar]
- 26.Mitchell A P. Control of meiotic gene expression in Saccharomyces cerevisiae. Microbiol Rev. 1994;58:56–70. doi: 10.1128/mr.58.1.56-70.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ozsarac N, Bhattacharyya M, Dawes I W, Clancy M J. The SPR3 gene encodes a sporulation-specific homologue of the yeast CDC3/10/11/12 family of bud neck microfilaments and is regulated by ABF1. Gene. 1995;164:157–162. doi: 10.1016/0378-1119(95)00438-c. [DOI] [PubMed] [Google Scholar]
- 28.Ozsarac N, Straffon M J, Dalton H E, Dawes I W. Regulation of gene expression during meiosis in Saccharomyces cerevisiae: SPR3 is controlled by both ABF1 and a new sporulation control element. Mol Cell Biol. 1997;17:1152–1159. doi: 10.1128/mcb.17.3.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park H D, Luche R M, Cooper T G. The yeast UME6 gene product is required for transcriptional repression mediated by the CAR1 URS1 repressor binding site. Nucleic Acids Res. 1992;20:1909–1915. doi: 10.1093/nar/20.8.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rubin-Bejerano I, Mandel S, Robzyk K, Kassir Y. Induction of meiosis in Saccharomyces cerevisiae depends on conversion of the transcriptional repressor Ume6 to a positive regulator by its regulated association with the transcriptional activator Ime1. Mol Cell Biol. 1996;16:2518–2526. doi: 10.1128/mcb.16.5.2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sherman F, Fink G, Hicks J B. Methods in yeast genetics: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1986. [Google Scholar]
- 32.Sikorski R S, Boeke J D. In vitro mutagenesis and plasmid shuffling: from cloned gene to mutant yeast. Methods Enzymol. 1991;194:302–318. doi: 10.1016/0076-6879(91)94023-6. [DOI] [PubMed] [Google Scholar]
- 33.Sikorski R S, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith H E, Su S S, Neigeborn L, Driscoll S E, Mitchell A P. Role of IME1 expression in regulation of meiosis in Saccharomyces cerevisiae. Mol Cell Biol. 1990;10:6103–6113. doi: 10.1128/mcb.10.12.6103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steber C M, Esposito R E. UME6 is a central component of a developmental regulatory switch controlling meiosis-specific gene expression. Proc Natl Acad Sci USA. 1995;92:12490–12494. doi: 10.1073/pnas.92.26.12490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stern B, Nurse P. A quantitative model for the cdc2 control of S phase and mitosis in fission yeast. Trends Genet. 1996;12:345–350. [PubMed] [Google Scholar]
- 37.Strich R, Surosky R T, Steber C, Dubois E, Messenguy F, Esposito R E. UME6 is a key regulator of nitrogen repression and meiotic development. Genes Dev. 1994;8:796–810. doi: 10.1101/gad.8.7.796. [DOI] [PubMed] [Google Scholar]
- 38.Su S S, Mitchell A P. Identification of functionally related genes that stimulate early meiotic gene expression in yeast. Genetics. 1993;133:67–77. doi: 10.1093/genetics/133.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vershon A K, Hollingsworth N M, Johnson A D. Meiotic induction of the yeast HOP1 gene is controlled by positive and negative regulatory sites. Mol Cell Biol. 1992;12:3706–3714. doi: 10.1128/mcb.12.9.3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vidal M, Strich R, Esposito R E, Gaber R F. RPD1 (SIN3/UME4) is required for maximal activation and repression of diverse yeast genes. Mol Cell Biol. 1991;11:6306–6316. doi: 10.1128/mcb.11.12.6306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wagner, M., P. Briza, M. Pierce, and E. Winter. SMK1 thresholds coordinate spore morphogenesis in yeast. Submitted for publication.
- 42.Wagner M, Pierce M, Winter E. The CDK-activating kinase CAK1 can dosage suppress sporulation defects of smk1 MAP kinase mutants and is required for spore wall morphogenesis in Saccharomyces cerevisiae. EMBO J. 1997;16:1305–1317. doi: 10.1093/emboj/16.6.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu L, Ajimura M, Padmore R, Klein C, Kleckner N. NDT80, a meiosis-specific gene required for exit from pachytene in Saccharomyces cerevisiae. Mol Cell Biol. 1995;15:6572–6581. doi: 10.1128/mcb.15.12.6572. [DOI] [PMC free article] [PubMed] [Google Scholar]